Abstract
Ketogenic diets have been widely used for weight loss and are increasingly used in the management of type 2 diabetes. Despite evidence that ketones have multiple positive effects on kidney function, common misconceptions about ketogenic diets, such as high protein content and acid load, have prevented their widespread use in individuals with impaired kidney function. Clinical trial evidence focusing on major adverse kidney events is sparse. The aim of this review is to explore the effects of a ketogenic diet, with an emphasis on the pleiotropic actions of ketones, on kidney health. Given the minimal concerns in relation to the potential renoprotective effects of a ketogenic diet, future studies should evaluate the safety and efficacy of ketogenic interventions in kidney disease.
Keywords: Diet, Nephrology, Kidney Diseases, Ketones
Introduction
Low carbohydrate eating patterns including a very low carbohydrate or ketogenic diet have been successfully used for weight loss and remitting type 2 diabetes (T2D). Among patients with T2D, the prevalence of chronic kidney disease (CKD), whether characterized as a reduced estimated glomerular filtration rate (eGFR) function or albuminuria is almost 40%.1 Yet, a ketogenic diet is cautioned against in individuals with impaired kidney function,2 in part, due to concerns about increased protein intake. The effect of protein intake in CKD is controversial, but high protein intake has been associated with hyperfiltration, increased acid excretion, and potentially, a decline in kidney function.3 4 However, protein intake on a well-formulated ketogenic diet (WFKD) is moderate to effectively permit nutritional ketosis. Dietary analysis of very low carbohydrate studies usually reports daily protein intake ranging from 0.6 g/kg to 1.4 g/kg,5–7 which is similar to that in the standard American diet and below the high protein threshold (≥2.0 g/kg) believed to be of concern.8 The Kidney Disease Outcomes Quality Initiative clinical practice guideline for nutrition in CKD not dependent on dialysis recommends a “low-protein diet providing 0.55–0.6 g of dietary protein/kg body weight/day, or a very low-protein diet providing 0.28–0.43 g of dietary protein/kg of body weight/day with additional keto acid/amino acid analogs to meet protein requirements (0.55–0.60 g/kg/day).”9 In contrast, Kidney Disease Improving Global Outcomes (KDIGO) 2022 CKD guideline recommended a slightly higher daily protein allowance of 0.8 g/kg/day for individuals with advanced CKD with or without T2D.10 The Modification of Diet in Renal Disease (MDRD) study, a landmark trial examining the effect of protein restriction among 585 patients with non-diabetic CKD, did not demonstrate a significantly slower progression of disease,11 and in fact a very low protein diet (0.28 g/kg/day) was associated with increased risk of death at a median follow-up of 3.2 years.12 The null findings from MDRD are one of numerous inconsistent results studying protein restriction in patients with CKD. Taken altogether, systematic reviews have suggested—at best—a modest benefit for patients on a low protein diet13 14 and given the aforementioned long-term data noting increased risk of death with very low protein diets, most nephrology experts are more comfortable with moderate protein restriction to the degree of 0.8 g/kg/day as recommended by the KDIGO 2022 guideline.
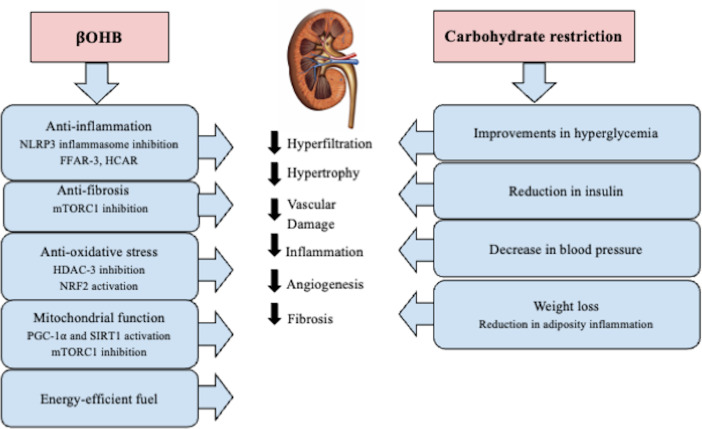
The impact of carbohydrate restriction interventions on kidney function is poorly understood. Existing studies consistently reported improvements in glycemic control, blood pressure, weight, and insulin resistance, all of which have favorable downstream implications for slowing kidney disease progression (figure 1). In addition, ketone bodies themselves have a myriad of physiologic and signaling effects that could elicit renoprotective effects. For example, the renoprotective effect of sodium-glucose cotransporter 2 inhibitors (SGLT2i) has been postulated to be partially mediated by the modest medication-induced ketosis.15–17 This low-grade ketosis induced by SGLT2i may directly or indirectly benefit the kidney by serving as an energy source during stress and kidney injury, and through its anti-inflammatory, antifibrotic, and antioxidant effects (figure 1).15 16 Given that SGLT2i-induced ketosis may be beneficial for the kidney, endogenously produced ketones resulting from a WFKD may prove to be another therapeutic option for diabetic nephropathy or kidney disease.18 19
Figure 1.
Summary of pleiotropic renal protective effect of ketones and carbohydrate restriction. BHB, β-hydroxybutyrate; mTORC1, mammalian target of rapamycin complex 1; NLRP3, NOD-, LRR-, and pyrin domain-containing 3; PGC-1ɑ, peroxisome proliferator-activator receptor γ (PPARγ) coactivator-1-alpha; FFAR-3, free fatty acid receptor-3; HCAR, hydroxycarboxylic acid receptor-2; HDAC-3, Histone deacetylase-3; NRF2, nuclear factor erythroid 2-related factor 2; SIRT1, silent information regulator transcript-1.
In this review, we explore the pleiotropic roles and signaling effects of ketones on kidney physiology, address potential concerns of ketogenic therapy, summarize the available literature on the effect of low carbohydrate diets on kidney function, and discuss future studies that could help address the gaps in knowledge and discrepancies in the literature.
Potential roles of ketones on kidney pathophysiology and disease
Ketones as an alternative energy-efficient fuel
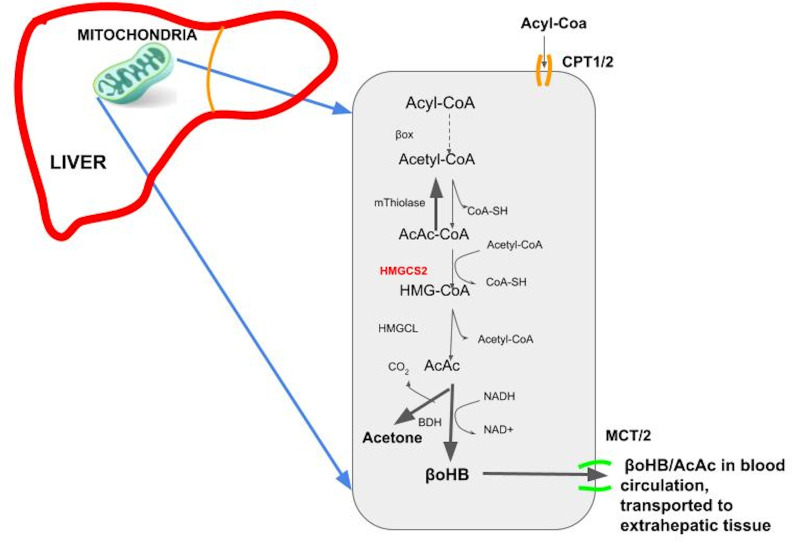
The human body naturally produces ketones, mostly in the liver, at varying rates that result in circulating ketones that span more than four orders of magnitude (<0.01 to >10 mM)20 21 depending primarily on a person’s carbohydrate intake and insulin level. Increased lipolysis and ketogenesis are upregulated in response to a low insulin-to-glucagon ratio, which occurs during calorie restriction/fasting, prolonged exercise, consumption of a ketogenic diet, or pathologic insulin deficiency. In the liver, when production of fatty acyl coenzyme A (CoA) increases during low insulin secretion and increased lipolysis, fatty acyl CoA is transported to the mitochondrial matrix, where it is then β-oxidized to produce acetyl-CoA.22 23 Acetyl-CoA is either converted to malonyl CoA or to acetoacetyl CoA (figure 2). Acetoacetyl CoA and acetyl-CoA are further condensed by a rate-limiting enzyme, 3-hydroxy 3-methylglutaryl-CoA synthase 2 (HMGCS2) to generate hydroxymethylglutaryl CoA (HMG-CoA). HMG-CoA is then converted into acetoacetate (AcAc) by hydroxymethylglutaryl coenzyme A lyase (HMGCL).22 23 Finally, AcAc is reduced to β-hydroxybutyrate (BHB) by BHB dehydrogenase (BDH) (figure 2). Both AcAc and BHB are released from the liver and transported in the blood circulation to extrahepatic tissues where they can have signaling effects and be metabolized and released or oxidized to produce energy. BHB is a vital energy source for the brain with uptake occurring in proportion to circulating levels. As such, during prolonged starvation, ketones can provide over half the brain’s energy requirements.24 Generally, glucose is considered the most efficient fuel since it produces more ATP per oxygen consumed with a phosphate/oxygen (P/O) ratio of 2.58.24 25 However, in a state of insulin resistance where glucose uptake and oxidation are impaired, BHB is an effective alternative dense energy molecule with a P/O ratio of 2.50. In contrast to free fatty acids (FFAs), another form of energy-dense fuel,25 26 BHB gives better ATP yield per oxygen consumed, is water soluble, and generates fewer reactive oxygen species (ROS).
Figure 2.
Ketogenesis pathway. AcAc, acetoacetate; BDH, BHB dehydrogenase; CoA, coenzyme A; HMGCL, hydroxymethylglutaryl coenzyme A lyase; HMG-CoA, hydroxymethylglutaryl CoA; HMGCS2, 3-hydroxy 3-methylglutaryl-CoA synthase 2; CPT1/2, carnitine palmitoyltransferase 1/2; MCT, moncarboxylate transporter.
The kidney is among the most metabolically active organs, with very high oxygen demand and the second-highest mitochondrial density after the myocardium.27 While oxidative metabolism is the principal source of energy in the kidney, the fuel substrates for metabolism differ across regions of the kidney. In the healthy kidney, both fatty acid oxidation (FAO) in the proximal tubules and glycolysis in the distal tubules support its metabolism. The renal cortex, especially the S1/S2 tubule segments, generates energy primarily from FFAs, lactate, and glutamate versus glucose.28 The outer medulla uses glucose, lactate, FFAs, and ketones for energy. However, in diseased kidneys, mitochondrial dysfunction has been reported as a key pathologic feature that contributes to disease initiation and progression.29 30 For example, in diabetic nephropathy (DN), hyperglycemia-induced flux of glycolysis increases oxygen demand with the by-product of amplified ROS.30 31 Excess glucose use in the kidney shifts the energy reliance from fatty acid metabolism to glycolysis, even in the proximal tubules.30 31
The renoprotective effect of SGLT2i in diabetic kidney disease is driven by amelioration of the pathologic metabolic shift from FAO to glycolysis. SGLT2i decreases reabsorption of excessive glucose, reduces energy production from glucose in the kidney, and increases fatty acid utilization in the kidney.32 Furthermore, the glycosuric effect of SGLT2i also augments the BHB level in the kidney mainly through increased production of ketones rather than reduced kidney clearance.33 The kidney is indeed an avid consumer of ketones.34 BHB serves as an important alternative source of energy for the kidney during metabolic imbalance. It can be effectively metabolized in all nephron regions, except the S1/S2 proximal tubule segments.35 During starvation or fasting, the BHB level in the kidney increases 20-fold and is used as a substrate for mitochondrial energy production.20 In the diabetic kidney disease mouse model, both SGLT2i and exogenous ketone treatment normalized the renal ATP levels by restoring its production and this intervention was also associated with kidney function improvement.36
Anti-inflammatory effect
Inflammation is critical in both acute kidney disease and CKD, especially through activation of inflammasomes such as NOD-, LRR-, and pyrin domain-containing 3 (NLRP3).37 Numerous studies highlight the link between DN and NLRP3 inflammasome activation, which negatively impacts podocyte function, escalates the expression of inflammatory markers like IL-1β, and is also linked with albuminuria and tubulointerstitial injury.38 39 Consequently, targeting NLRP3 inflammasome inhibition emerges as a promising approach for kidney disease treatment, despite concerns over the safety of current experimental drugs.40 BHB stands out for its wide-ranging anti-inflammatory actions, including its effect on inhibiting NLRP3 inflammasome activation.41 BHB successfully suppresses NLRP3 inflammasome activation in human monocytes and murine neutrophils in vitro and in animal models of NLRP3-mediated diseases.42 Likewise, the anti-inflammatory effect of SGLT2i in diabetic rats, characterized by subdued NLRP3 inflammasome activation and lower interleukin (IL)-1β and tumor necrosis factor (TNF)-ɑ levels, correlates with elevated BHB and reduced insulin levels in the bloodstream.43 BHB’s primary receptor is GPR109A (HCAR2), a G protein coupled receptor (GPCR) that acts by suppressing cyclic adenosine monophosphate (cAMP).44 Beyond NLRP3 inflammasome suppression, animal studies reveal BHB diminishes other proinflammatory cytokines, including IL-6, chemokine (C–C motif) ligand 2, and monocyte chemoattractant protein-1, through activation of GPR109A, partially influenced by BHB’s effect on nuclear factor kappa B translocation.45–47 In humans, ketogenic diets consistently reduce inflammation indicators. Individuals with T2D on a ketogenic diet show decreased serum C reactive protein and white cell counts,48 along with significant reductions in 15 out of 16 inflammatory/immune modulators after 1 and 2 years.49 This anti-inflammatory benefit aligns with prior findings that observed a greater reduction in 7 out of 14 inflammation/immune modulators with a ketogenic diet compared with a low-fat diet after 12 weeks.50
Antifibrotic effects
The antifibrotic effect of BHB is mainly mediated through the mammalian target of rapamycin complex 1 (mTORC1) pathway. In diabetic kidney disease, mTORC1 hyperactivation is associated with kidney dysfunction and increased fibrosis.43 In a mouse model of non-proteinuric diabetic kidney disease, SGLT2i, particularly empagliflozin conferred renal protection by increasing endogenous ketones and suppressing mTORC1 activation in the kidneys.36 The treatment with empagliflozin mirrored the effect of exogenous ketone supplementation, where both treatments reduced kidney damage as evident through lower plasma cystatin-C levels and decreased interstitial fibrosis.36 The renoprotective mechanism of SGLT2i hinges on the ketogenesis rate-limiting enzyme HMGCS2 highlighting ketone production’s central role in its antifibrotic effects.36 51
Antioxidative effects
Ketones, specifically BHB, act as an important signaling molecule influencing gene expression through various regulatory pathways. BHB notably inhibits class I histone deacetylase enzyme activity in kidney tissue, enhancing the expression of genes that respond to oxidative stress, including Foxo3a and Mt2.52 This confers protection against oxidative stress in human kidney cells and various animal models. Studies also show that a ketogenic diet or BHB treatment can activate the major detoxification and oxidative stress nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.53 In a spontaneous mouse model of T2D (db/db mice) treated with dapagliflozin, there was a noticeable reduction in the expression of genes related to oxidative stress compared with those treated with a standard vehicle or glimepiride.54 This reduction is associated with increased levels of BHB and NRF2 protein expression.54 Similarly, BHB treatment in human proximal tubular cells (HK-2) led to increased NRF2 expression and induced NRF2 nuclear translocation.55 Furthermore, a ketogenic diet has been reported to increase the expression of other antioxidants such as NAD(P)H dehydrogenase quinone I (NQO1) and superoxide dismutase (SOD1/2),56 and ameliorated paraquat (PQ)-induced elevated lipid peroxidation, toxicity, reduced antioxidant activity and decreased Nrf2 expression,57 highlighting its potential therapeutic role in combating oxidative stress and tissue hypoxia.
Mitochondrial dysfunction
Mitochondrial dysfunction is another key feature of both acute kidney failure and CKD.58 Ketogenic diet activates the expression of peroxisome proliferator-activator receptor γ (PPARγ) coactivator-1-alpha (PGC-1ɑ)59 and silent information regulator transcript-1 (SIRT1).60 61 PGC-1ɑ is the main transcription factor that controls the expression of genes involved in mitochondrial biogenesis and function, while SIRT1 activation protects organelle damage including the mitochondria and reduces oxidative stress. In a recent study on diabetic mice, a ketogenic diet improved mitochondrial function and capacity through its activation of PGC-1ɑ and SIRT1.62 Further, administration of exogenous BHB was found to increase PGC-1ɑ and mitochondrial copy number in rat kidneys.63 Human data on mitochondrial function are lacking, but we obtained skeletal muscle biopsies from physically active adults before and after a 12-week ketogenic diet and demonstrated that mitochondrial function and efficiency shifted towards fat oxidation while improving insulin sensitivity.64
Traditional concerns of a ketogenic diet on kidney function
Common misconceptions about ketogenic diets related to kidney health include potential adverse effects on acid–base and electrolyte balance and risk for kidney stones. The next section briefly discusses the typical renal metabolic response to a ketogenic diet that maintains pH and electrolyte status. Most work in this space has been done in the context of normal kidney function, so we mention how the situation in CKD may differ.
Electrolyte and acid–base imbalance
Ketogenic diets promote a natriuretic and diuretic effect similar to that demonstrated during starvation.65 66 This in part accounts for the typical rapid weight loss that occurs during the initiation of a ketogenic diet. If sodium intake is not commensurate with the additional loss of sodium, two deleterious outcomes are more likely to manifest: (1) Individuals may develop common signs and symptoms of hypovolemia, colloquially referred to as “Keto-Flu,” which include dizziness when standing, lethargy, and muscle spasms/cramps. (2) Counter-regulatory mechanisms are activated that include sympathetic and aldosterone stimulation that act to preserve plasma volume by increasing sodium reabsorption and a concomitant excretion of potassium and magnesium. These side effects can be eliminated with attention to proper electrolyte intake. For most individuals with normal kidney function consuming a ketogenic diet, it should be emphasized to ingest an additional 1–2 g sodium/day (4–5 g sodium/day total), a maintenance of 3–4 g/day potassium, and sufficient fluid intake.
In CKD, a decrease in viable nephrons and reduction in glomerular filtration rate (GFR) change the kidney’s normal physiology and sodium balance.67 68 Even though an adaptive fractional increase in sodium excretion per individual nephron unit compensates for the reduced number of working nephrons, the kidney’s inability to excrete sufficient amounts of sodium results in sodium retention, extracellular fluid expansion, and blood pressure increase.68 69 Likewise, the renin–angiotensin–aldosterone system is activated in CKD, further exacerbating sodium retention and causing vasoconstriction which could significantly raise blood pressure.69 Sodium retention and its association with blood pressure in CKD are often referred to as “sodium-sensitive hypertension.”70 Therefore, reducing salt intake is recommended to manage hypertension in patients with CKD.68 The natriuretic and diuretic effect of the ketogenic diet may help alleviate sodium retention and improve systemic and glomerular blood pressure. Low carbohydrate and ketogenic diet studies often report a reduction in systolic and diastolic blood pressure71 72 and blood pressure medication requirements.73 However, the current recommendation of sodium intake in a WFKD is based on individuals with normal kidney function.74 Recommendations for sodium and electrolyte intake for patients with CKD following a ketogenic diet should be individualized by a healthcare professional based on the patient’s renal function and electrolyte status. Future studies should assess the relationship between ketosis, sodium balance, and blood pressure.
Another misconception associated with ketogenic diets relates to promotion of acidosis owing to specific food items and the weakly acidifying effects of ketones, which could worsen kidney function, bone health, and kidney disease-associated endocrinopathies.75 76 In healthy subjects provided a carefully prepared ketogenic diet with mean BHB levels >2 mM, serum bicarbonate was modestly reduced but well within normal ranges.77 A ketogenic diet with mild ketosis (~1 mM) in individuals with normal kidney function has no significant impact on blood pH, serum bicarbonate level, and anion gap over 21 days78 and 4 months.79
When faced with an increased acid load, normal kidney function affords compensatory increases in ammonium excretion. In a somewhat mirrored perspective where the acid load is stable and the kidney function is reduced, the fewer working nephrons compensate with increased ammoniagenesis and excretion. This adaptation results in high intrarenal ammonia, which is thought to activate the alternative complement pathway eventually leading to tubulointerstitial fibrosis. Decreases in GFR to levels below 40–50 mL/min diminish the kidney’s ability to excrete more ammonium and overall acid80; hence, metabolic acidosis is more commonly encountered at this level of disease. In patients with kidney disease, clinicians commonly monitor steady-state serum bicarbonate levels to assess overall acid load. However, decreases in serum bicarbonate are often reported at a later stage of the disease and it is considered inadequate to reflect the overall acid load. Eubicarbonatemic hydrogen ion retention among patients with earlier CKD is increasingly an area of focus81; thus, studying ketogenic diet in all stages of CKD requires longer term study of acid excretion and the rate of kidney function decline.
Urinary acid excretion is favored as the gold standard for estimating acid load, and the prevailing wisdom was that an increased dietary acid load would burden the kidneys further and lead to more dysfunction. However, recent observations from the rich data collected in the Chronic Renal Insufficiency Cohort Study have demonstrated pitfalls to that simplistic view.82 83 Among patients with diabetes, higher levels of net acid excretion were associated with a lower risk of CKD progression. These studies suggested that the changes in acid excretion were diet-independent and may be elicited by changes in energy metabolism and endogenous acid production from insulin resistance.82 83 Currently, the effect of ketogenic diet on net acid excretion is unknown and this would be worthwhile exploring in patients with T2D and varying stages of CKD.
Kidney stones
Kidney stones, especially genetically driven stones, are associated with an increased risk of CKD.84 A recent meta-analysis reported a pooled kidney stone incidence of 5.9% among patients on a ketogenic diet followed for a median of 3.7 years,85 compared with a historical incidence rate of <0.3% per year in the general population.86 Most studies reporting risk of kidney stones were in children receiving a ketogenic diet therapy for epilepsy85 87–91 with higher incidence during long-term exposure (ie, 25% over 6 years,91 which is complicated by concurrent use of antiseizure medications (eg, carbonic anhydrase inhibitors) and other risk factors in this population. In adults with obesity, who are at higher kidney stone risk based on their higher adiposity,92 consuming a ketogenic diet over 2 years revealed no harmful effects on GFR, albuminuria, or fluid and electrolyte balance compared with a low-fat diet93; and there was one possible, but not confirmed, case of kidney stones out of 153 subjects.94
Uric acid stones are the most frequently reported by individuals on a ketogenic diet, followed by calcium oxalate stones or mixed stones with calcium and uric acid.85 A ketogenic diet transiently increases uric acid concentration 25%–50%, which usually peaks at 2–4 weeks, and gradually returns to prediet levels by 8 weeks.95–97 The initial rise in uric acid is concomitant with the rise in ketones, and it was postulated that the reason for this may be competition between uric acid and ketones for the same organic acid transporters, which are required for renal excretion.98–101 After several weeks, the kidney conserves ketones,102 presumably allowing for return of normal renal uric acid excretion and serum levels.
There may be effective strategies to mitigate the kidney stone risk in patients following a ketogenic diet. Increasing fluid intake to maintain dilute urine limits the possibility of mineral crystallization.103 Urine alkalinization, particularly addressing hypocitraturia, may inhibit supersaturation of calcium salts and aggregation.104 105 Moreover, studies of kidney stones have largely precluded patients with CKD where their urine parameters change alongside diminishing kidney function. A retrospective study of 811 patients with kidney stones noted that advancing kidney disease afforded reduced calcium stone formation, presumably due to reduced calciuria106 and increased uric acid stone formation.107 Metabolic acidosis resulting in acidic urine pH is common among individuals with CKD.108 Low urine pH is a well-known risk factor for forming uric acid kidney stones due to the low solubility of uric acidic in acidic conditions.109 110 At the same time, low urine pH leads to hypocitraturia which increases the risk of forming calcium oxalate kidney stones.111 Hence, future examination of how a ketogenic diet impacts the incidence of kidney stones among patients with T2D and CKD is paramount. Being aware of and addressing the potential kidney stone risk with well-established measures—such as urine alkalization, correcting hypocitraturia, and increasing fluid intake—is prudent. Additionally, understanding that diet-imposed change in risk through modulation of ammonia excretion, uricosuria, calciuria, citraturia, and other urinary parameters will assist with future guidance.
Current evidence on very low or low carbohydrate diet intervention and its effect on kidney function
Evidence from animal studies
Several rodent studies have specifically investigated the effects of a ketogenic diet on kidney function and disease. Two mouse studies reported benefit of ketogenic diet on DN, even reversing some of the key molecular features of DN. Poplawski et al assessed the effect of ketogenic diet on DN using both type 1 (Akita) and type 2 (db/db) murine diabetes models. In both models, the mice initially developed albuminuria on chow diet, and after transitioning to the ketogenic diet reversed and normalized urinary albumin/creatinine ratio (UACR) within 8 weeks.112 Furthermore, the expression of several stress-induced genes involved in oxidative stress and toxicity was completely normalized by ketogenic diet in both models, with an observed effect that was more consistently robust in the type 1 mouse model. Likewise, histopathologic features of glomerular sclerosis were also partially reversed by the ketogenic diet in the T2D mouse model.112 Jung et al examined db/db DN mice fed normal chow diet (dbNCD), high-fat diet (dbHFD), or ketogenic diet (dbKETO). dbKETO animals had lower UACR and blood urea nitrogen to creatinine ratio levels after 5 weeks compared with the dbNCD and dbHFD mice.55 Histologic analysis of the kidney showed that dbKETO mice had less fibrotic changes than the dbNCD and dbHCD mice suggesting that the dbKETO mice delayed progression of DN histologic phenotypes. Furthermore, in the same report, treatment of the human proximal tubular cell line (HK-2) with BHB led to activated autophagy by increasing the LC3 I to LC3 II ratio, phosphorylation of adenosine 5 monophosphate-activated protein kinase (AMPK), beclin, p62 degradation, NRF2 expression, and decreased glucose-induced ROS levels.55 Studies in a rat model of a genetic form of CKD, polycystic kidney disease, showed that a ketogenic diet not only slowed disease progression and preserved renal function in young animals but even partially reversed existing renal cystic disease in older animals.18 The treatment resulted in improvement of renal fibrosis and inhibition of mTORC1 and epithelial proliferation. Remarkably, the effects could be replicated by administering BHB in the drinking water in a dose-dependent manner, without any food changes.18 63 These results suggest that the actions of BHB may underlie most of the renoprotective mechanisms of nutritional ketosis, and that exogenous BHB can be effectively supplemented.
Evidence from clinical and observational studies
Clinical and observational studies that examined kidney function in response to low-carbohydrate diets ranging from <20 g/day to 30%–40% of energy expenditure are presented in online supplemental table 1. Three of the six randomized controlled trials (RCTs) reported no significant changes in kidney function in the low carbohydrate arm compared with the comparison diet group. Two of the three studies followed the participants with normal baseline eGFR for 52 weeks113 114 and the third study followed subjects with slightly lower baseline eGFR (<80) for 12 weeks.115 Another two RCTs reported renal benefit in the low carbohydrate arm with improvements in serum creatinine, cystatin C, eGFR, and albumin.93 116 The study by Tirosh et al reported greater eGFR improvement in those following a low-carbohydrate diet versus both a Mediterranean and low-fat diet.116
bmjdrc-2024-004101supp001.pdf (71.3KB, pdf)
The use of surrogate markers, especially serum creatinine-derived estimates of kidney function, is less accurate at higher eGFRs and may be mischaracterized amidst dietary intervention, highlighting the importance of studying major adverse kidney events and assessing cystatin C-derived kidney function estimates. Thus far, only one RCT has reported hard kidney endpoints including all-cause mortality that compared a carbohydrate-restricted, low-iron, polyphenol enriched diet (CR-LIPE) with a standard protein restriction diet (SPRD).117 The 191 participants in this study were followed for approximately 4 years. In this study, CR-LIPE significantly decreased doubling of serum creatinine (relative risk, 0.53, 95% CI 0.33 to 0.86, p<0.01), all-cause mortality (relative risk, 0.5, 95% CI 0.2 to 1.12) and also delayed end-stage renal disease and renal replacement therapy when compared with SPRD.117 However, the CR-LIPE intervention was a multimodal dietary intervention that included carbohydrate restriction (35% of the energy intake) as one of the dietary modifications along with low-iron availability and polyphenol enrichment in the diet. Future study involving major adverse kidney endpoints is warranted to confirm if a ketogenic diet has beneficial impact on kidney disease.
Presumably because eGFR is less accurate at healthier function (eGFR >80 mL/min), some of these studies have shown that the beneficial effect of low carbohydrate diet is greater in those with lower starting baseline eGFR. For example, the study by Tirosh et al reported that the increase in eGFR was greater in those with CKD stage 3 (a 7.1-point; 10% eGFR increase from baseline) than the whole cohort (+5.3% increase from baseline) in the low carbohydrate arm.116 While other studies included a range of baseline eGFRs, the subset of patients with more significant kidney dysfunction (eGFR <60 mL/min) exhibited a slower decline in function, and no deterioration was evident in participants with normal baseline eGFR.118 119 Furthermore, caution is warranted when interpreting creatinine-derived eGFR measurements because any change in skeletal muscle mass during a nutritional study may affect the endogenous production of creatinine independent of actual changes in renal function. Hence, corroboration with cystatin-C measurements would strengthen these observations. The single-arm prospective 12 weeks study on individuals with relatively advanced diabetes nephropathy (eGFR <40 mL/min) reported statistically significant improvements in eGFR, serum creatinine, and cystatin C.118 Three additional retrospective observational studies reported improvements in kidney function in individuals following a low carbohydrate diet120–122 (online supplemental table 1). One of these studies reported improvement in eGFR and decrease in UACR at an average follow-up of 30 months119 while the other two studies reported eGFR improvement in individuals with reduced kidney function at baseline (eGFR <90 in one study and eGFR <70 in the other study).121 122
In contrast, there were only two observational studies frequently cited when suggesting that a low carbohydrate diet is associated with adverse kidney outcomes. These studies did not focus on individuals adhering to a ketogenic diet or on those limiting their carbohydrate intake. For instance, Farhadnejad et al’s 2018 study, which was a population-based prospective analysis, investigated the association between different tertiles of low carbohydrate high protein (LCHP) scores and the incidence of CKD.123 Notably, none of the LCHP score tertiles in the study indicated a carbohydrate-restricted diet. Even in the tertile with the lowest LCHP score, carbohydrates contributed to 51.0% of the total energy, resembling the carbohydrate profile of a standard Western diet where 40%–60% of energy typically comes from carbohydrates. The other retrospective observational study by Li et al reported an association between elevated fasting ketone level with abnormal renal function124 in people with T2D who were admitted to the hospital, and who were not specifically eating a ketogenic diet. The association of ketones and renal function in this study is not relevant to dietary carbohydrate restriction in an ambulatory population.
Altogether, these clinical and observational studies show no harm from low or very low carbohydrate diets for people with diabetes in the setting of normal renal function, and a possible beneficial effect in the setting of moderately reduced renal function. The kidney function improvement observed in these studies may be an ancillary outcome associated with other improvements seen in these interventions including weight loss, glycemic control, or blood pressure improvement. Interestingly, Unwin et al reported no association between observed kidney function improvement with the magnitude of weight loss, improvement in blood pressure and HbA1c,120 while another study reported that the increase in eGFR was significantly associated with a decrease in fasting insulin and systolic blood pressure but not with the level of weight loss and protein intake in the intervention.116 In our previous study on patients with T2D following a very low carbohydrate intervention, there was a marginally significant increase in eGFR at 1 year.72 A post hoc analysis of these data revealed that a higher mean BHB at 1 year (β=5.04, p=0.005) was significantly associated with a greater increase in mean eGFR (unpublished data). Furthermore, in a subgroup analysis of 22 trial participants with an eGFR <60 mL/min/1.73 m2 at baseline who remained in the study for 2 years,72 the mean eGFR progressively increased from 51 mL/min/1.73 m2 to 60, 63, and 68 mL/min/1.73 m2 at 10 weeks, 1 year, and 2 years (unpublished data). Notably, the majority of the 22 participants reverted to stage 2 and no one progressed to stage 4 CKD. Evidently, a dose-dependent association exists between ketosis trajectory classes and the increase in total eGFR slope at 2 years.125 Participants with higher endogenous ketone concentration and longer duration of ketosis maintenance exhibited the greatest rise in the 2-year eGFR slope compared with those with lower endogenous ketone concentration and unsustainable ketosis maintenance.125 Hence, available evidence suggests that carbohydrate restriction and ketosis afford benefits to kidney function. It will be important to determine in future trials whether the improvement in kidney function translates to a sustained long-term reduction, or even reversal, in the progression of kidney disease.
Evidence from meta-analyses, systematic and narrative review
A recent review discussing the potential negative effect of purported ketogenic diets on kidney health focused on observational studies that compared low protein versus high protein diets that were not ketogenic or low carbohydrate diets,126 and raised concern about the association of albuminuria with high animal fat but only referred to observational studies that assessed high animal fat intake in the context of a Western diet126 negating the relevance of the studies cited for concern.
In contrast, systematic reviews and meta-analyses that assessed pooled effects of RCTs reported beneficial effects of low-carbohydrate diets. The meta-analysis by Oyabu et al evaluated nine RCTs with 861 participants in the low carbohydrate arm and 826 participants in the control group.127 Despite a large variation in the proportion of carbohydrate intake from 4% to 45% in the low carbohydrate arm of the nine studies with a study duration ranging from 6 to 24 months, the review revealed that there was a significant increase in eGFR in the low carbohydrate group versus control group.127 Another meta-analysis with 12 RCTs that only included patients with T2D reported no significant difference in the pooled eGFR and creatinine mean estimate between the lower carbohydrate diets (14%–45% of calories from carbohydrate) versus control diets over 5 weeks to 24 months.128 Similarly, another meta-analysis that included five RCTs with the low carbohydrate arm had carbohydrate intake <45%, and the studies ranging from 5 weeks to 24 months reported no difference in the pooled eGFR estimate between the control and low carbohydrate diets.129 The current evidence from systematic reviews and meta-analyses with a range of carbohydrate intake suggests that carbohydrate restriction is not associated with adverse effects on kidney function, or in some cases might be beneficial.
Evidence from genetically driven kidney disease
Individuals with autosomal dominant polycystic kidney disease (ADPKD) may benefit from calorie restriction or ketogenic diet.19 130 131 This chronic progressive condition is characterized by hyperproliferation, inflammation, fibrosis, and cyst growth, leading to deterioration of kidney function over time.19 132 mTOR is one of the main signaling pathways activated in ADPKD.132 A study of various polycystic kidney disease animal models showed that time-restricted feeding, administration of a ketogenic diet, or supplementation with exogenous BHB prevented kidney cyst disease progression by inhibiting cell proliferation, fibrosis, and cyst growth.18 63 Furthermore, the mTOR activity was inhibited in these animal models suggesting that blunting the signaling pathway inhibits cell proliferation, growth, and fibrosis in ADPKD.18 63 133 In humans, a retrospective observational study of ADPKD patients who self-initiated ketosis either using ketogenic or time-restricted diets reported improvement in eGFR after 6 months.134 A pilot study on 24 patients with ADPKD demonstrated the feasibility of the ketogenic diet, reporting high adherence rates and improvements in blood pressure, eGFR, and kidney pain.130 In another exploratory RCT, 66 participants with ADPKD were randomized to ketogenic, water fasting, or control diets. The study confirmed the feasibility of the therapy in the ketogenic arm (KD) and revealed significant improvements in eGFR, including both creatinine and cystatin C-derived eGFR in the KD group but no improvements were observed in the water fasting and control diets.131 Additionally, there were no significant differences in UACR and blood pressure among the three diets.131
Perspectives and future direction
There is a considerable body of research suggesting that a very low carbohydrate ketogenic diet is safe in individuals with moderately diminished kidney function, even in studies that had higher protein intake than what is recommended for kidney disease and diets that are not plant-based. The diet can be safely prescribed in patients with T2D for treating and remitting diabetes even if they have underlying stage 2 or 3 CKD or reduced kidney function. Beyond safety, mechanistic plausibility, preclinical data, and even some RCT studies suggest that carbohydrate-restricted diets may be beneficial in improving moderate kidney dysfunction and in reducing progression of CKD. The preliminary proof of concept from small and short duration studies in humans and animals suggests a very low carbohydrate diet could be an effective dietary intervention for patients with CKD. Furthermore, there are predeveloped ketogenic nutritional options to consider when we plan a future trial to assess the impact of ketogenic diet on patients with CKD, such as the recently developed program for treating ADPKD known as Ren.Nu. This program is a plant-focused ketogenic medical nutrition therapy, designed to avoid renal stressors like oxalate, inorganic phosphate, and purines/uric acid. It includes a medical food formulation, KetoCitra, containing BHB with alkaline citrate which helps antagonize kidney stone formation.129 130 Based on the findings from these different studies and currently available ketogenic medical therapy specific for kidney disease, there is a need for future larger and longer follow-up randomized controlled clinical trials on very low carbohydrate diet, including nutritional ketosis in patients with CKD with or without T2D on kidney hard endpoints including major adverse kidney events (a composite event of death, persistent renal decline >25% decline in eGFR, and a new initiation of dialysis) and other kidney-related outcomes to firmly establish the long-term effectiveness. For example, a head-to-head comparison of the safety and efficacy of ketogenic nutritional therapy versus SGLT2i pharmacologic intervention (that involves the same mechanism of raising ketone levels) could be of high interest. Weight loss from the diet can improve filtration and albuminuria. Thus, including other surrogate endpoints like eGFR slope and microalbuminuria in these studies have the potential to elucidate the degree to which weight loss and blood pressure improvement from the diet affects kidney function markers and also to explore if ketone levels independently have an impact on these markers and endpoints. Furthermore, these studies should also assess the diet’s overall safety in patients with T2D and CKD, specifically exploring its effect on net acid excretion, kidney stone formation, and maybe its beneficial effect on sodium retention hypertension. Finally, another important consideration in the clinical trial design for evaluating the efficacy of a very low carbohydrate diet in patients with CKD is understanding the diet’s additive role, especially how the diet interacts with currently available treatment drugs for patients with CKD including renin–angiotensin system blockade (angiotensin-converting enzyme inhibitor, ACEi and angiotensin receptor blockers, ARBs), SGLT2i, glucagon-like peptide-1 receptor agonists (GLP1-RA), and the non-steroidal mineralocorticoid receptor antagonists (finerenone).
Footnotes
Contributors: SJA, JV, and CGPR conceptualized the review topic and formulated the objectives; SJA conducted the comprehensive literature search, synthesized and interpreted the data from the collected literature; SJA drafted the original manuscript; JSV, TW, CGPR, and CV provided critical revisions and edits to the manuscript; ALM and GKS reviewed and edited the manuscript; All authors have read and agreed to the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: TW is an inventor on issued and pending patents filed by the University of California, Santa Barbara related to the topic of this article. TW is a founder and shareholder of Santa Barbara Nutrients, Inc., holds a managerial position, and has contributed to the development of the Ren.Nu ketogenic dietary program and the medical food KetoCitra. TW received speaker fees from Otsuka, was a scientific advisor of Chinook Therapeutics, and received research funding from Chinook Therapeutics. JSV is a cofounder and shareholder of Virta Health, serves as a science advisor for Simply Good Foods and Nutrishus Brands, and has authored books on ketogenic diets. SJA, CGPR, and GKS are employees and shareholders of Virta Health. ALM is a shareholder of Virta Health.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Feng XS, Farej R, Dean BB, et al. CKD prevalence among patients with and without type 2 diabetes: regional differences in the United States. Kidney Med 2022;4:100385. 10.1016/j.xkme.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. ElSayed NA, Aleppo G, Aroda VR, et al. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes—2023 . Diabetes Care 2023;46:S68–96. 10.2337/dc23-S005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jhee JH, Kee YK, Park S, et al. High-protein diet with renal hyperfiltration: a community-based prospective cohort study. Nephrol Dial Transplant 2020;35:98–106. [DOI] [PubMed] [Google Scholar]
- 4. Esmeijer K, Geleijnse JM, de Fijter JW, et al. Dietary protein intake and kidney function decline after myocardial infarction: the alpha Omega cohort. Nephrol Dial Transplant 2020;35:106–15. 10.1093/ndt/gfz015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yancy WS, Olsen MK, Guyton JR, et al. A low-carbohydrate ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia. Ann Intern Med 2004;140:769–77. 10.7326/0003-4819-140-10-200405180-00006 [DOI] [PubMed] [Google Scholar]
- 6. Stern L, Iqbal N, Seshadri P, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults. Ann Intern Med 2004;140:778–85. 10.7326/0003-4819-140-10-200405180-00007 [DOI] [PubMed] [Google Scholar]
- 7. Westman EC, Yancy WS, Mavropoulos JC, et al. The effect of a low-carbohydrate ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr Metab (Lond) 2008;5:36. 10.1186/1743-7075-5-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cuenca-Sánchez M, Navas-Carrillo D, Orenes-Piñero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Adv Nutr 2015;6:260–6. 10.3945/an.114.007716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 2020;76:S1–107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 10. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102:S1–127. 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 11. Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 1994;330:877–84. 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 12. Menon V, Kopple JD, Wang X, et al. Effect of a very low-protein diet on outcomes: long-term follow-up of the modification of diet in renal disease (MDRD) study. Am J Kidney Dis 2009;53:208–17. 10.1053/j.ajkd.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 13. Fouque D, Laville M. Low protein diets for chronic kidney disease in non-diabetic adults. Cochrane Database Syst Rev 2009;10:CD001892. 10.1002/14651858.CD001892.pub3 [DOI] [PubMed] [Google Scholar]
- 14. Fouque D, Laville M, Boissel JP, et al. Controlled low protein diets in chronic renal insufficiency: meta-analysis. BMJ 1992;304:216–20. 10.1136/bmj.304.6821.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hattori Y. Beneficial effects on kidney during treatment with sodium-glucose cotransporter 2 inhibitors. Heart Fail Rev 2021;26:947–52. 10.1007/s10741-020-10065-7 [DOI] [PubMed] [Google Scholar]
- 16. Gao YM, Feng ST, Wen Y, et al. Cardiorenal protection of SGLT2 inhibitors. EBioMedicine 2022;83:104215. 10.1016/j.ebiom.2022.104215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomita I, Kume S, Sugahara S, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab 2020;32:404–19. 10.1016/j.cmet.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 18. Torres JA, Kruger SL, Broderick C, et al. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab 2019;30:1007–23. 10.1016/j.cmet.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weimbs T, Saville J, Kalantar-Zadeh K. Ketogenic metabolic therapy for chronic kidney disease. Clin Kidney J 2024;17:sfad273. 10.1093/ckj/sfad273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Féry F, Balasse EO. Ketone body production and disposal in diabetic ketosis. Diabetes 1985;34:326–32. 10.2337/diabetes.34.4.326 [DOI] [PubMed] [Google Scholar]
- 21. Balasse EO, Féry F. Ketone body production and disposal. Diabetes Metab Rev 1989;5:247–70. 10.1002/dmr.5610050304 [DOI] [PubMed] [Google Scholar]
- 22. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017;25:262–84. 10.1016/j.cmet.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kolb H, Kempf K, Röhling M, et al. Ketone bodies: from enemy to friend and guardian angel. BMC Med 2021;19:313. 10.1186/s12916-021-02185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr 2006;26:1–22. 10.1146/annurev.nutr.26.061505.111258 [DOI] [PubMed] [Google Scholar]
- 25. Veech RL. The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions. Prostaglandins Leukot Essent Fatty Acids 2004;70:309–19. [DOI] [PubMed] [Google Scholar]
- 26. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study. Diabetes Care 2016;39:1115–22. 10.2337/dc16-0542 [DOI] [PubMed] [Google Scholar]
- 27. Wang Z, Ying Z, Bosy-Westphal A, et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr 2010;92:1369–77. 10.3945/ajcn.2010.29885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baverel G, Ferrier B, Martin M. Fuel selection by the kidney: adaptation to starvation. Proc Nutr Soc 1995;54:197–212. 10.1079/pns19950049 [DOI] [PubMed] [Google Scholar]
- 29. Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol 2018;14:291–312. 10.1038/nrneph.2018.9 [DOI] [PubMed] [Google Scholar]
- 30. Yao L, Liang X, Qiao Y, et al. Mitochondrial dysfunction in diabetic tubulopathy. Metabolism 2022;131:155195. 10.1016/j.metabol.2022.155195 [DOI] [PubMed] [Google Scholar]
- 31. Audzeyenka I, Bierżyńska A, Lay AC. Podocyte bioenergetics in the development of diabetic nephropathy: the role of mitochondria. Endocrinology 2022;163:bqab234. 10.1210/endocr/bqab234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cai T, Ke Q, Fang Y, et al. Sodium–glucose cotransporter 2 inhibition suppresses HIF-1Α-mediated metabolic switch. Cell Death Dis 2020;11:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferrannini E, Baldi S, Frascerra S, et al. Renal handling of Ketones in response to Sglt2 inhibition in type 2 diabetes. Diabetes Care 2017;40:771–6. 10.2337/dc16-2724 [DOI] [PubMed] [Google Scholar]
- 34. Cuenoud B, Hartweg M, Godin J-P, et al. Metabolism of exogenous D-beta-hydroxybutyrate. Front Nutr 2020;7:13. 10.3389/fnut.2020.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh P, McDonough AA, Thomson SC. Metabolic basis of solute transport. In: Brenner & Rectorʼs the kidney. 10th edn. Philadelphia, PA: Elsevier, 2016: 122–43. [Google Scholar]
- 36. Tomita I, Kume S, Sugahara S, et al. n.d. SGLT2 inhibition mediates protection from diabetic kidney disease. Cell Metab 32:404–19. 10.1016/j.cmet.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 37. Komada T, Muruve DA. The role of Inflammasomes in kidney disease. Nat Rev Nephrol 2019;15:501–20. 10.1038/s41581-019-0158-z [DOI] [PubMed] [Google Scholar]
- 38. Shahzad K, Bock F, Dong W, et al. Nlrp3-Inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int 2015;87:74–84. 10.1038/ki.2014.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hou Y, Lin S, Qiu J, et al. Nlrp3 Inflammasome negatively regulates podocyte autophagy in diabetic nephropathy. Biochem Biophys Res Commun 2020;521:791–8. 10.1016/j.bbrc.2019.10.194 [DOI] [PubMed] [Google Scholar]
- 40. Chi K, Geng X, Liu C, et al. Research progress on the role of Inflammasomes in kidney disease. Mediators Inflamm 2020;2020:8032797. 10.1155/2020/8032797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Youm Y-H, Nguyen KY, Grant RW, et al. The ketone metabolite β-hydroxybutyrate blocks Nlrp3 Inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–9. 10.1038/nm.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldberg EL, Asher JL, Molony RD, et al. b-Hydroxybutyrate deactivates neutrophil Nlrp3 inflammasome to relieve gout flares. Cell Rep 2017;18:2077–87. 10.1016/j.celrep.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim SR, Lee S-G, Kim SH, et al. Sglt2 inhibition modulates Nlrp3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 2020;11:2127. 10.1038/s41467-020-15983-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spigoni V, Cinquegrani G, Iannozzi NT, et al. Activation of G protein-coupled receptors by ketone bodies: clinical implication of the ketogenic diet in metabolic disorders. Front Endocrinol 2022;13:972890. 10.3389/fendo.2022.972890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gambhir D, Ananth S, Veeranan-Karmegam R, et al. Gpr109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest Ophthalmol Vis Sci 2012;53:2208–17. 10.1167/iovs.11-8447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu S-P, Li S-N, Wang J-F, et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kB activation. Mediators Inflamm 2014;2014:983401. 10.1155/2014/983401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fu SP, Liu BR, Wang JF, et al. b-Hydroxybutyric acid inhibits growth hormone-releasing hormone synthesis and secretion. J Neuroendocrinol 2015;27:212–22. 10.1111/jne.12256 [DOI] [PubMed] [Google Scholar]
- 48. Bhanpuri NH, Hallberg SJ, Williams PT, et al. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis. Cardiovasc Diabetol 2018;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Phinney S, Adams R, Athinarayanan S, et al. Broad spectrum effects of a ketogenic diet on inflammation and immune modulators in type 2 diabetes and prediabetes. J Endocr Soc 2020;4. 10.1210/jendso/bvaa046.2319 [DOI] [Google Scholar]
- 50. Forsythe CE, Phinney SD, Fernandez ML, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008;43:65–77. 10.1007/s11745-007-3132-7 [DOI] [PubMed] [Google Scholar]
- 51. Kogot-Levin A, Hinden L, Riahi Y, et al. Proximal tubule mTORC1 in diabetic nephropathy and its correction by SGLT2 inhibitors. Cell Rep 2020;32:107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate. Science 2013;339:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis 2010;40:238–44. 10.1016/j.nbd.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim MN, Moon JH, Cho YM. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence. Diabetes Obes Metab 2021;23:2561–71. [DOI] [PubMed] [Google Scholar]
- 55. Jung J, Park WY, Kim YJ, et al. 3-Hydroxybutyrate ameliorates the progression of diabetic nephropathy. Antioxidants (Basel) 2022;11:381. 10.3390/antiox11020381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greco T, Glenn TC, Hovda DA, et al. Ketogenic diet decreases oxidative stress. J Cereb Blood Flow Metab 2016;36:1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wei T, Tian W, Liu F, et al. Protective effects of exogenous β-hydroxybutyrate on paraquat toxicity in rat kidney. Biochem Biophys Res Commun 2014;447:666–71. 10.1016/j.bbrc.2014.04.074 [DOI] [PubMed] [Google Scholar]
- 58. Bhatia D, Capili A, Choi ME. Mitochondrial dysfunction in kidney injury, inflammation, and disease. Kidney Res Clin Pract 2020;39:244–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pathak SJ, Baar K. Ketogenic diets and mitochondrial function. Exerc Sport Sci Rev 2023;51:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McCarty MF, DiNicolantonio JJ, O’Keefe JH. Ketosis may promote brain macroautophagy. Med Hypotheses 2015;85:631–9. 10.1016/j.mehy.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 61. Scheibye-Knudsen M, Mitchell SJ, Fang EF, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab 2014;20:840–55. 10.1016/j.cmet.2014.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roberts MN, Wallace MA, Tomilov AA, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 2017;26:539–46. 10.1016/j.cmet.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torres JA, Holznecht N, Asplund DA, et al. A combination of SS-hydroxybutyrate and citrate ameliorates disease progression in a rat model of polycystic kidney disease. Am J Physiol Renal Physiol 2024;326:F352–68. 10.1152/ajprenal.00205.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Miller VJ, LaFountain RA, Barnhart E, et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle. Am J Physiol Endocrinol Metab 2020;319:E995–1007. 10.1152/ajpendo.00305.2020 [DOI] [PubMed] [Google Scholar]
- 65. Palmer BF, Clegg DJ. Starvation ketosis and the kidney. Am J Nephrol 2021;52:467–78. 10.1159/000517305 [DOI] [PubMed] [Google Scholar]
- 66. Saudek CD, Boulter PR, Arky RA. The natriuretic effect of glucagon and its role in starvation. J Clin Endocrinol Metab 1973;36:761–5. 10.1210/jcem-36-4-761 [DOI] [PubMed] [Google Scholar]
- 67. Shin J, Lee CH. The roles of sodium and volume overload on hypertension in chronic kidney disease. Kidney Res Clin Pract 2021;40:542–54. 10.23876/j.krcp.21.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Borrelli S, Provenzano M, Gagliardi I, et al. Sodium intake and chronic kidney disease. Int J Mol Sci 2020;21:4744. 10.3390/ijms21134744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Soi V, Yee J. Sodium homeostasis in chronic kidney disease. Adv Chronic Kidney Dis 2017;24:325–31. 10.1053/j.ackd.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 70. Bovée DM, Cuevas CA, Zietse R, et al. Salt-sensitive hypertension in chronic kidney disease: distal tubular mechanisms. Am J Physiol Renal Physiol 2020;319:F729–45. 10.1152/ajprenal.00407.2020 [DOI] [PubMed] [Google Scholar]
- 71. Unwin DJ, Tobin SD, Murray SW, et al. Substantial and sustained improvements in blood pressure, weight and lipid profiles from a carbohydrate restricted diet. Int J Environ Res Public Health 2019;16. 10.3390/ijerph16152680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hallberg SJ, McKenzie AL, Williams PT, et al. Effectiveness and safety of a novel care model for the management of type 2 diabetes. Diabetes Ther 2018;9:583–612. 10.1007/s13300-018-0373-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Athinarayanan SJ, Hallberg SJ, McKenzie AL, et al. Impact of a 2-year trial of nutritional ketosis on indices of cardiovascular disease risk in patients with type 2 diabetes. Cardiovasc Diabetol 2020;19:208. 10.1186/s12933-020-01178-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Phinney SD. Ketogenic diets and physical performance. Nutr Metab (Lond) 2004;1:2. 10.1186/1743-7075-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dhondup T, Qian Q. Electrolyte and acid-base disorders in chronic kidney disease and end-stage kidney failure. Blood Purif 2017;43:179–88. 10.1159/000452725 [DOI] [PubMed] [Google Scholar]
- 76. Arif H. Complications of chronic kidney disease: electrolyte and acid-base disorders. In: Approaches to Chronic Kidney Disease. Cham: Springer, 2022. [Google Scholar]
- 77. Phinney SD, Bistrian BR, Wolfe RR, et al. The human metabolic response to chronic ketosis without caloric restriction. Metab Clin Exp 1983;32:757–68. 10.1016/0026-0495(83)90105-1 [DOI] [PubMed] [Google Scholar]
- 78. Carr AJ, Sharma AP, Ross ML, et al. Chronic ketogenic low carbohydrate high fat diet has minimal effects on acid-base status in elite athletes. Nutrients 2018;10:236. 10.3390/nu10020236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gomez-Arbelaez D, Crujeiras AB, Castro AI, et al. Acid-base safety during the course of a very low-calorie-ketogenic diet. Endocrine 2017;58:81–90. 10.1007/s12020-017-1405-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pourafshar N, Pourafshar S, Soleimani M. Metabolic acidosis and progression of chronic kidney disease. Nephron 2018;138:222–8. 10.1159/000481892 [DOI] [PubMed] [Google Scholar]
- 81. Madias NE. Eubicarbonatemic hydrogen ion retention and CKD progression. Kidney Med 2021;3:596–606. 10.1016/j.xkme.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scialla JJ, Asplin J, Dobre M, et al. Higher net acid excretion is associated with a lower risk of kidney disease progression in patients with diabetes. Kidney Int 2017;91:204–15. 10.1016/j.kint.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brown L, Luciano A, Pendergast J, et al. Predictors of net acid excretion in the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis 2019;74:203–12. 10.1053/j.ajkd.2018.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rule AD, Bergstralh EJ, Melton LJ, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 2009;4:804–11. 10.2215/CJN.05811108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Acharya P, Acharya C, Thongprayoon C, et al. Incidence and characteristics of kidney stones in patients on ketogenic diet: a systematic review and meta-analysis. Diseases 2021;9:39. 10.3390/diseases9020039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Curhan GC. Epidemiology of stone disease. Urol Clin North Am 2007;34:287–93. 10.1016/j.ucl.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Furth SL, Casey JC, Pyzik PL, et al. Risk factors for urolithiasis in children on the ketogenic diet. Pediatr Nephrol 2000;15:125–8. 10.1007/s004670000443 [DOI] [PubMed] [Google Scholar]
- 88. Kossoff EH, Pyzik PL, Furth SL, et al. Kidney stones, carbonic anhydrase inhibitors, and the ketogenic diet. Epilepsia 2002;43:1168–71. 10.1046/j.1528-1157.2002.11302.x [DOI] [PubMed] [Google Scholar]
- 89. Herzberg GZ, Fivush BA, Kinsman SL, et al. Urolithiasis associated with the ketogenic diet. J Pediatr 1990;117:743–5. 10.1016/s0022-3476(05)83333-5 [DOI] [PubMed] [Google Scholar]
- 90. Sampath A, Kossoff EH, Furth SL, et al. Kidney stones and the ketogenic diet: risk factors and prevention. J Child Neurol 2007;22:375–8. 10.1177/0883073807301926 [DOI] [PubMed] [Google Scholar]
- 91. Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol 2006;48:978. 10.1017/S0012162206002143 [DOI] [PubMed] [Google Scholar]
- 92. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005;293:455. 10.1001/jama.293.4.455 [DOI] [PubMed] [Google Scholar]
- 93. Friedman AN, Ogden LG, Foster GD, et al. Comparative effects of low-carbohydrate high-protein versus low-fat diets on the kidney. Clin J Am Soc Nephrol 2012;7:1103–11. 10.2215/CJN.11741111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet. Ann Intern Med 2010;153:147–57. 10.7326/0003-4819-153-3-201008030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Volek JS, Gómez AL, Kraemer WJ. Fasting lipoprotein and postprandial triacylglycerol responses to a low-carbohydrate diet. J Am Coll Nutr 2000;19:383–91. 10.1080/07315724.2000.10718935 [DOI] [PubMed] [Google Scholar]
- 96. Phinney SD, Horton ES, Sims EAH, et al. Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric ketogenic diet. J Clin Invest 1980;66:1152–61. 10.1172/JCI109945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Phinney SD, Bistrian BR, Wolfe RR, et al. The human metabolic response to chronic ketosis. Metab Clin Exp 1983;32:757–68. 10.1016/0026-0495(83)90105-1 [DOI] [PubMed] [Google Scholar]
- 98. García-Nieto VM, Claverie-Martín F, Moraleda-Mesa T, et al. Gout associated with reduced renal excretion of uric acid. Nefrologia 2022;42:273–9. 10.1016/j.nefroe.2022.05.007 [DOI] [PubMed] [Google Scholar]
- 99. Goldfinger S, Klinenberg E, Seegmiller JE. Renal retention of uric acid induced by beta-hydroxybutyrate and acetoacetate. N Engl J Med 1965;272:351–5. 10.1056/NEJM196502182720705 [DOI] [PubMed] [Google Scholar]
- 100. Lecocq FR, McPhaul JJ. The effects of starvation, high fat diets, and ketone infusions on uric acid balance. Metabolism 1965;14:186–97. 10.1016/S0026-0495(65)80039-7 [DOI] [PubMed] [Google Scholar]
- 101. Ogryzlo MA. Hyperuricemia induced by high fat diets and starvation. Arthritis Rheum 1965;8:799–822. 10.1002/art.1780080443 [DOI] [PubMed] [Google Scholar]
- 102. Sapir DG, Owen OE. Renal conservation of ketone bodies during starvation. Metabolism 1975;24:23–33. 10.1016/0026-0495(75)90004-9 [DOI] [PubMed] [Google Scholar]
- 103. Agarwal N, Arkilo D, Farooq O, et al. Ketogenic diet: predictors of seizure control. SAGE Open Med 2017;5:2050312117712887. 10.1177/2050312117712887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McNally MA, Pyzik PL, Rubenstein JE, et al. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics 2009;124:e300–4. 10.1542/peds.2009-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet. Epilepsia 2009;50:304–17. 10.1111/j.1528-1167.2008.01765.x [DOI] [PubMed] [Google Scholar]
- 106. Cirillo M, Bilancio G, Cavallo P, et al. Reduced kidney function and relative hypocalciuria. J Clin Med 4133;9. 10.3390/jcm9124133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Simmons K, Nair H, Phadke M, et al. Risk factors for common kidney stones. Am J Nephrol 2023. [DOI] [PubMed] [Google Scholar]
- 108. Tangri N, Mathur V, Reaven NL, et al. Association of serum bicarbonate with the development of kidney stones in patients with chronic kidney disease: a retrospective cohort study. Clin Kidney J 2023;16:1113–21. 10.1093/ckj/sfad034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pazos Pérez F. Uric acid renal Lithiasis: new concepts. Contrib Nephrol 2018;192:116–24. 10.1159/000484286 [DOI] [PubMed] [Google Scholar]
- 110. Mulay SR, Evan A, Anders HJ. Molecular mechanisms of crystal-related kidney inflammation and injury. Nephrol Dial Transplant 2014;29:507–14. [DOI] [PubMed] [Google Scholar]
- 111. Haghighatdoost F, Sadeghian R, Clark CCT, et al. Higher dietary acid load and calcium oxalate kidney stones. J Ren Nutr 2021;31:467–74. [DOI] [PubMed] [Google Scholar]
- 112. Poplawski MM, Mastaitis JW, Isoda F, et al. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One 2011;6:e18604. 10.1371/journal.pone.0018604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tay J, Thompson CH, Luscombe-Marsh ND, et al. Effects of a very low carbohydrate diet on renal function in type 2 diabetes. Medicine (Baltimore) 2015;94:e2181. 10.1097/MD.0000000000002181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brinkworth GD, Buckley JD, Noakes M, et al. Renal function following long-term weight loss on a very-low-carbohydrate diet vs high-carbohydrate diet. Journal of the American Dietetic Association 2010;110:633–8. 10.1016/j.jada.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 115. Zainordin NA, Eddy Warman NA, Mohamad AF, et al. Safety and efficacy of very low carbohydrate diet in patients with diabetic kidney disease. PLoS ONE 2021;16:e0258507. 10.1371/journal.pone.0258507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tirosh A, Golan R, Harman-Boehm I, et al. Renal function following weight loss dietary strategies. Diabetes Care 2013;36:2225–32. 10.2337/dc12-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Facchini FS, Saylor KL. A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes 2003;52:1204–9. 10.2337/diabetes.52.5.1204 [DOI] [PubMed] [Google Scholar]
- 118. Friedman AN, Chambers M, Kamendulis LM, et al. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 2013;8:1892–8. 10.2215/CJN.04010413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bruci A, Tuccinardi D, Tozzi R, et al. Very low-calorie ketogenic diet: a safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients 2020;12:333. 10.3390/nu12020333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Unwin D, Unwin J, Crocombe D, et al. Renal function in patients following a low carbohydrate diet for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes 2021;28:469–79. [DOI] [PubMed] [Google Scholar]
- 121. Mitchell NS, Batch BC, Tyson CC. Changes in estimated glomerular filtration rate for patients prescribed a low Carb diet. Curr Opin Endocrinol Diabetes Obes 2021;28:480–7. 10.1097/MED.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 122. Wilmsen N, Pijl H, Geerlings W, et al. Effect of reverse diabetes2 now on kidney function in type 2 diabetes. BMJ Nutr Prev Health 2022;5:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Farhadnejad H, Asghari G, Emamat H, et al. Low-carbohydrate high-protein diet and chronic kidney diseases among Tehranian adults. J Ren Nutr 2019;29:343–9. [DOI] [PubMed] [Google Scholar]
- 124. Li Y, Zhang Y, Shen X, et al. The value of ketone bodies in the evaluation of kidney function in patients with type 2 diabetes mellitus. J Diabetes Res 2021;2021:5596125. 10.1155/2021/5596125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Athinarayanan SJ, Roberts CGP, Adams RN, et al. Two-year eGFR slope in people with type 2 diabetes on a very low carbohydrate diet. Diabetes 2023;72:410–P. 10.2337/db23-410-P [DOI] [Google Scholar]
- 126. Crosby L, Davis B, Joshi S, et al. Ketogenic diets and chronic disease: weighing the benefits against the risks. Front Nutr 2021;8:702802. 10.3389/fnut.2021.702802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Oyabu C, Hashimoto Y, Fukuda T, et al. Impact of low-carbohydrate diet on renal function: a meta-analysis. Br J Nutr 2016;116:632–8. 10.1017/S0007114516002178 [DOI] [PubMed] [Google Scholar]
- 128. Suyoto PST. Effect of low-carbohydrate diet on markers of renal function in type 2 diabetes: a meta-analysis. Diabetes Metabolism Res 2018;34:e3032. 10.1002/dmrr.3032 [DOI] [PubMed] [Google Scholar]
- 129. Couch GA. Comparison of low carbohydrate diets on renal and glucose function in type 2 diabetes: honors undergraduate theses. 691. 2020.
- 130. Bruen DM, Kingaard JJ, Munits M, et al. Ren.NU, a dietary program for individuals with autosomal-dominant polycystic kidney disease. Kidney Dialysis 2022;2:183–203. 10.3390/kidneydial2020020 [DOI] [Google Scholar]
- 131. Cukoski S, Lindemann CH, Arjune S, et al. Feasibility and impact of ketogenic dietary interventions in polycystic kidney disease. Cell Rep Med 2023;6:101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1 in polycystic kidney disease. Proc Natl Acad Sci U S A 2006;103:5466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nowak KL, Hopp K. Metabolic reprogramming in autosomal dominant polycystic kidney disease. CJASN 2020;15:577–84. 10.2215/CJN.13291019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Strubl S, Oehm S, Torres JA, et al. Ketogenic dietary interventions in autosomal dominant polycystic kidney disease. Clin Kidney J 2021;15:1079–92. 10.1093/ckj/sfab162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2024-004101supp001.pdf (71.3KB, pdf)
Data Availability Statement
No data are available.