Abstract
Background
Coronavirus disease 2019 (COVID-19) tends to have mild presentations in children. However, severe and critical cases do arise in the pediatric population with debilitating systemic impacts and can be fatal at times, meriting further attention from clinicians. Meanwhile, the intricate interactions between the pathogen virulence factors and host defense mechanisms are believed to play indispensable roles in severe COVID-19 pathophysiology but remain incompletely understood.
Data sources
A comprehensive literature review was conducted for pertinent publications by reviewers independently using the PubMed, Embase, and Wanfang databases. Searched keywords included “COVID-19 in children”, “severe pediatric COVID-19”, and “critical illness in children with COVID-19”.
Results
Risks of developing severe COVID-19 in children escalate with increasing numbers of co-morbidities and an unvaccinated status. Acute respiratory distress stress and necrotizing pneumonia are prominent pulmonary manifestations, while various forms of cardiovascular and neurological involvement may also be seen. Multiple immunological processes are implicated in the host response to COVID-19 including the type I interferon and inflammasome pathways, whose dysregulation in severe and critical diseases translates into adverse clinical manifestations. Multisystem inflammatory syndrome in children (MIS-C), a potentially life-threatening immune-mediated condition chronologically associated with COVID-19 exposure, denotes another scientific and clinical conundrum that exemplifies the complexity of pediatric immunity. Despite the considerable dissimilarities between the pediatric and adult immune systems, clinical trials dedicated to children are lacking and current management recommendations are largely adapted from adult guidelines.
Conclusions
Severe pediatric COVID-19 can affect multiple organ systems. The dysregulated immune pathways in severe COVID-19 shape the disease course, epitomize the vast functional diversity of the pediatric immune system and highlight the immunophenotypical differences between children and adults. Consequently, further research may be warranted to adequately address them in pediatric-specific clinical practice guidelines.
Keywords: Immunopathophysiology, MIS-C, Pediatric critical care, Severe pediatric COVID-19
Introduction
Contrary to most recognized respiratory pathogens, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative pathogen of COVID-19, typically leads to milder disease in pediatric cases than in adult patients [1, 2], but young children, particularly infants, may still be prone to contract the virus [3, 4], and a small number may develop severe or even critical illnesses [5, 6]. Children with severe COVID-19 may develop serious complications such as acute respiratory distress syndrome, myocarditis, acute renal failure, cardiogenic or septic shock, and multiorgan failure, and mortality can occur in extreme cases [7]. A nationwide surveillance study conducted in the United States during the peak of the pandemic recorded a COVID-19-related hospitalization rate of 48.2 per 100,000 population for children under 18 years of age from October 2020 to September 2021, of whom 26.4% required ICU admission, 6.2% required invasive mechanical ventilation, and 0.7% died while hospitalized [8]. The epidemiology of pediatric COVID-19 has also evolved substantially since the advent of successful vaccines, with new cases aggregating mostly in unvaccinated children or subgroups of children who are ineligible for immunization [9]. The picture is further complicated by the emergence of multisystem inflammatory syndrome in children (MIS-C), a distinctive post-infectious entity that accounted for a significant proportion of pediatric ICU admissions linked to COVID-19, and highlights the profound involvement of host immune response in the pathogenesis of severe pediatric COVID-19 and the associated conditions [10–13]. This review, therefore, aims to provide a holistic dissection of the diseases of interest with focuses on both the clinical and immunological standpoints.
Clinical approaches to severe pediatric COVID-19
Risk factors for developing severe COVID-19 in children and red flags for deterioration
The symptomology of clinically evident acute COVID-19 in children is similar to that in adults, which mostly involves the respiratory tract, with the most common presenting complaints being fever, coughs, coryzal symptoms including nasal congestion and rhinorrhea, and dyspnea, which may be accompanied by headaches, myalgia, generalized malaise, and possibly gastrointestinal symptoms such as nausea, vomiting, decreased oral intake, and diarrhea [14–16]. Identification and close monitoring of children at risk of severe COVID-19 represent a concrete first step in clinical assessment. The risk factors with the highest relative risks for severe COVID-19 in children are chronic lung diseases, obesity, diabetes, cardiovascular disease, neurological comorbidities including seizure disorders, and prematurity (among children below 2 years of age) [1, 17, 18], many of which are linked to endothelial impairment and a pro-inflammatory state [19], and odds of critical care admission and mortality increase in a step-wise manner with increased number of comorbidities [20].
Meanwhile, it is important to clarify the vaccination status in light of the well-rounded protective effects it offers against disease transmission, progression, and complications. For instance, a recent meta-analysis of 51 studies revealed that 2 doses of mRNA vaccines are 75.3% and 78% effective against COVID-19-associated hospitalizations and MIS-C, respectively, in children between the age of 5 and 11 years [21]. However, waning protection with the emergence of novel variants (e.g., Omicron)[22] and elapsed time since the last administered dose [23], especially for those aged 5–11 years as opposed to older children or adolescents [24, 25], need to be taken into consideration. It is worth noting that immunocompromised children, even if appropriately vaccinated as per the modified schedule, are still deemed at high risk of progression to severe COVID-19 due to lower response rates to vaccinations and vulnerability to SARS-CoV-2 pathogenicity [26].
Any clinical, biochemical, and radiological signs indicating deterioration, especially in at-risk children, should be promptly acknowledged and actioned upon, which include (1) increased respiratory rate; (2) poor responsiveness, drowsiness, and convulsions; (3) lymphopenia and/or thrombocytopenia; (4) hypo/hyperglycemia and/or hyperlactatemia; (5) markedly elevated inflammatory markers such as procalcitonin, C-reactive protein, and ferritin; (6) significant transaminitis and creatine kinase elevation; (7) pronounced abnormalities in coagulation function parameters; and (8) changes in head imaging such as cerebral edema or significant progression of pulmonary lesions on chest imaging [27].
Clinical characteristics of severe and critical pediatric COVID-19
Case definition
The case definition for severe pediatric COVID-19 may have slight variations across studies and guidelines, but it typically requires (1) a form of diagnostic certainty with a positive RT-PCR result for SARS-CoV-2 nucleic acids as the gold standard; (2) hospitalization as a result of COVID-19 related symptoms, thereby excluding cases managed in the outpatient setting; and (3) ICU admission, invasive mechanical ventilation, or circulatory support as the key indicators of disease severity, albeit inevitably limited by disparities in intervention thresholds between different centers and patient subgroups [1, 20]. Besides, deviations in respiratory and oxygenation indices have been used to delineate severe disease, including a blood oxygen saturation level (SpO2) of < 94% on room air under atmospheric pressure at sea level [27, 28] and significant elevations in age-adjusted respiratory rate [27], while conditions suggestive of other organ system dysfunctions are also proxy measures [10, 29].
Respiratory
The rate of viral pneumonia is determined to be 24% among children hospitalized with a COVID-19 diagnosis by a multi-center study in the US [30]. Necrotizing pneumonia (NP) is a disastrous complication of severe pediatric COVID-19, which stemmed from aggressive bacterial superinfections causing lung tissue liquefaction and cavitation, with the most common causative organisms being Staphylococcus aureus, Streptococcus pneumoniae, and Mycoplasma pneumoniae [31]. Specifically, Akuamoah-Boateng et al. reported the case of a 13-year-old boy with COVID-19 and convincing laboratory and radiologic features of NP presumably caused by Prevotella oris, which was not detected by the blood cultures prior to antimicrobial administration but returned positive on PCR of the surgical drainage sample of concurrent subdural empyemas [32]; and Brisca et al. reported a case of NP in a 4-month-old infant with of COVID-19 and concurrent central venous catheter-associated methicillin-susceptible Staphylococcus aureus bacteremia [33]. NP in both cases eventually resolved with appropriate level of respiratory support and multimodal pharmacotherapy.
Physicians should also be kept aware of COVID-19-related croup [34] that has shown increased incidences with Omicron variants [35, 36], manifests as vocal hoarseness, stridor, wheezing, or lung rales [34, 37, 38], and in critical cases may require endotracheal intubation and cardiopulmonary resuscitation, although COVID-19-associated croup is treated similarly to other viral causes of croup and respiratory distress is generally uncommon [39].
Additionally, a scrutinization of the Overcoming COVID-19 registry data has shown that acute respiratory distress syndrome (ARDS) was seen in approximately 10% of hospitalized children with severe acute COVID-19 and MIS-C [10], compared to 33% in admitted adults [40]. Despite lower incidences compared to adults, COVID-19-related pediatric ARDS required mechanical ventilation in most cases [41, 42], and the strategy of low tidal volume and limiting plateau pressure for lung protection has been routinely implemented [41, 43–45]. ARDS was also found to be independently associated with lower probability of discharge from PICU and hospital in a multivariable analysis of time to discharge [41], which reflects its protracted disease course and risk of lethality.
Cardiovascular
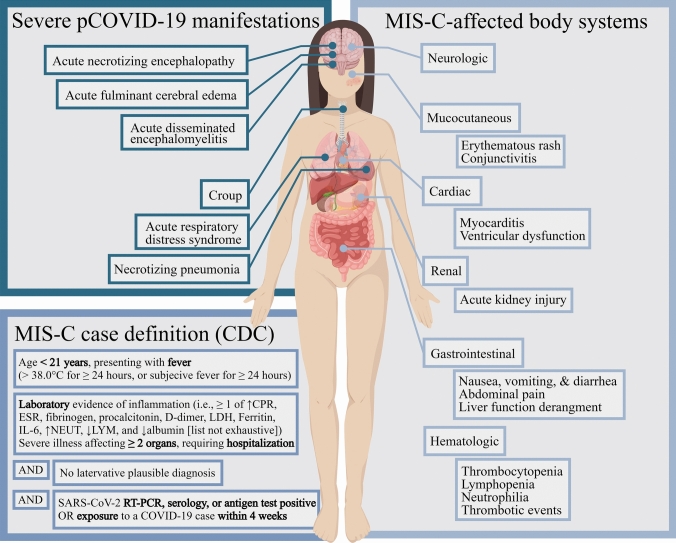
Cardiogenic shock [46], pericarditis [47], and myocardial injury [48–50] have all been reported in acute SARS-CoV-2 infection in children, and evidence of persistent cardiac injury could be detected 3–6 months on cardiac magnetic resonance imaging after severe pediatric COVID-19 [51]. However, a large proportion of COVID-19-related cardiovascular involvement in pediatric patients may be driven by indirect, non-cardiac insults, especially MIS-C [52]. 93% of the 283 children with MIS-C in a multicenter European study had myocardial injury, as reflected by elevated troponin levels [53], while reduced ejection fraction can be seen in approximately 30% of MIS-C patients [10, 53]. Even though SARS-CoV-2 RNA was detected with myocarditis on post-mortem cardiac biopsy of an 11-year-old child who died of MIS-C, suggesting direct viral invasion could be the inciting and perpetrating event in this particular case [54], a systemic inflammatory response is likely the principal driving force for myocarditis in most MIS-C cases [53, 55], which is known for its multiorgan manifestations (Fig. 1) and will be discussed in further details in connection with an evaluation of its elusive pathogenesis.
Fig. 1.
Potentially life-threatening manifestations of severe pediatric COVID-19 and organ systems implicated in MIS-C. The MIS-C diagnostic criteria are adapted from the Centers of Disease Control and Prevention of the United States. pCOVID-19 pediatric COVID-19, CRP C-reactive protein, ESR erythrocyte sedimentation rate, LDH lactate dehydrogenase, IL-6 interleukin 6, NEUT neutrophils, LYM lymphocytes, RT-PCR reverse transcription polymerase chain reaction
Neurologic
Non-specific neurologic symptoms are relatively common in children with COVID-19, whereas serious neurologic manifestations are much more infrequent, with an estimated prevalence of 3.8% among pediatric patients admitted to hospital with COVID-19 [56]. A significant proportion of them may be broadly categorized as neuroimmune disorders, among which numerous cases of acute disseminated encephalomyelitis [57–61], a demyelinating disease affecting the central nervous system, as well as Guillain–Barré syndrome [62, 63] have been reported. On the other hand, direct viral invasion of the central nervous system (CNS) by SARS-CoV-2 is rare but has been described [64], and disruption of the blood–brain barrier and host immunity secondary to COVID-19 may predispose patients to lethal CNS co-infections, including opportunistic ones by Mycobacterium Tuberculosis [61]. Furthermore, cytokine storm and systematic inflammation may be the driving force of life-threatening neurologic conditions seen in severe pediatric COVID-19 such as acute necrotizing encephalopathy, a para-infectious condition most commonly precipitated by viruses of the Orthomyxoviridae family that is characterized by multifocal symmetrical lesions, especially in bilateral thalami [65, 66], and acute fulminant cerebral edema, which can happen in previously healthy children, causing brain herniation and death within 24–48 hours of foretelling seizures [67, 68]. Moreover, a well-conducted US study utilizing surveillance data showed that 47% of the pediatric patients hospitalized with COVID-19-related illnesses who developed life-threatening neurologic conditions met the diagnostic criteria for MIS-C [11].
Pathogenic and immunologic determinants of disease severity
SARS-CoV-2 cellular binding and entry
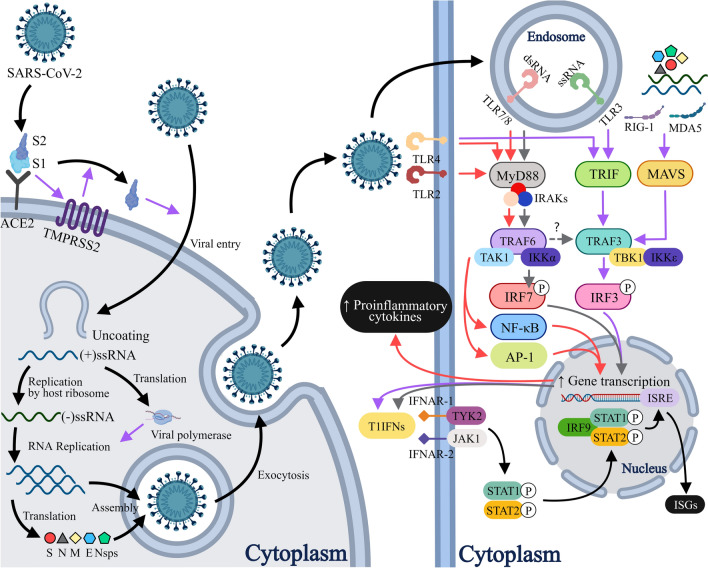
Like SARS-CoV, SARS-CoV-2 utilizes its spike (S) glycoprotein to facilitate entry into target cells via interaction with the angiotensin converting enzyme II (ACE2). The S1 subunit of the S protein binds to ACE2, which activates an accompanying host protease, most commonly transmembrane serine protease 2 (TMPRSS2), releasing the S2 subunit from the S1-S2 complex [69–72]. The S2 subunit then enables fusion of the viral envelope with the cellular membrane and consequently endocytosis of the viral components [73] (Fig. 2). SARS-CoV-2 cellular tropism is therefore largely determined by co-expression of ACE2 and TMPRSS2, which is present in cells from multiple tissue origins, including nasal secretory and ciliated cells [74], alveolar epithelial type II cells (AT2s) [75], enterocytes of the small and large intestines [76, 77], proximal tubular cells of the kidney [78], cardiomyocytes [79], and vascular endothelial cells [78, 80–82], suggesting clinical manifestations in the respective organ systems may be at least partially attributed to direct cellular invasion and damage. Though still debated, many have argued that relatively lower expression and distinct distribution pattern of these entry factors in infants and children may confer protection against severe disease [83–86].
Fig. 2.
ACE-2-TMPRSS2-mediated SARS-CoV-2 cell entry and type 1 interferon pathway. SARS-CoV-2-associated molecules are recognized by a wide range of PRRs located in different compartments, including TLR2, 4 and 6 on the plasma membrane, TLR3, 7 and 8 in endosomes, and RIG-1 and MDA-5 in the cytoplasm, each with its respective ligands. Ligand binding causes TLRs to dimerize and instigate the downstream signaling pathways in an MyD88-dependent or TRIF-dependent manner. Activation of TLR2, 4, 7, and 8 recruits the canonical adaptor protein MyD88, which sequentially mobilizes the IRAK complex, TRAF6, and TAK1. TAK1 is then capable of initiating the IKK-NFκB and the MAPK-AP1 pathways, stimulating production of various proinflammatory cytokines. In addition, activation of TLR7 or TLR8 also triggers IRAK, TRAF6, TRAF3, and IKKα-dependent phosphorylation and thus activation of IRF7. Contrarily, TRAF3 may be activated by TRIF recruitment following TLR3 and TLR4 activation, or MAVS recruitment secondary to RIG-1 or MDA5 activation. TRAF3 in turn gives rise to TBK1 and IKKε activation that potentiates IRF3. Both IRF3 and IRF7 act as transcription factors that promote T1IFN gene expression. T1IFNs bind to the heterodimeric IFNAR1/IFNAR2 receptor complex, which triggers the receptor-associated kinases TYK2 and JAK1 to phosphorylate STAT1 and STAT2 proteins. The phosphorylated STAT1 and STAT2 combine with IRF9 to form the ISGF3, which binds to IRSE in the nucleus to upregulate transcription of ISGs, exerting multitudinous antiviral effects. PPRs pattern recognition receptors, ACE2 angiotensin-converting enzyme 2, TMPRSS2 transmembrane serine protease 2, (+)/(–)ssRNA positive-/negative-sense single-stranded ribonucleic acid, S spike protein, N nucleocapsid protein, M membrane protein, E envelop protein, Nsps non-structural proteins, dsRNA double-stranded ribonucleic acid, TLR toll-like receptor, RIG-1 retinoic acid-inducible gene I, MDA5 melanoma differentiation-associated protein 5, MyD88 myeloid differentiation primary response factor 88, IRAKs interleukin-1 receptor-associated kinases, TRIF toll-interleukin-1 receptor-domain-containing adaptor-inducing interferon-β, MAVS mitochondrial antiviral signaling protein, TRAF tumor necrosis factor receptor-associated factor, TAK1 transforming growth factor-β activated kinase 1, IKK inhibitor of nuclear factor-κB (IκB) kinase, TBK1 TANK-binding kinase 1, IRF interferon regulatory factor, NF-κB nuclear factor kappa B, MAPK mitogen-activated protein kinase, AP-1 activator protein 1, T1IFNs type 1 interferons, IFNAR interferon-alpha receptor, TYK2 tyrosine kinase 2, JAK1 Janus kinase, STAT signal transducer and activator of transcription, ISGF3 interferon-stimulated gene factor 3, ISRE interferon-sensitive response element
Innate immunity as the first line of defense that arbitrates the course of disease
SARS-CoV-2 is encountered by a cascade of innate immune responses in vivo, which are crucial determinants of disease course in children [87–90]. Apart from ACE2 binding, the S protein, along with other viral constituents, may act as pathogen-associated molecular patterns (PAMPs) to activate pattern recognition receptors (PRRs), which drives the production of an array of cytokines that govern responses following the viral infection. Specifically, SARS-CoV-2 proteins, single-stranded genomic RNA, and double-stranded RNA (dsRNA) replication intermediates are sensed by various subtypes of toll-like receptor (TLRs) [91–94], and cytosolic RNA by the retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) such as RIG-I and melanoma differentiation-associated gene 5 (MDA-5) [95], which ultimately evoke potent interferon (IFN) responses.
Induction of type I IFNs and, to a lesser extent, type III IFNs constitutes the backbone of innate immunity against SARS-CoV-2, which exerts its far-reaching effects via the JAK/STAT signaling pathway to ultimately mobilize hundreds of IFN-stimulated genes (ISGs) with a myriad of direct and indirect (i.e., via recruitment of immune cells) anti-viral functions [96, 97] (Fig. 2). Age-associated features such as more robust mucosal IFN response [89, 98], more preformed cytosolic PRRs in cells populating the upper airways [99] and more efficient RIG-1 signaling [100] in the pediatric population are believed to correlate with milder presentation of COVID-19, whereas severe disease could result from blunted type 1 IFN responses [101, 102] as a consequence of in-born defects in type 1 IFN-mediated immunity [103–107], presence of IFN-neutralizing autoantibodies [108–110], and a multitude of inhibitory factors employed by SARS-CoV-2 such as its structural and non-structural proteins and the way it replicates inside enclosed membranes to evade host immune detection [96, 111–113].
Strikingly, around 10% of children hospitalized for COVID-19 pneumonia were found to have complete recessive deficiencies in one of the four type 1 IFN immunity-related molecules [107]. Inappropriate type I/III IFN responses failing to facilitate viral clearance at the point of initial contact, most commonly the upper airway, can precipitate paradoxical hyper-inflammation with delayed but sustained release of type I IFNs down the stream and may be accompanied by unchecked upregulation of pro-inflammatory cytokines that, in turn, mediate the deleterious pulmonary and systemic inflammation seen in severe and critical illnesses [73, 88, 96, 98, 102, 111, 114, 115]. Consequently, the therapeutic values of exogenous IFN formulations for COVID-19 are subject to the timing of administration. Available clinical trials found no added benefits of IFN beta-1a in hospitalized adults [116, 117], while outpatients were protected from emergency department visits and hospital admissions with a dose of pegylated interferon lambda administered within 7 days of symptom onset [118]. Relevant pediatric data are scarce.
Raging hyper-inflammation signifies severe COVID-19
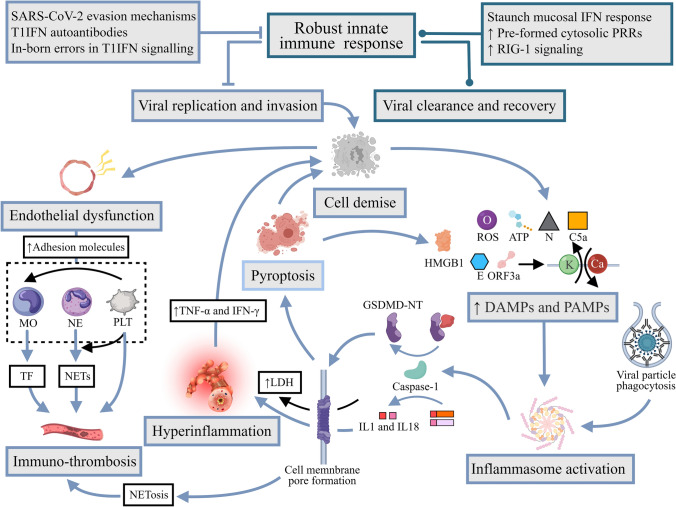
Dysregulated host inflammatory response gains pathogenic dominance over the viral burden itself with COVID-19 disease progression, as evidenced by otherwise no clear correlation between viral load and disease severity, including in the pediatric population [119–121]. In severe/critical COVID-19, complex and intertwined concurrent immunopathological proceedings (Fig. 3) ensue as a result of viral dissemination under the circumstances of waning innate immunity. AT2s, which are made susceptible due to the aforementioned dual ACE2/TMPRSS2 positivity, and other cells succumbing to viral invasion release PAMPs as well as danger-associated molecular patterns (DAMPs), which are hallmarks of cellular stress that elicit various forms of programmed cell death (RCD) [122, 123]. Inflammasomes are micrometer-level multiprotein signaling complexes that form in the cytoplasm via combining particular nucleotide-binding and oligomerization domain(NOD)-like receptors, a subtype of PRRs, with the respective adaptor molecules, secondary to priming and activation by PAMPs and DAMPs [124]. Inappropriate activation of inflammasomes has been demonstrated to play indispensable roles in linking different compartments of immunity and orchestrating the hyper-inflammatory reaction to SARS-CoV-2, with the decisive endpoint being the caspase-mediated interleukin-1b and interleukin-18 release through cell membrane-spanning pores formed by gasdermin-D oligomerization [125–128] (Fig. 3). Markers of inflammasome activation were indeed found elevated proportionately to disease severity in the sera of critically ill COVID-19 patients [126]. Pyroptosis, a form of inflammatory caspase-dependent RCD evident in as many as 6% of the monocytes in the peripheral blood of COVID-19 patients [129], is another well-recognized eventuality in the context of porous plasma membrane [130] leading to release of large intracellular molecules such as lactate dehydrogenase, which is pathognomonic for pyroptosis and a laboratory parameter of prognostic value in clinical practice [131, 132], as well as further outpouring of inflammatory cytokines and DAMPs, such as high mobility group box 1 [133]. The self-propagating vicious cycle of hyper-inflammation and cell death is thus established and culminates in dire clinical consequences. Systemically, severe COVID-19 can elicit a cytokine profile somewhat similar to that of the cytokine storms secondary to other etiologies [134–136]. Synergism of cytokines such as TNF-α and IFN-γ [137] has been demonstrated to be capable of inducing inflammatory cell death and thus may inflict tissue damage directly to end-organs and perpetuate the vicious cycle.
Fig. 3.
Hyperinflammation and immunothrombosis in severe COVID-19. Virus-related factors like the N protein and potassium efflux and calcium influx set off by envelope and ORF3a “viroporin” proteins, and host-related factors such as extracellular ATP, complement C5a, ROS, and phagocytosis of antibody-opsonized viral particles into otherwise ACE2− monocytes have all been proposed to trigger assembly of inflammasomes, most notably NLRP3, in myeloid-derived immune cells and pulmonary cells. Inflammasome activation recruits the caspase-1 canonically, which proteolytically potentiates pro-IL-1β and/or pro-IL-18 and concomitantly cleaves GSDMD into NT and CT fragments. GSDMD-NT oligomerization and translocation to plasma membrane create pores through which activated IL-1β and IL-18 can directly enter the extracellular space, mediating hyperinflammation, and simultaneously trigger pyroptosis, leading to LDH and HMGB1 release, among other pro-inflammatory DAMPs. Meanwhile, endothelial dysfunction in the hyperinflammatory milieu initiates immunothrombosis. Adhesion molecules are markers of activated endothelial cells over-expressed in COVID-19 that exhibit strong anchoring effects on monocytes and neutrophils, the former of which reciprocate with active TFs that directly institute the extrinsic coagulation pathway. Conversely, neutrophils may be prompted by direct SARS-CoV-2 entry, SAR-CoV-2-induced ROS generation, complement activation, and/or a self-sustaining loop of IL-8 production to undergo NETosis, defined by the release of large extracellular web-like structures termed NETs, which may also be triggered by the inflammasome/GSDMD pathway in COVID-19. NETs consist of decondensed chromatin embellished with histones and proteins that act as a scaffold for erythrocyte and platelet settling and fibrin deposition, while its constituents exert a range of pro-thrombotic effects with varying mechanisms. Furthermore, circulating platelets adopt a hyperactive state in the context of SARS-CoV-2 infection. Platelets recruited in response to NETs and other stimuli such as vWF on the activated endothelial cells in turn amplify NETosis via secretion of chemokines such as PF4, and may induce further expression of TF by monocytes and complementarily augment monocytic secretion of inflammatory cytokines. N protein nucleocapsid protein, ORF3a open reading frame 3a, ATP adenosine triphosphate, ROS reactive oxygen species, NLRP3 NOD-like receptor containing pyrin domain 3, IL-1β interleukin-1β, IL-18 interleukin-18, GSDMD gasdermin-D, NT N-terminal, CT C-terminal, LDH lactate dehydrogenase, HMGB1 high mobility group box 1, DAMPs damage-associated molecular patterns, TF transcription factor, IL-8 interleukin-8, NETs neutrophil extracellular traps, vWF von Willebrand Factor, PF4 platelet factor 4
Features of pediatric adaptive responses in COVID-19
The traits of cellular and humoral immunity specific to children may become apparent when challenged with SARS-CoV-2. Mobilization of T and B cell pathways synchronized with the innate defense in the upper airway in children, thereby preventing severe disease development from viral dissemination [138]. Lymphopenia is a much-feared consequence and indicator of poor prognosis consistently seen in hospitalized, ICU-admitted, and non-surviving COVID-19 patients [139–141], albeit less common in children [15], that may be partly attributed to the plethora of pro-inflammatory cytokines [88, 142, 143]. Nevertheless, adults were found to have stronger CD4+ and CD8+ T cell responses in the acute infective phase, which were proposedly a compensation for the inferior innate responses and, indeed, did not lead to favorable outcomes [98, 144, 145]. By the same token, some studies have shown that SARS-CoV-2 invokes a less vigorous antibody response in children that was restricted to S protein-specific IgG production, as opposed to anti-S IgM, IgG, and IgA as well as antibody formation against other viral proteins in adults [146–148]. Otherwise, there have been mixed results regarding the neutralizing activity of SARS-CoV-2-specific immunoglobulins derived from children [144, 149, 150], although they are generally longer lasting than their adult counterparts, offering protection from re-infection for months and beyond [147, 149, 151, 152].
Devastating cross-talk of the inflammatory and coagulation pathways
In the lungs, the highly inflammatory alveolar microenvironment is the harbinger of alveolar epithelial injury and dysfunction, supported by transcriptomic evidence of AT2 and AT1 exhaustion and demise in fatal COVID-19 [153–155]. The resultant denudation of the alveolar basement membrane and exposure of the underlying endothelial cells to the detrimental cocktail of hypoxia, viral content, apoptotic and necrotic debris, immune cells, cytokines, and chemokines trigger their activation, transformation to a leaky state due to cytoskeleton and intercellular junction alterations [156], and potentially direct cell death that, in combination with the increased epithelial permeability, precipitates leukocyte extravasation and accumulation of the proteinaceous edema characteristic of ARDS [73, 157].
In the meantime, loss of the usual quiescent endothelial cell phenotype under the influence of cytokine overdrive ignites the sophisticated and incompletely understood interplay between the immune and hemostatic mediators in an attempt to limit the spread of the pathogen that ultimately leads to the micro- and macro-thrombus formation frequently observed in severe/critical COVID-19 [158–161], as alveolar capillary microthrombi are found to be close to 10 times as prevalent in patients who died of COVID as in those who died of ARDS following H1N1 influenza [162]. The injurious positive feedback loop of immunothrombosis, which may notably involve NETosis [163, 164] (Fig. 3), eventually gives rise to the formation of the pathognomonic alveolar hyaline membranes in ARDS [157, 165]. The fibrin-rich exudates may significantly compromise the alveolar-capillary interface for gas exchange, resulting in the profound refractory hypoxemia seen in respiratory failure secondary to severe/critical COVID-19 [73, 165].
Similarly, there are evidently increased incidences of pulmonary emboli [166, 167], thrombosis, and angiopathy of the microvasculature that may contribute to multi-organ failure [168, 169], and extrapulmonary arterial and venous thromboembolic phenomena in adults [170, 171], and to a lesser degree, in children and adolescents with COVID-19, where nine (2.1%) of the 426 hospitalized pediatric patients with symptoms developed thrombotic events across seven children’s hospitals in the US [172]. Therefore, thrombo-inflammatory markers such as D-dimer are the most common abnormal laboratory findings in adult and pediatric COVID-19 [173], and may be predictive of disease course [174, 175].
MIS-C, a riddle unresolved
Since the emergence of SARS-CoV-2, surges in MIS-C cases appear to aggregate 3–6 weeks following peak incidences of COVID-19 cases in a heavily affected locale and whose symptomology bears resemblance to but is distinct from Kawasaki disease (KD), toxic shock syndrome (TSS), and macrophage activation syndrome (MAS) [176–178] (Fig. 1). MIS-C may share clinical features of KD including sustained high fevers, conjunctivitis, diffuse non-vesicular erythematous rash, and dry and cracked lips, but clearly diverges from KD in that it (1) is more frequently reported in older children of non-Asian descent with an average age of 9 years, no apparent gender bias, and an overall mortality rate of 2%, and increased age may be associated with risks of ICU admissions [12, 13], which are required for up to 65%–70% of all cases[179–181]; (2) has a markedly high rate of gastrointestinal (GI) tract (80%) and neurological involvement [11, 182], and cardiac manifestations as discussed but less likelihood of developing severe or persistent coronary artery aneurysms [10, 53, 55, 183, 184]; and (3) is notably associated with ferritinemia in severe disease and thrombocytopenia [185, 186].
Although there are commonalities of shock, multi-organ failure, endothelial damage and coagulopathy, neutrophilia, lymphocytopenia, elevated inflammatory markers between severe/critical pediatric COVID-19 and MIS-C, the latter is less likely to have primary respiratory implications and mostly arise in otherwise healthy children and adolescents devoid of major pre-existing co-morbidities who have already seroconverted with IgG predominance at time of MIS-C diagnosis and mild to no symptoms on initial infection or exposure [187]. MIS-C also carries distinguishing cytokine signatures including enrichment of the type II interferon (IFN-γ) and the downstream effector molecules [115, 188–192] that may explain its homogeneous features with MAS. Admittedly, the immunological landscape of MIS-C appears rather complex and only partially defined, and there has not been a unifying pathophysiological blueprint to account for the manifold stigmata of MIS-C, but much progress has been made in delineating the nuances in immunophenotypes that may shed light on their similarities and differences to other systemic inflammatory syndromes.
Given the overlaps between MIS-C with TSS, which has bacterial superantigens as the unequivocal trigger [193], and detection of SARS-CoV-2 in various organs on autopsy of patients who died of MIS-C [194], it is reasonable to hypothesize that SARS-CoV-2 proteins may possess superantigenic properties that set off the hyper-inflammatory response in MIS-C, where computational analysis has indeed identified a sequence motif exclusive to SARS-CoV-2 S protein capable of T cell activation that shows striking structural similarity to a segment of the staphylococcal enterotoxin B, the culprit responsible for TSS [195, 196]. The definitive reservoir of superantigens in MIS-C, however, has not yet been found, given the varied nasopharyngeal RT-PCR positivity status in MIS-C cases [197]. In accordance with the prominent gastrointestinal complaints in MIS-C, persistence of SARS-CoV-2 in the GI tract [198, 199] and subsequently the compromise to the intestinal barrier integrity have been posited as a potential route of antigenic entry and dissemination, as evidenced by elevation of enterocyte damage [200] and intestinal permeability [201] and inflammation [202] makers in MIS-C children, although studies have yielded conflicting results on whether antigenemia is present in most cases of MIS-C [115, 201, 203]. Moreover, numerous groups have unanimously demonstrated a phenomenon typical of superantigenic stimulation where, similar to TSS, MIS-C is characterized by extensive polyclonal proliferation of a specific T cell receptor (TCR) β-chain variable domain subset, namely TRBV11-2 in MIS-C [115, 200, 201, 204–207], and de-escalation of the expansion appears to coincide with abatement in inflammatory cytokine levels and clinical improvement with therapy, especially after glucocorticoid administration [115, 205], further supporting the potential roles of TRBV11-2-expressing T cells in pathogenesis. On the contrary, many studies have detected autoantibodies against endothelial, cardiac, gastrointestinal, and immune antigens [207–210] in MIS-C as the alternative pathogenic mediators that may provoke damage to the respective organ systems, although intravenous immunoglobulin therapies may be a confounding factor [115, 211].
In addition, several genetic predispositions have been identified for MIS-C including the specific combination of HLA class I alleles A02, B35, C04 that is associated with TRBV11-2 expansion [115, 204], albeit not supported by findings of many groups [200, 205, 206] possibly due to differing ethnic compositions of the study populations, as well as flaws in down-regulators of inflammation such as an autosomal recessive defect in the OAS-Rnase L pathway, which normally disposes of the cytosolic dsRNA that can stimulate production of pro-inflammatory cytokines [212], and nonsynonymous mutations in XIAP, CYBB and SOCS1 genes [213]. Ongoing immune profiling efforts are underway to advance mechanistic understanding of this perplexing disease entity, as with many other inflammatory conditions, through which more universally applicable insights may also be generated into the broader immune system as a whole.
Management, prognosis, and long-term sequelae
Fortunately, COVID-19 in the vast majority of children is self-limiting and would not qualify for treatment other than supportive care and symptomatic management until spontaneous resolution, and although pediatric clinical trial data are lacking, the number needed to treat for therapeutic benefits is considered to be higher in children than in adults [214].
Several pharmacological agents are available to reduce the risk of progression to severe disease in vulnerable outpatients, should the potential benefits be deemed to outweigh the risks. Paxlovid, a combination medication of nirmatrelvir and ritonavir available in oral formulations that can be conveniently taken in the outpatient setting, has been demonstrated in the cornerstone phase III trial involving 1219 adults at-risk for severe disease to have an astonishing 89% risk reduction in COVD-19-related hospitalizations or deaths if administered within 3 days of symptom onset [215]. Paxlovid appears still highly effective in the time of Omicron preeminence [216], but has only been authorized by the US Food and Drug Administration (FDA) for use in children aged 12 years or older and weighing 40 kg or greater, and is limited by its extensive interactions with other medications due to ritonavir being a potent CYP3A inhibitor [217]. Alternatively, remdesivir represents the sole FDA-authorized agent for prevention of disease progression in the community setting for at-risk and mildly to moderately symptomatic children with at least 28 days of age and a weight of at least 3 kg, with the logistical caveat of requiring intravenous dosage delivery on 3 consecutive days [218]. In contrast, clinical decisions regarding inpatient management of severe COVID-19-related diseases in children are not infrequently extrapolated from adult guidelines and made on a case-by-case basis given the scarcity of large-scale randomized controlled trials in the pediatric population from which evidence-based recommendations can be derived [16, 219, 220].
Despite the generally favorable outcomes even in the critically ill children [221], the full picture of the long-term impacts of COVID-19 on pediatric health may not be readily apparent at this stage given the recency of the pandemic. The term long COVID, sometimes also referred to as post-acute sequelae of COVID-19, has been coined as the diagnosis of exclusion to encompass the constellation of complaints after the acute stage of COVID-19 has settled [222]. One or more persistent symptoms are reported in 16.2% of children for 3 months or greater post-infection as estimated by a recently published meta-analysis [223]. Some of the most commonly reported symptoms range from persistent sore throat, fever, dyspnea, anosmia/ageusia, muscle weakness, and coughs to various vague and largely non-localizing neuropsychiatric troubles, including fatigue, mood and sleep disturbances, and mental dysfunction [223–226], seemingly so heterogeneous that categorizations in clinical phenotypes of diverse pathogenesis for subgroup analysis may be advisable [227]. Children with less than 5 years of age, underlying comorbidities, or admission to ICU during the acute phase of disease are found to be especially susceptible [228], but symptomatic or asymptomatic non-hospitalized patients are not precluded from developing the condition [223]. Of note, among the several pathophysiological hypotheses that have been put forward for long COVID are viral persistence [229], autoimmunity [230], endothelial dysfunction with microcirculation thrombosis [231, 232], and immune dysregulation with potential reactivation of latent viral infections such as Epstein-Barr virus and herpesviruses given the shared features with myalgic encephalomyelitis/chronic fatigue syndrome and dysautonomia including orthostatic intolerance and profound fatigue [233–235]. The rather limited understanding of long COVID at this point in time has prompted well-coordinated systematic research undertakings such as the RECOVER initiative to comprehensively delineate the disease, which notably has a branch of effort dedicated to children and young people [236].
Expert opinion and conclusions
Various measures can be implemented at different stages to minimize adverse outcomes associated with severe COVID-19 in children. Vaccination proves to be effective in reducing severe disease development and should be administered as per protocol, if not contraindicated.
Red flags for deterioration should be recognized and attended to without delay, especially in children with predisposing comorbidities. Management of severe disease should generally observe a holistic approach to account for the multi-system manifestations, where emphases are placed on airway and respiratory status optimization, hemodynamic support, modulation of the detrimental hyperinflammatory response, addressing the comorbid conditions and superinfections if any, and preservation of organ functions.
The contrasting susceptibilities and responses to SARS-CoV-2 and the variations in the disease course are, to a great extent, an attestation of the monumental differences in the ways pediatric and adult immune systems are programmed to resist a highly immunogenic viral threat. In conjunction with protective antibodies with longer lifespans and lower expression of SARS-CoV-2 entry factors, a swiftly induced T1IFN response of the optimal magnitude at the mucosal surface and upper airway serves as a powerful protective barrier for children against viral spread, consequently mitigating the risk of severe COVID-19, which may see indiscriminate firing of various proinflammatory apparatuses such as inflammasomes and NETs. Further research on severe pediatric COVID-19 and relevant conditions such as MIS-C and long COVID remains of scientific and clinical significance, as it offers a novel viewpoint for deciphering the age-specific characteristics of immunity. With ongoing intermittent outbreaks, clinicians must remain vigilant of the telltale signs of deterioration, especially in those children at risk, and rationalize the use of therapeutic measures for the best outcome, although more pediatric-specific clinical trials are required before recommendations with high level of evidence can be made, as unsuspecting application of findings in adult studies to pediatric patients is a fundamentally unscientific practice that disregards the unique qualities of pediatric immunity and physiology. Additionally, rapidly evolving SARS-CoV-2 strains and the immunological memory that has formed along the way will only make it more difficult to interpret earlier studies. In more general terms, the collective knowledge gained from the COVID-19 pandemic serves as a methodological construct for better understanding of and responding to similar communicable diseases with heavy pediatric disease burdens, such as influenza and mycoplasma pneumonia, as well as existing and emerging pathogens with pandemic potential.
Acknowledgements
We thank all staff involved with this study and the physicians at Children’s Hospital, Zhejiang University School of Medicine, and Dr. Zhaoming Ye at the Second Affiliated Hospital, Zhejiang University School of Medicine. The figures in this manuscript are created with Medpeer (www.medpeer.cn).
Author contributions
SYK and WC contributed equally to this work. Please see below the CRediT authorship contribution statement below for a detailed account of the roles undertaken by each author. Conceptualization: TLH, HLS, and SQ; funding acquisition: GZG; project administration: WC, LPQ, LR, HL, YJ, GZG and LHM; supervision: LPQ, HL, and YJ; visualization: SYK; writing—original draft: SYK and WC; writing—review and editing: SYK and TLH.
Funding
This work was supported by the Key Research and Development Program of Zhejiang (2023C03029).
Declarations
Conflict of interest
Author SQ is a member of the Editorial Board for World Journal of Pediatrics. The paper was handled by the other Editor and has undergone rigorous peer review process. Author SQ was not involved in the journal's review of, or decisions related to, this manuscript. No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors declared no conflicts of interest.
Ethical approval
This study did not involve the participation of human or animal subjects, and, therefore, was exempt from formal assessment by the ethics committee for clinical research of our center.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li-Su Huang, Email: huanglisu@xinhuamed.com.cn.
Lin-Hua Tan, Email: chtlh@zju.edu.cn.
References
- 1.Woodruff RC, Campbell AP, Taylor CA, Chai SJ, Kawasaki B, Meek J, et al. Risk factors for severe COVID-19 in children. Pediatrics. 2022;149:e2021053418. doi: 10.1542/peds.2021-053418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kompaniyets L, Bull-Otterson L, Boehmer TK, Baca S, Alvarez P, Hong K, et al. Post-COVID-19 symptoms and conditions among children and adolescents—United States, March 1, 2020-January 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:993–999. doi: 10.15585/mmwr.mm7131a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 4.Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021;39:667–677. doi: 10.1016/j.vaccine.2020.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otto WR, Geoghegan S, Posch LC, Bell LM, Coffin SE, Sammons JS, et al. The epidemiology of severe acute respiratory syndrome coronavirus 2 in a pediatric healthcare network in the United States. J Pediatric Infect Dis Soc. 2020;9:523–529. doi: 10.1093/jpids/piaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce CA, Herold KC, Herold BC, Chou J, Randolph A, Kane B, et al. COVID-19 and children. Science. 2022;377:1144–1149. doi: 10.1126/science.ade1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blatz AM, Randolph AG. Severe COVID-19 and multisystem inflammatory syndrome in children in children and adolescents. Crit Care Clin. 2022;38:571–586. doi: 10.1016/j.ccc.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delahoy MJ, Ujamaa D, Taylor CA, Cummings C, Anglin O, Holstein R, et al. Comparison of influenza and coronavirus disease 2019-associated hospitalizations among children younger than 18 years old in the United States: FluSurv-NET (October-April 2017–2021) and COVID-NET (October 2020-September 2021) Clin Infect Dis. 2023;76:e450–e459. doi: 10.1093/cid/ciac388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamid S, Woodworth K, Pham H, Milucky J, Chai SJ, Kawasaki B, et al. COVID-19-Associated Hospitalizations Among U.S. Infants Aged <6 Months - COVID-NET, 13 States, June 2021-August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1442–14428. doi: 10.15585/mmwr.mm7145a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams JY, Oster ME, Godfred-Cato SE, Bryant B, Datta SD, Campbell AP, et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: a retrospective surveillance study. Lancet Child Adolesc Health. 2021;5:323–331. doi: 10.1016/S2352-4642(21)00050-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merckx J, Cooke S, El Tal T, Bitnun A, Morris SK, Yeh EA, et al. Predictors of severe illness in children with multisystem inflammatory syndrome after SARS-CoV-2 infection: a multicentre cohort study. CMAJ. 2022;194:E513–E523. doi: 10.1503/cmaj.210873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020;16:223–231. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Zhao Z, Zhang T, Guo W, Guo W, Zheng J, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J Med Virol. 2021;93:1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021;106:440–448. doi: 10.1136/archdischild-2020-321385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JH, Choi SH, Yun KW. Risk factors for severe COVID-19 in children: a systematic review and meta-analysis. J Korean Med Sci. 2022;37:e35. doi: 10.3346/jkms.2022.37.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antoon JW, Grijalva CG, Thurm C, Richardson T, Spaulding AB, Teufel RJ, 2nd, et al. Factors associated with COVID-19 disease severity in US children and adolescents. J Hosp Med. 2021;16:603–610. doi: 10.12788/jhm.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druzak S, Iffrig E, Roberts BR, Zhang T, Fibben KS, Sakurai Y, et al. Multiplatform analyses reveal distinct drivers of systemic pathogenesis in adult versus pediatric severe acute COVID-19. Nat Commun. 2023;14:1638. doi: 10.1038/s41467-023-37269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harwood R, Yan H, Talawila Da Camara N, Smith C, Ward J, Tudur-Smith C, et al. Which children and young people are at higher risk of severe disease and death after hospitalisation with SARS-CoV-2 infection in children and young people: A systematic review and individual patient meta-analysis. EClinicalMedicine. 2022;44:101287. doi: 10.1016/j.eclinm.2022.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piechotta V, Siemens W, Thielemann I, Toews M, Koch J, Vygen-Bonnet S, et al. Safety and effectiveness of vaccines against COVID-19 in children aged 5–11 years: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7:379–391. doi: 10.1016/S2352-4642(23)00078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen-Stavi CJ, Magen O, Barda N, Yaron S, Peretz A, Netzer D, et al. BNT162b2 vaccine effectiveness against omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–236. doi: 10.1056/NEJMoa2205011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell AA, Kirsebom F, Stowe J, McOwat K, Saliba V, Ramsay ME, et al. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect Dis. 2022;22:581–583. doi: 10.1016/S1473-3099(22)00177-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fowlkes AL, Yoon SK, Lutrick K, Gwynn L, Burns J, Grant L, et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years—PROTECT Cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:422–428. doi: 10.15585/mmwr.mm7111e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorabawila V, Hoefer D, Bauer UE, Bassett MT, Lutterloh E, Rosenberg ES. Effectiveness of the BNT162b2 vaccine among children 5–11 and 12–17 years in New York after the Emergence of the Omicron Variant. medRxiv. 2022:2022.02.25.22271454.
- 26.National Institutes of Health. Special Considerations in People Who Are Immunocompromised. [Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/.
- 27.General Office of the National Health Commission, General Division of the State Administration of Traditional Chinese Medicine. Diagnosis and Treatment Plans for SARS-CoV-2 (Tentative 10th Edition). 2023. Available from: https://www.gov.cn/zhengce/zhengceku/2023-01/06/5735343/files/5844ce04246b431dbd322d8ba10afb48.pdf. Accessed 29 June, 2023.
- 28.National Institutes of Health. Clinical Spectrum of SARS-CoV-2 Infection. COVID-19 Treatment Guidelines. Updated March 6, 2023. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed 29 June 2023.
- 29.Fisler G, Izard SM, Shah S, Lewis D, Kainth MK, Hagmann SHF, et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10:171. doi: 10.1186/s13613-020-00790-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller CC, Cosgrove A, Shinde M, Rosen E, Haffenreffer K, Hague C, et al. Treatment and care received by children hospitalized with COVID-19 in a large hospital network in the United States, February 2020 to September 2021. PLoS ONE. 2023;18:e0288284. doi: 10.1371/journal.pone.0288284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Li L, Wang C, Zhang Y, Zhou Y. Necrotizing pneumonia in children: early recognition and management. J Clin Med. 2023;12:2256. doi: 10.3390/jcm12062256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akuamoah Boateng G, Ristagno EH, Levy E, Kahoud R, Thacker PG, Setter DO, et al. A complicated presentation of pediatric COVID-19 with necrotizing pneumonia and pulmonary artery pseudoaneurysms. Pediatr Pulmonol. 2021;56:4042–4044. doi: 10.1002/ppul.25631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisca G, Buratti S, Basso L, Miano M, Salvati P, Castagnola E, Moscatelli A. Necrotizing pneumonia and severe COVID-19 in an infant with catheter-related bloodstream infection by methicillin-sensitive Staphylococcus aureus. Pediatr Int. 2023;65:e15401. doi: 10.1111/ped.15401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewster RC, Parsons C, Laird-Gion J, Hilker S, Irwin M, Sommerschield A, et al. COVID-19-associated croup in children. Pediatrics. 2022;149:e2022056492. doi: 10.1542/peds.2022-056492. [DOI] [PubMed] [Google Scholar]
- 35.Iijima H, Kubota M, Ogimi C. Clinical characteristics of pediatric patients with COVID-19 between Omicron era vs. pre-Omicron era. J Infect Chemother. 2022;28:1501–1505. doi: 10.1016/j.jiac.2022.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi YY, Kim YS, Lee SY, Sim J, Choe YJ, Han MS. Croup as a manifestation of SARS-CoV-2 omicron variant infection in young children. J Korean Med Sci. 2022;37:e140. doi: 10.3346/jkms.2022.37.e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamali Aghdam M, Shabani Mirzaee H, Eftekhari K. Croup is one of the clinical manifestations of novel coronavirus in children. Case Rep Pulmonol. 2021;2021:8877182. doi: 10.1155/2021/8877182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassedy EA, Kim S, Silver AH. An unexpected cause of croup in a pediatric patient. Clin Pediatr. 2021;60:574–576. doi: 10.1177/00099228211053938. [DOI] [PubMed] [Google Scholar]
- 39.Park S, You J, Lee J, Park E. Two case reports of life-threatening croup caused by the sARS-CoV-2 omicron BA.2 variant in pediatric patients. J Korean Med Sci. 2022;37:e192. doi: 10.3346/jkms.2022.37.e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derespina KR, Kaushik S, Plichta A, Conway EE, Jr, Bercow A, Choi J, et al. Clinical manifestations and outcomes of critically ill children and adolescents with coronavirus disease 2019 in New York City. J Pediatr. 2020;226:55–63.e2. doi: 10.1016/j.jpeds.2020.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. doi: 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr. 2020;223:14–9.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khemani RG, Smith LS, Zimmerman JJ, Erickson S. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:S23–40. doi: 10.1097/PCC.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar M, Das B, Mahapatra MK, Roychowdhoury S, Das S, Konar MC. A retrospective analysis of clinical manifestations, management and outcome of acute respiratory distress syndrome associated with coronavirus disease-2019 infection in children. Indian J Crit Care Med. 2022;26:331–338. doi: 10.5005/jp-journals-10071-24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garau G, Joachim S, Duliere GL, Melissopoulou M, Boccar S, Fraipont V, et al. Sudden cardiogenic shock mimicking fulminant myocarditis in a surviving teenager affected by severe acute respiratory syndrome coronavirus 2 infection. ESC Heart Fail. 2021;8:766–773. doi: 10.1002/ehf2.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raymond TT, Das A, Manzuri S, Ehrett S, Guleserian K, Brenes J. Pediatric COVID-19 and pericarditis presenting with acute pericardial tamponade. World J Pediatr Congenit Heart Surg. 2020;11:802–804. doi: 10.1177/2150135120949455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trogen B, Gonzalez FJ, Shust GF. COVID-19-associated myocarditis in an adolescent. Pediatr Infect Dis J. 2020;39:e204–e205. doi: 10.1097/INF.0000000000002788. [DOI] [PubMed] [Google Scholar]
- 49.Gnecchi M, Moretti F, Bassi EM, Leonardi S, Totaro R, Perotti L, et al. Myocarditis in a 16-year-old boy positive for SARS-CoV-2. Lancet. 2020;395:e116. doi: 10.1016/S0140-6736(20)31307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesici S, Aykan HH, Orhan D, Bayrakci B. Fulminant COVID-19-related myocarditis in an infant. Eur Heart J. 2020;41:3021. doi: 10.1093/eurheartj/ehaa515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chochkova-Bukova LA, Funken D, Bukova M, Genova KZ, Ali S, Stoencheva S, et al. Cardiac MRI with late gadolinium enhancement shows cardiac involvement 3–6 months after severe acute COVID-19 similar to or worse than PIMS. Front Cardiovasc Med. 2023;10:1115389. doi: 10.3389/fcvm.2023.1115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jone PN, John A, Oster ME, Allen K, Tremoulet AH, Saarel EV, et al. SARS-CoV-2 infection and associated cardiovascular manifestations and complications in children and young adults: a scientific statement from the American Heart Association. Circulation. 2022;145:e1037–e1052. doi: 10.1161/CIR.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 53.Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 Infection in Europe. Circulation. 2021;143:21–32. doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 54.Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, Duarte-Neto AN, Soares Gomes-Gouvêa M, Viu Degaspare N, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. 2020;10:69. doi: 10.1186/s13613-020-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray STJ, Abdel-Mannan O, Sa M, Fuller C, Wood GK, Pysden K, et al. Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health. 2021;5:631–641. doi: 10.1016/S2352-4642(21)00193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siracusa L, Cascio A, Giordano S, Medaglia AA, Restivo GA, Pirrone I, et al. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital J Pediatr. 2021;47:123. doi: 10.1186/s13052-021-01066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Miranda Henriques-Souza AM, de Melo A, de Aguiar Coelho Silva Madeiro B, Freitas LF, Sampaio Rocha-Filho PA, Gonçalves FG. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021;63:141–145. doi: 10.1007/s00234-020-02571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akçay N, Bektaş G, Menentoğlu ME, Oğur M, Sofuoğlu A, Palabiyik FB, Şevketoğlu E. COVID-19-associated acute disseminated encephalomyelitis-like disease in 2 children. Pediatr Infect Dis J. 2021;40:e445–e450. doi: 10.1097/INF.0000000000003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLendon LA, Rao CK, Da Hora CC, Islamovic F, Galan FN. Post–COVID-19 Acute disseminated encephalomyelitis in a 17-month-old. Pediatrics. 2021;147:e2020049678. doi: 10.1542/peds.2020-049678. [DOI] [PubMed] [Google Scholar]
- 61.Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. The Lancet Child & Adolescent Health. 2021;5:167–177. doi: 10.1016/S2352-4642(20)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Curtis M, Bhumbra S, Felker MV, Jordan BL, Kim J, Weber M, Friedman ML. Guillain-Barré syndrome in a child with COVID-19 infection. Pediatrics. 2021;147:e2020015115. doi: 10.1542/peds.2020-015115. [DOI] [PubMed] [Google Scholar]
- 63.Orak SA, Kubur Ç, Atasever AK, Polat M. Two case reports and a literature review of typical GBS and rare GBS variants associated with COVID-19. Arch Pediatr. 2023;30:236–239. doi: 10.1016/j.arcped.2023.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes I, Karmirian K, Oliveira JT, Pedrosa CdSG, Mendes MA, Rosman FC, et al. SARS-CoV-2 infection of the central nervous system in a 14-month-old child: a case report of a complete autopsy. Lancet Reg Health Am. 2021;2:100046. [DOI] [PMC free article] [PubMed]
- 65.Lin X, Wang Y, Li X, Abdalla M, Zhang F, Dong C, et al. Acute necrotizing encephalopathy in children with COVID-19: a retrospective study of 12 cases. Front Neurol. 2023;14:1184864. doi: 10.3389/fneur.2023.1184864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu D, Zheng Y, Li Y, Peng M, Lin H, Wang K. Exploring the molecular and clinical spectrum of COVID-19-related acute necrotizing encephalopathy in three pediatric cases. J Hum Genet. 2023;68:769–775. doi: 10.1038/s10038-023-01171-z. [DOI] [PubMed] [Google Scholar]
- 67.Lin J-J, Tu Y-F, Chen S-J, Kuo Y-T, Jeng M-J, Hsin-Ju Ko M, Chiu C-H. Fatal fulminant cerebral edema in six children with SARS-CoV-2 omicron BA.2 infection in Taiwan. J Pediatric Infect Dis Society. 2022;12:99–103. doi: 10.1093/jpids/piac116. [DOI] [PubMed] [Google Scholar]
- 68.Botre A, Otiv M, Parekar A. Acute fulminant cerebral edema presenting as refractory status epilepticus in a SARS-CoV-2 PCR-positive child without pulmonary involvement. Indian J Pediatr. 2023;90:529. doi: 10.1007/s12098-023-04510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–84.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markus Hoffmann HKW, Schroeder S, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:1–10. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benton DJ, Wrobel AG, Xu P, Roustan C, Martin SR, Rosenthal PB, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588:327–330. doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peacock TP, Goldhill DH, Zhou J, Baillon L, Frise R, Swann OC, et al. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat Microbiol. 2021;6:899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- 73.Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol. 2022;20:270–284. doi: 10.1038/s41579-022-00713-0. [DOI] [PubMed] [Google Scholar]
- 74.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang J, Hume AJ, Abo KM, Werder RB, Villacorta-Martin C, Alysandratos KD, et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27:962–73.e7. doi: 10.1016/j.stem.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 77.Zang R, Castro MFG, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jansen J, Reimer KC, Nagai JS, Varghese FS, Overheul GJ, de Beer M, et al. SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29:217–31.e8. doi: 10.1016/j.stem.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang J, Chen T, Zhou Y. Mediators of SARS-CoV-2 entry are preferentially enriched in cardiomyocytes. Hereditas. 2021;158:4. doi: 10.1186/s41065-020-00168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. The Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–13.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar A, Narayan RK, Kumari C, Faiq MA, Kulandhasamy M, Kant K, Pareek V. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med Hypotheses. 2020;145:110320. doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Z, Guo L, Huang L, Zhang C, Luo R, Zeng L, et al. Distinct disease severity between children and older adults with coronavirus disease 2019 (COVID-19): impacts of ace2 expression, distribution, and lung progenitor cells. Clin Infect Dis. 2021;73:e4154–e4165. doi: 10.1093/cid/ciaa1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva MG, Falcoff NL, Corradi GR, Di Camillo N, Seguel RF, Tabaj GC, et al. Effect of age on human ACE2 and ACE2-expressing alveolar type II cells levels. Pediatr Res. 2023;93:948–952. doi: 10.1038/s41390-022-02163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muus C, Luecken MD, Eraslan G, Sikkema L, Waghray A, Heimberg G, et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat Med. 2021;27:546–559. doi: 10.1038/s41591-020-01227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuler BA, Habermann AC, Plosa EJ, Taylor CJ, Jetter C, Negretti NM, et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin Invest. 2021;131:e140766. doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chou J, Thomas PG, Randolph AG. Immunology of SARS-CoV-2 infection in children. Nat Immunol. 2022;23:177–185. doi: 10.1038/s41590-021-01123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diamond MS, Kanneganti TD. Innate immunity: the first line of defense against SARS-CoV-2. Nat Immunol. 2022;23:165–176. doi: 10.1038/s41590-021-01091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pierce CA, Sy S, Galen B, Goldstein DY, Orner E, Keller MJ, et al. Natural mucosal barriers and COVID-19 in children. JCI Insight. 2021;6:e148694. doi: 10.1172/jci.insight.148694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rotulo GA, Palma P. Understanding COVID-19 in children: immune determinants and post-infection conditions. Pediatr Res. 2023;94:434–442. doi: 10.1038/s41390-023-02549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng M, Karki R, Williams EP, Yang D, Fitzpatrick E, Vogel P, et al. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat Immunol. 2021;22:829–838. doi: 10.1038/s41590-021-00937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aboudounya MM, Heads RJ. COVID-19 and toll-like receptor 4 (TLR4): SARS-CoV-2 may bind and activate TLR4 to increase ACE2 expression, facilitating entry and causing hyperinflammation. Mediators Inflamm. 2021;2021:8874339. doi: 10.1155/2021/8874339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bortolotti D, Gentili V, Rizzo S, Schiuma G, Beltrami S, Strazzabosco G, et al. TLR3 and TLR7 RNA sensor activation during SARS-CoV-2 infection. Microorganisms. 2021;9:1820. doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y, Kuang M, Li J, Zhu L, Jia Z, Guo X, et al. SARS-CoV-2 spike protein interacts with and activates TLR41. Cell Res. 2021;31:818–820. doi: 10.1038/s41422-021-00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thorne LG, Reuschl A-K, Zuliani-Alvarez L, Whelan MVX, Turner J, Noursadeghi M, et al. SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 2021;40:e107826. doi: 10.15252/embj.2021107826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y-M, Shin E-C. Type I and III interferon responses in SARS-CoV-2 infection. Exp Mol Med. 2021;53:750–760. doi: 10.1038/s12276-021-00592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lazear HM, Schoggins JW, Diamond MS. Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 2019;50:907–923. doi: 10.1016/j.immuni.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoshida M, Worlock KB, Huang N, Lindeboom RGH, Butler CR, Kumasaka N, et al. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature. 2022;602:321–327. doi: 10.1038/s41586-021-04345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loske J, Röhmel J, Lukassen S, Stricker S, Magalhães VG, Liebig J, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol. 2022;40:319–324. doi: 10.1038/s41587-021-01037-9. [DOI] [PubMed] [Google Scholar]
- 100.Molony RD, Nguyen JT, Kong Y, Montgomery RR, Shaw AC, Iwasaki A. Aging impairs both primary and secondary RIG-I signaling for interferon induction in human monocytes. Sci Signal. 2017;10:eaan2392. doi: 10.1126/scisignal.aan2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–45.e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. [DOI] [PMC free article] [PubMed]
- 104.Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Maglorius Renkilaraj MRL, et al. X-linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol. 2021;6:eabl4348. [DOI] [PMC free article] [PubMed]
- 105.Abolhassani H, Landegren N, Bastard P, Materna M, Modaresi M, Du L, et al. Inherited IFNAR1 deficiency in a child with both critical COVID-19 pneumonia and multisystem inflammatory syndrome. J Clin Immunol. 2022;42:471–483. doi: 10.1007/s10875-022-01215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Made CI, Simons A, Schuurs-Hoeijmakers J, van den Heuvel G, Mantere T, Kersten S, et al. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324:663–673. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Q, Matuozzo D, Le Pen J, Lee D, Moens L, Asano T, et al. Recessive inborn errors of type I IFN immunity in children with COVID-19 pneumonia. J Exp Med. 2022;219:e20220131. [DOI] [PMC free article] [PubMed]
- 108.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. [DOI] [PMC free article] [PubMed]
- 109.Bastard P, Gervais A, Le Voyer T, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol. 2021;6:eabl4340. [DOI] [PMC free article] [PubMed]
- 110.Lemarquis A, Campbell T, Aranda-Guillén M, Hennings V, Brodin P, Kämpe O, et al. Severe COVID-19 in an APS1 patient with interferon autoantibodies treated with plasmapheresis. J Allergy Clin Immunol. 2021;148:96–98. doi: 10.1016/j.jaci.2021.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun. 2020;11:3810. doi: 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang W, Zhou Z, Xiao X, Tian Z, Dong X, Wang C, et al. SARS-CoV-2 nsp12 attenuates type I interferon production by inhibiting IRF3 nuclear translocation. Cell Mol Immunol. 2021;18:945–953. doi: 10.1038/s41423-020-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han L, Zhuang MW, Deng J, Zheng Y, Zhang J, Nan ML, et al. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5-MAVS, TLR3-TRIF, and cGAS-STING signaling pathways. J Med Virol. 2021;93:5376–5389. doi: 10.1002/jmv.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ravichandran S, Tang J, Grubbs G, Lee Y, Pourhashemi S, Hussaini L, et al. SARS-CoV-2 immune repertoire in MIS-C and pediatric COVID-19. Nat Immunol. 2021;22:1452–1464. doi: 10.1038/s41590-021-01051-8. [DOI] [PubMed] [Google Scholar]
- 115.Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28:1050–1062. doi: 10.1038/s41591-022-01724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:1365–1376. doi: 10.1016/S2213-2600(21)00384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. Repurposed antiviral drugs for Covid-19—interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reis G, Moreira Silva EAS, Medeiros Silva DC, Thabane L, Campos VHS, Ferreira TS, et al. Early treatment with pegylated interferon lambda for Covid-19. N Engl J Med. 2023;388:518–528. doi: 10.1056/NEJMoa2209760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yonker LM, Boucau J, Regan J, Choudhary MC, Burns MD, Young N, et al. Virologic Features of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children. J Infect Dis. 2021;224:1821–1829. doi: 10.1093/infdis/jiab509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kam KQ, Yung CF, Cui L, Tzer Pin Lin R, Mak TM, Maiwald M, Well Infant With Coronavirus Disease A, et al. With High Viral Load. Clin Infect Dis. 2019;2020(71):847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu M, Chen Y, Xia H, Wang C, Tan CY, Cai X, et al. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc Natl Acad Sci U S A. 2020;117:28336–28343. doi: 10.1073/pnas.2018030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Land WG. Role of DAMPs in respiratory virus-induced acute respiratory distress syndrome—with a preliminary reference to SARS-CoV-2 pneumonia. Genes Immun. 2021;22:141–160. doi: 10.1038/s41435-021-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Naqvi I, Giroux N, Olson L, Morrison SA, Llanga T, Akinade TO, et al. DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID-19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials. 2022;283:121393. doi: 10.1016/j.biomaterials.2022.121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vora SM, Lieberman J, Wu H. Inflammasome activation at the crux of severe COVID-19. Nat Rev Immunol. 2021;21:694–703. doi: 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2020;218:e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang M, Yu F, Chang W, Zhang Y, Zhang L, Li P. Inflammasomes: a rising star on the horizon of COVID-19 pathophysiology. Front Immunol. 2023;14:1185233. doi: 10.3389/fimmu.2023.1185233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sefik E, Qu R, Junqueira C, Kaffe E, Mirza H, Zhao J, et al. Inflammasome activation in infected macrophages drives COVID-19 pathology. Nature. 2022;606:585–593. doi: 10.1038/s41586-022-04802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Junqueira C, Crespo Â, Ranjbar S, de Lacerda LB, Lewandrowski M, Ingber J, et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature. 2022;606:576–584. doi: 10.1038/s41586-022-04702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38:1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kojima K, Yoon H, Okishio K, Tsuyuguchi K. Increased lactate dehydrogenase reflects the progression of COVID-19 pneumonia on chest computed tomography and predicts subsequent severe disease. Sci Rep. 2023;13:1012. doi: 10.1038/s41598-023-28201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Newton K, Dixit VM, Kayagaki N. Dying cells fan the flames of inflammation. Science. 2021;374:1076–1080. doi: 10.1126/science.abi5934. [DOI] [PubMed] [Google Scholar]
- 134.Yang L, Xie X, Tu Z, Fu J, Xu D, Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct Target Ther. 2021;6:255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–68.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mick E, Tsitsiklis A, Spottiswoode N, Caldera S, Serpa PH, Detweiler AM, et al. Upper airway gene expression shows a more robust adaptive immune response to SARS-CoV-2 in children. Nat Commun. 2022;13:3937. doi: 10.1038/s41467-022-31600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China Jama. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang Y-Q, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M. Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand J Immunol. 2021;93:e12967. doi: 10.1111/sji.12967. [DOI] [PubMed] [Google Scholar]
- 144.Pierce CA, Preston-Hurlburt P, Dai Y, Aschner CB, Cheshenko N, Galen B, et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12:eabd5487. doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cohen CA, Li APY, Hachim A, Hui DSC, Kwan MYW, Tsang OTY, et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat Commun. 2021;12:4678. doi: 10.1038/s41467-021-24938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dowell AC, Butler MS, Jinks E, Tut G, Lancaster T, Sylla P, et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol. 2022;23:40–49. doi: 10.1038/s41590-021-01089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rowntree LC, Nguyen THO, Kedzierski L, Neeland MR, Petersen J, Crawford JC, et al. SARS-CoV-2-specific T cell memory with common TCRαβ motifs is established in unvaccinated children who seroconvert after infection. Immunity. 2022;55:1299–315.e4. doi: 10.1016/j.immuni.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Renk H, Dulovic A, Seidel A, Becker M, Fabricius D, Zernickel M, et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat Commun. 2022;13:128. doi: 10.1038/s41467-021-27595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karron RA, Garcia Quesada M, Schappell EA, Schmidt SD, Deloria Knoll M, Hetrich MK, et al. Binding and neutralizing antibody responses to SARS-CoV-2 in very young children exceed those in adults. JCI Insight. 2022;7:e157963. doi: 10.1172/jci.insight.157963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garrido C, Hurst JH, Lorang CG, Aquino JN, Rodriguez J, Pfeiffer TS, et al. Asymptomatic or mild symptomatic SARS-CoV-2 infection elicits durable neutralizing antibody responses in children and adolescents. JCI Insight. 2021;6:e150909. doi: 10.1172/jci.insight.150909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stoddard CI, Sung K, Yaffe ZA, Weight H, Beaudoin-Bussières G, Galloway J, et al. Elevated binding and functional antibody responses to SARS-CoV-2 in infants versus mothers. Nat Commun. 2023;14:4864. doi: 10.1038/s41467-023-40554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Melms JC, Biermann J, Huang H, Wang Y, Nair A, Tagore S, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021;595:114–119. doi: 10.1038/s41586-021-03569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Delorey TM, Ziegler CGK, Heimberg G, Normand R, Yang Y, Segerstolpe Å, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. 2021;595:107–113. doi: 10.1038/s41586-021-03570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang S, Yao X, Ma S, Ping Y, Fan Y, Sun S, et al. A single-cell transcriptomic landscape of the lungs of patients with COVID-19. Nat Cell Biol. 2021;23:1314–1328. doi: 10.1038/s41556-021-00796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2017;6:32. doi: 10.21037/atm.2017.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Urata R, Ikeda K, Yamazaki E, Ueno D, Katayama A, Shin-Ya M, et al. Senescent endothelial cells are predisposed to SARS-CoV-2 infection and subsequent endothelial dysfunction. Sci Rep. 2022;12:11855. doi: 10.1038/s41598-022-15976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nicolai L, Leunig A, Brambs S, Kaiser R, Joppich M, Hoffknecht ML, et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemost. 2021;19:574–581. doi: 10.1111/jth.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104. doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Silva CMS, Wanderley CWS, Veras FP, Gonçalves AV, Lima MHF, Toller-Kawahisa JE, et al. Gasdermin-D activation by SARS-CoV-2 triggers NET and mediate COVID-19 immunopathology. Crit Care. 2022;26:206. doi: 10.1186/s13054-022-04062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Potere N, Garrad E, Kanthi Y, Di Nisio M, Kaplanski G, Bonaventura A, et al. NLRP3 inflammasome and interleukin-1 contributions to COVID-19-associated coagulopathy and immunothrombosis. Cardiovasc Res. 2023;119:2046–2060. doi: 10.1093/cvr/cvad084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miró Ò, Jiménez S, Mebazaa A, Freund Y, Burillo-Putze G, Martín A, et al. Pulmonary embolism in patients with COVID-19: incidence, risk factors, clinical characteristics, and outcome. Eur Heart J. 2021;42:3127–3142. doi: 10.1093/eurheartj/ehab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ameri P, Inciardi RM, Di Pasquale M, Agostoni P, Bellasi A, Camporotondo R, et al. Pulmonary embolism in patients with COVID-19: characteristics and outcomes in the Cardio-COVID Italy multicenter study. Clin Res Cardiol. 2021;110:1020–1028. doi: 10.1007/s00392-020-01766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang L, Feng X, Zhang D, Jiang C, Mei H, Wang J, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142:114–128. doi: 10.1161/CIRCULATIONAHA.120.046702. [DOI] [PubMed] [Google Scholar]
- 172.Whitworth H, Sartain SE, Kumar R, Armstrong K, Ballester L, Betensky M, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. doi: 10.1182/blood.2020010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mansourian M, Ghandi Y, Habibi D, Mehrabi S. COVID-19 infection in children: a systematic review and meta-analysis of clinical features and laboratory findings. Arch Pediatr. 2021;28:242–248. doi: 10.1016/j.arcped.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shi Q, Wang Z, Liu J, Wang X, Zhou Q, Li Q, et al. Risk factors for poor prognosis in children and adolescents with COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;41:101155. doi: 10.1016/j.eclinm.2021.101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Toraih EA, Hussein MH, Elshazli RM, Kline A, Munshi R, Sultana N, et al. Multisystem inflammatory syndrome in pediatric COVID-19 patients: a meta-analysis. World J Pediatr. 2021;17:141–151. doi: 10.1007/s12519-021-00419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Radia T, Williams N, Agrawal P, Harman K, Weale J, Cook J, Gupta A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. 2021;38:51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Santos MO, Gonçalves LC, Silva PAN, Moreira ALE, Ito CRM, Peixoto FAO, et al. Multisystem inflammatory syndrome (MIS-C): a systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr (Rio J) 2022;98:338–349. doi: 10.1016/j.jped.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Encinosa W, Moon K, Figueroa J, Elias Y. Complications, adverse drug events, high costs, and disparities in multisystem inflammatory syndrome in children vs COVID-19. JAMA Netw Open. 2023;6:e2244975. doi: 10.1001/jamanetworkopen.2022.44975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.LaRovere KL, Poussaint TY, Young CC, Newhams MM, Kucukak S, Irby K, et al. Changes in distribution of severe neurologic involvement in US pediatric inpatients with COVID-19 or multisystem inflammatory syndrome in children in 2021 vs 2020. JAMA Neurol. 2023;80:91–98. doi: 10.1001/jamaneurol.2022.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chang JC, Matsubara D, Morgan RW, Diorio C, Nadaraj S, Teachey DT, et al. Skewed cytokine responses rather than the magnitude of the cytokine storm may drive cardiac dysfunction in multisystem inflammatory syndrome in children. J Am Heart Assoc. 2021;10:e021428. doi: 10.1161/JAHA.121.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Felsenstein S, Duong P, Lane S, Jones C, Pain CE, Hedrich CM. Cardiac pathology and outcomes vary between Kawasaki disease and PIMS-TS. Clin Immunol. 2021;229:108780. doi: 10.1016/j.clim.2021.108780. [DOI] [PubMed] [Google Scholar]
- 185.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, Montin D. SARS-CoV-2-induced kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 187.Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. 2021;17:731–748. doi: 10.1038/s41584-021-00709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. doi: 10.1038/s41591-020-1054-6. [DOI] [PubMed] [Google Scholar]
- 189.Diorio C, Shraim R, Vella LA, Giles JR, Baxter AE, Oldridge DA, et al. Proteomic profiling of MIS-C patients indicates heterogeneity relating to interferon gamma dysregulation and vascular endothelial dysfunction. Nat Commun. 2021;12:7222. doi: 10.1038/s41467-021-27544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Esteve-Sole A, Anton J, Pino-Ramirez RM, Sanchez-Manubens J, Fumadó V, Fortuny C, et al. Similarities and differences between the immunopathogenesis of COVID-19–related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Investig. 2021;131:e144554. doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang JJ, Gaines SB, Amezcua ML, Lubell TR, Dayan PS, Dale M, et al. Upregulation of type 1 conventional dendritic cells implicates antigen cross-presentation in multisystem inflammatory syndrome. J Allergy Clin Immunol. 2022;149:912–922. doi: 10.1016/j.jaci.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kumar D, Rostad CA, Jaggi P, Villacis Nunez DS, Prince C, Lu A, et al. Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis (HLH) J Allergy Clin Immunol. 2022;149:1592–606.e16. doi: 10.1016/j.jaci.2022.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 194.Duarte-Neto AN, Caldini EG, Gomes-Gouvêa MS, Kanamura CT, de Almeida Monteiro RA, Ferranti JF, et al. An autopsy study of the spectrum of severe COVID-19 in children: From SARS to different phenotypes of MIS-C. eClinicalMedicine. 2021;35:100850. [DOI] [PMC free article] [PubMed]
- 195.Noval Rivas M, Porritt RA, Cheng MH, Bahar I, Arditi M. Multisystem inflammatory syndrome in children and long COVID: the SARS-CoV-2 viral superantigen hypothesis. Front Immunol. 2022;13:941009. doi: 10.3389/fimmu.2022.941009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Cheng MH, Zhang S, Porritt RA, Noval Rivas M, Paschold L, Willscher E, et al. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci U S A. 2020;117:25254–25262. doi: 10.1073/pnas.2010722117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Feleszko W, Okarska-Napierała M, Buddingh EP, Bloomfield M, Sediva A, Bautista-Rodriguez C, et al. Pathogenesis, immunology, and immune-targeted management of the multisystem inflammatory syndrome in children (MIS-C) or pediatric inflammatory multisystem syndrome (PIMS): EAACI Position Paper. Pediatr Allergy Immunol. 2023;34:e13900. doi: 10.1111/pai.13900. [DOI] [PubMed] [Google Scholar]
- 198.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mayordomo-Colunga J, Vivanco-Allende A, López-Alonso I, López-Martínez C, Fernández-Vega I, Gil-Peña H, Rey C. SARS-CoV-2 spike protein in intestinal cells of a patient with coronavirus disease 2019 multisystem inflammatory syndrome. J Pediatr. 2022;243:214–8.e5. doi: 10.1016/j.jpeds.2021.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoste L, Roels L, Naesens L, Bosteels V, Vanhee S, Dupont S, et al. TIM3+TRBV11–2 T cells and IFNγ signature in patrolling monocytes and CD16+ NK cells delineate MIS-C. J Exp Med. 2021;219:e20211381. doi: 10.1084/jem.20211381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131:e149633. doi: 10.1172/JCI149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rey-Jurado E, Espinosa Y, Astudillo C, Jimena Cortés L, Hormazabal J, Noguera LP, et al. Deep immunophenotyping reveals biomarkers of multisystemic inflammatory syndrome in children in a Latin American cohort. J Allergy Clin Immunol. 2022;150:1074–85.e11. doi: 10.1016/j.jaci.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sigal GB, Novak T, Mathew A, Chou J, Zhang Y, Manjula N, et al. Measurement of severe acute respiratory syndrome coronavirus 2 antigens in plasma of pediatric patients with acute coronavirus disease 2019 or multisystem inflammatory syndrome in children using an ultrasensitive and quantitative immunoassay. Clin Infect Dis. 2022;75:1351–1358. doi: 10.1093/cid/ciac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, et al. HLA class I–associated expansion of TRBV11–2 T cells in multisystem inflammatory syndrome in children. J Clin Investig. 2021;131:e146614. doi: 10.1172/JCI146614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moreews M, Le Gouge K, Khaldi-Plassart S, Pescarmona R, Mathieu A-L, Malcus C, et al. Polyclonal expansion of TCR Vβ 21.3+ CD4+ and CD8+ T cells is a hallmark of multisystem inflammatory syndrome in children. Sci Immunol. 2021;6:eabh1516. doi: 10.1126/sciimmunol.abh1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lam KP, Chiñas M, Julé AM, Taylor M, Ohashi M, Benamar M, et al. SARS-CoV-2-specific T cell responses in patients with multisystem inflammatory syndrome in children. Clin Immunol. 2022;243:109106. doi: 10.1016/j.clim.2022.109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ramaswamy A, Brodsky NN, Sumida TS, Comi M, Asashima H, Hoehn KB, et al. Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity. 2021;54:1083–95.e7. doi: 10.1016/j.immuni.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gruber CN, Patel RS, Trachtman R, Lepow L, Amanat F, Krammer F, et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–95.e14. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Consiglio CR, Cotugno N, Sardh F, Pou C, Amodio D, Rodriguez L, et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–81.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Pfeifer J, Thurner B, Kessel C, Fadle N, Kheiroddin P, Regitz E, et al. Autoantibodies against interleukin-1 receptor antagonist in multisystem inflammatory syndrome in children: a multicentre, retrospective, cohort study. The Lancet Rheumatology. 2022;4:e329–e337. doi: 10.1016/S2665-9913(22)00064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Burbelo PD, Castagnoli R, Shimizu C, Delmonte OM, Dobbs K, Discepolo V, et al. Autoantibodies against proteins previously associated with autoimmunity in adult and pediatric patients with COVID-19 and children With MIS-C. Front Immunol. 2022;13:841126. doi: 10.3389/fimmu.2022.841126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee D, Le Pen J, Yatim A, Dong B, Aquino Y, Ogishi M, et al. Inborn errors of OAS-RNase L in SARS-CoV-2-related multisystem inflammatory syndrome in children. Science. 2023;379:eabo3627. doi: 10.1126/science.abo3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Chou J, Platt CD, Habiballah S, Nguyen AA, Elkins M, Weeks S, et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C) J Allergy Clin Immunol. 2021;148:732–8.e1. doi: 10.1016/j.jaci.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.National Institutes of Health. Clinical Management of Children Summary. Accessed July 26, 2023. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-children/clinical-management-of-children-summary/.
- 215.Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 216.Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2022;76:e342–e349. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.The U.S. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for PAXLOVIDTM. 2021. Accessed July 26, 2023. https://www.fda.gov/media/155050/download.
- 218.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2021;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Harahsheh AS, Portman MA, Khoury M, Elias MD, Lee S, Lin J, McCrindle BW. Management of multisystem inflammatory syndrome in children: decision-making regarding a new condition in the absence of clinical trial data. Can J Cardiol. 2023;39:803–814. doi: 10.1016/j.cjca.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.National Institutes of Health. Therapeutic Management of Hospitalized Children With COVID-19. COVID-19 Treatment Guidelines. Updated July 21, 2023. Accessed July 28, 2023. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-children/hospitalized-children-therapeutic-management/.
- 221.González-Dambrauskas S, Vásquez-Hoyos P, Camporesi A, Díaz-Rubio F, Piñeres-Olave BE, Fernández-Sarmiento J, et al. Pediatric critical care and COVID-19. Pediatrics. 2020;146:e20201766. doi: 10.1542/peds.2020-1766. [DOI] [PubMed] [Google Scholar]
- 222.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jiang L, Li X, Nie J, Tang K, Bhutta ZA. A systematic review of persistent clinical features after SARS-CoV-2 in the pediatric population. Pediatrics. 2023;152:e2022060351. doi: 10.1542/peds.2022-060351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Matteudi T, Luciani L, Fabre A, Minodier P, Boucekine M, Bosdure E, et al. Clinical characteristics of paediatric COVID-19 patients followed for up to 13 months. Acta Paediatr. 2021;110:3331–3333. doi: 10.1111/apa.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kikkenborg Berg S, Palm P, Nygaard U, Bundgaard H, Petersen MNS, Rosenkilde S, et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc Health. 2022;6:614–623. doi: 10.1016/S2352-4642(22)00154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lopez-Leon S, Wegman-Ostrosky T, Ayuzo del Valle NC, Perelman C, Sepulveda R, Rebolledo PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. 2022;12:9950. doi: 10.1038/s41598-022-13495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gentilotti E, Górska A, Tami A, Gusinow R, Mirandola M, Rodríguez Baño J, et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. 2023;62:102107. doi: 10.1016/j.eclinm.2023.102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rao S, Lee GM, Razzaghi H, Lorman V, Mejias A, Pajor NM, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. 2022;176:1000–1009. doi: 10.1001/jamapediatrics.2022.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen B, Julg B, Mohandas S, Bradfute SB. Viral persistence, reactivation, and mechanisms of long COVID. Elife. 2023;12:e86015. doi: 10.7554/eLife.86015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wallukat G, Hohberger B, Wenzel K, Fürst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kruger A, Vlok M, Turner S, Venter C, Laubscher GJ, Kell DB, Pretorius E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol. 2022;21:190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kell DB, Laubscher GJ, Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Raj SR, Arnold AC, Barboi A, Claydon VE, Limberg JK, Lucci VM, et al. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res. 2021;31:365–368. doi: 10.1007/s10286-021-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Morrow AK, Malone LA, Kokorelis C, Petracek LS, Eastin EF, Lobner KL, et al. Long-term COVID 19 sequelae in adolescents: the overlap with orthostatic intolerance and ME/CFS. Curr Pediatr Rep. 2022;10:31–44. doi: 10.1007/s40124-022-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sukocheva OA, Maksoud R, Beeraka NM, Madhunapantula SV, Sinelnikov M, Nikolenko VN, et al. Analysis of post COVID-19 condition and its overlap with myalgic encephalomyelitis/chronic fatigue syndrome. J Adv Res. 2022;40:179–196. doi: 10.1016/j.jare.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Gross R, Thaweethai T, Rosenzweig EB, Chan J, Chibnik LB, Cicek MS, et al. Researching COVID to enhance recovery (RECOVER) pediatric study protocol: rationale, objectives and design. medRxiv. 2023. 2023.04.27.23289228. [DOI] [PMC free article] [PubMed]