Abstract
Background
Probiotics are intellectually rewarding for the discovery of their potential as a source of functional food. Investigating the economic and beauty sector dynamics, this study conducted a comprehensive review of scholarly articles to evaluate the capacity of probiotics to promote hair growth and manage dandruff.
Methods
We used the PRISMA 2020 with Embase, Pubmed, ClinicalTrials.gov, Scopus, and ICTRP databases to investigate studies till May 2023. Meta-analyses utilizing the random effects model were used with odds ratios (OR) and standardized mean differences (SMD).
Result
Meta-analysis comprised eight randomized clinical trials and preclinical studies. Hair growth analysis found a non-significant improvement in hair count (SMD = 0.32, 95 % CI -0.10 to 0.75) and a significant effect on thickness (SMD = 0.92, 95 % CI 0.47 to 1.36). In preclinical studies, probiotics significantly induced hair follicle count (SMD = 3.24, 95 % CI 0.65 to 5.82) and skin thickness (SMD = 2.32, 95 % CI 0.47 to 4.17). VEGF levels increased significantly (SMD = 2.97, 95 % CI 0.80 to 5.13), while IGF-1 showed a non-significant inducement (SMD = 0.53, 95 % CI -4.40 to 5.45). For dandruff control, two studies demonstrated non-significant improvement in adherent dandruff (OR = 1.31, 95 % CI 0.13–13.65) and a significant increase in free dandruff (OR = 5.39, 95 % CI 1.50–19.43). Hair follicle count, VEGF, IGF-1, and adherent dandruff parameters were recorded with high heterogeneity. For the systematic review, probiotics have shown potential in improving hair growth and controlling dandruff through modulation of the immune pathway and gut-hair axis. The Wnt/β-catenin pathway, IGF-1 pathway, and VEGF are key molecular pathways in regulating hair follicle growth and maintenance.
Conclusions
This review found significant aspects exemplified by the properties of probiotics related to promoting hair growth and anti-dandruff effect, which serve as a roadmap for further in-depth studies to make it into pilot scales.
Keywords: Probiotic, Hair loss, Alopecia, Hair growth, Dandruff, Scalp
1. Introduction
Hair loss and dandruff are widespread conditions, with dandruff affecting nearly half of the post-pubertal population [1] and male androgenetic alopecia being the most common form of hair loss in men, impacting 80 % of males by the age of 80 [2,94]. Hair refers to the filamentous outgrowth of the skin composed of keratinized cells, typically found on the scalp, and serves various functions, including insulation, protection, and sensory perception [3]. Hair growth, also known as hair follicle development or hair cycling, refers to the natural process by which hair strands grow from the hair follicles, undergo specific stages (anagen, catagen, and telogen), and eventually shed to allow new hair to emerge [3,4]. The pathophysiology of hair loss involves complex interactions between genetic factors [5], hormonal imbalances (such as increased levels of dihydrotestosterone) [6], inflammation [6], oxidative stress [7], immune dysregulation [8], and miniaturization [9] of hair follicles. These processes can lead to progressive inflammation, disruption of hair follicle cycling, and reduced hair density and loss [6]. On the other hand, dandruff is a common scalp condition characterized by the excessive shedding of dead skin cells from the scalp. It often leads to the presence of white or yellowish flakes, itchiness, and scalp irritation [1]. Dandruff is primarily characterized by an overgrowth of the yeast-like fungus Malassezia [[10], [11], [12]], which triggers an immune response, including inflammation [1,13], leading to increased cell turnover [14] and shedding of dead skin cells from the scalp, resulting in the formation of visible flakes and scalp irritation [1].
The micro-inflammatory component, in chronic hair loss processes in particular, is localized around the bulge stem cell niche, an important area for hair follicle cycling and renewal. The release of reactive oxygen species (ROS) and inflammatory mediators such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and histamine alters the immune environment of the follicle [7,15]. Although not immediately destructive, these inflammatory processes can dysregulate the normal hair cycle dynamics and stem cell renewal over time. One crucial factor is the role of inflammatory cytokines like type 1 T helper (Th1), type 2 T helper (Th2), type 17 T helper (Th17), and immune cell signaling in the pathogenesis of hair loss [16,17]. Specifically, the expression of genes related to the Wingless-related integration site (WNT) signaling pathway, such as WNT proteins and their receptors, plays a vital role in regulating hair growth [18]. These genes influence the expression of various growth factors like insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (bFGF), and vascular endothelial growth factor (VEGF), which are essential for initiating and maintaining the hair cycle's anagen (growth) phase [19,20]. Conversely, genes associated with the catagen (regression) phase of the hair cycle, such as transforming growth factor beta (TGF-β), IL-1, interleukin-2 (IL-2), interleukin-10 (IL-10), prostaglandin E2 (PEG-2) and TNF- α, are upregulated, leading to hair follicle apoptosis and regression [8,21]. These pro-apoptotic cytokines contribute to the premature entry of hair follicles into the catagen phase, resulting in hair shedding. Moreover, the genetic predisposition to hair loss is often influenced by hormones, such as androgens, sex hormones, prolactin, melatonin, and thyroid hormones. These hormones can bind to specific receptors in the hair follicles and modulate the expression of genes involved in hair growth and regulation [22]. Understanding the interplay between genes and proteins involved in hair loss can provide insights into potential therapeutic targets.
Recent evidence has emphasized the role of the gut-brain axis and the microbiome in hair loss and dandruff. Differences in the gut microbiome have been observed between individuals with these conditions and healthy controls, indicating their involvement in the underlying mechanisms [23]. The gut microbiome interacts with immune cells in the gastrointestinal tract, impacting the immune response [24]. Changes in the gut microbiome composition and metabolites affect T and B cell functions, influencing the severity of hair loss and dandruff in animal models [24,25]. During hair loss and dandruff onset, immunoglobulin (Ig) A + B cells from the intestine cross the blood-brain barrier, releasing antibodies that target specific bacterial strains related to these conditions [26]. This cross-reactivity may reduce neuroinflammation and alleviate symptoms. Transplanting fecal bacteria from individuals with hair loss and dandruff or healthy controls in animal models has shown symptom impact [26,27]. The disruption of miRNA-target interactions, characterized by a mismatch, results in abnormal T cell activation, impaired melanosome autophagy, and inhibited angiogenesis, ultimately contributing to hair follicle damage. The predominant metabolites produced by intestinal microbes are short-chain fatty acids (SCFAs), which play a regulatory role in T lymphocytes by influencing chromatin structure in the nucleus and promoting increased activity of gene products [28,29]. Modulating the gut microbiome and its metabolites, including SCFAs, may have the potential to prevent and treat hair loss and dandruff.
Contemporary research in hair growth and dandruff control employs diverse materials and methodologies. Regenerative approaches involve stem cell therapy [30] and the use of topicals enriched with growth factors and peptides, such as minoxidil [20], targeting hair follicle regeneration [30]. Genetic and molecular analyses aim to identify precise therapeutic targets, while convenient treatments like laser therapy [31] and microneedling [32] offer promising options. Specialized dermatologist-performed interventions include corticosteroid injections [33], hair transplants, platelet-rich plasma, and exosome treatments [34]. Prescription medications like finasteride and spironolactone provide pharmaceutical solutions, complemented by supplementation based on individual deficiencies [35]. In dandruff research, emphasis is on the scalp microbiome, anti-inflammatory agents [36], and advanced shampoo formulations with ingredients like zinc pyrithione, salicylic acid, sulfur, selenium sulfide, ketoconazole, and coal tar [37]. For severe or persistent dandruff, dermatologists may prescribe stronger shampoos or medications, considering potential underlying medical conditions such as seborrheic dermatitis, psoriasis, fungal infections, or eczema [38]. These treatments, including pharmaceutical solutions and advanced formulations, may exhibit side effects such as skin irritation, dryness, or, in the case of medications like finasteride and spironolactone, potential systemic effects that should be carefully considered and discussed with a healthcare professional [34,39], which highlight a need for a safer alternative treatment for these two problems.
Probiotics are live microorganisms that confer health benefits to the host when consumed in adequate amounts. The concept of probiotics dates back to the early 20th century when Nobel laureate Eli Metchnikoff proposed that the consumption of fermented milk products containing lactic acid bacteria could improve gut health and prolong life [40]. Colonizing the gut and enhancing the gut microbiome diversity can improve digestion, reduce inflammation, and boost the immune system [41]. Probiotics can also have beneficial effects on other parts of the body, including the skin and hair, with produced metabolites. These include short-chain fatty acids (SCFAs) [42], bacteriocins [43], exopolysaccharides (EPS) [44], and exosomes [45]. SCFAs, such as acetic, propionic, and butyric acid, have immunomodulatory properties and can impact inflammatory responses [46]. Bacteriocins are antimicrobial peptides produced by certain probiotic strains that can inhibit the growth of harmful bacteria [43]. EPS [44] and exosomes enriched in microRNAs [45] are involved in intercellular communication and may contribute to the modulation of immune and inflammatory processes.
Moreover, there is a considerable amount of scientific research highlighting the positive effects of probiotics on issues related to hair growth and dandruff. Recent preclinical studies have shown that both single and multi-strain probiotics can improve hair growth, balance immune responses and gut microbiome in various mouse and cell models related to hair growth and dandruff [18,47,48]. Additionally, clinical trials have revealed that taking single and multi-strain probiotics can positively influence the immune and inflammatory responses of patients dealing with hair loss and dandruff problems by regulating the composition of the scalp microbiota [49,50]. However, despite these promising findings, the evidence regarding the effects of probiotics on hair growth and dandruff is still limited and unclear. There is a need for more in-depth research to determine how effective probiotics are and to understand the mechanisms behind their potential role in managing hair growth and dandruff. Previous studies have also suggested a link between hair growth and dandruff conditions and the health of the scalp skin and gut. In this context, our meta-analysis is a pioneering effort, the first quantitative assessment of how probiotics might impact hair growth and dandruff-related factors. While probiotics have shown promise for promoting hair health, hair loss, and dandruff, the limited research in this area poses several limitations to our current understanding of their potential benefits and mechanisms. Future studies should address these limitations by investigating the effects of probiotics on hair health in humans, standardizing probiotic preparations and dosages, considering potential interactions with other factors that may impact hair health, and exploring potential mechanisms beyond the skin and gut microbiome.
Despite the growing interest in the use of probiotics for hair health, there is a lack of clinical trials investigating the effectiveness of probiotics in treating hair loss and dandruff. Therefore, the research question for this systematic review and meta-analysis was set as follows:
“Can probiotic supplementation improve hair health, particularly inducing hair growth and dandruff control?”
Our review plays an important role in contributing to the understanding of the intricate relationship between probiotics and hair health. By conducting a systematic review and meta-analysis, we provide a comprehensive synthesis of existing knowledge, offering important insights into probiotics impact on hair growth and dandruff management. As the first quantitative assessment in this field, our review not only aims to clarify this important relationship but also identifies gaps and limitations in current literature. Positioned at the intersection of dermatology and microbiome science, our work serves as an important foundational resource that will play a crucial role in guiding future research, informing clinical practices, and providing evidence-based insights for individuals seeking important interventions for common hair-related concerns using probiotic products.
2. Materials and methods
2.1. Literature search
A literature search on the efficacy of probiotics on hair growth and dandruff was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 protocol to find pertinent publications published up to May 2023. The database as information sources included Excerpta Medica Database (Embase), PubMed Central (Pubmed), ClinicalTrials.gov, Scopus, and International Clinical Trials Registry Platform (ICTRP). Probiotics, Lactobacillaceae, Lactobacillus, hair, hair loss, hair growth, alopecia, and dandruff were used as search terms, and filters were applied as in Table 1.
Table 1.
Summary of search results and filters applied in different databases.
| Database | Full search term | Filters applied | Paper numbers |
|---|---|---|---|
| Embase | ('Lactobacillaceae’ OR 'Lactobacillus' OR 'probiotic') AND ('hair' OR 'alopecia' OR 'hair loss' OR 'hair growth' OR 'dandruff') | Article | 133 |
| Article in press | |||
| Pubmed | (Lactobacillaceae OR Lactobacillus OR probiotic) AND (hair OR alopecia OR hair loss OR hair growth OR dandruff) | English | 71 |
| ClinicalTrials.gov | Condition or disease: hair Other terms: probiotic |
Completed Studies | 4 |
| Scopus | TITLE-ABS-KEY ((Lactobacillaceae) OR (Lactobacillus) OR probiotic) AND (hair OR alopecia OR "hair loss" OR "hair growth" OR dandruff)) | Article | 125 |
| English | |||
| ICTRP | Probiotic AND hair | With results only | 2 |
| Phase 4 |
2.2. Study selection and data extraction
2.2.1. Study selection
For the human component of this meta-analysis on the efficacy of probiotics in hair growth and dandruff control, the selection criteria for randomized controlled clinical trials (RCTs) adhered to the PICO framework outlined in Table 2. The study population (P) comprised healthy adult, patients with hair loss or dandruff who underwent an intervention (I) involving probiotics, with a comparison group (C) receiving a control or placebo. The primary outcome (O) of interest was the alteration in hair growth parameters, while secondary outcomes encompassed changes in dandruff-controlling perception parameters. The effectiveness of probiotics in controlling hair dandruff and enhancing hair growth among patients with dandruff and hair loss was assessed based on these outcomes.
Table 2.
The criteria of PICO of including and excluding studies of RCTs for meta-analysis.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Study design | Randomized controlled clinical trials published in peer-reviewed journals in English. | Observational studies, experimental studies, phase studies, case reports, reviews, abstracts, conference papers, study protocols for RCT, and studies that investigated probiotics' effect on hair growth, hair loss, and dandruff or did not report relevant outcome measures. |
| Population | Healthy adult human participants with ages between 18 and 60 or healthy adult human participants aged between 18 and 60 with stages of hair loss by a specific scale. | Not applicable. |
| Comparison | Control group or placebo group. | Not applicable. |
| Intervention | Probiotics, orally or topically administered, with no restriction on strains, doses, and frequency and duration of administration, provided information was reported. | Not applicable. |
| Outcome | Primary outcomes: changes in hair count and hair thickness measured by validated tools. Secondary outcomes: change in itchiness perception, free dandruff, and adherent dandruff measured by validated tools. | These data could not be calculated based on the information in the article such as lacking detailed data. |
Regarding cell and animal studies, preclinical investigations were deemed eligible if they fulfilled the following criteria: (1) studies conducted in cells, rats, or mice with regard to dandruff or hair loss; (2) intervention involving experimental administration of probiotics; and (3) assessment of hair loss or dandruff-related parameters and/or immune, inflammatory and growth markers such as VEGF and IGF-1 in animal models of dandruff or hair loss. Studies failing to meet any of these criteria were excluded.
Duplicate articles were identified and eliminated using EndNote X9 and additional manual procedures. Following the removal of duplicates, articles were screened based on titles and abstracts in accordance with the eligibility criteria. Full texts of potentially relevant articles were subsequently assessed for eligibility, and reasons for exclusion were documented. The entire process of study inclusion was conducted independently by two investigators (Trang Thi Minh Nguyen – T.T.M.N and Chang-Shik Yin – C.S.Y.). Discrepancies were primarily resolved through discussions to achieve a consensus, and in instances where agreement could not be reached, a third investigator (Tae-Hoo Yi – T.H.Y.) was consulted for further input.
2.2.2. Data extraction
Two independent reviewers (T.T.M.N and C.S.Y.) extracted and crosschecked data from eligible studies. For clinical trials, the extracted information included participant characteristics (age, sex, medical diagnostics, and comorbidities), sample size and follow-up time, and details of the intervention (probiotic strain, dose, and delivery method). Primary outcomes are related to hair count, hair thickness, hair growth, hair follicle strength, hair quality, hair diameter, gene expression related to hair growth (e.g., VEGF, IGF-1 …), and intestinal microbiota. Secondary outcomes include dandruff-controlling perception parameters (itching perception, scaling perception (adherent dandruff), cleaning perception (free dandruff), skin biophysical parameters (pH, hydration, transepidermal water loss (TEWL), sebum), glucose and lipid profile, scalp gloss, scalp redness, single closed patch test, and repeated closed patch test). Both are recorded as mean and standard deviation (SD) data if available.
For preclinical studies, the extracted information included study design, number of subjects per group, each group number, subject age and follow-up time, and details of the intervention (probiotic strain, dose, and delivery method). Outcomes related to hair regrowth, skin thickness, hair count, hair thickness, mRNA hair growth gene expression (e.g., VEGF, IGF-1 …), hair follicle strength, hair quality, hair diameter, Th1 immune responses, hair cell proliferation, gut microbiota regulation, intestinal tissue myeloperoxidase (MPO) content parameters in mean and standard deviation complement into primary outcome related to hair growth. Meanwhile, the antimicrobial effect on Staphylococcus aureus (S. aureus), Cutibacterium acnes (C. acnes), Candida albicans (C. albicans), Malassezia globosa (M. globosa), Malassezia restricta (M. restricta), and chemical analysis of anti-fungal compounds contribute to secondary outcome. Quantitative data were extracted and included in the meta-analyses for outcomes with data available from two or more studies.
2.3. Meta-analysis
For statistically significant homogeneous studies, the fixed-effects model was applied. In case the level of heterogeneity is large (I2 ≥ 40 %), the random-effects model recommended by DerSimonian and Laird [51] was used. This model takes into account the presence of heterogeneity and provides a more appropriate estimation of the overall effect size. To identify a significant level of heterogeneity, the I2 statistic with a cut-off of 50 % and the chi-squared test with a P value < 0.10 were both utilized. For primary outcomes related to hair growth, Standardized Mean difference (SMD) was used for all parameters related to hair growth since those outcomes are measured in a variety of ways. Meanwhile, the Odd Ratio (OR) was used for effect size due to the limited number of studies on secondary outcomes. According to Cohen's general interpretation of the SMD, an SMD of 0.2 denotes a minor effect, an SMD of 0.5 denotes a medium effect, and an SMD of 0.8 or higher denotes a substantial effect [52]. Standard deviations (SD) for changes were estimated using the formula SD = SE (Standard Error) × √N. The Review Manager version 5.4 (Revman 5.4) software was used to input each study's events, total or mean, and SD values and to perform data visualization. If the 95 % confidence interval (95 % CI) of the mean encompasses the value of 0 or the 95 % CI of the ratio encompasses the value of 1, the statistical findings would be considered non-significant [53]. Publication bias was not assessed using funnel plots due to the limited number of studies included in the meta-analyses.
2.4. Risk of bias and quality of evidence
Revman 5.4 was utilized to assess the quality of each clinical trial and preclinical studies included in the analysis. The tool evaluated seven domains of bias: Random sequence generation (selection bias); Allocation concealment (selection bias); Blinding of participants and personnel (performance bias); All outcomes, Blinding of outcome assessment (detection bias); All outcomes, Incomplete outcome data (attrition bias); All outcomes, Selective reporting (reporting bias); Other bias. Each domain was assigned a risk rating of high, low, or unclear. The risk of bias assessment was conducted independently by T.M.T.N. and C.S.Y., and any disagreements were resolved through discussions and consensus with other authors. No studies were excluded based on quality ratings. The quality assessment of the included studies was conducted independently by T.M.T.N. and C.S.Y., with any disagreements resolved through discussions involving a third investigator, T.H.Y.
3. Results
3.1. Features of included studies
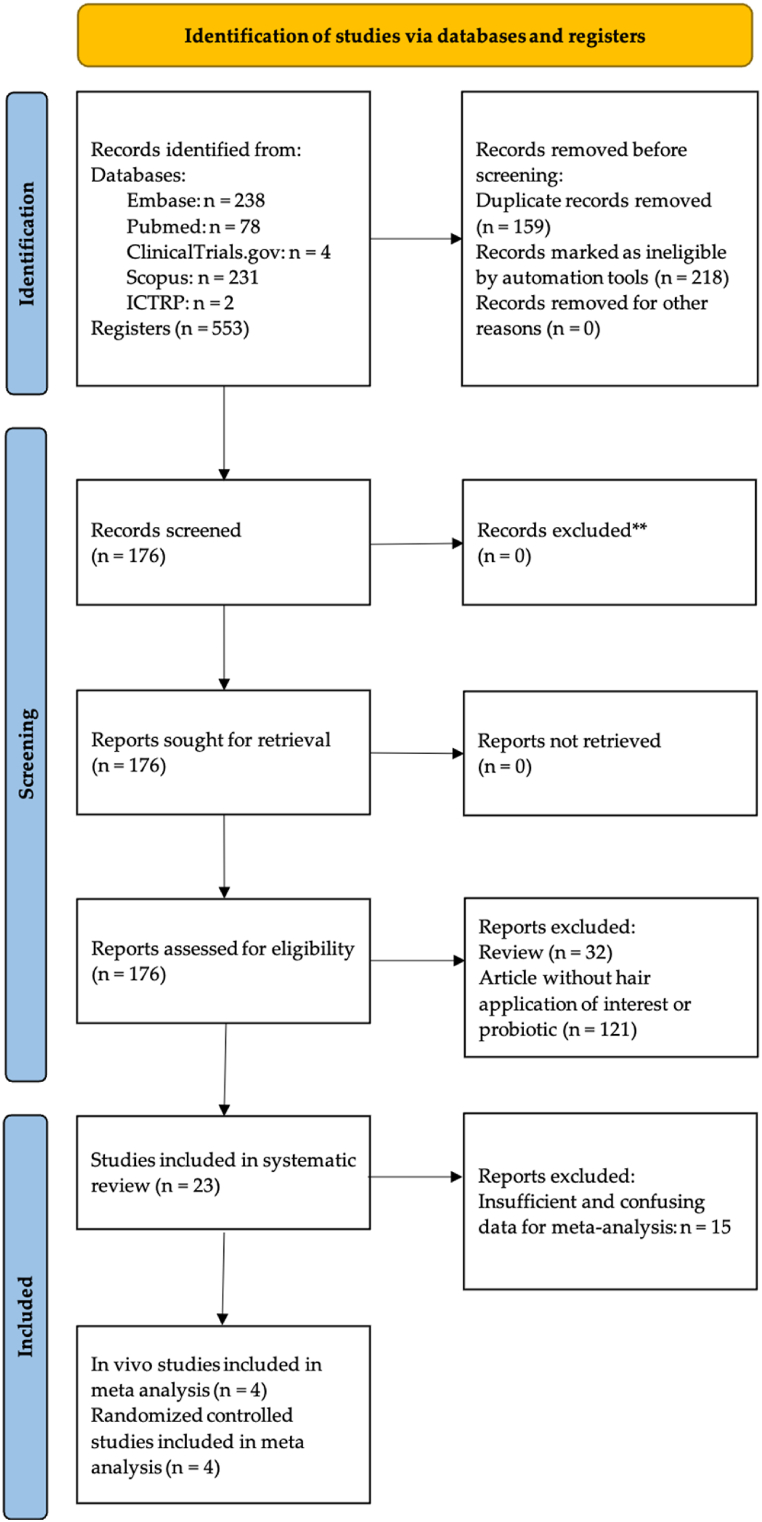
The search trials yielded a total of 553 records from online databases (Fig. 1). After removing 159 duplicates and filtering by automation tools, 176 articles remained, and based on the examination of titles and abstracts, 153 studies were excluded. A total of 8 clinical trial studies and 15 preclinical studies (with 2 papers including both clinical and preclinical data) were comprised for systematic analysis. Of the remaining 23 studies chosen for systematic review, 15 were excluded after assessing their full texts for meta-analysis. Ultimately, 4 RCTs and 4 preclinical studies were included for quantitative meta-analyses. The detailed process of article screening is summarized in Fig. 1.
Fig. 1.
PRISMA 2020 study selection flowchart for the systematic review and meta-analysis.
3.2. Description of included studies
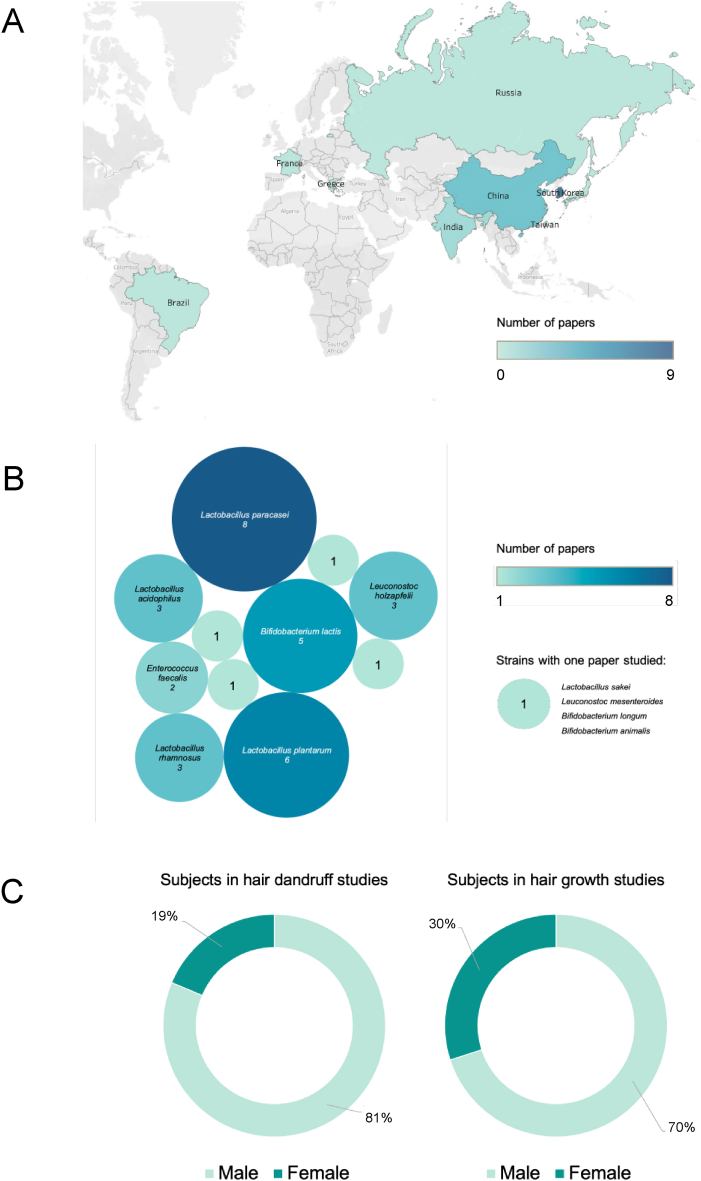
Table 3, Table 4 show the characteristics of the included studies in this systematic review and meta-analysis. Fig. 2A and B describes Korea with the most research in hair with probiotics and Lactobacillus paracasei (L. paracasei) being the most studied strain with 8 papers. Eight studies were clinical trials and were published between 2017 and 2023. The sample size ranged from 22 to 58 participants, with 322 in total, and all studies involved adult patients. Eight studies reported gender distribution (Fig. 2C) and the age range varied from 32.56 ± 10.28 to 46.43 ± 6.92 years with one paper did not report detailed data [54] (calculation being conducted in Supplement file 1 and 2). The intervention in the experimental group consisted of probiotics in all eight clinical trial studies. The follow-up period ranged from 2 to 24 weeks. The studies evaluated the effect of probiotics on hair health, including hair loss and dandruff. All studies used different probiotic strains, including Bifidobacterium lactis (B. lactis), Lactobacillus plantarum (L. plantarum), Leuconostoc holzapfelii (L. holzapfelii), and Rhynchosia volubilis Lour, among others. The measurement methods used in the included studies varied depending on the specific outcomes being evaluated. Regarding hair growth, Dr. Park in his recent study demonstrated increased hair count and thickness at 1 and 4 months [55]. A probiotic cocktail supplement improved hair density and reduced hair loss in 96.2 % of participants [50]. Furthermore, a Taiwan study by Liang in 2022 found a probiotic that boosted hair cell growth, root diameter, and overall hair quality, which showed potential influence on gene expression by reducing steroid 5α-reductase type I (SRD5A1), androgen receptor (AR), and TGF-β genes [54]. The systematic review on dandruff control yielded promising outcomes from various interventions of probiotics. L. paracasei NCC2461 ST11 showed significant improvements in itching perception for moderate to severe dandruff over a 4-week period [56]. B. lactis displayed noticeable itching improvement after 4 weeks, but not in a 2-week trial [57]. Probiotic supplementation relieved scalp itching in 73.1 % of participants over 12 weeks of treatment [50]. For scaling and cleaning perception, these parameters were suppressed by ST11 strain after 4 weeks [56], while B. lactis exhibited potential benefits only for scaling after 4 weeks [57]. Tsai's 2023 study [49] found decreased dandruff and oil secretion and increased hair growth with a heat-killed L. paracasei-containing shampoo.
Table 3.
Key features of clinical trial studies included hair dandruff and hair growth.
| Reference | Type of patient | Gender |
Age range (years) | Measurement system | Intervention | Main findings | |
|---|---|---|---|---|---|---|---|
| M | F | ||||||
| Reygagne 2017, France [56] | 58 healthy male, non-bald adults | 58 | 0 | 40 ± 9.39 ** | Itching perception Scaling perception (adherent dandruff) Cleaning perception (free dandruff) |
Food supplement containing L. paracasei NCC2461 ST11 |
Signs and symptoms of moderate to severe dandruff significantly improve in 8 weeks follow-up on the treatment group ST11 |
| Park 2020, Korea [55] | 46 men with stage II to V hair loss (Hamilton–Norwood) and women with stage I to III hair loss (Ludwig) | 23 | 23 | 45.35 ± 9.96 | Hair count Hair thickness |
80 mL of kimchi and cheonggukjang probiotic oral product containing:
|
Hair count and thickness significantly increased at:
|
| Yu 2022, China [50] | 26 adults presenting with hair loss and a high risk of metabolic | 10 | 16 | 33.6 ± 4.5 | Hair count Itching perception Skin biophysical:
|
Orally twice per day sachets (1.8 g per sachet of 18.1 billion CFU) containing:
|
After 12 weeks with probiotic supplementation:
|
| Yoon 2022, Korea [64] | 31 adults suffering from hair loss during the 12-week test period | 4 | 27 | 46.43 ± 6.92 ** | Hair count Hair thickness |
Inhaling L. holzapfelii extracellular vesicles ampoule | L. holzapfelii significantly improves the hair count and thickness after weeks 4th, 8th, and 12th of treatment |
| Liang 2022, Taiwan [54] | 50 healthy adults aged above 20 years old suffered from hair loss | N/A | N/A | 20 years and older | Hair growth, thickness, follicle strength Intestinal microbiota Scalp gloss, redness, health Hair diameter Dihydrotestosterone and testosterone in the blood Gene expression: SRD5A1, AR, TGF-β gene |
100 mg/day of L. plantarum TCI999 powder | TCI999 led to:
|
| Woo 2022, Korea [59] |
56 male adults with 2, 2A, or higher (Norwood–Hamilton) or 1 or higher (Ludwig) | 13 | 43 | 42.8 ± 5.21 | Hair count Hair thickness Hair quality Gene expression: VEGF, IGF-1, KGF, HGF, ALP |
Wash-off shampoo with L. plantarum KCTC 33133 fermenting Schisandra chinensis | Extract significantly increases the number of hairs at 8 weeks, 16 weeks, and 24 weeks compared to before product use, and a change in hair growth, a secondary efficacy evaluation variable in the clinical trial In vitro, hDPCs show an increase in IGF-1 gene expression in a dose-dependent manner |
| Alves 2023, Brazil [57] | 33 adults with varying dandruff levels | 16 | 17 | 32.56 ± 10.28 ** | Itching perception Scaling perception (adherent dandruff) Cleaning perception (free dandruff) |
1 % shampoo of Neoimuno from B. lactis CCT 7858 | Sample makes significant positive differences between the placebo and treatment group in 4 weeks in itching perception, scaling perception, and cleaning perception while 2 weeks has no effect |
| Tsai 2023, Taiwan [49] | 22 healthy adults | 8 | 14 | 37 ± 6.2 | Scalp sebum Cleaning perception (free dandruff) Hair density Single closed patch Repeated closed patch |
Twice a day base cream including heat-killed L. plantarum-GMNL6 (1 × 109 cells/g cream) |
Sample resulted in:
|
The table includes information on participant demographics, study design, and main findings. The gender breakdown is listed as M for males and F for females with mean and standard deviation data. High-Density Lipoprotein (HDL), Homeostatic Model Assessment of Insulin Resistance (HOMA-IR). N/A: Not available. **Supplement file 1.
Table 4.
Key features of preclinical studies included hair dandruff and hair growth.
| Reference | Model | Measurement system | Intervention | Main findings |
|---|---|---|---|---|
| Song 2012, Korea [58] | Topically treated on shaved 6-week-old C57BL/6 mice (4 groups/each group 5 subjects) | Hair regrowth Skin thickness |
G1: Normal G2: Negative control G3: Minoxidil G4: 10 % backryeoncho + L. rhamnosus |
Histological evaluation showed that the essence containing fermented products markedly increased the depth and size of the hair follicles, as compared to the minoxidil in 8 days. |
| Papista 2012, Greece [48] | Orally treated gluten-induced coeliac-like disease 10-week-old BALB/c mice (5 groups/each group 3 subjects) | Gene expression: IgA, COX-2, IFN, TNF-α, IL-10, IL-15, TG2 | G1: gluten-free food G2: gluten-containing commercial food G3: Saccharomyces boulardii KK1 (108 CFU) G4: L. paracasei DC205 G5: L. paracasei DC412 |
While an increase of IgA and IgG anti-gliadin antibodies (AGA) production as well as hair loss was observed in mice 6 month post gluten diet, G3 reduced expression of IgA, COX-2, IFN, TNF-α, IL-10, IL-15, but TG2 was increased in 7 days G3 reduced epithelial cell CD71 expression and Th1 immune responses and ameliorated the histopathological features of gluten-induced enteropathy in the gluten-induced mice |
| Kalenova 2015, Russia [60] | Orally treated burn-injured 12-week-old BALB/c mice (4 groups/each group 4 subjects) | Hair growth | G1: cellulose gel G2: solcoseryl G3: gel with Bacillus cereus IP5832 G4: gel with Bacillus spp. MG8 (2 × 106 CFU) |
G4 recorded stronger hair regrowth in the wound healing process than G3 in 13 days |
| Lee 2016, Korea [63] | Orally treated LPS-induced THP-1 cell 20-week-old inbred C57BL/6 mice (3 groups/each group 14 subjects) |
Gene expression on THP-1 cells: TNF-α Hair regrowth, follicles Gene expression on mice: TNF-α, IL-10, testosterone, insulin |
G1: Control G2: live L. reuteri BM36301 (106 CFU) G3: L. reuteri BM36304 |
G2 stimulated TNF-α in THP-1 cells Males mice G2 Increased testosterone levels in 20 weeks Males mice G3 experienced increased TNF-α and insulin Females mice G2 maintain lower serum TNF-α levels and higher counts of hair follicle and hair growth |
| Oike 2016, Japan [67] |
Orally treated shaved 8-week-old C57BL/6 mice (5 groups/each group 2 subjects) | Outer hair cell, spiral ganglion neurons cell survival rate Gene expression: Bak-1, Bax |
G1: Control G2: 0.05 % Lactococcus lactis H61 |
Outer hair cell, spiral ganglion neurons cell survival rate significantly increased by the intake of strain H61 in 6 months while downregulated bak1 gene |
| Woo 2019, Korea [47] |
HDPCs cell MG-63 cell Topically treated shaved 6-week-old C57BL/6 mice (6 groups/each group 4 subjects) |
Histological evaluation: - Hair growth mRNA hair growth gene expression: - VEGF - IGF-1 |
G1: Control G2: Hydrolysates of L. plantarum G3: Hydrolysates of L. plantarum by Alcalase G4: Hydrolysates of L. plantarum by bromelain G5: Hydrolysates of L. plantarum by Protamex G6: Minoxidil |
G2-6 produced hair growth superior to the growth obtained with 5 % minoxidil in hair growth experiments using C57BL/6 male mice with hair growth and increasing VEGF and IGF-1 in 14 days G2, 3, 4 show no toxicity to HDPCs cell and MG-63 cell |
| Hai 2019, China [65] |
Topically treated burn-injured 8-week-old female rats (4 groups/each group 3 subjects) | Antioxidative and antibacterial activity Hair regrowth and skin recovery Gene expression: TNF-α, IL-1β, and IL-4 |
G1: Control G2: 0.9 % normal saline G3: L. plantarum HM218749 aloe-fermented liquid G4: Bovec skin burn cream |
G3 markedly has an antioxidant effect and significantly inhibited the growth of pathogens G3 produced more eosinophils and fibroblasts and less vessel proliferation compared with G2 on the 14th day, shedding the scab and promoting hair growth, and had significantly reduced TNF-α and IL-1β and increased IL-4 G3 also regulated gut microbiota |
| Chae 2021, Korea [68] |
S. aureus C. acnes C. albicans M. globose M. restricta |
Indirect co-culture with LAB Plate agar overlay |
G1: Cell-free supernatant of L. plantarum APsulloc 331261 G2: L. plantarum APsulloc 331266 |
G1, 2 inhibits viability of S. aureus, C. acnes, C. albicans, M. globosa, and M. restricta was inhibited by indirect co-culture with APsulloc 331261 or APsulloc 331266 in a dose-dependent manner |
| Vairagkar 2021, India [11] | Malassezia furfur ATCC 44344 and Malassezia furfur 12078 | Agar well diffusion Minimum Inhibitory Concentration (MIC) assay Time kill of antifungal compounds RP‐HPLC and LC/HRESI-MS/MS of antifungal compounds |
Bacillus amyloliquefaciens MTCC 10456 | Sample fraction consisted of bacilysin, homologs of bacillomycin D, and members of the macrolactin family Synergism among the identified compounds was observed which enhanced the antagonistic activity against Malassezia spp. |
| Priya 2021, India [66] | Malassezia furfur 1374T | Agar well diffusion of LAB Minimum Inhibitory Concentration (MIC) assay GC/MS analysis |
G1: Positive control Climbazole (50 mg/mL) G2: Cell-free supernatant of L. rhamnosus G3: Cell-free supernatant of E. faecium G4: Cell-free supernatant of E. faecalis G5: Negative control (MRS broth) |
G2 has a 7 mm inhibition zone against Malassezia furfur with a MIC value of the extracellular fraction optimized to be 100 mg/mL GC/MS analysis revealed that all three extracellular bacterial isolates had propionic acid, lactic acid, phenol, 2,4-bis (1,1-dimethyl ethyl), hexadecanoic acid, octadecanoic acid, and 3-isobutyl hexahydro pyrrolo [1,2-a] pyrazine-1,4-dione |
| Nam 2021, Korea [69] |
HFDPC cell Orally treated shaved 7-week-old female C57BL/6 mice (3 groups/each group 6 subjects) |
HFDPC proliferation HFDPC growth factor secretion Histological evaluation: - Hair follicle, skin thickness mRNA hair growth gene expression: - VEGF, IGF-1 |
G1: Negative control G2: Medicinal yeast (positive control) (200 mg/kg) G3: L. paracasei HY7015 (108 CFU) |
HY7015 stimulated HFDPC proliferation in vitro and increased their secretion of VEGF and IGF-1 Oral administration of HY7015 promoted hair growth and hair follicle maturation in the dorsal skin, increased VEGF and IGF-1 levels in mouse serum in 7 weeks |
| Zhang 2022, China [70] |
Orally treated aspirin-induced 6-week-old BALB/c mice (10 groups/each group 4 subjects) | Intestinal tissue MPO content Gene expression: TNF-α, IL-6, intestinal tissue NF-κB p65 expression levels |
G1: Control G2: Aspirin G3: 2000 CFU Lactobacillus acidophilus LA-GHB1756 G4: 10000 CFU Lactobacillus acidophilus LA-GHB1756 |
G3, 4 decreased level of MPO content, TNF-α, IL-6, intestinal tissue NF-κB p65 compared to the aspirin-induced group and improved hair growth in 8 weeks |
| Wang 2022, China [71] | 95 healthy adults with varying dandruff levels aged 32.7 ± 7.2 | Scalp and hair surface microbiota Fungal and bacterial metagenomic Co‐occurrence/co‐exclusion relationships network analysis |
No intervention, only measure healthy scalp and hair microbiota |
L. plantarum and P. lactis from the scalp inhibit Staphylococcus epidermidis in vitro Healthy scalp microbiota, including Lactobacillus and Lactococcus, are co-excluded with other bacterial genera and Malassezia sp. |
| Baek 2022, Korea [62] | HDPCs cell Topically treated shaved 6-week-old C57BL/6 mice (5 groups/each group 3 subjects) |
HDPC proliferation Histological evaluation: skin thickness mRNA hair growth gene expression: - FGF2, FGF7, FGF10 - Wnt5a, Wnt5b, Wnt10b - VEGF A, VEGF B, IGF-1, IGF1-R |
G1: Control (PBS) G2: 5 % minoxidil G3: 0 mg/mL EF-2001 in acetone + olive oil (3:1) G4: 5 mg/mL EF-2001 G5: 50 mg/mL EF-2001 |
E. faecalis EF -2001 accelerated the progression of hair regrowth in mice and promoted hair-follicle conversion from telogen to anagen, likely by increasing the expression levels of growth factors (VEGF A, VEGF B, IGF-1, IGF1-R, FGF2, FGF7, FGF10) and marker genes (Wnt5a, Wnt5b, Wnt10b) after 14 days |
| Lee 2022, Korea [61] | HFDPC cell NIH3T3 cell Orally treated shaved 7-week-old C57BL/6 mice (5 groups/each group 6 subjects) |
HFDPC proliferation Histological evaluation:
|
G1: Negative control G2: Medicinal yeast (200 mg/kg) G3: Lycopus lucidus – LT (100 mg/kg) G4: L. paracasei HY7015 (108 CFU) G5: LT + HY7015 (100 mg/kg) |
Oral administration of G5 promoted hair regrowth as well as hair follicle maturation in the dermal skin of C57BL/6 mice and upregulated VEGF and IGF-1 growth factor levels in mouse serum in 5 weeks |
| Yoon 2022, Korea [64] | HFDPCs cell | HFDP Cell Migration and Proliferations mRNA Cisplatin-Induced HFDP Cells gene expression: CCK-8, Bcl-2, Bax, Capcase-3 mRNA hair growth gene expression: Wnt5a, Wnt10b, b-catenin, versican (VCAN), VSC, BAMBI, BMP-2, Lef1 Apoptosis and Induce Division via Control Cell Cycle in HFDP Cells |
G1: Control G2: Bifidobacterium longum (Bl) G3: B. animalis G4: L. acidophilus G5: human-scalp-derived-Leuconostoc holzapfelii (hs-Lh) G6: L. plantarum (Lp) |
G5 (1010 particles) extracellular vesicles increase cell proliferation, and migration, and regulate the cell cycle G5 modulates the mRNA expression of hair-growth-related genes in vitro, reduces apoptosis by modulating the cell cycle, and promotes hair growth by regulation via the Wnt/β-catenin signal transduction pathway |
| Tsai 2023, Taiwan [49] | Hs68 fibroblast cell B16F10 cells |
Collagen synthesis mRNA gene expression Hs68 fibroblast cell: Serine palmitoyltransferase small subunit A (SPTSSA) S. aureus biofilm |
G1: Control G2: Lipoteichoic acid from L. paracasei GMNL-653 G3: Peptidoglycan from L. paracasei GMNL-653 |
G2 enhanced collagen synthesis and the gene expression of SPTSSA, biofilm of S. aureus, and the proliferation of C. acnes in a dose-dependent manner |
THP-1: human acute monocytic leukemia cell, HFDPC: hair follicle dermal papilla cells, C57BL/6 mice: "C: strain's origin, the Bussey Institute for Research in Applied Biology, Harvard University, in 1921; 57: number of generations of inbreeding; BL: Black coat color of the mice; 6: sixth subline or substrain derived from the original C57BL strain", HDPCs: Human dermal papilla cells, NIH3T3: embryonic mouse fibroblast cell, MG-63: human osteosarcoma cell.
Fig. 2.
Summary of data result. A. Number of included papers being conducted in different countries; B. Number of included papers being conducted on each probiotic strain; C. Gender contributions in RCTs.
For preclinical studies, 15 preclinical studies in Table 4 evaluated the effects of probiotics on hair health. The studies utilized different strains of probiotics and were published between 2012 and 2023. Various measurement systems were employed in these studies to assess the outcomes. Regarding hair growth or regrowth, various interventions showed promising results. Song et al. (2012) [58] demonstrated fermented products' effectiveness in increasing hair follicle size beyond minoxidil, a common hair-growth substance. Woo et al. (2019) [59] also found hydrolysates of L. plantarum (LP) stimulated superior hair growth compared to minoxidil. The same effect goes with Kalenova et al. [60] observed stronger hair regrowth with Bacillus spp. MG8 gel when compared to the control group. Lee et al. [61] reported increased hair regrowth in mice treated with L. reuteri BM36301. For skin thickness, findings were explored by Song et al. [58] and Baek et al. [62], who suggested positive changes in skin thickness related to their interventions. For hair follicles, Lee et al. [63] showed increased follicle counts in mice treated with L. reuteri BM36301. Baek et al. (2022) [62] found Enterococcus faecalis (E. faecalis) EF-2001 may accelerate hair regrowth and follicle maturation. Lee et al. (2022) [61] observed hair regrowth and follicle maturation with L. paracasei HY7015 and Lycopus lucidus Turcz. (LT) administration. For hair growth mechanisms, gene expression changes were investigated in several studies. Cyclooxygenase-2 (COX-2), Interferon (IFN), Tumor Necrosis Factor-alpha (TNF-α), Interleukin (IL)-10 IL-15, IL-1β, CCK-8, Bcl-2, Bax, Capcase-3 are recorded to be suppressed while Transglutaminase 2 (TG2), insulin and testosterone, IL-4, growth factors (VEGF A, VEGF B, IGF-1, IGF1-R, FGF2, FGF7, FGF10) and marker genes (Wnt5a, Wnt5b, Wnt10b), b-catenin, versican (VCAN), Vascular Smooth Muscle Cell (VSC), BMP and Activin Membrane-Bound Inhibitor (BAMBI), Bone Morphogenetic Protein 2 (BMP-2), Lymphoid Enhancer-Binding Factor 1 (Lef1) are induced with probiotic treatment [48,[63], [64], [65]]. Yoon et al. (2022) [64] indicated gene expression modulation via the Wnt/β-catenin pathway with extracellular vesicles from L. holzapfelii. For dandruff control, preclinical studies in Table 4 highlight the potential of bacterial strains, especially Lactobacillus and Lactococcus species, to combat dandruff by inhibiting Malassezia furfur growth. Priya [66] and Vairagkar [11] found Lactobacillus rhamnosus (L. rhamnosus) and Lactobacillus amyloliquefaciens (L. amyloliquefaciens) fractions effectively inhibited Malassezia. These findings highlight the efficacy of these interventions in supporting scalp health and promoting hair growth.
3.3. Quality assessment of included 8 studies
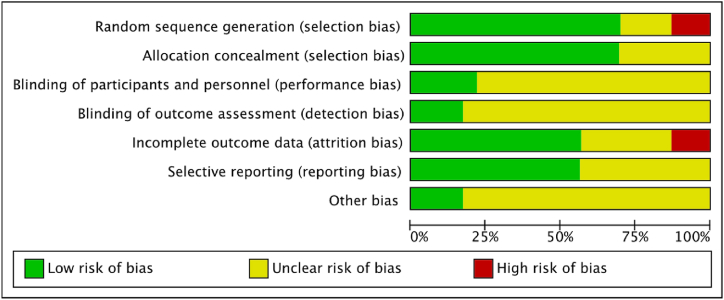
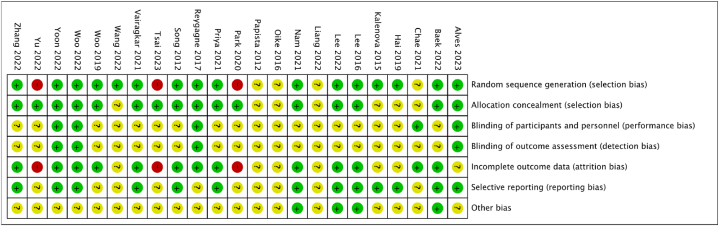
Fig. 3, Fig. 4 present a summary of the risk-of-bias assessment conducted on the included studies, both clinical trials and preclinical studies. Among them, 15 studies were classified as having a low and unclear risk of bias. However, 3 study was determined to have a high risk of bias in the Selection Bias and Incomplete outcome data domain due to the absence of baseline data in the published paper. Additional details and justifications for these judgments can be found in Supplement file 3. Additionally, for outcome assessment, approximately 50 % of the studies showed unclear risk of outcome data, suggesting a risk of detection bias. A comprehensive evaluation of study quality and risk of bias for all 23 included articles is included in Supplement file 3.
Fig. 3.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Fig. 4.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
3.4. Meta-analysis of included 8 studies
3.4.1. Effect on hair growth
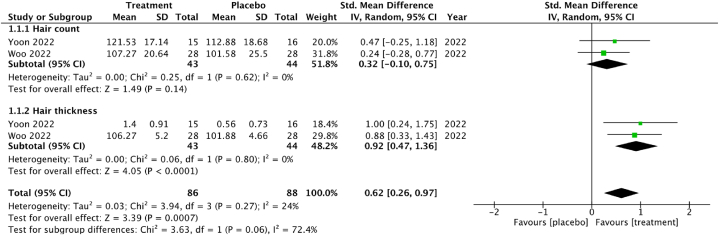
The primary outcomes of interest in this systematic review and meta-analysis included hair growth illustrated in Fig. 5, Fig. 6. This outcome was assessed in both RCTs and in vivo animal studies [62,69,72,73] included in the analysis. Hair regrowth was measured using various methods, including hair count, hair thickness, hair follicle count, skin thickness, VEGF, and IGF-1 parameters. For clinical studies in Fig. 5, Yoon 2022 [64] and Woo 2022 [59] studies involving 87 participants were included in the meta-analysis of hair growth outcomes. The pooled analysis showed low heterogeneity among the RCTs studies of I2 = 24 % (P = 0.27) and high heterogeneity in in-vivo studies with I2 = 68 % and P = 0.0005. The standardized mean difference (SMD) for hair growth, combining hair count and thickness, was 0.32 (95 % CI -0.10 to 0.75) and 0.92 (95 % CI 0.47 to 1.36), correspondingly indicating a significant effect of the intervention of hair thickness. The CI 95 % of hair count included 0, which demonstrates a non-significant increase. Subgroup analysis for hair count and thickness was not feasible due to the limited number of studies. Heterogeneity was not observed for hair thickness (I2 = 0 %, P = 0.62) and for hair count (I2 = 0 %, P = 0.80).
Fig. 5.
Forest plot of hair growth in RCT studies.
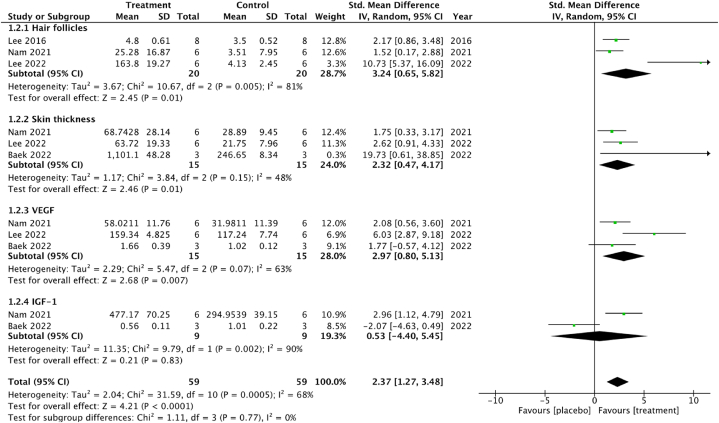
Fig. 6.
Forest plot of hair growth in preclinical studies.
For preclinical studies in Fig. 6, a meta-analysis of three in vivo studies involving 40 subjects was conducted to assess the effect of the intervention on hair follicle count. The pooled analysis showed a significant induction in hair follicle count following the intervention, with a standardized mean difference (SMD) of 3.24 (95 % CI 0.65 to 5.82). Substantial heterogeneity was observed among the included studies (I2 = 81 %, P = 0.005). Three studies with 30 subjects were included in the meta-analysis of skin thickness outcomes. The pooled analysis demonstrated a statistically significant development in skin thickness following the intervention, with an SMD of 2.32 (95 % CI 0.47 to 4.17). And heterogeneity was not notably observed among the studies (I2 = 48 %, P = 0.15). A meta-analysis of three studies involving 30 subjects was conducted to examine the effect of the intervention on VEGF levels. The pooled analysis revealed a significant increase in VEGF levels following the intervention, with an SMD of 2.97 (95 % CI 0.80 to 5.13). Substantial heterogeneity was observed across the studies (I2 = 63 %, P = 0.07). Two studies with 18 subjects were included in the meta-analysis of IGF-1 levels. The pooled analysis showed a moderate increase in IGF-1 levels, with an SMD of 0.53 (95 % CI -4.40 to 5.45) including 0 which demonstrated non-significant statistics. High heterogeneity was observed among the included studies (I2 = 90 %, P = 0.002).
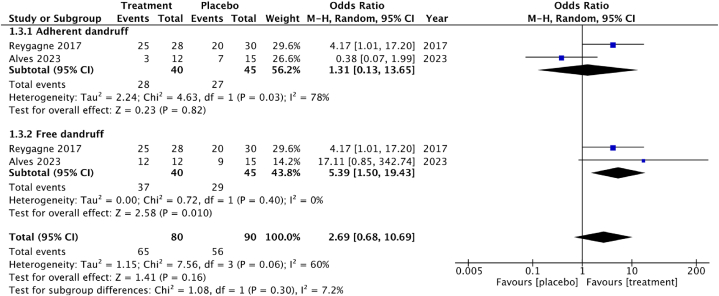
3.4.2. Effect on hair dandruff
The secondary outcome of this meta-analysis was dandruff control in Fig. 7. Dandruff control was evaluated through measurements such as scaling or peeling perception (adherent dandruff) and product quality cleaning perception (free dandruff). Regarding scaling perception (adherent dandruff), two studies [56,57] with 85 participants yielded an OR of 1.31 (95 % CI 0.13 to 13.65). A meta-analysis of two studies [56,57] on cleaning perception (free dandruff) included 85 participants and showed an OR of 5.39 (95 % CI 1.50 to 19.43). Adhere dandruff parameter has the Cl 95 % including 1 which indicated a non-significant increase. High heterogeneity was observed I2 = 78 % with P = 0.03 for adherent dandruff and no heterogeneity for I2 = 0 % with P = 0.4 of free dandruff parameter.
Fig. 7.
Forest plot of hair dandruff perception RCTs studies.
4. Discussions
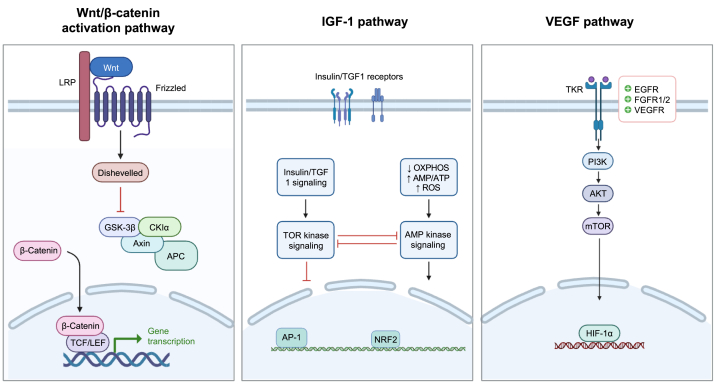
The present systematic review and meta-analysis aimed to assess the effects of probiotics on hair health, specifically focusing on hair growth and dandruff control. The analysis included a total of four clinical studies and four preclinical studies, providing valuable insights into the potential benefits of probiotic interventions in managing hair-related conditions, within the studied probiotic strains, L. paracasei, L. plantarum, and B. lactis are most commonly studied. Due to the complexity of probiotic live and dead cells, or their byproducts such as SCFA, bacteriocin, and peptidoglycan, the effects on hair health (Table 3, Table 4) are difficult to attribute to any specific substance. Further research is needed to differentiate the specificity and optimal composition of probiotic interventions for various hair-related conditions. The wide range of probiotic options also highlights the challenges in ensuring quality control, while simultaneously showcasing the potential for personalized medicine in skin microbiome. In terms of hair growth, the analysis included both clinical and preclinical studies. The meta-analysis of two clinical studies showed a statistically significant effect of probiotic interventions on hair growth, as measured by hair thickness, and a non-significant increase in hair count parameters. Preclinical studies provided additional support for the positive effects of probiotics on hair health. These studies consistently demonstrated a significant induction of hair follicle count and increased skin thickness and growth factors VEGF following probiotic intervention except for IGF-1 data (Fig. 5, Fig. 6). These findings suggest that probiotics have the potential to promote hair regrowth and improve overall hair and scalp health. However, it is important to consider the limitations of translating findings from animal studies to human populations. Three pathways involving different growth factors could be illustrated in Fig. 8.
Fig. 8.
Molecular diagram of hair growth pathways induced by Probiotics.
4.1. Probiotic intervention across growth and regression phases
In the context of hair follicle regulation of the systematic review, several molecules and genes were identified, each associated with different phases of the hair growth cycle. Among the active growth stage (anagen), various growth factors (VEGF A, VEGF B, IGF-1, IGF1-R, FGF2, FGF7, FGF10, and BMP-2) and hormones (Insulin and Testosterone) were observed to increase and play essential roles in promoting hair follicle proliferation and hair growth with the intervention of probiotic [48,62,64,65]. On the other hand, the catagen phase, representing the regression stage of the hair follicle, showed the downregulation of proteins associated with hair follicle apoptosis and regression with probiotic intervention, such as Transglutaminase 2 (TG2) [48], Versican (VCAN), Vascular Smooth Muscle Cell (VSC), BMP and Activin Membrane-Bound Inhibitor (BAMBI), and Lymphoid Enhancer-Binding Factor 1 (Lef1) [64]. Moreover, the increase of the cytokine Interleukin-4 (IL-4) with probiotics was observed suggesting its potential role in orchestrating hair follicle regression [65].
4.2. Wnt/β-catenin signaling: probiotic modulation of hair follicle development and stem cell activity
During the anagen phase of the hair growth cycle, marker genes like Wnt5a, Wnt5b, and Wnt10b were identified during the anagen phase, indicating their involvement in hair follicle development and maintenance. Wnt5a, Wnt5b, and Wnt10b [64] proteins induced by probiotics are actively involved in regulating hair follicle stem cell activity and promoting hair growth [18,74]. These Wnt proteins initiate the Wnt/β-catenin pathway, which plays a crucial role in the development and maintenance of hair follicles. Activation of the Wnt/β-catenin pathway begins with the binding of Wnt proteins to their cell surface receptors, Frizzled receptors, and coreceptors such as LRP5/6. This binding event triggers a series of intracellular signaling events that ultimately lead to the stabilization and accumulation of β-catenin in the cytoplasm [75]. In the absence of Wnt activation signaling, β-catenin is targeted for degradation by a protein complex called the destruction complex, which includes Axin, Adenomatous polyposis coli (APC), Glycogen synthase kinase-3 beta (GSK-3β), and Casein kinase 1 (CK1) [76]. This complex phosphorylates β-catenin, marking it for ubiquitination and subsequent degradation by the proteasome. However, when the Wnt/β-catenin pathway is activated by the binding of Wnt proteins, it disrupts the activity of the destruction complex [18,74,75]. This disruption prevents the phosphorylation and degradation of β-catenin, allowing it to accumulate in the cytoplasm. Accumulated β-catenin then translocates into the nucleus, where it interacts with transcription factors known as LEF/TCF (lymphoid enhancer-binding factor/T-cell factor) [77]. The binding of β-catenin to LEF/TCF proteins leads to the activation of target genes that are involved in critical cellular processes such as cell proliferation, cell differentiation, and hair follicle growth. The target genes activated by β-catenin-LEF/TCF complex can vary depending on the specific cellular context and the stage of hair follicle development. These genes include cyclin D1 [78], a key regulator of cell cycle progression, as well as other genes involved in cell adhesion, cell signaling, and hair follicle morphogenesis.
4.3. IGF-1 pathway dynamics in hair growth: probiotic regulation of cellular energy and metabolism
The IGF-1 pathway plays a significant role in hair growth and maintenance, and it can intersect with TOR kinase and AMP kinase to regulate these processes [79]. In the context of hair growth, IGF-1, along with its receptor IGF1-R, is involved in promoting the proliferation, survival, and differentiation of hair follicle cells during the anagen and telogen phases. IGF-1 binds to IGF1-R on the surface of hair follicle cells, initiating a cascade of intracellular signaling events. Downstream of IGF1-R activation, several signaling pathways are activated, including the phosphatidylinositol 3-kinase and protein kinase B (PI3K/Akt) and mitogen-activated protein kinase and extracellular signal-regulated kinases (MAPK/ERK) pathways [80]. The PI3K/Akt pathway promotes cell survival, growth, and metabolism, while the MAPK/ERK pathway is involved in cell proliferation, differentiation, and survival. TOR kinase, as a central regulator of cellular growth and metabolism, can be influenced by the IGF-1 pathway TOR kinase activation can stimulate protein synthesis, cell cycle progression, and other cellular activities necessary for hair follicle growth [81]. Additionally, AMP kinase [81], a cellular energy sensor, can also interact with the IGF-1 pathway. Activation of AMP kinase can modulate the IGF-1 pathway by regulating cellular energy availability and metabolism. These effects contribute to the overall energy balance and metabolic adaptations necessary for hair follicle growth. activation of the IGF-1 pathway can influence OXPHOS activity, leading to increased ATP production to meet the energy demands of hair follicle growth. The interaction between the IGF-1 pathway, TOR kinase, and AMP kinase affects the AMP/ATP ratio, modulating energy balance and resource allocation [82]. Additionally, the impact of the IGF-1 pathway on Reactive oxygen species (ROS) levels may be context-dependent, involving a balance between ROS signaling and oxidative stress [83]. These interconnected processes collectively contribute to the regulation of hair follicle growth and maintenance in response to energy availability and cellular redox status.
4.4. VEGF isoforms and hair growth: probiotic modulation for angiogenesis and blood supply
During the anagen and telogen phases of the hair growth cycle, VEGF (Vascular Endothelial Growth Factor) isoforms, specifically VEGF-A and VEGF-B, play a crucial role in angiogenesis and maintaining the blood supply to the hair follicles [47,84]. VEGF promotes the formation of new blood vessels, ensuring an adequate blood flow to support the needs of the hair follicles. The VEGF pathway involves the binding of VEGF to its receptors, VEGFR1 (Vascular Endothelial Growth Factor Receptor 1) and VEGFR2 (Vascular Endothelial Growth Factor Receptor 2), located on endothelial cells of blood vessels [84]. Upon binding, the receptors initiate signaling cascades that activate several downstream molecules, including TKR (Tyrosine Kinase Receptor), PI3K, AKT, and mTOR (Mammalian Target of Rapamycin) [85]. The activation of TKR leads to the recruitment and activation of PI3K. PI3K, in turn, generates the activation of AKT. Activated AKT promotes cell survival, proliferation, and growth by modulating various cellular processes, including protein synthesis, cell cycle progression, and metabolism. Furthermore, the activation of AKT can also lead to the activation of mTOR, a key regulator of cell growth and metabolism [86]. Upon activation by the VEGF pathway and other signaling inputs, mTOR can modulate the activity of transcription factors, such as HIF-1α (hypoxia-inducible factor 1-alpha) [87], which can translocate to the nucleus and regulate the expression of specific target genes. HIF-1α is involved in the cellular response to low oxygen levels (hypoxia) and is known to regulate genes related to angiogenesis, metabolism, and cell survival. This cascade of molecular events promotes blood vessel growth, vascular permeability, and nutrient supply to the hair follicles during the anagen and telogen phases, ensuring an optimal environment for hair growth and maintenance.
4.5. Probiotics for dandruff: metabolic benefits and microbiota insights
Regarding the effect of probiotics on dandruff control, the meta-analysis of two clinical studies suggested an improvement in scaling perception (adherent dandruff) non-significantly and cleaning perception (free dandruff) significantly in favor of the probiotic intervention (Fig. 7). These findings suggest that probiotics play a beneficial role in reducing dandruff symptoms and promoting a healthier scalp environment. Yu's 2022 study [50] revealed that probiotic supplementation over 12 weeks improved glucose and lipid profiles, suggesting potential metabolic benefits. Liang's 2022 study [54] reported changes in intestinal microbiota following TCI999 consumption, linking gut health to hair-related outcomes. Additionally, Woo and Tsai's studies [47,49] evaluated adverse events and scalp microbial species changes, providing insights into intervention safety and potential microbial effects. These findings present an exciting opportunity to explore the gut-hair axis and the potential role of probiotics in regulating scalp health and hair-related parameters. Further research in this area could lead to innovative therapeutic approaches for dandruff control, scalp health improvement, and hair growth promotion through the modulation of the gut microbiota.
4.6. Multi-pathway approach to dandruff control and scalp health by probiotics
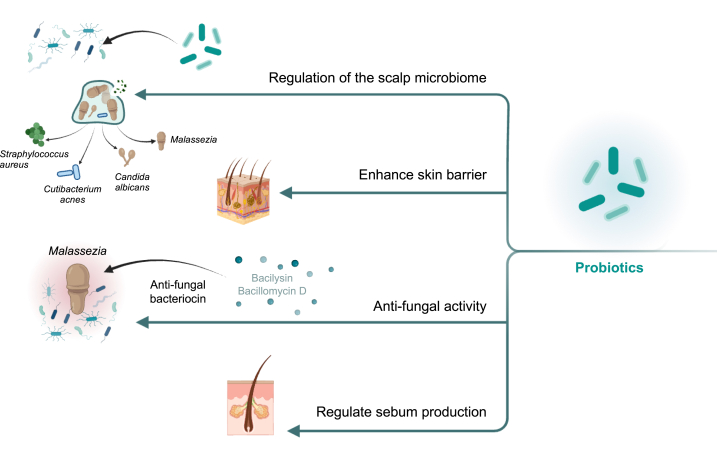
The control of hair dandruff through probiotics may involve multiple pathways that target the underlying causes of the condition (Fig. 9). One such pathway is the modulation of the skin microbiome. The scalp harbors a diverse microbial community, and an imbalance in this ecosystem can contribute to dandruff development [88]. Probiotics, including specific strains of bacteria and fungi, have been found to restore microbial balance by inhibiting the growth of dandruff-causing fungi such as Malassezia [11,49,66,68]. These beneficial microorganisms produce antimicrobial peptides [89,90] and metabolites [66] that create an unfavorable environment for the growth and proliferation of dandruff-causing pathogens. Another pathway involves strengthening the skin's natural barrier function. Probiotics have been shown to enhance the production of ceramides, which are lipids that play a crucial role in maintaining the scalp's moisture balance on the skin [49,91,92]. This helps prevent dryness, flaking, and itching associated with dandruff. By improving the integrity of the skin barrier, probiotics contribute to the overall health of the scalp and reduce the occurrence of dandruff. Furthermore, probiotics can modulate the immune response, regulating the excessive production of sebum, a common factor associated with dandruff [1]. Excessive sebum production can create an environment conducive to the growth of dandruff-causing microorganisms. Probiotics have been found to reduce inflammation and rebalance sebum production, leading to a healthier scalp environment and decreased dandruff symptoms [93]. In addition to their regulatory effects on the scalp microbiome, barrier function, and immune response, probiotics produce antimicrobial compounds that display antagonistic activity against dandruff-causing pathogens. These compounds, such as bacilysin and bacillomycin D [58], inhibit the growth of Malassezia species biofilm [49] and contribute to the control of dandruff. By targeting these pathways, probiotics offer a promising avenue for the development of effective and sustainable treatments for hair dandruff. The ability of probiotics to restore microbial balance, enhance skin barrier function, regulate sebum production, and produce antimicrobial compounds collectively contribute to their efficacy in controlling dandruff. However, further research is needed to optimize probiotic strains, dosages, and formulations for maximum effectiveness.
Fig. 9.
Dandruff control pathways through probiotics.
5. Limitations of study
Several limitations of this systematic review and meta-analysis should be acknowledged. Firstly, the included studies varied in terms of study design, probiotic strains used, intervention duration, and outcome measures assessed. This heterogeneity may introduce potential biases and limit the generalizability of the results. Additionally, the sample sizes and numbers of the included clinical studies were relatively small, which could impact the statistical power and precision of the findings. Furthermore, the quality assessment revealed a high risk of bias in one study due to incomplete outcome data, suggesting a need for further improvement in the reporting of study results.
Another limitation is the potential for publication bias, as the analysis relied on published studies and such studies as with negative results may have not been published. Moreover, the majority of the included studies were conducted in Korea, with only one study from Europe and one from South America. This geographical limitation raises the question of the generalizability of the findings to other populations and regions. Lastly, acknowledging the study limitation in the scarcity of papers compared to other systematic reviews, it highlights the recent surge of interest in probiotic-related studies in hair growth and dandruff, notably with the earliest identified paper dating back to 2012.
6. Conclusions
In conclusion, this systematic review and meta-analysis provide evidence supporting the potential benefits of probiotics in improving hair health, specifically in terms of dandruff control and hair growth. However, the heterogeneity among the included studies, limited sample sizes, potential publication bias, and geographical limitations should be considered when interpreting the results. Future well-designed studies including both clinical and preclinical approaches with larger sample sizes and standardized outcome measures are warranted to further investigate the effects of probiotics on hair health and to gain a better understanding of the underlying mechanisms.
Funding
This research was supported by the technology transfer and commercialization program through the INNOPOLIS Foundation funded by the Ministry of Science and ICT (No. 2022- GJ-RD-0021).
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
The data presented in this study are available in this paper.
CRediT authorship contribution statement
Chang-Shik Yin: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Formal analysis, Data curation, Conceptualization. Trang Thi Minh Nguyen: Writing – review & editing, Writing – original draft, Validation, Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Eun-Ji Yi: Resources, Methodology, Formal analysis, Data curation. Shengdao Zheng: Resources, Methodology, Formal analysis, Data curation. Arce Defeo Bellere: Software, Investigation. Qiwen Zheng: Visualization, Investigation, Formal analysis. Xiangji Jin: Visualization, Investigation, Formal analysis. Myeongju Kim: Visualization, Investigation. Sejic Park: Visualization, Investigation. Sarang Oh: Writing – review & editing, Resources, Data curation. Tae-Hoo Yi: Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Snowwhitefactory Co. Ltd.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e29539.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Mauldin E.A., Peters-Kennedy J. In: Jubb Kennedy., editor. vol. 1. Elsevier; 2016. Integumentary system; pp. 509–736.e1. (Palmer's Pathology of Domestic Animals). [DOI] [Google Scholar]
- 2.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009;19 doi: 10.1111/j.1467-2494.2006.00326.x. R132–R142. [1] C. Piérard-Franchimont, E. Xhauflaire-Uhoda, G.E. Piérard, Revisiting dandruff, Int J Cosmet Sci 28 (2006) 311–318. [DOI] [PubMed] [Google Scholar]
- 3.Mauldin E.A., Peters-Kennedy J. In: Jubb Kennedy., editor. vol. 1. Elsevier; 2016. Integumentary system; pp. 509–736.e1. (Palmer's Pathology of Domestic Animals). [DOI] [Google Scholar]
- 4.Schneider M.R., Schmidt-Ullrich R., Paus R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009;19:R132–R142. doi: 10.1016/j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Redler S., Messenger A.G., Betz R.C. Genetics and other factors in the aetiology of female pattern hair loss. Exp. Dermatol. 2017;26:510–517. doi: 10.1111/exd.13373. [DOI] [PubMed] [Google Scholar]
- 6.Sperling L.C. Evaluation of hair loss. Curr. Probl. Dermatol. 1996;8:99–136. doi: 10.1016/S1040-0486(96)80003-2. [DOI] [Google Scholar]
- 7.Prie B.E., Iosif L., Tivig I., Stoian I., Giurcaneanu C. Oxidative stress in androgenetic alopecia. J Med Life. 2016;9:79–83. [PMC free article] [PubMed] [Google Scholar]
- 8.Bain K.A., McDonald E., Moffat F., Tutino M., Castelino M., Barton A., Cavanagh J., Ijaz U.Z., Siebert S., McInnes I.B., Astrand A., Holmes S., Milling S.W.F. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease‐associated psychological morbidity. Br. J. Dermatol. 2019;bjd doi: 10.1111/bjd.18008. [DOI] [PubMed] [Google Scholar]
- 9.Whiting D.A. Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J. Am. Acad. Dermatol. 2001;45:S81–S86. doi: 10.1067/mjd.2001.117428. [DOI] [PubMed] [Google Scholar]
- 10.Rudramurthy S.M., Honnavar P., Dogra S., Yegneswaran P.P., Handa S., Chakrabarti A. Association of Malassezia species with dandruff. Indian J. Med. Res. 2014;139:431–437. [PMC free article] [PubMed] [Google Scholar]
- 11.Vairagkar U., Mirza Y. Antagonistic activity of antimicrobial metabolites produced from seaweed-associated Bacillus amyloliquefaciens MTCC 10456 against Malassezia spp. Probiotics Antimicrob Proteins. 2021;13:1228–1237. doi: 10.1007/s12602-021-09742-2. [DOI] [PubMed] [Google Scholar]
- 12.Bateman R.M., Md 36th international symposium on intensive care and emergency medicine : Brussels, Belgium. 15-18 march 2016. Crit. Care. 2016;20:94. doi: 10.1186/s13054-016-1208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seborrheic dermatitis and dandruff: a comprehensive review. J ClinInvestig Dermatol. 2015;3 doi: 10.13188/2373-1044.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganathan S., Mukhopadhyay T. Dandruff: the most commercially exploited skin disease. Indian J. Dermatol. 2010;55:130–134. doi: 10.4103/0019-5154.62734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soliman A.M., Barreda D.R. Acute inflammation in tissue healing. Indian J. Manag. Sci. 2022;24:641. doi: 10.3390/ijms24010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olayinka J.J.T., Richmond J.M. Immunopathogenesis of alopecia areata. Curr Res Immunol. 2021;2:7–11. doi: 10.1016/j.crimmu.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waśkiel-Burnat A., Osińska M., Salińska A., Blicharz L., Goldust M., Olszewska M., Rudnicka L. The role of serum Th1, Th2, and Th17 cytokines in patients with alopecia areata: clinical implications. Cells. 2021;10:3397. doi: 10.3390/cells10123397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., Xiao Q., Xiao J., Niu C., Li Y., Zhang X., Zhou Z., Shu G., Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Targeted Ther. 2022;7:3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozeki M. Promoted growth of murine hair follicles through controlled release of vascular endothelial growth factor. Biomaterials. 2002;23:2367–2373. doi: 10.1016/S0142-9612(01)00372-6. [DOI] [PubMed] [Google Scholar]
- 20.Choi N., Shin S., Song S., Sung J.-H. Minoxidil promotes hair growth through stimulation of growth factor release from adipose-derived stem cells. Indian J. Manag. Sci. 2018;19:691. doi: 10.3390/ijms19030691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann R., Eicheler W., Huth A., Wenzel E., Happle R. Cytokines and growth factors influence hair growth in vitro. Possible implications for the pathogenesis and treatment of alopecia areata. Arch. Dermatol. Res. 1996;288:153–156. doi: 10.1007/BF02505825. [DOI] [PubMed] [Google Scholar]
- 22.Grymowicz M., Rudnicka E., Podfigurna A., Napierala P., Smolarczyk R., Smolarczyk K., Meczekalski B. Hormonal effects on hair follicles. Int. J. Mol. Sci. 2020;21:5342. doi: 10.3390/ijms21155342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constantinou A., Kanti V., Polak-Witka K., Blume-Peytavi U., Spyrou G.M., Vogt A. The potential relevance of the microbiome to hair physiology and regeneration: the emerging role of metagenomics. Biomedicines. 2021;9:236. doi: 10.3390/biomedicines9030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Pellicer P., Navarro-Moratalla L., Núñez-Delegido E., Agüera-Santos J., Navarro-López V. How our microbiome influences the pathogenesis of alopecia areata. Genes. 2022;13 doi: 10.3390/genes13101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Pessemier B., Grine L., Debaere M., Maes A., Paetzold B., Callewaert C. Gut-skin Axis: current knowledge of the interrelationship between microbial dysbiosis and skin conditions. Microorganisms. 2021;9 doi: 10.3390/microorganisms9020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rea K., Dinan T.G., Cryan J.F. The microbiome: a key regulator of stress and neuroinflammation. Neurobiology of Stress. 2016;4:23–33. doi: 10.1016/j.ynstr.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W., Wan S., Xie B., Song X. Novel potential therapeutic targets of alopecia areata. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1148359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu M., Alashkar Alhamwe B., Santner-Nanan B., Miethe S., Harb H., Renz H., Potaczek D.P., Nanan R.K. Short-chain fatty acids augment differentiation and function of human induced regulatory T cells. Indian J. Manag. Sci. 2022;23:5740. doi: 10.3390/ijms23105740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gentile P., Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: Wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8:466. doi: 10.3390/cells8050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avci P., Gupta G.K., Clark J., Wikonkal N., Hamblin M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Laser Surg. Med. 2014;46:144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fertig R.M., Gamret A.C., Cervantes J., Tosti A. Microneedling for the treatment of hair loss? J. Eur. Acad. Dermatol. Venereol. 2018;32:564–569. doi: 10.1111/jdv.14722. [DOI] [PubMed] [Google Scholar]
- 33.Barton V.R., Toussi A., Awasthi S., Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J. Am. Acad. Dermatol. 2022;86:1318–1334. doi: 10.1016/j.jaad.2021.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nestor M.S., Ablon G., Gade A., Han H., Fischer D.L. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021;20:3759–3781. doi: 10.1111/jocd.14537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alikhan A., Sayed C., Alavi A., Alhusayen R., Brassard A., Burkhart C., Crowell K., Eisen D.B., Gottlieb A.B., Hamzavi I., Hazen P.G., Jaleel T., Kimball A.B., Kirby J., Lowes M.A., Micheletti R., Miller A., Naik H.B., Orgill D., Poulin Y. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part I: diagnosis, evaluation, and the use of complementary and procedural management. J. Am. Acad. Dermatol. 2019;81:76–90. doi: 10.1016/j.jaad.2019.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Wang C., Hsieh S., Hung Y., Chen H. Evaluation of a new‐formula shampoo containing 6% glycyrrhetinic acid complex for scalp seborrheic dermatitis: a pilot study. J. Cosmet. Dermatol. 2022;21:3423–3430. doi: 10.1111/jocd.14623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Squire R., Goode K. A randomised, single-blind, single-centre clinical trial to evaluate comparative clinical efficacy of shampoos containing ciclopirox olamine (1.5%) and salicylic acid (3%), or ketoconazole (2%, Nizoral ®) for the treatment of dandruff/seborrhoeic dermatitis. J. Dermatol. Treat. 2002;13:51–60. doi: 10.1080/095466302317584395. [DOI] [PubMed] [Google Scholar]
- 38.Dall'Oglio F., Nasca M.R., Gerbino C., Micali G. An overview of the diagnosis and management of seborrheic dermatitis. CCID. 2022;15:1537–1548. doi: 10.2147/CCID.S284671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angiolella L., Carradori S., Maccallini C., Giusiano G., Supuran C.T. Targeting Malassezia species for novel synthetic and natural antidandruff agents. Comput. Mater. Continua (CMC) 2017;24 doi: 10.2174/0929867324666170404110631. [DOI] [PubMed] [Google Scholar]
- 40.Metchnikoff, E., The prolongation of life: optimistic studies, in: The Prolongation of Life: Optimistic Studies, n.d..
- 41.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 42.Trompette A., Pernot J., Perdijk O., Alqahtani R.A.A., Domingo J.S., Camacho-Muñoz D., Wong N.C., Kendall A.C., Wiederkehr A., Nicod L.P., Nicolaou A., Von Garnier C., Ubags N.D.J., Marsland B.J. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022;15:908–926. doi: 10.1038/s41385-022-00524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simons A., Alhanout K., Duval R.E. Bacteriocins, antimicrobial peptides from bacterial origin: overview of their Biology and their impact against multidrug-resistant bacteria. Microorganisms. 2020;8:639. doi: 10.3390/microorganisms8050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oerlemans M.M.P., Akkerman R., Ferrari M., Walvoort M.T.C., De Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct.Foods. 2021;76 doi: 10.1016/j.jff.2020.104289. [DOI] [Google Scholar]
- 45.Pulliam L., Gupta A. Modulation of cellular function through immune-activated exosomes. DNA Cell Biol. 2015;34:459–463. doi: 10.1089/dna.2015.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markowiak-Kopeć P., Śliżewska K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo Y.M., Kim O.J., Jo E.S., Jo M.Y., Ahn M.Y., Lee Y.-H., Li C.-R., Lee S.-H., Choi J.-S., Ha J.M., Kim A. The effect of Lactobacillus plantarum hydrolysates promoting VEGF production on vascular growth and hair growth of C57BL/6 mice. Journal of Analytical Science and Technology. 2019;10 doi: 10.1186/s40543-019-0178-0. [DOI] [Google Scholar]
- 48.Papista C., Gerakopoulos V., Kourelis A., Sounidaki M., Kontana A., Berthelot L., Moura I.C., Monteiro R.C., Yiangou M. Gluten induces coeliac-like disease in sensitised mice involving IgA. CD71 and transglutaminase 2 interactions that are prevented by probiotics, Laboratory Investigation. 2012;92:625–635. doi: 10.1038/labinvest.2012.13. [DOI] [PubMed] [Google Scholar]
- 49.Tsai W.-H., Fang Y.-T., Huang T.-Y., Chiang Y.-J., Lin C.-G., Chang W.-W. Heat-killed Lacticaseibacillus paracasei GMNL-653 ameliorates human scalp health by regulating scalp microbiome. BMC Microbiol. 2023;23 doi: 10.1186/s12866-023-02870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu P., Teng X., Liu T., Li Y., Ni J., Xue S., Wang J. Effect of an oral probiotic formula on scalp and facial skin condition, glucose, and lipid metabolism. Functional Foods in Health and Disease. 2022;12:394–409. doi: 10.31989/ffhd.v12i7.944. [DOI] [Google Scholar]
- 51.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. Academic press; 2013. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 53.Lee D.K. Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol. 2016;69:555. doi: 10.4097/kjae.2016.69.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang C.H., Lin Y.H., Chan S.T. Lactiplantibacillus plantarum TCI999 probiotic promoted hair growth and regulated gut microbiome: double-blind, placebo-controlled trial. Journal of Probiotics & Health. 2022:1–6. doi: 10.35248/2329-8901.22.10.285. [DOI] [Google Scholar]
- 55.Park D.-W., Lee H.S., Shim M.-S., Yum K.J., Seo J.T. Do kimchi and Cheonggukjang probiotics as a functional food improve androgenetic alopecia? A clinical pilot study. World Journal of Men’s Health. 2020;38:95–102. doi: 10.5534/wjmh.180119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reygagne P., Bastien P., Couavoux M.P., Philippe D., Renouf M., Castiel-Higounenc I., Gueniche A. The positive benefit of Lactobacillus paracasei NCC2461 ST11 in healthy volunteers with moderate to severe dandruff. Benef. Microbes. 2017;8:671–680. doi: 10.3920/BM2016.0144. [DOI] [PubMed] [Google Scholar]
- 57.Alves de Jesus G.F., Rossetto M.P., Voytena A., Feder B., Borges H., da Costa Borges G., Feuser Z.P., Dal-Bó S., Michels M. Clinical evaluation of paraprobiotic-associated Bifidobacterium lactis CCT 7858 anti-dandruff shampoo efficacy: a randomized placebo-controlled clinical trial. Int. J. Cosmet. Sci. 2023 doi: 10.1111/ics.12850. [DOI] [PubMed] [Google Scholar]
- 58.Song J.H., Lee J.S., Choi H.J. Hair growth promoting effect of essence manufactured with products fermented by Lactobacillus rhamnosus and Backryeoncho (Opuntia ficus-indica var. sarboten) fruits in mice. Food Sci. Biotechnol. 2012;21:1101–1104. doi: 10.1007/s10068-012-0143-9. [DOI] [Google Scholar]
- 59.Woo Y.M., Seo J.Y., Kim S.-Y., Cha J.H., Cho H.D., Cha Y.K., Jeong J.T., Park S.M., Ryu H.S., Kim J.M., Kim M.H., Kim H.-T., Kim Y.-M., Joo K.S., Lee S.M., Lee J., Kim A. Hair growth effect of TS-SCLF from Schisandra chinensis extract fermented with Lactobacillus plantarum. Microbiology and Biotechnology Letters. 2022;50:533–547. doi: 10.48022/mbl.2206.06011. [DOI] [Google Scholar]
- 60.Kalenova L.F., Novikova M.A., Subbotin A.M. Effects of permafrost microorganisms on skin wound reparation. Bull. Exp. Biol. Med. 2015;158:478–482. doi: 10.1007/s10517-015-2789-9. [DOI] [PubMed] [Google Scholar]
- 61.Lee H., Kim H., Kim J.-H., Park S.-D., Shim J.-J., Lee J.-L. Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract promotes human dermal papilla cell cytoprotective effect and hair regrowth rate in C57bl/6 mice. Molecules. 2022;27 doi: 10.3390/molecules27238235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baek Y.-H., Lee J.-H., Chang S.-J., Chae Y., Lee M.-H., Kim S.-H., Han K.-I., Kim T.-J. Heat-killed Enterococcus faecalis EF-2001 induces human dermal papilla cell proliferation and hair regrowth in C57bl/6 mice. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23105413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee J., Yang W., Hostetler A., Schultz N., Suckow M.A., Stewart K.L., Kim D.D., Kim H.S. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16 doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoon Y.C., Ahn B.H., Min J.W., Lee K.R., Park S.H., Kang H.C. Stimulatory effects of extracellular vesicles derived from Leuconostoc holzapfelii that exists in human scalp on hair growth in human follicle dermal papilla cells. Curr. Issues Mol. Biol. 2022;44:845–866. doi: 10.3390/cimb44020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hai Z., Ren Y., Hu J., Wang H., Qin Q., Chen T. Evaluation of the treatment effect of aloe vera fermentation in burn injury healing using a rat model. Mediat. Inflamm. 2019;2019 doi: 10.1155/2019/2020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Priya K., Surabhi D., Sarath R., Ramya M. Isolation and characterization of active metabolites produced from probiotic isolates against dandruff causing Malassezia furfur (MTCC: 1374t) J. Microbiol. Biotechnol. Food Sci. 2021;10:1–5. doi: 10.15414/jmbfs.3522. [DOI] [Google Scholar]
- 67.Oike H., Aoki-Yoshida A., Kimoto-Nira H., Yamagishi N., Tomita S., Sekiyama Y., Wakagi M., Sakurai M., Ippoushi K., Suzuki C., Kobori M. Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci. Rep. 2016;6 doi: 10.1038/srep23556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chae M., Kim B.J., Na J., Kim S.-Y., Lee J.O., Kim Y.-J., Lee E., Cho D., Roh J., Kim W. Antimicrobial activity of Lactiplantibacillus plantarum APsulloc 331261 and APsulloc 331266 against pathogenic skin microbiota. Frontiers in Bioscience - Elite. 2021;13:237–248. doi: 10.52586/E881. [DOI] [PubMed] [Google Scholar]
- 69.Nam W., Kim H., Bae C., Kim J., Nam B., Kim J., Park S., Lee J., Sim J. Lactobacillus paracasei HY7015 promotes hair growth in a telogenic mouse model. J. Med. Food. 2021;24:741–748. doi: 10.1089/jmf.2020.4860. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L.-J., Tian X.-Y., Zhao J., Shi R.-W., Zhang Y., Guan H.-B. Effects of Lactobacillus acidophilus LA-GHB1756 on alleviating inflammatory bowel disease in mice caused by aspirin. Med. J. Chin. Peoples Liberation Army. 2022;47:976–983. doi: 10.11855/j.issn.0577-7402.2022.10.0976. [DOI] [Google Scholar]
- 71.Wang L., Yu T., Zhu Y., Luo Y., Dong F., Lin X., Zhao W., He Z., Hu S., Dong Z. Amplicon-based sequencing and co-occurrence network analysis reveals notable differences of microbial community structure in healthy and dandruff scalps. BMC Genom. 2022;23 doi: 10.1186/s12864-022-08534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee J., Yang W., Hostetler A., Schultz N., Suckow M.A., Stewart K.L., Kim D.D., Kim H.S. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee H., Kim H., Kim J.-H., Park S.-D., Shim J.-J., Lee J.-L. Lactobacillus paracasei HY7015 and Lycopus lucidus Turcz. Extract promotes human dermal papilla cell cytoprotective effect and hair regrowth rate in C57bl/6 mice. Molecules. 2022;27 doi: 10.3390/molecules27238235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi B.Y. Targeting wnt/β-catenin pathway for developing therapies for hair loss. Indian J. Manag. Sci. 2020;21:4915. doi: 10.3390/ijms21144915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacDonald B.T., He X. Frizzled and LRP5/6 receptors for Wnt/-catenin signaling. Cold Spring Harbor Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a007880. a007880–a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stamos J.L., Weis W.I. The -catenin destruction complex. Cold Spring Harbor Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a007898. a007898–a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo Q., Kim A., Li B., Ransick A., Bugacov H., Chen X., Lindström N., Brown A., Oxburgh L., Ren B., McMahon A.P. A β-catenin-driven switch in TCF/LEF transcription factor binding to DNA target sites promotes commitment of mammalian nephron progenitor cells. Elife. 2021;10 doi: 10.7554/eLife.64444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X., Lyle S., Liu Y., Solky B., Cotsarelis G. Differential expression of cyclin D1 in the human hair follicle. Am. J. Pathol. 2003;163:969–978. doi: 10.1016/S0002-9440(10)63456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Su H.Y., Hickford J.G., Bickerstaffe R., Palmer B.R. Insulin-like growth factor 1 and hair growth. Dermatol. Online J. 1999;5:1. [PubMed] [Google Scholar]
- 80.Hayashi Y., Yamamoto N., Nakagawa T., Ito J. Insulin-like growth factor 1 inhibits hair cell apoptosis and promotes the cell cycle of supporting cells by activating different downstream cascades after pharmacological hair cell injury in neonatal mice. Mol. Cell. Neurosci. 2013;56:29–38. doi: 10.1016/j.mcn.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Kim J., Shin J.Y., Choi Y.-H., Kang N.G., Lee S. Anti-hair loss effect of adenosine is exerted by cAMP mediated wnt/β-catenin pathway stimulation via modulation of Gsk3β activity in cultured human dermal papilla cells. Molecules. 2022;27:2184. doi: 10.3390/molecules27072184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louden E., Chi M.M., Moley K.H. Crosstalk between the AMP-activated kinase and insulin signaling pathways rescues murine blastocyst cells from insulin resistance. Reproduction. 2008;136:335–344. doi: 10.1530/REP-08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu M., Liu X., Wang Y., Sui Y., Liu F., Liu Z., Zou F., Zuo K., Wang Z., Sun W., Xu Q., Liu D., Liu J. Intrinsic ROS drive hair follicle cycle progression by modulating DNA damage and repair and subsequently hair follicle apoptosis and macrophage polarization. Oxid. Med. Cell. Longev. 2022;2022:1–35. doi: 10.1155/2022/8279269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melincovici C.S., Boşca A.B., Şuşman S., Mărginean M., Mihu C., Istrate M., Moldovan I.M., Roman A.L., Mihu C.M. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018;59:455–467. [PubMed] [Google Scholar]
- 85.Popescu A.M., Purcaru S.O., Alexandru O., Dricu A. New perspectives in glioblastoma antiangiogenic therapy, Wo. 2016;2:109–118. doi: 10.5114/wo.2015.56122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y., Fan Z., Wang X., Mo M., Zeng S.B., Xu R.-H., Wang X., Wu Y. PI3K/Akt signaling pathway is essential for de novo hair follicle regeneration. Stem Cell Res. Ther. 2020;11:144. doi: 10.1186/s13287-020-01650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bukowiecki J., Pförringer D., Thor D., Duscher D., Brett E. HIF-1α stimulators function equally to leading hair loss agents in enhancing dermal papilla growth. Skin Pharmacol. Physiol. 2020;33:309–316. doi: 10.1159/000512123. [DOI] [PubMed] [Google Scholar]
- 88.Grimshaw S.G., Smith A.M., Arnold D.S., Xu E., Hoptroff M., Murphy B. The diversity and abundance of fungi and bacteria on the healthy and dandruff affected human scalp. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boxberger M., Cenizo V., Cassir N., La Scola B. Challenges in exploring and manipulating the human skin microbiome. Microbiome. 2021;9:125. doi: 10.1186/s40168-021-01062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rademacher F., Gläser R., Harder J. Antimicrobial peptides and proteins: interaction with the skin microbiota. Exp. Dermatol. 2021;30:1496–1508. doi: 10.1111/exd.14433. [DOI] [PubMed] [Google Scholar]
- 91.Jung Y.-O., Jeong H., Cho Y., Lee E.-O., Jang H.-W., Kim J., Nam K., Lim K.-M. Lysates of a probiotic, Lactobacillus rhamnosus, can improve skin barrier function in a reconstructed human epidermis model. Indian J. Manag. Sci. 2019;20:4289. doi: 10.3390/ijms20174289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai W.-H., Chou C.-H., Chiang Y.-J., Lin C.-G., Lee C.-H. Regulatory effects of Lactobacillus plantarum -GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021;18:1114–1120. doi: 10.7150/ijms.51545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zareie E., Mansouri P., Hosseini H., Sadeghpour O., Shirbeigi L., Hejazi S., Emtiazy M. Effect of oral administration of Triphala, a polyphenol-rich prebiotic, on scalp sebum in patients with scalp seborrhea a randomized clinical trial. J. Dermatol. Treat. 2022;33:1011–1016. doi: 10.1080/09546634.2020.1800568. j.cub.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 94.Rathnayake D., Sinclair R. Male androgenetic alopecia. Expet Opin. Pharmacother. 2010;11:1295–1304. doi: 10.1517/14656561003752730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this paper.