Abstract
Expression of human T-cell leukemia virus type 1 (HTLV-1) is regulated both by the HTLV-1 Tax transactivator and by cellular transcriptional factors binding to the viral long terminal repeat (LTR), suggesting that cellular signals may play a role in regulating viral expression. Treatment of cells chronically infected with HTLV-1, which express low levels of HTLV-1 RNAs and Tax protein, with phorbol esters (i.e., phorbol12-myristate 13- acetate [PMA]), phytohemagglutinin (PHA), sodium butyrate, or combinations of cytokines resulted in induction of HTLV- 1 gene expression. PMA or PHA treatment following cotransfection of HTLV-1 Tax expression plasmids resulted in synergistic activation of HTLV-1 LTR-directed gene expression, apparently involving tyrosine ki- nase- mediated pathways. These results suggest that cellular activation stimuli may cooperate with HTLV-1 Tax to enhance expression of integrated HTLV-1 genomes and thus may play a role in the pathogenesis of HTLV-1 disease.
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia/lymphoma (ATL) (45, 63) and is also associated with a chronic, degenerative neurologic disease known as HTLV-1-associated myelopathy or tropical spastic paraparesis (HAM/TSP) (22, 41), as well as inflammatory arthritis, polymyositis, uveitis, and Sjögren’s syndrome. The development of these diseases is associated with long periods of clinical latency following HTLV-1 infection, prior to onset of symptons. The pathogenic mechanisms by which HTLV- 1 infection results in these diverse clinical syndromes are still not clear, and only a small percentage of HTLV-1-infected individuals actually develop HTLV-1-related disease processes (for reviews, see references 19 and 58). An important common denominator in the pathogenesis of these diverse syndromes appears to be induction of T-cell proliferation as a result of expression of the HTLV-1 Tax transactivator (for reviews, see references 55 and 64). HTLV-1 gene expression in vivo likely plays an important role in the steps leading to disease induction; however, it is still unclear how viral replication or expression of virally encoded proteins directly contributes to the development of HTLV-1-associated diseases. In HTLV-1-infected, asymptomatic individuals, easily detectable levels of circulating infected cells are seen; however, a further marked increase in the number of circulating infected cells is observed in HAM/TSP patients (12, 23, 30), with levels reported to be as high as one-fifth of the peripheral blood mononuclear cells or randomly cloned T cells (48). These high levels of viral infection are likely due to expansion of infected T cells under the influence of the viral Tax protein (59). These observations suggest that there may exist stimuli which modulate HTLV-1 gene expression in vivo and contribute to HTLV-1 pathogenesis. Furthermore, in situ PCR experiments showed that only approximately 10% of HTLV-1-infected lymphocytes were expressing HTLV-1 RNA (40), and recent studies of HTLV-1-infected T cells cloned randomly from patients demonstrated that most cell clones obtained were not producing detectable HTLV-1 RNAs (48), also suggesting that there may be important cellular controls of viral gene expression.
Transcription of HTLV-1 is regulated both by cellular transcription factors that bind directly to long terminal repeat (LTR) DNA and by a virally encoded transactivator, Tax, which augments HTLV-1 LTR transcription through the Tax response elements (TREs) located in the DNA of the U3 region of the HTLV-1 LTR (9, 43, 54, 56). Tax complexes with the cyclic AMP response element binding proteins (CREB/ATF), affecting the dimerization and binding of Tax-CREB complexes to TRE DNA (5, 44, 57, 65). Binding of CREB proteins to the LTR recruits the transcriptional coactivator, CBP, to the promoter to enhance transcription (31) and allows direct interactions of Tax with the basal transcriptional apparatus (10, 16). Full Tax-mediated activation may also involve other factors that associate with the HTLV LTR (33), such as the Ets proteins (24). Cellular transcriptional factors, such as Ets, Myb, AP1, and AP2, also can bind directly to the HTLV-1 LTR and activate HTLV-1 transcription (8, 15, 24, 29, 37).
Although binding sites for several inducible transcription factors have been mapped in the HTLV-1 LTR, and the expression of these transcription factors has been shown to transactivate the HTLV-1 LTR in vitro, the biological importance of these activation pathways remains to be determined. Furthermore, relatively little is known about activation of integrated HTLV-1 proviral gene expression in HTLV-1-infected cells. Increased expression of HTLV-1 Tax mRNA has been documented following culture of peripheral blood mononuclear cells from HAM/TSP patients and from the skin of ATL patients (52, 53), suggesting the possibility that HTLV-1 gene expression can be induced in vitro. It has also been reported that an HTLV-1 LTR-directed reporter gene transfected into HeLa cells can be activated when transfected cells are cocultured with T cells (such as Hut78 cells), suggesting that factors secreted by T cells may activate HTLV-1 gene expression (4). Recently, data demonstrating that induction of the cellular stress response may enhance HTLV-1 gene expression have been presented (2, 3). Thus, different cellular signals could play a role in activating expression of HTLV-1 proviruses.
To further study cellular activation stimuli that may affect HTLV-1 gene expression, we have utilized a series of HTLV-1 chronically infected cell lines exhibiting differing levels of expression of HTLV-1 proteins (17, 32). HTLV-1-infected cell lines used in these studies included 1657 cells (derived from an ATL patient), 1996 cells (derived from an asymptomatic carrier), and FS and A212 cells (both derived from HAM/TSP patients) (17, 35). Cells that had been maintained in RPMI 1640 medium with 15% fetal bovine serum (GIBCO-BRL) and 10% recombinant human interleukin-2 (IL-2; Boehringer-Mannheim) were treated with different compounds known to induce gene expression in T cells, including phytohemagglutinin-P (PHA) (5 μg/ml; Sigma), phorbol 12-myristate 13-acetate (PMA) (50 ng/ml; Sigma), and sodium butyrate (5 mM; Sigma), for 48 h and then examined for the expression of the HTLV-1 Tax protein by Western blot analysis as previously described (32). HTLV-1-transformed Hut102 cells, derived from an ATL patient (20), were grown in RPMI 1640 medium with 10% fetal bovine serum and were analyzed for induction by the same protocol. Basal tax expression was detectable in unstimulated Hut102, 1996, and 1657 cells; however, only very low to undetectable levels of Tax were observed in untreated FS and A212 cells (Fig. 1A, lanes 1). Sodium butyrate, a differentiating agent that enhances cellular gene expression by altering the chromatin structure and causing histone acetylation (49), strongly induced Tax protein expression in all cell lines compared to levels in nontreated cells (Fig. 1A, compare lanes 1 and 4 for each cell line). PMA, a phorbol ester that cooperates in T-cell activation through the protein kinase C (PKC) pathway and has been previously reported to activate the HTLV-1 LTR (47), induced tax expression in Hut102, FS, 1996, and 1657 cells but failed to induce expression of this gene in A212 cells (Fig. 1A, lanes 3). Treatment with PHA, a plant lectin that acts as a T-cell mitogen, activating resting T cells through the CD3 molecule on the T-cell membrane (61), strongly increased tax expression in FS cells but resulted in only low-level tax induction in 1996 cells and caused no apparent induction of expression of this gene in Hut102, 1657, or A212 cells (Fig. 1A, lanes 2).
FIG. 1.
Expression of Tax protein in HTLV-1 chronically infected cell lines following treatment with various T-cell activation stimuli and cytokines. (A) Expression of Tax protein in HTLV-1 chronically infected cell lines following treatment with PHA, PMA, or sodium butyrate. Western blot analysis of whole-cell extracts from HTLV-1-infected patient cell lines (17) and HTLV-1-transformed Hut102 cells was performed with antiserum directed against the HTLV-1 Tax protein. FS, 1996, 1657, A212, and Hut102 cells either were left untreated (lanes 1) or were treated with PHA at 5 μg/ml (lanes 2), PMA at 50 ng/ml (lanes 3), or sodium butyrate at 5 mM (lanes 4) for 48 h, and whole-cell extracts were then prepared in radioimmunoprecipitation assay (RIPA) buffer. Bacterially synthesized Tax protein was used as a positive control. The arrows indicate the position of the Tax protein. (B) Expression of Tax protein in FS cells following treatment with various activation stimuli and cytokines. Western blot analyses of whole-cell extracts from HTLV-1-infected FS cells, either untreated or treated with a variety of stimuli, including various differentiation and activation compounds and cytokines, were performed with antiserum directed against the HTLV-1 Tax protein. FS cells were treated as indicated for 48 h, and whole-cell extracts were prepared in RIPA buffer. Bacterially expressed Tax protein (Tax) was used as a positive control. The arrow indicates the position of the Tax protein. The numbers on the right indicate the positions of molecular size markers (in kilodaltons). IFN, interferon; LPS, lipopolysaccharide; HMBA, hexamethylene bisacetamide; NaB, sodium butyrate; −, untreated.
Since FS cells demonstrated significantly increased HTLV-1 Tax protein expression over background levels in response to all three stimuli initially examined (Fig. 1A), we next examined whether cytokines, as physiologic inducers of different cellular responses, would also induce expression of this protein. FS cells were treated with an array of stimuli, such as PHA, PMA, sodium butyrate, hexamethylene bisacetamide (6 mM; Sigma), lipopolysaccharide (0.1 ng/ml; Sigma), and combinations of IL-1β (2 ng/ml; Boehringer-Mannheim), IL-6 (100 or 1,000 U/ml; Boehringer-Mannheim), tumor necrosis factor alpha (TNF-α) (100 U/ml; Boehringer-Mannheim), and gamma interferon (100 U/ml; Boehringer-Mannheim), for 48 h, and Tax protein expression was detected by Western blot analysis (Fig. 1B). In addition to PMA, PHA, and sodium butyrate, which strongly increased Tax protein expression (three- to fourfold, as determined by densitometric analysis of the Western blot), as shown in the previous experiment, certain cytokine combinations, such as IL-1β plus either IL-6 or TNF-α and gamma interferon plus TNF-α, also resulted in modest (twofold, as determined by densitometry) but detectable induction of Tax protein expression, suggesting that combinations of cytokines may also be able to weakly induce HTLV-1 gene expression from latent states of viral gene expression.
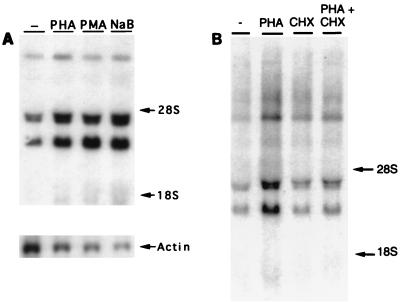
Northern blot analysis was performed to further characterize the activation of HTLV-1 gene expression in FS cells. Total cellular RNA (13) was prepared from FS cells treated with the activators 24 h prior to harvest and subjected to Northern blot hybridization (46), using a 32P-radiolabeled HTLV-1 LTR DNA fragment to detect levels of steady-state HTLV-1 RNA in untreated or stimulated cells (Fig. 2A). Untreated FS cells expressed easily detectable levels of full-length (8.4-kb) singly spliced env and doubly spliced tof-rof RNAs, as well as much lower levels of 1.6- to 2.0-kb tax-rex RNAs (14). Treatment of FS cells with PHA, PMA, or sodium butyrate resulted in readily observable increases in the levels of each of the different HTLV-1 RNA species (Fig. 2A), approximately three- to fourfold as quantitated by PhosphorImager analysis (Molecular Dynamics), including induction of the multiply spliced tax RNA at from very low to readily detectable levels. These data demonstrate that these stimuli increase the steady-state levels of HTLV-1 RNA, possibly through enhanced LTR transcription. Interestingly, addition of cycloheximide (100 μg/ml) to the PHA treatment regimen (Fig. 2B, lane 4) significantly reduced the activation of mRNA expression in FS cells compared to that resulting from PHA treatment alone (Fig. 2B, lane 2). These data suggest that new protein synthesis is required for PHA-induced activation of HTLV-1 RNA production.
FIG. 2.
Induction of HTLV-1 RNA expression in FS cells. (A) FS cells were treated with PHA (5 μg/ml), PMA (50 ng/ml), or sodium butyrate (NaB; 5 mM) for 24 h, and total RNA was prepared by the guanidinium isothiocyanate-acid phenol method (13). Fifteen micrograms of total cellular RNA was electrophoresed through denaturing agarose-formaldehyde gels, and Northern blot analysis was performed with a radiolabeled HTLV-1 LTR DNA fragment (a 500-bp XhoI-HindIII fragment from the pU3RCAT plasmid) as a probe. The positions of the 28S and 18S rRNAs are indicated. Hybridization to a mouse actin cDNA probe is shown at the bottom. (B) FS cells were treated with PHA (5 μg/ml), with or without the addition of cyclohexamide (CHX; 100 μg/ml), for 24 h, and total RNA was prepared. Northern blot analysis was performed as described for panel A. −, untreated.
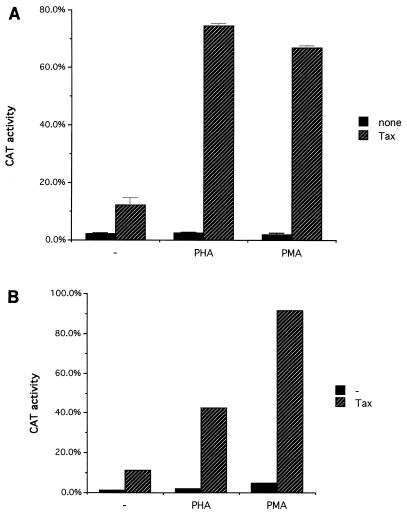
To clarify the possible mechanisms responsible for the increased HTLV-1 gene expression on treatment with T-cell-activating stimuli, transient transfections of T cells (Jurkat cells [Fig. 3A] and Hut78 cells [Fig. 3B]) with an HTLV-1 LTR-driven chloramphenicol acetyltransferase (CAT) reporter plasmid (pU3RCAT [56]) were performed by a DEAE-dextran procedure (18), and at 24 h posttransfection the cells were treated with 5 μg of PHA or 50 ng of PMA per ml. The cells were cultured for another 24 h before being harvested. Equal amounts (2 to 4 μg) of total cellular protein were used for the CAT activity assay (25). To examine possible cooperative effects, the transfections were performed with or without Tax protein coexpression (via cotransfection of pHTLV-1 Tax, a Tax expression vector directed by the HTLV-1 LTR [38]). In both cell lines, PHA or PMA treatment alone did not significantly affect HTLV-1 LTR-directed CAT gene expression (Fig. 3). Cotransfection of an HTLV-1 Tax-expressing plasmid with pU3RCAT resulted in an approximately 6- to 10-fold activation of CAT gene expression. PHA or PMA treatment in conjunction with Tax cotransfection resulted in a further 4- to 6-fold increase in HTLV-1 LTR-driven CAT activity compared with that resulting from Tax cotransfection alone (or 20- to 30-fold activation over that with LTR-CAT alone). Thus, LTR activation by PHA and PMA was Tax dependent and demonstrated synergistic stimulation with Tax transactivation. In these experiments, HTLV-1 Tax expression was directed by the HTLV-1 LTR. Therefore, one possible explanation for the observed results was that PHA or PMA treatment enhanced HTLV-1 LTR-directed Tax expression, thus further augmenting the expression of the HTLV-1 LTR-directed reporter gene through TRE elements in the LTR. Experiments utilizing cotransfection of a Tax expression vector directed by the cytomegalovirus immediate-early promoter also demonstrated synergistic activation of the LTR with PHA treatment (data not shown), suggesting that the effects seen in this experiment were not due simply to activation of the LTR-Tax plasmid but instead represented synergistic activation of HTLV-1 LTR activity by PHA or PMA, plus Tax.
FIG. 3.
Synergistic activation of the HTLV-1 LTR by simultaneous stimulation with Tax and either PMA or PHA. (A) The pU3RCAT plasmid (5 μg), containing the HTLV-1 LTR directing the expression of the CAT reporter gene, was transfected into Jurkat cells, with or without an HTLV-1 LTR-driven Tax-expressing vector (pHTLV-1 Tax; 1 μg); 24 h after transfection, the cells were treated with PHA (5 μg/ml) or PMA (50 ng/ml). CAT activities were assayed at 48 h after transfection. Average CAT activities from triplicate transfections are presented as percentages of chloramphenicol converted to the acetylated form, and the standard deviations are indicated by the error bars. (B) The pU3RCAT plasmid, containing HTLV-1 LTR-directed CAT reporter gene, was cotransfected into Hut78 cells, with or without pHTLV-1 Tax, the Tax expression plasmid. Stimuli were added 24 h after transfection. CAT activity was assayed 48 h after transfection.
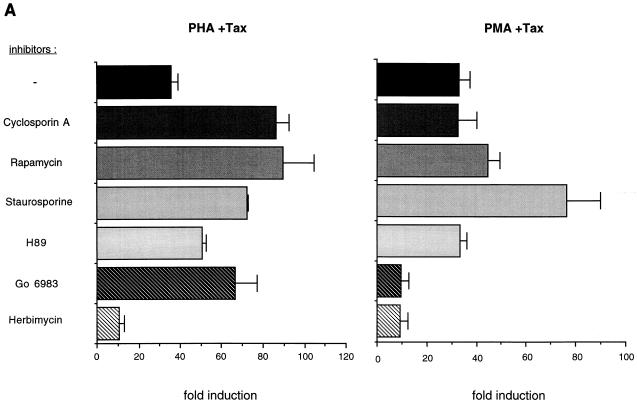
To identify signal transduction pathways that might mediate the activation of the HTLV-1 LTR by PHA-Tax and PMA-Tax, we transfected Jurkat T cells with an HTLV-1 LTR-luciferase reporter plasmid (constructed by inserting the XhoI-HindIII fragment containing the HTLV-1 LTR from pU3RCAT into the pGL3 luciferase expression vector [Promega]). Twenty-four hours following transfection, the transfected Jurkat cells were divided into aliquots and incubated with a series of signal transduction pathway inhibitors for 1 h prior to the addition of PHA or PMA. Cellular extracts were harvested and assayed for luciferase activity by the use of a luciferase assay system (Promega). The results are shown as fold induction of HTLV-1 luciferase activity over that observed after transfection of the HTLV-1 luciferase plasmid alone (Fig. 4A). The possible toxicities of these treatments were examined by quantifying viable cells by the MTT assay (36), and no significant decrease in cell viability was observed with any treatment compared to that in untreated transfected Jurkat cells (data not shown). The inhibitors used were as follows: the immunosuppressive compounds cyclosporin A (250 ng/ml; Sigma), which functions through the calcineurin pathway, and rapamycin (20 ng/ml; Calbiochem), which inhibits p70s6k, p34cdc2, and p33cdk2; the broad-specificity protein kinase inhibitor staurosporine (0.01 mM; Calbiochem); the protein kinase A-specific inhibitor H89 (1 mM; Calbiochem); the PKC-specific inhibitor Gö6983 (100 nM; Calbiochem); and the protein tyrosine kinase (PTK)-specific inhibitor herbimycin A (0.5 mg/ml; Sigma). Cyclosporin A, rapamycin, staurosporine, and H89 had no inhibitory effects on either PHA-Tax or PMA-Tax activation, suggesting the lack of involvement of the protein kinase A, calcineurin, and p70 S6 kinase-mediated pathways. As expected, Gö6983 inhibited the induction of HTLV-1 LTR by PMA-Tax with no toxicity, indicating the involvement of the PKC pathway in PMA activation of the HTLV-1 LTR (28). Gö6983 failed to inhibit PHA-Tax activation, however, suggesting that the involvement of PKC in PHA-Tax activation is unlikely. In contrast, herbimycin A, a PTK inhibitor, strongly reduced both PHA-Tax and PMA-Tax activation, suggesting the involvement of PTKs in the synergistic activation pathways induced by both PHA and PMA.
FIG. 4.
Role of signal transduction pathways in PHA-Tax and PMA-Tax activation of the HTLV-1 LTR. (A) Effects of signal transduction inhibitors on activation of the HTLV-1 LTR in transient transfections. The pHTLV-1 Luc plasmid (5 μg), containing the HTLV-1 LTR directing the expression of the luciferase reporter gene, was cotransfected into Jurkat cells with an HTLV-1 LTR-driven Tax-expressing vector (pHTLV-1 Tax; 1 μg); 24 h after transfection, the cells were treated with PHA (5 μg/ml) or PMA (50 ng/ml). The transfected cells were treated with inhibitors for 1 h prior to the addition of PMA or PHA. Inhibitors used were cyclosporin A (250 ng/ml), rapamycin (20 ng/ml), staurosporine (0.01 μM), H89 (1 μM), Gö6983 (100 nM), and herbimycin A (0.5 μg/ml). Cellular extracts were harvested and assayed for luciferase activity at 48 h after transfection. The results are presented as fold induction compared to the induction resulting from transfection of the pHTLV-1 Luc reporter plasmid alone, without treatment. Average fold inductions from triplicate transfections with standard deviations (error bars) are shown. (B) Effects of signal transduction inhibitors on activation of PHA- and PMA-induced Tax protein expression in HTLV-1 chronically infected FS cells. FS cells were treated with herbimycin A (0.5 μg/ml), Gö6983 (100 nM), H89 (1 μM), and staurosporine (0.01 μM) for 1 h prior to addition of PHA (5 μg/ml) or PMA (50 ng/ml). Western blot analyses of whole-cell extracts with antiserum directed against the HTLV-1 Tax protein were performed as described in the legend to Fig. 1. −, untreated.
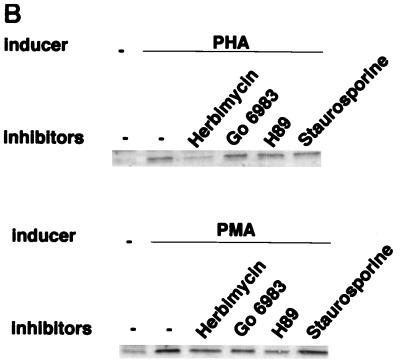
We further examined the potential role of signal transduction pathways in the activation of integrated HTLV-1 proviral gene expression. The effects of a series of kinase inhibitors on tax induction by PHA or PMA in HTLV-1 chronically infected FS cells were assessed. FS cells were treated with herbimycin A (0.5 mg/ml), Gö6983 (100 nM), H89 (1 mM), and staurosporine (0.01 mM) for 1 h prior to addition of PHA (5 mg/ml) or PMA (50 ng/ml). Cells were lysed 48 h after treatment, and the expression of Tax protein was detected by Western blot analysis (Fig. 4B). Consistent with the results observed in transient transfection studies, PHA-induced Tax expression was inhibited by herbimycin but not by Gö6983, H89, or staurosporine (Fig. 4B, top panel). Thus, PHA activation of the integrated HTLV-1 provirus in FS cells apparently also involves tyrosine kinase pathways. Somewhat surprisingly, the strong induction of Tax expression by PMA was not significantly inhibited by any kinase inhibitor tested (Fig. 4B, bottom panel), suggesting that the PMA induction pathways in FS cells may be distinct from those in transiently transfected Jurkat cells (Fig. 4A).
In these studies using HTLV-1 chronically infected cell lines, we identified cellular activation stimuli that induced expression of HTLV-1 RNA and Tax protein. These stimuli included both cellular activation and differentiation agents such as PHA, PMA, and sodium butyrate, which strongly induced HTLV-1 gene expression, as well as certain combinations of cytokines which resulted in more modest levels of HTLV-1 induction. Taken together, these results suggest that either direct immune stimulation of infected T cells or immune activation resulting in cytokine secretion could potentially activate HTLV-1 gene expression in infected cells and thus could play a role in HTLV-1 disease pathogenesis. In addition to the immune, differentiation, and cytokine activation pathways that we have described, the induction of cellular stress responses upon either heat stimulation or sodium arsenite treatment has also been shown to activate the expression of HTLV-1 genes from chronically infected cell lines (3). Induction of HTLV-1 expression by these agents was independent of Tax and was mediated by LTR DNA sequences containing the basal promoter without TREs (2) and thus appears to be mechanistically different from either the PMA or PHA stimulation that we have observed.
The mechanisms responsible for HTLV-1 gene activation by the different activating agents used in our studies are likely to be distinct. Sodium butyrate stimulates expression of many cellular and viral genes through the inhibition of histone deacetylases (50), resulting in hyperacetylation of histones, which has been shown to be associated with transcriptionally active chromatin (34). Interestingly, the CBP/p300 proteins associated with CREB/ATF and Tax (31) have been recently shown to be histone acetyltransferases (39); thus, histone deacetylation may represent a common pathway for activation of HTLV-1 gene expression, even by the HTLV-1 Tax protein and associated CREB/ATF complexes. Phorbol esters have been previously shown to activate the HTLV-1 LTR function in combination with HTLV-1 Tax (47) through a 60-bp element that includes the middle HTLV-1 LTR TRE. The TRE itself was not sufficient for tetradecanoyl phorbol acetate induction, suggesting that other cellular LTR-binding transcription factors, such as the phorbol ester-inducible AP1 (c-Jun) and AP2 transcription factors (27, 29, 37), may play a role. Our studies suggest that the PMA-mediated synergistic activation in Jurkat cells likely involves a PKC-mediated signal transduction pathway, although the pathway in FS cells may be somewhat different. Inhibition of the PKC pathway did not, however, block activation of the HTLV-1 LTR pathway by PHA and Tax, suggesting that to mediate HTLV-1 LTR activation, PMA and PHA function at least in part through different signal transduction pathways.
The T-cell mitogen PHA activated HTLV-1 gene expression in FS and 1996 cells but failed to induce HTLV-1 tax in A212 and 1657 cells. Many of the effects of PHA stimulation appear to parallel the effects of cross-linking of the CD3 molecule on the T-cell surface, and PHA activation requires an intact T-cell receptor complex (61); therefore, PHA activation of HTLV-1 gene expression may involve T-cell receptor components such as CD3. Fluorescence-activated cell sorter analysis of T-cell surface markers present on the different chronically infected cell lines demonstrated that CD3 expression was restricted to FS and 1996 cells, the two cell lines which exhibited induction of Tax protein expression following PHA treatment, and was not detectable on either A212 or 1657 cells (data not shown), raising the possibility that the effects of PHA on HTLV-1 gene expression may be mediated through CD3 signaling. Herbimycin A, an inhibitor of PTKs, inhibited the synergistic activation by PHA and Tax, raising the possibility that tyrosine kinases involved in T-cell activation, such as Lck, may play a role in the activation process. Herbimycin A also inhibited the synergistic activation induced by PMA and Tax, suggesting that a tyrosine kinase contributes to a downstream step in the synergistic activation of the HTLV-1 LTR, a step common to both the PHA-Tax and PMA-Tax activations of the LTR. Tyrosine kinases could regulate phosphorylation of components of the Tax-CREB/ATF-CBF complex or phosphorylation of downstream targets of this complex, such as Tafs or general transcription factors. It is not clear at this time which cellular transcription factors are required for the synergy with Tax in response to PHA treatment. Cellular Ets transcription factors are one family of transcription factors that may contribute to the activation by PHA and Tax, since transfection of Ets expression plasmids has been previously reported to induce HTLV-1 LTR expression, both alone and in combination with Tax (7, 24, 51); however, other factors, such as Myb or AP-2, could also play a role.
The pathogenesis of HTLV-1-associated disorders is complex, and only a subset of infected individuals progress to ATL, HAM/TSP, or an HTLV-1-associated autoimmune disorder. The time of viral infection apparently is one determinant of pathogenesis; however, it is likely that other host and viral factors also play a role. In particular, given the long latency periods associated with disease appearance, host factors such as the immune response as well as the intracellular regulation of viral gene expression may be important. The observation that immune system activation stimuli such as PHA and PMA as well as certain cytokines and cellular stress signals can induce enhanced HTLV-1 tax expression suggests that these stimuli may alter HTLV-1 pathogenesis in vivo. As is clear from our studies, different stimuli activate HTLV-1 gene expression to different degrees in different types of infected cells, possibly reflecting the effects of variable levels of cell surface receptors (such as for PHA) or of different integration sites in which cis-acting neighboring cellular DNA sequences might play a role in modulating the LTR autonomous response that we observed in our transfection studies. Despite the potential variability of such activation in vivo, the fact that PHA, PMA, and cytokine-induced stimulation of HTLV-1 gene expression could be readily identified in both transiently transfected as well as chronically infected cells suggests that this mechanism is likely to be operative in at least some of the large numbers of infected cells in vivo. The fact that HTLV-1-infected cells and particles have themselves been reported to induce immune system activation (21, 62) suggests that an autocrine stimulatory pathway, by which induction of HTLV-1 gene expression by immune system, cytokine, or stress activation could result in HTLV-1 production with further T-cell and viral activation, may be in operation.
For HTLV-1 infection, two different scenarios by which enhanced HTLV-1 gene expression may contribute to pathogenesis can be envisaged. In one model, increased HTLV-1 gene expression results in enhanced virus production and spread. This is similar to models proposed for human immunodeficiency virus (6, 42). Newly infected target cells then either become substrates for transformation into ATL cells or increase the likelihood of infection and proliferation of subsets of T cells, leading to the development of HAM/TSP. This model is unlikely in view of recent observations demonstrating that clonal expansion of HTLV-1-infected cells appears to account for increases in the HTLV-1 proviral load in vivo (11, 59, 60), strongly suggesting that extensive in vivo spread of HTLV-1 through multiple rounds of infection of new target cells may not be required for HTLV-1 pathogenesis (59). An alternative model suggests that the important effect of enhanced HTLV-1 gene expression is increased intracellular levels of the Tax protein. Tax protein expression is clearly associated with the induction of T-cell proliferation; retroviral and herpesvirus vectors expressing Tax can induce sustained proliferation of primary human T cells (1, 26), and the ability of randomly cloned HTLV-1-infected cells to proliferate in vitro is directly correlated with expression of Tax (48). Thus, an important effect of tax induction by immune or cytokine stimulation could be direct induction of proliferation of particular infected T cells rather than enhanced viral spread to uninfected cells. These cells activated for proliferation by induction of tax might contribute to the pool of cells from which ATL cells or, alternatively, cells responsible for the development of HAM/TSP could arise.
Acknowledgments
We thank T. Folks for helpful discussions and S. Marriott for purified Tax protein. Antiserum to HTLV-1 Tax was obtained through the NIH NIAID AIDS Research and Reference Reagent Program (contributed by K.-T. Jeang).
This work was funded by a Public Health Service research grant to A.B.R. from the National Cancer Institute (CA-68333) and by the New Jersey Commission on Science and Technology.
REFERENCES
- 1.Akagi T, Ono H, Nyunoya H, Shimotohno K. Characterization of peripheral blood T-lymphocytes transduced with HTLV-1 Tax mutants with different trans-activating phenotype. Oncogene. 1997;14:2071–2078. doi: 10.1038/sj.onc.1201045. [DOI] [PubMed] [Google Scholar]
- 2.Andrews J M, Newbound G C, Oglesbee M, Brady J N, Lairmore M D. The cellular stress response enhances human T-cell lymphotropic virus type 1 basal gene expression through the core promoter region of the long terminal repeat. J Virol. 1997;71:741–745. doi: 10.1128/jvi.71.1.741-745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews J M, Oglesbee M J, Trevino A V, Guyot D J, Newbound G C, Lairmore M D. Enhanced human T-cell lymphotropic virus type I expression following induction of the cellular stress response. Virology. 1995;208:816–820. doi: 10.1006/viro.1995.1218. [DOI] [PubMed] [Google Scholar]
- 4.Baier-Bitterlich G, Rappaport J, Baier G, Looney D, Wong-Staal F. Transcellular activation of the HTLV promoter by human hematopoietic cells. Oncogene. 1994;9:319–322. [PubMed] [Google Scholar]
- 5.Baranger A M, Palmer C R, Hamm M K, Glebler H A, Brauweiler A, Nyborg J K, Schepartz A. Mechanism of DNA-binding enhancement by the human T-cell leukemia virus transactivator Tax. Nature. 1995;376:606–608. doi: 10.1038/376606a0. [DOI] [PubMed] [Google Scholar]
- 6.Bednarik D P, Folks T M. Mechanisms of HIV-1 latency. AIDS. 1992;6:3–16. doi: 10.1097/00002030-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Bosselut R, Duvall J F, Gegonne A, Bailly M, Hemar A, Brady J, Ghysdael J. The product of the c-ets-1 proto-oncogene and the related Ets2 protein act as transcriptional activators of the long terminal repeat of human T cell leukemia virus HTLV-I. EMBO J. 1990;9:3137–3144. doi: 10.1002/j.1460-2075.1990.tb07511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosselut R, Lim F, Romand P C, Frampton J, Brady J, Ghysdael J. Myb protein binds to multiple sites in the human T cell lymphotropic virus type 1 long terminal repeat and transactivates LTR-mediated expression. Virology. 1992;186:764–769. doi: 10.1016/0042-6822(92)90044-p. [DOI] [PubMed] [Google Scholar]
- 9.Brady J, Jeang K-T, Duvall J, Khoury G. Identification of p40x-responsive regulatory sequences within the human T-cell leukemia virus type I long terminal repeat. J Virol. 1987;61:2175–2181. doi: 10.1128/jvi.61.7.2175-2181.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caron C, Rousset R, Beraud C, Moncollin V, Egly J M, Jalinot P. Functional and biochemical interaction of the HTLV-I Tax1 transactivator with TBP. EMBO J. 1993;12:4269–4278. doi: 10.1002/j.1460-2075.1993.tb06111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavrois M, Wain-Hobson S, Gessain A, Plumelle Y, Wattel E. Adult T-cell leukemia/lymphoma on a background of clonally expanding human T-cell leukemia virus type 1-positive cells. Blood. 1996;88:4646–4650. [PubMed] [Google Scholar]
- 12.Cho I, Sugimoto M, Mita S, Tokunaga M, Imamura F, Ando M. In vivo proviral burden and viral RNA expression in T cell subsets of patients with human T lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. Am J Trop Med Hyg. 1995;53:412–418. doi: 10.4269/ajtmh.1995.53.412. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Ciminale V, Pavlakis G N, Derse D, Cunningham C P, Felber B K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992;66:1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark N M, Smith M J, Hilfinger J M, Markovitz D M. Activation of the human T-cell leukemia virus type I enhancer is mediated by binding sites for Elf-1 and the pets factor. J Virol. 1993;67:5522–5528. doi: 10.1128/jvi.67.9.5522-5528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens K E, Piras G, Radonovich M F, Choi K S, Duvall J F, DeJong J, Roeder R, Brady J N. Interaction of the human T-cell lymphotropic virus type 1 Tax transactivator with transcription factor IIA. Mol Cell Biol. 1996;16:4656–4664. doi: 10.1128/mcb.16.9.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dezzutti C S, Rudolph D L, Roberts C R, Lal R B. Characterization of human T-lymphotropic virus type I- and II-infected T cell lines: antigenic, phenotypic, and genotypic analysis. Virus Res. 1993;29:59–70. doi: 10.1016/0168-1702(93)90125-7. [DOI] [PubMed] [Google Scholar]
- 18.Dorsett D L, Keshet I, Winocour E. Quantitation of a simian virus 40 nonhomologous recombination pathway. J Virol. 1983;48:218–228. doi: 10.1128/jvi.48.1.218-228.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franchini G. Molecular mechanisms of human T-cell leukemia/lymphotropic virus type 1 infection. Blood. 1995;86:3619–3639. [PubMed] [Google Scholar]
- 20.Gazdar A F, Carney D N, Bunn P A, Russell E K, Jaffe E S, Schechter G P, Guccion J G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980;55:409–417. [PubMed] [Google Scholar]
- 21.Gazzolo L, Duc Dodon M. Direct activation of resting lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- 22.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de Thé G. Antibodies to the human T lymphotropic virus type 1 in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 23.Gessain A, Saal F, Gout O, Daniel M-T, Flandrin G, de Thé G, Sigaux F. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood. 1990;75:428–433. [PubMed] [Google Scholar]
- 24.Gitlin S D, Dittmer J, Shin R C, Brady J N. Transcriptional activation of the human T-lymphotropic virus type I long terminal repeat by functional interaction of Tax1 and Ets1. J Virol. 1993;67:7307–7316. doi: 10.1128/jvi.67.12.7307-7316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type 1 X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imagawa M, Chiu R, Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathway: protein kinase C and cAMP. Cell. 1987;51:251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- 28.Jeang K-T, Boros I, Brady J, Radonovich M, Khoury G. Characterization of cellular factors that interact with the human T-cell leukemia virus type I p40x-responsive 21-base-pair sequence. J Virol. 1988;62:4499–4509. doi: 10.1128/jvi.62.12.4499-4509.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeang K-T, Chiu R, Santos E, Kim S J. Induction of the HTLV-1 LTR by Jun occurs through the Tax-responsive 21-bp elements. Virology. 1991;181:218–227. doi: 10.1016/0042-6822(91)90487-v. [DOI] [PubMed] [Google Scholar]
- 30.Kira J-i, Koyanagi Y, Yamada T, Itoyama Y, Goto I, Yamamoto N, Sasaki H, Sakaki Y. Increased HTLV-I proviral DNA in HTLV-1-associated myelopathy: a quantitative polymerase chain reaction study. Ann Neurol. 1991;29:194–201. doi: 10.1002/ana.410290214. [DOI] [PubMed] [Google Scholar]
- 31.Kwok R P, Laurance M E, Lundblad J R, Goldman P S, Shih H, Connor L M, Marriott S J, Goodman R H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. 1996;380:642–646. doi: 10.1038/380642a0. [DOI] [PubMed] [Google Scholar]
- 32.Lin H-C, Bodkin M, Lal R B, Rabson A B. Selective infection of human T-lymphotropic virus type 1 (HTLV-1)-infected cells by chimeric human immunodeficiency viruses containing HTLV-1 Tax response elements in the long terminal repeat. J Virol. 1995;69:7216–7225. doi: 10.1128/jvi.69.11.7216-7225.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marriott S J, Lindholm P F, Brown K M, Gitlin S D, Duvall J F, Radonovich M F, Brady J N. A 36-kilodalton cellular transcription factor mediates an indirect interaction of human T-cell leukemia/lymphoma virus type I TAX1 with a responsive element in the viral long terminal repeat. Mol Cell Biol. 1990;10:4192–4201. doi: 10.1128/mcb.10.8.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathis D J, Oudet P, Wasylyk B, Chambon P. Effect of histone acetylation on structure and in vitro transcription of chromatin. Nucleic Acids Res. 1978;5:3523–3547. doi: 10.1093/nar/5.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKendall R R, Oas J, Lairmore M D. HTLV-1-associated myelopathy endemic in Texas-born residents and isolation of virus from CSF cells. Neurology. 1991;41:831–836. doi: 10.1212/wnl.41.6.831. [DOI] [PubMed] [Google Scholar]
- 36.Mossman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 37.Muchardt C, Seeler J S, Nirula A, Gong S, Gaynor R. Transcription factor AP-2 activates gene expression of HTLV-1. EMBO J. 1992;11:2573–2581. doi: 10.1002/j.1460-2075.1992.tb05322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nerenberg M, Hinrichs S H, Reynolds R K, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987;237:1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- 39.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 40.Ohshima K, Hashimoto K, Izumo S, Suzumiya J, Kikuchi M. Detection of human T lymphotropic virus type I (HTLV-I) DNA and mRNA in individual cells by polymerase chain reaction (PCR), in situ hybridization (ISH), and reverse transcription (RT)-PCR ISH. Hematol Oncol. 1996;14:91–100. doi: 10.1002/(SICI)1099-1069(199606)14:2<91::AID-HON574>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 41.Osame M, Usuku K, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara H. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 42.Pantaleo G, Graziosi C, Fauci A S. The immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993;328:327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- 43.Paskalis H, Felber B K, Pavlakis G N. cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci USA. 1986;83:6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perini G, Wagner S, Green M R. Recognition of bZIP proteins by the human T-cell leukemia virus transactivator Tax. Nature. 1995;376:602–605. doi: 10.1038/376602a0. [DOI] [PubMed] [Google Scholar]
- 45.Poiesz B F, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from freshly cultured lymphocytes of a patient with cutaneous T cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabson A B, Hamagishi Y, Steele P E, Tykocinski M, Martin M A. Characterization of human endogenous retroviral envelope RNA transcripts. J Virol. 1985;56:176–182. doi: 10.1128/jvi.56.1.176-182.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radonovich M, Jeang K-T. Activation of the human T-cell leukemia virus type I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate and by Tax (p40x) occurs through similar but functionally distinct target sequences. J Virol. 1989;63:2987–2994. doi: 10.1128/jvi.63.7.2987-2994.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson J H, Hollsberg P, Windhagen A, Child L A, Hafler D A, Lever A M L. Variable immortalizing potential and frequent virus latency in blood-derived T-cell clones infected with human T-cell leukemia virus type 1. Blood. 1997;89:3303–3314. [PubMed] [Google Scholar]
- 49.Riggs M G, Whitaker R G, Neumann J R, Ingram V M. n-Butyrate causes histone modification in HeLa and Friend erythroleukemia cells. Nature. 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 50.Sealy L, Chalkley R. The effect of sodium butyrate on histone modification. Cell. 1978;14:115–121. doi: 10.1016/0092-8674(78)90306-9. [DOI] [PubMed] [Google Scholar]
- 51.Seeler J-S, Muchardt C, Podar M, Gaynor R. Regulatory elements involved in Tax-mediated transactivation of the HTLV-I LTR. Virology. 1993;196:442–450. doi: 10.1006/viro.1993.1500. [DOI] [PubMed] [Google Scholar]
- 52.Setoyama M, Fujiyoshi T, Mizoguchi S, Katahira Y, Yashiki S, Tara M, Kanzaki T, Sonoda S. HTLV-I messenger RNA is expressed in vivo in adult T-cell leukemia/lymphoma patients: an in situ hybridization study. Int J Cancer. 1994;57:760–764. doi: 10.1002/ijc.2910570525. [DOI] [PubMed] [Google Scholar]
- 53.Setoyama M, Katahira Y, Hamada T, Tashiro M, Yashiki S, Tanaka Y, Tozawa H, Sonoda S. Expression of human T-cell lymphotropic virus type 1 gene products in the short-term cultured skin tissues of an adult T-cell leukemia/lymphoma patient with cutaneous manifestations. J Dermatol. 1992;19:133–139. doi: 10.1111/j.1346-8138.1992.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 54.Shimotohno K, Takano M, Teruuchi T, Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci USA. 1986;83:8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith M R, Greene W C. Molecular biology of the type 1 human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Invest. 1991;87:761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sodroski J G, Rosen C A, Haseltine W A. Transacting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984;225:381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki T, Fujisawa J I, Toita M, Yoshida M. The trans-activator Tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci USA. 1993;90:610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchiyama T. Human T cell leukemia virus type 1 (HTLV-1) and human diseases. Annu Rev Immunol. 1997;15:15–37. doi: 10.1146/annurev.immunol.15.1.15. [DOI] [PubMed] [Google Scholar]
- 59.Wattel E, Cavrois M, Gessain A, Wain-Hobson S. Clonal expansion of infected cells: a way of life for HTLV-1. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:S92–S99. doi: 10.1097/00042560-199600001-00016. [DOI] [PubMed] [Google Scholar]
- 60.Wattel E, Vartanian J-P, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss A, Stobo J D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984;160:1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wucherpfenning K W, Hollsgerg P, Richard J H, Benjamin D, Hafler D A. T-cell activation by autologous human T-cell leukemia virus type I-infected T-cell clones. Proc Natl Acad Sci USA. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implications in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshida M, Suzuki T, Fujisawa J, Hirai H. HTLV-1 oncoprotein Tax and cellular transcription factors. Curr Top Microbiol Immunol. 1995;193:79–89. doi: 10.1007/978-3-642-78929-8_4. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L-J, Giam C-Z. Interaction of the human T-cell lymphotropic virus type I (HTLV-1) transcriptional activator Tax with cellular factors that bind specifically to the 21-base-pair repeats in the HTLV-I enhancer. Proc Natl Acad Sci USA. 1991;88:11445–11449. doi: 10.1073/pnas.88.24.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]