Abstract
Adeno-associated virus type 2 (AAV), a single-stranded, DNA-containing, nonpathogenic human parvovirus, has gained attention as a potentially useful vector for human gene therapy. However, the transduction efficiency of AAV vectors varies greatly in different cells and tissues in vitro and in vivo. We have recently documented that a cellular tyrosine phosphoprotein, designated the single-stranded D-sequence-binding protein (ssD-BP), plays an important role in AAV-mediated transgene expression (K. Y. Qing et al., Proc. Natl. Acad. Sci. USA 94:10879–10884, 1997) and that a strong correlation exists between the phosphorylation state of the ssD-BP and AAV transduction efficiency in vitro as well as in vivo (K. Y. Qing et al., J. Virol. 72:1593–1599, 1998). In this report, we document that treatment of cells with specific inhibitors of the epidermal growth factor receptor protein tyrosine kinase (EGF-R PTK) activity, such as tyrphostin, leads to significant augmentation of AAV transduction efficiency, and phosphorylation of the ssD-BP is mediated by the EGF-R PTK. Treatment of cells with EGF results in phosphorylation of the ssD-BP, whereas treatment with tyrphostin causes dephosphorylation of the ssD-BP and consequently leads to increased expression of the transgene. Furthermore, AAV transduction efficiency inversely correlates with expression of the EGF-R in different cell types, and stable transfection of the EGF-R cDNA causes phosphorylation of the ssD-BP, leading to significant inhibition in AAV-mediated transgene expression which can be overcome by the tyrphostin treatment. These data suggest that the PTK activity of the EGF-R is a crucial determinant in the life cycle of AAV and that further studies on the interaction between the EGF-R and the ssD-BP may yield new insights not only into its role in the host cell but also in the successful use of AAV vectors in human gene therapy.
Adeno-associated virus type 2 (AAV), a single-stranded, DNA-containing, nonpathogenic human parvovirus, has gained attention as a potentially useful vector for human gene therapy. The single-stranded viral genome is flanked at both ends by 145-nucleotide-long palindromic inverted terminal repeats of which 125 nucleotides form the hairpin structure and the remaining 20 nucleotides constitute the single-stranded region designated the D-sequence (4, 5, 51). The D-sequence at the 3′ end of the viral genome is termed the D(−) sequence whereas at the 5′ end, it is termed the D(+) sequence. We have previously shown that the D-sequence plays an important role in the efficient rescue, replication, and encapsidation of the AAV genome (56–58). More recently, we have identified a cellular tyrosine phosphoprotein, designated the single-stranded D-sequence-binding protein (ssD-BP), which preferentially interacts with the D(−) sequence in the AAV genome (47). We have also demonstrated that the tyrosine phosphorylation state of the ssD-BP correlates well with the efficiency of AAV-mediated transgene expression both in vitro and in vivo (45).
Two independent groups have presented evidence suggesting that following infection, the rate-limiting step for the efficient transduction by AAV is viral second-strand DNA synthesis (12, 13). In our proposed model (47), the tyrosine phosphorylated form of the ssD-BP inhibits viral second-strand DNA synthesis whereas the dephosphorylated form of the ssD-BP promotes it. Therefore, the higher the ratio of the dephosphorylated form to the phosphorylated form of the ssD-BP, the greater the transduction efficiency by AAV. We have also demonstrated that treatment of cells with genistein, a specific inhibitor of cellular protein tyrosine kinases (PTKs), which results in the accumulation of the dephosphorylated form of the ssD-BP, leads to a concomitant increase in the recombinant AAV-mediated transgene expression, transient as well as stable (45, 47). These studies have shown one of the ways by which the transduction efficiency of AAV vectors can be dramatically improved via manipulation of the phosphorylation state of the cellular ssD-BP.
Although treatment of target cells with genistein results in accumulation of the dephosphorylated form of the ssD-BP and consequently, leads to increased transduction efficiency by AAV vectors (45, 47), the genistein treatment is quite toxic since it inhibits all cellular tyrosine kinases. We hypothesized that the ssD-BP might be phosphorylated by a single tyrosine kinase, the specific inhibition of which would yield increased transduction efficiency by AAV vectors without being toxic to treated cells. In this pursuit, we performed systematic studies with a number of known specific inhibitors of cellular protein kinases and examined their effect on the recombinant AAV-mediated transgene expression. In this report, we present evidence that phosphorylation of the ssD-BP is catalyzed by the PTK activity associated with the cellular epidermal growth factor receptor (EGF-R), and therefore, is a crucial determinant of efficiency of transduction by recombinant AAV vectors. Thus, the EGF-R-ssD-BP interaction has important implications in the use of AAV vectors in human gene therapy.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
The human cervical carcinoma cell line HeLa, the human epidermoid carcinoma cell line A431, the human lung small-cell carcinoma cell line H69, the human erythroleukemia cell line K562, and the adenovirus-transformed human embryonic kidney cell line 293, were obtained from the American Type Culture Collection (Rockville, Md.). The human nasopharyngeal carcinoma cell line KB, and the human megakaryocytic leukemia cell line M07e were obtained from Asok C. Antony and Hal E. Broxmeyer, respectively (Indiana University School of Medicine, Indianapolis, Ind.). Monolayer cultures of HeLa, A431, KB, and 293 and suspension cultures of H69, M07e, and K562 were maintained in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal bovine serum and antibiotics. The recombinant AAV helper plasmid pAAV/Ad has been described previously (50) and was kindly provided by Richard J. Samulski (University of North Carolina, Chapel Hill). The recombinant plasmid pCHCEGFR, containing the human cytomegalovirus immediate-early promoter (CMVp)-driven cDNA for human EGF-R, has also been described previously (33) and was generously provided by Francis G. Kern (Georgetown University Medical Center, Washington, D.C.). Recombinant AAV plasmid pCMVp-lacZ containing the CMVp-driven β-galactosidase (lacZ) gene has been described elsewhere (40, 41). Recombinant AAV vector (vCMVp-lacZ) stocks were generated and purified by CsCl equilibrium density gradient centrifugation as previously described (24, 36, 40–45). Physical particle titers of recombinant vector stocks were determined by quantitative DNA slot blot analysis (23). The physical particle-to-infectious particle ratio (approximately 1,000:1) and the contaminating wild-type AAV-like particle titer (approximately 0.01%) in the recombinant vector stocks were determined as previously described (24, 55).
Cellular kinase inhibitors and treatment conditions.
Genistein, apigenin, tyrphostin 1, -23, -25, -46, -47, -51, and -63, AG126, AG1288, AG1295, AG1296, and AG1478 were obtained from Sigma Chemical Co. (St. Louis, Mo.). Staurosporine, LY294002, wortmannin, and tyrphostin A48 were obtained from CalBiochem (La Jolla, Calif.). Herbimycin A was obtained from Gibco-BRL Life Technologies (Grand Island, N.Y.). Stock solutions of genistein (150 mM), tyrphostin A48 (500 mM), staurosporine (1 mM), wortmannin (10 mM), herbimycin A (1 mM), LY294002 (200 mM) in dimethyl sulfoxide (DMSO), and hydroxyurea (HU) (1 M) in phosphate-buffered saline (PBS) were stored at −20°C and diluted into IMDM for use in experiments. Stock solutions of apigenin (500 mM), tyrphostin 1, -23, -25, -46, -47, -51, and -63, AG126, AG1288, AG1295, AG1296, and AG1478 (500 mM) in DMSO were stored at 4°C and diluted into IMDM for use in experiments. Cells were either mock treated or treated with various concentrations of genistein, apigenin, wortmannin, staurosporine, herbimycin A, LY294002, and tyrphostin separately for 2 h at 37°C. Chemical treatment with HU was for 16 h at 37°C. Following treatments, cells were washed twice with PBS and were either mock infected or infected with the recombinant AAV as follows.
Recombinant AAV transduction assay.
Approximately equivalent numbers of cells were washed once with IMDM and then infected with the recombinant vCMVp-lacZ vector at an infectious particle multiplicity of infection (MOI) of 2 or 4, as indicated. Forty-eight hours postinfection (p.i.), cells were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and the numbers of blue cells were determined as previously described (41–43).
Chemical toxicity assay.
Approximately 5 × 105 HeLa cells were seeded in 12-well dishes and allowed to adhere for 24 h. Cells were then treated with 150 μM genistein and 500 μM tyrphostin 1 or tyrphostin 23 or an equivalent volume of DMSO for 2 h, or with 10 mM HU for 16 h at 37°C followed by washing twice with IMDM and incubation at 37°C. Twenty-four hours posttreatment, cells were trypsinized and plated into five 10-cm dishes. Twelve days later, cells that led to colony formation were stained with methylene blue, and the numbers of colonies were determined as previously described (18).
Preparation of WCEs.
Whole-cell extracts (WCEs) were prepared as previously described by Muller (34). Total protein concentration was determined with the Bio-Rad protein assay kit (Hercules, Calif.).
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (47, 58). Briefly, 10 μg of each WCE was preincubated with 2 μg of poly(dI-dC), 2 μg of bovine serum albumin (BSA), and 12% glycerol in HEPES buffer (pH 7.9) for 10 min at 25°C. Following preincubation, 10,000 cpm of 32P-labeled D(−) sequence synthetic oligonucleotide (5′-AGGAACCCCTAGTGATGGAG-3′) was added to the reaction mixture and incubated for 30 min at 25°C. The bound complexes were separated from the free probe by electrophoresis on 4% polyacrylamide gels with recirculating Tris-acetate-EDTA buffer (pH 7.9) containing 6.72 mM Tris-HCl, 3.3 mM sodium acetate, and 1 mM EDTA. Following electrophoresis, gels were dried in vacuuo and autoradiographed with Kodak X-OMAT film. The ratios of dephosphorylated to phosphorylated forms of the ssD-BP in various cell types were determined by densitometric scanning of autoradiograms with a Digital Imaging System Alphaimager (Alpha Innotech Co., San Leandro, Calif.).
EGF-binding assay.
EGF-binding experiments were carried out as previously described by Livneh et al. (29) and Gamou et al. (16) with the following modifications. Briefly, 5 × 104 cells were washed twice with IMDM containing 1% BSA. One milliliter of IMDM containing 1% BSA was added to all cells either with 0.5 ng of 125I-EGF/ml obtained from Amersham (Arlington Heights, Ill.) alone or with 200-fold excess unlabeled EGF (Sigma Chemical Co.). Cells were incubated for 90 min at room temperature. Following incubation, cells were washed four times with IMDM containing 1% BSA and solubilized with 1 ml of 0.5 N NaOH for 30 min at 37°C. Radioactivity of lysates was determined in a Beckman Gamma counter. Specific binding was calculated as the total radioactivity minus the nonspecific (cell-associated) radioactivity.
AAV-binding assay.
AAV-binding experiments were carried out as previously described (41, 45). Briefly, 5 × 104 cells were washed twice with IMDM containing 1% BSA. One milliliter of IMDM containing 1% BSA was added to the cells with either 4 × 109 particles of [35S]methionine-labeled wild-type AAV alone or with 50-fold excess of unlabeled wild-type AAV particles for 90 min at 4°C. Following incubation, cells were washed four times with IMDM containing 1% BSA and solubilized with 1 ml of 0.5 N NaOH for 30 min at room temperature. Radioactivity of lysates was determined and specific binding was calculated as the total radioactivity minus the nonspecific (cell-associated) radioactivity as described above.
Stable transfection with the EGF-R expression plasmid.
Transfection of 293 cells with pCHCEGFR plasmid DNA was carried out using the Superfect reagent in accordance with the protocol provided by the vendor (Qiagen, Valencia, Calif.). Hygromycin was added at a final concentration of 300 μg/ml 48 h posttransfection, and individual hygromycin-resistant 293 cell clones were isolated after 14 days of selection.
In vitro phosphorylation assay.
In vitro phosphorylation assays were carried out as previously described (32, 59) with the following modifications. The complete reaction mixture contained 10 ng of the ssD-sequence affinity column-purified dephosphorylated ssD-BP from 293 cells, 20 mM HEPES, 4 mM MgCl2, 10 mM MnCl2, 50 mM NaOV, 200 μM ATP, and 0.8 U of EGF-R PTK (CalBiochem) with appropriate controls. The reaction mixtures were incubated at 30°C for 1 h, and used in an EMSA with the radiolabeled D(−) probe as described above.
In vitro DNA replication assay.
The appropriate AAV DNA substrate containing the 3′ hairpin structure were prepared and labeled with [γ-32P]ATP (3,000 Ci/mmol) by using T4 polynucleotide kinase as described previously (58). The labeled substrate was boiled, quickly chilled, and used in a DNA replication assay in the presence of all four unlabeled deoxynucleoside triphosphates and the Klenow fragment of Escherichia coli DNA polymerase I. Twenty nanograms of either the phosphorylated or the dephosphorylated form of the affinity column-purified ssD-BP was added to the reaction mixture and incubated for 15 min at 25°C prior to adding the Klenow enzyme to examine the effect of the ssD-BP on AAV DNA replication (second-strand DNA synthesis). The reaction mixtures were electrophoresed on 6% polyacrylamide gels. Gels were dried in vacuuo and autoradiographed at −70°C.
RESULTS
Inhibitors of EGF-R PTK increase the transduction efficiency of recombinant AAV.
Previously, we have shown that the inhibition of tyrosine phosphorylation of the ssD-BP by genistein, a specific inhibitor for all PTKs (1, 3, 7, 8), increased transduction efficiency by recombinant AAV (47). To investigate which kinase may be responsible for tyrosine phosphorylation of the ssD-BP, we studied the effects of various kinase inhibitors on the transduction efficiency of recombinant AAV. HeLa cells were treated with 100 nM to 1 μM herbimycin A (15), 100 nM to 1 μM staurosporine (9), 50 to 200 μM LY294002 (54), 500 nM to 10 μM wortmannin (38), 1 to 500 μM apigenin (25), 1 to 200 μM tyrphostin A48 (17), and 150 μM genistein for 2 h at 37°C. Following treatment, cells were infected with vCMVp-lacZ at an MOI of 2. Cells were then stained with X-Gal 48 h p.i. The results are summarized in Table 1. It is evident that, in addition to genistein, treatment with tyrphostin A48, a specific inhibitor for EGF-R PTK, caused an increase in the numbers of blue cells. These results suggest that EGF-R PTK may be involved in recombinant AAV-mediated transgene expression.
TABLE 1.
Effect of cellular protein kinase inhibitors on AAV-mediated transgene expression
| Inhibitora | Targetb | Fold-increase in AAV transductionc |
|---|---|---|
| Apigenin | MAP kinase | 0 |
| Genistein | Tyrosine kinases, PK-A, PK-C | 5.4 |
| Herbimycin A | pp60c-src | 0 |
| LY294002 | PI 3-kinase | 0 |
| Staurosporine | CaM kinase, MLC kinase, PK-A, PK-C, PK-G | 0 |
| Tyrphostin A48 | EGF-R tyrosine kinase | 8.1 |
| Wortmannin | MAP kinase, MLC kinase, PI 3-kinase, PI 4-kinase | 0 |
HeLa cells were treated with the indicated compounds at 37°C for 2 h at concentrations detailed in the text.
Abbreviations: MAP kinase, mitogen-activated protein kinase; PK-A, protein kinase A; PK-C, protein kinase C; PK-G, protein kinase G; PI 3-kinase, phosphatidylinositol 3 kinase; PI 4-kinase, phosphatidylinositol 4 kinase; CaM kinase, calmodulin-dependent protein kinase; MLC kinase, myosin light chain kinase.
Equivalent numbers of HeLa cells were either mock treated or treated with the highest concentration of the indicated compounds separately followed by infection with the vCMVp-lacZ vector at an MOI of 2, and percentages of cells expressing the transgene were determined 48 h p.i. as described in Materials and Methods.
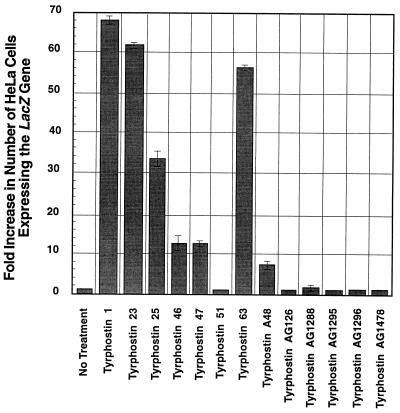
To further investigate the role of EGF-R PTK in recombinant AAV transduction, specific inhibitors for EGF-R PTK, tyrphostin 1, -23, -25, -46, -47, -51, and -63, and AG1478 (17, 26, 27, 30, 61), in addition to tyrphostin A48, were tested for their effects on recombinant AAV transduction. The following specific inhibitors were used as controls: for tumor necrosis factor alpha (TNF-α) production, AG126; for TNF-α cytotoxicity, AG1288 (37); and for platelet-derived growth factor receptor PTK, AG1295 and AG1296 (22). HeLa cells were treated for 2 h with 1 to 800 μM of each tyrphostin followed by infection with vCMVp-lacZ at an MOI of 2, as described above. The results are shown in Fig. 1. These results demonstrate that, of all the specific inhibitors tested, treatment with tyrphostin 1 resulted in the greatest increase in recombinant AAV-mediated transgene expression at the optimal concentration (without causing significant cytotoxicity) of 500 μM, followed by tyrphostin 23, -63, -25, -46, and -47. These results again emphasize the role the EGF-R PTK plays in AAV-mediated transgene expression. The varying degrees to which tyrphostin specific for EGF-R PTK affects AAV transduction efficiency may be due to the possible different mechanisms by which each compound inhibits the EGF-R PTK. It is interesting to note that we also observed an increase in recombinant AAV transduction efficiency with as little as 100 μM of tyrphostin 1, even though the 50% inhibitory concentration of tyrphostin 1 for EGF-R PTK is 1,250 μM (data not shown). In addition, treatment either with tyrphostin 1 or tyrphostin 23 consistently increased recombinant AAV transduction efficiency in many other cell lines, such as A431, K562, 293, and KB (data not shown). As expected, tyrphostin AG126 and AG1288, which are specific inhibitors for TNF-α production and TNF-α cytotoxicity, respectively, and tyrphostin AG1295 and AG1296, which are specific inhibitors of the platelet-derived growth factor receptor PTK, had no effect.
FIG. 1.
Comparative analyses of transduction efficiencies of vCMVp-lacZ in HeLa cells treated with 500 μM concentrations of various tyrphostins. Approximately equivalent numbers of HeLa cells were treated with each of the indicated compounds separately for 2 h and then infected with vCMVp-lacZ at an MOI of 2 under identical conditions. Forty-eight hours p.i., cells were fixed and stained with X-Gal, and the numbers of blue cells were determined as described in Materials and Methods.
Tyrphostin 1 and tyrphostin 23 are more effective and less toxic than HU and genistein.
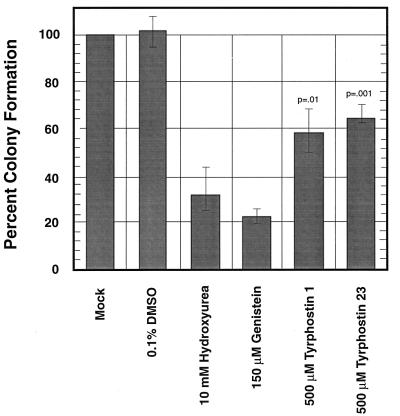
Treatment of cells with compounds such as genistein (45, 47) or HU (12, 48) has previously been shown to increase recombinant AAV transduction efficiency. To compare the effects of these compounds with that of tyrphostin, HeLa cells were either mock treated or treated with 150 μM genistein, 10 mM HU, 500 μM tyrphostin 1, or 500 μM tyrphostin 23, followed by infection with vCMVp-lacZ at an MOI of 2 as described above. The results are shown in Fig. 2. It is evident that treatment with either tyrphostin 1 or tyrphostin 23 resulted in a much greater increase in recombinant AAV transduction efficiency than treatment with either genistein or HU. We next wished to compare the relative toxicity of tyrphostin with that of HU or genistein. HeLa cells were treated with 500 μM tyrphostin 1 or tyrphostin 23, 150 μM genistein, or an equivalent volume of DMSO, for 2 h or 10 mM HU for 16 h, respectively. Following treatments, the numbers of viable cell colonies were determined as described in Materials and Methods. The results are shown in Fig. 3. It is evident that with reference to the mock-treated or DMSO-treated controls, both tyrphostin 1 and tyrphostin 23 are far less toxic than either genistein or HU. Tyrphostin 23, in particular, is the least toxic of the four treatments for HeLa cells. Thus, the tyrphostin treatment of primary cells may offer a physiological means to augment AAV transduction efficiency without causing a deleterious effect.
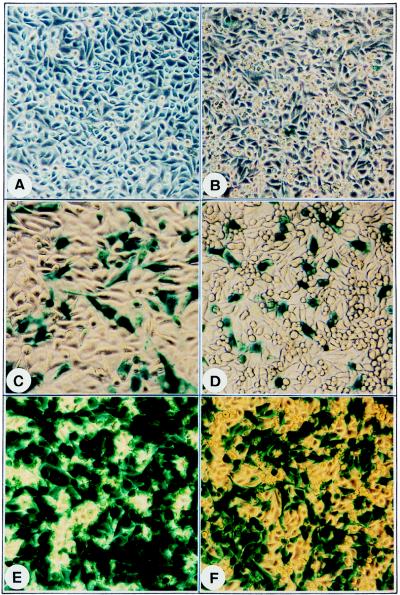
FIG. 2.
Comparative analyses of transduction efficiency of vCMVp-lacZ in HeLa cells (A) following either mock treatment (B) or treatment with HU (C), genistein (D), tyrphostin 1 (E), or tyrphostin 23 (F). Approximately equivalent numbers of HeLa cells were either mock treated or treated with the indicated compounds for 2 h and infected with the vCMVp-lacZ vector at an MOI of 2 under identical conditions. Forty-eight hours p.i., cells were fixed, stained with X-Gal, and photographed with a Nikon inverted light microscope. Magnification, ×80.
FIG. 3.
Effect of DMSO, HU, genistein, and tyrphostins on cell viability. Cytotoxicity assays with equivalent numbers of HeLa cells at optimal concentrations of each compound were performed under identical conditions as described in Materials and Methods. The P values for tyrphostin treatments compared with treatments with HU and genistein are indicated.
Tyrphostin treatment affects the phosphorylation state of the ssD-BP.
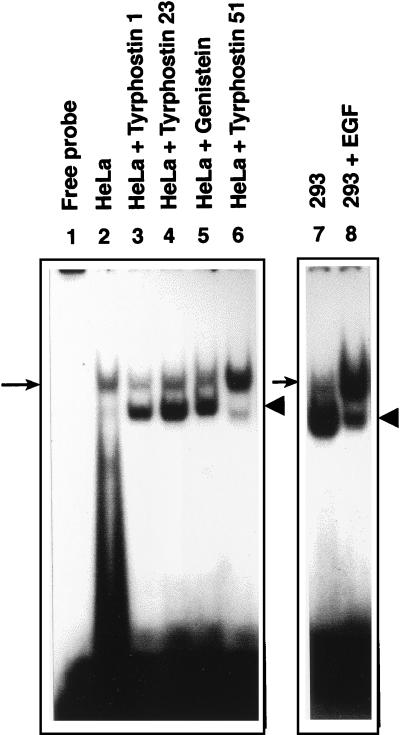
We have previously shown that recombinant AAV transduction efficiency correlates well with the phosphorylation state of the cellular ssD-BP (45). For example, HeLa cells, which are not readily transduced by recombinant AAV vectors, contain predominantly the phosphorylated form of the ssD-BP. In 293 cells, on the other hand, the ssD-BP is present predominantly in the dephosphorylated form, and these cells can be efficiently transduced by recombinant AAV vectors. Therefore, we next wished to examine the effects of tyrphostin 1 and tyrphostin 23 on the phosphorylation state of the ssD-BP in HeLa cells. Tyrphostin 51, which had little effect on AAV transduction (Fig. 1), was included as an appropriate control. Similarly, the effect of EGF on the phosphorylation state of the ssD-BP in 293 cells was also examined. In the first set of experiments, HeLa cells were treated separately with tyrphostin 1, tyrphostin 23, or tyrphostin 51 (500 μM each) and genistein (150 μM) for 2 h, followed by preparation of WCEs. WCEs were then analyzed by EMSA utilizing the D(−) probe. Fig. 4 demonstrates that with the exception of tyrphostin 51, all treatments caused a significant increase in the amount of dephosphorylated form of the ssD-BP in HeLa cells. For example, the ratios of dephosphorylated to phosphorylated forms of the ssD-BP in HeLa cells following each treatment, determined by densitometric analyses of autoradiographs, were as follows: mock treatment, 0.4 ± 0.2; tyrphostin 1, 2.1 ± 0.7; tyrphostin 23, 1.7 ± 0.5; genistein, 1.5 ± 0.5; and tyrphostin 51, 0.7 ± 0.2. Thus, consistent with our previous data (45), the amount of dephosphorylated ssD-BP for each treatment corresponded with the level of increase in transduction efficiency for each of the compounds. In the second set of experiments, 293 cells were either mock treated or treated with 100 ng of EGF in IMDM/ml for 1 h at 37°C immediately followed by preparation of WCEs. WCEs were then analyzed by EMSA utilizing the D(−) probe. The ssD-BP in 293 cells was present mostly in the dephosphorylated form in mock-treated 293 cells as observed previously (45), and the EGF treatment resulted in a significant increase in the phosphorylation of the ssD-BP. Taken together, these results strongly suggest that the EGF-R PTK plays a direct role in catalyzing the phosphorylation of the ssD-BP.
FIG. 4.
EMSA with WCE prepared from human HeLa and 293 cells. Equivalent amounts of WCE prepared from each indicated cell type were used in an EMSA with the D(−) probe as described in the text. The phosphorylated and dephosphorylated forms of the ssD-BP are indicated by the arrows and the arrowheads, respectively.
Recombinant AAV transduction efficiency correlates inversely with the EGF-R expression.
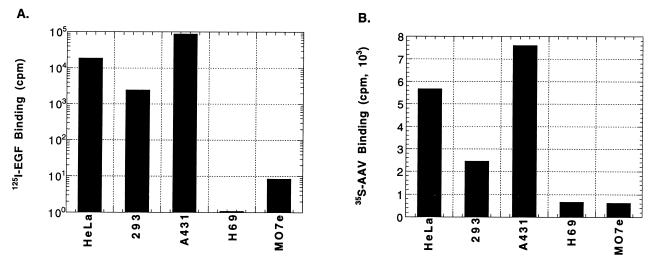
If EGF-R PTK is responsible for phosphorylating the ssD-BP, the efficiency of AAV-mediated transgene expression would be expected to be significantly lower in cells which express higher numbers of the EGF-R (A431 cells) than those which express fewer numbers of the EGF-R (H69 cells). Thus, AAV transduction efficiency would inversely correlate with the extent of the EGF-R expression. That is, the lower the level of EGF-R expression, the higher the transduction efficiency. This hypothesis was tested by using A431 and H69 cells, known to express very high and low numbers of the EGF-R, respectively. In addition, HeLa and 293 cells were infected with the vCMVp-lacZ vector at an MOI of 4 under identical conditions. Forty-eight hours p.i., the cells were stained with X-Gal. It was determined that, consistent with previously published data (45), the transduction efficiency in HeLa and 293 cells was approximately 4 and 20%, respectively, and less than 1% in A431 cells, as expected. However, contrary to the expectation, little transduction (<1%) in H69 was observed (data not shown). This apparent paradox was addressed by carrying out radiolabeled EGF and AAV binding assays. These data are shown in Fig. 5. It is clear that A431 cells bound the highest amounts of EGF (11, 19), followed by HeLa and 293 cells (panel A). As expected, EGF binding to H69 cells was negligibly small (16). The possibility that H69 cells do not express the receptor for AAV was substantiated by AAV binding assays, the results of which are shown in panel B. H69 cells fail to bind AAV, an observation consistent with that in M07e cells, an AAV receptor-negative cell line (42). A431 cells, on the other hand, express far greater numbers of the AAV receptor than HeLa or 293 cells. Thus, the low level of AAV-mediated transduction in A431 cells cannot be attributed to a lack of expression of AAV receptors in these cells.
FIG. 5.
Analyses of binding of EGF and AAV to different cell types. Equivalent numbers of HeLa, 293, A431, H69, and M07e cells were analyzed in binding assays using either 125I-EGF (A) or 35S-AAV (B) as described in Materials and Methods.
Phosphorylation state of the ssD-BP in A431 and H69 cells is insensitive to EGF treatment.
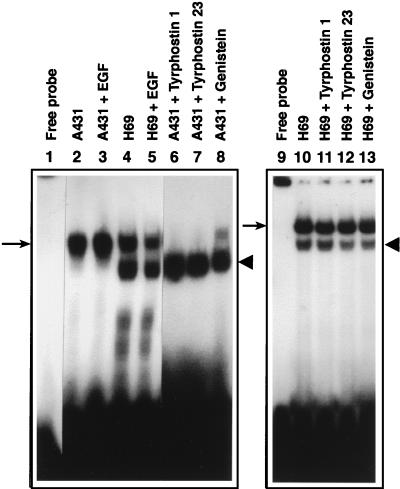
Since the EGF-R PTK appeared to catalyze phosphorylation of the ssD-BP, we next examined the effects of EGF as well as tyrphostin and genistein treatments on A431 and H69 cells. The rationale for these studies was that EGF treatment would have no effect on the phosphorylation state of the ssD-BP in either cell type because high levels of expression of the EGF-R in A431 cells would ensure that the ssD-BP would be present in its phosphorylated form, and H69 cells would fail to respond to EGF since little expression of the EGF-R occurs in these cells. Equivalent numbers of cells were either mock treated or treated with 100 ng of EGF/ml for 1 h at 37°C immediately followed by preparation of WCEs and analysis by EMSA utilizing the D(−) probe. As shown in Fig. 6, EGF treatment had no significant effect on the phosphorylation state of the ssD-BP in both cell types. In A431 cells, the ssD-BP was present predominantly in phosphorylated form due to high levels of expression of the EGF-R PTK. In H69 cells, on the other hand, both phosphorylated and dephosphorylated forms of the ssD-BP were detected. Interestingly, however, treatment with tyrphostin or genistein led to conversion to the dephosphorylated form of the ssD-BP, resulting in increased transduction in A431 cells (data not shown). Under identical conditions, however, these treatments had no effect on the phosphorylation state of the ssD-BP in H69 cells, and these cells could not be transduced by AAV since they lack the cell surface receptor for AAV. Although it is not readily apparent which of the cellular protein kinases phosphorylates the ssD-BP in H69 cells, these results are consistent with the conclusion that phosphorylation of the ssD-BP in A431 cells is catalyzed by the EGF-R PTK.
FIG. 6.
EMSA with WCE prepared from A431 and H69 cells following treatment with EGF, tyrphostin 1, tyrphostin 23, or genistein. Equivalent amounts of WCEs prepared from mock-treated A431 and H69 cells (lanes 2 and 4), from cells treated with EGF (lanes 3 and 5), and from A431 cells (lanes 6 to 8) and H69 cells (lanes 11 to 13) treated with tyrphostin 1, tyrphostin 23, and genistein, respectively, were used in an EMSA with the D(−) probe as described in Materials and Methods. The phosphorylated and dephosphorylated forms of the ssD-BP are indicated by the arrows and the arrowheads, respectively.
Stable transfection of EGF-R cDNA into 293 cells causes phosphorylation of the ssD-BP and results in inhibition of AAV-mediated transgene expression.
We also examined whether deliberate overexpression of the EGF-R PTK in 293 cells, which can be efficiently transduced by recombinant AAV vectors since they contain a predominantly dephosphorylated form of the ssD-BP (45), would cause phosphorylation of this protein and, consequently, lead to inhibition of AAV-mediated transgene expression in these cells. To test this, 293 cells were transfected with the EGF-R expression plasmid DNA, and a number of stably transfected clones were isolated as described in Materials and Methods. WCEs prepared from individual 293 cell clones were used in EMSAs to determine the ratios of dephosphorylated to phosphorylated ssD-BPs and were compared with that in control (untransfected) 293 cells. Replicate cultures were also evaluated for the efficiency of the recombinant vCMVp-lacZ vector-mediated transduction with or without prior treatment with tyrphostin 1, under identical conditions. These results are shown in Table 2. It is interesting to note that in each of the transfected 293 cell clones, the ratio of dephosphorylated to phosphorylated ssD-BPs was reduced to an average of 0.45 from more than 3.5 in the control 293 cells, which also led to a significant decrease in AAV transduction efficiency, from approximately 18% in control 293 cells to an average of about 2% in EGF-R-transfected 293 cell clones. Treatment with tyrphostin 1, on the other hand, resulted in an increase in AAV transduction efficiency to an average of approximately 22.5% in EGF-R-transfected 293 cell clones. These data strongly suggest that the EGF-R-ssD-BP interaction plays a crucial role in AAV-mediated transgene expression.
TABLE 2.
Effect of stable transfection of the EGF-R cDNA on the phosphorylation state of the ssD-BP and AAV-mediated transgene expression in 293 cells
| Cell or clone typea | Ratio of dephosphorylated to phosphorylated ssD-BPb | Efficiency of AAV-mediated transgene expressionc
|
|
|---|---|---|---|
| − Tyrphostin 1 | + Tyrphostin 1 | ||
| 293 | 3.5 ± 1.8 | 18.1 ± 2.4 | 33.8 ± 6.8 |
| 293-4 | 0.31 | 1.3 ± 0.2 | 17.7 ± 2.9 |
| 293-5 | 0.45 | 2.1 ± 1.1 | 12.6 ± 0.9 |
| 293-6 | 0.36 | 2.1 ± 0.3 | 19.7 ± 5.1 |
| 293-8 | 0.51 | 2.2 ± 0.7 | 24.6 ± 6.5 |
| 293-12 | 0.64 | 2.5 ± 1.0 | 37.8 ± 4.4 |
Cells were either used directly (293) or transfected with plasmid pCHCEGFR to obtain individual clones (293-4, -5, -6, -8, and -12) following selection with 300 μg of hygromycin/ml for 14 days.
Autoradiographic images of EMSA gels were scanned densitometrically and the ratios of dephosphorylated to phosphorylated ssD-BPs were determined as previously described (40) (P < 0.005).
Equivalent numbers of mock-transfected 293 cells or 293 cell clones transfected with pCHCEGFR plasmid were infected with vCMVp-lacZ vector at an MOI of 2, and percentage of cells expressing the transgene, with (+) and without (−) prior treatment with 500 μM tyrphostin 1, were determined 48 h p.i. as described in Materials and Methods.
Phosphorylation of the ssD-BP is mediated by the EGF-R PTK.
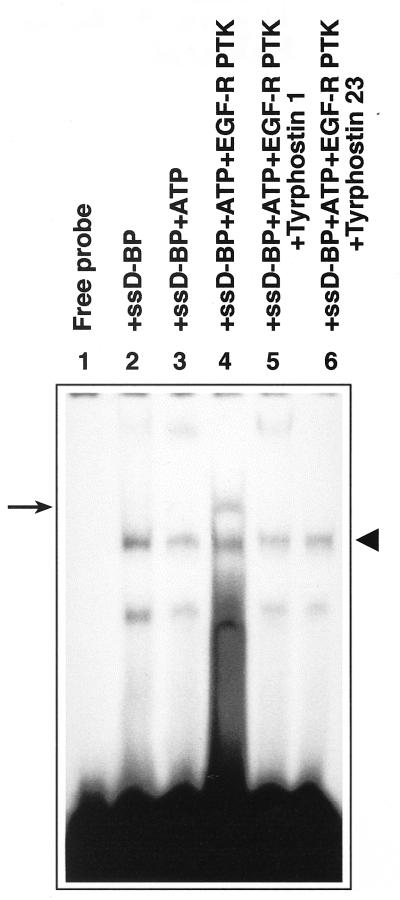
In order to unequivocally establish that tyrosine phosphorylation of the ssD-BP is indeed carried out by the EGFR-PTK, in vitro phosphorylation assays were performed with commercially available purified EGF-R PTK by using an ssD-sequence affinity column-purified dephosphorylated form of the ssD-BP from 293 cells followed by an EMSA as described in Materials and Methods. The results of these experiments are shown in Fig. 7. As is evident, incubation of the ssD-BP with the purified EGF-R PTK resulted in phosphorylation of this protein whereas incubation in the presence of ATP alone had no effect. More interestingly, in vitro phosphorylation of the ssD-BP by the EGF-R PTK was abrogated in the presence of tyrphostin 1 and tyrphostin 23. These results provide direct evidence that the ssD-BP is a downstream target of the EGF-R PTK.
FIG. 7.
In vitro phosphorylation of the ssD-BP by the EGF-R PTK. Equivalent amounts of the affinity column-purified ssD-BP from 293 cells were incubated either alone (lane 2) or in the presence of ATP (lane 3), ATP plus EGF-R PTK (lane 4), ATP plus EGF-R PTK plus tyrphostin 1 (lane 5), or ATP plus EGF-R PTK plus tyrphostin 23 (lane 6) followed by EMSA with the D(−) probe as described in Materials and Methods. The phosphorylated and dephosphorylated forms of the ssD-BP are indicated by the arrow and the arrowhead, respectively.
DISCUSSION
It has become increasingly clear that there are at least two major obstacles that need to be overcome to obtain high-efficiency transduction by AAV vectors (35, 36, 50). The first relates to the extent of expression of the cellular receptor for AAV (41), the identity of which was recently revealed (53), and the second concerns the rate-limiting step of the viral second-strand DNA synthesis (12, 13). Whereas overcoming the first obstacle must await a better understanding of molecular events involved in the AAV-receptor expression (46), we (45, 47) and others (2, 12, 13, 48) have suggested several ways in which the transduction efficiency of AAV vectors can be significantly increased by way of promoting the viral second-strand DNA synthesis. We have also presented evidence that a cellular protein, designated the ssD-BP, phosphorylated at tyrosine residues, plays a crucial role in viral second-strand DNA synthesis. We have established that the phosphorylation state of the ssD-BP correlates well with AAV-mediated transduction efficiency in vitro as well as in vivo (45).
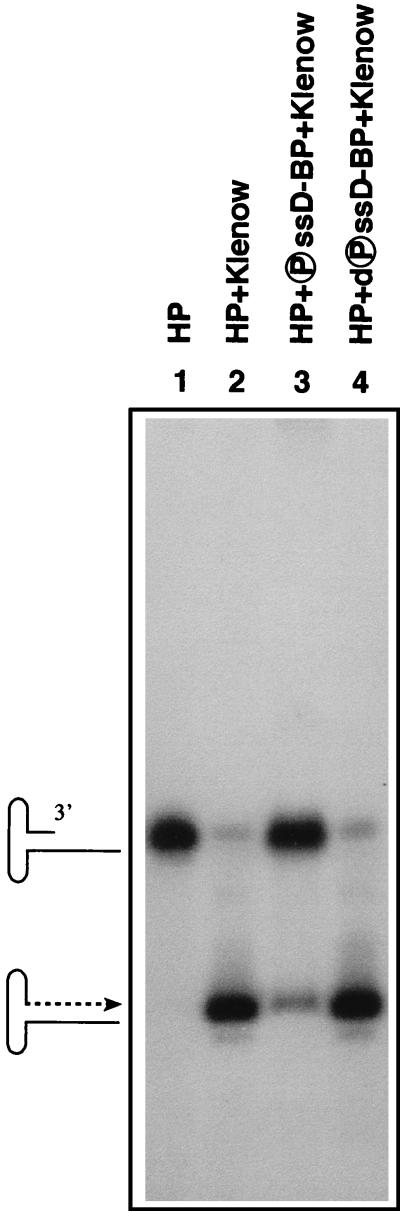
In the present studies, a systematic search for the PTK responsible for catalyzing the phosphorylation of the ssD-BP led to the identification of the EGF-R PTK since treatment of cells with tyrphostin, the specific inhibitors of the PTK activity of the EGF-R, resulted in a dramatic increase in AAV-mediated transgene expression. In particular, treatment with tyrphostin 1 consistently resulted in the greatest increase in AAV transduction efficiency. We believe that, in accordance with our model (47), the phosphorylated form of the ssD-BP blocks the viral second-strand DNA synthesis since treatment with tyrphostin prevents phosphorylation of the ssD-BP. In fact, this model was experimentally tested in in vitro DNA replication assays in which the effects of both phosphorylated and dephosphorylated forms of affinity column-purified ssD-BPs were examined as described in Materials and Methods. These data are depicted in Fig. 8. It is remarkable that, consistent with our hypothesized model (47), the AAV second-strand DNA synthesis is indeed inhibited by the phosphorylated ssD-BP, whereas the dephosphorylated ssD-BP has no significant effect under identical conditions.
FIG. 8.
Effects of phosphorylated and dephosphorylated forms of the ssD-BP on AAV second-strand DNA synthesis in in vitro replication assays. The 32P-labeled single-stranded AAV DNA with the 3′ hairpin primer (lane 1) was used as a substrate for DNA synthesis with the Klenow fragment of E. coli DNA polymerase I and unlabeled deoxynucleoside triphosphates (lane 2) as well as in the presence of either the phosphorylated ssD-BP (lane 3) or the dephosphorylated ssD-BP (lane 4) under identical conditions. DNA samples were fractionated on a 6% polyacrylamide gel and autoradiographed as described in Materials and Methods.
The possibility that tyrphostin treatment augments the promoter activity which leads to increased transgene expression was ruled out by experiments in which a double-stranded plasmid DNA containing the same CMVp-driven lacZ reporter gene was transfected in tyrphostin-treated cells, and no effect on the extent of transgene expression was observed (data not shown). Although the precise reason for lack of effect of tyrphostin 51, known to be specific for the EGF-R PTK, on AAV transduction efficiency in HeLa cells remains unclear, this treatment was insufficient to cause dephosphorylation of the ssD-BP. It is possible that each tyrphostin inhibits the EGF-R PTK by different mechanisms. It is also possible that tyrphostin 1 and tyrphostin 23, the two most active compounds, act on the downstream target(s) of the EGF-R PTK as well. Interestingly, however, there was a significant increase in the ratio of dephosphorylated to phosphorylated forms of the ssD-BP when the cells were treated with tyrphostin 1 and tyrphostin 23. The increase in this ratio, once again, strongly correlated with the efficiency of AAV-mediated transduction. Tyrphostin 51, on the other hand, failed to elicit a significant response. However, since other treatments, such as HU or expression of AdE4orf6 protein, which have been shown to increase AAV transduction efficiency, also result in an increase in the ratio of dephosphorylated to phosphorylated ssD-BP, it is possible that these treatments also involve the inhibition of the EGF-R PTK. Nevertheless, the possibility that in addition to the EGF-R PTK activity, the ssD-BP phosphorylation is mediated by a common downstream pathway affected by all treatments, cannot be discounted.
An additional interesting observation was that there appeared to be a strong correlation between the cellular EGF-R numbers and the extent of AAV binding. For example, A431 cells, which express the highest numbers of the EGF-R, also bound AAV most efficiently, and H69 cells, which do not express these receptors, failed to bind AAV (Fig. 5). The possibility that in addition to heparan sulfate proteoglycan for binding, AAV might utilize the EGF-R as a coreceptor for efficient entry did not escape our notice and was quickly examined, but unfortunately this could not be substantiated experimentally (46). It is also noteworthy that phosphorylated forms of the ssD-BP were detected in H69 cells that apparently lack the EGF-R PTK activity (Fig. 6). Moreover, the pattern of phosphorylation of the ssD-BP in H69 cells was not altered in response to treatment with genistein, tyrphostin 1, and tyrphostin 23. Further studies to determine whether the ssD-BP is phosphorylated by protein tyrosine kinases other than the EGF-R PTK and/or serine-threonine kinases in these cells are warranted.
EGF-R PTK can be activated upon EGF ligand binding (6). Treatment of 293 cells with EGF resulted in an increase in the amount of the phosphorylated form of the ssD-BP, again suggesting the involvement of the EGF-R PTK in the ssD-BP phosphorylation. However, treatment of 293 cells with EGF also resulted in increased transduction with vCMVp-lacZ (data not shown), an apparent paradox. It is possible that this may be due to EGF pushing cells toward the S-phase of cell cycle (10), since it has been previously reported that AAV vectors transduce cells in S-phase with greater than 200 times the frequency than cells that are quiescent (49). However, we believe that the rate of dephosphorylation of the ssD-BP in 293 cells may be high enough to negate the transient effect of EGF, since WCEs were prepared and analyzed immediately following the EGF-treatment whereas AAV-mediated transgene expression was evaluated 48 h p.i. Alternatively, it is possible that factors in addition to the ssD-BP phosphorylation state act in concert to influence the AAV transduction efficiency. It is noteworthy, however, that skeletal muscle and brain tissues, which have been shown to be extremely well transduced by recombinant AAV vectors in vivo (14, 20, 21, 31, 60), express little to no EGF-R (28, 52).
The toxicity assays demonstrated that both tyrphostin 1 and tyrphostin 23 were much less toxic to cells than other previously published treatments, such as genistein or HU. The low toxicity of these compounds as well as their ability to significantly increase recombinant AAV transduction efficiency may prove to be valuable for gene therapy. In preliminary experiments, treatment of primary human bone marrow-derived CD34+ hematopoietic progenitor cells with tyrphostin 1 and tyrphostin 23 was also less toxic than that with genistein (data not shown). Attempts to document the efficacy of tyrphostin treatment in augmenting AAV transduction efficiency in a murine model in vivo did not succeed, most probably because at low doses of tyrphostin, an effective threshold concentration could not be achieved. In vivo experiments with high-dose tyrphostin treatments were compromised due to the toxicity of DMSO, which was used as a solvent (data not shown).
In sum, our present studies have identified that the cellular EGF-R PTK catalyzes phosphorylation of the ssD-BP, a crucial player in AAV-mediated transduction. Further studies of the interactions between EGF-R and the ssD-BP and additional downstream target proteins should allow for a better understanding of molecular events involved in high-efficiency AAV transduction which, in turn, should lead to improvements in the optimal use of AAV vectors in human gene therapy.
ACKNOWLEDGMENTS
We thank Francis G. Kern and Richard J. Samulski for generously providing the recombinant pCHCEGFR and pAAV/Ad plasmids, respectively. We also thank Kelly Hiatt for expert technical assistance.
This research was supported in part by Public Health Service grants (HL-48342, HL-53586, HL-58881, and DK-49218, Centers of Excellence in Molecular Hematology) from the National Institutes of Health and by a grant from the Phi Beta Psi sorority. A.S. was supported by an Established Investigator Award from the American Heart Association.
REFERENCES
- 1.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- 2.Alexander I E, Russell D W, Spence A M, Miller A D. Effects of gamma irradiation on the transduction of dividing and nondividing cells in brain and muscle of rats by recombinant adeno-associated virus vectors. Hum Gene Ther. 1996;7:841–850. doi: 10.1089/hum.1996.7.7-841. [DOI] [PubMed] [Google Scholar]
- 3.Barnes S, Peterson G T. Biochemical targets of the isoflavone genistein in tumor cell lines. Soc Exp Biol Med. 1995;280:103–109. doi: 10.3181/00379727-208-43840. [DOI] [PubMed] [Google Scholar]
- 4.Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–307. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 5.Berns K I, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 6.Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995;19:413–430. doi: 10.1006/cbir.1995.1086. [DOI] [PubMed] [Google Scholar]
- 7.Carlo-Stella C, Regazzi E, Garau D, Mangoni L, Rizzo M T, Bonati A, Dotti G, Almici C, Rizzoli V. Effect of the protein tyrosine kinase inhibitor genistein on normal and leukaemic haemopoietic progenitor cells. Br J Haematol. 1996;93:551–557. doi: 10.1046/j.1365-2141.1996.d01-1694.x. [DOI] [PubMed] [Google Scholar]
- 8.Constantinou A, Huberman E. Genistein as an inducer of tumor cell differentiation: possible mechanisms of action. Soc Exp Biol Med. 1995;203:109–115. doi: 10.3181/00379727-208-43841. [DOI] [PubMed] [Google Scholar]
- 9.Couldwell W T, Hinton D R, He S, Chen T C, Sebat I, Weiss M H, Law R E. Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett. 1994;345:43–46. doi: 10.1016/0014-5793(94)00415-3. [DOI] [PubMed] [Google Scholar]
- 10.Faaland C A, Mermelstein F H, Hayashi J, Laskin J D. Rapid uptake of tyrphostin into A431 human epidermoid cells is followed by delayed inhibition of epidermal growth factor (EGF)-stimulated EGF receptor tyrosine kinase activity. Mol Cell Biol. 1991;11:2697–2703. doi: 10.1128/mcb.11.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabricant R N, DeLarco J E, Todaro G J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci USA. 1977;74:565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 15.Fukazawa H, Li P M, Yamamoto C, Murakami Y, Mizuno S, Uehara Y. Specific inhibition of cytoplasmic protein tyrosine kinases by herbimycin A in vitro. Biochem Pharmacol. 1991;42:1661–1671. doi: 10.1016/0006-2952(91)90500-5. [DOI] [PubMed] [Google Scholar]
- 16.Gamou S, Hunts J, Harigai H, Hirohashi H, Shimosato Y, Pastan I, Shimizu N. Molecular evidence for the lack of epidermal growth factor receptor gene expression in small cell lung carcinoma cells. Cancer Res. 1987;47:2668–2673. [PubMed] [Google Scholar]
- 17.Gazit A, Yaish P, Gilon C, Levitzki A. Tyrphostin I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 18.Ghiringhelli P D, Romanowski V. Quick methylene blue staining for visualizing virus plaques in titration experiments. BioTechniques. 1994;17:464–465. [PubMed] [Google Scholar]
- 19.Giard D J, Aaronson S A, Todaro G J, Arnstein P, Kersey J H, Dosik H, Parks W P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 20.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–153. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 21.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovalenko M, Gazit A, Böhmer A, Rorsman C, Rönnstrand L, Heldin C H, Waltenberger J, Böhmer F O, Levitzki A. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 23.Kube D M, Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic Acids Res. 1997;25:3375–3376. doi: 10.1093/nar/25.16.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kube D M, Ponnazhagan S, Srivastava A. Encapsidation of adeno-associated virus type 2 Rep proteins in wild-type and recombinant progeny virions: Rep-mediated growth inhibition of primary human cells. J Virol. 1997;71:7361–7371. doi: 10.1128/jvi.71.10.7361-7371.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo M L, Yang N C. Reversion of v-H-ras-transformed NIH 3T3 cells by apigenin through inhibiting mitogen activated protein kinase and its downstream oncogenes. Biochem Biophys Res Comm. 1995;212:767–775. doi: 10.1006/bbrc.1995.2035. [DOI] [PubMed] [Google Scholar]
- 26.Levitzki A. Tyrphostin: potential anti-proliferative agents and novel molecular tools. Biochem Pharmacol. 1990;40:913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- 27.Levitzki A, Gazit A, Osherov N, Posner I, Gilon C. Inhibition of protein tyrosine kinases by tyrphostin. Methods Enzymol. 1991;201:347–361. doi: 10.1016/0076-6879(91)01031-v. [DOI] [PubMed] [Google Scholar]
- 28.Lim R W, Hauschka S D. A rapid decrease in epidermal growth factor binding capacity accompanies the terminal differentiation of mouse myoblasts in vitro. J Cell Biol. 1984;98:739–747. doi: 10.1083/jcb.98.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livneh E, Prywes R, Kashles O, Reiss N, Sasson I, Mory Y, Ullrich A, Schlessinger J. Reconstitution of human epidermal growth factor receptors and its deletion mutants in cultured hamster cells. J Biol Chem. 1986;261:12490–12497. [PubMed] [Google Scholar]
- 30.Lyall R M, Zilberstein A, Gazit A, Gilon C, Levitzki A, Schlessinger J. Tyrphostin inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989;264:14503–14509. [PubMed] [Google Scholar]
- 31.McCown T J, Xiao X, Li J, Breese G R, Samulski R J. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 32.McGlynn E, Becker M, Mett H, Reutner S, Cozens R, Lydon N B. Large scale purification and characterization of a recombinant epidermal growth factor receptor protein tyrosine kinase. Eur J Biochem. 1992;207:265–275. doi: 10.1111/j.1432-1033.1992.tb17047.x. [DOI] [PubMed] [Google Scholar]
- 33.Miller D L, El-Ashry D, Cheville A L, Liu Y, McLeskey S W, Kern F G. Emergence of MCF-7 cells overexpressing a transfected epidermal growth factor receptor (EGFR) under estrogen-depleted conditions: evidence for growth and progression. Cell Growth Differ. 1994;5:1263–1274. [PubMed] [Google Scholar]
- 34.Muller M T. Binding of herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987;61:858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 36.Nahreini P, Woody M J, Zhou S Z, Srivastava A. Versatile adeno-associated virus 2-based vectors for constructing recombinant virions. Gene. 1993;124:257–262. doi: 10.1016/0378-1119(93)90402-o. [DOI] [PubMed] [Google Scholar]
- 37.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 38.Okada T, Sakuma L, Fukui Y, Hazeki O, Ui M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phophatidylinositol 3-kinase. J Biol Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- 39.Ponnazhagan S, Erikson D, Kearns W G, Zhou S Z, Nahreini P, Wang X-S, Srivastava A. Lack of site-specific integration of the recombinant adeno-associated virus 2 genomes in human cells. Hum Gene Ther. 1997;8:275–284. doi: 10.1089/hum.1997.8.3-275. [DOI] [PubMed] [Google Scholar]
- 40.Ponnazhagan S, Mukherjee P, Wang X-S, Qing K, Kube D M, Mah C, Kurpad C, Yoder M C, Srour E F, Srivastava A. Adeno-associated virus type 2-mediated transduction of primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol. 1997;71:8262–8267. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X-S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 42.Ponnazhagan S, Wang X-S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 43.Ponnazhagan S, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol. 1997;71:3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qing K, Bachelot T, Mukherjee P, Wang X-S, Peng L, Yoder M C, Leboulch P, Srivastava A. Adeno-associated virus type 2-mediated transfer of ecotropic retrovirus receptor cDNA allows ecotropic retroviral transduction of established and primary human cells. J Virol. 1997;71:5663–5667. doi: 10.1128/jvi.71.7.5663-5667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qing K, Khuntirat B, Mah C, Kube D M, Wang X-S, Ponnazhagan S, Zhou S Z, Dwarki V J, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qing, K. Y., C. Mah, J. Hansen, S. Z. Zhou, V. J. Dwarki, and A. Srivastava. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Submitted for publication. [DOI] [PubMed]
- 47.Qing K Y, Wang X-S, Kube D M, Ponnazhagan S, Bajpai A, Srivastava A. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci USA. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell D W, Alexander I E, Miller A D. DNA synthesis and topoisomerase inhibitors increase transduction by adeno-associated virus vectors. Proc Natl Acad Sci USA. 1995;92:5719–5723. doi: 10.1073/pnas.92.12.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell D W, Miller A D, Alexander I E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA. 1994;91:8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;36:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava A, Lusby E W, Berns K I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983;45:555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Styren S D, DeKosky S T, Rogers J, Mufson E J. Epidermal growth factor receptor expression in demented elderly: localization to vascular endothelial cells of brain, pituitary, and skin. Brain Res. 1993;615:181–190. doi: 10.1016/0006-8993(93)90028-l. [DOI] [PubMed] [Google Scholar]
- 53.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 55.Wang X-S, Khuntirat B, Qing K Y, Ponnazhagan S, Kube D M, Zhou S Z, Dwarki V J, Srivastava A. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination. J Virol. 1998;72:5472–5480. doi: 10.1128/jvi.72.7.5472-5480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X-S, Ponnazhagan S, Srivastava A. Rescue and replication signals of the adeno-associated virus 2 genome. J Mol Biol. 1995;250:573–580. doi: 10.1006/jmbi.1995.0398. [DOI] [PubMed] [Google Scholar]
- 57.Wang X-S, Ponnazhagan S, Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J Virol. 1996;70:1668–1677. doi: 10.1128/jvi.70.3.1668-1677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X-S, Qing K Y, Ponnazhagan S, Srivastava A. Adeno-associated virus type 2 DNA replication in vivo: mutation analyses of the D sequence in viral inverted terminal repeats. J Virol. 1997;71:3077–3082. doi: 10.1128/jvi.71.4.3077-3082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber W, Bertics P J, Gill G N. Immunoaffinity purification of the epidermal growth factor receptor. J Biol Chem. 1984;259:14631–14636. [PubMed] [Google Scholar]
- 60.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yaish P, Gazit A, Gilon C, Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988;242:933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]