Abstract
The airway is an important target for gene transfer to treat cystic fibrosis and other diseases that affect the lung. We previously found that marker gene expression did not persist in the bronchial epithelium following adeno-associated virus (AAV) vector administration to the rabbit lung. In an attempt to promote continued expression, we tested repeat vector administration, but no additional transduction was observed, and the block to transduction correlated with the appearance of neutralizing antibodies to the viral capsid. Here we show that mice exhibit a similar response but that treatment with anti-CD40 ligand antibody (MR1) and a soluble CTLA4-immunoglobulin fusion protein (CTLA4Ig) at the time of primary AAV vector exposure allowed successful repeat transduction and prevented production of neutralizing antibodies. We also tested the possibility that an immune response caused the loss of marker-positive cells in the epithelial population in rabbits by evaluating AAV vector expression in immunocompetent and immunodeficient mice. In contrast to results in rabbits, marker protein expression persisted in the lung in both groups of mice. AAV vector transduction occurred in alveolar cells, airway epithelial cells, and smooth muscle cells, and vector expression persisted for at least 8 months. Although data on persistence of AAV vector expression in the human lung are not available, it is likely that repeat transduction will be necessary either due to loss of expression or to the need for repeat administration to deliver effective amounts of AAV vectors. Results presented here indicate that transient immunosuppression will allow such repeat vector treatment of the lung.
Genetic diseases that affect the lung may be cured by the use of gene therapy. Among these diseases, cystic fibrosis affects one in 3,000 Caucasian births and leads to debilitating lung disease. Gene therapy directed to the epithelial cells of the lung could possibly alleviate the pulmonary pathology that is the primary cause of morbidity in cystic fibrosis. The complex architecture of the lung and the inability to remove and reimplant airway epithelial cells require that gene transfer be done in vivo, posing important challenges to the development of effective gene therapy.
Adeno-associated virus (AAV) vectors are appealing candidates for in vivo transduction of airway epithelial cells. AAV itself is quite stable under normal physiologic conditions and is naturally tropic for the airway epithelium. AAV vectors can be made without the inclusion of any viral regulatory or structural genes that might elicit an immune response. Their ability to integrate into the host chromosome (24, 28) promotes persistence of gene expression. AAV vectors can transduce nondividing cells in animals (1, 8, 16, 20, 21, 33, 36), an important feature for transduction of slowly dividing airway epithelial cells.
The potential use of AAV vectors for gene therapy has been evaluated in the rabbit lung. Expression of the human cystic fibrosis transmembrane regulator (CFTR) from an AAV vector was detected by antibody staining at 7 days after vector infusion, and persistent expression was detected by reverse transcription-PCR at 7 months in adult lungs (10). In addition, AAV vector transduction in the developing neonatal rabbit lung has been observed in a variety of airway and alveolar cell types (31, 38). We have obtained quantitative data regarding rates of AAV vector transduction in the airway epithelium of adult rabbits by using vectors that expressed either the β-galactosidase (β-Gal) or the human placental alkaline phosphatase (AP) protein (14). We found that AAV vector transduction efficiency could be quite high in some localized areas of the airway epithelium but that it was low overall. While other in vivo studies have shown persistence of AAV vector expression in brain, liver, and skeletal muscle (1, 8, 16, 20, 21, 33, 36), and we found persistent marker protein expression in smooth muscle in the rabbit lung, the expression in epithelial cells did not persist, suggesting the need for repeated administration of AAV vectors for long-term treatment of genetic disease. However, readministration of AAV vectors failed to generate further transduction events, and this result was correlated with the appearance of virus-neutralizing antibodies in serum samples from animals exposed to the AAV vectors (14). Consistent with our results with the rabbit lung, attempts to readminister AAV vectors in skeletal muscle have also resulted in little or no new transduction (8, 20, 36).
Here we have tested whether transient immunomodulation with a CTLA4-immunoglobulin fusion protein (CTLA4Ig) and/or with MR1 protein might allow repeat AAV vector transduction in the lung. B7 proteins on antigen-presenting cells can bind CD28 or CTLA4 proteins on T cells. Binding of the former leads to T-cell activation, which is particularly important for the primary response of naive T cells to novel antigens (6). Binding of the latter dampens T-cell activation. CTLA4Ig is a soluble molecule composed of the extracellular domain of CTLA4 fused to an immunoglobulin IgG Fc domain. It binds B7 ligands on antigen-presenting cells with a much greater affinity than does CD28 (25, 35), thereby blocking the binding of the B7 proteins by CD28 and inhibiting T-cell priming. The CD40 protein is expressed primarily on activated T cells and is critical to their ability to provide help for B-cell antibody responses (11). MR1, a monoclonal antibody to CD40 ligand, profoundly inhibits antibody production in mice (13). It has been demonstrated that administration of MR1 and CTLA4Ig during the primary exposure to adenovirus vectors facilitates persistence and readministration of the vector to mouse hepatocytes (19). Similarly, treatment with an antibody to CD4 at the time of primary vector exposure allowed transgene expression following readministration of AAV vectors to skeletal muscle (27). However, there is considerable evidence that the inflammatory response in the lung is to a large degree compartmentalized from the systemic inflammatory response (15, 22, 29). In addition, the lung, unlike the liver and muscle, is protected by a combination of mucosal and systemic defenses (26). Thus, conclusions reached regarding strategies to prolong vector expression in other tissues cannot be directly extrapolated to the lung.
In this study, we show that administration of MR1 and CTLA4Ig during the primary exposure to an AAV vector allows for successful readministration of AAV vectors to the mouse lung. Both agents were required for maximum effect. We also studied transduction rates and persistence of AAV vector expression in normal and immunodeficient mice in comparison to our previous studies in rabbits. We found that AAV vectors transduced a wider variety of lung cells in mice than in rabbits and that vector expression after a single administration was not affected by the immunocompetence of the host. Vector expression persisted for at least 8 months in many cell types, in contrast to the transient expression that we observed in rabbits (14).
MATERIALS AND METHODS
Cell culture.
The 293 (12) and IB3 (37) cells were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of amphotericin B per ml. Cells were cultured at 37°C in an atmosphere of 5% CO2 in air.
AAV vector production.
Recombinant AAV plasmids were propagated in the bacterial strain JC8111 (7). The AAV vector CWRAP (9) contains the AP cDNA driven from a Rous sarcoma virus promoter and enhancer sequence and was obtained from S. Chatterjee (City of Hope National Medical Center, Duarte, Calif.). CWRZn contains a cDNA that encodes a nuclear localizing β-Gal in place of the AP cDNA (14). The vectors will be referred to as AAV-AP and AAV-βgal, respectively.
Vector stocks were generated as previously described (14). Briefly, cells were infected with adenovirus 5, and then vector plasmid (4 μg) and an AAV packaging plasmid, either pAAV/Ad (12 μg) (32) or pMTrepCMVcap (2), were cotransfected by using the calcium phosphate transfection method. Vector stocks were purified by CsCl centrifugation and stored at −80°C. Vector titers were determined by using IB3 cells as targets for transduction and were equal to 108 AP-positive (AP+) focus-forming units (FFU) per ml for AAV-AP and 105 β-Gal-positive (β-Gal+) FFU per ml for AAV-βgal. Although there was a significant difference in the transducing titer between the AP and β-Gal vectors, the number of genome-containing particles was actually similar, about 1010 per ml. Vector stocks were characterized for the presence of infectious adenovirus by plaque assay (14), and none was detected (<100 PFU/ml). Vector stocks were assayed for contamination by replication-competent AAV by infectious center assay, and results were calculated as a percentage of the vector titer, also measured by infectious center assay (14). Vectors made by using the standard packaging plasmid pAAV/Ad (32) contained 1 to 5% replication-competent AAV, and vectors made with the more recently available pMTrepCMVcap packaging plasmid (2) contained no detectable replication-competent AAV (<0.00002%). We observed similar results with both types of vector preparations containing low or undetectable replication-competent AAV.
AAV vector delivery to mouse airways.
The studies were performed in accordance with the guidelines set forth by the Institutional Review Office of the Fred Hutchinson Cancer Research Center. BALB/c, C57BL/6 (B6), and 129/Sv mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Immunoglobulin M chain knockout mice (Igh−) on a B6 background, which lack mature B lymphocytes and do not produce antibody, were obtained from Jackson Laboratories and bred in our facility. Severe combined immunodeficient (SCID) mice on a B6 background were obtained from Jackson Laboratories, and recombinase-activating gene II (RagII) knockout mice were obtained on a mixed 129/Sv-B6 background that lack mature T and B lymphocytes were obtained from Taconic Laboratories (Germantown, N.Y.) and bred in our facilities. Nude mice, which are deficient in mature T lymphocytes, were obtained from Jackson Laboratories. (For a review of immunodeficient mouse strains, see reference 23).
Mice were sedated by an intraperitoneal injection of 48 mg of ketamine and 3.3 mg of xylazine per kg of body weight prior to intratracheal inoculation. The trachea was exposed by a skin incision above the trachea, and 100 μl of the vector was delivered from a 1-ml syringe with a 22-gauge needle. After vector delivery, the incision was sutured and the animals were awake within 1 h. When animals received vector by nasal aspiration, they were anesthetized in a jar containing cotton gauze soaked with metafane prior to administration to the nares of 100 μl of vector from a micropipette tip. Animals spontaneously inhaled the vector droplets. Animals revived within seconds after vector delivery by nasal aspiration. When immunomodulators were used, 0.2 mg of murine CTLA4Ig per animal and 0.25 mg of murine MR1 per animal (Bristol-Myers Squibb) (18, 19) were given by an intraperitoneal injection on days 0, 2, and 4. Hamster immunoglobulin (HIg; 0.25 mg per animal) and murine monoclonal antibody (L6; 0.26 mg per animal) were given as control molecules for the immunomodulating drugs. FK506 (3.7 mg/kg) was given daily by intraperitoneal injection. In the first readministration experiment, animals were given the first AAV vector by intratracheal injection on day 0 and the second AAV vector by nasal aspiration on day 60. The animals were sacrificed 3 weeks later. In the second readministration experiment, both AAV vectors were administered by nasal aspiration and the animals were sacrificed 2 weeks after the second administration of vector.
AP and β-Gal staining of mouse lung tissue.
At 1, 3, 8, 12, and 32 weeks following vector instillation, animals were anesthetized with 100 mg of ketamine and 6.6 mg of xylazine per kg. Blood samples were obtained by cardiac puncture. The chest was then opened, and the lungs were excised. An endotracheal tube was inserted into the trachea, and fixative (2% paraformaldehyde in phosphate-buffered saline [PBS]) at a pressure of 25 cm of H2O was instilled into the lungs. The trachea was then ligated, and the lungs were immersed in fixative for 3 h for the AP staining procedure or 1 h for the β-Gal staining procedure. After fixation, the lungs were drained and refilled with PBS three times, cut into 3-mm-thick slices, and rinsed three additional times with PBS for 30 min each on a rocker. For β-Gal staining, the tissue slices were stained overnight in X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining buffer (14) at room temperature. For AP staining, the tissue slices were placed in a 10-ml conical tube containing 7 ml of PBS and heated in a water bath at 69°C for 1.5 h. The tissue slices were then placed in AP staining buffer (14) overnight at room temperature.
Quantitation of transduction efficiency.
Stained tissue slices were cut into blocks (3 mm on a side) and embedded in paraffin. Serial sections were taken from each lung sample and stained with nuclear fast red (sections 1, 3, and 5) or hematoxylin and eosin (section 2). Quantitation of transduction efficiency was done by counting the number of AP+ cells per section on the slides stained with nuclear fast red. Two to three sections, approximately 1 cm2 of tissue each (5-μm thickness), were scored per lung. Values were expressed as AP+ cells per cm2. Cell categories were designated as follows: bronchial epithelial cells are cells in an airway epithelium having underlying cartilage, distal airway epithelial cells are cells in an airway epithelium that does not have underlying cartilage, alveolar cells are cells in the alveolar walls, smooth muscle cells are smooth muscle cells underlying airways, and vascular cells are smooth muscle cells in the walls of blood vessels.
Virus neutralization assay.
Serum samples from mice were incubated at 56°C for 30 min to inactivate complement. AAV-βgal was diluted in DMEM containing 1% fetal bovine serum to obtain 5 × 103 β-Gal FFU/ml (as determined on IB3 cells and equivalent to approximately 5 × 108 genome-containing particles of AAV-βgal). Heat-treated serum was added to 200 μl of diluted virus to achieve the desired dilution of serum. The virus-serum mixtures were incubated for 1 h at 37°C. Then, 1 ml of DMEM containing 5% fetal bovine serum was added to each sample. Each sample was split between two wells containing IB3 cells that had been plated at 5 × 104 cells per well (12-well plates) on the previous day. Two days following vector exposure, the cells were fixed for 15 min in 3.7% formaldehyde in PBS and rinsed three times in PBS. Cells were stained for β-Gal expression by incubation overnight in X-Gal staining buffer at room temperature.
Spleen cell proliferation.
Spleen cell suspensions were generated and cultured as previously described (18), except that serum-free HL-1 medium (BioWhittaker, Walkersville, Md.) was used instead of RPMI 1640 medium supplemented with 10% fetal calf serum because background uptake of [3H]thymidine is lower in HL-1 medium. Various concentrations of UV-inactivated AAV-AP were added to replicate wells containing 5 × 105 splenocytes per well. After 72 h, [3H]thymidine was added to the wells, and incorporation was determined 24 h later, as described previously (18).
Antibody responses to tetanus immunization.
To determine the capacity of mice to produce antibodies to a novel antigen, mice were immunized by intraperitoneal injection of 0.1 ml of tetanus-diphtheria vaccine (Connaught Laboratories, Swiftwater, Pa.). Antibodies to tetanus toxoid were assayed by an enzyme-linked immunosorbent assay. Plates were coated overnight with tetanus toxoid (Massachusetts Biological Laboratories, Boston, Mass.) in carbonate buffer (pH 9.6), blocked with PBS containing 3% bovine serum albumin and 0.05% Tween 20, washed, and incubated with serum samples that were diluted serially in 10% PBS, 0.3% Tween 20, and 0.01 M EDTA. The plates were then washed, incubated with isotype-specific, peroxidase-conjugated antisera, and developed as previously described (18). The reciprocal of the lowest dilution yielding an increase in optical density at 405 nm of 0.2 compared to that of the preimmune sample for that mouse was taken as the titer. The lowest detectable titer was 100; values less than this were assigned a titer of 50 for purposes of analysis.
RESULTS
Transduction by AAV vectors in the mouse lung occurs in many cell types and is not affected by the immunocompetence of the host.
Different strains of immunocompetent and immunodeficient mice were given an AAV vector that encoded AP (AAV-AP; 107 AP+ FFU per animal) to test whether AAV transduction efficiency was affected by the immunocompetence of the host. At 14 days after inoculation, transduced cells were observed throughout the mouse lung in all strains of mice (Fig. 1B and D). AP+ epithelial and smooth muscle cells were found in the bronchial epithelium and just below the epithelium (Fig. 1D). AP+ cells were also observed in the parenchyma of the lung (Fig. 1D). Treatment of mice with saline (Fig. 1A and C) or an AAV vector expressing β-Gal did not result in AP staining in the lung (data not shown).
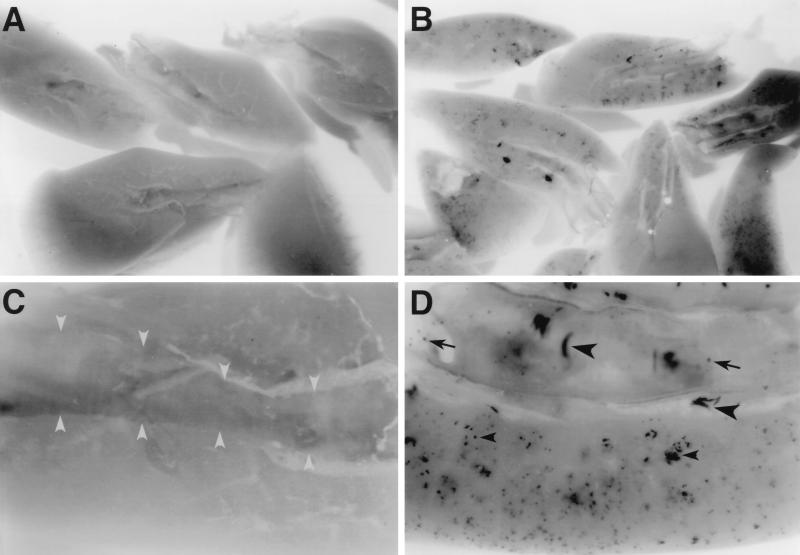
FIG. 1.
AAV vector transduction in the mouse lung. Mice were given saline (A and C) or 107 AP+ FFU of AAV-AP (made by using the AAV/Ad packaging plasmid) (B and D) by intratracheal inoculation. The lungs were excised and stained for AP expression 21 days after inoculation. (A and C) Saline-treated mouse lungs do not exhibit any AP+ cells. The bronchus is outlined by gray arrowheads in panel C. (B and D) AAV-AP-treated lungs show AP staining in epithelial cells (arrows) and smooth muscle cells (large arrowheads) of bronchial airways and in parenchymal cells (small arrowheads) of the lung. Original magnifications, ×8 (A and B) and ×32 (C and D).
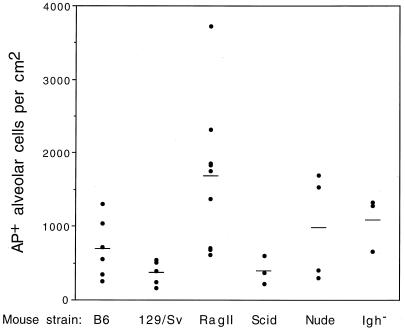
Histologic analysis of transduced mouse lungs showed that the stained cells were airway epithelial cells, alveolar cells, and smooth muscle cells underlying the epithelium or in vasculature (Fig. 2A through D). The alveolar cells comprised the majority of stained cells found in the lung. Indeed, AP+ cells in the other cell types occurred infrequently. Therefore, quantitation of stained alveolar cells was used to determine whether there was a difference in AAV vector transduction rates between the normal and immunodeficient strains of mice (Fig. 3). Animals that had combined immunodeficiencies exhibited transduction rates that were high (RagII), low (SCID), and moderate (nude). The parental strains of mice for the RagII-deficient animals, normal immunocompetent B6 mice and 129/Sv mice, exhibited moderate and low ranges of transduction rates, respectively. The immunoglobulin-deficient strain of mice (Igh−) exhibited a moderate transduction rate. Thus, while the transduction efficiency differed for different strains of mice, it was not positively correlated with immunodeficiency.
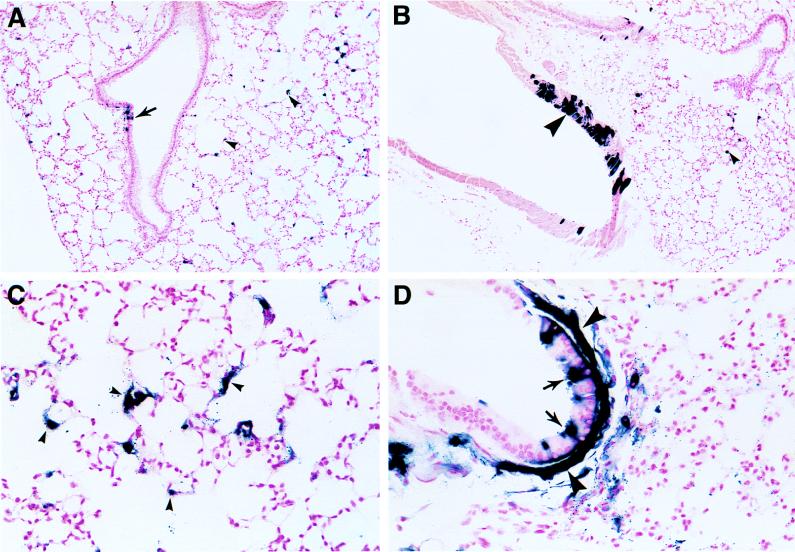
FIG. 2.
Histologic analysis of AAV vector-treated mouse lungs. Mice were given AAV-AP and were treated as described in the legend to Fig. 1. AP staining was performed 21 days after vector exposure. AP+ epithelial cells in distal airways are indicated in panels A and D by arrows. AP+ alveolar cells are designated by a small arrowheads in panes A, B, and C. AP+ smooth muscle cells were found beneath the airway epithelium (large arrowheads in panel D) and in vascular walls (large arrowhead in panel B). Original magnifications, ×100 (A and B) and ×400 (C and D).
FIG. 3.
Transduction by AAV vector in immunocompetent and immunodeficient strains of mice. Mice were treated as described in the legend to Fig. 1. AP staining was performed 21 days after vector exposure. Transduction efficiencies in individual animals (solid circles) and mean values (bars) are shown.
Relationship between AAV vector dose, transduction efficiency, and generation of neutralizing antibodies against AAV.
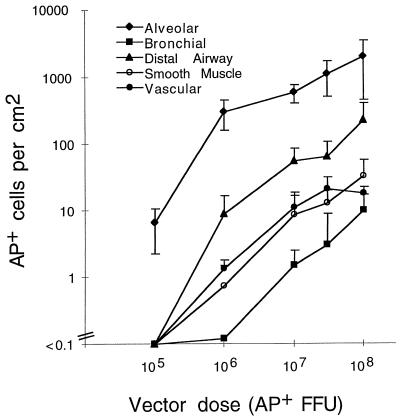
Increasing doses of the AAV-AP vector were given to mice in an effort to increase transduction in airway epithelial cells. Vector doses ranging from 105 to 108 AP+ FFU (containing no detectable replication-competent AAV) were given by nasal aspiration, and the mouse lungs were stained for AP expression 21 days after inoculation (n = 4 for each vector dose). Only alveolar cells stained for AP when animals were given a dose of 105 AP+ FFU of AAV-AP (Fig. 4). Transduction of the other cell categories occurred at low frequencies at 106 AP+ FFU and increased with higher doses of vector. At all doses, transduction of alveolar cells occurred more frequently than transduction of any of the other cell types (P ≤ 0.05). The least permissive cell category was the epithelial cells of the bronchial airway. These results indicate the minimum quantity of vector needed to detect transduction and show that increasing the vector dose resulted in a higher transduction rate in all cell categories.
FIG. 4.
Relationship of AAV vector dose to transduction efficiency in different cell populations of the mouse lung. B6 mice were given the indicated doses of the AAV-AP vector (made by using the pMTrepCMVcap packaging plasmid) by nasal aspiration. The lungs were excised 21 days after vector exposure and stained for AP expression. Arithmetic mean values ± standard deviations for the transduction rates are shown (n = 4 for each vector dose tested). Transduction rates for all cell types in animals receiving vector doses of ≥107 AP+ FFU were significantly different (P ≤ 0.05) from background.
A 5% transduction rate in distal airway epithelium was achieved at the highest dose tested (approximately 200 AP+ cells of 4,000 distal airway epithelial cells counted, representing 30 distal airways analyzed in one cross section of lung tissue). Additionally, about 4% of all alveolar cells were AP+ (2,000 AP+ alveolar cells of a total of 4.5 × 104 alveolar cells per cm2 of tissue; four microscope fields at ×1,000 magnification were scored per animal; n = 3). Because each tissue section was about 5 μm thick, there were an estimated 2,000 AP+ cells per 5 × 10−4 cm3 of tissue or 4 × 106 AP+ cells per cm3. The total number of AP+ alveolar cells was estimated to be about 4 × 106 per lung because a mouse lung had a volume of approximately 1 cm3 (as measured by volume displacement; n = 3). To calculate the particle-to-transducing unit ratio in vivo, the total number of genome-containing particles was divided by the estimated total number of AP+ cells (2 × 1010 genome-containing particles per 4 × 106 AP+ alveolar cells), giving a particle-to-transducing unit ratio of 5,000. This compares with a particle-to-transducing-unit ratio of 200 for the same vector measured in cultured cells (data not shown).
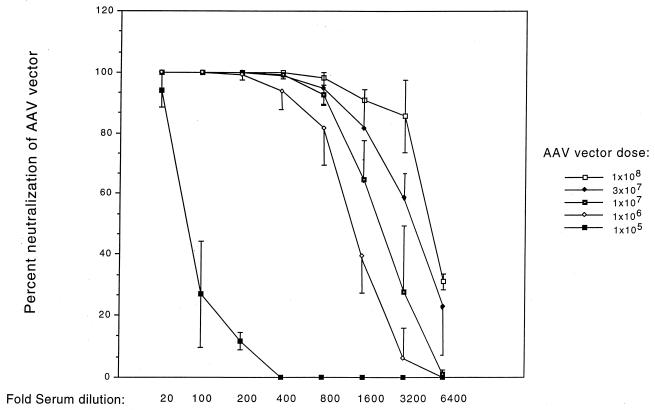
The amount of vector-neutralizing antibody in serum increased with higher vector doses and transduction rates (Fig. 5). Low neutralizing activity was detected in sera from animals given 105 AP+ FFU of AAV vector. Dilution of these sera 1:100 neutralized only 25% of the AAV vector. Higher neutralizing activity was observed in animals given 106 AP+ FFU or more. The sera from these animals achieved similar low levels of neutralization only after further dilution (1:1,600 to 1:6,400). These results show that a certain level of vector immunogen was required to generate a robust humoral immune response.
FIG. 5.
Relationship of AAV vector dose to generation of neutralizing antibodies against the AAV vector. Mice were treated as described in the legend to Fig. 4, and sera were obtained from animals 21 days after exposure to the AAV-AP vector. Percent neutralization of the AAV-βgal vector is shown. Duplicate assays were done for each serum dilution for all animals (n = 4 animals for each vector dose). Mean values ± standard deviations for percent neutralization at each serum dilution are shown.
AAV vector expression persists in all populations of transduced cells in the lungs of normal and immunodeficient mice.
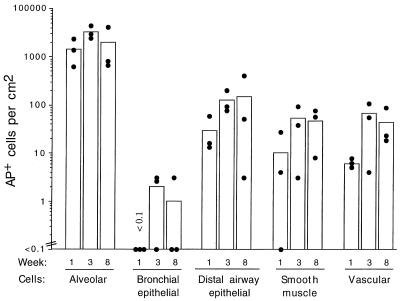
Persistence of expression was evaluated in immunodeficient mice to test whether loss of B- and T-cell-associated immune response functions resulted in persistence of marker gene expression in the epithelial population. RagII-deficient animals lack both T-cell- and B-cell-mediated pathways of immunity because they are deficient in a recombinase required for T-cell maturation (30). Nine RagII-deficient mice were given an AAV-AP vector by intratracheal injection, and three mice each were sacrificed at 1, 3, and 8 weeks after vector delivery (Fig. 6). The number of AP+ cells in each cell category was determined in histologic sections of the lung. At 7 days, AP+ cells occurred in all cell categories except the bronchial epithelium, and vector expression persisted in these cell populations for 8 weeks (the duration of the experiment). We found a few transduced bronchial epithelial cells in two of three animals at 3 weeks, and this low number was detectable for 8 weeks in one of three animals. These results showed that AAV vector expression can persist in both epithelial and smooth muscle cells of the lungs of RagII mice.
FIG. 6.
Persistence of AAV vector expression in the different cell populations of the RagII mouse lung. Mice were given 107 AP+ FFU of AAV-AP (made by using the AAV/Ad packaging plasmid) by intratracheal inoculation, groups of mice were sacrificed at the indicated times after exposure, and the lungs were stained for AP. Three animals in each group were analyzed. Means (bars) and individual values (solid circles) are shown.
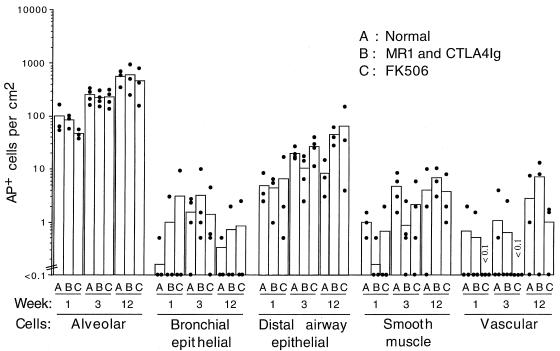
We next evaluated the persistence of AAV vector expression in normal immunocompetent B6 mice. We hypothesized that immunomodulation of normal mice would facilitate the persistence of AAV vector expression and perhaps even increase the initial transduction rate. Mice were given AAV vector alone, with MR1 and CTLA4Ig, or in conjunction with a daily administration of FK506. FK506 causes a general suppression of T-cell function (5), whereas MR1 and CTLA4Ig preferentially impair the response around the time of administration (18, 19). Animals were sacrificed at 1, 3, and 12 weeks following vector exposure (Fig. 7). The results for all groups of treated or control B6 mice were similar to the results for the RagII-deficient mice. At each time point, transduction rates in alveolar cells were higher than those in any of the other cell types, and persistence of marker protein expression occurred in all cell categories analyzed. Treatment of mice with FK506 or with MR1 and CTLA4Ig did not increase the initial transduction efficiency, the maximal transduction efficiency, or the persistence of AP+ cells. In one animal given FK506 for 12 weeks followed by 20 weeks without FK506, only a statistically insignificant drop in the number of AP+ cells was observed at 32 weeks (data not shown). Similar results were found at 32 weeks in two animals given MR1 and CTLA4Ig (data not shown).
FIG. 7.
Persistence of AAV vector expression in the different cell populations of the B6 mouse lung. Mice were given 107 AP+ FFU of AAV-AP (made by using the AAV/Ad packaging plasmid) by intratracheal inoculation. Animals were either untreated (A), treated with MR1 and CTLA4Ig (B), or treated with FK506 (C). Three animals were analyzed for weeks 1 and 12, and four animals were analyzed for week 3. Means (bars) and individual values (solid circles) are shown.
The persistence of vector expression in B6 mice suggested that there was little or no cellular immune response to the vector or the transgene product. To evaluate cellular immunity, splenocyte proliferation assays were done. Spleen cells from mice given the AAV vector alone proliferated weakly but detectably in response to AAV vector when assessed 21 days after primary administration of AAV vector (stimulation index, 5.5 ± 0.5; n = 2). In contrast, the response of splenocytes from mice treated with MR1 and CTLA4Ig at the time of vector exposure was less than that of those given vector only (stimulation index, 1.2 ± 0.2; n = 2; P < 0.01). However, both sets of animals exhibited transgene expression for at least 12 weeks.
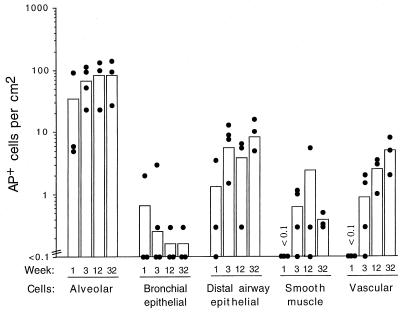
It has been reported that some strains of mice, such as the BALB/c and C3H/HeJ strains, clear adenovirus vector-transduced cells more rapidly than do other strains of mice (4). In addition, the B6 mice exhibited a longer persistence of therapeutic protein expression than the BALB/c mice when an adenovirus vector was used for gene transfer to liver cells. Therefore, persistence in normal BALB/c mice was evaluated (Fig. 8). Animals were given the AAV-AP vector, and AP+ lung cells were quantitated at 1, 3, 12, and 32 weeks (n = 4 for week 3; n = 3 for all other time points). At week 1, AP+ alveolar, bronchial epithelial, and airway epithelial cells were detected, and by week 3, transduced smooth muscle cells underlying the epithelium and in vasculature were also observed. Vector expression persisted for at least 32 weeks, the duration of the experiment. Although there seemed to be a drop in the values obtained in the bronchial epithelial cells and smooth muscle cells underlying the epithelium at 32 weeks, these values were not significantly different from those obtained at earlier time points. Indeed, AP+ cells were observed at 32 weeks in these cell types also. Taken together, the results in B6 and BALB/c mice show that AAV vector expression can persist in transduced lung cells of normal immunocompetent mouse for at least 8 months.
FIG. 8.
Persistence of AAV vector expression in the different cell populations of BALB/c mice. Mice were treated as described in the legend to Fig. 6. Three animals were analyzed for weeks 1, 12, and 32, and four animals were analyzed for week 3. Means (bars) and individual values (solid circles) are shown.
Transient immunosuppression by MR1 and CTLA4Ig allows repeat transduction.
The persistence of AAV vector expression in the mouse lung showed that in some species readministration of AAV vectors may not be required for several months. While it is not known whether AAV vector administration to lungs of humans will result in long-term vector expression similar to that seen in mice or will result in short-term expression similar to that seen in rabbits, it is likely that repeat transduction will be necessary to establish or maintain therapeutic levels of protein. To determine whether secondary transduction could be achieved, mice were treated with MR1 and CTLA4Ig or control antibodies (L6 and HIg) at the time of primary vector administration with AAV-βgal and were challenged 60 days later with AAV-AP (Table 1). No expression of AP was observed in mice given control antibody preparations, indicating that mice develop a strong neutralizing response against AAV vectors, as we observed in rabbits. In contrast, robust expression was observed in mice given MR1 and CTLA4Ig. Transduction rates were comparable to those seen in RagII-deficient mice and in B6 mice that received saline rather than AAV-βgal at the time of primary vector administration, with the exception of a slight drop in transduction of vascular cells in the MR1- and CTLA4Ig-treated mice and in the RagII-deficient mice in comparison to that of control animals that had not been exposed to an AAV vector.
TABLE 1.
Effect of immunosuppression on repeat transduction by an AAV vector in the mouse lunga
| Mouse strain | Primary vector | Secondary vector | No. of animals | Treatment | AP+ cells/cm2b
|
||||
|---|---|---|---|---|---|---|---|---|---|
| A | DA | BE | SM | V | |||||
| B6 | None (saline) | AAV-AP | 3 | None | 674 ± 120 | 43 ± 34 | 0.3 ± 0.5 | 5.5 ± 3.6 | 4.8 ± 2.9 |
| AAV-βgal | AAV-AP | 5 | L6 + HIg | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | |
| AAV-βgal | AAV-AP | 5 | MR1 + CTLA4Ig | 496 ± 268 | 49 ± 31 | 0.7 ± 1.1 | 5.5 ± 3.4 | 1.0 ± 1.0 | |
| AAV-βgal | None | 2 | None | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | |
| RagII | AAV-βgal | AAV-AP | 4 | None | 261 ± 194 | 20 ± 17 | <0.1 | 4.2 ± 2.6 | 0.4 ± 0.7 |
Animals were given saline or AAV-βgal (104 β-Gal+ FFU) by intratracheal administration in the presence or absence of immunomodulating drugs. Sixty days later, they received AAV-AP (107 AP+ FFU). On day 84, the animals were euthanized and their lungs were stained for AP expression.
Mean values ± standard deviations are shown. A, alveolar cells; DA, distal airway epithelial cells; BE, bronchial epithelium (defined as having underlying cartilage); SM, smooth muscle cells underlying airway epithelium; V, vascular smooth muscle cells.
Serum samples were obtained from mice on day 54 to assay for the presence of neutralizing activities prior to the second administration of AAV vector (Table 2). As expected, the serum samples from the saline-treated (control) mice and the vector-treated RagII mice did not have detectable neutralizing activities to AAV vector. Serum samples from B6 mice given MR1 and CTLA4Ig had undetectable or low neutralizing activities, whereas sera from B6 animals given L6 and HIg exhibited substantial neutralizing activities. Serum from one animal from the group treated with MR1 and CTLA4Ig had some neutralizing activity at a 1:20 dilution. AP+ cells were detected in this animal following the second administration, but the value was only 15% of that found in the other four treated animals (data not shown).
TABLE 2.
Neutralization of an AAV vector by sera from mice exposed to an AAV vector with or without immunosuppressiona
| Mouse strain | Primary vector | Treatment | No. of animals | Vector neutralization (%) for serum dilution indicated:
|
|
|---|---|---|---|---|---|
| 1:20 | 1:100 | ||||
| B6 | None (saline) | None | 3 | 0, 0, 0 | 0, 0, 0 |
| AAV-βgal | L6 + HIg | 4 | 100, 100, 100, 100 | 100, 100, 100, 100 | |
| AAV-βgal | MR1 + CTLA4Ig | 5 | 36, 0, 0, 0, 0 | 0, 0, 0, 0, 0 | |
| RagII | AAV-βgal | None | 4 | 0, 0, 0, 0 | 0, 0, 0, 0 |
Animals were given saline or AAV-βgal (as outlined in Table 1) in the presence or absence of immunomodulating drugs. Fifty-four days later, serum samples were obtained and analyzed for neutralizing activity to AAV.
Optimal transduction following readministration of an AAV vector requires both MR1 and CTLA4Ig.
The ability of either MR1 or CTLA4Ig alone to facilitate transduction following readministration was tested in a second experiment (Table 3). Animals were given the AAV-βgal vector by nasal inhalation, rechallenged with an AAV-AP vector on day 60, and sacrificed on day 74. Again, mice treated with MR1 and CTLA4Ig exhibited transduction in the lung after readministration of AAV vector. The number of AP+ cells in these mice was similar to that in the saline-treated control mice in most cell categories. As was noted in the first experiment, the values obtained for vascular cells from AAV-treated mice seemed lower than those for the saline control group. Although treatment with MR1 or CTLA4Ig alone facilitated secondary transduction, the numbers of AP+ cells were generally lower for mice in this treatment group than for mice treated with both MR1 and CTLA4Ig. CTLA4Ig seemed to be more effective than MR1 in facilitating AAV transduction by readministration.
TABLE 3.
Effect of CTLA4Ig and/or MR1 on repeat transduction by an AAV vector in the B6 mouse lunga
| Primary vector | Secondary vector | No. of animals | Treatment | AP+ cells/cm2b
|
||||
|---|---|---|---|---|---|---|---|---|
| A | DA | BE | SM | V | ||||
| None (saline) | AAV-AP | 3 | None | 389 ± 66 | 36 ± 5.6 | 1.0 ± 0.5 | 6.3 ± 7.9 | 17 ± 14 |
| AAV-βgal | AAV-AP | 4 | L6 + HIg | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| AAV-βgal | AAV-AP | 4 | MR1 + CTLA4Ig | 496 ± 66 | 46 ± 17 | 2.0 ± 2.1 | 7.7 ± 5.0 | 6.4 ± 4.7 |
| AAV-βgal | AAV-AP | 4 | CTLA4Ig | 173 ± 227 | 22 ± 25 | 0.2 ± 0.5 | 1.9 ± 2.8 | 0.1 ± 0.2 |
| AAV-βgal | AAV-AP | 5 | MR1 | 20 ± 25 | 0.9 ± 0.9 | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.1 ± 0.2 |
B6 mice were given saline or AAV-βgal (104 β-Gal+ FFU) by nasal aspiration in the presence or absence of immunomodulating drugs. Sixty days later, they were given AAV-AP (107 AP+ FFU). On day 84, the animals were euthanized and their lungs were stained for AP expression.
Mean values ± standard deviations are shown. A, alveolar cells; DA, distal airway epithelial cells; BE, bronchial epithelium (defined as having underlying cartilage); SM, smooth muscle cells underlying airway epithelium; V, vascular smooth muscle cells.
Neutralizing activity to AAV vector was detected in the sera of MR1- or CTLA4Ig-treated animals obtained on day 54, just prior to the second administration of AAV vector (Table 4). The CTLA4Ig-treated animal whose serum neutralized 4.7% of vector at a 1:20 dilution of serum (and 0% at a 1:100 dilution) had a number of AP+ cells similar to the average number for saline-treated control animals. Another animal whose serum neutralized 50% of vector at a 1:100 dilution of serum (and 100% at 1:20) had AP+ alveolar cell numbers at 40% of the mean value obtained for saline-treated control animals. Serum samples from two animals in the MR1-treated group that also exhibited a similar level of neutralizing activity against vector (10 and 38% neutralization of vector at a 1:100 dilution of serum) gave only 10% of the transduction rate seen in the saline-treated controls. Overall, MR1 or CTLA4Ig alone was not as effective in suppressing the generation of neutralizing antibodies, and this correlated with the lower transduction rates achieved with readministration.
TABLE 4.
Neutralization of an AAV vector by sera from B6 mice exposed to an AAV vector with or without CTLA4Ig and/or MR1 immunosuppressiona
| Primary vector | Treatment | No. of animals | Vector neutralization (%) for serum dilution indicatedb:
|
|
|---|---|---|---|---|
| 1:20 | 1:100 | |||
| None (saline) | None | 3 | 0, 0, 0 | 0, 0, 0 |
| AAV-βgal | L6 + HIg | 4 | 100, 100, 100, 100 | 100, 100, 100, 100 |
| AAV-βgal | MR1 + CTLA4Ig | 4 | 0, 0, 0, 0 | 0, 0, 0, 0 |
| AAV-βgal | CTLA4Ig | 4 | 100, 100, 4.7, 100 | 100, 50, 0, 100 |
| AAV-βgal | MR1 | 5 | 100, 100, 100, 100, 100 | 89, 38, 100, 100, 10 |
B6 mice were given saline or AAV-βgal (as described in Table 3) in the presence or absence of immunomodulating drugs. Fifty-four days later, serum samples were obtained and analyzed for neutralizing activity to AAV vector.
Neutralization values for serum samples from individual animals are given in the same order for each serum dilution.
The vector preparation used in the primary challenge in the first readministration experiment contained low levels of replication-competent AAV, whereas the preparation used in the primary challenge in the second experiment did not contain any detectable levels of replication-competent AAV. For the second challenge, both experiments used vector preparations that did not contain any detectable levels of replication-competent AAV. The neutralization results (Tables 2 and 4) show that substantial levels of neutralizing antibodies were generated to the primary AAV vector whether the preparation had a low or an undetectable level of replication-competent AAV. In addition, secondary transduction was blocked in both cases (Table 1, line 1; Table 3, line 2), showing that replication-competent AAV was not responsible for inducing this response.
Suppression of the humoral immune response by MR1 and CTLA4Ig is transient.
To determine whether persistent immunosuppression was correlated with the ability to successfully readminister AAV vector, the concentrations of MR1 and CTLA4Ig in the sera obtained on day 54 from animals in both readministration experiments were evaluated. Very low or undetectable levels of MR1 and CTLA4Ig were found at the time of readministration (data not shown). Additionally, all MR1- and CTLA4Ig-treated animals generated neutralizing activities to AAV vector after the second readministration (data not shown), demonstrating that they were capable of generating a humoral immune response to the vector. To unequivocally test the ability to generate a primary humoral immune response to a novel antigen, a tetanus vaccine was given intraperitoneally at the time of the second administration of AAV vector (Table 5). Antitetanus antibody was detected in all treatment groups. Although the MR1- and CTLA4Ig-treated group and the MR1-treated group showed lower mean titers than the control group, the values were not significantly different from those of the control group. These results showed that the initial regimen of MR1 and CTLA4Ig facilitated a successful second transduction even when the animals were immunocompetent at the time of the second challenge with AAV vector.
TABLE 5.
Antibody response to a novel antigen is detected in sera from animals challenged 60 days after treatment with MR1 and/or CTLA4Ig
| Expta | Treatment | No. of animals | IgG1 antitetanus antibody titerb |
|---|---|---|---|
| 1 | L6 + HIg | 3 | 550 ± 300 |
| MR1 + CTLA4Ig | 5 | 350 ± 300 | |
| 2 | L6 + HIg | 3 | 500 ± 200 |
| MR1 + CTLA4Ig | 4 | 300 ± 115 | |
| MR1 | 5 | 260 ± 134 | |
| CTLA4Ig | 4 | 812 ± 632 |
Animals in readministration experiment 1 were given 0.1 ml of tetanus-diphtheria vaccine (Connaught Laboratories) by intraperitoneal injection 60 days after administration of an AAV-βgal vector in conjunction with treatment using the indicated immunomodulating drug regimen (MR1 and CTLA4Ig) or control molecules (L6 and HIg). Sera were obtained when animals were euthanized on day 81. Experiment 2 was the same as experiment 1 except that sera were obtained on day 74.
IgG1 antitetanus antibody titers were determined with an enzyme-linked immunosorbent assay and are expressed as means ± standard deviations.
Although the suppression of humoral immune response to AAV vector by MR1 and CTLA4Ig was transient and the immune response to tetanus was intact at the time of secondary vector administration, a lower splenocyte proliferation response to the AAV vector was detected in comparison to that in nonimmunosuppressed animals even after the second administration of AAV vector. Mice given the AAV-βgal vector in conjunction with control antibodies (L6 and HIg) showed a response to AAV vector when assessed 14 days after administration of the second AAV vector (stimulation index, 3.1 ± 1.0; n = 3). In contrast, the response of splenocytes from mice treated with AAV vector in conjunction with MR1 and CTLA4Ig was lower (stimulation index, 1.4 ± 0.2; n = 4; P = 0.05 versus controls) and similar to values for naive mice (data not shown).
DISCUSSION
In a previous study of rabbits, we found that efficient AAV vector transduction occurred in the bronchial epithelium in the area where the balloon catheter was lodged, indicating that tissue trauma was necessary for efficient transduction in the epithelium of the large airways. Indeed, we saw a much lower efficiency of transduction in nonwounded epithelium at airway branches just adjacent to the site of injury in the rabbit lung. Thus, a much lower transduction rate was seen without injury. In the present study of mice, we observed a low level of transduction in the bronchial epithelium, where no injury occurred during vector delivery. Additionally, a small but dense area of AP+ epithelial cells was found in the tracheas of animals treated by intratracheal injection of the vector. This area of increased transduction was presumably due to tissue injury at the site of the intratracheal injection because animals that received vector by nasal aspiration did not show such foci of AP+ cells. Thus, the results in mice were consistent with the results in rabbits, showing that tissue injury was associated with more efficient transduction in the epithelium of the large airways.
The small airways of the rabbit lung have high endogenous levels of AP activity, and thus transduction of distal airways by a vector encoding AP was difficult to determine. The results with mice show that AAV vector transduction occurred in the distal airways, and transduction appeared to be more efficient in the epithelium of smaller, more distal airways than in the larger bronchial airways (P ≤ 0.05 for vector doses of 1 × 106 to 3 × 107) (Fig. 4). The epithelia of the smaller airways are actually more important than those of the upper airways as targets for cystic fibrosis gene therapy because the smaller airways are more prone to bacterial occlusion. Therefore, the data from mice gave information about transduction in this important site for cystic fibrosis gene therapy.
In addition to the epithelial cells of the large and small airways, alveolar cells were also transduced in the mouse. Indeed, the predominant cells transduced were alveolar. We observed that at later times, AP+ alveolar cells tended to occur in clusters as if they were derived from one transduced cell that proliferated. Alveolar type I and type II epithelial cells comprise the majority of epithelial cells in the lung parenchyma. It is most likely that the transduced cells were alveolar type II epithelial cells because the type II cells can proliferate whereas the type I cells are terminally differentiated and derived from type II cells (17). Further work is needed to determine the epithelial nature of the transduced alveolar cells. We observed little to no staining in the lung parenchyma of adult rabbits in our previous study, although transduction of alveolar epithelial cells was noted in a previous study of neonatal rabbits (38). The differences observed in the adult lungs suggest that there may be species or age differences in alveolar susceptibility to AAV transduction.
Cell types other than epithelial cells were also transduced. One conclusion from both this study of the adult mouse lung and a previous one of the adult rabbit lung (14) is that the smooth muscle cells were more permissive than the epithelial cells of the large airways to AAV vector transduction. This is consistent with the success of AAV vector transduction in skeletal muscle tissues (8, 20, 36). We observed staining in the smooth muscle cells underlying the airway epithelium and in the blood vessel walls of the mouse lung. At first, we speculated that intratracheal inoculation with a syringe needle created a wound site that allowed the vector to enter the blood system and be transported and deposited on vessel walls. However, transduction of these cells still occurred in animals that received vector by nasal aspiration. It is not immediately apparent how a viral vector delivered by nasal aspiration can circumvent physical barriers imposed by the basement membrane of the epithelium. The mechanism by which AAV vector can target vascular cells warrants further investigation.
AAV vector expression persisted in the lungs of immunocompetent mice in all cell types transduced. In the previous study of rabbits, we found a dramatic loss of AP+ cells in the epithelial cells of the large airways. Although the rates of transduction were low in uninjured mouse bronchial epithelium, the low numbers of AP+ cells persisted for at least 3 months. The vector preparations used in the rabbit studies contained low levels of replication-competent AAV that is generated during vector production by recombination of the AAV packaging plasmid with the AAV vector plasmid. Here, the studies on vector persistence in the mouse were also done with preparations of AAV that had low levels of replication-competent AAV. Therefore, the differences in persistence in the airway epithelium observed between rabbits and mice cannot be explained by the presence or absence of replication-competent AAV. Perhaps the rapid loss in rabbit bronchial epithelium may be due to a higher turnover rate in traumatized epithelium. It is known that after wounding, there is a wave of proliferation as resting epithelial cells are recruited to enter the cell cycle (3, 17). Terminal differentiation of the progeny cells is accelerated so that a pseudostratified epithelium is formed within days of wounding. It is conceivable that an accelerated cycling of cells will lead to a more rapid loss of progeny cells through differentiation and sloughing at the surface of the epithelium. The results in mice suggest that the cell turnover rate in the mouse lung is very low. Indeed, it has been reported that the generation time of mouse alveolar cells is about 125 days (17). Here, we observed persistence for 32 weeks, or two generations, and persistence was not affected by immunodeficiency or immunosuppression.
In contrast to differences in the persistence of vector expression in mice and rabbits, similar results concerning the readministration of AAV vectors and the production of neutralizing antibodies were obtained in both animal models, that is, one dose of an AAV vector can render animals resistant to subsequent transduction by AAV vectors and can stimulate the production of neutralizing antibodies in serum. Here we show that transient immunosuppression with both MR1 and CTLA4Ig during the primary exposure of mice to an AAV vector allowed in secondary transduction at a rate that was similar to the level found in primary administration of AAV vectors. Similarly, it has been reported that transient immunosuppression with an antibody to CD4 allowed transgene expression following readministration of AAV vector in the mouse muscle but at a level that was only 40% of maximal (27). Since production of neutralizing antibodies to AAV vectors correlated with the inability to obtain efficient secondary transduction, and lower doses of an AAV vector resulted in reduced antibody production, it is possible that effective secondary administration could be achieved by an initial administration of a low vector dose. However, administration of low vector doses (e.g., 105 AP+ FFU) to the lung would not be useful due to the minimal transduction achieved.
A recent report detailing the results of administration of an AAV vector containing a human CFTR cDNA (AAV-CFTR) to the maxillary sinus of humans (34) suggests that there may be limited immune response to AAV vectors in humans. No consistent change in AAV capsid antibodies in serum was observed after treatment with AAV-CFTR. However, all patients had preexisting AAV capsid antibodies, and the transduction rate could not be measured to determine whether there was an effect of these antibodies on AAV vector transduction. In addition, the vector dose used (up to 105 replication units of vector) appears to be at the lower end of what would stimulate an immune response in mice (Fig. 5; note that the assay of vector stocks for replication units most likely gives a value higher than that determined by the assay for focus-forming units that we use). Therefore, whether repeat transduction by AAV vectors will be possible in humans is unknown, but the animal data indicate that it will not be possible without immunosuppression.
The abilities of a vector to persist and be readministered successfully are two important goals for gene therapy to the lung. We have shown here that AAV vectors can satisfy these criteria in the mouse lung. An inoculum of 108 FFU achieved a 5% transduction rate in airway epithelial cells. It remains to be seen whether these promising results obtained in the mouse model can be duplicated in humans.
ACKNOWLEDGMENTS
This work was supported by grants from the Cystic Fibrosis Foundation (C.L.H. and T.A.S.) and grants DK47754 (C.L.H., C.B.W., and A.D.M.) and DK95006 (C.B.W.) from the National Institutes of Health.
REFERENCES
- 1.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 2.Allen J M, Debelak D J, Reynolds T C, Miller A D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J Virol. 1997;71:6816–6822. doi: 10.1128/jvi.71.9.6816-6822.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayers M M, Jeffery P K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- 4.Bar D, Tubb J, Ferguson D, Scaria A, Lieber A, Wilson C, Perkins J, Kay M A. Strain related variation in adenovirally mediated transgene expression from mouse hepatocytes in vivo: comparisons between immunocompetent and immunodeficient inbred strains. Gene Ther. 1995;2:151–155. [PubMed] [Google Scholar]
- 5.Bierer B E. Mechanisms of action of immunosuppressive agents: cyclosporin A, FK506, and rapamycin. Proc Assoc Am Physicians. 1995;107:28–40. [PubMed] [Google Scholar]
- 6.Bluestone J A. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 7.Boissy R, Astell C R. An Escherichia coli recBCsbcBrecF host permits the deletion resistant propagation of plasmid clones containing the 5′ palindrome of minute virus of mice. Gene. 1985;35:179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- 8.Fisher K J, Jooss K, Alson J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 9.Fisher-Adams G, Wong Jr K K, Podsakoff G, Forman S J, Chatterjee S. Integration of adeno-associated virus vectors in CD34+ human hematopoietic progenitor cells after transduction. Blood. 1996;88:492–504. [PubMed] [Google Scholar]
- 10.Flotte T R, Afione S A, Conrad C, McGrath S A, Solow R, Oka H, Zeitlin P Z, Guggino W B, Carter B J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci USA. 1993;90:10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foy T M, Aruffo A, Bajorath J, Buhlmann J E, Noelle R J. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 12.Graham F L, Smiley J. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 13.Grewal I S, Flavell R A. The CD40 ligand. At the center of the immune universe? Immunol Res. 1997;16:59–70. doi: 10.1007/BF02786323. [DOI] [PubMed] [Google Scholar]
- 14.Halbert C L, Standaert T A, Aitken M L, Alexander I E, Russell D W, Miller A D. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71:5932–5941. doi: 10.1128/jvi.71.8.5932-5941.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe H, Buhl R, Mastrangeli A. Organ specific cytokine therapy: local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-γ to the human lung. J Clin Invest. 1991;88:297–302. doi: 10.1172/JCI115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman S L. Cell proliferation in the mammalian lung. Int Rev Exp Pathol. 1980;22:131–191. [PubMed] [Google Scholar]
- 18.Kay M A, Holterman A, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 19.Kay M A, Meuse L, Gown A M, Linsley P, Hollenbaugh D, Aruffo A, Ochs H, Wilson C B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc Natl Acad Sci USA. 1997;94:4686–4691. doi: 10.1073/pnas.94.9.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of AAV vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolls J, Nelson S, Summer W. Recombinant cytokines and pulmonary host defense. Am J Med Sci. 1993;306:330–335. doi: 10.1097/00000441-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kokron C M, Bonilla F A, Oettgen H C. Searching for genes involved in the pathogenesis of primary immunodeficiency diseases: lessons from mouse knockouts. J Clin Immunol. 1997;17:109–126. doi: 10.1023/a:1027322314256. [DOI] [PubMed] [Google Scholar]
- 24.Kotin R M, Siniscalco M, Samulski R J, Zhu X, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linsley P A, Ledbetter J A. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 26.Lipscomb M F, Bice D E, Lyons C R, Schuyler M R, Wilkes D. The regulation of pulmonary immunity. Adv Immunol. 1995;59:369–455. doi: 10.1016/S0065-2776(08)60634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning W C, Zhou S, Bland M P, Escobedo J A, Dwarki V. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther. 1998;9:477–485. doi: 10.1089/hum.1998.9.4-477. [DOI] [PubMed] [Google Scholar]
- 28.McLaughlin S K, Collis P, Hermonat P L, Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson S, Bagby G, Bainton B. Compartmentalization of intraalveolar and systemic lipopolysaccharide-induced tumor necrosis factor and the pulmonary inflammatory response. J Infect Dis. 1989;159:189–194. doi: 10.1093/infdis/159.2.189. [DOI] [PubMed] [Google Scholar]
- 30.Oettinger M A. Activation of V(D)J recombination by RAG1 and RAG2. Trends Genet. 1992;8:413–416. doi: 10.1016/0168-9525(92)90323-v. [DOI] [PubMed] [Google Scholar]
- 31.Rubenstein R C, McVeigh U, Flotte T R, Guggino W B, Zeitlin P L. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther. 1997;4:384–392. doi: 10.1038/sj.gt.3300417. [DOI] [PubMed] [Google Scholar]
- 32.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Gown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 34.Wagner J A, Reynolds T, Moran M L, Moss R B, Wine J J, Flotte T R, Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 35.Wallace P M, Rodgers J N, Leytze G M, Johnson J S, Linsley P S. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA4Ig treatment. J Immunol. 1995;154:5885–5895. [PubMed] [Google Scholar]
- 36.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeitlin P L, Lu L, Rhim J, Cutting G, Stetten G, Kieffer K A, Craig R, Guggino W B. A cystic fibrosis bronchial epithelial cell line: immortalization by Adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991;4:313–319. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 38.Zeitlin P L, Chu S, Conrad C, McVeigh U, Ferguson K, Flotte T R, Guggino W B. Alveolar stem cell transduction by an adeno-associated viral vector. Gene Ther. 1995;2:623–631. [PubMed] [Google Scholar]