Abstract
Background
Several studies indicate that female obesity increases the risk of spontaneous abortion (SAB). Central adiposity, height, and location of typical weight gain have not been examined as risk factors for SAB.
Methods
We examined the associations between selected anthropometric factors and risk of SAB among 5132 women enrolled in a Danish Internet-based prospective cohort study of pregnancy planners. We used Cox proportional hazards regression models, with gestational weeks as the time scale, to compute hazard ratios (HRs) of SAB and 95% confidence intervals (CIs).
Results
After adjustment for potential confounders, the HRs for SAB among underweight (body mass index (BMI, kg/m2) <20), overweight (BMI: 25–29) and obese (BMI ≥30) women were 1.00 [95% CI: 0.81, 1.24], 0.90 [95% CI: 0.73, 1.09] and 1.23 [95% CI: 0.98, 1.54], respectively, compared with normal weight women (BMI 20–24). The association between obesity and SAB was stronger for early SAB (<8 weeks gestation); HR: 1.34 95% CI: 1.01, 1.77. The HR for height ≥174 cm vs. <166 cm was 0.81 [95% CI: 0.66, 1.00]. Increased waist-to-hip ratio (WHR) was inversely associated with risk of SAB (HR: 0.81; 95% CI: 0.63, 1.05). Waist circumference and location of typical weight gain were not appreciably associated with SAB risk.
Conclusions
This study confirms previous studies that have shown a small positive association between obesity and SAB risk. Our results suggest that obesity is a stronger risk factor for early pregnancy losses, and that small stature and low WHR are associated with an increased risk of SAB.
Keywords: obesity, body size, spontaneous abortion
The prevalence of obesity is increasing worldwide.1 In Denmark, the prevalence of obesity has exhibited a marked increase within the past 25 to 30 years, particularly for reproductive-aged women, of whom 9.7% were obese in 2006–2007.2 Studies have reported that both obesity3–6 and underweight7,8 are associated with an increased risk of spontaneous abortion (SAB).
Obesity is associated with disturbances in sex hormone metabolism, reproductive disorders,9 intrauterine and urinary tract infections,10 and disruptions in the follicular environment including increased inflammation.11 Although few studies have examined the risk of SAB among underweight women, lower maternal serum leptin levels and poor nutritional status have been hypothesized to increase SAB risk in this group.7,8
Using data from a Danish prospective cohort of pregnancy planners, we examined risk of SAB in relation to selected anthropometric factors, including body mass index (BMI), location of typical weight gain, height, waist circumference (WC), and waist-to-hip ratio (WHR).
Methods
Data collection
The Snart-Gravid study is an Internet-based prospective cohort study of time to pregnancy. Recruitment began in 2007 when an advertisement was placed on a Danish health-related website (www.netdoktor.dk) and a coordinated media strategy was launched.12–14 Enrollment and primary data collection were conducted via a self-administered questionnaire on the study website (www.snart-gravid.dk). Contact with participants was maintained through the study website and e-mail.
Before enrollment, participants completed a consent form and an online screening questionnaire to verify eligibility. Women eligible to participate in Snart-Gravid were aged 18–40 years, residents of Denmark, in a stable relationship with a male partner, not using fertility treatment, and trying to become pregnant. Participants were required to provide a valid e-mail address and their Civil Personal Registration (CPR) number, a unique 10-digit personal identification number assigned to each Danish resident. After 38 months of recruitment, 5921 women had enrolled in the study. The study was approved by the Danish Data Protection Board and the Institutional Review Board of Boston University Medical Campus.
The baseline questionnaire collected information on demographics, lifestyle and behavioral factors, and reproductive and medical history. Initially, women were randomised with equal probability to receive either a short- or long-form version of the baseline questionnaire. Because completion rates and missing data were similar for both questionnaires,14 after six months all new participants received the long-form baseline questionnaire. Participants were contacted every two months by e-mail with a reminder to fill out a follow-up questionnaire. Follow-up questionnaires assessed changes in exposures and pregnancy status, including whether any clinically recognised pregnancy losses had occurred. Follow-up continued until conception occurred or for a maximum of 12 months, whichever came first.
To obtain information on pregnancy outcomes among women in the cohort, we linked each woman's CPR number to the Danish National Registry of Patients (DNRP) and Danish Medical Birth Registry (DMBR) records through 2012. The DNRP provides information on hospital, emergency room, specialist, and outpatient encounters (including SAB and therapeutic abortion (TAB)). The DMBR gives information on all live and still births after 22 gestational weeks.15,16 Pregnancy losses and terminations occurring after the baseline enrollment date in the Snart Gravid cohort were identified using ICD-10 codes (DO03 for SAB and DO04 for TAB) in the DNRP. A validation study of DNRP data found that 30% of self-reported SABs were not registered in the DNRP;17 however, this study examined records from 1991– 1995, before the registry started to include data from outpatient clinics (≥1995). Another study that compared the DNRP data with data from individual medical records found a positive predictive value of 98.7%.16
Assessment of anthropometric measures
Participants' height (cm) and weight (kg) before conception were reported on the baseline questionnaire. Women were also asked ‘When you gain weight, where on your body do you mainly add the weight?’ Possible responses were ‘equally all over’, ‘waist/ stomach’, ‘hips/thighs’, ‘chest/shoulders’, and ‘do not gain weight’. On the long-form questionnaire (asked of 50% of participants in the first 6 months of the study (1201 of 2368 enrolled women) and all participants thereafter), women reported their WC (cm) at the level of the umbilicus and their hip circumference (cm) at its widest location. Respondents were asked if they had used a measuring tape to make these measurements (yes/no). We calculated waist-to-hip ratio as the waist circumference divided by hip circumference. We used BMI (kg/m2) to measure total adiposity adjusted for body size, WHR to measure the distribution of visceral to peripheral body fat, and WC to estimate the sum of abdominal visceral fat, subcutaneous fat, and muscle.18 Because taller women tend to have larger waist circumferences, we created a measure of height-adjusted WC by regressing WC on height and adding an amount equal to the predicted change in waist size corresponding to a participant's departure from the average height in our cohort.19
Assessment of spontaneous abortion
The outcome of interest was SAB. We used two sources of data to ascertain SABs: data from the DNRP and self-reported data from follow-up questionnaires. On the follow-up questionnaire, women who reported a pregnancy loss were asked to report the date of the loss and how long the pregnancy lasted (in weeks since the last menstrual period). The DNRP provided information on occurrences of SAB up to 22 gestational weeks and any TAB, the dates of these events, and the gestational age at which the pregnancy ended, as measured by ultrasound fetometry. For pregnancy losses recorded in both the registry and on a questionnaire, we used data from the DNRP (based on either early ultrasound fetometry or LMP) to measure gestational week of pregnancy loss. In Denmark, the first pregnancy-related ultrasound is typically performed at approximately 12 weeks of gestation. Therefore, gestational ages of SABs after 12 weeks are likely based on ultrasound. For SABs reported only on a Snart-Gravid follow-up questionnaire, gestational age was calculated the number of weeks from the LMP to the date of pregnancy loss, rounded to the nearest whole week. Among the women who had a SAB reported in both data sources, the correlation between gestational weeks reported on the questionnaire and in the registry was 0.71. The mean reported gestational age from the questionnaire was slightly lower (7.2 weeks) relative to that in the registry (7.3 weeks).
There were 163 women who reported a pregnancy on a Snart-Gravid follow-up questionnaire but did not have any data from that pregnancy recorded in either the hospital or birth registries. For this analysis, we assumed that these women had an early SAB. We used multiple imputation to impute a gestational age of SAB. Because the vast majority of SABs that occur after 12 weeks are recorded in the DNRP, we restricted the imputed values to be ≤12 weeks for each of these presumed SABs. Sensitivity analyses in which we excluded these 163 reported pregnancies produced results similar to those in our primary analysis (results not shown).
Assessment of confounders
Data on maternal age, parity, smoking status, alcohol and caffeine consumption, physical activity, and vocational training/education were reported on the baseline questionnaire. We estimated total metabolic equivalents (METs) per week by summing the METs from moderate physical activity (hours per week multiplied by 3.5 METs) and vigorous exercise (hours per week multiplied by 7.0 METs).20 Smoking status and consumption of alcohol and caffeine were updated on all subsequent follow-up questionnaires.
Eligibility
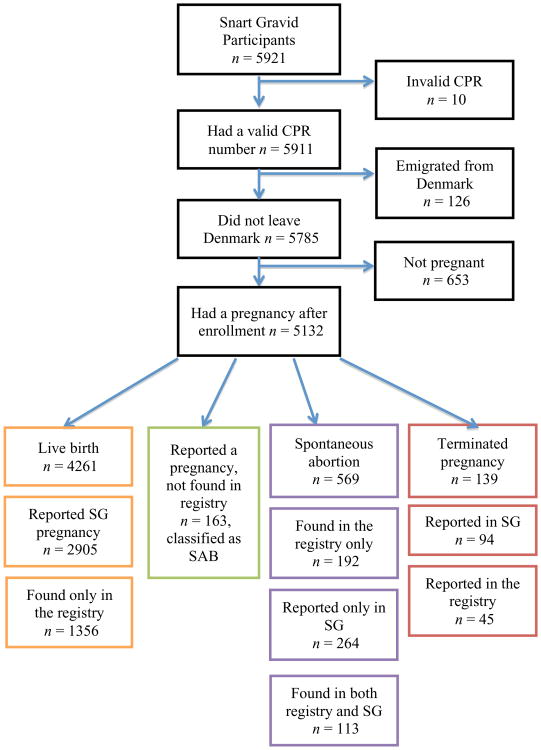
The present analysis was restricted to women who conceived a clinically-recognised pregnancy after enrollment in the cohort. Only the first pregnancy after enrollment in the study was included because anthropometric variables are known to change after childbirth21 and exposure information for subsequent pregnancies might be misclassified. In addition, we excluded 126 women because they were not living in Denmark during follow-up, 10 women who did not provide a valid CPR, and 653 (11%) women who did not conceive during the follow-up period (as indicated by the absence of pregnancy data on the follow-up questionnaires, the DNRP, and the DMBR), leaving a total of 5132 women in the analysis (see Figure 1).
Figure 1.
Flow chart of pregnancies observed in Snart Gravid participants.
Data analysis
We assessed the relationship between pre-pregnancy anthropometric factors and SAB using a time-to-event analysis. TABs were censored at the week of pregnancy termination and pregnancies lasting more than 22 weeks were censored at 22 weeks. We divided female BMI (kg/m2) into the following categories: <20, 20–24, 25–29, and ≥30, in accordance with WHO guidelines. The categories of WC and WHR were <75 cm, 75–79 cm, 80–86 cm, ≥87 cm and <0.75, 0.75– 0.79, 0.80–0.84, ≥0.85, respectively. These categories were determined based on clinical literature that has suggested higher risks of morbidity and mortality with higher WC and WHR.22 Height was categorised into quartiles of <166 cm, 166–169 cm, 170–173 cm, and ≥174 cm. The typical location of weight gain was analysed in the original categories described above. We examined the shape and magnitude of the relation between each continuous anthropometric variable and SAB risk by using restricted cubic splines.23
We used Cox proportional hazards regression models, with gestational weeks as the time scale, to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for anthropometric variables associated with SAB. We assumed that there was a true but unknown ordering for tied event times and used the ‘exact’ option in SAS PROC PHREG, which takes into account all possible orderings of event times. The HR may be interpreted as the average per-week risk of SAB for the exposed category divided by the corresponding risk for the reference category.
We selected potential confounders from a group of variables associated with SAB and the anthropometric variables at baseline, and those meeting qualitative criteria for confounding based on a review of the literature and the assessment of causal graphs. The variables included were maternal age, cigarette smoking, parity, vocational training/education, physical activity, alcohol and caffeine consumption. We controlled for potential confounders that changed the unadjusted HR by more than 5% in univariate analyses. Both unadjusted and adjusted models are presented in the tables, although there was little confounding. We compared models controlling for the baseline and most recent values of caffeine, alcohol, and smoking. Because they yielded similar estimates, we used baseline values. Additional models are presented simultaneously controlling for other measures of adiposity (BMI models adjusted for WC, height models adjusted for weight, and all other models adjusted for BMI).
In secondary analyses, we assessed whether the HRs were similar across strata of waiting time to pregnancy (<6 vs. ≥6 months), parity (nulliparous vs. parous), maternal age (<30 years vs. ≥30 years), and menstrual cycle regularity (yes vs. no), defined in the questionnaire as a woman's ability to predict when her next menstrual period will start. For these analyses, we fit separate models for the aforementioned dichotomous variables. Because the etiology of pregnancy loss likely differs for early and late losses,24 especially by karyotype, we also examined the association between each exposure and time timing of pregnancy losses (<8 vs. ≥8 weeks of gestation). Using separate Cox models for losses <8 and ≥8 weeks, we analysed the risk for all women to experience an ‘early’ loss and all women still carrying a fetus at the end of 8 weeks to have a ‘late’ loss. The time to event for ‘late’ losses began at 8 weeks of gestation and was right-censored at 22 gestational weeks. The choice of 8 weeks as a cut point was based on karyotype data showing a higher proportion of chromosomal abnormalities before 8 weeks.
We used multiple imputation methods to impute missing covariate, exposure, and outcome information.25 Missing data ranged from 0% for maternal age, time to pregnancy, and smoking status to 6% for number of glasses of dessert wine consumed per week. As a result of randomisation to long and short questionnaires during the first six months of enrollment, 30% of waist and hip measurements were missing from the baseline questionnaire. An additional 33% of waist and hip circumferences were missing due to nonresponse. We used PROC MI to create 5 imputed datasets based on 33 variables in the imputation model. We combined beta coefficients and standard errors across the imputed datasets using PROC MIANALYZE.
To assess departures from the proportional hazards assumption we plotted log-log survivor functions for each variable in categorical form. In the log-log survivor functions, parallel curves indicated proportional hazards. SAS statistical software (version 9.3) was used for all analyses.
Results
Among the 5132 women who reported a pregnancy or for whom there was registry-based evidence of a pregnancy after enrolling in the Snart-Gravid study, a total of 732 participants (14.3%) had a SAB. Overall, 26% of SABs were recorded only in the DNRP, 36% were reported only on a Snart Gravid follow-up questionnaire, 16% were documented in both sources, and 22% were women who reported a pregnancy on a follow-up questionnaire but had no outcome information in the registries. Baseline characteristics of study participants according to BMI and WHR are presented in Table 1. BMI was positively associated with parity, lower levels of vocational training, lower caffeine and alcohol consumption, and sedentary activity. WHR was positively associated with smoking more cigarettes per day. Pearson correlation coefficients were 0.31 for BMI vs. WHR, 0.84 for BMI vs. WC, and 0.64 for WHR vs. WC. The number of SABs by maternal age and BMI categories is presented in Table 2. In general, the risk of SAB increased with increasing maternal age, although results were not entirely consistent across categories of BMI.
Table 1. Age-adjusted distribution of baseline characteristicsa of 5132 women from the Snart Gravid study by body mass index and waist-to-hip ratio.
| Body mass index (kg/m2) | Waist-to-hip ratio | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| <20 | 20–24.9 | 25–29.9 | ≥30 | <0.75 | 0.75–0.79 | 0.80–0.84 | ≥0.85 | |
| Parity (%) | ||||||||
| Nulliparous | 68.6 | 71.5 | 61.3 | 54.8 | 76.6 | 72.5 | 64.0 | 58.3 |
| 1 birth | 24.2 | 21.5 | 28.6 | 34.7 | 18.6 | 19.4 | 26.8 | 32.9 |
| ≥2 births | 7.2 | 7.0 | 10.1 | 10.5 | 4.9 | 8.1 | 9.3 | 8.8 |
| Vocational training/education (%) | ||||||||
| No vocational training | 13.1 | 10.6 | 14.0 | 16.4 | 10.6 | 10.5 | 13.1 | 14.7 |
| Semi-skilled/basic training | 12.9 | 14.6 | 17.4 | 21.0 | 11.9 | 15.2 | 15.7 | 18.6 |
| Higher education (≤4 years) | 47.0 | 48.7 | 54.8 | 53.3 | 47.3 | 50.1 | 49.7 | 52.2 |
| Higher education (>4 years) | 27.0 | 26.2 | 13.9 | 9.3 | 30.2 | 24.2 | 21.5 | 14.6 |
| Smoking status (%) | ||||||||
| Non-smokers | 79.0 | 81.5 | 78.4 | 78.7 | 82.0 | 83.8 | 78.4 | 77.0 |
| <10 cigarettes/day | 10.6 | 10.3 | 10.6 | 9.7 | 10.8 | 8.7 | 10.8 | 10.8 |
| ≥10 cigs/day | 10.4 | 8.2 | 11.0 | 11.6 | 7.2 | 7.5 | 10.8 | 12.2 |
| Caffeine consumption (%) | ||||||||
| <100 mg/day | 48.0 | 45.6 | 47.1 | 54.1 | 44.8 | 48.7 | 46.6 | 48.2 |
| 100–199 mg/day | 17.3 | 17.0 | 18.0 | 17.8 | 18.1 | 16.7 | 16.4 | 18.2 |
| 200–299 mg/day | 13.2 | 14.0 | 14.3 | 10.1 | 15.1 | 13.7 | 12.8 | 12.7 |
| ≥300 mg/day | 21.5 | 23.4 | 20.6 | 17.9 | 22.0 | 21.0 | 24.3 | 20.9 |
| Alcohol categories (%) | ||||||||
| 0 drinks per week | 30.0 | 28.2 | 34.0 | 40.0 | 28.5 | 31.6 | 30.1 | 33.2 |
| 1–2 drink/week | 30.3 | 29.9 | 29.1 | 28.3 | 32.1 | 28.9 | 29.5 | 28.6 |
| 3–6 drinks/week | 30.1 | 30.2 | 26.7 | 22.7 | 29.2 | 29.6 | 28.6 | 27.6 |
| ≥7 drinks/week | 9.9 | 11.6 | 10.2 | 9.0 | 102 | 10.1 | 11.8 | 10.7 |
| METs categories (%) | ||||||||
| <10 METs | 14.5 | 12.6 | 19.4 | 21.1 | 12.8 | 13.1 | 15.9 | 19.6 |
| 10–19 METs | 30.7 | 30.9 | 33.4 | 38.4 | 30.4 | 32.8 | 33.1 | 32.6 |
| 20–39 METs | 35.6 | 37.8 | 34.2 | 26.6 | 36.9 | 36.1 | 35.1 | 33.9 |
| ≥40 METs | 19.2 | 18.7 | 13.1 | 11.0 | 19.9 | 18.0 | 16.0 | 13.9 |
| Regular menstrual cycles (%) | 71.1 | 76.6 | 77.0 | 70.4 | 76.7 | 75.0 | 75.1 | 73.8 |
| Time to pregnancy ≥6 months (%) | 55.6 | 52.8 | 58.7 | 65.2 | 52.6 | 53.6 | 54.9 | 61.1 |
Characteristics are presented as percentages within levels of BMI and WHR based on the first imputation dataset, and are standardised to age distribution of cohort at baseline.
Table 2. Numbera of spontaneous abortions and gestational weeks at risk by maternal age and body mass index.
| Age (Years) | Body mass index (kg/m2) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| <20 | 20–24 | 25–30 | ≥30 | Total | ||
| ≤20 | SAB | 4 | 7 | 4 | 4 | 19 |
| Gestational weeks | 523 | 1101 | 476 | 339 | 2439 | |
| Rate/1000 GW | 7.6 | 6.4 | 8.4 | 11.8 | 7.8 | |
| 21–24 | SAB | 18 | 44 | 16 | 16 | 94 |
| Gestational weeks | 2634 | 7248 | 3505 | 1914 | 15 301 | |
| Rate/1000 GW | 6.8 | 6.1 | 4.6 | 8.4 | 6.1 | |
| 25–28 | SAB | 39 | 123 | 41 | 42 | 245 |
| Gestational weeks | 5584 | 19 678 | 7055 | 3916 | 36 233 | |
| Rate/1000 GW | 7.0 | 6.3 | 5.8 | 10.7 | 6.8 | |
| 29–32 | SAB | 29 | 126 | 41 | 23 | 219 |
| Gestational weeks | 4112 | 16 647 | 5977 | 3414 | 30 150 | |
| Rate/1000 GW | 7.1 | 7.6 | 6.9 | 6.7 | 7.3 | |
| 33–36 | SAB | 13 | 69 | 24 | 14 | 120 |
| Gestational weeks | 1620 | 6770 | 2915 | 1440 | 12 745 | |
| Rate/1000 GW | 8.0 | 10.2 | 8.2 | 9.7 | 9.4 | |
| ≥37 | SAB | 5 | 16 | 9 | 5 | 35 |
| Gestational weeks | 519 | 1515 | 766 | 611 | 3411 | |
| Rate/1000 GW | 9.6 | 10.6 | 11.7 | 8.2 | 10.3 | |
| Age-Standardized rate/1000 GW | 7.2 | 7.3 | 6.5 | 9.0 | ||
| Age-Standardized rate difference | 0.0 | [ref] | −0.8 | 1.7 | ||
| (95% CI) | −1.6, 1.5 | −2.0, 0.6 | −0.2, 3.6 | |||
| Age-Standardized rate ratio | 0.99 | [ref] | 0.90 | 1.23 | ||
| (95% CI) | 0.80, 1.23 | 0.73, 1.09 | 0.99, 1.54 | |||
From the first imputation dataset.
After adjustment for all covariates except WC, the HRs for SAB among underweight, overweight, and obese women were 1.00 [95% CI: 0.81, 1.24], 0.90 [95% CI: 0.73, 1.09] and 1.23 [95% CI: 0.98, 1.54], respectively, compared with normal weight women. After controlling for WC, the HRs remained similar (Table 3). Results were also similar when a cut-point of 18.5 kg/m2 instead of 20 kg/m2 for underweight was used (results not shown). Figure 2 displays the association between BMI and the risk of SAB by gestational week using restricted cubic splines. The curve indicates little association with increasing BMI until a BMI of 30, and then a steep rise in the HR as BMI increases from 30 to 40. The shape of the spline curve is consistent with the findings from our categorical analyses.
Table 3. Hazard ratios for the association between body size and SAB when adjusting for potential confounders measured at baseline.
| Exposure | N SABa | Total GWsa | Unadjusted HR | Adjusted model HR [95% CI]b | Adjusted model with body size HR [95% CI]c |
|---|---|---|---|---|---|
| Body mass index (kg/m2) | |||||
| <20 | 108 | 14 992 | 0.99 | 1.00 [0.81, 1.24] | 0.93 [0.73, 1.19] |
| 20–24 | 385 | 52 959 | 1.00 [Reference] | ||
| 25–29 | 135 | 20 694 | 0.90 | 0.90 [0.73, 1.09] | 0.94 [0.71, 1.23] |
| ≥30 | 104 | 11 634 | 1.22 | 1.23 [0.98, 1.54] | 1.26 [0.87, 1.83] |
| Waist circumference | |||||
| <75 cm | 199 | 25 500 | 1.00 [Reference] | ||
| 75–79 cm | 165 | 23 120 | 0.94 | 0.92 [0.73, 1.16] | 0.91 [0.71, 1.17] |
| 80–86 cm | 150 | 23 115 | 0.85 | 0.82 [0.65, 1.03] | 0.80 [0.61, 1.06] |
| ≥87 cm | 218 | 28 544 | 1.00 | 0.97 [0.75, 1.25] | 0.88 [0.57, 1.36] |
| Waist-to-hip ratio | |||||
| <0.75 | 186 | 21 024 | 1.00 [Reference] | ||
| 0.75–0.79 | 175 | 24 316 | 0.86 | 0.85 [0.67, 1.06] | 0.84 [0.67, 1.06] |
| 0.80–0.84 | 166 | 24 695 | 0.79 | 0.76 [0.58, 1.00] | 0.75 [0.57, 0.99] |
| ≥0.85 | 205 | 30 244 | 0.85 | 0.81 [0.63, 1.05] | 0.78 [0.59, 1.03] |
| Height | |||||
| <166 cm | 233 | 31 550 | 1.00 [Reference] | ||
| 166–169 cm | 180 | 21 151 | 1.15 | 1.14 [0.93, 1.38] | 1.12 [0.92, 1.37] |
| 170–173 cm | 171 | 23 058 | 1.00 | 0.99 [0.81, 1.21] | 0.97 [0.79, 1.18] |
| ≥174 cm | 148 | 24 520 | 0.82 | 0.81 [0.66, 1.00] | 0.79 [0.63, 0.98] |
| Location of weight gain | |||||
| Equally all over | 198 | 27 464 | 1.00 [Reference] | ||
| Waist/stomach | 300 | 40 975 | 1.01 | 1.01 [0.84, 1.21] | 1.02 [0.85, 1.23] |
| Hips/thighs | 198 | 26 754 | 1.03 | 1.03 [0.84, 1.26] | 1.04 [0.85, 1.27] |
| Does not gain weight | 27 | 4204 | 0.88 | 0.88 [0.59, 1.32] | 0.89 [0.59, 1.35] |
| Chest/ shoulders | 9 | 882 | 1.18 | 1.15 [0.50, 2.65] | 1.15 [0.50, 2.65] |
Frequencies and gestational weeks (GW) from the first imputation dataset.
Adjusted for maternal age, physical activity, caffeine consumption, parity, vocational training/education, alcohol consumption, and smoking.
Adjusted for variables in footnote b and body size (BMI model adjusted for waist circumference, height model adjusted for weight; all other models adjusted for BMI.
Figure 2.
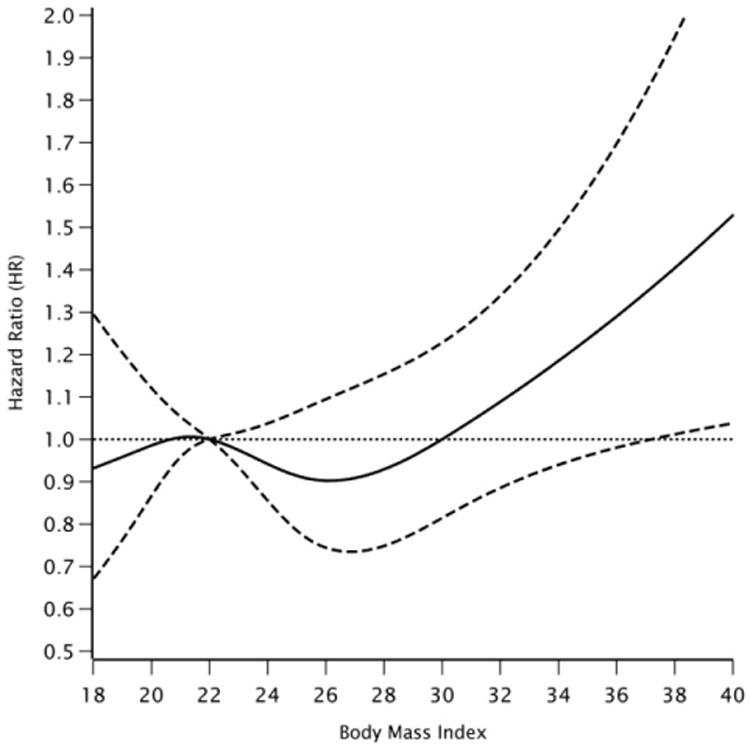
Relationship between BMI and risk of SAB <22 weeks fitted by restricted cubic splines.
Notes: Reference level for the hazard ratio is a BMI of 22 kg/m2. The dotted lines represent the corresponding 95% confidence intervals. Four knots at 19.5, 21.3, 25.4, and 30.5 kg/m2. The curve is adjusted for maternal age, smoking, parity, vocational training/education, physical activity, and alcohol and caffeine consumption.
WC was not materially associated with SAB risk in the fully adjusted model. With the addition of BMI to the model, increasing WC was associated with a slightly decreased risk of SAB. HRs for WC 75–79 cm, 80–86 cm, and ≥87 cm were 0.91 [95% CI: 0.71, 1.17], 0.80 [95% CI: 0.61, 1.06], and 0.88 [95% CI: 0.57, 1.36], respectively, relative to women with a WC <75 cm. Increasing WHR also was associated with a small decrease in SAB risk. HRs for WHR 0.75–0.79, 0.80– 0.84 and ≥0.85 were 0.85 [95% CI: 0.67, 1.06], 0.76 [95% CI: 0.58, 1.00], and 0.81 [95% CI: 0.63, 1.05], respectively, relative to WHR <0.75. The observed inverse relationship between WHR and SAB could reflect a higher risk among women with a small WHR. Further adjustment for BMI did not change the observed HRs noticeably (Table 3). HRs derived from a complete case analysis of WC and WHR were similar to the original results based on multiple imputation as were results among the 50% of women who used a tape measure to provide waist and hip circumferences (Supporting Information Table S1).
Women who were 174 cm or taller had a 19% decreased risk of SAB relative to women who were <166 cm (HR: 0.81; [95% CI: 0.66, 1.00]). Heights between 166–169 cm and 170–173 cm showed little association with SAB risk (Table 3). Further adjustment for weight did not produce substantially different HRs.
No appreciable differences in SAB risk were found among women who tended to gain weight in their waist/stomach and hips/thighs compared with women who tended to gain weight equally all over (Table 3). HRs were 0.88 for women who reported that they tended not to gain weight [95% CI: 0.59, 1.32] and 1.15 for women who gained weight in their chest/shoulders [95% CI: 0.50, 2.65], though there were few SABs in each of these groups (27 and 9, respectively). Adjustment for BMI had little effect on the observed results.
The risk of SAB earlier than 8 weeks of gestation was 1.34 times as high [95% CI: 1.01, 1.77] among obese women as normal weight women (Table 4). Underweight, WHR and WC were not appreciably associated with the risk of SAB earlier than 8 weeks of gestation. In contrast, obese women had no appreciable difference in risk of SAB at 8 weeks of gestation or later (HR: 0.99; [95% CI: 0.62, 1.59]) relative to normal weight women. Underweight was not materially associated with risk of SAB occurring at 8 gestational weeks or later (HR: 1.10; [95% CI: 0.72, 1.68]). The effects of height were similar for early and late losses. Higher WC and WHR were associated with lower risks of SAB at 8 weeks or later, but the magnitude of the associations was not consistent across categories (Table 4).
Table 4. Hazard ratios for the association of body size with early (<8 gestational weeks) and late SAB.
| Early SAB <8 weeks gestation | Late SAB >8 weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| NSABa | Total GWa | Unadjusted | Adjusted model HR [95% CI]b | Adjusted model with body size HR [95% CI]c | NSABa | Total GWa | Unadjusted | Adjusted model HR [95% CI]b | Adjusted model with body size HR [95% CI]c | |
| Body mass index (kg/m2) | ||||||||||
| <20 | 67 | 414 | 0.95 | 0.95 [0.70,1.28] | 0.92 [0.67,1.26] | 41 | 14 578 | 1.06 | 1.10 [0.72,1.68] | 0.95 [0.58,1.58] |
| 20–24.9 | 255 | 1685 | 1.00 [Reference] | 130 | 51 274 | 1.00 [Reference] | ||||
| 25–29.9 | 78 | 494 | 0.84 | 0.82 [0.62,1.10] | 0.87 [0.58,1.29] | 57 | 20 200 | 1.02 | 1.04 [0.70,1.54] | 1.07 [0.69,1.67] |
| ≥30 | 80 | 502 | 1.36 | 1.34 [1.01,1.77] | 1.41 [0.84, 2.35] | 24 | 11 132 | 0.95 | 0.99 [0.62,1.59] | 0.99 [0.56,1.74] |
| Waist circumference | ||||||||||
| <75 cm | 121 | 758 | 1.00 [Reference] | 78 | 24 742 | 1.00 [Reference] | ||||
| 75–79 cm | 114 | 754 | 1.04 | 1.03 [0.78,1.38] | 1.02 [0.77,1.35] | 51 | 22 366 | 0.78 | 0.74 [0.47,1.16] | 0.73 [0.44,1.19] |
| 80–86 cm | 97 | 679 | 0.88 | 0.87 [0.66,1.14] | 0.86 [0.63,1.18] | 53 | 22 436 | 0.78 | 0.74 [0.49,1.10] | 0.71 [0.43,1.17] |
| ≥87cm | 148 | 904 | 1.08 | 1.04 [0.79,1.38] | 0.91 [0.54,1.53] | 70 | 27 640 | 0.87 | 0.85 [0.55,1.30] | 0.81 [0.44,1.50] |
| Waist-to-hip ratio | ||||||||||
| <0.75 | 115 | 688 | 1.00 [Reference] | 71 | 20 336 | 1.00 [Reference] | ||||
| 0.75–0.79 | 116 | 812 | 0.93 | 0.92 [0.68,1.25] | 0.92 [0.67,1.25] | 59 | 23 504 | 0.75 | 0.72 [0.47,1.10] | 0.72 [0.47,1.10] |
| 0.80–0.84 | 110 | 693 | 0.80 | 0.78 [0.54,1.11] | 0.76 [0.53,1.10] | 56 | 24 002 | 0.78 | 0.73 [0.48,1.11] | 0.73 [0.48,1.11] |
| ≥0.85 | 139 | 902 | 0.92 | 0.89 [0.66,1.19] | 0.84 [0.62,1.16] | 66 | 29 342 | 0.72 | 0.69 [0.46,1.02] | 0.68 [0.45,1.02] |
| Height | ||||||||||
| <166 cm | 163 | 1081 | 1.00 [Reference] | 70 | 30 469 | 1.00 [Reference] | ||||
| 166−169 cm | 112 | 709 | 1.00 | 1.01 [0.77,1.31] | 1.00 [0.73,1.31] | 68 | 20 442 | 1.49 | 1.45 [0.99, 2.12] | 1.41 [0.96, 2.08] |
| 170−173 cm | 107 | 666 | 0.89 | 0.89 [0.69,1.15] | 0.87 [0.67,1.14] | 64 | 22 392 | 1.28 | 1.23 [0.85,1.79] | 1.18 [0.81,1.73] |
| ≥174 cm | 98 | 639 | 0.78 | 0.78 [0.60,1.02] | 0.77 [0.58,1.02] | 50 | 23 881 | 0.92 | 0.88 [0.59,1.33] | 0.83 [0.54,1.28] |
| Location of weight gain | ||||||||||
| Equally all over | 125 | 810 | 1.00 [Reference] | 73 | 26 654 | 1.00 [Reference] | ||||
| Waist/stomach | 191 | 1259 | 1.02 | 1.02 [0.81, 1.28 | 1.04 [0.83,1.32] | 109 | 39 716 | 0.99 | 0.99 [0.73,1.35] | 0.99 [0.73,1.35] |
| Hips/thighs | 142 | 882 | 1.13 | 1.14 [0.87,1.50] | 1.17 [0.88,1.54] | 56 | 25 872 | 0.84 | 0.84 [0.56,1.25] | 0.83 [0.55,1.26] |
| Does not gain weight | 16 | 107 | 0.83 | 0.82 [0.45,1.50] | 0.84 [0.45,1.57] | 11 | 4097 | 0.95 | 0.98 [0.45, 2.16] | 0.95 [0.42, 2.13] |
| Chest/shoulders | 6 | 37 | 1.10 | 1.06 [0.34, 3.31] | 1.07 [0.35, 3.31] | 3 | 845 | 1.29 | 1.29 [0.39, 4.19] | 1.27 [0.39, 4.17] |
Frequencies and gestational weeks (GW) from the first imputation dataset.
Adjusted for maternal age, physical activity, caffeine consumption, parity, vocational training, alcohol consumption, and smoking.
Adjusted for variables in footnote b and body size (BMI model adjusted for waist circumference, height model adjusted for weight; all other models adjusted for BMI).
Results for BMI stratified by TTP, parity, maternal age, and menstrual cycle regularity are shown in the web index. Briefly, the association between obesity and risk of SAB was stronger among women with shorter TTPs, women aged <30 years, and women with regular menstrual cycles. The HRs for SAB were 1.17 among underweight parous women (95% CI: 0.81, 1.69) and 0.92 among underweight nulliparous women (95% CI: 0.70, 1.20). Results for analyses of WHR, WC, height, and tendency to gain weight in relation to SAB did not differ much across strata of TTP, parity, maternal age, or cycle regularity (Supporting Information Table S2).
Comment
In this prospective cohort study of Danish pregnancy planners, we observed a small increased risk of SAB among obese women relative to normal weight women, especially for SAB occurring before 8 completed weeks of gestation. Decreased WHR and height were associated with a small increased risk of SAB. Underweight, WC, and location of weight gain were not materially associated with SAB risk.
Our results for obesity agree with previous studies that have shown increased risk of SAB among overweight and obese women.5,6,26–29 However, our study did not corroborate reports of an increased risk among underweight women,5,7 using two different cut-points for underweight (18.5 and 20 kg/m2). Our finding of an increased risk of SAB before 8 weeks of gestation among obese women agrees with one study,27 but differs from another,5 possibly because different definitions of early SAB (6–12 weeks gestation27 and <14 weeks gestation5) were used in each study. Differential left-truncation bias, which is common in other studies of SAB that recruit women who are already pregnant,30 is not a problem in our study because we enrolled women before conception. No previous study has investigated WHR, WC, height, and body fat distribution.
The present study did not collect daily readings of human chorionic gonadotropin (hCG), the earliest biologic marker of implantation, so it is inevitable that some early SABs were not identified. This limitation is common to most SAB studies. Missing early losses would have induced bias if body size affected early losses differently than later losses. It is also possible that women who were lost to follow-up or became pregnant a long time after completing Snart-Gravid had an overlooked SAB or a change in body size before the index pregnancy that was included in our study. The proportion of missed SABs is expected to be smaller within a population of pregnancy planners than among the general population because pregnancy planners are presumably more aware of their menstrual cycles. In support of this theory, 96% of the Snart-Gravid participants who conceived reported having used home pregnancy tests to confirm their pregnancies.31
Errors in fetal measurements of gestational age are likely, especially for obese women among whom gestational age tends to be underestimated.32 In this study, gestational age was measured two ways, depending on the source of the information. For SABs identified by questionnaire only, gestational age was measured using date of LMP. For all registry-confirmed SABs, gestational age was measured using early ultrasound fetometry or LMP. Ultrasounds at 17–18 gestational weeks have been found to be less accurate in obese women,32 which would lead to distortions in the association between obesity and timing of SAB. Among women with self-reported SABs, gestational age was based on date of the last menstrual period. This assessment can also be inaccurate among women with irregular cycles. Inaccurate gestational age may have led to either an overestimation or underestimation of the association, depending on how the date of conception was misclassified. In Denmark, the cut-point used to define SAB clinically is 22 weeks. Because many studies have measured SAB only up to 20 weeks, we re-analysed the results censoring at 20 weeks and found little difference in the effect estimates, likely because only one SAB occurred after 20 weeks.
Body size measurements were self-reported by participants, thus enhancing the potential for misclassification of the exposure. In a previous investigation based on this cohort,33 high concordance was found between height and weight measurements reported by participants and corresponding measurements taken during a physical exam (r = 0.96). However, self-reported data on WC and WHR were not validated in our cohort. Validation studies of similar populations have found that women tend to underestimate these measurements,34 though secondary analyses among women who used a tape measure in our cohort produced similar results. Tendency to gain weight was also not verified, but one study35 found that self-reported female body shape was consistent with anthropometric measures used to assess fat distribution. Exposure misclassification in this study is expected to be nondifferential and bias associations for the extreme categories toward the null.
Since the outcome data were collected from two independent data sources, we do not believe that dependent misclassification between exposure and outcome is a major concern. Nevertheless, because the exposure and covariates were collected using a self-administered questionnaire, there is a possibility that misclassification of these variables may be correlated with each other and lead to dependent misclassification and residual confounding.
The observed association between high BMI and increased risk of SAB is biologically plausible. Increased total protein, leptin, apolipoprotein A1, and by-products of chronic inflammation and dyslipidemia have been found in the follicular microenvironment of obese women compared with normal women, and they may affect the development of a viable fetus.36 A study of placental tissues showed that 90.9% of SAB placentas had high bacteria levels vs. only 16.7% of control placentas, a difference that could heighten inflammatory immune responses and lead to SAB10 but also may be caused by SAB. Decreased levels of estradiol have been found among reproductive-aged women with both very low and very high percentages of body fat.37 Lower levels of estradiol during the menstrual cycle have been associated with lower pregnancy rates38 and it is plausible that low estradiol levels negatively affect developing fetuses. In contrast, taller women have been found to have higher follicular-phase plasma estradiol levels compared with shorter women, which may explain the observed decrease in SAB risk.39 Increased abdominal obesity has been shown to be associated with decreased levels of sex hormone binding globulin (SHBG),9,29 independent of BMI. Residual confounding may contribute to the observed decreased risk of SAB among taller women. Other studies have found strong relationships between female height and higher socio-economic status, education, and other risk factors associated with both obesity and increased SAB risk.40
In summary, we found that obesity was associated with a small increased risk of SAB, and the association was stronger for early pregnancy losses (before 8 weeks of gestation). Low WHR was associated with a slight increase in risk of SAB. The association was mainly driven by a higher risk in the women with the smallest WHR; differences in the HRs across the higher categories of WHR were not as pronounced. Increasing height was also associated with a decrease in SAB risk. WC, underweight, and tendency to gain weight were not appreciably associated with SAB in this cohort.
Supplementary Material
Table S1. Complete case analysis using unimputed waist and hip measures compared with analyses among women who used a tape measure and multiple imputation analysis.
Table S2. Hazard ratios for the association between body size and SAB, stratified by time to pregnancy, parity, maternal age, and menstrual cycle regularity.
Hazard ratios for the association between body size and SAB, stratified by parity.
Hazard ratios and 95% confidence intervals the association between body size and SAB, stratified by age.
Hazard ratios and 95% confidence intervals for the association between body size and SAB, stratified cycle regularity.
Footnotes
Supporting information: Additional Supporting Information may be found in the online version of this article at the publisher's web-site.
References
- 1.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obesity Research. 2001;9(Suppl. 4):228S–233S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- 2.Frederiksen P, Jensen KE, Kjaer SK. Sociodemographic factors and risk-taking behaviour during adolescence and obesity among more than 40 000 Danes. Public Health Nutrition. 2012;17:162–169. doi: 10.1017/S1368980012004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroon B, Harrison K, Martin N, Wong B, Yazdani A. Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertility and Sterility. 2011;95:1827–1829. doi: 10.1016/j.fertnstert.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 4.Landres IV, Milki AA, Lathi RB. Karyotype of miscarriages in relation to maternal weight. Human Reproduction. 2010;25:1123–1126. doi: 10.1093/humrep/deq025. [DOI] [PubMed] [Google Scholar]
- 5.Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstetrics and Gynecology. 2005;106:250–259. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- 6.Metwally M, Saravelos SH, Ledger WL, Li TC. Body mass index and risk of miscarriage in women with recurrent miscarriage. Fertility and Sterility. 2010;94:290–295. doi: 10.1016/j.fertnstert.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage – results from a UK-population-based case-control study. International Journal of Obstetrics and Gynaecology. 2007;114:170–186. doi: 10.1111/j.1471-0528.2006.01193.x. [DOI] [PubMed] [Google Scholar]
- 8.Helgstrand S, Andersen AM. Maternal underweight and the risk of spontaneous abortion. Acta Obstetricia et Gynecologica Scandinavica. 2005;84:1197–1201. doi: 10.1111/j.0001-6349.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 9.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Human Reproduction Update. 2003;9:359–372. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- 10.Atay GA, Arsan S, Atasay B, Ensari A, Aysev D. The possible role of intrauterine infections in unexplained second trimester abortions and macerated stillbirths: a study from a single center. Journal of Perinatology. 2004;24:679–685. doi: 10.1038/sj.jp.7211167. [DOI] [PubMed] [Google Scholar]
- 11.Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, et al. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. Journal of Clinical Endocrinology and Metabolism. 2009;94:1533–1540. doi: 10.1210/jc.2008-2648. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort profile: the Danish Web-based Pregnancy Planning Study – ‘Snart-Gravid’. International Journal of Epidemiology. 2009;38:938–943. doi: 10.1093/ije/dyn191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen T, Riis AH, Hatch EE, Wise LA, Sørensen HT, et al. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid’. European Journal of Epidemiology. 2010;25:297–304. doi: 10.1007/s10654-010-9431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman KJ, Mikkelsen EM, Riis A, Sorensen HT, Wise LA, Hatch EE. Randomized trial of questionnaire length. Epidemiology (Cambridge, Mass) 2009;20:154. doi: 10.1097/EDE.0b013e31818f2e96. [DOI] [PubMed] [Google Scholar]
- 15.Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. Journal of Clinical Epidemiology. 1996;49:893–897. doi: 10.1016/0895-4356(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 16.Lohse SR, Farkas DK, Lohse N, Skouby SO, Nielsen FE, Lash TL, et al. Validation of spontaneous abortion diagnoses in the Danish National Registry of Patients. Clinical Epidemiology. 2010;2:247–250. doi: 10.2147/CLEP.S13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buss L, Tolstrup J, Munk C, Bergholt T, Ottesen B, Grønbaek M, et al. Spontaneous abortion: a prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstetricia et Gynecologica Scandinavica. 2006;85:467–475. doi: 10.1080/00016340500494887. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RW, Keil D, Gold EJ, Williams SM, Goulding A. Body mass index, waist girth, and waist-to-hip ratio as indexes of total and regional adiposity in women: evaluation using receiver operating characteristic curves. The American Journal of Clinical Nutrition. 1998;67:44–49. doi: 10.1093/ajcn/67.1.44. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk of colorectal adenoma in women (United States) Cancer Causes & Control. 1996;7:253–263. doi: 10.1007/BF00051301. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and Science in Sports and Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Abrams B, Heggeseth B, Rehkopf D, Davis E. Parity and body mass index in US women: a prospective 25-year study. Obesity (Silver Spring) 2013;21:1514–1518. doi: 10.1002/oby.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu S, Wang Z, Heshka S, Heo M, Faith MS, Heymsfield SB. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. The American Journal of Clinical Nutrition. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 23.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 24.Savitz DA, Hertz-Picciotto I, Poole C, Olshan AF. Epidemiologic measures of the course and outcome of pregnancy. Epidemiologic Reviews. 2002;24:91–101. doi: 10.1093/epirev/mxf006. [DOI] [PubMed] [Google Scholar]
- 25.Boots C, Stephenson MD. Does obesity increase the risk of miscarriage in spontaneous conception: a systematic review. Seminars in Reproductive Medicine. 2011;29:507–513. doi: 10.1055/s-0031-1293204. [DOI] [PubMed] [Google Scholar]
- 26.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Human Reproduction. 2004;19:1644–1646. doi: 10.1093/humrep/deh277. [DOI] [PubMed] [Google Scholar]
- 27.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertility and Sterility. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 28.Bellver J, Rossal LP, Bosch E, Zúñiga A, Corona JT, Meléndez F, et al. Obesity and the risk of spontaneous abortion after oocyte donation. Fertility and Sterility. 2003;79:1136–1140. doi: 10.1016/s0015-0282(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 29.Yeung EH, Zhang C, Hediger ML, Wactawski-Wende J, Schisterman EF. Racial differences in the association between sex hormone-binding globulin and adiposity in premenopausal women: the BioCycle study. Diabetes Care. 2010;33:2274–2276. doi: 10.2337/dc10-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. American Journal of Epidemiology. 2007;165:444–452. doi: 10.1093/aje/kwk027. [DOI] [PubMed] [Google Scholar]
- 31.Wise LA, Mikkelsen EM, Rothman KJ, Riis AH, Sørensen HT, Huybrechts KF, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. American Journal of Epidemiology. 2011;174:701–709. doi: 10.1093/aje/kwr130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallen B, Finnstrom O, Nygren KG, Olausson PO. Maternal and fetal factors which affect fetometry: use of in vitro fertilization and birth register data. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2013;170:372–376. doi: 10.1016/j.ejogrb.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Wise LA, Rothman KJ, Mikkelsen EM, Sorensen HT, Riis A, Hatch EE. An internet-based prospective study of body size and time-to-pregnancy. Human Reproduction. 2010;25:253–264. doi: 10.1093/humrep/dep360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology (Cambridge, Mass) 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Thoma ME, Hediger ML, Sundaram R, Stanford JB, Peterson CM, Croughan MS, et al. Comparing apples and pears: women's perceptions of their body size and shape. Journal of Women's Health (2002) 2012;21:1074–1081. doi: 10.1089/jwh.2012.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valckx SD, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, et al. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Human Reproduction. 2012;27:3531–3539. doi: 10.1093/humrep/des350. [DOI] [PubMed] [Google Scholar]
- 37.Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Human Reproduction. 2008;23:2555–2563. doi: 10.1093/humrep/den213. [DOI] [PubMed] [Google Scholar]
- 38.Li H, Nakajima ST, Chen J, Todd HE, Overstreet JW, Lasley BL. Differences in hormonal characteristics of conceptive vs. nonconceptive menstrual cycles. Fertility and Sterility. 2001;75:549–553. doi: 10.1016/s0015-0282(00)01765-9. [DOI] [PubMed] [Google Scholar]
- 39.Dorgan JF, Reichman ME, Judd JT, Brown C, Longcope C, Schatzkin A, et al. The relation of body size to plasma levels of estrogens and androgens in premenopausal women (Maryland, United States) Cancer Causes & Control. 1995;6:3–8. doi: 10.1007/BF00051674. [DOI] [PubMed] [Google Scholar]
- 40.Davey Smith G, Hart C, Upton M, Hole D, Gillis C, Watt G, et al. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. Journal of Epidemiology and Community Health. 2000;54:97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Complete case analysis using unimputed waist and hip measures compared with analyses among women who used a tape measure and multiple imputation analysis.
Table S2. Hazard ratios for the association between body size and SAB, stratified by time to pregnancy, parity, maternal age, and menstrual cycle regularity.
Hazard ratios for the association between body size and SAB, stratified by parity.
Hazard ratios and 95% confidence intervals the association between body size and SAB, stratified by age.
Hazard ratios and 95% confidence intervals for the association between body size and SAB, stratified cycle regularity.