Abstract
Objective. Commonly recommended drugs for adults and children include proton pump inhibitors (PPIs), proven effective for treating peptic diseases like stomach ulcers, GERD, and Helicobacter pylori infections in children over 1-year-old. Yet, prolonged PPI use carries higher risks of adverse reactions, prompting this study’s analysis. Methods. We have performed a systematic review of 30 articles, which include a total of 762 505 pediatric patients. Results. Adverse effects were encountered in 6.98% of the population. The 5 most common adverse effects were respiratory tract complications, gastrointestinal complications, urinary tract infections, asthma, and ENT infections. Conclusion. Hence, PPIs should be prescribed only when necessary, and physicians should prioritize patient education when considering their use.
Keywords: proton pump inhibitors, pediatric population, GI infections, secondary infections, adverse effects
Introduction
Proton pump inhibitors (PPI) are the most efficacious drugs for suppressing gastric acid secretion in the stomach’s parietal cells by inhibiting the H+/K+ ATPase.1,2 They have been proven useful in treating peptic disorders in children, such as gastric ulcers, gastroesophageal reflux disease (GERD), and Helicobacter pylori infections. 3 As of 2015, Rabeprazole, Lansoprazole, Pantoprazole, Omeprazole, Dexlansoprazole, and Esomeprazole are the PPIs that have obtained FDA approval. 2
Concerns have been expressed by gastroenterologists and FDA regulators about prolonged suppression of the proton pump in the pediatric age group due to the extended use of PPIs. 3 Tolia and Boyer reported the results of 32 to 47 months of PPI medication in 133 pediatric patients, with ages ranging from 0.1 to 17.6 years. Most patients received 2 daily doses of PPIs. During follow-up, hyperplasia of the parietal cells was noted in 0% to 16% of patients. Seventy-three percent of the children had increased levels of the hormone gastrin. Despite certain biochemical, histologic, and endoscopic alterations, long-term PPI medication seems to be effective, safe and well-tolerated in children. 4
Histamine H2 receptor antagonists (H2RAs) and PPIs are both commonly used to treat GERD, peptic ulcer disease, and dyspepsia. Although both PPIs and H2RAs act on parietal cells via a different mechanism of action, PPI acid suppression is more potent.4,5 PPIs (except rabeprazole) exhibit nonlinear pharmacokinetics in contrast to H2RAs, which follow linear pharmacokinetics in pediatric patients. 4
Long-term PPI use has been associated with an increased risk of community-acquired pneumonia, gastroenteritis, and Clostridium infection and causes headache, diarrhea, nausea, and rash in pediatric patients6,7 while H2RAs may lead to adverse effects like diarrhea, constipation, headache, and fatigue. 7
A 16-year (2000-2015) Danish register-based study, where the annual use of PPI in children (0 -17 years old) increased by 8-fold 8 and a study conducted in the United States reported a 7.5-fold rise in PPI use in infants. 9 Thus, over the past 3 decades, there has been a noticeable increase in the prescription of PPIs for children. Research indicates that PPIs are occasionally used for unsuitable causes and are overprescribed. A study conducted by Alosaily et al reported that the use of Omeprazole was deemed appropriate in only 38.6% of the population and there was an overuse of PPIs in the institution. 10 Proton pump inhibitors are regularly prescribed, sold over the counter, and frequently taken for longer periods than may be necessary from a therapeutic standpoint.
PPI use is accompanied by various adverse effects, as stated by numerous studies. In 1 such study by Cohen et al short-term side effects associated with PPI use were headache, nausea, and gastrointestinal symptoms 11 and PPI use was also found to be associated with C difficile infection, allergies like asthma, and autoimmune disease.12-14
Other adverse effects related to prolonged use of PPIs in adults and children are gastrointestinal diseases, infections, and hypomagnesemia15,16 but bone growth concerns and allergic symptoms are more common among children. 13
In a systematic review by van der Pol et al., headache was the most commonly reported treatment-related adverse effect of PPI use in children with GERD. Other adverse effects were diarrhea, abdominal pain, pharyngitis, and systemic infections. 17 However, there was not enough evidence to prove whether PPI in the pediatric population is safe or not. This review aims to assess the benefits and potential risks of PPIs for each patient individually and to encourage physicians to keep an eye out for side effects when administering a long-term PPI treatment.
Methods
The Systematic review was carried out according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The review was registered on PROSPERO (ID: CRD42023464370).
Data Sources and Search Strategy
A comprehensive search was conducted in PubMed, Scopus, and Web of Science databases without publication period restriction on September 3rd, 2023. The keywords used are summarized in Table 1. The search process was completed separately by 2 researchers. The studies’ significance level was further screened by appropriately evaluating the publications’ titles, abstracts, and full text. A total of 30 articles were included and reviewed.
Table 1.
Keywords Used for Searching Data Sources.
| Treatment terms | “Proton pump inhibitors” OR “Pantoprazole” OR “Lansoprazole” OR “Rabeprazole” OR “Omeprazole” OR “Dexlansoprazole” OR “Esomeprazole” |
| Population terms | “Paediatric population” OR “Children” OR “Child” OR “Kids” |
| Others | “Adverse effects” OR “Side effects” OR “Safety” OR “Complications” |
Eligibility Criteria
The studies were included or excluded as per the defined inclusion and exclusion criteria. The inclusion criteria followed were:
(1) The study was a randomized control trial, cohort studies, case-control studies, or other original research articles
(2) The study population constituted children between the age group of 0 and 18 years
(3) The study consists of the demographic details of the patients
(4) The study reports short- or long-term side effects associated with PPI use in children
The following exclusion criteria were considered:
(1) Non-original studies, including conference abstracts, review articles, protocols, case reports, animal studies, and editorials.
(2) Articles in a language other than English.
(3) Studies in which more than 1 intervention is given to the same individual
(4) Unavailability of full texts.
Study Selection
Revman software was used to organize the search results and remove duplicates. Eight authors independently screened 313 non-duplicated records and the conflicts were resolved after a discussion with DA, SSM, and AS.
Data Extraction
Required data were extracted by 8 authors of the research team as follows: first author name, year of the study, place of study, number of participants, mean age, gender, the disease being treated, drug used, route of administration, treatment duration, adverse effects, and immune cell changes. The results of the included articles are discussed in Table 2. The first author investigated the extracted data and settled any disagreements among the other authors.
Table 2.
Table Representing the Data Extracted From the Articles.
| First author | Year of study | Study design | Place of study | Participants | Mean age [Range] | Gender | Condition being treated | Drug | Route of administration | Treatment duration | Adverse effects (no of events) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tammara et al 18 | 2011 | Clinical trial | USA | 59 | N/A [1 month-6 years] | 64.28%B, 35.71% G | GERD | Pantoprazole (59) | Oral | 7 days (42) 28 days (17) | fever (6), diarrhea (8), gastroenteritis (3), rhinitis (3), GI infection (2), contact dermatitis (2), tooth disorder (2) vomiting (4), abdominal pain (2), nausea (2), increased cough (2), URTI (2). |
| Gutiérrez-Junquera et al 19 | 2018 | Prospective cohort study | Madrid | 57 | 11 [5.5-12.8] years | 73.7% B, 26.3% G | Eosinophilic esophagitis | Esomeprazole | N/A | 12 months | diarrhea (3), abdominal pain (1), urticaria (1), headache (1) |
| Duncan et al 20 | 2018 | Retrospective cohort study | USA | 293 | 8.8 months | 58.38% B, 41.62% G | Oropharyngeal dysphagia | lansoprazole, pantoprazole, omeprazole (149) | NA | 7.11 months | pneumonia, URTI, gastrointestinal infections, and sepsis |
| Gremse et al 21 | 2019 | Clinical trial | USA, Poland, Portugal, Mexico) | 62 | N/A [12-17 years] | 100% B | Esophagitis | Dexlansoprazole (62) | Oral | 36 weeks | headache (14), oropharyngeal pain (6), diarrhea (4), nasopharyngitis (7), abdominal pain (8), Pharyngitis (6), URTI (4), bronchitis (3), sinusitis (1), insomnia (3), erosive esophagitis (1) |
| Hassall et al 22 | 2006 | Cohort study | Canada | 166 | 8 [0.75-11.5] years | N/A | GERD, esophageal atresia | Omeprazole, lansoprazole. | NA | 3 years | nausea (2), diarrhea (1), skin rash (1), agitation (1), irritability (1), vomiting (1) |
| Gunasekaran et al 23 | 2002 | Clinical trail | USA | 63 | 14.1 [12-17] years | 50.8% B, 49.2% G | GERD | Lansoprazole (63) | Oral | 5 days | peripheral edema (1), maculopapular rash (1) urticaria (1), sensitive teeth (4), diarrhea (4), dizziness (4) |
| Haddad et al 24 | 2013 | Clinical trial | United States, Belgium, Denmark, France, Italy, Poland, Israel, South Africa, India | 127 | 5.7 [1-11] years | 56% B, 44% G | GERD | Rabeprazole (127) | Oral | 12 weeks | vomiting (18), (18), abdominal pain (15), diarrhea (14) |
| Haddad et al 25 | 2014 | Cohort study | United States, Belgium, Denmark, France, Italy, Poland, Israel, South Africa, India | 64 | 6 [1-12] years | 57.81% B, 42.19% G | GERD | Rabeprazole | N/A | 36 weeks | URTI (8), vomiting (7), abdominal pain (5), diarrhea (4), pyrexia (3), cough (2) |
| Kierkus et al 26 | 2006,2007 | Clinical trail | USA, Europe, Australia | 45 | N/A [1-11 months] | 53.3% B, 46.7% G | GERD | Pantoprazole | Oral | 5 days | anemia (4), constipation (3), vomiting (3), hypoxia (3), cough (4), rhinitis (4), fever (3), URTI (3), otitis media (3), infection (2), diarrhea (2), GERD (2), pharyngitis (2), contact dermatitis (2), eczema (2), rash (2) |
| Kuhn et al 27 | 2017 | Retrospective cohort study | Pennsylvania, USA | 526 | 9.9 years | 28.1% G, 71.9% B | N/A | Proton pump inhibitors (30) | N/A | N/A | Eosinophilic esophagitis (30) |
| James et al 28 | 2007 | Clinical trial | USA | 24 | 14.2 [12-16] years | 45.83% B, 54.17% G | GERD | Rabeprazole | Oral | 5-7 days | headache (4), nausea (2), asthma (1), fatigue (1), periorbital edema (1), diarrhea (1), dysmenorrhea (1), pharyngolaryngeal pain (1), proteinuria (1), polyuria (1). |
| Sandström et al 29 | 2012 | Clinical trial | USA | 59 | N/A [0-17 years] | 50.8% B, 49.2%G | GERD | Esomeprazole (59) | IV | 4 days | constipation (6), pyrexia (5), erythema (3), pruritus (3), rash (3), arthralgia (3), tachycardia (3) |
| Lassalle et al 30 | 2023 | Cohort study | France | 1 262 424 | N/A [1.8-6.2 years] | 53.4% B, 46.6% G | N/A | Proton pump inhibitors (606 645) | N/A | N/A | GI infections (9412), ENT infections (3700), LRTI (10 446), UTI (2798), skin infections (360), musculoskeletal system infections (203), nervous system infections (200), bacterial pathogen (3177), viral pathogen (14 598), traumatic Injuries (Excluding Fractures) (1106) |
| Gilger et al 31 | 2015 | Clinical trial | Belgium, France, Italy, USA | 109 | N/A [1-11 years] | 51.4% B, 48.6% G | GERD | Esomeprazole (108) | Oral | 53.4 days | diarrhea (3), headache (2), somnolence (2), flatulence (1), nausea (1), cough (1), asthenia (1), viral infection (1), arthralgia (1). |
| Kukulka et al 32 | 2011 | Clinical trial | USA | 36 | 14.6 [12-17] years | 38.9% B, 61.1% G | GERD | Dexlansoprazole (36) | Oral | 7 days | abdominal pain (4), vomiting (2), headache (1) |
| Fleishman et al 33 | 2020 | Retrospective cohort study | USA | 32 001 | 4 years [6 months-15.5 years] | 52.5% B, 47.5%G | N/A | Esomeprazole, lansoprazole, pantoprazole, omeprazole (431) | NA | 2 years | Fracture (431) |
| Zannikos et al 34 | 2011 | Clinical trial | US, Belgium | 28 | 6.7 [1-11] years | 57.1%B, 42.9%G | GERD | Rabeprazole (28) | oral | 5 day | vomiting (3), abdominal pain (3), diarrhea (2), hypergastrinemia (3), nausea (1), pancreatitis (1), regurgitation (1), toothache (1), volvulus (1), viral gastritis (1), streptococcus pharyngitis (2), URTI (1), cough (3), asthma (1), pyrexia (2), chills (1) |
| Turco et al 35 | 2005-2009 | Case control study | Italy | 68 | N/A [1.1-17.8 years] | 50.8% B, 49.2% G | Protracted diarrhea and abdominal pain | Proton pump inhibitors (19) | N/A | N/A | Clostridium difficle infection (19) |
| Righini Grunder et al 36 | 2017 | Cohort study | Montreal, Canada | 73 | 4.78 years [3.64-7.97] | 43.8% G, 56.2% B | Esophageal atresia | Proton pump inhibitors (73) | Oral | 2 weeks (43), Ongoing (30) | Regurgitation (49), eosinophilic esophagitis (15), pneumonia (15), bolus impaction (10) |
| Ward et al 37 | 2010 | Clinical trial | USA | 40 | 37.9 weeks | 75% B, 25% G | GERD | Pantoprazole | Oral | ≥5 days | constipation (2), anemia (2), hypoxia (2), rhinitis (2), and contact dermatitis (2). |
| Fiedorek et al 38 | 2005 | Clinical trail | United States | 87 | 14.1 [12-17] years | 39% B, 61% F | GERD | Lansoprazole (87) | Oral | 8 weeks | headache (20) abdominal pain (12), dizziness (3), asthenia (2), diarrhea (2), vomiting (2), nausea (3), anorexia (1) |
| Orenstein et al 39 | 2006-07 | Clinical trial | Poland, United States | 162 | 16 [4-49] weeks | 76.9%B, 23.07% G | GERD | Lansoprazole (81) | Oral | N/A | URTI (29), dermatitis (16), Eczema (16), constipation (12), GERD (12), ear infections (including otitis media) (15), Fever (15), Diarrhea (10), Rhinorrhea (10), LRTI (11), Candidiasis (8), Viral infection (8), Vomiting (6), Ileus (1), Dehydration (1), Epididymal infection (1), Arachnoid cyst (1), Cellulitis (1), Febrile convulsion (1), Klebsiella infection (1) |
| Omari et al 40 | 2007 | Clinical trial | Australia | 50 | N/A [1-24 months] | 62% B, 38% G | GERD | Esomeprazole (50) | Oral | 1 week | irritability (3), nasopharyngitis (3), vomiting (2), eczema (1), UTI (1), constipation (1), nasal congestion (1), rash (1), regurgitation (1) |
| Tolia et al 41 | 2002 | Clinical trial | USA | 66 | 7 [1-11] years | 61% B, 39% G | GERD, erosive esophagitis | Lansoprazole | N/A | 8-12 week | pharyngitis (15), gastroenteritis (8), headache (7), vomiting (7) |
| Tolia et al 42 | 2004, 2005 | Clinical trial | Belgium, France, Italy, and the United States | 109 | 5.7 [1-11] years | 51.3% B, 48.6% G | Erosive esophagitis | esomeprazole | oral | 8 weeks | diarrhea (3), headache (2), somnolence (2) |
| Winter et al 43 | 2010 | Clinical trial | United States,Canada, Europe, South Africa | 106 | 4.9 [1-11] months | 65.38% B, 34.61% G | GERD | Pantoprazole (52) | Oral | 4 weeks | URTI (7), rash (4), ↑CPK (3), otitis media (3), vomiting (3), diarrhea (2), cough (2), laryngitis 2 |
| Winter et al 44 | 2015 | Clinical trial | United States, France, Germany, Poland | 98 | 4.9 [1-11] months | 76.9% B, 23.07% G | GERD | Esomeprazole (39) | Oral | 2 weeks | URTI (6), pyrexia (5), rhinitis (4), diarrhea (4), nasopharyngitis (23) |
| Yanqin Li et al 45 | 2020 | Cohort study | China | 42 232 | 6.2 years [1 month-18 years] | 39% B, 61% G | All hospitalized pediatric patients | Proton pump inhibitors (11 496) | Oral | N/A | Hospital-acquired Acute Kidney Injury (HA-AKI) (962) |
| Wang et al 46 | 2021 | Cohort study | Sweden | 80 870 | 12.9 [1-17] years | 37% B, 63% G | N/A | Omeprazole (65 860), Esomeprazole (11 305), Pantoprazole (821), Lansoprazole (3219), Rabeprazole (6) | N/A | 3 years | Asthma (4428) [Omeprazole, 2854; Esomeprazole, 1250; Pantoprazole, 37; Lansoprazole, 305] |
| Wang et al 47 | 2022 | Cohort study | Sweden | 29 320 | 11.9 [7-17] years | 40.3% B, 59.7% G | N/A | Omeprazole (25 061), Esomeprazole (3328), Pantoprazole (209), Lansoprazole (865), Rabeprazole (0) | N/A | 1.6 years | Depression (273), Anxiety (432) [Esomeprazole, 97; Omeprazole, 843] |
Abbreviations: B, boys; G, girls; URTI, upper respiratory tract infection; LRTI, lower respiratory tract infection; UTI, urinary tract infection; CPK, creatinine phosphokinase.
Quality Assessment
Newland Ottawa Scale (NOS) for cohort and case-control studies was implemented to critically appraise the included studies. 48 The jaded scale was used for randomized control trials (RCT). The Risk of bias was assessed by 8 authors independently. The risk of bias analysis is made available in the Supplemental Material.
Statistical Analysis
All data were extracted onto a predesigned Excel sheet and represented in percentages, mean, and standard deviation for appropriate variables.
Results
Patient Characteristics
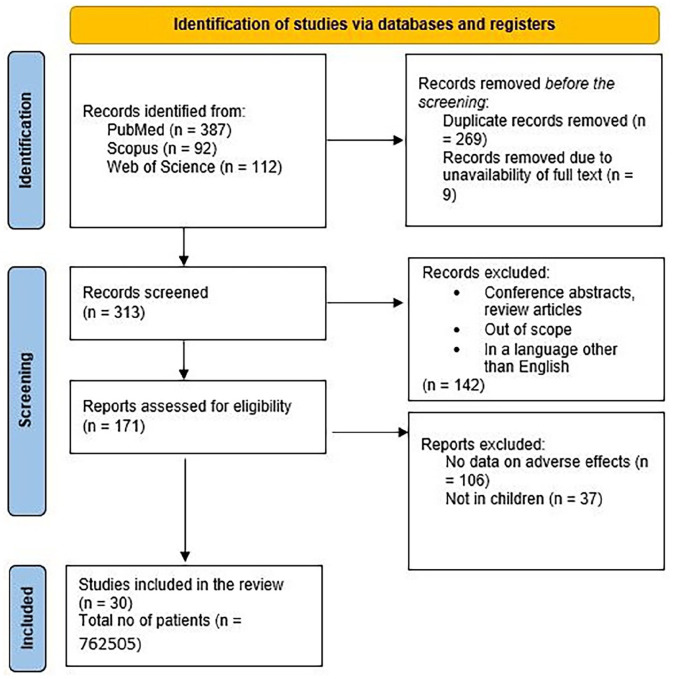
A total of 30 studies (18 clinical trials, 12 cohort studies) were included in the final analysis. All the included studies are hospital-based. Data from the included studies are presented in Table 2. The selection process of articles is shown in the PRISMA diagram (Figure 1).
Figure 1.
Search results from different databases.
A total of 762 505 patients were included in the review. Among the 30 studies, the number of males and females were 390 321 (51.2%) and 372 000 (48.8%), respectively. The mean age was 7.39 years (SD 4.69).
The drugs used in the studies were esomeprazole (n = 7, 20.5%), rabeprazole (n = 4, 11.7%), dexlamoprazole (n = 2, 5.8%), lansoprazole (n = 8, 23.5%), pantoprazole (n = 7, 20.5%), and omeprazole (n = 6, 17.6%). The most common route of administration was oral (n = 29), with one study using intravenous route of administration. The duration of administration of PPIs ranged from 4 days to 3 years. An increase in the duration of drug usage was associated with an increase in the occurrence of adverse effects.
Adverse Effects
A comprehensive analysis of 30 global studies has shown that children exposed to Proton Pump Inhibitors (PPIs) face an increased risk of adverse reactions. With 762 505 cases, a total of 53 309 side effects were reported, accounting for 6.99% of the total. Adverse effects reported in children <2 years of age accounted for 0.58% of the total side effects (n = 311).26,37,39,40,43,44 PPI usage has been associated with an elevated likelihood of secondary infections, hematological complications, and a spectrum of other adverse effects, encompassing bone fractures, psychiatric disorders, and asthma. Among these side effects, secondary infections were the most common.
Diarrhea was the most predominant GI side effect, impacting 67 patients18,19,22-26,28,31,34,38,39,42-44 (0.12% of total effects), followed by vomiting in 59 cases18,22,24-26,32,34,38,40,41,43 (0.11% of total effects), abdominal pain in 50 instances18,19,21,24,25,32,34,38 (0.09% of total effects), and less frequently, nausea in 11 cases18,22,28,31,34,38 (0.02% of total effects), and GERD (Gastroesophageal Reflux Disease) in 15 cases26,39 (0.03% of total effects). Other rare GI effects include hypergastrinemia, flatulence, regurgitation, and ileus.
Additionally, cutaneous side effects were observed in 53 patients. This category included skin rash in 12 cases22,23,26,29,40,43 (0.02% of total effects), urticaria in 2 cases19,23 (0.004% of total effects), eczema in 19 cases26,39,40 (0.03% of total effects), and dermatitis in 20 cases18,26,37,39 (0.037% of total effects).
Secondary Infections
Regarding secondary infections, among the 53 190 reported side effects, a substantial 84.6% (n = 44 997) were attributed to secondary infections, including bacterial, viral, and fungal infections.
Respiratory tract complications include18-21,23,25,26,30,34,36,37,39,40,43,44 (n = 10 583, 19.8%) URTI (Upper respiratory tract infections), LRTI (Lower respiratory tract infections), nasopharyngitis, bronchitis, rhinitis, pneumonia, and rhinorrhea. ENT infections (n = 3707, 6.95%), CNS infections (n = 200, 0.37%), skin infections (n = 361, 0.68%), and musculoskeletal infections (n = 203, 0.38%) were also reported. 30
Others
Studies identified fractures in 431 cases 30 (0.81%), as well as mental health problems such as anxiety (n = 432,0.81%) and depression (n = 273, 0.51%). 47 Additionally, 4429(8.33%) cases of asthma were reported among 80 870 children using PPIs.28,34,46 In more severe instances, 962 (1.8%) cases of acute kidney injury were noted among 11 496 children taking PPIs. 45 Furthermore, less common side effects in children on PPIs included headaches (n = 51, 0.09%), dizziness (n = 7, 0.01%), arthralgia (n = 4, 0.007%), irritability (n = 4, 0.0078%), tooth problems (n = 7, 0.0137%), and asthenia. Significant side effects associated with PPI use in the pediatric population are presented in Table 3.
Table 3.
Table Representing the Number of Events for Each Significant Side Effect Along With Their Proportion in Total Adverse Effects.
| Adverse effects | Number of events | Proportion % |
|---|---|---|
| Viral pathogen | 14 607 | 27.46% |
| Respiratory tract complications | 10 547 | 19.8% |
| Gastrointestinal complications | 9445 | 17.75% |
| Asthma | 4430 | 8.33% |
| ENT infection | 3721 | 6.99% |
| Bacterial pathogen | 3177 | 5.97% |
| Kidney or urinary tract infections | 2799 | 5.26% |
| Traumatic injuries | 1106 | 2.07% |
| Hospital acquired AKI | 962 | 1.8% |
| Anxiety | 433 | 0.81% |
| Fracture | 431 | 0.81% |
| Skin infections | 361 | 0.68% |
| Depression | 273 | 0.51% |
| Musculoskeletal infections | 203 | 0.38% |
| Nervous system infections | 200 | 0.37% |
| Diarrhea | 67 | 0.12% |
| Vomiting | 59 | 0.11% |
| Headache | 50 | 0.09% |
| Abdominal pain | 51 | 0.09% |
| Fever | 42 | 0.08% |
| Rash | 45 | 0.08% |
| Constipation | 24 | 0.05% |
| Eczema | 19 | 0.04% |
| Regurgitation | 16 | 0.03% |
| Cough | 14 | 0.03% |
| Nausea | 11 | 0.02% |
| Somnolence | 8 | 0.02% |
Side Effects Associated With Short and Long-Term Use
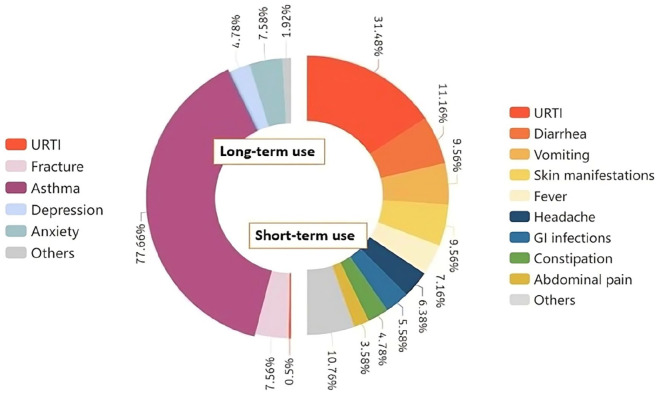
The common side effects reported in studies with short-term use of PPIs18,23,26,28,29,31,32,34,37,38,40-44 (<12 weeks) are upper respiratory tract infections (31.4%), diarrhea (11.16%), vomiting (9.56%), cutaneous manifestations (9.56%), fever (7.16%), headache (6.38%), GI infections (5.58%), constipation (4.78%), and abdominal pain (3.58%). Other short-term side effects are nausea, anemia, dysmenorrhea, arthralgia, tachycardia, GERD, anemia, and urinary tract infections. Asthma is the most common side effect reported in long-term use of PPIs (>12 weeks). Other side effects of long-term use are fractures (7.56%), anxiety (7.48%), and depression (4.78%). Some rare side effects like urticaria, insomnia, and erosive esophagitis were also found to be associated with long-term use.19-22,24,25,33,46,47 Various side effects associated with short and long-term use of PPIs are depicted in Figure 2.
Figure 2.
Pie chart depicting the adverse effects associated with short and long-term use of PPIs.
Abbreviation: URTI, upper respiratory tract infection.
Discussion
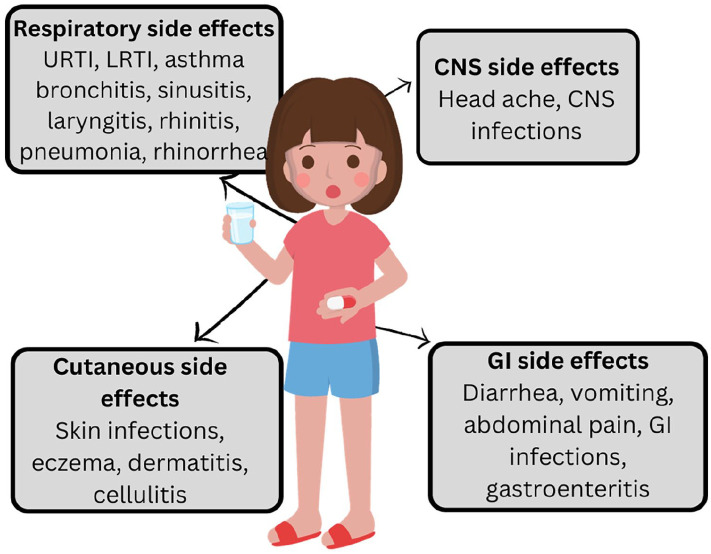
In the current systematic review on PPI usage in the pediatric population, 53 190 (6.98%) adverse effects were encountered out of 761 906 participants. The 5 most common adverse effects were respiratory tract complications, gastrointestinal complications, urinary tract infections, asthma, and ENT infection. Gastrointestinal, respiratory, skin, central nervous system, and musculoskeletal system infections, asthma, fractures, and psychiatric adverse effects were a few significant side effects of using PPI in the pediatric population, as depicted in Figure 3.
Figure 3.
Important side effects reported due to the use of PPIs in the pediatric population.
Proton pump inhibitors (PPIs) work by blocking the proton pump (H+/K+ ATPase) in the parietal cells of the stomach, which reduces acid generation in the stomach. Reduced stomach acid output may make it easier for ingested bacteria and other pathogens to survive and spread throughout the gut since stomach acid acts as a natural barrier against them. This increases the risk of acquiring GI infections. 49
There have been significant modifications in the GI microbiota linked to PPI use. Reduced stomach acidity may cause some bacteria to proliferate in the stomach and small intestine and may even alter the microbiological makeup of the colon. Studies have shown that, as compared to non-PPI users, oral and upper GI tract commensals are more abundant, while gut commensals are less abundant in PPI users. 50 In the case-control study of 19 children, the use of PPIs (proton pump inhibitors) was significantly higher in the Clostridioides difficile positive group compared to the negative group, with an odds ratio of 4.5 [95% confidence interval of 1.4-14.4] 35
This results in gastrointestinal infections, as reported in a few studies. In an RCT consisting of 42 participants, conducted by Tammara et al in 2011, pantoprazole use was associated with adverse effects of gastroenteritis (3, 8.33%) and GIT infections (2, 5.55%). 18 Similarly, in 2023, a cohort study conducted in France by Lassalle et al concluded that PPI use in infants causes adverse effects of digestive tract infection in about 9412 (1.5%) participants. 30
Overall, in the pediatric population, the most common organisms associated with PPI-related infections include Clostridioides difficile, which causes diarrhea and can lead to severe complications. Salmonella and Campylobacter are also the leading causes of bacterial gastroenteritis. Escherichia coli causes severe foodborne illnesses in children, leading to symptoms like diarrhea and abdominal pain. Some of the viruses, like rotavirus and norovirus, may also cause gastroenteritis in young children. 51
PPIs promote the colonization of bacteria like streptococcus and lactobacillus in the stomach by reducing gastric acid production which helps bacteria enter the lungs through invasion or aspiration, leading to respiratory tract infections. 52 This has been reported in a cohort study conducted in France in the year 2023 with total participants of 606 645, among which 10 446 (3.6%) lower respiratory tract infections have occurred as adverse effects of PPI. 30 A randomized control study for assessing the efficacy and safety of lansoprazole reported both upper respiratory tract infections (35%) and lower respiratory tract infections (13%) as adverse events following PPI use in children. 39 Similarly, Haddad et al while studying the efficacy and safety of rabeprazole maintenance therapy in children found upper respiratory tract infection as an adverse effect in 13% of the population 25 and among 54 adverse effects in a study by Tolia et al, the most common adverse effect was pharyngitis (23%). 41
Apart from Gastrointestinal and respiratory infections, various other systemic infections have also been reported in a few studies. In a cohort study conducted by Lassalle et al on 606 645 individuals, 360 (0.059%) individuals have shown skin infections, 203 (0.034%) cases of musculoskeletal infections, and, 200 (0.033%) cases of CNS infections were reported. 30 From this study, it is evident that the people who were on ongoing PPI therapy were at more risk than people without exposure. Among the past users, the median (IQR) interval between cessation of PPI use and the onset of major infections was 9.7 months. With increasing time elapsed since stopping PPI treatment (withdrawal since ≤3 months: aHR, 1.13; 95% CI, 1.10-1.16; withdrawal since >12 months: aHR, 1.03; 95% CI, 1.01-1.05), the risk of serious infections gradually decreased.
As discussed earlier, the use of proton pump inhibitors PPIs in children can result in the alteration of both intestinal and lung microbiomes by inhibiting gastric acid secretions. Dysbiosis, which refers to the disruption of the microbial balance resulting from the reduced diversity in specific microbiomes is known to provoke asthma flare. 46 This was reported in a cohort study among 80 870 participants, in which patients on PPI use had a higher incidence rate of asthma (21.8 events per 1000 person-years) compared with the non-initiators. Asthma risk was significantly increased across all age groups who initiated PPIs and was greatest among young children ≤6 months old (HR, 1.83; 95% CI, 1.65-2.03) and 6 months to <2 years old (HR, 1.91; 95% CI, 1.65-2.22; P < .001). 46 Similarly, in a randomized control study conducted by James et al, subjects with a mean age of 14.2 years were randomized to receive oral rabeprazole. The tolerability was assessed in terms of adverse events. 8.3% of the total cases had asthma as a treatment-emergent side effect. 28 To summarize, the PPI-exposed peers experienced a substantially greater rate of getting asthma (n = 4430, 8.33% of total adverse effects), in the current study.
This is also supported by a systemic review conducted by Robinson and Ruffner which reported that there is a relationship between the risk of allergic diseases in children, such as food allergies, asthma, and eosinophilic esophagitis, and PPI exposure during pregnancy and childhood. However, uncontrolled confounding is still a possibility, and further prospective research would be helpful to determine the exact extent of this effect. 53
A study by Wang et al reported that starting PPIs was associated with a 2.6-fold increased risk of depression and anxiety in children when compared to non-PPI users. The study cohort included 29 320 children who initiated PPI treatment and 29 320 matched children who did not. Among the study cohorts, 273 (0.93%) developed depression, and 432 (1.47%) developed anxiety. On the other hand, just 123 (0.42%) subjects developed depression and 168 (0.57%) developed anxiety among non-PPI users. PPI use was linked to both immediate and delayed risks of depression and anxiety. The amplitude of the connection was greater in younger age groups, increasing considerably with a longer duration of PPI usage, but was similar among individual PPIs. Furthermore, the risk was gradually reduced but remained considerable even 1 year after the PPIs were discontinued. 47
Although some researchers have suggested that PPI may cause decreased bone density and exacerbated osteoporosis, this impact is far from proven. Further confusion arises from the fact that elderly people seem to experience bone effects more readily, leading some to speculate that younger patients are better able to offset the effects of PPI on bone.
Examining a child’s fracture risk using PPI is complicated by the fact that the location and mechanism of pediatric fractures vary significantly from those of adult fractures. Because of this, it is essential to take fracture sites into account while researching children, since their presentation patterns may vary from those of adults. Malchodi et al in 2019 reported an elevated risk of fracture in early life in newborns exposed to PPI and histamine blockers, contrary to Freedberg et al in 2015 who showed no relationship between fracture risk and PPI use in children.54,55
PPI use in children has been recognized as an efficient intervention for Barrett’s esophagus, eosinophilic esophagitis (EoE), Zollinger-Ellison syndrome, Helicobacter pylori in conjunction with other medications, duodenal ulcers, gastroesophageal reflux disease (GERD), and non-steroidal anti-inflammatory-induced ulcer-related prophylaxis. 56 However, each year, the administration of PPIs increases in both Western and Eastern nations, leading to a potential risk of inappropriate utilization. The prevention of gastric duodenal ulcers in individuals without risk factors, stress ulcer prophylaxis in non-intensive care facilities, and overuse due to lack of awareness among patients are the main causes of PPI overuse. 57 Being offered over-the-counter increases the patient’s risk of long-term, unmonitored use. Prolonged use of PPIs causes various adverse effects in children, including, systemic infections, decreased bone density, hypersensitivity reactions, and increased risk of allergic diseases. 56 Mitigation of potential long-term PPI risk could be attempted by periodic evaluations of children on long-term PPI therapy, to make sure they are administered the lowest dosage necessary to control their illness. 55 Clinicians must evaluate carefully the situation in which they’re prescribing PPIs and whether or not they’re clinically indicated.
Limitations
The inference from our studies and the representativeness of our findings are compromised by the paucity of research on the use of proton pump inhibitors in the pediatric population. Owing to missing data, several original research articles were disqualified. The adverse effects associated with PPI use have been documented in very few articles. Even if the data from more comprehensive, well-designed research are not yet available, our analysis offers an up-to-date, exploratory summary of the available data.
Conclusion
PPIs are effective in treating both acute and chronic diseases, however, they are commonly overprescribed and freely accessible in many nations. Hence, proton pump inhibitors must be used judiciously in children after evaluating the benefits and adverse outcomes. Healthcare practitioners should take into account the possible risks associated with PPIs despite their evident benefits in the prevention and treatment of upper gastrointestinal symptoms in children. Thus, PPI must only be prescribed when indicated and physicians are encouraged to be mindful about educating patients while deciding whether to begin using PPIs. To corroborate these findings, further prospective studies in children with PPI therapy are essential.
Supplemental Material
Supplemental material, sj-docx-1-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health
Supplemental material, sj-docx-2-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health
Supplemental material, sj-docx-3-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health
Footnotes
Author Contributions: DA: Contributed to conception and design; Drafted the manuscript; critically revised the manuscript; Gave final approval; Agrees to be accountable for all aspects of work ensuring integrity and accuracy.
DJS: Contributed to analysis; Drafted the manuscript; Gave final approval.
MS: Contributed to analysis; Drafted the manuscript; Gave final approval.
RBS: Contributed to analysis; Drafted the manuscript; Gave final approval.
SSMA: Contributed to conception and design; critically revised the manuscript; Gave final approval: Agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MKM: Contributed to analysis; Drafted the manuscript; Gave final approval.
MS: Contributed to analysis; Drafted the manuscript; Gave final approval.
MSSV: Contributed to analysis; Drafted the manuscript; Gave final approval.
AS: Contributed to conception and design; Contributed to analysis; Drafted the manuscript; Gave final approval; Agrees to be accountable for all aspects of work ensuring integrity and accuracy.
SG: Contributed to analysis; Drafted the manuscript; Gave final approval.
SSR: Contributed to analysis; Drafted the manuscript; Gave final approval.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval and Informed Consent: Ethical approval or informed consent was not needed, as the current study is a review of published articles.
Registration and Protocol: Protocol was prepared. Registration is done on PROSPERO.
Provenance and Peer Review: Not commissioned, externally peer-reviewed.
Assistance With the Study: None
ORCID iDs: Deekshitha Alla  https://orcid.org/0000-0003-4234-6141
https://orcid.org/0000-0003-4234-6141
Dhruv Jayeshkumar Shah  https://orcid.org/0009-0008-6696-7704
https://orcid.org/0009-0008-6696-7704
Sai Santhosha Mrudula Alla  https://orcid.org/0000-0001-5700-6743
https://orcid.org/0000-0001-5700-6743
Mohan Sai Sunith Vegesna  https://orcid.org/0009-0009-2224-3525
https://orcid.org/0009-0009-2224-3525
Aradhya Singh  https://orcid.org/0009-0002-1936-6355
https://orcid.org/0009-0002-1936-6355
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Mössner J. The indications, applications, and risks of proton pump inhibitors. Dtsch Arztebl Int. 2016;113:477-483. doi: 10.3238/arztebl.2016.0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed A. Proton pump inhibitors (PPI). StatPearls - NCBI Bookshelf. Published May 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557385/ [Google Scholar]
- 3. Ward RM, Kearns GL. Proton pump inhibitors in pediatrics. Pediatr Drugs. 2013;15(2):119-131. doi: 10.1007/s40272-013-0012-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tolia V, Boyer K. Long-term proton pump inhibitor use in children: a retrospective review of safety. Dig Dis Sci. 2008;53(2):385-393. doi: 10.1007/s10620-007-9880-7 [DOI] [PubMed] [Google Scholar]
- 5. Tighe M, Afzal NA, Bevan A, et al. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database Syst Rev. 2014;2016(11):101723. doi: 10.1002/14651858.cd008550.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canani RB, Cirillo P, Roggero P, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117(5):e817-e820. doi: 10.1542/peds.2005-1655 [DOI] [PubMed] [Google Scholar]
- 7. Tighe MP, Afzal NA, Bevan A, Beattie RM. Current pharmacological management of gastro-esophageal reflux in children: an evidence-based systematic review. Pediatr Drugs. 2009;11(3):185-202. doi: 10.2165/00148581-200911030-00004 [DOI] [PubMed] [Google Scholar]
- 8. Aznar-Lou I, Reilev M, Lødrup AB, et al. Use of proton pump inhibitors among Danish children: a 16-year register-based nationwide study. Basic Clin Pharmacol Toxicol. 2019;124(6):704-710. doi: 10.1111/bcpt.13191 [DOI] [PubMed] [Google Scholar]
- 9. Barron JJ, Tan H, Spalding J, Bakst AW, Singer J. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421-427. doi: 10.1097/mpg.0b013e31812e0149 [DOI] [PubMed] [Google Scholar]
- 10. Alosaily YA, Alfallaj JM, Alabduljabbar JS, et al. Appropriateness of proton pump inhibitors use in noncritically ill hospitalized children in a tertiary hospital in Saudi Arabia. Saudi Pharm J. 2023;31(9):1157547. doi: 10.1016/j.jsps.2023.101723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. 2015;80(2):200-208. doi: 10.1111/bcp.12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anjewierden S, Han Z, Foster CB, Pant C, Deshpande A. Risk factors for Clostridium difficile infection in pediatric inpatients: a meta-analysis and systematic review. Infect Control Hosp Epidemiol. 2019;40(4):420-426. doi: 10.1017/ice.2019.23 [DOI] [PubMed] [Google Scholar]
- 13. Mitre E, Susi A, Kropp LE, et al. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018;172(6):e180315. doi: 10.1001/jamapediatrics.2018.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Räisänen L, Viljakainen H, Kolho KL. Exposure to proton pump inhibitors is associated with the development of pediatric autoimmune diseases. Front Pediatr. 2023;11:1157547. doi: 10.3389/fped.2023.1157547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Bruyne P, Ito S. Toxicity of long-term use of proton pump inhibitors in children. Arch Dis Child. 2018;103(1):78-82. doi: 10.1136/archdischild-2017-314026 [DOI] [PubMed] [Google Scholar]
- 16. Florentin M, Elisaf MS. Proton pump inhibitor-induced hypomagnesemia: a new challenge. World J Nephrol. 2012;1(6):151-154. doi: 10.5527/wjn.v1.i6.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Pol RJ, Smits MJ, van Wijk MP, et al. Efficacy of proton-pump inhibitors in children with gastroesophageal reflux disease: a systematic review. Pediatrics. 2011;127(5):925-935. doi: 10.1542/peds.2010-2719 [DOI] [PubMed] [Google Scholar]
- 18. Tammara BK, Sullivan JE, Adcock KG, et al. Randomized, open-label, multicentre pharmacokinetic studies of two dose levels of pantoprazole granules in infants and children aged 1 month through <6 years with gastro-oesophageal reflux disease. Clin Pharmacokinet. 2011;50(8):541-550. doi: 10.2165/11591900-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 19. Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. Long-term treatment with proton pump inhibitors is effective in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2018;67(2):210-216. doi: 10.1097/MPG.0000000000001952 [DOI] [PubMed] [Google Scholar]
- 20. Duncan DR, Mitchell PD, Larson K, McSweeney ME, Rosen RL. Association of proton pump inhibitors with hospitalization risk in children with oropharyngeal dysphagia. Otolaryngol Head Neck Surg. 2018;144(12):1116-1124. doi: 10.1001/jamaoto.2018.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gremse D, Gold BD, Pilmer B, et al. Dual delayed-release dexlansoprazole for healing and maintenance of healed erosive esophagitis: a safety study in adolescents. Dig Dis Sci. 2019;64(2):493-502. doi: 10.1007/s10620-018-5325-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassall E, Kerr W, El-Serag HB. Characteristics of children receiving proton pump inhibitors continuously for up to 11 years duration. J Pediatr. 2007;150(3):262-267, 267.e1. doi: 10.1016/j.jpeds.2006.08.078 [DOI] [PubMed] [Google Scholar]
- 23. Gunasekaran T, Gupta S, Gremse D, et al. Lansoprazole in Adolescents with Gastroesophageal Reflux Disease: Pharmacokinetics, Pharmacodynamics, Symptom Relief Efficacy, and Tolerability. J Pediatr Gastroenterol Nutr. 2002;35:S327-S335. https://journals.lww.com/jpgn/abstract/2002/11004/lansoprazole_in_adolescents_with_gastroesophageal.5.aspx [DOI] [PubMed] [Google Scholar]
- 24. Haddad I, Kierkus J, Tron E, et al. Efficacy and safety of rabeprazole in children (1-11 years) with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2013;57:798-807. [DOI] [PubMed] [Google Scholar]
- 25. Haddad I, Kierkus J, Tron E, et al. Maintenance of efficacy and safety of rabeprazole in children with endoscopically proven GERD. J Pediatr Gastroenterol Nutr. 2014;58(4):510-517. doi: 10.1097/MPG.0000000000000229 [DOI] [PubMed] [Google Scholar]
- 26. Kierkus J, Furmaga-Jablonska W, Sullivan JE, et al. Pharmacodynamics and safety of pantoprazole in neonates, preterm infants, and infants aged 1 through 11 months with a clinical diagnosis of gastroesophageal reflux disease. Dig Dis Sci. 2011;56(2):425-434. doi: 10.1007/s10620-010-1321-3 [DOI] [PubMed] [Google Scholar]
- 27. Kuhn BR, Young AJ, Justice AE, Chittoor G, Walton NA. Infant acid suppression use is associated with the development of eosinophilic esophagitis. Dis Esophagus. 2020;33(10):doaa073. doi: 10.1093/dote/doaa073 [DOI] [PubMed] [Google Scholar]
- 28. James L, Walson P, Lomax K, et al. Pharmacokinetics and tolerability of rabeprazole sodium in subjects aged 12 to 16 years with gastroesophageal reflux disease: an open-label, single- and multiple-dose study. Clin Ther. 2007;29(9):2082-2092. doi: 10.1016/j.clinthera.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 29. Sandström M, Davidson G, Tolia V, et al. Phase I, Multicenter, randomized, open-label study evaluating the pharmacokinetics and safety profile of repeated once-daily doses of intravenous esomeprazole in children 0 to 17 years of age. Clin Ther. 2012;34(8):1828-1838. doi: 10.1016/j.clinthera.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 30. Lassalle M, Zureik M, Dray-Spira R. Proton pump inhibitor use and risk of serious infections in young children. JAMA Pediatr. 2023;177(10):1028-1038. doi: 10.1001/jamapediatrics.2023.2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gilger M, Tolia V, Vandenplas Y, et al. Safety and tolerability of esomeprazole in children with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2015;60(Supplement 1):S16-S23. doi: 10.1097/mpg.0b013e318176b2cb [DOI] [PubMed] [Google Scholar]
- 32. Kukulka M, Wu J, Perez MC. Pharmacokinetics and safety of dexlansoprazole MR in adolescents with symptomatic GERD. J Pediatr Gastroenterol Nutr. 2012;54(1):41-47. doi: 10.1097/MPG.0b013e31822a323a [DOI] [PubMed] [Google Scholar]
- 33. Fleishman N, Richardson T, Attard T. The clinical characteristics of fractures in pediatric patients exposed to proton pump inhibitors. J Pediatr Gastroenterol Nutr. 2020;70(6):815-819. doi: 10.1097/MPG.0000000000002690 [DOI] [PubMed] [Google Scholar]
- 34. Zannikos PN, Doose DR, Leitz GJ, et al. Pharmacokinetics and tolerability of rabeprazole in children 1 to 11 years old with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2011;52(6):691-701. doi: 10.1097/MPG.0b013e318207834d [DOI] [PubMed] [Google Scholar]
- 35. Turco R, Martinelli M, Miele E, et al. Proton pump inhibitors as a risk factor for paediatric Clostridium difficile infection. Aliment Pharmacol Ther. 2010;31(7):754-759. doi: 10.1111/j.1365-2036.2009.04229.x [DOI] [PubMed] [Google Scholar]
- 36. Righini Grunder F, Petit LM, Ezri J, et al. Should proton pump inhibitors be systematically prescribed in patients with esophageal atresia after surgical repair? J Pediatr Gastroenterol Nutr. 2019;69(1):45-51. doi: 10.1097/MPG.0000000000002328 [DOI] [PubMed] [Google Scholar]
- 37. Ward RM, Tammara B, Sullivan SE, et al. Single-dose, multiple-dose, and population pharmacokinetics of pantoprazole in neonates and preterm infants with a clinical diagnosis of gastroesophageal reflux disease (GERD). Eur J Clin Pharmacol. 2010;66(6):555-561. doi: 10.1007/s00228-010-0811-8 [DOI] [PubMed] [Google Scholar]
- 38. Fiedorek S, Tolia V, Gold BD, et al. Efficacy and safety of lansoprazole in adolescents with symptomatic erosive and non-erosive gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2005;40(3):319-327. doi: 10.1097/01.mpg.0000155369.54464.41 [DOI] [PubMed] [Google Scholar]
- 39. Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. J Pediatr. 2009;154(4):514-520.e4. doi: 10.1016/j.jpeds.2008.09.054 [DOI] [PubMed] [Google Scholar]
- 40. Omari T, Davidson G, Bondarov P, et al. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2015;60(Suppl 1):S2-S8. doi: 10.1097/01.mpg.0000469415.50085.f7 [DOI] [PubMed] [Google Scholar]
- 41. Tolia V, 3rd, Fitzgerald J, Hassall E, et al. Safety of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2002;35(Suppl 4):S300-S307. doi: 10.1097/00005176-200211004-00002 [DOI] [PubMed] [Google Scholar]
- 42. Tolia V, Youssef NN, Gilger MA, Traxler B, Illueca M. Esomeprazole for the treatment of erosive esophagitis in children: an international, multicenter, randomized, parallel-group, double-blind (for dose) study. J Pediatr Gastroenterol Nutr. 2015;60(Supplement 1):S24-S30. doi: 10.1097/01.mpg.0000469419.29000.94 [DOI] [PubMed] [Google Scholar]
- 43. Winter H, Kum-Nji P, Mahomedy SH, et al. Efficacy and safety of pantoprazole delayed-release granules for oral suspension in a placebo-controlled treatment-withdrawal study in infants 1–11 months old with symptomatic GERD. J Pediatr Gastroenterol Nutr. 2010;50(6):609-618. doi: 10.1097/mpg.0b013e3181c2bf41 [DOI] [PubMed] [Google Scholar]
- 44. Winter H, Gunasekaran T, Tolia V, et al. Esomeprazole for the treatment of GERD in infants ages 1–11 months. J Pediatr Gastroenterol Nutr. 2015;60(Supplement 1):S9-S15. doi: 10.1097/mpg.0b013e3182496b35 [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Xiong M, Yang M, et al. Proton pump inhibitors and the risk of hospital-acquired acute kidney injury in children. Ann Transl Med. 2020;8(21):1438-1438. doi: 10.21037/atm-20-2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang YH, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Association between proton pump inhibitor use and risk of asthma in children. JAMA Pediatr. 2021;175(4):394-403. doi: 10.1001/jamapediatrics.2020.5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang YH, Wintzell V, Ludvigsson JF, Svanström H, Pasternak B. Proton pump inhibitor use and risk of depression and anxiety in children: nationwide cohort study. Clin Transl Sci. 2022;15(5):1112-1122. doi: 10.1111/cts.13225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Metaanalyses. Ottawa Hospital Research Institute; 2016. Accessed September 14, 2023. www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 49. Lambert AA, Lam JO, Paik JJ, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: A systematic review and meta-analysis. PLoS One. 2015;10(6):e0128004. doi: 10.1371/journal.pone.0128004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749-756. doi: 10.1136/gutjnl-2015-310861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rivera-Dominguez G. Pediatric Gastroenteritis. StatPearls - NCBI Bookshelf. Published April 3, 2023. https://www.ncbi.nlm.nih.gov/books/NBK499939/ [Google Scholar]
- 52. Vakil N. Acid inhibition and infections outside the gastrointestinal tract. Am J Gastroenterol. 2009;104(S2):S17-S20. doi: 10.1038/ajg.2009.47 [DOI] [PubMed] [Google Scholar]
- 53. Robinson LB, Ruffner MA. Proton pump inhibitors in allergy: benefits and risks. J Allergy Clin Immunol Pract. 2022;10(12):3117-3123. doi: 10.1016/j.jaip.2022.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Malchodi L, Wagner K, Susi A, Gorman G, Hisle-Gorman E. Early acid suppression therapy exposure and fracture in young children. Pediatrics. 2019;144(1):e20182625. doi: 10.1542/peds.2018-2625 [DOI] [PubMed] [Google Scholar]
- 55. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 56. Pasman EA, Ong B, Witmer CP, Nylund CM. Proton pump inhibitors in children: the good, the bad, and the ugly. Curr Allergy Asthma Rep. 2020;20(8):39. doi: 10.1007/s11882-020-00926-4 [DOI] [PubMed] [Google Scholar]
- 57. Savarino V, Marabotto E, Zentilin P, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11:1123-1134. doi: 10.1080/17512433.2018.1531703 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health
Supplemental material, sj-docx-2-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health
Supplemental material, sj-docx-3-gph-10.1177_2333794X241248967 for Safety of Proton Pump Inhibitors in Pediatric Population: A Systematic Review by Deekshitha Alla, Dhruv Jayeshkumar Shah, Muneesh Seepana, Rishabh Baskara Salian, Sai Santhosha Mrudula Alla, Midhun Krishna Mohanan, Mert Sabıroğlu, Mohan Sai Sunith Vegesna, Aradhya Singh, Srajan Gupta and Shushrutha Shivalingappa Rekha in Global Pediatric Health