Abstract
Enediyne antibiotics are a striking family of DNA-cleaving natural products with high degrees of cytotoxicity and structural complexity. Microbial genome sequences, which have recently accumulated, point to an untapped trove of “cryptic” enediynes. Most of the cognate biosynthetic gene clusters (BGCs) are sparingly expressed under standard growth conditions, making it difficult to characterize their products. Herein, we report a fluorescence-based DNA cleavage assay coupled with high-throughput elicitor screening for the rapid, targeted discovery of cryptic enediyne metabolites. We applied the approach to Streptomyces clavuligerus, which harbors two such BGCs with unknown products, identified steroids as effective elicitors, and characterized 10 cryptic enediyne-derived natural products, termed clavulynes A–J with unusual carbonate and terminal olefin functionalities, with one of these congeners matching the recently reported jejucarboside. Our results contribute to the growing repertoire of enediynes and provide a blueprint for identifying additional ones in the future.
Graphical Abstract

INTRODUCTION
Enediyne antibiotics are a fascinating group of natural products with a distinctive molecular architecture that consists of a 9- or 10-membered carbocycle with two acetylenic groups conjugated to an olefin.1,2 In addition to this latently reactive framework, enediynes typically harbor a chemical activation moiety as well as a targeting functionality, the latter of which facilitates binding to the minor groove of DNA.3–5 Upon cycloaromatization, the enediyne core undergoes Bergman or Myers–Saito rearrangement to generate a benzenoid diradical, which can cause site-specific double-stranded DNA cleavage.2,6–8 The first natural enediyne, neocarzinostatin, was reported in 1985, and the family has gradually grown since.9,10 Despite notable efforts, enediynes still remain rare as a mere 14 members have been structurally elucidated and 6 additional products have been characterized in cycloaromatized forms.2,11,12 Hundreds of enediyne biosynthetic gene clusters (BGCs) can be observed in microbial genomes;13,14 however, most are transcriptionally silent or sparingly expressed under standard laboratory growth conditions. As a result, identification of enediynes has typically required cultures in the hundred-to-thousand-liter range.12,15 New methods are thus needed to unlock the stores of cryptic enediyne natural products that can be detected bioinformatically.
Over the past decade, a suite of approaches have emerged for inducing silent BGCs, including manipulation of culture conditions, heterologous expression, genetic alterations, and chemical genetics approaches.16–18 As part of these efforts, we have contributed high-throughput elicitor screening (HiTES), a forward chemical genetics method that identifies small-molecule inducers of silent natural product BGCs.19 Dozens of cryptic metabolites have been identified using HiTES, which has been coupled to various read-outs, such as genetic reporters, mass spectrometry (MS), or antimicrobial assays.19–22 Given that enediyne antibiotics are potent DNA cleavers, combining HiTES with a suitable DNA cleavage assay seemed practical for detecting cryptic enediynes. Especially appealing are fluorescence resonance energy transfer (FRET)-based assays using a scission beacon, in which a donor fluorophore and an acceptor quencher are attached to the opposite ends of an oligonucleotide probe (Figure 1).23–25 The probe does not fluoresce when the chromophores are maintained in close proximity by a stem-loop structure. However, when the oligonucleotide is cleaved upon exposure to an enediyne, the quencher is separated from the fluorophore, thus giving rise to turn-on fluorescence and enabling identification of DNA-cleaving agents with exquisite sensitivity, with a reportedly lower limit of detection of 0.3 nM.25 Moreover, detection of heptaene, a possible biosynthetic intermediate identified during enediyne production, can be used as an additional read-out.26,27 We envisioned that these assays could facilitate detection and targeted discovery of cryptic enediynes.
Figure 1.

FRET-coupled HiTES workflow. Bacterial cultures are prepared in 96-well plates and subjected to HiTES to activate biosynthesis of cryptic secondary metabolites. The resulting culture supernatants are examined for DNA cleavage activity using FRET-based assays (top path). Production of heptaene, a key metabolite in enediyne biosynthesis, is monitored by HPLC-MS analysis as validation (bottom path).
Herein, we report the application of FRET-guided HiTES to Streptomyces clavuligerus, a well-characterized soil-dwelling model bacterium that harbors two predicted enediyne BGCs.13 HiTES identified steroids as strong inducers of DNA-cleaving metabolites, and FRET-based assays in conjunction with high-resolution mass spectrometry (HR-MS) facilitated the isolation of 10 cryptic enediyne natural products in their cycloaromatized forms, which we characterized structurally by extensive spectroscopic analyses. Of these, nine were novel congeners, whereas one was identical to the recently reported jejucarboside.12 Aside from adding to the repertoire of enediyne antibiotics, our results connect a previously unassigned enediyne BGC to its product in an already “drained” model strain. More broadly, the described approach can be employed toward the characterization of other cryptic DNA-cleaving natural products that are encoded in microbial genomes.
RESULTS AND DISCUSSION
Enediyne DNA Cleavage Assay.
We commenced by devising a high-throughput DNA cleavage assay. Based on the work of Thorson and colleagues, we generated a 24-mer nucleotide consisting of a T4 loop and 10 complementary base-pairs (bps) of double-stranded DNA.25 The 5′- and 3′-ends carried a fluorescein fluorophore and the azobenzene-based Black Hole Quencher 1a (BHQ1a), respectively. We tested the probe by incubating it with various control conditions and cleared supernatants derived from the stationary phase cultures of Streptomyces globisporus, the producer of the enediyne C-1027 (Figures S1 and S2).28,29 As expected, no fluorescence was detected in the control lacking S. globisporus, nor with culture supernatants of Streptomyces canus, which does not encode an enediyne BGC. The DNA intercalator ethidium bromide did not result in turn-on fluorescence either. With the filtered supernatants of S. globisporus, however, we detected time-dependent fluorescence emission, indicating that the assay was able to detect C-1027, which has been reported at approximately 6 mg/L culture under these conditions.29 These results are consistent with the lower limit of detection of 0.3 nM reported by Biggins et al., which is significantly more sensitive than HPLC-MS-based detection.25
FRET-Guided HiTES in S. clavuligerus.
The best test for a method that can reliably identify enediynes is application to a model strain that has been overexploited by extensive natural product mining. With this idea in mind, we selected S. clavuligerus, the industrial producer of clavulanic acid, as a proof of concept because its cultures have been mined for decades, but the strain still possesses two unassigned enediyne BGCs.13 We subjected the strain to HiTES by culturing it in 96-well plates and challenging it with a 400-membered library of functionally diverse small molecules (Figure 1). Because trace levels of sodium iodide (NaI) have been reported to enhance the yields of enediynes by up to ~300-fold,30 NaI was included in every well at a concentration of 3 μM, except in the control wells. After a six-day culture period, cell-free supernatants from each well were subjected to the FRET-based DNA cleavage assay, and the percent activity was subsequently quantified.
S. clavuligerus cultures in the presence of NaI showed enhanced DNA cleavage activity relative to the growth medium alone (Figure 2A), consistent with augmented enediyne biosynthesis. Of the 400 compounds, 19 elicitors that induced higher DNA cleavage activity by two standard deviations compared to the average of NaI-treated S. clavuligerus cultures were identified (Figures 2B and S3, Table S1). The structure–activity relationships among these 19 elicitors indicated that steroids, notably testosterone (1), estrone (5), cortisone, and danazol, were among the best inducers (Figures 2C and S3A). Steroids can play important roles in bacteria, functioning as signaling agents or carbon sources.31,32 Elicitation in S. clavuligerus may involve regulation of signal transduction pathways and/or an additional nutrient supply. Determination of the mode of elicitation is an elaborate process;33–35 further studies are necessary to elucidate the underlying mechanisms. Moreover, benserazide (2), lactulose (3), and N-methylhydroxynicotinic acid (4) also served as strong inducers. We confirmed that the top five elicitors were all inactive in the DNA cleavage assay (Figure S3B).
Figure 2.
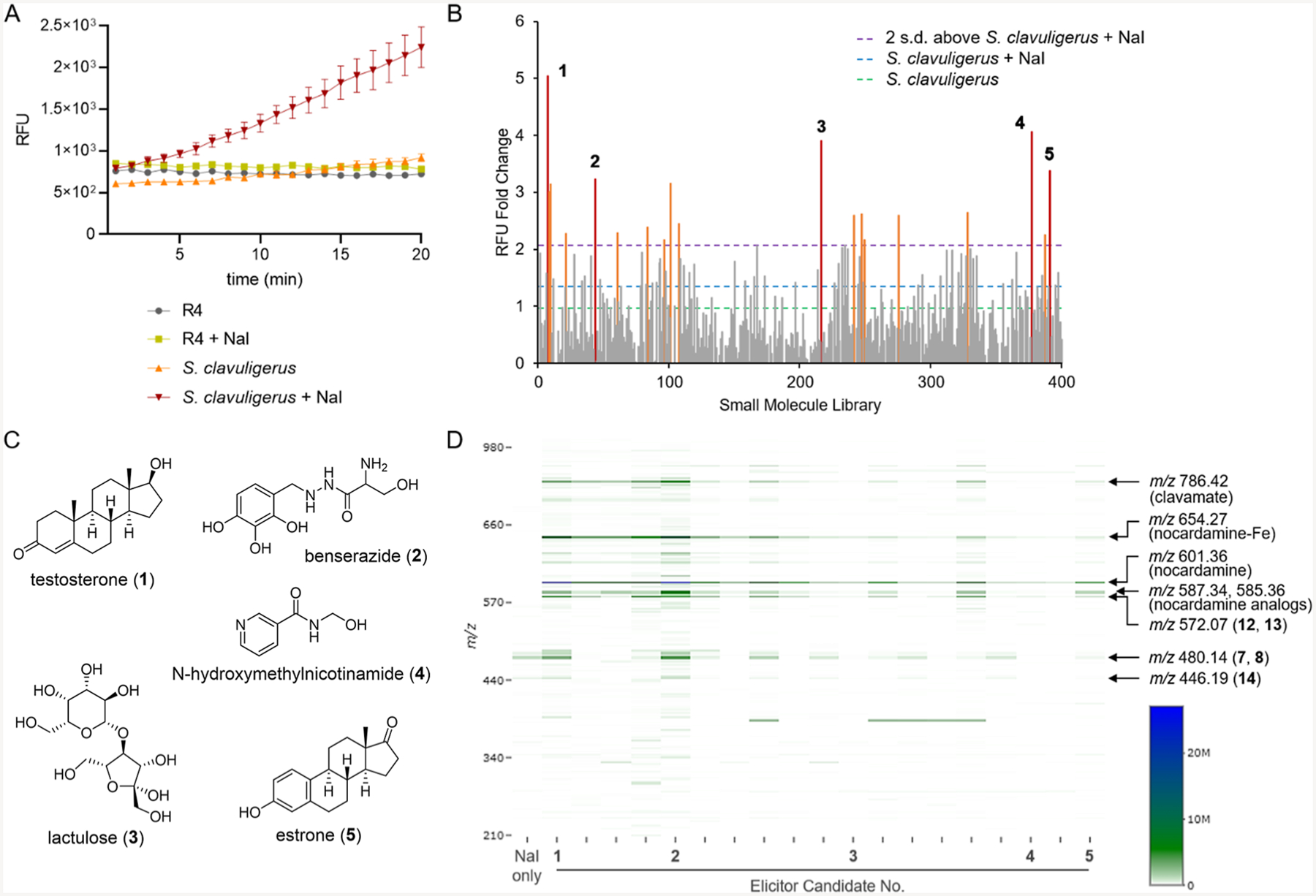
Induction of potential cryptic enediyne metabolites from S. clavuligerus by FRET-coupled HiTES. (A) Cleavage of the FRET DNA probe by S. clavuligerus cultures in the presence and absence of 3 μM NaI. Compared to negative controls (R4 medium or R4 medium + NaI), S. clavuligerus culture supernatants displayed modest fluorescence emission over time. The extent of DNA cleavage was considerably higher with NaI supplementation. (B) Relative DNA cleavage activities elicited from S. clavuligerus by 400 small-molecule inducers. Potential elicitors that resulted in DNA cleavage activities of >2 standard deviations above the mean of NaI-treated cultures are marked in orange with the top-five candidates numbered and shown in red. (C) Chemical structures of the top-five elicitors. See Figure S3 for structures of all candidate elicitors. (D) Cryptic secondary metabolome of S. clavuligerus as a function of 19 candidate elicitors. The plot displays elicitor-dependent production of cryptic metabolites with intensities of >2-fold of the unelicited control. These are marked by m/z and MS intensity according to the color bar and include nocardamine, its analogues, and several cryptic metabolites.
Enediyne natural products often contain one or more halogens.2,12 We therefore set out to search for halogenated metabolites present only in elicitor-treated conditions. S. clavuligerus was cultured in the presence of each of the 19 elicitors, the supernatants assessed by HPLC-Qtof-MS, and the resulting traces analyzed using MetEX, an in-house application for interrogating multi-dimensional HR-MS datasets.36 All detected ions above a selected threshold were subtracted from twice the average abundance of the unelicited control. The resulting data were arranged in a heatmap (Figure 2D), which shows the m/z (y-axis) and abundance as measured by the MS intensity of each induced metabolite (z-axis) as a function of elicitor (x-axis). Several known natural products were dereplicated, including nocardamine (m/z 601.36), demethylene-nocardamine (m/z 587.34), deoxynocardamine (m/z 585.36), and clavamate A (m/z 786.42).37 The heatmap pointed to cryptic metabolites in the m/z range of 400–600. Enhanced production was observed for metabolites with m/z 480.14, 446.19, and 572.07, all of which were not detected in the absence of NaI and elicitor (Table S2). We suspected that some of these newly induced metabolites were responsible for the observed DNA cleavage activity and that, as with previous results, the concentration of the active form of enediyne may be below the limit of HPLC-MS detection (~1 μM) but above that of the fluorescence-based DNA cleavage assay (~0.3 nM as reported by Biggins et al.).25
Prioritization and Validation.
Among the tabulated metabolites, m/z 480.14 stood out with significant induction in the presence of most elicitors (Figures 2D and 3A). Two apparently isomeric metabolites exhibited this m/z with different retention times (Figure 3B). Moreover, both showed mass isotope patterns characteristic of monochlorination, a common feature of enediynes (Figure 3B).1–4 The 1:1 abundance ratio of the two metabolites is consistent with the cycloaromatized products of the benzenoid diradical that can be quenched at two different carbons to give distinctly chlorinated regioisomers. Similar observations have been made with sporolides A and B as well as cyanosporasides A and B (Figure S4).38,39 Given that m/z 480.14 did not return matches in the Natural Product Atlas and consisted of chlorinated regioisomers, we prioritized it for downstream studies.40
Figure 3.

Identification of cryptic enediynes. (A) Comparative HPLC-MS profiles of S. clavuligerus cultures as a function of NaI and elicitors 1 and 2. Induced known metabolites (nocardamine and analogues) are shaded in gray and induced new metabolites, as judged by HR-MS, in blue. (B) Extracted ion chromatograms of m/z 480.14 from S. clavuligerus cultures in the presence or absence of 3 μM NaI and 28 μM 1; the inset shows the mass spectrum of 7 with the distinctive Cl isotope pattern. (C) Structure of 6, an analogue of 1. (D) Dose-dependent induction of m/z 480.14 upon co-treatment of S. clavuligerus with NaI and 6. Shown are averages of two independent biological replicates. (E) Validation of 3 μM NaI and 100 μM 6 as inducers of heptaene (shaded in blue) from S. clavuligerus and S. globisporus (as control). (F) The UV/Vis spectrum of the induced metabolite, shaded blue in panel E, is consistent with the heptaene intermediate.
HiTES identified 1 as the best elicitor. However, due to restrictions, this steroid was difficult to obtain. We therefore validated production of the putative enediyne with the structurally analogous and commercially available 11α-hydroxyprogesterone (6, Figure 3C). Flask cultures containing NaI and varying concentrations of 6 showed a clear dose-response in the production of the target compounds with m/z 480.14 (7 and 8) with a half-maximal elicitor concentration of approximately 6 μM and optimal production at 100 μM (Figure 3D). NaI, by itself, induced the otherwise completely silent signal to a detectable level, and provision of 6 led to an additional ~6-fold overproduction.
As further validation, we sought to detect the heptaene metabolite that has been shown to be diagnostic for active enediyne biosynthesis (Figure 1).26,27,41,42 A dose-dependent induction of heptaene with its distinctive UV/Vis signature was observed in the presence of NaI and 6, but not when these molecules were absent, thus providing additional support for the induced biosynthesis of DNA-cleaving metabolites (Figure 3E,F). Likewise, the stimulatory effects of 2, a non-steroidal elicitor, were confirmed in flask cultures where induction of 7 and 8 along with that of the heptaene product was detected (Figure S5).
Purification and Structural Elucidation of Clavulynes.
To characterize the target compounds, medium-scale production cultures (3 L) of S. clavuligerus were generated in the presence of NaI (3 μM) and 6 (100 μM), yielding 2.0 and 2.2 mg of pure 7 and 8, respectively, per L culture. The HR-MS data suggested a molecular formula of C23H26ClNO8. The 11 degrees of unsaturation required by the formula, coupled with NMR data, suggested that these metabolites are composed of five rings.
Extensive analysis of 1D/2D NMR spectra of 7 suggested the presence of an amino sugar connected to an aromatic chromophore (Figures 4A,B and S6 and Table S3). Strong HMBC correlations from the H-1s to C-3 and unique 3JHH coupling constants (17.5 and 11.0 Hz) between the two H-1s and H-2 were consistent with the presence of a terminal olefin (Figure 4A). A suite of HMBC correlations from H-4 to C-5 and C-6 as well as H-15 to C-4 and C-6 together with observed 1H/13C chemical shifts pointed to a chlorobenzene aromatic ring. COSY cross-peaks between H-9, H-10, and H-12 and additional HMBC correlations suggested a cyclopentene moiety. The connection between the chlorobenzene and the five-membered ring was established by HMBC signals from the methine proton H-7 to C-13 and C-14, which also confirmed the presence of an additional five-membered ring located between the two units. The carbonate functionality at C-11 was deduced based on a carbon chemical shift (155.0 ppm) and an HMBC correlation from H-9 to C-11. COSY correlations from H-1′ to H-2′ and the presence of a quaternary carbon at C-3′ (74.5 ppm) together with COSY and HMBC correlations and chemical shift analysis pointed to an unusual amino sugar. An HMBC cross-peak from H-1′ to C-8 confirmed the linkage between the aglycone and the amino sugar, completing the planar structure of 7.
Figure 4.

Characterization of clavulynes A-D. (A) Key NMR correlations used to solve the structures of 7–10 (see Tables S3 and S4). (B) Structures and numbering schemes for clavulynes A-D. (C) Relative configuration of the amino sugar based on ROESY correlations. (D) KEY ROESY correlations between the amino sugar and the aglycone of 7.
The absolute configuration of 7 was determined via 1D ROESY analysis and application of Snatzke’s method (Figure 4B–D).43–46 The magnitude of 1JCH coupling constants of 161.4 Hz for H-1′, measured from the 1JCH satellites in the gHMBC spectrum, suggested that the anomeric carbon is β-configured.47 A ROESY correlation between H-9 and H-1′ suggested a trans-configuration of the C-9 glycoside and C-8 hydroxyl bond. A large diaxial coupling constant (7.5 Hz) between H-1′ and H-2′ established an axial position for H-2′. ROESY correlations between H1′ and H-5′ and between H-4′ and H-5′ indicated axial and equatorial configurations, respectively. Other ROEs from H-7 to H-9, H-1′, and H-5′ and from H-12 to H-5′ determined the relative configuration of the aglycone with respect to the amino sugar moiety (Figure 4D). Lastly, C-2′ and C-3′ were determined to be R-configured based on Snatzke’s method (Figure S7).43 With that, the absolute configuration was fully determined to be 7R, 8R, 9S, 10S, 1′S, 2′R, 3′R, 4′S, and 5′R, thus completing the structure of 7. We have termed this compound clavulyne A; it is a new cycloaromatized enediyne natural product.
The 1H NMR spectrum of 8 was almost identical to that of 7 except for the two aromatic signals in the chlorobenzene ring (Figures 4B and S8, Table S3), which appeared as doublets in 8. Inspection of HMBC and COSY data revealed that these protons were ortho to each other with the chlorine atom now substituting C-15 (Figure 4B). The absolute configuration of 8 was determined to be identical to 7 by the analysis of circular dichroism (CD) spectroscopic data (Figure S9), thus completing the structure of 8, termed clavulyne B. In a recent, impressive effort by Oh and colleagues, this metabolite was isolated from 360 L cultures of a marine streptomycete and termed jejucarboside.12
Additional metabolites with m/z 512, clavulyne C (9) and D (10), were detected during the purification of 7 and 8 with mass isotope patterns consistent with monochlorinated metabolites. HR-MS analysis of 9 and 10 suggested the molecular formula C24H30ClNO9, and 1D/2D NMR spectra indicated that these compounds were analogues generated by ring opening of the cyclic carbonate in 7 and 8 (Figures 4A and S10, S11, Table S4). The most noticeable difference in their 1H NMR spectra is the appearance of a new methoxy peak at 3.73 ppm (Figures S12 and S13). Also, the methine peak at C-9 changed from δC 73.4/δH 5.28 in 7 to δC 68.0/δH 4.48 in 9, suggesting that the position is substituted by a hydroxyl group rather than the carbonate functionality. Absolute configurations of 9 and 10 were found to be identical to that of 7 based on their superimposable CD spectra (Figure S9).
Characterization of Additional Clavulyne Congeners.
Given that the tandem HR-MS (HR-MS/MS) analysis of clavulynes A–D gave rise to an m/z 174.11 fragment, corresponding to the amino sugar moiety, we screened the other 6-induced metabolites for this feature and identified m/z 446.19 (11), 572.07, (12 and 13), 462.18 (14), 420.21 (15), and 454.17 (16) as additional putative clavulyne congeners (Figures 3A and S14). These were also prioritized by metabolomic analysis (Figure 2D). We subsequently isolated 11 from large-scale cultures (8 L) and characterized its structure. 1H NMR and HR-MS data showed 11 (clavulyne E) to be the hydrogenated congener of 7 (Figures 5A and S15, Table S2). Trace amounts of this form, which arises from double H-atom transfer to the diradical intermediate, were reported for sporolide and cyanosporaside;38,39 it is confirmed here for the first time by NMR. Lastly, we were able to deduce the possible structures of the remaining metabolites based on HR-MS and HR-MS/MS analysis (Figure 5B–E and Table S2). 12 and 13 correspond to the iodinated variants with the halogen substituting at C-5 and C-15, respectively. 14 is proposed to be the C-15-hydroxy variant of 11, whereas 15 possesses a 1,2-diol moiety in the place of a carbonate functionality. 16 was identified as the chlorinated variant of 15. Interestingly, 12 and 13 were found in a 1:2 ratio, perhaps reflecting the steric constraints posed by radical quenching at C-15. Monobrominated products were reported for sporolide and cyanosporaside;38,39 these were not detected in our case. Together, we identified 10 cycloaromatized products, representing the first structurally characterized enediynes from S. clavuligerus.
Figure 5.

Identification of clavulyne derivatives and the biosynthetic locus. (A–E) HR-MS-extracted ion chromatograms (top) and chemical structures (bottom) for additional analogues of 7. Compared to vehicle (black traces), six additional peaks were induced upon co-treatment with 3 μM NaI and 28 μM 1 (red traces). The structure of 11 was determined by NMR and those of 12–16 were deduced based on HR-MS and HR-MS/MS analysis. (F) Proposed enediyne origin and its cyclization mechanism to yield clavulynes. (G) The clv and ene BGCs in S. clavuligerus. Genes are color-coded as shown. (H) Production of clavulynes, as shown for the extracted ion chromatogram of 7 and 8, occurs in wt and the Δene30-ene31 mutant, but not in Δclv22-clv23.
Putative Structure of the Original Enediyne and BGC Identification.
FRET-based DNA cleavage assays were performed for pure clavulynes A–D (Figure S16). As expected, however, these cycloaromatized metabolites were no longer able to cleave DNA. Nocardamine, a known but induced natural product identified by FRET-HiTES, was also not active (Figures 2D and S16). Given the supreme sensitivity of the FRET-based assay, we surmise that the assay monitored trace levels of the precursor form of clavulyne, which were below the level of detection by HPLC-MS. The lack of stability of the clavulyne precursor, the size of the carbon skeleton, and the para-arrangement of the halides upon cyclization all lead us to conclude that the precursor is a 9-membered ring enediyne (17, Figure 5F) that undergoes Bergman cyclization and quenching of a 1,4-benzenoid diradical to yield clavulynes. There is no obvious DNA-targeting moiety or chemical trigger. Clavulynes, therefore, fall in the class of C-1027, maduropeptin, and the precursors of sporolide, cyanosporaside, fijiolide, amycolamycin, and jejucarboside, wherein the position of a triggering system, if any, is not immediately obvious from the structure of the cyclized product.1,2,12
To identify the clavulyne BGC, we considered two candidate loci near the end of the second chromosome of S. clavuligerus (Figure 5G). Both encode pksE, which is diagnostic for enediyne BGCs, and exhibit overall 26 and 10% homology to the C-1027 cluster, respectively.28 The former, which we have termed clv, seemed more likely not only because of the greater homology to C-1027 but also because it encodes an N-methyltransferase (Clv33) and a glycosyltransferase (Clv36), which are necessary biosynthetic enzymes to generate the sugar moiety in clavulyne (Figure 5G and Table S6). Indeed, these enzymes are highly homologous to SgcA5 and SgcA6, the corresponding enzymes involved in the biosynthesis of C-1027.28 To test the role of the clv locus, we generated an insertional inactivation mutant by replacing the pksE and thioesterase genes (clv22-clv23) with an apramycin resistance marker. Upon treatment with NaI and 6, the deletion mutant was no longer capable of synthesizing any of the clavulyne congeners (Figures 5H and S17). The insertional mutant targeting the other BGC (Δene30-ene31), on the other hand, synthesized clavulynes in the presence of NaI and 6 (Figure 5H). Thus, clv is responsible for the production of clavulynes, setting the stage for future biosynthetic investigations. Moreover, the results suggest that S. clavuligerus can synthesize an additional enediyne natural product from the ene locus, which can now be explored.
CONCLUSIONS
Enediyne antibiotics are among the most potent cytotoxins known for their inhibitory concentrations in the sub-pM range.3 The supreme potency means that a little production goes a long way and, as a result, many enediynes are produced at titers that are difficult to detect even when the biosynthetic pathway is active, thus necessitating culture volumes >100 L.13 Moreover, as with most natural product BGCs, enediyne gene clusters may be poorly expressed or transcriptionally silent under standard growth conditions, thereby further hampering facile discovery of new compounds in spite of the hundreds of BGCs that can be located bioinformatically.13,14,48 To address these challenges, we combined a method for activation of silent BGCs and a suitable bioassay to identify cryptic enediyne antibiotics. Application to S. clavuligerus revealed testosterone and other steroids as potent inducers of the clv BGC and led to the discovery of clavulynes, the cycloaromatized form of a 9-membered enediyne. Whether these elicitors will be useful in unearthing additional enediynes and their mechanism of induction in S. clavuligerus remains to be determined. Our results show that even the heavily studied industrial clavulanic acid producer, S. clavuligerus, still has surprises in store when the appropriate discovery method is utilized.
Clavulynes are notable for several reasons: they appear to be a warhead-only enediyne lacking an easily identifiable triggering group and an obvious DNA-targeting moiety, though the amino sugar may be involved in this process. Like several other enediynes identified to date, they may be primed to undergo cycloaromatization in the absence of a triggering event, though we cannot exclude an activation process.38,39,49,50 Several other structural features will be of interest in the future, including the unusual amino sugar, the pair of vicinal diols, as well as the pendant terminal olefin. In C-1027, it has been proposed that the glycosidic aminomethyl group functions as a basic moiety to facilitate nucleophilic addition and trigger cycloaromatization.51,52 Whether the amino sugar in the precursor to clavulynes plays a similar role remains to be determined.53 All 9-membered ring enediynes have been proposed to originate from an analogous intermediate that carries a terminal olefin pendant to the carbocycle.42 This feature appears to be retained and not further modified in clavulynes. Its function, which may be analogous to that of the pendant cyanide in cyanosporasides, remains to be elucidated. The presence of the terminal olefin and the two vicinal diols, possibly arising from the hydrolysis of an epoxide precursor, provides additional lines of inquiries into clavulyne biosynthesis.2,54 Clavulynes represent a minimalistic enediyne with good production yields (when induced) from a genetically tractable model strain and an identified BGC reported herein, all of which make this compound family a favorable target for biosynthetic investigations. More broadly, our approach provides a blueprint for rapidly identifying new and cryptic enediynes and other DNA-cleaving agents that are encoded in microbial genomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank I. Pelczer for assistance with NMR data acquisition, V. Ying for assistance with optical rotation measurements, and the Edward C. Taylor 3rd Year Fellowship in Chemistry (to E.J.H.), a postdoctoral fellowship from the National Research Foundation in Korea (2020R1A6A3A03037782 to S.R.L.) and the National Institutes of Health (grant GM140034 to M.R.S.) for financial support.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.3c00281.
Complete materials and methods; HR-MS, HR-MS/MS, tabulated NMR data, and 1D/2D NMR spectra for new compounds reported; and analysis of gene deletion mutants (PDF)
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acschembio.3c00281
Contributor Information
Esther J. Han, Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States
Seoung Rak Lee, Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States.
Craig A. Townsend, Department of Chemistry, Johns Hopkins University, Baltimore, Maryland 21218, United States
Mohammad R. Seyedsayamdost, Department of Chemistry, Princeton University, Princeton, New Jersey 08544, United States; Department of Molecular Biology, Princeton University, Princeton, New Jersey 08544, United States
REFERENCES
- (1).Xi Z; Goldberg IH 7.15—DNA-damaging Enediyne Compounds. In Comprehensive Natural Products Chemistry; Barton SD, Nakanishi K, Meth-Cohn O, Eds.; Pergamon, 1999; pp 553–592. [Google Scholar]
- (2).Adhikari A; Teijaro CN; Townsend CA; Shen B 1.12—Biosynthesis of Enediyne Natural Products. In Comprehensive Natural Products III; Liu H-W, Begley TP, Eds.; Elsevier, 2020; pp 365–414. [Google Scholar]
- (3).Nicolaou KC; Smith AL; Yue EW Chemistry and biology of natural and designed enediynes. Proc. Natl. Acad. Sci. U.S.A 1993, 90, 5881–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Shen B; Liu W; Nonaka K Enediyne natural products: biosynthesis and prospect towards engineering novel antitumor agents. Curr. Med. Chem 2003, 10, 2317–2325. [DOI] [PubMed] [Google Scholar]
- (5).Liang ZX Complexity and simplicity in the biosynthesis of enediyne natural products. Nat. Prod. Rep 2010, 27, 499–528. [DOI] [PubMed] [Google Scholar]
- (6).Kraka E; Cremer D Enediynes, enyne-allenes, their reactions, and beyond. Wiley Interdiscip. Rev.: Comput. Mol. Sci 2014, 4, 285–324. [Google Scholar]
- (7).De Voss JJ; Townsend CA; Ding WD; Morton GO; Ellestad GA; Zein N; Tabor AB; Schreiber SL Site-specific atom transfer from DNA to a bound ligand defines the geometry of a DNA-calicheamicin .gamma.1I complex. J. Am. Chem. Soc 1990, 112, 9669–9670. [Google Scholar]
- (8).De Voss JJ; Hangeland JJ; Townsend CA Characterization of the in vitro cyclization chemistry of calicheamicin and its relation to DNA cleavage. J. Am. Chem. Soc 1990, 112, 4554–4556. [Google Scholar]
- (9).Lee MD; Dunne TS; Siegel MM; Chang CC; Morton GO; Borders DB Calichemicins, a novel family of antitumor antibiotics. 1. Chemistry and partial structure of calichemicin .gamma.1I. J. Am. Chem. Soc 1987, 109, 3464–3466. [Google Scholar]
- (10).Lee MD; Dunne TS; Chang CC; Ellestad GA; Siegel MM; Morton GO; McGahren WJ; Borders DB Calichemicins, a novel family of antitumor antibiotics. 2. Chemistry and structure of calichemicin .gamma.1I. J. Am. Chem. Soc 1987, 109, 3466–3468. [Google Scholar]
- (11).Low ZJ; Ma GL; Tran HT; Zou Y; Xiong J; Pang L; Nuryyeva S; Ye H; Hu JF; Houk KN; et al. Sungeidines from a Non-canonical Enediyne Biosynthetic Pathway. J. Am. Chem. Soc 2020, 142, 1673–1679. [DOI] [PubMed] [Google Scholar]
- (12).Im JH; Shin D; Ban YH; Byun WS; Bae ES; Lee D; Du YE; Cui J; Kwon Y; Nam SJ; et al. Targeted Discovery of an Enediyne-Derived Cycloaromatized Compound, Jejucarboside A, from a Marine Actinomycete. Org. Lett 2022, 24, 7188–7193. [DOI] [PubMed] [Google Scholar]
- (13).Rudolf JD; Yan X; Shen B Genome neighborhood network reveals insights into enediyne biosynthesis and facilitates prediction and prioritization for discovery. J. Ind. Microbiol. Biotechnol 2016, 43, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yan X; Ge H; Huang T; Hindra; Yang D; Teng Q; Crnovcic I; Li X; Rudolf JD; Lohman JR; et al. Strain Prioritization and Genome Mining for Enediyne Natural Products. mBio 2016, 7, e02104–e02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Lee MD; Manning JK; Williams DR; Kuck NA; Testa RT; Borders DB Calicheamicins, a novel family of antitumor antibiotics. 3. Isolation, purification and characterization of calicheamicins .BETA.1Br, GAMMA.1Br, ALPHA.2I, ALPHA.3I, BETA.1I, GAMMA.1I and .DELTA.1I. J. Antibiot 1989, 42, 1070–1087. [DOI] [PubMed] [Google Scholar]
- (16).Rutledge PJ; Challis GL Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol 2015, 13, 509–523. [DOI] [PubMed] [Google Scholar]
- (17).Zhang X; Hindra; Elliot MA Unlocking the trove of metabolic treasures: activating silent biosynthetic gene clusters in bacteria and fungi. Curr. Opin. Microbiol 2019, 51, 9–15. [DOI] [PubMed] [Google Scholar]
- (18).Covington BC; Xu F; Seyedsayamdost MR A Natural Product Chemist’s Guide to Unlocking Silent Biosynthetic Gene Clusters. Annu. Rev. Biochem 2021, 90, 763–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Seyedsayamdost MR High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. U.S.A 2014, 111, 7266–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Xu F; Wu Y; Zhang C; Davis KM; Moon K; Bushin LB; Seyedsayamdost MR A genetics-free method for high-throughput discovery of cryptic microbial metabolites. Nat. Chem. Biol 2019, 15, 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Li Y; Lee SR; Han EJ; Seyedsayamdost MR Momomycin, an Antiproliferative Cryptic Metabolite from the Oxytetracycline Producer Streptomyces rimosus. Angew. Chem., Int. Ed 2022, 61, No. e202208573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Moon K; Xu F; Seyedsayamdost MR Cebulantin, a Cryptic Lanthipeptide Antibiotic Uncovered Using Bioactivity-Coupled HiTES. Angew. Chem., Int. Ed 2019, 58, 5973–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Tyagi S; Kramer FR Molecular Beacons: Probes that Fluoresce upon Hybridization. Nat. Biotechnol 1996, 14, 303–308. [DOI] [PubMed] [Google Scholar]
- (24).Tyagi S; Bratu DP; Kramer FR Multicolor molecular beacons for allele discrimination. Nat. Biotechnol 1998, 16, 49–53. [DOI] [PubMed] [Google Scholar]
- (25).Biggins JB; Prudent JR; Marshall DJ; Ruppen M; Thorson JS A continuous assay for DNA cleavage: the application of ″break lights″ to enediynes, iron-dependent agents, and nucleases. Proc. Natl. Acad. Sci. U.S.A 2000, 97, 13537–13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Zhang J; Van Lanen SG; Ju J; Liu W; Dorrestein PC; Li W; Kelleher NL; Shen B A phosphopantetheinylating polyketide synthase producing a linear polyene to initiate enediyne antitumor antibiotic biosynthesis. Proc. Natl. Acad. Sci. U.S.A 2008, 105, 1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Bhardwaj M; Cui Z; Daniel Hankore E; Moonschi FH; Saghaeiannejad Esfahani H; Kalkreuter E; Gui C; Yang D; Phillips GN Jr.; Thorson JS; et al. A discrete intermediate for the biosynthesis of both the enediyne core and the anthraquinone moiety of enediyne natural products. Proc. Natl. Acad. Sci. U.S.A 2023, 120, No. e2220468120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Liu W; Christenson SD; Standage S; Shen B Biosynthesis of the enediyne antitumor antibiotic C-1027. Science 2002, 297, 1170–1173. [DOI] [PubMed] [Google Scholar]
- (29).Chen Y; Yin M; Horsman GP; Shen B Improvement of the enediyne antitumor antibiotic C-1027 production by manipulating its biosynthetic pathway regulation in Streptomyces globisporus. J. Nat. Prod 2011, 74, 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Cohen DR; Townsend CA Characterization of an Anthracene Intermediate in Dynemicin Biosynthesis. Angew. Chem., Int. Ed 2018, 57, 5650–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Maser E; Lanisnik Rizner T Steroids and microorganisms. J. Steroid Biochem. Mol. Biol 2012, 129, 1–3. [DOI] [PubMed] [Google Scholar]
- (32).Donova M Microbial Steroid Production Technologies: Current Trends and Prospects. Microorganisms 2021, 10, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Li A; Mao D; Yoshimura A; Rosen PC; Martin WL; Gallant E; Wuhr M; Seyedsayamdost MR Multi-Omic Analyses Provide Links between Low-Dose Antibiotic Treatment and Induction of Secondary Metabolism in Burkholderia thailandensis. mBio 2020, 11, e03210–e03219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Li A; Okada BK; Rosen PC; Seyedsayamdost MR Piperacillin triggers virulence factor biosynthesis via the oxidative stress response in Burkholderia thailandensis. Proc. Natl. Acad. Sci. U.S.A 2021, 118, No. e2021483118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wang R; Gallant E; Wilson MZ; Wu Y; Li A; Gitai Z; Seyedsayamdost MR Algal p-coumaric acid induces oxidative stress and siderophore biosynthesis in the bacterial symbiont Phaeobacter inhibens. Cell Chem. Biol 2022, 29, 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Covington BC; Seyedsayamdost MR MetEx, a Metabolomics Explorer Application for Natural Product Discovery. ACS Chem. Biol 2021, 16, 2825–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Han EJ; Lee SR; Hoshino S; Seyedsayamdost MR Targeted Discovery of Cryptic Metabolites with Antiproliferative Activity. ACS Chem. Biol 2022, 17, 3121–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Buchanan GO; Williams PG; Feling RH; Kauffman CA; Jensen PR; Fenical W Sporolides A and B: Structurally Unprecedented Halogenated Macrolides from the Marine Actinomycete Salinispora tropica. Org. Lett 2005, 7, 2731–2734. [DOI] [PubMed] [Google Scholar]
- (39).Oh DC; Williams PG; Kauffman CA; Jensen PR; Fenical W Cyanosporasides A and B, chloro- and cyano-cyclopenta-[a]indene glycosides from the marine actinomycete ″Salinispora pacifica. Org. Lett 2006, 8, 1021–1024. [DOI] [PubMed] [Google Scholar]
- (40).van Santen JA; Jacob G; Singh AL; Aniebok V; Balunas MJ; Bunsko D; Neto FC; Castano-Espriu L; Chang C; Clark TN; et al. The Natural Products Atlas: An Open Access Knowledge Base for Microbial Natural Products Discovery. ACS Cent. Sci 2019, 5, 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Belecki K; Crawford JM; Townsend CA Production of octaketide polyenes by the calicheamicin polyketide synthase CalE8: implications for the biosynthesis of enediyne core structures. J. Am. Chem. Soc 2009, 131, 12564–12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Horsman GP; Chen Y; Thorson JS; Shen B Polyketide synthase chemistry does not direct biosynthetic divergence between 9- and 10-membered enediynes. Proc. Natl. Acad. Sci. U.S.A 2010, 107, 11331–11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Di Bari L; Pescitelli G; Pratelli C; Pini D; Salvadori P Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem 2001, 66, 4819–4825. [DOI] [PubMed] [Google Scholar]
- (44).Gorecki M; Jablonska E; Kruszewska A; Suszczynska A; Urbanczyk-Lipkowska Z; Gerards M; Morzycki JW; Szczepek WJ; Frelek J Practical method for the absolute configuration assignment of tert/tert 1,2-diols using their complexes with Mo2(OAc)4. J. Org. Chem 2007, 72, 2906–2916. [DOI] [PubMed] [Google Scholar]
- (45).Li XC; Ferreira D; Ding Y Determination of Absolute Configuration of Natural Products: Theoretical Calculation of Electronic Circular Dichroism as a Tool. Curr. Org. Chem 2010, 14, 1678–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Snatzke G; Wagner U; Wolff HP Circulardichroism—LXXV11Part LXXIV: W. Schoenfelder and G. Snatzke, Isr. J. Chem.20 142 (1980).: Cottonogenic derivatives of chiral bidentate ligands with the complex [Mo2(O2CCH3)4]. Tetrahedron 1981, 37, 349–361. [Google Scholar]
- (47).Pretsch E; Bühlmann P; Affolter C Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin; New York, 2000; p 153. [Google Scholar]
- (48).Nett M; Ikeda H; Moore BS Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep 2009, 26, 1362–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lane AL; Nam SJ; Fukuda T; Yamanaka K; Kauffman CA; Jensen PR; Fenical W; Moore BS Structures and comparative characterization of biosynthetic gene clusters for cyanosporasides, enediyne-derived natural products from marine actinomycetes. J. Am. Chem. Soc 2013, 135, 4171–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Nam SJ; Gaudencio SP; Kauffman CA; Jensen PR; Kondratyuk TP; Marler LE; Pezzuto JM; Fenical W Fijiolides A and B, Inhibitors of TNF-α-Induced NFκB Activation, from a Marine-Derived Sediment Bacterium of the Genus Nocardiopsis. J. Nat. Prod 2010, 73, 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Myers AG; Harrington PM; Kwon BM Evidence for aminoglycoside participation in thiol activation of neocarzinostatin chromophore. Synthesis and reactivity of the epoxy dienediyne core. J. Am. Chem. Soc 1992, 114, 1086–1087. [Google Scholar]
- (52).Tanaka T; Hirama M; Fujita K.-i.; Imajo S; Ishiguro M Solution structure of the antitumour antibiotic neocarzinostatin, a chromophore–protein complex. J. Am. Chem. Soc 1993, 115, 1205–1207. [Google Scholar]
- (53).Chatterjee M; Smith PJ; Townsend CA The Role of the Aminosugar and Helix Binding in the Thiol-Induced Activation of Calicheamicin for DNA Cleavage. J. Am. Chem. Soc 1996, 118, 1938–1948. [Google Scholar]
- (54).Lin S; Horsman GP; Chen Y; Li W; Shen B Characterization of the SgcF epoxide hydrolase supporting an (R)-vicinal diol intermediate for enediyne antitumor antibiotic C-1027 biosynthesis. J. Am. Chem. Soc 2009, 131, 16410–16417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


