Abstract
Osteoarthritis (OA) commonly affects the knee and hip joints and accounts for 19.3% of disability-adjusted life years and years lived with disability worldwide (Refs 1, 2). Early management is important in order to avoid disability uphold quality of life (Ref. 3). However, a lack of awareness of subclinical and early symptomatic stages of OA often hampers early management (Ref. 4). Moreover, late diagnosis of OA among those with severe disease, at a stage when OA management becomes more complicated is common (Refs 5, 6, 7, 8). Established risk factors for the development and progression of OA include increasing age, female, history of trauma and obesity (Ref. 9). Recent studies have also drawn a link between OA and metabolic syndrome, which is characterized by insulin resistance, dyslipidaemia and hypertension (Refs 10, 11).
Keywords: biomarkers, diabetes mellitus, inflammation, metabolism, osteoarthritis
Introduction
Osteoarthritis (OA) commonly affects the knee and hip joints and accounts for 19.3% of disability-adjusted life years and years lived with disability worldwide (Refs 1, 2). Early management is important in order to avoid disability uphold quality of life (Ref. 3). However, a lack of awareness of subclinical and early symptomatic stages of OA often hampers early management (Ref. 4). Moreover, late diagnosis of OA among those with severe disease, at a stage when OA management becomes more complicated is common (Refs 5, 6, 7, 8). Established risk factors for the development and progression of OA include increasing age, female, history of trauma and obesity (Ref. 9). Recent studies have also drawn a link between OA and metabolic syndrome, which is characterized by insulin resistance, dyslipidaemia and hypertension (Refs 10, 11).
Diabetes mellitus (DM) is a prevalent non-communicable disease that affects more than 470 million people worldwide (Ref. 12). The presence of diabetes is believed to accelerate the progression of OA and further complicate the management of OA. This has led to the proposal of the ‘diabetes-induced-osteoarthritis’ (DM-OA) phenotype, which suggests that inflammation and oxidative stress predispose persons living with DM to OA (Ref. 13). DM manifests as a chronic hyperglycaemic state which induces further cartilage degeneration and joint inflammation, causing enrichment of advanced glycation end-products (AGEs) and matrix stiffening preventing optimal cushioning of the joint (Ref. 14). This process then contributes to the cycle of worsening of OA symptoms with resultant avoidance of physical inactivity and subsequent weight gain. As a consequence, metabolic dysregulation and joint symptoms persist or worsen (Refs 15, 16).
Biomarker profiles are now one of the tools for quantification of disease activity, for example, procalcitonin for medullary thyroid cancer and tricarboxylic acid from urine metabolites for gastrointestinal diseases (Refs 17, 18). Specifically, an increasing interest has been drawn towards biomarkers for OA with DM (Ref. 19). The identification of novel biomarkers for OA with DM may aid early diagnosis as a key towards improvement in disease outcomes through secondary preventive measures, prior to the onset of irreversible structural changes. Therefore, in this review, we aimed to identify the impact of DM on OA by examining the studied biomarker signatures.
Methods
Identification of relevant studies
Literature search was conducted initially in January 2022 and updated in December 2022. Articles containing the key words ‘osteoarthritis’ AND (‘diabetes mellitus’ OR ‘hyperglycemia’) AND ‘biomarker*’ NOT ‘animal model’ were identified from PubMed, Web of Science, EBSCO and the Cochrane library. Complete search syntax is documented in Supplementary Tables S2–S5. Additional full-text articles were identified through cross-referencing of review articles identified through EBSCO. Titles of articles identified were first screened using Rayyan.ai (by three authors independently: SM, AAA and SRS) (Ref. 20). Any disagreement in title screening was resolved through discussion. The abstracts of articles identified from the title search were then screened using Endnote™ (Clarivate, Philadelphia USA, London United Kingdom). The full text of articles for the selected abstracts was subsequently evaluated by two authors (AAA and SRS).
Articles reporting observational studies, including experimental and cross-sectional studies, that investigated any metabolite, intracellular or extracellular matrix component as potential biomarkers in OA with DM were selected. We included studies involving human subjects that utilized samples of synovial fluid, blood, bone and cartilage employing immunoassay, histological and high-performance liquid chromatography techniques.
Data extraction
AAA and SRS independently extracted data on author, year of publication, study design and population (sample size, gender, inclusion and exclusion criteria, as well as subject grouping), definitions for OA and DM, methodology, specimens collected and signature biomarkers evaluated using a standardized data extraction table. Quality assessment was performed using the modified Newcastle–Ottawa Quality Assessment Scale (Supplementary Tables S6 and S7) (Ref. 21).
Results
Study characteristics
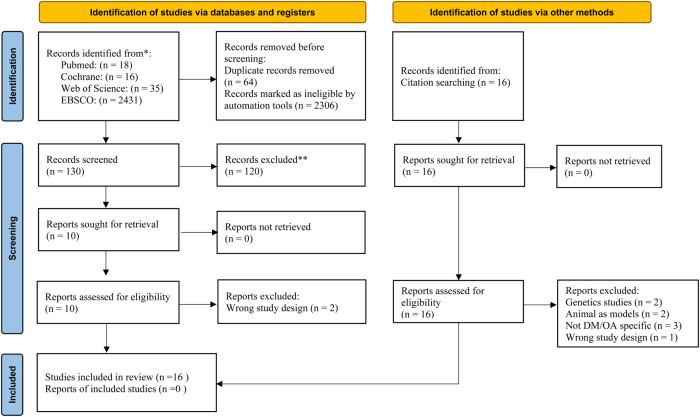
The database and reference search yielded 16 articles dated until April 2022. Eight articles were first identified from the 2500 potentially eligible articles extracted from PubMed, Web of Science, EBSCO and Cochrane library (Supplementary Figs S1–S4), while another eight articles were identified from cross-referencing. Figure 1 presents the PRISMA flow diagram illustrating the systematic selection of the articles.
Figure 1.
PRISMA flow diagram of study selection.
The study characteristics were heterogenous across the articles, with the study population sampled from various geographical locations. The total number of participants in each study ranged from 3 to 35 for the experimental studies and 15 to 143 for the cross-sectional studies (Table 1). The studies were conducted in nine countries, six in Europe (Portugal, Spain, Mexico, France, Germany and Finland), two in North America (Canada and the United States) and one in Asia (China). Ten articles identified the presence of DM or hyperglycaemia through medical history, fasting plasma glucose and glycated haemoglobin (HbA1c) levels, whereas DM was not defined by six articles that were based on primary cell cultures.
Table 1.
Study characteristics
| Author, year | Type of study (targeted/untargeted) (cross-sectional/ experimental) | Classification of biomarkers (BIPED) | Quality assessment | Metabolomic approach | Sample (cartilage, bone marrow, synovial tissue/ fluid, blood) | Number of samples |
|---|---|---|---|---|---|---|
| Luo et al. (Ref. 22) | Untargeted cross-sectional study
|
B | 8 | LC-MS/MS-based N-glycoproteomics analysis | Cartilage | OA + DM+ = 5 OA + DM− = 5 Control = 5 |
| Li et al. (Ref. 23) | Targeted cross-sectional study
|
B, P | 7 | Immunohistochemical staining | Synovial tissue | OA + DM+ = 10 OA + DM− = 10 |
| Scotece et al. (Ref. 24) | Targeted cross-sectional study
|
D | 8 | Human ELISA-linked immunosorbent assay | Cartilage | 100 OA |
| Silawal et al. (Ref. 25) | Targeted experimental study
|
P, D | 6 | Immunohistochemical staining | hACs | 5 OA cultures |
| Vertti et al. (Ref. 26) | Targeted cross-sectional study
|
B | 10 | Human ELISA-linked immunosorbent assay | Synovial fluid | OA + DM+ = 92 OA + DM− = 29 |
| D | Blood | OA + DM+ = 92 OA + DM− = 29 |
||||
| Eitner et al. (Ref. 27) | Targeted cross-sectional study
|
B, P | 9 | Human ELISA-linked immunosorbent assay | Synovial fluid | OA + DM+ = 23 OA + DM− = 47 |
| Serum | OA + DM+ = 23 OA + DM− = 47 |
|||||
| Zhang et al. (Ref. 28) | Targeted cross-sectional study
|
B, P | 8 | Mass spectrometry by flow injection analysis LC–MS/MS |
Synovial fluid | OA + DM+ = 46 OA + DM− = 38 |
| D | Plasma | OA + DM+ = 46 OA + DM− = 38 |
||||
| Hamada et al. (Ref. 29) | Targeted experimental study Targeted
|
B, P | 7 | Immunohistochemistry | Synovial tissue | OA + DM+ = 7 OA + DM− = 6 |
| D | Immunoprecipitation and western blot analysis | Synovial tissue | OA + DM+ = 7 OA + DM− = 6 |
|||
| D | qRT-PCR | Fibroblast-like-synoviocytes culture | OA + DM− = 4 | |||
| Ribeiro et al. (Ref. 30) | Targeted experimental study
|
D | 7 | Western blotting | Primary human chondrocytes (HC) | OA + DM+ = 4 OA + DM− = 4 Control = 2 |
| Zhang et al. (Ref. 31) | Untargeted cross-sectional study
|
B, P | 10 | Ultra-high performance liquid-chromatography - mass spectrometry | Synovial fluid | OA + DM+ = 29 OA + DM− = 43 Control = 46 |
| D | Plasma | OA + DM+ = 29 OA + DM− = 43 DM = 25 Control = 46 |
||||
| Laiguillon et al. (Ref. 32) | Targeted experimental study
|
D | 9 | Human ELISA-linked immunosorbent assay | Homogenous isolated cartilage samples | OA + DM+ = 5 OA + DM− = 5 |
| Tsai et al. (Ref. 33) | Targeted experimental study
|
D | 6 | qRT-PCR and western blot analysis | Human bone marrow-derived MSCs | 3 OA |
| Tsai et al. (Ref. 34) | Targeted experimental study
|
D | 7 | qRT-PCR and human ELISA-linked immunosorbent assay | Human synovial fibroblast culture | 35 OA |
| Rosa et al. (Ref. 35) | Targeted experimental study
|
7 | qRT-PCR | Cartilage chondrocyte culture | 11 OA 7 non-OA |
|
| Oren et al. (Ref. 36) | Targeted cross-sectional study
|
B, D, P | 10 | Human ELISA-linked immunosorbent assay | Serum | OA + DM+ = 10 OA + DM− = 10 |
| Synovial fluid | OA + DM+ = 10 OA + DM− = 10 |
|||||
| High-performance liquid chromatography | Bone and cartilage | OA + DM+ = 10 OA + DM− = 10 |
||||
| Rosa et al. (Ref. 37) | Targeted experimental study
|
D | 7 | qRT-PCR, western blot and human ELISA-linked immunosorbent assay | Cartilage chondrocyte culture | Non-OA = 15 OA = 18 |
ADAMTS4, a disintegrin and metalloproteinase with thrombospondin motifs 4; ADAMTS5, a disintegrin and metalloproteinase with thrombospondin motifs 5; AGEs, advanced glycation end-products; Akt, serine threonine kinase; ANGPTL2, angiopoietin-like protein 2; ASPN, asporin; ATF6, activating transcription factor 6; BGN, biglycan; BIPED, burden of disease, investigative, prognostic, efficacy of intervention and diagnostic; BSG, basigin; C8A, C8 alpha chain N437; CD47, cluster of differentiation 47; COL1A1, collagen type 1 alpha 1 chain; COL6A2, collagen type VI alpha 1 chain; CTSD, cathepsin D; FBLN7, ELISA, enzyme-linked immunosorbent assay; fibulin-7; FN1, fibronectin 1; GLUT-1, glucose transporter 1; GRP78, 78 kDa glucose-regulated protein; hAC, human articular chondrocyte; HbA1c, glycated haemoglobin; HIF-1α, hypoxia-inducible factor-1α; IGHM, immunoglobulin heavy constant mu; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IR, insulin receptor; JCAD, junctional cadherin 5-associated protein; LC3, microtubule-associated protein 1A/1B-light chain 3; LC–MS/MS, liquid chromatography coupled-tandem mass spectrometry; MG, methylglyoxal; MG-H1, free methylglyoxal-derived hydroimidazolone; MMP-1, matrix metalloproteinase-1; MMP-13, matrix metalloproteinase-13; MSC, mesenchymal stem cell; NF-κB p65, RelA of nuclear factor kappa-light-chain-enhancer of activated B cells; ROS, reactive oxygen species; p-rpS6, phosphorylated ribosomal S6; qRT-PCR, quantitative real-time polymerase chain reaction; RBP4, retinol binding protein 4; SF COMP, synovial fluid cartilage oligomeric matrix protein; Smad3, SMAD family member 3; SPARC, secreted protein acidic and rich in cysteine/osteonectin; SOX9, SRY-box transcription factor 9; THBS3, thrombospondin 3; TIMP-1, tissue inhibitor of metalloproteinase-1; TIMP-2, tissue inhibitor of metalloproteinase-2; TNC, tenascin C; TNF-α, tumour necrosis factor-alpha; VEGF, vascular endothelial growth factor.
Bold biomarkers indicate significant expression change.
The types of samples collected included blood, synovial fluid, bone and cartilage. Five studies used blood samples whereas nine studies collected cartilage or synovial tissues through total knee replacement surgeries and autopsies. The tissues collected were processed into primary cultures to measure in vitro cell expression under hyperglycaemic conditions. The results of this review were categorized according to the following classification: (1) DM-specific biomarkers, (2) cartilage-specific factors, (3) inflammatory mediators, (4) proteases, (5) cell homoeostasis regulators and (6) AGEs and phospholipids.
Biomarkers screening approach
Three of the 16 selected studies utilized metabolomic analysis and mass spectrometry techniques to screen for candidate markers (Table 1). Mass spectrometry was coupled with the separation techniques of flow-injection and liquid chromatography. Zhang et al. evaluated 168/186 biomarkers including 40 acylcarnitines (1 free carnitine), 20 amino acids, 9 biogenic amines, 87 glycerophospholipids, 11 sphingolipids and 1 hexose from plasma and synovial fluid, and eventually proposed plasma unsaturated phosphatidylcholines (PCs), PC ae C34:3 and PC ae C36:3 as possible OA with DM biomarkers after matching the two samples (Ref. 31). As a continuation of the previously untargeted metabolomic approach, AGEs and their precursor were quantified with liquid chromatography coupled-tandem mass spectrometry (LC–MS/MS) in order to identify markers associated with PC ae C34:3 and PC ae C36:3 concentrations (Ref. 28). Luo et al. compared the changes in N-glycosylated protein abundance from cartilages using LC–MS/MS-based N-glycoproteomics analysis and showed 1 upregulated and 16 downregulated N-glycosylated peptides between OA and OA with DM groups (Ref. 22).
Oren et al. performed high-performance liquid chromatography and enzyme-linked immunosorbent assay (ELISA) for tissue and fluid samples, respectively (Ref. 36). The remaining studies utilized immunological techniques for targeted biomarker detection (Table 1).
Eight studies measured biomarker expression through isolated cell cultures: isolated fibroblast-like synoviocytes and isolated chondrocytes, three of these studies compared results of those with OA only with those with comorbid DM (Refs 23, 29, 30, 32) and five studies isolated samples from individuals with OA only for further high-glucose stimulation culture (Refs 25, 33, 34, 35, 37). Studies by Hamada et al. and Tsai et al. isolated RNA from treated fibroblast-like synoviocytes, whereas Rosa et al. isolated RNA from chondrocyte cultures for quantitative real-time polymerase chain reaction which enabled quantitation of targeted biomarkers (Table 1).
Method of OA assessment
The articles utilized different criteria to determine the potential presence of OA utilized, including radiographic evidence with or without the Kellgren and Lawrence (KL) grading, the American College of Rheumatology (ACR) clinical criteria or planned total knee arthroplasty. Ten studies recruited participants with OA who were about to have total knee replacement in order to sample synovial fluid, bone or cartilage during their surgery. Six studies did not utilize the ACR clinical diagnostic criteria confirmed by an orthopaedic surgeon and radiographic evidence with KL grading. One of the studies excluded non-OA participants based on weight-bearing anteroposterior and lateral 30° knee flexion radiographic images (Ref. 26).
Candidate biomarkers
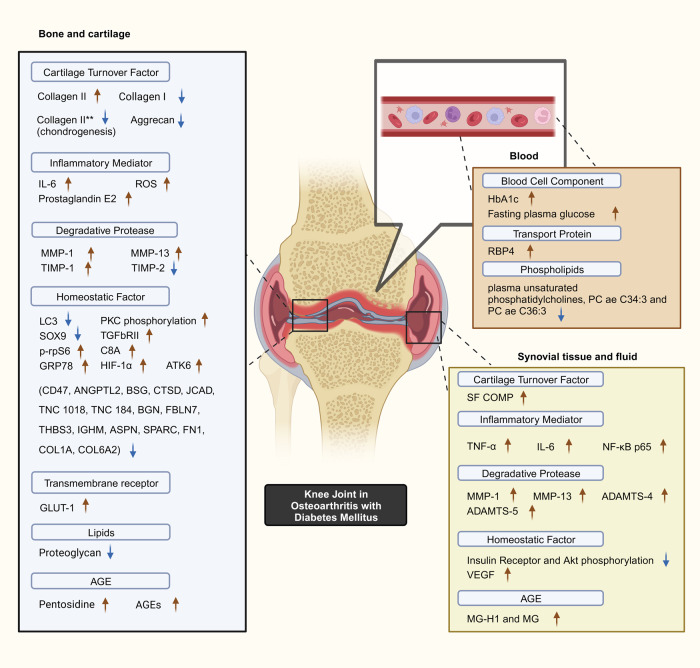
Selected studies quantified key biomarker expression in terms of the presence of significant increases or decreases corresponding to the presence of DM with OA as a constant. Minimal overlap existed between the studies with regards to biomarkers studied. Common OA and inflammation biomarkers are observed to be significantly influenced by the presence of DM (Fig. 2).
Figure 2.
Postulated DM–OA biomarker pathway. An illustrated diagram with the proposed pathway for biomarkers which differentiates the diabetes-OA phenotype from the classical OA phenotype. The biomarkers with significant different in expression magnitude are listed according to sample origin. Red arrows and blue arrows indicate upregulated and downregulated OA with DM biomarker expression as compared with OA, respectively. **Indicates collagen type II expressed by human bone marrow-derived mesenchymal cells during chondrogenesis capacity experiment. Created with BioRender.com. ADAMTS4, a disintegrin and metalloproteinase with thrombospondin motif 4; ADAMTS5, a disintegrin and metalloproteinase with thrombospondin motif 5; AGEs, advanced glycation end-products; Akt, serine threonine kinase; ANGPTL2, angiopoietin-like protein 2; ASPN, asporin; ATF6, activating transcription factor 6; BGN, biglycan; BSG, basigin; C8A, C8 alpha chain N437; CD47, cluster of differentiation 47; COL1A1, collagen type 1 alpha 1 chain; COL6A2, collagen type VI alpha 1 chain; CTSD, cathepsin D; FBLN7, fibulin-7; FN1, fibronectin 1; GLUT-1, glucose transporter; GRP78, 78 kDa glucose-regulated protein; HbA1c, glycated haemoglobin; HIF-1α, hypoxia-inducible factor-1α; IGHM, immunoglobulin heavy constant mu; IL-6, interleukin-6; LC3, microtubule-associated protein 1A/1B-light chain 3; JCAD, junctional cadherin 5-associated protein; MG, methylglyoxal; MG-H1, free methylglyoxal-derived hydroimidazolone; MMP-1, matrix metalloproteinase-1; MMP-13, matrix metalloproteinase-13; NF-κB p65, RelA of nuclear factor kappa-light-chain-enhancer of activated B cells; PKC, protein kinase C; p-rpS6, phosphorylated ribosomal S6; RBP4, retinol binding protein 4; ROS, reactive oxygen species; SF COMP, synovial fluid cartilage oligomeric matrix protein; Smad3, SMAD family member 3; SPARC, secreted protein acidic and rich in cysteine/osteonectin; SOX9, SRY-box transcription factor 9; THBS3, thrombospondin 3; TIMP-1, tissue inhibitor of metalloproteinase-1; TIMP-2, tissue inhibitor of metalloproteinase-2; TNC, tenascin C; TNF-α, tumour necrosis factor-alpha; TGFβRII, type II transforming growth factor-β receptor; VEGF, vascular endothelial growth factor.
DM-specific biomarkers
As presumed, HbA1c was higher in the group with both OA and diabetes as compared with the group without DM (Ref. 36). Remarkably, population having both DM and OA has significantly higher HbA1c than population without OA (Ref. 26). Next, glucose transporter 1 (GLUT-1) expression reduction in response to high-glucose cultivation was reported in normal chondrocytes but was not observed in OA chondrocytes (Refs 23, 37). Blunted insulin receptor (IR) and serine threonine kinase (Akt) phosphorylation was also observed in synovial cells in response to high-insulin levels, leading to significantly decreased human articular chondrocyte (hAC) proliferation (Refs 25, 29). Retinol binding protein 4 (RBP4) adipokine expression was detected within blood, synovial fluid and cartilage samples of 100 individuals with OA, which was associated with adiponectin, leptin, resistin, matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-3 (MMP-3), chitinase 3-like-1 and adipsin (Ref. 24).
Cartilage-specific factors
Synovial fluid cartilage oligomeric matrix protein (SF COMP) levels were significantly higher in 92 OA with DM subjects compared with 29 OA subjects. At baseline, Rosa et al. reported a 3.5-fold higher ratio of collagen type II to type I messenger-ribonucleic acid (mRNAs) expressions in normal chondrocytes as compared with OA origins. Next, transient increase of collagen type II mRNA levels was significant at 24 h in both normal and OA chondrocytes cultured in elevated glucose concentration, but soon reduced to level equivalent to regular glucose concentration cultures. Collagen II production was increased in OA chondrocytes when proceeded with transforming growth factor-β (TGF-β) stimulation (Ref. 35). On the other hand, Silawal et al. observed significantly lower collagen type II expression in hyperglycaemic hAC culture in response to interleukin-10 (IL-10) and high insulin when compared with normal glycaemic culture. Also, compared with normal glycaemic hAC culture, the decrease induced expression of non-specific dedifferentiation marker collagen type I in hyperglycaemic hAC culture decreased more to a significant level after treated with IL-10. Proteoglycan expression decreased in hAC cultured in IL-10-treated media regardless of glycaemic condition (Ref. 25). Chondrogenic capacity analysis of human bone marrow-derived mesenchymal stem cells (MSCs) reported significantly lower aggrecan mRNA expression at day 9 in high-glucose-maintained culture compared with low-glucose-maintained culture. At day 22, both aggrecan and collagen type II mRNA expression, but not collagen type IX, attained statistical significance lower expression in high-glucose-maintained culture (Ref. 33).
Inflammatory mediators
Tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) in synovial tissue have been found to be higher in individuals with OA and DM compared with individuals with OA (Ref. 23). This is consistent with the immunohistochemistry findings that observed higher TNF expression in fibroblast-like synoviocytes of comparable groups (Ref. 29). Greater IL-6 and prostaglandin E2 expression was also observed in interleukin-1β (IL-1β)-stimulated human OA with DM cartilage culture compared with OA cartilage culture (Ref. 32). Further, increased IL-6 expression is positively associated with pain in OA with DM (Ref. 27).
Reactive oxygen species (ROS) increased remarkably when cultured cartilage chondrocytes from individuals with OA were stimulated with IL-1β to promote glucose transportation, which is said to mimic defective GLUT-1 downregulation and intracellular accumulation of glucose. ROS production in OA chondrocytes was increased and more sustained in glucose-rich culture (Ref. 37).
Protease
MMP-13 and a disintegrin and metalloproteinase with thrombospondin motif 5 (ADAMTS5) were significantly upregulated in OA with DM synovial tissue compared with non-DM OA participants (Ref. 23). Similarly, primary OA fibroblast-like synoviocytes treated with TNF has shown increased MMP-1, MMP-13 and a disintegrin and metalloproteinase with thrombospondin motif 4 (ADAMTS4) expression under glucose-rich conditions. Nevertheless, the increased expressions reduced >50% after treating with insulin (Ref. 29). Exposure to glucose-rich conditions increased tissue inhibitor of metalloproteinase-2 (TIMP-2), MMP-1 and MMP-13 (mRNAs in OA chondrocytes), whereas only MMP-1 increment and tissue inhibitor of metalloproteinase-1 (TIMP-1) decrement was observed in normal chondrocytes (Ref. 35).
Cell homoeostasis regulators
Within the DM-OA phenotype, chondrocyte expression of microtubule-associated protein 1A/1B-light chain 3 (LC3) was significantly reduced whereas phosphorylated ribosomal S6 (p-rpS6) expressions significantly increased in comparison with both healthy as well as OA chondrocytes (Ref. 30). A reduction in chondrogenic expression was observed in human bone marrow-derived MSCs within high-glucose culture, with an increased protein kinase C (PKC) activation and type II TGF-β receptor (TGFβRII) expression (Ref. 33). High glucose also induces vascular endothelial growth factor (VEGF) production in human OA synovial fibroblast (Ref. 34). As endoplasmic reticulum stress-related proteins, both activating transcription factor 6 (ATF6) and 78 kDa glucose-regulated protein (GRP78) expression were found to be significantly higher in the OA group with DM as compared with the non-DM group (Ref. 23). The same research population also had higher hypoxia-inducible factor-1α (HIF-1α) in the OA with DM group, suggesting possible shared pathophysiology of OA with DM (Ref. 23).
hAC and chondrosarcoma cell lines used in IL-10-stimulated cell culture demonstrated reduced proliferation ability in hyperglycaemia and hyperinsulinaemia compared with normal glycaemic conditions. SRY-box transcription factor 9 (SOX9) synthesis under hyperglycaemic conditions was significantly reduced alongside proteoglycan (Ref. 25). Based on a chondrogenesis stimulation using high-glucose-maintained human bone marrow-derived MSCs, SOX9 mRNA expression that reflect chondrogenic capacity was significantly downregulated at day 9 as compared with low-glucose maintained culture, but the significance waned at day 22 (Ref. 33). Among the 17 N-glycosylated proteins that shown fold changes between OA and OA with DM group comparison, fibronectin 1 (FN1), collagen type I alpha 1 chain (COL1A1) and collagen type VI alpha 1 chain (COL6A2) played the most central role in the protein–protein interactions; the other significant N-glycosylated proteins that likely to participate in OA with DM pathogenesis is C8 alpha chain (C8α) N437 (Ref. 22).
AGEs and phospholipids
Three AGEs were identified as potential OA with DM biomarkers: free methylglyoxal-derived hydroimidazolone-1 (MG-H1), methylglyoxal (MG) and pentosidine. Zhang et al. observed a negative relationship between plasma in participants with both OA and DM and two unsaturated PCs, PC ae C34:3 and PC ae C36:3 (Ref. 31), and subsequently uncovered a positive upregulation of MG-H1 and MG in synovial fluid of participants with OA and DM (Ref. 28). Pentosidine was significantly higher in both the bone and cartilage of OA with DM individuals compared with non-DM OA individuals (Ref. 36). In addition, following high-GLUT-1 expression in Li et al.'s study, significantly higher accumulation of AGEs in subjects with OA and DM was found (Ref. 23).
Burden of disease, investigative, prognostic, efficacy of intervention and diagnostic classification
The burden of disease, investigative, prognostic, efficacy of intervention and diagnostic (BIPED) biomarker system has been widely used to classify OA biomarkers (Ref. 38). Table 1 lists the function of candidate biomarkers identified within the published articles using the BIPED classification. Burden of disease biomarkers may be useful for early detection whereas transcription factors and protein kinase involved in cartilage homoeostasis are considered indicators of OA with DM prognosis. No specific biomarker has been proposed as investigative and efficacy of intervention biomarkers as the selected criteria had not taken into account research on the effects of pharmacological agents.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale was used (Ref. 21), and to address heterogeneity, this was modified into separate versions for experimental and cross-sectional studies. The eight items within the scale assess the three domains: participant sampling, comparability and outcomes. Three studies (20%) were assigned the maximal score of 10, whereas two studies (13%) had a score of nine. Seven studies (46%) did not adequately address comparability for confounding factors and hyperglycaemic conditions whereas two studies (13%) controlled for basic confounding factors only (Table 1).
Discussion
This review has provided a comprehensive catalogue of investigated blood, synovial fluid, bone and cartilage biomarkers for OA with DM. Biomarkers were uniquely evaluated from the perspective of their physiological functions and general structures, in comparison with previous reviews which addressed signature biomarkers in OA; this review article has focused primarily on biomarkers involved in the contribution of DM to joint inflammation and degeneration within OA (Ref. 39). The presence of DM significantly alters biomarker expression in individuals with OA which can be distinguished from basic OA phenotype, the differences may in turn help unravel the mechanisms underlying the acceleration in OA development associated with DM (Ref. 11).
Although cartilage- and synovial-specific factors are present in both classical OA and OA with DM, the two phenotypes are differentiated by the magnitude of biomarker expression; for instance, higher SF COMP levels in OA with DM indicates greater articular cartilage degradation (Refs 40, 41). In contrast, synthesis of proteoglycan in OA hAC was lowered in hyperglycaemia with IL-10. The transient increase of collagen type II mRNA in high-glucose concentration could be rationalized by the depletion of glucose overtime, where mRNA expression at 72 h became similar to that in regular glucose concentration cultures (Ref. 25). Furthermore, the measurement of mRNA might not reflect collagen type II protein concentration (Ref. 42). Expression of collagen type II in OA culture significantly increased when treated with TGF and high-glucose level showing how glucose concentration affect chondrocyte anabolic and catabolic gene expression (Ref. 35). The expression, however, reduced significantly when cultured with high insulin and IL-10 even though IL-10 is known for its chondroprotective effects (Ref. 43), suggesting hyperglycaemia and hyperinsulinaemia impaired chondrocyte expression (Ref. 44). This suppressive capacity also being suggested for the diminished collagen type I observed in OA hAC culture after IL-10 and high-insulin treatment, which usually expressed in monolayer chondrocyte culture and indicates cartilage differentiation activity (Ref. 45). A paradoxical increase in TGF-stimulated collagen type II expression, conversely, is observed within high-glucose culture environments (Ref. 35). Chondrogenic capacity measurement of lower aggrecan and collagen type II mRNA expression in high-glucose-maintained human mesenchymal cells, which are also the two main components of articular extracellular matrix, suggested remarkable influence of high-glucose concentration on chondrogenesis and cartilaginous matrix production, leading to disrupted cartilage homoeostasis as in OA (Ref. 46).
Transcriptional factors and protein kinase expression involved in the chondrocyte life cycle are altered in OA with DM. Decreased LC3 and increased p-rpS6 expression are seen in the chondrocytes of individuals with both OA and DM, which has been attributed to defective autophagy (Refs 30, 47). In the absence of effective autophagy, dysfunctional organelles and macromolecules cannot be removed, which indicates a negative disease prognosis (Refs 48, 49). Higher PKC phosphorylation is observed with high-glucose-maintained MSCs prior to chondrogenesis (Ref. 33). PKC-mediated mitogen-activated protein kinase, activated by TGF-β-stimulated Wnt-5a overexpression, signals for chondrogenic differentiation into functional cells (Ref. 50). VEGF is hypothesized to mediate cartilage catabolism and endochondral ossification in OA (Ref. 51), and since VEGF upregulation is significant under hyperglycaemic conditions (Ref. 52), the higher VEGF expression in OA with DM compared with OA is coherent (Ref. 34). SOX9, a chondrocyte-protecting factor commonly downregulated in OA, is further reduced in OA with DM (Refs 25, 53).
The mechanism of endoplasmic reticulum stress to DM is hypothesized that nutrient stress and inflammatory cytokines induce unfolded protein response and endoplasmic reticulum stress by β-cell islets of Langerhans, which then stimulate inflammatory response (Ref. 54). The higher expression of ATF6 and GRP78 in the OA with DM group compared with the non-DM group was in line with their endoplasmic reticulum stress regulation roles (Ref. 55). The fact that endoplasmic reticulum stress is involved in OA pathological changes may explain the expression magnitude in OA with DM (Ref. 56).
Inflammation is now considered the key pathway for OA with DM (Ref. 57). The biomarkers identified in the published studies have, however, primarily been associated with insulin resistance and nutrient stress with only a few biomarkers linked with inflammation. Inflammatory mediators shown in Figure 2 have been identified as biomarkers for OA with DM. The chronic hyperglycaemic condition of DM is postulated to be associated with increased expression of inflammatory mediators which has been purported to arise from the interaction between AGEs and macrophages (Ref. 58). Receptor binding for advanced glycation end-products activates pro-inflammatory M1 macrophages to increase NF-κB transcriptional factor, which in turns further enhances TNF-α and IL-1β expression (Refs 59, 60, 61). Macrophage activation induces TIMPs, MMPs and ADAMTS production, which is responsible for the catabolic action on cartilage, the degradation subsequently releases damage-associated molecular pattern that stimulates inflammatory mediators' production in return (Refs 62, 63, 64, 65).
The novel biomarkers for early OA with DM detection are putatively DM-specific biomarkers. Blunted insulin-dependent phosphorylation of IRs and Akt were observed in the presence of DM, reflecting insulin resistance (Ref. 29). Under normal conditions, IRs undergo trans-autophosphorylation triggered through IR binding, activating the PI3K–PKB/Akt signalling cascade. On the contrary, blunted IR responsiveness and Akt phosphorylation result in defective glucose uptake (Ref. 66). Excessive nutrient stress hyperactivates the mammalian target of rapamycin complex 1, leading to a negative feedback loop that inhibits Akt (Ref. 67). Subsequently, a shift in anti-inflammatory M2-polarized macrophages towards M1-polarization, which is pro-inflammatory, is then observed (Refs 58, 68).
The common diabetes biomarkers glucose and HbA1c levels represent indicators of nutrient stress in OA with DM (Ref. 26). Insulin resistance stimulate ROS production by reducing AMP-activated protein kinase activity in macrophages, which then activates more HIF-1α and upregulates glycolysis in the attempt to restore cellular energy homoeostasis, with the eventual increase in glucose-6-phosphate and nicotinamide adenine dinucleotide phosphate production, further promoting ROS production (Refs 69, 70). Within a high-glucose environment, OA chondrocytes fails to downregulate GLUT-1, resulting in intracellular glucose accumulation and ROS production (Ref. 37). RBP4 that contributes to insulin resistance development could be associated with OA development through MMP expression signalling (Refs 71, 72, 73). Plasma unsaturated phosphatidylcholine depletion, linked to increased insulin resistance and reduced cartilage lubrication, has emerged as a potential indicator owing to its greater depletion in OA with DM (Refs 74, 75).
Next, biomarkers resulting from N-glycosylation post-translational modification (Ref. 76) have demonstrated significant fold changes in OA with DM cartilage (Ref. 22). FN1 is an extracellular matrix component with vital functions in regulating cell signalling, growth and differentiation (Ref. 77). Distinctly, upregulated FN1 N-glycosylation commonly reported in OA is observed to be downregulated in OA with DM (Refs 22, 78). Three N-glycosylated PI3K/Akt pathway proteins: FN1, COL1A1 and COL6A2 are downregulated in OA with DM (Refs 22, 79, 80), indicating pathological roles of PI3K/Akt signalling and collagen glycosylation may be different in OA and OA with DM (Ref. 81). Only C8α N437 is upregulated in OA with DM (Ref. 22). Notably, C8α involves in complement activation and complement complex formation, which is a key modulator in metabolic diseases (Ref. 82).
Limitation
The non-inclusive of articles not published in the English language limits the scope of this article in recommending putative OA with DM biomarkers. Next, the temporal relationship between OA and DM was not clarified during participant recruitments, and there were potential confusions between markers about their specificities towards OA or DM, raising doubt in the reflecting direction of the biomarkers. Moreover, identified signatures of the specimen harvested from surgical-removed cartilage and stimulation experiments with isolated chondrocytes are not complementary to an early preventive strategy. Future studies should therefore focus on populations with early-stage OA to identify putative diagnostic biomarkers for early OA with DM.
Conclusion
The novel biomarkers proposed in the scoping review comprise DM-specific biomarkers and cartilage cell homoeostasis regulators with expressions significantly altered in OA with DM as compared with OA. Future studies are required to evaluate the pathophysiology underlying OA with DM by examining the interaction of these biomarkers.
Supporting information
Seow et al. supplementary material
Supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/erm.2024.7.
click here to view supplementary material
Funding statement
This study was funded to the principal investigator S. M. by Fundamental Research Grant Scheme, Ministry of Higher Education, Malaysia, Grant/Award Number: FRGS/1/2021/SKK0/UKM/02/15.
Competing interests
None.
Ethical standards
Ethical approval had been provided for this study (Universiti Kebangsaan Malaysia [JEP-2022-2021]; Malaysia) by Professor Dr Mohd Shahrir Mohamed Said (UKM PPI/111/8/JEP 2022-001).
References
- 1.Ajit Singh DK et al. (2018) Knee associated problems and functional mobility among adults with knee osteoarthritis. Jurnal Sains Kesihatan Malaysia 16, 229–230. 10.17576/jskm-2018-16si-37 [DOI] [Google Scholar]
- 2.Safiri S et al. (2020) Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Annals of the Rheumatic Diseases 79, 819. 10.1136/annrheumdis-2019-216515 [DOI] [PubMed] [Google Scholar]
- 3.Mat S et al. (2022) Factors influencing quality of life among older persons living with osteoarthritis using 3 different definitions. Topics in Geriatric Rehabilitation 38, 26–34. 10.1097/TGR.0000000000000340 [DOI] [Google Scholar]
- 4.Luyten FP et al. (2012) Definition and classification of early osteoarthritis of the knee. Knee Surgery, Sports Traumatology, Arthroscopy 20, 401–406. 10.1007/s00167-011-1743-2 [DOI] [PubMed] [Google Scholar]
- 5.Bay-Jensen A-C et al. (2010) Which elements are involved in reversible and irreversible cartilage degradation in osteoarthritis? Rheumatology International 30, 435–442. 10.1007/s00296-009-1183-1 [DOI] [PubMed] [Google Scholar]
- 6.Jonsson H et al. (2016) Incidence and prevalence of total joint replacements due to osteoarthritis in the elderly: risk factors and factors associated with late life prevalence in the AGES-Reykjavik study. BMC Musculoskeletal Disorders 17, 1–8. 10.1186/s12891-016-0864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamsan SS et al. (2021) Healthcare utilization and knee osteoarthritis symptoms among urban older Malaysian. International Journal of Environmental Research and Public Health 18, 3777. 10.3390/ijerph18073777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh DKA et al. (2019) Diabetes, arthritis, urinary incontinence, poor self-rated health, higher body mass index and lower handgrip strength are associated with falls among community-dwelling middle-aged and older adults: pooled analyses from two cross-sectional Malaysian datasets. Geriatrics & Gerontology International 19, 798–803. 10.1111/ggi.13717 [DOI] [PubMed] [Google Scholar]
- 9.O'Neill TW, McCabe PS and McBeth J (2018) Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Practice & Research in Clinical Rheumatology 32, 312–326. 10.1016/j.berh.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Courties A, Berenbaum F and Sellam J (2019) The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Joint, Bone, Spine: Revue du Rhumatisme 86, 725–730. 10.1016/j.jbspin.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 11.Tan Q et al. (2021) Metabolic syndrome and osteoarthritis: possible mechanisms and management strategies. Medicine in Novel Technology and Devices 9, 100052. 10.1016/j.medntd.2020.100052 [DOI] [Google Scholar]
- 12.Lin X et al. (2020) Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific Reports 10, 14790. 10.1038/s41598-020-71908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courties A and Sellam J (2016) Osteoarthritis and type 2 diabetes mellitus: what are the links? Diabetes Research and Clinical Practice 122, 198–206. 10.1016/j.diabres.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 14.Verzijl N et al. (2003) AGEing and osteoarthritis: a different perspective. Current Opinion in Rheumatology 15, 616–622. 10.1097/00002281-200309000-00016 [DOI] [PubMed] [Google Scholar]
- 15.Schwarz S et al. (2018) The interrelation of osteoarthritis and diabetes mellitus: considering the potential role of interleukin-10 and in vitro models for further analysis. Inflammation Research 67, 1–16. 10.1007/s00011-017-1121-8 [DOI] [PubMed] [Google Scholar]
- 16.Shazwani N et al. (2010) Assessment of physical activity level among individuals with type 2 diabetes mellitus at Cheras Health Clinic, Kuala Lumpur. Malaysian Journal of Nutrition 16, 101–112. [PubMed] [Google Scholar]
- 17.Bay-Jensen AC et al. (2016) Osteoarthritis year in review 2015: soluble biomarkers and the BIPED criteria. Osteoarthritis and Cartilage 24, 9–20. 10.1016/j.joca.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 18.Bodaghi A, Fattahi N and Ramazani A (2023) Biomarkers: promising and valuable tools towards diagnosis, prognosis and treatment of COVID-19 and other diseases. Heliyon 9, e13323. 10.1016/j.heliyon.2023.e13323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Spil WE et al. (2019) Osteoarthritis phenotypes and novel therapeutic targets. Biochemical Pharmacology 165, 41–48. 10.1016/j.bcp.2019.02.037 [DOI] [PubMed] [Google Scholar]
- 20.Ouzzani M et al. (2016) Rayyan – a web and mobile app for systematic reviews. Systematic Reviews 5, 210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells GA et al. (2000) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford.
- 22.Luo Y et al. (2022) Protein N-glycosylation aberrations and glycoproteomic network alterations in osteoarthritis and osteoarthritis with type 2 diabetes. Scientific Reports 12, 6977. 10.1038/s41598-022-10996-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q et al. (2021) Hyperglycemia-induced accumulation of advanced glycosylation end products in fibroblast-like synoviocytes promotes knee osteoarthritis. Experimental & Molecular Medicine 53, 1735–1747. 10.1038/s12276-021-00697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scotece M et al. (2020) Novel adipokine associated with OA: retinol binding protein 4 (RBP4) is produced by cartilage and is correlated with MMPs in osteoarthritis patients. Inflammation Research 69, 415–421. 10.1007/s00011-020-01326-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silawal S et al. (2019) IL-10 could play a role in the interrelation between diabetes mellitus and osteoarthritis. International Journal of Molecular Sciences 20, 768. 10.3390/ijms20030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vertti RDA et al. (2019) Cartilage oligomeric matrix protein levels in type 2 diabetes associated with primary knee osteoarthritis patients. Genetic Testing and Molecular Biomarkers 23, 16–22. [DOI] [PubMed] [Google Scholar]
- 27.Eitner A et al. (2017) Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain 158, 1743–1753. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W et al. (2017) Hyperglycemia-related advanced glycation end-products is associated with the altered phosphatidylcholine metabolism in osteoarthritis patients with diabetes. PLoS ONE 12, e0184105. 10.1371/journal.pone.0184105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamada D et al. (2016) Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis & Rheumatology 68, 1392–1402. 10.1002/art.39561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ribeiro M et al. (2016) Insulin decreases autophagy and leads to cartilage degradation. Osteoarthritis and Cartilage 24, 731–739. 10.1016/j.joca.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 31.Zhang WD et al. (2016) Metabolomic analysis of human synovial fluid and plasma reveals that phosphatidylcholine metabolism is associated with both osteoarthritis and diabetes mellitus. Metabolomics 12, 1–10. [Google Scholar]
- 32.Laiguillon MC et al. (2015) Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis and Cartilage 23, 1513–1522. 10.1016/j.joca.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 33.Tsai TL, Manner PA and Li WJ (2013) Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthritis and Cartilage 21, 368–376. 10.1016/j.joca.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 34.Tsai C-H et al. (2013) High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochimica et Biophysica Acta – General Subjects 1830, 2649–2658. 10.1016/j.bbagen.2012.12.017 [DOI] [PubMed] [Google Scholar]
- 35.Rosa SC et al. (2011) Role of glucose as a modulator of anabolic and catabolic gene expression in normal and osteoarthritic human chondrocytes. Journal of Cellular Biochemistry 112, 2813–2824. [DOI] [PubMed] [Google Scholar]
- 36.Oren TW et al. (2011) Arthroplasty in veterans: analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. Journal of Rehabilitation Research & Development 48, 1195–1210. 10.1682/jrrd.2010.09.0186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosa SC et al. (2009) Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Research & Therapy 11, R80. 10.1186/ar2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus VB et al. (2011) Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthritis and Cartilage 19, 515–542. 10.1016/j.joca.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter DJ et al. (2014) Biomarkers for osteoarthritis: current position and steps towards further validation. Best Practice & Research in Clinical Rheumatology 28, 61–71. 10.1016/j.berh.2014.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arellano RD et al. (2017) Cartilage oligomeric matrix protein levels in synovial fluid in patients with primary knee osteoarthritis and healthy controls: a preliminary comparative analysis with serum cartilage oligomeric matrix protein. Archives of Rheumatology 32, 189–196. 10.5606/ArchRheumatol.2017.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plsikova Matejova J et al. (2020) A preliminary study of combined detection of COMP, TIMP-1, and MMP-3 in synovial fluid: potential indicators of osteoarthritis progression. Cartilage 13, 1421S–1430S. 10.1177/1947603520946385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Beyer A and Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550. 10.1016/j.cell.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 43.Ge J et al. (2020) IL-10 delays the degeneration of intervertebral discs by suppressing the p38 MAPK signaling pathway. Free Radical Biology and Medicine 147, 262–270. 10.1016/j.freeradbiomed.2019.12.040 [DOI] [PubMed] [Google Scholar]
- 44.Heywood HK et al. (2014) Culture expansion in low-glucose conditions preserves chondrocyte differentiation and enhances their subsequent capacity to form cartilage tissue in three-dimensional culture. BioResearch Open Access 3, 9–18. 10.1089/biores.2013.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marlovits S et al. (2004) Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. Journal of Bone and Joint Surgery 86–B, 286–295. 10.1302/0301-620X.86B2.14918 [DOI] [PubMed] [Google Scholar]
- 46.Zhang M, Grote C and Wang J (2019) Chapter 9 – Epigenetic mechanisms underlying the pathogenesis of osteoarthritis and their clinical relevance. In Sharma S (ed.), Prognostic Epigenetics. United States: Academic Press, pp. 245–268. [Google Scholar]
- 47.Zhou M et al. (2018) Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 35, 345–360. 10.1016/j.ebiom.2018.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caramés B et al. (2010) Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis and Rheumatism 62, 791–801. 10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López de Figueroa P et al. (2015) Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis & Rheumatology 67, 966–976. 10.1002/art.39025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matta C and Mobasheri A (2014) Regulation of chondrogenesis by protein kinase C: emerging new roles in calcium signalling. Cellular Signalling 26, 979–1000. 10.1016/j.cellsig.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 51.Zupan J et al. (2018) VEGF-A is associated with early degenerative changes in cartilage and subchondral bone. Growth Factors 36, 263–273. 10.1080/08977194.2019.1570926 [DOI] [PubMed] [Google Scholar]
- 52.Zhang Q et al. (2018) VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type-2 diabetes: a cohort study. Medicine 97, e0415. 10.1097/MD.0000000000010415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haseeb A et al. (2021) SOX9 keeps growth plates and articular cartilage healthy by inhibiting chondrocyte dedifferentiation/osteoblastic redifferentiation. Proceedings of the National Academy of Sciences 118, e2019152118. 10.1073/pnas.2019152118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shrestha N et al. (2021) Pathological β-cell endoplasmic reticulum stress in type 2 diabetes: current evidence. Frontiers in Endocrinology 12, 1–7. 10.3389/fendo.2021.650158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen J et al. (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Developmental Cell 3, 99–111. 10.1016/S1534-5807(02)00203-4 [DOI] [PubMed] [Google Scholar]
- 56.Wen Z et al. (2023) Endoplasmic reticulum stress in osteoarthritis: a novel perspective on the pathogenesis and treatment. Aging and Disease 14, 283–286. 10.14336/ad.2022.0725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berenbaum F (2011) Diabetes-induced osteoarthritis: from a new paradigm to a new phenotype. Annals of the Rheumatic Diseases 70, 1354. 10.1136/ard.2010.146399 [DOI] [PubMed] [Google Scholar]
- 58.Dickson BM et al. (2019) The burden of metabolic syndrome on osteoarthritic joints. Arthritis Research & Therapy 21, 289. 10.1186/s13075-019-2081-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapoor M et al. (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nature Reviews Rheumatology 7, 33–42. 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 60.Kierdorf K and Fritz G (2013) RAGE regulation and signaling in inflammation and beyond. Journal of Leukocyte Biology 94, 55–68. 10.1189/jlb.1012519 [DOI] [PubMed] [Google Scholar]
- 61.Rasheed Z, Akhtar N and Haqqi TM (2011) Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology 50, 838–851. 10.1093/rheumatology/keq380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H et al. (2017) New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthritis Research & Therapy 19, 248. 10.1186/s13075-017-1454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verma P and Dalal K (2011) ADAMTS-4 and ADAMTS-5: key enzymes in osteoarthritis. Journal of Cellular Biochemistry 112, 3507–3514. 10.1002/jcb.23298 [DOI] [PubMed] [Google Scholar]
- 64.Xue J et al. (2013) Tumor necrosis factor-α induces ADAMTS-4 expression in human osteoarthritis chondrocytes. Molecular Medicine Reports 8, 1755–1760. 10.3892/mmr.2013.1729 [DOI] [PubMed] [Google Scholar]
- 65.Zeng GQ et al. (2015) High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genetics and Molecular Research 14, 14811-14822. 10.4238/2015.November.18.46 [DOI] [PubMed] [Google Scholar]
- 66.Beg M et al. (2017) Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. eLife 6, e26896. 10.7554/eLife.26896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byles V et al. (2013) The TSC–mTOR pathway regulates macrophage polarization. Nature Communications 4, 2834. 10.1038/ncomms3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lumeng CN, Bodzin JL and Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation 117, 175–184. 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koo S-J et al. (2018) Pentose phosphate shunt modulates reactive oxygen species and nitric oxide production controlling Trypanosoma cruzi in macrophages. Frontiers in Immunology 9, 202. 10.3389/fimmu.2018.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salminen A, Kaarniranta K and Kauppinen A (2016) AMPK and HIF signaling pathways regulate both longevity and cancer growth: the good news and the bad news about survival mechanisms. Biogerontology 17, 655–680. 10.1007/s10522-016-9655-7 [DOI] [PubMed] [Google Scholar]
- 71.Li H et al. (2018) RBP4 regulates trophoblastic cell proliferation and invasion via the PI3K/AKT signaling pathway. Molecular Medicine Reports 18, 2873–2879. 10.3892/mmr.2018.9240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moraes-Vieira Pedro M et al. (2014) RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metabolism 19, 512–526. 10.1016/j.cmet.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scotece M et al. (2018) Novel adipokine associated with osteoarthritis: retinol binding protein 4 is produced by cartilage and correlates with matrix metalloproteinasesin osteoarthritis patients. Osteoarthritis and Cartilage 26, S126–S127. 10.1016/j.joca.2018.02.276 [DOI] [Google Scholar]
- 74.Chen Y, Crawford RW and Oloyede A (2007) Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles. Journal of Orthopaedic Surgery and Research 2, 14. 10.1186/1749-799X-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hills BA (2000) Role of surfactant in peritoneal dialysis. Peritoneal Dialysis International 20, 503–515. 10.1177/089686080002000505 [DOI] [PubMed] [Google Scholar]
- 76.Stanley P et al. (2022) N-Glycans, 4th edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, Chapter 9. [Google Scholar]
- 77.Shaoul E et al. (1995) Fibroblast growth factor receptors display both common and distinct signaling pathways. Oncogene 10, 1553–1561. [PubMed] [Google Scholar]
- 78.Wu Z et al. (2020) Identification of the key gene and pathways associated with osteoarthritis via single-cell RNA sequencing on synovial fibroblasts. Medicine 99, e21707. 10.1097/MD.0000000000021707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun K et al. (2020) The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis and Cartilage 28, 400–409. 10.1016/j.joca.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 80.Xue J-F et al. (2017) Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomedicine & Pharmacotherapy 89, 1252–1261. 10.1016/j.biopha.2017.01.130 [DOI] [PubMed] [Google Scholar]
- 81.Jürgensen HJ et al. (2011) A novel functional role of collagen glycosylation: interaction with the endocytic collagen receptor uPARAP/ENDO180. Journal of Biological Chemistry 286, 32736–32748. 10.1074/jbc.M111.266692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shim K et al. (2020) Complement activation in obesity, insulin resistance, and type 2 diabetes mellitus. World Journal of Diabetes 11, 1. 10.4239/wjd.v11.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seow et al. supplementary material
For supplementary material accompanying this paper visit http://doi.org/10.1017/erm.2024.7.
click here to view supplementary material