Abstract
Purpose:
BCI (H/I) has been shown to predict extended endocrine therapy (EET) benefit. We examined BCI (H/I) for EET benefit prediction in NSABP B-42, which evaluated extended letrozole therapy (ELT) in patients with hormone receptor-positive breast cancer after 5 years of ET.
Experimental Design:
A stratified Cox model was used to analyze RFI as the primary endpoint, with DR, BCFI, and DFS as secondary endpoints. Because of a nonproportional effect of ELT on DR, time-dependent analyses were performed.
Results:
The translational cohort included 2,178 patients (45% BCI (H/I)-High, 55% BCI (H/I)-Low). ELT showed an absolute 10-year RFI benefit of 1.6% (P = 0.10), resulting in an underpowered primary analysis (50% power). ELT benefit and BCI (H/I) did not show a significant interaction for RFI (BCI (H/I)-Low: 10 years absolute benefit 1.1% [HR, 0.70; 95% confidence interval (CI), 0.43–1.12; P = 0.13]; BCI (H/I)-High: 2.4% [HR, 0.83; 95% CI, 0.55–1.26; P = 0.38]; Pinteraction = 0.56). Time-dependent DR analysis showed that after 4 years, BCI (H/I)-High patients had significant ELT benefit (HR = 0.29; 95% CI, 0.12–0.69; P < 0.01), whereas BCI (H/I)-Low patients were less likely to benefit (HR, 0.68; 95% CI, 0.33–1.39; P = 0.29; Pinteraction = 0.14). Prediction of ELT benefit by BCI (H/I) was more apparent in the HER2- subset after 4 years (ELT-by-BCI (H/I) Pinteraction = 0.04).
Conclusions:
BCI (H/I)-High versus BCI (H/I)-Low did not show a statistically significant difference in ELT benefit for the primary endpoint (RFI). However, in time-dependent DR analysis, BCI (H/I)-High patients experienced statistically significant benefit from ELT after 4 years, whereas (H/I)-Low patients did not. Because BCI (H/I) has been validated as a predictive marker of EET benefit in other trials, additional follow-up may enable further characterization of BCI's predictive ability.
Translational Relevance.
Patients with hormone receptor-positive breast cancer remain at risk of recurrence well after the first 5 years from diagnosis. Extended endocrine therapy (EET) beyond 5 years modestly reduces that risk. There is need to identify biomarkers that predict risk of late recurrence and benefit from EET. The Breast Cancer Index (BCI) predicts risk of late recurrence and its component BCI (H/I) also predicts benefit from EET in patients with ER+, early-stage breast cancer. We evaluated BCI (H/I) in the NSABP B-42 trial, which evaluated extended letrozole therapy (ELT) in postmenopausal women with HR+ early-stage breast cancer. There was no statistically significant difference in ELT benefit by BCI (H/I) for the primary endpoint of recurrence-free interval. In time-dependent analysis for distant recurrence, BCI (H/I)-High patients experienced significant benefit from ELT after 4 years, but BCI (H/I)-Low patients did not. Additional follow-up may enable further characterization of BCI's predictive ability in B-42.
Introduction
Patients with hormone receptor-positive (HR+) breast cancer are at considerable risk of recurrence well after the first 5 years from diagnosis (1, 2). Extension of adjuvant endocrine therapy (ET) beyond 5 years has been shown to mitigate this enduring risk of late recurrence (3–11). Meaningful improvements in disease-free survival (DFS) have been achieved with extending adjuvant tamoxifen to 10 years compared with 5 years (3, 4) and with extending ET with an aromatase inhibitor (AI) after initial therapy with either tamoxifen, an AI, or the sequence of the two (5–11). However, no overall survival benefit has been observed with extended ET, except in the ATLAS trial, in which extending adjuvant tamoxifen to 10 years versus 5 years significantly improved breast cancer mortality and overall survival (3).
Randomized trials of ET have reported modest relative and absolute improvements in DFS from the extended ET in the range of 2% to 5% absolute risk reduction (3–11), prompting the need to identify subsets of patients who may receive preferential benefit from ET. Prognostic factors including clinicopathologic variables and algorithms (12), circulating tumor cells (13), as well as several commercially available genomic assays (14–17), have been used to aid extended ET decision making.
The Breast Cancer Index (BCI) is a gene expression-based signature with two components: the HOXB13/IL17BR (H/I) ratio and the molecular grade index (MGI), which interrogate estrogen signaling and proliferation pathways in breast cancer, respectively. The predictive component of BCI (BCI (H/I)) is based on the H/I ratio and has been shown to predict the likelihood of benefit from extended ET across various treatment regimens (18–21). The BCI prognostic score is an algorithmic combination of both components, which reports the individualized risk of overall (0–10 year) and late (post-5-year) distant recurrence (DR; refs. 17, 22, 23). BCI has recently been incorporated into both National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO) clinical practice guidelines as a biomarker for prediction of benefit from extended ET in patients with early-stage, HR+ breast cancer (24, 25).
In the NSABP B-42 parent trial, 3,966 postmenopausal women with HR+ early-stage breast cancer who were disease-free after 5 years of hormonal therapy (with an AI or ≥3 years of tamoxifen followed by an AI) were randomized to receive 5 additional years of either letrozole or placebo (11). Initial results of the trial after 6.9 years of median follow-up showed that extended letrozole therapy (ELT) improved DFS by 3.4% (HR, 0.85, 95% confidence interval [CI], 0.73–0.99) with a P value of 0.048, which did not reach the pre-specified significance level due to pre-planned interim analyses. Updated results after 10 years indicated a similar degree of benefit favoring ELT (absolute difference in DFS 3.3%: HR, 0.85; 95% CI, 0.74–0.96; P value = 0.01; ref. 26). Although a significant improvement of DFS was noted with extended AI therapy, the need to improve patient selection in this setting is evident.
The B-42 trial provides a suitable cohort to determine if BCI (H/I) can identify a subset of postmenopausal HR+ patients with breast cancer who may benefit from extending adjuvant ET with an AI, while sparing patients without benefit from the unnecessary prolonged ET that is often associated with significant side effects and toxicities. In this study, BCI (H/I) was evaluated as a predictor of benefit from ELT in the NSABP B-42 trial.
Materials and Methods
Patient population and translational study design
NSABP B-42 was a prospective phase III trial that enrolled 3,966 postmenopausal women with HR+ early-stage breast cancer who were disease-free after 5 years of ET (with an AI or ≥3 years of tamoxifen followed by an AI), then randomized patients to receive 5 additional years of either letrozole or placebo.
This study is a prospective-retrospective translational study of patients enrolled in the B-42 trial with the objective of validating the predictive performance of BCI (H/I) in the extended endocrine setting. The B-42 trial was approved by local human investigations committees/institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services. Written informed consent was required.
All eligible B-42 patients with clinical follow-up, appropriate consent, and available formalin-fixed paraffin-embedded (FFPE) primary tumor tissues were included in the study. Exclusion criteria included lack of invasive tumor, incorrect tumor specimen, and insufficient or poor RNA amplification. Gene expression analysis of FFPE tumor-derived RNA was performed as described (Biotheranostics Inc., A Hologic Company), blinded to clinical outcome (22). To unblind, BCI testing data were sent to the NSABP for merging with the clinical data prior to final analysis.
BCI molecular testing
BCI gene expression analysis by RT-PCR was conducted on FFPE primary tumor specimens in a CLIA-certified, CAP-accredited laboratory (Biotheranostics), blinded to clinical outcome as reported previously (22). Briefly, macro-dissection was performed on FFPE sections to enrich for tumor content before RNA extraction. Total RNA was reverse transcribed, and the resulting cDNA was pre-amplified by PCR using the PreAmp Master Mix Kit (Thermo Fisher Scientific) before TaqMan PCR analysis. Calculation of BCI (H/I) was carried out as described previously (18, 22, 27). Briefly, BCI (H/I) was calculated as the difference in reference genes normalized PCR cycle threshold (CT) values between HOXB13 and IL17BR genes, with a prespecified cut-point that was previously determined to optimally separate recurrence cases from non-recurrent cases in a case–control study. BCI (H/I) was normalized into a range between 0 and 10.
Statistical considerations
The primary objective of this study was to demonstrate whether BCI (H/I) status (High vs. Low) is predictive of benefit from 5 years of ELT in patients who comprised the translational cohort of the B-42 trial. It was hypothesized that patients with BCI (H/I)-High tumors will show a statistically significant benefit from ELT, whereas patients with BCI (H/I)-Low tumors will not derive benefit. Secondary objectives were to evaluate the predictive performance of BCI (H/I) status in clinical subsets defined by nodal status, HER2 status, and type of initial ET.
The primary endpoint for the study was recurrence-free interval (RFI), defined as the time from randomization to local, regional, or distant breast cancer recurrence. Predefined secondary endpoints included DR, defined as the time from randomization to DR; DFS, defined as the time from randomization to breast cancer recurrence, second primary cancer, or death; and breast cancer-free interval (BCFI), defined as the time from randomization to breast cancer recurrence or contralateral breast cancer as a first event. Patients who were otherwise event free were censored at the date of last clinical follow-up. In addition, for BCFI, other second primary cancers and death without evidence of recurrence were treated as censored events.
To determine whether the translational cohort was representative of the parent B-42 trial population, distributions of key patient and tumor characteristics were compared by chi-square tests. In addition, the treatment-specific patterns of outcome for RFI were compared between the translational cohort and the excluded B-42 population.
Differences in primary and secondary endpoints between ELT and placebo groups were assessed by the stratified log-rank tests controlling for the stratification variables of the parent trial (pathologic node status, prior use of tamoxifen as a component of initial adjuvant therapy, and lowest bone mineral density T score in the lumbosacral spine, total hip, or femoral neck). HRs and corresponding 95% confidence intervals were calculated on the basis of the stratified Cox proportional hazards model. Likelihood-ratio test, which compared a full Cox model with treatment by biomarker interaction versus a reduced model without the interaction, was used to evaluate treatment by BCI (H/I) interaction. Kaplan–Meier estimates were used for illustration purposes, with absolute benefit defined as the differences in 10-year Kaplan–Meier estimates of risk of event between ELT and placebo treatments. The assumption of proportionality of hazards between the treatment groups was tested for all endpoints. The violation of the assumption justified the post hoc time-dependent secondary analyses. All statistical analyses, including those of clinically relevant subgroups, were pre-specified in a statistical analysis plan (SAP). All P values were two-sided and not adjusted for multiple comparisons as per the SAP. Analyses were performed in SAS with the clinical cutoff date of April 30, 2020, with a median follow-up of 10.3 years (IQR, 9.6–11.1 years).
Data availability/sharing statement
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within one year after publication and will be accessible through the NCTN Data Archive. Please contact the corresponding author for the protocol, access to data prior to NCTN availability, or other information.
Results
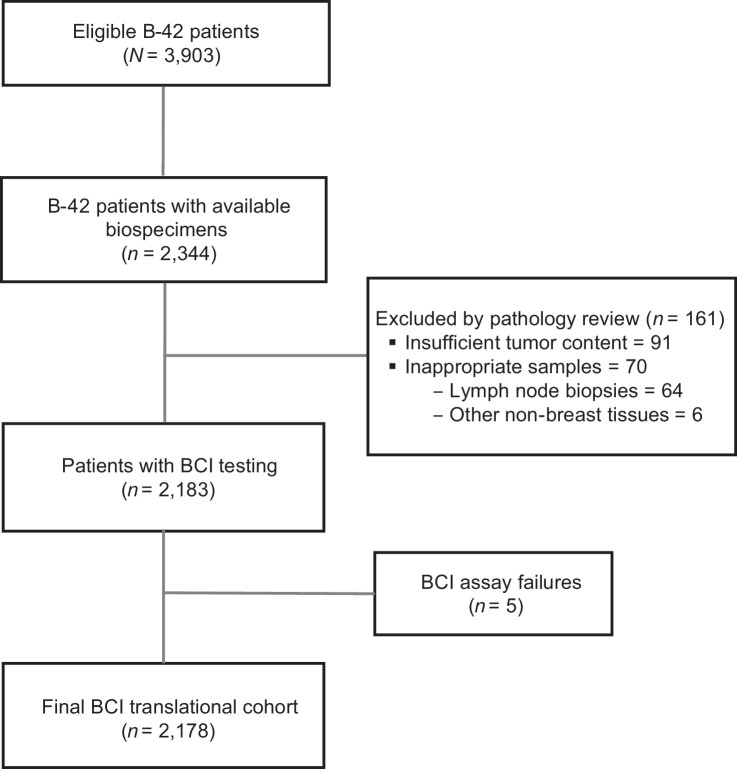
Among 3,903 eligible patients with follow-up, 2,344 patients had consented for future research and had tumor blocks available. Of those, 161 patients were excluded following pathology review because of insufficient tumor content (n = 91) or inappropriate samples (n = 70). Five samples failed the BCI assay resulting in a final translational BCI cohort of 2,178 patients, representing 56% of the parent B-42 trial (Fig. 1). In this cohort, 1,307 (60%) were node negative (N0) patients, 297 (13.6%) were HER2+, 1,342 (61.6%) received only AI as prior ET, and 836 (38.4%) received tamoxifen followed by AI sequential therapy prior to randomization (Table 1; Supplementary Table S1).
Figure 1.
Modified REMARK diagram. The diagram shows tumor block collection, specimen processing, and molecular testing, leading to a final analyzable BCI NSABP B-42 translational cohort of 2,178 patients.
Table 1.
Clinicopathologic and treatment characteristics of patients in the translational cohort versus remaining NSABP B-42 patients.
| Characteristics | Translational B-42 cohort N = 2,178 (%) | Remaining B-42 patients N = 1,725 (%) | P valuea |
|---|---|---|---|
| Age at randomization | 0.05 | ||
| <55 years | 320 (14.7) | 284 (16.5) | |
| 55–65 years | 984 (45.2) | 801 (46.4) | |
| 66–75 years | 662 (30.4) | 510 (29.6) | |
| >75 years | 212 (9.7) | 130 (7.5) | |
| Nodal status | <0.01 | ||
| Negative | 1,307 (60.0) | 933 (54.1) | |
| Positive | 871 (40.0) | 792 (45.9) | |
| Lowest BMD T-score | 1.00 | ||
| ≤−2.0 | 532 (24.4) | 422 (24.5) | |
| >−2.0 | 1,646 (75.6) | 1,303 (75.5) | |
| Prior tamoxifen | 0.32 | ||
| No | 1,342 (61.6) | 1,035 (60.0) | |
| Yes | 836 (38.4) | 690 (40.0) | |
| HER2 status | 0.01 | ||
| Positive | 297 (13.6) | 262 (15.2) | |
| Negative | 1,732 (79.5) | 1,309 (75.9) | |
| Unknown | 149 (6.8) | 154 (8.9) | |
| Surgery type | 0.36 | ||
| Lumpectomy | 1,339 (61.5) | 1,035 (60.0) | |
| Mastectomy | 839 (38.5) | 690 (40.0) | |
| Treatment | 1.00 | ||
| Placebo | 1,090 (50.0) | 863 (50.0) | |
| Letrozole | 1,088 (50.0) | 862 (50.0) |
Abbreviation: BMD, bone mineral density.
a P values comparing the translational cohort versus the remaining B-42 patients were calculated using Chi-square test.
In general, the clinicopathologic and treatment characteristics were similar between the BCI cohort and the remaining B-42 patients, except for pathological nodal status and HER2 status, in which the BCI cohort had a slightly lower proportion of node-positive and higher proportion of HER2-negative patients than the remaining B-42 population (Table 1). When comparing the BCI cohort to the excluded B-42 population, there was no statistically significant difference in the rate of RFI or in the ELT effect on RFI.
Among 2,178 patients in the BCI cohort, 971 (44.6%) were identified as BCI (H/I)-High and 1,207 (55.4%) as BCI (H/I)-Low. The distribution of RFI and of the secondary endpoints of DR, DFS, and BCFI, is summarized in Supplementary Table S2.
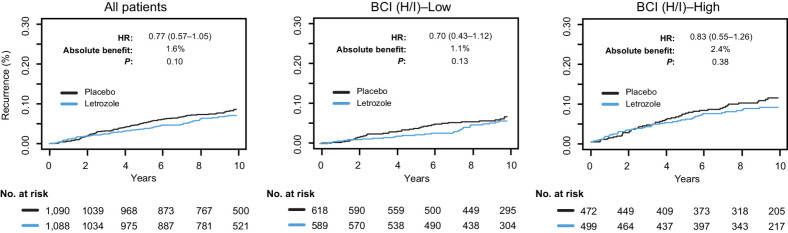
In the overall translational BCI cohort, ELT showed an absolute 10-year benefit of 1.6% for the primary RFI endpoint (HR, 0.77; 95% CI, 0.57–1.05; P = 0.10), resulting in 50% power for the primary analysis. There was no statistically significant ELT by BCI (H/I) interaction (Pinteraction = 0.56). The 10-year benefit of ELT in BCI (H/I)-Low was 1.1% (HR, 0.70; 95% CI, 0.43–1.12; P = 0.13) and in BCI (H/I)-High was 2.4% (HR, 0.83; 95% CI, 0.55–1.26; P = 0.38; Fig. 2). The results were similar when the predictive performance of BCI (H/I) status on RFI was investigated in independent subsets of patients by nodal status, HER2 status, and the type of initial ET. Similar findings were observed for the secondary endpoints of BCFI, DR, and DFS as for the primary endpoint (Supplementary Table S3).
Figure 2.
Kaplan–Meier analysis based on RFI comparing extended letrozole versus placebo in the overall BCI NSABP B-42 translational cohort and according to BCI (H/I) status.
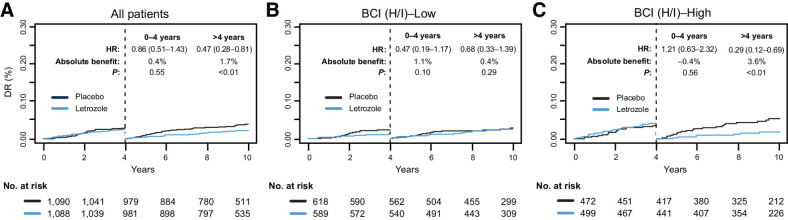
In the parent B-42 trial, the proportional hazards assumption between ELT and placebo groups was not satisfied for DR and a delayed treatment effect of ELT on DR was observed at approximately year 4 after randomization. The proportional hazards assumption between ELT and placebo groups was also not satisfied for DR for the BCI (H/I)-High group (P = 0.016). As a result, time-dependent secondary analyses for DR were performed by splitting the follow-up time into two intervals (prior to 4 years and after 4 years from randomization) per SAP. The proportional hazards assumption was satisfied on each of those two intervals. Before 4 years, there was no statistically significant ELT benefit in either BCI (H/I) group (Pinteraction = 0.09; Fig. 3A). After 4 years, BCI (H/I)-High patients had statistically significant ELT benefit (HR, 0.29; 95% CI, 0.12–0.69; P < 0.01), whereas BCI (H/I)-Low patients were less likely to benefit (HR, 0.68; 95% CI, 0.33–1.39; P = 0.29; Pinteraction = 0.14; Fig. 3B and C).
Figure 3.
Time-dependent Kaplan–Meier analysis based on DR comparing extended letrozole versus placebo in the overall BCI NSABP B-42 translational cohort for (A) all patients, (B) BCI (H/I)-Low, and (C) BCI (H/I)-High.
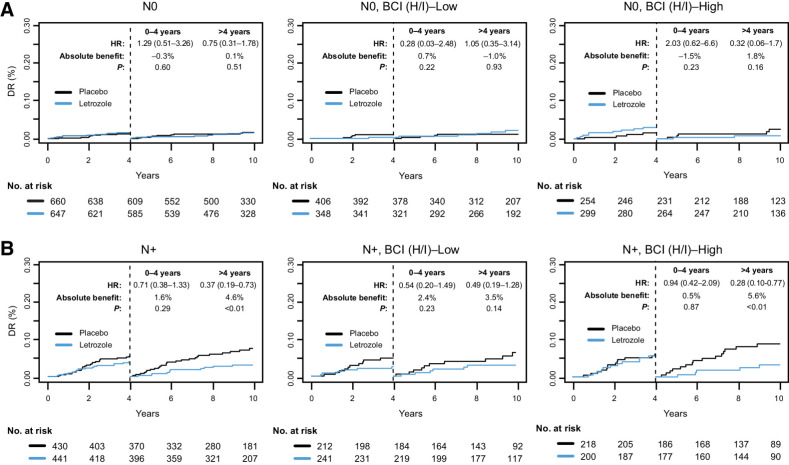
The time-dependent predictive performance of BCI (H/I) on DR was investigated in clinical patient subsets based on nodal status, HER2 status, and type of initial ET. In the first 4 years after randomization, no significant ELT benefit was observed in either the BCI (H/I)-Low or BCI (H/I)-High group (Figs. 4 and 5; Supplementary Fig. S1). However, in the period after 4 years following randomization, the BCI (H/I)-High group showed a substantially larger benefit from ELT than the BCI (H/I)-Low group. In the N0 subset, the BCI (H/I)-High group derived a nonsignificant 10-year absolute ELT benefit of 1.8%, conditional on remaining DR-free by year 4 (HR, 0.32; 95% CI, 0.06–1.68; P = 0.16), whereas no benefit was seen in BCI (H/I)-Low (absolute benefit: −1.0%; HR, 1.05; 95% CI, 0.35–3.14; P = 0.93; Fig. 4A). In the N+ subset, BCI (H/I)-High patients derived a significant absolute ELT benefit of 5.6% (HR, 0.28; 95% CI, 0.10–0.77; P < 0.01), whereas the benefit observed in the BCI (H/I)-Low patients was not statistically significant (absolute benefit: 3.5%; HR, 0.49; 95% CI, 0.19–1.28; P = 0.14; Fig. 4B). For those receiving only AI as their initial ET, the absolute benefit of ELT was more apparent, although not statistically significant for BCI (H/I)-High (absolute benefit: 3.2%; HR, 0.42; 95% CI, 0.15–1.19; P = 0.09) compared with BCI (H/I)-Low (absolute benefit: 0.5%; HR, 0.70; 95% CI, 0.29–1.68; P = 0.42; Supplementary Fig. S1A). Conversely, for those receiving tamoxifen as part of their initial ET, BCI (H/I)-High showed a statistically significant absolute benefit of 4.3% (HR, 0.16; 95% CI, 0.04–0.76; P < 0.01), whereas BCI (H/I)-Low derived no benefit (absolute benefit: 0.1%; HR, 0.63; 95% CI, 0.18–2.24; P = 0.47; Supplementary Fig. S1B). ELT-by-BCI (H/I) interaction terms were not statistically significant in the clinical subsets defined by either nodal status or by prior tamoxifen use.
Figure 4.
Time-dependent Kaplan–Meier analysis based on DR comparing extended letrozole verus placebo in subsets of patients with (A) node-negative (N0) or (B) node-positive (N+) disease in the overall BCI NSABP B-42 translational cohort and according to BCI (H/I) status.
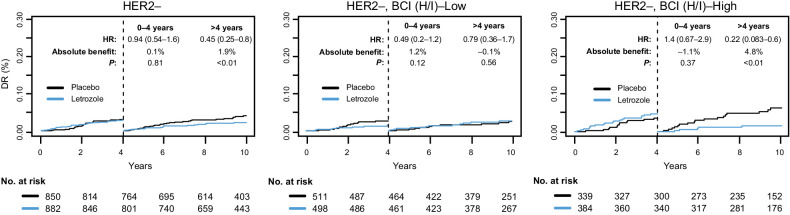
Figure 5.
Time-dependent Kaplan–Meier analysis based on DR comparing extended letrozole versus placebo in the patients with HER2– disease in the overall BCI NSABP B-42 translational cohort and according to BCI (H/I) status.
The limited number of HER2+ patients precluded a meaningful analysis of the ELT effect in that subgroup. For HER2− patients, there was no statistically significant difference in the ELT effect on DR between BCI (H/I)-High and BCI (H/I)-Low subgroups up to year 4 from randomization (Pinteraction = 0.07; Fig. 5). A statistically significant ELT-by-BCI (H/I) interaction was observed after 4 years (Pinteraction = 0.04). The 10-year absolute benefit of ELT, conditional on remaining DR-free by year 4, was 4.8% (HR, 0.22; 95% CI, 0.08–0.60; P < 0.01) for BCI (H/I)-High and −0.1% (HR, 0.79; 95% CI, 0.36–1.74; P = 0.56) for BCI (H/I)-Low (Fig. 5).
Discussion
BCI (H/I) has been previously validated in several prospective-retrospective studies to predict the likelihood of benefit from extended ET (Supplementary Table S4; refs. 18–20). In a nested case–control study within the NCIC MA.17 trial that evaluated ELT after 5 years of tamoxifen, expression of BCI (H/I) was evaluated for prediction of ELT benefit (18). High BCI (H/I) was significantly associated with a decrease in late recurrence in patients receiving ELT (OR, 0.35; 95% CI, 0.16–0.75; P = 0.007) in the univariable setting and in a model adjusted for standard clinicopathologic factors (OR, 0.33; 95% CI, 0.15–0.73; P = 0.006). Reduction in the absolute risk of recurrence at 5 years was 16.5% for patients with BCI (H/I)-High (P = 0.007). The interaction between BCI (H/I) and ELT was significant (Pinteraction = 0.03). The predictive ability of BCI (H/I) for extended ET with tamoxifen was further evaluated in 789 HR+, node-positive patients previously randomized in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial (19, 21). In that study, BCI (H/I)-High patients derived significant benefit from 10 years versus 5 years of tamoxifen treatment (HR, 0.33; 95% CI, 0.14–0.75; 9.7% absolute 12-year risk reduction based on RFI, P = 0.016). In contrast, BCI (H/I)-Low patients derived no benefit from extended ET (HR, 1.11; 95% CI, 0.76–1.64; −1.2% absolute 12-year risk reduction; P = 0.581). The predictive effect was also demonstrated in a significant treatment by biomarker interaction (Pinteraction = 0.037). Finally, further validation of BCI (H/I) as a predictive marker from extended ET was provided in the IDEAL trial (20). In that trial, BCI (H/I) was tested in primary tumor specimens from 908 patients randomized to receive 2.5 years versus 5 years of ELT after 5 years of ET with tamoxifen, an AI, or the sequence of the two. BCI (H/I)-High status predicted the benefit from longer ELT in the overall cohort (HR, 0.42; 95% CI, 0.21–0.84; P = 0.011) and in the prior AI-treated subset (HR, 0.34; 95% CI, 0.16–0.73; P = 0.004), whereas BCI (H/I)-Low patients did not derive significant benefit either in the overall cohort (HR, 0.95; 95% CI, 0.58–1.56; P = 0.84) or in the AI-treated subset (HR, 0.90; 95% CI, 0.53–1.55; P = 0.71; Pinteraction = 0.045 and Pinteraction = 0.025, respectively). These findings show the predictive ability of BCI (H/I) across multiple ET treatment modalities.
Overall BCI results in the B-42 translational cohort were inconsistent with previous findings regarding the predictive performance of BCI (H/I) on the ELT benefit on risk of recurrence. However, the time-dependent analysis of DR confirmed the BCI (H/I) predictive ability of ELT benefit for the period after 4 years following randomization with the statistically significant benefit in the BCI (H/I)-High group. The benefit was primarily observed in node-positive patients, in patients who received prior tamoxifen, and in patients with HER2- tumors. In the HER2- subset, significant ELT by BCI (H/I) interaction was demonstrated. The time-dependent secondary analyses for DR (before and after 4 years following randomization) were statistically warranted because the proportional hazards assumption between the treatment groups was not satisfied for DR in the BCI (H/I)-High group; Also, in the parent B-42 trial, a delayed treatment effect of ELT on DR was observed after approximately year 4 after randomization. A delayed treatment effect has been reported in other extended ET trials. For example, both the aTTom and ATLAS trials showed that recurrence risk reduction was time-dependent, with essentially no benefit seen with 10-year tamoxifen in years 5–9, followed by significant improvement in year 10 and onward (3, 28). The delayed treatment effect of extended ET might be attributed to a carry-over effect of the initial ET. In the Trans-aTTom study, statistical techniques that take the delayed treatment effect of primary adjuvant therapy into consideration were also adopted.
Of interest, compared to other extended ET studies, the treatment effect of ELT in BCFI observed in the B-42 trial was based to a large degree on a reduction in contralateral breast cancer (58% in B-42 vs. ∼20% in other extended ET studies, Supplementary Table S5), which could explain in part the lack of ELT benefit for DFS and BCFI in the BCI (H/I)-High group (Supplementary Fig. S2).
When the results of the B-42 BCI translational study are considered together with the results from the studies reviewed above, which evaluated the predictive ability of BCI (H/I) for extended ET benefit, the preponderance of evidence shows that BCI has demonstrated a predictive benefit for extended ET for three distinct groups: those who benefit from extended tamoxifen therapy after 5 years of tamoxifen, those who benefit from extended therapy with an AI after 5 years of tamoxifen, and those who benefit from extended therapy with an AI after 5 years of ET with either an AI or the sequence of tamoxifen followed by an AI.
A small ELT benefit on RFI observed in the parent B-42 trial, especially in the node-negative patients, limited the power of the translational analysis and may have in part contributed to the lack of predictive ability for the primary endpoint analysis. Other potential limitations include the retrospective nature of the BCI translational study as well as the lack of adjustment for multiplicity, which could have contributed to some of the results being statistically significant. In addition, the number of observed events was low in some of the subgroups, leading to wide confidence intervals. A strength of the study was the prospectively defined statistical analysis plan and the inclusion of 56% of patients enrolled in the parent B-42 trial in the translational study. The patients in B-42 continue to be followed for at least 15 years from randomization. Additional follow-up may enable further characterization of BCI (H/I) predictive performance in B-42.
Supplementary Material
Supplemental Figure SF1. Time-dependent Kaplan-Meier analysis based on Distant Recurrence comparing extended letrozole vs placebo in subsets of patients treated with initial adjuvant endocrine therapy with either A, AI only or B, a sequence of tamoxifen followed by AI in the overall Breast Cancer Index (BCI) NSABP B-42 translational cohort.
Supplemental Figure SF2. Exploratory Kaplan-Meier analysis based on A, any new primary cancer and B, new breast primary cancer comparing extended letrozole vs placebo in the overall Breast Cancer Index (BCI) NSABP B-42 translational cohort.
Acknowledgments
NCI U10CA180868, U10CA180822; UG1CA189867; Korea Health Technology R&D Project (to S. Paik). Funding was provided by The Breast Cancer Research Foundation to DCS (grants BCRF19-147, BCRF20-147, BCRF 21-147, and BCRF 22-147); Novartis, which provided letrozole and placebo to all study sites during the course of the study; and Biotheranostics Inc., A Hologic Company. The authors thank Wendy L. Rea, BA, for manuscript editing and preparation, and Ana A. Stephens, BA, for graphics assistance, both of whom are employees of NSABP, and were not compensated beyond their normal salaries for this work.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
E.P. Mamounas reports nonfinancial support from Hologic and Novartis during the conduct of the study as well as personal fees from Genentech/Roche, Merck, Exact Sciences, Tersera, Hologic, and Genzyme-Aventis outside the submitted work. H. Bandos reports grants from NCI U10CA180868, NCI U10CA180822, NCI UG1CA189867, and Biotheranostics Inc., A Hologic Company (paid to institution) during the conduct of the study as well as other support from Agendia (paid to institution) and Exact Sciences (paid to institution) outside the submitted work. Y. Zhang reports employment by and stock holdings of Hologic, the manufacturer of the biomarker analyzed in this work, and reports patents of the biomarker analyzed in this work, pending and issued. K. Treuner reports employment by and stock holdings of Hologic, the manufacturer of the biomarker analyzed in this work, and reports patents of the biomarker analyzed in this work, pending. P.C. Lucas reports other support from Amgen (stock) outside the submitted work and also has a patent for “Small molecules and their use as MALT1 inhibitors” (Patent No. PCT/US2022/021720), issued. C.E. Geyer reports grants from Biotheranostics and Novartis during the conduct of the study as well as grants and nonfinancial support from Genentech/Roche, AstraZeneca, Daiichi Sankyo, and Exact Sciences outside the submitted work. S.K. Chia reports personal fees from Novartis, Pfizer, and Eli Lilly as well as grants from Exact Sciences outside the submitted work. A.M. Brufsky reports personal fees from Myriad, Agendia, and Novartis during the conduct of the study as well as personal fees from Lilly, Pfizer, AstraZeneca, Gilead, Daiichi Sankyo, Seagen, Roche, Menarini-Stemline, Puma, and Merck outside the submitted work. S. Paik reports grants from NCI during the conduct of the study. S.M. Swain reports grants, personal fees, nonfinancial support, and other support from Genentech/Roche; personal fees from Biotheranostics, Sanofi, Lilly, Merck, Seagen, Napo Pharmaceuticals, Natera, and Molecular Templates; personal fees and other support from Astra Zeneca and Daichi Sankyo; and grants from Kailos Genetics outside the submitted work. D.C. Sgroi reports a patent for DCS and is a named inventor on a patent to use HOXB13/IL17BR and Molecular Grade Index assays to predict breast cancer outcome, issued, licensed, and with royalties paid from Biotheranostics/Hologic. C.A. Schnabel reports other support from Biotheranostics Inc., A Hologic Company, during the conduct of the study; also has patents related to BCI biomarker, pending and issued. N. Wolmark reports grants from NCI U10CA180868, NCI U10CA180822, and NCI UG1CA189867; nonfinancial support from Novartis; and other support from Biotheranostics Inc. during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
E.P. Mamounas: Conceptualization, data curation, investigation, methodology, writing–original draft, writing–review and editing. H. Bandos: Conceptualization, formal analysis, methodology, software, visualization, writing–original draft, writing–review and editing. P. Rastogi: Conceptualization, writing–review and editing. Y. Zhang: Conceptualization, formal analysis, methodology, resources, software, visualization, writing–review and editing. K. Treuner: Conceptualization, methodology, resources, writing–review and editing. P.C. Lucas: Methodology, resources, writing–review and editing. C.E. Geyer Jr: Conceptualization, writing–review and editing. L. Fehrenbacher: Conceptualization, investigation, writing–review and editing. S.K. Chia: Investigation, project administration, resources, supervision, writing–review and editing. A.M. Brufsky: Investigation, resources, writing–review and editing. J.M. Walshe: Investigation, resources, writing–original draft, writing–review and editing. G.S. Soori: Conceptualization, investigation, writing–original draft, writing–review and editing. S. Dakhil: Investigation, writing–review and editing. S. Paik: Conceptualization, funding acquisition, investigation, project administration, resources, writing–original draft, writing–review and editing. S.M. Swain: Conceptualization, writing–review and editing. D.C. Sgroi: Conceptualization, writing–review and editing. C.A. Schnabel: Conceptualization, methodology, resources, supervision, writing–review and editing. N. Wolmark: Conceptualization, funding acquisition, project administration, resources, visualization, writing–review and editing.
References
- 1. Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351:1451–67. [PubMed] [Google Scholar]
- 2. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017;377:1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013;381:805–16. Erratum in: Lancet 2013; 381:804. Erratum in: Lancet 2017; 389:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gray RG, Rea D, Handley K, Bowden SJ, Perry P, Earl HM, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. ASCO /J Clin Oncol 2013;31:18_Suppl 5–5. [Google Scholar]
- 5. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003;349:1793–802. [DOI] [PubMed] [Google Scholar]
- 6. Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clin Oncol 2008;26:1965–71. [DOI] [PubMed] [Google Scholar]
- 7. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, Putter H, van den Bosch J, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; Results of the IDEAL Trial (BOOG 2006–05). J Natl Cancer Inst 2018;110. [DOI] [PubMed] [Google Scholar]
- 9. Tjan-Heijnen VCG, van Hellemond IEG, Peer PGM, Swinkels ACP, Smorenburg CH, van der Sangen MJC, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol 2017;18:1502–11. Erratum in: Lancet Oncol 2017; 18:e642. [DOI] [PubMed] [Google Scholar]
- 10. Gnant M, Fitzal F, Rinnerthaler G, Steger GG, Greil-Ressler S, Balic M, et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N Engl J Med 2021;385:395–405. [DOI] [PubMed] [Google Scholar]
- 11. Mamounas EP, Bandos H, Lembersky BC, Jeong JH, Geyer CE, Rastogi P, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:88–99. Erratum in: Lancet Oncol 2019; 20(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dowsett M, Sestak I, Regan MM, Dodson A, Viale G, Thürlimann B, et al. Integration of clinical variables for the prediction of late distant recurrence in patients with estrogen receptor-positive breast cancer treated with 5 years of endocrine therapy: CTS5. J Clin Oncol 2018;36:1941–48. Erratum in: J Clin Oncol 2020; 38(6):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sparano JA, O'Neill A, Alpaugh K, Wolff AC, Northfelt DW, Dang C, et al. Circulating tumor cells (CTCs) five years after diagnosis are prognostic for late recurrence in operable stage II-III breast cancer. In: Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5–9; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78(4 Suppl):Abstr nr GS6–03. [Google Scholar]
- 14. Dubsky P, Brase JC, Jakesz R, Rudas M, Singer CF, Greil R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013;109:2959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Filipits M, Nielsen TO, Rudas M, Greil R, Stöger H, Jakesz R, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014;20:1298–305. [DOI] [PubMed] [Google Scholar]
- 16. Wolmark N, Mamounas EP, Baehner FL, Butler SM, Tang G, Jamshidian F, et al. Prognostic impact of the combination of recurrence score and quantitative estrogen receptor expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol 2016;34:2350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sgroi DC, Sestak I, Cuzick J, Zhang Y, Schnabel CA, Schroeder B, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013;14:1067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sgroi DC, Carney E, Zarrella E, Steffel L, Binns SN, Finkelstein DM, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013;105:1036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bartlett JMS, Sgroi DC, Treuner K, Zhang Y, Ahmed I, Piper T, et al. Breast Cancer Index and prediction of benefit from extended endocrine therapy in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial. Ann Oncol 2019;30:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noordhoek I, Treuner K, Putter H, Zhang Y, Wong J, Meershoek-Klein Kranenbarg E, et al. Breast Cancer Index predicts extended endocrine benefit to individualize selection of HR+ early-stage breast cancer patients for 10 years of endocrine therapy. Clin Cancer Res 2021;27:311–9. [DOI] [PubMed] [Google Scholar]
- 21. Bartlett JMS, Sgroi DC, Treuner K, Zhang Y, Piper T, Salunga RC, et al. Breast Cancer Index is a predictive biomarker of treatment benefit and outcome from extended tamoxifen therapy: final analysis of the Trans-aTTom study. Clin Cancer Res 2022;28:1871–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Schnabel CA, Schroeder BE, Jerevall P-LL, Jankowitz RC, Fornander T, et al. Breast Cancer Index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013;19:4196–205. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Schroeder BE, Jerevall PL, Ly A, Nolan H, Schnabel CA, et al. A novel Breast Cancer Index for prediction of distant recurrence in HR+ early-stage breast cancer with one to three positive nodes. Clin Cancer Res 2017;23:7217–24. [DOI] [PubMed] [Google Scholar]
- 24. Gradishar WJ. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer Version 5.2020; 2020. Accessed March 13, 2023.
- 25. Andre F, Ismaila N, Allison KH, Barlow WE, Collyar DE, Damodaran S, et al. Biomarkers for adjuvant endocrine and chemotherapy in early-stage breast cancer: ASCO guideline update. J Clin Oncol 2022;40:1816–37. [DOI] [PubMed] [Google Scholar]
- 26. Mamounas EP, Bandos H, Rastogi P, Lembersky BC, Jeong J-H, Geyer CE, et al. Ten-year update: NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-42 randomized trial: extended letrozole therapy in early-stage breast cancer. J Natl Cancer Inst 2023;115:1302–9. Erratum in: J Natl Cancer Inst 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma X-J, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 2004;5:607–16. [DOI] [PubMed] [Google Scholar]
- 28. Rea DW, Gray RG, Bowden SJ, Handley K, Earl HM, Poole CJ, et al. Overall and subgroup findings of the aTTom trial: a randomised comparison of continuing adjuvant tamoxifen to 10 years compared to stopping after 5 years in 6953 women with ER positive or ER untested early breast cancer. Eur J Cancer 2013;49:S402. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure SF1. Time-dependent Kaplan-Meier analysis based on Distant Recurrence comparing extended letrozole vs placebo in subsets of patients treated with initial adjuvant endocrine therapy with either A, AI only or B, a sequence of tamoxifen followed by AI in the overall Breast Cancer Index (BCI) NSABP B-42 translational cohort.
Supplemental Figure SF2. Exploratory Kaplan-Meier analysis based on A, any new primary cancer and B, new breast primary cancer comparing extended letrozole vs placebo in the overall Breast Cancer Index (BCI) NSABP B-42 translational cohort.
Data Availability Statement
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within one year after publication and will be accessible through the NCTN Data Archive. Please contact the corresponding author for the protocol, access to data prior to NCTN availability, or other information.