Abstract
Alpiniae oxyphylla fructus was extensively utilized both as dietary supplements and traditional herbal medicines for healthcare functions and has exhibited a positive impact on animal health. The present study aimed to investigate the effects of Alpiniae oxyphyllae fructus powder (AOP) on production performance, egg quality, egg yolk fatty acid composition, reproductive hormones, antioxidant capacity, immunity, anti-apoptosis ability, and intestinal health in hens. A total of 252 Hainan Wenchang laying hens (30-wk-old) were randomly divided into 3 groups with 6 replicates, a basic diet with 0 (CON), 1 g/kg AOP (AOP1), and 3 g/kg (AOP3) mixed AOP. The AOP supplementation was found to decrease the feed conversion ratio and embryo mortality but to increase the laying rate, average egg weight, and oviduct index linearly (p < 0.05). Furthermore, AOP treatment reduced the total saturated fatty acids and palmitic acid (C16:0) in the egg yolk while increasing eggshell strength, albumen height, and Haugh unit (p < 0.05). The serum levels of albumin and phosphorus were increased, whereas total cholesterol, triglycerides, and glucose levels decreased as a result of AOP treatment (p < 0.05). The inclusion of 3 g/kg AOP had higher 17 β-estradiol and follicle-stimulating hormone levels in serum, while it up-regulated follicle-stimulating hormone receptor and gonadotropin-releasing hormone expression in ovary (p < 0.05). Dietary AOP strengthened the expression of nuclear factor erythroid2-related factor 2 in ovary and increased the activity of superoxide dismutase and total antioxidant capacity, but had a lower malondialdehyde content in serum (p < 0.05). AOP at 3 g/kg up-regulated superoxide dismutase 1 and heme oxygenase 1 expression in jejunum and ovary (p < 0.05). Meanwhile, AOP supplementation down-regulated p53 expression in ovary and bcl-2-associated x expression in liver and jejunum, especially 3 g/kg of AOP had lower caspase-8 concentrations and down-regulated bcl-2-associated x and caspase-3 expression in ovary (p < 0.05). AOP treatment increased serum levels of immunoglobulin A and immunoglobulin M and upregulated interleukin-4 expression in the liver, while decreasing interleukin-1β expression in liver and ovary and nod-like receptor protein 3 expression in jejunum (p < 0.05). Dietary AOP increased the ratio of villus height to crypt depth but decreased crypt depth in jejunum, especially when 1 g/kg AOP increased expression levels of occludin, mucin-2, peptide-transporter 1, and sodium glucose cotransporter 1 in jejunum (p < 0.05). AOP treatment altered the composition of the cecal microbial community, as evidenced by increased abundance of Oscillospira and Phascolarctobacterium and reduced richness of Clostridiaceae_Clostridium. Dietary AOP supplementation enriched lipid, amino acid, and propanoate metabolism. Spearman's correlation analysis revealed that the genera Oscillospira, Blautia, and Megasphaera were related to laying performance and intestinal integrity. In brief, supplementation of AOP, especially at 3 g/kg, could improve production performance and egg quality of hens via modulating reproductive hormones, antioxidant capacity, immunity, intestinal barrier, and cecal microbiota. Overall, the present work recommends the dietary inclusion of AOP as a beneficial additive for improving the performance of hens.
Key words: alpiniae oxyphyllae fructus, production performance, egg quality, immunity, intestinal health
INTRODUCTION
The extensive use of antibiotics in chicken farming raised the risk of antibiotic residues in livestock products and fosters the growth of drug-resistant bacteria, which was extremely dangerous for human health and the environment (Al-Khalaifa et al., 2019). Wenchang chickens, which originated from Hainan island in the South China Sea, were known for their excellent meat quality and high prolificacy (Tang et al., 2009). Due to restrictions on antibiotic growth promoters for animals, intensive breeding of laying hens faced significant challenges, including reduced efficiency in production and impaired reproductive system function (Wang et al., 2018), a risk for intestinal barrier dysfunction, immune imbalance, and gut microbiota disturbance (Feng et al., 2021). Based on these facts, the search for safe and effective antibiotic alternatives or feed additives like medicinal plants to mitigated the impact of detrimental factors and ensure animal health was becoming increasingly urgent, especially in the context of the highly efficient production of hens.
Dried fruit from Alpinia oxyphylla Miq., commonly known as A. oxyphylla, was a dietetic Chinese herb (Zingiberaceae) that was extensively used in southern China to treat kidney asthenia, dyspepsia, diarrhea, and abdominal pain (Li et al., 2021). Alpiniae oxyphyllae fructus contained a variety of natural products, including flavonoids, essential oils, sesquiterpenes, diarylheptanoids, steroids, and glycosides (Chen et al., 2013; Zhang et al., 2018). Research has indicated that the primary active ingredients in A. oxyphylla include tectochrysin, nootkatone, chrysin, and protocatechuic acid (Xiao et al., 2023). Recent pharmacological studies have indicated that A. oxyphylla s exhibits a variety of biological effects, including anti-inflammatory (Wang et al., 2018), anti-apoptotic (Qi et al., 2019), antioxidant (El-Sonbaty et al., 2019), and anti-ulcer (Wang et al., 2012) properties. Procyanidin B-2 and epicatechin, the 2 primary chemical constituents of A. oxyphylla, have demonstrated 2,2-diphenyl-1-picryhydrazyl radical (DPPH) and 2,20 -azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid radical (ABTS) radical scavenging properties (Li et al., 2023). Additionally, a number of studies revealed that by enhancing antioxidant status, oregano essential oil reduced the detrimental effects of transportation on pigs (Zou et al., 2016). Earlier studies have demonstrated that flavonoids offer various beneficial effects, including enhancing immunity and improving gut morphology and function (Ali, 2015). One of the many natural compounds known as polyphenols regulated immune responses in the intestinal mucosa and enhanced protection against foreign infections through various mechanisms (Ding et al., 2018). The gut microbiome was essential for preserving gut health and had an effect on laying hens' general performance. In addition to providing vital micronutrients, amino acids, and short-chain fatty acids, it also aided in the intestinal epithelium's maturation and boosted the immune system's development by generating antimicrobial compounds and using competitive exclusion to stop pathogen invasion (Khan et al., 2020). Research has indicated that supplementation of ducks' diets with extracts from A. oxyphylla altered the intestinal microbial composition while preserving intestinal integrity (Ji et al., 2021). Furthermore, it has been discovered that flavonoids alter the gut microbiome's composition, inhibit the development of some diseases, and promote the proliferation of beneficial bacterial species (Oteiza et al., 2018).
However, while most studies on A. oxyphylla have focused on its beneficial effects on humans as traditional Chinese medicine, we aimed to explore its potential application as an additive in the animal production industry. As far as we know, little was known about the effects of AOP as feed additives or about its possible uses in the production of chickens. The purpose of this experiment was to ascertain how adding different concentrations of AOP to the diet affected the hens' immune system, microflora composition, nutrient transporters, anti-apoptotic ability, immune status, and ability to produce eggs and eggs of high quality.
MATERIALS AND METHODS
Preparation of AOP
Alpiniae oxyphyllae Fructus was provided by Anguo Chang'an Chinese Medicinal Herbs Co., Ltd (Hebei, China). Following drying, the material was ground into a powder using a pulverizer, sieved through an 80-mesh screen for filtration, and kept for later use at room temperature (25°C). Association of Official Analytical Chemists (AOAC) procedures were used to determine the proximate chemical composition of AOP, which includes calcium, total phosphorus, ash, crude protein, and ether extract (AOAC, 2010). The phenol-sulfuric acid method was used to identify the polysaccharides in AOP, with glucose acting as a reference (Yang et al., 2021). The NaNO2-Al (NO3)3-NaOH colorimetric method was used to measure the flavonoid content of AOP, with rutin acting as a reference (Park et al., 2008). Gallic acid was used as the standard in the Folin-ciocalteu method to calculate the polyphenol content of AOP (Jayaprakasha et al., 2001). The detailed nutritional levels and main bioactive compounds were presented in Table 1.
Table 1.
The nutritive composition and main bioactive compounds in dried powder of Alpiniae oxyphyllae Fructus.
| Nutritive ingredients | Content (%) | Bioactive compound | Content (%) |
|---|---|---|---|
| Crude protein | 7.45 | Polyphenols | 19.85 |
| Crude fat | 2.10 | Flavonoids | 10.56 |
| Crude fiber | 11.10 | Polysaccharides | 2.06 |
| Ash | 6.70 | ||
| Ca | 0.24 |
Experimental Birds, Diets, and Management
The Animal Care and Use Committee of South China Agricultural University (approval number: SYXK 2022–0136, Guangzhou, China) approved each experimental protocol.
A total of 252 healthy Wenchang breeder hens (30 wk old) were provided by Enping Jilong Industrial Co., Ltd (Jiangmen, China). The hens were evenly and randomly divided into 3 treatment groups, each consisting of 6 replicates of 14 breeder hens. The 3 groups received the following treatments: AOP1 (basal diet + 1 g/kg AOP), AOP3 (basal diet + 3 g/kg AOP), and CON (basal diet + 0 g/kg AOP). Table S1 displayed the ingredients and composition of the diets. After a week-long acclimation period, all breeder hens were fed the designated experimental diets for a duration of 8 wk (d 56). During the trial period, each hen received 85 g of food daily at 07:30 and had unrestricted access to water. Breeder hens were artificially inseminated every 3 d with 35 μL of pooled semen per bird, as per the protocol described by (Liu et al., 2019). The chicken coop was kept at 27.5 ± 2.5 °C and 78.5 ± 3.5%, respectively, in terms of temperature and relative humidity. A lighting schedule consisting of 16 h of light and 8 h of darkness was followed throughout the experiment.
Sample Collection
Six laying breeder hens were chosen at random from each group at the end of the trial (one per replicate). After a 12-h fast, 5 mL of blood was collected from the wing vein and centrifuged for 10 min at 4°C and 3,000 × g. The resultant serum was kept for additional examination at -80°C. Organ indices were determined by removing, weighing, and analyzing the liver, oviduct, and ovary tissues after euthanizing the chickens via cervical dislocation. Additionally, 2 sections of the jejunum were excised and preserved in a formalin solution for microscopic examination. Measurements were taken of the oviduct's length and the number of graded follicles, which included pre-ovulatory follicles (POF, >10 mm in diameter) and small yellow follicles (SYF, 8∼10 mm in diameter). Likewise, tissue samples from the liver and ovaries, as well as mucosal samples from the middle jejunum, were taken and preserved at -80°C for later examination. The cecal digesta samples were immediately collected on ice and stored at -80°C for 16S rRNA analysis.
Production Performance and Egg Quality
At the beginning of 32 wk of the trial, the daily feed intake and egg production were recorded. Egg production, average egg weight, egg mass, feed intake, and feed conversion ratio (FCR, feed/egg) were calculated at the end of 40 wk.
At the conclusion of the trial, twelve randomly selected eggs from each group were analyzed for egg quality, resulting in a total of 6 replicates per group, each comprising 2 eggs. The collected eggs were assessed for Haugh unit, yolk color, albumen height, and shell strength on a level surface using an egg quality analyzer (ET-6000, Nabel, Kyoto, Japan). An electronic balance was used to calculate the weight of the egg, yolk, and eggshell. Eggshell thickness was measured with a vernier caliper (Guilin Guanglu Measuring Instrument Co., Ltd., Guangxi, P.R. China), and the long and short diameters of the eggs were measured with a spiral micrometer (217-111, Nanjing Sucian Measuring Instrument Co., Ltd., Nanjing, China).
On the last 4 d of the experiment period, 120 eggs were collected from each group and kept at a temperature of 13°C and a relative humidity of 80%. All eggs were kept in the Bengbu Sanyuan Incubation Equipment Co., Ltd. (Anhui, China) and hatched under standard conditions of 70 to 80% humidity at 37.8°C with intermittent rotation. Additionally, from the 15th d of incubation until hatching, the eggs were sprayed with water once daily (Xia et al., 2020). The number of dead embryos and fertile eggs were determined by candling eggs after 19 d of incubation. Fertility, the ability of fertile eggs to hatch, the ability of set eggs to hatch, and embryo mortality were among the measured and recorded parameters.
Egg Yolk Fatty Acids Profile
At the conclusion of the experiment, 6 qualified eggs per group were retrieved (one egg per replicate × 6 replicates per group), and the method described by Zhang et al. (2023), was employed to assess the yolk fatty acid composition. Essentially, 200 mg of lyophilized egg yolk were mixed with 1 mL of n-hexane, 4 mL of methanolic HCl solution, and 1 mL of internal standard (1 mg/mL 11-carbon fatty acid methyl ester). Subsequently, the mixture was incubated at 80°C for 2.5 h. After cooling, the supernatant was collected following the addition of 5 mL of a 7% potassium carbonate solution. A capillary column and a flame ionization detector were fitted to a gas chromatograph (6,890 series, Agilent Technologies, Wilmington, DE) for the analysis of the fatty acids in the egg yolk.
Serum Biochemical Parameters
A fully automated biochemistry analyzer, the Mindray BS-380 (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China), along with the appropriate detection kits, were used to measure the serum concentrations of the following: albumin (ALB), glucose (GLU), calcium (Ca), phosphorus (P), triglycerides (TG), total cholesterol (TC), total protein (TP), albumin (ALB), and calcium (Ca).
Determination of Serum Reproductive Hormone, Antioxidant Capacity, Immunoglobulin and Caspases Level
Using commercial kits and following the manufacturer's instructions, the activities of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), total antioxidant capacity (T-AOC), and the concentration of malondialdehyde (MDA) in the serum were measured (Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Additionally, commercial ELISA kits from Shanghai Enzyme-linked Biotechnology Co., Ltd. (China) were utilized to quantify the content of immunoglobulin A (IgA), immunoglobulin M (IgM), caspase-8, follicle-stimulating hormone (FSH), and 17β-estradiol (E2) in the serum.
Hematoxylin and Eosin Staining
The jejunal tissue (2 cm from the mid-jejunum) of laying breeder hens was removed and fixed in 4% paraformaldehyde. Subsequently, the tissue was embedded in paraffin, sliced, dehydrated, and stained with hematoxylin and eosin. Villus height (VH) and crypt depth (CD) were measured using microscope image processing software (Image-Pro Plus 6.0, Media Cybernetics, MD). Finally, the ratio of villus height to crypt depth (VH/CD) was calculated.
Tissue RNA Extraction and qRT-PCR Analysis
Total RNA was isolated from tissues (liver, ovary, and jejunal mucosa) using Trizol (Vazyme Biotech Co., Ltd., Nanjing, China). Subsequently, cDNA synthesis was performed using the Takara PrimeScript RT Kit (Vazyme Biotech Co., Ltd., Nanjing, China). For quantitative real-time PCR (qRT-PCR), the ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China) was utilized following the manufacturer's instructions. The primer sequences for the target genes are provided in Table S2. After normalization to the housekeeping gene β-actin, fold changes were calculated, and mRNA abundance was quantified using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
16S rRNA Sequencing and Gut Microbiota Analysis
A The intestinal microbiota in the cecal content samples was examined through bacterial 16S rRNA gene sequencing, as described previously (Liu et al., 2023). D As directed by the manufacturer, the QIAamp DNA Stool Kit (Qiagen, Valencia, United States) was used to extract DNA from fecal samples. The primer sequences F: ACTCCTACGGGAGGCAGCA and R: GGACTACHVGGGTWTCTAAT were used to amplify the V3–V4 region of the microbial 16S rRNA gene by PCR. Sequencing was carried out by Shanghai Paisano Biotechnology Co., Ltd. (Shanghai, China) using the Illumina MiSeq gene sequencing platform. Microbial analysis was conducted using the Genescloud Platform (www.genescloud.cn). The overall composition and relative species abundances of intestinal microbial communities were assessed using the Kruskal-Wallis test. To determine statistical significance and biological relevance, a linear discriminant analysis effect size (LEfSe) study was conducted. Additionally, Kyoto encyclopedia of genes and genomes (KEGG) metabolic pathway analysis was performed to evaluate functional differences between groups. Predictions regarding the functional differences of cecal microbiota in hens at different laying phases were made using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2) (Parks et al., 2014). The repository names and accession numbers are listed below: https://www.ncbi.nlm.nih.gov/sra/PRJNA1040004.
Statistical Analysis
The data were examined using Duncan's multiple comparison test and one-way analysis of variance (ANOVA) using SPSS 22.0 statistical software (SPSS Institute Inc., Chicago, IL). Orthogonal polynomial comparisons were used to test the linear and quadratic responses to the AOP levels. Graphs were generated using GraphPad Prism 7.0 software (GraphPad; San Diego, CA). Trends were observed between p > 0.05 and p < 0.10, with significant findings defined as p < 0.01 or p < 0.05.
RESULTS
Production Performance of Hens
Table 2 displays the impact of dietary AOP on laying breeder hens' production performance. The feed conversion ratio of the hens dropped linearly (p < 0.05) as the inclusion of AOP increased in comparison to CON group. Moreover, with increasing levels of supplemented AOP, the laying rate and average egg weight of laying breeder hens increased linearly (p < 0.01). However, supplemental AOP had no significant effect on the proportion of qualifying eggs in hens (p > 0.05).
Table 2.
Effects of dietary AOP supplementation on the production performance of laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| Laying rate (%) | 63.52b | 64.28b | 66.36a | 0.448 | 0.016 | 0.006 | 0.403 |
| Average egg weight (g) | 43.77b | 44.22ab | 44.82a | 0.017 | 0.023 | 0.007 | 0.802 |
| Percentage of qualified eggs (%) | 95.51 | 95.92 | 96.30 | 0.252 | 0.465 | 0.224 | 0.983 |
| Feed conversion ratio (g/g) | 3.51a | 3.29b | 3.24b | 0.047 | 0.039 | 0.018 | 0.320 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are mean ± SEM of 84 hens. Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
Fertilizing Capacity and Hatchability of Hens
Table 3 shows the breeder hens' capacity for fertilization and hatchability. Supplementation with higher doses of AOP resulted in a significant linear decrease in embryo mortality (p < 0.01) and a linear increase in viable egg hatching and set egg hatchability (p < 0.05). However, there were no significant variations in the fertility of laid eggs in hens across the treatment groups (p > 0.05).
Table 3.
Fertilizing capacity and hatchability of laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| Embryo mortality (%) | 6.30a | 4.39ab | 1.75b | 0.687 | 0.014 | 0.004 | 0.759 |
| Fertility of set eggs (%) | 92.50 | 94.17 | 95.00 | 0.953 | 0.580 | 0.313 | 0.844 |
| Hatch of fertile eggs (%) | 93.70b | 95.61ab | 98.25a | 0.687 | 0.014 | 0.004 | 0.759 |
| Hatchability of set eggs (%) | 86.67b | 90.00ab | 93.33a | 1.143 | 0.048 | 0.015 | 1.000 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are means ± SEM (n = 4 plates of 30 eggs each). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
Reproductive Organs and Ovarian Follicle Development of Hens
Table 4 illustrates that the oviduct weight, number of SYF, and number of POF increased linearly (p < 0.05), while the oviduct index responded quadratically (p < 0.001). However, there were no significant variations observed in the ovarian weight, ovary index, oviduct length, or liver index between the 3 groups (p > 0.05).
Table 4.
Effects of dietary AOP on the reproductive organ and ovarian follicle development in laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| Liver index (%) | 1.81 | 1.88 | 1.82 | 0.053 | 0.852 | 0.943 | 0.581 |
| Oviduct weight (g) | 37.47b | 44.90a | 45.45a | 1.222 | 0.004 | 0.003 | 0.092 |
| Oviduct index (%) | 1.89c | 2.67a | 2.28b | 0.097 | 0.001 | 0.023 | 0.001 |
| Oviduct length (cm) | 51.00 | 54.67 | 54.83 | 1.406 | 0.480 | 0.289 | 0.571 |
| Ovarian weight (g) | 41.13 | 41.30 | 43.55 | 1.607 | 0.809 | 0.566 | 0.774 |
| Ovary index (%) | 2.07 | 2.20 | 2.18 | 0.082 | 0.815 | 0.605 | 0.718 |
| Number of SYF | 12.83b | 17.33a | 17.83a | 0.878 | 0.026 | 0.014 | 0.218 |
| Number of POF | 5.00b | 6.83ab | 7.50a | 0.422 | 0.031 | 0.012 | 0.453 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are means ± SEM (n = 6 hens). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; POF, preovulatory follicles; SYF, small yellow follicles.
Egg Quality of Laying Hens
Table 5 lists the egg quality of hens fed AOP. The egg shape index, yolk color, yolk percentage, eggshell thickness, eggshell ratio, and eggshell ratio did not significantly change with the introduction of AOP (p > 0.05). In addition, AOP inclusion resulted in significant linear and quadratic increases (p < 0.05) in eggshell strength, albumen height, and Haugh unit.
Table 5.
Egg quality of laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| Egg shape index | 1.30 | 1.31 | 1.27 | 0.008 | 0.217 | 0.169 | 0.276 |
| Eggshell thickness, mm | 0.312 | 0.308 | 0.314 | 0.004 | 0.392 | 0.813 | 0.618 |
| Eggshell strength (kg/cm2) | 3.50b | 4.58a | 4.28a | 0.159 | 0.013 | 0.006 | 0.021 |
| Eggshell ratio (%) | 12.70 | 13.33 | 13.46 | 0.192 | 0.232 | 0.113 | 0.529 |
| Albumen height (mm) | 2.57b | 3.78a | 3.35a | 0.173 | 0.011 | 0.048 | 0.019 |
| Yolk colour | 5.33 | 5.83 | 5.67 | 0.184 | 0.541 | 0.469 | 0.404 |
| Haugh unit | 48.74b | 62.87a | 57.11a | 1.870 | 0.005 | 0.045 | 0.007 |
| Yolk percentage (%) | 32.65 | 33.02 | 32.78 | 0.444 | 0.943 | 0.903 | 0.751 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are means ± SEM (n = 12 eggs). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
Fatty Acid Content of Egg Yolk
Table 6 shows the fatty acid content of egg yolks from laying breeder hens fed AOP. As AOP inclusion increased relative to the CON group, the levels of palmitic acid (PA, C16:0) and total saturated fatty acids (SFA) decreased (p < 0.001) both linearly and quadratically. Additionally, there was a linear decrease in behenic acid (BA, C22:0), a type of SFA, in the AOP group (p < 0.01). The levels of monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) in the egg yolk were similar across the 3 groups (p > 0.05).
Table 6.
Fatty acid content in egg yolk of laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| Saturated fatty acid, % | |||||||
| C14:0 | 0.047 | 0.036 | 0.048 | 0.004 | 0.369 | 0.824 | 0.114 |
| C15:0 | 0.007 | 0.004 | 0.006 | 0.001 | 0.330 | 0.454 | 0.205 |
| C16:0 | 2.316a | 0.744b | 0.342b | 0.252 | <0.001 | <0.001 | 0.037 |
| C17:0 | 0.025 | 0.020 | 0.027 | 0.002 | 0.430 | 0.829 | 0.218 |
| C18:0 | 1.624 | 1.245 | 1.638 | 0.111 | 0.282 | 0.959 | 0.119 |
| C22:0 | 0.017a | 0.013ab | 0.007b | 0.002 | 0.017 | 0.006 | 0.789 |
| Total SFA | 4.037a | 2.062b | 2.067b | 0.301 | 0.001 | 0.001 | 0.025 |
| Monounsaturated fatty acid, % | |||||||
| C14:1 | 0.008 | 0.007 | 0.006 | 0.001 | 0.645 | 0.399 | 0.709 |
| C16:1 | 0.375 | 0.347 | 0.321 | 0.025 | 0.699 | 0.407 | 0.986 |
| C17:1 | 0.010 | 0.008 | 0.010 | 0.001 | 0.457 | 0.897 | 0.223 |
| C18:1n-9 | 6.760 | 6.386 | 6.726 | 0.473 | 0.947 | 0.979 | 0.747 |
| C20:1 | 0.072 | 0.060 | 0.064 | 0.006 | 0.723 | 0.597 | 0.554 |
| C24:1 | 0.039 | 0.044 | 0.052 | 0.005 | 0.619 | 0.343 | 0.886 |
| Total MUFA | 7.263 | 6.851 | 7.718 | 0.500 | 0.947 | 0.950 | 0.751 |
| Polyunsaturated fatty acid, % | |||||||
| C20:2 | 0.019 | 0.020 | 0.018 | 0.002 | 0.834 | 0.769 | 0.607 |
| C18:3n-3 | 0.025 | 0.023 | 0.022 | 0.002 | 0.834 | 0.565 | 0.894 |
| C20:3n-3 | 0.262 | 0.117 | 0.189 | 0.033 | 0.214 | 0.359 | 0.132 |
| C20:5n-3 | 0.002 | 0.003 | 0.004 | 0.001 | 0.727 | 0.438 | 0.918 |
| C22:6n-3 | 0.222 | 0.196 | 0.212 | 0.020 | 0.881 | 0.849 | 0.649 |
| C18:2n-6 | 2.498 | 2.330 | 2.458 | 0.202 | 0.947 | 0.941 | 0.752 |
| C18:3n-6 | 0.022 | 0.018 | 0.021 | 0.002 | 0.699 | 0.869 | 0.416 |
| C20:3n-6 | 0.010 | 0.013 | 0.011 | 0.002 | 0.768 | 0.843 | 0.494 |
| C20:4n-6 | 0.101 | 0.211 | 0.141 | 0.029 | 0.321 | 0.581 | 0.166 |
| Total PUFA | 3.161 | 2.931 | 3.075 | 0.249 | 0.939 | 0.898 | 0.748 |
| n-3 | 0.512 | 0.339 | 0.426 | 0.048 | 0.374 | 0.484 | 0.228 |
| n-6 | 2.630 | 2.572 | 2.631 | 0.219 | 0.993 | 1.000 | 0.908 |
| n-6/n-3 | 5.784 | 7.571 | 7.172 | 0.641 | 0.524 | 0.404 | 0.447 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are means ± SEM (n = 6 eggs). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Serum Biochemical Index of Hens
Table 7 shows that AOP had no significant effect on serum AST and ALT concentrations (p > 0.05). However, serum concentrations of ALB and P increased linearly (p < 0.05) with increasing AOP inclusion, while serum concentrations of TC, TG, and GLU decreased linearly (p < 0.05). Serum concentrations of Ca tended to increase linearly with increasing AOP inclusion (p = 0.062). Furthermore, there was a noteworthy rise in serum TP contents (p < 0.05) in the AOP1 group when compared with the CON group.
Table 7.
Effects of dietary AOP supplementation on the serum biochemical index in laying breeder hens.
| Items | Treatments |
SEM |
p-value |
||||
|---|---|---|---|---|---|---|---|
| CON | AOP1 | AOP3 | AOP | Linear | Quadratic | ||
| AST (U/L) | 236.07 | 222.70 | 211.88 | 7.059 | 0.398 | 0.183 | 0.933 |
| ALT (U/L) | 4.92 | 4.08 | 3.75 | 0.470 | 0.608 | 0.341 | 0.811 |
| ALB (g/L) | 10.78b | 13.62a | 13.42a | 0.539 | 0.045 | 0.036 | 0.146 |
| TP (g/L) | 43.58b | 53.07a | 47.95ab | 1.415 | 0.013 | 0.134 | 0.008 |
| TC (mmol/L) | 4.65a | 2.74b | 2.87b | 0.346 | 0.025 | 0.018 | 0.132 |
| TG (mmol/L) | 11.38a | 4.61b | 5.49b | 0.882 | <0.001 | <0.001 | 0.004 |
| GLU (mmol/L) | 6.08a | 4.64b | 4.20b | 0.309 | 0.022 | 0.009 | 0.370 |
| Ca (mmol/L) | 3.63 | 3.98 | 3.92 | 0.067 | 0.059 | 0.062 | 0.115 |
| P (mmol/L) | 2.83b | 3.75a | 3.76a | 0.166 | 0.020 | 0.014 | 0.135 |
Means within a row with no common superscript differ significantly (p < 0.05). Values are means ± SEM (n = 6 hens). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; AST, aspartate amino transferase; ALT, alanine transaminase; ALB, albumin; TP, total protein; TC, total cholesterol; TG, triglyceride; GLU, glucose; Ca, calcium; P, phosphorus.
Reproductive Hormone Indices of Hens
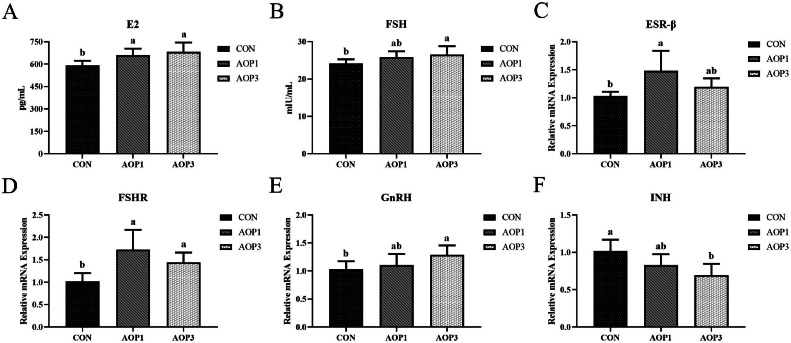
The effects of dietary supplements with AOP on serum hormone levels and the mRNA expression of hormone-related genes were shown in Figure 1. Regarding serum hormone levels, the contents of serum E2 and FSH increased linearly in laying breeder hens as the dietary levels of AOP increased (p < 0.05). Next, we examined the mRNA expression of hormone-related genes in the ovary. There was a significant linear and quadratic increase (p < 0.05) in the relative mRNA expression of FSHR and a linear increase (p < 0.05) in the expression of GnRH in the ovary with increasing levels of AOP in the diets. Furthermore, there was a linear down-regulation (p < 0.01) of the relative mRNA expression of INH in the ovary with AOP food supplementation. Additionally, the mRNA expression of ESR-β in the AOP group at 1 g/kg was significantly higher than that in the CON group (p < 0.05).
Figure 1.
Effects of dietary supplementation with AOP on hormone indices of laying breeder hens. (A) Serum E2. (B) serum FSH. (C–F) The mRNA expression of hormone-related genes (ESR-β, FSHR, GnRH, and INH) in ovary. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 6). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; E2, 17β-estradiol; FSH, follicle-stimulating hormone; ESR-β, estrogen receptor beta; FSHR, follicle-stimulating hormone receptor; GnRH, gonadotropin-releasing hormone; INH, inhibit 1.
Antioxidant Capacity of Hens
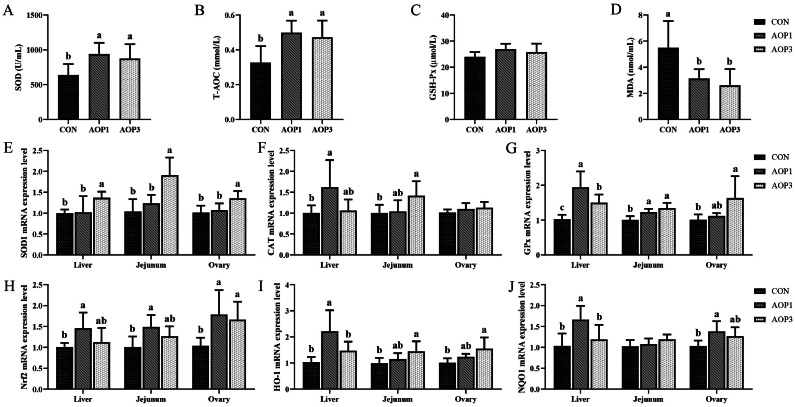
The effects of dietary supplementation with AOP on the antioxidant function of laying breeder hens were presented in Figure 2. The activity of serum SOD and T-AOC showed a linear increase (p < 0.05) with increasing levels of added AOP, while the MDA content exhibited a linear decrease (p < 0.05). However, there were no significant changes (p > 0.05) observed in the activity of serum GSH-Px. Additionally, we investigated the mRNA expression of genes associated with antioxidants in laying breeder chickens. The relative mRNA expression of SOD1 and GPx in the liver was linearly up-regulated (p < 0.05) by dietary supplementation with AOP, with this effect observed across increasing levels of AOP addition. Moreover, there was a significant linear increase (p < 0.05) in the relative mRNA expression of GPx, HO-1, SOD1, and CAT in the jejunum. When AOP was added to the diet at a rate of 1 g/kg, the relative mRNA expression of CAT, Nrf2, HO-1, and NQO1 in the liver, as well as Nrf2 expression in the jejunal mucosa, increased significantly (p < 0.05) compared to the CON group. Moreover, the relative mRNA expression of SOD1, GPx, Nrf2, and HO-1 exhibited a linear increase (p < 0.05) with increasing levels of AOP inclusion. Furthermore, there was a trend for NQO1 expression to increase linearly (p= 0.083) in the ovary. However, when AOP was added, there were no discernible effects (p> 0.05) on the relative mRNA expression of NQO1 in the mucosa of the jejunum and CAT in the ovaries of laying breeder hens.
Figure 2.
Effects of dietary supplementation with AOP on the antioxidant capacity of laying breeder hens. (A–D) Serum SOD, T-AOC, GSH-Px, and MDA. (E–J) The mRNA expression of antioxidant-related genes (SOD1, GPx, CAT, Nrf2, HO-1, and NQO1) in the liver, jejunum, and ovary, respectively. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 6). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; SOD, superoxide dismutase; T-AOC, total antioxidant capacity; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD1, copper and zinc superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; Nrf2, nuclear factor erythroid2-related factor 2; HO-1, heme oxygenase 1; NQO1, NAD(P)H quinone oxidoreductase 1.
Anti-Apoptosis Ability of Hens
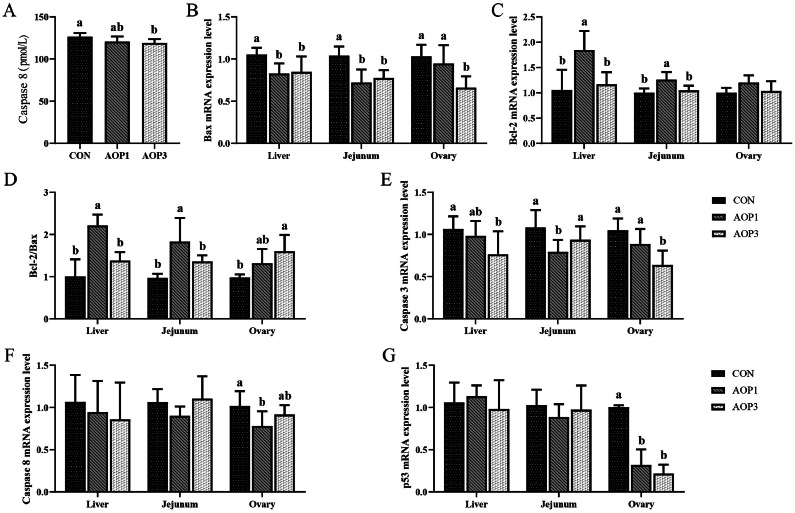
The effect of dietary supplementation with AOP on apoptosis indices within the laying breeder hens was shown in Figure 3. When increasing the amount of AOP supplementation, there was a significant linear decrease (p < 0.05) in the blood levels of caspase-8 in laying breeder hens. Additionally, we examined the mRNA expression of genes linked to apoptosis in laying breeder hens. AOP levels linearly altered the ratio of Bcl-2/Bax (p = 0.067), and the mRNA expressions of Bax and Caspase3 in the liver exhibited a linear decrease with increasing AOP levels (p < 0.05). Additionally, there was a linear decrease in the Bcl-2/Bax ratio (p = 0.093) and a linear decrease in the expression of Bax in the jejunal mucosa (p < 0.05) with increasing AOP levels. Furthermore, increasing AOP inclusion resulted in a linear decrease (p < 0.05) in mRNA expressions of Bax, Caspase3, and p53 in the ovary, while the Bcl-2/Bax ratio increased linearly (p < 0.05). Compared to the CON group, dietary AOP at 1 g/kg increased (p < 0.05) Bcl-2 expression in the liver and jejunal mucosa but reduced Caspase3 expression in the jejunal mucosa, as well as Caspase8 expression in the ovary. However, AOP supplementation had no discernible impact on the expression of Bcl-2 in the ovaries, as well as the expression of caspase8 and p53 in the liver and jejunal mucosa (p > 0.05).
Figure 3.
Effects of dietary supplementation with AOP on anti-apoptotic ability in laying breeder hens. (A) Serum caspase-8. (B–G) The mRNA expression of apoptosis-related genes (Bax, Bcl-2, Bcl-2/Bax, Caspase 3, Caspase 8, and p53) in liver, jejunum, and ovary, respectively. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 6). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; Bax, bcl2-associated x; Bcl-2, b-cell lymphoma-2.
Immune Function of Hens
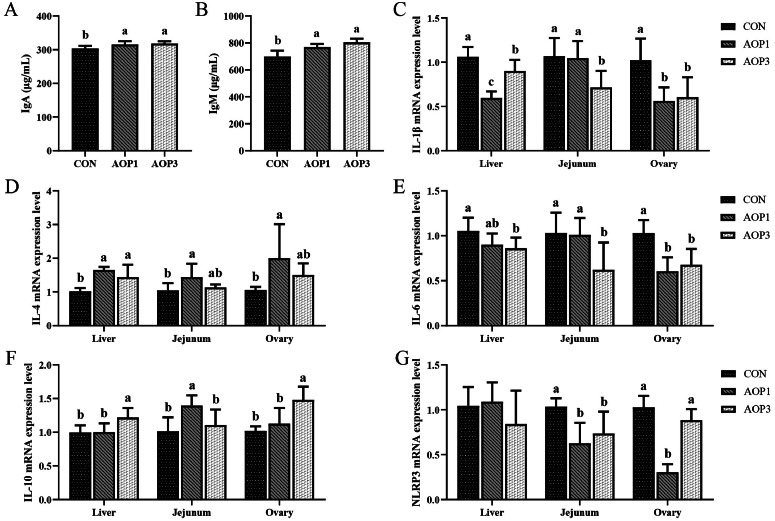
The effects of dietary supplementation with AOP on immune function in laying breeder hens were detailed in Figure 4. The content of serum IgA and IgM was enhanced by dietary AOP in a linear manner (p < 0.05) in proportion to serum immunoglobulin levels. Additionally, the mRNA expressions of IL-4 and IL-10 in the liver were considerably elevated (linear, p < 0.05) by dietary supplementation with AOP, whereas the mRNA expressions of IL-1β and IL-6 were down-regulated (linear, p < 0.05) with increasing AOP levels. However, NLRP3 expression in the liver did not significantly alter (p > 0.05). Additionally, as dietary AOP supplementation increased, the mRNA expressions of NLRP3, IL-6, and IL-1β in the jejunal mucosa decreased linearly (p < 0.05). Moreover, with increasing AOP levels, the mRNA expression of IL-β and IL-6 in the ovary was linearly down-regulated (p < 0.05), while the mRNA expression of IL-10 was linearly up-regulated (p < 0.05) by AOP supplementation. Specifically, dietary supplementation with AOP at 1 g/kg increased the expression of IL-10 in the jejunal mucosa and IL-4 in the liver and jejunal mucosa compared to the CON group, while it decreased the expression of NLRP3 in the ovary (p < 0.05).
Figure 4.
Effects of dietary supplementation with AOP on the immune function in laying breeder hens. (A–B) Serum immunoglobulin A (IgA) and immunoglobulin M (IgM). (C–G) The mRNA expression of inflammatory related genes (IL-1β, IL-4, IL-6, IL-10, and NLRP3) in liver, jejunum, and ovary, respectively. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 6). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; IgA, immunoglobulin A; IgM, immunoglobulin M; IL-1β, interleukin-1β; IL-4, interleukin-4; IL-6, interleukin-6; IL-10, interleukin-10; NLRP3, NOD-like receptor protein 3.
Intestinal Morphology and Barrier Genes of Hens
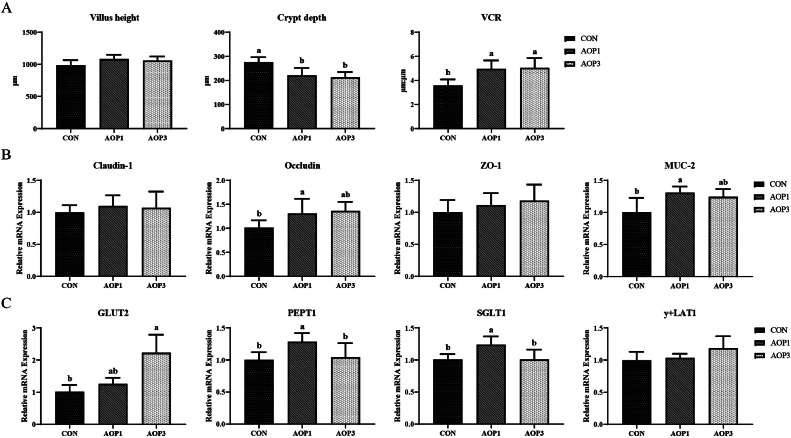
The effect of dietary supplementation with AOP on the jejunal morphology of laying breeder hens was presented in Figure S1. Additionally, in order to examine the effect of AOP on barrier function, we assessed the mRNA expression in the jejunal mucosa of tight junction proteins, mucin, and genes related to nutrient transport (Figure 5). While there were no significant changes (p > 0.05) in the height of the jejunal villi in laying breeder hens, there was a linear increase (p < 0.05) in the villus/crypt ratio (VCR) and a decrease (p < 0.05) in the depth of the jejunal crypt (Figure 5A). Additionally, there was a linear increase (p < 0.05) in the expression of jejunal occludin (Figure 5B) and in the expression of jejunal GLUT-2 and y+LAT1 (Figure 5C). The mRNA expression of mucin-2 (MUC-2), peptide transporter 1 (PEPT1), and sodium/glucose cotransporter 1 (SGLT1) in the jejunal mucosa was upregulated (p < 0.05) with dietary AOP inclusion at a level of 1 g/kg compared to the CON group. However, there were no significant differences in the mRNA expression of Claudin-1, ZO-1, or y+LAT1 between the treatment groups (p > 0.05).
Figure 5.
Effects of dietary supplementation with AOP on intestinal morphology and barrier genes and nutrient transport in laying breeder hens. (A) Villus height, crypt depth, and villus height to crypt depth ratio of the jejunum. (B) The mRNA expression of tight junction proteins and mucin (Claudin-1, Occludin, ZO-1, and MUC-2) in the jejunum. (C) The mRNA expression of nutrient transport related genes (GLUT2, PEPT1, SGLT1, and y+LAT1) in the jejunum. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 6). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP; ZO-1, zonula occludens-1; MUC-2, mucin-2; GLUT2, glucose transporter 2; PEPT1, peptide-transporter 1; SGLT1, sodium glucose cotransporter 1; y+LAT1, y(+)L-type amino acid transporter 1.
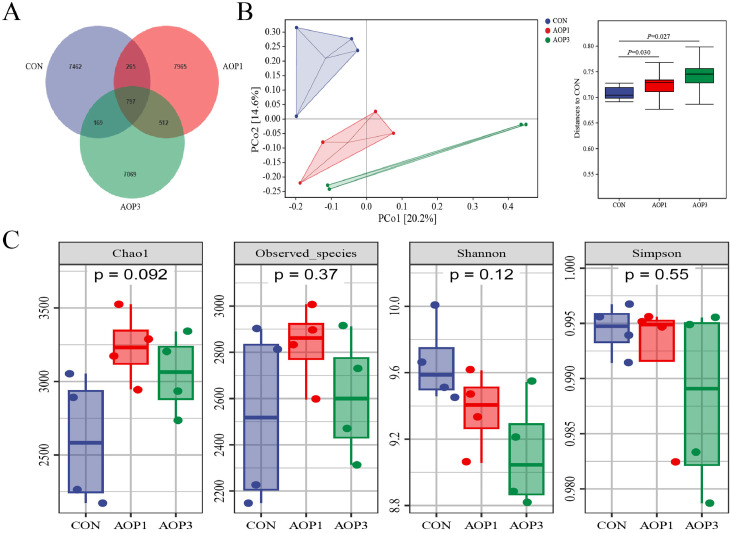
Microbial Diversity of Cecal Digesta
The sequencing analysis of fecal samples from each of the 3 groups revealed an average of 90,288 raw data per sample. After quality trimming and chimera checking, 52,747 high-quality sequences were obtained from each sample. Microbial diversity in the cecal digesta is affected by AOP, as shown in Figure 6. The Venn diagram analysis revealed 8,693 operational taxonomic units (OTU) in the CON group, while there were 9,539 and 8,547 OTUs in the AOP1 and AOP3 groups, respectively (Figure 6A). Out of these OTUs, 797 were shared by all 3 groups, indicating a degree of similarity in the microbial composition across the samples. The principal component analysis (PCA) plot demonstrated a significant distinction between the CON and AOP groups (CON vs. AOP1, R2 = 0.635, p = 0.030; CON vs. AOP3, R2 = 0.688, p = 0.027), indicating a notable influence of AOP supplementation on the intestinal microbiota (Figure 6B). When comparing the AOP1 and AOP3 groups to the CON group, there was a trend toward an increase in the Chao1 index of cecal bacterial microbiota, as indicated by the alpha diversity index (p = 0.092, Figure 6C). However, there were no significant differences (p >0.05) observed between the 3 groups in terms of the other index, according to the study's findings.
Figure 6.
Effects of AOP on cecal microbiota diversity in laying breeder hens. (A)Veen diagram. (B) Principal component analysis (PCA) scatterplot. (C) Bacterial alpha-diversity indices (Chao1, Observed_species, Shannon, and Simpson). * indicates a significant difference at p < 0.05 (n = 4). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
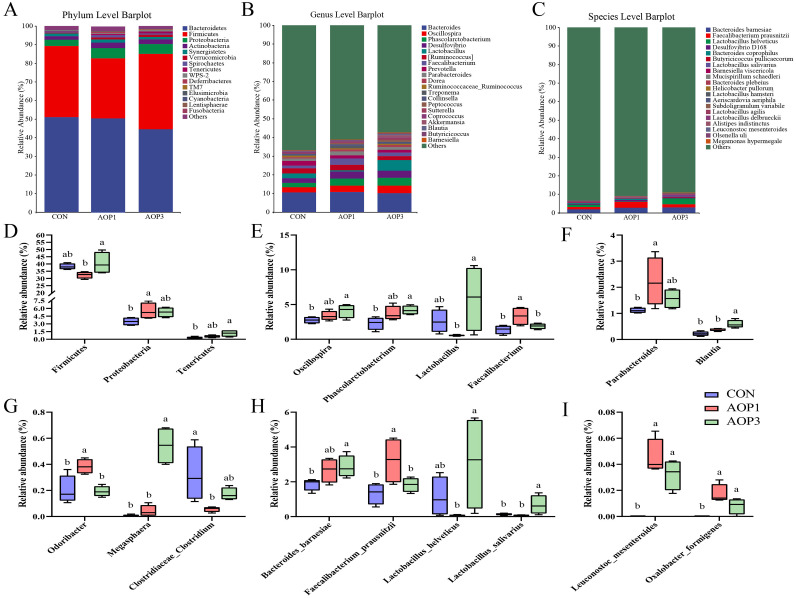
Composition of Cecal Digesta
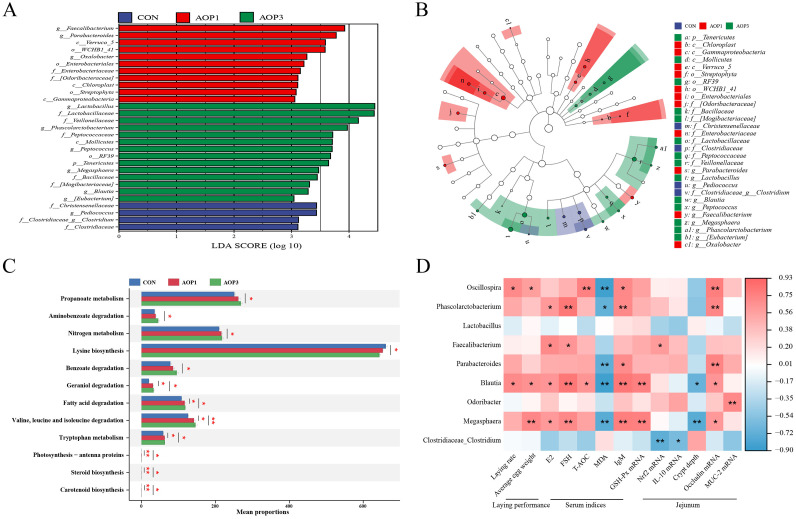
Figure 7 showed the composition of the microbiota at the phylum, genus, and species levels. Bacteroidetes, Firmicutes, and Proteobacteria were consistently found to be the 3 most prevalent bacterial phyla in each group of laying breeder hens (Figure 7A). Proteobacteria in the AOP1 group and Tenericutes in the AOP3 group had significantly higher relative abundances at the phylum level when compared to the CON group, as shown in Figure 7D (p < 0.05). Upon further examination at the genus level, we observed 20 bacteria with varying relative abundances (Figure 7B). AOP1 supplementation dramatically reduced the abundance of Clostridiaceae_Clostridium (p < 0.05) in the diet compared to the CON group but increased the abundance of Odoribacter, Paeabacteroides, and Faecalibacterium (p < 0.05). Similarly, the AOP3 group exhibited a significant increase in the abundance of Blautia, Megasphaera, Oscillospira, and Phascolarctobacterium (Figures 7E–7G). At the species level (Figure 7C), the AOP1 group showed an overrepresentation of Faecalibaterium prausnitzii (p < 0.05), while the AOP3 group exhibited an overrepresentation of Bacteroides barnesiae and Lactobacillus salvarius (p < 0.05). Furthermore, both the AOP1 and AOP3 groups demonstrated significantly higher relative abundances of Leuconostoc_mesenteroides and Oxalobacter_ formigenes in comparison to CON group (p < 0.05) (Figures 7H–7I). We employed linear discriminant analysis effect size (LEfSe) analysis (LDA score > 3) to pinpoint bacteria exhibiting notable variations between the 3 groups, aiming to explore and identify distinct bacterial taxa (Figure 8A). Christensenellaceae (family), Pediococcus (genus), and Clostridium (family) were more abundant in the CON group; Faecalibacterium (genus), Parabacteroides (genus), and Verruco_5 (class) were higher abundant in the AOP1 group; Lactobacillus (genus), Lactobacillaceae (family), and Veillonellaceae (family) were enriched in the AOP3 group. The cladogram subsequently depicted abundance values based on LEfSe, showcasing the taxa. Potential biomarkers were identified at 4, 11, and 14 distinct taxonomic levels of microorganisms in the CON, AOP1, and AOP3 groups, respectively (Figure 8B).
Figure 7.
Effects of dietary supplementation with AOP on the composition of the cecal microbiota in laying breeder hens. (A) Phylum level barplot. (B) Genus level barplot. (C) Species level barplot. Comparison of dominant cecal microbiota at phylum (D), genus (E–G), and species (H-I) levels. Different lowercase letters in the figure indicate statistically significant differences (p < 0.05, n = 4). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
Figure 8.
Changes in laying breeder hens cecal microbiota and functional prediction with AOP supplementation. (A) LEfSe bar based on phylum to genus level (LDA > 3). (B) Branching diagram of evolution scatterplot. (C) Functional profiles of the gut microbiota. (D) Spearman's correlation analysis between cecal microbiota and different indicators (laying performance, serum indices, and jejunal mucosa). * indicates a significant difference at p < 0.05 and ** indicates p < 0.01 (n = 4). Abbreviations: CON, control group, basal diet; AOP1, basal diet supplemented with 1 g/kg AOP; AOP3, basal diet supplemented with 3 g/kg AOP.
Prediction of Cecal Microbial Function
Fourteen metabolic pathways (at level 3) were influenced among the groups, as depicted in Figure 8C. These pathways encompassed 3 pathways for amino acid metabolism, 2 for energy metabolism, 2 for lipid metabolism, 2 for xenobiotic biodegradation and metabolism, 2 for terpenoid and polyketide metabolism, and one for carbohydrate metabolism. Furthermore, with the application of dietary AOP3, microbial gene abundance associated with energy metabolism (nitrogen metabolism), xenobiotic biodegradation and metabolism (aminobenzoate degradation and benzoate degradation), and carbohydrate metabolism (propanoate metabolism) significantly increased (p < 0.05) compared to the CON group. Conversely, it decreased (p < 0.05) in relation to amino acid metabolism (lysine biosynthesis). The metabolism of terpenoids and polyketides (including geraniol degradation and carotenoid biosynthesis), energy metabolism (including photosynthesis-antenna proteins), lipid metabolism (including fatty acid degradation and steroid biosynthesis), and amino acid metabolism (including tryptophan metabolism and valine, leucine, and isoleucine degradation) pathways were all enhanced (p < 0.05) when AOP1 and AOP3 were added to the diet of laying breeder hens.
Correlation Analysis of Altered Cecal Bacteria With Measured Parameters
The potential relationship of modifications in cecal microbiota at the genus level with laying performance, hormone indices, immune-related factors, antioxidant factors, and barrier function‐related factors was examined by applying Spearman correlation analysis (Figure 8D). Correlation analysis revealed significant associations between laying performance and the genera Oscillospira, Blautia, and Megasphaera (p < 0.05). Furthermore, the concentration of serum hormones (E2 and FSH) was strongly positively correlated with the genera Phascolarctobacterium, Fecalibacterium, Blautia, and Megasphaera (p < 0.05). Additionally, antioxidant and immune-related factors exhibited potential correlations with Oscillospira, Phascolarctobacterium, Parabacteroides, Blautia, and Megasphaera, while demonstrating a negative relationship with Clostridiaceae_Clostridium (p < 0.05). The barrier function‐related factors were possibly correlated with Blautia and Megasphaera, in addition to being positively correlated with Oscillospira, Phascolarctobacterium, Parabacteroides, and Odoribacter (p < 0.05).
DISCUSSION
Alpinia oxyphylla was an edible and medicinal plant with a diverse range of pharmacological effects and active ingredients. These effects include antioxidant, anti-inflammatory, anti-parasitic, and neuroprotective qualities (Zhang et al., 2018). Our recent study revealed that laying breeder hens fed AOP diets exhibited a reduced feed conversion ratio. This effect could be attributed to the capacity of A. oxyphylla to enhance digestion, thereby improving the gastrointestinal tract's ability to absorb and utilize nutrients. Moreover, significant enhancements were observed in the average egg weight, hatchability of set eggs, laying rate, and hatchability of viable eggs. However, the inclusion of 3 g/kg AOP in the diet led to a decrease in embryo mortality. According to (Yuan et al., 2016), incorporating tea polyphenols into the diet of layer chickens enhances laying hen productivity, egg quality, and resilience to adverse effects. In our study, Alpiniae oxyphyllae fructus powder contained a significant proportion of polyphenols (19.85%), followed by flavonoids (10.56%) and polysaccharides (2.06%). Hence, we hypothesized that the synergistic interaction among these compounds contributed to the beneficial effects of AOP on laying hen performance.
In laying hen production systems, egg quality was paramount, as it directly impacts customer acceptance and financial profitability (Korver, 2023). The notable increase in albumen height and Haugh unit observed in the AOP group could be attributed to the presence of polyphenolic components, showcasing the beneficial impact of dietary AOP inclusion on protein anabolism (Yuan et al., 2016). Similarly, significant reductions were observed in serum TC, TG, and GLU levels following AOP treatment (Ghasemi and Nari, 2019). Nootkatone, mainly from Alpiniae Oxyphyllae Fructus, was shown to dramatically increase glucose consumption and decrease intracellular TG accumulation in L02 cells; this was supported by in vitro data in a study conducted by (Yong et al., 2022). The incorporation of AOP into the diet at a concentration of 3 g/kg for laying hens was observed to have advantageous impacts on both the internal and external quality of eggs. Specifically, while serum Ca concentrations exhibited a tendency to increase linearly, there were elevations in eggshell strength and serum P concentrations in the AOP-supplemented diet. Phosphorus was a crucial nutrient required by chickens, participating in numerous metabolic processes, as highlighted in this study (Bello et al., 2020). Both calcium and phosphorus were indispensable for skeletal function and eggshell formation and maintenance (Bello et al., 2020; Zhang et al., 2023). The addition of AOP may enhance the absorption of these minerals from the digestive system, thereby improving their utilization within the body. On the other hand, the study findings indicated that dietary supplementation with AOP led to a reduction in the levels of total saturated fatty acids (SFA) in egg yolks, specifically palmitic acid (C16:0) and behenic acid (C22:0). Under certain physiological conditions and dietary factors, de novo lipogenesis (DNL) could be significantly stimulated, leading to elevated tissue levels of palmitic acid and disruption of the regulatory control over its tissue concentration (Wilke et al., 2009). Similarly, chickens supplemented with antioxidants derived from flaxseed, Rhus coriaria seed, or Zingiber officinale root powder exhibited a reduction in the saturated fatty acid content of their egg yolks (Gurbuz and Salih, 2017). The AOP intervention collectively improved both the external and internal quality of the eggs, thereby enhancing their suitability for further production or sale.
The ovary played a crucial role in determining a female's egg-laying capacity, and by enhancing ovarian function, it improved the ability of laying hens to produce eggs. The diet supplemented with AOP positively impacted the oviduct weight, oviduct index, number of segmented yellow follicles, and number of preovulatory follicles, as evidenced by this study. On the other hand, the reproductive system's layers experienced a complex series of biochemical and physiological changes alongside the formation and maturation of ovarian follicles. Hormones primarily induced follicle formation (Yang et al., 2018). The AOP group was found to have higher levels of serum FSH and E2 in comparison to the control group, according to the study. It was widely recognized that gonadotropins, particularly the pituitary gland's secreted LH and FSH, were vital in stimulating oocyte growth and follicular cell proliferation, essential processes for follicle formation (Oduwole et al., 2021). Moreover, in the ovary, the AOP-supplemented diet down-regulated INH expression while up-regulating FSHR and GnRH expression. According to reports, FSH could increase the weight and volume of follicles while decreasing the number of atresia small follicles (Ma et al., 2020). According to earlier studies, the high polyphenol content of F. velutipes stem waste raised serum hormone levels as well as the expression of genes and proteins linked to the follicle-stimulating hormone receptor (FSHR) and luteinizing hormone receptor (LHR) signal pathways (Sun et al., 2023). Taken together, these findings provide compelling evidence for the beneficial impact of AOP on the egg production capacity of laying breeder hens.
The laying process was closely associated with heightened susceptibility to inflammation and oxidative stress. Antioxidant enzymes, such as GSH-Px, CAT, and SOD, were pivotal in defending the organism against oxidative damage, which results from the production of reactive oxygen species (ROS) and the antioxidant defense system being out of balance (Mishra and Jha, 2019). Malondialdehyde (MDA), a byproduct of lipid peroxidation initiated by free radicals, serves as a marker to determine the level of oxidative stress, while T-AOC reflects the comprehensive antioxidant capacity (Cao et al., 2016; Liu et al., 2021). The findings from this study indicate that the supplementation of AOP led to an increase in the activity of T-AOC and SOD, alongside a decrease in the content of MDA in the serum. In a study by (Bayati and Yazdanparast, 2011), yakuchinone B, a component of Alpinia oxyphylla seeds, was demonstrated to reduce levels of ROS and inhibit the production of lipofuscin, thus mitigating cellular aging. In this study, GPx gene expression was significantly upregulated in the liver and jejunum upon dietary addition of 3 g/kg of AOP. Additionally, the expression of GPx in the ovary, CAT in the jejunum, and SOD1 in both the ovary and the jejunum was upregulated. (Liu et al., 2023) reported that extracts from Lonicera floss and Cnicus japonicus could improve egg quality by affecting the production of cytokines associated with inflammation, antioxidant status, and eggshell matrix proteins in the oviduct of laying hens. Additionally, the Nrf2 was widely recognized for its essential role in regulating antioxidant genes and coordinating various cellular defense mechanisms against oxidative stress (Liu et al., 2018). The activation of the Nrf2/HO-1 pathway reduced oxidative stress in tissues by increasing the expression of several antioxidant genes (Sahin et al., 2014). Consistent with these results, the present study showed that AOP supplementation up-regulated Nrf2 gene mRNA expression in the ovary. Specifically, at a dosage of 3 g/kg, AOP elevated the expression of HO-1 in both the ovaries and jejunum. Moreover, (Wang et al., 2013) reported that A. oxyphylla exhibited antioxidant activity, primarily attributed to its total phenolic components. Therefore, the antioxidant function of AOP may be the cause of the laying breeder hens' elevated antioxidant levels.
Cell apoptosis brought on by reproductive tract infections was one of the most frequent causes of reproductive dysfunction in laying hens (Li et al., 2016). Apoptosis, a regulated process of cell death, played crucial roles in development, maintaining balance, and eliminating damaged cells and tissues (Armstrong, 2007). Studies indicated that ovarian-cell apoptosis was more prevalent in breeder hens that produce fewer eggs (Wang et al., 2021). Bax exhibits pro-apoptotic activity, while Bcl-2 was the most prominent anti-apoptotic protein within the Bcl-2 family, serving as a crucial regulator of cellular death (Birkinshaw and Czabotar, 2017). Upon receiving an apoptotic signal, apoptotic initiators were activated, followed by a downstream apoptotic executor, such as caspase 3, which resulted in apoptosis (Boice and Bouchier-Hayes, 2020). The results of this experiment partially explained the process of apoptosis. The results showed that dietary AOP supplemented with 3 g/kg decreased the concentration of Caspase 8 in serum, decreased the expression of Bax and Caspase3, and increased the ratio of Bcl-2/Bax in ovary. Research indicated that Alpiniae oxyphyllae Fructus (AOF) decreased H2O2-induced damage to PC12 cells by upregulating Bcl-2 expression and downregulating Bax and caspase-3 expression in PC12 cells (Li et al., 2022). Based on these findings, it's plausible that AOP might modulate apoptosis in laying breeder hens.
Enhancing immunity was vital for shielding the body from parasite infections, cancer, viral infections, and other diseases (Yang et al., 2020). Serum immunoglobulin levels served as markers of the host's overall humoral immune function, and immunoglobulins such as IgG, IgM, and IgA were pivotal in safeguarding the host against infections and other harmful microorganisms (Lu et al., 2019). Our study results suggested that supplementing laying hens' diets with AOP could enhance their immunological well-being, as evidenced by increased levels of serum IgA and IgM.
T helper type 1 cytokines (e.g., IL-2, TNF-α, and IFN) were responsible for cell-mediated immune responses, while T helper type 2 cytokines (e.g., IL-4, IL-6, and IL-10) promoted B-cell growth, differentiation, and humoral immune responses (Kohut et al., 2002). Our study found an increase in IL-4 expression in the liver, a decrease in mRNA levels of IL-1β expression in the liver, IL-1β and IL-6 expression in the ovary, and NLRP3 in the jejunal mucosa. Additionally, there was a significant rise in the mRNA level of IL-10 in the liver and ovaries with dietary AOP at 3 g/kg. These findings suggested potent anti-inflammatory properties of AOP. Our findings align with a recent study demonstrating that the polysaccharides from A. oxyphyllae fructus elevated TNF-α, IL-6, IL-10, and TGF-β production, leading to the activation of RAW264.7 macrophages and enhancement of the Th2-type immune response (Yang et al., 2021). Our findings prompted speculation that AOP might modulate the immune systems of laying breeder hens.
Intestinal morphology, including villus height, crypt depth, and intestinal wall thickness, along with the V/C ratio, served as crucial indicators of intestinal health in animals as they directly impact the absorptive capacity of the intestinal mucosa (Wu et al., 2018). A greater VCR fosters an environment in the gut that was favorable to effective nutrition absorption and digestion (Montagne et al., 2003). In our observation of the jejunum, we found that AOP supplementation in the diet decreased crypt depth and augmented the VCR. Additionally, the AOP group displayed a markedly higher VH/CD ratio compared to the CON group. Plant-derived feed additives may improve the efficiency of nutrient absorption by increasing the ratio of villus height to crypt depth in the small intestine (Khattak et al., 2014). There was evidence that oregano essential oil improved intestinal nutrient absorption by increasing villus height and reducing crypt depth (Amer et al., 2021). We found that hens fed with 1 g/kg AOP enhanced the levels of mRNA for PEPT1 and SGLT1 in jejunal mucosa, which suggested that this additive might promote the absorption of nutrients. Tight junctions, comprising transmembrane proteins such as CLDN1, Occludin, and ZO1, served as critical physical barriers in maintaining intestinal barrier integrity. These proteins played a crucial role in regulating the permeability of the intestinal epithelium (Gong et al., 2020; Amevor et al., 2022). Additionally, mucin-2 has been demonstrated to be vital for preserving the integrity of the intestinal mucosal barrier, safeguarding the intestinal epithelium, and preventing infections (Mcguckin et al., 2011). When AOP was added to the diet at a concentration of 1 g/kg, there was a notable increase in the VCR and a decrease in crypt depth in the jejunum. These alterations correlated with elevated mRNA levels of MUC-2 and occludin in the jejunal mucosa. Prior studies investigating ducks administered with 30 or 130 mg/kg AOE have similarly demonstrated positive alterations in jejunal architecture, aligning with our observations and implying potential protective advantages for gut health and small intestine integrity (Ji et al., 2021). Overall, these findings provided support for the hypothesis that dietary AOP might enhance the physical barrier function of laying breeder hens.
It was well-established that the gut microbiota had a significant impact on the host's gastrointestinal system development, thereby exerting a substantial impact on immunological function, growth performance, energy balance, and nutrient utilization (Backhed et al., 2004; Yang et al., 2022). In our study, we examined the impact of dietary AOP on cecal microbial composition. Prior research has indicated that supplementation with 30 mg/kg of A. oxyphylla extract led to increased jejunal villus height, altered microbial composition, and demonstrated potential for preserving intestinal health in ducks (Ji et al., 2021). While no discernible variations were found between the AOP and CON groups regarding alpha diversity indices, the beta diversity analysis revealed a distinct separation, suggesting variations in microbial communities between the 2 groups. Bacteroidetes, Firmicutes, and Proteobacteria were found to dominate the microbial community in hens, which was consistent with the results of (Zhao et al., 2019), who found that Bacteroidetes and Firmicutes were the predominant phyla in the cecum, with a beneficial role in nutrient digestion and absorption (Mohd et al., 2015; Xiao et al., 2017). The composition of the cecal microbial community in the AOP group of hens changed as a result of our study's AOP dietary supplementation. Notably, there was an increase in the abundance of Oscillospira, Phascolarctobacterium, Faecalibacterium, Parabacteroides, Blautia, Odoribacter, and Megasphaera genera, alongside a reduction in the richness of Clostridiaceae_Clostridium at the genus level. Oscillospira, a genus of anaerobic bacteria within the Firmicutes phylum, includes species like O. ruminantium, known for its potential to produce butyrate (Gophna et al., 2016). Phascolarctobacterium, another butyrate-producing bacterium, was recognized for its anti-inflammatory properties and role in promoting intestinal epithelial integrity (Louis and Flint, 2009). Faecalibacterium, an essential butyrate producer in chicken ceca, contributed to gut health (Duncan et al., 2002). Blautia, identified as a functional genus with probiotic potential, has been associated with reducing inflammation, metabolic diseases, and exhibiting antimicrobial activity against specific microorganisms (van Leeuwen et al., 2020). These findings suggested that dietary supplementation with AOP might enhance intestinal barrier function by promoting the colonization of beneficial bacteria.
We conducted a Spearman's correlation analysis to investigate the relationship between the observed changes in intestinal architecture and cecal microbial composition. Our findings revealed correlations between the genera Oscillospira, Blautia, and Megasphaera with indicators of intestinal integrity and laying performance. Previous research has indicated the potential of Oscillospira to produce short-chain fatty acids (SCFA) like butyrate, positioning it as a promising candidate for future probiotic applications (Yang et al., 2021). Blautia, a type of probiotic bacteria, contributed to both the metabolism of carbohydrates and the synthesis of fatty acids (Liu et al., 2021). Megasphaera, known for its production of short-chain fatty acids (SCFA), essential amino acids, and vitamins, could adjust the host's immune response to favorably impact host health (Shetty et al., 2013; Carey et al., 2021). SCFAs, which were produced through bacterial fermentation, played crucial roles in maintaining gut integrity, regulating the immune system, reducing inflammation, and modulating epithelial gene expression (Parada et al., 2019). Therefore, it was plausible to suggest that supplementing the diet with AOP might enhance laying efficiency and promote gut health through a rise in the number of good bacteria and fostering favorable bacterial interactions.
CONCLUSIONS
In conclusion, our research indicated that adding AOP to the diet improved the productivity of laying breeder hens. The observed beneficial effects of AOP on broiler chickens were likely attributable to improvements in immunity, intestinal health, antioxidant capacity, and reproductive hormone levels. These findings suggested that AOP has potential as a chicken-produced antibiotic substitute.
ACKNOWLEDGMENTS
This work was supported by Hainan Province's Key Research and Development Project (ZDYF2023XDNY067) and National Key Research and Development Program (2022YFD1801103).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103770.
Appendix. Supplementary materials
REFERENCES
- Ali A. Flavonoids: health promoting phytochemicals for animal production-a review. J. Anim. Health Prod. 2015;3:6–13. [Google Scholar]
- Al-Khalaifa H., Al-Nasser A., Al-Surayee T., Al-Kandari S., Al-Enzi N., Al-Sharrah T., Ragheb G., Al-Qalaf S., Mohammed A. Effect of dietary probiotics and prebiotics on the performance of broiler chickens. Poult. Sci. 2019;98:4465–4479. doi: 10.3382/ps/pez282. [DOI] [PubMed] [Google Scholar]
- Amer S.A., Tolba S.A., AlSadek D., Abdel F.D., Hassan A.M., Metwally A.E. Effect of supplemental glycerol monolaurate and oregano essential oil blend on the growth performance, intestinal morphology, and amino acid digestibility of broiler chickens. BMC Vet. Res. 2021;17:312. doi: 10.1186/s12917-021-03022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amevor F.K., Cui Z., Du X., Ning Z., Deng X., Xu D., Shu G., Wu Y., Cao X., Shuo W., Tian Y., Li D., Wang Y., Zhang Y., Du X., Zhu Q., Han X., Zhao X. Supplementation of dietary quercetin and vitamin e promotes the intestinal structure and immune barrier integrity in aged breeder hens. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.860889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoac I. 18^ ed. AOAC International; 2010. Official Methods of Analysis of AOAC International. [Google Scholar]
- Armstrong J. Mitochondrial medicine: pharmacological targeting of mitochondria in disease. Br. J. Pharmacol. 2007;151:1154–1165. doi: 10.1038/sj.bjp.0707288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayati S., Yazdanparast R. Antioxidant and free radical scavenging potential of yakuchinone B derivatives in reduction of lipofuscin formation using H2O2-treated neuroblastoma cells. Iran Biomed. J. 2011;15:134–142. doi: 10.6091/IBJ.1010.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A., Dersjant-Li Y., Korver D.R. Effects of dietary calcium and available phosphorus levels and phytase supplementation on performance, bone mineral density, and serum biochemical bone markers in aged white egg-laying hens. Poult. Sci. 2020;99:5792–5801. doi: 10.1016/j.psj.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw R.W., Czabotar P.E. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin. Cell Dev. Biol. 2017;72:152–162. doi: 10.1016/j.semcdb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Boice A., Bouchier-Hayes L. Targeting apoptotic caspases in cancer. Biochimica et Biophysica Acta (BBA) – Mol. Cell Res. 2020;1867 doi: 10.1016/j.bbamcr.2020.118688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Xiao L., Liu G., Fang T., Wu X., Jia G., Zhao H., Chen X., Wu C., Cai J., Wang J. Dietary arginine and N-carbamylglutamate supplementation enhances the antioxidant statuses of the liver and plasma against oxidative stress in rats. Food Funct. 2016;7:2303–2311. doi: 10.1039/c5fo01194a. [DOI] [PubMed] [Google Scholar]
- Carey M.A., Medlock G.L., Alam M., Kabir M., Uddin M.J., Nayak U., Papin J., Faruque A., Haque R., Petri W.A., Gilchrist C.A. Megasphaera in the stool microbiota is negatively associated with diarrheal cryptosporidiosis. Clin. Infect. Dis. 2021;73:e1242–e1251. doi: 10.1093/cid/ciab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Li H.L., Li Y.H., Tan Y.F., Zhang J.Q. Quantitative analysis of the major constituents in Chinese medicinal preparation SuoQuan formulae by ultra fast high performance liquid chromatography/quadrupole tandem mass spectrometry. Chem. Cent. J. 2013;7:131. doi: 10.1186/1752-153X-7-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S., Jiang H., Fang J. Regulation of immune function by polyphenols. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/1264074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan S., Hold G., Harmsen H., Stewart C., Flint H. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002;52:2141–2146. doi: 10.1099/00207713-52-6-2141. [DOI] [PubMed] [Google Scholar]
- El-Sonbaty Y.A., Suddek G.M., Megahed N., Gameil N.M. Protocatechuic acid exhibits hepatoprotective, vasculoprotective, antioxidant and insulin-like effects in dexamethasone-induced insulin-resistant rats. Biochimie. 2019;167:119–134. doi: 10.1016/j.biochi.2019.09.011. [DOI] [PubMed] [Google Scholar]
- Feng J., Lu M., Wang J., Zhang H., Qiu K., Qi G., Wu S. Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Anim. Sci. Biotechnol. 2021;12:72. doi: 10.1186/s40104-021-00600-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi H., Nari N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels. J. Appl. Poult. Res. 2019;29:301–303. [Google Scholar]
- Gong Y., Xia W., Wen X., Lyu W., Xiao Y., Yang H., Zou X. Early inoculation with caecal fermentation broth alters small intestine morphology, gene expression of tight junction proteins in the ileum, and the caecal metabolomic profiling of broilers. J. Anim. Sci. Biotechnol. 2020;11:8. doi: 10.1186/s40104-019-0410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gophna U., Konikoff T., Nielsen H. Oscillospira and related bacteria – from metagenomic species to metabolic features. Environ. Microbiol. 2016;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- Gurbuz Y., Salih Y.G. Influence of sumac (Rhus Coriaria L.) and ginger (Zingiber officinale) on egg yolk fatty acid, cholesterol and blood parameters in laying hens. J. Anim. Physiol. Anim. Nutr. (Berl) 2017;101:1316–1323. doi: 10.1111/jpn.12652. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G., Singh R., Sakariah K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001;73:285–290. [Google Scholar]
- Ji F., Gu L., Rong G., Hu C., Sun W., Wang D., Peng W., Lin D., Liu Q., Wu H., Dai H., Zhou H., Xu T. Using extract from the stems and leaves of Yizhi (Alpiniae oxyphyllae) as feed additive increases meat quality and intestinal health in ducks. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.793698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Moore R.J., Stanley D., Chousalkar K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020;86 doi: 10.1128/AEM.00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2014;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohut M.L., Cooper M.M., Nickolaus M.S., Russell D.R., Cunnick J.E. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- Korver D.R. Review: current challenges in poultry nutrition, health, and welfare. Animal. 2023;17 doi: 10.1016/j.animal.2023.100755. Suppl. [DOI] [PubMed] [Google Scholar]
- Li J.T., Zhao Y.H., Lv Y., Su X., Mei W.L., Lu Y.P., Zheng P.H., Zhang Z.L., Zhang X.X., Chen H.Q., Dai H.F., Xian J.A. Evaluating the antioxidant properties of the leaves and stems of alpinia oxyphylla in vitro and its growth-promoting, muscle composition change, and antioxidative stress function on juvenile litopenaeus vannamei. Antioxidants (Basel) 2023;12 doi: 10.3390/antiox12101802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Du Q., Li N., Du S., Sun Z. Alpiniae oxyphyllae Fructus and Alzheimer's disease: an update and current perspective on this traditional Chinese medicine. Biomed. Pharmacother. 2021;135 doi: 10.1016/j.biopha.2020.111167. [DOI] [PubMed] [Google Scholar]
- Li R., Guo K., Liu C., Wang J., Tan D., Han X., Tang C., Zhang Y., Wang J. Strong inflammatory responses and apoptosis in the oviducts of egg-laying hens caused by genotype VIId Newcastle disease virus. BMC Vet. Res. 2016;12:255. doi: 10.1186/s12917-016-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Wang L., Zhang Q., Duan H., Qian D., Yang F., Xia J. Alpiniae oxyphyllae fructus possesses neuroprotective effects on H(2)O(2) stimulated PC12 cells via regulation of the PI3K/Akt signaling pathway. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.966348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhou J., Li Y., Ding Y., Lian J., Dong Q., Qu Q., Lv W., Guo S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.J., Wang J., He T.F., Liu H.S., Piao X.S. Effects of natural capsicum extract on growth performance, nutrient utilization, antioxidant status, immune function, and meat quality in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Mao B., Gu J., Jiaying W., Cui S., Wang G., Zhao J., Zhang H., Chen W. Blautia —a new functional genus with potential probiotic properties? Gut Microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1875796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Peng C., Qu X., Guo S., Chen J.F., He C., Zhou X., Zhu S. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019;103:182–190. doi: 10.1111/jpn.13022. [DOI] [PubMed] [Google Scholar]
- Liu X., Lin X., Zhang S., Guo C., Li J., Mi Y., Zhang C. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging (Albany NY) 2018;10:2016–2036. doi: 10.18632/aging.101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.P., Chao J.R., Xu P.T., Lv H.Y., Ding B.Y., Zhang Z.F., Li L.L., Guo S.S. Lonicera flos and Cnicus japonicus extracts improved egg quality partly by modulating antioxidant status, inflammatory-related cytokines and shell matrix protein expression of oviduct in laying hens. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhang X., Liu Y., Cao H., Han Q., Xie B., Fan L., Li X., Hu J., Yang G., Shi X. Effect of fermented corn-soybean meal on serum immunity, the expression of genes related to gut immunity, gut microbiota, and bacterial metabolites in grower-finisher pigs. Front. Microbiol. 2019;10:2620. doi: 10.3389/fmicb.2019.02620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Yao J., Zhou S., Mi Y., Tan X., Zhang C. Enhancing effect of FSH on follicular development through yolk formation and deposition in the low-yield laying chickens. Theriogenology. 2020;157:418–430. doi: 10.1016/j.theriogenology.2020.07.012. [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Linden S.K., Sutton P., Florin T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Mishra B., Jha R. Oxidative stress in the poultry gut: potential challenges and interventions. Front. Vet. Sci. 2019;6:60. doi: 10.3389/fvets.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd S.M., Sieo C.C., Chong C.W., Gan H.M., Ho Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut. Pathog. 2015;7:4. doi: 10.1186/s13099-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne L., Pluske J., Hampson D. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003;108:95–117. [Google Scholar]
- Oduwole O.O., Huhtaniemi I.T., Misrahi M. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza P.I., Fraga C.G., Mills D.A., Taft D.H. Flavonoids and the gastrointestinal tract: local and systemic effects. Mol. Aspects Med. 2018;61:41–49. doi: 10.1016/j.mam.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Parada V.D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Jung S., Kang S., Heo B., Arancibia P., Toledo F., Drzewiecki J., Namieśnik J., Gorinstein S. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107:640–648. [Google Scholar]
- Parks D., Tyson G., Philip H., Beiko R. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics (Oxford, England) 2014;30:3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Cheng X., Jing H., Yan T., Xiao F., Wu B., Bi K., Jia Y. Effect of Alpinia oxyphylla-Schisandra chinensis herb pair on inflammation and apoptosis in Alzheimer's disease mice model. J. Ethnopharmacol. 2019;237:28–38. doi: 10.1016/j.jep.2019.03.029. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Sahin N., Ali S., Bahcecioglu I.H., Guler O., Ozercan I., Ilhan N., Kucuk O. Orally administered lycopene attenuates diethylnitrosamine-induced hepatocarcinogenesis in rats by modulating Nrf-2/HO-1 and Akt/mTOR pathways. Nutr Cancer. 2014;66:590–598. doi: 10.1080/01635581.2014.894092. [DOI] [PubMed] [Google Scholar]
- Shetty S.A., Marathe N.P., Lanjekar V., Ranade D., Shouche Y.S. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. PLoS One. 2013;8:e79353. doi: 10.1371/journal.pone.0079353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Wu H., Xiao H., Nguepi Tsopmejio I.S., Jin Z., Song H. Effect of dietary Flammulina velutipes (Curt.: Fr.) stem waste on ovarian follicles development in laying hens. Ital. J. Anim. Sci. 2023;22:200–213. [Google Scholar]
- Tang H., Gong Y.Z., Wu C.X., Jiang J., Wang Y., Li K. Variation of meat quality traits among five genotypes of chicken. Poult. Sci. 2009;88:2212–2218. doi: 10.3382/ps.2008-00036. [DOI] [PubMed] [Google Scholar]
- van Leeuwen S.S., Te P.E., Chatziioannou A.C., Benjamins E., Haandrikman A., Dijkhuizen L. Goat milk oligosaccharides: their diversity, quantity, and functional properties in comparison to human milk oligosaccharides. J. Agric. Food Chem. 2020;68:13469–13485. doi: 10.1021/acs.jafc.0c03766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.Z., Yuan H.H., Bao X.L., Lan M.B. In vitro antioxidant and cytotoxic properties of ethanol extract of Alpinia oxyphylla fruits. Pharm. Biol. 2013;51:1419–1425. doi: 10.3109/13880209.2013.794844. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhang H., Bai S., Zeng Q., Su Z., Zhuo Y., Mao X., Yin H., Feng B., Liu J., Zhang K., Ding X. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Zhu J., Shen L. A new lignan with anti-tumour activity from Polygonum perfoliatum L. Nat. Prod. Res. 2012;27:568–573. doi: 10.1080/14786419.2012.682993. [DOI] [PubMed] [Google Scholar]
- Wang X.C., Wang X.H., Wang J., Wang H., Zhang H.J., Wu S.G., Qi G.H. Dietary tea polyphenol supplementation improved egg production performance, albumen quality, and magnum morphology of Hy-Line Brown hens during the late laying period. J Anim Sci. 2018;96:225–235. doi: 10.1093/jas/skx007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang M., Fan K., Li T., Yan T., Wu B., Bi K., Jia Y. Protective effects of Alpinae Oxyphyllae Fructus extracts on lipopolysaccharide-induced animal model of Alzheimer's disease. J Ethnopharmacol. 2018;217:98–106. doi: 10.1016/j.jep.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Wilke M.S., French M.A., Goh Y.K., Ryan E.A., Jones P.J., Clandinin M.T. Synthesis of specific fatty acids contributes to VLDL-triacylglycerol composition in humans with and without type 2 diabetes. Diabetologia. 2009;52:1628–1637. doi: 10.1007/s00125-009-1405-9. [DOI] [PubMed] [Google Scholar]
- Wu Y., Shao Y., Song B., Zhen W., Wang Z., Guo Y., Shahid M.S., Nie W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J Anim Sci Biotechnol. 2018;9:9. doi: 10.1186/s40104-017-0220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W.G., Chen W., Abouelezz K., Ruan D., Wang S., Zhang Y.N., Fouad A.M., Li K.C., Huang X.B., Zheng C.T. The effects of dietary Se on productive and reproductive performance, tibial quality, and antioxidant capacity in laying duck breeders. Poult Sci. 2020;99:3971–3978. doi: 10.1016/j.psj.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Pan M., Wang Y., Huang Y., Tsunoda M., Zhang Y., Wang R., Hu W., Yang H., Li L.S., Song Y. In vitro bloodbrain barrier permeability study of four main active ingredients from Alpiniae oxyphyllae fructus. J Pharm Biomed Anal. 2023;235 doi: 10.1016/j.jpba.2023.115637. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yang J.X., Chaudhry M.T., Yao J.Y., Wang S.N., Zhou B., Wang M., Han C.Y., You Y., Li Y. Effects of phyto-oestrogen quercetin on productive performance, hormones, reproductive organs and apoptotic genes in laying hens. J Anim Physiol Anim Nutr (Berl) 2018;102:505–513. doi: 10.1111/jpn.12778. [DOI] [PubMed] [Google Scholar]
- Yang J., Li Y., Wen Z., Liu W., Meng L., Huang H. Oscillospira - a candidate for the next-generation probiotics. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1987783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ma X., Yang C., Jiang S., Yang W., Jiang S. The combination of plant extracts and probiotics improved jejunal barrier and absorption capacity of weaned piglets. Agriculture. 2022;12:912. [Google Scholar]
- Yang X., Zhou S., Li H., An J., Li C., Zhou R., Teng L., Zhu Y., Liao S., Yang Y., Chen H., Chen Y. Structural characterization of Alpiniae oxyphyllae fructus polysaccharide 2 and its activation effects on RAW264.7 macrophages. Int. Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107708. [DOI] [PubMed] [Google Scholar]
- Yang X., Yang Y., Chen H., Xu T., Li C., Zhou R., Gao L., Han M., He X., Chen Y. Extraction, isolation, immunoregulatory activity, and characterization of Alpiniae oxyphyllae fructus polysaccharides. Int. J. Biol. Macromol. 2020;155:927–937. doi: 10.1016/j.ijbiomac.2019.11.051. [DOI] [PubMed] [Google Scholar]
- Yong Z., Zibao H., Zhi Z., Ning M., Ruiqi W., Mimi C., Xiaowen H., Lin D., Zhixuan X., Qiang L., Weiying L., Xiaopo Z. Nootkatone, a sesquiterpene ketone from alpiniae oxyphyllae fructus, ameliorates metabolic-associated fatty liver by regulating AMPK and MAPK signaling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.909280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.H., Zhang K.Y., Ding X.M., Luo Y.H., Bai S.P., Zeng Q.F., Wang J.P. Effect of tea polyphenols on production performance, egg quality, and hepatic antioxidant status of laying hens in vanadium-containing diets. Poult. Sci. 2016;95:1709–1717. doi: 10.3382/ps/pew097. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ge J., Gao F., Yang M., Li H., Xia F., Bai H., Piao X., Sun Z., Shi L. Rosemary extract improves egg quality by altering gut barrier function, intestinal microbiota and oviductal gene expressions in late-phase laying hens. J. Anim. Sci. Biotechnol. 2023;14:121. doi: 10.1186/s40104-023-00904-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.Q., Chang C., Chu Q., Wang H.H., Zhang J., Yan Z.X., Song Z.G., Geng A.L. Dietary calcium and non-phytate phosphorus levels affect the performance, serum biochemical indices, and lipid metabolism in growing pullets. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2022.102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zheng Y., Hu X., Hu X., Lv W., Lv D., Chen J., Wu M., Song Q., Shentu J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miquel: a review. J. Ethnopharmacol. 2018;224:149–168. doi: 10.1016/j.jep.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Li K., Luo H., Duan L., Wei C., Wang M., Jin J., Liu S., Mehmood K., Shahzad M. Comparison of the intestinal microbial community in ducks reared differently through high-throughput sequencing. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/9015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y., Xiang Q., Wang J., Wei H., Peng J. Effects of oregano essential oil or quercetin supplementation on body weight loss, carcass characteristics, meat quality and antioxidant status in finishing pigs under transport stress. Livest. Sci. 2016;192:33–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.