Abstract
Background
The review represents one in a family of four reviews focusing on a range of different interventions for drug‐using offenders. This specific review considers pharmacological interventions aimed at reducing drug use or criminal activity, or both, for illicit drug‐using offenders.
Objectives
To assess the effectiveness of pharmacological interventions for drug‐using offenders in reducing criminal activity or drug use, or both.
Search methods
We searched Fourteen electronic bibliographic databases up to May 2014 and five additional Web resources (between 2004 and November 2011). We contacted experts in the field for further information.
Selection criteria
We included randomised controlled trials assessing the efficacy of any pharmacological intervention a component of which is designed to reduce, eliminate or prevent relapse of drug use or criminal activity, or both, in drug‐using offenders. We also report data on the cost and cost‐effectiveness of interventions.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane.
Main results
Fourteen trials with 2647 participants met the inclusion criteria. The interventions included in this review report on agonistic pharmacological interventions (buprenorphine, methadone and naltrexone) compared to no intervention, other non‐pharmacological treatments (e.g. counselling) and other pharmacological drugs. The methodological trial quality was poorly described, and most studies were rated as 'unclear' by the reviewers. The biggest threats to risk of bias were generated through blinding (performance and detection bias) and incomplete outcome data (attrition bias). Studies could not be combined all together because the comparisons were too different. Only subgroup analysis for type of pharmacological treatment were done. When compared to non‐pharmacological, we found low quality evidence that agonist treatments are not effective in reducing drug use or criminal activity, objective results (biological) (two studies, 237 participants (RR 0.72 (95% CI 0.51 to 1.00); subjective (self‐report), (three studies, 317 participants (RR 0.61 95% CI 0.31 to 1.18); self‐report drug use (three studies, 510 participants (SMD: ‐0.62 (95% CI ‐0.85 to ‐0.39). We found low quality of evidence that antagonist treatment was not effective in reducing drug use (one study, 63 participants (RR 0.69, 95% CI 0.28 to 1.70) but we found moderate quality of evidence that they significantly reduced criminal activity (two studies, 114 participants, (RR 0.40, 95% CI 0.21 to 0.74).
Findings on the effects of individual pharmacological interventions on drug use and criminal activity showed mixed results. In the comparison of methadone to buprenorphine, diamorphine and naltrexone, no significant differences were displayed for either treatment for self report dichotomous drug use (two studies, 370 participants (RR 1.04, 95% CI 0.69 to 1.55), continuous measures of drug use (one study, 81 participants, (mean difference (MD) 0.70, 95% CI ‐5.33 to 6.73); or criminal activity (one study, 116 participants, (RR 1.25, 95% CI 0.83 to 1.88) between methadone and buprenorphine. Similar results were found for comparisons with diamorphine with no significant differences between the drugs for self report dichotomous drug use for arrest (one study, 825 participants, (RR 1.25, 95% CI 1.03 to 1.51) or naltrexone for dichotomous measures of reincarceration (one study, 44 participants, (RR 1.10, 95% CI 0.37 to 3.26), and continuous outcome measure of crime, (MD ‐0.50, 95% CI ‐8.04 to 7.04) or self report drug use (MD 4.60, 95% CI ‐3.54 to 12.74).
Authors' conclusions
When compared to non‐pharmacological treatment, agonist treatments did not seem effective in reducing drug use or criminal activity. Antagonist treatments were not effective in reducing drug use but significantly reduced criminal activity. When comparing the drugs to one another we found no significant differences between the drug comparisons (methadone versus buprenorphine, diamorphine and naltrexone) on any of the outcome measures. Caution should be taken when interpreting these findings, as the conclusions are based on a small number of trials, and generalisation of these study findings should be limited mainly to male adult offenders. Additionally, many studies were rated at high risk of bias.
Keywords: Adult, Female, Humans, Male, Criminals, Buprenorphine, Buprenorphine/therapeutic use, Crime, Crime/prevention & control, Heroin, Heroin/therapeutic use, Methadone, Methadone/therapeutic use, Naltrexone, Naltrexone/analogs & derivatives, Naltrexone/therapeutic use, Narcotics, Narcotics/therapeutic use, Opiate Substitution Treatment, Opiate Substitution Treatment/methods, Randomized Controlled Trials as Topic, Substance‐Related Disorders, Substance‐Related Disorders/drug therapy
Plain language summary
Pharmacological interventions for drug‐using offenders
Background
Drug‐using offenders by their nature represent a socially excluded group in which drug use is more prevalent than in the rest of the population. Pharmacological interventions play an important role in the rehabilitation of drug‐using offenders. For this reason, it is important to investigate what we know works when pharmacological interventions are provided for offenders.
Study characteristics
The review authors searched scientific databases and Internet resources to identify randomised controlled trials (where participants are allocated at random to one of two or more treatment groups) of interventions to reduce, eliminate, or prevent relapse of drug use or criminal activity of drug‐using offenders. We included males and female of any age or ethnicity.
Key results
We identified 14 trials of pharmacological interventions for drug‐using offenders. The interventions included: (1) naltrexone in comparison with routine parole, social psychological treatment or both; (2) methadone maintenance in comparison with different counselling options; and (3) naltrexone, diamorphine and buprenorphine in comparison with a non‐pharmacological alternative and in combination with another pharmacological treatment. Studies could not be combined all together because the comparisons were too different. When compared to non‐pharmacological, we found low quality evidence that agonist treatments are not effective in reducing drug use or criminal activity . We found low quality of evidence that antagonist treatment was not effective in reducing drug use but we found moderate quality of evidence that they significantly reduced criminal activity. When comparing the drugs to one another we found no significant differences between the drug comparisons (methadone versus buprenorphine, diamorphine and naltrexone) on any of the outcome measures suggesting that one pharmacological drug does not preside over another. One study provided some cost comparisons between buprenorphine and methadone, but data were not sufficient to generate a cost‐effectiveness analysis. In conclusion, we found that pharmacological interventions do reduce subsequent drug use and criminal activity (to a lesser extent). Additionally, we found individual differences and variation between the degree to which successful interventions were implemented and were able to sustain reduction of drug use and criminal activity.
Quality of evidence
This review was limited by the lack of information reported in this group of trials and the quality of the evidence was low. The evidence is current to May 2014.
Summary of findings
Summary of findings for the main comparison. Summary of findings for the main comparisons: Agonist pharmacological compared to no intervention for drug‐using offenders.
| Agonist pharmacological compared to no intervention for drug‐using offenders | ||||||
| Patient or population: drug‐using offenders Settings: criminal justice Intervention: Agonist pharmacological Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Agonist pharmacological | |||||
| Drug use (objective) hair and urine analyses Follow‐up: 3 months to 4 years | Study population | RR 0.72 (0.51 to 1) | 237 (2 studies) | ⊕⊕⊝⊝ low1,2 | ||
| 43 per 100 | 31 per 100 (22 to 43) | |||||
| Moderate | ||||||
| 50 per 100 | 36 per 100 (25 to 50) | |||||
| Drug use self reported dichotomous self report information Follow‐up: 3 months to 4 years | Study population | RR 0.61 (0.31 to 1.18) | 317 (3 studies) | ⊕⊕⊝⊝ low3,4 | ||
| 74 per 100 | 45 per 100 (23 to 88) | |||||
| Moderate | ||||||
| 74 per 100 | 45 per 100 (23 to 88) | |||||
| Drug use self reported continuous self report information Follow‐up: 9 months to 4 years | The mean drug use self reported continuous in the intervention groups was 0.62 standard deviations lower (0.85 to 0.39 lower) | 510 (3 studies) | ⊕⊕⊝⊝ low5,6 | SMD ‐0.62 (‐0.85 to ‐0.39) | ||
| Criminal activity dichotomous ‐ Arrests official records Follow‐up: median 9 months | Study population | RR 0.6 (0.32 to 1.14) | 62 (1 study) | ⊕⊕⊝⊝ low7, 10 | ||
| 55 per 100 | 33 per 100 (18 to 63) | |||||
| Moderate | ||||||
| 55 per 100 | 33 per 100 (18 to 63) | |||||
| Criminal activity dichotomous ‐ Re‐incarceration official records Follow‐up: 7 months to 4 years | Study population | RR 0.77 (0.36 to 1.64) | 472 (3 studies) | ⊕⊕⊝⊝ low8,9 | ||
| 66 per 100 | 51 per 100 (24 to 100) | |||||
| Moderate | ||||||
| 83 per 100 | 64 per 100 (30 to 100) | |||||
| Criminal activity continuous mean number of crime dayes Follow‐up: median 9 months | The mean criminal activity continuous in the intervention groups was 74.21 lower (133.53 to 14.89 lower) | 51 (1 study) | ⊕⊕⊝⊝ low7, 11 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Across the two studies 10 of the 18 risk of bias items in total were rated as unclear. 2 The total number of events across the two studies is less than 300. This is a threshold rule of thumb based on Muller et al Ann Intern Med. 2007; 146: 878‐881. 3 Across the three studies 17 items were rated as unclear out of a total of 27 items. 4 The P value for heterogenity is less than 0.05 and the I2 is 89% suggesting significant inconsistency between the studies. 5 Across the three studies 16 of the 27 items on risk of bias were rated as unclear 6 The P value for heterogenity is less than 0.05 and the I2 is 99% suggesting significant inconsistency across the studies. 7 6 of the 9 risk of bias items were rated as unclear 8 Across the three studies 17 of the 27 risk of bias items in total were rated as unclear 9 The P value for heterogenity is less than 0.05 and the I2 is 74% suggesting significant heterogenity.
10 only 1 study with 62 participants
11 only 1 study with 51 participants
Summary of findings 2. Summary of findings for the main comparisons: Antagonost (Naltrexone) compared to no pharmacological for drug‐using offenders.
| Antagonost(Naltrexone) compared to no pharmacological for drug‐using offenders | ||||||
| Patient or population: patients with drug‐using offenders Settings: criminal justice Intervention: Antagonost(Naltrexone) Comparison: no pharmacological | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No pharmacological | Antagonost(Naltrexone) | |||||
| Criminal activity dichotomous ‐ Reincarceration official records Follow‐up: 6 months | Study population | RR 0.4 (0.21 to 0.74) | 114 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 39 per 100 | 16 per 100 (8 to 29) | |||||
| Moderate | ||||||
| 44 per 100 | 17 per 100 (9 to 32) | |||||
| drug use (objective) urine screen Follow‐up: 30 days prior to 6 months | Study population | RR 0.69 (0.28 to 1.7) | 63 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 28 per 100 | 19 per 100 (8 to 48) | |||||
| Moderate | ||||||
| 28 per 100 | 19 per 100 (8 to 48) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Across the two studies 9 of the 18 risk of bias items were rated as unclear 2 5 of the 9 risk of bias items was rated as unclear
3 only 1 study with 63 participants
Background
This review represents part of a family of four reviews undertaken to closely examine what works in reducing drug use and criminal activity among drug‐using offenders. Overall, the four reviews contain over 100 trials, generating a number of publications and numerous comparisons (Perry 2013a; Perry 2013b; Perry 2013c). The four reviews represent specific interests in pharmacological interventions, non‐pharmacological interventions, female offenders and offenders with co‐occurring mental illness. All four reviews stem from an updated previous Cochrane systematic review (Perry 2006). In this set of four reviews, we consider the effectiveness of interventions based on two key outcomes and analyse the impact of setting and intervention type. Presented here is the revised methodology for this individual review, focusing on the impact of pharmacological interventions provided for drug‐using offenders.
Description of the condition
Offenders as a socially excluded group of people demonstrate significant drug use and subsequent health problems. Studies investigating the prevalence of drug dependence in UK prisons report variable results of 10% (Gunn 1991), 39% (Brooke 1996), and 33% (Mason 1997). Similar trends have been reported elsewhere. In France, 30% of prison inmates are heroin addicted, and in Australia, 59% of prison inmates report injecting (primarily heroin) drug use histories. In the US, it is recognised that many offenders are in need of treatment to tackle their drug use (Lo 2000).The link between drug use, subsequent health and social and criminological consequences is well documented in the literature (e.g. Michel 2005), and offenders have a high risk of death from opioid overdose within two weeks of release from incarceration (Bird 2003; Binswanger 2007). Substance use disorders are linked to criminal behaviour and are a significant burden on the criminal justice system. Approximately 30% of acquisitive crime is committed by individuals supporting drug use with the use of criminal acts (Magura 1995).
Description of the intervention
Internationally, methadone maintenance has been the primary choice for chronic opioid dependence in prisons and jails, including those in the Netherlands, Australia, Spain and Canada, and it is being increasingly implemented in the criminal justice setting (Moller 2007; Stallwitz 2007). The US has not generally endorsed the use of methadone treatment, and only 12% of correctional settings offer this option for incarcerated inmates (Fiscella 2004). Reasons for this lack of expansion suggest that public opinion and that of criminal justice system providers consider methadone treatment as substituting one addiction for another. In contrast, buprenorphine appears not to carry the same social stigma associated with methadone treatment and has been used in France, Austria and Puerto Rico (Catania 2003; Reynaud‐Maurupt 2005; Garcia 2007). Naltrexone treatment has shown some promising findings, but associated problems surrounding high attrition and low medication compliance in the community and high mortality rates (e.g. Gibson 2007; Minozzi 2011) pose concerns. Trials conducted in the criminal justice setting are still lacking, and continuity of care is considered crucial in the treatment of drug‐involved offenders who transition between prison and the community.
How the intervention might work
A growing body of evidence shows the effects of pharmacological interventions for drug use among the general population. Existing reviews have focused on naltrexone maintenance treatment for opioid dependence (Amato 2005; Lobmaier 2008; Minozzi 2011); and the efficacy of methadone (Marsch 1998; Faggiano 2003; Mattick 2009); and buprenorphine maintenance (Mattick 2009). Recent guidance has been provided from the National Institute for Health and Clinical Excellence on evidence‐based use of naltrexone, methadone and buprenorphine for the management of opioid dependence (NICE 2007a; NICE 2007b). Five Cochrane reviews (including 52 studies) reported on the effectiveness of opiate methadone therapies (Amato 2005). Findings showed that methadone maintenance therapies at appropriate doses were most effective in retaining participants in treatment and in suppressing heroin use, but evidence of effectiveness for other relevant outcome measures such as criminal activity was weak and was not systematically evaluated.
Systematic reviews evaluating treatment programs more generally for offender populations have focused on evaluating treatment in one setting such as community‐based programmes, (e.g. Mitchell, 2012a; Mitchell, 2012b); or have based their evidence on literature from one country (e.g. Germany or the US) (Chanhatasilpa 2000; Egg 2000); or a number of specific treatments (Mitchell 2006). Pharmacological systematic reviews of offender treatment appear to be sparse. We identified two previous reviews, one focusing on specific drug‐ and property‐related criminal behaviours in methadone maintenance treatment (Marsch 1998); and an evaluation of the effectiveness of opioid maintenance treatment (OMT) in prison and post‐release (Hedrich 2011). The later of these two reviews identified six experimental studies up until January 2011 (Hedrich 2011). The authors found that OMT in prison was significantly associated with reduced heroin use, injecting and syringe sharing. Use of pre‐release OMT was also found to have important implications for associated treatment uptake after release, but the impact on criminal activity was equivocal.
Why it is important to do this review
The current review provides a systematic examination of trial evidence relating to the effectiveness of pharmacological interventions for drug‐using offenders. We believe it is important to conduct this review because the evidence about pharmacological interventions for drug‐using offenders has not been evaluated in this manner before. In order to address this broad topic a series of questions will consider the effectiveness of different interventions in relation to criminal activity, drug misuse treatment setting and type of treatment. The review will additionally report descriptively on the costs and cost effectiveness of such treatment programs.
Objectives
To assess the effectiveness of pharmacological interventions for drug‐using offenders in reducing criminal activity or drug misuse or both. The review addressed the following questions:
Does any pharmacological treatment for drug‐using offenders reduce drug use?
Does any pharmacological treatment for drug‐using offenders reduce criminal activity?
Does the treatment setting (e.g. court, community, prison/secure establishment) affect outcome(s) of pharmacological treatments?
Does one type of pharmacological treatment perform better than one other?
Additionally, this review aimed to report on the cost and cost‐effectiveness of interventions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Types of participants
We included illicit drug‐misusing offenders in the review regardless of gender, age, ethnicity or psychiatric illness. Drug misuse includes individuals occasionally using drugs, or who are dependent on, or are known to abuse, drugs. Offenders are defined as individuals who were subject to the criminal justice system.
Types of interventions
Included interventions were designed, wholly or in part, to eliminate or prevent relapse to drug use or criminal activity, or both, among participants. We defined relapse as individuals who may have returned to an incarcerated setting, or had subsequently been arrested or had relapsed back into drug misuse, or both. We included a range of different types of interventions in the review.
Experimental interventions included in the review:
Any pharmacological intervention (e.g. buprenorphine, methadone)
Control interventions included in the review.
No treatment
Minimal treatment
Waiting list
Treatment as usual
Other treatment (e.g. pharmacological or psychosocial)
Types of outcome measures
Primary outcomes
For the purpose of our review we categorised our primary outcomes into those relating to dichotomous and continuous drug use or criminal activity, or both. Where papers reported a number of different follow‐up periods, we report the longest time period, as we felt that such measures provide the most conservative estimate of effectiveness. For specific meta‐analyses of sub‐groupings, we reviewed all reported follow‐up periods to select the most appropriate time period for combining comparable studies.
Drug use measures were reported as:
self‐report drug use (unspecified drug, specific drug use not including alcohol/tobacco, Addiction Severity Index drug composite scores); and
biological drug use (measured by drugs tested by urine or hair analysis).
Criminal activity as measured by:
self‐report or official report of criminal activity (including arrest for any offence, drug offences, reincarceration, convictions, charges and recidivism).
Secondary outcomes
Our secondary outcome reported on costs or cost‐effectiveness information. We used a descriptive narrative for these findings. We undertook a full critical appraisal based on the Drummond 1997 checklist for those studies presenting sufficient information.
Search methods for identification of studies
Electronic searches
Electronic searches
The update searches identified records from 2004 to May 2014.
CENTRAL (Issue 5, 2014).
MEDLINE (1966 to May 2014).
EMBASE (1980 to May 2014).
PsycINFO (1978 to April 2014).
Pascal (1973 to November 2004)a.
SciSearch (Science Citation Index) (1974 to April 2014).
Social SciSearch (Social Science Citation Index) (1972 to April 2014).
ASSIA (1987 to May 2014).
Wilson Applied Science and Technology Abstracts (1983 to October 2004)a.
Inside Conferences (1993 to November 2004)a.
Dissertation Abstracts (1961 to October 2004)a.
NTIS (1964 to April 2014).
Sociological Abstracts (1963 to April 2014).
HMIC (to April 2014).
PAIS (1972 to April 2014).
SIGLE (1980 to June 2004)b.
Criminal Justice Abstracts (1968 to April 2014).
LILACS (2004 to April 2014).
National Research Register (March 2004)c.
Current Controlled Trials (December 2009).
Drugscope (February 2004)-unable to access.
SPECTR (March 2004)d.
aUnable to access further to 2004 search. bDatabase not updated since original 2004 search. cNo longer exists. dNow Campbell Collaboration searched on line.
To update the original review (Perry 2006), the search strategy was restricted to studies that were published or unpublished from 2004 onwards. A number of original databases were not searched for this update (indicated by the key at the end of the database list). Pascal, ASSIA, Wilson Applied Science and Technology Abstracts, Inside Conferences and Dissertation Abstracts were not searched. These databases are available only via the fee‐charging DIALOG online host service: we did not have the resources to undertake these searches. The National Research Register no longer exists, and SIGLE has not been updated since 2005. Drugscope is available only to subscribing members. The original searches were undertaken by Drugscope staff.
Search strategies were developed for each database to exploit the search engine most effectively and to make use of any controlled vocabulary. Search strategies were designed to restrict the results to RCTs. No language restriction was placed on the search results. We included methodological search filters designed to identify trials. Whenever possible, filters retrieved from the InterTASC Information Specialists' Sub‐Group (ISSG) Search Filter Resource site (http://www.york.ac.uk/inst/crd/intertasc/) were used. If filters were unavailable from this site, search terms based on existing filters were used instead.
In addition to the electronic databases, a range of relevant Internet sites (Home Office, National Institute of Drug Abuse (NIDA) and European Association of Libraries and Information Services on Alcohol and Other Drugs (ELISAD)) were searched. Directory web sites, including OMNI (http://www.omni.ac.uk), were searched up until November 2011. The review did not place any language restrictions on identification and inclusion of studies in the review.
Details of the update search strategies and results and of the Internet sites searched are listed in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12; Appendix 13.
Searching other resources
Reference checking
We scrutinised the reference lists of all retrieved articles for further references, and also undertook searches of the catalogues of relevant organisations and research founders.
Personal communication
We contacted experts for their knowledge of other studies, published or unpublished, relevant to the review.
Data collection and analysis
Selection of studies
Two authors independently inspected the search hits by reading the titles and abstracts, and obtained each potentially relevant study located in the search as a full‐text article to independently assess them for inclusion. In the case of discordance, a third independent author arbitrated. One author undertook translation of articles not written in the English language.
The screening process was divided into two key phases. Phase one used the initial seven key questions reported in the original new reference review. These were:
Prescreening criteria: phase one
Is the document an empirical study? [If "no" exclude document.]
Does the study evaluate an intervention, a component of which is designed to reduce, eliminate or prevent relapse among drug‐using offenders?
Are the participants referred by the criminal justice system at baseline?
Does the study report pre‐programme and post‐programme measures of drug use?
Does the study report pre‐programme and post‐programme measures of criminal behaviour?
Is the study a randomised controlled trial?
Do the outcome measures refer to the same length of follow‐up for two groups?
After relevant papers from phase one had been identified, phase two screening was performed to identify papers reporting on pharmacological interventions. Criteria included the following.
Prescreening: phase two
Is the intervention a pharmacological intervention? [if "yes" include document]
Drug‐using interventions were implied if the programme targeted reduced drug use in a group of individuals. Offenders were individuals either residing in special hospitals, prisons, the community (i.e. under the care of the probation service) or diverted from court or placed on arrest referral schemes for treatment. We included studies in the review where the sample were not entirely drug‐using, but reported pre‐ and post‐measures. The study setting could change throughout the process of the study, e.g. offenders could begin in prison but progress through a work‐release project into a community setting. Finally, studies did not need to report both drug and criminal activity outcomes: if either of these was reported we included the study in the review.
Data extraction and management
We used data extraction forms to standardise the reporting of data from all studies obtained as potentially relevant. Two authors independently extracted data and subsequently checked them for agreement.
Assessment of risk of bias in included studies
Five independent review authors (AEP, JMG, MM‐SJ, MN, RW) assessed risk of bias in all included studies using risk of bias assessment criteria recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The risk of bias assessment for RCTs in this review was performed using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane Review involves the use of a two‐part tool that addresses six specific domains, namely, sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. The first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement related to the risk of bias for that entry in terms of low, high or unclear risk. To make these judgements, we used the criteria indicated by the Cochrane Handbook for Systematic Reviews of Interventions as adapted for the addiction field.
The domains of sequence generation and allocation concealment (avoidance of selection bias) were addressed in the tool by a single entry for each study.
Blinding of participants, personnel and outcome assessor (avoidance of performance bias and detection bias) was considered separately for objective outcomes (e.g. dropping out, using substance of abuse as measured by urinalysis, relapsing of participants at the end of follow‐up, engaging of participants in further treatments) and subjective outcomes (e.g. duration and severity of signs and symptoms of withdrawal, participant self‐reported use of substance, side effects, social functioning as integration at school or at work, family relationships).
Incomplete outcome data (avoidance of attrition bias) were considered for all outcomes except dropping out of treatment, which very often is the primary outcome measure in trials on addiction. See Appendix 14 for details.
For studies identified in the most recent search, the review authors attempted to contact study authors to establish whether a study protocol was available.
Measures of treatment effect
The mean differences (MD) were used for outcomes measured on the same scale and the standardised mean difference (SMD) for outcomes measured on different scales. Higher scores for continuous measures are representative of greater harm. We present dichotomous outcomes as risk ratios (RR), with 95% confidence intervals (CIs).
Unit of analysis issues
To avoid double counting of outcome measures (e.g. arrest and parole violation) and follow up time periods (e.g. 12, 18 months) all trials were checked to ensure that multiple studies reporting the same evaluation did not contribute towards multiple estimates of programme effectiveness. We followed Cochrane guidance and where appropriate we combined intervention and control groups to create a single pairwise comparison. Where this was not appropriate we selected one treatment arm and excluded the others.
Dealing with missing data
Where we found data was missing in the original publication, we attempted to contact the study authors via email to obtain the missing information.
Assessment of heterogeneity
Heterogenity was assessed using I² and Q statistics (Higgins 2011).
Data synthesis
The RevMan software package was used to perform a series of meta‐analyses for continuous and dichotomous outcome measures (Review Manager 2014). A random‐effects model was used to account for the fact that participants did not come from a single underlying population. A narrative review were performed to address each of the key questions outlined in the objectives. The narrative tables included a presentation of study details (e.g. author, year of publication, and country of study origin), study methods (e.g. random assignment), participants (e.g. number in sample, age, gender, ethnicity, age, mental health status), interventions (e.g. description, duration, intensity, setting), outcomes (e.g. description, follow‐up period, reporting mechanism), resource and cost information and resource savings (e.g. number of staff, intervention delivery, estimated costs, estimated savings), and notes (e.g. methodological and quality assessment information). For outcomes of criminal activity, data were sufficient to allow the review authors to divide this activity into "re‐arrest" and reincarceration categories.
Subgroup analysis and investigation of heterogeneity
A separate subgroup analysis of the studies was planned by different types of treatments and different settings.
Sensitivity analysis
When appropriate, sensitivity analyses were planned to assess the impact of studies with high risk of bias. Because of the overall high risk of bias of the included studies, this analysis was not conducted.
Results
Description of studies
Results of the search
Original review
The original searches spanned from database inception to October 2004. This identified a total of 8217 records after duplication. We acquired a total of 90 full text papers for assessment and excluded 66 papers, bringing 24 trials to the review (see Figure 1).
1.
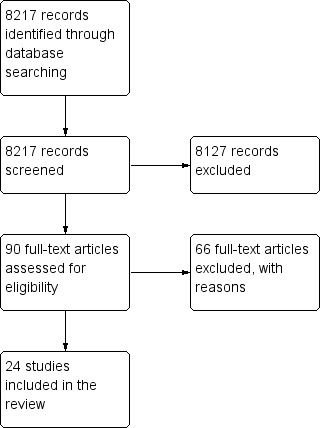
Study flow diagram of paper selection: Original Review
First update
The updated searches spanned from October 2004 until March 2013. This identified a total of 3896 records after duplication. We acquired a total of 115 full text papers for assessment and excluded 105 papers, bringing 10 new trials to the review (see Figure 2).
2.
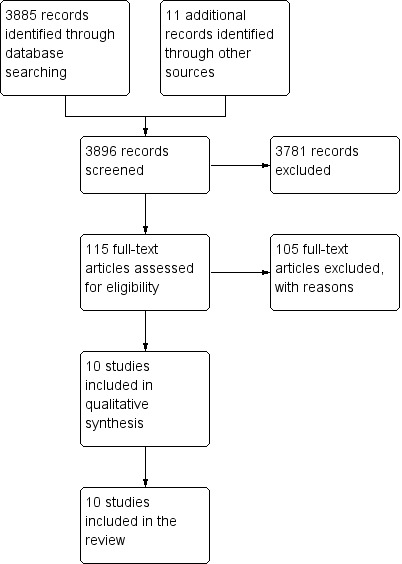
Study flow diagram of paper selection: First Update
Second update
The updated searches spanned from March 2013 until May 2014. This identified a total of 2092 records after duplication. We acquired a total of 72 full text papers for assessment and excluded 68 papers, bringing four new trials to the review making a total of 14 trials (see Figure 3).
3.
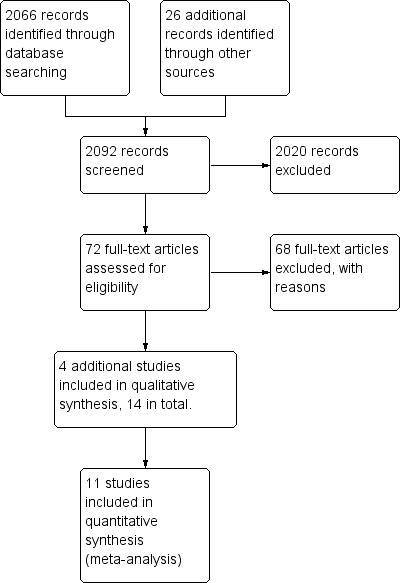
Study flow diagram of paper selection: Second Update
Included studies
The studies were published between 1969 and 2014 and represented 14 trials, including 2647 participants. The 14 trials consisted of 18 trial publications on different interventions (Bayanzadeh 2004; Brown 2013; Cornish 1997; Cropsey 2011; Coviello 2010; Dolan 2003; Dole 1969; Howells 2002; Kinlock 2005; Kinlock 2007; Lobmaier 2010; Lobmann 2007; Magura 2009; Wright 2011). Two trials represented data from multiple follow‐up publications. The Dolan studies published data on the primary study and four year follow‐up data (Dolan 2003); and Kinlock and colleagues reported on outcome measures and a secondary analysis of the data in two subsequent publications (see Kinlock 2007). See Table 3 for a summary of study information and outcomes.
1. Table 1 summary of outcomes and comparisons.
| Study | Setting | Intervention | Comparison group | Follow‐up period | Outcome type | Outcome description |
| Bayanzadeh 2004 | Prison | Methadone treatment in combination with CBT and widely focused on coping and problem‐solving skills. | Non‐methadone drugs plus standard psychiatric services and therapeutic medications | 6 months | Biological drug use Self‐report drug use |
Drug use yes/no Frequency of drug injections (percentage) Syringe sharing Morphine urine analysis |
| Brown 2013 | Community | Methadone | Primary care plus suboxone (buprenorphine and naloxone) | 6 months 12 months |
Biological drug use Self report drug use |
Frequency and pattern of daily drug use Addiction Severity Index (self report). Lite, HIV risk behaviours (RAB ‐ Risk Assessment Battery short version), and health services utilization. Urine drug screens were collected as a part of routine management. |
| Cornish 1997 | Community | Naltrexone | Routine parole/probation | 6 months and during 6 months of treatment | Criminal activity dichotomous | % reincarcerated during 6 months of follow‐up |
| Coviello 2010 | Community | Naltrexone | Psychosocial treatment only | 6 months | Biological drug use dichotomous Criminal activity dichotomous |
% positive urine drug screen opioids % positive urine drug screen cocaine % violating parole/probation |
| Cropsey 2011 | Community | Buprenorphine | Placebo | End of treatment 3 months |
Biological drug use dichotomous Self‐report drug use dichotomous |
% positive urine opiates % self‐report injection drug use |
| Dolan 2003 | Prison | Pharmacological (methadone) | Waiting list control | 4 months 2 months 3 months |
Biological drug use continuous Biological drug use dichotomous Self‐report drug use dichotomous |
% hair positive for morphine % self‐reported any injection % self‐reported heroin injection |
| Dole 1969 | Prison | Methadone | Waiting list control. | At between 7 and 10 months At 50 weeks |
Biological drug use continuous Biological drug use dichotomous Self‐report drug use dichotomous |
Heroin use Reincarceration Treatment retention Employment |
| Howells 2002 | Prison | Methadone and placebo | Lofexidine and placebo | Post treatment | Self report data on withdrawl Severity of psychological dependence |
Withdrawal symptom severity measured using two withdrawal scales: the 20‐item Withdrawal Problems Scale (WPS), and the eight item Short Opiate Withdrawal Scale (SOWS). Secondary outcome measures were rates and timing of withdrawal from the detoxification programme so that the relationship between failure to complete detoxification and severity of withdrawal symptoms could be measured. The Severity of Dependency Scale (SDS) was also used to assess the severity of psychological aspects of drug dependence. |
|
Kinlock 2007 |
Prison | Counselling + methadone initiation pre‐release(a) and post‐release (b) | Counselling only | 1 month 3 months 6 months 12 months |
Biological drug use dichotomous Self‐report drug use dichotomous Criminal activity dichotomous |
% positive for urine opioids % positive for urine cocaine % self‐reported 1 or more days heroin n used heroin for entire 180‐day follow‐up period Re‐incarcerated Self‐reported criminal activity |
| Kinlock 2005 | Prison | Prison based levo alpha acetyl methanol and transfer to methadone after release | untreated controls | During 9 months | Biological drug use dichotomous Self‐report drug use dichotomous Criminal activity dichotomous |
Heroin use Arrest Re incarceration Frequency of illegal activity Admission drug use Average weekly income |
| Lobmaier 2010 | Prison | Naltrexone | Methadone | 6 months | Criminal activity continuous Criminal activity dichotomous Self‐report drug use continuous |
Mean days of criminal activity % re‐incarcerated Mean days of heroin use Mean days of benzodiazepine use Mean days of amphetamine use |
| Lobmann 2007 | Community | Pharmacological (diamorphine) | Methadone | 12 months | Criminal activity dichotomous | % self‐reported criminal activity % police‐recorded offences |
| Magura 2009 | Prison | Buprenorphine | Methadone | 3 months | Criminal activity dichotomous Self‐report drug use continuous Self‐report drug use dichotomous |
% re‐incarcerated % arrested for property crime % arrested for drug possession Mean days of heroin use % any heroin/opioid use |
| Wright 2011 | Prison | Buprenorphine | Methadone | 1 month 3 months 6 months post detoxification |
Biological drug test Self report official drug records |
Abstinence from illicit opiates at 8 days post detoxification, as indicated by a urine test. If the participant had been released, local community drugs service records were accessed to verify abstinence. |
A number of studies produced different comparisons and were combined appropriately according to time point of measurement (e.g. 1 month, 3 months, 6 months, 12 months) and type of outcome.
Treatment regimens and settings
Thirteen studies used methadone as the intervention or for comparison (Bayanzadeh 2004; Brown 2013; Dolan 2003; Dole 1969; Howells 2002; Kinlock 2005; Kinlock 2007; Lobmaier 2010; Lobmann 2007; Magura 2009; Wright 2011). Brown 2013 compared specialist treatment plus suboxone or methadone versus primary care plus suboxone; Lobmann 2007 compared methadone with diamorphine; and Magura 2009 and Wright 2011 compared methadone with buprenorphine. One study compared methadone to lofexidine (Howells 2002). All other studies compared methadone maintenance with interventions where there was no drugs administration (waiting list or counselling alone).
Three studies used naltrexone in oral and implantation formats in comparison with probation or parole (Cornish 1997); psychosocial therapy (Coviello 2010); and methadone (Lobmaier 2010).
One study compared the use of buprenorphine with a placebo (Cropsey 2011).
The studies were categorised by setting; five studies were conducted in the community (Cornish 1997; Lobmann 2007; Coviello 2010; Cropsey 2011; Brown 2013); and the remainder in secure settings (Dole 1969; Dolan 2003; Bayanzadeh 2004; Kinlock 2005; Kinlock 2007; Magura 2009; Lobmaier 2010; Howells 2002; Wright 2011).
One study was conducted using a jail diversion scheme for either a drug treatment court or Treatment Alternative Program (TAP) (Brown 2013).
Different outcome measures were presented for each study, and just over half of all studies reported four or more outcome measures (see Table 3). Criminal justice and drug outcomes were measured by all studies except four. Cornish 1997 and Lobmann 2007 reported on criminal activity outcomes only; and Bayanzadeh 2004, Brown 2013, Dolan 2003, Cropsey 2011 and Wright 2011 reported on drug use only.
Countries in which the studies were conducted
Nine studies were published in the US, two in England, one in Iran, one in Australia, one in Norway and one in Germany.
Duration of trials
Most studies (n = 10) reported outcomes of six months or less, and the longest follow‐up period was four years.
Participants
The fourteen studies included adult drug‐using offenders: twelve of the fourteen studies used samples with a majority of men and one study used female offenders only (Cropsey 2011). In two studies, gender was not reported (Lobmann 2007; Wright 2011).
The average age of study participants ranged from 27 years to 40.9 years.
Excluded studies
We excluded 165 studies. See Characteristics of excluded studies for further details. Reasons for exclusion were: lack of criminal justice involvement in referral to the intervention; not reporting relevant drug or crime outcome measures or both at both the pre‐ and post‐intervention periods; allocation of participants to study groups that were not strictly randomised or did not contain original trial data. The majority of studies were excluded because the study population were not offenders. One study was excluded because follow‐up periods were not equivalent across study groups (Di Nitto 2002); and Berman 2004 was excluded because the intervention (acupuncture) did not measure our specified outcomes of drug use or criminal activity. One study reported the protocol of a trial only (Baldus 2011); while another only contained conference proceedings (Kinlock 2009a). We were unable to obtain the data for one trial (Cogswell 2011); or the full‐text version of another (Rowan‐Szal 2005).
Risk of bias in included studies
See Figure 4 and Figure 5 for further information.
4.
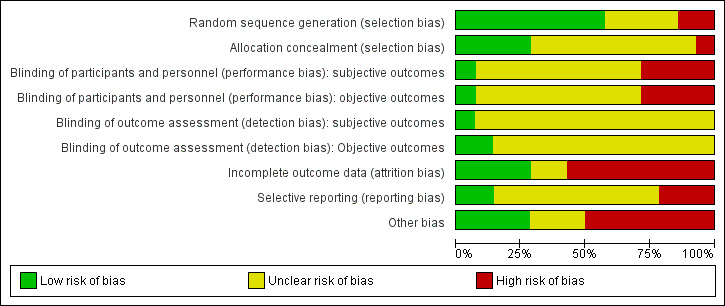
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.
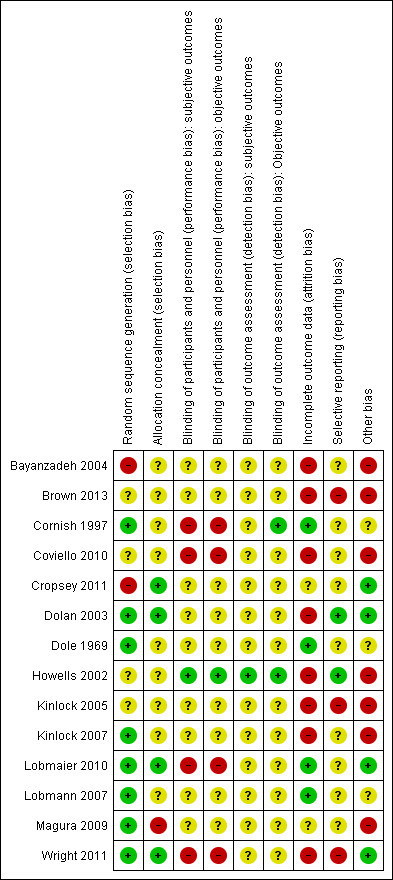
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation: All of the 14 included studies were described as randomised. In four studies, the reporting of this information was noted as unclear, as it was difficult to find an accurate description of the methodology used (Brown 2013; Coviello 2010; Howells 2002; Kinlock 2005). Two studies were reported at high risk of bias (Bayanzadeh 2004; Cropsey 2011); and the remaining eight studies at low risk of bias.
Allocation concealment: Of the 14 included studies, only four reported that the allocation process was concealed and were rated at low risk of bias (Cropsey 2011; Dolan 2003; Lobmaier 2010; Wright 2011). One study was rated at high risk of bias (Magura 2009). All of the remaining nine studies were rated as unclear, and the review author was not able to decide whether allocation concealment had occurred within the studies.
Blinding
Blinding was assessed across four dimensions considering performance and detection bias across subjective and objective measures (see Appendix 14). Nine studies were rated as unclear risk of bias providing no information on blinding across all four domains (Bayanzadeh 2004; Brown 2013; Cropsey 2011; Dolan 2003; Dole 1969; Kinlock 2005; Kinlock 2007; Lobmann 2007; Magura 2009). Four studies were rated at high risk of bias for participant and personnel blinding (Cornish 1997; Coviello 2010; Lobmaier 2010; Wright 2011). Cornish 1997 was rated at low risk of outcome assessors on objective measures.
Incomplete outcome data
Four studies were noted at low risk of bias (Cornish 1997; Dole 1969; Lobmaier 2010; Lobmann 2007); eight studies were noted at high risk of bias; and two studies were rated as unclear (Cropsey 2011; Magura 2009).
Selective reporting
Of the 14 studies, nine studies were rated as unclear, and two studies were rated at low risk (Dolan 2003; Howells 2002). Three studies were rated at high risk of bias (Brown 2013; Kinlock 2005; Wright 2011).
Other potential sources of bias
Threats to other bias within the study designs generally yielded mixed results. In total, seven studies were rated at high risk. Low risk was noted in four further studies (Cropsey 2011; Dolan 2003; Lobmaier 2010; Wright 2011); and three studies were rated as unclear (Cornish 1997; Dole 1969; Lobmann 2007).
Effects of interventions
Of the 14 studies, 11 were included in a series of meta‐analyses and the main comparisons are presented in the 'Summary of findings' tables (Table 1; Table 2). Three studies were not included in the meta‐analyses: Bayanzadeh 2004 because it compared methadone + CBT versus not further specified non‐pharmacological treatment, so it was not possible to ascertain the effect of methadone treatment alone; Brown 2013 because it compared specialist treatment plus suboxone or methadone versus primary care plus suboxone, so it was not possible to ascertain the effect of methadone or suboxone alone; moreover it did not assess the outcomes of interest; and Howells 2002 because it did not assess the outcomes of interest and repeated attempted contact with the authors asking for more information was unsuccesful. For those studies that were included we grouped them by drug and criminal activity outcomes (re‐arrest and reincarceration), setting (community and secure establishment), and intervention type (buprenorphine, methadone and naltrexone). Tests for heterogeneity at the 0.01 level revealed that across all meta‐analyses, the studies were found to be homogeneous.
1. Agonist pharmacological interventions vs no non‐pharmacological treatment
Drug use
See Table 1
For dichotomous measure, results did not show reduction in drug use for objective results (biological), two studies, 237 participants: (RR 0.72, 95% CI 0.51 to 1.00), low quality of evidence and for subjective (self‐report), three studies, 317 participants: (RR 0.61 95% CI 0.31 to 1.18), low quality of evidence. Also for continuous measures, self‐report drug use did not show differences, three studies, 510 participants: (SMD ‐0.62 95% CI ‐0.85 to ‐0.39), low quality of evidence, see Analysis 1.1; Analysis 1.2; and Analysis 1.3.
1.1. Analysis.
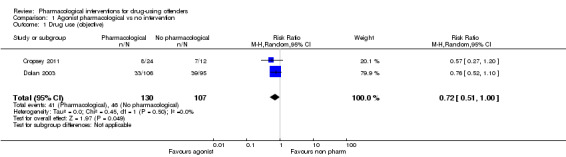
Comparison 1 Agonist pharmacological vs no intervention, Outcome 1 Drug use (objective).
1.2. Analysis.
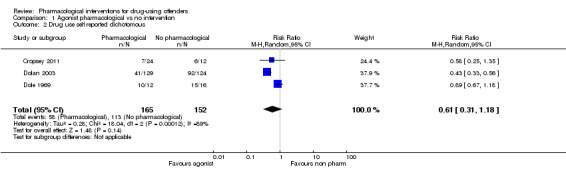
Comparison 1 Agonist pharmacological vs no intervention, Outcome 2 Drug use self reported dichotomous.
1.3. Analysis.
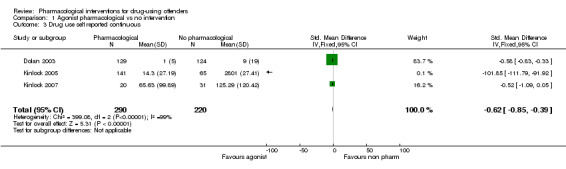
Comparison 1 Agonist pharmacological vs no intervention, Outcome 3 Drug use self reported continuous.
Criminal activity
See Table 1
All data come from studies assessing the efficacy of methadone treatment. Both for reincarceration three studies, 472 participants (RR 0.77, 95% CI 0.36 to 1.64) low quality of evidence; and re‐arrests, one study, 62 participants (RR 0.60, 95% CI 0.32 to 1.14), low quality of evidence, the studies did not show difference (see Analysis 1.4). The impact on criminal activities was evaluated also utilising continuous measures in one study, 51 participants: MD of ‐74.21 (95% CI ‐133.53 to ‐14.89), low quality of evidence, the result is in favour of pharmacological interventions, (see Analysis 1.5).
1.4. Analysis.
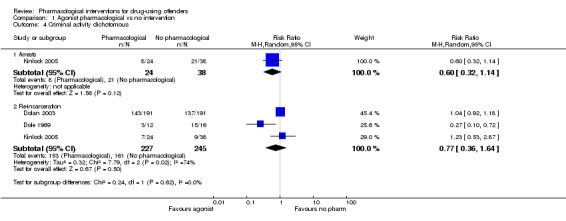
Comparison 1 Agonist pharmacological vs no intervention, Outcome 4 Criminal activity dichotomous.
1.5. Analysis.
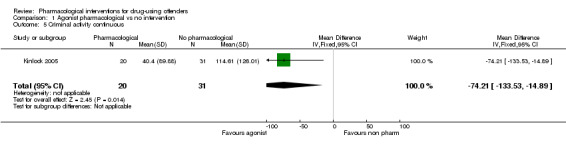
Comparison 1 Agonist pharmacological vs no intervention, Outcome 5 Criminal activity continuous.
2. Antagonist (Naltrexone) pharmacological treatment vs non ‐pharmacological treatment?
See Table 2
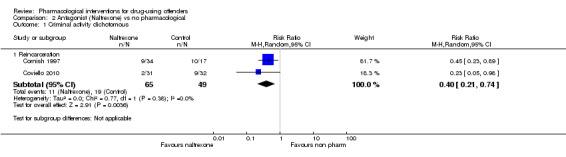
Two studies, 114 participants focused on the use of naltrexone versus no pharmacological treatment and subsequent criminal activity. The results indicate that naltrexone does appear to reduce subsequent reincarceration, with an RR of 0.40 (95% CI 0.21, 0.74), moderate quality of evidence, see Analysis 2.1
2.1. Analysis.
Comparison 2 Antagonist (Naltrexone) vs no pharmacological, Outcome 1 Criminal activity dichotomous.
One study, 63 participants (RR 0.69, 95% CI 0.28 to 1.70) did not show statistically significant difference, low quality of evidence, see Analysis 2.2,
2.2. Analysis.
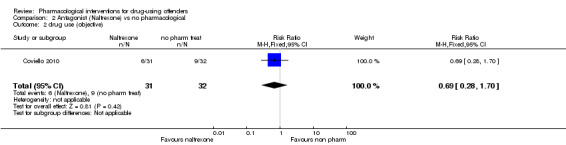
Comparison 2 Antagonist (Naltrexone) vs no pharmacological, Outcome 2 drug use (objective).
3. Methadone versus buprenorphine
Drug use
Two studies (Magura 2009; Wright 2011), showed a reduction in self report drug use for 370 participants using a dichotomous outcome (RR 1.04. 95% CI 0.69 to 1.55) altough the result is not statistically significant. Continuous outcomes, one study with 81 participants, (MD 0.70, 95% CI ‐5.33 to 6.73) see Analysis 3.1 and Analysis 3.2 .
3.1. Analysis.
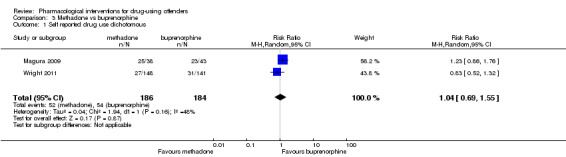
Comparison 3 Methadone vs buprenorphine, Outcome 1 Self reported drug use dichotomous.
3.2. Analysis.
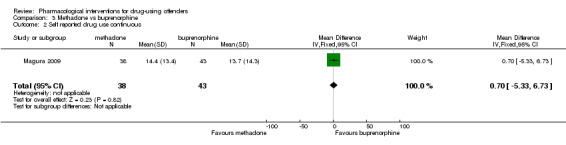
Comparison 3 Methadone vs buprenorphine, Outcome 2 Self reported drug use continuous.
Criminal activity
Magura 2009 showed a non‐statistically significant reduction in criminal activity for 116 participants (RR 1.25, 95% CI 0.83 to 1.88) see Analysis 3.3.
3.3. Analysis.
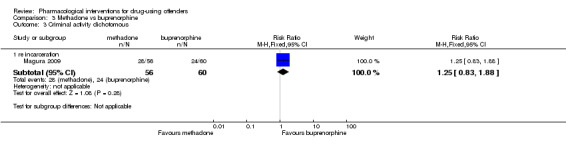
Comparison 3 Methadone vs buprenorphine, Outcome 3 Criminal activity dichotomous.
4. Methadone versus diamorphine
Drug use: the study did not assess this outcome
Criminal activity
Rearrest: One study, (Lobmann 2007) 825 participants shows a non‐statistically significant reduction in criminal activity for re‐arrests: (RR 1.25, 95% CI 1.03 to 1.51 see Analysis 4.1.
4.1. Analysis.
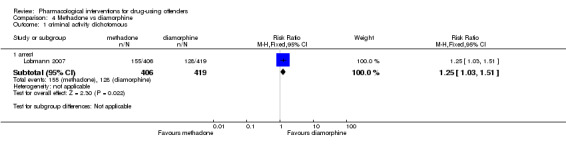
Comparison 4 Methadone vs diamorphine, Outcome 1 criminal activity dichotomous.
5. Methadone vs naltrexone
Drug use
Lobmaier 2010, 44 participants, showed a non‐statistically significant reduction in self reported drug use continuous MD 4.60 (95% CI ‐3.54 to 12.74) see Analysis 5.1.
5.1. Analysis.
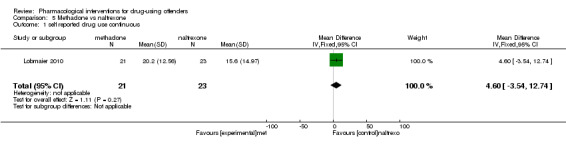
Comparison 5 Methadone vs naltrexone, Outcome 1 self reported drug use continuous.
Criminal activity
Lobmaier 2010, 44 participants, showed a non‐statistically significant reduction in dichotomous reincarceration, outcomes (RR 1.10, 95% CI 0.37 to 3.26) and continuous outcomes (MD ‐0.50, 95% CI ‐8.04 to 7.04) see Analysis 5.2; Analysis 5.3.
5.2. Analysis.
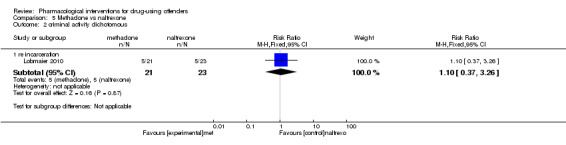
Comparison 5 Methadone vs naltrexone, Outcome 2 criminal activity dichotomous.
5.3. Analysis.
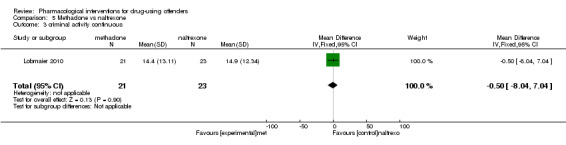
Comparison 5 Methadone vs naltrexone, Outcome 3 criminal activity continuous.
Does setting of intervention (community, prison/secure establishment) affect outcomes of pharmacological interventions?
All the studies comparing methadone versus non‐pharmacological intervention were conducted in a secure setting; the only study comparing buprenorphine with non‐pharmacological intervention was conducted in the community, as well as the two studies comparing naltrexone with non‐pharmacological treatment. In the other comparison only one study was included for each, so it was not possible to perform a subgroup analysis for setting of the intervention.
Cost and cost‐effectiveness
The Magura study noted differences in the costs of administering buprenorphine and methadone, but were not sufficient for us to conduct a full cost effectiveness appraisal (Magura 2009). The investigators estimated that about ten times as many inmates can be served with methadone as with buprenorphine with the same staff resources. This cost implication is also endorsed in the community, where physicians have difficulty in obtaining reimbursement for buprenorphine treatment for released inmates, making the continued use of buprenorphine problematic after release.
Discussion
Summary of main results
This systematic review provides evidence from 14 trials producing several meta‐analyses. Studies could not be combined all together because the comparisons were too different. Only subgroup analysis for type of pharmacological treatment was done. Findings of the effects of individual interventions on drug use and criminal activity show mixed results. When compared to non‐pharmacological, we found low quality evidence that agonist treatments are not effective in reducing drug use or criminal activity. We found low quality of evidence that antagonist treatment was not effective in reducing drug use but we found moderate quality of evidence that they significantly reduced criminal activity. When comparing the drugs to one another we found no significant differences between the drug comparisons (methadone versus buprenorphine, diamorphine and naltrexone) on any of the outcome measures suggesting that no one pharmacological drug is more effective than another. Two studies provided some cost comparisons, but data were not sufficient to generate a cost‐effectiveness analysis. In conclusion, we found that pharmacological interventions do reduce subsequent drug use and (to a lesser extent) criminal activity. Additionally, we found individual differences and variation on different outcome measures when pharmacological interventions were compared to a non‐pharmacological treatment but no significant differences when compared to another pharmacological treatment.
Buprenorphine
The Cropsey study specifically evaluated buprenorphine for opioid‐dependent women with HIV risk and found that buprenorphine given to participants in prison (followed by its use upon release into the community) was beneficial in preventing or delaying relapse to opioid use (Cropsey 2011). The findings of this study add to the growing body of evidence (which primarily includes men) suggesting that outcomes with buprenorphine are comparable with what others have found with both methadone and methadone maintenance (Lobmaier 2010). The findings however were not sustained post treatment, and most women had relapsed to active opioid treatment at the three‐month follow‐up point. Future studies on the use of buprenorphine in women should evaluate its impact on long‐term effects with the goal of assessing its effect on opioid abstinence and prevention of associated criminal activity (Cropsey 2011). Overall, the dosage of buprenorphine varied between studies; in one study, instances of 30 mg rising to 130 mg were reported (Lobmaier 2010). A meta‐analysis of buprenorphine dose and treatment outcome found that a higher dosage (16 to 32 mg per day) predicted better retention in treatment when compared with a lower dosage (Fareed 2012). Another Cochrane review (outside the prison environment) indicated that buprenorphine detoxification and maintenance studies concluded that completion of withdrawal treatment is possibly more likely when managed with buprenorphine compared to methadone although the difference was not statistically significant, leading the authors to conclude that more research is needed to evaluate the possible differences between the two medications (Gowing 2009). The Wright 2011 study in this review suggests that there is equal clinical effectiveness between buprenorphine and methadone in maintaining abstinence at eight days post detoxification in prison. As many prisoners are eventually released back into the community the authors note that GPs need to be aware of the few trials which compare two of the most common detoxification agents in the UK. The research currently supports the use of either buprenorphine or methadone within a detoxification setting (Wright 2011).
Methadone
Two studies showed a decrease in self‐report methadone treatment upon release into the community (Dole 1969; Magura 2009). The Dole study, albeit small, found that 3 of 12 prisoners who started using methadone before release were convicted of new crimes during an 11.5‐month follow‐up compared with 15 of 16 prisoners randomly assigned to a control condition (Dole 1969); and a larger, more recent study found that Rikers Island MMT programme in New York significantly facilitated entry and retention at six months in post release programmes (Magura 2009). In contrast, another study reported on opioid agonist maintenance by examining levo‐alpha‐acetylmethadol (LAAM) before prison release and found no significant differences with regard to subsequent arrest of participants who received LAAM and a control group at nine months post‐release (Kinlock 2005). Subsequent Kinlock studies involving evaluations of counselling only and counselling with transfer in comparison with counselling and methadone support the findings of Dole 1969 and Dolan 2003 suggesting that methadone programmes can provide effective opioid agonist therapy for prisoners with a history of heroin addiction but not arrest at 12 month post prison release (Kinlock 2007). Taken together, the findings also suggest that increased criminal activity and overdose death are disproportionately likely to occur within one month of release from incarceration. The authors conclude that making connections with drug treatment services at release from prison is likely to help sustain treatment for opiod addictions; such findings are supported by other studies which found that offering pre‐release MMT and payment assistance was significantly associated with increased enrolment in post‐release MMT and reduce time to enter community‐based MMT (e.g. Binswanger 2007). Additionally, in support of methadone treatment, the World Health Organisation has listed methadone as an essential medication and has strongly recommended that treatment should be made available in prison and supported subsequently within the community to significantly reduce the likelihood of adverse health and criminogenic consequences (Hergert 2005).
Dosage of methadone treatment varied across studies. For example, Magura 2009 reported problems with the use of suboptimal doses of methadone when higher doses were available. Investigators argue that higher doses appear to reflect participant preference because most did not intend to continue treatment after release. The Dolan study reported moderate doses of methadone (61 mg) and noted that outcomes might have improved if higher doses had been given (Dolan 2003). Significantly lower doses of methadone were noted in the Dole study, in which 10 mg of methadone per day was increased to a dosage of 35 mg per day (Dole 1969). Participants in the Kinlock 2005 study were medicated three times per week, starting at 10 mg and increasing by 5 mg every third medication day during incarceration to a target dose of 50 mg. Evidence from the Amato 2005 review suggests that low dosages of methadone maintenance lead to compromise in the effectiveness of treatment and that recommendations for dosage should be monitored at around 60 mg. Additional systematic review evidence considering the use of methadone and a tapered dose for the management of opioid withdrawal shows a wide range of programmes with differing outcome measures, making the application of meta‐analysis difficult (Amato 2013). The authors conclude that slow tapering with temporary substitution of long‐acting opioids can reduce withdrawal severity; however, most participants still relapsed to heroin use (Amato 2013).
Naltrexone
For evaluation of naltrexone, two studies (one pilot: Cornish 1997) and Coviello 2010, a subsequent larger replication trial, show that use of a larger sample size consisting of a diverse group of offenders resulted in no differences in criminal behaviour between naltrexone and treatment‐as‐usual groups. The authors note that one of the major differences between the two studies remains the extent and quality of supervision provided by parole officers. The authors suggest that for treatment to be successful, use of oral naltrexone by probationers and parolees requires more supervision than is typically available within the criminal justice system. Study authors reported instances of 35 mg of naltrexone rising to 300 mg (Coviello 2010). Other research evidence related to naltrexone use and mortality rates highlights possible concerns about the high risk of death after treatment. Gibson 2007 compared mortality rates associated with naltrexone and methadone by using retrospective data analysis of coronial participants between 2000 and 2003. Findings show that participants receiving naltrexone were up to 7.4 times more likely to die after receiving treatment when compared with those using methadone over the same time period. Although this study was not conducted in a population of prisoners, it is likely that such risks are comparable; therefore generalised use of naltrexone and associated subsequent supervision of those taking naltrexone in its oral form require careful consideration.
Overall completeness and applicability of evidence
Overall, the findings of this review suggest that pharmacological interventions have an impact on reducing self‐report drug use. Individual pharmacological drugs had differing effects, particularly in relation to subsequent drug use. Promising results highlight the use of methadone or buprenorphine (although this was only one study) within a prison environment but may be limited to shorter‐term outcomes when prisoners are released into the community. For naltrexone, the evidence is sparse and presents problems associated with different mechanisms of drug administration (e.g. oral versus implants). We can say little about the cost and cost‐effectiveness of these studies. One study reported some descriptive cost information, but the information was insufficient to generate a cost analysis (Magura 2009). In conclusion, high‐quality research is required to evaluate the processes involved in the engagement of offenders mandated to substance abuse programmes to enable us to understand better why one programme works and another does not.
Quality of the evidence
A number of limitations within each of the studies are highlighted by the authors. High dropout rates were noted in the methadone group after prison release in the Lobmaier study and appear to be more difficult to maintain in offender populations (Lobmaier 2010). Major limitations of the Coviello 2010 study included low treatment retention and low six‐month follow‐up rates. Most offenders did not return for the follow‐up evaluation because they could not be located (63%). Only two‐thirds of treated participants remained in treatment in the Dolan study (Dolan 2003). As a consequence, the study does not provide conclusive evidence regarding the efficacy of oral naltrexone in this offender sample. Attrition was also a problem in Kinlock 2005; this was due in part to the fact that individuals were being transferred to other prisons or were having their sentences extended because of pre‐existing charges (Kinlock 2005). Similiar problems of segregation and impact of sentence releases affected the sample size in the Bayanzadeh 2004 and Wright 2011 studies whereby transfer to other prison establishments with little prior warning made follow‐up data difficult to collect. Such attrition within studies threatens the comparability of experimental and control groups, thereby ensuring that any conclusions should be taken with considerable caution. In particular, the Bayanzadeh 2004 study noted some of the practical difficulties associated with contamination between experimental and control groups, given that the ideal would be to keep the groups apart. In contrast the pilot study by Brown 2013 produced a study retention rate of 80%; the authors note that this may be due to the coercive nature of participation in jail diversion programs in which successful completion may result in the dismissal or reduction of criminal charges. Although this finding is represented by only one study it suggests the possibility that completion of drug treatment programs might fare best when an incentive which effects sentence or charge outcome can be sustained.
Sample sizes were considered modest in a number of studies, with attrition presenting difficulties in interpretation of study findings. For example, 30% attrition at follow‐up producing possible threats to the internal validity of the study design in Magura 2009 and similar small sample sizes in the Lobmaier trial may have been too small to reveal any differences between the two treatment conditions (Lobmaier 2010). The Cropsey 2011 study identified a sample of 36 women and randomly allocated 15 to the intervention and 12 to the placebo group. Investigators note that although the potency of buprenorphine for control of opioid use is clearly demonstrated, a larger sample size may be needed to detect significant differences between groups on other variables of interest. Larger trials are therefore required to assess the possible advantages of one treatment over the other. Additionally, the study was limited to three months of treatment, and further studies should explore the provision of buprenorphine for longer periods of time to prolong opioid abstinence and prevent associated criminal activity. Similiar short follow‐up periods were noted in other trials, including Dolan 2003.
Potential biases in the review process
Despite limitations associated with the literature, two limitations in review methodology were achieved. Specifically, the original review included an additional five fee paying databases and one search using DrugScope. In this current review resources did not allow such extensive searching. Whislt the electronic databases searches have been updated to April 2014. the web site search has been updated to November 2011. As a result some literature may have been missed from this current review
Authors' conclusions
Implications for practice.
When compared to non‐pharmacological treatments, agonist treatments did not seem effective in reducing drug use or criminal activity Antagonist treatment was not effective in reducing drug use but significantly reduced criminal activity. When comparing the drugs to one another we found no significant differences between the drug comparisons (methadone versus buprenorphine, diamorphine and naltrexone) on any of the outcome measures. Caution should be taken when interpreting these findings, as the conclusions are based on a small number of trials, and generalisation of these study findings should be limited mainly to male adult offenders. Additionally, many studies were rated at high risk of bias because trial information was inadequately described.
Implications for research.
Several research implications can be identified from this review.
Generally, better quality research is required to evaluate the effectiveness of interventions with extended long‐term effects of aftercare following release into the community.
Buprenorphine research in the prison environment requires evidence of the long‐term impact and larger studies, currently an equivalence of buprenorphine and methadone exists.
Evidence for naltrexone is less convincing. Trials evaluating differences between oral and implantation naltrexone and associated supervision requirements under the criminal justice system are required.
Only one court diversion study was identified: exploration of some court diversionary schemes using different pharmacological interventions would be useful.
Future clinical trials should collect information from all sectors of the criminal justice system. This would enhance the heterogeneous nature of the included studies and would facilitate generalisation of study findings.
Evidence of comparable mortality rates in prisoners using pharmacological interventions (particularly after release) needs to be explored to assess the long‐term outcomes of such treatments.
The link between dosage, treatment retention and subsequent criminal activity should be examined across all three pharmacological treatment options. Evidence from other trial data suggests that dose has important implications for retention in treatment; in future studies, this should be considered alongside criminal activity outcomes.
Cost and cost‐effectiveness information should be standardized within trial evaluations; this will help policymakers to decide upon health versus criminal justice costs.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2015 | New citation required and conclusions have changed | In the previous version pharmacological interventions for drug‐using offenders appeared to reduce overall subsequent drug use and criminal activity (but to a lesser extent), while with the introduction of new studies agonist treatments did not seem effective in reducing drug use or criminal activity. |
| 29 July 2014 | New search has been performed | This latest update reflects an additional four new trials (and one ongoing trial) with new follow‐up data on two existing trials with searches conducted up until May 2014 |
History
Review first published: Issue 12, 2013
| Date | Event | Description |
|---|---|---|
| 27 January 2014 | Amended | Plain language summary title correction |
| 16 July 2012 | New search has been performed | This review has been updated using searches to 21 March 2013. The review represents one in a family of four reviews. The other reviews cover non‐ pharmacological interventions for drug‐using offenders and interventions for drug‐using female offenders and offenders with co‐occurring mental illness. This new review of pharmacological interventions with drug‐using offenders contains 17 randomised controlled trials. Six of the 17 trials are awaiting classification for the review; the remaining 11 trials represent a total of 2,678 participants. |
| 2 March 2012 | New search has been performed | The updated edit of this review produced a new document with additional findings reflecting searches up to 11 November 2011. Five new review authors have been added to this version of the review, including Steven Duffy, Rachael McCool, Matthew Neilson, Catherine Hewitt and Marrissa Martyn‐St James. |
| 19 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the help of the York Health Economics Consortium and The Health Sciences Department at the University of York and the Cochrane Drugs and Alcohol Group.
Appendices
Appendix 1. MEDLINE search strategy
| MEDLINE search |
| 1. exp "Substance‐Related‐Disorders"/ |
| 2. ((drug or substance) adj (abuse* or addict* or dependen* or misuse*)).ti,ab |
| 3. (drug* adj (treat* or intervention* or program*) |
| 4. substance near (treat* or intervention* or program*) |
| 5.(detox* or methadone) in ti,ab |
| 6. narcotic* near (treat* or intervention* or program*) |
| 7. 1 or 2 or 3 or 4 or 5 or 6 |
| 8. prison*. ti,ab |
| 9. exp "Prisoners"/ |
| 10. offender* or criminal* or inmate* or convict* or probation* or remand or felon*).ti,ab |
| 11. exp "Prisons"/ |
| 12. 8 or 9 or 10 or 11 |
| 13. 7 and 12 |
Appendix 2. EMBASE search strategy
| Embase search |
| 1. (detox$ or methadone or antagonist prescri$).ti,ab. |
| 2. detoxification/ or drug detoxification/ or drug withdrawal/ or drug dependence treatment/ or methadone/ or methadone treatment/ or diamorphine/ or naltrexone/ |
| 3. (diamorphine or naltrexone or therapeutic communit$).ti,ab. |
| 4. morality/ |
| 5. (motivational interview$ or motivational enhancement).ti,ab. |
| 6. (counselling or counseling).ti,ab. |
| 7. exp counseling/ |
| 8. (psychotherap$ or cognitive behavioral or cognitive behavioural).ti,ab. |
| 9. exp psychotherapy/ |
| 10. (moral adj3 training).ti,ab. |
| 11. (cognitive restructuring or assertiveness training).ti,ab. |
| 12. reinforcement/ or self monitoring/ or self control/ |
| 13. (relaxation training or rational emotive or family relationship therap$).ti,ab. |
| 14. social learning/ or withdrawal syndrome/ or coping behavior/ |
| 15. (community reinforcement or self monitoring or self control or self management or interpersonal skills).ti,ab. |
| 16. (goal$ adj3 setting).ti,ab. |
| 17. (social skills adj3 training).ti,ab. |
| 18. anger/ or lifestyle/ |
| 19. (basic skills adj3 training).ti,ab. |
| 20. (relapse adj3 prevent$).ti,ab. |
| 21. (craving adj3 (minimi$ or reduc$)).ti,ab. |
| 22. (trigger or triggers or coping skills or anger management or group work).ti,ab. |
| 23. (lifestyle adj3 modifi$).ti,ab. |
| 24. (high intensity training or resettlement or throughcare or aftercare or after care).ti,ab. |
| 25. aftercare/ or halfway house/ |
| 26. (brief solution or brief intervention$ or minnesota program$ or 12 step$ or twelve step$).ti,ab. |
| 27. (needle exchange or nes or syringe exchange or dual diagnosis or narcotics anonymous).ti,ab. |
| 28. self help/ or support group/ |
| 29. (self‐help or selfhelp or self help or outreach or bail support or arrest referral$).ti,ab. |
| 30. exp urinalysis/ or rehabilitation/ or rehabilitation center/ |
| 31. (diversion or dtto or dttos or drug treatment or testing order$ or carat or carats).ti,ab |
| 32. (combined orders or drug‐free or drug free).ti,ab. |
| 33. (peer support or evaluation$ or urinalysis or drug testing or drug test or drug tests).ti,ab. |
| 34. ((rehab or rehabilitation or residential or discrete) adj2 (service$ or program$)).ti,ab. |
| 35. (asro or addressing substance$ or pasro or prisons addressing or acupuncture or shock or boot camp or boot camps).ti,ab. |
| 36. (work ethic camp$ or drug education or tasc or treatment accountability).ti,ab |
| 37. exp acupuncture/ |
| 38. or/1‐36 |
| 39. (remand or prison or prisoner or prisoners or offender$ or criminal$ or probation or court or courts).ti,ab. |
| 40. (secure establishment$ or secure facilit$).ti,ab. |
| 41. (reoffend$ or reincarcerat$ or recidivi$ or ex‐offender$ or jail or jails or goal or goals).ti,ab. |
| 42. (incarcerat$ or convict or convicts or convicted or felon or felons or conviction$ or revocation or inmate$ or high security).ti,ab. |
| 43. criminal justice/ or custody/ or detention/ or prison/ or prisoner/ or offender/ or probation/ or court/ or recidivism/ or crime/ or criminal behavior/ or punishment/ |
| 44. or/39‐43 |
| 45. 38 and 44 |
| 46. (substance abuse$ or substance misuse$ or substance use$).ti,ab. |
| 47. (drug dependanc$ or drug abuse$ or drug use$ or drug misuse$ or drug addict$).ti,ab. |
| 48. (narcotics adj3 (addict$ or use$ or misuse$ or abuse$)).ti,ab. |
| 49. (chemical dependanc$ or opiates or heroin or crack or cocaine or amphetamines or addiction or dependance disorder or drug involved).ti,ab. |
| 50. substance abuse/ or drug abuse/ or analgesic agent abuse/ or drug abuse pattern/ or drug misuse/ or intravenous drug abuse/ or multiple drug abuse/ |
| 51. addiction/ or drug dependence/ or narcotic dependence/ or exp narcotic agent/ or narcotic analgesic agent/ |
| 52. opiate addiction/ or heroin dependence/ or morphine addiction/ |
| 53. cocaine/ or amphetamine derivative/ or psychotropic agent/ |
| 54. or/46‐53 |
| 55. 45 and 54 |
Appendix 3. PsycInfo search strategy
| PsycInfo |
| 1. (detoxification in de) or (drug withdrawal in de) |
| 2. (drug usage screening in de) or (methadone maintenance) in de |
| 3. explode "Narcotic‐Antagonists" in DE |
| 4. 1 or 2 or 3 |
| 5. (counseling in de) or (explode "psychotherapeutic‐counseling" in de) |
| 6. (explode "cognitive‐therapy" in de) or (explode "psychotherapeutic‐techniques" in de) |
| 7. (cognitive restructuring in de) or (assertiveness training in de) |
| 8. explode "relaxation‐therapy" in de |
| 9. (rational emotive therapy in de) or (rational‐emotive therapy in de) |
| 10. (explode "self monitoring" in de) or (explode self‐monitoring) in de |
| 11. (goal setting in de) or (self control in de) or (explode "self‐management" in de) |
| 12. (social skills in de) or (relapse prevention in de) or (craving in de) or (coping behavior in de) |
| 13. (anger control in de) or (explode "group‐psychotherapy" in de) or (brief psychotherapy in de) |
| 14. (explode "behavior‐modification" in de) or (posttreatment followup in de) or (aftercare in de) |
| 15. (halfway houses in de) or (twelve step programs in de) |
| 16. (dual diagnoses in de) or (explode "self help techniques" in de) or (outreach programs in de) or (court referrals in de) |
| 17. (peer pressure in de) or (urinalysis in de) |
| 18. (drug rehabilitation in de) or (residential care institutions in de) or (acupuncture in de) or (drug education in de) |
| 19. (detox* or methadone or antagonist prescri* or diamorphine or naltrexone or therapeutic communit*) in ti,ab |
| 20. (motivational interview* or motivational enhancemen* or counseling or psychotherapy or psychotherapies) in ti,ab |
| 21. (cognitive behav* or cognitive therapy or cognitive therapies or moral training or cognitive restructuring) in ti,ab |
| 22. (assertiveness training or relaxation training or relaxation therapy or relaxation therapies) in ti,ab |
| 23. (rational emotive therap* or rational emotive behav* therap* or family relationship therap* or community reinforcement) in ti,ab |
| 24. (self‐monitor* or self monitor* or goal setting or self control or self‐control or self management or self‐management) in ti,ab |
| 25. (interpersonal skills training or social skills training or basic skills training) in ti,ab |
| 26. (relapse with prevent*) in ti,ab |
| 27. (craving near reduc*) in ti,ab |
| 28. craving with (reduc* in ti,ab) |
| 29. (trigger* or coping skills or anger management or group work or lifestyle modif* or high intensity training or resettlement) in ti,ab |
| 30. (throughcare or aftercare or after care or brief solution* or brief intervention*) in ti,ab |
| 31. (minnesota or 12 step* or twelve step* or needle exchange or nes or syringe exchange or dual diagnosis) in ti,ab |
| 32. (narcotics anonymous or self‐help or self help or outreach or bail support or arrest referral*) in ti,ab |
| 33. (diversion or dtto* or testing order* or carat* or counseling assessment referral or combined order or combined orders or drug free wing* or drug free environment*) in ti,ab |
| 34. (peer support or user evaluations or urinalysis or urinalyses or mandatory drug test* or rehabilitation or discrete service* or discrete program*) in ti,ab |
| 35. (residential program* or residential scheme* or asro or addressing substance* or pasro or prisons addressing substance) in ti,ab |
| 36. (acupuncture or shock or boot camp* or work ethic or drug education or tasc or treatment accountability) in ti,ab |
| 37. or/4‐36 |
| 38. (secure facilities or convict* or revocation or inmate* or high security) in ti,ab |
| 39. (prisoners in de) or (explode "correctional‐institutions" in de) |
| 40. (perpetrators in de) or (explode criminals in de) |
| 41. (probation in de) or (parole in de) or (incarceration in de) or (recidivism in de) or (criminal conviction in de) or (crime in de) |
| 42. (remand or prison* or offender* or criminal* or probation or court or courts or secure establishment* or reoffend* or reincarcerat* or recidivi* or ex‐offender* or jail or jails or incarcerat*) in ti,ab |
| 43. (drug abuse in de) or (explode "inhalant‐abuse" in de) or (explode "drug‐dependency" in de) |
| 44. (polydrug abuse in de) or (drug abuse in de) or (intravenous drug usage in de) |
| 45. (narcotic drugs in de) or (heroin in de) or (cocaine in de) or (explode amphetamine in de) |
| 46. (substance abuse* or substance misuse* or substance user*) in ti,ab |
| 47. (drug dependen* or drug abuse* or drug misuse* or drug addict* or drug use) in ti,ab |
| 48. (narcotic abuse* or narcotic misuse* or chemical dependen* or opiate misuse* or opiate abuse*) in ti,ab |
| 49. (heroin use* or heroin addict* or heroin misuse* or heroin abuse*) in ti,ab |
| 50. (crack use* or crack addict* or crack misuse* or crack abuse*) in ti,ab |
| 51. (cocaine use* or cocaine addict* or cocaine misuse* or cocaine abuse*) in ti,ab |
| 52. (amphetamine* use* or amphetamine* addict* or amphetamine* misuse* or amphetamine* abuse*) in ti,ab |
| 53. (dependence disorder or drug involved or dug‐involved) in ti,ab |
| 54. #38 or #39 or #40 or #41 or #42 |
| 55. #4 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 |
| 56. #37 and #54 and #55 |
Appendix 4. SPECTRA search strategy
| SPECTRA search |
| 1. {remand} or {prison} or {offender} or {criminal} or {probation} or {court} or {tribunal} or {secure establishment} or {secure facilit} or {reoffend} or {reincarcerat} or {recidivi} or {ex‐offender} or {jail} or {incarcerat} or {convict} or {felon} or {reconvict} or {high security} or {law enforcement} {remand} or {prison} or {offender} or {criminal} or {probation} or {court} or {tribunal} or {secure establishment} or {secure facilit} or {reoffend} or {reincarcerat} or {recidivi} or {ex‐offender} or {jail} or {incarcerat} or {convict} or {felon} or {reconvict} or {high security} or {law enforcement} |
| 2. {substance} or {dependenc} or {drug abuse} or {drug use} or {drug misuse} or {addict} All indexed fields: {remand} or {prison} or {offender} or {criminal} or {probation} or {court} or {tribunal} or {secure establishment} or {secure facilit} or {reoffend} or {reincarcerat} or {recidivi} or {ex‐offender} or {jail} or {incarcerat} or {convict} or {felon} or {reconvict} or {high security} or {law enforcement} OR All unindexed fields: {remand} or {prison} or {offender} or {criminal} or {probation} or {court} or {tribunal} or {secure establishment} or {secure facilit} or {reoffend} or {reincarcerat} or {recidivi} or {ex‐offender} or {jail} or {incarcerat} or {convict} or {felon} or {reconvict} or {high security} or {law enforcement} AND All unindexed fields: {substance} or {dependenc} or {drug abuse} or {drug use} or {drug misuse} or {addict} or {narcotics} or {opiates} or {heroin} or {crack} or {cocaine} or {amphetamines} or {drug involved} or {substance‐related} or {amphetamine‐related} or {cocaine‐related} or {marijuana} or {opioid} or {street drug} or {designer drug} |
| 3. narcotics |
| 4. opiates |
| 5. heroin |
| 6. {crack} |
| 7. cocaine |
| 8. amphetamines |
| 9. drug involved |
| 10. substance‐related |
| 11. amphetamine‐related |
| 12. cocaine‐related |
| 13. marijuana |
| 14. opioid |
| 15. street drug |
| 16. designer drug |
| 17. 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 |
| 18. 1 AND 17 |
Appendix 5. PASCAL. SciSearch, Social SciSSciSearch, Wilson Applied Science and Technology Abstracts search strategy
| PASCAL search |
| 1. (DETOX? OR METHADONE OR ANTAGONIST()PRESCRI?)/TI,AB |
| 2. METHADONE/DE OR NALTREXONE/DE |
| 3. (DIAMORPHINE OR NALTREXONE)/TI,AB |
| 4. THERAPEUTIC()COMMUNITY/DE OR THERAPEUTIC()COMMUNIT?)/TI,AB |
| 5. (MOTIVATIONAL()INTERVIEW? OR MOTIVATIONAL()ENHANCEMENT)/TI,AB |
| 6. (COUNSELLING OR COUNSELING)/TI,AB |
| 7. COUNSELING/DE |
| 8. (PSYCHOTHERAP? OR COGNITIVE()BEHAVIORAL OR COGNITIVE()BEHAVIOURAL)/TI,AB |
| 9. PSYCHOTHERAPY!/DE |
| 10. (MORAL(3W)TRAINING)/TI,AB |
| 11. (COGNITIVE()RESTRUCTURING OR ASSERTIVENESS()TRAINING)/TI,AB |
| 12. ASSERTIVENESS/DE OR RELAXATION()TECHNIQUES/DE |
| 13. (RELAXATION()TRAINING OR RATIONAL()EMOTIVE OR FAMILY()RELATIONSHIP()THERAP?)/TI,AB |
| 14. FAMILY()RELATIONS/DE |
| 15. (COMMUNITY()REINFORCEMENT OR SELF()MONITORING OR SELF()CONTROL OR SELF()MANAGEMENT OR INTERPERSONAL()SKILLS)/TI,AB |
| 16. (GOAL?(3W)SETTING)/TI,AB |
| 17. (SOCIAL(3W)TRAINING)/TI,AB |
| 18. SOCIAL RESPONSIBILITY/DE |
| 19. (BASIC()SKILLS(3W)TRAINING)/TI,AB |
| 20. (RELAPSE(3W)PREVENT?)/TI,AB |
| 21. (CRAVING(3W)(MINIMI? OR REDUC?))/TI,AB |
| 22. (TRIGGER OR TRIGGERS OR COPING()SKILLS OR ANGER()MANAGEMENT OR GROUP()WORK)/TI,AB |
| 23. (LIFESTYLE(3W)MODIFI?)/TI,AB |
| 24. (HIGH()INTENSITY()TRAINING OR RESETTLEMENT OR THROUGHCARE OR AFTERCARE OR AFTER()CARE)/TI,AB |
| 25. ADAPTATION,‐PSYCHOLOGICAL!/DE OR ANGER/DE OR LIFE()STYLE/DE OR AFTER()CARE/DE OR HALFWAY()HOUSES/DE |
| 26. (BRIEF()SOLUTION OR BRIEF()INTERVENTION? OR MINNESOTA()PROGRAM? OR 12()STEP? OR TWELVE()STEP?)/TI,AB |
| 27. (NEEDLE()EXCHANGE OR NES OR SYRINGE()EXCHANGE OR DUAL()DIAGNOSIS OR NARCOTICS()ANONYMOUS)/TI,AB |
| 28. NEEDLE‐EXCHANGE()PROGRAMS/DE |
| 29. (SELF‐HELP OR SELFHELP OR SELF()HELP OR OUTREACH OR BAIL()SUPPORT OR ARREST()REFERRAL?)/TI,AB |
| 30. SELF‐HELP()GROUPS/DE OR URINALYSIS/DE OR SUBSTANCE()ABUSE()DETECTION/DE |
| 31. (DIVERSION OR DTTO OR DTTOS OR DRUG()TREATMENT OR TESTING()ORDER? ? OR CARAT OR CARATS)/TI,AB |
| 32. (COMBINED()ORDERS OR DRUG‐FREE OR DRUG()FREE)/TI,AB |
| 33. (PEER()SUPPORT OR EVALUATION? ? OR URINALYSIS OR DRUG()TESTING OR DRUG()TEST? ?)/TI,AB |
| 34. ((REHAB OR REHABILITATION OR RESIDENTIAL OR DISCRETE)(2W)(SERVICE? ? OR PROGRAM?))/TI,AB |
| 35. (ASRO OR ADDRESSING()SUBSTANCE? OR PASRO OR PRISONS()ADDRESSING OR ACUPUNCTURE OR SHOCK OR BOOT()CAMP OR BOOT()CAMPS)/TI,AB |
| 36. (WORK()ETHIC()CAMP? ? OR DRUG()EDUCATION OR TASC OR TREATMENT()ACCOUNTABILITY)/TI,AB |
| 37. ACUPUNCTURE‐THERAPY!/DE OR ACUPUNCTURE/DE OR HEALTH()EDUCATION/DE OR SUBSTANCE()ABUSE()TREATMENT()CENTERS/DE |
| 38. S1:S3 |
| 39. S4:S37 |
| 40. S38 AND S39 |
| 40. (REMAND OR PRISON OR PRISONER OR PRISONERS OR OFFENDER? ? OR CRIMINAL? ? OR PROBATION OR COURT OR COURTS)/TI,AB |
| 41. (SECURE()ESTABLISHMENT? ? OR SECURE()FACILIT?)/TI,AB |
| 42. (REOFFEND? OR REINCARCERAT? OR RECIDIVI? OR EX()OFFENDER? ? OR JAIL OR JAILS)/TI,AB |
| 43. (INCARCERAT? OR CONVICT OR CONVICTS OR CONVICTED OR FELON? ? OR CONVICTION? ? OR REVOCATION OR INMATE? ? OR HIGH()SECURITY)/TI,AB |
| 44. PRISONERS/DE OR LAW()ENFORCEMENT/DE OR JURISPRUDENCE/DE |
| 45. S40:S44 |
| 46. S40 AND S45 |
| 47. (SUBSTANCE()ABUSE? OR SUBSTANCE()MISUSE? OR SUBSTANCE()USE?)/TI,AB |
| 48. (DRUG()DEPENDANC? OR DRUG()ABUSE? OR DRUG()USE? OR DRUG()MISUSE? OR DRUG()ADDICT?)/TI,AB |
| 49. (NARCOTICS(3W)(ADDICT? OR USE? OR MISUSE? OR ABUSE?))/TI,AB |
| 50. (CHEMICAL()DEPENDANC? OR OPIATES OR HEROIN OR CRACK OR COCAINE OR AMPHETAMINES OR ADDICTION OR DEPENDENCE()DISORDER OR DRUG()INVOLVED)/TI,AB |
| 51. SUBSTANCE‐RELATED()DISORDERS/DE OR AMPHETAMINE‐RELATED()DISORDERS/DE OR COCAINE‐RELATED()DISORDERS/DE OR MARIJUANA ()ABUSE/DE |
| 52. OPIOID‐RELATED‐DISORDERS!/DE OR PHENCYCLIDINE()ABUSE/DE OR SUBSTANCE()ABUSE()INTRAVENOUS/DE |
| 53. STREET()DRUGS/DE OR DESIGNER()DRUGS/DE OR NARCOTICS/DE |
| 54. COCAINE!/DE OR AMPHETAMINES!/DE OR ANALGESICS()OPIOID/DE |
| 55. S47:S54 |
| 56. S46 AND S55 |
| 57. (DETOXIFICATION OR METHADONE OR ANTAGONIST‐PRESCRIBING)/DE FROM 144,34,434,7,99,65,35,6 |
| 58. (DIAMORPHINE OR NALTREXONE)/DE FROM 144,34,434,7,99,65,35,6 |
| 59. THERAPEUTIC‐COMMUNITY)/DE FROM 144,34,434,7,99,65,35,6 |
| 60. (MOTIVATIONAL‐INTERVIEW OR MOTIVATIONAL‐ENHANCEMENT)/DE FROM 144,34,434,7,99,65,35,6 |
| 61. (COUNSELLING OR COUNSELING)/DE FROM 144,34,434,7,99,65,35,6 |
| 62. (PSYCHOTHERAPY! OR COGNITIVE‐BEHAVIORAL OR COGNITIVE‐BEHAVIOURAL)/DE FROM 144,34,434,7,99,65,35,6 |
| 63. (MORAL‐TRAINING)/DE FROM 144,34,434,7,99,65,35,6 |
| 64. (COGNITIVE‐RESTRUCTURING OR ASSERTIVENESS‐TRAINING)/DE FROM 144,34,434,7,99,65,35,6 |
| 65. (RELAXATION‐TRAINING OR RATIONAL‐EMOTIVE OR FAMILY‐RELATIONSHIP‐THERAPY)/DE FROM 144,34,434,7,99,65,35,6 |
| 66. FAMILY‐RELATIONS/DE |
| 67. (COMMUNITY‐REINFORCEMENT OR SELF‐MONITORING OR SELF‐CONTROL OR SELF‐MANAGEMENT OR INTERPERSONAL‐SKILLS)/DE FROM 44,34,434,7,99,65,35,6 |
| 68. (GOAL‐SETTING)/DE FROM 144,34,434,7,99,65,35,6 |
| 69. (SOCIAL‐SKILLS‐TRAINING)/DE FROM 144,34,434,7,99,65,35,6 |
| 70. SOCIAL‐RESPONSIBILITY/DE |
| 71. (BASIC‐SKILLS‐TRAINING)/DE FROM 144,34,434,7,99,65,35,6 |
| 72. (RELAPSE‐PREVENTION)/DE FROM 144,34,434,7,99,65,35,6 |
| 73. CRAVING/DE FROM 144,34,434,7,99,65,35,6 |
| 74. (TRIGGER OR COPING‐SKILLS OR ANGER‐MANAGEMENT OR GROUP‐WORK)/DE FROM 144,34,434,7,99,65,35,6 |
| 75. (LIFESTYLE‐MODIFICATION)/DE FROM 144,34,434,7,99,65,35,6 |
| 76. (HIGH‐INTENSITY‐TRAINING OR RESETTLEMENT OR THROUGHCARE OR AFTERCARE OR AFTER‐CARE)/DE FROM 144,34,434,7,99,65,35,6 |
| 77. (BRIEF‐SOLUTION OR BRIEF‐INTERVENTIONS OR MINNESOTA‐PROGRAM OR 12‐STEP‐PROGRAM OR TWELVE‐STEP‐PROGRAM)/DE FROM 144,34,434,7,99,65,35,6 |
| 77. (NEEDLE‐EXCHANGE OR SYRINGE‐EXCHANGE OR DUAL‐DIAGNOSIS OR NARCOTICS‐ANONYMOUS)/DE FROM 144,34,434,7,99,65,35,6 |
| 79. (SELF‐HELP OR OUTREACH OR BAIL‐SUPPORT OR ARREST‐REFERRAL)/DE FROM 144,34,434,7,99,65,35,6 |
| 80. (DRUG‐TREATMENT OR TESTING‐ORDERS OR CARAT)/DE FROM 144,34,434,7,99,65,35,6 |
| 81. (COMBINED‐ORDERS OR DRUG‐FREE)/DE FROM 144,34,434,7,99,65,35,6 |
| 82. (PEER‐SUPPORT OR EVALUATION OR URINALYSIS OR DRUG‐TESTING OR DRUG‐TESTS)/DE FROM 144,34,434,7,99,65,35,6 |
| 83. (REHABILITATION OR RESIDENTIAL OR DISCRETE‐SERVICES)/DE FROM 144,34,434,7,99,65,35,6 |
| 84. (ASRO OR PASRO ACUPUNCTURE OR BOOT‐CAMP)/DE FROM 144,34,434,7,99,65,35,6 |
| 85. (WORK‐ETHIC‐CAMP OR DRUG‐EDUCATION OR TASC OR TREATMENT‐ACCOUNTABILITY)/DE FROM 144,34,434,7,99,65,35,6 |
| 86. (REMAND OR PRISON OR PRISONER OR PRISONERS OR OFFENDER OR OFFENDERS OR CRIMINAL OR CRIMINALS OR PROBATION OR COURT OR COURTS)/DE FROM 144,34,434,7,99,65,35,6 |
| 87. (SECURE‐ESTABLISHMENTS OR SECURE‐FACILITY)/DE FROM 144,34,434,7,99,65,35,6 |
| 88. (REOFFENDERS OR REINCARCERATION OR RECIDIVISM OR EX‐OFFENDERS OR JAILS)/DE FROM 144,34,434,7,99,65,35,6 |
| 89. (INCARCERATION OR CONVICT OR CONVICTS OR FELON OR FELONS OR CONVICTIONS OR REVOCATION OR INMATE OR INMATES OR HIGH‐SECURITY)/DE FROM 144,34,434,7,99,65,35,6 |
| 90. (SUBSTANCE‐ABUSE OR SUBSTANCE‐MISUSE OR SUBSTANCE‐USE)/DE FROM 144,34,434,7,99,65,35,6 |
| 91. (DRUG‐DEPENDANCE OR DRUG‐DEPENDENCY OR DRUG‐ABUSE OR DRUG‐MISUSE OR DRUG‐ADDICT OR DRUG‐ADDICTION)/DE FROM 144,34,434,7,99,65,35,6 |
| 92. (CHEMICAL‐DEPENDANCY OR OPIATE‐DEPENDENCY OR HEROIN‐DEPENDENCY OR CRACK‐DEPENDENCY OR COCAINE‐DEPENDENCY OR AMPHETAMINES OR ADDICTION OR DEPENDENCE‐DISORDER OR DRUG‐INVOLVED)/DE FROM 144,34,434,7,99,65,35,6 |
| 93. S40 OR S57:S85 |
| 94. S45 OR S86:S89 |
| 95. S55 OR S90:S92 |
| 96. S93 AND S94 AND S95 |
| 97. S96/1980‐2004 |
Appendix 6. The CENTRAL Register of Controlled trials search strategy
| CENTRAL search |
| 1. prison* |
| 2. offender* |
| 3. (criminal* or probation or court*) |
| 4. (secure next establishment*) |
| 5. reoffend* |
| 6. reincarcerat* |
| 7. recidiv* |
| 8. exoffend* |
| 9. (jail or jails or incarcerat*) |
| 10. (secure next facilit*) 10(secure next facilit*) |
| 11. (convict* or revocation or inmate* or (high next security)) |
| 12. PRISONERS |
| 13. LAW ENFORCEMENT |
| 14. JURISPRUDENCE |
| 15. CRIME |
| 16. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 |
| 17. SUBSTANCE‐RELATED DISORDERS |
| 18. ((substance or drug*) next (abuse* or misuse* or dependen*or use* or addict*)) |
| 19. (narcotics or chemical or opiate) next (dependen* or addict* or abuse* or misuse*)) |
| 20. ((heroin) next (addict* or dependen* or misuse* or abuse*)) |
| 21. ((crack) next (addict* or dependen* or misuse* or abuse* or use*)) |
| 22. ((cocaine next addict*) or (cocaine next dependenc*) or (cocaine next misuse*) or (cocaine next abuse*) or (cocaine next use*)) |
| 23. ((amphetamine*) next (addict* or dependen* or misuse* or abuse* or use*)) |
| 24. (addicts or (dependence next disorder) or (drug next involved)) |
| 25. (street next drugs) |
| 26. STREET DRUGS |
| 27. DESIGNER DRUGS |
| 28. NARCOTICS |
| 29. COCAINE |
| 30. AMPHETAMINES |
| 31. ANALGESICS ADDICTIVE |
| 32. ANALGESICS OPIOID |
| 33. PSYCHOTROPIC DRUGS |
| 34. opioid* or opiat* |
| 35. #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 |
| 35. (#16 and #35) |
Appendix 7. SIGLE search strategy
| SIGLE |
| 1. ((reoffend* or reincarcerat* or recidivi* or ex‐offend* or jail or jails or incarcerat* or secure facilit* or convict* or revocation or inmate*) in ti,ab) |
| 2. ((remand or prison* or offender* or criminal* or probation or court or courts or secure establishment*) in ti,ab |
| 3. ((drug dependenc* or drug addict* or narcotics abuse* or narcotics use* or narcotics misuse* or narcotics addict*) in ti,ab |
| 4. ((drug abuse* or drug misuse* or drug use*) in ti,ab |
| 5. ((substance abuse* or substance misuse* or substance use*) in ti,ab |
| 6. ((detox* or methadone maintenance or methadone prescri* or antagonist prescri* or dimorphine or naltrexone) in ti,ab |
| 7. ((dependence disorder or drug involved) in ti,ab |
| 8. ((amphetamine* abuse* or amphetamine* misuse* or amphetamine* use* or amphetamine* addict*) in ti,ab |
| 9. ((cocaine abuse* or cocaine misuse* or cocaine use* or cocaine addict*) in ti,ab |
| 10. ((crack abuse* or crack misuse* or crack use* or crack addict*) in ti,ab |
| 11. ((heroin abuse* or heroin misuse* or heroin use* or heroin addict*) in ti,ab |
| 12. ((chemical dependenc* or opiate abuse* or opiate misuse* or opiate use* or opiate addict*) in ti,ab |
| 13. #1 or #2 |
| 14. #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 |
| 15. #13 and #14 |
Appendix 8. Sociological Abstracts search strategy
| Sociological Abstrac |
| 1. remand in de |
| 2. detention in de |
| 3. prisoners in de |
| 4. prisons in de |
| 5. offenders in de |
| 6. parole in de |
| 7. probation in de |
| 8. correctional system in de |
| 9. courts in de |
| 10. imprisonment in de |
| 11. criminal justice in de |
| 12. criminal proceedings in de |
| 13. recidivism in de |
| 14. jail in de |
| 15. institutionalization (persons) in de |
| 16. conviction/convictions in de |
| 17. (remand or prison* or offender* or criminal* or probation or court or courts or secure establishment*) in ti,ab |
| 18. (reoffend* or reincarcerat* or recidivi* or ex‐offend* or jail or jails or incarcerat* or secure facilit* or convict* or revocation or inmate*) in ti,ab |
| 19. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 |
| 20. substance abuse in de |
| 21. explode "Drug‐Abuse" in DE |
| 22. "Drug‐Injection" in DE |
| 23. explode "Narcotic‐Drugs" in DE |
| 24. "Cocaine‐" in DE |
| 25. "Addiction‐" in DE |
| 26. explode "Psychedelic‐Drugs" in DE |
| 27. (substance abuse* or substance misuse* or substance use*) in ti,ab |
| 28. (drug abuse* or drug misuse* or drug use*) in ti,ab |
| 29. (drug dependenc* or drug addict* or narcotics abuse* or narcotics use* or narcotics misuse* or narcotics addict*) in ti,ab |
| 30. (chemical dependenc* or opiate abuse* or opiate misuse* or opiate use* or opiate addict*) in ti,ab |
| 31. (heroin abuse* or heroin misuse* or heroin use* or heroin addict*) in ti,ab |
| 32. (crack abuse* or crack misuse* or crack use* or crack addict*) in ti,ab |
| 33. (cocaine abuse* or cocaine misuse* or cocaine use* or cocaine addict*) in ti,ab |
| 34. (amphetamine* abuse* or amphetamine* misuse* or amphetamine* use* or amphetamine* addict*) in ti,ab |
| 35. (dependence disorder or drug involved) in ti,ab |
| 36. #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 |
| 37. #19 and #36 |
| 38. "Detoxification‐" in DE |
| 39. "Methadone‐Maintenance" in DE |
| 40. "Counseling‐" in DE |
| 41. "Psychotherapy‐" in DE |
| 42. "Assertiveness‐" in DE |
| 43. (detoxification in de) or (methadone maintenance in de) or (treatment programs in de) |
| 44. (counseling in de) or (psychotherapy in de) or (assertiveness in de) or (group therapy in de) or (goals in de) or (self control in de) |
| 45. (interpersonal communication in de) or (social interaction in de) or (social competence in de) or (coping in de) |
| 46. (social behavior in de) or (group work in de) or (lifestyle in de) |
| 47. (after care in de) or (support networks in de) or (self help in de) or (self help groups in de) or (outreach programmes in de) |
| 48. (outreach programs in de) or (referral in de) or (delinquency prevention in de) or (diversion/diversions in de) |
| 49. (peer groups in de) or (peer influence in de) or (drug use screening in de) or (rehabilitation in de) or (work experience in de) |
| 50. (detox* or methadone maintenance or methadone prescri* or antagonist prescri* or dimorphine or naltrexone) in ti,ab |
| 51. (therapeutic communit* or motivational interview* or motivational enhance* or counseling or counselling or psychotherapy or cognitive behavi*) in ti,ab |
| 52. (moral training or cognitive restructuring or assertiveness training or relaxation training) in ti,ab |
| 53. (rational‐emotive or rational emotive or family relationship therap* or community reinforcement or self monitoring or goal setting or self control training) in ti,ab |
| 54. (self management or interpersonal skills or social skills or basic skills or relapse prevent* or prevent* relapse or craving reduc* or reduc* craving) in ti,ab |
| 55. (trigger* or coping skills or anger management or group work or lifestyle modif* or high intensity training or resettlement or throughcare) in ti,ab |
| 56. (aftercare or after care or brief solution or brief intervention* or 12 step* or twelve step* or minnesota program* or needle exchange or nes) in ti,ab |
| 57. (syringe exchange or dual diagnosis or narcotics anonymous or self help or selfhelp or outreach or bail support) in ti,ab |
| 58. (arrest referral* or diversion or dtto or dttos or drug treatment or carat or carats or counseling assessment or combined orders) in ti,ab |
| 59. (drug‐free or drug free or peer support or evaluation* or urinalysis or drug testing or drug use screen* or rehabilitation or discrete service* or discrete program*) in ti,ab |
| 60. (residential program* or residential scheme* or residential service*) in ti,ab |
| 61. (asro or addressing substance or pasro or prisons addressing or acupuncture or shock or boot camp*) in ti,ab |
| 62. (work ethic or drug education or tasc or treatment accountability) in ti,ab |
| 63. #38 or #39 #or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 |
| 64. #37 and #63 |
Appendix 9. ASSIA search strategy
| ASSIA search |
| 1. remand |
| 2. prison or prisoner or prisoners |
| 3. offender* |
| 4. criminal* |
| 5. probation |
| 6. court or courts |
| 7. tribunal or tribunals |
| 8. secure establishment* |
| 9. secure facilit* |
| 10. reoffend* |
| 11. reincarcerat* |
| 12. recidivi* |
| 13. ex‐offender* |
| 14. jail or jails |
| 15. incarcerat* |
| 16. convict or convicts |
| 17. convicted |
| 18. felon or felons |
| 19. conviction* |
| 20. reconviction* |
| 21. high security |
| 22. law enforcement |
| 23. Substance abuse* or substance misuse* or substance use* |
| 24. drug dependanc* or drug abuse* or drug use* |
| 25. drug misuse* or drug addict* |
| 26. narcotics addict* narcotics use* narcotics misuse* narcotics abuse* |
| 27. chemical dependanc* |
| 28. opiates |
| 29. heroin |
| 30. crack |
| 31. cocaine |
| 32. amphetamines |
| 33. cocaine |
| 34. addiction |
| 35. dependence disorder* |
| 36. drug involved |
| 37. Substance‐related disorders |
| 38. amphetamine‐related disorders |
| 39. cocaine‐related disorders |
| 40. marijuana abuse |
| 41. opioid‐related disorders |
| 42. street drugs |
| 43. designer drugs |
| 44. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 |
| 45. 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 |
| 46. 44 and 45 |
Appendix 10. HMIC search strategy
| HMIC |
| 1. remand in de |
| 2. detention in de |
| 3. prisoners in de |
| 4. prisons in de |
| 5. offenders in de |
| 6. parole in de |
| 7. probation in de |
| 8. correctional system in de |
| 9. courts in de |
| 10. imprisonment in de |
| 11. criminal justice in de |
| 12. criminal proceedings in de |
| 13. recidivism in de |
| 14. jail in de |
| 15. institutionalization (persons) in de |
| 16. conviction/convictions in de |
| 17. (remand or prison* or offender* or criminal* or probation or court or courts or secure establishment*) in ti,ab |
| 18. (reoffend* or reincarcerat* or recidivi* or ex‐offend* or jail or jails or incarcerat* or secure facilit* or convict* or revocation or inmate*) in ti,ab |
| 19. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 |
| 20. substance abuse in de |
| 21. explode "Drug‐Abuse" in DE |
| 22. "Drug‐Injection" in DE |
| 23. explode "Narcotic‐Drugs" in DE |
| 24. "Cocaine‐" in DE |
| 25. "Addiction‐" in DE |
| 26. explode "Psychedelic‐Drugs" in DE |
| 27. (substance abuse* or substance misuse* or substance use*) in ti,ab |
| 28. (drug abuse* or drug misuse* or drug use*) in ti,ab |
| 29. (drug dependenc* or drug addict* or narcotics abuse* or narcotics use* or narcotics misuse* or narcotics addict*) in ti,ab |
| 30. (chemical dependenc* or opiate abuse* or opiate misuse* or opiate use* or opiate addict*) in ti,ab |
| 31. (heroin abuse* or heroin misuse* or heroin use* or heroin addict*) in ti,ab |
| 32. (crack abuse* or crack misuse* or crack use* or crack addict*) in ti,ab |
| 33. (cocaine abuse* or cocaine misuse* or cocaine use* or cocaine addict*) in ti,ab |
| 34. (amphetamine* abuse* or amphetamine* misuse* or amphetamine* use* or amphetamine* addict*) in ti,ab |
| 35. (dependence disorder or drug involved) in ti,ab |
| 36. #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 |
| 37. #19 and #36 |
Appendix 11. National Research Register search strategy
| NRR search |
| 1. REMAND |
| 2. PRISON* |
| 3. OFFENDER* |
| 4. ((CRIMINAL* or PROBATION) or COURT) or COURTS) |
| 5. (SECURE next ESTABLISHMENT*) |
| 6. REOFFEND* |
| 7. REINCARCERAT* |
| 8. RECIDIV* |
| 9. EXOFFEND* |
| 10. ((JAIL or JAILS) or INCARCERAT*) |
| 11. (SECURE next FACILIT*) |
| 12. (((CONVICT* or REVOCATION) or INMATE*) OR (HIGH next SECURITY)) |
| 13. PRISONERS:ME |
| 14. LAW‐ENFORCEMENT:ME |
| 15. JURISPRUDENCE:ME |
| 16. CRIME:ME |
| 17. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 |
| 18. #11 or #12 or #13 or #14 or #15 or #16 |
| 19. #17 or #18 |
| 20. ((SUBSTANCE next ABUSE*) or (SUBSTANCE next MISUSE*)) OR (DRUG NEXT DEPENDENC*)) OR (DRUG NEXT ABUSE*)) OR (DRUG NEXT MISUSE*)) OR (DRUG NEXT USE*)) OR (DRUG NEXT ADDICTION)) |
| 21. ((NARCOTICS or (CHEMICAL next DEPENDENC*)) OR (OPIATE NEXT ADDICT*)) OR (OPIATE NEXT DEPENDENC*)) OR (OPIATE NEXT ABUSE*)) OR (OPIATE NEXT MISUSE*)) |
| 22. ((HEROIN next ADDICT*) or (HEROIN next DEPENDENC*)) OR (HEROIN NEXT MISUSE*)) OR (HEROIN NEXT ABUSE*)) |
| 23. ((CRACK next ADDICT*) or (CRACK next DEPENDENC*)) OR (CRACK NEXT MISUSE*)) OR (CRACK NEXT ABUSE*)) OR (CRACK NEXT USE*)) |
| 24. ((COCAINE next ADDICT*) or (COCAINE next DEPENDENC*)) OR (COCAINE NEXT MISUSE*)) OR (COCAINE NEXT ABUSE*)) OR (COCAINE NEXT USE*)) |
| 25. ((AMPHETAMINE* next ADDICT*) or (AMPHETAMINE* next DEPENDENC*)) OR (AMPHETAMINE* NEXT MISUSE*)) OR (AMPHETAMINE* NEXT ABUSE*)) OR (AMPHETAMINE* NEXT USE*)) |
| 26. ((ADDICTS or (DEPENDENCE next DISORDER)) OR (DRUG NEXT INVOLVED)) |
| 27. (SUBSTANCE‐RELATED and DISORDERS:ME) |
| 28. SUBSTANCE‐RELATED‐DISORDERS:ME |
| 29. AMPHETAMINE‐ABUSE:ME |
| 30. COCAINE‐ABUSE:ME |
| 31. MARIJUANA‐ABUSE:ME |
| 32. OPIOID‐RELATED‐DISORDERS:ME |
| 33. PHENCYCLIDINE‐ABUSE:ME |
| 34. SUBSTANCE‐ABUSE‐INTRAVENOUS:ME |
| 35. SUBSTANCE‐WITHDRAWAL‐SYNDROME:ME |
| 36. (STREET next DRUGS) |
| 38. STREET‐DRUGS:ME |
| 39. DESIGNER‐DRUGS:ME |
| 40. NARCOTICS:ME |
| 41. (COCAINE:ME or AMPHETAMINES:ME) |
| 42. ANALGESICS‐ADDICTIVE:ME |
| 43. ANALGESICS‐OPIOID:ME |
| 44. PSYCHOTROPIC‐DRUGS:ME |
| 45. #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 |
| 46. 19 and 45 |
Appendix 12. PAIS search strategy
| PAIS |
| 1. ((reoffend* or reincarcerat* or recidivi* or ex‐offend* or jail or jails or incarcerat* or secure facilit* or convict* or revocation or inmate*) in ti,ab) |
| 2. ((remand or prison* or offender* or criminal* or probation or court or courts or secure establishment*) in ti,ab) |
| 3. ((drug dependenc* or drug addict* or narcotics abuse* or narcotics use* or narcotics misuse* or narcotics addict*) in ti,ab) |
| 4. ((drug abuse* or drug misuse* or drug use*) in ti,ab) or ((substance abuse* or substance misuse* or substance use*) in ti,ab) |
| 5. ((detox* or methadone maintenance or methadone prescri* or antagonist prescri* or dimorphine or naltrexone) in ti,ab) |
| 6. ((dependence disorder or drug involved) in ti,ab) |
| 7. ((amphetamine* abuse* or amphetamine* misuse* or amphetamine* use* or amphetamine* addict*) in ti,ab) |
| 8. ((cocaine abuse* or cocaine misuse* or cocaine use* or cocaine addict*) in ti,ab) |
| 9. ((crack abuse* or crack misuse* or crack use* or crack addict*) in ti,ab) |
| 10. ((heroin abuse* or heroin misuse* or heroin use* or heroin addict*) in ti,ab) |
| 11. ((chemical dependenc* or opiate abuse* or opiate misuse* or opiate use* or opiate addict*) in ti,ab) |
| 12. ((moral training or cognitive restructuring or assertiveness training or relaxation training) in ti,ab) |
| 13. ((therapeutic communit* or motivational interview* or motivational enhance* or counseling or counselling or psychotherapy or cognitive behavi*) in ti,ab) |
| 14. ((work ethic or drug education or tasc or treatment accountability) in ti,ab) |
| 15. ((asro or addressing substance or pasro or prisons addressing or acupuncture or shock or boot camp*) in ti,ab) |
| 16. ((arrest referral* or diversion or dtto or dttos or drug treatment or carat or carats or counseling assessment or combined orders) in ti,ab) |
| 17. ((residential program* or residential scheme* or residential service*) in ti,ab) |
| 18. ((syringe exchange or dual diagnosis or narcotics anonymous or self help or selfhelp or outreach or bail support) in ti,ab) |
| 19. ((drug‐free or drug free or peer support or evaluation* or urinalysis or drug testing or drug use screen* or rehabilitation or discrete service* or discrete program*) in ti,ab) |
| 20. ((aftercare or after care or brief solution or brief intervention* or 12 step* or twelve step* or minnesota program* or needle exchange or nes) in ti,ab) |
| 21. ((trigger* or coping skills or anger management or group work or lifestyle modif* or high intensity training or resettlement or throughcare) in ti,ab) |
| 22. ((self management or interpersonal skills or social skills or basic skills or relapse prevent* or prevent* relapse or craving reduc* or reduc* craving) in ti,ab) |
| 24. ((rational‐emotive or rational emotive or family relationship therap* or community reinforcement or self monitoring or goal setting or self control training) in ti,ab) |
| 25. #1 or #2 |
| 26. #3 or #4 or #5 or #6 or #7 or #8 or 9 or #10 or #11 |
| 27. #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 |
| 28. 25 and #26 and #27 |
Appendix 13. Criminal Justice Abstracts search strategy
| CJA search |
| 1. (substance abuse* or substance misuse* or substance use or substance users) in ti,ab,de |
| 2. substance related in ti,ab,de |
| 3. drug related in ti,ab,de |
| 4. (drug dependenc* or drug abuse* or drug misuse* or drug use or drug users or drug addiction) in ti,ab,de |
| 5. (narcotics use or narcotics users or narcotics abuse* or narcotics misuse* or chemical dependenc*) in ti,ab,de |
| 6. (opiates or heroin or crack or cocaine or amphetamines or addict or addicts or addicted or dependence disorder* or drug involved) in ti,ab,de |
| 7. (designer drugs or street drugs or polydrug misuse* or polydrug abuse*) in ti,ab,de |
| 8. #1 or #2 or #3 or #4 or #5 or #6 or #7 |
| 9. ((antagonist near prescri*) or diamorphine or naltrexone) in ti,ab,de |
| 10(therapeutic communit* or (motivational near interview*)) in ti,ab,de |
| 11. (motivational near enhancement) in ti,ab,de |
| 12. (counselling or counseling) in ti,ab,de |
| 13. (psychotherap* or cognitive behav* or behav* therap* or (moral near training)) in ti,ab,de |
| 14. (cognitive restructuring or (assertiveness near train*) or relaxation training) in ti,ab,de |
| 15. (rational emotive or family relationship therap*) in ti,ab,de |
| 16. (community reinforcement or self monitoring or goal setting or goalsetting) in ti,ab,de |
| 17. (self control near training) in ti,ab,de |
| 18. (self management) in ti,ab,de |
| 19. (interpersonal skills near training) in ti,ab,de |
| 20. ((social skills or basic skills) near training) in ti,ab,de |
| 21. ((relapse near prevent*) or (craving near reduc*)) in ti,ab,de |
| 22. (trigger* or coping skills or anger management or group work or (lifestyle near modif*)) in ti,ab,de |
| 23. (high intensity training or resettlement or throughcare or aftercare or after care) in ti,ab,de |
| 24. (brief solution* or brief intervention*) in ti,ab,de |
| 25. (minnesota in ti,ab) in ti,ab,de |
| 26. (12 step* or twelve step*) in ti,ab,de |
| 27. (needle exchange or nes or syringe exchange) in ti,ab,de |
| 28. (dual diagnosis or narcotics anonymous or self help or selfhelp or outreach) in ti,ab,de |
| 29. (bail support or bail program* or arrest referral* or diversion or dtto* or drug treatment) in ti,ab,de |
| 30. (carat or counselling assessment or counseling assessment) in ti,ab,de |
| 31. (combined order* or drug free wing* or drug free environment* or peer support) in ti,ab,de |
| 32. (user evaluations or urinalys* or urinanalys* or drug test* or rehab* or discrete service*) in ti,ab,de |
| 33. (discrete program* or residential program* or residential scheme*) in ti,ab,de |
| 34. (asro or addressing substance*) in ti,ab,de |
| 35. (pasro or prisons addressing) in ti,ab,de |
| 36. (acupuncture or shock or boot camp or boot camps or work ethic camp*) in ti,ab,de |
| 37. (drug education or tasc or treatment accountability) in ti,ab,de |
| 38. (detoxification or detox or methadone maintenance or (methadone near prescri*)) in ti,ab,de |
| 39. #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 |
| 40. #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 |
| 41. #39 or #40 |
| 42. #8 and #41 |
| 9. #42 and (PY > "1979") |
Appendix 14. Criteria for assessing risk of bias
| Item | Judgment | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention | |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled, randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following method was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear risk | Insufficient information to permit judgement of low or high risk This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement | |
| 3. Blinding of participants and providers (performance bias) Objective outcomes |
Low risk |
No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| 4. Blinding of participants and providers (performance bias) Subjective outcomes |
Low risk |
Blinding of participants and providers and unlikely that the blinding could have been broken; |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 5. Blinding of outcome assessor (detection bias) Objective outcomes |
Low risk |
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| 6.Blinding of outcome assessor (detection bias) Subjective outcomes |
Low risk |
No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; Blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk; | |
| 7. Incomplete outcome data (attrition bias) For all outcomes except retention in treatment or drop out |
Low risk |
No missing outcome data; Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; Missing data have been imputed using appropriate methods All randomised patients are reported/analysed in the group they were allocated to by randomisation irrespective of non‐compliance and co‐interventions (intention to treat) |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of drop out not reported for each group); | |
| 8 Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk | Not all of the study’s pre‐specified primary outcomes have been reported; One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub scales) that were not pre‐specified; One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; The study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
|
| Unclear risk | Insufficient information to permit judgement of low or high risk | |
| 9. Other bias * | Low risk | Evidence to suggest other problems identified with the study which might threaten the validity of the random allocation, attrition or data integrity and results of the trial. |
| High risk | Evidence to suggest that the trial might be underpowered/problems with the random allocation process leading to potential self selection bias/ issues of analysis not conducted using intent to treat analysis or evidence of missing data. Concerns of attrition and measurement error including reliance on self report measures. | |
| Unclear risk | insufficient information to permit judgement of low or high risk |
Data and analyses
Comparison 1. Agonist pharmacological vs no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Drug use (objective) | 2 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.00] |
| 2 Drug use self reported dichotomous | 3 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.18] |
| 3 Drug use self reported continuous | 3 | 510 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.85, ‐0.39] |
| 4 Criminal activity dichotomous | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Arrests | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.32, 1.14] |
| 4.2 Re‐incarceration | 3 | 472 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.36, 1.64] |
| 5 Criminal activity continuous | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐74.21 [‐133.53, ‐14.89] |
Comparison 2. Antagonist (Naltrexone) vs no pharmacological.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Criminal activity dichotomous | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Reincarceration | 2 | 114 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.74] |
| 2 drug use (objective) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.28, 1.70] |
Comparison 3. Methadone vs buprenorphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self reported drug use dichotomous | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.69, 1.55] |
| 2 Self reported drug use continuous | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐5.33, 6.73] |
| 3 Criminal activity dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 re incarceration | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.83, 1.88] |
Comparison 4. Methadone vs diamorphine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 criminal activity dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 arrest | 1 | 825 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.03, 1.51] |
Comparison 5. Methadone vs naltrexone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 self reported drug use continuous | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 4.60 [‐3.54, 12.74] |
| 2 criminal activity dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 re incarceration | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.37, 3.26] |
| 3 criminal activity continuous | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐8.04, 7.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayanzadeh 2004.
| Methods | Allocation: random assignment Randomisation method: high risk based on even and odd rows Similar on drug use: yes Similar on criminal activity: unknown Blinding methodology: high‐risk participants not blind Loss to follow‐up: inadequate information with some attrition in the control group/high risk | |
| Participants | 120 male participants 100% male Age range: 20 to 70 years Mean age: 35.7 years (SD 8.86) Participants had to have a history of opioid use for longer than one year, had to be dependent upon drugs and had to have a sentence length greater than 6 months. In addition, non‐death penalty inmates were excluded, and individuals had to be willing to engage in services |
|
| Interventions | Intervention group: The intervention group received methadone treatment in combination with CBT and widely focused on coping and problem‐solving skills. n = 60. The CBT training offered analysis on the role and thoughts on drug abuse, identification of high‐risk situations, relapse prevention resilience skills, family participation in treatment and motivational interviewing. Family education was arranged to coincide with weekly visiting hours and the harm reduction education was delivered once a week. Comparison group: The comparison group received non‐methadone drugs plus standard psychiatric services and therapeutic medications. An option for treatment using clonidine and psychoactive drugs was provided as part of this treatment alternative n = 60. |
|
| Outcomes | Drug use: yes/no Frequency of drug injections (percentage) Syringe sharing Morphine urine analysis All outcomes at six months |
|
| Notes | After random allocation, 20 participants who were allocated to the control group opted out of the research. This group of inmates were subsequently replaced by individuals from the general inmate population. No conflict of interest was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were categorised into one of four lists based on their previous history of drug abuse. The random allocation was then chosen, using even and odd row numbers from each list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After random allocation, 20 participants from the control group opted out of the research. At the end of the study attrition was high in both groups: for the intervention group n = 38 out of the original 60 allocated and for the control group n = 31 out of the original 60 allocated. |
| Selective reporting (reporting bias) | Unclear risk | Not clearly reported but problems with the research design are highlighted |
| Other bias | High risk | The authors note a number of operational difficulties, especially in relation to contamination across prison wings and the two intervention groups |
Brown 2013.
| Methods | Allocation: random assignment Randomisation method: not reported Similar on drug use: reported that there was no significant between‐group difference in any demographic variable. Variable and data not presented Similar on criminal activity: as above Blinding methodology: unclear risk, not reported Loss to follow‐up: high risk, study retention rate reported as 80%, but figure indicates 80% at week 24, 33% at week 52 and 26% at follow‐up | |
| Participants | 15 adults enrolled in either a drug treatment court (DTC) or Treatment Alternative Program (TAP). Participants were referred by the Clinical Assessment Unit at the Mental Health Centre of Dane County, where all potential jail diversion program participants receive initial clinical evaluation. Average age: 27.5 years 53.3 % male 80.0 % white % drug users, not reported % alcohol, not reported % psychiatric history, not reported Eligibility criteria: inclusion criteria were diagnosis of opioid dependence (via Mini International Neuropsychiatric Interview (MINI)), opioid positive urine drug screen, negative screening urine pregnancy test, and willingness to use appropriate birth control methods throughout the study. Exclusion criteria (via MINI and initial medical history and physical exam) were current alcohol or sedative dependence, pregnancy, women who were breastfeeding, complex psychiatric comorbidity, complex medical comorbidity, or pharmacotherapy with an agent contraindicated in combination with suboxone or methadone, according to drug labelling. |
|
| Interventions | Interventions: (I) specialist treatment facility plus suboxone (buprenorphine and naloxone) or (ii) specialist treatment facility plus methadone, n = 9 Control: (C) primary care plus suboxone (buprenorphine and naloxone), n = 6 Participation lasted 13.5 months, including a 12‐month treatment period and a one‐time follow‐up 6 weeks post‐treatment. |
|
| Outcomes | Primary outcomes included on‐going drug use measured by timeline follow‐back method (TLFB is a reliable, calendar‐based technique for retrospectively assessing the frequency and patterns of daily drug use) and use of the Addiction Severity Index (self report). Lite, HIV risk behaviours (RAB ‐ Risk Assessment Battery short version), and health services utilization. TLFB was administered at baseline, bi‐weekly for the first 6 months, and monthly thereafter. All other measures were assessed at baseline, month 6, month 12, and follow‐up. Urine drug screens were collected as a part of routine management in DTC and TAP. |
|
| Notes | The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR ‐ now the National Centre for Advancing Translational Sciences, NCATS) grant 1UL1RR025011, and grant 9U54TR000021. Funding was also provided by the Vilas Foundation. The authors report no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation noted no further information. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Small sample size (reported as a “pilot study”) with 80% completing the 24‐week assessment, 33% completing week 52 and 26% at follow‐up |
| Selective reporting (reporting bias) | High risk | Protocol reported as being available. However, on‐going drug use (frequency and patterns of daily drug use), health services utilization and urine tests are reported as being assessed, but no outcome data are reported. |
| Other bias | High risk | The authors report: “The higher baseline HIV risk in the specialist study condition, and, hence, greater potential for risk reduction, may have affected this result. In other words, the relatively low prevalence of global HIV risk behaviours in the primary care group may have contributed to a ‘floor effect’ or greater difficulty achieving improvement on this factor.” “Additionally, urine drug testing was not collected randomly DTC and TAP where severity of use affects frequency of testing. Hence, urine drug test results are likely to present a biased picture and be difficult to interpret in aggregate in this community‐based setting.” |
Cornish 1997.
| Methods | Allocation: random assignment, 2:1 ratio (naltrexone:control) Randomisation method: unclear Similar on drug use: yes Similar on criminal activity: unknown Blinding methodology: high risk Loss to follow‐up: unclear risk; some loss to follow‐up; volunteer participants | |
| Participants | 51 adults randomized, 68 indicated initial interest, of these 2 failed the naltrexone challenge and 15 did not return for completion of screening and enrollment. Average age: 39 years 90% male 24% white 62% African American 14% Latino |
|
| Interventions | Community‐based naltrexone programme and routine parole/probation (n = 34) vs routine parole/probation (n = 17) (I) Nalrexone programe: When a 0.8 naltrexone challenge was negative, the participant received 25 mg oral dose of naltrexone, if no signs of opioid withdrawal after 1 hour, this was followed by 25 mg daily for two days and 50 mg daily for the following three days. Aproximately 1 week after initiation participants were stabilized on naltrexone regimen of 100 mg on Tuesdays and 150 mg on Fridays. In addition, research staff obtained observed urine specimens and breathalyzer readings weekly (results of these were not shared with probation staff). (C) Routine parole/probation: Participants were required to attend three orientation and counseling sessions per week for the first 2 weeks of the study. Both groups received weekly parole/probation officer contact for the first 6 months and medication visits occured twice weekly. At 6‐month follow up participants were give a $25 incentive payment and at 9, 12, 15 and 18 month follow up participants were given $25 for keeping scheduled appointments. |
|
| Outcomes | Reincarceration for technical violation (official records) during the past 6 months at 6 months' follow‐up Mean percentage for opioid positive urine specimens per group |
|
| Notes | Work supported by NIDA Grant DA05186. No declarations of interest are noted by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individuals were assigned at a ratio of 2:1 to naltrexone vs control |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Study description suggests that participants were not blind: see p.531 |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Study description suggests that participants were not blind: see p.531 |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of urine samples were not shared with probation staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All allocated participants were reported in the analysis. Retention rates appeared to be similar; appears to be an ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Groups similar at baseline, but potential for volunteer bias |
Coviello 2010.
| Methods | Allocation: random Randomisation method: unknown/unclear Similar on drug use: significant difference in heroin use. Otherwise similar Similar on criminal activity: yes Blinding methodology: high risk Loss to follow‐up: inadequate/high risk |
|
| Participants | 111 adults Age range: 18 to 55 years; average age: 34 years 82% male 47% Caucasian 100% drug users Alcohol use not reported but participants excluded if severe alcohol dependence Psychiatric history not reported Eligibility criteria: consented, age 18 to 55 years, opioid dependence, otherwise good health, probation or parole for 6 months, 3 days opioid free |
|
| Interventions | Community pharmacological intervention vs treatment as usual (I) Oral naltrexone plus psychosocial treatment (n = 56) vs (C) psychosocial treatment only (n = 55) The (I) group was started on directly observed administration of naltrexone, increasing in dose from 25 mg to 300 mg and was also given psychosocial treatment. The (C) group was given a treatment regimen consisting of group therapy, individual therapy and case management, all of which the (I) group also received |
|
| Outcomes | Criminal activity (self‐reported) and criminal record data at 6 months Illicit drug use (self‐reported) during the 30 days before the interview at 6 months % positive urine drug screen for opioids % positive urine drug screen for cocaine |
|
| Notes | The study was supported by grant R01‐DA‐012268 from the National Institute on Drug Abuse, Bethesda, MD (Dr. Cornish). Declaration of Interest In the past 3 years, Dr. O’Brien has served as a consultant on one occasion to Alkermes, a company that makes a version of depot naltrexone. He is also conducting an NIH‐funded study of this medication in opioid addiction. The authors report no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method unclear. Note that randomisation was balanced by using six variables |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | page 4 'we did not use a placebo for participants'. The treatment as usual group were not blinded |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | page 4 'we did not use a placebo for participants'. The treatment as usual group were not blinded |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A large amount of attrition was noted in the first week, and only one‐third of participants remained at 6‐month follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | High risk | Blinding and attrition concerns throughout the study |
Cropsey 2011.
| Methods | Allocation: random assignment, random number table-first 9 people put on intervention Randomisation method: sealed envelopes opened at the end of treatment Similar on drug use: yes Similar on criminal activity: yes Blinding methodology: double‐blinded. Placebo was used and was not known to evaluators or dispensers during treatment Loss to follow‐up: partial-a few individuals not included in the final analysis | |
| Participants | 36 adults Mean age: 31.8 years (SD 8.4) 100% female 89% white 100 drug users Alcohol use: yes-percentage not available 54.3% prescribed medication for mental illness Eligibility criteria: adult women, opioid dependent, interest in treatment for opioid dependence, no contraindications for buprenorphine, due for release from residential treatment within the month, returning to the community, release to correct area |
|
| Interventions | Community‐based pharmacological intervention vs placebo (I) buprenorphine (n = 24) vs (C) placebo (n = 12) (I) group was started on 2 mg of buprenorphine, increased to target dose of 8 mg at discharge. Only 37.2% reached target dose at discharge. (Doses were lower than standard induction, as participants had been in a controlled environment for some time without access to opiates.) Doses were titrated up to a maximum of 32 mg per day in the community, as clinically indicated. Participants were assessed weekly for side effects, were given drug testing and were counselled by the study physician if using drugs. The treatment course was 12 weeks. The (C) group was given a placebo on the same regimen as the (I) group |
|
| Outcomes | % injection drug use and % urine opiates at end of treatment and at 3 months' follow‐up | |
| Notes | This project was supported by funding from NIDA R21DA019838
and product support from Reckitt Benckiser Pharmaceuticals Inc. The authors have no declarations of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | First 9 participants deliberately allocated to intervention for practical reasons; use of a random number table |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | This trial began as an open label trial then became a double blind trial of participants and providers on all outcomes. Some concerns about contamination issues with the placebo group but difficult to assess to what extent the blinding might have been affected. |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | This trial began as an open label trial then became a double blind trial of participants and providers on all outcomes. Some concerns about contamination issues with the placebo group but difficult to assess to what extent the blinding might have been affected. |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No evidence to provide information about whether the assessors were blind |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No evidence to provide information about whether the assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 8 individuals were not included in the final analysis after randomisation |
| Selective reporting (reporting bias) | Unclear risk | No information reported |
| Other bias | Low risk | No other concerns within the methodology |
Dolan 2003.
| Methods | Allocation: random allocation Randomisation method: low risk, cards drawn from sealed envelope Similar on drug use: yes Similar on criminal activity: yes Blinding methodology: high risk, treatment and comparator (methadone or wait list) would not permit blinding. No statement that the outcome assessment was blind (unclear risk) Loss to follow‐up: high risk, > 30% in both groups excluded from 4‐month follow‐up | |
| Participants | 382 adults and young offenders Mean age: 27 years (SD 6) 100% male Ethnicity: not reported 100% drug‐using Alcohol use: not reported Psychiatric history: not reported Eligibility criteria: prisoners with a heroin problem, as confirmed by a detailed interview, who have at least 4 months remaining on their prison sentence at time of interview | |
| Interventions | Secure establishment‐based pharmacological intervention versus waiting‐list control (I) methadone maintenance (n = 191) vs waiting‐list control (n = 191). (I) Participants were given 30 mg of methadone each day, increasing by 5 mg every 3 days until 60 mg was achieved; duration in treatment varied. Duration of waiting‐list was 4 months. At 5 months, all participants were offered methadone through the prison‐based methadone treatment programme. Released subjects who had been treated through the prison methadone programme were offered the opportunity to transfer to local community methadone programmes |
|
| Outcomes |
Dolan 2003: primary study Heroin use (hair analysis) and self‐reported heroin use during the past 2 months at 2 months' follow‐up Drug injecting during the past 3 months at 3 months' follow‐up. Syringe sharing and HIV/HCV seroconversion during the past 4 months at 4 months' follow‐up Dolan 2005: 4‐year follow up Long‐term outcomes at four years including mortality, reincarceration, hepatitis C seroconversion and HIV seroconversion |
|
| Notes | Funding was provided by the Commonwealth Department of Health and Family Services, Glaxo Wellcome, the NSW Department of Health and the National Drug and Alcohol Research Centre, UNSW. The authors have no declarations of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation by phone |
| Allocation concealment (selection bias) | Low risk | Allocation held by researcher not involved in recruiting or interviewing participants. Trial nurses had no access to lists |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | Treatment and comparator (methadone or wait list) would not permit blinding |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | Treatment and comparator (methadone or wait list) would not permit blinding. |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition > 30% in both groups and ITT not undertaken. At follow‐up, 129 (68%) treated and 124 (65%) control subjects who had been in continuous custody were reinterviewed. 29 treated and 33 control subjects had been released from prison and were excluded. No data on other participants not accounted for at follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes in objectives were reported in results |
| Other bias | Low risk | Baseline characteristics largely similar (p 61) Some control participants received Tx, some Tx not given; methadone tested by subgroup analysis |
Dole 1969.
| Methods | Allocation: random assignment Randomisation method: lottery method Similar on drug use: yes Similar on criminal activity: yes Blinding methodology: unclear and not reported Loss to follow‐up: adequate/low risk | |
| Participants | 32 males Heroin addicts 5 years or longer 5 or more previous convictions 15 European, 10 negro, 7 Puerto Rican With a population of heroin‐dependent prerelease prisoners |
|
| Interventions | Methadone (n = 12) vs waiting‐list control (n = 16). Methadone was prescribed on admission to a hospital unit where individuals were given 10 mg per day, gradually increasing to a dose of 35 mg |
|
| Outcomes | Heroin use Reincarceration Treatment retention Employment At 7 to 10 months, 50 weeks |
|
| Notes | Participants were chosen by a lottery based on release dates between January 1 and April 30 1968. Supported by grants from the Health Research Council and the New York State Narcotics Addiction Control Commission. No declarations of interest by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by lottery, no further details of the study method provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data on key outcomes |
| Selective reporting (reporting bias) | Unclear risk | Intention‐to‐treat analysis not reported |
| Other bias | Unclear risk | Representativeness of the small sample with no urine analysis in follow‐up of controls |
Howells 2002.
| Methods | Allocation: random assignment Randomisation method: method not reported Similar on drug use: yes Similar on criminal activity: not reported Blinding methodology: low risk, double‐blind with blinded outcome assessment Loss to follow‐up: high risk, 21 participants (27.63%) (13/32 lofexidine, 8/36 methadone) were withdrawn from the trial prematurely. | |
| Participants | 80 adult participants was planned, in the time available for the trial, 76 patients met eligibility criteria and gave their consent to participate. Of these, two patients immediately elected to withdraw from the trial. In error, six patients were entered into the trial for a second detoxification after completing the trial on the first occasion and then receiving a separate prison sentence following release. Four of these patients were randomised to the other drug on second entry. Average age: The ages of the lofexidine and methadone groups were similar (29.8 years [range 22 to 43] and 30.5 years [range 22 to 49] respectively, P = 0.65) 100% male % white not reported Use of heroin was reported by 97.1% (n = 66) of the participants during the previous month and 89.7% reported heroin to be their main problem substance. % alcohol not reported % psychiatric history not reported. Major psychiatric illness was an exclusion criterion. Eligibility criteria: Consenting patients were required to be under 55 years old and to meet DSM‐IV criteria for opioid dependence and induced withdrawal (American Psychiatric Association, 1994). Participant exclusion criteria were concurrent serious major psychiatric illness (schizophrenia, psychotic depression) or serious physical illness that would prevent participation in the trial. Opioid use was confirmed by urine screening for the presence of urinary opioid metabolites. |
|
| Interventions | Intervention: (I) Placebo syrup as a green aqueous solution and lofexidine peach‐coloured tablets twice daily for 10 days (n = 32) Control: (C) Methadone as a green liquid (1 mg/ml), and placebo peach‐coloured tablets, twice daily for 10 days. Following the manufacturer’s datasheet the Lofexidine (Britlofex) regimen consisted of an initial daily dose of 0.6 mg (with 0.2 mg administered in the morning and 0.4 mg at night) increasing by 0.4 mg daily (two tablets) until day 4. At this point the dose was maintained at 2 mg daily (five tablets twice a day) for 3 days. Over the next 3 days the dose was tapered by 0.4 mg per day. The gradual dose reduction was designed to prevent any possible rebound hypertension (n = 36). |
|
| Outcomes | The primary outcome measure was withdrawal symptom severity measured using two withdrawal scales: the 20‐item Withdrawal Problems Scale (WPS), and the eight item Short Opiate Withdrawal Scale (SOWS). The participants self‐completed the withdrawal scales each morning. Given limited item overlap between the two scales, a composite 28‐item total withdrawal symptoms scale was computed to facilitate presentation of results. To analyse the total daily scores for each scale, the following global indices were derived: the highest daily score observed and the time of the occurrence, the lowest daily score observed and the time of the occurrence, the total score summed over all 10 days of the trial. Secondary outcome measures were rates and timing of withdrawal from the detoxification programme so that the relationship between failure to complete detoxification and severity of withdrawal symptoms could be measured. The Severity of Dependency Scale (SDS) was also used to assess the severity of psychological aspects of drug dependence. |
|
| Notes | Britannia Pharmaceuticals provided the medication. No declarations of interest statement included in the trial report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors report “The pharmacist who made up the medication used a simple randomisation procedure to allocate each participant to one arm of the trial” but no further description is reported. |
| Allocation concealment (selection bias) | Unclear risk | The authors report “The independent pharmacy team at the prison oversaw the randomisation and blinding procedure…”, but no statement that allocation was concealed |
| Blinding of participants and personnel (performance bias) subjective outcomes | Low risk | “…both the patient and health centre clinicians were blind to the assigned treatment group” |
| Blinding of participants and personnel (performance bias) objective outcomes | Low risk | The authors report “The independent pharmacy team at the prison oversaw the randomisation and blinding procedure…”, but no statement that allocation was concealed |
| Blinding of outcome assessment (detection bias) subjective outcomes | Low risk | “…blinding was maintained during treatment of the patients and during data entry and analysis” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | “…blinding was maintained during treatment of the patients and during data entry and analysis” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Twenty‐one participants (27.63%) (13/32 lofexidine, 8/36 methadone) were withdrawn from the trial prematurely. ITT not used, data analysed per‐protocol |
| Selective reporting (reporting bias) | Low risk | The authors indicate that there was a protocol for the study (“Patient safety elements in the protocol were as follows:”) and primary and secondary outcomes are clearly defined. Outcome data for the primary and secondary outcomes are reported. |
| Other bias | High risk | The authors report “Four of these patients were randomised to the other drug on second entry. As a check on results, we repeated the analyses with the exclusion of these six cases. Whilst both the direction and magnitude of the results were unaltered we removed these cases from the dataset and the remaining results relate to the reduced sample of 68 patients.” |
Kinlock 2005.
| Methods | Allocation: random assignment Randomisation method: unclear Similar on drug use: yes Similar on criminal activity: yes Blinding methodology: unknown Loss to follow‐up: inadequate/high risk | |
| Participants | 126 adult males Age: 35.7 years (SD 6.8) 100% male 14% white 100% drug users Alcohol use: not reported Eligibility criteria: 3 months before anticipated release from prison, history of heroin dependence meeting DSM‐IV criteria | |
| Interventions | (I) Prison/secure establishment‐based levo‐alpha‐acetyl methanol + transfer to methadone maintenance after release (n = 20) vs (C) untreated controls (31) and withdrew before treatment (N = 13) (I) Participants medicated 3 times per week starting at 10 mg and increasing by 5 mg every third medication day during incarceration to a target dose of 50 mg. At release participants were advised to report to the program's communirty ased maintenance facility for continuing care. (C) Received community treatment referral information only. |
|
| Outcomes | Heroin use during 9‐month follow‐up (self‐report), arrests during 9‐month follow‐up (official records) and reincarceration during 9‐month follow‐up (official records), frequency of illegal activity, admission to drug use and average weekly income obtained from illegal activities, mean number of crime days. | |
| Notes | No funding information provided No declaration of interest by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information reported other than stated 'random' |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A considerable number of experimental participants declined medication after initial consent and randomisation to the experimental condition (see pp. 437 and 499). High attrition from the experimental group after random assignment and before treatment initiation required revision of the original two‐group study design for purposes of data analyses |
| Selective reporting (reporting bias) | High risk | Table 4, p. 446, indicates only selected outcomes. No ITT conducted |
| Other bias | High risk | Experimental and control groups could not be considered comparable (p. 449); therefore, the number of variables was restricted. Study groups were revised after attrition in treatment group. Groups were considered not to be comparable, and the number of variables assessed was restricted. Urine samples and treatment records available on experimental group only |
Kinlock 2007.
| Methods | Allocation: random assignment Randomisation method: block randomised Similar on drug use: unknown Similar on criminal activity: unknown Blinding methodology: high risk Loss to follow‐up: adequate | |
| Participants | 211 adult males
Age: group (a) 40.9 years (SD 7.6), (b) 40.3 years (7.0), (c) 39.8 years (7.0)
100% male
% white: group (a) 31.3%, (b) 19.7%, (c) 20%
100% drug users
Alcohol use not reported Psychiatric history not reported Eligibility criteria: (1) 3 to 6 months before release from prison; (2) meeting Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) criteria of heroin dependence at time of incarceration and being physiologically dependent during the year prior to incarceration; (3) no pending parole hearings and/or unadjudicated charges; (4) having a Baltimore city address post‐release; and (5) suitability for methadone maintenance as determined by medical evaluation. Inmates were excluded from study participation if they had any unadjudicated charges and/or pending parole hearings. |
|
| Interventions | (C) Counselling Only: counselling in prison and passive referral to community‐based drug treatment (n = 70) (I) Counselling + Transfer: counselling in prison and transfer to methadone maintenance in the community upon release beginning with 5 mg of methadone and increasing by 5 mg every eighth day to a target minimum dose of 60 mg (n = 70). (I) Counselling + Methadone: counselling and methadone in prison with transfer to methadone treatment in the community upon release, begininning with 5 mg dose of methadone and increasing by 5 mg every eighth day during incarceration to a target dose of 60 mg. Advised to report to the program's community‐based methadone program within 10 days of release for continuing care (n = 71). |
|
| Outcomes |
Kinlock 2007: primary study Urine test for opioids 1 month post‐release, urine test for cocaine 1 month post‐release, self‐report heroin use 1 month post‐release, self‐report cocaine use 1 month post‐release. Gordon 2008: 6 month follow up study Urine testing for opioids, cocaine and other illicit drugs 6 months post‐release, treatment record review, Addiction Severity Index (ASI) from baseline and follow up. Wilson 2012: follow up study Post‐release changes over time in the specific HIV risk behaviours in which the participants had a prior history of engaging. Participants were assessed at baseline (study entry in prison), and at 1‐, 3‐, 6‐, and 12‐month post‐release. The primary outcome measures at each time period were self‐reported participation in risky drug‐ and sex‐risk behaviours obtained from the Texas Christian University AIDS Risk Assessment (ARA). |
|
| Notes | Funding for this study was provided by Grant R01 DA 16237 from the National Institute on Drug Abuse (NIDA). No declarations of interest reported by the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Individuals in the counselling only group did not receive treatment |
| Selective reporting (reporting bias) | Unclear risk | No information reported |
| Other bias | High risk | Contamination of treatment groups |
Lobmaier 2010.
| Methods | Allocation: random Randomisation method: permuted block protocol Groups similar on drug use at baseline: yes Groups similar on criminal activity at baseline: yes Blinding methodology: not blinded-open label Loss to follow‐up: unknown |
|
| Participants | 46 adults Mean age: 35.1 years (SD 7) 93% male Ethnicity: unknown 100% drug users. 86.4% polydrug use Alcohol use: not reported Psychiatric history: not reported Eligibility criteria: inclusion: pre‐incarceration heroin dependence, at least 2 months sentence time remaining. Exclusion: untreated major depression or psychosis, severe hepatic impairment, already in agonist maintenance treatment, pregnant |
|
| Interventions | Secure establishment naltrexone intervention vs methadone treatment (I) Received 20‐pellet naltrexone implants around one month before release. Implants give sustained‐release naltrexone over 5 to 6 months (n = 23) vs (C) Initiated on 30 mg methadone per day at around one month pre‐release. Increased over typical period of three weeks to recommended dose of 80 to 130 mg (n = 21) |
|
| Outcomes | Mean days per month of criminal activity (self‐reported) at 6 months No. of days in prison (from official records of Norwegian prison) at 6 months Mean days per month using heroin, benzodiazepines and amphetamines (self‐reported) at 6 months |
|
| Notes | Funding was provided by the Research Council of Norway No declarations of interest by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation sequence performed at an independent centre using a permuted block protocol |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | p143 "the treatment conditions were not blind and may have increased risk if performance bias" |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | p143 "the treatment conditions were not blind and may have increased risk if performance bias" |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | No evidence |
| Other bias | Low risk | No other concerns |
Lobmann 2007.
| Methods | Allocation: random assignment Randomisation method: block randomised Similar on drug use: unknown Similar on criminal activity: unknown Blinding methodology: unknown Loss to follow‐up: adequate | |
| Participants | 1015 drug‐using offenders Age: 36 years (SD 6.7) % male not reported % white not reported 100% drug users Alcohol use not reported Eligibility criteria: min age 23 years, ICD‐10 opiate addiction, opiate addiction min 5 years, current daily heroin consumption, OTI scale health problems, not received therapy for addiction during past 6 months | |
| Interventions | Community‐based: diamorphine treatment (n = 500) vs methadone treatment (n = 515). | |
| Outcomes | 12 months follow up and outcomes. Drug use and criminal activity (self‐report and official records) |
|
| Notes | Article in German, single reviewer translation completed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all outcomes presented, limited attrition noted |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No information provided |
Magura 2009.
| Methods | Random allocation: to methadone or buprenorphine allocation initially on a 1:1 ratio and subsequently periodically based on 7:3 Randomisation method: inadequate, personnel aware of allocation Similar on drug use: yes Similar on criminal activity: yes Blinding methodology: unknown Loss to follow‐up: inadequate, up to 30% lost |
|
| Participants | 133 male inmates Age: group (a) 38.4 years (SD 7.9), (b) 40.7 years (9.1) 100% male 25% black, 64% Hispanic 100% drug users Alcohol use: not reported Eligibility criteria: inmates who were eligible for the Key Extended Entry Program (KEEP), 18 to 65 years old, sentenced to 10 to 90 days' jail time, and expected to reside locally post‐release | |
| Interventions | Prison/secure establishment based methadone (n = 56) vs buprenorphine (n = 77) (C) Methadone: Participants were given liquid methadone dispensed once daily usual maintenance dose was 30 mg which could be stepped up to a maximum of 70 mg if clinically indicated and participant agreed. (I) Buprenorphine: The sublingual combination burprenorphine/naloxone tablet was used for both induction and maintenance, initial dose of 4 mg which could be stepped up to 8 mg on the first day and could be stepped up to 32 mg on subsequent days. Participants observed until the tablet had dissolved. |
|
| Outcomes | Arrest (self‐report) during the past 12 months at 3‐month follow‐up for property crime, drug posession and % reincarcerated. Drug use past 30 days (self‐report), mean number of days heroin use post‐release at 3‐month follow‐up | |
| Notes | No funding information provided by the authors No declarations of interest reported by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator used. Allocation was originally 1:1, but loss in one group meant that treatment‐adaptive randomisation was used at a ratio of 7:3 |
| Allocation concealment (selection bias) | High risk | Project director was naive to allocation, but research assistant was not |
| Blinding of participants and personnel (performance bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) objective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some attrition occurred before medication was received by buprenorphine‐assigned participants. 30% of participants could not be interviewed at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No information reported |
| Other bias | High risk | Participants at one site received methadone suboptimal doses (30 mg). The study contained a modest sample size |
Wright 2011.
| Methods | Allocation: random allocation Randomisation method: low risk, generated using Microsoft Excel Similar on drug use: yes Similar on criminal activity: not reported Blinding methodology: high risk, open‐label Loss to follow‐up: high risk | |
| Participants | 439 eligible adults of whom 133 declined leaving 306 available for randomisation. Seventeen excluded at randomisation. 289 adults randomised and allocated The median age was 30.8 years (interquartile range (IQR), 26.9 to 34.9) % male, not reported – mixed sample (1 all‐female and 2 all‐male prisons) Methadone, 89.9 % white; buprenorphine, 93.6% white % drug users not reported % alcohol, not reported % Psychiatric history, not reported Eligibility criteria: Inclusion criteria: 21 to 65 years old; using illicit opiates as confirmed by urine test; expressing a wish to detoxify and remain abstinent; willing to give informed consent; and remaining in custody for at least 28 days. Exclusion criteria: contraindications to methadone or buprenorphine; medical conditions requiring emergency admission to hospital, thus precluding detoxification; currently undergoing detoxification from other addictive drugs whereby concurrent opiate detoxification would not be clinically indicated; and previously randomised into the trial |
|
| Interventions | Sublingual buprenorphine (n = 141) vs Oral methadone (n = 148) (I) Sublingual buprenorphine: prescribed daily within set dose limits of 8 mg for days 1 to 5, 6 mg for days 6 to 7, 4 mg for days 8 to 10 and subsequently descreasing to a limit of 0.4 on day 20. (C) Oral methadone (1mg/1ml mixture): prescribed daily within set dose limits of 30 mg for days 1 to 5, 25 mg for days 6 to 7, 22 mg for days 8 to 9, 20 mg for days 10 to 11 and subsequently descreasing to a limit of 2 mg on day 20. |
|
| Outcomes | The primary outcome was abstinence from illicit opiates at 8 days post detoxification, as indicated by a urine test. Secondary outcomes included abstinence status at 1, 3, and 6 months post detoxification, ascertained via urine test if the participant was still in prison. If the participant had been released, local community drugs service records were accessed to verify abstinence. Adverse events were recorded and a researcher was informed immediately of any serious adverse events, which were then reported to the regulatory authorities. These included overdose, self‐harm, or suicide attempt; inappropriate use of prescribed medication; or admission as a prison healthcare inpatient. |
|
| Notes | Funded by Department of Health, National Research and Development Programme on Forensic Mental Health Research Funding Scheme 2004. The authors state that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence (with random block size) was generated using Microsoft Excel RAND function |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, consecutively numbered envelopes concealing the name of the allocated intervention were prepared by a researcher who had no contact with participants. |
| Blinding of participants and personnel (performance bias) subjective outcomes | High risk | Open label “The prescribing doctor randomised by opening the next envelope and prescribing the intervention named inside. Both prisoner and doctor were blind to the intervention until this point.” |
| Blinding of participants and personnel (performance bias) objective outcomes | High risk | Open label “The prescribing doctor randomised by opening the next envelope and prescribing the intervention named inside. Both prisoner and doctor were blind to the intervention until this point.” |
| Blinding of outcome assessment (detection bias) subjective outcomes | Unclear risk | No statement regarding blinding of individual who undertook the biochemical urine tests. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No statement regarding blinding of individual who recorded self‐report or clinical notes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High levels of attrition. 50% buprenorphine and 45% methadone did not provide urine sample at day 8, 65% and 62% at 1 month, 80% and 85% at 3 months and 86% and 91% at 6 months. ITT undertaken assuming if no objective or subjective data available, participants were not abstinent. |
| Selective reporting (reporting bias) | High risk | Adverse events and reasons for withdrawal stated as being recorded but no outcome data reported. |
| Other bias | Low risk | No other concerns |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alemi 2010 | Does not concern pharmacological intervention |
| Alessi 2011 | Not original RCT. Data is from previous, older studies. |
| Andersson 2014 | Intervention not aimed at reducing drug use or criminal activity, or both |
| Anglin 1999 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Awgu 2010 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Azbel 2013 | Intervention not aimed at reducing drug use or criminal activity, or both. |
| Baldus 2011 | Study protocol only, author has since died. |
| Baltieri 2014 | Intervention not aimed at reducing drug use or criminal activity, or both. |
| Barnes 2012 | Not using a population of drug‐using offenders |
| Berman 2004 | The intervention was not aimed at reducing drug use or criminal activity or both in drug‐using offenders. |
| Black 2011 | Not offender population |
| Brady 2010 | Not RCT |
| Braithwaite 2005 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Breckenridge 2000 | Evaluated a DWI Court for alcoholic offenders, not illicit drug use, not a pharmacological intervention |
| Britt 1992 a‐d | Does not concern pharmacological intervention. |
| Brown 2001 | 3‐arm study in which only 2 arms were randomised ‐ 1 treatment arm and control arm. Results presented as both treatment arms combined vs control. |
| Burdon 2013 | Not a trial. |
| Carr 2008 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Carroll 2006 | Does not concern pharmacological intervention |
| Carroll 2011 | Not offender population |
| Carroll 2012 | Not a pharmacological intervention. |
| Chandler 2006 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Chaple 2014 | No pre‐ and post‐test measures of drug or crime, or both. |
| Clair 2013 | No data presented at pre‐ and post‐test outcomes for crime and drug |
| Cogswell 2011 | Population not offenders. |
| Cosden 2003 | Does not concern pharmacological intervention |
| Cosden 2005 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Coviello 2012 | Not a Randomised Controlled Trial |
| Cox 2013 | Not an offender population |
| Cropsey 2013 | Not a Randomised Controlled Trial |
| Cullen 2011 | Not a drug program aimed at reducing drug use/criminal activity in drug using offenders. |
| Cusack 2010 | Not a drug program aimed at reducing drug use/criminal activity in drug using offenders. |
| D'Amico 2013 | Does not present data for pre‐ and post‐test information on drug or crime measures, or both. |
| Dakof 2010 | Study population is mothers of offenders, not offenders themselves |
| Dana 2013 | Not an RCT |
| DeFulio 2013 | Not an RCT |
| Dembo 2000 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. The follow‐up periods reported for the different groups were not equivalent. |
| Deschenes 1994 | Does not concern pharmacological intervention |
| Di Nitto 2002 | The follow‐up periods reported for the different groups were not equivalent. |
| Diamond 2006 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Dugan 1998 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Evans 2012 | Not an RCT |
| Forsberg 2011 | Does not concern pharmacological intervention |
| Freudenberg 2010 | Does not concern pharmacological intervention |
| Friedman 2012 | Not an RCT |
| Frost 2013 | Not an RCT |
| Gagnon 2010 | Not offender population |
| Gil 2004 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Gordon 2012 | No relevant data; all analysis at baseline; no pre‐ and post‐test information on drug use or criminal activity, or both. |
| Gordon 2013 | No relevant data; all analysis secondary, not a primary RCT. |
| Gottfredson 2002 | Does not concern pharmacological intervention |
| Grohman 2002 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Grommon 2013a | Not a pharmacological intervention. |
| Grommon 2013b | Not a pharmacological intervention. |
| Guydish 2011 | Does not concern pharmacological intervention |
| Guydish 2014 | Not criminal justice population |
| Haapanen 2002 | Does not concern pharmacological intervention |
| Haasen 2010 | Not offender population |
| Hanlon 1999 | Does not concern pharmacological intervention |
| Harada 2012 | No data on pre‐ and post‐test outcomes for drug or criminal justice, or both. |
| Harrell 2001 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Henderson 2010 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Henggeler 1991 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Henggeler 1999 | Does not concern pharmacological intervention |
| Henggeler 2002 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Henggeler 2006 | Does not concern pharmacological intervention |
| Henggeler 2012 | Not a pharmacological intervention. |
| Hser 2011 | Unclear if study looks at offender population |
| Hser 2013 | Not a pharmacological intervention |
| Inciardi 2004 | Some participants were not randomly selected into the treatment groups. |
| Jain 2011 | Paper not available and not clear from abstract if looks at offender population |
| Johnson 2011 | Does not concern pharmacological intervention |
| Johnson 2012 | Does not concern pharmacological intervention |
| Jones 2013 | Not a pharmacological intervention |
| Jones, 2011 | Evaluated a DWI Court for alcoholic offenders, not illicit drug use |
| Katz 2007 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Kelly 2013 | Not a pharmacological intervention. |
| Kidorf 2013 | Not offender population |
| King 2014 | Not offender population |
| Kinlock 2008 | Not a pharmacological intervention. |
| Kinlock 2009a | Conference proceedings only |
| Kinlock 2009b | Not a pharmacological intervention |
| Kok 2013 | Not offender population |
| Law 2012 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Lee 2012 | No pre‐ and post‐test data for outcomes of drug or criminal justice measures, or both. |
| Liddle 2011 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods |
| Ling 2013 | Not offender population |
| Lobmann 2009 | No pre‐ and post‐outcome measures for drug or crime outcomes, or both |
| MacDonald 2007 | Evaluated a DWI Court for alcoholic offenders, not illicit drug use |
| Marlowe 2003 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Marlowe 2005 | Not a pharmacological intervention |
| Marlowe 2007 | Participants randomised to receive treatment were not randomised into the different treatment groups but were identified by level of risk. Not an RCT. |
| Marlowe 2008 | Does not concern pharmacological intervention |
| Marsch 2014 | Not offender population |
| Martin 1993 | Does not concern pharmacological intervention |
| Mbilinyi 2011 | Participants not recruited through criminal justice system |
| McKendrick 2007 | The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| McKenzie 2012 | Does not concern pharmacological intervention |
| Messina 2000 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Messina 2010 | No pharmacological interventions |
| Milloy 2011 | No pre‐ and post‐data for outcomes of crime or drug use, or both. |
| Needels 2005 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Nemes 1998 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Nemes 1999 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. The study did not report relevant drug or crime outcome measures, or both, at both the pre‐ and post‐intervention periods. |
| Nielsen 1996 | Does not concern pharmacological intervention |
| Nosyk 2010 | Not offender population |
| Petersilia 1992 | Does not concern pharmacological intervention |
| Petry 2005 | Not 100% criminal justice population. |
| Petry 2011 | Not offender population |
| Polsky 2010 | Not offender population |
| Prendergast 2003 | Does not concern pharmacological intervention |
| Prendergast 2008 | Does not concern pharmacological intervention |
| Prendergast 2009 | The study did not report relevant drug or crime outcome (or both) measures at both the pre‐ and post‐intervention periods |
| Prendergast 2011 | Does not concern pharmacological intervention |
| Proctor 2012 | No pharmacological interventions |
| Reimer 2011 | Not offender population |
| Robertson 2006 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention |
| Rosengard 2008 | The study did not report relevant drug or crime outcome (or both) measures at both the pre‐ and post‐intervention periods |
| Rossman 1999 | Does not concern pharmacological intervention |
| Rounsaville 2001 | No pre‐ and post‐test data presented on drug use or crime outcomes, or both |
| Rowan‐Szal 2005 | Population not offenders. |
| Rowan‐Szal 2009 | Not RCT |
| Rowe 2007 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Sacks 2004 | Does not concern pharmacological intervention |
| Sacks 2008 | Does not concern pharmacological intervention |
| Sacks 2011 | Does not concern pharmacological intervention |
| Sanchez‐Hervas 2010 | Population not offenders. |
| Schaeffer 2014 | Does not contain a pharmacological intervention |
| Schmiege 2009 | No data for pre‐ and post‐test outcome measures of drug or crime outcomes, or both. |
| Schwartz 2006 | Not offender population |
| Shanahan 2004 | This is not a pharmacological intervention |
| Sheard 2009 | The study did not report relevant drug or crime outcome (or both) measures at both the pre‐ and post‐intervention periods |
| Siegal 1999 | Not RCT |
| Sinha 2003 | Not a pharmacological intervention. |
| Smith 2010 | Does not concern pharmacological intervention |
| Solomon 1995 | Not an offender population. |
| Specka 2013 | Not an offender population. |
| Stanger 2009 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Staton‐Tindall 2009 | No control group; not an RCT. |
| Stein 2006 | No pre‐ and post‐test data for drug or crime outcome measures, or both. |
| Stein 2010 | Not offender population |
| Stein 2011 | Does not concern pharmacological intervention |
| Stevens 1998 | The population of the study was not 100% drug‐using offenders that were specifically referred by the criminal justice system to the intervention. |
| Svikis 2011 | Not clear if offender population |
| Taxman 2006 | Does not concern pharmacological intervention |
| Vagenas 2014 | No pre‐ and post‐test data on drug or crime outcome measures, or both |
| Vanderberg 2002 | No pre‐ and post‐test outcome data on crime or drug measures, or both |
| Villagrá Lanza 2013 | Does not concern pharmacological intervention |
| Walters 2014 | No data on pre‐ and post‐test information for drug or crime outcome measures, or both. |
| Wang 2010 | Participants not in criminal justice system |
| Webster 2014 | No data on pre‐ and post‐test information for drug or crime outcome measures, or both. |
| White 2006 | Randomisation broken as 40% of control arm were allowed to receive treatment (acupuncture) outside of the intervention. |
| Williams 2011 | Not RCT |
| Winstanley 2011 | Not clear if offender population |
| Witkiewitz 2010 | Not clear if offender population |
| Wolff 2012 | No data for pre‐ and post‐test outcomes of drug or crime measures, or both. |
| Zlotnick 2009 | Does not concern pharmacological intervention |
Characteristics of ongoing studies [ordered by study ID]
Springer 2015.
| Trial name or title | Naltrexone for opioid dependent released HIV+ criminal justice populations. Referred to as NEWHOPE. |
| Methods | Our specific aim is to conduct a placebo‐controlled RCT of depot NTX (d‐NTX) for HIV+ prisoners with OD who are transitioning to the community 150 subjects within CJS in New Haven, Hartford and Springfield. Subjects will be randomized 2:1 to d‐NTX or d‐placebo for 6 months and observed for 12 months. |
| Participants | HIV‐infected prisoners with opioid dependence who are treated with depot naltrexone as they are transitioning from the correctional to the community setting. 150 participants. |
| Interventions | Depot naltrexone versus placebo |
| Outcomes | 6 and 12 months HIV treatment (HIV‐1 RNA levels, CD4 count, ART adherence, retention in care), substance abuse (time to relapse to opioid use, % opioid negative urines, opioid craving), adverse side effects and HIV risk behavior (sexual and drug‐related risks) The public health relevance is that outcomes from this study will establish the efficacy, safety and tolerability of pharmacological therapy using naltrexone treatment among HIV+s and establish depot‐naltrexone treatment as an effective, evidence‐based treatment for opioid dependence for released HIV+ prisoners. |
| Starting date | 2012 |
| Contact information | Yale University |
| Notes |
Differences between protocol and review
The original review Perry 2006 has been split up into different reviews and so there is no dedicated protocol for this particular review
Contributions of authors
Searches were constructed and conducted by DF. Three independent review authors inspected the search hits by reading the titles and abstracts (AEP, MN, RW). Each potentially relevant study located in the search was obtained as a full article and was independently assessed for inclusion by two review authors. In the case of discordance, a third independent review author arbitrated. Where it was not possible to evaluate the study because of language problems or missing information, the studies were classified as 'translation/information required to determine decision' until a translation or further details were provided. Four review authors conducted data extraction for the papers (MM‐SJ, JMG, RW, and MN), and review author CG conducted data extraction and a narrative summary of the cost‐effectiveness studies. The results were compiled and organised by MM‐ST, MN, CH, RW and AEP, and all eight authors contributed towards the final draft text.
Sources of support
Internal sources
-
Reviewer from Cochrane Drugs and Alcohol Group, Other.
A reviewer from the Drugs and Alcohol Group provided the researchers with the results of a search strategy for three databases
External sources
The Department of Health funded the original review, UK.
Declarations of interest
Amanda E Perry have no interests to declare relating to this work
Matthew Neilson have no interests to declare relating to this work
Marrissa Martyn‐St James have no interests to declare relating to this work
Julie M Glanville have no interests to declare relating to this work
Dave Fox have no interests to declare relating to this work
Rebecca Woodhouse have no interests to declare relating to this work
Catherine Hewitt have no interests to declare relating to this work
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bayanzadeh 2004 {unpublished data only}
- Bayanzadeh SA. Final report of research project: a study of the effectiveness of psychopharmacological and psychological interventions in reducing harmful/high risk behaviours among substance user prisoners. Iran University of Medical Education and Health and Treatment Services, Tehran Psychology Institute. Centre for Psychological Health Research Polarity of Science, Education and Research 2004.
Brown 2013 {published data only}
- Brown R, Gassman M, Hetzel S, Berger L. Community‐based treatment for opioid dependent offenders: A pilot study. American Journal on Addictions 2013;22(5):500‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cornish 1997 {published data only}
- Cornish JW, Metzger D, Woody GE, Wilson D, McLellan AT, Vandergrift B, et al. Naltrexone pharmacotherapy for opioid dependent federal probationers. Journal of Substance Abuse Treatment 1997;14(6):529‐34. [DOI] [PubMed] [Google Scholar]
Coviello 2010 {published data only}
- Coviello DM, Cornish JW, Lynch KG, Alterman AI, O'Brien CP. A randomized trial of oral naltrexone for treating opioid‐dependent offenders. American Journal of Addiction 2010;19(5):422‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cropsey 2011 {published data only}
- Cropsey KL, Lane PS, Hale GJ, Jackson DO, Clark CB, Ingersoll KS, et al. Results of a pilot randomized controlled trial of buprenorphine for opioid dependent women in the criminal justice system. Drug and Alcohol Dependence 2011;119(3):172‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2003 {published data only}
- Dolan K A, Shearer J, MacDonald M, Mattick RP, Hall W, Wodak AD. A randomised controlled trial of methadone maintenance treatment versus wait list control in an Australian prison system. Drug and Alcohol Dependence 2003;72(1):59‐65. [DOI] [PubMed] [Google Scholar]
- Dolan KA, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four‐year follow‐up of imprisoned male heroin users and methadone treatment: mortality, re‐incarceration and hepatitis C infection. Addiction 2005;100(6):820‐828. [DOI: 10.1111/j.1360-0443.2005.01050.x] [DOI] [PubMed] [Google Scholar]
Dole 1969 {published data only}
- Dole VP, Robinson MD, Orraca J, Towns E, Searcy P, Caine E. Methadone treatment of randomly selected criminal addicts. New England Journal of Medicine 1969;280:1372‐5. [DOI] [PubMed] [Google Scholar]
Howells 2002 {published data only}
- Howells C, Allen S, Gupta J, Stillwell G, Marsden J, Farrell M. Prison based detoxification for opioid dependence: a randomised double blind controlled trial of lofexidine and methadone.. Drug & Alcohol Dependence 2002;67(2):169‐76. [DOI] [PubMed] [Google Scholar]
Kinlock 2005 {published data only}
Kinlock 2007 {published data only}
- Gordon MS, Kinlock TW, Schwartz RP, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post‐release. Addiction 2008b;103(8):1333‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months post release. Journal of Substance Abuse Treatment 2009;37(3):277‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, O'Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1‐month post‐release. Drug and Alcohol Dependence 2007;91(2‐3):220‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, O'Grady KE. A study of methadone maintenance for male prisoners: 3‐month postrelease outcomes. Criminal Justice Behavior 2008;35(1):34‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Kinlock TW, Gordon MS, O'Grady KE, Schwartz RP. Postprison Release HIV‐Risk Behaviors in a Randomized Trial of Methadone Treatment for Prisoners. American Journal on Addictions 2012;21(5):476‐87. [DOI: 10.1111/j.1521-0391.2012.00250.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobmaier 2010 {published data only}
- Lobmaier PP, Kunoe N, Gossop M, Katevoll T, Waal H. Naltrexone implants compared to methadone: outcomes six months after prison release. European Addiction Research 2010;16(3):139‐45. [DOI] [PubMed] [Google Scholar]
Lobmann 2007 {published data only}
- Lobmann R. Diamorphine substitution therapy and criminal activity. Sucht: Zeitschrift fur Wissenschaft und Praxis 2007; Vol. 53, issue 5:288‐95. [CN‐00627424]
Magura 2009 {published data only}
- Magura S, Lee JD, Hershberger J, Joseph H, Marsch L, Shropshire C, et al. Buprenorphine and methadone maintenance in jail and post‐release: a randomized clinical trial. Drug and Alcohol Dependence 2009; Vol. 99, issue 1‐3:222‐30. [DOI] [PMC free article] [PubMed]
Wright 2011 {published data only}
- Wright N, Sheard L. Comparison of methadone and buprenorphine for opiate detoxification (LEEDS trial): a randomised controlled trial. British Journal of General Practice 2011;61(593):772‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alemi 2010 {published and unpublished data}
- Alemi F, Haack M, Nemes S, Harge A, Baghi H. Impact of online counseling on drug use: a pilot study. Quality Management Health Care 2010;19(1):62‐9. [DOI] [PubMed] [Google Scholar]
Alessi 2011 {published data only}
- Alessi SM, Rash C, Petry NM. Contingency management is efficacious and improves outcomes in cocaine patients with pretreatment marijuana use. Drug and Alcohol Dependence 2011;118(1):62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersson 2014 {published data only}
- Andersson C, Vasiljevic Z, Hoglund P, Ojehagen A, Berglund M. Daily automated telephone assessment and intervention improved 1‐month outcome in paroled offenders. International Journal Offender Therapy Comparative Criminology 2014;online:1‐18. [DOI: 10.1177/0306624X14526800]] [DOI] [PubMed] [Google Scholar]
Anglin 1999 {published data only}
- Anglin MD, Longshore D, Turner S. Treatment alternatives to street crime ‐ An evaluation of five programs. Criminal Justice and Behavior 1999;26(2):168‐95. [Google Scholar]
Awgu 2010 {published data only}
- Awgu E, Magura S, Rosenblum A. Heroin‐dependent inmates' experiences with buprenorphine or methadone maintenance. Journal of Psychoactive Drugs 2010;42(3):339‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azbel 2013 {published data only}
- Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PLoS ONE 2013;8(3):e59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldus 2011 {published data only}
- Baldus C, Miranda A, Weymann N, Reis O, More K, Thomasius R. "CAN Stop"-implementation and evaluation of a secondary group prevention for adolescent and young adult cannabis users in various contexts-study protocol. BMC Health Services Research 2011;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baltieri 2014 {published data only}
- Baltieri D. A. Order of onset of drug use and criminal activities in a sample of drug‐abusing women convicted of violent crimes.. Drug & Alcohol Review 2014;33(2):202‐10. [DOI] [PubMed] [Google Scholar]
Barnes 2012 {published data only}
- Barnes GC, Hyatt JM, Ahlman LC, Kent DTL. The effects of low intensity supervision for lower risk probationers: updated results from a RCT. Journal of Crime and Justice 2012;35(2):200‐20. [Google Scholar]
Berman 2004 {published data only}
- Berman AH, Lundberg U, Krook AL, Gyllenhammar C. Treating drug using prison inmates with auricular acupuncture: a randomized controlled trial. Journal of Substance Abuse Treatment 2004;26(2):95‐102. [0740‐5472: (Print)] [DOI] [PubMed] [Google Scholar]
Black 2011 {published data only}
- Black S, Carey E, Webber A, Neish N, Gilbert R. Determining the efficacy of auricular acupuncture for reducing anxiety in patients withdrawing from psychoactive drugs. Journal of Substance Abuse Treatment 2011;41(3):279‐87. [DOI] [PubMed] [Google Scholar]
Brady 2010 {published data only}
- Brady LLC, Najavits LM, Toussaint D, Bonavota D, Veysey B. Does recent criminal involvement matter? A study of women with co‐occurring disorders in a multisite national trial. Mental Health and Substance Use: Dual Diagnosis 2010;3 (3):193‐202. [Google Scholar]
Braithwaite 2005 {published data only}
- Braithwaite RL, Stephens TT, Treadwell HM, Braithwaite K, Conerly R. Short‐term impact of an HIV risk reduction intervention for soon‐to‐be released inmates in Georgia. Journal of Health Care for the Poor and Underserved 2005;16(4Suppl B):130‐9. [CN‐00532300] [DOI] [PubMed] [Google Scholar]
Breckenridge 2000 {published data only}
- Breckenridge JF, Winfree LT, Maupin JR, Clason DL. Drunk drivers, DWI 'drug court' treatment and recidivism: Who fails?. Justice Research and Policy 2000;2:87‐105. [Google Scholar]
Britt 1992 a‐d {published data only}
- Britt IC, Gottfredson MR, Goldkamp JS. Drug testing and pretrial misconduct: an experiment on the specific deterrent effects of drug monitoring defendants on pretrial release. Journal of Research in Crime and Delinquency 1992;29(1):62‐78. [Google Scholar]
Brown 2001 {published data only}
- Brown BS, O'Grady KE, Battjes RJ, Farrell EE, Smith NP, Nurco DN. Effectiveness of a stand‐alone aftercare program for drug‐involved offenders. Journal of Substance Abuse Treatment 2001;21(4):185‐92. [DOI] [PubMed] [Google Scholar]
Burdon 2013 {published data only}
- Burdon WM, De Lore J, Dang J, Warda US, Prendergast ML. Psychosocial Functioning Among Inmates in Prison‐Based Drug Treatment: Results from Project BRITE. Journal of Experimental Criminology 2013;9(1):45–64. [DOI: 10.1007/s11292-012-9169-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carr 2008 {published data only}
- Carr CJ, Xu J, Redko C, Lane D, Rapp RC, Goris J, et al. Individual and system influences on waiting time for substance abuse treatment. Journal of Substance Abuse Treatment 2008;34(2):192‐201. [0740‐5472] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2006 {published data only}
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, et al. The use of contingency management and motivational/skills‐building therapy to treat young adults with marijuana dependence. Journal of Consulting and Clinical Psychology 2006;74(5):955‐66. [0022‐006X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2011 {published data only}
- Carroll KM, Kiluk BD, Nich C, Babuscio TA, Brewer JA, Potenza MN, et al. Cognitive function and treatment response in a randomized clinical trial of computer‐based training in cognitive‐behavioral therapy. Substance Use and Misuse 2011;46(1):23‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2012 {published data only}
- Carroll KM, Nich C, Lapaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less.. Addiction 2012;107(9):1650‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler 2006 {published data only}
- Chandler DW, Spicer G. Integrated treatment for jail recidivists with co‐occurring psychiatric and substance use disorders. Community Mental Health Journal 2006;42(4):405‐25. [0010‐3853: (Print)] [DOI] [PubMed] [Google Scholar]
Chaple 2014 {published data only}
- Chaple M, Sacks S, McKendrick K, Marsch LA, Belenko S, Leukefeld C, et al. Feasibility of a computerized intervention for offenders with substance use disorders: a research note. Journal of Experimental Criminology 2014;10:105‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clair 2013 {published data only}
- Clair M, Stein LA, Soenksen S, Martin RA, Lebeau R, Golembeske C. Ethnicity as a moderator of motivational interviewing for incarcerated adolescents after release.. Journal of Substance Abuse Treatment 2013;45(4):370‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cogswell 2011 {published data only}
- Cogswell J, Negley SK. The effect of autonomy-supportive therapeutic recreation programming on integrated motivation for treatment among persons who abuse substances. Therapeutic Recreation Journal 2011;45(1):1st Quarter:47‐61. [Google Scholar]
Cosden 2003 {published data only}
- Cosden M, Ellens JK, Schnell JL, Yamini‐Diouf Y, Wolfe MM. Evaluation of a mental health treatment court with assertive community treatment. Behavioral Sciences & the Law 2003;21(4):415‐27. [DOI] [PubMed] [Google Scholar]
Cosden 2005 {published data only}
- Cosden M, Ellens J, Schnell J, Yamini‐Diouf Y. Efficacy of a mental health treatment court with assertive community treatment. Behavioral Sciences & the Law 2005;23(2):199‐214. [0735‐3936: (Print)] [DOI] [PubMed] [Google Scholar]
Coviello 2012 {published data only}
- Coviello DM, Cornish JW, Lynch KG, Boney TY, Clark C, Lee JD, et al. A multisite pilot study of extended release injectable naltrexone treatment for previously opioid dependent parolees and probationers. Substance Abuse 2012;33(1):48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cox 2013 {published data only}
- Cox BR, Olney JJ, Lowery‐Gionta EG, Sprow GM, Rinker JA, Navarro M, et al. Repeated cycles of binge‐like ethanol (EtOH)‐drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence‐like phenotypes.. Alcoholism, Clinical and Experimental Research 2013;37(10):1688‐95.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cropsey 2013 {published data only}
- Cropsey KL, Lane PS, Perkins AC, Clark B, Hardy S, McCullumsmith C, et al. Buprenorphine and Medication management in a community corrections population: A pilot study. Journal of Addict Med 2013;7:210‐5. [DOI] [PubMed] [Google Scholar]
Cullen 2011 {published data only}
- Cullen AE, Soria C, Clarke AY, Dean K, Fahy T. Factors Predicting Dropout from the Reasoning and Rehabilitation Program with Mentally Disordered Offenders.. Criminal Justice and Behavior 2011;38(3):217‐30. [DOI: 10.1177/0093854810393659] [DOI] [Google Scholar]
Cusack 2010 {published data only}
- Cusack KJ, Morrissey JP, Cuddeback GS, Prins A, Williams DM. Criminal justice involvement, behavioral health service use, and costs of forensic assertive community treatment: a randomized trial. Community Mental Health Journal 2010;46(4):356‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Amico 2013 {published data only}
- D'Amico EJ, Hunter SB, Miles JN, Ewing BA, Osilla KC. A randomized controlled trial of a group motivational interviewing intervention for adolescents with a first time alcohol or drug offense.. Journal of Substance Abuse Treatment 2013;45(5):400‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dakof 2010 {published data only}
- Dakof GA, Cohen JB, Henderson CE, Duarte E, Boustani M, Blackburn A, et al. A randomized pilot study of the Engaging Moms Program for family drug court. Journal of Substance Abuse Treatment 2010;38(3):263‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dana 2013 {published data only}
- Dana D, Zary N, Peyman A, Behrooz A. Risk prison and hepatitis B virus infection among inmates with history of drug injection in Isfahan, Iran.. Scientific World Journal 2013;735761:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
DeFulio 2013 {published data only}
- DeFulio A, Stitzer M, Roll J, Petry N, Nuzzo P, Schwartz RP, et al. Criminal justice referral and incentives in outpatient substance abuse treatment.. Journal of Substance Abuse Treatment 2013;45(1):70‐5. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dembo 2000 {published data only}
- Dembo R, Ramirez GG, Rollie M, Schmeidler J, Livingston S, Hartsfield A. Youth recidivism twelve months after a family empowerment intervention: final report. Journal of Offender Rehabilitation 2000;31(3/4):29‐65. [Google Scholar]
Deschenes 1994 {published data only}
- Deschenes EP, Greenwood PW. Maricopa‐County Drug Court ‐ an innovative program for 1st‐time drug offenders on probation. Justice System Journal 1994;17(1):99‐115. [Google Scholar]
Diamond 2006 {published data only}
- Diamond G, Panichelli‐Mindel SM, Shrea D, Dennis M, Tims F, Ungemack J. Psychiatric syndromes in adolescents with marijuana abuse and dependency in outpatient treatment. Journal of Child & Adolescent Substance Abuse 2006; Vol. 15, issue 4:37‐54.
Di Nitto 2002 {published data only}
- Nitto DM, Webb DK, Rubin A. The effectiveness of an integrated treatment approach for clients with dual diagnoses. Research on Social Work Practice 2002;12(5):621‐41. [Google Scholar]
Dugan 1998 {published data only}
- Dugan JR, Everett RS. An experimental test of chemical dependency therapy for jail inmates. International Journal of Offender Therapy & Comparative Criminology 1998;42(4):360‐8. [Google Scholar]
Evans 2012 {published data only}
- Evans E, Jaffe A, Urada D, Anglin MD. Differential outcomes of court‐supervised substance abuse treatment among California parolees and probationers.. International Journal of Offender Therapy and Comparative Criminology 2012;56(4):539‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsberg 2011 {published data only}
- Forsberg LG, Ernst D, Sundqvist K, Farbring CA. Motivational Interviewing delivered by existing prison staff: a randomized controlled study of effectiveness on substance use after release. Substance Use and Misuse 2011;46(12):1477‐85. [DOI] [PubMed] [Google Scholar]
Freudenberg 2010 {published data only}
- Freudenberg N, Ramaswamy M, Daniels J, Crum M, Ompad DC, Vlahov D. Reducing drug use, human immunodeficiency virus risk, and recidivism among young men leaving jail: evaluation of the REAL MEN re‐entry program. Journal of Adolescent Health 2010;47(5):448‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2012 {published data only}
- Friedman SR, West BS, Pouget ER, Hall HI, Cantrell J, Tempalski B, et al. Metropolitan Social Environments and Pre‐HAART/HAART Era Changes in Mortality Rates (per 10,000 Adult Residents) among Injection Drug Users Living with AIDS.. PLoS ONE 2013;8(2):12. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frost 2013 {published data only}
- Frost M, Iacobacci B. Utilization of buprenorphine assisted opioid dependence treatment in a county drug court program.. Journal of Addiction Medicine 2013;7(4):E10. [Google Scholar]
Gagnon 2010 {published data only}
- Gagnon H, Godin G, Alary M, Bruneau J, Otis J. A randomized trial to evaluate the efficacy of a computer‐tailored intervention to promote safer injection practices among drug users. AIDS & Behavior 2010;14(3):538‐48. [DOI] [PubMed] [Google Scholar]
Gil 2004 {published data only}
- Gil AG, Wagner EF, Tubman JG. Culturally sensitive substance abuse intervention for Hispanic and African American adolescents: empirical examples from the Alcohol Treatment Targeting Adolescents in Need (ATTAIN) Project. Addiction 2004;99(Suppl 2):140‐50. [0965‐2140] [DOI] [PubMed] [Google Scholar]
Gordon 2012 {published data only}
- Gordon M, Kinlock TW, Couvillion KA, Schwartz RP, O'Grady K. A Randomized Clinical Trial of Methadone Maintenance for Prisoners: Prediction of Treatment Entry and Completion in Prison. Journal of Offender Rehabilitation 2012;51(4):222‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2013 {published data only}
- Gordon MS, Kinlock TW, Couvillion KA, Wilson ME, Schwartz RP, O'Grady KE. Gender Differences Among Prisoners With Pre‐Incarceration Heroin Dependence Participating in a Randomized Clinical Trial of Buprenorphine Treatment. Journal of Offender Rehabilitation 2013;52(5):376‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottfredson 2002 {published data only}
- Gottfredson DC, Exum ML. The Baltimore City drug treatment court: one‐year results from a randomized study. Journal of Research in Crime and Delinquency 2002;39(3):337‐56. [Google Scholar]
Grohman 2002 {published data only}
- Grohman K, Fals‐Stewart W, Bates ME. Cognitive rehabilitation for neuropsychologically impaired substance‐abusing patients: posttreatment outcomes [web page]. http://addictionandfamily.org [2004, 29 Oct] 2002.
Grommon 2013a {published data only}
- Grommon E, Cox SM, Davidson WS, Bynum TS. Alternative models of instant drug testing: Evidence from an experimental trial. Journal of Experimental Criminology 2013;9(2):145‐68. [DOI: ] [Google Scholar]
Grommon 2013b {published data only}
- Grommon E, Davidson WS, Bynum TS. A randomized trial of a multimodal community‐based prisoner reentry program emphasizing substance abuse treatment. Journal of Offender Rehabilitation 2013;52(4):287‐309. [DOI: ] [Google Scholar]
Guydish 2011 {published data only}
- Guydish J, Chan M, Bostrom A, Jessup M, Davis T, Marsh C. A randomized trial of probation case management for drug‐involved women offenders. Crime and Delinquency 2011;57(2):167‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guydish 2014 {published data only}
- Guydish J, Campbell BK, Manuel JK, Delucchi KL, Le T, Peavy KM, et al. Does treatment fidelity predict client outcomes in 12‐Step Facilitation for stimulant abuse?. Drug & Alcohol Dependence 2014;134:330‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haapanen 2002 {published data only}
- Haapanen R, Britton L. Drug testing for youthful offenders on parole: an experimental evaluation. Criminology and Public Policy 2002;1(2):217‐44. [Google Scholar]
Haasen 2010 {published data only}
- Haasen C, Verthein U, Eiroa‐Orosa FJ, Schäfer I, Reimer J. Is heroin‐assisted treatment effective for patients with no previous maintenance treatment? Results from a German randomised controlled trial. European Addiction Research 2010;16(3):124‐30. [DOI] [PubMed] [Google Scholar]
Hanlon 1999 {published data only}
- Hanlon TE, Bateman RW, O'Grady KE. The relative effects of three approaches to the parole supervision of narcotic addicts and cocaine abusers. Prison Journal 1999;79(2):163‐81. [Google Scholar]
Harada 2012 {published data only}
- Harada T. The randomized controlled trial of the prison‐based Japanese Matrix Program (J‐MAT) for methamphetamine abusers. Japanese Journal of Alcohol Studies and Drug Dependence 2012;47(6):298‐307. [PubMed] [Google Scholar]
Harrell 2001 {published data only}
- Harrell A, Roman J. Reducing drug use and crime among offenders: the impact of graduated sanctions. Journal of Drug Issues 2001;31(1):207‐32. [Google Scholar]
Henderson 2010 {published data only}
- Henderson CE, Dakof GA, Greenbaum PE, Liddle HA. Effectiveness of multidimensional family therapy with higher severity substance‐abusing adolescents: report from two randomized controlled trials. Journal of Consulting in Clinical Psychology 2010;78(6):885‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henggeler 1991 {published data only}
- Henggeler SW, Borduin CM, Melton GB, Mann BJ. Effects of multisystemic therapy on drug use and abuse in serious juvenile offenders: a progress report from two outcome studies. Family Dynamics of Addiction Quarterly 1991;1(3):40‐51. [Google Scholar]
Henggeler 1999 {published data only}
- Henggeler SW, Pickrel SG, Brondino MJ. Multisystemic treatment of substance‐abusing and dependent delinquents: outcomes, treatment fidelity, and transportability. Mental Health Services Research 1999;1(3):171‐84. [DOI] [PubMed] [Google Scholar]
Henggeler 2002 {published data only}
- Henggeler SW, Clingempeel WG, Brondino MJ, Pickrel SG. Four‐year follow‐up of multisystemic therapy with substance‐abusing and substance‐dependent juvenile offenders. Journal of the American Academy of Child & Adolescent Psychiatry 41;7:868‐74. [DOI] [PubMed] [Google Scholar]
Henggeler 2006 {published data only}
- Henggeler SW, Halliday‐Boykins CA, Cunningham PB, Randall J, Shapiro SB, Chapman JE. Juvenile drug court: enhancing outcomes by integrating evidence‐based treatments. Journal of Consulting in Clinical Psychology 2006;74(1):42‐54. [0022‐006X: (Print)] [DOI] [PubMed] [Google Scholar]
Henggeler 2012 {published data only}
- Henggeler SW, McCart MR, Cunningham PB, Chapman JE. Enhancing the effectiveness of juvenile drug courts by integrating evidence‐based practices. Journal of Consulting & Clinical Psychology 2012;80(2):264‐75. [DOI: 10.1037/a0027147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hser 2011 {published data only}
- Hser Y‐I, Li J, Jiang H, Zhang R, Du J, Zhang C, et al. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction 2011;106(10):1801‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hser 2013 {published data only}
- Hser Y‐I, Fu L, Wu F, Du J, Zhao M. Pilot trial of a recovery management intervention for heroin addicts released from compulsory rehabilitation in China. Journal of Substance Abuse Treatment 2013;44(1):78‐83. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inciardi 2004 {published data only}
- Inciardi JA, Martin SS, Butzin CA. Five‐year outcomes of therapeutic community treatment of drug‐involved offenders after release from prison. Crime & Delinquency 2004;50(1):88‐107. [0011‐1287] [Google Scholar]
Jain 2011 {published data only}
- Jain K, Jain R, Dhawan A. A double‐blind, double‐dummy, randomized controlled study of memantine versus buprenorphine in naloxone‐precipitated acute withdrawal in heroin addicts. J Opioid Manag 2011;7(1):11‐20. [DOI] [PubMed] [Google Scholar]
Johnson 2011 {published data only}
- Johnson JE, Friedmann PD, Green TC, Harrington M, Taxman FS. Gender and treatment response in substance use treatment‐mandated parolees. Journal of Substance Abuse Treatment 2011;40(3):313‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2012 {published data only}
- Johnson JE, Zlotnick C. Pilot study of treatment for major depression among women prisoners with substance use disorder. Journal of Psychiatric Research 2012;46(9):1174‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones, 2011 {published data only}
- Jones RK. Evaluation of the DUI court program in Maricopa County, Arizona.. Washington, DC: U.S. Department of Transportation. 2011; Vol. Report.
Jones 2013 {published data only}
- Jones CG. Early‐phase outcomes from a randomized trial of intensive judicial supervision in an Australian Drug Court. Criminal Justice and Behavior 2013;40(4):453‐68. [DOI: ] [Google Scholar]
Katz 2007 {published data only}
- Katz EC, Brown BS, Schwartz RP, King SD, Weintraub E, Barksdale W. Impact of role induction on long‐term drug treatment outcomes. Journal of Addictive Diseases 2007;26(2):81‐90. [CN‐00590052] [DOI] [PubMed] [Google Scholar]
Kelly 2013 {published data only}
- Kelly SM, O'Grady KE, Jaffe JH, Gandhi D, Schwartz RP. Improvements in outcomes in methadone patients on probation/parole regardless of counseling early in treatment. Journal of Addiction Medicine 2013;7(2):133‐8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kidorf 2013 {published data only}
- Kidorf M, Brooner RK, Gandotra N, Antoine D, King VL, Peirce J, et al. Reinforcing integrated psychiatric service attendance in an opioid‐agonist program: a randomized and controlled trial. Drug and Alcohol Dependence 2013;133(1):30‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2014 {published data only}
- King VL, Brooner RK, Peirce JM, Kolodner K, Kidorf MS. A randomized trial of Web‐based videoconferencing for substance abuse counseling.. Journal of Substance Abuse Treatment 2014;46(1):36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinlock 2008 {published data only}
- Kinlock TW, Gordon MS, Schwartz RP, O'Grady KE. A study of methadone maintenance for male prisoners: 3‐month postrelease outcomes. Criminal Justice and Behavior 2008; Vol. 35, issue 1:34‐47. [0093‐8548: (Print)] [DOI] [PMC free article] [PubMed]
Kinlock 2009a {published data only}
- Kinlock T, Gordon M, Schwartz R. Buprenorphine for prisoners: preliminary findings at one‐month post release. Conference Papers ‐‐ American Society of Criminology. 2009:1.
Kinlock 2009b {published data only}
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: Results at 12 months postrelease. Journal of Substance Abuse Treatment 2009; Vol. 37, issue 3:277‐85. [0740‐5472] [DOI] [PMC free article] [PubMed]
Kok 2013 {published data only}
- Kok T, Haan HA, Meer M, Najavits LM, DeJong CAJ. Efficacy of "seeking safety" in a Dutch population of traumatized substance‐use disorder outpatients: study protocol of a randomized controlled trial. BMC Psychiatry 2013;13(8):162‐70. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Law 2012 {published data only}
- Law FM, Guo GJ. Hope and recovery from substance abuse for female drug offenders in Taiwan. International journal of offender therapy and comparative criminology 2012;56(8):1258‐82. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee JD, Grossman E, Truncali A, Rotrosen J, Rosenblum A, Magura S, et al. Buprenorphine‐naloxone maintenance following release from jail. Subst Abus 2012;33(1):40‐7. [DOI: 10.1080/08897077.2011.620475] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liddle 2011 {published data only}
- Liddle HA, Dakof GA, Henderson C, Rowe C. Implementation outcomes of multidimensional family therapy‐detention to community: a reintegration program for drug‐using juvenile detainees. International Journal of Offender Therapy and Comparative Criminology 2011;55(4):587‐604. [DOI] [PubMed] [Google Scholar]
Ling 2013 {published data only}
- Ling Murtaugh K, Krishnamurti T, Davis AL, Reback CJ, Shoptaw S. Spend today, clean tomorrow: predicting methamphetamine abstinence in a randomized controlled trial. Health Psychology 2013;32(9):958‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lobmann 2009 {published data only}
- Lobmann R, Verthein U. Explaining the effectiveness of heroin‐assisted treatment on crime reductions. Law and Human Behavior 2009;33(1):83‐95. [DOI: 10.1007/s10979-008-9138-8] [DOI] [PubMed] [Google Scholar]
MacDonald 2007 {published data only}
- MacDonald JM, Morral AR, Raymond B, Eibner C. The efficacy of the Rio Hondo DUI court: A 2‐year field experiment. Evaluation Review 2007;31(4):4‐23. [DOI] [PubMed] [Google Scholar]
Marlowe 2003 {published data only}
- Marlowe DB, Festinger DS, Lee PA, Schepise MM, Hazzard JER, Merrill JC, et al. Are judicial status hearings a key component of drug court? During treatment data from a randomized trial. Criminal Justice and Behavior 2008;30(2):141‐62. [Google Scholar]
Marlowe 2005 {published data only}
- Marlowe DB, Festinger DS, Dugosh KL, Lee PA. Are judicial status hearings a "key component" of drug court? Six and twelve month outcomes. Drug and Alcohol Dependence 2005;79(2):145‐55. [DOI] [PubMed] [Google Scholar]
Marlowe 2007 {published data only}
- Marlowe DB, Festinger DS, Dugosh KL, Lee PA, Benasutti KM. Adapting judicial supervision to the risk level of drug offenders: discharge and 6‐month outcomes from a prospective matching study. Drug and Alcohol Dependence 2007;88(Suppl 2):S4‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marlowe 2008 {published data only}
- Marlowe DB, Festinger DS, Dugosh KL, Arabia PL, Kirby KC. An effectiveness trial of contingency management in a felony preadjudication drug court. Journal of Applied Behavior Analysis 2008;41(4):565‐77. [0021‐8855: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsch 2014 {published data only}
- Marsch LA, Guarino H, Acosta M, Aponte‐Melendez Y, Cleland C, Grabinski M, et al. Web‐based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. Journal of Substance Abuse Treatment 2014;46(1):43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1993 {published data only}
- Martin SS, Scarpitti SR. An intensive case management approach for paroled IV drug users. Journal of Drug Issues 1993;23(1):43‐59. [Google Scholar]
Mbilinyi 2011 {published data only}
- Mbilinyi LF, Neighbors C, Walker DD, Roffman RA, Zegree J, Edleson J, et al. A telephone intervention for substance‐using adult male perpetrators of intimate partner violence. Research on Social Work Practice 2011;21(1):43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKendrick 2007 {published data only}
- McKendrick K, Sullivan C, Banks S, Sacks S. Modified therapeutic community treatment for offenders with MICA disorders: antisocial personality disorder and treatment outcomes. Journal of Offender Rehabilitation 2006; Vol. 44, issue 2‐3:133‐59. [1050‐9674]
McKenzie 2012 {published data only}
- McKenzie M, Zaller N, Dickman SL, Green TC, Parihk A, Friedmann PD, et al. A randomized trial of methadone initiation prior to release from incarceration. Substance Abuse 2012;33(1):19‐29. [DOI: 10.1080/08897077.2011.609446] [DOI] [PMC free article] [PubMed] [Google Scholar]
Messina 2000 {published data only}
- Messina N, Wish E, Nemes S. Predictors of treatment outcomes in men and women admitted to a therapeutic community. American Journal of Drug & Alcohol Abuse 2000;26(2):207‐27. [DOI] [PubMed] [Google Scholar]
Messina 2010 {published data only}
- Messina N, Grella CE, Cartier J, Torres S. A randomized experimental study of gender‐responsive substance abuse treatment for women in prison.. Journal of Substance Abuse Treatment 2010;38(2):97‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milloy 2011 {published data only}
- Milloy MJS, Kerr T, Zhang R, Tyndall M, Montaner J. Randomised Trial of the Effectiveness of Naloxone. London: Department of Health, 2011. [Google Scholar]
Needels 2005 {published data only}
- Needels K, James‐Burdumy S, Burghardt J. Community case management for former jail inmates: its impacts on rearrest, drug use, and HIV risk. Journal of Urban Health 2005;82(3):420‐33. [1099‐3460: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemes 1998 {published data only}
- Nemes S, Wish E, Messina N. The District of Columbia Treatment Initiative (DCI) final report. College Park, MD: University of Maryland, National Evaluation Data and Technical Assistance Center (NEDTAC), 1998. [Google Scholar]
Nemes 1999 {published data only}
- Nemes S, Wish ED, Messina N. Comparing the impact of standard and abbreviated treatment in a therapeutic community: findings from the District of Columbia treatment initiative experiment. Journal of Substance Abuse Treatment 1999;17(4):339‐47. [DOI] [PubMed] [Google Scholar]
Nielsen 1996 {published data only}
- Farrell A. Women, crime and drugs: testing the effect of therapeutic communities. Women and Criminal Justice 2000;11(1):21‐48. [Google Scholar]
- Nielsen AL, Scarpitti FR, Inciardi JA. Integrating the therapeutic community and work release for drug‐involved offenders: the CREST program. Journal of Substance Abuse Treatment 1996;13(4):349‐58. [DOI] [PubMed] [Google Scholar]
Nosyk 2010 {published data only}
- Nosyk B, Geller J, Guh DP, Oviedo‐Joekes E, Brissette S, Marsh DC, et al. The effect of motivational status on treatment outcome in the North American Opiate Medication Initiative (NAOMI) study. Drug and Alcohol Dependence 2010;111(1‐2):161‐5. [DOI] [PubMed] [Google Scholar]
Petersilia 1992 {published data only}
- Petersilia J, Turner S, Deschenes EP. Intensive supervision programs for drug offenders. In: Byrne JM, Lurigio AJ editor(s). Smart Sentencing: The Emergence of Intermediate Sanctions. Thousand Oaks, CA: Sage Publications Inc, 1992:18‐37. [Google Scholar]
Petry 2005 {published data only}
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize‐based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry 2005;62(10):1148‐56. [DOI: 10.1001/archpsyc.62.10.1148] [DOI] [PubMed] [Google Scholar]
Petry 2011 {published data only}
- Petry NM, Ford J D, Barry D. Contingency management is especially efficacious in engendering long durations of abstinence in patients with sexual abuse histories. Psychology of Addictive Behaviors 2011;25(2):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polsky 2010 {published data only}
- Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE. Cost‐effectiveness of extended buprenorphine‐naloxone treatment for opioid‐dependent youth: data from a randomized trial. Addiction 2010;105(9):1616‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prendergast 2003 {published data only}
- Prendergast ML, Hall EA, Wexler HK. Multiple measures of outcome in assessing a prison‐based drug treatment program. Journal of Offender Rehabilitation 2003;37:65‐94. [Google Scholar]
Prendergast 2008 {published data only}
- Prendergast ML, Hall EA, Roll J, Warda U. Use of vouchers to reinforce abstinence and positive behaviors among clients in a drug court treatment program. Journal of Substance Abuse Treatment 2008;35(2):125‐36. [1873‐6483: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prendergast 2009 {published data only}
- Prendergast M, Greenwell L, Cartier J, Sacks J, Frisman L, Rodis E, et al. Adherence to scheduled sessions in a randomized field trial of case management: the Criminal Justice‐Drug Abuse Treatment Studies Transitional Case Management Study. Journal of Experimental Criminology 2009;5(3):273‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prendergast 2011 {published data only}
- Prendergast M, Frisman L, Sacks JY, Staton‐Tindall M, Greenwell L, Lin HJ, et al. A multi‐site, randomized study of strengths‐based case management with substance‐abusing parolees. Journal of Experimental Criminology 2011;7(3):225‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2012 {published data only}
- Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance‐dependent jail inmates. International Journal of Offender Therapy and Comparative Criminology 2012;56(2):317‐32. [DOI] [PubMed] [Google Scholar]
Reimer 2011 {published data only}
- Reimer J, Verthein U, Karow A, Schäfer I, Naber D, Haasen C. Physical and mental health in severe opioid‐dependent patients within a randomized controlled maintenance treatment trial. Addiction 2011;106(9):1647‐55. [DOI] [PubMed] [Google Scholar]
Robertson 2006 {published data only}
- Robertson JR, Raab GM, Bruce M, McKenzie JS, Storkey HR, Salter A. Addressing the efficacy of dihydrocodeine versus methadone as an alternative maintenance treatment for opiate dependence: a randomized controlled trial. Addiction 2006;101(12):1752‐9. [CN‐00577209] [DOI] [PubMed] [Google Scholar]
Rosengard 2008 {published data only}
- Rosengard C, Stein LAR, Barnett NP, Monti PM, Golembeske C, Lebeau‐Craven R, et al. Randomized clinical trial of motivational enhancement of substance use treatment among incarcerated adolescents. Journal of HIV/AIDS Prevention in Children and Youth 2008;8(2):45‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rossman 1999 {published data only}
- Rossman S, Sridharan S, Gouvis C, Buck J, Morley E. Impact of the Opportunity to Succeed (OPTS) Aftercare Program for Substance‐Abusing Felons: comprehensive Final Report. Washington DC: Urban Institute, 1999. [Google Scholar]
Rounsaville 2001 {published data only}
- Rounsaville BJ, Carroll KM, Onken LS. A Stage Model of Behavioral Therapies research: Getting started and moving on from stage I. Clinical Psychology‐Science and Practice 2001;8(2):133‐42. [DOI: 10.1093/clipsy/8.2.133] [DOI] [Google Scholar]
Rowan‐Szal 2005 {published data only}
- Rowan‐Szal GA, Bartholomew NG, Chatham LR, Simpson DD. A combined cognitive and behavioral intervention for cocaine‐using methadone clients. Journal of Psychoactive Drugs 2005;37(1):75‐84. [DOI] [PubMed] [Google Scholar]
Rowan‐Szal 2009 {published data only}
- Rowan‐Szal GA, Joe GW, Simpson D, Greener JM, Vance J. During‐treatment outcomes among female methamphetamine‐using offenders in prison‐based treatments. Journal of Offender Rehabilitation 2009;48(5):388‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowe 2007 {published data only}
- Rowe M, Bellamy C, Baranoski M, Wieland M, Connell MJO, Benedict P, et al. A peer‐support, group intervention to reduce substance use and criminality among persons with severe mental illness. Psychiatric Services 2007;58(7):955‐61. [1075‐2730] [DOI] [PubMed] [Google Scholar]
Sacks 2004 {published data only}
- Sacks S, Sacks JY, McKendrick K, Banks S, Stommel J. Modified TC for MICA inmates in correctional settings: crime outcomes. Behavioural Sciences and the Law 2004;22(4):477‐501. [DOI] [PubMed] [Google Scholar]
Sacks 2008 {published data only}
- Sacks JY, Sacks S, McKendrick K, Banks S, Schoeneberger M, Hamilton Z, et al. Prison therapeutic community treatment for female offenders: profiles and preliminary findings for mental health and other variables (crime, substance use and HIV risk). Journal of Offender Rehabilitation 2008;46(3‐4):233‐61. [1050‐9674] [Google Scholar]
Sacks 2011 {published data only}
- Sacks S, Chaple M, Sacks JY, McKendrick K, Cleland CM. Randomized trial of a reentry modified therapeutic community for offenders with co‐occurring disorders: crime outcomes. Journal of Substance Abuse Treatment 2012;42(3):247‐59. [DOI] [PubMed] [Google Scholar]
Sanchez‐Hervas 2010 {published data only}
- Sanchez‐Hervas E, Secades‐Villa R, Romaguera FZ, Fernandez GG, Gomez FJS, Garcia‐Rodriguez O. Behavioral therapy for cocaine addicts: outcomes of a follow‐up six month study. Revista Mexicana De Psicologia 2010;27(2):159‐67. [Google Scholar]
Schaeffer 2014 {published data only}
- Schaeffer CM, Henggeler SW, Ford JD, Mann M, Chang R, Chapman JE. RCT of a promising vocational/employment program for high‐risk juvenile offenders. Journal of Substance Abuse Treatment 2014;46(2):134‐43. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmiege 2009 {published data only}
- Schmiege SJ, Broaddus MR, Levin M, Bryan AD. Randomized Trial of Group Interventions to Reduce HIV/STD Risk and Change Theoretical Mediators Among Detained Adolescents. Journal of Consulting and Clinical Psychology 2009;77(1):38‐50. [DOI: 10.1037/A0014513] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2006 {published data only}
- Schwartz RP, Highfield DA, Jaffe JH, Brady JV, Butler CB, Rouse CO, et al. A randomized controlled trial of interim methadone maintenance. Archives of General Psychiatry 2006;63(1):102‐9. [DOI] [PubMed] [Google Scholar]
Shanahan 2004 {published data only}
- Shanahan M, Lancsar E, Haas M, Lind B, Weatherburn D, Chen S. Cost‐effectiveness analysis of the New South Wales adult drug court program. Evaluation Review 2004;28(1):3‐27. [DOI] [PubMed] [Google Scholar]
Sheard 2009 {published data only}
- Sheard L, Wright NM, El‐Sayeh CE, Adams C, Li R, Tompkins CN. The Leeds evaluation of efficacy of detoxification study (LEEDS) prisons project: a randomised controlled trial comparing dihydrocodeine and buprenorphine for opiate detoxification. Substance Abuse Treatment Prevention and Policy 2009;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegal 1999 {published data only}
- Siegal HA, Jichuan W, Carlson RG, Falck RS, Rahman AM, Fine RL. Ohio's prison‐based therapeutic community treatment programs for substance abusers: preliminary analysis of re‐arrest data. Journal of Offender Rehabilitation 1999;28(3/4):33‐48. [Google Scholar]
Sinha 2003 {published data only}
- Sinha R, Easton C, Renee‐Aubin L, Carroll KM. Engaging young probation‐referred marijuana‐abusing individuals in treatment: a pilot trial. American Journal on Addictions 2003;12(4):314‐23. [PubMed] [Google Scholar]
Smith 2010 {published data only}
- Smith DK, Chamberlain P, Eddy JM. Preliminary support for multidimensional treatment foster care in reducing substance use in delinquent boys. Journal of Child & Adolescent Substance Abuse 2010;19:343‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 1995 {published data only}
- Solomon P, Draine J. One‐Year Outcomes of a Randomized Trial of Case‐Management with Seriously Mentally‐Ill Clients Leaving Jail.. Evaluation Review 1995;19(3):256‐73. [DOI: 10.1177/0193841x9501900302] [DOI] [Google Scholar]
Specka 2013 {published data only}
- Specka M, Boning A, Kluwig J, Schifano F, Banger M, Lange W, et al. Can reinforcement‐based interventions to reduce drug use successfully be adapted to routine opioid maintenance treatment?. Annali dell Istituto Superiore di Sanita 2013;49(4):358‐64. [DOI] [PubMed] [Google Scholar]
Stanger 2009 {published data only}
- Stanger C, Budney AJ, Kamon JL, Thostensen J. A randomized trial of contingency management for adolescent marijuana abuse and dependence. Drug and Alcohol Dependence 2009;105(3):240‐7. [0376‐8716] [DOI] [PMC free article] [PubMed] [Google Scholar]
Staton‐Tindall 2009 {published data only}
- Staton‐Tindall M, McNees E, Leukefeld CG, Walker R, Thompson L, Pangburn K, et al. Systematic outcomes research for corrections‐based treatment: implications from the criminal justice Kentucky treatment outcome study. Journal of Offender Rehabilitation 2009;48(8):710‐24. [Google Scholar]
Stein 2006 {published data only}
- Stein LA, Monti PM, Colby SM, Barnett NP, Golembeske C, Lebeau‐Craven R, et al. Enhancing Substance Abuse Treatment Engagement in Incarcerated Adolescents. Psychol Serv 2006;3(1):25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2010 {published data only}
- Stein MD, Herman DS, Kettavong M, Cioe PA, Friedmann PD, Tellioglu T, et al. Antidepressant treatment does not improve buprenorphine retention among opioid‐dependent persons. Journal of Substance Abuse Treatment 2010;39(2):157‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2011 {published data only}
- Stein LA, Clair M, Lebeau R, Colby SM, Barnett NP, Golembeske CM, et al. Motivational interviewing to reduce substance‐related consequences: effects for incarcerated adolescents with depressed mood. Drug and Alcohol Dependence 2011;118(2‐3):475‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 1998 {published data only}
- Stevens SJ, Patton T. Residential treatment for drug addicted women and their children: Effective treatment strategies. Drugs & Society 1998;13(1‐2):235‐49. [Google Scholar]
Svikis 2011 {published data only}
- Svikis DS, Keyser‐Marcus L, Stitzer M, Rieckmann T, Safford L, Loeb P, et al. Randomized multi‐site trial of the Job Seekers' Workshop in patients with substance use disorders. Drug and Alcohol Dependence 2012;1(1,20):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taxman 2006 {published data only}
- Taxman FS, Meridith T. Risk, need, and responsivity (RNR): it all depends. Crime & Delinquency 2006;52(1):28‐51. [0095‐2990: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vagenas 2014 {published data only}
- Vagenas P, Paola A, Herme M, Lincoln T, Skiest DJ, Altice FL, et al. An evaluation of hepatic enzyme elevations among HIV‐infected released prisoners enrolled in two randomized placebo‐controlled trials of extended release naltrexone. Journal of Substance Abuse Treatment 2014;47(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderberg 2002 {published data only}
- Vanderberg SA. Motivational interviewing as a precursor to a substance abuse program for offenders. Doctoral thesis, Department of Psychology, Carlton University, Ottawa, Ontario 2002.
Villagrá Lanza 2013 {published data only}
- Villagrá Lanza P, González Menéndez A. Acceptance and Commitment Therapy for drug abuse in incarcerated women. Psicothema 2013;25(3):307‐12. [DOI] [PubMed] [Google Scholar]
Walters 2014 {published data only}
- Walters ST, Ondersma SJ, Ingersoll KS, Rodriguez M, Lerch J, Rossheim ME, et al. MAPIT: development of a web‐based intervention targeting substance abuse treatment in the criminal justice system. Journal of Substance Abuse Treatment 2014;46(1):60‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang EA, Moore BA, Sullivan LE, Fiellin DA. Effect of incarceration history on outcomes of primary care office‐based buprenorphine/naloxone. Journal of General Internal Medicine 2010;25(7):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Webster 2014 {published data only}
- Webster JM, Staton‐Tindall M, Dickson MF, Wilson JF, Leukefeld CG. Twelve‐month employment intervention outcomes for drug‐involved offenders.. American Journal of Drug and Alcohol Abuse 2014;40(3):200‐5. [DOI: 10.3109/00952990.2013.858722] [DOI] [PubMed] [Google Scholar]
White 2006 {published data only}
- White MD, Goldkamp JS, Robinson JB. Acupuncture in drug treatment: exploring its role and impact on participant behavior in the drug court setting. Journal of Experimental Criminology 2006;2(1):45‐65. [1573‐3750] [Google Scholar]
Williams 2011 {published data only}
- Williams K, Martin M, Martin D. Examining a drug court treatment program in New Jersey: a perspective from the field. Alcoholism Treatment Quarterly 2011;29(1):85‐90. [Google Scholar]
Winstanley 2011 {published data only}
- Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone‐maintained patients. Journal of Substance Abuse Treatment 2011;40(3):255‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witkiewitz 2010 {published data only}
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness‐based relapse prevention. Journal of Consulting in Clinical Psychology 2010;78(3):362‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2012 {published data only}
- Wolff N, Frueh BC, Shi J, Schumann BE. Effectiveness of cognitive‐behavioral trauma treatment for incarcerated women with mental illnesses and substance abuse disorders. Journal of Anxiety Disorders 2012;26(7):703‐10. [DOI: 10.1016/j.janxdis.2012.06.001] [DOI] [PubMed] [Google Scholar]
Zlotnick 2009 {published data only}
- Zlotnick C, Johnson J, Najavits LM. Randomized controlled pilot study of cognitive‐behavioral therapy in a sample of incarcerated women with substance use disorder and PTSD. Behavior Therapy 2009;40(4):325‐36. [0005‐7894] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Springer 2015 {unpublished data only}
- Springer SA. Naltrexone for opioid dependent released HIV+ Criminal Justice Populations.. (ongoing‐2015) to be confirmed.
Additional references
Amato 2005
- Amato L, Davoli M, Perucci CA, Ferri M, Faggiano F, Mattick RP. An overview of systematic reviews of effectiveness of opiate maintenance therapies: available evidence to information clinical practice and research. Journal of Substance Abuse Treatment 2005;28:321‐9. [DOI] [PubMed] [Google Scholar]
Amato 2013
- Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD003409.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Binswanger 2007
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison-a high risk of death for former inmates. New England Journal of Medicine 2007;356:157‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bird 2003
- Bird SM, Hutchinson SJ. Male drugs related deaths in the fortnight after release from prison: Scotland 1996‐99. Addiction 2003;98:185‐90. [DOI] [PubMed] [Google Scholar]
Brooke 1996
- Brooke D, Taylor C, Gunn J, Maden A. Point prevalence of mental disorder in unconvicted male prisoners in England and Wales. British Medical Journal 1996;313:1524‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catania 2003
- Catania H. Prison health needed in prisons. Harm reduction news: Newsletter of the International Harm Reduction Development Program of the Open Society Institute. Open Society Institute 2003; Vol. 4, issue 11:13.
Chanhatasilpa 2000
- Chanhatasilpa C, Mackenzie DL, Hickman LJ. The effectiveness of community‐based programs for chemically dependent offenders: a review and assessment of the research. Journal of Substance Abuse Treatment 2000;19:383‐93. [DOI] [PubMed] [Google Scholar]
Drummond 1997
- Drummond M, O'Brien B, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. 2nd Edition. Oxford, UK: Oxford University Press, 1997. [Google Scholar]
Egg 2000
- Egg R, Pearson FS, Cleland CM, Lipton DS. Evaluations of correctional treatment programs in Germany: a review and meta‐analysis. Substance Use and Misuse 2000;35(12‐14):1967‐2009. [DOI] [PubMed] [Google Scholar]
Faggiano 2003
- Faggiano F, Vigna‐Taglianti F, Versino E, Lemma P. Methadone maintenance at different dosages for opioid dependence. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002208] [DOI] [PubMed] [Google Scholar]
Fareed 2012
- Fareed A, Vayalapalli S, Casarella J, Drexler K. Effect of buprenorphine dose on treatment outcome. Journal of Addiction Disorders 2012;31(1):8‐18. [DOI] [PubMed] [Google Scholar]
Fiscella 2004
- Fiscella K, Moore A, Engerman J, Meldrum S. Jail management of arrestees/inmates enrolled in community methadone maintenance programs. Journal of Urban Health: Bulletin of the New York Academy of Medicine. 2004;81:645‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2007
- Garcia CA, Correa GC, Viver AD, Hernandez BS, Kinlock TW, Gordon MS, et al. Buprenorphine‐naloxone treatment for pre‐release opiod dependent inmates in Puerto Rico. Journal of Addiction Medicine 2007;1:126‐32. [DOI] [PubMed] [Google Scholar]
Gibson 2007
- Gibson A, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug and Alcohol Review 2007;26:405‐10. [DOI] [PubMed] [Google Scholar]
Gowing 2009
- Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002025.pub4] [DOI] [Google Scholar]
Gunn 1991
- Gunn J, Maden A, Swinton M. Treatment needs of prisoners with psychiatric disorders. British Medical Journal 1991;303:338‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedrich 2011
- Hedrich D, Alves P, Farrell M, Stover H, Moller L, Mayet S. The effectiveness of opioid maintenance treatment in prison settings: a systematic review. Addiction 2011;107:501‐17. [DOI] [PubMed] [Google Scholar]
Hergert 2005
- Hergert G. Methadone and buprenorphine added to the WHO list of essential medicines. HIV, AIDS Policy Law Review 2005;10:23‐4. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lo 2000
- Lo CC, Stephens RC. Drugs and prisoners: treatment needs on entering prison. American Journal of Drug and Alcohol Abuse 2000;26:229‐45. [DOI] [PubMed] [Google Scholar]
Lobmaier 2008
- Lobmaier P, Kornor H, Kunoe N, Bjorndal A. Sustained‐release naltrexone for opioid dependence. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006140.pub2] [DOI] [PubMed] [Google Scholar]
Magura 1995
- Magura S, Kang SY, Shapiro JL. Measuring cocaine use by hair analysis among criminally involved youth. Journal of Drug Issues 1995;25:683‐701. [Google Scholar]
Marsch 1998
- Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behaviours and criminality: a meta‐analysis. Addiction 1998;93(4):515‐32. [DOI] [PubMed] [Google Scholar]
Mason 1997
- Mason D, Birmingham L, Grubin D. Substance use in remand prisoners: a consecutive case study. British Medical Journal 1997;315:18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattick 2009
- Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michel 2005
- Michel L, Maguet O. Guidelines for substitution treatments in prison populations. Encephale 2005;31(1):92‐7. [DOI] [PubMed] [Google Scholar]
Minozzi 2011
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD001333.pub3] [DOI] [PubMed] [Google Scholar]
Mitchell 2006
- Mitchell O, Wilson D, MacKenzie D. The effectiveness of incarceration‐based drug treatment on criminal behaviour. Campbell Systematic Reviews 2006, issue 11. [DOI: 10.4073/csr.2006.11] [DOI]
Mitchell, 2012a
- Mitchell O, Mackenzie LD, Wilson D. The effectiveness of incarcerated based drug treatment on criminal behaviour: A systematic review. Campbell Systematic Reviews 2012; Vol. 18. [DOI: 10.4073/csr.2012.18] [DOI]
Mitchell, 2012b
- Mitchell O, Wilson D, Eggers A, Mackenzie LD. Drug Courts effects on criminal offending for juveniles and adults: A systematic review. Campbell Systematic Reviews 2012; Vol. 4. [DOI: 10.4073/csr.2012.4] [DOI]
Moller 2007
- Moller L, Gathere A, Juergens R, Stover H, Nikogosian H. Health in Prisons. A WHO Guide to the Essentials in Prison Health. Copenhagen: World Health Organization Regional Office for Europe, 2007.
NICE 2007a
- National Institute for Health and Clinical Excellence. NICE technology appraisal guidance 114 Methadone and buprenorphine for the management of opioid dependence. www.nice.org.uk/TA114. Accessed 20 July 2013. 2007. [ISBN 1‐84629‐338‐3]
NICE 2007b
- National Institute for Health and Clinical Excellence. NICE technology appraisal guidance 115 Naltrexone for the management of opioid dependence. www.nice.org.uk/TA115. Accessed 20 July 2013. 2007.
Perry 2006
- Perry A, Coulton S, Glanville J, Godfrey C, Lunn J, McDougall C, et al. Interventions for drug‐using offenders in the courts, secure establishments and the community. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005193.pub2] [DOI] [PubMed] [Google Scholar]
Perry 2013a
Perry 2013b
Perry 2013c
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reynaud‐Maurupt 2005
- Reynaud‐Maurupt C, Caer Y, Escaffre N, Gagneau M, Galinier A, Marzo NJ, et al. High‐dose buprenorphine substitution during incarceration. La Presse Médicale 2005;34:487‐90. [PubMed] [Google Scholar]
Stallwitz 2007
- Stallwitz A, Stover H. The impact of substitution treatment in prisons-a literature review. International Journal Drug Policy 2007;18:464‐74. [DOI] [PubMed] [Google Scholar]


