Abstract
Based on our current understanding of insular regions, effects of chronic alcohol use on the insula may affect the integration of sensory-motor, socio-emotional, and cognitive function. There is no comprehensive understanding about these differences in individuals with alcohol use disorder that accounts for both structural and functional differences related to chronic alcohol use. The purpose of this study was to investigate these variations in both the anterior and posterior insula in persons with alcohol use disorder. We investigated insula gray matter volume, morphometry, white matter structural connectivity, and resting state functional connectivity in 75 participants with alcohol use disorder (females = 27) and 75 age-matched healthy control participants (females = 39). Results indicated structural differences mostly in the anterior regions, while functional connectivity differences were observed in both the anterior and posterior insula in those with alcohol use disorder. Differing connectivity was observed with frontal, parietal, occipital, cingulate, cerebellar, and temporal brain regions. While these results align with prior studies showing differences primarily in anterior insular regions, they also contribute to the existing literature suggesting differences in anterior insular connectivity with brain regions shown to be engaged during higher cognitive and emotional tasks.
Keywords: alcohol use disorder, functional connectivity, human, insula, magnetic resonance imaging, structural connectivity
1 |. INTRODUCTION
Approximately 29.1% of Americans over the age of 18 have met criteria for alcohol use disorder (AUD) at some point in their lives (Grant et al., 2015). Prior studies have shown altered brain function and structure (both white matter and gray matter) associated with excessive alcohol use. These effects have been primarily observed in prefrontal and subcortical areas that are involved in executive functioning, emotion regulation, cognition, and memory. However, regions associated with impulsivity and related functions, such as parietal and temporal regions have also shown effects associated with alcohol abuse (Dupuy & Chanraud, 2016; Monnig et al., 2013).
Among all regions of the brain, the insula is considered one of the most involved. It is considered a multifunctional brain region that integrates sensory-motor, socio-emotional, and cognitive function (Uddin et al., 2017). In-vivo studies have shown that the insula can be primarily divided into two subregions: anterior and posterior, separated by the central sulcus that passes through this structure. The anterior region predominantly consists of pyramidal cells while the posterior region consists of mostly granule cells, thus characterizing the subregions with somewhat different functionality. The anterior insula also consists of von Economo neurons, which are large bipolar neurons involved in empathy, social awareness, and self-control (Allman et al., 2010). The anterior insula is associated with higher cognitive and emotional tasks, while the posterior insula is considered to facilitate somatosensory and viscerosensory functions (Nieuwenhuys, 2012). Moreover, animal studies (Mufson & Mesulam, 1982) that utilized tracer techniques as well as diffusion weighted imaging studies in humans (Cerliani et al., 2012; Cloutman et al., 2012; Ghaziri et al., 2017; Uddin et al., 2011) have identified that anterior portions of the insula have a greater number of connections to the frontal and cingulate areas, while posterior portions have a greater number of connections to parietal, temporal, and sensorimotor areas. Importantly, these findings have also been consistent with those from studies investigating functional connectivity of the insula (Cereda et al., 2002; Nomi et al., 2016).
Signatures of alcohol use have been observed in the insula, starting at the neurochemical level, where studies have shown a reduction in the insular glutamate/glutamine concentration with increased levels of alcohol compulsion and greater alcohol use severity (Betka et al., 2019). This change in levels of glutamate has been associated with selective neuronal death and atrophy (Tsai et al., 1995). Furthermore, the anterior, agranular insula consists of a high density of dopamine, opioid, and corticotrophin-releasing hormone receptors, all of which have a role in mediating drug-related reward and motivational responses (Baumgärtner et al., 2006; Hurd et al., 2001).
One widely accepted explanation of the role of the insula in addiction is that interoceptive signals resulting from the drugassociated physiological state initiate activation of the posterior insula. The posterior insula then, relays signals to the anterior insula where somatic-marker representation links the association to memory (Naqvi & Bechara, 2009; Naqvi et al., 2014). The association of drug cues with memory is a learning process, that is well established in persons with AUD. When one such person is presented with an alcohol-related stimulus, the representation of the alcohol-related memory initiates the physical sensation of craving that is processed by the posterior insula. Such response in the presence of drug cues is facilitated by the connections to brain regions that are associated with impulsive and reflective behavior such as the amygdala, anterior cingulate cortex (ACC), and hippocampus (Droutman et al., 2015).
Studies investigating the effects of alcohol on the structure of the insula have shown significant changes mostly in the anterior insula volume and morphometry (Grodin et al., 2013, 2017; Momenan et al., 2012; Senatorov et al., 2015), with only one study reporting changes in the posterior insula (Galandra et al., 2018). These studies indicate a reduction in insula volume and morphometry, that may be a result of chronic injury to von Economo neurons present in the anterior portion of the insula (Senatorov et al., 2015). Reduced anterior insula volume has been associated with higher levels of impulsivity and compulsivity measures in AUD (Grodin et al., 2017). Moreover, reductions in integrity of the white matter (WM) associated with the insula have been shown, in those with AUD (Hampton et al., 2019; Pfefferbaum et al., 2006). Although the exact mechanisms driving alcohol-induced reduction in WM volume are not fully understood, neuropathological and neuroimaging studies indicate that it may be due to alcohol toxicity, which results in damage to the myelin sheath or axonal size, leading to neural death (Oscar-Berman, 2000). WM-related damage has been observed in the corpus callosum, internal and external capsules, fornix, superior longitudinal fasciculus, frontolimbic, and fronto-cortical–striatal projects; essentially indicating widespread effects of alcohol use (Fortier et al., 2014; Hommer et al., 1996; Zahr & Pfefferbaum, 2017; Zou et al., 2018). Changes in WM have been linked to impairment in cognitive functioning such as decision-making, memory, executive function, and psychomotor performance in persons with AUD (Konrad et al., 2012; Le Berre et al., 2017; Pfefferbaum et al., 2010). While the research into WM microstructural changes in AUD is extensive, more research is needed to understand WM connectivity associated with the insula and its relation to alcohol misuse.
Evidence of effects of chronic alcohol use on the structure of the insula alone is insufficient to draw conclusions regarding how it may affect the function of the brain. Functional connectivity studies help elucidate these effects by providing insight into the interplay between structural and functional connectivity. Resting state functional connectivity studies have shown that individuals with AUD have increased between network, and decreased within network connectivity in executive control, default mode, basal ganglia, salience, and visual networks (Chanraud et al., 2011; Müller-Oehring et al., 2015; Zhu et al., 2017, 2018). Changes in these networks have been linked to changes in impulsivity, cognition, and memory and have also been found to be able to predict AUD severity (Fede et al., 2019). Resting state analyses investigating functional connectivity with the insula have shown reduced connectivity with frontal, parietal, and temporal areas in AUD, while one study also showed increased functional connectivity in the temporal, limbic, and orbito-frontal cortex in nontreatment seeking individuals with AUD (Halcomb et al., 2019; Sullivan & Pfefferbaum, 2013; Vergara et al., 2017).
Although there have been a number of studies investigating differences in insula structure and function in AUD, we do not yet have a complete understanding of this brain region. Moreover, the majority of research that investigated the impact of alcohol use on the anterior and posterior subregions of the insula has been limited to animal studies. The current study attempts to address these gaps in the literature by investigating various aspects of the anterior and posterior insula regions in AUD compared to healthy individuals. As such, we investigated (i) gray matter (GM) volume and morphometry of the insula, (ii) WM structural connectivity of insula, and (iii) resting state functional connectivity between the insula and the rest of the brain. To our knowledge, this is the first study that has investigated data with respect to the insula using multiple modalities collected from the same participant. Therefore, the results from this study may help shed light on the interplay between the structure and function of the insula in AUD. We hypothesize that results from this study will indicate significant structural and functional differences associated with the anterior insula in persons with AUD. Furthermore, we expect that overall the results will show insula-related connectivity (both structural and functional) impairment with frontal, parietal, and temporal brain regions.
2 |. MATERIALS AND METHODS
2.1 |. Participants
Seventy-five participants with alcohol use disorder (females = 27, mean age ± SD = 40 ± 10 years) and 75 age-matched healthy control participants (females = 39, mean age ± SD = 40 ± 11 years) enrolled in screening protocols at the National Institute on Alcohol Abuse and Alcoholism (NIAAA), underwent a structural magnetic resonance imaging (MRI) scan, a diffusion weighted imaging (DWI) scan, and a resting state MRI (rs-fMRI) scan. Participants in the AUD group were enrolled in the inpatient treatment program at NIAAA. All AUD participants were sober at the time of their scan. AUD participants were scanned at various points throughout their approximately 28 day stay after withdrawal symptoms had subsided. The mean days of abstinence between participants' last drink and their scan date was 23 days (Min = 7, Max = 52). All participants provided written informed consent prior to participating in this study. Behavioral data characteristic of drinking behavior such as the Lifetime Drinking History (LDH) (Skinner & Sheu, 1982), Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993), and the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 2007) Axis 1 Disorders or for DSM-5 (SCID-5) (First et al., 2015) was completed (Table 1). All participants in the AUD group were found to have either alcohol dependence (based on DSM-IV criteria) or moderate-to-severe AUD (based on DSM-5 criteria).
TABLE 1.
Demographics of participants (N = 150)
| AUD (n = 75) | Control (n = 75) | Statistics between AUD and control | |
|---|---|---|---|
| Audit | 28.91 (5.64) | 2.61 (2.26) | p = 2.20e−16** |
| Mean age (SD) | 40.23 (9.93) | 39.60 (10.7) | t = 0.36, p = .72 |
| Mean of years of education (SD) | 13.84 (2.43) | 16.60 (4.02) | t = −5.07, p = 1.18e−06** |
| Mean of drinking years (SD) | 13.04 (8.86) | 0.13 (0.77) | t = 11.97, p = 2.20e−16** |
| Smokers (%) | 53.33 | 1.33 | X2 = 51.05, p = 8.99e−13** |
p < .025
p < .001.
Participants with neurological disorders, history of psychotic symptoms, history of significant head trauma, claustrophobia, ferrous metal in the body, positive urine drug tests, and positive pregnancy tests (if applicable) were excluded from the study.
2.2 |. Data acquisition
All scans were collected on a 3 Tesla MRI scanner (Siemens Skyra) with a 20-channel head coil. In the scanner, participants completed a T1-weighted scan, a DWI scan, a fat suppressed T2-weighted scan, and a rs-fMRI scan. High-resolution T1-weighted 3-D structural scans were acquired for each participant using an MPRAGE sequence (128 axial slices, TR = 1900 ms TE = 30 ms, 256 × 256 matrix, 0.938 × 0.938 × 1.0 mm3). The DWI scan included 80 volumes (Gradient field strength = 50 mT/m, TR of 8600 ms, TE of 84.0 ms, 2.5 × 2.5 × 2.5 mm3 voxel size, 80 directions). The fat suppressed T2-weighted scan was collected to be used for EPI distortion correction later in the preprocessing pipeline as is described as best practice by Irfanoglu et al. (2012) (100 axial slices, TR = 11,770 ms TE = 90 ms, 150 × 192 matrix, 1.719 × 1.719 × 1.7 mm3). For the rs-fMRI scan, participants lay in the dark for 10 min with eyes open and no additional stimuli. This scan was acquired using an echo planar-imaging pulse sequence (TR: 2000 ms, TE: 30 ms, flip angle: 90°, FOV: 24 × 24 cm, 3.8 mm slice thickness, 36 slices, multi-slice mode: interleaved). Trained research assistants visually inspected all images prior to processing the data.
2.3 |. Data analysis
2.3.1 |. Insula volume analysis
T1-weighted structural images were processed using FreeSurfer version 5.3 (Fischl, 2012), an automated parcellation program to obtain insula volume measures. Briefly, the processing pipeline consists of motion correction, removal of non-brain tissue, automated Talairach transformation, segmentation of subcortical structures, intensity normalization, tessellation of the gray matter/white matter boundary, topology correction, and surface deformation. The images were then automatically parcellated based on cortical gyral and sulcal structure (for further details see (Fischl & Dale, 2000; Fischl et al., 2004)). Thereafter, AFNI toolbox (Cox, 1996) was used to translate all the volumetric and surface data into an AFNI compatible NIFTI format, to translate the FreeSurfer generated surfaces into standard meshes, and to create tissue-based maps. These maps were then used to calculate the volume of the anterior and posterior insula for each participant. In addition, the intracranial volume was also calculated, which was then used to normalize insula volume, in order to account for differences in brain sizes. A two-way MANCOVA with left and right insular volumes as the outcomes was used to assess differences between the groups while controlling for effects of age, sex, and years of education. The significance level of all analysis completed in the current study was set at p < .025, correcting for multiple comparisons for left and right regions.
2.3.2 |. Insula morphometry analysis
Insula morphometry was investigated using FSĽs voxel-based morphometry (Douaud et al., 2007) toolbox. First, whole-brain extraction was performed on the T1 mprage images. Afterwards, gray matter was segmented before being registered to the MNI152 standard space using nonlinear registration (Andersson et al., 2007). The resulting images were used to create a study-specific gray matter template, thereafter all native gray matter images were nonlinearly registered to this template and modulated to correct for local changes. The images were then smoothed with an isotropic 9 mm full-width at half-maximum Gaussian smoothing kernel. Finally, a two-way ANCOVA was used to examine group differences in morphometry for each insula ROI (Figure 1) using the randomize function, controlling for effects of age, sex, and years of education. The randomize was applied using 5000 permutations and significant clusters were identified using Threshold-Free Cluster Enhancement (TFCE) at a family-wise error (FEW) corrected threshold of p < .025.
FIGURE 1.
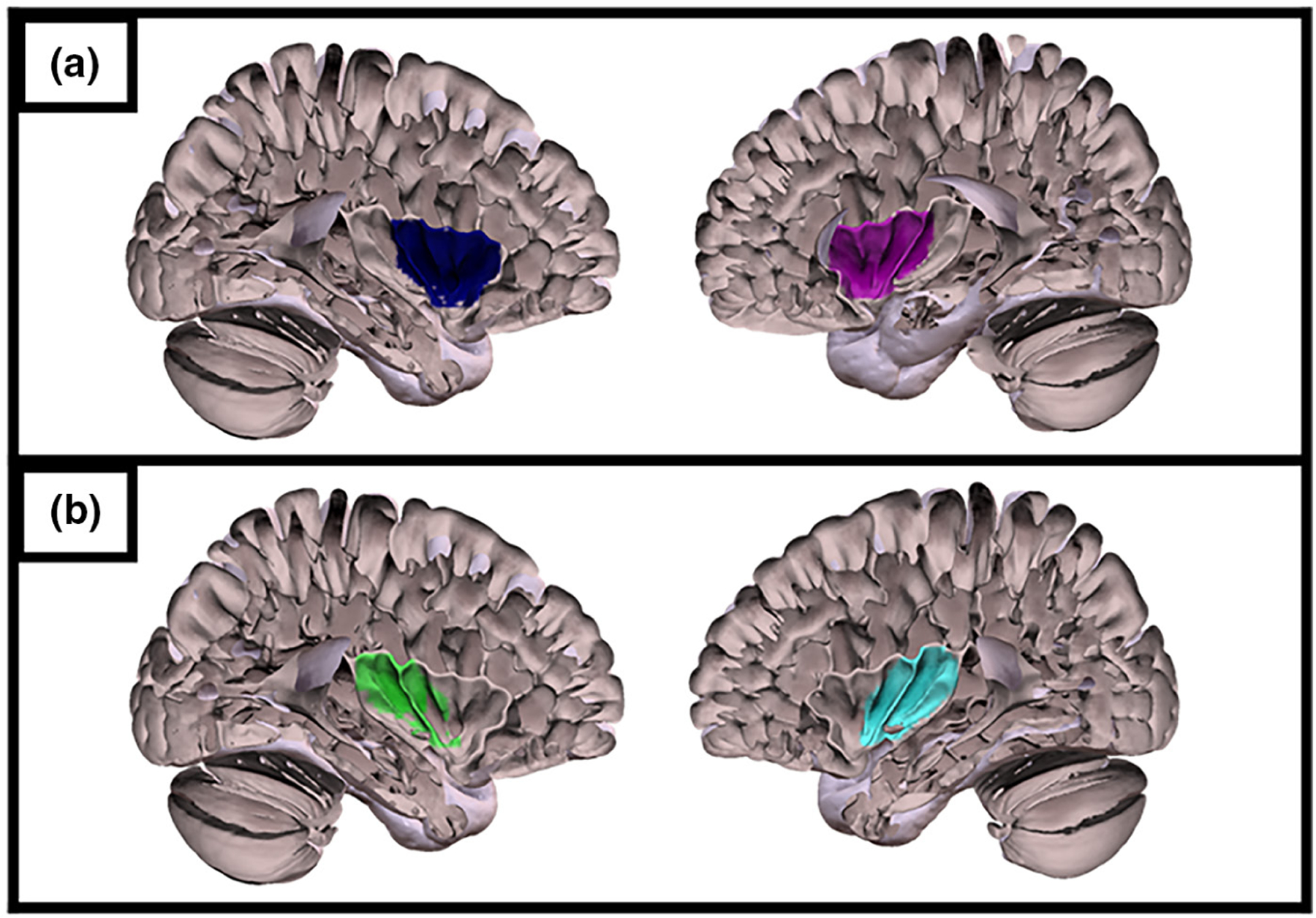
(a) Left and right anterior insula mask. (b) Left and right posterior insula mask.
2.3.3 |. Insula white matter connectivity
All diffusion images, T1w, and T2w images were converted to NIFTI format; Supplementary gradient files were created for the diffusion images by setting a coordinate of (0, 0, 0) at the volume's center of mass. Then, subjects' T1w and DWI images were aligned to their T2w reference volume. Next, any diffusion images with significant artifacts were removed before creating a whole-brain mask of the raw images. Images were closely examined to verify that none of the participants included in the analysis had >10% of volumes corrupted. Freesurfer software was then used to preprocess the T1w anatomical volume to create tissue maps and, perform skull-stripping, surface mesh estimation, and whole-brain segmentation and parcellation. Using TORTOISE's Diff_prep command, all DWI images were computed to b-matrix gradients, corrected for motion, eddy current distortions, and EPI related distortions, and reoriented into the T1w target space with b-matrix orientation. Afterwards the Diff_Calc function was used to provide an estimation of the diffusion tensor at each voxel.
2.3.4 |. Creating a group probabilistic map
First, the probabilistic tractography was generated between each insula ROI and the rest of the brain, for all subjects using AFNI software (FA threshold = 0.2, tracts per voxel threshold = 0.001, number of seeds per voxel per iteration = 5, number of Monte Carlo iterations = 1000). Afterwards, the probabilistic tractography for each subject was binarized and summed before thresholding at 25% to create a map. The map consisted of connecting fiber tracks present in at least 25% of all the subjects, in order to ensure that the map represented tractography of both AUD and healthy individuals. Group maps representing the WM connectivity between each of the insula ROIs and the rest of the brain were created (Figure 2).
FIGURE 2.
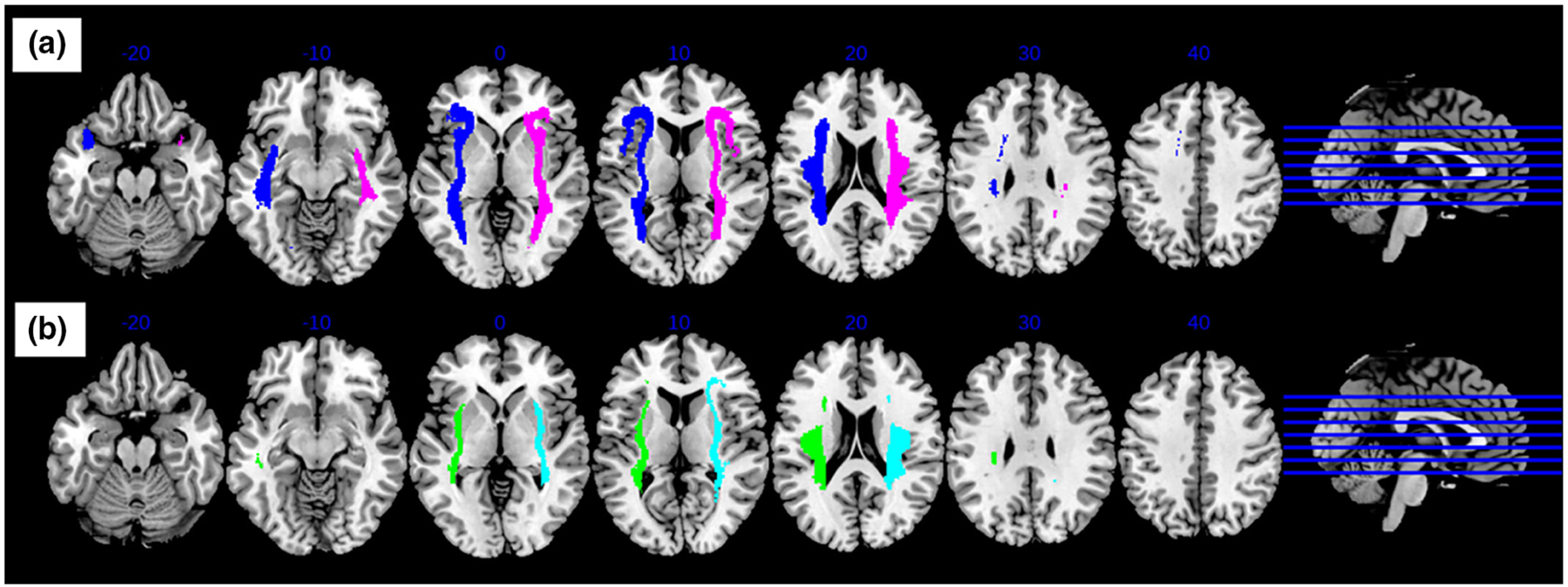
Group probabilistic maps for (a) left and right anterior insula, and (b) left and right posterior insula tractography. Tractography was generated using an insula seed and FA threshold = 0.2. Probabilistic maps were created using a 25% thresholding to ensure that the map represented tractography of both AUD and healthy individuals.
2.3.5 |. Tract-based spatial statistics analysis
Voxel-wise statistical analysis of the FA data was carried out using FSĽs tract-based spatial statistics (TBSS) (Smith et al., 2006). First, FA images were created using the AFNI toolbox as described above. Nonlinear registration was then carried out to align all FA data to a 1 mm3 standard space using a b-spline representation of the registration warp field. Next, the mean FA image was created and then used to create a mean FA skeleton which represents the centers of all tracts common to the subjects. An FA threshold of 0.2 was used in order to exclude voxels that are primarily gray matter or CSF. Each subjecťs aligned FA data were thereafter projected onto the skeleton created. Voxel-wise statistics were then performed on the resulting image using FSĽs randomize (Winkler et al., 2014) using 5000 iterations for nonparametric permutation testing and the group probabilistic map to mask the data. Similarly the mean diffusivity (MD) images were also analyzed. A two-way ANCOVA tested for differences between the two groups for each ROI, while covarying age, sex, and years of education. Significant clusters were identified using threshold-free cluster enhancement (TFCE) at a family-wise error (FEW)-corrected threshold of p < .025.
2.3.6 |. Insula resting state functional connectivity
All rs-fMRI data were preprocessed using AFNI (Cox, 1996). For each subject, the first three TRs were removed from the time course. Then, 3dDespike was applied to smooth spikes in the signal over the time course. Next, the cardiac and respiratory artifacts were removed from the data using 3dretroicor function. Time courses were shifted for each voxel to be aligned to the beginning of the TR by detrending then interpolating the time series. For each subject, volumes across the time series were aligned to the base volume and then to the skull-stripped anatomical image. These images were then mapped to standard Talairach space using the non-linear warping procedure. Finally, each volume was blurred with a 6-mm full-width at half-maximum Gaussian smoothing kernel.
After preprocessing the data, correlation maps between each insula seed mask and the rest of the brain were generated for each subject. Fisher's Z transformation formula was then applied to the correlation maps to reduce skewness and make the sampling distribution normal. Group differences were identified using an MANCOVA that explored functional connectivity with each insula ROI, covarying for age, sex, and years of education.
2.3.7 |. Correlation analysis
In an effort to better address the insula structure–function relationship, we performed an exploratory correlation analysis on the data. Associations were examined between GM morphometry, WM connectivity as measured by the mean FA value of significantly different WM clusters, and resting state connectivity measured by the connectivity coefficient between regions. These correlations were run within insular regions, that is that gray matter density in the left anterior insula was correlated only with structural and functional connectivity observed between the left anterior insula and demonstrated significant regions.
Associations between smoking status and the neuroimaging measures described above were examined in the AUD group as a secondary analysis. Results from this analysis can be found in the supplement. Pearson's correlation analysis was performed for both the anterior and posterior insula.
3 |. RESULTS
3.1 |. Insula gray matter volume and morphometry
Insula gray matter volume analysis revealed a significant effect of group for both anterior (F(2,144) = 8.42, p < .001), and posterior (F(2,144) = 11.14, p < .001) insula. None of the nuisance covariates were significant. Volumetric differences between AUD and control groups were observed in all four regions of the insula (Table 2). Insula morphometry analysis revealed significant differences (pFWE < .025) between groups, for the anterior insula in both hemispheres and left posterior insula (Figures 3 and 4). Collectively these results suggest higher insula gray matter volume and density in the control group.
TABLE 2.
Group means and between group differences in insula gray matter volume (normalized using total intracranial volume) obtained from ANCOVA analyses
| Region | Hemisphere | Healthy volunteers n = 75, mean ± SD | AUD participants n = 75, mean ± SD | Statistical comparison between groups |
|---|---|---|---|---|
| Anterior insula | Right | 1.55e−3 ± 2.53e−4 | 1.43e−3 ± 1.72e−4 | F = 12.25, p = 6.19e−4** |
| Left | 1.68e−3 ± 2.37e−4 | 1.55e−3 ± 1.86e−4 | F = 15.43, p = 1.32e−04** | |
| Posterior insula | Right | 9.52e−4 ± 1.77e−4 | 8.83e−4 ± 1.31e−4 | F = 7.28 p = 7.78e−3* |
| Left | 9.24e−4 ± 1.66e−4 | 8.14e−4 ± 1.29e−4 | F = 22.22, p = 5.65e−06** |
p < .025
p < .001.
FIGURE 3.
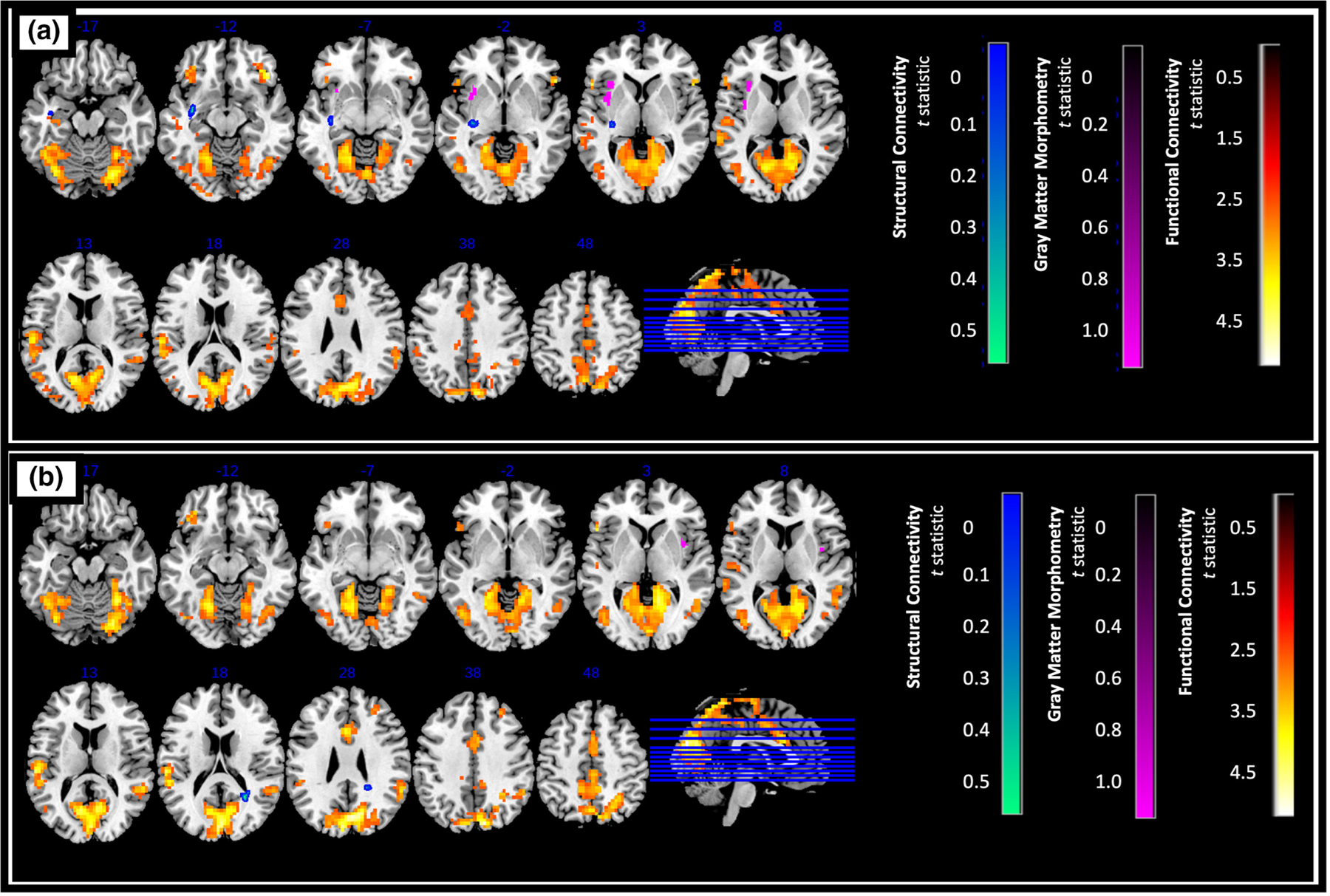
Difference in GM density, structural connectivity, and functional connectivity of (a) left anterior insula (b) right anterior insula (control > AUD, pFWE < .025, [FDR corrected, cluster size of k > 120 for functional connectivity analysis]). Higher t statistic and lower p-values indicate greater difference between the two groups.
FIGURE 4.
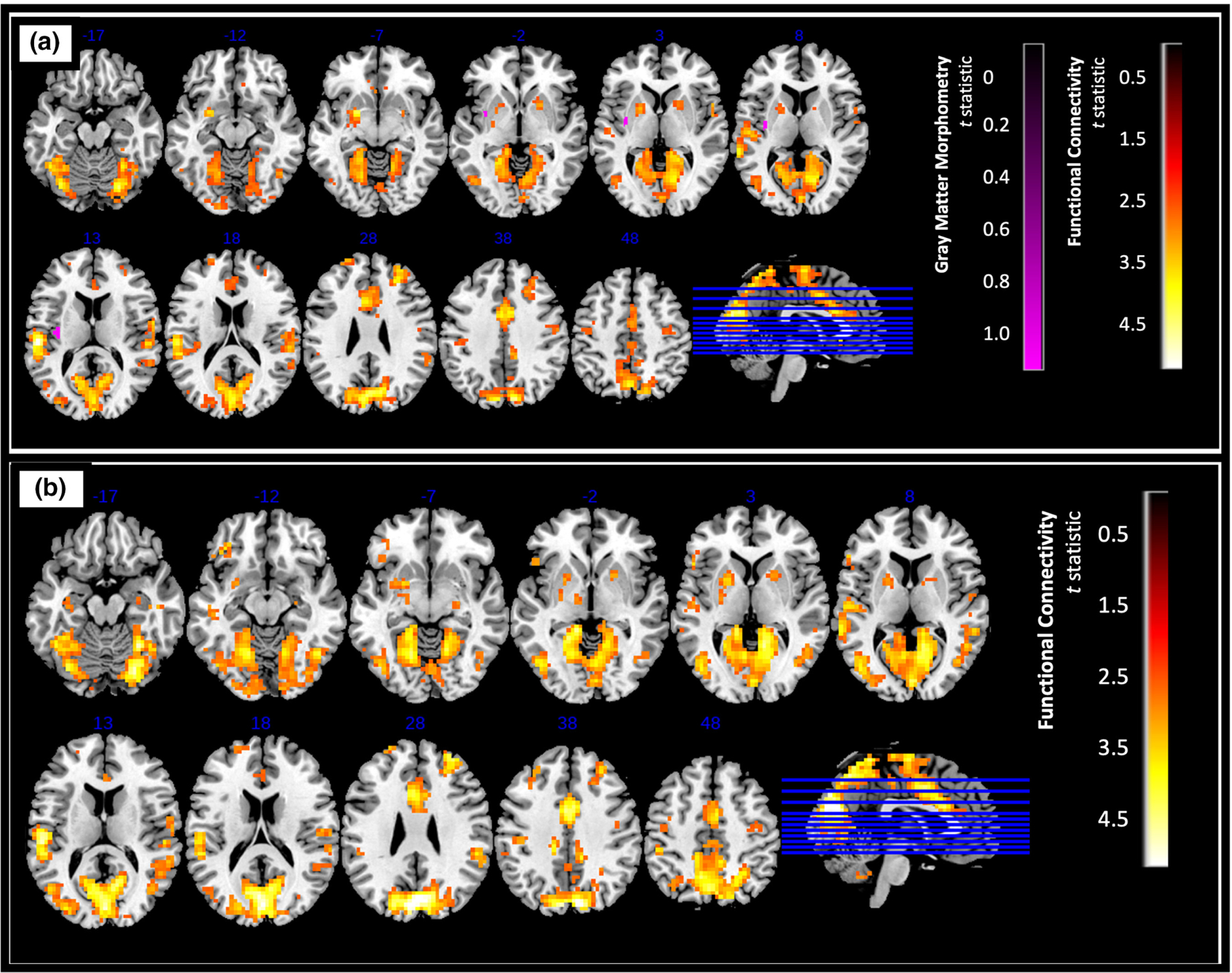
Difference in GM density and functional connectivity of (a) left posterior insula (b) right posterior insula (control > AUD, pFWE < .025, [FDR corrected, cluster size of k > 120 for functional connectivity analysis]). Higher t statistic and lower p-values indicate greater difference between the two groups.
The correlation analysis performed between smoking status and GM density in individuals with AUD revealed that the lower density of left anterior insula may be due to a combined effect of AUD and smoking, and that the actual differences in volume between the two groups may be greater if smokers were excluded from the study (Table S1). It is important to note that although this result was significant, the correlation itself was weak (r = 0.28).
3.2 |. Insula white matter connectivity
Group probabilistic maps created for the anterior insula revealed connectivity involving the inferior frontal, anterior temporal, and inferior parietal regions. The maps created for the posterior insula revealed connections involving the anterior temporal and inferior parietal regions.
The connectivity analysis showed significant differences between groups (control > AUD) for only the left and right anterior insula (pFWE < .025, Table 3), where connectivity was higher in the control group. The clusters that significantly differed in FA of the left anterior insula tractography between healthy controls and AUD groups belonged to the left inferior fronto-occipital fasciculus (IFOF), and left uncinate fasciculus. For the right anterior insula, the clusters belonged to the forceps major and right inferior fronto-occipital fasciculus. There were no structural connectivity differences observed in either left or right posterior insula between healthy controls and AUD groups. Connectivity analysis performed using the MD images complemented the FA results reported above. Results from this analysis can be found in the supplement.
TABLE 3.
Results from ANCOVA analyses showing regions with significantly higher fractional anisotropy in the control group relative to AUD group (control > AUD)
| ROI | Cluster | Region (JHU atlas) | Voxels | Peak coordinate (mm) |
Statistical comparison between groups (p-value) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Left anterior insula | A | Inferior fronto-occipital fasciculus L | 46 | −32 | −20 | −1 | .017* |
| B | Inferior fronto-occipital fasciculus L Uncinate fasciculus L | 36 | −36 | −8 | −13 | .023* | |
| C | Inferior fronto-occipital fasciculus L | 20 | −35 | −21 | −4 | .023* | |
| Right anterior insula | A | Inferior fronto-occipital fasciculus R Inferior longitudinal fasciculus R | 74 | 247 | −54 | 19 | .023* |
| B | Inferior fronto-occipital fasciculus R | 13 | 25 | −48 | 22 | .025* | |
p < .001;
3.3 |. Insula resting state functional connectivity
Overall, we found significantly greater resting state connectivity for both the anterior and posterior insula with frontal, parietal, temporal, and occipital regions in controls compared to AUD (p < .025, FDR corrected, a cluster size of k > 120 determined using 3dClustSim, Figures 3 and 4).
Specifically, significantly higher connectivity was observed between the left anterior insula and the left occipital cortex, middle cingulate gyrus, right cerebellum, left superior temporal gyrus, and left precuneus (Figure 3a), while higher right anterior insula connectivity was observed with the left and right cuneus, as well as the left superior temporal gyrus (Figure 3b). Connectivity between the left anterior insula and the right cerebellum is potentially even lower in AUD participants that also smoked (Table S1).
For the left posterior insula, higher connectivity was observed with the left cuneus, left superior temporal gyrus, left precuneus, right middle cingulate gyrus, and right cerebellum (Figure 4a). Very similarly, for the right posterior insula, increased connectivity was observed with the same regions (except the left precuneus) as the left posterior insula (Figure 4b).
3.4 |. Relationship between structural and functional measures
To better understand the relationship between observed differences in brain structure and function in those with AUD, we conducted an exploratory correlational analysis investigating the relationships between white matter connectivity, gray matter morphometry, and resting state functional connectivity in regions that demonstrated significant differences from the group-level analyses.
The results from this exploratory analysis provided limited evidence for relationships between these measures. All correlations were weak and only one remained significant at a threshold of p < .01. We observed a significant positive correlation between gray matter density in the right anterior insula and functional connectivity of the right anterior insula with the left superior temporal gyrus (r = 0.30, p < .01).
4 |. DISCUSSION
The current study investigated the morphometry, structural, and functional connectivity of anterior and posterior insula regions in AUD, and how they compare to healthy individuals. As hypothesized, there were significant differences in insula structure and connectivity of persons with AUD compared to controls. Furthermore, as expected, the results indicated differing connectivity with frontal, parietal, occipital, cerebellar, and temporal brain regions in AUD. While the design of this study does not allow for conclusions about causality, we observed a relationship between structural and functional measures in the AUD group that was not present in the control group. The following sections discuss these results in terms of group differences for both the anterior and posterior insula.
4.1 |. Differences in GM volume and morphometry
Insula GM volume analysis revealed significantly lower volume in the AUD group in the anterior and posterior insula in both hemispheres compared to the control group. Morphometry analysis revealed lower GM density in the AUD group in anterior insula in both left and right hemispheres, but only in the left hemisphere for the posterior insula. While reduced anterior insula volume and GM density is consistent with preliminary findings from a subset of the current sample (Grodin et al., 2013, 2017; Momenan et al., 2012) and prior literature (Senatorov et al., 2015), there is very little evidence showing differences in posterior insula volume. One study by Galandra and colleagues investigating the posterior insula showed AUD-related volume differences primarily in the right posterior insula (Galandra et al., 2018), instead of the left posterior insula as observed in the current study. However, this discrepancy and the lack of differences in posterior insula volume reported by other prior studies could be due to low power. Importantly, the WM and functional connectivity results of this study support the differences observed within the volumetric results. Alcohol misuse is known to affect cognitive functioning, and the posterior insula has been suggested to be associated with both affective-perceptual and cognitive–evaluative forms of empathy (Fan et al., 2011). Therefore, the effects of chronic alcohol use on the posterior insula may contribute to cognitive difficulties observed in other studies. Overall it is possible that such differences in function between the anterior and posterior insula (Kurth et al., 2010) influence how much each region is affected by alcohol misuse.
4.2 |. Insula WM tractography and differences in WM microstructure
As expected (Cerliani et al., 2012; Cloutman et al., 2012; Ghaziri et al., 2017; Uddin et al., 2011), the WM tractography generated for the anterior insular regions traversed the inferior frontal, superior temporal, and inferior parietal regions, while WM tractography for the posterior insular regions is involved in superior temporal and inferior parietal regions. This illustrates the influence of the insula on regions involved in executive functioning and goal-directed movement. It has been suggested that the anterior insula plays the role of integrating autonomic and visceral information into emotional and motivational functions, while the posterior insula integrates somatosensory, vestibular, and motor functions (Naqvi et al., 2014).
Overall, lower WM integrity (i.e., connectivity) in the brain has been observed in those with AUD compared to controls (Crespi et al., 2020; Pfefferbaum et al., 2009). There are asymmetric differences in the healthy brain, where WM connecting frontal, parietal, and temporal regions are left hemisphere biased, while WM connecting the posterior temporal and superior parietal lobe is right hemisphere biased (Barrick et al., 2006). As the results suggest, the primary difference in FA for the left anterior insula was observed in the regions belonging to the left inferior fronto-occipital fasciculus. Dysfunction of these pathways have been shown to be associated with emotionally driven responses and sensation seeking behavior (Fettes et al., 2017). Differences in FA for the right anterior insula were observed in regions belonging to the forceps major, right inferior longitudinal fasciculus, and right inferior fronto-occipital fasciculus. The peaks of these clusters with differing FA were situated in or around the right parietal area, indicating alcohol-related effects in WM pathways that also propagate transcallosally, between hemispheres. The right posterior parietal cortex exerts strong inhibitory activity over the contralateral homologous areas that is mediated by direct pathways located in the posterior corpus callosum (Koch et al., 2011). Moreover, lesion studies have shown that damage to these parietal areas can result in visuospatial neglect (Vallar et al., 2003). Collectively, these results suggest that differences to the insular WM tractography could implicate integration of cognitive, visuospatial attention, and goal-directed functions.
4.3 |. Differences in insular seed-based resting state functional connectivity
Similar to WM connectivity results, both left and right anterior insula resting state functional connectivity differences were observed with frontal, striatal, parietal, occipital, cingulate, cerebellar, and temporal regions in the AUD group. However, unlike structural connectivity findings, these differences were observed in both anterior and posterior regions. This is consistent with prior studies that tested resting state functional connectivity of the insula, and are considered impairments reflected in functional networks such as the fronto-parietal, default mode, and salience networks.
The resting fronto-parietal network is considered to represent the brain's control system, which regulates other systems in a goal-directed manner to promote and maintain homeostatic balance across distributed neural systems (Cole et al., 2013). Disruption of this system may indicate behavioral regulation difficulties present in persons with AUD. Changes to the default mode network have been linked with craving, negative mood, and relapse in addiction and impairment in integrating information from other brain regions for self-related decision making (Zhang & Volkow, 2019). Damage to the salience-detection network has been shown to impair attention and working memory in persons with AUD. Given that the insula is considered to integrate perceptual, cognitive, and autonomic information through this network (Galandra et al., 2018), lower connectivity between the insula and regions of this resting network may be deleterious for functioning of these systems.
Additionally, the middle cingulate cortex (MCC) emerged as a noteworthy region, showing lower functional connectivity with the posterior insula in those with AUD. The cingulate cortex and the insula are considered as two of the major components of the salience network. The MCC has been implicated in cognitive and sensorimotor function (Bush et al., 2000; Critchley et al., 2004), while the posterior insula has been shown to receive interoceptive information (Craig, 2002; Critchley et al., 2004). Alcohol abuse can lower connectivity of the MCC related to stress cue processing, which is also an indication of subsequent alcohol relapse (Zakiniaeiz et al., 2016). The MCC and insula are anatomically connected and form a vitality form circuit, which plays a role in the expression of actions (e.g., gentle, rude etc.) (Cesare et al., 2021). Although the existing literature about the role of MCC in AUD is limited, based on prior findings it is possible that an alteration in response to stressful cues contribute to deviations from expression of normal vitality forms in persons with AUD.
The superior temporal gyrus and cerebellum were also identified as regions with significantly lower connectivity with the insula. Co-activation of the insula and superior temporal gyrus has been linked to the decision-making process (Paulus et al., 2005). The insula and superior temporal gyrus have shown to be structurally connected (Ghaziri et al., 2017). It has been shown that smaller surface area of the superior temporal gyrus is associated with greater trait impulsivity in addiction (Kaag et al., 2014). Although the structural changes of the temporal lobe were not investigated in the current study, this literature gives us an understanding about how the decision-making process may be affected in this population. In addition, correlational analyses revealed an association between decreased gray matter density in the right anterior insula with lower insular functional connectivity with the superior temporal gyrus. While exploratory, this finding could suggest that the impact of alcohol in the left anterior insula may have a more direct effect on its functional connectivity with the superior temporal regions, potentially carrying implications for decision-making processes in individuals with AUD as discussed above. Literature regarding the connectivity between the insula and cerebellum are limited. However, the few existing studies have shown that white matter systems of the cerebellum are vulnerable to chronic alcohol abuse. Moreover, greater cerebellar deficit has been linked to poorer motor performance (Zhao et al., 2019). Direct structural connections exist between the insula and brain regions involved in performing motor movements (Uddin et al., 2017). Lower connectivity between the insula and cerebellum observed in the current study could possibly indicate poor motor performance in AUD. Further investigation is warranted to verify this potential relationship.
4.4 |. Limitations
Those in the AUD group were more likely to report tobacco smoking and comorbid psychiatric diagnoses than controls, who had very few psychiatric diagnoses or tobacco use. Since these comorbidities were observed almost entirely in those with AUD, we could not effectively control for them as separate from the effects of group. Although the smoking status analysis was able to provide some insight into the effects of smoking in AUD, future studies should give more consideration during recruitment to appropriately match groups.
In addition, while we included sex as a covariate in all analyses, we did not formally test the presence of sex differences. Prior research has shown that men typically have larger total brain volumes and larger volumes of several subregions, including the insula (Cosgrove et al., 2007; Ruigrok et al., 2014). Previous studies have also shown that men have lower gray matter volume, but higher gray matter density in insular regions (Cosgrove et al., 2007; Ruigrok et al., 2014). Additionally, there is some evidence for sex differences in functional connectivity of the insula with other brain regions (Kann et al., 2016). While some research has explored structural and functional sex differences in the context of AUD, results have been mixed and further research is needed to better understand the interaction between biological sex and observed differences associated with chronic alcohol use (Kisner et al., 2021; Verplaetse et al., 2021).
Since these findings are not based on a longitudinal study, conclusions cannot be drawn regarding whether lower functional connectivity observed with the insula is in fact a more direct result of lower structural integrity. In the absence of a longitudinal component any causal relationship drawn from these data should be treated with caution. Furthermore, a longitudinal study could test whether lower structure or function of the insular regions serves as a predisposition for the development of AUD. Any claims regarding possible relationships between cognitive/behavioral deficits and observed neural alterations is based on findings from other studies; these associations were not tested in the current study.
5 |. CONCLUSION
This is the first study of this sample size that assesses morphometric, structural, and functional connectivity of the insula associated with AUD. While functional differences were observed between AUD and healthy controls in both the anterior and posterior insula, the structural (morphometric and white matter connectivity) differences were mostly observed in the anterior regions. However, it seems that these structural differences in the anterior regions may contribute to functional differences mostly lateralized to the left hemisphere. These results conform with prior studies showing differences primarily in anterior insular regions. Importantly, based on existing knowledge of the functionality of the brain regions showing connectivity differences with the anterior insula, it is possible that the lower connectivity suggests alcohol-related effects on tasks requiring higher cognitive and emotional processing. A study constituting a larger sample size, better matched in demographics could serve to clarify these results. Furthermore, future studies testing longitudinal effects and relationships between neural correlates and behavioral measures are needed in order to better understand the effects of alcohol on the insula.
Supplementary Material
TABLE S1 Correlation of smoking status with insula structure and functional connectivity in AUD. Values in square brackets indicate the 95% confidence interval for each correlation. The confidence interval is a plausible range of population correlations that could have caused the sample correlation (Cumming, 2014). *p < .05. **p < .01.
TABLE S2 Two-sample t test results showing regions with significantly higher mean diffusivity in the AUD group compared to control (AUD > control, statistical significance indicated by *p < .025 and **p < .001).
Transparent Science Questionnaire for Authors
Significance.
Previous research has demonstrated differences in insular regions in those with alcohol use disorder (AUD) compared to healthy controls, using only structural or functional MR imaging. To the best of our knowledge, the present study is the first to utilize multiple MR modalities to simultaneously investigate the gray matter volume and morphometry, diffusion tensor imaging of white matter microstructure, and resting state functional connectivity alterations in insula in humans with chronic alcohol use. The findings reported here provide a more comprehensive insight into the impact of AUD on the structural and functional differences in the insula.
ACKNOWLEDGMENTS
We thank Samantha Fede, Carlos Cortez, Sarah Dean, Savita Madan, Nicholas Bustos, Sasha Brietzke, Nicole MacIlvane, Sophie Haven, and Jesse Honig for their assistance with data collection. We also acknowledge Nicholas Bustos, and Claire Hunt for their assistance with data preprocessing as well as Mike Kerich for technical support during preprocessing and analysis. This study was funded with support from the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (ZAIAA000123: PI Reza Momenan), National Institutes of Health (NIH).
Funding information
National Institute on Alcohol Abuse and Alcoholism, Grant/Award Number: ZAIAA000123
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
DECLARATION OF TRANSPARENCY
The authors, reviewers and editors affirm that in accordance to the policies set by the Journal of Neuroscience Research, this manuscript presents an accurate and transparent account of the study being reported and that all critical details describing the methods and results are present.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jnr.25113.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, & Hof PR (2010). The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Structure and Function, 214(5–6), 495–517. 10.1007/s00429-010-0254-0 [DOI] [PubMed] [Google Scholar]
- Andersson JL, Jenkinson M, & Smith S (2007). Non-linear registration, aka spatial normalisation FMRIB technical report (TR07JA2). FMRIB Analysis Group of the University of Oxford, 2(1), e21. [Google Scholar]
- Barrick TR, Lawes IN, Mackay CE, & Clark CA (2006). White matter pathway asymmetry underlies functional lateralization. Cerebral Cortex, 17(3), 591–598. 10.1093/cercor/bhk004 [DOI] [PubMed] [Google Scholar]
- Baumgärtner U, Buchholz HG, Bellosevich A, Magerl W, Siessmeier T, Rolke R, Höhnemann S, Piel M, Rösch F, Wester HJ, Henriksen G, Stoeter P, Bartenstein P, Treede RD, & Schreckenberger M (2006). High opiate receptor binding potential in the human lateral pain system. Neuroimage, 30(3), 692–699. 10.1016/j.neuroimage.2005.10.033 [DOI] [PubMed] [Google Scholar]
- Betka S, Harris L, Rae C, Palfi B, Pfeifer G, Sequeira H, Duka T, & Critchley H (2019). Signatures of alcohol use in the structure and neurochemistry of insular cortex: A correlational study. Psychopharmacology, 236(9), 2579–2591. 10.1007/s00213-019-05228-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. 10.1016/s1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Cereda C, Ghika J, Maeder P, & Bogousslavsky J (2002). Strokes restricted to the insular cortex. Neurology, 59(12), 1950–1955. 10.1212/01.wnl.0000038905.75660.bd [DOI] [PubMed] [Google Scholar]
- Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D'Arceuil H, & Keysers C (2012). Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human Brain Mapping, 33(9), 2005–2034. 10.1002/hbm.21338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, & Sullivan EV (2011). Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral Cortex, 21(10), 2272–2281. 10.1093/cercor/bhq297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, & Lambon Ralph MA (2012). The variation of function across the human insula mirrors its patterns of structural connectivity: Evidence from in vivo probabilistic tractography. NeuroImage, 59(4), 3514–3521. 10.1016/j.neuroimage.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, & Braver TS (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, & Staley JK (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry, 62(8), 847–855. 10.1016/j.biopsych.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Crespi C, Galandra C, Canessa N, Manera M, Poggi P, & Basso G (2020). Microstructural damage of white-matter tracts connecting large-scale networks is related to impaired executive profile in alcohol use disorder. NeuroImage: Clinical, 25, 102141. 10.1016/j.nicl.2019.102141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Di Cesare G, Marchi M, Lombardi G, Gerbella M, Sciutti A, & Rizzolatti G (2021). The middle cingulate cortex and dorso-central insula: A mirror circuit encoding observation and execution of vitality forms. Proceedings of the National Academy of Sciences of the United States of America, 118(44), e2111358118. 10.1073/pnas.2111358118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G, S. S., Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM, & James A (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain, 130, 2375–2386. [DOI] [PubMed] [Google Scholar]
- Droutman V, Read SJ, & Bechara A (2015). Revisiting the role of the insula in addiction. Trends in Cognitive Sciences, 19(7), 414–420. 10.1016/j.tics.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy M, & Chanraud S (2016). Chapter one—Imaging the addicted brain: Alcohol. In Zahr NM & Peterson ET (Eds.), International review of neurobiology (Vol. 129, pp. 1–31). Academic Press. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews, 35(3), 903–911. 10.1016/j.neubiorev.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Fede SJ, Grodin EN, Dean SF, Diazgranados N, & Momenan R (2019). Resting state connectivity best predicts alcohol use severity in moderate to heavy alcohol users. Neuroimage Clinical, 22, 101782. 10.1016/j.nicl.2019.101782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettes P, Schulze L, & Downar J (2017). Cortico-striatal-thalamic loop circuits of the orbitofrontal cortex: Promising therapeutic targets in psychiatric illness. Frontiers in Systems Neuroscience, 11, 25. 10.3389/fnsys.2017.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5-research version (SCID-5 for DSM-5, research version; SCID-5-RV). American Psychiatric Association. [Google Scholar]
- First MB, Williams JBW, Spitzer RL, & Gibbon M (2007). Structured clinical interview for DSM-IV-TR Axis I disorders, clinical trials version (SCID-CT). Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage, 62(2), 774–781. 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, & Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Fortier CB, Leritz EC, Salat DH, Lindemer E, Maksimovskiy AL, Shepel J, Williams V, Venne JR, Milberg WP, & McGlinchey R (2014). Widespread effects of alcohol on white matter microstructure. Alcoholism, Clinical and Experimental Research, 38(12), 2925–2933. 10.1111/acer.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galandra C, Basso G, Manera M, Crespi C, Giorgi I, Vittadini G, Poggi P, & Canessa N (2018). Salience network structural integrity predicts executive impairment in alcohol use disorders. Scientific Reports, 8(1), 14481. 10.1038/s41598-018-32828-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaziri J, Tucholka A, Girard G, Houde JC, Boucher O, Gilbert G, Descoteaux M, Lippé S, Rainville P, & Nguyen DK (2017). The corticocortical structural connectivity of the human insula. Cerebral Cortex, 27(2), 1216–1228. 10.1093/cercor/bhv308 [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, & Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on alcohol and related conditions III. JAMA Psychiatry, 72(8), 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Cortes CR, Spagnolo PA, & Momenan R (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug and Alcohol Dependence, 179, 100–108. 10.1016/j.drugalcdep.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodin EN, Lin H, Durkee CA, Hommer DW, & Momenan R (2013). Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co-morbid substance abuse. Neuroimage Clinical, 2, 469–476. 10.1016/j.nicl.2013.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcomb ME, Chumin EJ, Goñi J, Dzemidzic M, & Yoder KK (2019). Aberrations of anterior insular cortex functional connectivity in nontreatment-seeking alcoholics. Psychiatry Research: Neuroimaging, 284, 21–28. 10.1016/j.pscychresns.2018.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton WH, Hanik IM, & Olson IR (2019). Substance abuse and white matter: Findings, limitations, and future of diffusion tensor imaging research. Drug and Alcohol Dependence, 197, 288–298. 10.1016/j.drugalcdep.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, & Eckardt M (1996). Decreased corpus callosum size among alcoholic women. Archives of Neurology, 53(4), 359–363. 10.1001/archneur.1996.00550040099019 [DOI] [PubMed] [Google Scholar]
- Hurd YL, Suzuki M, & Sedvall GC (2001). D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. Journal of Chemical Neuroanatomy, 22(1–2), 127–137. 10.1016/s0891-0618(01)00122-3 [DOI] [PubMed] [Google Scholar]
- Irfanoglu MO, Walker L, Sarlls J, Marenco S, & Pierpaoli C (2012). Effects of image distortions originating from susceptibility variations and concomitant fields on diffusion MRI tractography results. Neuroimage, 61(1), 275–288. 10.1016/j.neuroimage.2012.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Crunelle CL, van Wingen G, Homberg J, van den Brink W, & Reneman L (2014). Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non-drug using controls. Drug and Alcohol Dependence, 144, 210–217. 10.1016/j.drugalcdep.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Kann S, Zhang S, Manza P, Leung HC, & Li CR (2016). Hemispheric lateralization of resting-state functional connectivity of the anterior insula: Association with age, gender, and a novelty-seeking trait. Brain Connectivity, 6(9), 724–734. 10.1089/brain.2016.0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisner MA, Sussman L, Manuweera T, Grodin EN, Fede SJ, Sarlls JE, & Momenan R (2021). Evaluating effects of sex and age on white matter microstructural alterations in alcohol use disorder: A diffusion tensor imaging study. Alcoholism, Clinical and Experimental Research, 45(9), 1790–1803. 10.1111/acer.14678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Bonnì S, Giacobbe V, Bucchi G, Versace V, Caltagirone C, & Bozzali M (2011). Asymmetry of parietal interhemispheric connections in humans. Journal of Neuroscience, 31(24), 8967–8975. 10.1523/jneurosci.6567-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thümmel M, Chai C, Pfeifer P, Stoeter P, Scheurich A, & Fehr C (2012). Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol and Alcoholism, 47(2), 118–126. 10.1093/alcalc/agr157 [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, & Eickhoff SB (2010). A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure & Function, 214(5–6), 519–534. 10.1007/s00429-010-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, & Sullivan EV (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism, Clinical and Experimental Research, 41(8), 1432–1443. 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenan R, Steckler LE, Saad ZS, van Rafelghem S, Kerich MJ, & Hommer DW (2012). Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Research, 204(2–3), 101–111. 10.1016/j.pscychresns.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Monnig MA, Tonigan JS, Yeo RA, Thoma RJ, & McCrady BS (2013). White matter volume in alcohol use disorders: A meta-analysis. Addiction Biology, 18(3), 581–592. 10.1111/j.1369-1600.2012.00441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, & Mesulam MM (1982). Insula of the old world monkey. II: Afferent cortical input and comments on the claustrum. Journal of Comparative Neurology, 212(1), 23–37. 10.1002/cne.902120103 [DOI] [PubMed] [Google Scholar]
- Müller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, & Schulte T (2015). The resting brain of alcoholics. Cerebral Cortex, 25(11), 4155–4168. 10.1093/cercor/bhu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, & Bechara A (2009). The hidden Island of addiction: The insula. Trends in Neurosciences, 32(1), 56–67. 10.1016/j.tins.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, & Bechara A (2014). The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Annals of the New York Academy of Sciences, 1316, 53–70. 10.1111/nyas.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R (2012). The insular cortex: A review. Progress in Brain Research, 195, 123–163. 10.1016/b978-0-444-53860-4.00007-6 [DOI] [PubMed] [Google Scholar]
- Nomi JS, Farrant K, Damaraju E, Rachakonda S, Calhoun VD, & Uddin LQ (2016). Dynamic functional network connectivity reveals unique and overlapping profiles of insula subdivisions. Human Brain Mapping, 37(5), 1770–1787. 10.1002/hbm.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M (2000). Neuropsychological vulnerabilities in chronic alcoholism. Review of NIAAA's Neuroscience and Behavioral Research Portfolio, 34, 149–158. [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, & Simmons AN (2005). Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage, 25(2), 607–615. 10.1016/j.neuroimage.2004.12.055 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, & Sullivan EV (2006). Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biological Psychiatry, 59(4), 364–372. 10.1016/j.biopsych.2005.06.025 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, & Sullivan EV (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biological Psychiatry, 65(8), 680–690. 10.1016/j.biopsych.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, & Sullivan EV (2010). Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: Selective relations to dissociable functions. Alcoholism, Clinical and Experimental Research, 34(7), 1201–1211. 10.1111/j.1530-0277.2010.01197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, & Suckling J (2014). A meta-analysis of sex differences in human brain structure. Neuroscience and Biobehavioral Reviews, 39(100), 34–50. 10.1016/j.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, & Grant M (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction, 88(6), 791–804. 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Damadzic R, Mann CL, Schwandt ML, George DT, Hommer DW, Heilig M, & Momenan R (2015). Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain, 138(Pt 1), 69–79. 10.1093/brain/awu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, & Sheu W-J (1982). Reliability of alcohol use indices: The lifetime drinking history and the MAST. Journal of Studies on Alcohol, 43(11), 1157–1170. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TE (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage, 31(4), 1487–1505. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2013). Neuropsychology and neuroimaging studies in alcohol-dependence. Revue de Neuropsychologie, 5(3), 187–199. 10.3917/rne.053.0187 [DOI] [Google Scholar]
- Tsai G, Gastfriend DR, & Coyle JT (1995). The glutamatergic basis of human alcoholism. American Journal of Psychiatry, 152(3), 332–340. 10.1176/ajp.152.3.332 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B, Ghaziri J, & Boucher O (2017). Structure and function of the human insula. Journal of Clinical Neurophysiology, 34(4), 300–306. 10.1097/wnp.0000000000000377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, & Menon V (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience, 31(50), 18578–18589. 10.1523/jneurosci.4465-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Bottini G, & Paulesu E (2003). Neglect syndromes: The role of the parietal cortex. Advances in Neurology, 93, 293–319. [PubMed] [Google Scholar]
- Vergara VM, Liu J, Claus ED, Hutchison K, & Calhoun V (2017). Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage, 151, 45–54. 10.1016/j.neuroimage.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Cosgrove KP, Tanabe J, & McKee SA (2021). Sex/gender differences in brain function and structure in alcohol use: A narrative review of neuroimaging findings over the last 10 years. Journal of Neuroscience Research, 99(1), 309–323. 10.1002/jnr.24625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, & Nichols TE (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, & Pfefferbaum A (2017). Alcohol's effects on the brain: Neuroimaging results in humans and animal models. Alcohol Research, 38, 183–206. [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, & Constable RT (2016). Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clinical, 13, 181–187. 10.1016/j.nicl.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, & Volkow ND (2019). Brain default-mode network dysfunction in addiction. Neuroimage, 200, 313–331. 10.1016/j.neuroimage.2019.06.036 [DOI] [PubMed] [Google Scholar]
- Zhao Q, Pfefferbaum A, Podhajsky S, Pohl K, & Sullivan E (2019). Accelerated aging and motor control deficits are related to regional deformation of central cerebellar white matter in alcohol use disorder. Addiction Biology, 25, e12746. 10.1111/adb.12746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, & Momenan R (2017). Model-free functional connectivity and impulsivity correlates of alcohol dependence: A resting-state study. Addiction Biology, 22(1), 206–217. 10.1111/adb.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Du X, Kerich M, Lohoff FW, & Momenan R (2018). Random forest based classification of alcohol dependence patients and healthy controls using resting state MRI. Neuroscience Letters, 676, 27–33. 10.1016/j.neulet.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Murray DE, Durazzo TC, Schmidt TP, Murray TA, & Meyerhoff DJ (2018). White matter microstructural correlates of relapse in alcohol dependence. Psychiatry Research: Neuroimaging, 281, 92–100. 10.1016/j.pscychresns.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Correlation of smoking status with insula structure and functional connectivity in AUD. Values in square brackets indicate the 95% confidence interval for each correlation. The confidence interval is a plausible range of population correlations that could have caused the sample correlation (Cumming, 2014). *p < .05. **p < .01.
TABLE S2 Two-sample t test results showing regions with significantly higher mean diffusivity in the AUD group compared to control (AUD > control, statistical significance indicated by *p < .025 and **p < .001).
Transparent Science Questionnaire for Authors
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


