Abstract
Nonalcoholic steatohepatitis (NASH) represents a severe disease subtype of nonalcoholic fatty liver disease (NAFLD) that is thought to be highly associated with systemic metabolic abnormalities. It is characterized by a series of substantial liver damage, including hepatocellular steatosis, inflammation, and fibrosis. The end stage of NASH, in some cases, may result in cirrhosis and hepatocellular carcinoma (HCC). Nowadays a large number of investigations are actively under way to test various therapeutic strategies, including emerging oligonucleotide drugs (e.g., antisense oligonucleotide, small interfering RNA, microRNA, mimic/inhibitor RNA, and small activating RNA) that have shown high potential in treating this fatal liver disease. This article systematically reviews the pathogenesis of NASH/NAFLD, the promising druggable targets proven by current studies in chemical compounds or biological drug development, and the feasibility and limitations of oligonucleotide-based therapeutic approaches under clinical or pre-clinical studies.
Keywords: MT: Oligonucleotides: Therapies and Applications, NASH, NAFLD, RNAi, ASO, siRNA, miRNA, oligonucleotide drug
Graphical abstract
Wang and colleagues summarize pre-clinical and clinical studies that are undertaken by utilizing various types of delivery approaches to testify promising oligonucleotide-related therapeutic strategies for nonalcoholic steatohepatitis.
Introduction
Nonalcoholic fatty liver disease (NAFLD) (Table 1 for abbreviations) is a clinicopathological syndrome characterized by hepatic steatosis, which lacks secondary causes of excessive fat deposition, such as alcohol.1 NAFLD and nonalcoholic steatohepatitis (NASH) have long been considered to be metabolic diseases, as in the majority of NASH patients the disease is also accompanied by metabolic abnormalities, including obesity, insulin resistance (IR) or type 2 diabetes (T2D), hypertriglyceridemia, and dyslipidemia.2,3,4,5,6 Despite obscure symptoms at a very early stage, NASH may gradually progress to cirrhosis and other end-stage liver diseases such as hepatocellular carcinoma (HCC), requiring eventual liver transplantation.7 As NASH has brought huge life-threatening concerns and economic burdens around the world, effective therapeutic approaches are urgently desired. It has been widely accepted that NASH results from numerous metabolic and pathologic alterations that proceed in parallel, including genetic predisposition, abnormal lipid metabolism, oxidative stress, lipid toxicity, mitochondrial dysfunction, inflammation, gut dysbiosis, and endoplasmic reticulum (ER) stress,8 which certainly raise great difficulties for single-action drug development. So far, the development of chemical drugs targeting thyroid hormone receptor β (Thr-β), glucagon-like peptide 1 receptor (Glp-1R), farnesoid X receptor (Fxr), and peroxisomal proliferator-activated receptor (PPAR) are at the forefront of the drug pipelines.9 Followed by the announced positive topline results of Thr-β agonist resmetirom (MGL-3196),10 the US Food and Drug Administration (FDA) approved resmetirom as the first-line medication for NASH patients with moderate to advanced liver fibrosis on March 14, 2024,11 greatly boosting confidence and demands in NASH-specific drug development.
Table 1.
List of abbreviations
| Abbreviation | Definition |
|---|---|
| 2ʹ-F | 2ʹ-fluoro |
| 2ʹ-OMe | 2ʹ-O-methyl |
| 2ʹ-MOE | 2ʹ-O-methoxyethyl |
| AASLD | American Association for the Study of Liver Diseases |
| AAV | adeno-associated virus |
| Acc | acetyl-coenzyme A carboxylase |
| AcNH | N-acetylamine |
| ADA-SCID | adenosine deaminase-deficient severe combined immunodeficiency |
| ADGRF1 | adhesion G-protein-coupled receptor F1 |
| ADR | adverse drug reaction |
| AdV | adenovirus |
| AEAA | aminoethyl anisamide |
| AEG-1 | astrocyte elevated gene 1 |
| AGO2 | Argonaute 2 protein |
| AHP | acute hepatic porphyria |
| ALT | alanine transaminase |
| AMLN | amylin liver nonalcoholic steatohepatitis |
| anti-miR | anti-miRNA oligonucleotide |
| APOC3 | apolipoprotein C 3 |
| ApoE | apolipoprotein E |
| ASGPR | asialoglycoprotein receptor |
| asiRNA | asymmetric siRNA |
| ASK1 | apoptosis signal-regulating kinase 1 |
| ASO | antisense oligonucleotide |
| AST | alanine aminotransferase |
| BDNF | brain-derived neurotrophic factor |
| BNA | bridged nucleic acid |
| CAR | chimeric antigen receptor |
| CCR2/5 | C-C chemokine receptor type 2/5 |
| CDAA | choline-deficient/amino acid-defined |
| CDHFD | choline-deficient high-fat diet |
| CE | cholesterol esters |
| CHREBP | carbohydrate response element binding protein |
| CLCF1 | cardiotrophin-like cytokine factor 1 |
| CMV | cytomegalovirus |
| CpG | cytosine phosphate-guanine |
| CPP | cell-penetrating peptide |
| CRN | Clinical Research Network |
| CYP7A1 | cholesterol 7α-hydroxylase |
| DAMP | damage-associated molecular pattern |
| DGAT2 | diacylglycerol acyltransferase 2 |
| DIO | diet-induced obese |
| DLinDMA | 1, 2-dilinoleyloxy-N,N-dimethyl-3-aminopropane |
| DLin-MC3-DMA | dilinoleylmethyl-4-dimethylaminobutyrate |
| DMD | Duchenne muscular dystrophy |
| DMN | dimethylnitrosamine |
| DNL | de novo lipogenesis |
| DSPC | distearolyphosphatidylcholine |
| dsRNA | double-stranded RNA |
| EASL | European Association for the Study of the Liver |
| ECM | extracellular matrix |
| ENA | ethylene-bridged nucleic acid |
| ER | endoplasmic reticulum |
| ESC | enhanced stabilization chemistry |
| ESC+ | enhanced stabilization chemistry-plus |
| ETC | electron transfer chain |
| FA | fatty acid |
| FASN | fatty acid synthase |
| FDA | US Food and Drug Administration |
| FFA | free fatty acid |
| FGF12/19 | fibroblast growth factor 12/19 |
| Fxr | farnesoid X receptor |
| GalNAc | N-acetylgalactosamine |
| GAN | Gubra amylin nonalcoholic steatohepatitis |
| GI | gastrointestinal |
| GIP | glucose-dependent insulinotropic polypeptide |
| GIP-R | insulinotropic polypeptide receptor |
| GLP-1R | glucagon-like peptide 1 receptor |
| GLU-R | glucagon receptors |
| GNA | glycol nucleic acid |
| GPCR | G-protein-coupled receptor |
| GWAS | genome-wide association study |
| HAO1 | hydroxyacid oxidase 1 |
| hATTR | hereditary transthyretin |
| HBV | hepatitis B virus |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HF/HS | high fat and sucrose |
| HFD | high-fat diet |
| HFFC | high-fat/fructose/cholesterol |
| HFHCD | high-fat/cholesterol diet |
| HMGB1 | high-mobility group box 1 |
| HSC | hepatic stellate cell |
| HSD17B13 | 17β-hydroxysteroid dehydrogenase 13 |
| HSP47 | 47-kDa heat-shock protein |
| HULC | highly upregulated in liver cancer |
| ICAM-1 | intercellular adhesion molecule 1 |
| ICC | interstitial cell of Cajal |
| Ihh | Indian hedgehog |
| IPF | idiopathic pulmonary fibrosis |
| IR | insulin resistance |
| JNK | c-Jun N-terminal kinase |
| KC | Kupffer cell |
| KLF11 | Krüppel-like factor 11 |
| LD | lipid droplet |
| LDLR | low-density lipoprotein receptor |
| LICA | ligand-conjugated antisense oligonucleotide |
| LNA | locked nucleic acid |
| lncRNA | long non-coding RNA |
| LNP | lipid nanoparticles |
| LPH | lipid-protamine-hyaluronic acid |
| LV | lentivirus |
| LXR | liver X receptor |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| MAP | mitogen-activated protein |
| MAPK | mitogen-activated protein kinase |
| MAPKKK | mitogen-activated protein kinase kinase kinase |
| MASH | metabolic dysfunction-associated steatohepatitis |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MCD | deficient in methionine and choline |
| MCJ | methylation-controlled J protein |
| miRNA | microRNA |
| MoMF | monocyte-derived macrophages |
| mRNA | messenger RNA |
| MST3 | mammalian sterile 20-like 3 |
| mtDNA | mitochondrial DNA |
| NAFLD | nonalcoholic fatty liver disease |
| NAS | nonalcoholic fatty liver disease activity scoring |
| NASH | nonalcoholic steatohepatitis |
| ncRNA | non-coding RNA |
| NDA | new drug application |
| NEAT1 | nuclear paraspeckle assembly transcript 1 |
| NF-кB | nuclear factor κB |
| OCA | obeticholic acid |
| Opn | osteopontin |
| PAMAM | poly-amidoamine |
| PANK | pantothenate kinase |
| PARP | potential poly(adenosine 5′-diphosphate ribose) polymerase |
| PCSK7 | proprotein convertase subtilisin/kexin type 7 |
| PEG | polyethylene glycol |
| PGC-1α | peroxisomal proliferator-activated receptor γ co-activator 1α |
| PH1 | primary hyperoxaluria type 1 |
| PKLR | pyruvate kinase L/R |
| PMO | phosphorodiamidate morpholino oligomers |
| PNA | peptide nucleic acid |
| PNPLA3 | patatin-like phospholipase domain-containing 3 |
| PO | phosphodiester |
| PPAR | peroxisomal proliferator-activated receptor |
| pri-miRNA | primary miRNA |
| PRR | pattern-recognition receptor |
| PS | phosphorothioate |
| RISC | RNA-induced silencing complex |
| RNAi | RNA interference |
| ROS | reactive oxygen species |
| SAHA | suberanilohydroxamic acid |
| SalB | salvianolic acid B |
| saRNA | small activating RNA |
| Scd | stearoyl-CoA dehydrogenase |
| SGLT-1/2 | sodium-glucose co-transporter 1/2 |
| shRNA | short hairpin RNA |
| siRNA | short interfering RNA |
| SIRT1 | silent information regulator 1 |
| SMS1 | sphingomyelin synthase 1 |
| SREBF2 | sterol-regulatory element binding factor 2 |
| SREBP1c | sterol-regulatory element binding protein 1c |
| STC | standard template chemistry |
| STK | serine/threonine protein kinase |
| T2D | type 2 diabetes |
| T3 | tri-iodothyronine |
| tcDNA | tricyclo-DNA |
| TG | triglyceride |
| THR-β | thyroid hormone receptor β |
| TLR | Toll-like receptor |
| TNF-α | tumor necrosis factor |
| TRBP | transactivation-responsive RNA-binding protein |
| TTR | transthyretin |
| TZD | thiazolidinedione |
| UNA | unlocked nucleic acid |
| UPR | unfolded protein response |
| UTR | untranslated region |
| VAP-1 | vascular adhesion protein-1 |
| VLDL | very-low-density lipoproteins |
| WAT | white adipose tissue |
| YAP | Yes-associated protein |
As an emerging drug-development strategy, oligonucleotide drugs have risen rapidly in recent years. Oligonucleotides refer to small DNA/RNA molecules with 8–50 nucleotides in length that bind to target RNA via Watson-Crick base pairing.12 Oligonucleotides can be normally used to inhibit gene expression through various mechanisms including RNA interference (RNAi), RNase H-mediated cleavage, and non-coding RNA (ncRNA) inhibition.13 Thanks to their potent gene-silencing capacity, oligonucleotides have been widely applied in gene therapy via both vehicle-based and vehicle-free approaches.13,14 Liver is considered an attractive organ for gene therapy due to natural hepatic tropism for many virus or non-viral vehicles.15,16 Therefore, oligonucleotide drugs are now rendering potential therapeutic options for patients with various metabolic liver diseases.15 For instance, givosiran and mipormersen are two oligonucleotide drugs approved for treating acute hepatic porphyria (AHP)17 and homozygous familial hypercholesterolemia (HoFH),18 which have encouraged attempts to develop oligonucleotides in the treatment of NASH. Here, we summarize the latest advances and perspectives in NASH/NAFLD pathogenesis, chemical compounds undergoing NASH-related clinical investigations, and recent innovations in liver-targeting therapeutic oligonucleotides for NAFLD/NASH.
Clinical presentation and diagnosis
In 1980 NASH was, for the first time, described as a nonalcoholic disease with similar pathological features and tendency to cirrhosis as alcoholic hepatitis.19 The consensus statement published in 2022 reported that the global prevalence of NAFLD in adults was estimated to range from 23% to 25%, among whom 1 in 5 were diagnosed with NASH.20 In the United States, the number of people with NASH was predicted to reach 19.53 million by 2039.21 Nowadays, NAFLD/NASH is considered to be greatly driven by altered metabolism, whereby many metabolic factors are involved.22 To further strengthen the consensus from the field, in June 2023, the European Association for the Study of the Liver (EASL) Congress announced the new nomenclatures MASH (metabolic dysfunction-associated steatohepatitis) and MASLD (metabolic dysfunction-associated steatotic liver disease) to replace NASH and NAFLD, respectively.23 Based on 14 histological features assigned in the NAFLD activity scoring (NAS) system that was designed by the Pathology Committee of the NASH Clinical Research Network (CRN), scores reaching 5 or above correlate with increased severities of NASH diagnosis.24 Additionally, according to the practice guidance from the American Association for the Study of Liver Diseases (AASLD), patients presenting more than 5% hepatocyte steatosis and lobular inflammation (regardless of liver fibrosis) but lacking excessive alcohol consumption are diagnosed with NASH.1 Noninvasive assessments (including “NAFLD fibrosis scoring” or “fibrosis-4 scoring,” magnetic resonance elastography, ultrasound elastography, and vibration-controlled transient elastography) are usually needed for patients with comorbid conditions, persistently elevated transaminases, and/or concern for cirrhosis.25 Liver biopsy, as the only method to distinguish simple liver fatty infiltration from NASH, should be considered once inconclusive results of fibrosis are obtained from the aforementioned diagnostic methods.25 However, patients typically do not undergo liver function tests or imaging diagnosis until symptoms occur, resulting in progressive NASH conditions ahead of the time of discovery. Therefore, NASH is also known as the “silent killer.”
Pathogenesis and current treatment approaches for NASH
NASH differs from simple steatosis by showing more significant hepatocyte apoptosis accompanied by increased inflammation.26 The “two-hit hypothesis” suggested that NASH development requires steatosis caused by triglyceride (TG) accumulation and oxidative stress-mediated lipid peroxidation.27 Further studies held the “non-triglyceride lipotoxicity hypothesis” by elucidating that TGs played protective roles throughout NASH progression, whereas liver injury was mainly caused by non-triglyceride lipotoxic metabolites.28 In many cases, liver inflammation prior to steatosis was observed, leading to the prevailing “multiple parallel hits hypothesis” that NASH is the result of multiple factors derived especially from adipose tissue and gut.29 Overload of fatty acids (FAs) in the liver has been shown to contribute to IR and lipotoxicity30,31,32 via disrupted mitochondria respiration33,34 and elevated reactive oxygen species (ROS) to cause hepatocyte death.35,36 The aforementioned cellular stress could stimulate pro-inflammatory and pro-fibrogenic responses of immune cells including monocyte-derived macrophages, resident Kupffer cells (KCs), and lymphocytes,37,38 which in turn promote extracellular matrix (ECM) production and fibrosis via activated hepatic stellate cells (HSCs).39 Moreover, toxic bile acid retention caused by disturbed hepatobiliary function has been found to be involved in NASH pathogenesis.40,41 Furthermore, recent studies have demonstrated the roles of bacterial metabolites and increased gut permeability in the progression of NAFLD/NASH.42,43
Nowadays, primary treatments of NAFLD still mainly focus on lifestyle intervention. For example, limiting fructose intake is thought to improve disease conditions, as daily fructose ingestion has been shown to associate with liver fibrosis in NAFLD patients.44 In addition, aerobic exercise and adequate sleep are beneficial.45,46 Nevertheless, the efficacies of such interventions mainly rely on individuals’ genetic backgrounds and/or self-discipline. Once the disease has progressed to fibrotic stages, lifestyle interventions are considered meaningless. Prior to the recent approval of resmetirom by the FDA, medications for NASH mainly aim at harnessing risk factors, including correcting dyslipidemia and hyperglycemia. For instance, vitamin E has been used in the treatment of NASH for its antioxidant properties.47 Some thiazolidinediones (TZDs), such as pioglitazone, have been shown to act as insulin sensitizers to improve metabolic status.48,49 However, the data also indicated the increased number of adverse events in pioglitazone-administered NASH patients by showing weight gain, dysregulated bone metabolism, and hemorrhagic stroke.48,49
Current chemical drug-developing strategies for NASH
Targeting lipid metabolism
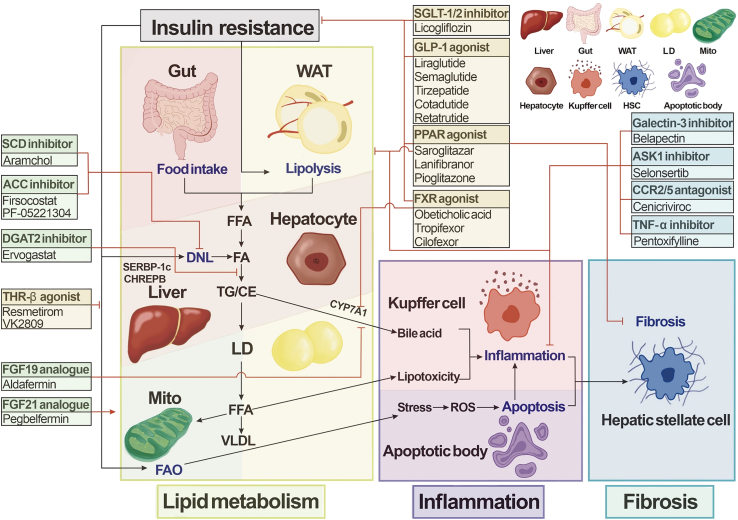
Dietary fat intake, plasma free fatty acid (FFA) absorption, and de novo lipogenesis (DNL) provide major sources for hepatic lipids. Once esterified to TGs and cholesterol esters (CEs), the excessive FAs are stored in lipid droplets (LDs), where fatty acid oxidation (FAO) and very-low-density lipoprotein (VLDL) secretion are important outlets for them50 (Figure 1). Any faulty step can render opportunities to develop liver steatosis.51,52 For instance, enhanced DNL promoted liver fat accumulation,53 while significantly elevated FFA levels were also shown in NAFLD patients.54 In addition, the enzymatic activity of β-hydroxyacyl-coenzyme A (CoA) dehydrogenase (the rate-limiting enzyme for β-oxidation in FAO) was shown to decrease during the progression of NAFLD/NASH.55 Therefore, key enzymes involved in this pathway (including stearoyl-CoA dehydrogenase [Scd], acetyl-CoA carboxylase [Acc], fatty acid synthase [Fasn], diacylglycerol acyltransferase 2 [Dgat2], and fibroblast growth factor 21 [Fgf21] and Fgf19) for generating liver FAs/TGs have been shown to serve as potential therapeutic targets to prevent NASH progression.
Figure 1.
Small-molecule drugs and biologics for NASH therapy
Several small-molecule drugs and biologics for nonalcoholic steatohepatitis (NASH) are now in development, including the projects now closed. Drugs are categorized according to their targets in the NASH pathogenesis. SCD, stearoyl-CoA dehydrogenase; ACC, acetyl-CoA carboxylase; DGAT2, diacylglycerol acyltransferase 2; SGLT-1/2, sodium-glucose co-transporter 1/2; GLP, glucagon-like peptide-1 receptor; PPAR, peroxisomal proliferator-activated receptor; THR-β, thyroid hormone receptor β; FXR, farnesoid X receptor; ASK1, apoptosis signal-regulating kinase 1; CCR2/5, C-C chemokine receptor type 2/5; FGF 19/21, fibroblast growth factor 19/21; WAT, white adipose tissue; FFA, free fatty acid; DNL, de novo lipogenesis; TG, triglyceride; CE, cholesteryl ester; SREBP-1c, sterol-regulatory element binding protein 1c; CHREBP, carbohydrate response element binding protein; CYP7A1, cholesterol 7α-hydroxylase; LD, lipid droplet; VLDL, very-low-density lipoproteins; FAO, fatty acid oxidation; mito, mitochondria; ROS, reactive oxygen species; HSC, hepatic stellate cell.
Based on this knowledge, Acc and Scd inhibitors were reported to ameliorate NASH by reducing steatosis, liver injury, inflammation, and fibrosis.56,57 A phase 2 study of liver-targeted Acc inhibitor firsocostat (GS-0976) has been completed (NCT02856555), showing decreased hepatic steatosis and fibrosis compared with placebo groups.58,59 Another Acc inhibitor, clesacostat (PF-05221304), was shown to possess an anti-steatosis effect in high-dose groups (NCT03248882).60 Meanwhile, the Scd inhibitor aramchol was reported to significantly alleviate liver fibrosis in NASH patients61 and is now awaiting the phase 3 study for formulation improvement (NCT04104321).62 Fasn is another enzyme in the DNL pathway, and the misregulated expression of this factor was found to mediate pro-inflammatory and fibrogenic signaling.63 Recently, TVB-2640 (denifanstat), a Fasn inhibitor, finished its phase 2 trial in NASH patients (NCT04906421). On the other hand, liver FAs can also be used for TG synthesis via Dgat2 catalysis64 (Figure 1). The phase 2 study of the Dgat2 selective inhibitor ervogastat (PF-06865571) co-administered with clesacostat in NAFLD patients has been completed with satisfactory results (NCT03776175).60 Fgf 21 and Fgf19 could serve as diagnostic markers for NASH.65 Fgf19 also regulates cholesterol 7α-hydroxylase (CYP7A1) gene transcription, which encodes the rate-limiting enzyme in bile acid synthesis.66 Fgf21/19 and their analogs were shown to reduce hepatic steatosis, inflammation, and fibrosis in NASH mouse models.67,68,69 Currently, Fgf21 analogs pegbelfermin (BMS-986036) and Fgf19 analog aldafermin (NGM282) were found to present significant therapeutic effects in NASH patients (NCT03486899 and NCT03912532).70,71,72,73,74,75
Targeting insulin resistance
IR results in higher insulin levels than normal because insulin-targeted tissues are less responsive in blood sugar regulation.76 It has been widely accepted that IR is involved in the progression of liver steatosis and fibrosis.77,78,79 IR-mediated lipid metabolism disturbance may contribute to NAFLD/NASH through promotion of white adipose tissue (WAT) lipolysis and liver DNL as well as altered mitochondrial FAO.80 For example, in NAFLD patients, serum FFA levels increased due to the failure of insulin-mediated lipolysis suppression.78 Meanwhile, hyperinsulinemia and hyperglycemia in NASH patients may activate sterol-regulatory element binding protein 1c (Srebp1c) and carbohydrate response element binding protein (Chrebp), respectively, to activate DNL-related gene expression in the liver.81 Mitochondrial FA β-oxidation may increase to adapt to the upregulated lipogenesis at an earlier stage, but decompensates to such changes, eventually leading to mitochondria damage, oxidative stress, and insulin signaling impairment.80 Currently, promising therapeutic targets involved in the clinical treatments of IR-related NASH include Glp-1R, Thr-β, sodium-glucose co-transporter 1/2 (Sglt1/2), PPAR, and Fxr.
As glucose-lowering drugs, Glp-1R agonists have been approved for treating T2D,82 and were also shown to protect lipid metabolism homeostasis and improve liver function.83,84,85,86 An FDA-approved long-acting Glp-1 analog, liraglutide, was shown in a phase 2 study (NCT01237119) to improve liver function and resolve pathological manifestations in NASH individuals with or without T2D.87,88 During single administration or in combinatory treatment with cilofexor or firsocostat, semaglutide has been shown to resolve hepatocyte inflammation and ballooning, alleviate liver steatosis, or even impede liver fibrosis in phase 2 studies (NCT02970942, NCT03987451, NCT03987074, and NCT04971785).89,90,91,92 A phase 3 research study of single administration of semaglutide in NASH is under way (NCT04822181). However, semaglutide and liraglutide were unfortunately shown to be associated with increased risk of gastrointestinal adverse events in weight control.93 Additionally, serving as dual agonists for both Glp-1Rs and glucose-dependent insulinotropic polypeptide (Gip) receptors (Gip-Rs), tirzepatide (LY3298176) and cotadutide (MEDI0382) are also undergoing clinical trials of NASH therapy (NCT04166773 and NCT04019561). Furthermore, efinopegdutide (MK-6024), the dual agonist for Glp-1Rs and glucagon receptors (Glu-Rs), has been granted a fast-track designation from the FDA recently for NASH treatment (NCT04944992). Retatrutide (LY3437943) is a triagonist of Glp-1Rs, Gip-Rs, and Glu-Rs. In recently published phase 2 results, retatrutide was demonstrated to resolve hepatic steatosis in obese patients with NASH (NCT04881760).94 The Thr-β ligand tri-iodothyronine (T3) has been shown to confer insulin-like effects by regulating functional gene expression in FA synthesis.95 The positive topline results of the Thr-β-selective agonist resmetirom (MGL-3196) in the phase 3 trial (NCT03900429) were announced in December 2023.10 Very recently, it has been approved by the FDA as the first NASH-specific drug for treating patients with moderate to advanced liver fibrosis.11 The phase 2 study of another Thr-β agonist, VK2809, is under way to treat histologically confirmed NASH patients (NCT04173065). Sglt1 and Sglt2 are glucose transporters that mediate uptake through the apical cell membrane.96 Sglt1 is mainly responsible for sodium-dependent glucose uptake in the small intestine, while Sglt2 is responsible for glucose reabsorption in renal proximal convoluted tubules.97,98 Licogliflozin (LIK066), a chemical compound inhibiting both Sglt1 and Sglt2, was found to improve the liver function in obese patients with NASH in a phase 2 study (NCT03205150).99 The PPAR family members PPAR-α, PPAR-β/δ, and PPAR-γ have also been demonstrated to link with NASH via regulating lipogenesis,100,101 FA transportation,102 and energy utilization,103,104,105 as well as lipotoxicity-related inflammation.106 Saroglitazar has been shown to act as a PPAR-α/γ agonist, decreasing liver fat content and alanine transaminase (ALT) in NAFLD/NASH patients (NCT03061721).107 Lanifibranor (IVA337), a pan-PPAR ligand that stimulates PPAR-α, -δ, and -γ, was reported to decrease the SAF (steatosis, activity, and fibrosis) score in patients with active NASH108,109 and is now in a phase 3 study (NCT04849728). However, the PPAR-γ-specific agonist pioglitazone was recently shown to have no increased benefit over placebo in NASH patients without diabetes (NCT00063622).110 The phase 3 study of elafibranor, which activates PPAR-α and PPAR-δ, was also terminated due to low efficacy (NCT02704403).111,112 It has been shown that the bile acid receptor Fxr downregulates Cyp7a1 expression to lower bile acid level.40,113,114 Fxr activation was also found to inhibit the expression of Srebp1c and facilitate TG homeostasis.115 The phase 3 study (NCT02548351) of obeticholic acid (OCA), an Fxr agonist that was shown to decrease IR in NAFLD patients,116 is now terminated. Tropifexor (LJN452) has been shown to downregulate alanine aminotransferase (AST) level and hepatic fat fraction in NASH patients,117 but its phase 2 study was terminated (NCT02855164). Cilofexor (GS-9674), another Fxr agonist, is now in a combination therapy study with tropifexor (NCT03449446).118
Targeting hepatocyte inflammation, fibrosis, and death
As mentioned earlier, increased serum FFAs and accumulated lipids in the liver could both cause liver steatosis, where lipotoxicity is considered one of the most critical mechanisms leading to the transition of NASH from NAFLD.119 Under such circumstances, hepatocyte apoptosis is induced by subsequent oxidative stress, ER stress, and other damage,120,121,122 which in turn cause inflammation and fibrosis via activated KCs and HSCs, respectively.123,124 Hence, anti-inflammation/anti-fibrosis strategies for treating NASH are considered effective by manipulating the targets including C-C chemokine receptor type 2/5 (Ccr2/5), tumor necrosis factor (TNF-α), vascular adhesion protein 1 (Vap-1), galectin-3, and apoptosis signal-regulating kinase 1 (Ask1).
It has been shown that Ccr2-mediated hepatic infiltration of monocyte-derived macrophages (MoMFs) could directly cause inflammation and activate HSCs.125 Ccr5, another member of the Ccr family expressed on HSCs, has also been shown to promote HSC migration, proliferation, and secretion.126,127 Cenicriviroc (CVC), a dual inhibitor of Ccr2/5, had its phase 3 clinical trials in treating NASH terminated early due to lack of efficacy (NCT03028740).128 Pentoxifylline (PTX), a methylxanthine derivative attenuating the production of pro-inflammatory cytokines including TNF-α,129 was shown to improve the histological features of NASH (NCT00590161) and is now in a phase 3 study (NCT05284448).130 Vap-1, also known as semicarbazide-sensitive amine oxidase, promotes the recruitment of pro-inflammatory cells to the liver.131 The phase 1 clinical trial of its inhibitor TERN-201 has been completed (NCT04897594). Moreover, galectin-3 is a glycan-binding protein that has been shown to activate HSCs or myofibroblasts, which contributes to tissue fibrogenesis.132,133,134,135,136 The galectin-3 inhibitor belapectin (GR-MD-02) was reported to reduce liver fibrosis in NASH patients in a phase 2 study (NCT02421094). Selonsertib (GS-4997) is a selective inhibitor targeting Ask1, a mitogen-activated protein (Map) kinase kinase kinase (Mapkkk), in response to various cytotoxic stresses.137 The therapeutic potential of selonsertib was shown in combination with firsocostat or cilofexor in a phase 2 study for treating bridging fibrosis or compensated cirrhosis due to NASH (NCT03449446 and NCT02781584).138 Notably, ER stress initiated by failed unfolded protein response (UPR) network is proven to be associated not only with metabolism disorders but also with inflammation and apoptosis.120 The AdipoR1/AdipoR2 dual agonist peptide JT003 was shown to regulate ER functions and improve liver fibrosis in mouse models.139 Another recent study has demonstrated that BGP-15, a potential poly (adenosine 5′-diphosphate ribose) polymerase (PARP) inhibitor, functioned in ER stress blockade and NASH mitigation when combined with olamkicept (sgp130Fc, an interleukin-6 trans-signaling blocker).140,141
Oligonucleotide drug-development strategies for liver diseases
Although major obstacles including relatively lower therapeutic efficacy and tissue specificity compared with conventional chemical compounds prevent the widespread application of oligonucleotide drugs, as of December 2023 dozens of oligonucleotide drugs have received regulatory approval from the FDA. Given the high perfusion rate, discontinuous sinusoidal endothelium, and abundant receptors in the liver, oligonucleotide drugs have long been considered as the alternative approach to treat liver metabolic diseases.142 Among these approved drugs, 11 target the liver. Intensive studies in oligonucleotide therapies have shed light on treating various liver diseases, including NASH. Learning from valuable results obtained in NASH-related chemical compounds and biologics development (Figure 1), oligonucleotide drugs have been designed to target critical factors residing in, but not limited to, the aforementioned pathways.
Type of oligonucleotides and the modes of action
As small synthetic nucleic acid polymers, oligonucleotides target messenger RNA (mRNA), ncRNA, or DNA via complementary base pairing while also interacting with certain proteins through three-dimensional binding.143 Currently, antisense oligonucleotide (ASO), small interfering RNA (siRNA), microRNA (miRNA) mimic or inhibitor, and small activating RNA (saRNA) are the most intensively studied oligonucleotide species, with diversified action modes, including expression inhibition or activation of functional genes and non-coding transcripts as well as mRNA splicing modulation.144
ASO
ASO is defined as a short, synthetic, single-stranded DNA, consisting of 8–50 nucleotides in length and designed to bind to RNA via Watson-Crick base pairing.145,146 Currently, ASOs make up more than 60% of oligonucleotide drugs undergoing active development.144 Fomivirsen is the first FDA-approved ASO drug developed for treating cytomegalovirus (CMV) retinitis.147 ASOs mainly function as expression inhibitors through the RNase H enzyme-mediated mRNA degradation pathway146 (Figure 2). Other studies suggested that ASOs might inhibit 5′ end capping and 3′ end polyadenylation once bound with pre-mRNAs, leading to the destabilization of RNAs.148 Additionally, it has been reported that ASOs could be designed to bind with the intron-exon boundaries of targeted pre-mRNAs for splicing regulation.148,149
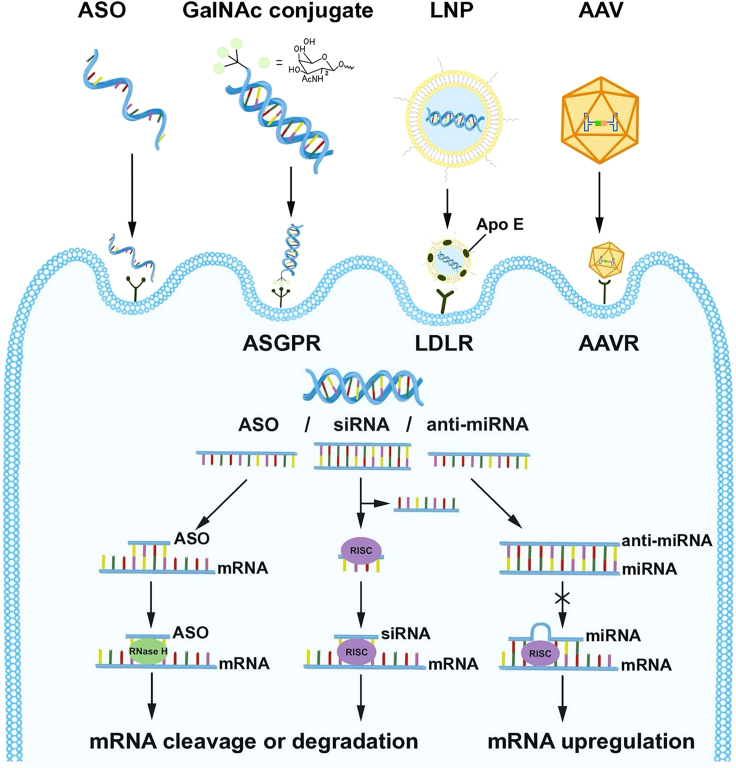
Figure 2.
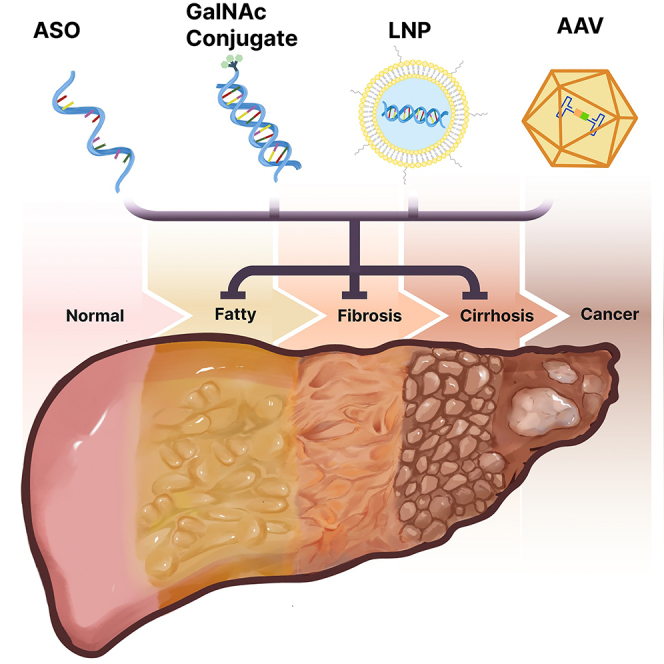
Hepatic delivery systems and action modes of oligonucleotides for liver diseases
GalNAc-ASO/siRNA conjugates are delivered to hepatocytes via the ASGPR expressed on hepatocyte surface. LNPs containing siRNA/miRNA are internalized via the LDLR expressed on the hepatocyte surface. AAV delivers shRNA and miRNA for gene knockdown via primary cell-surface glycoprotein receptors and secondary receptors or universal AAV receptor (AAVR). ASO binds to the target mRNA and attracts RNase H for mRNA degradation. siRNA is loaded into the RISC, leading to targeted mRNA cleavage or translation inhibition. miRNA complementary base pairs with the corresponding miRNA to block the cleavage of targeted mRNA via RISC. GalNAc, N-acetylgalactosamine; LNP, lipid nanoparticle; AAV. adeno-associated virus; LDLR, low-density lipoprotein receptor.
siRNA
siRNA refers to a 21- to 23-nt-long double-stranded RNA, usually with two free bases at the 3′ end.150 Matured siRNAs formed by cleavage of internalized exogenous long double-stranded RNAs (dsRNAs) or short hairpin RNAs (shRNAs), have been demonstrated to introduce cleavage or degradation on mRNA targets.151 Artificially designed siRNAs with perfect base-pair matching can be synthesized and transfected into host cells for gene transcription manipulation. In the cytosol, siRNA duplexes participate in the formation of RNA-induced silencing complex (RISC) with Argonaute 2 protein (Ago2), resulting in separated single strands.152 Once the RISC-bound antisense sequences specifically match the target mRNAs, mRNA cleavage is induced by Ago2, followed by RNase-mediated hydrolysis153 (Figure 2). Notably, because RISC-bound siRNAs are protected from nuclease degradation, they can render prolonged effects via siRNA recycling and repeated degradation of mRNAs.154
miRNA mimic or inhibitor
miRNAs were primarily discovered as endogenous ncRNAs involved in RNA-mediated gene silencing in mammalian cells.155 RNA polymerase II mediates miRNA transcription in the nucleus by forming primary miRNA (pri-miRNA) transcripts.156 These transcripts are cleaved by Drosha and co-factor protein Dgcr8, resulting in precursor miRNAs, namely pre-miRNA.157 Once translocated to the cytoplasm and further cleaved by Dicer along with transactivation-responsive RNA-binding protein (Trbp) to form miRNA duplex, one strand of miRNA binds with RISC, leading to translational inhibition or degradation on target mRNA.158,159 Due to their ability to manipulate mRNA abundance, synthetic miRNA mimics or inhibitors have been developed as applicable therapeutic approaches for various diseases. miRNA mimics are synthetic RNA duplexes containing strands identical to those of the corresponding miRNAs, facilitating the restoration or enhancement of miRNA functions.160 On the other hand, inhibiting miRNA function can be achieved by using anti-miRNA oligonucleotides (anti-miRs).161 Anti-miRs are single-stranded oligonucleotides structurally similar to ASOs, which have been shown to directly bind with the target miRNAs, displaying promising utilizations in miRNA therapeutics.161 Currently, phase 2 trials of miRNA mimics for keloid treatment (NCT03601052)162 and anti-miRs, known as miravirsen, for hepatitis C virus (HCV) therapy (NCT01200420) have been completed.163
saRNA
Unlike the gene-silencing oligonucleotides mentioned above, saRNAs are 21-nt double-stranded RNAs that interact with promoters to induce transcriptional activation in an Ago2-dependent manner.164,165 Although the mechanism of saRNAs has not yet been clarified, their therapeutic potential has been investigated.166,167 For instance, hepatocyte nuclear factor 4α (Hnf4α) is a crucial liver-specific transcription factor to mediate hepatocyte differentiation,168 liver morphogenesis,169 and lipid metabolism.170 Liver-specific deletion of HNF4α in mice displayed deleterious effects in increasing liver lipid accumulation.171 Huang et al. developed saRNA oligo-dendrimers targeting HNF4A P1 promoter to enhance HNF4A expression. The results showed favorable metabolic profile change with reduced liver TGs and IR improvement in high-fat diet (HFD)-fed rats, indicating that saRNA-mediated HNF4A activation may represent a new therapeutic strategy for NAFLD and IR.172
Modifications of synthetic oligonucleotides
To improve specific and effective delivery to target tissues, chemical modifications of synthetic oligonucleotides have been proven as necessary strategies. These modification strategies can be applied to nucleic acid backbone, ribose sugar, and nucleobase singly or in combination to enhance the stability and efficacy of oligonucleotide drugs.13 In particular, the modifications on oligonucleotide backbones have involved primarily replacing phosphodiester (PO) linkages with phosphorothioate (PS) linkages. In this process, sulfur atoms are utilized to substitute non-bridging oxygen atoms of the internucleotide phosphate group to increase nuclease resistance.173 Balancing the ratio between PO and PS linkages residing in the same oligonucleotide molecule is considered critical to reducing undesired effects such as prolonged retention and compromised target binding.174 Notably, Rp and Sp isomers are two configurations for PS linkages. It has been shown that PS linkages with the Sp configuration are more stable than its stereochemical counterparts.175 A study team from Wave Life Sciences demonstrated that the DNA region with an (RpSpSp)3 core within ASO Gapmer (described below) were more effective than a stereorandom arrangement in leading RNase H1-mediated degradation on target mRNAs.175 Moreover, 5′-phosphate terminal modifications were developed to enhance the efficacies of siRNAs, as the phosphorylated 5′ end of the guide strand was found to interact with the middle domain of Ago proteins.176 The newly developed 5′-phosphate analogs including 5ʹ-C-methyl, 5ʹ-methylenephosphonate, and 5ʹ-vinylphosphonate are shown to have conformations and steroidal electronic properties similar to those of natural phosphates while displaying resistance against dephosphorylases.177,178 Ribose sugar modifications are commonly designed to substitute the 2ʹ-hydroxyl group on RNA with 2ʹ-O-methyl (2ʹ -OMe), 2ʹ-O-methoxyethyl (2ʹ-MOE), or 2ʹ-fluoro (2ʹ-F), which have been verified to increase the half-lives of oligonucleotides in plasma and improve their binding affinities179,180,181,182 but cannot lead to RNase H activation.183,184 Bridged nucleic acids (BNAs) are featured by a linkage joining the 2ʹ oxygen to 4ʹ carbon between the ribose,185 including locked nucleic acid (LNA),186 2ʹ,4ʹ-constrained 2ʹ-O-ethyl (constrained ethyl) BNA (cEt),187 and 2ʹ-O,4ʹ-C-ethylene-bridged nucleic acid (ENA).188 The most commonly used LNA has been found to significantly improve the thermodynamic stability and nucleic acid recognition potential with increased melting temperature.189 Further studies have developed alternative chemistries to alter the original DNA or RNA structures, resulting in excellent resistance against various enzymes and unwanted aggregation once linked with charged bioconjugates, such as cationic cell-penetrating peptides (CPPs, described in “other bioconjugations”).190,191 For instance, peptide nucleic acids (PNAs) have aminoethylglycine backbones with acetyl linkers,192 while phosphorodiamidate morpholino oligomers (PMOs) have backbones consisting of morpholine rings that bear methylene groups.193 In addition, unlocked nucleic acids (UNAs) with unconnected 2ʹ and 3ʹ carbons,194 glycol nucleic acids (GNAs) using propylene glycol to alter ribose or deoxyribose,195 and tricyclo-DNAs (tcDNAs) with an additional ethylene bridge between the 3ʹ and 5ʹ carbons have also been tested.196 Strategies for nucleobase modifications have also been widely investigated. For instance, 2-thiouridine, pseudouridine (Psi), and dihydrouridine have been shown to enhance the thermodynamic stability and gene-silencing efficacy of particular siRNAs/ASOs.197
ASO
The earliest attempts at ASO modification mainly included PS linkage, leading to the advent of FDA-approved fomivirsen198 (Figure 3). However, studies have shown that PS may cause compromised interaction between ASO and target mRNA.199 As the second generation of ASOs, Gapmer is a short central DNA segment flanked by RNA-based sequences on both sides.13 Due to the hybrid structure that is resistant to nuclease and allows modifications on the RNA flanks, Gapmer has been shown to display improved target-binding ability.200,201 Inotersen, utilizing the Gapmer structure to target transthyretin (TTR) mRNA for the treatment of hereditary transthyretin (hATTR)-mediated amyloidosis, was successfully developed and approved by the FDA in 2018202 (Figure 3). More advanced strategies such as LNA, PNA, and PMO are prevalently adopted in recent ASO design.203 Eteplirsen and golodirsen, utilizing PMO technology, were approved by the FDA in 2016 and 2019, respectively, for the treatment of Duchenne muscular dystrophy (DMD)204,205 (Figure 3).
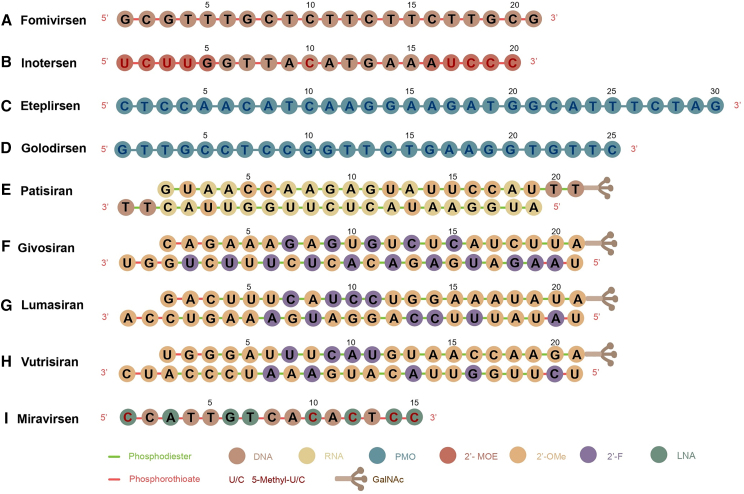
Figure 3.
Chemical modifications on major FDA-approved oligonucleotide drugs
Chemical modifications of the FDA-approved oligonucleotide drugs: (A) fomivirsen, (B) inotersen, (C) eteplirsen, (D) golodirsen, (E) patisiran, (F) givosiran, (G) lumasiran, (H) vutrisiran, and (I) miravirsen. Circles in different colors refer to different nucleotides and their derivatives, and short lines with different colors refer to phosphodiester or phosphorothioate linkages. PMO, phosphorodiamidate morpholino oligomers; 2ʹ-OMe, 2ʹ-O-methyl; 2ʹ-MOE, 2ʹ-O-methoxyethyl; 2ʹ-F, 2ʹ-fluoro; GalNAc, N-acetylgalactosamine; 5-Methyl-U, 5-methyluridine; 5-Methyl-C, 5-methylcytidine.
siRNA
The aforementioned PS backbone and ribose sugar-modification strategies180,181,182 have also been widely used in innovating siRNA drugs.206 For example, patisiran is a 2ʹ-OMe modified siRNA-based drug approved by the FDA in 2018 to silence mutated TTR expression in hATTR liver207,208 (Figure 3). Moreover, combinations of different modifications at specific sites are desirable.209,210,211 The FDA-approved anti-ALAS1 RNAi drug givosiran is an example that introduces both 2ʹ-OMe and 2ʹ-F modifications208 (Figure 3). Special modification patterns were developed by Alnylam Pharmaceuticals, including standard template chemistry (STC), enhanced stabilization chemistry (ESC), advanced ESC, and ESC-Plus (ESC+). STC pattern was designed as an alternative, with 2ʹ-OMe and 2ʹ-F modifications employed in both siRNA strands except three consecutive 2ʹ-F modifications placed at positions 9, 10, and 11 of the passenger strand and consecutive 2ʹ-OMe modifications placed at positions 11, 12, and 13 of the guide strand.206 Additionally, two PS linkages are added at the 3ʹ end of the guide strand. Although the new pattern invested siRNAs with higher stability and affinity, safety remains a significant concern.212 To reduce toxicity and further improve stability, fewer 2ʹ-F modifications and four more PS linkages were added to the ESC pattern.213 Givosiran, lumasiran, and vutrisiran are successful representatives of the ESC pattern17 (Figure 3). Thereafter, Alnylam explored multiple modification design variants by changing the proportion and position of 2ʹ-F and 2ʹ-OMe.206 Compared to the former ESC pattern, ESC+ introduced a GNA at position 7 of the guide strand, which was shown to reduce the off-target effects of N-acetylgalactosamine (GalNAc) siRNAs.214,215 Now the ESC+ pattern is applied to the development of new drugs, as seen in ALN-HBV02 for treating chronic hepatitis B virus (HBV) infection (NCT03672188) and zilebesiran for treating hypertension (NCT05103332).
miRNA mimic or inhibitor
For miRNA-based therapy, modifications including PS, LNA, and 2ʹ-OMe are widely utilized to protect oligonucleotides from RNase-mediated degradation. Because the guide strand in miRNA mimics needs to be recognized by RISC, fewer modifications (such as 2'-F modification) are available, while the passenger strand can be modified (such as 2ʹ-OMe) and linked to bioconjugations (such as cholesterol).216 Given that single-stranded anti-miRs are structurally similar to ASOs, most of the chemical modification strategies applied in ASOs could be utilized.217,218,219,220,221,222,223 Currently, miravirsen, an anti-miR-122 modified with LNA, was developed by Santaris Pharma for chronic HCV genotype 1 infection treatment224 (Figure 3). Studies have shown that by adding LNA modification, anti-miRs significantly antagonized the endogenous miRNAs.225,226 In addition, a variety of different sequences are designed as double-stranded domains or hairpin structures and added on both ends of anti-miR to improve the binding affinity and nuclease stability.227 Furthermore, Krützfeldt et al. innovated a special modification combination “antagomir” by using 2′-OMe sugar modification, PS backbone modification, and cholesterol conjugation on the 3′ end.228 Thanks to the specific, efficient, and long-lasting gene silencing, antagomir is now widely used in in vivo tests.229,230
Safety issues of synthetic oligonucleotides
The common adverse drug reactions (ADRs) of oligonucleotides reported in various clinical studies include injection-site reactions, headache, fever, and hypersensitivity,231 making oligonucleotide-mediated side effects a big concern.
Mechanistically, by base pairing with targeted mRNA sequences, oligonucleotides may cause on-target or off-target toxicities.232 On-target toxicities refer to exaggerated intended effect (e.g., too strong silencing of the targeted mRNA) and/or target-gene silencing in unwanted organs.232 To avoid such problems, tissue-specific delivery systems are needed, while accurate assessments of tissue-related expression pattern and biological function in disease-relevant cell lines or primary human cells should be conducted in pre-clinical investigations.233 On the other hand, off-target toxicities are adverse pharmacological effects caused by undesired silencing on unrelated transcripts.232 In terms of this issue, in silico screening and in vitro/in vivo targeting evaluation are widely used,234,235 while transcriptomics analysis to evaluate hybridization specificity is also suggested.236
Other toxicities independent of base pairing can cause inflammation responses, impaired coagulation, and abnormal complement activation, as well as tissue damage in kidney and liver.232 For example, most of the earlier generation of siRNA drugs, such as genasense for the treatment of melanoma, were shown to trigger unmethylated cytosine phosphate-guanine (CpG) motif-induced immune stimulation.237,238 ASO-based ISIS2302 targeting intercellular adhesion molecule 1 (ICAM-1) was found to inhibit coagulation in cynomolgus monkeys.239 To solve these issues, precise determinations of safe concentration and efficiency of oligonucleotides are imperative. Moreover, introducing novel chemical modifications (such as 2ʹ-hydroxyl group substitution and PMO) is currently being tested and applied. Intriguingly, several modification species aiming to increase binding affinity to mRNAs, such as LNAs, may also bring extra risks to off-target toxicities.240,241 Therefore, it is crucial to find the proper kinetics between oligonucleotide drug and its pharmacological target in the particular disease condition.
Hepatic delivery systems of oligonucleotides
The liver is the largest visceral organ in the body, with a unique circulatory system142 where a great number of metabolic targets are susceptible to be regulated by various therapeutic nucleic acids, including oligonucleotides.15 To develop effective delivery methods for liver-targeting oligonucleotides in clinical applications, intensive studies have utilized various approaches including chemical modifications, GalNAc conjugates, liposomes, and viral vectors.15 To date, the GalNAc-conjugate platform has been proven to be an accessible solution for hepatocyte-targeted oligonucleotides.242 Based on the sophisticated chemical modification technologies (STC, ESC, advanced ESC, and ESC+) in combination with GalNAc, Alnylam Pharmaceuticals has innovated a series of FDA-approved RNAi drugs (givosiran, lumasiran, and vutrisiran) and several oligonucleotide candidates currently undergoing clinical trials. On the other hand, lipid nanoparticles (LNPs) could achieve hepatocyte-specific delivery via apolipoprotein E (ApoE)/low-density lipoprotein receptor (LDLR) interaction.243,244 Despite the high transduction efficiency, virus-based delivery approaches are mainly used to demonstrate proof of concept for the therapeutic potential of certain oligonucleotides because of safety concerns.245 Collectively, liver-targeting oligonucleotide delivery platforms are becoming more mature and implementable, laying the foundation for the development of oligonucleotide drugs to treat NASH.
GalNAc conjugates
The asialoglycoprotein receptor (ASGPR) was discovered as a lectin in rabbits by Gilbert Ashwell and Anatol Morell in 1965.246 Galactose was later identified as a terminal sugar residue necessary for ASGPR binding, where the number and arrangement of galactose residues were significantly involved.247,248,249,250,251,252 By substituting an N-acetylamine (AcNH) to the OH group at C-2 position (Figure 2), the galactose derivative GalNAc was shown to be more rapidly endocytosed by hepatocytes at the sinusoidal surface.253,254,255 It then dissociates from ASGPR upon endosome lumen pH drop, resulting in degradation of GalNAc and membrane recycling of ASGPR.256,257 Rogers and Kornfeld initiated liver-targeted cargo delivery via ASGPR by transferring fetuin glycopeptide-coupled proteins into the rat liver.258 Subsequently, researchers sought to deliver different substances into hepatocytes through this pathway, including therapeutic glycolipids,259 chemotherapy drugs,260 and nucleotides.261,262 Hangeland et al. achieved the successful delivery of an oligodeoxynucleoside methylphosphonate neoglycopeptide conjugate, [YEE (ah-GalNAc) 3]-SMCC-AET-pUmpT7, into human hepatocellular carcinoma cells (HepG2) in 1995.263 Since then, the use of GalNAc conjugation to enhance the delivery efficiencies of ASOs and siRNAs has been constantly investigated and optimized.264,265,266 Prakash et al. developed a triantennary GalNAc-conjugated ASO, improving the potency of hepatocyte-targeted delivery by 10-fold in mice.267 Notably, GalNAc conjugated with ASOs or siRNAs now are shedding light on the clinical applications of liver-targeted oligonucleotide drugs. For instance, givosiran was designed to utilize the ESC-GalNAc delivery platform targeting ALAS1, a key enzyme gene upregulated in AHP.17 Lumasiran and vutrisiran were designed for liver-targeted gene silencing of hydroxyacid oxidase 1 (HAO1) in primary hyperoxaluria type 1 (PH1),268 and TTR in hATTR amyloidosis,269 respectively (Figure 3). Meanwhile, Ionis Pharmaceuticals is leading the ongoing ligand-conjugated ASO (LICA) program, which began with the GalNAc conjugation platform developed to achieve liver-targeted inhibition of TTR mRNA and apolipoprotein C3 (APOC3) mRNA.144,270,271
Other bioconjugations
In addition to GalNAc, other bioconjugations, including lipids, peptides, aptamers, and antibodies, have also been tested. Cholesterol and its derivatives, linked with the 3′ ends of passenger stands, are considered some of the most attractive lipid conjugates. Cholesterol-conjugated siRNAs have been shown to exhibit stronger binding to lipoproteins to enhance cellular transportation and uptake.272 Moreover, long-chain FAs and α-tocopherol are used to enhance siRNA delivery efficiencies to the liver.272,273 Peptide conjugates, such as CPPs, which are short cationic and/or amphipathic peptides typically equipped with fewer than 30 amino acids, have demonstrated the ability to cargo different molecules and traverse biological membranes via peptide-mediated uptake mechanisms.274 Therefore, CPPs are usually introduced to enhance the bioavailability and the target tissue uptake of oligonucleotides.275 Aptamers and antibodies are potentially optimal conjugates for delivering oligonucleotides into other cells and tissues due to their specific interactions with non-hepatocyte surface receptors.276,277
Lipid nanoparticles
LNPs, utilizing physiologically relevant lipids as nanocarriers, are considered low in toxicity and biocompatible.278 It has been demonstrated that LNPs can be internalized via the endocytosis process followed by endosomal escape to facilitate the release of oligonucleotides in the cytosol.279 LNPs typically consist of four lipid components: distearoylphosphatidylcholine (DSPC), cholesterol, ionizable cationic lipid, and polyethylene glycol (PEG)-lipid. DSPC and cholesterol are related to LNP structure formation.280 Ionizable cationic lipids are used to improve membrane fusion efficiencies and avoid immune responses via low surface charge at physiological pH,281 while PEG-lipids are added to control particle size and prevent aggregation.282,283 LNP-encapsulated siRNA cargoes have been shown to accumulate in hepatocytes, KCs, and sinusoids, while the strongest gene-silencing effect is typically achieved in hepatocytes.284 LNPs can be further modified to enhance binding specificities toward hepatocytes244 and HSCs285 by conjugating with GalNAc and vitamin A, respectively.
Intensive studies have shown the therapeutic potential of LNP-encapsulated oligonucleotides delivered to the liver for treating various diseases.282 For instance, the aforementioned patisiran is an approved LNP-RNAi drug207 that utilizes the ionizable cationic lipid dilinoleylmethyl-4-dimethylaminobutyrate (DLin-MC3-DMA) and results in more than two orders of silencing effect compared to the original 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane (DLinDMA).286 The PEG-lipid in this system is the shorter dimyristyl (C14) chain, which has been shown to mitigate the negative impacts of PEG shielding on siRNA silencing in vivo.287 Moreover, clinical trials are under way for the LNP-encapsulated siRNA ARB-001467 for treating HBV infection (NCT02631096) and BMS-986263 for treating liver fibrosis (NCT03420768).288 Notably, LNP-encapsulated siRNAs targeting high-mobility group box 1 (HMGB1)289 and methylation-controlled J protein (MCJ)290 have been tested in pre-clinical NASH models, respectively. In addition, LNPs have demonstrated the ability to deliver miRNA into the liver. For instance, an miR-30a-5p mimic was encapsulated into lipid-protamine-hyaluronic acid (LPH) nanoparticle modified with HSC-targeting aminoethyl anisamide (AEAA) to treat liver fibrosis in mice.291
Viral vectors as proof-of-concept research approaches
Since 1990, when retrovirus was first applied for clinical gene therapy of adenosine deaminase (ADA)-deficient severe combined immunodeficiency (ADA-SCID),292 viral vectors for the delivery of nucleotide agents have rapidly developed. Lentiviruses (LVs), adenoviruses (AdVs), and adeno-associated viruses (AAVs) are three major types of viral vehicles currently used.293 Due to relatively lower relevance to human diseases, compromised immunogenicity, and cytotoxicity, AAVs are nowadays considered safer viral vectors for in vivo expression of oligonucleotide molecules.294 In addition, tissue tropism varies greatly in different AAV serotypes,295 among which AAV8 has been shown to be a reliable vector to transduce for hepatocytes.296 Therefore, despite the controversies on AAVs as a suitable system for NASH therapy, this delivery platform has been intensively utilized in therapeutic target discovery. By introducing shRNA or pri-miRNA expressing cassettes that are driven by hepatocyte-specific promoters into viral vectors, AAVs can be used as a potent liver-targeted delivery approach for mRNA-modulating regions (e.g., siRNAs, miRNAs, and anti-miRNAs). For example, AAV-anti-miR-20b was shown to slow NAFLD progression by upregulating FAO and attenuating IR.297 AAV6-mediated in vivo expression of the shRNA against pyruvate kinase L/R (PKLR) was reported to lower L-type pyruvate kinase expression in the liver of mice fed a high-fat and sucrose (HF/HS) diet, leading to alleviated IR and reduced liver steatosis.298 AAV8 harboring shRNA against SMS1 (sphingomyelin synthase 1) was administered in mice fed a high-fat/cholesterol diet (HFHCD), resulting in lowered expression of pro-inflammatory factors and collagen type III α1.299
Current status of research in oligonucleotide drug developments for NASH
To date, various chemical compounds or small peptides have been developed to modulate a large number of potential therapeutic targets for NASH. Most of these target proteins mainly serve as enzymes or ligands/receptors, leaving insufficient pharmacological approaches applicable for other “less druggable” targets. Alternatively, emerging oligonucleotides are expected to modulate these targets through transcriptional regulation, offering new hopes for NASH treatments (Figure 4). In this context, we have summarized oligonucleotide therapeutics in NASH clinical trials and major pre-clinical studies (Tables 2 and 3).
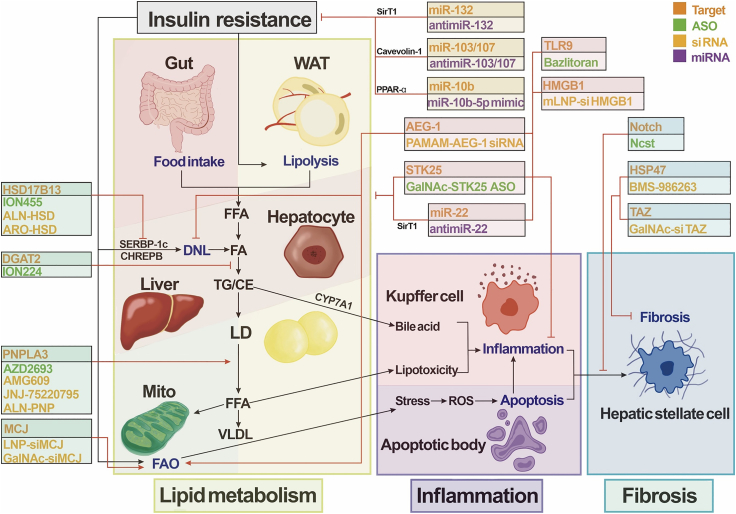
Figure 4.
Oligonucleotide drugs for NASH
Oligonucleotide drugs for nonalcoholic steatohepatitis (NASH) are now in development, including the projects now closed. Drugs are categorized according to their targets in the NASH pathogenesis. TLR9, Toll-like receptor 9; HSP47, 47-kDa heat-shock protein; STK25, serine/threonine protein kinase 25; HMGB1, high-mobility group box 1; TAZ, transcriptional co-activator with PDZ-binding motif; HSD17B13, 17β-hydroxysteroid dehydrogenase 13; DGAT2, diacylglycerol acyltransferase 2; PNPLA3, patatin-like phospholipase domain-containing 3; MCJ, methylation-controlled J protein; SirT1, silent information regulator 1; RTK, receptor tyrosine kinase; FAS, fatty acid synthase; AEG-1, astrocyte elevated gene 1; SREBP-1c, sterol-regulatory element binding protein 1c; CHREBP, carbohydrate response element binding protein; CYP7A1, cholesterol 7α-hydroxylase; PPAR, peroxisomal proliferator-activated receptor; Ihh, Indian hedgehog; WAT, white adipose tissue; FFA, free fatty acid; DNL, de novo lipogenesis; TG, triglyceride; CE, cholesteryl ester; LD, lipid droplet; VLDL, very-low-density lipoproteins; FAO, fatty acid oxidation; mito, mitochondria; ROS, reactive oxygen species; HSC, hepatic stellate cell.
Table 2.
Oligonucleotide therapeutics for NASH in clinical trials
| Name (company) | Targeted gene | Targeting agent | Disease | Latest status | ClinicalTrials.gov identifier | Reference |
|---|---|---|---|---|---|---|
| AZD2693 (Ionis Pharmaceuticals) | PNPLA3 | ASO/ASO-GalNAc conjugate | NASH | phase 2 | NCT04483947 | Ionis Pharmaceuticals306 |
| ION224 (Ionis Pharmaceuticals) | DGAT2 | ASO/ASO-GalNAc conjugate | NASH | phase 2 | NCT04932512 | Ionis Pharmaceuticals311 |
| ALN-HSD (Alnylam Pharmaceuticals) | HSD17B13 | siRNA/(ESC+)-GalNAc conjugate | NASH | phase 2 | NCT05519475 | Regeneron Pharmaceuticals352 |
| ARO-HSD (Arrowhead Pharmaceuticals) | HSD17B13 | siRNA/siRNA-GalNAc conjugate | NASH | phase 1 | NCT04202354 | Mak et al.353 |
| ION455 (Ionis Pharmaceuticals) | HSD17B13 | ASO/ASO-GalNAc conjugate | NASH | phase 1 | NCT05143905NCT05560607 | Ionis Pharmaceuticals354 |
| BMS-986263 (Bristol Myers Squibb) | HSP47 | retinoid-conjugated LNP containing siRNA | NASH | phase 2 | NCT04267393 | Lawitz et al.288 |
| AMG 609 (Amgen) | PNPLA3 | siRNA-GalNAc conjugate | NAFLD | phase 1 | NCT04857606 | N/A |
| JNJ-75220795 (Arrowhead Pharmaceuticals) | PNPLA3 | TRIM platform419 | fatty liver disease | phase 1 | NCT04844450NCT05039710 | Arrowhead Pharmaceuticals363 |
| ALN-PNP (Alnylam Pharmaceuticals) | PNPLA3 | siRNA (in ESC+/GalNAc platform) | NASH | phase 1 | NCT05648214 | N/A |
| AZD4076 (Regulus Therapeutics) | miR-103/107 | GalNAc-conjugated anti-miRNA | NAFLD/T2D | phase 1/2a | NCT02826525 | Regulus Therapeutics401 |
| NASH | phase 1 | NCT02612662 |
PNPLA3, patatin-like phospholipase domain-containing 3; DGAT2, diacylglycerol acyltransferase 2; HSD17B13, 17β-hydroxysteroid dehydrogenase 13; HSP47, 47-kDa heat-shock protein.
Table 3.
Oligonucleotide therapeutics in pre-clinical studies
| Name (company) | Targeted gene | Targeting agent | Targeting cell | Disease | Animal model | Reference |
|---|---|---|---|---|---|---|
| GalNAc-Stk25ASO (Sprint Bioscience AB) | STK25 | ASO/ASO-GalNAc conjugate | hepatocyte | NASH/T2D | murine | Cansby et al.316 |
| MST3-targeting ASO | MST3 | ASO | hepatocyte | NAFLD | murine | Caputo et al.318 |
| GalNAc-ASO-ADGRF1 | ADGRF1 | ASO-GalNAc conjugate | hepatocyte | NAFLD | murine | Wu et al.320 |
| GalNAc-ASO-PCSK7 | PCSK7 | ASO-GalNAc conjugate | hepatocyte | NAFLD | murine | Sachan et al.323 |
| AVO101 (Avogadro Pharmaceuticals) | TLR9 | ASO | N/A | NASH | primate | Shepard et al.329 |
| NCST | nicastrin | 2′-O-MOE modified ASO | hepatocyte | NASH | murine | Zhu et al.332 |
| LNP-siMCJ/GalNAc-siMCJ | MCJ | LNP/siRNA-GalNAc conjugate | hepatocyte | NASH | murine | Barbier-Torres et al.290 |
| PAMAM-AEG-1si | AEG-1 | nanoplexes conjugating PAMAM-PEG-Gal | hepatocyte | NASH | murine | Srivastava et al.370 |
| GalNAc-siTAZ | TAZ | GalNAc-siRNA | hepatocyte | NASH | murine | Wang et al.375 |
| mLNP-siHMGB1 | HMGB1 | mannose-modified siRNA loaded LNP | Kupffer cell | NASH | murine | Zhou et al.289 |
| OLX702A (OliX Pharmaceuticals) | N/A | asiRNA-GalNAc conjugate | N/A | NASH | primate | OliX Pharmaceuticals385 |
| anti-miR-132 (Regulus Therapeutics) | miR-132 | 2′-F and 2′-O-Me modified anti-miRNA | hepatocyte | NASH | murine | Papazyan et al.394 |
| RES-010 (Resalis Therapeutics) | miR-22 | LNA modified anti-miRNA | hepatocyte | NASH/NAFLD | murine | Thibonnier et al.406 |
| anti-miR-33 | miR-33 | amido-bridged nucleic acids (AmNAs)420 | hepatocyte | NASH | murine | Miyagawa et al.413 |
| MiR-10b-5p mimic (RosVivo Therapeutics) | miR-10b-5p | miRNA mimic | N/A | NAFLD/T2D/obesity/GI | N/A | RosVivo Therapeutics418 |
STK25, serine/threonine protein kinase 25; MST3, mammalian sterile 20-like 3; ADGRF1, adhesion G-protein-coupled receptor F1; PCSK7, proprotein convertase subtilisin/kexin type 7; TLR9, Toll-like receptor 9; MCJ, methylation-controlled J protein; AEG-1, astrocyte elevated gene 1; TAZ, transcriptional co-activator with PDZ-binding motif; HMGB1, high-mobility group box 1.
ASO
Patatin-like phospholipase domain-containing protein 3
Patatin-like phospholipase domain-containing 3 (PNPLA3) encodes Pnpla3 protein with TG hydrolase activity in hepatocytes.300 Amino acid substitution from isoleucine (I) to methionine (M) at position 148 (I148M) has been reported to have a robust association with various liver metabolic diseases including steatosis and fibrosis/cirrhosis,300 probably due to reduced enzymatic activity.301 Further studies have shown that ubiquitylation and proteasome-mediated Pnpla3 degradation were impaired by the I148M substitution, leading to the accumulation of mutated Pnpla3 in LDs and enhancing steatosis.302,303 Moreover, the overexpression of Pnpla3 I148M in an NAFLD mouse model upregulated the transcription of several marker genes involved in UPR and induced the accumulation of oxidized glutathione, suggesting its association with ER and oxidative stress.304 In a pre-clinical study, S-cEt-modified 16-mer ASOs were screened for optimal targeting on the mouse PNPLA3 gene.305 The resultant ASO was further modified by 5′ end conjugation with triantennary GalNAc.305 The potency of anti-PNPLA3 ASO-GalNAc in improving NAFLD conditions caused by mutated PNPLA3, including liver fibrosis, was proven.305 Furthermore, ASO/ASO-GalNAc conjugate AZD2693(ION839) was innovated by Ionis Pharmaceuticals and AstraZeneca to inhibit PNPLA3 expression.306 A phase 2 study of AZD2693 with NASH patients carrying Pnpla3 I148M has been launched (NCT05809934).
Diacylglycerol acyltransferase 2
Diacylglycerol acyltransferase 2 (Dgat2) catalyzes TG synthesis from diacylglycerol and fatty acyl CoA as substrates.64 DGAT2-knockout mice were found dead soon after birth due to lipopenic phenotypes, such as dysregulated energy metabolism and impaired skin barrier function.307 Given that TG accumulation is considered one of the key steps in NAFLD pathogenesis,27 ASO-mediated DGAT2 silencing was developed. Results showed that ASOs targeting DGAT2 significantly reduced hepatic lipid storage in rats, accompanied by lowered expressions of lipogenic genes (SREBP1c, ACC1, SCD1, and mtGPAT) and elevated expressions of oxidative/thermogenic genes (CPT1 and UCP2).308 A parallel study showed administrations of ASOs targeting DGAT2 in HFD-fed mice and ob/ob mice both efficiently reduced liver Dgat2, resulting in lowered intrahepatic TG level and attenuated hyperlipidemia, as well as reduction of hepatic steatosis.309 However, further studies using the NASH mouse model induced by a diet deficient in methionine and choline (MCD) showed that ASO-mediated DGAT2 silencing aggravated hepatic inflammation and fibrosis via elevated FFA-associated oxidative stress,310 indicating critical roles of non-TG lipid products initiating hepatotoxicity in NASH progression. ION224 is a DGAT2-targeting ASO innovated by Ionis Pharmaceuticals.311 The phase 2 clinical trial of ION224 (NCT04932512) was completed with positive results showing improvement in NAS score without worsening fibrosis in NASH patients.312
Other targets in lipid metabolism
LD-associated protein serine/threonine protein kinase 25 (Stk25) has been demonstrated to play an inhibitory role in regulating lipid oxidation and insulin sensitivity.313 Biopsy data showed a positive correlation between Stk25 abundance and fat content in human livers.314 In addition, STK25 transgenic mice displayed a dramatic increase in liver lipid deposition, hepatic IR, and steatohepatitis.314 Consistently, repressed NASH symptoms including liver steatosis and oxidative damage were found in STK25-knockout mice.315 Cansby et al. designed a triantennary GalNAc-conjugated ASO for hepatocyte-targeted STK25 silencing (GalNAc-STK25 ASO), which displayed alleviated NASH symptoms in mice under chronic exposure of dietary lipids without obvious systemic toxicity or local tolerability concerns.316 Currently, Sprint Bioscience and Gothenburg University are conducting GalNAc-STK25 ASO for NASH and T2D treatment in humans.
Mammalian sterile 20-like 3 (Mst3, also known as Stk24) is another LD-associated protein closely related to Stk25.317 Chemical modified ASOs targeting MST3 have also shown the capacity to ameliorate diet-induced NAFLD, including the reduced oxidative stress and ER stress biomarkers (4-hydroxynonenal, 8-oxoguanine, KDEL, and CHOP) in mouse livers.318
Adhesion G-protein-coupled receptor F1 (Adgrf1) belongs to the G-protein-coupled receptor (GPCR) family.319 Recent studies found that Adgrf1 acted as an upstream regulator of Scd1.320 Moreover, two GalNAc-conjugated ASO-ADGRF1s that bind to different regions of ADGRF1 mRNA have been found to improve glucose homeostasis, alleviating lipid abundance and liver damage in HFD-fed ADGRF1-overexpressed mice.320
Proprotein convertase subtilisin/kexin type 7 (PCSK7) encodes Pcsk7 as a transmembrane protease,321 whose single-nucleotide variation (rs236918) is linked with dyslipidemia and liver damage in NAFLD patients.322 The recent studies led by Sachan et al. have shown that GalNAc-ASO selected to target PCSK7 mRNA had the ability to accelerate the recovery of high-fat/fructose/cholesterol (HFFC) diet-induced mice exhibiting hepatic steatosis.323
Toll-like receptor 9
By serving as pattern-recognition receptors (PRRs), Toll-like receptors (TLRs) were demonstrated to recognize unwanted or mislocated DNA fragments, such as unmethylated CpG-DNA motifs from bacteria or virus genome, to initiate tissue inflammation.324 Meanwhile, a substantial amount of mitochondrial DNA (mtDNA) was also found in the plasma of NASH patients as well as HFD-fed mice,325 where mtDNA was shown to be released into extracellular milieu from injured hepatocytes.326 In line with these findings, the mRNA level of TLR9 (a member of the TLR family) was reported to increase in the livers of NASH patients and atherogenic diet-fed mouse models.327 Moreover, pro-inflammatory cytokines in the liver were demonstrated to be mediated by activated Tlr9 along within NASH progression, where Tlr9 antagonist IRS954 could block this process.325 TLR9 knockout led to less liver steatosis, fibrosis, and IR in mice fed with choline-deficient/amino acid-defined (CDAA) diet, probably due to suppressed interleukin-1β and nuclear factor κB (NF-κB) signaling.328 All of the above data strongly suggested critical roles of Tlr9 in NASH progression. AVO101, a phase 2-ready TLR9 ASO, was developed by Shepard et al. to display elevated adiponectin, lowered weight, and reduced NASH symptoms in a primate obesity model.329
Notch signaling pathway
The Notch signaling pathway is a conserved cellular process well known to be involved in organ formation and morphogenesis.330 Under physiological conditions, the Notch pathway was found to be required for bile duct development in nonparenchymal cells but inactive in hepatocytes.331 Interestingly, positive correlations between Notch activity in hepatocytes and NASH progression were observed in patients and diet-induced mouse NASH models.332 In addition, forced Notch activation was shown to promote secretion of the fibrogenic factor osteopontin (Opn), leading to the activation of HSC-mediated fibrosis.332 γ-Secretase is an enzyme catalyzing Notch intramembrane proteolysis to facilitate downstream reactions.333 Therefore, various approaches to inhibit γ-secretase have been considered to treat NASH. Given that the commonly used γ-secretase inhibitor (GSI) was found to cause goblet cell metaplasia,334 a liver-selective ASO to target NCST (the gene encoding one of the γ-secretase complex subunits for ligand-dependent Notch activation) was developed.332 Results showed suppressed HSC activation and collagen deposition along with lowered body weight and adiposity in mice.332 Moreover, the absence of intestinal toxicity during NCST ASO administration indicated its safety via specific targeting of the inappropriately activated Notch signaling in hepatocytes.332
Long non-coding RNAs
The essential roles of ncRNAs in NAFLD/NASH pathogenesis has been elucidated in recent studies.335 Long-ncRNAs (lncRNAs) are large ncRNA transcripts (longer than 200 nt), which are involved in post-transcriptional regulation by directly interacting with proteins or sponging miRNAs (protecting target mRNAs from miRNA binding and degradation).336,337
As a multi-functional lncRNA, nuclear paraspeckle assembly transcript 1 (NEAT1) has been demonstrated as a therapeutic target in several disease conditions. For instance, ASO-based NEAT1 silencing has been utilized in preventing post-stroke LD agglomeration.338 Since the level of this lncRNA was found to upregulate in NAFLD and liver fibrosis patients,339,340 silencing NEAT1 by shRNA or siRNA was shown to suppress liver fibrosis and inflammation probably through disrupting the binding with miR-122 and miR-506.340,341 Other studies reported reduced lipid accumulation by shRNA-mediated NEAT1 silencing by derepressing miR-146a-5p and miR-212-5p.339,342 These results indicated that NEAT1 is a promising lncRNA target for ASO-based NAFLD treatment through multi-target regulation.
Given that NASH is highly related to metabolic disorders including IR, diabetes, and diabetic complications, ASO-mediated treatments targeting metabolism-regulatory lncRNAs are thought to confer potential benefits to treat this syndrome.343 AstraZeneca developed a Glp-1-conjugated ASO targeting lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), to achieve pancreatic β cell-specific oligonucleotide uptake for treating diabetes,344,345,346 whose application could be transferred to improve dysregulated liver metabolism in NASH.
siRNA
17β-Hydroxysteroid dehydrogenase 13
Like PNPLA3 and STK25, 17β-hydroxysteroid dehydrogenase 13 (HSD17B13) also encodes an LD-associated protein mainly expressed in hepatocytes.347 Both the protein and mRNA levels of this gene were observed to be upregulated in human NAFLD liver samples.348,349 Additionally, individuals carrying HSD17B13 loss-of-function variant (rs72613567: T/A) were found to have reduced risks of NASH and cirrhosis.350,351 Furthermore, AdV-mediated overexpression of human HSD17B13 led to a fatty liver phenotype in mice,348 highlighting its role in promoting NAFLD/NASH pathogenesis. ALN-HSD, a GalNAc-conjugated siRNA, was developed by ESC+ GalNAc-conjugate technology to silence HSD17B13 expression.352 A phase 2 clinical study of subcutaneously administered ALN-HSD for NASH therapy (NCT05519475) is currently led by Alnylam Pharmaceuticals. Meanwhile, ARO-HSD (GSK4532990) siRNA developed by Arrowhead Pharmaceuticals has completed the phase 1 clinical trial (NCT04202354), showing good tolerance with lowered hepatic HSD17B13 expression as well as decreased serum ALT level in NASH patients.353 In addition, Ionis Pharmaceuticals and AstraZeneca developed ION455 (AZD7503) based on LICA ASO targeting HSD17B13 and is currently launching phase 1 studies (NCT05143905 and NCT05560607).354
47-kDa heat-shock protein
The 47-kDa heat-shock protein (HSP47) encodes an ER-resident chaperone, which binds to and stabilizes collagens/procollagens via Gly-Xaa-Arg repeats on triple-helical procollagen.355,356 Abnormalities in Hsp47 function have been thought to be associated with tissue fibrosis, such as CCl4-induced liver fibrosis and bleomycin-induced pulmonary fibrosis.357,358 Sato et al. showed successful HSC-specific delivery of siRNA targeting rat HSP47 homolog through vitamin A-coupled liposomes, which alleviated liver fibrosis and resolved collagen deposition in multiple liver disease models induced by dimethylnitrosamine (DMN), CCl4, and bile duct ligation, respectively.285 Encouraged by these results, HSP47 siRNA encapsulated in HSC-targeting vitamin A-coupled liposomes were tested in other organs, displaying dampened tissue fibrosis in pancreas, lung, lacrimal glands, and skin.359,360,361,362 BMS-986263 (ND-L02-s0201), a retinoid-conjugated LNP encapsulating HSP47 siRNA, has been used to target HSC-mediated liver fibrosis (NCT02227459) and myofibroblast-mediated idiopathic pulmonary fibrosis (IPF) (NCT03538301). It has been shown that fibrosis scores (METAVIR and Ishak) were significantly downregulated in HCV-infected patients with advanced liver fibrosis (NCT03420768).288 As of the latest update, a phase 2 clinical trial evaluating the safety and effectiveness of BMS-986263 in NASH patients with compensated cirrhosis is under way (NCT04267393).
PNPLA3
As mentioned earlier, the PNPLA3 I148M variant has been demonstrated as one of the key factors causing hepatocyte lipid accumulation.303 AMG 609 is essentially an siRNA that selectively targets the mutated allele. A phase 1 clinical trial evaluating the safety, tolerance, and liver fat changes upon subcutaneously administered AMG 609 has been launched (NCT04857606). Meanwhile, other siRNA drug candidates, JNJ-75220795 (ARO-PNPLA3)363 and ALN-PNP, designed for reducing PNPLA3 expression, are also undergoing phase 1 trials for NASH treatment (NCT04844450, NCT05039710, and NCT05648214).
Methylation-controlled J protein
MCJ (also called DnaJC1), located in the mitochondrial inner membrane, was identified as a co-chaperone to inhibit the functions of electron transfer chain (ETC) complex I.364 As the ETC serves as the outlet for products of FA β-oxidation, an excessive amount of MCJ may contribute to NAFLD development via abnormally increased FA accumulation.52 In fact increased MCJ expression has been reported in NAFLD patients, while reduction of liver steatosis and fibrosis were observed in MCJ-deficient mouse NASH models.290 In addition, loss of MCJ was shown to increase FA consumption by promoting biogenesis of respiratory supercomplexes,364,365 leaving electron leakage unchanged.364,365,366,367 Since the increase in ROS production from hyperactivated ETC normally impairs mitochondria and aggravates the tissue damage, it is believed that reduction of MCJ expression might be a feasible strategy to prevent NAFLD progression.368 LNP-siRNA targeting MCJ (LNP-siMCJ) was shown to result in reduced lipid accumulation, fibrosis, and hepatocyte damage in several NASH models mimicking different disease conditions.290 GalNAc-siRNA targeting MCJ (GalNAc-siMCJ) was also tested to achieve comparable therapeutic effects.290
Astrocyte elevated gene 1
Previous studies have shown the stimulatory roles of astrocyte elevated gene 1 (AEG-1) in the NF-κB pathway to induce inflammation in hepatocytes and macrophages.369 Srivastava et al. showed that Aeg-1 protein levels were significantly overexpressed in biopsy samples from NASH patients.370 In addition, spontaneous NASH-related pathological changes were observed in transgenic mice with hepatocyte-specific overexpression of AEG-1, whereas hepatocyte-specific AEG-1 knockout was shown to protect mice from HFD-induced NASH.370 The versatile functions of Aeg-1 in promoting NASH may be attributed to enhanced DNL and inflammation as well as downregulated FAO in the liver.370 Previously validated liver-targeted nanoplexes composing of poly-amidoamine (PAMAM) dendrimers, PEG, and lactobionic acid (PAMAM-PEG-Gal)371 were applied to encapsulate and deliver siRNAs that specifically silence AEG-1 (PAMAM-AEG-1si) in the HFD-induced mouse model, resulting in a significant alleviation of liver damage and downregulated serum AST/ALT, liver weight, and TG/cholesterol levels.370
Transcriptional co-activator with PDZ-binding motif
Transcriptional co-activator with a PDZ-binding motif (TAZ), encoding a transcriptional co-activator sharing homology with Yes-associated protein (Yap), was found to bind to the PPXY motif through its WW domain.372 TAZ is considered to be related to mesenchymal differentiation and development of multiple organs.373 Wang et al. observed elevated TAZ in the livers from NASH patients and MCD-induced murine models.374 In addition, AAV8-mediated liver-specific TAZ silencing was shown to reduce hepatic inflammation, hepatocyte death, and fibrosis in a NASH mouse model through repression of Indian hedgehog (Ihh)-mediated fibrogenic gene activation in HSCs.374 In the follow-up study, the same research group utilized TAZ siRNA conjugated with GalNAc (GalNAc-siTAZ) for therapeutic study. The results showed that GalNAc-siTAZ was able to prevent or even reverse NASH progression.375
High-mobility group box 1
Hmgb1 is known as a damage-associated molecular pattern (DAMP) released from nucleus in fat-laden hepatocytes and activated KCs to initiate the activation of the liver pro-inflammatory response as well as fibrosis.376,377,378 Plasma Hmgb1 level was found to be elevated in a diet-induced NASH mouse model379 and positively correlated with the severity of liver fibrosis in NASH patients.380 Salvianolic acid B (SalB), a compound that inhibits Hmgb1 nuclear translocation and release, was demonstrated to protect against NAFLD in rats.381 Zhou et al. developed a stable mannose-modified LNP delivery system carrying HMGB1-siRNA (mLNP-siHMGB1) to achieve specific HMGB1 silencing in KCs via mannose receptors on their surfaces.289,382 The results showed that mLNP-siHMGB1 reduced Hmgb1 protein in the liver, shifted KCs to M2 phenotype, attenuated fibrosis, and restored liver function in the NASH mouse model.289
OLX702A (by OliX Pharmaceuticals)
OLX702A is a GalNAc-conjugated asymmetric siRNA (asiRNA) drug with fewer off-target and side effects than other siRNAs.383 It was innovated by OliX Pharmaceuticals to target NASH-related genes found in a human NASH genome-wide association study (GWAS).384 The administration of OLX702A was shown to significantly reduce liver fat content in a non-human primate NASH model.385
Long non-coding RNAs
lncRNA HULC (highly upregulated in liver cancer) expression was found to be upregulated in HFD-induced rat models.386 Shen et al. demonstrated that siRNA plasmid targeting HULC in vivo significantly reduced lipid deposition, fibrosis, and hepatocyte apoptosis in the NAFLD rat model through the p38 mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase (JNK) pathways.386 Meanwhile, siRNAs targeting other metabolism-related lncRNAs can be potentially utilized in NASH treatments, such as the siRNA designed for silencing lncRNA NONRATT021972, which was shown to alleviate diabetic neuropathy in T2D rat models.387,388,389
miRNA mimic or inhibitor
MicroRNA-132
miR-132 levels were found to be significantly increased in NAFLD patients and murine NASH models, while transgenic mice with overexpressed miR-132 exhibited liver steatosis and hyperlipidemia.390 miR-132 was first demonstrated to inhibit Sirt1 expression through directly binding to SIRT1 3ʹ-untranslated region (UTR) in adipocytes.391 Sirt1 has been reported to regulate various transcription factors involved in inflammation, lipid metabolism, and insulin secretion (e.g., p53, NF-κB, PPAR-α, PPAR-γ, PPAR-γ co-activator 1α [Pgc-1α], and liver X receptor [Lxr]) via its deacetylase activity in specific sites.392,393 Therefore, anti-miR-132-mediated SIRT1 derepression could be applied as a potential approach in intervening metabolism-related diseases including NAFLD. Studies have shown that diet-induced obese (DIO) mice treated with anti-miR-132 displayed resolved liver steatosis as well as reduced liver FFA and serum LDL/VLDL.390 The above data collectively suggest that the downregulation of miR-132 has the potential to impede NASH progression. Regulus Therapeutics tested oligonucleotide-based miR-132 antagonists in DIO, choline-deficient high-fat diet (CDHFD), and amylin liver NASH (AMLN) models, showing promising efficacies in treatments.394
MicroRNA-103/107
Differing by only one nucleotide,395 miR-103 and miR-107 paralogs exist within the intron region of PANK, which encodes the pantothenate kinase (Pank).396 They were shown to be co-transcribed with this gene to regulate several target mRNAs involved in lipid and pyruvate metabolic pathways.396 Studies have shown that the levels of these two miRNAs were significantly upregulated in the livers of obese mice with steatosis,397 which induce impaired glucose homeostasis and insulin sensitivity by inhibiting the expression of cavevolin-1,398 a factor known to enhance insulin receptor signaling.399,400 Therefore, anti-miR-103/107 could function as an insulin sensitizer. A study conducted by Regulus Therapeutics showed that the administration of anti-miR-103/107 reduced TG level and liver steatosis in mice.401 Clinical trials of RG-125 (AZD4076), a GalNAc-conjugated anti-miR-103/107 designed for treating NAFLD/NASH, have been launched.401 In particular, the phase 1/2a study in T2D patients with NAFLD has been completed (NCT02826525), and a phase 1 study in patients with NASH is now under way (NCT02612662).
MicroRNA-22
miR-22 was previously reported as a tumor suppressor to regulate colon and liver cancer.402 Elevated miR-22 was observed in the serum of NAFLD patients.403 Further studies demonstrated that miR-22 expression was negatively correlated with Fgf21 levels in human or mice with fatty liver, as miR-22 directly targets FGFR1 3′ UTR and downregulates FGF21 transcription through decreasing the recruitment of PPAR-α and PGC-1α.404 In addition, miR-22 was also reported to affect lipogenesis and production of pro-inflammatory cytokines through silencing SIRT1 transcription,405 suggesting that miR-22 inhibition may have therapeutic potential for harnessing NAFLD and obesity by manipulating the metabolic gene-expression landscape. An anti-miR-22 drug candidate, APT-110, was shown to increase insulin sensitivity and effectively reduce hepatic steatosis in mice, suggesting the potential application in NAFLD treatment.406 Resalis Therapeutics is currently leading a pre-clinical study to inhibit miR-22 using LNA-based anti-miR-22 (RES-010) for treating NASH/NAFLD.407,408
MicroRNA-33
miR-33a was identified as an intronic miRNA located within SREBP2, encoding sterol regulatory element binding factor 2 (Srebf2), a transcriptional regulator targeting the expression of cellular cholesterol transporters in cholesterol metabolism.409 Studies have shown that the regulation of glucose homeostasis was improved and the development of fibrosis and inflammation was slowed in a hepatic miR-33a deficiency conditional knockout mouse model.410 In addition to miR-33a in mice, miR-33b is located in the intron of SREBP1 in humans,411 which is a crucial regulator in hepatic FA synthesis.412 Recently, studies have shown that anti-miR-33 treatments, especially anti-miR-33b, ameliorated liver dysfunction and improved the serum and liver lipid profile in Gubra amylin NASH (GAN) diet-induced mice with miR-33b knockin in the intron of SREBP1.413
Krüppel-like factor 11
Serum miR-10b levels in NASH patients have been shown to negatively correlate with the lobular inflammation score.414 Its expression was also observed to be significantly lower in the livers of mice with HFD-induced IR.415 Mechanistically, miR-10b-5p was shown to upregulate RTK (encoding receptor tyrosine kinase) expression through suppressing key transcription factor Krüppel-like factor 11 (Klf11) in interstitial cells of Cajal (ICCs) or pancreatic β cells.416,417 In pre-clinical studies, injection of the miR-10b-5p mimic successfully improved glucose homeostasis and gastrointestinal (GI) motility in mice,417 indicating the therapeutic potential of miR-10b-5p mimic in treating metabolic diseases. Led by RosVivo Therapeutics, the development of the miR-10b-5p mimic (RSVI-301) is currently under way for a group of metabolic diseases including NAFLD, T2D, obesity, and GI motility.418
Conclusions and future directions
Thanks to considerable advances in demonstrating underlying links between NASH and various pathological processes including dysregulated lipid metabolism, IR, inflammation, and fibrosis, abundant potential therapeutic targets have been uncovered. However, based on the results from particular clinical trials, Glp-1 analogs were found only to prevent the progression of liver steatosis but not to resolve the pathological changes especially in middle-to-late stage. Relying on advanced chemical modification strategies and a specific liver-targeted delivery system, oligonucleotide drugs are now becoming safe, stable, and selective in therapeutic applications of liver metabolic diseases, including NASH. However, unlike some of the metabolic diseases which are driven by single gene abnormality, NAFLD/NASH is considered a complex syndrome caused by alterations in multiple parameters. Therefore, one key question in this field is whether satisfactory therapeutic efficacy against NASH could be achieved by blocking any single target—in other words, whether multi-target therapies could be applied by combinatory administration of oligonucleotide drugs with conventional drugs. Besides, in addition to the involvement of hepatocytes, other cell types including HSCs and KCs also participate in these processes. However, effective oligonucleotide drug delivery for hepatic non-parenchymal cells remains an obstacle. Therefore, continuous inquiries into more accurate and selective delivery systems are needed. Depending on the results of current clinical and pre-clinical studies on oligonucleotide drugs, the effectiveness and safety of this emerging therapeutic strategy are believed to be open to further improvement, ultimately benefiting NASH patients.
Acknowledgments
This work is funded by the National Natural Science Foundation of China, China (grant no. 82270486 to F.X.) and the Science and Technology Bureau Fund of Sichuan Province, China (grant no. 2021YFS0051 to Y.W.).
Author contributions
S.L., Y.W., and F.X. wrote the original draft of the manuscript. S.L., F.X., and S.Z. prepared the figures and tables. J.L., G.G., J.X., and Y.W. revised the drafts and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Jun Xie, Email: jun.xie@umassmed.edu.
Yi Wang, Email: wangyi83@scu.edu.cn.
References
- 1.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Farrell G.C., Haczeyni F., Chitturi S. Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease. Adv. Exp. Med. Biol. 2018;1061:19–44. doi: 10.1007/978-981-10-8684-7_3. [DOI] [PubMed] [Google Scholar]
- 3.Ratziu V., Giral P., Charlotte F., Bruckert E., Thibault V., Theodorou I., Khalil L., Turpin G., Opolon P., Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 4.Rojano-Toimil A., Rivera-Esteban J., Manzano-Nuñez R., Bañares J., Martinez Selva D., Gabriel-Medina P., Ferrer R., Pericàs J.M., Ciudin A. When Sugar Reaches the Liver: Phenotypes of Patients with Diabetes and NAFLD. J. Clin. Med. 2022;11 doi: 10.3390/jcm11123286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treeprasertsuk S., Björnsson E., Enders F., Suwanwalaikorn S., Lindor K.D. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J. Gastroenterol. 2013;19:1219–1229. doi: 10.3748/wjg.v19.i8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.Noureddin M., Vipani A., Bresee C., Todo T., Kim I.K., Alkhouri N., Setiawan V.W., Tran T., Ayoub W.S., Lu S.C., et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am. J. Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caligiuri A., Gentilini A., Marra F. Molecular Pathogenesis of NASH. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17091575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chew N.W.S., Ng C.H., Truong E., Noureddin M., Kowdley K.V. Nonalcoholic Steatohepatitis Drug Development Pipeline: An Update. Semin. Liver Dis. 2022;42:379–400. doi: 10.1055/a-1877-9656. [DOI] [PubMed] [Google Scholar]
- 10.Madrigal Pharmaceuticals Madrigal Announces Positive Topline Results from the Pivotal Phase 3 MAESTRO-NASH Clinical Trial of Resmetirom for the Treatment of NASH and Liver Fibrosis. 2022. https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-announces-positive-topline-results-pivotal-phase-3
- 11.Madrigal Pharmaceuticals Madrigal Pharmaceuticals Announces FDA Approval of Rezdiffra™ (resmetirom) for the Treatment of Patients with Noncirrhotic Nonalcoholic Steatohepatitis (NASH) with Moderate to Advanced Liver Fibrosis. 2024. https://ir.madrigalpharma.com/news-releases/news-release-details/madrigal-pharmaceuticals-announces-fda-approval-rezdiffratm
- 12.Lakhia R., Mishra A., Patel V. Methods in Kidney Cell Biology - Part B. 2019. Manipulation of renal gene expression using oligonucleotides; pp. 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts T.C., Langer R., Wood M.J.A. Advances in oligonucleotide drug delivery. Nat. Rev. Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setten R.L., Rossi J.J., Han S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019;18:421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 15.Maestro S., Weber N.D., Zabaleta N., Aldabe R., Gonzalez-Aseguinolaza G. Novel vectors and approaches for gene therapy in liver diseases. JHEP Rep. 2021;3 doi: 10.1016/j.jhepr.2021.100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aravalli R.N., Belcher J.D., Steer C.J. Liver-targeted gene therapy: Approaches and challenges. Liver Transpl. 2015;21:718–737. doi: 10.1002/lt.24122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balwani M., Sardh E., Ventura P., Peiró P.A., Rees D.C., Stölzel U., Bissell D.M., Bonkovsky H.L., Windyga J., Anderson K.E., et al. Phase 3 Trial of RNAi Therapeutic Givosiran for Acute Intermittent Porphyria. N. Engl. J. Med. 2020;382:2289–2301. doi: 10.1056/NEJMoa1913147. [DOI] [PubMed] [Google Scholar]
- 18.Toth P.P. Emerging LDL therapies: Mipomersen-antisense oligonucleotide therapy in the management of hypercholesterolemia. J. Clin. Lipidol. 2013;7:S6–S10. doi: 10.1016/j.jacl.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 20.Lazarus J.V., Mark H.E., Anstee Q.M., Arab J.P., Batterham R.L., Castera L., Cortez-Pinto H., Crespo J., Cusi K., Dirac M.A., et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022;19:60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 21.Younossi Z.M., Paik J.M., Henry L., Yang J., Fernandes G., Stepanova M., Nader F. The Growing Economic and Clinical Burden of Nonalcoholic Steatohepatitis (NASH) in the United States. J. Clin. Exp. Hepatol. 2023;13:454–467. doi: 10.1016/j.jceh.2022.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bence K.K., Birnbaum M.J. Metabolic drivers of non-alcoholic fatty liver disease. Mol. Metab. 2021;50 doi: 10.1016/j.molmet.2020.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinella M.E., Lazarus J.V., Ratziu V., Francque S.M., Sanyal A.J., Kanwal F., Romero D., Abdelmalek M.F., Anstee Q.M., Arab J.P., et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023;79:1542–1556. doi: 10.1016/j.jhep.2023.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA. 2020;323:1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 26.Feldstein A.E., Canbay A., Angulo P., Taniai M., Burgart L.J., Lindor K.D., Gores G.J. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 27.Day C.P., James O.F. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Neuschwander-Tetri B.A. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Curr. Gastroenterol. Rep. 2010;12:49–56. doi: 10.1007/s11894-009-0083-6. [DOI] [PubMed] [Google Scholar]
- 29.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 30.Pagadala M., Kasumov T., McCullough A.J., Zein N.N., Kirwan J.P. Role of ceramides in nonalcoholic fatty liver disease. Trends Endocrinol. Metab. 2012;23:365–371. doi: 10.1016/j.tem.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petersen M.C., Shulman G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017;38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 33.Serviddio G., Bellanti F., Tamborra R., Rollo T., Romano A.D., Giudetti A.M., Capitanio N., Petrella A., Vendemiale G., Altomare E. Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur. J. Clin. Invest. 2008;38:245–252. doi: 10.1111/j.1365-2362.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 34.Serviddio G., Bellanti F., Vendemiale G., Altomare E. Mitochondrial dysfunction in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011;5:233–244. doi: 10.1586/egh.11.11. [DOI] [PubMed] [Google Scholar]
- 35.Sanyal A.J., Campbell-Sargent C., Mirshahi F., Rizzo W.B., Contos M.J., Sterling R.K., Luketic V.A., Shiffman M.L., Clore J.N. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf N.U., Sheikh T.A. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of Non-alcoholic fatty liver disease. Free Radic. Res. 2015;49:1405–1418. doi: 10.3109/10715762.2015.1078461. [DOI] [PubMed] [Google Scholar]
- 37.Wenfeng Z., Yakun W., Di M., Jianping G., Chuanxin W., Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann. Hepatol. 2014;13:489–495. [PubMed] [Google Scholar]
- 38.Dooley S., ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347:245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puche J.E., Saiman Y., Friedman S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013;3:1473–1492. doi: 10.1002/cphy.c120035. [DOI] [PubMed] [Google Scholar]
- 40.Radun R., Trauner M. Role of FXR in Bile Acid and Metabolic Homeostasis in NASH: Pathogenetic Concepts and Therapeutic Opportunities. Semin. Liver Dis. 2021;41:461–475. doi: 10.1055/s-0041-1731707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gillard J., Clerbaux L.A., Nachit M., Sempoux C., Staels B., Bindels L.B., Tailleux A., Leclercq I.A. Bile acids contribute to the development of non-alcoholic steatohepatitis in mice. JHEP Rep. 2022;4 doi: 10.1016/j.jhepr.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashiardes S., Shapiro H., Rozin S., Shibolet O., Elinav E. Non-alcoholic fatty liver and the gut microbiota. Mol. Metab. 2016;5:782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D., Gill S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 44.Abdelmalek M.F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R.J., Diehl A.M., Nonalcoholic Steatohepatitis Clinical Research Network Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Windt D.J., Sud V., Zhang H., Tsung A., Huang H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018;18:89–101. doi: 10.3727/105221617X15124844266408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koren D., Taveras E.M. Association of sleep disturbances with obesity, insulin resistance and the metabolic syndrome. Metabolism. 2018;84:67–75. doi: 10.1016/j.metabol.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Perumpail B.J., Li A.A., John N., Sallam S., Shah N.D., Kwong W., Cholankeril G., Kim D., Ahmed A. The Role of Vitamin E in the Treatment of NAFLD. Diseases. 2018;6 doi: 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rinella M.E. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 49.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland C., Musi N., et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 50.Seebacher F., Zeigerer A., Kory N., Krahmer N. Hepatic lipid droplet homeostasis and fatty liver disease. Semin. Cell Dev. Biol. 2020;108:72–81. doi: 10.1016/j.semcdb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Fujita K., Nozaki Y., Wada K., Yoneda M., Fujimoto Y., Fujitake M., Endo H., Takahashi H., Inamori M., Kobayashi N., et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 52.Miele L., Grieco A., Armuzzi A., Candelli M., Forgione A., Gasbarrini A., Gasbarrini G. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am. J. Gastroenterol. 2003;98:2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 53.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Zhao Y., Xu C., Hong Y., Lu H., Wu J., Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci. Rep. 2014;4:5832. doi: 10.1038/srep05832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore M.P., Cunningham R.P., Meers G.M., Johnson S.A., Wheeler A.A., Ganga R.R., Spencer N.M., Pitt J.B., Diaz-Arias A., Swi A.I.A., et al. Compromised hepatic mitochondrial fatty acid oxidation and reduced markers of mitochondrial turnover in human NAFLD. Hepatology. 2022;76:1452–1465. doi: 10.1002/hep.32324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross T.T., Crowley C., Kelly K.L., Rinaldi A., Beebe D.A., Lech M.P., Martinez R.V., Carvajal-Gonzalez S., Boucher M., Hirenallur-Shanthappa D., et al. Acetyl-CoA Carboxylase Inhibition Improves Multiple Dimensions of NASH Pathogenesis in Model Systems. Cell. Mol. Gastroenterol. Hepatol. 2020;10:829–851. doi: 10.1016/j.jcmgh.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kurikawa N., Takagi T., Wakimoto S., Uto Y., Terashima H., Kono K., Ogata T., Ohsumi J. A novel inhibitor of stearoyl-CoA desaturase-1 attenuates hepatic lipid accumulation, liver injury and inflammation in model of nonalcoholic steatohepatitis. Biol. Pharm. Bull. 2013;36:259–267. doi: 10.1248/bpb.b12-00702. [DOI] [PubMed] [Google Scholar]
- 58.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M., Wang L., Harting E., Tarrant J.M., McColgan B.J., et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2018;155:1463–1473.e6. doi: 10.1053/j.gastro.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lawitz E.J., Coste A., Poordad F., Alkhouri N., Loo N., McColgan B.J., Tarrant J.M., Nguyen T., Han L., Chung C., et al. Acetyl-CoA Carboxylase Inhibitor GS-0976 for 12 Weeks Reduces Hepatic De Novo Lipogenesis and Steatosis in Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2018;16:1983–1991.e3. doi: 10.1016/j.cgh.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 60.Calle R.A., Amin N.B., Carvajal-Gonzalez S., Ross T.T., Bergman A., Aggarwal S., Crowley C., Rinaldi A., Mancuso J., Aggarwal N., et al. ACC inhibitor alone or co-administered with a DGAT2 inhibitor in patients with non-alcoholic fatty liver disease: two parallel, placebo-controlled, randomized phase 2a trials. Nat. Med. 2021;27:1836–1848. doi: 10.1038/s41591-021-01489-1. [DOI] [PubMed] [Google Scholar]
- 61.Ratziu V., de Guevara L., Safadi R., Poordad F., Fuster F., Flores-Figueroa J., Arrese M., Fracanzani A.L., Ben Bashat D., Lackner K., et al. Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021;27:1825–1835. doi: 10.1038/s41591-021-01495-3. [DOI] [PubMed] [Google Scholar]
- 62.Galmed Pharmaceuticals Galmed updates business and clinical development strategy to better leverage Aramchol's anti-fibrotic effects. 2022. https://galmedpharma.investorroom.com/2022-05-17-Galmed-updates-business-and-clinical-development-strategy-to-better-leverage-Aramchols-anti-fibrotic-effects
- 63.O'Farrell M., Duke G., Crowley R., Buckley D., Martins E.B., Bhattacharya D., Friedman S.L., Kemble G. FASN inhibition targets multiple drivers of NASH by reducing steatosis, inflammation and fibrosis in preclinical models. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhatt-Wessel B., Jordan T.W., Miller J.H., Peng L. Role of DGAT enzymes in triacylglycerol metabolism. Arch. Biochem. Biophys. 2018;655:1–11. doi: 10.1016/j.abb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 65.Henriksson E., Andersen B. FGF19 and FGF21 for the Treatment of NASH-Two Sides of the Same Coin? Differential and Overlapping Effects of FGF19 and FGF21 From Mice to Human. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.601349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song K.H., Li T., Owsley E., Strom S., Chiang J.Y.L. Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology. 2009;49:297–305. doi: 10.1002/hep.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka N., Takahashi S., Zhang Y., Krausz K.W., Smith P.B., Patterson A.D., Gonzalez F.J. Role of fibroblast growth factor 21 in the early stage of NASH induced by methionine- and choline-deficient diet. Biochim. Biophys. Acta. 2015;1852:1242–1252. doi: 10.1016/j.bbadis.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou M., Learned R.M., Rossi S.J., DePaoli A.M., Tian H., Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol. Commun. 2017;1:1024–1042. doi: 10.1002/hep4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher F.M., Chui P.C., Nasser I.A., Popov Y., Cunniff J.C., Lundasen T., Kharitonenkov A., Schuppan D., Flier J.S., Maratos-Flier E. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology. 2014;147:1073–1083.e6. doi: 10.1053/j.gastro.2014.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanyal A., Charles E.D., Neuschwander-Tetri B.A., Loomba R., Harrison S.A., Abdelmalek M.F., Lawitz E.J., Halegoua-DeMarzio D., Kundu S., Noviello S., et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- 71.Abdelmalek M.F., Charles E.D., Sanyal A.J., Harrison S.A., Neuschwander-Tetri B.A., Goodman Z., Ehman R.A., Karsdal M., Nakajima A., Du S., et al. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp. Clin. Trials. 2021;104 doi: 10.1016/j.cct.2021.106335. [DOI] [PubMed] [Google Scholar]
- 72.Harrison S.A., Neff G., Guy C.D., Bashir M.R., Paredes A.H., Frias J.P., Younes Z., Trotter J.F., Gunn N.T., Moussa S.E., et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology. 2021;160:219–231.e1. doi: 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Harrison S.A., Abdelmalek M.F., Neff G., Gunn N., Guy C.D., Alkhouri N., Bashir M.R., Freilich B., Kohli A., Khazanchi A., et al. Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet. Gastroenterol. Hepatol. 2022;7:603–616. doi: 10.1016/S2468-1253(22)00017-6. [DOI] [PubMed] [Google Scholar]
- 74.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L., Kugelmas M., Bashir M.R., Jaros M.J., Ling L., et al. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 75.Rinella M.E., Lieu H.D., Kowdley K.V., Goodman Z.D., Alkhouri N., Lawitz E., Ratziu V., Abdelmalek M.F., Wong V.W.S., Younes Z.H., et al. A randomized, double-blind, placebo-controlled trial of aldafermin in patients with NASH and compensated cirrhosis. Hepatology. 2024;79:674–689. doi: 10.1097/HEP.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S.H., Park S.Y., Choi C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022;46:15–37. doi: 10.4093/dmj.2021.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fujii H., Kawada N., Japan Study Group Of Nafld J.-N. The Role of Insulin Resistance and Diabetes in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020;21:3863. doi: 10.3390/ijms21113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bugianesi E., Gastaldelli A., Vanni E., Gambino R., Cassader M., Baldi S., Ponti V., Pagano G., Ferrannini E., Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 79.Seppälä-Lindroos A., Vehkavaara S., Häkkinen A.M., Goto T., Westerbacka J., Sovijärvi A., Halavaara J., Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 80.Khan R.S., Bril F., Cusi K., Newsome P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 81.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., Okunade A.L., Patterson B.W., Nyangau E., Field T., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brunton S.A., Wysham C.H. GLP-1 receptor agonists in the treatment of type 2 diabetes: role and clinical experience to date. Postgrad. Med. 2020;132:3–14. doi: 10.1080/00325481.2020.1798099. [DOI] [PubMed] [Google Scholar]
- 83.Patel Chavez C., Cusi K., Kadiyala S. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists for the Management of NAFLD. J. Clin. Endocrinol. Metab. 2022;107:29–38. doi: 10.1210/clinem/dgab578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parlevliet E.T., Wang Y., Geerling J.J., Schröder-Van der Elst J.P., Picha K., O'Neil K., Stojanovic-Susulic V., Ort T., Havekes L.M., Romijn J.A., et al. GLP-1 receptor activation inhibits VLDL production and reverses hepatic steatosis by decreasing hepatic lipogenesis in high-fat-fed APOE∗3-Leiden mice. PloS one. 2012;7 doi: 10.1371/journal.pone.0049152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taher J., Baker C.L., Cuizon C., Masoudpour H., Zhang R., Farr S., Naples M., Bourdon C., Pausova Z., Adeli K. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol. Metab. 2014;3:823–833. doi: 10.1016/j.molmet.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Trevaskis J.L., Griffin P.S., Wittmer C., Neuschwander-Tetri B.A., Brunt E.M., Dolman C.S., Erickson M.R., Napora J., Parkes D.G., Roth J.D. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G762–G772. doi: 10.1152/ajpgi.00476.2011. [DOI] [PubMed] [Google Scholar]
- 87.Armstrong M.J., Hull D., Guo K., Barton D., Hazlehurst J.M., Gathercole L.L., Nasiri M., Yu J., Gough S.C., Newsome P.N., Tomlinson J.W. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J. Hepatol. 2016;64:399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., LEAN trial team. Abouda G., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 89.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., Sanyal A.J., Sejling A.S., Harrison S.A., NN9931-4296 Investigators A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 90.Loomba R., Abdelmalek M.F., Armstrong M.J., Jara M., Kjær M.S., Krarup N., Lawitz E., Ratziu V., Sanyal A.J., Schattenberg J.M., et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: a randomised, placebo-controlled phase 2 trial. Lancet. Gastroenterol. Hepatol. 2023;8:511–522. doi: 10.1016/S2468-1253(23)00068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romero-Gómez M., Armstrong M.J., Funuyet-Salas J., Mangla K.K., Ladelund S., Sejling A.S., Shrestha I., Sanyal A.J. Improved health-related quality of life with semaglutide in people with non-alcoholic steatohepatitis: A randomised trial. Aliment. Pharmacol. Ther. 2023;58:395–403. doi: 10.1111/apt.17598. [DOI] [PubMed] [Google Scholar]
- 92.Alkhouri N., Herring R., Kabler H., Kayali Z., Hassanein T., Kohli A., Huss R.S., Zhu Y., Billin A.N., Damgaard L.H., et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J. Hepatol. 2022;77:607–618. doi: 10.1016/j.jhep.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 93.Sodhi M., Rezaeianzadeh R., Kezouh A., Etminan M. Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss. JAMA. 2023;330:1795–1797. doi: 10.1001/jama.2023.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jastreboff A.M., Kaplan L.M., Frías J.P., Wu Q., Du Y., Gurbuz S., Coskun T., Haupt A., Milicevic Z., Hartman M.L., Retatrutide Phase 2 Obesity Trial Investigators Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial. N. Engl. J. Med. 2023;389:514–526. doi: 10.1056/NEJMoa2301972. [DOI] [PubMed] [Google Scholar]
- 95.Flores-Morales A., Gullberg H., Fernandez L., Ståhlberg N., Lee N.H., Vennström B., Norstedt G. Patterns of liver gene expression governed by TRbeta. Mol. Endocrinol. 2002;16:1257–1268. doi: 10.1210/mend.16.6.0846. [DOI] [PubMed] [Google Scholar]
- 96.Poulsen S.B., Fenton R.A., Rieg T. Sodium-glucose cotransport. Curr. Opin. Nephrol. Hypertens. 2015;24:463–469. doi: 10.1097/MNH.0000000000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorboulev V., Schürmann A., Vallon V., Kipp H., Jaschke A., Klessen D., Friedrich A., Scherneck S., Rieg T., Cunard R., et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghezzi C., Loo D.D.F., Wright E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–2097. doi: 10.1007/s00125-018-4656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harrison S.A., Manghi F.P., Smith W.B., Alpenidze D., Aizenberg D., Klarenbeek N., Chen C.-Y., Zuckerman E., Ravussin E., Charatcharoenwitthaya P., et al. Licogliflozin for nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a study. Nat. Med. 2022;28:1432–1438. doi: 10.1038/s41591-022-01861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernández-Alvarez A., Alvarez M.S., Gonzalez R., Cucarella C., Muntané J., Casado M. Human SREBP1c expression in liver is directly regulated by peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 2011;286:21466–21477. doi: 10.1074/jbc.M110.209973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferré P., Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm. Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 102.Aoyama T., Peters J.M., Iritani N., Nakajima T., Furihata K., Hashimoto T., Gonzalez F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha) J. Biol. Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 103.Janani C., Ranjitha Kumari B.D. PPAR gamma gene--a review. Diabetes Metab. Syndr. 2015;9:46–50. doi: 10.1016/j.dsx.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 104.Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol. Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Palomer X., Barroso E., Pizarro-Delgado J., Pena L., Botteri G., Zarei M., Aguilar D., Montori-Grau M., Vazquez-Carrera M. PPARbeta/delta: A Key Therapeutic Target in Metabolic Disorders. Int. J. Mol. Sci. 2018;19:913. doi: 10.3390/ijms19030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kostadinova R., Wahli W., Michalik L. PPARs in diseases: control mechanisms of inflammation. Curr. Med. Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 107.Gawrieh S., Noureddin M., Loo N., Mohseni R., Awasty V., Cusi K., Kowdley K.V., Lai M., Schiff E., Parmar D., et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology. 2021;74:1809–1824. doi: 10.1002/hep.31843. [DOI] [PubMed] [Google Scholar]
- 108.Francque S.M., Bedossa P., Ratziu V., Anstee Q.M., Bugianesi E., Sanyal A.J., Loomba R., Harrison S.A., Balabanska R., Mateva L., et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 109.Sven M F., Pierre B., Manal F A., Quentin M A., Elisabetta B., Vlad R., Philippe H.M., Bruno S., Jean-Louis J., Pierre B., Jean-Louis A. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp. Clin. Trials. 2020;98 doi: 10.1016/j.cct.2020.106170. [DOI] [PubMed] [Google Scholar]
- 110.Gastaldelli A., Harrison S., Belfort-Aguiar R., Hardies J., Balas B., Schenker S., Cusi K. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment. Pharmacol. Ther. 2010;32:769–775. doi: 10.1111/j.1365-2036.2010.04405.x. [DOI] [PubMed] [Google Scholar]
- 111.Eichenbaum N., Lavin H. GENFIT: Announces results from interim analysis of RESOLVE-IT phase 3 trial of elafibranor in adults with NASH and fibrosis. GENFIT, Loos. 2020;1–5 [Google Scholar]
- 112.Westerouen Van Meeteren M.J., Drenth J.P.H., Tjwa E.T.T.L. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH) Expert Opin. Investig. Drugs. 2020;29:117–123. doi: 10.1080/13543784.2020.1668375. [DOI] [PubMed] [Google Scholar]
- 113.Keitel V., Dröge C., Häussinger D. Targeting FXR in Cholestasis. Handb. Exp. Pharmacol. 2019;256:299–324. doi: 10.1007/164_2019_231. [DOI] [PubMed] [Google Scholar]
- 114.Li S., Hsu D.D.F., Li B., Luo X., Alderson N., Qiao L., Ma L., Zhu H.H., He Z., Suino-Powell K., et al. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 2014;20:320–332. doi: 10.1016/j.cmet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Moore D.D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 117.Sanyal A.J., Lopez P., Lawitz E.J., Lucas K.J., Loeffler J., Kim W., Goh G.B.B., Huang J.-F., Serra C., Andreone P., et al. Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial. Nat. Med. 2023;29:392–400. doi: 10.1038/s41591-022-02200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel K., Harrison S.A., Elkhashab M., Trotter J.F., Herring R., Rojter S.E., Kayali Z., Wong V.W.S., Greenbloom S., Jayakumar S., et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 119.Zámbó V., Simon-Szabó L., Szelényi P., Kereszturi E., Bánhegyi G., Csala M. Lipotoxicity in the liver. World J. Hepatol. 2013;5:550–557. doi: 10.4254/wjh.v5.i10.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang X.Q., Xu C.F., Yu C.H., Chen W.X., Li Y.M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Santos C.X.C., Tanaka L.Y., Wosniak J., Laurindo F.R.M. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 123.Kazankov K., Jørgensen S.M.D., Thomsen K.L., Møller H.J., Vilstrup H., George J., Schuppan D., Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 124.Carter J.K., Friedman S.L. Hepatic Stellate Cell-Immune Interactions in NASH. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.867940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lefere S., Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30–43. doi: 10.1016/j.jhepr.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seki E., De Minicis S., Gwak G.-Y., Kluwe J., Inokuchi S., Bursill C.A., Llovet J.M., Brenner D.A., Schwabe R.F. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Berres M.-L., Koenen R.R., Rueland A., Zaldivar M.M., Heinrichs D., Sahin H., Schmitz P., Streetz K.L., Berg T., Gassler N., et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Invest. 2010;120:4129–4140. doi: 10.1172/JCI41732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anstee Q.M., Neuschwander-Tetri B.A., Wong V.W.S., Abdelmalek M.F., Younossi Z.M., Yuan J., Pecoraro M.L., Seyedkazemi S., Fischer L., Bedossa P., et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials. 2020;89 doi: 10.1016/j.cct.2019.105922. [DOI] [PubMed] [Google Scholar]
- 129.Koppe S.W.P., Sahai A., Malladi P., Whitington P.F., Green R.M. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J. Hepatol. 2004;41:592–598. doi: 10.1016/j.jhep.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 130.Zein C.O., Yerian L.M., Gogate P., Lopez R., Kirwan J.P., Feldstein A.E., McCullough A.J. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology. 2011;54:1610–1619. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Weston C.J., Shepherd E.L., Claridge L.C., Rantakari P., Curbishley S.M., Tomlinson J.W., Hubscher S.G., Reynolds G.M., Aalto K., Anstee Q.M., et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Invest. 2015;125:501–520. doi: 10.1172/JCI73722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henderson N.C., Mackinnon A.C., Farnworth S.L., Poirier F., Russo F.P., Iredale J.P., Haslett C., Simpson K.J., Sethi T. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc. Natl. Acad. Sci. USA. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mackinnon A.C., Gibbons M.A., Farnworth S.L., Leffler H., Nilsson U.J., Delaine T., Simpson A.J., Forbes S.J., Hirani N., Gauldie J., Sethi T. Regulation of transforming growth factor-β1-driven lung fibrosis by galectin-3. Am. J. Respir. Crit. Care Med. 2012;185:537–546. doi: 10.1164/rccm.201106-0965OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Henderson N.C., Mackinnon A.C., Farnworth S.L., Kipari T., Haslett C., Iredale J.P., Liu F.T., Hughes J., Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 2008;172:288–298. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nomoto K., Nishida T., Nakanishi Y., Fujimoto M., Takasaki I., Tabuchi Y., Tsuneyama K. Deficiency in galectin-3 promotes hepatic injury in CDAA diet-induced nonalcoholic fatty liver disease. ScientificWorldJournal. 2012;2012 doi: 10.1100/2012/959824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maeda N., Kawada N., Seki S., Arakawa T., Ikeda K., Iwao H., Okuyama H., Hirabayashi J., Kasai K.i., Yoshizato K. Stimulation of proliferation of rat hepatic stellate cells by galectin-1 and galectin-3 through different intracellular signaling pathways. J. Biol. Chem. 2003;278:18938–18944. doi: 10.1074/jbc.M209673200. [DOI] [PubMed] [Google Scholar]
- 137.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lawitz E., Herring R., Younes Z., Gane E., Ruane P., Schall R.A., Jia C., Xu R., McColgan B., Djedjos S., et al. Proof of concept study of an apoptosis-signal regulating kinase (ASK1) inhibitor (selonsertib) in combination with an acetyl-CoA carboxylase inhibitor (GS-0976) or a farnesoid X receptor agonist (GS-9674) in NASH. J. Hepatol. 2018;68:S57. [Google Scholar]
- 139.Xu H., Zhao Q., Song N., Yan Z., Lin R., Wu S., Jiang L., Hong S., Xie J., Zhou H., et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020;11:5807. doi: 10.1038/s41467-020-19668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boslem E., Reibe S., Carlessi R., Smeuninx B., Tegegne S., Egan C.L., McLennan E., Terry L.V., Nobis M., Mu A., et al. Therapeutic blockade of ER stress and inflammation prevents NASH and progression to HCC. Sci. Adv. 2023;9 doi: 10.1126/sciadv.adh0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schreiber S., Aden K., Bernardes J.P., Conrad C., Tran F., Höper H., Volk V., Mishra N., Blase J.I., Nikolaus S., et al. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology. 2021;160:2354–2366.e11. doi: 10.1053/j.gastro.2021.02.062. [DOI] [PubMed] [Google Scholar]
- 142.Sehgal A., Vaishnaw A., Fitzgerald K. Liver as a target for oligonucleotide therapeutics. J. Hepatol. 2013;59:1354–1359. doi: 10.1016/j.jhep.2013.05.045. [DOI] [PubMed] [Google Scholar]
- 143.Lundin K.E., Gissberg O., Smith C.I.E., Zain R. Chemical Development of Therapeutic Oligonucleotides. Methods Mol. Biol. 2019;2036:3–16. doi: 10.1007/978-1-4939-9670-4_1. [DOI] [PubMed] [Google Scholar]
- 144.Moumné L., Marie A.-C., Crouvezier N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rinaldi C., Wood M.J.A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018;14:9–21. doi: 10.1038/nrneurol.2017.148. [DOI] [PubMed] [Google Scholar]
- 146.Bennett C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019;70:307–321. doi: 10.1146/annurev-med-041217-010829. [DOI] [PubMed] [Google Scholar]
- 147.Fomivirsen approved for CMV retinitis: first antisense drug. AIDS Treat. News. 1998;7:14–16. [PubMed] [Google Scholar]
- 148.Gheibi-Hayat S.M., Jamialahmadi K. Antisense Oligonucleotide (AS-ODN) Technology: Principle, Mechanism and Challenges. Biotechnol. Appl. Biochem. 2021;68:1086–1094. doi: 10.1002/bab.2028. [DOI] [PubMed] [Google Scholar]
- 149.Pagani F., Baralle F.E. Genomic variants in exons and introns: identifying the splicing spoilers. Nat. Rev. Genet. 2004;5:389–396. doi: 10.1038/nrg1327. [DOI] [PubMed] [Google Scholar]
- 150.Rao D.D., Vorhies J.S., Senzer N., Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 151.Ketting R.F., Fischer S.E., Bernstein E., Sijen T., Hannon G.J., Plasterk R.H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dana H., Chalbatani G.M., Mahmoodzadeh H., Karimloo R., Rezaiean O., Moradzadeh A., Mehmandoost N., Moazzen F., Mazraeh A., Marmari V., et al. Molecular Mechanisms and Biological Functions of siRNA. Int. J. Biomed. Sci. 2017;13:48–57. [PMC free article] [PubMed] [Google Scholar]
- 153.Rivas F.V., Tolia N.H., Song J.J., Aragon J.P., Liu J., Hannon G.J., Joshua-Tor L. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat. Struct. Mol. Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 154.Wittrup A., Ai A., Liu X., Hamar P., Trifonova R., Charisse K., Manoharan M., Kirchhausen T., Lieberman J. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015;33:870–876. doi: 10.1038/nbt.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jonas S., Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015;16:421–433. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 156.Carthew R.W., Sontheimer E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Han J., Lee Y., Yeom K.H., Kim Y.K., Jin H., Kim V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Correia de Sousa M., Gjorgjieva M., Dolicka D., Sobolewski C., Foti M. Deciphering miRNAs' Action through miRNA Editing. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iwakawa H.O., Tomari Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol. 2015;25:651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 160.Thorsen S.B., Obad S., Jensen N.F., Stenvang J., Kauppinen S. The therapeutic potential of microRNAs in cancer. Cancer J. 2012;18:275–284. doi: 10.1097/PPO.0b013e318258b5d6. [DOI] [PubMed] [Google Scholar]
- 161.Simonson B., Das S. MicroRNA Therapeutics: the Next Magic Bullet? Mini Rev. Med. Chem. 2015;15:467–474. doi: 10.2174/1389557515666150324123208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gallant-Behm C.L., Piper J., Lynch J.M., Seto A.G., Hong S.J., Mustoe T.A., Maari C., Pestano L.A., Dalby C.M., Jackson A.L., et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Invest. Dermatol. 2019;139:1073–1081. doi: 10.1016/j.jid.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 163.Ottosen S., Parsley T.B., Yang L., Zeh K., van Doorn L.J., van der Veer E., Raney A.K., Hodges M.R., Patick A.K. In vitro antiviral activity and preclinical and clinical resistance profile of miravirsen, a novel anti-hepatitis C virus therapeutic targeting the human factor miR-122. Antimicrob. Agents Chemother. 2015;59:599–608. doi: 10.1128/AAC.04220-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Meng X., Jiang Q., Chang N., Wang X., Liu C., Xiong J., Cao H., Liang Z. Small activating RNA binds to the genomic target site in a seed-region-dependent manner. Nucleic Acids Res. 2016;44:2274–2282. doi: 10.1093/nar/gkw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Place R.F., Noonan E.J., Földes-Papp Z., Li L.C. Defining features and exploring chemical modifications to manipulate RNAa activity. Curr. Pharm. Biotechnol. 2010;11:518–526. doi: 10.2174/138920110791591463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wei J., Zhao J., Long M., Han Y., Wang X., Lin F., Ren J., He T., Zhang H. p21WAF1/CIP1 gene transcriptional activation exerts cell growth inhibition and enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC Cancer. 2010;10:632. doi: 10.1186/1471-2407-10-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Junxia W., Ping G., Yuan H., Lijun Z., Jihong R., Fang L., Min L., Xi W., Ting H., Ke D., Huizhong Z. Double strand RNA-guided endogeneous E-cadherin up-regulation induces the apoptosis and inhibits proliferation of breast carcinoma cells in vitro and in vivo. Cancer Sci. 2010;101:1790–1796. doi: 10.1111/j.1349-7006.2010.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li J., Ning G., Duncan S.A. Mammalian hepatocyte differentiation requires the transcription factor HNF-4alpha. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- 169.Parviz F., Matullo C., Garrison W.D., Savatski L., Adamson J.W., Ning G., Kaestner K.H., Rossi J.M., Zaret K.S., Duncan S.A. Hepatocyte nuclear factor 4α controls the development of a hepatic epithelium and liver morphogenesis. Nat. Genet. 2003;34:292–296. doi: 10.1038/ng1175. [DOI] [PubMed] [Google Scholar]
- 170.Naiki T., Nagaki M., Shidoji Y., Kojima H., Imose M., Kato T., Ohishi N., Yagi K., Moriwaki H. Analysis of gene expression profile induced by hepatocyte nuclear factor 4alpha in hepatoma cells using an oligonucleotide microarray. J. Biol. Chem. 2002;277:14011–14019. doi: 10.1074/jbc.M105403200. [DOI] [PubMed] [Google Scholar]
- 171.Hayhurst G.P., Lee Y.H., Lambert G., Ward J.M., Gonzalez F.J. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang K.W., Reebye V., Czysz K., Ciriello S., Dorman S., Reccia I., Lai H.S., Peng L., Kostomitsopoulos N., Nicholls J., et al. Liver Activation of Hepatocellular Nuclear Factor-4α by Small Activating RNA Rescues Dyslipidemia and Improves Metabolic Profile. Mol. Ther. Nucleic Acids. 2020;19:361–370. doi: 10.1016/j.omtn.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Eckstein F. Nucleoside Phosphorothioates. J. Am. Chem. Soc. 1966;88:4292–4294. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- 174.Bennett C.F., Swayze E.E. RNA Targeting Therapeutics: Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 175.Iwamoto N., Butler D.C.D., Svrzikapa N., Mohapatra S., Zlatev I., Sah D.W.Y., Apponi L.H., Standley S.M., Standley S.M., Lu G., et al. Control of phosphorothioate stereochemistry substantially increases the efficacy of antisense oligonucleotides. Nat. Biotechnol. 2017;35:845–851. doi: 10.1038/nbt.3948. [DOI] [PubMed] [Google Scholar]
- 176.Frank F., Sonenberg N., Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–822. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- 177.Prakash T.P., Lima W.F., Murray H.M., Li W., Kinberger G.A., Chappell A.E., Gaus H., Seth P.P., Bhat B., Crooke S.T., Swayze E.E. Identification of metabolically stable 5'-phosphate analogs that support single-stranded siRNA activity. Nucleic Acids Res. 2015;43:2993–3011. doi: 10.1093/nar/gkv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haraszti R.A., Roux L., Coles A.H., Turanov A.A., Alterman J.F., Echeverria D., Godinho B.M.D.C., Aronin N., Khvorova A. 5΄-Vinylphosphonate improves tissue accumulation and efficacy of conjugated siRNAs in vivo. Nucleic Acids Res. 2017;45:7581–7592. doi: 10.1093/nar/gkx507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manoharan M. 2′-Carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim. Biophys. Acta. 1999;1489:117–130. doi: 10.1016/s0167-4781(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 180.Shen X., Corey D.R. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46:1584–1600. doi: 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Inoue H., Hayase Y., Imura A., Iwai S., Miura K., Ohtsuka E. Synthesis and hybridization studies on two complementary nona(2'-O-methyl)ribonucleotides. Nucleic Acids Res. 1987;15:6131–6148. doi: 10.1093/nar/15.15.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dowler T., Bergeron D., Tedeschi A.L., Paquet L., Ferrari N., Damha M.J. Improvements in siRNA properties mediated by 2'-deoxy-2'-fluoro-beta-D-arabinonucleic acid (FANA) Nucleic Acids Res. 2006;34:1669–1675. doi: 10.1093/nar/gkl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kawasaki A.M., Casper M.D., Freier S.M., Lesnik E.A., Zounes M.C., Cummins L.L., Gonzalez C., Cook P.D. Uniformly modified 2'-deoxy-2'-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- 184.Sproat B.S., Lamond A.I., Beijer B., Neuner P., Ryder U. Highly efficient chemical synthesis of 2'-O-methyloligoribonucleotides and tetrabiotinylated derivatives; novel probes that are resistant to degradation by RNA or DNA specific nucleases. Nucleic Acids Res. 1989;17:3373–3386. doi: 10.1093/nar/17.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Obika S., Nanbu D., Hari Y., Andoh J.-i., Morio K.-i., Doi T., Imanishi T. Stability and structural features of the duplexes containing nucleoside analogues with a fixed N-type conformation, 2′-O,4′-C-methyleneribonucleosides. Tetrahedron Lett. 1998;39:5401–5404. [Google Scholar]
- 186.Veedu R.N., Wengel J. Locked nucleic acid as a novel class of therapeutic agents. RNA Biol. 2009;6:321–323. doi: 10.4161/rna.6.3.8807. [DOI] [PubMed] [Google Scholar]
- 187.Seth P.P., Vasquez G., Allerson C.A., Berdeja A., Gaus H., Kinberger G.A., Prakash T.P., Migawa M.T., Bhat B., Swayze E.E. Synthesis and biophysical evaluation of 2',4'-constrained 2'O-methoxyethyl and 2',4'-constrained 2'O-ethyl nucleic acid analogues. J. Org. Chem. 2010;75:1569–1581. doi: 10.1021/jo902560f. [DOI] [PubMed] [Google Scholar]
- 188.Morita K., Hasegawa C., Kaneko M., Tsutsumi S., Sone J., Ishikawa T., Imanishi T., Koizumi M. 2'-O,4'-C-ethylene-bridged nucleic acids (ENA): highly nuclease-resistant and thermodynamically stable oligonucleotides for antisense drug. Bioorg. Med. Chem. Lett. 2002;12:73–76. doi: 10.1016/s0960-894x(01)00683-7. [DOI] [PubMed] [Google Scholar]
- 189.Koshkin A.A., Singh S.K., Nielsen P., Rajwanshi V.K., Kumar R., Meldgaard M., Olsen C.E., Wengel J. LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron. 1998;54:3607–3630. [Google Scholar]
- 190.Hudziak R.M., Barofsky E., Barofsky D.F., Weller D.L., Huang S.B., Weller D.D. Resistance of Morpholino Phosphorodiamidate Oligomers to Enzymatic Degradation. Antisense Nucleic Acid Drug. Dev. 1996;6:267–272. doi: 10.1089/oli.1.1996.6.267. [DOI] [PubMed] [Google Scholar]
- 191.Juliano R.L., Ming X., Nakagawa O. The chemistry and biology of oligonucleotide conjugates. Acc. Chem. Res. 2012;45:1067–1076. doi: 10.1021/ar2002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Nielsen P.E. PNA technology. Mol. Biotechnol. 2004;26:233–248. doi: 10.1385/MB:26:3:233. [DOI] [PubMed] [Google Scholar]
- 193.Moulton J.D. Using Morpholinos to Control Gene Expression. Curr. Protoc. Nucleic Acid Chem. 2017;68:4.30.1–4.30.29. doi: 10.1002/cpnc.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laursen M.B., Pakula M.M., Gao S., Fluiter K., Mook O.R., Baas F., Langklaer N., Wengel S.L., Wengel J., Kjems J., Bramsen J.B. Utilization of unlocked nucleic acid (UNA) to enhance siRNA performance in vitro and in vivo. Mol. Biosyst. 2010;6:862–870. doi: 10.1039/b918869j. [DOI] [PubMed] [Google Scholar]
- 195.Egli M., Schlegel M.K., Manoharan M. Acyclic (S)-glycol nucleic acid (S-GNA) modification of siRNAs improves the safety of RNAi therapeutics while maintaining potency. RNA. 2023;29:402–414. doi: 10.1261/rna.079526.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Renneberg D., Leumann C.J. Watson-Crick base-pairing properties of tricyclo-DNA. J. Am. Chem. Soc. 2002;124:5993–6002. doi: 10.1021/ja025569+. [DOI] [PubMed] [Google Scholar]
- 197.Sipa K., Sochacka E., Kazmierczak-Baranska J., Maszewska M., Janicka M., Nowak G., Nawrot B. Effect of base modifications on structure, thermodynamic stability, and gene silencing activity of short interfering RNA. RNA. 2007;13:1301–1316. doi: 10.1261/rna.538907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Azad R.F., Driver V.B., Tanaka K., Crooke R.M., Anderson K.P. Antiviral activity of a phosphorothioate oligonucleotide complementary to RNA of the human cytomegalovirus major immediate-early region. Antimicrob. Agents Chemother. 1993;37:1945–1954. doi: 10.1128/aac.37.9.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kibler-Herzog L., Zon G., Uznanski B., Whittier G., Wilson W.D. Duplex stabilities of phosphorothioate, methylphosphonate, and RNA analogs of two DNA 14-mers. Nucleic Acids Res. 1991;19:2979–2986. doi: 10.1093/nar/19.11.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dhuri K., Bechtold C., Quijano E., Pham H., Gupta A., Vikram A., Bahal R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020;9 doi: 10.3390/jcm9062004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Monia B.P., Lesnik E.A., Gonzalez C., Lima W.F., McGee D., Guinosso C.J., Kawasaki A.M., Cook P.D., Freier S.M. Evaluation of 2‘-modified oligonucleotides containing 2‘-deoxy gaps as antisense inhibitors of gene expression. J. Biol. Chem. 1993;268:14514–14522. [PubMed] [Google Scholar]
- 202.Benson M.D., Waddington-Cruz M., Berk J.L., Polydefkis M., Dyck P.J., Wang A.K., Planté-Bordeneuve V., Barroso F.A., Merlini G., Obici L., et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
- 203.Mansoor M., Melendez A.J. Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene Regul. Syst. Bio. 2008;2:275–295. doi: 10.4137/grsb.s418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Syed Y.Y. Eteplirsen: First Global Approval. Drugs. 2016;76:1699–1704. doi: 10.1007/s40265-016-0657-1. [DOI] [PubMed] [Google Scholar]
- 205.Heo Y.A. Golodirsen: First Approval. Drugs. 2020;80:329–333. doi: 10.1007/s40265-020-01267-2. [DOI] [PubMed] [Google Scholar]
- 206.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X.J. Therapeutic siRNA: state of the art. Signal Transduct. Target. Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Adams D., Gonzalez-Duarte A., O'Riordan W.D., Yang C.C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 208.Gangopadhyay S., Gore K.R. Advances in siRNA therapeutics and synergistic effect on siRNA activity using emerging dual ribose modifications. RNA Biol. 2022;19:452–467. doi: 10.1080/15476286.2022.2052641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Czauderna F., Fechtner M., Dames S., Aygün H., Klippel A., Pronk G.J., Giese K., Kaufmann J. Structural variations and stabilising modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31:2705–2716. doi: 10.1093/nar/gkg393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Song X., Wang X., Ma Y., Liang Z., Yang Z., Cao H. Site-Specific Modification Using the 2'-Methoxyethyl Group Improves the Specificity and Activity of siRNAs. Mol. Ther. Nucleic Acids. 2017;9:242–250. doi: 10.1016/j.omtn.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Koller E., Propp S., Murray H., Lima W., Bhat B., Prakash T.P., Allerson C.R., Swayze E.E., Marcusson E.G., Dean N.M. Competition for RISC binding predicts in vitro potency of siRNA. Nucleic Acids Res. 2006;34:4467–4476. doi: 10.1093/nar/gkl589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gillmore J.D., Falk R.H., Maurer M.S., Hanna M., Karsten V., Vest J., Gollob J., Hawkins P.N. Phase 2, open-label extension (OLE) study of revusiran, an investigational RNAi therapeutic for the treatment of patients with transthyretin cardiac amyloidosis. Orphanet J. Rare Dis. 2015;10:O21. [Google Scholar]
- 213.Janas M.M., Jiang Y., Schlegel M.K., Waldron S., Kuchimanchi S., Barros S.A. Impact of Oligonucleotide Structure, Chemistry, and Delivery Method on In Vitro Cytotoxicity. Nucleic Acid Ther. 2017;27:11–22. doi: 10.1089/nat.2016.0639. [DOI] [PubMed] [Google Scholar]
- 214.Janas M.M., Schlegel M.K., Harbison C.E., Yilmaz V.O., Jiang Y., Parmar R., Zlatev I., Castoreno A., Xu H., Shulga-Morskaya S., et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-02989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Schlegel M.K., Janas M.M., Jiang Y., Barry J.D., Davis W., Agarwal S., Berman D., Brown C.R., Castoreno A., LeBlanc S., et al. From bench to bedside: Improving the clinical safety of GalNAc–siRNA conjugates using seed-pairing destabilization. Nucleic Acids Res. 2022;50:6656–6670. doi: 10.1093/nar/gkac539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol. Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fabani M.M., Gait M.J. miR-122 targeting with LNA/2'-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA. 2008;14:336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Morcos P.A., Li Y., Jiang S. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 2008;45:613–614. doi: 10.2144/000113005. 616, 618 passim. [DOI] [PubMed] [Google Scholar]
- 219.Meister G., Landthaler M., Dorsett Y., Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lennox K.A., Sabel J.L., Johnson M.J., Moreira B.G., Fletcher C.A., Rose S.D., Behlke M.A., Laikhter A.L., Walder J.A., Dagle J.M. Characterization of modified antisense oligonucleotides in Xenopus laevis embryos. Oligonucleotides. 2006;16:26–42. doi: 10.1089/oli.2006.16.26. [DOI] [PubMed] [Google Scholar]
- 221.Davis S., Lollo B., Freier S., Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Esau C., Kang X., Peralta E., Hanson E., Marcusson E.G., Ravichandran L.V., Sun Y., Koo S., Perera R.J., Jain R., et al. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004;279:52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 223.Chan J.A., Krichevsky A.M., Kosik K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65:6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 224.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 225.Elmén J., Lindow M., Silahtaroglu A., Bak M., Christensen M., Lind-Thomsen A., Hedtjärn M., Hansen J.B., Hansen H.F., Straarup E.M., et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res. 2008;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Elmén J., Lindow M., Schütz S., Lawrence M., Petri A., Obad S., Lindholm M., Hedtjärn M., Hansen H.F., Berger U., et al. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452:896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 227.Vermeulen A., Robertson B., Dalby A.B., Marshall W.S., Karpilow J., Leake D., Khvorova A., Baskerville S. Double-stranded regions are essential design components of potent inhibitors of RISC function. RNA. 2007;13:723–730. doi: 10.1261/rna.448107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Krützfeldt J., Rajewsky N., Braich R., Rajeev K.G., Tuschl T., Manoharan M., Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 229.Murdaca G., Tonacci A., Negrini S., Greco M., Borro M., Puppo F., Gangemi S. Effects of AntagomiRs on Different Lung Diseases in Human, Cellular, and Animal Models. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20163938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Innao V., Allegra A., Pulvirenti N., Allegra A.G., Musolino C. Therapeutic potential of antagomiRs in haematological and oncological neoplasms. Eur. J. Cancer Care. 2020;29 doi: 10.1111/ecc.13208. [DOI] [PubMed] [Google Scholar]
- 231.Alhamadani F., Zhang K., Parikh R., Wu H., Rasmussen T.P., Bahal R., Zhong X.B., Manautou J.E. Adverse Drug Reactions and Toxicity of the Food and Drug Administration-Approved Antisense Oligonucleotide Drugs. Drug Metab. Dispos. 2022;50:879–887. doi: 10.1124/dmd.121.000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hirabayashi Y., Maki K., Kinoshita K., Nakazawa T., Obika S., Naota M., Watanabe K., Suzuki M., Arato T., Fujisaka A., et al. Considerations of the Japanese Research Working Group for the ICH S6 & Related Issues Regarding Nonclinical Safety Assessments of Oligonucleotide Therapeutics: Comparison with Those of Biopharmaceuticals. Nucleic Acid Ther. 2021;31:114–125. doi: 10.1089/nat.2020.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Goyenvalle A., Jimenez-Mallebrera C., van Roon W., Sewing S., Krieg A.M., Arechavala-Gomeza V., Andersson P. Considerations in the Preclinical Assessment of the Safety of Antisense Oligonucleotides. Nucleic Acid Ther. 2023;33:1–16. doi: 10.1089/nat.2022.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lindow M., Vornlocher H.P., Riley D., Kornbrust D.J., Burchard J., Whiteley L.O., Kamens J., Thompson J.D., Nochur S., Younis H., et al. Assessing unintended hybridization-induced biological effects of oligonucleotides. Nat. Biotechnol. 2012;30:920–923. doi: 10.1038/nbt.2376. [DOI] [PubMed] [Google Scholar]
- 235.Kamola P.J., Kitson J.D.A., Turner G., Maratou K., Eriksson S., Panjwani A., Warnock L.C., Douillard Guilloux G.A., Moores K., Koppe E.L., et al. In silico and in vitro evaluation of exonic and intronic off-target effects form a critical element of therapeutic ASO gapmer optimization. Nucleic Acids Res. 2015;43:8638–8650. doi: 10.1093/nar/gkv857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hagedorn P.H., Hansen B.R., Koch T., Lindow M. Managing the sequence-specificity of antisense oligonucleotides in drug discovery. Nucleic Acids Res. 2017;45:2262–2282. doi: 10.1093/nar/gkx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Weiner G.J., Liu H.M., Wooldridge J.E., Dahle C.E., Krieg A.M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc. Natl. Acad. Sci. USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Agrawal S., Kandimalla E.R. Antisense and siRNA as agonists of Toll-like receptors. Nat. Biotechnol. 2004;22:1533–1537. doi: 10.1038/nbt1042. [DOI] [PubMed] [Google Scholar]
- 239.Henry S.P., Novotny W., Leeds J., Auletta C., Kornbrust D.J. Inhibition of coagulation by a phosphorothioate oligonucleotide. Antisense Nucleic Acid Drug Dev. 1997;7:503–510. doi: 10.1089/oli.1.1997.7.503. [DOI] [PubMed] [Google Scholar]
- 240.Swayze E.E., Siwkowski A.M., Wancewicz E.V., Migawa M.T., Wyrzykiewicz T.K., Hung G., Monia B.P., Bennett C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007;35:687–700. doi: 10.1093/nar/gkl1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Burel S.A., Hart C.E., Cauntay P., Hsiao J., Machemer T., Katz M., Watt A., Bui H.H., Younis H., Sabripour M., et al. Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res. 2016;44:2093–2109. doi: 10.1093/nar/gkv1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Springer A.D., Dowdy S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther. 2018;28:109–118. doi: 10.1089/nat.2018.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Bisgaier C.L., Siebenkas M.V., Williams K.J. Effects of apolipoproteins A-IV and A-I on the uptake of phospholipid liposomes by hepatocytes. J. Biol. Chem. 1989;264:862–866. [PubMed] [Google Scholar]
- 244.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R., et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Maurya S., Sarangi P., Jayandharan G.R. Safety of Adeno-associated virus-based vector-mediated gene therapy—impact of vector dose. Cancer Gene Ther. 2022;29:1305–1306. doi: 10.1038/s41417-021-00413-6. [DOI] [PubMed] [Google Scholar]
- 246.Gilbert A. Sweet on science. Nat. Med. 2008;14:608. doi: 10.1038/nm0608-608. [DOI] [PubMed] [Google Scholar]
- 247.Sarkar M., Liao J., Kabat E.A., Tanabe T., Ashwell G. The binding site of rabbit hepatic lectin. J. Biol. Chem. 1979;254:3170–3174. [PubMed] [Google Scholar]
- 248.Novogrodsky A., Ashwell G. Lymphocyte mitogenesis induced by a mammalian liver protein that specifically binds desialylated glycoproteins. Proc. Natl. Acad. Sci. USA. 1977;74:676–678. doi: 10.1073/pnas.74.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Van Lenten L., Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. Development of a quantitative inhibition assay. J. Biol. Chem. 1972;247:4633–4640. [PubMed] [Google Scholar]
- 250.Baenziger J.U., Fiete D. Galactose and N-acetylgalactosamine-specific endocytosis of glycopeptides by isolated rat hepatocytes. Cell. 1980;22:611–620. doi: 10.1016/0092-8674(80)90371-2. [DOI] [PubMed] [Google Scholar]
- 251.Lee Y.C., Townsend R.R., Hardy M.R., Lönngren J., Arnarp J., Haraldsson M., Lönn H. Binding of synthetic oligosaccharides to the hepatic Gal/GalNAc lectin. Dependence on fine structural features. J. Biol. Chem. 1983;258:199–202. [PubMed] [Google Scholar]
- 252.Lee Y.C., Lee R.T. Carbohydrates in Chemistry and Biology. 2000. Interactions of Oligosaccharides and Glycopeptides with Hepatic Carbohydrate Receptors; pp. 549–561. [Google Scholar]
- 253.Harford J., Bridges K., Ashwell G., Klausner R.D. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. A pH-mediated nonlysosomal event. J. Biol. Chem. 1983;258:3191–3197. [PubMed] [Google Scholar]
- 254.Wall D.A., Wilson G., Hubbard A.L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980;21:79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]
- 255.Nie H., Qiu B., Yang Q.X., Zhao Y., Liu X.M., Zhang Y.T., Liao F.L., Zhang S.Y. Effect of gal/GalNAc regioisomerism in galactosylated liposomes on asialoglycoprotein receptor-mediated hepatocyte-selective targeting in vivo. J. Liposome Res. 2021;31:79–89. doi: 10.1080/08982104.2019.1682606. [DOI] [PubMed] [Google Scholar]
- 256.Geuze H.J., Slot J.W., Strous G.J., Lodish H.F., Schwartz A.L. Intracellular site of asialoglycoprotein receptor-ligand uncoupling: double-label immunoelectron microscopy during receptor-mediated endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 257.Bridges K., Harford J., Ashwell G., Klausner R.D. Fate of receptor and ligand during endocytosis of asialoglycoproteins by isolated hepatocytes. Proc. Natl. Acad. Sci. USA. 1982;79:350–354. doi: 10.1073/pnas.79.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Rogers J.C., Kornfeld S. Hepatic uptake of proteins coupled to fetuin glycopeptide. Biochem. Biophys. Res. Commun. 1971;45:622–629. doi: 10.1016/0006-291x(71)90462-1. [DOI] [PubMed] [Google Scholar]
- 259.Rensen P.C.N., van Leeuwen S.H., Sliedregt L.A.J.M., van Berkel T.J.C., Biessen E.A.L. Design and synthesis of novel N-acetylgalactosamine-terminated glycolipids for targeting of lipoproteins to the hepatic asialoglycoprotein receptor. J. Med. Chem. 2004;47:5798–5808. doi: 10.1021/jm049481d. [DOI] [PubMed] [Google Scholar]
- 260.Seymour L.W., Ferry D.R., Anderson D., Hesslewood S., Julyan P.J., Poyner R., Doran J., Young A.M., Burtles S., Kerr D.J., Cancer Research Campaign Phase I/II Clinical Trials committee Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002;20:1668–1676. doi: 10.1200/JCO.2002.20.6.1668. [DOI] [PubMed] [Google Scholar]
- 261.Wu G.Y., Wu C.H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- 262.Merwin J.R., Noell G.S., Thomas W.L., Chiou H.C., DeRome M.E., McKee T.D., Spitalny G.L., Findeis M.A. Targeted delivery of DNA using YEE(GalNAcAH)3, a synthetic glycopeptide ligand for the asialoglycoprotein receptor. Bioconjug. Chem. 1994;5:612–620. doi: 10.1021/bc00030a017. [DOI] [PubMed] [Google Scholar]
- 263.Hangeland J.J., Levis J.T., Lee Y.C., Ts'o P.O. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleoside methylphosphonates covalently linked with a neoglycopeptide, YEE(ah-GalNAc)3. Bioconjug. Chem. 1995;6:695–701. doi: 10.1021/bc00036a006. [DOI] [PubMed] [Google Scholar]
- 264.Biessen E.A., Vietsch H., Rump E.T., Fluiter K., Kuiper J., Bijsterbosch M.K., van Berkel T.J. Targeted delivery of oligodeoxynucleotides to parenchymal liver cells in vivo. Biochem. J. 1999;340:783–792. [PMC free article] [PubMed] [Google Scholar]
- 265.Matsuda S., Keiser K., Nair J.K., Charisse K., Manoharan R.M., Kretschmer P., Peng C.G., V Kel'in A., Kandasamy P., Willoughby J.L.S., et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 266.Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel'in A.V., Milstein S., et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 267.Prakash T.P., Graham M.J., Yu J., Carty R., Low A., Chappell A., Schmidt K., Zhao C., Aghajan M., Murray H.F., et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Garrelfs S.F., Frishberg Y., Hulton S.A., Koren M.J., O'Riordan W.D., Cochat P., Deschênes G., Shasha-Lavsky H., Saland J.M., Van't Hoff W.G., et al. Lumasiran, an RNAi Therapeutic for Primary Hyperoxaluria Type 1. N. Engl. J. Med. 2021;384:1216–1226. doi: 10.1056/NEJMoa2021712. [DOI] [PubMed] [Google Scholar]
- 269.Adams D., Tournev I.L., Taylor M.S., Coelho T., Planté-Bordeneuve V., Berk J.L., González-Duarte A., Gillmore J.D., Low S.C., Sekijima Y., et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30:1–9. doi: 10.1080/13506129.2022.2091985. [DOI] [PubMed] [Google Scholar]
- 270.Alexander V.J., Xia S., Hurh E., Hughes S.G., O'Dea L., Geary R.S., Witztum J.L., Tsimikas S. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur. Heart J. 2019;40:2785–2796. doi: 10.1093/eurheartj/ehz209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Coelho T., Ando Y., Benson M.D., Berk J.L., Waddington-Cruz M., Dyck P.J., Gillmore J.D., Khella S.L., Litchy W.J., Obici L., et al. Design and Rationale of the Global Phase 3 NEURO-TTRansform Study of Antisense Oligonucleotide AKCEA-TTR-L(Rx) (ION-682884-CS3) in Hereditary Transthyretin-Mediated Amyloid Polyneuropathy. Neurol. Ther. 2021;10:375–389. doi: 10.1007/s40120-021-00235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wolfrum C., Shi S., Jayaprakash K.N., Jayaraman M., Wang G., Pandey R.K., Rajeev K.G., Nakayama T., Charrise K., Ndungo E.M., et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25:1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 273.Nishina K., Unno T., Uno Y., Kubodera T., Kanouchi T., Mizusawa H., Yokota T. Efficient In Vivo Delivery of siRNA to the Liver by Conjugation of α-Tocopherol. Mol. Ther. 2008;16:734–740. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 274.Lehto T., Ezzat K., Wood M.J.A., El Andaloussi S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016;106:172–182. doi: 10.1016/j.addr.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 275.McClorey G., Banerjee S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines. 2018;6 doi: 10.3390/biomedicines6020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.McNamara J.O., Andrechek E.R., Wang Y., Viles K.D., Rempel R.E., Gilboa E., Sullenger B.A., Giangrande P.H. Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 277.Song E., Zhu P., Lee S.-K., Chowdhury D., Kussman S., Dykxhoorn D.M., Feng Y., Palliser D., Weiner D.B., Shankar P., et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 278.Meenakshi Sundaram D.N., Plianwong S., Kc R., Ostergaard H., Uludağ H. In Vitro Cytotoxicity and Cytokine Production by Lipid-Substituted Low Molecular Weight Branched PEIs Used for Gene Delivery. Acta Biomater. 2022;148:279–297. doi: 10.1016/j.actbio.2022.06.030. [DOI] [PubMed] [Google Scholar]
- 279.Perche F., Clemençon R., Schulze K., Ebensen T., Guzmán C.A., Pichon C. Neutral Lipopolyplexes for In Vivo Delivery of Conventional and Replicative RNA Vaccine. Mol. Ther. Nucleic Acids. 2019;17:767–775. doi: 10.1016/j.omtn.2019.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang W., Feng S., Ye Z., Gao H., Lin J., Ouyang D. Prediction of lipid nanoparticles for mRNA vaccines by the machine learning algorithm. Acta Pharm. Sin. B. 2022;12:2950–2962. doi: 10.1016/j.apsb.2021.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Semple S.C., Klimuk S.K., Harasym T.O., Dos Santos N., Ansell S.M., Wong K.F., Maurer N., Stark H., Cullis P.R., Hope M.J., Scherrer P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta. 2001;1510:152–166. doi: 10.1016/s0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 282.Witzigmann D., Kulkarni J.A., Leung J., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv. Drug Deliv. Rev. 2020;159:344–363. doi: 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Belliveau N.M., Huft J., Lin P.J., Chen S., Leung A.K., Leaver T.J., Wild A.W., Lee J.B., Taylor R.J., Tam Y.K., et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids. 2012;1:e37. doi: 10.1038/mtna.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Shi B., Keough E., Matter A., Leander K., Young S., Carlini E., Sachs A.B., Tao W., Abrams M., Howell B., Sepp-Lorenzino L. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. J. Histochem. Cytochem. 2011;59:727–740. doi: 10.1369/0022155411410885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sato Y., Murase K., Kato J., Kobune M., Sato T., Kawano Y., Takimoto R., Takada K., Miyanishi K., Matsunaga T., et al. Resolution of liver cirrhosis using vitamin A-coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat. Biotechnol. 2008;26:431–442. doi: 10.1038/nbt1396. [DOI] [PubMed] [Google Scholar]
- 286.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X., Hope M.J., Madden T.D., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 287.Mui B.L., Tam Y.K., Jayaraman M., Ansell S.M., Du X., Tam Y.Y.C., Lin P.J., Chen S., Narayanannair J.K., Rajeev K.G., et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of siRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids. 2013;2 doi: 10.1038/mtna.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Lawitz E.J., Shevell D.E., Tirucherai G.S., Du S., Chen W., Kavita U., Coste A., Poordad F., Karsdal M., Nielsen M., et al. BMS-986263 in patients with advanced hepatic fibrosis: 36-week results from a randomized, placebo-controlled phase 2 trial. Hepatology. 2022;75:912–923. doi: 10.1002/hep.32181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhou J.E., Sun L., Liu L., Jia Y., Han Y., Shao J., Wang J., Wang Y., Yu L., Yan Z. Hepatic macrophage targeted siRNA lipid nanoparticles treat non-alcoholic steatohepatitis. J. Control. Release. 2022;343:175–186. doi: 10.1016/j.jconrel.2022.01.038. [DOI] [PubMed] [Google Scholar]
- 290.Barbier-Torres L., Fortner K.A., Iruzubieta P., Delgado T.C., Giddings E., Chen Y., Champagne D., Fernández-Ramos D., Mestre D., Gomez-Santos B., et al. Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat. Commun. 2020;11:3360. doi: 10.1038/s41467-020-16991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hu M., Wang Y., Liu Z., Yu Z., Guan K., Liu M., Wang M., Tan J., Huang L. Hepatic macrophages act as a central hub for relaxin-mediated alleviation of liver fibrosis. Nat. Nanotechnol. 2021;16:466–477. doi: 10.1038/s41565-020-00836-6. [DOI] [PubMed] [Google Scholar]
- 292.Blaese R.M., Culver K.W., Miller A.D., Carter C.S., Fleisher T., Clerici M., Shearer G., Chang L., Chiang Y., Tolstoshev P., et al. T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 293.Bulcha J.T., Wang Y., Ma H., Tai P.W.L., Gao G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021;6 doi: 10.1038/s41392-021-00487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zincarelli C., Soltys S., Rengo G., Rabinowitz J.E. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol. Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 296.Nakai H., Fuess S., Storm T.A., Muramatsu S.I., Nara Y., Kay M.A. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J. Virol. 2005;79:214–224. doi: 10.1128/JVI.79.1.214-224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Lee Y.H., Jang H.-J., Kim S., Choi S.S., Khim K.W., Eom H.-j., Hyun J., Shin K.J., Chae Y.C., Kim H., et al. Hepatic MIR20B promotes nonalcoholic fatty liver disease by suppressing PPARA. Elife. 2021;10 doi: 10.7554/eLife.70472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chella Krishnan K., Floyd R.R., Sabir S., Jayasekera D.W., Leon-Mimila P.V., Jones A.E., Cortez A.A., Shravah V., Péterfy M., Stiles L., et al. Liver Pyruvate Kinase Promotes NAFLD/NASH in Both Mice and Humans in a Sex-Specific Manner. Cell. Mol. Gastroenterol. Hepatol. 2021;11:389–406. doi: 10.1016/j.jcmgh.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Koh E.H., Yoon J.E., Ko M.S., Leem J., Yun J.Y., Hong C.H., Cho Y.K., Lee S.E., Jang J.E., Baek J.Y., et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut. 2021;70:1954–1964. doi: 10.1136/gutjnl-2020-322509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Trépo E., Romeo S., Zucman-Rossi J., Nahon P. PNPLA3 gene in liver diseases. J. Hepatol. 2016;65:399–412. doi: 10.1016/j.jhep.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 301.Huang Y., Cohen J.C., Hobbs H.H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J. Biol. Chem. 2011;286:37085–37093. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.BasuRay S., Wang Y., Smagris E., Cohen J.C., Hobbs H.H. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proc. Natl. Acad. Sci. USA. 2019;116:9521–9526. doi: 10.1073/pnas.1901974116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.BasuRay S., Smagris E., Cohen J.C., Hobbs H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology. 2017;66:1111–1124. doi: 10.1002/hep.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Banini B.A., Kumar D.P., Cazanave S., Seneshaw M., Mirshahi F., Santhekadur P.K., Wang L., Guan H.P., Oseini A.M., Alonso C., et al. Identification of a Metabolic, Transcriptomic, and Molecular Signature of Patatin-Like Phospholipase Domain Containing 3-Mediated Acceleration of Steatohepatitis. Hepatology. 2021;73:1290–1306. doi: 10.1002/hep.31609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lindén D., Ahnmark A., Pingitore P., Ciociola E., Ahlstedt I., Andréasson A.C., Sasidharan K., Madeyski-Bengtson K., Zurek M., Mancina R.M., et al. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Mol. Metab. 2019;22:49–61. doi: 10.1016/j.molmet.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ionis Pharmaceuticals ION839. 2023. https://www.ionispharma.com/medicines/ionis-az6-2-5-lrxazd2693/
- 307.Stone S.J., Myers H.M., Watkins S.M., Brown B.E., Feingold K.R., Elias P.M., Farese R.V., Jr. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 308.Choi C.S., Savage D.B., Kulkarni A., Yu X.X., Liu Z.X., Morino K., Kim S., Distefano A., Samuel V.T., Neschen S., et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 309.Yu X.X., Murray S.F., Pandey S.K., Booten S.L., Bao D., Song X.Z., Kelly S., Chen S., McKay R., Monia B.P., Bhanot S. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 310.Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K., Bhanot S., Monia B.P., Li Y.X., Diehl A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 311.Ionis Pharmaceuticals ION224. 2023. https://www.ionispharma.com/medicines/ion224/
- 312.Ionis Pharmaceuticals Ionis announces positive results from Phase 2 study of ION224, an investigational medicine demonstrating clinical efficacy in the treatment of NASH/MASH. 2024. https://ir.ionispharma.com/news-releases/news-release-details/ionis-announces-positive-results-phase-2-study-ion224
- 313.Nerstedt A., Cansby E., Andersson C.X., Laakso M., Stančáková A., Blüher M., Smith U., Mahlapuu M. Serine/threonine protein kinase 25 (STK25): a novel negative regulator of lipid and glucose metabolism in rodent and human skeletal muscle. Diabetologia. 2012;55:1797–1807. doi: 10.1007/s00125-012-2511-7. [DOI] [PubMed] [Google Scholar]
- 314.Amrutkar M., Cansby E., Nuñez-Durán E., Pirazzi C., Ståhlman M., Stenfeldt E., Smith U., Borén J., Mahlapuu M. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. FASEB J. 2015;29:1564–1576. doi: 10.1096/fj.14-264937. [DOI] [PubMed] [Google Scholar]
- 315.Amrutkar M., Chursa U., Kern M., Nuñez-Durán E., Ståhlman M., Sütt S., Borén J., Johansson B.R., Marschall H.U., Blüher M., Mahlapuu M. STK25 is a critical determinant in nonalcoholic steatohepatitis. FASEB J. 2016;30:3628–3643. doi: 10.1096/fj.201600562R. [DOI] [PubMed] [Google Scholar]
- 316.Cansby E., Nuñez-Durán E., Magnusson E., Amrutkar M., Booten S.L., Kulkarni N.M., Svensson L.T., Borén J., Marschall H.U., Aghajan M., Mahlapuu M. Targeted Delivery of Stk25 Antisense Oligonucleotides to Hepatocytes Protects Mice Against Nonalcoholic Fatty Liver Disease. Cell. Mol. Gastroenterol. Hepatol. 2019;7:597–618. doi: 10.1016/j.jcmgh.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Cansby E., Kulkarni N.M., Magnusson E., Kurhe Y., Amrutkar M., Nerstedt A., Ståhlman M., Sihlbom C., Marschall H.U., Borén J., et al. Protein kinase MST3 modulates lipid homeostasis in hepatocytes and correlates with nonalcoholic steatohepatitis in humans. FASEB J. 2019;33:9974–9989. doi: 10.1096/fj.201900356RR. [DOI] [PubMed] [Google Scholar]
- 318.Caputo M., Kurhe Y., Kumari S., Cansby E., Amrutkar M., Scandalis E., Booten S.L., Ståhlman M., Borén J., Marschall H.U., et al. Silencing of STE20-type kinase MST3 in mice with antisense oligonucleotide treatment ameliorates diet-induced nonalcoholic fatty liver disease. FASEB J. 2021;35 doi: 10.1096/fj.202002671RR. [DOI] [PubMed] [Google Scholar]
- 319.Fredriksson R., Lagerström M.C., Höglund P.J., Schiöth H.B. Novel human G protein-coupled receptors with long N-terminals containing GPS domains and Ser/Thr-rich regions. FEBS Lett. 2002;531:407–414. doi: 10.1016/s0014-5793(02)03574-3. [DOI] [PubMed] [Google Scholar]
- 320.Wu M., Lo T.H., Li L., Sun J., Deng C., Chan K.Y., Li X., Yeh S.T.Y., Lee J.T.H., Lui P.P.Y., et al. Amelioration of non-alcoholic fatty liver disease by targeting adhesion G protein-coupled receptor F1 (Adgrf1) Elife. 2023;12 doi: 10.7554/eLife.85131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rousselet E., Benjannet S., Hamelin J., Canuel M., Seidah N.G. The proprotein convertase PC7: unique zymogen activation and trafficking pathways. J. Biol. Chem. 2011;286:2728–2738. doi: 10.1074/jbc.M110.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Dongiovanni P., Meroni M., Baselli G., Mancina R.M., Ruscica M., Longo M., Rametta R., Cespiati A., Pelusi S., Ferri N., et al. PCSK7 gene variation bridges atherogenic dyslipidemia with hepatic inflammation in NAFLD patients. J. Lipid Res. 2019;60:1144–1153. doi: 10.1194/jlr.P090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sachan V., Le Dévéhat M., Roubtsova A., Essalmani R., Laurendeau J.F., Garçon D., Susan-Resiga D., Duval S., Mikaeeli S., Hamelin J., et al. PCSK7: A novel regulator of apolipoprotein B and a potential target against non-alcoholic fatty liver disease. Metabolism. 2024;150 doi: 10.1016/j.metabol.2023.155736. [DOI] [PubMed] [Google Scholar]
- 324.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Garcia-Martinez I., Santoro N., Chen Y., Hoque R., Ouyang X., Caprio S., Shlomchik M.J., Coffman R.L., Candia A., Mehal W.Z. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J. Clin. Invest. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.An P., Wei L.-L., Zhao S., Sverdlov D.Y., Vaid K.A., Miyamoto M., Kuramitsu K., Lai M., Popov Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020;11:2362. doi: 10.1038/s41467-020-16092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mridha A.R., Haczeyni F., Yeh M.M., Haigh W.G., Ioannou G.N., Barn V., Ajamieh H., Adams L., Hamdorf J.M., Teoh N.C., Farrell G.C. TLR9 is up-regulated in human and murine NASH: pivotal role in inflammatory recruitment and cell survival. Clin. Sci. 2017;131:2145–2159. doi: 10.1042/CS20160838. [DOI] [PubMed] [Google Scholar]
- 328.Miura K., Kodama Y., Inokuchi S., Schnabl B., Aoyama T., Ohnishi H., Olefsky J.M., Brenner D.A., Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Shepard, C., Shapiro, D., and Landau, S.B. (2020). TLR9 oligonucleotide antagonist AVO101 causes dramatic elevations in adiponectin, followed by weight loss and NASH resolution in an obese primate model. The Liver Meeting Digital Experience™. AASLD.
- 330.Artavanis-Tsakonas S., Rand M.D., Lake R.J. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 331.Zong Y., Stanger B.Z. Molecular mechanisms of bile duct development. Int. J. Biochem. Cell Biol. 2011;43:257–264. doi: 10.1016/j.biocel.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhu C., Kim K., Wang X., Bartolome A., Salomao M., Dongiovanni P., Meroni M., Graham M.J., Yates K.P., Diehl A.M., et al. Hepatocyte Notch activation induces liver fibrosis in nonalcoholic steatohepatitis. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aat0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Shah S., Lee S.F., Tabuchi K., Hao Y.H., Yu C., LaPlant Q., Ball H., Dann C.E., 3rd, Südhof T., Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 334.van Es J.H., van Gijn M.E., Riccio O., van den Born M., Vooijs M., Begthel H., Cozijnsen M., Robine S., Winton D.J., Radtke F., Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 335.Ramezani M., Zobeiry M., Abdolahi S., Hatami B., Zali M.R., Baghaei K. A crosstalk between epigenetic modulations and non-alcoholic fatty liver disease progression. Pathol. Res. Pract. 2023;251 doi: 10.1016/j.prp.2023.154809. [DOI] [PubMed] [Google Scholar]
- 336.Bhattacharjee R., Prabhakar N., Kumar L., Bhattacharjee A., Kar S., Malik S., Kumar D., Ruokolainen J., Negi A., Jha N.K., Kesari K.K. Crosstalk between long noncoding RNA and microRNA in Cancer. Cell. Oncol. 2023;46:885–908. doi: 10.1007/s13402-023-00806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Statello L., Guo C.J., Chen L.L., Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021;22:96–118. doi: 10.1038/s41580-020-00315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Pan Y., Xin W., Wei W., Tatenhorst L., Graf I., Popa-Wagner A., Gerner S.T., Huber S.E., Kilic E., Hermann D.M., et al. Knockdown of NEAT1 prevents post-stroke lipid droplet agglomeration in microglia by regulating autophagy. Cell. Mol. Life Sci. 2024;81:30. doi: 10.1007/s00018-023-05045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hu M.J., Long M., Dai R.J. Acetylation of H3K27 activated lncRNA NEAT1 and promoted hepatic lipid accumulation in non-alcoholic fatty liver disease via regulating miR-212-5p/GRIA3. Mol. Cell. Biochem. 2022;477:191–203. doi: 10.1007/s11010-021-04269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yu F., Jiang Z., Chen B., Dong P., Zheng J. NEAT1 accelerates the progression of liver fibrosis via regulation of microRNA-122 and Kruppel-like factor 6. J. Mol. Med. 2017;95:1191–1202. doi: 10.1007/s00109-017-1586-5. [DOI] [PubMed] [Google Scholar]
- 341.Jin S.S., Lin X.F., Zheng J.Z., Wang Q., Guan H.Q. lncRNA NEAT1 regulates fibrosis and inflammatory response induced by nonalcoholic fatty liver by regulating miR-506/GLI3. Eur. Cytokine Netw. 2019;30:98–106. doi: 10.1684/ecn.2019.0432. [DOI] [PubMed] [Google Scholar]
- 342.Chen X., Tan X.R., Li S.J., Zhang X.X. LncRNA NEAT1 promotes hepatic lipid accumulation via regulating miR-146a-5p/ROCK1 in nonalcoholic fatty liver disease. Life Sci. 2019;235 doi: 10.1016/j.lfs.2019.116829. [DOI] [PubMed] [Google Scholar]
- 343.Feng S.D., Yang J.H., Yao C.H., Yang S.S., Zhu Z.M., Wu D., Ling H.Y., Zhang L. Potential regulatory mechanisms of lncRNA in diabetes and its complications. Biochem. Cell Biol. 2017;95:361–367. doi: 10.1139/bcb-2016-0110. [DOI] [PubMed] [Google Scholar]
- 344.Abdulle L.E., Hao J.L., Pant O.P., Liu X.F., Zhou D.D., Gao Y., Suwal A., Lu C.W. MALAT1 as a Diagnostic and Therapeutic Target in Diabetes-Related Complications: A Promising Long-Noncoding RNA. Int. J. Med. Sci. 2019;16:548–555. doi: 10.7150/ijms.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Wang Y., Ding H., Guo C., Bao Q., Li D., Xiong Y. LncRNA Malat1 regulates iPSC-derived β-cell differentiation by targeting the miR-15b-5p/Ihh axis. Cell. Signal. 2024;113 doi: 10.1016/j.cellsig.2023.110975. [DOI] [PubMed] [Google Scholar]
- 346.Ämmälä C., Drury W.J., 3rd, Knerr L., Ahlstedt I., Stillemark-Billton P., Wennberg-Huldt C., Andersson E.M., Valeur E., Jansson-Löfmark R., Janzén D., et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aat3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Horiguchi Y., Araki M., Motojima K. 17beta-Hydroxysteroid dehydrogenase type 13 is a liver-specific lipid droplet-associated protein. Biochem. Biophys. Res. Commun. 2008;370:235–238. doi: 10.1016/j.bbrc.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 348.Su W., Wang Y., Jia X., Wu W., Li L., Tian X., Li S., Wang C., Xu H., Cao J., et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 2014;111:11437–11442. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Ma Y., Belyaeva O.V., Brown P.M., Fujita K., Valles K., Karki S., de Boer Y.S., Koh C., Chen Y., Du X., et al. 17-Beta Hydroxysteroid Dehydrogenase 13 Is a Hepatic Retinol Dehydrogenase Associated With Histological Features of Nonalcoholic Fatty Liver Disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Luukkonen P.K., Tukiainen T., Juuti A., Sammalkorpi H., Haridas P.A.N., Niemelä O., Arola J., Orho-Melander M., Hakkarainen A., Kovanen P.T., et al. Hydroxysteroid 17-β dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI insight. 2020;5 doi: 10.1172/jci.insight.132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P., Liu Y., Kozlitina J., Stender S., Wood G.C., et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Regeneron Pharmaceuticals Regeneron And Alnylam Report Promising Data From Ongoing Phase 1 Study Of Aln-Hsd In NASH Patients And Healthy Volunteers. 2022. https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-alnylam-report-promising-data-ongoing-phase-1
- 353.Mak L.Y., Gane E., Schwabe C., Yoon K.T., Heo J., Scott R., Lee J.H., Lee J.I., Kweon Y.O., Weltman M., et al. A phase I/II study of ARO-HSD, an RNA interference therapeutic, for the treatment of non-alcoholic steatohepatitis. J. Hepatol. 2023;78:684–692. doi: 10.1016/j.jhep.2022.11.025. [DOI] [PubMed] [Google Scholar]
- 354.Ionis Pharmaceuticals ION455. 2023. https://www.ionispharma.com/medicines/ion455/
- 355.Nagata K. Hsp47: a collagen-specific molecular chaperone. Trends Biochem. Sci. 1996;21:22–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- 356.Ito S., Nagata K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017;62:142–151. doi: 10.1016/j.semcdb.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 357.Masuda H., Fukumoto M., Hirayoshi K., Nagata K. Coexpression of the collagen-binding stress protein HSP47 gene and the alpha 1(I) and alpha 1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J. Clin. Invest. 1994;94:2481–2488. doi: 10.1172/JCI117617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Razzaque M.S., Hossain M.A., Kohno S., Taguchi T. Bleomycin-induced pulmonary fibrosis in rat is associated with increased expression of collagen-binding heat shock protein (HSP) 47. Virchows Arch. 1998;432:455–460. doi: 10.1007/s004280050191. [DOI] [PubMed] [Google Scholar]
- 359.Ishiwatari H., Sato Y., Murase K., Yoneda A., Fujita R., Nishita H., Birukawa N.K., Hayashi T., Sato T., Miyanishi K., et al. Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut. 2013;62:1328–1339. doi: 10.1136/gutjnl-2011-301746. [DOI] [PubMed] [Google Scholar]
- 360.Otsuka M., Shiratori M., Chiba H., Kuronuma K., Sato Y., Niitsu Y., Takahashi H. Treatment of pulmonary fibrosis with siRNA against a collagen-specific chaperone HSP47 in vitamin A-coupled liposomes. Exp. Lung Res. 2017;43:271–282. doi: 10.1080/01902148.2017.1354946. [DOI] [PubMed] [Google Scholar]
- 361.Ohigashi H., Hashimoto D., Hayase E., Takahashi S., Ara T., Yamakawa T., Sugita J., Onozawa M., Nakagawa M., Teshima T. Ocular instillation of vitamin A-coupled liposomes containing HSP47 siRNA ameliorates dry eye syndrome in chronic GVHD. Blood Adv. 2019;3:1003–1010. doi: 10.1182/bloodadvances.2018028431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Yamakawa T., Ohigashi H., Hashimoto D., Hayase E., Takahashi S., Miyazaki M., Minomi K., Onozawa M., Niitsu Y., Teshima T. Vitamin A-coupled liposomes containing siRNA against HSP47 ameliorate skin fibrosis in chronic graft-versus-host disease. Blood. 2018;131:1476–1485. doi: 10.1182/blood-2017-04-779934. [DOI] [PubMed] [Google Scholar]
- 363.Arrowhead Pharmaceuticals Arrowhead Announces JNJ-75220795 in Development for NASH. 2021. https://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-announces-jnj-75220795-development-nash
- 364.Hatle K.M., Gummadidala P., Navasa N., Bernardo E., Dodge J., Silverstrim B., Fortner K., Burg E., Suratt B.T., Hammer J., et al. MCJ/DnaJC15, an endogenous mitochondrial repressor of the respiratory chain that controls metabolic alterations. Mol. Cell. Biol. 2013;33:2302–2314. doi: 10.1128/MCB.00189-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Champagne D.P., Hatle K.M., Fortner K.A., D'Alessandro A., Thornton T.M., Yang R., Torralba D., Tomás-Cortázar J., Jun Y.W., Ahn K.H., et al. Fine-Tuning of CD8(+) T Cell Mitochondrial Metabolism by the Respiratory Chain Repressor MCJ Dictates Protection to Influenza Virus. Immunity. 2016;44:1299–1311. doi: 10.1016/j.immuni.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Barbier-Torres L., Iruzubieta P., Fernández-Ramos D., Delgado T.C., Taibo D., Guitiérrez-de-Juan V., Varela-Rey M., Azkargorta M., Navasa N., Fernández-Tussy P., et al. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen-induced liver injury. Nat. Commun. 2017;8 doi: 10.1038/s41467-017-01970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Lenaz G., Genova M.L. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int. J. Biochem. Cell Biol. 2009;41:1750–1772. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 368.Ma Y., Lee G., Heo S.-Y., Roh Y.-S. Oxidative Stress Is a Key Modulator in the Development of Nonalcoholic Fatty Liver Disease. Antioxidants. 2021;11 doi: 10.3390/antiox11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Robertson C.L., Srivastava J., Siddiq A., Gredler R., Emdad L., Rajasekaran D., Akiel M., Shen X.N., Guo C., Giashuddin S., et al. Genetic deletion of AEG-1 prevents hepatocarcinogenesis. Cancer Res. 2014;74:6184–6193. doi: 10.1158/0008-5472.CAN-14-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Srivastava J., Robertson C.L., Ebeid K., Dozmorov M., Rajasekaran D., Mendoza R., Siddiq A., Akiel M.A., Jariwala N., Shen X.N., et al. A novel role of astrocyte elevated gene-1 (AEG-1) in regulating nonalcoholic steatohepatitis (NASH) Hepatology. 2017;66:466–480. doi: 10.1002/hep.29230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Rajasekaran D., Srivastava J., Ebeid K., Gredler R., Akiel M., Jariwala N., Robertson C.L., Shen X.N., Siddiq A., Fisher P.B., et al. Combination of Nanoparticle-Delivered siRNA for Astrocyte Elevated Gene-1 (AEG-1) and All-trans Retinoic Acid (ATRA): An Effective Therapeutic Strategy for Hepatocellular Carcinoma (HCC) Bioconjug. Chem. 2015;26:1651–1661. doi: 10.1021/acs.bioconjchem.5b00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Kanai F., Marignani P.A., Sarbassova D., Yagi R., Hall R.A., Donowitz M., Hisaminato A., Fujiwara T., Ito Y., Cantley L.C., Yaffe M.B. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Lei Q.Y., Zhang H., Zhao B., Zha Z.Y., Bai F., Pei X.H., Zhao S., Xiong Y., Guan K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Wang X., Zheng Z., Caviglia J.M., Corey K.E., Herfel T.M., Cai B., Masia R., Chung R.T., Lefkowitch J.H., Schwabe R.F., Tabas I. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016;24:848–862. doi: 10.1016/j.cmet.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wang X., Sommerfeld M.R., Jahn-Hofmann K., Cai B., Filliol A., Remotti H.E., Schwabe R.F., Kannt A., Tabas I. A Therapeutic Silencing RNA Targeting Hepatocyte TAZ Prevents and Reverses Fibrosis in Nonalcoholic Steatohepatitis in Mice. Hepatol. Commun. 2019;3:1221–1234. doi: 10.1002/hep4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Schuster S., Cabrera D., Arrese M., Feldstein A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018;15:349–364. doi: 10.1038/s41575-018-0009-6. [DOI] [PubMed] [Google Scholar]
- 377.Huebener P., Pradere J.-P., Hernandez C., Gwak G.-Y., Caviglia J.M., Mu X., Loike J.D., Schwabe R.F., Antoine D.J., Schwabe R.F. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J. Clin. Invest. 2015;125:539–550. doi: 10.1172/JCI76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Arriazu E., Ge X., Leung T.M., Magdaleno F., Lopategi A., Lu Y., Kitamura N., Urtasun R., Theise N., Antoine D.J., Nieto N. Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. 2017;66:1123–1137. doi: 10.1136/gutjnl-2015-310752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ganz M., Bukong T.N., Csak T., Saha B., Park J.K., Ambade A., Kodys K., Szabo G. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J. Transl. Med. 2015;13:193. doi: 10.1186/s12967-015-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Alisi A., Nobili V., Ceccarelli S., Panera N., De Stefanis C., De Vito R., Vitali R., Bedogni G., Balsano C., Cucchiara S., Stronati L. Plasma high mobility group box 1 protein reflects fibrosis in pediatric nonalcoholic fatty liver disease. Expert Rev. Mol. Diagn. 2014;14:763–771. doi: 10.1586/14737159.2014.928205. [DOI] [PubMed] [Google Scholar]
- 381.Zeng W., Shan W., Gao L., Gao D., Hu Y., Wang G., Zhang N., Li Z., Tian X., Xu W., et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci. Rep. 2015;5 doi: 10.1038/srep16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Melgert B.N., Olinga P., Van Der Laan J.M., Weert B., Cho J., Schuppan D., Groothuis G.M., Meijer D.K., Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719–728. doi: 10.1053/jhep.2001.27805. [DOI] [PubMed] [Google Scholar]
- 383.Chang C.I., Yoo J.W., Hong S.W., Lee S.E., Kang H.S., Sun X., Rogoff H.A., Ban C., Kim S., Li C.J., Lee D.K. Asymmetric shorter-duplex siRNA structures trigger efficient gene silencing with reduced nonspecific effects. Mol. Ther. 2009;17:725–732. doi: 10.1038/mt.2008.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.OliX Pharmaceuticals OliX Pharmaceuticals Announces Results from Preclinical Study of NASH Therapeutic Candidate. 2021. https://www.olixpharma.com/eng/pr/news.php?ptype=view&idx=237&page=1&code=news_eng&searchopt=content&searchkey=702A
- 385.OliX Pharmaceuticals OliX Announces Positive Preclinical Data in NASH Non-human Primate Models. 2022. https://www.olixpharma.com/eng/pr/news.php?ptype=view&idx=360&page=1&code=news_eng&searchopt=content&searchkey=702A
- 386.Shen X., Guo H., Xu J., Wang J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell. Physiol. 2019;234:18169–18179. doi: 10.1002/jcp.28450. [DOI] [PubMed] [Google Scholar]
- 387.Liu S., Zou L., Xie J., Xie W., Wen S., Xie Q., Gao Y., Li G., Zhang C., Xu C., et al. LncRNA NONRATT021972 siRNA regulates neuropathic pain behaviors in type 2 diabetic rats through the P2X7 receptor in dorsal root ganglia. Mol. Brain. 2016;9:44. doi: 10.1186/s13041-016-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Peng H., Zou L., Xie J., Wu H., Wu B., Zhu G., Lv Q., Zhang X., Liu S., Li G., et al. lncRNA NONRATT021972 siRNA Decreases Diabetic Neuropathic Pain Mediated by the P2X(3) Receptor in Dorsal Root Ganglia. Mol. Neurobiol. 2017;54:511–523. doi: 10.1007/s12035-015-9632-1. [DOI] [PubMed] [Google Scholar]
- 389.Song M., Zou L., Peng L., Liu S., Wu B., Yi Z., Gao Y., Zhang C., Xu H., Xu Y., et al. LncRNA NONRATT021972 siRNA normalized the dysfunction of hepatic glucokinase through AKT signaling in T2DM rats. Endocr. Res. 2017;42:180–190. doi: 10.1080/07435800.2017.1292522. [DOI] [PubMed] [Google Scholar]
- 390.Hanin G., Yayon N., Tzur Y., Haviv R., Bennett E.R., Udi S., Krishnamoorthy Y.R., Kotsiliti E., Zangen R., Efron B., et al. miRNA-132 induces hepatic steatosis and hyperlipidaemia by synergistic multitarget suppression. Gut. 2018;67:1124–1134. doi: 10.1136/gutjnl-2016-312869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Strum J.C., Johnson J.H., Ward J., Xie H., Feild J., Hester A., Alford A., Waters K.M. MicroRNA 132 regulates nutritional stress-induced chemokine production through repression of SirT1. Mol. Endocrinol. 2009;23:1876–1884. doi: 10.1210/me.2009-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Yang T., Fu M., Pestell R., Sauve A.A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006;17:186–191. doi: 10.1016/j.tem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 393.Colak Y., Ozturk O., Senates E., Tuncer I., Yorulmaz E., Adali G., Doganay L., Enc F.Y. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med. Sci. Monit. 2011;17 doi: 10.12659/MSM.881749. Hy5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Papazyan R., Kinberger G., Wang D., Lang G., Gogas K., Wright T., Zhu S. LBP-40-Development of Oligonucleotide-Based miR-132 Antagonists for the Treatment of NASH. J. Hepatol. 2019;70:e160–e161. [Google Scholar]
- 395.Mourelatos Z., Dostie J., Paushkin S., Sharma A., Charroux B., Abel L., Rappsilber J., Mann M., Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wilfred B.R., Wang W.X., Nelson P.T. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol. Genet. Metab. 2007;91:209–217. doi: 10.1016/j.ymgme.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Li S., Chen X., Zhang H., Liang X., Xiang Y., Yu C., Zen K., Li Y., Zhang C.Y. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J. Lipid Res. 2009;50:1756–1765. doi: 10.1194/jlr.M800509-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Trajkovski M., Hausser J., Soutschek J., Bhat B., Akin A., Zavolan M., Heim M.H., Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 399.Nystrom F.H., Chen H., Cong L.-N., Li Y., Quon M.J. Caveolin-1 Interacts with the Insulin Receptor and Can Differentially Modulate Insulin Signaling in Transfected Cos-7 Cells and Rat Adipose Cells. Mol. Endocrinol. 1999;13:2013–2024. doi: 10.1210/mend.13.12.0392. [DOI] [PubMed] [Google Scholar]
- 400.Otsu K., Toya Y., Oshikawa J., Kurotani R., Yazawa T., Sato M., Yokoyama U., Umemura S., Minamisawa S., Okumura S., Ishikawa Y. Caveolin gene transfer improves glucose metabolism in diabetic mice. Am. J. Physiol. Cell Physiol. 2010;298:C450–C456. doi: 10.1152/ajpcell.00077.2009. [DOI] [PubMed] [Google Scholar]
- 401.Regulus Therapeutics RG-125 (AZD4076), a microRNA therapeutic targeting microRNA 103/107 for the treatment of NASH in patients with type 2 diabetes/Pre-Diabetes, selected as clinical candidate by AstraZeneca. 2015. https://ir.regulusrx.com/2015-04-07-RG-125-AZD4076-,-a-microRNA-Therapeutic-Targeting-microRNA-103-107-for-the-Treatment-of-NASH-in-Patients-with-Type-2-Diabetes-Pre-Diabetes,-Selected-as-Clinical-Candidate-by-AstraZeneca
- 402.Yang F., Hu Y., Liu H.X., Wan Y.J.Y. MiR-22-silenced cyclin A expression in colon and liver cancer cells is regulated by bile acid receptor. J. Biol. Chem. 2015;290:6507–6515. doi: 10.1074/jbc.M114.620369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.López-Riera M., Conde I., Tolosa L., Zaragoza Á., Castell J.V., Gómez-Lechón M.J., Jover R. New microRNA Biomarkers for Drug-Induced Steatosis and Their Potential to Predict the Contribution of Drugs to Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2017;8:3. doi: 10.3389/fphar.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Hu Y., Liu H.X., Jena P.K., Sheng L., Ali M.R., Wan Y.J.Y. miR-22 inhibition reduces hepatic steatosis via FGF21 and FGFR1 induction. JHEP Rep. 2020;2 doi: 10.1016/j.jhepr.2020.100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yang Z., Qin W., Huo J., Zhuo Q., Wang J., Wang L. MiR-22 modulates the expression of lipogenesis-related genes and promotes hepatic steatosis in vitro. FEBS open bio. 2021;11:322–332. doi: 10.1002/2211-5463.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Thibonnier M., Esau C., Ghosh S., Wargent E., Stocker C. Metabolic and energetic benefits of microRNA-22 inhibition. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Panella R., Zanderigo F., Morandini F., Federico D., Vicentini E., Andreetta F., Toniolo A., Kauppinen S. Assessment of immunostimulatory responses to the antimiR-22 oligonucleotide compound RES-010 in human peripheral blood mononuclear cells. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1125654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Resalis Therapeutics Mechanism of Action. 2023. https://www.resalistherapeutics.com/science/mechanism-of-action/
- 409.Rayner K.J., Suárez Y., Dávalos A., Parathath S., Fitzgerald M.L., Tamehiro N., Fisher E.A., Moore K.J., Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Price N.L., Zhang X., Fernández-Tussy P., Singh A.K., Burnap S.A., Rotllan N., Goedeke L., Sun J., Canfrán-Duque A., Aryal B., et al. Loss of hepatic miR-33 improves metabolic homeostasis and liver function without altering body weight or atherosclerosis. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2006478118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Yamasaki T., Horie T., Koyama S., Nakao T., Baba O., Kimura M., Sowa N., Sakamoto K., Yamazaki K., Obika S., et al. Inhibition of microRNA-33b specifically ameliorates abdominal aortic aneurysm formation via suppression of inflammatory pathways. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-16017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Chen G., Liang G., Ou J., Goldstein J.L., Brown M.S. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl. Acad. Sci. USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Miyagawa S., Horie T., Nishino T., Koyama S., Watanabe T., Baba O., Yamasaki T., Sowa N., Otani C., Matsushita K., et al. Inhibition of microRNA-33b in humanized mice ameliorates nonalcoholic steatohepatitis. Life Sci. Alliance. 2023;6 doi: 10.26508/lsa.202301902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Celikbilek M., Baskol M., Taheri S., Deniz K., Dogan S., Zararsiz G., Gursoy S., Guven K., Ozbakır O., Dundar M., Yucesoy M. Circulating microRNAs in patients with non-alcoholic fatty liver disease. World J. Hepatol. 2014;6:613–620. doi: 10.4254/wjh.v6.i8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Zhao X., Chen Z., Zhou Z., Li Y., Wang Y., Zhou Z., Lu H., Sun C., Chu X. High-throughput sequencing of small RNAs and analysis of differentially expressed microRNAs associated with high-fat diet-induced hepatic insulin resistance in mice. Genes Nutr. 2019;14:6. doi: 10.1186/s12263-019-0630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Mathison A., Escande C., Calvo E., Seo S., White T., Salmonson A., Faubion W.A., Jr., Buttar N., Iovanna J., Lomberk G., et al. Phenotypic Characterization of Mice Carrying Homozygous Deletion of KLF11, a Gene in Which Mutations Cause Human Neonatal and MODY VII Diabetes. Endocrinology. 2015;156:3581–3595. doi: 10.1210/en.2015-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Singh R., Ha S.E., Wei L., Jin B., Zogg H., Poudrier S.M., Jorgensen B.G., Park C., Ronkon C.F., Bartlett A., et al. miR-10b-5p Rescues Diabetes and Gastrointestinal Dysmotility. Gastroenterology. 2021;160:1662–1678.e18. doi: 10.1053/j.gastro.2020.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.RosVivo Therapeutics Pipelines in Development. 2023. https://rosvivo.com/pipelines/
- 419.Arrowhead Pharmaceuticals Platforms that Accelerate Drug Discovery. 2023. https://arrowheadpharma.com/science/
- 420.Yahara A., Shrestha A.R., Yamamoto T., Hari Y., Osawa T., Yamaguchi M., Nishida M., Kodama T., Obika S. Amido-bridged nucleic acids (AmNAs): synthesis, duplex stability, nuclease resistance, and in vitro antisense potency. Chembiochem. 2012;13:2513–2516. doi: 10.1002/cbic.201200506. [DOI] [PubMed] [Google Scholar]