Abstract
Hyperpolarized (HP) carbon-13 (13C) MRI represents an innovative approach for noninvasive, real-time assessment of dynamic metabolic flux, with potential integration into routine clinical MRI. The use of [1-13C]pyruvate as a probe and its conversion to [1-13C]lactate constitute an extensively explored metabolic pathway. This review comprehensively outlines the establishment of HP 13C-MRI, covering multidisciplinary team collaboration, hardware prerequisites, probe preparation, hyperpolarization techniques, imaging acquisition, and data analysis. This article discusses the clinical applications of HP 13C-MRI across various anatomical domains, including the brain, heart, skeletal muscle, breast, liver, kidney, pancreas, and prostate. Each section highlights the specific applications and findings pertinent to these regions, emphasizing the potential versatility of HP 13C-MRI in diverse clinical contexts. This review serves as a comprehensive update, bridging technical aspects with clinical applications and offering insights into the ongoing advancements in HP 13C-MRI.
Keywords: Hyperpolarized, Carbon 13, Magnetic resonance imaging, Pyruvate, Lactate
INTRODUCTION
In routine clinical MRI, the proton (1H) nucleus serves as the signal source due to its nearly 100% natural abundance and substantial gyromagnetic ratio, rendering it exceptionally responsive to magnetic fields. However, carbon (C), another important nucleus of the human body, has also garnered substantial research interest. Although carbon-12 (12C) has an abundance of 98.9%, its ground-state nuclear spin is zero, rendering it inactive for MRI applications (Supplementary Fig. 1). In contrast, carbon-13 (13C) possesses a ground-state nuclear spin of 1/2, rendering it MRI-active. Nevertheless, 13C possesses only a quarter of the gyromagnetic ratio of 1H and a lower natural abundance of 1.1%. Consequently, to expand the utilization of 13C MRI in clinical scenarios, an essential process known as hyperpolarization is necessary to significantly enhance the signal of 13C.
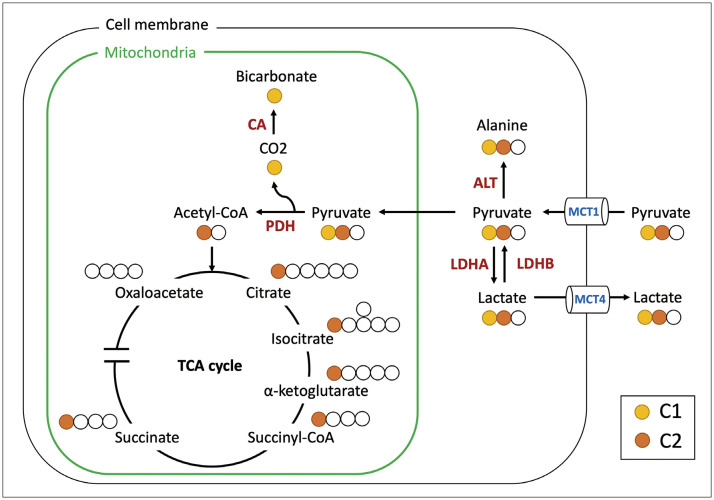
Dynamic nuclear polarization (DNP) is a hyperpolarization technique that has been successfully implemented in clinical human studies. It enhances the polarization of solid-state 13C labeled compounds by subjecting them to extremely low temperatures and high magnetic fields, followed by rapid liquid dissolution (Supplementary Fig. 2) [1]. Hyperpolarized carbon-13 (HP 13C)-MRI has emerged as a promising method for metabolic imaging with clinical potential, significantly boosting the signal-to-noise ratio by up to 50000-fold. This enhancement facilitates the detection of probe signals and downstream metabolites in clinical MRI scanners, illustrating their potential impact in clinical settings. The primary probe frequently used in HP 13C-MRI is [1-13C]pyruvate, in which the 13C label is positioned at the C1 site of the pyruvate molecule. Once transported to cells via the monocarboxylate transporter 1 (MCT1), this compound is converted by lactate dehydrogenase (LDH) into [1-13C]lactate. This metabolic pathway is a valuable indicator of the Warburg effect [2]. Concurrently, [1-13C]pyruvate can follow alternative metabolic routes, such as its conversion into [1-13C]alanine within the cytosol via alanine aminotransferase (ALT), or into 13C-bicarbonate within the mitochondria through pyruvate dehydrogenase (PDH). Notably, the C1 position of [1-13C]pyruvate remains uninvolved in the tricarboxylic acid (TCA) cycle. Consequently, monitoring the TCA flux can only be achieved indirectly by evaluating the pyruvate-to-bicarbonate flux. In contrast, [2-13C]pyruvate, specifically labeled at the C2 position, directly enters the TCA cycle, facilitating the direct observation of TCA intermediates, as illustrated in Figure 1.
Fig. 1. Principal metabolic pathways of [1-13C]pyruvate and [2-13C]pyruvate that were detected by hyperpolarized 13C-MRI. For comparison of the metabolic fates of pyruvate’s C1 and C2, the C1 and C2 13C positions are indicated in yellow and orange, respectively. CA = carbonic anhydrase, PDH = pyruvate dehydrogenase, ALT = alanine transaminase, MCT = monocarboxylate transporter, LDH = lactate dehydrogenase.
HP 13C-MRI provides unique benefits compared to other molecular imaging techniques, such as positron emission tomography (PET) [3]. Unlike PET, which depends on radioactive tracers, HP 13C-MRI employs non-radioactive compounds, thereby enhancing its safety and suitability for repeated imaging studies. Moreover, HP 13C-MRI provides sufficient spatial and temporal resolution, enabling the real-time visualization of metabolic flux in living organs. The acquisition of HP 13C-MRI takes only a few minutes and can be seamlessly integrated into routine clinical MRI scans. In contrast, the most commonly used radiotracer in PET/CT, 2-deoxy-2-[18F]fluoroglucose (FDG), captures only cellular glucose uptake and the initial glycolysis step [4]. This limitation prevents the monitoring of downstream metabolism. HP 13C-MRI, on the other hand, features high spectral resolution, allowing for discrimination of the metabolic fates of [1-13C]pyruvate. This capability enables the monitoring of metabolic pathways beyond the initial step of glycolysis, thereby providing a more comprehensive understanding of metabolism. A comparison between HP 13C-MRI and FDG-PET is shown in Table 1.
Table 1. Comparison between HP 13C-MRI and PET.
| HP 13C-MRI | PET | |
|---|---|---|
| Radiation | No | Yes |
| Procedure time, mins | 2−3 | 30 |
| Multiple tracer | Yes | No |
| Downstream metabolism | Yes | No |
| Cost | Very high | High |
| Clinical applications | Under research | Well established |
HP = hyperpolarized
To effectively implement HP 13C-MRI in clinical settings, specific hardware, pharmaceutical techniques, robust data acquisition approaches, and standardized analyses are essential. This article provides an in-depth review of the technical aspects and potential clinical uses of HP 13C-MRI.
Technical Considerations for Clinical HP 13C-MRI
Multidisciplinary Team for HP 13C-MRI
Initiating a clinical team for HP 13C-MRI necessitates the assembly of a proficient group of MRI physicists, pharmacists, and radiologists. Acquiring a research license for tracers is of paramount importance, particularly when focusing on the development of investigative medicinal products. Similar to the procedures followed for developing new PET tracers, the process of submitting an Investigational New Drug (IND) application for a novel 13C-labeled probe involves providing the FDA with comprehensive documentation, including chemistry, manufacturing, and control procedures; preclinical safety pharmacology and toxicology data; dosimetry data; and a clinical protocol. These submissions lay the groundwork for initiating human trials [5]. For probes that have already received IND approval, for example, [1-13C]pyruvate in the U.S., obtaining authorization for distribution in other countries still necessitates approval from the respective nation’s drug regulatory authorities. This ensures that safety and quality control standards are maintained during probe production at local manufacturing sites. In addition, to foster collaborative efforts, teams should establish a healthcare institution referral pathway and create a seamless network for research and development partnerships. While initial endeavors may revolve around research pursuits, the primary objective is to secure regulatory approval for diagnostic use, underscoring a commitment to compliance and upholding the highest standards of patient care throughout the process.
Clinical Workflow of HP 13C-MRI
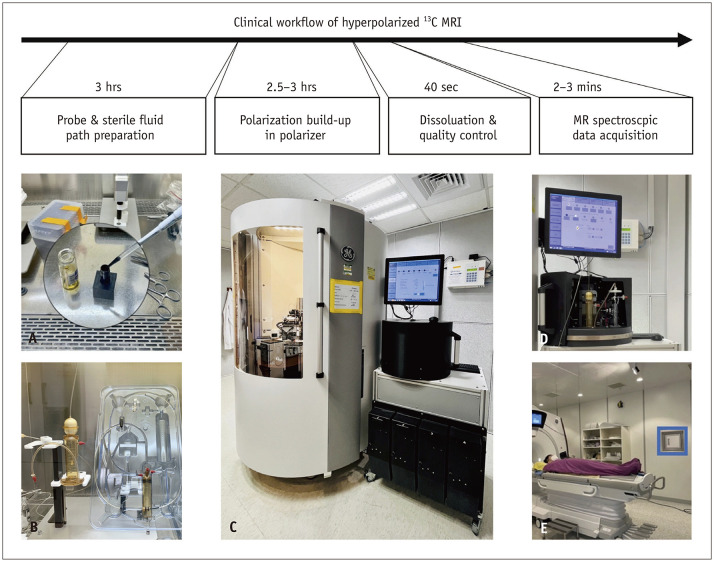
A clinical human HP 13C-MRI study requires specialized equipment, including a clinical polarizer to polarize 13C-labeled probes and a multinuclear MRI scanner equipped with specialized radiofrequency (RF) transmit-receive coils tailored for 13C acquisition [6]. The polarizer is typically positioned near the MRI scanner to reduce the probe transportation time. The clinical workflow of HP 13C-MRI is well established and involves 1) probe preparation, 2) hyperpolarization (DNP), 3) dissolution and quality control, 4) imaging acquisition, and 5) data analysis. The clinical workflow of HP 13C-MRI is summarized in Figure 2.
Fig. 2. Clinical workflow of hyperpolarized 13C-MRI. A: In a controlled, clean room equipped with a laminar flow hood, the drug was prepared by blending a 13C-labeled probe with EPAs and gadolinium. B: The pre-mixed drug was introduced into a sterile fluid path assembly, which included a dissolution syringe (containing sterile water for injection), a sample vial (housing the probe), an EPA filter, a receiver vessel (containing buffer solution), and an administration syringe for injection. C: A clinical polarizer, designed for human research, operates within a high magnetic field and an ultra-low-temperature environment. The buildup of polarization typically takes 2–3 hours to reach a polarization level of 20%–40%. D: Following the initiation of dissolution, superheated and pressurized water dissolved the solid-state sample, and the resulting solution underwent filtration and buffering processes. The solution, comprising the hyperpolarized probe, underwent meticulous quality control measures. E: The hyperpolarized probe was subsequently transported to an MRI scanner for administration in human subjects. The 13C signal was promptly and efficiently acquired using optimized pulse sequences. EPA = electron paramagnetic agent.
Probe Preparation
This process involves mixing good manufacturing practice-grade 13C-labeled probes with electron paramagnetic agents (EPAs) and a minimal quantity of gadolinium-binding contrast agent [7]. The mixture is then loaded onto a commercially available sterile fluid path designed for polarizer use. The preparation must adhere to sterile techniques and be conducted in a sterile clean room.
Hyperpolarization
Except for the initial investigation, the vast majority of clinical applications of HP 13C-MRI in human subjects utilize the SPINlab polarizer (GE Healthcare, Chicago, IL, USA) [6]. In a clinical polarizer, functioning at 0.8 K and 5T, the mixture exists in a solid state. Owing to the EPAs’ higher susceptibility to the magnetic field, an initial polarization build-up occurs within the EPAs. Subsequently, the polarization of the EPAs’ unpaired electrons is transferred to nearby 13C nuclei via microwave irradiation at a specific frequency [8]. This DNP process, taking approximately 2–3 hours, results in a 20%–40% polarization level, leading to a signal amplification exceeding four orders of magnitude [1].
Dissolution and Quality Control
After the dissolution is initiated, pressurized and superheated sterile water is used to rapidly dissolve the solid-state probe. The resulting solution is passed through a filter to remove the EPAs and neutralized with a buffer solution. Finally, the solution is passed through a sterile filter to remove potential pathogens. Before administration to the patient, the final solution undergoes rigorous quality control checks, including evaluation of temperature (25℃–37℃), pH (5–9), EPA concentrations (≤ 5 µM), pyruvate concentrations (200–280 mM), and polarization levels (≥ 10%). In addition, the integrity of the sterile filter is verified using a bubble point test, ensuring that it exceeds 50 pounds per square inch (PSI) [9]. These measures ensure that the imaging probe is safe, effective, and of the highest quality for clinical applications. Notably, the utilization of deuterium oxide (D2O) as a solvent for [1-13C]pyruvate has recently been demonstrated to be a safe and practical approach for maintaining polarization and enhancing the signal-to-noise ratio [10].
Imaging Acquisition
Prior to gathering data, it is essential to calibrate the 13C central frequency, fine-tune the transmit gain, and optimize the magnetic field through shimming [11,12]. After dissolution, the hyperpolarized state becomes transient and returns to its thermal equilibrium state after leaving the polarizer. This transition creates a limited time window of 2–3 min for signal acquisition, depending on the spin-lattice relaxation time (T1). To maximize this brief window, flip angles usually range from 10° to 30° to manage the nonrecoverable hyperpolarized magnetization. Using small flip angles helps preserve the magnetization for longer experiments, albeit at the cost of signal strength and resolution [13].
The following MRI sequences are commonly used in HP 13C-MRI. Fast spectroscopic imaging methods, such as echo planar spectroscopic imaging (EPSI), achieve a speed advantage with multi-echo readouts; however, they face compromises in spectral bandwidth, spatial resolution, and signal-to-noise ratio efficiency [14,15,16,17]. In contrast, model-based imaging, such as Iterative Decomposition of water and fat with Echo Asymmetry and Least-square estimation (IDEAL) chemical shift imaging, accelerates spectral encoding and reduces scan times when metabolite chemical shifts and the B0 field map are known. However, this method carries the risk of noise amplification and is vulnerable to motion and frequency shifts [18]. Another option, metabolite-specific imaging, is an extremely fast technique that uses spectral-spatial (SPSP) RF pulses to excite a single metabolite, followed by a rapid imaging readout, such as echoplanar or spiral [19,20]. The acquisition then shifts the center frequency to the next metabolite and cycles through the resonances of each metabolite of interest. The use of variable flip-angle schemes can be used to enhance the signal-to-noise ratio of the metabolic products because each metabolite is excited separately [21]. The advantages and challenges of these sequences are summarized in Table 2.
Table 2. Comparison of pulse sequences in hyperpolarized 13C-MRI.
| Sequence | Advantages | Challenges |
|---|---|---|
| Fast spectroscopic imaging | Faster by employing multi-echo readouts; still providing continuous spectral coverage; robust for frequency shifts | Limited spectral bandwidth, spatial resolution, and SNR efficiency; relatively slow because of the requirement for phase encoding; susceptible to motion |
| Model-based imaging | More efficient phase encoding because only relative metabolite chemical shift required | Requires prior knowledge of both metabolite chemical shifts and B0 field map; lower temporal resolution compared to metabolite-specific imaging; susceptible to both motion and frequency shifts |
| Metabolite-specific imaging | Fastest acquisition method for single metabolite; robust to motion | Requires spectra with sparce and well separated peaks; easy for variable flip angle schemes; susceptible to frequency shifts |
SNR = signal-to-noise ratio
Data Analysis
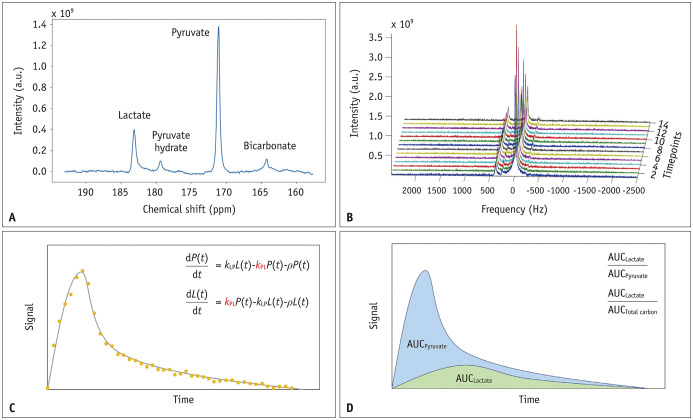
In the quantification of dynamic 13C spectral data acquired through HP 13C-MRI, two prevalent methodologies are often employed: kinetic modeling and model-free metrics (Fig. 3). Kinetic modeling entails the development of a mathematical model that delineates the rates at which 13C-labeled probes undergo conversion into products, accounting for factors such as relaxation and RF pulse effects. For example, for [1-13C]pyruvate, the pyruvate-to-lactate conversion rate constant, commonly denoted as kPL, is frequently used to characterize glycolytic activity [22]. Beyond the two-site exchange model, more intricate models have been devised to accommodate additional factors, such as vascular perfusion and cellular uptake, rendering them more physiologically realistic. However, because of the increased number of parameters, these models can become more susceptible to instability during model fitting [23].
Fig. 3. Representative data and key quantitative methods for pyruvate-to-lactate conversion in hyperpolarized 13C-MRI. A: A representative single timepoint 13C spectrum was dominated by the resonance from [1-13C]pyruvate (171 ppm), [1-13C]lactate (183 ppm), [1-13C]pyruvate hydrate (179 ppm), and [13C]bicarbonate (164 ppm). B: Stacked plot of dynamic 13C spectra with a temporal resolution of 2 s. C: Kinetic modeling involves creating a mathematical model to describe the conversion rates of 13C-labeled probes into various products. The conversion rate constant, often denoted as kPL, serves as a key parameter for characterizing the speed of the pyruvate-to-lactate flux. D: Metabolite ratios, often in the form of AUC ratios, employ the AUC of the product as the numerator and either the substrate or the total 13C signal as the denominator, offering an alternative approach to evaluate the pyruvate-to-lactate flux. AUC = area under the curve.
Model-free metrics have gained traction as viable alternatives to kinetic models. Among these metrics, metabolite ratios have emerged as the predominant choice, in which the substrate or total 13C signal is used as the denominator. In the context of dynamic 13C spectra, the area under the curve (AUC) is a fundamental tool for calculating ratios [24]. These ratios provide a straightforward approach for assessing metabolic conversion and possess inherent normalization, rendering them highly resilient to fluctuations in polarization levels, RF coil sensitivities, and perfusion. However, it is important to note that these AUC ratios may require correction in instances where variable flip angles are employed during the acquisition process.
Clinical Applications
The first human HP 13C-MRI using [1-13C]pyruvate was a significant milestone in medical imaging and cancer research. This study, conducted in 2013, involved patients with prostate cancer and demonstrated the feasibility and safety of this technique. Subsequently, this approach has been applied to various organs and diseases. Table 3 summarizes the clinical applications of HP 13C-MRI, and Supplementary Table 1 contains the in-depth quantitative results from human studies [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56].
Table 3. Summary of clinical applications from the human studies using hyperpolarized 13C-MRI.
| Authors | N | Targets | Clinical applications | |
|---|---|---|---|---|
| Brain | ||||
| Miloushev et al., 2018 [25] | 4 | Glioma and metastasis | 1st human brain tumor study | |
| Autry et al., 2020 [26] | 8 | Infiltrative glioma | Post radio-chemotherapy follow-up | |
| Autry et al., 2021 [27] | 10 | Pediatric brain tumor | Tumor vs. normal brain, safety profile | |
| Lee et al., 2021 [28] | 11 | Intracranial metastasis | Prediction of radiotherapy response | |
| Chen et al., 2021 [29] | 3 | Glioblastoma | Tumor vs. normal brain | |
| Zaccagna et al., 2022 [30] | 8 | Glioblastoma | Tumor vs. normal brain | |
| Grist et al., 2019 [31] | 4 | Normal brain | Technical feasibility in human brain | |
| Lee et al., 2020 [32] | 14 | Normal brain | Metabolite topography in human brain | |
| Hackett et al., 2020 [33] | 2 | Brain trauma | Traumatic brain injury | |
| Ma et al., 2022 [34] | 4 | Normal brain | Technical feasibility in human brain | |
| Uthayakumar et al., 2023 [35] | 35 | Brain aging | Metabolic changes in brain aging | |
| Heart and skeletal muscle | ||||
| Cunningham et al., 2016 [36] | 4 | Heart | 1st human healthy heart study | |
| Rider et al., 2020 [37] | 10 | Heart | Healthy heart vs. diabetic heart | |
| Park et al., 2020 [38] | 9 | Heart | Cardiotoxicity of doxorubicin | |
| Ma et al., 2022 [39] | 3 | Heart | End diastolic vs. end systolic | |
| Park et al., 2021 [40] | 9 | Calf skeletal muscle | Rest vs. exercise vs. recovery | |
| Body and oncology | ||||
| Woitek et al., 2020 [41] | 1 | Breast cancer | Post neoadjuvant chemotherapy follow-up | |
| Woitek et al., 2021 [42] | 7 | Breast cancer | Very early neoadjuvant chemotherapy response | |
| Tran et al., 2019 [43] | 1 | Renal cell carcinoma | Intratumoral metabolic heterogeneity | |
| Tang et al., 2021 [44] | 11 | Renal cell carcinoma | Tumor grade and histopathologic type | |
| Ursprung et al., 2022 [45] | 9 | Renal cell carcinoma | Prediction of tumor grade | |
| Lee et al., 2022 [46] | 13 | Liver, kidney, pancreas, spleen | Normal intrabdominal solid organs | |
| Stødkilde-Jørgensen et al., 2020 [47] | 2 | Pancreatic cancer | Tumor vs. normal | |
| Gordon et al., 2023 [48] | 6 | Pancreatic cancer | Tumor vs. normal, early response prediction | |
| Nelson et al., 2013 [49] | 31 | Prostate cancer | 1st human study, safety profile, tumor vs. normal | |
| Aggarwal et al., 2017 [50] | 1 | Prostate cancer | Early response for androgen deprivation therapy | |
| Chen et al., 2020 [51] | 6 | Prostate cancer, metastatic castration-resistant | Feasibility in bone and liver metastasis | |
| Granlund et al., 2020 [52] | 12 | Prostate cancer | Tumor grade of prostate cancer | |
| de Kouchkovsky et al., 2021 [53] | 1 | Prostate cancer, metastatic castration-resistant | Early immunotherapy response assessment | |
| Chen et al., 2022 [54] | 5 | Prostate cancer | Integration with MR/TRUS fusion-guided biopsy | |
| Sushentsev et al., 2022 [55] | 10 | Prostate cancer, intermediate risk | Metabolic phenotyping in intermediate risk prostate cancer | |
| Lin et al., 2024 [56] | 6 | Cervical cancer | Immune activation of spleen | |
N = number, TRUS = transrectal ultrasound
Brain
Tailored imaging and treatment strategies are essential for brain tumors because of their diverse behaviors, emphasizing the need for ongoing research and innovative approaches, such as HP 13C-MRI. Miloushev et al. [25] conducted the first study on the human brain, discovering non-uniform lactate production from pyruvate, with increased levels observed in the cortical and subcortical regions. Additionally, they noted variability in glycolytic activity across various gliomas and brain metastases. Meanwhile, Autry et al. [26] conducted a pivotal study of HP 13C-MRI in brain tumors involving five patients with glioma and three healthy volunteers. Their findings affirmed HP 13C-MRI’s reliability in providing data on pyruvate conversion to lactate and bicarbonate within the brain tissues. Notably, treatment with bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, increased pyruvate conversion rates, which was attributed to its impact on the blood-brain barrier.
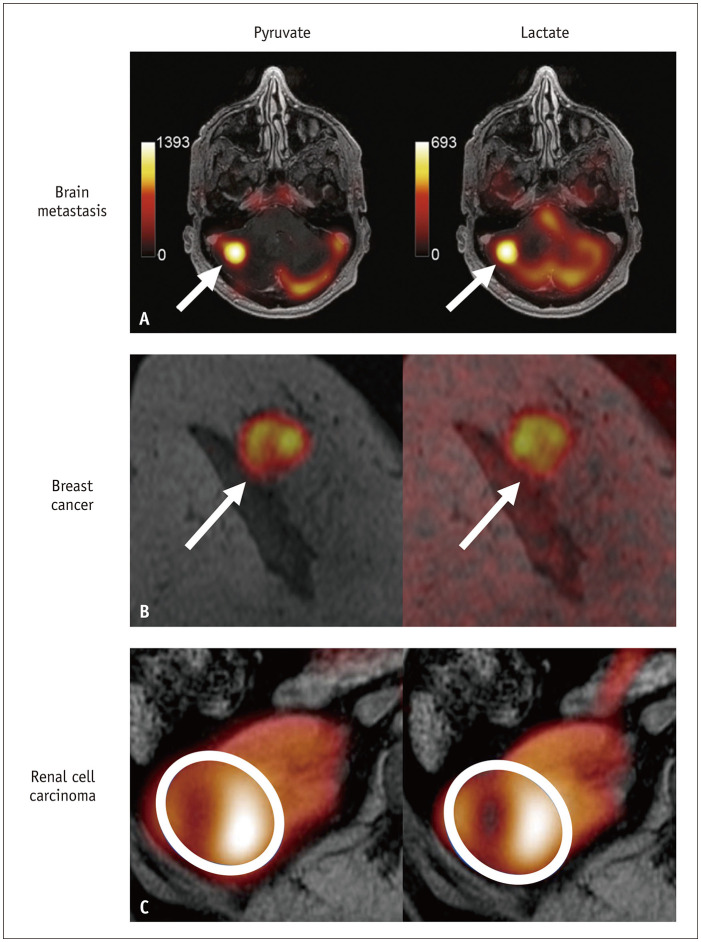
Gliomas with isocitrate dehydrogenase (IDH) mutations have a favorable prognosis. In IDH-mutant glioblastomas, higher pyruvate-to-lactate conversion rates were observed, with the contrast-enhancing region showing a higher conversion rate than the T2 hyperintense region [26,30]. Conversely, IDH wild-type glioblastomas show lower pyruvate-to-lactate conversion [29]. Nevertheless, multiple studies have consistently reported reduced bicarbonate production in brain tumors compared to normal brain tissue [26,29,30]. These studies indicate that HP 13C-MRI has the potential to evaluate intertumoral heterogeneity in tumor metabolism. Furthermore, the application of 13C-MRI extends beyond glioblastomas to pediatric brain tumors, including diffuse intrinsic pontine gliomas, demonstrating the safe and efficient use of this novel technique in pediatric patients [27]. Additionally, Lee et al. [28] studied intracranial brain metastases and used pre-treatment HP 13C-MRI lactate signals to predict tumor progression after stereotactic radiosurgery, achieving a positive predictive value of 0.8 (Fig. 4A).
Fig. 4. Examples of clinical applications of hyperpolarized 13C-MRI, where pyruvate and lactate maps are overlayed with anatomic 1H-MRI. A: Right cerebellar metastasis from renal cell carcinoma (arrows) (Adapted from Lee et al. J Neurooncol 2021;152:551-557 [28], with permission of CC BY 4.0 license). B: Right breast cancer (arrows) (Adapted from Woitek et al. Radiol Imaging Cancer 2020;2:e200017 [41], with permission of Radiological Society of North America). C: Renal cell carcinoma in the right kidney (Adapted from Ursprung et al. Cancers (Basel) 2022;14:335 [45], with permission of CC BY 4.0 license).
The blood–brain barrier’s permeability to pyruvate has been confirmed by the detection of both lactate and bicarbonate signals in the human brain following intravenous [1-13C]pyruvate injection, with metabolite signals being more prominent in the cortex than in the white matter [31,32,34]. An investigation demonstrated consistently elevated lactate and bicarbonate signals in specific cerebral regions, such as the precuneus, cuneus, and lingual gyrus, which were segmented according to the Human Brain Atlas. This highlights HP 13C-MRI’s capability to assess spatial metabolic variations in the human brain, suggesting its potential as an alternative to traditional functional brain MRI [32]. In patients with mild traumatic brain injury, focal changes at the injured site revealed decreased bicarbonate levels and increased lactate production without apparent anatomical alterations [33]. Uthayakumar et al. [35] employed HP 13C-MRI to measure the whole-brain metabolism in individuals of various ages. Their findings showed a 7% decrease in lactate and a 9% decrease in bicarbonate levels per decade, with distinct spatial variations in the rates of change among different brain regions.
Heart and Skeletal Muscle
Cunningham et al. [36] performed the first human heart HP 13C-MRI utilizing the electrocardiogram (EKG)-gated fast metabolic imaging technique (SPSP) in healthy volunteers. They observed the downstream metabolism of pyruvate with lactate in both chambers and the myocardium, and bicarbonate only in the myocardium. This finding highlights the significance of bicarbonate in the assessment of cardiac metabolism. Meanwhile, in a study comparing heart metabolism in healthy volunteers and patients with type 2 diabetes mellitus (DM), a significant reduction in pyruvate-to-bicarbonate flux was observed in the hearts of patients with type 2 DM, and an oral glucose challenge before HP 13C-MRI was found to enhance bicarbonate production [37]. Similarly, EKG gating’s importance in imaging heart metabolism was emphasized in a dual-phase acquisition study at the end systolic and diastolic phases, with the bicarbonate-to-lactate ratio being significantly smaller at end diastolic phase, influenced by coronary flow and cyclical metabolic changes [39]. Transitioning to monitoring tumor metabolism, HP 13C-MRI can assess cancer treatment-related adverse events, as observed in a study by Park et al. [38] investigating doxorubicin-induced cardiotoxicity in breast cancer patients. They observed a decrease in the bicarbonate signal after doxorubicin treatment, indicating its potential to improve patient care. Furthermore, in another study by Park et al. [40], the impact of exercise on pyruvate metabolism in human skeletal muscles was investigated. They found that in the imaged calf muscles, bicarbonate production increased 8.4-fold after exercise and returned to baseline after resting, whereas lactate continued to rise, increasing by 3.3-fold compared to baseline, even after resting.
Breast
Gallagher et al. [57] demonstrated HP 13C-MRI’s potential in breast cancer grading by analyzing lactate-to-pyruvate ratios (Lac/Pyr), with all grade 3 tumors showing lactate signals, but not grade 2 tumors. In subsequent studies aimed at the early prediction of treatment response, Lac/Pyr was found to increase 7–11 days after neoadjuvant chemotherapy, and a 20% threshold increase in Lac/Pyr effectively differentiated responders from non-responders (Fig. 4B) [41,42]. These studies underline HP 13C-MRI’s potential to enhance treatment response prediction in breast cancer.
Liver, Kidney, and Pancreas
Conducting HP 13C-MRI in the liver presents a challenge because of the dual perfusion system of the portal vein and hepatic artery. Peak perfusion of the liver occurs during the portovenous phase, typically 70–90 s after administration of the contrast medium. Unfortunately, this time limit is suboptimal for achieving the most advantageous acquisition window for the HP 13C signals. In addition, on HP 13C-MRI of the abdomen, it was evident that the liver exhibited a lower metabolite signal than the kidney, pancreas, and spleen. Nevertheless, the liver is a highly metabolic organ, displaying the highest rates of pyruvate-to-lactate and pyruvate-to-alanine conversion among intra-abdominal solid organs [46]. The use of HP 13C-MRI to study liver parenchymal diseases, including hepatic steatosis, fibrosis, and malignancies, is presently undergoing thorough investigation [58].
Studies have suggested that HP 13C-MRI can provide valuable metabolic information for diagnosing and grading renal tumors. Tran et al. [43] demonstrated intratumoral metabolic heterogeneity in human renal cell carcinoma (RCC). Similarly, Tang et al. [44] used HP 13C-MRI to assess the lactate-to-pyruvate ratio in various RCC subtypes, with chromophobe RCC having the highest ratio, followed by grade 3 clear-cell RCC and grade 2 clear-cell RCC. Additionally, Ursprung et al. [45] confirmed the correlation between increased kPL values and higher tumor grade in RCC (Fig. 4C) and noted that renal liposarcomas and pheochromocytomas had metabolic activity similar to that of grade 4 RCC, while benign oncocytomas had lower kPL values.
Despite the technical challenge of the decreased signal-to-noise ratio of the pancreas due to its central location in the abdomen, Stødkilde-Jørgensen et al. [47] successfully acquired metabolic signals from pancreatic cancers in two patients. The alanine signal primarily originated from the tumor and the lactate signal was distributed in both cancerous and normal pancreases. Subsequently, Gordon et al. [48] demonstrated comparable findings, revealing lower alanine-to-lactate ratios in cancerous pancreatic tissues than in healthy pancreatic tissues. Additionally, initial metabolic responses observed four weeks after post-chemotherapy were found to correlate with later changes in tumor size.
Prostate
Prostate cancer, the second most prevalent cancer among men worldwide, presents diagnostic and treatment complexities primarily owing to its varying clinical manifestations, ranging from indolent to highly aggressive tumors with the potential to metastasize. Nelson et al. [49] performed the first-in-human study of 31 patients with prostate cancer using [1-13C]pyruvate and demonstrated the safety and potential of characterizing tumor metabolism. They observed increased pyruvate-to-lactate conversion in cancerous areas, encouraging further HP 13C-MRI clinical trials. Subsequently, Chen et al. [51] extended the use of HP 13C-MRI to assess bone and liver metastases in prostate cancer. They found a higher pyruvate-to-lactate conversion in metastatic tumors than in primary tumors, indicating a higher tumor grade. Additionally, chemotherapy led to a reduction in pyruvate-to-lactate conversion in treated liver metastases.
Early response assessment is crucial in cancer care and in guiding treatment adjustments to enhance patient outcomes and survival. Aggarwal et al. [50] showed a substantial reduction in pyruvate-to-lactate flux in prostate cancer after anti-androgen therapy despite limited tumor size changes on T2-weighted images and modest changes in apparent diffusion coefficient images. HP 13C-MRI offers promise for early metabolic assessment and prediction of treatment responses. Assessing the treatment response in bone metastases using traditional imaging is challenging because of bone sclerosis and unreliable size measurements. A previous study demonstrated kPL reduction in bone metastases after 8 weeks of immune checkpoint inhibitor therapy, preceding observable size changes [53]. This study also highlighted the value of HP 13C-MRI for early response assessment.
Prostate cancer varies from low-risk indolent tumors to aggressive malignancies, and intratumoral heterogeneity challenges the accuracy of biopsies. HP 13C-MRI exhibits promise in assessing this complexity. Granlund et al. [52] showed that lactate levels increase with the Gleason tumor grade in prostate cancer, a process driven by MCT1 and associated with PTEN loss. Similarly, Sushentsev et al. [55] studied patients with intermediate-risk prostate cancer (Gleason 7; PSA < 20 ng/mL). They found a positive correlation between lactate signal-to-noise ratio and Gleason pattern 4, suggesting HP 13C-MRI’s value in distinguishing significant from indolent prostate cancers (Fig. 5A). Transitioning to alternative approaches, magnetic resonance imaging (MRI)-guided transrectal ultrasound (TRUS) biopsy combines MRI and TRUS guidance to obtain prostate tissue samples for cancer detection and treatment planning. Nevertheless, HP 13C-MRI is an emerging technique for the detection of hidden prostate cancer. Notably, Chen et al. [54] integrated HP 13C-MRI and conventional MRI to guide TRUS biopsy, achieving success in five patients using a kPL value above 0.02 as a cancer marker. As a result, half of the analyzed 13C-positive biopsy cores were found to be cancerous. This approach enhances the detection accuracy of MRI-guided TRUS biopsies.
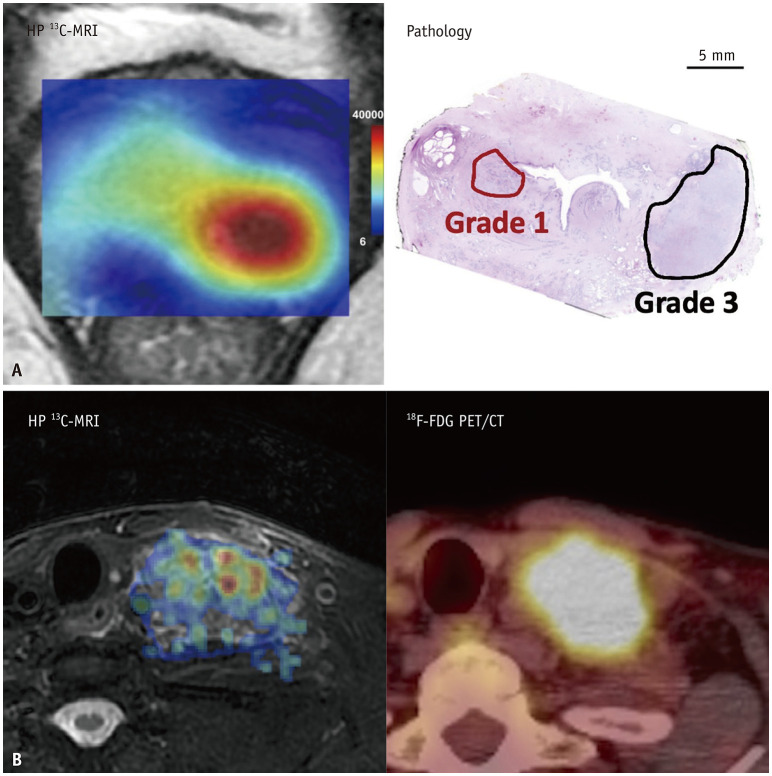
Fig. 5. Aligning HP 13C-MRI with established diagnostic procedures. A: The lactate signal-to-noise ratio map in the HP 13C-MRI (left panel) correlates to the histopathology analysis (right panel) and distinctly showcases a stronger lactate signal in the grade 3 prostate cancer located in the left peripheral zone, as opposed to the weaker signal observed in the grade 1 prostate cancer in the right transition zone, which illustrates of the correlation of the Warburg effect and the tumor grade (Adapted from Sushentsev et al. Nat Commun 2022;13:466 [55], with permission of CC BY 4.0 license). B: Comparative analysis of HP 13C-MRI and 18F-FDG PET/CT in assessing the left neck metastatic lymph node (level IV) from a patient with head and neck cancer. It shows a [1-13C]pyruvate map superimposed on a fat-suppressed T2-weighted image (right panel), alongside an 18F-FDG map overlaid on a CT scan (left panel). HP = hyperpolarized, FDG = 2-deoxy-2-[18F]fluoroglucose.
Future Directions of HP 13C-MRI
Beyond pyruvate, a diverse array of innovative agents has either received clinical approval or are currently considered to have clinical potential, as summarized in Table 4 [59,60,61,62,63,64,65,66,67,68]. For instance, the metabolic activity of the TCA cycle in healthy human brains was investigated by analyzing the conversion of [2-13C]pyruvate to [5-13C]glutamate [61]. Additionally, [13C,15N2]urea administered without subsequent metabolism has been identified as a valuable tool for assessing tissue perfusion dynamics [62,63]. The IDH mutation, a critical biomarker in glioblastoma, is specifically interrogated using [1-13C]α-ketoglutarate, which transforms into [1-13C]2-hydroxyglutarate [64]. Moreover, intratumoral pH is a crucial indicator of tumor aggressiveness, and [1-13C]1,2-glycerol carbonate serves as a non-invasive pH imaging probe [65,66]. Furthermore, [2-13C]dihydroxyacetone was designed to evaluate hepatic glycolysis, gluconeogenesis, and glycerol synthesis [67], whereas [1,4-13C2]fumarate has been used to probe necrosis [68].
Table 4. 13C-labeled probes and their potential applications.
| Probes | Potential clinical applications |
|---|---|
| [1-13C]pyruvate* | Glycolysis |
| [2-13C]pyruvate* [59,60,61] | Glycolysis, TCA cycle |
| [13C, 15N2]urea* [62,63] | Perfusion |
| [1-13C]α-ketoglutarate* [64] | TCA cycle, IDH |
| [1-13C]1,2-glycerol carbonate* [65] | pH imaging |
| [13C]bicarbonate [66] | pH imaging |
| [2-13C]dihydroxyacetone [67] | Hepatic glycolysis, gluconeogenesis, and glycerol synthesis |
| [1,4-13C2]fumarate [68] | Necrosis |
*Indicate probes that have been clinically validated for human use.
TCA = tricarboxylic acid cycle, IDH = isocitrate dehydrogenase
Introducing copolarization as an innovative technique allows the simultaneous investigation of multiple metabolic pathways in a single HP 13C-MRI. For instance, the copolarization of [1-13C]pyruvate and [13C,15N2]urea provides comprehensive data on both pyruvate metabolism and tumor perfusion [62,63]. Overall, as research progresses, the clinical utilization of HP 13C-MRI is expanding, as exemplified by the current exploration of HP 13C-MRI for evaluating the immune potential in the spleen [56] or neck lymph nodes (Fig. 5B).
CONCLUSIONS
In summary, HP 13C-MRI is an emerging technique with significant potential in the field of radiology. With its ability to monitor real-time metabolic changes, this imaging method can revolutionize disease diagnosis and treatment. Although still in its early developmental phases, initial studies have indicated its capacity to differentiate between benign and malignant tumors, monitor treatment responses, and potentially forecast patient outcomes. The ongoing evolution and increasing accessibility of this technology suggest a growing role for HP 13C-MRI in the diagnosis, treatment, and care of patients with cancer. However, further research is required to optimize its functionality and validate its clinical usefulness. Despite the need for refinement, the prospective advantages are evident, indicating a promising future for this technology.
Acknowledgments
The authors acknowledge the helps from Chun-Yu Su, Yu-Ying Yu, Rainie Liu, Hsin-Ju Chiang, Dr. Lan-Yan Yang, Dr. Yu-Chun Lin, Dr. Kung-Chu Ho, Dr. Rolf F Schulte, Dr. Chien-Yuan Eddy Lin, and GE Healthcare kindly provides investigational sequences in multi-nuclear spectroscopy (MNS) research package.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Ying-Chieh Lai, Ching-Yi Hsieh, Kuan-Ying Lu, Gigin Lin.
- Data curation: all authors.
- Formal analysis: Ying-Chieh Lai, Ching-Yi Hsieh, Kuan-Ying Lu, Gigin Lin.
- Funding acquisition: Ying-Chieh Lai, Ching-Yi Hsieh, Yu-Hsiang Juan, Shu-Hang Ng, Yung-Liang Wan, Gigin Lin.
- Investigation: Ying-Chieh Lai, Ching-Yi Hsieh, Gigin Lin.
- Methodology: Ying-Chieh Lai, Ching-Yi Hsieh, Gigin Lin.
- Project administration: Kuan-Ying Lu, Hsien-Ju Lee.
- Resources: Gigin Lin.
- Software: Ying-Chieh Lai, Ching-Yi Hsieh.
- Supervision: Gigin Lin.
- Validation: Ying-Chieh Lai, Ching-Yi Hsieh.
- Visualization: Ying-Chieh Lai, Ching-Yi Hsieh.
- Writing—original draft: Ying-Chieh Lai, Gigin Lin.
- Writing—review & editing: all authors.
Funding Statement: This study was funded by National Science and Technology Council, Taiwan (MOST 109-2628-B-182A-007-, MOST 110-2628-B-182A-018-, MOST 111-2628-B-182A-012-, MOST 111-2314-B-182A-042-, MOST 112-2314-B-182A-015-, NSTC 110-2314-B-182A-062-, NSTC 111-2314-B-182A-154- and NSTC 112-2314-B-182A-127-MY3) and Chang Gung Medical Foundation (CLRPG3K0024 and CMRPG3M0732).
Availability of Data and Material
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2024.0069.
References
- 1.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007;104:19773–19777. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher FA, Bohndiek SE, Kettunen MI, Lewis DY, Soloviev D, Brindle KM. Hyperpolarized 13C MRI and PET: in vivo tumor biochemistry. J Nucl Med. 2011;52:1333–1336. doi: 10.2967/jnumed.110.085258. [DOI] [PubMed] [Google Scholar]
- 4.Pantel AR, Ackerman D, Lee SC, Mankoff DA, Gade TP. Imaging cancer metabolism: underlying biology and emerging strategies. J Nucl Med. 2018;59:1340–1349. doi: 10.2967/jnumed.117.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosessian S, Duarte-Vogel SM, Stout DB, Roos KP, Lawson GW, Jordan MC, et al. INDs for PET molecular imaging probes-approach by an academic institution. Mol Imaging Biol. 2014;16:441–448. doi: 10.1007/s11307-014-0735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardenkjaer-Larsen JH, Leach AM, Clarke N, Urbahn J, Anderson D, Skloss TW. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 2011;24:927–932. doi: 10.1002/nbm.1682. [DOI] [PubMed] [Google Scholar]
- 7.Hu KN. Polarizing agents and mechanisms for high-field dynamic nuclear polarization of frozen dielectric solids. Solid State Nucl Magn Reson. 2011;40:31–41. doi: 10.1016/j.ssnmr.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovav Y, Feintuch A, Vega S. Theoretical aspects of dynamic nuclear polarization in the solid state.spin temperature and thermal mixing. Phys Chem Chem Phys. 2013;15:188–203. doi: 10.1039/c2cp42897k. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Hyperpolarized pyruvate 13C injection investigator’s brochure. [accessed on February 14, 2024]. Available at: https://imaging.cancer.gov/programs_resources/cancer-tracer-synthesis-resources/docs/c13_pyruvate_IB_PDF.pdf .
- 10.Deh K, Zhang G, Park AH, Cunningham CH, Bragagnolo ND, Lyashchenko S, et al. First in-human evaluation of [1-13C] pyruvate in D2O for hyperpolarized MRI of the brain: a safety and feasibility study. Magn Reson Med. 2024;91:2559–2567. doi: 10.1002/mrm.30002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacolick LI, Sun L, Vogel MW, Dixon WT, Hancu I. Fast radiofrequency flip angle calibration by Bloch-Siegert shift. Magn Reson Med. 2011;66:1333–1338. doi: 10.1002/mrm.22902. [DOI] [PubMed] [Google Scholar]
- 12.Schulte RF, Sacolick L, Deppe MH, Janich MA, Schwaiger M, Wild JM, et al. Transmit gain calibration for nonproton MR using the Bloch-Siegert shift. NMR Biomed. 2011;24:1068–1072. doi: 10.1002/nbm.1657. [DOI] [PubMed] [Google Scholar]
- 13.Larson PEZ, Gordon JW. Hyperpolarized metabolic MRI—acquisition, reconstruction, and analysis methods. Metabolites. 2021;11:386. doi: 10.3390/metabo11060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen YF, Kohler SJ, Chen AP, Tropp J, Bok R, Wolber J, et al. Imaging considerations for in vivo 13C metabolic mapping using hyperpolarized 13C-pyruvate. Magn Reson Med. 2009;62:1–10. doi: 10.1002/mrm.21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer D, Levin YS, Hurd RE, Glover GH, Spielman DM. Fast metabolic imaging of systems with sparse spectra: application for hyperpolarized 13C imaging. Magn Reson Med. 2006;56:932–937. doi: 10.1002/mrm.21025. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez MS, Lee J, Walker CM, Sandulache VC, Hennel F, Lai SY, et al. Radial spectroscopic MRI of hyperpolarized [1-13C] pyruvate at 7 tesla. Magn Reson Med. 2014;72:986–995. doi: 10.1002/mrm.25004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Lustig M, Larson PE. Concentric rings K-space trajectory for hyperpolarized 13C MR spectroscopic imaging. Magn Reson Med. 2016;75:19–31. doi: 10.1002/mrm.25577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiesinger F, Weidl E, Menzel MI, Janich MA, Khegai O, Glaser SJ, et al. IDEAL spiral CSI for dynamic metabolic MR imaging of hyperpolarized [1-13C]pyruvate. Magn Reson Med. 2012;68:8–16. doi: 10.1002/mrm.23212. [DOI] [PubMed] [Google Scholar]
- 19.Meyer CH, Pauly JM, Macovski A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med. 1990;15:287–304. doi: 10.1002/mrm.1910150211. [DOI] [PubMed] [Google Scholar]
- 20.Lau AZ, Chen AP, Hurd RE, Cunningham CH. Spectral-spatial excitation for rapid imaging of DNP compounds. NMR Biomed. 2011;24:988–996. doi: 10.1002/nbm.1743. [DOI] [PubMed] [Google Scholar]
- 21.Xing Y, Reed GD, Pauly JM, Kerr AB, Larson PE. Optimal variable flip angle schemes for dynamic acquisition of exchanging hyperpolarized substrates. J Magn Reson. 2013;234:75–81. doi: 10.1016/j.jmr.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bankson JA, Walker CM, Ramirez MS, Stefan W, Fuentes D, Merritt ME, et al. Kinetic modeling and constrained reconstruction of hyperpolarized [1-13C]-pyruvate offers improved metabolic imaging of tumors. Cancer Res. 2015;75:4708–4717. doi: 10.1158/0008-5472.CAN-15-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison C, Yang C, Jindal A, DeBerardinis RJ, Hooshyar MA, Merritt M, et al. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13C NMR. NMR Biomed. 2012;25:1286–1294. doi: 10.1002/nbm.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DK, Orton MR, Mariotti E, Boult JK, Panek R, Jafar M, et al. Model free approach to kinetic analysis of real-time hyperpolarized 13C magnetic resonance spectroscopy data. PLoS One. 2013;8:e71996. doi: 10.1371/journal.pone.0071996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miloushev VZ, Granlund KL, Boltyanskiy R, Lyashchenko SK, DeAngelis LM, Mellinghoff IK, et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Res. 2018;78:3755–3760. doi: 10.1158/0008-5472.CAN-18-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Autry AW, Gordon JW, Chen HY, LaFontaine M, Bok R, Van Criekinge M, et al. Characterization of serial hyperpolarized 13C metabolic imaging in patients with glioma. Neuroimage Clin. 2020;27:102323. doi: 10.1016/j.nicl.2020.102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Autry AW, Park I, Kline C, Chen HY, Gordon JW, Raber S, et al. Pilot study of hyperpolarized 13C metabolic imaging in pediatric patients with diffuse intrinsic pontine glioma and other CNS cancers. AJNR Am J Neuroradiol. 2021;42:178–184. doi: 10.3174/ajnr.A6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CY, Soliman H, Bragagnolo ND, Sahgal A, Geraghty BJ, Chen AP, et al. Predicting response to radiotherapy of intracranial metastases with hyperpolarized 13C MRI. J Neurooncol. 2021;152:551–557. doi: 10.1007/s11060-021-03725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Patel TR, Pinho MC, Choi C, Harrison CE, Baxter JD, et al. Preoperative imaging of glioblastoma patients using hyperpolarized 13C pyruvate: potential role in clinical decision making. Neurooncol Adv. 2021;3:vdab092. doi: 10.1093/noajnl/vdab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaccagna F, McLean MA, Grist JT, Kaggie J, Mair R, Riemer F, et al. Imaging glioblastoma metabolism by using hyperpolarized [1-13C]pyruvate demonstrates heterogeneity in lactate labeling: a proof of principle study. Radiol Imaging Cancer. 2022;4:e210076. doi: 10.1148/rycan.210076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grist JT, McLean MA, Riemer F, Schulte RF, Deen SS, Zaccagna F, et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. Neuroimage. 2019;189:171–179. doi: 10.1016/j.neuroimage.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CY, Soliman H, Geraghty BJ, Chen AP, Connelly KA, Endre R, et al. Lactate topography of the human brain using hyperpolarized 13C-MRI. Neuroimage. 2020;204:116202. doi: 10.1016/j.neuroimage.2019.116202. [DOI] [PubMed] [Google Scholar]
- 33.Hackett EP, Pinho MC, Harrison CE, Reed GD, Liticker J, Raza J, et al. Imaging acute metabolic changes in patients with mild traumatic brain injury using hyperpolarized [1-13C]pyruvate. iScience. 2020;23:101885. doi: 10.1016/j.isci.2020.101885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Pinho MC, Harrison CE, Chen J, Sun C, Hackett EP, et al. Dynamic 13C MR spectroscopy as an alternative to imaging for assessing cerebral metabolism using hyperpolarized pyruvate in humans. Magn Reson Med. 2022;87:1136–1149. doi: 10.1002/mrm.29049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uthayakumar B, Soliman H, Bragagnolo ND, Cappelletto NIC, Lee CY, Geraghty B, et al. Age-associated change in pyruvate metabolism investigated with hyperpolarized 13C-MRI of the human brain. Hum Brain Mapp. 2023;44:4052–4063. doi: 10.1002/hbm.26329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, et al. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circ Res. 2016;119:1177–1182. doi: 10.1161/CIRCRESAHA.116.309769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rider OJ, Apps A, Miller JJJJ, Lau JYC, Lewis AJM, Peterzan MA, et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circ Res. 2020;126:725–736. doi: 10.1161/CIRCRESAHA.119.316260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JM, Reed GD, Liticker J, Putnam WC, Chandra A, Yaros K, et al. Effect of doxorubicin on myocardial bicarbonate production from pyruvate dehydrogenase in women with breast cancer. Circ Res. 2020;127:1568–1570. doi: 10.1161/CIRCRESAHA.120.317970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma J, Malloy CR, Pena S, Harrison CE, Ratnakar J, Zaha VG, et al. Dual-phase imaging of cardiac metabolism using hyperpolarized pyruvate. Magn Reson Med. 2022;87:302–311. doi: 10.1002/mrm.29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JM, Harrison CE, Ma J, Chen J, Ratnakar J, Zun Z, et al. Hyperpolarized 13C MR spectroscopy depicts in vivo effect of exercise on pyruvate metabolism in human skeletal muscle. Radiology. 2021;300:626–632. doi: 10.1148/radiol.2021204500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woitek R, McLean MA, Gill AB, Grist JT, Provenzano E, Patterson AJ, et al. Hyperpolarized 13C MRI of tumor metabolism demonstrates early metabolic response to neoadjuvant chemotherapy in breast cancer. Radiol Imaging Cancer. 2020;2:e200017. doi: 10.1148/rycan.2020200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woitek R, McLean MA, Ursprung S, Rueda OM, Manzano Garcia R, Locke MJ, et al. Hyperpolarized carbon-13 MRI for early response assessment of neoadjuvant chemotherapy in breast cancer patients. Cancer Res. 2021;81:6004–6017. doi: 10.1158/0008-5472.CAN-21-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran M, Latifoltojar A, Neves JB, Papoutsaki MV, Gong F, Comment A, et al. First-in-human in vivo non-invasive assessment of intra-tumoral metabolic heterogeneity in renal cell carcinoma. BJR Case Rep. 2019;5:20190003. doi: 10.1259/bjrcr.20190003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang S, Meng MV, Slater JB, Gordon JW, Vigneron DB, Stohr BA, et al. Metabolic imaging with hyperpolarized 13C pyruvate magnetic resonance imaging in patients with renal tumors-initial experience. Cancer. 2021;127:2693–2704. doi: 10.1002/cncr.33554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ursprung S, Woitek R, McLean MA, Priest AN, Crispin-Ortuzar M, Brodie CR, et al. Hyperpolarized 13C-pyruvate metabolism as a surrogate for tumor grade and poor outcome in renal cell carcinoma—a proof of principle study. Cancers (Basel) 2022;14:335. doi: 10.3390/cancers14020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee PM, Chen HY, Gordon JW, Wang ZJ, Bok R, Hashoian R, et al. Whole-abdomen metabolic imaging of healthy volunteers using hyperpolarized [1-13C]pyruvate MRI. J Magn Reson imaging. 2022;56:1792–1806. doi: 10.1002/jmri.28196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stødkilde-Jørgensen H, Laustsen C, Hansen ESS, Schulte R, Ardenkjaer-Larsen JH, Comment A, et al. Pilot study experiences with hyperpolarized [1-13C]pyruvate MRI in pancreatic cancer patients. J Magn Reson Imaging. 2020;51:961–963. doi: 10.1002/jmri.26888. [DOI] [PubMed] [Google Scholar]
- 48.Gordon JW, Chen HY, Nickles T, Lee PM, Bok R, Ohliger MA, et al. Hyperpolarized 13C metabolic MRI of patients with pancreatic ductal adenocarcinoma. J Magn Reson Imaging. 2023 Dec 02; doi: 10.1002/jmri.29162. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal R, Vigneron DB, Kurhanewicz J. Hyperpolarized 1-[13C]-pyruvate magnetic resonance imaging detects an early metabolic response to androgen ablation therapy in prostate cancer. Eur Urol. 2017;72:1028–1029. doi: 10.1016/j.eururo.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen HY, Aggarwal R, Bok RA, Ohliger MA, Zhu Z, Lee P, et al. Hyperpolarized 13C-pyruvate MRI detects real-time metabolic flux in prostate cancer metastases to bone and liver: a clinical feasibility study. Prostate Cancer Prostatic Dis. 2020;23:269–276. doi: 10.1038/s41391-019-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Granlund KL, Tee SS, Vargas HA, Lyashchenko SK, Reznik E, Fine S, et al. Hyperpolarized MRI of human prostate cancer reveals increased lactate with tumor grade driven by monocarboxylate transporter 1. Cell Metab. 2020;31:105–114.e3. doi: 10.1016/j.cmet.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Kouchkovsky I, Chen HY, Ohliger MA, Wang ZJ, Bok RA, Gordon JW, et al. Hyperpolarized 1-[13C]-pyruvate magnetic resonance imaging detects an early metabolic response to immune checkpoint inhibitor therapy in prostate cancer. Eur Urol. 2022;81:219–221. doi: 10.1016/j.eururo.2021.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen HY, Bok RA, Cooperberg MR, Nguyen HG, Shinohara K, Westphalen AC, et al. Improving multiparametric MR-transrectal ultrasound guided fusion prostate biopsies with hyperpolarized 13C pyruvate metabolic imaging: a technical development study. Magn Reson Med. 2022;88:2609–2620. doi: 10.1002/mrm.29399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sushentsev N, McLean MA, Warren AY, Benjamin AJV, Brodie C, Frary A, et al. Hyperpolarised 13C-MRI identifies the emergence of a glycolytic cell population within intermediate-risk human prostate cancer. Nat Commun. 2022;13:466. doi: 10.1038/s41467-022-28069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin G, Hsieh CY, Lai YC, Wang CC, Lin Y, Lu KY, et al. Hyperpolarized [1-13C]-pyruvate MRS evaluates immune potential and predicts response to radiotherapy in cervical cancer. Eur Radiol Exp. 2024;8:46. doi: 10.1186/s41747-024-00445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallagher FA, Woitek R, McLean MA, Gill AB, Manzano Garcia R, Provenzano E, et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci U S A. 2020;117:2092–2098. doi: 10.1073/pnas.1913841117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye Z, Song B, Lee PM, Ohliger MA, Laustsen C. Hyperpolarized carbon 13 MRI in liver diseases: recent advances and future opportunities. Liver Int. 2022;42:973–983. doi: 10.1111/liv.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu S, Yoshihara HA, Bok R, Zhou J, Zhu M, Kurhanewicz J, et al. Use of hyperpolarized [1-13C]pyruvate and [2-13C]pyruvate to probe the effects of the anticancer agent dichloroacetate on mitochondrial metabolism in vivo in the normal rat. Magn Reson Imaging. 2012;30:1367–1372. doi: 10.1016/j.mri.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB J. 2009;23:2529–2538. doi: 10.1096/fj.09-129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung BT, Chen HY, Gordon J, Mammoli D, Sriram R, Autry AW, et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. J Magn Reson. 2019;309:106617. doi: 10.1016/j.jmr.2019.106617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qin H, Tang S, Riselli AM, Bok RA, Delos Santos R, van Criekinge M, et al. Clinical translation of hyperpolarized 13C pyruvate and urea MRI for simultaneous metabolic and perfusion imaging. Magn Reson Med. 2022;87:138–149. doi: 10.1002/mrm.28965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu X, Tang S, Mu C, Qin H, Cui D, Lai YC, et al. Development of specialized magnetic resonance acquisition techniques for human hyperpolarized [13C,13N2]urea + [1-13C]pyruvate simultaneous perfusion and metabolic imaging. Magn Reson Med. 2022;88:1039–1054. doi: 10.1002/mrm.29266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaumeil MM, Larson PE, Yoshihara HA, Danforth OM, Vigneron DB, Nelson SJ, et al. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat Commun. 2013;4:2429. doi: 10.1038/ncomms3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korenchan DE, Flavell RR, Baligand C, Sriram R, Neumann K, Sukumar S, et al. Dynamic nuclear polarization of biocompatible 13C-enriched carbonates for in vivo pH imaging. Chem Commun (Camb) 2016;52:3030–3033. doi: 10.1039/c5cc09724j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt Ri, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 67.Marco-Rius I, Wright AJ, Hu DE, Savic D, Miller JJ, Timm KN, et al. Probing hepatic metabolism of [2-13C]dihydroxyacetone in vivo with 1H-decoupled hyperpolarized 13C-MR. MAGMA. 2021;34:49–56. doi: 10.1007/s10334-020-00884-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallagher FA, Kettunen MI, Hu DE, Jensen PR, Zandt RI, Karlsson M, et al. Production of hyperpolarized [1,4-13C2] malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci U S A. 2009;106:19801–19806. doi: 10.1073/pnas.0911447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the study.