Abstract
Background and Objective
Glucose-Potassium Ratio (GPR) has emerged as a biomarker in several pathophysiological conditions. However, the association between GPR and long-term outcomes in stroke patients has not been investigated. Our study evaluated the applicability of baseline GPR as a predictive prognostic tool for clinical outcomes in ischemic stroke patients.
Methods
The multicenter retrospective cohort study included acute-subacute adult ischemic stroke patients who had their baseline serum GPR levels measured. Eligible patients were categorized into two sub-cohorts based on the baseline GPR levels (<1.67 vs. ≥ 1.67). The primary outcome was the incidence of 30-day hemorrhagic transformation, while stroke recurrence, and all-cause mortality within twelve months, were considered secondary.
Results
Among 4083 patients screened, 1047 were included in the current study. In comparison with GPR < 1.67 group, patients with ≥ 1.67 GPR had a significantly higher ratio of all-cause mortality within twelve months (aHR 2.07 [95 % CI 1.21–3.75] p = 0.01), and higher ratio of 30-day hemorrhagic transformation but failed to reach the statistical significance (aHR 1.60 [95 % CI 0.95–2.79], p = 0.08).
Conclusion
Overall, baseline GPR serum is an independent predictor of all-cause mortality within twelve months in patients with acute and subacute ischemic stroke. Further clinical studies are necessary to validate these findings.
Keywords: Brain Injury, Brain Ischemia, Glucose, Mortality, Potassium, Stroke
1. Introduction
The prevalence of acute ischemic stroke represents a major public health concern in Saudi Arabia, marked by significant incidence of morbidity and mortality rates (Al-Senani et al., 2019). In the past three decades, there has been a 70 % increase in global stroke incidence exceeding 12.2 million new stroke cases annually. Compared to western countries, the incidence of stroke in Saudi Arabia is considered low, which could be due to the predominance of the younger age groups in this region. However, by 2030, the incidence of first stroke is predicted to increase within the range of 57–67 % due to population growth in Saudi Arabia (Al-Senani et al., 2019). Despite the progress in stroke management, many patients continue to encounter poor outcomes and endure disabilities (Al Shoyaib et al., 2021, Alamri et al., 2021, Moraes et al., 2023). Given the odds reduction of mortality, clinical severity, and complications when stroke patients have earlier access to clinical care and earlier outcome prediction (Alharbi et al., 2022), finding novel and more predictive biomarkers for stroke outcome prediction will not only facilitate earlier diagnosis but also improve short and long-term clinical care (Glushakova et al., 2016), and help to mitigate the economic burden associated with stroke (Basri et al., 2021).
Numerous blood-based biomarkers have been proposed as potential predictors for acute ischemic stroke, including S100B, glutamate, glial fibrillary acidic protein, von Willebrand factor, intercellular adhesion molecule-1, interleukin-6, activated protein C, tumor necrosis factor-alpha, matrix metalloproteinase-9, tau protein, neurolysin, neuron-specific enolase, circulating CD34-positive cells, endothelial progenitor cells, blood glucose, and neuron-specific enolase (Huang et al., 2023, Jayaraman et al., 2020). Nevertheless, these indices have not found prevalent clinical utility, mainly due to the limited availability of testing tools or relatively modest predictive value. Therefore, exploring parameters that can be easily measured in blood samples and yield rapid results has been helpful in guiding clinical decision-making at the time of hospital admission. Examples include serum glucose and electrolytes such as sodium, calcium, chloride, and potassium (Piironen et al., 2012, Wang et al., 2021a).
Recently, a ratio that depends on serum glucose and potassium levels has emerged broadly to evaluate the two predictors as an alternative to only one indicator, thus avoiding false positive test results (Shibata et al., 2021). Studies have revealed that glucose and potassium display a complex interaction in the individual biological system. Following stress and brain trauma, catecholamines, cortisol, growth hormones, and cytokines are released due to the neuroendocrine response of the sympathetic nervous system (Dungan et al., 2009, Jayaraman et al., 2020). As a result, glucose levels rise, causing hyperglycemia, while potassium levels diminish, leading to hypokalemia (Lu et al., 2022, Thier, 1986). The Glucose-to-Potassium Ratio (GPR), which reflects glucose and potassium levels in the bloodstream, has emerged as a biomarker in various pathophysiological conditions sharing similarities with ischemic stroke, including aneurysmal subarachnoid hemorrhage (Calviere et al., 2022, Jung et al., 2021, Matano et al., 2019, Wang et al., 2021b), acute intracerebral hemorrhage (Wu et al., 2021), severe traumatic brain injury (Marini and Sein, 2023, Zhou et al., 2020), massive and non-massive pulmonary embolism (Boyuk, 2022), and delayed neuropsychiatric syndrome after carbon monoxide poisoning (Demirtaş et al., 2021). Moreover, a recent study has shown a positive correlation between serum GPR and the risk of 30-day mortality in patients with ischemic stroke (Lu et al., 2022).
Hemorrhagic transformation is the most serious complication in acute ischemic stroke patients, exacerbated by reperfusion with alteplase or endovascular therapy (Jickling et al., 2014). It occurs due to the interruption of the blood–brain barrier after ischemic stroke in response to peripheral blood extravasations; as a result, it increases mortality and deteriorates stroke outcomes (Kastrup et al., 2008). Multiple factors correlate with increased risk of hemorrhagic transformation, such as reperfusion therapy, high blood pressure, high glucose level, age, and inflammation (Spronk et al., 2021). A few reports showed an association between elevated glucose levels and hemorrhagic transformation in ischemic stroke patients (Laredo et al., 2020, Paciaroni et al., 2009, Yuan et al., 2021). A meta-analysis study reported that high glucose level on admission was associated with an increased rate of spontaneous intracranial hemorrhage following mechanical thrombectomy in patients with acute ischemic stroke (Zang et al., 2021). Moreover, previous studies reported that elevated serum GPR at the time of admission was notably associated with increased mortality, augmented risk of rebleeding, and poor outcomes three months after aneurysmal subarachnoid hemorrhage (Fujiki et al., 2017, Jung et al., 2021, Wang et al., 2021b).
However, the association between GPR and 30-day hemorrhagic transformation, 12-month stroke recurrence, and 12-month all-cause mortality in acute and subacute ischemic stroke patients has not been investigated; therefore, this study aims to evaluate the applicability of baseline GPR as a potential predictive prognostic tool of 30-day hemorrhagic transformation, 12-month stroke recurrence, or 12-month all-cause mortality in acute as well as subacute ischemic stroke.
2. Materials and methods
2.1. Study design
A multicenter retrospective cohort study was conducted between January 2016 and September 2022 at King Abdulaziz Medical City (KAMC) in Jeddah and Riyadh, Saudi Arabia. Patients were screened for eligibility based on the predefined criteria using the electronic medical record system (BESTCare 2.0). Eligible patients with acute and subacute ischemic stroke were categorized into two sub-cohorts based on the baseline GPR levels (<1.67 vs. ≥ 1.67). The glucose-potassium ratio GPR was determined by dividing the glucose and potassium levels (mmol/l). All patients were followed until they were discharged or died during their stay in the hospital except for the variables, pneumonia (up to seven days after stroke) and other complications checked for 12 months after injury.
The diagnosis of ischemic stroke was clinically confirmed through computerized tomography (CT), magnetic resonance Imaging (MRI), or a combination of both. Glucose (hexokinase method) and potassium (ion-selective electrodes method) serum concentrations were measured with an automatic multichannel analyzer (ARCHITECT c8000; Abbott Diagnostics) (Fotiou et al., 2015).
The King Abdullah Medical Research Center (KAIMRC) Institutional Review Board approved the study in Riyadh, Saudi Arabia (study number: NRJ22J/235/09). All methods were performed in accordance with the relevant guidelines and regulations. Considering the retrospective and observational nature of the study, we used only de-identified retrospective data, and the informed consent for patients was waived.
2.2. Study participants
All adult patients (≥18 years) with confirmed ischemic stroke using a CT scan or MRI who arrived at the emergency room within seven days of symptoms onset or were admitted at the stroke incident time during the study period were screened for eligibility. Patients were excluded if the GPR levels were not measured within three days of injury, were diagnosed with transient ischemic or hemorrhagic stroke, and had a follow-up period below one year (Fig. 1).
Fig. 1.
Flowchart of the study population and the selecting criteria of eligible patients with acute or subacute ischemic stroke.
2.3. Outcomes
The primary outcome was to evaluate the association between the patient’s serum GPR and 30-day hemorrhagic transformation. Other outcomes, such as stroke recurrence, and all-cause mortality within twelve months, were considered secondary.
2.4. Study setting
The study was conducted at two different canters of KAMC. KAMC is an academic referral tertiary care hospital with 509 beds in Jeddah, Saudi Arabia, and KAMC in Riyadh, Saudi Arabia, with 690 beds. The two centers provide clinical care to all National Guard military members and their families, as well as primary and specialized health care who work at the centers.
2.5. Data collection
The collected data included demographic data, hospital admission data, the National Institute of Health Stroke Scale (NIHSS) score, and stroke subtype (Alharbi et al., 2022). The stroke subtype was ranked according to the TOAST classification. The TOAST classification denotes five subtypes of ischemic stroke: 1) large-artery atherosclerosis, 2) small vessel occlusion, 3) cardioembolism, 4) stroke of other determined etiology, and 5) stroke of undetermined etiology (Adams et al., 1993). Initial stroke severity was assessed by the NIHSS method within 24 h of admission (Alharbi et al., 2022). Comorbidities, signs, and symptoms of stroke and complications, including ICU admission, were collected. Complications were followed up to one year following the date of injury except for pneumonia (seven days after stroke). Management at the time of stroke (Mechanical thrombectomy, tPA) was also collected.
Baseline lab results after stroke, inflammatory markers, complete blood count after stroke, and rehabilitation variables were compiled. We assessed the upper/lower and left/right motor impairment at the time of admission. Upon admission and discharge, we collected initial clinical severity NIHSS and functional independence and disability by modified Rankin Scale (mRS).
2.6. Statistical analysis
Descriptive analysis was conducted to summarize the demographic and clinical characteristics of study subjects. Frequencies with percentages were calculated for categorical variables. Means with standard deviations or median with interquartile range (IQR) were calculated for numerical variables. Comparisons of categorical variables between GPR levels (<1.67 vs. ≥ 1.67) were assessed using chi-square or Fisher’s exact test. While numerical variables were evaluated using an independent t-test or one-way ANOVA, as appropriate. Survival analyses of study endpoints were done using Kaplan Meier curves and the Log Rank test of significance for comparing GPR levels.
Cox proportional hazard regressions were used to identify factors associated with each of the study endpoints. The model was adjusted for (1) patient characteristics, (2) past medical history, (3) stroke characteristics and treatment. Hazard ratios (HR) with their 95 % confidence intervals (CIs) were calculated to determine the magnitude of associations. A p-value of less than 0.05 (two‐tailed) was considered statistically significant. All analyses were performed using SAS University Edition (SAS Institute, Cary, NC, USA).
3. Results
A total of 4083 S patients were screened, and 1047 patients met the eligibility criteria. Among the included patients, 721 (68.9 %) had a GPR of 1.67 and above.
3.1. Baseline characteristics of included ischemic stroke patients based on GPR level
Table 1 demonstrates the demographic and baseline characteristics of the total included sample. In the whole cohort, the mean age was 64.9 ± 12.9 years, and 59.9 % were male. In addition, hypertension (81.1 %) was the most common comorbidity, followed by diabetes mellitus (72.9 %), dyslipidemia (45.9 %), atrial fibrillation (19.8 %), and depression (13.4 %). In the bivariate analysis, comorbidities were comparable between the two groups except for hypertension (85.9 % vs. 70.3 %; p < 0.001), diabetes mellitus (85.6 % vs. 45.1 %; p < 0.001), and dyslipidemia (49.8 % vs. 37.1 %; p < 0.001), which was higher in patients with a GPR of 1.67 and above.
Table 1.
Demographic and baseline characteristics of patients stratified by GPR level.
| Characteristics | Total (N = 1,047) |
Glucose Potassium Ratio (GPR) Levels |
P | |
|---|---|---|---|---|
| <1.67 (N = 326) |
≥ 1.67 (N = 721) |
|||
| Age, mean ± SD | 64.9 ± 12.9 | 64.3 ± 14.6 | 65.3 ± 11.9 | 0.263 |
| Male, n (%) | 628 (59.9) | 195 (59.8) | 433 (60.1) | 0.942 |
| BMI, mean ± SD | 29.4 ± 7.3 | 29.2 ± 7.2 | 29.5 ± 7.3 | 0.605 |
| Stroke subtype, n (%) | ||||
| 1 | 471 (44.9) | 148 (45.4) | 323 (44.8) | 0.094 |
| 2 | 236 (22.5) | 77 (23.6) | 159 (22.1) | |
| 3 | 60 (5.7) | 23 (7.1) | 37 (5.1) | |
| 4 | 23 (2.2) | 8 (2.4) | 15 (2.1) | |
| 5 | 139 (13.3) | 29 (8.9) | 110 (15.2) | |
| Unknown/missing | 118 (11.3) | 41 (12.6) | 77 (10.7) | |
| tPA therapy, n (%) | 106 (10.1) | 35 (10.7) | 71 (9.9) | 0.674 |
| Mechanical thrombectomy, n (%) | 56 (5.4) | 20 (6.1) | 36 (4.9) | 0.453 |
| Prior stroke, n (%) | 397 (37.9) | 120 (36.8) | 277 (38.4) | 0.618 |
| Initial NIHSS, median (IQR) | 6 (3; 9) | 6 (3; 10) | 6 (3; 9) | 0.705 |
| Initial mRS, median (IQR) | 4 (1; 5) | 4 (1; 5) | 4 (1; 5) | 0.589 |
| Length of Stay (days), median (IQR) | 5.5 (3; 14) | 5 (3; 13) | 6 (3; 14) | 0.114 |
| ICU admission, n (%) | 309 (29.5) | 89 (27.3) | 220 (30.5) | 0.334 |
| History of comorbidities, n (%) | ||||
| HTN | 849 (81.1) | 229 (70.3) | 620 (85.9) | <0.001 |
| DM | 764 (72.9) | 147 (45.1) | 617 (85.6) | <0.001 |
| Dyslipidemia | 480 (45.9) | 121 (37.1) | 359 (49.8) | <0.001 |
| AF | 207 (19.8) | 65 (20.1) | 142 (19.7) | 0.896 |
| Dementia | 129 (12.3) | 36 (11.1) | 93 (12.9) | 0.407 |
| Depression | 140 (13.4) | 43 (13.2) | 97 (13.5) | 0.923 |
| Stroke signs and symptoms, n (%) | ||||
| LL Motor Impairment | 465 (44.4) | 144 (44.2) | 321 (44.5) | 0.895 |
| LR Motor Impairment | 411 (39.3) | 122 (37.4) | 289 (40.1) | 0.422 |
| UL Motor Impairment | 466 (44.5) | 139 (42.6) | 327 (45.4) | 0.407 |
| UR Motor Impairment | 420 (40.1) | 125 (38.3) | 295 (40.9) | 0.418 |
| Aphasia | 289 (27.6) | 100 (30.7) | 189 (26.2) | 0.126 |
| Dysarthria | 589 (56.3) | 179 (54.9) | 410 (56.9) | 0.589 |
| Stroke-related complications, n (%) | ||||
| Pneumonia | 228 (21.8) | 65 (19.9) | 163 (22.6) | 0.339 |
| Seizures | 138 (13.2) | 39 (11.9) | 99 (13.7) | 0.429 |
| Cerebral edema | 88 (8.4) | 24 (7.4) | 64 (8.9) | 0.421 |
| DVT-PE | 83 (7.9) | 23 (7.1) | 60 (8.3) | 0.486 |
| Impaired consciousness | 306 (29.2) | 95 (29.1) | 211 (29.3) | 0.956 |
Abbreviations: SD: Standard deviation; BMI: body mass index; tPA: Tissue-type plasminogen activator; NIHSS: The National Institutes of Health Stroke Scale; IQR: Interquartile range; mRS: the modified Rankin Scale; ICU: Intensive care unit; HTN: hypertension; DM: diabetes mellitus; AF: atrial fibrillation; LL: lower left; LR: lower right; UL: upper left; UR: upper right; DVT-PE: Deep vein thrombosis-Pulmonary embolism.
3.2. Initial clinical severity and ischemic stroke subtypes and complications distribution based on GPR level
The median stroke clinical severity at hospital arrival measured by the NIHSS score was 6 (3–9), and the median disability score measured by modified Rankin Scale (mRS) at arrival was 4 (1–5) (Table 1). Ischemic stroke subtype 1 was the most diagnosed stroke subtype (44.9 %). The most frequent stroke-related complications in the study population (Table 1) are impaired consciousness (29.2 %), pneumonia (21.8 %), seizures (13.2 %), cerebral edema (8.4 %), and deep vein thrombosis- pulmonary edema (7.9 %). Surprisingly, no significant differences were found in the initial clinical severity, functional independence, patients’ stroke subtypes, or complication incidence rates between the GPR groups (Table 1).
3.3. Association between GPR level and frequency of 30-day hemorrhagic transformation, 12-month stroke recurrence, and 12-month all-cause mortality
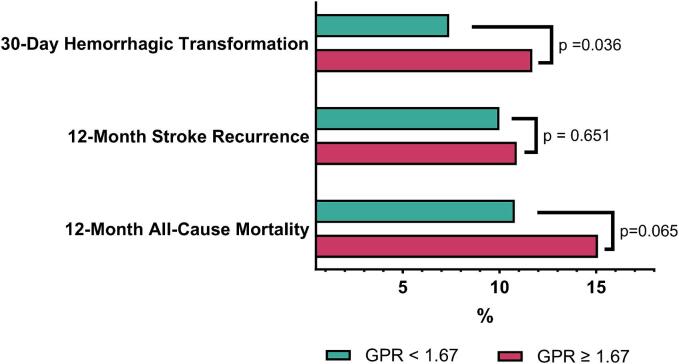
In the overall cohort, hemorrhagic transformation, stroke recurrence, and all-cause mortality accounted for 10.4 %, 10.7 %, and 13.7 % cases, respectively. Patients with baseline GPR ≥ 1.67 had significantly higher 30-day hemorrhagic transformation than the control (11.7 % vs. 7.4 %; p = 0.04). In addition, there was a higher rate of all-cause mortality within twelve months in patients with GPR ≥ 1.67 compared to patients with a GPR < 1.67 but the difference was insignificant (15.1 % vs. 10.8 %; p = 0.07). In contrast, stroke recurrence was comparable and not statistically significant between the two groups (10.9 % vs. 10 %; p-value = 0.65) (Fig. 2, Supplemental Table-1).
Fig. 2.
Comparison of study endpoints according to GPR levels.
3.4. Survival probability over days from hemorrhagic transformation, stroke recurrence, and all-cause mortality for higher and lower GPR groups
Fig. 3 compares GPR levels at the three primary endpoints. The Kaplan-Meier curves in Fig. 3 (a and b) demonstrate that the GPR ≥ 1.67 group has significantly higher all-cause mortality and hemorrhagic transformation (p = 0.04 and p = 0.02, respectively) compared to the GPR < 1.67 group. In Fig. 3 (c), however, the log-rank test indicates that there was no statistically significant difference between the two groups in the 12-month stroke recurrence rate (p = 0.66).
Fig. 3.
Kaplan-Meier curve comparing (a) 12-month all-cause mortality, (b) 30-Day Hemorrhagic Transformation, and (c) 12-month stroke recurrence between GRP levels.
3.5. GPR level predicts 30-day-hemorrhagic transformation and 12-month all-cause mortality, but not 12-month stroke recurrence
In the unadjusted Cox proportional hazard regression, the GPR ≥ 1.67 group had a significantly higher hazard ratio (HR) for 30-day hemorrhagic transformation (HR = 1.74 [95 % CI 1.10–2.89], p = 0.02) and 12-month all-cause mortality (HR = 1.48 [95 % CI 1.01–2.22], p = 0.04) compared to the GPR < 1.67 group. However, the two groups had no significant difference in the HR for 12-month stroke recurrence (HR = 1.09 [95 % CI 0.73–1.66], p = 0.68).
In the adjusted Cox proportional hazard regression analysis (Fig. 4), it was found that the GPR ≥ 1.67 group had a 2-fold increase in adjusted HR for 12-month all-cause mortality (aHR = 2.07 [95 % CI 1.21–3.75], p = 0.01) compared to the GPR < 1.67 group. However, there was no significant difference in the risk of 30-day hemorrhagic transformation (aHR = 1.60 [95 % CI 0.95–2.79], p = 0.08) or 12-month stroke recurrence (aHR = 1.16 [95 % CI 0.67–2.10], p = 0.68) between the two groups.
Fig. 4.
Forest plot of adjusted hazard ratios (with 95% confidence intervals) from multivariable cox proportional hazard regression analyses of study endpoints.
3.6. Other predictors of 30-day hemorrhagic transformation, 12-month stroke recurrence, and 12-month all-cause mortality
As shown in Fig. 4, other factors besides GPR significantly impact the incidence risk of the three primary endpoints. Admission to ICU and increasing age have been identified as independent factors that increase the risk of all-cause mortality by five-fold (aHR = 4.66 [95 % CI 2.78–8.04], p = 0.01) and 1.39-fold (aHR = 1.39 [95 % CI 1.15–1.69], p = 0.001) respectively. Additionally, the presence of comorbid atrial fibrillation in ischemic stroke patients was found to be an independent predictor of 12-month stroke recurrence (aHR = 1.98 [95 % CI 1.09–3.47], p = 0.02) by two-fold. The study also revealed that aHR for initial clinical severity (1.18 [95 % CI 1.09–1.26], p < 0.001) and disability score (1.14 [95 % CI 1.02–1.29], p = 0.02) are independent significant predictors of 12-month all-cause mortality. Initial stroke severity score aHR was also found to independently predict 30-day hemorrhagic transformation (aHR = 1.12 [95 % CI 1.03–1.21], p = 0.005), but not 12-month stroke recurrence (aHR = 1.04 [95 % CI 0.95–1.13], p = 0.35).
4. Discussion
This multicenter, retrospective cohort study investigated the association between GPR and 30-day hemorrhagic transformation, 12-month stroke recurrence, and 12-month all-cause mortality in patients with acute and subacute ischemic stroke. Our study's Cox proportional hazard regression analysis revealed a significantly higher incidence risk of 12-month all-cause mortality in patients with GPR ≥ 1.67. The stability of this association was confirmed by adjusting for other independent variables with clinical significance using multivariable Cox proportional hazard regressions. Our findings are consistent with a single-center retrospective cohort study that included 784 patients who arrived at an emergency in Norway between 2010 and 2015. Using the continuous model, the study found a significant positive correlation between GPR levels and 30-day mortality (OR 2.01 [95 % CI 1.12–3.61], p = 0.01) in ischemic stroke patients (Lu et al., 2022). The study confirmed the results by categorizing GPR into three tertiles (GPR: Tertile 1: ≤ 1.372; Tertile 2: 1.375–1.658; Tertile 3: ≥ 1.659). The Odd ratio (OR) and 95 % for 30-day mortality of tertile 1 (reference) were compared to tertile 2 (OR 1.24 [95 % CI 0.60–2.65], p = 0.56) and were significantly different when compared to tertile 3 (OR 2.15 [95 % CI 1.09–4.24], p = 0.02). These results align with the current study confirming the positive association of GPR with mortality.
Hyperglycemia is very common in patients with ischemic stroke, occurring in 30–40 % of patients, even among non-diabetics (Luitse et al., 2012). In our sample, around 36 % of non-diabetic patients had a ratio of GPR ≥ 1.67. Hyperglycemia in non-diabetic patients with acute illness is usually referred to as stress or transient hyperglycemia (Guo et al., 2021). Stress hyperglycemia following acute injury is caused by a complex interplay between catecholamines, cytokines, and cortisol (Dungan et al., 2009). In fact, stress hyperglycemia has been suggested to be associated with a more severe form of stroke. For instance, it was shown previously that hyperglycemia in non-diabetic ischemic stroke patients is related to a higher risk of 90-day stroke recurrence and poor functional outcome (Guo et al., 2021, Zonneveld et al., 2017).
In addition to hyperglycemia, hypokalemia was reported to occur frequently in stroke patients (20 %) (Gariballa et al., 1997). Lower potassium level was shown to be associated with increased mortality rates and poor outcomes in stroke patients (Gariballa et al., 1997). At normal conditions, potassium levels are transported intracellularly by active cellular uptake through the cell membrane and by the sodium/potassium adenosine triphosphatase pump (Na+/K + -ATPase) controlled by catecholamines (Gariballa et al., 1997). The increased catecholamines due to stroke leads to a decline in circulating blood potassium levels (Gariballa et al., 1997). The association between potassium levels and mortality rate was also suggested by studies showing high dietary potassium intake to protect against stroke-related mortality (Khaw & Barrett-Connor, 1987).
GPR is a novel biomarker to predict stroke patients' outcomes and can be measured easily in blood samples. High GPR levels have been shown to be associated with worsened clinical outcomes in neurological disorders such as traumatic brain injury (TBI) and acute intracerebral hemorrhage (Wu et al., 2021, Zhou et al., 2020). In our sample, there were no significant differences in stroke-related signs and symptoms and complications between the different GPR groups. However, the rate of 12-month all-cause mortality was significantly higher with high levels of GPR. This could be due to the mechanisms related to sympathetic activation. Activating the sympathetic system releases catecholamines, cytokines, cortisol, and growth hormones, resulting in hyperglycemia and hypokalemia. This finding may reflect the hypothalamic–pituitary–adrenal (HPA) axis activation, resulting in energy dysregulation (Herman et al., 2016). In addition, high cortisol levels result in the activation of the renin-angiotensin-aldosterone system, inducing low levels of potassium. This system was shown to play an essential role in the progression of ischemic stroke (Sokol et al., 2004).
In addition, the current study did not find a significant difference in the initial clinical severity upon hospital arrival between GPR groups. This further supports the concept that increased GPR contributes to worse outcomes, particularly if stress-induced hypoglycemia requires prolonged periods to manifest. This might explain that in most studies (Demirtaş et al., 2021, Jung et al., 2021, Katipoğlu and Demirtas, 2022), the adverse effects of high GPR can be seen on a chronic rather than acute basis.
The mRS is a commonly utilized global assessment tool for disability in stroke patients (Banks & Marotta, 2007). A previous study of long-term outcomes in hemorrhagic stroke or intracerebral hemorrhage found that serum GPR was predictive of poor prognosis as determined by an mRS score nearly 3-folds (OR 2.945 [95 % CI 1.099–7.889]) (Wu et al., 2021). These findings provide additional support for the prospective value of serum GPR levels in stroke prognosis for disability.
There were a few limitations to this retrospective observational study that should be noted. Firstly, due to its retrospective nature and the sample size, it was not possible to establish the causality between GPR levels and the primary outcomes. Secondly, the study did not explore the reproducibility of the results in non-diabetic or non-hypertensive populations. Thirdly, The timing of serum glucose and potassium measurements was not fixed, and there were no follow-up measurements. Lastly, the study did not assess other comorbidities that may have influenced glucose and potassium levels, such as kidney diseases and cancer, as many of these comorbidities were included in the study due to their frequent occurrence with stroke.
Overall, the present study has provided evidence supporting the association between measuring GPR levels within three days of experiencing symptoms of ischemic stroke and all-cause mortality within twelve months. The results indicate that GPR level can be effectively utilized as a predictor of mortality in patients with ischemic stroke. Further research is necessary to investigate the influence of diabetes and hypertension on GPR levels in ischemic stroke patients, to gain a more comprehensive understanding of this relationship. Future studies should investigate if changes in GPR levels can predict successful interventions of tPA or mechanical thrombectomy in ischemic stroke patients.
5. Ethics approval statement
The study was approved by the King Abdullah Medical Research Center (KAIMRC) Institutional Review Board in Riyadh, Saudi Arabia (study number: NRJ22J/235/09).
6. Patient consent statement
Considering the retrospective and observational nature of the study, we used only de-identified retrospective data, and the informed consent for patients was waived.
CRediT authorship contribution statement
Faisal F. Alamri: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. Daniyah A. Almarghalani: Writing – original draft, Writing – review & editing, Conceptualization. Eman A. Alraddadi: Writing – original draft, Writing – review & editing, Conceptualization. Abdullah Alharbi: Conceptualization, Data curation, Methodology, Writing – review & editing, Supervision. Hajar S. Algarni: Data curation, Supervision, Writing – original draft. Oyoon M. Mulla: Data curation, Writing – original draft. Abdullah M. Alhazmi: Data curation, Writing – original draft. Turki A. Alotaibi: Data curation, Writing – original draft. Deema H. Beheiry: Data curation, Writing – original draft. Abdullah S. Alsubaie: Data curation, Writing – original draft. Ahmed Alkhiri: Data curation, Formal analysis, Writing – review & editing. Yasser Alatawi: Conceptualization, Formal analysis, Methodology, Validation, Writing – review & editing. Mohammad S. Alzahrani: Formal analysis, Software, Validation, Writing – review & editing. Alqassem Y. Hakami: Investigation, Methodology, Resources, Writing – review & editing. Aser Alamri: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. Khalid Al Sulaiman: Investigation, Methodology, Validation, Visualization, Writing – review & editing.
Acknowledgements
The authors extend their appreciation to the King Salman Center For Disability Research for funding this work through Research Group no KSRG-2022-062.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2024.102082.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Adams H.P., Jr, Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., Marsh E., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Al Shoyaib A., Alamri F.F., Biggers A., Karamyan S.T., Arumugam T.V., Ahsan F., Mikelis C.M., Al-Hilal T.A., Karamyan V.T. Delayed exercise-induced upregulation of angiogenic proteins and recovery of motor function after photothrombotic stroke in mice. Neuroscience. 2021;461:57–71. doi: 10.1016/j.neuroscience.2021.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamri F.F., Al Shoyaib A., Syeara N., Paul A., Jayaraman S., Karamyan S.T., Arumugam T.V., Karamyan V.T. Delayed atomoxetine or fluoxetine treatment coupled with limited voluntary running promotes motor recovery in mice after ischemic stroke. Neural Regen. Res. 2021;16(7):1244–1251. doi: 10.4103/1673-5374.301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi A.R., Alali A.S., Samman Y., Alghamdi N.A., Albaradie O., Almaghrabi M., Makkawi S., Alghamdi S., Alzahrani M.S., Alsalmi M., Karamyan V.T., Al Sulaiman K., Aljuhani O., Alamri F.F. Vitamin D serum level predicts stroke clinical severity, functional independence, and disability-A retrospective cohort study. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.951283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Senani F., Al-Johani M., Salawati M., ElSheikh S., AlQahtani M., Muthana J., AlZahrani S., Shore J., Taylor M., Ravest V.S. A national economic and clinical model for ischemic stroke care development in Saudi Arabia: a call for change. Int. J. Stroke. 2019;14(8):835–842. doi: 10.1177/1747493019851284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks J.L., Marotta C.A. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- Basri R., Issrani R., Hua Gan S., Prabhu N., Khursheed Alam M. Burden of stroke in the Kingdom of Saudi Arabia: A soaring epidemic. Saudi Pharm J. 2021;29(3):264–268. doi: 10.1016/j.jsps.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyuk F. The Predictor potential role of the glucose to potassium ratio in the diagnostic differentiation of massive and non-massive pulmonary embolism. Clin. Appl. Thromb. Hemost. 2022;28 doi: 10.1177/10760296221076146. 10760296221076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviere L., Gathier C.S., Rafiq M., Koopman I., Rousseau V., Raposo N., Albucher J.F., Viguier A., Geeraerts T., Cognard C., Rinkel G.J.E., Vergouwen M.D.I., Olivot J.M. Rebleeding after aneurysmal subarachnoid hemorrhage in two centers using different blood pressure management strategies. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.836268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtaş E., Korkmaz İ., Tekin Y., Demirtaş E., Çaltekin İ. Assessment of serum glucose/potassium ratio as a predictor for delayed neuropsychiatric syndrome of carbon monoxide poisoning. Hum. Exp. Toxicol. 2021;40(2):207–213. doi: 10.1177/0960327120945773. [DOI] [PubMed] [Google Scholar]
- Dungan K.M., Braithwaite S.S., Preiser J.-C. Stress hyperglycaemia. Lancet. 2009;373(9677):1798–1807. doi: 10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiou M., Michaelidou A.M., Athanasiadis A.P., Menexes G., Symeonidou M., Koulourida V., Ganidou M., Theodoridis T.D., Tarlatzis B.C. Second trimester amniotic fluid glucose, uric acid, phosphate, potassium, and sodium concentrations in relation to maternal pre-pregnancy BMI and birth weight centiles. J. Matern. Fetal Neonatal Med. 2015;28(8):910–915. doi: 10.3109/14767058.2014.937692. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Matano F., Mizunari T., Murai Y., Tateyama K., Koketsu K., Kubota A., Kobayashi S., Yokota H., Morita A. Serum glucose/potassium ratio as a clinical risk factor for aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2017;129(4):870–875. doi: 10.3171/2017.5.JNS162799. [DOI] [PubMed] [Google Scholar]
- Gariballa S.E., Robinson T.G., Fotherby M.D. Hypokalemia and potassium excretion in stroke patients. J. Am. Geriatr. Soc. 1997;45(12):1454–1458. doi: 10.1111/j.1532-5415.1997.tb03195.x. [DOI] [PubMed] [Google Scholar]
- Glushakova O.Y., Glushakov A.V., Miller E.R., Valadka A.B., Hayes R.L. Biomarkers for acute diagnosis and management of stroke in neurointensive care units. Brain Circ. 2016;2(1):28–47. doi: 10.4103/2394-8108.178546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Wang G., Jing J., Wang A., Zhang X., Meng X., Zhao X., Liu L., Li H., Wang D., Wang Y., Wang Y. Stress hyperglycemia may have higher risk of stroke recurrence than previously diagnosed diabetes mellitus. Aging (Albany NY) 2021;13(6):9108–9118. doi: 10.18632/aging.202797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016;6(2):603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Wang Z., Huang Z.X., Liu Z. Biomarkers and the outcomes of ischemic stroke. Front. Mol. Neurosci. 2023;16:1171101. doi: 10.3389/fnmol.2023.1171101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S., Al Shoyaib A., Kocot J., Villalba H., Alamri F.F., Rashid M., Wangler N.J., Chowdhury E.A., German N., Arumugam T.V., Abbruscato T.J., Karamyan V.T. Peptidase neurolysin functions to preserve the brain after ischemic stroke in male mice. J. Neurochem. 2020;153(1):120–137. doi: 10.1111/jnc.14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jickling G.C., Liu D., Stamova B., Ander B.P., Zhan X., Lu A., Sharp F.R. Hemorrhagic transformation after ischemic stroke in animals and humans. J. Cereb. Blood Flow Metab. 2014;34(2):185–199. doi: 10.1038/jcbfm.2013.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.M., Paik J.H., Kim S.Y., Hong D.Y. Association of plasma glucose to potassium ratio and mortality after aneurysmal subarachnoid hemorrhage. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.661689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A., Gröschel K., Ringer T.M., Redecker C., Cordesmeyer R., Witte O.W., Terborg C. Early disruption of the blood–brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39(8):2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- Katipoğlu B., Demirtas E. Assessment of serum glucose potassium ratio as a predictor for morbidity and mortality of blunt abdominal trauma. Turk. J. Trauma Emerg. Surgery. 2022;28(2):134. doi: 10.14744/tjtes.2020.88945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw K.T., Barrett-Connor E. Dietary potassium and stroke-associated mortality. A 12-year prospective population study. N. Engl. J. Med. 1987;316(5):235–240. doi: 10.1056/NEJM198701293160502. [DOI] [PubMed] [Google Scholar]
- Laredo C., Renú A., Llull L., Tudela R., López-Rueda A., Urra X., Macías N.G., Rudilosso S., Obach V., Amaro S., Chamorro Á. Elevated glucose is associated with hemorrhagic transformation after mechanical thrombectomy in acute ischemic stroke patients with severe pretreatment hypoperfusion. Sci. Rep. 2020;10(1):10588. doi: 10.1038/s41598-020-67448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Ma X., Zhou X., Wang Y. The association between serum glucose to potassium ratio on admission and short-term mortality in ischemic stroke patients. Sci. Rep. 2022;12(1):8233. doi: 10.1038/s41598-022-12393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luitse M.J., Biessels G.J., Rutten G.E., Kappelle L.J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11(3):261–271. doi: 10.1016/S1474-4422(12)70005-4. [DOI] [PubMed] [Google Scholar]
- Marini J.I., Sein M.E. The Role of the Glucose Potassium Ratio in the Management of Traumatic Brain Injury. Korean J Neurotrauma. 2023;19(1):82–89. doi: 10.13004/kjnt.2023.19.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano F., Fujiki Y., Mizunari T., Koketsu K., Tamaki T., Murai Y., Yokota H., Morita A. Serum glucose and potassium ratio as risk factors for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2019;28(7):1951–1957. doi: 10.1016/j.jstrokecerebrovasdis.2019.03.041. [DOI] [PubMed] [Google Scholar]
- Moraes M.d.A., Jesus P.A.d., Muniz L.S., Baccin C.A., Barreto A.B.M., Sales R.S., Pires C.G.d.S., Teles C.A.D.S., Mussi F.C. Arrival time at a referral hospital and functional disability of people with stroke: a cohort study. Sao Paulo Med J. 2023;141:e2022510. doi: 10.1590/1516-3180.2022.0510.R1.27022023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciaroni M., Agnelli G., Caso V., Corea F., Ageno W., Alberti A., Lanari A., Micheli S., Bertolani L., Venti M. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc. Dis. 2009;28(2):119–123. doi: 10.1159/000223436. [DOI] [PubMed] [Google Scholar]
- Piironen K., Putaala J., Rosso C., Samson Y. Glucose and acute stroke: evidence for an interlude. Stroke. 2012;43(3):898–902. doi: 10.1161/STROKEAHA.111.631218. [DOI] [PubMed] [Google Scholar]
- Shibata A., Matano F., Saito N., Fujiki Y., Matsumoto H., Mizunari T., Morita A. Serum glucose-to-potassium ratio as a prognostic predictor for severe traumatic brain injury. J. Nippon Med. Sch. 2021;88(4):342–346. doi: 10.1272/jnms.JNMS.2021_88-506. [DOI] [PubMed] [Google Scholar]
- Sokol S.I., Portnay E.L., Curtis J.P., Nelson M.A., Hebert P.R., Setaro J.F., Foody J.M. Modulation of the renin-angiotensin-aldosterone system for the secondary prevention of stroke. Neurology. 2004;63(2):208–213. doi: 10.1212/01.wnl.0000130360.21618.d0. [DOI] [PubMed] [Google Scholar]
- Spronk E., Sykes G., Falcione S., Munsterman D., Joy T., Kamtchum-Tatuene J., Jickling G.C. Hemorrhagic Transformation in Ischemic Stroke and the Role of Inflammation. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.661955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier S.O. Potassium physiology. Am. J. Med. 1986;80(4A):3–7. doi: 10.1016/0002-9343(86)90334-7. [DOI] [PubMed] [Google Scholar]
- Wang J., Feng Q., Zhang Y., Qiu W., Gao H. Elevated glucose-potassium ratio predicts preoperative rebleeding in patients with aneurysmal subarachnoid hemorrhage. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.795376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Tian X., Gu H., Zuo Y., Meng X., Chen P., Li H., Wang Y. Electrolytes and clinical outcomes in patients with acute ischemic stroke or transient ischemic attack. Ann Transl Med. 2021;9(13):1069. doi: 10.21037/atm-21-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.Y., Zhuang Y.K., Cai Y., Dong X.Q., Wang K.Y., Du Q., Yu W.H. Serum glucose and potassium ratio as a predictive factor for prognosis of acute intracerebral hemorrhage. J. Int. Med. Res. 2021;49(4) doi: 10.1177/03000605211009689. 3000605211009689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Chen S., Ruan Y., Liu Y., Cheng H., Zeng Y., Chen Y., Cheng Q., Huang G., He W. The stress hyperglycemia ratio is associated with hemorrhagic transformation in patients with acute ischemic stroke. Clin. Interv. Aging. 2021:431–442. doi: 10.2147/CIA.S280808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L., Zhang D., Yao Y., Wang Y. Symptomatic intracranial hemorrhage in patients with admission hyperglycemia and diabetes after mechanical thrombectomy: A systematic review and meta-analysis. Am. J. Emerg. Med. 2021;45:23–28. doi: 10.1016/j.ajem.2021.02.032. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yang C.S., Shen L.J., Lv Q.W., Xu Q.C. Usefulness of serum glucose and potassium ratio as a predictor for 30-day death among patients with severe traumatic brain injury. Clin. Chim. Acta. 2020;506:166–171. doi: 10.1016/j.cca.2020.03.039. [DOI] [PubMed] [Google Scholar]
- Zonneveld T.P., Nederkoorn P.J., Westendorp W.F., Brouwer M.C., van de Beek D., Kruyt N.D., Investigators P. Hyperglycemia predicts poststroke infections in acute ischemic stroke. Neurology. 2017;88(15):1415–1421. doi: 10.1212/WNL.0000000000003811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.