Abstract
MicroRNAs (miRNAs) are a class of nonprotein-coding short transcripts that provide a layer of post-transcriptional regulation essential to many plant biological processes. MiR858, which targets the transcripts of MYB transcription factors, can affect a range of secondary metabolic processes. Although miR858 and its 187-nt precursor have been well studied in Arabidopsis (Arabidopsis thaliana), a systematic investigation of miR858 precursors and their functions across plant species is lacking due to a problem in identifying the transcripts that generate this subclass. By re-evaluating the transcript of miR858 and relaxing the length cut-off for identifying hairpins, we found in kiwifruit (Actinidia chinensis) that miR858 has long-loop hairpins (1,100 to 2,100 nt), whose intervening sequences between miRNA generating complementary sites were longer than all previously reported miRNA hairpins. Importantly, these precursors of miR858 containing long-loop hairpins (termed MIR858L) are widespread in seed plants including Arabidopsis, varying between 350 and 5,500 nt. Moreover, we showed that MIR858L has a greater impact on proanthocyanidin and flavonol levels in both Arabidopsis and kiwifruit. We suggest that an active MIR858L-MYB regulatory module appeared in the transition of early land plants to large upright flowering plants, making a key contribution to plant secondary metabolism.
Widespread long-loop precursors MIR858L efficiently generate miR858 to affect proanthocyanidin and flavonol metabolism, and the long-loop precursor MIR858L likely originated from the TT2-like MYB gene
Introduction
Plant microRNAs (miRNAs) have been a focus of study for more than 20 yr (Reinhart et al. 2002; Rhoades et al. 2002). However, there are still many classes of miRNAs to be identified across the diversity of plant families (Huang et al. 2017; Guo et al. 2022a). miRNAs normally come from single-stranded primary mRNAs (pri-miRNAs) containing a hairpin structure (Axtell 2013; Moran et al. 2017), which is considered to have evolved from an ancient siRNA mechanism that existed in a common ancestor of all eukaryotes (Moran et al. 2017). MiRNAs repress targets with a complementary sequence and then use miRNA-induced gene silencing complex to drive changes in plant development (D'Ario et al. 2017; Yu et al. 2017; Wang et al. 2020a), e.g. converting leaf age and shape (Nikovics et al. 2006; Gao et al. 2022), altering flowering time (Wu et al. 2009; Hu et al. 2014), or aiding in the resistance to biotic/abiotic stresses (Wu et al. 2017; Li et al. 2020). Plant miRNAs and animal miRNAs have apparently originated separately, which is supported by the observation that there are no common miRNA targets found between plants and animals (Allen et al. 2004; Baldrich et al. 2018). In addition, a two-step cleavage of pri-miRNA to mature miRNA is catalyzed by a RNase Dicer-like protein in plants, but in animal systems it is processed sequentially by Drosha and Dicer (Kurihara and Watanabe 2004; Moran et al. 2017; Yu et al. 2017; Wang et al. 2020a). Inverted duplication is one important pathway for the evolutionary origin of plant miRNAs (Allen et al. 2004; Baldrich et al. 2018), e.g. Arabidopsis (Arabidopsis thaliana) MIR161 and MIR163 are considered to have evolved relatively recently by inverted duplication of their target gene members (Allen et al. 2004).
The vast majority (∼98%) of miRNA hairpin structures are less than 336 nt, with an average length of 146 bp (Thakur et al. 2011). A few studies have found longer hairpin sequences that produce miRNAs that regulate specific traits, which are presumed to arise from an inverted duplication event. For example, the OGI locus of persimmon (Diospyros lotus) specifically appears in male plants, and has high similarity to the target gene MeGI (a homeodomain regulating anther fertility), which contributes to sex determination (Akagi et al. 2014). In wheat (Triticum durum) a 500-nt hairpin structure MIRW1 (microRNA specific to W1-COE), results in a reduction in waxes and the nonglaucous (waxy coating) trait (Huang et al. 2017). Notably, both these miRNAs with long-loop hairpins are species-specific, and it remains elusive as to whether long-loop hairpins exist widely.
miR858 is present in a regulatory module that often mediates the degradation of R2R3 MYB transcription factor transcripts (Dubos et al. 2010; Xia et al. 2012). miR858 binds to the mRNA encoding the MYB R3 domain (Xia et al. 2012) leading to transcript degradation. MYB transcription factors participate extensively in plant metabolism and growth, particularly secondary metabolism. R2R3 MYBs involved in regulating polyphenolic metabolism, which are also miRNA/siRNA targets, include the subgroup 6 clade of MYBs responsible for anthocyanin accumulation that gives color to plants or fruits (Yang et al. 2013; Tirumalai et al. 2019; Wang et al. 2022a), several MYBs controlling lignin synthesis (Lin et al. 2012; Wang et al. 2022b), and AtTT2 (AtMYB123), which is a key regulator of proanthocyanidin biosynthesis (Nesi et al. 2001; Xu et al. 2014). miR858 has been found in small RNA sequencing data of most eudicot species (Xia et al. 2012; Guan et al. 2014; Tirumalai et al. 2019; Lin et al. 2021), but study of its function and precursor is mostly limited to Arabidopsis (Sharma et al. 2016, 2020; Wang et al. 2016). A previous study showed that the miR858 precursor cannot be found in 168 out of 179 studied plant species via de-novo prediction (Guo et al. 2022b). Thus, finding the primary transcript of miR858 and re-analyzing the RNA secondary structures may help us understand the biogenesis of miR858 in plants.
In this study, we systematically examined small RNA sequencing data and found miR858 sequences in all examined seed plants, including gymnosperms, monocots, and eudicots. We found noncanonical hairpins of miR858 (1,100 to 2,100 nt) for kiwifruit (Actinidia chinensis), with a larger loop and longer stem. Such hairpins of miR858 were found to be widespread in seed plants, including the model plant Arabidopsis. The long-loop hairpin identified in Arabidopsis was more effective in miR858 biogenesis and had stronger repression on its mRNA target. Stable transgenic plants showed that long-loop precursor MIR858L had conserved repressive effects on proanthocyanidin and flavonol metabolism. Further evidence showed that the long-loop structure of MIR858L was catalyzed by the Dicer-like 1 complex, as is the case for most MIRNAs (MIRs). Sequence analysis suggests that the stems of MIR858L likely originated from TT2-like genes (an R2R3 MYB) and that insertion of transposable elements (TEs) has led to a variation of loop lengths in different seed plants.
Results
Mature miR858 is observed across seed plants
To investigate the distribution of miR858 within the plant kingdom, small RNA sequencing data were collected from 32 representative species spanning bryophytes to seed plants, which included a range of different tissues. This represents sequencing of 69 samples (detailed in Supplementary Data Set 1) with 70 GB of sequence (1.5 billion sequences), which revealed that miR858 homologs are absent in lycophytes and bryophytes, but can be found in basal angiosperms (Amborella trichopoda and Nymphaea colorata) and the gymnosperm ginkgo (Ginkgo biloba) (Fig. 1), suggesting ancestral miR858 likely pre-dates the split of angiosperms and gymnosperms. In addition, miR858 exists in the sequenced monocots (Fig. 1), while previous studies have rarely found miR858 in monocots (Chávez Montes et al. 2014; Chen et al. 2018). For most species, miR858s were found to be 21-nt small RNAs and highly conserved (Supplementary Fig. S1, Supplementary Data Set 2), reflecting high conservation under natural selection. Sequence alignment revealed that miR858 diverges into different types during its evolution, with the main variations in the first and fourth nucleotides (Supplementary Fig. S1).
Figure 1.
The distribution of mature miR858s in seed plants. Small RNA sequencing data for 32 representative land plants were screened for mature miR858. Mature miR858s were found in all examined seed plants, but not in lycophytes and bryophytes. Solid circles indicate where mature miR858 was identified in small RNA sequencing data of the corresponding species. In contrast, a hollow circle shows that mature miR858 was not found.
Noncanonical hairpins for miR858 were identified in kiwifruit
Our previous studies identified several miRNAs and their precursors in kiwifruit, but a miR858 precursor was not found (Wang et al. 2020b, 2022a). Small RNA sequencing data showed that miR858 exists in kiwifruit. Two types of miR858 were found from the sequencing data with one nucleotide difference, and these could be mapped to four loci in the kiwifruit reference genome (Red5 version, Pilkington et al. 2018), named ach-miR858a/b/c/d (Supplementary Fig. S2). In addition, ach-miR858c/d was highly expressed, with over 180,000 counts in this sequencing data set. We examined the potential hairpins for ach-miR858a-d through the canonical approach (i.e. applying existing rules of identification), which extended the flanking sequence to analyze the potential RNA secondary structures, and no hairpin structures were found (Supplementary Fig. S3). For this reason, precursor molecules for miR858 have not been previously identified in kiwifruit (Wang et al. 2020b, 2022a).
We analyzed full-length transcriptome data, and two alternatively spliced pri-MIR858c transcripts were found in the ach-miR858c locus, with isoform1 having a higher occurrence frequency (Supplementary Fig. S4A), which was confirmed by cloning from cDNA (Supplementary Fig. S4B). Therefore, ach-miR858c was most likely processed from two pri-ach-MIR858c transcripts that we had identified. To investigate the potential biogenesis, we analyzed the RNA secondary structure of the two variants using multiple pipelines (Mfold and RNAfold) (Zuker 2003; Lorenz et al. 2011). Both software analysis results revealed that two pri-ach-MIR858c variants contain a noncanonical hairpin structure with a large irregular terminal-loop and longer stem (Supplementary Fig. S5, A to D). The potential hairpin region is shown in Fig. 2A, termed ach-MIR858Lc, with ach-miR858c located at the 5′ stem. Furthermore, the proposed ach-miR858c*, which has a two-nucleotide 3′ overhang, was found in the small RNA sequencing data (Supplementary Fig. S6). The fragment containing the ach-MIR858Lc precursor was used for further experiments (Supplementary Fig. S7).
Figure 2.
A functional long-loop precursor ach-MIR858Lc identified in kiwifruit. A) An irregular loop is marked. ach-miR858c and ach-miR858c* are highlighted. B) The schematic diagram of the miRNA dual-luciferase assay for miR858. A artificial target (reverse complementary sequence of miR858) was inserted into the pGreen_dualluc_3′UTR_sensor vector, located behind firefly luciferase coding sequence. C) Dual-luciferase assay showing ach-MIR858Lc has a significant inhibitory effect on the artificial target. EV pSAK277 is used as control, and the REN/LUC ratio of EV is set as 1. D) Transient assays in N. benthamiana leaves. The data in (C) and (D) are plotted as means ± SE. n = 3 or 4 biological replicates.
To dissect the function of the long-loop hairpin, miRNA-dual-luciferase assays in Nicotiana benthamiana were performed. We inserted a reverse complementary sequence of miR858 behind the sequence encoding firefly luciferase as an artificial target (Fig. 2B). The assays in N. benthamiana showed that over-expression of ach-MIR858Lc almost entirely represses the firefly luciferase driven fluorescence signal (Fig. 2C). miRNA precursors function against their targets via the miRNAs they produce. RT-qPCR clearly showed that ach-miR858c accumulated when transiently over-expressing the long-loop hairpin in N. benthamiana leaves (Fig. 2D). By loosening the length requirements, potential hairpins for ach-miR858a/b/d had therefore been found, with all of them having a long-loop structure (Supplementary Fig. S8, A to C) and strong inhibitory effect (Supplementary Fig. S9). These results suggest that noncanonical hairpins could produce mature miR858 and repress their target.
The long-loop hairpin and short hairpin of miR858 in Arabidopsis
In the model plant Arabidopsis, two distinct miR858 precursors have been previously identified by extending the 200- to 300-nt upstream or downstream sequence of miR858 in the genome to analyze the potential hairpins (Fahlgren et al. 2007; Breakfield et al. 2012). Using the approach similar to that used in kiwifruit, we analyzed the published full-length Arabidopsis transcriptome library (Zhang et al. 2022b) to find the primary transcript of miR858. Interestingly, the long sequence that covers the previously identified ath-MIR858a and reverse complementary ath-MIR858b precursors (ath-MIR858b-rc) were found. Therefore, we proposed the long transcript as the pri-ath-MIR858-full (pri-ath-MIR858F) (Supplementary Fig. S10A); the presence of this long transcript was confirmed by RACE and cloning from cDNA (Supplementary Fig. S10, B and C). This pri-ath-MIR858F transcript has same sense-orientation as the precursor ath-MIR858a, but not ath-MIR858b (Supplementary Fig. S10A). Two software analysis results showed that pri-ath-MIR858F contained a long-loop structure like that observed for kiwifruit (Supplementary Fig. S11), which we termed ath-MIR858L. The short precursor ath-MIR858a (187-nt sequence from TAIR was used for prediction) formed a hairpin structure with a stem region SR1, consistent with the previous analysis (Fig. 3A). In contrast, the ath-MIR858L hairpin had a different hairpin-like structure with a larger loop and longer stem (stem region named SR2) resembling that in kiwifruit (Fig. 3A).
Figure 3.
A comparison of the secondary structure and function between short hairpin ath-MIR858a and long hairpin ath-MIR858L in Arabidopsis. A) The short and long precursors show a typical hairpin structure or a long-loop hairpin-like structure, respectively. The 3′ stem in ath-MIR858a and ath-MIR858L are termed SR1 and SR2 (Stem Region). miR858 generation region formed a stem within SR1 in ath-MIR858a. In the long-loop precursor, SR2 forms the stem, and SR1 becomes part of the loop in ath-MIR858L. B) Dual-luciferase assay showed that ath-MIR858L had a stronger inhibitory effect on the artificial target than ath-MIR858a. EV pSAK277 was used as control, and the REN/LUC ratio of EV was set as 1. C) Transient assays in N. benthamiana leaves. The expression of miR858 produced by ath-MIR858a was set as 1. D) Dual-luciferase assay of SR1mut (ath-MIR858L-SR1mut) and SR2mut (ath-MIR858L-SR2mut). EV pSAK277 was used as control, and the REN/LUC ratio of EV was set as 1. E) Levels of ath-miR858a when SR1mut and SR2mut are over-expressed. The expression of ath-miR858a produced by SR1mut was set as 1. The data in (B), (C), (D), and (E) were plotted as means ± SE. n = 3 or 4 biological replicates.
The published Arabidopsis small RNA sequencing data was analyzed (Xu et al. 2018). As expected, both ath-miR858a and ath-miR858b were found in the small RNA-seq data, with ath-miR858b being in the antisense strand (Supplementary Fig. S12). However, the miRNA-stars of short-hairpin ath-MIR858a and ath-MIR858b were not found from the sRNA-seq (Supplementary Fig. S12). On the contrary, proposed miRNA-star (ath-miR858*-2) of long-hairpin ath-MIR858L was clearly found with a two-nucleotide 3′ overhang (Supplementary Fig. S12), supporting our hypothesis that ath-MIR858F transcripts form the long-loop hairpin structure rather than polycistronic microRNA in vivo. Even though ath-MIR858a and ath-MIR858b appear to be polycistronic microRNAs, ath-MIR858b is actually from the antisense strand of the ath-MIR858F transcript. There is no miR858 sequence present in reverse complementary ath-MIR858b (termed ath-MIR858b-rc, partial of ath-MIR858F transcript). Furthermore, ath-MIR858-rc is unable to form a good hairpin (Supplementary Fig. S13). Therefore, the ath-MIR858F transcript does not contain two miR858 hairpin repeats. Meanwhile, the sequence of ath-miR858b can be found in ath-MIR858L with a one nucleotide position difference from that of ath-miR858a (Supplementary Fig. S12). The frequency of occurrence of ath-miR858a and b appeared highly correlated (Supplementary Fig. S14). We postulated that ath-miR858a and ath-miR858b are both generated from the 5′ stem of ath-MIR858L because of inaccurate cutting.
The fragments containing ath-MIR858a and ath-MIR858L hairpins were used for further experiments (Supplementary Fig. S15). MiRNA dual-luciferase assays in N. benthamiana were performed to test whether the two precursors have differing functions. Assays in N. benthamiana showed that ath-MIR858a could reduce by two-thirds the firefly luciferase activity compared to control, while long-length ath-MIR858L reduced the luminescence signal by 99% (Fig. 3B). This increased repression could be caused by the expression of mature miRNA. RT-qPCR results showed that the long-loop hairpin (ath-MIR858L) produced nearly 450-times the amount of ath-miR858a than the short hairpin (ath-MIR858a) (Fig. 3C), reflecting that both ath-MIR858a and ath-MIR858L can be processed to mature miR858, but that the long-loop ath-MIR858L has a higher efficiency in miR858 biogenesis.
To validate whether the stem region SR1 or SR2 in ath-MIR858L was necessary, we mutated the ath-miR858*-1 and ath-miR858*-2 sequences (named ath-MIR858L-SR1mut and ath-MIR858L-SR2mut) to disrupt the stem. RNA structure analysis showed that ath-MIR858L-SR1mut did not affect the complementation between 5′ stem and SR2 (Supplementary Fig. S16). Nevertheless, when ath-miR858*-2 sequence was mutated, RNA structure analysis showed that 5′ stem formed a stem structure with SR1 rather than SR2 (Supplementary Fig. S16). MiRNA dual-luciferase assays showed that SR1 (ath-MIR858L-SR1mut) did not influence the function of ath-MIR858L; on the contrary, a lack of SR2 in ath-MIR858L (ath-MIR858L-CR2mut) led to substantially lower efficiency of repression toward the target (Fig. 3D). The expression of mature miRNA was significantly reduced in ath-MIR858L-SR2mut (Fig. 3E). These results suggest that the 5′ stem and SR2 formed a stem structure which is critical for processing of the long-loop ath-MIR858L.
The generation efficiency of miR858 using different-length transcripts was then analyzed (Supplementary Fig. S17A). Full-length transcript (ath-MIR858F) had a similar processing efficiency with ath-MIR858L, which only contains the 3′ stem-SR2 but not the whole ath-MIR858b-rc (Supplementary Fig. S17A). Transcript that did not contain the 3′ stem-SR2 (ath-MIR858M) had extremely low production efficiency of miR858 (Supplementary Fig. S17B), indicating that the 3′ stem-SR2 is critical for miR858 biogenesis, which is consistent with the characteristic of polycistronic microRNAs where the first hairpin has higher production than the second one (Lunardon et al. 2021). We also observed that mutated 3′ stem-SR2, rather than 3′ stem-SR1, in the full-length transcript of ath-MIR858F had a similar production than that appeared in ath-MIR858L (Fig. 3E, Supplementary Fig. S18). These results suggest that the long-loop structure determines the miR858 biogenesis for long-length transcript ath-MIR858F. Polycistronic pri-miRNAs can initiate co-transcriptionally (Gonzalo et al. 2022), whereas we did not detect obvious cleavage of miR858a* (Supplementary Fig. S19, A to C), suggesting that long-length transcript hardly used the short hairpin ath-MIR858a to produce miR858 in vivo, excluding the potential of miR858 as a polycistronic miRNA. We also detected other cleavage signals that might be caused by inaccurate processing (Supplementary Fig. S19C). It has been reported that multibranched terminal loops will lead to abortive processing (Zhu et al. 2013), and the loop in the ath-MIR858F conforms to the characteristic of the multibranched terminal loop. We did not find any other miRNAs in this region (Supplementary Fig. S12). Additionally, we examined the potential local polycistronic tandem repeats of miR858 in kiwifruit, but no polycistronic miR858s were found even using several kiwifruit genomes (Red5, Hongyang V3, Hongyang T2T) (Pilkington et al. 2018; Wu et al. 2019; Yue et al. 2023).
Long-loop hairpin MIR858L modulates proanthocyanidin and flavonol metabolism via specific MYBs in both kiwifruit and Arabidopsis
Transient assays of ach-MIR858Lc and ath-MIR858L showed that these long-loop hairpins have a higher efficiency of miR858 biogenesis than ath-MIR858a (Figs. 2D and 3C). To test the effect of these precursors, in planta over-expression of ach-MIR858Lc and ath-MIR858a/L was performed in kiwifruit and Arabidopsis, respectively. RT-qPCR showed that over-expression of ach-MIR858Lc in kiwifruit was accompanied by increasing mature ach-miR858c (Fig. 4A). In contrast, when ath-MIR858L and ath-MIR858a were over-expressed in Arabidopsis, the expression of ath-MIR858L transcript was significantly lower than ath-MIR858a (Fig. 4B). Nevertheless, 35S:ath-MIR858L plants had significantly more mature ath-miR858a than 35S:ath-MIR858a plants (Fig. 4B). Although we used the same precursor sequence for ath-MIR858a according to Sharma et al. (2016), the expression of ath-miR858a in 35S:ath-MIR858a plants was only slightly higher than that in the wild-type (2- to 5-fold) (Fig. 4B). For polycistronic miRNAs, the first hairpin has a slightly higher efficiency than the second one (Lunardon et al. 2021). However, ath-MIR858L had more than 10,000 times greater processing efficiency than ath-MIR858a (Supplementary Fig. S20). These results validate that the long-loop structure for miR858 in Arabidopsis has a higher processing efficiency than ath-MIR858a, consistent with our transient results (Fig. 3C).
Figure 4.
MIR858L over-expression reduces proanthocyanidin and flavonol content in both kiwifruit and Arabidopsis. A) The expression of ach-MIR858Lc transcript and mature ach-miR858c in kiwifruit wild-type and over-expression plants. B) The expression of ach-MIR858a/L transcripts and mature ath-miR858a in Arabidopsis wild-type and over-expression plants. C) The seed color of Arabidopsis unstained and DMACA-stained. D) HPLC detection of proanthocyanidin and flavonol in kiwifruit leaves. E) HPLC detection of Arabidopsis proanthocyanidin and flavonol in silique and leaves, respectively. The data of (A), (B), (D), and (E) were plotted as means ± SE (n = 3 biological replicates). L refers to different transgenic lines. Statistical analysis was performed using the two-tailed Student's t-test. n.s. represents not significant. n.d. represents not detected.
No visible phenotypic differences were observed between the leaves of wild-type and transgenic kiwifruit plants (Supplementary Fig. S21A), or Arabidopsis plants (Supplementary Fig. S21B). The seed color of 35S:ath-MIR858a plants was similar to the wild type (Fig. 4C), as previously reported (Sharma et al. 2016). However, the seed color of 35S:ath-MIR858L plants is lighter than that of wild-type and 35S:ath-MIR858a plants (Fig. 4C). As the seed color of Arabidopsis is a result of the accumulation of proanthocyanidin, staining with p-dimethylaminocinnamaldehyde (DMACA) (Abrahams et al. 2002) was carried out to visualize proanthocyanidin levels. The stained seeds of wild-type and 35S:ath-MIR858a plants were dark. In contrast, the seeds of 35S:ath-MIR858L plants were only lightly stained, suggesting lower proanthocyanidin level (Fig. 4C). High-performance liquid chromatography was performed to detect proanthocyanidin and flavonol levels. The total proanthocyanidin and flavonol content in kiwifruit 35S:ach-MIR858Lc plants were significantly lower than that in wild type (Fig. 4D). In Arabidopsis, total proanthocyanidin content was reduced to ∼50% in 35S:ath-MIR858L while flavonol content fell to HPLC undetectable levels (Fig. 4E). Plants expressing the short precursor, 35S:ath-MIR858a, had no significant change in either proanthocyanidin content or flavonols (Fig. 4E).
We analyzed the targets of miR858 in kiwifruit and Arabidopsis. The genes encoding R2R3 MYB transcription factors AtTT2 (AtMYB123) and AtMYB12, which are involved in proanthocyanidin and flavonol regulation, are predicted targets (Xu et al. 2014; Sharma et al. 2016, 2020). The miRNA-dual-luciferase assays showed that both AtMYB123 and AtMYB12 were dramatically repressed by ath-MIR858L, and moderately repressed by ath-MIR858a (Supplementary Fig. S22A). The kiwifruit R2R3 MYB genes AcMYB123 (Acc28234) and AcMYB164 (Acc31558), which are homologs of Arabidopsis AtTT2 (AtMYB123) and AtMYB12, were also predicted targets of ach-miR858c (Supplementary Fig. S22B). miRNA-dual-luciferase results showed that AcMYB123 and AcMYB164 were significantly inhibited by kiwifruit ach-MIR858Lc (Supplementary Fig. S22C). These results show that MIR858L produces miR858 that regulates proanthocyanidin and flavonol metabolism via a mechanism conserved between diverse plant species.
The long-loop hairpin MIR858L is widespread in seed plants
After finding four long-loop hairpin-containing precursors of miR858 in kiwifruit, and a long-loop hairpin in Arabidopsis, a survey was made across seed-plant families. Sequence alignment showed that the 5′ and 3′ stem of kiwifruit ach-MIR858La/Lb/Lc/Ld and Arabidopsis ath-MIR858L were highly conserved (Supplementary Fig. S23), while the loop sequences were poorly conserved, suggesting that only 5′ or 3′ stems of any potential MIR858L will be conserved between different species. By adjusting the settings of where a 5′ or 3′ stem of any potential MIR858L can reside, we found precursors of miR858 in all examined seed plants, but not in Marchantia polymorpha (liverwort) and Selaginella moellendorffii (a lycophyte) (Fig. 5A, Supplementary Data Set 3). We did not find two miR858 repeats in identified hairpins and their flank sequences, further excluding the possibility of polycistronic tandem repeats (Supplementary Data Set 4, Supplementary File 1). In all species, precursors can form long-loop hairpin structures, with complementary stems of around 60 to 90 nt. For most species, their hairpin structures are long (Fig. 5A), with the shortest hairpin being approximately 350 nt, which is longer than the criteria for plant miRNA (300 nt) (Axtell and Meyers 2018). The miRNA hairpins described for kiwifruit and Arabidopsis, ach-MIR858La/Lb/Lc/Ld, and ath-MIR858L, were the longest hairpins within these species (Fig. 5B).
Figure 5.
Long-loop MIR858Ls are present widely in seed plants, while the long-loop does not influence the mode of processing and repressive action. A) The long-loop precursors of miR858 present in seed plants, but not in lycophytes and bryophytes. The solid line is the 300-nt recommended as a maximum length for plant miRNA hairpin precursors, according to Axtell and Meyers (2018). The bar on the right of phylogenetic tree shows the average length of putative hairpins in each species. B) The length of MIRs in Arabidopsis and kiwifruit, with MIR858Ls being the longest MIRs in Arabidopsis and kiwifruit. E) The expression of mature miR858 in control and after silencing of DCL1 in transient N. benthamiana assays. The expression of mature miR858 produced in control groups was set as 1. D) The loop-deleted hairpin MIR858LDel created by retaining 15 nt in the terminal loop, while the remaining loop nucleotides were deleted. EV pSAK277 was used as control, and the REN/LUC ratio of EV was set as 1. The data in (C) and (D) were plotted as means ± SE. n = 3 or 4 biological replicates.
To investigate how these long-loop hairpins are processed, we transiently expressed the long precursors while also silencing the Dicer-like1 (DCL1) gene in N. benthamiana, using RNAi (Fig. 5C), where the DCL1 was specifically silenced (Supplementary Fig. S24). RT-qPCR results showed that silencing DCL1 significantly reduced the levels of mature miR858 for precursors from both kiwifruit and Arabidopsis (Fig. 5C). In addition, in a published small RNA sequencing data set of the SERRATE (SE) Arabidopsis mutant (Wang et al. 2018), lacking a key component of miRNA processing (DCL1 complex), accumulation of ath-miR858a was significantly suppressed (Supplementary Fig. S25).
There is a wide variation between seed plants in the length of the loops in miR858 precursors (Fig. 5A). To test the effect of this loop, we deleted the large loop from ach-MIR858La/Lb/Lc/Ld and ath-MIR858L to create artificial short hairpins, termed ath-MIR858LDel and ach-MIR858LaDel/LbDel/LcDel/LdDel (Fig. 5D). RNA structure analysis revealed that the five loop-deleted hairpins formed typical short stem-loop structures as seen in most miRNA precursors (Supplementary Fig. S26). Transient assays of miRNA activity in N. benthamiana indicated that removing the loop did not alter their function (Fig. 5D), showing similar inhibitory effects as an artificial target to wild-type hairpins (Figs. 2C and 3B). The expression of miR858 was slightly reduced when the loop was deleted in ath-MIR858L and ach-MIR858Lc compared to normal precursors (Supplementary Fig. S27). Hence, even though the loop length is diverse in different species, it seems not to significantly affect the biogenesis of miR858 from MIR858L.
MIR858Ls likely originated from ancestral TT2-like R2R3 mYB genes
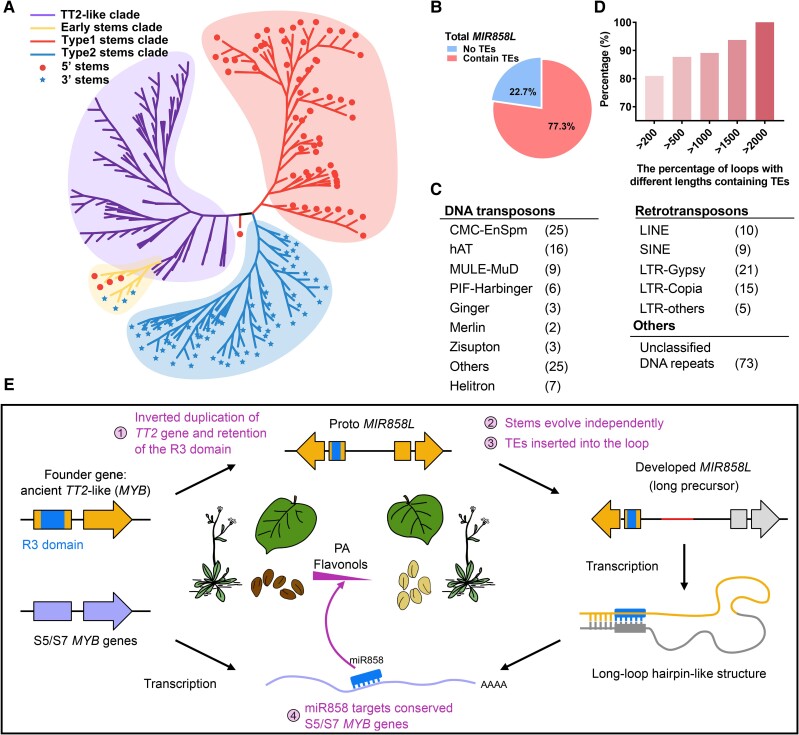
We analyzed the targets of miR858 in seed plants (Supplementary Data Set 5). Around 70% of targets belong to MYB transcription factors, most of which are R2R3 MYBs (Supplementary Fig. S28A). S5 (TT2-like) and S7 (MYB12-like) members were the main targets of miR858. Meanwhile, certain members of the closely related clade S4/6/12/15 (Dubos et al. 2010) are also targets of miR858 (Supplementary Fig. S28B). Several studies have reported that miRNAs probably originated from their targets (Allen et al. 2004; Hu et al. 2014; Huang et al. 2017). We found that the 5′ stems of ginkgo MIR858Ls are similar to the R3 domain of the ancient seed plant ginkgo TT2-like gene (MYB, Gb_03400) (Supplementary Fig. S29A). Furthermore, parts of both ginkgo 5′ and 3′ stems of MIR858Ls are highly similar (Supplementary Fig. S29B). This suggests that MIR858L evolved through partial duplication of its target TT2-like gene, which is observed for Arabidopsis MIR824 and wheat MIRW1 (Kutter et al. 2007; de Meaux et al. 2008; Huang et al. 2017). We examined the TT2-like genes (encoding R2R3 MYB transcription factors) of miR858s’ targets from representative seed plants to build a phylogenetic tree (Fig. 6A). The phylogenetic tree showed that the early stems of MIR858L in seed plants likely originated from its target TT2-like gene. Furthermore, MIR858L stems individually evolved into two classes which contain the majority of 5′ stems and 3′ stems, respectively.
Figure 6.
The origin, evolution, and development of miR858. A) Phylogenetic analysis of TT2-like genes (R2R3 MYBs) and the 5′ and 3′ stem (reverse complementary sequence) of MIR858Ls. Phylogenetic analysis suggests that early MIR858L stems likely originated from TT2-like genes, and developed stems were divided into two individual. Circles represent the 5′ stems of MIR858Ls, while stars represent the 3′ stems of MIR858Ls. The phylogenetic tree was drawn with ignore branch lengths. B) TEs are prevalent in the loops of MIR858Ls. C) The classification of TEs found in loops. D) The percentage of precursors containing TEs increases when the length of loops increases. E) Proto MIR858L originates from the ancient TT2-like gene (MYB) through the inverted duplication. Subsequently, evolution of the stem sequence and insertion of TEs promote the development of MIR858L, to regulate S5 and S7 MYB transcription factors in seed plants.
The hairpin length of MIR858Ls remains long in different species, although this varies between and within any examined species. However, the shortest hairpin is still more than 350 nt (Fig. 5A). We analyzed the correlation between loop length and genome size, but the correlation was low (R2 below 0.1, Supplementary Fig. S30). The average hairpin length of MIR858L in representative seed plants is 1,823 nt. Most other miRNAs (but not miR858) have a predominance of short precursors, usually less than 300 nt. This raises a question of why MIR858L precursors have evolved to include long loops. We found that 77.3% of MIR858L precursors possessed TEs in the loop sequences (Fig. 6B;Supplementary Data Set 6), and also found that some TEs in precursor loop might produce additional small RNAs. Because small RNAs are short and we used the multimapping method, it is hard to distinguish whether these small RNAs are coming from TEs or other locations with perfect matches. The ratio of the presence of TEs increases with the increasing length of the loops (Fig. 6C). Both DNA transposons and retrotransposons were found in the loop sequence (Fig. 6D), and several MIR858L precursors possessed multiple TEs insertions (Supplementary Fig. S31). Hence, TE insertion appears to be one of driving forces underlying the variation of the loop sequence in MIR858Ls of seed plants. We, therefore, propose a model whereby MIR858L originated from a TT2-like R2R3 MYB which then underwent evolution to form the long-loop hairpins of MIR858L in seed plants (Fig. 6E).
Discussion
miR858 is widespread in seed plants allowing regulation of proanthocyanidin and flavonol metabolism
miR858 was first identified in Arabidopsis (Fahlgren et al. 2007). Because miR858 targets genes encoding R2R3 MYBs that regulate the phenylpropanoid pathway, this miRNA has been a focus of a number of research initiatives. The majority of miR858s have been found in eudicots, with only a few in monocots, and thus miR858 has been proposed to be a less-conserved miRNA (Chávez Montes et al. 2014; Chen et al. 2018). We re-analyzed 69 small RNA sequencing libraries from the NCBI SRA database and found miR858 in all analyzed seed plants, but not in lycophytes and bryophytes (Fig. 1). The wide presence of miR858 contrasts with an absence of precursor MIR858. In this study, we found hairpins which could form a long-loop structure (Figs. 2A and 3A). Our results suggest that long-loop structures have a great impact on their targets and a high efficiency of miR858 biogenesis (Figs. 2D and 3C). The published Arabidopsis short precursor ath-MIR858a is a part of long precursor ath-MIR858L (Supplementary Fig. S12), which has a greater functional impact when acting as a long-loop hairpin (Fig. 4, C and E).
In Arabidopsis, ath-miR858a was confirmed to affect flavonoid biosynthesis via the regulation of MYB11, MYB12, and MYB111 (Sharma et al. 2016). Furthermore, miR858 can increase anthocyanin content via the cleavage of the transcript of the anthocyanin-related repressor gene MYBL2 (Wang et al. 2016). In other species miR858 has been implicated in a number of processes. Apple (Malus × domestica) miR858 was found to share partial targets with miR828 (Xia et al. 2012) and affect proanthocyanidins biosynthesis (Zhang et al. 2022a). Cotton (Gossypium hirsutum) miR858 targets the gene encoding MYB2, which regulates cotton fiber development (Guan et al. 2014). In grape (Vitis vinifera) and potato (Solanum tuberosum), miR858 was found to control flavonol biosynthesis (Tirumalai et al. 2019; Lin et al. 2021). Our target prediction revealed that miR858s mainly target genes encoding S5 and S7 MYB transcription factors (Supplementary Fig. S28). Thus, the function of miR858 to regulate proanthocyanidin and flavonol metabolism, appears relatively conserved in seed plants.
Using stable transformation of kiwifruit with 35S:ach-MIRR858Lc, flavonols were reduced, as has been reported in Arabidopsis with over-expression of ath-MIR858a (Sharma et al. 2016). However, we also observed a reduction in proanthocyanidin content, which was not observed in Arabidopsis (Sharma et al. 2016, 2020). The lines in Sharma et al. (2016) had high expression (20- to 80-fold) of mature ath-miR858a, but the mature ath-miR858a expression level was lower than that in wild-type siliques (by more than 200-fold). In contrast, over-expression of ath-MIR858L resulted in very high amounts of mature miR858 (6,000- to 10,000-fold). Over-expression of long-loop precursors significantly reduces proanthocyanidins, to produce visibly yellow seeds, as well as low flavonol contents (Fig. 4, D and E). In addition, the proanthocyanidin and flavonol-related regulator genes AtTT2 and AtMYB12 were greatly inhibited by ath-MIR858L (Supplementary Fig. S22). These results reinforce the functionality of miR858 long-loop structures.
Precursors of miR858 in seed plants and their biogenesis
The 5′ and 3′ stems of ath-MIR858L and ach-MIR858Ls have a high similarity (Supplementary Fig. S23), suggesting the presence of a general precursor of miR858 in other plants. Through scanning sequence similarity and allowing folding within long fragments, miR858 long-loop hairpins were found in all representative seed plants (Fig. 6A). This result helps explain the underestimation of miR858 precursors in most plants. For example, miR858 has been found in tomato (Solanum lycopersicum) (Jia et al. 2015), but a corresponding precursor has not been identified. In citrus (Citrus sinensis), a 77-nt hairpin for miR858 was predicted (Taylor et al. 2017), while we suggest a hairpin that is approximately 3,000 nt and has a similar long-loop structure to ath-MIR858L and ach-MIR858Ls. Transcripts previously annotated as long noncoding genes may well be miR858 precursors; we found that grape (XR_787088.2), citrus (XR_003063725.1), tomato (MH894932.1), and peach (Prunus persica) (XR_002269609.1) long noncoding genes contain miR858 long hairpins. Our results show widespread noncanonical hairpins, allowing unknown precursors for studied miRNAs to be identified.
Primary miRNA (pri-miRNA) loci are transcribed by Pol II (RNA polymerase II), then pri-miRNA transcripts are processed by the DCL1 complex through two-step cleavage to generate mature miRNAs (Kurihara and Watanabe 2004; Yu et al. 2017; Wang et al. 2020a). It has been found that a 706-nt precursor of ath-MIR869a is processed by DCL4 (Ben Amor et al. 2009). However, we found that transiently silencing DCL1 reduced the amount of mature miR858 produced from long hairpin by more than 90% (Fig. 5C). In addition, the accumulation of miR858 in the Arabidopsis SE mutant (a key component of the DCL1 complex) is greatly repressed (Wang et al. 2018) (Supplementary Fig. S25). This suggests that MIR858Ls share the same biosynthesis pathway with short precursor MIRs.
The RNA secondary structure of any precursor is critical for miRNA processing (Zhu et al. 2013; Wang et al. 2018). The lower-stem secondary structure of ath-MIR172a impacted mature miR172a biogenesis (Mateos et al. 2010; Werner et al. 2010), whereas random mutations in the loop region of ath-MIR172a have little effect. We modified the hairpins of ath-MIR858L and ach-MIR858Ls to create artificial hairpins ath-MIR858LDel and ach-MIR858LDels, which lose the long-loop sequences and form more standard hairpin structures (Supplementary Fig. S26). These loop-deleted hairpins possessed a similar repressive effect toward artificial targets (Fig. 5D). The pri-ath-MIR858F resembles a polycistronic miRNA, but our evidence (Supplementary Figs. S12, S17, and S19) shows that ath-MIR858F generates miR858 mainly through the long-loop hairpin.
The origin, evolution, and development of MIR858L loci in seed plants
Inverted duplication is regarded as one point of origin for plant miRNAs. Evidence suggests that miR161 and miR163 originated from inverted duplication of target genes (Allen et al. 2004). In wheat, miRW1 (which controls the glaucous trait) contains an inverted repeat with >80% identity to target gene W1-COE (Huang et al. 2017), indicating that miRW1 might originate from inverted duplication of W1-COE. Likewise, the small RNA locus OGI in persimmon shows similarity to the target gene MeGI that encodes a homeodomain transcription factor regulating anther fertility. The OGI gene contains a forward region and an inverted region which might be the embryonic form of a miRNA locus (Akagi et al. 2014). Ginkgo, an ancient seed plant, has long MIR858L precursors that appear to preserve characteristics of the ancestral MIR858L. The 5′ stem of gbi-MIR858Ls shows high similarity to a TT2-like homolog gene (Supplementary Fig. S29A). The fragment contains the region that encodes the R3 domain of the R2R3 MYB, consistent with miR858 target sites throughout the plant kingdom. In seed plants, phylogenetic analysis suggests that the 5′ stem sequences originate from TT2-like genes (Fig. 6A). Therefore, we propose that the origin of MIR858L is a segment of an ancient TT2-like gene which is an inverted duplication that forms the proto-MIR858L loci.
In our study, long-loop MIR858Ls were found to be widespread in seed plants and share the same biogenesis pathway with typical MIRs (Fig. 5, A and C). Unlike protein-encoding genes, noncoding genes are usually poorly conserved in different species (Chen et al. 2011). We found that transposable elements occur in the majority of MIR858Ls’ loop sequences (Fig. 6B), indicating that variation of loop length could be caused by TE insertion. In humans, more than two-thirds of long noncoding RNAs contain fragments of TE origin (Kapusta et al. 2013). In plants, TEs have been found contribute to noncoding RNA, estimated to be 20% to 50% of this class in Arabidopsis, rice (Oryza sativa) and maize (Zea mays) (Wang et al. 2017). Therefore, we suggest that TEs can be a major contributor to long-loop miRNA precursors.
Conclusion
miR858 and its long precursors are widespread in seed plants. These long precursors are processed via the DCL1 complex. In addition, the long precursor MIR858L was shown to be highly efficient in miR858 biogenesis, resulting in a stronger repression of it’s mRNA targets, which affect proanthocyanidin and flavonol metabolism in different species. Phylogenetic analysis suggests that the stems of MIR858L likely originated from TT2-like genes (an R2R3 MYB) and that insertion of TEs has led to variation of loop lengths in different seed plants.
Materials and methods
Plant material
The seeds of Arabidopsis (A. thaliana) Col-0 were placed in the dark at 4 °C for vernalization. After 2 d, seeds were placed in soil and then transferred to the growth room with 16 h:8 h light:dark at 22 °C, 50% relative humidity, and light intensity of ∼120 μmol m−2 s−1 (Sharma et al. 2016). The rosette leaves, silique, and seeds were collected and quickly frozen in liquid nitrogen for further use. The kiwifruit (A. chinensis) plants were grown in growth room with 16 h:8 h light:dark at 25 °C, 50% relative humidity, and LED white light intensity of ∼120 μmol m−2 s−1.
First-strand cDNA synthesis and RT-qPCR
Total RNA of kiwifruit and Arabidopsis was extracted via a modified CTAB method according to Wang et al. (2022a). For gene cloning, HiScript III 1st Strand cDNA Synthesis Kit (R312, Vazyme, China) was used to synthesize the cDNA. Firstly, 1 μg total RNA was incubated at 65 °C for 5 min, then 5 × g DNA wiper Mix was added at 42 °C for 2 min to remove genomic DNA. Then, first-strand cDNA was synthesized by HiScript III Enzyme with Oligo (dT)20 primer. For RT-qPCR, first-strand cDNA was obtained by HiScript III RT SuperMix for qPCR (R323, Vazyme, China) according to the manual. As for miRNA RT-qPCR, miRNAs were revised by specific stem-loop primers with miRNA First Strand cDNA Synthesis Kit (MR101; Vazyme, China) following Wang et al. (2022a).
Small nuclear RNA U6 gene was used as a control for mature miRNA. AtACT2 (Wang et al. 2016), actin (Yin et al. 2008), and NbPP2A (Liu et al. 2012) were used as housekeeping genes in Arabidopsis, kiwifruit, and N. benthamiana, respectively. qPCR was performed according to the following reaction: 10 μL LightCycler 480 SYBR Green I Mastermix (Roche), 2 μL diluted cDNA, 1 μL forward primer (10 μm), 1 μL reverse primer (10 μm) and 6 μL DEPC (Diethyl Pyrocarbonate)-treated water. Subsequently, qPCR was run in a LightCycler 480 instrument (Roche) with the following cycling conditions: 95 °C for 5 min; 50 cycles at 95 °C for 10 s, 60 °C for 10 s, and 75 °C for 15 s; then, automatic melting curve analysis was performed. Primers used for miRNA reverse transcription and RT-qPCR are listed in Supplementary Table S1.
Processing of sequencing data
Small RNA sequencing data for different species were collected from National Center for Biotechnology Information (NCBI), and the corresponding accession numbers are listed in Supplementary Data Set 1. The small RNA sequencing data were cleaned by removing the adaptor sequences using trim_galore (v0.6.7) (https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). Then, the sequences longer than 25-nt, less than 19-nt, or with a count less than 5 were removed. The remaining sequences were used to find the homolog of miR858 via Blast+(v 2.5.0) (Camacho et al. 2009). Bowtie (Langmead et al. 2009) was used to map the small RNA sequences to reference sequences with multi-mapping (-a -v 0). An Arabidopsis miRNA atlas was obtained from Xu et al. (2018) (NCBI: GSE79414). The Arabidopsis se-2 data was obtained from Arabidopsis Small RNA Database (Feng et al. 2020), and the corresponding sequencing data was from Wang et al. (2018) under accession number GSE108858 (NCBI). Arabidopsis TAIR10 and kiwifruit Red5/HYv3/HY-T2T were used as the reference genomes for analysis (Pilkington et al. 2018; Wu et al. 2019; Yue et al. 2023).
The full-length transcriptome libraries were collected from NCBI (SRR15498080 and SRR10389889). BLAST on the NCBI website was used to find the transcripts located in the ath-miR858a and ach-miR858s loci. The sequences with 5′ adaptor and poly(A) tail were regarded as full-length transcripts and were used for further analysis.
Prediction of RNA secondary structure
The 5′ or 3′ stem sequence of ath-MIR858L and ach-MIR858La/Lb/Lc/Ld was used to search for MIR858L homologs in different species via BLAST+(v 2.5.0) software with –task blastn algorithm (Camacho et al. 2009). The reference genomes used are listed in Supplementary Data Set 7. The RNA secondary structures were predicted by mFold and RNAfold with default parameters (Zuker 2003; Lorenz et al. 2011). The putative hairpins for different plants are listed in the Supplementary Data Set 3.
Sequence alignment and phylogenetic analysis
Multiple sequence alignment was performed using ClustalX (Thompson et al. 2002) with the reverse complementary sequences of ath-MIR858L and ach-MIR858La/Lb/Lc/Ld. The 5′ stem and reverse complementary 3′ stem sequences of gbi-MIR858La/Lb/Lc/Ld (121-nt fragments) were used to compare the similarity. The 5′ stem sequence of gbi-MIR858Ls and reverse complementary of Gb_03400 (MYB transcription factor, TT2-like gene) were used to find homologous regions. T-coffee was carried out to indicate the alignment quality (Di Tommaso et al. 2011).
The phylogenetic tree of 32 representative species was obtained from NCBI-Taxonomy-Common Tree. The alignment of 5′ and 3′ stem (121-nt fragment) of MIR858Ls and target TT2-like genes was performed by Mafft (v 7.310) with –auto parameter (Katoh and Standley 2013). Alignment sequences were used to build the phylogenetic tree with IQ-TREE2 (v2.2.5) via the maximum likelihood method, using MFP to choose the best model (the best model is TN + F + R5), and a bootstrap value of 1,000 (-m MFP -B 1000 –bnni -T AUTO) (Kalyaanamoorthy et al. 2017; Minh et al. 2020; Shen et al. 2020). The phylogenetic trees were drawn by iTOL (Letunic and Bork 2021). The nucleotide sequence alignments used to construct the phylogenetic tree are provided in Supplementary File S2, and the tree files are provided in Supplementary File S3.
Transient assays in N. benthamiana
The ath-MIR858a, ath-MIR858L, and ach-MIR858La/Lb/Lc/Ld sequences were cloned and inserted into pSAK277 vector (Ampomah-Dwamena et al. 2019). Using the NbDCL1-RNAi plasmid, the hairpin RNA construct was designed according to Helliwell and Waterhouse (2003) and inserted into pSAK277 vector. The specific fragment targeting NbDCL1 was predicted by VGIS tool (Sol Genomics Network, https://www.sgn.cornell.edu/). The recombinant plasmids were transferred into Agrobacterium tumefaciens strain EHA105 through electroporation as described by Zhang et al. (2018). The single gene or combination was infiltrated in N. benthamiana leaves, and empty vector (EV) pSAK277 was used as control. Three days after infiltration, the infected areas were collected. Subsequently, total RNA was extracted through CTAB method. All primers used for vector construction are listed in the Supplementary Table S2.
miRNA dual-luciferase assay
miRNA dual-luciferase assay is a tool to test the function of miRNA on their target sites (Liu et al. 2014). The reverse complement of ath-miR858 was constructed into 3′-UTR sensor vector used as the artificial target for miR858. Then the construct was transferred into A. tumefaciens strain GV3101. ath-MIR858a, ath-MIR858L, and ach-MIR858La/Lb/Lc/Ld were used as the effector. The fragments of ath-MIR858LDel, ath-MIR858L-CR1mut, ath-MIR858L-CR2mut, and ach-MIR858LaDel/LbDel/LcDel/LdDel were directly synthesized by HuaGene (Shanghai, China) constructed into the pSAK277 vector. A mixture of the 3′-UTR and the effectors at a 1:10 ratio was infiltrated in N. benthamiana leaves, with EV pSAK277 set as control. Three days after infection, the Promega GloMAX96 platform was used to detect the activities of firefly luciferase and renilla luciferase with Dual-Luciferase Reporter Assay System (Promega). The primers used for vectors construction are listed in the Supplementary Table S2.
Rapid amplification of cDNA ends experiment
Rapid amplification of cDNA ends (RACE) was carried out to isolate the full-length transcript of ath-MIR858a locus. Total RNA was isolated from Arabidopsis dcl1-9 homozygous mutant. 5′ and 3′ RACE cDNA was constructed by HiScript-TS 5′/3′ RACE Kit (RA101, Vazyme, China).
To identify whether ath-MIR858F was polycistronic miRNA or long-loop hairpin structure, 5′ RNA ligase-mediated RACE (5′ RLM-RACE) was performed to detect the processed fragment, according to Zhu et al. (2013). Total RNA was isolated from ath-MIR858F transient plants. 5′ RNA adapter was directly ligated with 5 μg of total RNA. After ligation, first-strand cDNAs were synthesized using HiScript III 1st Strand cDNA Synthesis Kit (R312, Vazyme, China) and a specific reverse primer. The PCR product around 350 bp was sequenced by Hi-Tom-seq (Liu et al. 2019). The primers used for RACE experiments are listed in the Supplementary Table S3.
Transformation for kiwifruit and Arabidopsis
The kiwifruit transformation was performed according to previous studies. The leaves of kiwifruit (A. chinensis “Donghong”) were cut into small pieces, and then the leaves were inoculated with A. tumefaciens strain carrying pSAK-ach-MIR858Lc. Regeneration and selection medium, shoot elongation medium, and rooting medium have been described in Wang et al. (2007) and Peng et al. (2019). The positive plants were further verified by PCR for DNA insertion and RT-qPCR.
pSAK277-ath-MIR858a and pSAK277-ath-MIR858L plasmids were used for overexpression in Arabidopsis. Flowering Arabidopsis (Col-0) plants were used for transformation through the floral dip method according to Clough and Bent (1998). The seeds of T0 plants were collected and selected on half-strength MS medium containing kanamycin (50 mg L−1). The plants that grew well on selection medium were transferred to soil. Extracted genomic DNA from positive plants to verify the DNA insertion and the expression of target genes were tested by RT-qPCR.
Measurement of proanthocyanidin and flavonol
Proanthocyanidin in Arabidopsis seeds was measured by DMACA staining, which is more sensitive to proanthocyanidin (PA) than vanillin (Abrahams et al. 2002). Seeds were stained with DMACA regent [2% (w/v) DMACA in 3 mL HCl/50% (w/v) methanol] for 3 d, and then the seeds were washed three times with 70% ethanol. Finally, the stained seeds were imaged under a microscope (ZEISS, Germany).
For proanthocyanidin measurement, approximately 200 mg ground samples (kiwifruit leaves and Arabidopsis silique) were used for the extraction according to previous protocols (Kennedy and Jones 2001; Jiang et al. 2015). Extraction solution (3 mL) (acetone:water:acetic acid = 70:29.5:0.5) was added to tissue powder and fully mixed. Then, the mixtures were incubated at 30b°C for 15 min, centrifuged at 13,000 rpm for 10 min, then the supernatant transferred into a new tube. These steps were repeated twice. Pooled supernatant was then extracted once by adding equal volume chloroform, and twice by equal volume hexane. The 400 μL extraction was dried in a vacuum vessel overnight. The pellets were dissolved by methanol reagent with 5% phloroglucinol (w/v), 1% ascorbic acid (w/v), and 0.2 N HCl. The mixture was then incubated at 50 °C for 100 min, and the reactions were stopped with 100 μL sodium acetate buffer (200 mm, pH 7.5). The proanthocyanidin was then detected by HPLC at 280 nm.
For the extraction of flavonol, around 100 mg of ground leaves (kiwifruit and Arabidopsis) were extracted in 1 mL of 50% methanol with 30 min sonication at room temperature, and the supernatant obtained by centrifugation at 12,000 rpm for 15 min. All samples were extracted twice, and the combined supernatant filtered through a 0.22 μm organic membrane prior to HPLC analysis (e2695 pump, 2998 PDA detector, Waters, USA) according to Xing et al. (2021). The mobile phases were 0.1% (v/v) formic acid–water (A) and 0.1% formic acid in acetonitrile (B) with a linear gradient program: 0/10, 5/10, 35/30, 40/90, 45/10, and 50/10 (min/B%). Standard rutin curves were used to estimate relative flavonol contents. Flavonol compounds were determined by their retention times and absorbances at 350 nm.
Transposable elements annotations
RepeatMasker (v 4.1.5) was used to predict the transposable elements in MIR858L precursors following the default parameters (-xsmall -cutoff 220). Transposable elements library for plants was collect from PlantRep (Luo et al. 2022). Simple repeats and low complexity regions were excluded from the analysis result.
miRNA target prediction
We used miR858 that we identified by small RNA sequencing to predict targets in seed plants. Prediction was performed with psRobot (v 1.2) with the following parameter: -ts 2.5 -fp 2 tp 16. The targets of miR858 are listed in Supplementary Data Set 5.
Statistical analysis
Significant differences were calculated using two-tailed Student's t-tests. The correlation analyses of Supplementary Figs. S14 and S30 were calculated by linear-regression analysis. Summaries of statistical analyses are shown in Supplementary Data Set 8.
Accession numbers
Sequence data of kiwifruit and Arabidopsis can be found in KGD (http://kiwifruitgenome.org/) and TAIR (https://www.arabidopsis.org/): AcMYB123 (Acc28234), AcMYB164 (Acc31558), AtTT2 (AT5G35550), ath-MIR858a (AT1G71002) and ath-MIR858b (AT1G09213).
Supplementary Material
Acknowledgments
We are grateful to Prof. Hongwei Guo from Southern University of Science and Technology providing Arabidopsis dcl1-9 mutant seeds. We thank Prof. Rong-hui Pan and Prof. Jian-hong Xu (College of Agriculture and Biotechnology, Zhejiang University, China) for discussion and suggestion.
Contributor Information
Wen-qiu Wang, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; School of Horticulture, Anhui Agricultural University, Hefei 230036, China.
Xiao-fen Liu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Yong-jing Zhu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Jia-zhen Zhu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The New Zealand Institute for Plant & Food Research Limited (Plant & Food Research), Mt Albert, Private Bag 92169, Auckland Mail Centre, Auckland 1142, New Zealand.
Chao Liu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Zhi-ye Wang, State Key Laboratory of Plant Physiology and Biochemistry, College of Life Science, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Xing-Xing Shen, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Andrew C Allan, The New Zealand Institute for Plant & Food Research Limited (Plant & Food Research), Mt Albert, Private Bag 92169, Auckland Mail Centre, Auckland 1142, New Zealand; School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland 1010, New Zealand.
Xue-ren Yin, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Author contributions
X.-r.Y., W.-q.W., and A.A. conceived and designed the study. W.-q.W. performed experiments with the help of X.-f.L., Y.-j.Z., J.-z.Z., C.L., Z.-y.W., and X.-X.S. W.-q.W. wrote the manuscript, X.-r.Y. and A.A. revised the manuscript.
Supplementary data
The following materials are available in the online version of this article.
Supplementary Figure S1. Mature miR858 sequences found in different species.
Supplementary Figure S2. Regions with potential to generate miR858 in kiwifruit
Supplementary Figure S3. RNA secondary structure of miR858 flanking sequences in kiwifruit
Supplementary Figure S4. Transcript features of the ath-miR858c locus
Supplementary Figure S5. RNA structure prediction results of two pri-ach-MIR858c variants
Supplementary Figure S6. Read counts of ach-miR858c and ach-miR858c*
Supplementary Figure S7. Fragment used for generating kiwifruit ath-MIR858Lc
Supplementary Figure S8. RNA structure prediction results of ach-MIR858La/Lb/Ld
Supplementary Figure S9. Kiwifruit ach-MIR858La/Lb/Ld has strong effects like ach-MIR858Lc
Supplementary Figure S10. Transcript features of the ath-MIR858a/b loci
Supplementary Figure S11. RNA structure prediction results of pri-ath-MIR858
Supplementary Figure S12. Arabidopsis small RNA mapping result
Supplementary Figure S13. RNA secondary structure of ath-MIR858b and ath-MIR858b-rc
Supplementary Figure S14. High correlation of ath-miR858a and ath-miR858b in Arabidopsis
Supplementary Figure S15. Fragments used for generating Arabidopsis ath-MIR858a and ath-MIR858L precursors
Supplementary Figure S16. RNA structure of ath-MIR858L-SR1mut and ath-MIR858L-SR2mut
Supplementary Figure S17. Expression of miR858 when using different transcript lengths
Supplementary Figure S18. Expression of miR858 in mutated ath-MIR858F
Supplementary Figure S19. Cleaving site detected by 5’ RLM-RACE
Supplementary Figure S20. Processing efficiency of ath-MIR858a and ath-MIR858L
Supplementary Figure S21. Appearance of transgenic kiwifruit and Arabidopsis
Supplementary Figure S22. Targets of ath-miR858 and ach-miR858c
Supplementary Figure S23. Sequence alignment of the stems of kiwifruit and Arabidopsis MIR858L
Supplementary Figure S24. Expression of NbDCL1/2/3/4 when silencing DCL1
Supplementary Figure S25. Expression of ath-miR858a was reduced in the Arabidopsis se-2 mutant
Supplementary Figure S26. Hairpin structure of MIR858LDel
Supplementary Figure S27. Expression of miR858 in ath-MIR858LDel and ach-MIR858LcDel
Supplementary Figure S28. Classification of miR858 targets in seed plants
Supplementary Figure S29. Sequence feature of gbi-MIR858Ls
Supplementary Figure S30. Correlation of genome size and the length of MIR858L
Supplementary Figure S31. Numbers of TE insertions in MIR858L precursors
Supplementary Table S1. Primers used for RT-qPCR
Supplementary Table S2. Primers used for vector construction
Supplementary Table S3. Primers used for RACE experiments
Supplementary Data Set 1. Small RNA data used for analysis
Supplementary Data Set 2. Mature miR858 sequence found from small RNA sequencing
Supplementary Data Set 3. Sequence of long-loop hairpin MIR858L in seed plants
Supplementary Data Set 4. Statistical information of miR858 repeats and hairpin in MIR858Ls
Supplementary Data Set 5. miR858 targets in different species
Supplementary Data Set 6. Insertion of TEs in the loop of MIR858Ls
Supplementary Data Set 7. Reference genomes used for finding MIR858Ls
Supplementary Data Set 8. Summary of statistical analyses
Supplementary File S1. Small RNA mapping results of MIR858Ls loci in seed plants
Supplementary File S2. Sequence alignment of 5’ stem, 3’ stem and TT2-like genes
Supplementary File S3. Phylogenetic tree of 5’ stem, 3’ stem and TT2-like genes
Funding
This research was supported by the National Key Research and Development Program of China (2018YFD1000200), the National Natural Science Foundation of China (32072635; 32102344), China Postdoctoral Science Foundation (PC2020076), Fruit New Varieties Breeding Project of Zhejiang Province (2021C02066-8), Pao Yu-Kong International Fund of Zhejiang University, the Fundamental Research Funds for the Central Universities of China (226–2022-00152; 226-2022-00215).
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Abrahams S, Tanner GJ, Larkin PJ, Ashton AR. Identification and biochemical characterization of mutants in the proanthocyanidin pathway in Arabidopsis. Plant Physiol. 2002:130(2):561–576. 10.1104/pp.006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi T, Henry IM, Tao R, Comai L. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science. 2014:346(6209):646–650. 10.1126/science.1257225 [DOI] [PubMed] [Google Scholar]
- Allen E, Xie Z, Gustafson AM, Sung GH, Spatafora JW, Carrington JC. Evolution of microRNA genes by inverted duplication of target gene sequences in Arabidopsis thaliana. Nat Genet. 2004:36(12):1282–1290. 10.1038/ng1478 [DOI] [PubMed] [Google Scholar]
- Ampomah-Dwamena C, Thrimawithana AH, Dejnoprat S, Lewis D, Espley RV, Allan AC. A kiwifruit (Actinidia deliciosa) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol. 2019:221(1):309–325. 10.1111/nph.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ. Classification and comparison of small RNAs from plants. Annu Rev Plant Biol. 2013:64(1):137–159. 10.1146/annurev-arplant-050312-120043 [DOI] [PubMed] [Google Scholar]
- Axtell MJ, Meyers BC. Revisiting criteria for plant microRNA annotation in the era of big data. Plant Cell. 2018:30(2):272–284. 10.1105/tpc.17.00851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrich P, Beric A, Meyers BC. Despacito: the slow evolutionary changes in plant microRNAs. Curr Opin Plant Biol. 2018:42:16–22. 10.1016/j.pbi.2018.01.007 [DOI] [PubMed] [Google Scholar]
- Ben Amor B, Wirth S, Merchan F, Laporte P, d’Aubenton-Carafa Y, Hirsch J, Maizel A, Mallory A, Lucas A, Deragon JM, et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009:19(1):57–69. 10.1101/gr.080275.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakfield NW, Corcoran DL, Petricka JJ, Shen J, Sae-Seaw J, Rubio-Somoza I, Weigel D, Ohler U, Benfey PN. High-resolution experimental and computational profiling of tissue-specific known and novel miRNAs in Arabidopsis. Genome Res. 2012:22(1):163–176. 10.1101/gr.123547.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009:10(1):421. 10.1186/1471-2105-10-421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chávez Montes RA, de Fatima Rosas-Cardenas F, De Paoli E, Accerbi M, Rymarquis LA, Mahalingam G, Marsch-Martinez N, Meyers BC, Green PJ, de Folter S. Sample sequencing of vascular plants demonstrates widespread conservation and divergence of microRNAs. Nat Commun. 2014:5(1):3722. 10.1038/ncomms4722 [DOI] [PubMed] [Google Scholar]
- Chen C, Zeng Z, Liu Z, Xia R. Small RNAs, emerging regulators critical for the development of horticultural traits. Hortic Res. 2018:5(1):63. 10.1038/s41438-018-0072-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Zhou H, Chen YQ, Qu LH, Gautheret D. Plant noncoding RNA gene discovery by “single-genome comparative genomics”. RNA. 2011:17(3):390–400. 10.1261/rna.2426511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998:16(6):735–743. 10.1046/j.1365-313x.1998.00343.x [DOI] [PubMed] [Google Scholar]
- D’Ario M, Griffiths-Jones S, Kim M. Small RNAs: big impact on plant development. Trends Plant Sci. 2017:22(12):1056–1068. 10.1016/j.tplants.2017.09.009 [DOI] [PubMed] [Google Scholar]
- de Meaux J, Hu JY, Tartler U, Goebel U. Structurally different alleles of the ath-MIR824 microRNA precursor are maintained at high frequency in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2008:105(26):8994–8999. 10.1073/pnas.0803218105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P, Moretti S, Xenarios I, Orobitg M, Montanyola A, Chang JM, Taly JF, Notredame C. T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011:39(suppl):W13–W17. 10.1093/nar/gkr245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010:15(10):573–581. 10.1016/j.tplants.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Fahlgren N, Howell MD, Kasschau K, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL, et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One. 2007:2(2):e219. 10.1371/journal.pone.0000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Zhang F, Zhang H, Zhao Y, Meyers BC, Zhai J. An online database for exploring over 2,000 Arabidopsis small RNA libraries. Plant Physiol. 2020:182(2):685–691. 10.1104/pp.19.00959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhang K, Cheng YJ, Yu S, Shang GD, Wang FX, Wu LY, Xu ZG, Mai YX, Zhao XY, et al. A robust mechanism for resetting juvenility during each generation in Arabidopsis. Nat Plants. 2022:8(3):257–268. 10.1038/s41477-022-01110-4 [DOI] [PubMed] [Google Scholar]
- Gonzalo L, Tossolini I, Gulanicz T, Cambiagno DA, Kasprowicz-Maluski A, Smolinski DJ, Mammarella MF, Ariel FD, Marquardt S, Szweykowska-Kulinska Z, et al. R-loops at microRNA encoding loci promote co-transcriptional processing of pri-miRNAs in plants. Nat Plants. 2022:8(4):402–418. 10.1038/s41477-022-01125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Pang M, Nah G, Shi X, Ye W, Stelly DM, Chen ZJ. Mir828 and miR858 regulate homoeologous MYB2 gene functions in Arabidopsis trichome and cotton fibre development. Nat Commun. 2014:5(1):3050. 10.1038/ncomms4050 [DOI] [PubMed] [Google Scholar]
- Guo Z, Kuang Z, Tao Y, Wang H, Wan M, Hao C, Shen F, Yang X, Li L. Miniature inverted-repeat transposable elements drive rapid microRNA diversification in angiosperms. Mol Biol Evol. 2022a:39:msac224. 10.1093/molbev/msac224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kuang Z, Zhao Y, Deng Y, He H, Wan M, Tao Y, Wang D, Wei J, Li L, et al. PmiREN2.0: from data annotation to functional exploration of plant microRNAs. Nucleic Acids Res. 2022b:50(D1):D1475–D1482. 10.1093/nar/gkab811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell C, Waterhouse P. Constructs and methods for high-throughput gene silencing in plants. Methods. 2003:30(4):289–295. 10.1016/S1046-2023(03)00036-7 [DOI] [PubMed] [Google Scholar]
- Hu JY, Zhou Y, He F, Dong X, Liu LY, Coupland G, Turck F, de Meaux J. miR824-Regulated AGAMOUS-LIKE16 contributes to flowering time repression in Arabidopsis. Plant Cell. 2014:26(5):2024–2037. 10.1105/tpc.114.124685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Feurtado JA, Smith MA, Flatman LK, Koh C, Cutler AJ. Long noncoding miRNA gene represses wheat beta-diketone waxes. Proc Natl Acad Sci U S A. 2017:114(15):E3149–E3158. 10.1073/pnas.1617483114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Shen J, Liu H, Li F, Ding N, Gao C, Pattanaik S, Patra B, Li R, Yuan L. Small tandem target mimic-mediated blockage of microRNA858 induces anthocyanin accumulation in tomato. Planta. 2015:242(1):283–293. 10.1007/s00425-015-2305-5 [DOI] [PubMed] [Google Scholar]
- Jiang W, Yin Q, Wu R, Zheng G, Liu J, Dixon RA, Pang Y. Role of a chalcone isomerase-like protein in flavonoid biosynthesis in Arabidopsis thaliana. J Exp Bot. 2015:66(22):7165–7179. 10.1093/jxb/erv413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods. 2017:14(6):587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta A, Kronenberg Z, Lynch VJ, Zhuo X, Ramsay L, Bourque G, Yandell M, Feschotte C. Transposable elements are major contributors to the origin, diversification, and regulation of vertebrate long noncoding RNAs. PLoS Genet. 2013:9(4):e1003470. 10.1371/journal.pgen.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013:30(4):772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JA, Jones GP. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J Agric Food Chem. 2001:49(4):1740–1746. 10.1021/jf001030o [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Watanabe Y. Arabidopsis micro-RNA biogenesis through dicer-like 1 protein functions. Proc Natl Acad Sci U S A. 2004:101(34):12753–12758. 10.1073/pnas.0403115101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter C, Schöb H, Stadler M, Meins FJ, Si-Ammour A. MicroRNA-mediated regulation of stomatal development in Arabidopsis. Plant Cell. 2007:19(8):2417–2429. 10.1105/tpc.107.050377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009:10(3):R25. 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021:49(W1):W293–W296. 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X, Yang J, He Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat Commun. 2020:11(1):5351. 10.1038/s41467-020-19186-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Singh RK, Navarre DA. R2R3-MYB transcription factors, StmiR858 and sucrose mediate potato flavonol biosynthesis. Hortic Res. 2021:8(1):25. 10.1038/s41438-021-00463-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Lin CC, Lin HH, Chen YC, Jeng ST. Micror828 regulates lignin and H2O2 accumulation in sweet potato on wounding. New Phytol. 2012:196(2):427–440. 10.1111/j.1469-8137.2012.04277.x [DOI] [PubMed] [Google Scholar]
- Liu D, Shi L, Han C, Yu J, Li D, Zhang Y. Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS One. 2012:7(9):e46451. 10.1371/journal.pone.0046451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang C, Jiao X, Zhang H, Song L, Li Y, Gao C, Wang K. Hi-TOM: a platform for high-throughput tracking of mutations induced by CRISPR/Cas systems. Sci China Life Sci. 2019:62(1):1–7. 10.1007/s11427-018-9402-9 [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang F, Axtell MJ. Analysis of complementarity requirements for plant microRNA targeting using a Nicotiana benthamiana quantitative transient assay. Plant Cell. 2014:26(2):741–753. 10.1105/tpc.113.120972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R, Bernhart SH, Honer Zu Siederdissen C, Tafer H, Flamm C, Stadler PF, and Hofacker IL. ViennaRNA package 2.0. Algorithms Mol Biol. 2011:6(1):26. 10.1186/1748-7188-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardon A, Kariuki SM, Axtell MJ. Expression and processing of polycistronic artificial microRNAs and trans-acting siRNAs from transiently introduced transgenes in Solanum lycopersicum and Nicotiana benthamiana. Plant J. 2021:106(4):1087–1104. 10.1111/tpj.15221 [DOI] [PubMed] [Google Scholar]
- Luo X, Chen S, Zhang Y. PlantRep: a database of plant repetitive elements. Plant Cell Rep. 2022:41(4):1163–1166. 10.1007/s00299-021-02817-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos JL, Bologna NG, Chorostecki U, Palatnik JF. Identification of microRNA processing determinants by random mutagenesis of Arabidopsis MIR172a precursor. Curr Biol. 2010:20(1):49–54. 10.1016/j.cub.2009.10.072 [DOI] [PubMed] [Google Scholar]
- Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol Biol Evol. 2020:37(5):1530–1534. 10.1093/molbev/msaa015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Agron M, Praher D, Technau U. The evolutionary origin of plant and animal microRNAs. Nat Ecol Evol. 2017:1(3):27. 10.1038/s41559-016-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 mYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001:13(9):2099–2114. 10.1105/TPC.010098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell. 2006:18(11):2929–2945. 10.1105/tpc.106.045617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Lin-Wang K, Cooney JM, Wang T, Espley RV, Allan AC. Differential regulation of the anthocyanin profile in purple kiwifruit (Actinidia species). Hortic Res. 2019:6(1):3. 10.1038/s41438-018-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkington SM, Crowhurst R, Hilario E, Nardozza S, Fraser L, Peng Y, Gunaseelan K, Simpson R, Tahir J, Deroles SC, et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genomics. 2018:19(1):257. 10.1186/s12864-018-4656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002:16(13):1616–1626. 10.1101/gad.1004402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. Prediction of plant microRNA targets. Cell. 2002:110(4):513–520. 10.1016/S0092-8674(02)00863-2 [DOI] [PubMed] [Google Scholar]
- Sharma A, Badola PK, Bhatia C, Sharma D, Trivedi PK. Primary transcript of miR858 encodes regulatory peptide and controls flavonoid biosynthesis and development in Arabidopsis. Nat Plants. 2020:6(10):1262–1274. 10.1038/s41477-020-00769-x [DOI] [PubMed] [Google Scholar]
- Sharma D, Tiwari M, Pandey A, Bhatia C, Sharma A, Trivedi PK. MicroRNA858 is a potential regulator of phenylpropanoid pathway and plant development. Plant Physiol. 2016:171(2):944–959. 10.1104/pp.15.01831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XX, Li Y, Hittinger CT, Chen XX, Rokas A. An investigation of irreproducibility in maximum likelihood phylogenetic inference. Nat Commun. 2020:11(1):6096. 10.1038/s41467-020-20005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RS, Tarver JE, Foroozani A, Donoghue PCJ. MicroRNA annotation of plant genomes—do it right or not at all. Bioessays. 2017:39(2):1600113. 10.1002/bies.201600113 [DOI] [PubMed] [Google Scholar]
- Thakur V, Wanchana S, Xu M, Bruskiewich R, Quick WP, Mosig A, Zhu XG. Characterization of statistical features for plant microRNA prediction. BMC Genomics. 2011:12(1):108. 10.1186/1471-2164-12-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics. 2002:Chapter 2:Unit 2.3. 10.1002/0471250953.bi0203s00 [DOI] [PubMed] [Google Scholar]
- Tirumalai V, Swetha C, Nair A, Pandit A, Shivaprasad PV. Mir828 and miR858 regulate VvMYB114 to promote anthocyanin and flavonol accumulation in grapes. J Exp Bot. 2019:70(18):4775–4791. 10.1093/jxb/erz264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Qu Z, Yang L, Zhang Q, Liu ZH, Do T, Adelson DL, Wang ZY, Searle I, Zhu JK. Transposable elements (TEs) contribute to stress-related long intergenic noncoding RNAs in plants. Plant J. 2017:90(1):133–146. 10.1111/tpj.13481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Atkinson R, Janssen B. The choice of Agrobacterium strain for transformation of kiwifruit. ISHS Acta Hortic. 2007:753(753):227–232. 10.17660/ActaHortic.2007.753.26 [DOI] [Google Scholar]
- Wang WQ, Allan AC, Yin XR. Small RNAs with a big impact on horticultural traits. Crit Rev Plant Sci. 2020a:39(1):30–43. 10.1080/07352689.2020.1741923 [DOI] [Google Scholar]
- Wang WQ, Moss SMA, Zeng L, Espley RV, Wang T, Lin-Wang K, Fu BL, Schwinn KE, Allan AC, Yin XR. The red flesh of kiwifruit is differentially controlled by specific activation-repression systems. New Phytol. 2022a:235(2):630–645. 10.1111/nph.18122 [DOI] [PubMed] [Google Scholar]
- Wang WQ, Wang J, Wu YY, Li DW, Allan AC, Yin XR. Genome-wide analysis of coding and non-coding RNA reveals a conserved miR164-NAC regulatory pathway for fruit ripening. New Phytol. 2020b:225(4):1618–1634. 10.1111/nph.16233 [DOI] [PubMed] [Google Scholar]
- Wang X, Yao S, Htet WPPM, Yue Y, Zhang Z, Sun K, Chen S, Luo K, Fan D. MicroRNA828 negatively regulates lignin biosynthesis in stem of Populus tomentosa through MYB targets. Tree Physiol. 2022b:42(8):1646–1661. 10.1093/treephys/tpac023 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Song Z, Zhang H. Repression of MYBL2 by both microRNA858a and HY5 leads to the activation of anthocyanin biosynthetic pathway in Arabidopsis. Mol Plant. 2016:9(10):1395–1405. 10.1016/j.molp.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma Z, Castillo-González C, Sun D, Li Y, Yu B, Zhao B, Li P, Zhang X. SWI2/SNF2 ATPase CHR2 remodels pri-miRNAs via serrate to impede miRNA production. Nature. 2018:557(7706):516–521. 10.1038/s41586-018-0135-x [DOI] [PubMed] [Google Scholar]
- Werner S, Wollmann H, Schneeberger K, Weigel D. Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr Biol. 2010:20(1):42–48. 10.1016/j.cub.2009.10.073 [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009:138(4):750–759. 10.1016/j.cell.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ma T, Kang M, Ai F, Zhang J, Dong G, Liu J. A high-quality Actinidia chinensis (kiwifruit) genome. Hortic Res. 2019:6(1):117. 10.1038/s41438-019-0202-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yang R, Yang Z, Yao S, Zhao S, Wang Y, Li P, Song X, Jin L, Zhou T. et al. et al. ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants. 2017:3(1):16203. 10.1038/nplants.2016.203 [DOI] [PubMed] [Google Scholar]
- Xia R, Zhu H, An YQ, Beers EP, Liu Z. Apple miRNAs and tasiRNAs with novel regulatory networks. Genome Biol. 2012:13(6):R47. 10.1186/gb-2012-13-6-r47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M, Cao Y, Ren C, Liu Y, Li J, Grierson D, Martin C, Sun C, Chen K, Xu C, et al. Elucidation of myricetin biosynthesis in Morella rubra of the Myricaceae. Plant J. 2021:108(2):411–425. 10.1111/tpj.15449 [DOI] [PubMed] [Google Scholar]
- Xu L, Hu Y, Cao Y, Li J, Ma L, Li Y, Qi Y. An expression atlas of miRNAs in Arabidopsis thaliana. Sci China Life Sci. 2018:61(2):178–189. 10.1007/s11427-017-9199-1 [DOI] [PubMed] [Google Scholar]
- Xu W, Grain D, Bobet S, Le Gourrierec J, Thevenin J, Kelemen Z, Lepiniec L, Dubos C. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 2014:202(1):132–144. 10.1111/nph.12620 [DOI] [PubMed] [Google Scholar]
- Yang F, Cai J, Yang Y, Liu Z. Overexpression of microRNA828 reduces anthocyanin accumulation in Arabidopsis. Plant Cell Tiss Org. 2013:115(2):159–167. 10.1007/s11240-013-0349-4 [DOI] [Google Scholar]
- Yin XR, Chen K, Allan AC, Wu RM, Zhang B, Lallu N, Ferguson IB. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J Exp Bot. 2008:59(8):2097–2108. 10.1093/jxb/ern067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Jia T, Chen X. The ‘how’ and ‘where’ of plant microRNAs. New Phytol. 2017:216(4):1002–1017. 10.1111/nph.14834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Chen Q, Wang Y, Zhang L, Ye C, Wang X, Cao S, Lin Y, Huang W, Xian H, et al. Telomere-to-telomere and gap-free reference genome assembly of the kiwifruit Actinidia chinensis. Hortic Res. 2023:10(2):uhac264. 10.1093/hr/uhac264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AD, Wang WQ, Tong Y, Li MJ, Grierson D, Ferguson I, Chen KS, Yin XR. Transcriptome analysis identifies a zinc finger protein regulating starch degradation in kiwifruit. Plant Physiol. 2018:178(2):850–863. 10.1104/pp.18.00427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Yang HJ, Qu D, Zhu ZZ, Yang YZ, Zhao ZY. The MdBBX22-miR858-MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel. Plant Biotechnol J. 2022a:20(9):1683–1700. 10.1111/pbi.13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Kuo R, Coulter M, Calixto CPG, Entizne JC, Guo W, Marquez Y, Milne L, Riegler S, Matsui A, et al. A high-resolution single-molecule sequencing-based Arabidopsis transcriptome using novel methods of iso-seq analysis. Genome Biol. 2022b:23(1):149. 10.1186/s13059-022-02711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhou Y, Castillo-González C, Lu A, Ge C, Zhao Y-T, Duan L, Li Z, Axtell MJ, Wang XJ, et al. Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nat Struct Mol Biol. 2013:20(9):1106–1115. 10.1038/nsmb.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003:31(13):3406–3415. 10.1093/nar/gkg595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.