Abstract
Rationale
Respiratory virus-induced inflammation is the leading cause of asthma exacerbation, frequently accompanied by induction of interferon-stimulated genes (ISGs). How asthma-susceptibility genes modulate cellular response upon viral infection by fine-tuning ISG induction and subsequent airway inflammation in genetically susceptible asthma patients remains largely unknown.
Objectives
To decipher the functions of gasdermin B (encoded by GSDMB) in respiratory virus-induced lung inflammation.
Methods
In two independent cohorts, we analysed expression correlation between GSDMB and ISGs. In human bronchial epithelial cell line or primary bronchial epithelial cells, we generated GSDMB-overexpressing and GSDMB-deficient cells. A series of quantitative PCR, ELISA and co-immunoprecipitation assays were performed to determine the function and mechanism of GSDMB for ISG induction. We also generated a novel transgenic mouse line with inducible expression of human unique GSDMB gene in airway epithelial cells and infected the mice with respiratory syncytial virus to determine the role of GSDMB in respiratory syncytial virus-induced lung inflammation in vivo.
Results
GSDMB is one of the most significant asthma-susceptibility genes at 17q21 and acts as a novel RNA sensor, promoting mitochondrial antiviral-signalling protein (MAVS)-TANK binding kinase 1 (TBK1) signalling and subsequent inflammation. In airway epithelium, GSDMB is induced by respiratory viral infections. Expression of GSDMB and ISGs significantly correlated in respiratory epithelium from two independent asthma cohorts. Notably, inducible expression of human GSDMB in mouse airway epithelium led to enhanced ISGs induction and increased airway inflammation with mucus hypersecretion upon respiratory syncytial virus infection.
Conclusions
GSDMB promotes ISGs expression and airway inflammation upon respiratory virus infection, thereby conferring asthma risk in risk allele carriers.
Shareable abstract
Asthma GWAS gene GSDMB promotes the induction of ISGs, mucus production and lung inflammation post-respiratory virus infection in vivo. In cellular models, GSDMB recognises and binds viral RNA, thereby activating viral RNA-induced MAVS-TBK1 signalling. https://bit.ly/48CVGxC
Introduction
Asthma affects as many as 334 million people worldwide [1]. Despite incomplete aetiology, asthma is a paradigmatic example of a complex genetic trait that emerges through the interplay of multiple genetic susceptibility variants with environmental cues in early life, particularly respiratory viral infection [2].
Upon viral infection, mitochondrial antiviral-signalling protein (MAVS)-TANK binding kinase 1 (TBK1) signalling, initiated by pattern recognition receptors, promotes the induction of interferon-stimulated genes (ISGs). Intracellular pattern recognition receptors such as retinoic acid-inducible gene I (RIG-I) or melanoma differentiation-associated gene 5 (MDA5) mediate viral nucleic acid-sensing and recruit MAVS to activate phosphorylation of TBK1 and interferon regulatory factor 3 (IRF3). Phosphorylated IRF3 then dimerises and translocates into the nucleus, transcriptionally activating interferon (IFN) production and subsequent induction of ISGs to restrict the spread of viral infection. Some ISGs are pro-inflammatory cytokines that trigger the innate immune response [3, 4]. As seen in asthmatic airway epithelium, upregulated ISG expression is strongly correlated with lung function and bronchodilator reversibility, which are key physiological measures of asthma, implicating MAVS-TBK1 signalling in the asthmatic epithelium [5, 6]. Furthermore, asthmatic human bronchial epithelial cells (HBEs) in vitro demonstrated greater induction of ISGs upon infection with respiratory syncytial virus (RSV) and expression levels of ISGs were correlated with the degree of airway obstruction in asthmatic children [5, 6]. These observations indicate that childhood asthma may be associated with an over-induction of ISGs in normal human bronchial epithelial cells (nHBEs) due to viral infection, especially in genetically susceptible children. However, how genetic determinants for asthma regulate MAVS-TBK1 signalling and subsequent induction of ISGs upon viral infection remains unexplored.
Common variants at 17q21, the most consistently reproducible asthma-susceptibility locus identified to date [2, 7, 8], determine the rate of asthma exacerbation, frequently triggered by respiratory viral infection [9]. The gene encoding gasdermin B, GSDMB on chromosome 17q21, is the gene most likely responsible for the asthma association [10]. Although GSDMB may trigger pyroptosis [11–14], programmed cell death possibly associated with lung inflammation [15], the functions of GSDMB in pyroptosis-independent lung inflammation remain unexplored.
Herein, we found that GSDMB promotes virus-induced ISG expression and subsequent inflammation by binding viral RNA and activating MAVS-TBK1 signalling in airway epithelium, which possibly explains the earlier epidemiological observation in asthma patients of the interaction between the 17q21 genome-wide association study (GWAS) locus and viral infection [9].
Methods
Cell lines and primary cells
HEK293T and BEAS-2B cells were maintained in DMEM (Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (Thermo Fisher Scientific, Pittsburgh, PA, USA). THP1, a human monocyte cell line, was cultured in RPMI 1640 medium with 10% fetal bovine serum (Thermo Fisher Scientific) and differentiated by preincubation in culture medium with 100 ng·mL−1 phorbol 12-myristate 13-acetate overnight. nHBEs were cultured in Small Airway Epithelial Cell Medium (Promocell, Heidelberg, Germany) with 1.0 μM A8301 (Tocris Bioscience, Bristol, UK), 0.5 μM CHIR99021 (Sigma-Aldrich, St Louis, MO, USA) and 5 μM Y27632 (Sigma-Aldrich) on plates precoated with laminin-enriched 804G-conditioned medium [16, 17].
Infection of rhinovirus 16-A and RSV line 19 in cells
The human rhinovirus 16-A (RV-A16) and RSV were generously provided by James Gern (University of Wisconsin School of Medicine and Public Health Madison) and Bruce D. Levy (Brigham and Women's Hospital at Harvard Medical School), respectively. Virus generation and expansion were based on protocols that have been previously described [18, 19]. BEAS-2B cells or nHBEs seeded in 6-well plates at 90% confluency were infected with RV-A16 (multiplicity of infection (MOI) of 1) or RSV (MOI of 1) for 72 h or 24 h, respectively. Cell pellets were collected for reverse transcription PCR and supernatants were collected for ELISA to measure levels of ISGs and IFNs.
Native PAGE analysis of oligomers
For native PAGE analysis of IRF3 oligomers, cellular protein extracts were prepared using low-salt lysis buffer polyinosinic-polycytidylic acid (poly(I:C)) transfection at indicated time points. Samples were mixed with Native Sample Buffer (Bio-Rad, Hercules, CA, USA) and then resolved in precast 4–20% Mini-PROTEAN TGX gel (Bio-Rad). The gel was incubated with TGS buffer (Thermo Fisher Scientific) for 30 min and then protein samples were transferred to polyvinylidene fluoride membranes (Bio-Rad) and further incubated with the appropriate antibodies followed by standard Western blotting protocol for protein detection. The G:Box system (Syngene, Bengaluru, Karnataka, India) was used for capturing images and band intensities were quantified using ImageJ software.
RSV infection in mice
For RSV infection, 6–8-week-old wild-type (WT) or Cc10-hGSDMB female mice were mated with genotype-matched males overnight. The next morning, female mice were examined and marked as pregnant in the presence of vaginal plugs (Day −21). Eight days later (Day −13), pregnant female mice received 2 mg·mL−1 doxycycline (Dox) (Sigma-Aldrich, cat. D9891-100G) in drinking water, protected from light, with 5% sucrose to mask the bitter taste. Dox and sucrose were dissolved in sterile water and renewed weekly. After weaning, pups received Dox (2 mg·mL−1) in drinking water. RSV line 19 was propagated and quantified as previously described [19]. WT and Cc10-hGSDMB neonatal mice were intranasally infected with RSV line 19 (2×104 pfu per mouse, n=5 mice per condition) or PBS at the age of postnatal Day 7, Day 14, Day 21 and Day 56. Mice were harvested 4 days after the last RSV infection, and lung tissues were collected for haematoxylin-and-eosin staining, immunofluorescence staining, Western blotting and quantitative PCR.
Periodic acid–Schiff staining
To detect the mucous amount in airways, lung slides were stained with periodic acid–Schiff (PAS) using Richard-Allan Scientific Periodic Acid–Schiff (PAS) kit (Thermo Fisher Scientific) as previously described [19] and images were captured using a light microscope (ZEISS Axio Imager 2, Oberkochen, Germany).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) and RStudio 3.6.2 (Posit Software, Boston, MA, USA). Two-way ANOVA and t-test were used to determine statistical significance. p-values <0.05 were considered statistically significant. Details on statistical analyses are described in the figure legends and the supplementary material.
A detailed Method section is also included in the supplementary material.
Results
GSDMB expression correlates with IFN signalling in human respiratory epithelial cells
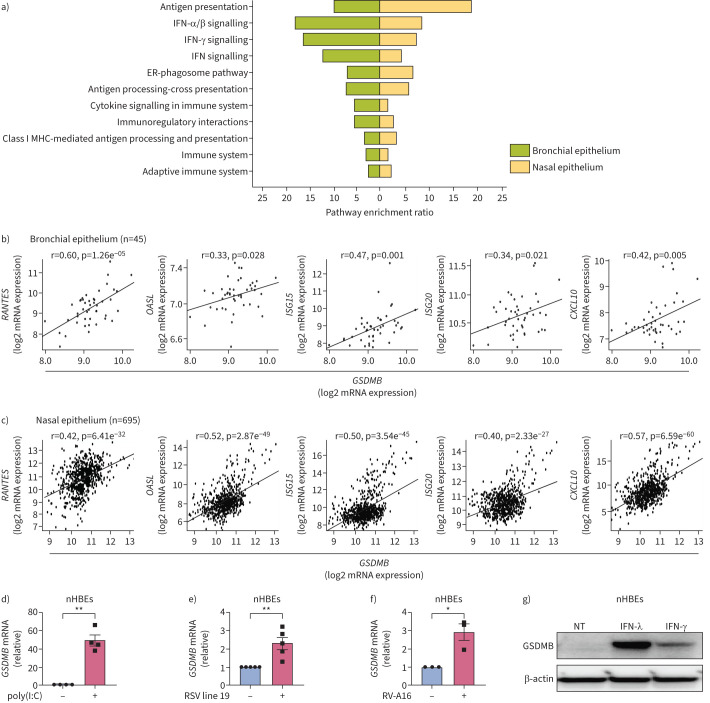
Using an unbiased transcriptome-wide search for genes that strongly correlated with GSDMB expression in human bronchial epithelial brushings (n=45, supplementary table E1) including asthma patients (n=28) from the Asthma BioRepository for Integrative Genomic Exploration (Asthma BRIDGE) [20, 21], we found that expression levels of 405 probe sets corresponding to 390 unique genes demonstrated significant correlation with expression of GSDMB at a false discovery rate (FDR) ≤0.001 (median r2=0.406, IQR 0.378–0.424). This set was strongly enriched for genes involved in IFN (and its subsets IFN-ɑ, IFN-β and IFN-γ) signalling (FDR ≤2.15e−04, figure 1a). We further replicated these findings in an independent, publicly accessible dataset of gene expression in nasal epithelial brushings from the Genes–Environments and Admixture in Latino Americans (GALA) II study (n=254 controls and 441 asthma patients) [22, 23], where expression of GSDMB was strongly correlated with genes enriched in IFN signalling (figure 1a). In both the Asthma BRIDGE and GALA II datasets, we observed a positive correlation of GSDMB with multiple ISGs (figure 1b, c), including RANTES, OASL, ISG15, ISG20 and CXCL10. In contrast, no correlation with ISGs was found for the other 17q21 asthma GWAS gene, ORDML3 (supplementary figure E1a). These results are consistent with previous publications showing lack of evidence for the roles of ORMDL3 in ISG expression [24] and lack of correlation between 17q21 asthma GWAS variants and expression of ORMDL3 in nasal epithelial cells [25].
FIGURE 1.
Gasdermin B (GSDMB) is closely related to interferon-stimulated gene (ISG) activation in respiratory epithelial cells. a) The Gene Ontology enrichment analysis of these genes significantly correlated with GSDMB expression in both Asthma BRIDGE (BioRepository for Integrative Genomic Exploration) samples (bronchial, green) and Genes–Environments & Admixture in Latino Americans (GALA) II study (nasal, yellow). b, c) Expression of RANTES, OASL, ISG15, ISG20 and CXCL10 significantly correlated with expression of GSDMB in Asthma BRIDGE samples (b) and GALA II study samples (c). d–f) Expression of GSDMB in normal human bronchial epithelial cells (nHBEs) transfected with polyinosinic-polycytidylic acid (poly(I:C)) for 6 h (d) or infected with respiratory syncytial virus (RSV) line 19 (multiplicity of infection=1) for 24 h (e) or rhinovirus 16-A (RV-A16) (multiplicity of infection=1) for 72 h (f). g) Protein levels of GSDMB were measured by Western blotting in nHBEs treated with interferon (IFN)-λ (500 ng·mL−1) or IFN-γ (500 ng·mL−1) for 24 h. For b and c, expression levels of each gene were log2-transformed and quantile-normalised. Then, the linear correlation between the expression levels of two genes was determined using a t-test for Pearson's correlation coefficient. For b–f, mean±sem shown from at least three independent biological replicates. ER: endoplasmic reticulum; MHC: major histocompatibility complex; NT: no treatment. *: p<0.05; **: p<0.01 (t-test).
Through positive feedback loops, many MAVS-TBK1 signalling components are themselves induced by IFNs to further amplify the immune response [26, 27]. Similar to the induction of GSDMB by cytokines in cancer cells [28], GSDMB expression is markedly increased upon transfection of poly(I:C) or infection with either RSV line 19 or RV-A16 in both primary bronchial epithelial cells (nHBEs) and bronchial epithelial BEAS-2B cell line (figure 1d–f and supplementary figure E1b–d). Consistently, marked induction of GSDMB protein in nHBEs was observed following IFN treatment (figure 1g). Notably, the induction of GSDMB by RV-A16 in BEAS-2B cells was significantly inhibited by the TBK1 inhibitor BX795, a key kinase activating the MAVS-TBK1 signalling pathway (supplementary figure E1e).
GSDMB augments double-stranded RNA-induced MAVS-TBK1 signalling in human airway epithelium
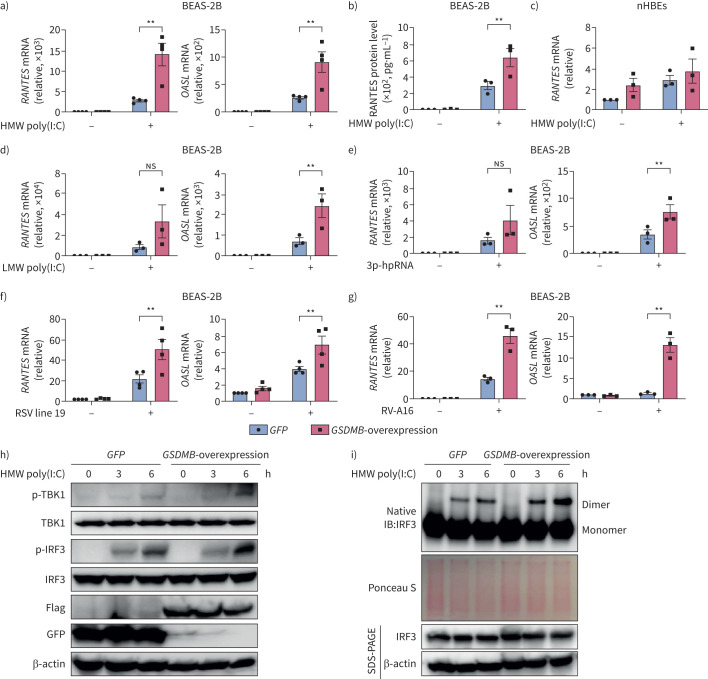
Given that the strongest risk of asthma is conferred by GSDMB-overexpressing haplotypes in childhood following infection with respiratory viruses [5] and the positive correlation of ISGs with GSDMB expression in respiratory epithelium, we hypothesise that GSDMB may regulate MAVS-TBK1 signalling. To test this, we overexpressed GSDMB (full-length) in the bronchial epithelial BEAS-2B cell line, with validation in nHBEs (figure 2) given increased expression of GSDMB in airway epithelial cells associated with asthma-risk alleles. During infection, single-stranded respiratory RNA viruses usually produce double-stranded RNA (dsRNA) mediator [29–31] to trigger series of downstream signalling cascades. We thus transfected cells with multiple synthetic analogues of dsRNA, including high molecular weight (HMW) and low molecular weight (LMW) poly(I:C) and 5′ triphosphate hairpin RNA, as mimics for RNA viruses in cellular models. In response to the various dsRNA tested, overexpression of GSDMB resulted in enhanced activation of ISGs, including RANTES and OASL, as well as the induction of IFNs (figure 2a, d, e and supplementary figure E2), with corresponding increases in protein levels of RANTES (figure 2b). Similar changes were observed in nHBEs (figure 2c and supplementary figure E2c). Of note, overexpression of GSDMB also led to greater induction of ISGs such as RANTES and OASL in BEAS-2B cells infected with the common childhood respiratory viruses RSV line 19 and RV-A16 (figure 2f, g), indicating that GSDMB enhances MAVS-TBK1 signalling in bronchial epithelial cells upon respiratory viral infection.
FIGURE 2.
Gasdermin B (GSDMB) promotes RNA mimic or RNA virus-induced interferon-stimulated gene (ISG) induction. a, b) mRNA levels of RANTES and OASL (a) or extracellular RANTES protein levels (b) were measured in BEAS-2B cells with overexpression of GSDMB and transfected with high molecular weight (HMW) polyinosinic-polycytidylic acid (poly(I:C)) for 6 h. c) Expression of RANTES in normal human bronchial epithelial cells (nHBEs) with overexpression of GSDMB after transfection of HMW poly(I:C) for 6 h. d, e) RNA level of RANTES and OASL in BEAS-2B cells with overexpression of GSDMB and transfected with low molecular weight (LMW) poly(I:C) (d) or 5′-triphosphate hairpin RNA (3p-hpRNA) for 6 h (e), respectively. f, g) Expression of RANTES and OASL in BEAS-2B cells with overexpression of GSDMB and infected with respiratory syncytial virus (RSV) line 19 (multiplicity of infection=1, 24 h) (f) or rhinovirus A16 (RV-A16) (multiplicity of infection=1, 72 h) (g). h) Measurements of interferon regulatory factor 3 (IRF3) and TANK binding kinase 1 (TBK1) phosphorylation by immunoblotting in green fluorescent protein (GFP)-overexpressing versus GSDMB-overexpressing cells. i) IRF3 dimerisation in BEAS-2B cells with overexpression of GSDMB after transfection of HMW poly(I:C) for 0, 3 and 6 h. Cells expressing GFP were used as controls. For a–g, mean±sem shown from at least three independent biological replicates. For all immunoblot data, representative results were shown from two independent biological experiments. ns: nonsignificant. **: p<0.01 (two-way ANOVA).
Additionally, the TBK1 inhibitor BX795 significantly mitigated GSDMB-enhanced ISG induction, suggesting that TBK1, a serine/threonine protein kinase pivotal for virus-induced IFN response [32], is required for GSDMB-regulated MAVS-TBK1 signalling (supplementary figure E3). Furthermore, the phosphorylation of TBK1 and IRF3, as well as phosphorylation-dependent IRF3 dimerisation, which are markers of MAVS-TBK1 signalling activation, were increased in GSDMB-overexpressing cells upon HMW poly(I:C) transfection (figure 2h, i).
GSDMB promotes MAVS-TBK1 signalling independent of pyroptosis, without signs of endoplasmic reticulum stress
Given that GSDMB was previously reported to promote inflammasome activation [28], which induces pyroptosis and may subsequently enhance MAVS-TBK1 signalling [31, 33], we further examined this possibility. Transfection of HMW poly(I:C) into the BEAS-2B cell line for 6 h showed no evidence of the typical pyroptosis characteristics, e.g. increased uptake of propidium iodide (supplementary figure E4a), rupture of the cell membrane (supplementary figure E4a), release of lactate dehydrogenase (supplementary figure E4b) and secretion of interleukin-1β (IL-1β) (supplementary figure E4c). However, 24 h after transfection of HMW poly(I:C), we indeed observed significantly increased cell death in GSDMB-overexpressing cells (supplementary figure E4b). Consistently, the caspase-1 inhibitor YVAD showed no effects on the induction of RANTES after transfection of HMW poly(I:C) (supplementary figure E4d), suggesting that GSDMB-regulated ISGs induction by poly(I:C) is independent of inflammasome activation in BEAS-2B cells. Moreover, pyroptosis-defective GSDMB isoform 1 [14] showed comparable induction of MAVS-TBK1 signalling as the isoform 3 of GSDMB (referred to as the full-length) (supplementary figure E4e), further supporting that GSDMB-induced activation of MAVS-TBK1 signalling is independent of pyroptosis.
To determine whether overexpression of GSDMB leads to endoplasmic reticulum (ER) stress that further regulates the IFN response [34, 35], we evaluated levels of ER stress in BEAS-2B cells with GSDMB overexpression. However, there was no evidence supporting the activation of ER stress (supplementary figure E5) because similar expression levels of splicing of X-box binding protein 1 (XBP1) were detected in both control and GSDMB-overexpressing cells.
GSDMB deficiency impairs RNA virus-induced MAVS-TBK1 signalling in human airway epithelium
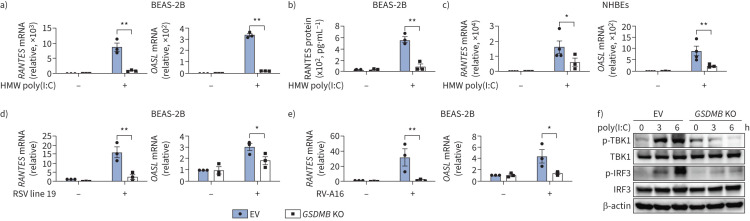
To further determine the role of GSDMB in regulating MAVS-TBK1 signalling, we generated BEAS-2B and nHBE GSDMB knockout (KO) cells using guide RNAs targeting GSDMB (supplementary figure E6a, d). GSDMB-deficient cells demonstrated impaired induction of ISGs following poly(I:C) transfection (figure 3a–c and supplementary figure E6b, c, e) or viral infection (figure 3d, e), suggesting that GSDMB is required for MAVS-TBK1 signalling in human airway epithelium. Likewise, the phosphorylation of TBK1 and IRF3 was diminished in GSDMB KO cells upon poly(I:C) transfection (figure 3f) as compared to vector-transfected control cells.
FIGURE 3.
Deficiency of gasdermin B (GSDMB) impairs induction of interferon-stimulated genes (ISGs) by RNA mimic or RNA virus. a) Expression of RANTES and OASL in BEAS-2B cells with or without knockout (KO) of GSDMB after transfection of high molecular weight (HMW) polyinosinic-polycytidylic acid (poly(I:C)) for 6 h. b) RANTES protein levels in supernatants were measured in BEAS-2B cells transfected with HMW poly(I:C) for 6 h. c) Expression of RANTES and OASL in normal human bronchial epithelial cells (nHBEs) with or without GSDMB KO after transfection of HMW poly(I:C) for 6 h. d, e) Expression of RANTES in empty vector (EV) or GSDMB KO BEAS-2B cells infected with respiratory syncytial virus (RSV) line 19 (multiplicity of infection=1, 24 h) (d) or rhinovirus 16-A (RV-A16) (multiplicity of infection=1, 72 h) (e). f) Measurements of interferon regulatory factor 3 (IRF3) and TANK binding kinase 1 (TBK1) phosphorylation by immunoblotting in EV versus GSDMB-overexpressing cells. EV-transfected cells were used as controls. Mean±sems shown from at least three independent biological replicates. *: p<0.05; **: p<0.01 (two-way ANOVA).
GSDMB interacts with MAVS
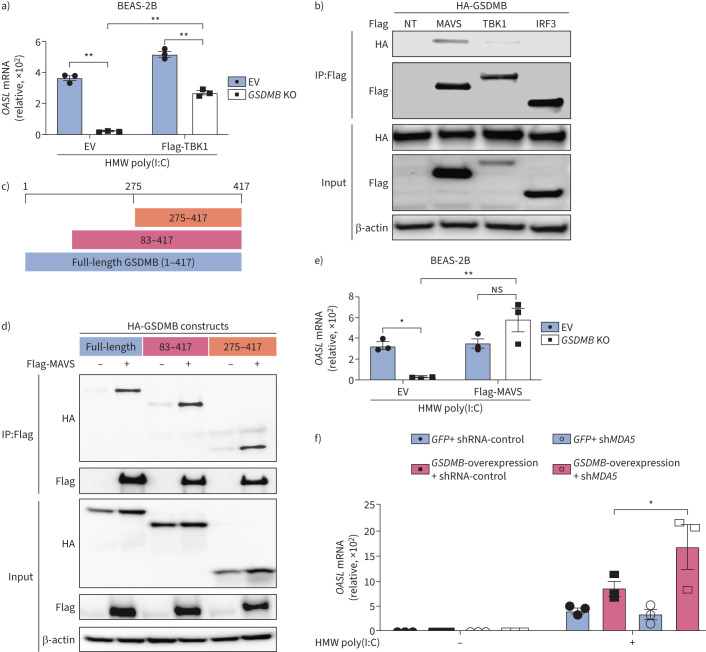
Given that inhibition of TBK1 with BX795 could significantly mitigate GSDMB-enhanced ISG induction (supplementary figure E5), while overexpression of TBK1 restored induction of ISGs in GSDMB KO BEAS-2B cells (figure 4a), we conclude that GSDMB functions upstream of TBK1. We next assessed for possible interactions of GSDMB with major components of MAVS-TBK1 signalling, including MAVS, TBK1 and IRF3. HEK293T cells were transfected with either Flag-tagged MAVS, TBK1 or IRF3 together with haemagglutinin-tagged GSDMB. Co-immunoprecipitation and immunoblot analyses revealed that GSDMB interacted with the adaptor molecule MAVS but not TBK1 or IRF3 (figure 4b). Furthermore, we found that the domain with amino acids 275–417 of GSDMB was responsible for its interaction with MAVS (figure 4c, d). Because overexpression of MAVS significantly rescued impaired ISG induction in GSDMB KO cells (figure 4e), GSDMB may function upstream of MAVS. MDA5, a well-known RNA sensor located upstream of MAVS, preferentially recognises HMW poly(I:C) [36]. We thus speculate that GSDMB potentially regulates MAVS-TBK1 signalling through MDA5. However, MDA5 deficiency failed to repress MAVS-TBK1 signalling in GSDMB-overexpressing cells, indicating that MDA5 is dispensable for GSDMB-induced MAVS-TBK1 signalling (figure 4f and supplementary figure E7a). Of note, GSDMB also promotes LMW dsRNA-induced MAVS-TBK1 signalling independent of RIG-I, a cytosolic RNA sensor for LMW poly(I:C) (supplementary figure E7b) [33]. In contrast, cell membrane receptor Toll-like receptor 3 (TLR3) failed to induce activation of MAVS-TBK1 signalling in BEAS-2B, suggesting that GSDMB-induced activation of MAVS-TBK1 signalling is independent of the TLR3 pathway (supplementary figure E7c).
FIGURE 4.
Gasdermin B (GSDMB) binds to mitochondrial antiviral-signalling protein (MAVS). a) Expression of OASL in control or GSDMB knockout (KO) BEAS-2B cells transfected with either empty vector (EV) or Flag-tagged TANK binding kinase 1 (TBK1), followed by high molecular weight (HMW) polyinosinic-polycytidylic acid (poly(I:C)) transfection for 6 h. b) Co-immunoprecipitation (IP) studies: anti-haemagglutinin (HA) immunoblots of either input or immunoprecipitated (IP:Flag) protein complex with anti-Flag from HEK293T cells transfected with HA-GSDMB and either Flag-MAVS, Flag-TBK1 or Flag-interferon regulatory factor 3 (IRF3). c) Schematic illustration of HA-GSDMB constructs used in co-IP assays in d. d) Flag-MAVS co-immunoprecipitated with various domains of HA-GSDMB in HEK 293T cells. e) Expression of OASL in control or GSDMB KO BEAS-2B cells transfected with EV or Flag-MAVS, followed by HMW poly(I:C) transfection for 6 h. f) Expression of OASL in GFP-overexpressing or GSDMB-overexpressing cells with MDA5 depletion, followed by transfection of HMW poly(I:C) for 6 h. For a, e, f, mean±sems shown from at least three independent biological replicates. For all immunoblot data, representative results are shown from two independent biological experiments. NT: no treatment; ns: nonsignificant; sh: small hairpin. *: p<0.05; **: p<0.01 (two-way ANOVA).
GSDMB binds to dsRNA
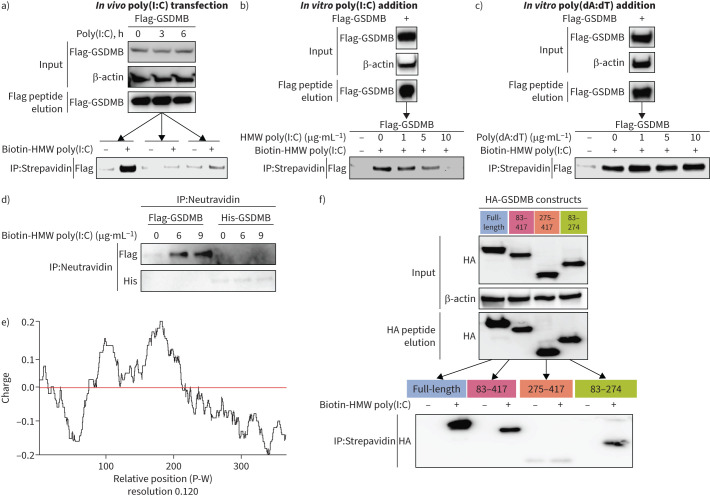
Because GSDMB functions upstream of MAVS independent of known RNA sensors, we speculated that GSDMB might act as an RNA sensor, activating the downstream MAVS-TBK1 signalling cascade. Motivated by a virtual ligand screening that identified a motif of GSDMB with nucleic acid binding properties (supplementary figure E8), we pursued this hypothesis through a series of in vitro binding experiments and found that immunoprecipitated GSDMB protein from BEAS-2B binds with biotin-labelled poly(I:C). Such binding was inhibited by either overexpressed or unlabelled poly(I:C) in a dose-dependent manner (figure 5a, b), while poly(dA:dT), a synthetic dsDNA analogue, was unable to compete (figure 5c). Given that GSDMB-interacting proteins may also contribute to the detected binding with poly(I:C), we thus incubated the pure GSDMB protein with different concentrations of biotin-labelled poly(I:C) in vitro. We observed that the pure GSDMB protein alone was sufficient to bind with poly(I:C) in a concentration-dependent manner, in contrast to its paralogue GSDMD protein (figure 5d). Indeed, part of GSDMB (amino acids 83–274) is positively charged (figure 5e) and is potentially capable of binding to nucleic acid, which was further confirmed experimentally when immunoprecipitated GSDMB domains (amino acids 83–274) interacted with biotin-labelled poly(I:C) (figure 5f).
FIGURE 5.
Gasdermin B (GSDMB) binds RNA as a potential RNA sensor. a) In vitro binding of biotin-high molecular weight (HMW) polyinosinic-polycytidylic acid (poly(I:C)) (3 µg·mL−1) and immunoprecipitated Flag-GSDMB from BEAS-2B cells with stable expression of Flag-GSDMB detected by immunoprecipitation (IP) with streptavidin beads and immunoblot analysis with anti-Flag antibody. b, c) Dose-dependent competitive binding of unlabelled HMW poly(I:C) (b), but not unlabelled poly(deoxyadenylic-deoxythymidylic) acid (poly(dA:dT) (c) with Flag-GSDMB against biotin-labelled poly(I:C) (3 µg·mL−1). d) Pure Flag-GSDMB instead of Flag-GSDMD protein binds to biotin-HMW poly(I:C) as shown by IP with neutravidin beads and immunoblotting analysis with anti-Flag antibody. e) Distribution of GSDMB electric charge was predicted based on each amino acid in the protein sequence. f) Haemagglutinin (HA)-GSDMB and its deletion mutants underwent IP and were eluted from BEAS-2B cells after transfection, followed by subsequent IP for their binding with biotin-labelled HMW poly(I:C). For all immunoblot data, representative results are shown from two independent biological experiments. His: histidine; P-W: position-window.
Human GSDMB promotes RSV-induced inflammation in mouse models
To further determine whether GSDMB can enhance MAVS-TBK1 signalling in vivo, we evaluated the impact of airway GSDMB expression using a murine model of respiratory viral infection. Because mice lack a GSDMB homologue, we generated a Dox-inducible humanised GSDMB mouse line with conditional expression of full-length (isoform 3) GSDMB restricted to the Cc10-expressing airway epithelium. Cc10-rtTA/Tet on-hGSDMB offspring (Cc10-hGSDMB) mice were born at term with normal appearance and normal lung histology.
Then, we validated the Dox-inducible expression of hGSDMB in airway epithelial cells in Cc10-hGSDMB mice (supplementary figure E9a–c). Importantly, the expression level of GSDMB in the lung tissues of DOX-treated Cc10-hGSDMB mice was comparable to that in normal human lung tissues, GSDMB-expressing BEAS-2B cells and nHBEs cultured at the air–liquid interface (supplementary figure E9d). As expected, expression of hGSDMB at relatively physiological levels in mouse airway epithelium showed no induction of ER stress (supplementary figure E9e).
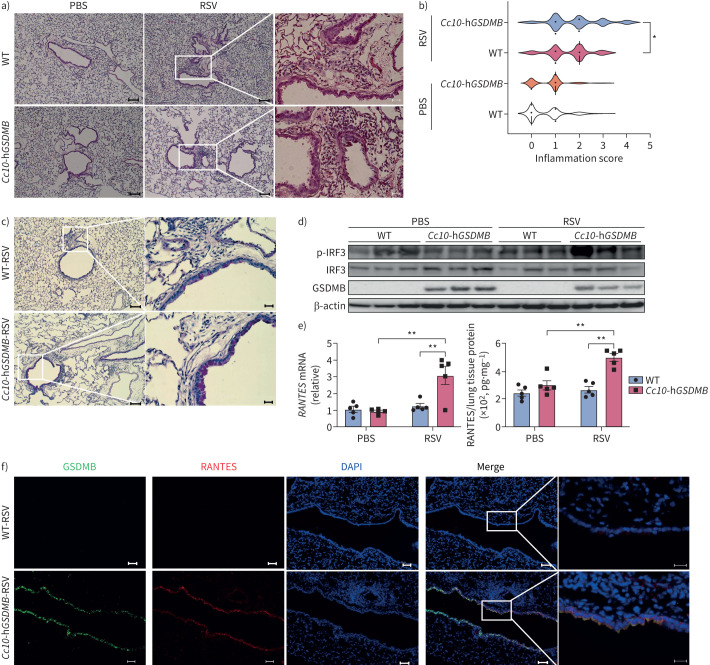
However, following RSV infection (supplementary figure E10a), the hGSDMB-expressing mice exhibited significantly greater immune cell infiltration and inflammation (figure 6a, b and supplementary figure E10b), as well as greater mucus hypersecretion (figure 6c), compared to infected WT neonatal mice. These findings were accompanied by a greater innate immune response in hGSDMB-expressing mice, as highlighted by increased levels of phosphorylated IRF3 (figure 6d), RANTES (figure 6e, f) and other ISGs compared to control mice lacking expression of hGSDMB (supplementary figure E10c).
FIGURE 6.
Human gasdermin B (hGSDMB) promotes respiratory syncytial virus (RSV)-induced mitochondrial antiviral-signalling protein (MAVS)-TANK binding kinase 1 (TBK1) signalling, inflammation and mucus production in mouse models. a) Representative haematoxylin and eosin (H&E) staining images of lung specimens from wild-type (WT), hGSDMB mice intranasally infected with 2×104 pfu RSV line 19 (n=5) or PBS (n=5), respectively. Scale bars: 100 μm or 20 μm (inset). b) Peri-airway inflammation was scored based on the number of immune cells infiltrating surrounding airways as shown by H&E staining. c) Periodic acid–Schiff staining in WT and hGSDMB mice. Scale bars: 100 μm or 20 μm (inset). d) Western blotting of GSDMB and activated interferon regulatory factor 3 (IRF3) in lungs from mice in a. e) mRNA and protein levels of RANTES in murine lungs were measured by quantitative PCR and ELISA. f) Immunofluorescence staining of GSDMB (green) and RANTES (red) in murine lung slides. Nuclei were counterstained with DAPI (blue). Scale bars: 50 μm or 20 μm (inset). *: p<0.05; **: p<0.01 (two-way ANOVA).
17q21 asthma GWAS variants associated with RV-induced ISG expression in human asthmatic airway epithelial cells
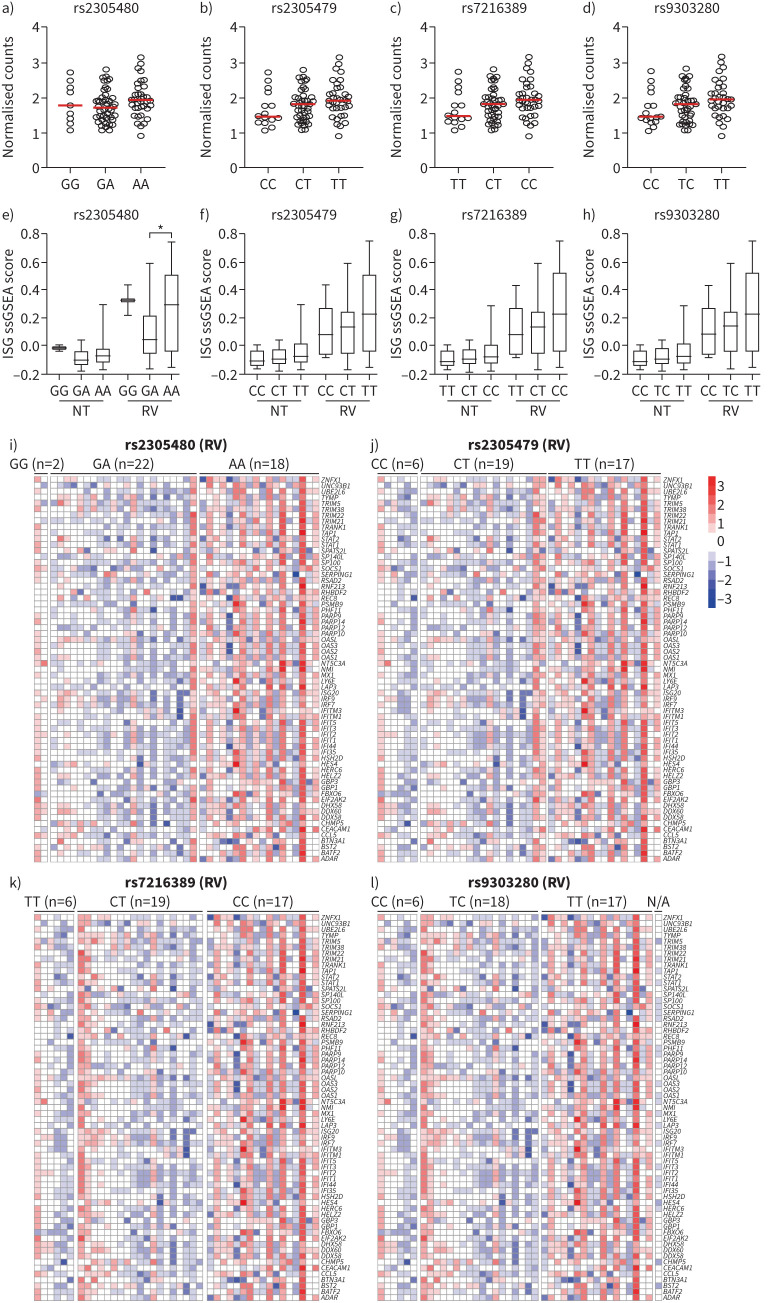
To further validate the significance of GSDMB in ISG induction in asthmatic samples from human airway epithelium, we explored a public dataset containing comprehensive genetic and gene expression data from human airway epithelial cells, specifically cells collected from endoscopic sinonasal surgeries then cultured under submerged conditions followed by infection with RV [37]. We then reanalysed gene expression data (n=95, 42 asthma patients and 53 non-asthma controls; supplementary table E2) in this dataset. For this analysis, we first chose a few previously reported asthma GWAS variants and determined their correlation with expression of GSDMB and ISGs induced by RV infection. We employed the dataset to calculate the single-sample gene set enrichment analysis (ssGSEA) score for ISGs. Cells carrying significant asthma variants (rs2305480, rs2305479, rs7216389 and rs9303280) that showed a trend of association towards increased expression of GSDMB also exhibited higher ssGSEA scores for ISGs (figure 7a–d) when infected with RV. These findings support that GSDMB genotypes correlate with induction of ISGs by viral infection, which was further supported by correlation analysis of GSDMB genotype with expression of each ISG in RV-infected asthmatic airway epithelial cells (figure 7e–h).
FIGURE 7.
The genotypes of gasdermin B (GSDMB) correlate with the expression of induced interferon-stimulated genes (ISGs) in airway epithelial cells of individuals with asthma after rhinovirus 16-A (RV) infection from a public dataset (GSE172368). a–d) Expression of GSDMB in healthy and asthmatic airway epithelial cells with different 17q21 genotypes: rs2305480 (a), rs2305479 (b), rs7216389 (c) and rs9303280 (d) without RV infection. e–h) Box plots showing genotypes of GSDMB and single-sample gene set enrichment analysis (ssGSEA) score for ISGs gene signature in asthmatic airway epithelial cells before and after RV infection by different genotypes of rs2305480 (e), rs2305479 (f), rs7216389 (g) and rs9303280 (h). i–l) Heatmap showing the expression of ISGs in RV-infected asthmatic airway epithelial cells with different genotypes in the GSDMB GWAS locus rs2305480 (i), rs2305479 (j), rs7216389 (k) and rs9303280 (l), scaled by each gene. NT: no treatment. *: p<0.05.
These consistent results, together with in vitro studies in bronchial epithelial cells and in vivo studies in mouse models, further corroborate GSDMB as an amplifier of the virus-induced activation of the innate immune response in airway epithelium.
Discussion
MAVS-TBK1 signalling is the essential first line of defence against respiratory infection through both primary intracellular and secondary extracellular immune responses. Upon respiratory viral infection, MAVS-TBK1 signalling is initiated by the RNA-sensing molecules RIG-I-like receptors (RLRs), including RIG-I and MDA5. Here, we demonstrate that GSDMB, as a novel RNA-binding protein, exhibits RLR-like properties and promotes the dsRNA-induced activation of MAVS-TBK1 signalling in human airway epithelium. GSDMB binds to MAVS to augment IFN expression and downstream signalling through the subsequent activation of TBK1 and IRF3. Consistent with this, conditional expression of human GSDMB restricted to the airway epithelium in mice resulted in a more pronounced inflammatory response after neonatal RSV infection. Together with our observation of the strong correlation of GSDMB expression with that of ISGs in two cohorts, these findings highlight the important role of GSDMB in augmenting MAVS-TBK1 signalling in the human airway epithelium upon viral infection.
Our work adds to the growing recognition of distinct functions of GSDMB in epithelial tissues, in contrast to its paralogues such as gasdermins A and D. GSDMB is one of six members of the gasdermin gene family, a set of genes that encode structurally similar pore-forming proteins that induce cytotoxic cell membrane permeability and pyroptosis [31, 38]. However, a non-pyroptosis-related function of GSDMB was reported in inflammatory bowel disease, distinct from those of its paralogues [39]. Among the gasdermin family, GSDMB is the only family member unique to mammals without a mouse or rat homologue, unlike GSDMC and GSDMD. It is noteworthy that the defensive mechanism against viral infection through the activation of MAVS-TBK1 signalling was functional in murine airway epithelial cells. This may very likely depend on the well-reported and conserved RNA sensors RIG-I and MDA5 to recognise viral RNA and activate downstream IFN production and ISG inductions, despite the lack of GSDMB in mice. It is conceivable that the acquisition of human airway epithelial expression of GSDMB may represent an evolutionary advantage, with robust MAVS-TBK1 signalling against viral defence. However, such adaptations may be a double-edged sword, resulting in higher sensitivity to a viral RNA-induced inflammatory response, potentially leading to stronger mucosal inflammation and chronic inflammatory lung disease, as seen in asthma patients carrying GSDMB risk alleles. Indeed, multiple studies profiling epithelial gene signatures following respiratory viral infection have demonstrated strong correlations between persistent expression of airway epithelial ISGs and adverse asthma phenotypes, including greater airway inflammation, reduced lung function and poorer asthma symptom scores [5, 6, 40–42].
In this study, we investigated how early childhood respiratory infection modifies the risk conferred by the 17q21 asthma-susceptibility locus, and uncovered a previously unrecognised function for GSDMB in the respiratory epithelial innate immune response that exhibits a novel function as a dsRNA-sensor that can activate MAVS-TBK1 signalling and induce robust expression of ISGs. These novel findings may motivate further investigation of GSDMB as a therapeutic target for immune modulation of both infectious and chronic inflammatory lung diseases. Nevertheless, this study has several limitations that may require further investigation in the future. First, the roles of IFN pathways during asthma pathogenesis remain controversial in the field. Both impaired and more robust IFN production have been reported in asthmatic bronchial epithelial cells. This may result from different disease stages, subtypes and subject endotypes. Second, while we were able to provide an explanation for the hyperactivation of IFN response in individuals with highly expressed GSDMB, the mechanism underlying this phenomenon is still unclear for patients with lower IFN production. In addition, it remains unanswered whether and which post-translational modification of GSDMB is responsible for RNA recognition and/or downstream binding of MAVS.
In summary, while investigating how early childhood respiratory infection modifies risk conferred by the 17q21 asthma-susceptibility locus, we uncovered a previously unrecognised function for GSDMB in the respiratory epithelial innate immune response, in that it exhibits RLR-like properties as a dsRNA-sensor that can induce robust expression of ISGs. In vitro and in vivo studies demonstrated that overexpression of GSDMB in airway epithelium results in greater airway inflammation upon infection with RSV. Together, these novel findings may motivate further investigation of GSDMB as a therapeutic target for immune modulation of both infectious and chronic inflammatory lung diseases.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: methods, tables, and detailed captions for supplementary figures. ERJ-01232-2023.Supplement (285.3KB, pdf)
Supplementary figure E1. ORMDL3 has no correlation with ISGs in asthmatic epithelial cells. ERJ-01232-2023.Figure_E1 (320KB, jpg)
Supplementary figure E2. GSDMB promotes interferon expression. ERJ-01232-2023.Figure_E2 (404.2KB, jpg)
Supplementary figure E3. GSDMB promotes MAVS-TBK1 signalling upstream of TBK1. ERJ-01232-2023.Figure_E3 (119.5KB, jpg)
Supplementary figure E4. The regulation of GSDMB on the MAVS-TBK1 signalling is independent of pyroptosis. ERJ-01232-2023.Figure_E4 (464.3KB, jpg)
Supplementary figure E5. GSDMB promotes MAVS-TBK1 signalling independent of ER stress. ERJ-01232-2023.Figure_E5 (149.6KB, jpg)
Supplementary figure E6. Knockout of GSDMB decreases IFN response. ERJ-01232-2023.Figure_E6 (235.1KB, jpg)
Supplementary figure E7. The functions of GSDMB on MAVS-TBK1 signalling are independent of MDA5 and RIG-I. ERJ-01232-2023.Figure_E7 (317KB, jpg)
Supplementary figure E8. Virtual ligand screening of GSDMB indicating potential binding of GSDMB with nucleic acid. ERJ-01232-2023.Figure_E8 (113.7KB, jpg)
Supplementary figure E9. Confirmation of Cc10-hGSDMB mice. ERJ-01232-2023.Figure_E9 (755.4KB, jpg)
Supplementary figure E10. GSDMB enhances RSV-induced IFN response and inflammation. ERJ-01232-2023.Figure_E10 (1.2MB, jpg)
Shareable PDF
Acknowledgements
We thank all subjects for their participation in this study. We thank Jack Elias (Brown University) and Chun Genun Lee (Brown University) for providing the Tet-on Cc10-rtTA constructs for generation of hGSDMB mice.
Footnotes
Author contributions: T. Liu, B.A. Raby and X. Zhou conceived and designed the project. T. Liu and X. Rui designed, performed and interpreted the experiments. F. Guo, S. Xu, N. Krishnamoorthyni, M.A. Perrella and B.D. Levy helped with animal experiments. S. Liu, N. Boyer, Y. Zhang, Y. Yu and Y. Zhou helped with cellular experiments. L. Gong, Y. Cao, J. Hecker and S. Chun helped with data analysis. S. Bates helped with plasmid generation. J-A. Park, M.A. Perrella, B.D. Levy, S.T. Weiss and H. Mou provided comments and suggestions. B.A. Raby and X. Zhou supervised the in vitro and in vivo aspects of the project, respectively. T. Liu, B.A. Raby and X. Zhou wrote the manuscript with input from all authors.
This article has an editorial commentary: https://doi.org/10.1183/13993003.02223-2023
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Support statement: This work was supported by the NIH (R01HL127200, R01HL148667, R01HL162783 and R01HL147148 to X. Zhou, and R01 HL123546 to B.A. Raby). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galanter J, Choudhry S, Eng C, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med 2008; 177: 1194–1200. doi: 10.1164/rccm.200711-1644OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol Rev 2013; 255: 25–39. doi: 10.1111/imr.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pervolaraki K, Rastgou Talemi S, Albrecht D, et al. Differential induction of interferon stimulated genes between type I and type III interferons is independent of interferon receptor abundance. PLoS Pathog 2018; 14: e1007420. doi: 10.1371/journal.ppat.1007420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakta NR, Christenson SA, Nerella S, et al. IFN-stimulated gene expression, type 2 inflammation, and endoplasmic reticulum stress in asthma. Am J Respir Crit Care Med 2018; 197: 313–324. doi: 10.1164/rccm.201706-1070OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altman MC, Reeves SR, Parker AR, et al. Interferon response to respiratory syncytial virus by bronchial epithelium from children with asthma is inversely correlated with pulmonary function. J Allergy Clin Immunol 2018; 142: 451–459. doi: 10.1016/j.jaci.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smit LA, Bouzigon E, Pin I, et al. 17q21 variants modify the association between early respiratory infections and asthma. Eur Respir J 2010; 36: 57–64. doi: 10.1183/09031936.00154509 [DOI] [PubMed] [Google Scholar]
- 8.Farzan N, Vijverberg SJ, Hernandez-Pacheco N, et al. 17q21 variant increases the risk of exacerbations in asthmatic children despite inhaled corticosteroids use. Allergy 2018; 73: 2083–2088. doi: 10.1111/all.13499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliskan M, Bochkov YA, Kreiner-Moller E, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 2013; 368: 1398–1407. doi: 10.1056/NEJMoa1211592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui H, Levin AM, Hu D, et al. Mapping the 17q12-21.1 locus for variants associated with early-onset asthma in African Americans. Am J Respir Crit Care Med 2021; 203: 424–436. doi: 10.1164/rccm.202006-2623OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong X, Zeng H, Zhou Z, et al. Structural mechanisms for regulation of GSDMB pore-forming activity. Nature 2023; 616: 598–605. doi: 10.1038/s41586-023-05872-5 [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Shivcharan S, Tian T, et al. Structural basis for GSDMB pore formation and its targeting by IpaH7.8. Nature 2023; 616: 590–597. doi: 10.1038/s41586-023-05832-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q, Shi P, Wang Y, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol 2019; 11: 496–508. doi: 10.1093/jmcb/mjy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panganiban RA, Sun M, Dahlin A, et al. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J Allergy Clin Immunol 2018; 142: 1469–1478. doi: 10.1016/j.jaci.2017.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LQ, Liu T, Yang S, et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat Commun 2021; 12: 2915. doi: 10.1038/s41467-021-23201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levardon H, Yonker LM, Hurley BP, et al. Expansion of airway basal cells and generation of polarized epithelium. Bio-Protoc 2018; 8: e2877. doi: 10.21769/BioProtoc.2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mou H, Vinarsky V, Tata PR, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 2016; 19: 217–231. doi: 10.1016/j.stem.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosser AG, Brockman-Schneider R, Amineva S, et al. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis 2002; 185: 734–743. doi: 10.1086/339339 [DOI] [PubMed] [Google Scholar]
- 19.Walker KH, Krishnamoorthy N, Bruggemann TR, et al. Protectins PCTR1 and PD1 reduce viral load and lung inflammation during respiratory syncytial virus infection in mice. Front Immunol 2021; 12: 704427. doi: 10.3389/fimmu.2021.704427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kothari PH, Qiu W, Croteau-Chonka DC, et al. Role of local CpG DNA methylation in mediating the 17q21 asthma susceptibility gasdermin B (GSDMB)/ORMDL sphingolipid biosynthesis regulator 3 (ORMDL3) expression quantitative trait locus. J Allergy Clin Immunol 2018; 141: 2282–2286. doi: 10.1016/j.jaci.2017.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Croteau-Chonka DC, Qiu W, Martinez FD, et al. Gene expression profiling in blood provides reproducible molecular insights into asthma control. Am J Respir Crit Care Med 2017; 195: 179–188. doi: 10.1164/rccm.201601-0107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson ND, Everman JL, Chioccioli M, et al. Single-cell and population transcriptomics reveal pan-epithelial remodeling in type 2-high asthma. Cell Rep 2020; 32: 107872. doi: 10.1016/j.celrep.2020.107872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajuthi SP, DeFord P, Li Y, et al. Type 2 and interferon inflammation regulate SARS-CoV-2 entry factor expression in the airway epithelium. Nat Commun 2020; 11: 5139. doi: 10.1038/s41467-020-18781-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Willis-Owen SAG, Spiegel S, et al. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med 2019; 199: 478–488. doi: 10.1164/rccm.201803-0438OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ober C, McKennan CG, Magnaye KM, et al. Expression quantitative trait locus fine mapping of the 17q12-21 asthma locus in African American children: a genetic association and gene expression study. Lancet Respir Med 2020; 8: 482–492. doi: 10.1016/S2213-2600(20)30011-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Wang W, Li Y, et al. RIG-I is a key antiviral interferon-stimulated gene against hepatitis E virus regardless of interferon production. Hepatology 2017; 65: 1823–1839. doi: 10.1002/hep.29105 [DOI] [PubMed] [Google Scholar]
- 27.Kang DC, Gopalkrishnan RV, Lin L, et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, MDA-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 2004; 23: 1789–1800. doi: 10.1038/sj.onc.1207300 [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z, He H, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020; 368: eaaz7548. 10.1126/science.aaz7548 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Renner DM, Comar CE, et al. SARS-CoV-2 induces double-stranded RNA-mediated innate immune responses in respiratory epithelial-derived cells and cardiomyocytes. Proc Natl Acad Sci USA 2021; 118: e2022643118. doi: 10.1073/pnas.2022643118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groskreutz DJ, Monick MM, Powers LS, et al. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol 2006; 176: 1733–1740. doi: 10.4049/jimmunol.176.3.1733 [DOI] [PubMed] [Google Scholar]
- 31.Liu T, Zhou YT, Wang LQ, et al. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J Allergy Clin Immunol 2019; 144: 777–787. doi: 10.1016/j.jaci.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 32.Hemmi H, Takeuchi O, Sato S, et al. The roles of two IκB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med 2004; 199: 1641–1650. doi: 10.1084/jem.20040520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Liu Q, Wu Y, et al. Zika virus elicits inflammation to evade antiviral response by cleaving cGAS via NS1-caspase-1 axis. EMBO J 2018; 37: e99347. doi: 10.15252/embj.201899347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu YP, Zeng L, Tian A, et al. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol 2012; 189: 4630–4639. doi: 10.4049/jimmunol.1102737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng L, Liu YP, Sha H, et al. XBP-1 couples endoplasmic reticulum stress to augmented IFN-β induction via a cis-acting enhancer in macrophages. J Immunol 2010; 185: 2324–2330. doi: 10.4049/jimmunol.0903052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu B, Peisley A, Richards C, et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 2013; 152: 276–289. doi: 10.1016/j.cell.2012.11.048 [DOI] [PubMed] [Google Scholar]
- 37.Soliai MM, Kato A, Helling BA, et al. Multi-omics colocalization with genome-wide association studies reveals a context-specific genetic mechanism at a childhood onset asthma risk locus. Genome Med 2021; 13: 157. doi: 10.1186/s13073-021-00967-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao F. Gasdermins: making pores for pyroptosis. Nat Rev Immunol 2021; 21: 620–621. doi: 10.1038/s41577-021-00602-2 [DOI] [PubMed] [Google Scholar]
- 39.Rana N, Privitera G, Kondolf HC, et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 2022; 185: 283–298. doi: 10.1016/j.cell.2021.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravi A, Koster J, Dijkhuis A, et al. Interferon-induced epithelial response to rhinovirus 16 in asthma relates to inflammation and FEV1. J Allergy Clin Immunol 2019; 143: 442–447. doi: 10.1016/j.jaci.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 41.Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50: 975–991. doi: 10.1016/j.immuni.2019.03.018 [DOI] [PubMed] [Google Scholar]
- 42.Li X, Christenson SA, Modena B, et al. Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol 2021; 147: 894–909. doi: 10.1016/j.jaci.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material: methods, tables, and detailed captions for supplementary figures. ERJ-01232-2023.Supplement (285.3KB, pdf)
Supplementary figure E1. ORMDL3 has no correlation with ISGs in asthmatic epithelial cells. ERJ-01232-2023.Figure_E1 (320KB, jpg)
Supplementary figure E2. GSDMB promotes interferon expression. ERJ-01232-2023.Figure_E2 (404.2KB, jpg)
Supplementary figure E3. GSDMB promotes MAVS-TBK1 signalling upstream of TBK1. ERJ-01232-2023.Figure_E3 (119.5KB, jpg)
Supplementary figure E4. The regulation of GSDMB on the MAVS-TBK1 signalling is independent of pyroptosis. ERJ-01232-2023.Figure_E4 (464.3KB, jpg)
Supplementary figure E5. GSDMB promotes MAVS-TBK1 signalling independent of ER stress. ERJ-01232-2023.Figure_E5 (149.6KB, jpg)
Supplementary figure E6. Knockout of GSDMB decreases IFN response. ERJ-01232-2023.Figure_E6 (235.1KB, jpg)
Supplementary figure E7. The functions of GSDMB on MAVS-TBK1 signalling are independent of MDA5 and RIG-I. ERJ-01232-2023.Figure_E7 (317KB, jpg)
Supplementary figure E8. Virtual ligand screening of GSDMB indicating potential binding of GSDMB with nucleic acid. ERJ-01232-2023.Figure_E8 (113.7KB, jpg)
Supplementary figure E9. Confirmation of Cc10-hGSDMB mice. ERJ-01232-2023.Figure_E9 (755.4KB, jpg)
Supplementary figure E10. GSDMB enhances RSV-induced IFN response and inflammation. ERJ-01232-2023.Figure_E10 (1.2MB, jpg)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01232-2023.Shareable (586.8KB, pdf)