Abstract
Factors relevant to the successful application of adeno-associated virus (AAV) vectors for liver-directed gene therapy were evaluated. Vectors with different promoters driving expression of human α-1-antitrypsin (α-1AT) were injected into the portal circulation of immunodeficient mice. α-1AT expression was stable but dependent on the promoter. Southern analysis of liver DNA revealed approximately 0.1 to 2.0 provirus copies/diploid genome in presumed head-to-tail concatamers. In situ hybridization and immunohistochemical analysis revealed expression in approximately 5% of hepatocytes clustered in the pericentral region. These results support the use of AAV as a vector for diseases treatable by targeting of hepatocytes.
The liver is an excellent target for in vivo gene therapy because hepatocytes are easily accessible to vectors injected into the circulation through large pores in liver capillaries (6). Nonviral gene delivery vehicles, based on liposomes and DNA-protein complexes, target hepatocytes in vivo, although gene transfer efficiency is low and expression is transient (20, 24). Recombinant adenoviruses target hepatocytes with higher efficiency but suffer from destructive cellular and blocking humoral immune responses (7, 11, 22).
Adeno-associated virus (AAV) has shown promise for in vivo gene therapy in a number of organs, such as skeletal muscle, central nervous system, and retina, where expression is efficient, stable, and associated with little inflammation or cellular immune response (1, 4, 5, 10, 12, 21). Results in the liver have been less consistent, with Snyder et al. providing the most impressive results by achieving sustained and therapeutic levels of factor IX in mice from an AAV vector utilizing a retroviral long terminal repeat (LTR) promoter (18).
The goal of this study was to further evaluate the potential of AAV as a vector for in vivo gene therapy of the liver. Studies were performed with human α-1-antitrypsin (α-1AT) as a reporter gene in murine models, because it allows quantitation of overall expression of the transgene through enzyme-linked immunosorbent assay (ELISA) of α-1AT in serum. Vectors in which α-1AT is driven from one of a number of promoters were constructed; they included (i) the 5′ flanking sequence and upstream enhancer sequences of the albumin gene (Alb), (ii) the 5′ flanking region of the immediate early gene of cytomegalovirus (CMV), (iii) the 5′ flanking region of the human phosphoglycerate kinase gene (PGK), (iv) the 5′ LTR of the Moloney murine leukemia virus, and (v) the chicken β-actin promoter fused to the enhancer region of the CMV immediate early gene (CB). Recombinant stocks of AAV for each vector were created by a transfection approach that does not require coinfection with adenovirus.
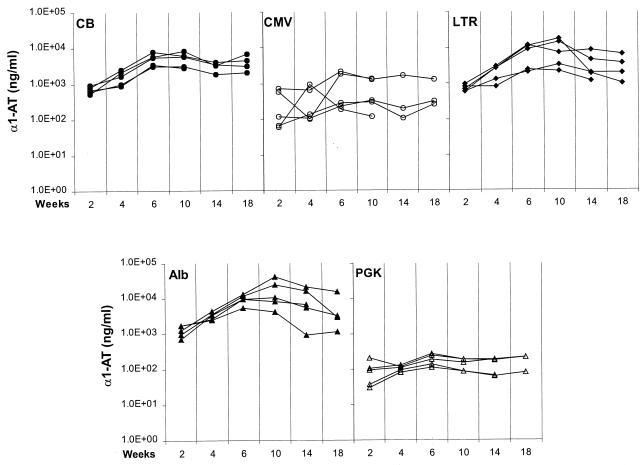
Hepatocytes were transduced in vivo following infusion of 1011 genome equivalents of AAV into the portal circulation of immunodeficient mice. Figure 1 shows the level of human α-1AT in serum of recipient mice for 18 weeks following gene transfer. The profile of expression with each vector was essentially the same, with the concentration of serum α-1AT increasing during the first 6 weeks and thereafter stabilizing for the duration of the experiment. Total production of α-1AT was consistent within each group but varied substantially between the different promoters. The highest expression was noted with the albumin and LTR constructs, with levels of α-1AT ranging from 5 to 50 μg/ml. The CMV enhanced β-actin promoter (CB) yielded intermediate levels of α-1AT (i.e., 1 to 10 μg/ml), whereas expression from the CMV and PGK promoters was substantially lower, at approximately 0.1 to 1 μg/ml. Additional experiments conducted with the albumin construct demonstrated a direct relationship between the dose of vector and transgene expression over a range of 3 × 108 to 1 × 1011 vector genomes (data not shown).
FIG. 1.
Levels of Human α-1AT in serum of mice after intraportal infusion of AAV vector. RAG-1 mice were injected with 1011 genomes of AAV vector, bearing different promoters, directly into the portal circulation via the spleen. Vector constructs contained the EcoRI fragment of pAT85 (from the American Type Culture Collection), spanning human α-1AT cDNA fused to the polyadenylation sequence from simian virus 40. Each panel presents serum α-1AT levels from multiple animals for vectors with different promoters measured up to 18 weeks after vector infusion. α-1AT was measured by an ELISA with α-1AT antibodies purchased from Sigma. The different promoters were as follows: CB, chicken β-actin promoter (−1 to +275) fused to enhancer sequences from the immediate early gene of CMV (23); CMV, promoter and enhancer of the immediate early gene of CMV spanning 795 bp of the 5′ flanking sequence; LTR, 2.3-kb Moloney murine leukemia virus LTR promoter isolated from pBR-MFG containing the LTR and intron (2); Alb, 2.4-kb murine albumin promoter from pAlbuPA2 (NotI-to-KpnI fragment) containing 300 bp of the 5′ flanking sequence and the enhancer region from −8.5 to −10.4 kb (16); PGK, 0.5-kb PGK promoter from PGK-neo (from M. McBurney and K. Jardine). Experiments were repeated with a number of different vector preparations (CB [n = 1], CMV, [n = 3], LTR [n = 1], Alb [n = 5], and PGK [n = 2]), all with identical results. Recombinant AAV based on AAV serotype 2 was produced by simultaneously transfecting three plasmids into 293 cells and purifying vector from the cell lysate by sequential cesium chloride sedimentation. The three plasmids were vector, pJWX500 (inverted terminal repeats deleted from wild-type AAV and a 500-bp stuffer inserted between p5 and rep), and PFΔ13 (the adenovirus [Ad] helper construct created by deleting the RsrII fragment from pFG140 [Microbix, Toronto, Canada]). These preparations contained varying levels of replication-competent AAV (rcAAV), ranging from 1/103 to 1/105 (rcAAV genomes/vector AAV genomes), and no detectable Ad based on PFU assay (<1 Ad PFU/1011 vector AAV genomes).
The impact of the promoter on the level of transgene expression, noted in our study, may explain some discrepancies in the literature. Two groups failed to demonstrate significant expression of lacZ in the liver with AAV vectors containing the CMV promoter (3, 17). The performance of the murine leukemia virus LTR is more complex. The high level of activity of the LTR demonstrated in our study is consistent with the data of Snyder et al., who achieved substantial levels of factor IX with a similar vector (18), but is discordant with the results of Koeberl et al., where expression of alkaline phosphatase and factor IX from the LTR was low (13).
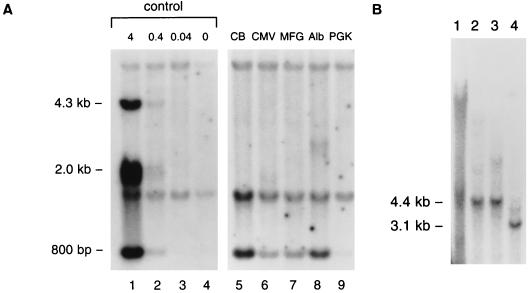
Additional studies were performed to evaluate the molecular state of the AAV genome in recipients. Livers were harvested from selected animals at 14 weeks after vector infusion and subjected to DNA hybridization analysis, the results of which are presented in Fig. 2. The total content of proviral DNA in liver was evaluated by digesting cellular DNA with enzymes that release an internal 800-bp fragment followed by DNA hybridization with human α-1AT used as a probe (Fig. 2A). Proviral DNA was detected in livers from each animal, ranging from approximately 0.1 to 2 proviral copies per diploid genome. Undigested DNA produced a smear without a discrete band (Fig. 2B, lane 1). DNA restricted with endonucleases that contain a single site within the provirus yielded discrete bands whose apparent molecular weights were equivalent to that of the provirus (Fig. 2B, lanes 2 and 3). A smaller band of equal intensity was observed when total cellular DNA was restricted with two endonucleases that together release a 3.1-kb internal fragment (Fig. 2B, lane 4). These data are most consistent with a model in which AAV exists as an integrated head-to-tail concatamer similar to what is seen with latent AAV infection (14, 15, 19) and muscle transduced with AAV vectors (4, 21).
FIG. 2.
Evaluation of the status of AAV vector genome in mouse liver by DNA hybridization analysis. RAG-1 mice were injected with 1011 vector genomes of AAV vectors containing different promoters. Livers were harvested at week 16 post-vector administration. DNA hybridization studies were performed with the whole cDNA of human α-1AT as a probe following the loading of 10 μg of restricted total cellular DNA per lane. (A) Lanes 1 to 4, control mouse genomic DNA spiked with 4, 0.4, 0.04, and 0 copies of human α-1AT plasmid digested with BamHI and EcoRV per diploid genome. This combination of enzymes releases an 800-bp fragment from the α-1AT cDNA. Lanes 5 to 9, genomic DNA from mice that received vectors with different promoters, as described in the legend to Fig. 1. DNA was digested with BamHI and EcoRV. The vector-specific fragment from DNA of transduced liver is 800 bp in length. The other two bands represent hybridization to endogenous mouse α-1AT sequence. (B) The liver genomic DNA from a mouse injected with vector expressing α-1AT from the Alb promoter was further analyzed. Lane 1, undigested genomic DNA; lane 2, genomic DNA digested with single-cut enzyme EcoRV; lane 3, genomic DNA digested with single-cut enzyme HindIII; lane 4, genomic DNA digested with both EcoRV and HindIII, which gives rise to a 3.1-kb internal fragment.
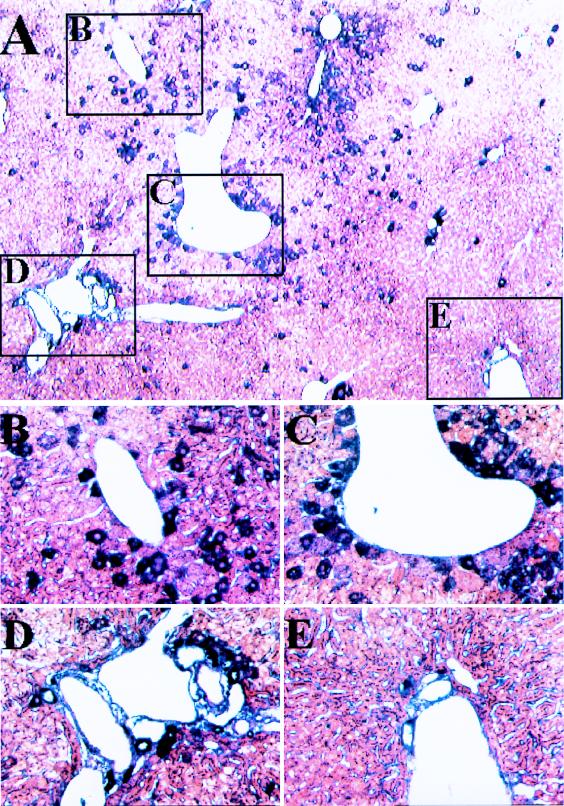
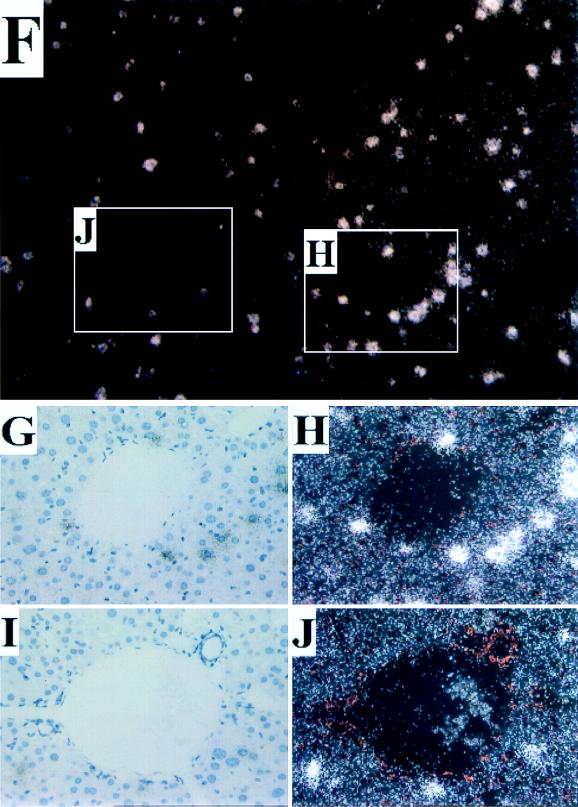
A number of histological analyses were performed to evaluate the frequency and distribution of AAV transduction following intraportal infusion. Immunohistochemical hybridization analysis with an antibody to human α-1AT probe revealed staining over ∼5% of hepatocytes throughout the liver (Fig. 3A to E) that was not found in mock-infected animals (data not shown). More transgene-expressing cells were clustered around central veins (Fig. 3B and C) than around portal triads (Fig. 3D and E). Similar frequency and distribution of transgene-expressing cells were found by in situ hybridization with a probe to human α-1AT (Fig. 3F to J). This does not agree with the findings of Snyder et al., who claimed expression of AAV-encoded tyrosine hydroxylase in cells around periportal regions (18). In our study, the pericentral localization of transduction was observed with each promoter, including the one used by Snyder et al., suggesting that other differences must play a role (e.g., method of delivery). The reasons for the observed gradient of transduction are unclear although not unexpected, considering that many genes demonstrate a gradient of expression along the portal to the central hepatic axis (9).
FIG. 3.
Localization of α-1AT expression in liver. The liver was harvested from a mouse 16 weeks after it received vector expressing α-1AT from the Alb promoter and was analyzed by histochemical staining for α-1AT protein by using rabbit anti-human α-1AT (Sigma) as the primary antibody and biotinylated goat anti-rabbit immunoglobulin (Sigma) and the Vector Labs alkaline phosphatase kit (Vector Laboratories, Burlingame, Calif.). Specific binding was visualized by the alkaline phosphatase colorimetric assay. (A) Low magnification (×40) illustrating representative pericentral (insets B and C) and periportal (insets D and E) regions. (B and C) High magnification (×200) of representative pericentral regions. (D and E) High magnification (×200) of periportal regions. The liver from a mouse that received the CB vector was harvested 18 weeks later, and paraffin sections were subjected to in situ hybridization with sense and antisense α-1AT probes. Sections were viewed under light and dark-field microscopy. Representative sections hybridizing to the antisense probe are shown. No signal was observed in sections hybridized with the sense probe. (F) Low magnification (×40) of a section of liver. Representative pericentral (G and H) and periportal (I and J) regions are demarcated. (H) High magnification (×200) of representative pericentral region. (J) High magnification (×200) of representative periportal region.
Data provided in this report and in the report of Snyder et al. (18) clearly support the use of AAV for targeting to hepatocytes genes that encode secreted proteins. What is the potential of AAV for treatment of metabolic diseases where the hepatocyte serves a catabolic or metabolic function, such as in familial hypercholesterolemia or urea cycle disorders? In situ hybridization and immunohistochemistry revealed transduction in 5% of hepatocytes following infusion of 1011 vector genomes of recombinant AAV. Increased catabolic/metabolic capacity would best be achieved if transgene expression was distributed throughout a greater number of hepatocytes. Transgene expression appears to be proportional to dose up to 1011 vector genomes per injection, suggesting that increased doses will lead to more transduced hepatocytes. Direct confirmation of this hypothesis will require improved methods of AAV production to allow injection of higher vector doses.
In summary, AAV appears to be a viable alternative to adenoviruses and nonviral vectors for in vivo gene therapy to the liver. The advantages of immune evasion that attend their use in muscle (8) need to be confirmed in the liver, although preliminary results in immunocompetent mice suggest that elimination of AAV-transduced hepatocytes by cytotoxic T lymphocytes will not be as much of a problem as has been observed with adenoviruses (data not shown). A better understanding of the mechanism and distribution of proviral integration is needed.
Acknowledgments
We acknowledge the Vector and Cell Morphology Cores of the Institute for Human Gene Therapy and the technical support of Marcia Houston-Leslie, Rosalind Barr, Melissa Casey, and Sarah Ehlen-Haecker.
This work was supported by grants from the NIH (P01 HD32649-04 and P30 DK47757-05 [to J. M. Wilson]) and by Genovo, Inc., a company J. M. Wilson founded and has equity in.
REFERENCES
- 1.Bennett J, Duan D, Engelhardt J, Maguire A M. Real-time non-invasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Investig Ophthalmol Vis Sci. 1997;38:2857–2863. [PubMed] [Google Scholar]
- 2.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher K J, Gao G-P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle-directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 5.Flannery J G, Zolotukhin S, Vaquero M I, LaVail M M, Muzyczka N, Hauswirth W W. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser R, Dobbs B R, Rogers G W T. Lipoproteins and the liver sieve: the role of fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;3:863–874. [PubMed] [Google Scholar]
- 7.Jaffe H A, Danel C, Longenecker G, Metzger M, Setoguchi Y, Rosenfeld M A, Gant T W, Thorgeirsson S S, Stratford-Perricaudet L D, Perricaudet M, Pavirani A, Lecocq J-P, Crystal R G. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992;1:372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- 8.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jungerman K. Zonation of metabolism and gene expression in liver. Histochemistry. 1995;103:81–91. doi: 10.1007/BF01454004. [DOI] [PubMed] [Google Scholar]
- 10.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 11.Kay M A, Graham F, Leland F, Woo S L C. Therapeutic serum concentrations of human alpha-1-antitrypsin after adenoviral-mediated gene transfer into mouse hepatocytes. Hepatology. 1995;21:815–819. [PubMed] [Google Scholar]
- 12.Kessler P D, Podsakoff G M, Chen X, McQuiston S A, Colosi P C, Matelis L A, Kurtzman G J, Byrne B J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koeberl D D, Alexander I E, Halbert C L, Russell D W, Miller A D. Persistent expression of human clotting factor IX from mouse liver after intravenous injection of adeno-associated virus vectors. Proc Natl Acad Sci USA. 1997;94:1426–1431. doi: 10.1073/pnas.94.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin S K, Collis P, Hermonat P L, Muzcyzka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988;62:1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzcyzka N. Use of adeno-associated virus as a general transduction vector in mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 16.Pinkert C A, Ornitz D M, Brinster R L, Palmiter R D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987;1:268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- 17.Ponnazahan S, Mukherjee P, Yoder M C, Wang X-S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1996;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 18.Snyder R O, Miao C H, Patijn G A, Spratt S K, Danos O, Nagy D, Brown A M, Winther B, Meuse L, Cohen L K, Thompson A R, Kay M A. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 19.Tratschin J-D, Miller I L, Smith M G, Carter B J. Adeno-associated virus vector for high-frequency integration, expression, and rescue of genes in mammalian cells. Mol Cell Biol. 1985;5:3251–3260. doi: 10.1128/mcb.5.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu G, Wu C H. Receptor-mediated gene delivery and expression in vivo. J Biol Chem. 1988;263:14621–14624. [PubMed] [Google Scholar]
- 21.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vectors. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Nunes F A, Berencsi K, Furth E E, Gönczöl E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye X, Robinson M B, Batshaw M L, Furth E E, Smith I, Wilson J M. Prolonged metabolic correction in adult ornithine transcarbamylase deficient mice with adenoviral vectors. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- 24.Zhu N, Liggett D, Liu Y, Debs R. Systemetric gene expression after intravenous DNA delivery into adult mice. Science. 1993;261:209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]