Abstract
Exogenous administration of inflammatory stimuli to humans and laboratory animals and chronic endogenous inflammatory states lead to motivational deficits and ultimately anhedonia, a core and disabling symptom of depression present in multiple other psychiatric disorders. Inflammation impacts neurotransmitter systems and neurocircuits in subcortical brain regions including the ventral striatum, which serves as an integration point for reward processing and motivational decision-making. Many mechanisms contribute to these effects of inflammation, including decreased synthesis, release and reuptake of dopamine, increased synaptic and extrasynaptic glutamate, and activation of kynurenine pathway metabolites including quinolinic acid. Neuroimaging data indicate that these inflammation-induced neurotransmitter effects manifest as decreased activation of ventral striatum and decreased functional connectivity in reward circuitry involving ventral striatum and ventromedial prefrontal cortex. Neurocircuitry changes in turn mediate nuanced effects on motivation that include decreased willingness to expend effort for reward while maintaining the ability to experience reward. Taken together, the data reveal an inflammation-induced pathophysiologic phenotype that is agnostic to diagnosis. Given the many mechanisms involved, this phenotype represents an opportunity for development of novel and/or repurposed pharmacological strategies that target inflammation and associated cellular and systemic immunometabolic changes and their downstream effects on the brain. To date, clinical trials have failed to capitalize on the unique nature of this transdiagnostic phenotype, leaving the field bereft of interpretable data for meaningful clinical application. However, novel trial designs incorporating established targets in the brain and/or periphery using relevant outcome variables (e.g., anhedonia) are the future of targeted therapy in psychiatry.
Significance Statement
Emerging understanding of mechanisms by which peripheral inflammation can affect the brain and behavior has created unprecedented opportunities for development of pharmacological strategies to treat deficits in motivation including anhedonia, a core and disabling symptom of depression well represented in multiple psychiatric disorders. Mechanisms include inflammation and cellular and systemic immunometabolism and alterations in dopamine, glutamate, and kynurenine metabolites, revealing a target-rich environment that nevertheless has yet to be fully exploited by current clinical trial designs and drugs employed.
I. Introduction
A wealth of knowledge regarding the impact of inflammation on the brain has yielded an opportunity to develop innovative pharmacological therapies that target the immune system and its effects on the brain to treat inflammation-related symptoms (Miller and Raison, 2016). Data demonstrate that inflammation affects neurotransmitter systems and relevant neurocircuits in the brain that ultimately regulate behaviors related to motivation, leading to anhedonia, a symptom of mood and anxiety disorders that is commonly found across a spectrum of psychiatric and medical illnesses (Miller and Raison, 2016). These behaviors and their link to the inflammatory response are believed to have grown out of evolutionary survival priorities of sickness or wounding that demanded a protective, reparative response with associated shifts in energy resources to fighting infection and wound healing and a reorientation of motivated behavior to energy conservation (Raison and Miller, 2013; Miller and Raison, 2016; Treadway et al., 2019a; Wang et al., 2019). In the modern world, in which pathogens and predators are largely mitigated, new threats have emerged, many of which are consequences of the Western lifestyle. Poor dietary habits, sedentary behavior, and chronic stress can fuel a persistent, nonresolving inflammatory state through obesity, metabolic syndrome, and diabetes, as well as related inflammatory illnesses including cardiovascular disease, cancer, neurodegenerative disorders, and accelerated aging (Christ and Latz, 2019; Furman et al., 2019). Thus, inflammatory states that in ancestral times may have promoted survival can become chronic and unremitting, leading to behavioral changes that serve as a pathophysiological substrate for shifts in reward processing that ultimately aid and abet anhedonia, a core symptom of depression that is also well represented in other psychiatric disorders, including anxiety disorders, post-traumatic stress disorder (PTSD), and schizophrenia. Anhedonia also has a prominent association with suicide, which represents a critical and pressing public health concern (Ducasse et al., 2018, 2020).
Pharmacological targets for inflammation and its effects on the brain encompass inflammation itself, including inflammatory cells and their inflammatory mediators (e.g., cytokines), their signaling pathways, and their metabolism of glucose and fatty acids, as well as the impact of inflammation on relevant neuronal and glial cells affecting neurotransmitter systems such as dopamine (DA), glutamate, and the kynurenine pathway (KP) and the neurocircuits they regulate (Haroon et al., 2012; Miller et al., 2017).
Taken together, the identification of pathophysiological mechanisms (inflammation and its effects on the brain) that lead to specific symptom’s dimensions (motivational deficits and anhedonia) across disorders provides a pivotal entrée into the future of drug development in psychiatry. Such a future embraces the concept of targeting treatments to transdiagnostic, biologically based subgroups of patients with common symptom presentations while moving away from drug development for currently conceived diagnostic groups based on consensus taxonomy and represented in the Diagnostic and Statistical Manual of Mental Disorders (DSM). This approach has become an exceedingly successful strategy in oncology, in which targeted treatments focus on specific pathologic signaling pathways, agnostic to tumor type (Baudino, 2015).
In this review, we will provide the background and foundation for identifying and characterizing the biologic subgroup of patients with increased inflammation. In the process, we will identify the many mechanisms involved in the effects of inflammation on the brain, ultimately revealing a multitude of pharmacological targets for treatment. Finally, we will discuss the shortcomings of the extant literature on the use of anti-inflammatory drugs to treat psychiatric disorders and will provide recommendations for future clinical trial design.
II. Inflammation in Depression
A. Increased Inflammatory Markers in Patients with Major Depression
The hypothesis that inflammation may play a role in psychiatric disorders evolved from early studies on patients with major depression. Based on sampling inflammatory markers in the peripheral blood of patients with depression, initial studies characterized increases in acute phase proteins that are primarily produced in the liver in response to inflammatory cytokines (Maes et al., 1992; Maes, 1995). These early reports were followed by a substantial literature represented by hundreds of studies that replicated and extended early findings demonstrating that, compared with controls, patients with depression exhibit mean increases in all of the inflammatory molecules that typify a chronic inflammatory response, including increases in peripheral blood inflammatory cytokines, chemokines, and adhesion molecules, as well the acute phase proteins. There have been numerous meta-analyses of this literature, and the inflammatory cytokines tumor necrosis factor (TNF) and interleukin (IL)-6 and the acute phase protein C-reactive protein (CRP) appear to be some of the most reliably elevated inflammatory biomarkers in patients with depression as a group and are robust to a range of confounds, including body mass index (BMI), smoking, age, and treatment status (Howren et al., 2009; Dowlati et al., 2010; Kohler et al., 2017; Osimo et al., 2020). Finally, a number of studies have shown that inflammatory biomarkers including CRP and IL-6 predict the subsequent development of depression (Valkanova et al., 2013; Osimo et al., 2019; Mac Giollabhui et al., 2020).
Examination of gene expression in peripheral blood immune cells of patients with major depression has also revealed activation of canonical inflammatory signaling pathways, including toll-like receptors (TLRs); nuclear factor κB (NF-kB); the NOD-, LRR-, and pyrin domain–containing protein (NLRP3) inflammasome complex, which via caspase-1 cleaves pro–IL-1β and IL-18 into their mature forms; markers of oxidative stress; and the inflammatory cytokines themselves (Hajebrahimi et al., 2014; Keri et al., 2014; Chen et al., 2017; Hung et al., 2014, 2017). Activation of these inflammatory pathways reflects the components of the fundamental innate immune response to microbe-derived pathogen-associated molecular patterns, possibly related to infection or the microbiome, and danger-associated molecular patterns (DAMPs), which are generated by host cells under stress (Fleshner and Crane, 2017).
A number of studies have also conducted whole-genome expression analyses in depressed versus control subjects using samples of whole blood, which include neutrophils, or gradient-isolated peripheral blood mononuclear cells (PBMCs), which do not contain neutrophils (Spijker et al., 2010; Yi et al., 2012; Mostafavi et al., 2014; Guilloux et al., 2015; Hori et al., 2016; Jansen et al., 2016; Le et al., 2018; Leday et al., 2018). Different array platforms have been employed, and in at least two cases, deep RNA sequencing was conducted, making comparison of these studies challenging at best. Results from this work generally indicate that peripheral blood immune cells from patients with depression exhibit increased activation of signaling pathways related to inflammation and the innate immune response, including genes enriched for IL-6 signaling and pathway enrichment of type 1 interferon (IFN) signaling (Mostafavi et al., 2014; Jansen et al., 2016). Of note, increased expression of a TNF receptor gene was within the top 15 of the strongest individual gene findings in one report (Mostafavi et al., 2014). Most relevant to the focus of the current review, in one small study, ingenuity pathway analysis of PBMCs in patients with depression versus controls (n ≅ 20 per group), revealed a gene network centered around TNF with NF-kB as a connecting hub that was associated with morphometric measures of the caudate (Savitz et al., 2013), a key brain region involved in both motivation and motor activity. Of relevance to immunometabolism (see below), one study on PBMCs from patients with postpartum depression found a positive association with multiple genes involved in energy metabolism, including glycolysis/gluconeogenesis and lipid metabolism, as well as pathways related to cytokine/cytokine receptor interactions and TLR signaling (Pan et al., 2018). Finally, in a recent report using multivariate mixture modeling and consensus clustering, four subgroups of patients with depression emerged, two of which exhibited increased inflammatory markers (IL-6 and CRP): one was dominated by immune cell subsets of predominately myeloid lineage (neutrophils and monocytes), and the other was characterized by an abundance of lymphoid cells (Lynall et al., 2020). Whether these subgroups represent discrete immunologic phenotypes or a progression from early innate immune responses that transition to greater representation of adaptive (lymphocytic) immune responses as the disease becomes more chronic (and potentially more treatment-resistant) remains unclear (Felger and Miller, 2020). Nevertheless, these data support the notion that there may be multiple immunophenotypes of inflammation in depression and possibly other psychiatric disorders, further suggesting that targeting specific immune pathways and immunologic subgroups may be especially relevant given heterogeneous populations of patients with psychiatric disorders with and without increased inflammation (Felger and Miller, 2020; Lynall et al., 2020).
In addition to the peripheral blood, increased inflammatory markers have also been described in the cerebrospinal fluid (CSF) of depressed subjects (Felger et al., 2020; Franzen et al., 2020), and postmortem studies have identified evidence of increased inflammatory signaling in brain parenchyma, represented by increased TLR expression and expression of inflammatory cytokines as well as evidence of immune cell trafficking to the brain and activation of microglia, the immune cells of the brain (Pandey et al., 2014, 2017; Torres-Platas et al., 2014a,b; Enache et al., 2019). Microglial activation has also been suggested by positron emission tomography (PET) in patients with depression using ligands to the translocator protein (TSPO), whose expression is increased in activated microglia (Setiawan et al., 2015; Richards et al., 2018; Enache et al., 2019). It should be noted, however, that although TSPO binds to activated microglia in neuroinflammatory states, it does not distinguish the many states and functions of activated microglia that include, for example, synaptic pruning that is unrelated to inflammation. TSPO ligands also bind to other activated cells in the brain, including astrocytes, vascular endothelial cells, and neurons, making interpretation of the results challenging (Notter et al., 2020).
1. Association with Treatment Response
In a meta-analysis and recent systematic review of the literature, increased inflammatory markers in depression have been associated with a reduced treatment response to conventional antidepressants, especially in ambulatory patients with depression (Strawbridge et al., 2015; Arteaga-Henríquez et al., 2019). For example, elevated biomarkers of inflammation including CRP at baseline have been associated with a poor response to selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) (Uher et al., 2014; Cattaneo et al., 2016; Jha et al., 2017). Moreover, patients who have failed multiple treatment trials have been found to exhibit increased inflammatory markers including IL-6, TNF, soluble TNF receptor 2, and CRP, and treatment-resistant versus treatment-responsive patients with depression were recently shown using binomial logistic models to exhibit a signature of mRNAs including P2X purinoceptor 7, IL-1β, TNF, and C-X-C motif ligand 12 (Haroon et al., 2018b; Chamberlain et al., 2019; Cattaneo et al., 2020). Interestingly, drugs that affect DAergic and noradrenergic pathways, including bupropion and nortriptyline, which also increases DA release in frontal cortex, have exhibited improved efficacy compared with SSRIs in patients with increased inflammation (see impact of inflammation on DA below) (Uher et al., 2014; Jha et al., 2017; Arteaga-Henríquez et al., 2019). Increased inflammatory markers have also been associated with increased responsiveness to electroconvulsive therapy and ketamine (Yang et al., 2015; Kruse et al., 2018). Taken together, these data suggest that inflammatory markers may help guide treatment selection in patients with depression. Nevertheless, most studies to date have examined the predictive capacity of inflammatory biomarkers using post hoc analyses. Few studies have a priori randomized patients on the basis of inflammation and determined treatment response (see below for further discussion of anti-inflammatory trial failures).
B. Induction of Depressive Symptoms after Administration of Inflammatory Stimuli
Much of the data that have substantiated the effects of inflammation on behavior has been derived from studies examining the impact of various inflammatory stimuli on symptoms of depression. Probably the most extensive literature in this regard comes from the administration of the inflammatory cytokine IFN-α to patients with cancer (malignant melanoma or renal cell carcinoma) or infectious diseases (primarily hepatitis C). IFN-α has been shown to induce a variety of depressive symptoms, with early appearance of neurovegetative symptoms including anhedonia, psychomotor slowing, and fatigue and later appearance of more cognitive symptoms including depressed mood and cognitive dysfunction (Capuron et al., 2002a; Capuron and Miller, 2004; Wichers et al., 2005; Su et al., 2019a). Depending on the dose of IFN-α, ∼30%–50% of patients meet symptom criteria for major depression, and comparisons between patients with IFN-α–induced depression and otherwise healthy depressed individuals reveal marked overlap in symptoms (Capuron et al., 2009). Aside from IFN-α, similar findings have been found in healthy controls acutely administered either typhoid or influenza vaccination or low-dose endotoxin (Brydon et al., 2008, 2019; Harrison et al., 2009, 2016; Eisenberger et al., 2010; Moieni et al., 2019a). In each case, depressive symptoms are induced, including symptoms of depressed mood, anhedonia, and psychomotor slowing. In combination, these data provide strong evidence that there is a cause-and-effect relationship between inflammation and depressive symptoms given that subjects did not exhibit depressive symptoms prior to inflammatory exposure and their behavior was significantly changed after administration of an acute or chronic inflammatory challenge. In addition, as discussed in detail below, these studies have provided the foundation for examining the neurotransmitter systems and neurocircuits that mediate the effects of inflammation on the brain and behavior.
C. Inhibition of Inflammation Reduces Depressive Symptoms
To complement the data demonstrating that administration of inflammatory stimuli can cause depressive symptoms, there is an emerging literature to suggest that blocking inflammation can reverse depressive symptoms. These studies again address the cause-and-effect relationship between inflammation and depression or depressive symptoms and provide the proof of concept of using anti-inflammatory drugs to treat patients with depression. The most convincing evidence in this regard is the reduction of depressive symptoms seen in patients with autoimmune and inflammatory disorders administered anticytokine therapies. For example, in a recent meta-analysis of the published literature and a mega-analysis of studies conducted by Janssen and GlaxoSmithKline, strong evidence was provided for the antidepressant efficacy of a variety of anticytokine therapies, with treatments targeting TNF, IL-6, and IL-12/23 showing the most reliable effects (Kappelmann et al., 2018; Wittenberg et al., 2020). Metaregression examining predictors of response revealed that severity of depressive symptoms at baseline was a better predictor of response than improvement in underlying disease activity (Kappelmann et al., 2018). Moreover, in the mega-analysis, results for IL-12/23 remained significant after controlling for physical response to treatment (Wittenberg et al., 2020). These results suggest that the effects of anticytokine therapy on disease activity do not fully account for results. Anticytokine treatments have also been studied in patients with major depression who were otherwise medically healthy (Raison et al., 2013; Salvadore, 2018; McIntyre et al., 2019). These studies have indicated responsiveness to treatment, but only in patients with evidence of high inflammation at baseline, and improvement has been primarily seen in symptoms of anhedonia (Raison et al., 2013; Salvadore, 2018; Lee et al., 2020). Other drugs with anti-inflammatory activity, including nonsteroidal anti-inflammatory drugs, statins, minocycline, omega 3 fatty acids, and others, have also been studied in depression (Kohler-Forsberg et al., 2019). These studies have multiple design issues, and all of these drugs have off-target effects, making it difficult to interpret the results (see discussion of clinical trials below). Indeed, in the largest randomized, controlled trial to date, the nonsteroidal anti-inflammatory drugs celecoxib or minocycline (a microglial stabilizer) both failed to separate from placebo in reducing depressive symptom scores in a heterogeneous sample of patients with depression and bipolar disorder (Husain et al., 2020).
D. Evolution of a Concept: Beyond Depression
1. An Inflammatory Subgroup
There was great initial excitement in the potential role of the immune system in depression. However, the relationship between inflammation and depression is now recognized as much more nuanced than originally appreciated. Although it had been suggested that depression might be an inflammatory disorder, it turns out that only a subgroup of patients with depression exhibit increased inflammation (Raison and Miller, 2011). This conclusion is not surprising given the well known intrinsic heterogeneity as it relates to patients with major depression, as well as the concept of depression. Few studies have directly examined this issue, but the estimate based on a recent meta-analysis of the literature is that approximately 30% of patients with depression exhibit high inflammation (as reflected by a CRP > 3 mg/l) (Osimo et al., 2019). This cutoff for increased inflammation is derived from categories of inflammatory risk for cardiovascular disease as recommended by the American Heart Association and Centers for Disease Control and Prevention (Ridker, 2003). CRP values between 1 and 3 mg/l are considered mild/moderate inflammation (inflammatory risk), whereas <1 mg/l is considered normal. Of note, given the relatively small percentage of patients with depression with high inflammation, it is not surprising that the response to anti-inflammatory drugs in heterogeneous samples of subjects with depression as noted above has been mixed.
A number of factors are associated with inflammation in depression, including obesity, metabolic syndrome, aging, medical disorders and their treatments, childhood trauma, and treatment resistance (Ambrosio et al., 2018; Haroon et al., 2018b; Lacey et al., 2020; Milaneschi et al., 2020; Miller et al., 2008). For example, 45% of patients enrolled in an anticytokine trial for treatment resistant depression exhibited a CRP > 3 mg/l (Raison et al., 2013). Other factors that are emerging as relevant to inflammation in depression and other neuropsychiatric symptoms are the microbiome and chronic infectious diseases including chronic viral illnesses such as cytomegalovirus and post-viral syndromes such as those after coronavirus disease 19 (Simanek et al., 2014; Jiang et al., 2015; Dinan and Cryan, 2019; Marshall, 2020).
2. Inflammation in Other Psychiatric Disorders
Aside from the recognition that only a relatively small percentage of patients with depression exhibit increased inflammation, it is also now appreciated that increased inflammation is not solely the purview of depression but is apparent in multiple other psychiatric disorders. For example, meta-analyses of the literature have demonstrated increased peripheral inflammatory markers including inflammatory cytokines, chemokines, and acute phase reactants in patients with bipolar disorder and schizophrenia, as well as anxiety disorders, PTSD, obsessive compulsive disorder, and personality disorders such as borderline personality disorder (Kahl et al., 2006; Goldsmith et al., 2016; Costello et al., 2019; Yang and Jiang, 2020). Indeed, data for a role of inflammation in schizophrenia and bipolar disorder are similar to those seen in depression (Goldstein et al., 2009; Najjar and Pearlman, 2015). Moreover, suicide has been linked with inflammation, possibly through its association with anhedonia (Ducasse et al., 2020).
3. A Transdiagnostic Pathway to Behavioral Pathology
Such reliable immunologic findings across disorders have challenged the field to think beyond depression and turn to a transdiagnostic phenomenology wherein inflammation plays a role in pathology agnostic to psychiatric diagnosis. Based on the rich literature of the impact of inflammatory mediators on neurotransmitter systems and specific neurocircuits in the brain, it has become increasingly apparent that inflammation is not about any given disorder but is a pathophysiological mechanism that can exist within any given population of subjects with similar consequences on the brain and behavior across disorders.
As detailed below, based on a variety of experimental strategies, inflammation has been shown to increase the reuptake and decrease the synthesis and release of monoamine neurotransmitters while decreasing the reuptake and increasing the release of glutamate (Miller and Raison, 2016). In conjunction with effects on growth factors and synaptic plasticity, the impact of inflammation on neurotransmitter metabolism appears to disrupt neurocircuitry in the basal ganglia, which is involved in motivation as well as motor activity (Fig. 1) (Miller and Raison, 2016). These effects of inflammation on the brain ultimately contribute to symptoms of anhedonia and psychomotor retardation, which characterize mood disorders. These symptoms also manifest themselves in other disorders, including the negative symptoms in schizophrenia or anhedonia in PTSD. Indeed, increased TNF and IL-6 were found to be associated with the deficit syndrome in schizophrenia, and total negative symptoms, blunted affect, and alogia correlated with TNF (Goldsmith et al., 2018). Similar results were found with psychomotor speed in patients with schizophrenia in which interactive effects with diagnosis were found for TNF and other inflammatory markers in association with multiple measures of psychomotor speed, including reduced performance on finger tapping and trail making tasks and the symbol coding test (Goldsmith et al., 2020b). In addition, increased inflammation in patients with PTSD as measured by a composite score of inflammatory markers previously associated with elevated CRP in both plasma and CSF was associated with altered functional connectivity in reward circuitry that in turn was correlated with anhedonia-related subscales from the Beck Depression Inventory (Felger et al., 2020; Mehta et al., 2020).
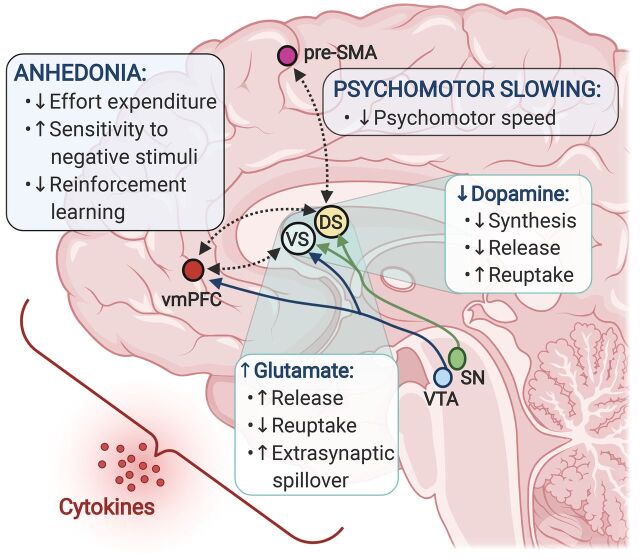
Fig. 1.
Inflammation-induced alterations in dopamine and glutamate converge to affect corticostriatal reward and motor circuitry and drive symptoms of anhedonia and psychomotor retardation. Peripheral innate immune activation and the release of inflammatory cytokines in patients with depression have been associated with elevated glutamate concentrations in basal ganglia regions, as well as decreased dopamine availability and reduced functional connectivity between the ventral and dorsal striatum and reward and motor-related cortical regions, ventromedial prefrontal cortex (vmPFC), and presupplementary motor area (pre-SMA). In turn, inflammation-related changes in both basal ganglia glutamate and corticostriatal connectivity correlated with symptoms of anhedonia and psychomotor retardation and may involve deficits in reward- or goal-directed behaviors such as reward anticipation, effort expenditure, reinforcement learning, and motor control, as well as heightened sensitivity to aversive stimuli. These effects on corticostriatal circuits may be mediated via inflammation-induced decreases in dopamine synthesis and release along with increased reuptake, resulting in overall reduction in dopaminergic signaling. In parallel, inflammatory cytokines promote glutamate release and spillover into the extrasynaptic space while impairing removal of glutamate via reuptake, ultimately contributing to loss of synaptic integrity and circuit dysfunction. DS, dorsal striatum; SN, substantia nigra; VS, ventral striatum; VTA, ventral tegmental area.
Data indicate that inflammation also impacts threat circuitry, including the dorsal anterior cingulate cortex (ACC), amygdala, insula, and hippocampus, which leads to anxiety, arousal, and alarm (Capuron et al., 2005; Miller et al., 2013; Davies et al., 2020). For example, endotoxin has been shown to enhance sensitivity of threat-related regions including the amygdala and dorsal ACC to negative social feedback (Muscatell et al., 2016) while exaggerating activation of the ventral striatum to positive social feedback and images of social support (Inagaki et al., 2015; Muscatell et al., 2016). Moreover, in a recent study, administration of IFN-α in patients with hepatitis C was shown to increase right amygdala reactivity to sad faces, whereas blockade of TNF in patients with inflammatory arthritis resulted in decreased reactivity in this same brain region (Davies et al., 2020). Finally, alterations in functional connectivity within threat circuitry such as the amygdala have been associated with symptoms of anxiety in patients with major depression (Mehta et al., 2018).
Taken together, these data support that inflammatory effects on the brain defy conventional diagnostic boundaries and contribute to common symptom clusters across disorders. As noted above, inflammation has multiple effects on neurocircuitry, including, notably, both reward and threat circuitry. This review will focus on the impact of inflammation on the brain leading to motivational deficits and anhedonia.
III. Mechanisms by which Inflammation Leads to Anhedonia
Regarding the mechanisms by which inflammatory exposure may lead to anhedonia, it is important to recognize that anhedonia is a complex construct. Multiple components of this construct can contribute to anhedonia, including decreases in effort-based motivation for reward, reward anticipation, reinforcement learning, and hedonic capacity (Treadway and Zald, 2011; Treadway et al., 2012; Berridge and Kringelbach, 2015; Cooper et al., 2018). Each of these components may be variably represented in the clinical presentation of anhedonia (Cooper et al., 2018), and as described in detail below, it appears that inflammation has preferential effects on effort-based motivation, reward anticipation, and reinforcement learning while having a lesser impact on the capacity to experience reward (i.e., consummatory reward processes). These findings may be a result of the well characterized effects of inflammation on DA and glutamate (see below) (Treadway and Zald, 2011; Cooper et al., 2018), whereas effects on opioidergic pathways that participate in consummatory reward processes (pleasure systems) in the brain have been less studied in this context (Berridge and Kringelbach, 2015). It should also be noted that within the diagnostic nomenclature of psychiatric disorders, symptoms like anhedonia (along with other psychiatric symptoms) must impair everyday function to rise to the level of clinical (diagnostic) relevance. Thus, inflammation can aid and abet anhedonia by affecting its contributing components but in many instances does not lead to frank dysfunction and disease (e.g., effects of typhoid and influenza vaccination or endotoxin). Whether anhedonia occurs as a clinically relevant symptom in the context of inflammation is a matter of degree (of inflammation) and/or the presence of additional contributing factors related to the regulation of reward processing, including genetic and environmental factors.
A. Impact of Inflammation on Reward-Related Brain Regions and Circuitry
1. Neuroimaging Studies
Much of the causal evidence for the effects of inflammation on neural circuits relevant to anhedonia has derived from studies on patients receiving chronic administration of IFN-α for hepatitis C virus (HCV) or malignant melanoma (Capuron et al., 2012; Dowell et al., 2016) or healthy volunteers exposed to low-dose endotoxin or typhoid vaccination (Fig. 1). For example, early studies using PET with fluorine-18–labeled fluorodeoxyglucose revealed evidence of increased resting-state glucose metabolism in basal ganglia nuclei in subcortical brain regions consistent with changes observed in Parkinson disease (PD) (Juengling et al., 2000; Capuron et al., 2007). Increased metabolism in the left putamen and left nucleus accumbens in turn was associated with anergia and fatigue (Capuron et al., 2007). Increased glucose metabolism in these selected basal ganglia nuclei in PD is believed to be secondary to increased oscillatory burst activity as a result of a loss of DA and an associated decreased postsynaptic dopamine 2 receptor (D2)-mediated inhibition of these brain nuclei (Wichmann and DeLong, 1999). Administration of levodopa has been shown to reduce this increased glucose metabolism in PD (Feigin et al., 2001), and interestingly, levodopa has been successfully used to reverse PD-like symptoms in patients administered IFN-α (Bersano et al., 2008).
Consistent with PET studies indicating reduced DA function as a result of IFN-α administration, functional magnetic resonance imaging has also revealed inflammation-induced alterations of responses in basal ganglia nuclei associated with motivation and motor activity. Four to 6 weeks of IFN-α treatment led to reduced activation of the bilateral ventral striatum in response to reward feedback and associated reward prediction error signaling in patients treated for HCV infection (Capuron et al., 2012). Reduced ventral striatal activation was in turn correlated with self-reported symptoms of reduced motivation. Administration of IFN-α has also been associated with acute alterations in striatal microstructure, which predicted symptoms of fatigue (Dowell et al., 2016).
Findings with IFN-α have been paralleled by functional magnetic resonance imaging studies involving exposure to acute immune challenges to healthy participants (Harrison et al., 2009; Eisenberger et al., 2010). For example, administration of endotoxin to healthy subjects led to blunted activation of the ventral striatal response in anticipation of reward cues in association with depressive symptoms (Eisenberger et al., 2010; Lasselin et al., 2020). Furthermore, endotoxin-induced decreases in ventral striatal activity in anticipation of reward were associated with plasma cytokine responses in female, but not male, healthy participants who completed a monetary reward task (Moieni et al., 2019b). Endotoxin administration has also been associated with decreased motivation for high-effort reward options with no effect on reward sensitivity (Draper et al., 2018). In healthy controls exposed to typhoid vaccination, decreased functional connectivity within mesolimbic circuitry including the nucleus accumbens and subgenual ACC was modulated by IL-6 and associated with vaccination-induced mood changes (Harrison et al., 2009). Increases in IL-6 after typhoid vaccination have also been correlated with slowed reaction times (Brydon et al., 2008), suggesting that cytokine-induced changes in the basal ganglia can contribute to both motivational deficits and psychomotor retardation. Of note, typhoid vaccination has also been shown to decrease ventral striatal encoding of reward prediction error and increase insula encoding of punishment prediction error in a probabilistic instrumental learning task (Harrison et al., 2016). Decreased ventral striatal reward prediction error signaling was also associated with IL-6 after a laboratory stressor in women (Treadway et al., 2017). These latter findings reflect an increasing appreciation of the nuanced effects of inflammation on reward processing in humans and laboratory animals (see below) that includes emerging evidence that inflammation may have a greater effect on effort-based motivation for reward, reward anticipation, reinforcement learning (reward prediction errors), and sensitivity to punishment/loss than consummatory reward processes, although the latter has yet to be fully explored in this context (Felger et al., 2013b; Nunes et al., 2014; Vichaya et al., 2014; Vichaya and Dantzer, 2018; Boyle et al., 2019). Moreover, effects on reward processing may be related to the dose and timing of the inflammatory challenge and reward processing task (in addition to the sex of the participant) (Larson, 2002; Lasselin et al., 2016; Lacourt et al., 2018).
In addition to exogenously administered cytokines and inflammatory challenges, endogenous inflammation has been associated with motivational deficits and alterations in reward circuitry in association with symptoms of anhedonia (Fig. 1). For example, in unmedicated, medically healthy patients with major depression, elevated plasma CRP concentrations were associated with decreased functional connectivity between ventral striatum and ventromedial prefrontal cortex (vmPFC), which in turn correlated with greater severity of anhedonia as assessed by the Snaith Hamilton Pleasure Scale and an anhedonia subscale of the Inventory of Depressive Symptoms Self-Reported (Felger et al., 2016). Additionally, plasma CRP also correlated with decreased functional connectivity between dorsal striatum and vmPFC and presupplementary motor area, which was associated with decreased speed on objective psychomotor tasks. Decreased functional connectivity in reward circuitry in relation to increased CRP has also been described in women exposed to trauma and in rhesus monkeys exposed to an obesogenic diet (Godfrey et al., 2020; Mehta et al., 2020). Taken together, these findings suggest that chronic, low-grade endogenous inflammation impacts mesolimbic reward circuitry and corticostriatal circuitry, involving both motivation and motor output, to impair different aspects of motivated behavior in depression (Fig. 1).
B. Impact of Inflammation on Neurotransmitter Systems Relevant to Reward Processing
Given the impact of inflammation on neurocircuitry relevant to reward processing, there has been interest in examining inflammation’s effects on the neurotransmitter systems that regulate reward circuits in the brain. Based on the anatomy and neurochemistry of the striatum, glutamatergic inputs and DA regulation of outputs of median spiny neurons have garnered considerable attention, especially regarding inflammation effects on motivational deficits and impaired reinforcement learning (Fig. 2).
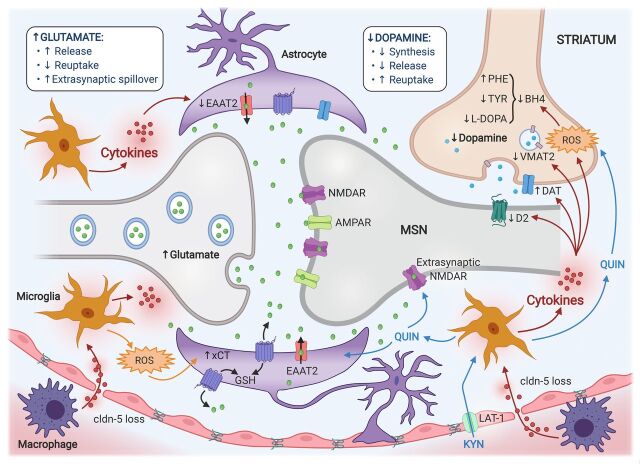
Fig. 2.
Potential mechanisms of inflammation effects on glutamatergic and dopaminergic neurotransmission and synaptic integrity in the microenvironment of the striatal medium spiny neuron (MSN). Glutamate is released into the synaptic cleft, where it binds to its postsynaptic receptors [e.g., NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPAR)] on the MSN, as well as presynaptic and astrocytic receptors. Inflammation and release of cytokines from peripheral immune cells (e.g., macrophages), or those produced in the brain by activated microglia and astrocytes, lead to elevated concentrations of synaptic glutamate by promoting its release and impairing its removal by reuptake mechanisms. This is in part achieved by the actions of cytokines to decrease the number and function of EAAT2. Furthermore, increased transport of KYN to the brain via the LAT-1, subsequent generation of toxic KYN metabolites (e.g., QUIN) by microglia, and downstream generation of reactive oxygen species (ROS) all contribute to additional glutamate release by activated astrocytes via promoting the activity of the xCT and reverse efflux via EAAT2. Finally, immune-induced astrocytic morphologic changes limit the astrocyte’s ability to sequester glutamate within the synapse, resulting in a spillover of the glutamate into the extrasynaptic space and diffusion toward extrasynaptic binding sites (e.g., NMDAR). Inflammation-induced glutamate excitotoxicity and oxidative stress may in turn impair the availability of dopamine, which is released by dopaminergic neurons and binds to D2‐like postsynaptic receptors located on the MSN. Cytokine- and QUIN-induced ROS contribute to oxidation of BH4, a cofactor required for the conversion of phenylalanine (PHE) to tyrosine (TYR) and TYR to L-DOPA, which are necessary precursors for the synthesis of dopamine. Furthermore, inflammatory cytokines may decrease the expression or function of the VMAT2 and/or increase the expression or function of the DAT. Finally, inflammatory cytokines may also decrease dopamine signaling by reducing D2 receptors. These effects of inflammation in the striatal microenvironment can be exacerbated by reduced blood-brain barrier integrity due to a loss of claudin (cldn)-5, allowing greater cytokine entry and leukocyte infiltration. GSH, glutathione.
1. Dopamine
The study of humans and nonhuman primates administered IFN-α has provided strong evidence that the impact of inflammation on reward circuitry and motivational deficits is mediated in part through reductions in DA signaling in the striatum. A series of studies combining pharmacological, neurochemical, and PET neuroimaging have demonstrated that inflammation impairs presynaptic DA function via multiple mechanisms, including reduced synthesis and release as well as increased reuptake. For example, using the radiolabeled DA precursor of levodopa ([18F]fluorodopa), 4 weeks of administration of IFN-α was found to increase uptake and decrease turnover of [18F]fluorodopa in the caudate, putamen, and ventral striatum of patients with HCV (Capuron et al., 2012). An increase in [18F]fluorodopa uptake after IFN-α indicates that inflammation may deplete DA and/or its precursor availability, whereas decreased [18F]fluorodopa turnover suggests impaired packaging/release of newly synthesized DA and/or increased reuptake.
Similar to findings from humans, rhesus monkeys chronically exposed to IFN-α reliably show immune activation and behavioral symptoms such as huddling, a depressive-like equivalent in nonhuman primates (Felger et al., 2007). Among animals that displayed depressive-like huddling behavior after IFN-α, significant reductions in the DA metabolite homovanillic acid (HVA) were found in the CSF, and decreased HVA was correlated with increased time spent huddling (Felger et al., 2007; Felger and Miller, 2012). Moreover, IFN-α led to anhedonia-like behavior in rhesus monkeys as indicated by decreased consumption of sucrose pellets only when effort was required (i.e., as assessed using a puzzle feeder task) but not when the sucrose pellets were freely available (Felger et al., 2015). These results mirror findings of intact reward sensitivity but reduced willingness to expend effort for reward in humans administered influenza vaccine (Boyle et al., 2019).
In vivo microdialysis in rhesus monkeys administered 4 weeks of IFN-α revealed decreased DA release in the striatum in response to both high K+, which leads to a voltage-dependent DA release, and amphetamine, which leads to stimulated DA release and decreased DA reuptake (Felger et al., 2013b). Decreased DA release during in vivo microdialysis was correlated with reduced effort-based motivation in the puzzle feeder task (Felger et al., 2013b). These data are consistent with results from rodents (rats) who also exhibit decreased DA in the ventral striatum (specifically the nucleus accumbens) using in vivo microdialysis after acute intraperitoneal injection of IL-6 (Yohn et al., 2016). Like in the monkeys, decreased DA in these animals was associated with decreased effort-based motivation (in the absence of decreased reward sensitivity), an effect that could be reversed by the stimulant methylphenidate (Yohn et al., 2016). In IFN-α–treated animals, reductions in striatal DA release were restored by local administration of the DA precursor levodopa via reverse microdialysis (Felger et al., 2015). Of note, decreased CSF DA in association with increased CRP has also been described in female rhesus monkeys exposed to an obesogenic diet, and decreased CSF DA was in turn associated with decreased functional connectivity between ventral striatum (nucleus accumbens) and vmPFC (Godfrey et al., 2020). Taken together, these data suggest that inflammation effects on DA availability likely occur via an impact on both the synthesis and release of DA.
DA synthesis depends on the conversion of the amino acid phenylalanine to tyrosine by phenylalanine hydroxylase and the subsequent conversion of tyrosine to L-DOPA by tyrosine hydroxylase (Fig. 2). There is strong evidence that inflammation impairs DA synthesis via decreasing the availability of tetrahydrobiopterin (BH4), an enzyme cofactor required for the activity of both phenylalanine hydroxylase and tyrosine hydroxylase. BH4 is sensitive to oxidative stress and also serves as a cofactor for nitric oxide synthase and can be usurped by the generation of nitric oxide during inflammation (Haroon et al., 2012). Of relevance to these synthetic pathways, patients with HCV treated with IFN-α for 4 weeks displayed increased plasma phenylalanine/tyrosine ratios (Zoller et al., 2012; Felger et al., 2013a), which were in turn associated with greater fatigue severity and lower concentrations of DA and HVA in CSF (Felger et al., 2013a). Furthermore, in IFN-α–treated patients, CSF levels of BH4 negatively correlated with CSF IL-6, whereas CSF concentrations of BH2, a breakdown product of BH4, were increased (Felger et al., 2013a). Low-grade inflammation in a medically healthy elderly population has also been associated with increased phenylalanine concentrations at the expense of tyrosine, and increases in the phenylalanine/tyrosine ratio were associated with greater neurovegetative symptoms, including sleep alterations, sickness, and motor symptoms (Capuron et al., 2011). Moreover, gene signatures in PBMCs of patients with depression with increased inflammation and anhedonia exhibited evidence of low tyrosine metabolism (Bekhbat et al., 2020).
In addition to effects on DA synthesis, inflammation can also target the packaging of DA into vesicles and DA reuptake (Felger and Treadway, 2017). Packaging of newly synthesized DA into synaptic vesicles for release is achieved by the vesicular monoamine transporter 2 (VMAT2). Of note, both IL-1 and TNF have been shown to decrease VMAT2 expression in rat enterochromaffin-like cells (Kazumori et al., 2004). In addition, similar to the well established impact of inflammatory cytokines and downstream p38 mitogen-activated protein kinase (MAPK) signaling pathways in increasing the expression and function of serotonin transporters and mediating, in part, endotoxin-induced depressive-like behavior in laboratory animals (Zhu et al., 2006, 2010), stimulation of MAPK signaling in a human embryonic kidney cell line increased DA transporter (DAT) activity, and inhibition of MAPK was associated with decreased DAT transport capacity in striatal synaptosomes (Moron et al., 2003). Interestingly, neither in vitro activation of MAPK nor in vivo administration of IFN-α to rhesus monkeys was associated with reduced DAT expression (Moron et al., 2003; Felger et al., 2013b), indicating that observed effects have been on the function but not the number of DAT. IFN-α administration to rhesus monkeys has also been associated with reduced D2 receptor binding as measured by PET using the D2 receptor tracer [11C]raclopride, suggesting that postsynaptic receptor changes may also contribute to inflammation effects on DA signaling (Felger et al., 2013b). Finally, it should be noted that there are inconsistencies in the literature. For example, peripheral administration of LPS in mice decreases DAT expression in striatal tissue (Lai et al., 2009), and enhanced stimulated release of DA in the striatum is found after acute endotoxin administration to healthy controls (Petrulli et al., 2017). These data suggest that there may be differences in the effects of acute versus chronic exposure to inflammation as well as differences among species.
2. Glutamate
Alterations in glutamate metabolism have been implicated in mood disorders (Sanacora et al., 2012). Indeed, a number of studies using magnetic resonance spectroscopy have found alterations in glutamate and glutamate metabolite levels in multiple brain regions of patients with depression (bipolar depression in particular) (Castillo et al., 2000; Frye et al., 2007; Hashimoto et al., 2007; Yoon et al., 2009; Yüksel and Öngür, 2010; Xu et al., 2013; Zwanzger et al., 2013). In addition, loss of glial elements including astrocytes and oligodendrocytes as well as excitatory amino acid transporters (EAATs), of which EAAT2 is responsible for 90% of the reuptake and ultimate recycling of glutamate (Kim et al., 2011), is one of the most reliable changes found in postmortem brain tissue from patients with mood disorders (Ongur et al., 1998; Hamidi et al., 2004; Rajkowska and Miguel-Hidalgo, 2007; Sanacora et al., 2012; Sanacora and Banasr, 2013). Probably the most dramatic evidence of the role of glutamate in the psychopathology of depression is the profound and rapid response of treatment-resistant patients with depression to ketamine, an antagonist of the glutamate N-methyl-d-aspartate (NMDA) receptor (Berman et al., 2000; aan het Rot et al., 2010; Duman et al., 2012).
Inflammatory cytokines have been shown to interact with glutamate pathways in several important ways that may contribute to increased extracellular glutamate and glutamate alterations in patients with depression (Haroon et al., 2017). Inflammatory cytokines such as TNF and IL-1β have been shown to decrease the expression of EAAT2 on relevant glial elements (astrocytes and oligodendrocytes) and increase the release of glutamate from astrocytes (Matute et al., 2006; Tilleux and Hermans, 2007; Ida et al., 2008) (Fig. 2). For example, TNF via NF-kB is associated with downregulation of EAAT2 expression on astrocytes and oligodendrocytes and, in excess, is directly toxic to these cells, further compromising glutamate reuptake (Buntinx et al., 2004; Korn et al., 2005; Matute et al., 2006; Li et al., 2008; Olmos and Lladó, 2014). In addition, TNF can lead to calcium elevation and astrocyte glutamate exocytosis through activation of prostaglandin E2 (Bezzi et al., 2001). Inflammation-induced increases in prostaglandin E2 as well as cyclooxygenase (COX)-2 can also lead to reverse efflux of glutamate through EAATs, further increasing synaptic and ultimately extrasynaptic glutamate (Bezzi et al., 2001; Petrelli and Bezzi, 2016). Interestingly, selective inhibition of EAAT2 with dihydrokainate led to reduced reward responses in rats, consistent with an anhedonic phenotype in these animals (Bechtholt-Gompf et al., 2010).
Inflammatory cytokines, including TNF, have also been shown reduce glutamine synthetase, which converts glutamate to glutamine, potentially leading to a buildup of intracellular and extracellular glutamate concentrations, which along with cytokine induction of nitrogen and oxygen free radicals (and oxidative stress), can lead to astrocyte death (Kazazoglou et al., 1996; Buntinx et al., 2004; Matute et al., 2006; Li et al., 2008). Induction of oxidative stress also enhances the function of xCT transporters. xCT transporters are expressed on astrocytes, microglia, and macrophages and exchange intracellular glutamate for extracellular cystine to generate the antioxidant glutathione (Haroon et al., 2017) (Fig. 2). In the process, glutamate is extruded into the extracellular space (Lewerenz et al., 2013). In addition, during immune activation and the release of ATP, binding of ATP to purinergic P2X7 receptors can lead to reverse efflux of glutamate from astrocytes (Haroon et al., 2017).
The confluence of the many effects of inflammation on glutamate can result in increased extrasynaptic glutamate that binds to extrasynaptic NMDA receptors that have been shown to decrease growth factors including brain-derived neurotrophic factor and increase excitotoxicity (Miller et al., 2009; Hardingham and Bading, 2010). Extrasynaptic glutamate also leads to synaptic spillover that can contribute to a loss of spatial precision of synaptic transmission, leading to chaotic, incoherent signaling activity (McCullumsmith and Sanacora, 2015). These effects of inflammatory cytokines on glutamate metabolism, reuptake, and release by astrocytes and oligodendrocytes as well as the fundamental integrity (and survival) of these glial elements provide an intriguing intersection of the inflammation and glutamate hypotheses of mood disorders including depression (Haroon et al., 2017). Indeed, in an animal model of LPS-induced depression, pretreatment with the NMDA antagonist ketamine prevented LPS-induced anhedonia as measured by the sucrose preference test (Walker et al., 2013). Moreover, in an animal model of treatment-resistant depression induced by chronic treatment with adrenocorticotropin, ketamine responsiveness was predicted by baseline elevations of peripheral biomarkers of inflammation including CRP and TNF (Walker et al., 2015).
Given the many mechanisms by which inflammatory mediators can impact glutamate metabolism, studies have examined glutamate in vivo in patients with depression exposed to increased inflammation using magnetic resonance spectroscopy. For example, patients with HCV administered IFN-α exhibited significant increases in glutamate within left basal ganglia and dorsal ACC (Haroon et al., 2014). The observed increases in left basal ganglia glutamate were further associated with reduced motivation, with the greatest effect in older individuals (>55 years) (Haroon et al., 2015). Increased endogenous inflammation (as measured by CRP) in patients with major depression was also correlated with increased left basal ganglia glutamate, which in turn was associated with self-reported symptoms of anhedonia and decreased psychomotor performance (Haroon et al., 2016). Using hierarchical clustering to generate groups with and without combined elevations in CRP and left basal ganglia glutamate (high and low CRP-glutamate groups), patients with high CRP-glutamate were found to exhibit increased anhedonia and psychomotor slowing as well as reductions in regional homogeneity (ReHo) in the left basal ganglia (Haroon et al., 2018a). ReHo is based on the analysis of brain oxygen level–dependent fluctuations in resting-state functional MRI and is an index of local coherence in neuronal activity (Haroon et al., 2018a). Thus, decreased ReHo may reflect the impact of inflammation on extrasynaptic glutamate and its potential to undermine precision synaptic transmission. Interestingly, generation of whole-brain ReHo contrast maps in high versus low CRP-glutamate groups led to 41 regions of interest that were decomposed into four subnetworks, one of which was a predictor of anhedonia and included several regions of interest within canonical reward and salience networks, including the vmPFC and dorsal and ventral striatal regions (Haroon et al., 2018a). Taken together, these data suggest that inflammation-induced changes in glutamate metabolism may have widespread effects on neuronal network integrity that are relevant to motivational deficits and, in combination with alterations in DA, may represent a fundamental mechanism of inflammation-induced behavioral alterations leading to anhedonia (Fig. 2).
IV. Metabolism, Inflammation, and Anhedonia
A growing appreciation for the impact of inflammation on metabolism has contributed to mounting interest in whether immune cell metabolic reprogramming and its relationship with altered systemic glucose and lipid metabolism may be linked to motivational deficits and ultimately anhedonia in depression and other psychiatric and medical disorders. Although the cause-and-effect relationship between cellular and systemic changes in metabolism is complex, it is clear that these processes engage in a feedforward loop in which immune cell activation, metabolic reprograming, and inflammatory cytokine production drive systemic metabolic disturbances including insulin resistance and hyperlipidemia and vice versa (Hotamisligil, 2017; Lercher et al., 2020).
A. Cellular Immunometabolism
Immune cells produce energy (ATP) to maintain their basic cellular functions by breaking down nutrients such as glucose, glutamine, and fatty acids. Depending on the type of cell and its activation status, immune cells use distinct biochemical cascades to accommodate their functional and resultant metabolic demands. Upon activation, innate immune cells such as macrophages and neutrophils rapidly undergo profound shifts in glucose metabolism. These shifts involve movement away from slow, energy-maximizing oxidative phosphorylation (OXPHOS) via mitochondrial respiration, which yields 34 ATP per molecule of glucose, to rapid but energetically inefficient glycolysis, which yields 2 ATP per molecule of glucose (Ganeshan and Chawla, 2014) (Fig. 3). This shift to aerobic glycolysis, referred to as the Warburg effect (also seen in cancer cells), allows limited but precipitous energy production and provides cellular building blocks necessary for rapid cellular proliferation including amino acids, nucleotides, lipids, and NADPH (Pearce and Pearce, 2013). Similar, but more nuanced immunometabolic shifts are required for activation and differentiation of lymphocytes (Fig. 3). Upon activation, naïve lymphocytes display a shift from OXPHOS and fatty acid oxidation (FAO) to a strategy of primarily glycolysis and glutaminolysis and, to some extent, OXPHOS to accommodate their effector/cytotoxic functions (Pearce and Pearce, 2013). Depending on the T-cell subset, this immunometabolic shift requires distinct signaling cascades including the mammalian target of rapamycin (mTOR) and MAPK signaling pathways (Bantug et al., 2018).
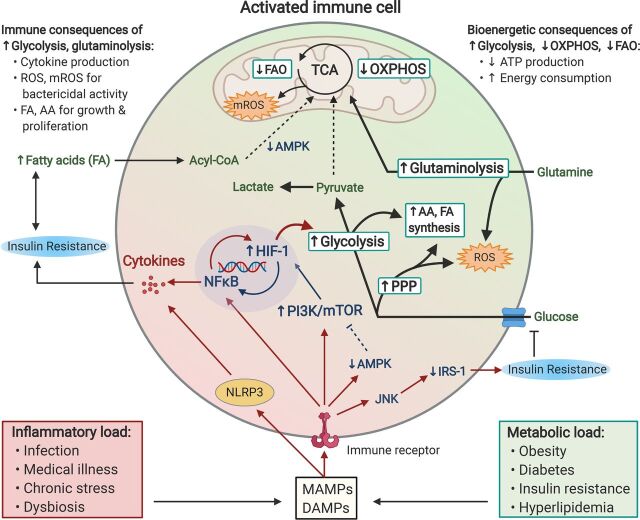
Fig. 3.
Chronic inflammation and metabolic dysfunction propagate pathologic immunometabolism in activated immune cells, leading to immune and bioenergetic consequences that may serve as a biomarker and pharmacological target for anhedonia. Excess inflammatory and metabolic load due to lifestyle and other factors lead to the release of DAMPs and microbe-associated molecular patterns (MAMPs), which bind to various immune receptors (e.g., cytokine, pattern recognition, and T-cell receptors) on immune cells that in turn activate NFκB and the NLRP3 inflammasome. Activated immune cells subsequently undergo rapid shifts in glucose, lipid, and amino acid metabolism that accommodate their cellular functions, including cytokine production, bactericidal activity, cellular growth, and proliferation. Immune cells primarily derive their energy via a two-step process whereby glucose is first metabolized into pyruvate via glycolysis, a reaction which yields 2 ATP. In resting leukocytes, most of the resulting pyruvate is then shuttled into the citric acid cycle (TCA) to enable OXPHOS, which drives the synthesis of up to 34 ATP per glucose molecule. In contrast, activated immune cells shift to aerobic glycolysis, whereby the majority of pyruvate is converted into lactate, thus dramatically reducing ATP generation via OXPHOS. Crucial to this proglycolytic shift is activation of the transcription factor HIF-1, which commits the cell to glycolysis, thus allowing limited but precipitous energy production. HIF-1 activity is driven by both NFκB transcription as well as DAMP/MAMP-induced activation of the PI3K-mTOR axis. Activated immune cells also rely on an ancillary glycolytic cascade, the pentose phosphate pathway (PPP), glutaminolysis, and the TCA cycle to produce reactive oxygen species (ROS) and mitochondrial ROS (mROS), necessary for their bactericidal actions. Intermediates from glycolysis and PPP additionally provide cellular building blocks [amino acid (AA), fatty acid (FA) synthesis] necessary for rapid cell proliferation. DAMPs and MAMPs also suppress the energy sensor AMP-activated protein kinase (AMPK), which promotes fatty acid (Acyl-CoA) transport into the TCA, thus limiting FAO, another major source of ATP. Together, reduced energy production resulting from a shift toward glycolysis and away from OXPHOS and FAO, along with the high energetic cost of chronic low-grade inflammation, creates a bioenergetic demand potentially relevant to anhedonia. This energy demand can be further exacerbated by concurrent metabolic dysfunction such as increased availability of free fatty acids, as well as insulin resistance due to inflammatory signaling via c-Jun N-terminal kinase (JNK) to inhibit insulin receptor substrate-1 (IRS-1) signaling, thus further limiting energy obtained through glycolysis.
Based on the metabolic reprogramming in activated immune cells, there is an increased metabolic/energy demand that may contribute to anhedonia through reorientation of reward processes dependent on effort expenditure (see below) (Treadway et al., 2019a). Consistent with this notion, high anhedonia in patients with depression with increased inflammation (versus low anhedonia in patients with depression with increased inflammation) is characterized by glycolysis-promoting pathways such as phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and HIF-1 signaling as well as insulin signaling and insulin resistance (Bekhbat et al., 2020) (Fig. 3). These pathway alterations, which were specific to patients with depression who exhibited both increased inflammation (CRP > 3 mg/l) and anhedonia, appeared to be represented primarily by monocytes in a transcript of origin analysis (Bekhbat et al., 2020). In addition, gene probes expressed in peripheral blood immune cells involved in metabolic networks, and pathways including HIF-1 and Akt negatively correlated with functional connectivity within reward circuitry in association with anhedonia (Goldsmith et al., 2020a). Gene expression analyses of peripheral blood immune cells also revealed that, in addition to inflammatory pathways, enrichment of genes related to a transcriptional control network involving hepatocyte nuclear factor 4α and other related networks involving glycolysis, gluconeogenesis, and lipid metabolism at baseline predicted subsequent antidepressant response to the TNF antagonist infliximab (Mehta et al., 2013). Infliximab reduced depressive symptoms in patients with treatment-resistant major depression who exhibited high baseline plasma CRP (CRP > 5 mg/l), and anhedonia was the most improved symptom (Raison et al., 2013). Taken together, these data indicate an intriguing relationship between metabolic reprograming toward glycolytic pathways in immune cells and anhedonia in patients with depression, especially those with increased inflammation.
B. Systemic Glucose and Lipid Metabolism
In conjunction with metabolic changes at the immune cell level, excess adiposity and high BMI promote inflammation via increased cytokine production from adipose tissue (Johnson et al., 2012) that in turn reprograms systemic glucose metabolism, contributing to insulin resistance (Ganeshan and Chawla, 2014) and leading to a state of hyperglycemia and dyslipidemia that further fuels inflammation and cytokine release (Shoelson et al., 2006) (Fig. 3). As noted above, fat-, carbohydrate-, and calorie-dense diets and sedentary behavior associated with the Western lifestyle contribute to both inflammation and metabolic disturbance and increase risk for inflammatory and metabolic diseases, cancer, and psychiatric disorders, all of which are associated with symptoms of anhedonia (Capuron et al., 2008; Shelton and Miller, 2010; Berk et al., 2013; Liu et al., 2014).
A significant proportion of patients with major depression with increased inflammatory markers exhibit evidence of metabolic disturbances including dyslipidemia and insulin resistance (Pan et al., 2012). Systemic insulin resistance and impaired glucose metabolism have been associated with anhedonia in patients with metabolic disorders and comorbid depression (Nefs et al., 2012; Hamer et al., 2019; Singh et al., 2019). In addition, recent work from our group showed that correlations between both inflammatory markers (e.g., CRP) and systemic evidence of glucose-related metabolic impairment in patients with depression was associated with lower functional connectivity within reward and motor circuits that were associated with symptoms of anhedonia and motor slowing (Goldsmith et al., 2020a). An interaction between CRP and the metabolic markers revealed that the greatest deficit in functional connectivity in reward circuits was observed in patients with both high inflammation and evidence of systemic metabolic dysfunction (similar to the results reported above regarding evidence of metabolic reprograming in immune cells).
Although depressive symptoms related to reduced motivation and motor activity have been proposed to drive inflammation through decreased voluntary energy expenditures (e.g., exercise) and associated weight gain (Lamers et al., 2018), our recent work involving anti-inflammatory treatment with infliximab suggests a causal role for inflammation in both symptoms of anhedonia (Raison et al., 2013) and metabolic disturbances in depression (Mehta et al., 2013; Bekhbat et al., 2018b). For example, both inflammatory and metabolic biomarkers in plasma were lower 2 weeks postinfliximab in patients with high inflammation at baseline in responders versus nonresponders (Mehta et al., 2013; Bekhbat et al., 2018b), consistent with changes in the inflammatory and metabolic-related genes. These findings suggest that inflammation (especially TNF signaling) and its associated shifts in metabolism within immune cells may synergize with systemic metabolic disturbances to contribute to symptoms of anhedonia. In the context of chronic low-grade inflammation, the energy demands resulting from this glycolytic shift are exacerbated by the actions of inflammatory cytokines, which promote insulin resistance and impaired glucose tolerance, thus additionally limiting energy obtained through glycolysis (Shoelson et al., 2006). Furthermore, as inflammation is an energetically costly process (Straub, 2017), chronic low-grade inflammation seen in depression may represent not only a state of great metabolic need but also a limited energy supply, which may contribute to impaired bioenergetic homeostasis (Treadway et al., 2019a).
In addition to an association between inflammation and both systemic and immunometabolic shifts related to glucose metabolism, altered systemic lipid metabolism may contribute to behavioral symptoms such as anhedonia. Although lipidomic studies in major depression have revealed changes in a wide range of lipid types, such as sphingolipids and phosphatidylcholines (Walther et al., 2018), studies examining both metabolic profiles and inflammation in patients with behavioral disturbances have been scarce. One targeted metabolomics study of IFN-α–treated patients with HCV found no changes in lipids including acylcarnitines, glycerophospholipids, sphingolipids, or sugars but a greater decrease in the branched-chain amino acid isoleucine in patients who developed depression compared with those who did not (Baranyi et al., 2018). With respect to symptoms of anhedonia, higher levels of triglycerides and total and low-density lipoprotein (LDL) cholesterol and lower levels of high-density lipoprotein cholesterol were observed in young adults with versus without anhedonia (Moreira et al., 2019). Moreover, the response to infliximab in patients with treatment-resistant depression (which was characterized by a reduction in anhedonia) was associated with higher baseline lipid biomarkers including total cholesterol, LDL cholesterol, non–high-density lipoprotein cholesterol, triglycerides, and nonesterified fatty acids, and infliximab significantly decreased these lipid markers in patients with a CRP > 5 mg/l (Bekhbat et al., 2018a). Of note, in contrast to these findings, associations between anhedonia and low levels of triglycerides and total or LDL cholesterol have also been reported (Loas et al., 2016; Su et al., 2019b).
Omega-3 polyunsaturated fatty acids (PUFAs) are thought to benefit both behavior and overall health in part through anti-inflammatory actions and are frequently found to be low in patients with major depression and other neuropsychiatric conditions. Evidence suggests that omega-3 PUFA deficiency may specifically be associated with reward deficits such as negative symptoms in schizophrenia (Sethom et al., 2010). Omega-3 PUFA deficiency during development in mice also led to impaired effort expenditure in adulthood, along with alterations in DA-relevant neural circuitry (Ducrocq et al., 2020). Related to immunometabolism, fatty acid oxidation is a primary metabolic strategy in some T-cell subsets (particularly Th17 and memory T cells) that dictates their transformation and activity (Bantug et al., 2018) (Fig. 3). As such, reduced fatty acid oxidation in diabetes has been shown to promote Th17-driven inflammation (Nicholas et al., 2019). Patients with major depression also have been shown to have reduced levels of acetyl l-carnitine, which plays a crucial role in transporting long-chain fatty acids for β oxidation in mitochondria (Nasca et al., 2018). Although these findings collectively suggest that systemic and immune cell changes in lipid metabolism may also contribute to the effects of inflammation on the brain and symptoms of anhedonia, future studies are needed to clarify the extent of this relationship.
C. Kynurenine Pathway Activation
Another metabolic pathway that may mediate the effects of inflammation on the brain and the development of anhedonia is the KP (Schwarcz, 2004; Haroon et al., 2012; Savitz, 2020). Inflammatory cytokines including IFN-γ and TNF as well as peripherally administered inflammatory stimuli including LPS and the bacillus Calmette-Guérin vaccine can activate the enzyme indoleamine 2,3-dioxygenase (IDO), which is expressed primarily in antigen-presenting cells such as macrophages and dendritic cells (O’Connor et al., 2009a; Dai and Zhu, 2010). Activation of IDO in turn leads to the breakdown of tryptophan into kynurenine (KYN), which is transported to the brain through the large neutral amino acid transporter (LAT)-2, where it is converted into neuroactive metabolites including kynurenic acid (KYNA) by astrocytes, oligodendrocytes, and neurons and quinolinic acid (QUIN) by microglia, tissue macrophages, and perivascular monocytes (Walker et al., 2019; Haroon et al., 2020). Although KYNA is an allosteric modulator of the NMDA receptor and can have neuroprotective effects as well as inhibitory effects on DA release (Wu et al., 2007), QUIN is an agonist at the NMDA receptor and can contribute to excitotoxicity, especially if interacting with the extrasynaptic NMDA receptor noted above (Savitz, 2020). QUIN also has been found to stimulate release and decrease reuptake of glutamate from astrocytes (Fig. 2). Based on studies in laboratory animals and humans, peripheral inflammatory mediators drive peripheral blood production of KP metabolites, including KYN, which are then transported to the brain (Raison et al., 2010; Walker et al., 2019; Haroon et al., 2020). A number of studies in patients with cancer or individuals administered IFN-α or endotoxin have demonstrated a relationship between evidence of IDO activation (e.g., Trp or KYN/Trp) and depressive symptoms, especially neurovegetative symptoms (Bonaccorso et al., 2002; Capuron et al., 2002b, 2003; Kruse et al., 2019).
Blockade of IDO in laboratory animals can reverse depressive-like symptoms including anhedonia after administration of LPS or bacillus Calmette-Guérin (O’Connor et al., 2009b; Salazar et al., 2012), and blockade of the transport of KYN into the brain by treatment with leucine, which competes with KYN for LAT-1, can abrogate LPS-induced depressive symptoms (Walker et al., 2019). QUIN plays an important role in these effects. For example, in animals in which the enzymes involved in QUIN synthesis have been knocked out (including kynurenine 3-monooxygenase and 3-hydroxyanthranilic acid dioxygenase knockout mice), reduced depressive symptoms are found after LPS administration (Parrott et al., 2016). In addition, in patients with depression, combined activation of both inflammation (as reflected by TNF) and the KP (as reflected by increased KYN/Trp) was associated with greater symptoms of anhedonia as well as greater depressive symptom severity and evidence of antidepressant treatment resistance (Haroon et al., 2020). Moreover, increased QUIN/KYNA has been associated with volume loss in the striatum and a trend association with anhedonia (Savitz et al., 2015; Savitz, 2020).
V. Access of Inflammatory Molecules to Reward-Related Brain Regions
Previous studies in laboratory animals have described a number of mechanisms by which peripheral inflammatory signals can access the brain (Miller et al., 2009). These mechanisms include 1) saturable transporters for a number of cytokines including TNF and IL-1 (Quan and Banks, 2007); 2) peripheral cytokine stimulation of visceral sensory afferent nerves, which via the nucleus of the solitary tract can activate catecholaminergic projections to brain regions including the hypothalamus and paraventricular nucleus, leading to activation of the hypothalamic-pituitary adrenal axis (Ericsson et al., 1994; Fleshner et al., 1998); 3) access to the brain via fenestrated capillaries in circumventricular organs including the median eminence, where they play a role in fever induction as well as activation of the hypothalamic-pituitary-adrenal axis (Quan and Banks, 2007); 4) release of cytokines by myeloid and lymphoid cells present in the brain meninges into the CSF, where they can then be transported into brain parenchyma via the glymphatic system (Da Mesquita et al., 2018); and 5) trafficking of immune cells to the brain, where they can interact with brain endothelial cells, leading to the release of cytokines and other inflammatory mediators in brain parenchyma (McKim et al., 2018).
Another pathway by which cytokines may access the brain is through disruption of the blood-brain barrier (BBB) as a function of stress and inflammation (Menard et al., 2017). This pathway is of particular relevance to anhedonia in that differential disruption of the BBB appears to be exclusive to brain regions related to motivation, including the nucleus accumbens. In a study using chronic social defeat stress (which also involves wounding and associated inflammation), male mice who were stress-sensitive, as reflected by decreased social interactions post–stress exposure, exhibited significant disruption of the BBB compared with stress-resilient and control mice (Menard et al., 2017). BBB disruption was mediated by a decrease in the expression of claudin 5, a key molecule involved in BBB integrity. As noted above, this effect in stress-sensitive versus stress-resilient and control animals was exclusive in the area of the nucleus accumbens and was associated with increased BBB permeability to IL-6 and was mediated by activation of TNF/NF-kB signaling pathways in endothelial cells (Dudek et al., 2020). Interestingly, similar decreases in claudin 5 expression were also demonstrated in the nucleus accumbens of postmortem brain samples of patients with depression versus control patients, with some evidence that antidepressants may mitigate this effect (Menard et al., 2017; Dudek et al., 2020). These data support a specific association between access of peripheral inflammatory mediators and key brain regions that are involved in motivational processing and anhedonia.
VI. Evolutionary Concepts Related to Anhedonia and the Immune System
A. Conservation of Internal Resources
The relationship between inflammation and anhedonia, especially as it relates to the willingness to expend effort, may be rooted in evolutionary survival priorities involving the conservation of energy resources for fighting infection and healing wounds in an ancestral environment that was rife with pathogens and predators (Raison and Miller, 2013). During infection or wounding, energy expenditures of the immune system can increase up to 30%–60% of the total daily amount (Straub, 2017). This energy demand is necessary to support a robust defensive response to external and/or internal threats and requires a shift away from growth and reproduction as well as metabolically intensive activities associated with goal pursuit, such as foraging, hunting, or physical aggression (Treadway et al., 2019a; Wang et al., 2019). Although modern day threats are not as dramatic or life threatening as those in ancestral times, the chronic inflammatory states driven by obesity, metabolic syndrome, dysbiosis, aging, psychologic stressors, and medical illnesses are equally relevant and can require energy expenditures of 10%–30% of the daily allotment (Straub, 2017). It has been suggested that the energy demands of chronic immune activation secondary to the shift in immunometabolism from oxidative phosphorylation to the relatively inefficient glycolysis (O'Neill et al., 2016) (described above) in conjunction with the inflammation-related systemic metabolic disturbances can in turn have an impact on valuations of future actions (Straub, 2017; Treadway et al., 2019a). Indeed, decision-making processes may incorporate peripheral estimates of the body’s current metabolic capacity to determine what is worth the effort. The metabolic demands of low-grade inflammation and associated alterations in systemic immunometabolism may thus lead to a reduced perceived ability to pursue a reward versus preference for reward (Treadway et al., 2019a).
Consistent with this framework, the mesolimbic DA system is well positioned to act as a central integrator of peripheral signals—including markers of increased inflammatory activity—to guide motivated behavior (Treadway et al., 2019b). The mesolimbic DA system comprises DA neurons located primarily in the ventral tegmental area of the midbrain (Haber, 2003; Haber and Knutson, 2010) that send dense afferents to the ventral striatum, where they can alter the sensitivity of striatal medium spiny neurons to cortical and subcortical glutamatergic afferents (Haber and Knutson, 2010; Berke, 2018) (Fig. 2). Robust evidence in laboratory animals and humans suggests that potentiation or attenuation of ventral striatal DA signaling can dramatically increase or decrease an individual’s willingness to work for rewards (Berridge, 2007; Salamone and Correa, 2012; Berke, 2018; Soder et al., 2020; Westbrook et al., 2020). Critically, midbrain DA neurons express a wide range of receptors for peripheral signaling molecules that convey messages about energy availability and body metabolism, including insulin (Stouffer et al., 2015; Ter Horst et al., 2018), leptin (Krügel et al., 2003; Fulton et al., 2006; Hommel et al., 2006), ghrelin (Symmonds et al., 2010; Sztainert et al., 2018), fatty acids, and steroid hormones (Shafiei et al., 2012), and manipulations of these signals have been found to alter effortful behavior. As such, shifts in cellular immunometabolism and inflammatory mediators, as well as associated disruptions in systemic glucose and lipid metabolism, can impact DA and glutamate signaling within the ventral striatum and reorganize behavioral priorities, especially as it relates to motivation and motor activity, to suit the demands of an activated immune system responding to external and/or internal environmental threats (Treadway et al., 2019a).
VII. Translational Implications: Treatment Targets
Based on the multiple mechanisms that are involved in inflammation and its many downstream effects on the brain, there is a target-rich environment for pharmacological interventions to address the impact of inflammation on anhedonia. Treatments relevant to these targets range from the immune system itself to the neurotransmitter systems affected and the metabolic pathways that may contribute (Table 1). It should be noted that targeting of neurotransmitter systems including DA and glutamate that directly mediate reward processing are more closely linked with the components of anhedonia described above. Nevertheless, given the apparent preferential effects of inflammation on these neurotransmitter systems and related reward circuitry, targeting inflammation more generally as a strategy to treat anhedonia is of utmost relevance to pharmacologic management of motivational deficits in clinical populations (albeit less specific than targeting the neurotransmitters and neurocircuits themselves). In this section, we will therefore first describe treatment considerations that address anhedonia via targeting inflammation itself followed by treatments that target the downstream neurotransmitter system pathways that ultimately mediate inflammation’s effects on reward processing and anhedonia. Finally, we will address the cellular and systemic metabolic pathways that are a cause and consequence of inflammation and may also contribute to inflammation-induced effects on motivation.
TABLE 1.
Pharmacological strategies to mitigate the impact of inflammation on anhedonia
| Immunotherapeutic Strategies |
|---|
| mAbs to cytokines, cytokine receptors, cellular adhesion molecules, and chemokine receptors |
| Inflammatory signaling pathway inhibitors (e.g., TLR4 inhibitors, JAK inhibitors—baricitinib, p38 MAPK inhibitors) |
| Inflammasome blockade (e.g., P2X7 receptor antagonists) |
| COX-2/prostaglandin inhibitors (e.g., celecoxib, aspirin) |
| Inhibitors of microglial activation (e.g., minocycline) |
| Neuroimmunomodulation [e.g., efferent vagal nerve stimulation; γ frequency light (flicker) exposure] |
| Neurotransmitter Strategies |
| Dopamine |
| Synthesis (e.g., sapropterin, folic acid, l-methylfolate, SAMe, levodopa) |
| Release (e.g., amphetamine, methamphetamine, lisdexamfetamine) |
| Reuptake (e.g., bupropion, methylphenidate, modafinil) |
| Agonists (e.g., aripiprazole, brexpiprazole, pramipexole) |
| Glutamate |
| Receptor antagonists (e.g., memantine, ketamine, esketamine, AXS-05) |
| Reuptake enhancers/glutamate stabilizers (e.g., riluzole) |
| Immunometabolic Strategies (Systemic and Cellular) |
| Glycolysis inhibitors (e.g., imatinib, lonidamine, rapamycin, dimethyl fumarate) |
| Antioxidants (e.g., N-acetyl cysteine) |
| Insulin sensitizers (e.g., pioglitazone, metformin) |
| Fatty acid metabolism (e.g., eicosapentaenoic acid, acetyl-l-carnitine) |
| Kynurenine Pathway Strategies |
| IDO inhibitors (e.g., indoximod) |
| Blockade of KYN transport into brain (e.g., leucine) |
mAb, monoclonal antibody.
A. Immune
Unfortunately, there are limited data on the exact immune pathways and cell types that drive inflammation and resultant symptoms such as anhedonia in psychiatric disorders. Indeed, as noted previously, clustering of patients into inflammatory subgroups yielded distinct populations of inflammation associated with myeloid cells in one case and lymphoid populations in the other (Lynall et al., 2020). Thus, much work needs to be done to further characterize the inflammatory substrate of behavioral changes, and until then, we will focus on the obvious immune targets and drugs associated with those targets.
1. Cytokine Inhibitors/Targeted Therapies
Cytokine inhibitors have provided proof of concept regarding the cytokine hypothesis of depression. As noted, a host of cytokine inhibitors have demonstrated efficacy regarding depressive symptoms in patients with autoimmune and inflammatory disorders (Kappelmann et al., 2018), and at least three studies have suggested some efficacy of anticytokine treatment in patients with depression who were otherwise medically healthy—in particular, those with evidence of increased inflammation at baseline (Raison et al., 2013; Salvadore, 2018; McIntyre et al., 2019).
Much of the data comes from patients treated with anti-TNF drugs (especially infliximab), consistent with the repeated demonstration of activation of TNF and its signaling pathways as a substrate for inflammation and its effects on the brain in psychiatric disorders. Anti–IL-1 and anti–IL-6 drugs have also shown efficacy in reducing depressive symptom severity in patients with autoimmune and inflammatory disorders (Kappelmann et al., 2018), and the anti–IL-6 drug sirukumab led to improvement in symptoms of anhedonia in otherwise healthy individuals with depression with increased inflammation (CRP > 3 mg/l) (Salvadore, 2018). Of note, studies in laboratory animals have indicated that cytokine antagonists, which are relatively large molecules, do not have to cross the BBB to be effective. Indeed, administration of infliximab to rats exposed to chronic mild stress blocked the development of depressive and anxiety-like behavior including anhedonia (Karson et al., 2013).
Also relevant to their efficacy in reducing depressive symptoms in autoimmune and inflammatory disorders, there is a vast array of other anticytokine therapies that selectively target T-cell cytokines including anti–IL-17 and anti–IL-12/23, which drives TH1 polarization (Griffiths et al., 2017; Wittenberg et al., 2020), as well as drugs that target downstream cytokine signaling pathways, such as baricitinib, which inhibits Janus kinase (JAK) 1 and JAK2 (Szollosi et al., 2018), and multiple drugs that inhibit p38 MAPK (Lee and Kim, 2017). Other relevant drugs in development include those that inhibit TLR-4 signaling and inhibitors of cell adhesion molecules and chemokine receptors, of which two drugs are Federal Drug Administration (FDA)-approved, including maraviroc, which inhibits C-C chemokine receptor 5 for prevention of human immunodeficiency virus, and plerixafor, an antagonist to C-X-C chemokine receptor 4 used to mobilize stem cells (Szollosi et al., 2018). In addition, drugs that can inhibit inflammasome activation via the P2X7 receptor are also being explored. Indeed, a P2X7 receptor antagonist is currently being tested in patients with depression with an incomplete response to a monoaminergic antidepressant and a CRP ≥ 1 mg/l (NCT04116606).
2. Cyclooxygenase Inhibitors
Significant attention has been paid to the selective COX-2 inhibitor celecoxib. The COX isozymes play integral roles in a number of physiologic functions, although the inducible COX-2 is most closely tied to inflammation and the immune response. Based on meta-analyses of the literature, celecoxib as either an add-on or monotherapy in individuals with depression/depressive symptoms has demonstrated an overall beneficial effect versus placebo for symptom reduction (Köhler et al., 2014; Köhler-Forsberg et al., 2019). Aspirin has shown similar efficacy, but for both drugs, sample sizes in studies have been relatively small, results have not been consistent, and the largest study to date found no efficacy for celecoxib versus placebo in patients with depression (Husain et al., 2020). Moreover, in several large population-based studies, including a double-blind, randomized, placebo-controlled trial in older adults, no protective effect against incident depression was found for aspirin (including low dose) or other COX inhibitors (Glaus et al., 2015; Veronese et al., 2018; Molero et al., 2019; Berk et al., 2020), although there is evidence in patients with cancer for some protection against depression with long-term, low-dose use of aspirin but not other COX inhibitors (Hu et al., 2020). Nevertheless, as discussed below, there are concerns regarding the design of these studies and the off-target effects of these drugs, including effects on the cadherin superfamily, a group of cell surface receptors that play a role in neuronal connections and interactions (Manabe et al., 2000). In addition, there is evidence in laboratory animals and humans that anti-inflammatory drugs including the COX inhibitors aspirin, ibuprofen, and naproxen can inhibit antidepressant responses to SSRIs, whose efficacy may be dependent in part on the local release of inflammatory cytokines (Warner-Schmidt et al., 2011; Raison et al., 2013). These data suggest that administration of anti-inflammatory drugs to patients with depression without inflammation may undermine response. Of note, one promising recent study with celecoxib identified responders post hoc as those who had increased TSPO binding at baseline, possibly suggestive of microglial activation (Attwells et al., 2020). Although interesting, however, this trial was open-label, and increased TSPO binding may reflect central nervous system (CNS) activation aside from neuroinflammation that could predispose to placebo responsiveness.
3. Minocycline
Minocycline, the second-generation tetracycline antibiotic, has also received considerable attention. Beyond its antimicrobial capabilities, numerous preclinical studies have demonstrated that minocycline possesses anti-inflammatory, antiapoptotic, neuroprotective, and anticancer effects (Soczynska et al., 2012; Garrido-Mesa et al., 2013). More specifically, preclinical studies have shown that minocycline selectively inhibits microglial activation (Kobayashi et al., 2013), with subsequent reduction in inflammatory biomarkers/cytokines including IL-1β, IL-6, TNF, and DAMPs (i.e., high-mobility group box 1 and netrin-1) (Du et al., 2019). It is purported that, through reductions in high-mobility group box 1 release, this microglial inhibition results in inhibition of KP activation and subsequent reduction in depressive-like behaviors in mouse chronic-stress models (Wang et al., 2018; Wang et al., 2020). Clinically, minocycline has generated mixed results in treatment of patients with depression, and a recent meta-analysis of the limited controlled trials available suggested benefit over placebo (Rosenblat and McIntyre, 2018). Nevertheless, the largest trial to date, failed to find that minocycline separated from placebo (Husain et al., 2020). Of note, however, one trial reported that higher blood IL-6 concentrations prior to treatment were predictive of a response to minocycline (Savitz et al., 2018). Unfortunately, without a reliable neuroimaging biomarker of microglial activation, it will remain difficult to interpret any effects of minocycline, especially given its effects on the microbiome.
4. Electroceuticals and Other Novel Neuroimmunomodulation Strategies
One innovative area of increasing interest is the use of neuromodulation strategies, such as targeting/stimulating the vagus nerve (vagal efferents) to reduce peripheral inflammation (Pavlov et al., 2020). For example, an elegant series of studies demonstrated that acetylcholine released after stimulation of vagal efferents can bind to the α7 subunit of nicotinic acetylcholine receptors to inhibit NF-kB and inflammatory signaling including the release of TNF from relevant immune cells such as macrophages (Tracey, 2002). Vagal nerve stimulation also leads to the generation of T cells that are capable of producing acetylcholine (Rosas-Ballina et al., 2011). This anti-inflammatory cholinergic reflex can be activated by electrically stimulating the vagus nerve, a treatment whose novel bioelectric platform has recently received designation as an FDA breakthrough device for the treatment of rheumatoid arthritis in patients nonresponsive to biologic drugs (Pavlov et al., 2020). Note that, although vagal nerve stimulation as currently used for depression has been reported to have immune effects (Perrin and Pariante, 2020), it is not designed to provide stimulation of efferent vagal nerve fibers, as in the device developed by Tracey and colleagues (Pavlov et al., 2020). Nevertheless, transcutaneous vagal nerve stimulation may increase efferent vagal output and has been shown to exhibit anti-inflammatory effects (Bremner et al., 2020). Other nonconvulsive neurostimulation used for depression (e.g., repetitive transcranial magnetic stimulation, transcranial direct current stimulation, and deep brain stimulation) have also been shown to have immunologic effects; however, there have been few studies, and sham-controlled comparator designs are required (Perrin and Pariante, 2020).
Another novel neuroimmunomodulation strategy is exposure to flickering lights, which in turn drive neural activity that can influence microglial activity. For example, exposure of mice to lights flickering at 40 Hz drives γ-frequency neural activity that in turn is associated with activation of NF-kB and MAPK pathways, as well as cytokine production (Garza et al., 2020). By adjusting the frequency of the flicker, the relative impact on microglial responses can be manipulated, leading to potential regulation of neuroinflammatory responses.
5. Other Agents
There are other drugs targeting the immune system in clinical trials, including phosphodiesterase inhibitors (pentoxifylline, NCT01625845) as well as various supplements, but these do not hold much promise to change the landscape of the other more exciting immunotherapies available or in development.
B. Neurotransmitter
Based on the studies described above, there is sound rationale for targeting the downstream effects of inflammation on relevant neurotransmitter systems, including DA and glutamate, which regulate fundamental gating processes in the ventral striatum that ultimately play pivotal roles in reward processing and motivation (Fig. 2).
1. Dopamine
Inflammation appears to affect all aspects of DA signaling, including DA synthesis, packaging and release, and reuptake, as well as postsynaptic modifications in DA receptor binding and expression.
a. Dopamine synthesis
BH4 is a pivotal cofactor for the enzymes that synthesize DA, and inflammation reduces BH4 availability through oxidation and excessive conversion to BH2 during generation of nitric oxide by nitric oxide synthase. Therefore, support of BH4 directly by sapropterin, a synthetic form of BH4, or through supplementation with folic acid, l-methyl folate, or S-adenosylmethionine (SAMe), which support the conversion of BH2 to BH4, are relevant strategies to reverse the effects of inflammation on DA synthesis (Stahl, 2007). Both l-methylfolate and SAMe have been studied in depression with limited efficacy, but the confounds in clinical trial designs described below make interpretation of the results difficult (Papakostas et al., 2010, 2012, 2014; Mischoulon et al., 2014; Sarris et al., 2018). However, in one study, post hoc analysis revealed that elevated baseline CRP, TNF, IL-6, and IL-8 along with BMI predicted improved responses to l-methylfolate in patients with depression with an inadequate response to conventional antidepressant treatment (Shelton et al., 2015).
Another strategy to address impaired DA synthesis is to provide the immediate precursor to DA, levodopa, as was done successfully in nonhuman primates to reverse IFN-α–induced impairments in DA release (Felger et al., 2015). Levodopa (in combination with carbidopa) is currently being examined in this regard (examining reward circuitry in patients with depression with low and high levels of peripheral inflammation before and after levodopa/carbidopa; NCT02513485). Of note, data from an aged and frail population with depression, which might be expected to exhibit increased inflammation, have shown a positive antidepressant response to levodopa/carbidopa administration (Rutherford et al., 2019).
b. Dopamine release
There are a number of medications that induce DA release, including amphetamine, methamphetamine, and lisdexamfetamine. Although there may be nuance in the specificity and exact mechanism of these drugs [including the additional release of norepinephrine (NE)], in general these monoamine releasing agents are substrates for VMAT2, causing inhibition of vesicular monoamine uptake and release of prestored vesicular monoamines (Partilla et al., 2006; Lizarraga et al., 2015), as well as inhibiting synaptic transporter function through trace amine-associated receptor 1-mediated mechanism (Xie et al., 2008; Xie and Miller, 2009; Rutigliano et al., 2018).
There is currently not convincing evidence to recommend these medications as an augmentation strategy or monotherapy for treatment of major depression (Pary et al., 2015; Giacobbe et al., 2018). However, their efficacy in depression has not been examined within the context of inflammation, and given impairment in DA release, these drugs should be a consideration. Indeed, it is worthwhile to note that despite several failed trials in depression (Richards et al., 2016), there have been positive trials that demonstrate efficacy for use of lisdexamfetamine as an SSRI augmentation strategy in mild depression with persistent cognitive dysfunction (Madhoo et al., 2014) and residual symptoms in moderate depression (Trivedi et al., 2013).
c. Dopamine reuptake
DAergic synaptic transmission can be enhanced through inhibition or reversal of synaptic reuptake. The DAT plays a key role in synaptic clearance of DA for purposes of vesicular sequestration/repackaging through VMAT2 in neurons or degradation via monoamine oxidase activity in glial cells (Meiser et al., 2013). Evidence also suggests that, particularly in the prefrontal cortex, the NE transporter (NET) may play a role in DA reuptake (Carboni et al., 1990). Targeting DAT (and to some extent NET) would thus putatively function to increase synaptic availability of DA for postsynaptic action.
A number of available medications, including bupropion and several members of the psychostimulant class, demonstrate DA reuptake inhibition. Bupropion, an FDA-approved medication for treatment of unipolar depression, functions primarily as a DAT and NET inhibitor. Bupropion has been shown to increase high-effort activity in rats (Randall et al., 2014), and clinical trials have demonstrated its efficacy as monotherapy for depression on par with other standard monotherapies such as SSRIs and SNRIs (Clayton et al., 2006), as well as an augmentation strategy for residual symptoms (Patel et al., 2016). Although evidence is limited, small trials have suggested a preferential response of symptoms of anhedonia to bupropion treatment (Tomarken et al., 2004; Thase et al., 2006). Moreover, as noted above, addition of bupropion to an SSRI led to an increased antidepressant response in patients with increased inflammation (CRP ≥ 1 mg/l), and a prospective clinical trial is underway comparing bupropion to escitalopram in patients with depression with CRP ≥ 2 mg/l (NCT04352101, see below).
In addition to bupropion, several psychostimulants with mechanistic DAT inhibition are available including methylphenidate and modafinil. As noted above, methylphenidate has been shown to abrogate the effects of IL-6 on effort-based motivation (Yohn et al., 2016). However, in randomized trials in depression, augmentation with methylphenidate has not demonstrated a statistical benefit over standard therapy alone (Patkar et al., 2006; Michelson et al., 2007; Ravindran et al., 2008; Pary et al., 2015). Methylphenidate has demonstrated some efficacy in treating cancer-related fatigue, which has been associated with increased inflammatory markers in multiple studies (Xiao et al., 2016, 2017; Rojí and Centeno, 2017; Bower, 2019). Clinical trials of modafinil have also demonstrated reduction in fatigue (Fava et al., 2005), although there is no clear consensus regarding relief of depression (Dunlop et al., 2007).
d. Dopamine agonists
DAergic receptors are treatment targets for numerous conditions including schizophrenia and Parkinson disease, in which DAergic signaling is pathologically disrupted. In this context, utilization of direct DA receptor agonists as augmenting strategies for management of depression have received attention. Included among these medications are several of the atypical antipsychotics as well as antiparkinsonian drugs that act as DA receptor agonists.
The body of evidence in support of atypical antipsychotics is larger than that for any other augmentation strategy in major depression. Meta-analyses of performed trials have identified that these agents are more effective than placebo for both response and remission (Nelson and Papakostas, 2009; Zhou et al., 2015), and two agents with DA receptor agonist activity—aripiprazole (Berman et al., 2007, 2009; Marcus et al., 2008; Fava et al., 2012) and brexpiprazole (Al Shirawi et al., 2017)—have received FDA approval for this indication. Although the typical antipsychotics are recognized as exerting their effect primarily through antagonism of the D2 receptor, newer-generation antipsychotics appear to have activity at multiple receptor sites. Both aripiprazole and brexpiprazole, for example, function as D2/3 partial agonists. As partial agonists, these medications can submaximally enhance DAergic signaling when inadequate amounts of endogenous ligand are present or alternatively prevent overstimulation when excess amounts of endogenous ligand are present. In the context of inflammation, in which presynaptic dopamine synthesis and release are impaired, the former function may be most relevant, although studies have yet to examine this possibility.
The antiparkinsonian agents, including pramipexole (nonergoline D2/3/4 agonist), ropinirole (D3 agonist), and bromocriptine (ergoline D2 agonist, serotonin receptor agonist), have also received attention in the literature for augmentation/treatment of treatment-resistant depression. Several studies have demonstrated efficacy for pramipexole in reducing depressive symptoms in patients with Parkinson disease (Lemke et al., 2006) and patients with major depression, with noted differential benefit for anhedonia and motor functioning (Corrigan et al., 2000; Cusin et al., 2013; Franco-Chaves et al., 2013). Pramipexole has also shown efficacy at higher doses (up to 4 mg/day) in patients with treatment resistance (Fawcett et al., 2016), an effect that has been hypothesized to be related to the increased inflammation in this population in conjunction with inflammation effects on DA (Escalona and Fawcett, 2017). Ropinirole, however, failed to separate from placebo in the treatment of patients with unipolar and bipolar depression (Gershon et al., 2019), despite prior studies indicating positive effects on depressive symptoms when comorbid with restless leg syndrome (Benes et al., 2011). Older studies reported some efficacy of bromocriptine equivalent to the antidepressant imipramine for treatment of depression (Bouras and Bridges, 1982); however, there is limited support for its use currently (Hori and Kunugi, 2013).
It should be noted that DA also has direct effects on immune responses, the nature of which is beyond the scope of this review. DA receptors are expressed on virtually every immune cell subpopulation, and DA has effects on multiple immune cell functions, including activation, proliferation, cellular adhesion, chemotaxis, and cytokine production, both centrally and peripherally (Sarkar et al., 2010; Matt and Gaskill, 2020). Immune cells such as T cells and myeloid cells also express all of the enzymatic machinery to produce and secrete DA (Matt and Gaskill, 2020). DA via the D2 receptor and its downstream signaling pathways has anti-inflammatory effects (Shao et al., 2013), and DA via D1 receptor signaling has been shown to reduce systemic and local inflammation through inhibition of the NLRP3 inflammasome and the release of nitric oxide (Färber et al., 2005; Yan et al., 2015). Thus, any drug that increases the availability of DA, including all of the DA-active agents described above, has the potential to affect the impact of inflammation on the brain through direct effects on the immune system. It should be noted, however, that many of the findings noted above have been generated in laboratory animal models, and the impact of DAergic agents at the doses used in clinical practice remains unclear. For example, although levodopa administration inhibited ex vivo mitogen-induced proliferation and dose-dependent stimulation of TNF in PBMCs (Bessler et al., 1999), peripheral indicators of systemic inflammation appeared to have no relationship to levodopa dose (Hassin-Baer et al., 2011; Andican et al., 2012). Moreover, bupropion augmentation for treatment nonresponders was not accompanied by changes in peripheral blood cytokine concentrations (i.e., soluble IL-2 receptor, IL-8, and TNF), despite improvement in depressive symptoms (Eller et al., 2009).
2. Glutamate
Given the potential role of glutamate in mood disorders, especially as reflected by the dramatic effects of the NMDA receptor antagonist ketamine on depressive symptoms (McGirr et al., 2015), there have been a number of drugs developed and in development for depression that target glutamate receptors, including not only NMDA receptors but also α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and metabotropic glutamate receptors (Wilkinson and Sanacora, 2019). Aside from ketamine, the best studied and most efficacious of these is the recently FDA-approved intranasal esketamine for augmentation of standard oral antidepressant therapy in management of major depression and depression with suicidal ideation/behavior (Fu et al., 2020; Ionescu et al., 2020). Esketamine, the (S)-enantiomer of ketamine, functions as an NMDA receptor antagonist and DAT inhibitor. Like its racemic mixture, and unlike standard antidepressant treatments, the antidepressant effect observed is rapid and robust in responders, with measurable responses within 24 hours of administration. Although limited work has been done regarding the immune system with esketamine, the response to ketamine has been tied to increased inflammatory markers prior to treatment in humans and in a laboratory animal model of antidepressant treatment resistance (Walker et al., 2015; Yang et al., 2015). These data support the notion that blockade of NMDA receptors might be a mechanism to reverse inflammation-induced increases in glutamate. In contrast to the success of ketamine and esketamine, other glutamate receptor modulators have not performed well in depression. For example, memantine, an NMDA antagonist that reduces cognitive decline in elderly patients with Alzheimer disease, has failed to show efficacy in depression in multiple studies (Kishi et al., 2017). In addition, adjunctive lanicemine (an NMDA channel blocker) did not separate from placebo in patients with depression over 12 weeks (Sanacora et al., 2017), and despite initial promise, the glutamate modulating agent GLYX-13 (rapastinel), which also acts as an NMDA receptor modulator, failed to separate from placebo in phase 3 trials in depression. There are a multitude of other drugs that modulate glutamate receptors in development, the review of which is beyond the scope of this manuscript [see (Wilkinson and Sanacora 2019)]. One compound of interest, however, is AXS-05, which is a combination of the NMDA receptor antagonists dextromethorphan and bupropion. Although bupropion is primarily being used to increase dextromethorphan blood levels through cytochrome P450 2D6 inhibition, bupropion nevertheless can inhibit the DAT, leading to an intriguing medication that can address both glutamate and DA pathologies seen in the context of inflammation. Phase 2 results appeared promising, with significant improvements in response and remission in depression at 6 weeks compared with bupropion alone, leading to FDA Breakthrough Therapy designation (Anderson et al., 2019). Nevertheless, as noted below, whether AXS-05 or other glutamate receptor modulators have special activity in patients with increased inflammation has not been tested.
Stabilization of glutamate is another mechanism of action that can mitigate the impact of inflammation on glutamate metabolism. Especially relevant in this regard is riluzole, which has been shown to decrease presynaptic glutamate release and enhance EAAT activity, thereby facilitating astrocytic glutamate reuptake and ultimately preventing extracellular accumulation and extrasynaptic spillover (Fumagalli et al., 2008; Banasr et al., 2010). Riluzole has also been shown to reverse anhedonia-like behavior in rats exposed to chronic, unpredictable stress (Banasr et al., 2010). Given these findings, a number of studies have sought to investigate riluzole in patients with depression. One small, open-label study found benefit in treatment-resistant patients with depression over the course of 6 weeks with riluzole monotherapy (Zarate et al., 2004), and another small trial similarly showed benefit with riluzole as an augmenting agent (Sanacora et al., 2007). In a study of maintenance treatment after ketamine infusion, riluzole demonstrated no statistically significant benefit over placebo, although the riluzole group did appear to have a longer time to relapse (17.2 days versus 9.8 days) (Ibrahim et al., 2012). Given the small size of these studies, it is difficult to draw conclusions; however, the results warrant further investigation for the utility of this medication class, especially in the context of inflammation.
C. Glucose and Lipid Metabolism
1. Glucose Metabolism
As the metabolic shift to aerobic glycolysis is a hallmark of cancer and a critical step for inflammatory activation of immune cells, opportunities to divert cellular metabolism from glycolysis and its end products, such as lactate, back to mitochondrial oxidation have been extensively explored. Components of the glycolytic cascade that have been frequently targeted are glucose transporters, as well as the glycolytic enzymes hexokinase (HK), 6-phosphofructokinase, pyruvate kinase, lactate dehydrogenase, and monocarboxylate transporters (Fortunato et al., 2018). The majority of compounds that inhibit glycolysis have been tested in cell lines or animal models, with the exception of a few agents currently approved for use in humans (Pelicano et al., 2006; Zhao et al., 2013).
Several clinically approved medications for cancer and autoimmune disorders are demonstrated to have antiglycolytic and/or pro-OXPHOS properties, which may primarily contribute to their therapeutic actions. For example, imatinib is an approved treatment of cancer that specifically inhibits tyrosine kinases, including the oncogene BCR-ABL, subsequently causing a decrease in activity of HK and glucose-6-phosphate dehydrogenase, enzymes that promote glycolysis and its ancillary pentose phosphate pathway. These actions decrease glycolytic activity and promote mitochondrial respiration (Gottschalk et al., 2004) while also decreasing inflammatory gene expression, including TNF, in a mouse model of nonalcoholic fatty liver disease (AlAsfoor et al., 2018). Other drugs such as lonidamine, which inhibits mitochondrially bound HK; AZD3965, a monocarboxylate transporter inhibitor; and several Akt inhibitors targeting the PI3K-Akt-mTOR pathway are currently in various stages of clinical trials for cancer and are being considered for the treatment of autoimmune disorders including rheumatoid arthritis (de Lartigue, 2017; Song et al., 2019). Of note, the mTORC1 inhibitor rapamycin has recently shown the capacity to prolong the effects of ketamine, which as noted previously, targets glutamate pathways, and its efficacy is greater in individuals with increased inflammation as well as its downstream effects on KP metabolites (Moaddel et al., 2018; Abdallah et al., 2020). First-line treatment with dimethyl fumarate in patients with multiple sclerosis has been shown to inhibit Warburg-like metabolism in immune cells via inhibiting the enzyme glyceraldehyde 3-phosphate dehydrogenase, which occupies a central position in the glycolytic pathway (Kornberg et al., 2018). Consistent with known differential reliance of immune cell subsets on aerobic glycolysis, dimethyl fumarate inhibited production of inflammatory cytokines and lactate in activated macrophages, Th1 and Th17 cells, while sparing the function of resting macrophages or regulatory T cells, suggesting that nuanced immunomodulation via metabolic targets may be possible in vivo. With respect to behavioral symptoms and anhedonia, promotion of OXPHOS and mitochondrial antioxidant defense systems is believed to contribute to ketamine’s antidepressant effect (Weckmann et al., 2014, 2017), and gene expression signatures related to mitochondrial respiration have been associated with long-term response to lithium in patients with bipolar disorder (Stacey et al., 2018).
Another agent that has received attention is N-acetyl cysteine (NAC). NAC as a precursor to the antioxidant glutathione reduces oxidative stress (reactive oxygen species) that can result from cellular immunometabolic shifts and inflammation and can improve mitochondrial function (Wright et al., 2015; Mocelin et al., 2019). NAC also has antidepressant effects on glutamate signaling through effects on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and the astroglial glutamate exchanger xCT (Linck et al., 2012; Nasca et al., 2017). Thus, NAC has been tested in depression with evidence of some benefit in a meta-analysis that includes a relatively small number of studies (five) and 291 subjects who received NAC. Clinical trials to treat depression using NAC are ongoing, including one trial with an inclusion criterion of a CRP > 0.85 mg/l (NCT02972398).
Insulin-sensitizing diabetes treatments that address systemic alterations in glucose metabolism and have been tested for antidepressant effects include thiazolidinediones (e.g., pioglitazone), which stimulate peroxisome proliferator–activated receptor-γ, and metformin, which promotes 5′-adenosine monophosphate–activated protein kinase, to lower circulating glucose and fatty acids. A meta-analysis in depression found that although metformin did not improve depressive symptoms, thiazolidinediones exerted an antidepressant effect in female patients only (Moulton et al., 2018).
2. Fatty Acids
Promotion of cellular fatty acid oxidation is another mechanism to address alterations in cellular immunometabolism to alleviate depressive symptoms. For example, blockade of carnitine palmitoyl transferase 1, which promotes the transport of acyl-carnitine for mitochondrial β oxidation, with etomoxir substantially reduced stress-induced anhedonia-like behavior in rats (Morkholt et al., 2017). In addition, systemic dietary supplementation with acetyl-l-carnitine induced both a rapid and enduring antidepressant effect in a rodent model of depressive behavior (Nasca et al., 2018), and supplementation with omega-3 PUFAs, particularly eicosapentaenoic acid (EPA), has demonstrated antidepressant efficacy, as indicated by several meta-analyses in major depression (Mocking et al., 2016; Liao et al., 2019). The antidepressant effects of PUFAs are thought to be mediated, at least in part, by effects on immune cells and reduced inflammation (Rapaport et al., 2016). As such, whereas patients with HCV who developed depression after IFN-α treatment had an “at risk” genotype for the phospholipase A2 gene in association with lower circulating EPA levels (Su et al., 2010), treatment with EPA prevented IFN-α–induced depression (Su et al., 2014). The enzyme phospholipase A2 regulates the balance between metabolism of fatty acid precursors into arachidonic acid, which promotes inflammation, or the anti-inflammatory omega-3 fatty acids. Elevated ratios of arachidonic acid to EPA and DHA have also been found to predict IFN-α–induced depression (Lotrich et al., 2013). Furthermore, among patients with depression who received 8 weeks of treatment with EPA, those with high CRP and IL-1ra at baseline displayed a greater antidepressant response (Rapaport et al., 2016).
These studies suggest that promoting fatty acid oxidation and increasing the availability of PUFAs as well as acetyl-l-carnitine may be fruitful strategies for targeting the immunometabolic mechanisms that sustain inflammation and contribute to anhedonia.
D. Kynurenine Metabolism
1. Indoleamine 2,3-Dioxygenase Inhibitors
IDO converts Trp, the primary precursor for serotonin, to KYN. Given the importance of serotonin in T-cell activation, there has been considerable interest in developing IDO inhibitors as a pharmacologic strategy to enhance T-cell function as a component of chemotherapeutic and other approaches to treating cancer (Prendergast et al., 2017). Several IDO inhibitors, including indoximod (1-methyl-d-tryptophan), have been developed for this purpose and are in clinical trials (Vacchelli et al., 2014). With the focus on oncology, there are currently no trials of an IDO inhibitor in any psychiatric disorder. In addition to IDO, other enzymes, including kynurenine monooxygenase and kynurenine aminotransferase, which maintain the relative balance between QUIN and KYNA, can be considered targets of treatment. Relative to this strategy, however, administration of a KYNA analog prodrug 4-Cl-kynurenine (4-Cl-KYN), which converts to the NMDAR glycine site antagonist 7-Cl-KYNA, failed to show efficacy in treatment-resistant depression (Park et al., 2020). Challenges in this area include defining the relevant target populations, related in part to which KP metabolite markers and their cutoff values and which inflammatory markers might help enrich future studies with subjects who are likely to respond, while also providing opportunities to establish target engagement (see below). Combinations of the peripheral blood KYN/Trp ratio and TNF have been used in this fashion, yielding a population of patients with depression with increased anhedonia and treatment resistance (Haroon et al., 2020).
2. Leucine
Leucine competes with KYN for LAT-1 and thus can inhibit transport of KYN into the brain, where it is converted into the neuroactive metabolites KYNA and QUIN (Haroon et al., 2020). Based on preclinical studies in laboratory animals (Walker et al., 2019), treatment with high-dose leucine (8 mg/d) for 2 weeks is currently being tested in patients with major depression, although inflammatory markers that have been associated with KP metabolites in the periphery and CNS will be examined only in post hoc analyses (with no a priori enrichment of the sample for peripheral blood inflammatory markers and/or KP metabolite concentrations) (NCT03079297).
E. Gut Microbiome
Although the majority of therapeutic focus remains on small-molecule and biologic drugs, the past several years have seen increased interest in evaluating the gut-brain axis as a mediator of psychiatric pathophysiology. The native microbiome and issues related to microbial dysbiosis have been implicated in a variety of salient neuropsychiatric conditions (Barbosa and Vieira-Coelho, 2020); however, the exact mechanism(s) by which the microbiome may impact brain function is unclear. Studies have repeatedly demonstrated the importance of microbiota in neurodevelopment (Warner, 2019) and have linked microbiome-produced bioactive metabolites to epigenetic changes and the host immune response/inflammatory state (Rooks and Garrett, 2016), each of which may have a putative role in mediating inflammation-induced behavioral changes. The relation to psychiatric illness has been underscored by a number of studies, such as the induction of depressive-like behavior in mice after fecal microbiota transplant (FMT) from donors with major depression (Zheng et al., 2016) and the significant improvements in reported depressive and anxious symptoms after FMT among patients with functional gastrointestinal disorders, with the related finding of a negative correlation between microbiota diversity and depression severity (Kurokawa et al., 2018). These findings have led to a number of trials examining prebiotics (dietary fibers that promote the health of the native microbiota) and probiotics (which are live microorganisms cultured for dietary supplementation) as interventions, although results have been modest, showing a small but significant improvement of symptoms with use of probiotics (Liu et al., 2019; Sanada et al., 2020). Given the relative importance of the gut-brain axis and the evidence suggesting its role in inflammation, continued research for more directed therapies such as FMT or predeveloped microbial ecosystems is warranted.
F. Future Drug Development
Although clinical neuroimaging, postmortem tissue analysis, and preclinical animal models provide insight into the impact of inflammation on the brain, there are limits inherent to these approaches. For example, there are human-specific aspects of neurodevelopment (Letinic and Rakic, 2001; Rakic, 2009; Clowry et al., 2010; Zeng et al., 2012; Hansen et al., 2013; Paredes et al., 2016; Hodge et al., 2019) that limit the generalizability and/or scope of studies undertaken with animal model systems and the feasibility of studying direct cellular and molecular correlates of imaging findings, making the approach to understanding pathophysiology necessarily coarse-grained. In recent years, however, induced pluripotent stem cell technology has emerged as a promising solution for preclinical neuropsychiatric disease modeling. Rapid progression in the field has led to development of methods for generating specific CNS cell types, including DAergic neurons (Swistowski et al., 2010), excitatory glutamatergic neurons (Li et al., 2009; Mariani et al., 2012), microglia (Abud et al., 2017), oligodendrocytes (Wang et al., 2013), and astrocytes (Santos et al., 2017). In addition, these approaches have been used to produce three-dimensional model systems that resemble subregions of the human brain (Eiraku et al., 2008; Mariani et al., 2015; Muguruma et al., 2015; Qian et al., 2016) and are capable of recapitulating features of human cortical development (Lancaster et al., 2013), providing previously inaccessible opportunities to model brain development/brain function in vitro. This method has already been applied to the study of schizophrenia (Stachowiak et al., 2017; Kathuria et al., 2020), and active research is expanding to conditions with more neurodiversity, including depression (Grossert et al., 2019) and anxiety (Soliman et al., 2017). Relevant to inflammation, studies in induced pluripotent stem cell–derived astrocytes have demonstrated a robust inflammatory response to IL-1β (Thelin et al., 2020), suggesting that these in vitro model systems can reveal how individual cell types respond to a neuroinflammatory milieu and how these responses may differ across patient populations. Thus, such a platform may be especially informative in isolating the various influences of specific inflammatory molecules and their downstream effects on cellular metabolism on relevant neurotransmitter systems including DA and glutamate.
VIII. Anti-Inflammatory Trial Failures
A. Shortcomings of Clinical Trials Using Anti-Inflammatory Drugs to Treat Depression
There are multiple targets and treatments that are relevant to pharmacological strategies to mitigate the impact of inflammation on the brain. A number of clinical trials have been conducted using drugs with putative anti-inflammatory properties to treat patients with psychiatric disorders or symptoms, many of which have been described above. These studies have primarily focused on patients with depression and schizophrenia (Xiang et al., 2017; Zheng et al., 2017; Deakin et al., 2018). Meta-analyses of this literature, including anticytokine therapies, COX-2 inhibitors, minocycline, statins, pioglitazone, and omega 3 PUFAs, have reported modest efficacy with some of these agents (Xiang et al., 2017; Zheng et al., 2017; Kohler-Forsberg et al., 2019; Liao et al., 2019; Cai et al., 2020), although the results of this literature is difficult, if not impossible, to interpret for reasons including the drugs used, the study designs, and the outcome variables (Table 2). Disregarding the numerous trials that have been conducted on patients with autoimmune and inflammatory disorders, in which results are confounded by the impact of anti-inflammatory treatment on the underlying disease process, there are relatively few interpretable studies, leaving the field bereft of data upon which to build the foundation for the ultimate translational and clinical impact of therapies that target inflammation and/or its downstream effects on the brain. In the case of COX-2 inhibitors and minocycline, for example, by far the largest trial to date failed to show any separation between patients with depression who received celecoxib or minocycline versus those who received placebo (Husain et al., 2020). This trial exemplifies all of the complications that have plagued clinical trials in the field, and if not addressed, there is significant risk that a potential therapeutic strategy for a disabling symptom (anhedonia) may be lost.
TABLE 2.
Problems and solutions for clinical trial failures of therapies targeting the impact of inflammation on the brain
| Methodologic Issues | |
|---|---|
| Sample | |
| Problem | Heterogenous populations with a limited percentage of patients with high inflammation |
| Solution | Enrich populations for high and/or low inflammation with a priori defined inflammation status at baseline |
| Design | |
| Problem | Randomized, double-blind, placebo-controlled clinical trial with no stratification |
| Solution | Match/mismatch design with patients stratified by inflammation status—drug targeting inflammation should only show efficacy in high-inflammation group Randomized, double-blind, placebo-controlled trial with stratification on inflammation or conducted solely on patients with high inflammation |
| Target engagement | |
| Problem | No measure of inflammatory or brain endpoint to establish drug effect on the target |
| Solution | Predetermined inflammatory markers in brain or periphery that establish that the drug has engaged the target |
| Outcome variables | |
| Problem | Overall symptom severity |
| Solution | Outcome variables tied to the known biologic effects of inflammation on behavior (e.g., anhedonia, psychomotor slowing, anxiety) |
1. Drugs
Aside from anticytokine treatments, which have selective effects on specific cytokines, all of the drugs that have been examined to date have an array of off-target effects. For example, celecoxib has effects on glucocorticoid receptor function and cadherin 11, an adhesion molecule involved in anxiety and fear-related responses as well as synaptic plasticity (Manabe et al., 2000; Hu et al., 2005). Minocycline is an antibiotic and therefore has a significant impact on the gut microbiome, which has been associated with depression and other behavioral states (Jiang et al., 2015; Dinan and Cryan, 2019). Statins and antidiabetic drugs have significant effects on metabolism of lipids and glucose, including insulin sensitivity, which can interact directly with neurons irrespective of any effects of inflammation (Stouffer et al., 2015; Ter Horst et al., 2018). Thus, although all of the drugs used in previous trials have putative anti-inflammatory effects, they also have multiple off-target effects, and based on current study designs, it is impossible to know which mechanism of action is leading to behavioral change.
2. Study Designs
Given that only a small percentage of depressed or other psychiatric patients in any given sample exhibit increased inflammation (Osimo et al., 2019), it makes little sense to conduct a trial of an anti-inflammatory drug in a heterogeneous population of psychiatric patients. Any drug response should be highly suspect because of off-target effects and the limited number of patients that are available to respond. Of note, only two published studies have enriched for increased inflammation (both using anticytokine therapies) (McIntyre et al., 2019; Salvadore, 2018). Most studies have used baseline inflammatory markers to predict treatment response in post hoc fashion. Even if post hoc, proof of concept has been established by mapping baseline inflammatory measures with subsequent treatment response. This has been successfully achieved in several studies in depression in which, for example, higher baseline plasma IL-6 concentrations predicted response to minocycline, higher baseline plasma CRP predicted response to infliximab and omega 3 PUFAs, and higher baseline microglial activation (as measured by PET using a TSPO ligand) predicted response to celecoxib (Rapaport et al., 2016; Savitz et al., 2018; Attwells et al., 2020). Multiple studies have also examined baseline inflammatory markers in a post hoc manner to predict response to conventional antidepressant therapies (targeting monoamines). For example, increased peripheral blood inflammatory markers such as CRP and mRNA for IL-1β, TNF, and migration inhibitory factor predicted treatment nonresponse to SSRIs and SNRIs, and in two studies, peripheral blood CRP predicted a differential response to the SSRI escitalopram versus bupropion and nortriptyline (Cattaneo et al., 2013; Uher et al., 2014; Strawbridge et al., 2015; Cattaneo et al., 2016; Jha et al., 2017; Liu et al., 2020). In these latter two studies, increased inflammation predicted a poor response to escitalopram and a better response to the these DAergic and NEergic drugs (Uher et al., 2014; Jha et al., 2017). Nevertheless, no trial to date has stratified patients as a function of baseline inflammatory status and determined whether those with high inflammation respond and those without do not. This match/mismatch design is the preferred design strategy for trials testing the efficacy of anti-inflammatory drugs, with enriching for high inflammation being a reasonable alternative. For example, an ongoing trial comparing bupropion versus escitalopram is underway in patients with high inflammation only, with the hypothesis that the DAergic drug bupropion will increase functional connectivity in reward circuitry along with improving motivational deficits compared with escitalopram (NCT04352101).
Given the opportunity to measure inflammatory markers as a function of treatment, it is also surprising that few studies have measured whether in fact the anti-inflammatory drug being tested is impacting inflammation. Indeed, in the failed celecoxib/minocycline trial noted above, no effect of celecoxib or minocycline was found on peripheral measures of inflammation, which does not instill confidence in the interpretation of a negative result (Husain et al., 2020). Peripheral and/or central markers (e.g., TSPO binding) of inflammation are an essential element of an interpretable trial. If there is no evidence that there is inflammatory target engagement (or engagement of downstream targets), the trial should be considered a failed trial.
3. Outcomes
Aside from measuring the impact of the selected drug on the pathway of interest (e.g., inflammation), outcome variables should be derived from what is known about the circuits and behaviors that are reproducibly affected by inflammation. In terms of behaviors, as indicated in this review, anhedonia as well as psychomotor retardation have been reliably associated with inflammation in terms of both the response to pro- and anti-inflammatory stimuli. In addition, reward circuitry has been identified as the mediator between endogenous inflammatory markers such as CRP and anhedonia and psychomotor speed (Felger et al., 2016). Thus, neuroimaging biomarkers such as functional connectivity within reward or motor circuitry can serve as relevant targets in the brain. A similar case can be made for threat circuitry as it relates to symptoms of anxiety, which have also been associated with inflammation (Davies et al., 2020). The challenge, however, is that the FDA and other regulatory bodies do not currently recognize individual symptom domains as appropriate for drug development, making the pharmaceutical approval process challenging if not impossible. Currently, only the major psychiatric disorders as defined in the DSM are recognized with some appreciation for treatment resistance in this context. Indeed, “the entire biomedical machinery for mental disorders (is) organized around DSM categories” (Cuthbert, 2015). Future efforts will clearly require advocacy for a broader appreciation of the subdomains of symptoms that cut across disorders and have a similar, well defined pathophysiological basis. This strategy has achieved great success in oncology, in which the FDA recognizes drugs for multiple cancer types that are labeled as “targeted therapies,” defined as drugs that target specific genes and proteins that are involved in the growth and survival of cancer cells (Baudino, 2015). Such an agnostic approach to diagnosis along with a greater appreciation of the emerging understanding of the genes and proteins that underlie the biologic bases of behaviors is what is needed in psychiatry and the approval of drugs that treat psychiatric disorders.
IX. Summary
An extensive amount of data has been amassed on the effects of inflammation on the brain. Attempts to curate, integrate and synthesize the extant literature in this review has required selecting and choosing data that build hypotheses that can be tested and hopefully translated to clinical practice. As noted, given the many mechanisms identified, there is a vast array of opportunities for drug development. Nevertheless, the current approach to drug approval in psychiatry by the various regulatory agencies including the FDA have yet to recognize and embrace patients who experience symptoms clusters that are the consequence of specific pathophysiologic processes. Only DSM diagnoses are recognized as relevant targets for approved treatments. This situation is despite the fact that treatment with anticytokine therapy for example, has shown efficacy for anhedonia in patients with evidence of increased inflammation, with a much lesser effect on overall depression severity. Nevertheless, the future of drug development in psychiatry lies with the identification of biologically defined subgroups with specific pathophysiologies and related symptoms that warrant targeted treatments. Until that time comes, we must understand that treatment trials targeting inflammation and/or its downstream targets on the brain in heterogeneous populations of patients with depression or other psychiatric diagnoses will generate data that will be difficult if not impossible to interpret.
Abbreviations
- ACC
anterior cingulate cortex
- Akt
protein kinase B
- BBB
blood-brain barrier
- BH4
tetrahydrobiopterin
- BMI
body mass index
- CNS
central nervous system
- CRP
C-reactive protein
- COX
cyclooxygenase
- CSF
cerebrospinal fluid
- D2
dopamine 2 receptor
- DA
dopamine
- DAMP
danger-associated molecular pattern
- DAT
DA transporter
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- EAAT
excitatory amino acid transporter
- EPA
eicosapentaenoic acid
- FAO
fatty acid oxidation
- FDA
Federal Drug Administration
- FMT
fecal microbiota transplant
- HCV
hepatitis C virus
- HIF-1
hypoxia-inducible factor-1
- HK
hexokinase
- HVA
homovanillic acid
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- JAK
Janus kinase
- KP
kynurenine pathway
- KYN
kynurenine
- KYNA
kynurenic acid
- LAT
large neutral amino acid transporter
- LDL
low-density lipoprotein
- L-DOPA
l-3,4-dihydroxyphenylalanine
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- NAC
N-acetyl cysteine
- NE
norepinephrine
- NET
NE transporter
- NF-kB
nuclear factor κB
- NLRP3
NOD-, LRR-, and pyrin domain–containing protein
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- OXPHOS
oxidative phosphorylation
- PBMC
peripheral blood mononuclear cell
- PD
Parkinson disease
- PET
positron emission tomography
- PI3K
phosphoinositide 3-kinase
- PTSD
post-traumatic stress disorder
- PUFA
polyunsaturated fatty acid
- QUIN
quinolinic acid
- ReHo
regional homogeneity
- SAMe
S-adenosylmethionine
- SNRI
serotonin norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
- Th
T helper
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TSPO
translocator protein
- VMAT2
vesicular monoamine transporter 2
- vmPFC
ventromedial prefrontal cortex
- xCT
cystine‐glutamate exchanger.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Lucido, Bekhbat, Goldsmith, Treadway, Haroon, Felger, Miller.
Footnotes
Research reported in this publication was supported by National Institutes of Health National Institute of Mental Health [R61MH121625, R01MH109637, R01MH108605, R21MH119622, R01MH107033, R01MH112076, K23MH114037, F32MH119750, R25MH101079] and National Center for Advancing Translational Sciences [UL1TR002378]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
M.T. is a paid consultant of Blackthorn Therapeutics and Avanir Pharmaceuticals. A.H.M. is a paid consultant of Behringer Ingelheim. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. The other authors have nothing to disclose.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiat 67:139–145. [DOI] [PubMed] [Google Scholar]
- Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, Sherif M, Ahn KH, D'Souza DC, Formica R, Southwick SM, Duman RS, Sanacora G, Krystal JH (2020) Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 45:990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abud EMRamirez RNMartinez ESHealy LMNguyen CHHNewman SAYeromin AVScarfone VMMarsh SEFimbres C, et al. (2017) iPSC-derived human microglia-like cells to study neurological diseases. Neuron 94:278–293.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Shirawi MI, Edgar NE, Kennedy SH (2017) Brexpiprazole in the treatment of major depressive disorder. Clin Med Ther 9:1–10. [Google Scholar]
- AlAsfoor S, Rohm TV, Bosch AJT, Dervos T, Calabrese D, Matter MS, Weber A, Cavelti-Weder C (2018) Imatinib reduces non-alcoholic fatty liver disease in obese mice by targeting inflammatory and lipogenic pathways in macrophages and liver. Sci Rep 8:15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrósio G, Kaufmann FN, Manosso L, Platt N, Ghisleni G, Rodrigues ALS, Rieger DK, Kaster MP (2018) Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 91:132–141. [DOI] [PubMed] [Google Scholar]
- Anderson A, Iosifescu DV, Jacobson M, Jones A, Kennon K, O’Gorman C, Stahl SM, Tabuteau H (2019) Efficacy and safety of AXS-05, an oral NMDA receptor antagonist with multimodal activity, in major depressive disorder: results of a phase 2, double-blind, active-controlled trial. W43, in ASCP Annual Meeting; 2019 May 29; Scottsdale, AZ; pp 28–31. [Google Scholar]
- Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiına S, Burcak G(2012) Plasma oxidative and inflammatory markers in patients with idiopathic parkinson’s disease. Acta Neurol Belg 112:155–9. [DOI] [PubMed] [Google Scholar]
- Arteaga-Henríquez G, Simon MS, Burger B, Weidinger E, Wijkhuijs A, Arolt V, Birkenhager TK, Musil R, Müller N, Drexhage HA (2019) Low-grade inflammation as a predictor of antidepressant and anti-inflammatory therapy response in MDD patients: a systematic review of the literature in combination with an analysis of experimental data collected in the EU-MOODINFLAME consortium. Front Psychiatry 10:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwells SSetiawan ERusjan PMXu CHutton CRafiei DVarughese BKahn AKish SJVasdev N, et al. (2020) Translocator protein distribution volume predicts reduction of symptoms during open-label trial of celecoxib in major depressive disorder. Biol Psychiatry 88:649–656. [DOI] [PubMed] [Google Scholar]
- Banasr M, Chowdhury GM, Terwilliger R, Newton SS, Duman RS, Behar KL, Sanacora G (2010) Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate-modulating drug riluzole. Mol Psychiatry 15:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantug GR, Galluzzi L, Kroemer G, Hess C (2018) The spectrum of T cell metabolism in health and disease. Nat Rev Immunol 18:19–34. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Meinitzer A, Rothenhäusler HB, Amouzadeh-Ghadikolai O, Lewinski DV, Breitenecker RJ, Herrmann M (2018) Metabolomics approach in the investigation of depression biomarkers in pharmacologically induced immune-related depression. PLoS One 13:e0208238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa RSD, Vieira-Coelho MA (2020) Probiotics and prebiotics: focus on psychiatric disorders - a systematic review. Nutr Rev 78:437–450. [DOI] [PubMed] [Google Scholar]
- Baudino TA (2015) Targeted cancer therapy: the next generation of cancer treatment. Curr Drug Discov Technol 12:3–20. [DOI] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Walther HV, Adams MA, CarlezonWA, Jr. , Ongur D, Cohen BM (2010) Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35:2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Chu K, Le NA, Woolwine BJ, Haroon E, Miller AH, Felger JC (2018a) Glucose and lipid-related biomarkers and the antidepressant response to infliximab in patients with treatment-resistant depression. Psychoneuroendocrinology 98:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, Felger JC (2020) Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high CRP and anhedonia. Brain Behav Immun 88:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes H, Mattern W, Peglau I, Dreykluft T, Bergmann L, Hansen C, Kohnen R, Banik N, Schoen SW, Hornyak M (2011) Ropinirole improves depressive symptoms and restless legs syndrome severity in RLS patients: a multicentre, randomized, placebo-controlled study. J Neurol 258:1046–1054. [DOI] [PubMed] [Google Scholar]
- Berk MWilliams LJJacka FNO’Neil APasco JAMoylan SAllen NBStuart ALHayley ACByrne ML, et al. (2013) So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk MWoods RLNelson MRShah RCReid CMStorey EFitzgerald SLockery JEWolfe RMohebbi M, et al. (2020) Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. JAMA Psychiatry 77:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018) What does dopamine mean? Nat Neurosci 21:787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Berman RMFava MThase METrivedi MHSwanink RMcQuade RDCarson WHAdson DTaylor LHazel J, et al. (2009) Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14:197–206. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:843–853. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191:391–431. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure systems in the brain. Neuron 86:646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersano A, Aghemo A, Rumi MG, Ballabio E, Candelise L, Colombo M (2008) Recovery after L-DOPA treatment in peginterferon and ribavirin induced parkinsonism. Eur J Intern Med 19:370–371. [DOI] [PubMed] [Google Scholar]
- Bessler H, Djaldetti R, Salman H, Bergman M, Djaldetti M(1999) IL-1β, IL-2, IL-6 AND TNF-α production by peripheral blood mononuclear cells from patients with parkinson's disease. Biomedicine & Pharmacotherapy 53:141–5. [DOI] [PubMed] [Google Scholar]
- Bezzi PDomercq MBrambilla LGalli RSchols DDe Clercq EVescovi ABagetta GKollias GMeldolesi J, et al. (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4:702–710. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M (2002) Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol 22:86–90. [DOI] [PubMed] [Google Scholar]
- Bouras N, Bridges PK (1982) Bromocriptine in depression. Curr Med Res Opin 8:150–153. [DOI] [PubMed] [Google Scholar]
- Bower JE (2019) The role of neuro-immune interactions in cancer-related fatigue: biobehavioral risk factors and mechanisms. Cancer 125:353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CC, Kuhlman KR, Dooley LN, Haydon MD, Robles TF, Ang YS, Pizzagalli DA, Bower JE (2019) Inflammation and dimensions of reward processing following exposure to the influenza vaccine. Psychoneuroendocrinology 102:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, Jung H, Shandhi MH, Beckwith J, Herring I, Rapaport MH, Murrah N, Driggers E, Ko Y-A, Alkhalaf ML, Soudan M, Song J, Ku BS, Shallenberger L, Hankus AN, Nye JA, Park J, Vaccarino V, Shah AJ, Inan OT, Pearce BD (2020) Transcutaneous vagal nerve stimulation blocks stress-induced activation of Interleukin-6 and interferon-γ in posttraumatic stress disorder: a double-blind, randomized, sham-controlled trial. Brain Behav Immun Health 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD (2008) Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry 63:1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Ameloot M (2004) Cytokine-induced cell death in human oligodendroglial cell lines: I. synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res 76:834–845. [DOI] [PubMed] [Google Scholar]
- Cai DB, Zheng W, Zhang QE, Ng CH, Ungvari GS, Huang X, Xiang YT (2020) Minocycline for depressive symptoms: a meta-analysis of randomized, double-blinded, placebo-controlled trials. Psychiatr Q 91:451–461. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH (2009) Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord 119:181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH (2002a) Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26:643–652. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH (2004) Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56:819–824. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH (2003) Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry 54:906–914. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH (2005) Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry 58:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH (2007) Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology 32:2384–2392. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH (2012) Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R (2002b) Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 7:468–473. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D (2011) Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 70:175–182. [DOI] [PubMed] [Google Scholar]
- Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V (2008) Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol Psychiatry 64:896–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G (1990) Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem 55:1067–1070. [DOI] [PubMed] [Google Scholar]
- Castillo M, Kwock L, Courvoisie H, Hooper SR (2000) Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. AJNR Am J Neuroradiol 21:832–838. [PMC free article] [PubMed] [Google Scholar]
- Cattaneo AFerrari CTurner LMariani NEnache DHastings CKose MLombardo GMcLaughlin APNettis MA, et al. ; Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium (2020) Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl Psychiatry 10:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Ferrari C, Uher R, Bocchio-Chiavetto L, Riva MA, Pariante CM; MRC ImmunoPsychiatry Consortium (2016) Absolute measurements of macrophage migration inhibitory factor and interleukin-1-β mRNA levels accurately predict treatment response in depressed patients. Int J Neuropsychopharmacol 19:pyw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM (2013) Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 38:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SRCavanagh Jde Boer PMondelli VJones DNCDrevets WCCowen PJHarrison NAPointon LPariante CM, et al. (2019) Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 214:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RA, Huang TL, Huang KW, Hung YY (2017) TNFAIP3 mRNA level is associated with psychological anxiety in major depressive disorder. Neuroimmunomodulation 24:271–275. [DOI] [PubMed] [Google Scholar]
- Christ A, Latz E (2019) The Western lifestyle has lasting effects on metaflammation. Nat Rev Immunol 19:267–268. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Croft HA, Horrigan JP, Wightman DS, Krishen A, Richard NE, Modell JG (2006) Bupropion extended release compared with escitalopram: effects on sexual functioning and antidepressant efficacy in 2 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 67:736–746. [DOI] [PubMed] [Google Scholar]
- Clowry G, Molnár Z, Rakic P (2010) Renewed focus on the developing human neocortex. J Anat 217:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA, Arulpragasam AR, Treadway MT (2018) Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci 22:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000) Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depress Anxiety 11:58–65. [DOI] [PubMed] [Google Scholar]
- Costello H, Gould RL, Abrol E, Howard R (2019) Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open 9:e027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusin C, Iovieno N, Iosifescu DV, Nierenberg AA, Fava M, Rush AJ, Perlis RH (2013) A randomized, double-blind, placebo-controlled trial of pramipexole augmentation in treatment-resistant major depressive disorder. J Clin Psychiatry 74:e636–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN (2015) Research Domain Criteria: toward future psychiatric nosologies. Dialogues Clin Neurosci 17:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S, Fu Z, Kipnis J (2018) The meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhu BT (2010) Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem 58:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KA, Cooper E, Voon V, Tibble J, Cercignani M, Harrison NA (2020) Interferon and anti-TNF therapies differentially modulate amygdala reactivity which predicts associated bidirectional changes in depressive symptoms. Mol Psychiatry [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue J (2017) Hallmark tumor metabolism becomes a validated therapeutic target. J Community Support Oncol 15:e47–e52. [Google Scholar]
- Deakin BSuckling JBarnes TREByrne KChaudhry IBDazzan PDrake RJGiordano AHusain NJones PB, et al. ; BeneMin Study team (2018) The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 5:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF (2019) Gut microbes and depression: still waiting for Godot. Brain Behav Immun 79:1–2. [DOI] [PubMed] [Google Scholar]
- Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, Harrison NA (2016) Acute changes in striatal microstructure predict the development of interferon-alpha induced fatigue. Biol Psychiatry 79:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. [DOI] [PubMed] [Google Scholar]
- Draper A, Koch RM, van der Meer JW, Aj Apps M, Pickkers P, Husain M, van der Schaaf ME (2018) Effort but not reward sensitivity is altered by acute sickness induced by experimental endotoxemia in humans. Neuropsychopharmacology 43:1107–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du B, Li H, Zheng H, Fan C, Liang M, Lian Y, Wei Z, Zhang Y, Bi X (2019) Minocycline ameliorates depressive-like behavior and demyelination induced by transient global cerebral ischemia by inhibiting microglial activation. Front Pharmacol 10:1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducasse D, Dubois J, Jaussent I, Azorin JM, Etain B, Gard S, Henry C, Bougerol T, Kahn JP, Aubin V, Bellivier F, Belzeaux R, Dubertret C, Dubreucq J, Llorca PM, Loftus J, Passerieux C, Polosan M, Samalin L, Leboyer M, Yrondi A, Bennabi D, Haffen E, Maruani J, Allauze E, Camus V, D'Amato T, Doumy O, Holtzmann J, Lançon C, Moliere F, Moirand R, Richieri RM, Horn M, Schmitt L, Stephan F, Genty JB, Vaiva G, Walter M, El-Hage W, Aouizerate B, Olié E, Courtet P (2020) Association between anhedonia and suicidal events in patients with mood disorders: a 3-year prospective study. Depress Anxiety 38:17–27. [DOI] [PubMed] [Google Scholar]
- Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, Olie E, Courtet P (2018) Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress Anxiety 35:382–392. [DOI] [PubMed] [Google Scholar]
- Ducrocq FWalle RContini AOummadi ACaraballo Bvan der Veldt SBoyer MLAby FTolentino-Cortez THelbling JC, et al. (2020) Causal Link between n-3 polyunsaturated fatty acid deficiency and motivation deficits. Cell Metab 31:755–772.e7. [DOI] [PubMed] [Google Scholar]
- Dudek KADion-Albert LLebel MLeClair KLabrecque STuck EFerrer Perez CGolden SATamminga CTurecki G, et al. (2020) Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci USA 117:3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G (2012) Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Crits-Christoph P, Evans DL, Hirschowitz J, Solvason HB, Rickels K, Garlow SJ, Gallop RJ, Ninan PT (2007) Coadministration of modafinil and a selective serotonin reuptake inhibitor from the initiation of treatment of major depressive disorder with fatigue and sleepiness: a double-blind, placebo-controlled study. J Clin Psychopharmacol 27:614–619. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR (2010) Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 68:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E(2009) Effects of bupropion augmentation on pro-inflammatory cytokines in escitalopram-resistant patients with major depressive disorder. J Psychopharmacol 23:854–858. [DOI] [PubMed] [Google Scholar]
- Enache D, Pariante CM, Mondelli V (2019) Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun 81:24–40. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Kovács KJ, Sawchenko PE (1994) A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci 14:897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalona R, Fawcett J (2017) Pramipexole in Treatment Resistant-Depression, Possible Role of Inflammatory Cytokines. Neuropsychopharmacology 42:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber K, Pannasch U, Kettenmann H (2005) Dopamine and noradrenaline control distinct functions in rodent microglial cells. Mol Cell Neurosci 29:128–138. [DOI] [PubMed] [Google Scholar]
- Fava M, Mischoulon D, Iosifescu D, Witte J, Pencina M, Flynn M, Harper L, Levy M, Rickels K, Pollack M (2012) A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom 81:87–97. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C (2005) A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry 66:85–93. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Rush AJ, Vukelich J, Diaz SH, Dunklee L, Romo P, Yarns BC, Escalona R (2016) Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am J Psychiatry 173:107–111. [DOI] [PubMed] [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, Moeller JR, Eidelberg D (2001) Metabolic correlates of levodopa response in Parkinson’s disease. Neurology 57:2083–2088. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH(2012) Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Front Neuroendocrinol 33:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH (2007) Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry 62:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le NA, Feinberg R, Tansey MG, Miller AH (2020) What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry 25:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Hernandez CR, Miller AH (2015) Levodopa reverses cytokine-induced reductions in striatal dopamine release. Int J Neuropsychopharmacol 18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH (2013a) Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun 31:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH (2016) Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 21:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Miller AH (2020) Identifying immunophenotypes of inflammation in depression: dismantling the monolith. Biol Psychiatry 88:136–138. [DOI] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, Freeman AA, Rye DB, Goodman MM, Howell LL, Miller AH (2013b) Chronic interferon-alpha decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology 38:2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Treadway MT (2017) Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology 42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Crane CR (2017) Exosomes, DAMPs and miRNA: features of stress physiology and immune homeostasis. Trends Immunol 38:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Goehler LE, Schwartz BA, McGorry M, Martin D, Maier SF, Watkins LR (1998) Thermogenic and corticosterone responses to intravenous cytokines (IL-1beta and TNF-alpha) are attenuated by subdiaphragmatic vagotomy. J Neuroimmunol 86:134–141. [DOI] [PubMed] [Google Scholar]
- Fortunato S, Bononi G, Granchi C, Minutolo F (2018) An update on patents covering agents that interfere with the cancer glycolytic cascade. ChemMedChem 13:2251–2265. [DOI] [PubMed] [Google Scholar]
- Franco-Chaves JA, Mateus CF, Luckenbaugh DA, Martinez PE, Mallinger AG, Zarate CA Jr (2013) Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study. J Affect Disord 149:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen AD, Lam TT, Williams KR, Nairn AC, Duman RS, Sathyanesan M, Kumar V, Carpenter LL, Newton SS (2020) Cerebrospinal fluid proteome evaluation in major depressive disorder by mass spectrometry. BMC Psychiatry 20:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye MA, Watzl J, Banakar S, O'Neill J, Mintz J, Davanzo P, Fischer J, Chirichigno JW, Ventura J, Elman S, Tsuang J, Walot I, Thomas MA (2007) Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology 32:2490–2499. [DOI] [PubMed] [Google Scholar]
- Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, Hough D, Manji H, Drevets WC, Canuso CM (2020) Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry 81:19m13191. [DOI] [PubMed] [Google Scholar]
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822. [DOI] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T (2008) Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol 578:171–176. [DOI] [PubMed] [Google Scholar]
- Furman DCampisi JVerdin ECarrera-Bastos PTarg SFranceschi CFerrucci LGilroy DWFasano AMiller GW, et al. (2019) Chronic inflammation in the etiology of disease across the life span. Nat Med 25:1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Chawla A (2014) Metabolic regulation of immune responses. Annu Rev Immunol 32:609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Mesa N, Zarzuelo A, Gálvez J (2013) Minocycline: far beyond an antibiotic. Br J Pharmacol 169:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza KM, Zhang L, Borron B, Wood LB, Singer AC (2020) Gamma visual stimulation induces a neuroimmune signaling profile distinct from acute neuroinflammation. J Neurosci 40:1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AA, Amiaz R, Shem-David H, Grunhaus L (2019) Ropinirole augmentation for depression: a randomized controlled trial pilot study. J Clin Psychopharmacol 39:78–81. [DOI] [PubMed] [Google Scholar]
- Giacobbe P, Rakita U, Lam R, Milev R, Kennedy SH, McIntyre RS (2018) Efficacy and tolerability of lisdexamfetamine as an antidepressant augmentation strategy: A meta-analysis of randomized controlled trials. J Affect Disord 226:294–300. [DOI] [PubMed] [Google Scholar]
- Glaus J, Vandeleur CL, Lasserre AM, Strippoli MP, Castelao E, Gholam-Rezaee M, Waeber G, Aubry JM, Vollenweider P, Preisig M (2015) Aspirin and statin use and the subsequent development of depression in men and women: results from a longitudinal population-based study. J Affect Disord 182:126–131. [DOI] [PubMed] [Google Scholar]
- Godfrey JRPincus MKovacs-Balint ZFeczko EEarl EMiranda-Dominguez OFair DAJones SRLocke JSanchez MM, et al. (2020) Obesogenic diet-associated C-reactive protein predicts reduced central dopamine and corticostriatal functional connectivity in female rhesus monkeys. Brain Behav Immun 88:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Bekhbat M, Le NA, Chen X, Woolwine BJ, Li Z, Haroon E, Felger JC (2020a) Protein and gene markers of metabolic dysfunction and inflammation together associate with functional connectivity in reward and motor circuits in depression. Brain Behav Immun 88:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, Miller BJ (2018) TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res 199:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DRMassa NPearce BDWommack ECAlrohaibani AGoel NCuthbert BFargotstein MFelger JCHaroon E, et al. (2020b) Inflammatory markers are associated with psychomotor slowing in patients with schizophrenia compared to healthy controls. NPJ Schizophr 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS (2009) Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry 70:1078–1090. [DOI] [PubMed] [Google Scholar]
- Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ (2004) Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res 10:6661–6668. [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Fava M, Miller AH, Russell J, Ball SG, Xu W, Acharya N, Rapaport MH (2017) Impact of ixekizumab treatment on depressive symptoms and systemic inflammation in patients with moderate-to-severe psoriasis: an integrated analysis of three phase 3 clinical studies. Psychother Psychosom 86:260–267. [DOI] [PubMed] [Google Scholar]
- Grossert A, Mehrjardi NZ, Bailey SJ, Lindsay MA, Hescheler J, Šarić T, Teusch N (2019) Ketamine increases proliferation of human iPSC-derived neuronal progenitor cells via insulin-like growth factor 2 and independent of the NMDA receptor. Cells 8:1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Bassi S, Ding Y, Walsh C, Turecki G, Tseng G, Cyranowski JM, Sibille E (2015) Testing the predictive value of peripheral gene expression for nonremission following citalopram treatment for major depression. Neuropsychopharmacology 40:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26:317–330. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajebrahimi B, Bagheri M, Hassanshahi G, Nazari M, Bidaki R, Khodadadi H, Arababadi MK, Kennedy D (2014) The adapter proteins of TLRs, TRIF and MYD88, are upregulated in depressed individuals. Int J Psychiatry Clin Pract 18:41–44. [DOI] [PubMed] [Google Scholar]
- Hamer JA, Testani D, Mansur RB, Lee Y, Subramaniapillai M, McIntyre RS (2019) Brain insulin resistance: a treatment target for cognitive impairment and anhedonia in depression. Exp Neurol 315:1–8. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Drevets WC, Price JL (2004) Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry 55:563–569. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR (2013) Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci 16:1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Bading H (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, Felger JC, Miller AH (2018a) Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry 8:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, Felger JC, Miller AH (2018b) Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology 95:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Felger JC, Woolwine BJ, Chen X, Parekh S, Spivey JR, Hu XP, Miller AH (2015) Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: preliminary findings. Brain Behav Immun 46:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, Hu XP, Miller AH (2016) Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry 21:1351–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Miller AH, Sanacora G (2017) Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology 42:193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH (2020) Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 45:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, Hu XP, Miller AH (2014) IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD (2009) Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 66:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, Critchley HD (2016) A neurocomputational account of how inflammation enhances sensitivity to punishments versus rewards. Biol Psychiatry 80:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M (2007) Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 62:1310–1316. [DOI] [PubMed] [Google Scholar]
- Hassin-Baer S, Cohen OS, Vakil E, Molshazki N, Sela B-A, Nitsan Z, Chapman J, Tanne D(2011) Is C-reactive protein level a marker of advanced motor and neuropsychiatric complications in parkinson’s disease?. J Neural Transm 118:539–543. [DOI] [PubMed] [Google Scholar]
- Hodge RDBakken TEMiller JASmith KABarkan ERGraybuck LTClose JLLong BJohansen NPenn O, et al. (2019) Conserved cell types with divergent features in human versus mouse cortex. Nature 573:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu Z-W, Gao X-B, Thurmon JJ, Marinelli M, DiLeone RJ (2006) Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810. [DOI] [PubMed] [Google Scholar]
- Hori H, Kunugi H (2013) Dopamine agonist-responsive depression. Psychogeriatrics 13:189–195. [DOI] [PubMed] [Google Scholar]
- Hori H, Sasayama D, Teraishi T, Yamamoto N, Nakamura S, Ota M, Hattori K, Kim Y, Higuchi T, Kunugi H (2016) Blood-based gene expression signatures of medication-free outpatients with major depressive disorder: integrative genome-wide and candidate gene analyses. Sci Rep 6:18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2017) Foundations of immunometabolism and implications for metabolic health and disease. Immunity 47:406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- Hu F, Wang X, Pace TW, Wu H, Miller AH (2005) Inhibition of COX-2 by celecoxib enhances glucocorticoid receptor function. Mol Psychiatry 10:426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Sjölander A, Lu D, Walker AK, Sloan EK, Fall K, Valdimarsdóttir U, Hall P, Smedby KE, Fang F (2020) Aspirin and other non-steroidal anti-inflammatory drugs and depression, anxiety, and stress-related disorders following a cancer diagnosis: a nationwide register-based cohort study. BMC Med 18:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YY, Kang HY, Huang KW, Huang TL (2014) Association between toll-like receptors expression and major depressive disorder. Psychiatry Res 220:283–286. [DOI] [PubMed] [Google Scholar]
- Hung YY, Lin CC, Kang HY, Huang TL (2017) TNFAIP3, a negative regulator of the TLR signaling pathway, is a potential predictive biomarker of response to antidepressant treatment in major depressive disorder. Brain Behav Immun 59:265–272. [DOI] [PubMed] [Google Scholar]
- Husain MIChaudhry IBKhoso ABHusain MOHodsoll JAnsari MANaqvi HAMinhas FACarvalho AFMeyer JH, et al. (2020) Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry 7:515–527. [DOI] [PubMed] [Google Scholar]
- Ibrahim L, DiazGranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H (2008) Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 432:232–236. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI (2015) The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun 44:247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, Hough D, Drevets WC, Manji H, Canuso CM (2020) Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmaco 24:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RPenninx BWMadar VXia KMilaneschi YHottenga JJHammerschlag ARBeekman Avan der Wee NSmit JH, et al. (2016) Gene expression in major depressive disorder. Mol Psychiatry 21:339–347. [DOI] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ, Trivedi MH (2017) Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, et al. (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Milner JJ, Makowski L (2012) The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol Rev 249:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K (2000) Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 152:383–389. [DOI] [PubMed] [Google Scholar]
- Kahl KG, Bens S, Ziegler K, Rudolf S, Dibbelt L, Kordon A, Schweiger U (2006) Cortisol, the cortisol-dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry 59:667–671. [DOI] [PubMed] [Google Scholar]
- Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM (2018) Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry 23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson A, Demirtaş T, Bayramgürler D, Balci F, Utkan T (2013) Chronic administration of infliximab (TNF-α inhibitor) decreases depression and anxiety-like behaviour in rat model of chronic mild stress. Basic Clin Pharmacol Toxicol 112:335–340. [DOI] [PubMed] [Google Scholar]
- Kathuria A, Lopez-Lengowski K, Jagtap SS, McPhie D, Perlis RH, Cohen BM, Karmacharya R (2020) Transcriptomic landscape and functional characterization of induced pluripotent stem cell-derived cerebral organoids in schizophrenia. JAMA Psychiatry 77:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazoglou T, Fleischer-Lambropoulos E, Geladopoulos T, Kentroti S, Stefanis C, Vernadakis A (1996) Differential responsiveness of late passage C-6 glial cells and advanced passages of astrocytes derived from aged mouse cerebral hemispheres to cytokines and growth factors: glutamine synthetase activity. Neurochem Res 21:609–614. [DOI] [PubMed] [Google Scholar]
- Kazumori H, Ishihara S, Rumi MA, Ortega-Cava CF, Kadowaki Y, Kinoshita Y (2004) Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am J Physiol Gastrointest Liver Physiol 286:G508–G514. [DOI] [PubMed] [Google Scholar]
- Kéri S, Szabó C, Kelemen O (2014) Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav Immun 40:235–243. [DOI] [PubMed] [Google Scholar]
- Kim KLee SGKegelman TPSu ZZDas SKDash RDasgupta SBarral PMHedvat MDiaz P, et al. (2011) Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol 226:2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Matsunaga S, Iwata N (2017) A meta-analysis of memantine for depression. J Alzheimers Dis 57:113–121. [DOI] [PubMed] [Google Scholar]
- Kobayashi KImagama SOhgomori THirano KUchimura KSakamoto KHirakawa ATakeuchi HSuzumura AIshiguro N, et al. (2013) Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler-Forsberg O, N Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME (2019) Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand 139:404–419. [DOI] [PubMed] [Google Scholar]
- Köhler CAFreitas THMaes Mde Andrade NQLiu CSFernandes BSStubbs BSolmi MVeronese NHerrmann N, et al. (2017) Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135:373–387. [DOI] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J (2014) Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry 71:1381–1391. [DOI] [PubMed] [Google Scholar]
- Korn T, Magnus T, Jung S (2005) Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J 19:1878–1880. [DOI] [PubMed] [Google Scholar]
- Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, Calabresi PA, Snyder SH (2018) Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360:449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U, Schraft T, Kittner H, Kiess W, Illes P (2003) Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol 482:185–187. [DOI] [PubMed] [Google Scholar]
- Kruse JL, Cho JH, Olmstead R, Hwang L, Faull K, Eisenberger NI, Irwin MR (2019) Kynurenine metabolism and inflammation-induced depressed mood: a human experimental study. Psychoneuroendocrinology 109:104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse JL, Congdon E, Olmstead R, Njau S, Breen EC, Narr KL, Espinoza R, Irwin MR (2018) Inflammation and improvement of depression following electroconvulsive therapy in treatment-resistant depression. J Clin Psychiatry 79:17m11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa SKishimoto TMizuno SMasaoka TNaganuma MLiang KCKitazawa MNakashima MShindo CSuda W, et al. (2018) The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: an open-label observational study. J Affect Disord 235:506–512. [DOI] [PubMed] [Google Scholar]
- Lacey RE, Pinto Pereira SM, Li L, Danese A (2020) Adverse childhood experiences and adult inflammation: single adversity, cumulative risk and latent class approaches. Brain Behav Immun 87:820–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacourt TE, Vichaya EG, Chiu GS, Dantzer R, Heijnen CJ (2018) The high costs of low-grade inflammation: persistent fatigue as a consequence of reduced cellular-energy availability and non-adaptive energy expenditure. Front Behav Neurosci 12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YT, Tsai YP, Cherng CG, Ke JJ, Ho MC, Tsai CW, Yu L (2009) Lipopolysaccharide mitagates methamphetamine-induced striatal dopamine depletion via modulating local TNF-alpha and dopamine transporter expression. J Neural Transm (Vienna) 116:405–415. [DOI] [PubMed] [Google Scholar]
- Lamers F, Milaneschi Y, de Jonge P, Giltay EJ, Penninx BWJH (2018) Metabolic and inflammatory markers: associations with individual depressive symptoms. Psychol Med 48:1102–1110. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA (2013) Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SJ (2002) Behavioral and motivational effects of immune-system activation. J Gen Psychol 129:401–414. [DOI] [PubMed] [Google Scholar]
- Lasselin J, Lekander M, Benson S, Schedlowski M, Engler H (2020) Sick for science: experimental endotoxemia as a translational tool to develop and test new therapies for inflammation-associated depression. Mol Psychiatry [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasselin J, Treadway MT, Lacourt TE, Soop A, Olsson MJ, Karshikoff B, Paues-Goranson S, Axelsson J, Dantzer R, Lekander M (2016) Lipopolysaccharide alters motivated behavior in a monetary reward task: a randomized trial. Neuropsychopharmacology 42:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TTSavitz JSuzuki HMisaki MTeague TKWhite BCMarino JHWiley GGaffney PMDrevets WC, et al. (2018) Identification and replication of RNA-Seq gene network modules associated with depression severity. Transl Psychiatry 8:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leday GGRVértes PERichardson SGreene JRRegan TKhan SHenderson RFreeman TCPariante CMHarrison NA, et al. ; MRC Immunopsychiatry Consortium (2018) Replicable and coupled changes in innate and adaptive immune gene expression in two case-control studies of blood microarrays in major depressive disorder. Biol Psychiatry 83:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Kim NJ (2017) Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 22:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Mansur RB, Brietzke E, Carmona NE, Subramaniapillai M, Pan Z, Shekotikhina M, Rosenblat JD, Suppes T, Cosgrove VE, Kramer NE, McIntyre RS (2020) Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav Immun 88:631–639. [DOI] [PubMed] [Google Scholar]
- Lemke MR, Brecht HM, Koester J, Reichmann H (2006) Effects of the dopamine agonist pramipexole on depression, anhedonia and motor functioning in Parkinson’s disease. J Neurol Sci 248:266–270. [DOI] [PubMed] [Google Scholar]
- Lercher A, Baazim H, Bergthaler A (2020) Systemic immunometabolism: challenges and opportunities. Immunity 53:496–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Rakic P (2001) Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci 4:931–936. [DOI] [PubMed] [Google Scholar]
- Lewerenz JHewett SJHuang YLambros MGout PWKalivas PWMassie ASmolders IMethner APergande M, et al. (2013) The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid Redox Signal 18:522–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ramenaden ER, Peng J, Koito H, Volpe JJ, Rosenberg PA (2008) Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J Neurosci 28:5321–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-J, Zhang X, Johnson MA, Wang Z-B, Lavaute T, Zhang S-C (2009) Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development 136:4055–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramaniapillai M, Fan B, Lu C, Mclntyer RS (2019) Efficacy of omega-3 PUFAs in depression: a meta-analysis. Transl Psychiatry 9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck VM, Costa-Campos L, Pilz LK, Garcia CR, Elisabetsky E (2012) AMPA glutamate receptors mediate the antidepressant-like effects of N-acetylcysteine in the mouse tail suspension test. Behav Pharmacol 23:171–177. [DOI] [PubMed] [Google Scholar]
- Liu CS, Carvalho AF, McIntyre RS (2014) Towards a “metabolic” subtype of major depressive disorder: shared pathophysiological mechanisms may contribute to cognitive dysfunction. CNS Neurol Disord Drug Targets 13:1693–1707. [DOI] [PubMed] [Google Scholar]
- Liu JJWei YBStrawbridge RBao YChang SShi LQue JGadad BSTrivedi MHKelsoe JR, et al. (2020) Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry 25:339–350. [DOI] [PubMed] [Google Scholar]
- Liu RT, Walsh RFL, Sheehan AE (2019) Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev 102:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga LE, Cholanians AB, Phan AV, Herndon JM, Lau SS, Monks TJ (2015) Vesicular monoamine transporter 2 and the acute and long-term response to 3,4-(±)-methylenedioxymethamphetamine. Toxicol Sci 143:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G, Dalleau E, Lecointe H, Yon V (2016) Relationships between anhedonia, alexithymia, impulsivity, suicidal ideation, recent suicide attempt, C-reactive protein and serum lipid levels among 122 inpatients with mood or anxious disorders. Psychiatry Res 246:296–302. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Sears B, McNamara RK (2013) Elevated ratio of arachidonic acid to long-chain omega-3 fatty acids predicts depression development following interferon-alpha treatment: relationship with interleukin-6. Brain Behav Immun 31:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall METurner LBhatti JCavanagh Jde Boer PMondelli VJones DDrevets WCCowen PHarrison NA, et al. ; Neuroimmunology of Mood Disorders and Alzheimer’s Disease (NIMA) Consortium (2020) Peripheral blood cell-stratified subgroups of inflamed depression. Biol Psychiatry 88:185–196. [DOI] [PubMed] [Google Scholar]
- Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB (2020) The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhoo M, Keefe RS, Roth RM, Sambunaris A, Wu J, Trivedi MH, Anderson CS, Lasser R (2014) Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology 39:1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 19:11–38. [DOI] [PubMed] [Google Scholar]
- Maes M, Scharpé S, Van Grootel L, Uyttenbroeck W, Cooreman W, Cosyns P, Suy E (1992) Higher alpha 1-antitrypsin, haptoglobin, ceruloplasmin and lower retinol binding protein plasma levels during depression: further evidence for the existence of an inflammatory response during that illness. J Affect Disord 24:183–192. [DOI] [PubMed] [Google Scholar]
- Manabe T, Togashi H, Uchida N, Suzuki SC, Hayakawa Y, Yamamoto M, Yoda H, Miyakawa T, Takeichi M, Chisaka O (2000) Loss of cadherin-11 adhesion receptor enhances plastic changes in hippocampal synapses and modifies behavioral responses. Mol Cell Neurosci 15:534–546. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM (2008) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28:156–165. [DOI] [PubMed] [Google Scholar]
- Mariani JCoppola GZhang PAbyzov AProvini LTomasini LAmenduni MSzekely APalejev DWilson M, et al. (2015) FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell 162:375–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, Horvath TL, Vaccarino FM (2012) Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA 109:12770–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M (2020) The lasting misery of coronavirus long-haulers. Nature 585:339–341. [DOI] [PubMed] [Google Scholar]
- Matt SM, Gaskill PJ (2020) Where is dopamine and how do immune cells see it?: dopamine-mediated immune cell function in health and disease. J Neuroimmune Pharmacol 15:114–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sánchez-Gómez MV (2006) Glutamate-mediated glial injury: mechanisms and clinical importance. Glia 53:212–224. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Sanacora G (2015) Regulation of extrasynaptic glutamate levels as a pathophysiological mechanism in disorders of motivation and addiction. Neuropsychopharmacology 40:254–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW (2015) A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med 45:693–704. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Subramaniapillai M, Lee Y, Pan Z, Carmona NE, Shekotikhina M, Rosenblat JD, Brietzke E, Soczynska JK, Cosgrove VE, Miller S, Fischer EG, Kramer NE, Dunlap K, Suppes T, Mansur RB (2019) Efficacy of adjunctive infliximab vs placebo in the treatment of adults with bipolar I/II depression: a randomized clinical trial. JAMA Psychiatry 76:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DBWeber MDNiraula ASawicki CMLiu XJarrett BLRamirez-Chan KWang YRoeth RMSucaldito AD, et al. (2018) Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry 23:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Raison CL, Woolwine BJ, Haroon E, Binder EB, Miller AH, Felger JC (2013) Transcriptional signatures related to glucose and lipid metabolism predict treatment response to the tumor necrosis factor antagonist infliximab in patients with treatment-resistant depression. Brain Behav Immun 31:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC (2018) Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun 73:725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta ND, Stevens JS, Li Z, Gillespie CF, Fani N, Michopoulos V, Felger JC (2020) Inflammation, reward circuitry and symptoms of anhedonia and PTSD in trauma-exposed women. Soc Cogn Affect Neurosci 15:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard CPfau MLHodes GEKana VWang VXBouchard STakahashi AFlanigan MEAleyasin HLeClair KB, et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson D, Adler LA, Amsterdam JD, Dunner DL, Nierenberg AA, Reimherr FW, Schatzberg AF, Kelsey DK, Williams DW (2007) Addition of atomoxetine for depression incompletely responsive to sertraline: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:582–587. [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Berk M, Penninx BWJH (2020) Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol Psychiatry 88:369–380. [DOI] [PubMed] [Google Scholar]
- Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR (2008) Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 26:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Felger JC (2017) Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology 42:334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon DPrice LHCarpenter LLTyrka ARPapakostas GIBaer LDording CMClain AJDurham KWalker R, et al. (2014) A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J Clin Psychiatry 75:370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddel RShardell MKhadeer MLovett JKadriu BRavichandran SMorris PJYuan PThomas CJGould TD, et al. (2018) Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl) 235:3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocelin R, Marcon M, D’ambros S, Mattos J, Sachett A, Siebel AM, Herrmann AP, Piato A (2019) N-acetylcysteine reverses anxiety and oxidative damage induced by unpredictable chronic stress in zebrafish. Mol Neurobiol 56:1188–1195. [DOI] [PubMed] [Google Scholar]
- Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhé HG, Schene AH (2016) Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry 6:e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Tan KM, Inagaki TK, Muscatell KA, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI (2019a) Sex differences in the relationship between inflammation and reward sensitivity: a randomized controlled trial of endotoxin. Biol Psychiatry Cogn Neurosci Neuroimaging 4:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero P, Ruiz-Estigarribia L, Lahortiga-Ramos F, Sánchez-Villegas A, Bes-Rastrollo M, Escobar-González M, Martínez-González MA, Fernández-Montero A (2019) Use of non-steroidal anti-inflammatory drugs, aspirin and the risk of depression: the “Seguimiento Universidad de Navarra (SUN)” cohort. J Affect Disord 247:161–167. [DOI] [PubMed] [Google Scholar]
- Moreira FPJansen KCardoso TAMondin TCVieira ISMagalhães PVDSKapczinski FSouza LDMda Silva RAOses JP, et al. (2019) Metabolic syndrome, depression and anhedonia among young adults. Psychiatry Res 271:306–310. [DOI] [PubMed] [Google Scholar]
- Mørkholt AS, Wiborg O, Nieland JGK, Nielsen S, Nieland JD (2017) Blocking of carnitine palmitoyl transferase 1 potently reduces stress-induced depression in rat highlighting a pivotal role of lipid metabolism. Sci Rep 7:2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JAZakharova IFerrer JVMerrill GAHope BLafer EMLin ZCWang JBJavitch JAGalli A, et al. (2003) Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci 23:8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi SBattle AZhu XPotash JBWeissman MMShi JBeckman KHaudenschild CMcCormick CMei R, et al. (2014) Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry 19:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Hopkins CWP, Ismail K, Stahl D (2018) Repositioning of diabetes treatments for depressive symptoms: a systematic review and meta-analysis of clinical trials. Psychoneuroendocrinology 94:91–103. [DOI] [PubMed] [Google Scholar]
- Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y (2015) Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep 10:537–550. [DOI] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, Irwin MR, Eisenberger NI (2016) Exposure to an inflammatory challenge enhances neural sensitivity to negative and positive social feedback. Brain Behav Immun 57:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161:102–112. [DOI] [PubMed] [Google Scholar]
- Nasca CBigio BLee FSYoung SPKautz MMAlbright ABeasley JMillington DSMathé AAKocsis JH, et al. (2018) Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci USA 115:8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasca CBigio BZelli Dde Angelis PLau TOkamoto MSoya HNi JBrichta LGreengard P, et al. (2017) Role of the astroglial glutamate exchanger xCT in ventral hippocampus in resilience to stress. Neuron 96:402–413.e5. [DOI] [PubMed] [Google Scholar]
- Nefs G, Pouwer F, Denollet J, Kramer H, Wijnands-van Gent CJ, Pop VJ (2012) Suboptimal glycemic control in type 2 diabetes: a key role for anhedonia? J Psychiatr Res 46:549–554. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991. [DOI] [PubMed] [Google Scholar]
- Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, Jones AR 4th, Raval F, Ip BC, Zhu M, et al. (2019) Fatty acid metabolites combine with reduced β oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab 30:447–461.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Schalbetter SM, Clifton NE, Mattei D, Richetto J, Thomas K, Meyer U, Hall J (2020) Neuronal activity increases translocator protein (TSPO) levels. Mol Psychiatry [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA, Baqi Y, Müller CE, Correa M, Salamone JD (2014) Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/ chow feeding choice task. Psychopharmacology (Berl) 231:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R (2009a) Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci 29:4200–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW (2009b) Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J Immunol 182:3202–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Kishton RJ, Rathmell J (2016) A guide to immunometabolism for immunologists. Nat Rev Immunol 16:553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G, Lladó J (2014) Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014:861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM (2019) Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med 49:1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD (2020) Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun 87:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Keum N, Okereke OI, Sun Q, Kivimaki M, Rubin RR, Hu FB (2012) Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care 35:1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DXu YZhang LSu QChen MLi BXiao QGao QPeng XJiang B, et al. (2018) Gene expression profile in peripheral blood mononuclear cells of postpartum depression patients. Sci Rep 8:10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN (2017) Inflammatory and innate immune markers of neuroprogression in depressed and teenage suicide brain. Mod Trends Pharmacopsychiatry 31:79–95. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y (2014) Toll-like receptors in the depressed and suicide brain. J Psychiatr Res 53:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M (2010) S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry 167:942–948. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Zajecka JM, Bottiglieri T, Roffman J, Cassiello C, Stahl SM, Fava M (2014) Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: results from a randomized clinical trial. J Clin Psychiatry 75:855–863. [DOI] [PubMed] [Google Scholar]
- Papakostas GIShelton RCZajecka JMEtemad BRickels KClain ABaer LDalton EDSacco GRSchoenfeld D, et al. (2012) L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry 169:1267–1274. [DOI] [PubMed] [Google Scholar]
- Paredes MFJames DGil-Perotin SKim HCotter JANg CSandoval KRowitch DHXu DMcQuillen PS, et al. (2016) Extensive migration of young neurons into the infant human frontal lobe. Science 354:aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LTKadriu BGould TDZanos PGreenstein DEvans JWYuan PFarmer CAOppenheimer MGeorge JM, et al. (2020) A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol 23:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC (2016) Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6:e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB (2006) Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther 319:237–246. [DOI] [PubMed] [Google Scholar]
- Pary R, Scarff JR, Jijakli A, Tobias C, Lippmann S (2015) A review of psychostimulants for adults with depression. Fed Pract 32:30S–37S. [PMC free article] [PubMed] [Google Scholar]
- Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK (2016) Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther Adv Psychopharmacol 6:99–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar AA, Masand PS, Pae C-U, Peindl K, Hooper-Wood C, Mannelli P, Ciccone P (2006) A randomized, double-blind, placebo-controlled trial of augmentation with an extended release formulation of methylphenidate in outpatients with treatment-resistant depression. J Clin Psychopharmacol 26:653–656. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Chavan SS, Tracey KJ (2020) Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb Perspect Med 10:a034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ (2013) Metabolic pathways in immune cell activation and quiescence. Immunity 38:633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646. [DOI] [PubMed] [Google Scholar]
- Perrin AJ, Pariante CM (2020) Endocrine and immune effects of non-convulsive neurostimulation in depression: a systematic review. Brain Behav Immun 87:910–920. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Bezzi P (2016) Novel insights into gliotransmitters. Curr Opin Pharmacol 26:138–145. [DOI] [PubMed] [Google Scholar]
- Petrulli JR, Kalish B, Nabulsi NB, Huang Y, Hannestad J, Morris ED (2017) Systemic inflammation enhances stimulant-induced striatal dopamine elevation. Transl Psychiatry 7:e1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ (2017) Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res 77:6795–6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian XNguyen HNSong MMHadiono COgden SCHammack CYao BHamersky GRJacob FZhong C, et al. (2016) Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Banks WA (2007) Brain-immune communication pathways. Brain Behav Immun 21:727–735. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH (2011) Is depression an inflammatory disorder? Curr Psychiatry Rep 13:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH (2013) The evolutionary significance of depression in pathogen host defense (PATHOS-D). Mol Psychiatry 18:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH (2013) A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 70:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ (2007) Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets 6:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (2009) Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci 10:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall PA, Lee CA, Podurgiel SJ, Hart E, Yohn SE, Jones M, Rowland M, López-Cruz L, Correa M, Salamone JD (2014) Bupropion increases selection of high effort activity in rats tested on a progressive ratio/chow feeding choice procedure: implications for treatment of effort-related motivational symptoms. Int J Neuropsychopharmacol 18:pyu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Nierenberg AA, Schettler PJ, Kinkead B, Cardoos A, Walker R, Mischoulon D (2016) Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof-of-concept study. Mol Psychiatry 21:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran AV, Kennedy SH, O’Donovan MC, Fallu A, Camacho F, Binder CE (2008) Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 69:87–94. [DOI] [PubMed] [Google Scholar]
- Richards C, McIntyre RS, Weisler R, Sambunaris A, Brawman-Mintzer O, Gao J, Geibel B, Dauphin M, Madhoo M (2016) Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: Results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Affect Disord 206:151–160. [DOI] [PubMed] [Google Scholar]
- Richards EMZanotti-Fregonara PFujita MNewman LFarmer CBallard EDMachado-Vieira RYuan PNiciu MJLyoo CH, et al. (2018) PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res 8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM (2003) Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107:363–369. [DOI] [PubMed] [Google Scholar]
- Rojí R, Centeno C (2017) The use of methylphenidate to relieve fatigue. Curr Opin Support Palliat Care 11:299–305. [DOI] [PubMed] [Google Scholar]
- Rooks MG, Garrett WS (2016) Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16:341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Ballina MOlofsson PSOchani MValdés-Ferrer SILevine YAReardon CTusche MWPavlov VAAndersson UChavan S, et al. (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, McIntyre RS (2018) Efficacy and tolerability of minocycline for depression: a systematic review and meta-analysis of clinical trials. J Affect Disord 227:219–225. [DOI] [PubMed] [Google Scholar]
- Rutherford BRSlifstein MChen CAbi-Dargham ABrown PJWall MWVanegas-Arroyave NStern YBailey VValente E, et al. (2019) Effects of L-DOPA monotherapy on psychomotor speed and [11C]raclopride binding in high-risk older adults with depression. Biol Psychiatry 86:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rutigliano G, Accorroni A, Zucchi R (2018) The case for TAAR1 as a modulator of central nervous system function. Front Pharmacol 8:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O’Connor JC (2012) Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav 62:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore GNash ABleys CHsu BSaad ZGause AMoyer JXi LManji HVan Nueten L, et al. (2018) A double-blind, placebo-controlled, multicenter study of sirukumab as adjunctive treatment to a monoaminergic antidepressant in adults with major depressive disorder, in American College of Neuropsychopharmacology 57th Annual Meeting; 2018 Dec 6; Hollywood, FL. [Google Scholar]
- Sanacora G, Banasr M (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ, Pathak S (2017) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology 42:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH (2007) Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry 61:822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada KNakajima SKurokawa SBarceló-Soler AIkuse DHirata AYoshizawa ATomizawa YSalas-Valero MNoda Y, et al. (2020) Gut microbiota and major depressive disorder: a systematic review and meta-analysis. J Affect Disord 266:1–13. [DOI] [PubMed] [Google Scholar]
- Santos RVadodaria KCJaeger BNMei ALefcochilos-Fogelquist SMendes APDErikson GShokhirev MRandolph-Moore LFredlender C, et al. (2017) Differentiation of inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Reports 8:1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S (2010) The immunoregulatory role of dopamine: an update. Brain Behav Immun 24:525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J, Byrne GJ, Bousman C, Stough C, Murphy J, MacDonald P, Adams L, Nazareth S, Oliver G, Cribb L, Savage K, Menon R, Chamoli S, Berk M, Ng C, Mischoulon D (2018) Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: an 8-week double-blind, randomized, controlled trial. Eur Neuropsychopharmacol 28:1126–1136. [DOI] [PubMed] [Google Scholar]
- Savitz J (2020) The kynurenine pathway: a finger in every pie. Mol Psychiatry 25:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JDantzer RMeier TBWurfel BEVictor TAMcIntosh SAFord BNMorris HMBodurka JTeague TK, et al. (2015) Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology 62:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Frank MB, Victor T, Bebak M, Marino JH, Bellgowan PS, McKinney BA, Bodurka J, Kent Teague T, Drevets WC (2013) Inflammation and neurological disease-related genes are differentially expressed in depressed patients with mood disorders and correlate with morphometric and functional imaging abnormalities. Brain Behav Immun 31:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JBTeague TKMisaki MMacaluso MWurfel BEMeyer MDrevets DYates WGleason ODrevets WC, et al. (2018) Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R (2004) The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol 4:12–17. [DOI] [PubMed] [Google Scholar]
- Sethom MM, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, Hechmi Z, Kaabachi N (2010) Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins Leukot Essent Fatty Acids 83:131–136. [DOI] [PubMed] [Google Scholar]
- Setiawan EWilson AAMizrahi RRusjan PMMiler LRajkowska GSuridjan IKennedy JLRekkas PVHoule S, et al. (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB (2012) Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology 37:2194–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao WZhang SZTang MZhang XHZhou ZYin YQZhou QBHuang YYLiu YJWawrousek E, et al. (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 494:90–94. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Miller AH (2010) Eating ourselves to death (and despair): the contribution of adiposity and inflammation to depression. Prog Neurobiol 91:275–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Pencina MJ, Barrentine LW, Ruiz JA, Fava M, Zajecka JM, Papakostas GI (2015) Association of obesity and inflammatory marker levels on treatment outcome: results from a double-blind, randomized study of adjunctive L-methylfolate calcium in patients with MDD who are inadequate responders to SSRIs. J Clin Psychiatry 76:1635–1641. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE (2014) Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology 50:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Leslie SM, Packer MM, Zaiko YV, Phillips OR, Weisman EF, Wall DM, Jo B, Rasgon N (2019) Brain and behavioral correlates of insulin resistance in youth with depression and obesity. Horm Behav 108:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soczynska JK, Mansur RB, Brietzke E, Swardfager W, Kennedy SH, Woldeyohannes HO, Powell AM, Manierka MS, McIntyre RS (2012) Novel therapeutic targets in depression: minocycline as a candidate treatment. Behav Brain Res 235:302–317. [DOI] [PubMed] [Google Scholar]
- Soder HE, Cooper JA, Lopez-Gamundi P, Hoots JK, Nunez C, Lawlor VM, Lane SD, Treadway MT, Wardle MC (2020) Dose-response effects of d-amphetamine on effort-based decision-making and reinforcement learning. Neuropsychopharmacology [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman MA, Aboharb F, Zeltner N, Studer L (2017) Pluripotent stem cells in neuropsychiatric disorders. Mol Psychiatry 22:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song GLu QFan HZhang XGe LTian RWang SFeng TPan JFeng J, et al. (2019) Inhibition of hexokinases holds potential as treatment strategy for rheumatoid arthritis. Arthritis Res Ther 21:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker S, Van Zanten JS, De Jong S, Penninx BW, van Dyck R, Zitman FG, Smit JH, Ylstra B, Smit AB, Hoogendijk WJ (2010) Stimulated gene expression profiles as a blood marker of major depressive disorder. Biol Psychiatry 68:179–186. [DOI] [PubMed] [Google Scholar]
- Stacey DSchubert KOClark SRAmare ATMilanesi EMaj CLeckband SGShekhtman TKelsoe JRGurwitz D, et al. (2018) A gene co-expression module implicating the mitochondrial electron transport chain is associated with long-term response to lithium treatment in bipolar affective disorder. Transl Psychiatry 8:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak EKBenson CANarla STDimitri AChuye LEBDhiman SHarikrishnan KElahi SFreedman DBrennand KJ, et al. (2017) Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl Psychiatry 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM (2007) Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr 12:739–744. [DOI] [PubMed] [Google Scholar]
- Stouffer MAWoods CAPatel JCLee CRWitkovsky PBao LMachold RPJones KTde Vaca SCReith MEA, et al. (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH (2017) The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat Rev Rheumatol 13:743–751. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ (2015) Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol 25:1532–1543. [DOI] [PubMed] [Google Scholar]
- Su KP, Huang SY, Peng CY, Lai HC, Huang CL, Chen YC, Aitchison KJ, Pariante CM (2010) Phospholipase A2 and cyclooxygenase 2 genes influence the risk of interferon-alpha-induced depression by regulating polyunsaturated fatty acids levels. Biol Psychiatry 67:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su KP, Lai HC, Peng CY, Su WP, Chang JP, Pariante CM (2019a) Interferon-alpha-induced depression: comparisons between early- and late-onset subgroups and with patients with major depressive disorder. Brain Behav Immun 80:512–518. [DOI] [PubMed] [Google Scholar]
- Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, Chang HC, Pariante CM (2014) Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry 76:559–566. [DOI] [PubMed] [Google Scholar]
- Su M, Li E, Tang C, Zhao Y, Liu R, Gao K (2019b) Comparison of blood lipid profile/thyroid function markers between unipolar and bipolar depressed patients and in depressed patients with anhedonia or suicidal thoughts. Mol Med 25:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, Zeng X (2010) Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28:1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmonds M, Emmanuel JJ, Drew ME, Batterham RL, Dolan RJ (2010) Metabolic state alters economic decision making under risk in humans. PLoS One 5:e11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi DE, Manzoor MK, Aquilato A, Jackson P, Ghoneim OM, Edafiogho IO (2018) Current and novel anti-inflammatory drug targets for inhibition of cytokines and leucocyte recruitment in rheumatic diseases. J Pharm Pharmacol 70:18–26. [DOI] [PubMed] [Google Scholar]
- Sztainert T, Hay R, Wohl MJA, Abizaid A (2018) Hungry to gamble? Ghrelin as a predictor of persistent gambling in the face of loss. Biol Psychol 139:115–123. [DOI] [PubMed] [Google Scholar]
- Ter Horst KWLammers NMTrinko ROpland DMFigee MAckermans MTBooij Jvan den Munckhof PSchuurman PRFliers E, et al. (2018) Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci Transl Med 10:eaar3752. [DOI] [PubMed] [Google Scholar]
- Thase ME, Clayton AH, Haight BR, Thompson AH, Modell JG, Johnston JA (2006) A double-blind comparison between bupropion XL and venlafaxine XR: sexual functioning, antidepressant efficacy, and tolerability. J Clin Psychopharmacol 26:482–488. [DOI] [PubMed] [Google Scholar]
- Thelin EPHall CETyzack GEFrostell AGiorgi-Coll SAlam ACarpenter KLHMitchell JTajsic THutchinson PJ, et al. (2020) Delineating astrocytic cytokine responses in a human stem cell model of neural trauma. J Neurotrauma 37:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, Hermans E (2007) Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85:2059–2070. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC (2004) Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord 78:235–241. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Giros B, Mechawar N (2014a) Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N (2014b) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Admon R, Arulpragasam AR, Mehta M, Douglas S, Vitaliano G, Olson DP, Cooper JA, Pizzagalli DA (2017) Association between interleukin-6 and striatal prediction-error signals following acute stress in healthy female participants. Biol Psychiatry 82:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH (2012) Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 121:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Cooper JA, Miller AH (2019a) Can’t or won’t? Immunometabolic constraints on dopaminergic drive. Trends Cogn Sci 23:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH (2011) Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Cutler AJ, Richards C, Lasser R, Geibel BB, Gao J, Sambunaris A, Patkar AA (2013) A randomized controlled trial of the efficacy and safety of lisdexamfetamine dimesylate as augmentation therapy in adults with residual symptoms of major depressive disorder after treatment with escitalopram. J Clin Psychiatry 74:802–809. [DOI] [PubMed] [Google Scholar]
- Uher RTansey KEDew TMaier WMors OHauser JDernovsek MZHenigsberg NSouery DFarmer A, et al. (2014) An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry 171:1278–1286. [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Aranda F, Eggermont A, Sautès-Fridman C, Tartour E, Kennedy EP, Platten M, Zitvogel L, Kroemer G, Galluzzi L (2014) Trial watch: IDO inhibitors in cancer therapy. OncoImmunology 3:e957994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkanova V, Ebmeier KP, Allan CL (2013) CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord 150:736–744. [DOI] [PubMed] [Google Scholar]
- Veronese N, Koyanagi A, Stubbs B, Solmi M, Fornaro M, Fernandes BS, Muller C, Thompson T, Carvalho AF, Maggi S (2018) Aspirin and incident depressive symptoms: a longitudinal cohort study over 8 years. Int J Geriatr Psychiatry 33:e193–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Dantzer R (2018) Inflammation-induced motivational changes: perspective gained by evaluating positive and negative valence systems. Curr Opin Behav Sci 22:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Hunt SC, Dantzer R (2014) Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology 39:2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA, Tye SJ (2015) Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant-resistance. Behav Brain Res 293:198–202. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Wing EE, Banks WA, Dantzer R (2019) Leucine competes with kynurenine for blood-to-brain transport and prevents lipopolysaccharide-induced depression-like behavior in mice. Mol Psychiatry 24:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther A, Cannistraci CV, Simons K, Durán C, Gerl MJ, Wehrli S, Kirschbaum C (2018) Lipidomics in major depressive disorder. Front Psychiatry 9:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Luan HH, Medzhitov R (2019) An evolutionary perspective on immunometabolism. Science 363:eaar3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Huang X, Pan X, Zhang T, Hou C, Su W-J, Liu L-L, Li J-M, Wang Y-X (2020) Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav Immun 88:132–143. [DOI] [PubMed] [Google Scholar]
- Wang B, Lian Y-J, Su W-J, Peng W, Dong X, Liu L-L, Gong H, Zhang T, Jiang C-L, Wang Y-X (2018) HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun 72:51–60. [DOI] [PubMed] [Google Scholar]
- Wang SBates JLi XSchanz SChandler-Militello DLevine CMaherali NStuder LHochedlinger KWindrem M, et al. (2013) Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P (2011) Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci USA 108:9262–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner BB (2019) The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res 85:216–224. [DOI] [PubMed] [Google Scholar]
- Weckmann KDeery MJHoward JAFeret RAsara JMDethloff FFiliou MDIannace JLabermaier CMaccarrone G, et al. (2017) Ketamine’s antidepressant effect is mediated by energy metabolism and antioxidant defense system. Sci Rep 7:15788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckmann K, Labermaier C, Asara JM, Müller MB, Turck CW (2014) Time-dependent metabolomic profiling of Ketamine drug action reveals hippocampal pathway alterations and biomarker candidates. Transl Psychiatry 4:e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook A, van den Bosch R, Määttä JI, Hofmans L, Papadopetraki D, Cools R, Frank MJ (2020) Dopamine promotes cognitive effort by biasing the benefits versus costs of cognitive work. Science 367:1362–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M (2005) Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. Psychol Med 35:433–441. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR (1999) Oscillations in the basal ganglia. Nature 400:621–622. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Sanacora G (2019) A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 24:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GMStylianou AZhang YSun YGupta AJagannatha PSWang DHsu BCurran MEKhan S, et al. ; MRC ImmunoPsychiatry Consortium (2020) Effects of immunomodulatory drugs on depressive symptoms: A mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 25:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DJ, Renoir T, Smith ZM, Frazier AE, Francis PS, Thorburn DR, McGee SL, Hannan AJ, Gray LJ (2015) N-Acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington’s disease. Transl Psychiatry 5:e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R (2007) Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm (Vienna) 114:33–41. [DOI] [PubMed] [Google Scholar]
- Xiang YQ, Zheng W, Wang SB, Yang XH, Cai DB, Ng CH, Ungvari GS, Kelly DL, Xu WY, Xiang YT (2017) Adjunctive minocycline for schizophrenia: a meta-analysis of randomized controlled trials. Eur Neuropsychopharmacol 27:8–18. [DOI] [PubMed] [Google Scholar]
- Xiao CBeitler JJHiggins KAConneely KDwivedi BFelger JWommack ECShin DMSaba NFOng LY, et al. (2016) Fatigue is associated with inflammation in patients with head and neck cancer before and after intensity-modulated radiation therapy. Brain Behav Immun 52:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA (2017) Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol Med 47:1733–1743. [DOI] [PubMed] [Google Scholar]
- Xie Z, Miller GM (2009) A receptor mechanism for methamphetamine action in dopamine transporter regulation in brain. J Pharmacol Exp Ther 330:316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Westmoreland SV, Miller GM (2008) Modulation of monoamine transporters by common biogenic amines via trace amine-associated receptor 1 and monoamine autoreceptors in human embryonic kidney 293 cells and brain synaptosomes. J Pharmacol Exp Ther 325:629–640. [DOI] [PubMed] [Google Scholar]
- Xu J, Dydak U, Harezlak J, Nixon J, Dzemidzic M, Gunn AD, Karne HS, Anand A (2013) Neurochemical abnormalities in unmedicated bipolar depression and mania: a 2D 1H MRS investigation. Psychiatry Res 213:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, Zhou R (2015) Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 160:62–73. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Jiang W (2020) Immune biomarkers alterations in post-traumatic stress disorder: A systematic review and meta-analysis. J Affect Disord 268:39–46. [DOI] [PubMed] [Google Scholar]
- Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K (2015) Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry 77:e19–e20. [DOI] [PubMed] [Google Scholar]
- Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z, Cui J, Shi T, Fang Y (2012) Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS One 7:e31283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE, Miguel NS, Correa M, Salamone JD (2016) Effort-related motivational effects of the pro-inflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology (Berl) 233:3575–3586. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Lyoo IK, Haws C, Kim TS, Cohen BM, Renshaw PF (2009) Decreased glutamate/glutamine levels may mediate cytidine's efficacy in treating bipolar depression: a longitudinal proton magnetic resonance spectroscopy study. Neuropsychopharmacology 34:1810–1818. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Öngür D (2010) Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, Charney DSManji HK (2004) An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry 161:171–174. [DOI] [PubMed] [Google Scholar]
- Zeng HShen EHHohmann JGOh SWBernard ARoyall JJGlattfelder KJSunkin SMMorris JAGuillozet-Bongaarts AL, et al. (2012) Large-scale cellular-resolution gene profiling in human neocortex reveals species-specific molecular signatures. Cell 149:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Butler EB, Tan M (2013) Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis 4:e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PZeng BZhou CLiu MFang ZXu XZeng LChen JFan SDu X, et al. (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. [DOI] [PubMed] [Google Scholar]
- Zheng W, Cai DB, Yang XH, Ungvari GS, Ng CH, Müller N, Ning YP, Xiang YT (2017) Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Psychiatr Res 92:139–146. [DOI] [PubMed] [Google Scholar]
- Zhou XKeitner GIQin BRavindran AVBauer MDel Giovane CZhao JLiu YFang YZhang Y, et al. (2015) Atypical antipsychotic augmentation for treatment-resistant depression: a systematic review and network meta-analysis. Int J Neuropsychopharmacol 18:pyv060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA (2006) The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology 31:2121–2131. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller H, Schloegl A, Schroecksnadel S, Vogel W, Fuchs D (2012) Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J Interferon Cytokine Res 32:216–220. [DOI] [PubMed] [Google Scholar]
- Zwanzger P, Zavorotnyy M, Gencheva E, Diemer J, Kugel H, Heindel W, Ruland T, Ohrmann P, Arolt V, Domschke K, Pfleiderer B (2013) Acute shift in glutamate concentrations following experimentally induced panic with cholecystokinin tetrapeptide–a 3T-MRS study in healthy subjects. Neuropsychopharmacology 38:1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]