Abstract
The issue of poor solubility of active pharmaceutical ingredients (APIs) has been a salient area of investigation and novel drug delivery systems are being developed to improve the solubility of drugs, enhance their permeability and thereby their efficacy. Several techniques for solubilization enhancement of poorly soluble drugs are often employed at various stages of pharmaceutical drug product development. One such delivery system is the therapeutic deep eutectic system (THEDES), which showed great potential in the enhancement of solubility and permeability of drugs and ultimately augmenting their bioavailability. THEDES are made by mixing drugs with deep eutectic solvents (DESs) in a definite molar ratio by the hit and trial method. The DESs are a new class of green solvents which are non-toxic, cheap, easy to prepare, biodegradable and have multiple applications in the pharmaceutical industry. The terminologies such as ionic liquids (ILs), DES, THEDES, and therapeutic liquid eutectic systems (THELES) have been very much in use recently, and it is important to highlight the pharmaceutical applications of these unexplored reservoirs in drug solubilization enhancement, drug delivery routes, and in the management of various diseases. This review is aimed at discussing the components, formulation strategies, and routes of administration of THEDES that are used in developing the formulation. Also, the major pharmaceutical applications of THEDES in the treatment of various metabolic and non-metabolic diseases are reviewed.
Keywords: Deep eutectic solvents, Therapeutic deep eutectic system, Solubility, Permeability, Bioavailability, Ionic liquids
1. Introduction
Solubility of pharmaceutical drugs and phytochemicals is a major concern in research and development, and it imposes challenges during drug delivery, permeability, and ultimately their bioavailability. It is warranted to develop advanced novel technologies to overcome these challenges in a cost-effective manner for ease of commercialization and high therapeutic outcomes [1,2]. The development of proper drug delivery systems to enhance drug applicability is considered crucial in the pharmaceutical field. The conventional drug development stages require different strategies to promote its solubilization during formulation development. It often requires the use of organic solvents, which are considered a serious threat to the environment as they can generate toxic and unstable products [3,4]. Often used in various steps of drug development and in complex chemical processes, these traditional organic solvents carry the risk of toxicity and pose serious threats to the environment as well as to living systems [5]. The growing awareness of a healthy environment has gained momentum, and researchers now prefer non-toxic, environment-friendly solvents such as deep eutectic solvents (DESs) as non-aqueous liquid vehicles for better acceptability and ease of use in various drug delivery systems.
The DESs act as solvents for various APIs to increase their solubility and permeability and ultimately enhance their bioavailability upon delivery [6,7]. Eutectic systems are defined classically as mixtures of two or more substances that interact via hydrogen bonds to lower the melting temperature of the mixture than of the components alone. A THEDES is one in which the API is dissolved, or one in which at least one of the constituents is an API. Owing to their intrinsic tunability and ability to transport large molecules over the skin, ionic liquids (ILs) and DESs have been attracting much attention for the transdermal delivery of APIs [8,9]. Additionally, with the advent of green chemistry concepts in the pharmaceutical sciences, several green solvents emerged and garnered the attention of scientists worldwide owing to their low cost, non-toxic nature, and environment-friendly properties. These solvents find numerous applications in drug delivery, solubility enhancement, and as drug stability carriers [10].
There are several examples of conventional drug delivery methods that can be enhanced by incorporating THEDES. Intravenous (IV) infusion is a common method of drug delivery that involves the direct injection of drugs into the bloodstream [11]. It allows for rapid and precise delivery of drugs and is often used for critical care, chemotherapy, or fluid replacement therapy. THEDES formulations can be explored as an alternative carrier system for IV infusion, enhancing the stability, solubility, and bioavailability of drugs. Implantable drug delivery systems are devices that are surgically implanted in the body to deliver drugs over an extended time [12,13]. They can provide sustained release of drugs and are often used for conditions requiring long-term treatment, such as contraception or pain management. THEDES can be incorporated into implantable systems to improve drug release characteristics and enhance therapeutic efficacy. Intramuscular (IM) injections involve the delivery of drugs into the muscle tissue. This method allows for the slow and sustained release of drugs into the bloodstream, providing a prolonged therapeutic effect. THEDES can be utilized to develop injectable formulations that improve drug stability, solubility, and controlled release characteristics [14]. Subcutaneous (SC) injections involve the delivery of drugs into the subcutaneous tissue, just below the skin [6,9].
This method provides a slower absorption rate compared to intravenous injections and is commonly used for insulin delivery and the administration of certain biologics. THEDES can be explored as a potential vehicle for subcutaneous drug delivery, enhancing the solubility, stability, and prolonged release of drugs [15]. Ocular drug delivery involves the administration of drugs to the eye for the treatment of various ocular diseases. Conventional methods include eye drops, ointments, and intraocular injections. THEDES can be investigated as an innovative approach for ocular drug delivery, improving drug retention, penetration, and sustained release within the ocular tissues [[16], [17], [18]]. Targeted drug delivery aims to deliver drugs specifically to the intended site of action, minimizing systemic side effects. Conventional targeted delivery systems include liposomes, nanoparticles, and micelles. THEDES can be explored as a potential carrier system for targeted drug delivery, offering advantages such as improved stability, enhanced drug loading capacity, and prolonged release profiles [9,10].
Basically, THEDES are prepared by hit and trial method, which is time-consuming and expensive. Therefore, a mechanistic idea of THEDES formation, including the possible intermolecular interactions between API and the excipient is necessary for delivery systems [19]. Being a recent concept of drug delivery, THEDES needs a thorough understanding of their fundamental aspects and all other important details concerning THEDES and eutecticity, aiming for improved drug dissolution, enhanced drug solubility, and improved drug penetration using DES having unique properties such as high tunability and high thermal and chemical stability [20]. This review highlights the applications of THEDES and NADES as promising alternatives to toxic organic liquids to achieve enhanced drug solubility and improved drug bioavailability in various formulations. This review summarizes the key findings and implications of THEDES-based pharmaceutical applications, emphasizing their potential to revolutionize drug delivery and improve patient outcomes. It highlights the significance of continued research and innovation in harnessing the full potential of THEDES in pharmaceutical sciences. By focusing on these aspects, this review contributes significantly to the existing literature on THEDES and provides valuable insights into their pharmaceutical applications.
2. DEEP eutectic systems (DES)
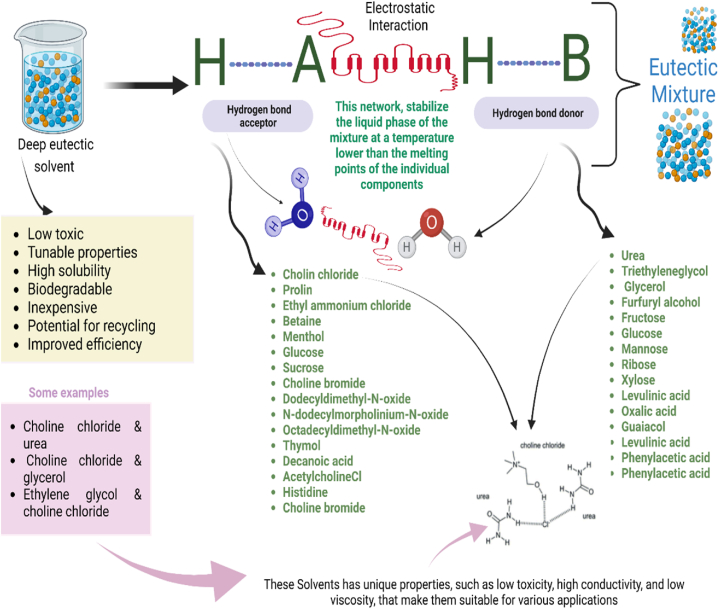
A DES is typically a mixture of two components, a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), which form strong intermolecular interactions, lowering the melting point of the mixture. A hydrogen bond is a type of intermolecular interaction that occurs when a hydrogen atom (H) is shared between two electronegative atoms, such as nitrogen, oxygen, or fluorine [21]. The hydrogen atom becomes partially positive charged, while the electronegative atom becomes partially negative charged, creating a dipole-dipole attraction. Hydrogen bonds are weaker bonds as compared to the covalent bonds; however, they can have a significant impact on the physical and chemical properties of a substance [22]. In a DES, the HBD and HBA components can form multiple hydrogen bonds with each other, creating a network of intermolecular interactions [23]. This network can stabilize the liquid phase of the mixture at a temperature lower than the melting points of the individual components. A common example of a DES is choline chloride-urea, which is made by combining choline chloride (HBD) and urea (HBA) in a 1:2 M ratio, forming strong hydrogen bonds.
Choline chloride is a quaternary ammonium salt that has a positively charged nitrogen atom, which can act as an HBD. Urea is a diamide that has two nitrogen atoms and a carbonyl group, which can act as HBA. When choline chloride and urea are mixed, the ammonium group of choline chloride forms hydrogen bonds with the carbonyl groups (C=O) of urea, creating a stable liquid phase at a temperature as low as 12 °C. The HBD/HBA concept can be applied to other types of DES as well. For example, a DES made from lactic acid (HBA) and choline chloride (HBD) could have a different ratio of components and different physical properties than choline chloride-urea. The HBD/HBA concept is a useful way to design and understand the properties of DESs, and it has implications in a wide range of fields, including chemistry, materials science, and biotechnology [19,20]. Fig. 1 depicts a pictorial representation of various components of THEDES with examples.
Fig. 1.
- DES and various compounds having HBA and HBD.
The term DES refers to a family of ILs that is typically made up of a mixture of different quaternary ammonium salts and HBDs such as alcohols, amides, and carboxylic acids. The charge delocalization that occurs during the creation of hydrogen bonds between the HBA and the HBD is what causes the constituents' melting points to fall. This can be regarded as one of the primary factors increasing DESs' solubility and absorption. Most APIs contain quaternary ammonium, amide, carboxylic acid, or alcohol polar functional groups. Although they can be utilized to form eutectic mixes, these tend to raise the melting points of the compounds. While this idea has been utilized previously, mixing quaternary ammonium salts with amides, carboxylic acids, and alcohols can lead to significant reductions in freezing points and the formation of so-called DES. These DESs quickly combine with water, which might improve API uptake [24]. Poised with remarkable properties of inherent tunability, biocompatibility, and good chemical and thermal stability, the DES has become a much-investigated topic of research in the pharmaceutical industry. The DESs are reported to be able to segregate bioactive compounds from natural sources as well as enhance the solubility and permeability of poorly soluble drugs, both of which are important for drug absorption and drug efficacy [25]. The ILs and DESs both possess unique properties of tunability and stability; however, DESs are now being considered as newer and cheaper alternatives to the expensive ILs. They can increase the drug's solubility and hence, its bioavailability inside the human body [26].
The ILs and DESs have potential pharmaceutical and biomedical applications such as in the solubilization of drugs, API delivery through complex biological barriers, protein and nucleic acid stabilization, and delivery of antimicrobial, antibacterial, and anticancer agents [27]. One of the promising IL/DES systems is choline geranate (CAGE), which is widely being used due to its biocompatibility and has garnered significant attention from researchers nowadays. CAGE has been used in the development of oral, transdermal, and subcutaneous drug delivery systems. It can solubilize both hydrophobic and hydrophilic drugs and can be used in the delivery of proteins and nucleic acids [28]. CAGE has numerous therapeutic applications as it facilitates drug delivery owing to its unique properties, making it an interesting therapeutic drug delivery system [29]. Numerous IL/DES and API/DES combinations and their applications in the pharmaceutical sciences are highlighted in Table 1.
Table 1.
- Various IL/DES and API/DES combinations and their applications in drug delivery.
| System | Molar Ratio | Application | Ref. |
|---|---|---|---|
| Choline-geranate (CAGE) | – | Oral, transdermal, and percutaneous delivery systems | [28,29] |
| Choline-geranate (CAGE), n-methyl-pyrrolidone, polyethylene glycol, and water | CAGE (1:2) | Self-emulsifying subcutaneous depot system for apomorphine | [30] |
| Choline chloride-mandelic acid | 1:2 | THEDES (Fast-dissolving delivery system) | [31] |
| Proline-glycerol | – | NADES (Cryoprotectant) | [32] |
| Ibuprofen-menthol | (10 and 20 wt %) | THEDES (Effective delivery) | [33] |
| Choline chloride and L-(+)-tartaric acid diethyl ester | 1:4 | Transdermal delivery of drugs | [34] |
| Choline chloride and urea Choline chloride and carboxylic acids Choline chloride and glycolic acid Choline chloride, glycolic acid, and oxalic acid |
ChCl:Gly acid (1:1 and 1:4) ChCl:Gly acid: Oxa acid (1:1.6:0.4) |
Solubilization of drugs | [35] |
| Choline chloride-geranate | – | Transdermal delivery of proteins in diabetics | [36] |
| Choline chloride-geranic acid | 1:2 | Transdermal delivery of ruxolitinib | [37] |
| Betaine – mandelic acid | 1:1 | Solubilization and permeability enhancement of natural products | [38] |
| Menthol – camphor | 1:1, 2:1, 3:1 and 4:1 | Enhanced percutaneous delivery of tacrolimus for atopic dermatitis | [39] |
3. Fabrication approaches for THEDES
3.1. Electrospinning technique
The electrospinning process is mainly used to fabricate nanostructures and core-shell structures by coaxial electrospinning. It is a multicomponent technique that can produce Janus fibers and three-layer fibers, depending on the electrospinning setup. Although the main application of this technique is in biopolymer manipulation, it has also been used in the production of thin gelatin fibers with smooth surfaces [31]. In lieu of developing a new drug delivery system, these gelatin fibers were used to encapsulate THEDES, which was prepared by mixing choline chloride and mandelic acid in a 1:2 M ratio. The THEDES was mixed with gelatin solution, and electrospun fibers were produced by the electrospinning technique and statistically optimized. Electrospun fast-dissolving gelatin fibers were found to be non-cytotoxic and showed a fast-dissolving release profile [31].
3.2. Self-emulsification technique
A deep eutectic-based system of a dopamine agonist, apomorphine, was formed by the self-emulsification technique. A homogenous mixture comprising choline-geranate, n-methyl-pyrrolidone, polyethylene glycol (PEG), and water was emulsified spontaneously into a microemulsion when administered via subcutaneous injection. Apomorphine was entrapped in the THEDES, thereby retarding its release and extending its pharmacokinetics. As a result, the three-injection per day dose of apomorphine could be reduced to every other day in patients with Parkinsonism [30].
3.3. Supercritical fluid technique
A controlled drug delivery system using the polymeric blends, starch: poly-ε-caprolactone (30:70) and menthol: ibuprofen-THEDES in different molar ratios was developed by employing the supercritical fluid technique at 20 MPa and 50 °C. Differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and micro-computed tomography (MCT) were used as characterization techniques in this study [33]. Additionally, in a recent study, THEDES of celecoxib (CEL) were produced by a novel supercritical CO2 technique for oral delivery and enhanced bioavailability. Different preparation methods, such as spray dying, evaporation crystallization, and supercritical fluid method were employed, and an enhanced dissolution profile was observed for supercritically processed CEL-THEDES [40]. The use of supercritical CO2 technique in combination with THEDES is a newer green approach to drug delivery systems. Recently, for the treatment of tuberculosis, 1-arginine-based THEDES were encapsulated in a lipidic matrix through supercritical CO2 technology by two different processes with different principles, i.e., Particle from Gas Saturated Solution (PGSS) and Rapid Expansion of Supercritical Solutions (RESS). The work involved a phase equilibrium study of THEDES and CO2 mixtures visually for the observation of physical state of isothermal mixture at certain compositions upon varying the pressure by mixture compression for transition of two-to-one phase corresponding to solubility measurements. No significant solubilization of THEDES in CO2 was observed, ruling out the possibility of using RESS method for encapsulation. However, phase equilibria studies allowed the selection of PGSS as a suitable technique for citric acid: l-arginine: H2O THEDES encapsulation in glycerol monostearate particles. Particles with mean size between 14 and 23 μm, an encapsulation efficiency of 75 %, and fast THEDES release, were obtained [40].
3.4. Nanoprecipitation technique
Lignin nanoparticles (LNPs) were prepared by dissolving lignin in DES with water as an anti-solvent through a self-assembling process through the nanoprecipitation technique. The lignin/DES nanotechnique was found to be simple and environment-friendly and resulted in the preparation of LNPs with sizes in the range of 20–200 nm. The prepared LNPs were characterized using various techniques, such as TEM, SEM, FTIR, and DLS. They also showed excellent dispersibility and prolonged stability [41].
3.5. Miscellaneous techniques
The production of single-walled carbon nanotubes (SWCNTs)-doping molecularly imprinted polymer nanocomposites-based systems with ILs and DES (choline chloride/ethylene glycol) for fenbufen delivery is reported as a controlled-release device [42]. In a recent study, a DES-based polymer monolith integrated with titanium dioxide nanotubes (TNTs) for specific protein recognition was reported to be an efficient material for the isolation of protein species [43].
4. Routes of THEDES delivery
THEDES can be administered to the patient via various routes of administration, such as oral, transdermal, intravenous, vaginal, and buccal routes. The potential of DESs and ILs to carry larger molecules over the skin has drawn the most attention of any delivery method, including THEDES-assisted transdermal delivery of medications. It has also been reported that the physicochemical properties of these DESs, such as viscosity and miscibility, can be fine-tuned and controlled through the selection of ions used in DES and their corresponding molar ratio.
4.1. Transdermal delivery
Transdermal drug delivery is a much safer and easier alternative to oral drug administration. But the biopharmaceutical issues of drugs, such as poor water solubility, make their inclusion into hydrophilic biopolymer systems very complicated for the formulator. Amongst various alternative formulation approaches, one approach is the formation of THEDES of that drug for transdermal drug delivery. Several ILs and DESs displayed promising results in enhancing the transdermal delivery of several small and large molecules. The most interesting of all are choline-based systems, such as the choline and geranic acid (CAGE) system being extensively used in THEDES transdermal delivery.
In lieu of this, THEDES of ibuprofen were prepared, and a solubility increase of up to 7917-fold was observed in DESs as compared to pure water. The formed THEDES were found to have enhanced solubility, improved permeability, were non-cytotoxic and no change in the anti-inflammatory activity of ibuprofen was observed [44]. The DESs are suitable solvents for anesthetic drug delivery in the liquid phase. An increase in the solubilization of prilocaine, procaine and bupivacaine in arginine-based DES was confirmed by quantum chemistry and classical molecular dynamics owing to the formation of hydrogen bonding [45]. Similarly, another study reported the incorporation of eutectic mixture of lidocaine and thymol into a microemulsion (ME) as an attractive and promising transdermal delivery for topical anesthetic therapy. Initially, a high dosage of lidocaine (10 %) was kneaded with thymol to form a lipophilic solution, and then it was readily incorporated into a ME using surfactants and co-surfactants. The THEDES in ME exhibited extended anesthetic activity and non-irritating effects on the skin [46]. Another interesting study revealed the use of skin needling combined with eutectic mixture of lidocaine and prilocaine (THEDES) to enhance the transdermal penetration of the local anesthetics. Improvised results in the level of pain sensation were observed in skin needling in conjunction with THEDES as compared to THEDES alone [47].
The DESs comprising the combination of ChCl and L-(+)-tartaric acid diethyl ester (L) in the molar ratio 1:4 were reported for transdermal delivery of diclofenac diethylamine. The formation of hydrogen bonds between the drug and the conformers was characterized using attenuated total reflectance-Fourier transform infrared (ATR-FTIR) and 1H nuclear magnetic resonance (1HNMR) spectroscopes. High solubility, percutaneous permeability, low cytotoxicity, and non-irritancy parameters were observed for this novel THEDES-TDDS [34]. THEDES of menthol and flurbiprofen were also reported previously, wherein the pure menthol is placed into silica hosts and sits as a fully amorphous substance within the pores of silica matrices. By incorporation into mesoporous silica hosts, this composite stabilizes the unstable amorphous menthol, which in turn regulates the drug's release profile [48].
A microemulsion-based THEDES of ionic liquid (imidazolium) and DES consisting of lidocaine and ibuprofen (oil phase) was recently prepared for the transdermal delivery of artemisinin. The prepared ME-THEDES showed a particle size of 41.95 ± 0.85 nm and a viscosity of 26.65 ± 0.13 mPa s, and in vitro transdermal assays using these particles through skin showed improved artemisinin transport. This enhancement in TDDS of artemisinin could be attributed to the disruption of the regular arrangement of keratin in the stratum corneum layer of skin. The findings from this study supported the fact that THEDES could be a promising vehicle for the transdermal delivery of other hydrophobic natural drugs [49]. The enhancement of skin permeation was also reported for the Chinese herb medicines in a DES-extract complex, which was prepared by co-heating the herb extracts with amino acids as the HBA and citric acid as the HBD. The DES extract incorporated into a hydrogel system proved to be a synergistic combination for the local treatment of rheumatoid arthritis [50]. The delivery of large biomolecules such as proteins, including bovine serum albumin (BSA), ovalbumin, and insulin, often imposes challenges due to their inherent low permeability across the skin. Their TDD was made easy using DESs, ChCl and geranate (CAGE). CAGE enhanced the permeation of these proteins into porcine skin ex vivo, making this delivery an alternative, noninvasive, and promising alternative to injectable insulin delivery in the treatment of diabetes [36].
In an innovative study on TDD of mesoporous silica nanoparticles (MSNPs), DES-MSNPs were prepared for noninvasive TDD using amino acid and citric acid as DESs for enhanced skin penetrability and sustained delivery of MSNPs [51]. Skin permeability of a model drug, ruxolitinib, was found to be enhanced by CAGE at a molar ratio of 1:2. As determined by 2D-NMR spectroscopy, mechanistic investigations revealed that the potency of ILs in the enhancement of TDD inversely correlates with the inter-ionic interactions [37]. Pharmaceutical DESs were prepared for imipramine HCl, catechol, and ascorbic acid for the controlled release of these pharmaceuticals using eutectic-modified gelatin. It was reported that the PDEs were able to increase the uptake of APIs, both oral and transdermal [52].
4.2. Oral delivery
The development of a novel amorphous solid dispersion based on NADES for the enhancement of anti-tumor RA-XII delivery, a unique natural cyclopeptide, by oral administration in rats was reported. It showed numerous advantages in oral cancer therapy being highly patient-compliant as it is non-invasive in usage. Ra-XII exhibited poor bioavailability in rats because of poor solubility and low permeability. A NADES comprising mandelic acid and betaine in a 1:1 M ratio was developed to deliver RA-XII efficiently by oral route. Approximately 17.54-fold increase in solubility and 10.35-fold improvement in permeability were observed for NADES solutions. The prepared NADES was converted to a solid dispersion formulation by the addition of a polymer (PVP K30). This strategy seemed to be novel and appropriate for improving the solubility, permeability, and ultimately the bioavailability of a natural product [38]. THEDES of celecoxib were prepared by the supercritical CO2 technique for oral delivery to enhance its bioavailability. The evaporation crystallization method, spray drying, and supercritical fluid techniques were the techniques employed. Enhanced dissolution was observed for samples processed with the supercritical technique as compared to others [40].
4.3. Buccal delivery
Buccal drug delivery is another non-invasive approach to administer therapeutics to the patients. Nonetheless, delivering proteins and peptides into the systemic circulation through the buccal route is still challenging. Chitosan was used as a mucoadhesive polymer, and DESs were used as transport facilitators to prepare a biodegradable polymeric patch containing insulin for its buccal delivery. The CAGE-insulin viscoelastic gel with two layers of biodegradable polymer sandwiched between them showed a 7-fold improvement in the total amount of insulin transported across the porcine buccal mucosa in an ex vivo setting [53].
4.4. Intravenous delivery
A DES-based formulation for intravenous delivery of verteporfin is reported, where the lipoidal DES comprising choline and oleate was used to enhance the solvation of verteporfin in stable nanocomplexes. The formulation showed improved cellular uptake of the drug as well as better retention, penetration, and tumor accumulation in vivo. This novel formulation could have great potential for intravenous delivery of chemotherapeutics [54].
4.5. Percutaneous delivery
A menthol/camphor-based eutectic system of tacrolimus for the treatment of atopic dermatitis aimed for percutaneous delivery was developed in a study. This promising nanoscale system enhanced the percutaneous delivery and treatment efficiency of the drug to effectively treat atopic dermatitis with reduced irritation [39].
4.6. Vaginal delivery
THEDES containing metronidazole has been prepared for the treatment of bacterial vaginosis by intravaginal application. A simple polycaprolactone (PCL) matrix was used to achieve an extended release of THEDES, and this was carried out using a bench scale twin-screw hot melt extruder. THEDES-PCL matrix system exhibited improved onset of action and a controlled release of drug over a period of 7 days, proving to be superior over oral therapy [55].
5. PHARMACEUTICAL applications of THEDES
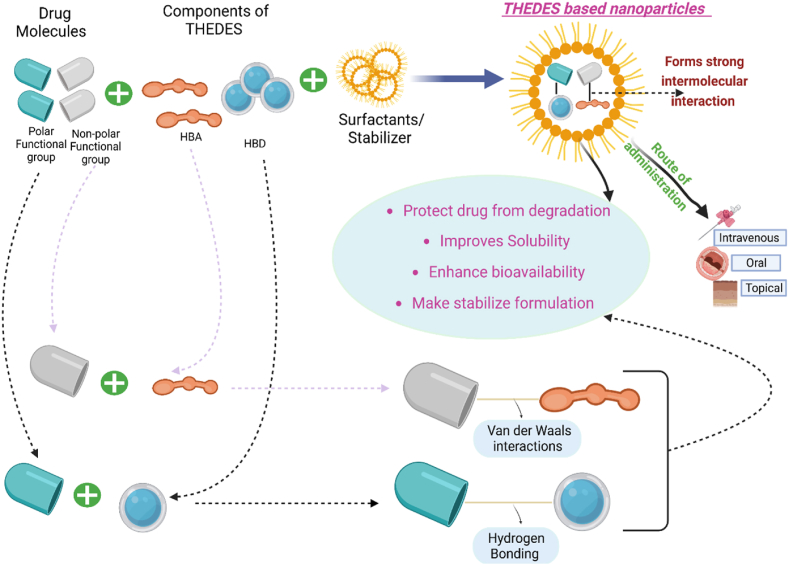
The development of novel drug delivery systems is an ever-evolving field where new ideas and concepts emerge to overcome various biopharmaceutical issues of existing and new drugs, such as solubility, permeability, and bioavailability. THEDES can be used as carriers for drug delivery by encapsulating drug molecules within nanoparticles made of THEDES. These nanoparticles can protect the drug molecules from degradation, improve their solubility, and enhance their bioavailability, making them more effective as pharmaceutical agents. The process of encapsulating drug molecules within THEDES-based nanoparticles typically involves mixing the drugs with components of THEDES (HBD and HBA), followed by the addition of a surfactant or stabilizer to form stable nanoparticles [14]. The resulting nanoparticles can then be administered to the patients through different routes of administration, such as oral, intravenous, and topical. Components in a deep eutectic solvent (THEDES) can improve the solubility of drugs that are poorly soluble by forming strong intermolecular interactions with the drug molecules. For instance, if a drug molecule has polar functional groups that can form hydrogen bonds, the HBD component of THEDES can form hydrogen bonds with these groups, stabilizing the drug molecule and preventing it from precipitating out of solution. Similarly, if a drug molecule has non-polar functional groups, the HBA component of a THEDES can form van der Waals interactions with these groups, solubilizing the drug molecule and preventing it from aggregating [56]. Various pharmaceutical applications of THEDES are summarized in Fig. 2.
Fig. 2.
- Various pharmaceutical applications of THEDES.
5.1. Dissolution enhancement of APIs
The DESs are reported to show high solvation capability for drugs based on strong solvent-solute intermolecular interactions. These highly tunable, non-toxic, and null-volatile solvents could be imagined as suitable solubilizing media and effective delivery vehicles for various APIs. This section summarizes the recent investigations performed on THEDES as solubilizing agents of various pharmaceutical drugs. The preparation of THEDES having menthol complexed with various APIs such as ibuprofen, benzoic acid, and phenylacetic acid is reported in the literature [57]. Eutectic mixture (EM) was prepared by the formation of H-bonds between menthol and the APIs, and these interactions were determined by NMR spectroscopy. The solubility and permeability of THEDES were compared with those of pure APIs. The solubility improvement up to 12-folds and increase in permeability up to 3-fold were observed with menthol-ibuprofen system in comparison to pure ibuprofen. This work provided enough evidence that by modifying the physical state of molecules from solid to liquid, THEDES had the efficiency to enhance the solubility, permeability, and ultimately the bioavailability of APIs [57]. Two distinct DESs based on choline chloride (HBA) and ascorbic acid, or polyethylene glycol (HBD), were used to improve the solubility and delivery of dapsone, a class IV drug. DSC, FT-IR, and NMR analyses were used to analyze the H-bond formation between the drug and DES components, indicating the development of eutectic systems between the two components [58].
Due to their low water solubility, sulfonamides were studied in a variety of natural deep eutectic solvents that included choline chloride and multi-hydroxyl-containing compounds to increase their solubility. When compared to water at 37 °C, the unimolar ratio of choline and glycerol exhibited maximum solubility up to 43 times. Quantum chemistry calculations based on the COSMO-RS procedure revealed that the interactions between the drug molecules sulfonamide and choline chloride were responsible for the increase in solubility [59]. THEDES were prepared with choline chloride (ChCl) or menthol in complexation with benzoic acid (BA), acetylsalicylic acid (AA), and phenylacetic acid (PA) in the following molar ratio: menthol: PA (2:1), menthol: PA (3:1), menthol: AA (3:1), menthol: BA (3:1), ChCl: AA (1:1), and ChCl: PA (1:1). THEDES were prepared and characterized extensively for structural features, thermal behavior, and dissolution rates. As compared to pure API alone, THEDES exhibited increased dissolution rates, highlighting the potential of THEDES as novel delivery systems [60].
A newly designed arginine-based THEDES for the solvation of lidocaine was also reported. The type and strength of intra- and inter-molecular bonding were examined in the intermolecular forces between lidocaine and the DES-HBDs and -HBAs [45]. In a study, curcumin was found to be dissolved most readily in NADES, made of glycerol and ChCl, which were identified as effective drug carriers. With the aid of quantum chemistry calculations, the reason for the increased solubility of curcumin in NADES in presence of intestinal fluids was attributed to direct inter-molecular interactions resulting in hetero-molecular pairings with glycerol and choline chloride [61]. The DESs, including ChCl (HBA) and urea or carboxylic acids (HBD), at their eutectic compositions were developed. Similarly, another DES based on glycolic acid and ChCl was reported, which was found effective in improving the solubility of numerous weakly basic, poorly water-soluble drugs in molar ratios of 1:1 to 1:4 at room temperature. It was able to enhance the solubility of itraconazole (6700-fold), piroxicam (430-fold), lidocaine (28-fold), and posaconazole (6400-fold) as compared to their aqueous solutions. Another novel ternary DES based on ChCl, glycolic acid, and oxalic acid at molar ratio 1:1.6:0.4 produced a striking and intriguing increase in the itraconazole solubility (53,600-fold) [35]. Another study aiming for the solubility improvement of tolbutamide and chlorpropamide by preparing THELES is reported. These two sulfonylurea compounds are poorly soluble in water. Drug-THELES were prepared by combining conformers in the molar ratios of 1:1 and 1:2, including l-tryptophan, tromethamine, citric acid, l(+)-arginine, malic acid, ascorbic acid, and β-aminobenzoic acid. An increase in the drug solubility without a decrease in their permeability was observed as compared to the pure APIs [62].
5.2. Maximizing drug delivery in disease treatment
Metabolic diseases are often associated with a high morbidity and mortality rate, making their treatment challenging for healthcare practitioners. Diseases including tuberculosis, cancer, diabetes mellitus, liver disorders, and atherosclerosis need effective and targeted delivery systems of their respective drugs to be treated properly. THEDES as a novel delivery system for APIs are easy to prepare, low production cost, high biodegradability, and high adjustability, making them a unique and interesting approach in the management of metabolic and non-metabolic disorders [63].
5.2.1. Tuberculosis
Tuberculosis disease generally requires a prolonged treatment schedule using various anti-tuberculosis drugs, which often leads to drug resistance issues. Successful THEDES-based delivery methods of anti-tuberculosis drugs have been reported in the literature. This could be a new breakthrough in the treatment of tuberculosis, which is one of the most socially burdened diseases [64]. A THEDES-based l-arginine and ethambutol system was prepared and characterized extensively by suitable techniques. Solubility studies revealed an improvement in the solubility of ethambutol upon incorporation into the THEDES system [65]. Similarly, using supercritical CO2 technology, the l-arginine-based THEDES was developed in a lipidic matrix for tuberculosis therapy, and the results revealed that the prepared lipid particles were non-cytotoxic in nature when studied using L929 fibroblasts [66].
5.2.2. Cancer
Cancer is a huge problem mankind is dealing with in this present time. It requires alternative therapies with fewer side effects and improved efficacy for its management and treatment. THEDES of anticancer drugs are unique, attractive, and portentous in terms of ease of preparation, stability profile, and cost-effectiveness. In support of this statement, a few studies from the literature were reviewed wherein anticancer drugs were delivered by incorporating them into THEDES. Temperature-responsive tunable entities with remarkable drug release potential were developed recently for doxorubicin using eutectic mixtures prepared from fatty acids (stearic and myristic acid) and liquid lipids such as oleic acid using hot melt encapsulation followed by sonication methods, which were further characterized for enhancement in permeability. The developed nanoformulation was found to be non-toxic as assessed using acute toxicity studies and hemolysis assays when compared to the free drug [67]. A new type of solid-lipid nanoparticle (SLN) of NADES and biotin-conjugated lysine-polyethylene glycol copolymer were prepared for paclitaxel (PTX) and 7-hydroxycoumarin (7-HC) and characterized for particle size, surface morphology, functional groups, and thermal stability. Both the drug-loaded novel SLN systems exhibited strong anticancer properties of drug molecules in cancer treatment [68].
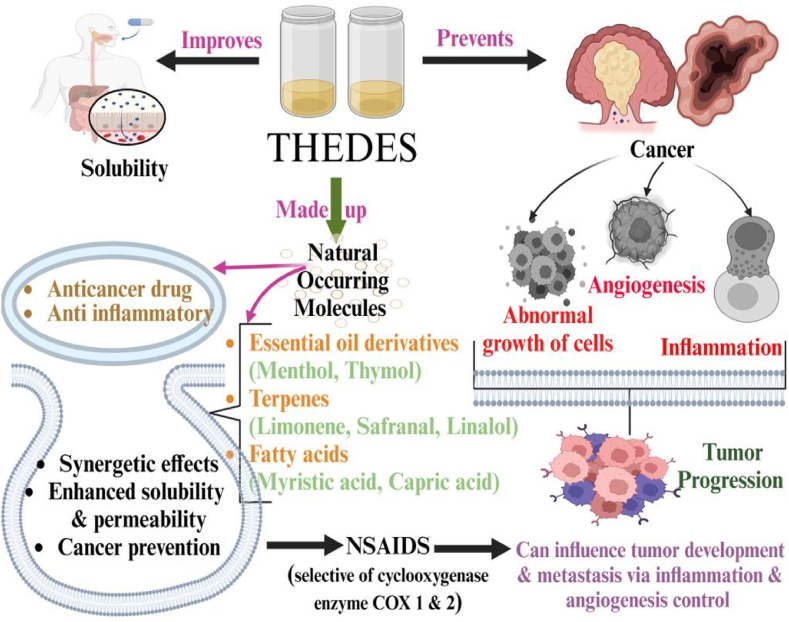
In another study aiming to treat cancer using THEDES, natural terpenes possessing anticancer properties, such as safranal, menthol, and linalool, were combined with non-steroidal anti-inflammatory drugs (NSAIDs), such as flurbiprofen, ibuprofen, and ketoprofen, as a promising treatment approach for cancer cells. The menthol-ibuprofen (3:1) combination was reported to improve the drug's solubility and bioavailability [69]. The THEDES of ibuprofen and perillyl alcohol were tested for their antimicrobial potential against various microorganisms, and the combination showed improved antimicrobial activities against most of the tested strains except P. aeruginosa. Also, the cytotoxicity of prepared THEDES was tested on in vitro colorectal cancer cell models, and it was found that the THEDES based on ibuprofen and perillyl alcohol could be a promising alternative to conventional therapies for colorectal cancer [70]. A THEDES-based on limonene (LIM), a portentous phytochemical compound reported to have anticancer potential, was also developed as a new possibility for cancer treatment. However, being non-selective towards cancer cells, it is considered as a highly toxic compound. The THEDES was prepared by gently mixing the saturated fatty acids and ibuprofen (IBU) or menthol with LIM to measure the anticancer potential of these systems. Various combinations, such as IBU: LIM (1:4), IBU: LIM (1:8), and menthol: LIM (1:1), were prepared and tested for antiproliferative properties against HT29 cell lines. The LIM: IBU (1:4) combination exhibited synergistic effects of DES, which can be useful in anticancer therapies [71]. Fig. 3 is a mechanistic representation of cancer prevention by THEDES.
Fig. 3.
Mechanistic representation of cancer prevention using THEDES.
5.2.3. Microbial infections
Benzalkonium chloride, a well-known antimicrobial agent with a variety of biomedical and pharmaceutical applications, was formulated in DES with acrylic acid and methacrylic acid. In this study, it was reported that an enhanced antimicrobial activity of benzalkonium chloride was observed for DES with acrylic acid towards S. aureus and C. albicans in an agar diffusion test. The DES also controlled and optimized the drug release profile, offering new insights into antimicrobial drug delivery [72]. A comparative study between two supersaturating drug delivery systems, eutectic mixture (EM) and amorphous solid dispersion, was performed recently to improve the release of griseofulvin (GSF) using different sweeteners such as saccharin (SAC), isomalt (ISO), and maltitol (MALT). Out of all formulations, the SAC-containing eutectic mixture (GSF-SAC-EM) showed maximum drug release in buffer solution as compared to the amorphous formulations using ISO and MALT sweeteners [73].
5.2.4. Amoebiasis
Recently, the effectiveness of malonic acid and salicylic acid-based DES on Acanthamoeba castellanii was also tested. Also in the cytotoxicity assays, minimal toxicity to human cells was observed, emphasizing the promising nature of these DES as effective therapy against A. castellanii [74].
5.2.5. Wound healing
Hydrophobic THEDES based on menthol and saturated fatty acids such as stearic acid, myristic acid, and lauric acid were reported recently for wound healing potential. The cytotoxicity evaluation of the menthol-stearic acid THEDES formulation was performed on HACaT cells, and it was found to be non-cytotoxic in nature with a remarkable wound healing potential [75]. Another study demonstrated the fabrication of green poly (vinyl alcohol) nanofibers using NADES for fast dissolution and delivery of acetylsalicylic acid (aspirin) and honey. The developed nanofibers were biocompatible and non-cytotoxic. Rapid and targeted drug delivery, enhanced solubility, and controlled drug concentration were the benefits of these fast-dissolving systems. The in vivo studies showed that the wound healing process was accelerated using PVA-DES-honey nanofibers and a remarkable improvement (85.2 %) in the wound healing rate on skin was observed after 6 days post-surgery. On the other hand, control (PVA) showed 68.2 %, whereas the PVA-DES nanofibers showed 76.3 % healing rate under similar conditions [76].
5.2.6. Hepatotoxicity
Silymarin-loaded eutectic mixtures with various ratios of poly-vinylpyrrolidone K30 were prepared and evaluated for their anti-inflammatory and hepatoprotective potentials. The solid dispersion of silymarin with PVP K30 was successfully formulated, which exhibited enhanced solubility (5-fold) in comparison to pure silymarin. Improved hepatoprotective and anti-inflammatory effects were observed for the developed formulation [77].
5.3. Drug delivery using natural deep eutectic systems (NADES)
NADES are a class of liquid mixtures formed by combining two or more naturally occurring components at a specific ratio, resulting in a eutectic point with unique physicochemical properties. Unlike conventional solvents, NADES are composed of natural compounds such as organic acids, amino acids, sugars, polyols, and their derivatives [16]. NADES are characterized by their ability to form a eutectic mixture, which exhibits a lower melting point compared to the individual components. This property arises due to the strong interactions between the components, such as hydrogen bonding and π-π stacking, which disrupt the crystal lattice and facilitate the formation of a liquid phase. One of the remarkable features of NADES is their versatility and tunability. By selecting different components and adjusting their ratios, the physicochemical properties of NADES, including viscosity, polarity, and melting point, can be tailored to meet specific application requirements [38]. This flexibility allows for the design of NADES with desired characteristics suitable for various applications, such as drug delivery, extraction processes, chemical synthesis, and biocatalysis.
NADES have gained significant interest in the field of drug delivery due to their biocompatibility, biodegradability, and ability to solubilize hydrophobic drugs or poorly water-soluble compounds. They can serve as alternative solvents or carriers for drug formulation, offering advantages such as improved drug solubility, enhanced bioavailability, and controlled release profiles. Moreover, NADES have shown cryoprotective properties, making them useful in preserving biological materials during freezing and thawing processes. Another advantage of NADES is their natural origin, which aligns with the growing demand for sustainable and environmentally friendly approaches in various industries. NADES can be derived from renewable resources, reducing reliance on synthetic solvents, and minimizing environmental impact. Cryopreservation is a preservation technique, and two synthetic cryoprotectants, dimethyl sulfoxide (DMSO) and glycerol, are commonly used for the process. Being toxic in nature, their use in clinical applications is limited.
NADES also act as natural cryoprotective agents (CPAs) in a way similar to the antifreeze proteins and sugars by protecting cell physiology in the cryopreservation process [78]. Recently, NADES were tested as cryoprotectants in two mammalian cell lines, L929 and HacaT cells, using the vitrification method, and they showed great potential as CPAs [79,80]. Also, in another study, a DES made from proline and glycerol emerged as an effective cryoprotectant that was found to be less toxic than its individual components [32]. NADES represent a promising platform for diverse applications, including drug delivery, biocatalysis, and extraction processes. Their unique properties, tunability, and natural origin make them an attractive alternative to conventional solvents, opening new avenues for innovative and sustainable solutions in various fields [[81], [82], [83], [84], [85]].
5.4. Fortifying bioactive compounds
The application of THEDES offers valuable opportunities for stabilizing bioactive compounds in various contexts where the preservation and efficacy of bioactive compounds are essential. THEDES demonstrate remarkable potential in protecting and enhancing the stability of sensitive bioactive molecules, including drugs, natural extracts, enzymes, and proteins. This stabilization capability has significant implications for improving the efficacy, shelf life, and delivery of bioactive compounds. One of the key areas where THEDES excels is drug stabilization. By encapsulating drugs within THEDES formulations, these systems can shield the drug molecules from degradation factors such as light, heat, oxygen, and pH changes. This protective environment ensures the maintenance of drug potency and efficacy over an extended period, contributing to enhanced therapeutic outcomes. Furthermore, THEDES can be instrumental in the preservation of natural extracts, which often contain delicate bioactive compounds prone to degradation. Incorporating THEDES as solvents or carriers for natural extracts safeguards the active constituents, prevents oxidation, and extends the shelf life of these extracts. This stabilization effect ensures the integrity and efficacy of the extracts for various applications, such as in the development of nutraceuticals or cosmeceuticals.
In addition to drug stabilization and natural extract preservation, THEDES also play a significant role in maintaining the stability of enzymes and proteins. These sensitive biomolecules require proper stabilization to retain their structure and activity. THEDES provide a favorable environment that protects enzymes and proteins, preventing denaturation and maintaining their stability during storage or application. This stabilization effect is particularly valuable in enzymatic reactions, biocatalysis, or the development of enzyme-based formulations. THEDES can also be integrated into controlled release systems, such as nanoparticles or hydrogels, to stabilize and control the release of bioactive compounds. The eutectic environment provided by THEDES protects the loaded compounds, preventing premature release or degradation. This controlled release system ensures a sustained and controlled release profile, enhancing the stability and therapeutic efficacy of the bioactive compounds.
In the realm of biopharmaceutical formulations, THEDES demonstrate their potential by stabilizing bioactive molecules, such as protein-based therapeutics, vaccines, or gene delivery systems. By ensuring the stability of these molecules, THEDES contributes to the integrity of biopharmaceutical formulations during storage and delivery, enhancing their effectiveness and therapeutic outcomes [[86], [87], [88], [89]]. Bioactive compounds have potential full biological activities and are therefore being used in alternative therapies for ages. They are widely used in the food, pharmaceutical, and cosmetic industries. Their extraction from natural sources is a very crucial step in the research and development stages. NADES are thus considered appropriate to extract these bioactive compounds owing to their high stabilizing abilities, low toxicity, biodegradability, and low environmental impact. Relevant studies conducted on NADES as extracting solvents for various bioactive compounds were reviewed. In one such study, NADES-curcumin with choline chloride and glycerol was prepared and utilized as an extraction tool to obtain the curcuminoids from their natural sources. It also served as an efficient stabilizer, which could prevent the degradation of curcumin from sunlight and offered beneficial properties [60].
The preparation of eutectic-based liposome of paeonol and lauric acid in a molar ratio of 1:1 was reported, and the developed THEDES exhibited enhanced water solubility in comparison to the pure APIs. The obtained liposomes were 120 nm in size, with an encapsulation efficiency of 84 %, proving to be a better strategy for delivering natural bioactives [90]. Similarly, carnosol, rosmarinic acid, carmosic acid, and caffeic acid were extracted from rosemary by the utilization of NADES using heat, stirring, and ultrasound-assisted extraction techniques. The extraction of carnosic acid and carnosol was achieved using menthol and lauric acid in a molar ratio of 2:1, whereas lactic acid and glucose in a molar ratio of 5:1 resulted in the extraction of rosmarinic acid. This work disclosed that employing two different systems simultaneously could assist in the extraction of different compounds using a single-step procedure under similar experimental conditions. The extracted bioactives were stable in NADES for a prolonged time, as quantified by HPLC at different time points [91]. In conclusion, the application of THEDES enables the stabilization of bioactive compounds, safeguarding them from degradation and enhancing their stability. This capability finds relevance in various fields, including drug development, natural extract preservation, enzyme stability, controlled release systems, and biopharmaceutical formulations. Harnessing the stabilization potential of THEDES contributes to improved efficacy, extended shelf life, and enhanced therapeutic outcomes in diverse biomedical applications.
5.5. When to consider THEDES technology and why?
While encountering the solubility issues of a poorly soluble drug during the formulation development stage, many modifications are carried out by the researcher to increase their oral bioavailability, such as the use of different routes of drug administration, use of new dosage forms, modifications in the drug itself, such as salts, esters, and prodrugs, or required modification in the pH of the medium. Other than these basic approaches, some more sophisticated techniques, such as the development of lipid nanoparticles (SLNs, NLCs), polymeric micelles, nanosuspension, and size reduction techniques, are also considered for the problematic drug. In lieu of this, a new and emerging trend in this discourse of increasing the solubility of a poorly soluble drug is the use of DES. With the use of DES, the issue of low solubility of certain APIs in aqueous media can be resolved [92].
THEDES can also be utilized as a platform for developing novel drug delivery systems, including nanocarriers and hydrogels, owing to their ability to solubilize a wide range of compounds and their compatibility with biological systems. Today, the growing global awareness of sustainability and its impact on the environment we live in drives us towards the use of eco-friendly solvents such as DES for our research purposes rather than toxic traditional organic solvents. DES are considered as non-toxic, safe, and green new-age solvents possessing a myriad of pharmaceutical applications [93]. Undoubtedly, novel drug delivery systems such as liposomes, hydrogels, and lipid nanoparticles are extensively being used to improve the solubility of poorly water soluble drugs, however there are unique advantages associated with THEDES, such as the ease of preparation, low toxicity profile, low cost of production, high stability, fine tunability, and biodegradability for the treatment of various metabolic diseases such as cancer and diabetes.
The DES constituted using xylitol, citric acid, sorbitol, and glucose (HBD) and choline chloride as HBA were prepared, and the solubility of APIs such as caffeine and furosemide in these DES was studied extensively. In the case of caffeine, an increase in solubility was observed, but not in the case of furosemide [94]. In another similar study, DES trends as a new approach to solubilize the drug. Ibuprofen is a poorly water-soluble drug and its solubility was increased using xylitol and choline chloride DES mixed with different proportions of water. The IBU-THEDES was found to be non-toxic in a cell viability study [95]. It was reported that the solubility and topical delivery of a class IV drug, dapsone, was enhanced using a DES based on choline chloride (CC, as HBA) and ascorbic acid or propylene glycol (AA, PG, as HBDs). The system CC:PG was able to dissolve dapsone at a high concentration (500 mg/mL) as compared to its solubility in water (380 mg/L). This increase in solubility could be attributed to the ability of DES's components to form hydrogen bonds with the drug, as confirmed by the FT-IR and 1H NMR techniques [57].
Until this study, it was considered that generally all the poorly soluble APIs dissolve in their respective DES, as we have seen in so many examples such as curcumin, baicalin, ibuprofen, etc. But a study conducted on six model drugs and thymol-decanoic acid DES concluded that out of six APIs, only three (ibuprofen, lenvatinib mesylate, and fenofibrate) were found to be fully dissolved in the thymol-decanoic acid DES system as a solution state, however, other APIs such as lurasidone hydrochloride, olaparib, and baicalein could not completely dissolve to form a true solution but remained only dispersed in DES as a colloidal system, giving rise to further queries and studies on the existence of APIs in DES [96]. A novel type of supersaturating drug delivery system was developed to reduce the risk of drug precipitation following oral administration. A polymer-embedded deep eutectic system with 15 % w/w polyvinyl pyrrolidone K30 (PVP) in l-carnitine: ethylene glycol (1:4, molar ratio) DES was made for the model drug indomethacin. A marked increase in the solubility of indomethacin in DES was observed (175.6 mg/mL), making PEDES a viable formulation approach [97].
Deep eutectic-based formulations for protein delivery after subcutaneous injections have been recently reported in the literature. Proteins as therapeutics are widely being used for the treatment of metabolic diseases, cancers, and diabetes, but large proteins suffer from poor absorption after subcutaneous injections. Deep eutectic based subcutaneous injection of monoclonal antibodies showed enhanced absorption by approximately 200 %, opening new vistas in the delivery of therapeutic proteins via the subcutaneous route [98].
For insights on the toxicity profile, a published review has summarized in detail all the previous studies on THEDES that have investigated their toxicity profile by various means, such as cell culture methods, animal models, and human trials. They concluded that THEDES are generally well tolerated and have a low toxicity profile; however, the same cannot be said for THEDES degradation products. The data is very scarce in support of them being labeled as non-toxic in nature [99]. Recently, published reviews have given valuable insights on the toxicity and regulatory aspects of THEDES. However, long-term toxicity data, biosafety profile, safety evaluations, and clinical trials are still ongoing for this new form of drug delivery [100,101]. Table 2 summarizes a few important examples present in the literature for solubility enhancement by the THEDES technique.
Table 2.
Solubility enhancement of APIs by incorporating in THEDES.
| APIs | DES/conformer composition | Solubility enhancement | Ref |
|---|---|---|---|
| Weakly basic drugs | Glycolic acid & choline chloride | Remarkable increase in solubility of these drugs: Itraconazole (6700-fold) Piroxicam (430-fold) Lidocaine (28-fold) Posaconazole (6400-fold) |
[35] |
| Itraconazole | Choline chloride, glycolic acid, and oxalic acid | Itraconazole (53600-fold) increase in solubility at DES molar ratio of 1:1.6:0.4. | [35] |
| Ibuprofen | Arginine and glycerol (1:4) | 7917-fold increase in solubility of IBU | [44] |
| Ibuprofen | Menthol | 12-fold increase in solubility of IBU | [57] |
| Dapsone | Choline chloride/Propylene glycol | Enhanced solubility (500 mg/mL) as compared to water (380 mg/L). | [58] |
| Sulfonamides | Choline chloride/multi hydroxyl compounds | ChCl/glycerol conferred 43 times higher solubility when compared with water at 37 °C. | [59] |
| Three APIs – acetylsalicylic acid, benzoic acid and phenylacetic acid | Choline chloride/Menthol | Enhanced dissolution rate profiles for APIs when complexed in THEDES form. | [60] |
| Curcumin | Choline chloride/glycerol | 12,000-fold higher solubility of curcumin than aqueous solution at RT. | [61] |
| Chlorpropamide and tolbutamide | Tromethamine | 188 times increase in solubility for chlorpropamide and 120 times for tolbutamide in THEDES than their respective crystalline APIs. | [62] |
| Ethambutol | l-Arginine | 27.5-fold increase in solubility | [65] |
| Ibuprofen | Perillyl alcohol | No marked solubility enhancement was observed using this eutectic mixture. | [70] |
| Silymarin | Polyvinylpyrrolidone K30 | 5-fold increase in solubility of silymarin THEDES as compared to pure drug. | [77] |
| Caffeine and furosemide | Xylitol, citric acid, sorbitol, and glucose (HBD) and choline chloride (HBA) | Solubility enhancement was observed for caffeine but not for furosemide. | [74] |
| Ibuprofen | Xylitol, choline chloride, and water | Improved solubility of ibuprofen in DES mixture. | [95] |
| Indomethacin | l-Carnitine: ethylene glycol (1:4) DES | Several thousand folds increase in solubility of indomethacin in selected DES (175.6 ± 3.4 mg/mL) was observed as compared to the aqueous solubility of drug (0.0011 mg/mL) at 37 °C. | [97] |
| Tadalafil | Choline chloride/malonic acid | High equilibrium solubility was observed for tadalafil in DES mixtures. | [102] |
6. Challenges and limitations of THEDES
Solvents are an important component in any drug delivery system, which can be used as extraction media, reaction media, solubilization media, and as vehicles for the delivery of drugs. The DESs belong to a new category of green solvents with interesting properties such as high solubilization capacity, non-inflammability, non-reactivity with water, low vapor pressure, and chemically tailorable characteristics, giving them versatile applications in all fields of science [103]. None of the formulation development strategies provide 100 % in terms of experimental successes, and they all have their own set of advantages and disadvantages, challenges, and limitations. The ionic liquids and DESs face challenging issues of biocompatibility, potential impact of the presence of impurities, and important aspects of understanding the microscopic interactions in ionic liquids, which is a crucial step in designing the task-specific solvents [27].
There are several limitations associated with the use of THEDES as drug delivery system that beset its use, and further research, development, and optimization is warranted to fully harness its potential in various applications. These limitations include the limited solubility of some APIs in the DESs, which can restrict their applications in certain formulations [96]. Also, the THEDES might be associated with stability issues, including phase separation or degradation of active compounds over time, especially under stressed environmental conditions. Although THEDES are generally considered safe, there may be concerns regarding the toxicity of certain ingredients used in their formulation intended for therapeutic applications [98]. The biocompatibility of THEDES also needs to be thoroughly evaluated for formulations that involve direct contact with biological tissues or are administered systemically [99]. Tedious, complex, and time-consuming regulatory approval processes for THEDES-based formulations, compatibility of the APIs with the ingredients or excipients, and a higher cost of production of THEDES, are other limitations associated with the use of THEDES in the drug delivery system.
Tedious experimental efforts are required to confirm the formation of THEDES between various APIs and excipient combinations using suitable techniques such as differential scanning calorimetry (DSC). These can be replaced with thermodynamic modeling, resulting in more effective and tailor-made formulations for successful drug delivery [19]. Despite their myriad applications, these ILs and DES are still not practically being used in commercial and viable formulations. Since this is a newer form of delivery system, more data related to clinical trials, long-term toxicity upon human administration, and safety evaluations are still warranted. However, interesting and positive findings obtained after the in vivo studies are encouraging enough to create new possibilities for delivering the drugs in their ionic forms [103]. Therefore, it is crucial for the formulator to consider these challenges and limitations of DESs in drug delivery prior to formulation development steps. The ability of THEDES to improve the bioavailability of APIs has significantly increased their popularity in the pharmaceutical industry; however, their biological performance has not been thoroughly examined yet [104].
7. Conclusions
In conclusion, THEDES can be regarded as any mixture of two or more components, where one of them can be an API, and these components, when mixed at a particular molar composition, become liquid at room temperature. This in turn enhances the solubilization of APIs into the system, even if they are poorly water-soluble, augmenting their bioavailability. THEDES can have a wide range of applications in drug delivery and are now being investigated as versatile drug delivery systems. THEDES can be administered using different routes, such as transdermal, oral, intravenous, buccal, and nasal administration, for enhanced drug bioavailability. Promising and interesting examples of THEDES are available in the medical, pharmaceutical, and biotechnological fields and were reviewed in this work. The last decade has witnessed several investigations aiming to utilize this novel drug delivery system to successfully deliver the poorly water-soluble drugs to their target. However, more pre-clinical and clinical studies are nevertheless required to fully establish their efficacy and toxicity in living systems.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number ISP-2024.
Data availability
No new data associated with the manuscript.
Ethical statement
Not applicable.
CRediT authorship contribution statement
Shamama Javed: Methodology, Data curation, Conceptualization, Validation, Writing – original draft. Bharti Mangla: Writing – original draft, Validation, Methodology, Data curation. Muhammad H. Sultan: Writing – review & editing, Project administration, Formal analysis, Data curation, Funding acquisition. Yosif Almoshari: Writing – review & editing, Supervision, Project administration. Durgaramani Sivadasan: Writing – review & editing, Resources, Methodology. Saad S. Alqahtani: Supervision, Project administration, Funding acquisition, Data curation. Osama A. Madkhali: Writing – review & editing, Methodology, Validation. Waquar Ahsan: Writing – review & editing, Validation, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number ISP-2024.
References
- 1.Paul R., Paul S. Exploration on the drug solubility enhancement in aqueous medium with the help of endo -functionalized molecular tubes: a computational approach. Phys. Chem. Chem. Phys. 2021;23:18999–19010. doi: 10.1039/d1cp01187a. [DOI] [PubMed] [Google Scholar]
- 2.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm. Sin. B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad K., Nazir M.A., Qureshi A.K., Hussain E., Najam T., Javed M.S., Shah S.S.A., Tufail M.K., Hussain S., Khan N.A., Shah H., Ashfaq M. Engineering of Zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater. Sci. Eng. B. 2020;262 doi: 10.1016/j.mseb.2020.114766. [DOI] [Google Scholar]
- 4.Huang X., Zafar A., Ahmad K., Hasan M., Tariq T., Gong S., Hassan S.G., Guo J., Javed H.U., Shu X. Biological synthesis of bimetallic hybrid nanocomposite: a remarkable photocatalyst, adsorption/desorption and antimicrobial agent. Appl Surf Sci Adv. 2023;17 doi: 10.1016/j.apsadv.2023.100446. [DOI] [Google Scholar]
- 5.Zainal-Abidin M.H., Hayyan M., Ngoh G.C., Wong W.F., Looi C.Y. Emerging frontiers of deep eutectic solvents in drug discovery and drug delivery systems. J Control Release. 2019 Dec 28;316:168–195. doi: 10.1016/j.jconrel.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Pedro S.N., Freire M.G., Freire C.S.R., Silvestre A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expet Opin. Drug Deliv. 2019;16(5):497–506. doi: 10.1080/17425247.2019.1604680. [DOI] [PubMed] [Google Scholar]
- 7.Morrison H.G., Sun C.C., Neervannan S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009;378(1–2):136–139. doi: 10.1016/j.ijpharm.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 8.Stott P.W., Williams A.C., Barry B.W. Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J Control Release. 1998;50(1–3):297–308. doi: 10.1016/s0168-3659(97)00153-3. [DOI] [PubMed] [Google Scholar]
- 9.Tanner E.E.L., Ibsen K.N., Mitragotri S. Transdermal insulin delivery using choline-based ionic liquids (CAGE) J Control Release. 2018;286:137–144. doi: 10.1016/j.jconrel.2018.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Álvarez M.S., Zhang Y. Sketching neoteric solvents for boosting drugs bioavailability. J Control Release. 2019;311–312:225–232. doi: 10.1016/j.jconrel.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Peterfreund R.A., Philip J.H. Critical parameters in drug delivery by intravenous infusion. Expet Opin. Drug Deliv. 2013;10(8):1095–1108. doi: 10.1517/17425247.2013.785519. [DOI] [PubMed] [Google Scholar]
- 12.Ezike T.C., Okpala U.S., Onoja U.L., Nwike C.P., Ezeako E.C., Okpara O.J., Okoroafor C.C., Eze S.C., Kalu O.L., Odoh E.C., Nwadike U.G., Ogbodo J.O., Umeh B.U., Ossai E.C., Nwanguma B.C. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9(6) doi: 10.1016/j.heliyon.2023.e17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart S.A., Domínguez-Robles J., Donnelly R.F., Larrañeta E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers. 2018;10(12):1379. doi: 10.3390/polym10121379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J., Li M., Duan L., Lin Y., Cui X., Yang Y., Wang C. Deep eutectic systems as novel vehicles for assisting drug transdermal delivery. Pharmaceutics. 2022;14(11):2265. doi: 10.3390/pharmaceutics14112265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayyan M. Versatile applications of deep eutectic solvents in drug discovery and drug delivery systems: perspectives and opportunities. Asian J. Pharm. Sci. 2023;18(2) doi: 10.1016/j.ajps.2023.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa N.R., Spelbos V.S., Witkamp G.J., Verpoorte R., Choi Y.H. Solubility and stability of some pharmaceuticals in natural deep eutectic solvents-based formulations. Molecules. 2021;26(9):2645. doi: 10.3390/molecules26092645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez G.M., Townley G.G., Martínez-Espinosa R.M. Controversy on the toxic nature of deep eutectic solvents and their potential contribution to environmental pollution. Heliyon. 2022;8(12) doi: 10.1016/j.heliyon.2022.e12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manyoni L., Redhi G. Separation potential of 1,5-pentanediol-based deep eutectic solvent: Infinite dilution activity coefficients and excess thermodynamic data. Heliyon. 2023;9(11) doi: 10.1016/j.heliyon.2023.e21516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolbert F., Brandenbusch C., Sadowski G. Selecting excipients forming therapeutic deep eutectic systems-A mechanistic approach. Mol. Pharm. 2019;16(7):3091–3099. doi: 10.1021/acs.molpharmaceut.9b00336. [DOI] [PubMed] [Google Scholar]
- 20.Chakraborty S., Chormale J.H., Bansal A.K. Deep eutectic systems: an overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021;610 doi: 10.1016/j.ijpharm.2021.121203. [DOI] [PubMed] [Google Scholar]
- 21.Karas L.J., Wu C.H., Das R., Wu J.I. Hydrogen bond design principles. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020;10(6) doi: 10.1002/wcms.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabłoński M. Intramolecular hydrogen bonding 2021. Molecules. 2021;26(20):6319. doi: 10.3390/molecules26206319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahanbakhsh Bonab P., Rastkar Ebrahimzadeh A., Jahanbin Sardroodi J. Insights into the interactions and dynamics of a DES formed by phenyl propionic acid and choline chloride. Sci. Rep. 2021;11(1):6384. doi: 10.1038/s41598-021-85260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu W., Qader I.B., Abbott A.P. Controlled release of pharmaceutical agents using eutectic modified gelatin. Drug Deliv Transl Res. 2022;12(5):1187–1194. doi: 10.1007/s13346-021-00998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Wu Y., Liu J., Wang W., Yang Q., Yang G. Deep eutectic solvents: recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022;622 doi: 10.1016/j.ijpharm.2022.121811. [DOI] [PubMed] [Google Scholar]
- 26.Tomé L.C., Mecerreyes D. Emerging ionic soft materials based on deep eutectic solvents. J. Phys. Chem. B. 2020;124(39):8465–8478. doi: 10.1021/acs.jpcb.0c04769. [DOI] [PubMed] [Google Scholar]
- 27.Curreri A.M., Mitragotri S., Tanner E.E.L. Recent advances in ionic liquids in biomedicine. Adv. Sci. 2021;8(17) doi: 10.1002/advs.202004819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riaz M., Akhlaq M., Naz S., Uroos M. An overview of biomedical applications of choline geranate (CAGE): a major breakthrough in drug delivery. RSC Adv. 2022;12(40):25977–25991. doi: 10.1039/d2ra03882j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felipe A., Lovenduski C.A., Baker J.L., Lindberg G.E. Long-ranged heterogeneous structure in aqueous solutions of the deep eutectic solvent choline and geranate at the liquid-vapor interface. Phys. Chem. Chem. Phys. 2022;24(22):13720–13729. doi: 10.1039/d2cp01530g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J., Gao Y., Zhao Z., Rodrigues D., Tanner E.E.L., Ibsen K., Sasmal P.K., Jaladi R., Alikunju S., Mitragotri S. A deep eutectic-based, self-emulsifying subcutaneous depot system for apomorphine therapy in Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2022;119(9) doi: 10.1073/pnas.2110450119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mano F., Martins M., Sá-Nogueira I., Barreiros S., Borges J.P., Reis R.L., Duarte A.R.C., Paiva A. Production of electrospun fast-dissolving drug delivery systems with therapeutic eutectic systems encapsulated in gelatin. AAPS PharmSciTech. 2017;18(7):2579–2585. doi: 10.1208/s12249-016-0703-z. [DOI] [PubMed] [Google Scholar]
- 32.Bryant S.J., Awad M.N., Elbourne A., Christofferson A.J., Martin A.V., Meftahi N., Drummond C.J., Greaves T.L., Bryant G. Deep eutectic solvents as cryoprotective agents for mammalian cells. J. Mater. Chem. B. 2022;10(24):4546–4560. doi: 10.1039/d2tb00573e. [DOI] [PubMed] [Google Scholar]
- 33.Aroso I.M., Craveiro R., Rocha Â., Dionísio M., Barreiros S., Reis R.L., Paiva A., Duarte A.R. Design of controlled release systems for THEDES-Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015;492(1–2):73–79. doi: 10.1016/j.ijpharm.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Lv J., Ou X., Fang Y., Wu M., Zheng F., Shang L., Lei K., Liu Y., Zhao Y. The study of deep eutectic solvent based on choline chloride and L-(+)-tartaric acid diethyl ester for transdermal delivery system. AAPS PharmSciTech. 2022;23(7):252. doi: 10.1208/s12249-022-02342-5. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Lee P.I. Investigation on drug solubility enhancement using deep eutectic solvents and their derivatives. Int. J. Pharm. 2016;505(1–2):283–288. doi: 10.1016/j.ijpharm.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee A., Ibsen K., Iwao Y., Zakrewsky M., Mitragotri S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv. Healthcare Mater. 2017;6(15) doi: 10.1002/adhm.201601411. [DOI] [PubMed] [Google Scholar]
- 37.Tanner E.E.L., Curreri A.M., Balkaran J.P.R., Selig-Wober N.C., Yang A.B., Kendig C., Fluhr M.P., Kim N., Mitragotri S. Design principles of ionic liquids for transdermal drug delivery. Adv. Mater. 2019;31(27) doi: 10.1002/adma.201901103. [DOI] [PubMed] [Google Scholar]
- 38.Liu M., Lai Z., Zhu L., Ding X., Tong X., Wang Z., Bi Q., Tan N. Novel amorphous solid dispersion based on natural deep eutectic solvent for enhancing delivery of anti-tumor RA-XII by oral administration in rats. Eur. J. Pharmaceut. Sci. 2021;166 doi: 10.1016/j.ejps.2021.105931. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Cao S., Yu K., Yang F., Yu X., Zhai Y., Wu C., Xu Y. Integrating tacrolimus into eutectic oil-based microemulsion for atopic dermatitis: simultaneously enhancing percutaneous delivery and treatment efficacy with relieving side effects. Int. J. Nanomed. 2019;14:5849–5863. doi: 10.2147/IJN.S212260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong S.H., Dinh L., Abuzar S.M., Lee E.S., Hwang S.J. Synthesis of celecoxib-eutectic mixture particles via supercritical CO2 process and celecoxib immediate release tablet formulation by quality by design approach. Pharmaceutics. 2022;14(8):1549. doi: 10.3390/pharmaceutics14081549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo T., Wang C., Ji X., Yang G., Chen J., Janaswamy S., Lyu G. Preparation and characterization of size-controlled lignin nanoparticles with deep eutectic solvents by nanoprecipitation. Molecules. 2021;26(1):218. doi: 10.3390/molecules26010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X.L., Yao H.F., Chai M.H., He W., Huang Y.P., Liu Z.S. Green synthesis of carbon nanotubes-reinforced molecularly imprinted polymer composites for drug delivery of fenbufen. AAPS PharmSciTech. 2018;19(8):3895–3906. doi: 10.1208/s12249-018-1192-z. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X., Chai M.H., Wei Z.H., Chen W.J., Liu Z.S., Huang Y.P. Deep eutectic solvents-based polymer monolith incorporated with titanium dioxide nanotubes for specific recognition of proteins. Anal. Chim. Acta. 2020;1139:27–35. doi: 10.1016/j.aca.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 44.Pedro S.N., Mendes M.S.M., Neves B.M., Almeida I.F., Costa P., Correia-Sá I., Vilela C., Freire M.G., Silvestre A.J.D., Freire C.S.R. Deep eutectic solvent formulations and alginate-based hydrogels as a new partnership for the transdermal administration of anti-inflammatory drugs. Pharmaceutics. 2022;14(4):827. doi: 10.3390/pharmaceutics14040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutiérrez A., Atilhan M., Aparicio S. Theoretical study on deep eutectic solvents as vehicles for the delivery of anesthetics. J. Phys. Chem. B. 2020;124(9):1794–1805. doi: 10.1021/acs.jpcb.9b11756. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y., Wang X., Wang X., Song Y., Wang X., Hao J. Design and development of lidocaine microemulsions for transdermal delivery. AAPS PharmSciTech. 2019;20(2):63. doi: 10.1208/s12249-018-1263-1. [DOI] [PubMed] [Google Scholar]
- 47.Fabbrocini G., De Vita V., Izzo R., Monfrecola G. The use of skin needling for the delivery of a eutectic mixture of local anesthetics. G. Ital. Dermatol. Venereol. 2014;149(5):581–585. [PubMed] [Google Scholar]
- 48.Cordeiro T., Castiñeira C., Mendes D., Danède F., Sotomayor J., Fonseca I.M., Gomes da Silva M., Paiva A., Barreiros S., Cardoso M.M., Viciosa M.T., Correia N.T., Dionisio M. Stabilizing unstable amorphous menthol through inclusion in mesoporous silica hosts. Mol. Pharm. 2017;14(9):3164–3177. doi: 10.1021/acs.molpharmaceut.7b00386. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y., Cao Y., Meng X., Li C., Wang H., Zhang S. Enhancement of transdermal delivery of artemisinin using microemulsion vehicle based on ionic liquid and lidocaine ibuprofen. Colloids Surf. B Biointerfaces. 2020;189 doi: 10.1016/j.colsurfb.2020.110886. [DOI] [PubMed] [Google Scholar]
- 50.Xiao S., Wang L., Han W., Gu L., Cui X., Wang C. Novel deep eutectic solvent-hydrogel systems for synergistic transdermal delivery of Chinese herb medicine and local treatments for rheumatoid arthritis. Pharm. Res. (N. Y.) 2022;39(10):2431–2446. doi: 10.1007/s11095-022-03239-5. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Z., Li M., Zheng L., Yang Y., Cui X., Xu T., Zhang W., Wang C. Noninvasive transdermal delivery of mesoporous silica nanoparticles using deep eutectic solvent. J Control Release. 2022;343:43–56. doi: 10.1016/j.jconrel.2022.01.019. [DOI] [PubMed] [Google Scholar]
- 52.Qu W., Qader I.B., Abbott A.P. Controlled release of pharmaceutical agents using eutectic modified gelatin. Drug Deliv Transl Res. 2022;12(5):1187–1194. doi: 10.1007/s13346-021-00998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaidya A., Mitragotri S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J Control Release. 2020;327:26–34. doi: 10.1016/j.jconrel.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Kim J., Shi Y., Kwon C.J., Gao Y., Mitragotri S. A deep eutectic solvent-based approach to intravenous formulation. Adv. Healthcare Mater. 2021;10(18) doi: 10.1002/adhm.202100585. [DOI] [PubMed] [Google Scholar]
- 55.Li S., Culkin A., Jones D.S., Andrews G.P. Development of Polycaprolactone-Based metronidazole matrices for intravaginal extended drug delivery using a mechanochemically prepared therapeutic deep eutectic system. Int. J. Pharm. 2021;593 doi: 10.1016/j.ijpharm.2020.120071. [DOI] [PubMed] [Google Scholar]
- 56.Shah P.A., Chavda V., Hirpara D., Sharma V.S., Shrivastav P.S., Kumar S. Exploring the potential of deep eutectic solvents in pharmaceuticals: challenges and opportunities. J. Mol. Liq. 2023;390 doi: 10.1016/j.molliq.2023.123171. [DOI] [Google Scholar]
- 57.Duarte A.R., Ferreira A.S., Barreiros S., Cabrita E., Reis R.L., Paiva A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017;114:296–304. doi: 10.1016/j.ejpb.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Trombino S., Siciliano C., Procopio D., Curcio F., Laganà A.S., Di Gioia M.L., Cassano R. Deep eutectic solvents for improving the solubilization and delivery of dapsone. Pharmaceutics. 2022;14(2):333. doi: 10.3390/pharmaceutics14020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cysewski P., Jeliński T. Optimization, thermodynamic characteristics and solubility predictions of natural deep eutectic solvents used for sulfonamide dissolution. Int. J. Pharm. 2019;570 doi: 10.1016/j.ijpharm.2019.118682. [DOI] [PubMed] [Google Scholar]
- 60.Aroso I.M., Silva J.C., Mano F., Ferreira A.S., Dionísio M., Sá-Nogueira I., Barreiros S., Reis R.L., Paiva A., Duarte A.R. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016;98:57–66. doi: 10.1016/j.ejpb.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Jeliński T., Przybyłek M., Cysewski P. Natural deep eutectic solvents as agents for improving solubility, stability and delivery of curcumin. Pharm. Res. (N. Y.) 2019;36(8):116. doi: 10.1007/s11095-019-2643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sarraguça M.C., Ribeiro P.R.S., Nunes C., Seabra C.L. Solids turn into liquids-liquid eutectic systems of Pharmaceutics to improve drug solubility. Pharmaceuticals. 2022;15(3):279. doi: 10.3390/ph15030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C., Chen X., Wei C., Wang H., Gao H. Deep eutectic solvents as active pharmaceutical ingredient delivery systems in the treatment of metabolic related diseases. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.794939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira F., Santos F., Duarte A.R.C. Therapeutic deep eutectic systems towards the treatment of tuberculosis and colorectal cancer: opportunities and challenges. Molecules. 2021;26(22):7022. doi: 10.3390/molecules26227022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santos F., P S., Leitão M.I., C Duarte A.R. Properties of therapeutic deep eutectic solvents of l-arginine and ethambutol for tuberculosis treatment. Molecules. 2018;24(1):55. doi: 10.3390/molecules24010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roda A., Santos F., Matias A.A., Paiva A., Duarte A.R.C. Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis. J. Supercrit. Fluids. 2020;161 doi: 10.1016/j.supflu.2020.104826. [DOI] [Google Scholar]
- 67.Parveen F., Madni A., Torchilin V.P., Rehman M., Jamshaid T., Filipczak N., Rai N., Khan M.M., Khan M.I. Investigation of eutectic mixtures of fatty acids as a novel construct for temperature-responsive drug delivery. Int. J. Nanomed. 2022;17:2413–2434. doi: 10.2147/IJN.S359664. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 68.Sun X., Pradeepkumar P., Rajendran N.K., Shakila H., Houreld N.N., Al Farraj D.A., Elnahas Y.M., Elumalai N., Rajan M. Natural deep eutectic solvent supported targeted solid-liquid polymer carrier for breast cancer therapy. RSC Adv. 2020;10(61):36989–37004. doi: 10.1039/d0ra03790g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pereira J., Miguel Castro M., Santos F., Rita Jesus A., Paiva A., Oliveira F., Duarte A.R.C. Selective terpene based therapeutic deep eutectic systems against colorectal cancer. Eur. J. Pharm. Biopharm. 2022;175:13–26. doi: 10.1016/j.ejpb.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Silva E., Oliveira F., Silva J.M., Matias A., Reis R.L., Duarte A.R.C. Optimal design of THEDES based on perillyl alcohol and ibuprofen. Pharmaceutics. 2020;12(11):1121. doi: 10.3390/pharmaceutics12111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pereira C.V., Silva J.M., Rodrigues L., Reis R.L., Paiva A., Duarte A.R.C., Matias A. Unveil the anticancer potential of Limomene based therapeutic deep eutectic solvents. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-51472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J., Xue J., Dong X., Yu Q., Baker S.N., Wang M., Huang H. Antimicrobial properties of benzalkonium chloride derived polymerizable deep eutectic solvent. Int. J. Pharm. 2020;575 doi: 10.1016/j.ijpharm.2019.119005. [DOI] [PubMed] [Google Scholar]
- 73.França M.T., Martins Marcos T., Costa P.F.A., Bazzo G.C., Nicolay Pereira R., Gerola A.P., Stulzer H.K. Eutectic mixture and amorphous solid dispersion: two different supersaturating drug delivery system strategies to improve griseofulvin release using saccharin. Int. J. Pharm. 2022;615 doi: 10.1016/j.ijpharm.2022.121498. [DOI] [PubMed] [Google Scholar]
- 74.Siddiqui R., Makhlouf Z., Akbar N., Khamis M., Ibrahim T., Khan A.S., Khan N.A. Antiamoebic properties of salicylic acid-based deep eutectic solvents for the development of contact lens disinfecting solutions against Acanthamoeba. Mol. Biochem. Parasitol. 2022;250 doi: 10.1016/j.molbiopara.2022.111493. [DOI] [PubMed] [Google Scholar]
- 75.Silva J.M., Pereira C.V., Mano F., Silva E., Castro V.I.B., Sá-Nogueira I., Reis R.L., Paiva A., Matias A.A., Duarte A.R.C. Therapeutic role of deep eutectic solvents based on menthol and saturated fatty acids on wound healing. ACS Appl. Bio Mater. 2019;2(10):4346–4355. doi: 10.1021/acsabm.9b00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q., Lin Z., Zhang W., Huang T., Jiang J., Ren Y., Zhang R., Li W., Zhang X., Tu Q. Fabrication of green poly(vinyl alcohol) nanofibers using natural deep eutectic solvent for fast-dissolving drug delivery. RSC Adv. 2021;11(2):1012–1021. doi: 10.1039/d0ra08755f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sherikar A., Siddique M.U.M., More M., Goyal S.N., Milivojevic M., Alkahtani S., Alarifi S., Hasnain M.S., Nayak A.K. Preparation and evaluation of silymarin-loaded solid eutectic for enhanced anti-inflammatory, hepatoprotective effect: in vitro-in vivo Prospect. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/1818538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcantonini G., Bartolini D., Zatini L., Costa S., Passerini M., Rende M., Luca G., Basta G., Murdolo G., Calafiore R., Galli F. Natural cryoprotective and Cytoprotective agents in cryopreservation: a Focus on Melatonin. Molecules. 2022;27(10):3254. doi: 10.3390/molecules27103254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jesus A.R., Meneses L., Duarte A.R.C., Paiva A. Natural deep eutectic systems, an emerging class of cryoprotectant agents. Cryobiology. 2021;101:95–104. doi: 10.1016/j.cryobiol.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jesus A.R., Duarte A.R.C., Paiva A. Use of natural deep eutectic systems as new cryoprotectant agents in the vitrification of mammalian cells. Sci. Rep. 2022;12(1):8095. doi: 10.1038/s41598-022-12365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hornberger K., Li R., Duarte A.R., Hubel A. Natural deep eutectic systems for nature‐inspired cryopreservation of cells. AIChE J. 2021;67(2) [PMC free article] [PubMed] [Google Scholar]
- 82.Jesus A.R., Duarte A.R., Paiva A. Use of natural deep eutectic systems as new cryoprotectant agents in the vitrification of mammalian cells. Sci. Rep. 2022;12(1):8095. doi: 10.1038/s41598-022-12365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Friesen J.B., McAlpine J.B., Lankin D.C., Chen S.N., Pauli G.F. Natural deep eutectic solvents: properties, applications, and perspectives. J. Nat. Prod. 2018;81(3):679–690. doi: 10.1021/acs.jnatprod.7b00945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faggian M., Sut S., Perissutti B., Baldan V., Grabnar I., Dall'Acqua S. Natural deep eutectic solvents (NADES) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: possible application in nutraceuticals. Molecules. 2016;21(11):1531. doi: 10.3390/molecules21111531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cannavacciuolo C., Pagliari S., Frigerio J., Giustra C.M., Labra M., Campone L. Natural deep eutectic solvents (NADESs) combined with sustainable extraction techniques: a review of the green chemistry approach in food analysis. Foods. 2022;12(1):56. doi: 10.3390/foods12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Socas-Rodríguez B., Torres-Cornejo M.V., Álvarez-Rivera G., Mendiola J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021;11(11):4897. doi: 10.3390/app11114897. [DOI] [Google Scholar]
- 87.Dheyab A.S., Abu Bakar M.F., AlOmar M., Sabran S.F., Muhamad Hanafi A.F., Mohamad A. Deep eutectic solvents (DESs) as green extraction media of beneficial bioactive phytochemicals. Separations. 2021;8(10):176. doi: 10.3390/separations8100176. [DOI] [Google Scholar]
- 88.Alu'datt M.H., Alrosan M., Gammoh S., Tranchant C.C., Alhamad M.N., Rababah T., Zghoul R., Alzoubi H., Ghatasheh S., Ghozlan K., Tan T.C. Encapsulation-based technologies for bioactive compounds and their application in the food industry: a roadmap for food-derived functional and health-promoting ingredients. Food Biosci. 2022;50 doi: 10.1016/j.fbio.2022.101971. [DOI] [Google Scholar]
- 89.Iyer P.V., Ananthanarayan L. Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem. 2008;43(10):1019–1032. doi: 10.1016/j.procbio.2008.06.004. [DOI] [Google Scholar]
- 90.Wu J., Yang S., Yin T., Wang X. Eutectic-based liposome as a potential delivery system of paeonol. RSC Adv. 2021;11(62):39343–39348. doi: 10.1039/d1ra06907a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vieira C., Rebocho S., Craveiro R., Paiva A., Duarte A.R.C. Selective extraction and stabilization of bioactive compounds from rosemary leaves using a biphasic NADES. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.954835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oyoun F., Toncheva A., Henríquez L.C., Grougnet R., Laoutid F., Mignet N., Alhareth K., Corvis Y. Deep eutectic solvents: an eco-friendly design for drug engineering. ChemSusChem. 2023 doi: 10.1002/cssc.202300669. [DOI] [PubMed] [Google Scholar]
- 93.Prabhune A., Dey R. Green and sustainable solvents of the future: deep eutectic solvents. J. Mol. Liq. 2023;379 doi: 10.1016/j.molliq.2023.121676. [DOI] [Google Scholar]
- 94.Lomba L., Polo A., Alejandre J., Martínez N., Giner B. Solubility enhancement of caffeine and furosemide using deep eutectic solvents formed by choline chloride and xylitol, citric acid, sorbitol or glucose. J. Drug Deliv. Sci. Technol. 2023;79 doi: 10.1016/j.jddst.2022.104010. [DOI] [Google Scholar]
- 95.Lomba L., Garralaga M.P., Werner A., Giner B., Baptista P.M., Sánchez-Romero N. Ibuprofen solubility and cytotoxic study of deep eutectic solvents formed by xylitol, choline chloride and water. J. Drug Deliv. Sci. Technol. 2023;82 doi: 10.1016/j.jddst.2023.104327. [DOI] [Google Scholar]
- 96.Zhu J., Wei Y., Zhang J., Qian S., Gao Y., Heng W. Are all poorly soluble drugs dissolved in deep eutectic solvents true solutions? J. Colloid Interface Sci. 2023;645:813–822. doi: 10.1016/j.jcis.2023.04.164. [DOI] [PubMed] [Google Scholar]
- 97.Panbachi S., Beranek J., Kuentz M. Polymer-embedded deep eutectic solvents (PEDES) as a novel bio-enabling formulation approach. Eur. J. Pharmaceut. Sci. 2023;186 doi: 10.1016/j.ejps.2023.106463. [DOI] [PubMed] [Google Scholar]
- 98.Curreri A.M., Kim J., Dunne M., Angsantikul P., Goetz M., Gao Y., Mitragotri S. Deep eutectic solvents for subcutaneous delivery of protein therapeutics. Adv. Sci. 2023;10(7) doi: 10.1002/advs.202205389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.García C.B., Concha J., Culleré L., Lomba L., Sangüesa E., Ribate M.P. Has the toxicity of therapeutic deep eutectic systems been assessed? Appl. Sci. 2023;13:5980. doi: 10.3390/app13105980. [DOI] [Google Scholar]
- 100.Abdelquader M.M., Li S., Andrews G.P., Jones D.S. Therapeutic deep eutectic solvents: a comprehensive review of their thermodynamics, microstructure and drug delivery applications. Eur. J. Pharm. Biopharm. 2023;186:85–104. doi: 10.1016/j.ejpb.2023.03.002. [DOI] [PubMed] [Google Scholar]
- 101.Qader I.B., Prasad K. Recent developments on ionic liquids and deep eutectic solvents for drug delivery applications. Pharm. Res. (N. Y.) 2022;39:2367–2377. doi: 10.1007/s11095-022-03315-w. [DOI] [PubMed] [Google Scholar]
- 102.Emami S., Shayanfar A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharmaceut. Dev. Technol. 2020;25(7):779–796. doi: 10.1080/10837450.2020.1735414. [DOI] [PubMed] [Google Scholar]
- 103.Alkhawaja B., Al-Akayleh F., Al-Khateeb A., Nasereddin J., Ghanim B.Y., Bolhuis A., Jaber N., Al-Remawi M., Qinna N.A. Deep eutectic liquids as a topical vehicle for tadalafil: Characterisation and potential wound healing and antimicrobial activity. Molecules. 2023;28(5):2402. doi: 10.3390/molecules28052402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lv J., Wu P., Fang Y., Zhang W., Liu D., Wu M., Shang L., Li H., Zhao Y. Deep eutectic solvents based on L-arginine and 2-hydroxypropyl-β-cyclodextrin for drug carrier and penetration enhancement. AAPS PharmSciTech. 2023;24(7):187. doi: 10.1208/s12249-023-02638-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data associated with the manuscript.