Abstract
Cachexia, a systemic wasting condition, is considered a late consequence of diseases, including cancer, organ failure, or infections, and contributes to significant morbidity and mortality. The induction process and mechanistic progression of cachexia are incompletely understood. Refocusing academic efforts away from advanced cachexia to the etiology of cachexia may enable discoveries of new therapeutic approaches. Here, we review drivers, mechanisms, organismal predispositions, evidence for multi-organ interaction, model systems, clinical research, trials, and care provision from early onset to late cachexia. Evidence is emerging that distinct inflammatory, metabolic, and neuro-modulatory drivers can initiate processes that ultimately converge on advanced cachexia.
Cachexia, a disabling wasting condition of lean body mass, is one of the most common and challenging whole body response syndromes and occurs during the progression of many diseases. With the emergence of powerful techniques to capture deep data over time and a resurgence of interest in multi-organ connectivity, research into this complex physiological disease response is ready to turn from a focus on advanced cachexia to the process that initiates the change of a previously healthy organism to a cachectic one. In this review, we aim to summarize the literature on the etiology of cachexia, while also providing an overview of the available model systems and clinical research efforts.
Epidemiology
With an estimated annual death rate of 2 million people worldwide, cachexia is one of the main contributors to human morbidity and mortality 1. When the term Cachexia (“kakos hexis = bad state”) was first coined in early Greek literature, it was considered a consequence of disease or aging leading to a lack of physical “conditioning” and, therefore, of broad relevance across seemingly different underlying causalities. During the 19th century tuberculosis-induced cachexia became widely recognized with specialist sanatoriums focused on supportive care. Glentworth R. Butler extended cachexia to cancer in 1906, describing “cancer cachexiae” as characterized by “debility, emaciation, anemia, and a dirty yellowish-brown or brown-green complexion” 2. In 1915, Howard C. Taylor highlighted the similarities of cachexia between diseases: “There is however, nothing that is distinctive about cancerous cachexia. Any of the known changes and a similar picture may be produced by other diseases… The symptoms of the cachexia are gradual but progressive…The emaciation is a late symptom…with the loss of appetite, and nausea” 3. In modern biomedical literature, there is no universal definition of cachexia, but in the context of cancer cachexia, it has been defined as “a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment”4 - a wide definition that may apply to cachexia caused by non-cancer conditions. Recent epidemiological data continue to associate cachexia with several seemingly unrelated, but potentially convergent diseases. Patients with cancer, such as lung, colon and pancreatic cancer, have a high risk of developing cachexia, sometimes estimated to be as high as 80% in pancreatic cancer 5. Cachexia is also common in patients with end-stage renal failure (ESRF)(25–50%), chronic obstructive pulmonary disease (COPD)(25%), chronic heart failure, AIDS, sepsis, and rheumatologic disorders 6,7. Given its incidence and prevalence, universally negative impact on prognosis and quality of life (QoL), and contribution to poor tolerance of and reduced response to treatment for the underlying disease, cachexia presents an area of major global public health burden and an urgent unmet need.
What patients with cachexia have in common is an unresolved underlying disease process - a wound that does not heal. They suffer from involuntary weight loss, more specifically muscle and fat loss that may expand to loss of other tissues (e.g., heart and bone), in the context of anorexia, coupled with fatigue and sickness behavior such as anhedonia. The apparent multiplicity of diseases that lead to cachexia suggest that either multiple processes converge on an advanced state or multiple early diseases converge on a common pathway of disease progression to an advanced state. In both cases, an unresolved continued process consequential to one or several distinct disease-mediated systemic perturbations may drive a systemic effect that results in cachexia (Figure 1). The pre-convergence processes could define therapeutically relevant cachexia subtypes. Therefore, the lack of certainty about the underlying mechanisms driving the processes of cachexia requires additional work to identify the initiators and understand how they alter the communication and function within and among multiple organ systems.
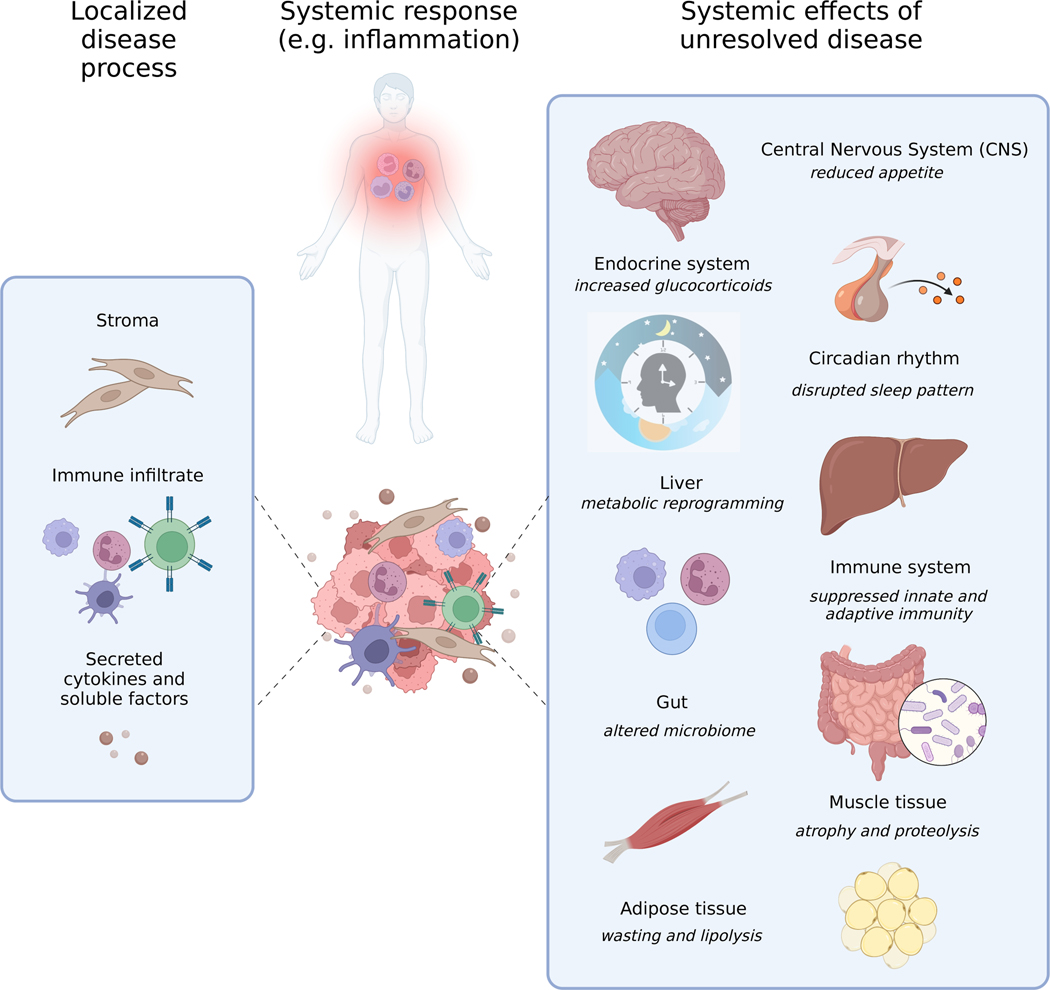
Figure 1. Interconnected causes and consequences of disease- or cancer-associated cachexia at organ level.
Examples of host cell contributors to and organ level consequences of cachexia inducing inflammatory and non-inflammatory processes are illustrated.
Diseased cell-, tumor-, and host-derived cachexia mediators
Some features of the cachexia syndrome, such as metabolic, hormonal, behavioral and inflammatory changes, have been linked to specific molecular mediators. Here, we collate these mediators and discuss their role during cachexia development and the importance of examining their interconnectivity.
Inflammatory mediators
The process of inflammation has long been associated with cancer cachexia. Injury to a tissue from a variety of insults (e.g., trauma, infection) results in the clinical phenotype of inflammation - features recognized since antiquity that include redness, heat, swelling, pain, and loss of function. The neurohumoral output from a local injury, including a rapid elevation in circulating cytokines generated by the activated immune system, can progress systemically. This triggers broader molecular, cellular, neuronal and behavioral responses that result in a more systemic inflammatory phenotype (e.g., fatigue, anhedonia, pain, weight loss).
The evidence that unresolved inflammation is a driver of one type of cachexia 8–11 comes from the detection of multiple inflammatory factors in tissues and the circulation of patients with cachexia (Table 1). These factors have mostly been investigated during periods of detectable weight loss and their longitudinal trajectories frequently remain unclear. They can be produced directly by disease-driving cells (e.g., infected, damaged or cancerous cells), by cells recruited to the microenvironment of the cellular lesion (e.g., fibroblasts and/or immune cells), and by other tissues at an organismal level (Figure 2B). Given that all these processes may amplify each other, changes in these factors will be dynamic. This is likely relevant to the progression of disease to cachexia.
Table 1.
Selected ongoing and completed clinical trials for patients with cachexia.
| Clinical Trial | Drug/Treatment | Mechanism | Phase | Population | Endpoint | Results | |
|---|---|---|---|---|---|---|---|
| Underlying disease | Stage | ||||||
| POWER (NCT00467844)147 | Enobosarm | Selective androgen receptor modulator | Phase 2 | Non-obese male (>45 years) and female (postmenopausal) patients with cancer | ≥ 2% weight loss in the previous 6 months | Change in total lean body mass from baseline, assessed by dual-energy x-ray absorptiometry | Significant increases in total lean body mass by day 113 or end of study (enobosarm 1mg median 1.5 kg, range 2.1–12.6, p=−0.0012; enobosarm 3 mg median 1kg, 4.8–11.5, p=0.046) |
| ROMANA (NCT00219817 and NCT00267358)148 | Anamorelin | Ghrelin receptor agonist | Phase 2 | Patients with advanced or incurable cancer | Weight loss ≥ 5% | Lean body mass changes by dual-energy x-ray absorptiometry over the 12-week treatment period | Lean body mass increased by a least-squares mean of 1.89 kg (95% CI 0.84–2.95) |
| NCT03743064, NCT03743051 | Phase 3 | Patients with advanced Non-Small Cell Lung Cancer (NSCLC) | Body mass index < 20 kg/m2 with involuntary weight loss of >2% within 6 months prior to screening | Changes in weight and 5-item Anorexia Symptom Subscale | Active. Not recruiting. | ||
| Loprinzi et al. 1999 149 | Dexamethasone | Corticosteroid | Phase 3 | Patients with advanced incurable cancer | Weight loss of ≥5 pounds withing the previous 2 months or estimated daily caloric intake of less than 20 cal/kg | O’Brien global test | Patients treated with megestrol acetate improved on more variables than patients in the other treatment arms |
| Fluoxymesterone | Synthetic testosterone | ||||||
| Megestrol acetate | Synthetic progesterone | ||||||
| Nelson et al. 1994 150 | Dronabinol | Orexigenic agent | Phase 2 | Patients with incurable cancer | Loss of 5 pounds or more during 2 months and/or a daily intake of less than 20 cal/kg | Changes in weight and appetite | Weight not improves compared to megestrol acetate arm |
| Fearon et al. 2006 151 | Eicosapentaenoic acid diester | Pure omega-3 fatty acid | Phase 2 | Clinical diagnosis of gastrointestinal or lung cancer | 5% or more loss of pre-illness stable weight | Survival, weight, and other nutritional variables | No statistically significant improvements. Relative to placebo, mean weight increased by 1.2 kg with 2 g EPA (95% CI, 0 kg to 2.3 kg) and by 0.3 kg with 4 g EPA (≥ 0.9 kg to 1.5 kg) |
| NCT01127386 152 | Lenalidomide | Immune-modulator | Phase 2 | Advances and incurable solid tumor with inflammatory cachexia | Weight loss ≥2 % in 2 months or ≥ 5% in 6 months, CRP ≥ 30 mg/L, Granul. ≥ 1.5 × 109/L, platelet ≥ 100 × 109/L, serum creatinine ≤ 2 mg/dL | Lean body mass and handgrip strength | No treatment response on muscle mass and muscle strength was observed with lenalidomide |
| NCT04803305 | anti-GDF-15 | Growth/differentiation factor-15 blockade | Phase 1 | Patients with advanced non-small cell lung, pancreatic, colorecta, prostate, breast or ovarian cancer | Anorexia as defined by a score of ≤ in the Cancer-Related Cachexia Symptom Assessment Appetite 7-day recall scale | Changes in appetite | Completed. No results published. |
| PINNACLES (NCT04725474) | Phase 2 | Patients with metastatic pancreatic adenocarcinoma | Incidence and Severity of Treatment-Emergent Adverse Events | Recruitment ongoing | |||
| GDFATHER (NCT04725474) | Phase 2 | Patients with advanced-stage or recurrent pancreatic adenocarcinoma | Life expectancy > 3 months | Adverse Events, determination of dose-limiting toxicity (DLT) and MTD, assessment of toxicities | |||
| MENAC (NCT02330926) | Exercise routine, nutritional supplementation and anti-inflammatory | Multimodal intervention | Phase 3 | Patients with lung, pancreatic, non-small cell lung (stage III or IV), or pancreatic (stage III or IV) cancer | Karnofsky Performance Status > 70 | Changes in body weight | Active. Not recruiting |
| NCT04906746 | Ruxolitinib | JAK1/2 inhibitor | Phase 1 | Patietns with non-small cell lung cancer and cachexia | Stage IV | Identification of any DLT | Recruitment ongoing |
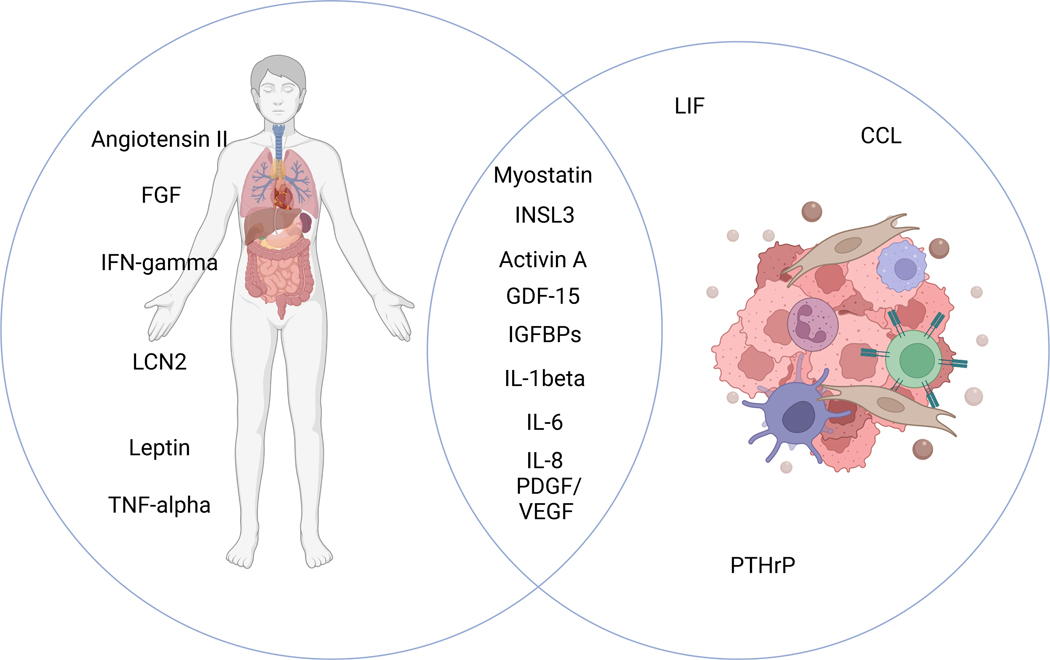
Figure 2. Specific molecular mediators of organ modulation in cancer progression and cachexia.
(A) Effect organ matrix for cachexia mediators. (B) Venn diagram of tumor- or host-derived cachexia mediators.
One of the first factors associated with cachexia was tumor necrosis factor alpha (TNF-alpha), historically termed cachexin, a pro-inflammatory cytokine with muscle and fat tissue catabolic and anorexic effects10. TNF-alpha is associated with cancer cachexia and other cachexia-inducing inflammatory conditions such as rheumatoid arthritis. Trials in patients with cachexia-investigating therapies either blocking the TNF-alpha receptor or neutralizing TNF-alpha itself (NCT00046904, NCT00060502, NCT00244192) have not detected clinical benefit.
Interleukin-6 (IL-6) superfamily members, including IL-6 and leukemia inducible factor (LIF), are among the most reported cachexia-inducing factors. Elevated circulating levels of IL-6 and its upstream regulator interleukin 1 (IL-1) have been widely associated with cancer-associated cachexia in both animal models and humans. In mice, IL-6 alters metabolic organ functions such as reducing the liver’s ketogenic capacity to respond to diminished food intake 11 and promoting adipose tissue browning 12. In non-cachectic conditions, IL-6 acts on the brain and influences energy balance 13. While reversibility of some IL-6 effects has been demonstrated in murine models, a clinical trial in patients with lung cancer and cachexia that blocked IL-6 signaling using ALD518, a humanized anti-IL-6 antibody, showed only mild improvements (NCT00866970), suggesting that late interventions may not be clinically beneficial.
The chemokine CCL2 (monocyte chemoattractant protein-1; MCP-1) that directs CCR2-driven migration of macrophages and can be produced by endothelial cells, fibroblasts, and macrophages has also been linked to cachexia in mice 14. CCL2 promotes liver inflammation, neuroinflammation, weight loss, and metabolic changes in muscle and white adipose tissue (WAT) 15–18. These reports indicate that persistent CCL2 production may sustain inflammatory changes, resulting in systemic metabolic alterations and reduced food intake that leads to cancer cachexia.
Lipocalin-2 (LCN2), a glycopeptide involved in coordinating the host response to inflammation 19, has been described in animal models as having an anorexigenic effect by acting through the central melanocortin system within the hypothalamus 20. Melanocortin 4 receptor antagonists protect from the cachexia of chronic kidney disease and the melanocortin 3 receptor is involved in lean body mass distribution 20,21, suggesting that the melanocortin pathways may have translational potential for anabolic interventions in cachexia.
This selection of example inflammatory molecules indicates that no single factor is the sole causative agent for cachexia. Different molecules may drive cachexia in different circumstances and/or that combinations of molecular factors and their connectivity may be relevant. For example, CCL2 macrophage activation may drive IL-6 production or vice versa, and similar interactions occur with TNF-alpha. It is, therefore, likely that several upstream inflammatory cascades ultimately converge on the clinical phenotype of cachexia. Reflecting this notion, the JAK 1/2 inhibitor ruxolitinib is currently in a Phase 2 (NCT04906746) clinical trial to assess the efficacy of targeting inflammatory signaling pathways activated in cachexia.
Although there is an array of potential cachexia-inducing factors, no single cell population has emerged as causative in cachexia. Infected cells or cancer cells may directly produce cachexia-inducing factors and so may immune cells or cells that modulate the immune response. For example, changes in cancer-associated fibroblast (CAFs), such as loss of myofibroblastic CAFs (myCAFs) and enrichment of IL-6 producing CAFs (iCAFs), lead to cachexia in pancreatic cancer mouse models. Since iCAFs are dependent on the activation of the JAK/STAT signaling pathway for their formation in pancreatic ductal adenocarcinoma (PDAC), JAK/STAT inhibitors that cause loss of iCAFs may ameliorate the cachectic phenotype 22. Similarly, depletion of fibroblast activation protein-α-positive (FAP+) stromal cells leads to loss of muscle mass and cachexia 23, and genetic depletion of alpha-smooth muscle actin-positive (alphaSMA+) cells, which include myofibroblastic CAFs, following tumor formation leads to a reduction in body weight in mouse models of pancreatic cancer 24.
Mediators that regulate tissue homeostasis
Apart from inflammatory molecules, multiple important other initiating factors of cachexia have been discovered. These include imbalances between circulating molecules that maintain skeletal muscle mass and mediators that primarily affect the central nervous system (CNS).
Much of the research that has been performed on circulating and local factors focuses on muscle homeostasis. Follistatin deficiency secondary to fibroblast depletion in the muscle is a potent inducer of cachexia 23. Activation of the activin receptor AcvR2B in skeletal muscle cells by agonists such as activin A or myostatin powerfully induces the catabolic processes of autophagy and proteolysis25. Treatment with a soluble activin A decoy receptor has been suggested as a therapeutic intervention in preclinical work 26, and a human monoclonal anti-ActR2 antibody tested in a clinical study (NCT01433263) led to promising results in preserving lean body mass. However, a clinical trial investigating LY2495655, an anti-myostatin antibody, in patients with pancreatic cancer (NCT01505530) was terminated due to its detrimental effect on survival 27.
Metabolic mediators
Metabolic mediators may also drive inflammation-independent cachexia subtypes. For example, tumor-secreted insulin growth factor binding proteins (IGFBPs) can stimulate catabolism in nutrient-rich tissues by blocking insulin/IGF-1 signaling and promoting insulin resistance 28. Among individuals without cancer, insulin resistance reduces metabolic flexibility, impairs muscle protein synthesis, and increases energy expenditure. In cross-sectional studies, patients with lung cancer often have insulin resistance 29. However, it is unknown how longitudinal changes in insulin resistance, metabolic flexibility, muscle protein synthesis, and energy expenditure relate to changes in muscle mass, muscle quality, and weight loss in patients with cancer.
Cancer also imposes systemic metabolic changes that have not yet been clearly linked to a specific factor. For example, lung cancer can induce diurnal and metabolic changes in the liver that promote gluconeogenesis and inhibit fatty acid metabolism 30. These effects may be due to hepatic inflammation or could be secondary to elevated levels of catabolic hormones such as glucocorticoids. Furthermore, unbiased metabolomic assessments have identified correlations between weight loss and plasma amino acids and phospholipids of unknown source 31.
Mediators that target the brain
Centrally acting circulating molecules such as the Growth Differentiation Factor 15 (GDF-15) have been shown to promote cachexia. GDF-15 is produced in response to cell stress and may have evolved to mediate food aversion in response to toxin exposure 32. It binds mostly to a small number of neurons in the brain stem, outside the blood brain barrier, that express its specific receptor GFRAL. In some conditions, including numerous cancers, GDF-15 levels are elevated and associated with reduced food intake and body weight in humans and mice 33. Moreover, GDF-15 potently activates the hypothalamic-pituitary adrenal (HPA) axis and increases circulating glucocorticoid levels 34. Since glucocorticoids are powerfully catabolic to skeletal muscle, GDF-15 could, via the brain, contribute to the two key features of cachexia, i.e., reduced food intake and selective loss of skeletal muscle. Recently, antibody-mediated blockade of the GDF15-GFRAL pathway has been reported to be efficient in reversing cancer cachexia in preclinical murine models 35. The role GDF-15 plays in anorexia, lipolysis, and muscle wasting has provided a strong rationale to study anti-GDF-15 agents in ongoing human clinical trials (NCT04803305, NCT04068896, NT04725474).
Reciprocal interactions between cachexia-inducing diseases and host organ systems
The physiology of cancer progression may be viewed as a continuously and increasingly perturbed state that involves behavioral changes, dysregulation of the neuroendocrine system, including changes in sleep and circadian rhythm, systemic immune dysregulation, and skeletal muscle and adipose tissue wasting – all of which occur in patients and model systems with cachexia (Figure 1 and 2). While the cachexia-inducing disease process may be locally confined, the hallmarks of cachexia are systemically mediated, indicating that the underlying mechanisms of cause and propagation likely involve modulation of networks that affect all body systems and inter-organ and organ-body communication. One way to approach this connectivity is from the perspective of the energetic imbalance, i.e., a relative lack of energy intake compared to energy expenditure of the system, that ultimately results in overt weight loss during advanced cachexia.
Neuroendocrine interactions
The central nervous system (CNS) makes important contributions to the control of energy homeostasis, and the hypothalamus is widely acknowledged as an area that integrates circulating signals generated in the periphery with potential relevance to cachexia-inducing disease. The hypothalamus’s central melanocortinergic system, and specifically MC3R, plays a role in sensing the body’s nutritional status. It helps co-coordinate the acquisition and retention of calories and their disposition into processes such as growth, reproduction and the acquisition of lean mass 21. Lack of an appropriate response to peripheral inputs leads to diminished appetite and promotes catabolic stimuli (i.e., reduced energy intake, increased energy expenditure, increased muscle proteolysis, and adipose tissue wasting). Moreover, the CNS regulates endocrine organ function (e.g., the release of hormones). Systemic release of glucocorticoids is a well described event in cachexia that occurs in response to the activation of the HPA axis by stressors and induces skeletal muscle atrophy and catabolism. Suppression of the hypothalamic–pituitary–gonadal (HPG) axis significantly decreases testosterone levels, contributing to several cachexia-related signs and symptoms like fatigue, weight loss and muscle catabolism 36. Additionally, hypogonadism has been linked to systemic inflammation and shortened survival in advanced pancreatic cancer 37.
Immunological and metabolic interactions
Inflammation and the immune system response in cachexia are host physiological processes directed to targeting the localized disease. However, tumor-infiltrating myeloid cells can differentiate into myeloid-derived suppressor cells (MDSC), and together with tumor-associated macrophages and lymphocytes (TAMs and TILs) promote angiogenesis and metastasis and eventually contribute to an immunosuppressive environment that favors cachexia 38.
In the periphery, hepatic inflammation and activation of the acute phase response are biosynthetically and bioenergetically costly – when left unresolved, they contribute to systemic metabolic and energetic imbalance. This affects all aspects of intermediary metabolism, including carbohydrate, protein, fat, and energy metabolism. Elevated levels of glucocorticoids and increased gluconeogenesis as well as inhibited fatty acid metabolism and suppressed ketogenesis in the liver are other examples of cancer-induced metabolic changes 11. Host-produced factors such as the hunger hormone Ghrelin, that originates in the stomach, could counteract anorexia. However, development of resistance to its own function ultimately worsens unresolved anorexia. Cachexia often features insulin resistance leading to reduced metabolic flexibility, impaired muscle protein synthesis and increased energy expenditure 29.
Skeletal muscle (SkM) and WAT are the body’s main reservoirs for amino acids and lipids, respectively, and both inflammation and imbalances of factors that maintain muscle mass can increase rates of protein breakdown. During times of stress when food intake is low and nutrient demands increase, such as with the reduced caloric intake associated with cancer cachexia, SkM and WAT activate catabolic processes and distribute stored nutrients to the rest of the body so that they can then be used for energy generation and promote survival. Mechanisms underlying SkM wasting may include upregulation of ERK1/2 and p38 MAPKs 39, activation of autophagy, loss of molecular motor protein MyHC-II 40, production of reactive oxygen species (ROS) that impair myotube morphometry 41, systemic inflammation and production of pro-inflammatory cytokines that induce skeletal muscle atrophy, as well as glucocorticoid release. WAT wasting is caused predominantly by increased lipolysis and reduced fat deposition. Lack of circadian rhythmicity in the expression of transcription factors that regulate fatty acid catabolism during cachexia contributes to lipid metabolism imbalance 42. If left unresolved, progressive catabolism of SkM and WAT leads to physical deterioration, and death. Moreover, tumors seemingly alter host nutrient availability, exchange, and use to favor their own metabolic demands. Identifying the fundamental nature of this tumor-mediated host metabolic reprograming will reveal new tools for the diagnosis and treatment of cachexia.
Interaction with the microbiome
Intestinal microbiota can coordinate hormonal communication between adipose tissue and skeletal muscle to protect from cachexia development during inflammation and infection43. Furthermore, changes in the intestinal microbiome ecology, known as dysbiosis, have been shown to influence cachexia due to gut barrier dysfunction 44, and intestinal pathogens can limit the cachectic response by controlling inflammatory signaling along the gut-brain axis to regulate feeding behavior 45. Thus, manipulation of beneficial bacteria in the gut microbiota has been explored as treatment 46.
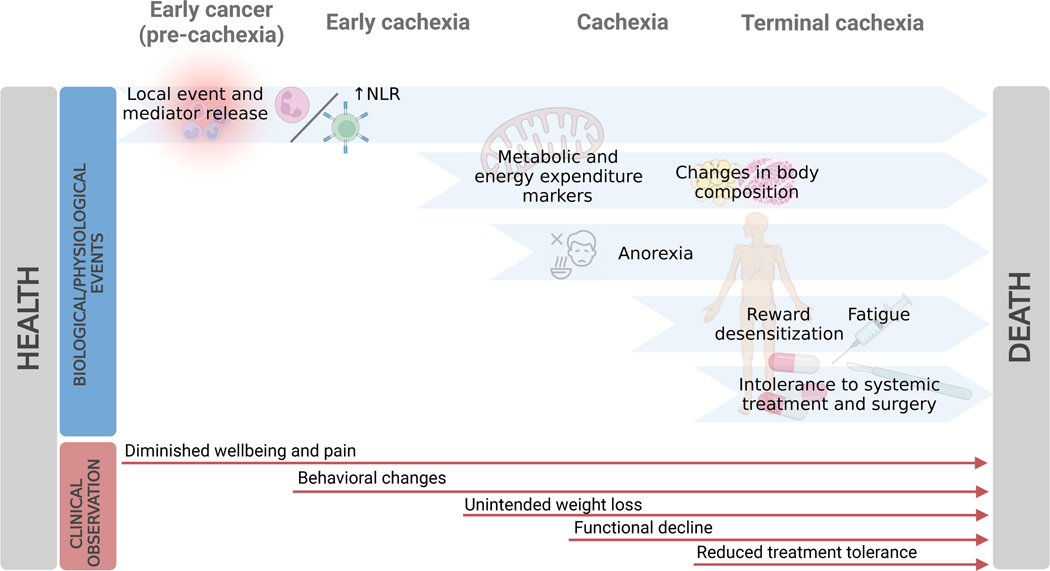
Taken together, these findings suggest a potential sequence of events which lends itself to systematic scientific study: a local insult promotes a systemic response that would normally lead to its resolution, but if the original insult is not cleared (e.g., advanced cancer) this persistent systemic response becomes destructive. It promotes central reduction in nutrient intake behavior and altered peripheral nutrient processing, leading to changes in body composition, fatigue and functional decline, which in turn diminishes tolerance to therapeutic interventions targeting the underlying disease and, ultimately, to cachexia and death (Figure 3).
Figure 3.
Longitudinal progression of biological phenomena and clinical observations from early cancer to advanced cachexia.
Host and iatrogenic contributors to cachexia
The interaction between cachexia molecular drivers and organ responsiveness occurs in a host organism. Individuals may have genetic or acquired characteristics that protect from or predispose to cachexia. This is an important dimension for understanding cachexia, because genetic or acquired cachexia-related traits will synergize or antagonize with the progression of disease-induced cachexia. Some genetic signatures related to inflammation and the renin-angiotensin system have been linked to cachexia susceptibility 47. Moreover, experiments in cachectic animals treated with dextran sulfate sodium to cause intestinal injury have demonstrated that strains purchased from different suppliers or vivarium rooms have more or less rapid onset of body weight loss and variations in the degree of skeletal muscle atrophy 43. Variations in the intestinal microbiota ecology are sufficient to generate differences in cachexia onset and severity, while the specific contribution of genetic differences remains unknown.
Sex-based differences
Distinct body composition, fat distribution, insulin sensitivity, glucose and lipid metabolism, and energy substrate utilization are biological differences between male and female metabolism. Innate metabolic divergences such as these may well influence the susceptibility and development of metabolic syndromes such as cachexia. Nevertheless, much of the preclinical and clinical research of cachexia has focused on its consequences in skeletal muscle tissue. Muscle depletion is more prevalent in males than in females with cancer 48 and cachectic male patients have increased muscle fatigue and greater reduction in handgrip strength compared to cachectic female patients 49. This difference could be attributed to estrogen status, since estrogen signaling is a regulator of muscle contractility and anabolism, and can impact morphology, fatigability and function of myofibers. Hormone replacement therapy can mitigate strength loss in postmenopausal females 50. In addition, estrogens modulate inflammation, and while pro-inflammatory cytokines such as IL-6 induce and accelerate cachexia in tumor-bearing male mice, IL-6 does not impact tumor-bearing female mice 51. Estrogens have a direct effect on hypothalamic neurons regulating physical activity and energy expenditure in females 52. Furthermore, hypogonadism has been associated with cachexia development 37. Testosterone is a potent anabolic factor contributing to muscle mass and its therapeutic potential for cancer-related weight loss has been assessed in a randomized trial, demonstrating improved lean body mass without an effect on survival 53.
However, differences between the male and female cachectic phenotype may also be due to variations in the distribution and metabolic preference of muscle fiber types. Type II muscle fibers account for the majority of male muscle mass and have a glycolytic phenotype, whereas females have predominantly Type I muscle fibers that are oxidative. Data from preclinical models and patients with cancer suggest that Type II glycolytic myofibers are more sensitive to cancer-induced muscle wasting than Type I myofibers 54. Thus, this inherent fiber difference is associated with differential fatigue susceptibility in males and females, and although direct causality has not been established it may drive some of the sex-dependent differential responses during muscle wasting and cachexia.
Sex-driven differences lead to some treatments being only effective in males or females in preclinical models 26. This is potentially relevant for differential outcome in clinical interventions in patients with cancer. A better understanding of how sex-based differences impact cachexia is needed and would benefit the development of therapeutics that improve quality of life and survival.
Aging
A gradual decrease in muscle mass and strength is estimated to start at the age of 30, with the rate of decline increasing after 60 years of age, and by the age of 80, 30% of muscle mass is estimated to be lost 55. Moreover, decrease in appetite and anhedonia are associated with aging and could be explained by changes in various neurotransmitters and brain circuitry, and hormones, which may then result in the frequently observed food intake decline 56.
A major feature of aging is a loss of physiological reserve and coordinated tuning of the organism, which would synergize with the predisposition to cachexia. This is perhaps captured in the concept of “Inflammaging”, the sustained increased levels of circulating pro-inflammatory molecules, which is associated with diminishing organ function, and progressive sarcopenia (loss of muscle: from Greek sarx: flesh, penia: poverty). In murine models, aging causes an energetic imbalance towards catabolism that interferes with homeostatic signaling, and ultimately causes high susceptibility to chronic morbidity, disability, frailty, and premature death. Data from the Rotterdam Study demonstrate that the neutrophil-to-lymphocyte ratio (NLR) is an independent risk indicator for survival in the elderly, and even in the general population over the age of 45. This highlights the clinical value of NLR as an early marker of disease progression and suggests that it may be a proxy measure of the aging process 9. Recent studies link metabolic dysregulation and chronic aging-associated inflammation in a reversible manner 57, opening new interventional avenues for anti-aging treatments.
Other factors, including reduced peripheral or central responsiveness for nutritional and lean body homeostasis may synergize with disease processes in aging and cachexia. Altogether, the phenotype and processes associated with old age mirrors those observed in patients with end-stage cachexia, pointing to convergent biological phenomena and underlying mechanisms that occur at different rates and may potentiate each other, an area that warrants further research.
Anti-cancer therapy-induced cachexia
Physical barriers that impede the digestion and absorption of food may directly contribute to cachexia. Anatomical obstructions of the gastrointestinal tract secondary to tumor progression, malabsorption due to infections or treatment side effects, surgical- or radiotherapy-induced alterations of the digestive system that result in strictures, anatomical removal of organ parts that aid nutrient uptake and neurohormonal imbalances may all contribute to whole-body wasting from caloric deficiency. These disease- or treatment-related developments may negatively synergize with the molecular mechanisms that cause cachexia.
Some anti-cancer treatments, such as chemotherapy, can cause muscle wasting, weakness, and fatigue in patients, thus exacerbating cachexia and worsening prognosis. A chemotherapy-induced increase in circulating GDF-15 peptide causes anorexia, nausea and emesis 58. These symptoms may be exacerbated by an underlying decreased glomerular filtration rate (GFR) in chronic kidney disease (CKD) or organ failure in the elderly. For example, endogenous formaldehyde toxicity-induced GDF-15 production in the proximal renal tubule promotes cachexia in a murine model of Cockayne syndrome A 59. Moreover, dexamethasone, a synthetic glucocorticoid prescribed as a supportive care co-medication for patients with cancer, induces muscle atrophy and dysfunction when used long-term 60. While dexamethasone is a well-established effective antiemetic drug for patients receiving chemotherapy, it induces a positive feedback loop that accelerates wasting. However, administration of dexamethasone reduces IL-6 secretion, ameliorates inflammation in muscle cells, and promotes appetite 61. This warrants further investigation, as glucocorticoids may have a dose-dependent and dose scheduling dependent effect on skeletal muscle and the cachectic phenotype.
Cachexia and more specifically lean muscle mass depletion is associated with worse immunotherapy responses and increased treatment-related toxicities 62. For example, a Phase 2 clinical trial assessing the efficacy of lenalidomide, an immunomodulatory drug, on lean body mass and handgrip strength in advanced solid tumor patients with inflammatory cachexia showed no treatment response (NCT01127386). Moreover, systemic inflammation and cancer-induced release of pro-inflammatory cytokines may not only directly contribute to muscle breakdown but also influence immune response and, consequently, immunotherapy effectiveness 63.
Early disease progression to advanced cachexia
To date, perhaps led by the literal interpretation of hexis (state), definitions and research of cachexia focus on contrasting health with advanced cachexia. More recently, the pre-cachectic state has been recognized as a state when early clinical and metabolic signs are present in the absence of weight loss 4. However, a successive chain of events must occur that connects the healthy state with advanced cachexia. Molecular, organ-, and host-level contributory factors and aspects continue to emerge. A better understanding of the exact hierarchical sequence of events and functional roles is necessary to guide attempts to treat cachexia successfully or prevent its onset. The early dynamics of tumor-host interaction, the pattern of change for circulating cachectogenic factors and metabolites, longitudinal organ-specific changes, and causal relationships must be characterized over the disease’s course and progression to cachexia (Figure 3). For example, early markers of protein breakdown, namely branched chain amino acids (BCAA), have been identified in patients with pancreatic cancer, who are at very high risk of developing cachexia, and could therefore be useful biomarkers to identify groups at risk of developing cachexia 64.
In the clinic and in mice, evidence of the liver’s acute phase response can be detected long before overt weight loss and development of cachexia is observed. In some patients who eventually develop cachexia, elevations of circulating C-reactive protein (CRP), a key marker of systemic inflammation and a response gene of IL-6, are detected at earlier stages of cancer and show a strong relationship with functional decline during cachexia development. Likewise, an elevated NLR is an early biological event in cancer, and neutrophilia may play an adaptive role in host metabolic homeostasis during cancer progression to directly influence weight loss 9.
Modest increases in glucose oxidation and desensitization to insulin have also been frequently observed in patients with cancer and can become more notable with cancer progression towards cachexia 29. These processes are driven by cytokines such as TNF-alpha, IL-1 and IL-6, and hormones such as glucocorticoids, which accelerate whole-body catabolism and lead to changes in body composition.
The patterns of progression for inflammation-independent cachexia are not established. However, they are likely to contribute to the increase over time of fatigue, depression and anhedonia, a cluster of symptoms and behavioral changes in the cachectic syndrome that contribute to diminished patient wellbeing. Understanding the progressive sequence of events that lead to cachexia as well as their relationship to the normal biology of the body, dynamics, and effect on the causal disease state are essential for diagnosis, stratification, prevention and therapy of cachexia.
Established model systems for cachexia research
While human sampling cannot capture the gradual transition from early stages of diseases to advanced cachexia, research using pre-clinical models can be used for that purpose. Well-characterized animal models are needed to demonstrate the efficacy of prospective treatments and to continue to explore etiologies of disease, potentially identifying new therapeutic targets (Figure 4).
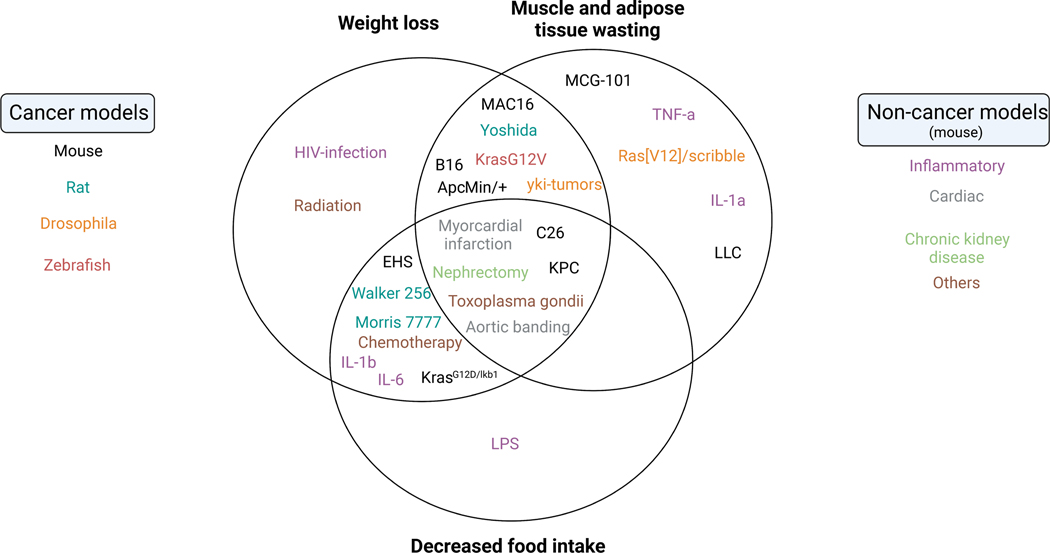
Figure 4. Key examples of established pre-clinical model systems for systemic inflammation predisposing or leading to cachexia.
(A) Venn diagram of physiological cachexia defining aspects of different cancer and non-cancer model systems. (B) Summary categorization of model systems. Note: The assignment of model systems is based on assessment of consensus from the current literature. It is likely that future characterization of model systems will lead to refined allocations.
Thus far, in vivo pre-clinical models of cachexia have been used to investigate multiple aspects of the syndrome, such as higher energy expenditure, which has been extensively described in the C26 mouse model of colorectal cancer cachexia 65, and brown adipose tissue (BAT) thermogenic activity, which despite being present in only 7% of patients with cancer, is greater in cachectic rats with Yoshida sarcoma66. Muscle and WAT wasting, as well as browning of WAT, have been described in multiple mouse models, including genetically engineered mouse models (GEMMs) of lung, pancreatic and skin cancer, diethylnitrosamine (DEN)-induced models of liver cancer, and subcutaneous models of melanoma (B16), lung (LLC) and colorectal (C26) cancers 67–69. IL-6 inhibition prevents browning and weight loss in the K5-SOS and the C26 models 12,70. Measurements of glucose uptake flux by tumors and tissues in the MAC16 model led to identification of the tumor as the second major consumer of glucose, only after the brain. MAC16 tumors also have a higher cycling flux through the triglyceride-fatty acid cycle, but protein synthesis rates are unchanged 71,72. These findings demonstrate a direct impact of the tumor on host nutrition in animal models, but their relevance in comparatively smaller human tumors is not yet clear.
Studies in various in vivo models describe severe systemic hypoglycemia, impaired hepatic ketogenesis, increased plasma triglycerides, VLDL and LDL, and modified ceramides as the main metabolic consequences of cancer cachexia in the host, making them potential therapeutic targets for prevention and reversal of cancer cachexia 11,73,74. In addition, patient-derived xenograft (PDX) and organoid models created from surgically resected tumors from cachectic patients recapitulate both local and systemic aspects of the wasting syndrome and are valuable models to investigate heterogeneity in cachexia.
The fruit fly Drosophila has emerged as an attractive model to address some of the outstanding questions in cachexia research because of 1) The conservation of signaling pathways and hormonal control of metabolism; 2) The tools available; and 3) The availability of organ wasting/cachexia models 75,76. A wealth of genetic tools is available for Drosophila studies of organ wasting. In particular, new cachectic factors can be identified using genome-wide tissue-specific RNAi or CRISPR screens. In addition, tissue-specific proximity labeling methods using biotin ligases can identify secreted factors from various tissues 77. Importantly, several Drosophila tumor models, based on the expression of oncogenes or loss of tumor suppressors in tissues such as the gut, imaginal discs, in either larvae and adults, are available 75,76. Already, studies of these models have identified tumor-derived factors involved in wasting and have provided insights into their roles in tumor-induced metabolic dysregulation. Among them are insulin-binding protein (ImpL2), receptor tyrosine kinase ligands (Pvf1/PDGF-VEGF, Bnl/FGF), Matrix Metallopeptidase (MMP1), and inflammatory cytokines (Unpaireds/IL-6, Eiger/TNF-alpha)75,76. Studies from flies support the emerging concept that cachexia is more than one disease as the nature of cachectic factors in many cases depends on the type of tumors analyzed. Moving forward, fly models will not only help obtain a system-level understanding of cachectic factors throughout the entire organism, but also allow various studies such as characterization of the role of microbiota in cachexia and how tumors affect feeding, olfactory and gustatory behaviors.
Observations of animal responses to non-cancer-associated cachexia have informed our understanding of the pathophysiology of this wasting syndrome, highlighting inflammation as one of the most relevant aspects of cachexia. Preclinical models of acute or chronic inflammation, such as injection of lipopolysaccharide (LPS) or specific inflammatory cytokines (TNF-α, IL-6 or IL-1) exhibit an intense decrease in food intake and increase in resting energy expenditure as seen in disease-associated cachexia (Figure 3). Cardiac cachexia is often a comorbidity in heart failure patients 78, partly caused by the release of inflammatory mediators in response to bacterial toxins absorbed through an edematous bowel wall. The use of surgical techniques either to cause cardiac muscle infarction or limit left ventricular output allow for the replication of the main clinical findings of cardiac cachexia. As with heart failure, cachexia in patients with chronic kidney disease (CKD) is thought to be due to increased inflammation. Animal models of CKD focus on surgical approaches that increase uremia as a means of inducing changes in food intake and body composition. Models of both cardiac cachexia and CKD-associated cachexia, as well as models of radiation- and chemotherapy-induced cachexia, have been used to demonstrate the efficacy of melanocortin-inhibitors and ghrelin on improving appetite, weight gain and lean body mass 79.
The mechanism of muscle protein catabolism in cachexia has been studied in vitro using C2C12 myoblasts and myotubes 80, muscle stem cells derived from C26 tumor-bearing mice 81, and engineered skeletal muscle 82. These models show great potential for research aimed to enhance regenerative processes in the muscle.
The preclinical model systems mentioned above – despite being useful, informative and instrumental in advancing cachexia research – have restrictions that may contribute to the unsuccessful clinical translation of some treatment approaches. These limitations include the inability to fully recapitulate the human disease (nonspontaneous development or lack of a tumor microenvironment), the fact that the tumor models often reach large tumor sizes (sometimes >10% of the entire body mass), the absence of an adaptive immune system in case of PDX models, or induction of cachexia on a rapid timescale (e.g., a few weeks).
Prevention and reversal of cachexia
Cachexia is preventable and potentially reversible. In murine models, treatments that reverse muscle mass loss and anorexia significantly prolong survival 26. Patients with early-stage cancer or with infections do not develop cachexia if they are cured. Occasionally, patients with cachexia who respond strongly to treatment of the underlying disease or undergo surgical resection of the cachexia-inducing tumor demonstrate recovery of lean body mass 83. However, as seen in clinical trials to date (Table 1), reversibility of cachexia, especially if the treatment is aimed at preventing the end organ damage of body fat loss and skeletal muscle atrophy, is a great challenge.
Cachexia prevents patients with advanced cancer from getting adequate treatments, thus, early intervention in cachexia would be highly advantageous. Most patients with advanced cachexia are too weak to tolerate standard doses of anticancer therapies and instead, succumb to accelerated death resulting from respiratory and cardiac failure due to weakened diaphragm and cardiac muscles. Consequently, a substantial portion of deaths in advanced cancer stem not necessarily from cancer itself, but from cachexia (Figure 3). Anti-cachexia treatments may synergize with cancer-directed treatments to the benefit of patients. A specific therapeutic example could be the synergy of patient reconditioning and normalization of HPA axis function in the context of cancer immunotherapy.
The multiple triggers of cancer cachexia and the amalgam of metabolic conditions that result from a tumor-initiated imbalance in whole body metabolism also suggest some value in exploring diet. Clinical guidelines for nutrition support during cachexia mostly focus on the later and end stages of disease and research approaches to nutrition and cachexia typically test a defined meal or supplement strategy. A better understanding of the early dynamics in tumor versus host metabolism is thus vital to the design of optimally targeted nutrition support earlier in disease process. Some nutritional interventions, such as a ketogenic diet, may disrupt tumor metabolism or synergize with chemotherapy, but on the other hand, may challenge host metabolism 11. Enteral nutrition may result in weight stability of patients with pancreatic cancer, but its feasibility is unclear 84. Total parental nutrition has had minimal or no effect in delaying cachexia in small trials 85, perhaps indicating that nutrient utilization is impaired in addition to nutrient uptake. Building on a Phase 2 feasibility trial, the MENAC trial (NCT02330926) is an active Phase 3 randomized-controlled trial (RCT) investigating the use of a home-based exercise routine, nutritional supplementation, and anti-inflammatories (EPA/NSAID). Additional clarity with respect to nutrient-gene interactions, the gut microbiota, the circadian clock, and biological rhythms in feeding and metabolism must be established to facilitate best deployment of nutritional strategies.
Dimensions for enhanced clinical cachexia research and patient care
Cachexia therapeutic development is at a critical juncture. Despite 60 years of investigation, there are no effective U.S Food and Drug Administration (FDA)-approved treatments for cachexia. Early cachexia clinical trials focused on evaluating drugs that simply stimulated appetite but did not significantly improve other aspects of this wasting syndrome. The past decade has seen advances in our understanding of cachexia pathophysiology, resulting in the development of drugs targeting presumed cachectogenic mechanisms. Unfortunately, these more recent strategies have still proven only partially effective or led to unsuccessful clinical trials (Table 1).
Gaps in our understanding of mechanisms and longitudinal courses of cachexia are likely to be the root of suboptimal endpoints, enrolment and dosing of patients in failed clinical trials. Cachexia manifests non-uniformly across patients, and there may be multiple cachexia subtypes. Mechanistic biomarker-driven patient stratification, better longitudinal understanding of the processes leading to advanced cachexia and refined trial design may improve therapeutic developments and lead to impactful clinical management guidelines (Table 2). For example, emerging circulating molecules, such as PLA2G7, may have potential as biomarkers for standardized early detection of cachexia, therapeutic efficacy and patient stratification 86.
Table 2.
Monitoring and measuring approaches for pathophysiological parameters in cachexia.
| Parameter | Sub-parameter | Clinical test | Pre-clinical test |
|---|---|---|---|
| Functional impairment | Cardiorespiratory fitness | 6-minute walk test | Calorimetry and telemetry |
| Mobility balance | Timed up and go (TUG) | Rotarod performance test | |
| Leg strength and speed | Short physical performance battery (SPPB) test | Whole-limb grip strength and treadmill speed | |
| Upper extremity strength | 30-second arm curl test | Forelimb grip strength | |
| Free-living activity and behavior | Triaxial actigraph | IR photocell technology for axis detection of animal motion in cage | |
| Anorexia | Calories, protein intake and diet quality | Food frequency questionnaires | Food intake monitoring and access control |
| Quality of life | Fatigue, pain, anxiety and depression | Patient-reported outcomes (PROs) | Treadmill fatigue test |
| Body composition | Skeletal muscle and adipose tissue density | Longitudinal CT scans | Longitudinal CT scans |
| Biomarkers | Blood markers for metabolic, endocrine and inflammatory state | Standardized panel of markers | Standardized panel of markers |
Imaging utilization
In addition to longitudinal tracking of circulating molecules and underlying disease with repeat sampling, the distribution and utilization of nutrients and the composition of lean body mass and organs can be monitored longitudinally in pre-clinical and clinical research using radiological methods. Utilizing this approach for research and care in cancer cachexia is prudent because, in the clinic, computed tomography (CT) is used for routine surveillance of tumor burden in cancer care and for diagnoses of non-cancerous cachexia inducing diseases. From routine scans, cross sectional muscle area size at the height of third lumbar vertebra (L3) correlate with whole-body muscle and adipose tissue volumes – their analyses can be automated for reproducible routine measurement and identification of high-risk groups in trials and care 87. Dynamic metabolic tracking offered by magnetic resonance imaging (MRI) and by positron emission tomography (PET) offers further research and care advances. Functional brain MRI has been used to demonstrate that pre-treatment dysfunction in executive networks is associated with post-treatment fatigue and cognitive dysfunction more strongly than receipt of chemotherapy 88. MRI and PET have also been used to track choline and glucose uptake in the brain and lungs and depletion of triglycerides as well as altered liver gluconeogenesis in murine cancer models. In patients, tumor glucose uptake positively correlates with energy expenditure and weight loss 89, while low liver uptake of glucose associates with poor cachexia-associated survival 90. New and sensitive (x40 fold improvement) total-body PET scanners that can image the full body in a single scan and measure multiple metabolic substrates bring new possibilities to image dynamic metabolic networks and identify interactions between organs, with examples including tumor-liver-muscle or brain-gut axes, as well as to image other metabolic pathways such as beta-oxidation in BAT, fatty acid synthesis in the tumor and liver, and the Cori and Cahill cycles in the liver and muscle. Radiological imaging, therefore, has potential for early detection of the metabolic alterations that precede changes in body composition. These new imaging approaches may also be applied to monitoring the effectiveness of new interventions in cachexia.
Patient stratification and trial enrolment
Patients have been traditionally dichotomized into either the presence or absence of cachexia. However, the view that all cachexia can be treated with one standard approach is likely oversimplified. Data from cross-sectional and retrospective studies suggest that distinct subtypes of cachexia with variable clinical phenotypes exist. Recently, Gagnon et al. demonstrated that there are at least two clinical subtypes of cancer cachexia, based on each patient’s inflammation and anorexic symptoms 91. The Inflammatory-Necrotic/Anorexic group had shorter median survival (13.9 vs. 27.7 months) than the Non-Inflammatory/Non-Anorexic group. Other studies have used clustering approaches to describe body composition changes observed in patients with cachexia, and three distinct descriptive clusters were found 92: muscle and fat wasting, fat wasting alone, and no wasting. Those with no wasting had the best survival outcomes, followed by those with fat wasting alone. Those with both muscle and fat wasting had the least favorable outcomes.
In addition to specific and concrete categorization of cachexia populations and a deeper understanding of the molecular and physiologic underpinnings of each cachexia subtype, evidence of how clinical and functional metrics change over time are urgently needed to advance the field. Large prospective observational trials in the clinic as well as correlative deep phenotyping and mechanistic sequential research in pre-clinical models, both focused on understanding the natural trajectory of cachexia, would meet this unmet need and inform future more targeted, precise and, hopefully, positive cachexia clinical trials (Figure 5). For example, the REVOLUTION study is a longitudinal observational study recruiting patients from a palliative care service with advanced cancer following changes in body composition, function, quality of life and inflammatory markers 93.
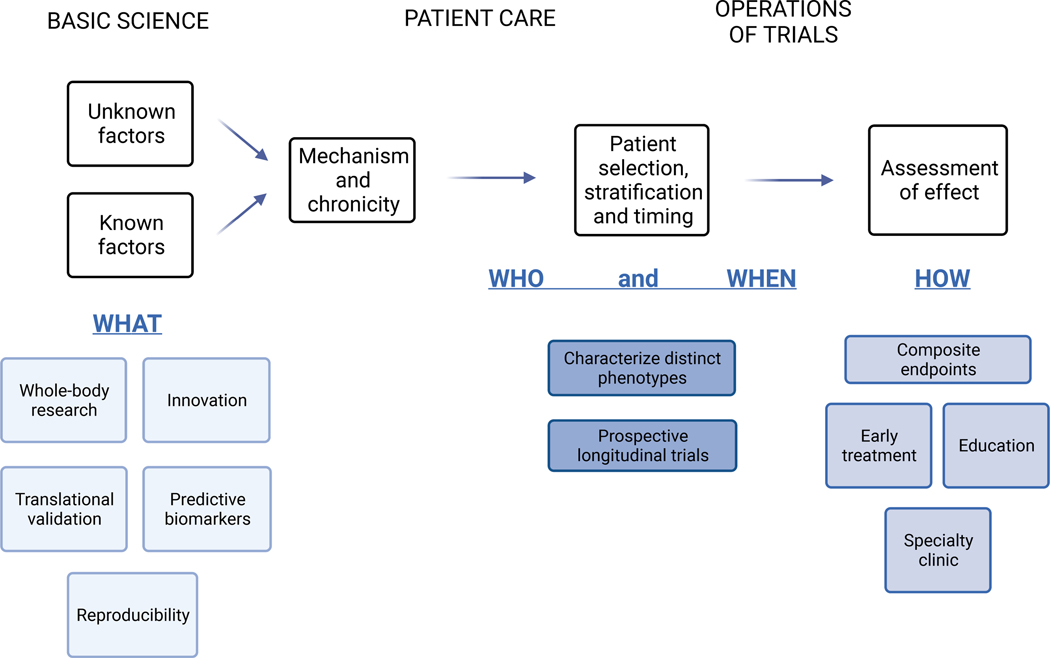
Figure 5. Potential and established contributory areas for the advancement of cachexia research and care.
Selected aspects relating to the full translational research enterprise from pre-clinical mechanistic work to clinical team building and trial design are listed.
A further challenge is that cachexia clinically resembles the consequences of other clinical entities that affect the whole organism, such as malnutrition, sarcopenia or frailty of old age. Therefore, more specific and concrete categorization of cachexia populations, a deeper understanding of the molecular and physiologic underpinnings of each cachexia subtype (genetic, microenvironmental and histologic), and evidence of how clinical and functional metrics change over time are urgently needed to advance the field. Rather than broadening the scope of cachexia trials, cachexia intervention trials should perhaps focus more on the target population and trial endpoints.
In terms of trial operation, most cachexia clinical trial programs enroll patients who have experienced >5% body weight loss. Weight loss, however, does not fully correlate with skeletal muscle loss 94, nor does it fully characterize the effect of cachexia on physical functioning, quality of life, and overall survival 95. Moreover, cachexia-induced weight loss is a late manifestation of the wasting syndrome, therefore, the whole-body’s metabolism is likely to have already reprogrammed at the time of enrolment. Interventions most likely to succeed may have to be delivered sooner, perhaps at cancer diagnosis when patients are in the early stages of cachexia; however, there continues to be no effective biomarkers for this state. The modified Glasgow Prognostic Score (mGPS) is a relevant tool that may help clinical identification and staging of cachexia in patients with cancer 96.
Trial Endpoints
An additional clinical trial challenge is a lack of agreement on the endpoints for cancer cachexia treatments. It remains uncertain if bodyweight per se represents an endpoint that is sufficiently clinically meaningful. Notably, in one study using CT, the traditional definition of >5% body weight loss underestimated cachexia at 56.6%, while CT-based body composition analysis detected tissue loss of >5% in 81% of patients 92. Endpoints that directly reflect how patients feel, function, or survive are most informative. Historically, regulatory agencies have required co-primary endpoints that quantify lean tissue and objectively measure physical functioning for cancer cachexia treatment indications. Enobosarm (POWER; NCT00467844) and anamorelin (ROMANA; NCT00219817 and NCT00267358) are examples of drugs that showed improved lean body mass but no changes in physical function and were consequently not approved by the FDA. Anamorelin has been approved for use in treating cancer cachexia in Japan but not in the U.S. and continues to be evaluated in Phase 3 clinical trials (NCT03743064 and NCT03743051), though its primary endpoints have shifted to focus more on weight and anorexia. Recently, a composite endpoint approach has been adopted that combines a measure of body habitus (e.g., body weight or body composition) with a patient-reported outcome (NCT02138422) 97. Such a composite endpoint approach could enable the quantification of clinical benefit across various types of interventions (e.g., lifestyle, pharmacotherapy, etc.) in a clinically meaningful manner.
Robustly addressing these challenges and coupling endpoints to mechanistic studies will reduce common barriers to developing and approving effective interventions for cachexia prevention and treatment (Figure 5). These discoveries would dramatically enhance the provision of evidence-based, patient-oriented cancer care. The foundation research is instrumental, but not sufficient. Outcome work in chronic disease management has demonstrated benefit from involvement of an informed care team in patient support. Thus, a cachexia-focused clinical effort should not solely focus on interventions. Education of a multidisciplinary team, awareness of malnutrition and other conditions that could mimic or exacerbate cachexia, and close collaborations with the teams that address the underlying diseases that drive cachexia, as well as involvement and support of patient caregivers and social networks of patients are essential for best care.
CONCLUSION:
Cachexia is the ultimate consequence of a variety of unresolved diseases, including infections, chronic inflammatory diseases, and cancers. Distinct causes for cachexia suggest cachexia subtypes driven by inflammatory mechanisms, imbalances of molecules that maintain tissue homeostasis, and suppression of appetite and nutrient intake. In addition, multi-organ interaction, organismal predisposition to cachexia due to genetics, age, sex, and treatments are emerging as relevant contributors to cachexia.
The robust research foundation on advanced cachexia and the availability of model systems that allow longitudinal tracking of specific molecular processes across organs together open the door to expand our understanding of the processes that lead to the development of cachexia. The combination of pre-clinical and clinical research and a comprehensive understanding of the unifying and distinct processes that drive earlier cachexia events, such as initiation and progression, will enable better clinical intervention and patient-centered care in the years to come.
Cachexia is a common systemic wasting condition with high morbidity and mortality associated with many diseases, including cancers and infections. This review summarizes and connects molecular mediators, driver mechanisms, organismal predispositions, model systems, and clinical research for cachexia. It highlights potential cachexia subtypes and the process of cachexia induction.
ACKNOWLEDGEMENTS:
We thank the three reviewers of our manuscript for constructive feedback and suggestions. TJ acknowledges funding from Cancer Grand Challenges, Cancer Research UK (C42738/A24868), The Mark Foundation for Cancer Research (33300111), Cold Spring Harbor Laboratory (CSHL), and developmental funds from CSHL Cancer Center Support Grant 5P30CA045508.
Footnotes
DECLARATION OF INTERESTS:
S.OR. receives remuneration for scientific advisory services provided to Pfizer, AstraZeneca. Courage Therapeutics and Third Rock Ventures.
M.J-H. has consulted for, and is a member of, the Achilles Therapeutics Scientific Advisory Board and Steering Committee, has received speaker honoraria from Pfizer, Astex Pharmaceuticals, Oslo Cancer Cluster, and is co-inventor on a European patent application relating to methods to detect lung cancer PCT/US2017/028013).
All authors, other than JG and DMcC, are members of the CANCAN Grand Challenge Team funded by Cancer Research UK and the National Cancer Institute. The funders had no role in writing or reviewing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Farkas J, von Haehling S, Kalantar-Zadeh K, Morley JE, Anker SD, and Lainscak M. (2013). Cachexia as a major public health problem: Frequent, costly, and deadly. J. Cachexia. Sarcopenia Muscle 4, 173–178. 10.1007/s13539-013-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler G. (1906). The diagnostics of internal medicine. 2nd editio. (D Appleton & Co; ). [Google Scholar]
- 3.Taylor H. (1915). Cancer: its study and prevention. (Lea & Febiger; ). [Google Scholar]
- 4.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, et al. (2011). Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 12, 489–495. 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, and Martignoni ME (2008). Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 12, 1193–1201. 10.1007/s11605-008-0505-z. [DOI] [PubMed] [Google Scholar]
- 6.Wagner PD (2008). Possible mechanisms underlying the development of cachexia in COPD. Eur. Respir. J. 31, 492–501. 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 7.Zanders L, Kny M, Hahn A, Schmidt S, Wundersitz S, Todiras M, Lahmann I, Bandyopadhyay A, Wollersheim T, Kaderali L, et al. (2022). Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J. Cachexia. Sarcopenia Muscle 13, 713–727. 10.1002/jcsm.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldenburg HSA, Rogy MA, Lazarus DD, van Zee KJ, Keeler BP, Chizzonite RA, Lowry SF, and Moldawer LL (1993). Cachexia and the acute-phase protein response in inflammation are regulated by interleukin-6. Eur. J. Immunol. 23, 1889–1894. 10.1002/eji.1830230824. [DOI] [PubMed] [Google Scholar]
- 9.Petruzzelli M, Ferrer M, Schuijs MJ, Kleeman SO, Mourikis N, Hall Z, Perera D, Raghunathan S, Vacca M, Gaude E, et al. (2022). Early Neutrophilia Marked by Aerobic Glycolysis Sustains Host Metabolism and Delays Cancer Cachexia. Cancers (Basel). 14, 1–21. 10.3390/cancers14040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schakman O, Dehoux M, Bouchuari S, Delaere S, Lause P, Decroly N, Shoelson SE, and Thissen JP (2012). Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am. J. Physiol. - Endocrinol. Metab. 303, 2012. 10.1152/ajpendo.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, and Fearon DT (2016). Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 24, 672–684. 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J, Swarbrick M, Rose-John S, Rincon M, Robertson G, et al. (2014). A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447. 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Wallenius V, Wallenius K, Dickson SL, Jansson J-O, Ahrén B, Wallenius V, Ohlsson C, and Carlsten H. (2002). Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79. [DOI] [PubMed] [Google Scholar]
- 14.Burfeind KG, Zhu X, Norgard MA, Levasseur PR, Huisman C, Buenafe AC, Olson B, Michaelis KA, Torres ERS, Jeng S, et al. (2020). Circulating myeloid cells invade the central nervous system to mediate cachexia during pancreatic cancer. Elife 9, 1–27. 10.7554/eLife.54095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose S, and Cho J. (2013). Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 36, 1039–1050. 10.1007/s12272-013-0161-z. [DOI] [PubMed] [Google Scholar]
- 16.Le Thuc O, Cansell C, Bourourou M, Denis RG, Stobbe K, Devaux N, Guyon A, Cazareth J, Heurteaux C, Rostène W, et al. (2016). Central CCL2 signaling onto MCH neurons mediates metabolic and behavioral adaptation to inflammation. EMBO Rep. 17, 1738–1752. 10.15252/embr.201541499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luciano-Mateo F, Cabré N, Fernández-Arroyo S, Baiges-Gaya G, Hernández-Aguilera A, Rodríguez-Tomàs E, Muñoz-Pinedo C, Menéndez JA, Camps J, and Joven J. (2020). Chemokine C–C motif ligand 2 overexpression drives tissue-specific metabolic responses in the liver and muscle of mice. Sci. Rep. 10, 1–12. 10.1038/s41598-020-68769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sell H, Dietze-Schroeder D, Kaiser U, and Eckel J. (2006). Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 147, 2458–2467. 10.1210/en.2005-0969. [DOI] [PubMed] [Google Scholar]
- 19.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MS, Strong RK, Akira S, and Aderem A. (2004). Lipocalin 2 mediates an innate immune response to bacterial infection by sequestering iron. Nature 432, 913–917. 10.1038/nature03021.Published. [DOI] [PubMed] [Google Scholar]
- 20.Mosialou I, Shikhel S, Liu J-M, Maurizi A, Luo N, He Z, Huang Y, Zong H, Friedman RA, Barasch J, et al. (2021). MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature 543, 613–615. 10.1016/b978-0-12-820649-2.00156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam BYH, Williamson A, Finer S, Day FR, Tadross JA, Gonçalves Soares A, Wade K, Sweeney P, Bedenbaugh MN, Porter DT, et al. (2021). MC3R links nutritional state to childhood growth and the timing of puberty. Nature 599, 436–441. 10.1038/s41586-021-04088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, and Tuveson DA (2019). IL-1-induced JAK/STAT signaling is antagonized by TGF-β to shape CAF heterogeneity in pancreatic ductal adenocarcinoma 10.1158/2159-8290.CD-18-0710.IL-1-induced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJB, et al. (2013). Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 210, 1137–1151. 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson T, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al. (2014). Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Diminished Survival. Cancer Cell 25, 719–734. 10.1016/j.ccr.2014.04.005.Depletion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busquets S, Toledo M, Orpí M, Massa D, Porta M, Capdevila E, Padilla N, Frailis V, López-Soriano FJ, Han HQ, et al. (2012). Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J. Cachexia. Sarcopenia Muscle 3, 37–43. 10.1007/s13539-011-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Queiroz AL, Dantas E, Ramsamooj S, Murthy A, Ahmed M, Zunica ERM, Liang RJ, Murphy J, Holman CD, Bare CJ, et al. (2022). Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat. Commun. 13. 10.1038/s41467-022-32135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golan T, Geva R, Richards D, Madhusudan S, Lin BK, Wang HT, Walgren RA, and Stemmer SM (2018). LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J. Cachexia. Sarcopenia Muscle 9, 871–879. 10.1002/jcsm.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding G, Li X, Hou X, Zhou W, Gong Y, Liu F, He Y, Song J, Wang J, Basil P, et al. (2021). Rev-erb in GABAergic Neurons Controls Diurnal Hepatic Insulin Sensitivity. 592, 763–767. 10.1038/s41586-021-03358-w.Rev-erb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winter A, MacAdams J, and Chevalier S. (2012). Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin. Nutr. 31, 765–773. 10.1016/j.clnu.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Goncalves MD, Hwang SK, Pauli C, Murphy CJ, Cheng Z, Hopkins BD, Wu D, Loughran RM, Emerling BM, Zhang G, et al. (2018). Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc. Natl. Acad. Sci. U. S. A. 115, E743–E752. 10.1073/pnas.1714703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J, Alshehri A, Ramage MI, Stephens NA, Mullen AB, Boyd M, Ross JA, Wigmore SJ, Watson DG, and Skipworth RJE (2019). Plasma metabolomics identifies lipid and amino acid markers of weight loss in patients with upper gastrointestinal cancer. Cancers (Basel). 11, 1–11. 10.3390/cancers11101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel S, Alvarez-Guaita A, Melvin A, Rimmington D, Dattilo A, Miedzybrodzka EL, Cimino I, Maurin AC, Roberts GP, Meek CL, et al. (2019). GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 29, 707–718.e8. 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockhart SM, Saudek V, and O’Rahilly S. (2020). Gdf15: A hormone conveying somatic distress to the brain. Endocr. Rev. 41, 610–642. 10.1210/ENDREV/BNAA007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimino I, Kim H, Tung YCL, Pedersen K, Rimmington D, Tadross JA, Kohnke SN, Neves-Costa A, Barros A, Joaquim S, et al. (2021). Activation of the hypothalamic-pituitary-adrenal axis by exogenous and endogenous GDF15. Proc. Natl. Acad. Sci. U. S. A. 118, 1–10. 10.1073/pnas.2106868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suriben R, Chen M, Higbee J, Oeffinger J, Ventura R, Li B, Mondal K, Gao Z, Ayupova D, Taskar P, et al. (2020). Antibody-mediated inhibition of GDF15–GFRAL activity reverses cancer cachexia in mice. Nat. Med. 26, 1264–1270. 10.1038/s41591-020-0945-x. [DOI] [PubMed] [Google Scholar]
- 36.Burney BO, and Garcia JM (2012). Hypogonadism in male cancer patients. J. Cachexia. Sarcopenia Muscle 3, 149–155. 10.1007/s13539-012-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skipworth RJE, Moses AGW, Sangster K, Sturgeon CM, Voss AC, Fallon MT, Anderson RA, Ross JA, and Fearon KCH (2011). Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support. Care Cancer 19, 391–401. 10.1007/s00520-010-0832-y. [DOI] [PubMed] [Google Scholar]
- 38.Miller Mj., Laird BJA, and Skipworth RJE (2019). The immunological regulation of cancer cachexia and its therapeutic implications. J. Cancer Metastasis Treat. 2019, 1–11. 10.20517/2394-4722.2019.001. [DOI] [Google Scholar]
- 39.Barreto R, Waning DL, Gao H, Liu Y, Zimmers TA, and Bonetto A. (2016). Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7, 43442–43460. 10.18632/oncotarget.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amrute-Nayak M, Pegoli G, Holler T, Lopez-Davila AJ, Lanzuolo C, and Nayak A. (2021). Chemotherapy triggers cachexia by deregulating synergetic function of histone-modifying enzymes. J. Cachexia. Sarcopenia Muscle 12, 159–176. 10.1002/jcsm.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rybalka E, Timpani CA, Cheregi BD, Sorensen JC, Nurgali K, and Hayes A. (2018). Chemotherapeutic agents induce mitochondrial superoxide production and toxicity but do not alter respiration in skeletal muscle in vitro. Mitochondrion 42, 33–49. 10.1016/j.mito.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Tsoli M, Schweiger M, Vanniasinghe AS, Painter A, Zechner R, Clarke S, and Robertson G. (2014). Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS One 9. 10.1371/journal.pone.0092966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schieber AMP, Lee YM, Chang MW, Leblanc M, Collins B, Downes M, Evans RM, and Ayres JS (2015). Disease tolerance mediated by commensal E. coli via inflammasome and IGF-1 signaling. Science (80-. ). 350, 558–563. 10.1126/science.aac6468.Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni Y, Lohinai Z, Heshiki Y, Dome B, Moldvay J, Dulka E, Galffy G, Berta J, Weiss GJ, Sommer MOA, et al. (2021). Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 15, 3207–3220. 10.1038/s41396-021-00998-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao S, Schieber AMP, O’Connor CP, Leblanc M, Michel D, and Ayres JS (2017). Pathogen-mediated inhibition of anorexia promotes host survival and transmission. Cell 168, 503–516. 10.1016/j.cell.2017.01.006.Pathogen-mediated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, Teliousis K, Ibrahim YM, Mirabal S, and Erdman SE (2016). Beneficial bacteria inhibit cachexia. Oncotarget 7, 11803–11816. 10.18632/oncotarget.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johns N, Stretch C, Tan BHL, Solheim TS, Sørhaug S, Stephens NA, Gioulbasanis I, Skipworth RJE, Deans DAC, Vigano A, et al. (2017). New genetic signatures associated with cancer cachexia as defined by low skeletal muscle index and weight loss. J. Cachexia. Sarcopenia Muscle 8, 122–130. 10.1002/jcsm.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallengren O, Iresjö BM, Lundholm K, and Bosaeus I. (2015). Loss of muscle mass in the end of life in patients with advanced cancer. Support. Care Cancer 23, 79–86. 10.1007/s00520-014-2332-y. [DOI] [PubMed] [Google Scholar]
- 49.Stephens NA, Gray C, MacDonald AJ, Tan BH, Gallagher IJ, Skipworth RJE, Ross JA, Fearon KCH, and Greig CA (2012). Sexual dimorphism modulates the impact of cancer cachexia on lower limb muscle mass and function. Clin. Nutr. 31, 499–505. 10.1016/j.clnu.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Pöllänen E, Ronkainen PHA, Horttanainen M, Takala T, Puolakka J, Suominen H, Sipilä S, and Kovanen V. (2010). Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Horm. IGF Res. 20, 372–379. 10.1016/j.ghir.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Hetzler KL, Hardee JP, Puppa MJ, Narsale AA, Sato S, Davis JM, and Carson JA (2015). Sex differences in the relationship of IL-6 signaling to cancer cachexia progression. Biochim. Biophys. Acta - Mol. Basis Dis. 1852, 816–825. 10.1016/j.bbadis.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krause WC, Rodriguez R, Gegenhuber B, Matharu N, Rodriguez AN, Padilla-Roger AM, Toma K, Herber CB, Correa SM, Duan X, et al. (2021). Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature 599, 131–135. 10.1038/s41586-021-04010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright TJ, Dillon EL, Durham WJ, Chamberlain A, Randolph KM, Danesi C, Horstman AM, Gilkison CR, Willis M, Richardson G, et al. (2018). A randomized trial of adjunct testosterone for cancer-related muscle loss in men and women. J. Cachexia. Sarcopenia Muscle 9, 482–496. 10.1002/jcsm.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, and Pessin JE (2013). Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 243–250. 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, and Roubenoff R. (2000). Aging of skeletal muscle: A 12-yr longitudinal study. J. Appl. Physiol. 88, 1321–1326. 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 56.Leibowitz SF (1988). Brain neurotransmitters and eating behavior in the elderly. Neurobiol. Aging 9, 20–22. 10.1016/S0197-4580(88)80007-1. [DOI] [PubMed] [Google Scholar]
- 57.He M, Chiang H-H, Luo H, Zheng Z, Qiao Q, Wang L, Tan M, Ohkubo R, Mu W-C, Zhao S, et al. (2020). An Acetylation Switch of the NLRP3 Inflammasome Regulates Aging-associated Chronic Inflammation and Insulin Resistance Ming. Cell Metab. 31, 580–591. 10.1016/j.cmet.2020.01.009.An. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breen DM, Kim H, Bennett D, Calle RA, Collins S, Esquejo RM, He T, Joaquim S, Joyce A, Lambert M, et al. (2020). GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 32, 938–950.e6. 10.1016/j.cmet.2020.10.023. [DOI] [PubMed] [Google Scholar]
- 59.Mulderrig L, Garaycoechea JI, Tuong ZK, Millington CL, Dingler FA, Ferdinand JR, Gaul L, Tadross JA, Arends MJ, O’Rahilly S, et al. (2021). Aldehyde-driven transcriptional stress triggers an anorexic DNA damage response. Nature 600, 158–163. 10.1038/s41586-021-04133-7. [DOI] [PubMed] [Google Scholar]
- 60.Cea LA, Balboa E, Puebla C, Vargas AA, Cisterna BA, Escamilla R, Regueira T, and Sáez JC (2016). Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta - Mol. Basis Dis. 1862, 1891–1899. 10.1016/j.bbadis.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 61.Chang WT, Hong MY, Chen CL, Hwang CY, Tsai CC, and Chuang CC (2021). Mutant glucocorticoid receptor binding elements on the interleukin-6 promoter regulate dexamethasone effects. BMC Immunol. 22, 1–11. 10.1186/s12865-021-00413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daly LE, Power DG, O’Reilly Á, Donnellan P, Cushen SJ, O’Sullivan K, Twomey M, Woodlock DP, Redmond HP, and Ryan AM (2017). The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 116, 310–317. 10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muhammed A, Fulgenzi CAM, Dharmapuri S, Pinter M, Balcar L, Scheiner B, Marron TU, Jun T, Saeed A, Hildebrand H, et al. (2022). The systemic inflammatory response identifies patients with adverse clinical outcome from immunotherapy in hepatocellular carcinoma. Cancers (Basel). 14. 10.3390/cancers14010186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, Yuan C, Bao Y, Townsend MK, Tworoger SS, et al. (2014). Elevated circulating branched chain amino acids are an early event in pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198. 10.1038/nm.3686.Elevated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka Y, Eda H, Tanaka T, Udagawa T, Ishikawa T, Horii I, Ishitsuka H, Kataoka T, and Taguchi T. (1990). Experimental Cancer Cachexia Induced by Transplantable Colon 26 Adenocarcinoma in Mice. Cancer Res. 50, 2290–2295. [PubMed] [Google Scholar]
- 66.Oudart H, Calgari C, Andriamampandry M, Le Maho Y, and Malan A. (1995). Stimulation of brown adipose tissue activity in tumor-bearing rats. Can. J. Physiol. Pharmacol. 73, 1625–1631. 10.1139/y95-724. [DOI] [PubMed] [Google Scholar]
- 67.Rohm M, Schäfer M, Laurent V, Üstünel BE, Niopek K, Algire C, Hautzinger O, Sijmonsma TP, Zota A, Medrikova D, et al. (2016). An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 22, 1120–1130. 10.1038/nm.4171. [DOI] [PubMed] [Google Scholar]
- 68.Tsoli M, Moore M, Burg D, Painter A, Taylor R, Lockie SH, Turner N, Warren A, Cooney G, Oldfield B, et al. (2012). Activation of thermogenesis in brown adipose tissue and dysregulated lipid metabolism associated with cancer cachexia in mice. Cancer Res. 72, 4372–4382. 10.1158/0008-5472.CAN-11-3536. [DOI] [PubMed] [Google Scholar]
- 69.Xu PC, You M, Yu SY, Luan Y, Eldani M, Caffrey TC, Grandgenett PM, O’Connell KA, Shukla SK, Kattamuri C, et al. (2022). Visceral adipose tissue remodeling in pancreatic ductal adenocarcinoma cachexia: the role of activin A signaling. Sci. Rep. 12, 1–14. 10.1038/s41598-022-05660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, and Spiegelman BM (2014). Tumor-derived PTHrP Triggers Adipose Tissue Browning and Cancer Cachexia. Nature 513, 100–104. 10.1038/nature13528.Tumor-derived. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plumb JA, Fearon KCH, Carter KB, and Preston T. (1991). Energy expenditure and protein synthesis rates in an animal model of cancer cachexia. Clin. Nutr. 10, 23–29. 10.1016/0261-5614(91)90077-P. [DOI] [PubMed] [Google Scholar]
- 72.Beck SA, and Tisdale MJ (2004). Effect of cancer cachexia on triacylglycerol/fatty acid substrate cycling in white adipose tissue. Lipids 39, 1187–1189. 10.1007/s11745-004-1346-8. [DOI] [PubMed] [Google Scholar]
- 73.O’Connell TM, Ardeshirpour F, Asher SA, Winnike JH, Yin X, George J, Guttridge DC, He W, Wysong A, Willis MS, et al. (2008). Metabolomic analysis of cancer cachexia reveals distinct lipid and glucose alterations. Metabolomics 4, 216–225. 10.1007/s11306-008-0113-7. [DOI] [Google Scholar]
- 74.Morigny P, Zuber J, Haid M, Kaltenecker D, Riols F, Lima JDC, Simoes E, Otoch JP, Schmidt SF, Herzig S, et al. (2020). High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J. Cachexia. Sarcopenia Muscle 11, 1459–1475. 10.1002/jcsm.12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bilder D, Ong K, Hsi TC, Adiga K, and Kim J. (2021). Tumour–host interactions through the lens of Drosophila. Nat. Rev. Cancer 21, 687–700. 10.1038/s41568-021-00387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Y, Li JSS, Rodiger J, Comjean A, Attrill H, Antonazzo G, Brown NH, Hu Y, and Perrimon N. (2022). FlyPhoneDB: an integrated web-based resource for cell–cell communication prediction in Drosophila. Genetics 220. 10.1093/genetics/iyab235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Droujinine IA, Meyer AS, Wang D, Udeshi ND, Hu Y, Rocco D, McMahon JA, Yang R, Guo JJ, Mu L, et al. (2021). Proteomics of protein trafficking by in vivo tissue-specific labeling. Nat. Commun. 12. 10.1038/s41467-021-22599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J, Schwarz P, and Faber J. (2013). Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine 43, 626–634. 10.1007/s12020-012-9836-3. [DOI] [PubMed] [Google Scholar]
- 79.Liu YL, Malik NM, Sanger GJ, and Andrews PLR (2006). Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother. Pharmacol. 58, 326–333. 10.1007/s00280-005-0179-0. [DOI] [PubMed] [Google Scholar]
- 80.Gomes-Marcondes M.C. c., Smith HJ, Cooper JC, and Tisdale MJ (2002). Development of an in-vitro model system to investigate the mechanism of muscle protein catabolism induced by proteolysis-inducing factor. Br. J. Cancer 86, 1628–1633. 10.1038/sj.bjc.6600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inaba S, Hinohara A, Tachibana M, Tsujikawa K, and Fukada S. (2018). Muscle regeneration is disrupted by cancer cachexia without loss of muscle stem cell potential. PLoS One 13, 1–15. 10.1371/journal.pone.0205467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shahriyari M, Islam MR, Sakib SM, Rinn M, Rika A, Krüger D, Kaurani L, Gisa V, Winterhoff M, Anandakumar H, et al. (2022). Engineered skeletal muscle recapitulates human muscle development, regeneration and dystrophy. J. Cachexia. Sarcopenia Muscle, 3106–3121. 10.1002/jcsm.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams JP, Phillips BE, Smith K, Atherton PJ, Rankin D, Selby AL, Liptrot S, Lund J, Larvin M, and Rennie MJ (2012). Effect of tumor burden and subsequent surgical resection on skeletal muscle mass and protein turnover in colorectal cancer patients. Am. J. Clin. Nutr. 96, 1064–1070. 10.3945/ajcn.112.045708. [DOI] [PubMed] [Google Scholar]
- 84.Gresham G, Placencio-Hickok VR, Lauzon M, Nguyen T, Kim H, Mehta S, Paski S, Pandol SJ, Osipov A, Gong J, et al. (2021). Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient-reported outcomes in pancreatic cancer cachexia. J. Cachexia. Sarcopenia Muscle 12, 1959–1968. 10.1002/jcsm.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amano K, Maeda I, Ishiki H, Miura T, Hatano Y, Tsukuura H, Taniyama T, Matsumoto Y, Matsuda Y, Kohara H, et al. (2021). Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin. Nutr. 40, 1168–1175. 10.1016/j.clnu.2020.07.027. [DOI] [PubMed] [Google Scholar]
- 86.Morigny P, Kaltenecker D, Zuber J, Machado J, Mehr L, Tsokanos FF, Kuzi H, Hermann CD, Voelkl M, Monogarov G, et al. (2021). Association of circulating PLA2G7 levels with cancer cachexia and assessment of darapladib as a therapy. J. Cachexia. Sarcopenia Muscle 12, 1333–1351. 10.1002/jcsm.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cespedes Feliciano EM, Popuri K, Cobzas D, Baracos VE, Beg MF, Khan AD, Ma C, Chow V, Prado CM, Xiao J, et al. (2020). Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J. Cachexia. Sarcopenia Muscle 11, 1258–1269. 10.1002/jcsm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Askren MK, Jung M, Berman MG, Zhang M, Therrien B, Peltier S, Ossher L, Hayes DF, Reuter-Lorenz PA, and Cimprich B. (2014). Neuromarkers of Fatigue and Cognitive Complaints Following Chemotherapy for Breast Cancer: A Prospective fMRI Investigation. Breast Cancer Res Treat. 147, 445–455. 10.1007/s10549-014-3092-6.Neuromarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mitamura A, Kaneta T, Miyata G, Takanami K, Hiraide T, Fukuda H, Takahashi S, and Satomi S. (2011). Positive correlations between tumor uptake on FDG PET and energy expenditure of patients with esophageal cancer. Ann. Nucl. Med. 25, 241–246. 10.1007/s12149-010-0456-9. [DOI] [PubMed] [Google Scholar]
- 90.Nakamoto R, Okuyama C, Ishizu K, Higashi T, Takahashi M, Kusano K, Kagawa S, and Yamauchi H. (2019). Diffusely Decreased Liver Uptake on FDG PET and Cancer-Associated Cachexia With Reduced Survival. Clin. Nucl. Med. 44, 634–642. 10.1097/RLU.0000000000002658. [DOI] [PubMed] [Google Scholar]
- 91.Murphy J, Simonyan D, and Penafuerte C. (2021). Cancer anorexia-cachexia syndrome is characterized by more than one inflammatory pathway. Poster Present. 6th Annu. Cancer Cachexia Conf. Bridg. Mol. Adv. to Clin. Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kays JK, Shahda S, Stanley M, Bell TM, O’Neill BH, Kohli MD, Couch ME, Koniaris LG, and Zimmers TA (2018). Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia. Sarcopenia Muscle 9, 673–684. 10.1002/jcsm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Patton R, Cook J, Haraldsdottir E, Brown D, Dolan RD, McMillan DC, Skipworth RJE, Fallon M, and Laird BJA (2021). REVOLUTION (Routine EValuatiOn of people LivIng with caNcer)—Protocol for a prospective characterisation study of patients with incurable cancer. PLoS One 16, 1–9. 10.1371/journal.pone.0261175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roeland EJ, Ma JD, Nelson SH, Seibert T, Heavey S, Revta C, Gallivan A, and Baracos VE (2017). Weight loss versus muscle loss: re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support. Care Cancer 25, 365–369. 10.1007/s00520-016-3402-0. [DOI] [PubMed] [Google Scholar]
- 95.Fearon KC, Voss AC, and Hustead DS (2006). Definition of cancer cachexia: Effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am. J. Clin. Nutr. 83, 1345–1350. 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 96.Silva G.A. da, Wiegert EVM, Calixto-Lima L, and Oliveira LC (2020). Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin. Nutr. 39, 1587–1592. 10.1016/j.clnu.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 97.Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, et al. (2017). MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 18, 192–201. 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 98.Matthys P, Dukmans R, Proost P, Van Damme J, Heremans H, Sobis H, and Billiau A. (1991). Severe cachexia in mice inoculated with interferon-γ-producing tumor cells. Int. J. Cancer 49, 77–82. 10.1002/ijc.2910490115. [DOI] [PubMed] [Google Scholar]
- 99.Chiappalupi S, Sorci G, Vukasinovic A, Salvadori L, Sagheddu R, Coletti D, Renga G, Romani L, Donato R, and Riuzzi F. (2020). Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J. Cachexia. Sarcopenia Muscle 11, 929–946. 10.1002/jcsm.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zimmers TA, McKillop IH, Pierce RH, Yoo JY, and Koniaris LG (2003). Massive liver growth in mice induced by systemic interleukin 6 administration. Hepatology 38, 326–334. 10.1053/jhep.2003.50318. [DOI] [PubMed] [Google Scholar]
- 101.Rupert JE, Narasimhan A, Jengelley DHA, Jiang Y, Liu J, Au E, Silverman LM, Sandusky G, Bonetto A, Cao S, et al. (2021). Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J. Exp. Med. 218. 10.1084/jem.20190450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding G, Xiang X, Hu Y, Xiao G, Chen Y, Binari R, Comjean A, Li J, Rushworth E, Fu Z, et al. (2021). Coordination of tumor growth and host wasting by tumor-derived Upd3. Cell Rep. 36. 10.1016/j.celrep.2021.109553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mace TA, Bloomston M, and Lesinski GB (2013). Pancreatic cancer-associated stellate cells: A viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology 2, 5–7. 10.4161/onci.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Engineer DR, and Garcia JM (2012). Leptin in anorexia and cachexia syndrome. Int. J. Pept. 2012. 10.1155/2012/287457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tanaka M, Suganami T, Kim-Saijo M, Toda C, Tsuiji M, Ochi K, Kamei Y, Minokoshi Y, and Ogawa Y. (2011). Role of central Leptin signaling in the starvation-induced alteration of B-cell development. J. Neurosci. 31, 8373–8380. 10.1523/JNEUROSCI.6562-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kandarian SC, Nosacka RL, Delitto AE, Judge AR, Judge SM, Ganey JD, Moreira JD, and Jackman RW (2018). Tumour-derived leukaemia inhibitory factor is a major driver of cancer cachexia and morbidity in C26 tumour-bearing mice. J. Cachexia. Sarcopenia Muscle 9, 1109–1120. 10.1002/jcsm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walton KL, Chen JL, Arnold Q, Kelly E, La M, Lu L, Lovrecz G, Hagg A, Colgan TD, Qian H, et al. (2019). Activin A-Induced Cachectic Wasting Is Attenuated by Systemic Delivery of Its Cognate Propeptide in Male Mice. Endocrinology 160, 2417–2426. 10.1210/en.2019-00257. [DOI] [PubMed] [Google Scholar]
- 108.Antsiferova M, Huber M, Meyer M, Piwko-Czuchra A, Ramadan T, MacLeod AS, Havran WL, Dummer R, Hohl D, and Werner S. (2011). Activin enhances skin tumourigenesis and malignant progression by inducing a pro-tumourigenic immune cell response. Nat. Commun. 2. 10.1038/ncomms1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lodge W, Zavortink M, Golenkina S, Froldi F, Dark C, Cheung S, Parker BL, Blazev R, Bakopoulos D, Christie EL, et al. (2021). Tumor-derived MMPs regulate cachexia in a Drosophila cancer model. Dev. Cell 56, 2664–2680.e6. 10.1016/j.devcel.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Song W, Kir S, Hong S, Hu Y, Wang X, Binari R, Tang H, Chung V, Banks AS, Spiegelman B, et al. (2020). Tumor-derived ligands trigger tumor growth and host wasting via differential MEK activcation. 48, 277–286. 10.1016/j.devcel.2018.12.003.Tumor-derived. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garfield BE, Crosby A, Shao D, Yang P, Read C, Sawiak S, Moore S, Parfitt L, Harries C, Rice M, et al. (2019). Growth/differentiation factor 15 causes TGFβ-activated kinase 1-dependent muscle atrophy in pulmonary arterial hypertension. Thorax 74, 164–176. 10.1136/thoraxjnl-2017-211440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, Vion J, Boulet N, Fontaine C, Marquès M, et al. (2020). Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight 5. 10.1172/jci.insight.131870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kwon Y, Song W, Droujinine IA, Hu Y, Asara JM, and Perrimon N. (2015). Systemic Organ Wasting Induced by Localized Expression of the Secreted Insulin/IGF Antagonist ImpL2. Dev Cell 176, 139–148. 10.1016/j.devcel.2015.02.012.Systemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laird BJ, McMillan D, Skipworth RJE, Fallon MT, Paval DR, McNeish I, and Gallagher IJ (2021). The Emerging Role of Interleukin 1β (IL-1β) in Cancer Cachexia. Inflammation 44, 1223–1228. 10.1007/s10753-021-01429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fried SK, and Zechner R. (1989). Cachectin/tumor necrosis factor decreases human adipose tissue lipoprotein lipase mRNA levels, synthesis, and activity. J. Lipid Res. 30, 1917–1923. 10.1016/s0022-2275(20)38211-0. [DOI] [PubMed] [Google Scholar]
- 116.Yeom E, Shin H, Yoo W, Jun E, Kim S, Hong SH, Kwon DW, Ryu TH, Suh JM, Kim SC, et al. (2021). Tumour-derived Dilp8/INSL3 induces cancer anorexia by regulating feeding neuropeptides via Lgr3/8 in the brain. Nat. Cell Biol. 23, 172–183. 10.1038/s41556-020-00628-z. [DOI] [PubMed] [Google Scholar]
- 117.Elattar S, Dimri M, and Satyanarayana A. (2018). The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 32, 4727–4743. 10.1096/fj.201701465RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Callaway CS, Delitto AE, Patel R, Nosacka RL, Dâ€TMLugos AC, Delitto D, Deyhle MR, Trevino JG, Judge SM, and Judge AR (2019). IL-8 released from human pancreatic cancer and tumor-associated stromal cells signals through a CXCR2-ERK1/2 axis to induce muscle atrophy. Cancers (Basel). 11, 1–20. 10.3390/cancers11121863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang S-P, Walsh AM, Baxi V, Pendya D, Baradet T, et al. (2020). Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692. 10.1038/s41591-020-0856-x.Elevated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshida T, Tabony AM, Galvez S, Mitch WE, Higashi Y, Sukhanov S, and Delafontaine P. (2013). Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int J Biochem Cell Biol 23, 1–7. 10.1016/j.biocel.2013.05.035.Molecular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tessitore L, Bonelli G, and Baccino FM (1987). Early development of protein metabolic perturbations in the liver and skeletal muscle of tumour-bearing rats. A model system for cancer cachexia. Biochem. J. 241, 153–159. 10.1042/bj2410153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Q, Yan C, Wang X, and Gong Z. (2019). Leptin induces muscle wasting in a zebrafish kras-driven hepatocellular carcinoma (HCC) model. DMM Dis. Model. Mech. 12, 38240. 10.1242/dmm.038240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Reilly B, Vander AJ, and Kluger MJ (1988). Effects of chronic infusion of lipopolysaccharide on food intake and body temperature of the rat. Physiol. Behav. 42, 287–291. 10.1016/0031-9384(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 124.Gould KE, Taffet GE, Michael LH, Christie RM, Konkol DL, Pocius JS, Zachariah JP, Chaupin DF, Daniel SL, Sandusky GE, et al. (2002). Heart failure and greater infarct expansion in middle-aged mice: A relevant model for postinfarction failure. Am. J. Physiol. - Hear. Circ. Physiol. 282, 25. 10.1152/ajpheart.00206.2001. [DOI] [PubMed] [Google Scholar]
- 125.Cheung W, Yu PX, Little BM, Cone RD, Marks DL, and Mak RH (2005). Role of leptin and melanocortin signaling in uremia-associated cachexia. J. Clin. Invest. 115, 1659–1665. 10.1172/JCI22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nelson K, Walsh D, and Sheehan F. (2002). Cancer and chemotherapy-related upper gastrointestinal symptoms: The role of abnormal gastric motor function and its evaluation in cancer patients. Support. Care Cancer 10, 455–461. 10.1007/s00520-002-0340-9. [DOI] [PubMed] [Google Scholar]
- 127.Ohe Y, Podack ER, Olsen KJ, Miyahara Y, Miura K, Saito H, Koishihara Y, Ohsugi Y, Ohira T, Nishio K, et al. (1993). Interleukin-6 cdna transfected lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shorten survival in syngenic mice. Br. J. Cancer 67, 939–944. 10.1038/bjc.1993.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morrison SD (1972). Feeding Response to Change in Absorbable Food Fraction During Growth of Walker 256 Carcinosarcoma. Cancer Res. 32, 968–972. [PubMed] [Google Scholar]
- 129.Plata-Salamán CR, Oomura Y, and Kai Y. (1988). Tumor necrosis factor and interleukin-1β: suppression of food intake by direct action in the central nervous system. Brain Res. 448, 106–114. 10.1016/0006-8993(88)91106-7. [DOI] [PubMed] [Google Scholar]
- 130.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, and Tuveson DA (2005). Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483. 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 131.Wang G, Biswas AK, Ma W, Kandpal M, Coker C, Grandgenett PM, Hollingsworth MA, Jain R, Tanji K, López-Pintado S, et al. (2018). Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770–781. 10.1038/s41591-018-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Voltarelli FA, Frajacomo FT, de Souza Padilha C, Testa MTJ, Cella PS, Ribeiro DF, de Oliveira DX, Veronez LC, Bisson GS, Moura FA, et al. (2017). Syngeneic B16F10 melanoma causes cachexia and impaired skeletal muscle strength and locomotor activity in mice. Front. Physiol. 8, 6–13. 10.3389/fphys.2017.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fong Y, Moldawer L, and Michael M. (1989). Cachectin / TNF redistribution or IL-alpha induces cachexia with redistribution of body proteins. Am. J. Physiol. 256, R659–65. [DOI] [PubMed] [Google Scholar]
- 134.Bibby MC, Double JA, Ali SA, Fearon KC, Brennan RA, and Tisdale MJ (1987). Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J. Natl. Cancer Inst. 78, 539–545. [PubMed] [Google Scholar]
- 135.Grubbs B, Rogers W, and Cameron I. (1979). Total parenteral nutrition and inhibition of gluconeogenesis on tumor-host responses. Oncology 36, 216–223. 10.1159/000225345. [DOI] [PubMed] [Google Scholar]
- 136.Figueroa-Clarevega A, and Bilder D. (2015). Malignant Drosophila tumors interrupt signaling to induce cachexia-like wasting. Dev Cell 176, 139–148. 10.1016/j.devcel.2015.03.001.Malignant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scarlett JM, Bowe DD, Zhu X, Batra AK, Grant WF, and Marks DL (2010). Genetic and pharmacologic blockade of central melanocortin signaling attenuates cardiac cachexia in rodent models of heart failure. J. Endocrinol. 206, 121–130. 10.1677/JOE-09-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Marks DL, Ling N, and Cone RD (2001). Role of the central melanocortin system in cachexia. Cancer Res. 61, 1432–1438. [PubMed] [Google Scholar]
- 139.Kent S, Rodriguez F, Kelley KW, and Dantzer R. (1994). Reduction in food and water intake induced by microinjection of interleukin-1β in the ventromedial hypothalamus of the rat. Physiol. Behav. 56, 1031–1036. 10.1016/0031-9384(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 140.Lonnroth C, Svaninger G, Gelin J, Cahlin C, Iresjo BM, Cvetkovska E, Edstrom S, Andersson M, Svanberg E, and Lundholm K. (1995). Effects related to indomethacin prolonged survival and decreased tumor growth in a mouse tumor model with cytokine dependent cancer cachexia. Int. J. Oncol. 7, 1405–1413. 10.3892/ijo.7.6.1405. [DOI] [PubMed] [Google Scholar]
- 141.Talbert EE, Cuitino MC, Ladner KJ, Rajasekerea PV, Siebert M, Shakya R, Leone GW, Ostrowski MC, Paleo B, Weisleder N, et al. (2017). Modeling Human Cancer-Induced Cachexia. Physiol. Behav. 176, 139–148. 10.1016/j.celrep.2019.07.016.Modeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.White JP, Puppa MJ, Narsale A, and Carson JA (2013). Characterization of the male ApcMin/+ mouse as a hypogonadism model related to cancer cachexia. Biol. Open 2, 1346–1353. 10.1242/bio.20136544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wisse BE, Frayo RS, Schwartz MW, and Cummings DE (2001). Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology 142, 3292–3301. 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 144.Hatter JA, Kouche YM, Melchor SJ, Ng K, Bouley DM, Boothroyd JC, and Ewald SE (2018). Toxoplasma gondii infection triggers chronic cachexia and sustained commensal dysbiosis in mice. PLoS One 13, 1–16. 10.1371/journal.pone.0204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, and Nakazato M. (2003). Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem. Biophys. Res. Commun. 301, 275–279. 10.1016/S0006-291X(02)03028-0. [DOI] [PubMed] [Google Scholar]
- 146.Rytik PG, Kutcherov II, Muller WEG, Poleschuk NN, Duboiskaya GP, Kruzo M, and Podolskaya IA (2004). Small animal model of HIV-1 infection. J. Clin. Virol. 31, 83–87. 10.1016/j.jcv.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 147.Crawford J, Prado CMM, Johnston MA, Gralla RJ, Taylor RP, Hancock ML, and Dalton JT (2016). Study Design and Rationale for the Phase 3 Clinical Development Program of Enobosarm, a Selective Androgen Receptor Modulator, for the Prevention and Treatment of Muscle Wasting in Cancer Patients (POWER Trials). Curr. Oncol. Rep. 18. 10.1007/s11912-016-0522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, and Fearon KC (2016). Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531. 10.1016/S1470-2045(15)00558-6. [DOI] [PubMed] [Google Scholar]
- 149.Loprinzi BCL, Kugler JW, Sloan JA, Mailliard JA, Krook JE, Wilwerding MB, Rowland KM, Camoriano JK, Novotny PJ, Christensen BJ, et al. (1999). Randomized Comparison of Megestrol Acetate Versus Dexamethasone Versus Fluoxymesterone for the Treatment of Cancer Anorexia/Cachexia. J. Clin. Oncol. 17, 3299–3306. [DOI] [PubMed] [Google Scholar]
- 150.Nelson K, Walsh D, Deeter P, and Sheehan F. (1994). A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J. Palliat. Care 10, 14–18. 10.1177/082585979401000105. [DOI] [PubMed] [Google Scholar]
- 151.Fearon KCH, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, and Murray GD (2006). Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J. Clin. Oncol. 24, 3401–3407. 10.1200/JCO.2005.04.5724. [DOI] [PubMed] [Google Scholar]
- 152.Blum D, Hertler C, Oberholzer R, Wolf-Linder S, Joerger M, Driessen C, and Strasser F. (2022). Lenalidomide in cancer cachexia: a randomized trial of an anticancer drug applied for anti-cachexia. JCSM Rapid Commun. 5, 68–76. 10.1002/rco2.54. [DOI] [Google Scholar]