Abstract
Gastric cancer (GC), a leading cause of cancer-related mortality, remains a significant challenge despite recent therapeutic advancements. In this study, we explore the potential of targeting cell surface glucose-regulated protein 94 (GRP94) with antibodies as a novel therapeutic approach for GC. Our comprehensive analysis of GRP94 expression across various cancer types, with a specific focus on GC, revealed a substantial overexpression of GRP94, highlighting its potential as a promising target. Through in vitro and in vivo efficacy assessments, as well as toxicological analyses, we found that K101.1, a fully human monoclonal antibody designed to specifically target cell surface GRP94, effectively inhibits GC growth and angiogenesis without causing in vivo toxicity. Furthermore, our findings indicate that K101.1 promotes the internalization and concurrent downregulation of cell surface GRP94 on GC cells. In conclusion, our study suggests that cell surface GRP94 may be a potential therapeutic target in GC, and that antibody-based targeting of cell surface GRP94 may be an effective strategy for inhibiting GRP94-mediated GC growth and angiogenesis.
Keywords: Cell surface glucose-regulated protein 94, Downregulation, Gastric cancer, Human antibody, Internalization
INTRODUCTION
Gastric cancer (GC) is a significant global health concern, ranking as the fifth most frequently diagnosed cancer and the fourth leading cause of cancer-related mortality worldwide (1). Despite considerable progress in cancer biology and therapeutic approaches, GC management remains difficult. Current treatment strategies encompass multifaceted interventions tailored to consider critical factors such as disease stage, overall patient health, and individualized therapy plans. Standard care includes surgical tumor excision and adjuvant chemotherapy regimens with drugs including capecitabine, oxaliplatin, paclitaxel, irinotecan, and 5-fluorouracil (5-FU) (2, 3). However, these treatments frequently cause debilitating adverse effects, severely affecting patients’ quality of life. Moreover, the prognosis for individuals with advanced GC remains poor, with a median survival period of less than a year (4, 5).
Conventional chemotherapeutic agents often face limitations, including side effects, cancer recurrence, and drug resistance. Therefore, the development of novel therapeutic agents is necessary to enhance the clinical outcome of cancer patients (6). In recent decades, therapeutic monoclonal antibodies (mAbs) have gained interest due to their precision in targeting specific molecular markers. Several mAbs, including trastuzumab (anti-human epidermal growth factor 2 [HER2]), ramucirumab (anti-vascular endothelial growth factor receptor 2), and nivolumab (anti-programmed death-1), have received approval from the US Food and Drug Administration (FDA) for GC treatment (7-9). The primary first-line treatment for advanced HER2-positive GC involves trastuzumab and chemotherapy. However, this treatment has only improved overall survival (OS) by 2.7 months, from 11.1 to 13.8 months. Importantly, it is most effective in 15-20% of GC patients with HER2 overexpression (7). Ramucirumab has been shown to improve OS by 1.4 months compared to the placebo group (3.8 months), with a median of 5.2 months (8). Additionally, the FDA has approved nivolumab for combination therapy with chemotherapy, which increases OS by 3.3 months compared to chemotherapy alone (14.4 vs. 11.1 months) (9).
Despite efforts to develop targeted therapies, the molecular heterogeneity of GC cells has hindered their application in clinical trials. The complexity of GC extends beyond clinical challenges, encompassing profound morphological and molecular diversity arising from genomic and epigenetic alterations. This heterogeneity causes an increase in drug resistance and highly aggressive cell subpopulations within the tumor microenvironment, diminishing the efficacy of current treatments (10, 11). Given these formidable challenges, there is an urgent need to explore novel therapeutic strategies. This involves studying the intricate molecular aspects of GC, identifying promising therapeutic targets, and developing precise and effective treatment modalities.
One promising target is glucose-regulated protein 94 (GRP94), a member of the heat shock protein 90 (HSP90) family (12). Under normal conditions, GRP94 is predominantly expressed in the endoplasmic reticulum (ER) in response to ER stress, stabilizing protein folding of misfolded polypeptides as a molecular chaperone (13). However, it is well-known that cancer cells exhibit the overexpression of GRP94. Elevated GRP94 levels are hallmarks of several types of cancers including colorectal and lung cancers, correlating with advanced disease stages and poor prognosis (14-16). Interestingly, cancer cells also increase the levels of cell surface GRP94, forming stable complexes with critical membrane proteins such as HER2, urokinase receptor, and estrogen receptor-alpha36 in plasma membranes. These complexes play an essential role in cancer progression and metastasis (17-19). In our previous study, we reported that GRP94 overexpression in colorectal cancer (CRC) is closely associated with an increase in microvessel density (MVD) (15). Furthermore, using a novel fully human mAb specifically targeting GRP94 that we have developed, we elucidated that antibody-based targeting of GRP94 is effective in reducing tumor growth and angiogenesis through internalization and concomitant downregulation of cell surface GRP94 on endothelial cell surfaces (15). These results also provide evidence revealing the potential role of GRP94 during cancer progression and metastasis.
This study aimed to explore the role of cell surface GRP94 as a potential molecular target in GC and to assess the suitability of targeting cell surface GRP94 for potential therapeutic applications. Our research employed histopathological and bioinformatics analyses to understand the significance of GRP94 expression in GC. Furthermore, we assessed the efficacy, toxicity profile, and mode of action of a novel anti-GRP94 human mAb developed for this purpose. The primary focus of our investigation was to evaluate the antibody’s capability to inhibit GC cell growth and angiogenesis. Therefore, we suggest that cell surface GRP94 could be a potential therapeutic target in GC for antibody therapy, emphasizing the potential of antibody-based targeting.
RESULTS
Comprehensive assessment reveals a significant increase in the expression of GRP94 in GC
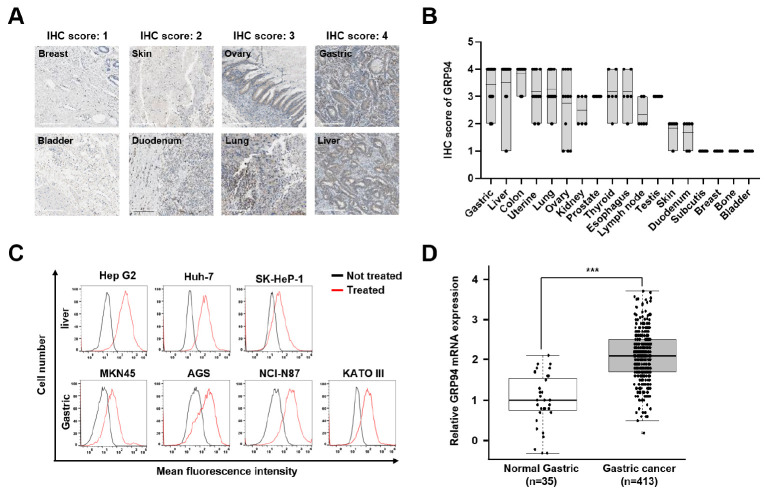
To comprehensively assess GRP94 expression across various cancer types, we analyzed immunohistochemistry (IHC) results and categorized GRP94 expression scores from tumor tissue microarrays (TMAs). Our analysis revealed strong expression (score 4) in CRC, liver cancer (LC), and GC, whereas bone and bladder cancers exhibited minimal expression (score 1) (Fig. 1A, B). These findings are consistent with our previous research, which had already confirmed GRP94 overexpression in CRC tissues compared to normal mucosa (15).
Fig. 1.
Analysis of GRP94 expression levels across different cancer types. (A) IHC staining was employed to assess GRP94 expression in tumor tissues of various cancer types. Representative images were categorized based on the IHC score for each cancer type (200× magnification). (B) The dot plot graph illustrates the conversion of IHC images for each cancer type into IHC scores, which encompass both intensity and the proportion of GRP94 expression levels. (C) Flow cytometry analysis of GRP94 expression in LC (upper) and GC (lower) cell lines. Cells were stained with (red) or without (black) anti-GRP94 polyclonal antibodies. (D) Examination of GRP94 mRNA levels in 413 GC tissues (light gray) and 35 normal gastric tissues (white) samples using Cancer Genome Atlas (TCGA) Stomach Adenocarcinoma (STAD) RNA-seq data (ICGC Database Release 28). Statistical analysis was performed using a two-tailed Student’s t-test. ***P < 0.001.
Building on this foundation, we expanded our investigation to include cell surface GRP94 in GC and LC cells that had the highest IHC scores, excluding CRC. Using flow cytometry with a commercially available anti-GRP94 polyclonal antibody, we confirmed the presence of distinct GRP94 expression in all tested human GC (MKN45, AGS, NCI-N87, KATO III) and LC (Hep G2, Huh-7, SK-Hep-1) cell lines (Fig. 1C).
To elucidate the clinical relevance of GRP94 in GC, we also evaluated GRP94 mRNA expression levels in GC patients. Specifically, this analysis was based on the TCGA gastric adenocarcinoma (STAD) RNA-seq data obtained from the International Cancer Genome Consortium (ICGC) database (release 28). As shown in Fig. 1D, a clear distinction in gene expression was evident between normal gastric tissues and GC tissues, with GRP94 expression being notably higher in cancer tissues. Taken together, these results indicate a close association between GRP94 expression and GC, suggesting its role in GC pathogenesis.
K101.1 promotes internalization and downregulates cell surface GRP94 expression in GC cells
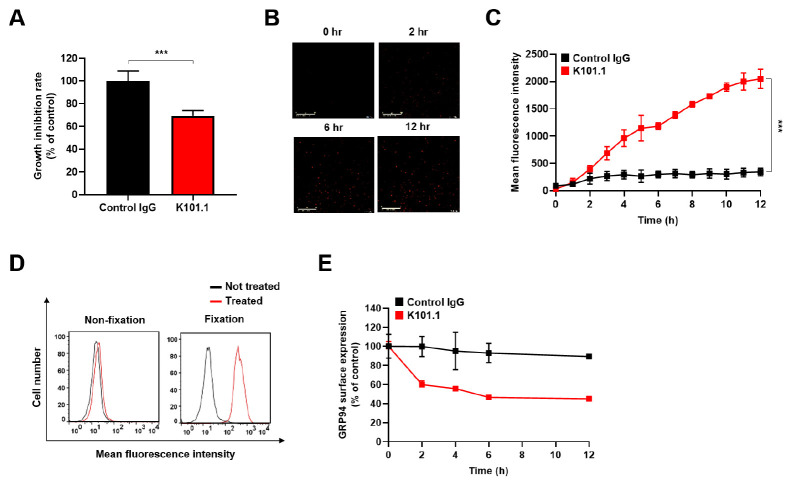
In our previous study, we developed a phage display-derived anti-GRP94 human mAb called K101.1, which specifically inhibits angiogenesis mediated by cell surface GRP94 (15). To assess the specific binding of K101.1 to cell surface GRP94 on GC cells, we performed flow cytometry experiments, where K101.1 was preincubated in the presence or absence of rhGRP94. The results indicated a substantial decrease in K101.1 binding to the cell surface of GC cells when preincubated with rhGRP94, suggesting the specific binding of K101.1 to cell surface GRP94 on GC cells ( Supplementary Fig. 1). To evaluate the impact of K101.1 on GC cell growth in vitro, NCI-N87 cells were cultured in the presence of control immunoglobulin G (IgG) and K101.1, and the cell growth was measured. The results showed a significant reduction in the growth of NCI-N87 cells in the presence of K101.1 (Fig. 2A).
Fig. 2.
Effect of K101.1 and evaluation of K101.1-induced internalization and downregulation of cell surface GRP94 on NCI-N87 cells. (A) NCI-N87 GC cells were seeded into a 96-well culture plate and treated with control IgG (black) and K101.1 (red) for 60 h. Cell viability was assessed by measuring absorbance at 450 nm. (B) Real-time tracking of antibody internalization in NCI-N87 cells treated with FabFluor-pH red-labeled antibodies using the Incucyte SX1 live cell imaging system. Representative images of antibody internalization at 12 h are displayed. Scale bar = 400 μm. (C) Monitoring of the internalization kinetics of FabFluor-pH red-labeled control IgG (black) and K101.1 (red) antibodies over a 12 h period. (D) Flow cytometry was performed to evaluate the downregulation of cell surface GRP94 by K101.1. PFA-fixed or unfixed NCI-N87 cells treated with (red) or without (black) K101.1 for 4 h were stained with fluorescent-labeled anti-GRP94 polyclonal antibody, and flow cytometry analysis was performed. (E) Quantification of the time-dependent decrease in cell surface GRP94 on NCI-N87 cells over time in the presence of control IgG (black) or K101.1 (red) as determined using cell ELISA with an anti-GRP94 polyclonal antibody. Data were subjected to statistical analysis using a two-tailed Student’s t-test. ***P < 0.001.
To understand the mode of action of K101.1 on GC cells, we investigated the internalization process induced by K101.1 in NCI-N87 cells. We treated the cells with FabFluor-conjugated K101.1 to observe its potential mechanism. The internalization of K101.1 was monitored over time, and the results demonstrated that K101.1 facilitated internalization, whereas the control IgG did not exhibit such effects (Fig. 2B, C).
Next, to examine whether K101.1 can modulate the expression of GRP94 on the cell surface, we conducted flow cytometry analysis and compared the levels of GRP94 expression on paraformaldehyde (PFA)-fixed and unfixed NCI-N87 cells, both in the presence and absence of K101.1. Before K101.1 treatment, PFA-fixed NCI-N87 cells were used to monitor cell surface GRP94 under native conditions. Our results clearly demonstrated that K101.1 significantly reduced the levels of cell surface GRP94 on NCI-N87 cells, while exhibiting no impact on PFA-fixed NCI-N87 cells (Fig. 2D).
To further validate the mechanism of action of K101.1 in NCI-N87 cells, we measured GRP94 expression on the cell surface individually after treatment with control IgG or K101.1, using cell enzyme‐linked immunosorbent assay (ELISA) with a commercially available anti-GRP94 polyclonal antibody. The results showed that over time, K101.1 reduced GRP94 expression on the surface of NCI-N87 cells by 44%, whereas control IgG had no such effect (Fig. 2E). Overall, these findings suggest that K101.1 effectively targets cell surface GRP94 in GC cells, promoting the internalization and downregulation of GRP94 expression.
K101.1 effectively inhibits the growth of GC without causing significant in vivo toxicity in a mouse xenograft model
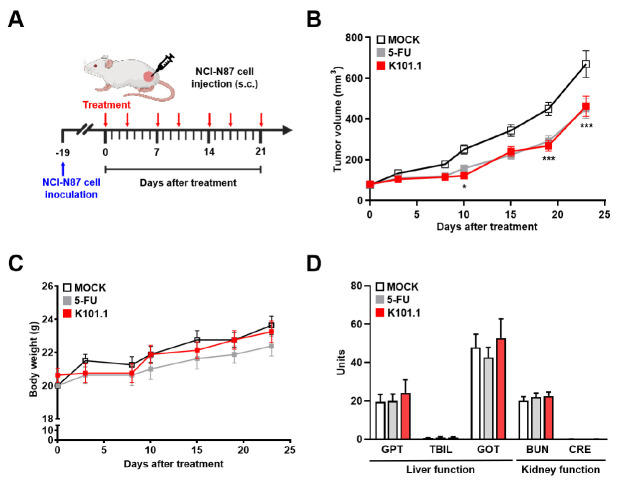
To assess the efficacy of K101.1 in vivo, we used a mouse xenograft model established by subcutaneously injecting NCI-N87 cells. The mice were treated twice a week for three weeks with phosphate-buffered saline (PBS) and 10 mg/kg K101.1 through intravenous injections, as well as with 40 mg/kg 5-FU, which was administered intraperitoneally (Fig. 3A). Here, we confirmed that negative controls, including intravenous injection of control IgG and intraperitoneal injection of PBS and 0.1% DMSO in PBS, had no impact on tumor growth rate in the previously established NCI-N87 xenograft model (data not shown). The results demonstrated a significant reduction in tumor growth upon K101.1 treatment compared to the control group (Fig. 3B), suggesting the potential effectiveness of antibody-based targeting of GRP94 in suppressing GC growth.
Fig. 3.
Efficacy and toxicological evaluation of K101.1 in a mouse xenograft model. (A) Schematic representation of the drug treatment protocols used in NCI-N87 GC xenograft animal models. (B) NCI-N87 tumor xenograft mouse models received either monotherapy with K101.1 (red) or 5-FU (gray). The mice were randomly divided into groups (n = 14) and administered intravenous injections of PBS and 10 mg/kg of K101.1, as well as intraperitoneal injections of 40 mg/kg of 5-FU twice a week for three weeks. Tumor volumes were monitored in the MOCK, 5-FU, and K101.1 treatment groups until day 23. P-values were calculated using two-way ANOVA with Bonferroni’s multiple comparison test. *P < 0.05, ***P < 0.001. (C) Regular recording of the total body weights of mice treated with 5-FU (gray), or K101.1 (red) was conducted twice weekly. (D) Biochemical analyses of GPT, TBIL, GOT, BUN, and CRE concentrations in blood samples collected on day 23 after treatment, providing insight into potential liver and kidney toxicity.
In addition to evaluating the anti-tumor efficacy, we also monitored the potential toxicity of K101.1 in mice by assessing liver and kidney functions and tracking changes in body weight. Throughout the period of drug administration period, we observed no significant changes in body weight. In addition, indicators of liver function, including glutamic pyruvic transaminase (GPT), total bilirubin (TBIL), and glutamic oxaloacetic transaminase (GOT), and kidney function, such as blood urea nitrogen (BUN) and creatinine (CRE), did not show any noticeable toxic effects compared to the controls (Fig. 3C, D). These results suggest that K101.1 does not induce severe toxicity in vivo.
K101.1 suppresses tumor angiogenesis and promotes apoptosis in GC
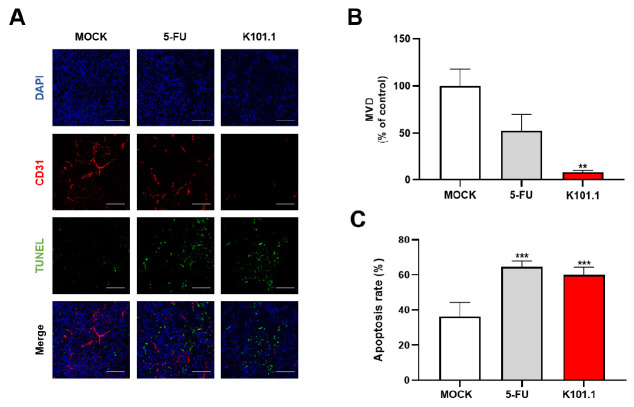
To analyze the effect of K101.1 on tumor angiogenesis and apoptosis in GC, we performed in vivo fluorescence IHC using NCI-N87 GC tissue samples obtained from each treatment group. MVD was quantified using CD31, a reliable endothelial marker. A significant reduction in MVD was observed in the K101.1-treated group compared to the control group (Fig. 4A, B). The 5-FU-treated group served as a positive control. This observation demonstrates that K101.1 strongly inhibits the formation of new blood vessels in GC, which is a crucial process in tumor growth.
Fig. 4.
IHC analysis of microvessel density (MVD) and apoptosis in tumor tissue. (A) MVD and apoptosis in tissues obtained from each group were determined using IHC with anti-CD31 antibody (red) and TUNEL staining (green), respectively. The fluorescent images were acquired using confocal microscopy. DAPI staining (blue) was used to detect nuclear morphology. Scale bar = 100 μm. (B) CD31 positivity per field in 5 samples for each group, expressed as a graph bar. (C) TUNEL positivity per field in 5 samples for each group, expressed as a graph bar. All values represent the mean ± SEM of quadruplicate measurements from two independent experiments. Data were analyzed using a two-tailed Student’s t-test. **P < 0.01, ***P < 0.001. DAPI, 4’,6-diamidino-2-phenylindole, dihydrochloride.
Simultaneously, we observed a significant increase in apoptosis in the K101.1-treated group, which was comparable to the 5-FU-treated group. This effect was confirmed through the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, indicating that K101.1 plays a role in promoting apoptosis in GC tissues (Fig. 4C). Collectively, these results demonstrate that K101.1 effectively inhibits GC growth by suppressing angiogenesis and promoting apoptosis.
DISCUSSION
In the field of cancer research, several studies have demonstrated a significant correlation between GRP94 overexpression and poor prognostic outcomes in various types of cancer, including GC (14-16, 20, 21). Additional investigations have also explored the functional role of GRP94 in tumor progression and metastasis, using diverse modulators such as siRNA and chemical inhibitors (22, 23). However, the specific role of cell surface GRP94 in GCs has not been clearly understood. Therefore, targeting cell surface GRP94 with a mAb could provide valuable insights into its role in GC. In the present study, we propose, for the first time, that cell surface GRP94 may be a potential therapeutic target in GC, and that antibody-based targeting of cell surface GRP94 holds promise as an effective strategy for modulating GRP94-overexpressing GC.
GC is a highly heterogeneous disease associated with high morbidity and mortality rates. The molecular diversity inherent in GC presents a significant obstacle to achieving favorable clinical outcomes through existing therapeutic strategies (10, 11). Therefore, there is an urgent need to identify a precise therapeutic target for a comprehensive understanding of GC pathogenesis and the subsequent development of targeted interventions. In this study, we propose the cell surface GRP94 as a potential therapeutic target in GC. Our hypothesis is supported by multiple lines of evidence. Initially, our IHC analysis of a TMA revealed a significant upregulation of GRP94 protein in GC compared to various other cancer types. Subsequently, to further explore the clinical relevance of GRP94 in GC, we examined mRNA expression levels using data from the ICGC. The analysis revealed higher GRP94 mRNA levels in GC tissues compared to normal gastric tissues. Furthermore, a study by Zheng et al. established a strong correlation between GRP94 expression and key aspects of GC, including onset, growth, invasion, metastasis, and poor prognosis (24). Lastly, our investigation demonstrated that the GRP94-targeting antibody, K101.1, effectively reduced GC growth and angiogenesis without inducing in vivo toxicity.
This investigation builds upon our prior study, which identified the role of K101.1 in inhibiting tumor growth and angiogenesis by targeting cell surface GRP94 in CRC (15). Previously, we utilized phage display technology for biopanning to isolate single-chain variable fragment (scFv) clones specific to rhGRP94 from a human synthetic scFv library. Following DNA sequencing, four scFv clones with distinct complementarity-determining region sequences were converted to IgGs. Among them, we selected one IgG clone, K101.1, which exhibited a high production yield (> 200 mg/L) and strong affinity (approximately 2.12 nM) to rhGRP94. Through several in vitro studies, we found that K101.1 inhibits angiogenesis by inducing rapid internalization and concomitant downregulation of cell surface GRP94 on human umbilical vein endothelial cells (HUVECs). Additionally, in the HCT116 CRC xenograft model, K101.1 suppressed GRP94-mediated tumor growth and angiogenesis in CRC tissues without causing significant toxicity (15). Shifting our focus to GC, which is characterized by significant GRP94 upregulation, we sought to evaluate the therapeutic potential of K101.1. In vitro experiments using NCI-N87 cells treated with K101.1 revealed a significant reduction in cell growth. In our xenograft model using the NCI-N87 GC cell line, the K101.1-treated groups exhibited significant inhibition of tumor growth, comparable to the effects of the conventional chemotherapeutic agent 5-FU. Moreover, we observed a significant reduction in MVD in tumor tissue, indicating K101.1’s ability to inhibit tumor angiogenesis, which is an essential mechanism of tumorigenesis. Additionally, K101.1 induced cell death in tumor tissue, suggesting its potential to suppress cancer growth. These findings suggest that antibody-based targeting of cell surface GRP94 using K101.1 may effectively suppress tumor growth and angiogenesis in GRP94-expressing GCs. This implication broadens the horizons of GRP94-targeted therapies and offers potential for cancer treatments.
In the pursuit of developing effective therapeutic antibodies, the internalization of the specific antibody-antigen complex is a crucial mechanism for therapeutic efficacy (25). The subsequent downregulation and degradation of target molecules on the cell surface through internalization can significantly influence tumor growth by disrupting interactions with various signaling molecules associated with the target molecule (26). Trastuzumab, a well-established internalizing antibody, effectively reduces HER2 expression in breast cancer cells, thereby inhibiting downstream oncogenic pathways (27). The results of our study, including antibody internalization assays and flow cytometry analyses, indicate that the specific binding of K101.1 induces time-dependent internalization of cell surface GRP94 in NCI-N87 human GC cells, leading to the subsequent downregulation of GRP94 expression on the cell surface. Additionally, our flow cytometry and cell-based ELISA analyses confirmed the specific reduction of GRP94 surface expression in GC cells by K101.1. This mode of action suggests that K101.1 may effectively inhibit cell surface GRP94-mediated cell signaling in GC cells, which is closely associated with GC progression and metastasis. Furthermore, we observed a significant reduction in GC cell growth induced by K101.1. These results lead us to speculate that K101.1’s anti-tumor activity may be, at least in part, attributed to its direct inhibition of GC cell growth through internalization and concomitant downregulation of cell surface GRP94 on GC cells. However, we cannot exclude the possibility that this anti-tumor activity may also arise from the impact of GRP94-mediated angiogenesis, achieved through the downregulation of cell surface GRP94 on endothelial cells in GC. This possibility is supported by our previously published findings, which indicate that K101.1 inhibits angiogenesis by inducing internalization and simultaneous downregulation of cell surface GRP94 in HUVECs. The K101.1-induced decrease in cell surface GRP94 expression of HUVECs, which leads to the disruption of tube formation, supports the notion that K101.1 suppresses GRP94-mediated angiogenesis. Moreover, our immunoblot analysis demonstrated that K101.1 did not affect the phosphorylation of protein kinase B (also known as Akt) and extracellular signal-regulated kinase in HUVECs, crucial downstream signaling molecules involved in VEGF-induced angiogenesis. These observations suggest that the antiangiogenic effect of K101.1 is independent of VEGF signaling, but is rather related to the GRP94-mediated pathway. Consequently, K101.1 specifically inhibited HUVECs tube formation in vitro through internalization-dependent downregulation of cell surface GRP94 on HUVECs, reduced MVD, and eventually decreased tumor growth in HCT116 CRC xenograft models (15). Consistent with our previous report, we observed a significant reduction in MVD in the tumor tissue of K101.1-treated NCI-N87 xenograft models. Overall, our findings support the potential of antibody-based targeting of cell surface GRP94 in GC, highlighting the importance of inhibiting cell surface GRP94-mediated signaling pathways involved in tumor angiogenesis and apoptosis.
In conclusion, our study utilizing K101.1, an internalizing antibody that specifically targets cell surface GRP94 in GC cells, provides several lines of evidence to support the role of cell surface GRP94 as a potential therapeutic target in GC for antibody therapy. Furthermore, antibody-based targeting of cell surface GRP94 may effectively inhibit tumor growth and angiogenesis in GC. While our findings represent a significant advancement in GC therapeutics, further studies are needed to confirm these observations. In the near future, we plan to expand our investigations to identify the downstream signaling pathway of GRP94, in which K101.1 is involved in anti-angiogenesis. Additionally, we aim to comprehensively assess the safety and efficacy of K101.1 for the successful treatment of human GC patients.
MATERIALS AND METHODS
Materials and methods are available in the supplemental material.
Funding Statement
ACKNOWLEDGEMENTS The authors declare that financial support was received for the research, authorship, and/or publication of this article from the Bio & Medical Technology Development Program of the National Research Foundation of Korea (grant numbers: NRF-2019M3E5D5065844 and NRF-2020M3A9I2107093) and the Korea Health Technology R&D Project of the Korea Health Industry Development Institute (grant number: HI22C0360) funded by the Korean government. The schematic representations in Fig. 3A were created using BioRender (https://www.biorender.com/).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hong YS, Song SY, Lee SI, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344–1347. doi: 10.1093/annonc/mdh343. [DOI] [PubMed] [Google Scholar]
- 3.Sastre J, Garcia-Saenz JA, Diaz-Rubio E. Chemotherapy for gastric cancer. World J Gastroenterol. 2006;12:204–213. doi: 10.3748/wjg.v12.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Xie D, Chen X, Hu T, Lu S, Han Y. Prognostic value of the site of distant metastasis and surgical interventions in metastatic gastric cancer: a population-based study. Technol Cancer Res Treat. 2020;19:1533033820964131. doi: 10.1177/1533033820964131.c4d0b6ab8c4f4a67bf50ff7473f1e5a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbarali HI, Muchhala KH, Jessup DK, Cheatham S. Chemotherapy induced gastrointestinal toxicities. Adv Cancer Res. 2022;155:131–166. doi: 10.1016/bs.acr.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Reilly M, Mellotte G, Ryan B, O'Connor A. Gastrointestinal side effects of cancer treatments. Ther Adv Chronic Dis. 2020;11:2040622320970354. doi: 10.1177/2040622320970354.0640f8fdff6047a292e543a05c3b85ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 8.Shimodaira Y, Elimova E, Wadhwa R, et al. Ramucirumab for the treatment of gastroesophageal cancers. Expert Opin Orphan Drugs. 2015;3:737–746. doi: 10.1517/21678707.2015.1040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sexton RE, Hallak MNA, Uddin MH, Diab M, Azmi AS. Gastric cancer heterogeneity and clinical outcomes. Technol Cancer Res Treat. 2020;19:1533033820935477. doi: 10.1177/1533033820935477.7084db465f554c94b98de5dd13709236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho SWT, Tan P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019;110:3405–3414. doi: 10.1111/cas.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marzec M, Eletto D, Argon Y. GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Shinogle HE, Galeva NA, Dobrowsky RT, Blagg BSJ. Endoplasmic reticulum-resident heat shock protein 90 (HSP90) isoform glucose-regulated protein 94 (GRP94) regulates cell polarity and cancer cell migration by affecting intracellular transport. JBC. 2016;291:8309–8323. doi: 10.1074/jbc.M115.688374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim K, Lee HW, Lee EH, et al. Differential expression of HSP90 isoforms and their correlations with clinicopathologic factors in patients with colorectal cancer. Int J Clin Exp Pathol. 2019;12:978–986. [PMC free article] [PubMed] [Google Scholar]
- 15.Cho YB, Kim JW, Heo K, et al. An internalizing antibody targeting of cell surface GRP94 effectively suppresses tumor angiogenesis of colorectal cancer. Biomed Pharmacother. 2022;150:113051. doi: 10.1016/j.biopha.2022.113051. [DOI] [PubMed] [Google Scholar]
- 16.Duan XF, Xin YW. Overexpression of molecule GRP94 favors tumor progression in lung adenocarcinoma by interaction with regulatory T cells. Thorac Cancer. 2020;11:704–712. doi: 10.1111/1759-7714.13321.09676ff4d8bb4b4489f4003dc22ca7ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Z, Zhen H, Zou F, Wang X, Chen Y, Liu L. Involvement of the Akt signaling pathway in ER-α36/GRP94-mediated signaling in gastric cancer. Oncol Lett. 2014;8:2077–2080. doi: 10.3892/ol.2014.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel PD, Yan P, Seidler PM, et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–684. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou J, Li X, Li C, et al. Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol Oncol. 2015;9:1312–1323. doi: 10.1016/j.molonc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei PL, Huang CY, Tai CJ, et al. Glucose-regulated protein 94 mediates metastasis by CCT8 and the JNK pathway in hepatocellular carcinoma. Tumor Biol. 2016;37:8219–8227. doi: 10.1007/s13277-015-4669-3. [DOI] [PubMed] [Google Scholar]
- 21.Fu Z, Deng H, Wang X, Yang X, Wang Z, Liu L. Involvement of ER-α36 in the malignant growth of gastric carcinoma cells is associated with GRP94 overexpression. Histopathology. 2013;63:325–333. doi: 10.1111/his.12171. [DOI] [PubMed] [Google Scholar]
- 22.Lin TY, Chang JT, Wang HM, et al. Proteomics of the radioresistant phenotype in head-and-neck cancer: Gp96 as a novel prediction marker and sensitizing target for radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:246–256. doi: 10.1016/j.ijrobp.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Samuni Y, Ishii H, Hyodo F, et al. Reactive oxygen species mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin and its analogs. Free Radic Biol Med. 2010;48:1559–1563. doi: 10.1016/j.freeradbiomed.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng HC, Takahashi H, Li XH, et al. Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol. 2008;39:1042–1049. doi: 10.1016/j.humpath.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 26.Cornel AM, Mimpen IL, Nierkens S. MHC class I downregulation in cancer: underlying mechanisms and potential targets for cancer immunotherapy. Cancers. 2020;12:1760. doi: 10.3390/cancers12071760.f7b564c2c0ad4a92a467d73eb568b00a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng J, Liang M, Carvalho MF, et al. Molecular mechanism of HER2 rapid internalization and redirected trafficking induced by anti-HER2 biparatopic antibody. Antibodies. 2020;9:49. doi: 10.3390/antib9030049.d23b66fb5b1140e39792ceb15857ee05 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.