Abstract
Objectives
Risk prediction for patients with polymyositis/dermatomyositis-associated interstitial lung disease (PM/DM-ILD) is challenging due to heterogeneity in the disease course. We aimed to develop a mortality risk prediction model for PM/DM-ILD.
Methods
This prognostic study analysed patients with PM/DM-ILD admitted to Nanjing Drum Hospital from 2016 to 2021. The primary outcome was mortality within 1 year. We used a least absolute shrinkage and selection operator (LASSO) logistic regression model to identify predictive laboratory indicators. These indicators were used to create a laboratory risk score, and we developed a mortality risk prediction model by incorporating clinical factors. The evaluation of model performance encompassed discrimination, calibration, clinical utility and practical application for risk prediction and prognosis.
Results
Overall, 418 patients with PM/DM-ILD were enrolled and randomly divided into development (n=282) and validation (n=136) cohorts. LASSO logistic regression identified four optimal features in the development cohort, forming a laboratory risk score: C reactive protein, lactate dehydrogenase, CD3+CD4+ T cell counts and PO2/FiO2. The final prediction model integrated age, arthralgia, anti-melanoma differentiation-associated gene 5 antibody status, high-resolution CT pattern and the laboratory risk score. The prediction model exhibited robust discrimination (area under the receiver operating characteristic: 0.869, 95% CI 0.811 to 0.910), excellent calibration and valuable clinical utility. Patients were categorised into three risk groups with distinct mortality rates. The internal validation, sensitivity analyses and comparative assessments against previous models further confirmed the robustness of the prediction model.
Conclusions
We developed and validated an evidence-based mortality risk prediction model with simple, readily accessible clinical variables in patients with PM/DM-ILD, which may inform clinical decision-making.
Keywords: dermatomyositis, polymyositis, risk factors, autoimmunity
WHAT IS ALREADY KNOWN ON THIS TOPIC?
Interstitial lung disease (ILD) is a serious and frequently fatal complication observed in patients with polymyositis/dermatomyositis (PM/DM). The clinical course, response to treatment and overall prognosis of PM/DM-ILD patients exhibit substantial heterogeneity. The early identification of mortality risk in PM/DM-ILD patients remains a formidable challenge within the clinical realm.
WHAT THIS STUDY ADDS?
This large cohort study is to develop a pretreatment prediction model, utilising baseline clinical and laboratory indicators, to accurately forecast mortality risk in Asian PM/DM-ILD patients. Patients were categorised into three risk groups with distinct mortality rates. This model is intended to facilitate early personalised treatment decisions and guide clinical management effectively.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Relying solely on a single biomarker is inherently restrictive and may lack precision due to variations in cohort sizes and differing cut-off values for biomarkers. In contrast, adopting this comprehensive approach that integrates baseline clinical and laboratory indicators accurately forecasting mortality risk in Asian PM/DM-ILD patients, guiding clinial management effectively.
Introduction
Interstitial lung disease (ILD) is an important cause of morbidity and mortality in patients with polymyositis/dermatomyositis (PM/DM).1–3 PM/DM-associated ILD can be broadly classified into two categories: rapid progressive ILD (RP-ILD) and non-rapid progressive ILD (nonRP-ILD), each presenting distinct pathological features.4 The clinical course, response to treatment and overall prognosis of PM/DM-ILD patients exhibit substantial heterogeneity. RP-ILD, characterised by its rapid onset within a matter of days or weeks, frequently proves refractory to conventional therapies such as corticosteroids and immunosuppressive agents, resulting in unfavourable outcomes. In contrast, non-RP-ILD represents a milder variant with a more favourable prognosis, often responding well to immunosuppressive treatments.5–8 Consequently, the early identification of mortality risk in PM/DM-ILD patients remains a formidable challenge within the clinical realm. Achieving accurate prognostication holds paramount importance for guiding clinical decision-making processes.
Numerous studies have investigated baseline parameters associated with RP-ILD in PM/DM patients, encompassing immunological factors, laboratory markers and imaging characteristics.9–11 Among PM/DM patients, the presence of myositis-specific antibodies (MSAs) has been linked to specific clinical manifestations, physical examination findings and disease prognosis. Notably, antimelanoma differentiation-associated gene 5 (MDA5) antibodies stand out as a pivotal biomarker for predicting RP-ILD and adverse outcomes.12 13 Conversely, patients harbouring anti-aminoacyl-tRNA-synthetase antibodies (ARS) are often associated with nonRP-ILD, a form of ILD that demonstrates sensitivity to immunosuppressive therapy but tends to recur following treatment reduction.14 15
However, it is important to note that while anti-MDA5 antibodies are valuable indicators for identifying RP-ILD in patients with PM/DM, more than half of those with anti-MDA5 antibodies still progress to nonRP-ILD.16 Additionally, anti-ARS-positive patients may also experience RP-ILD, which can be fatal.15 17 These observations underscore the insufficiency of MSAs alone in accurately predicting disease trajectory and outcomes. Other laboratory markers, including C reactive protein (CRP), peripheral blood lymphocyte counts and KL-6 levels, not only reflect disease activity but also exhibit significant correlations with prognosis and treatment response.18 19 Nonetheless, previous studies have certain limitations, notably the development of these biomarkers based on limited cohort sizes or isolated case reports. Relying solely on a single biomarker is inherently restrictive and may lack precision due to variations in cohort sizes and differing cut-off values for biomarkers. In contrast, adopting a comprehensive approach that integrates multiple factors for a personalised assessment of clinical characteristics holds promise. Consequently, the primary aim of our current study is to develop a pretreatment prediction model, utilising baseline clinical and laboratory indicators, to accurately forecast mortality risk in Asian patients with PM/DM-ILD. This model is intended to facilitate early personalised treatment decisions and guide clinical management effectively.
Patients and methods
Study population
Patients exhibited some or all of the relevant symptoms associated with ILD, including cough, chest tightness and exertional dyspnoea. These patients were admitted to the hospital for the first time due to clinical symptoms and imaging findings indicative of interstitial pneumonia changes. Patients with PM/DM-ILD admitted to the Department of Respiratory and Critical Care Medicine in Nanjing Drum Tower Hospital between December 2016 and December 2021 were enrolled. Patients with overlapping syndromes or malignant tumours, such as rheumatoid arthritis, systemic sclerosis, Sjogren’s syndrome and lung cancer, were excluded. All the patients were over 18 years old and were followed up for at least 12 months. The requirement for informed consent was waived due to the nature of the retrospective study and the anonymous processing of individual data. The study protocol was approved by the Ethics Committee of the Nanjing Drum Tower Hospital. This retrospective prognostic study adhered to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis reporting guidelines and was conducted following the principles of the Declaration of Helsinki.
Diagnostic criteria of PM/DM-ILD
PM/DM diagnosis was established based on the Bohan and Peter diagnostic criteria in1975 and EULAR/ACR classification criteria in 2017.20 21 Patients were considered to have ILD if they met following criteria: (1) demonstrated respiratory symptoms, such as dry cough, wheezing and dyspnoea on exertion; (2) exhibited physical signs like Velcro rales; (3) abnormal chest high-resolution CT (HRCT) revealed ground-glass opacity, consolidations, reticulations, honeycombs, etc; (4) lung function test indicated decreased restrictive ventilation and diffuse function, defined as total lung capacity and carbon monoxide lung diffusion below 80% of predicted values; (5) with the exclusion of infection, drug-induced ILD and exposure to the environment.22 The diagnosis of RP-ILD was made according to the revised diagnostic criteria described by Collard et al, in 2016, with some modifications.23 Briefly, RP- ILD was diagnosed with the presence of any of the following criteria: acute worsening or development of dyspnoea less than 1 month requiring hospitalisation; HRCT findings of new extent of interstitial abnormalities increased more than 20% including ground-glass opacity and/or consolidation superimposed on a background; the oxygen partial pressure reduced more than 10 mm Hg in arterial blood gas analysis. Two expert radiologists evaluated the presence of ILD independently.
Variables of interest and outcomes
Clinical data prior to the initial treatment were extracted from original (or electronic) medical records. In this study, the detection of MSAs and MAAs for all the patients was performed using an immunoblot assay conducted by the central lab on admission. Demographic information, including the age, gender and disease course, was recorded; additionally, clinical features, such as heliotrope sign, Gottron sign, Arthralgia and muscle weakness, were also recorded. Experimental data included routine blood tests (white blood counts (WBC), platelet, lymphocyte), lactic dehydrogenase (LDH), CRP, creatine kinase (CK), arterial blood gas analysis (PaO2, PaCO2), partial pressure of arterial oxygen/fraction of inspiration oxygen (PaO2/FiO2). If available, forced vital capacity predicted (FVC%) and diffusing capacity of the lung for carbon monoxide predicted (DLCO%) were recorded when patients were first admitted to the hospital.
The experimental data included routine blood tests (WBC, platelet, lymphocyte), CD4+ T counts, LDH, CRP, IgG, erythrocyte sedimentation rate, CK, arterial blood gas analysis (PaO2, PaCO2, PaO2/FiO2), lung function (FVC%, DLCO%), which were chosen as candidate laboratory biomarkers for developing a risk score for the following reasons: (1) their predictive roles in PM/DM-ILD outcomes have been previously reported in the literature; (2) relative laboratory assay systems have been established and these indicators are highly accessible. Additionally, all the patients underwent chest HRCT at admission. The imaging appearances observed were mainly categorised into non-specific interstitial pneumonia (NSIP), organising pneumonia (OP) patterns or usual interstitial pneumonia (UIP) patterns.
Mortality data are collected mainly through two methods. For patients who die during hospitalisation, electronic medical records are consulted for confirmation. For discharged patients who did not die in the hospital, including those who are cured, improved, or not improved, we conduct regular follow-ups through the contact information provided by the patients, once every 3 months in the first year and once every 6 months thereafter. The outcomes of death for discharged patients are primarily determined through narratives from family members, and the causes of death as described by them are also recorded.
Statistical analysis
Follow-up information, including survival and death outcomes, was collected by telephone calls retrospectively and prospectively. Continuous variables were presented using medians and IQRs, and their comparison involved the Mann-Whitney U test. Categorical variables were expressed in terms of frequencies and percentages, and their comparison relied on either the χ2 test or Fisher’s exact test. To address missing data, multiple imputations were conducted using a multivariable imputation by chained equations algorithm. Survival analysis was conducted using the Kaplan-Meier method and compared with the log-rank test. The methodology used for constructing and validating the prediction model in this study mainly refers to the literature.24 The study population was randomly split into development and validation cohorts at a ratio of 2:1. The derivation of the prediction model encompassed two key steps. Initially, we employed a least absolute shrinkage and selection operator (LASSO) logistic regression model, along with 10-fold cross-validation, to reduce model complexity and identify the optimal predictive features among the candidate laboratory indicators in the development cohort. Subsequently, we constructed a laboratory risk score based on the coefficients associated with these optimal features, as determined by the LASSO logistic regression using lambda.1se. In the second step, the laboratory risk score and potential prognostic clinical features were incorporated into a stepwise multivariate logistic regression model. The model selection process relied on the likelihood ratio test, guided by Akaike’s information criterion (AIC) as the stopping criterion. The model with the smallest AIC was chosen as the optimal model and further used to formulate the final prediction model. Dominance analysis was used to determine the relative contribution of each independent predictor to the prediction model.25 We assessed the prediction model’s performance in terms of discrimination, calibration and clinical usefulness. Discriminative ability was quantified using the area under the receiver operating characteristic (AUC) curves. We performed bootstrapping with 1000 replicates to obtain bias-corrected AUCs and their respective 95% CI. Calibration was scrutinised using the Hosmer–Lemeshow goodness-of-fit test, Brier score, and observed versus predicted graphs. Clinical usefulness was evaluated through decision curve analysis (DCA). Finally, the robustness of the model’s performance was further evaluated through internal validation, sensitivity analyses and comparative assessments against previous models.
All statistical analyses were conducted using R V.4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
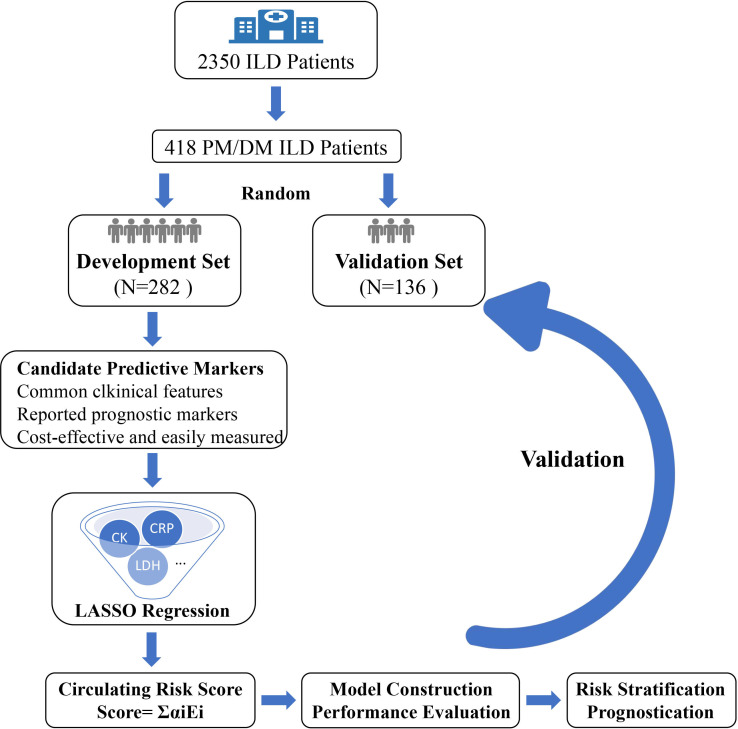
The study flowchart is shown in figure 1. Overall, 418 eligible patients were enrolled in the analysis. The study population was randomly divided into development (n=282) and validation (136) cohorts aiming at the ratio of 2:1. The baseline characteristics of all patients and of those in each cohort are summarised in table 1. The patients were predominantly women (63%) with a median age of 56.0 (IQR, 50.0–65.8) years. All patients received corticosteroid treatment as their initial therapy, and 88% underwent combination therapy involving two or more treatment approaches (online supplemental table S1). A total of 91 deaths were recorded at the follow-up cut-off, with the majority of patients (86, 94.5%) succumbing to PM/DM-ILD and PM/DM-ILD-related infection. Other causes of death included cerebrovascular accident (1), cardiovascular accidents (2) and unknown reasons (2). The pattern of missing values is visualised in online supplemental figure S1. The patient characteristics after the imputation of missing values are found in online supplemental table S2.
Figure 1.
Study flowchart. DM, dermatomyositis; ILD, interstitial lung disease; LASSO, least absolute shrinkage and selection operator; PM, polymyositis.
Table 1.
Patient characteristics
| Characteristic | Overall (N=418) | Development cohort (N=282) | Validation cohort (N=136) | P value |
| Age | 56.0 (50.0–65.8) | 55.5 (50.0–66.0) | 56.0 (50.0–65.0) | 0.496 |
| Gender | 0.811 | |||
| Female | 264 (63%) | 177 (63%) | 87 (64%) | |
| Male | 154 (37%) | 105 (37%) | 49 (36%) | |
| Smoking | 0.175 | |||
| Absent | 310 (76%) | 204 (74%) | 106 (80%) | |
| Present | 97 (24%) | 71 (26%) | 26 (20%) | |
| Unknown | 11 | 7 | 4 | |
| Cough | 0.630 | |||
| Absent | 61 (15%) | 43 (16%) | 18 (14%) | |
| Present | 337 (85%) | 227 (84%) | 110 (86%) | |
| Unknown | 20 | 12 | 8 | |
| Breathless | 0.740 | |||
| Absent | 37 (10%) | 26 (11%) | 11 (9.4%) | |
| Present | 327 (90%) | 221 (89%) | 106 (91%) | |
| Unknown | 54 | 35 | 19 | |
| Fever | 0.430 | |||
| Absent | 286 (69%) | 196 (70%) | 90 (66%) | |
| Present | 130 (31%) | 84 (30%) | 46 (34%) | |
| Unknown | 2 | 2 | 0 | |
| Heliotrope sign | 0.056 | |||
| Absent | 354 (85%) | 232 (83%) | 122 (90%) | |
| Present | 63 (15%) | 49 (17%) | 14 (10%) | |
| Unknown | 1 | 1 | 0 | |
| Gottron sign | 0.454 | |||
| Absent | 219 (53%) | 144 (51%) | 75 (55%) | |
| Present | 198 (47%) | 137 (49%) | 61 (45%) | |
| Unknown | 1 | 1 | 0 | |
| Myasthenia | 0.588 | |||
| Absent | 334 (80%) | 223 (79%) | 111 (82%) | |
| Present | 83 (20%) | 58 (21%) | 25 (18%) | |
| Unknown | 1 | 1 | 0 | |
| Arthralgia | 0.169 | |||
| Absent | 326 (78%) | 214 (76%) | 112 (82%) | |
| Present | 90 (22%) | 66 (24%) | 24 (18%) | |
| Unknown | 2 | 2 | 0 | |
| HRCT | 0.258 | |||
| NSIP | 156 (37%) | 100 (35%) | 56 (41%) | |
| OP or UIP | 262 (63%) | 182 (65%) | 80 (59%) | |
| MDA5 | 0.228 | |||
| Negative | 272 (65%) | 178 (63%) | 94 (69%) | |
| Positive | 146 (35%) | 104 (37%) | 42 (31%) | |
| RO52 | 0.072 | |||
| Negative | 161 (39%) | 117 (41%) | 44 (32%) | |
| Positive | 257 (61%) | 165 (59%) | 92 (68%) | |
| WBC | 7.4 (5.4–10.0) | 7.2 (5.4–10.0) | 7.7 (5.4–10.0) | 0.724 |
| PLT | 226.5 (173.0–285.8) | 227.0 (173.3–295.3) | 226.0 (172.0–276.8) | 0.467 |
| Lymphocyte | 1.1 (0.8–1.7) | 1.2 (0.7–1.7) | 1.1 (0.8–1.7) | 0.840 |
| CD3+CD4+ T | 0.4 (0.2–0.6) | 0.4 (0.2–0.6) | 0.3 (0.2–0.5) | 0.150 |
| Unknown | 3 | 3 | 0 | |
| LDH | 298.5 (236.5–422.3) | 292.0 (232.8–408.3) | 320.5 (246.5–439.3) | 0.058 |
| Unknown | 2 | 2 | 0 | |
| CRP | 6.7 (3.8–21.4) | 6.7 (3.8–20.7) | 6.7 (3.9–21.6) | 0.805 |
| IgG | 12.0 (9.6–14.7) | 12.3 (9.7–14.8) | 11.3 (9.4–14.6) | 0.123 |
| Unknown | 5 | 3 | 2 | |
| ESR | 27.5 (16.0–44.0) | 28.0 (16.0–45.8) | 26.0 (13.0–41.5) | 0.158 |
| CK | 49.0 (30.0–102.3) | 47.5 (30.0–94.5) | 55.0 (32.8–123.8) | 0.161 |
| Unknown | 2 | 2 | 0 | |
| FVC% | 61.8 (50.7–71.5) | 62.1 (52.0–71.5) | 61.2 (47.3–71.4) | 0.506 |
| Unknown | 185 | 125 | 60 | |
| FEV1% | 67.2 (55.9–75.8) | 67.4 (56.4–75.0) | 67.2 (53.6–77.8) | 0.608 |
| Unknown | 189 | 128 | 61 | |
| DLCO% | 53.0 (43.0–65.0) | 51.7 (43.2–64.6) | 53.6 (41.1–67.5) | 0.786 |
| Unknown | 185 | 125 | 60 | |
| PO2 | 73.0 (64.0–85.0) | 73.0 (64.0–86.0) | 71.5 (63.8–82.8) | 0.483 |
| Unknown | 1 | 1 | 0 | |
| PCO2 | 35.4 (32.8–39.8) | 35.5 (33.0–39.6) | 35.2 (32.2–39.8) | 0.988 |
| Unknown | 45 | 31 | 14 | |
| PaO2/FiO2 | 310.0 (220.0–376.0) | 319.0 (220.0–380.0) | 305.0 (218.8–376.0) | 0.248 |
| Unknown | 1 | 1 | 0 |
Data are given as median (IQR) or n (%). A Gottron’s sign and inverse Gottron’s sign were pooled in data collection.
CK, creatine kinase; CRP, C reactive protein; DLCO%, diffusing capacity of the lung for carbon monoxide predicted; ESR, erythrocyte sedimentation rate; FiO2, fraction of inspiration O2; FVC%, forced vital capacity predicted; HRCT, high-resolution CT; IgG, immunoglobulin G; LDH, lactate CT; MDA5, melanoma differentiation-associated gene 5; NSIP, non-specific interstitial pneumonia; OP, organising pneumonia; PLT, platelets; UIP, usual interstitial pneumonitis; WBC, white cell counts.
rmdopen-2023-003850supp001.pdf (528.3KB, pdf)
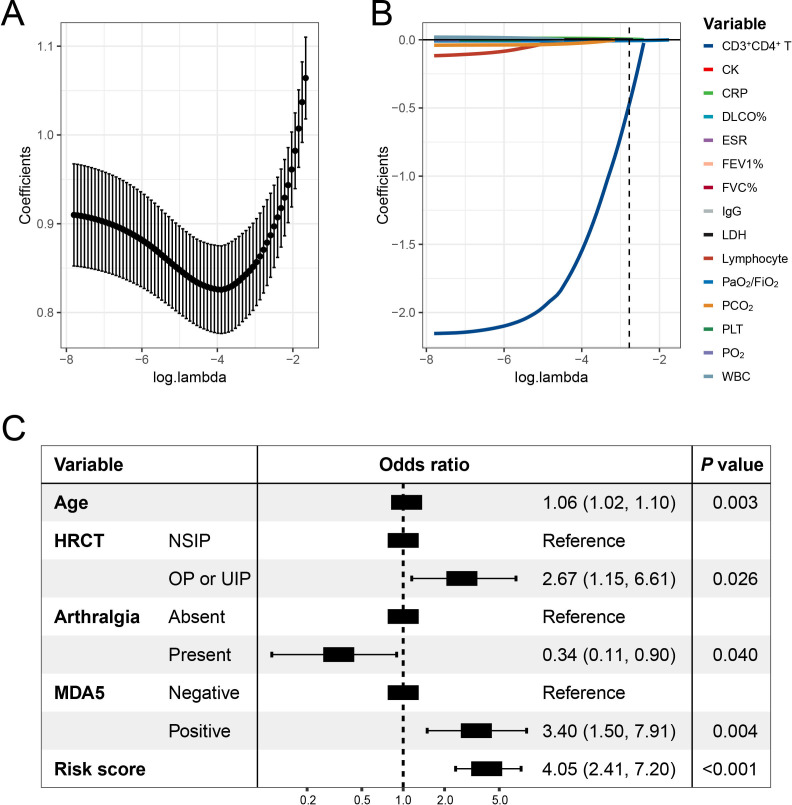
Construction of the laboratory risk score
LASSO logistic regression streamlined the initial array of 15 candidate laboratory indicators to the four most informative predictive indicators, namely CRP, LDH, CD3+CD4+ T-cell counts and PO2/FiO2 (figure 2A,B). Consequently, we computed a laboratory risk score for each patient using the selected features and their respective coefficients obtained from the optimal LASSO logistic regression model. The formula for calculation is as follows: laboratory risk score=CRP *0.0046+LDH * 0.00015 CD4 T-counts *0.47 - PaO2/FiO2 *0.0062. Univariate and multivariate associations between 15 candidate markers and 1-year mortality risk in patients with PM/DM-ILD are summarised in online supplemental table S3.
Figure 2.
The optimal features were selected using a LASSO logistics regression using 10-fold cross-validation via lambda.1se criteria in the development cohort (A). LASSO coefficient profiles of the candidate laboratory indicators (B). Summary of results obtained from the optimal logistic regression model selected by the stepwise multivariable logistics regression mode (C). CK, creatine kinase; CRP, C reactive protein; DLCO%, diffusing capacity of the lung for carbon monoxide predicted; ESR, erythrocyte sedimentation rate; FiO2, fraction of inspiration O2; FVC%, forced vital capacity predicted; HRCT, high-resolution CT; IgG, immunoglobulin G; LASSO, least absolute shrinkage and selection operator; LDH, lactate CT; MDA5, melanoma differentiation-associated gene 5; NSIP, non-specific interstitial pneumonia; OP, organising pneumonia; PLT, platelets; UIP, usual interstitial pneumonia; WBC, white cell counts.
Construction of the mortality risk prediction model
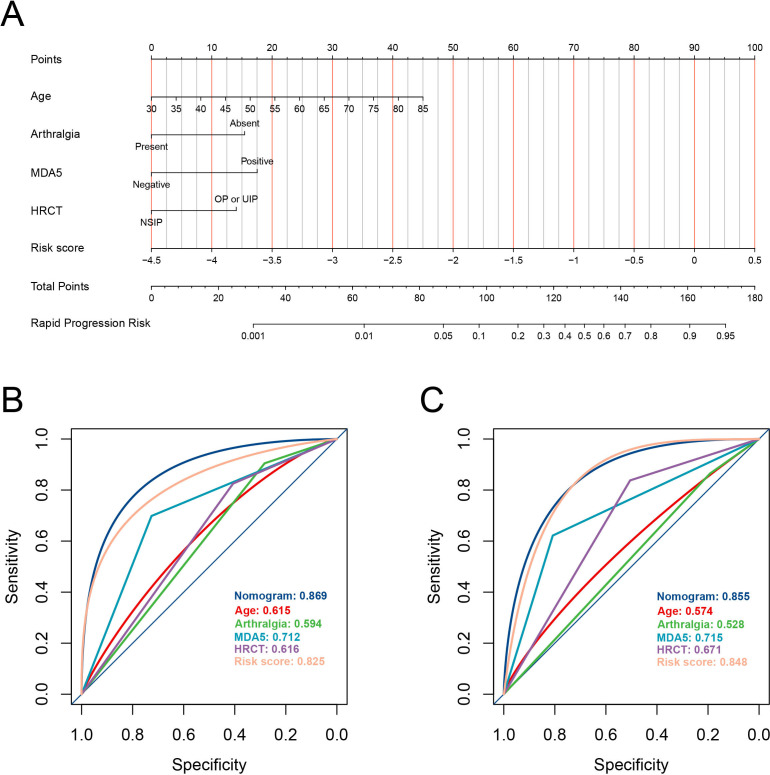
In the development cohort, the laboratory risk score, in conjunction with other candidate clinical features, underwent stepwise multivariate logistic regression modelling. We selected the final model, which exhibited no collinearity issues (online supplemental figure S2) and had the lowest AIC of 209, for model construction (figure 2C). Notably, the laboratory risk score emerged as a significant independent predictor (OR: 4.05, 95% CI 2.41 to 7.20; p<0.001). Additionally, several other indicators, including age, arthralgia, HRCT pattern and anti-MDA5 antibody status, were retained in this model. Dominance analysis demonstrated that the laboratory risk score was the greatest contributor to the mortality within 1 year (average contribution as measured by r2m, 0.179; online supplemental figure S3), followed by anti-MDA5 antibody (0.075), age (0.036), HRCT (0.027) and anti-MDA5 antibody (0.025). To facilitate practical application, we devised a prediction tool incorporating these factors, visually represented as a nomogram (figure 3A). Moreover, for user convenience, we have provided an accessible online calculator via the following website: https://shuangyiliu.shinyapps.io/PMDMILD/.
Figure 3.
The mortality risk prediction model for patients with polymyositis/dermatomyositis-associated interstitial lung disease is presented as a nomogram (A). Discrimination was evaluated using the area under the receiver operating characteristic curves for both the prediction model and other indicators in the development cohort (B) and the internal validation cohort (C). HRCT, high-resolution CT; MDA5, melanoma differentiation-associated gene 5; NSIP, non-specific interstitial pneumonia; OP, organising pneumonia; UIP, usual interstitial pneumonia.
Assessment for model performance
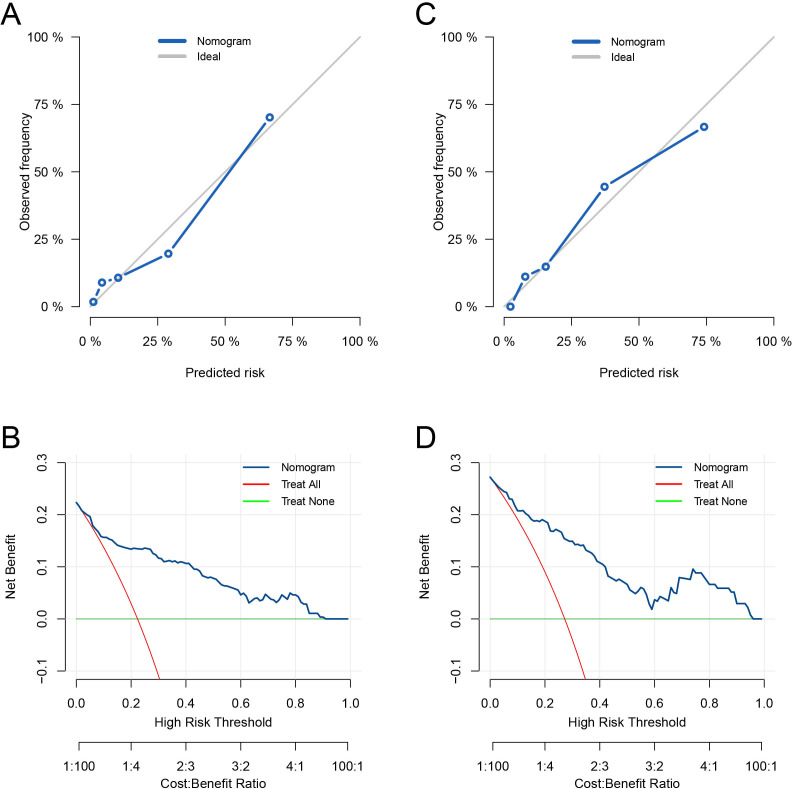
The mortality risk prediction model demonstrated robust discriminative performance in the development cohort, achieving an AUC of 0.869 (95% CI 0.811 to 0.910). Importantly, this AUC value was notably superior to that of other variables (all p<0.05, as shown in figures 3B). The model’s calibration was deemed acceptable, as evidenced by the Hosmer-Lemeshow test yielding a p value of 0.074, a Brier score of 0.11 and the observed versus predicted graphs (figure 4A). DCA further underscored the model’s utility. It revealed that the prediction model provided greater net benefits when compared with both the ‘treat-all-patients’ and ‘treat-no-patients’ strategies across all threshold probabilities (figure 4B). Internal validation using an independent cohort confirmed the model’s robust discriminative capacity (AUC: 0.855, 95% CI 0.780 to 0.917; figures 3C), its well-calibrated nature (Hosmer-Lemeshow test, p=0.670; Brier score, 0.13; observed vs predicted graphs, figure 4C) and its clinical utility (figure 4D).
Figure 4.
Calibration was examined via observed versus predicted graphs in the development cohort (A) and the internal validation cohort (C). Clinical utility was assessed through decision curve analysis in the development cohort (B) and the internal validation cohort (D).
Risk stratification of patients with PM/DM-ILD
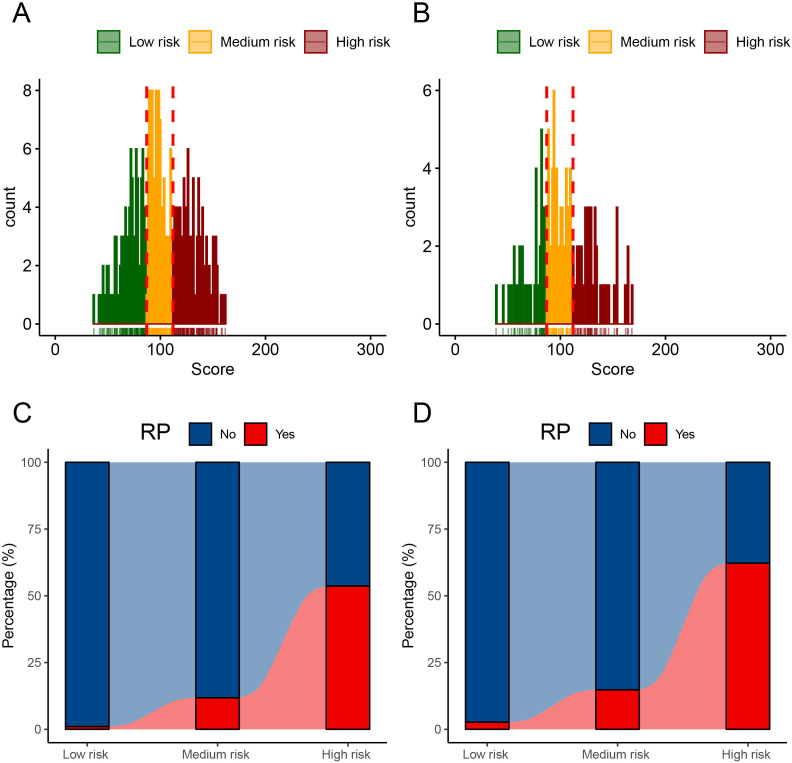
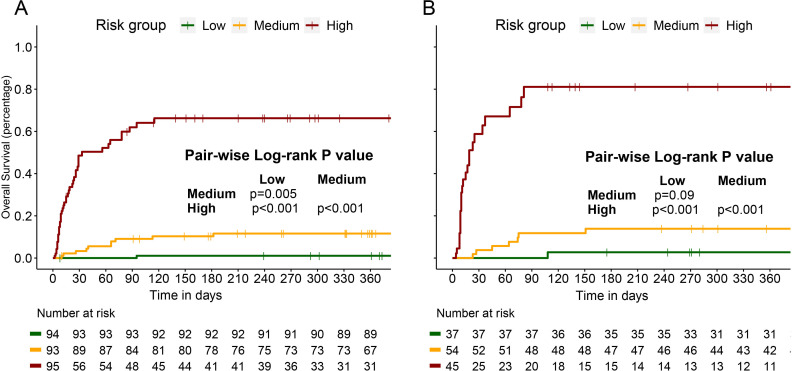
The distribution of the nomogram scores in both the development and validation cohorts is depicted in figure 5A,B. Patients were categorised into three risk groups, each associated with distinct 1-year mortality rates, based on score trisection values (87 and 112) determined during the training cohort analysis. Patients with higher nomogram scores showed a pronounced correlation with an elevated risk of 1-year mortality, a relationship clearly evident in both the development cohort (low risk: 1.1%, medium risk: 11.8%, high risk: 53.7%; Cochran-Armitage test for trend: p<0.001; figure 5C) and the internal validation cohort (low risk: 2.7%, medium risk: 14.8%, high risk: 62.2%; Cochran-Armitage test for trend: p<0.001; figure 4D). Survival analyses underscored the disparities in 1-year survival probabilities among patients in different risk groups. In the development cohort, the 1-year survival probabilities varied significantly (low vs medium vs high: 98.9% vs 89.1% vs 51.6%; figure 6A), and a similar pattern was observed in the internal validation cohort (low vs medium vs high: 97.3% vs 87.0% vs 44.4%; figure 6B).
Figure 5.
The distribution of nomogram scores in the development cohort (A) and internal validation cohort (B). Patients with higher nomogram scores were associated with significantly higher 1 year mortality risk in the development cohort (C) and internal validation cohort (D). RP, rapid progressive.
Figure 6.
Survival analyses with log-rank test revealed that patients with polymyositis/dermatomyositis-associated interstitial lung disease in different risk groups had distinct 1 year survival probabilities in the development cohort (A) and internal validation cohort (B).
Sensitivity analyses and comparative assessments against previous models
We conducted a series of sensitivity analyses to verify the model’s robustness in extreme scenarios without anti-MDA5 antibody data, across patients included at different periods, and among those receiving varied treatment modalities (online supplemental figure S4). We also compared the current model with the existing FLAW and FALIR models. The current model showed higher predictive accuracy than the other two models (online supplemental figure S5). Considering the variations in inclusion criteria for patients and laboratory biases among various testing indicators, these results need to be interpreted with caution.
Discussion
PM/DM is a clinically heterogeneous disease with a high incidence of ILD and a poor prognosis. Despite aggressive treatments, some patients experience respiratory failure, which is a leading contributor to death in patients with PM/DM-ILD, particularly in those with amyopathic dermatomyositis (CADM)-ILD.26 27 This study aimed to distinguish high mortality risk subgroups in a relatively large Chinese PM/DM-ILD cohort. We successfully distinguished clinical as well as laboratory indicators of susceptibility to death at the time of diagnosis and developed an evidence-based prediction model for assessing mortality risk in Chinese patients with PM/DM-ILD. In addition to the well-known significant contribution of anti-MDA5 antibodies to poor survival in patients with PM/DM-ILD,17 28 29 this study also identified other indicators such as CRP, LDH, CD3+CD4+ T-cell count, PO2/FiO2 and HRCT patterns, which further enhanced the ability to predict survival in patients with PM/DM-ILD, leading to a new prediction model. This prediction model demonstrated a consistent utility when applied to a single institution using two different cohorts. To the best of our knowledge, this prediction model offers several advantages over relying solely on anti-MDA5 antibody testing. It provided a more comprehensive risk stratification by assigning a specific score to each PM/DM-ILD patient. This model had the potential to guide individualised treatment strategies by accurately identifying patients with PM/DM-ILD with the highest risk of death, leading to reducing mortality.
Our results indicated that death of the disease was most likely to occur in patients classified as the high-risk according to the prediction model. The leading cause of death was related to rapid progression of the disease. The significant risk factors for mortality identified in this prediction model were not solely limited to biomarkers but may also reflect the persistent pathogenic processes underlying PM/DM-ILD.
Notably, MDA5, a double-stranded RNA-specific helicase, is an essential target of MSAs and involved in the synthesis of type I IFN and the activation of NF-κB.30 31 Patients with anti-MDA5 antibody are at a higher risk of developing RP-ILD, which is also strongly associated with unfavourable treatment outcomes in patients with PM/DM-ILD.13 18 However, the pathogenic mechanisms of anti-MDA5 antibodies in this disease are still in their infancy. Recent studies have demonstrated that the lung biopsy pathology in anti-MDA5 +DM-ILD predominantly exhibits diffuse alveolar damage (DAD) pattern.32 Wang et al find a correlation between high levels of anti-MDA5 antibodies and the severity, as well as unfavourable outcomes, in patients with COVID-19. The inflammation storm triggered by MDA5 antibody leads to DAD, contributing to acute respiratory distress syndrome (ARDS).33 A previous study by Yun et al demonstrates that anti-MDA5 antibodies induce epithelial cell damage, resulting in release of inflammatory cytokines through the formation of neutrophil extracellular traps.34 In conclusion, acute lung damage in anti-MDA5+ DM-ILD shows similarities to COVID-19, leading to the development of ARDS and potentially fatal outcomes.
The obviously elevated levels of CRP were observed in non-survivors. CRPs, as a serum inflammatory cytokine, are mainly produced under the control of IL-6 signalling.35 The recent studies demonstrate raised serum CRP participate in disease progression in PM/DM-ILD.36 37 Therefore, we believed that the identified serum biomarkers would participate in persistent pathogenic process of PM/DM-ILD, including DAD and excessive activation of inflammatory signalling pathway. Anti-MDA5 Ab and CRP acted as the important risk factors for poor survival, which were incorporated into the prediction model.
Lymphocyte-mediated immunity is considered to play an important role in the pathogenesis of PM/DM-ILD.38 Huang et al have reported that a lower blood lymphocyte count is closely associated with the development of anti-MDA5+ DM-ILD. More importantly, there is a significant reduction in CD4 T cell counts within the peripheral blood lymphocyte subgroups, which is associated with exacerbation of ILD.39 Similarly, Chen et al indicated that a noticeable decrease in peripheral CD4+T cell counts was closely associated with incidence of acute/subacute interstitial pneumonia and poor prognosis of anti-MDA5+ DM-ILD.40 CD3+CD4+ T cells, acting as T helper lymphocytes, play a crucial role in inflammatory diseases. Their reduction in patients with PM/DM ILD suggests an immune response imbalance.41 Nevertheless, further fundamental research is needed to elucidate the pathophysiological mechanisms of abnormal T cells in peripheral blood during the progression of PM/DM-ILD. Consistent with previous studies, our results indicated an obvious decrease in peripheral CD3+CD4+ T cell counts in PM/DM ILD patients, which could be used for clinical assessment of disease prognosis.
In the current study, HRCT pattern was identified as meaningful indicators of poor outcomes. OP was the most frequently observed radiological pattern, followed by NSIP. OP was closely associated with the rapid progress and death in PM/DM ILD patients. On the other hand, patients with UIP are typically rare and also related with unfavourable outcomes. The presence of honeycomb is the primary manifestation of UIP pattern that is associated with pulmonary fibrosis that is resistant to immunosuppressive and steroid therapy.42 In contrast, NSIP and OP patterns are sensitive to steroid therapy, resulting in the improvement of pulmonary lesions.43 However, in the case of anti MDA5+ DM-ILD patients, the presence of OP pattern was exceptional but proved to be an independent predictor of 90-day mortality. 44 Yu et al also reported that the combination of OP and anti-MDA5 antibodies was associated with the development of RP-ILD in patients with PM/DM ILD.45 This specific OP pattern observed at admission was linked to DAD and excessive inflammatory reaction, which contributed to disease progression, ultimately leading to death.
In addition, lung function tests play key role to evaluate the severity of the disease. FVC is related to the survival of anti MDA5+DM ILD. However, some patients could not complete lung function tests due to respiratory failure at admission. PaO2/FiO2 is also proved a well-conceived tool for evaluating severity of ILD.28 Moreover, some clinical features, such as arthralgia, were markedly found in survivors of patients with PM/DM-ILD. Hence, some clinical manifestations were also incorporated in developing the prediction tool. Therefore, such clinical features were also included in establishing the mortality risk prediction model. The prediction model we proposed could help us better understand the severity of the disease and guide clinical decision-making and individual treatment. Additionally, this model has the potential to assist in the clinical research design in patients with PM/DM-ILD based on varying risks of mortality.
There are several limitations in our study. First, our ability to conduct a comprehensive analysis of clinical parameters was restricted due to the lack of data, such as pulmonary function. It is understandable that some serious patients with respiratory failure could not complete pulmonary function tests. Second, the retrospective nature and single-centre study may lead to selection bias. Further multicentre external studies are needed to confirm the predictive capabilities and generalisability of our prediction model in the future. Finally, given that each circulating biomarker in the prediction could potentially change over time, whether the use of a mortality laboratory risk score as a dynamic monitoring indicator for treatment response in PM/DM-ILD patients requires further investigation.
In summary, we have effectively developed a predictive tool for patients with PM/DM-ILD by using baseline clinical and laboratory parameters. This tool can assess an individual’s risk of mortality and assist healthcare professionals in their decision-making process regarding the care of newly diagnosed patients with PM/DM-ILD.
rmdopen-2023-003850supp002.pdf (104.4KB, pdf)
Footnotes
Contributors: YX and XG had full access to all the data in the study and took responsibility for the data’s integrity and the data analysis’s accuracy. XG, WL and RW contributed equally to this study and shared the first authorship. Concept and design: YX, HJ and XG. Acquisition, analysis or interpretation of data: all authors. Drafting of the manuscript: XG, WL and RW. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: all authors. Obtained funding: YX, WL and XG. Administrative, technical or material support: YX and HJ. Supervision: YX, XG, HJ. YX was involved in the interpretation of the data and in drafting the article or revising it for critically important content. YX is the guarantor, responsible for the overall content. All authors read and approved the final version of submitted manuscript.
Funding: This work was supported by the National Natural Science Foundation of China (Grant Number 82303338), the Special Fund for Clinical Research from the Affiliated Drum Tower Hospital of Nanjing University Medical School (2021-LCYJ-PY-05), the Special Fund for Clinical Research (Single Disease Database) from the Affiliated Drum Tower Hospital of Nanjing University Medical School (2021-LCYJ-DBZ-06), Nanjing Special Fund for Health Science and Technology Development (YKK23081, ZKX22021), the Postdoctoral Research Foundation of China (Grant Number 2023T160146, 2023M740835), the Postdoctoral Fellowship Program of CPSF and the Grant of State Key Laboratory of Respiratory Disease (Grant Number SKLRD-Z-202401).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971–82. 10.1016/S0140-6736(03)14368-1 [DOI] [PubMed] [Google Scholar]
- 2. Johnson C, Pinal-Fernandez I, Parikh R, et al. Assessment of mortality in autoimmune Myositis with and without associated interstitial lung disease. Lung 2016;194:733–7. 10.1007/s00408-016-9896-x [DOI] [PubMed] [Google Scholar]
- 3. Yamasaki Y, Yamada H, Ohkubo M, et al. Longterm survival and associated risk factors in patients with adult-onset idiopathic inflammatory Myopathies and Amyopathic dermatomyositis: experience in a single Institute in Japan. J Rheumatol 2011;38:1636–43. 10.3899/jrheum.101002 [DOI] [PubMed] [Google Scholar]
- 4. Kameda H, Takeuchi T. Recent advances in the treatment of interstitial lung disease in patients with polymyositis/dermatomyositis [DOI] [PubMed]
- 5. Mukae H, Ishimoto H, Sakamoto N, et al. Clinical differences between interstitial lung disease associated with clinically Amyopathic dermatomyositis and classic dermatomyositis. Chest 2009;136:1341–7. 10.1378/chest.08-2740 [DOI] [PubMed] [Google Scholar]
- 6. Kawasumi H, Gono T, Kawaguchi Y, et al. Recent treatment of interstitial lung disease with idiopathic inflammatory Myopathies. Clin Med Insights Circ Respir Pulm Med 2015;9:9–17. 10.4137/CCRPM.S23313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morisset J, Johnson C, Rich E, et al. Management of Myositis-related interstitial lung disease. Chest 2016;150:1118–28. 10.1016/j.chest.2016.04.007 [DOI] [PubMed] [Google Scholar]
- 8. Go DJ, Park JK, Kang EH, et al. Survival benefit associated with early cyclosporine treatment for dermatomyositis-associated interstitial lung disease. Rheumatol Int 2016;36:125–31. 10.1007/s00296-015-3328-8 [DOI] [PubMed] [Google Scholar]
- 9. Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-Kd polypeptide, CADM-140, in Japanese patients with clinically Amyopathic dermatomyositis. Arthritis Rheum 2005;52:1571–6. 10.1002/art.21023 [DOI] [PubMed] [Google Scholar]
- 10. Arai S, Kurasawa K, Maezawa R, et al. Marked increase in serum KL-6 and surfactant protein D levels during the first 4 weeks after treatment predicts poor prognosis in patients with active interstitial pneumonia associated with polymyositis/dermatomyositis. Mod Rheumatol 2013;23:872–83. 10.1007/s10165-012-0756-0 [DOI] [PubMed] [Google Scholar]
- 11. Tanizawa K, Handa T, Nakashima R, et al. The Prognostic value of HRCT in Myositis-associated interstitial lung disease. Respir Med 2013;107:745–52. 10.1016/j.rmed.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 12. Gui X, Shenyun S, Ding H, et al. Anti-Ro52 antibodies are associated with the prognosis of adult idiopathic inflammatory myopathy-associated interstitial lung disease. Rheumatology 2022;61:4570–8. 10.1093/rheumatology/keac090 [DOI] [PubMed] [Google Scholar]
- 13. Moghadam-Kia S, Oddis CV, Sato S, et al. Antimelanoma differentiation-associated gene 5 antibody: expanding the clinical spectrum in North American patients with dermatomyositis. J Rheumatol 2017;44:319–25. 10.3899/jrheum.160682 [DOI] [PubMed] [Google Scholar]
- 14. Koreeda Y, Higashimoto I, Yamamoto M, et al. Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl-trna synthetase autoantibodies. Intern Med 2010;49:361–9. 10.2169/internalmedicine.49.2889 [DOI] [PubMed] [Google Scholar]
- 15. Shi J, Li S, Yang H, et al. Clinical profiles and prognosis of patients with distinct Antisynthetase Autoantibodies. J Rheumatol 2017;44:1051–7. 10.3899/jrheum.161480 [DOI] [PubMed] [Google Scholar]
- 16. Sato S, Masui K, Nishina N, et al. Initial predictors of poor survival in Myositis-associated interstitial lung disease: a Multicentre cohort of 497 patients. Rheumatology 2018;57:1212–21. 10.1093/rheumatology/key060 [DOI] [PubMed] [Google Scholar]
- 17. Huang K, Aggarwal R. Antisynthetase syndrome: A distinct disease spectrum. J Scleroderma Relat Disord 2020;5:178–91. 10.1177/2397198320902667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Wu G, Gao D, et al. Factors associated with interstitial lung disease in patients with polymyositis and dermatomyositis: A systematic review and meta-analysis. PLoS ONE 2016;11:e0155381. 10.1371/journal.pone.0155381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Yang CS, Li YJ, et al. Predictive factors of rapidly progressive-interstitial lung disease in patients with clinically Amyopathic dermatomyositis. Clin Rheumatol 2016;35:113–6. 10.1007/s10067-015-3139-z [DOI] [PubMed] [Google Scholar]
- 20. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–7. 10.1056/NEJM197502202920807 [DOI] [PubMed] [Google Scholar]
- 21. Lundberg IE, Tjärnlund A, Bottai M, et al. European League against rheumatism/American college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory Myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955–64. 10.1136/annrheumdis-2017-211468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaefer-Prokop C, Prokop M, Fleischmann D, et al. High-resolution CT of diffuse interstitial lung disease: key findings in common disorders. Eur Radiol 2001;11:373–92. 10.1007/s003300000648 [DOI] [PubMed] [Google Scholar]
- 23. Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis: an international working group report. Am J Respir Crit Care Med 2016;194:265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 24. Gui X, Li W, Yu Y, et al. Prediction model for the pretreatment evaluation of mortality risk in anti-Melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with interstitial lung disease. Front Immunol 2022;13:978708. 10.3389/fimmu.2022.978708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Azen R, Traxel N. Using dominance analysis to determine Predictor importance in logistic regression. J Educat Behav Stat 2009;34:319–47. 10.3102/1076998609332754 [DOI] [Google Scholar]
- 26. Lega JC, Reynaud Q, Belot A, et al. Idiopathic inflammatory Myopathies and the lung. Eur Respir Rev 2015;24:216–38. 10.1183/16000617.00002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sontheimer RD. Would a new name hasten the acceptance of Amyopathic dermatomyositis (dermatomyositis Siné Myositis) as a distinctive subset within the idiopathic inflammatory Dermatomyopathies spectrum of clinical illness J Am Acad Dermatol 2002;46:626–36. 10.1067/mjd.2002.120621 [DOI] [PubMed] [Google Scholar]
- 28. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and Prognostic factor in anti-Melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 2010;49:1713–9. 10.1093/rheumatology/keq149 [DOI] [PubMed] [Google Scholar]
- 29. Chen Z, Cao M, Plana MN, et al. Utility of anti-Melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 2013;65:1316–24. 10.1002/acr.21985 Available: https://acrjournals.onlinelibrary.wiley.com/toc/21514658/65/8 [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi O, Akira S. Mda5/RIG-I and virus recognition. Curr Opin Immunol 2008;20:17–22. 10.1016/j.coi.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 31. Hamaguchi Y, Kuwana M, Hoshino K, et al. Clinical correlations with dermatomyositis-specific Autoantibodies in adult Japanese patients with dermatomyositis: a multicenter cross-sectional study. Arch Dermatol 2011;147:391–8. 10.1001/archdermatol.2011.52 [DOI] [PubMed] [Google Scholar]
- 32. Ikeda S, Arita M, Morita M, et al. Interstitial lung disease in clinically Amyopathic dermatomyositis with and without anti-MDA-5 antibody: to lump or split? BMC pulmonary medicine BMC Pulm Med 2015;15:159. 10.1186/s12890-015-0154-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang G, Wang Q, Wang Y, et al. Presence of anti-Mda5 antibody and its value for the clinical assessment in patients with COVID-19: A retrospective cohort study. Front Immunol 2021;12:791348. 10.3389/fimmu.2021.791348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng Y, Zhang S, Zhao Y, et al. Neutrophil extracellular traps may contribute to interstitial lung disease associated with anti-Mda5 autoantibody positive dermatomyositis. Clin Rheumatol 2018;37:107–15. 10.1007/s10067-017-3799-y [DOI] [PubMed] [Google Scholar]
- 35. Deichmann M, Benner A, Waldmann V, et al. Interleukin-6 and its Surrogate C-reactive protein are useful serum markers for monitoring Metastasized malignant Melanoma. J Exp & Clin Cancer Res 2000;19:301–7. [PubMed] [Google Scholar]
- 36. Gono T, Kawaguchi Y, Ozeki E, et al. Serum Ferritin correlates with activity of anti-Mda5 antibody-associated acute interstitial lung disease as a complication of dermatomyositis. Mod Rheumatol 2011;21:223–7. 10.1007/s10165-010-0371-x [DOI] [PubMed] [Google Scholar]
- 37. Isoda K, Takeuchi T, Kotani T, et al. Pre-treatment Ferritin level and alveolar-arterial oxygen gradient can predict mortality rate due to acute/subacute interstitial pneumonia in dermatomyositis treated by cyclosporine a/Glucocorticosteroid combination therapy: a case control study. PLoS One 2014;9:e89610. 10.1371/journal.pone.0089610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malmström V, Venalis P, Albrecht I. T cells in Myositis. Arthritis Res Ther 2012;14:230. 10.1186/ar4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang W, Ren F, Luo L, et al. The characteristics of lymphocytes in patients positive for anti-Mda5 antibodies in interstitial lung disease. Rheumatology 2020;59:3886–91. 10.1093/rheumatology/keaa266 [DOI] [PubMed] [Google Scholar]
- 40. Chen F, Wang D, Shu X, et al. Anti-Mda5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012;32:3909–15. 10.1007/s00296-011-2323-y [DOI] [PubMed] [Google Scholar]
- 41. Wang DX, Lu X, Zu N, et al. Clinical significance of peripheral blood lymphocyte Subsets in patients with polymyositis and dermatomyositis. Clin Rheumatol 2012;31:1691–7. 10.1007/s10067-012-2075-4 [DOI] [PubMed] [Google Scholar]
- 42. Marie I. Morbidity and mortality in adult polymyositis and dermatomyositis. Curr Rheumatol Rep 2012;14:275–85. 10.1007/s11926-012-0249-3 [DOI] [PubMed] [Google Scholar]
- 43. Debray M-P, Borie R, Revel M-P, et al. Interstitial lung disease in anti-synthetase syndrome: initial and follow-up CT findings. Eur J Radiol 2015;84:516–23. 10.1016/j.ejrad.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 44. Tanizawa K, Handa T, Nakashima R, et al. The Prognostic value of HRCT in Myositis-associated interstitial lung disease. Respiratory Medicine 2013;107:745–52. 10.1016/j.rmed.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 45. Zuo Y, Ye L, Liu M, et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology (Oxford) 2020;59:2829–37. 10.1093/rheumatology/keaa034 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003850supp001.pdf (528.3KB, pdf)
rmdopen-2023-003850supp002.pdf (104.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.