This study revealed the enhanced therapeutic effects and mechanisms of Exendin-4–engineered MSCs for type 2 diabetes treatment.
Abstract
Mesenchymal stem cell (MSC)-based therapy to combat diabetic-associated metabolic disorders is hindered by impoverished cell survival and limited therapeutic effects under high glucose stress. Here, we genetically engineered MSCs with Exendin-4 (MSC-Ex-4), a glucagon-like peptide-1 (GLP-1) analog, and demonstrated their boosted cellular functions and antidiabetic efficacy in the type 2 diabetes mellitus (T2DM) mouse model. Mechanistically, MSC-Ex-4 achieved self-augmentation and improved survival under high glucose stress via autocrine activation of the GLP-1R-mediated AMPK signaling pathway. Meanwhile, MSC-Ex-4-secreted Exendin-4 suppressed senescence and apoptosis of pancreatic β cells through endocrine effects, while MSC-Ex-4-secreted bioactive factors (e.g., IGFBP2 and APOM) paracrinely augmented insulin sensitivity and decreased lipid accumulation in hepatocytes through PI3K-Akt activation. Furthermore, we encapsulated MSC-Ex-4 in 3D gelatin microscaffolds for single-dose administration to extend the therapeutic effect for 3 months. Together, our findings provide mechanistic insights into Exendin-4-mediated MSCs self-persistence and antidiabetic activity that offer more effective MSC-based therapy for T2DM.
INTRODUCTION
Diabetes has heretofore afflicted more than 436 million people worldwide, and this number is estimated to reach 700 million by 2045 (1). Type 2 diabetes mellitus (T2DM), which accounts for around 90% of diabetes cases, is characterized by insulin resistance and hyperglycemia, resulting from obesity, lack of exercise, unhealthy diet, and heredity (1). Insulin resistance occurs when cells in the liver, muscle, and adipose tissue become unresponsive to insulin and lead to a failure of glucose uptake (2). Therefore, pancreatic β cells will compensate for insulin resistance through an augmented insulin production, which eventually leads to the exhaustion of β cells and irreversible hyperglycemia (3). Consequently, prolonged exposure to the chronic hyperglycemia suppresses proliferation and induces apoptosis of β cells, which results in diminished β cell mass and β cell dysfunction (4). Besides, T2DM is immensely associated with hepatic dysfunction, providing that more than 90% of obese patients with T2DM have metabolic-associated fatty liver disease (MAFLD) (5). Hepatocytes play a prominent role in glucose and lipid homeostasis by directing nutrients to storage as glycogen and triglycerides (TGs). During hepatic insulin resistance state, insulin fails to suppress gluoconeogenesis but accelerates fatty acid synthesis in the hepatocytes, thus raising hepatic glucose production and TG accumulation (6). Despite β cell and hepatocyte dysfunction, hyperglycemia and hypertriglyceridemia exacerbate the insulin resistance state in muscle and adipose tissues while engendering dysfunction of other organs and tissues. Therefore, T2DM is inextricably linked to a vast range of complications including coronary heart disease, stroke, and retinopathy (7).
Besides change in the lifestyle, antidiabetic drugs should be applied to obtain a better maintenance of normoglycemia in patients with T2DM. Glucagon-like peptide-1 (GLP-1) is an incretin hormone that assists to control the glycemic excursions through augmentation of insulin and inhibition of glucagon secretion by interacting with GLP-1 receptors (GLP-1R) (8). However, GLP-1 is rarely used for T2DM therapy due to its short half-life, which is rapidly degraded by dipeptidyl peptidase-4 within minutes. The first approved GLP-1R agonist for T2DM treatment, Exendin-4, which is a 39–amino acid peptide, is a GLP-1 analog with a longer half-life of 2.4 hours. It enhances the β cell mass through inhibition of cell apoptosis and promotion of cell proliferation and thereby increases the amount of insulin secretion (9). Besides, it was proved that Exendin-4 is a competent drug candidate to reduce body weight, as well as ameliorate diabetes and MAFLD (10). Albeit the improvement of Exendin-4 in regulating blood glucose and insulin response, its plasma half-life is still limited due to renal elimination. Therefore, a twice-daily administration is needed, causing undesired fluctuations in plasma concentrations and intermittent activation of GLP-1R (10).
Despite the benefits brought by the above-mentioned antidiabetic drug therapy, some patients still failed to restore normoglycemia or suffered from versatile side effects such as hypoglycemia, diarrhea, nausea, and vomiting (11). In recent years, cell-based therapies have emerged as alternative approaches to combat a plethora of refractory diseases including T2DM (12). In particular, mesenchymal stem/stromal cells (MSCs) have demonstrated their therapeutic effects to ameliorate hyperglycemia, insulin resistance, and systemic inflammation caused by T2DM in several preclinical and clinical attempts (13–15), thus providing a new regime for the treatment of T2DM. Meanwhile, technological advances are still in high demand for successful translation of MSC-based therapy into the clinical treatment of T2DM. One of the main barriers to overcome is the reduced proliferation and survival rate of MSCs after in vivo administration (16). Therefore, several strategies such as biomaterial encapsulation (17), genetic engineering, and preconditioning of MSCs (18) have been investigated to improve the survival rate, delay clearance kinetics, and sustain MSC-secreted factors in vivo. Besides, it is critical to optimize the administration route of MSCs as MSCs administered intravenously are mostly trapped in the lungs and subsequent tissues, resulting in attenuated therapeutic effects (19). Furthermore, a comprehensive understanding of the therapeutic mechanisms of MSCs in T2DM remains elusive. MSCs were proved to promote the production of endogenous insulin and stimulate the proliferation of β cells (20). Other studies also showed that MSCs could increase glucose transporter (GLUT) expression and enhance the phosphorylation of insulin receptor substrate 1 (IRS-1), protein kinase B (Akt), and adenosine 5′-monophosphate (AMP)–activated protein kinase (AMPK) in insulin-targeting tissues such as liver and pancreas, thereby improving insulin resistance (21, 22). Besides, MSCs are well known for their ability to modulate immune responses, which is vital to ameliorate the systemic inflammation caused by T2DM (23).
Given the above deficiencies of Exendin-4 and MSCs in treating T2DM, researchers have explored to synergize the therapeutic benefits of Exendin-4 and MSCs. Habib et al. (24) revealed that coadministration of both MSCs and free Exendin-4 outperformed the free Exendin-4 alone in promoting β cell proliferation. MSCs have been also genetically modified with GLP-1, which have shown advantages in therapeutic efficacy over the wild-type MSCs in T2DM treatment (25). However, it should be stressed that these combinatorial therapies are inherited with numerous deficiencies. For examples, the therapeutic effects and duration of the single-dose free Exendin-4 are limited when administrated with MSCs. Besides, it is expected that the GLP-1–modified MSCs can hardly result in marked improvement in the therapeutic efficacy of MSCs, considering that GLP-1 has only a half-life of 2 min and requires a high effective dosage in treating T2DM (26), along with the diminished survival rate of MSCs after in vivo administration. None of these studies have systematically investigated the mechanisms of how the “biologics plus cells” combinatory approaches are synergized to augment their therapeutic benefits in T2DM treatment.
Here, on the basis of the finding that human MSCs expressed GLP-1R, we constructed Exendin-4 genetically engineered MSCs (MSC-Ex-4) through lentiviral transduction system to test our hypothesis that the secreted Exendin-4 from MSC-Ex-4 could activate AMPK signaling pathway via GLP-1R–mediated autocrine activation, thus potentially promoting the self-persistence by elongating their survival under high-glucose stress and augmenting the antidiabetic efficacy (Fig. 1A). We also examined the potential mechanisms regarding the endocrine effects of MSC-Ex-4 to protect the pancreatic β cells and the paracrine effects of MSC-Ex-4 to improve hepatocyte functions. Aside from the MSC-Ex-4 secreted Exendin-4, we postulated that the other secretomes of MSC-Ex-4 could reduce cellular senescence and apoptosis while promoting proliferation of pancreatic β cells, as well as improving insulin sensitivity and reducing lipid accumulation in hepatocytes through phosphatidylinositol 3-kinase (PI3K)–Akt signaling pathway (Fig. 1A). Last, we delivered the free MSC-Ex-4 systematically with multiple doses and assisted the MSC-Ex-4 with injectable three-dimensional (3D) gelatin microscaffolds (GMs) as cell encapsulation and delivery vehicles to achieve long-acting therapeutic benefit with single-dose local administration (Fig. 1B).
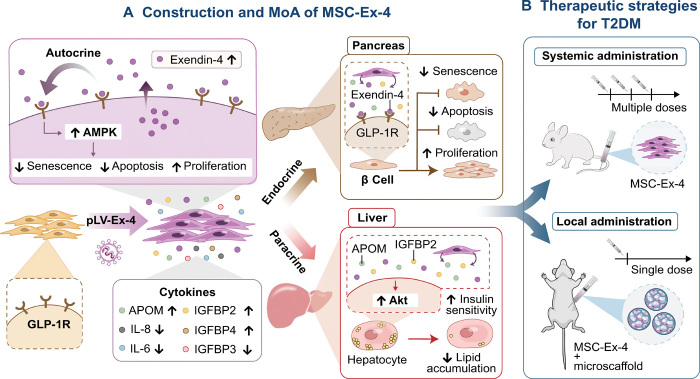
Fig. 1. Schematic representation for the construction of MSC-Ex-4, mechanism of actions (MoA), and the therapeutic strategies for T2DM treatment.
(A) Exendin-4 genetically engineered MSCs (MSC-Ex-4) were constructed through lentiviral transduction system for the delivery of Exendin-4 gene into the AD-MSCs and thus enabled continuous production of Exendin-4 and induction of versatile secretomes, including APOM and IGFBP2. Specifically, the secreted Exendin-4 from MSC-Ex-4 could interact with their own GLP-1R through autocrine signaling and activate AMPK signaling pathway, which could potentially promote their self-persistence by elongating their survival under high-glucose stress and augment the anti-diabetic efficacy. Besides, we delineated that MSC-Ex-4 could protect the pancreatic β cells through endocrine effects while improve hepatocytes functions though paracrine effects. Aside from Exendin-4, we postulated that the other secretomes of MSC-Ex-4, especially IGFBP2 and APOM, could reduce senescence and apoptosis of pancreatic β cells, as well as improve insulin sensitivity and reduce lipid accumulation in hepatocytes through PI3K-Akt signaling pathway. IL-8, interleukin-8. (B) Last, we adopted versatile therapeutic strategies for T2DM treatment in vivo. We delivered the free MSC-Ex-4 systematically with multiple doses and assisted the MSC-Ex-4 with injectable 3D gelatin microscaffolds (GMs) as cell encapsulation and delivery vehicles to achieve long-acting therapeutic benefit with single-dose local administration.
RESULTS
MSC-Ex-4 displayed higher cell proliferation, resistance to cellular senescence, and apoptosis under high-glucose treatment
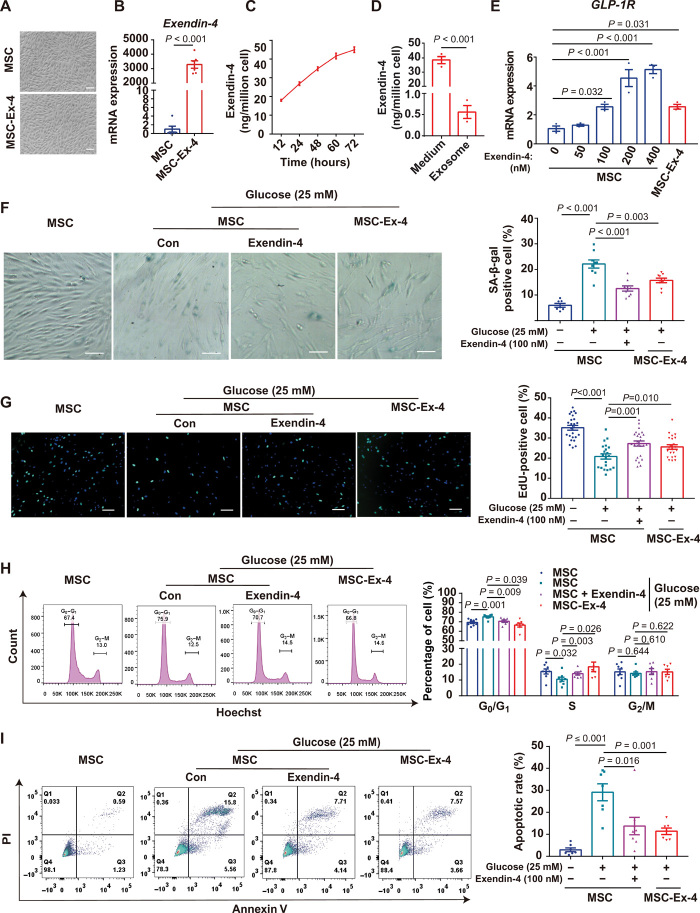
Lentiviral transduction was applied to overexpress Exendin-4 in human adipose-derived MSCs (AD-MSCs) (Fig. 2A), resulting in approximately 3000-fold increase in Exendin-4 mRNA expression (Fig. 2B). Compared with the wild-type MSCs, MSC-Ex-4 did not exhibit significant difference in proliferation (fig. S1A) and cell viability (fig. S1B), as well as expression of key functional genes (e.g., stemness-related, growth factors and inflammation-related genes) (fig. S1C) and MSC surface markers (e.g., CD44, CD73, CD90, and CD105) (fig. S1D), indicating that the genetic modification of Exendin-4 did not disrupt the typical features of MSCs. Furthermore, 1 million MSC-Ex-4 secreted about 15 ng of Exendin-4 to the conditioned medium (CM) every 24 hours (Fig. 2C). To investigate whether Exendin-4 was secreted directly into medium or presented in exosomes, we isolated the secreted exosomes by ultracentrifugation, which confirmed that the Exendin-4 peptides were secreted to the extracellular medium rather than in the form of exosomes (Fig. 2D and fig. S1, E and F). Furthermore, the mRNA expression of GLP-1R was elevated in both the MSC-Ex-4 and the free Exendin-4–treated MSCs in a concentration-dependent manner (Fig. 2E), suggesting a potential positive feedback for Exendin-4–mediated MSC enhancement.
Fig. 2. MSC-Ex-4 displayed higher cell proliferation, resistance to cellular senescence, and apoptosis under high-glucose stress.
(A) Morphology of MSCs and MSC-Ex-4. Representative micrographs with 100-μm scale bars. (B) Exendin-4 mRNA expression in MSCs transfected with lentivirus system. (C) Quantification of Exendin-4 continuously secreted by the MSC-Ex-4 to conditioned medium (CM). (D) Quantification of Exendin-4 in the CM and exosomes. (E) GLP-1R mRNA expression in MSCs and MSC-Ex-4. (F) MSCs and MSC-Ex-4 were induced to senescence with 25 mM glucose for 5 days, and the MSCs were pretreated with 100 nM Exendin-4 for 24 hours. In situ staining of SA-β-gal with cells was performed using a senescence β-galactosidase staining kit. Representative micrographs with 100-μm scale bars. (G) EdU staining of Exendin-4 (100 nM) pretreated MSCs and MSC-Ex-4 after induced with 25 mM glucose for 5 days. Representative micrographs with 100-μm scale bars. (H) Cell cycle of Exendin-4 (100 nM) pretreated MSCs and MSC-Ex-4 were presented by G0/G1, S, and G2/M phase after induced with 25 mM glucose for 5 days. (I) MSCs and MSC-Ex-4 were induced to apoptosis by 0.125 mM hydrogen peroxide (H2O2), and the MSCs were pretreated with 100 nM Exendin-4 for 24 hours. All statistical analyses are presented on the right panel of data. For all charts, data are presented as means ± SEM (standard error of the mean); data are not statistically significant when P values are not shown; n ≥ 3 per group. PI, propidium iodide.
Given that T2DM is characterized by hyperglycemia, which potentially disturbs the functions of MSCs in vivo (27), we investigated the effects of glucose on MSC proliferation and evaluated the protective effects of the MSC-Ex-4 and free Exendin-4 to MSCs. After 5 days of stimulation under 25 mM glucose, MSC proliferation was markedly suppressed with apparent cell senescence (fig. S1G). Reduced senescence (Fig. 2F) and improved proliferation (Fig. 2G) of the MSC-Ex-4 and the MSCs pretreated with 100 nM free Exendin-4 for 24 hours were achieved, which were further confirmed by 5-Ethynyl-2’-deoxyuridine (EdU) staining (Fig. 2G) and flow cytometry analysis showing the cell cycle shifting from G0/G1 phase to S phase (Fig. 2H). Furthermore, the MSC-Ex-4 and the Exendin-4–pretreated MSCs also showed increased resistance to oxidative stress–induced apoptosis by hydrogen peroxide (H2O2) (Fig. 2I). Overall, these results indicated that both Exendin-4 gene modification and free Exendin-4 could improve MSC functionalities with more resistance to senescence and apoptosis under high-glucose stress.
Exendin-4 improved MSC-Ex-4 functionality via GLP-1R–AMPK activation
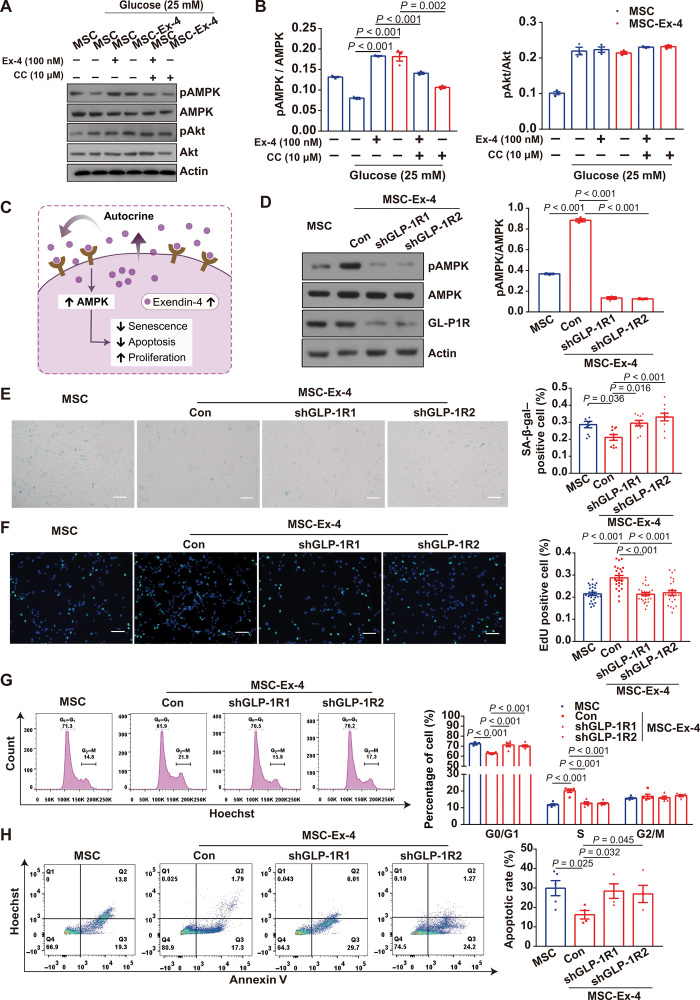
Next, we explored the potential mechanisms for the improved functionality of MSC-Ex-4. Exendin-4 was shown to activate GLP-1R and its downstream signaling pathways (e.g., Akt and AMPK) of β cells to modulate the cellular functions (28). Meanwhile, in vitro studies showed that both free Exendin-4 and GLP-1 could protect MSCs from reactive oxygen species or glucose/serum deprivation–induced apoptosis through PI3K-Akt signaling pathways (29). Therefore, we investigated the expression of phosphorylated Akt (pAkt) and phosphorylated AMPK (pAMPK) under high-glucose stress. Compared to MSCs, the Exendin-4–pretreated MSCs and the MSC-Ex-4 exhibited higher expression of pAMPK, which could be inhibited by the inhibitor of AMPK, compound C. Meanwhile, the expression of pAkt displayed no difference between groups (Fig. 3, A and B), indicating that the Exendin-4 gene modification promoted the activation of AMPK, but not AKT, signaling pathways in the MSC-Ex-4.
Fig. 3. Exendin-4 improved MSC-Ex-4 functionality via autocrine GLP-1R–AMPK activation.
(A) pAMPK and pAkt expression in Exendin-4 (100 nM) pre-treated MSC or the MSC-Ex-4 after stimulated with 25 mM glucose for 5 days. The inhibitor of AMPK, compound C (CC), was used to inactive the AMPK signaling in both MSCs and MSC-Ex-4. (B) Statistical analysis of pAMPK/AMPK and pAkt/Akt from immunoblots as shown in (A). (C) Schematic representation for the GLP-1R–mediated autocrine activation of AMPK signaling pathway with improved functions of the MSC-Ex-4. (D) pAMPK expression in MSC-Ex-4 after two short hairpin RNA (shRNA), namely, shGLP-1R1 and shGLP-1R2, was used to knockdown GLP-1R in MSC-Ex-4. (E) The apoptotic MSC-Ex-4 were stained with SA-β-gal after knockdown of GLP-1R with shGLP-1R1 and shGLP-1R2. (F) EdU staining of MSC-Ex-4 after GLP-1R knockdown (GLP-1RKD) with shGLP-1R1 and shGLP-1R2. Representative micrographs with 100-μm scale bars. (G) Cell cycle of MSC-Ex-4 after GLP-1RKD with shGLP-1R1 and shGLP-1R2 were presented by G0/G1, S, and G2/M phases. (H) The apoptotic rate of MSC-Ex-4 after GLP-1RKD with shGLP-1R1 and shGLP-1R2. All statistical analyses are presented on the right panel of data. Representative micrographs with 100-μm scale bars. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
Akin to the interaction between Exendin-4 and GLP-1R of the MSC-Ex-4, we hypothesized that the Exendin-4 secreted by MSC-Ex-4 may activate their own GLP-1R and AMPK signaling pathway via autocrine signaling (Fig. 3C). To validate this hypothesis, we used two short hairpin RNA (shRNA) to knockdown GLP-1R in MSC-Ex-4, which resulted in decreased pAMPK expression (Fig. 3D) and impaired antisenescence function (Fig. 3E). Meanwhile, GLP-1R knockdown (GLP-1RKD) reduced the advantages of MSC-Ex-4 in proliferation (Fig. 3F), cell cycle shifting (Fig. 3G), and antiapoptotic effects (Fig. 3H) over the wild-type MSCs under high-glucose stress. We further tested the different responses of the GLP-1RKD MSCs and the wild-type MSCs when treated with the CM of MSC-Ex-4. The increase in AMPK activation (fig. S2A), as well as improved antisenescence effects (fig. S2B), cell proliferation (fig. S2C), cell cycle shifting (fig. S2D), and antiapoptotic effects (fig. S2E), were all eliminated in the GLP-1RKD MSCs, which were treated with the MSC-Ex-4 CM. Together, these results demonstrated that Exendin-4 secreted by the MSC-Ex-4 could activate AMPK signaling pathway via GLP-1R–mediated autocrine activation, which could augment self-functionalities and achieve self-persistence under stressed microenvironment.
MSC-Ex-4 promoted cell proliferation and resistance to oxidative stress of β cells in vitro
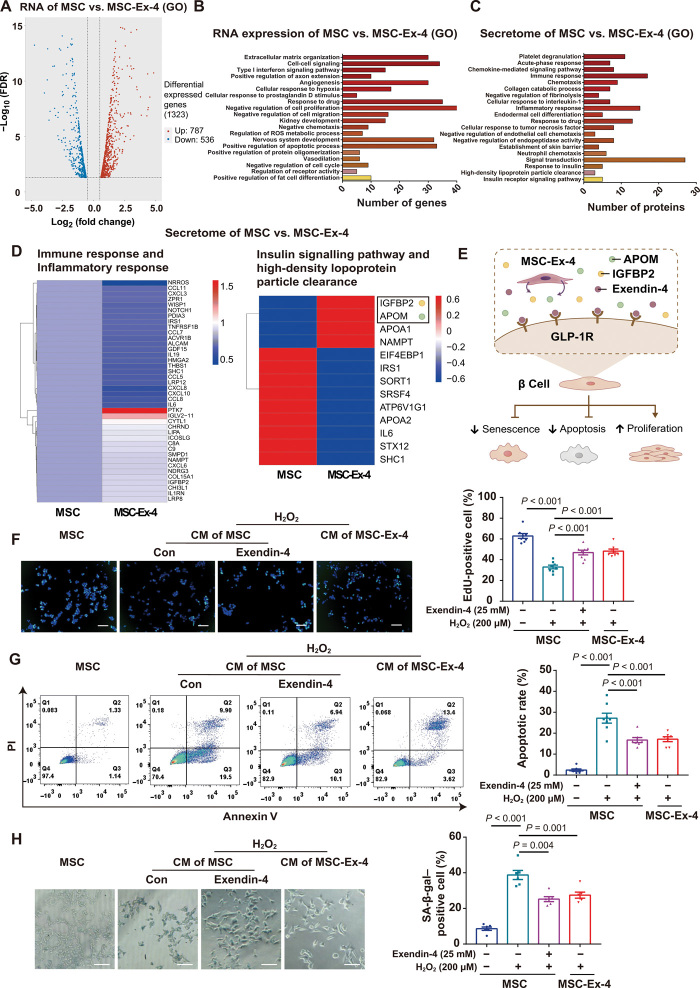
We next compared the RNA expression and secretomes between MSCs and MSC-Ex-4 under glucose stimulation (25 mM). A total of 1323 differentially expressed genes (DEGs) were identified, with 787 up-regulated and 536 down-regulated genes (Fig. 4A). Gene ontology (GO) enrichment analysis suggested that most differential genes were associated with cell proliferation, cell cycle, and cell apoptosis (Fig. 4B), which was aligned with the aforementioned findings regarding the self-persistence of MSC-Ex-4 (Fig. 2). Meanwhile, the MSC secretomes have been proved to play a regulatory role in vivo through the paracrine effects. Analysis of differentially expressed secretomes showed that these secretomes modulated cell signaling pathway, immune responses, and cell metabolism of MSC-Ex-4 (Fig. 4, C and D). Among them, insulin-like growth factor–binding protein 2 (IGFBP2) and apolipoprotein (APOM) were secreted more abundantly by MSC-Ex-4 compared to the wild-type MSCs (Fig. 4D), indicating their possible regulatory role in ameliorating T2DM. Apart from the secretomes, Exendin-4 could protect islet β cells, promote cell proliferation, and resist cell apoptosis in patients with T2DM (30). Therefore, we investigated the protective effects of MSCs and MSC-Ex-4 CM on β cell line Min6 with oxidative stress–induced senescence and apoptosis by H2O2 (Fig. 4E). The results indicated that Exendin-4 and the MSC-Ex-4 CM could enhance the proliferation of β cells (Fig. 4F) and resisted the oxidative stress–induced cell apoptosis (Fig. 4G) and cell senescence (Fig. 4H). Together, these data indicated that the MSC-Ex-4 might be able to protect β cells from the hostile T2DM microenvironment via an endocrine signaling, resembling the functions of Exendin-4 peptides.
Fig. 4. MSC-Ex-4 promoted β cell proliferation and resistance to oxidative stress in vitro.
(A and B) Differential expression gene between MSCs and MSC-Ex-4 was analyzed by RNA sequencing (RNA-seq). FDR, false discovery rate. (A) Volcano diagram of differential expression genes. (B) GO analysis of significantly enriched genes in MSC-Ex-4 cells. (C and D) Differential expression proteins between MSCs and MSC-Ex-4 secretomes were analyzed by proteomics. (C) GO analysis of significantly enriched proteins in MSC-Ex-4 cells. (D) Heatmap view of differential expression proteins involved in regulating immune response and metabolic regulation in MSCs and MSC-Ex-4 secretomes. (E) Schematic representation for the protective effects of MSC-Ex-4 on β cell in vitro. (F to H) Senescent or apoptotic Min6 treated with CM from MSCs or MSC-Ex-4. (F) EdU staining and the percentages of EdU-positive cells. (G) Annexin V–PI staining and the percentages of apoptotic cells. (H) SA-β-gal staining and the percentages of SA-β-gal–positive cells. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
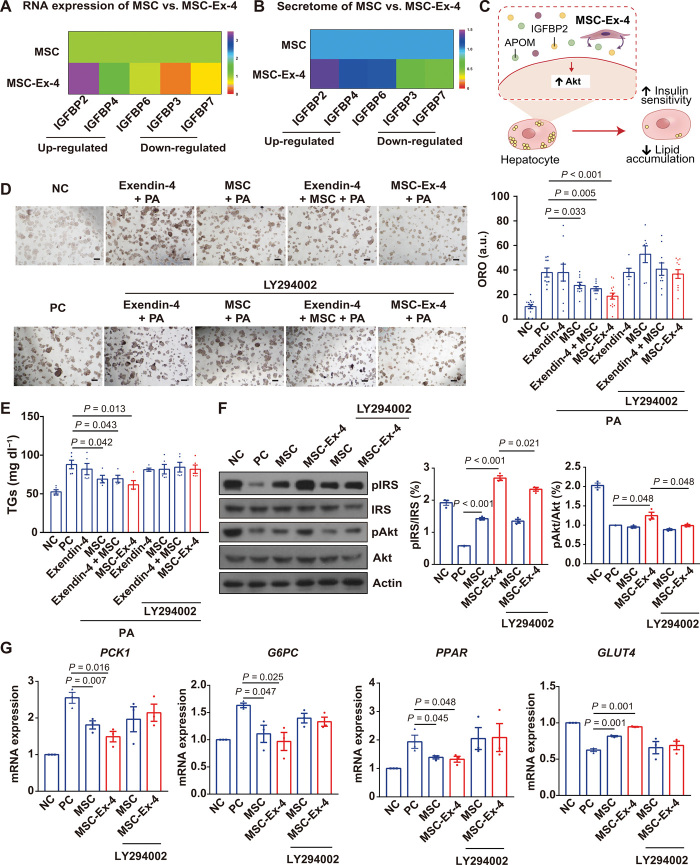
MSC-Ex-4 promoted insulin sensitivity and inhibited lipid accumulation of PA–induced HepG2 in vitro via paracrine signaling
Liver is the major metabolic organ in the body with 80% of liver mass comprised of hepatocytes (31), which play a crucial role in modulating insulin sensitivity and fatty acid accumulation. Meanwhile, MSCs have been reported to be capable of regulating insulin sensitivity and fatty acid metabolism (21, 32). Because previous studies indicated the absence of GLP-1R expression in hepatocytes (33), we first confirmed the absence of GLP-1R in hepatocytes by analyzing existing single-cell sequencing data, including RNA expression of GLP-1R in different cell types of liver (fig. S3, A and B) and their tissue specificity from The Human Protein Atlas (v20.proteinatlas.org/ENSG00000112164-GLP1R/celltype/liver) (fig. S3C) (34). Accordingly, we revealed a minimum expression of GLP-1R on HepG2 (a hepatic cell line) by Western blotting (fig. S3D). The absence of GLP-1R expression in hepatocytes indicated that the regulatory effect of MSC-Ex-4 on hepatocytes was mainly attributed to their paracrinely secreted therapeutic factors, other than the secreted Extendin-4. Next, to search for the key regulatory factors in paracrinely secreted factors, we reanalyzed the RNA sequencing (RNA-seq) and secretomes, both of which indicated a differential expression of APOM and IGFBP family, with the up-regulation of IGFBP2, IGFBP4, and IGFBP6 and down-regulation of IGFBP3 and IGFBP7 (Fig. 5, A and B). APOM could regulate the metabolism of liver lipids, particularly TGs (35), while the top up-regulated protein, IGFBP2, was associated with the regulation of insulin sensitivity and lipid accumulation of mice through the activation of PI3K-Akt signaling pathway (36). Therefore, we hypothesized that bioactive factors, including, but not limited to, APOM and IGFBP2, secreted by the MSC-Ex-4 were capable of regulating glycolipid metabolism of hepatocytes through PI3K-Akt signaling pathway (Fig. 5C).
Fig. 5. MSC-Ex-4 promoted insulin sensitivity and inhibited lipid accumulation in PA-induced HepG2 in vitro via paracrine signaling.
(A) Heatmap showing the expression of IGFBP gene family in MSCs and MSC-Ex-4. (B) Heatmap showing the expression of secreted IGFBP proteins in MSCs and MSC-Ex-4. (C) Schematic representation for the protective effects of MSC-Ex-4 secretome containing bioactive factors (e.g., IGFBP2 and APOM) on hepatocytes in vitro. (D to G) To induce insulin resistance in vitro, HepG2 was treated with 0.1 mM PA for 24 hours. (D) Oil Red O (ORO) staining and the percentages of ORO-positive cells. a.u., arbitrary units. (E) TG levels in each group. (F) Immunoblotting analysis of PI3K-Akt signaling pathway (pIRS and pAkt) and statistical analysis. (G) qPCR analysis of Akt downstream genes related to glucose and lipid metabolism. PCK1, phosphoenolpyruvate carboxykinase 1; G6PC, glucose-6-phosphatase catalytic subunit; PPAR, peroxisome proliferator-activated receptor. NC, negative control; PC, positive control. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
Palmitic acid (PA)–treated HepG2 with induced insulin resistance and lipid accumulation were cocultured with the wild-type MSCs and the MSC-Ex-4. Oil Red O–stained lipid accumulation and intracellular TG in HepG2 could be effectively ameliorated by the MSCs and the MSC-Ex-4 rather than the free Exendin-4 peptide, with the MSC-Ex-4 exhibiting the best therapeutic effect. Notably, addition of LY294002, an inhibitor of PI3K-Akt signaling pathway, totally abolished the therapeutic effects of the MSC-Ex-4 and the MSCs (Fig. 5, D and E). Mechanistically, activation of key members of the PI3K-Akt pathway in HepG2, IRS, and Akt was investigated by Western blot. The MSC-Ex-4 increased the phosphorylation of IRS and Akt more significantly than MSCs. As expected, LY294002 could effectively diminish the phosphorylation of IRS and AKT in HepG2 induced by MSC-Ex-4 (Fig. 5F). In accordance, compared to MSCs, MSC-Ex-4 greatly reduced the expression of genes in HepG2 related to glycolipid metabolism, including phosphoenolpyruvate carboxykinase 1, glucose-6-phosphatase catalytic subunit, and peroxisome proliferator-activated receptor, and increased the expression of GLUT4, which could be effectively abolished by LY294002 (Fig. 5G).
MSC-Ex-4 ameliorated insulin resistance more efficiently than MSCs in vivo
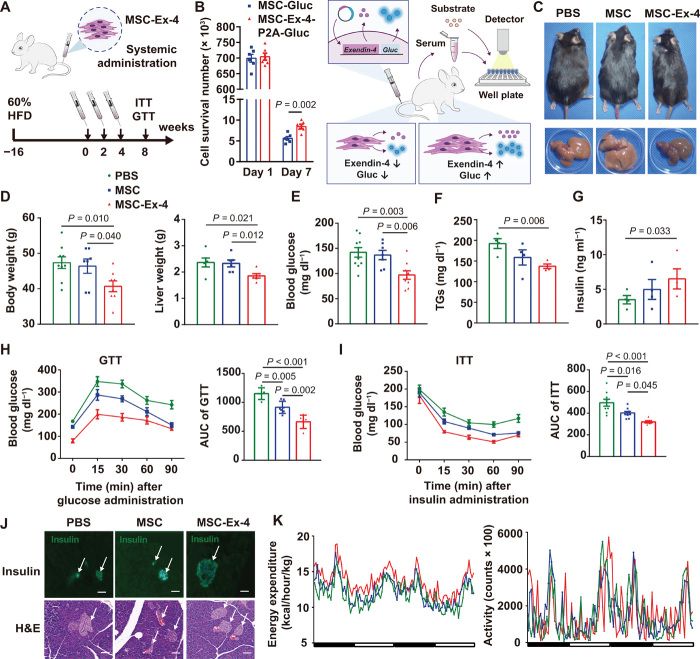
To prove the therapeutic superiority of the MSC-Ex-4 in alleviating metabolic disorders, we first tracked the survival of Akaluc-transfected MSCs after systemic administration, regarding that Akaluc was proved to be much brighter than the conventional luciferase system for in vivo imaging (37). MSCs were mainly trapped in the lungs after intravenous injection and finally resided in liver after 5 days (fig. S4A), while the bioluminescent signals persisted for 15 days (fig. S4, A and B). Next, we monitored the metabolic phenotypes of recipient mice fed with 60% high-fat diet (HFD) for 16 weeks, resulting in high-blood glucose (fig. S4C) and increased body weight (fig. S4D) before the MSC or the MSC-Ex-4 administration, and the same diet was continued for 4 weeks after the cell administration. MSCs showed a higher survival ratio in HFD mice than regular-diet mice after 1 week (fig. S4E). Concentration of the free Exendin-4 peptides decreased rapidly within 16 hours after intravenous injection, while the secreted Exendin-4 in mice received with the MSC-Ex-4 could retain for 7 days and reach a peak concentration on day 5 (fig. S4F). In addition, the blood glucose level changed inversely according to the changes of the Exendin-4 concentration in vivo (fig. S4G).
To guarantee the therapeutic efficacy, we adopted a multidose strategy by intravenous injection of 1 × 106 cells per mouse three times every 2 weeks to the HFD mice (Fig. 6A). To estimate the survival of MSC-Ex-4 and MSCs in vivo, we constructed MSC–Gaussia princeps luciferase (Gluc) and MSC-Ex-4-P2A-Gluc (Fig. 6B). Gluc is a secreted luciferase whose activity in the blood could represent the number of MSCs in vivo (fig. S4H) (38). Compared with the MSC-Gluc, around 3.45 × 103 additional MSC-Ex-4-P2A-Gluc were estimated to survive in the mice in 7 days after injection, suggesting that the Exendin-4 secreted by the MSC-Ex-4-P2A-Gluc could prolong the lifetime of MSCs in vivo (Fig. 6B). Metabolic phenotypes were monitored in 1 month after the third injection. The body and liver weight of the mice receiving the MSC-Ex-4 were significantly decreased by 15 and 20%, respectively, than those receiving the phosphate-buffered saline (PBS) and the MSCs (Fig. 6, C and D). Meanwhile, the concentrations of circulating glucose (Fig. 6E) and TG (Fig. 6F) were decreased up to 25%, and the serum insulin (Fig. 6G) was increased by 20% in the MSC-Ex-4–treated group compared to the MSC and PBS groups. Likewise, the glucose tolerance (Fig. 6H) and insulin sensitivity (Fig. 6I) of the MSC-Ex-4–treated group displayed greater improvements than the MSC-treated group. In addition, improvement in pancreatic islet survival and proliferation was exemplified with increased numbers of islets and insulin-secreted β cells in pancreas of the MSC-Ex-4–treated group (Fig. 6J).
Fig. 6. MSC-Ex-4 administration improved insulin sensitivity and metabolism of HFD mice.
(A) Schematic protocol for administration of PBS, MSCs, and MSC-Ex-4 into HFD mice. (B) Schematic illustration for detection of cell survival in vivo by Gaussia princeps luciferase (Gluc). Quantification of cell number survived in mice received with MSC-Ex-4-P2A-Gluc and MSC-Gluc on days 1 and 7. (C) Gross appearance of mice and the corresponding livers after treatment with PBS, MSCs, and MSC-Ex-4. (D) Body and liver weight after treatment. (E to G) Serum glucose (E), insulin (F), and TG (G) concentration in HFD mice 4 weeks after administration with PBS, MSCs, and MSC-Ex-4. (H and I) GTT (H) and ITT (I) in HFD mice 4 weeks after treatment; quantification (right) of area under curve in GTT and ITT. (J) H&E staining and immunostaining for insulin of murine islets (arrows) after treatment. Data are representative images with 100-μm scale bars. (K) Energy expenditure (left) and activity (right) of mice 4 weeks after treatment. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
Next, we monitored the energy expenditure and activity by housing the mice in metabolic cage systems, which showed that the HFD mice treated with MSC-Ex-4 experienced more energy consumption, independent of their exercise status (Fig. 6K). Furthermore, according to the serum-based biochemical index analysis, the levels of urea (fig. S5A) and lactic acid dehydrogenase (LDH) (fig. S5B) were significantly reduced, while the levels of creatinine (fig. S5C) and creatine kinase-MB (CK-MB; fig. S5D) did not change significantly in the MSC-Ex-4 mice, suggesting that MSC-Ex-4 has a certain protective effect on the kidney and heart. Meanwhile, no evident toxicity and immune rejection was observed after administration of the wild-type MSCs and the MSC-Ex-4, which were indicated by the blood cell count (fig. S5, E and F) and other blood parameters from hematology analysis (fig. S5G) and hematoxylin and eosin (H&E) staining of adipose, muscle, heart, and lung tissues (fig. S5H). Overall, we conducted comprehensive evaluations to verify the advantages of the MSC-Ex-4 in alleviating metabolic syndrome of HFD mice compared to the wild-type MSCs.
MSC-Ex-4 ameliorated lipid accumulation in vivo through PI3K-Akt pathway
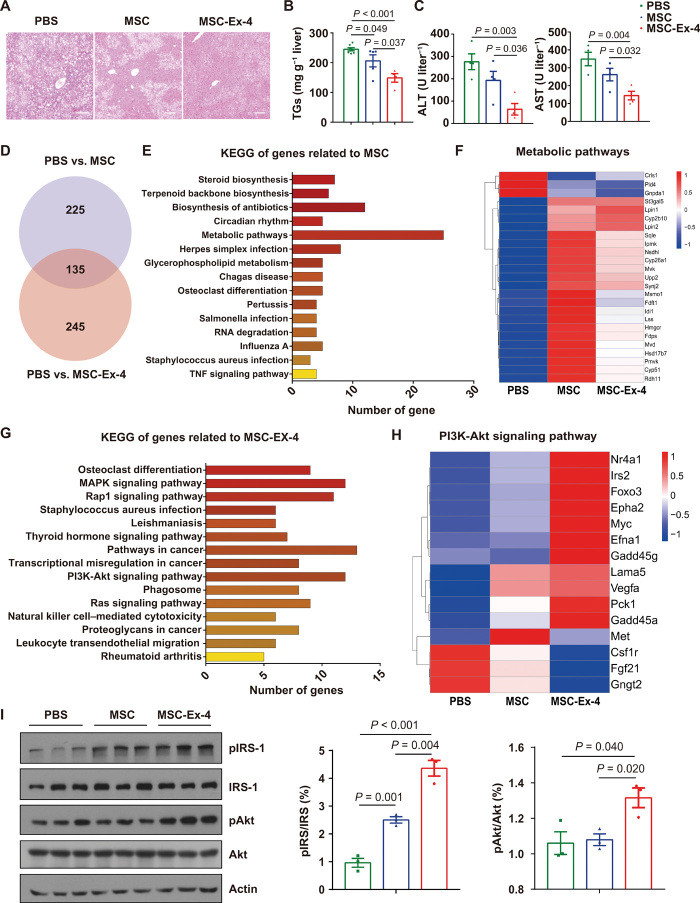
Because the systematically administered MSC-Ex-4 were mainly resided in the liver (fig. S3A), we investigated their therapeutic benefits for liver function improvement in T2DM mice. Compared to the wild-type MSCs, MSC-Ex-4 could alleviate fatty liver phenotypes more effectively (Fig. 7A), markedly decreased the concentrations of TGs (Fig. 7B), and improved the liver functions (Fig. 7C). To compare the therapeutic mechanisms of the wild-type MSCs and the MSC-Ex-4 in alleviating insulin sensitivity and fatty liver, we analyzed the differential transcriptomic of mice liver. A total of 360 DEGs were identified between PBS and MSC group, while 380 DEGs were identified between the PBS and the MSC-Ex-4 group (Fig. 7D). Among them, 135 DEGs were overlapped (Fig. 7D), and the expression levels of these 135 overlapped DEGs in the MSC and the MSC-Ex-4 group were both higher than the PBS group (fig. S6A), indicating various shared therapeutic targets of the MSC and the MSC-Ex-4 treatment in the livers. The shared targets were mainly enriched in the regulation of metabolic pathway according to the analysis by Kyoto Encyclopedia of Genes and Genomes (KEGG) (Fig. 7E), which could be attributed to the therapeutic alleviation of fatty liver by the MSCs and the MSC0Ex-4 therapy (Fig. 7F).
Fig. 7. MSC-Ex-4 ameliorated lipid accumulation in liver through PI3K-Akt pathway.
HFD mice 4 weeks after administration with PBS, MSCs, and MSC-Ex-4. (A) H&E staining of murine liver. (B) Liver TG levels. (C) Amino transferase (ALT) and aspartate aminotransferase (AST) levels in serum. (D) Venn diagram showing differential expression genes in MSCs (compared to PBS) and MSC-Ex-4 (compared to PBS). (E) GO analysis (biological process) of 135 overlapped genes related to MSCs function. TNF, tumor necrosis factor. (F) Heatmap view of 25 overlapped genes related to MSC function involved in regulating metabolic pathways. (G) GO analysis (biological process) of 245 genes in PBS versus MSC-Ex-4 without the overlapped 135 genes in PBS versus MSCs. (H) Heatmap view of 15 genes related to MSC-Ex-4 function involved in PI3K-Akt signaling pathway. (I) Immunoblot analysis of PI3K-Akt signaling pathway (pIRS and pAkt) and statistical analysis. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
Meanwhile, gene expression levels of the 245 nonoverlapped DEGs in the PBS and the MSC-Ex-4 group were all higher in the MSC-Ex-4 group compared to those of the wild-type MSCs (fig. S6B), indicating the distinctive mechanism of action of the MSC-Ex-4. KEGG analysis of these 245 genes revealed that PI3K-Akt signaling pathway (Fig. 7G) and genes related to glycolipid metabolism were markedly changed (Fig. 7H), which might be attributed to the therapeutic superiority of the MSC-Ex-4 compared to the MSCs. These findings were in accordance with the results of RNA-seq and secretome analysis by proteomics in vitro, which indicated that the paracrine factors (e.g., APOM and IGFBP) secreted by the MSC-Ex-4 could regulate the insulin resistance and TG accumulation through the activation of PI3K-Akt signaling pathway in hepatocytes. Furthermore, we investigated the expression of IRS and Akt in PI3K-Akt signaling pathway in liver tissues and revealed that both the wild-type MSCs and the MSC-Ex-4 increased the transcription level of IRS, with a higher expression in the MSC-Ex-4 group. Akt activation by MSCs was not distinct, while MSC-Ex-4 markedly increased the expression of pAkt. Collectively, these results provided mechanistic insights associated with the improved therapeutic efficacy of the MSC-Ex-4, as compared to the wild-type MSCs, to enhance the insulin sensitivity and reduce the lipid accumulation in the liver.
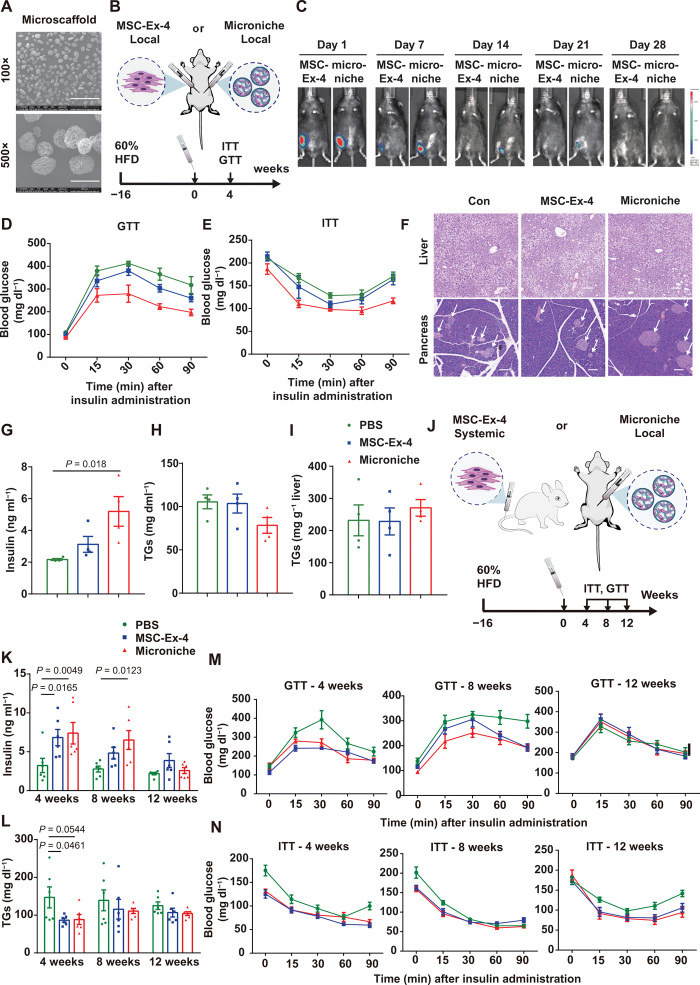
MSCEx4 encapsulated in 3D GMs exhibited augmented and long-term metabolic benefits in HFD mice
Our previous studies have demonstrated that GMs could be applied as injectable cell-loading vehicles and microniches to promote the survival and paracrine secretion of MSCs in vivo, thus leading to an augmented therapeutic efficacy (17, 39, 40). To strengthen the therapeutic benefit of MSC-Ex-4, we loaded MSC-Ex-4 in the porous 3D GMs (microniche) (Fig. 8A), which were cultured for 2 days in vitro to construct an amiable microniche for MSC cultivation. The scanning electron microscope image of the microscaffolds revealed their spherical and porous structure. Around 12,000 microniches loaded with 1 × 106 MSC-Ex-4 were subcutaneously injected into the HFD mice in comparison with the same number of free MSC-Ex-4, to investigate whether single-dose administration could alleviate the metabolic syndrome in T2DM (Fig. 8B). In vivo imaging revealed that the MSC-Ex-4-P2A-Gluc microscaffold resided in the body until day 21, while the free MSC-Ex-4-P2A-Gluc were not detectable (Fig. 8C), indicating that microscaffold encapsulation could prolong the survival of MSCs. Meanwhile, no distinction in body (fig. S7A) and liver weight (fig. S7B), as well as the postprandial blood glucose (fig. S7C), were observed among all groups after 4 weeks, whereas the glucose tolerance (Fig. 8D and fig. S7D) and insulin sensitivity (Fig. 8E and fig. S7E) of the microniche group were significantly improved. Likewise, the number of pancreatic islets (Fig. 8F) and the serum insulin (Fig. 8G) in the microniche group were consistently increased after 4 weeks, while the circulating TG (Fig. 8H), lipid droplets accumulation in liver (Fig. 8I), and overall metabolism (fig. S7F) were not significantly varied among all groups.
Fig. 8. MSCEx4 encapsulated in 3D GMs exhibited augmented and long-term therapeutic benefits in HFD mice.
(A) Scanning electron microscope image of the GMs. Data are representative images with 1-mm scale bars. (B) Schematic protocol for comparison of therapeutic effects between free MSC-Ex-4 and MSC-Ex-4 encapsulated with GM (microniche) after single-dose subcutaneous injection into HFD mice. (C) In vivo bioluminescent imaging of MSC-Ex-4 and microniche resided in mice until day 28. (D to G) Glucose tolerance (D), insulin sensitivity (E), serum insulin (F), and circulating TG (G) of mice after treatment. (H) H&E staining of the livers and pancreas (arrows indicate the islet β cells). (I) Lipid droplets accumulated in liver were extracted and quantified using TG reagent, which were calculated using the manufacturer’s instructions after treatment. (J) Schematic protocol for comparison of therapeutic effects between single-dose systematic administration of MSC-Ex-4 and subcutaneous transplantation of microniche into HFD mice. (K and L) Serum insulin (K) and circulating TG (L) of HFD mice 4, 8, and 12 weeks after treatment. (M and N) GTT (M) and ITT (N) in HFD mice 4, 8, and 12 weeks after treatment. For all charts, data are presented as means ± SEM; data are not statistically significant when P values are not shown; n ≥ 3 per group.
To further investigate the long-term therapeutic benefits of MSC-Ex-4, we monitored the HFD-fed mice subcutaneously transplanted with the MSC-Ex-4 microscaffold (microniche) and systematically administered with the free MSC-Exendin-4 for up to 12 weeks (Fig. 8J). A twofold increase in insulin secretion was achieved in the MSC-Ex-4 and the microniche groups compared to the PBS control in 4 weeks, which could be sustained for 8 weeks (Fig. 8K). On the other hand, the TG lowering effect of the MSC-Ex-4 and the microniche groups could be sustained for 4 weeks (Fig. 8L), in accordance with the improved glucose lowering effect (Fig. 8M and fig. S7G) and insulin sensitivity (Fig. 8N and fig. S7H), compared to the PBS recipients. In particular, we compared the blood glucose levels of mice after 6-hour fasting and ascertained that the glucose lowering effects of both the MSC-Ex-4 and the microniche group could persist for 8 weeks. Meanwhile, after 16-hour fasting, only the blood glucose level of the microniche group decreased significantly in 8 weeks, indicating a better performance in modulating hyperglycemia compared with the free MSC-Ex-4 (fig. S7I). After 8 weeks of transplantation, both groups showed amended insulin sensitivity (Fig. 8N and fig. S7H), but only the microniche group had significantly improved glucose tolerance (Fig. 8M and fig. S7G). Insulin sensitivity was improved in both the MSC-Ex-4 and the microniche groups after 12 weeks of transplantation (Fig. 8N and fig. S7H). Together, these results substantiated that the combinatorial approach of microscaffold and MSC-Ex-4 could significantly augment the therapeutic effects of single-dose MSC-Ex-4 for 12 weeks, thus providing a promising strategy for ameliorating the metabolic syndromes in HFD mice.
DISCUSSION
In this study, we synergized the genetic engineering and biomaterial encapsulation to equip MSCs with constitutively secreted Exendin-4 and GM-based microniche, which resulted in improved cell functionality and antidiabetic activity. Major conclusions were summarized as follows. (i) The Exendin-4–engineered MSCs exhibited improved viability and increased resistance to high-glucose–induced senescence and oxidative stress–induced apoptosis compared to the wild-type MSCs. (ii) The continuously secreted Exendin-4 improved the MSC-Ex-4 function by activation of the GLP-1R–AMPK pathway in an autocrine manner. (iii) The secretory factors by MSC-Ex-4, including IGFBP2 and APOM, promoted pancreatic β cell regeneration, repair, and hepatic insulin sensitivity while inhibiting hepatic lipid accumulation in vitro and in vivo through activation of the PI3K-Akt signaling pathway. (iv) The microniche-based delivery strategy further augmented the therapeutic efficacy of single-dose MSC-Ex-4 therapy. Our data demonstrated the preclinical therapeutic potential of the Exednin-4–engineered MSCs assisted by microscaffold encapsulation in the prevention and treatment of metabolic dysfunction, including T2DM and MAFLD, which are major healthcare challenges in modern society.
Although Exendin-4 and GLP-1 only share 50% of sequence similarity, Exendin-4 is a full agonist of GLP-1R and exhibits higher binding affinity [dissociation constant (Kd) = 6 nM] than GLP-1 peptide (Kd value > 500 nm) by 400 times (26). Besides, Exendin-4 is much more potent than GLP-1 in differentiating pancreatic precursor cells into insulin- and glucagon-producing islet cells (41). Following activation of the GLP-1R, Exendin-4 initiates cyclic AMP (cAMP)–dependent second messenger pathways, including protein kinase A and exchange protein directly activated by cAMP 2 (Epac2), thereby promoting the insulin secretion by triggering Ca2+-induced exocytosis of insulin-containing vesicles from the pancreatic β cells (9). In addition, Exendin-4 increases the mass of β cell islets and elevates the insulin secretion by promoting the expression of IRS-2 and stimulates Akt phosphorylation (42). We showed that the constitutive GLP-1R activation by MSC-Ex-4–secreted Exendin-4 could delay cell senescence in both the β cells and the MSC-Ex-4, which is predominantly mediated by AMPK activation. Therefore, the Exendin-4-AMPK axis demonstrated in the MSC-Ex-4, which is not shown in the wild-type MSCs, may represent a previously unidentified target for MSCs protection.
MSCs are considered as one of the most promising cell types in cell therapies for various diseases including diabetes. Nevertheless, the therapeutic efficiency and safety concerns of MSCs remain major challenges. Our study authenticated that Exendin-4–engineered MSCs improved hyperglycemia, hyperlipidemia, and insulin resistance simultaneously, suggesting an innovative strategy in treating T2DM and the related complications. Other than the therapeutic effects for diabetes, Exendin-4 has been reported to exhibit cardioprotective, anti-inflammatory, nephroprotective, and neuroprotective effects (41). Therefore, it is reasonable to speculate that Exendin-4 genetically modified human stem or progenitor cells could surpass the wild-type counterparts, which offer a wide range of therapeutic strategies well beyond those described here. However, regardless the tail vein or subcutaneous injection, the survival time of MSCs in vivo did not exceed 1 month, while their therapeutic effect is maintained for a longer time (43). Besides the secreted Exendin-4, multiple secretomes produced by the MSC-Ex-4 may also contribute to the outstanding therapeutic benefits over the wild-type MSCs and other GLP-1 agonists. APOM, a carrier of sphingosine 1-phosphate, is an important TG metabolism–related protein that participates in the development of hepatic steatosis and insulin resistance. Although recent study indicated that APOM could exert a protective effect against lipid metabolic disorder and insulin resistance through the activation of AMPK and Akt pathways in HFD mice, it has not been reported that APOM can be secreted by Exendin-4–engineered MSCs (35, 44). Besides, albeit IGFBP2 was found to be secreted by MSCs (45), it still remains elusive whether IGFBP2 secretion is causally linked to the antidiabetic activity of MSCs.
To investigate the optimal cell delivery routes and dosages, we evaluated the pharmacokinetics and diverse effects of systemic and local administration, together with single- and multiple-dose administration of MSC-Ex-4 in vivo. We revealed that the MSC-Ex-4 could reach the liver after systemic administration, thus alleviating the diabetic and fatty liver simultaneously. However, systematically administered MSCs could not persist for longer time due to the lung barrier effects and instant blood-mediated inflammatory reaction, which limited the cell retention and homing and accelerated the immune clearance, thus hampered their life span and therapeutic capacity. Therefore, multiple administrations are needed to augment the pharmacokinetics of MSCs in vivo for extended therapeutic effects. In present study, the microscaffold-based delivery strategy was used to foster the therapeutic capacity of MSC-Ex-4, revealing an elongated survival time of MSC-Ex-4, followed by a higher efficiency in maintaining glucose tolerance and insulin sensitivity compared to the free MSC-Ex-4. Together, these findings provided a promising new avenue for the combination of Exendin-4–engineered MSCs and 3D microniches in ameliorating metabolic dysfunction including diabetes and MAFLD as well as other related complications (e.g., diabetic foot ulcers).
It should be noted that this study has a number of limitations to be overcome in the future. We and others have used C57BI/6J mice as the recipients for human MSC transplantation (46), which might limit the therapeutic benefits of MSCs due to the immune rejection of the host. T2DM is a chronic inflammatory process (47) with compromised immune response to foreign substances (48), which prolonged the survival time for human MSCs in HFD mice (fig. S3). Therefore, application of allogenic or autologous MSCs is expected to further improve the therapeutic effects. Besides the primary MSCs, MSCs derived from human pluripotent stem cells, including embryonic stem cells and induced pluripotent stem cells (iPSCs), have been shown as alternative and unlimited sources, which should be further examined for T2DM therapy (49). Moreover, the lentiviral transduction system used in the current study might raise safety concerns such as the immunogenic toxicity of the viral vectors, insertional oncogenesis, and the mutational integration into the host genome (50). More precise gene modification tools such as CRISPR technology should be used to enhance the specificity, efficacy, and safety of gene editing in future MSC therapy of T2DM (51).
MATERIALS AND METHODS
Study design
This study was designed to investigate the enhanced therapeutic effects and mechanisms of MSC-Ex-4 and MSC-Ex-4–loaded 3D GMs for T2DM treatment in mouse models. To this end, MSCs were engineered with Exendin-4 through the lentiviral transduction system, and the production of Exendin-4 was confirmed. In vitro experiments, including cell senescence, apoptosis, and proliferation assays, were performed to study the therapeutic effects and mechanisms of action of MSC-Ex-4 on themselves, pancreatic β cells (Min6), and hepatocytes (HepG2). GLP-1R was knockdown by using two shRNA to confirm the hypothesis that secreted Exendin-4 from the MSC-Ex-4 activated their own GLP-1R via autocrine signaling. Besides, we conducted RNA-seq and secretomes analysis by proteomics to investigate the paracrine and endocrine effects of the MSC-Ex-4 in liver and pancreas to ameliorate insulin resistance and lipid accumulation. For all in vitro studies, three independent experiments with at least three samples per condition were performed. To assess the therapeutic effects of MSC-Ex-4 in vivo, a T2DM mouse model was obtained by feeding the 4-week-old male C57Bl/6J mice with HFD for 16 weeks. The MSC-Ex-4 was encapsulated in the 3D GM to prolong cell viability and therapeutic effects in vivo. In vivo and ex vivo experiments, including glucose tolerance test (GTT), insulin tolerance test (ITT), bioluminescence tracking, and H&E staining, were conducted to examine the performance of MSC-Ex-4 and their mechanisms of actions in ameliorating metabolic disorder. In all experiments, mice were assigned to the individual groups of five to eight randomly, and no animals or samples were excluded from the study. All mice were euthanized after termination of the experiments. In vitro experiments were not blinded, while in vivo and ex vivo experiments were blinded. One-way analysis of variance (ANOVA) was used when comparing three or more groups of data, and Student’s t test was used when determining two groups of data. All quantification data are presented as mean values ± SEM.
Plasmids and recombinant cell construction
The Gila monster Exendin-4 complementary DNA (cDNA) sequence (EU790959) was obtained from the National Center for Biotechnology Information browser. Exendin-4 coding sequence:
5′-atgcatggtgaaggaacatttaccagtgacttgtcaaaacagatggaagaggaggcagtgcggttatttattgagtggcttaagaacggaggaccaagtagcggggcacctccgccatcg-3′.
The Gila monster Exendin-4 cDNA coding sequence was cloned into lentivirus mammalian expression vector pLV-CMV-puro empty plasmids. For lentiviral production, a lentiviral expression vector was cotransfected with second-generation lentivirus packing vectors pVSVG and pΔ8.9 into 293FT cells using Neofect (Neofect biotech). Seventy-two hours after transfection, supernatant was collected and centrifuged with 19,500 rpm for 2.5 hours. Pellets were resuspended by Opti-MEM medium to a total volume of 200 μl. The suspension was added to the six-well plate seeded with MSCs, along with polyberene (8 μg/ml) to assist the viral infection. After 100% confluence was reached, the cell passaging was conducted. Twenty-four hours after infection, puromycin was added to a final concentration of 2.5 μg/ml for selection.
The template of pcDNA3-Venus-Akaluc was obtained from Atsushi Miyawaki. The template of pCMV-Gluc was a gift from W. Tan in Chinese Center for Disease Control and Prevention. shRNA1 (TRCN0000004705) and shRNA2 (TRCN0000004706) of GLP-1R were obtained from MISSION shRNA Library. The process of recombinant cell construction was the same as previously described.
CM preparation
MSCs or MSC-Ex-4 were seeded into T75 culture plate to reach 80% confluence, when MSC medium (M001, viral therapy) was replaced with 10 ml of fetal bovine serum–free MSC medium (RP02010, Nuwacell). After 2 days of culture, medium was harvested and centrifuged at 4000g for 30 min by concentration with Millipore Amicon Ultra 3000 NMW. Then, the concentrated medium was diluted to original volume by fresh Dulbecco’s modified Eagle’s medium (DMEM) or MSC medium for Min6 or MSC pretreatment, respectively.
Induction of apoptosis
MSCs and Min6 were cultured on 12-well plates with a total number of 1 × 104 and 1 × 105 cells per well, respectively. Cells were pretreated with 100 nM Exendin-4 peptide and MSC-Ex-4 CM. After overnight culture, MSCs were treated with 25 mM glucose for 5 days. Next, MSCs were treated with 0.125 mM H2O2 and Min6 were treated with 0.4 mM H2O2. Cells were costained with annexin V–fluorescein isothiocyanate and propidium iodide (PI) (C1062L, Beyotime) for 30 min, and cell apoptosis rates were measured using a BD LSRFortessa SORP flow cytometer (BD Biosciences).
Induction of senescence
MSCs were cultured on 12-well plates with a total number of 1 × 104 cells per well. Cells were pretreated with 100 nM Exendin-4 peptide and MSC-Ex-4 CM. After overnight culture, MSCs were treated with 25 mM glucose for 5 days. In situ staining of senescence-associated β-galactosidase (SA-β-gal) with cells was performed using a senescence β-galactosidase staining kit (C0602, Beyotime) following the manufacturer’s instructions. Cells were considered positive when the cytoplasm was stained with SA-β-gal.
Min6 were cultured on 12-well plates with a total number of 1 × 105 cells per well. Cells were pretreated with 100 nM Exendin-4 peptide and MSC-Ex-4 CM. After overnight culture, Min6 were treated with 200 μM H2O2 for 24-hour culture and replaced with fresh DMEM. After 3 days further culture, in situ staining of SA-β-gal with cells was performed using a senescence β-galactosidase staining kit according to the manufacturer’s instructions.
EdU cell proliferation
MSCs and Min6 were cultured on 12-well plates with a total number of 1 × 104 and 1 × 105 cells per well respectively. Cells were pretreated with Exendin-4 peptides or CM as same as the senescence assay. Cells were stained with BeyoClick EdU-488 (C0071S, Beyotime) for 2 hours according to the manufacturer’s instruction, and fluorescence was measured at 488 and 346 nm using fluorescent microscopy.
Cell cycles
MSCs and Min6 were cultured into 12-well plates with a total number of 1 × 104 and 1 × 105 cells per well, respectively. Cells were pretreated with Exendin-4 peptides or CM as same as the senescence assay. Cells were fixed by 70% ethyl alcohol and further stained with PI (50 μg/ml; diluted in 1% TritonX-100) for 30 min. Cell cycles were measured using BD LSRFortessa SORP (BD Biosciences), and data were analyzed by FlowJo (BD Biosciences).
Coculturing of cells
HepG2 were cultured into 6.5-mm Transwell (3422, Corning) with a total number of 1 × 105 cells and 1 × 104 MSCs or MSC-Ex-4 were seeded into 8.0-μm pore polycarbonate membrane insert. After overnight culture, Exendin-4 were added into the corresponding wells to reach 100 nM. After 24 hours of coculture, palmitic acid (PA) was added into cocultured system, and HepG2 were harvested after another 24 hours for Oil Red O staining, quantitative polymerase chain reaction (qPCR), or Western blots.
Oil Red O staining
Preparation of PA was as previously described (52). Briefly, PA powder was added to a 10% solution of fatty acid–free bovine serum albumin (BSA) and dissolved by shaking gently overnight at 37°C to yield an 8 mM solution of PA complexed to BSA. The final molar ratio of free fatty acid to BSA was 5.2:1. HepG2 cells were exposed to 0.1 mM PA or BSA as a control for 24 hours. Cells were stained with an Oil Red O Staining kit (DL0011, Leagene) to examine the amount of fat accumulation in the cells.
Western blots
Briefly, cells or mouse tissues were homogenized in radioimmunoprecipitation assay buffer (PS0012, Leagene). Protein concentrations were determined using the BCA Protein Assay Kit (P0010S, Beyotime). Samples were loaded on 10% SDS–polyacrylamide gel electrophoresis gels and then transferred to nitrocellulose membranes. Immunoblotting was done in primary antibody dilution buffer (A1810, Solarbio) with the corresponding antibodies. The antibodies were purchased as follows: anti–GLP-1R (PA5-97790, Invitrogen), anti–phospho-Akt (AP0304, ABclonal), anti-Akt (A5523, ABclonal), anti–phospho-AMPK (2535S, Cell Signaling Technology), anti-AMPK (5831S, Cell Signaling Technology), anti–phospho–IRS-1 (3203S, Cell Signaling Technology), anti–IRS-1 (3407S, Cell Signaling Technology), and anti–β-actin (4970S, Cell Signaling Technology).
Gene expression analysis
Cells, or tissues (e.g., livers), were homogenized in TRIzol reagent (R401-01, Vazyme) before RNA extraction. cDNA was synthesized from 1 μg of DNA-free total RNA using reverse transcriptase (R222-01, Vazyme). Gene-specific transcription was analyzed in triplicate by qPCR using AceQ qPCR SYBR Green Master Mix (Q121-02, Vazyme) on a CFX96 instrument (Bio-Rad). All genes were normalized to glyceraldehyde-3-phosphate dehydrogenase. Relative expression levels were calculated using the 2−ΔΔCT method. Primers are listed as in Table 1.
Table 1. List of primer sequences used for qPCR analysis in this study.
| Name | Forward | Reverse |
| Exendin-4 | CGCTCTAGAATGCATGGTGAAGGAACATTTAC | CGCGGATCCTTACGATGGCGGAGGTGCCCCGC |
| PCK1 | GGATGTGGCCAGGATCGAAA | ACGTACATGGTGCGACCTT |
| G6PC | GACTGGCTCAACCTCGTCTT | TAGTATACACCTGCTGTGCC |
| GLUT4 | TCTCCAACTGGACGAGCAAC | CAGCAGGAGGACCGCAAATA |
| PPAR | GACCTCAGACAGATTGTCAC | AGTCCTTGTAGATCTCCTGC |
| FASN | AATGCCTTGTTCCCACCTGT | GCTGTGGTCCCACTTGATGA |
| COL1A1 | TTTGGATGGTGCCAAGGGAG | CACCATCATTTCCACGAGCA |
| VEGF | CTTGCCTTGCTGCTCTACCT | GCAGTAGCTGCGCTGATAGA |
| EGF | GCAGATGGGTCAATGCAACC | AGCTTCGCTCCATTACCTGG |
| HGF | GACGCAGCTACAAGGGAACA | CCCGATAGCTCGAAGGCAAA |
| NANOG | TGAACCTCAGCTACAAACAG | CTGGATGTTCTGGGTCTGGT |
| SOX2 | ACACCAATCCCATCCACACT | GCAAACTTCCTGCAAAGCTC |
| OCT4 | AGCGAACCAGTATCGAGAAC | GCCTCAAAATCCTCTCGTTG |
| GLP-1R | ACCAGTGGGATGGGGATGGGCTCCTC | CAGCAGCCCTCTGCCTCATA |
| IL6 | ACTCACCTCTTCAGAACGAATTG | CCATCTTTGGAAGGTTCAGGTTG |
| IL8 | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| P53 | CCTCAGCATCTTATCCGAGTGG | TGGATGGTGGTACAGTCAGAGC |
| TF/CD142/F3 | ACTCCCCAGAGTTCACACCT | TCCCGGAGGCTTAGGAAAGT |
| β-Actin | TCATGAAGATCCTCACCGAG | CATCTCTTGCTCGAAGTCCA |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
Animal model
All animal experiments were kept to a strict protocol approved by the Animal Ethics Committee of the center of Biomedical Analysis (Institutional Animal Care and Use Committee), Tsinghua University, which is accredited by Association for Assessment and Accreditation of Laboratory Animal Care International. Male C57Bl/6J mice, 4 weeks of age, were obtained from the Tsinghua University Laboratory Animal Research Center. For HFD feeding experiments, regular diet was replaced with a diet containing 60 kcal% fat (H10060, Huafukang) for 16-week induction.
GTT and ITT
Blood glucose values were determined using a Sinocare automatic glucometer. GTTs were performed by glucose intraperitoneal administration (1 g/kg) after overnight fasting. ITTs were performed by intraperitoneal injection of human regular insulin (Novolin, Novo Nordisk) (1 U/kg) after 6-hour fasting. Blood glucose collected from tail vain was measured on 15, 30, 60, and 90 min.
Insulin, TG, and biochemistry index
After 3 weeks of treatment, mice were euthanized to analyze the therapeutic efficacy. Blood samples were obtained from the tail vein and centrifuged at 2000g and 4°C for 30 min. Insulin and TG were measured using an insulin enzyme-linked immunosorbent assay (ELISA) kit (10-1247-01, Mercodia) and TG reagent (T2449, Sigma-Aldrich), which were calculated according to the manufacturer’s instructions. The number of red blood cells, white blood cells, and platelets (PLT) and the concentration of hemoglobin, hematocrit, mean corpuscular, mean corpuscular hemoglobin, PLT count, and plateletcrit were measured using a fully automatic hematology analyzer (Sysmex). Plasma alanine amino transferase, aspartate aminotransferase, albumin, creatine kinase isoenzymes (CK-MB), lactate dehydrogenase (LDH), blood urea nitrogen, and creatinine levels were measured using automatic biochemistry analyzer (Hitachi).
Exendin-4 concentration measurement
Supernatant of MSC-Ex-4 was collected on 12, 24, 48, 60, and 72 hours. Blood samples collected on different time points were obtained from the tail vein and centrifuged at 700g and 4°C for 20 min to collect the serum. The concentration of Exendin-4 in the collected supernatants or serum was then measured using an exenatide ELISA kit (EK-070-94, Phoenix Pharmaceuticals) and calculated on the basis of standard curves for Exendin-4.
Bioluminescence monitoring of MSC-Akaluc and MSC-Gluc
MSCs used for tail vein injection were engineered to stably express luciferase by lentiviral mediated gene transfer (MSC-Akaluc and MSC-Gluc). To visualize MSC-Akaluc, mice were intraperitoneally injected with 100 μl of TokeOni (15 mg/ml; 808350, Sigma-Aldrich) 5 min before imaging. HFD mice and normal mice were intravenously injected with 1 × 106 MSC-Akaluc suspended in 100 μl of PBS. Mice were imaged using an IVIS Lumina II imaging system (Caliper Life Sciences) to observe the distribution of MSCs in vivo. To establish standard curves for correlation of cell number and luminescence, mice were injected with 102,103, 104, 105, and 106 MSC-Gluc via tail vein. Serum (100 μl) was collected after 24 hours for bioluminescence measurement upon addition of 1 μl of 40 μM coelenterazine (S7777, Selleck) as substrate.
Exosomes isolation and characterization
The CM was collected and centrifuged at 1000g for 10 min to remove cell debris, followed by centrifugation at 2000g for 10 min and 10,000g for 30 min. The supernatant was then ultracentrifuged at 100,000g for 70 min (L-80XP; Beckman Coulter). The exosome-enriched fraction was resuspended with PBS, ultracentrifuged at 100,000g for 70 min (Max-XP; Beckman Coulter), and diluted with 100 μl of PBS. The morphology and size distribution of exosomes were identified by using transmission electron microscopy (H-7650B, Hitachi) and nanosight tracking analysis (NanoSight LM14, Malvern).
Scanning electron microcopy characterization and cell loading within the GMs
The GMs (3D FloTrix, cytoniche) were fabricated as described previously (17, 53). GMs were gold-coated for 90 s before scanning electron microscopy (FEI Quanta 200) imaging for the microstructure evaluation. The pore diameter distribution was evaluated from scanning electron microscope images of seven different regions using ImageJ software (National Institutes of Health). A 200 μl of MSC suspension was subsequently pipetted onto 20 mg of tightly packed GMs, which were automatically absorbed to hydrate the porous structures and then maintained in CO2 incubator at 37°C for 2 hours to allow for cell attachment. After that, 8 ml of culture medium was supplemented for long-term culture. Cells (1 × 106) were collected for subcutaneous injection.
H&E staining
After 3-week treatment, mice were euthanized to analyze the therapeutic efficacy. Livers were isolated, fixed in 4% paraformaldehyde (PFA), and embedded in paraffin for standard H&E staining. For tissue histology, mouse tissues were fixed in 4% PFA and embedded in paraffin. Sections (5 μm) were used for H&E staining.
Immunofluorescence
Mouse tissues were isolated and embedded in optimal cutting temperature compound (Leica) for immunohistochemistry (frozen). The antibodies were purchased as follows: anti-insulin (A2090, ABclonal).
RNA sequencing
RNA-seq analysis was conducted on cells or liver isolated from mice of different groups, using 1 μg of total RNA for each sample. RNA-seq was conducted by Biomarker Technology Corporation (Beijing, China). Differential expression genes were identified by DESeq2 package, and functional enrichment for GO and KEGG were performed with GOstats package.
Secretome analysis
Cells were seeded into T75 culture plate. When cells reach 80% confluence, cells were washed by PBS, and the medium was replaced by 8 ml of RPMI 1640 basic medium (350-046-CL, Wisent). After 24 hours of culture, medium was harvested, centrifuged, and filtered with 0.45-μm filters. The supernatants were then mixed with cold acetone (12377, Tongguang Chem) in a ratio of 5:1 (v/v), followed by incubation at −20°C. After 4 hours of incubation, the samples were centrifuged at 7500 rpm for 20 min, and the supernatants were removed. The remaining protein pellets were collected and resuspended with 300 μl of 8 M urea buffer (BDH4602, VWR). Each 100 μg of proteins were reduced with dithiothreitol (D9760, Sigma-Aldrich), alkylated with iodoacetamide (I6125, Sigma-Aldrich), and digested with sequencing grade–modified trypsin (V5111, Promega). The resulted peptides solution was desalted with Sep-Pak C18 (Waters) and labeled with tandem mass tag reagent (Thermo Fisher Scientific). Then, the resulted peptides were divided into 12 fractions through first-dimension everse phase liquid chromatography separation.
Liquid chromatography–tandem mass spectrometry (LC-MS/MS)–based quantitative secretome analysis was performed using Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) and online-coupled nano–high-performance liquid chromatography system (Thermo Fisher Scientific). Peptides were separated by gradient elution in elution buffer at a flow rate of 0.25 μl/min for 2 hours, containing mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The mass spectrometer with Xcalibur software (version 3.0) was fixed to data-dependent acquisition mode, followed by the acquisition of full-scan mass spectrum. Data-dependent MS2 scans were performed for 20 times at higher-energy collisional-based fragmentation. Data of each LC-MS/MS run were examined through the SEQUEST search engine from Proteome Discoverer software (version 2.1, Thermo Fisher Scientific) against the UniProt database.
Statistical analysis
Statistical analysis was performed by GraphPad Prism. Significant differences between groups were checked using one-way ANOVA following Tukey’s multiple comparisons test or unpaired, two-tailed Student’s t test. One-way ANOVA was used when comparing three or more groups of data, and Student’s t test was used when determining two groups of data, as indicated in the figure legend. Unless indicated otherwise, all quantification data are presented as mean values ± SEM. Experiments have at least three independent replicates.
Acknowledgments
We would like to thank all members of Y.D.’s laboratory for great support. Funding: This work was financially supported by National Key R&D Program of China (2017YFA0104901), National Natural Science Foundation of China (82061148010), the Beijing Municipal Science and Technology Commission (Z181100001818005), and China Postdoctoral Science Foundation (2019 M650717). Author contributions: Y.Z. designed and performed experiments, analyzed and interpreted data, drafted the manuscript, and coordinated the project. S.G. performed experiments, analyzed and interpreted data, and drafted the manuscript. K.L. constructed the plasmids and recombinant cells, prepared the figures, and drafted the manuscript. Z.W. analyzed and interpreted the data of RNA-seq and secretome analysis. W.L. and X.Y. helped in the production of GMs and cell encapsulation. J.L. performed scanning electron microscopy characterization of microscaffolds. B.W. performed Western blotting of GLP-1R. Y.D. provided overall intellectual guidance, edited the manuscript, and is the principal investigator of the supporting grants. All authors read and approved the manuscript. Competing interests: Y.D. is scientific advisor, and X.Y and W.L. are employees of Beijing CytoNiche Biotechnology Co. Ltd. Y.D., Y.Z., and S.G. were listed as the inventors on a patent application for MSC-Ex-4 for T2DM therapy. The initial filing was assigned the Chinese patent application no. 201910836166.X. All other authors declare that they have no competing interests. Data and materials availability: All data pertaining to this study are present in the paper and/or in the Supplementary Materials. The pcDNA3-Venus-Akaluc can be provided by RIKEN BioResource Center pending scientific review and a completed material transfer agreement. Requests for the pcDNA3-Venus-Akaluc should be submitted to dnabank.brc@riken.jp. Requests for other information and reagents may be directed and will be fulfilled by the corresponding author, Y.D. (duyanan@tsinghua.edu.cn).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/27/eabi4379/DC1
REFERENCES AND NOTES
- 1."IDF diabetes atlas 9th edition", IDF Atlas 2019 International Diabetes Federation (2019).
- 2.Taylor R., Insulin resistance and type 2 diabetes. Diabetes 61, 778 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogihara T., Mirmira R. G., An islet in distress: β cell failure in type 2 diabetes. J. Diabetes Investig. 1, 123–133 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donath M. Y., Ehses J. A., Maedler K., Schumann D. M., Ellingsgaard H., Eppler E., Reinecke M., Mechanisms of β-cell death in type 2 diabetes. Diabetes 54 (Suppl 2), S108–S113 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Perry R. J., Samuel V. T., Petersen K. F., Shulman G. I., The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M. S., Goldstein J. L., Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 7, 95–96 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Eppens M. C., Craig M. E., Cusumano J., Hing S., Chan A. K. F., Howard N. J., Silink M., Donaghue K. C., Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 29, 1300–1306 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Drucker D. J., Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Doyle M. E., Egan J. M., Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol. Ther. 113, 546–593 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen A., Lund A., Knop F. K., Vilsbøll T., Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 14, 390–403 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Chaudhury A., Duvoor C., Reddy Dendi V. S., Kraleti S., Chada A., Ravilla R., Marco A., Shekhawat N. S., Montales M. T., Kuriakose K., Sasapu A., Beebe A., Patil N., Musham C. K., Lohani G. P., Mirza W., Clinical review of antidiabetic drugs: Implications for Type 2 diabetes mellitus management. Front. Endocrinol. 8, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks A., Futrega K., Liang X., Hu X., Liu X., Crawford D. H. G., Doran M. R., Roberts M. S., Wang H., Concise review: Quantitative detection and modeling the in vivo kinetics of therapeutic mesenchymal stem/stromal cells. Stem Cells Transl. Med. 7, 78–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bi S., Nie Q., Wang W. Q., Zhu Y. L., Ma X. M., Wang C. M., Zhang B. C., Li H. Y., Zhang Q., Chen G., Human umbilical cord mesenchymal stem cells therapy for insulin resistance: A novel strategy in clinical implication. Curr. Stem Cell Res. Ther. 13, 658–664 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Bhansali S., Dutta P., Kumar V., Yadav M. K., Jain A., Mudaliar S., Bhansali S., Sharma R. R., Jha V., Marwaha N., Khandelwal N., Srinivasan A., Sachdeva N., Hawkins M., Bhansali A., Efficacy of autologous bone marrow-derived mesenchymal stem cell and mononuclear cell transplantation in type 2 diabetes mellitus: A randomized, placebo-controlled comparative study. Stem Cells Dev. 26, 471–481 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Sun X., Hao H., Han Q., Song X., Liu J., Dong L., Han W., Mu Y., Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther 8, 241 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyun J. S., Tran M. C., Wong V. W., Chung M. T., Lo D. D., Montoro D. T., Wan D. C., Longaker M. T., Enhancing stem cell survival in vivo for tissue repair. Biotechnol. Adv. 31, 736–743 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Liu W., Liu F., Zeng Y., Zuo S., Feng S., Qi C., Wang B., Yan X., Khademhosseini A., Bai J., Du Y., Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc. Natl. Acad. Sci. 111, 13511–13516 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ocansey D. K. W., Pei B., Yan Y., Qian H., Zhang X., Xu W., Mao F., Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 18, 42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mastrolia I., Foppiani E. M., Murgia A., Candini O., Samarelli A. V., Grisendi G., Veronesi E., Horwitz E. M., Dominici M., Challenges in clinical development of mesenchymal stromal/stem cells: Concise review. Stem Cells Transl. Med. 8, 1135–1148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess D., Li L., Martin M., Sakano S., Hill D., Strutt B., Thyssen S., Gray D. A., Bhatia M., Bone marrow–derived stem cells initiate pancreatic regeneration. Nat. Biotechnol. 21, 763–770 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Si Y., Zhao Y., Hao H., Liu J., Guo Y., Mu Y., Shen J., Cheng Y., Fu X., Han W., Infusion of mesenchymal stem cells ameliorates hyperglycemia in type 2 diabetic rats: Identification of a novel role in improving insulin sensitivity. Diabetes 61, 1616–1625 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie M., Hao H. J., Cheng Y., Xie Z. Y., Yin Y. Q., Zhang Q., Gao J. Q., Liu H. Y., Mu Y. M., Han W. D., Adipose-derived mesenchymal stem cells ameliorate hyperglycemia through regulating hepatic glucose metabolism in type 2 diabetic rats. Biochem. Biophys. Res. Commun. 483, 435–441 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Singer N. G., Caplan A. I., Mesenchymal stem cells: Mechanisms of inflammation. Annu. Rev. Pathol. 6, 457–478 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Habib H. A., Heeba G. H., Khalifa M. M. A., Effect of combined therapy of mesenchymal stem cells with GLP-1 receptor agonist, exenatide, on early-onset nephropathy induced in diabetic rats. Eur. J. Pharmacol. 892, 173721 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Chang Y., Dong M., Wang Y., Yu H., Sun C., Jiang X., Chen W., Wang X., Xu N., Liu W., Jin N., GLP-1 gene-modified human umbilical cord mesenchymal stem cell line improves blood glucose level in type 2 diabetic mice. Stem Cells Int. 2019, 4961865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Runge S., Schimmer S., Oschmann J., Schiødt C. B., Knudsen S. M., Jeppesen C. B., Madsen K., Lau J., Thøgersen H., Rudolph R., Differential structural properties of GLP-1 and exendin-4 determine their relative affinity for the GLP-1 receptor N-terminal extracellular domain. Biochemistry 46, 5830–5840 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Zhang D., Lu H., Chen Z., Wang Y., Lin J., Xu S., Zhang C., Wang B., Yuan Z., Feng X., Jiang X., Pan J., High glucose induces the aging of mesenchymal stem cells via Akt/mTOR signaling. Mol. Med. Rep. 16, 1685–1690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natalicchio A., Biondi G., Marrano N., Labarbuta R., Tortosa F., Spagnuolo R., D’Oria R., Carchia E., Leonardini A., Cignarelli A., Perrini S., Laviola L., Giorgino F., Long-term exposure of pancreatic β-cells to palmitate results in SREBP-1C-dependent decreases in GLP-1 receptor signaling via CREB and AKT and insulin secretory response. Endocrinology 157, 2243–2258 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Zhou H., Li D., Shi C., Xin T., Yang J., Zhou Y., Hu S., Tian F., Wang J., Chen Y., Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci. Rep. 5, 12898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu J., Wei Q., Zheng H., Meng X., Zhang J., Wang D., Exendin-4 promotes survival of mouse pancreatic β-cell line in lipotoxic conditions, through the extracellular signal-related kinase 1/2 pathway. J. Diabetes Res. 2016, 5294025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kholodenko I. V., Yarygin K. N., Cellular mechanisms of liver regeneration and cell-based therapies of liver diseases. Biomed. Res. Int. 2017, 8910821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee C. W., Hsiao W. T., Lee O. K., Mesenchymal stromal cell-based therapies reduce obesity and metabolic syndromes induced by a high-fat diet. Transl. Res. 182, 61–74.e8 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Panjwani N., Mulvihill E. E., Longuet C., Yusta B., Campbell J. E., Brown T. J., Streutker C., Holland D., Cao X., Baggio L. L., Drucker D. J., GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− Mice. Endocrinology 154, 127–139 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran P., Dobie R., Wilson-Kanamori J. R., Dora E. F., Henderson B. E. P., Luu N. T., Portman J. R., Matchett K. P., Brice M., Marwick J. A., Taylor R. S., Efremova M., Vento-Tormo R., Carragher N. O., Kendall T. J., Fallowfield J. A., Harrison E. M., Mole D. J., Wigmore S. J., Newsome P. N., Weston C. J., Iredale J. P., Tacke F., Pollard J. W., Ponting C. P., Marioni J. C., Teichmann S. A., Henderson N. C., Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X., Zhang P., Gao J., Huang Q., Autophagy dysregulation caused by ApoM deficiency plays an important role in liver lipid metabolic disorder. Biochem. Biophys. Res. Commun. 495, 2643–2648 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm F., Kässner F., Schmid G., Kratzsch J., Laner A., Wabitsch M., Körner A., Kiess W., Garten A., Phosphatidylinositol 3-kinase (PI3K) signalling regulates insulin-like-growth factor binding protein-2 (IGFBP-2) production in human adipocytes. Growth Hormon. IGF Res. 25, 115–120 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Iwano S., Sugiyama M., Hama H., Watakabe A., Hasegawa N., Kuchimaru T., Tanaka K. Z., Takahashi M., Ishida Y., Hata J., Shimozono S., Namiki K., Fukano T., Kiyama M., Okano H., Kizaka-Kondoh S., McHugh T. J., Yamamori T., Hioki H., Maki S., Miyawaki A., Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 359, 935–939 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Wurdinger T., Badr C., Pike L., de Kleine R., Weissleder R., Breakefield X. O., Tannous B. A., A secreted luciferase for ex vivo monitoring of in vivo processes. Nat. Methods 5, 171–173 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qi C., Li Y., Badger P., Yu H., You Z., Yan X., Liu W., Shi Y., Xia T., Dong J., Huang C., Du Y., Pathology-targeted cell delivery via injectable micro-scaffold capsule mediated by endogenous TGase. Biomaterials 126, 1–9 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Zeng Y., Chen C., Liu W., Fu Q., Han Z., Li Y., Feng S., Li X., Qi C., Wu J., Wang D., Corbett C., Chan B. P., Ruan D., Du Y., Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 59, 53–65 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Yap M. K. K., Misuan N., Exendin-4 from heloderma suspectum venom: From discovery to its latest application as type II diabetes combatant. Basic Clin. Pharmacol. Toxicol. 124, 513–527 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Tourrel C., Bailbe D., Lacorne M., Meile M.-J., Kergoat M., Portha B., Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the β-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes 51, 1443 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Parekkadan B., Milwid J. M., Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurano M., Tsukamoto K., Shimizu T., Kassai H., Nakao K., Aiba A., Hara M., Yatomi Y., Protection against insulin resistance by apolipoprotein m/sphingosine-1-phosphate. Diabetes 69, 867–881 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Meier R. P., Mahou R., Morel P., Meyer J., Montanari E., Muller Y. D., Christofilopoulos P., Wandrey C., Gonelle-Gispert C., Bühler L. H., Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 62, 634–641 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Du L., Lin L., Li Q., Liu K., Huang Y., Wang X., Cao K., Chen X., Cao W., Li F., Shao C., Wang Y., Shi Y., IGF-2 preprograms maturing macrophages to acquire oxidative phosphorylation-dependent anti-inflammatory properties. Cell Metab. 29, 1363–1375.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Berbudi A., Rahmadika N., Tjahjadi A. I., Ruslami R., Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 16, 442–449 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Socarrás T. O., Vasconcelos A. C., Campos P. P., Pereira N. B., Souza J. P. C., Andrade S. P., Foreign body response to subcutaneous implants in diabetic rats. PLOS ONE 9, e110945 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang B., Yan L., Wang X., Li E., Murphy K., Vaccaro K., Li Y., Xu R.-H., Concise review: Mesenchymal stem cells derived from human pluripotent cells, an unlimited and quality-controllable source for therapeutic applications. Stem Cells 37, 572–581 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Thomas C. E., Ehrhardt A., Kay M. A., Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 4, 346–358 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Sharma G., Sharma A. R., Bhattacharya M., Lee S.-S., Chakraborty C., CRISPR-Cas9: A preclinical and clinical perspective for the treatment of human diseases. Mol. Ther. 29, 571–586 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi-Barve S., Barve S. S., Amancherla K., Gobejishvili L., Hill D., Cave M., Hote P., McClain C. J., Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 46, 823–830 (2007). [DOI] [PubMed] [Google Scholar]
- 53.Yan X., Zhang K., Yang Y., Deng D., Lyu C., Xu H., Liu W., Du Y., Dispersible and dissolvable porous microcarrier tablets enable efficient large-scale human mesenchymal stem cell expansion. Tissue Eng. Part C Methods 26, 263–275 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/27/eabi4379/DC1