Abstract
The blood-brain barrier (BBB) presents a formidable challenge in delivering therapeutic agents to the central nervous system. Ultrasound-mediated BBB disruption has emerged as a promising non-invasive technique to enhance drug delivery to the brain. This manuscript reviews fundamental principles of ultrasound-based techniques and their mechanisms of action in temporarily permeabilizing the BBB. Clinical trials employing ultrasound for BBB disruption are discussed, summarizing diverse applications ranging from the treatment of neurodegenerative diseases to targeted drug delivery for brain tumors. The review also addresses safety considerations, outlining the current understanding of potential risks and mitigation strategies associated with ultrasound exposure, including real-time monitoring and assessment of treatment efficacy. Among the large number of studies, significant successes are highlighted thus providing perspective on the future direction of the field.
Keywords: Ultrasound, Blood-brain barrier, Alzheimer's, Neuro-oncology
Introduction
The blood-brain barrier (BBB) is the first line of defense against potential toxins and pathogens. This selective barrier comprises endothelial cells, pericytes, and astrocytes that create tight junctions, only allowing specific molecules to enter the brain parenchyma [1]. Notably, lipophilic and uncharged molecules less than 400 Da (Da), unbound to plasma proteins, and without membrane transporters can diffuse freely [2]. For example, O2, CO2, water, and small lipid-soluble molecules easily cross the BBB via diffusion (i.e., passive diffusion) [3]. While the BBB preserves homeostasis in the brain, it also hinders the delivery of therapeutics to the central nervous system (CNS). Specifically, certain molecules do not enter the brain parenchyma due to unfavorable physicochemical properties such as molecular size, weight, polarity, and lipophilicity [4]. Therefore, the development of next generation neurotherapeutics faces a bottleneck in their eventual translation into clinical practice. For example, despite the promising efficacy of Trastuzumab in breast metastasis preclinical models, its molecular size limits effective distribution in the brain parenchyma [5,6].
The known entry pathways across the BBB independent of diffusion include transcytosis (i.e., receptor-mediated, carrier-mediated, and adsorptive-mediated), paracellular, and vector-mediated [7]. The initial attempts to disrupt, or “open” the BBB (BBBO) included the use of intra-arterial osmotic molecules (i.e., intra-arterial mannitol), chimeric peptides, and pro-inflammatory cytokines, but these approaches lacked specificity [8]. Currently, the preferred delivery strategies for neurotherapeutics include convection-enhanced, intraventricular, intrathecal, intranasal, and intravenous administration, utilizing specific receptors (such as insulin and transferrin) [9]. While these routes are effective in delivering selective therapeutics, investigators often face a tradeoff between high specificity and the risk of adverse events related to the invasiveness of the delivery route. For example, highly selective routes such as convection-enhanced delivery involve direct injections into the brain, which are associated with a certain degree of morbidity and adverse events. The ability to non-invasively, reversibly, enhance drug delivery to the brain in a spatially localized or targeted fashion thus represents a significant enabling technique for vast classes of neuropharmacological interventions with the potential ability to improve therapeutic efficacy across the whole spectrum of neurological diseases.
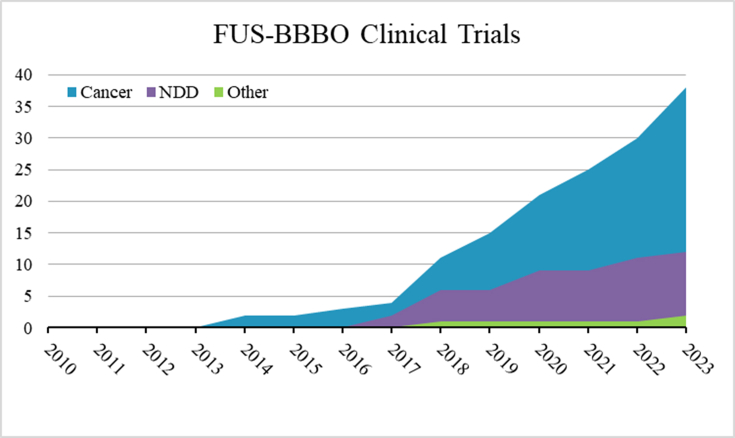
Transcranial focused ultrasound (FUS) has become an FDA-approved treatment for neurological disorders such as essential tremor and tremor-dominant Parkinson's disease and is currently being tested for epilepsy [10], neuropathic pain [11], and psychiatric disorders [12]. While the existing FUS applications involve tissue ablation, the ability for BBBO (FUS-BBBO), combined with intravascular contrast agents (e.g. conventional clinically approved microbubbles), offers an exciting opportunity to deliver neurotherapeutics into the brain. Extensive research in preclinical models has shown FUS-BBBO to be safe, reproducible, and repeatable. Given the substantial potential benefits of incisionless precision therapeutic delivery to the brain, enthusiasm around this approach has led to quick adaption for human applications and is currently being tested in an increasing number of clinical trials (Fig. 1). This review examines the technical considerations for FUS-BBBO, emphasizing the current research to deliver therapeutics optimally. In the second part of this review, we discuss the current applications of FUS-BBBO in clinical trials involving neurodegenerative disorders, tumors, and other applications.
Fig. 1.
The cumulative number of clinical trials registered on clinicaltrials.gov by year for studies related to ultrasound blood-brain barrier disruption for cancer applications (Cancer), neurodegenerative diseases (NDD), or other applications. Trials found by searching clinicaltrials.gov for the keywords “ultrasound” and “blood-brain barrier” were included and unrelated trials were removed.
Fundamentals of FUS-BBBO
Ultrasound-mediated blood-brain barrier disruption is driven by the interaction of acoustic pressure waves with intravascularly administered cavitation enhancing agents (referred to here as contrast agents). Everything from the composition and structure of the contrast agents [13] to the shape and characteristics of the acoustic pulse [14] may factor into the efficacy and safety of the administered therapy. A full description of all considerations for therapeutic ultrasound in the brain is beyond the scope of this review, but a brief overview will familiarize the reader with the major concepts necessary to discuss the current preclinical and clinical findings.
The relevant ultrasound parameters are frequency, incident energy (described as peak negative pressure), duty cycle and treatment duration [15]. Similarly, the properties of interest for the contrast agents include the shell formulation, size, dose, and half-life [16]. Careful combinations of contrast agents and FUS have allowed the noninvasive delivery of small molecules, proteins, antibodies, viral vectors, and nanoparticles across the BBB in preclinical studies [[17], [18], [19], [20]]. Through a deep understanding of these factors, the technique of BBBO can be tailored for the predictable delivery of specific therapeutics into the brain. While a vast parameter space has been covered in preclinical experiments, efforts are underway to standardize FUS-BBBO parameters in humans [15].
Microbubbles
Microbubbles are a key element in the translation of acoustic energy to mechanical effects that are required for BBBO. Originally developed as highly reflective vascular contrast agents for ultrasound imaging, microbubbles are stable in circulation for up to 10 min and are used diagnostically as a blood pool agent. Microbubbles are typically 1–3 μm in diameter and consist of a poorly water-soluble gas core stabilized by shell-forming agents at the surface. A variety of microbubble shell compositions are clinically approved, ranging from polymers to proteins to lipids [21]. Under the low acoustic energies of diagnostic imaging applications, microbubbles produce little to no bioeffects. Therapeutically, however, microbubbles can act as substantial local amplifiers of acoustic energy – increasing local cavitation at their location in the acoustic field. As a compressible entity, microbubbles expand and contract when exposed to an acoustic pressure wave, creating microstreaming fluid flow and shear stress in the immediate vicinity. When this oscillation occurs inside a blood vessel, it can temporarily and reversibly disrupt the integrity of the endothelium, allowing endogenous and exogenous molecules to pass across the vascular barrier [22,23]. Although other types of cavitation enhancing agents such as phase-change nanodroplets have also been explored for BBBO in pre-clinical studies [[24], [25], [26], [27]], only microbubbles are currently approved for use in humans.
Microbubble dose correlates directly with increasing BBBO if other variables remain constant [28]. Comparisons between microbubble sizes suggest that the total injected gas volume is more important for BBBO than microbubble size or concentration alone [29]. Practically, contrast agent dosing limitations (originally developed for contrast imaging indications) have dictated the microbubble administration, typically given as an IV infusion for consistent treatment throughout the procedure. Therapeutic applications may benefit from higher doses, and higher limits are being investigated [30]. The oscillating microbubbles produce a characteristic acoustic response in proportion to both dose and pressure (i.e., incident ultrasound energy), which can be monitored via passive cavitation detection. Cavitation monitoring feedback is used for real-time parameter regulation and dose estimation to maximize efficacy and minimize risk [[31], [32], [33], [34]].
Pressure
Peak negative pressure (PNP) describes the pressure amplitude of the negative cycle of an acoustic wave. Ultrasound frequency and PNP together are used to calculate the Mechanical Index (MI), a measure of acoustic power that is related to the likelihood of tissue effects from ultrasound alone and a safety regulated parameter for ultrasound diagnostic imaging [35]. The MI is often used to compare acoustic pressures at different frequencies. Increasing PNP increases the magnitude of the size change of the microbubbles. At low and moderate pressures, microbubbles will oscillate continually without collapsing (referred to as stable cavitation), creating fluid flow and shear stress sufficient to open the BBB. At higher pressures, the bubble can be driven to dissolution or collapse (inertial cavitation), which additionally produces shock waves and microjets [36] and is associated with a higher incidence of undesired bioeffects. For BBB disruption, PNP is the primary parameter determining efficacy and safety [37]. Microbubbles undergoing stable or inertial cavitation produce unique acoustic responses which can be detected and quantified by secondary ultrasound receivers. This passive cavitation dose (PCD) provides real-time feedback of the intensity and type of microbubble-related activity and correlates with both safety and efficacy [38,39]. Generally, stable cavitation increases with increasing pressure, until the inertial cavitation threshold is reached. Stable cavitation is sufficient to cause BBBO, while inertial cavitation is minimized to avoid tissue damage.
Clinically, pressure is a major control variable which can often be adjusted during the procedure. Due to differences in skull thickness and tissue properties for each patient, pressure is often optimized for each targeted area. For systems employing cavitation feedback, a brief pressure ramp is employed to identify the inertial cavitation threshold, allowing the subsequent treatment to proceed in the stable cavitation regime [40] regulated by device-specific automatic algorithms. As implanted devices bypass the skull and treat a fixed area, they can be pre-programmed for a set pressure based on initial device calibration [41]. Studies in implanted devices have demonstrated the FUS-BBBO threshold in humans to be around 0.8 MI [42].
Frequency
The frequency of ultrasound is an important parameter for BBB disruption as it largely determines two fundamental treatment parameters – focal spot size and penetration depth. Lower frequency waves experience less attenuation, and can thus generate higher pressures at locations deeper in tissue. Furthermore they are less aberrated by the skull morphology and thus more easily retain their ability to focus through the skull which increases the repeatability of the focal spot size, location, and magnitude. However, higher frequencies can be focused on a smaller point to improve treatment specificity. Preclinically, BBB disruption has been demonstrated with relatively high frequencies (in animal models, frequencies ≥ 1 MHz are often used, though a wider range has been investigated) as the thin skull and relatively small brain anatomy of rodents and non-human primates allow for shallow targeting with less concern of attenuation related heating or pressure loss. In contrast, human clinical systems used for BBBO typically operate in the 200–500 kHz range, with 1.5 MHz considered the upper safety limit of the ultrasound fundamental frequency to minimize the risk of heating-related tissue damage [3,43]. Most clinical studies use fixed frequencies of 0.22 MHz, 0.5 MHz, or 1.05 MHz, based on three leading commercial versions [21]. Transcranial devices use lower frequencies to deliver through the attenuating and diffracting skull, whereas an implantable system can use higher frequencies.
Pulse length
Diagnostic imaging typically uses single or several cycle ultrasound pulses, however, longer pulses consisting of tens to thousands of cycles can be employed for therapeutic applications. The pulse length is the length of the ultrasound transmission, typically given either in cycles or in seconds. As ultrasound pressure waves are directional and impart some of their momentum to objects in the medium they travel through, each cycle has the potential to push tissue and microbubbles in the direction of the wave, a property described as acoustic radiation force (ARF). Along with pressure, increasing pulse length can increase ARF, which can be leveraged to image the acoustic tissue stiffness [44] or to push bubbles into the vessel wall to improve ligand binding in ultrasound molecular imaging [45]. Similarly, long pulses driving bubbles into the vascular endothelium can increase both the magnitude and duration of BBB disruption in mice [46], as well as the likelihood of tissue damage [47]. The duty cycle is the ratio of the ultrasound “on time” to “off time”, usually given in percent. Pulse lengths and duty cycle should be adjusted to ensure microbubbles are perfusing the treated area between pulses. The time it takes to perfuse the vessels fully may be very different between healthy and tumor vasculature [48], though this can be assessed using acoustic monitoring. In clinical studies, an approximate 1% duty cycle is used for BBBO, but this parameter can vary according to the fundamental frequency and the sonication duration [49].
Preclinical mechanistic and safety evaluations
Experiments in the preclinical space help identify the biomechanical and biochemical changes that can accompany BBBO. Initially, safety studies focused on minimizing red blood cell extravasation and neuronal apoptosis [50,51], where the relationships of pressure, duty cycle, and microbubble dose as they contribute to efficacy and safety were established. Following the identification of safe limits, mechanistic studies sought to identify precisely how BBBO occurs. While the mechanisms are still being explored, increases in both paracellular and transcellular transport have been observed [22]. When microbubbles are stimulated with ultrasound while in direct contact with endothelial cells, they can create a calcium wave, causing cells to temporarily contract, which can open tight junctions [52], and histological analysis has revealed increased tight junction protein expression following BBBO, suggesting tight junction disruption.
Due to the heterogeneity of vasculature in the brain, it has been determined that different brain regions may require different ultrasound parameters for BBBO. For example, studies in macaques have shown that the same parameters sufficient for BBBO in grey matter structures did not cause permeability changes in white matter [53]. In humans, different cavitation dose prescriptions are being used for treating specific areas such as the hippocampus (higher dose) or the thalamus, parietal, or frontal lobes (lower dose) [54].
Investigations into the biochemical changes accompanying FUS-BBBO revealed an increase in a range of inflammatory cytokines, effectively creating a biochemical state of sterile inflammation without edema that resolves over time [51,55]. Contributing to this is the extravasation of serum albumin into the parenchyma, which initiates glial clearance mechanisms [55]. This mechanism could explain the observed cognitive improvement in some PD subjects [56] and amyloid beta (Aβ) clearance in Alzheimer's patients [57] who received BBBO without any therapeutic agents. However, as discussed later, the possibility of ultrasound neuromodulation cannot be ruled out.
Neuromodulation refers to the direct stimulation of nerves and neuronal tissue and has been evaluated with electrical, magnetic or ultrasonic stimuli (i.e., deep brain stimulation) [58,59]. Ultrasound neuromodulation is a rapidly growing field that utilizes acoustic pressure waves to act on cell membranes and mechanosensitive ion channels directly to induce neuronal activity [60], eliciting both excitatory and inhibitory responses [61]. Ultrasound neuromodulation can be achieved without microbubbles and has been clinically evaluated in patients with a variety of conditions, such as epilepsy, chronic pain, and Alzheimer's disease [61]. As microbubbles can enhance local ultrasound effects, it is hypothesized that neuromodulation could accompany FUS-BBBO, with preclinical investigations suggesting that it may occur at certain pressures [62]. Ultrasound neuromodulation has utility both as a mechanism to assess brain functionality and to affect durable neurocognitive changes [63], and may have additional utility in new applications in conjunction with FUS-BBBO.
Hardware and devices
One of the main challenges of using ultrasound for blood-brain barrier disruption is the precise delivery of ultrasound energy through the skull. Delivery of ultrasound through the bones of the skull is complicated by substantial attenuation and distortion, resulting in low pressures reaching brain tissue and phase aberration, which can shift the target region [12]. With the aforementioned critical parameters in mind, different hardware and device designs have been developed to meet various clinical needs. A comprehensive discussion of clinical systems has recently been published [15]. An overview of technical approaches and available systems are provided herein.
The most straightforward approach is to bypass the skull. Implantable ultrasonic devices (such as the Carthera SonoCloud) are installed through a cranial window and affixed rigidly to the skull, allowing the repeated delivery of ultrasound to the same location over time. With a fixed focus and position, implantable devices do not require concomitant image guidance for treatment. As implantable devices do not require transcranial ultrasound transmission, they can utilize higher frequencies (∼1 MHz), which provides a smaller focal region for precision treatment. While no specific indication or designation has yet been determined, a utility of implantable systems is ease of repeated pairing with an infusion of drugs and microbubbles in an outpatient setting, which is amenable to current chemotherapy workflows.
The second category of BBB hardware represents transcranial systems. These systems can utilize a singular transducer or an array of transducers around the skull, which can be focused on the targeted treatment area. Acoustic coupling to the patient is achieved with a water jacket or acoustic standoff, and no longer requires the head to be shaved prior to treatment [57]. Transcranial systems utilize lower frequency ultrasound (∼200–500 kHz) to overcome the transmission challenges presented by the skull. Transcranial systems typically employ image guidance via MRI or CT to select the appropriate treatment area. The NeuroAccess platform being developed by Cordance Medical and the NaviFUS system (NaviFUS Inc.) use a pre-acquired image and neuronavigational positioning system, which allows accurate treatment without the need for real-time imaging. Alternatively, the Insightec Exablate Neuro is a hemispheric array which is integrated with the MRI, allowing for additional feedback and safety monitoring, including gadolinium enhancement post-BBBO.
The FDA Breakthrough Devices program was established in 2018 to speed up development and premarket approval for certain designated systems with high impact potential. As of the time of this article, the SonoCloud-9 system has received Breakthrough Device designation for the treatment of GBM, and the transcranial systems Exablate Neuro and NeuroAccess have received the same designation for both BBB disruption for the treatment of brain tumors and liquid biopsy.
Building on preclinical work, the FUS-BBBO is being clinically tested in several neurological disorders. The following review summarizes the clinical trial results in patients with Alzheimer's Disease (AD), brain tumors, Parkinson's disease, and others.
Clinical Evaluations in Alzheimer's Disease
Alzheimer's disease is the most common neurodegenerative disorder [64], characterized by the deposition of amyloid-β plaque and hyperphosphorylated tau. Several immunotherapy medications have been tested recently and shown to reduce plaque burden and modestly slow cognitive decline [65,66]. While these immunotherapy treatments await implementation in clinical practice, current research aims to efficiently deliver these medications across the BBB to reduce the treatment duration and potentially improve spatial specificity (Table 1). It is possible that BBB dysfunction may itself be associated with the pathogenesis of Alzheimer's disease [67], which may warrant additional caution for interventions for AD which involve BBB compromise.
Table 1.
BBB disruption in patients with Alzheimer's Disease.
| Author Name | Trial Dates | Target | Ultrasound Equipment | Volume of Tissue for BBB Opening | Safety Results | Other Pertinent Findings |
|---|---|---|---|---|---|---|
| Mehta et al. [69] | October 2018 and May 2019 | The hippocampus was the targeted region, with three target locations for each subject within two weeks. | MRI-guided, low-intensity 220 kHz FUS transducer | The volume for each target location was 5 × 5 × 7 mm. | No adverse effects were observed, and BBB closing occurred within 24 h. | Enhancement was observed in the downstream veins from the target location. |
| Mehta et al. [54] | November 2019 and July 2022 | The hippocampal, parietal, and frontal regions of the brain. There were 77 target sites in total among the participants. | MRI-guided, low-intensity 220 kHz FUS transducer | Treatment volume varied between the different brain locations. | BBB closure occurred within 24 and only minor adverse effects were detected, with no severe hemorrhages. | There were alterations to the downstream veins from the treatment sites. |
| Lipsman et al. [40] | Between March and September 2023 | Right frontal lobe | MRgFUS with a 220 kHz FUS transducer | Target locations were about 9 mm × 9 mm, in a 3 × 3 grid | No significant adverse effects or cognitive decline within three months post-treatment | There was no change in amyloid-beta volume in the patients post-treatment. |
| Rezai et al. [68] | Dates not provided | The hippocampus and entorhinal cortex. BBB opened between 14 and 71% of the hippocampal volume. | MRI-guided, low-intensity 220 kHz FUS transducer | The total volume opened per patient ranged from 318 mm3 to 873 mm3. | No adverse effects, hemorrhaging, or cognitive decline were observed. BBB was effectively opened and safely closed within 24 h of the intervention. | There was no area outside of the targeted region that was exposed to BBB opening. |
| Rezai et al. (2024) [30] | Dates not provided | Nondominant frontal lobe, parietal, temporal lobes and hippocampus in different patients. Six treatments over 26 weeks. | MRI-guided, low-intensity 220 kHz FUS transducer | Opening of 10 cm3 in the first patient, 20 cm3 in the second and 40 cm3 in the third. | No adverse events, BBB opened and restored within 24–48 h. | Treated with aducanumab, average of 32% amyloid beta reduction in treated regions after 26 weeks |
In patients with AD, temporary and repeated opening of the BBB with FUS is safe. A phase I clinical trial conducted at the University of Toronto included five patients with early Alzheimer's Disease. Using the Insightec's 220 kHz system, the BBB was opened repeatedly and restored within 24 h in all patients with no significant adverse effects or cognitive decline in three months post FUS-BBBO [40]. Aβ levels were tracked before and after, but no significant changes were observed. In a phase II clinical trial conducted between October 2018 and May 2019 at West Virginia University and Cornell University, three female subjects with early AD received FUS BBB opening in the unilateral hippocampus with target volumes of 5 × 5 × 7 mm repeated every two weeks for three consecutive cycles [68]. Following the opening, the BBB integrity was determined by serial contrast-enhanced MRI. Results indicated no adverse effects, and all three patients had BBB closure in the hippocampus within 24 h [69]. Encouraged by the observed safety, the treatment volumes were expanded to bilateral hemispheres and larger volumes, and 10 subjects (55–76 years old) with mild AD underwent three FUS-BBBO treatments, two weeks apart, at West Virginia University and Cornell University. This clinical trial reported the effects of BBB opening at multiple locations in the parietal and frontal regions of the brain, as well as the hippocampus, with spatially distinct target locations [57]. FUS-BBBO treatment was accurate and effective (BBBO observed in all 30 treatments) and restoration occurred within 24 h from the FUS treatment at each timepoint. Only minor adverse effects were detected, with no hemorrhages visible on MRI. Contrast enhancement along the cerebral veins was also reported, which was thought to be related to contrast clearance via the brain's glymphatic system, as had been reported previously [70]. There was no cognitive or clinical decline in any patients who received the FUS treatment, and PET quantification of Aβ after the three treatments showed a reduction in standardized uptake value ratio (SUVR, a measure of Aβ) of 5% (±4%) in the targeted region compared to levels prior to the first treatment. The results demonstrate that FUS-BBBO was safe and reproducible in both cortical and subcortical regions, with larger (up to 30 cm3) treatment volumes, in AD patients.
Another phase II clinical trial from Yonsei University replicated similar safety results in 6 AD subjects with a target volume between 318 mm3 and 873 mm3 in the hippocampus centered in locations with high amyloid plaque burden. The researchers reported BBBO in an average of 95% of the target volume after FUS treatment and without any area outside the target being exposed to BBB disruption [71]. Currently, investigators are focusing on combining FUS-BBBO with anti-amyloid immunotherapy. Results from a limited 3 patient trial pairing FUS-BBBO with aducanumab infusions were recently reported [30]. In a dose escalation study, patients received 6 treatments (two at each dosage level of 1 mg/kg, 3 mg/kg and 6 mg/kg) of aducanumab combined with FUS-BBBO, with barrier openings as large as 40 cm3 being investigated. Amyloid beta in the brain was quantified periodically via 18F-florbetaben PET imaging for each patient, in addition to longitudinal neurologic, cognitive and behavioral assessments. Substantial reductions in Aβ were observed in the FUS-treated region compared to homologous regions at the 26-week timepoint, with treated regions seeing an average 32% reduction in SUVR from the original assessment while untreated regions stayed largely the same. This small proof-of-concept trial was not powered to detect clinical cognitive changes. No serious neurological adverse events were observed during the 6-month treatment period.
Clinical Evaluations in Brain Tumors
While FUS was initially tested for ablation in brain tumors, the more recent work has focused on FUS-BBBO to deliver agents of interest (Table 2). The first reported clinical results of FUS-BBBO employed an implantable device to bypass the skull. The prospective, open label, single-center, single-arm phase 1/2a dose escalation study conducted at the Assistance Publique – Hôpitaux de Paris (AP-HP) University Hospital La Pitié-Salpêtrière evaluated FUS-BBBO using an implantable 1.05 MHz transducer through a pressure range of 0.5 MPa (which corresponded to the FUS-BBBO threshold in rodent studies) up to 1.1 MPa for the delivery of carboplatin to recurrent glioblastoma [42]. The 10 mm diameter transducer was implanted within the skull bone over the tumor area as visualized on MRI, either during debulking surgery or as a dedicated procedure under local anesthesia. Monthly treatments were administered with a microbubble bolus at a total sonication treatment time of 150 s, followed by carboplatin infusion no later than 60 min after FUS-BBBO. BBBO was determined by MRI immediately following FUS treatment. No BBBO was observed via gadolinium extravasation in patients exposed to 0.5 and 0.65 MPa US (3 and 6 sonications respectively), but 8 of 11 sonications at 0.8 MPa resulted in BBBO, with increasing quality of disruption occurring at 0.95 and 1.1 MPa (producing BBBO in 6 of 7 sonications and 14 of 14 sonications respectively). Notably, monthly treatments were tolerated in all patients, and patients maintained speech and movement capacity during and after the procedure when treating language or motor areas. This investigation demonstrated higher acoustic pressures were necessary to achieve FUS-BBBO in humans than in rodent models. While not powered for efficacy, 9 patients who had confirmed BBBO of grade 2 or higher showed no detectable tumor progression on MRI evaluation for the duration of the study.
Table 2.
BBB disruption in patients with brain tumors.
| Author Name | Trial Dates | Target | Ultrasound Equipment | Volume of Tissue for BBB Opening | Safety Results | Other Pertinent Findings |
|---|---|---|---|---|---|---|
| Mainprize et al. [74] | 2015 to 2017 | High-grade gliomas in the right frontal, right temporal, and right parieto-occipital | Low-intensity, transcranial MRgFUS BBB | Each patient received between 2 and 5 targets, each with a volume of about 486 mm3 | No adverse events as a result of the FUS treatment. One patient developed from surgical resection. BBB opening was successfully opened and closed. | Chemotherapy delivery was higher in areas treated with FUS-BBBO, compared to the non-sonicated regions. |
| Anastasiadis et al. [72] | Not provided in the paper | Frontal and temporal lobe | Transcranial MRI-guided micro bubble FUS | Tumors had a mean volume of 18.2 cm3, while the average treatment volume for the first three patients was 0.57 cm3, and for the fourth patient was 10.08 cm3 | BBB was opened safely in the target location without opening other regions of the BBB. There were no hemorrhages or tissue damage. No gliosis was observed fifteen days post-treatment. | FUS-BBBO can be used to improve the delivery of drugs into gliomas. |
| Chen et al. [73] | Not provided in the paper | Four patients had frontal lobe tumors, one had a temporo-insular tumor, and the sixth had an occipital lobe tumor. | NaviFUS with 500 kHz | 3 × 3 mm targets, 5 mm part with a SonoVue dosage of 4.8 mL. | 36 adverse events occurred within one-month post-treatment. There were no deaths, swelling, hemorrhaging, or neurological changes in any of the patients. | Deviation from the target location was less than 3 mm for each patient, and live MRI guidance was not used during this procedure. Higher cavitation dosage resulted in larger BBB opening at the target location. |
| Meng et al. [75] | Not provided in the paper | 4 patients with Her2+ breast cancer, 1–3 infratentorial or supratentorial lesions each | Insightec ExAblate neuro 220 kHz | Mean sonication volume was 27 cm3 | Well tolerated with no serious adverse events. Three grade 1 adverse events were attributable to frame placement and positioning. | Trastuzumab delivery with longitudinal monitoring |
| Carpentier et al. [42] | July 2014 to January 2016 | Various, infiltrated brain and intratumoral | CarThera SonoCloud implantable 1.05 MHz | ∼5 cm3 region in the beam path of the device | No serious adverse events reported. | Threshold pressure for BBBO identified in humans |
A phase I clinical trial at the University of Maryland evaluated the safety and feasibility of targeted FUS BBB opening in patients with low-grade gliomas confirmed by the uptake of fluorescein, which was visualized by direct observation during surgery and histology of the resected specimen [72]. The trial included four subjects with a mean age of 32 years, good Karnofsky performance scores, and an intact neurological status. In addition, all the tumors were in the frontal or temporal lobe. Following the FUS BBB opening, all the patients underwent surgical resection, and the surgical specimens were analyzed. The analyses showed a 2.2-fold increase of fluorescein within the FUS-treated areas compared to the untreated non-enhancing tumors (P < 0.01). No serious side effects were observed postoperatively, and the median follow-up of the patients was 15.7 months with no tumor recurrence [72]. A second study conducted a prospective open-label, single-center, phase 1 pilot study assessing the safety of targeted BBB opening in patients with recurrent glioblastoma while using navigation-guided FUS. Seven days after the FUS treatment, the patients underwent surgical resection of their tumors. This study included six patients with a mean age of 49.5 years. Unlike the previous study, this trial observed thirty-six adverse events, including two serious adverse events (hyponatremia and hypernatremia). However, the adverse events were not related to FUS-BBBO. In all patients, BBB closure occurred safely and within 24 h of the initial opening, with no immunological response noted [73].
At the University of Toronto, a phase I, single-arm, open-label design clinical trial involving five patients with malignant high-grade glioma tested the safety and ability to deliver chemotherapy after FUS-BBBO [74]. One patient received intravenous doxorubicin, while the remaining four patients received oral temozolomide one day prior to their surgical resection. Following the procedure, there were no new clinical or radiological adverse effects, such as hemorrhage or edema, indicating the procedure to be safe. In addition, the BBB was opened in all five patients and closed within 24 h. Compared to non-sonicated areas, there was a 15–50% increase in contrast enhancement in locations subjected to FUS-BBBO. Another clinical trial, with four patients, studied the safety of delivering trastuzumab in conjunction with FUS-BBBO in four patients with Her2-positive breast cancer brain metastases. The results from the trial indicated no adverse effects, including no changes in their cognitive state as well as no hemorrhaging or edema. In addition, there was an increase in drug delivery to the metastases by about 87% as measured by SPECT imaging [75]. These observations indicate that FUS could potentially be used in conjunction with chemotherapy to increase targeted delivery to the tumors. However, further studies must be done to study the long-term clinical effects of the FUS treatment [74]. Given this initial success of FUS-BBBO in brain tumor patients, the upcoming trials are expected to define pathways for future clinical translation.
In addition to using FUS-BBBO for enhanced therapeutic delivery, another possibility gaining interest in the neuro-oncology space is the ability to collect cell-free circulating DNA for ongoing tumor surveillance. Proof-of-concept studies using FUS-BBBO to release DNA from tumors demonstrated improved cancer detection in liquid biopsy in preclinical models [76]. This possibility was tested in nine patients with glioblastoma who underwent FUS-BBBO resulting in 38 procedures at an average total target volume of 7.8 cm3. There were no adverse events recorded, and there was a significant increase in the quantity of circulating cell free DNA after FUS-BBBO compared to without [77].
Additional Applications for FUS Opening of BBB
The utility of FUS-BBBO as a non-invasive therapeutic delivery strategy have led to interest in treating a wide range of neurological disorders. While Alzheimer's disease and cancer therapy are to date the most clinically investigated applications, FUS-BBBO trials for other indications are increasing (Table 3).
Table 3.
Additional clinical investigations into BBB Opening.
| Author Name | Trial Dates | Target | Ultrasound Equipment | Volume of Tissue for BBB Opening | Safety Results | Other Pertinent Findings |
|---|---|---|---|---|---|---|
| Gasca-salas et al. [56] | October 2018 to May 2019 | Parieto-occipito-temporal junction | Magnetic resonance guided FUS and intravenous microbubble administration | Target volumes were about 6 × 6 mm, and 3 mm apart in a 2 × 2 grid. | No serious side effects during the procedures. Post-procedure SWAN images showed hypodensities which persisted for two months in the patient who received the highest ultrasound dose. | The patients showed improvement in several cognitive tests. |
| Zhang et al. [81] | Not applicable | Hippocampus | Precise intracerebral non-invasive guided surgery (PING) MRI-guided FUS |
Full bilateral hippocampus in Sprague Dawley rats | There was edema in three of the rats, but no bleeding. Some rats had severe atrophy in the hipoccampus | Significant decrease in seizure activity was recorded. However, when treatment area increased, status epilepticus developed post-operatively. |
| Meng et al. [78] | Not provided in the paper | Putaminal region of the brain | ExAblate neuro MR guided FUS, 220 kHz | Average volume of 3.4 cm3 covered in two treatments for each patient. This makes up about 66% of the putaminal volume. | No serious adverse effects, including no edema. Few mild to moderate adverse events, including two patients who developed short-lasting dyskinesia. | Temporary BBBO achieved, with closure shortly after. |
| Abrahao et al. [79] | Not provided in the paper | Primary motor cortex | ExAblate neuro MR guided FUS, 220 kHz | Total target volume of 350 mmˆ3 with 2–4 round of FUS-BBBO | Gadolinium leakage at the target occurred in all of the patients almost immediately after FUS treatment. There were no serious adverse effects recorded. | BBBO was safely achieved, as well as closure indicating reversibility of BBBO. |
In addition to AD, Gasca-Salas et al. conducted a prospective, single-arm, non-randomized proof of concept phase 1 clinical trial, assessing the safety and feasibility of FUS-BBBO in patients with Parkinson's disease dementia [56]. Five patients were recruited from October 2018 to May 2019. The patients received two treatment sessions at 2–3 weeks intervals. The BBB opening was targeted at the parietal-occipital-temporal junction and determined to be successful in 8 out of 10 treatments. There were no serious side effects during the procedures. Post-procedure susceptibility-weighted angiography (SWAN) images showed round SWAN hypodensities in three patients, typically interpreted to represent petechial hemorrhage. These hypodensities persisted for two months with an initial indication of long-lasting hypodensities in subjects receiving higher cavitation doses. Of note, the patients showed improvement in several cognitive tests, but these results should be regarded with caution since no statistical testing of the cognitive scores were reported, the number of patients was small, the changes were not uniform, and the study did not have a control group [56]. An open label phase I clinical trial delivered glucocerebrosidase intravenously and in escalating doses, along with FUS-BBBO in four patients with Parkinson's Disease associated with mutations of GB-1 gene. The procedure occurred every two weeks, for two consecutive cycles. In all the patients, BBB was successfully opened to deliver the deficient enzyme with no serious adverse effects. Mild to moderate adverse events were reported, including two patients who did develop short-lasting dyskinesia after FUS-BBBO [78]. The safety of FUS-BBBO was recently evaluated in patients with amyotrophic lateral sclerosis (ALS). Abrahao et. determined the accuracy and targeted drug delivery to the primary motor cortex. BBBO was successful in each of the four patients, as evidenced by gadolinium leakage at the target site immediately after FUS treatment. The leakage normalized within 24 h for all the patients, and there were no serious adverse effects. The integrity of the BBB restored successfully in all of the patients, which indicated that BBB opening was safe and reversible in patients with ALS [79].
Focused ultrasound ablation been assessed for safety and efficacy in epilepsy therapy [10], and although clinical studies specifically using FUS-BBBO for epilepsy have yet to be conducted, it is an area of developing interest [80]. Zhang et al. used Precise Intracerebral Non-invasive Guided Surgery (PING) to treat spontaneous recurrent seizures induced by pilocarpine in rats. This technique used MRI-guided FUS and intravenous microbubbles for targeted and reversible BBBO, to deliver a systemically administered neurotoxin (Quinolinic Acid) to select brain regions. By crossing BBB, the quinolinic acid focally destroyed the neurons involved in epilepsy in the targeted area. The animals treated with PING in the intermediate aspect of the hippocampus had a significant decrease in seizure activity. In contrast, when the area of treatment was extended to include the septal hippocampus, the animals developed status epilepticus post procedure [81].
FUS-BBBO presents an exciting opportunity to enhance access of neurotherapeutics into the brain parenchyma. Current efforts are focused on optimizing its technique while simultaneously testing the potential paths for clinical translation. Given the promising initial results, we expect FUS to be a major enabling technology in solving long-standing limitations to the safe and efficient delivery of neurotherapeutics to the brain.
Author Contributions
Phillip G. Durham: Conceptualization, Writing – Original Draft, Writing – Review & Editing, Visualization. Alexandra Butnariu: Writing – original draft. Rizk Alghorazi: Writing – original draft. Gianmarco Pinton: Writing – Review & Editing. Vibhor Krishna: Conceptualization, Writing – Original Draft, Writing – Review & Editing. Paul A. Dayton: Writing – Review & Editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Paul A. Dayton reports a relationship with Triangle Biotechnology that includes: equity or stocks. Paul A. Dayton has patent licensed to Revvity. Paul A. Dayton has patent pending to Triangle Biotechnology. Paul A. Dayton has patent licensed to SonoVascular. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This article is a review and does not describe new original research from the authors. However, the authors do receive funding from the National Institutes of Health related to BBBO and FUS in the brain, including R01MH132022 and R21CA286897 which support Dr. Dayton, R01NS125386 to Dr. Krishna, and R01NS113285 to Dr. Pinton.
Contributor Information
Vibhor Krishna, Email: vibhor_krishna@med.unc.edu.
Paul A. Dayton, Email: padayton@email.unc.edu.
References
- 1.Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B.V. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5) doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1) doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasielewska J.M., White A.R. Focused ultrasound-mediated drug delivery in humans – a path towards translation in neurodegenerative diseases. Pharmaceut Res. 2022;39(3) doi: 10.1007/s11095-022-03185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren K.E. Beyond the blood: brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;8(JUL) doi: 10.3389/fonc.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardridge W.M. Drug transport across the blood-brain barrier. J Cerebr Blood Flow Metabol. 2012;32(11) doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrell-Hall T.B., Nounou M.I., El-Amrawy F., Griffith J.I.G., Lockman P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget. 2017;8(48) doi: 10.18632/oncotarget.19634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong C., Chang W.S. Preclinical research on focused ultrasound-mediated blood–brain barrier opening for neurological disorders: a review. Neurol Int. 2023;15(1) doi: 10.3390/neurolint15010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hersh D.S., Kim A.J., Winkles J.A., Eisenberg H.M., Woodworth G.F., Frenkel V. Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery. 2016;79(5) doi: 10.1227/NEU.0000000000001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J.B., di Ianni T., Vyas D.B., Huang Z., Park S., Hosseini-Nassab N., et al. Focused ultrasound for noninvasive, focal pharmacologic neurointervention. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishna V., Mindel J., Sammartino F., Block C., Dwivedi A.K., Van Gompel J.J., et al. A phase 1 open-label trial evaluating focused ultrasound unilateral anterior thalamotomy for focal onset epilepsy. Epilepsia. 2023;64(4) doi: 10.1111/epi.17535. [DOI] [PubMed] [Google Scholar]
- 11.Jeanmonod D., Werner B., Morel A., Michels L., Zadicario E., Schiff G., et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1) doi: 10.3171/2011.10.FOCUS11248. [DOI] [PubMed] [Google Scholar]
- 12.Krishna V., Sammartino F., Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology advances in diagnosis and treatment. JAMA Neurol. 2018;75(2) doi: 10.1001/jamaneurol.2017.3129. [DOI] [PubMed] [Google Scholar]
- 13.Song K.-H., Harvey B.K., Borden M.A. State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics. 2018;8(16):4393–4408. doi: 10.7150/thno.26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDannold N., Vykhodtseva N., Hynynen K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood-brain barrier disruption. Ultrasound Med Biol. Jun. 2008;34(6):930–937. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhi K., Barzegar-Fallah A., Banstola A., Rizwan S.B., Reynolds J.N.J. Ultrasound-mediated blood–brain barrier disruption for drug delivery: a systematic review of protocols, efficacy, and safety outcomes from preclinical and clinical studies. Pharmaceutics. 2022;14(4) doi: 10.3390/pharmaceutics14040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersh A.M., Bhimreddy M., Weber-Levine C., Jiang K., Alomari S., Theodore N., et al. Applications of focused ultrasound for the treatment of glioblastoma: a new frontier. Cancers. Oct. 2022;14(19):4920. doi: 10.3390/cancers14194920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thombre R., Mess G., Kempski Leadingham K.M., Kapoor S., Hersh A., Acord M., et al. Towards standardization of the parameters for opening the blood–brain barrier with focused ultrasound to treat glioblastoma multiforme: a systematic review of the devices, animal models, and therapeutic compounds used in rodent tumor models. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.1072780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dauba A., Delalande A., Kamimura H.A.S., Conti A., Larrat B., Tsapis N., et al. Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics. Nov. 2020;12(11):1125. doi: 10.3390/pharmaceutics12111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janowicz P.W., Leinenga G., Götz J., Nisbet R.M. Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody. Sci Rep. Jun. 2019;9(1):1–9. doi: 10.1038/s41598-019-45577-2. 2019 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Floc’h J., Lu H.D., Lim T.L., Démoré C., Prud’homme R.K., Hynynen K., et al. Transcranial photoacoustic detection of blood-brain barrier disruption following focused ultrasound-mediated nanoparticle delivery. Mol Imag Biol. Apr. 2020;22(2):324. doi: 10.1007/S11307-019-01397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes A., Khan D.S., Alkins R. Current and emerging systems for focused ultrasound-mediated blood–brain barrier opening. Ultrasound Med Biol. 2023;49(7) doi: 10.1016/j.ultrasmedbio.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Burgess A., Shah K., Hough O., Hynynen K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert Rev Neurother. 2015;15(5) doi: 10.1586/14737175.2015.1028369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheikov N., McDannold N., Vykhodtseva N., Jolesz F., Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30(7) doi: 10.1016/j.ultrasmedbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Wu S.-Y., Fix S.M., Arena C.B., Chen C.C., Zheng W., Olumolade O.O., et al. Focused ultrasound-facilitated brain drug delivery using optimized nanodroplets: vaporization efficiency dictates large molecular delivery. Phys Med Biol. Jan. 2018;63(3) doi: 10.1088/1361-6560/aaa30d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lea-Banks H., Hynynen K. Sub-millimetre precision of drug delivery in the brain from ultrasound-triggered nanodroplets. J Contr Release. Oct. 2021;338:731–741. doi: 10.1016/J.JCONREL.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Bérard C., Desgranges S., Dumas N., Novell A., Larrat B., Hamimed M., et al. Perfluorocarbon nanodroplets as potential nanocarriers for brain delivery assisted by focused ultrasound-mediated blood–brain barrier disruption. Pharmaceutics. Jul. 2022;14(7):1498. doi: 10.3390/pharmaceutics14071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C.C., Sheeran P.S., Wu S.Y., Olumolade O.O., Dayton P.A., Konofagou E.E. Targeted drug delivery with focused ultrasound-induced blood-brain barrier opening using acoustically-activated nanodroplets. J Contr Release. 2013;172(3):795–804. doi: 10.1016/j.jconrel.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.N. A. Lapin, K. Gill, B. R. Shah, and R. Chopra, “Consistent opening of the blood brain barrier using focused ultrasound with constant intravenous infusion of microbubble agent,” Sci Rep|, vol. 10, p. 16546, 123AD, doi: 10.1038/s41598-020-73312-9. [DOI] [PMC free article] [PubMed]
- 29.Song K.-H., Fan A.C., Hinkle J.J., Newman J., Borden M.A., Harvey B.K. Microbubble gas volume: a unifying dose parameter in blood-brain barrier opening by focused ultrasound. Theranostics. 2017;7(1):144–152. doi: 10.7150/thno.15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezai A.R., D’Haese P.-F., Finomore V., Carpenter J., Ranjan M., Wilhelmsen K., et al. Ultrasound blood–brain barrier opening and aducanumab in Alzheimer’s disease. N Engl J Med. Jan. 2024;390(1):55–62. doi: 10.1056/NEJMoa2308719. [DOI] [PubMed] [Google Scholar]
- 31.Marquet F., Teichert T., Wu S.Y., Tung Y.S., Downs M., Wang S., et al. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0084310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S.Y., Sanchez C.S., Samiotaki G., Buch A., Ferrera V.P., Konofagou E.E. Characterizing focused-ultrasound mediated drug delivery to the heterogeneous primate brain in vivo with acoustic monitoring. Sci Rep. 2016;6 doi: 10.1038/srep37094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess M.T., Apostolakis I., Konofagou E.E. Power cavitation-guided blood-brain barrier opening with focused ultrasound and microbubbles. Phys Med Biol. 2018;63(6) doi: 10.1088/1361-6560/aab05c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novell A., Kamimura H.A.S., Cafarelli A., Gerstenmayer M., Flament J., Valette J., et al. A new safety index based on intrapulse monitoring of ultra-harmonic cavitation during ultrasound-induced blood-brain barrier opening procedures. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-66994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsberg F., Shi W.T., Merritt C.R.B., Dai Q., Solcova M., Goldberg B.B. 2005. On the usefulness of the mechanical Index displayed on clinical ultrasound scanners for predicting contrast microbubble destruction.https://onlinelibrary.wiley.com/doi/pdf/10.7863/jum.2005.24.4.443 [Online]. Available: [DOI] [PubMed] [Google Scholar]
- 36.Lentacker I., De Smedt S.C., Sanders N.N. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. May 2009;5(11):2161–2170. doi: 10.1039/b823051j. [DOI] [Google Scholar]
- 37.Chen H., Konofagou E.E. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J Cerebr Blood Flow Metabol. Jul. 2014;34(7):1197–1204. doi: 10.1038/jcbfm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tung Y.S., Vlachos F., Choi J.J., Deffieux T., Selert K., Konofagou E.E. In vivo transcranial cavitation threshold detection during ultrasound-induced blood-brain barrier opening in mice. Phys Med Biol. 2010;55(20) doi: 10.1088/0031-9155/55/20/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pouliopoulos A.N., Kwon N., Jensen G., Meaney A., Niimi Y., Burgess M.T., et al. Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood-brain barrier opening in non-human primates. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-94188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipsman N., Meng Y., Bethune A.J., Huang Y., Lam B., Masellis M., et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Idbaih A., Canney M., Belin L., Desseaux C., Vignot A., Bouchoux G., et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. Jul. 2019;25(13):3793–3801. doi: 10.1158/1078-0432.CCR-18-3643/74166/AM/SAFETY-AND-FEASIBILITY-OF-REPEATED-AND-TRANSIENT. [DOI] [PubMed] [Google Scholar]
- 42.Carpentier A., Canney M., Vignot A., Reina V., Beccaria K., Horodyckid C., et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. Jun. 2016;8(343):343re2. doi: 10.1126/scitranslmed.aaf6086. 343re2. [DOI] [PubMed] [Google Scholar]
- 43.Pajek D., Hynynen K. The application of sparse arrays in high frequency transcranial focused ultrasound therapy: a simulation study. Med Phys. 2013;40(12) doi: 10.1118/1.4829510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taljanovic M.S., Gimber L.H., Becker G.W., Latt L.D., Klauser A.S., Melville D.M., et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. May 2017;37(3):855–870. doi: 10.1148/rg.2017160116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borden M.A., Sarantos M.R., Stieger S.M., Simon S.I., Ferrara K.W., Dayton P.A. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol Imag : official journal of the Society for Molecular Imaging. 2006;5(3) doi: 10.2310/7290.2006.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batts A., Ji R., Noel R., Schoder A.K., Konofagou E. IEEE international ultrasonics symposium, IUS. 2021. The effect of pulse length on theranostic ultrasound-mediated blood-brain barrier opening volume, closing timeline, and cavitation mapping in vivo. [DOI] [Google Scholar]
- 47.Shin J., Kong C., Cho J.S., Lee J., Koh C.S., Yoon M.S., et al. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters. Neurosurg Focus. 2018;44(2) doi: 10.3171/2017.11.FOCUS17627. [DOI] [PubMed] [Google Scholar]
- 48.Gillies R.J., Schornack P.A., Secomb T.W., Raghunand N. Causes and effects of heterogeneous perfusion in tumors. Neoplasia. 1999;1(3) doi: 10.1038/sj.neo.7900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorick C.M., Breza V.R., Nowak K.M., Cheng V.W.T., Fisher D.G., Debski A.C., et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv Drug Deliv Rev. 2022:191. doi: 10.1016/j.addr.2022.114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baseri B., Choi J.J., Tung Y.S., Konofagou E.E. Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol. 2010;36(9) doi: 10.1016/j.ultrasmedbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung O., Thomas A., Burks S.R., Dustin M.L., Frank J.A., Ferrer M., et al. Neuroinflammation associated with ultrasound-mediated permeabilization of the blood–brain barrier. Trends Neurosci. 2022;45(6) doi: 10.1016/j.tins.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beekers I., Mastik F., Beurskens R., Tang P.Y., Vegter M., van der Steen A.F.W., et al. High-resolution imaging of intracellular calcium fluctuations caused by oscillating microbubbles. Ultrasound Med Biol. 2020;46(8) doi: 10.1016/j.ultrasmedbio.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 53.McDannold N., Arvanitis C.D., Vykhodtseva N., Livingstone M.S. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14) doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta R.I., Carpenter J.S., Mehta R.I., Haut M.W., Wang P., Ranjan M., et al. Ultrasound-mediated blood–brain barrier opening uncovers an intracerebral perivenous fluid network in persons with Alzheimer’s disease. Fluids Barriers CNS. 2023;20(1) doi: 10.1186/s12987-023-00447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovacs Z.I., Kim S., Jikaria N., Qureshi F., Milo B., Lewis B.K., et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci USA. Jan. 2017;114(1):E75–E84. doi: 10.1073/PNAS.1614777114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gasca-Salas C., Fernández-Rodríguez B., Pineda-Pardo J.A., Rodríguez-Rojas R., Obeso I., Hernández-Fernández F., et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat Commun. 2021;12(1) doi: 10.1038/s41467-021-21022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezai A.R., Ranjan M., Haut M.W., Carpenter J., D’Haese P.-F., Mehta R.I., et al. Focused ultrasound–mediated blood-brain barrier opening in Alzheimer’s disease: long-term safety, imaging, and cognitive outcomes. J Neurosurg. Nov. 2022:1–9. doi: 10.3171/2022.9.JNS221565. [DOI] [PubMed] [Google Scholar]
- 58.Velasco F. Neuromodulation. Arch Med Res. May 2000;31(3):232–236. doi: 10.1016/S0188-4409(00)00063-1. [DOI] [PubMed] [Google Scholar]
- 59.Gildenberg P.L. Evolution of neuromodulation. Stereotact Funct Neurosurg. 2005;83(2–3):71–79. doi: 10.1159/000086865. [DOI] [PubMed] [Google Scholar]
- 60.Kamimura H.A.S., Conti A., Toschi N., Konofagou E.E. Ultrasound neuromodulation: mechanisms and the potential of multimodal stimulation for neuronal function assessment. Frontiers in Physics. May 01, 2020;8:1–9. doi: 10.3389/fphy.2020.00150. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T., Pan N., Wang Y., Liu C., Hu S. Transcranial focused ultrasound neuromodulation: a review of the excitatory and inhibitory effects on brain activity in human and animals. Front Hum Neurosci. 2021;15 doi: 10.3389/fnhum.2021.749162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu P.C., Liu H.L., Lai H.Y., Lin C.Y., Tsai H.C., Pei Y.C. Neuromodulation accompanying focused ultrasound-induced blood-brain barrier opening. Sci Rep. 2015;5 doi: 10.1038/srep15477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riis T.S., Feldman D.A., Vonesh L.C., Brown J.R., Solzbacher D., Kubanek J., et al. Durable effects of deep brain ultrasonic neuromodulation on major depression: a case report. J Med Case Rep. Oct. 2023;17(1):449. doi: 10.1186/s13256-023-04194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirtz D., Thurman D.J., Gwinn-Hardy K., Mohamed M., Chaudhuri A.R., Zalutsky R. How common are the ‘common’ neurologic disorders? Neurology. Jan. 2007;68(5):326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 65.Mintun M.A., Lo A.C., Duggan Evans C., Wessels A.M., Ardayfio P.A., Andersen S.W., et al. Donanemab in early Alzheimer’s disease. N Engl J Med. May 2021;384(18):1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 66.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. Jan. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 67.Tijms B.M., Vromen E.M., Mjaavatten O., Holstege H., Reus L.M., van der Lee S., et al. Cerebrospinal fluid proteomics in patients with Alzheimer’s disease reveals five molecular subtypes with distinct genetic risk profiles. Nat Aging. Jan. 2024;4(1):33–47. doi: 10.1038/s43587-023-00550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rezai A.R., Ranjan M., D’Haese P.F., Haut M.W., Carpenter J., Najib U., et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc Natl Acad Sci U S A. 2020;117(17) doi: 10.1073/pnas.2002571117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta R.I., Carpenter J.S., Mehta R.I., Haut M.W., Ranjan M., Najib U., et al. Blood-brain barrier opening with MRI-guided focused ultrasound elicits meningeal venous permeability in humans with early alzheimer disease. Radiology. Jan. 2021:200643. doi: 10.1148/radiol.2021200643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meng Y., Abrahao A., Heyn C.C., Bethune A.J., Huang Y., Pople C.B., et al. Glymphatics visualization after focused ultrasound-induced blood–brain barrier opening in humans. Ann Neurol. Dec. 2019;86(6):975–980. doi: 10.1002/ana.25604. [DOI] [PubMed] [Google Scholar]
- 71.Park S.H., Baik K., Jeon S., Chang W.S., Ye B.S., Chang J.W. Extensive frontal focused ultrasound mediated blood–brain barrier opening for the treatment of Alzheimer's disease: a proof-of-concept study. Transl Neurodegener. Dec. 2021;10(1):44. doi: 10.1186/s40035-021-00269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anastasiadis P., Gandhi D., Guo Y., Ahmed A.K., Bentzen S.M., Arvanitis C., et al. Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci U S A. Sep. 2021;118(37) doi: 10.1073/pnas.2103280118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen K.T., Chai W.Y., Lin Y.J., Lin C.J., Chen P.Y., Tsai H.C., et al. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci Adv. 2021;7(6) doi: 10.1126/sciadv.abd0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mainprize T., Lipsman N., Huang Y., Meng Y., Bethune A., Ironside S., et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9(1) doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng Y., Reilly R.M., Pezo R.C., Trudeau M., Sahgal A., Singnurkar A., et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci Transl Med. Oct. 2021;13(615) doi: 10.1126/scitranslmed.abj4011. [DOI] [PubMed] [Google Scholar]
- 76.Zhu L., Cheng G., Ye D., Nazeri A., Yue Y., Liu W., et al. Focused ultrasound-enabled brain tumor liquid biopsy. Sci Rep. Apr. 2018;8(1):6553. doi: 10.1038/s41598-018-24516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meng Y., Pople C.B., Suppiah S., Llinas M., Huang Y., Sahgal A, et al. MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol. Oct. 2021;23(10):1789–1797. doi: 10.1093/neuonc/noab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng Y., Pople C.B., Huang Y., Jones R.M., Ottoy J., Goubran M., et al. Putaminal recombinant glucocerebrosidase delivery with magnetic resonance – guided focused ultrasound in Parkinson’s disease: a phase I study. Mov Disord. Oct. 2022;37(10):2134–2139. doi: 10.1002/mds.29190. [DOI] [PubMed] [Google Scholar]
- 79.Abrahao A., Meng Y., Llinas M., Huang Y., Hamani C., Mainprize T., et al. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. Sep. 2019;10(1):4373. doi: 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bex A., Bex V., Carpentier A., Mathon B. Therapeutic ultrasound: the future of epilepsy surgery? Rev Neurol. 2022;178(10) doi: 10.1016/j.neurol.2022.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y., Buckmaster P.S., Qiu L., Wang J., Keunen O., Ghobadi S.N., et al. Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy. Exp Neurol. 2021;343 doi: 10.1016/j.expneurol.2021.113761. [DOI] [PMC free article] [PubMed] [Google Scholar]