Abstract
The native lactococcal plasmid pKR223 encodes two distinct phage resistance mechanisms, a restriction and modification (R/M) system designated LlaKR2I and an abortive infection mechanism (Abi) which affects prolate-headed-phage proliferation. The nucleotide sequence of a 16,174-bp segment of pKR223 encompassing both the R/M and Abi determinants has been determined, and sequence analysis has validated the novelty of the Abi system, which has now been designated AbiR. Analysis of deletion and insertion clones demonstrated that AbiR was encoded by two genetic loci, separated by the LlaKR2I R/M genes. Mechanistic studies on the AbiR phenotype indicated that it was heat sensitive and that it impeded phage DNA replication. These data indicated that AbiR is a novel multicomponent, heat-sensitive, “early”-functioning Abi system and is the first lactococcal Abi system described which is encoded by two separated genetic loci.
Lactococci are widely used in the manufacture of numerous fermented dairy products. The nonsterile nature of many of these processes renders them particularly vulnerable to phage infection. Many strains have evolved natural phage resistance mechanisms and can be divided into four classes based on their mode of action: adsorption blocking, phage DNA penetration blocking, restriction and modification (R/M), and abortive infection (Abi) (reviewed in reference 15). While mechanisms of the first two classes act at the cell surface, R/M and Abi mechanisms act intracellularly. The complementary action of resistance mechanisms can provide strains with a significant barrier to phage infection.
Abi systems refer to any phage defense, other than R/M systems, which prevents phage proliferation after the phage DNA has entered the host cell intact. These systems encompass a diverse variety of mechanisms, which may act to prevent phage DNA replication, transcription, translation, processing, or assembly or lysis of the cell. Since 1984, when pNP40, which encodes an Abi system, was first reported (22), numerous native Abi-encoding plasmids have been reported. Currently, the genetic determinants of 17 Abi systems have been described, and all but two of them were encoded on native lactococcal plasmids (reviewed in reference 10). The majority of these Abi phage defense systems were found to be encoded by single genes, except for AbiE (13), AbiG (25), and AbiL (6), which are encoded by two genes. Comparisons of Abi sequences with existing database sequences have offered little insight into the molecular basis for their modes of action. In addition, comparing these sequences with each other has generally indicated evolutionary, diverse origins, presumably due to the multitude of potential mechanisms of action for this type of defense. The notable exceptions are AbiD (24), AbiDI (2), and AbiF (13), which exhibit significant similarity to each other, pointing to the presence of a family of Abi mechanisms. In addition, AbiA, which was identified as being encoded by pTR2030 (19) and also by pCI829 (5), shares homology with AbiK (9). A discernible molecular feature common to all lactococcal Abi systems characterized to date is that the G+C content of the encoding loci is significantly lower than the typical G+C content of lactococcal DNA.
The native lactococcal phage resistance plasmid pKR223 was isolated from Lactococcus lactis subsp. lactis bv. diacetylactis KR2 and was shown to mediate two distinct mechanisms of resistance to bacteriophage infection, an R/M system and an Abi system (20, 23). The sequence and genetic characterization of the R/M system (designated LlaKR2I) have been reported previously (31). The Abi system impeded proliferation of prolate-headed phage, and preliminary analysis indicated that the genetic determinants for its expression may not all be contained in one locus (23, 30). In this study we furthered the characterization of this novel phage defense system and determined the loci on the plasmid which are necessary for its expression.
Bacteria, plasmids, phage, and growth conditions.
The L. lactis strains used in this study included L. lactis subsp. lactis bv. diacetylactis KR2, an industrial starter strain containing seven plasmids, including pKR223, which encodes the LlaKR2I R/M system and an Abi mechanism (20); L. lactis subsp. cremoris LM0230, a plasmid-free derivative of L. lactis subsp. cremoris C2 (7) (formerly called L. lactis subsp. lactis); and GBK17, an LM0230 transformant with a 19-kb HpaII fragment of pKR223 cloned into the HpaII site of pGB301 in a construct designated pGBK17 (20). Escherichia coli XL1Blue MRF′ (Stratagene, La Jolla, Calif.) served as the host for the construction of pUC19 chimeras containing fragments of pGBK17 for sequencing. Plasmid pMN7 is an SphI deletion of pGBK17 and is R/M and Abi positive (23). Plasmid pMN5 is a partial EcoRI deletion of pGBK17 and is R/M positive and Abi negative (23). Plasmid pDOT64 is the LlaKR2I R/M system cloned in pCI372 (31). Plasmid pTRKH2 is a 6.9-kb E. coli/Lactococcus shuttle cloning vector (27). The prolate-headed bacteriophage φc2, which was obtained from the culture collection of L. L. McKay, was used for monitoring the Abi phenotype encoded by pKR223 and its derivatives. Growth conditions for lactococci and E. coli, as well as for phage assays, were as described previously (31).
Confirmation of the pKR223 Abi phenotype.
Previous studies indicated that a 19-kb region of pKR223 encoded an R/M system and another phage defense system which did not impede absorption of the phage to the cells. As it also resulted in an approximately twofold reduction in the burst size of the phage, it was termed an Abi system (20). To exclude the possibility that this system might be impeding injection of the phage DNA into the cell, cell survival studies were undertaken. Unlike Abi systems, which undergo cell death following phage infection, DNA injection blocking systems do not result in cell death (14). The effect of the Abi mechanism on cell survival was evaluated by infecting a mid-log-phase suspension of L. lactis LM0230(pGBK17) in M17G containing 5 mM CaCl2 with φc2, at a multiplicity of infection of >1, and monitoring the survival of cells over a 3-h period. As L. lactis LM0230(pGBK17) infected with methylated φc2 resulted in 100% cell death, it can be concluded that the phage defense system is an Abi mechanism.
Sequence analysis of the pKR223 Abi system.
Previous deletion studies of this 19-kb region of pKR223 indicated that the R/M and Abi systems were located on a 16.2-kb HpaII/SphI fragment (23). This fragment was subcloned in a variety of fragments in pUC19, and the nucleotide sequence of both DNA strands was determined. This segment included a 4.6-kb EcoRV/XbaI fragment, which encodes the LlaKR2I R/M system comprising an endonuclease gene (llaKR2IR) and a methyltransferase gene (llaKR2IM) which are divergently transcribed with respect to each other, with a complete copy of the lactococcal insertion sequence IS982, located in the intergenic region (31). Sequencing reactions were performed with an ABI Prism dye terminator cycle sequencing kit using AmpliTaq DNA polymerase, and the products were separated using an ABI 377 automatic sequencer (Applied Biosystems, Foster City, Calif.). DNA sequences were compiled and analyzed using DNASTAR software (DNASTAR, Madison, Wis.) and were compared to database sequences using the BLAST suite of programs (1).
In addition to the LlaKR2I R/M genes and two IS982 elements, the sequence organization revealed seven additional open reading frames (ORFs) and a 5′ truncated ORF (Fig. 1). The truncated ORF, ORF7, spanned the HpaII site used in the construction of pGBK17, and the deduced truncated protein sequence is homologous to members of the CBXX/CBQX superfamily of proteins with greatest identity (44% over 214 amino acids) to the C-terminal end of SpoVJ from Bacillus subtilis (11). The deduced protein sequence of ORF6 shared similarity with a gene of unknown function located on the 3′ end of an operon encoding the LlaI R/M system on pTR2030 (28), with 30% identity observed over 168 amino acids. Based on homologies to known protein sequences, ORF5 was predicted to belong to the resolvase family of site-specific recombinases, which have a role in recombination, either between inverted repeat sequences contributing to the inversion of a DNA segment or with repeat sequences in different DNA molecules permitting either cointegration or excision events to occur. The deduced resolvase protein shares 92% identity with a putative protein from the lactococcal antibiotic-resistant plasmid pK214 (29). The largest ORF, ORF3, was over 2.5 kb in length and was capable of encoding a putative SNF-like helicase homolog. The greatest identity (41% over 569 amino acids) was observed with a deduced protein from Bacillus cereus (26). Members of the SNF2 family of proteins have been implicated in a variety of cellular processes including transcriptional regulation, recombination, and a variety of DNA repair mechanisms. All members of the SNF2 family of proteins contain sequence motifs characteristic of the DNA and RNA helicase protein families. These related families are characterized by a number of conserved sequence motifs, including a special version of the B motif of ATP-binding proteins which is present as either DEAD, DEAH, or DEXH (3, 4, 17). The presence of characteristic helicase motifs (including a DEVH box) in the C-terminal end of ORF3 suggests that this protein may be regarded as a DEXH-box ATP-dependent helicase. ORF2 (C-terminal region) exhibited the greatest sequence similarity (23% over 212 amino acids) to Pip (phage infection protein), which is a chromosomally encoded transmembrane protein in L. lactis that is required for φc2 infection (16). However, the region of Pip similar to the predicted ORF2 protein does not correspond to the putative transmembrane region but to the large central region of the protein, which is theoretically oriented outside the membrane. As the biological function of Pip is not currently known, it is not possible to predict functional analogies for the predicted ORF2 protein. The deduced protein sequences of ORF1, ORF4, and ORF8 did not reveal any significant homologies.
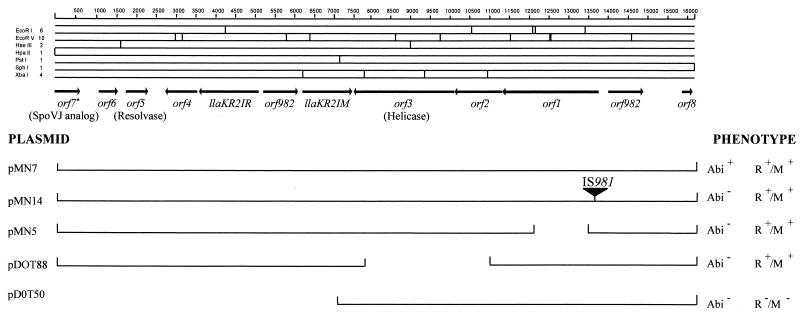
FIG. 1.
Restriction map and gene organization of the 16,174-bp phage resistance region of plasmid pKR223. The organization and sequence of the LlaKR2I R/M system between base positions 3653 and 7537 have previously been reported (31). Predicted functions for ORF3, ORF5, and ORF7 based on sequence comparisons are indicated. Below the gene map, the organization of the deletion and insertion plasmids used in the Abi characterization is represented, together with their respective phenotypes. ∗, a 5′ truncated ORF.
ORF1 to ORF3 are codirectionally transcribed, with no significant secondary structure observed within these three ORFs. A putative rho-independent transcriptional terminator was located between ORF3 and the countertranscribed methyltransferase gene, llaKR2IM. Upstream of ORF1, a second copy of IS982 was observed which was 98.8% identical to the IS element located between the LlaKR2I restriction and modification genes and which contained identical 16-bp perfect inverted repeats at its left and right termini. The region between IS982 and ORF8 contained a number of significant secondary structures, including 16-bp perfect inverted repeats (separated by 16 nucleotides), which are characteristic of the left and right termini of IS982 elements (Fig. 2). An additional 17-bp perfect inverted repeat structure with similarities to the putative rho-independent terminator structure located after the plasmid-borne nisin resistance gene, nsr (12), was located 55 bp downstream. These two sets of inverted repeat structures appear to form the boundaries between DNA fragments from a variety of sources, as DNA segments bordering these two sets of inverted repeat structures displayed significant identity to segments from different plasmids. This modular organization suggests that the inverted repeat structures are associated with a number of DNA recombination events in this region. It is interesting to speculate that the putative resolvase encoded by ORF5, which belongs to a family of site-specific recombinases known to promote DNA arrangements between inverted repeat sequences, may be responsible for some of these arrangements.
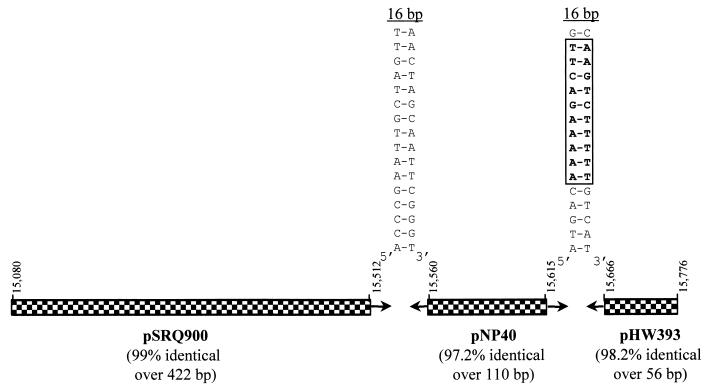
FIG. 2.
Modular organization of DNA segments from the intervening region between IS982 and ORF8 which are homologous to segments from different plasmids and which are separated by inverted repeat structures. Portions of the plasmids pSRQ900 (8), pNP40 (12), and pHW393 (21) which share homology with pKR223 are represented by checkered boxes. The 16-bp inverted repeat located between segments similar to fragments of pSRQ900 and pNP40 are identical to the left and right termini of IS982. A perfect 16-bp inverted repeat located between segments similar to fragments of pNP40 and pHW393 shared similarities with the putative transcriptional terminator structure (10 bp highlighted in boldface and boxed) located after the nisin resistance gene (12). The nucleotide positions of the homologous segments in the 16,174-bp HpaII/SphI fragment of pKR223 are indicated above the segments.
Experimental analysis of ORFs involved in the Abi phenotype.
Previous work by McKay et al. (23) found that during transformation of L. lactis LM0230 with pGBK17, an unexpected transformant was observed in which pGBK17 had acquired an extra piece of DNA and had lost the Abi phenotype. This extra piece of DNA was linked to the presence of an insertion sequence, IS981 (30), and its precise insertion point corresponds to a site within ORF1, indicating that either the disruption of this ORF or possible polar effects on the codirectionally transcribed ORFs, ORF2 and ORF3, accounted for the loss of the Abi phenotype (Fig. 1). Precise mapping of the deletion plasmid pMN5 substantiated the likely involvement of ORF1, as the deletion in this plasmid was contained within ORF1 and resulted in the abolition of the Abi phenotype. To determine if ORF2 and ORF3 may be involved in the Abi phenotype, pGBK17 was partially digested with XbaI and religated. This resulted in the isolation of deletion plasmid pDOT88, which had a deletion extending over ORF2 and ORF3 (Fig. 1). As this construct was Abi negative, it indicated that either one or both ORFs were implicated in the phage resistance mechanism.
To establish if the three ORFs exhibited any Abi phenotype, an 8.9-kb PstI/SphI segment from pGBK17 was subcloned in pTRKH2 and introduced into L. lactis LMO230. This construct, pDOT50, also did not exhibit any detectable Abi phenotype (Fig. 1). As this subclone contained all the DNA from the right of the R/M system (as positioned in Fig. 1), this result indicated that while several and possibly all three of the contiguous ORFs are involved in the Abi phenotype, an additional element(s) from the left of the R/M system is also required for the Abi phenotype.
The overall sequence organization of the phage resistance region of pKR223 points to an Abi mechanism interrupted by an R/M system. If the R/M system did insert into pKR223 to divide the genes encoding the Abi mechanism, then this suggests that ORF4 may be involved in the Abi mechanism. Evidence in support of this is the fact that ORF4 is the only ORF located to the left of the LlaKR2I R/M system (as oriented in Fig. 1) that has a low G+C content (30.5%), which is a characteristic feature of lactococcal Abi systems. To date, all genes associated with Abi mechanisms have a G+C content that is atypically lower (24 to 31%) than the average lactococcal G+C content (37%). ORF1, ORF2, and ORF3 also have atypically low G+C contents (29.8, 32.5, and 31.6%, respectively). As all other ORFs have largely typical G+C contents (34.4 to 39.1%), ORF4 may be associated with the pKR322 Abi mechanism. However, this has yet to be investigated.
Mode of action.
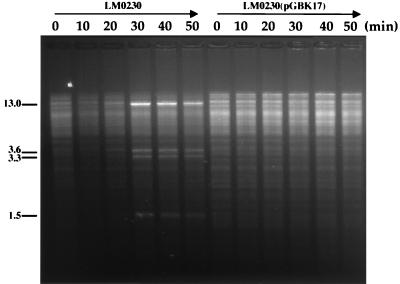
The mode of action of the pKR223 Abi mechanism was investigated essentially as outlined by Hill et al. (18). The bacteriophage φc2 was first propagated on a lactococcal host harboring the methyltransferase gene, llaKR2IM, to protect the phage DNA from digestion by the LlaKR2I restriction endonuclease. Following infection of L. lactis LM0230(pGBK17) with this phage and subsequent isolation of total genome DNA at various times postinfection, it was found that relative to the plasmid-free phage-sensitive host, pGBK17 significantly reduced the replication of phage DNA (Fig. 3). Therefore, these data indicate that the pKR223 Abi system targets an early stage in the φc2 developmental cycle, and the mechanism can therefore be classified as “early.”
FIG. 3.
Depiction of the replication of the φc2 genome in L. lactis LM0230 and in the same strain containing pGBK17 over 50 min. All lanes are digested with EcoRI. The numbers on the left refer to the expected size of the φc2 fragments, in kilobases.
Of the characterized Abi systems reported to date, this is the fourth one where the mechanism can be classified as “early.” As two of the other three (Abi A and K) were found to be ineffective during growth of L. lactis at 39 to 40°C, the pKR223 Abi phenotype was tested at the maximum growth temperature for strain LM0230, 39°C. At this temperature, there was essentially no significant Abi phenotype, as evidenced by infection with φc2.
The results of this study clearly indicate that this Abi system is a novel, multicomponent phage resistance mechanism which is encoded by two genetic loci that are separated by LlaKR2I R/M, and the system has been designated AbiR. It protects L. lactis cells from φc2 infection by impeding its DNA replication. However, similar to many lactococcal phage defense systems, AbiR is relatively ineffective during growth at elevated temperatures. Understanding the molecular mechanisms for the temperature sensitivity of AbiR will allow strategies to be developed to overcome this limitation.
Nucleotide sequence accession number.
The 16,174-bp nucleotide sequence presented here has been deposited in the GenBank database and has been assigned the accession number AF216814.
Acknowledgments
This work was supported in part by the United States Department of Agriculture, Minnesota-South Dakota Dairy Foods Research Center, Dairy Management Inc., and the Minnesota Agricultural Experimental Station.
Footnotes
Paper number 001180011 of the Scientific Journal Series of the Minnesota Agricultural Experiment Station.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Anba J, Bidnenko E, Hillier A, Ehrlich D, Chopin M-C. Characterization of the lactococcal abiDI gene coding for phage abortive infection. J Bacteriol. 1995;177:3818–3823. doi: 10.1128/jb.177.13.3818-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bork P, Koonin E. An expanding family of helicases within the “DEAD/H” superfamily. Nucleic Acids Res. 1993;21:751–752. doi: 10.1093/nar/21.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson M, Laurent B C. The SNF/SW1 family of global transcriptional activators. Curr Opin Struct Biol. 1994;6:396–402. doi: 10.1016/0955-0674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 5.Coffey A G, Fitzgerald G F, Daly C. Cloning and characterization of the determinant for abortive infection of bacteriophage from plasmid pCI829. J Gen Microbiol. 1991;137:1355–1362. doi: 10.1099/00221287-137-6-1355. [DOI] [PubMed] [Google Scholar]
- 6.Deng Y M, Liu C Q, Dunn N W. Genetic organization and functional analysis of a novel phage abortive infection system, AbiL, from Lactococcus lactis. J Biotechnol. 1999;67:135–149. doi: 10.1016/s0168-1656(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou J D, McKay L L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977;130:257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emond E, Dion E, Walker S A, Vedamuthu E R, Kondo J K, Moineau S. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl Environ Microbiol. 1998;64:4748–4756. doi: 10.1128/aem.64.12.4748-4756.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emond E, Holler B J, Boucher I, Vandenbergh P A, Vedamuthu E R, Kondo J K, Moineau S. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl Environ Microbiol. 1997;63:1274–1283. doi: 10.1128/aem.63.4.1274-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forde A, Fitzgerald G F. Bacteriophage defense systems in lactic acid bacteria. Antonie van Leeuwenhoek Int. J Gen Mol Microbiol. 1999;76:89–113. [PubMed] [Google Scholar]
- 11.Foulger D, Errington J. Sequential activation of dual promoters by different sigma factors maintains SpoVJ expression during successive developmental stages of Bacillus subtilis. Mol Microbiol. 1991;5:1363–1373. doi: 10.1111/j.1365-2958.1991.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Froseth B R, McKay L L. Molecular characterization of the nisin resistance region of Lactococcus lactis subsp. lactis biovar diacetylactis DRC3. Appl Environ Microbiol. 1991;57:804–811. doi: 10.1128/aem.57.3.804-811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garvey P, Fitzgerald G F, Hill C. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl Environ Microbiol. 1995;61:4321–4328. doi: 10.1128/aem.61.12.4321-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvey P, Hill C, Fitzgerald G F. The lactococcal plasmid pNP40 encodes a third bacteriophage resistance mechanism, one which affects phage DNA penetration. Appl Environ Microbiol. 1996;62:676–679. doi: 10.1128/aem.62.2.676-679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvey P, van Sinderen D, Twomey D P, Hill C, Fitzgerald G F. Molecular genetics of bacteriophage and natural phage defense systems in the genus Lactococcus. Int Dairy J. 1995;5:905–947. [Google Scholar]
- 16.Geller B L, Ivey R G, Trempy J E, Hettinger-Smith B. Cloning of a chromosomal gene required for phage infection of Lactococcus lactis subsp. lactis C2. J Bacteriol. 1993;175:5510–5519. doi: 10.1128/jb.175.17.5510-5519.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorbalenya A E, Koonin E V. Helicases: amino acid sequence comparisons and structure-function relationships. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]
- 18.Hill C, Massey I J, Klaenhammer T R. Rapid method to characterize lactococcal bacteriophage genomes. Appl Environ Microbiol. 1991;57:283–288. doi: 10.1128/aem.57.1.283-288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill C, Miller L A, Klaenhammer T R. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl Environ Microbiol. 1990;56:2255–2258. doi: 10.1128/aem.56.7.2255-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laible N J, Rule P L, Harlander S K, McKay L L. Identification and cloning of plasmid deoxyribonucleic acid coding for abortive infection from Streptococcus lactis ssp. diacetylactis KR2. J Dairy Res. 1987;70:2211–2219. [Google Scholar]
- 21.Madsen A, Josephsen J. Cloning and characterization of the lactococcal plasmid-encoded type II restriction/modification system, LlaDII. Appl Environ Microbiol. 1998;64:2424–2431. doi: 10.1128/aem.64.7.2424-2431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKay L L, Baldwin K A. Conjugative 40-megadalton plasmid in Streptococcus lactis subsp. diacetylactis DRC3 is associated with resistance to nisin and bacteriophage. Appl Environ Microbiol. 1984;47:68–74. doi: 10.1128/aem.47.1.68-74.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKay L L, Bohanon M J, Polzin K M, Rule P L, Baldwin K A. Localization of separate genetic loci for reduced sensitivity towards small isometric-headed bacteriophage sk1 and prolate-headed bacteriophage c2 on pGBK17 from Lactococcus lactis subsp. lactis KR2. Appl Environ Microbiol. 1989;55:2702–2709. doi: 10.1128/aem.55.10.2702-2709.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLandsborough L A, Kolaetis K M, Requena T, McKay L L. Cloning and characterization of the abortive infection genetic determinant abiD isolated from pBF61 of Lactococcus lactis subsp. lactis KR5. Appl Environ Microbiol. 1995;61:2023–2026. doi: 10.1128/aem.61.5.2023-2026.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Connor L, Coffey A, Daly C, Fitzgerald G F. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl Environ Microbiol. 1996;62:3075–3082. doi: 10.1128/aem.62.9.3075-3082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okstad O A, Hegna I, Lindback T, Rishovd A-L, Kolsto A-B. Genome organization is not conserved between Bacillus cereus and Bacillus subtilis. Microbiology. 1999;145:621–631. doi: 10.1099/13500872-145-3-621. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan D J, Klaenhammer T R. High- and low-copy-number Lactococcus shuttle cloning vectors with features for clone screening. Gene. 1993;137:227–231. doi: 10.1016/0378-1119(93)90011-q. [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan D J, Zagula K, Klaenhammer T R. In vivo restriction by LlaI is encoded by three genes, arranged in an operon with llaIM, on the conjugative Lactococcus plasmid pTR2030. J Bacteriol. 1995;177:134–143. doi: 10.1128/jb.177.1.134-143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perreten V, Schwarz F, Boeglin B, Cresta L, Dasen G, Teuber M. Antibiotic resistance spread in food. Nature. 1998;391:801–802. doi: 10.1038/39767. [DOI] [PubMed] [Google Scholar]
- 30.Polzin K M, McKay L L. Identification, DNA sequence, and distribution of IS981, a new, high-copy-number insertion sequence in lactococci. Appl Environ Microbiol. 1991;57:734–743. doi: 10.1128/aem.57.3.734-743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twomey D P, McKay L L, O'Sullivan D J. Molecular characterization of the Lactococcus lactis LlaKR2I restriction-modification system and effect of an IS982 element positioned between the restriction and modification genes. J Bacteriol. 1998;180:5844–5854. doi: 10.1128/jb.180.22.5844-5854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]