Abstract
People are exposed to high concentrations of antibacterial agent cetylpyridinium chloride (CPC) via food and personal care products, despite little published information regarding CPC effects on eukaryotes. Here, we show that low-micromolar CPC exposure, which does not cause cell death, inhibits mitochondrial ATP production in primary human keratinocytes, mouse NIH-3T3 fibroblasts, and rat RBL-2H3 immune mast cells. ATP inhibition via CPC (EC50 1.7 μM) is nearly as potent as that caused by canonical mitotoxicant CCCP (EC50 1.2 μM). CPC inhibition of oxygen consumption rate (OCR) tracks with that of ATP: OCR is halved due to 1.75 μM CPC in RBL-2H3 cells and 1.25 μM in primary human keratinocytes. Mitochondrial [Ca2+] changes can cause mitochondrial dysfunction. Here we show that CPC causes mitochondrial Ca2+ efflux from mast cells via an ATP-inhibition mechanism. Using super-resolution microscopy (fluorescence photoactivation localization) in live cells, we have discovered that CPC causes mitochondrial nanostructural defects in live cells within 60 min, including the formation of spherical structures with donut-like cross section. This work reveals CPC as a mitotoxicant despite widespread use, highlighting the importance of further research into its toxicological safety.
Keywords: cetylpyridinium chloride, antibacterial agent, mitochondria, super-resolution microscopy, keratinocyte, mast cell, mitochondrial calcium, ATP, oxygen consumption rate, fluorescence photoactivation localization microscopy (FPALM), mitotoxicity, quaternary ammonium compound
Graphical abstract

1. Introduction
The health impacts of chemical contaminants in food and personal care products are of critical importance. Cetylpyridinium chloride (CPC) is a quaternary ammonium compound (QAC) and antimicrobial used in personal care products at a concentration of ~1,500 μM - 3,000 μM (Rawlinson et al., 2008) since the 1930s (ACS, 2021). CPC is also used directly on human food in agricultural processing (HHS, 2004) and in cleaning products. For example, CPC is used on meat (İlhak et al., 2018) and vegetables (Wang et al., 2001). Thus, human and environmental exposure must occur; however, little is known about human CPC body burden.
CPC is likely absorbed (Voutchkova et al., 2010) and bioavailable (Klaassen and Casarett, 2019; Van Leeuwen et al., 2015). However, to our knowledge, no publications to date have directly measured CPC levels in humans. A report of the European Union Scientific Committee on Consumer Safety provided an estimate that consumers may daily receive 0.114 mg CPC per kg of body weight (Bernauer et al., 2015) although their estimation relied on the assumption that some CPC exposures are only 10% bioavailable. If CPC is highly absorbable and bioavailable as noted in the publications above, this burden would be closer to 1 mg CPC per kg of body weight. Extrapolation of this 0.114 mg/kg value, taking into account a mouse pharmacokinetics study of CPC (one-time oral administration of 1 mg/kg CPC led to a peak ~0.3 μM CPC in blood) (Pottel et al., 2020) would suggest human CPC blood levels of ~0.03 μM due to personal care products—or ~0.3 μM with high CPC bioavailability. Related QAC (CPC was not measured in the study) have been found in randomly-sampled human blood at levels up to 0.15 μM (Hrubec et al., 2021), and CPC remains in human saliva (following typical mouthwash usage) at high-micromolar concentrations for hours and at 1 μM 24 h following expectoration (Bonesvoll and Gjermo, 1978). CPC may be more prevalent than other QACs, in products and in the human body, due to its popular use during the SARS-CoV-2 pandemic (Alemany et al., 2022; Sánchez Barrueco et al., 2022) and its use in food processing. Consumption of CPC-treated chicken may lead to blood CPC concentration of roughly 0.3 μM for hours after each meal (Obeng et al., 2023), in addition to other exposures. In this study, we exposed cells to low-μM CPC doses for ~1 hr, to simulate the estimated levels in human fluids.
Classically, CPC has been employed for antibacterial applications in over-the-counter medicinal products (PubChem, 2023) like anti-gingivitis mouthwashes, toothpastes, lozenges, lip balms, and others (Mao et al., 2020). CPC helps to fight plaque (Sreenivasan et al., 2013), gingivitis (Teng et al., 2016), halitosis (Yaegaki and Sanada, 1992), and bacterial infections (Latimer et al., 2015; Schaeffer et al., 2011). Due to these oral care properties, including potential effects on bone signaling (Zheng et al., 2013), the addition of CPC to dental restorations is also being explored (Tomino et al., 2016; Yamamoto et al., 2022). CPC is used in numerous other cosmetics (hair coloring and styling products, deodorant, hair conditioners, and cosmetic biocides) and in the active pharmaceutical industry as an intermediate (PubChem, 2023).
The antibacterial properties of CPC stem from both its positively-charged headgroup and its hydrophobic tail: CPC acts as a detergent that lyses bacteria-- only when above its critical micelle concentration of ~600 μM - 900 μM (Abezgauz et al., 2010; Mandal and Nair, 2002; Shahinas et al., 2015; Shi et al., 2011; Varade et al., 2005). However, the CPC mitotoxicity we report in this study is not caused by CPC’s detergent activity because all experiments were conducted with CPC doses ~100-fold lower than the critical micelle concentration, at which doses CPC cannot act as a detergent. Additionally, under the currect experimental conditions, CPC likely does not cause membrane permeability, as indicated by lactate dehydrogenase release (Raut et al., 2022) and plasma membrane potential assays (Obeng et al., 2023).
A new potential positive use for CPC, as an antiviral, seems to be emerging. CPC displays antiviral activity through direct interaction with virus particles (Bañó-Polo et al., 2022; Koch-Heier et al., 2021; Popkin et al., 2017; Seo et al., 2019). For example, direct CPC exposure is virucidal to influenza viruses in vitro (EC50: 15–37 μM; exposure: 5 min) (Popkin et al., 2017). Moreover, mortality of influenza-infected mice (Popkin et al., 2017) and of zebrafish (0.1 μM for 1 hr) was reduced by CPC exposure (Raut et al., 2022). This suggests that CPC has potential use as an antiviral agent against influenza.
Further antiviral CPC actions are known or under current investigation. A small-scale human study indicated that CPC may treat upper respiratory infections (Mukherjee et al., 2017). CPC in mouthwash (Muñoz-Basagoiti et al., 2020) and in lozenge form (Steyer et al., 2021) is effective against SARS-CoV2 (Alemany et al., 2022): CPC lyses viral particles (Bañó-Polo et al., 2022; Koch-Heier et al., 2021) and inhibits viral fusion (Muñoz-Basagoiti et al., 2020). CPC is under investigation as a treatment against SaRS-CoV2 (Sánchez Barrueco et al., 2022). Because both SARS-CoV-2 (Guarnieri et al., 2023) and CPC are toxic to mitochondria, a risk-benefit analysis of use of this drug to combat Covid-19 may be advised. For example, the benefits of CPC may outweigh the risk of CPC during an active exposure/infection period with SARS-CoV-2.
While these beneficial antibacterial and antiviral effects are well-documented, CPC does lack antibacterial efficacy compared to plain cleansers, in hand soap and hand sanitizer formulations; this insufficiency led the FDA to effectively ban its presence in these products in 2016 (Wolf, 2016) and 2019 (Gottlieb, 2019), respectively. However, as noted above, CPC is still permitted as a food additive in chicken processing (FDA, 2020) and in numerous personal care and household products (PubChem, 2023). Despite a continuing range of uses, little is known about CPC effects on eukaryotes. Thus, there is a growing need for studies of CPC toxicology.
Despite a lack of data on potential adverse human health effects of CPC exposure, there are a few known eukaryotic effects (Burnell, 2022). Intriguingly, QAC compounds have been shown to be toxic to mitochondria in living humans: extracted white blood cells exhibited decreased oxygen consumption rates with increasing QAC exposure levels (Hrubec et al., 2021). However, the Hrubec study did not examine CPC specifically. CPC structure alone suggests toxicity because its 16-carbon tail is the length found to be maximally toxic in QACs compared to other chain lengths (Moss and Mayo-Bean, 2010). Additionally, CPC is a lipophilic cation, a type of chemical known to be targeted to and to accumulate in mitochondria (Murphy, 2008).
Mitochondria produce ATP through oxidative phosphorylation. This process is dependent on the functioning of mitochondrial complexes I-IV (the electron transport chain, ETC). Complexes I, III, and IV use Gibbs free energy derived from the transfer of electrons from reduced electron carriers through the chain to terminal electron acceptor oxygen (respiration) in order to actively pump protons against their concentration gradient from the mitochondrial matrix into the intermembrane space. These protons flow down their electrochemical gradient back into the matrix through ATP synthase, which drives the formation of ATP. Thus, changes in oxygen consumption rate (OCR) can indicate toxicity to mitochondria.
Use of media containing glutamine and galactose (instead of glucose) satisfies cells’ ATP energy and biosynthetic (Reitzer et al., 1979) needs via mitochondrial oxidative phosphorylation instead of cytosolic glycolysis (Lowry and Passonneau, 1969; Mulhausen and Mendicino, 1970; Rossignol et al., 2004). In contrast, if glucose is present, the cells used in this study will preferentially make ATP from glycolysis (Nelson and Cox, 2017).
Control of mitochondrial Ca2+ dynamics has been linked to immune function (Vig and Kinet, 2009), nervous system function (Kawamoto et al., 2012), and the regulation of mitochondrial metabolism (Lee et al., 2023).
Normal mitochondrial nanostructure, typically involving networks of fused mitochondria, is essential for healthy ATP production, exchange of proteins and lipids, and cellular respiration. Mitochondria undergo fission in response to stressors, such as to clear dysfunctional mitochondria (Youle and Van Der Bliek, 2012). Mitochondrial uncouplers such as carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and carbonyl cyanide p-triflouromethoxyphenylhydrazone (FCCP) increase fission (Giedt et al., 2012; Griparic et al., 2007), and induce mitochondrial swelling (Hanstein, 1976), and cause formation of donut-shaped mitochondria (Liu and Hajnoczky, 2011). To assess whether CPC impacts mitochondrial nanostructure, this study uses the live-cell super-resolution method fluorescence photoactivation localization microscopy (FPALM). Environmentally-induced mitochondrial dysfunction can lead to various health problems, including cardiac disease (Schwarz et al., 2014), obesity (Heinonen et al., 2015), and Alzheimer’s disease (Yan et al., 2013), to name a few.
A few publications have provided evidence of CPC mitochondrial toxicity (Chávez and Concepcion, 1982; Datta et al., 2017b; Liu et al., 2023; Saladino et al., 1971). The first utilized isolated rat mitochondria and found that CPC reduced OCR (Chávez and Concepcion, 1982). Use of isolated mitochondria, which are fragile, for mitotoxicity studies, is known to exaggerate toxic effects (Picard et al., 2011). A second study used whole cells rather than isolated mitochondria: human Leber’s hereditary optic neuropathy (LHON) osteosarcoma cytoplasmic hybrid cells (cybrids) (Datta et al., 2017b). LHON cells contain a mutation of complex I which amplifies test chemical toxicity compared to normal cells (Baracca et al., 2005; Datta et al., 2017a; Kirches, 2011). In this cell type, CPC decreased ATP production following a 24 hr exposure (Datta et al., 2017b); the accompanying cell death measurement was qualitative. Furthermore, the one experiment showing mitochondrial complex I inhibition utilized a 100 μM dose of CPC, which, although exposure was brief (<10 min), is a concentration several-fold higher than the amount known to cause significant cytotoxicity to mammalian cells in 1 hr (Raut et al., 2022). This finding raises the question of the levels of cell death accompanying the reported mitotoxicity (Datta et al., 2017b). Thus, CPC mitotoxicity results in LHON cells are suggestive, but the question of CPC effects on mitochondria at relevant doses in normal cells/tissues remains. A third study did use primary cells (toad bladder) but also employed very high doses of CPC, 100 μM. This dosage of CPC affected OCR (Saladino et al., 1971). Electron microscopy indicated mitochondrial deformation albeit at cytotoxic doses (Saladino et al., 1971). Electron microscopy reveals mitochondrial ultrastructure but requires sample processing which may complicate toxicant data interpretation. A fourth study also used electron microscopy to assess CPC effects on mitochondria and did use relevant doses in normal mouse tissues (Liu et al., 2023).
In the current study, we use relevant (low μM) doses, normal intact cells (including primary human cells), and a live cell super-resolution microscopy method which does not require harsh sample processing. Additionally, we examine the underlying biochemical mechanisms of action with respect to mitochondrial calcium levels and we put the CPC results in the context of data from known mitotoxicants.
As part of a larger study of CPC inhibition of immune cell, including mast cell, signaling (Raut et al., 2022), we unexpectedly discovered CPC mitotoxicity, the topic of this manuscript. CPC inhibits function (degranulation) of mast cells (Raut et al., 2022), a process which is ATP-dependent (Burgoyne and Morgan, 2003). Mast cells are enriched at environmental interfaces (Blank and Benhamou, 2013; Kuby, 1997; Theoharides et al., 2012), so they are poised for exposure to CPC via inhalation, food ingestion, and product application. Found in most tissues (Blank and al., 2007; Dvorak, 1986; Farrell and al., 1995; Theoharides and Sant, 1991) even the brain (Silver and Curley, 2013), mast cells play key roles in both nervous-system (Theoharides et al., 2016) and immune-related functions (Dawicki and Marshall, 2007; Krystel-Whittemore et al., 2015). The cell line we utilized to model human mast cells was the rat basophilic leukemia (RBL-2H3) cell line, which express the high-affinity immunoglobin E (IgE) receptor, FcεRI, and serve as an appropriate human mast cell model (Falcone et al., 2018; Mohr and Fewtrell, 1987) for toxicology (Alsaleh et al., 2016; Thrasher et al., 2013; Zaitsu et al., 2007). Mast cell function within the body hinges on their ability to undergo degranulation, which is an antigen (Ag)-dependent process that triggers the release of granules containing bioactive chemicals.
Use of multiple cell types affords opportunities to assess CPC effects on cells involved in different physiological functions, from different species, from different body tissues that may be differentially exposed, and to compare cell lines to primary cells. Mast cells, modeled here with immortalized RBL-2H3 cells, are derived from rat, involved in numerous physiological functions, and ubiquitous in the body, as noted above. Primary human keratinocytes are a predominant cell type in epidermal tissue, playing barrier protection and immune roles, and thus are a model for cells at interfaces between the human body and the environment through which exposure to CPC occurs. Another cell type used to confirm effects of CPC exposure is the immortalized NIH-3T3 cell line, mouse embryonic fibroblasts. Fibroblasts play varied roles in several organ tissues (Plikus et al., 2021). All three cell types (NIH-3T3, primary human keratinocytes, and RBL-2H3) used in this study were previously utilized to evaluate mitotoxicity of antibacterial agent triclosan (TCS)(Weatherly et al., 2018; Weatherly et al., 2016).
CPC cytotoxicity has previously been assessed in NIH-3T3 and RBL-2H3 cells. CPC (≤ 15 μM) was found to be non-cytotoxic via trypan blue-exclusion, lactate dehydrogenase release (Raut et al., 2022), and plasma membrane potential integrity (Obeng et al., 2023). In the current study, we have conducted further cytotoxicity tests to ensure that all dosing regimens used do not cause cell death.
In this study, super-resolution imaging of live cells (Hess et al., 2007) via FPALM (Hess et al., 2006) was employed to visualize the nanoscale details of mitochondrial structure, with the Dendra2TOM20 outer mitochondrial membrane marker. FPALM breaks the ~250 nm diffraction limit of conventional microscopy resolution by a factor of ~10X, detailing mitochondrial features which are blurred in conventional microscopy. The advantage of FPALM over traditional electron microscopy is the ability to image living cells and to do so without the heavy processing required by electron microscopy, which may confound interpretation of toxicant effects. FPALM allows localization of individual molecules labelled with fluorescent probes to capture the overall cellular distribution (Hess et al., 2006).
We aim to discover CPC effects specifically on mitochondrial Ca2+ via our development of a new plate reader-based mitochondrial-Ca2+ assay and nanostructural changes by the novel use of FPALM. Here, we examined the effects of CPC in human, rat, and mouse cells on mitochondrial function: ATP production, oxygen consumption, mitochondrial Ca2+ buffering, and nanostructural integrity. Our work demonstrates that CPC potently impairs mitochondrial function and structure at doses as low as 3000-fold lower than the dosages currently used in personal care and food products.
2. Methods
2.1. Chemicals and Reagents
Cetylpyridinium chloride (CPC; 99% purity, VWR; CAS no. 123–03-5) was prepared, including concentration determination, as previously detailed in aqueous solution (Raut et al., 2022), using a method that avoids organic solvents that could cause adverse cell effects. For the mitochondrial-Ca2+ assay, CPC was dissolved into Tyrode’s buffer (made with either galactose or glucose) (Hutchinson et al., 2011), then 1 g/L bovine serum albumin (BSA; MilliporeSigma; CAS no. 9048–46-8) was added as in (Raut et al., 2022); this is called BT. For all other experiments, CPC was dissolved into cell culture water (CCW; VWR; Molecular Biology Grade) as a base for preparation of ToxGlo Media, which was used in all experiments other than mitochondrial Ca2+. CPC does not absorb UV–Vis light beyond approximately 280 nm (Bernauer et al., 2015) and thus will not interfere via absorption with probes used in this study (which all use excitation wavelengths of 360 nm or higher, as noted in sections below).
2.2. Preparation of ToxGlo Media with Glucose or Galactose
CPC solution prepared in CCW was combined with additional ingredients including L-glutamine, to create ToxGlo Media with 5.6 mM glucose or galactose, using the published recipe (Weatherly et al., 2016). Glucose or galactose ToxGlo Media was made fresh on each day of experimentation with the final component BSA added, then media were brought to pH 7.4. The pH of 0 μM CPC control media was measured first, to avoid CPC cross-contamination.
2.3. Cell Culture
Three cell types were cultured as previously described: RBL-2H3 mast cells (Hutchinson et al., 2011), NIH-3T3 mouse fibroblast cells (Curthoys et al., 2019), and primary adult human epidermal keratinocytes (Weatherly et al., 2016).
2.4. Cytotoxicity and ATP Production Assay
Cytotoxicity and ATP Production were measured with the use of a Mitochondrial ToxGlo™ Assay Kit (Promega), as described in (Weatherly et al., 2016), adapted for CPC. RBL-2H3 cells, NIH-3T3 cells, or primary human keratinocytes were assayed. For the 60 min CPC treatments ahead of cytotoxicity assessment, pre-warmed CPC at 2X concentration in ToxGlo Media was added to cells, resulting in 1X [CPC] dissolved in glucose or galactose ToxGlo Media for cell exposures. Samples serving as positive controls for the cytotoxicity measurement received 72 μg/mL membrane-permeabilizer digitonin (Promega) in respective ToxGlo Media type (glucose or galactose). Following this 60 min treatment at 37°C/5% CO2, cytotoxicity was measured as in (Weatherly et al., 2016). Validity of the assay was determined by digitonin wells’ fluorescence values ≥ 2x of those of control wells. Cells were next incubated with ATP reagent at room temperature for 5 min before luminescence was measured. All replicates were normalized to 100% of the average of the untreated control (0 μM CPC) on a given experimental day.
2.5. Oxygen Consumption Rate Assay
Oxygen consumption rates of RBL-2H3 mast cells and of primary human keratinocytes were assessed via the Oxygen Consumption Rate Assay Kit (Cayman Chemical Company) as published (Weatherly et al., 2016). The same glucose-free, galactose ToxGlo Media as in the Mitochondrial ToxGlo Assay was made on the second day of experimentation and used to prepare CPC treatments. Quadruplet cell samples were assayed for each of the following four conditions: CPC with MitoXpress-Xtra probe, CPC without probe, no CPC with probe, and no CPC without probe. Probe-containing wells received 2.38% (v/v) of the probe. Foil plate covers (Zymo Research) were used to seal wells from the ambient environment and to eliminate oxygen entry into the wells. Phosphorescence measurements were taken for 3 hr at 10 min intervals at 37°C, 360 ± 40 nm excitation, 645 ± 15 nm emission, gain 90, bottom reading, and at normal speed.
The average of phosphorescence values from wells containing no probe was subtracted from the average values of corresponding wells containing probe; this process was repeated for each CPC dose and time point. The 30–180 min data points were utilized, to avoid initial (t = 0, 10, 20 min) phosphorescence drift due to plate warming. A linear regression line was fit to the 30–180 min data and was plotted, in order to determine oxygen consumption rate (raw phosphorescence units per minute; RFU/min). Accepted experiments met the criteria of having a control (“0 μM CPC”) with 1.) an RFU increase > 60 and 2.) an R2 linear regression fit value > 0.75. Areas under the curve were calculated with GraphPad Prism for each experiment, normalized to control (“0 μM CPC”), and then averaged across experiments.
2.6. RBL-2H3 Cell Degranulation Assay
Degranulation assay was performed as detailed in (Weatherly et al., 2013) adapted for use with CPC with protocol modifications as follow: CPC treatments (60 min) were delivered in ToxGlo Media with glucose or galactose, and DNP-BSA antigen was administered at 0.001 μg/mL.
2.7. Mitochondrial Ca2+ assay with Genetically Encoded Indicator CEPIA2mt
In the current study, mitochondrial Ca2+ was assayed with reporter protein construct CEPIA2mt rather than with organic dyes. Fluorescent reporter construct proteins possess structure which protects the internal fluorophore from photophysical interference by exogenous agents (Brejc et al., 1997; Weatherly et al., 2018; Weatherly et al., 2016).
RBL-2H3 cells were transfected with pCMV CEPIA2mt construct, a gift from Masamitsu Iino (Addgene plasmid # 58218; http://n2t.net/addgene:58218 ; PRID:Addgene_58218) (Suzuki et al., 2014) via an RBL-2H3-specific Amaxa Nucleofector Transfection Kit T (Lonza), as done in (Weatherly et al., 2018). The construct was utilized to measure levels of mitochondrial Ca2+ in response to CPC treatment. “Mock” transfected cells underwent the same electroporation process but without plasmid DNA. Following electroporation, cells were added to 200 μL phenol red-free RBL media at 100,000 cells/well in a black, clear-bottom, tissue culture-treated, 96-microwell Greiner Bio-One® plate and grown at 37°C/5% CO2 overnight. The next day, cell media was dumped, and cells were washed twice with fresh control (0 μM CPC) BT. The control BT used was made as noted in “Chemicals and Reagents,” with the glucose (5.6 mM) substituted for galactose (5.6 mM or 10 mM) in a series of experiments to assay the difference in mitochondrial Ca2+ based on cellular ability to perform glycolysis. The Mock-transfected cell wells, the “No CPC” cell wells, and “60 min CPC” exposed cells then received 200 μL control BT while “90 min CPC” exposed cells received 200 μL (5 or 10 μM) CPC in BT. The plate was incubated in the 37°C/5% CO2 incubator for 30 min. Following this incubation, the plate was dumped, and cells received 200 μL (5 or 10 μM) CPC (“90 min CPC” and “60 min CPC” exposed cells) or control BT without CPC (Mock and “No CPC”). Cell fluorescence was immediately read for 60 min at 485 ± 20 nm excitation, 528 ± 20 nm emission, gain 120, bottom reading, and at normal speed for 42 sec intervals. The average fluorescence of “Mock” transfected cells (which is average background fluorescence from the cells, the plate, and the BT) was subtracted from each curve at each respective time point from those of different treatment groups. Additionally, transfection using plasmid preparations of differing concentrations of DNA with differing levels of DNA purity adds variation to the total amount of transfection and subsequent fluorescence of the CEPIA2mt construct. Accepted experiments met the criterion that the “No CPC” group had 0 min, “Mock”-subtracted raw fluorescence values (RFU) of > 4,000; this criterion indicated that reporter construct transfection levels were sufficient. Areas under the curve were calculated with GraphPad Prism for each experiment, normalized to control (“No CPC”), and then averaged across experiments.
2.8. Sample Preparation for Imaging and Processing
NIH-3T3 cells were grown in complete NIH-3T3 media (DMEM with 4.5g/L Glucose and 4mM L-Glutamine (Lonza), 10%, iron-fortified Bovine Calf Serum (Sigma Aldrich), Penicillin (100 I.U./mL)-Streptomycin (100 μg/mL) (ATCC). Solutions were made in advance using Millipore Stericups. Cells were plated into 35 mm MatTek dishes (35 mm, coverglass diameter 20 mm) with complete NIH-3T3 media (without phenol red and without any antibiotics) and grown in 80,000 cells/MatTek dish for ~24h in 37°C/5% CO2 cell culture incubator. Then confluent NIH-3T3 cells were transfected with 1 μg Dendra2Tom20/MatTek dish in OptiMEM (reduced serum medium, with L-glutamine, without phenol red, Gibco) and Lipofectamine 3000 and incubated for 6 hours at 37°C/5% CO2. After incubation, the previous OptiMEM media/DNA mixture was removed and replaced with new complete NIH-3T3 media (without phenol red and without any antibiotics) and incubated for 12–16 hours at 37°C/5% CO2 until ready for imaging. Prior to imaging, media was removed, and cells were washed with pre-warmed control ToxGlo galactose media and pre-exposed with either 0 μM CPC or 5 μM CPC in ToxGlo galactose media for 60 minutes, at 37°C/5% CO2. After 60 minutes of exposure to either 0 μM CPC or 5 μM CPC media, imaging was performed such that 5 cells were imaged per MatTek dish for up to 30 minutes after removal from the incubator.
2.9. Illumination Path for Cell Selection
Cells were chosen based on observation of intracellular green fluorescence under excitation of inactive Dendra2 by a mercury lamp light passed through a beam expander (BE10 10X, ThorLabs), an excitation bandpass filter (476/10, Noran Microscopes), and a +300 mm lens. After collection by the 60X 1.45NA oil objective, the Dendra2 fluorescence was bandpass-filtered (ET525/50 m, Chroma) before reaching the oculars. After switching off the lamp, changing to the laser acquisition filter set, and opening the laser shutter, the fluorescence from the photoactivated Dendra2 molecules was brought into focus within a few seconds and then acquisition was initiated.
2.10. Illumination Pathway for FPALM Acquisition
Methods used for single-color super-resolution imaging and localization follow previously published methods (Curthoys et al., 2019; Hess et al., 2006; Weatherly et al., 2018) using temporal median background subtraction (Piccardi, 2004) for imaging of mitochondria (Weatherly et al., 2018). A 558 nm laser (CrystalLaser LC, CL558–100) was focused with a convex lens (f = +350 mm, Newport Corporation) into the back aperture of an inverted microscope (1X71, Olympus) and through a 60X 1.45 NA oil objective lens (Olympus) to yield 23.7mW total power and an average intensity of 6.3 kW/cm2 at the sample. Fluorescence emission from Dendra2TOM20 molecules was captured through the same objective, passed through a dichroic (405/488/561/635 nm multiband, Semrock), two 561 nm notch filters, and one 405 nm notch filter (Stopline, Semrock), and a bandpass filter (HQ590/75 M, Chroma). A 2x telescope (lenses with f = +200 mm and f = +400 mm, Newport Corporation) resulted in a measured camera pixel size equivalent to 109 nm at the sample. Image stacks of 10,000 frames were acquired with an EMCCD (iXon+ DU897DCSBV, Andor Scientific) with EM gain of 200 and an exposure time of 10.79 ms/frame. The use of the 558 nm beam as both the readout and activation laser improved signal to noise ratio, and as sufficient localizations were detected, this was the only beam used. Acquired images were analyzed using standard localization methods using MATLAB (MathWorks Inc.), including intensity threshold for localization, fitting with 2D Gaussian, use of tolerances to exclude localizations with poor fit quality, and rendering (Curthoys et al., 2019; Gudheti et al., 2013; Hess et al., 2006).
2.11. Morphological Analysis
Shape Frequency Analysis Unmodified renders of the localization data of both the 0 μM CPC and 5 μM CPC samples were assigned randomized file names, then analyzed by three separate researchers (one who had not seen the images before), who counted the number of donut-like spherical shapes observed in each of the images. The files were then mapped back to the original images and counts determined for each of the 0 μM CPC and 5 μM CPC samples, then averaged. Fourier transforms of images were used to quantify differences in spatial distributions between 0 μM CPC and 5 μM CPC samples on average. Spatial patterns were plotted to highlight conformational disparities induced under different treatment conditions. Please see Supplemental methods for more details.
2.12. Statistical Analyses
Using GraphPad Prism, statistical significance was determined by one-way ANOVA with a Tukey’s post-hoc test for the assays of cytotoxicity/ATP production, oxygen consumption rate (also one-tailed paired t-test), degranulation, mitochondrial Ca2+, and changes in mitochondrial shape extracted from FPALM images. ATP inhibition EC50 was also determined by Prism software. The Mann-Whitney test was used for donut counting data in GraphPad Prism. The plots generated from the FFT data were compared using a Kolmogorov-Smirnov test.
3. Results
3.1. CPC potently inhibits ATP production in RBL-2H3, NIH-3T3, and primary human keratinocyte cells
CPC concentrations of 10 μM and lower are known to be non-cytotoxic to RBL-2H3 and NIH-3T3 cells for 60 minutes via lactate dehydrogenase and trypan blue exclusion assays (Raut et al., 2022), but, due to the 90 minute total incubation time and differences in cell media used for this assay, a cytotoxicity assay component was necessary to support the ATP results.
In galactose media, the EC50 of ATP production when RBL-2H3 cells were exposed to CPC for 90 min was 1.7 μM, via GraphPad Prism statistical analysis (Figure 1A). Although at 10 μM CPC, levels of ATP in glucose media were 88% ± 3% (SEM) of the control (Figure 1A), no statistically significant decrease in ATP was found due to CPC exposure in the presence of glucose. This difference between CPC effects on ATP production in galactose vs. glucose media indicates that CPC’s effect is on mitochondrial ATP production, specifically–indicating that CPC is a mitochondrial toxicant. In these RBL-2H3 cell experiments, no statistically-significant effect on cytotoxicity was seen across media type (glucose or galactose) or concentration of CPC assayed for 90 min.
Figure 1. Effects of CPC on RBL-2H3 mast cell, NIH-3T3 mouse fibroblast, and primary human keratinocyte cytotoxicity and ATP production in glucose and galactose media.
RBL-2H3 mast cell assay was carried out in glucose or in glucose-free, galactose-containing media (A), whereas NIH-3T3 mouse fibroblast (B) and primary human keratinocyte (C) assays were conducted solely in galactose media, to cause cells to produce ATP in mitochondria. All cell types were exposed to varied CPC doses for 90 min prior to cytotoxicity measurements. Fluorescence and luminescence values were normalized as percentages of untreated control average values and shown as mean ± SEM. Figures are derived from at least three independent experiments with duplicates or triplicates for each CPC dosage. Statistically significant results indicated by *p < 0.05, **p < 0.01, ***p < 0.001 compared to control (0 μM CPC), one-way ANOVA followed by Tukey’s post-hoc test.
We confirmed that CPC had no significant effect on the fluorescence or luminescence signals of the Mitochondrial ToxGlo assay, confirming that the observed CPC effects on ATP production are due to a true cellular effect (Supplemental Figures S1 & S2).
To test whether this CPC mitotoxicity is a universal effect rather than one specific solely to rat mast RBL-2H3 cells, ATP production was also measured in NIH-3T3 mouse fibroblasts and in primary human keratinocytes. While in galactose media, the EC50 of ATP production of NIH-3T3 cells exposed to CPC for 90 min was 10 μM (Figure 1B) and in primary human keratinocytes was 1.2 μM (Figure 1C). CPC did not cause cytotoxicity under the tested conditions in both NIH-3T3 (Figure 1B) and primary human keratinocytes (Figure 1C). At 10 μM CPC, levels of ATP in galactose media decreased 76% ± 1% (SEM) in NIH-3T3 cells (Figure 1B), 92% ± 2% (SEM) in primary human keratinocytes (Figure 1C), when compared to the control. Overall, these data suggest that CPC is a mitochondrial toxicant in various cell types, from three species, including a primary cell type.
3.2. CPC dampens O2 consumption rate in primary human keratinocytes and in RBL-2H3 cells
To determine the effect that CPC has on O2 consumption rate (OCR), the OCR kit from Cayman Chemical Company was used. This kit relies on the use of a phosphorescent probe that is quenched by O2; thus, O2-consuming mitochondrial respiration reduces O2 over time, leading to an increase in phosphorescence reading. We previously developed and validated this assay with RBL-2H3 cells (Weatherly et al., 2016). To validate the experiments, we assayed CPC effects on the experimental signal (Supplemental Figures S3-S5). CPC does not change the background (no-probe) phosphorescence of samples with cells (Supplemental Figure S3). CPC does not change the OCR probe’s phosphorescence of samples without cells (Supplemental Figure S4), showing that the OCR cellular data (Figure 2) is truly due to CPC effects on cellular oxygen consumption rates. Additionally, we confirmed that CPC does not interfere with the phosphorescent probe signal at the start of the OCR experiments, with or without cells (Supplemental Figure S5).
Figure 2. Oxygen consumption rate in CPC-treated RBL-2H3 cells and primary human keratinocytes in galactose media.
OCR was measured in cells exposed to CPC for 3 hr with data fit to a linear line, with best-fit slope (RFU/min) based on 10 min incremental measurements (t = 30 to t = 180). Representative graph of RBL-2H3 OCR data (A). Slope linear line of best fit for 1.75 μM and 5 μM CPC normalized to untreated (0 μM CPC) control, shown as mean ± SEM, **p < 0.01 and ***p < 0.001, determined by one-way ANOVA by Tukey’s post-hoc test. (B). Representative graph of primary human keratinocyte OCR data (C) Slope of linear line of best fit for 1.25 μM normalized to untreated (0 μM CPC) control, shown as mean ± SEM, **p < 0.01, determined by one-tailed paired t-test with 95% Confidence Interval (D). Data gathered from three experiments, each with at least three replicates per sample.
The averaged, background-subtracted value for RBL-2H3 cells was plotted over time and shown as a representative figure (Figure 2A). A reduced rate of increase in phosphorescence over time is seen with CPC, when compared to the 0 μM control line (Figure 2A). The slopes of the lines which represent the OCR, in the 0 μM, 1.75 μM, and 5 μM CPC groups from t = 30 min to 180 min, were determined and normalized to that of the 0 μM control group (Figure 2B). The OCR for the 5 μM CPC-treated RBL-2H3 cells decreased to 34% ± 2% (SEM) of the untreated control, representing roughly a two-thirds decrease in oxygen consumption, while that of the 1.75 μM CPC-treated was 51% ± 8% (SEM), representing roughly a one-half decrease in oxygen consumption (Figure 2B). The averaged, background-subtracted value for primary human keratinocyte was plotted over time and shown as a representative figure (Figure 2C). The slope of the line in the 0 μM and 1.25 μM CPC groups from t = 30 min to 180 min were determined and normalized to that of the 0 μM control group (Figure 2D). The OCR for the 1.25 μM CPC-treated primary human keratinocytes was 32.1 % ± 0.1% (SEM) of the untreated control, representing a roughly two-thirds decrease in oxygen consumption (Figure 2D).
3.3. CPC inhibits RBL-2H3 degranulation under mitochondrial ToxGlo assay conditions
To assess differences in CPC’s ability to inhibit RBL-2H3 cells degranulation under ToxGlo assay conditions, the RBL-2H3 degranulation assay (Figure 1 in (Raut et al., 2022)) was repeated without a pre-incubation. The dose of antigen (Ag) utilized, 0.001 μg/mL, was chosen because it elicited a moderate absolute degranulation response when compared to the maximal possible granule release when in the absence of CPC: absolute degranulation in glucose media was 32% ± 4% (SEM), and in galactose media was 23.4% ± 0.5% (SEM).
There were no significant changes in levels of CPC suppression of degranulation between the glucose and galactose groups: levels of degranulation at 10 μM CPC were reduced to 51% ± 3% (SEM) of the control value when in glucose media and to 57% ± 2% (SEM) of the control value in galactose media (Figure 3). In both media types, inhibition of degranulation was dose-responsive, with significance beginning at 1 μM for the cells in glucose media and at 5 μM for those in galactose media (Figure 3). Previously, we found that 10 μM CPC inhibits degranulation to 30% ± 6% (SEM) of control values when in glucose-BT buffer (Raut et al., 2022), indicating that CPC causes less inhibition of degranulation under the ToxGlo assay conditions. In a previous study (Raut et al., 2022), we showed that CPC inhibition of degranulation is a true cellular effect, not an interference of CPC with the enzymatic reaction and fluorophore used to assess degranulation.
Figure 3. Relative degranulation levels of RBL-2H3 cells after CPC treatment in glucose and galactose media.
Cells primed with IgE for 60 min followed by treatment with CPC + 0.001 μg/mL antigen (Ag) for 60 min. CPC concentrations ranged between 0 and 10 μM. Degranulation values were normalized to control (0 μM CPC + Ag). Data shown as mean ± SEM of three independent experiments with triplicates of each sample in each experiment. n.s. = not significantly different for galactose vs glucose buffer conditions. Significance vs 0 μM CPC control shown by *p < 0.05 and ***p < 0.001, determined by one-way ANOVA followed by Tukey’s post-hoc test.
3.4. CPC lowers mitochondrial Ca2+ levels in galactose-containing buffer
To assess whether CPC affects mitochondrial Ca2+ levels, RBL-2H3 cells were transfected with the mitochondrial-targeted, fluorescent construct CEPIA2mt and tested in a novel plate reader-based assay that allows for relatively rapid assessment of numerous treatment conditions. CPC addition led to decreased reporter fluorescence levels, under a variety of exposure conditions: both 5 μM (Figure 4A) and 10 μM (Figure 4B–4C) CPC concentrations; both “60 min CPC’’ and “90 min CPC” exposure timings (Figures 4A–4C); and in both 5.6 mM (Figures 4A–4B) or 10 mM (Figure 4C) galactose buffer. In “90 min CPC” representative graphs (Figures 4A–4C), refilling of Ca2+ into the mitochondrial matrix is apparent at the later time points. AUC analyses of the galactose-buffer groups revealed statistically significant decreases in reporter fluorescence due to CPC under all conditions tested (various CPC timing and dosing and galactose concentrations as indicated in Figure 4D).
Figure 4. Effects of CPC on RBL-2H3 cell mitochondrial Ca2+ levels in galactose and glucose media.
RBL cells were transfected with fluorescent CEPIA2mt construct and plated overnight. Cells were placed in galactose or glucose BSA-Tyrodes (BT) media and exposed to one of the conditions: “60 min CPC” means cells had no pre-exposure to CPC and were only exposed to CPC during the 60 minute measurement displayed the graph. “90 min CPC” means cells were pre-exposed to CPC for 30 minutes prior to, as well as during, the 60 minute measurement displayed on the graph. Representative CEPIA2mt fluorescence intensity curves of 5.6 mM galactose BT with 5 μM (A) and 5.6 mM galactose BT with 10 μM CPC (B) as well as 10 mM galactose BT with 10 μM CPC (C). Areas under the fluorescence curves (AUC) were determined and normalized to 0 μM CPC control for the various galactose (Gal) and CPC concentration combinations as indicated (D). Representative CEPIA2mt fluorescence intensity curves of 5.6 mM glucose BT with 10 μM CPC (E). AUC for the glucose BT group was determined and normalized to 0 μM CPC (F). All values presented are mean ± SEM from at least three independent experiments, each with three replicates per CPC treatment. *p < 0.05, **p < 0.01, one-way ANOVA followed by Tukey’s post-hoc test.
In contrast, CPC addition to cells in glucose-containing buffer had no effect on mitochondrial Ca2+ levels, as indicated by a lack of change of CEPIA2mt reporter fluorescence (Figure 4E). AUC analysis of 10 μM CPC exposure (60 or 90 min) in glucose-containing buffer indicates no significant effects (Figure 4F).
3.5. CPC disrupts mitochondrial morphology as measured with super-resolution microscopy
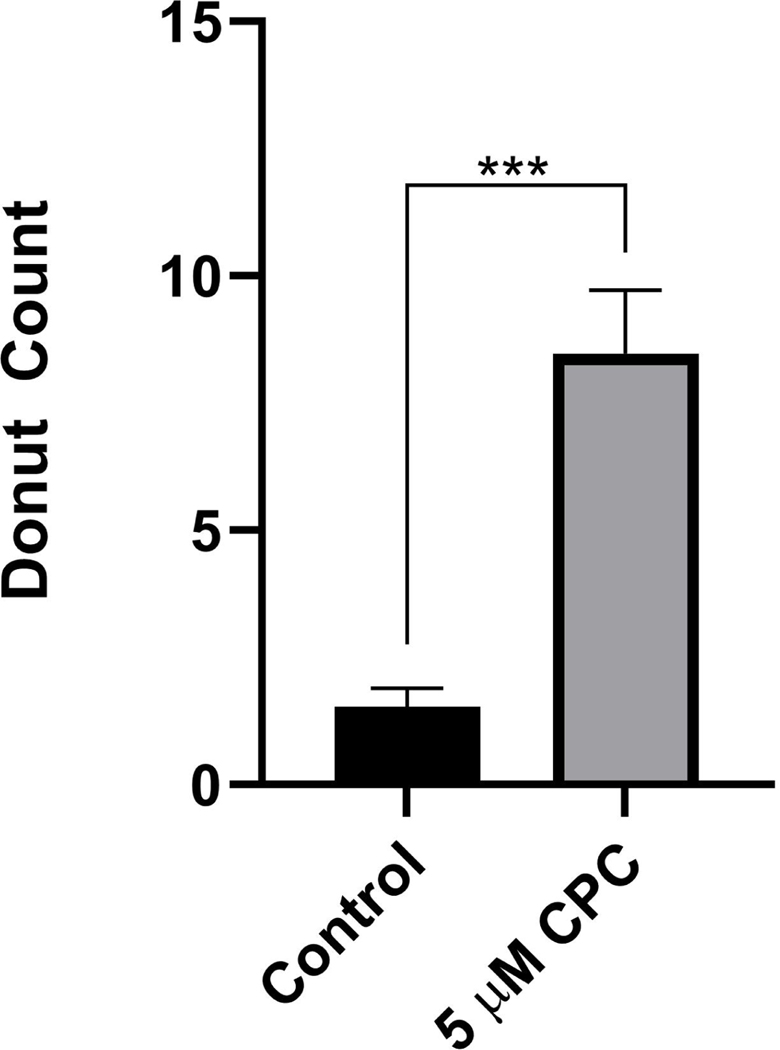
FPALM was utilized to precisely assess CPC effects on mitochondrial morphology at the nanoscale. A photoactivatable fluorescent marker of the outer mitochondrial membrane, Dendra2Tom20, was imaged in NIH-3T3 cells treated with 0 μM CPC (Control) or 5 μM CPC in galactose media. CPC induces a donut-like morphology in the mitochondria of NIH-3T3 cells (representative images in Figure 5). The presence of the donuts in the control and 5 μM CPC groups was quantified based on the number of observed donuts in each generated image (Figure 6).
Figure 5. Representative super-resolution microscopy images of NIH-3T3 cell mitochondria structural effect following 0 μM CPC (Control) and 5 μM CPC exposure in galactose media for 60 min.
FPALM images of live NIH-3T3 cell mitochondria expressing outer-membrane marker Dendra2TOM20 (green) in Control 0 μM CPC (A) and 5 μM CPC (B) treated cells. The horizontal and vertical axes are defined as X (right), and Y (up), respectively. Shown are representative images from two days of imaging during which n = 30 of control cells and n = 30 of 5 μM CPC treated cells were imaged. Scale bars = 1 μm.
Figure 6. Mitochondrial donut formation in super-resolution microscopy images of NIH-3T3 cells following 0 μM CPC (Control) and 5 μM CPC in galactose media exposure for 60 min.
Live NIH-3T3 cells expressing mitochondria outer-membrane marker Dendra2TOM20 were imaged using FPALM. Donut shapes were observed in images recorded by three separate researchers via a blinded, randomized analysis in control (n = 25) and 5 μM CPC (n = 25). The average counts for each image were gathered and mapped back to the control or 5 μM groups. Significance is represented by ***p < 0.001 as determined by a Mann-Whitney test.
The morphological variation was quantified using the Fast Fourier Transforms of FPALM renders (Figure 7), where the control group can be observed to have a more symmetric distribution of spatial frequencies compared to the 5 μM CPC group. The donut shapes present in the sample had specific features and spatial frequencies typically within the range of 0.1–0.45 μm−1 (Figure 7). Furthermore, when donut-like shapes were observed, two of the sides of the donut (nominally defined as X; Figures 5 and 7) were typically observed to have brighter fluorescence than the other two sides (nominally defined as Y; Figures 5 and 7). Thus, the differences in the histogram of spatial frequencies as a function of X and Y were able to capture the differences in mitochondrial morphology (Figure 7C). The probability distributions for the dimensional ratios were statistically significant (p < 10−10) using a Kolmogorov-Smirnov test) comparing control and 5 μM CPC.
Figure 7. Fourier Transform Analysis of NIH-3T3 Mitochondrial Morphology following 0 μM CPC (Control) and 5 μM CPC exposure in galactose media for 60 min.
Averaged Fourier transforms of cellular images for control (n = 25) (A) and 5 μM CPC (n = 25) (B) samples after thresholding with a scale bar representing a wavenumber k of 1 μm-1. Images were of live NIH-3T3 cells transfected with Dendra2TOM20 and generated via FPALM rendering. Green circles in (A-B) represent an estimated region for features between 190–320 nm in size as the spacing for some of the most relevant structural features. This was used to estimate a lower threshold for features to be included, and an upper threshold was added to remove features at longer length scales represented at the center of the images. The white rectangles in (A-B) correspond to the regions selected for calculation in (C). The graph in (C) plots the fraction of points within the rectangle spanning the X-direction contained within a certain distance from the center as a ratio with the same fraction in the Y-direction. This was used to quantify the elongation of the shape at different distances with a near circular shape corresponding to a ratio of 1. A Kolmogorov-Smirnov test returned a significance of p < 10−10 for the two curves and similarly for the two curves starting at a distance ≥ 2 μm−1 to assure enough data was collected before comparison.
4. Discussion
Likely due to its continual use since the 1930s (ACS, 2021), modern comprehensive toxicology studies have not been conducted to evaluate potential risks of CPC despite its continued existence in many consumer/cleaning/food applications. Its widespread use raises the importance of identifying any potential harmful effects, including mitotoxicity, to inform risk assessors. Extensive searching of the literature indicates that CPC effects on human health or on biochemical or physiological processes in eukaryotic organisms have not been widely explored. Human epidemiology studies of CPC are currently lacking, apart from those looking for positive uses such as against SARS-CoV-2. There are a few emerging published articles of toxicity in eukaryotic cells and model organisms, including the previous mitochondrial toxicity studies (Chávez and Concepcion, 1982; Datta et al., 2017b; Liu et al., 2023; Saladino et al., 1971) and a report showing CPC-induced rat lung damage (Kim et al., 2021; Lin et al., 1991). While prescription drugs are tightly regulated for safety in humans by the Food & Drug Administration (FDA), there are many over-the-counter personal care and consumer products containing unregulated chemicals that have never undergone toxicity testing. The Toxic Substances Control Act (TSCA) of 1976, which authorizes the Environmental Protection Agency to regulate toxic chemicals, effectively legacied tens of thousands of chemicals from regulation at its start and has exempted others through the years (MacKendrick, 2018). Triclosan, for example, was widely used in consumer products for decades until a number of studies by epidemiologists and toxicologists revealed toxic effects (Weatherly and Gosse, 2017).
In this study, we have revealed the mitochondrial toxicity of the common personal care and food chemical CPC towards multiple cell types, including primary human keratinocytes. We have shown that CPC inhibits mitochondrial ATP production (Figure 1), inhibits OCR (Figure 2), and causes efflux of mitochondrial Ca2+ (Figure 4). The mitochondrial toxicity of CPC seems to be more potent than its inhibition of mast cell signal transduction (Figure 3).
Using live-cell super-resolution microscopy, we show that CPC deforms mitochondria at the nanoscale, inducing a toroidal, “donut” morphology (Figures 5–7). Another recent study of CPC mitochondrial effects, using subcutaneous injections for 2 days of relevant CPC doses in mice, also revealed CPC deformation of mitochondrial ultrastructure (Liu et al., 2023). Liu et al. used electron microscopy and found evidence of mitochondrial swelling (Liu et al., 2023), which is consistent with the “donut” shapes we observed (Liu and Hajnoczky, 2011). The formation of “donuts” is a mode of fission of mitochondrial networks. Mitochondrial shape is key for healthy mitochondrial function and plays a role in disease development (Youle and Van Der Bliek, 2012). Abnormal mitochondrial structures including donuts are associated with disease etiology (Youle and Van Der Bliek, 2012), including insulin resistance (Jheng et al., 2012), bipolar disorder (Cataldo et al., 2010), memory problems in monkeys (Hara et al., 2014), Parkinson’s disease (Bhandari et al., 2014; Cui et al., 2010; Pozo Devoto and Falzone, 2017), and problems with embryonic development (Chen et al., 2003). In fact, the recent CPC paper (Liu et al., 2023) found CPC-induced impaired embryonic development and oocyte loss in neonate mice.
Donut patterns observed in 5 μM CPC samples tended to have an x- or y-directional bias at certain distances (Figure 7B). As the Fourier transforms are averaged over the set of cells (n = 25 per treatment type), it is assumed that directional bias from cell orientation will be averaged over as in the 0 μM control (Figure 7A). Membrane-associated fluorophores have often been observed to be preferentially excited by a given direction of laser polarization when their transition dipoles align with that laser polarization, leading to asymmetric intensity distributions in images of spherical structures (Brasselet, 2011; Krebs et al., 2005). Here, we expect that the membrane-associated TOM20-Dendra2 molecules are preferentially excited by a similar process, leading to the asymmetric fluorescence signals when spherical (donut-like) membranes are present (Figure 5). The Fourier transform of the images is then expected to be asymmetric when the donuts are present, and this is confirmed by the Fourier transforms of experimental images (Figure 7A and B). Hence, we quantify the asymmetry of the Fourier transform as a measure of the degree to which the donut shapes are present on the average, as a function of CPC exposure.
The mechanism underlying CPC disruption of mitochondrial nanostructure and formation of donuts (Figure 5) is unknown. Mitochondrial uncouplers including CCCP, FCCP, and TCS also cause donut formation, in multiple cell types (Ding et al., 2012; Giedt et al., 2012; Liu and Hajnoczky, 2011; Weatherly et al., 2018). However, because CPC possesses no ionizable proton and thus cannot act via an uncoupling process, its biochemical process of structural deformation is necessarily distinct from that of uncouplers. Because CPC may be an electron transport chain (ETC) inhibitor (Chávez and Concepcion, 1982; Datta et al., 2017b; Hess et al., 2006), CPC’s mechanism may be related to its ETC inhibition: antimycin A and rotenone also induce donut formation, potentially via mitochondrial reactive oxygen species (ROS) generation (Ahmad et al., 2013; Bulthuis et al., 2019; Giedt et al., 2012; Plecita-Hlavata et al., 2008). Another potential mechanistic explanation for CPC-induced donuts involves CPC (3 & 10 μM; 2 hr) activation of AMP-activated protein kinase (AMPK), which has been demonstrated in liver cells (Allen et al., 2020). Interestingly, inositol, which prevents aberrant AMPK activation, also prevents mitochondrial fission (Hsu et al., 2021). Because CPC both activates AMPK (Allen et al., 2020) and causes donut formation (Figure 5), AMPK stimulation may be involved in the mechanism underlying CPC formation of mitochondrial donuts.
To compare the mitochondrial toxicity of CPC to that of known mitotoxicants, in Table 1 we list EC50 values for inhibition of mitochondrial ATP production in RBL-2H3 cells, in galactose media, measured under nearly identical conditions. As a striking example, the canonical mitochondrial uncoupler CCCP exhibits an EC50 of 1.2 μM in this cell line in the same media type as the CPC experiments (with the only differences being media without BSA and with an incubation time of 2 hr in the CCCP experiments) (Weatherly et al., 2016), indicating that CPC (EC50 of 1.7 μM) is basically as mitotoxic as CCCP. Another canonical mitochondrial toxicant, 2,4-dinitrophenol (DNP) (Loomis and Lipmann, 1948), has an EC50 of ~314 μM, which is ~184-fold less potent as a mitotoxicant than CPC; DNP was a diet drug that worked via mitochondrial uncoupling and that was banned in 1938 in the USA due to its severe health effects (Grundlingh et al., 2011). Another antibacterial agent recently determined to be mitotoxic (Weatherly et al., 2016), TCS, has an EC50 of ~8.6 μM, which is also less potent than CPC, by ~5-fold.
Table 1.
Comparison of mitochondrial toxicants' potency to that of CPC.
CPC reduces O2 consumption rate (Figure 2). In RBL-2H3 cells, CPC at the ~EC50 value for mitochondrial ATP suppression, 1.75 μM, suppressed OCR to 51% ± 8% of that of untreated cells (Figure 2B), supporting the concept that CPC’s suppression of ATP production as a direct consequence of its ETC inhibition. This magnitude of decrease is comparable to the amount of increase in OCR seen in RBL-2H3 cells exposed to canonical uncoupler CCCP (1 μM): 150% ± 10% (SEM) (Weatherly et al., 2016), although CPC depresses OCR while CCCP stimulates OCR due to differences in mechanisms of toxicity. Also, in primary human keratinocytes, CPC at the ~EC50 value for mitochondrial ATP suppression, 1.25 μM, suppressed OCR to 32.1 % ± 0.1% of that of untreated cells (Figure 2D), lending further support to the concept of CPC’s suppression of ATP production as a direct consequence of inhibition of oxygen consumption (Figure 2D). This CPC mitochondrial toxicity of mitochondrial ATP and OCR inhibition may be universal, based on the 3 cell types (from different species and tissues) tested in this study plus those tested previously (Datta et al., 2017b; Liu et al., 2023; Saladino et al., 1971).
The mechanism of CPC suppression of mitochondrial Ca2+ levels (Figure 4) is unknown. However, while mitochondrial proton ionophore uncouplers increase mitochondrial Ca2+ while concurrently suppressing ATP levels (and decreasing mitochondrial membrane potential [MMP]) (Weatherly et al., 2018; Weatherly et al., 2016), CPC exposure leads to decreases of both mitochondrial Ca2+ and ATP, indicating a mechanism of mitochondrial Ca2+ modulation distinct from that of uncouplers.
We found that CPC suppresses mitochondrial Ca2+ concentration only when mitochondrial ATP production was also inhibited (i.e., in galactose media, not in glucose media, which allows glycolytic production of ATP; Figure 1). These data suggest that CPC causes mitochondrial Ca2+ efflux because it inhibits mitochondrial ATP production. One potential mechanism could be that CPC’s reduction of ATP levels results in malfunction of the mitochondrial Ca2+ uniporter (MCU), which works in energized cells to transport Ca2+ (when its cytosolic concentration is > 1 μM) into the mitochondrial matrix (Fan et al., 2020). For example, positively-charged ruthenium complexes inhibit MCU, albeit via an unknown mechanism (Woods et al., 2019; Ying et al., 1991). Another putative mechanism could be that CPC’s reduction of mitochondrial ATP production results in impairment of the ATP-dependent (Erecińska and Dagani, 1990) plasma membrane transporter Na+/K+-ATPase, which works in energized cells to maintain plasma membrane potential by catalyzing the exchange of three intracellular Na+ cations for two extracellular K+ cations (Nelson and Cox, 2017). Impairment of Na+/K+-ATPase would lead to cytosol buildup of Na+, then passive flow of Na+ down its concentration gradient through the cation-porous (Nelson and Cox, 2017) outer mitochondrial membrane, into the intermembrane space. At the inner mitochondrial membrane, the excess Na+ may then flow passively into the matrix via the ATP-independent Na+/Li+/Ca2+ exchanger (NCLX) (Palty et al., 2004). This inflow of Na+ could then cause mitochondrial Ca2+ efflux via NCLX (Palty et al., 2010), resulting in the decreased mitochondrial Ca2+ levels observed (Figure 4). Indeed, cytosolic Na+ increases can lead to efflux in matrix Ca2+ levels in cardiomyocytes (Liu and O’Rourke, 2008). NCLX function is reliant on MMP (Kostic et al., 2018). Such questions–CPC effects on MCU, NCLX, MMP, as well as on other Ca2+ transporters–will be topics of future research.
Apparent recovery of mitochondrial Ca2+ levels, for example in groups pre-exposed for 30 min to CPC, begins at ~30 min of the plate reader time course, equating to a 60 min total of CPC exposure (Figure 4A-C). This refilling of mitochondrial Ca2+ occurs under conditions when mitochondrial ATP production is strongly inhibited (Figure 1). When mitochondrial ATP is not being produced, ATP synthase is not functioning, and thus H+ is not flowing back into the matrix through ATP synthase, and the matrix becomes relatively more alkaline (Nelson and Cox, 2017). In RBL-1 cells, MCU function is pH-dependent: MCU is activated at higher matrix pH levels (Moreau and Parekh, 2008). Thus, the observed mitochondrial Ca2+ recovery at later exposure times (Figure 4) may be due to activation of MCU.
Does CPC’s mitotoxic inhibition of ATP production (Figure 1) explain CPC’s inhibition of mast cell function (Raut et al., 2022)? We have found that mast cell degranulation is inhibited by CPC to the same degree, regardless of whether ATP is concurrently inhibited (in galactose media) or not inhibited (in glucose media) (Figure 3). Therefore, CPC’s ability to inhibit mast cell degranulation is independent of mitochondrial toxicity. This finding shows that CPC has additional cellular signal transduction targets of inhibition, to be probed in future work. The results in Figure 3 also demonstrate that CPC’s mitochondrial toxicity exceeds the potency of CPC’s immune cell signaling interference: the EC50 is ~10 μM CPC for inhibition of degranulation (Figure 3) whereas the EC50 is 1.7 μM for CPC’s inhibition of mitochondrial ATP production (Figure 1), a value ~6-fold lower under the same exposure conditions. Thus, CPC appears to be ~6-fold more potently toxic to mitochondria than to general cellular signal transduction. However, if CPC inhibits ATP production through mitochondrial complex I inhibition (Datta et al., 2017b), this process could mask increased degranulation inhibition: mitochondrial complex I inhibition can lead to increased levels of ROS (Fato et al., 2009), which are known stimulators of mast cell degranulation (Swindle et al., 2004). Future research will examine CPC effects on various types of ROS, in mitochondria and elsewhere, to investigate this mechanistic avenue. Furthermore, the antiestrogenic effect of CPC after 24 hr exposure could interfere with the known stimulation of degranulation through estrogen exposure (Zaitsu et al., 2007), implicating a potential connection between estrogenic and immunogenic signaling inhibition. In the timeframe of a 90 minute exposure to CPC, other mechanisms are likely at play to cause levels of ATP lowered by ~90% at 10 μM (Figure 1) without cytotoxicity.
In conclusion, we have shown that CPC acts as a mitotoxicant in multiple types of living cells including primary human cells, at concentrations potentially achieved in human tissues and fluids following typical exposures (Bonesvoll and Gjermo, 1978; Obeng et al., 2023). Longer-term exposures to CPC will be the subject of further investigation in our laboratory. CPC causes inhibition of mitochondrial ATP production, oxygen consumption, mitochondrial Ca2+ buffering (as revealed via a novel experimental method), and disruption of mitochondrial nanostructural integrity, as shown via FPALM super-resolution microscopy. These data provide insight into mechanisms underlying CPC disruption of mitochondrial function at doses relevant to human exposure via consumer and food products. In summary, we show that CPC is a mitotoxicant in mammalian cells (rat, mouse, and human), similarly potent as canonical mitotoxicant CCCP and more potent than other previously determined mitotoxicants, DNP and TCS.
Supplementary Material
Antibacterial cetylpyridinium chloride inhibits mitochondrial ATP production
Antibacterial cetylpyridinium chloride inhibits oxygen consumption rate
Antibacterial cetylpyridinium chloride causes mitochondrial Ca2+ efflux
Cetylpyridinium chloride is nearly as potent as canonical mitotoxicant CCCP
Super-resolution microscopy reveals nanoscale cetylpyridinium chloride mitodefects
Acknowledgments
We thank Roger Sher for the Dendra2-TOM20 construct, Komala Shivanna for providing access to shared lab materials and resources, David Winski for insightful discussions, Matthew Parent for MATLAB scripts, Patrick Fleming and Marissa Paine for help in ordering materials and lab support, Patricia Byard and Timothy Campbell for administrative assistance, Dr. Melody Neely and Caitlin Wiafe-Kwakye for allowing our lab to use their nanodrop, as well as Dr. Melissa Maginnis and Avery Bond for providing use of equipment.
This project was funded primarily by the University of Maine System Research Reinvestment Fund Grant Program Track 1 Rural Health and Wellbeing Grand Challenge (PI: Gosse), by a UMaine Medicine Seed Grant (PI: Gosse), and from a Bioscience Association of Maine (BioME) Seed Grant. This research was also supported the National Institutes of Health: National Institute of General Medical Sciences award numbers 1R15GM139070 (PI: Hess) and P20GM103423 (an Institutional Development Award). The Maine Technological Asset Fund (MTAF 1106 and 2061, PI: Hess) provided equipment. University of Maine student funding that supported this work includes a Graduate Student Government Grant, the Center for Undergraduate Research (CUGR), the Charlie Slavin Research fund of The Honors College.
Footnotes
Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing – original draft; Writing – review & editing
Sasha R. Weller: Conceptualization; Data Curation; Funding acquisition; Investigation; Methodology; Validation; Writing – Original Draft; Writing – Review & Editing
John E. Burnell: Conceptualization; Data Curation; Funding acquisition; Investigation; Methodology; Validation; Writing – Original Draft
Brandon M. Aho: Conceptualization; Data Curation; Formal Analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – Original Draft; Writing – Review & Editing
Bright Obeng: Formal analysis; Investigation; Methodology; Validation; Visualization
Emily L. Ledue: Investigation; Validation; Writing – review & editing
Juyoung K. Shim: Investigation; Validation; Writing – review & editing
Samuel T. Hess: Conceptualization; Data Curation; Formal analysis; Funding Acquisition; Investigation; Project Administration; Resources; Software; Supervision; Visualization; Writing – Original Draft; Writing – Review & Editing
Julie A. Gosse: Conceptualization; Data curation; Formal analysis; Funding Acquisition; Investigation; Methodology; Project Administration; Resources; Supervision; Validation; Writing – Original Draft; Writing – Review & Editing
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abezgauz L, Kuperkar K, Hassan PA, Ramon O, Bahadur P, Danino D, 2010. Effect of Hofmeister anions on micellization and micellar growth of the surfactant cetylpyridinium chloride. J Colloid Interface Sci 342, 83–92. [DOI] [PubMed] [Google Scholar]
- ACS, 2021. Cetylpyridinium chloride, Molecule of the Week Archive. Am. J. Pharm, ACS Chemistry for Life. [Google Scholar]
- Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A, 2013. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis 4, e461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany A, Perez-Zsolt D, Raich-Regue D, Munoz-Basagoiti J, Ouchi D, Laporte-Villar C, Baro B, Henriquez N, Prat N, Gianinetto MO, Gutierrez MV, Sanchez-Paniagua MG, Henriquez NL, Vicente JM, Ara J, Rodriguez-Arias MA, Puig J, Blanco I, Lopez CC, Hernandez A, Bordoy AE, Redondo CE, Soler VG, Gimenez M, Blanc V, Leon R, Gispert J, Cpc-Covid G, Clotet B, Izquierdo-Useros N, Mitja O, 2022. Cetylpyridinium Chloride Mouthwash to Reduce Shedding of Infectious SARS-CoV-2: A Double-Blind Randomized Clinical Trial. J Dent Res, 220345221102310. [DOI] [PubMed] [Google Scholar]
- Allen SA, Datta S, Sandoval J, Tomilov A, Sears T, Woolard K, Angelastro JM, Cortopassi GA, 2020. Cetylpyridinium chloride is a potent AMP-activated kinase (AMPK) inducer and has therapeutic potential in cancer. Mitochondrion 50, 19–24. [DOI] [PubMed] [Google Scholar]
- Alsaleh NB, Persaud I, Brown JM, 2016. Silver Nanoparticle-Directed Mast Cell Degranulation Is Mediated through Calcium and PI3K Signaling Independent of the High Affinity IgE Receptor. PLoS One 11, e0167366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañó-Polo M, Martínez-Gil L, Sánchez Del Pino MM, Massoli A, Mingarro I, Léon R, Garcia-Murria MJ, 2022. Cetylpyridinium chloride promotes disaggregation of SARS-CoV-2 virus-like particles. J Oral Microbiol 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracca A, Solaini G, Sgarbi G, Lenaz G, Baruzzi A, Schapira AH, Martinuzzi A, Carelli V, 2005. Severe impairment of complex I-driven adenosine triphosphate synthesis in leber hereditary optic neuropathy cybrids. Arch Neurol 62, 730–736. [DOI] [PubMed] [Google Scholar]
- Bernauer U, Coenraads P, Degen G, Dusinska M, Lilienblum W, Nielsen E, Rastogi SC, Rousselle C, van Benthem J, 2015. Opinion on cetylpyridinium chloride - submission II (P97), pp. 1–54. [Google Scholar]
- Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW 2nd, , 2014. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res 114, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank U, al., e., 2007. Mast cells and inflammatory kidney disease. 217, 79–95. [DOI] [PubMed] [Google Scholar]
- Blank U, Benhamou M, 2013. Deciphering new molecular mechanisms of mast cell activation. Front Immunol 4, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonesvoll P, Gjermo P, 1978. A comparison between chlorhexidine and some quaternary ammonium compounds with regard to retention, salivary concentration and plaque-inhibiting effect in the human mouth after mouth rinses. Archs oral Biol. 23, 289–294. [DOI] [PubMed] [Google Scholar]
- Brasselet S, 2011. Polarization-resolved nonlinear microscopy: application to structural molecular and biological imaging. Advances and Optics and Phontonics 3, 205–271. [Google Scholar]
- Brejc K, Sixma TK, Kitts PA, Kain SR, Tsien RY, Ormo M, Remington SJ, 1997. Structural basis for dual excitation and photoisomerization of the Aequorea victoria green fluorescent protein. Proc Natl Acad Sci U S A 94, 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulthuis EP, Adjobo-Hermans MJW, Willems P, Koopman WJH, 2019. Mitochondrial Morphofunction in Mammalian Cells. Antioxid Redox Signal 30, 2066–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A, 2003. Secretory granule exocytosis. Physiol Rev 83, 581–632. [DOI] [PubMed] [Google Scholar]
- Burnell JE, 2022. Mitochondrial Toxicity of Antimicrobial Agent Cetylpyridinium Chloride Inhibits Mast Cell University of Maine Honors College. Univeristy of Maine [Google Scholar]
- Cataldo AM, McPhie DL, Lange NT, Punzell S, Elmiligy S, Ye NZ, Froimowitz MP, Hassinger LC, Menesale EB, Sargent LW, Logan DJ, Carpenter AE, Cohen BM, 2010. Abnormalities in mitochondrial structure in cells from patients with bipolar disorder. Am J Pathol 177, 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez E, Concepcion B, 1982. Anisotropic Action of Cetyl Pyridinium Chloride on Rat Heart Mitochondria Archives of Biochemistry and Biophysics 213, 81–86. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC, 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Tang X, Christian WV, Yoon Y, Tieu K, 2010. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. J Biol Chem 285, 11740–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys NM, Mlodzianoski MJ, Parent M, Butler MB, Raut P, Wallace J, Lilieholm J, Mehmood K, Maginnis MS, Waters H, Busse B, Zimmerberg J, Hess ST, 2019. Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering. Biophys J 116, 893–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA, 2017a. The Eye Drop Preservative Benzalkonium Chloride Potently Induces Mitochondrial Dysfunction and Preferentially Affects LHON Mutant Cells, Investigative Ophthalmology & Visual Science pp. 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, He G, Tomilov A, Sahdeo S, Denison MS, Cortopassi G, 2017b. In Vitro Evaluation of Mitochondrial Function and Estrogen Signaling in Cell Lines Exposed to the Antiseptic Cetylpyridinium Chloride. Environ Health Perspect 125, 087015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawicki W, Marshall JS, 2007. New and emerging roles for mast cells in host defence. Curr Opin Immunol 19, 31–38. [DOI] [PubMed] [Google Scholar]
- Ding WX, Li M, Biazik JM, Morgan DG, Guo F, Ni HM, Goheen M, Eskelinen EL, Yin XM, 2012. Electron microscopic analysis of a spherical mitochondrial structure. J Biol Chem 287, 42373–42378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak AM, 1986. Mast-cell degranulation in human hearts. N Engl J Med 315, 969–970. [DOI] [PubMed] [Google Scholar]
- Erecińska M, Dagani F, 1990. Relationships between the Neuronal Sodium/Potassium Pump and Energy Metabolism Effects of K +, Na +, and Adenosine Triphosphate in Isolated Brain Synaptosomes. J Gen Physiol 95, 591–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone FH, Wan D, Barwary N, Sagi-Eisenberg R, 2018. RBL cells as models for in vitro studies of mast cells and basophils. Immunol Rev 282, 47–57. [DOI] [PubMed] [Google Scholar]
- Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, Liao M, Tsai MF, Feng L, 2020. Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex. Nature 582, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell DJ, al., e., 1995. Intrahepatic mast cells in chronic liver diseases. Hepatology 22, 1175–1181. [DOI] [PubMed] [Google Scholar]
- Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, Lenaz G, 2009. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta 1787, 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2020. Code of Federal Regulations 21. [Google Scholar]
- Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR, 2012. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med 52, 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, 2019. Safety and effectiveness of consumer antiseptic rubs; topical antimicrobial drug products for over-the-counter human use, in: F. Reg (Ed.), pp. 14847–14864. [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM, 2007. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 178, 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM, 2011. 2,4-dinitrophenol (DNP): a weight loss agent with significant acute toxicity and risk of death. J Med Toxicol 7, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri JW, Dybas JM, Fazelinia H, Kim MS, Frere J, Zhang Y, Soto Albrecht Y, Murdock DG, Angelin A, Singh LN, Weiss SL, Best SM, Lott MT, Zhang S, Cope H, Zaksas V, Saravia-Butler A, Meydan C, Foox J, Mozsary C, Bram Y, Kidane Y, Priebe W, Emmett MR, Meller R, Demharter S, Stentoft-Hansen V, Salvatore M, Galeano D, Enguita FJ, Grabham P, Trovao NS, Singh U, Haltom J, Heise MT, Moorman NJ, Baxter VK, Madden EA, Taft-Benz SA, Anderson EJ, Sanders WA, Dickmander RJ, Baylin SB, Wurtele ES, Moraes-Vieira PM, Taylor D, Mason CE, Schisler JC, Schwartz RE, Beheshti A, Wallace DC, 2023. Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts. Science Translational Medicine 15, eabq1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudheti MV, Curthoys NM, Gould TJ, Kim D, Gunewardene MS, Gabor KA, Gosse JA, Kim CH, Zimmerberg J, Hess ST, 2013. Actin mediates the nanoscale membrane organization of the clustered membrane protein influenza hemagglutinin. Biophys J 104, 2182–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein WG, 1976. Uncoupling of oxidative phosphorylation. Biochimica et biophysica acta 462, 129–148. [DOI] [PubMed] [Google Scholar]
- Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH, 2014. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc Natl Acad Sci U S A 111, 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K, Hakkarainen A, Lundbom J, Lundbom N, Vuolteenaho K, Moilanen E, Kaprio J, Rissanen A, Suomalainen A, Pietilainen KH, 2015. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes 64, 3135–3145. [DOI] [PubMed] [Google Scholar]
- Hess ST, Girirajan TP, Mason MD, 2006. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J 91, 4258–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess ST, Gould TJ, Gudheti MV, Maas SA, Mills KD, Zimmerberg J, 2007. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci U S A 104, 17370–17375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HHS, 2004. Rules and Regulations: Secondary Direct Food Additives Permitted in Food for Human Consumption, in: D.O.H.A.H.S.F.A.D. Administration (Ed.), 04–7399, pp. 17297–17298. [Google Scholar]
- Hrubec TC, Seguin RP, Xu L, Cortopassi GA, Datta S, Hanlon AL, Lozano AJ, McDonald VA, Healy CA, Anderson TC, Musse NA, Williams RT, 2021. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol Rep 8, 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Zhang X, Wang G, Zhang W, Cai Z, Pan BS, Gu H, Xu C, Jin G, Xu X, Manne RK, Jin Y, Yan W, Shao J, Chen T, Lin E, Ketkar A, Eoff R, Xu ZG, Chen ZZ, Li HY, Lin HK, 2021. Inositol serves as a natural inhibitor of mitochondrial fission by directly targeting AMPK. Mol Cell 81, 3803–3819 e3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson LM, Trinh BM, Palmer RK, Preziosi CA, Pelletier JH, Nelson HM, Gosse JA, 2011. Inorganic arsenite inhibits IgE receptor-mediated degranulation of mast cells. J Appl Toxicol 31, 231–241. [DOI] [PubMed] [Google Scholar]
- İlhak O, İncili GK, Durmuşoğlu H, 2018. Effect of some chemical decontaminants on the survival of Listeria monocytogenes and Salmonella Typhimurium with different attachment times on chicken drumstick and breast meat. Journal of food science and technology 55, 3093–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS, 2012. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol 32, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto EM, Vivar C, Camandola S, 2012. Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Yoo J, Lim YM, Kim EJ, Yoon BI, Kim P, Yu SD, Eom IC, Shim I, 2021. Comprehensive pulmonary toxicity assessment of cetylpyridinium chloride using A549 cells and Sprague-Dawley rats. J Appl Toxicol 41, 470–482. [DOI] [PubMed] [Google Scholar]
- Kirches E, 2011. LHON: Mitochondrial Mutations and More. Curr Genomics 12, 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen CDE, Casarett LJT, 2019. Casarett and Doull’s Toxicology. [Google Scholar]
- Koch-Heier J, Hoffmann H, Schindler M, Lussi A, Planz O, 2021. Inactivation of SARS-CoV-2 through Treatment with the Mouth Rinsing Solutions ViruProX((R)) and BacterX((R)) Pro. Microorganisms 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic M, Katoshevski T, Sekler I, 2018. Allosteric Regulation of NCLX by Mitochondrial Membrane Potential Links the Metabolic State and Ca(2+) Signaling in Mitochondria. Cell Rep 25, 3465–3475 e3464. [DOI] [PubMed] [Google Scholar]
- Krebs MR, Bromley EH, Rogers SS, Donald AM, 2005. The mechanism of amyloid spherulite formation by bovine insulin. Biophys J 88, 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN, Wood JG, 2015. Mast Cell: A Multi-Functional Master Cell. Front Immunol 6, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuby J, 1997. Immunology, 3rd ed. W.H. Freeman, San Francisco, CA. [Google Scholar]
- Latimer J, Munday JL, Buzza KM, Forbes S, Sreenivasan PK, McBain AJ, 2015. Antibacterial and anti-biofilm activity of mouthrinses containing cetylpyridinium chloride and sodium fluoride. BMC Microbiol 15, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Duron HE, Chaudhuri D, 2023. Beyond the TCA cycle: new insights into mitochondrial calcium regulation of oxidative phosphorylation. Biochem Soc Trans 51, 1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GH, Voss KA, Davidson TJ, 1991. Acute inhalation toxicity of cetylpyridinium chloride. Food Chem Toxicol 29, 851–854. [DOI] [PubMed] [Google Scholar]
- Liu R, Mu X, Gao R, Geng Y, Zhang Y, Chen X, Yin X, Wang H, Li F, He J, 2023. Maternal exposure to cetylpyridinium chloride impairs oogenesis by causing mitochondria disorder in neonates. Environ Toxicol Pharmacol 102, 104239. [DOI] [PubMed] [Google Scholar]
- Liu T, O’Rourke B, 2008. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res 103, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hajnoczky G, 2011. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ 18, 1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis WF, Lipmann F, 1948. Reversible inhibition of the coupling between phosphorylation and oxidation. J. Biol. Chem 173. [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, 1969. Phosphoglucomutase kinetics with the phosphates of fructose, glucose, mannose, ribose, and galactose. J Biol Chem 244, 910–916. [PubMed] [Google Scholar]
- MacKendrick N, 2018. Better Safe Than Sorry: How Consumers Navigate Exposure to Everyday Toxics. Univ. of California Press. [Google Scholar]
- Mandal AB, Nair BU, 2002. Cyclic voltammetric technique for the determination of the critical micelle concentration of surfactants, self-diffusion coefficient of micelles, and partition coefficient of an electrochemical probe. The Journal of Physical Chemistry 95, 9008–9013. [Google Scholar]
- Mao X, Auer DL, Buchalla W, Hiller K, Maisch T, Hellwig E, Al-Ahmad A, Cleplik F, 2020. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob Agents Chemother 64, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr FC, Fewtrell C, 1987. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol 104, 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau B, Parekh AB, 2008. Ca2+ -dependent inactivation of the mitochondrial Ca2+ uniporter involves proton flux through the ATP synthase. Curr Biol 18, 855–859. [DOI] [PubMed] [Google Scholar]
- Moss KJR, Mayo-Bean K, 2010. TSA NEW CHEMICALS PROGRAM (NCP) CHEMICAL CATEGORIES, in: O.o.P.P.a.T.U.S.E.P. Agency (Ed.). [Google Scholar]
- Mukherjee PK, Esper F, Buchheit K, Arters K, Adkins I, Ghannoum MA, Salata RA, 2017. Randomized, double-blind, placebo-controlled clinical trial to assess the safety and effectiveness of a novel dual-action oral topical formulation against upper respiratory infections. BMC Infect Dis 17, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhausen H, Mendicino J, 1970. A kinetic study of the effect of alpha-D-galactose, alpha-D-mannose, alpha-D-glucosamine, N-acetyl-alpha-D-glucosamine, and alpha-D-ribose diphosphate on the activity of phosphoglucomutase. J Biol Chem 245, 4038–4046. [PubMed] [Google Scholar]
- Muñoz-Basagoiti J, Perez-Zsolt D, León R, Blanc V, Raïch-Regué D, Cano-Sarabia M, Trinité B, Pradenas E, Blanco J, Gispert J, Clotet B, Izquierdo-Useros N, 2020. Cetylpyridinium chloride-containing mouthwashes reduce in vitro SARS- CoV-2 infectivity. BioRxiv. [DOI] [PubMed] [Google Scholar]
- Murphy MP, 2008. Targeting lipophilic cations to mitochondria. Biochim Biophys Acta 1777, 1028–1031. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Cox MM, 2017. Lehninger Principles of Biochemistry, 7th ed. W.h.freeman, Macmilan Learning. [Google Scholar]
- Obeng B, Potts CM, West BE, Burnell JE, Fleming PJ, Shim JK, Kinney MS, Ledue EL, Sangroula S, Baez Vazquez AY, Gosse JA, 2023. Pharmaceutical agent cetylpyridinium chloride inhibits immune mast cell function by interfering with calcium mobilization. Food Chem Toxicol 179, 113980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I, 2004. Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem 279, 25234–25240. [DOI] [PubMed] [Google Scholar]
- Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I, 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci U S A 107, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT, 2011. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6, e18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccardi M, 2004. Background subtraction techniques: a review*. IEEE International Conference on Systems, Man and Cybernetics, 3099–3104. [Google Scholar]
- Plecita-Hlavata L, Lessard M, Santorova J, Bewersdorf J, Jezek P, 2008. Mitochondrial oxidative phosphorylation and energetic status are reflected by morphology of mitochondrial network in INS-1E and HEP-G2 cells viewed by 4Pi microscopy. Biochim Biophys Acta 1777, 834–846. [DOI] [PubMed] [Google Scholar]
- Plikus MV, Wang X, Sinha S, Forte E, Thompson SM, Herzog EL, Driskell RR, Rosenthal N, Biernaskie J, Horsley V, 2021. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 184, 3852–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin DL, Zilka S, Dimaano M, Fujioka H, Rackley C, Salata R, Griffith A, Mukherjee PK, Ghannoum MA, Esper F, 2017. Cetylpyridinium Chloride (CPC) Exhibits Potent, Rapid Activity Against Influenza Viruses in vitro and in vivo. Pathog Immun 2, 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottel J, Armstrong D, Zou L, Fekete A, Huang XP, Torosyan H, Bednarczyk D, Whitebread S, Bhhatarai B, Liang G, Jin H, Ghaemi SN, Slocum S, Lukacs KV, Irwin JJ, Berg EL, Giacomini KM, Roth BL, Shoichet BK, Urban L, 2020. The activities of drug inactive ingredients on biological targets. Science 369, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo Devoto VM, Falzone TL, 2017. Mitochondrial dynamics in Parkinson’s disease: a role for alpha-synuclein? Dis Model Mech 10, 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PubChem, 2023. PubChem Compound Summary for CID 31239, Cetylpyridinium Chloride. National Center for Biotechnology Information, https://pubchem.ncbi.nlm.nih.gov/compound/Cetylpyridinium-Chloride (accessed 6 November 2023).
- Raut P, Weller SR, Obeng B, Soos BL, West BE, Potts CM, Sangroula S, Kinney MS, Burnell JE, King BL, Gosse JA, Hess ST, 2022. Cetylpyridinium chloride (CPC) reduces zebrafish mortality from influenza infection: Super-resolution microscopy reveals CPC interference with multiple protein interactions with phosphatidylinositol 4,5-bisphosphate in immune function. Toxicol Appl Pharmacol 440, 115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson A, Pollington S, Walsh TF, Lamb DJ, Marlow I, Haywood J, Wright P, 2008. Efficacy of two alcohol-free cetylpyridinium chloride mouthwashes - a randomized double-blind crossover study. J Clin Periodontol 35, 230–235. [DOI] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM, Kennell D, 1979. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. Journal of Biological Chemistry 254, 2669–2676. [PubMed] [Google Scholar]
- Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, Capaldi RA, 2004. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64, 985–993. [DOI] [PubMed] [Google Scholar]
- Saladino AJ, Hawkins HK, Trump BF, 1971. Ion Movements in Cell Injury Efects of the Cationic Detergent Cetyl Pyridinium Chloride on the Ultrastructure and Function of the ToadBladder. Am J Pathol 64, 271–294. [PMC free article] [PubMed] [Google Scholar]
- Sánchez Barrueco Á, Mateos-Moreno MV, Martínez-Beneyto Y, García-Vázquez E, Campos González A, Zapardiel Ferrero J, Bogoya Castaño A, Alcalá Rueda I, Villacampa Aubá JM, Cenjor Español C, Moreno-Parrado L, Ausina-Márquez V, García-Esteban S, Artacho A, López-Labrador FX, Mira A, Ferrer MD, 2022. Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial. Emerging Microbes & Infections 11, 1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer LM, Szewczyk G, Nesta J, Vandeven M, Du-Thumm L, Williams MI, Arvanitidou E, 2011. In vitro antibacterial efficacy of cetylpyridinium chloride-containing mouthwashes. J Clin Dent 22, 183–186. [PubMed] [Google Scholar]
- Schwarz K, Siddiqi N, Singh S, Neil CJ, Dawson DK, Frenneaux MP, 2014. The breathing heart - mitochondrial respiratory chain dysfunction in cardiac disease. Int J Cardiol 171, 134–143. [DOI] [PubMed] [Google Scholar]
- Seo HW, Seo JP, Cho Y, Ko E, Kim YJ, Jung G, 2019. Cetylpyridinium chloride interaction with the hepatitis B virus core protein inhibits capsid assembly. Virus Res 263, 102–111. [DOI] [PubMed] [Google Scholar]
- Shahinas D, Debnath A, Benedict C, McKerrow JH, Pillai DR, 2015. Heat shock protein 90 inhibitors repurposed against Entamoeba histolytica. Front Microbiol 6, 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Luo HQ, Li NB, 2011. Determination of the critical premicelle concentration, first critical micelle concentration and second critical micelle concentration of surfactants by resonance Rayleigh scattering method without any probe. Spectrochim Acta A Mol Biomol Spectrosc 78, 1403–1407. [DOI] [PubMed] [Google Scholar]
- Silver R, Curley JP, 2013. Mast cells on the mind: new insights and opportunities. Trends Neurosci 36, 513–521. [DOI] [PubMed] [Google Scholar]
- Sreenivasan PK, Haraszthy VI, Zambon JJ, 2013. Antimicrobial efficacy of 0.05% cetylpyridinium chloride mouthrinses. Lett Appl Microbiol 56, 14–20. [DOI] [PubMed] [Google Scholar]
- Steyer A, Marušić M, Kolenc M, Triglav T, 2021. A Throat Lozenge with Fixed Combination of Cetylpyridinium Chloride and Benzydamine Hydrochloride Has Direct Virucidal Effect on SARS-CoV-2. Covid 1, 435–446. [Google Scholar]
- Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M, 2014. Imaging intraorganellar Ca(2+) at subcellular resolution using CEPIA. Nat Commun 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle EJ, Metcalfe DD, Coleman JW, 2004. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol Chem 279, 48751–48759. [DOI] [PubMed] [Google Scholar]
- Teng F, He T, Huang S, Bo CP, Li Z, Chang JL, Liu JQ, Charbonneau D, Xu J, Li R, Ling JQ, 2016. Cetylpyridinium chloride mouth rinses alleviate experimental gingivitis by inhibiting dental plaque maturation. Int J Oral Sci 8, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D, 2012. Mast cells and inflammation. Biochim Biophys Acta 1822, 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR, 1991. Bladder mast cell activation in interstitial cystitis. Seminars in urology 9, 74–87. [PubMed] [Google Scholar]
- Theoharides TC, Stewart JM, Panagiotidou S, Melamed I, 2016. Mast cells, brain inflammation and autism. Eur J Pharmacol 778, 96–102. [DOI] [PubMed] [Google Scholar]
- Thrasher SM, Scalfone LK, Holowka D, Appleton JA, 2013. In vitro modelling of rat mucosal mast cell function in Trichinella spiralis infection. Parasite Immunol 35, 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomino M, Nagano K, Hayashi T, Kuroki K, Kawai T, 2016. Antimicrobial efficacy of gutta-percha supplemented with cetylpyridinium chloride. J Oral Sci 58, 277–282. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen MP, Rosema NA, Versteeg PA, Slot DE, Van Winkelhoff AJ, Van der Weijden GA, 2015. Long-term efficacy of a 0.07% cetylpyridinium chloride mouth rinse in relation to plaque and gingivitis: a 6-month randomized, vehicle-controlled clinical trial. Int J Dent Hyg 13, 93–103. [DOI] [PubMed] [Google Scholar]
- Varade D, Joshi T, Aswal VK, Goyal PS, Hassan PA, Bahadur P, 2005. Effect of salt on the micelles of cetyl pyridinium chloride. Colloids and Surfaces A: Physicochemical and Engineering Aspects 259, 95–101. [Google Scholar]
- Vig M, Kinet JP, 2009. Calcium signaling in immune cells. Nat Immunol 10, 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutchkova AM, Ferris LA, Zimmerman JB, Anastas PT, 2010. Toward molecular design for hazard reduction – fundamental relationships between chemical properties and toxicity. 66, 1031–1039. [Google Scholar]
- Wang H, Li Y, Slavik MF, 2001. Efficacy of cetylpyridinium chloride in immersion treatment for reducing populations of pathogenic bacteria on fresh-cut vegetables. Journal of Food Protection 64, 2071–2074. [DOI] [PubMed] [Google Scholar]
- Weatherly LM, Gosse JA, 2017. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev 20, 447–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Kennedy RH, Shim J, Gosse JA, 2013. A microplate assay to assess chemical effects on RBL-2H3 mast cell degranulation: effects of triclosan without use of an organic solvent. J Vis Exp, e50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Nelson AJ, Shim J, Riitano AM, Gerson ED, Hart AJ, de Juan-Sanz J, Ryan TA, Sher R, Hess ST, Gosse JA, 2018. Antimicrobial agent triclosan disrupts mitochondrial structure, revealed by super-resolution microscopy, and inhibits mast cell signaling via calcium modulation. Toxicol Appl Pharmacol 349, 39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly LM, Shim J, Hashmi HN, Kennedy RH, Hess ST, Gosse JA, 2016. Antimicrobial agent triclosan is a proton ionophore uncoupler of mitochondria in living rat and human mast cells and in primary human keratinocytes. J Appl Toxicol 36, 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf KJ, 2016. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use, in: F. Reg (Ed.), pp. 61106–61130. [PubMed] [Google Scholar]
- Woods JJ, Nemani N, Shanmughapriya S, Kumar A, Zhang M, Nathan SR, Thomas M, Carvalho E, Ramachandran K, Srikantan S, Stathopulos PB, Wilson JJ, Madesh M, 2019. A Selective and Cell-Permeable Mitochondrial Calcium Uniporter (MCU) Inhibitor Preserves Mitochondrial Bioenergetics after Hypoxia/Reoxygenation Injury. ACS Cent Sci 5, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaegaki K, Sanada K, 1992. Biochemical and clinical factors influencing oral malodor in periodontal patients. J Periodontol 63, 783–789. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yoshihara K, Nagaoka N, Van Meerbeek B, Yoshida Y, 2022. Novel composite cement containing the anti-microbial compound CPC-Montmorillonite. Dent Mater 38, 33–43. [DOI] [PubMed] [Google Scholar]
- Yan MH, Wang X, Zhu X, 2013. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying WL, Emerson J, Clarke MJ, Sanadi DR, 1991. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry 30, 4949–4952. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Van Der Bliek AM, 2012. Mitochondrial fission, fusion, and stress. Science 337, 1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, Brooks EG, Watson CS, Goldblum RM, Midoro-Horiuti T, 2007. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol 44, 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Chen L, Noh ALSM, Yim M, 2013. Cetylpyridinium Chloride Inhibits Receptor Activator of Nuclear Factor-κB Ligand-Induced Osteoclast Formation. Biol. Pharm. Bull. 36, 509–514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.