Abstract
Background
Canine babesiosis is a clinically significant tick‐transmitted disease caused by several species of the intraerythrocytic protozoan parasite Babesia, which result in a wide range of clinical manifestations, from mild, transient infection to serious disease and even death.
Objectives
The current study aimed to estimate the global prevalence and associated risk factors of Babesia in dogs.
Methods
Multiple databases (PubMed, Scopus, ProQuest, Web of Science and Google Scholar) were searched for relevant literature published from January 2000 up to December 2022. The statistical analyses were performed based on the R software (version 3.6) meta‐package.
Results
Out of 23,864 publications, 229 studies met the inclusion criteria. The pooled prevalence of canine babesiosis was 0.120 (95% CI; 0.097–0.146). The highest pooled prevalence was found in Europe (0.207, 95% CI; 0.097–0.344). Among several species, Babesia canis was the most prevalent parasite (0.216, 95% CI; 0.056–0.441). The highest pooled prevalence of Babesia in dogs was observed in the summer season (0.097, 95% CI; 0.040–0.174).
Conclusions
Regular screening and appropriate control strategies are recommended for the prevention of transmission of tick‐borne disease transmission among dogs.
Keywords: canine babesiosis, meta‐analysis, tick‐borne diseases, worldwide
Canine babesiosis is a clinically significant tick‐transmitted disease caused by several species of Babesia parasites. Babesia canis was the most prevalent parasite. Owned dogs had higher prevalence rate than stray/shelter dogs.
1. INTRODUCTION
Vector‐borne diseases (VBDs) are included among the emerging and re‐emerging infections, representing health concern for humans, livestock, wildlife, and companion animals (Kuleš et al., 2017). They cause significant economic losses due to high mortality rates and, as a result, decreases profit in the global livestock industry (Lew‐Tabor & Valle, 2016).
Several factors, including global development, urbanization, climate change, increased international trade, and animal travel and mobility, influence the epidemiology and distribution of VBDs (Baneth et al., 2012; Harrus & Baneth, 2005). Ticks are well‐known vectors for a broad range of microbial pathogens of both public health and veterinary importance (Bajer, Kowalec, et al., 2022; Efstratiou et al., 2021).
Babesiosis is a globally distributed tick‐borne disease caused by intra‐erythrocytic protozoa Babesia (Solano‐Gallego et al., 2016). In 1895, the disease was first observed in dogs in northern Italy (Penzhorn, 2020). The life cycle of Babesia parasites develops through the transmission of sporozoites from the salivary glands of ixodid ticks (the main vectors) to their vertebrate hosts, where the merozoites appear in red blood cells after the occurrence of asexual replication (merogony) (Antunes et al., 2017; Jalovecka et al., 2019; Vannier & Krause, 2020).
Dogs are considered the most frequent companion animals worldwide. The transmission of zoonotic pathogens from these animals to the human population is an inevitable concern (Dantas‐Torres et al., 2020; Eslahi et al., 2021; Omidinia et al., 2020). However, the canine Babesia species are assumed to have no zoonotic importance.
Currently Babesia vogeli, B. canis and Babesia rossi are categorized as the large species of canine Babesia (Panti‐May & Rodiguez‐Vivas, 2020). These three species that previously were described as sub‐species are distinct genetically and also differ in the severity of clinical symptoms that they cause, their tick vectors and geographic distribution (Depoix et al., 2002; Solano‐Gallego & Baneth, 2011; Zahler et al., 1998). Until now, three small species of Babesia, including B. gibsoni, B. conradae and B. vulpes, are recognized to infect dogs (Teodorowski et al., 2022). The occurrence of non‐canine Babesia (B. caballi), which is most dominantly found in horses is confirmed to infect dogs (Beck et al., 2009).
Although there have been recent studies estimating the regional and global prevalence of babesiosis, there is no comprehensive study for the disease in dogs on a worldwide scale. Therefore, this review and meta‐analysis aimed to estimate the global prevalence of babesiosis in dogs and assess the associated risk factors.
2. MATERIALS AND METHODS
2.1. Search strategy
The present study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis checklist (PRISMA) (Page et al., 2021). The searching process was performed using multiple databases (Scopus, PubMed, ProQuest, Web of Science, and Google Scholar).Moreover, a hand search was carried out for the articles that were published from 2000 until December 2022. Search terms using AND and/or OR Boolean operators were as follows: Babesia spp., Babesiosis, B. vogeli, B. canis, B. rossi, B. gibsoni, B. conradae, B. vulpes, B. caballi, tick‐borne protozoan diseases, tick‐borne pathogens, blood protozoan parasites, dog, puppies, prevalence, frequency, global, worldwide. In addition to removing duplicates and irrelevant papers, the reference lists of the collected publications were checked for further studies that could not be located through database searches. The assessment of full‐text articles as well as the screening of the titles and abstracts of each article was performed independently by two authors.
2.2. Screening and eligibility of the study
All retrieved articles were primarily imported into the EndNote citation manager software (version 8, Thomson Reuters, Stamford, CT, USA) to sort and eliminate the duplicates. A Microsoft Excel version 2016 was applied to collect the following details from retrieved articles: first author's name, year of publication, countries, continent, time of sampling, sample size, number of positive samples, type of Babesia, climate (https://www.britannica.com/science/Koppen‐climate‐classification), seasons, diagnostic method (s) and stray/animal shelter dogs (Table S1, Figure S4 and Table 1).
TABLE 1.
Sub‐group analysis based on seasons, diagnostic method, and stray/animal shelter dogs in included studies.
| Variables | No studies | Sample size | Infected | Pooled prevalence (95% CI) | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 | τ 2 | p‐Value | |||||
| Seasons | |||||||
| Spring | 12 | 13704 | 551 | 0.080 (0.030–0.149) | 95 | 0.034 | <0.001 |
| Summer | 10 | 12367 | 396 | 0.097 (0.040–0.174) | 97 | 0.030 | <0.001 |
| Autumn | 9 | 12511 | 363 | 0.085 (0.022–0.179) | 95 | 0.042 | <0.001 |
| Winter | 10 | 12252 | 527 | 0.089 (0.023–0.188) | 96 | 0.049 | <0.001 |
| Diagnostic method | |||||||
| Blood smear | 36 | 54871 | 3322 | 0.128 (0.074–0.194) | 98 | 0.073 | <0.001 |
| ELISA | 8 | 4086 | 406 | 0.108 (0.069–0.154) | 89 | 0.008 | <0.001 |
| IFA | 18 | 11617 | 1245 | 0.198 (0.115–0.297) | 99 | 0.059 | <0.001 |
| PCR | 110 | 44502 | 4054 | 0.117 (0.088–0.149) | 98 | 0.059 | <0.001 |
| Stray/animal shelter dogs | |||||||
| Stray/animal shelter dogs | 27 | 5302 | 459 | 0.116 (0.066–0.178) | 95 | 0.044 | <0.001 |
| Owned dogs | 76 | 28113 | 3306 | 0.139 (0.104–0.180) | 98 | 0.054 | <0.001 |
Abbreviations: ELISA, enzyme‐linked immunosorbent assays; IFA, indirect fluorescent antibody.
2.3. Inclusion and exclusion criteria
The inclusion criteria considered for the current study were as follows: (1) All published observational studies (cross‐sectional, case–control and cohort) reporting the prevalence of Babesia in dogs, (2) availability of full‐text and abstract in English, (3) peer‐reviewed original articles, (4) availability of data regarding the total sample size and the number of positive cases, (5) articles published until December 2022. Those studies with sample size lower than 30, papers with non‐original data, review articles, case reports, case series, letters, editorials, publications with unclear or undetermined results were excluded from the analysis of the present study. Furthermore, those papers that reported Babesia infection in humans and in animals other than dogs were excluded.
2.4. Quality assessment
The included studies were evaluated for quality using the Newcastle‐Ottawa Scale (Table S2) (Eslahi et al., 2023; Modesti et al., 2016). Three factors made up the scoring system: selection (maximum of five stars), comparability (maximum of two stars) and result (maximum of three stars).
2.5. Data synthesis and statistical analysis
The global pooled prevalence of Babesia in dogs was estimated with a 95% confidence interval. To estimate the pooled prevalence, a Freeman–Tukey double arcsine transformation was applied using a random‐effects model. We employed Begg's rank test to identify potential publication bias. Additionally, publication bias was assessed using the Luis Furuya‐Kanamori (LFK) index and the Doi plot (Barendregt & Doi, 2016). In order to specify the impact of year of publication on the prevalence, a meta‐regression analysis was applied. An LFK index within the range of outside ±2, ±2 and ±1 is regarded as significantly/major asymmetrical, slightly/minor asymmetrical and asymmetrical symmetrical (absence of publication bias), respectively. A Freeman–Tukey double arcsine transformation for the random‐effects model was applied to calculate the overall prevalence. In order to assess the magnitude of heterogeneity among included studies, cochrane's Q test and inconsistency index (I 2 statistics) was used considering I 2 values of 25, 50 and 75% as low, medium and high heterogeneity, respectively. A p‐value lower than 0.05 was interpreted as statistically significant. All statistical analyses conducted herein were based on meta‐package of R (version 3.6.1) (Team, 2020).
3. RESULTS
3.1. Literature search selection and data extraction
The systematic search performed in the current study yielded a total of 23,864 articles. Totally, 292 full‐text papers were considered to be evaluated for eligibility. Among these publications, we excluded 5 studies due to insufficient data, 6 studies with overlapping data, 7 studies with sample size lower than 30 and 45 studies with no original data, including letters, reviews, workshops and theses. Finally, we included 229 papers, which were eligible according to the critical appraisal criteria (Figure 1).
FIGURE 1.
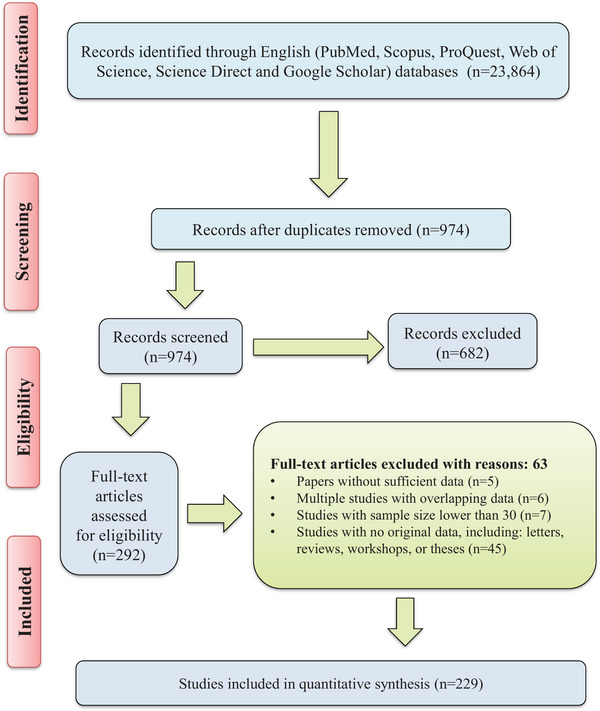
Flow diagram of the study design process.
3.2. Pooled prevalence
The global pooled prevalence of Babesia in dogs was 0.120 (95% CI; 0.097–0.146) with a higher estimated pooled prevalence in owned dogs (0.139, 95% CI; 0.104–0.180) than stray/shelter dogs (0.116, 95% CI; 0.066–0.178) (Figure S1 and Table 1). The prevalence of Babesia in dogs has been documented in 61 countries of the world. A highest number of publications were related to India (29 studies). Our country‐based analysis showed that Slovakia (0.908, 95% CI; 0.826–0.948) following Bosnia and Herzegovina (0.825, 95% CI; 0.730–0.897) showed the highest pooled prevalence (Figure S2). A map was created using QGIS3 software (https://qgis.org/en/site/) to demonstrate the prevalence of Babesia in dogs in different geographical regions of the world (Figure 2).
FIGURE 2.
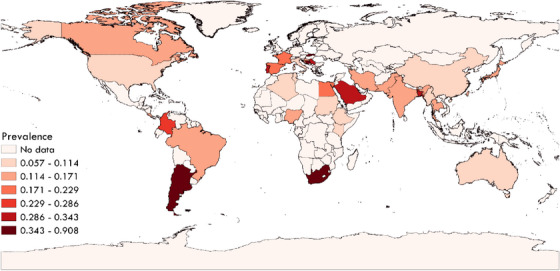
Global prevalence of Babesia in dogs in different geographical regions of the world based on the included studies.
The continent‐based estimates ranged from 0.207% to 0.074% that included a prevalence rate of 0.207 (99% CI; 0.097–0.344) for Europe, 0.135 (95% CI; 0.079–0.202) for South America, 0.104 (95% CI; 0.065–0.152) for Africa, 0.103 (95% CI; 0.062–0.154) for North America, 0.097 (95% CI; 0.072–0.126) for Asia and 0.074 (95% CI; 0.043–0.112) for Oceania (Figure S3). The present study showed that the highest pooled prevalence was attributable to the regions with tropical wet climate (0.156, 95% CI; 0.081–0.249), especially in summer (0.097, 95% CI; 0.040–0.174) (Figure S4 and Table 1). The global pooled prevalence of Babesia in dogs based on species or genus was as follows; 0.216 (95% CI; 0.056–0.441) for B. canis, 0.101 (95% CI; 0–0.677) for B. conradea, 0.089 (95% CI; 0.021–0.193) for B. rossi, 0.088 (95% CI; 0.065–0.113) for B. gibsoni, 0.063 (95% CI; 0.045–0.083) for B. vogeli, 0.035 (95% CI; 0.001–0.105) for B. vulpes and 0.005 (95% CI; 0–0.019) for B. caballi (Figure S5).
The estimated pooled prevalence based on reports of Babesia spp. in dogs was 0.123 (95% CI; 0.093–0.155) (Figure S6). The prevalence of Babesia spp. in dogs in different continents ranged from 0.189 to 0.023 including 0.189 (95% CI; 0.101–0.296) for South America, 0.154 (95% CI; 0.094–0.225) for Africa, 0.149 (95% CI; 0.090–0.219) for Europe, 0.133 (95% CI; 0.063–0.223) for Oceania, 0.058 (95% CI; 0.026–0.101) for Asia and 0.023 (95% CI; 0.001–0.063) for North America (Figure S7).
The highest pooled prevalence based on the detection method was related to the studies that utilized indirect fluorescent antibody (IFA) (0.198, 95% CI; 0.115–0.297) (Table 1).
3.3. Meta‐regression, publication bias and quality assessment
The meta‐regression analysis indicated that there was a statistically significant correlation between prevalence and year of publication (slop: 15.93, p < 0.05) (Figure S8).
There was a major asymmetry in the Doi plot (LFK index: 3.04). Furthermore, a highly significant publication bias was detected using the Linear regression plot (t = 6.03, p < 0.0001) (Figure S9).
The finding of the quality assessment indicated that, among 229 studies, 46 had a total score of 4–6 points (moderate level), and 183 had a total score of 7–9 points (high level) (Table S2).
4. DISCUSSION
The current systematic review and meta‐analysis study brings together, for the first time, true global prevalence data on Babesia parasites in dogs.
Slovakia followed by Bosnia and Herzegovina were the regions with highest prevalence of babesiosis in dogs, which was expected, as previous investigations indicated that B. canis is the commonly detected species in symptomatic dogs in European regions (Ćoralić et al., 2018). However, these estimates based on the country presented herein must be interpreted cautiously as they were related to single study for each country.
Babesiosis is a serious infection in dogs in subtropical and tropical regions (Kuo et al., 2020). B. canis occurs in the countries with a temperate climate, especially as Europe (Aktas et al., 2015; Øines et al., 2010). Similarly, the findings of the meta‐analysis presented herein suggested that climatic conditions have a substantial role in the prevalence of babesiosis in dogs with the highest pooled prevalence in regions with a tropical wet climate. Temperature and humidity are the potential factors that are advantageous to cause increased prevalence in tropical regions, as higher humidity and temperature are favorable to the development of life cycle of both vectors and parasites.
B. canis, the most prevalent parasite observed in the study, is the cause of a great number of clinical babesiosis cases in dogs in Europe (Solano‐Gallego et al., 2016). The clinical presentations associated with this species may vary from mild transient disease to acute illness with potential of mortality. Most of the canine babesiosis cases occurred in the central Europe revealed that they were complicated with a high mortality rate (Matijatko et al., 2012).
Europe was the continent with highest pooled prevalence. B. canis is endemic in the central Europe, and Baltic region. Germany and Poland from central Europe as well as Lithuania and Latvia from Baltic region are the newly documented endemic regions for canine babesiosis (Berzina et al., 2013; Paulauskas et al., 2014; Schäfer et al., 2021; Seleznova et al., 2020).
However, in central and northeast European regions, the disease is an emerging one (Bajer, Beck, et al., 2022; Pawełczyk et al., 2022). The geographical distribution of Babesia parasites in these regions is remarkably diverse, which highly depends on the distribution of the vector, the type of detection techniques, the species of Babesia, the country and cases under investigation (Solano‐Gallego et al., 2016). The fast growth of tourism with companion animals, particularly domestic dogs and cats, might have also contributed to the recent trend of increasing the prevalence of canine babesiosis in Europe. Moreover, it can be due to the diagnostic methods with higher sensitivity (e.g. molecular‐based techniques), which are applied in veterinary clinics (Pawełczyk et al., 2022). In European regions, the Dermacentor reticulatus is the vector for transmission of Babesia species in dogs and the spread of B. canis to new regions have a significant relationship with the wide distribution of this tick species (Drehmann et al., 2020; Dwużnik‐Szarek et al., 2021; Hornok et al., 2016; Rubel et al., 2016).
Higher prevalence of the infection was observed in dogs in summer. The peak activity of D. reticulatus is at the end of the spring and autumn. However, a highest number of clinical cases of the infection were reported in spring in Central Europe (Hornok et al., 2016). It is proposed that in environments with similar ecological features, the seasonal pattern of canine babesiosis is not solely determined by the availability of the appropriate vector. It is also affected by the early activity of ticks infected with Babesia (Hornok et al., 2016). In addition, D. reticulatus was also known to exist in western Slovakia (Majlathova et al., 2011), the country with the highest pooled prevalence for canine babesiosis in our study. The vectors responsible for the transmission of the Babesia species are as follows; Haemaphysalis elliptica (and probably H. leachi) for B. rossi (Penzhorn, 2020; Penzhorn et al., 2020), Rhipicephalus sanguineus for B. vogeli (Penzhorn, 2020), H. longicornis for B. gibsoni (Liu et al., 2018) and probably D. reticulatus for B. caballi (Daněk et al., 2022). Further studies are required for the identification of potential vector of B. conradae. However, the parasite was detected in the salivary glands of R. sanguineus (Dear et al., 2018; Liu et al., 2018).
The recent changes in the spread of D. reticulatus to new endemic regions of the world apparently reflects the important role of climate change and the possibility that specific local climatic conditions are responsible for the abrupt seasonal variations that changes the incidence of babesiosis in dogs (Leschnik et al., 2008). South America that showed the highest prevalence rate showcases a range of weather and climate conditions, incorporating tropical, subtropical and extratropical characteristics (Garreaud et al., 2009).
The occurrence of Babesia spp. in dogs of Latin America and the Caribbean exhibited considerable variability. The prevalence rates were 1.4% in Peru (Temoche et al., 2018), 2.2% in Venezuela (Criado‐Fornelio et al., 2007), 3.3% in Brazil (Silva et al., 2012), 5.5% in Colombia (Vargas‐Hernández et al., 2012) and 7.7% in Argentina (Mascarelli et al., 2016). In contrast, higher prevalence rates were reported in Nicaragua (15.4%) (Wei et al., 2014) and Brazil (23.4%) (Jojima, 2008). The lack of awareness regarding animal welfare and disease issues, economic constraints leading to restricted access to proper veterinary care and the absence of responsible practices in pet ownership are the factors that facilitated the transmission and persistence of tick‐borne diseases in this region. Moreover, socioeconomic and ecological elements, such as globalization, the rise in international trade, tourism and travel, climate change impacts, heightened mobility of dogs, alterations in landscape use and interactions with wildlife, have altered both the distribution of ticks and the patterns of infection for canine babesiosis (Panti‐May & Rodiguez‐Vivas, 2020).
The overall prevalence of Babesia parasites was higher in owned dogs than in stray/shelter dogs. This could be attributed to the comparatively higher number of studies documenting infections in owned dogs. Furthermore, the unrestrained access of owned dogs to public areas, infrequent application of ectoparasiticides and the advanced monitoring of owned dogs are among the factors that could potentially raise the detection rate and reduce the underestimation of infection cases in these animals compared to stray dogs.
Babesiosis is generally associated with fever, mild‐to‐severe anemia and thrombocytopenia, enlarged lymph nodes and spleen, jaundice and pigmenturia. The range of clinical signs and the severity of the disease is highly related to the species of Babesia causing infection along with the other factors such as age, concurrent infection and immune‐compromised situation (e.g. splenectomy and immunosuppressive treatment). The regular manifestations in canine babesiosis are anorexia, lethargy, weakness, pale mucous membranes, hypoalbuminemia and hyperbilirubinemia (Irwin, 2009; Solano‐Gallego et al., 2016; Solano‐Gallego & Baneth, 2011).
In our findings, the highest prevalence was related to IFA method. Despite the probable occurrence of cross‐reactivity between different Babesia species and other protozoa, IFA and the enzyme‐linked immunosorbent assays (ELISA) are commercially available and frequently utilized for diagnosis of babesiosis in dogs (Solano‐Gallego & Baneth, 2011). The assessment through IFA identifies antibodies against Babesia in the blood samples of animals that either are infected or have been exposed to the pathogen. It stands out as the most sensitive indirect approach for identifying occult and chronic babesiosis, as well as instances of low‐level parasitemia (Bicalho et al., 2004; Hartmann et al., 2013). Despite the appropriate sensitivity and ease of application provided by IFA, its specificity is reduced (Alvarez et al., 2019). The absence of standardized antigenic targets, potential cross‐reactivity and the difficulties in determination of positivity thresholds are the drawbacks (Garcia et al., 2022). For the accurate detection of B. canis, rBcMSA1 and rBcSA1 exhibit promising serodiagnostic antigens for indirect ELISA and rapid immunochromatographic tests (ICTs) (Zhou et al., 2016). Moreover, thrombospondin‐related adhesive protein (TRAP) is a recombinant protein derived from B. gibsoni can be used as an alternative for whole parasite antigen due to its high sensitivity and specificity (Solano‐Gallego & Baneth, 2011). Among the different molecular approaches, cPCR, nested PCR and multiplex PCR assays are considered as main methods with regard to the quality in detection of DNA in blood samples along with a newly customized portable real‐time PCR platform (Galon et al., 2022; Kuo et al., 2020).
5. LIMITATIONS
The present study has the following limitations:
The analyses presented herein may have been impacted by publication bias due to the lack of data or the limited number of published literatures from some geographic regions.
The present study was limited to publications in English language.
A significant number of studies included herein, used direct blood smear as a diagnostic method for detecting parasites, which may be associated with lower sensitivity and specificity and a high number of reports that did not specifically detect.
The parasite at the species level.
Despite these limitations, the current study provides the most comprehensive estimates of the prevalence of Babesia in dogs from a global perspective.
6. CONCLUSION
Understanding and addressing the risk factors associated with Babesia in dogs are crucial for an effective prevention and management of the infection. Geographical location can increase the risk of infection in dogs, as certain regions have a higher prevalence of ticks carrying Babesia parasites.
The findings of the present study underscore the need for investigations in a broader range of geographical areas. Dogs in regions with a warm humid continental climate showed a higher prevalence of the infection, emphasizing the necessity of sufficient strategies for animal health and biosecurity measures in these regions. The infection was most prevalent in owned dogs, which is crucial for owners to implement effective tick prevention measures, maintain awareness of regional risks, and seek veterinary care promptly if any symptoms of babesiosis are observed. Regular check‐ups and consultations with veterinarians can also contribute to the early detection and management of the disease. The limitations of diagnostic techniques and the standardization of current methods must also be taken into account by reference laboratories. The surveillance and control sectors should consider the priorities of each region, as they may have substantial differences between developed and developing countries.
AUTHOR CONTRIBUTIONS
Conceptualization: Aida Vafae Eslahi, Milad Badri and Panagiotis Karanis. Data curation: Meysam Olfatifar, Aida Vafae Eslahi and Milad Badri. Formal analysis: Meysam Olfatifar. Funding acquisition: Milad Badri. Investigation: Amir Abdoli, Leila Zaki, Behzad Bijani, Majid Pirestani and Kareem Hatam‐Nahavandi. Methodology: Meysam Olfatifar, Aida Vafae Eslahi, Leila Zaki and Milad Badri. Project administration: Amir Abdoli, Aida Vafae Eslahi, Milad Badri and Panagiotis Karanis. Software: Meysam Olfatifar. Supervision: Amir Abdoli, Milad Badri and Panagiotis Karanis. Validation: Meysam Olfatifar and Milad Badri. Visualization: Milad Badri and Panagiotis Karanis. Writing—original draft: Amir Abdoli, Aida Vafae Eslahi, Milad Badri and Panagiotis Karanis. Writing—review and editing: Aida Vafae Eslahi, Milad Badri and Panagiotis Karanis.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflicts of interest concerning the research or authorship.
FUNDING INFORMATION
Medical Microbiology Research Center, Qazvin University of Medical Sciences, Qazvin, Iran, under the contract no.: IR.QUMS.REC.1401.351
ETHICS STATEMENT
Ethical approval was required and provided for this study, as stated by our institutional review board.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1427.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Abdoli, A. , Olfatifar, M. , Badri, M. , Zaki, L. , Bijani, B. , Pirestani, M. , Hatam‐Nahavandi, K. , Eslahi, A. V. , & Karanis, P. (2024). A global systematic review and meta‐analysis on the babesiosis in dogs with special reference to Babesia canis . Veterinary Medicine and Science, 10, e1427. 10.1002/vms3.1427
Amir Abdoli, Behzad Bijani and Leila Zaki contributed equally to this work.
Contributor Information
Milad Badri, Email: badri22.milad@gmail.com.
Aida Vafae Eslahi, Email: Vafaeeslahia@gmail.com.
Panagiotis Karanis, Email: Karanis.p@unic.ac.cy.
DATA AVAILABILITY STATEMENT
All data are included in the manuscript or as supplementary files.
REFERENCES
- Aktas, M. , Özübek, S. , Altay, K. , Ipek, N. D. S. , Balkaya, İ. , Utuk, A. E. , Kırbas, A. , Şimsek, S. , & Dumanlı, N. (2015). Molecular detection of tick‐borne rickettsial and protozoan pathogens in domestic dogs from Turkey. Parasites & Vectors, 8, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, J. A. , Rojas, C. , & Figueroa, J. V. (2019). Diagnostic tools for the identification of Babesia sp. in persistently infected cattle. Pathogens, 8, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes, S. , Rosa, C. , Couto, J. , & Ferrolho, J. , Domingos, A. (2017). Deciphering Babesia–vector interactions. Frontiers in Cellular and Infection Microbiology, 7, 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer, A. , Beck, A. , Beck, R. , Behnke, J. M. , Dwużnik‐Szarek, D. , Eichenberger, R. M. , Farkas, R. , Fuehrer, H.‐P. , Heddergott, M. , Jokelainen, P. , Leschnik, M. , Oborina, V. , Paulauskas, A. , Radzijevskaja, J. , Ranka, R. , Schnyder, M. , Springer, A. , Strube, C. , Tolkacz, K. , & Walochnik, J. (2022). Babesiosis in southeastern, central and northeastern Europe: An emerging and re‐emerging tick‐borne disease of humans and animals. Microorganisms, 10, 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajer, A. , Kowalec, M. , Levytska, V. A. , Mierzejewska, E. J. , Alsarraf, M. , Poliukhovych, V. , Rodo, A. , Wkeżyk, D. , & Dwużnik‐Szarek, D. (2022). Tick‐borne pathogens, Babesia spp. and Borrelia burgdorferi sl, in sled and companion dogs from central and north‐eastern Europe. Pathogens, 11, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baneth, G. , Bourdeau, P. , Bourdoiseau, G. , Bowman, D. , Breitschwerdt, E. , Capelli, G. , Cardoso, L. , Dantas‐Torres, F. , Day, M. , Dedet, J.‐P. , Dobler, G. , Ferrer, L. , Irwin, P. , Kempf, V. , Kohn, B. , Lappin, M. , Little, S. , Maggi, R. , Miró, G. , … Weston, S. (2012). Vector‐borne diseases‐constant challenge for practicing veterinarians: Recommendations from the CVBD World Forum. Parasites & Vectors, 5, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt, J. J. , & Doi, S. A. (2016). MetaXL user guide (Version, 4, 2011–2016). MetaXL. [Google Scholar]
- Beck, R. , Vojta, L. , Mrljak, V. , Marinculić, A. , Beck, A. , Živičnjak, T. , & Cacciò, S. M. (2009). Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. International Journal for Parasitology, 39, 843–848. [DOI] [PubMed] [Google Scholar]
- Berzina, I. , Capligina, V. , Baumanis, V. , Ranka, R. , Cirule, D. , & Matise, I. (2013). Autochthonous canine babesiosis caused by Babesia canis canis in Latvia. Veterinary Parasitology, 196, 515–518. [DOI] [PubMed] [Google Scholar]
- Bicalho, K. A. , Ribeiro, M. F. B. , & Martins‐Filho, O. A. (2004). Molecular fluorescent approach to assessing intraerythrocytic hemoprotozoan Babesia canis infection in dogs. Veterinary Parasitology, 125, 221–235. [DOI] [PubMed] [Google Scholar]
- Ćoralić, A. , Gabrielli, S. , Zahirović, A. , Stojanović, N. M. , Milardi, G. L. , Jažić, A. , Zuko, A. , Čamo, D. , & Otašević, S. (2018). First molecular detection of Babesia canis in dogs from Bosnia and Herzegovina. Ticks and Tick‐Borne Diseases, 9, 363–368. [DOI] [PubMed] [Google Scholar]
- Criado‐Fornelio, A. , Rey‐Valeiron, C. , Buling, A. , Barba‐Carretero, J. C. , Jefferies, R. , & Irwin, P. (2007). New advances in molecular epizootiology of canine hematic protozoa from Venezuela, Thailand and Spain. Veterinary Parasitology, 144, 261–269. [DOI] [PubMed] [Google Scholar]
- Daněk, O. , Hrazdilová, K. , Kozderková, D. , Jirkuu, D. , & Modr`y, D. (2022). The distribution of Dermacentor reticulatus in the Czech Republic re‐assessed: Citizen science approach to understanding the current distribution of the Babesia canis vector. Parasites & Vectors, 15, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantas‐Torres, F. , Alves, L. C. , & Uilenberg, G. (2017). Babesiosis. In Marcondes, C. B. (Ed.), Arthropod borne diseases (1st ed., pp. 347–354). Springer. [Google Scholar]
- Dantas‐Torres, F. , Ketzis, J. , Mihalca, A. D. , Baneth, G. , Otranto, D. , Tort, G. P. , Watanabe, M. , Linh, B. K. , Inpankaew, T. , Castro, P. D. J. , Borrás, P. , Arumugam, S. , Penzhorn, B. L. , Ybañez, A. P. , Irwin, P. , & Traub, R. J. (2020). TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Veterinary Parasitology, 283, 109167. [DOI] [PubMed] [Google Scholar]
- Dear, J. D. , Owens, S. D. , Lindsay, L. L. , Biondo, A. W. , Chomel, B. B. , Marcondes, M. , & Sykes, J. E. (2018). Babesia conradae infection in coyote hunting dogs infected with multiple blood‐borne pathogens. Journal of Veterinary Internal Medicine, 32, 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoix, D. , Carcy, B. , Jumas‐Bilak, E. , Pages, M. , Precigout, E. , Schetters, T. P. M. , Ravel, C. , & Gorenflot, A. (2002). Chromosome number, genome size and polymorphism of European and South African isolates of large Babesia parasites that infect dogs. Parasitology, 125, 313–321. [DOI] [PubMed] [Google Scholar]
- Drehmann, M. , Springer, A. , Lindau, A. , Fachet, K. , Mai, S. , Thoma, D. , Schneider, C. R. , Chitimia‐Dobler, L. , Bröker, M. , & Dobler, G. , Mackenstedt, U. , & Strube, C. (2020). The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—Evidence of a continuing spread of Dermacentor reticulatus . Frontiers in Veterinary Science, 7, 578220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwużnik‐Szarek, D. , Mierzejewska, E. J. , Rodo, A. , Goździk, K. , Behnke‐Borowczyk, J. , Kiewra, D. , Kartawik, N. , & Bajer, A. (2021). Monitoring the expansion of Dermacentor reticulatus and occurrence of canine babesiosis in Poland in 2016–2018. Parasites & Vectors, 14, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiou, A. , Karanis, G. , & Karanis, P. (2021). Tick‐borne pathogens and diseases in Greece. Microorganisms, 9, 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslahi, A. V. , Mowlavi, G. , Houshmand, E. , Pirestani, M. , Majidiani, H. , Nahavandi, K. H. , Johkool, M. G. , & Badri, M. (2021). Occurrence of Dioctophyme renale (Goeze, 1782) in road‐killed canids of Iran and its public health implication. Veterinary Parasitology, Regional Studies and Reports, 24, 100568. [DOI] [PubMed] [Google Scholar]
- Eslahi, A. V. , Olfatifar, M. , Zaki, L. , Pirestani, M. , Sotoodeh, S. , Farahvash, M. A. , Maleki, A. , & Badri, M. (2023). The worldwide prevalence of intestinal helminthic parasites among food handlers: A systematic review and meta‐analysis. Food Control, 148, 109658. [Google Scholar]
- Galon, E. M. , Zafar, I. , Ji, S. , Li, H. , Ma, Z. , & Xuan, X. (2022). Molecular reports of ruminant Babesia in southeast Asia. Pathogens, 11, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, K. , Weakley, M. , Do, T. , & Mir, S. (2022). Current and future molecular diagnostics of tick‐borne diseases in cattle. Veterinary Sciences, 9, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreaud, R. D. , Vuille, M. , Compagnucci, R. , & Marengo, J. (2009). Present‐day south American climate. Palaeogeography, Palaeoclimatology, Palaeoecology, 281, 180–195. [Google Scholar]
- Harrus, S. , & Baneth, G. (2005). Drivers for the emergence and re‐emergence of vector‐borne protozoal and bacterial diseases. International Journal for Parasitology, 35, 1309–1318. [DOI] [PubMed] [Google Scholar]
- Hartmann, K. , Addie, D. , Belák, S. , Boucraut‐Baralon, C. , Egberink, H. , Frymus, T. , Gruffydd‐Jones, T. , Hosie, M. J. , Lloret, A. , Lutz, H. , Marsilio, F. , Möstl, K. , Pennisi, M. G. , Radford, A. D. , Thiry, E. , Truyen, U. , & Horzinek, M. C. (2013). Babesiosis in cats: ABCD guidelines on prevention and management. Journal of Feline Medicine and Surgery, 15, 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornok, S. , Kartali, K. , Takács, N. , & Hofmann‐Lehmann, R. (2016). Uneven seasonal distribution of Babesia canis and its two 18S rDNA genotypes in questing Dermacentor reticulatus ticks in urban habitats. Ticks and Tick‐Borne Diseases, 7, 694–697. [DOI] [PubMed] [Google Scholar]
- Irwin, P. J. (2009). Canine babesiosis: From molecular taxonomy to control. Parasites & Vectors, 2, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalovecka, M. , Sojka, D. , Ascencio, M. , & Schnittger, L. (2019). Babesia life cycle‐when phylogeny meets biology. Trends in Parasitology, 35, 356–368. [DOI] [PubMed] [Google Scholar]
- Jojima . (2008). Ocorrência e caracterização molecular de espécies de Babesia em cães de uma população hospitalar da região de Londrina, PR. Revista Brasileira de Parasitologia Veterinária, 17, 277–283. [PubMed] [Google Scholar]
- Kuleš, J. , Potocnakova, L. , Bhide, K. , Tomassone, L. , Fuehrer, H.‐P. , Horvatić, A. , Galan, A. , Guillemin, N. , Nižić, P. , Mrljak, V. , & Bhide, M. (2017). The challenges and advances in diagnosis of vector‐borne diseases: where do we stand? Vector‐Borne and Zoonotic Diseases, 17, 285–296. [DOI] [PubMed] [Google Scholar]
- Kuo, C. Y. , Zhao, C. , Cheng, T. , Tsou, C. C. , Li, Y. C. , Zhang, Y. , Hsieh, M. C. , Haung, S.‐B. , & Chen, W.‐Y. (2020). Rapid identification of Babesia canis and Babesia gibsoni (Asian genotype) in canine blood samples using a customized portable real‐time PCR analyzer and TaqMan‐based assay. Ticks and Tick‐borne Diseases, 11, 101362. [DOI] [PubMed] [Google Scholar]
- Leschnik, M. , Kirtz, G. , Tichy, A. , & Leidinger, E. (2008). Seasonal occurrence of canine babesiosis is influenced by local climate conditions. International Journal of Medical Microbiology, 298, 243–248. [Google Scholar]
- Lew‐Tabor, A. E. , & Valle, M. R. (2016). A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks and Tick‐Borne Diseases, 7, 573–585. [DOI] [PubMed] [Google Scholar]
- Liu, M. , Adjou Moumouni, P. F. , Asada, M. , Hakimi, H. , Masatani, T. , Vudriko, P. , Lee, S.‐H. , Kawazu, S. , Yamagishi, J. , & Xuan, X. (2018). Establishment of a stable transfection system for genetic manipulation of Babesia gibsoni . Parasites & Vectors, 11, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlathova, V. , Majlath, I. , Vichova, B. , Gul'ová, I. , Derdakova, M. , Sesztakova, E. , & Pet'ko, B. (2011). Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector‐Borne Zoonotic Dis, 11, 1447–1451. [DOI] [PubMed] [Google Scholar]
- Mascarelli, P. E. , Tartara, G. P. , Pereyra, N. B. , & Maggi, R. G. (2016). Detection of Mycoplasma haemocanis, Mycoplasma haematoparvum, Mycoplasma suis and other vector‐borne pathogens in dogs from Córdoba and Santa Fé. Argentina Parasites & Vectors, 9, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matijatko, V. , Torti, M. , & Schetters, T. P. (2012). Canine babesiosis in Europe: how many diseases? Trends in Parasitology, 28, 99–105. [DOI] [PubMed] [Google Scholar]
- Modesti, P. A. , Reboldi, G. , Cappuccio, F. P. , Agyemang, C. , Remuzzi, G. , Rapi, S. , Perruolo, E. , Parati, G. , & ESH Working Group on CV Risk in Low Resource Settings . (2016). Panethnic differences in blood pressure in Europe: A systematic review and meta‐analysis. PLoS ONE, 11, e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Øines, Ø. , Storli, K. , & Brun‐Hansen, H. (2010). First case of babesiosis caused by Babesia canis canis in a dog from Norway. Veterinary Parasitology, 171, 350–353. [DOI] [PubMed] [Google Scholar]
- Omidinia, N. , Zibaei, M. , Hosseini, H. , Pourrostami, K. , Vafae Eslahi, A. , & Badri, M. (2020). Human hydatidosis in Alborz Province: A 5‐year retrospective epidemiological analysis of hospitalized cases (2014–2019). Annals of Parasitology, 66, 587–592. [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , & Moher, D. (2021). Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. Journal of Clinical Epidemiology, 134, 103–112. [DOI] [PubMed] [Google Scholar]
- Panti‐May, J. A. , & Rodiguez‐Vivas, R. I. (2020). Canine babesiosis: A literature review of prevalence, distribution, and diagnosis in Latin America and the Caribbean. Veterinary parasitology, Regional Studies and Reports, 21, 100417. [DOI] [PubMed] [Google Scholar]
- Paulauskas, A. , Radzijevskaja, J. , Karvelienė, B. , Grigonis, A. , Aleksandravičienė, A. , Zamokas, G. , Babickaitė, L. , Sabūnas, V. , & Petkevičius, S. (2014). Detection and molecular characterization of canine babesiosis causative agent Babesia canis in the naturally infected dog in Lithuania. Veterinary Parasitology, 205, 702–706. [DOI] [PubMed] [Google Scholar]
- Pawełczyk, O. , Kotela, D. , Asman, M. , Witecka, J. , Wilhelmsson, P. , Bubel, P. , & Solarz, K. (2022). The first records of canine babesiosis in dogs from Dermacentor reticulatus—Free zone in Poland. Pathogens, 11, 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzhorn, B. L. (2020). Don't let sleeping dogs lie: Unravelling the identity and taxonomy of Babesia canis, Babesia rossi and Babesia vogeli . Parasites & Vectors, 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzhorn, B. L. , Harrison‐White, R. F. , & Stoltsz, W. H. (2020). Completing the cycle: Haemaphysalis elliptica, the vector of Babesia rossi, is the most prevalent tick infesting black‐backed jackals (Canis mesomelas), an indigenous reservoir host of B. rossi in South Africa. Ticks and Tick‐borne Diseases, 11, 101325. [DOI] [PubMed] [Google Scholar]
- Rubel, F. , Brugger, K. , Pfeffer, M. , Chitimia‐Dobler, L. , Didyk, Y. M. , Leverenz, S. , Dautel, H. , & Kahl, O. (2016). Geographical distribution of Dermacentor marginatus and Dermacentor reticulatus in Europe. Ticks and Tick‐Borne Diseases, 7, 224–233. [DOI] [PubMed] [Google Scholar]
- Schäfer, I. , Helm, C. , Marsboom, C. , Hendrickx, G. , Kohn, B. , Krücken, J. , Samson‐Himmelstjerna, G. , & Müller, E. (2021). Infections with Babesia spp. in dogs living in Germany (2007–2020). Journal of Veterinary Internal Medicine, 35, 3199. [Google Scholar]
- Seleznova, M. , Kivrane, A. , Namina, A. , Krumins, R. , Aleinikova, D. , Lazovska, M. , Akopjana, S. , Capligina, V. , & Ranka, R. (2020). Babesiosis in Latvian domestic dogs, 2016–2019. Ticks and Tick‐Borne Diseases, 11, 101459. [DOI] [PubMed] [Google Scholar]
- Silva, A. B. , Costa, A. P. , De Sá, J. C. , Costa, F. B. , dos Santos, A. C. G. , & de Guerra, R. M. S. N. C. (2012). Detecção molecular de Babesia canis vogeli em cães e em Rhipicephalus sanguineus na mesorregião do oeste maranhense, nordeste brasileiro. Ciência Animal Brasileira/Brazilian Animal Science, 13, 388–395. [Google Scholar]
- Solano‐Gallego, L. , & Baneth, G. (2011). Babesiosis in dogs and cats‐expanding parasitological and clinical spectra. Veterinary Parasitology, 181, 48–60. [DOI] [PubMed] [Google Scholar]
- Solano‐Gallego, L. , Sainz, Á. , Roura, X. , Estrada‐Peña, A. , & Miró, G. (2016). A review of Canine babesiosis: The European perspective. Parasites & Vectors, 9, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R.C. (2020). R Core Team R. R: A language and environment for statistical computing R foundation for statistical computing . Team R.C. [Google Scholar]
- Temoche, L. C. , Assad, R. , Seabra‐Junior, E. S. , Lemos, T. D. , & Almosny, N. (2018). Frequency of Babesia vogeli in domestic dogs in the metropolitan area of Piura, Acta Veterinaria Brno, 87, 255–260. [Google Scholar]
- Teodorowski, O. , Kalinowski, M. , Winiarczyk, D. , Dokuzeylül, B. , Winiarczyk, S. , & Adaszek, Ł. (2022). Babesia gibsoni infection in dogs—A European perspective. Animals, 12, 730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier, E. , & Krause, P. J. (2020). Babesiosis. In Hunter's tropical medicine and emerging infectious diseases (pp. 799–802). Elsevier. [Google Scholar]
- Vargas‐Hernández, G. , André, M. R. , Faria, J. L. M. , Munhoz, T. D. , Hernandez‐Rodriguez, M. , Machado, R. Z. , & Tinucci‐Costa, M. (2012). Molecular and serological detection of Ehrlichia canis and Babesia vogeli in dogs in Colombia. Veterinary Parasitology, 186, 254–260. [DOI] [PubMed] [Google Scholar]
- Wei, L. , Kelly, P. , Ackerson, K. , Zhang, J. , El‐Mahallawy, H. S. , Kaltenboeck, B. , & Wang, C. (2014). First report of Babesia gibsoni in Central America and survey for vector‐borne infections in dogs from Nicaragua. Parasites & Vectors, 7, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahler, M. , Schein, E. , Rinder, H. , & Gothe, R. (1998). Characteristic genotypes discriminate between Babesia canis isolates of differing vector specificity and pathogenicity to dogs. Parasitology Research, 84, 544–548. [DOI] [PubMed] [Google Scholar]
- Zhou, M. , Cao, S. , Luo, Y. , Liu, M. , Wang, G. , Moumouni, P. F. A. , Jirapattharasate, C. , Iguchi, A. , Vudriko, P. , Terkawi, M. A. , Löwenstein, M. , Kern, A. , Nishikawa, Y. , Suzuki, H. , Igarashi, I. , & Xuan, X. (2016). Molecular identification and antigenic characterization of a merozoite surface antigen and a secreted antigen of Babesia canis (BcMSA1 and BcSA1). Parasites & Vectors, 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
Data Availability Statement
All data are included in the manuscript or as supplementary files.


