Abstract
RNA-dependent protein kinase PKR is an important regulator of gene expression in interferon (IFN)-treated and virus-infected cells. The 50-kb gene encoding human PKR kinase (pkr) is inducible by IFN. Transfection analyses, using chloramphenicol acetyltransferase (CAT) as the reporter in constructs possessing various 5′-flanking fragments of the human pkr gene, led to the identification of a functional TATA-less promoter that directed IFN-inducible transcription. Sequence determination and mutational analysis of the pkr promoter region revealed, in addition to a functional copy of the IFN-stimulated response element (ISRE) responsible for inducibility by type I IFN, a novel 15-bp element required for optimal promoter activity mediated by the ISRE. This element (5′ GGGAAGGCGGAGTCC 3′), designated KCS for kinase-conserved sequence, is exactly conserved between the human and mouse pkr promoters in sequence and position relative to the ISRE. We have now carried out an extensive mutational analysis of the 15-bp KCS element. Site-directed mutagenesis was performed, whereby every base pair position within the KCS element was replaced by each of the other three alternatives. Forty-five substitution mutants were analyzed for promoter activity by transient transfection analysis of untreated and IFN-treated human cells. The results establish 5′ NNRRRGG(C,A,T)GGRGYYN 3′, where R stands for purine and Y stands for pyrimidine, as the consensus sequence for the KCS element, both for basal and for IFN-inducible promoter activity. KCS-binding proteins were detected by electrophoretic mobility shift analysis (EMSA). Competition EMSA established that constitutively expressed nuclear proteins bound the KCS element selectively; KCS protein binding activity correlated with promoter activity in the transient transfection reporter assay.
The RNA-dependent protein kinase (PKR) is an interferon (IFN)-inducible enzyme (5, 27, 29, 32). PKR catalyzes the phosphorylation of the α subunit of eukaryotic protein synthesis initiation factor 2; this modulation causes an inhibition of mRNA translation (5, 10, 29). PKR is also involved in the modulation of cytokine signaling and transcriptional activation via the NF-κB and STAT factors (13, 39). Because of these fundamental biochemical activities, PKR affects a range of biological processes. For example, PKR plays a central role in the antiviral actions of IFN (28) and is implicated in the control of cell growth and differentiation as well as apoptosis (5, 16). The expression and function of PKR are regulated in several ways including transcriptional induction by IFN treatment (12, 19, 33, 35), translational inhibition by an autoregulatory mechanism (1, 36), posttranslational activation by RNA-dependent autophosphorylation (3, 18, 24, 27, 34, 37), and posttranslational modulation via homomeric and heteromeric protein-protein interactions (2, 4, 6, 15, 20, 21).
The induction by IFN of pkr gene transcription above the basal level of synthesis is well established, initially from Northern blot analysis and nuclear run-on analyses (19, 35) and more recently by transient transfection analyses with the isolated promoter from the human and mouse pkr genes (11, 12, 33). The gene encoding the PKR kinase consists of 17 exons and spans about 50 kb on human chromosome 2 (11). Transient transfection analyses, using chloramphenicol acetyltransferase (CAT) as the reporter in plasmid constructions possessing various 5′-flanking fragments of the human pkr gene, led to the identification of a functional TATA-less promoter that directed IFN-inducible transcription (12). Sequence determination and mutational analysis of the pkr promoter region revealed a consensus and functional copy of the IFN-stimulated response element (ISRE) responsible for the inducibility of many different genes by type I IFNs (31, 38). In addition to the ISRE, a novel 15-nucleotide (nt) element which was required for optimal promoter activity was identified (12). This newly identified element (5′ GGGAAGGCGGAGTCC 3′) was designated KCS, for kinase-conserved sequence, because it was exactly conserved in sequence and position between the human and mouse pkr promoters and so far is unique to the pkr promoters (12, 33).
In this communication we elucidate by extensive mutagenesis the consensus sequence for the newly identified KCS element and we establish by competition electrophoretic mobility shift assay (EMSA) the presence of KCS-binding proteins. A family of 45 KCS substitution mutants were generated and tested for basal and IFN-inducible promoter activity. The consensus sequence of the KCS element required for basal promoter activity in untreated cells was identical to that required for inducible activity in IFN-treated cells. EMSA revealed that constitutively expressed nuclear proteins selectively bound the wild-type KCS element, but not mutated forms of KCS which were deficient in promoter activity, in transient transfection assays.
MATERIALS AND METHODS
Oligonucleotide-directed mutagenesis of the KCS element within the pkr promoter.
The isolation of the functional promoters for the IFN-inducible RNA-dependent protein kinase genes from both λ-phage human placenta and P1-phage human fibroblast foreskin genomic libraries has been previously reported (12). Single site-directed nucleotide substitutions within the KCS element of the pkr promoter were prepared by a PCR-based method for site-directed mutagenesis. For each KCS sub mutant, PCR (25) was performed with native Taq DNA polymerase under conditions specified by the manufacturer (Perkin-Elmer). The PCR products were engineered to possess appropriate restriction sites that facilitated the subcloning of the mutant KCS fragment into the parent 503 wild-type pkr promoter construct.
The plus primers for PCR were the oligonucleotides used for mutagenesis. They were 5′ CTGCAGGGAAGGCGGAGTCC 3′ for positions 1 through 12 of the KCS element and 5′ CTGCAGGGAAGGCGGAGTCCAAGGGG 3′ for positions 13, 14, and 15 of KCS, where the 15 nt of the KCS element are shown in boldface and underlined. The KCS element nucleotides were preceded by a 5′ PstI restriction site that corresponds to the PstI site found naturally at that position in the pkr promoter (12). The custom oligonucleotide primers used for mutagenesis were obtained commercially from BioSynthesis (Lewisville, Tex.) or were synthesized with a Millipore Cyclone Plus automated DNA synthesizer. The mutagenic primers were prepared in a doped manner, in which each position of the KCS element was singly altered in a manner that permitted all three non-wild-type nucleotides to be available for incorporation. For example, the three KCS sub mutants in which the wild-type G nucleotide at position 1 of the KCS element was replaced with a C, A, or T were generated with an oligonucleotide mixture of primers with the sequence 5′ CTGCA(C,A,T)GGAAGGCGGAGTCC 3′. The minus primer was the pCAT-Basic (−) oligonucleotide 5′ CAACGGTGGTATATCCAG 3′. The template for the PCR was the SmaI-PstI fragment from the pkr promoter (12).
Construction of reporter gene plasmids.
CAT reporter plasmids with mutated KCS elements were derived from the 503 wild-type parent human pkr promoter construct (12). The 503 parent plasmid contains the 503-nt SacII-SacII restriction fragment from the 5′-flanking region of the human pkr gene, which possesses the KCS element and ISRE, inserted into the CAT-Basic promoterless plasmid (Promega) that contains the CAT gene (see Fig. 1 schematic). KCS substitution (sub) mutants were subsequently made in the background of the 503 parent reporter plasmid by exchanging the wild-type 63-bp PstI-SacII fragment with the corresponding mutant PstI-SacII fragment that possessed the site-directed base pair substitution within the KCS element (Fig. 1). The structures of all pkr promoter mutant constructions were confirmed by Sanger sequencing (30).
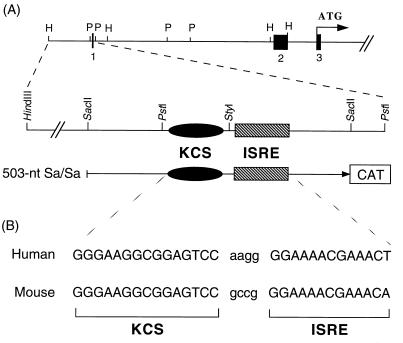
FIG. 1.
Physical map of the human pkr promoter region that contains the KCS element and ISRE. (A) Schematic diagram of the 5′-flanking region of the human pkr gene. Exons are indicated to scale in the upper diagram as filled boxes; introns and the 5′-flanking promoter region are indicated by the solid line. The entire human gene spans about 50 kb and contains 17 exons (11). The 503 wild-type human pkr promoter plasmid consists of the SacII-SacII fragment inserted into pCAT-Basic. The relative positions of the KCS element and ISRE are indicated in the lower diagram of the SacII-SacII fragment. (B) The sequences of the human (12) and the mouse (33) pkr promoters in the region that includes the 15-bp KCS element and the 13-bp ISRE.
The methods utilized for construction of the transcription vector plasmids were essentially those described by Sambrook et al. (26). Chemicals were reagent grade, and enzymes were obtained from New England Biolabs unless otherwise specified. The GenBank accession number for the 5′-flanking genomic sequence of the human pkr gene including the promoter region is U51035; the accession numbers for the sequences of the 17 exons, including the 5′-untranslated region, are U50632 to U50648.
Cell maintenance and IFN treatment.
Human amnion U cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with fetal bovine serum (HyClone) at 5% (vol/vol), 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Where indicated (see Fig. 2 and 3), treatment was with 1,000 IU of alpha IFN (IFN-α) per ml for 24 h. Parallel cultures were left untreated as controls.
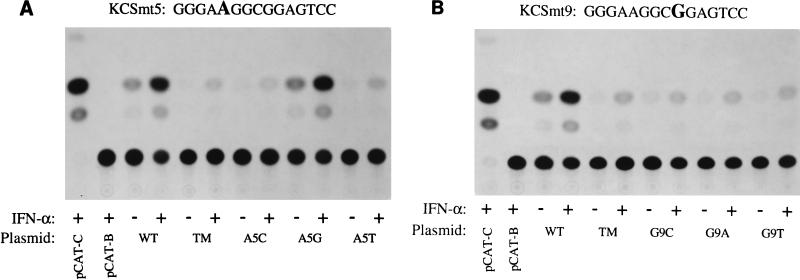
FIG. 2.
Autoradiogram showing the expression of CAT activity in cells transfected with pkr promoter constructs that contained representative KCS substitution mutants compared to that in cells containing the wild-type KCS sequence. Human amnion U cells were transfected with the indicated 503-nt Sa/Sa pkr promoter CAT reporter plasmids that contained single-nucleotide substitutions at either nucleotide position 5 (KCSmt5) or position 9 (KCSmt9) of the KCS element. The autoradiogram shown in panel A is representative of results obtained with KCSmt5 constructs, in which the A at nucleotide position 5 of the KCS element was replaced with either C (A5C), G (A5G), or T (A5T). The autoradiogram shown in panel B is representative of results obtained with KCSmt9 constructs, in which the G at nucleotide position 9 of the KCS element was replaced with either C (G9C), A (G9A), or T (G9T). Transfected cells were either left untreated (−) or were treated with IFN-α (+) prior to harvest and analysis of CAT activity. To control for transfection efficiency, cells were cotransfected with the pRSV2-βgal construct as an internal reference. The 503 pkr-CAT reporter constructions contained either the wild-type (WT) KCS element or a KCS element with triple-substitution mutation (TM) C8A, G9C, G10T. pCAT-Control, the CAT reporter gene linked to the simian virus 40 promoter and enhancer; pCAT-Basic, the promoterless plasmid vector alone without inserted human genomic DNA.
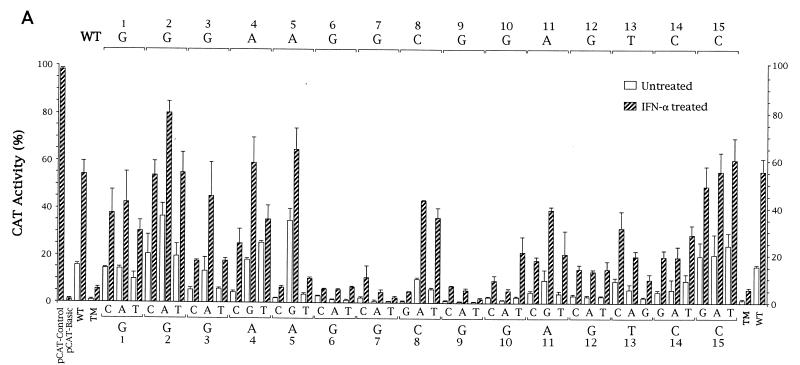
FIG. 3.
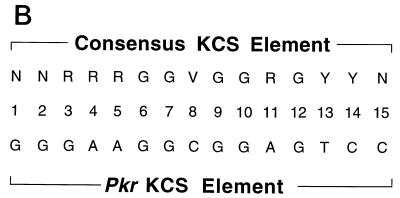
Functional analysis of KCS element substitution mutants. (A) Three different single-nucleotide substitutions were generated by site-directed mutagenesis at each of the 15 positions of the KCS element. PCR-derived fragments possessing the 45 specified mutations in KCS were subcloned into the IFN-inducible 503-nt Sa/Sa human pkr promoter CAT reporter plasmid that possesses the wild-type ISRE. Promoter activities were determined as described for Fig. 2 by transient transfection of human U cells. Activities are shown as percentages of the conversion of [14C]chloramphenicol to the acetylated derivatives. The averages and standard errors of the means were determined from three to five independent transfections for each of the KCS substitution mutants. pCAT-Control, the CAT reporter gene linked to the simian virus 40 promoter and enhancer; pCAT-Basic, the promoterless plasmid vector without inserted human genomic DNA; WT, the parent 503 pkr reporter construct containing the wild-type KCS element; TM, 503 construct containing a KCS element with a triple-substitution (C8A, G9C, G10T) mutation. (B) The consensus sequence of the KCS element as deduced from the family of single-site-substitution mutants analyzed in panel A, where R stands for purine, Y stands for pyrimidine, and V stands for c, a, t. The sequence of the KCS element from the human and mouse pkr promoters is from Fig. 1.
Transfection and reporter assays.
Transient transfection and CAT reporter analyses were carried out as previously described (12). Briefly, for the transient expression assay of pkr promoter function, U cells (60-mm-diameter dishes) at a density of approximately 5 × 105 cells per plate were transfected by the DEAE-dextran-chloroquine phosphate transfection method (17) using 10 μg of the pkr-CAT reporter gene construct and 5 μg of internal reference plasmid pRSV2-βgal. For comparison purposes, the pCAT-Control (Promega) plasmid containing the simian virus 40 promoter and enhancer and the pCAT-Basic promoterless plasmid were routinely analyzed in transfection experiments. All DNA plasmids used in transfections were purified by cesium chloride equilibrium centrifugation and were analyzed by agarose gel electrophoresis to verify plasmid integrity. Treatment with IFN was carried out beginning at approximately 24 h posttransfection. For the analysis of CAT and β-galactosidase activities, cell cultures were harvested 65 h after transfection, extracts were prepared by repeated freeze-thaw cycles, and enzymatic assays were performed as described previously (12, 26). The protein concentrations of extracts were determined by the Bradford method (Bio-Rad). CAT activity was quantified after thin-layer chromatography (TLC) by measurement of the 14C-acetylated chloramphenicol products formed, either by using a Beckman LS1801 liquid scintillation system to determine the radioactivity associated with the excised product spots localized by utilizing an autoradiogram of the TLC plate or by using a Bio-Rad GS525 molecular imager system. CAT activity values, calculated as percentages of the conversion of [14C]chloramphenicol to the acetylated derivatives, were normalized by β-galactosidase activity to control for variation in transfection efficiency. The results are reported as averages ± standard errors of the means determined from three to five independent transfections. Activities of the mutants were normalized to those of the 503 wild-type parent.
EMSA.
For protein-DNA binding reactions, nuclear extracts were prepared (9) from untreated or IFN-treated human U cells and incubated (∼10 μg of protein) in 25 μl of total reaction mixture containing 1 μg of poly(dI-dC), 2 mM Tris (pH 7.6), 0.2 mM EDTA, 8 mM NaCl, 0.8% glycerol, 0.3 mM β-mercaptoethanol, and 5 ng of the 32P-labeled KCS(WT) oligonucleotide probe. The probe was added last, and the reaction mixture was incubated for 20 min at room temperature. The reaction mixture was analyzed by gel electrophoresis with a 5% native polyacrylamide gel and 0.5× Tris-borate-EDTA that had been prerun at 4°C for 30 min. Electrophoresis was allowed to continue for approximately 80 min; the gel was then dried and exposed to X-ray film to obtain an autoradiographic image. Quantification of specific gel-shifted complexes was performed with a Bio-Rad GS525 molecular imager system. The following oligodeoxynucleotides were used, double stranded, as the 32P-end-labeled probe or unlabeled competitor in the gel shift analyses (mutations are underlined and in boldface): wild-type KCS [KCS(WT)], CTGCAGGGAAGGCGGAGTCCAAGG; wild-type ISRE [ISRE(WT)], CCAAGGGGAAAACGAAACTGCAG; mutant KCS(mt6A), CTGCAGGGAAAGCGGAGTCCAAGG; and mutant KCS(mt9T), CTGCAGGGAAGGCTGAGTCCAAGG.
Materials.
Unless otherwise specified, materials and reagents were as described previously (12, 33).
RESULTS
Transient transfection analyses using reporter constructions possessing 5′-flanking fragments from the human pkr gene (11) led to the identification of a functional TATA-less promoter that directed IFN-inducible transcription in human cells (12). The promoter for the pkr gene contains, in addition to the well-established 13-bp ISRE necessary for IFN-inducible transcription of most type I IFN-regulated genes (7, 31, 38), a novel 15-bp element that we designated KCS for kinase-conserved sequence. The KCS element is exactly conserved in sequence, distance, and position relative to the ISRE in the human and mouse pkr promoters (12). The KCS element is found immediately upstream of the ISRE in the 5′-flanking region of the human pkr gene as shown by the schematic diagram in Fig. 1. The KCS element is required for optimal transcriptional activity of the pkr promoter.
Functional analysis of the KCS element.
In order to define the nucleotide sequence necessary for transcriptional activation mediated by the KCS element, we carried out a systematic mutational analysis of the 15-bp region. At each of the 15 nt positions of the KCS element which are exactly conserved between the human and the mouse pkr promoters (Fig. 1B), three single-nucleotide-substitution mutants were generated by site-directed mutagenesis. A total of 45 KCS sub single mutants were prepared and subcloned into the background of the 503 promoter CAT construction which contains a wild-type ISRE. These KCS sub single mutants then were examined for their abilities to support basal as well as IFN-inducible transcription. Shown in Fig. 2 are autoradiograms that illustrate the promoter activities obtained with two sets of the single-nucleotide-substitution mutants: KCSmt5 (Fig. 2A), in which the base at position 5 of the KCS element (A) is replaced with C, G, or T, and KCSmt9 (Fig. 2B), in which the base at position 9 (G) is replaced with C, A, or T. Results obtained with the 503 KCS sub mutants were compared to those obtained with parallel cultures transfected with the parent 503 wild-type construction, the 503 triple-mutant construction, and with vector controls. The 503 wild-type construction reproducibly showed strong promoter activity that was IFN inducible (Fig. 2 and 3). As a negative control, the promoterless pCAT-Basic plasmid vector lacking the human pkr 503 insert exhibited a very low level of CAT activity (<2% conversion). The triple-substitution mutant (C8A, G9C, G10T) within the KCS element also showed a very low level of CAT activity, both in the absence and the presence of IFN treatment, as previously reported (12). pCAT-Control, the positive control plasmid which contains the simian virus 40 promoter and enhancer, displayed high CAT activity levels (>90% conversion) in our transfection assay. Neither pCAT-Basic nor pCAT-Control showed IFN inducibility (data not shown).
When human amnion U cells were transfected with the KCSmt5 constructs and then either left untreated or treated with IFN-α, the A5G mutant exhibited strong promoter activity. The CAT activity present in extracts prepared from IFN-treated cells transfected with the KCS A5G mutant was comparable to that of the wild-type construct in IFN-treated cells (Fig. 2A). By contrast, the activities of the KCS A5C and A5T mutants were greatly reduced relative to that of the wild-type construct. The levels of activity of the A5C and A5T single-substitution KCS mutants were low, both in untreated and IFN-treated cells, and were comparable to that of the KCS triple-substitution mutant (12) included as a reference control. The constitutive activity of the A5G mutant observed in the absence of IFN treatment was curiously about twofold higher than that of the wild-type parent. The three KCSmt9 single-substitution constructs, G9C, G9A, and G9T, all displayed poor promoter activity both in the absence and the presence of IFN treatment (Fig. 2B).
Consensus nucleotide sequence deduced from the KCS substitution mutants.
Forty-five site-directed mutations within the conserved 15-bp KCS were generated, corresponding to the three possible single-nucleotide substitutions for the wild-type nucleotide at each of the 15 positions conserved between the human (12) and mouse (33) pkr promoter sequences (Fig. 3). The family of single-nucleotide-substitution KCS sub mutants was analyzed for promoter activity in the transient transfection assay of both untreated and IFN-treated cells.
The results obtained from three to five independent transfections for each of the KCS substitution mutants in the background of the 503-nt Sa/Sa human pkr promoter CAT reporter plasmid are summarized in Fig. 3A. Several points emerge. First, a G at positions 6, 7, and 9 of the KCS core was absolutely essential for promoter activity, both basal and IFN inducible. Substitution of the wild-type G with a C, A, or T at these positions dramatically reduced pkr promoter activity. Likewise, a G was strongly preferred, although not essential, at positions 10 and 12. Second, substitution of the wild-type C with a G at position 8 of the KCS was the only change at this position which did not result in the retention of significant basal and inducible promoter activity. Third, a purine was preferred over a pyrimidine at positions 3, 4, 5, and 11 of the KCS sequence, whereas a pyrimidine was preferred over a purine at positions 13 and 14. The most pronounced difference between a purine and pyrimidine was found at position 5. Fourth, positions 1, 2, and 15 of the conserved 15-bp KCS sequence did not show a preference for any particular nucleotide. No significant change in basal or IFN-inducible activity was observed relative to that of the wild type for any of the sub mutants at these positions. These results are summarized in Fig. 3B, which shows the consensus nucleotide sequence deduced from the family of single-site KCS substitution mutants.
Computer analyses of the KCS consensus sequence deduced from the saturation mutagenesis results (Fig. 3) were performed with the FINDPATTERNS program and transcription factor databases, as well as with the TRANSFAC database (22). The sequence data banks revealed that so far the 15-nt KCS element seems to be unique to the human and mouse pkr promoters. However, the 5′ portion of the element corresponding to the sequence 5′ GGGAAGG 3′ conforms to a low-affinity-level binding site for the Sp1 transcription factor (14).
The KCS element is selectively bound by nuclear proteins.
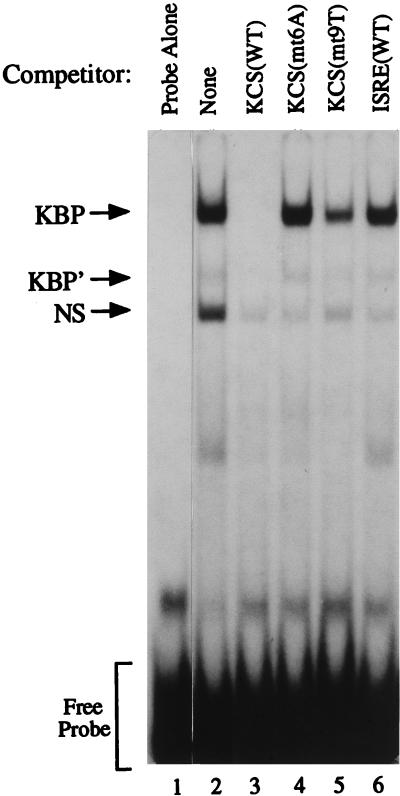
Results of the systematic mutational analysis of the 15-bp KCS element (Fig. 3) established the importance of the element for transcriptional activation of the pkr promoter in the transient transfection assay, both in untreated and IFN-treated cells. In an attempt to detect the existence in cells of KCS-binding protein factors, EMSA was carried out. A wild-type 32P-labeled KCS oligonucleotide was used as the probe, with nuclear extracts prepared from human amnion U cells either untreated or treated with IFN. Two major band shift complexes were observed, as shown by the results in Fig. 4 obtained with extracts from untreated U cells. The complexes, designated KBP and NS (Fig. 4, lane 2), were not observed with the probe alone (lane 1). Nuclear extracts prepared from IFN-treated U cells were comparable to those from untreated U cells in their ability to form the KBP and NS complexes with the wild-type KCS probe (data not shown). Similar results were obtained with extracts prepared from human HepG2 cells (data not shown).
FIG. 4.
Competition analysis of KCS-binding protein complex formation by EMSA. Nuclear extract prepared from human amnion U cells (∼10 μg of protein) was incubated with a 32P-labeled KCS oligonucleotide probe in the absence of competitors (lanes 1 and 2) or in the presence of a 100-fold molar excess of the following unlabeled double-stranded DNA synthetic oligomers: KCS(WT) (lane 3); KCS(mt6A) mutant (lane 4); KCS(mt9T) mutant (lane 5); and ISRE(WT) (lane 6). KBP and KBP′, KCS-binding protein complexes; NS, nonspecific binding.
Competition analysis revealed that the KCS-binding activity was selective. A 100-fold molar excess of unlabeled wild-type KCS oligonucleotide efficiently competed the formation of the KBP complex (lane 3). By contrast, neither two mutant KCS oligomers (lanes 4 and 5) nor a wild-type ISRE oligomer (lane 6) significantly affected KBP complex formation (Fig. 4). All unlabeled oligonucleotides examined as competitors prevented the formation of the nonspecific (NS) complex. The KCS(mt6A) and KCS(mt9T) mutants, which did not significantly compete the formation of the KBP complex (Fig. 4), also were unable to support significant promoter activity in the transient transfection assay (Fig. 3A). These results demonstrate the presence of constitutively expressed proteins that selectively bind the KCS element. Furthermore, the results correlate KCS protein binding activity with the constitutive activator function that KCS possesses.
DISCUSSION
This work is concerned with the precise definition of the sequence of the novel DNA element designated KCS (12) required to support optimal basal as well as IFN-inducible transcriptional activation of the pkr gene promoter encoding the RNA-dependent protein kinase PKR. The KCS element was originally identified by comparison of the sequence for the human pkr promoter (12) with that of the mouse pkr promoter (33). The absolute conservation of sequence, distance, and position of the 15-bp KCS element relative to the ISRE suggested a possible functional role for the newly recognized element. Preliminary deletion analysis indicated that the KCS element was required for optimal basal pkr promoter activity as well as IFN-inducible transcriptional activation mediated by the ISRE (12). Our results reported herein describing the systematic substitution analysis of the KCS element firmly establish that the KCS region of the pkr promoter is of central importance, both for basal transcription in the absence of IFN treament and for IFN-inducible transcriptional activation mediated by IFN-α treatment. We identified the nucleotide positions within the conserved 15-bp KCS sequence which are absolutely essential in order to sustain activity of the pkr promoter in human cells, and we established the presence of selective KCS-binding proteins. Single-base-pair substitutions at defined positions within the 15-bp KCS region completely abolished promoter activity, both basal and IFN inducible, whereas substitutions at other positions either had no significant effect or a minimal effect on activity. These results are consistent with an important functional role for the KCS element in pkr promoter function, both in the absence and the presence of cytokine treatment.
Single-base-pair substitutions at positions 6, 7, and 9 of the KCS element completely abolished promoter activity in human cells transiently transfected with CAT reporter constructs that contained the natural wild-type ISRE. These findings revealed that the presence of a wild-type ISRE together with the other elements within a 500-bp region of the pkr promoter was insufficient to confer significant promoter activity, either basal or IFN inducible, in the absence of a functional KCS element. EMSA revealed the presence of specific KCS-binding proteins. The binding of nuclear proteins to the KCS element was not dependent upon IFN treatment and correlated with the activity of the pkr promoter in transfected human U cells. Consistent with the role of the KCS element in providing basal promoter activity, nuclear extracts from untreated cells contained proteins that bound the KCS element specifically. A single major band shift complex (KBP complex) was detected with the KCS(WT) probe and extracts prepared from either human U or HepG2 cells. The complex was efficiently competed by an unlabeled KCS(WT) oligomer, but not by KCS mutant oligomers KCS(mt6A) and KCS(mt9T) or by the ISRE(WT) oligomer (Fig. 4). Both the basal and the IFN-inducible promoter activities of the single-nucleotide-substitution mutants KCS(mt6A) and KCS(mt9T) were severely impaired in the 503 pkr promoter background (Fig. 3). Although the full magnitude of the induced activity of the pkr promoter was dependent upon the KCS element, the fold induction for most of the KCS mutant constructs produced by IFN treatment varied only slightly from the ∼3.5-fold observed for the wild-type promoter, consistent with the potential role of the KCS element in providing basal promoter activity.
In IFN-α-treated cells, two families of factors are known to bind at the ISRE. The multiprotein transcriptional activator complex ISGF3, composed of Stat1, Stat2, and p48, binds to the ISRE, and the IFN regulatory factor family of proteins to which the p48 DNA-binding protein component of the ISGF3 complex belongs also binds to the ISRE (7, 8, 23, 31, 40). Presently available information does not support the notion of obligate interactions between the KCS element and ISRE and their cognate DNA-binding proteins. Nucleotide substitutions within the KCS element affected both the basal and the IFN-inducible activities of the pkr promoter in the transient transfection reporter assay (Fig. 3). And the activity of proteins that selectively bound to the KCS element was not dependent upon IFN treatment.
It is now of utmost importance to attempt to identify the protein factors that interact with the KCS element. Although computer analyses of the KCS consensus sequence deduced from saturation mutagenesis revealed that the 5′ portion of the element corresponded to a low-affinity binding site for the Sp1 transcription factor (14, 22), preliminary footprint analysis did not show protection of the KCS element by purified Sp1 protein (33a). Attempts to directly identify the proteins which bind to the KCS element are under way. The elucidation by systematic mutational analysis of the positions within the 15-bp KCS sequence that are of primary importance for pkr promoter activity hopefully will facilitate the expression cloning and affinity purification of KCS-binding proteins as well as the subsequent elucidation of the roles that they may play in transcriptional activation under various conditions of cytokine treatment and virus infection.
ACKNOWLEDGMENT
This work was supported in part by research grant AI-20611 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
REFERENCES
- 1.Barber G N, Wambach M, Wong M L, Dever T E, Hinnebusch A G, Katze M G. Translational regulation by the interferon-induced double-stranded RNA-activated 68-kDa protein kinase. Proc Natl Acad Sci USA. 1993;90:4621–4625. doi: 10.1073/pnas.90.10.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benkirane M, Neurveut C, Chun R F, Smith S M, Samuel C E, Gatignol A, Jeang K-T. Oncogenic potential of TAR RNA binding protein TRBP and its regulatory interaction with RNA-dependent protein kinase PKR. EMBO J. 1997;16:611–624. doi: 10.1093/emboj/16.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bevilacqua P C, George C X, Samuel C E, Cech T R. Binding of the protein kinase PKR to RNAs with secondary structure defects: role of the tandem A-G mismatch and noncontiguous helixes. Biochemistry. 1998;37:6303–6316. doi: 10.1021/bi980113j. [DOI] [PubMed] [Google Scholar]
- 4.Carpick B W, Graziano V, Schneider D, Maitra R K, Lee X, Williams B R G. Characterization of the solution complex between the interferon-induced, double-stranded RNA-activated protein kinase and HIV-1 trans-activating region RNA. J Biol Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 5.Clemens M J, Elia A. The double-stranded RNA-dependent protein kinase PKR: structure and function. J Interferon Cytokine Res. 1997;17:503–524. doi: 10.1089/jir.1997.17.503. [DOI] [PubMed] [Google Scholar]
- 6.Cosentino G P, Venkatesan S, Serluca F C, Green S R, Mathews M B, Sonenberg N. Double-stranded-RNA-dependent protein kinase and TAR RNA-binding protein form homo- and heterodimers in vivo. Proc Natl Acad Sci USA. 1995;92:9445–9449. doi: 10.1073/pnas.92.21.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darnell J E. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 8.Darnell J E, Kerr I M, Stark G R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 9.Dignam J D, Martin P L, Shastry B S, Roeder R G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- 10.Hershey J W B. Protein phosphorylation controls translation rates. J Biol Chem. 1989;264:20823–20826. [PubMed] [Google Scholar]
- 11.Kuhen K L, Shen X, Carlisle E R, Richardson A L, Weier H-U G, Tanaka H, Samuel C E. Mechanism of interferon action. Structural organization of the human Pkr gene encoding an interferon-inducible RNA-dependent protein kinase (PKR) and differences from its mouse homolog. Genomics. 1996;36:197–201. doi: 10.1006/geno.1996.0446. [DOI] [PubMed] [Google Scholar]
- 12.Kuhen K L, Samuel C E. Isolation of the interferon-inducible RNA-dependent protein kinase Pkr promoter and identification of a novel DNA element within the 5′-flanking region of the human and mouse Pkr genes. Virology. 1997;227:119–130. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Yang Y-L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissman C, Williams B R G. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutoh E, Margot J B, Schwander J. Genomic structure and regulation of the promoter of the rat insulin-like growth factor binding protein-2 gene. Mol Endocrinol. 1993;7:1205–1216. doi: 10.1210/mend.7.9.7504179. [DOI] [PubMed] [Google Scholar]
- 15.Lee T G, Tang N, Thompson S, Miller J, Katze M G. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lengyel P. Tumor-suppressor genes: news about the interferon connection. Proc Natl Acad Sci USA. 1993;90:5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthman H, Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983;11:1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack S J, Thomis D C, Samuel C E. Mechanism of interferon action. Identification of an RNA binding domain within the N-terminal region of the human RNA-dependent P1/eIF-2α protein kinase. Virology. 1992;188:47–56. doi: 10.1016/0042-6822(92)90733-6. [DOI] [PubMed] [Google Scholar]
- 19.Meurs E, Chong K, Galabru J, Thomas N S, Kerr I M, Williams B R G, Hovanessian A G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 20.Ortega L G, McCotter M D, Henry G L, McCormack S J, Thomis D C, Samuel C E. Mechanism of interferon action. Biochemical and genetic evidence for the intermolecular association of the RNA-dependent protein kinase PKR from human cells. Virology. 1996;215:31–39. doi: 10.1006/viro.1996.0004. [DOI] [PubMed] [Google Scholar]
- 21.Patel R C, Stanton P K, Sen G C. Specific mutations near the amino terminus of double-stranded RNA-dependent protein kinase (PKR) differentially affect its double-stranded RNA binding and dimerization properties. J Biol Chem. 1996;271:25657–25663. doi: 10.1074/jbc.271.41.25657. [DOI] [PubMed] [Google Scholar]
- 22.Quandt K, Frech C, Karas H, Wingender E, Weiner T. Matind and Matinspector—new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qureshi S A, Salditt-Georgieff M, Darnell J E. Tyrosine-phosphorylated Stat1 and Stat2 plus a 48-kDa protein all contact DNA in forming the interferon-stimulated-gene factor 3. Proc Natl Acad Sci USA. 1995;92:3829–3833. doi: 10.1073/pnas.92.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romano P R, Garcia-Barrio M T, Zhang X, Wang Q, Taylor D R, Zhang F, Herring C, Mathews M B, Qin J, Hinnenbush A G. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2α kinases PKR and GCN2. Mol Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saiki R K, Scharf S, Faloona F, Mullis K B, Horn G T, Erlich H A, Arnheim N. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985;230:1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Samuel C E. Mechanism of interferon action. Phosphorylation of protein synthesis initiation factor eIF-2 in interferon-treated human cells by a ribosome-associated protein kinase possessing site-specificity similar to hemin-regulated rabbit reticulocyte kinase. Proc Natl Acad Sci USA. 1979;76:600–604. doi: 10.1073/pnas.76.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 29.Samuel C E. The eIF-2α protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem. 1993;268:7603–7606. [PubMed] [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schindler C, Darnell J E. Transcriptional responses to polypeptide ligands. The Jak-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 32.Sen G C, Lengyel P. The interferon system: a bird’s eye view of its biochemistry. J Biol Chem. 1992;267:5017–5020. [PubMed] [Google Scholar]
- 33.Tanaka H, Samuel C E. Mechanism of interferon action. Structure of the mouse PKR gene encoding the interferon-inducible RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7995–7999. doi: 10.1073/pnas.91.17.7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Tanaka, H., and C. E. Samuel. Unpublished data.
- 34.Taylor D R, Lee S B, Romano P R, Marshak D R, Hinnenbush A G, Esteban M, Mathews M B. Autophosphorylation sites participate in the activation of the double-stranded RNA-activated protein kinase. Mol Cell Biol. 1996;16:6295–6302. doi: 10.1128/mcb.16.11.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomis D C, Doohan J P, Samuel C E. Mechanism of interferon action. cDNA structure, expression and regulation of the interferon-induced, RNA-dependent P1/eIF-2α protein kinase from human cells. Virology. 1992;188:33–46. doi: 10.1016/0042-6822(92)90732-5. [DOI] [PubMed] [Google Scholar]
- 36.Thomis D C, Samuel C E. Mechanism of interferon action: autoregulation of RNA-dependent P1/eIF-2α protein kinase (PKR) expression in transfected mammalian cells. Proc Natl Acad Sci USA. 1992;89:10837–10841. doi: 10.1073/pnas.89.22.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomis D C, Samuel C E. Mechanism of interferon action: evidence for intermolecular autophosphorylation and autoactivation of the interferon-induced, RNA-dependent protein kinase PKR. J Virol. 1993;67:7695–7700. doi: 10.1128/jvi.67.12.7695-7700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams B R G. Transcriptional regulation of interferon-stimulated genes. Eur J Biochem. 1991;200:1–11. doi: 10.1111/j.1432-1033.1991.tb21041.x. [DOI] [PubMed] [Google Scholar]
- 39.Wong A H-T, Tam N W N, Yang Y-L, Cuddihy A R, Li S, Kirchoff S, Hauser H, Decker T, Koromilas A E. Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. EMBO J. 1997;16:1291–1304. doi: 10.1093/emboj/16.6.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J J, Vinkemeier U, Gu W, Chakravarti D, Horvath C M, Darnell J E., Jr Two contact regions between Stat1 and CBP/p300 in interferon gamma signaling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]