Abstract
Nuclear receptors are ligand-activated transcription factors and include the receptors for steroid hormones, lipophilic vitamins, sterols, and bile acids. These receptors serve as targets for development of myriad drugs that target a range of disorders. Classically defined ligands that bind to the ligand-binding domain of nuclear receptors, whether they are endogenous or synthetic, either activate receptor activity (agonists) or block activation (antagonists) and due to the ability to alter activity of the receptors are often termed receptor “modulators.” The complex pharmacology of nuclear receptors has provided a class of ligands distinct from these simple modulators where ligands display agonist/partial agonist/antagonist function in a tissue or gene selective manner. This class of ligands is defined as selective modulators. Here, we review the development and pharmacology of a range of selective nuclear receptor modulators.
I. Introduction
The nuclear receptor (NR) superfamily constitutes a group of 48 transcription factors in humans, which includes the receptors for steroid hormones, thyroid hormone, lipophilic vitamins, and cholesterol metabolites (Evans, 1988; Mangelsdorf et al., 1995) (Table 1). Approximately half of NRs are classified as orphan receptors because they do not have well characterized ligands (O’Malley and Conneely, 1992; Mangelsdorf and Evans, 1995; Giguere, 1999; Kliewer et al., 1999). The NRs regulate a wide range of physiologic and developmental processes, and virtually all the NRs that have identified ligands are well-characterized targets for the development of drugs to treat myriad diseases including cancer, diabetes, atherosclerosis, inflammation, and endocrine/reproductive disorders.
TABLE 1.
Human Nuclear Receptor Superfamily
| Common Symbol | Endogenous Ligand | |
|---|---|---|
| Subfamily 1 | ||
| NR1A1 | TRα | Thyroxine |
| NR1A2 | TRβ | Thyroxine |
| NR1B1 | RARα | Retinoic acid |
| NR1B2 | RARβ | Retinoic acid |
| NR1B3 | RARγ | Retinoic acid |
| NR1C1 | PPARα | Fatty acids |
| NR1C2 | PPARδ | Fatty acids |
| NR1C3 | PPARγ | Fatty acids |
| NR1D1 | REV-ERBα | Heme |
| NR1D2 | REV-ERBβ | Heme |
| NR1F1 | RORα | Oxysterols |
| NR1F2 | RORβ | Oxysterols |
| NR1F3 | RORγ | Oxysterols |
| NR1H2 | LXRβ | Oxysterols |
| NR1H3 | LXRα | Oxysterols |
| NR1H4 | FXR | Bile acids |
| NR1I1 | VDR | Vitamin D |
| NR1I2 | PXR | Xenobiotics |
| NR1I3 | CAR | Xenobiotics |
| Subfamily 2 | ||
| NR2A1 | HNF4α | |
| NR2A2 | HNF4γ | |
| NR2B1 | RXRα | 9-Cis-retinoic acid |
| NR2B2 | RXRβ | 9-Cis-retinoic acid |
| NR2B3 | RXRγ | |
| NR2C1 | TR2 | |
| NR2C2 | TR4 | |
| NR2E1 | TLX | |
| NR2E3 | COUPTF1 | |
| NR2F1 | COUPTF2 | |
| NR2F6 | EAR2 | |
| Subfamily 3 | ||
| NR3A1 | ERα | Estradiol |
| NR3A2 | ERβ | Estradiol |
| NR3B1 | ERRα | |
| NR3B2 | ERRβ | |
| NR3B3 | ERRγ | |
| NR3C1 | GR | Glucocorticoids |
| NR3C2 | MR | Mineralocorticoids |
| NR3C3 | PR | Progestins |
| NR3C4 | AR | Androgens |
| Subfamily 4 | ||
| NR4A1 | NGFIB | |
| NR4A2 | NURR1 | |
| NR4A3 | NOR1 | |
| Subfamily 5 | ||
| NR5A1 | SF-1 | |
| NR5A2 | LRH-1 | |
| Subfamily 6 | ||
| NR6A1 | GCNF | |
| Subfamily 0 | ||
| NR0B1 | DAX1 | |
| NR0B2 | SHP |
REV-ERB, reverse strand ERBA oncogene; FXR, farnesoid X receptor; CAR, constitutive androstane receptor; HNF, hepatocyte nuclear factor; TLX, Tailless gene homolog; COUPTF, chicken ovalbumin upstream promoter transcription factor; EAR, erbA-related gene; ERR, estrogen receptor-related orphan receptor; NGFIB, nerve growth factor-induced gene B; NURR, nuclear receptor related 1; NOR, neuron-derived orphan receptor; SF1, steroidogenic factor 1; GCNF, germ cell nuclear factor; DAX1, dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1; SHP, short heterodimer partner.
A. Discovery of Nuclear Receptors
Biochemical evidence for the existence of cellular receptors for steroid hormones was first demonstrated by the use of radiolabeled estrogens and examination of specific binding within estrogen-responsive tissues (Glascock and Hoekstra, 1959; Jensen and Jacobson, 1960, 1962). Autoradiographic analysis suggested that these estrogen receptors (ERs) were nuclear in localization rather than associated with the plasma membrane as were the other receptors that were being characterized at the time (Jensen et al., 1967). Localization of ER within the nucleus and experiments that suggested that the receptor was associated with chromatin (King et al., 1966; Maurer and Chalkley, 1967; Teng and Hamilton, 1968; Shyamala and Gorski, 1969; Mainwaring, 1971; Mainwaring and Peterken, 1971; Spelsberg et al., 1971; Steggles et al., 1971) were consistent with the early suggestions that estrogen action might be associated with regulation of RNA synthesis (Mueller et al., 1958). Studies in the early to mid-1960s suggested that ER was a protein, and in 1966, Toft and Gorski were the first to biochemically isolate a steroid hormone receptor (Toft and Gorski, 1966). Characterization of purified ER as well as other steroid receptors over the next two decades led to the following key observations: 1) steroid receptors stimulate mRNA synthesis of specific genes by stimulating RNA polymerase activity (Gorski, 1964; Greenman et al., 1965; Means and Hamilton, 1966; O’Malley et al., 1968a,b, 1972a,b; O’Malley and McGuire, 1968; Comstock et al., 1972; Glasser et al., 1972; Means et al., 1972; Mohla et al., 1972; Rosenfeld et al., 1972; Chan et al., 1973; Parks et al., 1974; Ringold et al., 1975, 1977; Scolnick et al., 1976; Young et al., 1977); 2) steroid receptors bind to double-stranded DNA via specific base sequences (hormone response elements) (King and Gordon, 1972; Musliner and Chader, 1972; Toft, 1972; Andre and Rochefort, 1973; Beato et al., 1973; Gehring and Tomkins, 1974; Yamamoto and Alberts, 1974, 1975; Yamamoto et al., 1974); and 3) hormone response elements are located in the promoters of steroid-responsive target genes.
Characterization of purified steroid receptor proteins by limited proteolysis clearly indicated that the receptors were composed of several domains that retained specific functions (e.g., DNA binding or steroid binding) when examined in isolation (Wrange and Gustafsson, 1978; Carlstedt-Duke et al., 1982). Molecular cloning of the glucocorticoid receptor (GR) in 1985 provided the first glimpse into the genetic organization of a nuclear receptor (Hollenberg et al., 1985; Weinberger et al., 1985a). On the basis of the predicted amino acid sequence and domain structure of the receptor, the location of the DNA-binding domain (DBD) and steroid-binding domain (ligand-binding domain; LBD) was correctly predicted (Weinberger et al., 1985b). The cDNAs for all of the steroid receptors were rapidly identified, including the estrogen receptor (ER) (Walter et al., 1985; Green et al., 1986; Greene et al., 1986; Krust et al., 1986; Kumar et al., 1986), the progesterone receptor (PR) (Conneely et al., 1986, 1987a; Jeltsch et al., 1986; Loosfelt et al., 1986; Gronemeyer et al., 1987; Misrahi et al., 1987), the mineralocorticoid receptor (MR) (Arriza et al., 1987; Patel et al., 1989), and the androgen receptor (AR) (Chang et al., 1988a,b; Lubahn et al., 1988; Trapman et al., 1988; Tilley et al., 1989). Comparison of the sequences of all of these receptors demonstrated a highly conserved structure that was defined as six subregions (regions A through F; Fig. 1) based on degree of homology (Krust et al., 1986). The amino-terminal region, considered the A/B region, was the most divergent among the receptors and was shown to contain a hormone-independent transactivation function in many receptors (activation function 1; AF-1). Region C, the most conserved domain, is rich in cysteines and basic amino acids, with the position of the cysteines absolutely conserved among the receptors consistent with their function in coordination of Zn2+ ions within the two zinc-finger structures located within this DNA binding domain. The D region, also termed the “hinge domain” due to its localization between the DBD and the LBD, is a relatively short region with a low degree of conservation. The hinge domain plays a role in modulation of DNA binding for some receptors. The E region encompasses the LBD and contains the ligand-dependent transactivation domain (activation function 2; AF-2). A region further toward the carboxy-terminal to the E region, referred to as the F domain, was noted in only some receptors; to date, it still has an unclear role in receptor function.
Fig. 1.

Nuclear receptor domain structure and mechanism of action. (A) Nuclear receptors display a conserved modulator domain architecture with an N-terminal AF-1 region (A/B region), followed by zinc-finger DBD (C region), a hinge domain (D region), an LBD containing the AF-2 region (E region), and some receptors have a C-terminal F domain. (B) Mechanistically, nuclear receptors are regulated by small molecule ligands, which generally stabilize the receptor into a conformation suitable to bind coregulator proteins (coactivators or corepressors). Ligands can also modulate posttranslational modification of the receptor. Ultimately, these events have an impact on the expression of receptor-specific target genes by modulating coregulator recruitment at specific DNA-response element sites in the target gene promoter. (C) Schematic illustrating the principle of selective receptor modulation.
Significant homology between the steroid receptors and the v-erbA oncogene led to the prediction that the c-erbA gene may encode a receptor for a steroid or steroid-like molecule (Weinberger et al., 1985a; Green et al., 1986). Functional analysis of the protein encoded by the c-erbA gene verified this prediction when it was shown to encode a receptor for thyroid hormone (TH) (Sap et al., 1986; Weinberger et al., 1986b). Receptors for a range of lipophilic vitamins were soon identified as belonging to the same class of molecules, including vitamin A (retinoic acid) (Giguere et al., 1987; Petkovich et al., 1987) and vitamin D (McDonnell et al., 1987; Baker et al., 1988). A number of additional “receptors” also were identified based on sequence homology to the steroid receptors but with no known ligands. These include v-erbA-related receptor and estrogen-related receptor, which were identified based on low-stringency cross-hybridization strategies (Giguere et al., 1988; Miyajima et al., 1988). More orphan receptors would be identified with this method; however, in addition, a number would be identified with other methods including biochemical methods (e.g., chicken ovalbumin upstream promoter transcription factor) (Wang et al., 1989), positional cloning (e.g., DAX-1) (Zanaria et al., 1994; Burris et al., 1996), or interaction cloning [e.g., receptor-interacting protein (RIP) 14, RIP15 and SHP] (Seol et al., 1995, 1996). Since their initial characterization as orphan nuclear receptors, endogenous ligands have been identified for many of these including bile acids for farnesoid X receptor (Makishima et al., 1999; Parks et al., 1999), oxysterols for liver X receptors (LXRs) (Janowski et al., 1996; Lehmann et al., 1997), 9-cis-retinoic acid for retinoic acid X receptor (RXR) (Heyman et al., 1992; Levin et al., 1992; Mangelsdorf et al., 1992), heme for REV-ERB (Raghuram et al., 2007; Yin et al., 2007), xenobiotics for pregnane X receptor (PXR) and constitutive active/androstane receptor (Willson and Kliewer, 2002), and fatty acids for peroxisome proliferator-activated receptors (PPARs) (Keller et al., 1993; Yu et al., 1995).
B. Ligand Regulation of Nuclear Receptor Function
As indicated earlier, NRs typically function as ligand-dependent transcription factors. A number of orphan NRs still have no characterized endogenous or synthetic ligands; however, this review focuses on the action of ligands on NRs, so only those receptors that have characterized ligands will be discussed. NRs recognize specific DNA-response elements in the promoters/enhancers of their cognate target genes where they can respond to ligands by altering their ability to recruit a range of other transcriptional proteins that alter the rate of gene expression (Fig. 1). Of course, the various NRs have distinct preferences for both ligands and DNA sequences that they recognize as response elements within the genome, providing for the distinct functions of the 48 members of this class of transcription factors. Most of the NRs function as dimers, either homodimers (as is the case for the steroid receptors) or heterodimers with a common NR, the RXR. A subset of NRs also functions as monomers and include many of the orphan members of the superfamily.
Some NRs, including several steroid hormone receptors, undergo a translocation from the cytoplasm to the nucleus that is ligand dependent, as discussed in the previous section. In this case, they are unable to exert an effect on the transcription of target genes in the absence of ligands. Most NRs appear to bind their response elements both in the absence and presence of ligand, and in many cases may take an active role in the regulation of target gene regulation even in the absence of ligand. One clear example of this is the thyroid hormone receptor (TR), which can actively silence target gene transcription in the absence of TH but transforms into a transcriptional activator in the presence of TH (Cheng et al., 2010). These types of functional transformations are due to induction of conformational changes that alter the array of protein cofactors to which the NR binds, as will be discussed in detail later.
Based on the observation of transcriptional interference or “squelching” of transactivation activity of PR by overexpression of ER, it was proposed that limiting the amount of accessory or coactivator proteins was required for NRs to regulate transcription (Meyer et al., 1989). It became clear that NRs compete for coactivator proteins within the cell, which mediates activation of transcription, and it also appeared that distinct transactivation domains may use distinct coactivator proteins (Tasset et al., 1990). Interestingly, transcriptional silencing mediated by TR or the retinoic acid receptor (RAR) could also be squelched, suggesting the existence of proteins that mediated suppression of target gene transcription (corepressors) (Baniahmad et al., 1995). By use of biochemical methods, putative intermediary proteins that interacted with the ER’s LBD were identified that were potential coactivators as they only interacted in the presence of an agonist, antagonists blocked the agonist-dependent interaction, and the proteins could not interact with transcriptionally defective ERs (Halachmi et al., 1994). Similar putative coactivators were identified as GR-interacting proteins that display ligand-dependent interaction (Eggert et al., 1995). The first coactivator, steroid receptor coactivator 1 (SRC1), was cloned using a two-hybrid system using the LBD of PR as bait and shown to function as a coactivator for a range of NRs (Onate et al., 1995). SRC1 displayed all of the expected characteristics of an authentic coactivator, including agonist-dependent interaction with the LBD, which could be blocked by an antagonist, and the ability to rescue squelched NRs (Onate et al., 1995). It rapidly became clear that SRC1 was only the first of a family of coactivators, which includes SRC2 and SRC3 (Hong et al., 1996, 1997; Voegel et al., 1996; Anzick et al., 1997; Chen et al., 1997; Li et al., 1997a; Takeshita et al., 1997; Torchia et al., 1997). Additionally, a range of other classes of coactivators were soon characterized, and the list of various proteins with transcriptional coactivator activity is now in the hundreds (Horwitz et al., 1996; Xu et al., 1999; McKenna and O’Malley, 2002, 2010; Lonard and O’Malley, 2006, 2007; Lonard et al., 2007). Corepressor proteins were also identified, such as nuclear receptor corepressor 1 (NCoR1) and silencing mediator for retinoid and thyroid hormone receptor (SMRT) (Chen and Evans, 1995; Horlein et al., 1995), but this class of proteins appears to be far fewer in number than coactivators. These coactivator/corepressor proteins display an array of activities associated with regulation of transcription, including histone acetyltransferase activity, histone deacetylase activity, arginine methyltransferase activity, ubiquitin ligase activity, and ATP-dependent chromatin-remodeling activity.
Structural studies of the LBD of NRs have provided significant information about how ligands modulate the structure of the receptor leading to recruitment of these cofactors. The LBDs of NRs display a very conserved tertiary structure, which is a globular domain composed almost exclusively of α-helices arranged in a three-layer “sandwich” (Wurtz et al., 1996). NR ligands bind to a ligand-binding pocket (LBP) within the interior of this globular domain, which is consistent with the typical hydrophobic character of NR ligands. There are 11 α-helices within the globular structure that vary in size between various NRs. Helix 12 (H12) forms a mobile “lid” over the entrance to the LBP and contains critical residues for the function of AF-2. Ligand-dependent positioning of H12 proves to be critical for formation of the coactivator-binding surface of the LBD that allows for ligand-dependent recruitment of coactivator proteins. In the absence of ligand, H12 is believed to be quite mobile, but ligand binding stabilizes the position of H12 against the globular domain so as to complete the formation of a hydrophobic groove on the surface of the LBD that recognizes a signature motif found on many coactivator proteins known as an NR box or LXXLL motif (L = leucine, X = any amino acid) (Feng et al., 1998; Heery et al., 1997; Savkur and Burris, 2004). The coactivator-binding surface of the LBD is composed of helices 3, 5, and 12 and serves as a docking site for the α-helical NR box (Fig. 2). The hydrophobic leucine side chains of the NR box become buried within the hydrophobic LBD groove while absolutely conserved glutamic acid (H12) and lysine (H3) residues form a charge clamp by forming hydrogen bonds with the peptide backbones of the leucines flanking the NR box (Nolte et al., 1998). Unexpectedly, corepressors were found to use a similar signature sequence for recognition of the LBD, a longer amphipathic α-helical sequence known as a CoRNR box (Fig. 3) (Hu and Lazar, 1999; Hu et al., 2001). As one would anticipate, H12 cannot be in the agonist position, as we indicated earlier, for effective CoRNR box binding to the LBD; in fact, agonist binding places H12 into a position that physically precludes binding of the CoRNR box motif. This was predicted before a corepressor was cloned or the structure of the LBD was determined, as deletion of the AF-2 region (later determined to be H12) of TR created a constitutive silencing receptor, which indicated that AF-2 (H12) was required for displacement of a putative corepressor protein (Baniahmad et al., 1995).
Fig. 2.
Coactivator binding surface in the LBD composed of a surface formed at the intersection of several structural elements in the LBD, including helices 3, 4, and 12. This surface is called the AF-2 site. Coactivator regions containing a canonical LXXLL motif bind to this surface, docking the three hydrophobic leucine side chains (show in yellow) into a hydrophobic groove. Two key residues in the LBD form a “charge clamp” that helps stabilize this interaction, including a Lysine residue on helix 3 and a Glutamic acid residue on helix 12, which make interactions with the backbone of the LXXLL motif peptide.
Fig. 3.

Corepressor binding surface in the LBD. This surface is similar to the coactivator-binding surface but uses a conserved LXXIIXXXI motif to interact with the LBD. The conserved hydrophobic residues (shown in yellow) bind in a hydrophobic groove formed the intersection of helices 3 and 4, but this motif does not engage helix 12 via the “charge clamp.”
C. Nuclear Receptor Modulators and Selective Modulators
Because of the role that many of the NRs play in disease, these receptors have been a rich source for the development of small molecule synthetic ligands that either mimic the action of typical endogenous ligands (agonists) or block the action of endogenous ligands (antagonists). For the vast majority of receptors, agonist binding to the LBD results in a conformational change that leads to recruitment of coactivator proteins, resulting in increased transcription of target genes. An antagonist (in the purist sense, or as some would call it, a “neutral” antagonist) simply binds to the LBD and prevents the conformational change that an agonist would cause, thus preventing coactivator recruitment and subsequent stimulation of transcription. This is the simplest case, but the pharmacology of the NRs turns out to be much more complex. Partial agonists bind to the LBD, and the resulting conformational change provides only a partial activation of transcription, which is likely due to less proficient recruitment of coactivators. Partial agonists will also compete with full agonists so as to reduce the level of transactivation intrinsic to that of the full agonist. Inverse agonists are also commonly found, and they are particularly important when a receptor has some degree of basal activity (and basal level of recruitment of coactivator); binding of this class of ligand results in a conformational change that reduces the basal level of activity (reduces basal coactivator binding). In many cases, it has become clear that the conformation induced by inverse agonists may also result in recruitment of corepressor proteins, resulting in active silencing of target gene transcription. In this case, a particular NR may not need to display any basal coactivator binding for the inverse agonist to cause a decrease in target gene transcription.
The resulting positioning of H12 in response to ligand binding is the critical determinate of the function of the ligand (agonist versus antagonist), and this has been clearly shown in several cocrystal structures of NR LBDs with various ligands. An example is shown in Fig. 4 for ER. When ER is bound to an agonist (estradiol), H12 is positioned in a manner to complete the formation of the coactivator-binding groove, which allows for recruitment of the NR box of coactivator proteins. In the case of antagonist binding, such as raloxifene or ICI 182,780 (fulvestrant), the LBD assumes a conformation that does not allow coactivator NR box recognition. In the case of raloxifene binding, H12 is placed in such a position so as to physically block NR box binding (Brzozowski et al., 1997; Shiau et al., 1998); in the case of ICI 182,780 binding, the long aliphatic extension of this compound itself exits the LBP entryway and folds along the coactivator binding groove, thus preventing NR box recognition (Pike et al., 1999, 2001).
Fig. 4.
Agonist and antagonist LBD conformations observed in ER crystal structures. Crystal structures of the ER LBD have suggested structural features contributing to ligand-induced agonism and antagonism. (A) The natural agonist, 17β-estradiol, docks in the LBP and positions helix 12 into a conformation referred to as the agonist or active conformation. This conformation forms the coactivator-binding surface as described in Fig. 2. (B) In contrast, when raloxifene is bound in the LBP, the position of helix 12 is rotated with respect to the agonist conformation such that it binds in the AF-2 coactivator-binding surface and thus blocks binding of coactivators via the LXXLL motif. This is referred to as the antagonist, repressive, or inactive conformation. (C) Other ligands such as ICI 182,780 can physically block the AF-2 coactivator-binding surface and do not stabilize helix 12; thus, these ligands are termed pure antagonists. In these panels, helix 12 is blue, and ligands are black.
Significantly more complex is the situation of NR ligands classified as selective modulators. Selective NR modulators display tissue and/or target gene specificity in terms of their agonist, antagonist, or inverse agonist activity. The first examples of selective modulators were identified targeting ER, where compounds such as tamoxifen function as an antagonist in breast tissue but as an agonist in bone and uterus. It is clear that the tissue specificity can be altered, given that compounds such as the selective ER modulator (SERM) raloxifene were identified that had the clinically superior tissue specificity profile of antagonist in the breast and uterus but agonist in bone. These types of NR ligands are the focus of this review; in the subsequent sections, the pharmacologic profile of major modulators for several NRs are described in detail. The mechanism of action that underlies the tissue/target gene specificity is still not clear, but several sound theories have been proposed. It is highly likely that various selective modulators function via distinct mechanisms and that one mechanism will not be able to explain how they all display their unique pharmacologic profiles. Before turning to specific selective modulators, the potential mechanisms that may be responsible for modulator pharmacology will be discussed.
It is quite clear that various modulators with distinct tissue/target gene specificity profiles induce unique conformations within the LBD of the NRs. In many cases ER is used as a model because the pharmacology of selective modulators is most well developed with this particular receptor. Using techniques such as phage display or hydrogen deuterium exchange mass spectroscopy (HDXMS), it has been demonstrated that binding of distinct classes of SERMs results in distinct conformations on the surface of the LBD (Chang et al., 1999; Norris et al., 1999; Dai et al., 2008, 2009). In fact, we were able to predict the tissue specificity profile of various SERMS using LBD conformation information gleaned from HDXMS data (Dai et al., 2009). The unique conformations induced by the various selective modulators are believed to be associated with distinct patterns of recruitment of coactivators and corepressors that lead to the tissue/target gene specificity profile. We and others were also able to demonstrate this by showing that ER LBD bound to various selective modulators displayed distinct affinities for different coactivators and/or NR boxes (Gee et al., 1999; Bramlett et al., 2001). Various cell types express different levels of the coactivators and corepressors that may lead to the tissue-specific activity. Variation of the ratio of coactivator to corepressor in a particular cell type modulates the agonist/antagonist activity of the SERM tamoxifen (Smith et al., 1997). SRC1-dominant expression over SMRT led to tamoxifen displaying significant agonist activity, whereas in the reverse conditions, tamoxifen functioned as an antagonist (Smith et al., 1997). Shang and Brown (2002) took this one step farther by comparing two SERMs, raloxifene and tamoxifen, that display distinct tissue specificity profiles. In mammary cells, both these SERMS are effective in recruitment of corepressor to target promoters (hence both are antagonists), but in uterine cells SRC1 is at a much higher level of expression than in mammary cells and tamoxifen is much more sensitive to SRC1 than raloxifene in terms of “transformation” into an agonist. This results in tamoxifen effectively recruiting SRC1 to various ER target genes in uterine cells (agonist) but not mammary cells, whereas raloxifene is not effective in recruitment of SRC1 in either cell type (antagonist). In cases such as ER, where receptor subtypes (ERα and ERβ) mediate the action of the modulators, distinct pharmacologic action at the various subtypes may also play a role in tissue selective action. Receptor subtypes are often expressed differentially; as a single modulator may function differently on the various subtypes, distinct tissue specificity profiles may emerge (Barkhem et al., 1998).
There are examples of differential, target-gene specific actions of a single modulator within a single cell type (Bramlett and Burris, 2003). The distinct action of the selective modulator cannot be attributed to differential expression of cofactor proteins or receptor subtypes in this case. One potential mechanism that may explain these actions is the target gene itself may convey specific information to the LBD so that it may respond differentially to various selective modulator ligands. For example, ER has been demonstrated to alter its affinity for various coactivators depending on the DNA-response element to which it is bound (Hall et al., 2002). In addition, the specific DNA-response element to which GR is bound has been shown to play an important role in its selection of coactivators (Meijsing et al., 2009). Furthermore, the relatively recent X-ray structure of an intact NR heterodimer (PPARγ/RXRα) complexed with DNA (Fig. 5) demonstrated that the DBD of RXRα made critical contacts with the LBD of PPARγ that were required for function of the complex, suggesting that critical communication between DNA, DBD, and LBD occurs. HDXMS data with intact vitamin D receptor (VDR)/RXRα on DNA (Fig. 6) demonstrated that binding of the dimer to DNA resulted in major conformational alterations in H12, clearly demonstrating the potential for DNA to relay critical information to regions of the LBD that are important for coactivator recognition (Zhang et al., 2011).
Fig. 5.
Intact structure of PPARγ/RXRα complex on DNA. At the time of this review, only one crystal structure has been reported of an intact nuclear receptor complex: PPARγ/RXRα bound to DNA, ligands, and coactivator peptides. In this structure, the receptors form an asymmetric complex where the LBD of PPARγ contacts not only the LBD of RXRα through the canonical helix 11 heterodimerization surface but also contacts the RXRα DBD, suggesting a feature that could allow molecular communication between the LBD (the site of ligand binding) and DBD (the site of DNA binding). The N-terminal AF-1 region of the receptors was not captured in this complex due to its highly dynamic properties. In this figure, PPARγ is orange, RXRα is blue, ligands are green, and DNA is white.
Fig. 6.

HDX study of intact VDR/RXRα complex on DNA. HDX is a powerful technique to monitor the effect of ligand and DNA binding on the dynamics of intact receptor complexes. HDX studies on the intact VDR/RXRα complex were the first to show that binding of the heterodimer to DNA can allosterically permeate changes in receptor dynamics, not only in the DBD but extending to the AF-2 regions in the LBD of the receptors. In this figure, regions colored blue show protection from HDX upon binding DNA, whereas regions colored yellow show increased HDX upon binding DNA. Ligand binding to the LBD was also observed to affect the dynamics of the DBD (not shown).
The molecular events underlying selective NR modulator pharmacology are myriad, and this discussion has only touched on a few highlights of various mechanisms that have been implicated in this unique profile of ligands that target the NRs. Posttranslational modifications of receptors and regulation of the modifications has been shown to lead to target-gene specific regulation; additionally, the nongenomic actions (referring to actions of NRs independent of their classic action as transcription factors, i.e., genomic action) of various receptors are likely to also play a role in the action of at least some of the selective NR modulators that have been identified to date. This review will summarize some of the key breakthroughs that have been made in the development of selective modulators for a range of NRs and will discuss how many of them have been effective in targeting human diseases.
II. Selective Estrogen Receptor Modulators
A. Estrogen Receptor Structure
The estrogen receptors (ERs) have a deep history in the realm of selective modulators, whose ligands are referred to as selective estrogen receptor modulators (SERMs). ERs were discovered in the laboratory of Elwood Jensen in the 1950s and 1960s when experiments suggested that estrogenic effects observed in the uterus were mediated by a specific receptor. Since this discovery, the field of ER pharmacology has exploded, resulting in large numbers of synthetic ligands to explore the biology of ERs. In 1996, a second ER isoform, ERβ (ESR2; NR3A2), was discovered in the laboratory of Jan-Ake Gustafsson; the ER discovered earlier is now known as ERα (ESR1; NR3A1) (Kuiper et al., 1996).
ERs display the canonical domain organization found in other members of the nuclear receptor superfamily. However, the ERs have longer N-terminal (A/B domain; contains the AF-1 region) and C-terminal (F domain) domains. The F domain in particular appears to have an effect on the function of ER ligands, though the exact molecular mechanisms are not well understood (Kojetin et al., 2008; Skafar and Zhao, 2008). In addition, ER splice variants have been shown to influence the pharmacology of synthetic ER compounds, and site-specific phosphorylation of ER has been shown to modulate the gene-specific transcriptional response (Taylor et al., 2010; Duplessis et al., 2011).
The natural ligand for the ERs is the steroid hormone 17β-estradiol, which binds to ERs with high affinity (Kd ∼0.2 nM) and activates the receptors, resulting in increased transcription of genes containing estrogen response element (ERE) promoter sequences (5′-GGTCANNNTGACCT-3′) (Maurer and Notides, 1987). ERs can function as homodimers (ERα-ERα; ERβ-ERβ) or heterodimers (ERα-ERβ) (Cowley et al., 1997). The ER LBD is probably the most studied NR domain in terms of NR structure, and, as discussed earlier and illustrated in Fig. 4, these structural studies have served as a model for understanding the molecular basis of NR agonism and antagonism.
B. Estrogen Receptor Function
Both ERs are widely expressed, although ERα is more abundant in breast, endometrial, ovarian, and hypothalamus tissues, whereas ERβ is more abundant in brain, bone, endothelial, heart, intestine, kidney, lung, and prostate tissues. Furthermore, ERα and ERβ have both overlapping and nonoverlapping functions, which combine with the tissue-specific differences in the relative abundance of the receptors. This suggests that the specific targeting of ER isoforms could prove useful in the therapeutic treatment of diseases with perhaps limited cross-talk to other tissues or ER isoform-specific genes—essentially the primary basis of selective ligand modulation. The actions of ERβ in many cases oppose the actions of ERα, suggesting a role for a single ligand to have different functional outcomes depending on the specific ER target (Matthews and Gustafsson, 2003). ERs work in concert with a variety of coregulator proteins, many of which are differentially expressed in various tissues, providing what is considered the molecular basis of SERM activity: ligand-dependent and tissue-specific coregulator recruitment.
A variety of ER splice variants and mutations have been identified, some of which from were obtained from clinical samples from patients with cancer and other diseases (Herynk and Fuqua, 2004). The mutations are primarily found in AF-1 and LBD/AF-2. Many of the natural ER mutations found in clinical samples were in fact derived from tissues deemed to be ER negative in terms of their therapeutic classification using a ligand-binding assay. This suggests that natural mutations in ERs can affect the ligand-binding properties of ERs. Thus, while some cancers might be classified as ER negative, this could mean either that ER is not expressed or that a mutation has rendered ER incapable of binding ligand or has reduced its binding affinity and thus other modes of treatment are warranted.
Because of their broad tissue expression patterns and important roles in development and physiology, ERs are drug targets for a variety of diseases (Riggs and Hartmann, 2003). Breast tissue development and physiology is substantially influenced by ERs, and treatment of ER-positive breast cancer is currently one of the primary clinical uses of SERMs. In these treatments, SERMs are given as an adjuvant therapy after a primary therapy such as a mastectomy or lumpectomy and radiation therapy. ER-based adjuvant cancer therapies focus on blocking estrogen action in these tissues. As will be discussed more later, because many breast cancers occur in postmenopausal women, who may also suffer from diseases such as osteoporosis and hot flashes, SERMs that display antagonist blocking action in the breast and agonist action in the bone serve in a sense a dual role in these patients.
As discussed previously, estrogens also have a strong influence in bone, which expresses both ER isoforms (Bord et al., 2001; Riggs et al., 2002). ERα is highly expressed in developing cortical bone, and ERβ levels are higher in developing cancellous bone. One of the primary diseases affecting bone is osteoporosis, which is in part caused by estrogen deficiencies in the body. Cancellous bone (ERβ rich) is less rigid compared with cortical bone (ERα rich) and is affected more in osteoporosis than cortical bone, supporting the selective targeting of ERβ for osteoporosis treatment.
In the cardiovascular system, estrogens and SERMs provide a generally favorable serum lipid profile. Estrogen increases high-density lipoprotein (HDL) and triglycerides and lowers low-density lipoprotein (LDL) levels. SERMs can display both similar and ligand-specific effects on lipids. SERMs generally decrease LDL and triglyceride levels, but some increase HDL (toremifene) while others increase HDL levels (Saarto et al., 1996). Estrogen and SERMs thus generally provide a benefit in cardiovascular disease in postmenopausal women, among whom it is estimated that about one-third die from coronary artery disease (Wenger, 1997).
In the central nervous system (CNS), where ERβ expression levels are generally high, ERβ function and targeting has been linked to several brain processes, including cognition and memory (McEwen and Alves, 1999). In addition, further, studies have implicated ER agonism in affording relief from hot flashes associated with the postmenopause period in women (Marttunen et al., 1998; Johnston et al., 2000).
C. Selective Estrogen Receptor Modulators
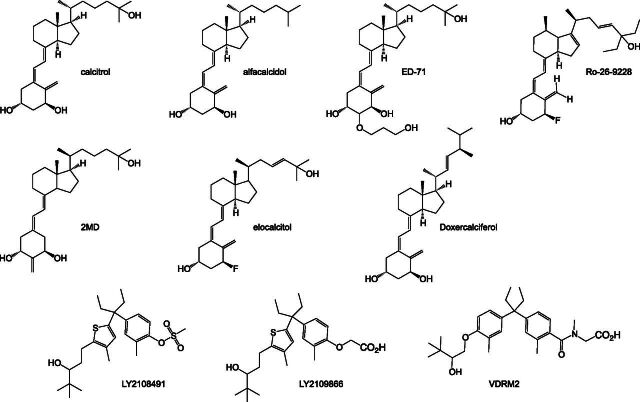
SERMs have been useful for clinical treatment of a variety of disorders, including breast cancer, osteoporosis, and menopausal health symptoms via hormone replacement therapy. However, the need for better SERM therapeutics is illustrated by the complex pharmacology of tamoxifen (Fig. 7), a commonly prescribed SERM for breast cancer treatment. Tamoxifen displays mixed pharmacology in different tissues, as it acts as an antagonist in breast tissue but as an agonist in bone and the uterus. Thus, patients who take tamoxifen as a breast cancer therapy receive benefits in the breast where it acts as an ER antagonist and in the bone where it acts as an ER agonist (increased bone mineral density); however, patients also experience negative side effects, such as in the uterus where tamoxifen acts as an agonist that increases cell proliferation.
Fig. 7.
ER modulators.
The notion that the same drug can have such opposing actions in different tissues, not all of which are favorable for the patient, supports the need for SERMs with better tissue-selective pharmacologic properties. An ideal SERM may be one that antagonizes in breast and endometrial tissues, but agonizes in bone and CNS tissues (O’Regan and Jordan, 2001). On a molecular level, an ideal SERM may be one that recruits a particular coregulator protein over another, perhaps in a gene/promoter-specific manner.
Tamoxifen, perhaps the most well known SERM, is used in the treatment of ER-positive breast cancer. Tamoxifen was discovered by ICI Pharmaceuticals (now AstraZeneca, London, UK) under the internal name ICI46474 (Harper and Walpole, 1967a,b; Jordan, 2006). It is a chemical derivative of the first nonsteroidal antiestrogen MER25, which was discovered in the 1950s and identified as an inhibitor of estrogen actions (Lerner et al., 1958). Both these compounds are chemical derivatives of the highly potent synthetic nonsteroidal estrogen diethylstilbestrol (Furr and Jordan, 1984). Early studies revealed that tamoxifen could compete with estrogens, in terms of preventing the accumulation of [3H]estradiol in ER target tissues (e.g., uterine, vaginal, and mammary) of mice, rats, and humans (Emmens, 1971; Lunan and Green, 1974; Jordan, 1975; Jordan and Dowse, 1976). These studies also provided insight into the pharmacokinetic/pharmacodynamic properties of tamoxifen, including its conversion into the metabolite 4-hydroxytamoxifen (Fig. 7), which is the primary form that binds to ERs in vivo (Rochefort et al., 1979; Rochefort and Borgna, 1981). Tamoxifen shows maximal levels in tissues approximately 6-hours after dosing, with elevated plasma levels detected for the entire duration of the experiment (120 hours) (Major et al., 1976). Additional biochemical studies in the form of [3H]estradiol competition assays validated that tamoxifen binds directly to ER and competes with 17β-estradiol. Later, structural validation via X-ray crystallography confirmed this interaction and provided molecular details concerning the interactions between ERα with 17β-estradiol and tamoxifen (Brzozowski et al., 1997; Shiau et al., 1998). Namely, helix 12 in the LBD acts as a molecular switch, adopting an “active” conformation in the presence of an agonist such as diethylstilbestrol or 17β-estradiol, allowing the interaction of coregulator proteins with the AF-2 coregulator-binding surface. In the presence of 4-hydroxytamoxifen, helix 12 rotates and occupies the AF-2 coregulator-binding surface, resulting in transcriptional antagonism.
As already described, tamoxifen displays a mixed agonist/antagonist pharmacologic profile in different tissues. Generally, this is a good property for SERMs, in the sense that it is possible to develop compounds with differential actions that could potentially benefit one tissue while not affecting others. However, for tamoxifen these differential effects provide significant side effects. Notable evidence suggests that the source of this mixed pharmacology depends on the tissue-selective expression of coregulator proteins, which interact with ERs at the promotor and enhance or repress transcription of ER-dependent genes. A primary example is the tamoxifen-dependent recruitment steroid receptor coactivator 1 (SRC1/NCOA1) (Shang and Brown, 2002). SRC1 is expressed at higher levels in the uterus and lower levels in breast tissue. Tamoxifen induces SRC1 recruitment to ERα-dependent gene promoters in uterine tissue, which is not the case for other SERMs including raloxifene. However, like many other SERMs, tamoxifen induces corepressor recruitment to ER gene promotors in breast tissue.
Raloxifene (Fig. 7) was discovered and is marketed by Eli Lilly and Company (Indianapolis, IN) as a SERM used in the prevention of osteoporosis in postmenopausal women. Raloxifene also shows efficacy in reducing the risk of invasive breast cancer without affecting the risk of primary coronary events (Barrett-Connor et al., 2006). Similar to tamoxifen, when raloxifene binds to the ER LBD, it induces a conformation that repositions helix 12 to the AF-2 surface and blocks coactivator binding (Brzozowski et al., 1997). Additionally, raloxifene preserves or increases bone density and inhibits the growth of breast cancer (Clemens et al., 1983; Gottardis and Jordan, 1987; Jordan et al., 1987; Black et al., 1994; Sato et al., 1994, 1995; Turner et al., 1994; Anzano et al., 1996). Raloxifene does not agonize endometrial growth to as large an extent as tamoxifen (Gottardis et al., 1990). The reduced level of agonism in uterine tissue is likely a consequence of its inability to stimulate SRC1 recruitment to ER gene promotors in endometrial cells as is the case for tamoxifen (Shang and Brown, 2002). However, both tamoxifen and raloxifene induce recruitment of corepressors to ER gene promotors in breast cancer cells (Shang and Brown, 2002).
Lasofoxifene (Fig. 7), discovered in a collaboration between Pfizer (New York, NY) and Ligand Pharmaceuticals (La Jolla, CA), is currently under development for the prevention and treatment of osteoporosis and the treatment of vaginal atrophy. Lasofoxifene is a potent SERM that does not agonize uterine cell growth but decreases total cholesterol, fat body mass, and bone loss in female rats, and displays similar properties in male rats (Ke et al., 1998, 2000; Rosati et al., 1998). In addition, lasofoxifene increases vaginal mucus formation without inducing cell proliferation. This suggests that it would be useful in the treatment of vaginal and vulvar atrophy in postmenopausal women (Wang et al., 2006). It is thought that these effects are likely due to the ability of lasofoxifene to increase the expression levels of ERβ and AR in vaginal tissues, which other SERMs do not (Wang et al., 2006). Clinical data support the use of lasofoxifene in the prevention and treatment of osteoporosis and treatment of vaginal atrophy in postmenopausal women without an increased risk of endometrial cancer but with an increased risk of venous thromboembolic events (Bachmann et al., 2005; McClung et al., 2006b; Taylor, 2009; Cummings et al., 2010; Gennari et al., 2010). From a structural perspective, much like tamoxifen and raloxifene, lasofoxifene displaces helix 12 in the LBD from the agonist position to block the AF-2 coregulator-binding surface (Vajdos et al., 2007).
Toremifene (Fig. 7) is a tamoxifen analog licensed under the brand name Fareston (marketed by GTx) as a treatment approved by the U.S. Food and Drug Administration (FDA) for advanced metastatic breast cancer. It is also currently under development for prevention of prostate cancer. Clinical studies have shown that toremifene is equally as efficacious as tamoxifen in the treatment of metastatic breast cancer and may have fewer negative side effects (Holli, 2002; Lewis et al., 2010). Biochemical studies have shown that toremifene downregulates the expression of breast cancer resistance protein (BCRP), which is a multidrug resistance transporter for a variety of antitumor agents (Zhang et al., 2010).
Fulvestrant (ICI 182,780) (Fig. 7) is a selective estrogen receptor downregulator (SERD) initially discovered by ICI Pharmaceuticals and marketed under the brand name Faslodex by AstraZeneca. Recently, fulvestrant has been used for the treatment of metastatic breast cancer in postmenopausal women (Wakeling et al., 1991). Fulvestrant is considered a pure ER antagonist, as it has no ER agonist effects. Interestingly, fulvestrant has a greater binding affinity than estradiol for ER, and is more effective than tamoxifen at inhibiting MCF-7 breast cancer cell proliferation and tumor progression in patients. Fulvestrant also has a much longer half-life when compared with tamoxifen (Wakeling et al., 1991; Bundred et al., 2002; Robertson et al., 2003; Robertson and Harrison, 2004). Fulvestrant has been shown to be effective in tamoxifen sensitive and insensitive cell lines (Coopman et al., 1994; Osborne et al., 1994). The mechanism of action for fulvestrant involves the direct blocking of the ER AF-2 coregulator-binding surface, impairing receptor dimerization and increasing receptor degradation and turnover, perhaps via fulvestrant-dependent interaction with cytokeratins 8 and 18 (CK8, CK18) and colocalization to proteasomes (Robertson, 2001; Long and Nephew, 2006; Long et al., 2010). It has been suggested that resistance to fulvestrant is mediated by the tyrosine kinase c-ABL (Zhao et al., 2011a). Clinical trials revealed that fulvestrant is well tolerated in breast cancer patients without the negative side effects associated with most ER SERMS such as partial agonist activity in the uterus (Addo et al., 2002; Vergote and Robertson, 2004).
Clomifene (clomiphene) (Fig. 7) is marketed under the brand names Clomid and Serophene. Since its introduction in the 1960s, clomifene has been used in the management of infertility via induction of ovulation (Goldstein et al., 2000). Clomifene is a mixture E and double-bond Z isomers, one of which is an ER agonist/antagonist and the other a strict antagonist (Glasier, 1990). In terms of isoform specificity, clomifene has been shown to agonize and antagonize ERα but only antagonize ERβ (Kurosawa et al., 2010). The mechanism of action ascribed to clomifene in infertility treatment involves antagonism in the hypothalamus, which increases levels of gonadotropin-releasing hormone (GnRH) and subsequently increases follicle-stimulating hormone (FSH) and luteinizing hormone (LH) secretion (Dickey and Holtkamp, 1996; Tarlatzis and Grimbizis, 1998). It has also been suggested that clomifene may inhibit the estradiol-dependent proliferation of endometrial epithelial cells by inhibiting the recruitment of SRC1 to ERα and thus estradiol-dependent ER transactivation (Amita et al., 2010). A summary of several clinical trials revealed that clomifene is as effective as tamoxifen in inducing ovulation for infertility management, although results from another study suggest that the actions on gonadotropin-releasing hormone levels may be nongenomic in nature (Garas et al., 2004; Steiner et al., 2005).
Ormeloxifene (centchroman) (Fig. 7) is a SERM that has been licensed under the brand names Saheli, Novex-DS, Centron, and Sevista by Torrent Pharmaceuticals (Ahmedabad, India) and later HLL Lifecare (Thiruvananthapuram, Kerala, India) for birth control and dysfunctional uterine bleeding. Ormeloxifene displays higher affinity and selectivity for ERα (Ki = 250 nM) versus ERβ (Ki = 750 nM) (Blesson et al., 2006). Early studies reported on the anti-inflammatory properties of ormeloxifene in acute and chronic models of inflammation, and both estrogenic and antiestrogenic effects in the uterus at low and high doses, respectively (Dhawan and Srimal, 1973; Kamboj et al., 1973). Ormeloxifene shows estrogenic activity in the uterus and fallopian tubes, which was suggested to contribute to its antifertility efficacy (Imam et al., 1975). Structure-activity relationship analysis revealed regions of ormeloxifene important for ER binding and function (Salman et al., 1983). Pharmacokinetic data have revealed the half-life of a single dose of ormeloxifene (approximately 170 hours) as well as the tissue distribution of ormeloxifene and its metabolite 7-desmethyl ormeloxifene (Paliwal et al., 1989; Lal et al., 1995; Paliwal and Gupta, 1996). Ormeloxifene induces caspase-dependent apoptosis in both ER-positive (MCF-7) and ER-negative (MDA-MB-231) breast cancer cell lines (Nigam et al., 2008). However, the ER dependency of these cell lines is in reference to ERα. Two ERβ variants are expressed in these cells, suggesting an ERβ-mediated effect. Ormeloxifene appears to function by inhibiting the interaction of the coactivator SRC1 with ERα while enhancing the interaction with the coactivator RIP140 and corepressor NCoR as well as interaction of NCoR with ERβ in the rat uterus (Daverey et al., 2009). Antimutagenic effects from treatment with ormeloxifene have also been described, as it reduces sister chromatid exchange and chromosome aberrations in female Swiss albino mice exposed to genotoxic compounds (Giri et al., 1999). A phase 2 trial revealed that ormeloxifene has efficacy in the treatment of advanced breast cancer (Misra et al., 1989).
Femarelle (DT56a) is a SERM used for the treatment of menopause and bone health (Somjen et al., 2007). Femarelle is an extract of tofu and flaxseed oil containing phytoestrogens, and is thus sometimes referred to as the “Tofu pill” or a “natural” SERM. The exact identity of the pharmacologically active phytoestrogens in the extract are unclear, but femarelle competes with 17β-estradiol and stimulates or agonizes creatine kinase activity, which is an estrogenic marker, in rat skeletal tissue in a manner similar to 17β-estradiol (Malnick et al., 1983; Somjen and Yoles, 2003; Somjen et al., 2007). However, unlike 17β-estradiol, femarelle does not agonize estrogenic activity in the uterus or other reproductive tissues (Somjen and Yoles, 2003; Oropeza et al., 2005). In addition, femarelle does not effect cell proliferation in the human MCF-7 breast cancer cell line (Yoles and Lilling, 2007). Femarelle shows efficacy in preserving bone mineral density (BMD) in both female rats and clinically in postmenopausal women without risk of thrombogenicity (Yoles et al., 2003, 2004; Somjen et al., 2005, 2006; Nachtigall et al., 2011). Femarelle and other ER agonists have been shown to abolish fat cell content in rat bone marrow. It has also been suggested that femarelle can relieve menopausal vasomotor symptoms not by affecting hormone levels or the endometrium directly, but rather in brain responsiveness via estrogenic action on the brain involved in regulating mood, cognition, and homeostasis (Pluchino et al., 2009).
Bazedoxifene (WAY-140424) (Fig. 7), discovered as a result of collaboration between Wyeth (Madison, NJ) and Ligand Pharmaceuticals, has been approved by the FDA under the brand name Conbriza for the prevention and treatment of osteoporosis in postmenopausal women. Bazedoxifene was discovered using a stringent preclinical selection process, as it was understood that the discovery of a new ligand would be compared with the already established SERM raloxifene (Komm and Lyttle, 2001; Anonymous 2008). Bazedoxifene displays general properties of a dual ERα/ERβ SERM, with Ki values of 0.1 and 0.3 nM, respectively, with no cross reactivity with other nuclear receptors. In its early discovery, it displayed the prototypical SERM gene-selective activation phenotype, where it antagonized expression on a 2×-ERE (estrogen response element) promoter, but agonized expression driven by a hepatic lipase promoter in the same cell line (Komm and Lyttle, 2001). Bazedoxifene antagonizes estrogen-stimulated proliferation of MCF-7 breast cancer cells with little to no effects in uterine and CNS tissue, and also maintains bone density, reduces cholesterol in rats, and causes regression of endometriosis in mice (Komm and Lyttle, 2001; Komm et al., 2005; Ronkin et al., 2005; Kulak et al., 2011). Bazedoxifene inhibits the proliferation of estrogen-dependent (MCF-7 and T47D) and estrogen-independent (MCF-7:5C and MCF-7:2A) cell lines (Lewis-Wambi et al., 2011). Its ability to antagonize growth of MCF-7:5C cells in particular is unique among SERMs and occurs as a result of downregulation of ERα (via protein degradation) and suppressing cyclin D1 expression. Bazedoxifene has also shown some efficacy in inflammation, where it reduces lipopolysaccharide (LPS)-induced expression of interleukin-6 (IL-6) and interferon-γ-inducible protein-10 (IP-10) through ER-dependent inhibition of nuclear factor κB (NF-κB) p65 transactivation (Cerciat et al., 2010).
During early phase 1 and 2 clinical trials, it was shown that bazedoxifene did not increase hot flashes, which is a common side effect of SERMs. Bazedoxifene also preserved bone density and lowered cholesterol levels (Komm and Lyttle, 2001). It appears that this SERM does not stimulate but rather antagonizes endometrial growth in postmenopausal women (Ronkin et al., 2005). Other clinical trials revealed that bazedoxifene treatment prevents bone loss and reduces bone turnover with similar efficacy to raloxifene in postmenopausal women who have normal-to-low BMD without increasing mammographic breast density, the incidence of hot flashes (Miller et al., 2008; Silverman et al., 2008, 2012; Archer et al., 2009; Harvey et al., 2009; Kanis et al., 2009; Pinkerton et al., 2009a; Christiansen et al., 2010; Bachmann et al., 2011; de Villiers et al., 2011; Xu et al., 2011). Bazedoxifene also displays efficacy for treating vasomotor symptoms and preventing endometrial hyperplasia in postmenopausal women (Pickar et al., 2009; Pinkerton et al., 2009b).
Preclinical data have suggested that the combination treatment of bazedoxifene and conjugated estrogens (CE) might lead to a more favorable benefit-risk profile in the treatment of menopause symptoms, including favorable vasomotor, lipid, and skeletal response with minimal stimulation in the uterus (Kharode et al., 2008; Peano et al., 2009). This combination therapy also shows efficacy in the treatment of osteoporosis in a manner that prevents uterine growth, with decreased uterine wet weight and lower cholesterol levels (Komm et al., 2011). In particular, gene expression analysis via microarray experiments have revealed that a subset of CE-inducible genes were antagonized by bazedoxifene alone or in combination with CE (Chang et al., 2010b). The combination of bazedoxifene and CE [BZA-CE; or tissue-selective estrogen complex (TSEC)] displays efficacy and was determined to be safe in treating menopausal symptoms, decreasing bone turnover and bone loss in postmenopausal women at risk for osteoporosis, and treating vulvar/vaginal atrophy (Lindsay et al., 2009; Lobo et al., 2009; Utian et al., 2009; Bachmann et al., 2010; Kagan et al., 2010).
Arzoxifene (LY353381) (Fig. 7), developed by Eli Lilly and Company, is structurally related to raloxifene; however, early studies revealed improved in vivo potency and efficacy (Palkowitz et al., 1997; Sato et al., 1998a). In addition, whereas raloxifene shows more specificity for ERα versus ERβ (21 versus 560 nM), arzoxifene displays properties of a dual ERα/ERβ SERM (22 versus 66 nM) (Overk et al., 2007). Arzoxifene binds directly to the ER and prevents increased body weight and cholesterol levels in ovariectomized rats with similar efficacy as estrogen and raloxifene but with more potency than raloxifene in reducing these parameters (Sato et al., 1998a; Suh et al., 2001). In addition, arzoxifene prevented bone loss in ovariectomized rats with an efficacy similar to parathyroid hormone (PTH), including during long-term dosing (Sato et al., 1998b; Ma et al., 2002). It has been reported that arzoxifene displays negligible effects in uterine cells and increased efficacy in antagonizing uterine hypertrophy stimulated by estrogen when compared with tamoxifen (Sato et al., 1998a; Suh et al., 2001). Other studies have revealed that arzoxifene shows some efficacy in activating insulin-like growth factor I in uterine signaling. This causes an increase in proliferating cell nuclear antigen expression and the number of mitotic cells in the uterus, as well as simulation of EnCa101 endometrial tumors that were previously stimulated with estrogen or tamoxifen (Klotz et al., 2000; Dardes et al., 2001). Arzoxifene was also shown to act as a chemoprotective agent in a rat model for mammary carcinogenesis (Suh et al., 2001). It was shown that arzoxifene was 30–100 times more potent than raloxifene in regulating these in vivo parameters. Arzoxifene also displayed several advantages for use as a SERM over raloxifene, including preventing increased body weight, cholesterol, and bone loss, while also acting as an antagonist in the uterus (Sato et al., 1998a). Arzoxifene antagonizes estrogen-stimulated growth in ER-positive MCF-7 human breast cancer cells in a manner similar to tamoxifen, including the propensity for cross-resistance, although resistance to either modulator is independent of the other (Schafer et al., 2001; Detre et al., 2003; Freddie et al., 2004b). This suggests that if a patient becomes resistant to one SERM, another may be beneficial for future treatments (Freddie et al., 2004a).
Resistance to arzoxifene has been linked to overexpression of cyclin D1, which occurs in approximately 40% of all breast cancer patients, in a manner that converts arzoxifene from an antagonist to an agonist as a result of a ligand-dependent increase in the stabilization of SRC1 complexed with ERα (Zwart et al., 2009). Arzoxifene has also been shown to synergize with the retinoid LG100268 (6-[1-(3,5,5,8,8-pentamethyl-6,7-dihydronaphthalen-2-yl)cyclopropyl]pyridine-3-carboxylic acid), which binds to RXRs, in prevention and treatment rat models for breast cancer at concentrations with treatment with one ligand alone displayed negligible effects (Suh et al., 2002; Liby et al., 2006). The mechanism by which the combined treatment of arzoxifene and LG100268 influences apoptosis in breast cancer involves the induction of transforming growth factor β (TGFβ) by arzoxifene and inhibition of NF-κB and phosphatidylinositol 3′ kinase signaling by LG100268 (Rendi et al., 2004). One study suggests that arzoxifene is an agonist of ERβ within the serotonin neuron, increasing the expression of tryptophyan hydroxylase and the serotonin reuptake transporter (SERT) (Bethea et al., 2002).
A phase 1 clinical trial of arzoxifene determined that daily oral dosing was safe and well tolerated; and combined with all previous studies, this suggests that arzoxifene may be useful in the treatment of metastatic breast cancer (Munster et al., 2001; Fabian et al., 2004). A phase 2 clinical trial determined that arzoxifene is effective in treating tamoxifen-sensitive and tamoxifen-refractory patients with advanced or metastatic breast cancer (Chan, 2002; Baselga et al., 2003; Buzdar et al., 2003). Other phase 2 trials have revealed that arzoxifene is effective in the treatment of recurrent or advanced endometrial cancer, as well as suppressing bone turnover in the treatment of osteoporosis (Burke and Walker, 2003; McMeekin et al., 2003). However, phase 3 clinical trials revealed that arzoxifene did not significantly differ in terms of clinical output compared with the standard mode of treatment with tamoxifen for advanced and metastatic breast cancer (Deshmane et al., 2007). In fact, the results revealed that tamoxifen produced longer survival and time-to-treatment failure rates. Despite this, a clinical study revealed that arzoxifene might still be useful for treatment of bone loss in postmenopausal women at doses that do not have a significant effect in the uterus and endometrium (Jackson et al., 2008). Another phase 3 trial revealed that although arzoxifene displayed significantly greater effects on BMD and turnover compared with raloxifene, it did not translate into greater nonvertebral fracture efficacy or a more favorable adverse event profile (Kendler et al., 2012).
Afimoxifene (Fig. 7) is the 4-hydroxy derivative of tamoxifen, the active metabolite of tamoxifen. Afimoxifene is typically formulated as a gel containing 4-hydroxytamoxifen, and shows clinical efficacy in the treatment of cyclical mastalgia (breast pain/discomfort) in premenopausal women (Mansel et al., 2007).
SERM drug discovery has been categorized under three broad categories. Tamoxifen is generally considered to be a first-generation SERM as it was the one of the first discovered and is perhaps the most used clinically. Because tamoxifen has less than optimal tissue specificity, such as agonist activity in the uterus, discovery efforts concentrated on second-generation SERMs. Raloxifene is considered a second-generation SERM, and it was selected for its selective tissue specificity, such as its antagonist activity in the uterus, as compared with tamoxifen. The third-generation of SERMs include the compounds discovered after raloxifene that display further improvements in tissue specificity. An example is LSN2120310, (R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)phenyl]-5H-6-oxachrysen-2-ol, which is based on the raloxifene chemical scaffold; it displays agonist activity in the bone and antagonist activity in the breast and uterus, but unlike tamoxifen and raloxifene it may actually prevent hot flashes (Wallace et al., 2006). Going forward, the next generation of SERMs will build upon our understanding of ligand-specific regulation of individual genes within a single tissue, not only displaying tissue-specific agonist or antagonist activity but also differential upregulation or downregulation of specific genes within a single cell type (Bramlett and Burris, 2003).
III. Vitamin D Receptor Modulators
A. Vitamin D Receptor Structure
Similar to other NRs, the 427-amino acid vitamin D receptor (VDR) protein can be functionally divided into three regions with well-characterized functions. The short amino terminus, also referred to as the A/B domain, contains the ligand-independent transactivation function AF-1. However, unlike many other NRs, the A/B domain is small, consisting of only 20 amino acids. The AF-1 region with the A/B domain is not well developed in VDR, and it remains to be determined whether this region plays a significant role in VDR-mediated transactivation (Sone et al., 1991). The central region of VDR contains the DBD consisting of two zinc-finger motifs, which target the receptor to vitamin D3 response elements (VDREs), followed by a flexible “hinge” region (D domain). This section contains the nuclear localization sequence, which allows the entry of the RXR/VDR heterodimer into the nucleus. The carboxy terminus of VDR contains a multifunctional domain harboring the LBD, the RXR heterodimerization motif, and a ligand-dependent transactivation function (AF-2). When ligand binds to VDR, a conformational change ensues, resulting in the enhancement of RXR/VDR heterodimer formation (Cheskis and Freedman, 1994; Prufer et al., 2000; Pinette et al., 2003; Sutton and MacDonald, 2003). There is only one VDR isoform encoded by a single gene in both humans and other organisms.
The amino acid residues 165–215 in the VDR LBD is an “insertion” domain that is poorly conserved between different species and does not appear to have any biologic significance. Additionally, this region has made resolving the crystal structure extremely difficult. In 2000, the Moras laboratory resolved the crystal structure of a mutant VDR LBD, lacking residues 165–215, bound to 1,25-(OH)2D3, thus proving that VDR bound this ligand and was capable of transactivation (Rochel et al., 2000). The crystal structure VDR’s LBD resembles that of other NRs, displaying a three-stranded β- and 12-α helices arranged to form three layers that completely encompass the ligand, resting in a hydrophobic core. The C-terminal helix 12 contributes to transactivation by forming the bottom portion of a surface that has a high affinity for coactivator molecules (Renaud and Moras, 2000; Xu and O’Malley, 2002). Several laboratories published follow-up studies describing the VDR LBD bound to four superagonist analogs of 1,25-(OH)2D3 or other known agonists, thereby confirming the original report (Tocchini-Valentini et al., 2001, 2004; Shaffer and Gewirth, 2002; Ciesielski et al., 2004; Vanhooke et al., 2004). All the human VDR X-ray crystal structures subsequently cited are of the mutant VDR LBD protein, as residues 165–215 form an “undefined” loop in the hinge region of domain D. This region has been removed to facilitate crystal growth, and studies have found that deletion of this region does not influence VDR LBD structure or genomic function (Rochel et al., 2001).
The crystal structure of the receptor-ligand complex has revealed many aspects of VDR biology. The β-sheet residues contact the ligand and Trp-286, which is specific to VDR, plays an essential role in positioning of the ligand (Rochel et al., 2000). The ligand-binding pocket is composed of mainly hydrophobic residues. The crystal structure has revealed that bound 1,25-(OH)2D3 curves around helix H3, with its A ring interacting with the C terminus of helix H5, and the 25-hydroxyl end is close to helices H7 and H11 (Rochel et al., 2000). Furthermore, Rochel et al. (2000) determined that the positioning of helix H12 is critical for coactivator binding and transcriptional activation of VDR, and the position of helix H12 is stabilized by a number of hydrophobic contacts and polar interactions.
Hydrogen/deuterium exchange (HDX) coupled with mass spectrometry is a rapid and sensitive approach for characterization of protein folding, protein-protein interactions, and protein-ligand interactions. This technique has provided complementary information to that gained by X-ray crystallography (Hamuro et al., 2006; Chandra et al., 2008; Hsu et al., 2009; Iacob et al., 2009). HDX was used to probe the conformational dynamics of the LBD of VDR in complex with three ligands, one being 1,25-(OH)2D3. While HDX analysis did not provide direct information about the position of H12, it did indicate that ligand binding 1,25-(OH)2D3 did stabilize this domain, thereby aiding the ability of the receptor to stimulate the classic AF-2-dependent VDR transactivation (Zhang et al., 2011).
B. Vitamin D Receptor Function
VDR has traditionally been associated with calcemic activities (modulation of calcium and phosphate homeostasis involved in the development and maintenance of bone) (Mizwicki and Norman, 2009). VDR is a ligand-dependent transcription factor with the hormonally active form of vitamin D3, the secosteroid 1α-25-dihydroxyvitamin D3 [1,25-(OH)2D3], or calcitriol as its natural ligand (Mizwicki and Norman, 2009). However, the scope of VDR biology has expanded due to the observations that VDR is present in cells other than those of the intestine, bone, kidney, and parathyroid gland leading to the conclusion that there an noncalcemic actions of VDR ligands that regulate a wide range of physiologic cellular responses including cell proliferation, differentiation, and immunomodulation.
Thirty-seven tissues are known to possess a VDR, the expression of which, coupled with the increased evidence involving VDR in processes other than mineral homeostasis, has prompted the generation and testing of therapeutic VDR ligands in inflammation, dermatological conditions, osteoporosis, cancers, secondary hyperparathyroidism, and autoimmune diseases (Corbett et al., 2006; Norman, 2008). These efforts have led to the development of VDR ligands for the treatment of psoriasis, secondary hyperparathyroidism, and osteoporosis (Nagpal et al., 2005). While the therapeutic potential for targeting VDR is immense, the limiting factor for the clinical application of VDR ligands has been the increased incidence of hypercalcemia/hypercalciuria (Cheskis and Freedman, 1994; Crofts et al., 1998; Prufer et al., 2000; Thompson et al., 2002; Dong et al., 2003). Therefore, there is an unmet clinical need for the identification of VDR ligands that exhibit an improved therapeutic index while limiting the untoward side effects. The goal of this section is to review the biologic actions of vitamin D and its analogs with future perspectives for the next generation of noncalcemic vitamin D3 analogs.
In 1919, Sir Edward Mellanby’s observation that rickets was caused by a nutritional deficiency led to the isolation of a fat-soluble antirachitic substance in fish oil and other foods that was further identified as vitamin D2 (Mellanby, 1919). Concurrently, Huldschinsky (1919) and Hess and Unger (1921) discovered that exposing children to ultraviolet (UV) light could cure them of rickets and that antirachitic activity could be induced in various foods by ultraviolet radiation. Subsequent studies led to the structural identification of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) as secosterols, which were derived from the photolytic cleavage of the B rings of ergosterol and 7-dehydrocholesterol. These two sterols were considered the biologically active forms of vitamin D until the mid-1960s when 25-hydroxyvitamin D3 [25-(OH)D3] was found to be the major circulating metabolite of vitamin D3, produced primarily in the liver. Subsequently, 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], a metabolite more polar than 25-(OH)D3, was identified and is now known to be the most active metabolite of vitamin D.
In 1968, the discovery of a high-affinity receptor for 1,25-(OH)2D3 in the intestine of vitamin D-deficient chicks further advanced vitamin D research (Haussler and Norman, 1969). This 50–70 kDa protein, found to be associated with nuclear chromatin, displayed saturable binding of 1,25-(OH)2D3 and had specificity for other vitamin D metabolites. Cloning of VDR revealed considerable similarity to other members of the NR superfamily, as it possessed the characteristic two zinc-finger motifs in the DBD. This sequence homology suggested that VDR was also a ligand-activated transcription factor. Although VDR was initially detected in classic vitamin D target organs, including intestine, bone, kidney, and parathyroid glands, all involved in mineral homeostasis, VDR has since been demonstrated to be present in many other tissues and cell types as well.
Adequate availability of vitamin D3 depends on the photochemical production of vitamin D3 in the skin plus the dietary intake of vitamin D3. Few food sources naturally contain significant amounts of vitamin D2 and D3, but many foods are now fortified with the vitamin, so minimum daily requirements are easily met. Vitamin D3 does not have significant biologic activity and must be metabolized to its active form 1,25-(OH)2D3, which occurs in a two-step process. The first step toward the activation of vitamin D3 occurs primarily in the liver, where vitamin D3 is hydroxylated at carbon 25 by 25-hydroxylase, yielding 25-hydroxycholecalciferol [25(OH)D3], or calcidiol. The resulting 25(OH)D3 is the more stable metabolite and is transported by the vitamin D-binding protein (DBP) to the kidneys for the final step in vitamin D bioactivation, where 25(OH)D3 serves as a substrate for the 25(OH)2D3-1α-hydroxylase enzyme. The conversion of 25(OH)D3 results in the steroid hormone 1,25-(OH)2D3, or calcitriol, the active circulating metabolite, which is released into the general circulatory system (Henry and Norman, 1974; Bikle et al., 1975; Goltzman et al., 2004; Chlon et al., 2008). DBP also delivers 25(OH)D3 to at least 10 other organs and tissues that have low levels of the 25(OH)2D3-1α-hydroxylase enzyme, which results in the paracrine or local production of 1,25-(OH)2D3 (Norman, 2008). This restricted production produces limited quantities of 1,25-(OH)2D3 in the local environment to regulate a given biologic effect. The potency of 1,25-(OH)2D3 requires the circulating levels to be tightly regulated (Dreier et al., 2008; Nemere and Hintze, 2008). Control of serum usually involves joint reciprocal changes in the rate of synthesis and degradation. Collectively, the VDR-containing tissues define locations where 1,25-(OH)2D3 can initiate biologic processes via receptor-ligand complexes to produce biologic responses through genomic mechanisms.
The natural ligand for VDR is the conformationally flexible secosteroid 1,25-(OH)2D3, which binds VDR with an affinity KD in the range of 0.1 nM to 5 nM (Wecksler and Norman, 1980a,b; Wecksler et al., 1980a,b). The parent vitamin D3 binds to VDR with an affinity of >100 μM. VDR functions as a heterodimer with another NR, the RXR. RXR, a nuclear receptor for 9-cis-retinoic acid, is an obligate partner of VDR in mediating 1,25-(OH)2D3 action (Yu et al., 1991; Kliewer et al., 1992; Pinette et al., 2003; Sutton and MacDonald, 2003). In the absence of ligand, the majority of VDR is present in the cytoplasm (Barsony et al., 1990). Upon ligand binding, VDR undergoes a conformational change that promotes RXR-VDR heterodimerization and complex nuclear translocation (Cheskis and Freedman, 1994; Prufer et al., 2000). Once in the nucleus, the RXR-VDR complex binds to VDREs present in the promoter regions of responsive genes. Canonical VDREs are a direct repeat of 5′-AGG/TTCA-3′ motifs or a minor variation of this motif separated by three nucleotides and commonly referred to as direct repeat-3 motifs. Upon binding to VDREs on VDR target genes, the ligand-bound heterodimer recruits or dissociates the coactivator/corepressor proteins that ultimately modulate the transcriptional activity of the complex. The molecular details surrounding much of RXR/VDR transactivation have been described, including the chromatin environment, interacting protein partners, and temporal kinetics, all of which are highly diverse, depending on the genomic target and cellular context in which the regulation is occurring (Meyer et al., 2007).
Ligand binding increases the RXR/VDR heterodimer interaction with coactivators, transcriptional proteins that mediate induction of gene transcription. Ligand binding induces a conformational change in the receptor, creating a hydrophobic cleft that renders NRs receptive to coactivator binding through their NR boxes, distinct amino acid sequences (LXXLL motifs). Unable to bind DNA itself, coactivator proteins enhance transcriptional activity through a range of enzymatic activities and protein-protein interactions. Several coactivators have been identified that interact with VDR, including those of the steroid receptor coactivator family (SRC1, SRC2, and SRC3) as well as CBP [cAMP response element binding protein (CREB) binding protein]/p300, pCAF (p300/CBP-associated factor), and thyroid receptor interacting protein 1/Sug1, to name a few (Lee et al., 1995; Hermanson et al., 2002). VDR also directly interacts with certain components of the basal transcriptional machinery, including TF-IIB, TF-IIA, and TAF (Lavigne et al., 1999; Mengus et al., 1997, 2000; Barry et al., 2003).
A multiprotein complex that functions as a transcriptional coactivator for VDR is the vitamin D interacting protein (DRIP) complex (Rachez et al., 1998). The RXR/VDR heterodimer recruits the DRIP complex by ligand-mediated recruitment of DRIP 205, a component of the complex. Ligand-dependent targeted recruitment of the VDR-DRIP and VDR-SRC complex occurs in a sequential manner. The VDR-SRC complex is recruited to the VDR responsive promoter to promote the destabilization of the nucleosomal core, allowing the VDR-DRIP complex to bind to the unwound DNA and interact with basal transcriptional machinery (Rachez et al., 2000).
In the absence of ligand, corepressor proteins bind VDR, which results in chromatin compaction and gene silencing. Three corepressors, NCoR-1, NCoR-2, and Hairless, have been found to interact with VDR (Hermanson et al., 2002). Motifs related to NR boxes are present in the corepressors called CoRNR boxes (I/LXXI/VI motifs). These motifs are shown to be essential for the interaction of corepressors with unliganded NRs (Hu and Lazar, 1999; Webb et al., 2000; Hu et al., 2001). The number of cofactors and corepressors identified to date gives insight into the complexity of VDR activity and have increased our understanding of tissue- and gene-selective transcription mediated by VDR and its natural and synthetic ligands.
By its very definition, a transcription factor modulates gene expression, and VDR is no exception to that rule. However, in addition to its ability to positively regulate the expression of genes, VDR can also negatively regulate the expression of other genes and antagonize the action of other transcription factors. Genes that are positively regulated by VDR ligands include those that regulate extracellular bone matrix formation, bone remodeling, adhesion molecules, differentiation, antiproliferation, and metabolism. Interestingly, genes known to be downregulated in response to VDR ligands are involved in hyperproliferation and anti-inflammatory functions, and may indicate why VDR ligands have therapeutic effects in inflammatory diseases. VDR ligands have been demonstrated to inhibit the expression of cytokines and chemokines, including IL-12, IL-2, tumor necrosis factor α (TNFα), and interferon γ (INF-γ), to name a few (Nagpal et al., 2005). Genes involved in mineral homeostasis, including PTH and PTH-related peptide (PTHrP), are also downregulated by VDR ligands (Kremer et al., 1991; Falzon, 1996). Negative regulation of PTH, PTHrP, and Rel B appears to occur through a negative DNA motif called a negative VDRE (nVDRE) (Demay et al., 1992; Nishishita et al., 1998; Dong et al., 2003). Finally, VDR can compete with nuclear factor of activated T cells-1 (NFAT1) for binding to the NFAT1–activator protein (AP-1) enhancer motif where it then interacts with c-Jun. The displacement of AP-1 leads to the inhibition of gene expression. Both RXR/VDR heterodimers and VDR monomers have been demonstrated to be involved in the inhibition (Alroy et al., 1995; Harant et al., 1997; Takeuchi et al., 2002).
Vitamin D metabolism is strictly regulated to perform its principle function: govern calcium and phosphate homeostasis. This regulation occurs in the “classic” mineral-regulating organs: the intestine, bone, parathyroid glands, and kidneys. Elegant genetic studies in VDR-deficient mice have provided insight into the physiologic function of VDR as well as confirmed its crucial role in the regulation of bone development. Although they are phenotypically normal at birth, VDR−/− mice develop hypocalcemia, hyperparathyroidism, osteomalacia (rickets), and alopecia after weaning (Li et al., 1997b; Yoshizawa et al., 1997; Kato et al., 1999; Van Cromphaut et al., 2001; Zeitz et al., 2003). These mice die between 4 and 6 months of age. However, all symptoms are abolished in these animals when they are fed a diet rich in calcium phosphate and lactose, with the exception of the hair abnormalities. These findings suggest that intestinal calcium absorption is critical for 1,25-(OH)2D3 action on bone and calcium homeostasis.
We will summarize the role of vitamin D and VDR activity in the classic mineral-regulating organs. The intestine absorbs dietary calcium and phosphate. This occurs along the length of the entire intestine, but calcium transport occurs primarily in the duodenum and phosphate absorption in the jejunum and ileum. The absorption of calcium is facilitated by the presence of bile salts, which increase the absorption efficiency to 50%. The most crucial function of the active form of vitamin D in mineral homeostasis is to enhance the small intestine’s efficiency in absorbing dietary calcium and phosphate. Both hypocalcemia and hypophosphatemia increase the production of 1,25-(OH)2D3, thereby increasing the intestine’s efficiency in absorbing calcium and phosphate. Vitamin D deficiency causes calcium malabsorption and a negative calcium and phosphate balance. In addition, 1,25-(OH)2D3 directly stimulates intestinal calcium and phosphate transport by acting on duodenal enterocytes to induce the calcium transport protein 1, which channels calcium from the intestinal lumen into the cell (Bronner, 2003).
In skeletal development, once the epiphyses and metaphyses fuse, longitudinal growth ceases. From this point forward, bone mineralization and turnover occur to maintain skeletal strength and integrity. Vitamin D is essential for the development and maintenance of a mineralized skeleton. Bone is one of the major target organs of vitamin D, and VDR ligands regulate bone formation and resorption. Vitamin D deficiency results in rickets in the young and osteomalacia in adults. Also, 1,25-(OH)2D3 stimulates the mobilization of calcium stores from bone by inducing the dissolution of bone mineral and matrix (Holick, 1996). Therefore 1,25-(OH)2D3 can enhance bone formation and growth. The primary target cells of 1,25-(OH)2D3 are osteoblasts and osteoblast precursors. The effects of 1,25-(OH)2D3 are mediated by VDR and lead to the expression of several genes associated with osteoblast proliferation and differentiation.
VDR is expressed at high levels in primary osteoblasts and various osteoblast cell lines. Thus, 1,25-(OH)2D3 is classically considered to be a stimulator of bone resorption because it induces osteoclastogenesis by enhancing the expression of receptor activator of NF-κB ligand (RANKL) in bone marrow and stromal cells (Suda et al., 1999). Additionally, the human and mouse RANKL promoter contains a functional VDRE that demonstrates RXR-VDR heterodimer-mediated ligand-dependent activation (Kitazawa and Kitazawa, 2002; Kitazawa et al., 2003).
Given the profound role of VDR in bone formation; it is no surprise that vitamin D insufficiency, particularly in the elderly, results in osteoporosis and is thought to be a major factor in fracture risk (Chapuy et al., 1997). However, there is a large body of evidence suggesting that VDR plays a role in regulating the neuromuscular system, and that a combination VDR action in bone and muscle increases the risk of falls and fractures in the elderly (Fraix, 2012). Support for VDR in muscle comes from analyses of myoblast cell lines generated from VDR−/− mice. These studies revealed that VDR−/− mice had fiber sizes 20% smaller than wild-type controls. VDR−/− mice also exhibited increased expression of the myogenic transcription factors myf5, E2A, and myogenin (Endo et al., 2003). These findings support a direct role for VDR transcriptional regulation of skeletal muscle. Because calcium is a critical modulator of skeletal muscle function, it is reasonable to hypothesize that VDR would have a significant impact on muscle function and fracture risk (Hamilton, 2010).
The parathyroid glands act as a calcium sensor in the body and hence play a central role in calcium homeostasis. The parathyroid glands sense the circulating levels of calcium in the body and secrete PTH, a calciotropic hormone that stimulates the release of calcium from bone. PTH can stimulate 1,25-(OH)2D3 synthesis in the kidneys, whereas calcium is the dominant regulator of PTH synthesis and secretion. Therefore, PTH and calcium regulate the synthesis and secretion of each other and act as an important feedback loop in regulating calcium homeostasis.
In the kidney, vitamin D regulates calcium and phosphate resorption and controls its own synthesis and degradation. Perhaps the most important effect of 1,25-(OH)2D3 in the kidney is the suppression of 1α-hydroxylase activity and the stimulation of 24-hydroxylase activity. This homeostatic feedback loop ensures that the proper amount of 1,25-(OH)2D3 will be released by the kidney. Under conditions of hypocalcemia or hypophosphatemia, renal VDR expression is decreased to prevent feedback until mineral levels are normalized.
C. Vitamin D Receptor Modulators
Osteoporosis is a common metabolic disease characterized by decreased bone mass due to deterioration of bone tissue and loss of spatial architecture, thus resulting in increase bone fragility and risk of developing fractures. Osteoporosis involves the loss of both organic and mineral contents of the bone. Various conditions that can lead to osteoporosis include estrogen or androgen deficiency, glucocorticoid excess, hyperthyroidism, hyperparathyroidism, and/or severe inactivity. A number of antiresorptive agents that treat these various conditions are currently on the market and prevent further bone loss, but they do not rebuild bone once it has been lost. Currently, there is only one FDA-approved anabolic bone-building agent for the treatment of osteoporosis, recombinant human parathyroid hormone (1-34) [rhPTH(1-34)], also known as teriparatide. This agent rebuilds bone mass and restores bone architecture while reducing the risk of vertebral and nonvertebral bone fractures in osteoporotic patients (Neer et al., 2001). However, teriparatide use is limited because daily subcutaneous injections are required, which may prove difficult for the elderly. Additionally, its high cost, the warnings regarding osteosarcoma, and the restriction of therapy to a maximum 2 years adds to this drug’s limitations. Therefore, the need for bioactive bone-building agents with reduced side effects persists.
A second, new antiresorptive agent emerged from the discovery that RANKL is the principal regulator of osteoclastic bone resorption. From this discovery came the development of denosumab, a fully human monoclonal antibody to RANKL (McClung et al., 2006a). This agent, which is administered at 60 mg subcutaneously every 6 months, has recently received regulatory approval for the treatment of women with postmenopausal osteoporosis who are at high risk for fracture (Lewiecki et al., 2010). Denosumab has been shown to inhibit the resorptive component of the bone-remodeling system, increase BMD, and reduce the risk of vertebral fractures, hip fractures, and nonvertebral fractures in women with postmenopausal osteoporosis (Hattner et al., 1965; Gallagher and Sai, 2010).
The most commonly used osteoporosis treatments are antiresorptive agents, including bisphosphonates and selective estrogen modulators (Lewiecki et al., 2010). Less effective antiresorptive agents include calcitriol, 1-α-hydroxyvitamin D3 [1α(OH)D3] (Fig. 8), and 1α,25-dihydroxyvitamin D3 [1,25-(OH)2D3]. VDR ligands regulate both bone formation and resorption, and 1α,25-(OH)2D3 increases the expression and/or protein levels of osteocalcin and osteopontin in osteoblasts, thus supporting a role in bone matrix formation. Therefore, designing drugs to target VDR for the treatment of osteoporosis and osteomalacia is a viable option. However, the use of VDR ligands is limited by its margin of safety, as there is a high risk of developing side effects such as hypercalcemia and hypercalciuria. These side effects result from increased calcium absorption in the intestine, leading to increased plasma and urine levels of calcium that can ultimately result in the mineralization of soft tissue and kidney stone formation. Osteoporosis is a debilitating disease, with a growing prevalence among the elderly population. Therefore, there is an urgent need for bone-building agents that are orally bioavailable yet have reduced side effects (Nagpal et al., 2005).
Fig. 8.
VDR modulators.
Several VDR ligands, both natural and synthetic, have been used for the treatment of osteoporosis and osteomalacia, including calcitriol, alfacalcidol, ED-71 [1α,25-dihydroxy-2β-(3-hydroxypropoxy) vitamin D3], Ro-26-9228 [1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3], and 2MD [2-methylene-19-nor-(20S)-1α,25(OH)2D3] (Fig. 8). These ligands enhance their beneficial bone anabolic effects by enhancing intestinal calcium absorption and by inhibiting the synthesis of PTH. Several reports have demonstrated the prevention and decrease of vertebral fractures and an increase in total body and spine BMD in osteoporotic patients after calcitriol (1,25-(OH)2D3) treatment. However, 2 years of calcitriol treatment resulted in a 57% increase in intestinal calcium absorption, a 100% increase in urinary calcium, and a 32% decrease in PTH serum levels. Several of the patients treated with calcitriol also developed hypercalciuria. Clearly, the treatment of osteoporosis with calcitriol is limited by its margin of safety, which appears to be very narrow (Sairanen et al., 2000).
Prodrug and medicinal chemistry approaches have been explored in an effort to identify less calcemic vitamin D3 analogs or VDRMs (selective VDR modulators) that are suitable for the treatment of osteoporosis. Using the prodrug approach, alfacalcidol (1α-hydroxyvitamin D3), a precursor to 1,25-(OH)2D3, was identified. This precursor gets enzymatically converted to the active hormone 1,25-(OH)2D3 in the liver by the action of 25-hydroxylase. Alfacalcidol is superior to 1,25-(OH)2D3 because enterocytes, the intestinal absorptive cells found in the small intestine and colon, lack 25-hydroxylase. Therefore, alfacalcidol’s actions are reduced compared with calcitriol because it does not induce intestinal calcium absorption in the first pass when it is absorbed from the intestine. It was determined that in vivo, this analog is converted rapidly to 1,25-(OH)2D3 in both the liver and bone. Thus, treatment with alfacalcidol reduced the incidence of vertebral fractures and increased bone mass in several clinical trials (Lau and Baylink, 1999). The success of this drug is believed to be the result of the ability to administer higher doses of alfacalcidol than calcitriol before hypercalcemia occurs (Lau and Baylink, 1999; Shiraishi et al., 1999).
Calcitriol and alfacalcidol (Fig. 8) are currently used in Japan for treatment of osteoporosis. Clinical evidence of improvement of fracture rates after alfacalcidol and 1,25-(OH)2D3 treatment has been published, although the results from these studies are still being debated (Orimo et al., 1987; Gallagher et al., 1989; Ott and Chesnut, 1989; Gallagher and Goldgar, 1990; Papadimitropoulos et al., 2002; O’Donnell et al., 2008). This mode of treatment is viable in Japan and other countries outside the United States because their diets have modest calcium intake, making the side effects more manageable and hypercalcemia and hypercalciuria less common. In a controlled environment, the use of 1,25-(OH)2D3 and alfacalcidol incurs few side effects and can effectively treat osteoporosis.
Not only does the risk of osteoporosis increase with age, it is well recognized that muscle strength declines with age (Iannuzzi-Sucich et al., 2002). This inverse correlation has led to many cross-sectional studies evaluating the role of vitamin D in moderating the age-related decline in muscle function and the increased risk of falls. One study found that reduced 1,25-(OH)2D3 levels correlated with an increased risk of falls in the elderly (Gerdhem et al., 2005). Another study demonstrated a dose-dependent (800 U/day) impact of vitamin D supplementation with the reduction of fall rates in the elderly (Broe et al., 2007). Several other studies have demonstrated similar benefits with vitamin D supplementation (Hamilton, 2010). However, the direct effect of vitamin D on the neuromuscular system in aged individuals has yet to be evaluated. Some postulate that the effect of vitamin D on muscle may be due to parathormone and not to a direct action of vitamin D on muscle (Annweiler et al., 2010). This mechanism needs to be addressed, but it is still conceivable that use of VDR ligands may present more benefit in the treatment of osteoporosis than originally thought by acting on both bone and muscle.
Several synthetic analogs of 1,25-(OH)2D3 have been developed in hopes of reducing the occurrence of hypercalcemia. One such analog, ED-71 [1α,25-dihydroxy-2β-(3-hydroxypropoxy) vitamin D3] (Fig. 8), bears a hydroxypropoxy substituent at the 2β-position. This analog has one-eighth the binding affinity to VDR and a 2.7-fold greater affinity for DBP (Kubodera et al., 2003). In studies using normal, ovariectomized, and prednisolone-treated rats, ED-71 increased calcium resorption in the gut, decreased bone resorption, and increased bone mineralization (Tanaka et al., 1996; Ono et al., 1998). The osteopenic ovariectomized (OVX) rat is a model of postmenopausal osteoporosis that has been predictive of clinical efficacy for potential therapeutics and is required by the regulatory agencies for consideration of new therapies. ED-71 has been found to be as effective as PTH in several rat studies using OVX rats. In a 5-week trial, a dose of 0.08 μg/kg per day of ED-71 decreased bone resorption and increased bone mass without inducing hypercalcemia. In phase 1 clinical trials, ED-71 given orally at doses of 0.1–1.0 μg in healthy human male volunteers for 15 days resulted in a dose-dependent increase in urinary calcium with no sustained hypercalcemia more than 10.4 mg/dl or hypercalciuria more than 400 mg/day. As a result of this trial, a second phase 2 trial with ED-71 began. Varying doses of ED-71 (0.25, 0.5, 0.75, and 1.0 μg/day) were administered for 24 weeks to osteoporosis patients. There was a dose-dependent increase in lumbar spine and hip BMD with effects better than those obtained from estrogen-treated patients or results from studies using alfacalcidol or 1,25-(OH)2D3. Ultimately ED-71 was well tolerated, and a clinical dose of 0.75 μg/day was effective (Kubodera et al., 2003).
One of the first tissue- and cell-type selective secosteroidal VDRMs identified was Ro-26-9228 (1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3) (Fig. 8). This analog was identified based on the observation that specific structural changes to 1,25-(OH)2D3 led to alterations of VDR transcriptional output (Peleg et al., 2002). In an OVX rat model of osteoporosis, Ro-26-9228 demonstrated 17- to 27-fold improved therapeutic index over 1,25-(OH)2D3 (Peleg et al., 2002), leading to a 3-fold separation between BMD effect versus hypercalciuria over 1,25-(OH)2D3. More importantly, Ro-26-9228 was less potent than 1,25-(OH)2D3 in inducing the expression of 24-hydroxylase, calbindin D-9k, and calcium pump 1 in the duodena of OVX rats but was as efficacious as 1,25-(OH)2D3 in enhancing the expression of osteocalcin, osteopontin, TGFβ1, and TGFβ2 in trabecular bone (Peleg et al., 2002, 2003). An increase in BMD was accompanied by a decrease in type I collagen degradation products in the urine. Tissue selective action was observed, as Ro-26-9228 was less potent than 1,25-(OH)2D3 in inducing the expression of a VDRE-containing reporter in intestinal Caco-2 cells, but both compounds were equally efficacious in osteoblastic MG-63 cells (Peleg et al., 2002, 2003). Further characterization of Ro-26-9228 revealed that its preference for osteoblasts over intestinal cells was based on differences in the ability of the VDRs from these two cell types to recruit coactivators to the transcriptional complex (Ismail et al., 2004).
Another vitamin D analog, 2MD [2-methylene-19-nor-(20S)-1α,25(OH)2D3], which was modified at the 2-carbon position of the A ring, is highly potent, exhibiting an affinity for VDR equal to that of 1,25-(OH)2D3 (Sicinski et al., 1998) (Fig. 8). This analog has been found to be potent stimulator of bone formation in vitro and in vivo, with a preferential activity on bone over intestine (Sicinski et al., 1998). Analyses have found that 2MD is at least 30-fold more effective than 1,25-(OH)2D3 in stimulating osteoblast-mediated bone calcium mobilization, while being only slightly more potent in intestinal calcium transport. Over a 23-week period, 2MD (7 pmol/day) caused a 9% increase in total body bone mass in OVX rats whereas 1,25-(OH)2D3 (500 pmol, 3 times a week) only prevented bone loss and did not facilitate an increase in bone mass (Shevde et al., 2002). Of great importance is the fact that 2MD, at concentrations of 0.01 nM, stimulated osteoblastic bone formation, whereas 1,25-(OH)2D3 at 100 nM did not (Shevde et al., 2002). Further work characterizing 2MD revealed that it stimulates the expression of several vitamin D-sensitive genes, including 25-hydroxyvitamin D3-24 hydroxylase (Cyp24), osteopontin, and RANKL, while suppressing osteoprotegerin at concentrations 2 logs lower than 1,25-(OH)2D3 (Yamamoto et al., 2003). Also, 2MD was more potent than 1,25-(OH)2D3 at inducing the interaction of VDR with RXR and the coactivators SRC1 and DRIP205, suggesting that the potency of 2MD is due to its ability to enhance specific DNA binding to VDR (Yamamoto et al., 2003). This enhancement is due to the modification of carbon 20 stereochemistry to the unnatural S-configuration. Further studies have confirmed that this configuration, in the presence of a full-length side chain, improves binding of specific proteins to the VDR transcriptional complex (Schwinn and DeLuca, 2007). Furthermore, replacement of the 20-methyl with hydrogen did not affect VDR binding or transcriptional activity, but it did eliminate the mobilization of calcium from bone while leaving intestinal calcium transport intact (Barycki et al., 2009). A clinical trial was performed on groups of osteopenic women (placebo, 220 ng of 2MD, and 440 ng of 2MD) to measure the effect of daily oral treatment with 2MD on BMD, serum markers of bone turnover, and safety for 1 year. Although 2MD was generally well tolerated, the results were contrary to those obtained in OVX rats. Treatment with 2MD in osteopenic women did not change BMD, although it did increase markers of both bone formation and bone resorption. Although the lack of change in BMD was discouraging, the investigators concluded that 2MD likely stimulated both bone formation and bone resorption, thus increasing bone remodeling (DeLuca et al., 2011).
To date, most of the synthetic VDR ligands generated and used clinically have a secosteroidal backbone and exert their effects on several tissues, including those that can lead to hypercalcemia, as seen by administration of VDR ligands resulting in hypercalcemia by increasing calcium absorption from the intestine. Nonsteroidal structures have provided SERMS that are agonist in bone and transcriptionally inactive (antagonist) in breast and uterine cells, therefore, synthesis of VDR ligands that were tissue selective was warranted (Lin and Huebner, 2000; Smith and O’Malley, 2004). Ma et al. (2006) describe the synthesis of two nonsecosteroidal analogs of vitamin D, the VDRMs LY2108491 and LY2109866 (Fig. 8), which function as potent and efficacious agonists to VDR in keratinocytes, human peripheral blood mononuclear cells (PBMCs), and osteoblasts, but exhibited attenuated transcriptional activity in intestinal cells. In vivo, these drugs presented with reduced hypercalcemia as well as an improved therapeutic index relative to 1,25-(OH)2D3. Compared with other vitamin D analogs, LY2108491 and LY2109866 were more tissue selective than Ro-26-9228 (Peleg et al., 2002, 2003).
Although many of the analogs presented here have proven efficacy over calcitriol and alfacalcidol, they still have been shown to elevate calcium in sera and in urine in a dose-dependent manner, leading to concerns about hypercalcemia and hypercalciuria. In addition, these agents are not orally bioavailable and do not rebuild bone once it has been lost. With this in mind, Sato et al. (2010) identified VDRM2, a nonsecosteroidal, tissue-selective, orally bioavailable VDR ligand. This analog induced VDR–RXR heterodimerization (EC50 7.1 nM) and stimulated the expression of the bone genes (osteocalcin) in a manner similar to ED-71 and alfacalcidol. Using the osteopenic OVX rat model, VDRM2 was evaluated 5 times in an 8-week study. It was generally well tolerated and restored bone mass, spatial architecture, and bone strength, but it was not as potent as ED71 or 1,25-(OH)2D3. However, hypercalcemia was not observed in animals until 4.6 μg/kg, indicating a therapeutic safety margin of 57-fold between bone efficacy and hypercalcemia, which is significantly greater than ED-71, 1,25-(OH)2D3, and alfacalcidol (Sato et al., 2010). If the data from this study are relevant to the clinical trials with ED-71, VDRM2 may have clinical efficacy to treat osteoporosis as well as other disorders, either alone or in combination with other approved therapies.
Secondary hyperparathyroidism (SHPT) refers to the excessive secretion of PTH in response to low blood calcium levels. Suboptimal levels of vitamin D lead to a reduction in intestinal calcium absorption, increased PTH production, and parathyroid cell proliferation (Sprague and Coyne, 2010). This disorder is prevalent in patients with chronic renal failure. Control of SHPT is important in managing the course of chronic kidney disease (CKD). Studies have demonstrated that if the hypocalcemia can be addressed, SHPT will resolve.
Inactive forms of vitamin D, ergocalciferol and cholecalciferol, have been demonstrated to significantly increase 25-dihydroxyvitamin D and 1,25-(OH)2D3 levels in patients with stages 3 and 4 CKD while suppressing, but not normalizing PTH levels (Al-Aly et al., 2007; Chandra et al., 2007; Zisman et al., 2007). However, as CKD progresses, the ability of these vitamin D supplements to suppress SHPT is reduced (Al-Aly et al., 2007; Zisman et al., 2007). Additionally, the biologically active VDR agonists, calcitriol and paricalcitol, suppress PTH in a dose-related fashion regardless of the stage CKD. Paricalcitol is an analog of calcitriol but presents fewer calcemic and phosphatemic effects. In a randomized, prospective, phase 3 study, 0.04–0.24 μg of IV paricalcitol for 32 weeks, showed similar or improved PTH suppression and fewer hypercalcemic episodes compared with 0.01–0.06 μg of IV calcitriol (Sprague et al., 2003).
Doxercalciferol (Fig. 8), a prohormone that can be converted in the liver into a VDR agonist, was originally investigated as a treatment of osteoporosis, but it induced hypercalcemia at higher doses, precluding it from use for osteoporosis (Gallagher et al., 1994). However, doxercalciferol later demonstrated its efficacy by reducing PTH in hemodialysis patients, albeit with elevated serum calcium and phosphorus levels (Frazao et al., 2000; Maung et al., 2001). Thus, the use of VDR agonists can suppress SHPT in CDK patients and is associated with significantly better survival. Use of some VDR agonists over others is warranted, as episodes of hypercalcemia and/or hyperphosphatemia were more frequent in calcitriol recipients (Kovesdy et al., 2008; Shoben et al., 2008).
CKD leads to increased cardiovascular mortality in nondialyzed patients. Studies evaluating patients with stage 3 and 5 CKD have demonstrated excessive coronary artery calcification (Kramer et al., 2005). One mechanism by which VDR agonists inhibit vascular calcification is through inhibition of the inflammatory response associated with the calcification process. The effects of VDR activation on inflammation will be discussed in greater detail later in this review. A second mechanism is currently evolving regarding the effects of VDR agonists on vascular calcification. This mechanism involves an imbalance between calcification inhibitors and activators in serum and its effect on the differentiation of specific cells. As vascular smooth muscle cells and osteoblasts are derived from a similar mesenchymal precursor cell, the increased uremic toxins present in the serum of dialysis patients may lead to this imbalance and drive the differentiation of the mesenchymal precursors toward a more osteoblast cell type. VDRs are present in vascular smooth muscle cells, and activation of these receptors has been shown to inhibit the synthesis of type 1 collagen (Bellows et al., 1999). Therefore, VDR agonists may help restore the balance between inhibitory factors and inducing factors present in vessels and the circulation.
Decreased vitamin D activity increases renin expression, renin levels, atrial natriuretic peptide levels, and angiotensin II levels, and causes hypertension and cardiac myocyte hypertrophy in mouse models. Indeed, VDR−/− mice also showed defects in the renin-angiotensin system (Li et al., 2002). These data suggest that VDR is a negative regulator of this system and may play a critical role in blood pressure homeostasis. Thus, intravenous treatments with calcitriol (twice weekly, 2 mg) in patients on hemodialysis caused regression of myocardial hypertrophy (Kim et al., 2006a). Furthermore, treatment of nephrectomized rats with paricalcitol was associated with suppression of renin, renin receptor, angiotensin, and angiotensin II type I receptors (Aihara et al., 2004). Therefore, hypertension and the deterioration of renal function were significantly improved with treatment of VDR agonists.
It has been well established that the active form of vitamin D, 1,25-(OH)2D3, plays a central role in calcium and bone regulation. However, recent evidence suggests that vitamin D exerts many immunomodulatory functions. Cells other than those of the intestine, bone, and kidney possess VDR, 1α-hydroxylase, and 24-hydroxylase, including immune cells, which can produce the active form of vitamin D and respond in an autocrine or paracrine fashion. In fact, many animal studies and early epidemiologic and clinical studies support a role for VDR action in maintaining the immune balance. The effects of 1,25-(OH)2D3 on the immune system include decreased TH1/TH17 CD4+ T cells and cytokines, increased T-regulatory cells, downregulation of T cell-cell driven IgG production, and inhibition of dendritic cell differentiation (Kamen and Tangpricha, 2010).
Vitamin D has been used as a form of treatment for infection for over 150 years, beginning with the observation that cod-liver oil, an excellent source of vitamin D, induced favorable results in patients with tuberculosis (Kamen and Tangpricha, 2010). Since that time, vitamin D and its analogs have been proven to be widely effective in treating various inflammatory conditions, some of which we will describe here.
Multiple sclerosis, a chronic autoimmune disease of the CNS, is characterized by inflammatory cell infiltration and subsequent axonal demyelination in localized areas, known as multiple sclerosis lesions. Experimental autoimmune encephalomyelitis (EAE) is a widely used animal model for human multiple sclerosis as EAE presents with similar pathologies to the human disease. Specific self-antigen reactive T-cell subsets, T helper 1 (TH1) and T helper 17 (TH17), have been demonstrated to play a critical role in both the induction and onset of the disease. TH1 and TH17 cell differentiation is controlled by antigen stimulation and cytokines, particularly IL-12 or TGFβ and IL-6, which subsequently drives the TH1 or TH17 specific transcription factors T-bet (T-box expressed in T cells) or RORα and RORγt (retinoic acid receptor–related orphan receptor), respectively. Therefore, controlling and or inhibiting TH1 and TH17 cell development would be beneficial in the treatment of multiple sclerosis. With their regulatory effects on cell proliferation, VDR ligands are an attractive strategy for the control of these T helper subsets.
Vitamin D3 has a crucial effect on the immune response. In fact, several groups have established that 1,25-(OH)2D3 inhibits both TH1 and TH17 cell differentiation in vitro (Mattner et al., 2000; Chang et al., 2010a). Using the EAE model, in vivo use of 1,25-(OH)2D3 (3 μg/kg) inhibited both TH1 and TH17 mediated disease induction, as evidenced by decreased inflammatory infiltration and reduced demyelination of the brain and spinal cord (Mattner et al., 2000; Chang et al., 2010a). In addition, 1,25-(OH)2D3 has been shown to inhibit macrophage accumulation in the CNS during EAE development; thus, 1,25-(OH)2D3 is acting on various cell types, leading to the protective effects seen after administration of vitamin D3 (Nashold et al., 2000).
Despite the beneficial effects seen with vitamin D3 treatment in the EAE model, marked hypercalcemia was observed in the treated mice, which is a major impediment in the clinical development of vitamin D3 and its analogs for the treatment of autoimmune diseases. In an attempt to advance these findings toward a more clinical use, one group identified compound A, a nonsecosteroidal VDRM, that was transcriptionally less active in intestinal cells yet retained its modulatory effects on T-helper cells. Daily oral administration of compound A to mice with EAE (10 μg/kg per day) led to inhibition of the induction and progress of the disease compared with treatment with 1,25-(OH)2D3 (0.05 μg/kg per day). Analysis of spinal cords from the mice demonstrated that there were reduced demyelination areas compared with the vehicle control. More importantly, serum calcium levels were within the normal range after compound A treatment versus 1,25-(OH)2D3. Ex vivo stimulation of total splenocytes from diseased mice revealed that compound A inhibited both TH1 and TH17 cell differentiation (Na et al., 2011).
Irritable bowel disease (IBD), including ulcerative colitis and Crohn’s disease, is an immune-mediated disorder of unknown etiology that affects the gastrointestinal tract. Specifically, proinflammatory cytokine–producing T cells are associated with IBD in humans (Aranda et al., 1997; Bregenholt and Claesson, 1998). Several mouse models of spontaneous and induced colitis have demonstrated that VDR expression is required to control inflammation. To this end, treatment with 1,25-(OH)2D3 has been shown to ameliorate spontaneous colitis by direct and indirect inhibition of TNFα (Zhu et al., 2005) in a mouse model of inflammatory bowel disease. Furthermore, in a pilot clinical study of IBD patients, the VDR agonists alfacalcidol (twice daily doses of 0.25 μg) and cholecalciferol (1000 U daily) had proven short-term beneficial effects on bone metabolism and disease severity after 1 year of administration (Miheller et al., 2009). By performing modifications in the side chain of 1,25-(OH)2D3, one group described the VDR agonist ZK156979 (22-ene-25-oxa-vitamin D), which at normocalcemic doses effectively improved the symptoms of colitis induced by 2,4,6-trinitrobenzene sulfonic acid by inhibiting TNFα production and increasing levels of anti-inflammatory cytokines. In vivo experiments performed in rats showed that ZK156979 exhibited 100-fold lower hypercalcemic activity than 1,25-(OH)2D3 (Daniel et al., 2005, 2006).
Use of another VDR analog, TX527 [19-nor-14,20-bisepi-23-yne-1,25(OH)2D3], proved efficacious at inhibiting cell proliferation and TNFα production in vitro in human PBMCs from Crohn’s disease patients (Stio et al., 2007). Through introduction of two moieties in 1,25-(OH)2D3, 20-cyclopropyl or 16-ene, one group characterized a potent anti-inflammatory VDR agonist, BXL-62 [1α,25(OH)2-16-ene-20-cyclopropyl-vitamin D3]. BXL-62 resulted in a marked increase in anti-inflammatory cytokines in PBMCs from healthy subjects in vitro and did not induce hypercalcemia in mice after 4 days of oral treatment (1 μg/kg) (Laverny et al., 2009). Further analysis of this analog demonstrated in vitro inhibition of proinflammatory cytokines in the PBMCs and lamina propria mononuclear cells from patients with IBD while still showing the capacity to induce VDR primary response genes, including CYP24A1 and CAMP, at lower concentrations than 1,25-(OH)2D3. In vivo analysis demonstrated amelioration of experimental colitis (1 μg/kg daily for 4 days) (Laverny et al., 2010). Currently, the clinical use of 1,25-(OH)2D3 and its analogs has been hindered by its adverse effects on calcium homeostasis. Perhaps the generation of or use of novel VDR analogs similar to those we have described will yield more promising therapeutics for the treatment of IBD.
Psoriasis is a recurrent, inflammatory skin disorder affecting approximately 2% of the population. Additionally, a small percentage of psoriasis sufferers develop psoriatic arthritis, with inflammation and swelling in the hands, feet, and joints. Psoriasis is characterized by keratinocyte hyperproliferation, abnormal keratinocyte differentiation, and immune cell infiltration, specifically CD8+ T cells and CD4+ T cells, into the epidermis and dermis, respectively (Nagpal et al., 2005). The notion that vitamin D and its analogs could be used as a therapeutic for psoriasis was generated from an observation that a patient undergoing treatment of osteoporosis with oral 1α-hydroxyvitamin D3 demonstrated remission of psoriatic lesions (Morimoto and Kumahara, 1985). Subsequent clinical studies using oral 1α-hydroxyvitamin D3, oral and topical calcitriol, and topical 1,24-(OH)2D3 (tacalcitol) have yielded promising results in which 70–80% of the patients showed marked improvement while 20–25% of the patients have complete clearance of lesions (Nagpal et al., 2001). Topical calcitriol has shown safety and efficacy at 3 μg/g, but 15 μg/g exhibits an increased risk for hypercalciuria (Langner et al., 2001; Nagpal et al., 2001).
By inducing minor modification of the secosteroidal backbone, medicinal chemists have tried to develop analogs of 1,25-(OH)2D3 with decreased hypercalcemia. One analog, calcipotriol, is metabolized quickly in the blood and results in 100–200 times less calcemia than 1,25-(OH)2D3 (Kragballe, 1995). Use of twice daily applications of calcipotriol leads to 70% improvement in patients when used for 6 to 8 weeks (Kragballe, 1995). One side effect, occurring in approximately 20% of patients, was cutaneous irritation. However, topical calcipotriol was tolerated better than topical steroids for psoriasis treatments. The mechanism of action for these VDR agonists appears to be via decreased proinflammatory cytokine expression in cutaneous T cells, as well as decreased T cell and keratinocyte proliferation. Clearly, the development of more efficacious oral and topical VDR agonists with improved side-effect profiles is warranted for the treatment of psoriasis patients.
Current therapies for graft rejection involve the use of immunosuppressive drugs that specifically target the immune cells, yielding numerous side effects. Among the most significant of these side effects are opportunistic infections and transplant-related malignancies, due in part to the weakened immune system of transplant recipients. Immunosuppressants, which aim to decrease immunologic rejection of the transplant, inadvertently handicap the ability of the immune system as a whole. The immune system thus has a decreased capacity to protect the individual from microorganisms and cancerous cells. These side effects make immunosuppression difficult for the patient and significantly affect quality of life, as immunosuppressant therapy is often lifelong.
VDR agonists may be useful as potential dose-reducing agents for conventional immunosuppressants because they behave as immunoregulators and help to inhibit allograft rejection, as has been demonstrated in several models of both acute and chronic allograft rejection (Adorini, 2002; Amuchastegui et al., 2005). Both VDR agonists, elocalcitol and BXL-01-0029, a prodrug of BXL-2198 (1,25-dihydroxy-16,23Z-diene-26,27-hexafluoro-19-nor vitamin D3, also known as BXL-219 or Ro 26-2198), were used in models of allograft rejection and were demonstrated to retain their VDR activity while inducing less hypercalcemia. One study looked at BXL-01-0029 efficacy on the suppression of proinflammatory stimuli in isolated human cardiomyocytes and purified CD4+ T cells. BXL-01-0029 inhibited INFγ and TNFα-induced CXC chemokine ligand 10 (CXCL10) secretion in human isolated cardiomyocytes as well as CXC chemokine ligand 10 (CXCL10) secretion and gene expression in CD4+ T cells (Sottili et al., 2009). Another study set out to investigate whether VDR activation, via BXL-01-229 and elocalcitol, would be useful in kidney allograft rejection. BXL-01-229 proved to be the most potent drug in isolated human proximal tubule endothelial cells (human renal tubular cells) and could potentially be used as a dose-reducing agent for conventional immunosuppressors of kidney rejection management (Sagrinati et al., 2010).
The past two decades have yielded valuable insight into the physiologic, molecular, structural, and biochemical actions of 1,25-(OH)2D3 and VDRMs on VDR activity. These studies have validated the therapeutic potential of VDRMs for various diseases, including those occurring in both classic and nonclassic VDR tissues. Despite this recent progress, the major hurdle still hindering the clinical use of VDRMs is the resulting hypercalcemia/hypercalciuria that occurs with VDRM treatment.
What is clear from recent studies is that in and of itself, ligand binding may alter DNA-binding properties such that different classes of ligands may alter DNA-binding abilities and give unique pharmacologic profiles that may account for the tissue- and gene-specific effects that some ligands have over others. In addition, the sequence of the DBD itself may relay information to the LBD to alter its conformation, suggesting that DNA-response element–dependent responsiveness may either activate or repress target-gene transcription, depending on the specific DNA sequence to which VDR is binding. Finally, it is obvious from X-ray crystallographic and HDX studies that specific moieties within each VDRM confer stability of the LBD upon ligand binding. One clear example is the 25 hydroxyl group in 1,25-(OH)2D3, which results in H12 stability upon ligand binding. With the recent generation of VDRMs, which confer tissue specificity but do not display hypercalcemia, even more exciting compounds are sure to be discovered.
IV. Thyroid Hormone Receptor Modulators
A. Thyroid Hormone Receptor Structure
The thyroid hormones (THs) triiodothyronine (T3) and thyroxine (T4) bind to and activate the nuclear thyroid receptors TRα and TRβ. As the other members of the nuclear receptor family, TRs also act by inducing or reducing the expression of target genes by binding specific sequences in the genome. The use of radioactivity-labeled TH has demonstrated binding activity in cells and nuclear fractions, and thus reflects the first evidence of a TH receptor (Samuels and Tsai, 1973). Differential binding activity could be shown among various tissues, with high binding in the liver, kidney, pituitary gland, heart, and brain (Oppenheimer et al., 1974).
The TRα gene was subsequently cloned and identified as a cellular homolog of the virally encoded oncogene v-erbA, c-erbA (or ERBAα) (Sap et al., 1986; Weinberger et al., 1986a). Also, the TRβ gene turned out to be previously known as ERBAβ. In humans. THRA (or ERBAα or NR1A1) encodes TRα1, TRα2 and TRα3, which are generated by alternative splicing (Mitsuhashi et al., 1988). Only one of these gene products, TRα1, displays T3-binding activity. DBD–deficient but T3-binding TRα proteins have also been described to occur through alternative promoter usage (Plateroti et al., 2001). The biologic significance of the TRα proteins lacking either DNA- or hormone-binding activity needs to be clarified. The THRB (or ERBAβ or NR1B1) gene codes for the isoforms TRβ1, TRβ2, and TRβ3 (Hodin et al., 1990; Bradley et al., 1992; Williams, 2000; Cheng et al., 2010). All three have DNA- and TH-binding capacity and differ only in the amino terminal domains.
The TRs can bind to their DNA-response elements in the target genes as homodimers, monomers, or as heterodimers together with another nuclear receptor, RXR (Lazar, 1993; Zhang and Lazar, 2000; Yen, 2001). TRs share the typical domain organization of the protein with the other nuclear receptors, where the hormone-binding E/F domain is located in the C terminal, the DNA-binding C domain is centrally located, and the N terminal A/B domain is where coregulator proteins are recruited. The TRs have a high degree of homology, with the most variation found in the A/B domain (Yen, 2001; Cheng et al., 2010).
B. Thyroid Hormone Receptor Action
The endogenous ligands of the thyroid hormone receptors TRα and TRβ are commonly known as thyroid hormones. Studies dating back to the 19th century showed that transplantation of sheep thyroid gland tissue could rapidly improve the state of a patient suffering from what later became known as hypothyroidism (Murray, 1891). Accordingly, extracts from thyroid glands showed an improving effect on the same disease condition when injected into patients. During this time, similar treatments were also found to be effective against childhood cretinism, characterized by developmental deficits such as mental and growth retardation (Osler, 1897). This condition is now known as TH deficiency. In the same century, it was realized that overt activity of the thyroid gland was the cause of a pathologic state later to be known as hyperthyroidism (Baumann, 1895–1896). In 1915, almost 100 years ago, Kendall reported on the isolation of an iodine-containing compound from the thyroid gland, now known as TH; to date, it is the only known molecule in terrestrial organisms that contains covalently incorporated iodine atoms (Kendall, 1915).
The thyroid gland produces two main forms of TH, T4 (3,5,3′5′-tetraiodo-l-thyronine), and T3 (3,5,3′-tetraiodo-l-thyronine). Thyroxine (T4), the main product of the thyroid gland, displays less bioactivity but a longer half-life compared with T3. The thyroid gland is the only site in the body where T4 is produced, whereas only around 20% of T3, the more active variant, is generated in this tissue. THs are highly pleiotropic hormones and have the capacity to regulate development, growth, and cellular metabolism in most tissues (Oetting and Yen, 2007; Moreno et al., 2008).
Different carriers in the blood, including transthyretin, serum albumin, and thyroxine-binding globulin, transport TH (Schussler, 2000; Hamilton and Benson, 2001). The uptake of TH into target tissues is thought to occur mainly as an active process through the monocarboxylate anion transporters MCT8 and MCT10, and also the organic anion transporter 1c, and possibly other transporters as well (Heuer and Visser, 2009; van der Deure et al., 2010).
The majority of the T3 is generated in the peripheral tissues by enzymatic modification of T4 by deiodinases. In adults, the type I and type II iodothyronine 5′-deiodinases (DIO1 and DIO2) remove the 5′-iodine from T4 on the outer ring, generating T3 (Gereben et al., 2008). A third deiodinase, DIO3, can inactivate both T4 and T3 through deiodination of the inner ring. The enzyme encoded by the DIO1 gene can act both on the inner and outer ring and is therefore able to both produce T3 as well as inactivate T3 (Gereben et al., 2008). These deiodinases display different tissue distribution; DIO1 is expressed mainly in the peripheral organs including the liver and kidney; DIO2 is present in a variety of tissues, with high expression in the pituitary gland, brain, and brown adipose tissue; and DIO3 is found in placenta, brain, and skin. These enzymes thus play an important role in regulating the local and also systemic levels of TH. In addition, sulfation and glucuronidation can also modify the structure of TH, thereby contributing to maintaining their bioactive levels (Robbins, 1981; Kester et al., 2002).
A higher order of TH-level control is regulated by the classic hypothalamus-pituitary-thyroid axis (Yen, 2001; Chiamolera and Wondisford, 2009). Thyrotropin-releasing hormone produced in the hypothalamus induces the expression of thyroid-stimulating hormone (TSH, or thyrotropin) in the anterior pituitary gland. TSH then activates the production of TH in the follicular cells of the thyroid gland. TH exert control over of their own production through a feedback loop where circulating TH can repress the TSH levels by acting on both hypothalamus and the pituitary gland. TSH displays a circadian secretion profile, with highest levels at the dark phase (Eisenberg et al., 2010; Kessler et al., 2010). This implies an intricate network of control mechanisms to keep the local and systemic levels of TH within narrow concentration limits, under normal physiologic conditions. Indeed, if these concentration limits are not kept, severe disease may occur as a result. Symptoms of hypothyroidism, when levels of TH are too low, are exemplified by a generally suppressed metabolic profile with weight gain, decreased body temperature, decreased cold tolerance, mental retardation, mood disorders, constipation, myxedema, elevated serum cholesterol levels, decreased lipolysis, decreased sterol excretion. Additionally, hypothyroidism is associated with an increased risk of coronary artery disease (Bartuska and Dratman, 1973; Boyages and Halpern, 1993; Shagam, 2001; Cappola and Ladenson, 2003).
Individuals with hyperthyroidism largely display symptoms opposite to that of hypothyroidism, with elevated circulating TH levels, weight loss, intolerance to heat, sweating, hyperdefecation, muscle wasting, fatigue, anxiety, osteoporosis, and markedly increased heart rate (tachycardia) with increased atrial arrhythmia and heart failure (Shagam, 2001; Bettendorf, 2002; Sharma et al., 2011). Hyperthyroid subjects also present with lower serum total cholesterol levels, with both LDL and HDL fractions reported to be reduced (Scottolini et al., 1980; Muls et al., 1982; Lee et al., 2004). Regarding changes in serum triglyceride levels, for this group of patients no consistency can be found because elevated, reduced, and unaltered levels have been reported (Tulloch et al., 1973; Muls et al., 1982; Friis and Pedersen, 1987).
Almost every cell expresses some variant of the TRs, but the levels for a specific variant depend on the type of tissue and stage of development (Bradley et al., 1992; Cheng et al., 2010). TRα1 is found at highest levels in skeletal muscle and brown adipose tissue but is expressed in kidney, heart, and brain. TRα1 appears early during development. TRβ1 is the predominant isoform in the liver and kidney, and is also found at high levels in brain, heart, and thyroid tissues. TRβ1 is expressed in later stages of development, as opposed to TRα1. TRβ2 is located to the hypothalamus, anterior pituitary, inner ear, and retina (Hodin et al., 1990; Bradley et al., 1992, 1994; Cook et al., 1992; Sjoberg et al., 1992; Cheng et al., 2010). TRβ3 is predominantly expressed in liver, kidney, and lung (Yen, 2001).
Mutations in the THRB gene are known to be associated with a pathologic condition termed resistance to thyroid hormone syndrome (RTH) (Refetoff et al., 1967; Refetoff, 1994; Olateju and Vanderpump, 2006). RTH shares several features with hypothyroidism, but high levels of TH are detected. The THRB mutations found in RTH seem to infer a dominant negative property to the receptor function. Subjects with RTH frequently display greater fat mass, insulin resistance, and lower HDL cholesterol levels compared with normal subjects; however, the RTH syndrome can be subdivided into different categories, which display different symptoms.
A rather complex mix of information on the specific roles of the individual TR isoforms has resulted from work on mice with ablated and mutated variants of TR (Forrest et al., 1996a,b; Wikstrom et al., 1998; Marrif et al., 2005). Currently, at least seven mutant alleles for THRA and nine for THRB exist, either as knockout or knockin mutations (Flamant et al., 2006). A substantial redundancy in target gene activation seems to be present when comparing different TR isoforms (Flores-Morales et al., 2002; Yen, 2003). However, distinct magnitudes of target gene activation have been described for different TR isoforms, relating to specific subsets of genes (Flores-Morales et al., 2002; Yen, 2003). It has also been demonstrated that one TR isoform will activate specific genes when bound to ligand, whereas another TR variant will repress the same genes under the same conditions (Ng et al., 1995; Sjoberg and Vennstrom, 1995; Langlois et al., 1997; Wan et al., 2005). Like other NRs, TRs can also regulate gene expression in the unliganded state (Sjoberg and Vennstrom, 1995). This is manifested by the observations that hypothyroidism gives rise to more severe developmental defects than do either TRα gene deletion or combined deletion of both TRα and TRβ genes. These observations indicate mechanisms driven by corepressor complexes recruited to the unliganded TRα receptor.
Mice that lack the TRα1 form display problems with maintaining a stable body temperature and also present with bradycardia. Mice with defective TRα1 and TRα2 have low serum TH, retarded growth, and intestinal defects. TRβ-deficient mice show a defect in the feedback control exerted by TH on the hypothalamus-pituitary-thyroid axis; they have high thyrotropin-releasing hormone and TSH levels and consequent increased circulating TH levels (Forrest et al., 1996a,b; Wikstrom et al., 1998; Marrif et al., 2005). TRβ-deficient mice also display auditory and visual defects, including deafness and colorblindness, goiter, and defective hepatic response to triiodothyronine, including inability to regulate cholesterol breakdown to bile acids (Forrest et al., 1996a,b; Wikstrom et al., 1998; Gullberg et al., 2000, 2002; Marrif et al., 2005). Elevated TH is also found in mice lacking both TRα and TRβ (Vennstrom et al., 2010). Some of the phenotypes may become more pronounced in mice deficient in both TRα and TRβ. Importantly, many neurologic functions and behavioral patterns have been shown to be aberrant in both TRα and TRβ mutant mice (Vennstrom et al., 2010; Patel et al., 2011). This is in line with an important role for TRs in the development and function of the neural tissues (Patel et al., 2011).
The work describing the X-ray crystal structure of the TRα LBD with bound ligand was pioneering in the NR field, because it was the first NR LBD structure to be determined, and it revealed unexpected features of a NR bound to its ligand (Wagner et al., 1995). Most strikingly, it was discovered that the LBD was folded in a conformation so that it enclosed the hormone, which had not been envisioned previously.
C. Selective Thyroid Hormone Receptor Modulators
Pathologic conditions involving metabolic disorders, including cardiovascular disease, diabetes, and massive obesity (i.e., metabolic syndrome), continue to be a major cause of death despite massive efforts. With the expected reduction of life span and quality of life for the younger generation as a consequence, and an enormous cost burden to future society, the need for improved therapies targeting these disorders cannot be underestimated.
Although some of the effects exerted by TH involve an improved LDL cholesterol profile, increased metabolic rate, and reduced adiposity, interest has been long standing in separating these beneficial effects of TH from the harmful and deleterious effects. The efforts to design synthetic ligands for TRs must be careful not to mimic the deleterious effects of TRα on increased heart rate or of TH-induced bone turnover and muscle wasting. Instead, the focus for the design of synthetic selective TR modulators has been on creating TRβ-specific compounds because this TR is responsible for the beneficial effects seen in lipid lowering. Moreover, studies have demonstrated that hepatic TRβ receptors are largely responsible for these effects, hence defining another level of selectivity in designing TR drugs.
Studies of the structures of TRα and TRβ have found it a rather cumbersome task to discover TRβ-selective compounds, and only a single amino acid differs in the two LBDs of TRα and TRβ (Darimont et al., 1998; Wagner et al., 2001). Nevertheless, work with designing TRβ-selective compounds has been ongoing for considerable time, and in recent years has become remarkably successful.
In the 1950s, human trials with TH infusions showed that TH therapy could lower serum cholesterol levels (Strisower et al., 1954, 1955; Galioni et al., 1957). Later, animal and human studies with TH analogs, including triac, tertac, triprop and 3,5-diiodothyropropionic acid, likewise demonstrated a capacity to reduce serum lipids. However, these analogs were not specific for either TR, so the adverse effects caused by these compounds made further studies directed at metabolic effects not feasible (Lerman and Pitt-Rivers, 1956; Rawson et al., 1959; Hill et al., 1960; Leeper et al., 1961; Pennock et al., 1992; Sherman and Ladenson, 1992).
A large human trial using a TH variant was included in the Coronary Drug Project to test the effects of various lipid-lowering drugs on men who had undergone at least one myocardial infarction (Coronary Drug Project Research Group, 1972). The study was terminated due to a higher number of deaths in the group receiving the d-thyroxine (d-T4). However, a serum cholesterol-lowering effect was seen in that group. It was later discovered that the d-thyroxine may have been contaminated with the more bioactive form l-thyroxine (l-T4), so conclusions drawn from this arm of the study may have been blurred by the severity of condition of the patients at the start of the study (Young et al., 1984). These results markedly reduced the interest at the time in continued studies employing T4 as a lipid-lowering drug.
More recently, several synthetic TH analogs have been identified with the capability to reduce serum cholesterol without adverse heart effects. One of these compounds was L-94901, 3,5-dibromo-3-pyridazinone-l-thyronine (Barlow et al., 1989) (Fig. 9) from GlaxoSmithKline (Research Triangle Park, NC). L-94901 lowered cholesterol while not affecting the heart (Ness et al., 1998). The mechanism likely involves a liver-selective uptake, as L-94901 binds both TRs with similar affinity. CGH 509A, the conjugation of the primary bile acid cholic acid to l-triiodothyronine, has been evaluated as to gain a preferential liver uptake (Stephan et al., 1992). CGH 509A was able to reduce serum cholesterol in rats, although it is less potent than l-T3 alone, without adverse effects on heart or T4 lowering (Stephan et al., 1992).
Fig. 9.
TR modulators.
Another TH analog with cardiac-sparing properties, CGS 23425, N-[3,5-dimethyl-4-(4′-hydroxy-3′-isopropylphenoxy)-phenyl]-oxamic acid (Fig. 9), was shown to lower LDL in hypercholesterolemic rats and to increase the number of LDL receptors, decrease apolipoprotein B (apoB)-100 levels, and increase apolipoprotein A1 (apoAI) expression (Taylor et al., 1997; Wada et al., 2000).
Based on insights from the structural studies on the interaction TRs with ligands, the synthetic high-affinity ligand GC-1, 3,5-dimethyl-4(4′-hydroxy-3′-isopropylbenzyl)-phenoxy) acetic acid (Fig. 9), was designed through a rational approach by Scanlan and Baxter and colleagues (Chiellini et al., 1998). GC-1, also known as sobetirome or QRX-431, was originally intended to improve the synthesis of TH analogs. Referring to T3, the three iodines were replaced by methyl and isopropyl groups, the biaryl ether linkage was replaced by a methylene linkage, and the amino acid side chain was replaced by an oxyacetic-acid side chain (Chiellini et al., 1998). GC-1 was demonstrated to bind TRs with an affinity similar to that of T3 itself. Moreover, this TH analog bound TRβ with the same affinity as T3 but displayed a 10-fold lower affinity for TRα compared with T3. Later studies revealed that the oxyacetic acid side chain is responsible for the TRβ selectivity by utilization of the only nonconserved amino acid residue, Asn331, in the TRβ ligand-binding pocket, which is replaced by Ser277 in TRα (Wagner et al., 2001; Yoshihara et al., 2003).
A second level of specificity could also be attributed to GC-1 when it was found to show a much higher accumulation in the liver compared with other organs, including the heart, because TRβ is the predominant form in the liver and also is largely responsible for the lipid-lowering activities by THs (Trost et al., 2000). The exact mechanisms of liver selectivity are not clear but likely involve a higher degree of hepatic first-pass extraction.
Since its appearance, GC-1 has become the best-studied selective TR agonist. It has been demonstrated to lower serum cholesterol in several animal models, which include hypothyroid and euthyroid mice, cholesterol-fed mice and rats, and also cynomolgus monkeys, with up to 90% reductions in rats and 40% in monkeys (Trost et al., 2000; Grover et al., 2004; Johansson et al., 2005). In each case, the LDL fractions are mostly affected. No major effects were seen on the heart in GC-1-treated primates or rodents at cholesterol-lowering doses. Serum triglycerides were reduced by GC-1 treatment in mice; in rodents, decreased fat mass and increased metabolic rate and steatosis were found (Johansson et al., 2005; Perra et al., 2008; Villicev et al., 2007). Importantly, GC-1 was found to induce breakdown of cholesterol to bile acids through induction of the rate-limiting enzyme in bile acid synthesis Cyp7a1 (Johansson et al., 2005).
KB141, 3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenoxy]phenyl acetic acid (Fig. 9), is a TRβ selective agonist that that uses the same principles as GC-1 to achieve its higher affinity for TRβ (Grover et al., 2003, 2005, 2007). This agonist has approximately 15-fold higher binding affinity to TRβ than TRα. KB141 is not selectively enriched in the liver, but is capable of lowering serum lipids in several rodent models and cynomolgus monkeys like GC-1 (Grover et al., 2007). In addition to inducing bile acid synthesis by regulating Cyp7a1, KB141 was also shown to induce hepatic LDL receptor expression in the mouse (Erion et al., 2007). Both GC-1 and KB141 reduced the serum content of the atherogenic Lp(a) in monkeys (Grover et al., 2003, 2004). Additionally, KB141 lowers weight and adiposity, and reduces serum triglycerides and blood glucose in rodent models of obesity-induced diabetes; however, KB141 has not been tested further in clinical trials (Erion et al., 2007; Bryzgalova et al., 2008).
The drug MB07811, (2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane (Fig. 9), is a liver-selective prodrug that upon enzymatic activation in the liver becomes a selective TRβ agonist (Erion et al., 2007). MB07811 lowers both serum cholesterol and triglycerides in rodents (Cable et al., 2009). Moreover, this drug displayed additive effects in statin-treated rabbits, dogs, and monkeys with regards to cholesterol (Ito et al., 2009). MB07811 also induces LDL receptors and Cyp7a1 in rodents (Erion et al., 2007; Cable et al., 2009).
The liver selective TR agonist T-0681, sodium;3-[4-[3-[(4-fluorophenyl)-hydroxymethyl]-4-hydroxyphenoxy]-3,5-dimethylanilino]-3-oxopropanoate (also known as KAT-681), has recently been demonstrated to have beneficial effects on atherosclerotic lesions in cholesterol-fed rabbits and mice deficient in apolipoprotein E (Hayashi et al., 2004; Tancevski et al., 2009). T-0681 induced the LDL and HDL receptors in rodents and in addition was found to significantly increase fecal excretion of macrophage-derived neutral and acidic sterols (Tancevski et al., 2010). Notably, no apparent positive effects on the process of reverse cholesterol transport could be found in CETP transgenic mice in the same study.
GC-1, 3,5-dimethyl-4(4′-hydroxy-3′-isopropylbenzyl)-phenoxy) acetic acid (also known as sobetirome or QRX-431, licensed to QuatRx Pharmaceuticals, Ann Arbor, MI), has been tested in human subjects during a phase I clinical trial that was performed in 2008 (Lin et al., 2008). The study showed that the compound was well tolerated at all doses tested. In particular, at a daily dose of 100 µg, GC-1 reduced serum LDL cholesterol levels approximately 41% in healthy volunteers without effects on heart rate or thyroid axis.
In a continued effort to develop a selective TRβ agonist, Karo Bio AB and collaborators discovered KB2115, or 3-[[3,5-dibromo-4-[4-hydroxy-3-(1-methylethyl)-phenoxy]-phenyl]-amino]-3-oxopropanoic acid, also called eprotirome (Berkenstam et al., 2008) (Fig. 9). So far, no in vitro or animal data for KB2115 have been published so far. Compared with KB141, KB2115 appears to undergo a high hepatic extraction. In the first human study with subjects aged 18–60 years who were not pregnant, had a body mass index of 25–35, and had a total serum cholesterol >5.0 mM, KB2115 was administrated daily at doses of 100 and 200 µg for 14 days; the study found a 40% reduction of total serum cholesterol as well as LDL cholesterol (Berkenstam et al., 2008). Treatment with KB2115 also induced CYP7A1, affecting bile acid synthesis and cholesterol regulation, as seen in previous rodent studies. However, no effect was seen on cholesterol synthesis in this study. Additionally, the doses used did not appear to cause adverse effects in skeletal muscle or heart parameters or changes in body weight or oxygen consumption.
This study was followed up by a phase 2, multicenter, randomized, placebo-controlled, double-blind human trial with the KB2115 compound (Ladenson et al., 2010). Subjects in the study were between 18 and 65 years of age, not pregnant, and had received treatment with simvastatin or atorvastatin for at least 3 months before entry in study but had an serum LDL cholesterol level >3.0 mM. Subjects already under treatment with statins who received a daily dose of KB2115 of 100 µg for 12 weeks showed an approximately 30% reduction of serum LDL. Similar reductions were found in atherogenic apolipoprotein B and Lp(a) lipoprotein serum levels, and no apparent side effects were reported on bone or heart. However, decreased levels of T4, but not T3 and thyrotropin, were evident in subjects receiving KB2115. Currently, phase 3 studies are underway to further assess the efficacy and safety of KB2115. Approximately 1100 patients with familial heterozygous hypercholesterolemia, who frequently do not meet their treatment goals with common cholesterol-lowering regimens including statins, are being treated in this study (Karo Bio AB, 2010; http://www.karobio.com/research-development/en-substans-mot-hoga-blodfetter).
In summary, the progress with establishing selective TR modulators has accelerated the last years, and several compounds now being tested in human clinical studies, with human hypercholesterolemia as the primary indication. These astonishing achievements have been reached despite initial structural and functional studies pointing at a very narrow window for selective drug design aimed at TRβ. The studies with TRβ-selective compounds have also contributed insights into previously unanticipated properties of NR interaction with ligands. It will be interesting to see whether the findings demonstrating TRβ agonist effects on parameters including blood glucose and fat reduction in animal experiments can be translated to human clinical studies in the future. Current lipid-lowering regimens are in many cases insufficient, so novel therapies that can synergize with the drugs in present use are highly sought after in clinical practice.
V. Selective Androgen Receptor Modulators
A. Androgen Receptor Structure
The AR belongs to nuclear receptor subfamily 3, group C (NR3C4), which also includes steroid receptors GR, MR, and PR. AR plays a major role in the development and maintenance of the male reproductive organs by mediating the physiologic response to testosterone and dihydrotestosterone. Although a single gene localized on the X-chromosome codes for AR, two isoforms of AR have been identified. AR-A is an 87-kDa protein, consisting of a truncated A/B resulting from proteolytic cleavage of 187 amino acids; AR-B is considered the full-length protein at 110 kDa. Similar to other nuclear receptors, AR consists of three major structural and functional domains: the amino-terminal domain contains the activation AF-1 and is the major transactivation domain, the DBD, and the LBD that contains second transactivation function (AF-2) (Jenster et al., 1991; Auwerx et al., 1999). In the absence of ligands, the molecular chaperone complex is critical for AR to maintain in a stable, inactive, intermediate conformation. The 70-kDa heat shock protein (Hsp70) functions as a negative regulator of transactivation of AR, but Hsp40 is necessary for hormone binding to the AR. Ligand binding causes a sequential dissociation of molecular chaperones from AR and activates the receptor. Receptor activation leads to exposure of the nuclear localization signal, which results in Hsp90-dependent translocation of the AR to the nucleus and AR homodimer formation. The AR dimers bind the androgen-responsive element in the promoter of target genes, recruit coregulators, and initiate transcription (Prescott and Coetzee, 2006).
B. Androgen Receptor Function
AR is necessary for male sexual differentiation and maintaining sexual function. It is also important in maintaining skeletal muscle mass and strength, BMD, hematopoiesis, and cognitive behavior (Heemers and Tindall, 2007). Additionally, AR has been shown to have a significant role in development and metabolism, as most mutations in AR are associated with disease. For example, androgen insensitivity syndrome is caused by a mutation of the AR gene, and a CAG repeat (trinucleotide repeat) in the first exon of the AR gene is expanded in Kennedy’s disease (Holterhus et al., 2005; Galani et al., 2008; Palazzolo et al., 2008). Somatic AR mutations are also commonly found in prostate cancer (Newmark et al., 1992).
AR requires the binding of endogenous ligands to perform its physiologic function. Testosterone is the major androgen, as it represents 90% of the androgens available to bind to AR. Approximately 5–8% of testosterone is reduced to dihydrotestosterone (DHT) by type-2 5α-reductase in the prostate and hair follicles. The remaining endogenous androgens, which include dehydroepiandrosterone (DHEA), androstenediol, and androstenedione, are produced by the adrenal cortex and can be converted into testosterone in peripheral tissues (Rainey et al., 2002; Davison and Bell, 2006). Circulating testosterone and DHT are critical for the development and maintenance of male accessory reproductive organs, the control of male sexual performance, and the maintenance of male secondary characteristics involving muscle, bone, and hair (Mooradian et al., 1987).
As men grow older, their testosterone level gradually declines. Low levels of androgens can also result from testicular diseases, inflammation, and chemotherapy used to treat cancer. Diseases affecting the hypothalamus and pituitary glands can also decrease the level of androgens in men. Often, a lower level of androgens can lead to a decreased sex drive, erectile dysfunction, fatigue, decreased physical activity, loss of muscle mass and strength, and bone weakness, which results in poor quality of life (Bhasin and Jasuja, 2009).
Physicians have used androgen replacement therapy for many years to treat male disorders. Unfortunately, the clinical application of androgen is not widespread because natural androgens and their derivatives tend to have low efficacy by oral administration, or are inconvenient to administer by intramuscular injection or implant of testosterone and/or testosterone esters (Negro-Vilar, 1999). The anabolic effects of androgens can also be used to treat loss of muscle mass and osteoporosis caused by aging and chronic diseases (Bhasin and Jasuja, 2009). Unfortunately, long-term and high-dose use of androgens is associated with adverse effects, including erythrocytosis, increased body weight, leg edema, and aggressive behavior. With the limitation of natural androgens and derivatives, the development of selective androgen receptor modulators (SARM) has attracted attention from pharmaceutical companies (Negro-Vilar, 1999). In the early 1940s, the development of SARMs focused on the modification of the testosterone molecule to improve oral bioavailability and/or tissue selectivity. A collaboration between the University of Tennessee and Ligand Pharmaceuticals developed the first nonsteroidal SARMs in 1998 (Dalton et al., 1998; Edwards et al., 1998). Since then, many major pharmaceutical companies have developed a large number of nonsteroidal SARMs.
C. Selective Androgen Receptor Modulators
The development of SARMs is based on four pharmacophores: aryl-propionamide, bicyclic hydantoin, quinoline, and tetrahydroquinoline analogs. In contrast with testosterone, the synthetic SARMs are not substrates for aromatase or 5-reductase (Chen et al., 2005). SARMs have been demonstrated to act as full agonists in anabolic organs (e.g., muscle and bone), but they only act as partial agonists in androgenic tissues (e.g., prostate and seminal vesicles). SARMs may provide opportunities to treat primary or secondary hypogonadism, osteoporosis, frailty, chronic sarcopenia, and cachexia as well as contributing to rehabilitation, anemia, hormonal male contraception, and sexual desire disorders. This section describes some of the SARMs currently used in treatment of AR-derived diseases.
S-4 or andarine, (2S)-3-(4-acetamido-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide (Fig. 10), was developed using the nonsteroidal androgen antagonist bicalutamide as the lead compound (Gao et al., 2005). Pharmacokinetics studies demonstrated that S-4 is rapidly absorbed, slowly cleared, and has a moderate volume of distribution in rats (Kearbey et al., 2004). S-4 also showed in vivo both androgenic and anabolic activity that is tissue selective. Although S-4 appears to be less potent and efficacious than testosterone propionate in its androgenic activity, its anabolic activity appears greater due to its ability to increase muscle mass and strength in castrated rats. It also restored castration-induced loss of lean body mass and significantly increased BMD (Gao et al., 2005; Kearbey et al., 2007, 2009).
Fig. 10.
AR modulators.
Another SARM, ostarine [(2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide], also known as MK-2866 (Fig. 10), was developed by GTx and is currently the most advanced clinical candidate since the completion of phase 2 trials for chronic sarcopenia and cancer cachexia. The chemical composition of ostarine can be found in patent databases, but GTx has not formally disclosed the structure. Ostarine treatment leads to increased lean body mass and improved muscle function but has no apparent effects on the prostate, skin, or pituitary gland (Zilbermint and Dobs, 2009).
S-23, (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (Fig. 10), another SARM developed by GTx, acts as a full agonist in vitro. In vivo rodent studies performed with S-23 show the anabolic activity of this SARM. In castrated male mice, S-23 was able to increase the body weight of the levator ani muscle to the intact control level, while its effects on the prostate were kept at a much lower level. S-23 also suppresses the LH level in the serum of intact rats; when combined with estradiol benzoate, S-23 acted as an effective, reversible regimen of hormonal male contraception in rats (Jones et al., 2009).
Ligand Pharmaceuticals developed the SARM LGD-3303 [9-chloro-2-ethyl-1-methyl-3-(2,2,2-trifluoroethyl)-3H-pyrrolo-[3,2-f] quinolin-7(6H)-one] (Fig. 10). Similar to other SARMs, LGD-3303 can act as a full agonist on muscle but is a partial agonist on the preputial gland and ventral prostate. LGD-3303 significantly increased the muscle weight of castrated male rats above the intact level while maintaining the ventral prostate weight at a lower level than that of intact animals. Additionally, tissue-selective activity was not affected by different dose methods. LGD-3303 also demonstrated the ability to increase muscle weight, bone density, and bone mineral content in OVX female rats (Vajda et al., 2009). With LGD-3303 treatment, female rats with previous sexual experience showed an enhanced sexual preference for males. LGD-3303 may offer a potential therapy for women with sexual desire disorders (Vajda et al., 2009).
LGD-2226 [6-(bis-(2,2,2-trifluoroethyl)amino)-4-trifluoromethyl-1H-quinolin-2-one] (Fig. 10) is a nonaromatizable, highly selective AR ligand also developed by Ligand Pharmaceuticals. LGD-2226 alters the conformation of the AR LBD in a different pattern compared with testosterone and fluoxymesterone. In a cell-based assay, LGD-2226 showed full agonist activity on bone and muscle. This anabolic activity was further confirmed in animal models, where bone strength above control levels was noted. Oral dosing of rats with LGD-2226 significantly increased the animals’ sexual function (Miner et al., 2007b).
Bristol-Myers Squibb Pharmaceutical Research Institute (Princeton, NJ) developed BMS-564929, (7R,7aS)-2-chloro-4-(7-hydroxy-1,3-dioxotetrahydropyrrolo[1,2-c]imidazol-2-yl)-3-methylbenzonitrile (Fig. 10), which may be the first once daily, orally available treatment of age-related musculoskeletal decline in men. BMS-564929 is a subnanomolar AR agonist that does not appear to exhibit significant interactions with sex hormone-binding globulin or aromatase. Cell-based assays suggest that BMS-564929 is approximately 20-fold more potent in muscle cells than in prostate cells, which correlates with in vivo rodent studies (Ostrowski et al., 2007). In vivo studies suggest that BMS-564929 is substantially more potent than testosterone in stimulating muscle growth of castrated animals and is more selective for muscle versus prostate. Additional studies have demonstrated that the binding of BMS-564929 to the LBD of AR initiates differential cofactor recruitment, which may be the molecular basis for tissue selectivity and potency of this SARM. BMS-564929 has advanced through preclinical safety testing and is now in phase 1 clinical trials for age-related functional decline (Ostrowski et al., 2007).
Kaken Pharmaceutical (Tokyo, Japan) generated the SARM S-40503, 2-[4-(dimethylamino)-6-nitro-1,2,3,4-tetrahydroquinolin-2-yl]-2-methylpropan-1-ol (Fig. 10), using tetrahydroquinolines as the scaffold. This compound has nanomolar affinity with AR. When administrated into orchiectomized rats, S-40503 increased the BMD of the femur and the muscle weight of the levator ani but had little effect on prostate weight. The osteoanabolic activity of S-40503 also worked on female OVX rats. The compound significantly increased the BMD and biomechanical strength of the femoral cortical bone (Hanada et al., 2003).
AC-262536, 4-(3-hydroxy-8-aza-bicyclo[3.2.1]octyl)-naphthalene-1-carbonitrile (Fig. 10), was identified by Acadia Pharmaceuticals (San Diego, CA) as a potent and selective AR ligand with partial agonist activity relative to testosterone. AC-262536 significantly improves anabolic parameters in castrated male rats. The compound stimulated the growth of the levator ani muscle and suppressed elevated LH levels. AC-262536 has weak androgenic effects compared with testosterone (Piu et al., 2008).
Clearly, significant advances have been made in the development of tissue-specific AR ligands. With the demand for therapeutics to slow the aging process in men, development of improved compounds will certainly remain an active area of research.
VI. Selective Glucocorticoid Receptor Modulators
A. Glucocorticoid Receptor Structure
The GR is a glucocorticoid-activated member of the NR superfamily of transcription factors. GR is ubiquitously expressed and exerts diverse effects on physiologic processes, including endocrine homeostasis and regulation of metabolism, development, stress, and immune responses. Only partial or incomplete glucocorticoid resistance in humans has been reported to date, suggesting that complete loss of GR signaling is incompatible with life. Support for this comes from studies using targeted deletion of GR in rodents, which causes lethality at birth (Reichardt et al., 1998b; Benecke et al., 2000). Both Addison’s disease and Cushing’s syndrome/disease are associated with aberrant glucocorticoid activity. Addison’s disease results when the body fails to produce sufficient glucocorticoids, whereas a high level of cortisol in the blood causes Cushing’s.
The predominant glucocorticoid found in humans is cortisol, a member of the steroid class of hormones, which was first introduced as a therapeutic agent in 1948 for its anti-inflammatory and immunosuppressive activities (Kirwan et al., 1999). The initial success observed with cortisol led to the search for novel, synthetic glucocorticoid derivatives. Synthetic glucocorticoids are the most potent anti-inflammatory agents available, and they are used to treat a wide variety of allergic and inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. However, their use is limited due to the range and severity of their side effects. Long-term use can lead to pleiotropic side effects, including fat redistribution, obesity, and osteoporosis. Therefore, the goal is to develop select GR ligands that preserve the beneficial anti-inflammatory activity yet minimize the side-effect profile.
The human GR is encoded by one gene, NR3C1, which is located at chromosome 5q31-32. It is 777 amino acids in length and highly conserved across species. Like all NRs, GR consists of a highly variable N-terminal domain (A/B domain), a DBD containing two zinc-finger motifs (C domain), a hinge region (D domain), and a C-terminal LBD (E domain). GR possesses two AF domains responsible for regulating transcriptional activity. The N-terminal region of GR, located between amino acids 77 and 262, contains the AF-1 domain, which plays an important role in the communication between itself and the molecules necessary for transcription (Almlof et al., 1997). The AF-1 domain, while relatively unfolded in the basal state, forms a complex helical structure in response to cofactor binding (Kumar et al., 2004). AF-1 is capable of constitutively regulating 60–80% of transcriptional activity in the absence of ligand (Dahlman-Wright et al., 1994). AF-1 interacts with coregulatory proteins essential for optimal GR activation, but the mechanism through which this interaction occurs has yet to be determined, given that the AF-1 lacks the canonical LXXLL motif (Kumar and Thompson, 2012). The DBD of GRα corresponds to amino acids 420–480 and contains two zinc-finger motifs through which GRα binds to glucocorticoid-responsive elements (GREs) (Howard et al., 1990; Schule et al., 1990). The optimal recognition DNA sequence is an inverted hexameric palindrome (AGAACANNNTGTTCT) separated by three base pairs (Lieberman et al., 1993). The DBD contains two perpendicularly oriented α-helices, one of which is responsible for DNA recognition (Luisi et al., 1991). Alteration of the three-dimensional structure of the DBD can occur depending on the base sequence of the GRE to which it binds, influencing different transcriptional responses and suggesting that DNA is a sequence-specific allosteric modulator of GR-induced transcriptional activity (Meijsing et al., 2009). Finally, the LBD corresponds to amino acids 481–777 and plays a critical role in the ligand-induced activation of GRα. Interaction of the LBD with cytosolic proteins maintains the integrity of the domain and allows for ligand binding. Ligand binding induces a conformational change in the LBD, leading to the dissociation of its accessory proteins, and unveils GRα’s nuclear localization sequences, enabling GRα to translocate to the nucleus and initiate gene transcription. The LBD also contains a second transactivation domain, termed AF-2, the activity of which is ligand dependent. The AF-1 recognizes canonical LXXLL motifs through conformational rearrangement of helix 12. While either the AF-1 or AF-2 domains are capable of regulating transcriptional activity, full GR-mediated transcription requires synergy between the AF-1 and AF-2. Interestingly, this synergy requires ligand binding (Hittelman et al., 1999).
Despite being the first nuclear receptor as well as the first transcription factor cloned, the crystal structure of GR bound to ligand took some time to solve due to a longstanding problem of expressing and purifying active GR protein. However, a single-point mutation, F602S, in the GR LBD allowed for robust expression of a soluble GR LBD, enabling it to be determined in complex with dexamethasone and a coactivator motif derived from the cofactor transcriptional mediator/intermediary factor 2 (TIF2) (Bledsoe et al., 2002). Cofactors such as SRC1 and TIF2 contain three LXXLL motifs, whereas previous crystal structures of LBD/coactivator complexes were solved with the first or the second LXXLL motif. The LBD contains 11 α-helices and 4 β-strands, which form into a three-layer helical domain. Helices 1 and 3, and 7 and 10 form two sides of a helical sandwich with the middle helices (4, 5, 8, and 9) shaping the top half of the protein and creating a cavity for ligand. The AF-2 domain packs against helices 3, 4, and 10 forming an “agonist bound” structure. Following the AF-2 is a small conserved β-strand between helices 8 and 9 (Bledsoe et al., 2002). This β-strand plays an important role in GR activity stabilizing the AF-2 domain. When deleted, the receptor loses its activity (Zhang et al., 1996).
These studies demonstrated that the GR LBD adopts a surprising structure, involving the formation of an intramolecular β-sheet such that GR LBD monomers are arranged in a unique dimer conformation. The central hydrophobic interface lies in the β turn of strands 3 and 4. An extensive network of hydrogen bonds surrounds the hydrophobic interface, which may play a key role in stabilizing the GR dimer configuration (Bledsoe et al., 2002). Additionally, the GR dimer forms a second charge clamp that interacts with residues only present in the third LXXLL motif of coactivators. Mutational analysis of the two charge clamps has revealed that both are critical for transactivation in vivo, providing an explanation for the preferential binding of this motif to the receptor (Ding et al., 1998).
In the crystal structure, dexamethasone is bound in the bottom half of the LBD, occupying only 65% of the volume (Bledsoe et al., 2002). The ligand is oriented with its A-ring toward the β-strands 1 and 2 and its D-ring toward the AF-2. The A-ring carbonyl forms direct hydrogen bonds with Arg611 and Gln570. The side chain of Asn564 is oriented in such a way as to allow it to form hydrogen bonds to the C-ring 11-hydroxyl and 24-hydroxyl. Furthermore, the 21-hydroxyl and 22-carbonyl form hydrogen bonds with residues Q642 and T739, respectively (Bledsoe et al., 2002). Interestingly, the presence of dexamethasone revealed an additional side pocket formed by the structural rearrangement of helices 6 and 7. Structure analysis determined that dexamethasone made direct contact with the AF-2 helix (Lys753) and the loop preceding the AF-2 (I747 and Phe749), suggesting that this interaction, in addition to the extensive hydrogen bond network between GR and ligand, aids in stabilizing the AF-2 helix in the active conformation. These interactions are likely the molecular basis for ligand-dependent GR activation (Bledsoe et al., 2002).
B. Glucocorticoid Receptor Action
Glucocorticoids have potent anti-inflammatory and immunosuppressive properties. The GR is expressed in a wide variety of lymphoid cells, including T and B cells, and macrophages. Because of its expression pattern, GR is an extremely attractive drug target for the generation of therapeutics aimed at ameliorating inflammatory diseases caused by aberrant T cells, B cells, and macrophage functions. During an inflammatory response, activation of GR leads to a decrease in proinflammatory cytokine expression via a mechanism that involves both activation and repression of gene transcription, termed “transactivation” and “transrepression,” respectively. As is the case with most NRs, transactivation occurs when ligand-bound NRs bind their respective DNA-response elements, resulting in an increased rate of gene expression. Transrepression is a process where one protein represses or inhibits the activation of a second protein through protein-protein interactions, and it was first observed with GR, where GR was found to bind to and inhibit the transcriptional activity of the transcription factors AP-1 and NF-κB (Lucibello et al., 1990; Herrlich and Ponta, 1994). The anti-inflammatory effects of GR are thought to be mediated through both of these events, although whether transactivation plays a role in this effect is still under debate (Clark, 2007; Newton and Holden, 2007). Interestingly, glucocorticoids still inhibit inflammatory processes in a mouse model where GR is defective in its dimerization and DNA-binding potential (GRdim), which solidifies transrepression as a critical mechanism underlying the immunosuppressive effects of these hormones (Reichardt et al., 1998a; Tuckermann et al., 1999).
Glucocorticoids also have major effects on energy balance and carbohydrate metabolism. The term “glucocorticoid” is derived from the initial observation that this hormone was involved in glucose metabolism. During fasting, cortisol stimulates several processes that lead to the increase and maintenance of normal blood glucose concentrations. GR activity is involved in the expression of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase. These enzymes are involved in gluconeogenesis, which accounts for the diabetogenic effects of glucocorticoids and also leads to the synthesis of glucose from other nonhexose organic molecules including pyruvate, lactate, glycerol, and amino acids (van Raalte et al., 2009). GR activity is also the basis for the muscle-wasting effects associated with long-term glucocorticoid use (Carballo-Jane et al., 2004). GR activity increases the expression of key enzymes in gluconeogenesis and increases the availability of amino acids essential for this process (Pilkis and Granner, 1992). A second mechanism involved in glucocorticoid action results in the conservation of glucose for neural tissues, such that glucose uptake is inhibited by muscle and adipose tissue (McMahon et al., 1988). Additionally, glucocorticoids stimulate lipolysis in adipose tissue. The fatty acids released from this process are used for the production of energy in tissues, including muscle. The glycerol released from lipolysis provides a substrate for gluconeogenesis. Finally, glucocorticoids inhibit leptin signaling, which is an important hormone in the maintenance of body weight and reproductive function (Zakrzewska et al., 1997).
Various synthetic glucocorticoids are available for therapeutic use. The chemical structures of many of these compounds are based on the natural corticosteroids, but modifications have been made to the structures to improve efficacy compared with endogenous hormones, while reducing mineralcorticoid-like actions via MR (Schäcke et al., 2007). Prednisone and prednisolone were generated by introducing a C=C double bond into the first aromatic ring of cortisol, improving potency and diminishing mineralcorticoid activity (Lutsky et al., 1979). Further improvement occurred when 1) a fluoro atom was introduced at position 9α, yielding fludrocortisone, 2) an addition of a hydroxyl group at position 16α yielded triamcinolone, 3) an addition of a methyl group at position 16α yielded dexamethasone and betamethasone, or 4) a methyl group was added at position 6α, which derived methylprednisolone (Brattsand et al., 1982; Gessi et al., 2010). These modifications were essential to improve affinity for GR, minimize binding to the MR, and increase half-life, thus increasing in vivo potency. However, despite their anti-inflammatory effects, these “improvements” did not minimize the side-effect profile typically seen with classic glucocorticoid use, hence the search for novel selective GR modulators that retain the potent anti-inflammatory effects of classic glucocorticoids but have negligible side effects.
Systemic glucocorticoids most commonly used are hydrocortisone, prednisolone, methylprednisolone, and dexamethasone. They display good oral bioavailability, are eliminated/metabolized in the liver, and are excreted by the renal system. However, due to their profound effects on numerous tissues and organ systems, the therapeutic use of glucocorticoids is often associated with a number of adverse side effects. The side effects are often dose and duration dependent and include such symptoms as alterations of fluid and electrolyte imbalance, edema, weight gain, hypertension, muscle weakness, development of diabetes, and osteoporosis. Some of these side effects may be severe and/or life threatening. Numerous approaches have been made to optimize the effects of the glucocorticoid action and minimize the overall impact of the side effects, including optimization of dosing regimens, increasing nuclear receptor selectivity, and direct delivery to the site of action. Despite these approaches, the current therapies still suffer from significant risk of side effects. Therefore, the quest for the development of novel GR targets that will allow for longer and higher dosing regimens while minimizing side effects continues (Buttgereit et al., 2005).
As discussed in a previous section of this review, various synthetic ligands are available for therapeutic use, many of which are similar in structure to the natural corticosteroids. Modifications to the natural structure have been made to optimize pharmacokinetics, therapeutic potential, and minimize some of the adverse side effects. While these modifications to the natural structure have failed to minimize the negative side effects, there has been increased interest in identifying new compounds that would function as such. These efforts have led to the generation of compounds that can be grouped into four different classes: selective glucocorticoid receptor modulators (SGRMs), gene-selective compounds, dissociated compounds, and soft steroids.
C. Selective Glucocorticoid Receptor Modulators
SGRMs and selective GR agonists are general terms used to describe compounds that retain their anti-inflammatory activity but have impaired activity affecting bone metabolism or glucose and lipid metabolism. Therefore, these compounds result in an improved therapeutic index in vivo over classic glucocorticoids (Miner, 2002; Schäcke et al., 2002).
The major goal for a number of years has been to identify truly dissociated glucocorticoids, but the first attempt to characterize a “dissociative” compound resulted in the identification of several steroid based compounds, RU24858, RU40066, and RU24782 (Fig. 11), that were capable of separating transcriptional activation from repression in vitro. Vayssiere et al. (1997) demonstrated that these compounds, specifically RU24858, not only bound GR with high affinity, they exerted strong AP-1 inhibition with little to no agonist activity compared with dexamethasone-treated cells in a variety of in vitro assays. However, while RU24858 was very efficient at inhibiting both AP-1- and NF-κB-mediated gene induction in vivo, and was as potent an anti-inflammatory as prednisolone in a rat asthma model, it also induced side effects similar to prednisolone itself: loss of body weight and induction of osteoporosis (Belvisi et al., 2001). Why RU24858 is effective in vitro but not in vivo remains to be determined. One possibility is that because the compound is similar in structure to classic glucocorticoids, it may be metabolized in vivo to yield a derivative, which behaves like a classic glucocorticoid.
Fig. 11.
GR modulators.
Another novel GR ligand, A276575 [2,5-dihydro-9-hydroxy-10-methoxy-2,2,4-trimethyl-5-(1-methylcyclohexen-3-y1)-1H-[1]benzopyrano[3,4-f]quinoline; Fig. 11], discovered by Abbott Laboratories (North Chicago, IL), displayed high repression but very low transactivation activities, unlike traditional glucocorticoids. Much like dexamethasone, and with a high affinity for GR, A276575 was a potent anti-inflammatory as it inhibited IL-1β and IL-6 production in human skin fibroblasts and human lung epithelial cells, and inhibited Con A-induced proliferation of human PBMCs. Interestingly, A276575 was a racemic mixture, containing 7:1 (−)-Syn to antidiastereomers, and the (+)-enantiomers were 10-fold weaker than their respective (−)-enantiomers. Additionally, the (−)-Syn enantiomer of A276575 was inactive in repressing regulated and normal T cell expressed and secreted production whereas the (−)-Anti enantiomer was highly active against regulated and normal T cell expressed and secreted production (Gessi et al., 2010). These data suggest that even subtle effects of ligand can impact receptor function. However, this compound displayed undesirable properties, including a high affinity for PR and superagonist activity in mouse mammary tumor virus (MMTV)-PR-B transfection assays, which precluded it from further use in vivo (Gessi et al., 2010). Further modifications of this compound failed to separate the desired transcriptional repression from the undesirable transactivation. Although these compounds may not have been truly “dissociative,” they present as valuable tools to dissect the differential effects on GR-mediated gene regulation. In fact, evaluation of these molecules revealed that the substitution pattern on the C-5 aryl group profoundly affected the compound’s functional activity. Therefore, a series of compounds were developed to investigate the effects of the C-5 aliphatic substitution, leading to the discovery of a gene-selective compound, AL-438 [10-methoxy-5-(2-propenyl)-2,5-dihydro-2,2,4-trimethyl-1H-(1)benzopyrano(3,4-f)quinoline].
Gene-selective compounds act on the receptor to influence gene expression in a gene-specific or promoter-specific manner. For instance, some genes might be activated, some might be repressed, but the resulting profile is different than that of classic glucocorticoids (Coghlan et al., 2003). During the search for dissociative compounds, many gene-selective compounds were designed by default. Screening activities identified AL-438 (Fig. 11), a derivative of benzorano[3,4f]quinoline that appears to have partial agonism at GR with repressive activity as well. AL-438 had an almost identical binding affinity for GR as prednisolone (Kassel et al., 2004). In vitro characterization of this compound demonstrated that AL-438 efficiently inhibits the production of IL-6 and E-selectin in transactivation assays (Coghlan et al., 2003). Because of its in vitro success, AL-438 was tested in vivo in both acute and chronic models of inflammation. In the carrageenan-induced paw edema assay in the rat, a model for acute inflammation, AL-438 demonstrated almost equivalent efficacy in reducing paw edema relative to prednisolone treatment. Because glucocorticoids demonstrate desirable effects on joint swelling, synovitis, and periosteal new bone formation, the rat adjuvant-induced arthritis model was used to study the effects of AL-438 in a chronic model of inflammation. AL-438 had an efficacy equal to that of prednisolone at 30 mg/kg per day, despite lower potency. Additionally, mice treated with AL-438 exhibited grooming behavior equivalent to nonadjuvant-treated controls, meaning that the behavior of AL-438-treated animals is similar to that of healthy control animals, whereas the prednisolone-treated animals still exhibited signs of stress. Furthermore, AL-438 demonstrated a decreased potential to increase blood glucose, a marker for diabetes induction, and less likely to induce osteoporosis, both side effects associated with long-term GR treatment (Coghlan et al., 2003).
Molecular analysis of AL-438 elucidated the mechanism by which this improved therapeutic potential was observed, differential cofactor recruitment. AL-438 exhibits gene-specific regulation, capable of only fully regulating a subset of the genes normally regulated by GR. Using a two-hybrid assay, prednisolone was able to efficiently induce the interaction with both peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) and glucocorticoid receptor-interacting protein (GRIP-1), whereas AL-438 was only able to induce the interaction with GRIP-1, with an efficacy equal to prednisolone. The ability of AL-438 to recruit PGC-1 was significantly reduced to that of prednisolone (Coghlan et al., 2003). Because PGC-1 is involved in the glucocorticoid regulation of hepatic glucose production, the loss of this interaction may explain why AL-438 causes less hyperglycemia in vivo compared with prednisolone. Although these data did not characterize a fully dissociative compound, they did demonstrate that structural changes induced by AL-438 versus prednisolone are very different, and that these differences are responsible for not only cofactor interaction but altered pharmacology as well. These data not only suggest that the mechanisms of transactivation and transrepression are diverse processes, but that it is possible to achieve therapeutic benefit without complete separation between activation and repression.
After extensive high-throughput screening using a GR-dependent cotransfection assay and extensive medicinal chemistry efforts, LGD-5552, (5Z)-5-[(2-fluoro-3-methylphenyl)methylidene]-10-methoxy-2,2,4-trimethyl-1H-chromeno[3,4-f]quinolin-9-ol (Fig. 11), was identified and synthesized. LGD-5552 is a nonsteroidal compound similar in size to prednisolone. It exhibits selective binding to human GR, antagonizes prednisolone-induced transcriptional activation of the MMTV promoter, and displays agonistic properties in repressing IL-1β/TNF-induced activation of E-selectin and IL-6 promoters. In an adjuvant-induced arthritis model, a strong repression of serum monocyte chemoattractant protein 1 and a reduced mRNA expression of joint ankle cyclooxygenase 2 was observed after LGD-5552 treatment. In contrast to prednisolone, the anti-inflammatory cytokine IL-10 was upregulated by LGD-5552 treatment. Furthermore, LGD-5552 binding to the LBD resulted in fundamental changes to the outer receptor structure, which altered the ability of GR to interact with coactivators and corepressors. The conformational change resulted in effects on gene expression in a gene-specific manner, leading to differential gene responses. LGD-5552 demonstrates selectivity on bone growth, blood pressure, and thymus organ weight and remains a powerful anti-inflammatory agent (Miner et al., 2007a; Lopez et al., 2008).
“Dissociated” compounds completely dissociate transactivation from transrepression by GR. Compounds in this class fail to globally induce GR-mediated transactivation, but still significantly repress gene transcription. In an effort to identify GR agonists with a dissociative profile, several hundred compounds were screened at Bayer Schering Pharma AG (Berlin-Wedding, Germany). ZK216348, 3-dihydro-1-benzofuran-7-yl)-2-hydroxy-4-methyl-N-(4-methyl-1-oxo-2,3-benzoxazin-6-yl)-2-(trifluoromethyl)pentanamide (Fig. 11), was obtained from this screen as it did not induce tyrosine amino transferase (TAT) activity but was able to repress IL-8 expression (Schäcke et al., 2004). ZK216348 showed higher potency and fewer side effects compared with prednisolone after subcutaneous injection in mice. Similar anti-inflammatory effects were observed with this compound after systemic treatment. Although ZK216348 did not increase blood glucose in rats in a dose-dependent manner, no differences in ACTH suppression were observed compared with glucocorticoid treatment (Schäcke et al., 2004).
The effect of ZK216348 on osteoprotegerin (OPG) and RANKL in osteoblastic cells was evaluated as both are pivotal proteins in the regulation of bone mass. RANKL stimulates bone resorption by increasing osteoclast differentiation, and OPG is a decoy receptor for RANKL and inhibits bone resorption. Dexamethasone, prednisolone, deflazacort, RU24858, and other RU compounds all inhibited OPG production by a maximum of 70–80% whereas AL438 and ZK216348 inhibited OPG production by a maximum of 40–50% at 1μM (Humphrey et al., 2006). Therefore, these data suggest that these compounds may induce less bone loss than traditional glucocorticoid treatment. These data also suggest that there is a difference in how these two compounds cause GR to repress OPG compared with traditional glucocorticoids. Further studies need to be performed to determine whether ZK216348 and AL-438 recruit cofactors differently than prednisolone to both OPG and RANKL promoters. However, because of its improved safety profile, ZK216348 is a promising alternative for the treatment of inflammatory disorders.
The first example of a dissociated compound isolated from a natural source was Compound A (CpdA). This molecule is a stable analog of the hydroxyl phenyl aziridine precursor found in the Namibian shrub Salsola tuberculatiformis. This compound lacks a steroidal structure, but it is efficient at downregulating NF-κB-driven genes via GR binding. What is most intriguing about CpdA is that it does not stimulate GRE-driven genes, suggesting that it is a completely dissociated compound (De Bosscher et al., 2005). CpdA interferes with the DNA-binding capability of NF-κB and also directly inhibits the transcriptional capacity of the NF-κB p65 subunit via activated GR. CpdA is an equally effective anti-inflammatory agent in vivo as dexamethasone, but it presents with a significantly better side-effect profile because it does not stimulate hyperglycemia (De Bosscher et al., 2005).
Recent evidence suggests that the transcriptional activity of GR upon agonist stimulation is correlated with an increase in the phosphorylation status of Ser211 in the N-terminus of GR (Wang et al., 2002). Interestingly, in contrast to dexamethasone, CpdA did not affect the phosphorylation of Ser211, suggesting that CpdA may induce a subtly different conformational change in GR than classic glucocorticoids. Thus, the phosphorylation status of Ser211 may reflect differences in transrepression versus transactivation and may be a valid screening method for the identification of dissociative compounds. Furthermore, it has been demonstrated that CpdA could effectively suppress experimental autoimmune neuritis, a helper T cell−mediated autoimmune demyelinating inflammatory disease of the peripheral nervous system, suggesting that CpdA could be a potent candidate for treatment of autoimmune neuropathies (Zhang et al., 2009).
The idea of completely dissociated GR compounds is enticing, but mouse models containing GR mutants suggest that complete abolishment of GR activity may not yield the anticipated results. For example, a study using the GRdim/dim mice demonstrated that while anti-inflammatory effects were seen in these mice, they still developed osteoporosis after a 2-week systemic treatment with prednisolone (Rauch et al., 2010). These results suggest that some but not all negative side effects might be eliminated with the complete dissociation of transactivation with transrepression. Additionally, GR transactivates some genes known to negatively regulate the immune system, including thymosin β4 sulfoxide (Young et al., 1999), glucocorticoid-induced leucine zipper (Berrebi et al., 2003), and macrophage migration factor (Calandra et al., 1995). Complete dissociation of transactivation from transrepression would disrupt these genes anti-inflammatory activities. Furthermore, the ability of GR to act as a “coactivator” via tethering does not appear to be affected in the GRdim/dim mice. For example, STAT5, a transcription factor that regulates the expression of many proinflammatory cytokines, is not affected in the dimerization and DNA-binding mutant (Stocklin et al., 1996). Thus, the quest for dissociated anti-inflammatory glucocorticoids with reduced side effects, although attractive, has some limitations.
“Soft steroids” refer to a class of corticosteroids that act at or near the site of administration but are rapidly inactivated by enzymes, thereby reducing systemic exposure and activity. Such compounds are used topically or inhaled for dermatologic diseases and asthma, respectively (Lee and Ko, 1999; Belvisi and Hele, 2003). Glucocorticoids that follow this principle have been designed. These drugs act locally by enzymes in the skin (methylprednisolone aceponate) or in the lungs (ciclesonide, butixocort 21-propionate) and show a low systemic exposure. These glucocorticoids potently inhibit proinflammatory cytokines and chemokines at the site of administration, while eliciting limited systemic responses (Welker et al., 1996; Gunther et al., 1998; O’Connell, 2003).
A selective, nonsteroidal GR modulator that was identified by Bayer Schering Pharma well suited for local application was ZK-245186, (2R)-1,1,1-trifluoro-4-(5-fluoro-2,3-dihydro-1-benzofuran-7-yl)-4-methyl-2-[[(2-methylquinolin-5-yl)amino]methyl]pentan-2-ol (Fig. 11), also known as BOL-303242. This compound is expected to have a favorable therapeutic index due to its low systemic availability because of low metabolic stability and high systemic clearance. ZK-245186 was examined in a variety of in vitro and in vivo ocular models. Primary ocular cell cultures were challenged with either LPS or interleukin-1β (IL-1β), and the effects of ZK-245186 on NF-κB and mitogen-activated protein kinases (MAPK) were assessed by Luminex technology. ZK-245186 significantly reduced LPS- or IL-1β-induced inflammatory cytokine release in a dose-dependent manner. This compound also showed potency and activity similar to dexamethasone, with IC50 values in the nanomolar range (Zhang et al., 2009). Furthermore, the anti-inflammatory actions of ZK-245186 were demonstrated in in vitro assays to inhibit T-cell cytokine secretion and proliferation. Using a T cell-mediated contact allergy model, ZK-245186 showed anti-inflammatory efficacy after topical application similar to that of classic glucocorticoids. ZK-245186 also demonstrates a better safety profile than classic glucocorticoids, as growth inhibition and induction of skin atrophy after long-term application was decreased (Schäcke et al., 2009).
Mapracorat, formerly known as ZK-245186 or BOL-303242, was also evaluated in vivo in models of dry eye and ocular disease. Mapracorat inhibited IL-6, IL-8, and monocyte chemoattractant protein 1 secretion from human corneal epithelial cells with potency similar to dexamethasone. Mapracorat treatment also decreased NF-κB and AP-1 activity (Cavet et al., 2010). In experimental models of dry eye and postoperative inflammation, mapracorat improved ocular inflammation similar to dexamethasone but demonstrated reduced effects in intraocular pressure and body weight (Shafiee et al., 2011). Collectively, these data indicate that ZK-245186/BOL-303242/Mapracorat shows efficacy similar to that of traditional steroids while exhibiting improved side-effect profiles. Currently, ZK-245186 is under phase 2 clinical trials for atopic dermatitis and eye inflammation (Schäcke et al., 2009).
While glucocorticoids are widely used as potent anti-inflammatory agents, their initial discovery was based on the fact that their primary role is to regulate glucose metabolism. Glucocorticoids raise blood glucose levels by antagonizing insulin action, thereby inhibiting the disposal of glucose and promoting hepatic glucose production. This vicious circle is a hallmark of type 2 diabetes. GR antagonism has been a validated strategy for regulating hepatic glucose output in vitro, in animal disease models, and in humans. Most of the GR antagonist validation studies have used mifepristone (RU486), as this drug has been demonstrated to normalize glucose levels and increase insulin sensitivity. However, long-term systemic GR antagonism is not a viable approach for the treatment of type 2 diabetes as long-term use can lead to symptoms of adrenal insufficiency (nausea, vomiting, exhaustion). In addition, generalized GR antagonism results in activation of the hypothalamic-pituitary-adrenal axis, causing stimulation of the adrenal cortex and increasing cortisol secretion, all undesirable symptoms from a patient’s perspective.
With this in mind, several researchers have attempted to design selective hepatic GR antagonists. Using a novel in vivo assay that simultaneously evaluated both heptic and systemic GR blockade, researchers at Abbott Laboratories identified A-348441 [4-[(3S,7R,12S)-7,12-dihydroxy-3-[2-[4-[(8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-11-yl]-N-methylanilino]ethoxy]-2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid] (von Geldern et al., 2004). This compound was the first identified liver-selective GR antagonist and was proven to reduce glucose levels and improve lipid profiles in an animal model of diabetes (von Geldern et al., 2004). This compound was so promising that it has since reached clinical trials. The clinical phase 1 program of A-348441, now known as KB3305, has recently been completed. The results of this trial show a pronounced, clinically relevant, and statistically significant lowering of fasting plasma glucose levels compared with baseline and placebo. There was also a statistically significant improvement in glucose tolerance tests. The side-effect profile was acceptable as no serious adverse reactions were reported. The results of the trial are still being analyzed, but it is still intriguing as well as exciting that such a drug has been identified. The ability to create a tissue-specific compound not only paves the way to generate other tissue-specific GR modulators, but also proves that separation of GR function is possible.
Our knowledge of the molecular events surrounding GR activation has greatly increased in the past decade, leading to the generation of several therapeutic selective GR modulators, but the identification of a truly dissociative compound still remains at large. However, characterization of the GRdim mice should give us some pause in regards to the plausibility of dissociative compounds. The idea of separating transactivation and transrepressive activities of GR is extremely enticing, but data from the GRdim mouse have suggested that some of the negative side effects associated with GR transactivation may not be ameliorated: inhibition of bone formation still occurred in these mice. Additionally, a full anti-inflammatory response did not occur in the GRdim mice, again suggesting that some transactivation events may be beneficial as some anti-inflammatory genes are induced by GR transactivation (Grose et al., 2002; Kleiman and Tuckermann, 2007; Tuckermann et al., 2007).
Despite the restrictions surrounding dissociating transactivation and transrepression, there have been several successful SGRMs/dissociative compounds identified, including AL-438, ZK216348, and ZK245186. Animal studies have revealed an improved ratio between therapeutic efficacy and side effects. However, only clinical trials will define the benefit-risk ratio in humans. With this in mind, rather than searching for more “dissociative” compounds, perhaps identifying “differential” compounds with the most favorable functional profiles is a more realistic approach.
VII. Selective Peroxisome Proliferator-Activated Receptor γ Modulators
A. Peroxisome Proliferator-Activated Receptor γ Structure
PPARγ (NR1C3) can be grouped with other nuclear receptors that heterodimerize with RXR (Germain et al., 2006). Alternative splicing of the PPARG gene transcript produces two protein isoforms in humans. Isoform 2 (PPARγ2) is 505 amino acids and is highly expressed in adipocytes; isoform 1 (PPARγ1) lacks the first 28 amino acids at the N-terminus and is expressed in a wider variety of tissues (Tontonoz et al., 1994; Mukherjee et al., 1997). From this point forward, stated residue numbers will refer to human PPARγ2. PPARγ contains structural domains similar to other NRs, a DBD and a LBD. Heterodimerization with RXRα allows tight binding of PPARγ to DNA through the DBD (Yu et al., 1991; Kliewer et al., 1992). Similar to other transcription factors, PPARγ regulates transcription of target genes near a particular DNA sequence motif, the PPAR response element (PPRE) (Tugwood et al., 1992). Along with several coregulators, PPARγ regulates transcriptional expression of genes in a ligand-dependent manner, including p300, CBP, RIP140, SRC1, SRC2, PGC1, NCOR, TRAP 220, and PRIP (Yuan et al., 1998; Zhu et al., 2000; Wu et al., 2003; Yu et al., 2005; Gelman et al., 2007; McKenna and O’Malley, 2010; Pochetti et al., 2010). Similar to other nuclear receptors, PPARγ also contains two regions that are heavily involved in activation of transcription. The N-terminal AF-1 domain, of which, a portion (residues 29–136) is capable of transcriptional activation alone, and the C-terminal AF-2 domain (Adams et al., 1997; Li et al., 2000).
Individual reports appear contradictory, but a meta analysis of the effects of a natural variant of the N-terminal region (P12A) in humans suggests it is weakly associated with higher body mass index (Masud et al., 2003). Phosphorylation of Ser112 decreases PPARγ transcription and adipogenesis, whereas reduced phosphorylation of Ser112 (through a P113Q allele) appears to be associated with obesity and increased insulin sensitivity in humans (Hu et al., 1996; Adams et al., 1997; Camp and Tafuri, 1997; Ristow et al., 1998; Camp et al., 1999). These reports indicate that the N-terminus of PPARγ affects ligand-dependent and independent transcriptional activity, but the exact mechanisms by which it does this are unknown. It is likely that AF-1 interaction with coregulators and or C-terminal PPARγ is involved.
The PPARγ C terminus contains the LBD, which consists of 275 residues (230–505), and contains the AF-2 region, consisting of 12 α-helices and a small β-sheet region. The LBP is large (∼1300 Å3) and T shaped to accommodate PPARγ agonists, such as the thiazolidinedione drugs (TZD), which stabilize helix 12 (the AF-2 helix) (Nolte et al., 1998; Kallenberger et al., 2003; Bruning et al., 2007). Based on crystal-structure analysis, stabilization of PPARγ is accomplished by the movement of helix 12 into contact with helices 3–4 (∼305–358), helix 11 (∼459–487), and the ligand (Nolte et al., 1998). The movement of helix 12 into this position forms a hydrophobic patch bound on either end by charged residues on the surface of the AF-2 surface within the LBD. It is thought that this is a key change that allows coactivators to bind to the AF-2 surface, displacing corepressors and activating transcription. Antagonists appear to sterically hinder helix 12’s completion of the AF-2 surface allowing corepressors to bind (Nettles and Greene, 2005). However, HDXMS data suggest that some ligands achieve reduced insulin resistance without significant stabilization of helix 12, likely through prevention of Ser273 phosphorylation (Hamuro et al., 2006; Bruning et al., 2007; Choi et al., 2010).
B. Peroxisome Proliferator-Activated Receptor γ Function
Many genetic PPARγ manipulations have been performed in mice that have yielded contradictory results; but, in general, they emphasize the importance of PPARγ in numerous tissues to insulin sensitivity (Barak and Kim, 2007). The loss of one or both alleles of PPARγ by dominant-negative effects reduces adipose tissue and leads to insulin resistance in humans (Barroso et al., 1999; Francis et al., 2006). Somatic loss-of-function mutations have been found in human colon cancer (Sarraf et al., 1999).
PPARγ is widely known as the target for the two TZDs rosiglitazone and pioglitazone, which are used clinically to increase insulin sensitivity in type 2 diabetes mellitus, a disease with a significant worldwide health impact (Wild et al., 2004). In addition to insulin sensitization, agonism of PPARγ with rosiglitazone or pioglitazone can lead to side effects in humans, including heart failure and edema, weight gain, increased bone fracture in women, and anti-inflammation (Haffner et al., 2002; Bongartz et al., 2005; Dormandy et al., 2005; Kahn et al., 2006; Erdmann et al., 2007; Lago et al., 2007; Home et al., 2009; Loke et al., 2009). There are differences between the TZDs. Pioglitazone reduces nonfatal myocardial infarction and produces a healthier lipid profile while rosiglitazone does not appear to have these properties (Dormandy et al., 2005; Home et al., 2009). Based on the observed adverse effects, the U.S. FDA has severely limited rosiglitazone use; its European counterpart has entirely banned the use of rosiglitazone (Grether et al., 2010). A recent report found that long-term use of pioglitazone is weakly associated with increased bladder cancer in humans, which has prompted the FDA to add new warnings to pioglitazone packaging and to modify prescription guidelines (Lewis et al., 2011; http://www.fda.gov/drugs/drugsafety/ucm259150.htm). Currently, the FDA requires 2-year carcinogenicity studies in preclinical models before the clinical trials, which last more than 6 months and involve PPAR ligands (Aoki, 2007). A PPARγ ligand with significantly fewer negative effects than the TZDs but with similar efficacy in insulin sensitization is needed.
Many studies elucidating PPARγ-dependent effects and their mechanisms have been published, which supports ongoing efforts toward a safer PPARγ ligand. PPARγ plays an integral role in fat metabolism, where it is both necessary and sufficient for differentiation of precursor cells into adipocytes (Tontonoz et al., 1994; Barak et al., 1999; Rosen et al., 1999). This function may be mediated by endogenous PPARγ ligands, including the nitrated fatty acids linoleic acid (LNO2) and oleic acid (OA-NO2), which are potent natural PPARγ agonists that induce adipocyte differentiation (Baker et al., 2005; Schopfer et al., 2005; Li et al., 2008). The formation of smaller adipocytes, as found in lean subjects, along with other changes in fat cells brought on by changes in PPARγ activity may help increase insulin sensitivity (Tontonoz and Spiegelman, 2008; Kawai and Rosen, 2010). However, PPARγ’s proadipocyte role appears to negatively affect bone density and strength as PPARγ agonism can block osteoblast differentiation and cause osteoblast precursors to form adipocytes. This inhibits bone formation, causing increased bone marrow adipocity and apparently increasing the probability of bone fractures in women who take TZDs (Loke et al., 2009; Shockley et al., 2009; Kawai and Rosen, 2010). In addition to negative effects in bone density, renal PPARγ appears to directly affect Na+ excretion, which is likely a factor in edema and plasma volume expansion induced by TZDs (Guan et al., 2005; Savkur and Miller, 2006). It has also been shown that PPARγ in the brain that has been agonized by TZDs may be responsible for increased calorie intake, leading to fat weight gain (Ryan et al., 2011). Interestingly, PPARγ ligands also suppress many cytokines and chemokines, with some reports showing clear PPARγ-dependent modulation of inflammation by its ligands (Ricote and Glass, 2007). Suppression of inflammation via PPARγ can involve direct interactions with NF-κB and NFAT, or some other indirect mechanisms (Chung et al., 2000; Yang et al., 2000; Ricote and Glass, 2007; Tontonoz and Spiegelman, 2008; Zhao et al., 2011b). Sumoylated (K395) PPARγ can block degradation of transcriptional repressors on NF-κB and AP-1 responsive genes in macrophages (Pascual et al., 2005). PPARγ’s ability to suppress inflammation may be an important part of a PPARγ drug’s efficacy in increasing insulin sensitivity in diabetes (Kallenberger et al., 2003; Hotamisligil, 2006; Tontonoz and Spiegelman, 2008).
Although both clinical data and PPARγ biology point out that inducing all or most of PPARγ’s effects is not desirable, inducing a subset of effects such as insulin sensitization, anti-inflammation, and antiproliferation would be (Han and Roman, 2007; Straus and Glass, 2007). This is the goal of selective PPARγ modulator (SPPARγM) development. Many PPARγ ligands that elicit fewer negative effects of PPARγ agonism while still increasing insulin sensitivity have been developed (Rangwala and Lazar, 2002; Higgins and DePaoli, 2010). Much of the current preclinical work suggests distinct PPARγ ligands can cause different outcomes in vivo. One prominent clinical example demonstrates that two PPARγ ligands can produce differing clinical results in humans: pioglitazone lowers triglyceride levels while rosiglitazone does not (Chiquette et al., 2004; Goldberg et al., 2005). A conceptual model of PPARγ function is emerging that offers some insight into how SPPARγMs may tune PPARγ to produce selective effects (Wu et al., 2003). The binding of a particular ligand (or no ligand) by PPARγ produces a unique equlibria of conformations and dynamics that favors/disfavors interaction with a particular set of protein kinases, ubiquitin ligases, or coregulators, thus producing ligand-specific transcriptional effects (Oberfield et al., 1999; Rocchi et al., 2001; Fujimura et al., 2005; Pascual et al., 2005; Burgermeister et al., 2006; Kim et al., 2006c; Motani et al., 2009; Choi et al., 2010). Many of the SPPARγMs do show distinct cofactor binding from rosiglitazone or pioglitazone; however, the right combination of coregulator recruitment necessary for desired physical effects remains unclear. Extensive PPARγ and nuclear receptor research has enabled development of ligands that show some SPPARγM characteristics. The goal of the SPPARM concept, a ligand that shows clear separation of weight gain, edema, and bone loss from insulin sensitization, has yet to enter clinical practice.
C. Selective Peroxisome Proliferator-Activated Receptor γ Modulator
GW0072, 4-[4-[(2S,5S)-5-[2-(dibenzylamino)-2-oxoethyl]-2-heptyl-4-oxo-1,3-thiazolidin-3-yl]butyl]benzoic acid (Fig. 12), is one of the first published PPARγ partial agonists that induces less transcriptional activity from a PPRE-containing reporter plasmid in a cell-based assay than the TZDs (Lehmann et al., 1995; Rocchi et al., 2001). Oberfield et al. (1999) found that GW0072 did not occupy the end of the T-binding pocket associated with interaction with helix 12, which may explain its partial agonism (15–25%), despite a binding affinity comparable to rosiglitazone. It also antagonized the activity of rosiglitazone in transcription, coregulator recruitment, and fat-cell differentiation assays. However, both rosiglitazone and GW0072 recruited far less NCoR to PPARγ compared with vehicle in a mammalian two-hybrid assay (Oberfield et al., 1999). Additionally, GW0072 recruited the PGC1α peptide to a similar degree as rosiglitazone, despite recruiting far fewer SRC1, SRC2, and SRC3 peptides (Burgermeister et al., 2006). Recruitment of PGC1α likely promotes insulin sensitization, as it is upregulated with exercise and likely contributes to some of the observed benefits of exercise on metabolic syndrome, so a strong case for the benefits of PGC1α can be made (Handschin and Spiegelman, 2008). Furthermore, skeletal muscle from PGC1α+/− mice shows a significant increase in expression of several proinflammatory cytokines and a reduced expression of PGC1α, leading to chronic inflammation, which would be expected to reduce insulin sensitivity (Hotamisligil, 2006; Handschin et al., 2007; Handschin and Spiegelman, 2008). Therefore, retaining PGC1α recruitment similar to rosiglitazone is likely beneficial.
Fig. 12.
PPARγ modulators.
Lack of SRC2 is implicated in inhibiting obesity, while SRC1−/− mice become obese more readily, so it is difficult to determine what effect reduced recruitment of both these factors would have (Picard et al., 2002). Rosiglitazone both blocked osteoblast formation and promoted adipocyte differentiation, while GW0072 blocked osteoblast formation but did not promote adipocyte differentiation (Lecka-Czernik et al., 2002). Unpublished observations in insulin-resistant Zucker rats have indicated that GW0072 lowered plasma insulin and triglyceride levels as much as a full agonist with less weight gain (Willson et al., 2001). Therefore, GW0072 does more than simply block the binding of rosiglitazone, changing the state of PPARγ from that characterized by the exogenous ligand free form to one that releases NCoR and binds PGC1α, blocks osteoblast differentiation, and possibly increases insulin sensitivity.
Fmoc-l-leucine (F-L-Leu) (Fig. 12) is a PPARγ-selective partial agonist that induces transcription up to 85% that of rosiglitazone. Two F-L-Leu molecules bind to one PPARγ molecule with a Ki of 15 μM. A mammalian two-hybrid assay using full-length cofactors showed that the coactivator SRC2 (TIF-2) interacts with rosiglitazone-bound PPARγ but not with F-L-Leu-bound PPARγ, whereas both F-L-Leu and rosiglitazone have similar binding of SRC1 (Rocchi et al., 2001). These full-length data differ from peptide recruitment assay results that have demonstrated that SRC 1, 2, and 3 peptides are all less recruited to PPARγ by F-L-Leu than by rosiglitazone (Rocchi et al., 2001; Burgermeister et al., 2006). The lack of SRC2 recruitment and the maintained SRC1 recruitment (compared with rosiglitazone) by F-L-Leu-bound PPARγ may be at least partially responsible for the reduced weight gain in F-L-Leu-treated mice. This can be seen in SRC2−/− mice, which are resistant to obesity and have reduced adipocyte size, increased plasma leptin concentration, and enhanced adaptive thermogenesis, while the SRC1−/− mice are obesity prone (Rocchi et al., 2001; Picard et al., 2002). F-L-Leu induced adipocyte differentiation and was able to increase glucose tolerance in db/db diet-induced glucose-intolerant mice. However, F-L-Leu was also able to enhance glucose tolerance in normal mice while rosiglitazone did not. F-L-Leu also demonstrated anti-inflammatory efficacy in a 2,3,6-trinitrobenzene sulfonic acid-induced colitis mouse model (Rocchi et al., 2001).
MK0533, (2R)-2-(3-3-[(4-methoxyphenyl)carbonyl]-2-methyl-6-(trifluoromethoxy)-1H-indol-1-ylphenoxy)butanoic acid (Fig. 12), is a partial agonist that had comparable effects on blood glucose reduction to those of pioglitazone and rosiglitazone in obese diabetic db/db mice. It also increased brown adipose tissue as compared with vehicle in Sprague-Dawley rats, but not as significantly as rosiglitazone. In addition, while rosiglitazone increased plasma volume, extracellular fluid, and heart weight after 7 days of treatment, MK0533 did not produce significant increases in those same parameters in Zucker fa/fa rats (Acton et al., 2009). Although these preclinical data showed promise, a phase 2 clinical trial for MK0533 by Merck was terminated due to lack of glycemic and body fluid benefits over pioglitazone (Merck; http://clinicaltrialsgov/ct2/show/NCT00543959).
PA-082, 1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-4-[4-(2-methoxy-phenyl)-piperidin-1-ylmethyl]-isoquinoline (Fig. 12), induced less recruitment of SRC1, SRC2 and SRC3, but nearly equal recruitment of PGC1α as compared with rosiglitazone in a fluorescent resonance energy transfer peptide recruitment assay with a profile very similar to GW0072 and F-L-Leu. This assay also demonstrated that, like rosiglitazone and GW0072, PA-082 caused displacement of NCoR. PA-082’s selective recruitment of PGC1α may be important to either its positive effects on glucose uptake and insulin signaling or lack of triglyceride accumulation in adipocytes in vitro. This same group stated unpublished observations that administration of PA-082 to db/db mice resulted in lower plasma triglyceride levels without body or liver weight gain (Burgermeister et al., 2006).
FK614, 3-(2,4-dichlorobenzyl)-2-methyl-N-(pentylsulfonyl)-3-H-benzimidazole-5-carboxamide (Fig. 12), is a partial agonist, inducing 75% of the transcription induced by several TZDs while displaying different cofactor binding. FK614 recruited less SRC1, p300, CBP to PPARγ than the TZDs while equaling their PGC1α, PRIP, and PPAR-binding protein recruitment and NCoR and SMRT displacement (Fujimura et al., 2005, 2006a,b). In db/db mice, FK614 reduced plasma triglyceride levels and plasma glucose to levels comparable to rosiglitazone at the same dosage (3.2 mg/kg). FK614 also reduced plasma insulin and glucose in ob/ob mice similar to pioglitazone at 1/3 the dose. Interestingly, FK614 reduced red blood cell (RBC) counts in female rats (and not male) at doses of 320 mg/kg while rosiglitazone affected RBC counts in both male and female rats at 32 and 100 mg/kg (Minoura et al., 2004). A reduction in RBC count can indicate an increase in blood plasma volume, a known side effect of rosiglitazone. Thus, FK614 appears to have effects comparable to those of rosiglitazone and pioglitazone on plasma glucose levels in ob/ob mice and insulin sensitivity in Zucker fatty rats with less propensity for increasing plasma volume/edema (Minoura et al., 2004, 2005, 2007). In white adipose tissue, FK614 had similar effects to pioglitazone, increasing the number of small adipocytes to near lean controls and slightly decreasing the number of large adipocytes (Minoura et al., 2007). However, both rosiglitazone and FK614 trended toward weight gain in Zucker fatty rats (Minoura et al., 2005). Astellas Pharma (Toyko, Japan) terminated development of FK614 in 2005 due to lack of benefits over existing PPAR agonists (http://www.astellas.com/en/ir/library/pdf/h_pre2006_en.pdf).
Another SPPARγM, INT-131 (T131, AMG131), N-(3,5-dichloro-4-quinolin-3-yloxyphenyl)-2,4-dihydroxybenzenesulfonamide (Fig. 12), induces ∼20% of the expression of rosiglitazone in a PPRE plasmid reporter assay while binding more tightly to PPARγ than rosiglitazone. INT-131 binds to the PPARγ LBD in a manner distinct from rosiglitazone, with the most notable difference being that it apparently does not interact with helix 12 (AF-2 Helix). It also displays decreased TRAP220, SRC2, SRC3, CPB, and p300 peptide binding when compared with rosiglitazone. Conversely, it shows similar TIF1 and RIP140 peptide binding and dramatically increased, near-unbound levels of NCor and SMRT corepressor binding compared with rosiglitazone (Motani et al., 2009). The differences in binding and subsequent coregulator recruitment translate into cell culture and in vivo differences between rosiglitazone and INT-131. Unlike rosiglitazone, INT-131 weakly promotes differentiation of preadipocytes and antagonizes the rosiglitazone-induced differentiation. Additionally, INT-131 causes similar glucose tolerance and weight gain in Zucker fatty rats; however, plasma volume, and heart and lung weight are less in comparison with rosiglitizone (Motani et al., 2009). In a phase 2b clinical trial, INT-131 was tested at 1 mg and 10 mg per day for 4 weeks. The 1 mg/day and 10 mg/day groups both showed significant improvement over placebo in fasting plasma glucose. The 1 mg/day group had no significant changes in hematocrit or weight, but the 10 mg/day group had significantly decreased hematocrit (indicative of increased plasma volume) and significantly increased weight along with clinical evidence of edema in 6 of 24 patients. Thus, the 1 mg/day group did not display significant safety risks but did have a significant improvement in fasting plasma glucose; the 10 mg/day produced significant unwanted effects (Dunn et al., 2011). Whether INT-131 can succeed in separating edema, heart failure, weight gain, and bone marrow adipocity from adequate insulin sensitization better than the TZDs in humans is still questionable.
In 2006, Kim et al. (2006a,b) described the novel nonthiazolidinedione compound KR-62980, 1-(trans-methylimino-N-oxy)-6-(2-morpholinoethoxy)-3-phenyl-1H-indene-2-carboxylic acid ethyl ester (Fig. 12), that induces only 30% of the transcriptional activity compared with rosiglitazone in HIH3T3 cells using a PPARγ LBD GAL4 assay. KR-62980 also causes little differentiation of C3H10T1/2 cells, almost no induction of the aP2 transcription, and blocks differentiation and aP2 transcription induced by rosiglitazone. Additionally, KR-62980 allows for recruitment of SRC2 similar to rosiglitazone in a mammalian two-hybrid assay, but dramatically reduces TRAP220 recruitment and slightly reduces association of AIB1 and SRC1 compared with rosiglitazone. A 14-day high-fat diet in C57BL/6J mice treated with KR-62980 improved plasma glucose similar to rosiglitazone. It was interesting to note that KR-62980 also significantly reduced the high-fat diet-induced body fat and heart weight gain, but treatment with rosiglitazone did not appear to affect this (Kim et al., 2006b). KR-62980 also appears to have several anti-inflammatory effects, including IL-4 and IFNγ suppression, and improved outcomes when administered in the context of an allergic asthma model (Won et al., 2010). Both KR-62980 and rosiglitazone showed neuroprotective and antiproliferative effects in a breast cancer cell line (Kim et al., 2006b, 2011). Interestingly, pioglitazone significantly reduced the risk of breast cancer in the PROactive Trial (Dormandy et al., 2005). Further investigation into the antiadipogenic mechanism of KR-62980 revealed that TAZ (transcriptional coactivator with PDZ-binding motif) is necessary for KR-62980 to block rosiglitazone-induced adipocyte differentiation in 3T3-L1 cells (Jung et al., 2009). TAZ had been previously shown to bind PPARγ and to function to block adipocyte and induce osteoblast differentiation from mesenchymal stem cells (Hong et al., 2005). KR-62980 increased transiently expressed TAZ nuclear localization in Cos7 cells. It also increased the interaction of TAZ and PPARγ in an immunoprecipitation assay using 3T3-L1 adipocytes, and decreased binding of PPRE containing DNA to PPARγ in 3T3-L1 adipocyte nuclear protein extract (Jung et al., 2009). An abstract from the American Society for Bone and Mineral Research reported that KR-62980 has pro-bone-formation effects and helped maintain bone mass in ovariectomized mice (Bae et al., 2007). These data suggest that KR-62980 increases PPARγ TAZ interaction, which inhibits adipocyte differentiation and induces osteoblast differentiation, while still decreasing inflammation and improving plasma glucose levels in mice on a high-fat diet. Separation of bone loss from insulin sensitization has not been clearly demonstrated, and it may be difficult given that the pro-adipocyte-differentiation effects of PPARγ appear to be partly responsible for both insulin sensitization and bone loss. It appears possible that KR-62980 can clearly separate these two activities. The apparent insulin sensitization effects combined with pro-osteogenic and antiadipogenic activities would make KR-62980 a strong and rather unique candidate for further development.
CLX-0921 (THR0921) (Fig. 12) is structurally similar to polyphenols found in the bark of plants of the Pterocarpus genus, which have been traditionally used as a remedy for diabetes within the ayurvedic system of medicine. In vitro assays using 3T3-L1 cells demonstrated that CLX-0921 recruited equivalent CBP coregulator at 10-fold higher concentrations than rosiglitazone and produced half the triglyceride accumulation of rosiglitazone. CLX-0921 also induced glycogen synthesis in HepG2 cells while rosiglitazone did not. In ob/ob mice, a dose of 10 mg/kg per day of CLX-0921 and rosiglitazone improved blood glucose levels to a similar degree over vehicle. However, CLX-0921 did produce weight gain in Zucker diabetic fatty rats, but not ob/ob or db/db mice, along with reductions in blood glucose at a daily dose of 50 mg/kg for all groups (Dey et al., 2003). In a collagen-induced arthritis model, CLX-0921 treatment appeared to improve the clinical score. Spleen cells from arthritic mice treated with CLX-0921 secreted less INFγ, TNFα, and IL1-β than untreated arthritic mice upon challenge with LPS or collagen (INFγ and IL1-β) (Tomita et al., 2006).
Several recent reports have introduced new concepts that impact SPPARγM development and may aid in the development of SPPARγMs with a wider separation of positive and negative effects. Choi et al. (2010) offered new insight into the complex link between PPARγ and insulin sensitization. They reported that phosphorylation of Ser273 of PPARγ by CDK5 is inhibited by rosiglitazone and that Ser273 phosphorylation was strongly anticorellated with in vivo insulin sensitization in a small human trial (Choi et al., 2010). Rosiglitazone shares the ability to block Ser273 phosphorylation with a partial agonist (MRL-24), which is also capable of robust insulin sensitization. The apparent mechanism by which both these drugs block phosphorylation is through stabilization of the β-sheet region near Ser273, which presumably disfavors interaction with CDK5 (Choi et al., 2010). A previous report showed that stabilization of this β-sheet region is achieved by three other partial and full agonists as well, while only the two full agonists stabilized helix 12 (Bruning et al., 2007). These data indicate that stabilization of helix 12 appears necessary for full transcriptional activity but not to induce insulin sensitization. Whether Ser273 phosphorylation is a bystander effect or is a cause of increased insulin sensitivity is yet to be determined. However, development of drugs that specifically block Ser273 phosphorylation without causing transcriptional activation of PPARγ may provide a new direction for future SPPARγM development. MRL-24 is a step in this direction, as it binds to PPARγ with higher affinity than rosiglitazone and blocks Ser273 phosphorylation more effectively, yet induces only 20% of the transcription of rosiglitazone. It also provides as good or better glucose correction than rosiglitazone in db/db or DIO mice while inducing less heart and body weight gain (Acton et al., 2005; Choi et al., 2010). Another recently reported ligand, SR1664 (Fig. 12), goes a step farther, showing no significant induction of transcription from a PPRE containing reporter plasmid while providing robust insulin sensitization with no indication of edema or weight gain (Choi et al., 2011).
Two other reports by separate groups point to the importance of brain PPARγ for weight gain and a portion of insulin sensitivity using different approaches (Lu et al., 2011; Ryan et al., 2011). Ryan et al. (2011) found that injection of a small amount of rosiglitazone or viral delivery of a constitutively active PPARγ into the brain increased feeding and weight gain. Weight gain and increased feeding induced by oral dosing of much larger amounts of rosiglitazone were blocked by ventricular injection of GW9662, 2-chloro-5-nitro-N-phenylbenzamide (Fig. 12), a PPARγ antagonist, which also decreased feeding and weight in rats fed a high-fat diet (Ryan et al., 2011). Lu and coworkers found that both treated and untreated mice with a brain-specific knockout of PPARγ gained less weight on a high-fat diet and rosiglitazone, and had increased activity compared with controls. Interestingly, the PPARγ brain-specific knockout mice did show increases in insulin sensitivity from rosiglitazone treatment. However, the treatment did not reduce basal and insulin-stimulated hepatic glucose production (Lu et al., 2011). Thus, it appears that blood-brain barrier permeability of PPARγ ligands affects weight gain and possibly insulin sensitization. Perhaps modifying existing ligands to make them less able to penetrate the brain could reduce weight gain and leave insulin sensitization largely intact.
No SPPARγM has reached the clinic, but preclinical research indicates that new PPARγ ligands can potentially separate edema, weight gain, and bone loss from insulin sensitization to a greater extent than TZDs. More preclinical analysis of the effect of SPPARγMs on cancer models and bone are needed. SPPARγMs will hopefully result in more effective diabetes treatments, and their anti-inflammatory effects may be useful in treating other diseases as well.
VIII. Liver X Receptor Modulators
A. Liver X Receptor Structure
The LXRs were originally isolated using low-stringency hybridization screening of cDNA libraries and yeast two-hybrid technology. The LXRα isoform was independently identified by two groups and was initially named RLD-1 and LXR, whereas four groups identified the LXRβ isoform, applying the names UR, NER, OR-1, and RIP-15 (Apfel et al., 1994; Shinar et al., 1994; Song et al., 1994; Seol et al., 1995; Teboul et al., 1995; Willy et al., 1995). Further sequence analysis showed that there was approximately 77% amino acid identity in both the DBD and LBD between human LXRα and LXRβ, suggesting that they are closely related. LXRs are highly conserved between humans and rodents, but also share the highest homology with the Drosophila ecdysone receptor and VDR (Willy et al., 1995). Janowski et al. (1996) found a unique class of meiosis-activating sterols through ligand-screening techniques as the first activators of LXRs. However, the major breakthrough in elucidating the physiologic role of the LXRs came with the finding that oxysterols serve as their endogenous ligands (Janowski et al., 1996; Lehmann et al., 1997). A specific group of mono-oxidized derivatives of cholesterol were later identified as the most potent ligands through screening of the cholesterol metabolic pathway; these derivatives are 24(S)-hvdroxycholesterol, 22(R)-hydroxycholesterol, and 24(S),25-epoxycholesterol (Forman et al., 1997; Janowski et al., 1996; Lehmann et al., 1997).
LXRs share the conserved structure of the NRs, and a closer look at the LXRβ LBD reveals that it adopts the canonical fold of nuclear hormone receptors, consisting of three layers of α-helices and possessing a small three-stranded β-sheet (s0, s1, and s2), similar to a set of other, structurally most closely related NRs [VDR, retinoic acid receptor γ (RARγ), TRβ, PPARα, PPARγ, RXRα, RORα, RORβ, and PXR] (Wurtz et al., 1996; Ribeiro et al., 1998; Klaholz et al., 2000; Cronet et al., 2001; Stehlin et al., 2001; Tocchini-Valentini et al., 2001; Watkins et al., 2001; Xu et al., 2001). The ligand-binding pocket of LXRβ is a large hydrophobic cavity that is surrounded by H3, H5, H6, H7, H11, and H12. Its volume of 800 Å3 is slightly larger than the size of the cavity reported for RORα and RORβ (722 Å3 and 766 Å3, respectively) (Stehlin et al., 2001; Kallen et al., 2002). This pocket also shows considerable plasticity, in that it can accommodate ligands of different structures, most likely due to adjustments of the rotational conformers of several residues, without significant effects on the overall structure of the protein. In the agonist conformation of the receptor LBD, an entrance to the ligand cavity exists between H5 and the loop between s1 and s2, similar to the situation in PPARγ (Gampe et al., 2000). The 11 residues between H1 and H3, which are not visible in the electron density, are proximal to this region, and their flexibility might be necessary for ligand access to the binding pocket. Mainly hydrophobic residues, except for residue His435, form the cavity. Known agonists such as T0901317 take advantage of this by binding to His435 close to H12; others such as GW3965 orient themselves with the charged group in the opposite direction. However, both induce a fixed agonist conformation of helix 12 (also called the AF-2 domain), resulting in a transcriptionally active receptor (Farnegardh et al., 2003). Residues lining the ligand-binding pocket of LXRβ are also completely conserved in LXRα. LXRα adopts the classic NR fold; in the structure, helix 12 is positioned in an agonistic position, as seen in the presence of GW3965 (Bennett et al., 2008). Binding of an agonist to the LBD of LXRs induces a cation–π interaction between His421 and Trp443 (LXRα numbering), thus stabilizing the C-terminal helix 12 to form a binding groove for specific protein coactivators (Williams et al., 2003).
LXR activity is regulated by dimerization, and LXRs carry a sequence signature for homo- and heterodimerization (Gampe et al., 2000; Greschik and Moras, 2003). Whether LXRs can effectively signal as homodimers is still controversial; however, it is clear that LXRs are readily activated through heterodimerization with the RXR (Peet et al., 1998a,b; Tamura et al., 2000; Anderson et al., 2003; Lalloyer et al., 2009). The LXR–RXR heterodimer binds to a sequence called the LXRE (LXR-responsive element) in the regulatory regions of target genes. The LXR-responsive element is known to consist of direct repeats of a core motif, AGGTCA, separated by four nucleotides (termed DR4) (Willy et al., 1995; Chawla et al., 2001). LXR–RXR heterodimer bound to the DR4-response element can be transcriptionally activated by either LXR or RXR ligands, which are referred to as “permissive heterodimers” (Zelcer and Tontonoz, 2006). LXR can also be activated by heterodimerization alone, in the absence of ligand, via a mechanism termed dimerization-induced activation. Currently, this is a unique mechanism of NR activation that has not been described for NRs other than LXR (Wiebel and Gustafsson, 1997). Dimerization-induced activation requires the AF-2 domain of LXR, but not the RXR AF-2 domain. The DNA-bound LXR-RXR heterodimer recruits SRC1, which then induces a conformational change to the LXR (Wiebel et al., 1999). This is reminiscent of the structural changes induced by the binding of ligand, termed the phantom-ligand effect (Wiebel and Gustafsson, 1997). In this model of LXR transactivation, which encompasses the permissive heterodimer model and the allosteric effects of RXR heterodimerization, the LXR–RXR heterodimer is activated by dimerization, and exhibits dual-ligand permissiveness and synergism (Wiebel and Gustafsson, 1997; Wiebel et al., 1999; Schulman and Heyman, 2004). However, the precise role of RXR in the interaction of the LXR–RXR heterodimer with coactivators remains poorly understood. In particular, it is unclear whether RXR is a direct binding partner of specific NR boxes of coactivators or functions as an allosteric activator of LXR by stimulating the interaction of LXR with coactivators.
In absence of ligand, LXRs are acetylated at residues Lys432 in LXRα and Lys433 in LXRβ and constitutively bound to RXR (Li et al., 2007). Upon oxysterol binding to the LXR LBD, the carboxy terminal domain is modified to release the corepressors, nuclear receptor corepressor (NCoR), or silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) (Hu et al., 2003). Helix 12 conforms to block the ligand in its binding pocket. Interaction of coactivators, such as activating signal cointegrator-2 (ASC-2), changes the conformation of the AF-2 domain, allowing the chromatin to be in a permissive state to initiate transcription (Svensson et al., 2003; Lee et al., 2008). Corepressor proteins that inhibit transcription can also competitively block this coactivator binding. Coactivators are generally characterized by an LXXLL sequence element, whereas corepressors contain an LXX(I/L)XXL sequence element (Greschik and Moras, 2003; Savkur and Burris, 2004).
B. Liver X Receptor Function
The expression patterns of LXRα and LXRβ demonstrate significant overlap. LXRα has highest expression in liver along with kidney, intestine, fat, lung, macrophages, and spleen while LXRβ is ubiquitously. LXR plays varied physiologic roles consistent with its expression pattern. Thus, agonists for LXR have been shown to suppress hepatic gluconeogenesis, improve insulin sensitivity, and suppress inflammatory and proliferative responses of vascular cells (Nomiyama and Bruemmer, 2008). Targeting this receptor has proven to be of therapeutic importance in the treatment of atherosclerosis, diabetes, cancer, cardiovascular disease, autoimmune disorders, and Alzheimer’s disease (Joseph et al., 2002; Tangirala et al., 2002; Cao et al., 2003; Laffitte et al., 2003; Koldamova and Lefterov, 2007; Riddell et al., 2007; Nomiyama and Bruemmer, 2008; Xu et al., 2009; Chuu, 2011).
Despite their therapeutic potential in hypercholesterolemia and atherosclerotic vascular disease, potent synthetic LXR ligands have problematic, unwanted side effects, including hypertriglyceridemia, largely because of the activation of SREBP1c expression. Hence, the former effects need to be promoted by such a drug, and the latter left unaffected or even antagonized (Steffensen and Gustafsson, 2004). Because of the undesirable effects caused by first-generation LXR agonists, LXRα and LXRβ-selective agonists and selective LXR modulators need to be developed that will help elucidate isoform-specific functions of LXR. In all, further research on the molecular mechanism of LXR is needed, which may facilitate treatment of clinical cardiovascular disease with more suitable drugs. Clearly, as yet we are at the beginning of what promises to be an extremely interesting period of continuous revelations of new functions of the two LXR isoforms, and it is likely that novel approaches to treat diseases will become apparent. The goal of this section is thus to elucidate the biologic aspects of LXR in the generation of selective, therapeutically improved, next-generation selective LXR modulators.
C. Selective Liver X Receptor Modulators
Several natural LXR ligands have been elucidated in mammals, the most important being oxysterols derived by enzymatic pathways or through exogenous nutritional supply (Lehmann et al., 1997; Schroepfer, 2000). Other natural activating oxysterols include 22(R)-hydroxycholesterol in steroidogenic tissues, 24(S)-hyroxycholesterol in the brain and plasma, 25-epoxycholesterol in the liver, and 27-hydroxycholesterol in macrophages, which are known to activate both isoforms of LXR (Janowski et al., 1996; Lehmann et al., 1997; Bjorkhem, 2009). Another cholesterol-derived molecule, follicular fluid meiosis activating sterol, is also a potent activator of LXRα. Desmosterol, 6α-hydroxylated bile acids, and various compounds derived from plants and fungi are also potent activators of LXR (Song et al., 2000; Bramlett et al., 2003; Huang et al., 2005; Jayasuriya et al., 2005; Ondeyka et al., 2005; Yang et al., 2006).
LXR is widely known to be targeted by two nonsteroidal synthetic agonists: T0901317 [N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide] and GW3965 [2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2,2-diphenylethyl)amino]propoxy]phenyl]acetic acid] (Fig. 13). Both have been demonstrated to inhibit atherosclerosis progression and even promote lesion regression in mouse models via a dual mechanisms of activation of reverse cholesterol transport and repression of inflammation (Schultz et al., 2000; Collins et al., 2002; Joseph et al., 2002; Terasaka et al., 2003; Levin et al., 2005). Thus, selective LXR modulators have gained worldwide attention and have high potential for treatment of atherosclerosis. LXR agonists also induce the hepatic SREBP1c expression, which in turn induces expression of fatty acid synthetic genes, causing hepatic steatosis and hypertriglyceridemia in animals (Schultz et al., 2000; Houck et al., 2004; Shenoy et al., 2004; Mitro et al., 2007; Kumar et al., 2010; Solt et al., 2011). Moreover, LXR agonists appear to increase LDL cholesterol levels through upregulation of cholesteryl ester transfer protein (CETP) (Groot et al., 2005). These issues have lead to a considerable hindrance in the development of LXR agonists. In the ideal situation, scientists are in search of tissue-selective LXR modulators (e.g., specificity for macrophages over liver) or even against specific genes (e.g., ABCA1/G1 over SREBP1c). Alternatively, a selective modulator to LXRβ (relatively low expression in the liver) than to LXRα might have improved therapeutic implications in plasma lipids and steatosis.
Fig. 13.
LXR modulators.
Extensive medicinal chemistry research has enabled the development of ligands with some SLiM (selective LXR modulator) characteristics. T0901317 was the first high affinity synthetic LXR ligand identified which caused an ATP binding cassette (ABC) transporter (ABCA1) induction and cholesterol efflux, but also induced hypertriglyceridemia and fatty liver (Schultz et al., 2000). The LXR agonist GW3965 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2,2-diphenylethyl)amino]propoxy]phenyl] acetic acid) has also been reported to induce less hepatic steatosis although it is still clearly significant (Miao et al., 2004; Grefhorst et al., 2005).
Makishima’s group identified YT-32, (22E)-ergost-22-ene-1α,2β diol (Fig. 13), a phytosterol of fungal cell membranes, as a potent LXR activator both in vivo as well as in vitro (Kaneko et al., 2003). Along with the natural function in lowering plasma cholesterol, it did not appear to have the adverse side effects seen with other LXR agonists. LXR-623 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)indazole), an indazole, was also tested in the first reported phase 1 clinical trials; it had adverse CNS side effects, so the study was terminated (DiBlasio-Smith et al., 2008; Katz et al., 2009).
Differential coactivator and corepressor recruitment might also be an important determinant to the tissue specificity of LXRs, as they are with other NRs (e.g., the SERMs tamoxifen and raloxifene) (Shang and Brown, 2002). Similarly, the relative expression levels of coregulators might be important determinants for tissue selectivity (Shang and Brown, 2002). For LXRα, SRC1 and DRIP are recruited to similar levels with T1317 and GW3965. In contrast, GW3965 recruits CBP to a lesser extent when compared with T1317. It is tempting to speculate that coactivators like SRC1 and DRIP might mediate the intestinal effects on gene regulation while CBP mediates liver effects of these two ligands through LXRα.
Current efforts are focused on identification of molecules specific for each isoform, but the close similarity in the LBD of LXRα and LXRβ prevents the development of α or β selective compounds. Thus, identifying SLiMs (selective LXR modulators) that inhibit the progression of atherosclerosis by increasing reverse cholesterol transport without the detrimental lipogenic effects in liver is an important challenge for the future advancement in this field.
It has been postulated that a LXRβ selective compound may have a beneficial outcome on the lipid profile for a ligand by dissociating the favorable and unfavorable effects of LXR agonists. Although there have been a few examples of compounds showing a modest level of LXRα selectivity, obtaining a potent LXRβ-selective compound has proven to be more challenging. Analysis of the structure-activity relationship (SAR) and X-ray crystallographic data suggests that the rational design of a LXRβ-selective compound will not be trivial. WAY-252623 (LXR-623) (Fig. 13) is one such indazole-based selective LXR modulator with an IC50 of 179 and 24 nM for LXRα and LXRβ, respectively (Katz et al., 2009). The X-ray structure of LXR-623 bound to LXRβ revealed that N-1 indazole nitrogen forms a hydrogen bond with His435, while the 7-trifluoromethyl group makes electrostatic interactions with the same residue; this makes it different from GW3965 and explains its good in vivo efficacy (Li et al., 2010). It is a novel partial LXR agonist with an improved therapeutic index as profiled in several animal models expressing CETP, including hamsters and cynomolgus monkeys, and is orally bioavailable (Groot et al., 2005). Katz et al. recently presented results from a single ascending-dose study of the safety, pharmacokinetics, and pharmacodynamics of LXR-623 in human participants. LXR-623 was absorbed rapidly after oral administration with peak concentrations (Cmax) achieved at approximately 2 hours, which increased in a dose-proportional manner (Katz et al., 2009). LXR activation resulted in a dose-dependent increase in ABCA1 and ABCG1 expression. The effect of LXR-623 concentration on ABCA1 and ABCG1 expression was further characterized via a population pharmacokinetic-pharmacodynamic analysis, yielding ED50 estimates of 526 ng/ml and 729 ng/ml, respectively (Katz et al., 2009). Additionally, LXR-623 demonstrated significant efficacy for reducing lesion progression in the murine LDLR−/− atherosclerosis model with no significant adverse effects such as an increase in hepatic lipogenesis, performing significantly better than GW3965 in the same experiment (Quinet et al., 2009). LXR-623 also displayed little lipogenic potential or neutral lipid effects in CETP-expressing species such as the Syrian hamster, suggesting reduced effects on steady-state HDL levels due to enhanced transfer of HDL cholesterol to LDL for clearance by liver via reverse cholesterol transport process (Quinet et al., 2009). In nonhuman primates with normal lipid levels, LXR-623 significantly reduced total (50–55%) and LDL cholesterol (70–77%) in a time- and dose-dependent manner. Significant decreases in LDL cholesterol levels were observed at less than 7 days and reached peak levels by day 28. The results were even better than the conventional reductions observed with 20 mg/kg of simvastatin alone. Treatment with LXR-623 led to hepatic downregulation of several lipogenic genes, including FAS, LDLR, INSIG1, and SREBF1, without causing the adverse effects seen with other LXR agonists.
LXRs are widely expressed in the brain, and LXR agonists have been known to induce cholesterol efflux from neurons and glia by upregulation of ABCA1, and also to upregulate apolipoprotein E for potential beneficial effects on Alzheimer’s disease by lowering β-amyloid (Cao et al., 2007). Unfortunately, LXR-623 displayed adverse CNS effects at higher doses (150 and 300 mg) in a phase 1 clinical trial and was discontinued (Katz et al., 2009).
T0901317, a nonsteroidal synthetic LXR agonist, facilitated the discovery of biologic signaling pathways that are regulated by LXRs (Schultz et al., 2000; Michael et al., 2005). Similar to other older generation LXR agonists, it causes increased expression of lipogenic genes including Fas and SREBP1c. A crystal structure of T0901317 bound to hLXRβ was thus used to design a series of substituted N-phenyl tertiary amines, which led to the identification of the lead LXR ligand GSK-9772 [4-[[N-butyl-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)anilino]methyl]-2,6-dichlorophenol] through profiling in binding and functional assays (Chao et al., 2008). Nuclear receptor selectivity and functional assays showed that GSK-9772 was 100-fold more selective to LXR than either AR, GR, PR, MR, ERα/β, farnesoid X receptor, or PPARα/γ/δ with little cross-reactivity to PXR (EC50 = 250 nM). It had a higher affinity for LXRβ (IC50 = 30 nM) than LXRα (IC50 = 180 nM), and dissociated anti-inflammatory signaling from lipogenic activities in human macrophage and liver cell lines. Mechanistic studies showed that GSK-9772, like first-generation LXR agonist GW3965, effectively suppressed the expression of proinflammatory target genes via a SUMOylation-dependent mechanism. This resulted in a greater than 10-fold selectivity for transrepression versus transactivation. Cofactor profiling also revealed key differences between first-generation LXR agonist T1317 and GSK-9772 by the use of mammalian two-hybrid profiling assays with more than 60 amino acid fragments of SRC1 and NCoR, indicative of LXR stabilization in a basal, repressed state by GSK-9772 (Chao et al., 2008). The LXR ligand GSK-9772 thus serves as a valuable chemical tool for exploring nuclear receptor transrepression and will provide an opportunity for the future discovery of selective LXR modulators with improved therapeutic indexes.
Recent advances in medical research have lead scientists to develop drugs that modulate nuclear receptors in a tissue and/or gene-specific way. Unlike other SERMs and natural ER ligands, raloxifene does not cause adverse effects of the endometrial activation of ER (Black et al., 1994; Clemett and Spencer, 2000). This example of tissue-selective activation of an NR has fueled the quest for selective NR modulators for LXRs. Phenex Pharmaceuticals (Ludwigshafen, Germany) developed two such recently discovered LXR ligands that induce differential cofactor recruitment patterns. Each of them shows different LXRβ–cofactor interaction signaling in cofactor profiles using the yeast two-hybrid system when compared with conventional agonists such as T901317 and GW3965 (Kremoser et al., 2007). Unfortunately their poor pharmacokinetic properties precluded their testing in various animal models of disease.
Despite its history as a difficult drug target, LXR remains a potential therapeutic target for atherosclerosis, diabetes, and other common diseases. Target validation with better definition of therapeutic relevance and a better understanding of the ligand-induced activities that produce tissue-selective and gene-specific beneficial effects should enable the development of safer drugs with minimized unwanted side effects.
IX. Selective Progesterone Receptor Modulators
A. Progesterone Receptor Structure
The PR (NR3C2) is encoded by a single gene on chromosome 11q22 that uses two separate promoters and start sites to generate PR-A and PR-B isoforms (Conneely et al., 1987b; Law et al., 1987; Misrahi et al., 1987; Gadkar-Sable et al., 2005). The PR-A isoform is 769 amino acids, whereas the PR-B isoform is 933 amino acids, with the additional 165 amino acids in PR-B located in the N terminus (Kastner et al., 1990). This unique N-terminal region in PR-B contains a transactivation function known as TAF-3. The identical portions of PR include the part of the N-terminal region that includes the AF-1, DBD, the hinge region, and the LBD composed of the LBP, the AF-2, the coactivator/corepressor interaction surface, and the dimerization motif. The two forms of PR are identical in their DNA-binding and ligand-binding properties, and it is likely that PR homodimers and heterodimers exist. The A form is considerably less transcriptionally active in cotransfection assays than the B form, and it has been demonstrated that PR A may act as a repressor of the more active PR B (Vegeto et al., 1993).
Williams and Sigler (1998) published the first structure of the PR LBD, and the LBD contained the standard 10 α-helices in the helical sandwich but lacked helix 2, although helices 10 and 11 were shown to be contiguous. Progesterone (P4) makes contact with residues in helices 3, 5, 7, 11, and 12 as well as with the β-turn. PR has a C-terminal extension in the LBD (amino acids 922–933) that is essential for P4 binding, similar to the C-terminal extension in glucocorticoid and androgen receptors. A hydrogen bond is formed between the side chain amino group of Gln725 and the 3-keto group of progesterone, which is conserved in all steroid receptors except ER. Hydrogen bonding and van der Waals contacts stabilize the side chain for interaction with P4. Interestingly, this structure showed that the PR homodimer structure is smaller and less stable than previously published RXR and ER dimer structures. Other structures have been solved with PR LBD bound to synthetic ligands, including metribolone, mometasone furoate, norethindrone, tanaproget, mifepristone, levonorgestrel, and several pyrrolidine ligands (Matias et al., 2000; Madauss et al., 2004; Zhang et al., 2005; Petit-Topin et al., 2009; Raaijmakers et al., 2009; Thompson et al., 2009; Kallander et al., 2010). Two other structures were published showing PR bound to asoprisnil and corepressor proteins, specifically NCoR and SMRT (Madauss et al., 2007). The structure of the PR DBD bound to DNA has also been shown, and demonstrates a similar mode of DNA binding as other NRs (Roemer et al., 2006).
Agonist-bound PR forms a dimer that is capable of translocation to the nucleus and binding to progesterone receptor DNA-response elements (PREs) within the genome. The PRE sequence is a palindromic sequence consisting of two inverted hexameric half-sites separated by three base pairs, known as an IR3 [inverted repeat with 3-base pair spacer] sequence. An optimal PRE of −7RGNACANRNTGTNCY+7 was inferred from binding studies involving MMTV and various point mutations and substitutions in the MMTV sequence (Lieberman et al., 1993). In the crystal structure of PR bound to DNA, it was observed that the C-terminal extension of the DBD of PR was able to interact with DNA in the minor groove. This extended region of interaction may be important physiologically because many PR target genes have weak half-site or palindromic sequences that are different from the classic consensus sequence for nuclear hormone receptors. It also has been proposed that the sequence of the 3-base pair spacer region could affect PR binding by altering DNA structural features (Roemer et al., 2006).
Agonist binding to the LBD induces a conformational change, leading to repositioning of helix 12. This shift produces a hydrophobic cleft that facilitates coactivator binding. Coactivator proteins typically contain LXXLL motifs, known as NR boxes, that interact with the exposed hydrophobic cleft of PR. The recruitment of coactivator proteins leads to transcriptional activation of PR target genes. Although PR-A and PR-B are similarly expressed in most human tissues and over 300 coregulators are reported to interact with PR, it is the tissue-specific expression of coregulators that determines the effects of progesterone (Lonard et al., 2007; Scarpin et al., 2009).
Corepressor proteins have also been shown to interact with PR when antagonists, such as RU486 or other selective modulators, are bound to PR. Crystal structures have shown the binding of NCoR and SMRT to both PR-A and PR-B, but this was in the presence of the selective modulator asoprisnil (Madauss et al., 2007). Overexpression of these corepressor proteins in breast and endometrial cancers has been observed, but their role in cancer development remains unclear (Kurebayashi et al., 2000; Kershah et al., 2004).
B. Progesterone Receptor Function
PR plays an essential role in the regulation of reproductive tissue response to progestins. PR is a ligand-dependent transcription factor, whose most prominent endogenous ligand is the steroid hormone progesterone (P4, pregn-4-ene-3,20-dione). P4 is produced predominantly in the ovaries, adrenal glands, and placenta (during pregnancy). Similar to other steroid hormones, the biosynthesis of P4 initiates with the cholesterol molecule. Cholesterol is converted to pregnenolone in a two-step process: a double oxidation at C-20 and C-22 followed by the oxidation of the C-22 diol. These oxidation steps are catalyzed by the cytochrome P450 side chain cleavage enzyme. The 3-hydroxyl group of pregnenolone is then oxidized to form a keto group and subjected to a keto/enol tautomerization by 3β-hydroxysteroid dehydrogenase/Δ5-Δ4-isomerase to yield progesterone.
Progesterone secreted by the corpus luteum after ovulation acts to stimulate differentiation of the uterine endometrium into a glandular structure capable of accepting a fertilized egg via PR. If pregnancy occurs, P4 secretion is elevated during the entire course of the pregnancy, as it is essential during this time. While the corpus luteum continues to produce P4, the placenta is the main site of P4 production after the second month of pregnancy. P4 and PR also play an important role in the mammary glands where they act to suppress lactogenesis until late in pregnancy, when P4 levels decrease.
Having a clear role in reproductive health and female fertility, both PRs are widely expressed in the female reproductive system. Interestingly, they are also expressed in the brain, bone, pancreas, testes, and lower urinary tract (Viale et al., 1992; Graham and Clarke, 1997, 2002; Bland, 2000; Han et al., 2009; Tincello et al., 2009). Examination of the phenotype of PR-null animals has provided significant insight into the explicit functions of the two PR isoforms. Embryos carrying a PR-null mutation (PRKO) developed normally through adulthood, but PRKO female mutant adults were unable to ovulate and exhibited abnormal sexual behavior. The mutants also displayed uterine hyperplasia, inflammation, and abnormal mammary gland development (Lydon et al., 1995). Later work focused on ablating individual PR isoforms to determine the functional differences between PR-A and PR-B. In PR-A knockout mice, superovulation in response to gonadotropin treatment was impaired, but it was not completely absent as is seen in the PRKO mice. The regulation of ADAMTS-1 and cathepsin-L was also unaffected, demonstrating that PR-B is functional in ovarian tissues, unlike what is observed in the PRKO mice. Therefore, PR-B is not entirely responsible for ovulation, with either PR-A functioning as the dominant factor or a heterodimeric interaction between PR isotypes being required. Additionally, embryo implantation is impaired in PR-A knockout mice, which led to the discovery that PR-B regulates only a subset of the genes required for uterine receptivity to implantation. It was also found that PR-B is responsible for uterine proliferative activity, and that PR-A can tissue selectively inhibit both estrogen- and progesterone-induced proliferation in the uterus. In mammary epithelium, progesterone-induced proliferation and differentiation can be mediated solely by PR-B (Conneely et al., 2001). Studies using PR-B knockout mice showed that mammary ductal morphogenesis was reduced, but other ovarian, uterine, and thymic responses were intact due to the presence of PR-A (Conneely et al., 2002).
C. Selective Progesterone Receptor Modulators
The initial clinical efforts for antagonizing PR focused on compounds such as mifepristone, which terminated pregnancies; however, the current research model incorporates molecular substitutions into the hormone-based structure to both agonize and antagonize PR in a tissue-selective manner. This mixed agonist-antagonist selective modulator actually has only a minimal effect on pregnancy termination in mammals, thus removing the stigma associated with targeting PR for clinical applications.
Mifepristone (RU486) (Fig. 14) was synthesized as part of a chemical screen to develop GR antagonists at Roussel-Uclaf (Paris, France) in the early 1980s; it is currently sold in the United States under the tradename Mifeprex through Danco Laboratories (New York, NY). After its discovery as a PR antagonist, mifepristone was investigated for use as an abortion agent, emergency contraceptive, and antiproliferative therapeutic (Chabbert-Buffet et al., 2005). Because PR controls reproductive organs by hormone signaling and transcriptional regulation, other applications of mifepristone have been studied in several phase 2 clinical trials, including treatment of uterine fibroids, endometriosis, depression, glaucoma, and breast, ovarian, and even prostate cancers. Additionally, this compound has been used to treat Cushing’s syndrome without noticeable adverse effects.
Fig. 14.
PR modulators.
Mifepristone typically functions as a competitive PR antagonist in the presence of P4 (full agonist of PR). Structurally, mifepristone is a 19-nor steroid containing a p-(dimethylamino)phenyl substitution at the 11β-position—this is responsible for stabilizing PR in an inactive conformation, which favors the binding of corepressor protein complexes to downregulate gene expression of PR target genes. Additionally, mifepristone contains a hydrophobic 1-propynyl substitution that increases its binding affinity for PR more than twice that of P4. As mentioned earlier, mifepristone was developed as a GR antagonist, and it thus retains some binding affinity for GR and also displays weak AR antagonist activity. Studies have shown that treatment with mifepristone at doses of 1 mg/kg or greater in women effectively antagonizes the effects of P4 via PR in the endometrial and myometrial tissues, resulting in reduced endometrial proliferation, menstrual bleeding, or amenorrhea and inhibition of ovulation (Brown et al., 2002; Chabbert-Buffet et al., 2005). Interestingly, rodents treated with mifepristone display the typical estrogenic effects of modifying uterine and/or endometrial morphology (Spitz et al., 1998; Chabbert-Buffet et al., 2005). However, continued treatment with doses greater than 4.5 mg/kg in women and other primates often leads to an antiglucocorticoid effect, resulting in increased levels of cortisol and ACTH. Additionally, animal studies have shown that administration of very high doses (up to 100 mg/kg) resulted in antiandrogenic effects (Heikinheimo et al., 2003).
In its current use as a medicinal abortion agent, mifepristone works by binding to PR and inhibiting transcription of its target genes. This, in turn, causes a cascade of physiologic effects, including the release of endogenous prostaglandins, cervical dilation, and endometrial degeneration, which ultimately results in trophoblast detachment. Aside from this, low-dose treatment with mifepristone has been shown clinically to have daily contraceptive potential by inhibiting the surge of LH, which prevents ovulation. There is also evidence that mifepristone may block follicular maturation, hinder tubal function and oocyte maturation, and inhibit fertilization (Brown et al., 2002; Gemzell-Danielsson et al., 2003; Chabbert-Buffet et al., 2005). When administered to men, mifepristone appears to hinder the maturation of gametes, thus showing potential as a male contraceptive agent (Gemzell-Danielsson et al., 2003). Long-term trials have been conducted with 50 women given a daily dose of either 2 or 5 mg of mifepristone as the only contraceptive agent. After 200 months of mifepristone exposure, no pregnancies were reported, contributing to the idea that mifepristone can be used safely as a daily contraceptive (Brown et al., 2002; Heikinheimo et al., 2003; Chabbert-Buffet et al., 2005, 2012). Although mifepristone has been shown to be an effective contraceptive agent and is used for the termination of pregnancy, its use as an emergency contraceptive agent has yet to be approved in the United States.
Mifepristone has also been used to treat prostate cancer, Cushing’s disease, and even human immunodeficiency virus (Chabbert-Buffet et al., 2005, 2012; Taplin et al., 2008). In one study, men with metastatic prostate cancer were treated with 200 mg/day mifepristone for approximately 85 days. This treatment appeared to inhibit GR and cause an increase in adrenal androgens, testosterone, and DHT (Taplin et al., 2008). These results suggest that mifepristone has limited activity in men with prostate cancer, but studies for the potential use of mifepristone as a prostate cancer therapeutic are currently ongoing. Cushing’s disease is a rare disorder with significant morbidity due to metabolic and cardiovascular complications as well as increased susceptibility to infections. Surgery followed by pituitary irradiation or bilateral adrenalectomy is the current method of treatment of this disease. Hypercortisolism can often be managed by radiotherapy, chemotherapy, or by several approved drugs. Unfortunately, the current drug regimen often leads to numerous unwanted side effects, including hepatotoxicity, hypertension, and intolerance—for this reason, new effective methods of treating this disease without the adverse affects are currently being explored. Mifepristone is one drug being studied to treat Cushing’s disease due to its GR antagonist effects, but it is yet to be discovered whether its strong PR effects will inhibit its development as a Cushing’s disease treatment (Castinetti et al., 2009).
As described earlier, targeting PR clinically as an abortion agent has been the subject of controversy for many years in the United States and several other countries. For this reason, some hesitation in the development of newer PR antagonistic agents has occurred. Recent studies, however, have shown novel mixed agonist/antagonist compounds that can selectively stimulate or inhibit PR action in a tissue-dependent manner. Several compounds are still under development, but the FDA has approved the use of a few selective progesterone receptor modulators (SPRMs) for emergency contraceptive treatment and as a potential therapeutic for the treatment of leiomyomas, endometriosis, and breast cancer.
Ulipristal acetate (CDB-2914) (Fig. 14), currently marketed by Watson Pharmaceuticals (Parsippany, NJ) and HRA Pharma (Paris, France), is an analog of 19-nor-progesterone with an 11β-aryl substitution that is used for emergency contraception. Animal studies have revealed that when given orally, ulipristal acetate is rapidly absorbed from the gut and metabolized in the liver via CYP3A4, CYP1A2, and CYP2D6. The two metabolites of ulipristal acetate have demonstrated pharmacologic activity, but that activity is significantly less than the original compound (Richardson and Maltz, 2012). Like mifepristone, ulipristal acetate acts upon the PR as a potent antagonist, with mixed reports of agonist activity for this receptor (Chabbert-Buffet et al., 2005; Nieman et al., 2011; Donnez et al., 2012a,b; Richardson and Maltz, 2012). Several studies have shown that ulipristal acetate can also bind GR, similar to mifepristone, but does not appear to have any binding affinity for ER, AR, or MR (Chabbert-Buffet et al., 2005; Donnez et al., 2012a,b).
Clinically, ulipristal acetate is currently being evaluated as a therapeutic agent for postmenopausal endometriosis. The expression of both PR isoforms and their coregulators (SRC1, NCoR, and SMRT) in the endometrium has been described in many studies (Wang et al., 1998a,b; Stratton et al., 2010). The subnuclear localization of the PR isoforms has been shown to vary during the menstrual cycle, but the physiologic relevance within the endometrial tissue is still undetermined. Although reports are conflicting, one study suggested that endometrial hyperplasia was more frequent in postmenopausal women treated for 6 weeks with oral estradiol at 1 mg/day plus 10 mg/day or 50 mg/day of ulipristal acetate, than in those treated with estradiol plus placebo or medroxyprogesterone. Based on this finding, it appears that PR antagonism by ulipristal acetate can increase the estradiol-induced effects of endometrial proliferation in postmenopausal women (Passaro et al., 2003; Levens et al., 2008; Spitz, 2009; Stratton et al., 2010; Nieman et al., 2011).
While research is still underway to determine the effectiveness of ulipristal for the treatment of endometrial hyperplasia and other PR-mediated reproductive disorders, it is currently FDA approved for use as an emergency contraceptive. The primary mechanism of action is to delay or prevent ovulation by competitively binding the PR and preventing the binding of P4 (Li et al., 2004; Fine, 2011; Chabbert-Buffet et al., 2012; Richardson and Maltz, 2012). The pharmacodynamics of this drug are well understood and depend on the timing of ulipristal administration with regards to the menstrual cycle. When taken during the midfollicular phase, ulipristal causes a reduction in estradiol concentration and an inhibition of folliculogenesis. Ulipristal can also be administered during the peak of LH levels to delay follicular rupture, or during the early luteal phase to reduce endometrial thickness, which decreases the likelihood of implantation (Levens et al., 2008; Stratton et al., 2010; Nieman et al. 2011; Richardson and Maltz, 2012).
Another frequent ailment that appears to affect 70–80% of women within reproductive age is uterine leiomyomas, more commonly called fibroids (Kim and Sefton, 2012). Unfortunately, the cause of leiomyoma development is poorly understood despite its prevalence, although race, age, diet, obesity, alcohol consumption, and oral contraceptive use appear to play a significant role in leiomyoma development (Levens et al., 2008; Nieman et al. 2011; Kim and Sefton, 2012). The symptoms associated with fibroids typically lead to an adverse affect on quality of life and fertility, so the most common treatments are hysterectomy, myomectomy, uterine-artery ablation, and other invasive techniques. While the main mitogenic factor in the uterus is estrogen, new clinical evidence suggests that P4 plays a significant role in the development and growth of fibroids following initial somatic mutation events in the uterus (Donnez et al., 2012a,b; Kim and Sefton, 2012). Thus, selective modulation of PR using SPRMs such as ulipristal acetate may lead to an effective noninvasive treatment. Ishikawa et al. developed a mouse model showing the role of P4/PR in leiomyomas (Ishikawa et al., 2010; Kim and Sefton, 2012). Human leiomyoma cells were grafted into nonobese diabetic-scid IL2Rγ-null female OVX mice and were supplemented with estrogen, P4, or both in the form of subcutaneous hormone pellets. This study demonstrated that leiomyomas formed only in the presence of both estrogen and P4; however, tumor volume, progression, and proliferation solely depended on PR function. Additionally, the maintenance of leiomyomas depended on the activation of PR by P4; therefore, SPRMs, including mifepristone and ulipristal acetate, could be used to reduce the presence of leiomyomas (Ishikawa et al., 2010; Kim and Sefton, 2012). Another study recently published in the New England Journal of Medicine describes the use of ulipristal acetate as a limited fibroid therapeutic (Donnez et al., 2012a,b). A 13-week placebo-controlled study was performed on women with planned hysterectomy surgery due to leiomyomas. The women treated with 5 mg/day or 10 mg/day of ulipristal acetate showed significant reductions in hemoglobin and hematocrit levels, fibroid size, and pain-associated with the fibroids (Donnez et al., 2012a,b). Although this study was focused on the use of ulipristal acetate as a preoperative treatment, it may be possible to modify this compound for use as an oral fibroid therapy to reduce the number of women who undergo invasive surgical treatment.
Another drug currently in phase 2 and 3 trials for the treatment of leiomyomas is asoprisnil (Fig. 14). Marketed by TAP Pharmaceutical Products, asoprisnil is a steroid-based hydrophobic oxime with mixed agonist/antagonist PR activity (DeManno et al., 2003; Chabbert-Buffet et al., 2005). Studies in primates have shown that treatment with asoprisnil can inhibit endometrial gland proliferation, resulting in endometrial atrophy. When administered to women in doses up to 25 mg/day, asoprisnil reduced the intensity and duration of uterine bleeding associated with leiomyomas in a dose-dependent manner (Chwalisz et al., 2004, 2005). More importantly, after 3 months of treatment with asoprisnil, a significant reduction in the volume of fibroids and associated symptoms was reported for these women. Additionally, the women treated with asoprisnil showed no significant changes in ovarian estrogens or serum concentration changes in cortisol, suggesting that asoprisnil does not have GR affinity at clinically relevant doses, unlike other SPRMs (DeManno et al., 2003; Chwalisz et al., 2005, 2007). While these results are promising for the use of SPRMs as a possible treatment of fibroids, further analysis is needed to determine their long-term efficacy and safety as an effective alternative to the historical treatment of fibroids. It may be possible for women with symptomatic fibroids to avoid surgical procedures with the further development of this drug.
In addition to treating leiomyomas, asoprisnil is also in clinical studies for the treatment of endometriosis. Primates (human and nonhuman) have highly specialized endometrial tissue composed of several cell types, which allow for the cyclic changes of regeneration and proliferation, secretory differentiation, and vasoconstriction due to hormone-dependent menstrual cycling (Chwalisz et al., 2005, 2007). Endometrial tissue, which is primarily dependent on estrogen signaling, has a significantly high density of PR that, when activated, has been shown to downregulate ER in target tissues and reduce estrogen-induced protooncogenes in the endometrium. This is especially important when targeting proliferative effects in the endometrium. A 39-week study in cynomolgus monkeys displayed the antiproliferative effects of asoprisnil and its main metabolite asoprisnil ecamate when administered orally in daily doses up to 480 mg/kg (Chwalisz et al., 2004, 2005). As a result of asoprisnil treatment, suppression of menstrual activity and a significant reduction in endometrial proliferation were observed (Brenner and Slayden, 2005; Chwalisz et al., 2005). As earlier studies have shown, asoprisnil has no effect on ER, suggesting that the effects of treatment described are due to its binding PR. Currently, the effects of asoprisnil treatment are being evaluated in humans by morphologic, immunohistochemical, and molecular studies (DeManno et al., 2003; Brenner and Slayden, 2005; Chwalisz et al., 2005; Chabbert-Buffet et al., 2012). Treatment of leiomyomas and endometriosis with asoprisnil appears promising, but the mechanisms associated with these effects are still unclear. It is necessary to continue long-term studies with asoprisnil to fully understand how SPRMs act to alleviate reproductive syndromes.
The development of SPRMs for the treatment of progesterone-dependent diseases is continuing in the United States. One of the more recent selective PR modulators, telapristone acetate or CDB-4124 (Fig. 14), was developed by the National Institutes of Health to treat chronic symptoms associated with uterine fibroids and endometriosis (Morris et al., 2011). Telapristone acetate, a 21-substituted-19-nor-progestin, was designed in an attempt to decrease the undesirable effects of mifepristone while still having a strong binding affinity for PR (Wiehle et al., 2011). Similar to other SPRMs, telapristone acetate blocks the action of P4 at the molecular and receptor level, and has been shown to exhibit potent antiprogesterone effects. Unlike its precursors mifepristone and ulipristal acetate, telapristone acetate does not appear to affect GR, as indicated by a lack of measurable changes to serum cortisol levels. It was also shown that telapristone acetate did not show affinity for binding ER and did not affect estrogen or progesterone levels in serum (Morris et al., 2011; Wiehle et al., 2011). Pharmacokinetic data from mouse studies have demonstrated that the cytochrome P450 pathway, mainly CYP3A4 and CYP3A5, metabolizes telapristone acetate, but additional studies are warranted to evaluate the metabolic pathways of this compound (Morris et al., 2011).
Clinical trials continue to look at the use of telapristone acetate as a treatment of fibroids and endometriosis. Premenopausal women with symptomatic uterine fibroids were treated for 3 months with 12.5, 25, or 50 mg/day telapristone. Treatment with telapristone resulted in a reduction of fibroid size and associated symptoms over the placebo treatments. The serum concentrations of estrogen and progesterone were also monitored, and they remained physiologically preserved for both groups. These data also suggest that the efficacy and safety of telapristone acetate might be more advantageous than other currently available treatments (Wiehle et al., 2008). Studies of the effects of telapristone acetate on proliferation and apoptosis in the uterine tissue have also been performed. Gene and protein expression of proliferating cell nuclear antigen, Klf11, Bcl-2, and caspase-3 were analyzed from fibroid cells and nonproliferative myometrial cells. In leiomyoma cells collected from patients, Bcl-2 mRNA expression was reduced at both 48 and 72 hours in a dose-dependent manner after treatment with telapristone acetate, whereas KLF11 mRNA and protein expression were increased. No changes in Bcl-2 and KLF11 expression were observed in myometrial cells. Another study using the immortalized cell line 53F2 treated with up to 1000 nM/l for 48 hours showed the treated cells were unable to induce apoptosis as determined by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) assays, but other agents were able to induce the apoptotic pathway. These cell studies suggest that treatment with telapristone acetate may not function through the apoptotic pathway to reduce fibroids, and that signaling strictly through PR may be the sole mechanism (Luo et al., 2010). Phase 2 clinical trials are also currently ongoing to study the effects of telapristone acetate as a treatment for premenopausal women with endometriosis. While the mechanism of how telapristone acetate effects endometrial tissue has yet to be determined, trials have shown that it is sufficient in reducing pain associated with endometriosis in premenopausal women, possibly because it modifies the vasculature of the endometrial tissue (Spitz, 2009; Chabbert-Buffet et al., 2012). Because endometriosis is still a poorly understood disorder that affects many women, it is important to continue the efforts in developing SPRMs such as telapristone acetate that may lead to reduced pain and a better quality of life.
Aside from these studies, telapristone acetate has also been implicated in reducing the carcinogenesis of breast cancer by suppressing proliferation and inducing apoptosis. Clinical trials have shown that postmenopausal women undergoing hormone replacement therapy (estrogen and P4) developed a higher incidence of breast cancer than those taking a placebo (Chlebowski et al., 2003a,b). Animal experiments have shown that treatment with P4 increases the incidence of spontaneous mammary tumors (Wiehle et al., 2011). Additionally, studies with PR knockout mice have shown that it is PR and not ER that is specifically important for mammary carcinogenesis.
In a long-term carcinogenicity study, rats treated with 20, 70, and 200 mg/kg telapristone acetate daily were observed for 24 months to determine toxicity and changes in body weight from treatment, and whether telapristone acetate can function as an inhibitor of carcinogenicity in rats cotreated with injections of the carcinogen N-methyl-N-nitrourea (MNU). There were very few changes in animal survival between the control and telapristone acetate-treated animals, indicating that this compound has low toxicity when used over the natural life span of the animals. Additionally, there were no significant changes in body weight or organ composition between the groups (Wiehle et al., 2011). More importantly, this study revealed that telapristone acetate was able to inhibit N-methyl-N-nitrourea-induced mammary carcinogenesis. Cell proliferation and apoptotic markers were evaluated in tumors from control and telapristone acetate-treated rats. The control group showed a significant increase in the expression of proliferative markers when compared with the telapristone acetate group, and similar decrease in apoptosis in the same tumor cells. In addition, it was also shown that treatment with telapristone acetate reduced PR signaling while having no effect on ER, estrogen, or estradiol, similar to earlier studies with this drug (Wiehle et al., 2011). These data suggest that the inhibitory effect observed from telapristone acetate on mammary carcinogenesis is due to PR modulation (Wiehle et al., 2011; Chabbert-Buffet et al., 2012). More studies are certainly needed to demonstrate that telapristone acetate can effectively inhibit and/or reduce mammary carcinogenesis in primates, but it may also be useful to determine whether this compound can be administered in combination with tamoxifen or another SERM to modulate both PR and ER signaling in metastatic breast and other PR/ER-dependent cancers.
The main challenge in developing clinical applications for SPRMs is the potential effects on GR and changes in corticosteroids as well as the effects on AR and potential toxicity. Development of an SPRM with high binding affinity for PR with a decreased incidence of adverse effects remains desirable to further study the prevention and treatment of common ailments such as leiomyomatas, endometriosis, fertility and reproductive disorders, and breast cancer.
Abbreviations
- A276575
2,5-dihydro-9-hydroxy-10-methoxy-2,2,4-trimethyl-5-(1-methylcyclohexen-3-y1)-1H-[1]benzopyrano[3,4-f]quinoline
- A-348441
4-[(3S,7R,12S)-7,12-dihydroxy-3-[2-[4-[(8S,11R,13S,14S,17S)-17-hydroxy-13-methyl-3-oxo-17-prop-1-ynyl-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-11-yl]-N-methylanilino]ethoxy]-2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
- AC-262536
4-(3-hydroxy-8-aza-bicyclo[3.2.1]octyl)-naphthalene-1-carbonitrile
- A/B
amino-terminal domain
- AF
activation function
- AL-438
10-methoxy-5-(2-propenyl)-2,5-dihydro-2,2,4-trimethyl-1H-(1)benzopyrano(3,4-f)quinoline
- AP
activator protein
- AR
androgen receptor
- BMD
bone mineral density
- BMS-564929
(7R,7aS)-2-chloro-4-(7-hydroxy-1,3-dioxotetrahydropyrrolo[1,2-c]imidazol-2-yl)-3-methylbenzonitrile
- BXL-2198
1,25-dihydroxy-16,23Z-diene-26,27-hexafluoro-19-nor vitamin D3
- BXL-01-0029
1,3-di-O-acetyl-1,25-dihydroxy-16,23Z-diene-26,27-hexafluoro-19-nor-cholecalciferol
- BXL-62
1α,25(OH)2-16-ene-20-cyclopropyl-vitamin D3
- CBP
CREB–binding protein
- CDB-2914
ulipristal acetate
- CDB-4124
telapristone acetate
- CE
conjugated estrogens
- CETP
cholesteryl ester transfer protein
- CGH 509A
2-(4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl)-2-(4-(3,7,12-trihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)acetic acid
- CGS 23425
N-[3,5-dimethyl-4-(4′-hydroxy-3′-isopropylphenoxy)-phenyl]-oxamic acid
- CKD
chronic kidney disease
- CLX-0921
(THR0921), (E)-methyl 3-(3,5-dimethoxyphenyl)-2-(4-(4-((2,4-dioxothiazolidin-5-yl)methyl)phenoxy)phenyl)acrylate
- CNS
central nervous system
- CpdA
Compound A
- CREB
cAMP response element-binding protein
- Cyp24
25-hydroxyvitamin D3-24 hydroxylase
- DBD
DNA-binding domain
- DBP
vitamin D-binding protein
- DHT
dihydrotestosterone
- DIO1
type I iodothyronine 5′-deiodinase
- DIOII
type II iodothyronine 5′-deiodinase
- DRIP
vitamin D interacting protein
- DT56a
femarelle
- EAE
experimental autoimmune encephalomyelitis
- ED-71
1α,25-dihydroxy-2β-(3-hydroxypropoxy) vitamin D3
- ER
estrogen receptor
- ERBAα
v-erbA, c-erbA
- FDA
Food and Drug Administration
- FK614
3-(2,4-dichlorobenzyl)-2-methyl-N-(pentylsulfonyl)-3-H-benzimidazole-5-carboxamide
- F-L-Leu
Fmoc-l-leucine
- GC-1
3,5-dimethyl-4(4′-hydroxy-3′-isopropylbenzyl)-phenoxy) acetic acid
- GR
glucocorticoid receptor
- GRE
glucocorticoid-responsive element
- GSK-9772
4-[[N-butyl-4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)anilino]methyl]-2,6-dichlorophenol
- GW0072
4-[4-[(2S,5S)-5-[2-(dibenzylamino)-2-oxoethyl]-2-heptyl-4-oxo-1,3-thiazolidin-3-yl]butyl]benzoic acid
- GW3965
2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-(2,2-diphenylethyl)amino]propoxy]phenyl] acetic acid
- GW9662
2-chloro-5-nitro-N-phenylbenzamide
- HDL
high-density lipoprotein
- HDX
hydrogen/deuterium exchange
- HDXMS
hydrogen deuterium exchange mass spectroscopy
- Hsp
heat shock protein
- IBD
irritable bowel disease
- ICI 182,780
fulvestrant
- IL
interleukin
- INF-γ
interferon γ
- INT-131
N-(3,5-dichloro-4-quinolin-3-yloxyphenyl)-2,4-dihydroxybenzenesulfonamide
- KAT-681
sodium
- 3-[4-[3-[(4-fluorophenyl)-hydroxymethyl]-4-hydroxyphenoxy]-3
5-dimethylanilino]-3-oxopropanoate
- KB141
3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenoxy]phenyl acetic acid
- KB2115
3-[[3,5-dibromo-4-[4-hydroxy-3-(1-methylethyl)-phenoxy]-phenyl]-amino]-3-oxopropanoic acid
- KR-62980
1-(trans-methylimino-N-oxy)-6-(2-morpholinoethoxy)-3-phenyl-1H-indene-2-carboxylic acid ethyl ester
- L-94901
3,5-dibromo-3-pyridazinone-l-thyronine
- LBD
ligand-binding domain
- LBP
ligand-binding pocket
- LDL
low-density lipoprotein
- LG100268
6-[1-(3,5,5,8,8-pentamethyl-6,7-dihydronaphthalen-2-yl)cyclopropyl]pyridine-3-carboxylic acid
- LGD-2226
6-(bis-(2,2,2-trifluoroethyl)amino)-4-trifluoromethyl-1H-quinolin-2-one
- LGD-3303
9-chloro-2-ethyl-1-methyl-3-(2,2,2-trifluoroethyl)-3H-pyrrolo-[3,2-f] quinolin-7 (6H)-one
- LGD-5552
(5Z)-5-[(2-fluoro-3-methylphenyl)methylidene]-10-methoxy-2,2,4-trimethyl-1H-chromeno[3,4-f]quinolin-9-ol
- LH
luteinizing hormone
- LPS
lipopolysaccharide
- LSN
(R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)phenyl]-5H-6-oxachrysen-2-ol
- LXR
liver X receptor
- LXR-623
2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)indazole
- LXRM
liver X receptor modulator
- LY353381
arzoxifene
- MB07811
(2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane
- 2MD
2-methylene-19-nor-(20S)-1α,25(OH)2D3
- MK0533
(2R)-2-(3-3-[(4-methoxyphenyl)carbonyl]-2-methyl-6-(trifluoromethoxy)-1H-indol-1-ylphenoxy)butanoic acid
- MK-2866
(2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide
- MMTV
mouse mammary tumor virus
- MR
mineralocorticoid receptor
- MRL-24
3-(3-((1-(4-methoxybenzoyl)-2-methyl-5-(trifluoromethoxy)-1H-indol-3-yl)methyl)phenyl)-2-methylpropanoic acid
- NCoR
nuclear receptor corepressor
- NFAT1
nuclear factor of activated T cells 1
- NF-κB
nuclear factor κB
- NR
nuclear receptor
- 1α(OH)D3
1-α-hydroxyvitamin D3
- 1,25-(OH)2D3
1α,25-dihydroxyvitamin D3
- OPG
osteoprotegerin
- OVX
ovariectomized
- P4
pregn-4-ene-3,20-dione
- PA-082
1-(3,4-dimethoxy-benzyl)-6,7-dimethoxy-4-[4-(2-methoxy-phenyl)-piperidin-1-ylmethyl]-isoquinoline
- PBMC
peripheral blood mononuclear cell
- PPARγ
peroxisome proliferator-activated receptor γ
- PPRE
peroxisome proliferator-activated receptor response element
- PR
progesterone receptor
- PRE
progesterone receptor DNA-response element
- PRKO
progesterone receptor knockout/null mutation
- PTH
parathyroid hormone
- PXR
pregnane X receptor
- QRX-431
3,5-dimethyl-4(4′-hydroxy-3′-isopropylbenzyl)-phenoxy) acetic acid
- RANKL
receptor activator of nuclear factor κB ligand
- RBC
red blood cell
- RIP
receptor-interacting protein
- Ro-26-9228
1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3
- RAR
retinoid acid receptor
- ROR
retinoic acid receptor–related orphan receptor
- RTH
resistance to thyroid hormone syndrome
- RU 24858
3-((9R,10S,11S,13S,16R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-17-yl)-3-oxopropanenitrile
- RU24782
(9R,10S,11S,13S,16R)-9-fluoro-11-hydroxy-10,13,16-trimethyl-17-(2-(methylthio)acetyl)-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one
- RU40066
(10S,13S,17S)-17-hydroxy-10,13-dimethyl-17-(prop-1-yn-1-yl)-6,7,8,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthren-3-one
- RU486
mifepristone
- RXR
retinoic acid X receptor
- S-4
(2S)-3-(4-acetamido-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide
- S-23
(S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide
- S-40503
2-[4-(dimethylamino)-6-nitro-1,2,3,4-tetrahydroquinolin-2-yl]-2-methylpropan-1-ol
- SARM
selective androgen receptor modulator
- SeGRA
selective glucocorticoid receptor agonist
- SERM
selective estrogen receptor modulator
- SGRM
selective glucocorticoid receptor modulator
- SHPT
secondary hyperparathyroidism
- SLXRM
selective liver X receptor modulator
- SMRT
silencing mediator for retinoid and thyroid hormone receptor
- SPPARγM
selective peroxisome proliferator-activated receptor γ modulator
- SPRM
selective progesterone receptor modulator
- SRC
steroid receptor coactivator
- SREBP1c
sterol regulatory element-binding protein 1c
- T-0681
- 3-[4-[3-[(4-fluorophenyl)-hydroxymethyl]-4-hydroxyphenoxy]-3
5-dimethylanilino]-3-oxopropanoate
- SR1664
(S)-4′-((5-((1-(4-nitrophenyl)ethyl)carbamoyl)-1H-indol-1-yl)methyl)-[1,1′-biphenyl]-2-carboxylic acid
- T0901317
N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide
- T3
triiodothyronine
- T4
thyroxine
- TAZ
transcriptional coactivator with PDZ-binding motif
- TDZ
thiazolidinedione drug
- TGFβ
transforming growth factor β
- TH
thyroid hormone
- TH
T helper
- TIF
transcriptional mediators/intermediary factor
- TNFα
tumor necrosis factor α
- TR
thyroid receptor
- TSH
thyroid-stimulating hormone
- VDR
vitamin D receptor
- VDRE
vitamin D3 response element
- VDRM
vitamin D receptor modulator
- WAY-140424
bazedoxifene
- WAY-252623
2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)indazole
- YT-32
(22E)-ergost-22-ene-1α,2β diol
- ZK156979
22-ene-25-oxa-vitamin D
- ZK216348
3-dihydro-1-benzofuran-7-yl)-2-hydroxy-4-methyl-N-(4-methyl-1-oxo-2,3-benzoxazin-6-yl)-2-(trifluoromethyl)pentanamide
- ZK-245186
(2R)-1,1,1-trifluoro-4-(5-fluoro-2,3-dihydro-1-benzofuran-7-yl)-4-methyl-2-[[(2-methylquinolin-5-yl)amino]methyl]pentan-2-ol
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Burris, Solt, Wang, Crumbley, Banerjee, Griffett, Lundasen, Hughes, Kojetin.
Footnotes
References
- Acton JJ, 3rd, Akiyama TE, Chang CH, Colwell L, Debenham S, Doebber T, Einstein M, Liu K, McCann ME, Moller DE, et al. (2009) Discovery of (2R)-2-(3-3-[(4-methoxyphenyl)carbonyl]-2-methyl-6-(trifluoromethoxy)-1H-indol-1-ylphenoxy)butanoic acid (MK-0533): a novel selective peroxisome proliferator-activated receptor gamma modulator for the treatment of type 2 diabetes mellitus with a reduced potential to increase plasma and extracellular fluid volume. J Med Chem 52:3846–3854. [DOI] [PubMed] [Google Scholar]
- Acton JJ, 3rd, Black RM, Jones AB, Moller DE, Colwell L, Doebber TW, Macnaul KL, Berger J, Wood HB. (2005) Benzoyl 2-methyl indoles as selective PPARγ modulators. Bioorg Med Chem Lett 15:357–362. [DOI] [PubMed] [Google Scholar]
- Adams M, Reginato MJ, Shao DL, Lazar MA, Chatterjee VK. (1997) Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272:5128–5132. [DOI] [PubMed] [Google Scholar]
- Addo S, Yates RA, Laight A. (2002) A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers. Br J Cancer 87:1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L. (2002) 1,25-Dihydroxyvitamin D3 analogs as potential therapies in transplantation. Curr Opin Investig Drugs 3:1458–1463. [PubMed] [Google Scholar]
- Aihara K, Azuma H, Akaike M, Ikeda Y, Yamashita M, Sudo T, Hayashi H, Yamada Y, Endoh F, Fujimura M, et al. (2004) Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J Biol Chem 279:35798–35802. [DOI] [PubMed] [Google Scholar]
- Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ. (2007) Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis 50:59–68. [DOI] [PubMed] [Google Scholar]
- Almlöf T, Gustafsson JA, Wright APH. (1997) Role of hydrophobic amino acid clusters in the transactivation activity of the human glucocorticoid receptor. Mol Cell Biol 17:934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy I, Towers TL, Freedman LP. (1995) Transcriptional repression of the interleukin-2 gene by vitamin D3: direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol Cell Biol 15:5789–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amita M, Takahashi T, Tsutsumi S, Ohta T, Takata K, Henmi N, Hara S, Igarashi H, Takahashi K, Kurachi H. (2010) Molecular mechanism of the inhibition of estradiol-induced endometrial epithelial cell proliferation by clomiphene citrate. Endocrinology 151:394–405. [DOI] [PubMed] [Google Scholar]
- Amuchastegui S, Daniel KC, Adorini L. (2005) Inhibition of acute and chronic allograft rejection in mouse models by BXL-628, a nonhypercalcemic vitamin D receptor agonist. Transplantation 80:81–87. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Choe SE, Yukhananov RY, Hopfner RL, Church GM, Pratt RE, Dzau VJ. (2003) Identification of a novel set of genes regulated by a unique liver X receptor-alpha-mediated transcription mechanism. J Biol Chem 278:15252–15260. [DOI] [PubMed] [Google Scholar]
- Andre J, Rochefort H. (1973) Specific effect of estrogens on an interaction between the uterine estradiol receptor and DNA. FEBS Lett 29:135–140. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Montero-Odasso M, Schott AM, Berrut G, Fantino B, Beauchet O. (2010) Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous ] (2008) Bazedoxifene: bazedoxifene acetate, TSE 424, TSE-424, WAY 140424. Drugs R D 9:191–196. [DOI] [PubMed] [Google Scholar]
- Anzano MA, Peer CW, Smith JM, Mullen LT, Shrader MW, Logsdon DL, Driver CL, Brown CC, Roberts AB, Sporn MB. (1996) Chemoprevention of mammary carcinogenesis in the rat: combined use of raloxifene and 9-cis-retinoic acid. J Natl Cancer Inst 88:123–125. [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. (1997) AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968. [DOI] [PubMed] [Google Scholar]
- Aoki T. (2007) Current status of carcinogenicity assessment of peroxisome proliferator-activated receptor agonists by the US FDA and a mode-of-action approach to the carcinogenic potential. J Toxicol Pathol 20:197–202. [Google Scholar]
- Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. (1994) A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14:7025–7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. (1997) Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol 158:3464–3473. [PubMed] [Google Scholar]
- Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, Yuen CK, Levine AB, Chines AA, Constantine GD. (2009) Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 16:1109–1115. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275. [DOI] [PubMed] [Google Scholar]
- Auwerx J, Baulieu E, Beato M, Becker-Andre M, Burbach PH, Camerino G, Chambon P, Cooney A, Dejean A, Dreyer C, et al. Nuclear Receptors Nomenclature Committee (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Bobula J, Mirkin S. (2010) Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 13:132–140. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Crosby U, Feldman RA, Ronkin S, Constantine GD. (2011) Effects of bazedoxifene in nonflushing postmenopausal women: a randomized phase 2 trial. Menopause 18:508–514. [DOI] [PubMed] [Google Scholar]
- Bachmann G, Gass M, Kagan R, Moffett A, Barcomb L, Symons J. (2005) Lasofoxifene (LASO), a next generation selective estrogen response modulator (SERM) improves dyspareunia in postmenopausal women with vaginal atrophy (VA) [abstract]. Menopause 12:238. [Google Scholar]
- Bae M, Cheon H, Lee J, Heo J, Kim H. (2007) KR62980, a novel compound, enhances bone formation through promoting osteoblastogenesis and inhibiting osteoclastogenesis [abstract M387]. J Bone Miner Res 22 (Suppl 1):S204. [Google Scholar]
- Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW. (1988) Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA 85:3294–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, et al. (2005) Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem 280:42464–42475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A, Leng XH, Burris TP, Tsai SY, Tsai MJ, O’Malley BW. (1995) The tau 4 activation domain of the thyroid hormone receptor is required for release of a putative corepressor(s) necessary for transcriptional silencing. Mol Cell Biol 15:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Kim S. (2007) Genetic manipulations of PPARs: effects on obesity and metabolic disease. PPAR Res 2007:12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–595. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. (1998) Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol 54:105–112. [DOI] [PubMed] [Google Scholar]
- Barlow JW, Raggatt LE, Lim CF, Munro SL, Topliss DJ, Stockigt JR. (1989) The thyroid hormone analogue SKF L-94901: nuclear occupancy and serum binding studies. Clin Sci (Lond) 76:495–501. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK, Raloxifene Use for The Heart (RUTH) Trial Investigators (2006) Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355:125–137. [DOI] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VEF, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TDM, Lewis H, Schafer AJ, et al. (1999) Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402:880–883. [DOI] [PubMed] [Google Scholar]
- Barry JB, Leong GM, Church WB, Issa LL, Eisman JA, Gardiner EM. (2003) Interactions of SKIP/NCoA-62, TFIIB, and retinoid X receptor with vitamin D receptor helix H10 residues. J Biol Chem 278:8224–8228. [DOI] [PubMed] [Google Scholar]
- Barsony J, Pike JW, DeLuca HF, Marx SJ. (1990) Immunocytology with microwave-fixed fibroblasts shows 1 alpha,25-dihydroxyvitamin D3-dependent rapid and estrogen-dependent slow reorganization of vitamin D receptors. J Cell Biol 111:2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartuska DG, Dratman MB. (1973) Evolving concepts of thyroid function. Med Clin North Am 57:1117–1133. [DOI] [PubMed] [Google Scholar]
- Barycki R, Sicinski RR, Plum LA, Grzywacz P, Clagett-Dame M, Deluca HF. (2009) Removal of the 20-methyl group from 2-methylene-19-nor-(20S)-1alpha,25-dihydroxyvitamin D3 (2MD) selectively eliminates bone calcium mobilization activity. Bioorg Med Chem 17:7658–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Llombart-Cussac A, Bellet M, Guillem-Porta V, Enas N, Krejcy K, Carrasco E, Kayitalire L, Kuta M, Lluch A, et al. (2003) Randomized, double-blind, multicenter trial comparing two doses of arzoxifene (LY353381) in hormone-sensitive advanced or metastatic breast cancer patients. Ann Oncol 14:1383–1390. [DOI] [PubMed] [Google Scholar]
- Baumann E. (1895–1896) Ueber das normale Vorkommen von Jod im Thierkörper. (I. Mittheilung). Z Physiol Chemie 21:319–330. [Google Scholar]
- Beato M, Kalimi M, Konstam M, Feigelson P. (1973) Interaction of glucocorticoids with rat liver nuclei. II. Studies on the nature of the cytosol transfer factor and the nuclear acceptor site. Biochemistry 12:3372–3379. [DOI] [PubMed] [Google Scholar]
- Bellows CG, Reimers SM, Heersche JNM. (1999) Expression of mRNAs for type-I collagen, bone sialoprotein, osteocalcin, and osteopontin at different stages of osteoblastic differentiation and their regulation by 1,25 dihydroxyvitamin D3. Cell Tissue Res 297:249–259. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Hele DJ. (2003) Soft steroids: a new approach to the treatment of inflammatory airways diseases. Pulm Pharmacol Ther 16:321–325. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Wicks SL, Battram CH, Bottoms SEW, Redford JE, Woodman P, Brown TJ, Webber SE, Foster ML. (2001) Therapeutic benefit of a dissociated glucocorticoid and the relevance of in vitro separation of transrepression from transactivation activity. J Immunol 166:1975–1982. [DOI] [PubMed] [Google Scholar]
- Benecke A, Chambon P, Gronemeyer H. (2000) Synergy between estrogen receptor alpha activation functions AF-1 and AF-2 mediated by transcription intermediary factor TIF2. EMBO Rep 1:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Carswell EL, Cooke AJ, Edwards AS, Nimz O. (2008) Design, structure activity relationships and X-Ray co-crystallography of non-steroidal LXR agonists. Curr Med Chem 15:195–209. [DOI] [PubMed] [Google Scholar]
- Berkenstam A, Kristensen J, Mellström K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F, et al. (2008) The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 105:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, Maillot MC, Portier A, Couderc J, Galanaud P, et al. (2003) Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood 101:729–738. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Mirkes SJ, Su A, Michelson D. (2002) Effects of oral estrogen, raloxifene and arzoxifene on gene expression in serotonin neurons of macaques. Psychoneuroendocrinology 27:431–445. [DOI] [PubMed] [Google Scholar]
- Bettendorf M. (2002) Thyroid disorders in children from birth to adolescence. Eur J Nucl Med Mol Imaging 29 (Suppl 2):S439–S446. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Jasuja R. (2009) Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care 12:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Murphy EW, Rasmussen H. (1975) The ionic control of 1,25-dihydroxyvitamin D3 synthesis in isolated chick renal mitochondria. The role of calcium as influenced by inorganic phosphate and hydrogen-ion. J Clin Invest 55:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I. (2009) Are side-chain oxidized oxysterols regulators also in vivo? J Lipid Res 50 (Suppl):S213–S218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR, et al. (1994) Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland R. (2000) Steroid hormone receptor expression and action in bone. Clin Sci (Lond) 98:217–240. [PubMed] [Google Scholar]
- Bledsoe RK, Montana VG, Stanley TB, Delves CJ, Apolito CJ, McKee DD, Consler TG, Parks DJ, Stewart EL, Willson TM, et al. (2002) Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110:93–105. [DOI] [PubMed] [Google Scholar]
- Blesson CS, Awasthi S, Kharkwal G, Daverey A, Dwivedi A. (2006) Modulation of estrogen receptor transactivation and estrogen-induced gene expression by ormeloxifene-a triphenylethylene derivative. Steroids 71:993–1000. [DOI] [PubMed] [Google Scholar]
- Bongartz T, Coras B, Vogt T, Schölmerich J, Müller-Ladner U. (2005) Treatment of active psoriatic arthritis with the PPARγ ligand pioglitazone: an open-label pilot study. Rheumatology (Oxford) 44:126–129. [DOI] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, Compston J. (2001) Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab 86:2309–2314. [DOI] [PubMed] [Google Scholar]
- Boyages SC, Halpern JP. (1993) Endemic cretinism: toward a unifying hypothesis. Thyroid 3:59–69. [DOI] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS., 3rd (1992) Spatial and temportal expression of alpha- and beta-thyroid hormone receptor messenger RNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci 12:2288–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Towle HC, Young WS., 3rd (1994) Alpha and beta thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci USA 91:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett KS, Burris TP. (2003) Target specificity of selective estrogen receptor modulators within human endometrial cancer cells. J Steroid Biochem Mol Biol 86:27–34. [DOI] [PubMed] [Google Scholar]
- Bramlett KS, Houck KA, Borchert KM, Dowless MS, Kulanthaivel P, Zhang YY, Beyer TP, Schmidt R, Thomas JS, Michael LF, et al. (2003) A natural product ligand of the oxysterol receptor, liver X receptor. J Pharmacol Exp Ther 307:291–296. [DOI] [PubMed] [Google Scholar]
- Bramlett KS, Wu YF, Burris TP. (2001) Ligands specify coactivator nuclear receptor (NR) box affinity for estrogen receptor subtypes. Mol Endocrinol 15:909–922. [DOI] [PubMed] [Google Scholar]
- Brattsand R, Thalén A, Roempke K, Kallström L, Gruvstad E. (1982) Influence of 16 alpha, 17 alpha-acetal substitution and steroid nucleus fluorination on the topical to systemic activity ratio of glucocorticoids. J Steroid Biochem 16:779–786. [DOI] [PubMed] [Google Scholar]
- Bregenholt S, Claesson MH. (1998) Increased intracellular Th1 cytokines in scid mice with inflammatory bowel disease. Eur J Immunol 28:379–389. [DOI] [PubMed] [Google Scholar]
- Brenner RM, Slayden OD. (2005) Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin Reprod Med 23:74–81. [DOI] [PubMed] [Google Scholar]
- Broe KE, Chen TC, Weinberg J, Bischoff-Ferrari HA, Holick MF, Kiel DP. (2007) A higher dose of vitamin d reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J Am Geriatr Soc 55:234–239. [DOI] [PubMed] [Google Scholar]
- Bronner F. (2003) Mechanisms of intestinal calcium absorption. J Cell Biochem 88:387–393. [DOI] [PubMed] [Google Scholar]
- Brown A, Cheng L, Lin S, Baird DT. (2002) Daily low-dose mifepristone has contraceptive potential by suppressing ovulation and menstruation: a double-blind randomized control trial of 2 and 5 mg per day for 120 days. J Clin Endocrinol Metab 87:63–70. [DOI] [PubMed] [Google Scholar]
- Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR. (2007) Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15:1258–1271. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, et al. (2008) Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol 111:262–267. [DOI] [PubMed] [Google Scholar]
- Brzozowski AM, Pike ACW, Dauter Z, Hubbard RE, Bonn T, Engström O, Öhman L, Greene GL, Gustafsson J-Å, Carlquist M. (1997) Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758. [DOI] [PubMed] [Google Scholar]
- Bundred NJ, Anderson E, Nicholson RI, Dowsett M, Dixon M, Robertson JF. (2002) Fulvestrant, an estrogen receptor downregulator, reduces cell turnover index more effectively than tamoxifen. Anticancer Res 22:2317–2319. [PubMed] [Google Scholar]
- Burgermeister E, Schnoebelen A, Flament A, Benz J, Stihle M, Gsell B, Rufer A, Ruf A, Kuhn B, Märki HP, et al. (2006) A novel partial agonist of peroxisome proliferator-activated receptor-gamma (PPARγ) recruits PPARγ-coactivator-1alpha, prevents triglyceride accumulation, and potentiates insulin signaling in vitro. Mol Endocrinol 20:809–830. [DOI] [PubMed] [Google Scholar]
- Burke TW, Walker CL. (2003) Arzoxifene as therapy for endometrial cancer. Gynecol Oncol 90:S40–S46. [DOI] [PubMed] [Google Scholar]
- Burris TP, Guo WW, McCabe ER. (1996) The gene responsible for adrenal hypoplasia congenita, DAX-1, encodes a nuclear hormone receptor that defines a new class within the superfamily. Recent Prog Horm Res 51:241–259, discussion 259–260. [PubMed] [Google Scholar]
- Buttgereit F, Burmester GR, Lipworth BJ. (2005) Optimised glucocorticoid therapy: the sharpening of an old spear. Lancet 365:801–803. [DOI] [PubMed] [Google Scholar]
- Buzdar A, O’Shaughnessy JA, Booser DJ, Pippen JE, Jr, Jones SE, Munster PN, Peterson P, Melemed AS, Winer E, Hudis C. (2003) Phase II, randomized, double-blind study of two dose levels of arzoxifene in patients with locally advanced or metastatic breast cancer. J Clin Oncol 21:1007–1014. [DOI] [PubMed] [Google Scholar]
- Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, Linemeyer DL, Erion MD. (2009) Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 49:407–417. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. (1995) MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377:68–71. [DOI] [PubMed] [Google Scholar]
- Camp HS, Tafuri SR. (1997) Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem 272:10811–10816. [DOI] [PubMed] [Google Scholar]
- Camp HS, Tafuri SR, Leff T. (1999) c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-gamma1 and negatively regulates its transcriptional activity. Endocrinology 140:392–397. [DOI] [PubMed] [Google Scholar]
- Cao G, Bales KR, DeMattos RB, Paul SM. (2007) Liver X receptor-mediated gene regulation and cholesterol homeostasis in brain: relevance to Alzheimer’s disease therapeutics. Curr Alzheimer Res 4:179–184. [DOI] [PubMed] [Google Scholar]
- Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, Zhang Y, Stayrook KR, Suen C, Otto KA, et al. (2003) Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J Biol Chem 278:1131–1136. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Ladenson PW. (2003) Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab 88:2438–2444. [DOI] [PubMed] [Google Scholar]
- Carballo-Jane E, Pandit S, Santoro JC, Freund C, Luell S, Harris G, Forrest MJ, Sitlani A. (2004) Skeletal muscle: a dual system to measure glucocorticoid-dependent transactivation and transrepression of gene regulation. J Steroid Biochem Mol Biol 88:191–201. [DOI] [PubMed] [Google Scholar]
- Carlstedt-Duke J, Okret S, Wrange O, Gustafsson JA. (1982) Immunochemical analysis of the glucocorticoid receptor: identification of a third domain separate from the steroid-binding and DNA-binding domains. Proc Natl Acad Sci USA 79:4260–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castinetti F, Fassnacht M, Johanssen S, Terzolo M, Bouchard P, Chanson P, Do Cao C, Morange I, Picó A, Ouzounian S, et al. (2009) Merits and pitfalls of mifepristone in Cushing’s syndrome. Eur J Endocrinol 160:1003–1010. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Harrington KL, Ward KW, Zhang JZ. (2010) Mapracorat, a novel selective glucocorticoid receptor agonist, inhibits hyperosmolar-induced cytokine release and MAPK pathways in human corneal epithelial cells. Mol Vis 16:1791–1800. [PMC free article] [PubMed] [Google Scholar]
- Cerciat M, Unkila M, Garcia-Segura LM, Arevalo MA. (2010) Selective estrogen receptor modulators decrease the production of interleukin-6 and interferon-gamma-inducible protein-10 by astrocytes exposed to inflammatory challenge in vitro. Glia 58:93–102. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Meduri G, Bouchard P, Spitz IM. (2005) Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update 11:293–307. [DOI] [PubMed] [Google Scholar]
- Chabbert-Buffet N, Pintiaux A, Bouchard P. (2012) The immninent dawn of SPRMs in obstetrics and gynecology. Mol Cell Endocrinol 358:232–243. [DOI] [PubMed] [Google Scholar]
- Chan L, Means AR, O’Malley BW. (1973) Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci USA 70:1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. (2002) Arzoxifene in breast cancer. Eur J Cancer 38 (Suppl 6):S55–S56. [DOI] [PubMed] [Google Scholar]
- Chandra P, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V. (2007) Efficacy of cholecalciferol (vitamin D-3) therapy in correcting vitamin D insufficiency and secondary hyperparathyroidism in patients with chronic kidney disease: a randomised, placebo controlled study [abstract]. Am J Kidney Dis 49:B34. [Google Scholar]
- Chandra V, Huang P, Hamuro Y, Raghuram S, Wang Y, Burris TP, Rastinejad F. (2008) Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature 456:350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Norris JD, Grøn H, Paige LA, Hamilton PT, Kenan DJ, Fowlkes D, McDonnell DP. (1999) Dissection of the LXXLL nuclear receptor-coactivator interaction motif using combinatorial peptide libraries: discovery of peptide antagonists of estrogen receptors alpha and beta. Mol Cell Biol 19:8226–8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST. (1988a) Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324–326. [DOI] [PubMed] [Google Scholar]
- Chang CS, Kokontis J, Liao ST. (1988b) Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci USA 85:7211–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. (2010a) 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of TH17 cells to protect against experimental autoimmune encephalomyelitis. PLoS ONE 5:e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS. (2010b) Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol 118:117–124. [DOI] [PubMed] [Google Scholar]
- Chao EY, Caravella JA, Watson MA, Campobasso N, Ghisletti S, Billin AN, Galardi C, Wang P, Laffitte BA, Iannone MA, et al. (2008) Structure-guided design of N-phenyl tertiary amines as transrepression-selective liver X receptor modulators with anti-inflammatory activity. J Med Chem 51:5758–5765. [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. (2001) Nuclear receptors and lipid physiology: opening the X-files. Science 294:1866–1870. [DOI] [PubMed] [Google Scholar]
- Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. (1997) Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580. [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM. (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457. [DOI] [PubMed] [Google Scholar]
- Chen JY, Kim J, Dalton JT. (2005) Discovery and therapeutic promise of selective androgen receptor modulators. Mol Interv 5:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SY, Leonard JL, Davis PJ. (2010) Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis B, Freedman LP. (1994) Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol 14:3329–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamolera MI, Wondisford FE. (2009) Minireview: thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology 150:1091–1096. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RCJ, Scanlan TS. (1998) A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol 5:299–306. [DOI] [PubMed] [Google Scholar]
- Chiquette E, Ramirez G, Defronzo R. (2004) A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med 164:2097–2104. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, et al. WHI Investigators (2003a) Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA 289:3243–3253. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Kim JA, Col NF. (2003b) Estrogen deficiency symptom management in breast cancer survivors in the changing context of menopausal hormone therapy. Semin Oncol 30:776–788. [DOI] [PubMed] [Google Scholar]
- Chlon TM, Taffany DA, Welsh J, Rowling MJ. (2008) Retinoids modulate expression of the endocytic partners megalin, cubilin, and disabled-2 and uptake of vitamin D-binding protein in human mammary cells. J Nutr 138:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Blüher M, et al. (2010) Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature 466:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Kamenecka TM, Busby SA, Chalmers MJ, Kumar N, Kuruvilla DS, Shin Y, He Y, Bruning JB, et al. (2011) Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 477:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C, Chesnut CH, 3rd, Adachi JD, Brown JP, Fernandes CE, Kung AW, Palacios S, Levine AB, Chines AA, Constantine GD. (2010) Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskelet Disord 11:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. (2000) Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem 275:32681–32687. [DOI] [PubMed] [Google Scholar]
- Chuu CP. (2011) Modulation of liver X receptor signaling as a prevention and therapy for colon cancer. Med Hypotheses 76:697–699. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, DeManno D, Garg R, Larsen L, Mattia-Goldberg C, Stickler T. (2004) Therapeutic potential for the selective progesterone receptor modulator asoprisnil in the treatment of leiomyomata. Semin Reprod Med 22:113–119. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Elger W, Stickler T, Mattia-Goldberg C, Larsen L. (2005) The effects of 1-month administration of asoprisnil (J867), a selective progesterone receptor modulator, in healthy premenopausal women. Hum Reprod 20:1090–1099. [DOI] [PubMed] [Google Scholar]
- Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, Elger W, Winkel CA. (2007) A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril 87:1399–1412. [DOI] [PubMed] [Google Scholar]
- Ciesielski F, Rochel N, Mitschler A, Kouzmenko A, Moras D. (2004) Structural investigation of the ligand binding domain of the zebrafish VDR in complexes with 1alpha,25(OH)2D3 and Gemini: purification, crystallization and preliminary X-ray diffraction analysis. J Steroid Biochem Mol Biol 89–90:55–59. [DOI] [PubMed] [Google Scholar]
- Clark AR. (2007) Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol 275:79–97. [DOI] [PubMed] [Google Scholar]
- Clemens JA, Bennett DR, Black LJ, Jones CD. (1983) Effects of a new antiestrogen, keoxifene (LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin levels. Life Sci 32:2869–2875. [DOI] [PubMed] [Google Scholar]
- Clemett D, Spencer CM. (2000) Raloxifene: a review of its use in postmenopausal osteoporosis. Drugs 60:379–411. [DOI] [PubMed] [Google Scholar]
- Coghlan MJ, Jacobson PB, Lane B, Nakane M, Lin CW, Elmore SW, Kym PR, Luly JR, Carter GW, Turner R, et al. (2003) A novel antiinflammatory maintains glucocorticoid efficacy with reduced side effects. Mol Endocrinol 17:860–869. [DOI] [PubMed] [Google Scholar]
- Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, et al. (2002) Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem 45:1963–1966. [DOI] [PubMed] [Google Scholar]
- Comstock JP, Rosenfeld GC, O’Malley BW, Means AR. (1972) Estrogen-induced changes in translation, and specific messenger RNA levels during oviduct differentiation. Proc Natl Acad Sci USA 69:2377–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely OM, Dobson AD, Tsai MJ, Beattie WG, Toft DO, Huckaby CS, Zarucki T, Schrader WT, O’Malley BW. (1987a) Sequence and expression of a functional chicken progesterone receptor. Mol Endocrinol 1:517–525. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. (1987b) The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun 149:493–501. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. (2002) Reproductive functions of progesterone receptors. Recent Prog Horm Res 57:339–355. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. (2001) Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol 179:97–103. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Sullivan WP, Toft DO, Birnbaumer M, Cook RG, Maxwell BL, Zarucki-Schulz T, Greene GL, Schrader WT, O’Malley BW. (1986) Molecular cloning of the chicken progesterone receptor. Science 233:767–770. [DOI] [PubMed] [Google Scholar]
- Cook CB, Kakucska I, Lechan RM, Koenig RJ. (1992) Expression of thyroid hormone receptor beta 2 in rat hypothalamus. Endocrinology 130:1077–1079. [DOI] [PubMed] [Google Scholar]
- Coopman P, Garcia M, Brünner N, Derocq D, Clarke R, Rochefort H. (1994) Anti-proliferative and anti-estrogenic effects of ICI 164,384 and ICI 182,780 in 4-OH-tamoxifen-resistant human breast-cancer cells. Int J Cancer 56:295–300. [DOI] [PubMed] [Google Scholar]
- Corbett ST, Hill O, Nangia AK. (2006) Vitamin D receptor found in human sperm. Urology 68:1345–1349. [DOI] [PubMed] [Google Scholar]
- Coronary Drug Project Research Group (1972) The Coronary Drug Project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. JAMA 220:996–1008. [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG. (1997) Estrogen receptors alpha and beta form heterodimers on DNA. J Biol Chem 272:19858–19862. [DOI] [PubMed] [Google Scholar]
- Crofts LA, Hancock MS, Morrison NA, Eisman JA. (1998) Multiple promoters direct the tissue-specific expression of novel N-terminal variant human vitamin D receptor gene transcripts. Proc Natl Acad Sci USA 95:10529–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronet P, Petersen JFW, Folmer R, Blomberg N, Sjöblom K, Karlsson U, Lindstedt EL, Bamberg K. (2001) Structure of the PPARα and -γ ligand binding domain in complex with AZ 242; ligand selectivity and agonist activation in the PPAR family. Structure 9:699–706. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Ensrud K, Delmas PD, LaCroix AZ, Vukicevic S, Reid DM, Goldstein S, Sriram U, Lee A, Thompson J, et al. PEARL Study Investigators (2010) Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362:686–696. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Almlöf T, McEwan IJ, Gustafsson JA, Wright AP. (1994) Delineation of a small region within the major transactivation domain of the human glucocorticoid receptor that mediates transactivation of gene expression. Proc Natl Acad Sci USA 91:1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SY, Burris TP, Dodge JA, Montrose-Rafizadeh C, Wang Y, Pascal BD, Chalmers MJ, Griffin PR. (2009) Unique ligand binding patterns between estrogen receptor alpha and beta revealed by hydrogen-deuterium exchange. Biochemistry 48:9668–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai SY, Chalmers MJ, Bruning J, Bramlett KS, Osborne HE, Montrose-Rafizadeh C, Barr RJ, Wang Y, Wang M, Burris TP, et al. (2008) Prediction of the tissue-specificity of selective estrogen receptor modulators by using a single biochemical method. Proc Natl Acad Sci USA 105:7171–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JT, Mukherjee A, Zhu ZX, Kirkovsky L, Miller DD. (1998) Discovery of nonsteroidal androgens. Biochem Biophys Res Commun 244:1–4. [DOI] [PubMed] [Google Scholar]
- Daniel C, Radeke HH, Sartory NA, Zahn N, Zuegel U, Steinmeyer A, Stein J. (2006) The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. J Pharmacol Exp Ther 319:622–631. [DOI] [PubMed] [Google Scholar]
- Daniel C, Schlauch T, Zügel U, Steinmeyer A, Radeke HH, Steinhilber D, Stein J. (2005) 22-ene-25-oxa-vitamin D: a new vitamin D analogue with profound immunosuppressive capacities. Eur J Clin Invest 35:343–349. [DOI] [PubMed] [Google Scholar]
- Dardes RC, Bentrem D, O’Regan RM, Schafer JM, Jordan VC. (2001) Effects of the new selective estrogen receptor modulator LY353381.HCl (Arzoxifene) on human endometrial cancer growth in athymic mice. Clin Cancer Res 7:4149–4155. [PubMed] [Google Scholar]
- Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, Fletterick RJ, Yamamoto KR. (1998) Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev 12:3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daverey A, Saxena R, Tewari S, Goel SK, Dwivedi A. (2009) Expression of estrogen receptor co-regulators SRC-1, RIP140 and NCoR and their interaction with estrogen receptor in rat uterus, under the influence of ormeloxifene. J Steroid Biochem Mol Biol 116:93–101. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R. (2006) Androgen physiology. Semin Reprod Med 24:71–77. [DOI] [PubMed] [Google Scholar]
- De Bosscher K, Vanden Berghe W, Beck IME, Van Molle W, Hennuyer N, Hapgood J, Libert C, Staels B, Louw A, Haegeman G. (2005) A fully dissociated compound of plant origin for inflammatory gene repression. Proc Natl Acad Sci USA 102:15827–15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers TJ, Chines AA, Palacios S, Lips P, Sawicki AZ, Levine AB, Codreanu C, Kelepouris N, Brown JP. (2011) Safety and tolerability of bazedoxifene in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled phase 3 trial. Osteoporos Int 22:567–576. [DOI] [PubMed] [Google Scholar]
- DeLuca HF, Bedale W, Binkley N, Gallagher JC, Bolognese M, Peacock M, Aloia J, Clagett-Dame M, Plum L. (2011) The vitamin D analogue 2MD increases bone turnover but not BMD in postmenopausal women with osteopenia: results of a 1-year phase 2 double-blind, placebo-controlled, randomized clinical trial. J Bone Miner Res 26:538–545. [DOI] [PubMed] [Google Scholar]
- DeManno D, Elger W, Garg R, Lee R, Schneider B, Hess-Stumpp H, Schubert G, Chwalisz K. (2003) Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids 68:1019–1032. [DOI] [PubMed] [Google Scholar]
- Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. (1992) Sequences in the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin D3 receptor and mediate transcriptional repression in response to 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 89:8097–8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane V, Krishnamurthy S, Melemed AS, Peterson P, Buzdar AU. (2007) Phase III double-blind trial of arzoxifene compared with tamoxifen for locally advanced or metastatic breast cancer. J Clin Oncol 25:4967–4973. [DOI] [PubMed] [Google Scholar]
- Detre S, Riddler S, Salter J, A’Hern R, Dowsett M, Johnston SR. (2003) Comparison of the selective estrogen receptor modulator arzoxifene (LY353381) with tamoxifen on tumor growth and biomarker expression in an MCF-7 human breast cancer xenograft model. Cancer Res 63:6516–6522. [PubMed] [Google Scholar]
- Dey D, Medicherla S, Neogi P, Gowri M, Cheng J, Gross C, Sharma SD, Reaven GM, Nag B. (2003) A novel peroxisome proliferator-activated gamma (PPAR gamma) agonist, CLX-0921, has potent antihyperglycemic activity with low adipogenic potential. Metabolism 52:1012–1018. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Srimal RC. (1973) Anti-inflammatory and some other pharmacological effects of 3,4-trans-2,2-dimethyl-3-phenyl-4-(p-(beta-pyrrolidinoethoxy)-phenyl)-7-methoxy-chroman (Centchroman). Br J Pharmacol 49:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBlasio-Smith EA, Arai M, Quinet EM, Evans MJ, Kornaga T, Basso MD, Chen L, Feingold I, Halpern AR, Liu QY, et al. (2008) Discovery and implementation of transcriptional biomarkers of synthetic LXR agonists in peripheral blood cells. J Transl Med 6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey RP, Holtkamp DE. (1996) Development, pharmacology and clinical experience with clomiphene citrate. Hum Reprod Update 2:483–506. [DOI] [PubMed] [Google Scholar]
- Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ, Stallcup MR. (1998) Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol 12:302–313. [DOI] [PubMed] [Google Scholar]
- Dong XY, Craig T, Xing NZ, Bachman LA, Paya CV, Weih F, McKean DJ, Kumar R, Griffin MD. (2003) Direct transcriptional regulation of RelB by 1α,25-dihydroxyvitamin D3 and its analogs: physiologic and therapeutic implications for dendritic cell function. J Biol Chem 278:49378–49385. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, et al. PEARL I Study Group (2012a) Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366:409–420. [DOI] [PubMed] [Google Scholar]
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, Baró F, Nouri K, Selvaggi L, Sodowski K, Bestel E, et al. PEARL II Study Group (2012b) Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med 366:421–432. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, et al. PROactive investigators (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289. [DOI] [PubMed] [Google Scholar]
- Dreier R, Günther BK, Mainz T, Nemere I, Bruckner P. (2008) Terminal differentiation of chick embryo chondrocytes requires shedding of a cell surface protein that binds 1,25-dihydroxyvitamin D3. J Biol Chem 283:1104–1112. [DOI] [PubMed] [Google Scholar]
- Dunn FL, Higgins LS, Fredrickson J, DePaoli AM, INT131-004 Study Group (2011) Selective modulation of PPARγ activity can lower plasma glucose without typical thiazolidinedione side-effects in patients with type 2 diabetes. J Diabetes Complications 25:151–158. [DOI] [PubMed] [Google Scholar]
- Duplessis TT, Williams CC, Hill SM, Rowan BG. (2011) Phosphorylation of estrogen receptor α at serine 118 directs recruitment of promoter complexes and gene-specific transcription. Endocrinology 152:2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP, West SJ, Pooley CLF, Marschke KB, Farmer LJ, Jones TK. (1998) New nonsteroidal androgen receptor modulators based on 4-(trifluoromethyl)-2(1H)-pyrrolidino[3,2-g] quinolinone. Bioorg Med Chem Lett 8:745–750. [DOI] [PubMed] [Google Scholar]
- Eggert M, Möws CC, Tripier D, Arnold R, Michel J, Nickel J, Schmidt S, Beato M, Renkawitz R. (1995) A fraction enriched in a novel glucocorticoid receptor-interacting protein stimulates receptor-dependent transcription in vitro. J Biol Chem 270:30755–30759. [DOI] [PubMed] [Google Scholar]
- Eisenberg MC, Santini F, Marsili A, Pinchera A, DiStefano JJ., 3rd (2010) TSH regulation dynamics in central and extreme primary hypothyroidism. Thyroid 20:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmens CW. (1971) Compounds exhibiting prolonged antioestrogenic and antifertility activity in mice and rats. J Reprod Fertil 26:175–182. [DOI] [PubMed] [Google Scholar]
- Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. (2003) Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144:5138–5144. [DOI] [PubMed] [Google Scholar]
- Erdmann E, Dormandy J, Wilcox R, Massi-Benedetti M, Charbonnel B. (2007) PROactive 07: pioglitazone in the treatment of type 2 diabetes: results of the PROactive study. Vasc Health Risk Manag 3:355–370. [PMC free article] [PubMed] [Google Scholar]
- Erion MD, Cable EE, Ito BR, Jiang HJ, Fujitaki JM, Finn PD, Zhang BH, Hou JZ, Boyer SH, van Poelje PD, et al. (2007) Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 104:15490–15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM. (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian CJ, Kimler BF, Anderson J, Tawfik OW, Mayo MS, Burak WE, Jr, O’Shaughnessy JA, Albain KS, Hyams DM, Budd GT, et al. (2004) Breast cancer chemoprevention phase I evaluation of biomarker modulation by arzoxifene, a third generation selective estrogen receptor modulator. Clin Cancer Res 10:5403–5417. [DOI] [PubMed] [Google Scholar]
- Falzon M. (1996) DNA sequences in the rat parathyroid hormone-related peptide gene responsible for 1,25-dihydroxyvitamin D3-mediated transcriptional repression. Mol Endocrinol 10:672–681. [DOI] [PubMed] [Google Scholar]
- Färnegårdh M, Bonn T, Sun S, Ljunggren J, Ahola H, Wilhelmsson A, Gustafsson JA, Carlquist M. (2003) The three-dimensional structure of the liver X receptor beta reveals a flexible ligand-binding pocket that can accommodate fundamentally different ligands. J Biol Chem 278:38821–38828. [DOI] [PubMed] [Google Scholar]
- Feng W, Ribeiro RC, Wagner RL, Nguyen H, Apriletti JW, Fletterick RJ, Baxter JD, Kushner PJ, West BL. (1998) Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280:1747–1749. [DOI] [PubMed] [Google Scholar]
- Fine PM. (2011) Update on emergency contraception. Adv Ther 28:87–90. [DOI] [PubMed] [Google Scholar]
- Flamant F, Baxter JD, Forrest D, Refetoff S, Samuels H, Scanlan TS, Vennström B, Samarut J. (2006) International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev 58:705–711. [DOI] [PubMed] [Google Scholar]
- Flores-Morales A, Gullberg H, Fernandez L, Ståhlberg N, Lee NH, Vennström B, Norstedt G. (2002) Patterns of liver gene expression governed by TRβ. Mol Endocrinol 16:1257–1268. [DOI] [PubMed] [Google Scholar]
- Forman BM, Ruan BF, Chen J, Schroepfer GJ, Jr, Evans RM. (1997) The orphan nuclear receptor LXRα is positively and negatively regulated by distinct products of mevalonate metabolism. Proc Natl Acad Sci USA 94:10588–10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest D, Erway LC, Ng L, Altschuler R, Curran T. (1996a) Thyroid hormone receptor beta is essential for development of auditory function. Nat Genet 13:354–357. [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. (1996b) Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J 15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Fraix M. (2012) Role of the musculoskeletal system and the prevention of falls. J Am Osteopath Assoc 112:17–21. [PubMed] [Google Scholar]
- Francis GA, Li G, Casey R, Wang J, Cao H, Leff T, Hegele RA. (2006) Peroxisomal proliferator activated receptor-gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3). BMC Med Genet 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazão JM, Elangovan L, Maung HM, Chesney RW, Acchiardo SR, Bower JD, Kelley BJ, Rodriguez HJ, Norris KC, Robertson JA, et al. (2000) Intermittent doxercalciferol (1α-hydroxyvitamin D2) therapy for secondary hyperparathyroidism. Am J Kidney Dis 36:550–561. [DOI] [PubMed] [Google Scholar]
- Freddie CT, Christensen GL, Lykkesfeldt AE. (2004a) A new MCF-7 breast cancer cell line resistant to the arzoxifene metabolite desmethylarzoxifene. Mol Cell Endocrinol 220:97–107. [DOI] [PubMed] [Google Scholar]
- Freddie CT, Larsen SS, Bartholomaeussen M, Lykkesfeldt AE. (2004b) The effect of the new SERM arzoxifene on growth and gene expression in MCF-7 breast cancer cells. Mol Cell Endocrinol 219:27–36. [DOI] [PubMed] [Google Scholar]
- Friis T, Pedersen LR. (1987) Serum lipids in hyper- and hypothyroidism before and after treatment. Clin Chim Acta 162:155–163. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Kimura C, Oe T, Takata Y, Sakuma H, Aramori I, Mutoh S. (2006a) A selective peroxisome proliferator-activated receptor gamma modulator with distinct fat cell regulation properties. J Pharmacol Exp Ther 318:863–871. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Sakuma H, Konishi S, Oe T, Hosogai N, Kimura C, Aramori I, Mutoh S. (2005) FK614, a novel peroxisome proliferator-activated receptor gamma modulator, induces differential transactivation through a unique ligand-specific interaction with transcriptional coactivators. J Pharmacol Sci 99:342–352. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Sakuma H, Ohkubo-Suzuki A, Aramori I, Mutoh S. (2006b) Unique properties of coactivator recruitment caused by differential binding of FK614, an anti-diabetic agent, to peroxisome proliferator-activated receptor gamma. Biol Pharm Bull 29:423–429. [DOI] [PubMed] [Google Scholar]
- Furr BJ, Jordan VC. (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 25:127–205. [DOI] [PubMed] [Google Scholar]
- Gadkar-Sable S, Shah C, Rosario G, Sachdeva G, Puri C. (2005) Progesterone receptors: various forms and functions in reproductive tissues. Front Biosci 10:2118–2130. [DOI] [PubMed] [Google Scholar]
- Galani A, Kitsiou-Tzeli S, Sofokleous C, Kanavakis E, Kalpini-Mavrou A. (2008) Androgen insensitivity syndrome: clinical features and molecular defects. Hormones (Athens) 7:217–229. [DOI] [PubMed] [Google Scholar]
- Galioni EF, Gofman JW, Guzvich P, Pouteau J, Rubinger JH, Strisower B. (1957) Long-term effect of dried thyroid on serum-lipoprotein and serum-cholesterol levels. Lancet 272:120–123. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Bishop CW, Knutson JC, Mazess RB, DeLuca HF. (1994) Effects of increasing doses of 1 α-hydroxyvitamin D2 on calcium homeostasis in postmenopausal osteopenic women. J Bone Miner Res 9:607–614. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Goldgar D. (1990) Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med 113:649–655. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Riggs BL, Recker RR, Goldgar D. (1989) The effect of calcitriol on patients with postmenopausal osteoporosis with special reference to fracture frequency. Proc Soc Exp Biol Med 191:287–292. [DOI] [PubMed] [Google Scholar]
- Gallagher JC, Sai AJ. (2010) Molecular biology of bone remodeling: implications for new therapeutic targets for osteoporosis. Maturitas 65:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe RT, Jr, Montana VG, Lambert MH, Miller AB, Bledsoe RK, Milburn MV, Kliewer SA, Willson TM, Xu HE. (2000) Asymmetry in the PPARγ/RXRα crystal structure reveals the molecular basis of heterodimerization among nuclear receptors. Mol Cell 5:545–555. [DOI] [PubMed] [Google Scholar]
- Gao WQ, Reiser PJ, Coss CC, Phelps MA, Kearbey JD, Miller DD, Dalton JT. (2005) Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology 146:4887–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garas A, Trypsianis G, Kallitsaris A, Milingos S, Messinis IE. (2004) Effects of clomiphene and raloxifene on gonadotrophin secretion in postmenopausal women: evidence of nongenomic action. Clin Endocrinol (Oxf) 61:256–262. [DOI] [PubMed] [Google Scholar]
- Gee AC, Carlson KE, Martini PG, Katzenellenbogen BS, Katzenellenbogen JA. (1999) Coactivator peptides have a differential stabilizing effect on the binding of estrogens and antiestrogens with the estrogen receptor. Mol Endocrinol 13:1912–1923. [DOI] [PubMed] [Google Scholar]
- Gehring U, Tomkins GM. (1974) A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell 3:301–306. [DOI] [PubMed] [Google Scholar]
- Gelman L, Feige JN, Desvergne B. (2007) Molecular basis of selective PPARγ modulation for the treatment of type 2 diabetes. Biochim Biophys Acta 1771:1094–1107. [DOI] [PubMed] [Google Scholar]
- Gemzell-Danielsson K, Mandl I, Marions L. (2003) Mechanisms of action of mifepristone when used for emergency contraception. Contraception 68:471–476. [DOI] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, De Paola V, Nuti R. (2010) Lasofoxifene: evidence of its therapeutic value in osteoporosis. Core Evid 4:113–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. (2005) Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA Study of Elderly Women. Osteoporos Int 16:1425–1431. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC. (2008) Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci 65:570–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Staels B, Dacquet C, Spedding M, Laudet V. (2006) Overview of nomenclature of nuclear receptors. Pharmacol Rev 58:685–704. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Borea PA. (2010) Glucocorticoid’s pharmacology: past, present and future. Curr Pharm Des 16:3540–3553. [DOI] [PubMed] [Google Scholar]
- Giguère V. (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725. [DOI] [PubMed] [Google Scholar]
- Giguere V, Ong ES, Segui P, Evans RM. (1987) Identification of a receptor for the morphogen retinoic acid. Nature 330:624–629. [DOI] [PubMed] [Google Scholar]
- Giguère V, Yang N, Segui P, Evans RM. (1988) Identification of a new class of steroid hormone receptors. Nature 331:91–94. [DOI] [PubMed] [Google Scholar]
- Giri AK, Mukhopadhyay A, Sun J, Hsie AW, Ray S. (1999) Antimutagenic effects of centchroman—a contraceptive and a candidate drug for breast cancer in multiple mutational assays. Mutagenesis 14:613–620. [DOI] [PubMed] [Google Scholar]
- Glasier AF. (1990) Clomiphene citrate. Baillieres Clin Obstet Gynaecol 4:491–501. [DOI] [PubMed] [Google Scholar]
- Glascock RF, Hoekstra WG. (1959) Selective accumulation of tritium-labelled hexoestrol by the reproductive organs of immature female goats and sheep. Biochem J 72:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser SR, Chytil F, Spelsberg TC. (1972) Early effects of oestradiol-17 on the chromatin and activity of the deoxyribonucleic acid-dependent ribonucleic acid polymerases (I and II) of the rat uterus. Biochem J 130:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Tan MH, Khan MA, Perez AT, Jacober SJ, GLAI Study Investigators (2005) A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28:1547–1554. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L., Jr (2000) A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update 6:212–224. [DOI] [PubMed] [Google Scholar]
- Goltzman D, Miao DS, Panda DK, Hendy GN. (2004) Effects of calcium and of the vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol 89-90:485–489. [DOI] [PubMed] [Google Scholar]
- Gorski J. (1964) Early estrogen effects on the activity of uterine ribonucleic acid polymerase. J Biol Chem 239:889–892. [PubMed] [Google Scholar]
- Gottardis MM, Jordan VC. (1987) Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res 47:4020–4024. [PubMed] [Google Scholar]
- Gottardis MM, Ricchio ME, Satyaswaroop PG, Jordan VC. (1990) Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res 50:3189–3192. [PubMed] [Google Scholar]
- Graham JD, Clarke CL. (1997) Physiological action of progesterone in target tissues. Endocr Rev 18:502–519. [DOI] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. (2002) Expression and transcriptional activity of progesterone receptor A and progesterone receptor B in mammalian cells. Breast Cancer Res 4:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. (1986) Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature 320:134–139. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. (1986) Sequence and expression of human estrogen receptor complementary DNA. Science 231:1150–1154. [DOI] [PubMed] [Google Scholar]
- Greenman DL, Wicks WD, Kenney FT. (1965) Stimulation of ribonucleic acid synthesis by steroid hormones. II. High molecular weight components. J Biol Chem 240:4420–4426. [PubMed] [Google Scholar]
- Grefhorst A, van Dijk TH, Hammer A, van der Sluijs FH, Havinga R, Havekes LM, Romijn JA, Groot PH, Reijngoud DJ, Kuipers F. (2005) Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am J Physiol Endocrinol Metab 289:E829–E838. [DOI] [PubMed] [Google Scholar]
- Greschik H, Moras D. (2003) Structure-activity relationship of nuclear receptor-ligand interactions. Curr Top Med Chem 3:1573–1599. [DOI] [PubMed] [Google Scholar]
- Grether U, Klaus W, Kuhn B, Maerki HP, Mohr P, Wright MB. (2010) New insights on the mechanism of PPAR-targeted drugs. ChemMedChem 5:1973–1976. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Turcotte B, Quirin-Stricker C, Bocquel MT, Meyer ME, Krozowski Z, Jeltsch JM, Lerouge T, Garnier JM, Chambon P. (1987) The chicken progesterone receptor: sequence, expression and functional analysis. EMBO J 6:3985–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot PHE, Pearce NJ, Yates JW, Stocker C, Sauermelch C, Doe CP, Willette RN, Olzinski A, Peters T, d’Epagnier D, et al. (2005) Synthetic LXR agonists increase LDL in CETP species. J Lipid Res 46:2182–2191. [DOI] [PubMed] [Google Scholar]
- Grose R, Werner S, Kessler D, Tuckermann J, Huggel K, Durka S, Reichardt HM, Werner S. (2002) A role for endogenous glucocorticoids in wound repair. EMBO Rep 3:575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. (2004) Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-l-thyronine. Endocrinology 145:1656–1661. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellstrom K, Malm J. (2005) Development of the thyroid hormone receptor beta-subtype agonist KB-141: a strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovasc Drug Rev 23:133–148. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellström K, Malm J. (2007) Therapeutic potential for thyroid hormone receptor-beta selective agonists for treating obesity, hyperlipidemia and diabetes. Curr Vasc Pharmacol 5:141–154. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B, et al. (2003) Selective thyroid hormone receptor-beta activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA 100:10067–10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. (2005) Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med 11:861–866. [DOI] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Forrest D, Angelin B, Vennström B. (2000) Thyroid hormone receptor beta-deficient mice show complete loss of the normal cholesterol 7α-hydroxylase (CYP7A) response to thyroid hormone but display enhanced resistance to dietary cholesterol. Mol Endocrinol 14:1739–1749. [DOI] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Saltó C, Forrest D, Angelin B, Vennström B. (2002) Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol 16:1767–1777. [DOI] [PubMed] [Google Scholar]
- Günther C, Kecskes A, Staks T, Täuber U. (1998) Percutaneous absorption of methylprednisolone aceponate following topical application of Advantan lotion on intact, inflamed and stripped skin of male volunteers. Skin Pharmacol Appl Skin Physiol 11:35–42. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Greenberg AS, Weston WM, Chen HZ, Williams K, Freed MI. (2002) Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation 106:679–684. [DOI] [PubMed] [Google Scholar]
- Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. (1994) Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455–1458. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP, Korach KS. (2002) Allosteric regulation of estrogen receptor structure, function, and coactivator recruitment by different estrogen response elements. Mol Endocrinol 16:469–486. [DOI] [PubMed] [Google Scholar]
- Hamilton B. (2010) Vitamin D and human skeletal muscle. Scand J Med Sci Sports 20:182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA, Benson MD. (2001) Transthyretin: a review from a structural perspective. Cell Mol Life Sci 58:1491–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamuro Y, Coales SJ, Morrow JA, Molnar KS, Tuske SJ, Southern MR, Griffin PR. (2006) Hydrogen/deuterium-exchange (H/D-Ex) of PPARγ LBD in the presence of various modulators. Protein Sci 15:1883–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Roman J. (2007) Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs 18:237–244. [DOI] [PubMed] [Google Scholar]
- Han Y, Feng HL, Sandlow JI, Haines CJ. (2009) Comparing expression of progesterone and estrogen receptors in testicular tissue from men with obstructive and nonobstructive azoospermia. J Androl 30:127–133. [DOI] [PubMed] [Google Scholar]
- Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N. (2003) Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol Pharm Bull 26:1563–1569. [DOI] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, et al. (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1α knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117:3463–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. (2008) The role of exercise and PGC1α in inflammation and chronic disease. Nature 454:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harant H, Andrew PJ, Reddy GS, Foglar E, Lindley IJD. (1997) 1α,25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-κB-mediated interleukin-8 gene expression. Eur J Biochem 250:63–71. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. (1967a) Mode of action of I.C.I. 46,474 in preventing implantation in rats. J Endocrinol 37:83–92. [DOI] [PubMed] [Google Scholar]
- Harper MJ, Walpole AL. (1967b) A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J Reprod Fertil 13:101–119. [DOI] [PubMed] [Google Scholar]
- Harvey JA, Holm MK, Ranganath R, Guse PA, Trott EA, Helzner E. (2009) The effects of bazedoxifene on mammographic breast density in postmenopausal women with osteoporosis. Menopause 16:1193–1196. [DOI] [PubMed] [Google Scholar]
- Hattner R, Epker BN, Frost HM. (1965) Suggested sequential mode of control of changes in cell behaviour in adult bone remodelling. Nature 206:489–490. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Norman AW. (1969) Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci USA 62:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Ohnota H, Tamura T, Kuroda J, Shibata N, Akahane M, Moriwaki H, Machida N, Mitsumori K. (2004) Inhibitory effects of KAT-681, a liver-selective thyromimetic, on development of hepatocellular proliferative lesions in rats induced by 2-acetylaminofluorene and partial hepatectomy after diethylnitrosamine initiation. Arch Toxicol 78:460–466. [DOI] [PubMed] [Google Scholar]
- Heemers HV, Tindall DJ. (2007) Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 28:778–808. [DOI] [PubMed] [Google Scholar]
- Heery DM, Kalkhoven E, Hoare S, Parker MG. (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736. [DOI] [PubMed] [Google Scholar]
- Heikinheimo O, Kekkonen R, Lähteenmäki P. (2003) The pharmacokinetics of mifepristone in humans reveal insights into differential mechanisms of antiprogestin action. Contraception 68:421–426. [DOI] [PubMed] [Google Scholar]
- Henry HL, Norman AW. (1974) Studies on calciferol metabolism. IX. Renal 25-hydroxy-vitamin D3-1 hydroxylase. Involvement of cytochrome P-450 and other properties. J Biol Chem 249:7529–7535. [PubMed] [Google Scholar]
- Hermanson O, Glass CK, Rosenfeld MG. (2002) Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol Metab 13:55–60. [DOI] [PubMed] [Google Scholar]
- Herrlich P, Ponta H. (1994) Mutual cross-modulation of steroid/retinoic acid receptor and AP-1 transcription factor activities: a novel property with practical implications. Trends Endocrinol Metab 5:341–346. [DOI] [PubMed] [Google Scholar]
- Herynk MH, Fuqua SA. (2004) Estrogen receptor mutations in human disease. Endocr Rev 25:869–898. [DOI] [PubMed] [Google Scholar]
- Hess AF, Unger LJ. (1921) The cure of infantile rickets by artificial light and by sunlight. Proc Soc Exp Biol Med 18:298. [Google Scholar]
- Heuer H, Visser TJ. (2009) Minireview: pathophysiological importance of thyroid hormone transporters. Endocrinology 150:1078–1083. [DOI] [PubMed] [Google Scholar]
- Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. (1992) 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell 68:397–406. [DOI] [PubMed] [Google Scholar]
- Higgins LS, Depaoli AM. (2010) Selective peroxisome proliferator-activated receptor gamma (PPARγ) modulation as a strategy for safer therapeutic PPARγ activation. Am J Clin Nutr 91:267S–272S. [DOI] [PubMed] [Google Scholar]
- Hill SR, Jr, Barker SB, McNEIL JH, Tingley JO, Hibbett LL. (1960) The metabolic effects of the acetic and propionic acid analogs of thyroxine and triiodothyronine. J Clin Invest 39:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittelman AB, Burakov D, Iñiguez-Lluhí JA, Freedman LP, Garabedian MJ. (1999) Differential regulation of glucocorticoid receptor transcriptional activation via AF-1-associated proteins. EMBO J 18:5380–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin RA, Lazar MA, Chin WW. (1990) Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Invest 85:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick MF. (1996) Vitamin D and bone health. J Nutr 126 (Suppl):S1159–S1164. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. (1985) Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 318:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holli K, Finnish Breast Cancer Group (2002) Tamoxifen versus toremifene in the adjuvant treatment of breast cancer. Eur J Cancer 38 (Suppl 6):S37–S38. [DOI] [PubMed] [Google Scholar]
- Holterhus PM, Werner R, Hoppe U, Bassler J, Korsch E, Ranke MB, Dörr HG, Hiort O. (2005) Molecular features and clinical phenotypes in androgen insensitivity syndrome in the absence and presence of androgen receptor gene mutations. J Mol Med (Berl) 83:1005–1013. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJV, RECORD Study Team (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Garabedian MJ, Stallcup MR. (1997) GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol 17:2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Kohli K, Trivedi A, Johnson DL, Stallcup MR. (1996) GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc Natl Acad Sci USA 93:4948–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309:1074–1078. [DOI] [PubMed] [Google Scholar]
- Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK, et al. (1995) Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L. (1996) Nuclear receptor coactivators and corepressors. Mol Endocrinol 10:1167–1177. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. (2006) Inflammation and metabolic disorders. Nature 444:860–867. [DOI] [PubMed] [Google Scholar]
- Houck KA, Borchert KM, Hepler CD, Thomas JS, Bramlett KS, Michael LF, Burris TP. (2004) T0901317 is a dual LXR/FXR agonist. Mol Genet Metab 83:184–187. [DOI] [PubMed] [Google Scholar]
- Howard KJ, Holley SJ, Yamamoto KR, Distelhorst CW. (1990) Mapping the HSP90 binding region of the glucocorticoid receptor. J Biol Chem 265:11928–11935. [PubMed] [Google Scholar]
- Hsu YH, Burke JE, Li S, Woods VL, Jr, Dennis EA. (2009) Localizing the membrane binding region of Group VIA Ca2+-independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry. J Biol Chem 284:23652–23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu ED, Kim JB, Sarraf P, Spiegelman BM. (1996) Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science 274:2100–2103. [DOI] [PubMed] [Google Scholar]
- Hu X, Lazar MA. (1999) The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature 402:93–96. [DOI] [PubMed] [Google Scholar]
- Hu X, Li SZ, Wu J, Xia CS, Lala DS. (2003) Liver X receptors interact with corepressors to regulate gene expression. Mol Endocrinol 17:1019–1026. [DOI] [PubMed] [Google Scholar]
- Hu X, Li Y, Lazar MA. (2001) Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol 21:1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang THW, Razmovski-Naumovski V, Salam NK, Duke RK, Tran VH, Duke CC, Roufogalis BD. (2005) A novel LXR-alpha activator identified from the natural product Gynostemma pentaphyllum. Biochem Pharmacol 70:1298–1308. [DOI] [PubMed] [Google Scholar]
- Huldschinsky K. (1919) Heilung von Rachitis durch Ku nstliche Hohensonne. Deutsch Med Woch 45:712–713. [Google Scholar]
- Humphrey EL, Williams JHH, Davie MWJ, Marshall MJ. (2006) Effects of dissociated glucocorticoids on OPG and RANKL in osteoblastic cells. Bone 38:652–661. [DOI] [PubMed] [Google Scholar]
- Iacob RE, Pene-Dumitrescu T, Zhang J, Gray NS, Smithgall TE, Engen JR. (2009) Conformational disturbance in Abl kinase upon mutation and deregulation. Proc Natl Acad Sci USA 106:1386–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannuzzi-Sucich M, Prestwood KM, Kenny AM. (2002) Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol A Biol Sci Med Sci 57:M772–M777. [DOI] [PubMed] [Google Scholar]
- Imam SK, Srivastava K, Dasgupta PR, Kar AB. (1975) Effect of 3,4-trans-2,2-dimethyl-3-phenyl-4-P-(beta-pyrrolidinoethoxy) phenyl -7-methoxy chroman (centchroman) on the biochemistry of the fallopian tube and uterus of rhesus monkeys (Macaca mulatta). Contraception 11:309–316. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. (2010) Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151:2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail A, Nguyen CV, Ahene A, Fleet JC, Uskokovic MR, Peleg S. (2004) Effect of cellular environment on the selective activation of the vitamin D receptor by 1α,25-dihydroxyvitamin D3 and its analog 1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26-9228). Mol Endocrinol 18:874–887. [DOI] [PubMed] [Google Scholar]
- Ito BR, Zhang BH, Cable EE, Song X, Fujitaki JM, MacKenna DA, Wilker CE, Chi B, van Poelje PD, Linemeyer DL, et al. (2009) Thyroid hormone beta receptor activation has additive cholesterol lowering activity in combination with atorvastatin in rabbits, dogs and monkeys. Br J Pharmacol 156:454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Cheung KL, Buzdar AU, Robertson JF. (2008) Arzoxifene: the evidence for its development in the management of breast cancer. Core Evid 2:251–258. [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728–731. [DOI] [PubMed] [Google Scholar]
- Jayasuriya H, Herath KB, Ondeyka JG, Guan ZQ, Borris RP, Tiwari S, de Jong W, Chavez F, Moss J, Stevenson DW, et al. (2005) Diterpenoid, steroid, and triterpenoid agonists of liver X receptors from diversified terrestrial plants and marine sources. J Nat Prod 68:1247–1252. [DOI] [PubMed] [Google Scholar]
- Jeltsch JM, Krozowski Z, Quirin-Stricker C, Gronemeyer H, Simpson RJ, Garnier JM, Krust A, Jacob F, Chambon P. (1986) Cloning of the chicken progesterone receptor. Proc Natl Acad Sci USA 83:5424–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, Desombre ER, Kawashima T, Suzuki T, Kyser K, Jungblut PW. (1967) Estrogen-binding substances of target tissues. Science 158:529–530. [DOI] [PubMed] [Google Scholar]
- Jensen EV, Jacobson HI. (1960) Fate of steroid estrogens in target tissues, Academic Press, New York. [Google Scholar]
- Jensen EV, Jacobson HI. (1962) Basic guides to the mechanism of estrogen action. Recent Prog Horm Res 18:387–414. [Google Scholar]
- Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. (1991) Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol 5:1396–1404. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, Angelin B, Parini P. (2005) Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 102:10297–10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CC, Jr, Bjarnason NH, Cohen FJ, Shah A, Lindsay R, Mitlak BH, Huster W, Draper MW, Harper KD, Heath H, 3rd, et al. (2000) Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three-year data from 2 double-blind, randomized, placebo-controlled trials. Arch Intern Med 160:3444–3450. [DOI] [PubMed] [Google Scholar]
- Jones A, Chen JY, Hwang DJ, Miller DD, Dalton JT. (2009) Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception. Endocrinology 150:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC. (1975) Prolonged antioestrogenic activity of ICI 46, 474 in the ovariectomized mouse. J Reprod Fertil 42:251–258. [DOI] [PubMed] [Google Scholar]
- Jordan VC. (2006) Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol 147 (Suppl 1):S269–S276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, Dowse LJ. (1976) Tamoxifen as an anti-tumour agent: effect on oestrogen binding. J Endocrinol 68:297–303. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Phelps E, Lindgren JU. (1987) Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat 10:31–35. [DOI] [PubMed] [Google Scholar]
- Joseph SB, McKilligin E, Pei LM, Watson MA, Collins AR, Laffitte BA, Chen MY, Noh G, Goodman J, Hagger GN, et al. (2002) Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA 99:7604–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Lee MS, Jang EJ, Ahn JH, Kang NS, Yoo SE, Bae MA, Hong JH, Hwang ES. (2009) Augmentation of PPARγ-TAZ interaction contributes to the anti-adipogenic activity of KR62980. Biochem Pharmacol 78:1323–1329. [DOI] [PubMed] [Google Scholar]
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. (2010) A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 17:281–289. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, et al. ADOPT Study Group (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443. [DOI] [PubMed] [Google Scholar]
- Kallander LS, Washburn DG, Hoang TH, Frazee JS, Stoy P, Johnson L, Lu Q, Hammond M, Barton LS, Patterson JR, et al. (2010) Improving the developability profile of pyrrolidine progesterone receptor partial agonists. Bioorg Med Chem Lett 20:371–374. [DOI] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. (2002) X-ray structure of the hRORα LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORα. Structure 10:1697–1707. [DOI] [PubMed] [Google Scholar]
- Kallenberger BC, Love JD, Chatterjee VKK, Schwabe JWR. (2003) A dynamic mechanism of nuclear receptor activation and its perturbation in a human disease. Nat Struct Biol 10:136–140. [DOI] [PubMed] [Google Scholar]
- Kamboj VP, Singh MM, Kar AB. (1973) Effect of some non-steroidal antifertility agents on biochemistry of the uterus and uterine fluid in rats. Indian J Exp Biol 11:479–483. [PubMed] [Google Scholar]
- Kamen DL, Tangpricha V. (2010) Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med (Berl) 88:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko E, Matsuda M, Yamada Y, Tachibana Y, Shimomura I, Makishima M. (2003) Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. J Biol Chem 278:36091–36098. [DOI] [PubMed] [Google Scholar]
- Kanis JA, Johansson H, Oden A, McCloskey EV. (2009) Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone 44:1049–1054. [DOI] [PubMed] [Google Scholar]
- Kassel O, Schneider S, Heilbock C, Litfin M, Göttlicher M, Herrlich P. (2004) A nuclear isoform of the focal adhesion LIM-domain protein Trip6 integrates activating and repressing signals at AP-1- and NF-κB-regulated promoters. Genes Dev 18:2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Takeyama K, Kitanaka S, Murayama A, Sekine K, Yoshizawa T. (1999) In vivo function of VDR in gene expression-VDR knock-out mice. J Steroid Biochem Mol Biol 69:247–251. [DOI] [PubMed] [Google Scholar]
- Katz A, Udata C, Ott E, Hickey L, Burczynski ME, Burghart P, Vesterqvist O, Meng X. (2009) Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol 49:643–649. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. (2010) PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat Rev Endocrinol 6:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke HZ, Paralkar VM, Grasser WA, Crawford DT, Qi H, Simmons HA, Pirie CM, Chidsey-Frink KL, Owen TA, Smock SL, et al. (1998) Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology 139:2068–2076. [DOI] [PubMed] [Google Scholar]
- Ke HZ, Qi H, Crawford DT, Chidsey-Frink KL, Simmons HA, Thompson DD. (2000) Lasofoxifene (CP-336,156), a selective estrogen receptor modulator, prevents bone loss induced by aging and orchidectomy in the adult rat. Endocrinology 141:1338–1344. [DOI] [PubMed] [Google Scholar]
- Kearbey JD, Gao WQ, Fisher SJ, Wu D, Miller DD, Dalton JT. (2009) Effects of selective androgen receptor modulator (SARM) treatment in osteopenic female rats. Pharm Res 26:2471–2477. [DOI] [PubMed] [Google Scholar]
- Kearbey JD, Gao WQ, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT. (2007) Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res 24:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearbey JD, Wu D, Gao W, Miller DD, Dalton JT. (2004) Pharmacokinetics of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro- 3-trifluoromethyl-phenyl)-propionamide in rats, a non-steroidal selective androgen receptor modulator. Xenobiotica 34:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. (1993) Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci USA 90:2160–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall EC. (1915) The isolation in crystalline form of the compound containing iodin, which occurs in the thyroid. Its chemical nature and physiologic activity. JAMA 64:2042–2043. [PubMed] [Google Scholar]
- Kendler DL, Palacios S, Cox DA, Stock J, Alam J, Dowsett SA, Zanchetta J. (2012) Arzoxifene versus raloxifene: effect on bone and safety parameters in postmenopausal women with osteoporosis. Osteoporos Int 23:1091–1101. [DOI] [PubMed] [Google Scholar]
- Kershah SM, Desouki MM, Koterba KL, Rowan BG. (2004) Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol Oncol 92:304–313. [DOI] [PubMed] [Google Scholar]
- Kessler L, Nedeltcheva A, Imperial J, Penev PD. (2010) Changes in serum TSH and free T4 during human sleep restriction. Sleep 33:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester MHA, Kaptein E, Van Dijk CH, Roest TJ, Tibboel D, Coughtrie MWH, Visser TJ. (2002) Characterization of iodothyronine sulfatase activities in human and rat liver and placenta. Endocrinology 143:814–819. [DOI] [PubMed] [Google Scholar]
- Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. (2008) The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 149:6084–6091. [DOI] [PubMed] [Google Scholar]
- Kim HW, Park CW, Shin YS, Kim YS, Shin SJ, Kim YS, Choi EJ, Chang YS, Bang BK. (2006a) Calcitriol regresses cardiac hypertrophy and QT dispersion in secondary hyperparathyroidism on hemodialysis. Nephron Clin Pract 102:c21–c29. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Sefton EC. (2012) The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol 358:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KR, Lee JH, Kim SJ, Rhee SD, Jung WH, Yang SD, Kim SS, Ahn JH, Cheon HG. (2006b) KR-62980: a novel peroxisome proliferator-activated receptor gamma agonist with weak adipogenic effects. Biochem Pharmacol 72:446–454. [DOI] [PubMed] [Google Scholar]
- Kim KY, Cho HS, Lee SH, Ahn JH, Cheon HG. (2011) Neuroprotective effects of KR-62980, a new PPARγ agonist, against chemical ischemia-reperfusion in SK-N-SH cells. Brain Res 1372:103–114. [DOI] [PubMed] [Google Scholar]
- Kim KY, Kim SS, Cheon HG. (2006c) Differential anti-proliferative actions of peroxisome proliferator-activated receptor-gamma agonists in MCF-7 breast cancer cells. Biochem Pharmacol 72:530–540. [DOI] [PubMed] [Google Scholar]
- King RJ, Gordon J. (1972) Involvement of DNA in the acceptor mechanism for uterine oestradiol receptor. Nat New Biol 240:185–187. [DOI] [PubMed] [Google Scholar]
- King RJ, Gordon J, Cowan DM, Inman DR. (1966) The intranuclear localization of [6,7-3H]-oestradiol-17-beta in dimethylbenzanthracene-induced rat mammary adenocarcinoma and other tissues. J Endocrinol 36:139–150. [DOI] [PubMed] [Google Scholar]
- Kirwan JR, Bálint G, Szebenyi B. (1999) Anniversary: 50 years of glucocorticoid treatment in rheumatoid arthritis. Rheumatology (Oxford) 38:100–102. [DOI] [PubMed] [Google Scholar]
- Kitazawa R, Kitazawa S. (2002) Vitamin D3 augments osteoclastogenesis via vitamin D-responsive element of mouse RANKL gene promoter. Biochem Biophys Res Commun 290:650–655. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kajimoto K, Kondo T, Kitazawa R. (2003) Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J Cell Biochem 89:771–777. [DOI] [PubMed] [Google Scholar]
- Klaholz BP, Mitschler A, Belema M, Zusi C, Moras D. (2000) Enantiomer discrimination illustrated by high-resolution crystal structures of the human nuclear receptor hRARγ. Proc Natl Acad Sci USA 97:6322–6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman A, Tuckermann JP. (2007) Glucocorticoid receptor action in beneficial and side effects of steroid therapy: lessons from conditional knockout mice. Mol Cell Endocrinol 275:98–108. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM. (1999) Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757–760. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. (1992) Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature 355:446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Korach KS, Diaugustine RP. (2000) Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: requirement of estrogen receptor-alpha. Endocrinology 141:3430–3439. [DOI] [PubMed] [Google Scholar]
- Kojetin DJ, Burris TP, Jensen EV, Khan SA. (2008) Implications of the binding of tamoxifen to the coactivator recognition site of the estrogen receptor. Endocr Relat Cancer 15:851–870. [DOI] [PubMed] [Google Scholar]
- Koldamova R, Lefterov I. (2007) Role of LXR and ABCA1 in the pathogenesis of Alzheimer’s disease—implications for a new therapeutic approach. Curr Alzheimer Res 4:171–178. [DOI] [PubMed] [Google Scholar]
- Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. (2005) Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology 146:3999–4008. [DOI] [PubMed] [Google Scholar]
- Komm BS, Lyttle CR. (2001) Developing a SERM: stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci 949:317–326. [DOI] [PubMed] [Google Scholar]
- Komm BS, Vlasseros F, Samadfam R, Chouinard L, Smith SY. (2011) Skeletal effects of bazedoxifene paired with conjugated estrogens in ovariectomized rats. Bone 49:376–386. [DOI] [PubMed] [Google Scholar]
- Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. (2008) Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med 168:397–403. [DOI] [PubMed] [Google Scholar]
- Kragballe K. (1995) Calcipotriol: a new drug for topical psoriasis treatment. Pharmacol Toxicol 77:241–246. [DOI] [PubMed] [Google Scholar]
- Kramer H, Toto R, Peshock R, Cooper R, Victor R. (2005) Association between chronic kidney disease and coronary artery calcification: the Dallas Heart Study. J Am Soc Nephrol 16:507–513. [DOI] [PubMed] [Google Scholar]
- Kremer R, Karaplis AC, Henderson J, Gulliver W, Banville D, Hendy GN, Goltzman D. (1991) Regulation of parathyroid hormone-like peptide in cultured normal human keratinocytes. Effect of growth factors and 1,25 dihydroxyvitamin D3 on gene expression and secretion. J Clin Invest 87:884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremoser C, Albers M, Burris TP, Deuschle U, Koegl M. (2007) Panning for SNuRMs: using cofactor profiling for the rational discovery of selective nuclear receptor modulators. Drug Discov Today 12:860–869. [DOI] [PubMed] [Google Scholar]
- Krust A, Green S, Argos P, Kumar V, Walter P, Bornert JM, Chambon P. (1986) The chicken oestrogen receptor sequence: homology with v-erbA and the human oestrogen and glucocorticoid receptors. EMBO J 5:891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubodera N, Tsuji N, Uchiyama Y, Endo K. (2003) A new active vitamin D analog, ED-71, causes increase in bone mass with preferential effects on bone in osteoporotic patients. J Cell Biochem 88:286–289. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. (1996) Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak J, Jr, Fischer C, Komm B, Taylor HS. (2011) Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology 152:3226–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez R, Burris TP, Griffin PR. (2010) The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-alpha/gamma inverse agonist. Mol Pharmacol 77:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Thompson EB. (2012) Folding of the glucocorticoid receptor N-terminal transactivation function: dynamics and regulation. Mol Cell Endocrinol 348:450–456. [DOI] [PubMed] [Google Scholar]
- Kumar R, Volk DE, Li JQ, Lee JC, Gorenstein DG, Thompson EB. (2004) TATA box binding protein induces structure in the recombinant glucocorticoid receptor AF-1 domain. Proc Natl Acad Sci USA 101:16425–16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Green S, Staub A, Chambon P. (1986) Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO J 5:2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J, Otsuki T, Kunisue H, Tanaka K, Yamamoto S, Sonoo H. (2000) Expression levels of estrogen receptor-alpha, estrogen receptor-beta, coactivators, and corepressors in breast cancer. Clin Cancer Res 6:512–518. [PubMed] [Google Scholar]
- Kurosawa T, Hiroi H, Momoeda M, Inoue S, Taketani Y. (2010) Clomiphene citrate elicits estrogen agonistic/antagonistic effects differentially via estrogen receptors alpha and beta. Endocr J 57:517–521. [DOI] [PubMed] [Google Scholar]
- Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. (2010) Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362:906–916. [DOI] [PubMed] [Google Scholar]
- Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, et al. (2003) Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci USA 100:5419–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago RM, Singh PP, Nesto RW. (2007) Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 370:1129–1136. [DOI] [PubMed] [Google Scholar]
- Lal J, Asthana OP, Nityanand S, Gupta RC. (1995) Pharmacokinetics of centchroman in healthy female subjects after oral administration. Contraception 52:297–300. [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Pedersen TA, Gross B, Lestavel S, Yous S, Vallez E, Gustafsson JA, Mandrup S, Fiévet C, Staels B, et al. (2009) Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol 29:1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois MF, Zanger K, Monden T, Safer JD, Hollenberg AN, Wondisford FE. (1997) A unique role of the beta-2 thyroid hormone receptor isoform in negative regulation by thyroid hormone. Mapping of a novel amino-terminal domain important for ligand-independent activation. J Biol Chem 272:24927–24933. [DOI] [PubMed] [Google Scholar]
- Langner A, Stapór W, Ambroziak M. (2001) Efficacy and tolerance of topical calcitriol 3 microg g(-1) in psoriasis treatment: a review of our experience in Poland. Br J Dermatol 144 (Suppl 58):11–16. [DOI] [PubMed] [Google Scholar]
- Lau KHW, Baylink DJ. (1999) Vitamin D therapy of osteoporosis: plain vitamin D therapy versus active vitamin D analog (D-hormone) therapy. Calcif Tissue Int 65:295–306. [DOI] [PubMed] [Google Scholar]
- Laverny G, Penna G, Uskokovic M, Marczak S, Maehr H, Jankowski P, Ceailles C, Vouros P, Smith B, Robinson M, et al. (2009) Synthesis and anti-inflammatory properties of 1α,25-dihydroxy-16-ene-20-cyclopropyl-24-oxo-vitamin D3, a hypocalcemic, stable metabolite of 1α,25-dihydroxy-16-ene-20-cyclopropyl-vitamin D3. J Med Chem 52:2204–2213. [DOI] [PubMed] [Google Scholar]
- Laverny G, Penna G, Vetrano S, Correale C, Nebuloni M, Danese S, Adorini L. (2010) Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett 131:49–58. [DOI] [PubMed] [Google Scholar]
- Lavigne AC, Mengus G, Gangloff YG, Wurtz JM, Davidson I. (1999) Human TAF-(II)55 interacts with the vitamin D3 and thyroid hormone receptors and with derivatives of the retinoid X receptor that have altered transactivation properties. Mol Cell Biol 19:5486–5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law ML, Kao FT, Wei Q, Hartz JA, Greene GL, Zarucki-Schulz T, Conneely OM, Jones C, Puck TT, O’Malley BW, et al. (1987) The progesterone receptor gene maps to human chromosome band 11q13, the site of the mammary oncogene int-2. Proc Natl Acad Sci USA 84:2877–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar MA. (1993) Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev 14:184–193. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. (2002) Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology 143:2376–2384. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Ko DH. (1999) A novel approach to the discovery of non-systemic anti-inflammatory steroids; antedrug. Arch Pharm Res 22:279–287. [DOI] [PubMed] [Google Scholar]
- Lee JW, Ryan F, Swaffield JC, Johnston SA, Moore DD. (1995) Interaction of thyroid-hormone receptor with a conserved transcriptional mediator. Nature 374:91–94. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee J, Lee SK, Lee JW. (2008) Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to the liver X receptors. Mol Endocrinol 22:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Suh JY, Rhee EJ, Park JS, Sung KC, Kim SW. (2004) Plasma CRP, apolipoprotein A-1, apolipoprotein B and Lpa levels according to thyroid function status. Arch Med Res 35:540–545. [DOI] [PubMed] [Google Scholar]
- Leeper RD, Mead AW, Money WL, Rawson RW. (1961) Metabolic effects and therapeutic applications of triiodothyropropionic acid. Clin Pharmacol Ther 2:13–21. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su J-L, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, et al. (1997) Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272:3137–3140. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. (1995) An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem 270:12953–12956. [DOI] [PubMed] [Google Scholar]
- Lerman J, Pitt-Rivers R. (1956) Physiologic activity of triiodo-and tetraiodo-thyroacetic acid in human myxedema. J Clin Endocrinol Metab 16:1470–1479. [DOI] [PubMed] [Google Scholar]
- Lerner LJ, Holthaus FJ, Jr, Thompson CR. (1958) A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology 63:295–318. [DOI] [PubMed] [Google Scholar]
- Levens ED, Potlog-Nahari C, Armstrong AY, Wesley R, Premkumar A, Blithe DL, Blocker W, Nieman LK. (2008) CDB-2914 for uterine leiomyomata treatment: a randomized controlled trial. Obstet Gynecol 111:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AA, Sturzenbecker LJ, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, et al. (1992) 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature 355:359–361. [DOI] [PubMed] [Google Scholar]
- Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. (2005) Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol 25:135–142. [DOI] [PubMed] [Google Scholar]
- Lewiecki EM, Diaz Curiel M, Borges JL, Kung A, Brandi ML, Dimai HP. (2010) New and emerging therapies for osteoporosis. J Osteoporos 2010:318320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Chagpar AB, Shaughnessy EA, Nurko J, McMasters K, Edwards MJ. (2010) Excellent outcomes with adjuvant toremifene or tamoxifen in early stage breast cancer. Cancer 116:2307–2315. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. (2011) Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care 34:916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Kim HR, Curpan R, Grigg R, Sarker MA, Jordan VC. (2011) The selective estrogen receptor modulator bazedoxifene inhibits hormone-independent breast cancer cell growth and down-regulates estrogen receptor α and cyclin D1. Mol Pharmacol 80:610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Gomes PJ, Chen JD. (1997a) RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA 94:8479–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Pascual G, Glass CK. (2000) Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol Cell Biol 20:4699–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lonard DM, O’Malley BW. (2004) A contemporary understanding of progesterone receptor function. Mech Ageing Dev 125:669–678. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. (2007) SIRT1 deacetylates and positively regulates the nuclear receptor LXR. Mol Cell 28:91–106. [DOI] [PubMed] [Google Scholar]
- Li XL, Yeh V, Molteni V. (2010) Liver X receptor modulators: a review of recently patented compounds (2007–2009). Expert Opin Ther Pat 20:535–562. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang J, Schopfer FJ, Martynowski D, Garcia-Barrio MT, Kovach A, Suino-Powell K, Baker PR, Freeman BA, Chen YE, et al. (2008) Molecular recognition of nitrated fatty acids by PPAR gamma. Nat Struct Mol Biol 15:865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kong J, Wei MJ, Chen ZF, Liu SQ, Cao LP. (2002) 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. (1997b) Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Rendi M, Suh N, Royce DB, Risingsong R, Williams CR, Lamph W, Labrie F, Krajewski S, Xu X, et al. (2006) The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res 12:5902–5909. [DOI] [PubMed] [Google Scholar]
- Lieberman BA, Bona BJ, Edwards DP, Nordeen SK. (1993) The constitution of a progesterone response element. Mol Endocrinol 7:515–527. [DOI] [PubMed] [Google Scholar]
- Lin VH, Klepp HM, and Hanley RM (2008) Sobetirome is a thyroid hormone receptorβ- and liver-selective thyromimetic that can effect substantial LDL-C lowering without significant changes in heart rate or the thyroid axis in euthyroid men; in 90th Annual Meeting Of the Endocrine Society, 2008 June 15–18; San Francisco, CA. OR36–OR33, The Endocrine Society, Chevy Chase, MD. [Google Scholar]
- Lin X, Huebner V. (2000) Non-steroidal ligands for steroid hormone receptors. Curr Opin Drug Discov Devel 3:383–398. [PubMed] [Google Scholar]
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. (2009) Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 92:1045–1052. [DOI] [PubMed] [Google Scholar]
- Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, Constantine G. (2009) Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 92:1025–1038. [DOI] [PubMed] [Google Scholar]
- Loke YK, Singh S, Furberg CD. (2009) Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonard DM, Lanz RB, O’Malley BW. (2007) Nuclear receptor coregulators and human disease. Endocr Rev 28:575–587. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. (2006) The expanding cosmos of nuclear receptor coactivators. Cell 125:411–414. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. (2007) Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700. [DOI] [PubMed] [Google Scholar]
- Long X, Fan M, Nephew KP. (2010) Estrogen receptor-alpha-interacting cytokeratins potentiate the antiestrogenic activity of fulvestrant. Cancer Biol Ther 9:389–396. [DOI] [PubMed] [Google Scholar]
- Long X, Nephew KP. (2006) Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem 281:9607–9615. [DOI] [PubMed] [Google Scholar]
- Loosfelt H, Atger M, Misrahi M, Guiochon-Mantel A, Meriel C, Logeat F, Benarous R, Milgrom E. (1986) Cloning and sequence analysis of rabbit progesterone-receptor complementary DNA. Proc Natl Acad Sci USA 83:9045–9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López FJ, Ardecky RJ, Bebo B, Benbatoul K, De Grandpre L, Liu S, Leibowitz MD, Marschke K, Rosen J, Rungta D, et al. (2008) LGD-5552, an antiinflammatory glucocorticoid receptor ligand with reduced side effects, in vivo. Endocrinology 149:2080–2089. [DOI] [PubMed] [Google Scholar]
- Lu M, Sarruf DA, Talukdar S, Sharma S, Li PP, Bandyopadhyay G, Nalbandian S, Fan WQ, Gayen JR, Mahata SK, et al. (2011) Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med 17:618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. (1988) Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science 240:327–330. [DOI] [PubMed] [Google Scholar]
- Lucibello FC, Slater EP, Jooss KU, Beato M, Müller R. (1990) Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J 9:2827–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB. (1991) Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 352:497–505. [DOI] [PubMed] [Google Scholar]
- Lunan CB, Green B. (1974) 3H-oestradiol uptake in vivo by human uterine endometrium: effect of tamoxifen (I.C.I. 46,474). Clin Endocrinol (Oxf) 3:465–480. [DOI] [PubMed] [Google Scholar]
- Luo X, Yin P, Coon V JS, Cheng YH, Wiehle RD, Bulun SE. (2010) The selective progesterone receptor modulator CDB4124 inhibits proliferation and induces apoptosis in uterine leiomyoma cells. Fertil Steril 93:2668–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutsky BN, Berkenkopf J, Fernandez X, Monahan M, Shue HJ, Tiberi RL, Green MJ. (1979) Novel class of potent topical anti-inflammatory agents: 17-benzoylated, 7 alpha-halogeno substituted corticosteroids. Arzneimittelforschung 29:1662–1667. [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. (1995) Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278. [DOI] [PubMed] [Google Scholar]
- Ma Y, Khalifa B, Yee YK, Lu J, Memezawa A, Savkur RS, Yamamoto Y, Chintalacharuvu SR, Yamaoka K, Stayrook KR, et al. (2006) Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest 116:892–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YL, Bryant HU, Zeng Q, Palkowitz A, Jee WS, Turner CH, Sato M. (2002) Long-term dosing of arzoxifene lowers cholesterol, reduces bone turnover, and preserves bone quality in ovariectomized rats. J Bone Miner Res 17:2256–2264. [DOI] [PubMed] [Google Scholar]
- Madauss KP, Deng SJ, Austin RJ, Lambert MH, McLay I, Pritchard J, Short SA, Stewart EL, Uings IJ, Williams SP. (2004) Progesterone receptor ligand binding pocket flexibility: crystal structures of the norethindrone and mometasone furoate complexes. J Med Chem 47:3381–3387. [DOI] [PubMed] [Google Scholar]
- Madauss KP, Grygielko ET, Deng SJ, Sulpizio AC, Stanley TB, Wu C, Short SA, Thompson SK, Stewart EL, Laping NJ, et al. (2007) A structural and in vitro characterization of asoprisnil: a selective progesterone receptor modulator. Mol Endocrinol 21:1066–1081. [DOI] [PubMed] [Google Scholar]
- Mainwaring WI. (1971) Interaction of androgenic steroids with prostatic chromatin. Biochem J 124:42P–43P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwaring WI, Peterken BM. (1971) A reconstituted cell-free system for the specific transfer of steroid—receptor complexes into nuclear chromatin isolated from the rat ventral prostate gland. Biochem J 125:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major JS, Green B, Heald PJ. (1976) Interactions of oestradiol-17beta and tamoxifen in the uterus of the pregnant rat. J Endocrinol 71:315–324. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. (1999) Identification of a nuclear receptor for bile acids. Science 284:1362–1365. [DOI] [PubMed] [Google Scholar]
- Malnick SD, Shaer A, Soreq H, Kaye AM. (1983) Estrogen-induced creatine kinase in the reproductive system of the immature female rat. Endocrinology 113:1907–1909. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. (1992) Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev 6:329–344. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. (1995) The RXR heterodimers and orphan receptors. Cell 83:841–850. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. (1995) The nuclear receptor superfamily: the second decade. Cell 83:835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansel R, Goyal A, Nestour EL, Masini-Etévé V, O’Connell K, Afimoxifene (4-OHT) Breast Pain Research Group (2007) A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat 106:389–397. [DOI] [PubMed] [Google Scholar]
- Marrif H, Schifman A, Stepanyan Z, Gillis MA, Calderone A, Weiss RE, Samarut J, Silva JE. (2005) Temperature homeostasis in transgenic mice lacking thyroid hormone receptor-alpha gene products. Endocrinology 146:2872–2884. [DOI] [PubMed] [Google Scholar]
- Marttunen MB, Hietanen P, Tiitinen A, Ylikorkala O. (1998) Comparison of effects of tamoxifen and toremifene on bone biochemistry and bone mineral density in postmenopausal breast cancer patients. J Clin Endocrinol Metab 83:1158–1162. [DOI] [PubMed] [Google Scholar]
- Masud S, Ye S, SAS Group (2003) Effect of the peroxisome proliferator activated receptor-gamma gene Pro12Ala variant on body mass index: a meta-analysis. J Med Genet 40:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias PM, Donner P, Coelho R, Thomaz M, Peixoto C, Macedo S, Otto N, Joschko S, Scholz P, Wegg A, et al. (2000) Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J Biol Chem 275:26164–26171. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson J-Å. (2003) Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv 3:281–292. [DOI] [PubMed] [Google Scholar]
- Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. (2000) Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D3. Eur J Immunol 30:498–508. [DOI] [PubMed] [Google Scholar]
- Maung HM, Elangovan L, Frazão JM, Bower JD, Kelley BJ, Acchiardo SR, Rodriguez HJ, Norris KC, Sigala JF, Rutkowski M, et al. (2001) Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1α-hydroxyvitamin D2) in dialysis patients with secondary hyperparathyroidism: a sequential comparison. Am J Kidney Dis 37:532–543. [PubMed] [Google Scholar]
- Maurer HR, Chalkley GR. (1967) Some properties of a nuclear binding site of estradiol. J Mol Biol 27:431–441. [DOI] [PubMed] [Google Scholar]
- Maurer RA, Notides AC. (1987) Identification of an estrogen-responsive element from the 5′-flanking region of the rat prolactin gene. Mol Cell Biol 7:4247–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, et al. AMG 162 Bone Loss Study Group (2006a) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831. [DOI] [PubMed] [Google Scholar]
- McClung MR, Siris E, Cummings S, Bolognese M, Ettinger M, Moffett A, Emkey R, Day W, Somayaji V, Lee A. (2006b) Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause 13:377–386. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O’Malley BW. (1987) Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 235:1214–1217. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. (1999) Estrogen actions in the central nervous system. Endocr Rev 20:279–307. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465–474. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. (2010) SnapShot: NR coregulators. Cell 143:172–172.e1. [DOI] [PubMed] [Google Scholar]
- McMahon M, Gerich J, Rizza R. (1988) Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev 4:17–30. [DOI] [PubMed] [Google Scholar]
- McMeekin DS, Gordon A, Fowler J, Melemed A, Buller R, Burke T, Bloss J, Sabbatini P. (2003) A phase II trial of arzoxifene, a selective estrogen response modulator, in patients with recurrent or advanced endometrial cancer. Gynecol Oncol 90:64–69. [DOI] [PubMed] [Google Scholar]
- Means AR, Comstock JP, Rosenfeld GC, O’Malley BW. (1972) Ovalbumin messenger RNA of chick oviduct: partial characterization, estrogen dependence, and translation in vitro. Proc Natl Acad Sci USA 69:1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means AR, Hamilton TH. (1966) Early estrogen action: concomitant stimulations within two minutes of nuclear RNA synthesis and uptake of RNA precursor by the uterus. Proc Natl Acad Sci USA 56:1594–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR. (2009) DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 324:407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellanby E. (1919) An experimental investigation on rickets. Lancet 196:407–412. [Google Scholar]
- Mengus G, Gangloff YG, Carré L, Lavigne AC, Davidson I. (2000) The human transcription factor IID subunit human TATA-binding protein-associated factor 28 interacts in a ligand-reversible manner with the vitamin D3 and thyroid hormone receptors. J Biol Chem 275:10064–10071. [DOI] [PubMed] [Google Scholar]
- Mengus G, May M, Carré L, Chambon P, Davidson I. (1997) Human TAF-(II)135 potentiates transcriptional activation by the AF-2s of the retinoic acid, vitamin D3, and thyroid hormone receptors in mammalian cells. Genes Dev 11:1381–1395. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Zella LA, Nerenz RD, Pike JW. (2007) Characterizing early events associated with the activation of target genes by 1,25-dihydroxyvitamin D3 in mouse kidney and intestine in vivo. J Biol Chem 282:22344–22352. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P. (1989) Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433–442. [DOI] [PubMed] [Google Scholar]
- Miao B, Zondlo S, Gibbs S, Cromley D, Hosagrahara VP, Kirchgessner TG, Billheimer J, Mukherjee R. (2004) Raising HDL cholesterol without inducing hepatic steatosis and hypertriglyceridemia by a selective LXR modulator. J Lipid Res 45:1410–1417. [DOI] [PubMed] [Google Scholar]
- Michael LF, Schkeryantz JM, Burris TP. (2005) The pharmacology of LXR. Mini Rev Med Chem 5:729–740. [DOI] [PubMed] [Google Scholar]
- Miheller P, Muzes G, Hritz I, Lakatos G, Pregun I, Lakatos PL, Herszényi L, Tulassay Z. (2009) Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis 15:1656–1662. [DOI] [PubMed] [Google Scholar]
- Miller PD, Chines AA, Christiansen C, Hoeck HC, Kendler DL, Lewiecki EM, Woodson G, Levine AB, Constantine G, Delmas PD. (2008) Effects of bazedoxifene on BMD and bone turnover in postmenopausal women: 2-yr results of a randomized, double-blind, placebo-, and active-controlled study. J Bone Miner Res 23:525–535. [DOI] [PubMed] [Google Scholar]
- Miner JN. (2002) Designer glucocorticoids. Biochem Pharmacol 64:355–361. [DOI] [PubMed] [Google Scholar]
- Miner JN, Ardecky B, Benbatoul K, Griffiths K, Larson CJ, Mais DE, Marschke K, Rosen J, Vajda E, Zhi L, et al. (2007a) Antiinflammatory glucocorticoid receptor ligand with reduced side effects exhibits an altered protein-protein interaction profile. Proc Natl Acad Sci USA 104:19244–19249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, López FJ, Marschke KB, Rosen J, Schrader W, Turner R, et al. (2007b) An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology 148:363–373. [DOI] [PubMed] [Google Scholar]
- Minoura H, Takeshita S, Ita M, Hirosumi J, Mabuchi M, Kawamura I, Nakajima S, Nakayama O, Kayakiri H, Oku T, et al. (2004) Pharmacological characteristics of a novel nonthiazolidinedione insulin sensitizer, FK614. Eur J Pharmacol 494:273–281. [DOI] [PubMed] [Google Scholar]
- Minoura H, Takeshita S, Kimura C, Hirosumi J, Takakura S, Kawamura I, Seki J, Manda T, Mutoh S. (2007) Mechanism by which a novel non-thiazolidinedione peroxisome proliferator-activated receptor gamma agonist, FK614, ameliorates insulin resistance in Zucker fatty rats. Diabetes Obes Metab 9:369–378. [DOI] [PubMed] [Google Scholar]
- Minoura H, Takeshita S, Yamamoto T, Mabuchi M, Hirosumi J, Takakura S, Kawamura I, Seki J, Manda T, Ita M, et al. (2005) Ameliorating effect of FK614, a novel nonthiazolidinedione peroxisome proliferator-activated receptor gamma agonist, on insulin resistance in Zucker fatty rat. Eur J Pharmacol 519:182–190. [DOI] [PubMed] [Google Scholar]
- Misra NC, Nigam PK, Gupta R, Agarwal AK, Kamboj VP. (1989) Centchroman—a non-steroidal anti-cancer agent for advanced breast cancer: phase-II study. Int J Cancer 43:781–783. [DOI] [PubMed] [Google Scholar]
- Misrahi M, Atger M, d’Auriol L, Loosfelt H, Meriel C, Fridlansky F, Guiochon-Mantel A, Galibert F, Milgrom E. (1987) Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun 143:740–748. [DOI] [PubMed] [Google Scholar]
- Mitro N, Vargas L, Romeo R, Koder A, Saez E. (2007) T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett 581:1721–1726. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T, Tennyson GE, Nikodem VM. (1988) Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci USA 85:5804–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima N, Kadowaki Y, Fukushige S, Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K, Yamamoto T. (1988) Identification of two novel members of erbA superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res 16:11057–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizwicki MT, Norman AW. (2009) The vitamin D sterol-vitamin D receptor ensemble model offers unique insights into both genomic and rapid-response signaling. Sci Signal 2:re4. [DOI] [PubMed] [Google Scholar]
- Mohla S, DeSombre ER, Jensen EV. (1972) Tissue-specific stimulation of RNA synthesis by transformed estradiol-receptor complex. Biochem Biophys Res Commun 46:661–667. [DOI] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. (1987) Biological actions of androgens. Endocr Rev 8:1–28. [DOI] [PubMed] [Google Scholar]
- Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. (2008) Metabolic effects of thyroid hormone derivatives. Thyroid 18:239–253. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Kumahara Y. (1985) A patient with psoriasis cured by 1 alpha-hydroxyvitamin D3. Med J Osaka Univ 35:51–54. [PubMed] [Google Scholar]
- Morris D, Podolski J, Kirsch A, Wiehle R, Fleckenstein L. (2011) Population pharmacokinetics of telapristone (CDB-4124) and its active monodemethylated metabolite CDB-4453, with a mixture model for total clearance. AAPS J 13:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani A, Wang ZL, Weiszmann J, McGee LR, Lee G, Liu QX, Staunton J, Fang ZX, Fuentes H, Lindstrom M, et al. (2009) INT131: a selective modulator of PPAR gamma. J Mol Biol 386:1301–1311. [DOI] [PubMed] [Google Scholar]
- Mueller GC, Herranen AM, Jervell KF. (1958) Studies on the mechanism of action of estrogens. Recent Prog Horm Res 14:95–129. [PubMed] [Google Scholar]
- Mukherjee R, Jow L, Croston GE, Paterniti JR., Jr (1997) Identification, characterization, and tissue distribution of human peroxisome proliferator-activated receptor (PPAR) isoforms PPARγ2 versus PPARγ1 and activation with retinoid X receptor agonists and antagonists. J Biol Chem 272:8071–8076. [DOI] [PubMed] [Google Scholar]
- Muls E, Blaton V, Rosseneu M, Lesaffre E, Lamberigts G, De Moor P. (1982) Serum lipids and apolipoproteins A-I, A-II, and B in hyperthyroidism before and after treatment. J Clin Endocrinol Metab 55:459–464. [DOI] [PubMed] [Google Scholar]
- Münster PN, Buzdar A, Dhingra K, Enas N, Ni L, Major M, Melemed A, Seidman A, Booser D, Theriault R, et al. (2001) Phase I study of a third-generation selective estrogen receptor modulator, LY353381.HCL, in metastatic breast cancer. J Clin Oncol 19:2002–2009. [DOI] [PubMed] [Google Scholar]
- Murray GR. (1891) Note on the treatment of myxoedema by hypodermic injections of an extract of the thyroid gland of a sheep. BMJ 2:796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musliner TA, Chader GJ. (1972) Estradiol-receptors of the rat uterus: interaction of the cytoplasmic estrogen-receptor with DNA in vitro. Biochim Biophys Acta 262:256–263. [DOI] [PubMed] [Google Scholar]
- Na S, Ma Y, Zhao J, Schmidt C, Zeng QQ, Chandrasekhar S, Chin WW, Nagpal S. (2011) A nonsecosteroidal vitamin D receptor modulator ameliorates experimental autoimmune encephalomyelitis without causing hypercalcemia. Autoimmune Dis 2011:132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtigall MJ, Jessel RH, Flaumenhaft R, Nachtigall R, Yoles I, Naftolin F, Nachtigall LE. (2011) The selective estrogen receptor modulator DT56a (Femarelle) does not affect platelet reactivity in normal or thrombophilic postmenopausal women. Menopause 18:285–288. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Lu J, Boehm MF. (2001) Vitamin D analogs: mechanism of action and therapeutic applications. Curr Med Chem 8:1661–1679. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na SQ, Rathnachalam R. (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26:662–687. [DOI] [PubMed] [Google Scholar]
- Nashold FE, Miller DJ, Hayes CE. (2000) 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol 103:171–179. [DOI] [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, et al. (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441. [DOI] [PubMed] [Google Scholar]
- Negro-Vilar A. (1999) Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab 84:3459–3462. [DOI] [PubMed] [Google Scholar]
- Nemere I, Hintze K. (2008) Novel hormone “receptors”. J Cell Biochem 103:401–407. [DOI] [PubMed] [Google Scholar]
- Ness GC, Lopez D, Chambers CM, Newsome WP, Cornelius P, Long CA, Harwood HJ., Jr (1998) Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochem Pharmacol 56:121–129. [DOI] [PubMed] [Google Scholar]
- Nettles KW, Greene GL. (2005) Ligand control of coregulator recruitment to nuclear receptors. Annu Rev Physiol 67:309–333. [DOI] [PubMed] [Google Scholar]
- Newmark JR, Hardy DO, Tonb DC, Carter BS, Epstein JI, Isaacs WB, Brown TR, Barrack ER. (1992) Androgen receptor gene mutations in human prostate cancer. Proc Natl Acad Sci USA 89:6319–6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton R, Holden NS. (2007) Separating transrepression and transactivation: a distressing divorce for the glucocorticoid receptor? Mol Pharmacol 72:799–809. [DOI] [PubMed] [Google Scholar]
- Ng L, Forrest D, Haugen BR, Wood WM, Curran T. (1995) N-terminal variants of thyroid hormone receptor beta: differential function and potential contribution to syndrome of resistance to thyroid hormone. Mol Endocrinol 9:1202–1213. [DOI] [PubMed] [Google Scholar]
- Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, Blithe D, Wesley R, Armstrong A. (2011) Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: a randomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 95:767–772.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam M, Ranjan V, Srivastava S, Sharma R, Balapure AK. (2008) Centchroman induces G0/G1 arrest and caspase-dependent apoptosis involving mitochondrial membrane depolarization in MCF-7 and MDA MB-231 human breast cancer cells. Life Sci 82:577–590. [DOI] [PubMed] [Google Scholar]
- Nishishita T, Okazaki T, Ishikawa T, Igarashi T, Hata K, Ogata E, Fujita T. (1998) A negative vitamin D response DNA element in the human parathyroid hormone-related peptide gene binds to vitamin D receptor along with Ku antigen to mediate negative gene regulation by vitamin D. J Biol Chem 273:10901–10907. [DOI] [PubMed] [Google Scholar]
- Nolte RT, Wisely GB, Westin S, Cobb JE, Lambert MH, Kurokawa R, Rosenfeld MG, Willson TM, Glass CK, Milburn MV. (1998) Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 395:137–143. [DOI] [PubMed] [Google Scholar]
- Nomiyama T, Bruemmer D. (2008) Liver X receptors as therapeutic targets in metabolism and atherosclerosis. Curr Atheroscler Rep 10:88–95. [DOI] [PubMed] [Google Scholar]
- Norman AW. (2008) From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 88:491S–499S. [DOI] [PubMed] [Google Scholar]
- Norris JD, Paige LA, Christensen DJ, Chang CY, Huacani MR, Fan D, Hamilton PT, Fowlkes DM, McDonnell DP. (1999) Peptide antagonists of the human estrogen receptor. Science 285:744–746. [DOI] [PubMed] [Google Scholar]
- O’Connell EJ. (2003) Review of the unique properties of budesonide. Clin Ther 25 (Suppl C):C42–C60. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Moher D, Thomas K, Hanley DA, Cranney A. (2008) Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J Bone Miner Metab 26:531–542. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Aronow A, Peacock AC, Dingman CW. (1968a) Estrogen-dependent increase in transfer RNA during differentiation of the chick oviduct. Science 162:567–568. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, McGuire WL. (1968) Studies on the mechanism of estrogen-mediated tissue differentiation: regulation of nuclear transcription and induction of new RNA species. Proc Natl Acad Sci USA 60:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley BW, McGuire WL, Middleton PA. (1968b) Altered gene expression during differentiation: population changes in hybridizable RNA after stimulation of the chick oviduct with oestrogen. Nature 218:1249–1251. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Rosenfeld GC, Comstock JP, Means AR. (1972a) Induction of specific translatable messenger RNA’s by oestrogen and progesterone. Acta Endocrinol Suppl (Copenh) 168:381–395. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Rosenfeld GC, Comstock JP, Means AR. (1972b) Steroid hormone induction of a specific translatable messenger RNA. Nat New Biol 240:45–48. [DOI] [PubMed] [Google Scholar]
- O’Regan RM, Jordan VC. (2001) Tamoxifen to raloxifene and beyond. Semin Oncol 28:260–273. [DOI] [PubMed] [Google Scholar]
- Oberfield JL, Collins JL, Holmes CP, Goreham DM, Cooper JP, Cobb JE, Lenhard JM, Hull-Ryde EA, Mohr CP, Blanchard SG, et al. (1999) A peroxisome proliferator-activated receptor gamma ligand inhibits adipocyte differentiation. Proc Natl Acad Sci USA 96:6102–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oetting A, Yen PM. (2007) New insights into thyroid hormone action. Best Pract Res Clin Endocrinol Metab 21:193–208. [DOI] [PubMed] [Google Scholar]
- Olateju TO, Vanderpump MPJ. (2006) Thyroid hormone resistance. Ann Clin Biochem 43:431–440. [DOI] [PubMed] [Google Scholar]
- O’Malley BW, Conneely OM. (1992) Orphan receptors: in search of a unifying hypothesis for activation. Mol Endocrinol 6:1359–1361. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. (1995) Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357. [DOI] [PubMed] [Google Scholar]
- Ondeyka JG, Jayasuriya H, Herath KB, Guan ZQ, Schulman M, Collado J, Dombrowski AW, Kwon SS, McCallum C, Sharma N, et al. (2005) Steroidal and triterpenoidal fungal metabolites as ligands of liver X receptors. J Antibiot (Tokyo) 58:559–565. [DOI] [PubMed] [Google Scholar]
- Ono Y, Kawase A, Watanabe H, Shiraishi A, Takeda S, Higuchi Y, Sato K, Yamauchi T, Mikami T, Kato M, et al. (1998) Syntheses and preventive effects of analogues related to 1α,25-dihydroxy-2β-(3-hydroxypropoxy)vitamin D3 (ED-71) on bone mineral loss in ovariectomized rats. Bioorg Med Chem 6:2517–2523. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL, Surks MI. (1974) Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology 95:897–903. [DOI] [PubMed] [Google Scholar]
- Orimo H, Shiraki M, Hayashi T, Nakamura T. (1987) Reduced occurrence of vertebral crush fractures in senile osteoporosis treated with 1 alpha (OH)-vitamin D3. Bone Miner 3:47–52. [PubMed] [Google Scholar]
- Oropeza MV, Orozco S, Ponce H, Campos MG. (2005) Tofupill lacks peripheral estrogen-like actions in the rat reproductive tract. Reprod Toxicol 20:261–266. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Jarman M, McCague R, Coronado EB, Hilsenbeck SG, Wakeling AE. (1994) The importance of tamoxifen metabolism in tamoxifen-stimulated breast tumor growth. Cancer Chemother Pharmacol 34:89–95. [DOI] [PubMed] [Google Scholar]
- Osler W. (1897) Sporadic cretinism in America. Trans Congr Am Physicians Surg 4:169–206. [Google Scholar]
- Ostrowski J, Kuhns JE, Lupisella JA, Manfredi MC, Beehler BC, Krystek SR, Jr, Bi YZ, Sun CQ, Seethala R, Golla R, et al. (2007) Pharmacological and x-ray structural characterization of a novel selective androgen receptor modulator: potent hyperanabolic stimulation of skeletal muscle with hypostimulation of prostate in rats. Endocrinology 148:4–12. [DOI] [PubMed] [Google Scholar]
- Ott SM, Chesnut CH., 3rd (1989) Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med 110:267–274. [DOI] [PubMed] [Google Scholar]
- Overk CR, Peng KW, Asghodom RT, Kastrati I, Lantvit DD, Qin Z, Frasor J, Bolton JL, Thatcher GR. (2007) Structure-activity relationships for a family of benzothiophene selective estrogen receptor modulators including raloxifene and arzoxifene. ChemMedChem 2:1520–1526. [DOI] [PubMed] [Google Scholar]
- Palazzolo I, Gliozzi A, Rusmini P, Sau D, Crippa V, Simonini F, Onesto E, Bolzoni E, Poletti A. (2008) The role of the polyglutamine tract in androgen receptor. J Steroid Biochem Mol Biol 108:245–253. [DOI] [PubMed] [Google Scholar]
- Paliwal JK, Gupta RC. (1996) Tissue distribution and pharmacokinetics of centchroman. A new nonsteroidal postcoital contraceptive agent and its 7-desmethyl metabolite in female rats after a single oral dose. Drug Metab Dispos 24:148–155. [PubMed] [Google Scholar]
- Paliwal JK, Gupta RC, Grover PK, Asthana OP, Srivastava JS, NityaNand S. (1989) High performance liquid chromatographic (HPLC) determination of centchroman in human serum and application to single-dose pharmacokinetics. Pharm Res 6:1048–1051. [DOI] [PubMed] [Google Scholar]
- Palkowitz AD, Glasebrook AL, Thrasher KJ, Hauser KL, Short LL, Phillips DL, Muehl BS, Sato M, Shetler PK, Cullinan GJ, et al. (1997) Discovery and synthesis of [6-hydroxy-3-[4-[2-(1-piperidinyl)ethoxy]phenoxy]-2-(4-hydroxyphenyl)]b enzo[b]thiophene: a novel, highly potent, selective estrogen receptor modulator. J Med Chem 40:1407–1416. [DOI] [PubMed] [Google Scholar]
- Papadimitropoulos E, Wells G, Shea B, Gillespie W, Weaver B, Zytaruk N, Cranney A, Adachi J, Tugwell P, Josse R, et al. Osteoporosis Methodology Group and The Osteoporosis Research Advisory Group (2002) Meta-analyses of therapies for postmenopausal osteoporosis. VIII: Meta-analysis of the efficacy of vitamin D treatment in preventing osteoporosis in postmenopausal women. Endocr Rev 23:560–569. [DOI] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284:1365–1368. [DOI] [PubMed] [Google Scholar]
- Parks WP, Scolnick EM, Kozikowski EH. (1974) Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science 184:158–160. [DOI] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaro MD, Piquion J, Mullen N, Sutherland D, Zhai S, Figg WD, Blye R, Nieman LK. (2003) Luteal phase dose-response relationships of the antiprogestin CDB-2914 in normally cycling women. Hum Reprod 18:1820–1827. [DOI] [PubMed] [Google Scholar]
- Patel J, Landers K, Li H, Mortimer RH, Richard K. (2011) Thyroid hormones and fetal neurological development. J Endocrinol 209:1–8. [DOI] [PubMed] [Google Scholar]
- Patel PD, Sherman TG, Goldman DJ, Watson SJ. (1989) Molecular cloning of a mineralocorticoid (type I) receptor complementary DNA from rat hippocampus. Mol Endocrinol 3:1877–1885. [DOI] [PubMed] [Google Scholar]
- Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. (2009) Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 150:1897–1903. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Janowski BA, Mangelsdorf DJ. (1998a) The LXRs: a new class of oxysterol receptors. Curr Opin Genet Dev 8:571–575. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. (1998b) Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell 93:693–704. [DOI] [PubMed] [Google Scholar]
- Peleg S, Ismail A, Uskokovic MR, Avnur Z. (2003) Evidence for tissue- and cell-type selective activation of the vitamin D receptor by Ro-26-9228, a noncalcemic analog of vitamin D3. J Cell Biochem 88:267–273. [DOI] [PubMed] [Google Scholar]
- Peleg S, Uskokovic M, Ahene A, Vickery B, Avnur Z. (2002) Cellular and molecular events associated with the bone-protecting activity of the noncalcemic vitamin D analog Ro-26-9228 in osteopenic rats. Endocrinology 143:1625–1636. [DOI] [PubMed] [Google Scholar]
- Pennock GD, Raya TE, Bahl JJ, Goldman S, Morkin E. (1992) Cardiac effects of 3,5-diiodothyropropionic acid, a thyroid hormone analog with inotropic selectivity. J Pharmacol Exp Ther 263:163–169. [PubMed] [Google Scholar]
- Perra A, Simbula G, Simbula M, Pibiri M, Kowalik MA, Sulas P, Cocco MT, Ledda-Columbano GM, Columbano A. (2008) Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J 22:2981–2989. [DOI] [PubMed] [Google Scholar]
- Petit-Topin I, Turque N, Fagart J, Fay M, Ulmann A, Gainer E, Rafestin-Oblin ME. (2009) Met909 plays a key role in the activation of the progesterone receptor and also in the high potency of 13-ethyl progestins. Mol Pharmacol 75:1317–1324. [DOI] [PubMed] [Google Scholar]
- Petkovich M, Brand NJ, Krust A, Chambon P. (1987) A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 330:444–450. [DOI] [PubMed] [Google Scholar]
- Picard F, Géhin M, Annicotte JS, Rocchi S, Champy MF, O’Malley BW, Chambon P, Auwerx J. (2002) SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111:931–941. [DOI] [PubMed] [Google Scholar]
- Pickar JH, Yeh IT, Bachmann G, Speroff L. (2009) Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 92:1018–1024. [DOI] [PubMed] [Google Scholar]
- Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engström O, Ljunggren J, Gustafsson JA, Carlquist M. (1999) Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J 18:4608–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike AC, Brzozowski AM, Walton J, Hubbard RE, Thorsell AG, Li YL, Gustafsson JA, Carlquist M. (2001) Structural insights into the mode of action of a pure antiestrogen. Structure 9:145–153. [DOI] [PubMed] [Google Scholar]
- Pilkis SJ, Granner DK. (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54:885–909. [DOI] [PubMed] [Google Scholar]
- Pinette KV, Yee YK, Amegadzie BY, Nagpal S. (2003) Vitamin D receptor as a drug discovery target. Mini Rev Med Chem 3:193–204. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Archer DF, Utian WH, Menegoci JC, Levine AB, Chines AA, Constantine GD. (2009a) Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 16:1102–1108. [DOI] [PubMed] [Google Scholar]
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. (2009b) Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 16:1116–1124. [DOI] [PubMed] [Google Scholar]
- Piu F, Gardell LR, Son T, Schlienger N, Lund BW, Schiffer HH, Vanover KE, Davis RE, Olsson R, Bradley SR. (2008) Pharmacological characterization of AC-262536, a novel selective androgen receptor modulator. J Steroid Biochem Mol Biol 109:129–137. [DOI] [PubMed] [Google Scholar]
- Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. (2001) Functional interference between thyroid hormone receptor alpha (TRα) and natural truncated TRΔα isoforms in the control of intestine development. Mol Cell Biol 21:4761–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino N, Merlini S, Cubeddu A, Giannini A, Bucci F, Casarosa E, Cela V, Angioni S, Luisi M, Genazzani AR. (2009) Brain-region responsiveness to DT56a (Femarelle) administration on allopregnanolone and opioid content in ovariectomized rats. Menopause 16:1037–1043. [DOI] [PubMed] [Google Scholar]
- Pochetti G, Mitro N, Lavecchia A, Gilardi F, Besker N, Scotti E, Aschi M, Re N, Fracchiolla G, Laghezza A, et al. (2010) Structural insight into peroxisome proliferator-activated receptor gamma binding of two ureidofibrate-like enantiomers by molecular dynamics, cofactor interaction analysis, and site-directed mutagenesis. J Med Chem 53:4354–4366. [DOI] [PubMed] [Google Scholar]
- Prescott J, Coetzee GA. (2006) Molecular chaperones throughout the life cycle of the androgen receptor. Cancer Lett 231:12–19. [DOI] [PubMed] [Google Scholar]
- Prüfer K, Racz A, Lin GC, Barsony J. (2000) Dimerization with retinoid X receptors promotes nuclear localization and subnuclear targeting of vitamin D receptors. J Biol Chem 275:41114–41123. [DOI] [PubMed] [Google Scholar]
- Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, Clerin V, Resmini C, Keith JC, Berrodin TJ, Feingold I, et al. (2009) LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res 50:2358–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers HC, Versteegh JE, Uitdehaag JC. (2009) The X-ray structure of RU486 bound to the progesterone receptor in a destabilized agonistic conformation. J Biol Chem 284:19572–19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C, Gamble M, Chang CPB, Atkins GB, Lazar MA, Freedman LP. (2000) The DRIP complex and SRC-1/p160 coactivators share similar nuclear receptor binding determinants but constitute functionally distinct complexes. Mol Cell Biol 20:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C, Suldan Z, Ward J, Chang CPB, Burakov D, Erdjument-Bromage H, Tempst P, Freedman LP. (1998) A novel protein complex that interacts with the vitamin D3 receptor in a ligand-dependent manner and enhances VDR transactivation in a cell-free system. Genes Dev 12:1787–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. (2007) Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. (2002) Dissecting human adrenal androgen production. Trends Endocrinol Metab 13:234–239. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA. (2002) The dawn of the SPPARMs? Sci STKE 2002:pe9. [DOI] [PubMed] [Google Scholar]
- Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, et al. (2010) Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab 11:517–531. [DOI] [PubMed] [Google Scholar]
- Rawson RW, Money WL, Kroc RL, Kumaoka S, Benua RS, Leeper RD. (1959) A dissociation of thyroid hormonal effects by structural alterations of the thyroxine molecule. Am J Med Sci 238:261–273. [DOI] [PubMed] [Google Scholar]
- Refetoff S. (1994) Resistance to thyroid hormone: an historical overview. Thyroid 4:345–349. [DOI] [PubMed] [Google Scholar]
- Refetoff S, DeWind LT, DeGroot LJ. (1967) Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab 27:279–294. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, et al. (1998a) DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93:531–541. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Wessely O, Gass P, Schmid W, Schütz G. (1998b) Analysis of glucocorticoid signalling by gene targeting. J Steroid Biochem Mol Biol 65:111–115. [DOI] [PubMed] [Google Scholar]
- Renaud JP, Moras D. (2000) Structural studies on nuclear receptors. Cell Mol Life Sci 57:1748–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendi MH, Suh N, Lamph WW, Krajewski S, Reed JC, Heyman RA, Berchuck A, Liby K, Risingsong R, Royce DB, et al. (2004) The selective estrogen receptor modulator arzoxifene and the rexinoid LG100268 cooperate to promote transforming growth factor beta-dependent apoptosis in breast cancer. Cancer Res 64:3566–3571. [DOI] [PubMed] [Google Scholar]
- Ribeiro RCJ, Apriletti JW, Wagner RL, Feng WJ, Kushner PJ, Nilsson S, Scanlan TS, West BL, Fletterick RJ, Baxter JD. (1998) X-ray crystallographic and functional studies of thyroid hormone receptor. J Steroid Biochem Mol Biol 65:133–141. [DOI] [PubMed] [Google Scholar]
- Richardson AR, Maltz FN. (2012) Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception. Clin Ther 34:24–36. [DOI] [PubMed] [Google Scholar]
- Ricote M, Glass CK. (2007) PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 1771:926–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Comery TA, Kouranova E, Lo CF, Warwick HK, Ring RH, Kirksey Y, Aschmies S, Xu J, et al. (2007) The LXR agonist TO901317 selectively lowers hippocampal Aβ42 and improves memory in the Tg2576 mouse model of Alzheimer’s disease. Mol Cell Neurosci 34:621–628. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. (2003) Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med 348:618–629. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ., 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302. [DOI] [PubMed] [Google Scholar]
- Ringold GM, Yamamoto KR, Bishop JM, Varmus HE. (1977) Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci USA 74:2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold GM, Yamamoto KR, Tomkins GM, Bishop M, Varmus HE. (1975) Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell 6:299–305. [DOI] [PubMed] [Google Scholar]
- Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. (1998) Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med 339:953–959. [DOI] [PubMed] [Google Scholar]
- Robbins J. (1981) Factors altering thyroid hormone metabolism. Environ Health Perspect 38:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JF. (2001) ICI 182,780 (Fulvestrant)—the first oestrogen receptor down-regulator—current clinical data. Br J Cancer 85 (Suppl 2):11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JF, Harrison M. (2004) Fulvestrant: pharmacokinetics and pharmacology. Br J Cancer 90 (Suppl 1):S7–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JF, Odling-Smee W, Holcombe C, Kohlhardt SR, Harrison MP. (2003) Pharmacokinetics of a single dose of fulvestrant prolonged-release intramuscular injection in postmenopausal women awaiting surgery for primary breast cancer. Clin Ther 25:1440–1452. [DOI] [PubMed] [Google Scholar]
- Rocchi S, Picard F, Vamecq J, Gelman L, Potier N, Zeyer D, Dubuquoy L, Bac P, Champy MF, Plunket KD, et al. (2001) A unique PPARγ ligand with potent insulin-sensitizing yet weak adipogenic activity. Mol Cell 8:737–747. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Borgna JL. (1981) Differences between oestrogen receptor activation by oestrogen and antioestrogen. Nature 292:257–259. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Garcia M, Borgna JL. (1979) Absence of correlation between antiestrogenic activity and binding affinity for the estrogen receptor. Biochem Biophys Res Commun 88:351–357. [DOI] [PubMed] [Google Scholar]
- Rochel N, Tocchini-Valentini G, Egea PF, Juntunen K, Garnier JM, Vihko P, Moras D. (2001) Functional and structural characterization of the insertion region in the ligand binding domain of the vitamin D nuclear receptor. Eur J Biochem 268:971–979. [DOI] [PubMed] [Google Scholar]
- Rochel N, Wurtz JM, Mitschler A, Klaholz B, Moras D. (2000) The crystal structure of the nuclear receptor for vitamin D bound to its natural ligand. Mol Cell 5:173–179. [DOI] [PubMed] [Google Scholar]
- Roemer SC, Donham DC, Sherman L, Pon VH, Edwards DP, Churchill ME. (2006) Structure of the progesterone receptor-deoxyribonucleic acid complex: novel interactions required for binding to half-site response elements. Mol Endocrinol 20:3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkin S, Northington R, Baracat E, Nunes MG, Archer DF, Constantine G, Pickar JH. (2005) Endometrial effects of bazedoxifene acetate, a novel selective estrogen receptor modulator, in postmenopausal women. Obstet Gynecol 105:1397–1404. [DOI] [PubMed] [Google Scholar]
- Rosati RL, Da Silva Jardine P, Cameron KO, Thompson DD, Ke HZ, Toler SM, Brown TA, Pan LC, Ebbinghaus CF, Reinhold AR, et al. (1998) Discovery and preclinical pharmacology of a novel, potent, nonsteroidal estrogen receptor agonist/antagonist, CP-336156, a diaryltetrahydronaphthalene. J Med Chem 41:2928–2931. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617. [DOI] [PubMed] [Google Scholar]
- Rosenfeld GC, Comstock JP, Means AR, O’Malley BW. (1972) Estrogen-induced synthesis of ovalbumin messenger RNA and its translation in a cell-free system. Biochem Biophys Res Commun 46:1695–1703. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. (2011) A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med 17:623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarto T, Blomqvist C, Ehnholm C, Taskinen MR, Elomaa I. (1996) Antiatherogenic effects of adjuvant antiestrogens: a randomized trial comparing the effects of tamoxifen and toremifene on plasma lipid levels in postmenopausal women with node-positive breast cancer. J Clin Oncol 14:429–433. [DOI] [PubMed] [Google Scholar]
- Sagrinati C, Sottili M, Mazzinghi B, Borgogni E, Adorini L, Serio M, Romagnani P, Crescioli C. (2010) Comparison between VDR analogs and current immunosuppressive drugs in relation to CXCL10 secretion by human renal tubular cells. Transpl Int 23:914–923. [DOI] [PubMed] [Google Scholar]
- Sairanen S, Kärkkäinen M, Tähtelä R, Laitinen K, Mäkelä PP, Lamberg-Allardt C, Välimäki MJ. (2000) Bone mass and markers of bone and calcium metabolism in postmenopausal women treated with 1,25-dihydroxyvitamin D (Calcitriol) for four years. Calcif Tissue Int 67:122–127. [DOI] [PubMed] [Google Scholar]
- Salman M, Ray S, Agarwal AK, Durani S, Setty BS, Kamboj VP, Anand N. (1983) Antifertility agents. 38. Effect of the side chain and its position on the activity of 3,4-diarylchromans. J Med Chem 26:592–595. [DOI] [PubMed] [Google Scholar]
- Samuels HH, Tsai JS. (1973) Thyroid hormone action in cell culture: domonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci USA 70:3488–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B. (1986) The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, Aaltonen LA, de la Chapelle A, Spiegelman BM, Eng C. (1999) Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell 3:799–804. [DOI] [PubMed] [Google Scholar]
- Sato M, Kim J, Short LL, Slemenda CW, Bryant HU. (1995) Longitudinal and cross-sectional analysis of raloxifene effects on tibiae from ovariectomized aged rats. J Pharmacol Exp Ther 272:1252–1259. [PubMed] [Google Scholar]
- Sato M, Lu JL, Iturria S, Stayrook KR, Burris LL, Zeng QQ, Schmidt A, Barr RJ, Montrose-Rafizadeh C, Bryant HU, et al. (2010) A nonsecosteroidal vitamin D receptor ligand with improved therapeutic window of bone efficacy over hypercalcemia. J Bone Miner Res 25:1326–1336. [DOI] [PubMed] [Google Scholar]
- Sato M, McClintock C, Kim J, Turner CH, Bryant HU, Magee D, Slemenda CW. (1994) Dual-energy x-ray absorptiometry of raloxifene effects on the lumbar and femora of ovariectomized rats. J Bone Miner Res 9:715–724. [DOI] [PubMed] [Google Scholar]
- Sato M, Turner CH, Wang T, Adrian MD, Rowley E, Bryant HU. (1998a) LY353381.HCl: a novel raloxifene analog with improved SERM potency and efficacy in vivo. J Pharmacol Exp Ther 287:1–7. [PubMed] [Google Scholar]
- Sato M, Zeng GQ, Rowley E, Turner CH. (1998b) LY353381 x HCl: an improved benzothiophene analog with bone efficacy complementary to parathyroid hormone-(1-34). Endocrinology 139:4642–4651. [DOI] [PubMed] [Google Scholar]
- Savkur RS, Burris TP. (2004) The coactivator LXXLL nuclear receptor recognition motif. J Pept Res 63:207–212. [DOI] [PubMed] [Google Scholar]
- Savkur RS, Miller AR. (2006) Investigational PPAR-gamma agonists for the treatment of type 2 diabetes. Expert Opin Investig Drugs 15:763–778. [DOI] [PubMed] [Google Scholar]
- Scarpin KM, Graham JD, Mote PA, Clarke CL. (2009) Progesterone action in human tissues: regulation by progesterone receptor (PR) isoform expression, nuclear positioning and coregulator expression. Nucl Recept Signal 7:e009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäcke H, Berger M, Rehwinkel H, Asadullah K. (2007) Selective glucocorticoid receptor agonists (SEGRAs): novel ligands with an improved therapeutic index. Mol Cell Endocrinol 275:109–117. [DOI] [PubMed] [Google Scholar]
- Schäcke H, Hennekes H, Schottelius A, Joroch S, Lehmann M, Schmees N, Rehwinkel H, Asadullah K. (2002) SEGRAs: a novel class of anti-inflammatory compounds. Ernst Schering Res Found Workshop 40:357–371. [DOI] [PubMed] [Google Scholar]
- Schäcke H, Schottelius A, Döcke WD, Strehlke P, Jaroch S, Schmees N, Rehwinkel H, Hennekes H, Asadullah K. (2004) Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA 101:227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäcke H, Zollner TM, Döcke WD, Rehwinkel H, Jaroch S, Skuballa W, Neuhaus R, May E, Zügel U, Asadullah K. (2009) Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol 158:1088–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JM, Lee ES, Dardes RC, Bentrem D, O’Regan RM, De Los Reyes A, Jordan VC. (2001) Analysis of cross-resistance of the selective estrogen receptor modulators arzoxifene (LY353381) and LY117018 in tamoxifen-stimulated breast cancer xenografts. Clin Cancer Res 7:2505–2512. [PubMed] [Google Scholar]
- Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. (2005) Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci USA 102:2340–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroepfer GJ., Jr (2000) Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev 80:361–554. [DOI] [PubMed] [Google Scholar]
- Schüle R, Rangarajan P, Kliewer S, Ransone LJ, Bolado J, Yang N, Verma IM, Evans RM. (1990) Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell 62:1217–1226. [DOI] [PubMed] [Google Scholar]
- Schulman IG, Heyman RA. (2004) The flip side: identifying small molecule regulators of nuclear receptors. Chem Biol 11:639–646. [DOI] [PubMed] [Google Scholar]
- Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li LP, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. (2000) Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schussler GC. (2000) The thyroxine-binding proteins. Thyroid 10:141–149. [DOI] [PubMed] [Google Scholar]
- Schwinn MK, DeLuca HF. (2007) Differential recruitment of coactivators to the vitamin D receptor transcriptional complex by 1α,25-dihydroxyvitamin D3 analogs. Arch Biochem Biophys 465:443–451. [DOI] [PubMed] [Google Scholar]
- Scolnick EM, Young HA, Parks WP. (1976) Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology 69:148–156. [DOI] [PubMed] [Google Scholar]
- Scottolini AG, Bhagavan NV, Oshiro TH, Abe SY. (1980) Serum high-density lipoprotein cholesterol concentrations in hypo- and hyperthyroidism. Clin Chem 26:584–587. [PubMed] [Google Scholar]
- Seol W, Choi HS, Moore DD. (1995) Isolation of proteins that interact specifically with the retinoid X receptor: two novel orphan receptors. Mol Endocrinol 9:72–85. [DOI] [PubMed] [Google Scholar]
- Seol W, Choi HS, Moore DD. (1996) An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science 272:1336–1339. [DOI] [PubMed] [Google Scholar]
- Shaffer PL, Gewirth DT. (2002) Structural basis of VDR-DNA interactions on direct repeat response elements. EMBO J 21:2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee A, Bucolo C, Budzynski E, Ward KW, López FJ. (2011) In vivo ocular efficacy profile of mapracorat, a novel selective glucocorticoid receptor agonist, in rabbit models of ocular disease. Invest Ophthalmol Vis Sci 52:1422–1430. [DOI] [PubMed] [Google Scholar]
- Shagam JY. (2001) Thyroid disease: an overview. Radiol Technol 73:25–40, quiz 42–44, 66. [PubMed] [Google Scholar]
- Shang Y, Brown M. (2002) Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468. [DOI] [PubMed] [Google Scholar]
- Sharma MSM, Aronow WS, Patel L, Gandhi K, Desai H. (2011) Hyperthyroidism. Med Sci Monit 17:RA85–RA91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SD, Spencer TA, Mercer-Haines NA, Alipour M, Gargano MD, Runge-Morris M, Kocarek TA. (2004) CYP3A induction by liver X receptor ligands in primary cultured rat and mouse hepatocytes is mediated by the pregnane X receptor. Drug Metab Dispos 32:66–71. [DOI] [PubMed] [Google Scholar]
- Sherman SI, Ladenson PW. (1992) Organ-specific effects of tiratricol: a thyroid hormone analog with hepatic, not pituitary, superagonist effects. J Clin Endocrinol Metab 75:901–905. [DOI] [PubMed] [Google Scholar]
- Shevde NK, Plum LA, Clagett-Dame M, Yamamoto H, Pike JW, DeLuca HF. (2002) A potent analog of 1alpha,25-dihydroxyvitamin D3 selectively induces bone formation. Proc Natl Acad Sci USA 99:13487–13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. (1998) The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95:927–937. [DOI] [PubMed] [Google Scholar]
- Shinar DM, Endo N, Rutledge SJ, Vogel R, Rodan GA, Schmidt A. (1994) NER, a new member of the gene family encoding the human steroid hormone nuclear receptor. Gene 147:273–276. [DOI] [PubMed] [Google Scholar]
- Shiraishi A, Higashi S, Ohkawa H, Kubodera N, Hirasawa T, Ezawa I, Ikeda K, Ogata E. (1999) The advantage of alfacalcidol over vitamin D in the treatment of osteoporosis. Calcif Tissue Int 65:311–316. [DOI] [PubMed] [Google Scholar]
- Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. (2008) Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19:1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. (2009) PPARγ2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem 106:232–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G, Gorski J. (1969) Estrogen receptors in the rat uterus. Studies on the interaction of cytosol and nuclear binding sites. J Biol Chem 244:1097–1103. [PubMed] [Google Scholar]
- Sicinski RR, Prahl JM, Smith CM, DeLuca HF. (1998) New 1γ,25-dihydroxy-19-norvitamin D3 compounds of high biological activity: synthesis and biological evaluation of 2-hydroxymethyl, 2-methyl, and 2-methylene analogues. J Med Chem 41:4662–4674. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Chines AA, Kendler DL, Kung AW, Teglbjaerg CS, Felsenberg D, Mairon N, Constantine GD, Adachi JD. (2012) Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis: results of a 5-year, randomized, placebo-controlled study. Osteoporos Int 23:351–363. [DOI] [PubMed] [Google Scholar]
- Silverman SL, Christiansen C, Genant HK, Vukicevic S, Zanchetta JR, de Villiers TJ, Constantine GD, Chines AA. (2008) Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis: results from a 3-year, randomized, placebo-, and active-controlled clinical trial. J Bone Miner Res 23:1923–1934. [DOI] [PubMed] [Google Scholar]
- Sjöberg M, Vennström B. (1995) Ligand-dependent and -independent transactivation by thyroid hormone receptor beta 2 is determined by the structure of the hormone response element. Mol Cell Biol 15:4718–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg M, Vennström B, Forrest D. (1992) Thyroid hormone receptors in chick retinal development: differential expression of mRNAs for alpha and N-terminal variant beta receptors. Development 114:39–47. [DOI] [PubMed] [Google Scholar]
- Skafar DF, Zhao C. (2008) The multifunctional estrogen receptor-alpha F domain. Endocrine 33:1–8. [DOI] [PubMed] [Google Scholar]
- Smith CL, Nawaz Z, O’Malley BW. (1997) Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol Endocrinol 11:657–666. [DOI] [PubMed] [Google Scholar]
- Smith CL, O’Malley BW. (2004) Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev 25:45–71. [DOI] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang YJ, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, et al. (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472:491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen D, Katzburg S, Knoll E, Hendel D, Stern N, Kaye AM, Yoles I. (2007) DT56a (Femarelle): a natural selective estrogen receptor modulator (SERM). J Steroid Biochem Mol Biol 104:252–258. [DOI] [PubMed] [Google Scholar]
- Somjen D, Katzburg S, Lieberherr M, Hendel D, Yoles I. (2006) DT56a stimulates gender-specific human cultured bone cells in vitro. J Steroid Biochem Mol Biol 98:90–96. [DOI] [PubMed] [Google Scholar]
- Somjen D, Katzburg S, Livne E, Yoles I. (2005) DT56a (Femarelle) stimulates bone formation in female rats. BJOG 112:981–985. [DOI] [PubMed] [Google Scholar]
- Somjen D, Yoles I. (2003) DT56a (Tofupill/Femarelle) selectively stimulates creatine kinase specific activity in skeletal tissues of rats but not in the uterus. J Steroid Biochem Mol Biol 86:93–98. [DOI] [PubMed] [Google Scholar]
- Sone T, Kerner S, Pike JW. (1991) Vitamin D receptor interaction with specific DNA. Association as a 1,25-dihydroxyvitamin D3-modulated heterodimer. J Biol Chem 266:23296–23305. [PubMed] [Google Scholar]
- Song C, Hiipakka RA, Liao SS. (2000) Selective activation of liver X receptor alpha by 6alpha-hydroxy bile acids and analogs. Steroids 65:423–427. [DOI] [PubMed] [Google Scholar]
- Song C, Kokontis JM, Hiipakka RA, Liao SS. (1994) Ubiquitous receptor: a receptor that modulates gene activation by retinoic acid and thyroid hormone receptors. Proc Natl Acad Sci USA 91:10809–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottili M, Cosmi L, Borgogni E, Sarchielli E, Maggi L, Francalanci M, Vannelli GB, Ronconi E, Adorini L, Annunziato F, et al. (2009) Immunomodulatory effects of BXL-01-0029, a less hypercalcemic vitamin D analogue, in human cardiomyocytes and T cells. Exp Cell Res 315:264–273. [DOI] [PubMed] [Google Scholar]
- Spelsberg TC, Steggles AW, O’Malley BW. (1971) Progesterone-binding components of chick oviduct. 3. Chromatin acceptor sites. J Biol Chem 246:4188–4197. [PubMed] [Google Scholar]
- Spitz IM. (2009) Clinical utility of progesterone receptor modulators and their effect on the endometrium. Curr Opin Obstet Gynecol 21:318–324. [DOI] [PubMed] [Google Scholar]
- Spitz IM, Bardin CW, Benton L, Robbins A. (1998) Early pregnancy termination with mifepristone and misoprostol in the United States. N Engl J Med 338:1241–1247. [DOI] [PubMed] [Google Scholar]
- Sprague SM, Coyne D. (2010) Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol 5:512–518. [DOI] [PubMed] [Google Scholar]
- Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. (2003) Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 63:1483–1490. [DOI] [PubMed] [Google Scholar]
- Steffensen KR, Gustafsson JA. (2004) Putative metabolic effects of the liver X receptor (LXR). Diabetes 53 (Suppl 1):S36–S42. [DOI] [PubMed] [Google Scholar]
- Steggles AW, Spelsberg TC, Glasser SR, O’Malley BW. (1971) Soluble complexes between steroid hormones and target-tissue receptors bind specifically to target-tissue chromatin. Proc Natl Acad Sci USA 68:1479–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlin C, Wurtz JM, Steinmetz A, Greiner E, Schüle R, Moras D, Renaud JP. (2001) X-ray structure of the orphan nuclear receptor RORbeta ligand-binding domain in the active conformation. EMBO J 20:5822–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AZ, Terplan M, Paulson RJ. (2005) Comparison of tamoxifen and clomiphene citrate for ovulation induction: a meta-analysis. Hum Reprod 20:1511–1515. [DOI] [PubMed] [Google Scholar]
- Stephan ZF, Yurachek EC, Sharif R, Wasvary JM, Steele RE, Howes C. (1992) Reduction of cardiovascular and thyroxine-suppressing activities of L-T3 by liver targeting with cholic acid. Biochem Pharmacol 43:1969–1974. [DOI] [PubMed] [Google Scholar]
- Stio M, Martinesi M, Bruni S, Treves C, Mathieu C, Verstuyf A, d’Albasio G, Bagnoli S, Bonanomi AG. (2007) The vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn’s disease. J Steroid Biochem Mol Biol 103:51–60. [DOI] [PubMed] [Google Scholar]
- Stöcklin E, Wissler M, Gouilleux F, Groner B. (1996) Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728. [DOI] [PubMed] [Google Scholar]
- Stratton P, Levens ED, Hartog B, Piquion J, Wei Q, Merino M, Nieman LK. (2010) Endometrial effects of a single early luteal dose of the selective progesterone receptor modulator CDB-2914. Fertil Steril 93:2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DS, Glass CK. (2007) Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol 28:551–558. [DOI] [PubMed] [Google Scholar]
- Strisower B, Gofman JW, Galioni E, Rubinger JH, O’Brien GW, Simon A. (1955) Effect of long-term administration of desiccated thyroid on serum lipoprotein and cholesterol levels. J Clin Endocrinol Metab 15:73–80. [DOI] [PubMed] [Google Scholar]
- Strisower B, Gofman JW, Galioni EF, Almada AA, Simon A. (1954) Effect of thyroid extract on serum lipoproteins and serum cholesterol. Metabolism 3:218–227. [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357. [DOI] [PubMed] [Google Scholar]
- Suh N, Glasebrook AL, Palkowitz AD, Bryant HU, Burris LL, Starling JJ, Pearce HL, Williams C, Peer C, Wang Y, et al. (2001) Arzoxifene, a new selective estrogen receptor modulator for chemoprevention of experimental breast cancer. Cancer Res 61:8412–8415. [PubMed] [Google Scholar]
- Suh N, Lamph WW, Glasebrook AL, Grese TA, Palkowitz AD, Williams CR, Risingsong R, Farris MR, Heyman RA, Sporn MB. (2002) Prevention and treatment of experimental breast cancer with the combination of a new selective estrogen receptor modulator, arzoxifene, and a new rexinoid, LG 100268. Clin Cancer Res 8:3270–3275. [PubMed] [Google Scholar]
- Sutton ALM, MacDonald PN. (2003) Vitamin D: more than a “bone-a-fide” hormone. Mol Endocrinol 17:777–791. [DOI] [PubMed] [Google Scholar]
- Svensson S, Ostberg T, Jacobsson M, Norström C, Stefansson K, Hallén D, Johansson IC, Zachrisson K, Ogg D, Jendeberg L. (2003) Crystal structure of the heterodimeric complex of LXRα and RXRβ ligand-binding domains in a fully agonistic conformation. EMBO J 22:4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. (1997) TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem 272:27629–27634. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Murata Y, Sadow P, Hayashi Y, Seo H, Xu JM, O’Malley BW, Weiss RE, Refetoff S. (2002) Steroid receptor coactivator-1 deficiency causes variable alterations in the modulation of T3-regulated transcription of genes in vivo. Endocrinology 143:1346–1352. [DOI] [PubMed] [Google Scholar]
- Tamura K, Chen YE, Horiuchi M, Chen Q, Daviet L, Yang ZY, Lopez-Ilasaca M, Mu H, Pratt RE, Dzau VJ. (2000) LXRα functions as a cAMP-responsive transcriptional regulator of gene expression. Proc Natl Acad Sci USA 97:8513–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Nakamura T, Nishida S, Suzuki K, Takeda S, Sato K, Nishii Y. (1996) Effects of a synthetic vitamin D analog, ED-71, on bone dynamics and strength in cancellous and cortical bone in prednisolone-treated rats. J Bone Miner Res 11:325–336. [DOI] [PubMed] [Google Scholar]
- Tancevski I, Demetz E, Eller P, Duwensee K, Hoefer J, Heim C, Stanzl U, Wehinger A, Auer K, Karer R, et al. (2010) The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice. PLoS ONE 5:e8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancevski I, Wehinger A, Demetz E, Hoefer J, Eller P, Huber E, Stanzl U, Duwensee K, Auer K, Schgoer W, et al. (2009) The thyromimetic T-0681 protects from atherosclerosis. J Lipid Res 50:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, et al. (2002) Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci USA 99:11896–11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin ME, Manola J, Oh WK, Kantoff PW, Bubley GJ, Smith M, Barb D, Mantzoros C, Gelmann EP, Balk SP. (2008) A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int 101:1084–1089. [DOI] [PubMed] [Google Scholar]
- Tarlatzis BC, Grimbizis G. (1998) Future use of clomiphene in ovarian stimulation. Will clomiphene persist in the 21st century? Hum Reprod 13:2356–2358. [DOI] [PubMed] [Google Scholar]
- Tasset D, Tora L, Fromental C, Scheer E, Chambon P. (1990) Distinct classes of transcriptional activating domains function by different mechanisms. Cell 62:1177–1187. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Stephan ZF, Steele RE, Wong NCW. (1997) Beneficial effects of a novel thyromimetic on lipoprotein metabolism. Mol Pharmacol 52:542–547. [DOI] [PubMed] [Google Scholar]
- Taylor HS. (2009) Designing the ideal selective estrogen receptor modulator—an achievable goal? Menopause 16:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Martin-Hirsch PL, Martin FL. (2010) Oestrogen receptor splice variants in the pathogenesis of disease. Cancer Lett 288:133–148. [DOI] [PubMed] [Google Scholar]
- Teboul M, Enmark E, Li Q, Wikström AC, Pelto-Huikko M, Gustafsson JA. (1995) OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci USA 92:2096–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng CS, Hamilton TH. (1968) The role of chromatin in estrogen action in the uterus, I. The control of template capacity and chemical composition and the binding of H3-estradiol-17 beta. Proc Natl Acad Sci USA 60:1410–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. (2003) T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett 536:6–11. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Jurutka PW, Whitfield GK, Myskowski SM, Eichhorst KR, Dominguez CE, Haussler CA, Haussler MR. (2002) Liganded VDR induces CYP3A4 in small intestinal and colon cancer cells via DR3 and ER6 vitamin D responsive elements. Biochem Biophys Res Commun 299:730–738. [DOI] [PubMed] [Google Scholar]
- Thompson SK, Washburn DG, Frazee JS, Madauss KP, Hoang TH, Lapinski L, Grygielko ET, Glace LE, Trizna W, Williams SP, et al. (2009) Rational design of orally-active, pyrrolidine-based progesterone receptor partial agonists. Bioorg Med Chem Lett 19:4777–4780. [DOI] [PubMed] [Google Scholar]
- Tilley WD, Marcelli M, Wilson JD, McPhaul MJ. (1989) Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci USA 86:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tincello DG, Taylor AH, Spurling SM, Bell SC. (2009) Receptor isoforms that mediate estrogen and progestagen action in the female lower urinary tract. J Urol 181:1474–1482. [DOI] [PubMed] [Google Scholar]
- Tocchini-Valentini G, Rochel N, Wurtz JM, Mitschler A, Moras D. (2001) Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc Natl Acad Sci USA 98:5491–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G, Rochel N, Wurtz JM, Moras D. (2004) Crystal structures of the vitamin D nuclear receptor liganded with the vitamin D side chain analogues calcipotriol and seocalcitol, receptor agonists of clinical importance. Insights into a structural basis for the switching of calcipotriol to a receptor antagonist by further side chain modification. J Med Chem 47:1956–1961. [DOI] [PubMed] [Google Scholar]
- Toft D. (1972) The interaction of uterine estrogen receptors with DNA. J Steroid Biochem 3:515–522. [DOI] [PubMed] [Google Scholar]
- Toft D, Gorski J. (1966) A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci USA 55:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Kakiuchi Y, Tsao PS. (2006) THR0921, a novel peroxisome proliferator-activated receptor gamma agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther 8:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu ED, Spiegelman BM. (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79:1147–1156. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. (2008) Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312. [DOI] [PubMed] [Google Scholar]
- Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. (1997) The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677–684. [DOI] [PubMed] [Google Scholar]
- Trapman J, Klaassen P, Kuiper GG, van der Korput JA, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Brinkmann AO. (1988) Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun 153:241–248. [DOI] [PubMed] [Google Scholar]
- Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang HJ, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, et al. (2000) The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 141:3057–3064. [DOI] [PubMed] [Google Scholar]
- Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, Clausen BE, Stride B, Förster I, Habenicht AJR, et al. (2007) Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest 117:1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckermann JP, Reichardt HM, Arribas R, Richter KH, Schütz G, Angel P. (1999) The DNA binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J Cell Biol 147:1365–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. (1992) The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J 11:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch BR, Lewis B, Fraser TR. (1973) Triglyceride metabolism in thyroid disease. Lancet 1:391–394. [DOI] [PubMed] [Google Scholar]
- Turner CH, Sato M, Bryant HU. (1994) Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology 135:2001–2005. [DOI] [PubMed] [Google Scholar]
- Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. (2009) Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas 63:329–335. [DOI] [PubMed] [Google Scholar]
- Vajda EG, López FJ, Rix P, Hill R, Chen Y, Lee KJ, O’Brien Z, Chang WY, Meglasson MD, Lee YH. (2009) Pharmacokinetics and pharmacodynamics of LGD-3303 [9-chloro-2-ethyl-1-methyl-3-(2,2,2-trifluoroethyl)-3H-pyrrolo-[3,2-f]quinolin-7(6H)-one], an orally available nonsteroidal-selective androgen receptor modulator. J Pharmacol Exp Ther 328:663–670. [DOI] [PubMed] [Google Scholar]
- Vajdos FF, Hoth LR, Geoghegan KF, Simons SP, LeMotte PK, Danley DE, Ammirati MJ, Pandit J. (2007) The 2.0 A crystal structure of the ERα ligand-binding domain complexed with lasofoxifene. Protein Sci 16:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JGJ, Stockmans I, Van Herck E, Kato S, Bindels RJM, Collen D, Carmeliet P, Bouillon R, et al. (2001) Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Deure WM, Peeters RP, Visser TJ. (2010) Molecular aspects of thyroid hormone transporters, including MCT8, MCT10, and OATPs, and the effects of genetic variation in these transporters. J Mol Endocrinol 44:1–11. [DOI] [PubMed] [Google Scholar]
- van Raalte DH, Ouwens DM, Diamant M. (2009) Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest 39:81–93. [DOI] [PubMed] [Google Scholar]
- Vanhooke JL, Benning MM, Bauer CB, Pike JW, DeLuca HF. (2004) Molecular structure of the rat vitamin D receptor ligand binding domain complexed with 2-carbon-substituted vitamin D3 hormone analogues and a LXXLL-containing coactivator peptide. Biochemistry 43:4101–4110. [DOI] [PubMed] [Google Scholar]
- Vayssiere BM, Dupont S, Choquart A, Petit F, Garcia T, Marchandeau C, Gronemeyer H, Resche-Rigon M. (1997) Synthetic glucocorticoids that dissociate transactivation and AP-1 transrepression exhibit antiinflammatory activity in vivo. Mol Endocrinol 11:1245–1255. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. (1993) Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 7:1244–1255. [DOI] [PubMed] [Google Scholar]
- Vennstrom B, Houng L and Forrest D (2010) Thyroid Hormone Receptors. [DOI] [PubMed]
- Vergote I, Robertson JF. (2004) Fulvestrant is an effective and well-tolerated endocrine therapy for postmenopausal women with advanced breast cancer: results from clinical trials. Br J Cancer 90 (Suppl 1):S11–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viale G, Doglioni C, Gambacorta M, Zamboni G, Coggi G, Bordi C. (1992) Progesterone receptor immunoreactivity in pancreatic endocrine tumors. An immunocytochemical study of 156 neuroendocrine tumors of the pancreas, gastrointestinal and respiratory tracts, and skin. Cancer 70:2268–2277. [DOI] [PubMed] [Google Scholar]
- Villicev CM, Freitas FRS, Aoki MS, Taffarel C, Scanlan TS, Moriscot AS, Ribeiro MO, Bianco AC, Gouveia CHA. (2007) Thyroid hormone receptor beta-specific agonist GC-1 increases energy expenditure and prevents fat-mass accumulation in rats. J Endocrinol 193:21–29. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. (1996) TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- von Geldern TW, Tu N, Kym PR, Link JT, Jae HS, Lai CQ, Apelqvist T, Rhonnstad P, Hagberg L, Koehler K, et al. (2004) Liver-selective glucocorticoid antagonists: a novel treatment for type 2 diabetes. J Med Chem 47:4213–4230. [DOI] [PubMed] [Google Scholar]
- Wada Y, Matsubara S, Dufresne J, Hargrove GM, Stephan ZF, Steele RE, Wong NCW. (2000) Effects of a thyromimetic on apolipoprotein B-100 in rats. J Mol Endocrinol 25:299–308. [DOI] [PubMed] [Google Scholar]
- Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. (1995) A structural role for hormone in the thyroid hormone receptor. Nature 378:690–697. [DOI] [PubMed] [Google Scholar]
- Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. (2001) Hormone selectivity in thyroid hormone receptors. Mol Endocrinol 15:398–410. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. (1991) A potent specific pure antiestrogen with clinical potential. Cancer Res 51:3867–3873. [PubMed] [Google Scholar]
- Wallace OB, Lauwers KS, Dodge JA, May SA, Calvin JR, Hinklin R, Bryant HU, Shetler PK, Adrian MD, Geiser AG, et al. (2006) A selective estrogen receptor modulator for the treatment of hot flushes. J Med Chem 49:843–846. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M, et al. (1985) Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA 82:7889–7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan W, Farboud B, Privalsky ML. (2005) Pituitary resistance to thyroid hormone syndrome is associated with T3 receptor mutants that selectively impair β2 isoform function. Mol Endocrinol 19:1529–1542. [DOI] [PubMed] [Google Scholar]
- Wang A, Lu C, Qiao J. (1998a). The effects of FSH,LH and insulin on steroids production by granulosa cells from polycystic ovaries syndrome [article in Chinese]. Zhonghua Yi Xue Za Zhi 78:830–832. [PubMed] [Google Scholar]
- Wang A, Lu C, Qiao J. (1998b) Effects of human chorionic gonadotropine and insulin on androgen production by cultured thecal cells from patients with polycystic ovary syndrome [article in Chinese]. Zhonghua Fu Chan Ke Za Zhi 33:280–283. [PubMed] [Google Scholar]
- Wang LH, Tsai SY, Cook RG, Beattie WG, Tsai MJ, O’Malley BW. (1989) COUP transcription factor is a member of the steroid receptor superfamily. Nature 340:163–166. [DOI] [PubMed] [Google Scholar]
- Wang XN, Simmons HA, Salatto CT, Cosgrove PG, Thompson DD. (2006) Lasofoxifene enhances vaginal mucus formation without causing hypertrophy and increases estrogen receptor beta and androgen receptor in rats. Menopause 13:609–620. [DOI] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. (2002) Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem 277:26573–26580. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR. (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333. [DOI] [PubMed] [Google Scholar]
- Webb P, Anderson CM, Valentine C, Nguyen P, Marimuthu A, West BL, Baxter JD, Kushner PJ. (2000) The nuclear receptor corepressor (N-CoR) contains three isoleucine motifs (I/LXXII) that serve as receptor interaction domains (IDs). Mol Endocrinol 14:1976–1985. [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. (1980a) Biochemical properties of 1 alpha, 25-dihydroxyvitamin D receptors. J Steroid Biochem 13:977–989. [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Norman AW. (1980b) A kinetic and equilibrium binding study of 1 alpha,25-dihydroxyvitamin D3 with its cytosol receptor from chick intestinal mucosa. J Biol Chem 255:3571–3574. [PubMed] [Google Scholar]
- Wecksler WR, Ross FP, Mason RS, Posen S, Norman AW. (1980a) Biochemical properties of the 1 alpha, 25-dihydroxyvitamin D3 cytoplasmic receptors from human and chick parathyroid glands. Arch Biochem Biophys 201:95–103. [DOI] [PubMed] [Google Scholar]
- Wecksler WR, Ross FP, Mason RS, Norman AW. (1980b) Biochemical properties of the 1 alpha, 25-dihydroxyvitamin D3 cytosol receptors from human and chicken intestinal mucosa. J Clin Endocrinol Metab 50:152–157. [DOI] [PubMed] [Google Scholar]
- Weinberger C, Giguere V, Hollenberg S, Rosenfeld MG, Evans RM. (1986a) Human steroid receptors and erbA proto-oncogene products: members of a new superfamily of enhancer binding proteins. Cold Spring Harb Symp Quant Biol 51:759–772. [DOI] [PubMed] [Google Scholar]
- Weinberger C, Hollenberg SM, Ong ES, Harmon JM, Brower ST, Cidlowski J, Thompson EB, Rosenfeld MG, Evans RM. (1985a) Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science 228:740–742. [DOI] [PubMed] [Google Scholar]
- Weinberger C, Hollenberg SM, Rosenfeld MG, Evans RM. (1985b) Domain structure of human glucocorticoid receptor and its relationship to the v-erb-A oncogene product. Nature 318:670–672. [DOI] [PubMed] [Google Scholar]
- Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM. (1986b) The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646. [DOI] [PubMed] [Google Scholar]
- Welker P, Lippert U, Nürnberg W, Krüger-Krasagakes S, Möller A, Czarnetzki BM. (1996) Glucocorticoid-induced modulation of cytokine secretion from normal and leukemic human myelomonocytic cells. Int Arch Allergy Immunol 109:110–115. [DOI] [PubMed] [Google Scholar]
- Wenger NK. (1997) Coronary heart disease: an older woman’s major health risk. BMJ 315:1085–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebel FF, Gustafsson JA. (1997) Heterodimeric interaction between retinoid X receptor alpha and orphan nuclear receptor OR1 reveals dimerization-induced activation as a novel mechanism of nuclear receptor activation. Mol Cell Biol 17:3977–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebel FF, Steffensen KR, Treuter E, Feltkamp D, Gustafsson JA. (1999) Ligand-independent coregulator recruitment by the triply activatable OR1/retinoid X receptor-alpha nuclear receptor heterodimer. Mol Endocrinol 13:1105–1118. [DOI] [PubMed] [Google Scholar]
- Wiehle R, Lantvit D, Yamada T, Christov K. (2011) CDB-4124, a progesterone receptor modulator, inhibits mammary carcinogenesis by suppressing cell proliferation and inducing apoptosis. Cancer Prev Res (Phila) 4:414–424. [DOI] [PubMed] [Google Scholar]
- Wiehle RD, Goldberg J, Brodniewicz T, Jarus-Dziedzic K, Jabiry-Zieniewicz Z. (2008) Effects of a new progesterone receptor modulator, cdb-4124, on fibroid size and uterine bleeding. US Obstet Gynecol 5:17–20. [Google Scholar]
- Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B. (1998) Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor alpha 1. EMBO J 17:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- Williams GR. (2000) Cloning and characterization of two novel thyroid hormone receptor beta isoforms. Mol Cell Biol 20:8329–8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Bledsoe RK, Collins JL, Boggs S, Lambert MH, Miller AB, Moore J, McKee DD, Moore L, Nichols J, et al. (2003) X-ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine-tryptophan switch. J Biol Chem 278:27138–27143. [DOI] [PubMed] [Google Scholar]
- Williams SP, Sigler PB. (1998) Atomic structure of progesterone complexed with its receptor. Nature 393:392–396. [DOI] [PubMed] [Google Scholar]
- Willson TM, Kliewer SA. (2002) PXR, CAR and drug metabolism. Nat Rev Drug Discov 1:259–266. [DOI] [PubMed] [Google Scholar]
- Willson TM, Lambert MH, Kliewer SA. (2001) Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem 70:341–367. [DOI] [PubMed] [Google Scholar]
- Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. (1995) LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev 9:1033–1045. [DOI] [PubMed] [Google Scholar]
- Won HY, Min HJ, Ahn JH, Yoo SE, Bae MA, Hong JH, Hwang ES. (2010) Anti-allergic function and regulatory mechanisms of KR62980 in allergen-induced airway inflammation. Biochem Pharmacol 79:888–896. [DOI] [PubMed] [Google Scholar]
- Wrange O, Gustafsson JA. (1978) Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis. J Biol Chem 253:856–865. [PubMed] [Google Scholar]
- Wu Y, Chin WW, Wang Y, Burris TP. (2003) Ligand and coactivator identity determines the requirement of the charge clamp for coactivation of the peroxisome proliferator-activated receptor gamma. J Biol Chem 278:8637–8644. [DOI] [PubMed] [Google Scholar]
- Wurtz JM, Bourguet W, Renaud JP, Vivat V, Chambon P, Moras D, Gronemeyer H. (1996) A canonical structure for the ligand-binding domain of nuclear receptors [published correction appears in Nat Struct Biol 3:206]. Nat Struct Biol 3: 87–94. [DOI] [PubMed] [Google Scholar]
- Xu HE, Lambert MH, Montana VG, Plunket KD, Moore LB, Collins JL, Oplinger JA, Kliewer SA, Gampe RT, Jr, McKee DD, et al. (2001) Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA 98:13919–13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, O’Malley BW. (2002) Molecular mechanisms and cellular biology of the steroid receptor coactivator (SRC) family in steroid receptor function. Rev Endocr Metab Disord 3:185–192. [DOI] [PubMed] [Google Scholar]
- Xu J, Wagoner G, Douglas JC, Drew PD. (2009) Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol 86:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Glass CK, Rosenfeld MG. (1999) Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev 9:140–147. [DOI] [PubMed] [Google Scholar]
- Xu L, Tsai KS, Kim GS, Wu Y, Vincendon P, Chines AA, Constantine GD. (2011) Efficacy and safety of bazedoxifene in postmenopausal Asian women. Osteoporos Int 22:559–565. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shevde NK, Warrier A, Plum LA, DeLuca HF, Pike JW. (2003) 2-Methylene-19-nor-(20S)-1,25-dihydroxyvitamin D3 potently stimulates gene-specific DNA binding of the vitamin D receptor in osteoblasts. J Biol Chem 278:31756–31765. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Alberts B. (1975) The interaction of estradiol-receptor protein with the genome: an argument for the existence of undetected specific sites. Cell 4:301–310. [DOI] [PubMed] [Google Scholar]
- Yamamoto KR, Alberts B. (1974) On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem 249:7076–7086. [PubMed] [Google Scholar]
- Yamamoto KR, Stampfer MR, Tomkins GM. (1974) Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci USA 71:3901–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CD, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F, Westover EJ, Covey DF, Mangelsdorf DJ, Cohen JC, et al. (2006) Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem 281:27816–27826. [DOI] [PubMed] [Google Scholar]
- Yang XY, Wang LH, Chen TS, Hodge DR, Resau JH, DaSilva L, Farrar WL. (2000) Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. J Biol Chem 275:4541–4544. [DOI] [PubMed] [Google Scholar]
- Yen PM. (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142. [DOI] [PubMed] [Google Scholar]
- Yen PM. (2003) Molecular basis of resistance to thyroid hormone. Trends Endocrinol Metab 14:327–333. [DOI] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, et al. (2007) Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1786–1789. [DOI] [PubMed] [Google Scholar]
- Yoles I, Lilling G. (2007) Pharmacological doses of the natural phyto-SERM DT56a (Femarelle) have no effect on MCF-7 human breast cancer cell-line. Eur J Obstet Gynecol Reprod Biol 130:140–141. [DOI] [PubMed] [Google Scholar]
- Yoles I, Yogev Y, Frenkel Y, Hirsch M, Nahum R, Kaplan B. (2004) Efficacy and safety of standard versus low-dose Femarelle (DT56a) for the treatment of menopausal symptoms. Clin Exp Obstet Gynecol 31:123–126. [PubMed] [Google Scholar]
- Yoles I, Yogev Y, Frenkel Y, Nahum R, Hirsch M, Kaplan B. (2003) Tofupill/Femarelle (DT56a): a new phyto-selective estrogen receptor modulator-like substance for the treatment of postmenopausal bone loss. Menopause 10:522–525. [DOI] [PubMed] [Google Scholar]
- Yoshihara HAI, Apriletti JW, Baxter JD, Scanlan TS. (2003) Structural determinants of selective thyromimetics. J Med Chem 46:3152–3161. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, et al. (1997) Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396. [DOI] [PubMed] [Google Scholar]
- Young HA, Shih TY, Scolnick EM, Parks WP. (1977) Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol 21:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Lawrence AJ, MacLean AG, Leung BP, McInnes IB, Canas B, Pappin DJC, Stevenson RD. (1999) Thymosin beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat Med 5:1424–1427. [DOI] [PubMed] [Google Scholar]
- Young WFJ, Jr, Gorman CA, Jiang NS, Machacek D, Hay ID. (1984) L-thyroxine contamination of pharmaceutical D-thyroxine: probable cause of therapeutic effect. Clin Pharmacol Ther 36:781–787. [DOI] [PubMed] [Google Scholar]
- Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN. (2005) The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J Biol Chem 280:13600–13605. [DOI] [PubMed] [Google Scholar]
- Yu K, Bayona W, Kallen CB, Harding HP, Ravera CP, McMahon G, Brown M, Lazar MA. (1995) Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J Biol Chem 270:23975–23983. [DOI] [PubMed] [Google Scholar]
- Yu VC, Delsert C, Andersen B, Holloway JM, Devary OV, Näär AM, Kim SY, Boutin JM, Glass CK, Rosenfeld MG. (1991) RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell 67:1251–1266. [DOI] [PubMed] [Google Scholar]
- Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. (1998) The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA 95:7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska KE, Cusin I, Sainsbury A, Rohner-Jeanrenaud F, Jeanrenaud B. (1997) Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes 46:717–719. [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom TM, Guioli S, Guo W, Lalli E, Moser C, Walker AP, McCabe ER, et al. (1994) An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372:635–641. [DOI] [PubMed] [Google Scholar]
- Zeitz U, Weber K, Soegiarto DW, Wolf E, Balling R, Erben RG. (2003) Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 17:509–511. [DOI] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P. (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 116:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chalmers MJ, Stayrook KR, Burris LL, Wang Y, Busby SA, Pascal BD, Garcia-Ordonez RD, Bruning JB, Istrate MA, et al. (2011) DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat Struct Mol Biol 18:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Lazar MA. (2000) The mechanism of action of thyroid hormones. Annu Rev Physiol 62:439–466. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liang X, Danielsen M. (1996) Role of the C terminus of the glucocorticoid receptor in hormone binding and agonist/antagonist discrimination. Mol Endocrinol 10:24–34. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Wei L, Li G, Yu J, Gao Y, Gao P, Zhang X, Wei F, Yin D, et al. (2010) Transcriptional modulation of BCRP gene to reverse multidrug resistance by toremifene in breast adenocarcinoma cells. Breast Cancer Res Treat 123:679–689. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Olland AM, Zhu Y, Cohen J, Berrodin T, Chippari S, Appavu C, Li S, Wilhem J, Chopra R, et al. (2005) Molecular and pharmacological properties of a potent and selective novel nonsteroidal progesterone receptor agonist tanaproget. J Biol Chem 280:28468–28475. [DOI] [PubMed] [Google Scholar]
- Zhang ZR, Zhang ZY, Schluesener HJ. (2009) Compound A, a plant origin ligand of glucocorticoid receptors, increases regulatory T cells and M2 macrophages to attenuate experimental autoimmune neuritis with reduced side effects. J Immunol 183:3081–3091. [DOI] [PubMed] [Google Scholar]
- Zhao H, Lo YH, Yu L, Wang SC. (2011a) Overcoming resistance to fulvestrant (ICI182,780) by downregulating the c-ABL proto-oncogene in breast cancer. Mol Carcinog 50:383–389. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang LJ, Zhang M, Wang P, Zhang L, Yuan C, Qi JN, Qiao Y, Kuo PC, Gao CJ. (2011b) Peroxisome proliferator-activated receptor gamma negatively regulates IFN-beta production in Toll-like receptor (TLR) 3- and TLR4-stimulated macrophages by preventing interferon regulatory factor 3 binding to the IFN-beta promoter. J Biol Chem 286:5519–5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Mahon BD, Froicu M, Cantorna MT. (2005) Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol 35:217–224. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Kan LX, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK. (2000) Isolation and characterization of peroxisome proliferator-activated receptor (PPAR) interacting protein (PRIP) as a coactivator for PPAR. J Biol Chem 275:13510–13516. [DOI] [PubMed] [Google Scholar]
- Zilbermint MF, Dobs AS. (2009) Nonsteroidal selective androgen receptor modulator Ostarine in cancer cachexia. Future Oncol 5:1211–1220. [DOI] [PubMed] [Google Scholar]
- Zisman AL, Hristova M, Ho LT, Sprague SM. (2007) Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol 27:36–43. [DOI] [PubMed] [Google Scholar]
- Zwart W, Rondaij M, Jalink K, Sharp ZD, Mancini MA, Neefjes J, Michalides R. (2009) Resistance to antiestrogen arzoxifene is mediated by overexpression of cyclin D1. Mol Endocrinol 23:1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]