Abstract
Background:
Hodgkin lymphoma (HL) survivors experience neurocognitive impairment despite receiving no central nervous system-directed therapy, though little is known about underlying mechanisms.
Methods:
HL survivors (n=197) and age-, sex- and race/ethnicity-frequency-matched community controls (n=199) underwent standardized neurocognitive testing, and serum collection. Luminex multiplex or ELISA assays measured markers of inflammation and oxidative stress. Linear regression models compared biomarker concentrations between survivors and controls and with neurocognitive outcomes, adjusting for age, sex, race, body mass index, anti-inflammatory medication, and recent infections.
Results:
HL survivors (mean[SD] current age 36[8] years, 22[8] years post-diagnosis) demonstrated higher concentrations of interleukin-6 (IL-6), high-sensitivity c-reactive protein (hs-CRP), oxidized low-density lipoprotein, and glutathione peroxidase (GPx), compared to controls (p’s<0.001). Among survivors, higher concentrations of IL-6 were associated with worse visuomotor processing speed (p=0.046). hs-CRP ≥3 mg/L was associated with worse attention, processing speed, memory, and executive function (p’s<0.05). Higher concentrations of malondialdehyde were associated with worse focused attention and visual processing speed (p’s<0.05). Homocysteine was associated with worse short-term recall (p=0.008). None of these associations were statistically significant among controls. Among survivors, hs-CRP partially mediated associations between cardiovascular or endocrine conditions and visual processing speed, while IL-6 partially mediated associations between pulmonary conditions and visuomotor processing speed.
Conclusions:
Neurocognitive function in long-term survivors of HL appears to be associated with inflammation and oxidative stress, both representing potential targets for future intervention trials.
Introduction
An estimated 30-40% of long-term survivors of childhood cancer not exposed to central nervous system- (CNS) directed therapy experience neurocognitive impairment.[1] Hodgkin lymphoma (HL) is the most common childhood cancer treated without CNS-directed therapy and survivors of HL are more likely to self-report impaired attention and memory compared to siblings: up to 30% of HL survivors are impaired on neurocognitive tests of memory and attention.[2, 3] However, the biological mechanisms of neurocognitive impairment in survivors treated without CNS-directed therapy are poorly understood.[4] Identifying these mechanisms will inform the development of targeted interventions, both preventive and restorative.
The etiology of neurocognitive impairment in long-term survivors is likely multifactorial and may be facilitated by chronic low-grade inflammation and oxidative stress.[5] Studies in animal models and among persons with cancer indicate that chemotherapy and radiation activate inflammatory and oxidative stress pathways, which are associated with worse neurocognitive function during and shortly after exposure.[5-10] Peripheral inflammation can increase the permeability of the blood brain barrier, increasing brain exposure to pro-inflammatory cytokines and activating microglia. This process results in neuroinflammation, decreased neurogenesis, and ultimately neurocognitive impairment.[8, 11, 12] Accumulation of reactive oxygen species can lead to lipid peroxidation, protein oxidation, impaired folate physiology, DNA damage, and increased inflammation (e.g. TNF-α), which can ultimately lead to neuronal damage and neurocognitive impairment.[5, 13] Little is known about the concentrations of these markers or their associations with measures of neurocognitive function in survivors of childhood cancer treated without CNS-directed therapy.
Long-term survivors of childhood cancer have a high-burden of chronic health conditions which are associated with impaired neurocognitive functioning. [14, 15] Specifically, we have previously demonstrated that treatment-related cardiopulmonary disease is associated with neurocognitive impairment in survivors of childhood HL[2, 3, 16]. Inflammation, oxidative stress, and endothelial damage are associated with cardiopulmonary disease in the general population, suggesting they are a potential mechanism by which cardiopulmonary disease affects neurocognitive function.[17, 18] However, little is understood about how inflammation, oxidative stress, and endothelial damage may contribute to the association between cardiopulmonary conditions and neurocognitive function in survivors of childhood cancer.
This study aimed to evaluate biomarkers of inflammation, oxidative stress, and endothelial damage and their associations with neurocognitive function in long-term survivors of childhood cancer not exposed to CNS-directed therapies. Survivors of HL were chosen due to successful non-CNS-directed treatments and a high burden of morbidity and excess mortality among survivors.[19-21] We hypothesized HL survivors would have higher concentrations of inflammatory, oxidative stress, and endothelial damage biomarkers compared to community controls, and that these would be associated with worse neurocognitive functioning. We also hypothesized biomarkers would partially mediate the association between cardiopulmonary disease and neurocognitive dysfunction.
Materials and Methods
Participants
Survivors of HL were recruited from the St. Jude Lifetime Cohort (SJLIFE)[22] to an ancillary protocol (R01CA174794).[16] SJLIFE is a dynamic longitudinal cohort established to facilitate prospective assessment of health outcomes among long-term childhood cancer survivors. Survivors are eligible to enroll in SJLIFE once they at least five years post-diagnosis, and biospecimen or assessment data is not available at diagnosis. For this ancillary protocol, eligibility included treatment with thoracic radiation <21 years of age, currently ≥18 years old, and ≥10 years post-diagnosis. Exclusion criteria included cranial radiation, intrathecal or high-dose antimetabolite chemotherapy (rare in pediatric HL), history of head injury, genetic neurologic/heart disorder associated with neurocognitive impairment but not HL, and current pregnancy. Survivors who relapsed remained eligible, however those who underwent autologous stem cell transplant were ineligible. Non-cancer community controls were recruited from the survivor’s network or the Memphis, TN area (where St. Jude Children’s Research Hospital is located).[22] Controls were frequency matched (1:1) on age (+/−5 years), sex, and race/ethnicity. Survivors and controls were recruited between 2013 and 2017. Of the 204 HL survivors and 205 controls who completed the protocol, 197 and 199, respectively, had stored serum samples available and were included in these analyses (Supplemental Figure 1). This study was conducted in accordance with U.S. Common Rule and other ethical guidelines (e.g. Declaration of Helsinki); it was approved by the institutional review board and participants provided written informed consent.
Procedures
Participants completed a neurocognitive assessment including tests of intelligence/academics[23], attention[24, 25], processing speed[26, 27], memory[28, 29], and executive function.[24] Scores were referenced to national normative data to generate age-adjusted Z-scores. Within 3 days of neurocognitive testing, participants had a physical examination, laboratory, echocardiography, and pulmonary function testing.[22, 30]
Fasting blood samples were processed and serum stored in 0.5mL aliquots at −80° Celsius. We designed a panel of inflammatory, oxidative stress, and endothelial markers based on reported associations with neurocognition in cancer and non-cancer populations.[10, 31-36] Markers of inflammation included high sensitivity c-reactive protein (hs-CRP), interleukin-6 (IL-6), interferon gamma (IFN-ɤ), and tumor necrosis factor alpha (TNF-α); oxidative stress markers included homocysteine, oxidized low density lipoprotein (OxLDL), malondialdehyde, and glutathione peroxidase (GPx); cardiac endothelial damage markers included N-terminal-pro hormone brain natriuretic peptide (NTproBNP), Troponin I, and Troponin T. Absolute lymphocyte and platelet counts were included as markers of immune function and inflammation. hs-CRP was measured using a quantitative immunoturbidimetry assay and homocysteine was measured using a quantitative enzymatic assay at ARUP laboratories (Salt Lake City, UT). All other assays were completed by the CLIA licensed Cytokine Reference Laboratory (University of Minnesota) on Luminex multiplex or ELISA platforms (Supplemental Methods).
Participants self-reported demographics, smoking status, recent infections (within two weeks, e.g. sinus infection) and current medication (non-steroidal and steroidal anti-inflammatory medications; e.g., ibuprofen, prednisone). A stadiometer measured height and an electronic scale measured weight to calculate body mass index (BMI; kg/m2). Treatment history including chemotherapy (cumulative doses), surgical procedures, and radiation (fields/doses) was abstracted from medical records. Chronic health conditions (CHC) were graded using the SJLIFE-modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.3.[37] We considered five composite groups of grade 2+ CHC: 1) vascular (hypertension or dyslipidemia), 2) cardiac, 3) pulmonary, 4) endocrine, and 5) neurologic conditions [16].
Statistical Analyses
Biomarker values falling below the lower limit of detection (<LLOD) were imputed from a uniform distribution, U(0, LLOD). The process was repeated 20 times (multiple imputation, n=20) [34] for each biomarker. MIANALYZE procedure was used to summarize the parameter estimates from 20 independent analyses. Notably, >90% of the samples were <LLOD for INF-ɤ and NT-proBNP, therefore these biomarkers were excluded from analyses. All analyses were completed using SAS 9.4 (SAS Institute, Cary, N.C.) and MPLUS version 7.11 (MUTHEN & MUTHEN, Los Angeles, CA). All tests were two sided and considered statistically significant at p<0.05.
Linear regression compared natural-log-transformed values of biomarker concentrations in survivors and controls, adjusting for a priori identified potential confounders including age at evaluation, sex, race, BMI, current anti-inflammatory medication use (yes/no), and recent infection.[38, 39] Linear regression models among the survivors examined associations between treatment exposures and log-transformed biomarkers, adjusted for the same covariates. To enhance clinical significance, only neurocognitive outcomes where survivors performed significantly worse than controls after adjustment for multiple comparisons were examined (See Phillips et al [16] and Supplemental Table 1). We converted biomarker concentrations to tertiles derived from the control distribution, except for hs-CRP where the clinically validated cut point of 3 mg/L was used[18]. Mean differences in neurocognitive z-scores across the three tertiles of biomarker concentrations were compared using linear regression models adjusted for the covariates noted above and were repeated in controls for reference.
Because the mechanism underlying cancer-related neurocognitive impairment is unclear and involves several biomarkers, in secondary analyses we used elastic net variable selection[40] to evaluate multiple biomarkers simultaneously, aiming to identify the most important predictors of neurocognitive function. This approach of variable selection is robust to extreme correlations among predictors and avoids the potential problem of multiple testing issues in choosing the most parsimonious model. Among Hodgkin lymphoma survivors only, the GLMSELECT procedure was used for variable selection using elastic net for each of 20 imputed datasets. Parameters selected more than 70% (n=14) of the time were included into a final model. Linear regression (GENMOD procedure with normal distribution and identify link function) was then conducted using 20 datasets and MIANALYSE was used to summarize parameter estimates.
Based on preliminary observations that neurocognitive outcomes in HL were associated with smoking and cardiopulmonary disease[2, 3, 16], we aimed to examine if biomarkers mediated these associations. These analyses were considered exploratory given the small sample size and cross-sectional nature of our data. Among Hodgkin lymphoma survivors only, structural equation modeling explored mediation for any association where 1) current smoking or grade 2+ CHC was associated with the neurocognitive outcome, 2) biomarker was associated with the neurocognitive outcome, and 3) current smoking or grade 2+ CHC was associated with the biomarker. These analyses were considered exploratory and hypothesis generating, therefore we report associations statistically significant at p<0.10. IL-6 was the only imputed biomarker where significant mediation was observed, however, there was no variability in effect estimates across 20 imputed data sets and the results of one randomly chosen dataset are reported.
Data Sharing Statement:
Additional data on the biomarker assays used is available in the supplemental methods. The raw data underlying this article will be shared on reasonable request to the corresponding author.
Results
Biomarker Concentrations in HL Survivors and Controls
HL survivors were on average, 35.4 years old and 20.5 years from diagnosis (Table 1). After adjusting for covariates, survivors had higher concentrations of IL-6, hs-CRP, OxLDL, and GPx in addition to higher platelet counts compared to controls (Table 2). Approximately 45% of survivors had a hs-CRP ≥3 mg/L compared to 28% in controls (p<0.001). After adjustment for covariates, chest radiation >30 Gy was associated with significantly higher log-transformed concentrations of hs-CRP (β=0.65, 95%CI 0.07, 1.23) and homocysteine (β=0.23, 95%CI 0.07,0.39) compared to those with <24 Gy (Supplemental Table 2). Higher doses of vincristine were associated with significantly higher concentrations of hs-CRP (β=1.11, 95%CI 0.02, 2.20, per 100 mg/m2). Notably, there were no significant difference in biomarker concentrations between survivors with and without a subsequent neoplasm.
Table 1:
Sample characteristics
| Hodgkin Lymphoma Survivors N=197 |
Controls N=199 |
p-value1 | |
|---|---|---|---|
| N (%) | N (%) | ||
| Male | 95(48.2) | 94(47.2) | 0.84 |
| White | 167(84.8) | 171(85.9) | 0.75 |
| Smoking Status | |||
| Current | 38(19.3) | 29(14.6) | |
| Former | 33(16.8) | 42(21.1) | |
| Never | 126(64.0) | 128(64.3) | 0.32 |
| Body Mass Index (BMI) | |||
| Underweight | 7(3.6) | 2(1.0) | |
| Normal weight | 50(25.4) | 62(31.2) | |
| Overweight | 69(35.0) | 58(29.1) | |
| Obese | 71(36.0) | 77(38.7) | 0.15 |
| Recent Infection | 12 (6.1) | 14 (7.0) | 0.72 |
| Anti-Inflammatory Medication2 | 46 (23.4) | 37 (18.6) | 0.24 |
| Age at Assessment (Years) | |||
| Median (min, max) | 35.41 (22.63, 64.64) | 35.38 (18.28, 65.89) | 0.98 |
| Age at Diagnosis (Years) | |||
| Median (min, max) | 14.84 (3.01,22.60) | - | - |
| Time Since Diagnosis (Years) | |||
| Median (min, max) | 20.52 (10.68,45.62) | - | - |
| Treatment Exposures 3 | |||
| Anthracyclines (mg/m2) | 148 (75.1) | - | - |
| Median (min, max) | 157.20(91.50-400) | - | - |
| IV Methotrexate (mg/m2) | 81 (41.1) | - | - |
| Median (min, max) | 122.88(20.28-167.66) | - | - |
| Vincristine (mg/m2) | 133 (67.5) | - | - |
| Median (min, max) | 8.28(1.56-73.27) | - | - |
| Alkylating Agents (mg/m2) | 131 (66.5) | - | - |
| Median (min, max) | 6896.8(1189-28980) | - | - |
| Bleomycin (yes) | 56(28.4) | - | - |
| Prednisone (yes) | 135 (68.9) | ||
| Chest Radiation | |||
| <24 Gy | 48(24.4) | ||
| 24-30 Gy | 108(54.8) | - | - |
| >30 Gy | 41(20.8) | - | - |
| Neck Radiation | |||
| ≥30 Gy | 34(17.3) | - | - |
| 20 to <30 Gy | 143(72.6) | - | - |
| <20 Gy | 20(10.2) | - | - |
| Relapsed | 14(7.1) | - | - |
| Subsequent Malignancy4 | 40(20.3) | - | - |
Gy: Gray
p-value from Chi-square tests or two sided t-tests
anti-inflammatory medications included any non-steroidal anti-inflammatory drugs (NSAID, e.g. ibuprofen) and steroid medication
Mean(SD) and median (range) for all chemo and radiation doses were based on those who received treatment exposure
subsequent malignancies included 6 genitourinary tumors (2 uterine, 3 cervical, 1 testicular), 11 non-melanoma skin cancers, 10 thyroid tumors, 10 breast tumors, 1 lymphoma, and 2 parotid tumors.
Table 2:
Descriptive characteristics of biomarkers among survivors of Hodgkin lymphoma (HL) and community controls.
| HL Survivors | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Direction of Harmful Effect |
n | Mean (SD) | Median (min-max) | n | Mean (SD) | Median (min-max) | p-value1 |
| Inflammation | ||||||||
| Interleukin-6 (IL-6, pg/mL)2 | Higher | 197 | 1.94 (9.76) | 0.55 (0.0004-126.64) | 199 | 0.51 (0.95) | 0.32 (0.0002-11.15) | <0.001 |
| Tumor necrosis factor-α (TNF α, pg/mL)3 | Higher | 197 | 5.66 (3.90) | 5.43 (0.0375-37.29) | 199 | 4.84 (2.48) | 4.93 (0.0180-12.44) | 0.087 |
| High-sensitivity C-reactive protein (hs-CRP, mg/L)4 | Higher | 192 | 5.15 (9.32) | 2.45 (0.20-83.10) | 193 | 3.38 (6.17) | 1.20 (0.0137-46.10) | <0.001 |
| Oxidative Stress | ||||||||
| Oxidized low-density-lipoprotein (OxLDL, mU/L) | Higher | 197 | 80.28 (42.03) | 70.06 (11.721-255.57) | 199 | 62.51 (31.95) | 55.28 (13.030-185.39) | <0.001 |
| Malondialdehyde (uM)5 | Higher | 197 | 0.32 (0.53) | 0.05 (0.0005-5.12) | 199 | 0.42 (0.83) | 0.06 (0.0006-10.05) | 0.200 |
| Glutathione peroxidase (GPx, U/L) | Lower | 197 | 251.38 (134.7) | 262.50 (12.700-623.40) | 199 | 195.95 (112.0) | 196.10 (5.4526-625.70) | <0.001 |
| Endothelial Damage | ||||||||
| Troponin T6 (pg/mL) | Higher | 197 | 37.13 (64.63) | 19.11 (0.0854-531.70) | 199 | 36.99 (57.21) | 6.14 (0.0614-386.31) | 0.150 |
| Troponin I7 (pg/mL) | Higher | 197 | 197.47 (428.8) | 6.30 (0.0621-3116.7) | 199 | 234.36 (431.9) | 39.20 (0.0815-3229.2) | 0.180 |
| Immune/Hematological Markers | ||||||||
| Absolute lymphocyte count8 (109cells/liter) | Higher | 195 | 2294.70 (1032) | 2100.0 (606.0-8272.0) | 198 | 2087.40 (617.3) | 2000.0 (802.0-4300.0) | 0.170 |
| Platelets9 (109 cells/liter) | Higher | 193 | 286.40 (79.24) | 274.00 (129.0-624.00) | 189 | 255.52 (58.00) | 249.00 (131.0-499.00) | <0.001 |
| Homocysteine10 (μmol/L) | Higher | 190 | 10.49 (5.38) | 9.30 (4.00-47.70) | 193 | 9.77 (3.03) | 9.00 (5.00-28.00) | 0.118 |
Model examined difference in natural log transformed biomarkers in survivors and controls adjusted for age at evaluation, sex, race, smoking status, BMI, current anti-inflammatory use, and recent infection. Biomarker concentrations below the lower limit of detection (LLOD) were imputed using a range from 0 to the LLOD and multiple imputation.
28 (14%) HL <LLOD and 46 (23%) controls <LLOD
3 (2%) HL <LLOD and 10 (5%) controls <LLOD
Measured as part of routine clinical care, 5 HL survivors were missing data, 6 controls were missing data and 12 (6%) controls <LLOD
109 (55%) HL <LLOD and 106 (53%) controls <LLOD
72 (37%) HL <LLOD and 109 (55%) controls <LLOD
100 (51%) HL <LLOD and 91 (46%) controls <LLOD
measured as part of routine clinical care, 2 HL survivors and 1 control missing data
measured as part of routine clinical care, 4 HL survivors and 10 controls missing data
measured as part of routine clinical care 7 HL survivors and 6 controls missing data..
Associations between Biomarkers and Neurocognitive Function
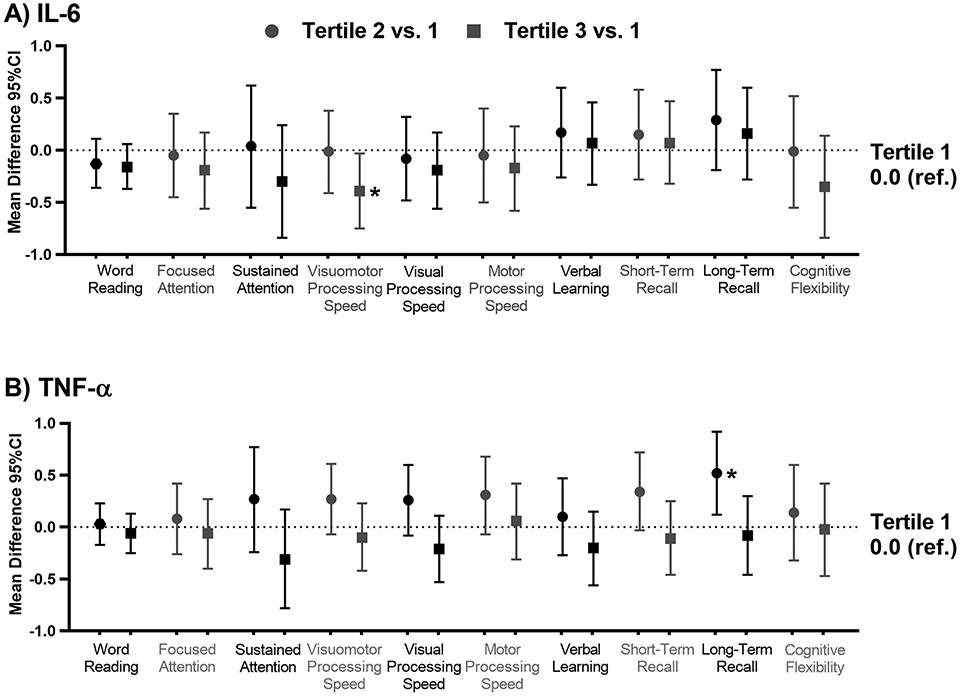
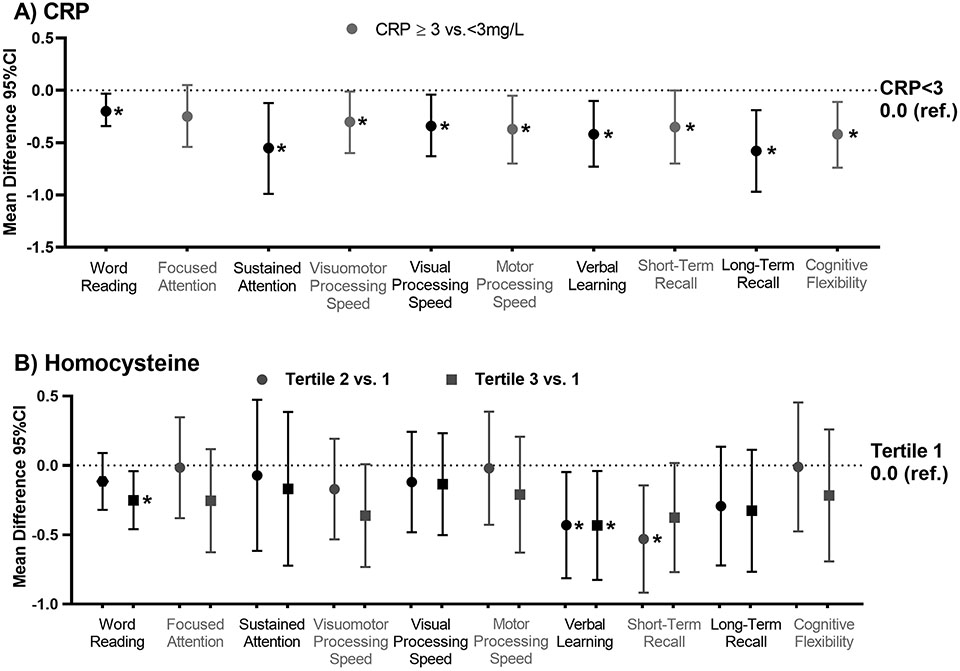
Among HL survivors, IL-6 was associated with worse visual-motor processing speed; those in the third tertile performed 0.39 standard deviation worse than those in the first (95%CI −0.75, −0.03, p=0.037; Figure 1). This association was smaller and not statistically significant among community controls (Supplemental Table 3). The second tertile of TNF-a was associated with a worse score on long-term recall compared to those in the first (Figure 1). Survivors with hs-CRP ≥3 mg/L had worse performance on tests of word reading, sustained attention, visual-motor, motor, and visual processing speed, verbal learning, short- and long-term recall and cognitive flexibility (p’s<0.05; Figure 2, Supplemental Table 3). Similar associations were not identified in controls.
Figure 1: Associations between inflammatory biomarkers and neurocognitive outcomes among survivors of Hodgkin lymphoma.
The figures below represent the mean difference and 95% confidence interval (CI) in neurocognitive z-score comparing the second tertile (circles) and the third tertile (squares) to the first tertile (0.0 ref.) for A) interleukin-6 (IL-6) and B) tumor necrosis factor alpha (TNF-α). Models are adjusted for age at evaluation, sex, race, body mass index, current anti-inflammatory use, and recent infection.
Figure 2: Associations between hs-CRP and Homocysteine and neurocognitive outcomes among survivors of Hodgkin lymphoma.
The figures below represent the mean difference and 95% confidence interval (CI) in neurocognitive z-score comparing the second tertile (circles) and the third tertile (squares) to the first tertile (1.0 ref.) for A) c-reactive protein (CRP) and B) homocysteine. Models are adjusted for age at evaluation, sex, race, body mass index, current anti-inflammatory use, and recent infection.
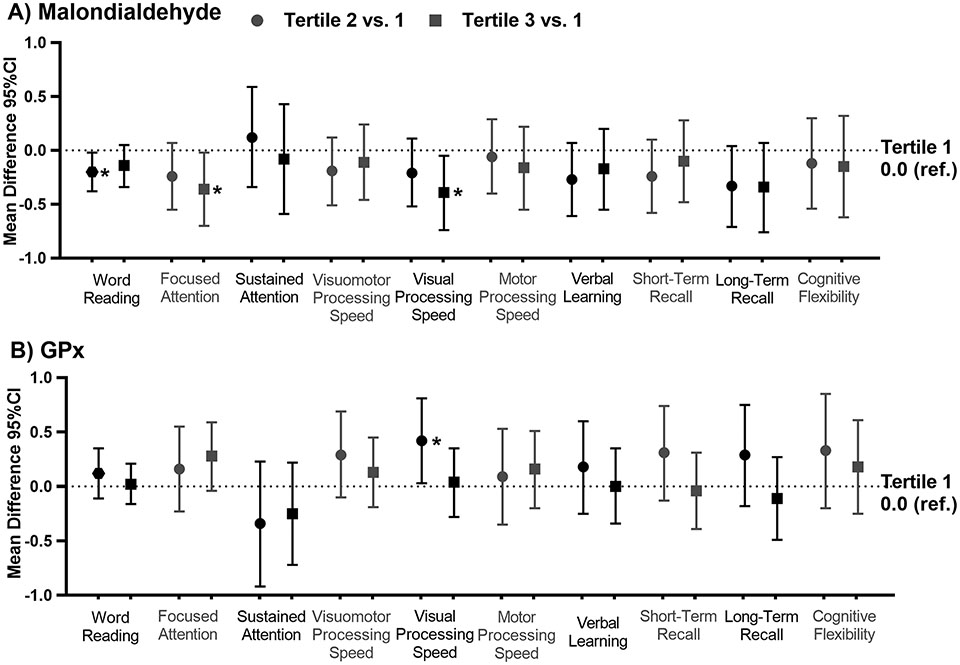
Higher homocysteine concentrations were associated with worse performance on word reading, verbal learning and short-term recall (p’s<0.05; Figure 2, Supplemental Table 3). Apart from verbal learning, these associations were not noted among controls. Higher concentrations of malondialdehyde were associated with worse performance on word reading, focused attention, and visual processing speed among HL survivors (p’s<0.05; Figure 3, Supplemental Table 3). Survivors in the second tertile of GPx, an enzyme that helps to clear reactive oxygen species, performed better on visual processing speed than those in the first (p=0.038). These associations were not noted among community controls.
Figure 3: Associations between oxidative stress biomarkers and neurocognitive outcomes among survivors of Hodgkin lymphoma.
The figures below represent the mean difference and 95% confidence interval (CI) in neurocognitive z-score comparing the second tertile (circles) and the third tertile (squares) to the first tertile (1.0 ref.) for A) Malondialdehyde (Malondialdehyde) and B) glutathione peroxidase (GPx). Models are adjusted for age at evaluation, sex, race, body mass index, current anti-inflammatory use, and recent infection.
Multiple-Biomarker Models
After elastic net multi-biomarker variable selection, hs-CRP remained significantly associated with worse performance on word reading (p=0.006), motor (p=0.010), visual (p=0.010) and visuomotor (p=0.048) processing speed, sustained attention (p=0.010), short-term recall (p=0.011), and cognitive flexibility (p=0.011) (Table 3). Homocysteine remained associated with worse performance on word reading (p=0.027), focused attention (p=0.013), and cognitive flexibility (p=0.008). Malondialdehyde was associated with worse visual processing speed (p=0.033). GPx was also associated with better performance on focused attention (p=0.029). Interestingly, higher platelet count was associated with better performance on all memory tests (verbal learning p=0.011, short-term recall p=0.004, long-term recall p=0.037).
Table 3:
Mean difference (95% confidence intervals) in neurocognitive z-scores associated with natural log-transformed biomarkers and covariates derived from multivariate models with elastic net variable selection among survivors of Hodgkin lymphoma.
| Attention | Processing Speed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Focused | Sustained | Visuomotor | Motor | Visual | ||||||
| β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | |
| CRP>3 mg/L | −0.51(−0.90, −0.12) | 0.010 | −0.27 (−0.53, −0.001) | 0.048 | −0.38(−0.67, −0.09) | 0.010 | −0.35(−0.61, −0.08) | 0.010 | ||
| IL-6 | ||||||||||
| Homocysteine | −0.50(−0.90, −0.10) | 0.013 | −0.39(−0.95, 0.18) | 0.181 | −0.48(−0.88, −0.08) | 0.019 | −0.45(−0.90, −0.01) | 0.045 | ||
| OxLDL | ||||||||||
| GPx | 0.20(0.02, 0.38) | 0.029 | ||||||||
| Malondialdehyde | −0.09(−0.17, −0.01) | 0.033 | ||||||||
| Lymphocyte Count | ||||||||||
| Platelets | ||||||||||
| Troponin T | ||||||||||
| Age (years) | ||||||||||
| White (vs. Other) | 0.30(0.00, 0.60) | 0.053 | 0.64(0.28, 1.00) | 0.009 | ||||||
| Female (vs. Male) | 0.25(−0.02, 0.52) | 0.075 | 0.36(0.09, 0.63) | 0.010 | 0.39(0.00, 0.79) | 0.051 | 0.35(0.09, 0.61) | <0.001 | ||
| Anti-inflammatory (vs. no) | −0.30(−0.61, 0.01) | 0.057 | ||||||||
| Recent Infection (vs. no) | −0.63(−1.41, 0.16) | 0.12 | ||||||||
| Body Mass Index (kg/m2) | ||||||||||
| Memory | Executive Function | Academics | ||||||||
| Verbal Learning | Short-Term Recall | Long-Term Recall | Cognitive Flexibility | Word Reading | ||||||
| β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | β(95%CI) | p | |
| CRP>3 mg/L | −0.36(−0.64, −0.08) | 0.011 | −0.28(−0.60, 0.04) | 0.087 | −0.43(−0.76, −0.10) | 0.011 | −0.23(−0.39, −0.06) | 0.006 | ||
| IL-6 | ||||||||||
| Homocysteine | −0.66(−1.14, −0.18) | 0.008 | −0.25(−0.46, −0.03) | 0.027 | ||||||
| OxLDL | 0.24(−0.01, 0.5) | 0.064 | ||||||||
| GPx | ||||||||||
| Malondialdehyde | ||||||||||
| Lymphocyte Count | 0.11(−0.08, 0.29) | 0.251 | ||||||||
| Platelets | 0.69(0.16, 1.22) | 0.011 | 0.77(0.25, 1.30) | 0.004 | 0.63(0.04, 1.21) | 0.037 | ||||
| Troponin T | 0.03(−0.02, 0.07) | 0.270 | ||||||||
| Age (years) | 0.01(0.00, 0.02) | 0.059 | ||||||||
| White (vs. Other) | 0.55(0.10, 0.99) | 0.016 | 0.35(0.15, 0.56) | 0.001 | ||||||
| Female (vs. Male) | −0.22(−0.50, 0.05) | 0.115 | ||||||||
| Anti-inflammatory (vs. no) | −0.54(−0.88, −0.19) | 0.002 | −0.30(−0.69, 0.08) | 0.124 | ||||||
| Recent Infection (vs. no) | ||||||||||
| Body Mass Index (kg/m2) | 0.02(0.01, 0.03) | 0.003 | ||||||||
Results from separate multivariable models for each neurocognitive outcome where elastic net variable section was used; all the variables above were entered into the model and the most appropriate model based on model fit criteria such as BIC or aBIC is selected. All biomarkers were natural log transformed and operationalized continuously. The estimates from final models are presented, if a cell is empty that variable was not selected for the final model.
Mediation of CHC Associations
Mediation analyses were considered exploratory; therefore, we report indirect effects of borderline significance (p<0.10) with full results available in Supplemental Table 4. hs-CRP partially mediated the association between grade 2+ vascular conditions and worse performance on sustained attention (39.6% variance mediated), motor processing speed (17.4%), and visual processing speed (70.9%). hs-CRP also mediated the association between endocrine conditions and visual processing speed (32.1%). hs-CRP partially mediated the association between pulmonary conditions and visual processing speed (25.7%) while IL-6 partially mediated the association between pulmonary conditions and visuomotor-processing speed (36.8%).
Discussion
In this, the first assessment of serum biomarkers in non-CNS-treated survivors of childhood cancer, we provide data to support the hypothesis that inflammation, oxidative stress, and folate physiology contribute to cancer-related neurocognitive impairment in long-term survivors of childhood HL. Compared to matched community controls, survivors of HL have higher concentrations of inflammation and oxidative stress 20 years post-diagnosis which are associated with neurocognitive dysfunction. hs-CRP was broadly associated with worse neurocognitive function, while IL-6 was specifically associated with worse visuomotor processing speed. Moreover, hs-CRP and IL-6 mediated associations between vascular or pulmonary CHCs and measures of attention and processing speed. Malondialdehyde and homocysteine were associated with worse neurocognitive function in multiple domains, which were not observed in community controls. Antineoplastic treatments may inhibit folate-dependent processing resulting in the accumulation of homocysteine in addition to significant oxidative damage. These data suggest systemic inflammation and oxidative stress is chronic in long-term survivors and may serve as a target for interventions to alleviate late cancer-related neurocognitive dysfunction.
Similar to our findings, Cheung et al. report IL-6 and hs-CRP were associated with visuomotor processing speed and cognitive flexibility in adolescent survivors of acute lymphoblastic leukemia (ALL).[41] IL-6 and hs-CRP are also associated with deficits in processing speed and executive functions among breast cancer survivors.[6, 10, 42, 43] In our study, after adjustment for known confounders, hs-CRP was associated with worse performance on multiple measures of attention, processing speed, memory, and executive function. Importantly, these associations could not be replicated in community controls. These data implicate inflammation in the etiology of cancer-related neurocognitive impairment among non-CNS treated survivors of childhood cancer. High peripheral concentrations of pro-inflammatory cytokines can increase the permeability of the blood brain barrier, allowing cytokines to enter the CNS and activate microglia-related neuroinflammation, which decreases neurogenesis and can lead to neurocognitive impairment.[8, 11, 12] Assays for hs-CRP are routinely available, making it a useful biomarker to identify survivors at risk for neurocognitive dysfunction who may benefit most from an anti-inflammatory intervention to mitigate neurocognitive impairment.
We provide new data that suggest chronic inflammation may be important to the underlying causal mechanisms by which cardiopulmonary morbidity impacts processing speed dysfunction in survivors. We previously demonstrated that cardiovascular and pulmonary health conditions were associated with neurocognitive impairment among survivors of childhood HL.[2, 3, 16] This study expands the previous work to understand how cardiopulmonary disease may impact neurocognitive function, suggesting it may be through increased peripheral inflammation. However, the relationship between inflammation and cardiopulmonary disease is likely complex and bidirectional. Therefore, longitudinal studies are warranted to confirm the temporal relationship between these factors. It is possible that behavioral interventions targeting cardiopulmonary health, combined with an anti-inflammatory pharmaceutical intervention, may positively impact neurocognitive functioning. In fact, preliminary data from an ongoing clinical trial of adult cancer patients demonstrate exercise and low-dose ibuprofen may improve attention.[44]
Accumulation of reactive oxygen species (ROS) can lead to lipid peroxidation, protein oxidation, DNA damage, and increased inflammation (e.g. TNF-α), leading to neuronal damage and neurocognitive impairment.[5, 13] We demonstrate that malondialdehyde was associated with worse visual processing speed, while glutathione, an enzyme that clears ROS, was associated with better visual processing speed. These associations were not seen in controls, suggesting HL may be uniquely susceptible to the effects of oxidative stress on the CNS. This may be due to altered metabolism. Two studies of ALL survivors report polymorphisms in glutathione S-transferase genes are associated with worse memory, attention, or processing speed.[45, 46] One study also reported polymorphisms in the methionine synthase gene, an enzyme involved in homocysteine metabolism, were associated with decreased attention and response speed and elevated homocysteine levels were associated with impaired cognitive flexibility.[45, 47] In our study higher concentrations of serum homocysteine were associated with worse performance on tests of verbal learning and short-term recall. Mild hyperhomocysteinemia, due to impaired folate physiology, has been associated with vascular damage, inflammation, and oxidative stress all of which can contribute to neurocognitive impairment.[48, 49] Although only a small subset of survivors (7%) had elevated homocysteine levels, future research is needed to examine how polymorphisms related to homocysteine metabolism modify reported associations as well as the utility of folate therapy to improve neurocognitive function.
This study provides new data on biomarkers of inflammation and oxidative stress and associations with neurocognitive function among survivors of childhood cancer treated without CNS directed therapies. Because many of the biomarkers measured do not have clinically validated reference ranges, the inclusion of a control group strengthens the interpretation of our findings. Where most of the existing literature has examined one biomarker at a time, we were able to replicate associations in models examining multiple markers simultaneously, strengthening the specificity of associations. We were also able to account for many potential confounders, such as medications, infections, and BMI. Because neurocognitive function and biomarkers were measured simultaneously, we cannot comment on the temporal association between them, and results should be interpreted cautiously with respect to causality. Future longitudinal analyses are planned to confirm if inflammation and oxidative stress contribute to neurocognitive decline and to examine the trajectories of biomarkers as they relate to the trajectories of neurocognitive function. While this is the largest study of biomarkers of neurocognitive dysfunction in non-CNS-treated survivors of childhood cancer, we were likely underpowered to detect some effects, larger studies may be needed to detect subtle associations. This may have contributed to our inability to detect statistically significant associations in the third tertile when the second tertile was significant. There may also be a threshold effect where these associations become apparent and dissipate thereafter, which would explain the several associations noted only in the second tertile. This needs to be replicated in additional, larger studies. While our sample is representative of the epidemiology of pediatric Hodgkin lymphoma in the US (Supplemental Table 5), these findings should be replicated in more diverse samples. Additionally, future work should include patient reported assessments of neurocognitive function that may detect more subtle, yet impactful neurocognitive impairments. Lastly, because of our limited sample size and exploratory hypotheses, we did not perform a formal adjustment for multiple comparisons, rather we used elastic net models to consider multiple markers while accounting for multiple comparisons.
In summary, long-term survivors of childhood HL had higher concentrations of biomarkers of inflammation and oxidative stress that were associated with worse neurocognitive performance. These data provide insight into the underlying mechanisms of and potential targets to improve neurocognitive function in survivors of childhood cancer. Future research will help elucidate longitudinal associations between these biomarkers and long-term neurocognitive decline.
Supplementary Material
Translational Relevance.
Survivors of childhood Hodgkin lymphoma experience significant neurocognitive dysfunction, despite never receiving therapy directed at the central nervous system. In the current study, we demonstrate that biomarkers of inflammation and oxidative stress are associated with worse neurocognitive function in long-term survivors of childhood Hodgkin lymphoma. Further, inflammatory markers mediated associations between cardiopulmonary conditions and worse neurocognitive functioning. Therefore, these data suggest that inflammation and oxidative stress represent targets for future intervention trials to improve neurocognitive functioning in long-term survivors of Hodgkin lymphoma.
Acknowledgements:
We would like to thank the participants of the SJLIFE cohort for their time and participation in this study. This work was supported by the National Cancer Institute at the National Institutes of Health (grant numbers K00CA222742 [Dr. A. Williams], K99CA256356 [Dr. A. Williams], R01CA174794 [Drs. K. Krull and N. Sabin], and U01CA195547 [Drs. M. Hudson and K. Ness]) and the American Lebanese Syrian Associated Charities (ALSAC, all). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Funding Agency:
The funding agency was not involved in the design, conduct, analysis or interpretation of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- HL
Hodgkin lymphoma
- CNS
Central Nervous System
- ELISA
enzyme-linked immunoassay
- SD
standard deviation
- hs-CRP
high-sensitivity C-reactive protein
- GPx
glutathione peroxidase
- BMI
body mass index
- IL-6
interleukin-6
- IFN-ɤ
interferon gamma
- TNF-α
tumor necrosis factor alpha
- OxLDL
oxidized low density lipoprotein
- NTproBNP
N-terminal-pro hormone brain natriuretic peptide
- SJLIFE
St. Jude Lifetime Cohort
- CHC
chronic health conditions
- LLOD
lower limit of detection
Footnotes
The authors declare no potential conflicts of interest.
Author Disclosures: The authors have nothing to disclose.
Disclaimers: No disclaimers exist.
Prior Presentations of these Data: These data were presented, in part, at the International Cancer and Cognition Task Force meeting in February of 2020 as well as the American Society of Hematology annual meeting in December of 2019.
References
- 1.Ahles TA, Hurria A. New Challenges in Psycho-Oncology Research IV: Cognition and cancer: Conceptual and methodological issues and future directions. Psychooncology 2018;27(1):3–9. [DOI] [PubMed] [Google Scholar]
- 2.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol 2012;30(29):3618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AM, Mirzaei Salehabadi S, Xing M, et al. Modifiable Risk Factors for Neurocognitive and Psychosocial Problems After Hodgkin Lymphoma. Blood 2021; 10.1182/blood.2021013167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams AM, Cole PD. Biomarkers of Cognitive Impairment in Pediatric Cancer Survivors. J Clin Oncol 2021; 10.1200/jco.20.02436:Jco2002436. [DOI] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 2007;7(3):192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol 2015;26(7):1446–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams ALM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. Journal of Neuroimmunology 2018;314:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren X, Boriero D, Chaiswing L, et al. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochimica et Biophysica Acta - Molecular Basis of Disease 2019;1865:1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005;104(4):788–93. [DOI] [PubMed] [Google Scholar]
- 10.Patel SK, Wong AL, Wong FL, et al. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. J Natl Cancer Inst 2015;107(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers JS, Pierce J, Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncol Nurs Forum 2008;35(6):916–20. [DOI] [PubMed] [Google Scholar]
- 12.Schrepf A, Lutgendorf SK, Pyter LM. Pre-treatment effects of peripheral tumors on brain and behavior: neuroinflammatory mechanisms in humans and rodents. Brain Behav Immun 2015;49:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariani E, Polidori MC, Cherubini A, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: An overview. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 2005;827:65–75. [DOI] [PubMed] [Google Scholar]
- 14.Williams AM, Cheung YT, Hyun G, et al. Childhood Neurotoxicity and Brain Resilience to Adverse Events during Adulthood. Ann Neurol 2021;89(3):534–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung YT, Brinkman TM, Li C, et al. Chronic Health Conditions and Neurocognitive Function in Aging Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 2018;110(4):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips NS, Mulrooney DA, Williams AM, et al. Neurocognitive Impairment Associated with Chronic Morbidity in Long-term Survivors of Hodgkin Lymphoma. Blood Adv 2023; 10.1182/bloodadvances.2023010567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006;113(19):2335–62. [DOI] [PubMed] [Google Scholar]
- 18.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 19.Delaney A, Howell CR, Krull KR, et al. Progression of Frailty in Survivors of Childhood Cancer: a St. Jude Lifetime Cohort Report. JNCI: Journal of the National Cancer Institute 2021; 10.1093/jnci/djab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016;17(9):1325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams AM, Liu Q, Bhakta N, et al. Rethinking Success in Pediatric Oncology: Beyond 5-Year Survival. J Clin Oncol 2021; 10.1200/jco.20.03681:Jco2003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell CR, Bjornard KL, Ness KK, et al. Cohort Profile: The St. Jude Lifetime Cohort Study (SJLIFE) for paediatric cancer survivors. International Journal of Epidemiology 2020;50(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Acheivement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 24.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 2004;19(2):203–14. [DOI] [PubMed] [Google Scholar]
- 25.Conners CK, Connelly V, Campbell S, MacLean M. Conners' Continuous Performance Test, 2nd ed. North Tonawanda, NY: Multi-Health Systems Inc. ; 2003. [Google Scholar]
- 26.Wechsler D. Abbreviated Scale of Intelligence. San Antonio, TX: Pyschological Corporation; 1999. [Google Scholar]
- 27.Strauss E, Sherman EM, Spreen, ). A Compendium of Neuropsychological Tests: Adminstration, Norms, and Commentary, 3rd ed. . London, UK: Oxford University Press; 2006. [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: Pyschological Corporation; 2008. [Google Scholar]
- 29.Delis DC, Kramer JH, Kaplan E. California Verbal Learning Test, 2nd ed. San Antonio, TX; 2000. [Google Scholar]
- 30.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer 2011;56(5):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Velpen IF, Feleus S, Bertens AS, et al. Hemodynamic and serum cardiac markers and risk of cognitive impairment and dementia. Alzheimers Dement 2017;13(4):441–453. [DOI] [PubMed] [Google Scholar]
- 32.Beydoun MA, Dore GA, Canas JA, et al. Systemic Inflammation Is Associated With Longitudinal Changes in Cognitive Performance Among Urban Adults. Front Aging Neurosci 2018;10:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe Y, Kitamura K, Nakamura K, et al. Elevated C-Reactive Protein Is Associated with Cognitive Decline in Outpatients of a General Hospital: The Project in Sado for Total Health (PROST). Dement Geriatr Cogn Dis Extra 2016;6(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun 2013;30 Suppl:S99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YT, Chang WN, Tsai NW, et al. The roles of biomarkers of oxidative stress and antioxidant in Alzheimer's disease: a systematic review. Biomed Res Int 2014;2014:182303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomykala KL, Ganz PA, Bower JE, et al. The association between pro-inflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav 2013;7(4):511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev 2017;26(5):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontes JD, Yamamoto JF, Larson MG, et al. Clinical correlates of change in inflammatory biomarkers: The Framingham Heart Study. Atherosclerosis 2013;228(1):217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frijhoff J, Winyard PG, Zarkovic N, et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid Redox Signal 2015;23(14):1144–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Cheung YT, Brinkman TM, Mulrooney DA, et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer 2017;123(17):3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun 2013;30 Suppl:S109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon DE, Cohen R, Chen H, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J Neuroimmunol 2016;301:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janelsins MC, Shayne M, Huston A, et al. Phase II study of exercise and low-dose ibuprofen for cancer-related cognitive impairment (CRCI) during chemotherapy. Journal of Clinical Oncology 2021;39(15_suppl):12016–12016. [Google Scholar]
- 45.Krull KR, Bhojwani D, Conklin HM, et al. Genetic Mediators of Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia. Journal of Clinical Oncology 2013;31(17):2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole PD, Finkelstein Y, Stevenson KE, et al. Polymorphisms in Genes Related to Oxidative Stress Are Associated With Inferior Cognitive Function After Therapy for Childhood Acute Lymphoblastic Leukemia. J Clin Oncol 2015;33(19):2205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krull KR, Cheung YT, Liu W, et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34(22):2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Q, Wang L, Si X, et al. Current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur J Pharmacol 2021; 10.1016/j.ejphar.2021.174168:174168. [DOI] [PubMed] [Google Scholar]
- 49.Wyse ATS, Bobermin LD, Dos Santos TM, et al. Homocysteine and Gliotoxicity. Neurotox Res 2021;39(3):966–974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.