Abstract
The coronavirus disease 2019 (COVID-19) pandemic has caused changes in the global health system, causing significant setbacks in healthcare systems worldwide. This pandemic has also shown resilience, flexibility, and creativity in reacting to the tragedy. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection targets most of the respiratory tract, resulting in a severe sickness called acute respiratory distress syndrome that may be fatal in some individuals. Although the lung is the primary organ targeted by COVID-19 viruses, the clinical aspect of the disease is varied and ranges from asymptomatic to respiratory failure. However, due to an unorganized immune response and several affected mechanisms, the liver may also experience liver cell injury, ischemic liver dysfunction, and drug-induced liver injury, which can result in respiratory failure because of the immune system’s disordered response and other compromised processes that can end in multisystem organ failure. Patients with liver cirrhosis or those who have impaired immune systems may be more likely than other groups to experience worse results from the SARS-CoV-2 infection. We thus intend to examine the pathogenesis, current therapy, and consequences of liver damage concerning COVID-19.
Keywords: Autoimmune liver disease, COVID-19, Clinical manifestation of liver, Drug-induced liver injury, SARS-CoV-2
Core Tip: The coronavirus disease 2019 (COVID-19) pandemic has imposed an unprecedented burden on public health and healthcare globally. It can decompensate pre-existing liver disease or induce acute liver injury. Its presence in hepatocytes directly exhibits cytopathic action and damages the liver because of hypoxia, inflammation, and medication toxicity. The pathophysiology of COVID-19-related liver involvement includes viral cytotoxicity, immunological dysregulation's secondary effect, hypoxia brought on by respiratory failure, ischemia damage from vascular endotheliitis, heart failure, or drug-induced liver injury. This study focuses on the pathophysiology, available treatments, and outcomes of liver injury in relation to COVID-19.
INTRODUCTION
The continuing infection caused by coronaviruses, which exploded in 2019, has accelerated into a pandemic problem worldwide. The disease is mainly a respiratory tract viral infection caused by newly emerging strains of coronaviruses. More than 60 million confirmed coronavirus disease 2019 (COVID-19) cases, including almost 1.5 million deaths, have been reported globally in 189 countries since its inception up to October 2022[1]. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease infection has mainly targeted the respiratory tract system. It can cause severe disease with acute respiratory distress syndrome, which seems to increase its potential fatality in some infected patients[2]. However, lately, the notion that COVID-19 is a systemic infection and inflammatory disease is gaining eye-catching attention, as the disease is showing a systemic feature that affects other visceral organs, including the liver and gastrointestinal tract[3-7]. The chief viral receptor for SARS-CoV-2 infection is angiotensin-converting enzyme 2[8,9]. After virus attachment to the host cell, the host transmembrane serine protease-2 (TMPRSS2) primes the viral S protein[10]. The viral RNA forms two major polyproteins in the host cytosol: protein phosphatase 1 alpha and protein phosphatase 1, which is further converted into 16 non-structural proteins (nsp1 to nsp16)[11]. Phosphatase 1, unlike SARS-CoV-2 infection-induced lung system and myocardial injury, the clinical manifestation of liver organ involvement has been a point of contention since the beginning of the COVID-19 pandemic[3,12-18]. The debate includes potential pathophysiology mechanisms where active viral replication of SARS-CoV-2 in the liver produces liver cytotoxicity and drug-induced liver injury, exacerbating underlying liver disease[19,20]. The incidence of increased concentration of liver transaminase enzymes aspartate aminotransferase and alanine aminotransferase in COVID-19 patients ranges from 2.5% to 76.3%[21-24]. As the liver is humans' primary metabolic and detoxifying organ, the therapeutic efficacy and safety profile could alter the moderate loss of hepatic function. As such, mechanistic insights causing liver injury linked to COVID-19 are required. To date, there is little comprehensive evidence of underlying histopathological changes. In addition to vascular abnormalities that include liver cell necrosis, mild lobular and portal inflammation, unbalanced portal vein branches producing intrahepatic, typically ductular proliferation, and hepatic steatosis (microvesicular) seem to be regularly observed in the livers of diseased patients[25-29]. Although through real-time reverse transcription polymerase chain reaction (PCR), viral RNA has been detected in the liver among major organs excluding the respiratory tract[13], a classic hepatic picture has not yet been reported. Hepatic tropism and direct cytopathic effects are the potential mechanisms for infections associated with liver injury[19,26-29]. S protein is the principal mediator for the entrance of SARS-CoV-2, which interconnects with the host angiotensin-converting enzyme 2 (ACE2) receptor and TMPRSS2 receptors specifically[30]. However, other factors, such as ganglioside[31], can affect how S protein and ACE2 interact. Recently, Ou and colleagues evaluated the capacity of S protein-containing pseudovirions to infect several cell lines. Interestingly, viral vectors encoding the S protein were more effective at transfecting the HuH7 hepatocyte cell line and the Calu3 human lung cancer cell line than the control pseudovirions[32]. As permissive cell types for coronavirus infection are recognized, hepatocyte cell lines such as HuH7 cells have emerged as viable positive controls in SARS-CoV-2 immunostaining[33]. These findings indicate that SARS-CoV-2 infection of human liver ductal teratoma may be possible. Viral replication may occur inside the bile duct epithelium despite the discovery of noticeably greater ACE2; no direct proof of SARS-CoV-2 cholangiocellular infection has been revealed in infectious patients with COVID-19. The detection and identification of SARS-CoV-2 viral RNA or proteins in bile, which are largely generated by hepatocytes and cholangiocytes, are indirect indications of SARS-CoV-2, cholangiocellular infection, and ongoing direct interaction between biliary fluids with cholangiocellular apical membrane. However, only a single case report indicates SARS-CoV-2 RNA is present in bile[34].Activating hepatic stellate cells is crucial as the primary source of cellular fibrosis in developing chronic liver disorders[35]. Hepatocellular and cholangiocellular damage caused directly or indirectly by COVID-19 may produce a proinflammatory milieu that leads to the activation of hepatic stellate cells and, as a result, the development of fibrosis. Fibrosis has been reported in patients with non-alcoholic fatty liver disease or underlying chronic liver disease (CLD). Long-term follow-up studies are required to establish hepatic fibrosis as a potential long-term side effect of COVID-19, especially in patients with pre-existing liver illnesses, even though the current evidence suggests minor and transient liver damage associated with the virus. SARS-CoV-2 can cause cytopathic consequences such as host lipid metabolism and mitochondrial dysfunction. Hepatic steatosis in patients may be caused by cytokine storm-induced immunopathology, activity, and adverse medication reactions such as corticosteroids. Microvesicular steatosis is often brought on by abnormalities in mitochondrial oxidation, either hereditary or acquired; mammalian target of rapamycin (mTOR), which serves as the primary regulator for autophagy, also stimulates de novo lipogenesis[36-40] through mechanisms reliant on viral non-structural protein 6, which is substantially husbanded in SARS-CoV-2 and has been demonstrated in hijacking the autophagy pathway[41-43]. Additionally, it has been discovered that cells infected with Middle East Respiratory Syndrome, HuH7, exhibit hyperactivation of the mTOR pathway, which prevents viral replication when inhibited by the drug rapamycin[44]. Given the most recent findings that SARS-CoV-2 infection limits autophagy[45],it is probable that the mTOR-dependent infection mechanisms of SARS-CoV-2, SARS-CoV, and MERS-CoV are related. Furthermore, it has been demonstrated that interleukin 6 (IL-6) stimulation dramatically boosts mTOR activity[46]. The most common factor in COVID-19-associated acute respiratory distress syndrome (ARDS) is the need for invasive ventilation, substantial doses of positive end-expiratory pressure, and vasoconstrictor therapy due to hemodynamic instability[47-50].
MECHANISM OF SARS-COV-2 INFECTION
The entry of SARS-CoV-2 virus into host cells requires dense glycosylated spike (S) protein containing two functional components, S1 and S2. The S2 subunit controls the fusion of the viral and cell membranes, whereas the S1 subunit controls virus binding to the host cell receptors[51,52]. The serine protease TMPRSS2 must first prime the S protein. TMPRSS2 breaks down the S protein at the S1/S2 and S2 subunit sites[8]. The receptor-binding domain (RBD), composed of about 300 amino acids, is a trimer of separate monomers that comprise the S protein, which is roughly 1300 amino acids long. The RBD on S protein plays a specific role that involves direct participation when host receptors are recognized[53-55]. Zhou et al[9] showed that S protein and ACE2 binding are necessary for infection in HeLa cells. Walls and associates also discovered the activity of human ACE2 as a SARS-CoV-2 functional receptor[56]. In a metaphor, the virus S protein can unlock the human body's ACE2 lock. The SARS-CoV-2 S protein has 10-20 times the affinity of ACE2 compared to SARS-CoV, as reported by surface plasma resonance analysis[53]. This is a significant finding that could help explain the virus's high infectivity rate. Many studies have reported the activity of human ACE2 as a SARS-CoV-2 functional receptor[56,57].
HEPATOPATHY/RISK FACTOR
There are insufficient data regarding the influence of hepatitis B on COVID-19, which has a high incidence, particularly in Asia and China. As per the studies, 2% of severe COVID-19 cases involved hepatitis B infection compared to mild COVID-19 cases (0.06%)[58]. Patients who have a history of hepatitis B or C infection are more likely to develop severe hepatitis, similar to the higher susceptibility for developing severe immunodeficiency infection faced by patients with cirrhosis[3,59-61]. Compared to others, patients with liver disease more commonly develop leukopenia and lymphocytosis, as they suffer from conditions such as leukocytosis with neutrophilia and elevated C-reactive protein[62]. People with related gastrointestinal neoplasms express a higher degree of angiotensin 2 receptors, contributing to the risk of SARS-CoV-2 infection since this enzyme provides an entry for the virus to enter the cells. However, this could be regulated by systemic inflammatory responses and gastrointestinal tract level, as angiotensin 2 modulates this response[63,64]. Monocyte chemoattractant protein-1, known to aggravate COVID-19 and promote steatohepatitis, is higher in the serum of coronavirus-infected patients[18].
ETIOLOGY OF LIVER INJURY IN COVID-19-INFECTED PATIENTS
Viral immunologic injury
SARS-CoV-2 has also been successfully detected in blood and fecal samples from COVID-19 patients, indicating potential intestinal involvement of the virus[65-67]. Through tests with HeLa cells that produced ACE2, Zhou et al[9] demonstrated that ACE2 is the cell receptor for the entrance of SARS-CoV-2 into host cells. It was recently found that cholangiocytes might express ACE2 at a level up to 20 times greater than hepatocytes. SARS-CoV-2 may be able to infect cholangiocytes and lead to bile duct dysfunction, as per the expression pattern of ACE2. Viral immunologic damage may be one of the causes of liver injury since cholangiocytes are involved in immune responses and liver regeneration on a multifaceted and crucial level. In individuals with COVID-19, levels of alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT), which indicate bile duct damage, do not significantly rise despite the clinical data showing and demonstrating elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) in these individuals. Additionally, the autopsy report revealed no viral inclusions in the liver tissue[68].
Damage of cholangiocytes by SARS-CoV-2
According to research by Qi et al[63], the epithelial cells of the bile duct express the ACE2 receptor 20 times more than liver cells[69,70]. This indicates that SARS-CoV-2 may damage and infect bile duct cells directly, which could eventually result in bile duct dysfunction. Epithelial cells for bile ducts are crucial for liver regeneration and the immune system; thus, liver damage may result when SARS-CoV-2 infects them and results in cholestasis[69,70]. Increased concentrations of ALP and GGT are reliable signs of damage to bile duct epithelial cells[71]. More studies are necessary to conclusively connect liver damage to the bile duct cell damage brought on by the SARS-CoV-2 infection. Researchers have hypothesized a potential mechanism for SARS-COV-2-induced liver damage. Infection of liver cells occurs when hepatic parenchymal cells, which are produced from bile duct epithelial cells, compensate for the hyperplasia of ACE2 expression in the liver tissues[72]. Nonspecific liver inflammation can cause an increase in cytokines and inflammatory biomarkers, including IL-2, IL-6, IL-7, and IL-10, interferon gamma (IFN-g) inducible protein 10, and tumor necrosis factor alpha (TNF-α)[12,73], which can cause severe damage (e.g., hepatomegaly, elevated serum transaminase, high bilirubin, hepatic encephalopathy, and even liver failure). In case inflammatory response syndrome worsens without appropriate management, COVID-19 patients may experience multiple organ failure and death[12,74].
Hypoxic injury
The liver is susceptible to cardiovascular abnormalities because of composite vascular supply and substantial metabolic activity. The condition known as ischemic hepatitis, also referred to as hypoxic hepatitis, is frequently found in critically ill patients and is the consequence of underlying circulatory, cardiac, or respiratory failure that can result in passive congestion or reduced hepatic perfusion[75,76]. Systemic stress causes a compensatory reduction in peripheral and splanchnic blood flow, which in turn, causes a reduction in hepatic blood flow, and as a result, hepatocellular hypoxia, particularly in zone 3[12,77]. Cell injury via lipid peroxidation can occur when reactive oxygen species are generated by re-exposing ischemic hepatocytes to oxygen in a condition called reperfusion injury[78]. Furthermore, Kupffer cells can trigger the reunion and activation of polymorphonuclear leukocytes by producing cytokines as a response to ischemia[78].
Drug-induced damage to the liver
In the interim, a variety of different medications including antiviral (lopinavir /ritonavir, remdesivir), antibiotic (macrolides), antimalarial/antirheumatic (hydroxychloroquine), immunomodulating (tocilizumab, corticosteroids), and antipyretic (acetaminophen) medications, are being used in clinical studies or an off-label manner. However, most medications (e.g., ritonavir and remdesivir) have already shown hepatotoxic potential. Also included is corticosteroid treatment, which the World Health Organization currently advises for patients with severe SARS-CoV-2 infection[79]. These findings are further supported by the Human Protein Atlas database, which demonstrates that the greatest pattern of ACE2 expression throughout human intestinal cells have a variety of cell types (information accessible at https://www.prote inatl as.org/ENSG0 00001 30234-ACE2/tissue). Additionally, it has been proven that human intestine organoids are susceptible to SARS-CoV and SARS-CoV-2 infections[80]. The most common clinical symptoms, including fever, coughing, exhaustion, and shortness of breath, were highlighted in early clinical trials. Later investigations were able to unearth evidence that COVID-19 also has extrapulmonary manifestations. Because the liver is the primary organ for metabolism and detoxification, it is essential to maintain optimal liver function when utilizing any of the COVID-19 therapeutic modalities. It is well known that liver damage results from hepatic inflammation, including activation of innate immune cells and cytokine production[81]. An old drug called chloroquine has recently been tested in treatments of COVID-19 patients after showing indications of being a potential treatment. The drug’s superior efficacy in viral control was well demonstrated when concurrent clinical trials on chloroquine conducted in 10 hospitals across China showed successful inhibition of viral replication[82]. The pharmacodynamics of this drug in treating COVID-19 may show involvement of the arresting of cytokine storms, the activation of CD8 T cells or prevention of endocytosis-mediated uptake of the virus[83,84]. The COVID-19 virus primarily affects the lungs[74]. However, it can also harm the liver through a variety of mechanisms, including an disorganized immune response, virus-related liver cell damage, drug-induced liver injury (DILI), and ischemic liver dysfunction in the context of multiorgan failure[85]. Patients with cirrhosis and those with impaired immune systems may be more likely to experience negative consequences after contracting SARS-CoV-2[21]. The direct cytopathic impact of COVID-19, DILI, an uncontrolled immunological response, or sepsis are only a few of the possible causes of liver damage[86]. In COVID-19 patients who also experience diarrhea, SARS-CoV-2 RNA has been found in blood and stool samples, indicating that the liver is likely implicated in the etiology of this illness[67]. The underlying condition might become worse because of COVID-19. CLD increases the risk of death, especially in critically ill patients, by causing hepatic decompensation or acute-on-chronic liver failure[56,86,87]. In severe COVID-19 infections, liver damage is more frequently caused by an inflammatory cytokine storm[86,88] than by the virus itself in a direct cytotoxic manner[86]. While in the final stage of SARS, the continued interaction between the lung and systemic inflammation causes multiorgan vascular dysfunction and a cytokine storm, and prothrombotic factors aggregate and produce thrombosis due to the bone marrow and liver acute phase responses[89,90].The human body's primary organ for drug metabolism is the liver. The medications used to treat COVID-19 individuals may harm the liver. According to Kulkarni et al[21], there is drug-induced liver damage as frequently as 25.4% of the time. In the United States, antimalarial drugs, including chloroquine and hydroxychloroquine, have received emergency authorization to treat COVID-19. However, because hydroxychloroquine concentrates in the liver, individuals with hepatitis or other hepatic illnesses, as well as those taking other medications known to be hepatotoxic, should exercise caution[91]. Along with antimalarial drugs, antiviral drugs such as lopinavir-ritonavir, remdesivir, and favipiravir have been utilized to treat COVID-19. According to one case study, remdesivir was the drug that produced the most instances of hepatotoxicity, with 23% of patients reporting elevated levels of liver enzymes linked to the drug[92]. A 50-year-old man who developed ARDS and died from severe COVID-19 had his first COVID-19-related autopsy. The liver’s autopsy revealed minor lobular and portal activity, as well as moderate microvesicular steatosis. The SARS-CoV-2 virus or liver damage brought on by drugs is thought to be the source of the injury. There was an increase in proinflammatory CCR6+T-helper 17 (Th17) in CD4 T cells and cytotoxic granules in CD8 cells. Hepatocyte dysfunction may also be caused by decreased CD4 and CD8 cell numbers[93]. The clinical findings of patients with and without a history of non-alcoholic fatty liver disease (NAFLD) were compared. The results showed that patients with NAFLD had a longer viral shedding time (P = 0.0001) and a greater probability of disease development. According to research published by Sonzogni and associates[94], a hepatic biopsy from one of these patients revealed microvesicular steatosis along with overactivation of T cells, which raised the potential for collateral damage of the liver caused by virally induced cytotoxic T cells. The main finding of the study revealed that patients with severe and non-severe COVID-19 may be at combined risk for elevated blood ALT levels. In adult patients with severe and non-severe COVID-19, the secondary outcomes included the risk of the variant parameters namely elevated AST, hyperbilirubinemia, and hypoalbuminemia. The amount of GGT from the included prior studies was also evaluated using a pooled mean difference (MD). Serum ALT or AST values over 40 U/L were deemed to be high. As opposed to hypoalbuminemia, defined as a serum albumin level below 40 g/L, hyperbilirubinemia is defined as a total bilirubin (TBIL) level greater than 17 mmol/L[95]. Immune-mediated injury, or ischemic hepatitis, can result from a significant systemic inflammatory response brought on by COVID-19. Current therapies include lopinavir/ritonavir, hydroxychloroquine, and remdesivir, all of which have the potential to be hepatotoxic and increase the risk of DILI[96,97]. The underlying chronic hepatitis B may reactivate while using tocilizumab or other immunosuppressive options. Although it has been hypothesized that viral replication within the infected hepatocytes might directly induce cytotoxicity, SARS-CoV-2 viral inclusions have not been found in the liver[68]. The clinical signs and symptoms of COVID-19 might be anything from asymptomatic to respiratory failure. Fever (50%) and cough (38%) are the symptoms that patients experience most frequently. Patients have also mentioned experiencing other symptoms such as headaches, nausea, vomiting, and diarrhea[98,99]. Inflammatory cytokines including IL-1, IL-6, IL-8, and TNF-α increase COVID-19 severity. When there is a serious infection, abnormal liver enzyme and bilirubin levels are discovered, which makes it challenging to treat individuals with liver disease who are also on immunosuppressants, notably cirrhosis or autoimmune hepatitis[100-102]. Conversely, investigations have indicated a relationship between elevated LDH levels, creatinine kinase, and myoglobin in critically ill COVID-19-infected patients. Therefore, it is proposed that elevations of aminotransferase levels might also be due to other conditions apart from liver damage. Nevertheless, COVID-19 infection could cause myositis similar to that caused by severe flu. A liver biopsy of a patient who died due to COVID-19 infection revealed the presence of microvesicular steatosis as well as mild lobular and portal inflammation[17,103,104]. Purely hepatocellular and cholestatic types of drug-induced liver damage are clinical conditions. Patients with COVID-19 who have abnormal liver function are typically described as having hepatocellular damage. The primary signs and symptoms of COVID-19 are fever, cough, exhaustion, and dyspnea. Because acetaminophen is a common constituent in antipyretic medications, some COVID-19 patients may have a history of using them. These substances are known to be typical medications that can directly poison hepatocytes. The use of several patented Chinese medications to prevent COVID-19 infection may potentially induce liver damage. Recent liver pathology findings from analyzing COVID-19 patients point to the possibility of drug-induced liver damage, as the data revealed the presence of mild lobular inflammation and moderate microvascular steatosis[97]. Antiviral medications such as ribavirin, in addition to altering liver function, may also induce or worsen tissue hypoxia through hemolysis, which might raise blood liver enzyme levels. Before receiving COVID-19 medication, patients with chronic liver diseases such as hepatitis B or C may already have increased transaminase levels, which might increase their chance of developing drug-induced liver damage. As a result, individuals with COVID-19 who also have basic liver disorders as comorbidities are clinically treated with antipyretic medications, traditional herbal remedies, or antiviral medications. Multiple proinflammatory cytokines and inflammatory indicators, including TNF, IL-2, IL-6, IL-7, IL-18, granulocyte-colony stimulating factor, IFN, and ferritin, can be abundantly released when a cytokine storm occurs[12]. Fulminant and deadly hypercytokinemia may start a series of events that result in tissue damage or organ failure, including liver failure[105].
Autoimmune liver disease
The clinical effect of pre-existing immunosuppression on the severity of COVID-19 is still an intricate topic. The hazards that some illness groups confront are a source of worry. For instance, individuals with rheumatoid diseases and inflammatory bowel diseases have been linked to greater disease severity while using maintenance corticosteroids and thiopurines, respectively[106,107]. However, the effect of immunosuppression on the course of the illness in patients who have had solid organ transplantation, such as liver transplantation (LT), resembles that of non-immunosuppressed persons (described below)[108,109]. Additionally, interval meta-analyses have confirmed that immunosuppressed individuals do not have a noticeably higher chance of contracting severe SARS-CoV-2 infections[110,111]. SARS-CoV-2 is a member of the same family as SARS-CoV and MERS-CoV, the Coronaviridae. They are pathogenic and have a similar structure to the genomic sequence of SARS-CoV-2, which is 80% and 50% identical to that of SARS-CoV and MERS-CoV, respectively[51]. One week after the start of the illness, the presence of severe symptoms, including dyspnea and hypoxemia, might be a marker of severe pneumonia, which can result in ARDS, multiple organ dysfunction syndromes, and even mortality[112]. Due to its similar genetic lineage B, the new coronavirus reacts to the same receptor as both beta-coronaviruses[113]. The RBD, a ligand that interacts with the host cell surface receptor, is present in the glycoprotein (S protein) located on the virion envelope S, allowing for the fusing of membranes, viral penetration, and viral multiplication[113,114]. Although the target receptor is mainly expressed in type II pneumocyte of the lungs, the predominance of extrapulmonary symptoms suggests that SARS-CoV-2 infection may potentially impact other organs. It has been established by transcriptomics and immunohistochemical investigations that the lower respiratory tract, heart, lungs, ileum, esophagus, kidney, and bladder contain the largest percentage (>1%) of ACE2 receptors. Other organs such as the liver, stomach, brain, pancreas, arteries, endothelium, breast, uterus, oral and nasal mucosa, and ovary have lower ACE2 expression[115-117]. The majority of SARS-CoV-2 infection risk factors are associated with metabolic syndrome. As a vital organ for lipid metabolism, the liver is a critical factor in determining metabolic disorders and glucose metabolism. As a result, several studies have related severe COVID-19 to metabolic dysfunction-associated fatty liver disease (MAFLD)[12]. Even when compared to the meta-analysis statistics described before[28], patients with underlying hepatic problems were older and afflicted with numerous additional comorbidities, such as hypertension (68%) or diabetes (48%). The previously mentioned SARS-CoV-2 target receptor has variable distribution in the liver. Several published reviews have determined its presence in cholangiocytes and lack in Kupffer or sinusoidal endothelial cells[118,119]. It has been discovered that, among those over 65, sex has no bearing on the likelihood of this complication[120,121]. The connection between low testosterone and clinical outcomes, likewise fatty liver and atherosclerosis, metabolic syndrome, type 2 diabetes, and obesity, is one hypothesis that has been proposed. Female sex hormones have a preventive effect against these diseases in younger women[122]. Individuals with chronic hepatitis C, alcoholic liver cirrhosis, alcoholic liver damage, and chronic hepatitis B were shown to have the lowest association. By contrast, those with non-alcoholic liver disease and non-alcoholic cirrhosis had the strongest association. Additionally, SARS-CoV-2-infected individuals who also had pre-existing liver illnesses showed greater hospitalization and mortality rates than other patient groups[123]. Age, having non-liver cancer, and having higher baseline blood creatinine levels are all risk factors for patient mortality[124]. Immunosuppressants used to lower transplant rejection risk may make patients more susceptible to COVID-19, but they may also reduce the uncontrolled inflammatory response that results from SARS-CoV-2 infection. Additionally, long-term immunosuppressant usage may lengthen the viral shedding period, extending the duration of the communicable period[125]. For patients with acute liver failure, a high Model for End-Stage Liver Disease score, or hepatocellular carcinoma (HCC) at the Milan criteria's top limits, most societies advise against transplantation. Both organ donors and receivers should be checked for SARS-CoV-2 infection as an additional precaution[126]. SARS-CoV-2 vaccines are being developed at an unprecedented rate. Since the start of the pandemic, 126 new vaccines have been put into clinical development, as per the World Health Organization (WHO) COVID-19 vaccine tracer. WHO has given the go-ahead for seven of them for commercial usage. BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna), based on the mRNA encoding SARS-CoV-2 spiny glycoprotein variations, are the most widely used COVID-19 vaccines. Other vaccines include ChAdOx1 nCov-19 (AZD1222, the Oxford-AstraZeneca) and Ad26.COV2.S (Johnson & Johnson/Janssen) is an adenoviral vector-based vaccine. A recombinant human adenovirus type 26 vector encoding the SARS-CoV-2 S protein is used in the Johnson & Johnson/Janssen vaccination. Conversely, the Oxford-AstraZeneca vaccine is an adenoviral vector that is replication-free and carries a full-length, codon-optimized gene that encodes the SARS-CoV-2 S protein in chimpanzees. Since none of the three vaccinations contain live viruses, they cannot even trigger viral replication in immunocompromised people[127]. The COVID-19 vaccine should be taken into consideration for patients with hepatocellular carcinoma (HCC) who are receiving locoregional or systemic therapy without delaying their course of therapy[128-130]. Similarly, those with chronic liver disease who use antiviral medicines for the treatment of hepatitis B or hepatitis C should not stop taking those medications while getting the COVID-19 vaccination. Hepatopathy has already been linked to the severity of illness brought on by two additional extremely pathogenic coronavirus strains: MERS-CoV and SARS-CoV-1. Many factors affect the pathogenesis including hypoxic hepatitis (secondary to respiratory failure), hepatic congestion associated with mechanical ventilation (high levels of positive end-expiratory pressure (PEEP), virally induced intrahepatic cytotoxic T cells and Kupffer cells, and drug toxicity. Hepatic impairment has been linked to the most severe COVID-19 instances when thrombocytopenia, activated coagulation, and fibrinolysis are present[74]. In COVID-19 patients, abnormalities in the liver’s biochemistry have been observed. ALT and AST show moderate increases that range from 14% to 53%[12,74,120] and are frequently seen in these anomalies. Patients with more severe symptoms than those with mild to moderate symptoms, particularly those who require admission to an intensive care unit, may have higher rates of transaminase increase[131]. The SARS-CoV-2 gene was the target of PCR testing to diagnose COVID-19 in samples of the nasal or pharynx collected before admission. All patients underwent at least one chest computed tomography scan after admission. These requirements must be satisfied for release from the hospital: two negative reverse transcriptase-PCR findings for SARS-CoV-2 respiratory samples obtained at least 24 h apart, remission of respiratory symptoms, improvement in lung inflammation, and normal body temperature for at least 3 d[132,133]. A fatty liver was seen in 40.0% of all mild cases[17]. Hepatic dysfunction should be of concern, according to growing research, as it is prevalent in COVID-19 patients[134]. As per meta-analysis, 27.4% of COVID-19 patients had liver dysfunction[135]. As per the WHO report dated March 5, 2021, there have been 115289961 COVID-19 cases documented globally. Where the Americas made up the majority (44%), Europe came in second (34%), and Southeast Asia came in third (12%)[8,136]. Except for individuals under 1-year-old, children with COVID-19 often only have minor symptoms affecting the upper respiratory tract[6]. The most common technique of diagnosis for COVID-19 infection is PCR testing using a nasal swab sample. At the same time, presumptive diagnoses can also be made using clinical, laboratory, and imaging data[8]. Direct invasion and cytokine storms are the two key phases in the pathogenic processes of SARS-CoV-2. Direct invasion is the phase of SARS-CoV-2 infection where the virus penetrates target cells by binding to the ACE2 receptor through the viral structural S protein[137]. Pneumocytes, gastrointestinal epithelia, vascular endothelium, the liver, and nasal and bronchial epithelial cells all have the ACE2 receptor[138,139]. Type 2 transmembrane serine protease (TMPRSS2) in host target cells promotes viral uptake, particularly in alveolar epithelial type II cells[93]. Some infected individuals may undergo cytokine storms, an extrapulmonary systemic hyperinflammation syndrome[12]. Interleukin (IL-2, IL-6, IL-7, Il-10) and TNF-α are just a few of the cytokine types that may see an increase in levels as a result of a cytokine storm[140]. A cytokine storm also increases inflammatory biomarkers such as granulocyte colony-stimulating factor, IFN-g inducible protein 10, and monocyte chemo-attractant protein 1[141]. Significantly increased cytokine levels are a serious issue since they can lead to serious illness[142]. Serum ferritin, IL-6, and IL-10 were found to be powerful predictors of severe COVID-19 illness by a meta-analysis[23]. The most prevalent pre-existing liver diseases in COVID-19 patients are cirrhosis, HCC, autoimmune hepatitis, chronic hepatitis B, chronic hepatitis C, MAFLD, alcohol-related liver disease, and autoimmune hepatitis[21,143,144]. Certain underlying hepatic conditions may impact the prognosis for COVID-19. It may be the underlying mechanism because MAFLD is a proinflammatory hypercoagulable condition linked to severe illness and thrombosis in COVID-19 patients[145]. The best course of action for people with end-stage chronic liver disease or immediate liver failure is LT[146,147]. The primary biochemical markers used in the diagnosis of liver damage include ALT, AST, TBIL, ALP, GGT, albumin, and prothrombin time (PT)[148].
Gastrointestinal damage caused by lung infection
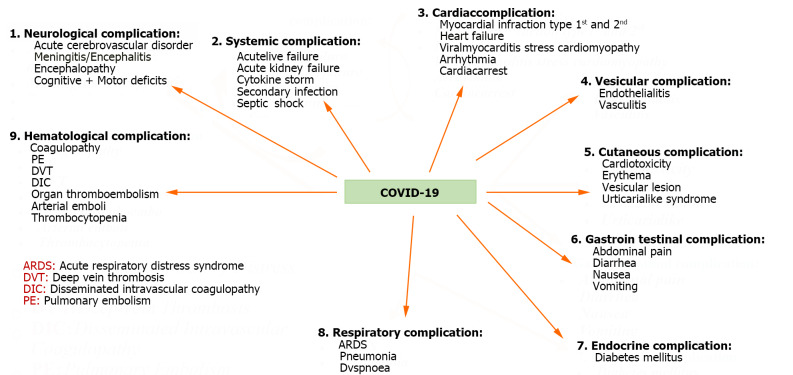
The potential gastric complications have been summarized in Figure 1. Immunity and chronic enteritis depend on CD4+ T cell infiltration of the intestinal mucosa. The entrance of CD4+ T lymphocytes into small intestinal cells is known to be facilitated by the C-C chemical receptor type 9 (CCR9)[149]. CCR9+ CD4+ T lung-derived cells expanded after viral infection, according to Wang et al[18]. Chemokine (C-C motif) ligand 25 can be incorporated by the small intestinal epithelium[150], which, therefore, encourages the recruitment of CCR9+ CD4+ T cells into the small intestine[151]. It damages the intestinal flora's balance and the gut immune system. As a result of the high levels of IL-17A produced, Th17 cell polarization and neutrophil recruitment were induced in the gut[152]. As a result, various gastrointestinal symptoms, such as diarrhea and intestinal immunological damage, might manifest. Additionally, intestinal inflammation may cause intestinal flora and cytokines to enter the circulation and travel to the lungs, influencing the immune system[153,154]. However, there are times when these antiviral immunological processes manage to get beyond the regulatory system, as shown in some patients. It ultimately helps to cause viral infection-induced multi-organ failure, which includes liver failure. Significant tissue and organ damage can also result from a host immune system's overreaction when stimulated. Significant tissue and organ damage can also result from an overreaction of the host immune system caused by the activation of a systemic inflammatory state brought on by elevated cytokine production. The latter condition, called a "cytokine storm", is recognized to harm tissue significantly[18]. Due to their advanced age and pre-existing medical conditions such as diabetes, asthma, and cardiovascular disease, COVID-19 patients experience more severe consequences, which increases their death rate. Through multisystem inflammation, COVID-19 can harm the liver directly or indirectly. As a result, pre-existing liver illnesses may raise patients' risk of developing severe COVID-19[155]. The three primary causes of COVID-19-induced liver injury are ischemia and hypoxia. Other considerations include pre-existing liver illness (such as hepatitis steatosis, cholangitis, thrombosis, Kupffer cell proliferation, and liver dysfunction), severe inflammatory responses/sepsis, and the direct cytotoxic action of the virus on cholangiocytes (through ACE2 receptors)[156]. Individuals with severe COVID-19 are at a significant mortality risk for hypoxic liver injury (HLI), which is not an uncommon occurrence. HLI is mainly caused by lung and heart failure and is related to immune-mediated inflammation. Patients with HLI are at a significant risk of death due to numerous organ failures. Comparing HLI instances to non-HLI cases, there is a statistically significant increase in the levels of (TBIL), (CRP), procalcitonin and IL-6. Additionally, compared to non-HLI patients, HLI patients had a considerably lower median survival time[157]. Elevated levels of ferritin, CRP, IL-6, LDH, and cytokine storm (known as cytokine release syndrome) are the hallmarks of this condition[158]. Damage in the liver is triggered by dysfunctional monocytes and macrophages[159]. Hepatotoxicity can be treated successfully with hydroxychloroquine, either alone/or in combination with lopinavir/ritonavir, remdesivir, azithromycin, umifenovir, darunavir, baricitinib, IFN-b, and imatinib. These medications are now often used for off-label COVID-19 therapy in several nations due to their quick accessibility[160].
Figure 1.
Potential complications due to coronavirus disease 2019. COVID-19: Coronavirus disease 2019.
COVID-19 AND LIVER-ASSOCIATED CLINICAL FEATURES
According to reports from China, patients who recuperated from severe COVID-19 symptoms experienced symptoms including pigmentation and a darker complexion. The primary factor for pigmentation and darkening of the skin is multiple organ damage, particularly liver disease[161,162]. Through three distinct pathways, liver dysfunction, aberrant liver function, and liver injury-poor liver function can readily change pigmentation. When the amount of estrogen rises, liver dysfunction prevents the inactivation of estrogen; it reduces the inhibition of thiamine on tyrosinase, which then enhances the transformation of tyrosine into melanin[163]. When the liver cannot properly metabolize the melanocyte-stimulating hormone released by the anterior pituitary gland, the body produces more melanin[164,165]. Liver damage increases blood iron levels, and if that blood is given to the facial skin, it may create a darkened face[166,167].The most prevalent comorbidities experienced by the patients in a clinical review that included 331 critically ill COVID-19 patients were hypertension (136 cases, or 41.1%), CHD (66 cases, or 19.9%), and diabetes (60 cases, or 18.1%). The majority of the patients’ treatments included inhaling oxygen (262 cases, or 79.2%), antibacterial therapy (256 cases, or 77.3%), adjuvant corticosteroid therapy (211 cases, or 63.7%), gamma globulin (146 cases, or 44.1%), mechanical ventilation (120 cases, or 36.3%), and muscle relaxants (37 cases, or 11.2%). A total of 273 (82.5%) of the 331 critically ill patients received antiviral medication including oseltamivir, arbidol, lopinavir/ritonavir, ganciclovir, and IFN. According to an investigation, 36 (13.2%) of the patients in this group experienced liver damage complications[168]. This research suggests that lopinavir may make ritonavir more likely to cause liver damage or vice versa. The reactive metabolites and drug-induced oxidative stress are thought to play a role in the molecular mechanism of hydroxychloroquine-induced liver damage, or the inflammatory processes after the viral infection itself may have an ad hoc or synergistic impact[169]. Azithromycin, hydroxychloroquine, and lopinavir/ritonavir use are linked to many liver damage pathways, including drug-induced oxidative stress[170]. Due to the many medications involved, providing a consistent molecular idea suited for all pharmaceuticals under consideration is impractical. However, the molecular mechanisms through which a single medication, such as acetaminophen, damages the liver through cytochrome P450, particularly its isoform 2E1, are well understood and may be applied to individuals who take an excessive amount of the drug.
Contrary to the majority of other medicines that generate unanticipated idiosyncratic liver disease, acetaminophen's intrinsic liver harm is predictable. Polypharmacy, a well-documented therapeutic strategy employed for COVID-19 patients (for instance, patients who were treated with up to 18 different medicines), poses a more significant risk for DILI than the use of a single drug from a molecular perspective. If two or more hepatotoxic medicines were administered concurrently for therapy, polypharmacy increased the incidence of DILI by a ratio of up to six[171]. SARS-CoV-2 can cause an asymptomatic infection or a condition that might be fatal[172,173]. Since the COVID-19 pandemic began in 2019, there have been worries that people with pre-existing CLD may be more likely to experience worse health outcomes after contracting SARS-CoV-2. This is particularly significant considering that advanced age, obesity, diabetes, and severe COVID-19 and CLD share risk factors[68,174]. Progressively rising levels of ALT, AST, ALP, and LDH were found during hospitalization in the first case of COVID-19 reported in the United States.
By contrast, the bilirubin level with PT remained normal[62]. Males are more likely than females to experience liver impairment, according to Xie et al's[57] analysis of liver function in patients not receiving critical care. Fifty-two patients from the COVID-19 trial who required mechanical breathing or help with at least 60% of the inspired oxygen fraction were included in the research. According to the research, 29% of the patients had liver damage, 15% had acute renal damage, and another 15% had cardiac damage[174]. The liver damage caused by COVID-19 is hepatocellular rather than cholestatic, and as a result, it primarily manifests as increases in ALT, AST, and LDH levels. Hepatocellular damage markers such as AST are also linked with mortality risk in COVID-19 patients[175].
HEMATOLOGICAL AND HEPATIC VARIATIONS
Changes in blood cellularity are observed during the early onset of the disease. A study with 1099 participants showed that 83.2%, 36.2%, and 33.7% of the patients had lymphopenia, thrombocytopenia, and leukopenia, respectively. These patients showed a rise in ALT and AST values, which indicated liver damage, particularly in severe COVID-19 cases[3,58,62,176]. It is speculated that the virus's attachment to ACE2 can directly cause cytopathic damage, which can lead to liver damage. Patients with COVID-19 also have cholangiocytes with high ACE2 expression. In addition to an extended PT, hypoproteinemia and coagulation abnormalities are further COVID-19 hepatic symptoms. This phenomenon may even be secondary to the use of hepatotoxic medicines as the cause of acute viral hepatitis, which is thought to be the source of viral tropism in the liver tissue[172]. In disseminated intravascular coagulation conditions, an increase in the D dimer and thrombocytopenia was observed in 36.2%-46.4% of critically ill patients, and a poorer prognosis was identified[172].
THERAPEUTIC RECOMMENDATIONS FOR COVID-19: VACCINES, LABEL, AND OFF-LABEL MEDICATIONS FOR PATIENT CARE
Classical antimalarial drugs include chloroquine, aminoquinolines, and hydroxychloroquine, which are polymerase inhibitors. The drug kills the malaria parasite by accelerating the formation of poisonous heme within the parasite through heme polymerase inhibition. It is believed that the function of medicines in treating COVID-19 is to stop the virus from entering host cells by limiting the glycosylation of host receptors and by inhibiting the generation of viral proteins through endosomal acidification. The WHO recommends the use of hydroxychloroquine or chloroquine together with lopinavir/ritonavir for COVID-19 therapy, regardless of the severity and length of the disease. On the other hand, regardless of the severity of the disease, remdesivir and systemic corticosteroids are suggested as prospective pharmacological options for conditional use in routine care of hospitalized COVID-19 patients (WHO/2019-nCoV/therapeutics/2020.1). The WHO recognizes this type of off-label pharmaceutical usage as being nation-specific. In several nations, clinicians provide COVID-19 therapeutic options that have yet to receive official approval (MEURI; http://www.who.int/). COVID-infected patients have been treated with off-label, compassionate-use treatments such as IFN and the repurposed medication Kaletra, which is an authorized combination of the virus, steroids, anti-IL-6 inhibitors, and protease inhibitors lopinavir and ritonavir, chloroquine, and azithromycin[177].
Coronaviruses are positive-sense single-stranded RNA viruses with an envelope measuring 80-220 nm in diameter. The virus is known as a coronavirus because it has an envelope measuring 20-nm-long spikes that resemble the sun's corona when seen under an electron microscope. Both humans and animals can become ill as a result of the infection. Among the RNA viruses that are currently known, it has the largest genome[178]. The coronavirus nucleoprotein (N) covers the RNA genome to create a coiled tubular shape. The viral envelope (E) that surrounds this helical nucleocapsid contains two or three structural proteins, including the S structural protein, which serves as the target for neutralizing antibodies, and the matrix protein (M), which is incorporated in the envelope. Numerous beta-coronavirus strains also include hemagglutinin esterase. There are four structural proteins, namely N, E, M, and S and RNA-dependent RNA polymerase (RdRp), which are encoded by the five essential genes in coronaviruses. The highly conserved arrangement of these genes is 5'-RdRp-S-E-M-N-3'[179].
It was observed that 51.1% of all confirmed cases were males. Eighty percent of the confirmed cases that were reported either had either no pneumonia or mild to moderate. In comparison, 15% showed severe pneumonia and 6% required critical care due to respiratory failure, shock, and multiple organ failure. The COVID-19 fatality rate is 3.8% across all of China, with fatality rates of 5.8% in Wuhan City and 0.7% across the remainder of mainland China. Old age (60 years and above) and medical comorbidities, namely hypertension, diabetes mellitus, cardiovascular disease, chronic pulmonary illness, or cancer, are risk factors for acquiring severe pneumonia or dying from it. Laboratory testing found leukopenia, lymphopenia, and mildly raised C-reactive protein in COVID-19 cases, but patients having severe pneumonia showed higher levels of leukocytes, neutrophils, and creatinine kinase. According to computed tomography of the chest, both lung fields exhibit a ground glass appearance, interstitial infiltration, or numerous patchy consolidations[180]. Some individuals had the rapid onset of severe pneumonia, pulmonary edema, acute respiratory distress syndrome, acute respiratory failure, and multiple organ failure. Chen et al[163] were the first to detect abnormal liver enzymes in infected patients. Forty-three cases (43.4%) of Wuhan's confirmed patients had elevated blood levels of the enzymes AST, ALT, and lactic dehydrogenase. Except for one instance, which had extremely high levels of aminotransferases, most had a modest increase in aminotransferase. None, however, was seen to have liver failure or apparent intrahepatic cholestasis[181]. The biggest subfamily of S viruses in the Nidovirales family is the coronaviruses. In the past 20 years, the virus has been responsible for three major outbreaks, including the most recent pandemic that the SARS-CoV-1 brought on. In 2002, Guangdong Province in China had the first outbreak of the 21st century. SARS-CoV-1 formed a severe form of SARS and caused 8098 fatalities (9.6%) worldwide[182-186]. The lung is the main organ affected by pneumonia caused by COVID-19. Typical respiratory symptoms, including dyspnea, coughing up sputum, exhaustion, ARDS, respiratory failure, and even death, affect the majority of COVID-19 patients. Contrarily, extrapulmonary clinical manifestations can affect a variety of other organs, including the cardiovascular system (i.e. acute coronary syndrome, arrhythmias, pericarditis, myocarditis,) the kidneys (acute kidney injury and acute tubular necrosis) and the liver[187].
ABNORMALITIES IN LIVER FUNCTION TEST PREVALENCE AMONG COVID-19 PATIENTS
There are few studies particularly investigating the clinical characteristics of liver failure in COVID-19 patients. These investigations revealed a rising trend in the liver enzyme levels seen in individuals who were severely ill or who did not survive. The percentage of patients with elevated ALT, AST, and GGT levels was 82%, 75%, and 72%, respectively. According to a recent finding, the incidence of abnormal liver test results was 76.3% and that of liver damage was 21.5% among hospitalized COVID-19 patients[188]. In that research, 26.7% of patients with abnormal liver tests indicated a tendency toward developing severe pneumonia within 2 wk of hospitalization, and individuals had higher levels of abnormal liver tests. Patients diagnosed with severe COVID-19 had considerably more liver damage than those with non-severe COVID-19. The scientists also noted that individuals with hepatocyte types had noticeably increased chances of suffering from severe COVID-19. In a different investigation, the levels of ALT, AST, GGT, ALP, and TBIL were significantly greater in dead patients than in healthy ones[173].
POSSIBLE IMPACT OF DRUGS ON LIVER FUNCTION IN COVID-19 PATIENTS
It is hypothesized that COVID-19 individuals who use certain medications may suffer from liver damage. For instance, individuals with COVID-19 may have liver damage as a result of taking various medications including antibiotics, antivirals, and antipyretics, as well as analgesics and traditional Chinese medicine[174]. It was observed that medications like ACE inhibitors (ACEis) and angiotensin II receptor blockers (ARBs) may impair liver functioning in COVID-19 patients. In research, participants who used ACEis or ARBs throughout their hospitalization had higher liver enzyme levels; however, the rise was not statistically significant in those who were not hospitalized[189]. Serum GGT, a possible cholangiocyte damage diagnostic marker, is up to 72% higher in individuals with severe COVID-19[173,185]. According to a preliminary investigation, the ACE2 receptor is highly expressed in cholangiocytes[189]. These data suggest that SARS-CoV-2 may bind to cholangiocytes that are ACE2-positive and cause liver damage[188].
Nevertheless, liver tissue from a patient who passed away from COVID-19 did not contain any viral inclusions[190]. A significant factor in the liver damage brought on by COVID-19 may be dysfunctional control of the innate immune response. The following potential processes could cause COVID-19 patients’ livers to become damaged: (1) immune-mediated inflammation including cytokine storm and hypoxia caused by pneumonia; (2) direct cytotoxicity due to active viral replication in the liver cells; (3) drug-induced liver damage, including the potential hepatotoxicity of uminefovir in patients with severe COVID-19, concerning antiviral medications like lopinavir/ritonavir, chloroquine as remdesivir, and tocilizumab; (4) patients who have a history of chronic hepatic illness are more susceptible to acquiring hepatic damage from this viral infection; and (5) reactivation of pre-existing hepatic disease hepatitis B virus reactivation could occur as a result of using biological medications such as tocilizumab and baricitinib, which have the potential to cause liver impairment. Another unanswered question is whether SARS-CoV-2 infection worsens cholestasis in an individual or with underlying cholestatic hepatic disorders. More mechanistic research is needed to better understand how viruses enter and replicate in liver cells and the possible liver effects of the prescriptions used to treat COVID-19[188].
MANAGEMENT AND PREVENTION
Preventing liver damage in COVID-19
All COVID-19 patients should have their liver biochemical markers monitored, including ALT/AST, albumin, bilirubin, and PT, for possible risk of liver damage. For instance, if serum AST and LDH levels increase while the ALT level is normal, skeletal muscle or myocardial damage should be diagnosed rather than a liver injury because chronic liver illness is a significant medical burden among older COVID-19 patients; doctors are encouraged to give attention to the treatment of pre-existing liver disease. Antiviral medications for the treatment of chronic hepatitis B should not be stopped to prevent the virus from reactivating. However, anti-HBV medicines should also be considered for patients receiving glucocorticoid therapy[12,13,18,172].Those receiving glucocorticoids or immunosuppressants as part of their treatment for autoimmune liver disease should be closely monitored because of their compromised immune systems. People with cirrhosis also require close monitoring for the development of complications and secondary infections[15,18]. Due to the ability of the new coronavirus to trigger a cytokine storm and a sequence of immunological responses, certain COVID-19 patients may quickly advance to multiple organ failure or death, septic shock, and acute respiratory distress syndrome. Therefore, prompt treatment in severe instances is necessary to avoid any additional liver damage. During COVID-19 clinical management, a variety of antiviral drugs, nonsteroidal anti-inflammatory drugs, traditional Chinese herbs, and glucocorticoids may be used. As such, it is advisable to streamline treatment and minimize the use of redundant medication types, doses, and durations to lower the risk of drug-induced liver injury[191].
COVID-19 liver injury management
The primary COVID-19 treatments currently employed include intensive care, treating hypoxemia with oxygenation assistance/mechanical ventilation, providing continuous renal replacement therapy for cytokine storm syndrome, maintaining sufficient blood volume, and other supportive therapies (Table 1). These therapies are essential for preventing and treating multiple organ failures, including liver damage[192].
Table 1.
Future directions and preventative strategies for coronavirus disease 2019
|
Aspect
|
Actions
|
| Prevention | Monitor liver biochemical markers (ALT/AST, bilirubin, prothrombin time and albumin, prothrombin time) to detect liver damage |
| Differentiate liver injury from other conditions (e.g., skeletal muscle or myocardial damage) | |
| Focus on the treatment of pre-existing patients with liver disease | |
| Consider continuation of antiviral medications for chronic hepatitis B to prevent reactivation | |
| Consider anti-HBV medications for patients receiving glucocorticoid therapy | |
| Cautiously monitor the COVID-19 course in patients with autoimmune liver disease on glucocorticoids or immunosuppressants | |
| Intensively monitor individuals with cirrhosis for complications and secondary infections due to immunocompromised state | |
| Reduce the risk of drug-induced liver impairment by streamlining the treatment and avoiding redundant pharmaceutical types, doses, and durations | |
| Management | Provide intensive care and supportive therapies to prevent and treat patients with multiple organ failure, including liver damage |
| Correct hypoxemia with oxygenation support or mechanical ventilation | |
| Continuous renal replacement therapy for cytokine storm syndrome | |
| Maintain adequate blood volume | |
| Monitor liver enzymes and other liver function markers regularly | |
| Implications | The COVID-19 pandemic has impacted the management of CLD and delayed screening and follow-up appointments |
| Future directions | Social isolation practices may lead to decompensation, mental health impairment, and malnutrition in CLD patients |
| COVID-19 can cause liver damage, potentially through direct harm, immune-mediated hepatotoxicity, or cytokine storm | |
| Liver involvement may be associated with the severity of COVID-19 | |
| Obesity and comorbid conditions like diabetes or hypertension increase the risk of liver disease and worsen SARS-CoV-2 infection | |
| Liver dysfunction is a potential risk factor for mortality in COVID-19 patients | |
| Liver cells may be directly infected by SARS-CoV-2, leading to liver dysfunction | |
| Histological characteristics of liver infection include significant apoptosis and binuclear hepatocytes |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CLD: Chronic liver disease; COVID-19: Coronavirus disease 2019; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
RESULTS AND DIRECTIONS FOR THE FUTURE
The COVID-19 pandemic has caused considerable setbacks in several healthcare services, particularly the management of CLD, and has been a historically severe worldwide health disaster. According to Besur et al[179], the pandemic delayed CLD screening and frequent follow-up appointments, which had an impact on CLD prevention and treatment and worsened the prognosis for CLD patients. Late detection of CLD consequences such as HCC may have an impact on these patient’s clinical outcomes. Social isolation practices have increased the likelihood that CLD patients may have decompensation, mental health impairment, and malnutrition[186]. Additionally, individuals with COVID-19 reported experiencing recurrent gastrointestinal issues[74]. In individuals with severe COVID-19, research found an abnormally high level of aminotransferase that may not have a hepatic origin[76]. The study shows a consistent link between the severity of COVID-19 and liver damage, although the mechanisms behind this damage are still unknown, given the multifaceted nature of the condition. One possible condition is hepatocyte apoptosis[187]. It was noted that while Frank's steatohepatitis symptoms were only present in two instances, macrovesicular steatosis, which displays a fat distribution unusual for NAFLD, was widespread in patients (75%). COVID-19 may have exacerbated steatosis in some individuals. This discovery is in line with those made public by previous investigations. For instance, a team connected to the Centers for Disease Control revealed that 50% of the livers from autopsies under study had steatosis[188]. ACE2 and TMPRSS2 are primarily used by SARS-CoV-2 as the docking and entrance receptor on host cells for cellular entry[189]. Additionally, it has been proposed that myositis, rather than liver damage, maybe the cause of the high aminotransferase level in COVID-19 individuals[191]. A reliable predictor of death was hypoalbuminemia[192]. Even at therapeutic quantities, acetaminophen, a common medication used to treat COVID-19 symptoms, can affect aminotransferase levels[193]. Compared to individuals without NAFLD, patients with NAFLD showed quicker disease development and a longer viral shedding time[194]. Obese patients with NAFLD also showed an increased risk for severe disease[195]. High PEEP may cause comparable hemodynamic changes in the liver of mechanically ventilated patients[176,187]. Most reports and research concentrate largely on the causes and side effects of cardiovascular damage due to the Kawasaki-like presentation of multisystem inflammatory syndrome in children (MIS-C)[188,174]. Recently, the assessment of COVID-19 severity or MIS-C presentation has incorporated liver involvement and the use of liver enzymes as potential prognostic markers[175]. Adults who have comorbid conditions, including obesity, diabetes, or hypertension, may develop non-alcoholic hepatosteatosis (fatty liver disease). The condition worsens patients’ SARS-CoV-2 infection[176]. According to research published in September 2020 by Zhou et al[186], younger children were more commonly affected by liver involvement in COVID-19 instances than older children. Young age-related liver immaturity is thought to be the cause[182]. Acute COVID-19 cases were thought to be caused by direct liver damage from hepatotropic viral invasion because of the reported ACE2 receptors on the surface of liver and bile duct epithelial cells[183]. By contrast, MIS-C[184] has a precise immune-mediated hepatotoxicity mechanism. When the COVID-19 infection is severe, “cytokine storm” and multiorgan dysfunction are types of liver involvement where cytokines encourage the increase and release of liver enzymes[185]. The progression of the reported situation is comparable to sepsis-associated liver dysfunction, in which increased levels of hepatic markers and bilirubin, as well as reduced synthesis function, result in hypoproteinemia and coagulation abnormalities[195]. Additionally, as part of the cytokine storm, IL-6 participation in COVID-19-related liver failure has been demonstrated[176]. The subfamily of SARS-CoV-2 has four genera, namely alpha-coronavirus, beta-coronavirus, gamma-coronavirus, and delta-coronavirus, according to genomic and phylogenetic studies[172]. Early in December 2019, Wuhan, China, reported the first case of pneumonia with a previously unidentified origin after being identified as a new beta-coronavirus using high-throughput sequencing research; the case was designated SARS-CoV-2. The WHO formally proclaimed the SARS-CoV-2 virus a pandemic of worldwide concern following its sudden global outbreak[148]. Early liver impairment in COVID-19 individuals raises their mortality risk. Cholestasis was detected in 151 patients (42.5%) and hepatocellular damage in 101 patients (28.5%). It was shown that severely sick individuals were more likely to have liver dysfunction[149]. In all, 9.6% of the chosen group had an elevated ALP level > 150 U/L and a cholestatic pattern of liver damage. High levels of ALT/AST, GGT, ALP, and TBIL were found to be the aberrant liver functions that were observed in a different investigation of cholestatic liver damage carried out at the Shanghai Public Health Clinical Center from January 20 to December 31, 2020[150]. The most frequent cancer is HCC, nevertheless. Patients with HCC also have underlying CLD, such as chronic hepatitis B or C virus infection, NAFLD, and alcoholic liver damage[151]. According to reports, COVID-19 infections are very likely to occur in cancer patients. A study in a Chinese hospital showed that 28 of 1276 confirmed COVID-19 cases had cancers, with two having HCC[152]. SARS-CoV-2 infection of liver cells may be directly connected to liver dysfunction in COVID-19 patients. About 2% to 10% of COVID-19 patients who had diarrhea contain SARS-CoV-2 RNA in their blood and stool, suggesting the possibility of exposure to the liver virus. The upper respiratory tract, lung tissue, and liver cholangiocytes are considered the main target sites for SARS-CoV-2 and SARS-CoV due to their affinity for the ACE2 receptor. In these tissues, the virus multiplies and shows symptoms[183] and the reported conspicuous cytopathy. Disturbed levels of liver enzymes, an elevated alveolar-arterial oxygen gradient and GGT level, a reduced level of albumin, and the presence of circulating CD4+ T cells and B lymphocytes are all indicators of SARS-CoV-2 infection and significant apoptosis with binuclear hepatocytes are the main histological characteristics of COVID-19 liver infection[195].
CONCLUSION
COVID-19 has been shown to impact additional organs in addition to the respiratory system, with the liver being one of the most often afflicted organs. Several factors, such as virus-associated immunological liver injury, direct cholangiocyte destruction resulting in liver injury, hypoxia injury, DILI, and autoimmune liver disease, can result in liver damage.
ACKNOWLEDGEMENTS
The authors want to thank the organizations that provided support and facilities.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 25, 2023
First decision: January 5, 2024
Article in press: February 28, 2024
Specialty type: Infectious diseases
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li H, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Cai YX
Contributor Information
Lokjan Singh, Department of Microbiology, Karnali Academy of Health Science, Teaching Hospital, Jumla 21200, Karnali, Nepal.
Anil Kumar, Department of Microbiology, Karnali Academy of Health Science, Teaching Hospital, Jumla 21200, Karnali, Nepal.
Maya Rai, Department of Microbiology, Karnali Academy of Health Science, Teaching Hospital, Jumla 21200, Karnali, Nepal.
Bibek Basnet, Health Sciences, Asian College of Advance Studies, Purbanchal University, Satdobato 24122, Lalitpur, Nepal.
Nishant Rai, Department of Biotechnology, Graphic Era (Deemed to be University), Dehradun 248002, Uttarakhand, India.
Pukar Khanal, Department of Pharmacology & Toxicology, KLE College of Pharmacy, Belagavi, KLE Academy of Higher Education and Research, Belagavi 590010, Karnataka, India.
Kok-Song Lai, Division of Health Sciences, Abu Dhabi Women's College, Higher Colleges of Technology, Abu Dhabi 41012, United Arab Emirates.
Wan-Hee Cheng, Health and Life Sciences, INTI International University, Nilai 71800, Malaysia.
Ahmed Morad Asaad, Department of Microbiology, College of Medicine, Zagazig University, Zagazig 44519, Egypt.
Shamshul Ansari, Division of Health Sciences, Abu Dhabi Women's College, Higher Colleges of Technology, Abu Dhabi 41012, United Arab Emirates. shamshulansari483@yahoo.com.
References
- 1.John Hopkins University and Medicine. COVID-19 Map. John Hopkins Corona virus Resource Center. 2020; March; Suppl: 8-19 COVID-19 Map - Johns Hopkins Coronavirus Resource Center (jhu.edu) [Google Scholar]
- 2.Xie M, Chen Q. Insight into 2019 novel coronavirus - An updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver international: official journal of the International Association for the Study of the Liver, 2020; 40:1278–1281. [DOI] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID-19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta-Analysis. Trop Med Infect Dis. 2020;5 doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CM, Chen XY, Cai YP, XiaJA, ZhouX, XuS, HuangHP, ZhangL, DuCL, ZhangYY, SongJ, WangSJ, ChaoYC, YangZY, Xu J, ChenDC, XiongWN, XuL, ZhouF, JiangJJ, BaiCX, ZhengJH, SongYL Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeckmans J, Rodrigues RM, Demuyser T, Piérard D, Vanhaecke T, Rogiers V. COVID-19 and drug-induced liver injury: a problem of plenty or a petty point? Arch Toxicol. 2020;94:1367–1369. doi: 10.1007/s00204-020-02734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav DK, Singh A, Zhang Q, Bai X, Zhang W, Yadav RK, Zhiwei L, Adhikari VP, Liang T. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulkarni AV, Tevethia HV, Arab JP, Candia R, Premkumar M, Kumar P, Sharma M, Reddy DN, Padaki NR. Efficacy and safety of obeticholic acid in liver disease-A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45:101675. doi: 10.1016/j.clinre.2021.101675. [DOI] [PubMed] [Google Scholar]
- 22.Paliogiannis P, Zinellu A. Bilirubin levels in patients with mild and severe Covid-19: A pooled analysis. Liver Int. 2020;40:1787–1788. doi: 10.1111/liv.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshanshad R, Roshanshad A, Fereidooni R, Hosseini-Bensenjan M. COVID-19 and liver injury: Pathophysiology, risk factors, outcome and management in special populations. World J Hepatol. 2023;15:441–459. doi: 10.4254/wjh.v15.i4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome: Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55:105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, Du R. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han D, Fang Q, Wang X. SARS-CoV-2 was found in the bile juice from a patient with severe COVID-19. J Med Virol. 2021;93:102–104. doi: 10.1002/jmv.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 35.Shieh WJ, Hsiao CH, Paddock CD, Guarner J, Goldsmith CS, Tatti K, Packard M, Mueller L, Wu MZ, Rollin P, Su IJ, Zaki SR. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36:303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tandra S, Yeh MM, Brunt EM, Vuppalanchi R, Cummings OW, Ünalp-Arida A, Wilson LA, Chalasani N. Presence and significance of microvesicular steatosis in nonalcoholic fatty liver disease. J Hepatol. 2011;55:654–659. doi: 10.1016/j.jhep.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21:183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prentice E, Jerome WG, Yoshimori T, Mizushima N, Denison MR. Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cottam EM, Maier HJ, Manifava M, Vaux LC, Chandra-Schoenfelder P, Gerner W, Britton P, Ktistakis NT, Wileman T. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy. 2011;7:1335–1347. doi: 10.4161/auto.7.11.16642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cottam EM, Whelband MC, Wileman T. Coronavirus NSP6 restricts autophagosome expansion. Autophagy. 2014;10:1426–1441. doi: 10.4161/auto.29309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kindrachuk J, Ork B, Hart BJ, Mazur S, Holbrook MR, Frieman MB, Traynor D, Johnson RF, Dyall J, Kuhn JH, Olinger GG, Hensley LE, Jahrling PB. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gassen NC, PapiesJ, BajajT, DethloffF, EmanuelJ, WeckmannK, HeinzDE, HeinemannN, Lennarz M, Richter A, Niemeyer D, CormanVM, Giavalisco P, Drosten C, MüllerMA Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. bioRxiv. 2020 [Google Scholar]
- 44.Song Y, Lim JY, Lim T, Im KI, Kim N, Nam YS, Jeon YW, Shin JC, Ko HS, Park IY, Cho SG. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J Stem Cells. 2020;12:1032–1049. doi: 10.4252/wjsc.v12.i9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu H, Tong Z, Ma P, Hu M, Peng Z, Wu W, Du B China Critical Care Clinical Trials Group (CCCCTG) Intensive care during the coronavirus epidemic. Intensive Care Med. 2020;46:576–578. doi: 10.1007/s00134-020-05966-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, Du B, Aboodi M, Wunsch H, Cecconi M, Koh Y, Chertow DS, Maitland K, Alshamsi F, Belley-Cote E, Greco M, Laundy M, Morgan JS, Kesecioglu J, McGeer A, Mermel L, Mammen MJ, Alexander PE, Arrington A, Centofanti JE, Citerio G, Baw B, Memish ZA, Hammond N, Hayden FG, Evans L, Rhodes A. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. bioRxiv. 2020 doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 52.Jiang S, Hillyer C, Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu YQ, Cao J, Zhang XY, Gao H, Wang YY, Wang J, Zhang JL, Shen GH, Jiang XY, Yang J, Zheng XC, Xu JQ, Zhang CC, Lan F, Qu D, Zhao Y, Xu GL, Xie YH, Luo M, Lu ZJ. Interaction network of SARS-CoV-2 with host receptome through spike protein. bioRxiv. :2020. [Google Scholar]
- 55.Sarin SK. "Fast, faster, and fastest: science on the run during COVID-19 drama"-"do not forget the liver". Hepatol Int. 2020;14:454–455. doi: 10.1007/s12072-020-10042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao R, Rieder F, Ben-Horin S, Kaplan GG, Ng SC, Wong GL, Ghosh S, Chen MH. Implications of COVID-19 for patients with pre-existing digestive diseases: an update. Lancet Gastroenterol Hepatol. 2021;6:258–260. doi: 10.1016/S2468-1253(21)00025-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li LY, Wu W, Chen S, Gu JW, Li XL, Song HJ, Du F, Wang G, Zhong CQ, Wang XY, Chen Y, Shah R, Yang HM, Cai Q. Digestive system involvement of novel coronavirus infection: Prevention and control infection from a gastroenterology perspective. J Dig Dis. 2020;21:199–204. doi: 10.1111/1751-2980.12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5:335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: a systematic review and meta-analysis. Hepatol Int. 2020;14:621–637. doi: 10.1007/s12072-020-10074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790–1796. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263–268. doi: 10.14218/JCTH.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lightsey J, Rockey DC. Current concepts in ischemic hepatitis. Current Opinion in Gastroenterology 2017; 33:158-163. [DOI] [PubMed] [Google Scholar]
- 70.Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265:174–189. doi: 10.1097/00000441-197303000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Rosser BG, Gores GJ. Liver cell necrosis: Cellular mechanisms and clinical implications, Gastroenterology, Volume 108, Issue 1, 1995, Pages 252-275, ISSN 0016-5085. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization. Corticosteroids for COVID-19. Living guidance. September; 2020. Corticosteroids for COVID-19 (who.int) [Google Scholar]
- 73.McDonald B, Kubes P. Innate Immune Cell Trafficking and Function During Sterile Inflammation of the Liver. Gastroenterology. 2016;151:1087–1095. doi: 10.1053/j.gastro.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 74.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 75.Hu TY, Frieman M, Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat Nanotechnol. 2020;15:247–249. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan GW, Gao L, Wang JW, Wen XJ, Mao TH, Peng SW, Zhang T, Chen XM, Lu FM. [Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia] Zhonghua Gan Zang Bing Za Zhi. 2020;28:100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 77.Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci. 2004;61:2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509–519. doi: 10.1177/2050640620924157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polidoro RB, Hagan RS, de Santis Santiago R, Schmidt NW. Overview: Systemic Inflammatory Response Derived From Lung Injury Caused by SARS-CoV-2 Infection Explains Severe Outcomes in COVID-19. Front Immunol. 2020;11:1626. doi: 10.3389/fimmu.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imbiakha B, Sahler JM, Buchholz DW, Ezzatpour S, Jager M, Choi A, Monreal IA, Byun H, Adeleke RA, Leach J, Whittaker G, Dewhurst S, Rudd BD, Aguilar HC, August A. Adaptive immune cells are necessary for SARS-CoV-2-induced pathology. Sci Adv. 2024;10:eadg5461. doi: 10.1126/sciadv.adg5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sonzogni A, Previtali G, Seghezzi M, Alessio MG, Gianatti A, Licini L, Zerbi P, Carsana L, Rossi RS, Lauri E, Pellegrinelli A, Nebuloni M. Liver and COVID 19 Infection: A Very Preliminary Lesson Learnt from Histological Post-mortem Findings in 48 patients. 2020. [Google Scholar]
- 86.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ozma MA, Maroufi P, Khodadadi E, Köse Ş, Esposito I, Ganbarov K, Dao S, Esposito S, Dal T, Zeinalzadeh E, Kafil HS. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez Med. 2020;28:153–165. [PubMed] [Google Scholar]
- 88.D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agarwal A, Chen A, Ravindran N, To C, Thuluvath PJ. Gastrointestinal and Liver Manifestations of COVID-19. J Clin Exp Hepatol. 2020;10:263–265. doi: 10.1016/j.jceh.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ishiguro T, Kobayashi Y, Shimizu Y, Uemura Y, Toriba R, Takata N, Ueda M. Prognostic factors of virus-associated pneumonia other than COVID-19 in adults. Respir Med. 2024;221:107497. doi: 10.1016/j.rmed.2023.107497. [DOI] [PubMed] [Google Scholar]
- 91.Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, Izadi Z, Jacobsohn L, Katz P, Lawson-Tovey S, Mateus EF, Rush S, Schmajuk G, Simard J, Strangfeld A, Trupin L, Wysham KD, Bhana S, Costello W, Grainger R, Hausmann JS, Liew JW, Sirotich E, Sufka P, Wallace ZS, Yazdany J, Machado PM, Robinson PC COVID-19 Global Rheumatology Alliance. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ungaro RC, Brenner EJ, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, Ng SC, Rahier JF, Reinisch W, Steinwurz F, Underwood FE, Zhang X, Colombel JF, Kappelman MD. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70:725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sonzogni A, Licini L, D’Antiga L. Pathology of Allograft Liver Dysfunction. In: D'Antiga, L. (eds) Pediatric Hepatology and Liver Transplantation. Springer, Cham. 2019. [Google Scholar]
- 95.Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J Infect. 2020;81:e93–e95. doi: 10.1016/j.jinf.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tassone D, Thompson A, Connell W, Lee T, Ungaro R, An P, Ding Y, Ding NS. Immunosuppression as a risk factor for COVID-19: a meta-analysis. Intern Med J. 2021;51:199–205. doi: 10.1111/imj.15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dixit SB, Zirpe KG, Kulkarni AP, Chaudhry D, Govil D, Mehta Y, Jog SA, Khatib KI, Pandit RA, Samavedam S, Rangappa P, Bandopadhyay S, Shrivastav O, Mhatre U. Current Approaches to COVID-19: Therapy and Prevention. Indian J Crit Care Med. 2020;24:838–846. doi: 10.5005/jp-journals-10071-23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yachimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355–1365.e4. doi: 10.1016/j.cgh.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y, Liu S, Cheng B, Zhang M, Wang L, Niu S, Yao Z, Deng X, Zhou F, Wei W, Li Q, Chen X, Chen W, Yang Q, Wu S, Fan J, Shu B, Hu Z, Wang S, Yang XP, Liu W, Miao X, Wang Z. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 102.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. doi: 10.1007/s10096-020-04138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. doi: 10.1016/j.aohep.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768–771.e3. doi: 10.1053/j.gastro.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naaraayan A, Nimkar A, Hasan A, Pant S, Durdevic M, Elenius H, Nava Suarez C, Jesmajian S. Analysis of Male Sex as a Risk Factor in Older Adults With Coronavirus Disease 2019: A Retrospective Cohort Study From the New York City Metropolitan Region. Cureus. 2020;12:e9912. doi: 10.7759/cureus.9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377–390. doi: 10.3748/wjg.v27.i5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kelly DM, Akhtar S, Sellers DJ, Muraleedharan V, Channer KS, Jones TH. Testosterone differentially regulates targets of lipid and glucose metabolism in liver, muscle and adipose tissues of the testicular feminised mouse. Endocrine. 2016;54:504–515. doi: 10.1007/s12020-016-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Q, Davis PB, Xu R. COVID-19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine. 2021;31:100688. doi: 10.1016/j.eclinm.2020.100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heimbach JK, Taner T. SARS-CoV-2 infection in liver transplant recipients: collaboration in the time of COVID-19. Lancet Gastroenterol Hepatol. 2020;5:958–960. doi: 10.1016/S2468-1253(20)30293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987–2999. doi: 10.3748/wjg.v26.i22.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H, Al-Jahdali HH, Baharoon SA, Arabi YM. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72:516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, Cao Y, Ding R, Wang JJ, Cheng C, Xie W. Pattern of liver injury in adult patients with COVID-19: a retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. doi: 10.1186/s40779-020-00256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wan J, Wang X, Su S, Zhang Y, Jin Y, Shi Y, Wu K, Liang J. Digestive symptoms and liver injury in patients with coronavirus disease 2019 (COVID-19): A systematic review with meta-analysis. JGH Open. 2020;4:1047–1058. doi: 10.1002/jgh3.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization. WHO coronavirus disease (COVID19) situation dashboard. 2020; cited 6 March 2021 COVID-19 cases | WHO COVID-19 dashboard. [Google Scholar]
- 118.Tezer H, Bedir Demirdağ T. Novel coronavirus disease (COVID-19) in children. Turk J Med Sci. 2020;50:592–603. doi: 10.3906/sag-2004-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18–24. doi: 10.1016/j.ejim.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsumita T, Takeda R, Maishi N, Hida Y, Sasaki M, Orba Y, Sato A, Toba S, Ito W, Teshirogi T, Sakurai Y, Iba T, Naito H, Ando H, Watanabe H, Mizuno A, Nakanishi T, Matsuda A, Zixiao R, Lee JW, Iimura T, Sawa H, Hida K. Viral uptake and pathophysiology of the lung endothelial cells in age-associated severe SARS-CoV-2 infection models. Aging Cell. 2024;23:e14050. doi: 10.1111/acel.14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu H, Zhu H, Yuan C, Yao C, Luo W, Shen X, Wang J, Shao J, Xiang Y. Clinical and Immune Features of Hospitalized Pediatric Patients With Coronavirus Disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. doi: 10.1001/jamanetworkopen.2020.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 123.Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612–620. doi: 10.1007/s12072-020-10078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ji D, Zhang M, Qin E, Zhang L, Xu J, Wang Y, Cheng G, Wang F, Lau G. Letter to the Editor: Obesity, diabetes, non-alcoholic fatty liver disease and metabolic dysfunction associated fatty liver disease are proinflammatory hypercoagulable states associated with severe disease and thrombosis in Covid-19. Metabolism. 2021;115:154437. doi: 10.1016/j.metabol.2020.154437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.El Kassas M, Alboraie M, Al Balakosy A, Abdeen N, Afify S, Abdalgaber M, Sherief AF, Madkour A, Abdellah Ahmed M, Eltabbakh M, Salaheldin M, Wifi MN. Liver transplantation in the era of COVID-19. Arab J Gastroenterol. 2020;21:69–75. doi: 10.1016/j.ajg.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Choudhary NS, Dhampalwar S, Saraf N, Soin AS. Outcomes of COVID-19 in Patients with Cirrhosis or Liver Transplantation. J Clin Exp Hepatol. 2021;11:713–719. doi: 10.1016/j.jceh.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xing QQ, Dong X, Ren YD, Chen WM, Zeng DY, Cai YY, Hong MZ, Pan JS. Liver Chemistries in Patients With COVID-19 Who Were Discharged Alive or Died: A Meta-analysis. Hepatol Commun. 2021;5:12–23. doi: 10.1002/hep4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211:2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The Role of Thymus-Expressed Chemokine and Its Receptor CCR9 on Lymphocytes in the Regional Specialization of the Mucosal Immune System1. J Immunol. 2000:165: 5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 130.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood. 2006;107:3447–3454. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- 131.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.SOHU News. Doctor blackened after successful rescue? Expert: It could be multiple organ damage [in Chinese]. (2020). Available from: https://www.sohu.com/a/389423079_160789 .
- 133.Mahdiabadi S, Rajabi F, Tavakolpour S, Rezaei N. Immunological aspects of COVID-19-related skin manifestations: Revisiting pathogenic mechanism in the light of new evidence. Dermatol Ther. 2022;35:e15758. doi: 10.1111/dth.15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jee SH, Lee SY, Chiu HC, Chang CC, Chen TJ. Effects of estrogen and estrogen receptor in normal human melanocytes. Biochem Biophys Res Commun. 1994;199:1407–1412. doi: 10.1006/bbrc.1994.1387. [DOI] [PubMed] [Google Scholar]
- 135.Burra P. Liver abnormalities and endocrine diseases. Best Pract Res Clin Gastroenterol. 2013;27:553–563. doi: 10.1016/j.bpg.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 136.Videira IF, Moura DF, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruan X, Lu X, Wang K, Zhang B, Wang J, Li Y, Xu Z, Yan F. Liver injury after antiviral treatment of critically ill patients with COVID-19: a single-centered retrospective cohort study. Ann Palliat Med. 2021;10:2429–2438. doi: 10.21037/apm-20-1581. [DOI] [PubMed] [Google Scholar]
- 138.Brito CA, Barros FM, Lopes EP. Mechanisms and consequences of COVID-19 associated liver injury: What can we affirm? World J Hepatol. 2020;12:413–422. doi: 10.4254/wjh.v12.i8.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ferron PJ, Gicquel T, Mégarbane B, Clément B, Fromenty B. Treatments in Covid-19 patients with pre-existing metabolic dysfunction-associated fatty liver disease: A potential threat for drug-induced liver injury? Biochimie. 2020;179:266–274. doi: 10.1016/j.biochi.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.de Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58:71–80. doi: 10.1111/j.1365-2125.2004.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romani L, Tomino C, Puccetti P, Garaci E. Off-label therapy targeting pathogenic inflammation in COVID-19. Cell Death Discov. 2020;6:49. doi: 10.1038/s41420-020-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cherry J, Demmler-Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. Feigin and Cherry's textbook of pediatric infectious disease. 8th ed. Philadelphia (PA): Elsevier Inc., 2019:1846-54. Feigin and Cherry's Textbook of Pediatric Infectious Diseases | ScienceDirect. [Google Scholar]
- 143.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 144.Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G, Zhao K, Ouyang S. Liver injury in COVID-19: clinical features and treatment management. Virol J. 2021;18:121. doi: 10.1186/s12985-021-01593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guerra JVS, Dias MMG, Brilhante AJVC, Terra MF, García-Arévalo M, Figueira ACM. Multifactorial Basis and Therapeutic Strategies in Metabolism-Related Diseases. Nutrients. 2021;13 doi: 10.3390/nu13082830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kukla M, Skonieczna-Żydecka K, Kotfis K, Maciejewska D, Łoniewski I, Lara LF, Pazgan-Simon M, Stachowska E, Kaczmarczyk M, Koulaouzidis A, Marlicz W. COVID-19, MERS and SARS with Concomitant Liver Injury-Systematic Review of the Existing Literature. J Clin Med. 2020;9 doi: 10.3390/jcm9051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sanz F, Gimeno C, Lloret T, Tormo N, Briones M, Fernández E, Cervera A, Aguar M, Chiner E, Blanquer J. Relationship between the presence of hypoxemia and the inflammatory response measured by C-reactive protein in bacteremic pneumococcal pneumonia. Eur Respir J. 2011;38:2492–2496. [Google Scholar]
- 148.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 149.Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de Wilde AH, Snijder EJ, Kikkert M, van Hemert MJ. Host Factors in Coronavirus Replication. Curr Top Microbiol Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elrobaa IH, New KJ. COVID-19: Pulmonary and Extra Pulmonary Manifestations. Front Public Health. 2021;9:711616. doi: 10.3389/fpubh.2021.711616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wu ZH, Yang DL. A meta-analysis of the impact of COVID-19 on liver dysfunction. Eur J Med Res. 2020;25:54. doi: 10.1186/s40001-020-00454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bernal-Monterde V, Casas-Deza D, Letona-Giménez L, de la Llama-Celis N, Calmarza P, Sierra-Gabarda O, Betoré-Glaria E, Martínez-de Lagos M, Martínez-Barredo L, Espinosa-Pérez M, M Arbones-Mainar J. SARS-CoV-2 Infection Induces a Dual Response in Liver Function Tests: Association with Mortality during Hospitalization. Biomedicines. 2020;8 doi: 10.3390/biomedicines8090328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yan B, Freiwald T, Chauss D, Wang L, West E, Mirabelli C, Zhang CJ, Nichols EM, Malik N, Gregory R, Bantscheff M, Ghidelli-Disse S, Kolev M, Frum T, Spence JR, Sexton JZ, Alysandratos KD, Kotton DN, Pittaluga S, Bibby J, Niyonzima N, Olson MR, Kordasti S, Portilla D, Wobus CE, Laurence A, Lionakis MS, Kemper C, Afzali B, Kazemian M. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abg0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Q, Bastard P, Liu Z, Le Pen J, Moncada-Velez M, Chen J, Ogishi M, Sabli IKD, Hodeib S, Korol C, Rosain J, Bilguvar K, Ye J, Bolze A, Bigio B, Yang R, Arias AA, Zhou Q, Zhang Y, Onodi F, Korniotis S, Karpf L, Philippot Q, Chbihi M, Bonnet-Madin L, Dorgham K, Smith N, Schneider WM, Razooky BS, Hoffmann HH, Michailidis E, Moens L, Han JE, Lorenzo L, Bizien L, Meade P, Neehus AL, Ugurbil AC, Corneau A, Kerner G, Zhang P, Rapaport F, Seeleuthner Y, Manry J, Masson C, Schmitt Y, Schlüter A, Le Voyer T, Khan T, Li J, Fellay J, Roussel L, Shahrooei M, Alosaimi MF, Mansouri D, Al-Saud H, Al-Mulla F, Almourfi F, Al-Muhsen SZ, Alsohime F, Al Turki S, Hasanato R, van de Beek D, Biondi A, Bettini LR, D'Angio' M, Bonfanti P, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Oler AJ, Tompkins MF, Alba C, Vandernoot I, Goffard JC, Smits G, Migeotte I, Haerynck F, Soler-Palacin P, Martin-Nalda A, Colobran R, Morange PE, Keles S, Çölkesen F, Ozcelik T, Yasar KK, Senoglu S, Karabela ŞN, Rodríguez-Gallego C, Novelli G, Hraiech S, Tandjaoui-Lambiotte Y, Duval X, Laouénan C COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group, Snow AL, Dalgard CL, Milner JD, Vinh DC, Mogensen TH, Marr N, Spaan AN, Boisson B, Boisson-Dupuis S, Bustamante J, Puel A, Ciancanelli MJ, Meyts I, Maniatis T, Soumelis V, Amara A, Nussenzweig M, García-Sastre A, Krammer F, Pujol A, Duffy D, Lifton RP, Zhang SY, Gorochov G, Béziat V, Jouanguy E, Sancho-Shimizu V, Rice CM, Abel L, Notarangelo LD, Cobat A, Su HC, Casanova JL. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 (6515):eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, Dorgham K, Philippot Q, Rosain J, Béziat V, Manry J, Shaw E, Haljasmägi L, Peterson P, Lorenzo L, Bizien L, Trouillet-Assant S, Dobbs K, de Jesus AA, Belot A, Kallaste A, Catherinot E, Tandjaoui-Lambiotte Y, Le Pen J, Kerner G, Bigio B, Seeleuthner Y, Yang R, Bolze A, Spaan AN, Delmonte OM, Abers MS, Aiuti A, Casari G, Lampasona V, Piemonti L, Ciceri F, Bilguvar K, Lifton RP, Vasse M, Smadja DM, Migaud M, Hadjadj J, Terrier B, Duffy D, Quintana-Murci L, van de Beek D, Roussel L, Vinh DC, Tangye SG, Haerynck F, Dalmau D, Martinez-Picado J, Brodin P, Nussenzweig MC, Boisson-Dupuis S, Rodríguez-Gallego C, Vogt G, Mogensen TH, Oler AJ, Gu J, Burbelo PD, Cohen JI, Biondi A, Bettini LR, D'Angio M, Bonfanti P, Rossignol P, Mayaux J, Rieux-Laucat F, Husebye ES, Fusco F, Ursini MV, Imberti L, Sottini A, Paghera S, Quiros-Roldan E, Rossi C, Castagnoli R, Montagna D, Licari A, Marseglia GL, Duval X, Ghosn J HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort, Tsang JS, Goldbach-Mansky R, Kisand K, Lionakis MS, Puel A, Zhang SY, Holland SM, Gorochov G, Jouanguy E, Rice CM, Cobat A, Notarangelo LD, Abel L, Su HC, Casanova JL. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. [Google Scholar]
- 160.Casanova JL, Abel L. Mechanisms of viral inflammation and disease in humans. Science. 2021;374:1080–1086. doi: 10.1126/science.abj7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348–364. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 165.Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, Lyu J, Dai ZM. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shajahan SR, Kumar S, Ramli MDC. Unravelling the connection between COVID-19 and Alzheimer's disease: a comprehensive review. Front Aging Neurosci. 2023;15:1274452. doi: 10.3389/fnagi.2023.1274452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang X, Zhao J, Yan Q, Zhang S, Wang Y, Li Y. A case of COVID-19 patient with the diarrhea as initial symptom and literature review. Clin Res Hepatol Gastroenterol. 2020;44:e109–e112. doi: 10.1016/j.clinre.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cao X, Xie Y, Zhou C, Mu H. Clinical characteristics associated with recurrent viral RNA positivity in patients within two weeks after recovering from the first SARS-CoV-2 infection. Biomol Biomed. 2024;24:196–204. doi: 10.17305/bb.2023.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095–2103. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 171.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Acetaminophen. [Updated 2016 Jan 28]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548162/
- 174.Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. doi: 10.1016/j.metabol.2020.154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kotzampassi K, Paramythiotis D, Eleftheriadis E. Deterioration of visceral perfusion caused by intra-abdominal hypertension in pigs ventilated with positive end-expiratory pressure. Surg Today. 2000;30:987–992. doi: 10.1007/s005950070018. [DOI] [PubMed] [Google Scholar]
- 176.Kredel M, Muellenbach RM, Johannes A, Brederlau J, Roewer N, Wunder C. Hepatic effects of lung-protective pressure-controlled ventilation and a combination of high-frequency oscillatory ventilation and extracorporeal lung assist in experimental lung injury. Med Sci Monit. 2011;17:BR275–BR281. doi: 10.12659/MSM.881974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ronco C, Navalesi P, Vincent JL. Coronavirus epidemic: preparing for extracorporeal organ support in intensive care. Lancet Respir Med. 2020;8:240–241. doi: 10.1016/S2213-2600(20)30060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Besur S, Begum R, Gunabushanam V, Bonkovsky HL. Liver disease in the Era of Coronavirus Disease 19 (COVID-19) pandemic. Ann ClinGastroenterol Hepatol. 2020;4:52–57. [Google Scholar]
- 180.Righi FA, Vander Heide RS, Graham RP, Aubry MC, Trejo-Lopez JA, Bois MC, Roden AC, Reichard R, Maleszewski JJ, Alexander MP, Quinton RA, Jenkins SM, Hartley CP, Hagen CE. A case-control autopsy series of liver pathology associated with novel coronavirus disease (COVID-19) Ann Diagn Pathol. 2024;68:152240. doi: 10.1016/j.anndiagpath.2023.152240. [DOI] [PubMed] [Google Scholar]
- 181.Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, Goldsmith CS, Silva-Flannery L, Seixas JN, Reagan-Steiner S, Uyeki T, Denison A, Bhatnagar J, Shieh WJ, Zaki SR COVID-19 Pathology Working Group. Pathology and Pathogenesis of SARS-CoV-2 Associated with Fatal Coronavirus Disease, United States. Emerg Infect Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rowley AH. Is Kawasaki disease an infectious disorder? Int J Rheum Dis. 2018;21:20–25. doi: 10.1111/1756-185X.13213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20:453–454. doi: 10.1038/s41577-020-0367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 186.Zhou YH, Zheng KI, Targher G, Byrne CD, Zheng MH. Abnormal liver enzymes in children and infants with COVID-19: A narrative review of case-series studies. Pediatr Obes. 2020;15:e12723. doi: 10.1111/ijpo.12723. [DOI] [PubMed] [Google Scholar]
- 187.Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522–532. doi: 10.4254/wjh.v13.i5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Woźnica EA, Inglot M, Woźnica RK, Łysenko L. Liver dysfunction in sepsis. Adv Clin Exp Med. 2018;27:547–551. doi: 10.17219/acem/68363. [DOI] [PubMed] [Google Scholar]
- 189.Malik YS, Sircar S, Bhat S, Sharun K, Dhama K, Dadar M, Tiwari R, Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arshad MI, Khan HA, Aslam B, Khan JA. Appraisal of One Health approach amid COVID-19 and zoonotic pandemics: insights for policy decision. Trop Anim Health Prod. 2020;53:11. doi: 10.1007/s11250-020-02479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fu L, Fei J, Xu S, Xiang HX, Xiang Y, Hu B, Li MD, Liu FF, Li Y, Li XY, Zhao H, Xu DX. Liver Dysfunction and Its Association with the Risk of Death in COVID-19 Patients: A Prospective Cohort Study. J Clin Transl Hepatol. 2020;8:246–254. doi: 10.14218/JCTH.2020.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chan SL, Kudo M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer. 2020;9:491–502. doi: 10.1159/000510765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kudo M, Kurosaki M, Ikeda M, Aikata H, Hiraoka A, Torimura T, Sakamoto N. Treatment of hepatocellular carcinoma during the COVID-19 outbreak: The Working Group report of JAMTT-HCC. Hepatol Res. 2020;50:1004–1014. doi: 10.1111/hepr.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Przekop D, Gruszewska E, Chrostek L. Liver function in COVID-19 infection. World J Hepatol. 2021;13:1909–1918. doi: 10.4254/wjh.v13.i12.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]