Abstract
Objective:
To investigate the associations among underweight body mass index (BMI), pregnancy, and obstetric outcomes among women using assisted reproductive technology (ART).
Design:
Retrospective cohort study using national data and log binomial regression.
Setting:
Not applicable.
Patient(s):
Women undergoing IVF in the United States from 2008 to 2013.
Intervention(s):
None.
Main Outcome Measure(s):
Pregnancy outcomes (intrauterine pregnancy, live birth rates) per transfer, miscarriage rate per pregnancy, and low birth weight and preterm delivery rates among singleton and twin pregnancies.
Result(s):
For all fresh autologous in vitro fertilization (IVF) cycles in the United States from 2008 to 2013 (n = 494,097 cycles, n = 402,742 transfers, n = 180,855 pregnancies) reported to the national ART Surveillance System, compared with normal weight women, underweight women had a statistically significant decreased chance of intrauterine pregnancy (adjusted risk ratio [aRR] 0.97; 95% confidence interval [CI], 0.96–0.99) and live birth (aRR 0.95; 95% CI, 0.93–0.98) per transfer. Obese women also had a statistically decreased likelihood of both (aRR 0.94; 95% CI, 0.94–0.95; aRR 0.87; 95% CI, 0.86–0.88, respectively). Among cycles resulting in singleton pregnancy, both underweight and obese statuses were associated with increased risk of low birth weight (aRR 1.39; 95% CI, 1.25–1.54, aRR 1.26; 95% CI, 1.20–1.33, respectively) and preterm delivery (aRR 1.12; 95% CI, 1.01–1.23, aRR 1.42; 95% CI, 1.36–1.48, respectively). The association between underweight status and miscarriage was not statistically significant (aRR 1.04; 95% CI, 0.98–1.11). In contrast, obesity was associated with a statistically significantly increased miscarriage risk (aRR 1.23; 95% CI, 1.20–1.26).
Conclusion(s):
Among women undergoing IVF, prepregnancy BMI affects pregnancy and obstetric outcomes. Underweight status may have a limited impact on pregnancy and live-birth rates, but it is associated with increased preterm and low-birth-weight delivery risk. Obesity negatively impacts all ART and obstetric outcomes investigated.
Keywords: IVF, miscarriage, outcomes, preterm, underweight
As the obesity epidemic continues to plague the United States, numerous reports have been published and recommendations made regarding the negative impact of obesity on fertility (1), assisted reproductive technology (ART) effectiveness (2–9), and pregnancy and obstetric outcomes (5, 10). By contrast, limited and conflicting data exist on the impact of being underweight (body mass index [BMI] <18.5 kg/m2), admittedly a less common problem, on fertility and the effectiveness of ART. A few small studies to date have evaluated the impact of low BMI on ART outcomes and have not found a statistically significant difference in underweight women as compared with their normal-weight counterparts (11–14). Nonetheless, many clinicians recommend weight gain in women with low BMI who desire in vitro fertilization (IVF) treatment based on small retrospective studies that have reported a lower absolute clinical pregnancy rate among underweight women using ART (11, 15).
Many clinicians are aware of the association between obesity and miscarriage; however, existing studies suggest that both extremities of BMI, both underweight and obese statuses, may be associated with increased miscarriage risk in the general population (16) and in the ART population (17). Additionally, prepregnancy underweight status coupled with poor weight gain has been associated with worse obstetric outcomes such as preterm delivery, preterm premature rupture of membranes, and low birth weight in the general population (18–21).
To our knowledge, the impact of prepregnancy underweight status on IVF and perinatal outcomes has not been investigated among a large cohort of ART-conceived pregnancies. We used National ART Surveillance System (NASS) data from 2008 through 2013 to investigate the association between BMI and ART on pregnancy and obstetric outcomes. The overweight BMI categories were included to put the underweight results in perspective. We hypothesized that underweight status, like overweight status, would be associated with an increased risk of adverse ART and obstetric outcomes. We also calculated trends in BMI among women undergoing ART during the 5-year interval.
MATERIALS AND METHODS
The Centers for Disease Control and Prevention’s National ART Surveillance System (NASS), a federally mandated, validated system that includes over 98% of all ART cycles performed in the United States, was used to characterize the relationship between BMI and obstetric outcomes of ART (22). The National ART Surveillance System (NASS) includes information from all 50 states and Puerto Rico on patient demographics, medical and obstetric history, and infertility diagnoses, detailed parameters of each treatment cycle, and, if applicable, the resultant pregnancy outcome (Fertility Clinic Success Rate and Certification Act of 1992 [FCSRCA], Public Law No. 102–493, October 24, 1992) (22). Notably, height and weight were added as collected variables in NASS in 2007.
This study included all fresh autologous (nondonor) ART cycles reported to NASS between 2008 and 2013 with BMI data available. Donor and frozen cycles were excluded to limit the heterogeneity of the study group and to minimize confounding. Among all fresh autologous ART cycles from 2008–2013 (n = 602,640 cycles), height and weight were reported for 82.0% (n = 494,097 cycles). Height, weight, or both height and weight were missing for 108,543 cycles; 16.4% of all cycles (n = 98,640) had missing height data, and 16.9% (n = 102,030) had missing weight data. The patents’ BMI was calculated as reported weight in kilograms per meter squared (reported height) at time of cycle start.
We began by describing trends in BMI over the 6-year study period. The number and percentage of all ART cycles for which the woman was underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI 25.0–29.9 kg/m2), or obese (BMI ≥ 30 kg/m2) were calculated for each year. Simple linear regression where the outcome was the percentage and the explanatory variable was the calendar year was used to check for trend.
Among all fresh autologous IVF cycles for which BMI could be calculated during the study period (n = 494,097), we described patient and cycle characteristics in each of the BMI categories. Next, we calculated cancellation rates per cycle and pregnancy outcomes, namely, intrauterine pregnancy rate and live-birth rate (≥ 20 weeks) per noncancelled cycle for which a transfer was performed (n = 402,742 cycles). Among cycles resulting in intrauterine pregnancy (n = 180,855 cycles), we calculated the miscarriage rate. Among singleton (n = 126,552) and twin (n = 49,499) gestations, we calculated preterm (<37 weeks) and low-birth-weight (<2,500 g) delivery rates. A twin pregnancy in which one twin was <2,500 g was considered a preterm delivery.
Using log-binomial regression to estimate the relative risk, we investigated the relationship between BMI and pregnancy outcomes, first for underweight versus normal weight, and then for obese versus normal weight. A similar process was repeated to explore the relationship between degree of thinness (severe thinness BMI <16.0 kg/m2, moderate thinness BMI 16.0–16.9 kg/m2, and mild thinness BMI 17.0–18.49 kg/m2) and obstetric outcomes as compared with normal weight. Of the considered potential confounders (age, number of prior pregnancies, cycle history, stimulation type, number of oocytes retrieved, use of intracytoplasmic sperm injection, use of assisted hatching, number of embryos transferred, stage of embryo at transfer, number of supernumerary embryos cryopreserved, infertility diagnosis as specifically diminished ovarian reserve, male factor infertility, endometriosis, ovulatory dysfunction, tubal factor infertility, uterine factor infertility, and unexplained), backward elimination with α level of 0.05 was used to determine and retain only statistically significant confounders. Race/ethnicity was not considered in the primary models due to the large amount of missing data (33.9%). However, a sensitivity analysis of only those cycles for which race/ethnicity was reported was performed. Finally, we calculated pregnancy and live-birth rates per noncancelled cycle resulting in transfer and the miscarriage rate per cycle that resulted in pregnancy among all fresh autologous IVF cycles from 2008–2013 by single unit of BMI (range <15.0 to ≥ 40 kg/m2).
All analyses were conducted using SAS version 9.3 (SAS Institute Inc). This study was approved by an institutional review board of the Centers for Disease Control and Prevention.
RESULTS
Over the study period, the percentage of cycles involving underweight women statistically significantly decreased from 2.9% to 2.6% while the percentage of cycles in which the woman was obese statistically significantly increased from 17.8% to 19.0%. The majority (55.0%) of women for all study years were of normal weight. Among 494,097 ART cycles started between 2008 and 2013 for which BMI was reported, 13,678 (2.8%) of the cycles involved underweight women with a low BMI, and 91,646 (18.5%) of cycles involved obese women (Table 1).
TABLE 1.
Patient and cycle characteristics, fresh autologous IVF cycles, 2008–2013.
| Underweight < 18.5 (kg/m2) | Normal weight 18.5–24.9 (kg/m2) | Overweight 25.0–29.9 (kg/m2) | Obese >30 (kg/m2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | n | % | n | % | n | % | n | % | Chi square |
| Totals | 13,678 | 2.8 | 271,985 | 55.0 | 116,788 | 23.6 | 91,646 | 18.5 | |
| Age (y) | |||||||||
| <35 | 6,656 | 48.7 | 116,697 | 42.9 | 47,278 | 40.5 | 37,421 | 40.8 | < .0001 |
| 35–37 | 2,880 | 21.1 | 57,970 | 21.3 | 24,829 | 21.3 | 19,763 | 21.6 | |
| 38–40 | 2,397 | 17.5 | 54,459 | 20.0 | 24,907 | 21.3 | 19,728 | 21.5 | |
| ≥41 | 1,745 | 12.8 | 42,859 | 15.8 | 19,774 | 16.9 | 14,734 | 16.1 | |
| Race/ethnicity | |||||||||
| Only non-Hispanic white | 5,494 | 40.2 | 129,235 | 47.5 | 53,618 | 45.9 | 43,235 | 47.2 | < .0001 |
| Only non-Hispanic black | 229 | 1.7 | 6,975 | 2.6 | 8,238 | 7.1 | 8,668 | 9.5 | |
| Only Aslan Pacific Islander | 2,459 | 18.0 | 28,729 | 10.6 | 8,337 | 7.1 | 3,465 | 3.8 | |
| Only Hispanic | 521 | 3.8 | 13,287 | 4.9 | 7,420 | 6.4 | 6,087 | 6.6 | |
| Other | 15 | 0.1 | 380 | 0.1 | 220 | 0.2 | 197 | 0.2 | |
| Missing | 4,960 | 36.3 | 93,379 | 34.3 | 38,955 | 33.4 | 29,994 | 32.7 | |
| Infertility diagnosis | |||||||||
| Diminished ovarian reserve | 3,666 | 26.8 | 72,114 | 26.5 | 28,918 | 24.8 | 19,752 | 21.6 | < .0001 |
| Male factor | 4,865 | 35.6 | 97,851 | 36.0 | 44,160 | 37.8 | 35,221 | 38.4 | < .0001 |
| Endometriosis | 1,634 | 11.9 | 31,735 | 11.7 | 12,220 | 10.5 | 7,562 | 8.3 | < .0001 |
| Ovulatory dysfunction | 1,707 | 12.5 | 30,727 | 11.3 | 15,633 | 13.4 | 22,153 | 24.2 | < .0001 |
| Tubal factor | 1,551 | 11.3 | 35,576 | 13.1 | 22,122 | 18.9 | 18,795 | 20.5 | < .0001 |
| Uterine factor | 605 | 4.4 | 12,877 | 4.7 | 6,527 | 5.6 | 5,241 | 5.7 | < .0001 |
| Unexplained | 2,111 | 15.4 | 42,587 | 15.7 | 14,592 | 12.5 | 9,242 | 10.1 | < .0001 |
| Maximum serum FSH (mIU/mL) | |||||||||
| <5 | 1,895 | 13.9 | 38,657 | 14.2 | 18,309 | 15.7 | 18,070 | 19.7 | < .0001 |
| 5.1–9.9 | 5,670 | 41.5 | 118,853 | 43.7 | 51,523 | 44.1 | 39,317 | 42.9 | |
| ≥10.0 | 3,053 | 22.3 | 53,771 | 19.8 | 19,845 | 17.0 | 11,711 | 12.8 | |
| Missing | 3,060 | 22.4 | 60,704 | 22.3 | 27,111 | 23.2 | 22,548 | 24.6 | |
| No. of prior ART cycles | |||||||||
| 0 | 7,540 | 55.1 | 149,502 | 55.0 | 65,592 | 56.2 | 51,706 | 56.4 | < .0001 |
| 1 | 2,673 | 19.5 | 54,107 | 19.9 | 23,367 | 20.0 | 19,133 | 20.9 | |
| 2+ | 3,464 | 25.3 | 68,332 | 25.1 | 27,810 | 23.8 | 20,792 | 22.7 | |
| No. of prior pregnancies | |||||||||
| 0 | 6,733 | 49.3 | 124,554 | 45.9 | 49,547 | 42.5 | 38,851 | 42.4 | < .0001 |
| 1 | 3,632 | 26.6 | 72,717 | 26.8 | 30,775 | 26.4 | 23,933 | 26.1 | |
| 2+ | 3,293 | 24.1 | 74,388 | 27.4 | 36,327 | 31.1 | 28,756 | 31.4 | |
| No. of prior spontaneous abortions | |||||||||
| 0 | 10,023 | 73.7 | 190,476 | 70.4 | 78,972 | 67.9 | 61,383 | 67.2 | < .0001 |
| 1 | 2,449 | 18.0 | 52,682 | 19.5 | 23,566 | 20.3 | 18,666 | 20.4 | |
| 2+ | 1,135 | 8.3 | 27,604 | 10.2 | 13,791 | 11.9 | 11,304 | 12.4 | |
| No. of prior preterm births | |||||||||
| 0 | 13,133 | 97.0 | 261,854 | 97.1 | 112,104 | 96.7 | 87,470 | 96.0 | < .0001 |
| 1 | 379 | 2.8 | 7,161 | 2.7 | 3,448 | 3.0 | 3,228 | 3.5 | |
| 2+ | 33 | 0.2 | 760 | 0.3 | 430 | 0.4 | 400 | 0.4 | |
| No. of prior full-term births | |||||||||
| 0 | 10,162 | 74.7 | 200,843 | 74.1 | 84,184 | 72.3 | 67,360 | 73.7 | < .0001 |
| 1 | 2,766 | 20.3 | 54,897 | 20.3 | 23,228 | 20.0 | 17,113 | 18.7 | |
| 2+ | 680 | 5.0 | 15,293 | 5.6 | 9,031 | 7.8 | 6,933 | 7.6 | |
| Stimulation type | |||||||||
| None (natural cycle) | 245 | 1.8 | 3,241 | 1.2 | 1,029 | 0.9 | 504 | 0.6 | < .0001 |
| Oral meds + gonadotropins | 178 | 1.3 | 3,662 | 1.4 | 1,472 | 1.3 | 1,074 | 1.2 | |
| Gonadotropins only (antagonist) | 5,915 | 44.3 | 115,248 | 43.4 | 49,185 | 43.1 | 38,151 | 42.5 | |
| Gonadotropins only (no suppression) | 376 | 2.8 | 7,189 | 2.7 | 3,109 | 2.7 | 2,346 | 2.6 | |
| Gonadotropins only (flare) | 1,508 | 11.3 | 32,241 | 12.1 | 14,124 | 12.4 | 11,021 | 12.3 | |
| Gonadotropins only (standard agonist) | 5,138 | 38.5 | 104,016 | 39.2 | 45,341 | 39.7 | 36,668 | 40.9 | |
| No. of oocytes retrieved | |||||||||
| 0–4 | 1,880 | 15.2 | 33,757 | 13.8 | 14,487 | 13.9 | 11,661 | 14.3 | < .0001 |
| 5–9 | 3,505 | 28.3 | 69,930 | 28.5 | 29,732 | 28.4 | 23,675 | 29.1 | |
| >10 | 6,999 | 56.5 | 141,665 | 57.7 | 60,332 | 57.7 | 46,023 | 56.6 | |
| Ovarian hyperstimulation | 190 | 1.4 | 3,570 | 1.3 | 1,343 | 1.1 | 896 | 1.0 | < .0001 |
| Used ICSIa | 8,298 | 74.6 | 163,615 | 73.7 | 70,599 | 74.3 | 55,631 | 74.7 | < .0001 |
| Used assisted hatchinga | 4,671 | 42.0 | 93,362 | 42.0 | 39,901 | 42.0 | 31,889 | 42.8 | .0013 |
| No. of embryos transferred | |||||||||
| 1 | 2,297 | 20.6 | 38,881 | 17.5 | 15,054 | 15.8 | 10,696 | 14.4 | < .0001 |
| 2 | 5,860 | 52.7 | 117,945 | 53.1 | 50,745 | 53.4 | 40,139 | 53.9 | |
| 3 | 2,080 | 18.7 | 43,600 | 19.6 | 19,803 | 20.8 | 16,223 | 21.8 | |
| >3 | 887 | 8.0 | 21,659 | 9.8 | 9,448 | 9.9 | 7,425 | 10.0 | |
| Embryo stage at transfera,b | |||||||||
| Days 2–3 | 6,060 | 54.5 | 122,554 | 55.2 | 52,818 | 55.6 | 42,498 | 57.1 | < .0001 |
| Days 5–6 | 4,817 | 43.3 | 94,847 | 42.7 | 40,186 | 42.3 | 30,261 | 40.6 | |
| No. of supernumerary embryos cryopreserveda | |||||||||
| 0 | 6,644 | 59.9 | 134,924 | 61.0 | 58,468 | 61.7 | 46,893 | 63.2 | < .0001 |
| 1–2 | 1,834 | 16.5 | 36,493 | 16.5 | 15,349 | 16.2 | 11,714 | 15.8 | |
| 3–4 | 1,303 | 11.7 | 23,996 | 10.8 | 10,109 | 10.7 | 7,582 | 10.2 | |
| 5+ | 1,316 | 11.9 | 25,937 | 11.7 | 10,773 | 11.4 | 8,050 | 10.8 | |
Note: ART = assisted reproduction technology; FSH = follicle-stimulating hormone; ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization.
Per noncancelled cycle resulting in transfer.
Does not sum to 100% due to exclusion of transfers on days other than 2, 3, 5, or 6.
Among ART cycles performed between 2008 and 2013 for which we have BMI information, a larger percentage of underweight women as compared with women in other BMI categories were under 35 years old, of Asian or Pacific Islander origin, had an infertility diagnosis of endometriosis, diminished ovarian reserve, or tubal factor infertility, had a maximum serum follicle-stimulating hormone (FSH) value of ≥ 10.0 mIU/mL, and had no prior pregnancies (see Table 1). As compared with women in the other BMI categories, obese women more frequently were of non-Hispanic Black race, held a diagnosis of ovulatory dysfunction or tubal factor infertility, had a maximum FSH concentration of ≤ 5.0 mIU/mL, had a history of two or more prior pregnancies, and had a history of two or more spontaneous abortions. Most frequently among all BMI categories, gonadotropin-releasing hormone antagonist protocols were used, 10 or more oocytes were retrieved, two embryos were transferred, cleavage-stage (days 2 to 3) embryos were transferred, intracytoplasmic sperm injection was used, assisted hatching was not performed, and no embryos were cryopreserved.
Among all cycles, the cancellation rates were comparable in underweight and normal BMI groups, but obesity as compared with normal BMI was associated with a slight but statistically significant increased risk of cancellation (adjusted risk ratio [aRR] 1.05; 95% confidence interval [CI], 1.03–1.07) (Table 2). Among noncancelled transfers in comparison to women with normal BMI, underweight women had a statistically significantly decreased chance of intrauterine pregnancy (aRR 0.97; 95% CI, 0.96–0.99) and live birth (aRR 0.95; 95% CI, 0.93–0.98) per transfer, as did obese women (aRR 0.94; 95% CI, 0.94–0.95 and aRR 0.87; 95% CI, 0.86–0.88, respectively).
TABLE 2.
ART cycle, transfer, and pregnancy outcomes by body mass index, fresh autologous IVF cycles, 2008–2013.
| Underweight | Obese | Normal weight (Reference) | |||||
|---|---|---|---|---|---|---|---|
| Outcome | n (%) | RR (95% Cl) | aRR (95% Cl) | n (%) | RR (95% Cl) | aRR (95% Cl) | n (%) |
| Among cycles | |||||||
| Cancellation | 2,554 (18.7) | 1.02 (0.98–1.05) | 1.03 (0.99–1.07) | 17,163 (18.7) | 1.02 (1.00–1.04) | 1.05 (1.03–1.07) | 49,900 (18.4) |
| Among transfers | |||||||
| Intrauterine pregnancy | 4,969 (44.7) | 0.97 (0.95–0.99) | 0.97 (0.96–0.99) | 31,252 (42.0) | 0.91 (0.90–0.92) | 0.94 (0.94–0.95) | 102,227 (46.1) |
| Live birth (≥20 wk) | 4,126 (37.2) | 0.97 (0.95–0.99) | 0.95 (0.93–0.98) | 24,451 (32.9) | 0.86 (0.85–0.87) | 0.87 (0.86–0.88) | 84,923 (38.3) |
| Among all pregnancies | |||||||
| Miscarriage (<20 wk) | 731 (14.8) | 0.99 (0.92–1.06) | 1.04 (0.98–1.11) | 6,093 (19.6) | 1.3 (1.27–1.34) | 1.23 (1.20–1.26) | 15,264 (15.0) |
| Among singleton pregnancies | |||||||
| Low birth weight <2,500 g | 345 (11.9) | 1.38 (1.24–1.53) | 1.39 (1.25–1.54) | 1,888 (11.3) | 1.30 (1.24–1.37) | 1.26 (1.20–1.33) | 5,134 (8.6) |
| Preterm delivery <37 wk | 355 (12.0) | 1.11 (1.00–1.22) | 1.12 (1.01–1.23) | 2,732 (16.0) | 1.48 (1.42–1.54) | 1.42 (1.36–1.48) | 6,543 (10.8) |
| Among twin pregnancies | |||||||
| Low birth weight <2,500 g | 869 (80.3) | 1.15 (1.11–1.18) | 1.14 (1.10–1.17) | 4,506 (67.1) | 0.96 (0.94–0.98) | 0.95 (0.94–0.97) | 15,958 (70.1) |
| Preterm delivery <37 wk | 675 (61.1) | 1.04 (0.99–1.10) | 1.04 (0.99–1.09) | 4,282 (62.3) | 1.06 (1.04–1.09) | 1.06 (1.03–1.08) | 13,574 (58.5) |
Note: Adjusted models are controlled for age, number of prior pregnancies, cycle history, stimulation type, number of oocytes retrieved, use of intracytoplasmic sperm injection, use of assisted hatching, number of embryos transferred, stage of embryo at transfer, number of supernumerary embryos cryopreserved, and infertility diagnosis specifically diminished ovarian reserve, male factor infertility, endometriosis, ovulatory dysfunction, tubal factor infertility, uterine factor infertility, and unexplained. aRR = adjusted risk ratio; Cl = confidence interval; IVF = in vitro fertilization; RR = risk ratio.
Among cycles resulting in pregnancy, the association between low BMI and miscarriage was not statistically significant (aRR 1.04; 95% CI, 0.98–1.11). In contrast, obesity as compared with normal weight was associated with a statistically significantly increased miscarriage risk (aRR 1.23; 95% CI, 1.20–1.26). Among cycles resulting in singleton pregnancy, both underweight and obese statuses were associated with increased risk of low-birth-weight (aRR 1.39; 95% CI, 1.25–1.54 and aRR 1.26; 95% CI, 1.20–1.33, respectively) and preterm delivery (aRR 1.12; 95% CI, 1.01–1.23 and aRR 1.42; 95% CI, 1.36–1.48, respectively).
Among cycles resulting in twin pregnancy, underweight as compared with normal weight status was associated with increased risk of low birth weight (aRR 1.14; 95% CI, 1.10–1.17) but not preterm delivery (aRR 1.04; 95% CI, 0.99–1.09). Obese weight was associated with increased risk of preterm delivery (aRR 1.06; 95% CI, 1.03–1.08) and low birth weight (aRR 0.95; 95% CI, 0.94–0.97). Of all the twin live births (n = 40,832), 7,990 (19.6%) women delivered at <34 weeks’ gestation. Among twin live births in underweight women, 224 (20.2%) delivered at <34 weeks. Among twin live births in normal weight and obese women, 4,343 (18.7%) and 1,466 (21.3%) delivered at <34 weeks, respectively.
Additionally, a sensitivity analyses incorporating race/ethnicity was performed. It noted no statistically significant difference in the adjusted relative risk in any of the comparisons (results not shown).
An analysis of severe, moderate, and mild thinness as compared with normal weight found no clinically significant differences between degree of thinness and cancellation rate or ART pregnancy outcomes (intrauterine pregnancy, live birth, and miscarriage) except for a decreased chance of live birth for moderate thinness compared with normal weight (aRR 0.92; 95% CI, 0.86–0.99), a decreased likelihood of intrauterine pregnancy for mild thinness compared with normal weight (aRR 0.98; 95% CI, 0.96–0.99), and a decreased chance of live birth for mild thinness compared with normal weight (aRR 0.96; 95% CI, 0.94–0.98) (Table 3). All degrees of thinness among both singleton and twin pregnancies were associated with an increased risk of low-birth-weight delivery as compared with normal weight women; however, only severe thinness among twin pregnancies was associated with increased risk of preterm delivery.
TABLE 3.
Pregnancy outcomes by degree of underweight body mass index, fresh IVF cycles, 2008–2013.
| Severe thinness < 16.0 kg/m2 | Moderate thinness 16.0–16.99 kg/m2 | Mild thinness 17.0–18.49 kg/m2 | Normal weight (Reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | n (%) | RR (95% CI) | aRR (95% CI) | n (%) | RR (95% CI) | aRR (95% CI) | n (%) | RR (95% CI) | aRR (95% CI) | n (%) |
| Among cycles | ||||||||||
| Cancellation | 87 (18.6) | 1.02 (0.84–1.23) | 1.03 (0.85–1.24) | 265 (18.5) | 1.01 (0.91–1.13) | 1.05 (0.94–1.17) | 2,202 (18.7) | 1.02 (0.98–1.06) | 1.03 (0.99–1.07) | 49,900 (18.4) |
| Among transfers | ||||||||||
| Intrauterine pregnancy | 160 (42.1) | 0.91 (0.81–1.03) | 0.94 (0.85–1.03) | 528 (45.4) | 0.99 (0.92–1.05) | 0.96 (0.92–1.01) | 4,281 (44.7) | 0.97 (0.95–0.99) | 0.98 (0.96–0.99) | 102,227 (46.1) |
| Live birth (≥20 wk) | 136 (35.8) | 0.93 (0.82–1.07) | 0.91 (0.8–1.03) | 441 (37.9) | 0.99 (0.92–1.06) | 0.92 (0.86–0.99) | 3,549 (37.2) | 0.97 (0.94–1.00) | 0.96 (0.94–0.98) | 84, 923 (38.3) |
| Among all pregnancies | ||||||||||
| Miscarriage (<20 wk) | 22 (13.8) | 0.92 (0.62–1.35) | 1.01 (0.70–1.48) | 79 (15.0) | 1.0 (0.82–1.23) | 1.06 (0.86–1.29) | 630 (14.8) | 0.99 (0.92–1.06) | 1.04 (0.97–1.12) | 15,264 (15.0) |
| Among singleton pregnancies | ||||||||||
| Low birth weight <2,500 g | 13 (13.5) | 1.57 (0.95–2.60) | 1.58 (0.96–2.63) | 50 (16.3) | 1.89 (1.46–2.44) | 1.93 (1.50–2.49) | 282 (11.3) | 1.31 (1.17–1.46) | 1.32 (1.18–1.47) | 5,134 (8.6) |
| Preterm delivery <37 wk | 15 (15.5) | 1.43 (0.90–2.28) | 1.51 (0.95–2.41) | 34 (10.6) | 0.98 (0.72–1.35) | 1.02 (0.74–1.40) | 306 (12.0) | 1.11 (1.00–1.23) | 1.12 (1.00–1.24) | 6,543 (10.8) |
| Among twin pregnancies | ||||||||||
| Low birth weight <2,500 g | 34 (91.9) | 1.31 (1.19–1.44) | 1.27 (1.16–1.39) | 94 (84.7) | 1.21 (1.12–1.31) | 1.18 (1.09–1.27) | 741 (79.3) | 1.13 (1.09–1.17) | 1.12 (1.09–1.16) | 15,958 (70.1) |
| Preterm delivery < 37 wk | 28 (73.7) | 1.26 (1.04–1.52) | 1.25 (1.04–1.51) | 68 (59.7) | 1.02 (0.88–1.19) | 1.01 (0.87–1.18) | 579 (60.8) | 1.04 (0.99–1.09) | 1.04 (0.98–1.09) | 13,574 (58.5) |
Note: aRR = adjusted risk ratio; CI = confidence interval; IVF = in vitro fertilization; RR = risk ratio.
When the pregnancy, live-birth, and miscarriage rates were explored against the unit value of BMI, a range of optimal BMI was clearly visible (Fig. 1). The pregnancy rate was highest in women whose BMI was between 19.0 and 22.9 kg/m2 (46.1% to 46.3%) and fell with increasing BMI to 38.8% in BMI ≥ 40 kg/m2 (see Fig. 1A). Similarly, the live-birth rate was highest in women whose BMI was between 19.0 and 22.9 kg/m2 (38.6% to 38.8%) and fell with increasing BMI to a nadir of 29.4% in BMI ≥ 40 kg/m2. The miscarriage rate increased with increasing BMI from 12.3% among women with BMI 15.0–15.9 kg/m2 to 22.0% among women with BMI ≥ 40 kg/m2 (see Fig. 1B).
FIGURE 1.
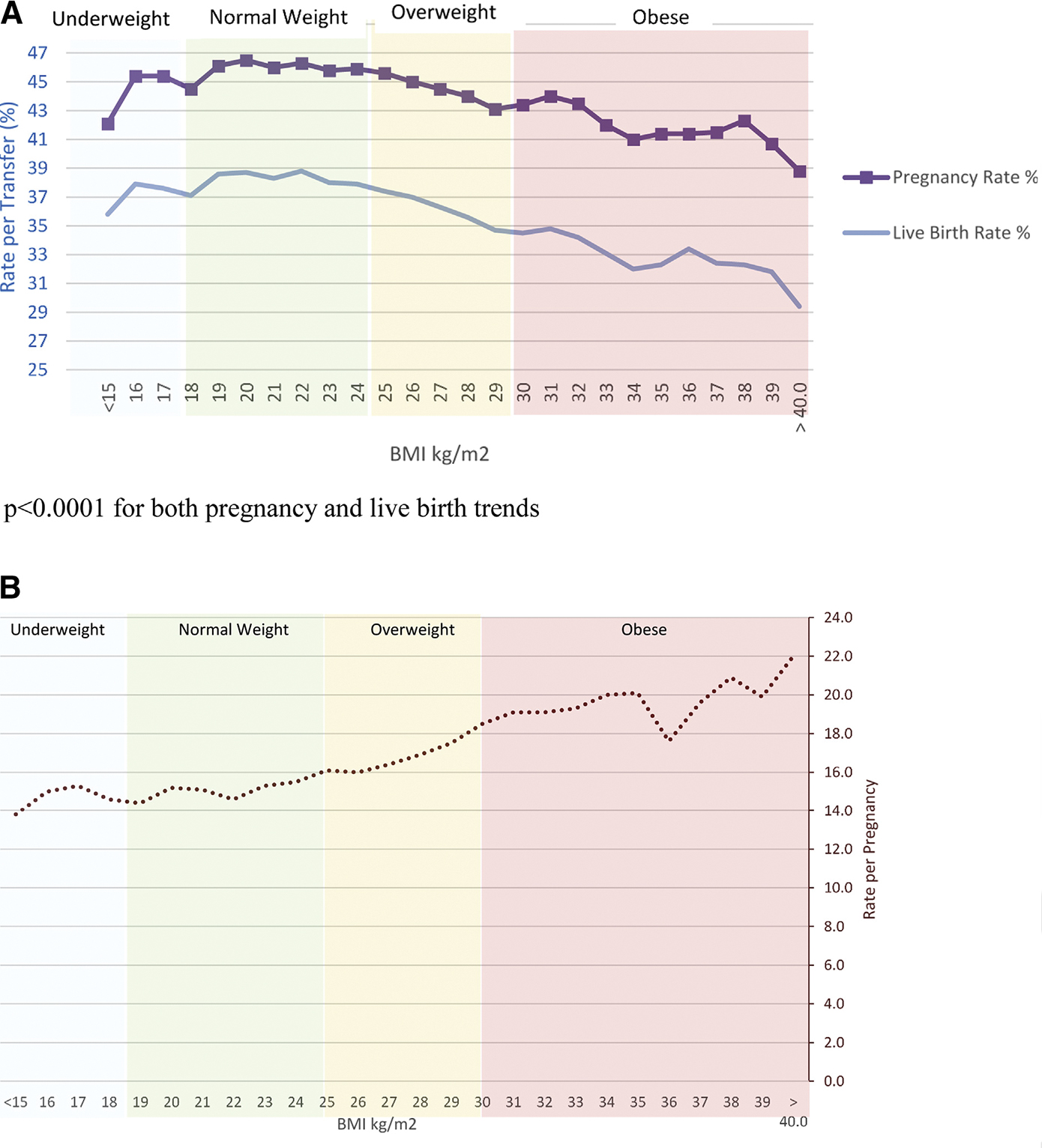
(A) Pregnancy and live-birth rate per transfer by body mass index, fresh autologous IVF cycles, 2008–2013. (B) Miscarriage rate among all pregnancies by body mass index, fresh autologous IVF cycles, 2008–2013.
DISCUSSION
Over the study period, the majority of women for all study years were of normal weight; the percentage of cycles involving underweight women statistically significant decreased while the percentage of cycles in which the female was obese statistically significantly increased. The best outcomes were observed among women of normal weight; for those of abnormal weight, obesity was associated with greater risk of adverse obstetric and obstetric outcomes than was underweight status.
According to the National Center for Health Statistics, among the general adult population during the study period the percentage of underweight women ranged from 1.6% to 1.7% while the percentage of obese women ranged from 33.7% to 34.9% (23, 24). The percentage of obese women (18.5%) within the ART population is smaller for obese women than in the general population. Part of this difference may be attributable to purposeful patient selection; women with BMIs outside the normal range may be discouraged from using reproductive services.
As compared with normal weight women, underweight women had a similar absolute percentage chance of intrauterine pregnancy, live birth, and miscarriage after IVF. After adjusting for possible confounders, the adjusted relative risks for these ART outcomes were statistically significant but likely of limited clinical significance, as they very closely approached 1. These findings, in a large cohort of women, support those of several smaller studies that suggested no statistically significant impact of low BMI on the ART outcomes of pregnancy and live birth (12–15). Our results do, however, contradict the reported association of low BMI with increased miscarriage risk in the ART population (17). The adjusted relative risk of delivering a low-birth-weight or preterm infant, singleton or twin, was elevated among underweight women, a finding consistent with prior studies that suggest that underweight women have an increased likelihood of poor obstetric outcomes, including preterm birth and low-birth-weight delivery (19–21). Our study is among the first to examine this relationship in the IVF population. Notably, we were unable to control for maternal weight gain during pregnancy, which also contributes to the risk of preterm birth and low-birth-weight possibly due to nutritional deficiencies. The fact that the impact of underweight maternal status on preterm delivery was less notable among twin pregnancies may reflect the underlying increased risk of preterm delivery associated with all twin pregnancies independent of maternal weight at time of conception.
In contrast to the findings for underweight women, the absolute percentage chance of ART success, pregnancy, and live birth was statistically significantly lower among the obese women as compared with the normal weight women. Obesity was also associated with a statistically significantly increased risk of miscarriage. These findings are consistent with multiple prior studies that suggest an association between obesity and impaired fertility (1), worse ART outcomes (2–9), and a statistically significantly increased miscarriage and obstetric risk (25, 26).
Our study is limited by its cycle-based rather than patient-based nature, by the lack of some patient medical information such as tobacco use, nonfertility-related medical history, obstetric complications, interpregnancy interval, pregnancy weight gain, and the lack of embryo quality data. To minimize the effects of lack data on embryo quality, we were able to control for the number of supernumerary embryos cryopreserved, which has been shown to correlate with embryo quality (27) and number of prior failed IVF cycles. Additionally, the study is limited by the quality of height and weight data entered by clinicians and by the fact that 18% of BMI data are missing. Potential bias exists in that the group that comprises the missing data may be different from those for whom we have data; however, we have no reason to believe that the two groups are inherently different.
This study is among the first of its size to focus on the impact of low BMI on ART outcomes. It is strengthened not only by the large sample size but also by its generalizability in that it includes all reporting clinics in the United States. We were also able to control for patient and ART cycle characteristics that impact pregnancy and obstetric outcomes, and a sensitivity analysis that incorporated BMI noted no statistically significant differences in adjusted relative risks.
CONCLUSION
Among women undergoing IVF, prepregnancy BMI affects pregnancy and obstetric outcomes. Although underweight status may have limited impact on ART success (namely, pregnancy and live-birth rates), it is associated with increased risk of preterm and low-birth-weight delivery. Obese status negatively impacts all favorable outcomes except birthweight among singletons. Independent of pregnancy weight gain, prepregnancy BMI is a modifiable characteristic that has obstetric implications. Whenever feasible, particularly among the ART population that is afforded preconception counseling, physicians should encourage women to reach a normal BMI before attempting conception.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
J.F.K. has nothing to disclose. A.D.K. has nothing to disclose. H.S.H. has nothing to disclose. S.C. has nothing to disclose. D.M.K. has nothing to disclose. D.J.J. has nothing to disclose.
REFERENCES
- 1.Talmor A, Dunphy B. Female obesity and infertility. Best Pract Res Clin Obstet Gynaecol 2014. [DOI] [PubMed] [Google Scholar]
- 2.Caillon H, Fréour T, Bach-Ngohou K, Colombel A, Denis MG, Barrière P, et al. Effects of female increased body mass index on in vitro fertilization cycles outcome. Obes Res Clin Pract 2015;9:382–8. [DOI] [PubMed] [Google Scholar]
- 3.Zhang JJ, Feret M, Chang L, Yang M, Merhi Z. Obesity adversely impacts the number and maturity of oocytes in conventional IVF not in minimal stimulation IVF. Gynecol Endocrinol 2015:1–5. [DOI] [PubMed] [Google Scholar]
- 4.Rehman R, Hussain Z, Fatima SS. Effectofweightstatusonpregnancyoutcome in intra cytoplasmic sperm injection. Iran J Reprod Med 2013;11:717–24. [PMC free article] [PubMed] [Google Scholar]
- 5.Dickey RP, Xiong X, Gee RE, Pridjian G. Effect of maternal height and weight on risk of preterm birth in singleton and twin births resulting from in vitro fertilization: a retrospective cohort study using the Society for Assisted Reproductive Technology Clinic Outcome Reporting System. Fertil Steril 2012;97:349–54. [DOI] [PubMed] [Google Scholar]
- 6.Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R, et al. Female obesity adversely affects assisted reproductive technology (ART) pregnancy and live birth rates. Hum Reprod 2011;26:245–52. [DOI] [PubMed] [Google Scholar]
- 7.Comstock IA, Kim S, Behr B, Lathi RB. Increased body mass index negatively impacts blastocyst formation rate in normal responders undergoing in vitro fertilization. J Assist Reprod Genet 2015;32:1299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellver J, Pellicer A, Garcia-Velasco JA, Ballesteros A, Remohi J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril 2013;100:1050–8. [DOI] [PubMed] [Google Scholar]
- 9.Moragianni VA, Jones SM, Ryley DA. The effect of body mass index on the outcomes of first assisted reproductive technology cycles. Fertil Steril 2012;98:102–8. [DOI] [PubMed] [Google Scholar]
- 10.Wei YM, Yang HX, Zhu WW, Liu XY, Meng WY, Wang YQ, et al. Risk of adverse pregnancy outcomes stratified for pre-pregnancy body mass index. J Matern Fetal Neonatal Med 2015:1–5. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Yang D, Zhang Q. Impact of overweight and underweight on IVF treatment in Chinese women. Gynecol Endocrinol 2010;26:416–22. [DOI] [PubMed] [Google Scholar]
- 12.Fedorcsak P, Dale PO, Storeng R, Ertzeid G, Bjercke S, Oldereid N, et al. Impact of overweight and underweight on assisted reproduction treatment. Hum Reprod 2004;19:2523–8. [DOI] [PubMed] [Google Scholar]
- 13.Wittemer C, Ohl J, Bailly M, Bettahar-Lebugle K, Nisand I. Does body mass index of infertile women have an impact on IVF procedure and outcome? J Assist Reprod Genet 2000;17:547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lashen H, Ledger W, Bernal AL, Barlow D. Extremes of body mass do not adversely affect the outcome of superovulation and in-vitro fertilization. Hum Reprod 1999;14:712–5. [DOI] [PubMed] [Google Scholar]
- 15.Wang JX, Davies M, Norman RJ. Body mass and probability of pregnancy during assisted reproduction treatment: retrospective study. BMJ 2000;321:1320–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn KA, Hatch EE, Rothman KJ, Mikkelsen EM, Brogly SB, Sorensen HT, et al. Body size and risk of spontaneous abortion among danish pregnancy planners. Paediatr Perinat Epidemiol 2014;28:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veleva Z, Tiitinen A, Vilska S, Hyden-Granskog C, Tomas C, Martikainen H, et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod 2008;23:878–84. [DOI] [PubMed] [Google Scholar]
- 18.Hoellen F, Hornemann A, Haertel C, Reh A, Rody A, Schneider S, et al. Does maternal underweight prior to conception influence pregnancy risks and outcome? Vivo 2014;28:1165–70. [PubMed] [Google Scholar]
- 19.Sharifzadeh F, Kashanian M, Jouhari S, Sheikhansari N. Relationship between pre-pregnancy maternal BMI with spontaneous preterm delivery and birth weight. J Obstet Gynaecol 2015;35:354–7. [DOI] [PubMed] [Google Scholar]
- 20.Lynch AM, Hart JE, Agwu OC, Fisher BM, West NA, Gibbs RS. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol 2014;210:428.e1–9. [DOI] [PubMed] [Google Scholar]
- 21.Masho SW, Bishop DL, Munn M. Pre-pregnancy BMI and weight gain: where is the tipping point for preterm birth? BMC Pregnancy Childbirth 2013;13:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. 2010 Assisted reproductive technology fertility clinic success rates report. Atlanta: Centers for Disease Control and Prevention. 2012. Available at: http://www.cdc.gov/art/ART2010/PDFs/ART_2010_Clinic_Report-Full.pdf. Accessed September 20, 2016. [Google Scholar]
- 23.Fryar CD, Ogden CL. Prevalence of underweight among adults aged 20 and over: United States, 1960–1962 through 2011–2012. National Center for Health Statistics, Centers for Disease Control and Prevention. Last updated: September 2014. Available at: http://www.cdc.gov/nchs/data/hestat/underweight_adult_11_12/underweight_adult_11_12.htm. Accessed September 20, 2016.
- 24.Fryar CD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. National Center for Health Statistics, Centers for Disease Control and Prevention. Last updated: September 2014. Available at: http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm. Accessed September 20, 2016.
- 25.Schummers L, Hutcheon JA, Bodnar LM, Lieberman E, Himes KP. Risk of adverse pregnancy outcomes by prepregnancy body mass index: a population-based study to inform prepregnancy weight loss counseling. Obstet Gynecol 2015;125:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill MJ, Richter KS, Heitmann RJ, Lewis TD, DeCherney AH, Graham JR, et al. Number of supernumerary vitrified blastocysts is positively correlated with implantation and live birth in single-blastocyst embryo transfers. Fertil Steril 2013;99:1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


