Abstract
Objectives
Chronic dyspnea, a distressing symptom in patients with complex chronic conditions, is linked to higher risks of mortality. This study aimed to identify nursing interventions that could improve self-management for complex chronic patients, thereby enhancing control over chronic dyspnea. The findings intend to guide nursing care strategies that promote self-management among this population.
Methods
We searched the databases Medline, Scopus, Web of Science, CINAHL, Cochrane Database of Systematic Reviews (CDSR), and Joanna Briggs Institute (JBI) databases were searched in December 2023. We included adult patients with complex chronic conditions with chronic dyspnoea. The team screened articles collaboratively, using Rayyan software. A qualitative appraisal was performed according to JBI Critical Appraisal Checklist tools. The review protocol is registered under the number CRD42023456021.
Results
Our review included 18 studies that explored a variety of interventions for chronic dyspnea. We identified pharmacological interventions (such as oxygen therapy and inhalation treatments) and non-pharmacological approaches (including educational programs, breathing exercises, fluid intake management, body awareness techniques, peer support, emotional intelligence training, and the use of web applications). Those interventions empower patients, improve their ability to fulfill life roles, mitigate emotional distress, and improve overall quality of life. Nursing care can be crucial in enabling individuals to achieve independence and autonomy in self-care.
Conclusions
Promoting self-management for chronic dyspnea in complex chronic patients requires a holistic approach, encompassing multidisciplinary interventions, individualized self-care education, peer engagement, and technological support. Current research on self-management inadequately addresses interventions targeting patient behaviour change. It highlights the need to delve deeper into the self-management process. Further research is needed to expand the evidence base and refine these interventions.
Keywords: Chronic disease, Dyspnea, Nursing, Patients, Self-management
What is known?
-
•
In the context of global health challenges posed by chronic conditions and aging, chronic dyspnea is a common symptom among patients with complex chronic conditions such as heart failure, chronic obstructive pulmonary disease (COPD), asthma, lung tumors, and interstitial lung disease.
-
•
Dyspnea is a multidimensional experience influenced by physiological, psychological, social, and environmental factors, and it significantly impacts patients’ quality of life and functional capacity.
-
•
Nursing-led self-management interventions are crucial for improving symptoms and overall wellbeing in patients with complex chronic diseases.
What is new?
-
•
Promoting the self-management of chronic dyspnea requires a comprehensive approach, considering factors like lifestyle, health status, resources, environment, and healthcare system-related aspects.
-
•
Pharmacological and non-pharmacological approaches are essential interventions nurses may develop to encourage dyspnea self-management.
-
•
Empowering patients and their families to manage symptoms actively and customizing personalized self-care strategies are essential elements in managing chronic dyspnea and complex chronic conditions.
1. Introduction
In the context where chronic conditions and aging pose an increasingly substantial global health challenge [1,2], chronic dyspnea emerges as a frequently encountered symptom among patients with complex chronic conditions, such as heart failure, chronic obstructive pulmonary disease (COPD), asthma, lung tumors, and interstitial lung disease [3]. In complex chronic conditions like these, symptoms and intricate self-management needs are linked, underscoring the need for healthcare strategies that address the multifaceted aspects of this complexity [[4], [5], [6]].
Complex patients are grappling with multiple medical, social, and psychological conditions, contributing to the intricacies of their clinical presentation. This complexity poses challenges in clinical management, care coordination, and achieving satisfactory health outcomes. The definition of complex patients is subject to variation in scientific publications, signaling the need for standardization to enhance study comparability and streamline identification in clinical practice [7]. This publication centers on the precursors to this complexity, such as multiple chronic conditions, intricate social needs, polypharmacy, self-care challenges, and nuanced interactions within the healthcare system [8]. These precursors play a substantial role in shaping the overall complexity, whether in isolation or combined. In this review, we recognized the significance of multiple chronic conditions as a key contributor to complexity. Patients with complex chronic diseases have a higher risk of drug adverse events [9] and experience frequent acute deterioration, increasing hospitalization risk [10]. In this regard, complexity refers to the interconnected patient needs affecting healthcare provision and decision-making across various dimensions, including health, social, economic, behavioural, and relational aspects [[4], [5], [6],11].
Dyspnea is a subjective experience of breathing discomfort and distress in breathing, comprising distinct sensations varying in intensity. It arises from numerous physiological, psychological, social, and environmental factors and can trigger secondary physiological and behavioural reactions [12,13]. Dyspnea encompasses a multidimensional range of experiences intricately tied to nearly every facet of a patient’s physiological and psychological condition. Dyspnea is not a singular experience; rather, it encompasses a diverse spectrum of sensations (such as air hunger, heightened effort, and rapid breathing) that are distinctly subjective [14]. Dyspnea is an overarching term encompassing various discernible subjective perceptions, such as difficult breathing, suffocation sensation, and air need [15]. Considerable individual variation exists among patients in their perception of dyspnea and the consequent degree of impairment, surpassing the boundaries of the underlying cause. It is commonly characterized as chronic when it lasts more than four weeks [15].
The patient’s emotional state, present mood, overall anxiety level, anticipation of dyspnea, and heightened focus on one’s breathing all shape the perception of dyspnea [16]. Based on Dame Cicely Saunders’ concept of total pain in the 1960s, a total dyspnea model reveals a self-perpetuating cycle in which dyspnea interacts with and is impacted by a patient’s physical, psychological, spiritual, and social dimensions [17]. In this sense, dyspnea can significantly impair patients’ quality of life and functional capacity, leading to increased healthcare utilization and associated costs [14,[18], [19], [20]]. The dyspnea phenomenon is significantly associated with mortality among the adult population. As measured by the modified Medical Research Council (mMRC) Dyspnea Scale, progressive increments in dyspnea severity correlate with escalating mortality risks, from 26% (mMRC grade 1) to 155% (mMRC grade 4) [21]. The extent of respiratory comfort is shaped by a dynamic interplay involving the patient’s coping approach, their tendency to seek assistance, the clinician’s responsiveness to breathlessness, and the concurrent management of the underlying medical condition [14].
Managing chronic dyspnea requires a comprehensive approach addressing the enabling factors and barriers, such as lifestyle, health status, resources, environment, and healthcare system [22]. Nursing-led self-management interventions are pivotal in empowering patients to participate in their care and optimize their symptom control [[22], [23], [24]]. Dyspnea, as well as self-management, are essential focuses of nursing care [23,24]. Self-management has been defined as the proactive engagement in activities initiated by individuals to uphold their own life, health, and wellbeing. This involves developing the skills necessary to create, implement, assess, and adjust a personalized plan for lifestyle changes [26]. Self-management encompasses medical, emotional, and role management, demanding patients cultivate six essential skills: decision-making, action planning, fostering a patient–provider partnership, self-tailoring, resource utilization, and problem-solving [27]. Recent advancements in designing health behaviour interventions have underscored the significance of incorporating theory and categorizing intervention components, known as Behavior Change Techniques (BCTs) [28], and aligning these components with change strategies. Despite the growing number of primary research in the field, there are still significant gaps in understanding the effectiveness of self-management interventions for patients with complex needs [[29], [30], [31]]. Additionally, although exploring chronic dyspnea self-management is vital to understanding how patients and families cope with chronic breathlessness, inform targeted interventions, and improve patient care [13,14], a thorough comprehension of the complexities involved in its self-care management still eludes us [6,32,33].
Therefore, an integrative review becomes crucial to consolidate existing knowledge, identify gaps, and pave the way for a more nuanced understanding of self-management. Such an approach has the potential to enhance symptom control, increase patient and family engagement, and explore personalized self-care strategies designed to address the unique needs of patients with complex chronic conditions and persistent dyspnea. This knowledge will inform the development of sustainable self-management and family management interventions, particularly for patients with multiple chronic conditions [22]. This study aimed to identify interventions that enhance self-management, tailored to complex chronic patients, to improve chronic dyspnea control. The insights will inform nursing care strategies to support self-management in this population.
2. Methods
We conducted an integrative literature review following the steps outlined by Whittemore and Knafl [34]: 1) problem identification, 2) literature search, 3) data evaluation, 4) data analysis, and 5) presentation of findings.
2.1. Problem identification
In the first step, we formulated a broad purpose and/or review question that guides the review process [33,34]. The research question was constructed using the PIO strategy, where “P” represents the population, “I” stands for the Intervention, and “O” represents the study outcomes. The following guiding question was formulated: “What interventions (I) promote self-management (O) in patients with complex chronic conditions with chronic dyspnea (P)?”
2.2. Literature search
In the second step, Medline (via EBSCOhost), CINAHL (via EBSCOhost), Cochrane Database of Systematic Reviews (via EBSCOhost), Scopus, Web of Science, and Joanna Briggs Institute (JBI) were searched on 15 December 2023. According to the following eligibility criteria, the search strategy was the same for all databases. 1) Population: inclusion criteria encompassed adult complex patients with multiple chronic conditions, specifically focusing on chronic dyspnea. Pediatric patients and articles addressing acute dyspnea were systematically excluded. 2) Interventions: we considered interventions aimed at promoting dyspnea self-management, including pharmacological and non-pharmacological approaches. 3) Outcomes: articles focused on primary outcomes related to dyspnea perception, knowledge, skills, and health-related behaviors for chronic dyspnea self-management. Secondary outcomes included overall health status, access to healthcare services, and long-term individual and family outcomes, such as quality of life [22]. The study’s linguistic inclusivity extended to Portuguese, English, Spanish, and French articles. We included a broad spectrum of quantitative and qualitative studies, while systematic reviews, protocol studies, opinion articles, comments, and editorials were excluded. There were no restrictions on publication dates.
We employed specific search terms that were required to appear in the title or the abstract. Following the starting question, the terms were combined using the boolean operators OR or AND: (“Dyspnea” OR “Dyspnoea” OR “breathlessness” OR “shortness of breath”) AND (“Chronic-complex-patients” OR “chronic illness” OR “multiple long-term conditions” OR “Multiple Chronic Conditions” OR “complexity” OR “Comorbidity” OR “Co-morbidities” OR “chronically ill older adults” OR “complex multimorbidity” OR “chronic patient” OR “Multimorbidity” OR “Chronic Disease” OR “complex care”) AND (Self-management OR self-care). The search strategy was the same for all databases.
Upon identifying all relevant references, the Rayyan software (Rayyan Systems Inc., Cambridge, MA, USA) was employed to facilitate collaborative efforts within the team [35]. The research team was divided into smaller groups of two or three members. Three researchers (TS, AC, and HRH) collaborated on the screening process, while other researchers (DS, JP, JT, and JF) conducted independent screening validation.
2.3. Data evaluation
In the third stage, the articles were subsequently screened based on eligibility criteria, and their quality and level of evidence were evaluated using JBI Critical Appraisal Checklist tools [36]. These tools facilitate evaluating methodological quality and bias risk in studies. Studies are evaluated according to their typology, considering predefined criteria. High-quality studies typically score higher than 70% on the total components of the critical appraisal tools, indicating rigor and reliability in the findings [36].
Using the Rayyan software, the process began with removing duplicate entries. Subsequently, the title and abstract of each retrieved study underwent independent assessment by two researchers (all team members). This assessment determined if the studies aligned with the predetermined eligibility criteria.
A decision tree approach was adopted to guide manual screening, starting with evaluating the study type. If this criterion was met, we examined the target population. Following the fulfilment of these criteria, scrutiny was extended to assess the intervention and the outcome.
Articles that successfully fulfilled the eligibility criteria underwent a comprehensive full-text review. In cases where two screeners have differing opinions regarding whether an article meets the eligibility criteria, they address this disparity through discussion and deliberation. A third individual (HRH) acted as an arbitrator to finalize the disputed article if a consensus could not be reached. Additionally, the librarian played a role in identifying articles that were not fully accessible.
2.4. Data analysis
In line with the methodological rigor advocated by Whittemore and Knafl [34] for integrative reviews, our data analysis followed the author’s method, encompassing data reduction, data display, data comparison, conclusion drawing, and verification.
Three researchers (TS, AC, and HRH) were engaged in the data reduction and display phases. During data reduction, manuscripts were categorized according to research type and intervention type to establish a logical and easily analyzable system. Subsequently, generic data from primary sources were extracted and coded across various subsections: study/location, study aims, design/sample, intervention components, outcomes, key findings, and level of evidence. This systematic approach was presented in table format to compare primary sources systematically based on their specific characteristics.
In constructing the data display, which addressed our research inquiry, a new table was devised to present findings on self-management components and behaviours categorized by the type of intervention (pharmacological or non-pharmacological).
Data comparison involved all authors, entailing a systematic and comparative discussion of the data in the tables above. This thorough process led to the development of an expanded version of the “self- and family management of chronic conditions framework” [22]. This expanded framework illustrates the patterns and relationships involved in dyspnea self-management, as depicted in Appendix A.
Drawing conclusions and verification received meticulous attention from the manuscript’s authors, which is evident in the results narrative and scientific integrity of the discussion.
2.5. Compliance with ethical standards
We comply with the ethical standards set for literature review research. All utilized sources were appropriately cited and referenced, demonstrating respect for the copyrights and intellectual integrity of the original authors. The review protocol is registered under the number CRD42023456021.
3. Results
Following the database search, 228 articles were retrieved.
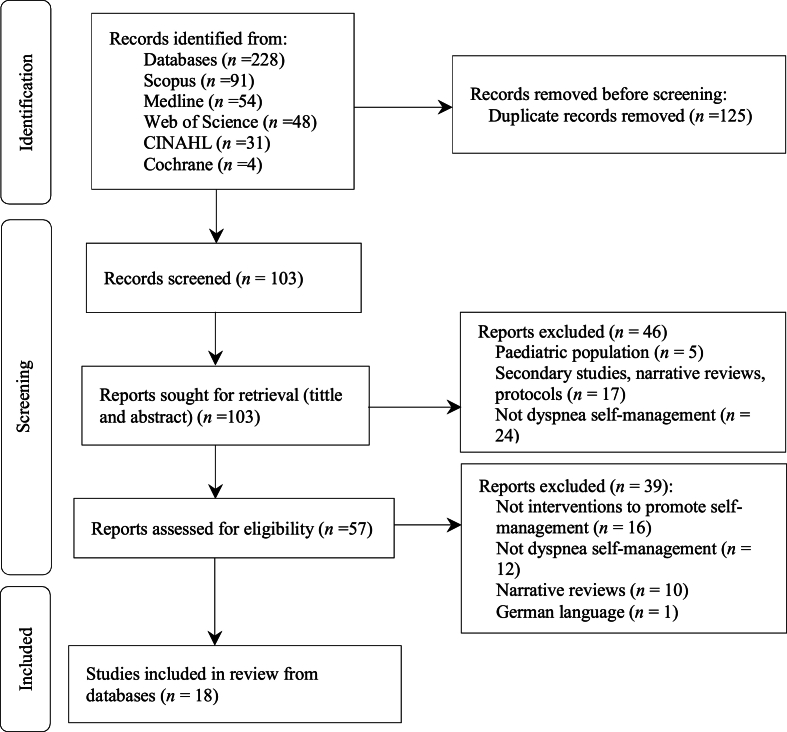
Upon the initial phase of exclusion, targeting duplicate articles, a thorough assessment ensued, encompassing the scrutiny of titles and abstracts. Subsequently, an exhaustive review of full-text articles was conducted, culminating in the identification of 18 final studies for inclusion in the review (Fig. 1).
Fig. 1.
Flowchart of the study selection process.
3.1. Characteristics of included studies
Our sample integrates studies with different levels of evidence [36]. Following JBI Critical Appraisal Tools [36], our sample was comprised mainly of studies of moderate quality, with only two studies [37,38] rated as low quality (Appendix B). Notably, moderate-quality randomized trials [18,[39], [40], [41], [42]] consistently exhibited shortcomings in blinding procedures for outcome assessors, participants, and treatment administrators. The most significant limitations in cohort studies [[35], [36], [37], [38]] were identifying and handling confounding factors and strategies for managing incomplete follow-up. In cross-sectional studies [[43], [44], [45]], the main concern was the validity and reliability of exposure measurement. The limitations of included studies raise concerns about potential bias in outcome assessment, participant responses, and the robustness of causal inferences. Validity and reliability issues in exposure measurement also doubt the integrity of study findings.
3.2. Self-management of chronic dyspnea
The sample presents articles originating from the USA (n = 8), Australia (n = 2), England (n = 2), Spain (n = 2), Canada (n = 1), Saudi Arabia (n = 1), Turkey (n = 1), and South Korea (n = 1). The selected articles encompass a temporal range of publications from 1999 to 2022. The studies predominantly target an aging population with chronic conditions, notably COPD, bronchiectasis, asthma, heart failure, hypertension, stroke, type 2 diabetes, chronic kidney disease, idiopathic pulmonary fibrosis, and mental health conditions (Table 1). The selected studies were subjected to a comprehensive analysis encompassing qualitative and quantitative dimensions.
Table 1.
The characteristics of the included studies (n = 18).
| Study and location | Aim of study | Design and sample | Intervention components | Outcomes | Key findings | Level of evidence |
|---|---|---|---|---|---|---|
| Cevirme et al., 2020, Turkey [18] | To evaluate the impact of dyspnea and chronic self-care management outcomes of an education-based intervention program compared to routine care. |
|
|
|
|
1 |
| Davis et al., 2006, USA [37] | To determine the effect of the intervention on self-efficacy and the relationship between domain-specific self-efficacy, walking performance, and symptom severity in patients with COPD. |
|
|
|
|
1 |
| Mark et al., 2011, USA [38] | To measure the effect of PLB training delivered via Skype on dyspnea, physical activity, health-related quality of life, and self-efficacy |
|
|
|
|
1 |
| Lorig et al., 2006, USA [39] | To determine the effectiveness of an Internet-based CDSMP |
|
|
|
|
1 |
| Lorig et al., 1999, USA [40] | To evaluate the effectiveness of a self-management program for chronic diseases designed to be used with a heterogeneous group of patients with chronic diseases; explore the differential effectiveness of the intervention for individuals with specific diseases and comorbidities. |
|
|
|
|
1 |
| Lavery et al., 2011, England [41] | To investigate the efficacy of a disease-specific Expert Patient Programme (EPP) compared with usual care in patients with bronchiectasis. |
|
|
|
|
1 |
| Cameron-Tucker et al., 2014, Australia [42] | To investigate both the efficacy of the CDSMP itself in COPD and the addition of supervised exercise to the CDSMP on physical capacity measured by the 6MWD. |
|
|
|
|
1 |
| Kim & Park, 2020, South Korea [43] | To evaluate the level of dyspnea and the self-management interventions used to alleviate dyspnea in lung cancer patients with concurrent pneumoconiosis, particularly oxygen therapy and bronchodilator treatment; to determine the factors associated with such self-management and to provide a basis for developing an applicable and safe treatment plan for alleviating dyspnea. |
|
|
|
|
4 |
| Stenekes et al., 2008, Canada [44] | To survey the population of cystic fibrosis patients in the Canadian Maritimes to gather self-reported assessment and self-management of pain, dyspnea, and cough information. |
|
|
|
|
4 |
| Benzo et al., 2016, USA [45] | To investigate the association between emotional intelligence and two meaningful outcomes in COPD: quality of life and self-management abilities |
|
|
|
|
4 |
| Moreno et al., 2018, Spain [47] | To determine the impact of an educational program to improve the management of COPD on the perception of quality of life, exercise capacity, degree of dyspnea, and clinical risk of COPD patients. |
|
|
|
|
3 |
| Lorig et al., 2008, USA [48] | To evaluate the effectiveness of an online self-management program for residents with long-term conditions. |
|
|
|
|
3 |
| Lorig et al., 2001, USA [49] |
|
|
|
|
|
3 |
| Lee et al., 2022, Australia [50] | To attain consensus from experts in PF and people living with the disease on the essential components and format of a PF self-management package. |
|
|
|
|
5 |
| Hermosa et al., 2020, Spain [51] | To provide further evidence to support prospective recording of daily symptoms as a useful strategy for detecting COPD exacerbations via the Prevexair smartphone app. It also aimed to analyze daily adherence and the frequency and characteristics of acute COPD exacerbations recorded with Prevexair. |
|
|
|
|
3 |
| Alharbey et al., 2019, Saudi Arabia [52] | To design an innovative mobile health (mHealth) application system called “MyLung” that provides complete solutions to increase self-awareness and promote better self-care management. |
|
|
|
|
2 |
| Reilly et al., 2022, England [53] | To explore the accessibility and willingness of patients with chronic breathlessness to use an internet-based breathlessness self-management intervention (SELF-BREATHE). |
|
|
|
|
5 |
| Dansky & Vasey, 2009, USA [55] | Evaluate the impact of telehealth-based disease management system on health and functional status related to patients’ self-management of heart failure, utilization of health services, and patients’ satisfaction. |
|
Knowledge/skills: Teleheath system after discharge from formal home health services (transmit the disease management program to the patient, collect clinical data and patients’ responses to questions, and transmit these data back to the healthcare provider). |
|
|
1 |
Note: 6MWD = 6-min walk distance. CDSMP = chronic disease self-management program. COPD = chronic obstructive pulmonary disease. RCT = randomized controlled trial. SpO2 = peripheral capillary oxygen saturation. PLB = pursed lips breathing. PF = pulmonary fibrosis. Levels of evidence for effectiveness according to JBI: Level 1 = experimental designs; Level 2 = quasi-experimental designs; Level 3 = observational – analytic designs; Level 4 = observational-descriptive studies; Level 5 = expert opinion and bench research.
The findings have been organized into thematic categories, allowing for a coherent and systematic presentation of the results. These categories provide insights into the various interventions promoting self-management of chronic dyspnea. These findings unveil a confluence of pharmacological and non-pharmacological modalities, converging to empower patients in exerting pivotal agency over their chronic disorders. This empowerment is reflected in the domains of perpetuating essential life roles, mitigating deleterious emotional states, bolstering symptom management, and augmenting the quality of life [43] (Table 2).
Table 2.
The components and behaviors for chronic dyspnea self-management.
| Self-management components | Self-management behaviors | ||
|---|---|---|---|
| Pharmacological Interventions | Use of medication | Oxygen therapy [43]S; [44]S; [50]KS | Medication adherence |
| Oral therapy (Antibiotics, Ibuprofen) [44]S; [50]K | |||
| Inhalation therapy [43]S; [44]S; [47]S; [50]K | |||
| Non-pharmacological interventions | Educational programs | CDSMP [40]K,S; [49]K,S (exercise; use of cognitive symptom management techniques; nutrition; fatigue and sleep management; use of community resources; use of medications; dealing with the emotions of fear, anger, and depression; communication with others including health professionals; problem-solving; decision-making) | Recognition and monitoring of dyspnea; Actively seeking health information (health literacy); Smoking cessation/smoke-free environment; Medication adherence; Maintain a healthy lifestyle (sleep, physical activity, nutrition); Manage emotions; Care planning (avoid trigger factors); Incorporating breathing exercises into daily routine. |
| Internet-based CDSMP [39]K,S; [48]K,S | |||
| CDSMP + physical activity [42]K,S; [47]K,S | |||
| The dyspnea self-management program [37]K,S | |||
| The expert patients programme [41]K,S (causes of bronchiectasis, disease process, medical investigations, dealing with symptoms, airway clearance techniques, exacerbations, health promotion, and support available) | |||
| Education-base intervention program [18]K,S: hospital education; home visits + education; telephone monitoring and guidance) | |||
| Supervised exercise sessions [42]K,S | |||
| Punctual Educational sessions activity [38]K,S | |||
| Breathing exercises | Rest and catch breath activity [44]S | Incorporating breathing exercises into daily routine; Conserve energy |
|
| Deep inspiration followed by prolonged expiration [44]S | |||
| Positive expiratory pressure/physiotherapy [44]S | |||
| Pursed-lips breathing [37]S | |||
| Diaphragmatic breathing [37]S; [38]S | |||
| Moisturize the mouth | Drink water [44]S | Avoiding oral dehydration; Care planning |
|
| Body awareness | Exempt from physical activity [44]S | Recognition and monitoring of dyspnea | |
| Symptoms Monitoring [37]S (heart rate, dyspnoea, and oxygen saturation monitoring during exercise) | |||
| Emotional intelligence | Emotional intelligence [45]S; [50]S | Manage emotions | |
| Web application | Mobile app “My Lung” [52]K,S (understanding about COPD and ways to avoid risk-related factors; self-monitor their symptoms and vitals, including peripheral capillary oxygen saturation) | Engage with digital health platforms; Actively seeking health information (health literacy); Recognition and monitoring of dyspnea; Measure SPO2; Care planning |
|
| Mobile app “Prevexair” [51]S (recording daily symptoms) | |||
| Internet-based breathlessness self-management intervention [53]S (“Self-Breathe”) | |||
| Teleheath system after discharge [55]K,S | |||
Note: CDSMP= chronic disease self-management program. SpO2 = peripheral capillary oxygen saturation. Superscript K = empowering patients through knowledge. Superscript S = empowering patients through skills.
3.2.1. Pharmacological interventions
Medication use represents a crucial component in the self-management of dyspnea, contributing significantly to alleviating symptoms and the overall wellbeing of individuals. Education on medication management empowers patients to take an active role in their health, fostering a sense of control and autonomy in the face of respiratory challenges. Managing dyspnea requires individuals to comprehend the principles of oxygen therapy and skillfully administer it based on prescribed guidelines. Similarly, mastering inhalation techniques, including using inhalers, is vital in optimizing respiratory function and dyspnea control [43,44,46,47].
Pharmacological interventions, such as oxygen therapy, antibiotics, ibuprofen, or inhalation-based treatments comprising bronchodilators and corticosteroids, emerge as a pivotal pathway for managing dyspnoea symptoms [43,44,46,47]. Short-term supplemental oxygen therapy has demonstrated noteworthy efficacy in augmenting exercise performance, thus presenting a viable means to mitigate the physiological impact of breathlessness during physical exertion.
In the broader context, bronchodilator utilization has shown an upward trajectory in response to comorbidities, cardiopulmonary dysfunction, heightened respiratory distress, and compromised activities of daily living [43,44,46]. However, a notable concern surfaces within the literature, as evidenced by Kim and Park’s [43] study, indicating that a significant proportion of participants, approximately 70.9%, engaged in excessive usage of inhaled bronchodilators and corticosteroids. Such behavior raises concerns regarding potential adverse effects and emphasizes the critical importance of prudent and informed management of pharmacological interventions to circumvent unwarranted complications and challenges.
3.2.2. Non-pharmacological interventions
In non-pharmacological interventions, diverse interventions emerge, collectively establishing a comprehensive framework for self-management. These interventions encompass a spectrum of modalities, each contributing distinct components to the holistic management of dyspnea.
3.2.2.1. Educational programs
Educational programs are pivotal in bestowing individuals with the requisite knowledge and skills to adeptly navigate the intricacies of their conditions [18,37,[39], [40], [41], [42],[47], [48], [49]]. The chronic disease self-management program (CDSMP), grounded in the theory of self-efficacy, stands out as a prominent exemplar in this domain, emphasizing problem-solving skills, decision-making, and fostering self-confidence [39,48]. One notable iteration of this program, the CDSMP-Online, was developed to extend the reach to a broader population of individuals with chronic illnesses while closely mirroring the original CDSMP framework [39,48]. This web-based program encompasses interactive online instructions, discussion groups, and the content from the Living a Healthy Life with Chronic Conditions.
A study by Lorig et al. [49] demonstrated that participants who engaged in the CDSMP experienced significant improvements in health status, health behaviors, and self-efficacy over a year. Notably, the utilization of the program correlated with a reduction in emergency department visits, indicating a potential avenue for reducing healthcare utilization costs. The transformative potential of such interventions, which empower individuals to manage their chronic conditions proactively, highlights their viability and efficacy, urging healthcare systems to consider their integration.
In these programs, self-management of dyspnoea is inherently integrated within a comprehensive approach to managing chronic conditions, and its significance is contingent upon its recognition within this holistic context.
Davis et al. [37] introduced the concept of a dyspnoea self-management program (DSP) structured around three distinct interventions differentiated by exercise type and intensity. Participants were equipped with various self-management interventions, including respiratory exercises and techniques such as diaphragmatic breathing and pursed-lip exhalation. The results showcased improvements in self-efficacy for walking and managing dyspnea, elucidating the potency of self-efficacy enhancement as a crucial outcome of self-management interventions.
An innovative approach emerged with the Specialized Patient Empowerment Program (SPEP) elucidated by Lavery et al. [41]. This group-based intervention prioritized enhancing self-efficacy through a comprehensive range of processes encompassing action planning, feedback, role modeling, problem-solving, symptom interpretation, and decision-making. The positive outcomes of SPEP underscored the potential of patient empowerment programs in chronic disease self-management.
The importance of education and rehabilitation in the context of respiratory frequency was explored by Blánquez Moreno et al. [47]. Their intervention, the respiratory frequency education and rehabilitation program, incorporated fundamental principles of respiratory physiology, respiratory physiotherapy exercises, and education on proper inhaler usage. The study demonstrated statistically significant and clinically relevant improvements in fatigue, exercise capacity, and dyspnea, signifying the potential of education and rehabilitation initiatives in enhancing self-management interventions for individuals with chronic respiratory conditions.
The results show that using cognitive interventions to manage symptoms led to significant improvements in various aspects of health, including reducing the feeling of breathlessness [18,[37], [38], [39], [40], [41], [42],[47], [48], [49]]. The group that received the program intervention also had fewer visits to healthcare providers [40,47,[40], [47], [48], [49],[40], [47], [48], [49]]. Patients’ self-efficacy in managing shortness of breath improved significantly during program implementation [37,41].
Furthermore, participants who learned to control symptom exacerbation reported better self-management of breathlessness, improved quality of life, and more independence in daily activities [38].
While designed to augment knowledge, educational programs exhibit a discernible association with enhanced capabilities in dyspnea management. Evidence suggests that participation in these programs contributes to the broadening of theoretical understanding but also correlates with tangible improvements in practical skills. This dual impact underscores the comprehensive nature of educational interventions, wherein the benefits extend beyond knowledge acquisition to include the cultivation of effective strategies for mitigating dyspnea symptoms in real-world scenarios [18,27,37,[39], [40], [41], [42],[47], [48], [49]].
3.2.2.2. Respiratory exercises
Respiratory exercises hold promise as a conduit for augmenting respiratory muscle strength, thereby fostering enhanced breathing patterns and overall pulmonary function [37,38,44]. The study’s findings reveal a range of exercises that facilitate dyspnea self-management: rest and catch breath (involves intentionally pausing to regain one’s breath, enhancing respiratory control); deep inspiration followed by prolonged expiration (this technique entails taking a deep breath followed by a deliberate and extended exhalation); pursed-lips breathing (with this exercise, individuals inhale through the nose and exhale through pursed lips); diaphragmatic breathing (characterized by the engagement of the diaphragm during inhalation). These exercises offer a means for patients to actively manage their dyspnea, potentially leading to improved symptom control and overall wellbeing.
3.2.2.3. Moisturize the mouth
The significance of mouth hydration practices, frequently underestimated but intrinsically linked to optimal respiratory wellbeing, is a fundamental facet of dyspnea self-management [44].
3.2.2.4. Body awareness
Moreover, cultivating bodily awareness through techniques like mindfulness and conscious breathing gave individuals the tools to manage their dyspnoea experience competently [44]. Self-monitoring of symptoms can be employed as a strategy to enhance bodily awareness [37].
3.2.2.5. Emotional intelligence
Building upon the foundation of self-efficacy, Benzo et al. [45] delved into the association between emotional intelligence and significant outcomes in COPD, including quality of life and self-management capacity. Emotional intelligence emerged as a trainable competence capable of addressing the emotional dimensions that intertwine with chronic disease management. The study unveiled a robust, independent link between emotional intelligence and self-efficacy and various quality-of-life domains. The incorporation of emotional intelligence, synergistically aligned with cognitive-behavioral interventions, accentuates the importance of addressing the psychological dimensions that intertwine with the dyspnea encounter [45,50].
3.2.2.6. Web applications
Noteworthy technological advancements have engendered web applications that present a novel avenue for engagement and support, facilitating real-time monitoring, personalized interventions, and access to informational reservoirs [[51], [52], [53]].
Emphasizing the significance of early detection and prompt intervention, Alharbey et al. [52] explored the potential of mobile health (mHealth) applications in mitigating exacerbations in COPD. Utilizing mHealth, these applications enable ongoing monitoring and higher awareness levels, alerting patients and healthcare professionals to potential exacerbations and facilitating immediate self-management and intervention.
In the realm of innovative applications, Hermosa et al. [51] introduced Prevexair, a smartphone application designed to promote daily symptom tracking as an intervention for exacerbation detection in COPD. Users track data and receive messages about healthy lifestyle behaviors, disease understanding, medication adherence, and symptom monitoring, which are aggregated into graphical representations. The study confirmed the feasibility and acceptability of daily app engagement among motivated COPD patients.
Reilly et al. [53] delved into the accessibility and motivation of individuals with chronic dyspnea using a web-based self-management intervention named Self-Breathe. This platform, offering a repository of knowledge about dyspnea and non-pharmacological interventions, was positively received by participants as a novel concept fulfilling an unmet need, showcasing the potential of digital interventions in self-management.
According to some studies [41,42,54], nurses are crucial in supporting the self-management process for chronic dyspnea in complex, chronically ill patients. They contribute significantly by enabling interventions through their capacity to promote knowledge acquisition, mastery of essential skills, medication adherence, facilitation of respiratory exercises, monitoring of dyspnea, and fostering body awareness [25] (Appendix A).
These areas of intervention are readily identifiable within the framework outlined by the International Council of Nurses [25]. Nurses play a pivotal role in promoting self-management of chronic dyspnoea by addressing interventions that empower patients with knowledge, skills, and emotional support, ultimately improving their ability to self-manage chronic dyspnoea and enhance their overall wellbeing.
3.3. Dyspnea self-management outcomes
In the short term, proficient dyspnea self-management yields multifaceted benefits (Appendix A). Individuals quickly improve their ability to recognize symptoms and accurately interpret changes in breathing, enabling timely interventions. This process expands patients’ understanding of respiratory conditions and cultivates informed decision-making [18,37,[39], [40], [41], [42],[47], [48], [49]].
Positive health behavioral changes occurring at various levels enrich the landscape of dyspnea self-management. These include maintaining medication adherence [43,44,46,47], actively seeking health information, pursuing smoking cessation, embracing a healthy lifestyle, managing emotions, creating care plans, incorporating breathing exercises, conserving energy [18,37,[39], [40], [41], [42],45,[47], [48], [49], [50]], avoiding oral dehydration [44], recognizing and monitoring dyspnea, measuring SPO2, and engaging with digital health platforms [[51], [52], [53],55].
In the short term, dyspnea self-management success relies on swift improvements in self-efficacy [38,39,41,48,52], motivation [18,37,[39], [40], [41], [42],[47], [48], [49]], reduced stress perception [39,48], increased body awareness [37,44], and enhanced emotional intelligence [45,50]. These immediate enhancements lay the groundwork for a resilient and empowered approach to dyspnea self-management.
In the long term, sustained dyspnea self-management exhibits profound implications across various dimensions, influencing health status outcomes. At the individual level, the enduring impact is reflected in improved quality of life [38,41,42,45,47,50,51] and optimized functional status [18,43,53,55]. Families also experience positive outcomes, witnessing an elevated quality of life due to the individual’s commitment to dyspnea self-management. Furthermore, the effects may extend to healthcare utilization, with a reduction in unscheduled physician visits and hospitalizations [18,37,[39], [40], [41], [42],[47], [48], [49]].
4. Discussion
The evidence in chronic dyspnea self-management is limited, marked by significant heterogeneity in the identified interventions. While this presents challenges in developing precise clinical guidelines, it underscores the intricacy of the dyspnea phenomenon. It illuminates the primary components of an intervention program aimed at enhancing dyspnea self-management, particularly emphasizing the nurse’s intervention.
The pharmacological and non-pharmacological interventions exhibited favorable results in chronic dyspnea self-management. Symptom management is a key outcome of self and family management in complex chronic conditions, as described by Grey et al. [22]. According to their framework, effective symptom management hinges on personal and familial factors such as knowledge about the condition, beliefs, psychological wellbeing, motivation, and lifestyle patterns. Individual health status, encompassing comorbidities, illness severity, symptoms, treatment side effects, and cognitive function, also plays a significant role in chronic condition management. Moreover, available resources (financial, equipment, and community), environmental factors (including home, work, and community environments), as well as the healthcare system’s accessibility, the ability to navigate it, and relationships with healthcare providers all exert substantial influence on patients’ and families’ capacity to manage chronic conditions. In this sense, the interventions identified in our review for chronic dyspnea self-management should be understood from an integrative perspective, considering the individual as a unique whole with their specificities, idiosyncrasies, and circumstances integrated within their family, community, and healthcare system. Every pharmacological and non-pharmacological intervention should be applied while considering barriers and facilitators to its implementation, considering the patient’s background [22].
Pharmacological interventions, such as oxygen therapy and inhalation-based treatments, have shown efficacy in mitigating dyspnea symptoms and enhancing problem-solving and decision-making skills for medical treatment management [43,44,46]. Short-term supplemental oxygen therapy has been found to improve exercise performance and reduce physiological impacts during physical exertion [44]. However, additional oxygen for managing dyspnea in patients without hypoxemia has not been definitively established. Patients need to be aware of the potential adverse effects of oxygen supplementation. It might be more effective and cost-efficient to focus on symptom control through medications, exercise, behavioral therapy, anxiety management, and the use of fans [56].
Inhalers can be utilized as adjuvant therapy to opioids to limit opioid use and augment responses to dyspnea [57]. Bronchodilators, such as short- and long-acting beta-2 agonists, anticholinergics, and inhaled corticosteroids, should be considered for treating mild to moderate dyspnea. Notably, a lack of knowledge of proper inhaler use can hinder dyspnea relief [58].
Informed management of pharmacological interventions is critical to avoid unwarranted complications and challenges. Therefore, interventions aimed at supporting patients with information and skills to actively engage in their healthcare, such as effective communication with healthcare providers, identifying relevant information, symptom management, adopting health behaviors, and adhering to treatment plans, are increasingly acknowledged as indispensable components of chronic condition management [22].
Patient education, employing cognitive and/or behavioral strategies, has been a foundational approach in previous studies for supporting the self-management of chronic illnesses. These approaches, delivered mainly by nurses, include enhancing patients’ understanding of their condition and treatment, enabling independent symptom monitoring, promoting personalized action plans for addressing worsening symptoms or exacerbations, providing psychological coping and stress management techniques, and reinforcing accountability for medication adherence and lifestyle choices [59].
The issue of information is also relevant in the field of non-pharmacological interventions. Patients need adequate education and support to effectively self-manage their symptoms [60,61]. This involves providing information on the nature of dyspnea, its causes, and interventions for managing and preventing exacerbations. To facilitate self-management, healthcare providers should also ensure patients can access appropriate resources and tools, such as educational materials, self-monitoring devices, and action plans [60,61].
The findings of our review highlight the self-management process from an empowerment and self-efficacy perspective [40,41,43,50,51]. Through dyspnea self-management, patients are encouraged to take an active role in recognizing and addressing their breathlessness rather than solely relying on healthcare providers for interventions. By actively managing their symptoms, individuals develop a sense of control and ownership over their health. This can lead to improved self-confidence and a better ability to cope with dyspnea. Focused on dyspnea, it can improve symptom control, decrease healthcare utilization, and enhance patient satisfaction. Those results are consistent with previous research that stresses the importance of patient activation, health literacy, and self-efficacy as self-management components [62].
The present review examined multiple articles proving the positive effect of pulmonary rehabilitation educational programs on dyspnea control [37,38,47]. Those results align with recent investigations, like the work of Smith et al. [63], that conducted a systematic review to assess pulmonary rehabilitation educational programs. The findings suggested that pulmonary rehabilitation is an intervention that significantly enhances the health of individuals with chronic respiratory diseases. The authors identified that educational sessions generally encompass lung function, pathophysiological changes associated with specific respiratory diseases, medication use, psychological support, interventions for dyspnea control, the role of rehabilitation exercises, and appropriate approaches to acute exacerbations. The studies demonstrated positive effects on self-management of chronic diseases, quality of life, and people’s knowledge.
Also, Schultz et al. [64] concluded that pulmonary rehabilitation in individuals diagnosed with asthma had positive changes in asthma symptom control, such as quality of life improvement, increased distance in the 6-min walk test, enhanced lung function parameters, reduced dyspnea, anxiety, and depression, smoking cessation, disease self-management skills, and medication adherence.
The management of dyspnea, stemming from a program that integrates different focus areas, emphasizes the importance of not viewing dyspnea as an isolated phenomenon. Various possible causes and associated behaviors and comorbidities need to be considered comprehensively. These results reinforce the Grey et al. [22] framework, which states that it is necessary to focus on illness needs for any self-management situation. This involves learning and acquiring knowledge about chronic dyspnea, understanding its causes, treatment options, and self-care strategies to empower better management. Patients and families should actively participate in prescribed treatments, medications, and lifestyle changes healthcare providers recommend. Also, adopting a healthy lifestyle, managing comorbid conditions, and activating available resources when needed are mandatory actions to improve overall wellbeing and control dyspnea. Smoking history, including secondhand exposure, increases the risk of developing dyspnoea [15,65]. Consequently, managing dyspnea may need behavioral changes, such as smoking cessation.
Our findings underscore using breathing exercises as a strategy for self-managing chronic dyspnea. This result aligns with existing research, where breathing exercises have shown promise in enhancing dyspnea management and improving health-related quality of life [66,67].
Like any other chronic medical condition, managing chronic dyspnea demands adaptation, emotional processing, adjustment, seamless integration into daily life, and the quest for meaning [22]. Breathlessness has a pervasive effect on patients and their careers. The extent of respiratory distress is shaped by the interplay among the patient’s coping approach, their propensity to seek assistance, and their clinician’s receptiveness to addressing breathlessness, all while managing the underlying medical condition [14]. Our results show that emotional intelligence is associated with self-management abilities, which means that is crucial to address not only the physical aspects of chronic illness but also paying attention to emotional wellbeing [45]. Certain factors like age, being male, having higher education, experiencing less stress, and having fewer depressive and anxiety symptoms are associated with better emotional coping. For people with chronic illnesses, it is essential to note that managing emotions might be linked to chronic disease and worse physical function [68].
Sharing peer experiences can facilitate patient learning and support individual self-management. By openly discussing and exchanging these experiences, patients can gain valuable insights into what strategies, coping mechanisms, or lifestyle changes have proven effective in their unique journey of self-managing their health conditions, including chronic dyspnea [69].
The sensation of dyspnea, or shortness of breath, can catalyze heightened body awareness. Our findings underscore that this increased awareness of one’s bodily sensations can play a constructive role in managing chronic dyspnea. In essence, when individuals become more attuned to the signals their bodies send during episodes of dyspnea, they are better equipped to respond effectively to manage and alleviate the symptoms associated with this chronic condition. Previous study has concluded the existence of interferences between dyspnea and cognitive functions, suggesting that respiratory-visual stimulation should be tested as a non-pharmacological approach to dyspnea treatment [70].
Enhancing body awareness is pivotal in fostering an independent ability to monitor symptoms such as dyspnea. This heightened awareness allows individuals to recognize early signs of breathing difficulties, enabling them to take proactive measures to address the symptoms before they escalate. Moreover, it empowers individuals to use self-management techniques, like controlled breathing exercises, to mitigate symptoms independently, reducing their reliance on external assistance.
This review, in line with the most recent research [71,72], indicates that mobile applications offer substantial clinical benefits to patients experiencing breathlessness and can serve as valuable tools to support clinical practice. Mobile apps have become increasingly sophisticated, offering features such as real-time symptom tracking, medication reminders, and personalized breathing exercises. These apps empower patients to take an active role in their self-management by providing a user-friendly interface and access to valuable health information.
This integrative review employs a rigorous methodology to synthesize knowledge on chronic dyspnea self-management. The thoughtful integration of qualitative and quantitative data enhances our understanding of the phenomenon, providing comprehensive insights into self-management strategies. Despite these strengths, the prevalence of moderate-quality evidence and challenges related to blinding procedures, confounding factors, and exposure measurement validity underscore the necessity for a nuanced interpretation of the study’s findings. This limitation highlights the importance of further research to establish effective interventions.
5. Conclusion
This integrative literature review emphasizes the need for thorough self-management interventions addressing chronic dyspnea in patients with complex conditions. It highlights the combined use of pharmacological and non-pharmacological methods, revealing significant gaps in research and application. The study underscores the essential role of nurses in patient education and care coordination, emphasizing a holistic approach aimed at enhancing patient care standards and more effective practices to improve patient quality of life.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Helga Rafael Henriques: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration. Andreia Correia: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Tatiana Santos: Conceptualization, Methodology, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. José Faria: Methodology, Validation, Formal analysis, Writing - review & editing. Diana Sousa: Methodology, Validation, Formal analysis, Writing - review & editing. Joana Portela: Methodology, Validation, Formal analysis, Writing - review & editing. Joana Teixeira: Methodology, Validation, Formal analysis, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
The authors would like to thank the Documentation Center of ESEL for her library support in locating articles.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2024.03.008.
Appendices. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization . 2023. World health statistics 2023: monitoring health for the sustainable development Goals.https://www.who.int/publications/i/item/9789240074323 [Google Scholar]
- 2.World Health Organization . 2022. Aging and health.https://www.who.int/news-room/fact-sheets/detail/ageing-and-health [Google Scholar]
- 3.Wahls S.A. Causes and evaluation of chronic dyspnea. Am Fam Physician. 2012;86:173–182. https://www.aafp.org/pubs/afp/issues/2012/0715/p173.html [PubMed] [Google Scholar]
- 4.Sevick M.A., Trauth J.M., Ling B.S., Anderson R.T., Piatt G.A., Kilbourne A.M., et al. Patients with complex chronic diseases: Perspectives on supporting self-management. J Gen Intern Med. 2007;22:438–444. doi: 10.1007/s11606-007-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinaz Romana G., Kislaya I., Salvador M.R., Gonçalves S.C., Nunes B., Dias C. Multimorbidity in Portugal: results from the first National health Examination Survey. Acta Med Port. 2019;32:30–37. doi: 10.20344/amp.11227. [in Portuguese] [DOI] [PubMed] [Google Scholar]
- 6.Gobeil-Lavoie A.-P., Chouinard M.-C., Danish A., Hudon C. Characteristics of self-management among patients with complex health needs: a thematic analysis review. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-028344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolaus S., Crelier B., Donzé J.D., Aubert C.E. Definition of patient complexity in adults: a narrative review. J Comorb Multimorb. 2022;25(12) doi: 10.1177/26335565221081288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning E., Gagnon M. The complex patient: a concept clarification. Nurs Health Sci. 2017;19:13–21. doi: 10.1111/nhs.12320. [DOI] [PubMed] [Google Scholar]
- 9.Clua-Espuny J.L., González-Henares M.A., Queralt-Tomas M.L.L., Gil-Guillen V.F. Polypharmacy and mortality among chronic complex outpatients: results of community-based prospective study. International Journal of Medical Research and Pharmaceutical Sciences. 2016;3(11):59–74. doi: 10.5281/zenodo.168345. [DOI] [Google Scholar]
- 10.Peirce S.C., Hardisty A.R., Preece A.D., Elwyn G. Designing and implementing telemonitoring for early detection of deterioration in chronic disease: Defining the requirements. Health Inf J. 2011;17:173–190. doi: 10.1177/1460458211409717. [DOI] [PubMed] [Google Scholar]
- 11.European Commission . 2020. Strengthening NHS reform by empowering health professionals in Portugal. Patient Complexity Index. Report on methodological properties, statistical robustness and deployment simulation results.https://www.acss.min-saude.pt/wp-content/uploads/2022/01/EY-SRSS-Primary-Health-relatorio-analise-ICU-final.pdf [Google Scholar]
- 12.Kong C.W., Wilkinson T.M.A. Predicting and preventing hospital readmission for exacerbations of COPD. ERJ Open Res. 2020;6(2):325–2019. doi: 10.1183/23120541.00325-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parshall M.B., Schwartzstein R.M., Adams L., Banzett R.B., Manning H.L., Bourbeau J., et al. An Official American Thoracic Society Statement: Update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson A., Barclay-Klingle N., Galvin K., Johnson M.J. Living with breathlessness: a systematic literature review and qualitative synthesis. Eur Respir J. 2018;51(2) doi: 10.1183/13993003.01477-2017. [DOI] [PubMed] [Google Scholar]
- 15.Berliner D., Schneider N., Welte T., Bauersachs J. The differential Diagnosis of dyspnea. Dtsch Arztebl Int. 2016;113(49):834–845. doi: 10.3238/arztebl.2016.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayen A., Herigstad M., Pattinson K.T.S. Understanding dyspnea as a complex individual experience. Maturitas. 2013;76(1):45–50. doi: 10.1016/j.maturitas.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Kamal A.H., Maguire J.M., Wheeler J.L., Currow D.C., Abernethy A.P. Dyspnea review for the palliative care professional: assessment, burdens, and etiologies. J Palliat Med. 2011;14(10):1167–1172. doi: 10.1089/jpm.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Çevirme A., Gökçay G. The impact of an Education-Based Intervention Program (EBIP) on dyspnea and chronic self-care management among chronic obstructive pulmonary disease patients. Saudi Med J. 2020;41(12):1350–1358. doi: 10.15537/smj.2020.12.25570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bausewein C., Booth S., Gysels M., Higginson I.J. Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev. 2008;16(2) doi: 10.1002/14651858.CD005623.pub3. [DOI] [PubMed] [Google Scholar]
- 20.Miner B., Tinetti M.E., Van Ness P.H., Han L., Leo-Summers L., Newman A.B., et al. Dyspnea in community-dwelling older persons: a multifactorial geriatric health condition. J Am Geriatr Soc. 2016;64:2042–2050. doi: 10.1111/jgs.14290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi D.K., Rhodes J., Ferris R., Banka R., Clarke A., Mishra E.K. vol. 15. 2023. (Breathlessness predicts mortality in adults: a systematic review and meta-analysis). 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grey M., Schulman-Green D., Knafl K., Reynolds N.R. A revised self- and family management framework. Nurs Outlook. 2015;63:162–170. doi: 10.1016/j.outlook.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Alharbi E.R., Wazqar D.Y., Sofar S.M. A quasi-experimental study of the effect of a comprehensive blended health educational program on self-management practices among patients with chronic obstructive pulmonary disease. Heart Lung. 2022;56:133–141. doi: 10.1016/j.hrtlng.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Sebastião B.F., Hortelão R.M., Granadas S.S., Faria J.M., Pinto J.R., Henriques H.R. Air quality self-management in asthmatic patients with COPD: an integrative review for developing nursing interventions to prevent exacerbations. Int J Nurs Sci. 2024;11(1):46–56. doi: 10.1016/j.ijnss.2023.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.International Council of Nurses . 2019. International classification for nursing practice (ICNP Browser®)https://www.icn.ch/icnp-browser [Google Scholar]
- 26.Orem D.E. sixth ed. 2001. Nursing: concepts of practice. [Google Scholar]
- 27.Lorig K.R., Holman H.R. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 28.Michie S., Richardson M., Johnston M., Abraham C., Francis J., Hardeman W., et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 29.Smith S.M., Wallace E., O'Dowd T., Fortin M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2016;3(3):CD006560. doi: 10.1002/14651858.CD006560.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäger M., Zangger G., Bricca A., Dideriksen M., Smith S.M., Midtgaard J., et al. Mapping interventional components and behavior change techniques used to promote self-management in people with multimorbidity: a scoping review. Health Psychol Rev. 2024;18(1):165–188. doi: 10.1080/17437199.2023.2182813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwerink M., Brusse-Keizer M., van der Valk P.D., Zielhuis G.A., Monninkhof E.M., van der Palen J., et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;3:CD002990. doi: 10.1002/14651858.CD002990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steindal S.A., Torheim H., Oksholm T., Christensen V.L., Lee K., Lerdal A., et al. Effectiveness of nursing interventions for breathlessness in people with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Adv Nurs. 2019;75:927–945. doi: 10.1111/jan.13902. [DOI] [PubMed] [Google Scholar]
- 33.Yorke J., Johnson M.J., Punnett G., Smith J., Blackhall F., Lloyd Williams M., et al. Respiratory distress symptom intervention for non-pharmacological management of the lung cancer breathlessness–cough–fatigue symptom cluster: randomised controlled trial. BMJ Support Palliat Care. 2022;13(e3):e1181–e1190. doi: 10.1136/spcare-2022-003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whittemore R., Knafl K. The integrative review: updated methodology. J Adv Nurs. 2005;52:546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- 35.Toronto C.E., Remington R., editors. A step-by-step guide to conducting an integrative review. Springer; 2020. [DOI] [Google Scholar]
- 36.Aromataris E., Munn Z., editors. JBI manual for evidence synthesis. JBI; 2020. https://jbi-global-wiki.refined.site/space/MANUAL [Google Scholar]
- 37.Davis A.H.T., Carrieri-Kohlman V., Janson S.L., Gold W.M., Stulbarg M.S. Effects of treatment on two types of self-efficacy in people with chronic obstructive pulmonary disease. J Pain Symptom Manag. 2006;32:60–70. doi: 10.1016/j.jpainsymman.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Mark D.D., Ikehara C., Matsuura C., Hara K., Li D. Validating the impact of teaching pursed-lips breathing with skype. J Hospice Palliat Nurs. 2013;15:424–432. doi: 10.1097/NJH.0000000000000015. [DOI] [Google Scholar]
- 39.Lorig K.R., Ritter P.L., Laurent D.D., Plant K. Internet-based chronic disease self-management. Med Care. 2006;44:964–971. doi: 10.1097/01.mlr.0000233678.80203.c1. [DOI] [PubMed] [Google Scholar]
- 40.Lorig K.R., Sobel D.S., Stewart A.L., Brown B.W., Bandura A., Ritter P., et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization. Med Care. 1999;37:5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Lavery K.A., O'Neill B., Parker M., Elborn J.S., Bradley J.M. Expert patient self-management program versus usual care in bronchiectasis: a randomized controlled trial. Arch Phys Med Rehabil. 2011;92:1194–1201. doi: 10.1016/j.apmr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 42.Cameron-Tucker H., Wood-Baker R., Owen C., Joseph L., Walters E. Chronic disease self-management and exercise in COPD as pulmonary rehabilitation: a randomized controlled trial. Int J Chronic Obstr Pulm Dis. 2014:513. doi: 10.2147/COPD.S58478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J.W., Park E.Y. Self-management of oxygen and bronchodilators to relieve the dyspnoea of lung cancer with pneumoconiosis. Int J Palliat Nurs. 2020;26:167–174. doi: 10.12968/ijpn.2020.26.4.167. [DOI] [PubMed] [Google Scholar]
- 44.Stenekes S.J., Hughes A., Grégoire M.-C., Frager G., Robinson W.M., McGrath P.J. Frequency and self-Management of pain, dyspnea, and cough in cystic fibrosis. J Pain Symptom Manag. 2009;38:837–848. doi: 10.1016/j.jpainsymman.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 45.Benzo R.P., Kirsch J.L., Dulohery M.M., Abascal-Bolado B. Emotional Intelligence: a novel outcome associated with wellbeing and self-management in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13:10–16. doi: 10.1513/AnnalsATS.201508-490OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J.Y.T., Tikellis G., Khor Y.H., Holland A.E. Developing a self-management package for pulmonary fibrosis: an international Delphi study. ERJ Open Res. 2022;8:349–2022. doi: 10.1183/23120541.00349-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blánquez Moreno C., Colungo Francia C., Alvira Balada M.C., Kostov B., González-de Paz L., Sisó-Almirall A. Effectiveness of an educational program for respiratory rehabilitation of chronic obstructive pulmonary disease patients in primary care in improving the quality of life, symptoms, and clinical risk. Atención Primaria. 2018;50:539–546. doi: 10.1016/j.aprim.2017.03.019. [in Spanish] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lorig K.R., Ritter P.L., Dost A., Plant K., Laurent D.D., Mcneil I. The expert patients programme online, a 1-year study of an Internet-based self-management programme for people with long-term conditions. Chron Illness. 2008;4:247–256. doi: 10.1177/1742395308098886. [DOI] [PubMed] [Google Scholar]
- 49.Lorig K.R., Sobel D.S., Ritter P.L., Laurent D., Hobbs M. Effect of a self-management program on patients with chronic disease. Effect Clin Pract. 2001;4:256–262. [PubMed] [Google Scholar]
- 50.Lee J.Y.T., Tikellis G., Khor Y.H., Holland A.E. Developing a self-management package for pulmonary fibrosis: an international Delphi study. ERJ Open Res. 2022 Dec 27;8(4):349–2022. doi: 10.1183/23120541.00349-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hermosa J.L.R., Fuster Gomila A., Puente Maestu L., Amado Diago C.A., Callejas González F.J., Malo De Molina Ruiz R., et al. Compliance and utility of a smartphone app for the detection of exacerbations in patients with chronic obstructive pulmonary disease: cohort Study. JMIR Mhealth Uhealth. 2020;8 doi: 10.2196/15699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alharbey R., Chatterjee S. An mHealth Assistive System “MyLung” to Empower Patients with chronic obstructive pulmonary disease: design science research. JMIR Form Res. 2019;3 doi: 10.2196/12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reilly C.C., Bristowe K., Roach A., Maddocks M., Higginson I.J. “You can do it yourself and you can do it at your convenience”: internet accessibility and willingness of people with chronic breathlessness to use an internet-based breathlessness self-management intervention during the COVID-19 pandemic. ERJ Open Res. 2022;8:557–2021. doi: 10.1183/23120541.00557-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Y., Bhattarai M., Kuo W.C., Bratzke L.C. Relationship between resilience and self-care in people with chronic conditions: a systematic review and meta-analysis. J Clin Nurs. 2023;32(9–10):2041–2055. doi: 10.1111/jocn.16258. [DOI] [PubMed] [Google Scholar]
- 55.Dansky K., Vasey J. Managing heart failure patients after formal homecare. Telemedicine and E-Health. 2009;15:983–991. doi: 10.1089/tmj.2009.0064. [DOI] [PubMed] [Google Scholar]
- 56.Baldwin J., Cox J. Treating dyspnea. Med Clin. 2016;100:1123–1130. doi: 10.1016/j.mcna.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Senderovich H., Yendamuri A. Management of breathlessness in palliative care: inhalers and dyspnea—a literature review. Rambam Maimonides Med J. 2019;10 doi: 10.5041/RMMJ.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Severino R. Breathlessness management in palliative care: pharmacological and non-pharmacological interventions: integrative review of literature. Revista investigação em enfermagem. 2020;9–23 https://www.sinaisvitais.pt/images/stories/Rie/RIE31_s2.pdf [in Portuguese] [Google Scholar]
- 59.Dineen-Griffin S., Garcia-Cardenas V., Williams K., Benrimoj S.I. Helping patients help themselves: a systematic review of self-management support strategies in primary health care practice. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Effing T., Monninkhof E., Valk P.V.D., Palen J.V.D., Herwaarden C.V., Partidge M., et al. Self-management education for patients with chronic obstructive pulmonary disease. Cochrane Database System. Rev. 2007;4 doi: 10.1002/14651858.CD002990.pub2. [DOI] [PubMed] [Google Scholar]
- 61.Jonkman N.H., Westland H., Trappenburg J.C.A., Groenwold R.H.H., Bischoff E.W.M.A., Bourbeau J., et al. Characteristics of effective self-management interventions in patients with COPD: individual patient data meta-analysis. Eur Respir J. 2016;48:55–68. doi: 10.1183/13993003.01860-2015. [DOI] [PubMed] [Google Scholar]
- 62.Yadav U.N., Lloyd J., Hosseinzadeh H., Baral K.P., Harris M.F. Do chronic obstructive pulmonary diseases (COPD) self-management interventions consider health literacy and patient activation? A systematic review. J Clin Med. 2020;9:646. doi: 10.3390/jcm9030646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith R., Osadnik C.R., Lee A.L. Educational topics and their rationale for inclusion within pulmonary rehabilitation – a systematic review. Patient Educ Counsel. 2020;103:1997–2008. doi: 10.1016/j.pec.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Schultz K., Seidl H., Jelusic D., Wagner R., Wittmann M., Faller H., et al. Effectiveness of pulmonary rehabilitation for patients with asthma: study protocol of a randomized controlled trial (EPRA) BMC Pulm Med. 2017;17:49. doi: 10.1186/s12890-017-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aktan R., Ozalevli S., Ozakbas S. Effects of cigarette smoking on respiratory problems and functional levels in multiple sclerosis patients. Mult Scler Relat Disord. 2018;25:271–275. doi: 10.5281/zenodo.168345. [DOI] [PubMed] [Google Scholar]
- 66.Hanada M., Kasawara K.T., Mathur S., Rozenberg D., Kozu R., Hassan S.A., et al. Aerobic and breathing exercises improve dyspnea, exercise capacity and quality of life in idiopathic pulmonary fibrosis patients: systematic review and meta-analysis. J Thorac Dis. 2020;12:1041–1055. doi: 10.21037/jtd.2019.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ubolnuar N., Tantisuwat A., Thaveeratitham P., Lertmaharit S., Kruapanich C., Mathiyakom W. Effects of breathing exercises in patients with chronic obstructive pulmonary disease: systematic review and meta-analysis. Ann Rehabil Med. 2019;43:509–523. doi: 10.5535/arm.2019.43.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wierenga K.L., Lehto R.H., Given B. Emotion regulation in chronic disease populations: an integrative review. Res Theor Nurs Pract. 2017;31:247–271. doi: 10.1891/1541-6577.31.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindblad A., Hedberg B., Nygårdh A., Petersson C. “An expanded window of understanding a changed everyday life”—experiences from patients with long-term conditions after attending group learning sessions. J Patient Exp. 2020;7:1022–1028. doi: 10.1177/2374373520937167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allard E., Canzoneri E., Adler D., Morélot-Panzini C., Bello-Ruiz J., Herbelin B., et al. Interferences between breathing, experimental dyspnoea and bodily self-consciousness. Sci Rep. 2017;7:9990. doi: 10.1038/s41598-017-11045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sunjaya A.P., Sengupta A., Martin A., Di Tanna G.L., Jenkins C. Efficacy of self-management mobile applications for patients with breathlessness: systematic review and quality assessment of publicly available applications. Respir Med. 2022;201 doi: 10.1016/j.rmed.2022.106947. [DOI] [PubMed] [Google Scholar]
- 72.Guerreiro M.P., Angelini L., Henriques H.R., El Kamali M., Baixinho C., Balsa J., et al. Conversational agents for health and wellbeing across the life course: protocol for an evidence map. JMIR Res Protoc. 2021;10(9) doi: 10.2196/26680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.