Abstract
The pleomorphic adenoma gene1 (PLAG1) encodes a DNA-binding, C2H2 zinc-finger protein which acts as a transcription factor that regulates the expression of diverse genes across different organs and tissues; hence, the name pleomorphic. Rearrangements of the PLAG1 gene, and/or overexpression, are associated with benign tumors and cancers in a variety of tissues. This is best described for pleomorphic adenoma of the salivary glands in humans. The most notable expression of PLAG1 occurs during embryonic and fetal development, with lesser expression after birth. Evidence has accumulated of a role for PLAG1 protein in normal early embryonic development and placentation in mammals. PLAG1 protein influences the expression of the ike growth factor 2 (IGF2) gene and production of IGF2 protein. IGF2 is an important mitogen in ovarian follicles/oocytes, embryos, and fetuses. The PLAG1-IGF2 axis, therefore, provides one pathway whereby PLAG1 protein can influence embryonic survival and pregnancy. PLAG1 also influences over 1,000 other genes in embryos including those associated with ribosomal assembly and proteins. Brahman (Bos indicus) heifers homozygous for the PLAG1 variant, rs109815800 (G > T), show greater fertility than contemporary heifers with either one, or no copy, of the variant. Greater fertility in heifers homozygous for rs109815800 could be the result of early puberty and/or greater embryonic survival. The present review first looks at the broader roles of the PLAG1 gene and PLAG1 protein and then focuses on the emerging role of PLAG1/PLAG1 in embryonic development and pregnancy. A deeper understanding of factors which influence embryonic development is required for the next transformational increase in embryonic survival and successful pregnancy for both in vivo and in vitro derived embryos in cattle.
Keywords: cattle, embryo, pleomorphic adenoma gene, PLAG1
This review explores how the pleomorphic adenoma gene1 (PLAG1) which is associated with cancers may also be fundamentally important in embryonic development and the establishment of pregnancy in mammals.
Introduction
The major cause of reproductive loss in cattle is the failure of embryos to progress to implantation and pregnancy. Fertilization rates in both beef and dairy cattle are in the order of 85% to 100%; however, only 40% to 60% of embryos establish a pregnancy (Diskin et al., 2016; Lockhart et al., 2023). In recent reviews, we have argued that the next transformational change in reproductive efficiency will require a deeper understanding of the biology of early embryo development in cattle (D’Occhio et al., 2019b, 2020a, b; Campanile et al., 2021). This applies to both natural mating and assisted reproduction. A critically important feature of early embryo development is the dialogue between embryo and uterus in the period before embryo attachment and during implantation (Hantak et al., 2014; Rizos et al., 2017; Sponchiado et al., 2017, 2019, 2020; Aguilera et al., 2022; Binelli et al., 2022; Cajas et al., 2022; Tesfaye et al., 2022). Factors involved in embryo-uterine communication include the transforming ß superfamily (D’Occhio et al., 2020a), cell-cell adhesion molecules (D’Occhio et al., 2019b), kisspeptin (D’Occhio et al., 2020b) and immune factors (Campanile et al., 2021), among others. Our reviews, and those of others, have noted the complexity of events associated with early embryo development, attachment of the conceptus to the uterine epithelium, and implantation. The reviews have identified major gaps in our understanding of early embryo development in cattle. The gaps in knowledge largely explain the relatively modest progress over the past 40 yr in reducing high embryo loss in cattle. High embryo loss applies to both in vivo and in vitro derived embryos. Embryo loss is comparable after natural mating, artificial insemination, or embryo transfer (Hansen, 2020). The transfer of a bovine embryo to a recipient at day 7 of development avoids the relatively large loss of embryos that occurs in the first 7 d after fertilization. However, there is still considerable loss between the transfer on day 7, and day 21, when embryo attachment has commenced (Hansen, 2020). Therefore, the transfer of a bovine embryo on day 7 of early development does not overcome all the embryo losses in cattle that occur before implantation.
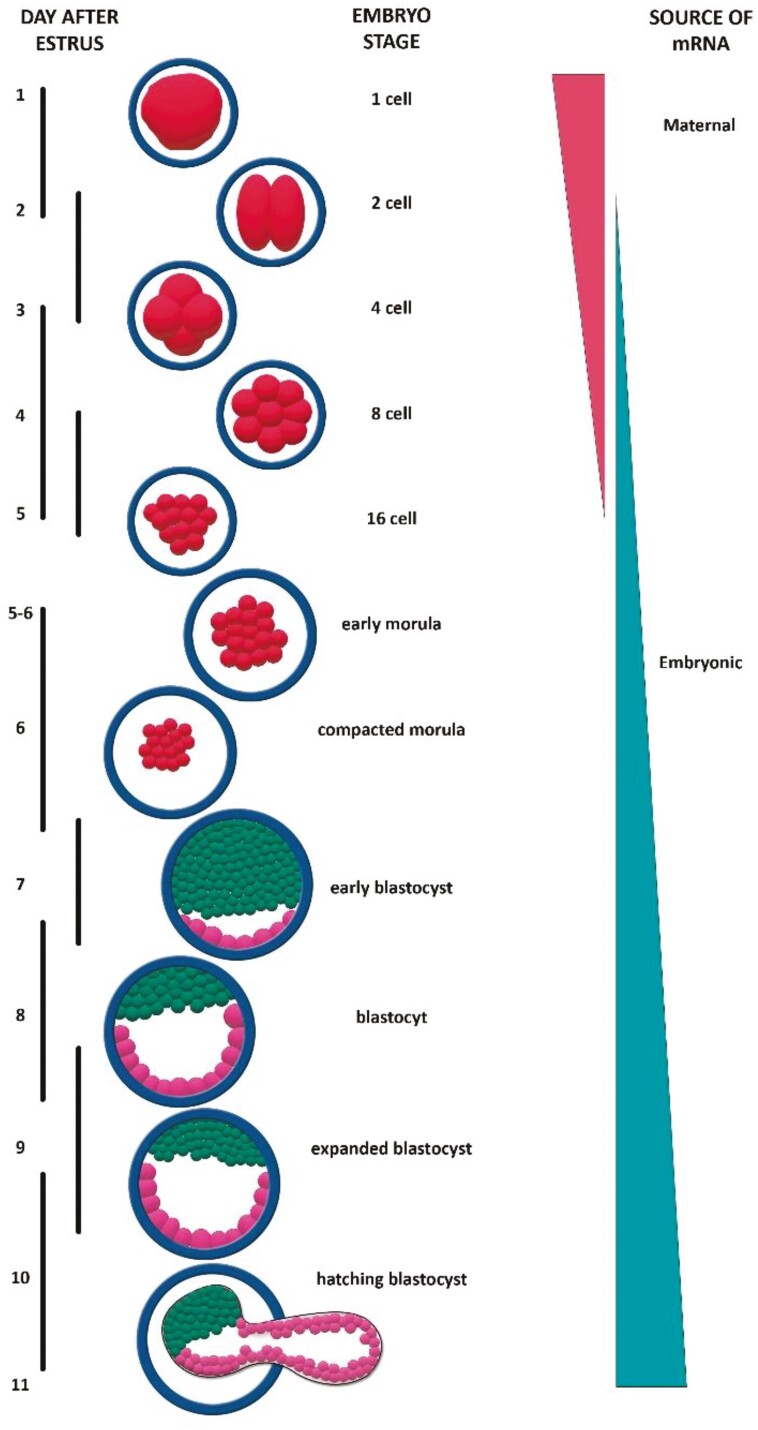
The present review seeks to build on our earlier articles and looks at the potential role of the pleomorphic adenoma gene1 (PLAG1) in early embryonic development. The PLAG1 gene encodes a DNA-binding, C2H2 zinc-finger protein which acts as a transcription factor that regulates the expression of diverse genes across different organs and tissues (Voz et al., 2004; Abdollahi, 2007; Wagner and Zhang, 2011; Adnani et al., 2018). The most notable expression of PLAG1 occurs during embryonic and fetal development with lesser expression after birth (Hensen et al., 2004; Tang et al., 2013; Habib et al., 2018; Madissoon et al., 2019; Li et al., 2020a). There is a paucity of information on the transcriptional regulation of the PLAG1 gene. The neurogenic factor Hmga2 induces expression of PLAG1 in neuronal progenitor cells (Sakai et al., 2019) while microRNA-141 shows translational regulation of PLAG1 mRNA (Tang et al., 2013). In early embryos, PLAG1 protein was reported to act at conserved Alu/B1 elements in the promotor region of over 1,000 genes associated with ribosomal assembly and protein synthesis (Madissoon et al., 2019). Rearrangements of the PLAG1 gene, and/or overexpression, are associated with benign tumors and neoplasia in different tissues (Matsuyama et al., 2011). This is best described for pleomorphic adenomas of the salivary glands in humans, which gave the gene its name (Voz et al., 1998, 2000; Åström et al., 1999; Debiec-Rychter et al., 2001; Hensen et al., 2002; Declercq et al., 2005; Asp et al., 2006; Van Dyck et al., 2007; Skálová et al., 2021). There is evidence of a role for PLAG1 protein in normal early embryonic development and placentation. In mice, oocytes with low amounts of maternal PLAG1 transcripts showed a delay in zygotic genome activation, and 2-cell-stage embryonic development (Madissoon et al., 2019). The PLAG1 gene is maternally imprinted and an ongoing role for PLAG1 protein during embryonic development may depend on the expression of paternal PLAG1 (Moore & Haig, 1991; O’Doherty et al., 2012; Barlow & Bartolomei, 2014; Plasschaert & Bartolomei, 2014; Adhami et al., 2015; Jiang et al., 2015; Lafontaine et al., 2020). In cattle, minor activation of the embryonic genome occurs at the 2-cell embryo stage, with major activation at the 4- to 8-cell stage (Telford et al., 1990; Memili et al., 1998; Memili & First, 1999, 2000; Dean et al., 2001; Kaňka et al., 2003; Meirelles et al., 2004; Ruddock et al., 2004; Gad et al., 2012; Ozawa et al., 2012; Graf et al., 2014a, b; O’Doherty et al., 2015; Jukam et al., 2017; Jiang et al., 2018; Lavagi et al., 2018; Duan et al., 2019; Halstead et al., 2020; Ivanova et al., 2020; Figure 1). PLAG1 is polymorphic in cattle and any potential action of PLAG1 protein on ongoing embryonic development may depend on the nature of the paternal PLAG1 allele. PLAG1 can influence the production of ike growth factor 2 (IGF2), H19, leukemia inhibitory factor (LIF), ß-catenin, and cytokines. These factors are all variously associated with embryonic development, uterine attachment, and implantation (Niemann & Wrenzycki, 1999; Han et al., 2003; Gabory et al., 2009; Agrogiannis et al., 2014; Jiang et al., 2015; Smith et al., 2015; Sferruzzi-Perri et al, 2017; Campanile et al., 2021; Llobat, 2021; Willhelm et al., 2021; Zhou et al., 2021; Sandovici et al., 2022). The role of LIF and other cytokines, and the LIF receptor, in embryonic development and implantation is comprehensively discussed in earlier reviews which are complemented by the present review (Guzeloglu-Kayisli et al., 2009; Robertson et al., 2018; Campanile et al., 2021; Namiki et al., 2023). The role of catenins during early vertebrate development through cell adhesion in association with cadherins (Stepniak et al, 2009; D’Occhio et al., 2019b) and intracellular signaling in the Wnt/β-catenin pathway (Valenta et al., 2012; Liu et al., 2022) also have been well documented. In cattle, polymorphisms of the PLAG1 gene are linked with fetal and postnatal growth and adult phenotypes including fertility (PLAG1 and Phenotype in Cattle below).
Figure 1.
Zygotic genome activation in cattle. PLAG1 is maternally imprinted and PLAG1 protein derived from paternally expressed PLAG1 could potentially be present in embryos from the 2 to 4 cell stage.
The approach adopted in the present review is to first provide a general background on the PLAG1 gene and PLAG1 protein. We then consider relationships between PLAG1 polymorphisms and phenotypes in cattle. This is followed by a focus on the role of PLAG1/PLAG1 in early embryonic development. In keeping with our earlier reviews, this review seeks to build awareness of the complex biology of embryonic development. Our consistent argument has been that a deeper understanding is needed of the factors that impact early embryo development before a meaningful transformational change can be made in the efficiency of both natural mating and assisted reproduction in cattle.
Discovery of PLAG1 Gene and PLAG1 Protein
The PLAG1 gene and PLAG1 protein were described from 1997 to 1998 (Table 1). The seminal report showed the PLAG1 gene to be associated with a chromosome translocation at 8q12 that was linked with pleomorphic adenomas of the salivary glands in humans (Kas et al. 1997a, b). The same laboratory described two related human proteins, PLAGL1 and PLAGL2. The protein PLAGL2 also binds to DNA and has similar properties as PLAG1 protein (Kas et al. 1998). The PLAG1/PLAG1 family members were subsequently assigned various names based on the association of PLAG1 mutations with different phenotypes in different species (Table 1). In the absence of PLAG1 gene rearrangement, and/or overexpression of PLAG1, PLAG1 protein can have antiproliferative activity and tumor suppression. Hence, the regulated expression of PLAG1 is associated with normal cellular function in different tissues, while overexpression is linked with benign tumors and malignancies (Zatkova et al., 2004). Overexpression of PLAG1 leads to overproduction of PLAG1, rather than changes in the structure of PLAG1 protein. PLAG1 stimulates the IGF2 gene and excess production of IGF2 is considered one mechanism linked to tumors and cancers (Voz et al., 2000, 2004; Zatkova et al., 2004; Akhtar et al., 2012).
Table 1.
Discovery of the pleomorphic adenoma gene (PLAG1) family members
| Name | Function described | Year | Species | Reference |
|---|---|---|---|---|
| PLAG1: pleomorphic adenoma gene | Activation in salivary gland tumorigenesis | 1997 | Human | Kas et al. 1997a, b |
| * LOT1: lost-on-transformation | Decreased or lost expression in transformed ovarian epithelia cells that developed into malignant ovarian tumors | 1997 | Rat | Abdollahi et al. 1997a, b; see also Abdollahi 2007 |
| * ZAC/ ZAC1: zinc-finger protein found to regulate apoptosis and cell cycle arrest | Induction of apoptosis and G1 cell cycle arrest and inhibition of tumor growth | 1997 | Mouse | Spengler et al. 1997 |
|
*
PLAGL1: PLAG1 like zinc-finger 1 PLAGL2: PLAG1 like zinc-finger protein 2 |
Identified by screening mouse embryo and human fetal kidney cDNA libraries using PLAG1 open reading frames (ORF) | 1998 | Human, Mouse | Kas et al. 1998 |
*Same PLAG/PLAG family member.
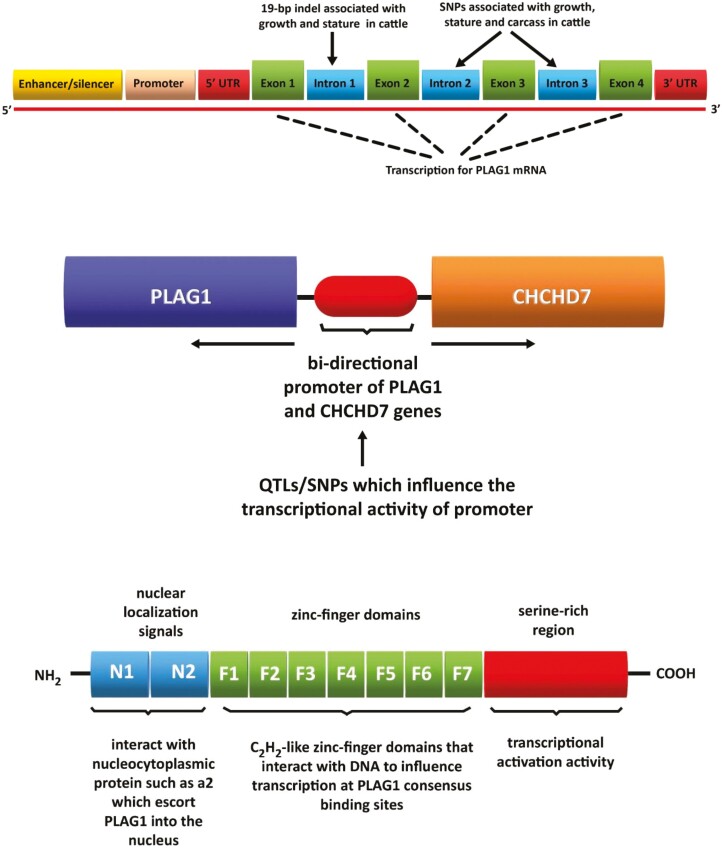
In humans, the PLAG1 gene comprises 6 exons and 5 introns. PLAG1 has yet to be fully described in cattle and is presently thought to comprise 3 introns and 4 exons (Van Dyck et al., 2007; Figure 2). In cattle, a 19-base pair insertion/deletion (19-bp indel) at Exon 1, and single-nucleotide polymorphisms at Exons 3 and 4, are associated with growth, stature, and carcass traits (Karim et al. 2011; Littlejohn et al. 2011; Zhong et al., 2019; Figure 2). PLAG1 mutations were also associated with age at puberty and circulating levels of IGF1 in heifers (Fortes et al., 2013). PLAG1 is located within the same quantitative trait loci as the coiled-coil-helix-coiled-coil-helix domain containing 7 (CHCHD7) gene, which is also associated with growth and stature in several species including cattle (Li et al., 2020a; Xu et al., 2020). Both genes share the same bi-directional promoter and SNPs known to influence the transcriptional activity of the promoter impact the expression of PLAG1 and CHCHD7 (Karim et al., 2011; Fink et al., 2017; Figure 2). PLAG1 protein is comprised of three regions with distinct functions: a region with nuclear translocation signals for the transfer of PLAG1 to the nucleus; C2H2-like zinc-finger domains that interact with DNA to influence transcription; a serine-rich region that has transcriptional activation activity (Braem et al., 2002; Hensen et al., 2002; Figure 2).
Figure 2.
The putative structure of the PLAG1 gene in cattle and variants of PLAG1 associated with different phenotypes. Indel, insertion/deletion; SNPs, single-nucleotide polymorphisms (top); the common bi-directional promoter of the PLAG1 and CHCHD7 genes. QTLs/SNPs in the promoter influence the transcriptional activity of PLAG1 and CHCHD7 and phenotypes in cattle including growth and stature. QTLs, quantitative trait loci; SNPs, single-nucleotide polymorphisms (middle); and the structure of PLAG1 protein and domains associated with translocation to the nucleus and binding to DNA. PLAG1 typically binds to the promoter of target genes to influence transcription (bottom).
PLAG1 and Phenotype in Cattle
The most studied relationships between PLAG1/PLAG1 and phenotype in cattle are for growth and stature (Karim, et al., 2011; Pryce et al., 2011; Visscher & Goddard, 2011; Boitard et al., 2016; Takasuga, 2016; Taye et al., 2017; Utsunomiya et al., 2017; Bouwman et al., 2018). As noted above, PLAG1 is most noticeably expressed during fetal development and PLAG1 polymorphisms are linked with differences in birth weight and calving ease in cattle (Littlejohn et al., 2011; Pausch et al., 2011; Utsunomiya et al., 2013). PLAG1 polymorphisms are also associated with growth, mature body size, and stature, in different breeds of cattle including Holstein-Friesian (Littlejohn et al., 2011; Zhao et al., 2015), Holstein Friesian × Jersey (Karim et al., 2011), Chinese (Xu et al., 2018; Hou et al., 2019; Zhong et al., 2019; Zhou et al., 2019; Li et al., 2020b), European (Randhawa et al., 2015; Zhao et al., 2015), African (Randhawa et al., 2015), and Japanese Black (Hoshiba et al., 2013; Sasaki et al., 2013). Other commercially important production traits in cattle linked with PLAG1 polymorphisms are carcass weight and meat yield (Nishimura et al., 2012; Hoshiba et al., 2013; Bolormaa et al., 2015; Song et al., 2016; Hay & Roberts, 2018; Zhang et al., 2019), milk quality (Zhao et al., 2015; Fink et al., 2017), and adaptation (Porto-Neto et al., 2014; Boitard et al., 2016). PLAG1 influences growth and production traits in goats (Wei et al., 2021) and sheep (Wu et al., 2019; Pan et al., 2022).
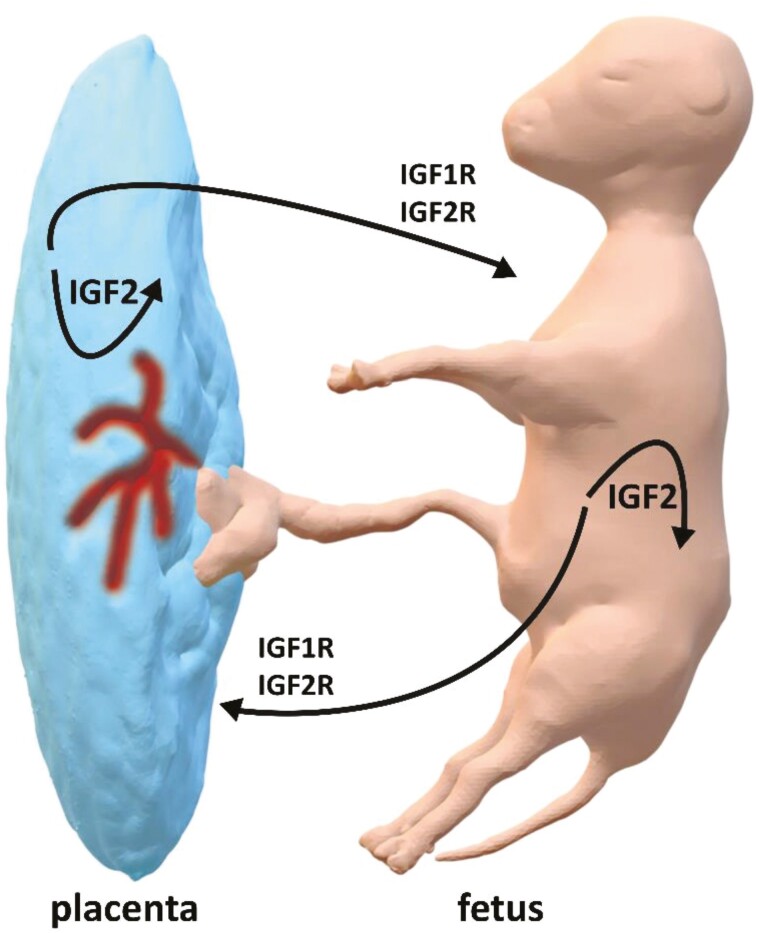
A major target for PLAG1 protein is the IGF2 gene and PLAG1 binding sites are present in the promoter of IGF2 (Voz et al., 2000, 2004; Zatkova et al., 2004; Van Dyck et al., 2007; Akhtar et al., 2012; Wang et al., 2013). IGF2 codes for the IGF2 protein which is an important fetal mitogen (O’Dell & Day, 1998; Curchoe et al., 2005; Berkowicz et al., 2010; Bergman et al., 2013). It is generally accepted that growth in cattle is at least partly associated with variants of PLAG1, and differential regulation of IGF2 by PLAG1 protein (Bolormaa et al., 2015). IGF2 is produced by placental tissue and acts in both the placenta and fetus (Constância et al., 2002; Figure 3). The developing fetus likewise produces IGF2 which acts at the fetus and placenta (Akhtar et al., 2012; Agrogiannis et al., 2014; Sandovici et al., 2022). Inactivation of PLAG1 is associated with reduced IGF2 and fetal growth retardation (Hensen et al., 2004; Varrault et al., 2006; Habib et al., 2018). Aberrant imprinting of PLAG1 and overexpression is associated with the large fetus syndrome (Chen et al., 2015). Relationships between PLAG1, IGF1 and phenotype have been described for cattle (Fortes et al., 2013).
Figure 3.
Insulin-like growth factor 2 (IGF2) is produced by the fetus and placenta and has both local and reciprocal action between the fetus and placenta. IGF2 can bind to both IGF1 and IGF2 receptors on target cells.
PLAG1/PLAG1 and Reproduction
Puberty
Age at puberty is a highly important trait which is linked to lifetime fertility in female cattle (Hawken et al., 2012; Wathes et al., 2014; D’Occhio et al., 2019a). Mutations on chromosome 14 (BTA14), in proximity to PLAG1, were reported to be associated with puberty in Zebu (Bos indicus) heifers including Brahman (Hawken et al., 2012; Fortes et al., 2013) and Nellore (Mota et al., 2020). Heifers with delayed puberty linked to various PLAG1 mutations are heavier at puberty. Over 36 yr, we have subjected a herd of Brahman (Bos indicus) females to uncompromising selection for fertility (Collins Belah Valley [CBV] Brahman, Belah Valley Cattle Station, Marlborough, Central Queensland, Australia). Females remain in this herd only if they conceive, wean a calf, and reconceive in successive years starting with their first mating (Collins A. Snr., J. E. Kinder, and M. J. D’Occhio, unpublished). Days-to-calving (DTC), defined as the number of days from the start of mating to subsequent calving, is the most important measure of fertility in Brahman and the key driver of profit in beef production. Herd records are used to calculate estimates of genetic differences between animals for DTC and these are expressed as estimated breeding value (EBV) or estimated progeny difference. Female cattle with a low DTC EBV show early puberty as heifers and resume cyclic ovarian function sooner after calving. The DTC EBV for the CBV Brahman herd is −16.8 d compared with the Australian Brahman breed average DTC EBV of −3.2 d. The latter demonstrates a strong genetic component for high fertility of the CBV Brahman herd. It was recently shown that maiden heifers in the CBV Brahman herd that were homozygous for the PLAG1 variant rs109815800 (G > T) conceived earlier and had greater fertility than contemporary heifers with either one or no copies of the variant (Engle & Hayes, 2022). Heifers with two copies of the variant had a smaller stature than heifers with one or no copies (Engle & Hayes, 2022).
Ovarian follicles and embryonic and fetal development
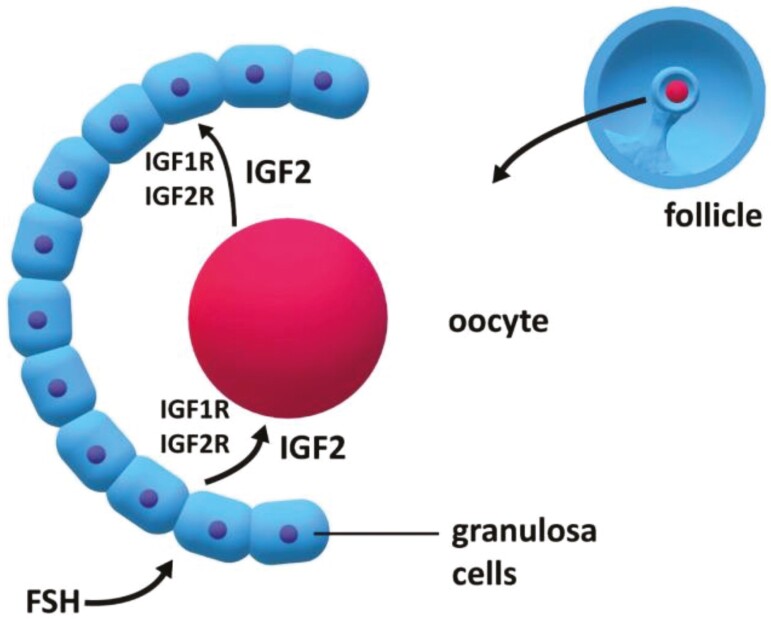
In addition to an effect on age at puberty, PLAG1/PLAG1 have been broadly associated with reproductive function in fish and mammals (Pendeville et al., 2006; Juma et al., 2016, 2017, 2018; Wong et al., 2020a, b). The relationship between PLAG1 and IGF2 in growth and development, which is discussed above, can be extended to ovarian function and embryonic development in cattle (Neirijnck et al., 2019). IGF2 is expressed in growing ovarian follicles and has important mitogenic actions on both the follicle and oocyte (Hunter et al., 2004; Spicer & Aad, 2007; Brogan et al., 2010; Aad et al., 2013; Baumgarten et al., 2015; Tkachenko et al., 2021; Figure 4). Oocytes also produce IGF2 which influences the function of oocytes and follicles (Willhelm et al., 2021; Figure 4). IGF2 is additionally expressed by early embryos and the uterus and is involved in autocrine, paracrine, and endocrine events associated with embryonic growth, attachment, and implantation (Robinson et al., 2000; Willhelm et al., 2021; Figure 5). IGF2 is maternally imprinted similar to PLAG1 (DeChiara et al., 1991; Giannoukakis et al., 1993; Dindot et al., 2004; Gebert et al., 2006, 2009; Sandovici et al., 2022). As noted above, in early embryos PLAG1 protein acts at the promotor region of over 1,000 genes including IGF2 (Madissoon et al., 2019). Mouse embryos lacking maternal PLAG1 transitioned slowly from the 2- to 4-cell stage of development (Madissoon et al., 2019). Embryos that transition through early cell divisions in a timely manner have a greater likelihood of surviving and establishing a pregnancy. In mice that lacked maternal PLAG1 the gene was expressed ectopically from the paternal allele earlier than would otherwise occur (Madissoon et al., 2019).
Figure 4.
Insulin-like growth factor 2 (IGF2) is produced by oocytes and granulosa cells of follicles and has a local and reciprocal action in oocytes and follicles. IGF2 is an important mitogen and can bind to both IGF1 and IGF2 receptors at target cells. The IGF2 gene is influenced by PLAG1 protein which provides a mechanism for PLAG1 to be associated with oocyte and follicular function.
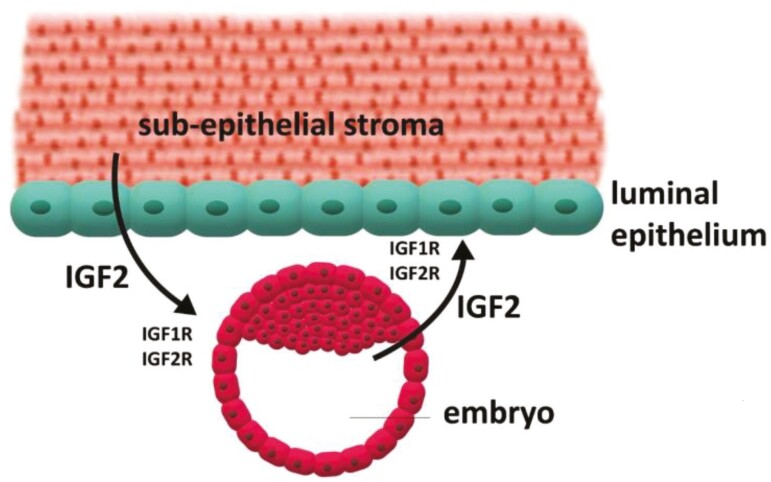
Figure 5.
Insulin-like growth factor 2 (IGF2) is produced by the embryo and uterine stroma and has a local and reciprocal action in embryos and uterus. IGF2 is an important mitogen and can bind to both IGF1 and IGF2 receptors at target cells. The IGF2 gene is influenced by PLAG1 protein which provides a mechanism for PLAG1 to be associated with embryonic and uterine function.
MicroRNAs (miRNAs) have been implicated in the function of PLAG1/PLAG1 in early development (Maccani & Marsit, 2011; Kochhar et al., 2021). For example, miRNA-141 downregulates PLAG1 translation which is associated with fetal growth retardation (Tang et al., 2013). Based on the relationship between PLAG1 and expression of the IGF2 gene discussed above, it was concluded that miRNA-141 downregulation of PLAG1 results in reduced IGF2, and suppressed fetal growth (Varrault et al., 2006; Tang et al., 2013; Saha et al., 2015). There is a lack of information on the specific localization of PLAG1 expression in the embryos and uterus and this is an area that warrants investigation.
PLAG1/PLAG1 and Embryos Survival in Cattle
As noted above, the failure of embryos to progress to implantation and pregnancy is the major cause of reproductive loss in cattle. A deeper understanding of the factors which support embryonic development, attachment, and implantation, is key to improving embryo survival and achieving a transformational increase in reproductive success in female cattle. The factors are both genetic and non-genetic, although these are clearly interrelated. As noted above, Brahman (Bos indicus) heifers homozygous for the PLAG1 variant, rs109815800 (G > T), show greater fertility than contemporary heifers with either one or no copy of the variant. Greater fertility in heifers homozygous for rs109815800 could be due to an earlier age at puberty and/or an increased propensity for embryo survival. The latter would mean that homozygous heifers require fewer matings to achieve pregnancy; typical embryo loss in cattle is in the order of 40% to 60%. Another PLAG1 variant, rs109231213, appears to be associated with central mechanisms of puberty in heifers (Fortes et al., 2013, 2016; DeAtley et al., 2018). Based on the information provided in this review, it is plausible that PLAG1/PLAG1 have a role in embryonic development and survival in cattle. This is supported by the important roles of IGF2 in follicles/oocytes, embryos, and fetuses, and the regulation of IGF2/IGF2 by PLAG1. A role in central mechanisms associated with puberty in cattle is also plausible.
Notwithstanding the body of evidence that links PLAG1/PLAG1 with IGF2 and embryonic development and reproduction generally, it is noted that some of the relationships in this review could be considered associations and further research is needed to demonstrate additional cause-and-effect relationships.
Summary
The present review has looked at the emerging roles of PLAG1/PLAG1 in embryonic development, placentation, and fetal growth. The most notable expression of PLAG1 occurs during embryonic and fetal development, with lesser expression after birth. Overexpression of PLAG1 is associated with the large calf syndrome in cattle and under-expression is linked to fetal growth restriction in cattle and humans. The overexpression of PLAG1 later in life is typically associated with the formation of solid tumors and cancers. Hence, the expression of PLAG1 is finely balanced, and disruption in expression at different stages in life shifts PLAG1 from having beneficial effects to adverse outcomes. PLAG1/PLAG1 influence the expression of the IGF2 gene, and the IGF2 protein is an important mitogen in reproduction. The PLAG1-IGF2 axis, therefore, provides a mechanistic basis for an effect of PLAG1 on ovarian follicles/oocytes, embryos, and fetuses. Our own work involving the selection of Brahman (Bos indicus) female cattle for fertility over a period of 35 yr has led to a herd in which heifers homozygous for the PLAG1 variant, rs109815800, have greater fertility than contemporary heifers with either one or no copy of the variant (Collins A. Snr, J. E. Kinder, B. J. Hayes, and M. J. D’Occhio, unpublished). PLAG1/PLAG1 would therefore appear to have important roles in embryonic development and pregnancy in cattle similar to other mammals.
Acknowledgments
We thank the many postgraduates who contributed generously to research and thinking that is included in this article. We also sincerely thank Dr Fábio de Moraes Francisco for producing the figures.
Glossary
Abbreviations
- CBV
Collins Belah Valley
- CHCHD7
coiled-coil-helix-coiled-coil-helix domain containing 7
- EBV
estimated breeding value
- IGF2
insulin-like growth factor 2
- LIF
leukemia inhibitory factor
- PLAG1
pleomorphic adenoma gene 1
Contributor Information
Michael J D’Occhio, School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Sydney, NSW, Australia.
Giuseppe Campanile, Department of Veterinary Medicine and Animal Production, University of Naples Federico II, Naples, Italy.
Pietro S Baruselli, Faculty of Veterinary Medicine and Animal Science, Department of Animal Reproduction, University of Sao Paulo, Sao Paulo, Brazil.
Laercio R Porto Neto, CSIRO, Agriculture and Food, Brisbane, QLD, Australia.
Ben J Hayes, Queensland Alliance for Agriculture and Food Innovation, The University of Queensland, Brisbane, QLD, Australia.
Alf Collins Snr, CBV Brahman, Marlborough, Central Queensland, QLD, Australia.
Marina R S Fortes, School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD, Australia.
Conflict of interest statement
Alf Collins Snr is a Brahman seedstock producer and the owner of cattle ranch Collins Belah Valley, Marlborough, Central Queensland. All other authors declare no real or perceived conflicts of interest.
Literature Cited
- Aad, P. Y., S. E. Echternkamp, and L. J. Spicer. 2013. Possible role of IGF2 receptors in regulating selection of 2 dominant follicles in cattle selected for twin ovulations and births. Domest Anim. Endocrinol. 45:187–195. doi: 10.1016/j.domaniend.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Abdollahi, A. 2007. Lot1 (Zac1/PLAGL1) and its family members: mechanisms and functions. J. Cell. Physiol. 210:16–25. doi: 10.1002/jcp.20835 [DOI] [PubMed] [Google Scholar]
- Abdollahi, A., D. Roberts, A. K. Godwin, D. C. Schultz, G. Sonoda, J. R. Testa, and T. C. Hamilton. 1997a. Identification of a zinc-finger gene at 6q25: a chromosomal region implicated in development of many solid tumors. Oncogene 14:1973–1979. doi: 10.1038/sj.onc.1201034 [DOI] [PubMed] [Google Scholar]
- Abdollahi, A., A. K. Godwin, P. D. Miller, L. A. Getts, D. C. Schultz, T. Taguchi, J. R. Testa, and T. C. Hamilton. 1997b. Identification of a gene containing zinc-finger motifs based on lost expression in malignantly transformed rat ovarian surface epithelial cells. Cancer Res. 57:2029–2034. [PubMed] [Google Scholar]
- Adhami, H. A., B. Evano, A. Le Digarcher, C. Gueydan, E. Dubois, H. Parrinello, C. Dantec, T. Bouschet, A. Varrault, and L. Journot. 2015. A systems-level approach to parental genomic imprinting: the imprinted gene network includes extracellular matrix genes and regulates cell cycle exit and differentiation. Genet. Res. 25:353–367. doi: 10.1101/gr.175919.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnani, L., R. Dixit, X. Chen, A. Balakrishnan, H. Modi, Y. Touahri, C. Logan, and C. Schuurmans. 2018. Plag1 and Plagl2 have overlapping and distinct functions in telencephalic development. Biol. Open. 7:bio038661. doi: 10.1242/bio.038661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrogiannis, G. D., S. Sifakis, E. S. Patsouris, and A. E. Konstantinidou. 2014. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol. Med. Rep. 10:579–584. doi: 10.3892/mmr.2014.2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera, C., A. E. Velásquez, Y. Wong, M. A. Gutierrez-Reinoso, J. Cabezas, B. Melo-Baez, F. Castro, and L. Rodriguez-Álvarez. 2022. Preimplantation bovine embryos secrete extracellular vesicles that participate in embryo-maternal communication. Reprod. Fertil. Dev. 34:234–234. doi: 10.1071/rdv34n2ab1 [DOI] [PubMed] [Google Scholar]
- Akhtar, M., C. Holmgren, A. Göndör, M. Vesterlund, C. Kanduri, C. Larsson, and T. J. Ekström. 2012. Cell type and context-specific function of PLAG1 for IGF2 P3 promotor activity. Int. J. Oncol. 41:1959–1966. doi: 10.3892/ijo.2012.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp, J., F. Persson, M. Kost-Alimova, and G. Stenman. 2006. CHCHD7-PLAG1 and TCEA1-PLAG1 gene fusions resulting from cryptic, intrachromosomal 8q rearrangements in pleomorphic salivary gland adenomas. Genes Chromosomes Cancer. 45:820–828. doi: 10.1002/gcc.20346 [DOI] [PubMed] [Google Scholar]
- Åström, A. K., M. L. Voz, K. Kas, E. Röijer, B. Wedell, N. Mandahl, W. Van de Ven, J. Mark, and G. Stenman. 1999. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: identification of SII as a new fusion partner gene. Cancer Res. 59:918–923. [PubMed] [Google Scholar]
- Barlow, D. P., and M. S. Bartolomei. 2014. Genomic imprinting in mammals. Cold Spring Harbor Pers. Biol. 6:a018382. doi: 10.1101/cshperspect.a018382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten, S. C., S. M. Convissar, A. M. Zamah, M. A. Fierro, N. J. Winston, B. Scoccia, and C. Stocco. 2015. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J. Clin. Endocrinol. Metab. 100:E1046–E1055. doi: 10.1210/jc.2015-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman, D., M. Halje, M. Nordin, and W. Engström. 2013. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 59:240–249. doi: 10.1159/000343995 [DOI] [PubMed] [Google Scholar]
- Berkowicz, E. W., D. A. Magee, K. M. Sikora, D. P. Berry, D. J. Howard, M. P. Mullen, R. D. Evans, C. Spillane, and D. E. MacHugh. 2010. Single nucleotide polymorphisms at the imprinted bovine insulin-like growth factor 2 (IGF2) locus are associated with dairy performance in Irish Holstein-Friesian cattle. J. Dairy Res. 78:1–8. doi: 10.1017/S0022029910000567 [DOI] [PubMed] [Google Scholar]
- Boitard, S., M. Boussaha, A. Capitan, D. Rocha, and B. Servin. 2016. Uncovering adaptation from sequence data: lessons from genome resequencing for four cattle breeds. Genetics 203:433–450. doi: 10.1534/genetics.115.181594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolormaa, S., J. E. Pryce, Y. Zhang, A. Reverter, W. Barendse, B. J. Hayes, and M. E. Goddard. 2015. Non-adaptive genetic variation in growth, carcass and fertility traits in beef cattle. Genet. Sel. Evol. 47:26. doi: 10.1186/s12711-015-0114-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman, A. C., H. D. Daetwyler, A. J. Chamberlain, C. Hurtado Ponce, M. Sargolzaei, F. Schenkel, G. Sahana, A. Govignon-Gion, S. Boitard, M. Dolezal, et al. 2018. Meta-analysis of genome-wide association studies for cattle stature identifies common genes that regulate body size in mammals. Nat. Genet. 50:362–367. doi: 10.1038/s41588-018-0056-5 [DOI] [PubMed] [Google Scholar]
- Braem, C. V., K. Kas, E. Meyen, M Debiec-Rychter, W. J. M. Van de Vent, M. L. Voz. 2002. Identification of a karyopherin α2 recognition site in PLAG1, which functions as a nuclear localization signal. J. Biol. Chem. 277:19673–19678. doi: 10.1074/jbc.m112112200 [DOI] [PubMed] [Google Scholar]
- Brogan, R. S., S. Mix, M. Puttabyatappa, C. A. Van de Voort, and C. L. Chaffin. 2010. Expression of the IGF and insulin systems in the luteinizing macaque ovarian follicle. Fertil. Steril. 93:1421–1429. doi: 10.1016/j.fertnstert.2008.12.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajas, Y. N., K. Cañon-Beltrán, M. G. M. de la Blanca, J. M. Sánchez, B. Fernandez-Fuertes, E. M. González, and D. Rizos. 2022. Role of reproductive fluids and extracellular vesicles in embryo-maternal interaction during early pregnancy in cattle. Reprod. Fertil. Dev. 34:117–138. doi: 10.1071/RD21275 [DOI] [PubMed] [Google Scholar]
- Campanile, G., P. S. Baruselli, A. Limone, and M. J. D’Occhio. 2021. Local action of cytokines and immune cells in communication between the conceptus and uterus during the critical period of early embryo development, attachment and implantation - implications for embryo survival in cattle: a review. Theriogenology. 167:1–12. doi: 10.1016/j.theriogenology.2021.02.020 [DOI] [PubMed] [Google Scholar]
- Chen, Z., D. E. Hagen, C. G. Elsik, T. Ji, C. J. Morris, L. E. Moon, and R. M. Rivera. 2015. Characterization of global loss of imprinting in fetal growth syndrome induced by assisted reproduction. Proc. Nat. Acad. Sci. 112:4618–4623. doi: 10.1073/pnas.1422088112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constância, M., M. Hemberger, J. Hughes, W. Dean, A. Ferguson-Smith, R. Fundele, F. Stewart, G. Kelsey, A. Fowden, C., Sibley, et al. 2002. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417:945–948. doi: 10.1038/nature00819 [DOI] [PubMed] [Google Scholar]
- Curchoe, C., S. Zhang, Y. Bin, X. Zhang, L. Yang, D. Feng, M. O’Neill, and X. C. Tian. 2005. Promoter-specific expression of the imprinted IGF2 gene in cattle (Bos taurus). Biol. Reprod. 73:1275–1281. doi: 10.1095/biolreprod.105.044727 [DOI] [PubMed] [Google Scholar]
- D’Occhio, M. J., P. S. Baruselli, and G. Campanile. 2019a. Influence of nutrition, body condition and metabolic status on reproduction in female cattle: a review. Theriogenology. 125:277–284. doi: 10.1016/j.theriogenology.2018.11.010 [DOI] [PubMed] [Google Scholar]
- D’Occhio, M. J., G. Campanile, L. Zicarelli, J. A. Visintin, and P. S. Baruselli. 2019b. Adhesion molecules in gamete transport, fertilization, early embryonic development, and implantation – role in establishing a pregnancy in cattle: a review. Mol. Reprod. Dev. 87:206–222. doi: 10.1002/mrd.23312 [DOI] [PubMed] [Google Scholar]
- D’Occhio, M. J., G. Campanile, and P. S. Baruselli. 2020a. Transforming growth factor-β superfamily and interferon-τ in ovarian function and embryo development in female cattle: a review of biology and application. Reprod. Fertil. Dev. 32:539–352. doi: 10.1071/rd19123 [DOI] [PubMed] [Google Scholar]
- D’Occhio, M. J., G. Campanile, and P. S. Baruselli. 2020b. Peripheral action of kisspeptin at reproductive tissues - role in ovarian function and embryo implantation and relevance to assisted reproductive technology in livestock: a review. Biol. Reprod. 103:1157–1170. doi: 10.1093/biolre/ioaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean, W., F. Santos, M. Stojkovic, V. Zakhartchenko, J. Walter, E. Wolf, and W. Reik. 2001. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc. Natl. Acad. Sci. U.S.A. 98:13734–13738. doi: 10.1073/pnas.241522698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAtley, K. L., M. L. Colgrave, A. Cánovas, G. Wijffels, R. L. Ashley, G. A. Silver, G. Rincon, J. F. Medrano, A. Islas-Trejo, M. R. S. Fortes, et al. 2018. Neuropeptidome of the hypothalamus and pituitary gland of Indicine × Taurine heifers: evidence of differential neuropeptide processing in the pituitary gland before and after puberty. J. Prot. Res. 17:1852–1865. doi: 10.1021/acs.jproteome.7b00875 [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter, M., I. Van Valckenborgh, C. V. Broeck, A. Hagemeijer, W. J. M. Van de Ven, K. Kas, B. Van Damme, and M. L. Voz. 2001. Histologic localization of PLAG1 (pleomorphic adenoma gene 1) in pleomorphic adenoma of the salivary gland: cytogenetic evidence of common origin of phenotypically diverse cells. Lab. Invest. 81:1289–1291. doi: 10.1038/labinvest.3780342 [DOI] [PubMed] [Google Scholar]
- DeChiara, T. M., E. J. Robertson, and A. Efstratiadis. 1991. Parental imprinting of the mouse insulin-like growth factor II. Cell 64:849–859. doi: 10.1016/0092-8674(91)90513-x [DOI] [PubMed] [Google Scholar]
- Declercq, J., F. Van Dyck, C. V. Braem, I. C. Van Valckenborgh, M. Voz, M. Wassef, L. Schoonjans, W. Van Damme, L. Fiette, and W. J. M. Van de Ven. 2005. Salivary gland tumors in transgenic mice with targeted PLAG1 proto-oncogene overexpression. Can. Res. 65:4544–4553. doi: 10.1158/0008-5472.can-04-4041 [DOI] [PubMed] [Google Scholar]
- Dindot, S. V., P. W. Farin, C. E. Farin, J. Romano, S. Walker, C. Long, and J. A. Piedrahita. 2004. Epigenetic and genomic imprinting analysis in nuclear transfer derived Bos gaurus/Bos taurus hybrid fetuses. Biol. Reprod. 71:470–478. doi: 10.1095/biolreprod.103.025775 [DOI] [PubMed] [Google Scholar]
- Diskin, M. G., S. M. Waters, M. H. Parr, and D. A. Kenny. 2016. Pregnancy losses in cattle: potential for improvement. Reprod. Fertil. Dev. 28:83–93. doi: 10.1071/RD15366 [DOI] [PubMed] [Google Scholar]
- Duan, J. E., Z. C. Jiang, F. Alqahtan, I. Mandolu, H. Dong, X. Zheng, S. L. Marjani, J. Chen, and X. C. Tian. 2019. Methylome dynamics of bovine gametes and in vitro early embryos. Front. Genet. 10:512. doi: 10.3389/fgene.2019.00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle, B. N., and B. J. Hayes. 2022. Genetic variation in PLAG1 is associated with early fertility in Australian Brahman cattle. J. Anim. Sci. 100:1–8. doi: 10.1093/jas/skac084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink, T., K. Tiplady, T. Lopdell, T. Johnson, R. G. Snell, R. J. Spelman, S. R. Davis, and M. D. Littlejohn. 2017. Functional confirmation of PLAG1 as the candidate causative gene underlying major pleiotropic effects on body weight and milk characteristics. Sci. Rep. 7:44793. doi: 10.1038/srep44793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes, M. R. S., K. Kemper, S. Sasazaki, A. Reverter, J. E. Pryce, W. Barendse, R. Bunch, R. McCulloch, B. Harrison, S. Bolormaa, et al. 2013. Evidence for pleiotropism and recent selection in the PLAG1 region in Australian beef cattle. Anim. Genet. 44:636–647. doi: 10.1111/age.12075 [DOI] [PubMed] [Google Scholar]
- Fortes, M. R. S., L. T. Nguyen, L. R. P. Neto, A. Reverter, S. S. Moore, S. A. Lehnert, and M. G. Thomas. 2016. Polymorphisms and genes associated with puberty in heifers. Theriogenology. 86:333–339. doi: 10.1016/j.theriogenology.2016.04.046 [DOI] [PubMed] [Google Scholar]
- Gabory, A., M. A. Ripoche, A. Le Digarcher, F. Watrin, A. Ziyyat, T. Forné, H. Jammes, J. F. X. Ainscough, M. A. Surani, L. Journot, et al. 2009. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development 136:3413–3421. doi: 10.1242/dev.036061 [DOI] [PubMed] [Google Scholar]
- Gad, A., M. Hoelker, U. Besenfelder, V. Havlicek, U. Cinar, F. Rings, E. Held, I., Dufort, M. A. Sirard, K. Schellander, et al. 2012. Molecular mechanisms and pathways involved in bovine embryonic genome activation and their regulation by alternative in vivo and in vitro culture conditions. Biol. Reprod. 87:1–13. doi: 10.1095/biolreprod.112.099697 [DOI] [PubMed] [Google Scholar]
- Gebert, C., C. Wrenzycki, D. Herrmann, D. Gröger, R. Reinhardt, P. Hajkova, A. Lucas-Hahn, J. Carnwath, H. Lehrach, and H. Niemann. 2006. The bovine IGF2 gene is differentially methylated in oocyte and sperm DNA. Genomics 88:222–229. doi: 10.1016/j.ygeno.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Gebert, C., C. Wrenzycki, D. Herrmann, D. Gröger, J. Thiel, R. Reinhardt, H. Lehrach, P. Hajkova, A. Lucas-Hahn, J. W. Carnwath, et al. 2009. DNA methylation in the IGF2 intragenic DMR is re-established in a sex-specific manner in bovine blastocysts after somatic cloning. Genomics 94:63–69. doi: 10.1016/j.ygeno.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Giannoukakis, N., C. Deal, J. Paquette, C. G. Goodyer, and C. Polychronakos. 1993. Parental genomic imprinting of the human IGF2 gene. Nat. Genet. 4:98–101. doi: 10.1038/ng0593-98 [DOI] [PubMed] [Google Scholar]
- Graf, A., S. Krebs, M. Heininen-Brown, V. Zakhartchenko, H. Blum, and E. Wolf. 2014a. Genome activation in bovine embryos: review of the literature and new insights from RNA sequencing experiments. Anim. Reprod. Sci. 149:46–58. doi: 10.1016/j.anireprosci.2014.05.016 [DOI] [PubMed] [Google Scholar]
- Graf, A., S. Krebs, V. Zakhartchenko, B. Schwalb, H. Blum, and E. Wolf. 2014b. Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl. Acad. Sci. U.S.A. 111:4139–4144. doi: 10.1073/pnas.1321569111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli, O., U. A. Kayisli, and H. S. Taylor. 2009. The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin. Reprod. Med. 27:62–79. doi: 10.1055/s-0028-1108011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib, W. A., F. Brioude, T. Edouard, J. T. Bennett, A. Lienhardt-Roussie, F. Tixier, J. Salem, T. Yuen, S. Azzi, Y. Le Bouc, et al. 2018. Genetic disruption of the oncogenic HMGA2-PLAG1-IGF2 pathway causes fetal growth restriction. Genet. Med. 20:250–258. doi: 10.1038/gim.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead, M. M., X. Ma, C. Zhou, R. M. Schultz, and P. J. Ross. 2020. Chromatin remodeling in bovine embryos indicates species-specific regulation of genome activation. Nat. Commun. 11:4654. doi: 10.1038/s41467-020-18508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, D. W., S. J. Song, J. Uhum, J. T. Do, N. H. Kim, K. S. Chung, and H. T. Lee. 2003. Expression of IGF2 and IGF receptor mRNA in bovine nuclear transferred embryos. Zygote 11:245–252. doi: 10.1017/s0967199403002296 [DOI] [PubMed] [Google Scholar]
- Hansen, P. J. 2020. The incompletely fulfilled promise of embryo transfer in cattle – why aren’t pregnancy rates greater and what can we do about it? J. Anim. Sci. 98:1–20. doi: 10.1093/jas/skaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantak, A. M., I. C. Bagchi, and M. K. Bagchi. 2014. Role of uterine stromal-epithelial crosstalk in embryo implantation. International J. Dev. Biol. 58:139–146. doi: 10.1387/ijdb.130348mb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawken, R. J., J. D. Zhang, M. R. S. Fortes, E. Collis, W. C. Barris, N. J. Corbet, P. J. Williams, G. Fordyce, R. G. Holroyd, J. R. W. Walkley, et al. 2012. Genome-wide association studies of female reproduction in tropically adapted beef cattle. J. Anim. Sci. 90:1398–1410. doi: 10.2527/jas.2011-4410 [DOI] [PubMed] [Google Scholar]
- Hay, E. H., and A. Roberts. 2018. Genome-wide association study for carcass traits in a composite beef cattle. Liv. Sci. 213:35–43. doi: 10.1016/j.livsci.2018.04.018 [DOI] [Google Scholar]
- Hensen, K., I. C. C. Van Valckenborgh, K. Kas, W. J. M. Van de Ven, and M. L. Voz. 2002. The tumorigenic diversity of the three PLAG family members is associated with different DNA binding capacities. Can. Res. 62:1510–1617. [PubMed] [Google Scholar]
- Hensen, K., C. Braem, J. Declercq, F. Van Dyck, M. Dewerchin, L. Fiette, C. Denef, and W. J. M. Van de Ven. 2004. Targeted disruption of the murine Plag1 proto-oncogene causes growth and reduced fertility. Dev. Growth Differ. 46:459–470. doi: 10.1111/j.1440-169x.2004.00762.x [DOI] [PubMed] [Google Scholar]
- Hoshiba, H., K. Setoguchi, T. Watanabe, A. Kinoshita, K. Mizoshita, Y. Sugimoto, and A. Takasuga. 2013. Comparison of the effects explained by variation in the bovine PLAG1 and NCAPG genes on the daily body weight gain, linear skeletal measurements and carcass traits in Japanese Black steers from a progeny testing program. Anim. Sci. J. 84:529–534. doi: 10.1111/asj.12033 [DOI] [PubMed] [Google Scholar]
- Hou, J., K. Qu, P. Jia, Q. Hanif, J. Zhang, N. Chen, R. Dang, H. Chen, B. Huang, and C. Lei. 2019. A SNP in PLAG1 is associated with body height trait in Chinese cattle. Anim. Genet. 51:87–90. doi: 10.1111/age.12872 [DOI] [PubMed] [Google Scholar]
- Hunter, M. G., R. S. Robinson, G. E. Mann, and R. Webb. 2004. Endocrine and paracrine control of follicular development and ovulation rate in farm animals. Anim. Reprod. Sci. 82-83:461–477. doi: 10.1016/j.anireprosci.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Ivanova, E., S. Canovas, S. Garcia-Martinez, R. Romar, J. S. Lopes, D. Rizos, M. J. Sanchez-Calabuig, F. Krueger, S. Andrews, F. Perez-Sanz, et al. 2020. DNA methylation changes during preimplantation development reveals interspecies differences and reprogramming events at imprinted genes. Clin. Epigenetics. 12:64. doi: 10.1186/s13148-020-00857-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z., H. Dong, X. Zheng, S. L. Marjani, D. M. Donovan, J. Chen, and X. C. Tian. 2015. mRNA levels of imprinted genes in bovine in vivo oocytes, embryos and cross-species comparisons with humans, mice and pigs. Sci. Rep. 5:17898. doi: 10.1038/srep17898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Z., J. Lin, H. Dong, X. Zheng, S. L. Marjani, J. Duan, Z. Ouyang, J. Chen, and X. C. Tian. 2018. DNA methylomes of bovine gametes and in vivo produced preimplantation embryos. Biol. Reprod. 99:949–959. doi: 10.1093/biolre/ioy138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam, D., A. M. Shariati, and J. M. Skotheim. 2017. Zygotic genome activation in vertebrates. Dev. Cell 42:316–332. doi: 10.1016/j.devcel.2017.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juma, A. R., P. E. Damdimopoulou, S. V. H. Grommen, W. J. M. Van de Ven, and B. De Groef. 2016. Emerging role of PLAG1 as a regulator of growth and reproduction. J. Endocrinol. 228:R45–R56. doi: 10.1530/joe-15-0449 [DOI] [PubMed] [Google Scholar]
- Juma, A. R., S. V. H. Grommen, M. K. O’Bryan, A. E. O’Connor, D. J. Merriner, N. E. Hall, S. R. Doyle, P. E. Damdimopoulou, D. Barriga, A. H. Hart, et al. 2017. PLAG1 deficiency impairs spermatogenesis and sperm motility in mice. Sci. Rep. 7:5317. doi: 10.1038/s41598-017-05676-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juma, A. R., N. E. Hall, J. Wong, J. G. Gasperoni, Y. Watanabe, A. Sahota, P. E. Damdimopoulou, S. V. H. Grommen, and B. De Groef. 2018. PLAG1 expression and target genes in the hypothalamo-pituitary system in male mice. Mol. Cell. Endocrinol. 478:77–83. doi: 10.1016/j.mce.2018.07.009 [DOI] [PubMed] [Google Scholar]
- Kaňka, J., A. Bryova, V. Duranthon, J. F. Oudin, N. Peynot, and J. P. Renard. 2003. Identification of differentially expressed mRNAs in bovine preimplantation embryos. Zygote 11:43–52. doi: 10.1017/s0967199403001060 [DOI] [PubMed] [Google Scholar]
- Karim, L., H. Takeda, L. Lin, T. Druet, J. A. C. Arias, D. Baurain, N. Cambisano, S. R. Davis, F. Farnir, B. Grisart, et al. 2011. Variants modulating the expression of a chromosomal domain encompassing PLAG1 influence bovine stature. Nat. Genet. 43:405–413. doi: 10.1038/ng.814 [DOI] [PubMed] [Google Scholar]
- Kas, K., E. Roijer, M. Voz, E. Meyen, G. Stenman, and W. J. M. Van de Ven. 1997a. A 2-Mb YAC contig and physical map covering the chromosome 8q12 breakpoint cluster region in pleomorphic adenomas of the salivary glands. Genomics 43:349–358. doi: 10.1006/geno.1997.4819 [DOI] [PubMed] [Google Scholar]
- Kas, K., M. L. Voz, E. Röijer, A. K. Åström, E. Meyen, G. Stenman, and W. J. M. Van de Ven. 1997b. Promoter swapping between the genes for a novel zing finger protein and β-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat. Genet. 15:170–174. doi: 10.1038/ng0297-170 [DOI] [PubMed] [Google Scholar]
- Kas, K., M. L. Voz, K. Hensen, E. Meyen, and W. J. M. Van de Ven. 1998. Transcriptional activation capacity of the novel PLAG family of zinc finger proteins. J. Biol. Chem. 273:23026–23032. doi: 10.1074/jbc.273.36.23026 [DOI] [PubMed] [Google Scholar]
- Kochhar, P., M. Vukku, R. Rajashekhar, and A. Mukhopadhyay. 2021. microRNA signatures associated with fetal growth restrictions: a systematic review. Eur. J. Clin. Nutr. 76:1088–1102. doi: 10.1038/s41430-021-01041-x [DOI] [PubMed] [Google Scholar]
- Lafontaine, S., R. Labrecque, J. M. Palomino, P. Blondin, and M. A. Sirard. 2020. Specific imprinted genes demethylation in association with oocyte donor’s age and culture conditions in bovine embryos assesses at day 7 and 12 post insemination. Theriogenology. 158:321–330. doi: 10.1016/j.theriogenology.2020.09.027 [DOI] [PubMed] [Google Scholar]
- Lavagi, I., S. Krebs, K. Simmet, A. Beck, V. Zakhartchenko, E. Wolf, and H. Blum. 2018. Single-cell RNA sequencing reveals developmental heterogeneity of blastomeres during major genome activation in bovine embryos. Sci. Rep. 8:4071. doi: 10.1038/s41598-018-22248-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., X. Wang, H. Chen, L. Qu, and X. Lan. 2020a. A 17-bpInDel(rs668420586) within goat CHCHD7 gene located in the growth-related QTL affecting body measurement traits. 3 Biotech. 10:441. doi: 10.1007/s13205-020-02434-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., M. Wu, H. Zhao, L. Fan, Y. Zhang, T. Yuan, S. He, P. Wang, Y. Zhang, X. Sun, et al. 2020b. The PLAG1 mRNA expression analysis among genetic variants and relevance to growth traits in Chinese cattle. Anim. Biotechnol. 31:504–511. doi: 10.1080/10495398.2019.1632207 [DOI] [PubMed] [Google Scholar]
- Littlejohn, M., T. Grala, K. Sanders, C. Walker, G. Waghorn, K. Macdonald, W. Coppieters, M. Georges, R. Spelman, E. Hillerton, et al. 2011. Genetic variation in PLAG1 associates with early life body weight and peripubertal weight and growth in Bos taurus. Anim. Genet. 43:591–594. doi: 10.1111/j.1365-2052.2011.02293.x [DOI] [PubMed] [Google Scholar]
- Liu, J., Q. Xiao, J. Xiao, C. Niu, Y. Li, X. Zhang, Z. Zhou, G. Shu, and G. Yin. 2022. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 7:3. doi: 10.1038/s41392-021-00762-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobat, L. 2021. Pluripotency and growth factors in early embryonic development of mammals: a comparative approach. Vet. Sci. 8:78. doi: 10.3390/vetsci8050078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, K. N., J. N. Drum, A. Z. Balboula, C. M. Spinka, T. E. Spencer, and M. S. Ortega. 2023. Sire modulates developmental kinetics and transcriptome of bovine embryo. Reproduction. 166:337–348. doi: 10.1530/REP-23-0030 [DOI] [PubMed] [Google Scholar]
- Maccani, M. A., and C. J. Marsit. 2011. Exposure and fetal growth-associated miRNA alterations in the human placenta. Clin Epigenetics. 2:401–404. doi: 10.1007/s13148-011-0046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madissoon, E., A. Damdimopoulos, S. Katayama, K. Krjutškov, E. Einarsdottir, K. Mamia, B. De Groef, O. Hovatta, J. Kere, and P. Damdimopoulou. 2019. Pleomorphic adenoma gene 1 is needed for timely zygotic genome activation and early embryo development. Sci. Rep. 9:8411. doi: 10.1038/s41598-019-44882-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama, A., M. Hisaoka, and H. Hashimoto. 2011. PLAG1 expression in cutaneous mixed tumors: an immunohistochemical and molecular genetic study. Virchows Arch. 459:539–545. doi: 10.1007/s00428-011-1149-z [DOI] [PubMed] [Google Scholar]
- Meirelles, F. V., A. R. Caetano, Y. F. Wantanabe, P. Ripamonte, S. F. Carambula, G. K. Merighe, and S. M. Garcia. 2004. Genome activation and developmental block in bovine embryos. Anim. Reprod. Sci. 8:13–20. doi: 10.1016/j.anireprosci.2004.05.012 [DOI] [PubMed] [Google Scholar]
- Memili, E., and N. L. First. 1999. Control of gene expression at the onset of bovine embryonic development. Biol. Reprod. 61:1198–1207. doi: 10.1095/biolreprod61.5.1198 [DOI] [PubMed] [Google Scholar]
- Memili, E., and N. L. First. 2000. Zygotic and embryonic gene expression in cow: a review of timing and mechanisms of early gene expression as compared with other species. Zygote 8:87–96. doi: 10.1017/s0967199400000861 [DOI] [PubMed] [Google Scholar]
- Memili, E., T. Dominko, and N. L. First. 1998. Onset of transcription in bovine oocytes and preimplantation embryos. Mol. Reprod. Dev. 51:36–41. doi: [DOI] [PubMed] [Google Scholar]
- Moore, T., and D. Haig. 1991. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 7:45–49. doi: 10.1016/0168-9525(91)90230-N [DOI] [PubMed] [Google Scholar]
- Mota, L. F. M., F. B. Lopes, G. A. F. Júnior, G. J. M. Rosa, A. F. B. Magalhães, R. Carvalheiro, and L. G. Albuquerque. 2020. Genome-wide scan highlights the role of candidate genes on phenotypic plasticity for age at first calving in Nellore heifers. Sci. Rep. 10:6481. doi: 10.1038/s41598-020-63516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki, T., J. Terakawa, H. Karakama, M. Noguchi, H. Murakami, Y. Hasegawa, O. Ohara, T. Daikoku, J. Ito, and N. Kashiwazaki. 2023. Uterine epithelial Gp130 orchestrates hormone response and epithelial remodeling for successfulembryo attachment in mice. Sci. Rep. 13:854. doi: 10.1038/s41598-023-27859-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neirijnck, Y., M. D. Ppaioannou, and S. Nef. 2019. The insulin/IGF system in mammalian sexual development and reproduction. Int. J. Mol. Sci. 20:4440. doi: 10.3390/ijms20184440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann, H., and C. Wrenzycki. 1999. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 53:21–34. doi: 10.1016/s0093-691x(99)00237-x [DOI] [PubMed] [Google Scholar]
- Nishimura, S., T. Watanabe, K. Mizoshita, K. Tatsuda, T. Fujita, N. Watanabe, Y. Sugimoto, and A. Takasuga. 2012. Genome-wide association study identified three major QTL for carcass weight including the PLAG1-CHCHD7 QTN for stature in Japanese Black cattle. BMC Genet. 13:40. doi: 10.1186/1471-2156-13-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell, S. D., and I. N. M. Day. 1998. Molecules in focus insulin-like growth factor II (IGF-II). Int. J. Biochem. Cell Biol. 30:767–771. doi: 10.1016/S1357-2725(98)00048-X [DOI] [PubMed] [Google Scholar]
- O’Doherty, A. M., L. C. O’Shea, and T. Fair. 2012. Bovine DNA methylation imprints are established in an oocyte size-specific manner, which are coordinated with the expression of the DNMT3 family proteins. Biol. Reprod. 86:1–10. doi: 10.1095/biolreprod.111.094946 [DOI] [PubMed] [Google Scholar]
- O’Doherty, A. M., D. A. Magee, L. C. O’Shea, N. Forde, M. E. Beltram, S. Mamo, and T. Fair. 2015. DNA methylation dynamics at imprinted genes during bovine pre-implantation embryo development. BMC Dev. Biol. 15:13. doi: 10.1186/s12861-015-0060-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa, M., M. Sakatani, J. Q. Yao, S. Shanker, F. Yu, R. Yamashita, S. Wakabayashi, K. Nakai, K. B. Dobbs, M. J. Sudano, et al. 2012. Global gene expression of the inner cell mass and trophectoderm of the bovine blastocyst. BMC Dev. Biol. 12:33. doi: 10.1186/1471-213x-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y., M. Wang, H. Wu, Z. Akhatayeva, X. Lan, P. Fei, C. Mao, and F. Jiang. 2022. Indel mutations of sheep PLAG1 gene and their associations with growth traits. Anim. Biotechnol. 33:1459–1465. doi: 10.1080/10495398.2021.1906265 [DOI] [PubMed] [Google Scholar]
- Pausch, H., K. Flisikowski, S. Jung, R. Emmerling, C. Edel, K. U. Götz, and R. Fries. 2011. Genome-wide association study identifies two major loci affecting calving ease and growth-related traits in cattle. Genetics 187:289–297. doi: 10.1534/genetics.110.124057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendeville, H., B. Peers, K. Kas, and M. L. Voz. 2006. Cloning and embryonic expression of zebrafish PLAG genes. Gene Expr. Patterns 6:267–276. doi: 10.1016/j.modgep.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Plassachaert, R. N., and M. S. Bartolomei. 2014. Genomic imprinting in development, growth, behavior and stem cells. Development 141:1805–1813. doi: 10.1242/dev.101428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Neto, L. R., A. Reverter, K. C. Prayaga, E. K. F. Chan, D. J. Johnston, R. J. Hawken, G. Fordyce, J. F. Garcia, T. S. Sonstegard, S. Bolormaa, et al. 2014. The genetic architecture of climatic adaptation of tropical cattle. PLoS One 9:e113284. doi: 10.1371/journal.pone.0113284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce, J. E., B. J. Hayes, S. Bolormaa, and M. E. Goddard. 2011. Polymorphic regions affecting human height also control stature in cattle. Genetics 187:981–984. doi: 10.1534/genetics.110.123943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa, I. A. S., M. S. Khatkar, P. C. Thomson, and H. W. Raadsma. 2015. Composite selection signals for complex traits exemplified through bovine stature using multibreed cohorts of European and African Bos taurus. G3 (Bethesda). 5:1391–1401. doi: 10.1534/g3.115.017772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos, D., V. Maillo, M. J. Sánchez-Calabuig, and P. Lonergan. 2017. The consequences of maternal-embryonic cross talk during the periconception period on subsequent embryonic development. Adv. Exp. Med. Biol. 1014:69–86. doi: 10.1007/978-3-319-62414-3_4 [DOI] [PubMed] [Google Scholar]
- Robertson, S. A., P. Y. Chin, J. G. Femia, and H. M. Brown. 2018. Embryonic cytokines – potential loss in embryo loss and fetal programming. J. Reprod. Immunol. 125:80–88. doi: 10.1016/j.jri.2017.12.003 [DOI] [PubMed] [Google Scholar]
- Robinson, R. S., G. E. Mann, T. S. Gadd, G. E. Lamming, and D. C. Wathes. 2000. The expression of the IGF system in the bovine uterus throughout the oestrous cycle and early pregnancy. J. Endocrinol. 165:231–243. doi: 10.1677/joe.0.1650231 [DOI] [PubMed] [Google Scholar]
- Binelli, M., F. A. C. C. Silva, C. C. Rocha, T. Martins, M. Sponchiado, V. Van Hoeck, A. Cordeiro, M. Campbell, J. L. M. R. Leroy, F. Peñagaricano, et al. 2022. Endometrial receptivity in cattle: the mutual reprogramming paradigm. Anim. Reprod. 19:e20220097. doi: 10.1590/1984-3143-AR2022-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddock, N. T., K. J. Wilson, M. A. Cooney, N. A. Korfiatis, R. T. Tecirlioglu, and A. J. French. 2004. Analysis of imprinted messenger RNA expression during bovine preimplantation development. Biol. Reprod. 70:1131–1135. doi: 10.1095/biolreprod.103.022236 [DOI] [PubMed] [Google Scholar]
- Saha, S., J. Choudhury, and R. Ain. 2015. MicroRNA-141-3p and miR-200a-3p regulate insulin-like growth factor 2 during mouse placental development. Mol. Cell. Endocrinol. 414:186–193. doi: 10.1016/j.mce.2015.07.030 [DOI] [PubMed] [Google Scholar]
- Sakai, H., Y. Fujii, N. Kuwayama, K. Kawaji, Y. Gotoh, and Y. Kishi. 2019. Plag1 regulates neuronal expression and neuronal differentiation of neocortical neuronal progenitor cells. Genes Cells. 24:650–666. doi: 10.1111/gtc.12718 [DOI] [PubMed] [Google Scholar]
- Sandovici, I., A. Georgopoulou, V. Pérez-Garcá, A. Hufnagel, J. López-Tello, B. Y. H. Lam, S. N. Schiefer, C. Gaudreau, F. Santos, K. Hoelle, et al. 2022. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev. Cell 57:63–79. doi: 10.1016/j.devcel.2021.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, S., T. Ibi, T. Wantanabe, T. Matsuhash, S. Ikeda, and Y. Sugimoto. 2013. Variants of the3’ UTR of general transcription factor IIF, polypeptide 2 affect female calving efficiency in Japanese Black cattle. BMC Genet. 14:41. doi: 10.1186/1471-2156-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri, A. N., I. Sandovici, M. Constancia, and A. L. Fowden. 2017. Placental phenotype and the insulin-like growth factors: resource allocation to fetal growth. J. Physiol. 595:5057–5093. doi: 10.1113/JP273330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skálová, A., A. Agaimy, T. Vanecek, M. Baněčková, J. Laco, N. Ptáková, P. Šteiner, H. Majewska, W. Biernat, L. Corcione, et al. 2021. Molecular profiling of clear cell myoepithelial carcinoma of salivary glands with EWSR1 rearrangement identifies frequent PLAG1 gene fusions but no EWSR1 fusion transcripts. Am. J. Surg. Pathol. 45:1–13. doi: 10.1097/PAS.0000000000001591 [DOI] [PubMed] [Google Scholar]
- Smith, L. C., J. Therrien, F. Fillion, F. Bressan, and F. V. Meirelles. 2015. Epigenetic consequences of artificial reproductive technologies to the bovine imprinted genes SNRPN, H19/IGF2, and IGF2R. Front. Genet. 6:58. doi: 10.3389/fgene.2015.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y., L. Xu, Y. Chen, L. Zhang, H. Gao, B. Zhu, H. Niu, W. Zhang, J. Xia, X. Gao, et al. 2016. Genome-wide association study reveals the PLAG1 gene for knuckle, biceps and shank weight in Simmental beef cattle. PLoS One 11:e0168316. doi: 10.1371/journal.pone.0168316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler, D., M. Villalba, A. Hoffmann, C. Pantaloni, S. Houssami, J. Bockaert, and L. Journot. 1997. Regulation of apoptosis and cell cycle arrest by Zac1, a novel zinc finger protein expressed in the pituitary gland and the brain. EMBO J. 16:2814–2825. doi: 10.1093/emboj/16.10.2814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer, L. J., and P. Y. Aad. 2007. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol. Reprod. 77:18–27. doi: 10.1095/biolreprod.106.058230 [DOI] [PubMed] [Google Scholar]
- Sponchiado, M., N. S. Gomes, P. K. Fonyes, T. Martins, M. Collado, A. A. Pastore, G. Pugliesi, M. F. G. Nogueira, and M. Binelli. 2017. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS One 12:e017954. doi: 10.1371/journal.pone.0175954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponchiado, M., A. M. Gonella-Diaza, C. C. Rocha, E. G. Lo Turco, G. Pugliesi, J. L. M. R. Leroy, and M. Binelli. 2019. The pre-hatching bovine embryo transforms the uterine luminal metabolite composition in vivo. 2019. Sci. Rep. 9:8354. doi: 10.1038/s41598-019-44590-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponchiado, M., W. F. A. Marei, G. T. S. Beemster, P. E. J. Bols, M. Binelli, and J. L. M. R. Leroy. 2020. Molecular interactions at the bovine embryo-endometrial epithelium interface. Reproduction. 160:887–903. doi: 10.1530/REP-20-0344 [DOI] [PubMed] [Google Scholar]
- Stepniak, E., G. L. Radice, and V. Vasioukhin. 2009. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb. Perspect. Biol. 1:a002949. doi: 10.1101/cshperspect.a002949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasuga, A. 2016. PLAG1 and NCAPG-LCORL in livestock. Anim. Sci. J. 87:159–167. doi: 10.1111/asj.12417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, Q., W. Wu, X. Xu, L. Huang, Q. Gao, H. Chen, H. Sun, Y. Xia, J. Sha, X. Wang, et al. 2013. miR-141 contributes to fetal growth restriction by regulating PLAG1 expression. PLoS One 8:e58737. doi: 10.1371/journal.pone.0058737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taye, M., W. Lee, S. Jeon, J. Yoon, T. Dessie, O. Hanotte, O. A. Mwai, S. Kemp, S. Cho, S. J. Oh, et al. 2017. Exploring evidence of positive selection signatures in cattle breeds selected for different traits. Mamm. Genome 28:528–541. doi: 10.1007/s00335-017-9715-6 [DOI] [PubMed] [Google Scholar]
- Telford, N. A., A. J. Watson, and G. A. Schultz. 1990. Transition from maternal to embryonic control in early mammalian development: a comparison of several species. Mol. Reprod. Dev. 26:90–100. doi: 10.1002/mrd.1080260113 [DOI] [PubMed] [Google Scholar]
- Tesfaye, D., N. Menjivar, and S. Gebremedhn. 2022. Current knowledge and the future potential of extracellular vesicles in mammalian reproduction. Reprod. Fert. Develop. 34:174–189. doi: 10.1071/rd21277 [DOI] [PubMed] [Google Scholar]
- Tkachenko, O. Y., S. Wolf, M. S. Lawson, A. Y. Ting, J. K. Rodrigues, F. Xu, C. V. Bishop, R. L. Stouffer, and J. Xu. 2021. Insulin-like growth factor 2 is produced by antral follicles and promotes preantral follicle development in macaques. Biol. Reprod. 104:602–610. doi: 10.1093/biolre/ioaa227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya, Y. T., A. S. do Carmo, R. Carvalheiro, H. H. R. Neves, M. C. Matos, L. B. Zavarez, A. M. P. O’Brien, J. Sölkner, J. C. McEwan, J. B. Cole, et al. 2013. Genome-wide association study for birth weight in Nellore cattle points to previously described orthologous genes affecting human and bovine height. BMC Genet. 14:52. doi: 10.1186/1471-2156-14-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsunomiya, Y. T., M. Milanesi, A. T. H. Utsunomiya, R. B. P. Torrecilha, E. S. Kim, M. S. Costa, T. S. Aguiar, S. Schroeder, A. S. do Carmo, R. Carvalheiro, et al. 2017. A PLAG1 mutation contributed to stature recovery in modern cattle. Sci. Rep. 7:17140. doi: 10.1038/s41598-017-17127-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta, T., G. Hausmann, and K. Basler. 2012. The many faces and functions of β-catenin. EMBO J. 31:2714–2736. doi: 10.1038/emboj.2012.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck, F., J. Declercq, C. V. Braem, and W. J. M. Van de Ven. 2007. PLAG1, the prototype of the PLAG gene family: versatility in tumour development (review). Int. J. Oncol. 30:765–774. doi: 10.3892/ijo.30.4.765 [DOI] [PubMed] [Google Scholar]
- Varrault, A., C. Gueydan, A. Delalbre, A. Bellmann, S. Houssami, C. Aknin, D. Severac, L. Chotard, M. Kahli, A. Le Digarcher, et al. 2006. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev. Cell 11:711–722. doi: 10.1016/j.devcel.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Visscher, P. M., and M. E. Goddard. 2011. Cattle gain stature. Nat. Genet. 43:397–398. doi: 10.1038/ng.819 [DOI] [PubMed] [Google Scholar]
- Voz, M. L., A. K. Åström, K. Kas, J. Mark, G. Stenman, and W. J. M. Van de Ven. 1998. The recurrent translocation t(5;8)(p13;q12) in pleomorphic adenomas results in upregulation of PLAG1 gene expression under control of the LIFR promoter. Oncogene 16:1409–1416. doi: 10.1038/sj.onc.1201660 [DOI] [PubMed] [Google Scholar]
- Voz, M. L., N. S. Agten, W. J. M. Van de Ven, and K. Kas. 2000. PLAG1, the main translocation target in pleomorphic adenoma of the salivary glands, is a positive regulator of IGF-II. Cancer Res. 60:106–113. [PubMed] [Google Scholar]
- Voz, M. L., J. Mathys, K. Hensen, H. Pendeville, I. Van Valckenborgh, C. Van Huffe, M. Chavez, B. Van Damme, B. De Moor, Y. Moreau, et al. 2004. Microarray screening for targets of the proto-oncogene PLAG1. Oncogene 23:179–191. doi: 10.1038/sj.onc.1207013 [DOI] [PubMed] [Google Scholar]
- Wagner, G., and J. Zhang. 2011. The pleiotropic structure of the genotype–phenotype map: the evolvability of complex organisms. Nat. Rev. Genet. 12:204–213. doi: 10.1038/nrg2949 [DOI] [PubMed] [Google Scholar]
- Wang, Y., W. Shang, X. Lei, S. Shen, H. Zhang, Z. Wang, L. Huang, Z. Yu, H. Ong, X. Yin, et al. 2013. Opposing functions of PLAG1 in pleomorphic adenoma: a microarray analysis of PLAG1 transgenic mice. Biotechnol. Lett. 35:1377–1385. doi: 10.1007/s10529-013-1213-7 [DOI] [PubMed] [Google Scholar]
- Wathes, D. C., G. E. Pollott, K. F. Johnson, H. Richardson, and J. S. Cooke. 2014. Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal. 8:91–104. doi: 10.1017/S1751731114000755 [DOI] [PubMed] [Google Scholar]
- Wei, Z., K. Wang, H. Wu, Z. Wang, C. Pan, H. Chen, and X. Lan. 2021D. etection of 15-bp deletion mutation within PLAG1 gene and its effects on growth traits in goats. Animals. 11:2064. doi: 10.3390/ani11072064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhelm, B. R., E. Ticiani, K. Campagnolo, G. B. de Oliveira, K. de Mattos, C. A. P. Bello, F. L. Ongaratto, P. Rodriguez-Villamil, L. Relly, J. P. M. Alves, et al. 2021. Promoter-specific expression of the imprinted IGF2 gene in bovine oocytes and pre-implantation embryos. Reprod. Dom. Anim. 56:857–863. doi: 10.1111/rda.13925 [DOI] [PubMed] [Google Scholar]
- Wong, J., A. R. Juma, S. C. Tran, J. G. Gasperoni, S. V. H. Grommen, and B. De Groef. 2020a. Deficiency of the transcription factor PLAG1 results in aberrant coiling and morphology of the epididymis. Asian J. Androl. 22:342–347. doi: 10.4103/aja.aja_87_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, J., A. Damdimopoulos, P. Damdimopoulou, J. G. Gasperoni, S. C. Tran, S. V. H. Grommen, B. De Groef, and S. Dworkin. 2020b. Transcriptome analysis of the epididymis from Plag1 deficient mice suggests dysregulation of sperm maturation and extracellular matrix genes. Dev. Dyn 249:1500–1513. doi: 10.1002/dvdy.254 [DOI] [PubMed] [Google Scholar]
- Wu, H., Y. Pan, Q. Zhang, Y. Cao, J. Li, H. Chen, Y. Cai, X. Sun, and X. Lan. 2019. Insertion/deletion (InDel) variations in sheep PLAG1 gene locating in growth-related major QTL are associated with adult body weight and morphometric traits. Small. Ruminant. Res. 178:63–69. doi: 10.1016/j.smallrumres.2019.08.003 [DOI] [Google Scholar]
- Xu, W., H. He, L. Zheng, J. W. Xu, C. Z. Lei, G. M. Zhang, R. H. Dang, H. Niu, X. L. Qi, H. Chen, et al. 2018. Detection of 19-bp deletion within PLAG1 gene and its effect on growth traits in cattle. Gene 675:144–149. doi: 10.1016/j.gene.2018.06.041 [DOI] [PubMed] [Google Scholar]
- Xu, H., H. Li, Z. Wang, A. Abudureyimu, J. Yang, X. Cao, X. Lan, R. Zang, and Y. Cai. 2020. A deletion downstream of the CHCHD7 gene is associated with growth traits in sheep. Animals (Basel). 10:1472. doi: 10.3390/ani10091472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatkova, A., J. M. Rouillard, W. Hartmann, B. J. Lamb, R. Kuick, M. Eckart, D. von Schweinitz, A. Koch, C. Fonatsch, T. Pietsch, et al. 2004. Amplification and overexpression of the IGF2 regulator PLAG1 in hepatoblastoma. Genes Chromosomes Cancer 39:126–137. doi: 10.1002/gcc.10307 [DOI] [PubMed] [Google Scholar]
- Zhang, R., J. Miao, Y. Song, W. Zhang, L. Xu, Y. Chen, L. Zhang, H. Gao, B. Zhu, J. Li, et al. 2019. Genome-wide association study identifies the PLAG1-OXR1 region on BTA14 for carcass meat yield in cattle. Physiol. Genom. 51:137–144. doi: 10.1152/physiolgenomics.00112.2018 [DOI] [PubMed] [Google Scholar]
- Zhao, F., S. McParland, F. Kearney, L. Du, and D. P. Berry. 2015. Detection of selection signatures in dairy and beef cattle using high-density genomic information. Genet. Sel. Evol. 47:49. doi: 10.1186/s12711-015-0127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, J. L., J. W. Xu, J. Wang, Y. F. Wen, H. Niu, L. Zheng, H. He, K. Peng, P. Hea, S. Y. Shi, et al. 2019. A novel SNP of PLAG1 gene and its association with growth traits in Chinese cattle. Gene 689:166–171. doi: 10.1016/j.gene.2018.12.018 [DOI] [PubMed] [Google Scholar]
- Zhou, Z., B. Huang, Z. Lai, S. Li, F. Wu, K. Qu, Y. Jia, J. Hou, J. Liu, C. Lei, et al. 2019. The distribution characteristics of a 19-bp indel of the PLAG1 gene in Chinese cattle. Animals (Basel). 9:1082. doi: 10.3390/ani9121082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C., M. Lv, P. Wang, C. Guo, Z. Ni, H. Bao, Y. Tang, H. Cai, J. Lu, W. Deng, et al. 2021. Sequential activation of uterine epithelial IGF1R by stromal IGF1 and embryonic IGF2 directs normal uterine preparation for embryo implantation. J. Mol. Cell. Biol. 13:646–661. doi: 10.1093/jmcb/mjab034 [DOI] [PMC free article] [PubMed] [Google Scholar]