Abstract
Sooty mangabeys naturally infected with simian immunodeficiency virus (SIV) do not develop immunodeficiency despite the presence of viral loads of 105 to 107 RNA copies/ml. To investigate the basis of apathogenic SIV infection in sooty mangabeys, three sooty mangabeys and three rhesus macaques were inoculated intravenously with SIVmac239 and evaluated longitudinally for 1 year. SIVmac239 infection of sooty mangabeys resulted in 2- to 4-log-lower viral loads than in macaques and did not reproduce the high viral loads observed in natural SIVsmm infection. During acute SIV infection, polyclonal cytotoxic T-lymphocyte (CTL) activity coincident with decline in peak plasma viremia was observed in both macaques and mangabeys; 8 to 20 weeks later, CTL activity declined in the macaques but was sustained and broadly directed in the mangabeys. Neutralizing antibodies to SIVmac239 were detected in the macaques but not the mangabeys. Differences in expression of CD38 on CD8+ T lymphocytes or in the percentage of naive phenotype T cells expressing CD45RA and CD62L-selection did not correlate with development of AIDS in rhesus macaques. In macaques, the proportion of CD4+ T lymphocytes expressing CD25 declined during SIV infection, while in mangabeys, CD25-expressing CD4+ T lymphocytes increased. Longitudinal evaluation of cytokine secretion by flow cytometric analysis of unstimulated lymphocytes revealed elevation of interleukin-2 and gamma interferon in a macaque and only interleukin-10 in a concurrently infected mangabey during acute SIV infection. Differences in host responses following experimental SIVmac239 infection may be associated with the divergent outcome in sooty mangabeys and rhesus macaques.
Lentivirus infection in nonhuman primate species does not always lead to AIDS. Animals that are natural hosts for lentiviruses generally remain asymptomatic, while the virus isolate from such hosts, when transferred to susceptible species results in AIDS (12). One such example is that of sooty mangabeys (Cercocebus torquatus atys), an Old World primate species that naturally harbors simian immunodeficiency virus (SIV) without developing AIDS (9). SIV isolates from asymptomatic sooty mangabeys induce AIDS when experimentally inoculated into rhesus (Macaca mulatta) and pig-tailed (M. nemestrina) macaques (16, 38). Evidence suggests that simian AIDS in captive rhesus macaques occurred as a result of cross-species infection from SIV-infected sooty mangabeys (35, 44) and that human immunodeficiency virus type 2 (HIV-2) entered the human population via zoonotic transmission from sooty mangabeys (8).
The basis of apathogenic SIV infection in sooty mangabeys is not known. We and others have found that plasma viral RNA levels in naturally infected sooty mangabeys approximate those detected in SIV-infected macaques with end-stage AIDS and range between 105 and 107 RNA copies/ml (17a, 54). In vitro, CD4+ T lymphocytes and macrophages of sooty mangabeys support SIV replication to the same or greater extent than cells from rhesus macaques (69). Other instances of lentivirus infection not leading to AIDS are generally associated with low viral loads. In asymptomatic HIV-infected chimpanzees, <5 infectious cells per million circulating peripheral blood mononuclear cells (PBMC) are detected and plasma viral RNA ranges from <104 to 20 × 104 copies/ml (21). Similarly, in HIV-infected long-term nonprogressors, viral loads are also low, plasma HIV RNA generally being <104 copies/ml (6, 55).
Analysis of the host immune system may also yield clues to the basis of apathogenic lentivirus infection. Studies in asymptomatic HIV-infected chimpanzees have documented the presence of neutralizing antibodies, CD8+ T-cell-mediated suppression of HIV replication, preserved CD4+ T-lymphocyte function, and an absence of increased apoptosis or immune activation in circulating PBMC (7, 13, 22, 47). In HIV-infected long-term nonprogressors, protection from disease is similarly associated with low viral loads and strong humoral and/or cellular virus-specific immune responses (4a, 6, 20, 30, 46, 55, 59). In naturally SIV-infected sooty mangabeys, on the other hand, recent studies have disclosed the surprising finding of the occurrence of an asymptomatic state associated with persistent, high-level viremia and relatively weak CTL activity (17a).
A comparative study of host responses to SIV infection in primate species with pathogenic or apathogenic outcomes may shed light on host determinants associated with lentivirus-induced immunodeficiency. Several studies have reported phenotypic and functional differences in PBMC of rhesus macaques and sooty mangabeys. Cytokine secretion profiles of cloned T-cell lines from normal and SIV-infected animals show a predominantly Th1-type secretion pattern in rhesus macaques and a Th2 pattern in sooty mangabeys (3). Natural killer cells in normal sooty mangabeys have significantly greater cytolytic activity and their phenotype is CD8+ and CD16−, while in rhesus macaques the phenotype is CD16+ CD8− or CD16+ CD8lo (1, 49). Published data on SIV-specific immunity in sooty mangabeys are sparse and largely confined to humoral and CD4+ T-cell responses. SIV-specific antibodies that cross-react with other SIV isolates have been identified by Western blotting, though neutralizing antibodies are weak or undetectable (14, 15). Proliferative responses have been detected with a cellular Western blot assay or when autologous macrophages pulsed with UV-irradiated inactivated SIV were used as antigen-presenting cells (2, 50). CD8+ T cells from naturally infected sooty mangabeys secrete a soluble factor that can inhibit in vitro SIV replication of exogenously infected PBMC (31, 51).
There are no published studies on SIV-specific cytotoxic T-lymphocyte (CTL) activity in SIV-infected sooty mangabeys. We have recently shown that in naturally infected sooty mangabeys, SIV-specific CTL activity, while not detectable in fresh PBMC, is detected to variable degrees after in vitro antigen-specific stimulation (17a). These observations suggest absent or low levels of in vivo-activated CTL, with preservation of an expandable pool of memory CTL. The association of high-level viremia with absent or ineffective activated CTL in the periphery could indicate an underlying anergic state with its advent early in the course of SIV infection.
In order to study early immune events following SIV infection and to concurrently compare cellular and humoral immune responses in apathogenic and pathogenic lentivirus infection, we instituted a prospective longitudinal study of SIV infection in rhesus macaques and sooty mangabeys. Three uninfected adult sooty mangabeys and three uninfected age-matched rhesus macaques were infected, one pair at a time, with SIVmac239, a pathogenic molecular clone of SIV. SIV-specific CTL activity, plasma neutralizing antibody titers, plasma viral load, and T-cell phenotype were determined sequentially for 1 year or until death in all animals. In addition, in one pair of infected animals, ex vivo intracellular cytokine production in PBMC following SIV infection was measured by flow cytometry. SIVmac239 infection of sooty mangabeys resulted in an asymptomatic chronic infection with viral loads approximately 2 to 4 logs lower than in naturally infected sooty mangabeys or SIVmac239-infected macaques. Striking differences were noted between SIV-infected sooty mangabeys and rhesus macaques with respect to outcome, viral load, strength and breadth of CTL activity, maintenance of T-cell homeostasis, and cytokine secretion, observations that may have a bearing on host determinants of resistance to pathogenic SIV infection.
MATERIALS AND METHODS
Animals.
Sooty mangabeys and rhesus macaques used in the study were housed at the Yerkes Regional Primate Research Center and maintained in accordance with the federal guidelines (44a). Sooty mangabeys reared separate from other SIV-infected animals have remained SIV seronegative. Prior to enrollment, the absence of SIV infection was confirmed by negative SIV PCR of plasma and negative HIV-2 serology over a period of at least 1 year. Three uninfected sooty mangabeys and three age-matched rhesus macaques (age range 6 to 10 years) were infected intravenously with 17.3 ng of p27 SIVmac239 (kindly provided by Ronald Desrosiers, New England Regional Primate Research Center [NERPRC]). The animals were infected, one pair at a time, at intervals of 4 to 8 months, each pair consisting of one sooty mangabey and an age-matched rhesus macaque.
Blood was collected at weekly intervals for the first month, bimonthly during the second month of infection, and thereafter at monthly intervals for 1 year or until the animal died.
Generation of B-LCL.
Transformed B-lymphoblastoid cell lines (B-LCL), for use as major histocompatibility complex (MHC)-matched stimulator and target cells in CTL assays, were established for each animal. B cells were transformed by incubating PBMC at 37°C in a 5% CO2 incubator with herpesvirus papio derived from the supernatant of S594 cells (provided by Norman Letvin, Beth Israel Hospital, Boston, Mass.). B-LCL were propagated in RPMI 1640 medium (Gibco) supplemented with 20% fetal bovine serum (Sigma Chemical Co., St. Louis, Mo.), 10 mM HEPES (Gibco), 2 mM l-glutamine (Gibco), 50 IU of penicillin (Gibco) per ml, and 50 μg of streptomycin (Gibco) per ml.
Stimulation of effector cells for CTL assays.
Blood for CTL assays was collected in cell preparation tubes (Vacutainer CPT; Becton Dickinson Vacutainer Systems, Franklin Lakes, N.J.), which contain sodium heparin, sodium diatrizoate, and a polyester gel plug. The tubes were spun at 1,500 × g for 20 min within 3 h of blood collection and shipped overnight at room temperature to NERPRC. This allowed immediate isolation of PBMC from whole blood by density gradient centrifugation. A polyester gel plug separates the PBMC from erythrocytes and granulocytes during transportation. PBMC shipped overnight were suspended at 2 × 106 cells/ml in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10 mM HEPES, 2 mM l-glutamine, 50 IU of penicillin per ml, and 50 μg of streptomycin per ml (R-10 medium). In the initial part of the study, heparinized blood was shipped overnight and PBMC were then separated by gradient centrifugation over sodium diatrizoate (Ficoll 1077; Sigma). Blood samples for CTL assays after 18 weeks of SIV infection for the first pair of infected animals (FYg and RFi-1) and for all data points for the second (FLg and RLk-1) and third (FWl and RGa-3) pairs of infected animals were transported in CPT tubes.
For antigen-specific stimulation, autologous B-LCL were infected at a multiplicity of infection of 5 PFU/cell with recombinant vaccinia viruses vAbT388-6-1, expressing the Gag and Pol proteins of SIVmac251 and Env protein of SIVmac239, and with vAbT306-6-1, expressing the Nef protein of SIVmac239 (provided by D. Panicali, Therion Biologics, Cambridge, Mass.). After overnight incubation, infected B-LCL were inactivated with long-wave UV irradiation (Fisher model UV; 350- to 400-nm wavelength) in the presence of psoralen (10 μg/ml; HRI Associates). Cells were UV irradiated at a distance of 3.5 cm from the light source, washed three times, and then used as stimulators. PBMC were cultured with stimulator cells at a responder-to-stimulator ratio of 10:1 in R-10 medium and incubated at 37°C in a 5% CO2 incubator. Cells were fed with R-10 medium twice a week, and recombinant human interleukin-2 (IL-2 [kindly donated by M. Gately, Hoffman-LaRoche]; 10 IU per ml) was added after 4 to 5 days. CTL assays were performed 10 to 14 days after stimulation.
Chromium release assay.
Target cells consisted of autologous or allogeneic B-LCL infected with individual recombinant vaccinia viruses expressing SIV proteins. Recombinant vaccinia viruses used to infect target cells included the control vaccinia virus NYCBH, vAbT252 (encoding the SIVmac251 p55 Gag and Protease proteins; Therion), vAbT258 (encoding the SIVmac251 Pol proteins reverse transcriptase, endonuclease, and protease; Therion), rVV-239 (encoding the SIVmac239 Env protein, provided by M. Mulligan) (56), and vAbT306 (encoding the SIVmac239 Nef protein; Therion). Target cells were infected overnight at a multiplicity of infection of 5 to 10 PFU/cell and then labeled with 50 μCi of 51C (DuPont NEN, Wilmington, Del.) per 106 cells. Target cells (104 cells/well) were dispensed in duplicate for each effector/target ratio into 96-well U-bottom plates (Costar). Chromium release was assayed after 5 h of incubation at 37°C in a 5% CO2 incubator. Plates were spun at 1,000 rpm for 10 min at 4°C, after which 30 μl of supernatant was harvested from each well onto wells of a LumaPlate-96 (Packard) and allowed to dry overnight. Emitted radioactivity was measured in a 1450 MicroBeta Plus liquid scintillation counter (Wallac, Turku, Finland). Spontaneous release was measured from wells containing only target cells and medium. Maximum release was measured from wells containing target cells and 0.1% Triton X-100 (Sigma). Percent specific cytotoxicity was calculated as {(test release − spontaneous release)/(maximum release − spontaneous release)} × 100. Spontaneous release of target cells was <25% in all assays.
CD8+ and CD4+ lymphocyte separation.
CD8+ T lymphocytes were isolated from stimulated PBMC by depletion of CD4+ T lymphocytes, using magnetic beads (CD4 Dynabeads; Dynal, Oslo, Norway) at a bead/cell ratio of 20:1 for 60 min at 4°C. The supernatant enriched for CD8+ T lymphocytes was collected with a magnetic separation device (Dynal). CD4+ T lymphocytes attached to the magnetic beads were released from the beads by incubation with a commercial polyclonal antibody against papain-digested Fab fragments of mouse immunoglobulin (DETACHaBEADS; Dynal) with continuous shaking at 37°C for 1 h. Detached CD4+ T lymphocytes were separated from the beads over a magnetic separation device (Dynal) and were >98% pure as assessed by flow cytometry. Similarly, negatively selected CD8+ T-lymphocyte populations contained <7% CD4+ T lymphocytes. Fractionated T lymphocytes were suspended in R-10 medium and used the same day in CTL assays.
Whole-virus ELISA.
Antibodies to lysed whole-virus antigen were measured by enzyme-linked immunosorbent assay (ELISA), using published methodology (67). Briefly, 96-well flat-bottom plates were coated for 2 h at 37°C with detergent-disrupted pelleted SIVmac239 diluted in a carbonate buffer and washed three times with phosphate-buffered saline containing 1% Tween 20. The plates were successively incubated after washes with a 1:20 dilution of serum at 37°C for 2 h, alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) at 37°C for 1 h, and finally the color substrate p-nitrophenyl phosphate (67). The color reaction was stopped after 30 min at room temperature by adding 3 N NaOH, and absorbance was analyzed at 405 nm on a Perkin-Elmer plate reader.
Neutralization assay.
Plasma stored at −80°C was thawed, heat inactivated at 56°C for 30 min, and used in neutralization assays. Neutralizing antibodies to SIVmac239 were measured with a sensitive assay system recently described by Means et al. (39). CEMx174 cells harboring a secreted alkaline phosphatase (SEAP) reporter gene under the control of a portion of the SIVmac239 long terminal repeat (pLNSIV-SEAP) were used to detect the degree of neutralization. Neutralization assays were set up in 96-well plates as described previously (39). Briefly, twofold dilutions of test serum were incubated in duplicate with 1 ng of p27 of primary SIVmac239 stock in a final volume of 100 μl for 1 h at room temperature with occasional mixing. At the end of incubation, 15,000 cells harboring pLNSIV-SEAP were added in a volume of 100 μl to each well, plates were incubated at 37°C in a CO2 incubator, and SEAP activity was checked after 52 to 72 h by using a chemiluminescence assay (39). Endogenous phosphatase activity as measured in control wells containing only the parental cell line without the SEAP construct was subtracted from each test value. Percent neutralization was calculated by dividing the counts per second of each test well by the average maximum SEAP value obtained from wells containing mock-neutralized virus. Positive control serum (pooled from five SIVmac239-infected macaques infected >20 weeks) and negative control serum (pooled from five SIV-naive rhesus macaques) were run concurrently with the test samples.
Determination of plasma viral RNA.
Blood plasma RNA concentration was determined with a SIV quantitative competitive reverse transcription-PCR assay based on a previously described method (62). Plasma was separated from acid citrate dextran-anticoagulated whole blood by centrifugation at 1,000 × g for 15 min and stored at −70°C within 3 h of phlebotomy. First-time-thawed plasma was centrifuged at 39,500 × g for 1 h to concentrate SIV virions, and RNA was extracted with TRIZOL reagent (Gibco/BRL, Grand Island, N.Y.). Competitor RNA was derived from in vitro transcription of SIVmac239 gag segments containing a 40-bp insertion to allow competitor-derived amplicons to be distinguished during polyacrylamide gel electrophoresis. The concentration of competitor RNA was determined after column purification by spectrophotometric absorbance after gel analysis confirmed RNA length integrity. Reverse transcription and PCR were performed as previously described (62) except that the PCR primers were 5′-AGA AAG CCT GTT GGA IAA CAA AGA AGG-3′ and 5′-CTC AGT ITG TTT CAC TTT CTC TTC TGC GTG-3′. The RNA copy number was calculated from the competitor-template equivalence point by cubic regression. Assay results based on these conditions correlated highly with SIV bDNA analysis (Chiron, Emeryville, Calif.) of SIVmac239-infected specimens as well as by limit of dilution PCR analysis of both SIVmac239- and SIVsmm-infected specimens (data not shown). The assay has a lower limit of quantitation of 103 copies per ml of plasma. The coefficient of variation of the assay was <5% in duplicate analysis of 17 specimens with 104 to 108 SIV RNA copies/ml. Plasma specimens from each pair of animals were analyzed in parallel.
Immunophenotyping.
Two-, three-, and four-color flow cytometry was used for sequential determination of the number and phenotype of T-lymphocyte subsets during the course of SIV infection. The fluorescent conjugates used were fluorescein isothiocyanate (FITC), phycoerythrin (PE), and peridinin chlorophyl protein (PerCP) on a FACScan (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, Calif.) and FITC, PE, PerCP or Red613, and allophycocyanin (APC) on a FACSVantage (BDIS).
The percentage of CD4+ and CD8+ subsets of T lymphocytes in the peripheral blood was determined longitudinally, by three-color flow cytometry, in all six animals during SIV infection. Immunophenotyping was performed from the day of SIV infection, at weekly intervals for the first month and thereafter, bimonthly or monthly for 1 year or until death, in four animals (pairs FLg plus RLk-1 and FWl plus RGa-3). In two animals (FYg and RFi-1, the first pair to be infected), three-color immunophenotyping was initiated only 13 weeks after SIV infection. The distribution of naive and memory CD4+ and CD8+ T lymphocytes, as well as the percentage of activated CD38-expressing CD8+ T lymphocytes in the peripheral blood, was determined longitudinally in the first two pairs of animals (FYg plus RFi-1 and FLg plus Rlk-1), using multiparametric four-color flow cytometry. The surface expression of the T-cell activation markers CD25, CD69, and HLA DR was also assessed sequentially on CD4+ and CD8+ peripheral T lymphocytes, using two-color flow cytometry, in all six animals for 1 year after SIV infection.
The antibody and fluorochrome combinations were as follows: (i) anti-rhesus CD3 monoclonal antibody (MAb) conjugated to PE together with anti-human CD4 and anti-human CD8 MAbs (CD4-FITC and CD8-PerCP); (ii) CD38-FITC, CD8-PerCP, and CD3-biotin strepavidin APC; (iii) CD45RA-FITC and CD62L-selectin (CD62L)-PE in combination with CD3-biotin strepavidin APC and CD8-PerCP or CD3-biotin strepavidin Red613 (Gibco) and CD4-APC (custom conjugated); (iv) CD4- or CD8-PE and CD25-FITC; (v) CD4- or CD8-PE and CD69-FITC; and (vi) CD4- or CD8-PE and HLA DR-FITC. Unless otherwise specified, antibodies were obtained from BDIS. All antibodies except anti-CD3 were MAbs of anti-human specificity that cross-react with rhesus antigens of the same specificity. Rhesus anti-CD3 (6G12) was kindly provided by Johnson Wong, Massachusetts General Hospital (27).
Surface staining of PBMC was carried out by standard procedures. Briefly, 0.5 × 106 to 1 × 106 PBMC were washed with phosphate-buffered saline containing 2% fetal calf serum and incubated with the conjugated antibodies for 30 min at 4°C. Stained cells were fixed in 2% paraformaldehyde and analyzed on a BDIS FACScan (two and three color) or FACSVantage (three or four color), depending on the conjugate combination used.
Statistical analysis.
Longitudinal changes in T-lymphocyte subsets during SIV infection and the slope of rise and fall of primary SIV viremia were analyzed by regression analysis. Regression coefficients and plots were generated by using statistical software (StatView; Abacus Concepts, Inc., Berkeley, Calif.). Simple regression analysis was used to determine the association between changes in T lymphocytes and progressing SIV infection. Absolute numbers or proportions of CD4+ and CD8+ T lymphocytes expressing specific cell surface markers were grouped by animal species, designated as dependent variables, and plotted against the independent variable of time after SIV infection.
A two-tailed, two-sample unequal-variance Student t test was used to calculate the P value for differences in mean plasma viremia between the sooty mangabeys and rhesus macaques at each measured time point.
Intracellular cytokine staining of PBMC.
Intracellular cytokine secretion in PBMC was assessed by multiparametric flow cytometric analysis using published methodology (52, 60). MAbs to human cytokines were used to detect cytokine secretion within PBMC of rhesus macaques and sooty mangabeys. In preliminary experiments, using PBMC from normal animals stimulated in vitro with mitogens, we showed that anti-human cytokine MAbs to IL-2 (clone MQ1-17H12), IL-4 (clone 8D4-8), human and viral IL-10 (clone JES3-9D7), gamma interferon (IFN-γ; clone 4S.B3), and tumor necrosis factor alpha (clone MAb 11) were cross-reactive with rhesus macaques and sooty mangabeys (63a). All anti-human cytokine MAbs were conjugated to PE and obtained from Pharmingen (San Diego, Calif.). Appropriate isotype antibodies (PE-conjugated mouse IgG1, rat IgG1, and rat IgG2a) were routinely used as negative controls.
PBMC were isolated from heparinized blood by density gradient centrifugation, suspended at 106 cells per ml in R-10 medium, and incubated with monensin (10 μM) for 4 h at 37°C. Monensin is an ionophore which disrupts intracellular Na+ and H+ gradients and leads to inhibition of Golgi transport (41). This results in intracellular accumulation of secreted proteins including cytokines and an amplified staining signal. Additionally, as a positive control, at each time point, an aliquot of PBMC was stimulated with phorbol 12-myristate 13-acetate (PMA; 10 ng/ml; Sigma) and the hemicalcium salt of calcium ionophore A23187 (250 ng/ml; Sigma) for 4 h. Cells were first surface stained with anti-CD4 (FITC) and anti-CD8 (PerCP) and then fixed with paraformaldehyde. Fixed cells were permeabilized with saponin and stained intracellularly with anti-human cytokine or isotype-matched antibodies conjugated to PE.
Since anti-human cytokine antibodies were being used to detect cytokines of two nonhuman primate species, the staining specificity was confirmed by blocking experiments. Incubation of cells with a molar excess of unconjugated anti-IL-2 or anti-IFN-γ MAb for 30 min prior to staining abolished a positive signal (data not shown).
RESULTS
Longitudinal analysis of SIV-specific CTL activity and viral load in SIVmac239-infected sooty mangabeys and rhesus macaques.
To investigate the relationship between CTL activity, viral load, and disease in SIV infection, nonhuman primate species with pathogenic or apathogenic outcomes of SIV infection were studied concurrently. Three SIV naive sooty mangabeys and three naive rhesus macaques were infected with a pathogenic strain of SIV and followed up longitudinally for 1 year or until death.
Initial CTL studies in our first pair of infected animals revealed absent or weak SIV-specific CTL activity (≤10% specific lysis) in the rhesus macaque RFi-1 (Fig. 1), a surprising finding in light of previous reports of the frequent occurrence of SIV-specific CTL during primary SIV infection (53, 68). However, the low level of SIV-specific CTL activity in the first 12 weeks of infection in this macaque is likely to have been due to technical reasons. During this period, heparinized blood was being transported to NERPRC, and PBMC were separated from whole blood 24 to 36 h after blood collection. To investigate the cause for low-level CTL activity in this macaque, we evaluated the effect of delayed isolation of PBMC from whole blood on CTL activity. Testing of an SIVΔnef-infected rhesus macaque with strong SIV-specific CTL activity (≥25% specific lysis at an effector/target ratio of <20:1), we found that isolation of PBMC after overnight storage of whole blood at room temperature reproducibly resulted in a two- to threefold loss of CTL activity. This loss of CTL activity did not occur when PBMC were separated prior to overnight storage (data not shown). Thus, all subsequent studies (all data points for the second and third pairs of infected animals and after 16 weeks of SIV infection for FYg and RFi-1, the first pair of infected animals) were performed with PBMC separated prior to overnight blood transport. Increased CTL activity was detected in RFi-1 after a change to CPT tubes at 16 weeks.
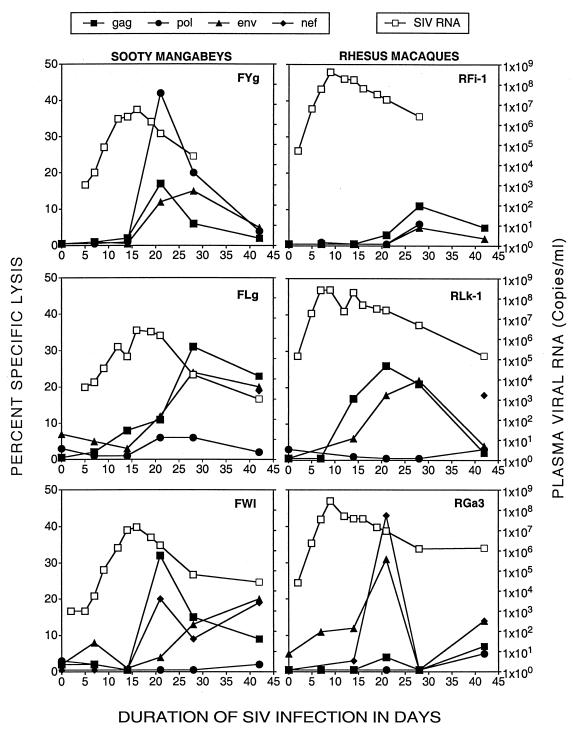
FIG. 1.
SIV-specific CTL activity and plasma SIV RNA during acute SIVmac239 infection in three sooty mangabeys (FYg, FLg, and FWl) and three rhesus macaques (RFi-1, RLk-1, and RGa-3). Adjacent pairs were infected concurrently.
Following in vitro antigen-specific stimulation of PBMC, vigorous SIV-specific CTL activity was detected 7 to 21 days after SIV infection in both sooty mangabeys and rhesus macaques. CTL activity peaked at 21 to 28 days and was preceded by a sharp decline in peak plasma viral RNA levels (Fig. 1). It is noteworthy that vigorous SIV-specific CTL activity was detected in the sooty mangabey FYg (concurrently infected with RFi-1) despite delayed separation of PBMC, which may reflect more vigorous CTL activity in the mangabey than in the macaque RFi-1.
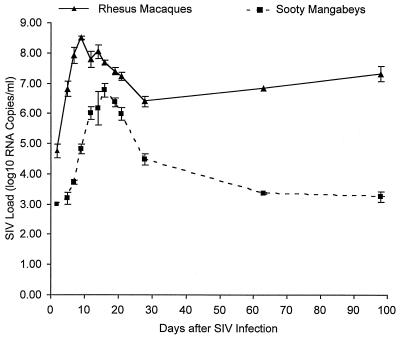
During the first 6 weeks of SIV infection, there were significant differences in viral kinetics between the rhesus macaques and sooty mangabeys (Fig. 1 and 2). Peak plasma viremia in the rhesus macaques occurred 7 days earlier and was 1 to 2 logs higher than in the sooty mangabeys (Fig. 2). Mean plasma viremia at all measured time points was significantly lower in the mangabeys (P = 0.0005 at days 5 and 9 and <0.05 after day 14). After the emergence of CTL activity, a greater degree of viral suppression was observed in sooty mangabeys. The slope of the decay in plasma viremia was significantly greater in the mangabeys, (−0.213 ± 0.029 log10 RNA copies/day [mean ± standard deviation {SD}]) than in the macaques (−0.093 ± 0.031 log10 RNA copies/day; P = 0.014) and resulted in a higher set point of plasma viremia in the macaques (Fig. 2 and 3). By 38 to 42 weeks, plasma SIV RNA had become undetectable in two of three sooty mangabeys (lower limit of detection of the assay, 1,000 RNA copies/ml), while the concurrently infected rhesus macaques had 3- to 4-log-higher levels of SIV RNA (Fig. 3).
FIG. 2.
Dynamics of primary SIV viremia in the first 100 days following SIVmac239 infection in three rhesus macaques and three sooty mangabeys. Plasma SIV viremia was quantitated by competitive RNA PCR at 2, 5, 7, 9, 12, 14, 16, 19, 21, 28, 63, and 98 days following SIV infection. Each data point with error bars represents the mean and standard error of the mean viral load. The differences in level of viremia between the rhesus macaques and sooty mangabeys at each measured time point were statistically significant (P value by Student’s t test = 0.0005 at days 5 and 9 and <0.05 after day 14). The slope of rise and decay in plasma viremia was calculated from data points in the linear portions of the curve (days 2 to 7 and 16 to 21 in the macaques; days 7 to 12 and 19 to 28 in the rhesus macaques). The slope of rise was 0.636 ± 0.015 log10 RNA copies/day (mean ± SD) in the macaques and 0.449 ± 0.075 log10 RNA copies/day in the mangabeys (P value by Student’s t test = 0.066). The slope of decay was −0.093 ± 0.031 log10 RNA copies/day (mean ± SD) in the macaques and −0.213 ± 0.029 log10 RNA copies/day in the mangabeys (P value by Student’s t test = 0.014).
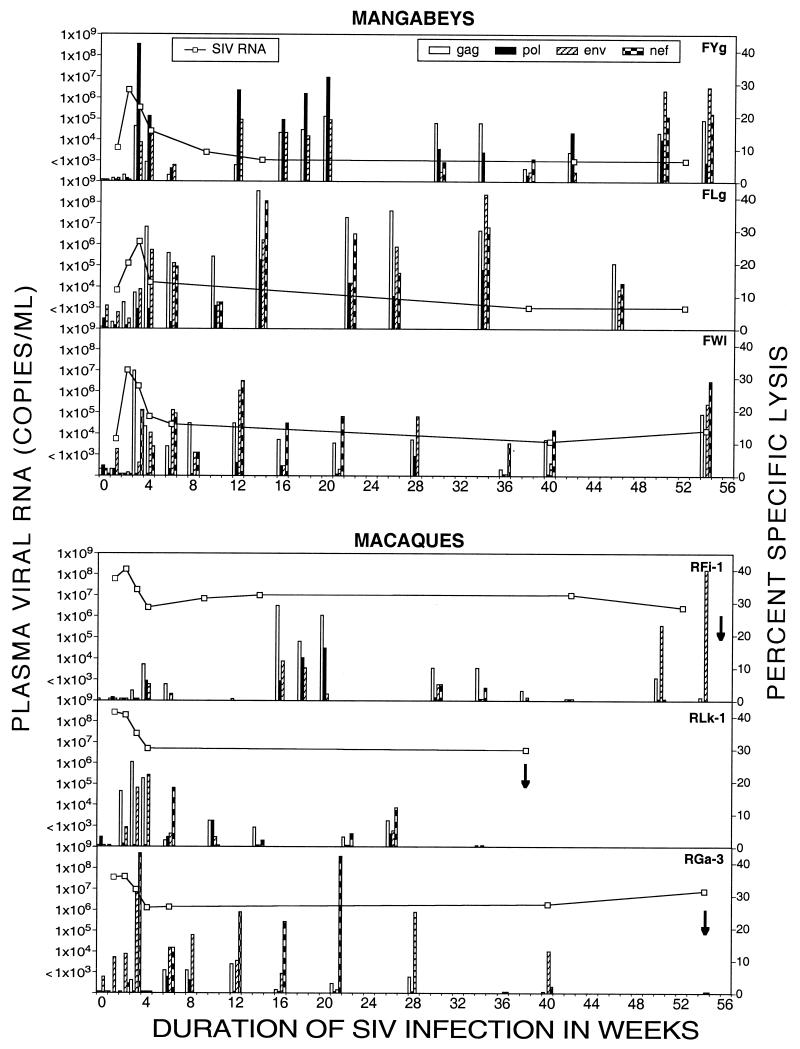
FIG. 3.
SIV-specific CTL activity and plasma SIV RNA during acute and chronic SIVmac239 infection in three sooty mangabeys (FYg, FLg, and FWl) and three rhesus macaques (RFi-1, RLk-1, and RGa-3). Arrows denote the time at which an animal died. SIV-specific CTL activity to Gag, Pol, Env, and Nef is shown as columns. The absence of a column(s) at a particular time point denotes that CTL activity was not tested at that time point. Undetectable CTL activity is depicted by 0.5% specific lysis. Viral loads below the threshold of detection (1,000 RNA copies/ml) are plotted as 1,000 copies/ml.
SIV-specific CTL activity declined in all six animals at 6 to 8 weeks following SIV infection and reemerged at 12 to 16 weeks (Fig. 3). The late CTL responses were qualitatively and quantitatively different in the sooty mangabeys and rhesus macaques. Vigorous CTL activity (>20% specific lysis) was sustained throughout the follow-up period in the sooty mangabeys and was polyclonal (Fig. 3). The SIV-specific CTL response in individual sooty mangabeys had a broad specificity that encompassed SIV structural and regulatory proteins. Even though the specificity varied with time and between animals, strong CTL activity directed toward two or more SIV proteins was sustained at all time points (Fig. 3). In contrast, in the rhesus macaques, SIV-specific CTL activity after 20 weeks of SIV infection either was absent or, when present, had a narrow specificity, being directed toward a single SIV protein (Env or Nef) (Fig. 3).
At 11 to 13 months after SIV infection, three of three macaques had been euthanized after onset of simian AIDS while all three sooty mangabeys were alive and healthy. Thus, experimental SIVmac239 infection in sooty mangabeys resulted in low viral loads and persistent strong CTL activity, while in rhesus macaques, it resulted in high viral loads and less sustained CTL activity.
SIV-specific CTL activity in sooty mangabeys is MHC restricted and mediated by CD8+ T lymphocytes.
The CTL response in SIV-infected rhesus macaques is MHC restricted and mediated by CD8+ T lymphocytes (40). To determine the phenotype of SIV-specific CTL in sooty mangabeys, PBMC following antigen-specific stimulation were fractionated into CD4+ and CD8+ T-lymphocyte fractions by using anti-CD4-coated magnetic beads. Specific lysis comparable to or greater than that for bulk CTL was detected with the CD8+ but not the CD4+ T-lymphocyte fraction (data not shown). The CTL lysis was MHC restricted, since bulk CTL and the CD8+ T-lymphocyte fraction lysed autologous but not allogeneic target cells infected with recombinant vaccinia virus expressing SIV proteins (data not shown). No SIV-specific CTL was detectable in PBMC from SIV-seronegative mangabeys (data not shown).
Longitudinal analysis of neutralizing antibodies to SIVmac239 in SIV-infected sooty mangabeys and rhesus macaques.
To assess the temporal relationship of humoral immunity and viral replication in SIV-infected sooty mangabeys and rhesus macaques, neutralizing antibodies to the homologous virus SIVmac239 were examined in sequential plasma samples from each animal. A recently described sensitive assay which uses CEMx174 cell lines expressing SEAP under the control of a portion of the SIV long terminal repeat was used for detection of neutralizing antibodies to a primary stock of SIVmac239 (39). With this assay, neutralizing antibodies to SIV are detected in most SIVmac239-infected rhesus macaques after 20 weeks of SIV infection (references 39 and 39a).
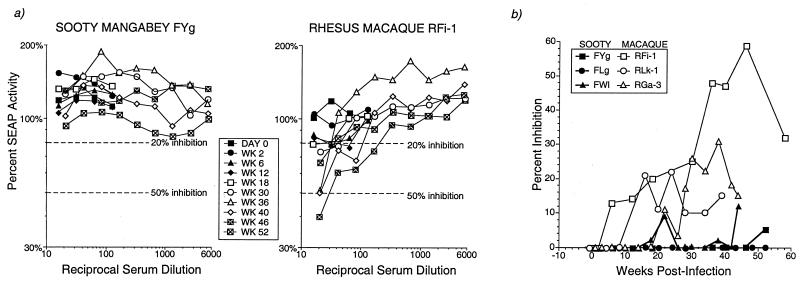
Neutralizing antibodies to SIVmac239 were not detected in the sera of any of the three SIVmac239-infected sooty mangabeys up to 1 year after SIV infection (Fig. 4 and Table 1). In contrast, two of three macaques showed rising neutralizing antibody titers starting at 20 weeks after SIV infection (Fig. 4b). In one macaque, the 50% neutralization titers at 46 weeks of SIV infection were comparable to that of positive control pooled sera from SIV-infected rhesus macaques (Table 1). Neutralization titers were appreciably lower in the other two macaques (Table 1 and Fig. 4b).
FIG. 4.
Neutralizing antibodies to SIVmac239 in SIV-infected sooty mangabeys and rhesus macaques. (a) Representative assay in one sooty mangabey and one rhesus macaque. (b) Percent inhibition of 1:20 dilution sera in three sooty mangabeys and three rhesus macaques during SIV infection.
TABLE 1.
Neutralization titers to SIVmac239 in sera of SIVmac239-infected sooty mangabeys and rhesus macaques
| Animal | Neutralization titera
|
|
|---|---|---|
| 50% inhibition | 20% inhibition | |
| Sooty mangabeys | ||
| FYg | <1:15 | <1:15 |
| FLg | <1:15 | <1:15 |
| FWl | <1:15 | <1:15 |
| Rhesus macaques | ||
| RFi-1 | 1:30 | 1:180 |
| RLk-1 | <1:15 | 1:15 |
| RGa3 | <1:15 | 1:100 |
| Control macaque serab | ||
| Pooled positive serum | 1:30 | 1:160 |
| Pooled negative serum | <1:15 | <1:15 |
Titers shown, determined by a SEAP assay as described in Materials and Methods, are the maximum reached for individual animals at any time point after SIV infection. In individual animals, sera from at least 10 time points, ranging from 0 to 52 weeks after SIV infection, were titered against SIVmac239. Maximum neutralization titers were observed only after 24 weeks of SIV infection.
Pooled positive serum was obtained from five SIVmac239-infected rhesus macaques infected for at least 20 weeks. Pooled negative serum was from five SIV-naive rhesus macaques.
SIV-specific virus binding antibodies were also measured by ELISA. Anti-SIV antibodies first became detectable by 1 to 3 weeks in the rhesus macaques and by 3 to 8 weeks in the sooty mangabeys (data not shown). The antibodies persisted at moderate to high levels and were detectable at equal levels in the sooty mangabeys and rhesus macaques after 12 weeks of infection (data not shown). In the sooty mangabeys, high levels of anti-SIV antibodies were detected at time points (38 to 42 weeks of SIV infection) when neutralizing antibodies were not detected.
T-lymphocyte homeostasis in SIV-infected sooty mangabeys and rhesus macaques.
Progressive CD4+ T lymphocytopenia is a hallmark of HIV/SIV-induced immunodeficiency. Declining total T-lymphocyte counts due to a failure of T-lymphocyte homeostasis have also been shown to precede the onset of AIDS in humans (36). Using three-color flow cytometry, we longitudinally determined the percentages of total, CD4+, and CD8+ T lymphocytes in sooty mangabeys and rhesus macaques. The inclusion of the rhesus-specific CD3 MAb allowed us to rigorously define CD4+ and CD8+ T lymphocytes as cells coexpressing CD3 and CD4 or CD3 and CD8, respectively. It also enabled us to follow peripheral T-lymphocyte homeostasis in infected animals by measuring total T lymphocytes (i.e., CD3+ T lymphocytes) and to evaluate other unusual T-lymphocyte populations in the peripheral blood, i.e., cells that do not express either CD4 or CD8 (double negative) or that express both markers (double positive).
In all SIV-infected macaques, the percentage of CD4+ T lymphocytes in the peripheral blood progressively declined to ≤20% (Fig. 5). In contrast, the percent of circulating CD4+ T lymphocytes remained fairly stable throughout SIV infection in the sooty mangabeys. In one sooty mangabey (FLg), CD4+ T lymphocytes declined sharply at 2 weeks but subsequently stabilized at >75% of the pre-SIV infection value (Fig. 5). Using simple regression analysis and regression plots, a significant inverse relationship between CD4+ T lymphocyte counts and duration of SIV infection was detected in the rhesus macaques (R = 0.756, P = <0.0001) but not in the sooty mangabeys (R = 0.191, P = 0.0959). Changes in peripheral CD8+ T-lymphocyte counts during SIV infection were not significant (rhesus macaque, R = 0.187, P = 0.1132; sooty mangabey, R = 0.197, P = 0.0856). The ratio of CD4+ to CD8+ T lymphocytes in the peripheral blood declined significantly in the macaques (R = 0.52, P = <0.0001) but was unaltered in the mangabeys (R = 0.045, P = 0.6969).
FIG. 5.
T-cell homeostasis during acute and chronic SIVmac239 infection in sooty mangabeys and rhesus macaques. The percentage of circulating T-lymphocyte subsets was determined longitudinally during SIV infection in all six animals by three-color flow cytometry. In the pair of animals FYg and RFi-1, immunophenotyping was performed only after 13 weeks of SIV infection.
T-cell homeostasis, as measured by the percentage of CD3+ lymphocytes (total T lymphocytes) in peripheral blood, was maintained in three of three mangabeys and in only one of three macaques (Fig. 5). In the early phase of SIV infection, despite CD4+ T lymphocytopenia, the total T-lymphocyte pool was maintained by reciprocal increases in the other T-lymphocyte subsets, chiefly CD3+ CD8+ lymphocytes (Fig. 5). In two of three macaques that progressed to AIDS, a progressive decline in total peripheral T lymphocytes was observed 4 to 6 weeks following SIV infection and was associated with CD4+ and CD8+ T lymphocytopenia (Fig. 5).
Interestingly, in the SIV-infected sooty mangabeys, 15 to 20% of the circulating CD3+ T lymphocytes did not express CD4 or CD8 on their surface. This cell population was also seen in the peripheral blood of uninfected sooty mangabeys (data not shown) and did not quantitatively change during SIV infection. We explored the possibility that the CD3+ CD4− CD8− lymphocytes were the equivalent of murine double-negative T lymphocytes that express the NK1.1 antigen (10, 64). By flow cytometry and reverse transcription-PCR, these cells did not have a restricted T-cell receptor Vα repertoire, nor did they predominantly express Vα24 (data not shown). We also did not find evidence for the characteristic cytokine secretion profile of NK1.1 cells, i.e., high levels of IL-4 and IFN-γ with little or no secretion of IL-2, in this cell population (data not shown). Such double-negative T lymphocytes were rarely seen in normal macaques. In SIV-infected macaques, transient increases in circulating CD3+ CD4− CD8− T lymphocytes were seen at time points when the percentages of both CD4+ and CD8+ T lymphocytes had declined.
Flow cytometric determination of the activation status of circulating T lymphocytes in SIV-infected sooty mangabeys and rhesus macaques.
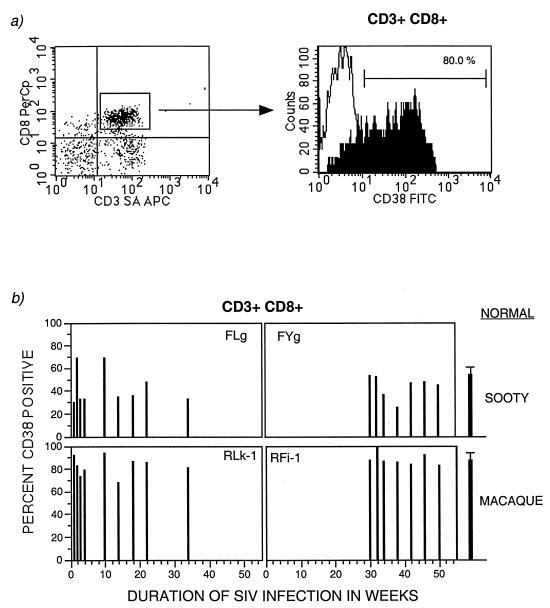
There is evidence that in HIV-infected humans, chronic immune activation of CD8+ T lymphocytes, particularly as reflected by increased numbers or surface expression of CD38, is a strong predictor of progression to AIDS (33, 34). We investigated the status of circulating activated CD8+ T lymphocytes during SIV infection in rhesus macaques and sooty mangabeys. PBMC were surface stained with a conjugated anti-human CD38 MAb along with anti-CD3 and anti-CD8 MAbs. In SIV-infected rhesus macaques, greater than 80% of the peripheral CD3+ CD8+ T lymphocytes expressed CD38, while less than 50% of such cells were present in the sooty mangabeys (Fig. 6). However, surprisingly, similar percentages of CD38+-expressing CD8+ T lymphocytes were also seen in normal macaques and sooty mangabeys, and there was no change in the number or fluorescence intensity of CD38 during SIV infection.
FIG. 6.
Surface expression of CD38 on CD3+ CD8+ T lymphocytes in peripheral blood during SIV infection in two sooty mangabeys (FLg and FYg) and two rhesus macaques (RLk-1 and RFi-1). PBMC were immunophenotyped with CD38, CD3, and CD8 and analyzed by three-color flow cytometry. (a) Analysis of CD38 on gated CD3+ CD8+ T lymphocytes. (b) Longitudinal evaluation of the proportion of CD38-expressing CD3+ CD8+ T lymphocytes. Data points from day 0 to week 34 are available for FLg and RLk-1 and from week 30 to week 50 for FYg and RFi-1. The last data point reflects the time of death of RLk-1 and RFi-1. The mean normal values were obtained from immunophenotyping uninfected sooty mangabeys (n = 7) and uninfected rhesus macaques (n = 7).
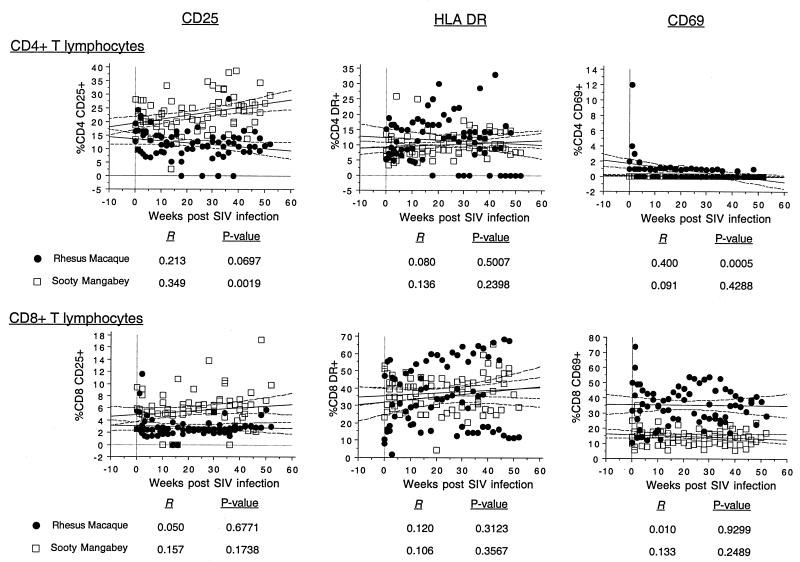
We also longitudinally examined the expression of the lymphocyte activation markers CD25, HLA DR, and CD69 on CD4+ and CD8+ T lymphocytes by using two-color flow cytometry. Simple regression analysis was used to generate regression plots to determine the association between SIV infection and the proportion of circulating activated T lymphocytes in sooty mangabeys and rhesus macaques (Fig. 7). The proportion of circulating CD8+ T lymphocytes expressing either CD25, CD69, or HLA DR did not change significantly during SIV infection (Fig. 7). In macaques, a decline in CD25-expressing CD4+ T lymphocytes was observed, while in the mangabeys there was a significant increase in CD25-expressing CD4+ T lymphocytes (Fig. 7). A transient increase in the proportion of CD4+ and CD8+ T lymphocytes expressing CD69 or CD25 was observed 7 days after SIV infection in the macaques (Fig. 7).
FIG. 7.
Longitudinal analysis of association between SIV infection and peripheral T lymphocytes expressing CD25, HLA DR, or CD69 in SIV infection. Regression plots were generated by simple regression analysis using time after SIV infection as the independent variable and the mean and 95% confidence intervals of the proportion of CD4+ or CD8+ T lymphocytes expressing the activation marker as the dependent variable. The data were generated from three sooty mangabeys and three rhesus macaques.
Longitudinal analysis of distribution of naive and memory T-lymphocyte subsets in sooty mangabeys and rhesus macaques during the course of SIV infection.
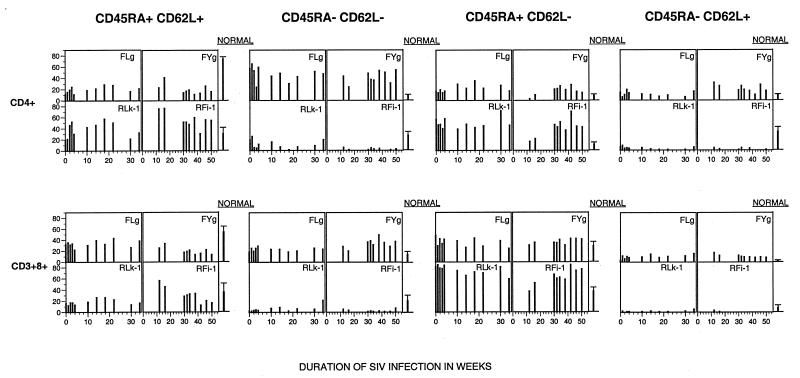
Studies of HIV-infected humans have demonstrated that T lymphocytes of the naive phenotype (defined as coexpressing CD45RA and CD62L) decrease during disease progression (57). Naive CD8+ T lymphocytes declined in adult HIV-infected individuals progressing to AIDS but were preserved in nonprogressors (57). To determine whether pathogenic SIV infection in rhesus macaques could be similarly distinguished from apathogenic SIV infection in sooty mangabeys, we investigated the proportions of naive and memory CD8+ and CD4+ T lymphocytes in peripheral blood longitudinally in two sooty mangabeys and two rhesus macaques during SIV infection.
Using multiparametric flow cytometry, the percentages of naive and memory phenotype CD4+ and CD8+ T lymphocytes were determined sequentially from the onset of SIV infection in one pair of infected animals (FLg and RLk-1) and from 12 weeks onward in another pair (FYg and RFi-1). In this longitudinal analysis of a limited number of animals, we did not observe an association between disease progression and decreased naive T lymphocytes in the peripheral blood (Fig. 8). At 34 weeks of infection, the percentages of circulating naive CD4+ (19 and 21%) and CD8+ (21 and 38%) T lymphocytes in the two SIV-infected sooty mangabeys were lower than the mean normal values obtained from six uninfected sooty mangabeys (CD4, 74% ± 3.9% [mean ± SD]; CD8, 54% ± 10.6% [Fig. 8]), while in the two SIV-infected rhesus macaques, the percentages of circulating naive CD4+ T lymphocytes (47 and 32%) were comparable to, and the percentages of circulating naive CD8+ T lymphocytes (37 and 16%) were lower than, the mean normal values obtained from six uninfected macaques (CD4, 30% ± 11%; CD8, 54% ± 10.6% [Fig. 8]).
FIG. 8.
Longitudinal analysis of naive and memory phenotype of CD4+ and CD8+ T lymphocytes in two rhesus macaques and two sooty mangabeys during SIV infection. PBMC were immunophenotyped and analyzed on a FACSVantage (BDIS), using four-color flow cytometry. The animal pairs FLg plus RLk-1 and FYg plus RFi-1 were infected concurrently. Data points from day 0 to week 34 are available for FLg plus RLk-1 and from week 10 to week 50 for FYg plus RFi-1. The last data point reflects the time of death of RLk-1 and RFi-1. The mean normal values were obtained from immunophenotyping uninfected sooty mangabeys (n = 7) and uninfected rhesus macaques (n = 7).
Striking differences in the memory phenotype of CD4+ and CD8+ T lymphocytes were observed between SIV-infected sooty mangabeys and rhesus macaques (Fig. 8). In both macaques studied, CD45RA− memory T lymphocytes (particularly CD62L+) were virtually absent during SIV infection, resulting in >90% of the memory pool of T lymphocytes being comprised of CD45RA+ CD62L− cells (Fig. 8). In contrast, CD45RA− T lymphocytes, especially of the phenotype CD45RA− CD62L−, were preserved in both SIV-infected sooty mangabeys and made up >50% of the memory pool of CD4+ T lymphocytes.
Differential patterns of cytokine secretion in sooty mangabeys and rhesus macaques following acute infection with SIVmac239.
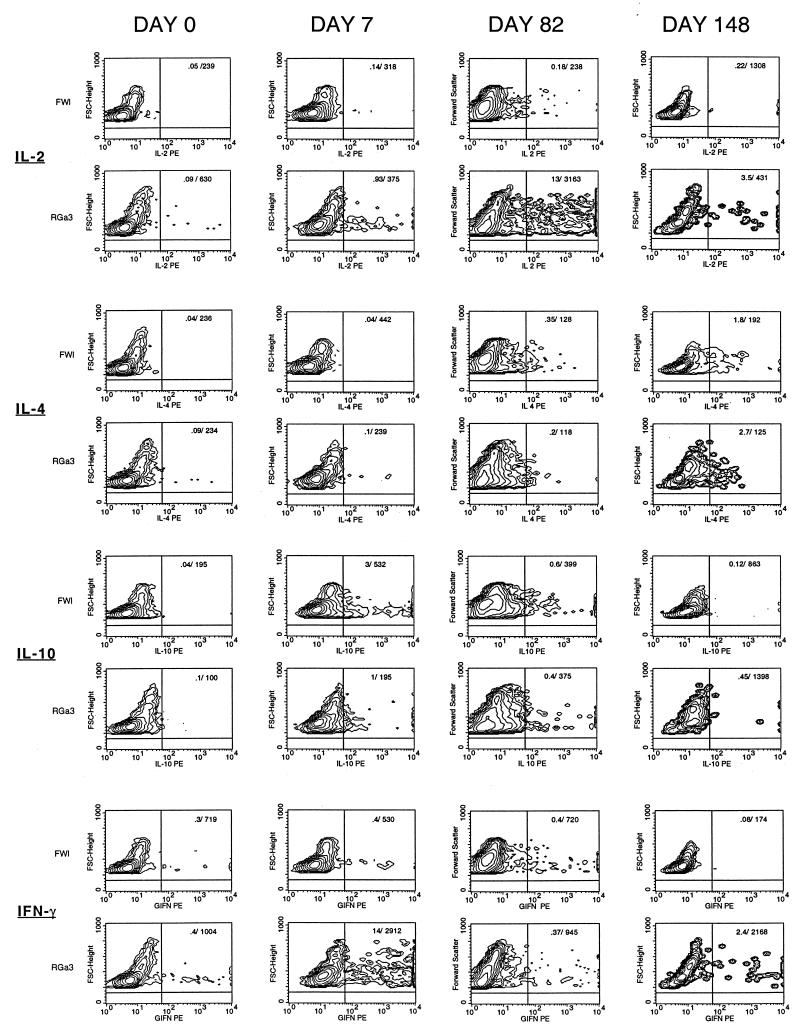
To investigate the possibility that differences in cytokine secretion correlate with differences in SIV-associated pathogenicity, constitutive cytokine secretion in ex vivo PBMC was examined longitudinally in one sooty mangabey and one rhesus macaque (FWl and RGa-3, the third pair of infected animals) from the onset of SIV infection. Cytokine secretion was assessed at the single-cell level by flow cytometric measurement of intracellular cytokines stained with directly conjugated anticytokine antibodies by using published methodology (52, 60). We used PBMC incubated with monensin alone (in the absence of mitogenic or CD3 stimulation) to analyze ex vivo cytokine secretion in unstimulated PBMC following acute SIV infection. One week after SIV infection, unstimulated PBMC from the rhesus macaque showed a 10-fold increase in the percentages of IL-2- and IL-10-secreting cells and a 35-fold increase in the number of IFN-γ secreting cells (Fig. 9). In contrast, only increased IL-10 production (eightfold increase in number and threefold increase in mean fluorescence intensity) was evident in the sooty mangabey (Fig. 9). Increased IL-2 and/or IFN-γ secretion persisted in the rhesus macaque even after the onset of CD4+ T lymphocytopenia and was evident until 21 weeks (Fig. 9). At 21 weeks, there was a concomitant increase in IL-4 which persisted until death of the animal (data not shown). The late increase in IL-4 was not accompanied by increases in IL-2 and IFN-γ and may denote a Th2 shift. In the sooty mangabey, 2- to 60-fold increases in IL-4 were observed after 6 weeks of infection and were not associated with increases in IL-2 or IFN-γ (Fig. 9 and data not shown). This pattern of absent or Th2 type of cytokine secretion pattern in the sooty mangabey was sustained till 40 weeks of SIV infection (Fig. 9).
FIG. 9.
Longitudinal comparison of intracellular cytokine secretion in PBMC during SIV infection in one sooty mangabey (FWl) and a concurrently infected rhesus macaque (RGa-3). Contour plots showing intracellular IL-2, IL-4, IL-10, and IFN-γ in unstimulated PBMC at days 0, 7, 82, and 148 following SIV infection. The numbers in the right upper quadrant of each plot are the values for the percentage/geometric mean channel fluorescence of cytokine-positive cells in the lymphocyte gate.
The use of CD4 and CD8 surface staining concomitant with intracellular cytokine staining allowed us to delineate the T lymphocytes responsible for cytokine secretion. In the rhesus macaque, increased secretion of IL-2 and IFN-γ was contributed largely by CD8+ and CD4+ CD8+ T lymphocytes (data not shown). Even though cytokine secretion was also detected in CD4+ T lymphocytes, the mean fluorescence intensity was 5- to 10-fold higher in CD8+ and CD4+ CD8+ T lymphocytes than in CD4+ T lymphocytes (data not shown).
The differences in cytokine secretion between the rhesus macaque and the sooty mangabey were observed only with fresh PBMC that had not been stimulated in vitro and hence are likely to reflect the actual in vivo effect of SIV infection in the two species. In vitro stimulation of PBMC with PMA and calcium ionophore prior to cytokine staining resulted in a 20- to 100-fold enhancement of cytokine staining signal. However, the differential pattern of cytokine secretion evident with unstimulated PBMC was no longer apparent (data not shown). In vitro stimulation also resulted in decreased cytokine secretion in already activated PBMC. Thus, at day 82, unstimulated rhesus PBMC showed marked increases in intracellular IL-2 (Fig. 9) which was absent after stimulation with PMA and calcium ionophore (data not shown). This may have been due to apoptosis following stimulation of already activated cells.
DISCUSSION
In this prospective study, we examined host immune events during early and late SIV infection in two nonhuman primate species with divergent outcomes of SIV infection. Pathogenic SIV infection in rhesus macaques was associated with high SIV viral loads, limited SIV-specific CTL activity, low-level neutralizing antibodies, progressive CD4+ T lymphocytopenia, loss of T-lymphocyte homeostasis, and increased constitutive production of IL-2 and IFN-γ. In contrast, SIVmac239 infection in sooty mangabeys did not result in disease and was associated with low SIV viral loads, sustained and broadly directed SIV-specific CTL activity, undetectable neutralizing antibodies, maintenance of T-lymphocyte homeostasis and CD4+ T-lymphocyte counts, and increased production of IL-10.
The features of SIVmac239 infection in sooty mangabeys were in striking contrast to natural SIVsmm infection in sooty mangabeys, where absence of disease is associated with persistent high viremia (17a, 54). We have previously investigated SIV-specific CTL responses in such naturally infected sooty mangabeys. In a cross-sectional analysis, 12 naturally infected sooty mangabeys housed at the Yerkes Regional Primate Research Center had high SIV viral loads and undetectable circulating activated SIV-specific CTL activity (17a). SIV-specific memory CTL were detected consistently in only 7 of 12 mangabeys and when detected, the bulk CTL response was generally weaker (≤20% specific lysis) than in SIVmac239-infected mangabeys. No correlation between viral load and the strength or presence of in vitro CTL activity was observed. Since early viral and host immune events can predict subsequent disease progression in HIV and SIV infection (32, 45), we undertook a prospective comparison of SIV infection in sooty mangabeys and rhesus macaques. We chose SIVmac239 for experimental SIV infection, as it is a pathogenic molecular clone of SIV in rhesus macaques, and its use enabled us to control for virus heterogeneity as a confounding factor determining the outcome of infection. Further, it enhanced our capacity to detect CTL responses due to the availability of vaccinia virus recombinants expressing SIV proteins of the homologous virus.
The contrasting features of SIVmac239 and natural SIVsmm infection in sooty mangabeys prevents any conclusions regarding early immune events following natural SIV infection in sooty mangabeys. However, it does allow us to draw two interesting conclusions. First, since vigorous and broad CTL responses were present in SIVmac239-infected mangabeys, limited CTL responses in naturally SIV infected sooty mangabeys are unlikely to be due to a genetically determined inability to respond to SIV antigens. Second, in spite of differences in viral load and CTL activity between SIVmac239 and natural SIVsmm infection in sooty mangabeys, the outcome was the same: SIV infection did not lead to AIDS, nor did it perturb T-lymphocyte homeostasis. These observations may be consistent with the hypothesis of immunopathology in HIV infection proposed by Zinkernagel and Hengartner (70). Thus, the absence of disease in the setting of a high virus load and weak immune response may reflect protection from immunopathology, while the absence of disease in the setting of a low viral load and strong host immune response could indicate adequate control of viral replication.
The reason for low viral loads in SIVmac239-infected sooty mangabeys are likely to be multifactorial. One possibility is that SIVmac239, a virus derived from a molecular clone that was selected because of its ability to cause disease in rhesus macaques (28), replicates poorly in sooty mangabeys. Although SIVmac239 was derived from a virus that originally had its origin in sooty mangabeys (35), it is likely to reflect the process of adaptation to replication in rhesus macaques. In addition, most molecular clones of SIV are relatively impaired in their ability to replicate in nonhuman primates and are nonpathogenic (11, 23, 24, 26, 29, 37). Evidence in support of this hypothesis is the delayed onset and low level of primary viremia in all three sooty mangabeys, which was evident as early as 2 to 5 days after SIV infection. It is unlikely that virus-specific immune responses could have controlled viral replication at such early time points. Subsequently, the temporal association between the onset of CTL activity and fall in peak plasma viremia in macaques and mangabeys, and the presence of a sustained, broadly directed CTL response in association with a greater slope of decay and low-level steady state viremia in mangabeys, suggests that CTL activity contributed to suppression of viral replication. It is conceivable that suboptimal viral replication facilitated the development of enhanced immune responses in sooty mangabeys, resulting in an inverse association between CTL activity and SIV viral load. Neutralizing antibodies were detected in macaques but not mangabeys and hence are unlikely to have played a role in controlling viral replication.
The presence of a Th1-to-Th2 switch in HIV infection and its role in the development of AIDS remains controversial (42). We used flow cytometry to investigate species-specific differences in patterns of cytokine secretion by longitudinally evaluating intracellular cytokines ex vivo in PBMC at the single-cell level. Direct examination of PBMC without subjecting them to in vitro stimulation allowed a more representative evaluation of the in vivo effect of SIV infection on cytokine secretion. Further, the use of flow cytometry enabled us to determine the phenotype of cytokine-secreting cells. At day 7 after SIV infection, in the one rhesus macaque studied, there was a 35-fold increase in IFN-γ secreting cells in the peripheral blood. The source of the increased IFN-γ was chiefly CD8+ and to a lesser extent CD8+ CD4+ T lymphocytes. At this time point, 14% of the circulating CD8+ T lymphocytes were secreting IFN-γ. Based on the recent observations of Butz and Bevan (5) and Murali-Krishna et al. (43), who documented that 50 to 70% of the expanded pool of CD8+ T lymphocytes during acute lymphocytic choriomeningitis virus infection are virus specific, these cells may represent SIV-specific activated CD8+ T lymphocytes. Surprisingly, an expansion of IFN-γ-secreting CD8+ T lymphocytes was not observed in the concurrently infected sooty mangabey, despite the presence of a strong SIV-specific CTL response in this animal. Instead, there was a moderate increase in IL-10-secreting cells, the chief source of which were CD4− CD8− cells. It is possible that we missed the time point for detecting expansion of IFN-γ-secreting CD8+ T lymphocytes in the sooty mangabey, since SIV-specific CTL activity was detected later than in the rhesus macaque. However, up to 12 weeks of infection, the pattern of cytokine secretion in the rhesus macaque was a predominantly Th1 pattern, while in the sooty mangabey, modest increases of IL-4 and/or IL-10 were observed. An increase in IL-4 was observed after 20 weeks in the macaque. Our observations indicate that at least in the sooty mangabeys, the presence of IL-10 but not IFN-γ or IL-2 secretion during acute infection can be associated with lack of disease and strong SIV-specific CTL responses. Our results are in contrast to published data on cytokine production in SIV infection in cynomolgus macaques (4). Unstimulated PBMC from cynomolgus macaques infected with an attenuated SIV (pC8 clone of SIVmac32H), but not those infected with pathogenic SIVmac251, showed increased levels of IL-2, IL-4, and IFN-γ mRNA during acute SIV infection (4). The reason for the contrasting pattern observed in the SIVmac239-infected macaque in our study is unclear, though it may be related to differences in virus strain, host species, or the techniques used to assess cytokine production. It is also important that our preliminary observations be confirmed in a larger number of animals. Increased IFN-γ production has been found in chimpanzees infected with pathogenic HIV strains and in HIV-infected humans. In two HIV-infected chimpanzees that developed CD4+ T lymphocytopenia, transient increases in IFN-γ mRNA were observed in unstimulated PBMC at the time of increased HIV viremia (65). Constitutively increased IFN-γ mRNA has also been observed in PBMC and lymph nodes of HIV-infected humans throughout the course of infection (19). In a study of cytokine expression in primary HIV infection, two patterns of IFN-γ secretion were observed (18). In one, the level of IFN-γ expression was low throughout primary infection. In the other, there was a peak in IFN-γ expression in CD8+ T cells which coincided with oligoclonal expansions of certain Vβ subsets of CD8+ T cells (18).
In HIV-infected humans, a decline in naive CD8+ T lymphocytes (57) and an increase in surface expression of CD38 on CD8+ T lymphocytes (33) are features of progressive infection. In our study, such an association was not observed during longitudinal evaluation of SIV infection in two sooty mangabeys and two rhesus macaques. Even though numbers of CD38-expressing CD8+ T lymphocytes were greater in SIV-infected rhesus macaques than in sooty mangabeys, this difference was also present in uninfected macaques and mangabeys. The significance of the high proportion of peripheral blood CD8+ T lymphocytes expressing CD38 in uninfected macaques is not known. Our finding that a high percentage of CD8+ T lymphocytes in rhesus macaques expressed CD38 independent of SIV infection is consistent with previous published data (17). The reason for these immune markers not serving as surrogates of disease progression in SIV infection may be twofold. One possibility is that the phenotypic classification of activation or naive and memory cell markers based on human data is not functionally applicable in sooty mangabeys or rhesus macaques; another possibility is that it reflects a species-specific or virus-specific difference in host response.
Certain phenotypic differences were observed between sooty mangabeys and rhesus macaques with increasing duration of SIV infection. In rhesus macaques, the proportion of CD4+ T lymphocytes expressing CD25 declined during SIV infection, and the decline was independent of CD4+ T lymphocytopenia. The decrease in activated CD4+ T lymphocytes in SIV-infected macaques may reflect selective loss of activated CD4+ T lymphocytes to productive SIV infection. Alternatively, similar to findings for HIV infection, it could reflect a functional impairment in the ability of infected CD4+ T lymphocytes to upregulate CD25 or CD69 (25, 48). A significant increase in CD25-expressing CD4+ T lymphocytes was observed in sooty mangabeys during SIV infection. The cause of this increase is unclear. A similar observation was reported for gut-associated lymphoid tissue of SIV-infected macaques (63). Intestinal CD4+ T lymphocytes expressing CD25 declined in SIVmac239-infected but increased in SIVmac239Δnef-infected rhesus macaques (63). It is possible that this signifies the presence of lymphocyte activation without lytic infection in attenuated SIV infection.
A selective loss of memory CD4+ T lymphocytes with a CD45RA+ phenotype was seen in the macaques but not the mangabeys. There is evidence that HIV and SIV preferentially replicate in memory CD4+ T lymphocytes (58, 61, 66). The selective decrease in the CD45RA− memory subset of CD4+ T lymphocytes in SIV-infected rhesus macaques may reflect the susceptibility of different memory populations of CD4+ T lymphocytes to SIV infection or differences in expression of memory markers associated with disease progression.
In conclusion, SIVmac239 infection in sooty mangabeys and rhesus macaques resulted in divergent outcomes and was associated with several differences in host response to SIV infection. Strong CTL responses and control of viral replication were associated with an absence of disease in SIVmac239-infected sooty mangabeys, though different mechanisms may be operative in maintaining an asymptomatic state in naturally infected mangabeys with high viral loads. Differences in T-cell phenotype and cytokine secretion were observed in macaques and mangabeys, but their role in conferring resistance or susceptibility to AIDS following SIV infection is not clear. Future studies in this animal model may shed light on host determinants of apathogenic lentivirus infection.
ACKNOWLEDGMENTS
We thank Ellen Lockwood, Pamela Carrol, Nirmala Bandrapalli, and Susan Czajak for technical assistance; Ann Brodie Hill, Mary Ann DeMaria, and Michael Rosenzweig for help with flow cytometry; Mark Mulligan for providing the rVV-239 vector; Brian Wilson for helpful discussions and gift of Vα24 MAb; Ron Veazey for help with the intracellular cytokine staining protocol; and Ron Desrosiers for providing SIVmac239.
This work was supported by Public Health Services grants RR 00168 and AI 38559 (A.K. and P.I.), RR 0165 (R.M.G. and H.M.), and AI 27763 (R.M.G.) and by a new investigator award from the University of California Universitywide AIDS Research Program (R.M.G.).
REFERENCES
- 1.Ahmed-Ansari A, Brodie A R, Fultz P N, Anderson D C, Sell K W, McClure H M. Flow microfluorometric analysis of peripheral blood mononuclear cells from nonhuman primates: correlation of phenotype with immune function. Am J Primatol. 1989;17:107–131. doi: 10.1002/ajp.1350170202. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed-Ansari A, Powell J D, Jensen P E, Yehuda-Cohen T, McClure H M, Anderson D, Fultz P N, Sell K W. Requirements for simian immunodeficiency virus antigen-specific in vitro proliferation of T cells from infected rhesus macaques and sooty mangabeys. AIDS. 1990;4:399–407. doi: 10.1097/00002030-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ansari A A, Mayne A, Hunt D, Sundstrom J B, Villinger F. TH1/TH2 subset analysis. I. Establishment of criteria for subset identification in PBMC samples from nonhuman primates. J Med Primatol. 1994;23:102–107. doi: 10.1111/j.1600-0684.1994.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste O, Vaslin B, Grand R L, Cheret A, Matheux F, Theodoro F, Cranage M P, Dormont D. Comparative interleukin-2/interferon-γ and IL-4/IL-10 responses during acute infection of macaques inoculated with attenuated nef-truncated or pathogenic SIVmac251 virus. Proc Natl Acad Sci USA. 1996;93:3658–3663. doi: 10.1073/pnas.93.8.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Buchbinder, S., et al. Unpublished data.
- 5.Butz E A, Bevan M J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 7.Castro B A, Walker C M, Eichberg J W, Levy J A. Suppression of human immunodeficiency virus replication by CD8+ cells from infected and uninfected chimpanzees. Cell Immunol. 1991;132:246–255. doi: 10.1016/0008-8749(91)90023-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Telfer P, Gettie A, Reed P, Zhang L, Ho D D, Marx P A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cichutek K, Norley S. Lack of immune suppression in SIV-infected natural hosts. AIDS. 1993;7:S25–S35. [PubMed] [Google Scholar]
- 10.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant Va24-JaQ/Vb11 T cell receptor is expressed in all individuals by clonally expanded CD4-8-T cells. J Exp Med. 1994;180:1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denesvre C, Grand R L, Boissin-Cans F, Chakrabarti L, Hurtrel B, Vaslin B, Dormont D, Sonigo P. Highly attenuated SIVmac142 is immunogenic but does not protect against SIVmac251 challenge. AIDS Res Hum Retroviruses. 1995;11:1397–1406. doi: 10.1089/aid.1995.11.1397. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers R C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 13.Eichberg J W, Zarling J M, Alter H J, Levy J A, Berman P W, Gregory T, Lasky L A, McClure J, Cobb K E, Moran P A, Hu S-L, Kennedy R C, Chanh T C, Dreesman G R. T-cell responses to human immunodeficiency virus (HIV) and its recombinant antigens in HIV-infected chimpanzees. J Virol. 1987;61:3804–3808. doi: 10.1128/jvi.61.12.3804-3808.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fultz P N, McClure H M, Anderson D C, Swenson R B, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fultz P N, Stricker R B, McClure H M, Anderson D C, Switzer W M, Horaist C. Humoral response to SIV/SMM infection macaque and mangabey monkeys. J Acquired Immune Defic Syndr. 1990;3:319–329. [PubMed] [Google Scholar]
- 16.Fultz P N, Anderson D C, McClure H M, Dewhurst S, Mullins J I. SIVsmm infection of macaque and mangabey monkeys: correlation between in vivo and in vitro properties of different isolates. Dev Biol Stand. 1990;72:253–258. [PubMed] [Google Scholar]
- 17.Giorgi J V, Hultin L E, Desrosiers R C. The immunopathogenesis of retroviral diseases: no immunophenotypic alterations in T, B, and NK cell subsets in SIVmac239-challenged rhesus macaques protected by SIVDnef vaccination. J Med Primatol. 1996;25:186–191. doi: 10.1111/j.1600-0684.1996.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 17a.Grant, R., et al. Unpublished data.
- 18.Graziosi C, Gantt K R, Vaccarezza M, Demarest J F, Daucher M B, Saag M S, Shaw G M, Quinn T C, Cohen O J, Welbon C C, Pantaleo G, Fauci A S. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graziosi C, Pantaleo G, Gantt K R, Fortin J-P, Demarest J F, Cohen O J, Sekaly R, Fauci A S. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 20.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 21.Heeney J, Bogers W, Buijs L, Dubbes R, Haaft P T, Koornstra W, Niphuis H, Nara P, Teeuwsen V. Immune strategies utilized by lentivirus infected chimpanzees to resist progression to AIDS. Immunol Lett. 1996;51:45–52. doi: 10.1016/0165-2478(96)02554-0. [DOI] [PubMed] [Google Scholar]
- 22.Heeney J, Jonker R, Koornstra W, Dubbes R, Niphuis H, Di-Rienzo A-M, Gougeon M-L, Montagnier L. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 23.Heeney J L, Holterman L, Haaft P T, Dubbes R, Koornstra W, Teeuwsen V, Bourquin P, Norley S, Niphuis H. Vaccine protection and reduced virus load from heterologous macaque-propagated SIV challenge. AIDS Res Hum Retroviruses. 1994;10:S117–S121. [PubMed] [Google Scholar]
- 24.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson N, Parkin J M. Dysregulation of the interleukin-2 receptor alpha- and beta-chain expression in CD4 and CD8 T cells in HIV infection. Cytometry. 1997;30:289–295. [PubMed] [Google Scholar]
- 26.Johnson P R, Goldstein S, London W T, Fomsgaard A, Hirsch V M. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and african green monkeys. J Med Primatol. 1990;19:279–286. [PubMed] [Google Scholar]
- 27.Kawai T, Wong J, MacLean J, Cosimi A B, Wee S. Characterization of a monoclonal antibody (6G12) recognizing the cynomolgus monkey CD3 antigen. Transplant Proc. 1994;26:1845–1846. [PubMed] [Google Scholar]
- 28.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 29.Kestler H W, III, Li Y, Naidu Y M, Butler C V, Ochs M F, Jaenel G, King N W, Daniel M D, Desrosiers R C. Comparison of simian immunodeficiency virus isolates. Nature. 1988;331:619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- 30.Klein M R, Baalen C A v, Holwerda A M, Garde S R K, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knuchel M, Bednarik D P, Chikkala N, Ansari A A. Biphasic in vitro regulation of retroviral replication by CD8+ cells from nonhuman primates. J Acquired Immune Defic Syndr. 1994;7:438–446. [PubMed] [Google Scholar]
- 32.Lifson J D, Nowak M A, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Brown C, Schneider D, Wahl L, Lloyd A L, Williams J, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J Virol. 1997;71:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z Y, Cumberland W G, Hultin L E, Prince H E, Detels R, Giorgi J V. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z Y, Hultin L E, Cumberland W G, Schmid I, Matud J L, Detels R, Giorgi J V. Elevated relative fluorescence intensity of CD38 antigen expression on CD8+ T cells is a marker of poor prognosis in HIV infection—results of 6 years of follow-up. Cytometry. 1996;26:1–7. doi: 10.1002/(SICI)1097-0320(19960315)26:1<1::AID-CYTO1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Mansfield K G, Lerche N W, Gardner M B, Lackner A A. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol. 1995;24:116–122. doi: 10.1111/j.1600-0684.1995.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 36.Margolick J B, Munoz A, Donnenberg A D, Park L P, Galai N, Giorgi J V, O’Gorman M R G, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. Nat Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 37.Marthas M L, Sutjipto S, Higgins J, Lohman B, Torten J, Luciw P A, Marx P A, Pedersen N C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990;64:3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClure H M, Anderson D C, Fultz P N, Ansari A A, Lockwood E, Brodie A. Spectrum of disease in macaque monkeys chronically infected with SIV/SMM. Vet Immunol Immunopathol. 1989;21:13–24. doi: 10.1016/0165-2427(89)90126-8. [DOI] [PubMed] [Google Scholar]
- 39.Means R E, Greenough T, Desrosiers R C. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol. 1997;71:7895–7902. doi: 10.1128/jvi.71.10.7895-7902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39a.Means, R. E. Personal communication.
- 40.Miller M D, Lord C I, Stallard V, Mazzara G P, Letvin N L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990;144:122–128. [PubMed] [Google Scholar]
- 41.Mollenhauer H M, Morre D J, Rowe L D. Alterations of intracellular traffic by monensin: mechanism, specificity and relationship to toxicity. Biochim Biphys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mossman T R. Cytokine patterns during the progression to AIDS. Science. 1994;265:193–194. doi: 10.1126/science.8023139. [DOI] [PubMed] [Google Scholar]
- 43.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 44.Murphey-Corb M, Martin L N, Rangan S R S, Baskin G B, Gormus B J, Wolf R H, Andes W A, West M, Montelaro R C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 44a.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH) 85–23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 45.Pantaleo G, Demarest J F, Schacker T, Vaccarezza M, Cohen O J, Daucher M, Graziosi C, Schnittman S S, Quinn T C, Shaw G M, Perrin L, Tambussi G, Lazzarin A, Sekaly R P, Soudeyns H, Corey L, Fauci A S. The qualitative nature of the primary immune response to HIV infection is a prognosticator of disease progression independent of the initial level of plasma viremia. Proc Natl Acad Sci USA. 1997;94:254–258. doi: 10.1073/pnas.94.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K, Margolick J B, Buchbinder S, Giorgi J V, Fauci A S. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 47.Peeters M, Janssens W, Haesvelde M V, Fransen K, Willems B, Heyndrickx L, Kestens L, Piot P, Groen G V D, Heeney J. Virologic and serologic characteristics of a natural chimpanzee lentivirus infection. Virology. 1995;211:312–315. doi: 10.1006/viro.1995.1407. [DOI] [PubMed] [Google Scholar]
- 48.Perfetto S P, Hickey T E, Blair P J, Maino V C, Wagner K F, Zhou S, Mayers D L, St. Louis D, June C H, Siegel J N. Measurement of CD69 induction in the assessment of immune function in asymptomatic HIV-infected individuals. Cytometry. 1997;30:1–9. doi: 10.1002/(sici)1097-0320(19970215)30:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Powell J D, McClure H M, Anderson D, Fultz P N, Sell K W, Ahmed-Ansari A. Phenotypic and functional differences in NK and LAK cells in the peripheral blood of sooty mangabeys and rhesus macaques. Cell Immunol. 1989;124:107–118. doi: 10.1016/0008-8749(89)90115-9. [DOI] [PubMed] [Google Scholar]
- 50.Powell J D, Villinger F, Yehuda-Cohen T, Vuchetich M, McClure H M, Sell K W, Ahmed-Ansari A. Identification of SIV/SMM viral proteins that induce T cell response in experimentally infected rhesus macaques and naturally infected sooty mangabeys by the cellular western blot assay. J Med Primatol. 1990;19:227–238. [PubMed] [Google Scholar]
- 51.Powell J D, Yehuda-Cohen T, Villinger F, McClure H M, Sell K W, Ahmed-Ansari A. Inhibition of SIV/SMM replication in vitro by CD8+ cells from SIV/SMM infected seropositive clinically asymptomatic sooty mangabeys. J Med Primatol. 1990;19:239–249. [PubMed] [Google Scholar]
- 52.Prussin C, Metcalfe D D. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995;188:117–128. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 53.Reimann K A, Tenner-Racz K, Racz P, Montefiori D C, Yasutomi Y, Lin W, Ransil B J, Letvin N L. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994;68:2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rey-Cuille M-A, Berthier J-L, Bomsel-Demontoy M-C, Chaduc Y, Montagnier L, Hovanessian A G, Chakrabarti L A. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol. 1998;72:3872–3886. doi: 10.1128/jvi.72.5.3872-3886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinaldo C, Huang X-L, Fan Z, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ritter G D J, Mulligan M J, Lydy S L, Compans R W. Cell fusion activity of the simian immunodeficiency virus envelope protein is modulated by the intracytoplasmic domain. Virology. 1993;197:255–264. doi: 10.1006/viro.1993.1586. [DOI] [PubMed] [Google Scholar]
- 57.Roederer M, Dubs J G, Anderson M T, Raju P A, Herzenberg L A, Herzenberg L A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Investig. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roederer M, Raju P A, Mitra D K, Herzenberg L A, Herzenberg L A. HIV does not replicate in naive CD4 T cells stimulated with CD3/CD28. J Clin Investig. 1997;99:1555–1564. doi: 10.1172/JCI119318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 60.Schauer U, Jung T, Krug N, Frew A. Measurement of intracellular cytokines. Immunol Today. 1996;17:305–306. doi: 10.1016/0167-5699(96)30020-0. [DOI] [PubMed] [Google Scholar]
- 61.Spina C A, Prince H E, Richman D D. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staprans S I, Corliss B C, Guthrie J L, Feinberg M B. Quantitative methods to monitor viral load in simian immunodeficiency virus infections. In: Adolph K W, editor. Viral genome methods. Boca Raton, Fla: CRC Press; 1996. pp. 167–184. [Google Scholar]
- 63.Veazey R S, DeMaria M, Chalifoux L V, Shvetz D E, Pauley D R, Knight H L, Rosenzweig M, Johnson R P, Desrosiers R C, Lackner A A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 63a.Veazey, R. S., and A. Kaur. Unpublished data.
- 64.Vicari A P, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 65.Villinger F, Brar S S, Brice G T, Chikkala N F, Novembre F J, Mayne A E, Bucur S, Hillyer C D, Ansari A A. Immune and hematopoietic parameters in HIV-1-infected chimpanzees during clinical progression towards AIDS. J Med Primatol. 1997;26:11–18. doi: 10.1111/j.1600-0684.1997.tb00314.x. [DOI] [PubMed] [Google Scholar]
- 66.Willerford D M, Gale M J, Benveniste R E, Clark E A, Gallatin W M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that include memory cells. J Immunol. 1990;144:3779–3783. [PubMed] [Google Scholar]
- 67.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple-deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yasutomi Y, Reimann K A, Lord C I, Miller M D, Letvin N L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993;67:1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yehuda-Cohen T, Powell J D, Villinger F, McClure H M, Sell K W, Ahmed-Ansari A. Comparison of SIV/SMM replication in CD4+ T cell and monocyte/macrophage cultures from rhesus macaques and sooty mangabeys. J Med Primatol. 1990;19:251–267. [PubMed] [Google Scholar]
- 70.Zinkernagel R M, Hengartner H. T-cell mediated immunopathology versus direct cytolysis by virus: implications for HIV and AIDS. Immunol Today. 1994;15:262–268. doi: 10.1016/0167-5699(94)90005-1. [DOI] [PubMed] [Google Scholar]