Abstract
Viral infections of the central nervous system (CNS) cause variable outcomes from acute to severe neurological sequelae with increased morbidity and mortality. Viral neuroinvasion directly or indirectly induces encephalitis via dysregulation of the immune response and contributes to the alteration of neuronal function and the degeneration of neuronal cells. This review provides an overview of the cellular and molecular mechanisms of virus-induced neurodegeneration. Neurotropic viral infections influence many aspects of neuronal dysfunction, including promoting chronic inflammation, inducing cellular oxidative stress, impairing mitophagy, encountering mitochondrial dynamics, enhancing metabolic rewiring, altering neurotransmitter systems, and inducing misfolded and aggregated pathological proteins associated with neurodegenerative diseases. These pathogenetic mechanisms create a multidimensional injury of the brain that leads to specific neuronal and brain dysfunction. The understanding of the molecular mechanisms underlying the neurophathogenesis associated with neurodegeneration of viral infection may emphasize the strategies for prevention, protection, and treatment of virus infection of the CNS.
Keywords: Neurodegeneration, Viral infection, CNS infection, Neurotropic virus, Oxidative stress, Apoptosis, Autophagy, Mitophagy, Neuroinflammation, Neuronal metabolism, Neurotransmission system, Alzheimer’s disease
Background
Neurodegeneration is the progressive atrophy and loss of function of neurons, glial cells, and the neural networks in the brain and spinal cord. This degeneration and death of neurons often leads to several neurodegenerative diseases that cause complex and serious medical conditions that worsen over time with motor, cognitive, and autonomic dysfunction, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and prion diseases [1]. Different neuropathologic mechanistic processes cause neuronal death in neurodegenerative diseases including the oxidative stress due to the overproduction of free radicals with the decline of cellular antioxidant defense systems. Excessive free radical formation induces mitochondrial dysfunction, mitochondrial dynamic imbalance, and impaired bioenergetics which contribute to the redox imbalance that can cause malfunctioning of the endoplasmic reticulum (ER), resulting in the abnormal protein accumulation, calcium accumulation, and intrinsic cell death pathway activation [2]. Severe or prolonged stress activates cell death pathways, including autophagy and apoptosis [3]. Neuronal loss in neurodegenerative diseases is often associated with the deposition of extra- and intracellular misfolded proteins that are toxic to neurons, impair mitochondrial redox activity, and increases the generation of oxidative stress that activates apoptosis signaling to trigger cell death [4]. The activation of an immune response by microglia and astrocytes which occurs in response to the cytotoxic consequences of the aggregation of misfolded proteins induces the production of several inflammatory factors that promote chronic neuroinflammation associated with the progression of neuronal loss in the CNS [5]. These interrelated mechanisms are involved in the death and dysfunction of neurons in neurodegenerative diseases.
Neurotropic viruses are common causes of CNS infections, both acute and chronic viral neurologic syndromes, including meningitis, encephalitis, encephalomyelitis, and myelitis [6]. Viruses can enter the CNS via two major routes: blood circulation (viremia) and crossing the blood–brain barrier (BBB) or entering via peripheral nerve endings. Viral entry into the brain through the BBB can occur through three different mechanisms: the transcellular pathway (virus passage through infected endothelial cells), the “Trojan Horse” pathway (virus transport using infected immune cells), or the paracellular pathway (virus entry through disruption of junction proteins, the actin cytoskeleton, or the basal lamina) [7, 8]. The invasion of neurotropic viruses into the CNS is associated with neurodegeneration through a variety of mechanisms. Viruses primarily infect neurons through interaction with host attachment factors and receptors, followed by either direct fusion to the plasma membrane (enveloped viruses) or endocytosis (non-enveloped viruses) and subsequent delivery of the viral genome (DNA or RNA) to the cytosol or the nucleus of the infected cell [9]. Upon entry, the virus replicates within neuronal cells, and the accumulation of viral antigens induces an increase in oxidative stress which leads to the activation of both innate and adaptive immune responses. Viral components are recognized by host pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), NOD-like receptors (NLRs), Retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), and cytosolic DNA sensors (CDS) which are widely expressed in the CNS cells including microglia, neurons, astrocytes, oligodendrocytes, epithelial cells, and innate immune cells [7]. Activation of PRR signaling triggers the production of inflammatory mediators in order to clear viral invasion. However, chronic activation of these receptors can cause inflammatory damage [10]. Neurotropic virus infection induces neuronal damage through direct killing, cell lysis, increased free radical release, perturbation of the cellular stress response, cellular activation of neuroinflammation, and induction of apoptotic signaling leading to neuronal cell death [11, 12]. In addition, viruses can infect neuroglial cells, which results in the activation of astrocytes and microglia, leading to the production of numerous proinflammatory cytokines. The overproduction of proinflammatory cytokines induces an increase in the permeability of the BBB, which allows the virus to easily enter the CNS. Moreover, the increase in proinflammatory cytokines induces neuroinflammation, which leads to pathological changes such as cellular infiltration, perivascular cuffing meningeal disruption, neuronal shrinkage, and plaque formation in brain tissues. Viral infections disrupt the cellular function of neurons, such as metabolic pathways and neurotransmitter synthesis, which result in neuronal and brain dysfunctions. Viral infections implicate pathogenic etiology in neurodegenerative diseases. In this review, we provide and summarize the molecular mechanisms of neuropathogenesis associated with neurotropic virus-induced neurodegeneration.
Viral Infection Induces Oxidative Stress
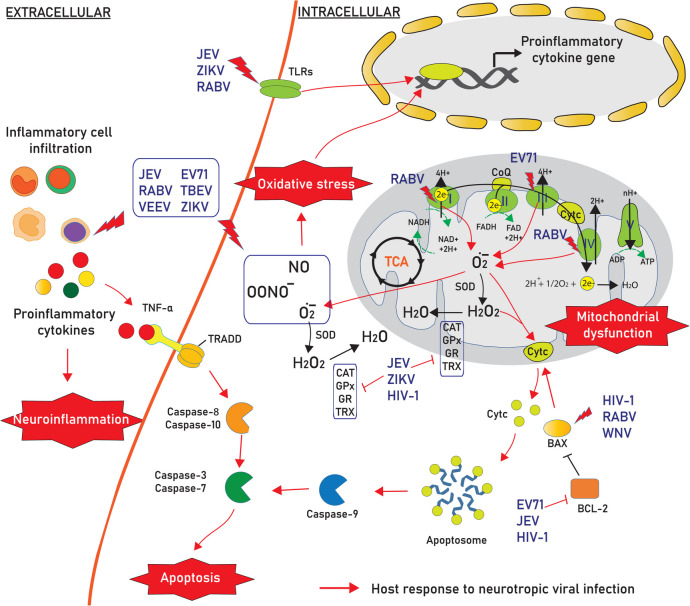
Infection with neurotropic viruses promotes oxidative stress, which is associated with excessive production of reactive oxygen species (ROS) and insufficient cellular antioxidant defenses. The disturbance in the oxidant–antioxidant balance leads to potential cellular damage in the host cell. ROS can damage cell components such as nucleic acids, lipids, and proteins and subsequently disturb their functions, contributing to neurodegeneration conditions in the CNS. Mitochondria are the major source of intracellular ROS and antioxidant enzymes, which maintain redox balance. Neurotropic viruses causing both acute and slow infection can induce the generation of ROS, which can initiate the lipid peroxidation process and play a critical role in cellular death (Fig. 1).
Fig. 1.
Neurotropic viral infection induces neurodegeneration through a variety of cellular mechanisms. Viral infection affects several host cell response mechanisms to attenuate neuronal functions, including (i) increasing reactive oxygen species (ROS) by interfering with the electron transport system and disrupting the production of antioxidants, (ii) promoting neuroinflammation by increasing proinflammatory cytokine secretion from infiltrated inflammatory cells and infected neuronal cells such as microglia and astrocytes, which then (iii) activates the apoptosis signaling pathway by inducing both intrinsic and extrinsic signaling pathways leading to neuronal death in the CNS. Abbreviation: Cyt c, cytochrome c; CoQ, coenzyme Q; BCL-2, B-cell lymphoma 2; BAX, BCL-2 associated X; FAD, flavin adenine dinucleotide; FADH, flavin adenine dinucleotide hydride; NADH, reduced nicotinamide adenine dinucleotide; NAD+, nicotinamide adenine dinucleotide; TNF-α, tumor necrosis factor alpha; TRADD, TNF receptor-associated death domain; CAT, catalase; SOD, superoxide dismutase; GPx, glutathione peroxidase; GR, glutathione reductase; TRX, thioredoxin; TLRs, toll-like receptors; TCA, tricarboxylic acid
Japanese encephalitis virus (JEV; family Flaviviridae) infection can cause Japanese encephalitis in humans with a high fatality rate in severe cases, and ~30–50% of survivors experience serious permanent neurologic sequelae or psychiatric sequelae [13, 14]. JEV infection increases the level of ROS production, which increases neuronal cell death triggered by both mature viruses and replication-incompetent virions [15]. ROS overproduction together with a decrease in membrane fluidity in JEV-infected neuronal cells causes severe cytopathic effects and subsequently contributes to neuronal cell death [16]. JEV infection increases the levels of superoxide anions (O2.-), nitric oxide (NO), and peroxynitrite (OONO-) in neurons and glial cells [17–19]. Excessive O2.- production during viral infection was also observed in neuronal cells infected with other members of Flaviviridae family, including West Nile virus (WNV) [20] and dengue virus type 2 (DENV-2) [21], leading to host cell apoptosis.
Venezuelan equine encephalitis virus (VEEV; family Togaviridae) causes severe zoonotic disease in humans, and approximately 4–14% of cases develop serious neurological complications and 1% develop lethal encephalitis [22–24]. VEEV infection significantly increases in ROS levels in astocytoma U87MG cells [25]. An increase in NO formation together with the activation of inducible nitric oxide synthase (iNOS) was observed in the brains of mice infected with VEEV [26]. The overproduction and activation of malondialdehyde (MDA), a lipid peroxidation marker, was detected in the brains of JEV-infected animals [27]. Lipid peroxidation induced by VEEV has been shown to increase the concentration of thiobarbituric acid reactive substances (TBARS), a byproduct of lipid peroxidation, in the mouse brain [26].
Rabies virus (RABV; family Rhabdoviridae) is a deadly virus that infects the CNS and causes encephalitis, ultimately resulting in death in mammals [28]. RABV infection is shown to increase the ROS production in mouse neuroblastoma cells [29]. The viral component of RABV plays a critical role in the induction of oxidative stress [30]. Immunostaining for 4-hydroxy-2-nonenal (4-HNE) indicated evidence of lipid peroxidation associated with oxidative stress that causes axonal injury, as shown by axonal swelling and reduced axonal growth in the dorsal root ganglion of RABV-infected mice [31]. Another member of the Rhabdoviridae family, Chandipura virus (CHPV), an enveloped RNA virus that causes acute encephalitis mainly in children, has been reported to induce neuronal apoptosis by stimulating oxidative stress. CHPV infection increases the intracellular Ca2+ secretion, which further increases ROS, superoxide production levels, and mitochondrial dysfunction within CHPV-infected cells and causes neuronal death in vitro and in vivo [32–34].
Enterovirus 71 (EV71; family Picornaviridae) is a major causative agent of hand, foot, and mouth disease (HFMD) with fatal neurological complications in young children. EV71 infection can lead to increased ROS generation and activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which in turn enhances EV71 infection in neural cells [35–37].
Oxidative stress is also seen in viruses causing latent or slow infections, such as herpes simplex virus (HSV; family Herpesviridae) and human immunodeficiency virus (HIV; family Retroviridae). Herpes simplex encephalitis is caused by herpes simplex virus type 1 (HSV-1) and is a common cause of sporadic focal encephalitis worldwide [38]. Intracellular ROS generation in response to HSV-1 infection was observed in microglia [39] and neural cells [40]. HSV-1 infection causes oxidative stress and induces the release of bioactive lipid peroxidation byproducts, MDA/hydroxyalkenals (HAEs), in cultured mouse neural cells, which is necessary for virus replication [40]. HIV causes immunodeficiency, which leads to acquired immunodeficiency syndrome (AIDS). HIV infects the CNS and enhances neurotoxicity, which directly harms the brain and manifests as HIV-associated neurocognitive disorders (HANDs) [41–43]. Several component proteins of human immunodeficiency virus type-1 (HIV-1) enhance ROS production in neuronal cells, including neurons, microglial cells, and astrocytes, by different mechanisms [44–46]. The HIV-1 transactivator of transcription (Tat) protein induces the production of ROS and significantly induces DNA breakage [47]. Increased levels of nitroxidative stress marker proteins such as NADPH oxidase, cytochrome P450-2E1 (CYP2E1), and iNOS are observed in HIV-1 transgenic rat brains [48].
ZIKV targeting of neuronal cells causes neurological complications such as congenital microcephaly, Guillain–Barré syndrome, transverse myelitis, and meningoencephalitis [49, 50]. ZIKV impairs mitochondrial structure and function, as shown by the decrease in oxygen flux coupled with adenosine triphosphate synthesis [51]. ZIKV infection leads to the increased production of ROS, which is associated with DNA breakage in neural cells in vivo and in vitro [51, 52]. ZIKV infection resulted in a significant increase in lipid peroxidation, as observed by the high levels of MDA and carbonyl protein in human glioblastoma-infected cells [52].
The increase in free radicals and lipid peroxidation induced by neurotrophic viruses demonstrates that oxidative stress contributes as a key factor in the pathogenesis of neurodegeneration in viral infection of the CNS.
Virus Infection Disturbs the Production of Antioxidants
The elevation of cellular oxidative stress due to increases in free radicals and lipid peroxidation was associated with an opposite decrease in antioxidant activities. Viral infections stimulate ROS production and inhibit antioxidant enzyme levels [53, 54] (Fig. 1). The antioxidant defense mechanism is impaired by a decline in intracellular antioxidant levels, such as superoxide dismutase 1 (SOD-1), thioredoxin 1 (TRX-1), and glutathione (GSH), and a reduction in catalase (CAT) and glutathione peroxidase (GPx) activities in neuronal cells and several brain regions during JEV infection [16, 27]. EV71 diminished the ratio of GSH to its disulfide form, glutathione disulfide (GSSG), an indicator of oxidative stress [55]. ZIKV-induced oxidative stress is correlated with decreased levels of SOD and CAT activities in U87-MG cells and the brains of C57BL/6 mice [52]. Infection with HSV-1 has been reported to induce the depletion of GSH [56]. Exposure of rat brain endothelial cells to HIV-1 envelope glycoprotein GP120 (gp120) and Tat significantly decreases the levels of intracellular GSH, GPx, and glutathione reductase (GR) and the ratio of GSH/GSSG [57]. The decrease in antioxidant enzyme activities after ZIKV infection was found to be associated with the negative regulation of nuclear factor erythroid 2-related factor 2 (NRF2)/antioxidant response element (ARE) signaling [52]. This evidence suggests that depletion of the antioxidant enzymatic system occurs during viral infection.
Virus Infection Induces Mitochondrial and Endoplasmic Reticulum Stresses
Mitochondrial oxidative stress is involved in pathological statuses and is the major source of excessive amounts of ROS in infected cells. ROS accumulation in cells is a direct reflection of disruption in mitochondrial electron transport chain (ETC) function and redox imbalance in the ER lumen, leading to the accumulation of unfolded proteins and in turn increasing oxidative stress, which results in increased cellular damage and apoptosis. An increase in ETC-related protein activity was observed after viral infection (Fig. 1). RABV infection significantly alters a variety of mitochondrial parameters, such as increases in maximal uncoupled respiration and complex IV respiration and mitochondrial complex I and complex IV activities in neurons [58]. The activity of mitochondrial complex I, a site of ROS production, was correlated with the high level of ROS in mouse neuroblastoma cells [29]. The increase in the generation of ROS and oxidative stress caused by the specific 139–172 region of the RABV phosphoprotein that interacts with complex I in mitochondria causes mitochondrial dysfunction [29]. RABV infection induces mitochondrial ETC dysfunction, resulting in oxidative stress and degenerative changes in neuronal processes (involving both dendrites and axons) in infected mice [31, 59, 60]. Experiments using inhibitors of several mitochondrial proteins showed that EV71 mainly induces ROS generation at a site of the mitochondrial ETC distal to mitochondrial complex III [35]. Increased mitochondrial oxidative stress by EV71 infection can cause mitochondrial morphological changes and exhibit functional anomalies, such as a decrease in mitochondrial electrochemical potential and a lower respiratory control ratio of mitochondria in glioblastoma cells [35].
Together with mitochondrial oxidative stress, ER stress usually occurs simultaneously to generate cellular stress in virus-infected cells. The ER is responsible for the correct three-dimensional conformation of protein folding and maturation. The formation of disulfide bonds produces large amounts of ROS and depletes GSH, contributing to the redox imbalance that can cause malfunctioning of the ER, including massive protein production, loss of Ca2+ homeostasis, inhibition of N-linked glycosylation, and accumulation of mutant proteins [61, 62]. Viral replication can cause an increase in viral protein synthesis demand to overcome the ER folding capacity, leading to the massive production of misfolded viral proteins that accumulate in the ER lumen and trigger ER stress [63]. JEV infection induces ER stress as evidence of the detection of the excessive proliferation of ER membranes together with the amount of viral proteins and the induction of unfolded protein response (UPR) signaling in neuronal N18 and NT-2 cells [16]. In human neural stem cells (hNS1 cells), JEV infection promoted the expression of ER stress-related proteins such as glucose-regulated protein 78 kDa (GRP78), heat shock protein (HSP) 60, HSP70, and HSP90 [64]. JEV infection activates several ER stress sensors, including protein kinase RNA-like ER kinase (PERK), activating transcription factor 4 (ATF4), and C/EBP homologous protein (CHOP) under acute or prolonged ER stress. The JEV-induced UPR provokes CHOP/growth arrest and DNA damage-inducible protein (GADD153) and triggers the activation of p38 mitogen-activated protein kinase (MAPK), which enhances JEV-induced apoptosis via the activation of the caspase cascade [16]. JEV nonstructural protein 4B (NS4B) activates the PERK/ATF4/CHOP neuronal apoptosis pathway both in vitro and in vivo [65].
WNV infection activates multiple branches of ER stress-mediated UPR pathways, leading to transcriptional and translational induction of UPR target genes [63]. Activation of ATF6 and PERK pathways was induced during WNV infection in SK-N-MC neuroblastoma cells, resulting in CHOP activation and downstream apoptosis [66]. The transcriptomic analysis shows that the components of the UPR-related genes such as PERK, ATF4, and DDIT3 (encoded CHOP) and early growth response 1 (EGR1) are activated in VEEV-infected human astrocytoma cells [67]. The activation of EGR1 is regulated by extracellular-signal-regulated kinase (ERK) and PERK pathways, which are important in contributing to neural cell death in VEEV infection [68].
ZIKV infection activates ER stress by significantly increasing the expression of ER stress markers in neural cells [69]. ZIKV upregulates UPR-related genes in the cerebral cortex of infected postmortem human fetuses as well as in cultured human neural stem cells and in the mouse embryonic brain [70]. Transfection with EV71 viral envelope protein 1 (VP1) increased translation initiation factor 2α (eIF2α) kinase phosphorylates Serine51 (Ser51) phosphorylation, which represented to ER stress activation in mouse brainstem neurons [71]. In mouse Schwann cells, the overexpression of VP1 also induced ER stress, leading to the upregulation of peripheral myelin protein 22 (PMP22) [72]. These observations indicate the role of ER stress in the pathogenesis induced by viral infection.
Virus Infection Induces Alteration of Autophagy
The cellular stress response induced by virus infection is also related to the induction of autophagy in infected cells [73]. CHPV infection shows an increase in microtubule-associated protein 1 light chain 3 beta (LC3B), an autophagic marker, associated with overproduction of ROS and mitochondria dysfunction in HT-22 mouse hippocampal neuronal cell [32]. Infection with EV71 shows an increase in LC3-II protein as well as the formation of LC3 aggregates, autophagosomes, and amphisomes in several infected brain tissues of mice [74, 75]. Immunofluorescence staining indicated the colocalization of EV71 proteins with LC3 and mannose-6-phosphate receptor (MPR, endosome marker) proteins, which indicates amphisome formation accompanied by autophagic flux in EV71-infected SK-N-SH cells [74]. The EV71 VP1 protein is an important neurovirulence protein that induces autophagy by regulating the mammalian target of rapamycin (mTOR) signaling pathway to promote viral replication [75–77]. Phosphorylated mTOR, phosphorylated 70-kDa S6 kinase (p70S6K), and peroxisomal acyl-coenzyme A oxidase 1 (ACOX1) are signaling pathways involved in EV71-induced autophagy and neural cell apoptosis when downregulated [36, 75, 76]. In HIV-1 associated encephalitis, increases in several autophagic markers, such as Beclin-1, autophagy-related gene (Atg)-5, Atg-7, and LC3-II, have been observed in frontal cortex postmortem brains [78]. The SK-N-SH cells treated with gp120 from C-X-C motif chemokine receptor 4 (CXCR4) and C-C motif chemokine receptor 5 (CCR5)-tropic HIV-1 virus exhibit the accumulation of autophagic proteins and autophagosomes [78]. Autophagy in HAND has also been evaluated. HIV-1 proteins such as Nef and Tat alter neural autophagy in different manners related to the autophagosome formation and autophagic flux, which may contribute to HAND [79–81]. The JEV-activated autophagy has been explored in both N2a neuroblastoma cells and mouse brains, and it demonstrated an increase in LC3-II protein accumulation and induction of autophagosome formation [82].
Virus Infection Induces Impairment of Mitophagy and Mitochondrial Dynamics
In addition, viruses induced impairment of mitochondrial dynamics and triggered mitophagy, a specific autophagy form for the removal of damaged mitochondria, directly and indirectly, and controlled the mitophagic process via different strategies [83]. The accumulation of ROS by VEEV TC-83 infection alters mitochondrial dynamics by affecting the expression of dynamin-related protein 1 (DRP1), a protein that plays a role in mitochondrial fission, resulting in an increase in mitochondrial fractions in infected astrocytes [25]. PTEN-induced kinase 1 (PINK1) and Parkin, proteins associated with mitophagy, appear to be enriched in mitochondrial fractions, indicating that mitochondrial damage contributes to the apoptosis of infected cells [25]. As shown in human primary neurons, HIV-1 gp120 and Tat proteins induce the mitochondrial fission process via DRP1 as a result of neural mitochondrial fragmentation and then activate mitophagy markers such as LC3B and Beclin-1 and recruit PINK and Parkin sequestosome 1 (SQSTM1) to damaged mitochondria [84–87]. However, HIV-1 proteins are found to inhibit mitophagic flux in human primary neurons by impairing the delivery of mitochondria to the lysosomal compartment, leading to incomplete neuronal mitophagy, which causes neuronal damage [84]. Dysregulation of autophagy and mitophagy and alteration of mitochondrial dynamics may be important and contribute to the pathogenesis of neurotropic virus infection.
Virus Infection Activates the Apoptosis Signaling Pathway
Oxidative stress elicits the loss of mitochondrial membrane potential and results in the induction of the intrinsic apoptosis pathway [16, 88, 89]. The expression of apoptotic protein markers has been observed in several neurotropic virus-infected cells (Fig. 1). Overexpression of caspase-3 is observed in the brains of pediatric patients with HIV-1 encephalitis and corresponds to increases in DNA fragmentation, a marker of apoptotic cells [90]. In the HIV-1 model, the levels of BCL2 associated X (BAX) and activated caspase-3 were significantly elevated in the hippocampus and were associated with neuronal cell death in HIV-1 transgenic rats [48]. HIV-1 gp120 protein induces apoptosis in neurons and microglial cells in association with the activation of MAPK pathways mediated by ERK and JNK and lowering the expression of B-cell lymphoma 2 (BCL-2) [42, 91, 92]. BCL-2 is a major target of HIV-induced changes that are modulated to different degrees during HIV infection, resulting in either a proapoptotic or an antiapoptotic phenotype [93]. VEEV infection causes neuronal injury ranging from nuclear chromatin condensations to nuclear and cellular fragmentation, indicating apoptotic cell death [94]. The positron emission tomography (PET) with a tracer targeting the caspase-3 substrate revealed an increase in apoptosis and a decrease in BBB integrity in VEEV-infected mouse brains [95]. EV71 markedly reduces BCL-2 expression but induces an increase in the mRNA expression of several apoptosis-promoting factors, such as BAX, CASP7 (caspase-7), CASP3 (caspase-3), and cleaved caspase-3 [96]. EV71 infection triggers the translocation of cytochrome c (Cyt c) from mitochondria to the cytosol, and caspase-9 is activated, leading to neural cell death and indicating that the mitochondria-mediated intrinsic apoptotic pathway is activated by EV71 [97, 98]. EV71 also induces cell cycle arrest of SH-SY5Y cells through stimulation of endogenous microRNA let-7b expression after EV71 infection [96]. The invasion of RABV into the CNS shows morphologic changes of apoptosis and marked increases in BAX in the hippocampus and cerebral cortex [99–103]. The marked apoptotic are present in neurons, glial cells, and perivascular mononuclear cells within the white matter of the cerebellum of dogs with the positive detection of BCL-2 and BAX protein [104]. The morphological changes and apoptosis of spinal neurons and dorsal root spinal ganglion cells are present in the late period of infection [105]. The replication of RABV in mouse neuroblastoma cells increases the level of BAX and caspase activation, which induces the degradation of poly ADP-ribose polymerase (PARP), leading to destruction of the DNA [106]. WNV proteins such as capsid and nonstructural protein 3 (NS3) induce apoptotic features in CNS cells via BAX-dependent apoptosis that triggers mitochondria-outer-membrane-permeabilization [107–111]. WNV replication decreased cell viability and induced upregulation of BAX expression and the release of Cyt c from the mitochondria and formation of apoptosomes, followed by the activation of the effector caspase-3, the initiator caspases-8 and -9 then cleavage of the PARP in which induces neuronal cell death [66, 108, 111–114]. JEV infection promoted increased the expression of BAX [89, 115], cleaved PARP levels [88], and the activation of caspase-3 activity [116], were observed in JEV-infected cells (Fig. 1). JEV infection also increased Cyt c release and caspase 3 activation in cultured neuronal cells and in infected mouse brain, inducing neuronal death, and increasing the mortality rate of mice [19, 115, 117]. A reduction in BCL-2 expression levels was found in JEV-infected neuronal cells and the mouse brain [115]. Several studies have also reported the overexpression of BCL-2 levels at the early stage of JEV infection, which helps to the induce host cell survival in order to facilitate viral persistence [117–121]. However, the studies showed that the increase in BCL-2 during JEV infection failed to inhibit the infected cells from undergoing apoptosis and failed to block viral replication [117, 119, 121, 122]. Moreover, our recent study with a high multiplicity of infection (MOI) of Beijing-1 infection remarkably induced neuronal cell death via inducing the proteolysis of endogenous p21 BAX to generate more apoptogenic molecules of p18 BAX during the late stage of JEV infection [117]. The accumulation of cleaved p18 BAX might be due to the activation of calpain protease, which increases the intrinsic cytotoxic properties of this proapoptotic molecule and effectively induces Cyt c release from mitochondria, leading to the activation of the caspase cascade, which results in the induction of intrinsic apoptosis [123]. Thus, the intrinsic apoptosis pathway plays an important role in the pathogenesis of neurotropic virus infection.
Virus Infection Induces Neuroinflammation
Neuroinflammation is an important feature of neurodegenerative disorders. Normally, neuroinflammatory processes play a protective role in response to CNS injury by several factors, including viral infections. Neurotropic viral infection of the CNS can cause the activation of microglia and astrocytes, inducing the production of several neuroinflammatory factors that promote chronic inflammation and increasing the progression of CNS diseases by the virus [124].
Inflammation is a hallmark of virus encephalitis. During JEV infection, various peripheral immune cell types infiltrate the CNS and affect the integrity of the BBB, which is a major regulator of neuroinflammation and viral propagation into the brain [125, 126]. In addition, peripheral immune cells infiltrating brain-resident cells interact with JEV upon infection of the brain. Microglial cells are the resident macrophages of the CNS, can be productively infected by JEV, and might serve as a reservoir for the virus [127]. The activation of microglia has been proposed to play a major role in neuronal cell death through the release of proinflammatory mediators [125, 128, 129]. The upregulation of TLR3, TLR7, and RIG-I signaling, including ERK, p38MAPK, activator protein 1 (AP-1), and nuclear factor kappa B (NF-κB), are triggered by JEV infection and induces the activation of microglia [116, 129] (Fig. 1). JEV induces encephalopathy by activating microglial cells and astrocytes that can increase the levels of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukins (IL-6, IL-8 and IL-10), and RANTES (regulated on activation, normal T-cell expressed and secreted) [130, 131].
Overproduction of chemokines and proinflammatory cytokines are key factors that play an important role in the pathogenesis of flavivirus infections. In the brain, the release of chemokines regulates the migration of leukocytes from the peripheral into the site of infection. Both clinical and experimental models suggest the involvement of neuroinflammation in dengue virus disease [132]. Intracranial injection of DENV is associated with the induction of interferon-beta (IFN-β), interferon-gamma (IFN-γ), TNF-α, chemokine (C-C motif) ligand 2 (CCL-2), CCL-5, C-X-C motif chemokine (CXCL)1, CXCL2, and IFN-stimulated gene (ISG) expression including viperin, Ifi27l2a, IRF7, and CXCL10 [133, 134]. DENV-2 and DENV-3 infections enhance of the infiltration of CD8+ and CD4+ T cells and neutrophils in the brain [132–134]. High levels of chemokines and proinflammatory cytokines such as IL-6, IL-1β, IFNγ, and TNF-α were associated with the severity of neuronal damage and the high mortality rate of mice during JEV infection [19, 131, 135–137]. TNF-α has a major effect on the induction of neuronal cell death in JEV infection by inducing the extrinsic apoptosis pathway via TNF receptor-associated death domain (TRADD) [89] and triggering inflammatory cells to release other cytokines, resulting in neuronal cell death [19, 131]. Moreover, IL-6, CXCL10, CCL-2, and CCL-5 downregulated the expression of BBB tight junction proteins, causing an increase in BBB permeability [136]. In addition, IL-1β activates the expression of adhesion molecules on epithelial cells, leading to inflammatory cell infiltration and the subsequent generation of numerous proinflammatory mediators, which ultimately cause neuronal cell death and irreversible brain damage [131, 136].
In another encephalitis virus, VEEV, PET tracer was used to target 18-kDa translocator protein (TSPO) to study the accumulation of macrophages and microglia/astrocyte activation; the results demonstrated an increase in neuroinflammation in the cortex, thalamus, striatum, hypothalamus, hippocampus, olfactory bulb, brain stem, and cerebellum of VEEV, and a decrease in BBB integrity was observed in VEEV-infected mouse brains [95]. VEEV-infected microglia produce several proinflammatory cytokines as a result of direct infection, including IFNγ, IL-1α, IL-1β, IL-6, and IL-12 [138].
The secretion of the proinflammatory cytokines, IFNγ, IL-6, and IL-1β in the cerebrospinal fluid (CSF) was increased in the acute stage of CNS involvement from EV71 infection [139, 140]. The histological studies of EV71-infected autopsy tissues showed obvious inflammation in the spinal cord gray matter, brainstem, hypothalamus, and subthalamic and dentate nuclei with perivascular cuffs, variable edema, neuronophagia, and microglial infiltration [141, 142]. EV71 infection increases TNF-α, IL-1β and RANTES production, which triggers bystander damage to neurons involving the tyrosine kinase/MAPKs/NF-κB signaling cascade during EV71 infection [143].
Tick-borne encephalitis virus (TBEV; family Flaviviridae) is an important tick-transmitted virus that causes tick-borne encephalitis (TBE) in Eurasia [144]. Increased levels several cytokines and chemokines have been detected in CSF or serum samples from TBE patients with higher ratios of IL-12:IL-4 and IL-12:IL-10, reflecting the global pro-inflammatory cytokine balance [145–148]. TBEV disrupts the BBB and infects neurons, astrocytes, and oligodendrocytes, inducing neuroinflammation followed by neuronal death [149–151]. TBEV infection upregulates the expression of several pathogen recognition receptors, pro-inflammatory cytokines, and interferon-stimulated genes in neuronal/glial cells [152, 153]. TBEV-infected mice exhibited time-dependent increases in serum and brain tissue concentrations of multiple cytokines/chemokines such as CXCL10/IP-10, CXCL1, G-CSF IL-6, and RANTES [153, 154]. Thus, neurons and astrocytes are potential sources of pro-inflammatory cytokines in TBEV infection (Fig. 1).
The CNS is the major target of ZIKV infection. In addition to the induction of massive neuronal cell damage, ZIKV-mediated neuroinflammation is the key to causing neurological pathology via an excessive inflammatory response, particularly in neonates. The relationship between ZIKV-induced neuroinflammation and postnatal microcephaly has been revealed in animal models and fatal cases of ZIKV-associated microcephaly [155, 156]. Brain histology demonstrated a pattern of inflammation, such as microglial activation, astrogliosis, vascular edema, lymphocytic infiltration, neuronal necrosis, neuronophagy, calcifications and apoptosis, which disrupted neural progenitor cells (NPCs) and neurovascular developments [155–157]. During ZIKV infection, activated microglia and astrocytes are mainly responsible for the production of several proinflammatory mediators, which are correlated with high expression of apoptosis markers in the brain and leakage of the BBB [155, 156, 158]. The molecular mechanism of ZIKV-mediated inflammation has been explored in vitro and in vivo [158–160]. TLR3 signaling was induced by ZIKV infection in both microglia and astrocytes, leading to increased NF-κB and PERK phosphorylation, which triggered the high production of several proinflammatory cytokines involved in the inflammatory process, such as IL-6, IL-1α, IL-4, IL-10, IL-8, monocyte chemoattractant protein-1 (MCP-1), RANTES, IFN-β, and transforming growth factor beta (TGF-β) [158] (Fig. 1). In addition, overproduction of IL-6, macrophage inflammatory protein (MIP)-1α, MIP-1β, MCP-1, TNF-α, IL-1Rα, IL-1α, IL-1β, IL-8, and IL-12p70 was observed in ZIKV-infected primary human fetal brain cells [160]. ZIKV infection induced the activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome in glioblastoma cells [159]. Therefore, the brain damage by uncontrolled inflammation is an important pathogenesis of ZIKV infection.
In addition, in the RABV-infected brain, microglial cells and astrocytes were significantly increased in the areas of the nerve cells that showed apoptosis [100, 103, 161, 162]. The neuronal damage from RABV infection is caused by the high production of inflammatory cytokines in primary astrocytes and microglia [163]. The induction of inflammation in the mouse brain by RABV is observed by the overexpression of TLR3, TLR4, MIP-1α, RANTES, IP-10, MCP-1, TNF-α, and IL-6, which increases RABV pathogenicity [162, 164]. These observations demonstrate that the persistent and high-level expression of chemokines, excessive infiltration, and accumulation of inflammatory cells in the CNS, and severe enhancement of BBB permeability are the major features associated with the neuropathology by neurotropic infection.
Viral Infection Alters Brain Metabolism
Over the past decade, it has become clear that viruses rely on host cell machinery to facilitate their replication. One of these mechanisms is based on the alteration of host cell metabolism, including glycolysis, the pentose phosphate pathway (PPP), the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation, to facilitate optimal viral propagation. In the brain, it is probably more important to acknowledge that virus infection affects neuron and astrocyte metabolism, which disrupts brain function.
Virus Infection Disturbs Neuron Metabolism
Brain development requires a controlled cellular metabolism for neural stem cell proliferation, differentiation, and maturation. Neural stem cell proliferation appears to have a specialized metabolism and is more dependent on glycolysis than oxidative phosphorylation for energy production. In contrast, neuronal differentiation is strongly manipulated by metabolic rewiring from glycolysis to oxidative phosphorylation [165]. Increasing evidence indicates that ZIKV infection suppresses neuron stem cell proliferation and induces premature differentiation, causing microcephaly of the newborn during pregnancy [166–168].
In recent publications, a multiomics study comprising the combination of metabolomic data with the information from transcriptomics and proteomics has been used to investigate and model the role of ZIKV-induced microcephaly. It demonstrated the metabolic alterations of nicotinamide adenine dinucleotide (NAD+)-related pathways including the TCA cycle, amino acid metabolism, and mitochondrial oxidative phosphorylation [169]. The mechanisms governing ZIKV disturbance of host cell metabolism are not yet fully understood. An activation of MAPK and cyclic guanidine monophosphate (GMP)-protein kinase G signaling upon ZIKV infection likely has a critical impact on the pathogenesis of ZIKV-induced microcephaly [169]. Initial studies demonstrated that NAD+ metabolism may be highly dependent on MAPK-mediated nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2) degradation, thereby promoting axon degeneration [170, 171]; this suggests that ZIKV infection alters cellular metabolism via the MAPK-NMNAT2-NAD+ axis (Fig. 2). These signatures of metabolic reprogramming are consistent with the findings that ZIKV in both wild-type and mutant strains disrupts glycolytic flow into the TCA cycle, leading to mitochondrial dysfunction, which triggers inflammation and neuronal cell death [172]. In addition, due to the reduction in glycolysis and the TCA cycle seen in ZIKV-infected host cells, there is a dependence on the PPP (Fig. 2). For example, there is actually an increase in metabolic intermediates, such as guanidine diphosphate and GMP, in the PPP of ZIKV-infected cells [172]. Trends toward a decreased glucose contribution to the TCA cycle and an increased contribution to PPP cells to sustain viral propagation were also observed in infected C6/36 mosquitoes [173], suggesting that ZIKV infection in neurons may divert the carbon substrate into the PPP to promote viral replication.
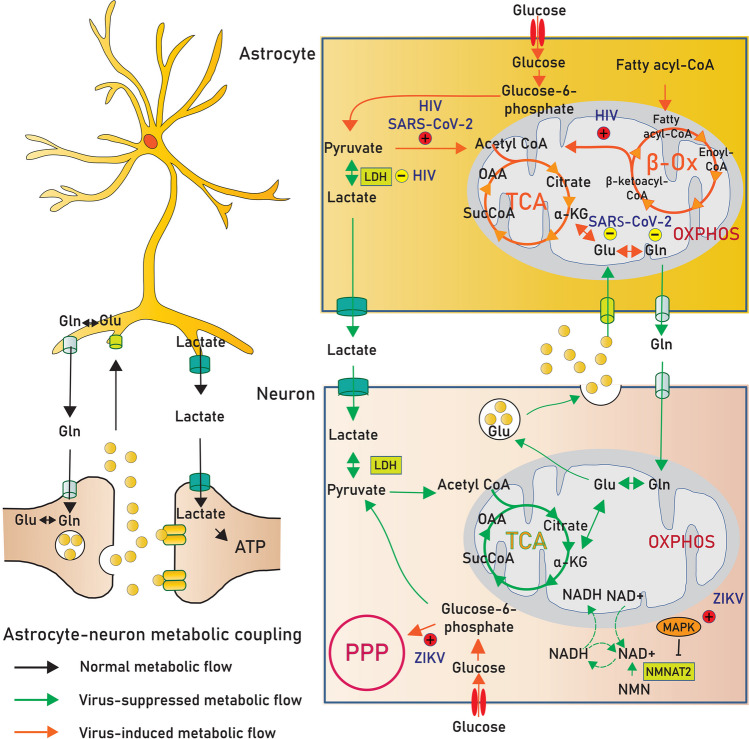
Fig. 2.
Cellular metabolism in the brain is altered by neurotrophic virus infection. Upon infection, there is a systemic metabolic alteration in neuron and astrocyte. The astrocyte to neuron lactate shuttle is inhibited and subsequently enhanced TCA cycle and oxidative phosphorylation in astrocytes. To fuel active TCA cycle, virus may activate fatty acid β-oxidation and glutamine metabolism. Within the neuron, the metabolic flow of glycolysis, TCA cycle, and oxidative phosphorylation is disrupted and selectively increased pentose phosphate pathway to sustain viral replication. Reduction of lactate production from astrocyte and dysregulation of neuron metabolism cooperatively induce neuron dysfunction. Abbreviation: α-KG, alpha ketoglutarate; β-Ox, beta oxidation; Gln, glutamine; Glu, glutamate; LDH, lactate dehydrogenase; NMN, nicotinamide mononucleotide; PPP, pentose phosphate pathway; OAA, oxaloacetate; OXPHOS, oxidative phosphorylation; SucCoA, succinyl-CoA; TCA, tricarboxylic acid
Virus Infection Disrupts Astrocyte-Neuron Metabolic Cooperation
The metabolism of neurons is functionally linked with astrocytes that provide energetic support to fuel the active neuron. While neuronal cells have robust aerobic glycolysis by converting glucose into acetyl-CoA for the production of substrates for the TCA cycle and oxidative phosphorylation, astrocytes, despite the presence of oxygen, favor the production of lactate. Astrocytes utilize glucose as their main energy source, and approximately 60% of glucose is converted to lactate [174], which is constitutively released to the extracellular milieu and taken up by neuronal cells to supply their high metabolic demand. This cooperative function of astrocytes and neurons is known as the astrocyte-to-neuron lactate shuttle (ANLS) [175]. Astrocyte-neuron metabolic cooperation is tightly regulated, but disrupting this ANLS by viral infection may cause abnormalities in brain function.
Neurological complications in HIV are strongly associated with cognitive impairment. Several studies have shown that induction of viral protein accelerates cellular damage in the CNS. HIV Tat release from infected cells can activate astrocytes and damage surrounding neurons [176, 177]. In fact, a number of studies have provided details about the potential mechanism underlying the role of HIV Tat in astrocyte metabolic shift and neurotoxicity (Fig. 2). First, the decrease in lactate dehydrogenase activity in Tat-activated astrocytes led to a reduction in extracellular lactate levels, which impaired neuronal energy metabolism and function [178]. Second, the cellular response of astrocytes to Tat is a metabolic shift from aerobic glycolysis to mitochondrial respiration mediated by the mitochondrial Ca2+ uniporter (MCU) regulating Ca2+ uptake. Targeting MCU has been found to rescue glycolysis and normalize extracellular lactate levels in astrocytes [178]. Third, astrocyte cellular stress mediated by Tat utilizes fatty acids as the energy source to support mitochondrial respiration [178]. Finally, HIV Tat impacts oxidative injury in astrocytes by reducing glutathione synthase. These changes in turn activate AMPK and increase glycolytic enzymes along with oxidative phosphorylation [179].
Neurological symptoms are more commonly observed in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; family Coronaviridae) infection. A number of studies have indicated that the foci of SARS-CoV-2 infection are also observed in astrocytes. The study shows that infected astrocytes alter key proteins and metabolites involved in glycolysis, gluconeogenesis, and the PPP. The decrease in lactate and pyruvate was due to the induction of oxidative metabolism in infected cells [180]. To supply the metabolic intermediates used in oxidative phosphorylation, infected astrocytes tend to alter other metabolic pathways, including glutamine metabolism. Intriguingly, among glutamine metabolic intermediates, glutamine, glutamate, and gamma-aminobutyric acid (GABA) are gradually decreased following SARS-CoV-2 infection [180, 181]. As astrocytes cooperate with neurons to maintain the glutamate-glutamine cycle in the brain, a reduction in glutamine metabolism in astrocytes may shape brain activities.
Virus Infection Affects Neurotransmission System
Previous reports have demonstrated the involvement of viral infection in neuronal dysfunction by affecting neurotransmitter systems. Neurotransmitters are endogenous chemical messengers by which neurons communicate with each other to enable the brain to regulate a variety of functions through the process of synaptic transmission. Alterations in neurotransmitter levels have been observed in some neurotropic viral infections and are correlated with the impairment of specific brain functions. Dopamine is the main catecholamine neurotransmitter that controls voluntary movement, cognition, and endocrine regulation [182]. JEV infection significantly increases dopamine production and modulates the rate-limiting enzyme of dopamine biosynthesis, with an increase in phosphorelated tyrosine hydroxylase levels at the early stage of infection [183]. JEVs exploit dopamine-mediated neuronal communication to increase the susceptibility of dopamine D2 receptors (D2R)-expressing cells to JEV infection, which causes damage to dopaminergic neuron-rich areas such as the thalamus and midbrain, leading to neuronal loss and increasing the fatality rate of JEV-infected mice [183]. The marked decline in catecholamine levels, including norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and serotonin, which are unrecoverable, has been reported in the brain, and it leads to the impairment of locomotor activity in JEV-infected rats [184]. The cholinergic system plays an important role in cognitive function. The decrease in acetylcholinesterase (AChE) activity together with damage to different brain regions is associated with the transient dysfunction of learning ability in JEV-infected rats [185, 186]. The decline in muscarinic cholinergic signaling in several brain regions of JEV-infected rats includes the expression levels of cholinergic receptor muscarinic 2 and choline acetyltransferase, and the reduction in total muscarinic cholinergic binding is correlated with transient spatial learning and memory impairment [186].
The balance of glutamate, the most abundant excitatory neurotransmitter, and GABA, an inhibitory neurotransmitter in the CNS, plays a crucial role in several brain functions, including synaptic signaling, cognition, pain, and motor stimuli [187–189]. HIV infection causes the imbalance of these two neurotransmitters and is correlated with neuronal and glial dysfunction as well as cognitive impairment in the brains of HIV-seropositive patients with prolonged antiretroviral treatment [190–193].
Glutamate-mediated excitohypertoxicity is an important mechanism of neuronal injury by viral infection. Several studies have revealed the effect of HIV-1 proteins on neurotoxicity induced by glutamatergic system dysregulation [194]. The HIV-1 Tat protein induces the formation of a macromolecular complex involving N-methyl-d-aspartic acid (NMDA) receptors that promotes apoptosis in neurons and astrocytes [195–197]. Dysregulation of glutamate can contribute to HIV-associated neurocognitive disorder [198, 199]. Similar to JEV infection, the increase in glutamate-mediated excitotoxicity activity is correlated with the increase in oxidative stress observed in the brain region responsible for memory and learning impairment in JEV-infected animals [200]. The increased glutamate levels and decreased levels of its NMDA receptors are associated with the activation of TNF-α signaling and the presence of redox imbalance in neural cells, which promote neuronal death [200, 201]. These results suggest that neurotropic virus infection can alter the neurotransmission systems that lead to the dysregulation of brain function and contribute to virus-induced neuropathogenesis.
Virus Infection Accelerates Alzheimer's Disease-Like Pathological Features
Alzheimer’s disease (AD) is the most common cause of dementia that leads to the death of elderly persons. Although age is the most important risk factor for the AD, however, several infections have been suggested to increase the risk of AD. AD is characterized by progressive impairment of synaptic function and degeneration in the brain. Two major pathological features associated with AD include amyloid and tau aggregates in the brain. Amyloid beta (Aβ) plaque accumulation is the main hallmark observed in the brains of AD patients [202]. Aβ is produced by the proteolytic process of the amyloid precursor protein (APP) that occurs at biological membrane. The cleavage of APP by α-secretase or enzymes from the disintegrin and metalloproteinase domain proteins (ADAM) family occurs within the Aβ region on APP; therefore this pathway does not produce Aβ peptide in neurons and called nonamyloidogenic pathway [203]. After α-secretase cleavage, soluble APP α (sAPPα) and α-C-terminal fragment (α-CTF, CTF-83 or C83) are generated. The α-CTF is further cleaved by γ-secretase complex releasing small fragment of extracellular p3 and the amino-terminal APP intracellular domain (AICD) which is rapidly degraded. In nonamyloidogenic pathway, the AICD is rapidly degraded [204]. On the other hand, the amyloidogenic pathway initiates with the cleavage of APP by β-secretase or β-site APP-cleaving enzyme 1 (BACE1), releasing the sAPPβ and leaving the β-C-terminal fragment (β-CTF, CTF-99 or C99) at the membrane. The processing of γ-secretase in this pathway generates two protein products including Aβ and AICD, which are different from another pathway by acting as transcriptional regulators of several target genes. Because γ-secretase can cleave many positions within CTF99, it can create different lengths of Aβ peptide, and the predominant forms are Aβ42 and Aβ40. The insoluble extracellular Aβ aggregates into plaques and can accumulate in the brain [205].
Another neuropathological feature of AD is the accumulation of neurofibrillary tangles (NFTs). NFTs are the result of hyperphosphorylation of the microtubule-stabilizing protein tau. Tau protein is highly expressed in neuronal cells and plays a role in axonal microtubule stabilization, modulation of axonal transport, and neuronal polarity. Tau phosphorylation occurs at serine and threonine sites that are regulated by balancing multiple kinases, such as glycogen synthase kinase 3 beta (GSK-3β) and cyclin-dependent kinase-5 (Cdk-5). Excessive kinase and reduced phosphatase activities cause hyperphosphorylated tau to detach and self-aggregate and cause microtubules to become destabilized [206]. Moreover, GSK-3β, a protein kinase, is not only involved in tau hyperphosphorylation but also linked to other aspects of AD pathogenesis, including Aβ production and mitochondrial dysfunction [207]. Tau deposition in the brain has been suggested to be the consequence of Aβ plaque accumulation [208]. The synergy of Aβ and NFT accumulation is distributed to various neurodegenerative mechanisms, including cholinergic degeneration, synaptic impairment, and neuroinflammation, which are partially responsible for cognitive and behavioral deficits [209].
As demonstrated in several studies, viral infections are related to the induction of pathological hallmarks of AD in association with neuronal damage, synaptic dysfunction, and impairment of cognitive functions. Most of the studies have shown the link between latent viral infections and the risk of developing AD. Latent infections from viruses such as HSV and HIV cause long-term activation of the immune system that leads to chronic neuroinflammation and neurodegeneration in the CNS [210–212]. Previous observations reveal a possible indication of HIV and HSV infections as a risk factor associated with AD by distribution of APP and tau-processing homeostasis [213, 214].
The presence of high levels of HSV and its genome have been found in brain tissues, especially in the frontal and temporal cortices and in the CSF of AD patients [211, 215–219]. HSV-1 is a neurotropic virus that lives long within the host and may reactivate after latency and penetrate the BBB into the limbic system and other brain areas that are most often affected in AD [218, 220]. HSV-1 infection causes the alteration of APP processing and has potential causality in the involvement in AD pathogenesis. HSV-1 induces multiple cleavages of APP and promotes intracellular accumulation of various neurotoxic species of Aβ [221–223]. The upregulation of BACE1 and the γ-secretase subunit (nicastrin) in the cultured neuronal and glial cells after HSV-1 infection indicates the induction of the amyloidogenic pathway by HSV-1, which leads to a dramatic increase in the intracellular levels of Aβ40 and Aβ42 [224] and in the autophagic compartments [223]. HSV-1 infection modulates the process of autophagy, as shown by the failure of the fusion of autophagosomes containing Aβ with lysosomes, indicating the impaired degradation of Aβ localized in the autophagic vesicles, which contributes to the accumulation of Aβ characteristic of AD [223]. Moreover, HSV-1 infection is associated with the inhibition of the nonamyloidogenic pathway by decreasing α-secretase activity in HSV-1-infected neuroblastoma cells [223]. Oxidative stress induced by HSV-1 potentiates the accumulation of intracellular Aβ and further inhibits its extracellular secretion, which disrupts the autophagic flux in the infected cells [225]. Furthermore, HSV-1 causes functional changes in cortical neurons that induce activity- and Ca2+-mediated APP phosphorylation and intracellular Aβ production [226]. Aβ protein deposits are present in the brains of mice infected with HSV-1 and show cognitive impairment in recurrent HSV-1-infected mice [224, 227]. The induction of APP amyloidogenic processing by HSV-1 also leads to the accumulation and nuclear translocation of AICD in HSV-1-infected neuronal cells [228]. This increase in AICD products in neurons has been observed to bind at the promoter and regulate the transcription of the neprilysin (NEP) gene, whose products are involved in the Aβ clearance process at early stages of infection. In addition, AICD regulates the expression of the GSK3β gene, which plays a role in tau phosphorylation during the late stage of HSV-1 infection [228]. Moreover, the increase in intracellular Aβ accumulation and GSK-3 activation induces synaptic dysfunction, as observed by the reduction in presynaptic proteins (i.e., synapsin-1 and synaptophysin) and the depressed synaptic transmission in cultured cortical neurons infected with HSV-1 [229]. HSV-1 infection affects tau processing by increasing the kinetics of tau aggregation and NFT formation. In the brains of HSV-1-infected mice, p-tau and its cleaved fragments are significantly increased in the cortex and hippocampus compared with noninfected mice [227, 230]. Infection with HSV-1 also induces an increase in tau cleavage, a marker of early neurodegeneration, by the activation of caspase-3 activity in neural cells, which indicates the involvement mechanism of HSVs in the alteration of tau processing homeostasis [227, 231]. In addition, HSV-1 reactivations cause hyperphosphorylation of tau at several sites, including serine 202, threonine 212, serine 214, serine 396, and serine 404, by the induction of GSK-3β and protein kinase A (PKA) activity [229, 231–233]. The HSV-1 protein can activate the PI3K/AKT signaling pathway to facilitate viral infection, protein synthesis, and reactivation [234, 235]. The PI3K/AKT pathways are known to regulate GSK-3 activation, which is involved in several cellular functions, including APP and tau hyperphosphorylation [236, 237]. The PI3K/AKT/GSK-3 pathways may be a crucial mechanism involved in the progressive accumulation of AD pathological hallmarks in HSV-1 infection. In the case of herpes simplex virus type 2 (HSV-2) infection, HSV-2 causes a marked accumulation of Aβ40 and Aβ42 in human SK-N-MC neuroblastoma cells [238]. HSV-2 infection decreases the levels of secreted APPα and α-CTF, which indicates the disruption of the APP nonamyloidogenic pathway [238]. Moreover, HSV-2 also induces tau phosphorylation in neuronal cells [238]. Latent infection with HSVs poses a risk of developing AD pathological features in the brain [211].
Epidemiologic studies have implicated the possible involvement of varicella zoster virus (VZV) in AD/dementia [239]. VZV (family Orthoherpesviridae) is an exclusively human neurotropic alphaherpesvirus that causes varicella (chicken pox) upon primary infection after which the virus establishes latency in ganglionic neurons along the entire neuraxis and the reactivation causes zoster (shingles) [240]. VZV causes gliosis and increased levels of several proinflammatory cytokines in human-induced neural stem cells (hiNSC). VZV infection of hiNSCs quiescently infected with HSV-1 leads to HSV-1 reactivation, and the increases in Aβ and p-tau accumulation were observed [239]. This suggests that VZV may reside latently in the brain and, upon reactivation, cause direct damage leading to AD through induced inflammation, which leads to neuroinflammation and reactivation of HSV-1 in the brain and consequent AD-like changes [239]. This report supports the indirect role of VZV in AD/dementia via reactivation of HSV-1 in the brain.
In HIV infection, Aβ deposition is one of the pathologic features found in the brains of HIV patients with prolonged antiretroviral treatment and aging [241, 242]. In long-term HIV infections, the alteration of the accumulation of Aβ42, total tau, and p-tau levels in CSF of HAND cases is similar to that of AD patients [243]. HIV viral proteins may be continually produced, increasing the risk of aged patients developing AD. HIV-1 Tat protein induces Aβ accumulation, tau phosphorylation, and subsequent neuronal death, causing slow cognitive and motor movements, seizures, and premature death [244–247]. HIV-1 Tat protein reduces clearance of Aβ42 from the brain to the blood and promotes nuclear entry of Aβ as well as inflammatory responses in human brain endothelial cells [248]. Furthermore, HIV-1 Tat protein enhances the cleavage of APP by β-secretase, as shown by the increased levels of β-CTF and reduced levels of α-CTF, resulting in elevated levels of Aβ42 in HIV-1-infected neurons [246, 249]. HIV-1 Tat protein induces impairment of endolysosome structure and function and influences the pathways of Aβ generation, degradation, phagocytosis, and transport, which contribute to HIV-1 neuropathogenesis [249, 250]. Tau processing and HIV-1 Tat protein accelerate tau phosphorylation via multiple mechanisms that lead to the formation of NFTs in HIV transgenic animals [48, 244, 250]. Tau hyperphosphorylation is found in the hippocampus of HIV-1 patients, and high levels of hyperphosphorylated tau are marked in prolonged anti-retroviral therapy-treated subjects [242]. Thus, neurotoxic viral proteins could be a risk factor for brain damage subsequent to AD and/or HIV-associated cognitive impairment.
Viral Infection Induces Proteins to Misfold and Aggregate
The aggregation of aberrantly folded specific proteins is a pathogenic mechanism underlying several neurodegenerative diseases. The misfolded proteins are involved in neurodegenerative diseases including Aβ, p-tau, alpha-synuclein (α-syn), transactive response DNA-binding protein 43 (TDP-43), and prion protein (PrP), share common structural, biological, and biochemical characteristics, as well as similar mechanisms of aggregation and self-propagation. Virus infection induces the deposition of Aβ and p-tau that increases the risk of developing AD, which we have already discussed in the previous section.
Several neuroinvasive viruses have shown a potential relationship to develop PD. PD is a second most common neurodegenerative disorder characterized by progressive loss of dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies (LBs) together with a reduction of dopamine concentration in the striatum [251]. Abnormal proteinaceous aggregates of α-syn, a major component in LBs, play a causative role in PD pathogenesis [252]. Several studies show that patients with WNV, JEV, DENV, HIV, influenza-A, hepatitis C virus (HCV), hepatitis B virus (HBV), and SARS-CoV-2 infections are at significant risk for developing Parkinson’s symptoms, including tremor, cogwheel rigidity, bradykinesia, and wide gait [253–258]. The increase in the amount of α-syn protein found in the primary neurons [259] and in the brains of patients with acute WNV encephalitis [259] and in the brains of HIV patients [260]. HIV-1 Vpr protein triggers the accumulation of α-syn in neurons [261]. Recent in vitro study showed that SARS-CoV-2 spike protein and nucleocapsid protein were found to accelerate the upregulation and aggregation of α-syn, which was associated with the LBs formation [262, 263].
The accumulation of intracellular ubiquitin inclusion bodies in motor neurons in the brain and spinal cord is one of the neuropathologic hallmarks of ALS. TDP-43 is a major component of inclusion bodies in pathological deposits [264, 265]. A number of reports have shown that TDP-43 plays a major role in virus entry, replication, and latency in several viruses [266]. TDP-43 proteinopathy, which is associated with neuronal dysfunction, is induced by several viral infections [266]. The TDP-43 levels were found to increase in the serum of WNV [267] and SARS-CoV-2 [268] patients in association with the high levels of inflammatory markers. Phosphorylated TDP-43 (pTDP-43) inclusion bodies have been found in the brains of SARS-CoV-2 infection [269]. TDP-43 has been shown to be potentially involved in both HIV-1 [270] as well as in HSV-2 [271] latency and cell permittivity, suggesting a possible role for TDP-43 in HIV-1 and HSV-2-associated neurodegeneration in latent infection.
Aggregation and spread of a cellular prion protein (PrPC) throughout the brain also play an important role in the pathogenesis of AD. PrPC is converted into an aggregated neurotoxic isoform called scrapie prion protein (PrPSc) that causes the neuronal death in prion diseases such as Creutzfeldt-Jakob disease in humans, which shared several neuropathological similarities links to AD [272–274]. In the hippocampus and temporal cortex of AD brains, PrPC has been shown to co-localize with Aβ plaques [275, 276]. PrPC functions as a receptor for Aβ42 oligomers and mediates the Aβ-induced synaptic dysfunction [277]. The binding of Aβ oligomers to PrPC activates an Aβ-induced signaling cascade involving metabotropic glutamate receptor 5 (mGluR5), tyrosine-protein kinase Fyn, proline-rich tyrosine kinase 2 (Pyk2), and eukaryotic elongation factor 2 kinase (eEF2K) that links Aβ accumulation and tau hyperphosphorylation, resulting in the synaptic failure and neuronal death in the CNS [277–282]. PrP expression is upregulated in vitro by infection with a variety of viruses including HCV, HIV-1, human adenovirus type 5, Epstein–Barr virus, murine leukemia virus, and vesicular stomatitis virus [283]. Recent report shows that influenza A virus infection causes PrPC to misfold into PrPSc in mouse neuroblastoma cells [284]. PrPC is significantly elevated in the both neuronal cells and CSF of HIV-1-infected individuals with neurocognitive impairment and mediates neuroinflammation by inducing chemokine release by astrocytes [285]. This evidence suggests that viral infection promotes PrP misfolding and formation of infectious prions in associated with the progression of CNS neurodegeneration, particularly in the pathogenesis of AD.
Thus, viral infection is involved in several specific neurodegeneration-related protein misfolding and subsequent processes of protein aggregate propagation in the CNS leading to neuronal dysfunction, neuroinflammation, and neuronal death that contribute to neurodegenerative disease.
Conclusion
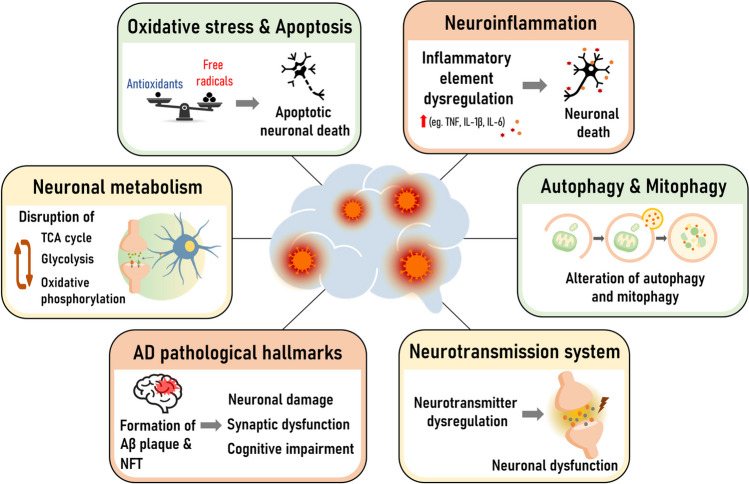
The diagnosis and treatment of CNS infection are urgent needs and challenging. Currently, there are limited or no effective antiviral drugs available for viral infection of the CNS. Neurotropic viruses can cause CNS disease through a variety of molecular mechanisms (Fig. 3), including direct or indirect immune activation, which contributes to the degeneration of neuronal cells. Viral infection promotes an imbalance between free radicals and antioxidants, which increases cellular oxidative stress and leads neuronal cells to undergo programmed cell death by apoptosis. Viruses encounter the cellular recycling process and induce impairment of mitophagy and mitochondrial dynamics in host cells. Dysregulation of mitochondrial homeostasis by viruses affects neuronal metabolism and disrupts brain function. Alterations in neurotransmitter systems and the presence of pathological hallmarks of AD can be observed in neurotropic viral infections in correlation with the specific destruction of brain functions. The fastest protection or cessation of neuronal damage by virus invasion can help to relieve symptoms, which could enhance the survivor rate and reduce severe neurological sequelae. An understanding of the neuropathogenesis of viral CNS infection could support intensive diagnosis and treatment strategies by targeting CNS molecular mechanisms and might be useful for the screening of novel antiviral agents that are essential to improving the management of these neurotropic viral infections.
Fig. 3.
Schematic representation showing the molecular mechanisms associated with neurodegeneration of viral infection in the CNS. Abbreviation: AD, Alzheimer’s disease; Aβ, amyloid beta; NFT, neurofibrillary tangle; TCA, tricarboxylic acid
Acknowledgements
We would like to thank Nattaporn Pakpian for drawing the some of the graphics.
Abbreviations
- 4-HNE
4-hydroxy-2-nonenal
- AchE
Acetylcholinesterase
- ACOX1
Peroxisomal acyl-coenzyme A oxidase 1
- AD
Alzheimer’s disease
- ADAM
Disintegrin and metalloproteinase domain proteins
- AICD
Amino-terminal APP intracellular domain
- AIDS
Acquired immunodeficiency syndrome
- ALS
Amyotrophic lateral sclerosis
- ANLS
Astrocyte-to-neuron lactate shuttle
- AP-1
Activator protein 1
- APP
Amyloid precursor protein
- ARE
Antioxidant response element
- ATF4
Activating transcription factor 4
- Atg
Autophagy-related gene
- Aβ
Amyloid beta
- BACE1
β-site APP-cleaving enzyme 1
- BAX
BCL-2 associated X
- BBB
Blood–brain barrier
- BCL-2
B-cell lymphoma 2
- Ca2+
Calcium ion
- CAT
Catalase
- CCL-2
Chemokine (C-C motif) ligand 2
- CCL-5
Chemokine (C-C motif) ligand 5
- CCR5
C-C motif chemokine receptor 5
- Cdk-5
Cyclin-dependent kinase-5
- CDS
Cytosolic DNA sensors
- CHOP
C/EBP homologous protein
- CHPV
Chandipura virus
- CLRs
C-type lectin receptors
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- CXCL10
C-X-C motif chemokine 10
- CXCR4
C-X-C motif chemokine receptor 4
- CYP2E1
Cytochrome P450-2E1
- Cyt c
Cytochrome c
- D2R
Dopamine D2 receptors
- DENV
Dengue virus
- DENV-2
Dengue virus type 2
- DENV-3
Dengue virus type 3
- DRP1
Dynamin-related protein 1
- eEF2K
Eukaryotic elongation factor 2 kinase
- EGR1
Early growth response 1
- eIF2α
Eukaryotic translation initiation factor 2α kinase
- ER
endoplasmic reticulum
- ERK
Extracellular-signal-regulated kinase
- ETC
Electron transport chain
- EV71
Enterovirus 71
- GABA
Gamma-aminobutyric acid
- GADD153
Growth arrest and DNA damage-inducible protein
- GMP
Cyclic guanidine monophosphate
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GRP78
Glucose-regulated protein 78 kDa
- GSH
Glutathione
- GSK-3β
Glycogen synthase kinase 3 beta
- HAEs
Hydroxyalkenals
- HANDs
HIV-associated neurocognitive disorders
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HD
Huntington’s disease
- HFMD
Hand, foot, and mouth disease
- HIV
Human immunodeficiency virus
- HSP
Heat shock protein
- HSV
Herpes simplex virus
- HSV-1
Herpes simplex virus type 1
- HSV-2
Herpes simplex virus type 2
- IFNγ
Interferon gamma
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- JEV
Japanese encephalitis virus
- LBs
Lewy bodies
- LC3B
Microtubule-associated protein 1 light chain 3 beta
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MCU
Mitochondrial Ca2+ uniporter
- MDA
Malondialdehyde
- mGluR5
Metabotropic glutamate receptor 5
- MIP-1α
Macrophage inflammatory protein 1 alpha
- MOI
Multiplicity of infection
- MPR
Mannose-6-phosphate receptor
- mTOR
Mammalian target of rapamycin
- NAD+
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NFTs
Neurofibrillary tangles
- NF-κB
Nuclear factor kappa B
- NLRP3
NLR family pyrin domain containing 3
- NLRs
NOD-like receptors
- NMDA
N-methyl-d-aspartic acid
- NMNAT2
MAPK-mediated nicotinamide mononucleotide adenylyltransferase 2
- NO
Nitric oxide
- NPCs
Neural progenitor cells
- NRF2
Nuclear factor erythroid 2-related factor 2
- NS4B
Nonstructural protein 4B
- O2.-
Superoxide anions
- OONO-
Peroxynitrite
- p70S6K
Phosphorylated 70-kDa S6 kinase
- PARP
Poly ADP-ribose polymerase
- PD
Parkinson’s disease
- PERK
Protein kinase RNA-like ER kinase
- PET
Positron emission tomography
- PINK1
PTEN-induced kinase 1
- PKA
Protein kinase A
- PMP22
Peripheral myelin protein 22
- PPP
Pentose phosphate pathway
- PrP
Prion protein
- PrPC
Cellular prion protein
- PrPSc
Scrapie prion protein
- PRRs
Pattern recognition receptors
- pTDP-43
Phosphorylated TDP-43
- Pyk2
Proline-rich tyrosine kinase 2
- RABV
Rabies virus
- RANTES
Regulated on activation, normal T-cell expressed, and secreted
- RIG-I
Retinoic acid-inducible gene I
- RLRs
Retinoic acid-inducible gene-I-like receptors
- ROS
Reactive oxygen species
- sAPPα
Soluble APP alpha
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- Ser51
Serine51
- SOD-1
Superoxide dismutase 1
- SQSTM1
Sequestosome 1
- TBARS
Thiobarbituric acid reactive substances
- TBEV
Tick-borne encephalitis virus
- TCA
Tricarboxylic acid cycle
- TDP-43
Transactive response DNA-binding protein 43
- TGF-β
Transforming growth factor beta
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- TNF-β
Tumor necrosis factor beta
- TRADD
TNF receptor-associated death domain
- TRX-1
Thioredoxin 1
- TSPO
Translocator protein
- UPR
Unfolded protein response
- VEEV
Venezuelan equine encephalitis virus
- VP1
Viral envelope protein 1
- VZV
Varicella zoster virus
- WNV
West Nile virus
- ZIKV
Zika virus
- α-CTF
α-C-terminal fragment
- α-syn
Alpha-synuclein
- β-CTF
β-C-terminal fragment
Author Contributions
The contribution of each author was as follows: PG suggested and supervised the idea. PW worked on the concept and outline of the review. PW executed the idea and wrote together with TC. TC was responsible for the design and drawing of the main artwork. PW and PG wrote and finalized the final manuscript. All authors have reviewed and agreed to the final manuscript.
Funding
This research project is supported by the Thailand Research Fund (RSA6280035) and Specific League Funds from Mahidol University for PW.
Data Availability
Not applicable
Declarations
Conflict of Interest
The authors declare no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003;111:3–10. doi: 10.1172/JCI200317522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li PA, Hou X, Hao S. Mitochondrial biogenesis in neurodegeneration. J Neurosci Res. 2017;95:2025–2029. doi: 10.1002/jnr.24042. [DOI] [PubMed] [Google Scholar]
- 3.Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14:457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moujalled D, Strasser A, Liddell JR. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021;28:2029–2044. doi: 10.1038/s41418-021-00814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9:42. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wongchitrat P, Shukla M, Sharma R, Govitrapong P, Reiter RJ. Role of melatonin on virus-induced neuropathogenesis-a concomitant therapeutic strategy to understand SARS-CoV-2 infection. Antioxidants (Basel) 2021;10:47. doi: 10.3390/antiox10010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh H, Koury J, Kaul M. Innate immune sensing of viruses and its consequences for the central nervous system. Viruses. 2021;13:170. doi: 10.3390/v13020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feige L, Zaeck LM, Sehl-Ewert J, Finke S, Bourhy H. Innate immune signaling and role of glial cells in herpes simplex virus- and rabies virus-induced encephalitis. Viruses. 2021;13:2364. doi: 10.3390/v13122364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen FS. How Viruses Invade Cells. Biophys J. 2016;110:1028–1032. doi: 10.1016/j.bpj.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, Miranda-Saksena M, Saksena NK. Viruses and neurodegeneration. Virol J. 2013;10:172. doi: 10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unni SK, et al. Japanese encephalitis virus: from genome to infectome. Microbes Infect. 2011;13:312–321. doi: 10.1016/j.micinf.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Fischer M, Lindsey N, Staples JE, Hills S. Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 15.Lin RJ, Liao CL, Lin YL. Replication-incompetent virions of Japanese encephalitis virus trigger neuronal cell death by oxidative stress in a culture system. J Gen Virol. 2004;85:521–533. doi: 10.1099/vir.0.19496-0. [DOI] [PubMed] [Google Scholar]
- 16.Mishra MK, Ghosh D, Duseja R, Basu A. Antioxidant potential of minocycline in Japanese encephalitis virus infection in murine neuroblastoma cells: correlation with membrane fluidity and cell death. Neurochem Int. 2009;54:464–470. doi: 10.1016/j.neuint.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava R, Kalita J, Khan MY, Misra UK. Free radical generation by neurons in rat model of Japanese encephalitis. Neurochem Res. 2009;34:2141–2146. doi: 10.1007/s11064-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 18.Raung SL, Kuo MD, Wang YM, Chen CJ. Role of reactive oxygen intermediates in Japanese encephalitis virus infection in murine neuroblastoma cells. Neurosci Lett. 2001;315:9–12. doi: 10.1016/S0304-3940(01)02300-X. [DOI] [PubMed] [Google Scholar]
- 19.Lixia H, Jun C, Song H, FaHu Y, Jinwen T. Neuroprotective effect of (-)-tetrahydropalmatine in Japanese encephalitis virus strain GP-78 infected mouse model. Microb Pathog. 2018;114:197–203. doi: 10.1016/j.micpath.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Verma S, et al. Role of oxidative stress in West Nile virus (WNV)-induced apoptosis. FASEB J. 2006;20:A1073–A1073. doi: 10.1096/fasebj.20.5.A1073-b. [DOI] [Google Scholar]
- 21.Jan JT, et al. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: arachidonic acid, superoxide anion, and NF-kappaB are sequentially involved. J Virol. 2000;74:8680–8691. doi: 10.1128/JVI.74.18.8680-8691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver SC, et al. Re-emergence of epidemic Venezuelan equine encephalomyelitis in South America. VEE Study Group. Lancet. 1996;348:436–440. doi: 10.1016/S0140-6736(96)02275-1. [DOI] [PubMed] [Google Scholar]
- 23.Bowen GS, Fashinell TR, Dean PB, Gregg MB. Clinical aspects of human Venezuelan equine encephalitis in Texas. Bull Pan Am Health Organ. 1976;10:46–57. [PubMed] [Google Scholar]
- 24.Ronca SE, Dineley KT, Paessler S. Neurological sequelae resulting from encephalitic alphavirus infection. Front Microbiol. 2016;7:959. doi: 10.3389/fmicb.2016.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keck F, et al. Altered mitochondrial dynamics as a consequence of Venezuelan Equine encephalitis virus infection. Virulence. 2017;8:1849–1866. doi: 10.1080/21505594.2016.1276690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valero N, MarinaEspina L, Bonilla E, Mosquera J. Melatonin decreases nitric oxide production and lipid peroxidation and increases interleukin-1 beta in the brain of mice infected by the Venezuelan equine encephalomyelitis virus. J Pineal Res. 2007;42:107–112. doi: 10.1111/j.1600-079X.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S, Misra UK, Kalita J, Khanna VK, Khan MY. Imbalance in oxidant/antioxidant system in different brain regions of rat after the infection of Japanese encephalitis virus. Neurochem Int. 2009;55:648–654. doi: 10.1016/j.neuint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Bourhy H, Gubala AJ, Weir RP, Boyle DB. Animal Rhabdoviruses. In: Mahy BWJ, Van Regenmortel MHV, editors. Encyclopedia of Virology. Third. Oxford: Academic Press; 2008. pp. 111–121. [Google Scholar]
- 29.Kammouni W, et al. Rabies virus phosphoprotein interacts with mitochondrial Complex I and induces mitochondrial dysfunction and oxidative stress. J Neurovirol. 2015;21:370–382. doi: 10.1007/s13365-015-0320-8. [DOI] [PubMed] [Google Scholar]
- 30.Kammouni W, Wood H, Jackson AC. Serine residues at positions 162 and 166 of the rabies virus phosphoprotein are critical for the induction of oxidative stress in rabies virus infection. J Neurovirol. 2017;23:358–368. doi: 10.1007/s13365-016-0506-8. [DOI] [PubMed] [Google Scholar]
- 31.Jackson AC, Kammouni W, Zherebitskaya E, Fernyhough P. Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J Virol. 2010;84:4697–4705. doi: 10.1128/JVI.02654-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma AK, Ghosh S, Basu A. Chandipura Virus induced neuronal apoptosis via calcium signaling mediated oxidative stress. Front Microbiol. 2018;9:1489. doi: 10.3389/fmicb.2018.01489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh S, Dutta K, Basu A. Chandipura virus induces neuronal death through Fas-mediated extrinsic apoptotic pathway. J Virol. 2013;87:12398–12406. doi: 10.1128/JVI.01864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh S, et al. Network analysis reveals common host protein/s modulating pathogenesis of neurotropic viruses. Sci Rep. 2016;6:32593. doi: 10.1038/srep32593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng ML, Weng SF, Kuo CH, Ho HY. Enterovirus 71 induces mitochondrial reactive oxygen species generation that is required for efficient replication. PLoS One. 2014;9:e113234. doi: 10.1371/journal.pone.0113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You L, et al. Enterovirus 71 induces neural cell apoptosis and autophagy through promoting ACOX1 downregulation and ROS generation. Virulence. 2020;11:537–553. doi: 10.1080/21505594.2020.1766790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tung WH, Hsieh HL, Lee IT, Yang CM. Enterovirus 71 induces integrin beta1/EGFR-Rac1-dependent oxidative stress in SK-N-SH cells: role of HO-1/CO in viral replication. J Cell Physiol. 2011;226:3316–3329. doi: 10.1002/jcp.22677. [DOI] [PubMed] [Google Scholar]
- 38.Rozenberg F. Chapter 122 - Acute viral encephalitis. In: Dulac O, Lassonde M, Sarnat HB, editors. Handbook of Clinical Neurology. (Elsevier; 2013. pp. 1171–1181. [DOI] [PubMed] [Google Scholar]
- 39.Hu S, Sheng WS, Schachtele SJ, Lokensgard JR. Reactive oxygen species drive herpes simplex virus (HSV)-1-induced proinflammatory cytokine production by murine microglia. J Neuroinflammation. 2011;8:123. doi: 10.1186/1742-2094-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavouras JH, et al. Herpes simplex virus type 1 infection induces oxidative stress and the release of bioactive lipid peroxidation by-products in mouse P19N neural cell cultures. J Neurovirol. 2007;13:416–425. doi: 10.1080/13550280701460573. [DOI] [PubMed] [Google Scholar]
- 41.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstein PS, et al. HIV-1 gp120 and drugs of abuse: interactions in the central nervous system. Curr HIV Res. 2012;10:369–383. doi: 10.2174/157016212802138724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 44.Guo L, et al. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS One. 2013;8:e70565. doi: 10.1371/journal.pone.0070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah A, Kumar S, Simon SD, Singh DP, Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013;4:e850. doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ivanov AV, et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozzi SJ, et al. PACAP27 is protective against tat-induced neurotoxicity. J Mol Neurosci. 2014;54:485–493. doi: 10.1007/s12031-014-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho YE, Lee MH, Song BJ. Neuronal cell death and degeneration through increased nitroxidative stress and tau phosphorylation in HIV-1 transgenic rats. PLoS One. 2017;12:e0169945. doi: 10.1371/journal.pone.0169945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munoz LS, Parra B, Pardo CA, S. Neuroviruses emerging in the Americas, neurological implications of Zika virus infection in adults. J Infect Dis. 2017;216:S897–S905. doi: 10.1093/infdis/jix511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acosta-Ampudia Y, et al. Autoimmune neurological conditions associated with Zika virus infection. Front Mol Neurosci. 2018;11:116. doi: 10.3389/fnmol.2018.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ledur PF, et al. Zika virus infection leads to mitochondrial failure, oxidative stress and DNA damage in human iPSC-derived astrocytes. Sci Rep. 2020;10:1218. doi: 10.1038/s41598-020-57914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida LT, et al. Zika virus induces oxidative stress and decreases antioxidant enzyme activities in vitro and in vivo. Virus Res. 2020;286:198084. doi: 10.1016/j.virusres.2020.198084. [DOI] [PubMed] [Google Scholar]
- 53.Camini FC, da Silva Caetano CC, Almeida LT, de Brito Magalhaes CL. Implications of oxidative stress on viral pathogenesis. Arch Virol. 2017;162:907–917. doi: 10.1007/s00705-016-3187-y. [DOI] [PubMed] [Google Scholar]
- 54.Deramaudt TB, Dill C, Bonay M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med Mal Infect. 2013;43:100–107. doi: 10.1016/j.medmal.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Ho H-Y, et al. Glucose-6-phosphate dehydrogenase deficiency enhances enterovirus 71 infection. J Gen Virol. 2008;89:2080–2089. doi: 10.1099/vir.0.2008/001404-0. [DOI] [PubMed] [Google Scholar]
- 56.Nucci C, et al. Imbalance in corneal redox state during herpes simplex virus 1-induced keratitis in rabbits. Effectiveness of Exogenous Glutathione Supply. Exp Eye Res. 2000;70:215–220. doi: 10.1006/exer.1999.0782. [DOI] [PubMed] [Google Scholar]
- 57.Price TO, Ercal N, Nakaoke R, Banks WA. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005;1045:57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 58.Alandijany T, Kammouni W, Roy Chowdhury SK, Fernyhough P, Jackson AC. Mitochondrial dysfunction in rabies virus infection of neurons. J NeuroVirology. 2013;19:537–549. doi: 10.1007/s13365-013-0214-6. [DOI] [PubMed] [Google Scholar]
- 59.Scott CA, Rossiter JP, Andrew RD, Jackson AC. Structural abnormalities in neurons are sufficient to explain the clinical disease and fatal outcome of experimental rabies in yellow fluorescent protein-expressing transgenic mice. J Virol. 2008;82:513–521. doi: 10.1128/JVI.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jackson AC, Kammouni W, Fernyhough P. Chapter 8 - Role of Oxidative Stress in Rabies Virus Infection. In: Jackson AC, editor. Advances in Virus Research. (Academic Press; 2011. pp. 127–138. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 62.Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosh Roy S, Sadigh B, Datan E, Lockshin RA, Zakeri Z. Regulation of cell survival and death during Flavivirus infections. World. J Biol Chem. 2014;5:93–105. doi: 10.4331/wjbc.v5.i2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee S, et al. Japanese encephalitis virus induces human neural stem/progenitor cell death by elevating GRP78, PHB and hnRNPC through ER stress. Cell Death Dis. 2018;8:e2556–e2556. doi: 10.1038/cddis.2016.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Q, et al. Japanese encephalitis virus induces apoptosis and encephalitis by activating the PERK pathway. J Virol. 2019;93:e00887–e00819. doi: 10.1128/JVI.00887-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medigeshi GR, et al. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81:10849–10860. doi: 10.1128/JVI.01151-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baer A, et al. Venezuelan equine encephalitis virus induces apoptosis through the unfolded protein response activation of EGR1. J Virol. 2016;90:3558–3572. doi: 10.1128/JVI.02827-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dahal B, et al. EGR1 upregulation following Venezuelan equine encephalitis virus infection is regulated by ERK and PERK pathways contributing to cell death. Virology. 2020;539:121–128. doi: 10.1016/j.virol.2019.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tan Z, et al. ZIKV infection activates the IRE1-XBP1 and ATF6 pathways of unfolded protein response in neural cells. J Neuroinflammation. 2018;15:275. doi: 10.1186/s12974-018-1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gladwyn-Ng I, et al. Stress-induced unfolded protein response contributes to Zika virus-associated microcephaly. Nat Neurosci. 2018;21:63–71. doi: 10.1038/s41593-017-0038-4. [DOI] [PubMed] [Google Scholar]
- 71.Hu DD, et al. Glucocorticoids prevent enterovirus 71 capsid protein VP1 induced calreticulin surface exposure by alleviating neuronal ER stress. Neurotox Res. 2017;31:204–217. doi: 10.1007/s12640-016-9670-0. [DOI] [PubMed] [Google Scholar]
- 72.Li P-Q, et al. Enterovirus 71 structural viral protein 1 promotes mouse Schwann cell autophagy via endoplasmic reticulum stress-mediated peripheral myelin protein 22 upregulation. bioRxiv. 2018;10(1101/314468):314468. [Google Scholar]
- 73.Mehrbod P, et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10:376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee YR, Wang PS, Wang JR, Liu HS. Enterovirus 71-induced autophagy increases viral replication and pathogenesis in a suckling mouse model. J Biomed Sci. 2014;21:80. doi: 10.1186/s12929-014-0080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J Med Virol. 2009;81:1241–1252. doi: 10.1002/jmv.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu ZW, et al. Enterovirus 71 VP1 protein regulates viral replication in SH-SY5Y cells via the mTOR autophagy signaling pathway. Viruses. 2019;12:11. doi: 10.3390/v12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Too IH, et al. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci Rep. 2016;6:36983. doi: 10.1038/srep36983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou D, Masliah E, Spector SA. Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis. 2011;203:1647–1657. doi: 10.1093/infdis/jir163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fields JA, et al. The anticancer drug sunitinib promotes autophagyand protects from neurotoxicity in an HIV-1 Tat model of neurodegeneration. J Neurovirol. 2017;23:290–303. doi: 10.1007/s13365-016-0502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheney L, et al. HIV Nef and antiretroviral therapy have an inhibitory effect on autophagy in human astrocytes that may contribute to HIV-associated neurocognitive disorders. Cells. 2020;9:1426. doi: 10.3390/cells9061426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fields J, et al. HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci. 2015;35:1921–1938. doi: 10.1523/JNEUROSCI.3207-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin R, et al. Japanese encephalitis virus activates autophagy as a viral immune evasion strategy. PLoS One. 2013;8:e52909. doi: 10.1371/journal.pone.0052909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang L, Qin Y, Chen M. Viral strategies for triggering and manipulating mitophagy. Autophagy. 2018;14:1665–1673. doi: 10.1080/15548627.2018.1466014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teodorof-Diedrich C, Spector SA. Human immunodeficiency virus type 1 gp120 and Tat induce mitochondrial fragmentation and incomplete mitophagy in human neurons. J Virol. 2018;92:e00993–18. doi: 10.1128/JVI.00993-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Teodorof-Diedrich C, Spector SA (2020) Human immunodeficiency virus type-1 and methamphetamine mediated mitochondrial damage and neuronal degeneration in human neurons. J Virol. 10.1128/JVI.00924-20 [DOI] [PMC free article] [PubMed]
- 86.Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 2018;4:8. doi: 10.1038/s41420-017-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Thangaraj A, et al. HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy. 2018;14:1596–1619. doi: 10.1080/15548627.2018.1476810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsao CH, et al. Japanese encephalitis virus infection activates caspase-8 and -9 in a FADD-independent and mitochondrion-dependent manner. J Gen Virol. 2008;89:1930–1941. doi: 10.1099/vir.0.2008/000182-0. [DOI] [PubMed] [Google Scholar]
- 89.Swarup V, Das S, Ghosh S, Basu A. Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J Neurochem. 2007;103:771–783. doi: 10.1111/j.1471-4159.2007.04790.x. [DOI] [PubMed] [Google Scholar]
- 90.James HJ, et al. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- 91.Lannuzel A, et al. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y, et al. Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res. 2012;1431:13–22. doi: 10.1016/j.brainres.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 93.Chandrasekar AP, Cummins NW, Badley AD. The role of the BCL-2 family of proteins in HIV-1 pathogenesis and persistence. Clin Microbiol Rev. 2019;33:e00107–19. doi: 10.1128/CMR.00107-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jackson AC, Rossiter JP. Apoptotic cell death is an important cause of neuronal injury in experimental Venezuelan equine encephalitis virus infection of mice. Acta Neuropathol. 1997;93:349–353. doi: 10.1007/s004010050626. [DOI] [PubMed] [Google Scholar]
- 95.Bocan TM, et al. Characterization of brain inflammation, apoptosis, hypoxia, blood-brain barrier integrity and metabolism in Venezuelan equine encephalitis virus (VEEV TC-83) exposed mice by in vivo positron emission tomography imaging. Viruses. 2019;11:1052. doi: 10.3390/v11111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Du X, et al. Enterovirus 71 induces apoptosis of SHSY5Y human neuroblastoma cells through stimulation of endogenous microRNA let-7b expression. Mol Med Rep. 2015;12:953–959. doi: 10.3892/mmr.2015.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang C, et al. Intrinsic apoptosis and proinflammatory cytokines regulated in human astrocytes infected with enterovirus 71. J Gen Virol. 2015;96:3010–3022. doi: 10.1099/jgv.0.000235. [DOI] [PubMed] [Google Scholar]
- 98.Luo Z, et al. EV71 infection induces neurodegeneration via activating TLR7 signaling and IL-6 production. PLoS Pathog. 2019;15:e1008142. doi: 10.1371/journal.ppat.1008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jackson AC, Rossiter JP. Apoptosis plays an important role in experimental rabies virus infection. J Virol. 1997;71:5603–5607. doi: 10.1128/jvi.71.7.5603-5607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rutherford M, Jackson AC. Neuronal apoptosis in immunodeficient mice infected with the challenge virus standard strain of rabies virus by intracerebral inoculation. J Neurovirol. 2004;10:409–413. doi: 10.1080/13550280490523643. [DOI] [PubMed] [Google Scholar]
- 101.Jackson AC. Apoptosis in experimental rabies in bax-deficient mice. Acta Neuropathol. 1999;98:288–294. doi: 10.1007/s004010051082. [DOI] [PubMed] [Google Scholar]
- 102.Fu ZF, Jackson AC. Neuronal dysfunction and death in rabies virus infection. J Neurovirol. 2005;11:101–106. doi: 10.1080/13550280590900445. [DOI] [PubMed] [Google Scholar]
- 103.Kojima D, et al. Lesions of the central nervous system induced by intracerebral inoculation of BALB/c mice with rabies virus (CVS-11) J Vet Med Sci. 2010;72:1011–1016. doi: 10.1292/jvms.09-0550. [DOI] [PubMed] [Google Scholar]
- 104.Sevil Atalay V, Mehmet Fatih B, Ali O, Mehmet Eray A, Fatma Sayin I. Apoptosis in natural rabies virus infection in dogs. Journal of. Vet Res. 2016;60:227–231. doi: 10.1515/jvetres-2016-0034. [DOI] [Google Scholar]
- 105.Kojima D, et al. Pathology of the spinal cord of C57BL/6J mice infected with rabies virus (CVS-11 strain) J Vet Med Sci. 2009;71:319–324. doi: 10.1292/jvms.71.319. [DOI] [PubMed] [Google Scholar]
- 106.Ubol S, Sukwattanapan C, Utaisincharoen P. Rabies virus replication induces Bax-related, caspase dependent apoptosis in mouse neuroblastoma cells. Virus Res. 1998;56:207–215. doi: 10.1016/S0168-1702(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 107.Parquet MC, Kumatori A, Hasebe F, Morita K, Igarashi A. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 2001;500:17–24. doi: 10.1016/S0014-5793(01)02573-X. [DOI] [PubMed] [Google Scholar]
- 108.Kleinschmidt MC, Michaelis M, Ogbomo H, Doerr HW, Cinatl J., Jr Inhibition of apoptosis prevents West Nile virus induced cell death. BMC Microbiol. 2007;7:49. doi: 10.1186/1471-2180-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Marle G, et al. West Nile virus-induced neuroinflammation: glial infection and capsid protein-mediated neurovirulence. J Virol. 2007;81:10933–10949. doi: 10.1128/JVI.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang JS, et al. Induction of inflammation by West Nile virus capsid through the caspase-9 apoptotic pathway. Emerg Infect Dis. 2002;8:1379–1384. doi: 10.3201/eid0812.020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramanathan MP, et al. Host cell killing by the West Nile virus NS2B-NS3 proteolytic complex: NS3 alone is sufficient to recruit caspase-8-based apoptotic pathway. Virology. 2006;345:56–72. doi: 10.1016/j.virol.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 112.Chu JJH, Ng ML. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J Gen Virol. 2003;84:3305–3314. doi: 10.1099/vir.0.19447-0. [DOI] [PubMed] [Google Scholar]
- 113.Samuel MA, Morrey JD, Diamond MS. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J Virol. 2007;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng BH, Wang T (2019) West Nile Virus Induced Cell Death in the Central Nervous System. Pathogens 8:215 [DOI] [PMC free article] [PubMed]
- 115.Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase 3 induction, and viral replication following Japanese encephalitis. J Neurochem. 2008;105:1582–1595. doi: 10.1111/j.1471-4159.2008.05238.x. [DOI] [PubMed] [Google Scholar]
- 116.Kitidee K, et al. Antiviral effect of melatonin on Japanese encephalitis virus infection involves inhibition of neuronal apoptosis and neuroinflammation in SH-SY5Y cells. Sci Rep. 2023;13:6063. doi: 10.1038/s41598-023-33254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wongchitrat P, et al. Elevation of cleaved p18 Bax levels associated with the kinetics of neuronal cell death during Japanese encephalitis virus infection. Int J Mol Sci. 2019;20:5016. doi: 10.3390/ijms20205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Obaidi MMJ, et al. Japanese encephalitis virus disrupts blood-brain barrier and modulates apoptosis proteins in THBMEC cells. Virus Res. 2017;233:17–28. doi: 10.1016/j.virusres.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Okamoto T, et al. Regulation of apoptosis during flavivirus infection. Viruses. 2017;9:243. doi: 10.3390/v9090243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee CJ, Liao CL, Lin YL. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J Virol. 2005;79:8388–8399. doi: 10.1128/JVI.79.13.8388-8399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liao C-L, et al. Antiapoptotic but not antiviral function of human bcl-2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J Virol. 1998;72:9844. doi: 10.1128/JVI.72.12.9844-9854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liao CL, et al. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gao G, Dou QP. N-terminal cleavage of Bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes Bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem. 2001;80:53–72. doi: 10.1002/1097-4644(20010101)80:1<53::AID-JCB60>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 124.Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lannes N, Summerfield A, Filgueira L. Regulation of inflammation in Japanese encephalitis. J Neuroinflammation. 2017;14:158. doi: 10.1186/s12974-017-0931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hsieh JT, Rathore APS, Soundarajan G, St John AL. Japanese encephalitis virus neuropenetrance is driven by mast cell chymase. Nat Commun. 2019;10:706. doi: 10.1038/s41467-019-08641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lannes N, et al. Interactions of human microglia cells with Japanese encephalitis virus. Virol J. 2017;14:8. doi: 10.1186/s12985-016-0675-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ghoshal A, et al. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55:483–496. doi: 10.1002/glia.20474. [DOI] [PubMed] [Google Scholar]
- 129.Jiang R, et al. Roles of TLR3 and RIG-I in mediating the inflammatory response in mouse microglia following Japanese encephalitis virus infection. J Immunol Res. 2014;2014:787023. doi: 10.1155/2014/787023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen CJ, et al. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol. 2010;91:1028–1037. doi: 10.1099/vir.0.013565-0. [DOI] [PubMed] [Google Scholar]
- 131.Ye J, et al. Etanercept reduces neuroinflammation and lethality in mouse model of Japanese encephalitis. J Infect Dis. 2014;210:875–889. doi: 10.1093/infdis/jiu179. [DOI] [PubMed] [Google Scholar]
- 132.Niranjan R, Muthukumaravel S, Jambulingam P. The involvement of neuroinflammation in dengue viral disease: importance of innate and adaptive immunity. Neuroimmunomodulation. 2019;26:111–118. doi: 10.1159/000501209. [DOI] [PubMed] [Google Scholar]
- 133.Al-Shujairi WH, et al. Intracranial injection of dengue virus induces interferon stimulated genes and CD8+ T cell infiltration by sphingosine kinase 1 independent pathways. PLoS One. 2017;12:e0169814. doi: 10.1371/journal.pone.0169814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amaral DC, et al. Intracerebral infection with dengue-3 virus induces meningoencephalitis and behavioral changes that precede lethality in mice. J Neuroinflammation. 2011;8:23. doi: 10.1186/1742-2094-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han YW, et al. Distinct dictation of Japanese encephalitis virus-induced neuroinflammation and lethality via triggering TLR3 and TLR4 signal pathways. PLoS Pathog. 2014;10:e1004319. doi: 10.1371/journal.ppat.1004319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li F, et al. Viral Infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol. 2015;89:5602–5614. doi: 10.1128/JVI.00143-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Das S, Mishra MK, Ghosh J, Basu A. Japanese encephalitis virus infection induces IL-18 and IL-1beta in microglia and astrocytes: correlation with in vitro cytokine responsiveness of glial cells and subsequent neuronal death. J Neuroimmunol. 2008;195:60–72. doi: 10.1016/j.jneuroim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 138.Keck F, et al. Mitochondrial-directed antioxidant reduces microglial-induced inflammation in murine in vitro model of TC-83 infection. Viruses. 2018;10:606. doi: 10.3390/v10110606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lin TY, Hsia SH, Huang YC, Wu CT, Chang LY. Proinflammatory cytokine reactions in enterovirus 71 infections of the central nervous system. Clin Infect Dis. 2003;36:269–274. doi: 10.1086/345905. [DOI] [PubMed] [Google Scholar]
- 140.Wang SM, et al. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin Microbiol Infect. 2007;13:677–682. doi: 10.1111/j.1469-0691.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 141.Wong KT, et al. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J Neuropathol Exp Neurol. 2008;67:162–169. doi: 10.1097/nen.0b013e318163a990. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y, et al. Pathogenesis study of enterovirus 71 infection in rhesus monkeys. Lab Invest. 2011;91:1337–1350. doi: 10.1038/labinvest.2011.82. [DOI] [PubMed] [Google Scholar]
- 143.Chang CY, et al. Enterovirus 71 infection caused neuronal cell death and cytokine expression in cultured rat neural cells. IUBMB Life. 2015;67:789–800. doi: 10.1002/iub.1434. [DOI] [PubMed] [Google Scholar]
- 144.Bogovic P, Strle F. Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases. 2015;3:430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zajkowska J, et al. Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE) Adv Med Sci. 2011;56:311–317. doi: 10.2478/v10039-011-0033-z. [DOI] [PubMed] [Google Scholar]
- 146.Grygorczuk S, et al. Increased concentration of interferon lambda-3, interferon beta and interleukin-10 in the cerebrospinal fluid of patients with tick-borne encephalitis. Cytokine. 2015;71:125–131. doi: 10.1016/j.cyto.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 147.Bardina SV, Lim JK. The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol Res. 2012;54:121–132. doi: 10.1007/s12026-012-8333-3. [DOI] [PubMed] [Google Scholar]
- 148.Palus M, et al. Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: identification of novel inflammatory markers with implications for pathogenesis. J Med Virol. 2015;87:885–892. doi: 10.1002/jmv.24140. [DOI] [PubMed] [Google Scholar]
- 149.Palus M, et al. Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology. 2017;507:110–122. doi: 10.1016/j.virol.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 150.Selinger M, et al. Integrative RNA profiling of TBEV-infected neurons and astrocytes reveals potential pathogenic effectors. Comput Struct Biotechnol J. 2022;20:2759–2777. doi: 10.1016/j.csbj.2022.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Potokar M, Korva M, Jorgacevski J, Avsic-Zupanc T, Zorec R. Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS One. 2014;9:e86219. doi: 10.1371/journal.pone.0086219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fares M, et al. Pathological modeling of TBEV infection reveals differential innate immune responses in human neurons and astrocytes that correlate with their susceptibility to infection. J Neuroinflammation. 2020;17:76. doi: 10.1186/s12974-020-01756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pokorna Formanova P, et al. Changes in cytokine and chemokine profiles in mouse serum and brain, and in human neural cells, upon tick-borne encephalitis virus infection. J Neuroinflammation. 2019;16:205. doi: 10.1186/s12974-019-1596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang X, et al. Tick-borne encephalitis virus induces chemokine RANTES expression via activation of IRF-3 pathway. J Neuroinflammation. 2016;13:209. doi: 10.1186/s12974-016-0665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shao Q, et al. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 2016;143:4127–4136. doi: 10.1242/dev.143768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.de Sousa JR, et al. Correlation between apoptosis and in situ immune response in fatal cases of microcephaly caused by Zika virus. Am J Pathol. 2018;188:2644–2652. doi: 10.1016/j.ajpath.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 157.Panganiban AT et al (2020) A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier, and upregulates CXCL12 in adult macaques. Brain Pathol. 10.1111/bpa.12873 [DOI] [PMC free article] [PubMed]
- 158.Ojha CR, et al. Toll-like receptor 3 regulates Zika virus infection and associated host inflammatory response in primary human astrocytes. PLoS One. 2019;14:e0208543. doi: 10.1371/journal.pone.0208543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tricarico PM, Caracciolo I, Crovella S, D'Agaro P. Zika virus induces inflammasome activation in the glial cell line U87-MG. Biochem Biophys Res Commun. 2017;492:597–602. doi: 10.1016/j.bbrc.2017.01.158. [DOI] [PubMed] [Google Scholar]
- 160.Lum FM, et al. Zika virus infects human fetal brain microglia and induces inflammation. Clin Infect Dis. 2017;64:914–920. doi: 10.1093/cid/ciw878. [DOI] [PubMed] [Google Scholar]
- 161.Shuangshoti S, et al. Reduced viral burden in paralytic compared to furious canine rabies is associated with prominent inflammation at the brainstem level. BMC Vet Res. 2013;9:31. doi: 10.1186/1746-6148-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Farahtaj F, et al. Natural infection with rabies virus: a histopathological and immunohistochemical study of human brains. Osong Public Health Res Perspect. 2019;10:6–11. doi: 10.24171/j.phrp.2019.10.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y, et al. Interferon-lambda attenuates rabies virus infection by inducing interferon-stimulated genes and alleviating neurological inflammation. Viruses. 2020;12:405. doi: 10.3390/v12040405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao L, Toriumi H, Kuang Y, Chen H, Fu ZF. The roles of chemokines in rabies virus infection: overexpression may not always be beneficial. J Virol. 2009;83:11808–11818. doi: 10.1128/JVI.01346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iwata R, Vanderhaeghen P. Regulatory roles of mitochondria and metabolism in neurogenesis. Curr Opin Neurobiol. 2021;69:231–240. doi: 10.1016/j.conb.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li C, et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell. 2016;19:120–126. doi: 10.1016/j.stem.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 167.Tiwari SK, et al. Zika virus depletes neural stem cells and evades selective autophagy by suppressing the Fanconi anemia protein FANCC. EMBO Rep. 2020;21:e49183. doi: 10.15252/embr.201949183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gabriel E, et al. Recent Zika virus isolates induce premature differentiation of neural progenitors in human brain organoids. Cell Stem Cell. 2017;20:397–406 e395. doi: 10.1016/j.stem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 169.Pang H, et al. Aberrant NAD(+) metabolism underlies Zika virus-induced microcephaly. Nat Metab. 2021;3:1109–1124. doi: 10.1038/s42255-021-00437-0. [DOI] [PubMed] [Google Scholar]
- 170.Walker LJ et al (2017) MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 6:e22540 [DOI] [PMC free article] [PubMed]
- 171.Yang J, et al. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell. 2015;160:161–176. doi: 10.1016/j.cell.2014.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yau C, et al. Dysregulated metabolism underpins Zika-virus-infection-associated impairment in fetal development. Cell Rep. 2021;37:110118. doi: 10.1016/j.celrep.2021.110118. [DOI] [PubMed] [Google Scholar]
- 173.Thaker SK, et al. Differential metabolic reprogramming by zika virus promotes cell death in human versus mosquito cells. Cell Metab. 2019;29:1206–1216 e1204. doi: 10.1016/j.cmet.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bouzier-Sore AK, Pellerin L. Unraveling the complex metabolic nature of astrocytes. Front Cell Neurosci. 2013;7:179. doi: 10.3389/fncel.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bonvento G, Bolanos JP. Astrocyte-neuron metabolic cooperation shapes brain activity. Cell Metab. 2021;33:1546–1564. doi: 10.1016/j.cmet.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 176.Fan Y, He JJ. HIV-1 Tat Induces unfolded protein response and endoplasmic reticulum stress in astrocytes and causes neurotoxicity through glial fibrillary acidic protein (GFAP) activation and aggregation. J Biol Chem. 2016;291:22819–22829. doi: 10.1074/jbc.M116.731828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci. 2004;27:296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 178.Natarajaseenivasan K, et al. Astrocytic metabolic switch is a novel etiology for cocaine and HIV-1 Tat-mediated neurotoxicity. Cell Death Dis. 2018;9:415. doi: 10.1038/s41419-018-0422-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sivalingam K, Cirino TJ, McLaughlin JP, Samikkannu T. HIV-Tat and cocaine impact brain energy metabolism: redox modification and mitochondrial biogenesis influence NRF transcription-mediated neurodegeneration. Mol Neurobiol. 2021;58:490–504. doi: 10.1007/s12035-020-02131-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Crunfli F, Carregari VC, Veras FP, Silva LS, Nogueira MH, Antunes ASLM, Vendramini PH, Valença AGF, Brandão-Teles C, Zuccoli GDS, Reis-de-Oliveira G, Silva-Costa LC, Saia-Cereda VM, Smith BJ, Codo AC, de Souza GF, Muraro SP, Parise PL, Toledo-Teixeira DA, Santos de Castro ÍM, Melo BM, Almeida GM, Firmino EMS, Paiva IM, Silva BMS, Guimarães RM, Mendes ND, Ludwig RL, Ruiz GP, Knittel TL, Davanzo GG, Gerhardt JA, Rodrigues PB, Forato J, Amorim MR, Brunetti NS, Martini MC, Benatti MN, Batah SS, Siyuan L, João RB, Aventurato ÍK, Rabelo de Brito M, Mendes MJ, da Costa BA, Alvim MKM, da Silva Júnior JR, Damião LL, de Sousa IMP, da Rocha ED, Gonçalves SM, Lopes da Silva LH, Bettini V, Campos BM, Ludwig G, Tavares LA, Pontelli MC, Viana RMM, Martins RB, Vieira AS, Alves-Filho JC, Arruda E, Podolsky-Gondim GG, Santos MV, Neder L, Damasio A, Rehen S, Vinolo MAR, Munhoz CD, Louzada-Junior P, Oliveira RD, Cunha FQ, Nakaya HI, Mauad T, Duarte-Neto AN, Ferraz da Silva LF, Dolhnikoff M, Saldiva PHN, Farias AS, Cendes F, Moraes-Vieira PMM, Fabro AT, Sebollela A, Proença-Modena JL, Yasuda CL, Mori MA, Cunha TM, Martins-de-Souza D (2022) Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A 119(35):e2200960119. 10.1073/pnas.2200960119 [DOI] [PMC free article] [PubMed]
- 181.L. G. de Oliveira et al., SARS-CoV-2 infection impacts carbon metabolism and depends on glutamine for replication in Syrian hamster astrocytes. 10.1101/2021.10.23.465567 %J bioRxiv, 2021.2010.2023.465567 (2021). [DOI] [PMC free article] [PubMed]
- 182.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 183.Simanjuntak Y, Liang JJ, Lee YL, Lin YL. Japanese encephalitis virus exploits dopamine D2 receptor-phospholipase C to target dopaminergic human neuronal cells. Front Microbiol. 2017;8:651. doi: 10.3389/fmicb.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Misra UK, et al. A study of motor activity and catecholamine levels in different brain regions following Japanese encephalitis virus infection in rats. Brain Res. 2009;1292:136–147. doi: 10.1016/j.brainres.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 185.Chauhan PS, Khanna VK, Kalita J, Misra UK. Japanese encephalitis virus infection results in transient dysfunction of memory learning and cholinesterase inhibition. Mol Neurobiol. 2017;54:4705–4715. doi: 10.1007/s12035-016-9963-6. [DOI] [PubMed] [Google Scholar]
- 186.Chauhan PS, Misra UK, Kalita J, Chandravanshi LP, Khanna VK. Memory and learning seems to be related to cholinergic dysfunction in the JE rat model. Physiol Behav. 2016;156:148–155. doi: 10.1016/j.physbeh.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 187.Ford TC, Nibbs R, Crewther DP. Increased glutamate/GABA+ ratio in a shared autistic and schizotypal trait phenotype termed social disorganisation. Neuroimage Clin. 2017;16:125–131. doi: 10.1016/j.nicl.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bonansco C, Fuenzalida M. plasticity of hippocampal excitatory-inhibitory balance: missing the synaptic control in the epileptic brain. Neural Plast. 2016;2016:8607038. doi: 10.1155/2016/8607038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. doi: 10.1111/j.1471-4159.2006.03913.x. [DOI] [PubMed] [Google Scholar]
- 190.Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed. 2009;22:326–331. doi: 10.1002/nbm.1329. [DOI] [PubMed] [Google Scholar]
- 191.Young AC, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014;83:1592–1600. doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging. 2010;32:1045–1053. doi: 10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ferrarese C, et al. Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology. 2001;57:671–675. doi: 10.1212/WNL.57.4.671. [DOI] [PubMed] [Google Scholar]
- 194.Gorska AM, Eugenin EA. the glutamate system as a crucial regulator of CNS toxicity and survival of HIV reservoirs. Front Cell Infect Microbiol. 2020;10:261. doi: 10.3389/fcimb.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eugenin EA, et al. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Prendergast MA, et al. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;954:300–307. doi: 10.1016/S0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- 197.Kim HJ, Martemyanov KA, Thayer SA. Human immunodeficiency virus protein Tat induces synapse loss via a reversible process that is distinct from cell death. J Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang Y, et al. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J Neurosci. 2011;31:15195–15204. doi: 10.1523/JNEUROSCI.2051-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vazquez-Santiago FJ, Noel RJ, Jr, Porter JT, Rivera-Amill V. Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol. 2014;20:315–331. doi: 10.1007/s13365-014-0258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chauhan PS, Misra UK, Kalita J. A study of glutamate levels, NR1, NR2A, NR2B receptors and oxidative stress in rat model of Japanese encephalitis. Physiol Behav. 2017;171:256–267. doi: 10.1016/j.physbeh.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 201.Chen CJ, et al. Glutamate released by Japanese encephalitis virus-infected microglia involves TNF-alpha signaling and contributes to neuronal death. Glia. 2012;60:487–501. doi: 10.1002/glia.22282. [DOI] [PubMed] [Google Scholar]
- 202.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Choy RW, Cheng Z, Schekman R. Amyloid precursor protein (APP) traffics from the cell surface via endosomes for amyloid beta (Abeta) production in the trans-Golgi network. Proc Natl Acad Sci U S A. 2012;109:E2077–E2082. doi: 10.1073/pnas.1208635109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Multhaup G, Huber O, Buee L, Galas MC. amyloid precursor protein (APP) metabolites APP intracellular fragment (AICD), Abeta42, and Tau in nuclear roles. J Biol Chem. 2015;290:23515–23522. doi: 10.1074/jbc.R115.677211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.O'Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer's disease. Annu Rev Neurosci. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 207.Reddy PH. Amyloid beta-induced glycogen synthase kinase 3beta phosphorylated VDAC1 in Alzheimer's disease: implications for synaptic dysfunction and neuronal damage. Biochim Biophys Acta. 2013;1832:1913–1921. doi: 10.1016/j.bbadis.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lewis J, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 209.Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 210.Laval K, Enquist LW. The potential role of herpes simplex virus type 1 and neuroinflammation in the pathogenesis of Alzheimer's disease. Front Neurol. 2021;12:658695. doi: 10.3389/fneur.2021.658695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sait A, Angeli C, Doig AJ, Day PJR. viral involvement in Alzheimer's disease. ACS Chem Neurosci. 2021;12:1049–1060. doi: 10.1021/acschemneuro.0c00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Borrajo A, Spuch C, Penedo MA, Olivares JM, Agis-Balboa RC. Important role of microglia in HIV-1 associated neurocognitive disorders and the molecular pathways implicated in its pathogenesis. Ann Med. 2021;53:43–69. doi: 10.1080/07853890.2020.1814962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Harris SA, Harris EA. Molecular mechanisms for herpes simplex virus type 1 pathogenesis in Alzheimer's disease. Front Aging Neurosci. 2018;10:48. doi: 10.3389/fnagi.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Canet G, et al. HIV Neuroinfection and Alzheimer's disease: similarities and potential links? Front Cell Neurosci. 2018;12:307. doi: 10.3389/fncel.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009;217:131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 216.Deatly AM, Haase AT, Fewster PH, Lewis E, Ball MJ. human herpes virus infections and Alzheimer's disease. Neuropathol Appl Neurobiol. 1990;16:213–223. doi: 10.1111/j.1365-2990.1990.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 217.Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer's disease. J Pathol. 2002;197:395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 218.Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer's disease patients. J Med Virol. 2005;75:300–306. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- 219.Readhead B, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron. 2018;99:64–82 e67. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ball MJ. Limbic predilection in Alzheimer dementia: is reactivated herpesvirus involved? Can J Neurol Sci. 1982;9:303–306. doi: 10.1017/S0317167100044115. [DOI] [PubMed] [Google Scholar]
- 221.De Chiara G, et al. APP processing induced by herpes simplex virus type 1 (HSV-1) yields several APP fragments in human and rat neuronal cells. PLoS One. 2010;5:e13989. doi: 10.1371/journal.pone.0013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cairns DM, et al. A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Sci Adv. 2020;6:eaay8828. doi: 10.1126/sciadv.aay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Santana S, Recuero M, Bullido MJ, Valdivieso F, Aldudo J. Herpes simplex virus type I induces the accumulation of intracellular beta-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol Aging. 2012;33(430):e419–e433. doi: 10.1016/j.neurobiolaging.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 224.Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci Lett. 2007;429:95–100. doi: 10.1016/j.neulet.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 225.Santana S, Sastre I, Recuero M, Bullido MJ, Aldudo J. Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS One. 2013;8:e75842. doi: 10.1371/journal.pone.0075842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Piacentini R, et al. HSV-1 promotes Ca2+ -mediated APP phosphorylation and Abeta accumulation in rat cortical neurons. Neurobiol Aging. 2011;32(2323):e2313–e2326. doi: 10.1016/j.neurobiolaging.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 227.De Chiara G, et al. Recurrent herpes simplex virus-1 infection induces hallmarks of neurodegeneration and cognitive deficits in mice. PLoS Pathog. 2019;15:e1007617. doi: 10.1371/journal.ppat.1007617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Civitelli L, et al. Herpes simplex virus type 1 infection in neurons leads to production and nuclear localization of APP intracellular domain (AICD): implications for Alzheimer's disease pathogenesis. J Neurovirol. 2015;21:480–490. doi: 10.1007/s13365-015-0344-0. [DOI] [PubMed] [Google Scholar]
- 229.Piacentini R, et al. Herpes simplex virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-beta protein accumulation. Sci Rep. 2015;5:15444. doi: 10.1038/srep15444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Martin C, et al. Inflammatory and neurodegeneration markers during asymptomatic HSV-1 reactivation. J Alzheimers Dis. 2014;39:849–859. doi: 10.3233/JAD-131706. [DOI] [PubMed] [Google Scholar]
- 231.Lerchundi R, et al. Tau cleavage at D421 by caspase-3 is induced in neurons and astrocytes infected with herpes simplex virus type 1. J Alzheimers Dis. 2011;23:513–520. doi: 10.3233/JAD-2010-101386. [DOI] [PubMed] [Google Scholar]
- 232.Alvarez G, Aldudo J, Alonso M, Santana S, Valdivieso F. Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J Neurosci Res. 2012;90:1020–1029. doi: 10.1002/jnr.23003. [DOI] [PubMed] [Google Scholar]
- 233.Wozniak MA, Frost AL, Itzhaki RF. Alzheimer's disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis. 2009;16:341–350. doi: 10.3233/JAD-2009-0963. [DOI] [PubMed] [Google Scholar]
- 234.Liu X, Cohen JI. The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside. Virology. 2015;479-480:568–577. doi: 10.1016/j.virol.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zheng K, et al. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio. 2014;5:e00958–e00913. doi: 10.1128/mBio.00958-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ploia C, et al. Role of glycogen synthase kinase-3beta in APP hyperphosphorylation induced by NMDA stimulation in cortical neurons. Pharmaceuticals (Basel) 2010;3:42–58. doi: 10.3390/ph3010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Medina M, Garrido JJ, Wandosell FG. Modulation of GSK-3 as a therapeutic strategy on Tau pathologies. Front Mol Neurosci. 2011;4:24. doi: 10.3389/fnmol.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kristen H, et al. Herpes simplex virus type 2 infection induces AD-like neurodegeneration markers in human neuroblastoma cells. Neurobiol Aging. 2015;36:2737–2747. doi: 10.1016/j.neurobiolaging.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 239.Cairns DM, Itzhaki RF, Kaplan DL. potential involvement of varicella zoster virus in Alzheimer's disease via reactivation of quiescent herpes simplex virus type 1. J Alzheimers Dis. 2022;88:1189–1200. doi: 10.3233/JAD-220287. [DOI] [PubMed] [Google Scholar]
- 240.Gershon AA, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015;1:15016. doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Green DA, et al. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- 242.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- 243.Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- 244.Giunta B, et al. HIV-1 Tat contributes to Alzheimer's disease-like pathology in PSAPP mice. Int J Clin Exp Pathol. 2009;2:433–443. [PMC free article] [PubMed] [Google Scholar]
- 245.Kim BO, et al. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kim J, Yoon JH, Kim YS. HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One. 2013;8:e77972. doi: 10.1371/journal.pone.0077972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Soliman ML, Geiger JD, Chen X. Caffeine blocks HIV-1 Tat-induced amyloid beta production and Tau phosphorylation. J Neuroimmune Pharmacol. 2017;12:163–170. doi: 10.1007/s11481-016-9707-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Naghavi MH. "APP"reciating the complexity of HIV-induced neurodegenerative diseases. PLoS Pathog. 2018;14:e1007309. doi: 10.1371/journal.ppat.1007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging. 2013;34:2370–2378. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hategan A, Masliah E, Nath A. HIV and Alzheimer's disease: complex interactions of HIV-Tat with amyloid beta peptide and Tau protein. J Neurovirol. 2019;25:648–660. doi: 10.1007/s13365-019-00736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 252.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 253.Sejvar JJ, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 254.Hsu TW, et al. Dengue virus infection and risk of Parkinson's disease: a nationwide longitudinal study. J Parkinsons Dis. 2022;12:679–687. doi: 10.3233/JPD-212938. [DOI] [PubMed] [Google Scholar]
- 255.Wang H, et al. Bacterial, viral, and fungal infection-related risk of Parkinson's disease: meta-analysis of cohort and case-control studies. Brain Behav. 2020;10:e01549. doi: 10.1002/brb3.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta. 2009;1792:714–721. doi: 10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Dehner LF, Spitz M, Pereira JS. Parkinsonism in HIV infected patients during antiretroviral therapy - data from a Brazilian tertiary hospital. Braz J Infect Dis. 2016;20:499–501. doi: 10.1016/j.bjid.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Merello M, Bhatia KP, Obeso JA. SARS-CoV-2 and the risk of Parkinson's disease: facts and fantasy. Lancet Neurol. 2021;20:94–95. doi: 10.1016/S1474-4422(20)30442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Beatman EL, et al. Alpha-synuclein expression restricts RNA viral infections in the brain. J Virol. 2015;90:2767–2782. doi: 10.1128/JVI.02949-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Khanlou N, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15:131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Santerre M, et al. HIV-1 Vpr protein impairs lysosome clearance causing SNCA/alpha-synuclein accumulation in neurons. Autophagy. 2021;17:1768–1782. doi: 10.1080/15548627.2021.1915641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Semerdzhiev SA, Fakhree MAA, Segers-Nolten I, Blum C, Claessens M. Interactions between SARS-CoV-2 N-Protein and alpha-Synuclein accelerate amyloid formation. ACS Chem Neurosci. 2022;13:143–150. doi: 10.1021/acschemneuro.1c00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Wu Z, Zhang X, Huang Z, Ma K. SARS-CoV-2 proteins interact with alpha synuclein and induce Lewy body-like pathology in vitro. Int J Mol Sci. 2022;23:3394. doi: 10.3390/ijms23063394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Swarup V, et al. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011;134:2610–2626. doi: 10.1093/brain/awr159. [DOI] [PubMed] [Google Scholar]
- 265.Saberi S, Stauffer JE, Schulte DJ, Ravits J. Neuropathology of amyotrophic lateral sclerosis and its variants. Neurol Clin. 2015;33:855–876. doi: 10.1016/j.ncl.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rahic Z, Buratti E, Cappelli S. Reviewing the potential links between viral infections and TDP-43 proteinopathies. Int J Mol Sci. 2023;24:1581. doi: 10.3390/ijms24021581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Constant O, Barthelemy J, Nagy A, Salinas S, Simonin Y. West Nile virus neuroinfection in humans: peripheral biomarkers of neuroinflammation and neuronal damage. Viruses. 2022;14:756. doi: 10.3390/v14040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Laudanski K et al (2021) Dynamic changes in central and peripheral neuro-injury vs. neuroprotective serum markers in COVID-19 are modulated by different types of anti-viral treatments but do not affect the incidence of late and early strokes. Biomedicines 9:1791 [DOI] [PMC free article] [PubMed]
- 269.Paidas MJ, Cosio DS, Ali S, Kenyon NS, Jayakumar AR. Long-term sequelae of COVID-19 in experimental mice. Mol Neurobiol. 2022;59:5970–5986. doi: 10.1007/s12035-022-02932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rathore A, et al. CRISPR-based gene knockout screens reveal deubiquitinases involved in HIV-1 latency in two Jurkat cell models. Sci Rep. 2020;10:5350. doi: 10.1038/s41598-020-62375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Cabrera JR, Rodriguez-Izquierdo I, Jimenez JL, Munoz-Fernandez MA. Analysis of ALS-related proteins during herpes simplex virus-2 latent infection. J Neuroinflammation. 2020;17:371. doi: 10.1186/s12974-020-02044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 273.Hainfellner JA, et al. Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol. 1998;96:116–122. doi: 10.1007/s004010050870. [DOI] [PubMed] [Google Scholar]
- 274.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Voigtlander T, et al. Marked increase of neuronal prion protein immunoreactivity in Alzheimer's disease and human prion diseases. Acta Neuropathol. 2001;101:417–423. doi: 10.1007/s004010100405. [DOI] [PubMed] [Google Scholar]
- 276.Zafar S, et al. Prion protein interactome: identifying novel targets in slowly and rapidly progressive forms of Alzheimer's disease. J Alzheimers Dis. 2017;59:265–275. doi: 10.3233/JAD-170237. [DOI] [PubMed] [Google Scholar]
- 277.Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Crestini A, et al. Prions and neurodegenerative diseases: a focus on Alzheimer's disease. J Alzheimers Dis. 2022;85:503–518. doi: 10.3233/JAD-215171. [DOI] [PubMed] [Google Scholar]
- 279.Roberson ED, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kong C, et al. Binding between prion protein and abeta oligomers contributes to the pathogenesis of Alzheimer's disease. Virol Sin. 2019;34:475–488. doi: 10.1007/s12250-019-00124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Zhou J, Liu B. Alzheimer's disease and prion protein. Intractable Rare Dis Res. 2013;2:35–44. doi: 10.5582/irdr.2013.v2.2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Brody AH, Strittmatter SM. synaptotoxic signaling by amyloid beta oligomers in Alzheimer's disease through prion protein and mGluR5. Adv Pharmacol. 2018;82:293–323. doi: 10.1016/bs.apha.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lathe R, Darlix JL. Prion protein PrP nucleic acid binding and mobilization implicates retroelements as the replicative component of transmissible spongiform encephalopathy. Arch Virol. 2020;165:535–556. doi: 10.1007/s00705-020-04529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hara H, et al. Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells. Sci Rep. 2021;11:10109. doi: 10.1038/s41598-021-89586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Roberts TK, et al. PrPC, the cellular isoform of the human prion protein, is a novel biomarker of HIV-associated neurocognitive impairment and mediates neuroinflammation. Am J Pathol. 2010;177:1848–1860. doi: 10.2353/ajpath.2010.091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable