Abstract
Chronic pain, a complex and debilitating condition, poses a significant challenge to both patients and healthcare providers worldwide. Conventional pharmacological interventions often prove inadequate in delivering satisfactory relief while carrying the risks of addiction and adverse reactions. In recent years, electric neuromodulation emerged as a promising alternative in chronic pain management. This method entails the precise administration of electrical stimulation to specific nerves or regions within the central nervous system to regulate pain signals. Through mechanisms that include the alteration of neural activity and the release of endogenous pain-relieving substances, electric neuromodulation can effectively alleviate pain and improve patients' quality of life. Several modalities of electric neuromodulation, with a different grade of invasiveness, provide tailored strategies to tackle various forms and origins of chronic pain. Through an exploration of the anatomical and physiological pathways of chronic pain, encompassing neurotransmitter involvement, this narrative review offers insights into electrical therapies’ mechanisms of action, clinical utility, and future perspectives in chronic pain management.
Keywords: Chronic pain, Pain mechanisms, Neuromodulation, Deep Brain Stimulation, Direct Cortical Stimulation, Spinal Cord Stimulation, Motor Cortex Stimulation, Peripheral Nerve Stimulation, Transcranial Focused Ultrasound
Introduction
The International Association for the Study of Pain (IASP) defines pain as "an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage" [1]. In 2013, the IASP established a task force to develop and update a classification of pain disorders. This effort led to the inclusion of a chronic pain classification in the 2019 edition of the International Classification of Diseases (ICD-11), formally adopted by the World Health Organization [2, 3]. Chronic pain is defined by a duration of at least three months or beyond the usual healing period. It can be further categorized into nociceptive, neuropathic, and nociplastic pain types [4]. Chronic pain presents a significant public health challenge, affecting millions of patients and incurring substantial medical expenses and lost productivity [5, 6]. According to the Global Burden of Disease Study, tension-type headache, migraine, low back pain, neck pain, diabetic neuropathy are among the most common and prevalent chronic pain syndromes in the population, increasing their years lived with disability [6].
In a certain percentage of patients, conventional treatments, including surgery, pharmacological therapies, combined with psychological, physical and occupational therapies, fail [7–9]. In these patients, neuromodulation may have a role in treatment. Neuromodulation refers to a broad category of techniques or therapies that modulate the activity of the nervous system to achieve therapeutic effects through chemical (i.e. pharmacological) interventions or electrical stimulation (i.e. neuromodulation). Neurostimulation, which involve modifying or stimulating nerve activity through targeted electrical at specific neurological sites, is increasingly utilized in patients with chronic pain of varied origins [10]. These modalities represent the evolving landscape for chronic pain management, each offering unique advantages and considerations [11].
This review aims to provide a comprehensive understanding of the neuroanatomy and neurophysiology of chronic pain, with an emphasis on the role of electrical neuromodulation in its treatment.
Neuroanatomy and neurophysiology of chronic pain
Pain pathways are intricate and dynamic systems comprising sensory, cognitive, and behavioral components. These mechanisms have evolved to recognize, integrate, and coordinate protective responses to noxious stimuli. They encompass both primitive spinal reactions and the nuanced emotional experiences consciously identified as pain in humans [12].
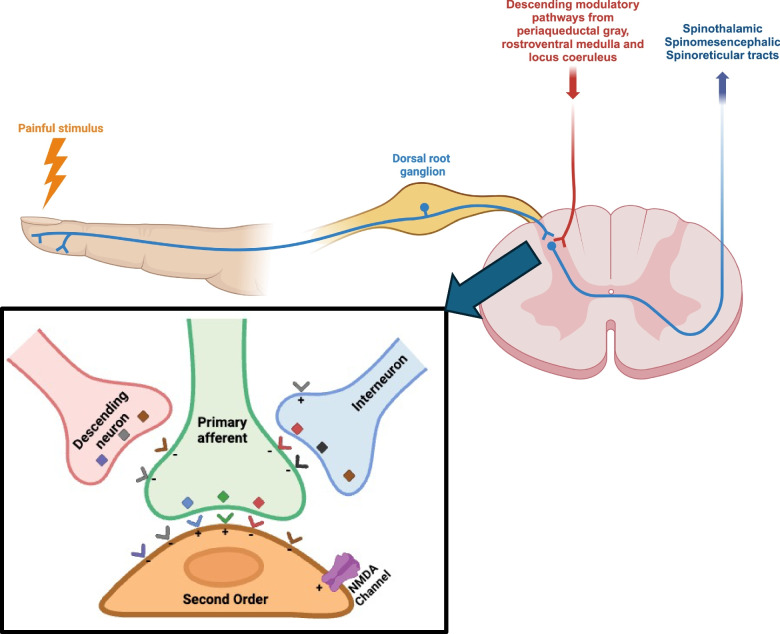
In addition to the peripheral pain pathways, which include receptors and neural fibers, the central nervous system (CNS) pain pathways involve a network of structures. These structures comprise the spinal cord, thalamus, amygdala, hypothalamus, periaqueductal grey (PAG) matter, basal ganglia, insular and cingulate cortices, as well as the sensory and motor cortices [13]. Figure 1 illustrates a schematic representation of pain pathways.
Fig. 1.
Anatomical pathways of chronic pain and neurotransmitters. The figure depicts the ascending and descending anatomical pathways of pain modulation. In the inner panel, neurotransmitters (depicted as squares) and receptors (depicted as V shapes) are illustrated. These include GABA (red), glycine (black), opiates (brown), norepinephrine (gray), glutamate (green), substance P (light blue), and serotonin (violet), each with their respective inhibitory (-) or excitatory ( +) activity indicated
Pain signaling from periphery to ascending pathways
The intricate process of a painful stimulus begins in the periphery with specialized nociceptors activated by stimuli like pressure, extreme temperatures, or tissue damage. Nociceptors transmit the pain stimulus to primary afferent fibers, responsible for carrying sensory information from the periphery to the CNS. Classified based on diameter and conduction velocity, two basic types of nerve fibers exist: myelinated Type A and unmyelinated Type C. Pain transmission occurs through two types: rapid (associated with Aδ-fibers) and slow (related to polymodal receptors and C fibers), each transmitting pain at different speeds. The cell bodies of sensory neurons are situated in the dorsal root ganglia (DRG); these neurons are capable of encoding and transmitting information derived from external stimuli. As these primary afferent fibers approach the spinal cord, they enter through the dorsal roots (i.e., dorsal root entry zone) [12, 13]. Inside the spinal cord, unmyelinated and small myelinated axons project laterally to enter the Lissauer tract, a marginal zone or layer of the dorsal horn. Here, they synapse with neurons in the dorsal horn. Aδ fibers ascend three to four segments in the Lissauer tract before terminating in the Rexed’s lamina I, II outer, or V. In contrast, C fibers typically ascend one segment before ending in lamina II.
One of the principal conduits in this relay of pain signals is the spinothalamic tract. This major ascending pathway serves as a dedicated highway for transmitting pain signals to the brain. The second-order neurons of the spinothalamic tract, crucial intermediaries in this relay, undergo a remarkable anatomical phenomenon known as decussation. These neurons cross over to the contralateral side of the spinal cord before ascending towards the thalamus, a central hub for sensory processing [14]. The thalamus acts as a sensory relay station, receives the pain signals, and orchestrates their distribution to various cortical areas. From the thalamus, projections fan out to regions of the brain responsible for the conscious perception of pain. These cortical areas, intricately connected and functioning in concert, give rise to the multifaceted experience of pain that encompasses its sensory, emotional, and cognitive dimensions [14].
The spinoreticular tract, diverging from traditional sensory pathways, projects to the reticular formation in the brainstem, influencing emotional and autonomic aspects of pain perception. Additionally, the spinocerebellar tract, known for proprioceptive transmission, also contributes to nociception and motor responses to pain, further intertwining sensory perception and motor output [13].
Pain modulation from descending pathways
As pain signals ascend from the periphery to the brain through the intricate pathways, a parallel network of descending modulatory pathways operates in concert, finely tuning the transmission and perception of pain, maintaining a delicate balance between the intensity of noxious stimuli and the perceptual experience of pain.
One of the most prominent players in the descending modulatory orchestra is the descending inhibitory pathway. This pathway exerts its influence through a series of neural structures, i.e. the PAG, situated around the cerebral aqueduct in the midbrain, and the rostroventral medulla (RVM), a region placed in the brainstem. These regions control the release of endogenous opioids and other neurotransmitters that act as pain inhibitors [15, 16]. In particular, the PAG integrates information from various brain regions; its activation largely reduces perception of pain [17].
Descending from the PAG, the inhibitory signals make their way to the RVM. The RVM acts as a relay station, forwarding the inhibitory commands to the spinal cord. Within the spinal cord, these commands target nociceptive neurons, the primary relay stations for pain signals. The release of endogenous opioids, such as enkephalins and endorphins, is a hallmark of this inhibitory process [18]. These opioids bind to receptors on nociceptive neurons, effectively dampening their activity and reducing the transmission of pain signals. Beyond endogenous opioids, neurotransmitters like serotonin and norepinephrine also play crucial roles in the descending inhibitory pathway. The release of these neurotransmitters contributes to the overall suppression of nociceptive signals. Serotonin is known for its involvement in pain modulation and mood regulation, highlighting the intricate interplay between sensory and emotional components in pain perception[18].
The descending inhibitory pathway not only reduces the transmission of pain signals but also contributes to the phenomenon of "pain gating". The Gate Control Theory, proposed by Melzack and Wall, suggests that non-nociceptive inputs can modulate pain perception [19].
The dorsal column of the spinal cord comprises various neural fibers, with certain types such as Aδ and C fibers being triggered by painful stimuli, while the larger Aβ fibers serve to suppress the transmission of these signals. According to the "Gate Control Theory," the interplay between the activity of Aδ and C fibers versus that of Aβ fibers dictates the perception of painful stimuli [7].
RVM is also known for the presence of "on-cells," which release neurotransmitters such as Substance P, glutamate, and norepinephrine. These neurotransmitters have the effect of amplifying the activity of nociceptive neurons in the spinal cord rather than inhibiting them [18].
Neurotransmitters of pain
Multiple neurotransmitters participate in both transmitting and modulating pain signals, exerting effects that can either enhance or suppress the perception of pain [20].
Substance P, a neuropeptide functioning as an excitatory neurotransmitter, is primarily synthesized and secreted by nociceptive neurons, transmitting and modulating pain signals throughout the CNS [21]. In the spinal cord, Substance P is released both at synaptic junctions and non-synaptic sites within the dorsal horn. It binds to tachykinins (NK1, NK2 and NK3) receptors located on lamina 1 neurons and the dendrites of lamina 5 neurons [22]. This interaction facilitates the transmission of pain signals from peripheral nerves to the CNS and assists in relaying these signals to higher brain regions responsible for processing pain perception. Additionally, Substance P can induce the release of other neurotransmitters, such as glutamate, thereby intensifying the pain signals [23]. Moreover, Substance P contributes to the emotional and affective dimensions of pain perception, influencing brain areas associated with mood regulation, stress responses, and emotional processing [24].
Glutamate is another excitatory neurotransmitter in the CNS involved in the conduction of pain signals by activating amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) and N-Methyl-D-Aspartate (NMDA) receptors on postsynaptic neurons in the dorsal horn of the spinal cord [25]. This activation induces the depolarization of postsynaptic neurons, generating action potentials and transmitting pain signals along ascending pathways to the brain centers [26]. NMDA receptors are crucial for amplifying and modulating pain signals, especially in chronic pain conditions. Unlike AMPA receptors, NMDA receptors necessitate both glutamate binding and postsynaptic depolarization for full activation of their ion channels. This property allows NMDA receptors to integrate synaptic activity and contribute to the phenomenon of central sensitization, wherein pain signals are amplified and prolonged in chronic pain conditions [27].
Calcitonin Gene-Related Peptide (CGRP) is a neuropeptide synthetized and released from sensory nerves in response to noxious stimuli, inflammation, and tissue injury. CGRP is a potent vasodilator, and it contributes to inflammation and hypersensitivity [28]. In addition, it enhances the excitability of sensory neurons and promotes the release of other neurotransmitters, such as substance P [29]. CGRP has received significant attention in the context of migraine since elevated levels have been found in the blood and cerebrospinal fluid during attacks. Therefore, CGRP has become a target for novel treatments for these patients [30].
Norepinephrine is released from descending pathways (particularly from the locus coeruleus), having both inhibitory and excitatory effects on pain transmission. Its net effect depends on the receptors it acts upon, with α2-adrenergic receptors generally inhibiting pain signals [31, 32]. Direct stimulation of the PAG or RVM elevates norepinephrine levels in the cerebrospinal fluid, then modulating pain transmission in the spinal cord by inhibition of the release of glutamate and substance P [33].
Serotonin, also known as 5-hydroxytryptamine (5-HT), is another neurotransmitter involved in pain modulation. It exhibits both excitatory and inhibitory effects on pain transmission, which are contingent upon the receptor subtype activated and the neural pathways engaged [34]. 5-HT exerts its effects through multiple receptor subtypes (including 5-HT1, 5-HT2, 5-HT3, and so on), differently distributed throughout the nervous system and with distinct functional properties. Of note, 5-HT is characterized by an excitatory or inhibitory effect on pain transmission according to the activated subtype of receptors [35]. For example, 5-HT2 and 5-HT3 receptors enhance pain transmission by increasing neuronal excitability and amplifying the signaling of pain pathways. Conversely, 5-HT1 receptors suppress the release of excitatory neurotransmitters, dampen neuronal activity, and reduce the transmission of pain signals, resulting in pain relief [34]. It's worth noting that in the typical condition, 5-HT concentrations are relatively low, with a certain level of facilitation mediated by 5-HT3 and 5-HT2 receptors. Subsequent elevations in spinal 5-HT then result in the inhibitory actions of the 5-HT7 receptor [35].
5-HT exerts regulatory effects on pain pathways across various levels of the nervous system. Apart from its role in the spinal cord, it also impacts pain processing in several brain regions associated with pain and anxiety, including the thalamus, frontal cortex, amygdala, and brainstem [36].
Gamma-Aminobutyric Acid (GABA) is the principal inhibitory neurotransmitter in the CNS. GABA produces its inhibitory effects by inducing hyperpolarization of postsynaptic neurons, thereby increasing the negativity of the neuron's membrane potential. This hyperpolarization reduces the likelihood of the neuron generating an action potential, leading to decreased excitability and limiting the propagation of signals along the neural pathway [37]. Within pain modulation, GABAergic activity mitigates pain signals, regulating both the intensity and duration of pain perception [24]. In the spinal cord, GABA is released onto postsynaptic neurons subsequent to receiving inputs from primary nociceptive neurons, exerting inhibitory control [38]. Conversely, GABA modulates various regions in the brain such as the thalamus, where it inhibits transmission to the cortex, as well as the somatosensory cortex, insula, and anterior cingulate cortex. Furthermore, GABA influences descending pain pathways including the PAG and RVM [39]. Additionally, GABAergic inhibition extends to the emotional and affective dimensions of pain processing, impacting brain regions like the amygdala and prefrontal cortex, thus modulating emotions such as fear, anxiety, and depression [24].
Endorphins and enkephalins, endogenous opioids synthesized in diverse CNS locales such as the hypothalamus, pituitary gland, and spinal cord, serve as neurotransmitters and neuromodulators, predominantly triggered by stress, pain, and physical exertion [40]. A key function of these compounds is pain signal inhibition, facilitated by their binding to mu (μ), delta (δ), and kappa (κ) opioid receptors on neurons in the spinal cord and brain, initiating intracellular cascades that lead to neuronal suppression [41]. Within the spinal cord, endorphins and enkephalins hinder the release of substance P and glutamate while diminishing the excitability of pain-transmitting neurons, thereby modulating the transmission of pain signals from the periphery to the brain. Moreover, these opioids exert their analgesic effects across various brain regions, including the PAG, RVM, thalamus, and limbic system [42].
Peripheral and central sensitization
Peripheral sensitization is a condition defined by the IASP as a state of “Increased responsiveness and diminished threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields” [43, 44]. This phenomenon occurs because of chemical mediators released by nociceptors and various non-neuronal cells, such as mast cells, basophils, platelets, macrophages, neutrophils, endothelial cells, keratinocytes, and fibroblasts, at the site of tissue injury or inflammation. A plethora of signaling molecules is involved in this process, including protons, ATP, prostaglandins (PGE2), thromboxanes, leukotrienes, endocannabinoids, growth factors (neurotrophins, granulocyte- or granulocyte-macrophage colony-stimulating factors), cytokines (IL6, IL1β, TNFα), chemokines, neuropeptides (CGRP, substance P, bradykinin, histamine), lipids, and various proteases [45–49].
Conversely, central sensitization is a complex phenomenon defined by an amplification of neural signaling within the CNS that elicits pain hypersensitivity [50]. In particular, it is marked by lasting changes in the excitability of second-order neurons within the spinal cord, induced by increased afferent activity. This results in significant alterations to the somatosensory system itself [51]. The profound significance of this concept was emphasized by the description of the long-term potentiation in the hippocampus, revealing that synchronous high-frequency input enhances synaptic efficacy [52]. Central sensitization has been postulated to contribute in several chronic pain syndromes, such as rheumatoid arthritis, osteoarthritis, temporomandibular disorders, fibromyalgia, musculoskeletal disorders, headache, neuropathic pain, complex regional pain syndrome [50, 53].
Neurostimulation techniques
The fundamental principle of neurostimulation in chronic pain revolves around precisely delivering electrical impulses to modulate nervous system activity, thereby modifying pain perception, and providing relief. This approach aims to interrupt or dampen pain signals along neural pathways, by either stimulating specific nerves to block pain transmission or influencing the brain's processing of pain signals [54]. More precisely, in the complexity of anatomical and physiological mechanisms of chronic pain, neurostimulation, in its various forms, interferes with the hyperexcitability of circuits involved in pain processing (such as the spinal cord stimulation and peripheral nerve stimulation) [55, 56], enhances endogenous pain inhibition (such as the motor cortex stimulation and the transcranial magnetic stimulation) [57–59], or modulates the affective component of pain [54, 60, 61]. Neurostimulation is an alternative or adjunctive approach to manage chronic pain, particularly when medical or surgical treatments have been ineffective or associated with undesirable side effects [54].
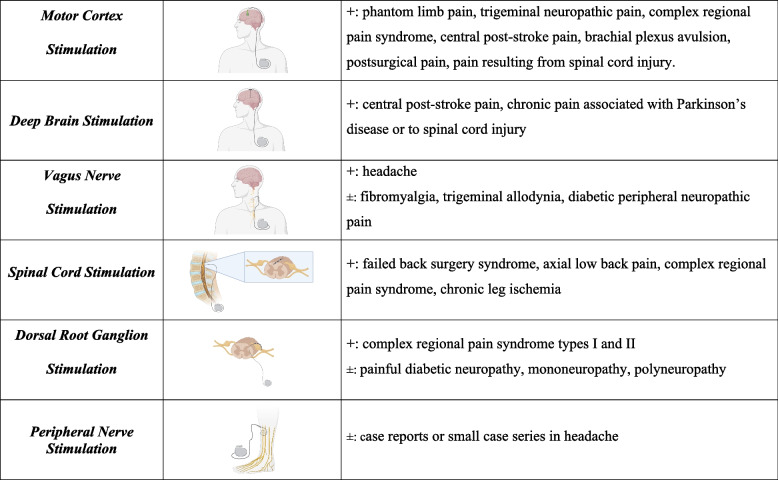
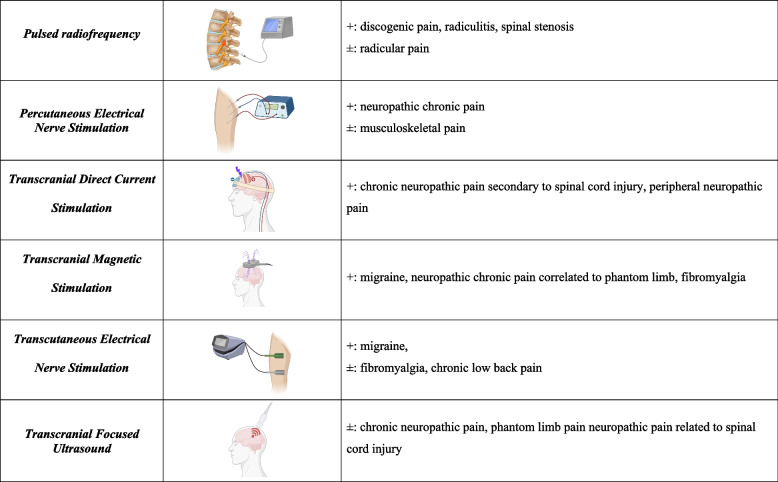
Neurostimulation therapies can be classified by invasiveness. Invasive methods involve surgical implantation of electrodes or devices, including motor cortex stimulation, deep brain stimulation, vagus nerve stimulation, spinal cord stimulation, and peripheral nerve stimulation (Figure 2). Minimally invasive modalities, like pulsed radiofrequency therapy and percutaneous electrical nerve stimulation are less intrusive but involve some intervention (Figure 3). Lastly, noninvasive techniques, such as transcranial direct current stimulation, transcranial magnetic stimulation, transcutaneous electrical nerve stimulation, transcranial focused ultrasound, do not require any surgical intervention or implantation and are typically administered externally to the body (Figure 3) [54].
Fig. 2.
Invasive neurostimulation techniques. The invasive techniques for neurostimulation of chronic pain are depicted alongside their current indications with clear ( +) or unclear ( ±) evidence. The specific characteristics of each modality are explained in the text
Fig. 3.
Mini-invasive and non-invasive neurostimulation techniques. The mini-invasive (i.e. pulsed radiofrequency therapy and percutaneous electrical nerve stimulation) and non-invasive (i.e. transcranial direct current stimulation, transcranial magnetic stimulation, transcutaneous electrical nerve stimulation and transcranial focused ultrasound) techniques for neurostimulation of chronic pain are schematized in the representation alongside their current indications with clear ( +) or unclear ( ±) evidence. The specific characteristics of each modality are explained in the text
Motor cortex stimulation
Motor Cortex Stimulation (MCS) consists in the implantation of an array of electrodes directly onto the motor cortex of the brain contralateral to the painful side [62, 63]. Particularly, after craniotomy, the electrode array is positioned in the precentral gyrus or central sulcus corresponding to the painful area either in the epidural or subdural spaces [64, 65]. Although there is not an evidence-based difference, opting for subdural lead placement may be more reasonable due to the reduced distance between the leads and the brain cortex compared to epidural placement [66].
MCS is theorized to alleviate pain by influencing both the emotional dimension of pain and inhibiting pain signals at different neural levels. Specifically, MCS relieves pain by activating the perigenual cingulate and orbitofrontal cortex, thus modulating the emotional component of pain. Simultaneously, MCS inhibits pain impulses at the spinal cord level by activating the PAG [67].
The research of MCS as a potential treatment for various neuropathic chronic pain populations has yielded inconsistent results. This variability is primarily attributable to small sample sizes and study designs of lower quality, suggesting that MCS is efficient only in specific subgroups of patients. Noteworthy, MCS has demonstrated effectiveness in addressing chronic pain associated with phantom limb pain, trigeminal neuropathic pain, complex regional pain syndrome, central post-stroke pain, brachial plexus avulsion, postsurgical pain, and pain resulting from spinal cord injury [66, 68–74].
Deep brain stimulation
Deep Brain Stimulation (DBS) consists in the surgical implantation of thin electrodes within specific area of the brain to be stimulated. In case of chronic pain, electrodes are generally positioned in the PAG, in the periventricular gray matter, the ventralposterior medial or the ventral posterior lateral nuclei of the thalamus (contralateral to the side of pain), the anterior cingulate cortex or the posterior insula [75, 76]. The positioning generally required a frame-based or frame-less stereotactic technique, targeting the area with a magnetic resonance imaging on the day before surgery and, in some centers, with intraoperative microelectrode recordings. Electrodes are then connected to an impulse generator that neuromodulate the target area [77].
When PAG or thalamus are stimulated, DBS seems to promote the secretions of endogenous opioids (i.e. beta-endorphin and methionine enkephalin) [78, 79]. Beside these reports, the other underlying mechanisms are still obscure.
Clinical studies have demonstrated a significant improvement in patients with central post-stroke pain [80, 81], in chronic pain associated with Parkinson’s disease [82, 83] and in those patients with spinal cord injury [84]. The most favorable outcomes in DBS, characterized by effects surpassing 50%, were observed when stimulating the somatosensory thalamus in individuals experiencing peripheral neuropathic pain [85, 86]. Nevertheless, the current trend indicates a decline in the number of patients undergoing implantation, primarily attributed to the emergence of new and less invasive treatment modalities. In addition, although considered safe, DBS is affected by adverse event in 8–9% of patients, including lead fractures, wound infections, intra-operative seizure and postoperative burr hole site erosion [87]. It is noteworthy that individuals exhibiting severe, debilitating neuropathic pain accompanied by verifiable pathology, resistant to conventional treatments, appear to be the most suitable candidates for this approach.
Vagus nerve stimulation
Vagus Nerve Stimulation (VNS) is a therapeutic method that employs electrical impulses to activate the vagus nerve, a component of the autonomic nervous system. Although widely recognized for its efficacy in treating epilepsy [88] and depression [89], VNS has also been investigated in chronic pain [90]. VNS involves the surgical placement of a stimulator device, delivering regular electrical impulses, connected to electrodes stimulating the vagus nerve. More recently, transcutaneous VNS and minimally invasive form of percutaneous VNS have been also developed [90].
The vagus nerve’s afferent pathway initiates from vagal afferents that supply the cervical, thoracic, and abdominal organs. Primarily traversing the nucleus of the solitary tract, it ultimately reaches higher brain regions, including the thalamus, hypothalamus, parabrachial nucleus, PAG, amygdala, and locus coeruleus [91–94]. The stimulation of vagus nerve modulates the descending serotoninergic and noradrenergic neurons, modulating the central sensitization and reducing the pain transmission [90]. VNS also induces anti-inflammatory effects through diverse pathways, such as the cholinergic anti-inflammatory pathway, the hypothalamic–pituitary–adrenal axis pathway, and the production of specialized pro-resolving mediators. Specifically, VNS initiates the release of acetylcholine from Vagus Nerve efferents in the coeliac ganglia and norepinephrine from the splenic nerve, subsequently promoting further release of acetylcholine [95]. Following its release, acetylcholine binds to the α7 nicotinic Ach receptors present on macrophages, leading to a decrease in cytokine production [95, 96]. Additionally, VNS activates the hypothalamic–pituitary–adrenal axis, triggering the release of adrenocorticotropic hormone. This hormone, in turn, acts on the adrenal glands, promoting the synthesis of cortisol [97]. Furthermore, VNS has the potential to induce the production of specialized pro-resolving mediators [98], which act on receptors expressed on immune cells, gliacytes, and neurons, thereby exerting a modulatory effect on inflammation and neuroinflammation [99–101].
Therapeutic effects on chronic pain have been casually discovered during the application of VNS for epilepsy. Some epileptic patients reported a reduction in headache frequency and intensity that after VNS implantation [102–104]. Following these first reports, several studies demonstrated the efficiency of VNS in preventing and reducing the intensity of headache, either with invasive implantation [105] and during transcutaneous stimulation [106–109]. Given the efficacy in modulation of central sensitization [90] and in depressive disease [89], VNS was reported to successfully reduce symptoms in patients with fibromyalgia [110], although these results were not further confirmed by other investigations [111]. VNS has been also investigated in other scenarios of chronic neuropathic pain (i.e. trigeminal allodynia and diabetic peripheral neuropathic pain) in animal models [112–115]. However, data in patients still lack and clinical studies are needed to validate the positive findings reported by animal studies.
Spinal cord stimulation
Spinal Cord Stimulation (SCS) is a neuromostimulation technique designed to address and alleviate neuropathic chronic pain. This method involves an implanted generator that produces pulsed electrical signals, which are then delivered to a specific area of the spinal cord through electrodes implanted within the epidural space [7]. Advancements in SCS techniques and equipment are ongoing, with dedicated literature available elsewhere [7]. SCS operates through neurophysiological and neurochemical mechanisms rooted in the "gate control theory” for pain transmission [7, 19].
SCS disrupts the processing of nociceptive signals through the lateral spinothalamic tract, influencing supraspinal brain centers like the ventral posterior nucleus of the thalamus, somatosensory cortex, cingulate cortex, and insula [116, 117]. Orthodromically, SCS depolarizes Aβ fibers cranially, controlling supraspinal centers such as the cuneate and gracile nuclei [118, 119]. After supraspinal integration, descending feedback loops from the locus coeruleus [120], nucleus raphe magnus [121], and rostral ventromedial medulla [122] modulate and control the spinal nociceptive signal at the "spinal gate" via serotonergic and noradrenergic projections to the dorsal horn [118, 119].
In terms of neurochemical mechanisms, SCS modulates gamma-aminobutyric acid (GABA) [123], serotonin [124], acetylcholine and norepinephrine [125, 126]. Studies in animal models show increased intraspinal release of GABA [127, 128] and attenuation of glutamate and aspartate responses [129]. Notably, GABA type "b" receptors play a crucial role, suggesting the potential for intrathecal baclofen administration to enhance SCS analgesia [130]. SCS also elevates serotonin and substance P release [124], enhances dynorphin and enkephalin expression [131], and decreases neuronal excitability and spinal pain transmission by activating 5-HT2A, 5-HT3, and 5-HT4 receptors [132]. Additionally, SCS promotes analgesia by modulating cholinergic and adrenergic neurotransmission, releasing acetylcholine and noradrenaline in the dorsal horn of the spinal cord [125, 126].
SCS is considered for those patients experiencing chronic pain, particularly when other conventional treatments (including pharmacological therapies, surgical treatments or physiotherapy) have proven ineffective or are associated with intolerable side effects. To define the indication of SCS implantation, the selection of the patient is fundamental for its success [133]. Firstly, SCS should be contemplated within two years of symptom onset, following the ineffectiveness of all standard therapies [134, 135]. It should be mentioned that the time between initial pain diagnosis and SCS implantation is still debated. In a study by Kumar et al., a time to SCS treatment < 2 years was characterized by a higher long term success rate (around 85%), declining precipitously at the lengthening of the diagnosis to implantation interval [136]. Another study by the same group reported that the greatest improvement in pain relief was obtained in case of SCS implantation within one year from the onset of symptoms [137]. However, several studies have shown a symptom-to-implantation waiting time from 5 to 6 years [138, 139]. Secondly, individuals with underlying psychiatric conditions, complete cognitive impairment, psychological comorbidities, or substance abuse are ineligible for SCS implantation [140]. However, if there is partial cognitive impairment, SCS may be considered, with a preference for non-rechargeable over rechargeable implantable pulse generators [134]. Thirdly, SCS is a viable option for neuropathic pain conditions (e.g., failed back surgery syndrome, arachnoiditis, complex regional pain syndrome, causalgia, peripheral neuropathy, chronic radiculopathy), while its efficacy is limited for nociceptive symptoms or central neuropathic pain [134]. SCS is strongly recommended in specific scenarios, including failed back surgery syndrome without neurologic progression [141], axial low back pain [142] and complex regional pain syndrome [143]. Furthermore, SCS is recommended for chronic refractory angina unresponsive to maximal medical therapy, bypass surgery, and percutaneous angioplasty of the legs [144], as well as for peripheral artery disease or non-reconstructable critical leg ischemia [145]. In particular, SCS is employed in patients experiencing chronic leg ischemia that is not suitable for open surgical or endovascular intervention [146]. This condition is typically characterized by either rest pain alone (Fontaine stage III) or the presence of rest pain accompanied by arterial ulcers less than 3 cm in diameter (Fontaine stage IV) [147].
Dorsal root ganglion stimulation
Dorsal root ganglion (DRG) stimulation resembles SCS, but with devices specifically design to target the first-order sensory neurons located in the DRG. The DRG's advantageous position within the epidural space, immersed in cerebral spinal fluid, makes it an ideal target for stimulation, allowing precise targeting of chronic pain. DRG stimulation applies an electrical field directly over the nuclei of primary afferent neurons, enabling modulation prior to signal propagation within the spinal cord. This modulation potentially includes inhibition at synapses with second-order neurons in the dorsal horn [148].
Recent pre-clinical and clinical research is shedding light on the mechanisms and therapeutic effects of DRG stimulation, highlighting differences compared to SCS. Unlike SCS, DRG stimulation does not elevate the inhibitory GABA neurotransmitter in either the dorsal horn or the DRG itself [149, 150]. The most accepted mechanism of DRG stimulation involves impeding the transmission of afferent pain signals and ectopic action potential transmission, supported by basic science research [151–153]. However, its effects on multi-dermatomal conditions with a single lead placement may depend on orthodromic propagation, a mechanism often discussed in SCS but less so in DRG stimulation [154, 155]. DRG stimulation is believed to activate Aδ-, Aβ-, and C-fiber low threshold mechanoreceptor fibers, utilizing the endogenous opioid system to modulate touch and pain processes at clinically utilized frequencies [156–158]. Furthermore, DRG stimulation has effects on the sympathetic nervous system, including antidromic effects in the treatment of peripheral vascular disease, reduction in blood pressure, and attenuation of neuroinflammation [159–162].
In 2019, the Neuromodulation Appropriateness Consensus Committee highlighted the significant efficacy of DRG stimulation in treating both complex regional pain syndrome types I and II, as well as focal neuropathic pain with identifiable pathology [163]. In fact, a large prospective randomized controlled trial, comparing SCS to DRG stimulation in patients affected by complex regional pain syndrome types I and II, demonstrated that 81.2% of DRG patients achieved ≥ 50% pain relief compared to 55.7% in the SCS arm [164].
Today the DRG stimulation is mainly accepted for the treatment of complex regional pain syndrome types I and II, although it has been attempted in several other forms of neuropathic chronic pain (including painful diabetic neuropathy, mononeuropathy, polyneuropathy) with for these latter a low-quality study and limited evidence [148, 165].
Peripheral nerve stimulation
Peripheral Nerve Stimulation (PNS) consists in the delivery of electrical impulses through electrodes surgically implanted near peripheral nerves, to modulate the transmission of pain signals along these nerves [166]. As per the SCS, PNS engages neurophysiological and neurochemical mechanisms based on the "gate control theory" of pain transmission, as above discussed [7, 19].
PNS can be implanted in various anatomical regions, including the upper extremities (brachial plexus, suprascapular nerve, axillary nerve, radial nerve, median nerve, or ulnar nerve), lower extremities (sciatic nerve, obturator nerve, femoral nerve, lateral femoral cutaneous nerve, genicular nerve, saphenous nerve, common peroneal nerve, tibial nerve, sural nerve, and superficial peroneal nerve), as well as other nerves in the head (occipital nerve, trigeminal nerve, and supraorbital nerve), trunk, abdomen, back, or pelvis (medial branch nerve, ilioinguinal nerve, iliohypogastric nerve, genitofemoral nerve, cluneal nerve, and pudendal nerve) [166].
The available evidence on PNS in the head and neck region has predominantly centered in case reports or small case series around the stimulation of occipital nerves for the treatment of headache disorders with a positive therapeutic effect [167–170]. On the opposite, the evidence for facial pain is still weak, lacking high quality studies [171–174]. Indeed, the current guidelines do not recommend PNS for chronic facial pain, whereas they state that PNS can be considered in case of chronic migraine headache after failure of conventional treatments [175].
According to the guidelines [175], PNS can also be considered as a treatment option for mononeuropathies affecting the upper extremity, failed back surgery syndrome, and chronic low back pain (supported by substantial evidence). Additionally, it may be explored in cases of radiculopathy and post-herpetic neuralgia, although the evidence for these conditions is limited. Furthermore, PNS shows promise in managing neuropathic pain in the lower extremities, including post-amputation pain, as well as in individuals with Complex Regional Pain Syndrome Type I/II or peripheral causalgia (while acknowledging that SCS remains the preferred neurostimulation option) [175].
Pulsed radiofrequency therapy
Pulsed Radiofrequency Therapy (PRF) is a medical intervention employed in the management of chronic pain conditions. In contrast to continuous radiofrequency, which subjects target nerves or tissues to sustained high temperatures (70–90 °C) through electrical stimulation [176], PRF utilizes electrical pulses delivered to specific nerves via an electrode generating localized thermal effects without causing substantial tissue damage [177]. Specifically, PRF employs a radiofrequency current characterized by alternately repeated electrical stimulation with a short duration (around 20 ms) followed by a resting phase (around 480 ms). This alternating pattern induces a temperature on the target tissue that remains below 42 °C [178].
PRF induces a targeted and prolonged reduction in spinal sensitization mediated by C-fibers. As a result, it effectively inhibits the transmission of pain signals from peripheral nerves to the central nervous system [179, 180]. PRF is reversible and temporary, it provides relief for a variable duration, and the procedure may be repeated if necessary [178].
PRF is commonly applied to peripheral nerves, DRG, or other nerve structures contributing to chronic pain. Therefore, PRF is often used to modulate neural activity and disrupt pain signals without causing tissue damage, whereas continuous radiofrequency creates thermal lesions that disrupt nerve function and alleviate pain [176–178]. Based on these different effects, PRF is commonly employed for conditions where nerve-related pain is the primary concern, such as neuropathic pain syndromes or neuralgias. On the opposite, continuous radiofrequency is frequently used for conditions characterized by localized pain arising from specific anatomical structures, such as facet joints or sacroiliac joints [176–178].
The most important efficacy of PRF has been shown in the treatment of postherpetic neuralgia [181] targeting the areas near the DRG via the angulus costae [182] or paravertebral puncture [183] or targeting the intercostal nerves [184, 185].
In recent findings, the literature has indicated limited and weak evidence for the use of PRF on the lumbar DRG for radicular pain [186]; in facets’ pain, PRF has been also considered to be inferior to continuous radiofrequency and it should be used only in selected cases [187]. Similar conclusions have been also drawn for cervical radicular pain and lumbar face pain [187]. On the opposite, intradiscal PRF seems to be a promising technique in case of discogenic pain, radiculitis, or spinal stenosis, although further studies are still required to confirm this indication [187].
Percutaneous electrical nerve stimulation
Percutaneous Electrical Nerve Stimulation (PENS) is a procedure using thin needles inserted through the skin to deliver electrical impulses to specific nerves or tissues [188]. The objective is to alleviate persistent and chronic pain by stimulation of peripheral sensory nerves through the insertion of needles in the outer layers of the skin, characterized by high resistance [188].
Today, the evidence in favor of PENS is quite weak. PENS (alone or in adjunct to other treatments) was shown to not provide a sufficient advantage in the control of musculoskeletal pain [189]. In one study including 17 patients with neuropathic pain due to spinal cord injury, PENS reduced the symptoms in 63% of cases [190]. However, no other studies have been conducted so far in this population, and the indication still lacks clear evidence. Another trial conducted in a population of patients with chronic low back pain secondary to degenerative disk disease, PENS was more effective in reducing pain and post-treatment function, as compared to Transcutaneous Electrical Nerve Stimulation (TENS) or other exercise therapies [191]. Finally, in a randomized double-blind crossover trial on 31 patients with neuropathic chronic pain, preliminary findings indicated that PENS may offer effective short-term pain relief [192].
Unfortunately, the underlying mechanisms of action of PENS are still few investigated, although they are thought to rely in the Gate Control Theory [7]. Despite its potential benefits in pain management, the precise indications for PENS, as well as the most effective treatment parameters, remain topics of discussion and investigation within the medical community. Additionally, comparative studies evaluating PENS against other therapeutic modalities are limited, leaving uncertainties about its relative efficacy and place in treatment algorithms [189, 193, 194]. To address these gaps in knowledge, future research efforts should prioritize well-designed studies that investigate the clinical utility of PENS across various pain conditions. These studies should aim to delineate the specific patient populations most likely to benefit from PENS, identify optimal stimulation parameters, and compare its efficacy and safety to other established treatments [189, 193, 194]. Furthermore, incorporating mechanistic studies to elucidate the underlying pathways through which PENS exerts its therapeutic effects can provide valuable insights into its mode of action and potential advantages over alternative interventions.
Transcranial direct current stimulation
Transcranial Direct Current Stimulation (tDCS) involves the non-invasive delivery of low-intensity electrical currents to specific brain regions for a duration of 20–30 min. These currents induce polarity-dependent shifts in resting membrane potential, thereby modulating neuronal activity at the site of stimulation and its associated structures [195, 196]. Typically, two electrodes, one anode positioned over the primary motor cortex and the other a cathode over the contralateral supraorbital region, are placed on the scalp. This setting generates a current flow through the prefrontal cortex, the cingulate cortex, the insula, and deeper structures such as thalamic and brainstem nuclei. The current flow modulates the rest membrane potential of axons, promoting the alleviation of pain [197].
To date, the efficacy of tDCS is limited to treatment of chronic neuropathic pain secondary to spinal cord injury [195] or for peripheral neuropathic pain [198]. Another recent meta-analysis has demonstrated that tDCS does not reduce the pain and related disability in non-specific chronic low-back pain syndrome, limiting the support for its use in this field [199]. Furthermore, a recent systematic review has assessed the efficacy of tDCS in migraine, reporting a positive effect on treatment success, although the included studies were quite heterogeneous and the sample size was small; therefore, these limitations precluded any definitive conclusion on the real efficacy of tDCS in this field of application [200].
Transcranial magnetic stimulation
Repetitive Transcranial Magnetic Stimulation (rTMS) is a non-invasive neurostimulation technique, applying a magnetic field externally to the scalp to induce electrical currents in targeted regions of the brain beneath the coil [201]. TMS operates on Faraday's principle of electromagnetic induction, where a rapid electric current passing through a coil for a brief duration, typically 1 ms, generates a potent electromagnetic field [202]. In TMS, the magnetic field is painlessly administered to the scalp, and cortical axons serve as the electrical conductors that receive the induced electric current [202].
The analgesic effect is obtained by stimulating the primary motor cortex (precentral gyrus), with a stimulation frequency ranging between 5 to 20 Hz, contralaterally to the side of pain localization [203, 204]. Therefore, rTMS has the capacity to regulate cortical hyperexcitability and pain pathways, including descending inhibitory pathways [205, 206]. These pathways exert an influence on supraspinal pain tracts and regions of the brain associated with social-affective functions, such as the right temporal lobe [207, 208].
rTMS has been applied in different populations of patients with chronic pain. In patients with migraine, rTMS decreases the intensity of the attack soon after the treatment application, particularly in those patients with cephalgia after traumatic brain injury [209]. High frequency rTMS on motor cortex has also proved to provide a positive temporary effect on pain in patients with neuropathic chronic pain correlated to phantom limb [210] and to manage pain alongside cognition and sleep disturbances in patients of fibromyalgia [211].
Finally, rTMS provides the additional purpose of anticipating the individual responsiveness of patients who may undergo subsequent implantation of a device for MCS [212]. In fact, a preoperative trial utilizing rTMS has demonstrated the ability to predict satisfactory pain relief in approximately 80 to 90% of cases where MCS implantation is performed [213, 214].
Transcutaneous electrical nerve stimulation
Transcutaneous Electrical Nerve Stimulation (TENS) is a non-invasive therapeutic technique based on the application of low-voltage electrical currents to the skin surface, through electrodes placed on or near the area of pain or along the nerve pathways [215]. The principles of TENS stay in the Gate Control Theory (see above) [7].
TENS is commonly employed for various types of pain. TENS has been studied to treat chronic low back pain. Two large randomized controlled trials were firstly conducted with contrasting results, mainly due to the different design, population, and modulation settings [216, 217]. A very recent systematic review, including the whole literature on this topic (i.e. 17 randomized controlled trials with a total of 1027 adults), has suggested that TENS may marginally reduce the perceived chronic low back pain for a short period, to an extent that cannot be considered clinically relevant [218]. TENS has also been tested in 43 patients with fibromyalgia, demonstrating that one 30-min application improved the pain perception during movement, but not at rest [219]. Then, several studies have been conducted so far in patients with fibromyalgia; the most recent pooled data analysis suggests that TENS improves pain symptoms in this population of patients [220]. A modified system for TENS specifically developed to stimulate the terminal supraorbital branch of the trigeminal nerve has been also demonstrated to prevent [221] or to treat attacks of episodic migraine [222]. However, the current evidence is still weak to recommend its use in this scenario.
Transcranial focused ultrasound
Transcranial Focused Ultrasound (tFUS) for chronic pain represents an emerging non-invasive neurostimulation method that utilizes focused ultrasound waves to modulate brain activity for therapeutic purposes [223]. This technique is characterized by a high spatial resolution, targeting any site of the peripheral or central nervous system [223].
tFUS induces mechanical vibrations and thermal effects influencing the neurons excitability, altering the synaptic transmission, and affecting the excitatory/inhibitory balance of neurotransmitters [224]. tFUS may also release endorphins or other neurochemicals involved in pain modulation [224]. In the application for chronic pain treatment, tFUS is centered to specific areas involved in the pain perception, such as the somatosensory cortex, thalamus, or limbic system [225–227].
Today, tFUS is approved in Europe for neuromodulation in neuropathic chronic pain not responsive to conventional pharmacological treatments [228]. Indeed, data available are limited to chronic neuropathic pain, phantom limb pain, neuropathic pain related to spinal cord injury in clinical and preclinical settings [229–231].
Future perspectives of neurostimulation
Although the growing literature investigating the complexity of pain transmission and the biochemical effects of neurostimulation, clear indications and strong recommendations still lack. The complexity of anatomical and physiological pathways of pain modulation, together with the various etiopathogenesis and pathophysiology of different forms of chronic pain, should probably require a personalized approach to neurostimulation.
Patients can develop chronic pain through various pathophysiological pathways. However, any single treatment, whether pharmacological or involving neurostimulation, has a finite number of mechanisms of action. Consequently, it may only be effective for a specific subset of patients. Monotherapy, whether it's pharmacological, neurostimulatory, surgical, or psychological, is thus likely to result in some individuals who do not respond adequately. These individuals, termed "non-responders," have underlying disease mechanisms that do not align with the mechanism of action of the chosen therapy [212, 232].
Novel approaches to neurostimulation, inspired by the principles of the personalized and precision medicine, have been proposed for other diseases, like tinnitus [233–237], Parkinson’s disease [238, 239], epilepsy [240] and depression [241, 242]. A recent publication explores the reasoning and basis for personalized electrical neurostimulation in individuals suffering from chronic neuropathic pain [212].Noninvasive methods of neurostimulation may predict the response of every single patient to more invasive techniques such a MCS or DBS [212]. Beside the prediction of responsiveness to a specific treatment, the study of clinical phenotypes [243], brain networks and oscillatory patterns [244–247], and patient’s genotype with the corresponding single nucleotide polymorphism [248–251] may also guide the clinician in the adoption of the best strategy for every single patient. In addition, the differences in neuropathophysiological processes underlying various painful syndromes should be taken into consideration when selecting a neurostimulation strategy, as recently suggested [252].
It should also be considered that technical advances (such as microelectronics, feedback-based system design, biomimetic stimulation patterns), and the integration of genomics with device-based therapies are revolutionizing the conceptualization of neuromodulation, with a trend towards miniaturization and noninvasive therapies [253].
In this complex scenario, we believe that the scientific literature should be directed on the way to better clarify those underlying anatomical and pathophysiological mechanisms that are still obscure. Once these bases are clearer, more detailed, and better focused studies could be specifically designed to assess and to define the criteria of choice of one technique over another one, for every single chronic pain manifestation.
Meanwhile, the approach to a patient suffering from chronic pain necessitating neurostimulation demands meticulous evaluation, taking into account the underlying condition, individual characteristics (including psychological factors), and prior treatment modalities. Given the absence of comprehensive cost-effectiveness studies, except for SCS, physicians may opt to transition from non-invasive to more invasive techniques known to be efficacious for that particular painful condition.
Conclusions
The electrical neuromodulation of pain comprises various techniques targeting distinct neural pathways involved in pain modulation. Despite numerous studies and trials, determining the most effective technique for each patient remains challenging. Patient selection plays a pivotal role in achieving optimal outcomes, considering factors such as the specific type and origin of chronic pain. Additionally, the invasiveness of the chosen treatment should align and be proportional with the patient's clinical status. Further trials are essential to refine these considerations. Meanwhile, a personalized approach to neurostimulation should guide clinicians in selecting the most appropriate treatment for individual patients.
Acknowledgements
none.
Abbreviations
- 5-HT
5-Hydroxytryptamine
- AMPA
Amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- CGRP
Calcitonin Gene-Related Peptide
- CNS
Central Nervous System
- DBS
Deep Brain Stimulation
- DRG
Dorsal Root Ganglion
- GABA
Gamma-Aminobutyric Acid
- IASP
International Association for the Study of Pain
- MCS
Motor Cortex Stimulation
- NMDA
N-Methyl-D-Aspartate
- PAG
PeriAqueductal Grey
- PENS
Percutaneous Electrical Nerve Stimulation
- PNS
Peripheral Nerve Stimulation
- PRF
Pulsed Radiofrequency Therapy
- rTMS
Repetitive Transcranial Magnetic Stimulation
- RVM
RostroVentral Medulla
- SCS
Spinal Cord Stimulation
- tDCS
Transcranial Direct Current Stimulation
- TENS
Transcutaneous Electrical Nerve Stimulation
- tFUS
Transcranial Focused Ultrasound
- VNS
Vagus Nerve Stimulation
Authors’ contributions
GG, ADT, FL and DLT substantial contributed to the conception and design of the work. All authors acquired data and information from the study literature, and they wrote the drafted manuscript. GG, ADT, FL and DLT wrote and revised the final manuscript, and they critically reviewed it for scientific and intellectual content. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Funding
None.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Prof. Federico Longhini contributed to the development of a new helmet for mechanical ventilation, and he is designated as inventor (European Patent number 3320941) not related to the present manuscript. He also received speaking fees from Draeger, Intersurgical and Fisher & Paykel. Prof. Federico Longhini acts also as Associate Editor for the Journal of Anesthesia, Analgesia and Critical Care. The remaining authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Giusy Guzzi and Attilio Della Torre these authors equally contributed.
References
- 1.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nugraha B, Gutenbrunner C, Barke A, Karst M, Schiller J, Schafer P, Falter S, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: functioning properties of chronic pain. Pain. 2019;160(1):88–94. doi: 10.1097/j.pain.0000000000001433. [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160(1):19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 4.Schug SA, Lavand'homme P, Barke A, Korwisi B, Rief W, Treede RD. The IASP classification of chronic pain for ICD-11: chronic postsurgical or posttraumatic pain. Pain. 2019;160(1):45–52. doi: 10.1097/j.pain.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 5.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basanez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabe E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fevre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leon FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzzi G, Della Torre A, La Torre D, Volpentesta G, Stroscio CA, Lavano A, Longhini F (2022) Spinal Cord Stimulation in Chronic Low Back Pain Syndrome: Mechanisms of Modulation, Technical Features and Clinical Application. Healthcare (Basel) 10 (10). 10.3390/healthcare10101953 [DOI] [PMC free article] [PubMed]
- 8.Pereira A, Herrero-Trujillano M, Vaquero G, Fuentes L, Gonzalez S, Mendiola A, Perez-Medina T (2022) Clinical Management of Chronic Pelvic Pain in Endometriosis Unresponsive to Conventional Therapy. J Pers Med 12 (1). 10.3390/jpm12010101 [DOI] [PMC free article] [PubMed]
- 9.Pirvulescu I, Biskis A, Candido KD, Knezevic NN. Overcoming clinical challenges of refractory neuropathic pain. Expert Rev Neurother. 2022;22(7):595–622. doi: 10.1080/14737175.2022.2105206. [DOI] [PubMed] [Google Scholar]
- 10.Knotkova H, Hamani C, Sivanesan E, Le Beuffe MFE, Moon JY, Cohen SP, Huntoon MA. Neuromodulation for chronic pain. Lancet. 2021;397(10289):2111–2124. doi: 10.1016/S0140-6736(21)00794-7. [DOI] [PubMed] [Google Scholar]
- 11.Moisset X, Lanteri-Minet M, Fontaine D. Neurostimulation methods in the treatment of chronic pain. J Neural Transm (Vienna) 2020;127(4):673–686. doi: 10.1007/s00702-019-02092-y. [DOI] [PubMed] [Google Scholar]
- 12.Bourne S, Machado AG, Nagel SJ. Basic anatomy and physiology of pain pathways. Neurosurg Clin N Am. 2014;25(4):629–638. doi: 10.1016/j.nec.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Hermosillo DC, Gonzalez-Hermosillo LM, Villasenor-Almaraz M, Ballesteros-Herrera D, Moreno-Jimenez S, Corona-Cedillo R, Velasco-Campos F, Carrillo-Ruiz JD, Roldan-Valadez E. Current concepts of pain pathways: a brief review of anatomy, physiology, and medical imaging. Curr Med Imaging. 2023 doi: 10.2174/1573405620666230519144112. [DOI] [PubMed] [Google Scholar]
- 14.Hodge CJ, Jr, Apkarian AV. The spinothalamic tract. Crit Rev Neurobiol. 1990;5(4):363–397. [PubMed] [Google Scholar]
- 15.de Mello Rosa GH, Ullah F, de Paiva YB, da Silva JA, Branco LGS, Corrado AP, Medeiros P, Coimbra NC, Franceschi Biagioni A. Ventrolateral periaqueductal gray matter integrative system of defense and antinociception. Pflugers Arch. 2022;474(4):469–480. doi: 10.1007/s00424-022-02672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468(3):425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- 17.Mokhtar M, Singh P. Neuroanatomy Periaqueductal Gray. 2024. [PubMed] [Google Scholar]
- 18.Comitato A, Bardoni R (2021) Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits. Int J Mol Sci 22 (1). 10.3390/ijms22010414 [DOI] [PMC free article] [PubMed]
- 19.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 20.Ossipov MH. The perception and endogenous modulation of pain. Scientifica (Cairo) 2012;2012:561761. doi: 10.6064/2012/561761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zieglgansberger W. Substance P and pain chronicity. Cell Tissue Res. 2019;375(1):227–241. doi: 10.1007/s00441-018-2922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10. [PubMed] [Google Scholar]
- 23.Graefe SB, Rahimi N, Mohiuddin SS. Biochemistry, Substance P. 2024. [PubMed] [Google Scholar]
- 24.Yang S, Chang MC (2019) Chronic Pain: Structural and Functional Changes in Brain Structures and Associated Negative Affective States. Int J Mol Sci 20 (13). 10.3390/ijms20133130 [DOI] [PMC free article] [PubMed]
- 25.Zhou Y, Danbolt NC. Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 2014;121(8):799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 27.Dedek A, Hildebrand ME. Advances and Barriers in Understanding Presynaptic N-Methyl-D-Aspartate Receptors in Spinal Pain Processing. Front Mol Neurosci. 2022;15:864502. doi: 10.3389/fnmol.2022.864502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granstein RD, Wagner JA, Stohl LL, Ding W. Calcitonin gene-related peptide: key regulator of cutaneous immunity. Acta Physiol (Oxf) 2015;213(3):586–594. doi: 10.1111/apha.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wattiez AS, Sowers LP, Russo AF. Calcitonin gene-related peptide (CGRP): role in migraine pathophysiology and therapeutic targeting. Expert Opin Ther Targets. 2020;24(2):91–100. doi: 10.1080/14728222.2020.1724285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80(2):53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Summers RJ, McMartin LR. Adrenoceptors and their second messenger systems. J Neurochem. 1993;60(1):10–23. doi: 10.1111/j.1471-4159.1993.tb05817.x. [DOI] [PubMed] [Google Scholar]
- 33.Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31(1):123–136. doi: 10.1016/0304-3959(87)90012-1. [DOI] [PubMed] [Google Scholar]
- 34.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol. 2011;22(5–6):390–404. doi: 10.1097/FBP.0b013e328349aae4. [DOI] [PubMed] [Google Scholar]
- 35.Bannister K, Dickenson AH. The plasticity of descending controls in pain: translational probing. J Physiol. 2017;595(13):4159–4166. doi: 10.1113/JP274165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charnay Y, Leger L. Brain serotonergic circuitries. Dialogues Clin Neurosci. 2010;12(4):471–487. doi: 10.31887/DCNS.2010.12.4/ycharnay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Leon AS, Tadi P. Biochemistry Gamma Aminobutyric Acid. 2024. [PubMed] [Google Scholar]
- 38.Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol Rev. 2012;92(1):193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francois A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A Brainstem-Spinal Cord Inhibitory Circuit for Mechanical Pain Modulation by GABA and Enkephalins. Neuron. 2017;93(4):822–839.e826. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snyder SH, Pasternak GW. Historical review: Opioid receptors. Trends Pharmacol Sci. 2003;24(4):198–205. doi: 10.1016/S0165-6147(03)00066-X. [DOI] [PubMed] [Google Scholar]
- 42.Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R (2018) General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int J Mol Sci 19 (8). 10.3390/ijms19082164 [DOI] [PMC free article] [PubMed]
- 43.Parraga JP, Castellanos A (2023) A Manifesto in Defense of Pain Complexity: A Critical Review of Essential Insights in Pain Neuroscience. J Clin Med 12 (22). 10.3390/jcm12227080 [DOI] [PMC free article] [PubMed]
- 44.Gangadharan V, Kuner R. Pain hypersensitivity mechanisms at a glance. Dis Model Mech. 2013;6(4):889–895. doi: 10.1242/dmm.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangadharan V, Wang R, Ulzhofer B, Luo C, Bardoni R, Bali KK, Agarwal N, Tegeder I, Hildebrandt U, Nagy GG, Todd AJ, Ghirri A, Haussler A, Sprengel R, Seeburg PH, MacDermott AB, Lewin GR, Kuner R. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J Clin Invest. 2011;121(4):1608–1623. doi: 10.1172/JCI44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schweizerhof M, Stosser S, Kurejova M, Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW, Brugger S, Dickenson A, Simone DA, Kuner R. Hematopoietic colony-stimulating factors mediate tumor-nerve interactions and bone cancer pain. Nat Med. 2009;15(7):802–807. doi: 10.1038/nm.1976. [DOI] [PubMed] [Google Scholar]
- 48.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28(52):14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 50.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 52.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nijs J, George SZ, Clauw DJ, Fernandez-de-Las-Penas C, Kosek E, Ickmans K, Fernandez-Carnero J, Polli A, Kapreli E, Huysmans E, Cuesta-Vargas AI, Mani R, Lundberg M, Leysen L, Rice D, Sterling M, Curatolo M. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021;3(5):e383–e392. doi: 10.1016/S2665-9913(21)00032-1. [DOI] [PubMed] [Google Scholar]
- 54.Yu K, Niu X, He B (2020) Neuromodulation Management of Chronic Neuropathic Pain in The Central Nervous system. Adv Funct Mater 30 (37). 10.1002/adfm.201908999 [DOI] [PMC free article] [PubMed]
- 55.Li SL, Li J, Xu HC, Liu YC, Yang TT, Yuan H. Progress in the efficacy and mechanism of spinal cord stimulation in neuropathological pain. Ibrain. 2022;8(1):23–36. doi: 10.1002/ibra.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deer TR, Eldabe S, Falowski SM, Huntoon MA, Staats PS, Cassar IR, Crosby ND, Boggs JW. Peripherally Induced Reconditioning of the Central Nervous System: A Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation. J Pain Res. 2021;14:721–736. doi: 10.2147/JPR.S297091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Giannoni-Luza S, Pacheco-Barrios K, Cardenas-Rojas A, Mejia-Pando PF, Luna-Cuadros MA, Barouh JL, Gnoatto-Medeiros M, Candido-Santos L, Barra A, Caumo W, Fregni F. Noninvasive motor cortex stimulation effects on quantitative sensory testing in healthy and chronic pain subjects: a systematic review and meta-analysis. Pain. 2020;161(9):1955–1975. doi: 10.1097/j.pain.0000000000001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopes PSS, Campos ACP, Fonoff ET, Britto LRG, Pagano RL. Motor cortex and pain control: exploring the descending relay analgesic pathways and spinal nociceptive neurons in healthy conscious rats. Behav Brain Funct. 2019;15(1):5. doi: 10.1186/s12993-019-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klein MM, Treister R, Raij T, Pascual-Leone A, Park L, Nurmikko T, Lenz F, Lefaucheur JP, Lang M, Hallett M, Fox M, Cudkowicz M, Costello A, Carr DB, Ayache SS, Oaklander AL. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain. 2015;156(9):1601–1614. doi: 10.1097/j.pain.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mariano TY, Burgess FW, Bowker M, Kirschner J, Van't Wout-Frank M, Jones RN, Halladay CW, Stein M, Greenberg BD. Transcranial Direct Current Stimulation for Affective Symptoms and Functioning in Chronic Low Back Pain: A Pilot Double-Blinded, Randomized. Placebo-Controlled Trial Pain Med. 2019;20(6):1166–1177. doi: 10.1093/pm/pny188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado AG, Baker KB, Plow E, Malone DA. Cerebral stimulation for the affective component of neuropathic pain. Neuromodulation. 2013;16(6):514–518. doi: 10.1111/j.1525-1403.2012.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78(3):393–401. doi: 10.3171/jns.1993.78.3.0393. [DOI] [PubMed] [Google Scholar]
- 63.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol. 1991;14(1):131–134. doi: 10.1111/j.1540-8159.1991.tb04058.x. [DOI] [PubMed] [Google Scholar]
- 64.Lavano A, Guzzi G, M DER, Romano M, Della Torre A, Vescio G, Deodato F, Lavano F, Volpentesta G, Minimally invasive motor cortex stimulation for Parkinson's disease. J Neurosurg Sci. 2017;61(1):77–87. doi: 10.23736/S0390-5616.16.03246-X. [DOI] [PubMed] [Google Scholar]
- 65.Tronnier V, Rasche D. Epidural and subdural stimulation. Handb Clin Neurol. 2013;116:343–351. doi: 10.1016/B978-0-444-53497-2.00028-0. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X, Hu Y, Tao W, Zhu H, Xiao D, Li Y. The Effect of Motor Cortex Stimulation on Central Poststroke Pain in a Series of 16 Patients With a Mean Follow-Up of 28 Months. Neuromodulation. 2017;20(5):492–496. doi: 10.1111/ner.12547. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: From phenomenology to mechanisms. Neuroimage. 2007;37(Suppl 1):S71–79. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 68.Ramos-Fresnedo A, Perez-Vega C, Domingo RA, Cheshire WP, Middlebrooks EH, Grewal SS. Motor Cortex Stimulation for Pain: A Narrative Review of Indications, Techniques, and Outcomes. Neuromodulation. 2022;25(2):211–221. doi: 10.1016/j.neurom.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 69.Hamani C, Fonoff ET, Parravano DC, Silva VA, Galhardoni R, Monaco BA, Navarro J, Yeng LT, Teixeira MJ, de Andrade DC. Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain. 2021;144(10):2994–3004. doi: 10.1093/brain/awab189. [DOI] [PubMed] [Google Scholar]
- 70.Henssen D, Kurt E, van Walsum AVC, Kozicz T, van Dongen R, Bartels R. Motor cortex stimulation in chronic neuropathic orofacial pain syndromes: a systematic review and meta-analysis. Sci Rep. 2020;10(1):7195. doi: 10.1038/s41598-020-64177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parravano DC, Ciampi DA, Fonoff ET, Monaco B, Navarro J, Yeng LT, Teixeira MJ, Hamani C. Quality of Life After Motor Cortex Stimulation: Clinical Results and Systematic Review of the Literature. Neurosurgery. 2019;84(2):451–456. doi: 10.1093/neuros/nyy060. [DOI] [PubMed] [Google Scholar]
- 72.Lavano A, Guzzi G, Chirchiglia D. Cortical neuromodulation for neuropathic pain and Parkinson disease: Where are we? Neurol Neurochir Pol. 2018;52(1):75–78. doi: 10.1016/j.pjnns.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Rasche D, Tronnier VM. Clinical Significance of Invasive Motor Cortex Stimulation for Trigeminal Facial Neuropathic Pain Syndromes. Neurosurgery. 2016;79(5):655–666. doi: 10.1227/NEU.0000000000001353. [DOI] [PubMed] [Google Scholar]
- 74.Sokal P, Harat M, Zielinski P, Furtak J, Paczkowski D, Rusinek M. Motor cortex stimulation in patients with chronic central pain. Adv Clin Exp Med. 2015;24(2):289–296. doi: 10.17219/acem/40452. [DOI] [PubMed] [Google Scholar]
- 75.Szymoniuk M, Chin JH, Domagalski L, Biszewski M, Jozwik K, Kamieniak P. Brain stimulation for chronic pain management: a narrative review of analgesic mechanisms and clinical evidence. Neurosurg Rev. 2023;46(1):127. doi: 10.1007/s10143-023-02032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergeron D, Obaid S, Fournier-Gosselin MP, Bouthillier A, Nguyen DK (2021) Deep Brain Stimulation of the Posterior Insula in Chronic Pain: A Theoretical Framework. Brain Sci 11 (5). 10.3390/brainsci11050639 [DOI] [PMC free article] [PubMed]
- 77.Mancini F, Guzzi G, Castrioto CF, Cavallo MA, Conti A, Conti C, Della Torre A, Esposito F, Iorio G, Landi A, Lanotte M, Lavano A, Locatelli M, Longhi M, Marruzzo D, Mondani M, Morace R, Pellizzari M, Piacentino M, Picozzi P, Romeo F, Sarrubbo S, Servello D, Somma T, Trezza A, Tringali G, Tufo T, Ricciuti RA. Deep brain stimulation for Parkinson's disease in practice: results of the survey by the Italian Neurosurgery Society. J Neurosurg Sci. 2022;66(6):526–534. doi: 10.23736/S0390-5616.22.05751-4. [DOI] [PubMed] [Google Scholar]
- 78.Sims-Williams H, Matthews JC, Talbot PS, Love-Jones S, Brooks JC, Patel NK, Pickering AE. Deep brain stimulation of the periaqueductal gray releases endogenous opioids in humans. Neuroimage. 2017;146:833–842. doi: 10.1016/j.neuroimage.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young RF, Bach FW, Van Norman AS, Yaksh TL. Release of beta-endorphin and methionine-enkephalin into cerebrospinal fluid during deep brain stimulation for chronic pain. Effects of stimulation locus and site of sampling. J Neurosurg. 1993;79(6):816–825. doi: 10.3171/jns.1993.79.6.0816. [DOI] [PubMed] [Google Scholar]
- 80.Gopalakrishnan R, Burgess RC, Malone DA, Lempka SF, Gale JT, Floden DP, Baker KB, Machado AG. Deep brain stimulation of the ventral striatal area for poststroke pain syndrome: a magnetoencephalography study. J Neurophysiol. 2018;119(6):2118–2128. doi: 10.1152/jn.00830.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lempka SF, Malone DA, Jr, Hu B, Baker KB, Wyant A, Ozinga JGt, Plow EB, Pandya M, Kubu CS, Ford PJ, Machado AG, Randomized clinical trial of deep brain stimulation for poststroke pain. Ann Neurol. 2017;81(5):653–663. doi: 10.1002/ana.24927. [DOI] [PubMed] [Google Scholar]
- 82.Belasen A, Rizvi K, Gee LE, Yeung P, Prusik J, Ramirez-Zamora A, Hanspal E, Paiva P, Durphy J, Argoff CE, Pilitsis JG. Effect of low-frequency deep brain stimulation on sensory thresholds in Parkinson's disease. J Neurosurg. 2017;126(2):397–403. doi: 10.3171/2016.2.JNS152231. [DOI] [PubMed] [Google Scholar]
- 83.Marques A, Chassin O, Morand D, Pereira B, Debilly B, Derost P, Ulla M, Lemaire JJ, Durif F. Central pain modulation after subthalamic nucleus stimulation: A crossover randomized trial. Neurology. 2013;81(7):633–640. doi: 10.1212/WNL.0b013e3182a08d00. [DOI] [PubMed] [Google Scholar]
- 84.Son BC, Kim DR, Kim HS, Lee SW. Simultaneous trial of deep brain and motor cortex stimulation in chronic intractable neuropathic pain. Stereotact Funct Neurosurg. 2014;92(4):218–226. doi: 10.1159/000362933. [DOI] [PubMed] [Google Scholar]
- 85.Rasche D, Rinaldi PC, Young RF, Tronnier VM. Deep brain stimulation for the treatment of various chronic pain syndromes. Neurosurg Focus. 2006;21(6):E8. doi: 10.3171/foc.2006.21.6.10. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto T, Katayama Y, Obuchi T, Kano T, Kobayashi K, Oshima H, Fukaya C. Thalamic sensory relay nucleus stimulation for the treatment of peripheral deafferentation pain. Stereotact Funct Neurosurg. 2006;84(4):180–183. doi: 10.1159/000094958. [DOI] [PubMed] [Google Scholar]
- 87.Cruccu G, Garcia-Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, Taylor R, Tronnier V, Truini A, Attal N. EAN guidelines on central neurostimulation therapy in chronic pain conditions. Eur J Neurol. 2016;23(10):1489–1499. doi: 10.1111/ene.13103. [DOI] [PubMed] [Google Scholar]
- 88.Gouveia FV, Warsi NM, Suresh H, Matin R, Ibrahim GM. Neurostimulation treatments for epilepsy: Deep brain stimulation, responsive neurostimulation and vagus nerve stimulation. Neurotherapeutics. 2024;21(3):e00308. doi: 10.1016/j.neurot.2023.e00308. [DOI] [PubMed] [Google Scholar]
- 89.Jung B, Yang C, Lee SH. Vagus Nerves Stimulation: Clinical Implication and Practical Issue as a Neuropsychiatric Treatment. Clin Psychopharmacol Neurosci. 2024;22(1):13–22. doi: 10.9758/cpn.23.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shao P, Li H, Jiang J, Guan Y, Chen X, Wang Y. Role of Vagus Nerve Stimulation in the Treatment of Chronic Pain. NeuroImmunoModulation. 2023;30(1):167–183. doi: 10.1159/000531626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the Uses of Vagal Nerve Stimulation in Chronic Pain Management. Curr Pain Headache Rep. 2015;19(12):54. doi: 10.1007/s11916-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 92.Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46(1):17–23. [PubMed] [Google Scholar]
- 93.Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 94.Morest DK. Experimental study of the projections of the nucleus of the tractus solitarius and the area postrema in the cat. J Comp Neurol. 1967;130(4):277–300. doi: 10.1002/cne.901300402. [DOI] [PubMed] [Google Scholar]
- 95.Vida G, Pena G, Deitch EA, Ulloa L. alpha7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89(2):337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 97.Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R141–147. doi: 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]
- 98.Tao X, Lee MS, Donnelly CR, Ji RR. Neuromodulation, Specialized Proresolving Mediators, and Resolution of Pain. Neurotherapeutics. 2020;17(3):886–899. doi: 10.1007/s13311-020-00892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chaudhry SR, Lendvai IS, Muhammad S, Westhofen P, Kruppenbacher J, Scheef L, Boecker H, Scheele D, Hurlemann R, Kinfe TM. Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): A proof-of-concept study. Brain Stimul. 2019;12(3):643–651. doi: 10.1016/j.brs.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 100.Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, Lam K, Simon B, Baker DG. Noninvasive Transcutaneous Vagus Nerve Stimulation Decreases Whole Blood Culture-Derived Cytokines and Chemokines: A Randomized, Blinded. Healthy Control Pilot Trial Neuromodulation. 2016;19(3):283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- 101.Tal M. A Role for Inflammation in Chronic Pain. Curr Rev Pain. 1999;3(6):440–446. doi: 10.1007/s11916-999-0071-4. [DOI] [PubMed] [Google Scholar]
- 102.Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4(9):530–534. doi: 10.1016/j.jpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 103.Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22(6):482–484. doi: 10.1046/j.1468-2982.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 104.Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55(8):1167–1171. doi: 10.1212/wnl.55.8.1167. [DOI] [PubMed] [Google Scholar]
- 105.Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man and back. Acta Neurol Belg. 2005;105(2):62–67. [PubMed] [Google Scholar]
- 106.Najib U, Smith T, Hindiyeh N, Saper J, Nye B, Ashina S, McClure CK, Marmura MJ, Chase S, Liebler E, Lipton RB. Non-invasive vagus nerve stimulation for prevention of migraine: The multicenter, randomized, double-blind, sham-controlled PREMIUM II trial. Cephalalgia. 2022;42(7):560–569. doi: 10.1177/03331024211068813. [DOI] [PubMed] [Google Scholar]
- 107.Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S, Simmons KA, Mullin C, Liebler EJ, Goadsby PJ, Saper JR. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: The EVENT study. Neurology. 2016;87(5):529–538. doi: 10.1212/WNL.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16:61. doi: 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34(12):986–993. doi: 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 110.Lange G, Janal MN, Maniker A, Fitzgibbons J, Fobler M, Cook D, Natelson BH. Safety and efficacy of vagus nerve stimulation in fibromyalgia: a phase I/II proof of concept trial. Pain Med. 2011;12(9):1406–1413. doi: 10.1111/j.1526-4637.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kutlu N, Ozden AV, Alptekin HK, Alptekin JO. The Impact of Auricular Vagus Nerve Stimulation on Pain and Life Quality in Patients with Fibromyalgia Syndrome. Biomed Res Int. 2020;2020:8656218. doi: 10.1155/2020/8656218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang S, Li S, Zhai X, Rong P, He J, Liu L, He X, Liu W. Transcutaneous auricular vagal nerve stimulation releases extrapineal melatonin and reduces thermal hypersensitivity in Zucker diabetic fatty rats. Front Neurosci. 2022;16:916822. doi: 10.3389/fnins.2022.916822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, Sun C, Rong P, Zhai X, Zhang J, Baker M, Wang S. Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Mol Pain. 2018;14:1744806918787368. doi: 10.1177/1744806918787368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang S, Zhai X, Li S, McCabe MF, Wang X, Rong P. Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS ONE. 2015;10(4):e0124195. doi: 10.1371/journal.pone.0124195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oshinsky ML, Murphy AL, Hekierski H, Jr, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–1042. doi: 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saade NE, Tabet MS, Banna NR, Atweh SF, Jabbur SJ. Inhibition of nociceptive evoked activity in spinal neurons through a dorsal column-brainstem-spinal loop. Brain Res. 1985;339(1):115–118. doi: 10.1016/0006-8993(85)90627-4. [DOI] [PubMed] [Google Scholar]
- 117.Bantli H, Bloedel JR, Thienprasit P. Supraspinal interactions resulting from experimental dorsal column stimulation. J Neurosurg. 1975;42(3):296–300. doi: 10.3171/jns.1975.42.3.0296. [DOI] [PubMed] [Google Scholar]
- 118.Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16(3):217–225. doi: 10.1007/s11916-012-0260-4. [DOI] [PubMed] [Google Scholar]
- 119.Sivanesan E, Maher DP, Raja SN, Linderoth B, Guan Y. Supraspinal Mechanisms of Spinal Cord Stimulation for Modulation of Pain: Five Decades of Research and Prospects for the Future. Anesthesiology. 2019;130(4):651–665. doi: 10.1097/ALN.0000000000002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. Exploration of supraspinal mechanisms in effects of spinal cord stimulation: role of the locus coeruleus. Neuroscience. 2013;253:426–434. doi: 10.1016/j.neuroscience.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 121.Saade NE, Atweh SF, Privat A, Jabbur SJ. Inhibitory effects from various types of dorsal column and raphe magnus stimulations on nociceptive withdrawal flexion reflexes. Brain Res. 1999;846(1):72–86. doi: 10.1016/s0006-8993(99)02003-x. [DOI] [PubMed] [Google Scholar]
- 122.Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience. 2013;247:134–144. doi: 10.1016/j.neuroscience.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 123.Stiller CO, Linderoth B, O'Connor WT, Franck J, Falkenberg T, Ungerstedt U, Brodin E. Repeated spinal cord stimulation decreases the extracellular level of gamma-aminobutyric acid in the periaqueductal gray matter of freely moving rats. Brain Res. 1995;699(2):231–241. doi: 10.1016/0006-8993(95)00911-9. [DOI] [PubMed] [Google Scholar]
- 124.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31(2):289–296. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 125.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139(1):136–145. doi: 10.1016/j.pain.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 126.Song Z, Meyerson BA, Linderoth B. Muscarinic receptor activation potentiates the effect of spinal cord stimulation on pain-related behavior in rats with mononeuropathy. Neurosci Lett. 2008;436(1):7–12. doi: 10.1016/j.neulet.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 127.Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma-aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery. 1996;39(2):367–374. doi: 10.1097/00006123-199608000-00026. [DOI] [PubMed] [Google Scholar]
- 128.Baba H, Yoshimura M, Nishi S, Shimoji K. Synaptic responses of substantia gelatinosa neurones to dorsal column stimulation in rat spinal cord in vitro. J Physiol. 1994;478(Pt 1):87–99. doi: 10.1113/jphysiol.1994.sp020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 130.Lind G, Schechtmann G, Winter J, Meyerson BA, Linderoth B. Baclofen-enhanced spinal cord stimulation and intrathecal baclofen alone for neuropathic pain: Long-term outcome of a pilot study. Eur J Pain. 2008;12(1):132–136. doi: 10.1016/j.ejpain.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 131.Wang YY, Wu SX, Liu XY, Wang W, Li YQ. Effects of c-fos antisense oligodeoxynucleotide on 5-HT-induced upregulation of preprodynorphin, preproenkephalin, and glutamic acid decarboxylase mRNA expression in cultured rat spinal dorsal horn neurons. Biochem Biophys Res Commun. 2003;309(3):631–636. doi: 10.1016/j.bbrc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 132.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152(7):1666–1673. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 133.Nagel SJ, Lempka SF, Machado AG. Percutaneous spinal cord stimulation for chronic pain: indications and patient selection. Neurosurg Clin N Am. 2014;25(4):723–733. doi: 10.1016/j.nec.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 134.Sitzman BT, Provenzano DA. Best Practices in Spinal Cord Stimulation. Spine (Phila Pa) 2017;42(Suppl 14):S67–S71. doi: 10.1097/BRS.0000000000002220. [DOI] [PubMed] [Google Scholar]
- 135.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–550. doi: 10.1111/ner.12208. [DOI] [PubMed] [Google Scholar]
- 136.Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481–496. doi: 10.1227/01.NEU.0000192162.99567.96. [DOI] [PubMed] [Google Scholar]
- 137.Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566–578. doi: 10.1227/NEU.0b013e3182181e60. [DOI] [PubMed] [Google Scholar]
- 138.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. The effects of spinal cord stimulation in neuropathic pain are sustained: a 24-month follow-up of the prospective randomized controlled multicenter trial of the effectiveness of spinal cord stimulation. Neurosurgery. 2008;63(4):762–770. doi: 10.1227/01.NEU.0000325731.46702.D9. [DOI] [PubMed] [Google Scholar]
- 139.Kumar K, Rizvi S, Nguyen R, Abbas M, Bishop S, Murthy V. Impact of wait times on spinal cord stimulation therapy outcomes. Pain Pract. 2014;14(8):709–720. doi: 10.1111/papr.12126. [DOI] [PubMed] [Google Scholar]
- 140.Fama CA, Chen N, Prusik J, Kumar V, Wilock M, Roth S, Pilitsis JG. The Use of Preoperative Psychological Evaluations to Predict Spinal Cord Stimulation Success: Our Experience and a Review of the Literature. Neuromodulation. 2016;19(4):429–436. doi: 10.1111/ner.12434. [DOI] [PubMed] [Google Scholar]
- 141.Grider JS, Manchikanti L, Carayannopoulos A, Sharma ML, Balog CC, Harned ME, Grami V, Justiz R, Nouri KH, Hayek SM, Vallejo R, Christo PJ. Effectiveness of Spinal Cord Stimulation in Chronic Spinal Pain: A Systematic Review. Pain Physician. 2016;19(1):E33–54. doi: 10.36076/ppj/2016.19.E33. [DOI] [PubMed] [Google Scholar]
- 142.Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, Amirdelfan K, Morgan DM, Brown LL, Yearwood TL, Bundschu R, Burton AW, Yang T, Benyamin R, Burgher AH. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: The SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123(4):851–860. doi: 10.1097/ALN.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 143.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Suppl):S13–19. doi: 10.1016/j.jpainsymman.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 144.Eldabe S, Thomson S, Duarte R, Brookes M, deBelder M, Raphael J, Davies E, Taylor R. The Effectiveness and Cost-Effectiveness of Spinal Cord Stimulation for Refractory Angina (RASCAL Study): A Pilot Randomized Controlled Trial. Neuromodulation. 2016;19(1):60–70. doi: 10.1111/ner.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amann W, Berg P, Gersbach P, Gamain J, Raphael JH, Ubbink DT. Spinal cord stimulation in the treatment of non-reconstructable stable critical leg ischaemia: results of the European Peripheral Vascular Disease Outcome Study (SCS-EPOS) Eur J Vasc Endovasc Surg. 2003;26(3):280–286. doi: 10.1053/ejvs.2002.1876. [DOI] [PubMed] [Google Scholar]
- 146.Naoum JJ, Arbid EJ. Spinal cord stimulation for chronic limb ischemia. Methodist Debakey Cardiovasc J. 2013;9(2):99–102. doi: 10.14797/mdcj-9-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21(5–6):499–533. [PubMed] [Google Scholar]
- 148.Chapman KB, Sayed D, Lamer T, Hunter C, Weisbein J, Patel KV, Dickerson D, Hagedorn JM, Lee DW, Amirdelfan K, Deer T, Chakravarthy K. Best Practices for Dorsal Root Ganglion Stimulation for Chronic Pain: Guidelines from the American Society of Pain and Neuroscience. J Pain Res. 2023;16:839–879. doi: 10.2147/JPR.S364370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Franken G, Douven P, Debets J, Joosten EAJ. Conventional Dorsal Root Ganglion Stimulation in an Experimental Model of Painful Diabetic Peripheral Neuropathy: A Quantitative Immunocytochemical Analysis of Intracellular gamma-Aminobutyric Acid in Dorsal Root Ganglion Neurons. Neuromodulation. 2021;24(4):639–645. doi: 10.1111/ner.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Koetsier E, Franken G, Debets J, Heijmans L, van Kuijk SMJ, Linderoth B, Joosten EA, Maino P. Mechanism of dorsal root ganglion stimulation for pain relief in painful diabetic polyneuropathy is not dependent on GABA release in the dorsal horn of the spinal cord. CNS Neurosci Ther. 2020;26(1):136–143. doi: 10.1111/cns.13192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chao D, Zhang Z, Mecca CM, Hogan QH, Pan B. Analgesic dorsal root ganglionic field stimulation blocks conduction of afferent impulse trains selectively in nociceptive sensory afferents. Pain. 2020;161(12):2872–2886. doi: 10.1097/j.pain.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gemes G, Koopmeiners A, Rigaud M, Lirk P, Sapunar D, Bangaru ML, Vilceanu D, Garrison SR, Ljubkovic M, Mueller SJ, Stucky CL, Hogan QH. Failure of action potential propagation in sensory neurons: mechanisms and loss of afferent filtering in C-type units after painful nerve injury. J Physiol. 2013;591(4):1111–1131. doi: 10.1113/jphysiol.2012.242750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 154.Chapman KB, Groenen PS, Vissers KC, van Helmond N, Stanton-Hicks MD. The Pathways and Processes Underlying Spinal Transmission of Low Back Pain: Observations From Dorsal Root Ganglion Stimulation Treatment. Neuromodulation. 2021;24(4):610–621. doi: 10.1111/ner.13150. [DOI] [PubMed] [Google Scholar]
- 155.Smits H, van Kleef M, Holsheimer J, Joosten EA. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013;13(2):154–168. doi: 10.1111/j.1533-2500.2012.00579.x. [DOI] [PubMed] [Google Scholar]
- 156.Chapman KB, Yousef TA, Foster A, M DS-H, van Helmond N, Mechanisms for the Clinical Utility of Low-Frequency Stimulation in Neuromodulation of the Dorsal Root Ganglion. Neuromodulation. 2021;24(4):738–745. doi: 10.1111/ner.13323. [DOI] [PubMed] [Google Scholar]
- 157.Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG. Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron. 2017;93(1):179–193. doi: 10.1016/j.neuron.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 158.Sandkuhler J, Chen JG, Cheng G, Randic M. Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci. 1997;17(16):6483–6491. doi: 10.1523/JNEUROSCI.17-16-06483.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chapman KB, Kloosterman J, Schor JA, Girardi GE, van Helmond N, Yousef TA. Objective Improvements in Peripheral Arterial Disease from Dorsal Root Ganglion Stimulation: A Case Series. Ann Vasc Surg. 2021;74(519):e517–519e516. doi: 10.1016/j.avsg.2021.01.069. [DOI] [PubMed] [Google Scholar]
- 160.Sverrisdottir YB, Martin SC, Hadjipavlou G, Kent AR, Paterson DJ, FitzGerald JJ, Green AL. Human Dorsal Root Ganglion Stimulation Reduces Sympathetic Outflow and Long-Term Blood Pressure. JACC Basic Transl Sci. 2020;5(10):973–985. doi: 10.1016/j.jacbts.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gravius N, Chaudhry SR, Muhammad S, Bostrom A, Gravius S, Randau T, Scheele D, Westhofen P, Kruppenbacher J, Stoffel-Wagner B, Maier C, Weidlich A, Yearwood TL, Chakravarthy KV, Kramer JM, Hurlemann R, Kinfe TM. Selective L4 Dorsal Root Ganglion Stimulation Evokes Pain Relief and Changes of Inflammatory Markers: Part I Profiling of Saliva and Serum Molecular Patterns. Neuromodulation. 2019;22(1):44–52. doi: 10.1111/ner.12866. [DOI] [PubMed] [Google Scholar]
- 162.Pan B, Zhang Z, Chao D, Hogan QH. Dorsal Root Ganglion Field Stimulation Prevents Inflammation and Joint Damage in a Rat Model of Rheumatoid Arthritis. Neuromodulation. 2018;21(3):247–253. doi: 10.1111/ner.12648. [DOI] [PubMed] [Google Scholar]
- 163.Deer TR, Pope JE, Lamer TJ, Grider JS, Provenzano D, Lubenow TR, FitzGerald JJ, Hunter C, Falowski S, Sayed D, Baranidharan G, Patel NK, Davis T, Green A, Pajuelo A, Epstein LJ, Harned M, Liem L, Christo PJ, Chakravarthy K, Gilmore C, Huygen F, Lee E, Metha P, Nijhuis H, Patterson DG, Petersen E, Pilitsis JG, Rowe JJ, Rupert MP, Skaribas I, Sweet J, Verrills P, Wilson D, Levy RM, Mekhail N. The Neuromodulation Appropriateness Consensus Committee on Best Practices for Dorsal Root Ganglion Stimulation. Neuromodulation. 2019;22(1):1–35. doi: 10.1111/ner.12845. [DOI] [PubMed] [Google Scholar]
- 164.Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail N. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669–681. doi: 10.1097/j.pain.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.D'Souza RS, Kubrova E, Her YF, Barman RA, Smith BJ, Alvarez GM, West TE, Abd-Elsayed A. Dorsal Root Ganglion Stimulation for Lower Extremity Neuropathic Pain Syndromes: An Evidence-Based Literature Review. Adv Ther. 2022;39(10):4440–4473. doi: 10.1007/s12325-022-02244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ong Sio LC, Hom B, Garg S, Abd-Elsayed A (2023) Mechanism of Action of Peripheral Nerve Stimulation for Chronic Pain: A Narrative Review. Int J Mol Sci 24 (5). 10.3390/ijms24054540 [DOI] [PMC free article] [PubMed]
- 167.Mekhail NA, Estemalik E, Azer G, Davis K, Tepper SJ. Safety and Efficacy of Occipital Nerves Stimulation for the Treatment of Chronic Migraines: Randomized, Double-blind. Controlled Single-center Experience Pain Pract. 2017;17(5):669–677. doi: 10.1111/papr.12504. [DOI] [PubMed] [Google Scholar]
- 168.Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, Sharan AD, Narouze S, Mogilner AY, Trentman TL, Ordia J, Vaisman J, Goldstein J, Mekhail N. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2015;35(4):344–358. doi: 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 169.Silberstein SD, Dodick DW, Saper J, Huh B, Slavin KV, Sharan A, Reed K, Narouze S, Mogilner A, Goldstein J, Trentman T, Vaisman J, Ordia J, Weber P, Deer T, Levy R, Diaz RL, Washburn SN, Mekhail N. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2012;32(16):1165–1179. doi: 10.1177/0333102412462642. [DOI] [PubMed] [Google Scholar]
- 170.Saper JR, Dodick DW, Silberstein SD, McCarville S, Sun M, Goadsby PJ. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalalgia. 2011;31(3):271–285. doi: 10.1177/0333102410381142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Warner NS, Schaefer KK, Eldrige JS, Lamer TJ, Pingree MJ, Bendel MA, Warner MA, Rho RH, Mauck WD. Peripheral Nerve Stimulation and Clinical Outcomes: A Retrospective Case Series. Pain Pract. 2021;21(4):411–418. doi: 10.1111/papr.12968. [DOI] [PubMed] [Google Scholar]
- 172.Colini Baldeschi G, Dario A, De Carolis G, Luxardo N, Natale M, Nosella P, Papa A, Raggi M, Reverberi C. Peripheral Nerve Stimulation in the Treatment of Chronic Pain Syndromes From Nerve Injury: A Multicenter Observational Study. Neuromodulation. 2017;20(4):369–374. doi: 10.1111/ner.12539. [DOI] [PubMed] [Google Scholar]
- 173.Ellis JA, Mejia Munne JC, Winfree CJ. Trigeminal branch stimulation for the treatment of intractable craniofacial pain. J Neurosurg. 2015;123(1):283–288. doi: 10.3171/2014.12.JNS14645. [DOI] [PubMed] [Google Scholar]
- 174.Schoenen J, Jensen RH, Lanteri-Minet M, Lainez MJ, Gaul C, Goodman AM, Caparso A, May A. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: a randomized, sham-controlled study. Cephalalgia. 2013;33(10):816–830. doi: 10.1177/0333102412473667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Strand N, D'Souza RS, Hagedorn JM, Pritzlaff S, Sayed D, Azeem N, Abd-Elsayed A, Escobar A, Huntoon MA, Lam CM, Deer TR. Evidence-Based Clinical Guidelines from the American Society of Pain and Neuroscience for the Use of Implantable Peripheral Nerve Stimulation in the Treatment of Chronic Pain. J Pain Res. 2022;15:2483–2504. doi: 10.2147/JPR.S362204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Vatansever D, Tekin I, Tuglu I, Erbuyun K, Ok G. A comparison of the neuroablative effects of conventional and pulsed radiofrequency techniques. Clin J Pain. 2008;24(8):717–724. doi: 10.1097/AJP.0b013e318173c27a. [DOI] [PubMed] [Google Scholar]
- 177.West M, Wu H. Pulsed radiofrequency ablation for residual and phantom limb pain: a case series. Pain Pract. 2010;10(5):485–491. doi: 10.1111/j.1533-2500.2009.00353.x. [DOI] [PubMed] [Google Scholar]
- 178.Park D, Chang MC. The mechanism of action of pulsed radiofrequency in reducing pain: a narrative review. J Yeungnam Med Sci. 2022;39(3):200–205. doi: 10.12701/jyms.2022.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang RY, Liao CC, Tsai SY, Yen CT, Lin CW, Chen TC, Lin WT, Chang CH, Wen YR. Rapid and Delayed Effects of Pulsed Radiofrequency on Neuropathic Pain: Electrophysiological, Molecular, and Behavioral Evidence Supporting Long-Term Depression. Pain Physician. 2017;20(2):E269–E283. [PubMed] [Google Scholar]
- 180.Sluijter ME, van Kleef M. Pulsed radiofrequency. Pain Med. 2007;8(4):388–389. doi: 10.1111/j.1526-4637.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 181.Lin CS, Lin YC, Lao HC, Chen CC. Interventional Treatments for Postherpetic Neuralgia: A Systematic Review. Pain Physician. 2019;22(3):209–228. doi: 10.36076/ppj/2019.22.209. [DOI] [PubMed] [Google Scholar]
- 182.Ke M, Yinghui F, Yi J, Xeuhua H, Xiaoming L, Zhijun C, Chao H, Yingwei W. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician. 2013;16(1):15–25. [PubMed] [Google Scholar]
- 183.Pi ZB, Lin H, He GD, Cai Z, Xu XZ. Randomized and controlled prospective trials of Ultrasound-guided spinal nerve posterior ramus pulsed radiofrequency treatment for lower back post-herpetic neuralgia. Clin Ter. 2015;166(5):e301–305. doi: 10.7417/T.2015.1882. [DOI] [PubMed] [Google Scholar]
- 184.Wang D, Zhang K, Han S, Yu L. PainVision(R) Apparatus for Assessment of Efficacy of Pulsed Radiofrequency Combined with Pharmacological Therapy in the Treatment of Postherpetic Neuralgia and Correlations with Measurements. Biomed Res Int. 2017;2017:5670219. doi: 10.1155/2017/5670219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saxena AK, Lakshman K, Sharma T, Gupta N, Banerjee BD, Singal A. Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag. 2016;6(3):217–227. doi: 10.2217/pmt.16.3. [DOI] [PubMed] [Google Scholar]
- 186.Park S, Park JH, Jang JN, Choi SI, Song Y, Kim YU. Pulsed radiofrequency of lumbar dorsal root ganglion for lumbar radicular pain: a systematic review and meta-analysis. Pain Pract. 2024 doi: 10.1111/papr.13351. [DOI] [PubMed] [Google Scholar]
- 187.Facchini G, Spinnato P, Guglielmi G, Albisinni U, Bazzocchi A. A comprehensive review of pulsed radiofrequency in the treatment of pain associated with different spinal conditions. Br J Radiol. 2017;90(1073):20150406. doi: 10.1259/bjr.20150406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Deer T, Shah A, Slavin K, Vorenkamp KE, Shah S, Leong M, McRoberts WP. Birds of a Feather Redux: Defining Ways to Stimulate the Peripheral Nervous System. J Pain Res. 2023;16:1219–1224. doi: 10.2147/JPR.S409158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Plaza-Manzano G, Gomez-Chiguano GF, Cleland JA, Arias-Buria JL, Fernandez-de-Las-Penas C, Navarro-Santana MJ. Effectiveness of percutaneous electrical nerve stimulation for musculoskeletal pain: a systematic review and meta-analysis. Eur J Pain. 2020;24(6):1023–1044. doi: 10.1002/ejp.1559. [DOI] [PubMed] [Google Scholar]
- 190.Kopsky DJ, Ettema FW, van der Leeden M, Dekker J, Stolwijk-Swuste JM. Percutaneous nerve stimulation in chronic neuropathic pain patients due to spinal cord injury: a pilot study. Pain Pract. 2014;14(3):252–259. doi: 10.1111/papr.12064. [DOI] [PubMed] [Google Scholar]
- 191.Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, Gajraj NM, Huber PJ, Gatchel RJ. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. JAMA. 1999;281(9):818–823. doi: 10.1001/jama.281.9.818. [DOI] [PubMed] [Google Scholar]
- 192.Raphael JH, Raheem TA, Southall JL, Bennett A, Ashford RL, Williams S. Randomized double-blind sham-controlled crossover study of short-term effect of percutaneous electrical nerve stimulation in neuropathic pain. Pain Med. 2011;12(10):1515–1522. doi: 10.1111/j.1526-4637.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 193.Rodriguez Lagos L, Arribas-Romano A, Fernandez-Carnero J, Gonzalez-Zamorano Y, Laguarta Val S. Effects of Percutaneous and Transcutaneous Electrical Nerve Stimulation on Endogenous Pain Mechanisms in Patients with Musculoskeletal Pain: A Systematic Review and Meta-Analysis. Pain Med. 2023;24(4):397–414. doi: 10.1093/pm/pnac140. [DOI] [PubMed] [Google Scholar]
- 194.Beltran-Alacreu H, Serrano-Munoz D, Martin-Caro Alvarez D, Fernandez-Perez JJ, Gomez-Soriano J, Avendano-Coy J. Percutaneous Versus Transcutaneous Electrical Nerve Stimulation for the Treatment of Musculoskeletal Pain. A Systematic Review and Meta-Analysis. Pain Med. 2022;23(8):1387–1400. doi: 10.1093/pm/pnac027. [DOI] [PubMed] [Google Scholar]
- 195.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS) Clin Neurophysiol. 2017;128(1):56–92. doi: 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- 196.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1(3):206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 197.DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. 2015;9:89. doi: 10.3389/fnana.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baptista AF, Fernandes A, Sa KN, Okano AH, Brunoni AR, Lara-Solares A, Jreige Iskandar A, Guerrero C, Amescua-Garcia C, Kraychete DC, Caparelli-Daquer E, Atencio E, Piedimonte F, Colimon F, Hazime FA, Garcia JBS, Hernandez-Castro JJ, Cantisani JAF, Karina do Monte-Silva K, Lemos Correia LC, Gallegos MS, Marcolin MA, Ricco MA, Cook MB, Bonilla P, Schestatsky P, Galhardoni R, Silva V, Delgado Barrera W, Caumo W, Bouhassira D, Chipchase LS, Lefaucheur JP, Teixeira MJ, de Andrade DC, Latin American and Caribbean consensus on noninvasive central nervous system neuromodulation for chronic pain management (LAC(2)-NIN-CP) Pain Rep. 2019;4(1):e692. doi: 10.1097/PR9.0000000000000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alwardat M, Pisani A, Etoom M, Carpenedo R, Chine E, Dauri M, Leonardis F, Natoli S. Is transcranial direct current stimulation (tDCS) effective for chronic low back pain? A systematic review and meta-analysis. J Neural Transm (Vienna) 2020;127(9):1257–1270. doi: 10.1007/s00702-020-02223-w. [DOI] [PubMed] [Google Scholar]
- 200.Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial Magnetic and Direct Current Stimulation (TMS/tDCS) for the Treatment of Headache: A Systematic Review. Headache. 2019;59(3):339–357. doi: 10.1111/head.13479. [DOI] [PubMed] [Google Scholar]
- 201.Hamid P, Malik BH, Hussain ML. Noninvasive Transcranial Magnetic Stimulation (TMS) in Chronic Refractory Pain: A Systematic Review. Cureus. 2019;11(10):e6019. doi: 10.7759/cureus.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 203.Garcia-Larrea L. Non-invasive cortical stimulation for drug-resistant pain. Curr Opin Support Palliat Care. 2023;17(3):142–149. doi: 10.1097/SPC.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 204.Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) Clin Neurophysiol. 2014;125(11):2150–2206. doi: 10.1016/j.clinph.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 205.Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115(7):1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 206.Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19(7):1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- 207.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203(1):31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 208.Yoo WK, You SH, Ko MH, Tae Kim S, Park CH, Park JW, Hoon Ohn S, Hallett M, Kim YH. High frequency rTMS modulation of the sensorimotor networks: behavioral changes and fMRI correlates. Neuroimage. 2008;39(4):1886–1895. doi: 10.1016/j.neuroimage.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 209.Stephens E, Dhanasekara CS, Montalvan V, Zhang B, Bassett A, Hall R, Rodaniche A, Robohm-Leavitt C, Shen CL, Kahatuduwa CN. Utility of Repetitive Transcranial Magnetic Stimulation for Chronic Daily Headache Prophylaxis: A Systematic Review and Meta-Analysis. Curr Pain Headache Rep. 2024 doi: 10.1007/s11916-024-01210-0. [DOI] [PubMed] [Google Scholar]
- 210.Knorst GRS, Souza PR, Araujo A, Knorst SAF, Diniz DS, Filho H. Transcranial magnetic stimulation in the treatment of phantom limb pain: a systematic review. Arq Neuropsiquiatr. 2024;82(1):1–10. doi: 10.1055/s-0044-1779051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tiwari VK, Kumar A, Nanda S, Chaudhary S, Sharma R, Kumar U, Kumaran SS, Bhatia R. Effect of neuronavigated repetitive Transcranial Magnetic Stimulation on pain, cognition and cortical excitability in fibromyalgia syndrome. Neurol Sci. 2024 doi: 10.1007/s10072-024-07317-x. [DOI] [PubMed] [Google Scholar]
- 212.Ciampi de Andrade D, Garcia-Larrea L. Beyond trial-and-error: Individualizing therapeutic transcranial neuromodulation for chronic pain. Eur J Pain. 2023;27(9):1065–1083. doi: 10.1002/ejp.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Andre-Obadia N, Mertens P, Lelekov-Boissard T, Afif A, Magnin M, Garcia-Larrea L. Is Life better after motor cortex stimulation for pain control? Results at long-term and their prediction by preoperative rTMS. Pain Physician. 2014;17(1):53–62. doi: 10.36076/ppj.2014/17/53. [DOI] [PubMed] [Google Scholar]
- 214.Andre-Obadia N, Peyron R, Mertens P, Mauguiere F, Laurent B, Garcia-Larrea L. Transcranial magnetic stimulation for pain control Double-blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clin Neurophysiol. 2006;117(7):1536–1544. doi: 10.1016/j.clinph.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 215.Sluka KA, Walsh D. Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness. J Pain. 2003;4(3):109–121. doi: 10.1054/jpai.2003.434. [DOI] [PubMed] [Google Scholar]
- 216.Cheing GL, Hui-Chan CW. Transcutaneous electrical nerve stimulation: nonparallel antinociceptive effects on chronic clinical pain and acute experimental pain. Arch Phys Med Rehabil. 1999;80(3):305–312. doi: 10.1016/s0003-9993(99)90142-9. [DOI] [PubMed] [Google Scholar]
- 217.Deyo RA, Walsh NE, Martin DC, Schoenfeld LS, Ramamurthy S. A controlled trial of transcutaneous electrical nerve stimulation (TENS) and exercise for chronic low back pain. N Engl J Med. 1990;322(23):1627–1634. doi: 10.1056/NEJM199006073222303. [DOI] [PubMed] [Google Scholar]
- 218.Verville L, Hincapie CA, Southerst D, Yu H, Bussieres A, Gross DP, Pereira P, Mior S, Tricco AC, Cedraschi C, Brunton G, Nordin M, Connell G, Shearer HM, Wong JJ, Hofstetter L, Romanelli A, Guist B, To D, Stuber K, da Silva-Oolup S, Stupar M, Myrtos D, Lee JGB, DeSouza A, Munoz Laguna J, Murnaghan K, Cancelliere C. Systematic Review to Inform a World Health Organization (WHO) Clinical Practice Guideline: Benefits and Harms of Transcutaneous Electrical Nerve Stimulation (TENS) for Chronic Primary Low Back Pain in Adults. J Occup Rehabil. 2023;33(4):651–660. doi: 10.1007/s10926-023-10121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Dailey DL, Rakel BA, Vance CGT, Liebano RE, Amrit AS, Bush HM, Lee KS, Lee JE, Sluka KA. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154(11):2554–2562. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gikaro JM, Bigambo FM, Minde VM, Swai EA. Efficacy of electrophysical agents in fibromyalgia: A systematic review and network meta-analysis. Clin Rehabil. 2023;37(10):1295–1310. doi: 10.1177/02692155231170450. [DOI] [PubMed] [Google Scholar]
- 221.Schoenen JE. Migraine prevention with a supraorbital transcutaneous stimulator: A randomized controlled trial. Neurology. 2016;86(2):201–202. doi: 10.1212/01.wnl.0000479686.32453.cc. [DOI] [PubMed] [Google Scholar]
- 222.Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J. Acute migraine therapy with external trigeminal neurostimulation (ACME): A randomized controlled trial. Cephalalgia. 2019;39(1):3–14. doi: 10.1177/0333102418811573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.di Biase L, Falato E, Di Lazzaro V. Transcranial Focused Ultrasound (tFUS) and Transcranial Unfocused Ultrasound (tUS) Neuromodulation: From Theoretical Principles to Stimulation Practices. Front Neurol. 2019;10:549. doi: 10.3389/fneur.2019.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang T, Guo B, Zuo Z, Long X, Hu S, Li S, Su X, Wang Y, Liu C. Excitatory-inhibitory modulation of transcranial focus ultrasound stimulation on human motor cortex. CNS Neurosci Ther. 2023;29(12):3829–3841. doi: 10.1111/cns.14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mishra A, Yang PF, Manuel TJ, Newton AT, Phipps MA, Luo H, Sigona MK, Reed JL, Gore JC, Grissom WA, Caskey CF, Chen LM. Disrupting nociceptive information processing flow through transcranial focused ultrasound neuromodulation of thalamic nuclei. Brain Stimul. 2023;16(5):1430–1444. doi: 10.1016/j.brs.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nussel M, Zhao Y, Knorr C, Regensburger M, Stadlbauer A, Buchfelder M, Del Vecchio A, Kinfe T. Deep Brain Stimulation, Stereotactic Radiosurgery and High-Intensity Focused Ultrasound Targeting the Limbic Pain Matrix: A Comprehensive Review. Pain Ther. 2022;11(2):459–476. doi: 10.1007/s40122-022-00381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Todd N, McDannold N, Borsook D. Targeted manipulation of pain neural networks: The potential of focused ultrasound for treatment of chronic pain. Neurosci Biobehav Rev. 2020;115:238–250. doi: 10.1016/j.neubiorev.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Meyerson BA. Neurosurgical approaches to pain treatment. Acta Anaesthesiol Scand. 2001;45(9):1108–1113. doi: 10.1034/j.1399-6576.2001.450910.x. [DOI] [PubMed] [Google Scholar]
- 229.Jeanmonod D, Werner B, Morel A, Michels L, Zadicario E, Schiff G, Martin E. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg Focus. 2012;32(1):E1. doi: 10.3171/2011.10.FOCUS11248. [DOI] [PubMed] [Google Scholar]
- 230.Martin E, Jeanmonod D, Morel A, Zadicario E, Werner B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann Neurol. 2009;66(6):858–861. doi: 10.1002/ana.21801. [DOI] [PubMed] [Google Scholar]
- 231.Richards DE, Tyner CF, Shealy CN. Focused ultrasonic spinal commissurotomy: experimental evaluation. J Neurosurg. 1966;24(4):701–707. doi: 10.3171/jns.1966.24.4.0701. [DOI] [PubMed] [Google Scholar]
- 232.Bouwense SA, Olesen SS, Drewes AM, van Goor H, Wilder-Smith OH. Pregabalin and placebo responders show different effects on central pain processing in chronic pancreatitis patients. J Pain Res. 2015;8:375–386. doi: 10.2147/JPR.S84484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Jeon SY, Choi JH, Kang SS, An YH, Shim HJ (2023) Personalized Neuromodulation: A Novel Strategy for Improving Tinnitus Treatment. J Clin Med 12 (22). 10.3390/jcm12226987 [DOI] [PMC free article] [PubMed]
- 234.Schoisswohl S, Langguth B, Hebel T, Vielsmeier V, Abdelnaim MA, Schecklmann M (2022) Personalization of Repetitive Transcranial Magnetic Stimulation for the Treatment of Chronic Subjective Tinnitus. Brain Sci 12 (2). 10.3390/brainsci12020203 [DOI] [PMC free article] [PubMed]
- 235.Schoisswohl S, Langguth B, Hebel T, Abdelnaim MA, Volberg G, Schecklmann M (2021) Heading for Personalized rTMS in Tinnitus: Reliability of Individualized Stimulation Protocols in Behavioral and Electrophysiological Responses. J Pers Med 11 (6). 10.3390/jpm11060536 [DOI] [PMC free article] [PubMed]
- 236.Schoisswohl S, Langguth B, Schecklmann M. Short-Term Tinnitus Suppression With Electric-Field Guided rTMS for Individualizing rTMS Treatment: A Technical Feasibility Report. Front Neurol. 2020;11:86. doi: 10.3389/fneur.2020.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kreuzer PM, Poeppl TB, Rupprecht R, Vielsmeier V, Lehner A, Langguth B, Schecklmann M. Individualized Repetitive Transcranial Magnetic Stimulation Treatment in Chronic Tinnitus? Front Neurol. 2017;8:126. doi: 10.3389/fneur.2017.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Boutet A, Madhavan R, Elias GJB, Joel SE, Gramer R, Ranjan M, Paramanandam V, Xu D, Germann J, Loh A, Kalia SK, Hodaie M, Li B, Prasad S, Coblentz A, Munhoz RP, Ashe J, Kucharczyk W, Fasano A, Lozano AM. Predicting optimal deep brain stimulation parameters for Parkinson's disease using functional MRI and machine learning. Nat Commun. 2021;12(1):3043. doi: 10.1038/s41467-021-23311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gilron R, Little S, Perrone R, Wilt R, de Hemptinne C, Yaroshinsky MS, Racine CA, Wang SS, Ostrem JL, Larson PS, Wang DD, Galifianakis NB, Bledsoe IO, San Luciano M, Dawes HE, Worrell GA, Kremen V, Borton DA, Denison T, Starr PA. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson's disease. Nat Biotechnol. 2021;39(9):1078–1085. doi: 10.1038/s41587-021-00897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Di Maio R. Neuronal mechanisms of epileptogenesis. Front Cell Neurosci. 2014;8:29. doi: 10.3389/fncel.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.van Rooij SJH, Arulpragasam AR, McDonald WM, Philip NS. Correction: Accelerated TMS - moving quickly into the future of depression treatment. Neuropsychopharmacology. 2024;49(1):297. doi: 10.1038/s41386-023-01714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.d'Andrea G, Mancusi G, Santovito MC, Marrangone C, Martino F, Santorelli M, Miuli A, Di Carlo F, Signorelli MS, Clerici M, Pettorruso M, Martinotti G (2023) Investigating the Role of Maintenance TMS Protocols for Major Depression: Systematic Review and Future Perspectives for Personalized Interventions. J Pers Med 13 (4). 10.3390/jpm13040697 [DOI] [PMC free article] [PubMed]
- 243.Andre-Obadia N, Mertens P, Gueguen A, Peyron R, Garcia-Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology. 2008;71(11):833–840. doi: 10.1212/01.wnl.0000325481.61471.f0. [DOI] [PubMed] [Google Scholar]
- 244.Bastuji H, Cadic-Melchior A, Ruelle-Le Glaunec L, Magnin M, Garcia-Larrea L. Functional connectivity between medial pulvinar and cortical networks as a predictor of arousal to noxious stimuli during sleep. Eur J Neurosci. 2024;59(4):570–583. doi: 10.1111/ejn.15958. [DOI] [PubMed] [Google Scholar]
- 245.Radulescu I, Dragoi AM, Trifu SC, Cristea MB. Neuroplasticity and depression: Rewiring the brain's networks through pharmacological therapy (Review) Exp Ther Med. 2021;22(4):1131. doi: 10.3892/etm.2021.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sampathkumar V, Miller-Hansen A, Sherman SM, Kasthuri N (2021) Integration of signals from different cortical areas in higher order thalamic neurons. Proc Natl Acad Sci U S A 118 (30). 10.1073/pnas.2104137118 [DOI] [PMC free article] [PubMed]
- 247.Cury RG, Teixeira MJ, Galhardoni R, Silva V, Iglesio R, Franca C, Arnaut D, Fonoff ET, Barbosa ER, Ciampi de Andrade D. Connectivity Patterns of Subthalamic Stimulation Influence Pain Outcomes in Parkinson's Disease. Front Neurol. 2020;11:9. doi: 10.3389/fneur.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vetterlein A, Monzel M, Reuter M. Are catechol-O-methyltransferase gene polymorphisms genetic markers for pain sensitivity after all? - A review and meta-analysis. Neurosci Biobehav Rev. 2023;148:105112. doi: 10.1016/j.neubiorev.2023.105112. [DOI] [PubMed] [Google Scholar]
- 249.Hong H, Kim RY, Song Y, Suh C, Lee H, Lyoo IK, Yoon S, Lim SM, Lee S. Genetic profile for dopamine signaling predicts brain functional reactivity to repetitive transcranial magnetic stimulation. Eur Arch Psychiatry Clin Neurosci. 2023;273(1):99–111. doi: 10.1007/s00406-022-01436-2. [DOI] [PubMed] [Google Scholar]
- 250.Ojala J, Vanhanen J, Harno H, Lioumis P, Vaalto S, Kaunisto MA, Putaala J, Kangasniemi M, Kirveskari E, Makela JP, Kalso E. A Randomized, Sham-Controlled Trial of Repetitive Transcranial Magnetic Stimulation Targeting M1 and S2 in Central Poststroke Pain: A Pilot Trial. Neuromodulation. 2022;25(4):538–548. doi: 10.1111/ner.13496. [DOI] [PubMed] [Google Scholar]
- 251.Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A, Forssell H, Hietala J, Hagelberg N, Pertovaara A, Parkkola R, Jaaskelainen S. Right secondary somatosensory cortex-a promising novel target for the treatment of drug-resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain. 2015;156(7):1276–1283. doi: 10.1097/j.pain.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 252.Motzkin JC, Kanungo I, D'Esposito M, Shirvalkar P. Network targets for therapeutic brain stimulation: towards personalized therapy for pain. Front Pain Res (Lausanne) 2023;4:1156108. doi: 10.3389/fpain.2023.1156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fishman MA, Antony A, Esposito M, Deer T, Levy R. The Evolution of Neuromodulation in the Treatment of Chronic Pain: Forward-Looking Perspectives. Pain Med. 2019;20(Suppl 1):S58–S68. doi: 10.1093/pm/pnz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.