Abstract
Exercise has many beneficial effects that provide health and metabolic benefits. Signaling molecules are released from organs and tissues in response to exercise stimuli and are widely termed exerkines, which exert influence on a multitude of intricate multi-tissue processes, such as muscle, adipose tissue, pancreas, liver, cardiovascular tissue, kidney, and bone. For the metabolic effect, exerkines regulate the metabolic homeostasis of organisms by increasing glucose uptake and improving fat synthesis. For the anti-inflammatory effect, exerkines positively influence various chronic inflammation-related diseases, such as type 2 diabetes and atherosclerosis. This review highlights the prospective contribution of exerkines in regulating metabolism, augmenting the anti-inflammatory effects, and providing additional advantages associated with exercise. Moreover, a comprehensive overview and analysis of recent advancements are provided in this review, in addition to predicting future applications used as a potential biomarker or therapeutic target to benefit patients with chronic diseases.
Keywords: Exerkines, Exercise factors, Metabolism, Inflammation, Chronic diseases
Introduction
Exercise plays an important role in promoting metabolism, maintaining health, eliminating inflammation, and preventing many diseases, spanning chronic metabolic disorders, cardiovascular diseases, neurological conditions, and cancers. The promising effects of exercise on metabolism have been observed due to its well-known impact on skeletal muscle and the metabolic adaptations in a diverse array of other tissues. However, for a long period, people’s understanding of the benefits of exercise was attributed to skeletal muscle contractions and energy expenditure. Thus, the molecular mechanisms underlying the comprehensive regulatory effects of exercise on various tissues and organs in the body are still unclear.
Skeletal muscle plays a crucial role during exercise, not only as a motor organ but also in posture control, movement, and energy regulation. Furthermore, skeletal muscle is also considered the largest endocrine organ, since the exercise factors released during muscle contraction play a crucial regulatory role in enhancing physical and mental health (Thyfault & Bergouignan, 2020). Since the last century, researchers have been looking for a link between muscle contractions and systemic improvements that mediate metabolic changes in other organs, such as the liver and adipose tissue, in the form of “exercise factors” (Pedersen et al., 2007). For example, amounts of interleukin-6 (IL-6) were released into the circulation from exercising leg, as evidenced by experiments involving single-legged exercise. Since then, the tally of identified exercise-linked signaling molecules has considerably expanded (Steensberg et al., 2000). The “exercise factors”, indicating the substances released from skeletal muscle and other tissues when doing training, are known as “exerkines”, which are concerned with regulating a variety of systemic adaptive processes during exercise (Safdar, Saleem & Tarnopolsky, 2016). The current interpretation of the term exerkine is defined as a signaling molecule that releases in response to acute and/or chronic exercise stimuli and exerts its effects through endocrine, paracrine, and/or autocrine pathways (Chow et al., 2022; Robbins & Gerszten, 2023).
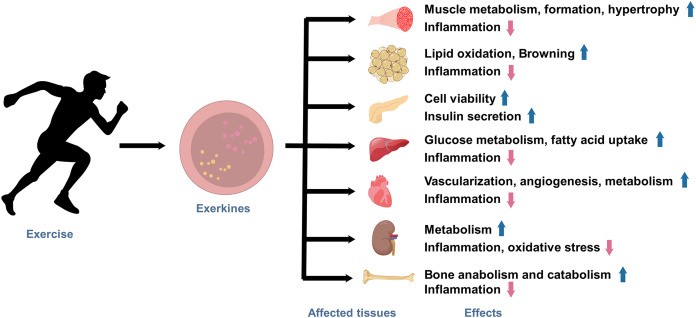
Under the state of active exercise, various tissues within the body secrete a diverse array of exerkines. These exercise factors encompass a broad range of biologically functional molecules, including peptides, acids, lipids, metabolites, and more. Exerkines are synthesized, secreted by multiple tissues and organs, and released into the circulation in response to exercise stimuli, thus regulating various physiological and pathological processes in human body, ultimately affecting metabolism and promoting physical health (Fig. 1). The studies on exerkines have extended into exercise humoral factors, including the myokines by skeletal muscle (Laurens, Bergouignan & Moro, 2020), hepatokines by the liver (Ingerslev et al., 2017), adipokines by white adipose tissue (WAT), and batokines by brown adipose tissue (BAT) (Lafontan et al., 2000). More than hundreds of exerkines have been reported (Thyfault & Bergouignan, 2020), and some of them are well-recognized, like apelin, adiponectin, myostatin (MSTN), IL-6, interleukin-15 (IL-15), irisin, fibroblast growth factor 21 (FGF-21), secreted protein acidic and rich in cysteine (SPARC), brain-derived neurotrophic factor (BDNF), leptin, and so on.
Figure 1. The effects of exercise-induced exerkines on tissues and organs.
Arrows indicate: ↑, possible promoting effect; ↓, possible inhibiting effect.
In recent years, growing evidence indicates that aerobic exercise plays a pivotal role as an effective therapy for the anti-inflammatory strategy (Papagianni et al., 2023). These studies highlight the profound impact of exercise on modulating the body’s inflammatory processes, further underscoring the importance of physical activity in promoting overall health and well-being (Joisten et al., 2019; Lavin et al., 2020). However, the molecular mechanisms by which exerkines promote metabolic and anti-inflammatory effects in the body are still unexplored.
This comprehensive review aims to explore the role of exerkines in the regulation of metabolism and inflammation. We aim to clarify the molecular mechanisms behind the multifaceted effects of exercise-induced factors on the human body. Furthermore, this review focuses on exerkines, their regulation and potential roles in the metabolic homeostasis of organisms and inflammation.
Survey methodology
We used the keywords as follows: exerkines, exercise factors, metabolism, inflammation, chronic diseases, metabolic diseases, apelin, adiponectin, myostatin, interleukin-6, interleukin-15, irisin, fibroblast growth factor 21, secreted protein acidic and rich in cysteine, brain-derived neurotrophic factor, and leptin to conduct a literature search of the PubMed database. The articles that were not associated with exerkines, metabolism, or inflammation were excluded.
Exercise and exerkines
Exercise is a non-pharmacological intervention that exerts profound effects on the release of exerkines. Exerkine secretion is intricately modulated by the intensity, type, and duration of exercise activity, resulting in a complex interplay of physiological responses. Moderate-intensity training is an effective exercise for increasing apelin levels, which can stimulate protein synthesis (Besse-Patin et al., 2014; Vinel et al., 2018). Moderate-intensity aerobic training can lead to a decrease in MSTN (Riahy, 2024), and 8 weeks of moderate-intensity endurance training result in increasing IL-6 and IL-15 and decreasing myogenesis (Banitalebi et al., 2019). An acute increase in circulating IL-6 is induced following high-intensity interval exercise (Cullen et al., 2016; Ostrowski, Schjerling & Pedersen, 2000). Aerobic exercise significantly reduces the inflammatory load in type 2 diabetes by improving circulating levels of factors such as resistin, TNF-α, and IL-6 (Papagianni et al., 2023). On the other hand, resistance training, characterized by high-intensity muscle contractions, suppresses the release of MSTN (Khalafi et al., 2023). Moreover, exercise-induced factors respond to endurance differently from high-intensity interval training (HIIT). For instance, FGF-21, known for its role in regulating energy homeostasis, has been found to increase following acute moderate-intensity exercise (Sargeant et al., 2018), which suggests a potential link between FGF-21 release and sustained aerobic effort.
The duration of exercise also plays a pivotal role in the secretion of exercise factors. Longer continuous durations of exercise are associated with the sustained release of specific exerkines, contributing to metabolic adaptations. On the other hand, high-intensity interval training with shorter duration had no effect on IL-6 and IL-15 secretion (He et al., 2018), whereas 6 to 12 weeks seems to be the key time when these underwent significant changes (Banitalebi et al., 2019; Bugera et al., 2018). During prolonged exercise, the secretion of IL-6 is significantly elevated, which is attributed to the repetitive muscle contractions and increased energy demands associated with continuous activities like long-distance running. IL-6, as an energy sensor and modulator of inflammation, orchestrates metabolic adaptations during extended exercise bouts (Nash et al., 2023). Short bursts of high-intensity exercise, often seen in HIIT, elicit rapid changes in the release of certain exerkines. For instance, HIIT has been linked to increasing levels of irisin and BDNF. Irisin is released in response to brief periods of intense activity and has been associated with improved glucose and fatty acid uptake (Archundia-Herrera et al., 2017). Similarly, the neurotrophic factor BDNF, which is rapidly released in response to the acute stress of high-intensity exercise, may be resistant to oxidative damage (Freitas et al., 2018).
Therefore, different intensities, modalities, and durations of exercise produce different effects and consequently different secretion factors. This underscores the complexity of the connections between exercise and physiological responses, providing valuable insights for tailoring exercise prescriptions to achieve specific metabolic goals.
Role of exercise and exerkines in metabolism
Exercise is a powerful modulator of metabolism, exerting its effects through intricate mechanisms involving both localized and systemic factors. Metabolism is a complex set of biochemical processes that regulate energy production, utilization, and storage within the body. The recognition of exerkines, bioactive molecules released by exercise stimuli, has expanded new dimensions to our understanding of how exercise acts on metabolism.
Exercise triggers a cascade of metabolic adaptations aimed at sustaining energy demands during physical activity. Muscle contraction during exercise leads to increased energy expenditure, creating a demand for substrates such as glucose and fatty acids. To meet this demand, exercise enhances the uptake and utilization of glucose in skeletal muscle tissue. This process is carried out by increased translocation of glucose transporter proteins to the cell membrane, facilitated by factors like AMP-activated protein kinase (AMPK) (Friedrichsen et al., 2013). As a signal transducer for metabolic adaptation, the AMPK pathway is activated by exercise, which in turn inhibits the biosynthetic and anabolic pathways to conserve ATP and stimulates catabolic pathways to restore cellular energy storage (Egan & Zierath, 2013; Hargreaves & Spriet, 2018). Additionally, exercise promotes mitochondrial biogenesis, boosting oxidative capacity within muscle cells (Perry & Hawley, 2018). These adaptations improve the efficiency of energy production and utilization, contributing to overall metabolic health. Exerkines also have a significant impact on mediating the metabolic effects of physical activity. IL-6, a prominent myokine, exemplifies this role. IL-6 is released during muscle contraction and acts as a signaling molecule that coordinates energy metabolism. It enhances lipolysis in adipose tissue (Frühbeck et al., 2014), leading to increased availability of fatty acids for energy production. Furthermore, IL-6 improves glucose uptake and utilization, promoting glucose homeostasis and insulin sensitivity (Ikeda et al., 2016). Meanwhile, irisin contributes to energy metabolism by influencing BAT activation. This process connects to metabolic disorders, as changes in BAT activity are associated with improved insulin sensitivity and weight management (Boström et al., 2012).
Exercise-induced metabolic adaptations impact various organs and systems. Exerkines have a potential role in coordinating a systemic cascade of effects that contribute to overall health and metabolic homeostasis. Exercise exerts regulatory effects on the endocrine system. For instance, irisin, a myokine released during physical activity, influences adipose tissue and pancreatic function. The liver, for example, responds to exercise by altering gluconeogenesis and glycogen metabolism, which may be regulated by the involvement of IL-6 released by contracting muscles (Schmidt-Arras & Rose-John, 2016). Similarly, exercise influences pancreatic function, enhancing insulin secretion and sensitivity (Tsuchiya et al., 2013). A study showed that the improvement of glucose homeostasis in patients with type 2 diabetes after chronic aerobic exercise may be more closely related to the amelioration of pancreatic β-cell function by factors secreted from contracting skeletal muscles (Solomon et al., 2013). Skeletal muscles and bones are intimately cross-linked, and exercise plays a critical role in maintaining their physiologic function. Exerkines regulate the function of osteoblasts, osteoclasts, and osteocytes, improving metabolic disorders. Irisin and β-aminoisobutyric acid are motility factors that promote bone anabolism, whereas MSTN promotes bone catabolism and affects bone remodeling by influencing osteoblast and osteoclast activity (Cariati et al., 2021). Regular physical activity, contributes to bone mineral density preservation and reduces the risk of osteoporosis by downregulating MSTN expression and enhancing osteoblast function.
As signaling molecules, exerkines are released from tissues into the circulation, followed by exercise stimuli, producing a variety of effects and regulating metabolism in multiple ways. Recognizing the prominent role of exerkines in translating exercise-induced signals into systemic metabolic adaptations opens new avenues for targeted interventions to combat metabolic disorders.
Role of exercise and exerkines in inflammation
Chronic low-grade inflammation is increasingly recognized as a pathogenic factor playing a crucial role in various metabolic disorders and chronic diseases, which is reflected by increased C-reactive protein (CRP) concentrations and systemic levels of some cytokines (Petersen & Pedersen, 2005). Studies have confirmed the association between low-grade systemic inflammation and chronic diseases such as metabolic syndrome, type 2 diabetes, and atherosclerosis (Handschin & Spiegelman, 2008; Hotamisligil, 2017). Exerkines have demonstrated profound anti-inflammatory effects across organs and systems, offering promising avenues for mitigating inflammatory-driven pathologies.
Exercise has been consistently associated with anti-inflammatory effects, as summarized in other reviews (Gleeson et al., 2011; Suzuki, 2019). Engaging in regular exercise has been shown to reduce systemic inflammation by lowering CRP and IL-6 (Wärnberg et al., 2010), which is attributed to the direct influence of exercise on immune cells, particularly monocytes and macrophages. Exercise training leads to a phenotypic shift in these cells towards an anti-inflammatory M2 phenotype (Oliveira et al., 2013), dampening the overall pro-inflammatory reaction. Exerkines regulate the inflammatory landscape of the body and also provide a direction for other therapeutic interventions. For instance, irisin possesses anti-apoptotic and anti-inflammatory effects, so that can inhibit the production of NLRP3 inflammasomes, which reduces the inflammatory response involved in pyrotic cell death in sepsis, and it resists oxidative stress (Li et al., 2021). Similarly, MSTN inhibition by exercise can contribute to the attenuation of inflammation by affecting macrophage polarization (Dong et al., 2016).
Adipose tissue is a pivotal site where inflammation and metabolism intersect; its regulation of metabolic inflammation is particularly influenced by exercise. Exerkines may alleviate the degree of inflammation in adipose tissue. SPARC is an exerkine that can induce the “browning” of WAT, converting it into metabolically active beige adipose tissue (Ghanemi, Yoshioka & St-Amand, 2023). This transformation not only enhances lipid metabolism but also reduces inflammation through mechanisms like AMPK activation and downregulation of NF-κB signaling. The cardiovascular system is profoundly impacted by inflammation, which contributes to the development of atherosclerosis and cardiovascular diseases. Exerkines exhibit a protective effect by modulating inflammation within the cardiovascular system. For instance, FGF-21 has emerged as a potent cardio-protective exerkine that improves lipid profiles, reduces oxidative stress, and exerts anti-inflammatory effects on endothelial cells (Huang, Xu & Cheung, 2017). Inflammation disrupts endocrine homeostasis and contributes to insulin resistance, a hallmark of metabolic disorders, while exerkines repair the damage to the endocrine system by regulating insulin sensitivity and glucose homeostasis. For example, exerkine apelin enhances glucose uptake in skeletal muscle cells and improves insulin sensitivity, thus counteracting inflammation-driven insulin resistance (Palmer, Irwin & O’Harte, 2022). Oxidative stress is associated with inflammation in patients with chronic kidney disease, which is associated with decreased levels of BDNF (Afsar & Afsar, 2022). BDNF releases from muscle contraction, reaches the brain, and mediates redox regulation, activating signaling pathways that regulate the expression of antioxidant molecules such as tropomyosin-related kinase receptor B and nuclear factor-erythroid 2-related factor 2 (Ishii & Mann, 2018; Vilela et al., 2017). BAIBA is a myogenic factor that can prevent oxidative stress-induced apoptosis of osteoblasts and reduce bone and muscle loss in the body to protect bones (Hamrick & McGee-Lawrence, 2018). The immune system’s response to exercise is modulated by exerkines, creating a unique cross-talk between physical activity and immunomodulation. IL-6, for instance, exhibits both pro-inflammatory and anti-inflammatory effects, depending on the exercise conditions. IL-6 can induce an anti-inflammatory milieu by inducing the production of anti-inflammatory cytokines, while it inhibits the production of the pro-inflammatory cytokine tumor necrosis factor-α (TNF-α) under certain conditions (Pedersen, 2007). Understanding the role of exercise and exerkines in anti-inflammatory responses has significant clinical implications. Harnessing the anti-inflammatory effects of exercise and exerkines could offer novel strategies for managing chronic inflammatory diseases such as type 2 diabetes, cardiovascular diseases, and obesity; tailored exercise regimens and exerkine-based interventions could be developed to specifically target inflammatory pathways, thereby reducing the risk of disease progression.
Features of selected exerkines
As discussed above, exercise induces the release of various exerkines, which play crucial roles in mediating the systemic effects on metabolism and inflammation (Table 1). Regarding their biological functions, exerkines can affect human health through autocrine, paracrine, and endocrine pathways (Giudice & Taylor, 2017; Pedersen, 2013). In the following context, the functions of different exerkines, their biological significance, and the explored mechanisms are elucidated.
Table 1. Examples of systemic effects of exerkines.
| Name | Sources | Target tissues | Systemic effects |
|---|---|---|---|
| Apelin | Skeletal muscle | WAT, bone, β-cells, brain | Glucose homeostasis, anti-inflammatory, insulin secretion |
| Adiponectin | WAT | Many tissues: liver, muscle | Enhances glucose and lipid metabolism, anti-inflammatory |
| MSTN | Many tissues, especially skeletal muscle and WAT | Many tissues: skeletal muscle, bone | Blunts skeletal muscle growth and glucose uptake |
| IL-6 | Primarily skeletal muscle | Many tissues: muscle, adipose tissue | Multiple effects: anti-inflammation, muscle atrophy, fatty acid oxidation |
| IL-15 | Many tissues, especially immune cells | Many tissues, especially immune cells | Regulates immune cell functioning, fat metabolism, improves glucose homeostasis, skeletal muscle growth |
| Irisin | Skeletal muscle, adipose tissue | skeletal muscle, adipocytes, β-cells | Improves insulin secretion, fatty acid oxidation, browning |
| FGF-21 | Many tissues, especially liver; WAT | Many tissues: liver, adipose tissue | Energy metabolism, muscle growth, mitochondrial biogenesis, anti-inflammation, |
| SPARC | Many tissues: muscle, adipose tissue | Many tissues: muscle, adipose tissue | Regulates cell function, tissue remodeling, metabolic homeostasis |
| BDNF | Skeletal muscle, brain | Skeletal muscle, brain | Muscle metabolism, fatty acid oxidation, anti-inflammation |
| Leptin | Adipose tissue | Adipose tissue, immune cells | Regulating inflammatory responses, glucose uptake, fatty acid oxidation |
Note:
WAT, white adipose tissue; MSTN, myostatin; IL-6, interleukin-6; IL-15, interleukin-15; FGF-21, fibroblast growth factor; SPARC, secreted protein acidic and rich in cysteine; BDNF, brain-derived neurotrophic factor.
Apelin
Apelin, a peptide in skeletal muscle, has gained attention as an essential regulator of metabolic and cardiovascular functions. It is a multifunctional exerkine that acts through autocrine and paracrine mechanisms. The secretion of apelin increases during muscle contraction, and its release is closely linked to the adaptations that occur in skeletal muscle during physical activity (Vinel et al., 2018). Apelin binds to the G-protein-coupled receptor apelin receptor and activates intracellular signaling pathways, including AMPK pathways (Bertrand, Valet & Castan-Laurell, 2015; Luo et al., 2021). These signaling cascades contribute to enhanced insulin sensitivity in adipose and skeletal muscle tissues, promoting glucose uptake and utilization (Hu et al., 2016). Studies have indicated that acute and chronic exercise lead to an upregulation of apelin secretion, suggesting its involvement in exercise-induced metabolic responses (Besse-Patin et al., 2014; Kazemi & Zahediasl, 2018). But in patients with newly diagnosed type 2 diabetes, plasma apelin levels decreased (Zhang et al., 2009b). However, a meta-analysis provides evidence that apelin circulatory levels are higher in type 2 diabetic subjects than in normal controls (Noori-Zadeh et al., 2019). Thus, there are controversies in the literature regarding the regulation of apelin in subjects with altered glucose metabolism. Most studies, however, support the physiological role of this peptide in the regulation of glucose homeostasis (Dray et al., 2008). Apelin exerts its effects on muscle cells in both humans and rodents. It enhances muscle metabolism by stimulating AMPK-dependent mitochondrial biogenesis, fostering autophagy, and reducing inflammation (Magliulo et al., 2022). Apelin’s actions extend beyond metabolism, it also exerts angiogenic effects in skeletal muscle by enhancing the formation of new blood vessels (Chen et al., 2015) and improving oxygen and nutrient supply to muscle tissues during exercise (Li et al., 2022a). This increased blood flow aids in muscle recovery and adaptation to physical demands. Additionally, apelin also exerts vasodilatory effects that improve blood flow to exercising muscles, enhancing exercise performance (Wysocka, Pietraszek-Gremplewicz & Nowak, 2018). In conclusion, apelin could be involved in many physiological processes, e.g., blood pressure (Tatemoto et al., 2001), angiogenesis (Zhang et al., 2016b), energy metabolism (Bertrand, Valet & Castan-Laurell, 2015), and participate in pathological processes, e.g., obesity (Boucher et al., 2005) and diabetes (Li et al., 2006). In summary, apelin represents a vital link between exercise and metabolic adaptations in skeletal muscle.
Adiponectin
Adiponectin, an adipokine secreted primarily by adipose tissue, has emerged as a crucial mediator of metabolic effects. Exercise influences adiponectin secretion, leading to significant physiological changes in skeletal muscle and other tissues. Adiponectin is known for its insulin-sensitizing and anti-inflammatory properties (Martinez-Huenchullan et al., 2020). Adiponectin binds to specific receptors, including AdipoR1 and AdipoR2, that are present in various target tissues (Khoramipour et al., 2021; Peng et al., 2018). The role of adiponectin in mediating exercise-induced metabolic alternations has been deeply explored. The activation of ADIPOR1 and ADIPOR2 leads to the activation of AMPK and peroxisome proliferator-activated receptor type alpha (PPAR-α) signaling pathways (Khoramipour et al., 2021). In skeletal muscle, adiponectin stimulates glucose uptake and enhances insulin sensitivity through the activation of AMPK (Martin et al., 2005). The increased glucose uptake improves glycemic indices, proving that adiponectin is an essential mediator of improvements in glucose metabolism. Animal studies have shown that adiponectin knockout mice display impaired glucose tolerance and reduced insulin sensitivity, highlighting the critical role of adiponectin in glucose homeostasis (Maeda et al., 2002). Adiponectin promotes fatty acid oxidation and inhibits lipid synthesis in skeletal muscle and the liver through the activation of PPAR-α (Combs & Marliss, 2014; Tilg & Moschen, 2010), which results in enhanced lipid utilization and an improved lipid profile, contributing to exercise-induced metabolic adaptations. Adiponectin exerts potent anti-inflammatory effects in various tissues, including skeletal muscle, which inhibits the production of pro-inflammatory cytokines (such as TNF-α and IL-6) and enhances the production of anti-inflammatory cytokines like interleukin-10 (IL-10) (Yanai & Yoshida, 2019; Zhang et al., 2009a). This balance in cytokine production helps in the resolution of inflammation and prevents chronic low-grade inflammation, commonly associated with metabolic disorders (Zaidi et al., 2021). Furthermore, studies in humans have shown increased circulating levels of adiponectin following exercise, particularly aerobic exercise (Yu et al., 2017). The aerobic exercise training indeed resulted in improved insulin sensitivity and increased adiponectin levels in individuals with type 2 diabetes (Balducci et al., 2010).
MSTN
MSTN, which is also named growth differentiation factor 8 (GDF-8), is a member of the transforming growth factor-beta (TGF-β) superfamily (McPherron, Lawler & Lee, 1997). It is primarily produced and secreted by skeletal muscle cells and acts as a potent negative regulator of muscle growth and development. The main function of MSTN is to inhibit the proliferation and differentiation of muscle cells. During exercise, MSTN expression is transiently suppressed, allowing for muscle hypertrophy and adaptation. MSTN suppresses the expression of genes involved in muscle hypertrophy and protein synthesis through binding to activating type IIB receptors, and subsequent activation of the downstream components of the canonical TGF-β family signaling pathway (Guo, Li & Xiao, 2020; Rodriguez et al., 2014). MSTN plays a crucial role in muscle homeostasis, and its dysregulation has been associated with various muscle-related disorders, including sarcopenia and muscle-wasting diseases (White & LeBrasseur, 2014). Intense physical activity, particularly resistance training, reduces MSTN expression, leading to a potential increase in muscle growth and performance (Allen, Hittel & McPherron, 2011). Exercise-induced mechanical stress and muscle contraction appear to be important stimuli for downregulating MSTN expression. Apart from its well-known functions in regulating muscle growth, recent studies have shed light on MSTN’s influence on muscle metabolism. It has been found that MSTN negatively impacts mitochondrial biogenesis and oxidative metabolism in skeletal muscle (Gonzalez-Gil & Elizondo-Montemayor, 2020). Moreover, the inhibition of MSTN leads to upregulation of the activity of peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1α) in muscle and stimulates mitochondrial biosynthesis (Leal, Lopes & Batista, 2018). This inhibition of mitochondrial biogenesis by MSTN is thought to further limit energy production and impair muscle endurance during physical activity. Studies have shown that MSTN can suppress the levels of pro-inflammatory cytokines in skeletal muscle (Zhang et al., 2011). This anti-inflammatory effect is important for mitigating muscle damage induced by exercise and promoting muscle recovery. Overall, MSTN serves as a critical regulator of muscle function and metabolism. The downregulation of MSTN caused by exercise may facilitate muscle growth, enhancing muscle performance. However, the potential inhibitory impact of MSTN on mitochondrial biogenesis and its anti-inflammatory properties contributing to muscle recovery indicate its complex role in skeletal muscle.
IL-6
IL-6 is a multifunctional cytokine with diverse roles in the immune system and inflammation regulation. Its functions extend beyond immunological responses, as it also plays a critical role in metabolism and exercise-related adaptations. During exercise, IL-6 is released from contracting skeletal muscles into the bloodstream, increases its circulating levels as a myokine, and exerts autocrine and paracrine effects (Pedersen & Febbraio, 2008). IL-6 binds to its receptor, triggering the Janus kinase signal transducer and activator of transcription (JAK/STAT) and MAPK/ERK signaling pathways, which can regulate glucose metabolism, lipid metabolism, and immune responses (Hoene & Weigert, 2008). IL-6 enhances glucose uptake in skeletal muscle and adipose tissue, supporting fuel mobilization during exercise. One well-studied downstream target of IL-6 signaling is the activation of AMPK in skeletal muscle (Kelly et al., 2009). AMPK is a master regulator of cellular energy homeostasis, and its activation by IL-6 leads to increased glucose uptake and enhanced fatty acid oxidation in muscle cells (Docherty et al., 2022; Jørgensen, Richter & Wojtaszewski, 2006). This metabolic reprogramming is crucial for coping with the increased energy demands during exercise. In addition to its metabolic effects, IL-6 plays a role in mediating the crosstalk between different organs and tissues during exercise. Studies have shown that IL-6 contributes to the regulation of glucose metabolism in the liver. It increases hepatic glucose output, which is associated with maintaining blood glucose levels during prolonged exercise (Pedersen & Fischer, 2007). Interestingly, IL-6 also exhibits anti-inflammatory effects during exercise. It stimulates the release of anti-inflammatory cytokines, which counteract the pro-inflammatory response to exercise-induced tissue damage. This anti-inflammatory action contributes to tissue repair and recovery following exercise. One study showed that exercise induced a significant increase in IL-6 and IL-10, which in turn inhibited TNF-α and stimulated IL-1 receptor antagonists to exert a direct anti-inflammatory effect (Pedersen, 2017). Another study demonstrated that exercise-induced classical anti-inflammatory mediators (IL-10, IL-8, and IL-6) are directly associated with myokine responses during the post-competition and recovery periods, utilize inflammatory mediators during muscle repair and regeneration, and influence the kinetics of muscle tissue repair (de Sousa et al., 2021). Overall, IL-6 is a key player in the complex network of exercise-induced adaptations. Its functions extend beyond inflammation regulation, encompassing metabolic, immune, and tissue repair processes. The intricate signaling pathways and crosstalk involved in IL-6’s actions during exercise highlight its importance in orchestrating the body’s response to physical activity.
IL-15
IL-15 is an anabolic factor that belongs to the family of interleukins. IL-15 has a variety of functions, including improving the balance of glucose and oxidative metabolism, lipid metabolism, and participation in the inflammatory response (Nadeau & Aguer, 2019; Nadeau et al., 2019). During physical activity, skeletal muscle is a significant source of IL-15 (Nadeau et al., 2019). IL-15 is released from skeletal muscle and acts in both autocrine and paracrine ways. IL-15 activates various signaling pathways, including the JAK/STAT and PI3K/AKT pathways, promoting glucose uptake and lipid synthesis in muscle cells and contributing to cell differentiation and proliferation (Krolopp, Thornton & Abbott, 2016; Ye, 2015). High-intensity and prolonged exercise have been shown to increase circulating levels of IL-15 (Rinnov et al., 2014). IL-15 promotes muscle hypertrophy and enhances muscle protein synthesis, suggesting its role in exercise-induced muscle adaptations (Nielsen et al., 2007). Moreover, IL-15’s influence on metabolism involves the activation of AMPK signaling pathway, which regulates cellular energy balance and protein synthesis (Nelke et al., 2019). These pathways are predominant in regulating energy homeostasis during exercise (Quinn, 2008). For example, studies have reported that IL-15 administration can enhance endurance capacity and increase fat oxidation, indicating its potential as a therapeutic target for improving exercise outcomes and metabolic conditions (Quinn et al., 2013). Furthermore, IL-15 possesses anti-inflammatory properties that contribute to its overall immunomodulatory effects (Waldmann, 2013). It can suppress the production of pro-inflammatory cytokines and promote the secretion of anti-inflammatory molecules, such as IL-10 (Loell & Lundberg, 2011). Additionally, IL-15 promotes the recruitment and activation of natural killer and memory CD8+ T cells, enhancing immune responses during exercise and potentially protecting against inflammation-related diseases (Zhang et al., 2021). In general, IL-15 is a multifunctional cytokine with vital roles in immune regulation and muscle metabolism. Exercise-induced IL-15 release contributes to immune cell mobilization, muscle growth, and anti-inflammatory responses. The interaction between exercise and IL-15 is a complex interplay that involves various signaling pathways and physiological processes.
Irisin
Irisin is generated by cleaving the fibronectin type III domain-containing protein 5 (FNDC5) and was first identified in 2012 as a myokine that is secreted by skeletal muscle in response to exercise (Boström et al., 2012). Irisin has been found to increase the expression of uncoupling protein 1 (UCP1) and other thermogenic genes in WAT, which enhances the conversion of white adipocytes into brown-like adipocytes with higher thermogenic capacity (Lee et al., 2014). This process, known as browning, can increase energy expenditure and improve insulin sensitivity (Gamas, Matafome & Seiça, 2015). Furthermore, irisin has been shown to improve glucose homeostasis by increasing glucose uptake in muscle cells (Lee et al., 2015; Perakakis et al., 2017). Additionally, exercise-associated increases in irisin contribute to insulin sensitivity by modulating pancreatic beta-cell proliferation (Perakakis et al., 2017). Exercise can significantly increase circulating irisin levels, which is believed to be mediated by the activation of PGC-1α and FNDC5 in skeletal muscle (Boström et al., 2012; Liang & Ward, 2006). A study revealed a notable rise in irisin levels and UCP1 expression following resistance exercise in rats, leading to enhanced thermogenesis in WAT (Reisi et al., 2016). The increase in irisin levels after exercise may contribute to the beneficial effects of exercise on energy metabolism and thermogenesis. Furthermore, a study has demonstrated that in diabetes, renal protection induced by physical exercise may be associated with an elevation in muscle and serum irisin, as well as enhanced AMPK activity in kidney (Formigari et al., 2022). This anti-inflammatory effect of irisin may support protection against chronic inflammation-related diseases, such as obesity and type 2 diabetes (Korta, Pocheć & Mazur-Biały, 2019; Zhang et al., 2022). In brief, the regulation of irisin levels by exercise and its multifaceted effects on metabolism and inflammation make it an intriguing target in the field of exercise physiology and metabolic health.
FGF-21
FGF-21 is an endocrine factor produced from liver, muscle, and other tissues, that can affect adipose tissue through the FGF receptor-1 and β-Klotho complex (Suzuki et al., 2008). It plays a role in regulating metabolic homeostasis, muscle growth, inflammation, and premature aging (Guo, Li & Xiao, 2020). FGF-21 activates the ERK1/2 and PI3K/AKT signaling pathways, increases glucose uptake and fatty acid oxidation in skeletal muscle and adipose tissue (Pan et al., 2019). Furthermore, several studies have shown that in fasting, FGF-21 is overexpressed and mediates fatty acid metabolism and ketone body synthesis (Badman et al., 2007; Potthoff et al., 2009). In addition, FGF-21 can promote brown adipose differentiation by increasing the expression of UCP1 and PGC-1α, leading to heat production in adipose tissue and skeletal muscle and inhibiting adipogenesis (Bilski et al., 2022; Jimenez et al., 2018). In high-fat diet-fed rats, FGF-21 administration decreases weight gain and visceral fat, enhances insulin sensitivity, and improves lipid metabolism. All of the data support the conclusion that FGF21 has positive effects on metabolism (Charoenphandhu et al., 2017). On the other side, FGF-21 binds to liver kinase B1 and activates the AMPK pathway, elevating PGC1-α, which enhances mitochondrial respiratory function and the oxidative capacity of adipocytes, thereby promoting energy expenditure (Chau et al., 2010; Salminen, Kauppinen & Kaarniranta, 2017). A close relationship between exercise and FGF-21 has been explored. For instance, a meta-analysis study demonstrated that acute exercise transiently increased circulating concentrations of FGF21 (Khalafi et al., 2021). A transient increase in FGF21 in an exercise intensity-dependent manner was also observed following acute aerobic training and may support the greater metabolic benefit of high-intensity exercise (Willis et al., 2019). Additionally, 12 weeks of aerobic training may increase FGF21 sensitivity (Li et al., 2022b). However, some recent studies have identified the possible adverse effects of FGF-21 at high circulating plasma concentrations, such as the promotion of muscle atrophy (Oost et al., 2019). FGF-21 has important anti-inflammatory effects not only in adipose tissue, but also in a variety of tissues/cells, such as cardiac tissue and macrophages (Wang et al., 2018; Zhang et al., 2016a). Therefore, as an exerkine with multiple biological functions, further studies are needed to fully understand the mechanism of action of FGF-21 during exercise.
SPARC
SPARC, also known as osteonectin, is a matricellular protein involved in extracellular matrix regulation. Exercise-induced changes in SPARC expression can influence metabolic homeostasis, inflammation reduction, extracellular matrix remodeling, and collagen maturation (Ghanemi, Yoshioka & St-Amand, 2021). The interplay between exercise and SPARC involves various signaling pathways and physiological processes, contributing to its diverse effects on skeletal muscle and beyond. SPARC may regulate glucose metabolism by activating AMPK, thereby promoting glucose transporter 4 expression (Aoi et al., 2019; Song et al., 2010). By comparing to SPARC knockout mice, impaired systemic metabolism and reduced phosphorylation within AMPK and protein kinase B in skeletal muscle have been observed. Additionally, SPARC inhibits the expression of adipogenic and lipogenic proteins and elevates lipolytic and fatty acid oxidative proteins (Mukherjee et al., 2020). In terms of its anti-inflammatory role, SPARC can act as an immunomodulatory factor during exercise-induced inflammation. It can regulate the recruitment and activation of immune cells, such as macrophages (Riley & Bradshaw, 2020). SPARC is involved in promoting cell migration, proliferation, and differentiation, which has a greater impact on matrix remodeling (Chlenski & Cohn, 2010). SPARC also regulates extracellular matrix (ECM) composition, which is a key factor influencing inflammatory cell activation in tissues, implying that SPARC can potentially regulate inflammatory progression and ECM remodeling (Riley & Bradshaw, 2020). SPARC can also modulate the expression of cytokines and chemokines by influencing the production and activity of metalloproteinases (Ng et al., 2013; Soehnlein & Lindbom, 2010). Overall, exercise-induced changes in SPARC expression highlight its vital role in muscle metabolism, tissue repair, and inflammation regulation.
BDNF
BDNF is a growth factor that plays a crucial role in supporting the survival, growth, and maintenance of neurons in the central and peripheral nervous systems (Kowiański et al., 2018). It is widely expressed in various tissues, including the brain, skeletal muscle, and adipose tissue, where it exerts its pleiotropic effects on neural function, metabolism, and inflammation. Acute and chronic exercise can increase BDNF levels in the brain and peripheral tissues (Inoue et al., 2020; Marinus et al., 2019; Wang et al., 2022), then take beneficial effects in skeletal muscle through AMPKα-PGC1α-mediated mitochondrial function and β-oxidation-mediation (Matsumoto et al., 2021). Moreover, BDNF stimulation can increase mitochondrial content and cellular respiration in skeletal muscle via the AMPK-PGC-1α pathway (Ahuja et al., 2022; Wood et al., 2018; Yang et al., 2019). In addition to this, the function of BDNF in stimulating mitochondrial biosynthesis was detected during neuronal dendrite formation (Cheng et al., 2012). BDNF activates AMPK and Acetyl-CoA carboxylase in skeletal muscle, leading to increased fatty acid oxidation, which is essential for maintaining energy balance during exercise and promoting metabolic health (Brandt & Pedersen, 2010). BDNF also exhibits potent anti-inflammatory properties. One study demonstrated that mature BDNF promoted the conversion of M1 to M2 macrophages and inhibited the secretion of inflammatory cytokines, triggering macrophage migration to the site of injury (Sasaki et al., 2021; Yu et al., 2022). BDNF can also influence immune cell function, leading to a balanced and controlled inflammatory response during exercise and recovery. The beneficial effects of exercise-induced BDNF release extend beyond its neurotrophic and metabolic functions. Therefore, in the future, it will be necessary to consider additional roles for BDNF in the prevention or treatment of metabolic and neurological disorders.
Leptin
Leptin is a classical lipotropic factor, but is expressed in skeletal muscle. It acts as a signal to activate the brain directly or indirectly, particularly in the hypothalamus, to modulate appetite and satiety, thereby influencing food intake and energy expenditure (D’Souza et al., 2017). Leptin also has various functions beyond appetite regulation, including its involvement in immune function, reproduction, and bone metabolism. Endurance exercise-induced energy expenditure is associated with reduced circulating leptin levels (Zaccaria et al., 2002). Leptin can activate AMPK in skeletal muscle, a key cellular energy sensor that regulates metabolic pathways to enhance fatty acid oxidation and glucose uptake (Minokoshi et al., 2002). The action of leptin can promote the secretion of IL-6 and TNF-α (La Cava & Matarese, 2004), whereas exposure to inflammatory stimuli can increase leptin expression in adipose tissue and circulating leptin, forming a feedback link that promotes inflammatory reactions (Landman et al., 2003). Exercise-induced changes in leptin levels may influence immune function and inflammatory responses. However, the interaction between exercise and leptin is complex, and conflicting findings have been reported regarding the effects of exercise on leptin levels and metabolic health.
Future directions
In addition to the several exerkines described above, hormones, neurotransmitters, or metabolites are also associated with exercise. For instance, catecholamines (Kjaer, Secher & Galbo, 1987), lactate (Nalbandian & Takeda, 2016), etc., may serve as exercise factors with endocrine signaling, mediating the beneficial effects in the regulation of energy homeostasis. Related exercise factors such as vascular endothelial growth factor may enhance angiogenesis and neovascularization as well as improve blood pressure, endothelial function, and overall health in the cardiovascular system (Kim et al., 2000). Peptides, kinases, lipids, and their metabolites have been analyzed in skeletal muscle and adipose tissue by a variety of approaches in the search for new exercise factors. Although hundreds of exercise factors have been identified, the study of skeletal muscle and adipose tissue secretions is still an emerging field.
While identifying various exerkines and their effects is crucial, there is still a need for a more comprehensive understanding of the intricate signaling pathways that exerkines engage in. It is worth focusing on unraveling the downstream mechanisms by which exerkines modulate metabolic processes and inflammation. Additionally, the therapeutic potential of exerkines in various diseases requires further exploration. Clinical trials involved in the investigation of exerkine-based interventions for conditions such as metabolic disorders, cardiovascular diseases, and cancer are highly expected. Furthermore, exerkines also show a promising target as biomarkers for predicting disease risk, progression, and treatment response. Developing accurate and reliable assays to measure exerkine levels enables their applications as diagnostic and prognostic tools. Investigating the cross-talk between different exerkines can uncover synergistic or antagonistic effects that impact metabolism and inflammation. More in-depth investigations into their molecular pathways, tissue-specific actions, and potential adverse effects are warranted.
Conclusion
Exerkines emerge as pivotal molecules that bridge the gap between exercise, metabolism, and inflammation. Small changes induced by exercise can create a cascading effect on the whole body. Regular exercise has many beneficial effects on metabolism and inflammation regulation. Through deep exploration, we clarify that exerkines function as orchestrators of metabolic homeostasis and inflammatory response modulation rather than mere messengers. Their capacity to enhance energy metabolism while simultaneously mitigating inflammation is a remarkable point for drug development in the future. The effects of exerkines are deeply influenced by the intricacies of exercise modality, intensity, and duration. These effects, in fact, set obstacles in the way of obtaining the full map of their potential functions. To a certain extent, our understanding of the mechanisms is merely rudimentary in connection with the benefits of exercise and the variability. In conclusion, exerkines present great potential as targets to be explored for the reduction of inflammatory effects, enhancement of metabolism, and healthy improvement.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Nihong Zhou conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Lijing Gong conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Enming Zhang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Xintang Wang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review.
References
- Afsar & Afsar (2022).Afsar B, Afsar RE. Brain-derived neurotrophic factor (BDNF): a multifaceted marker in chronic kidney disease. Clinical and Experimental Nephrology. 2022;26(12):1149–1159. doi: 10.1007/s10157-022-02268-z. [DOI] [PubMed] [Google Scholar]
- Ahuja et al. (2022).Ahuja P, Ng CF, Pang BPS, Chan WS, Tse MCL, Bi X, Kwan HR, Brobst D, Herlea-Pana O, Yang X, Du G, Saengnipanthkul S, Noh HL, Jiao B, Kim JK, Lee CW, Ye K, Chan CB. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy. 2022;18(6):1367–1384. doi: 10.1080/15548627.2021.1985257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, Hittel & McPherron (2011).Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Medicine & Science in Sports & Exercise. 2011;43(10):1828–1835. doi: 10.1249/MSS.0b013e3182178bb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoi et al. (2019).Aoi W, Hirano N, Lassiter DG, Björnholm M, Chibalin AV, Sakuma K, Tanimura Y, Mizushima K, Takagi T, Naito Y, Zierath JR, Krook A. Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. The FASEB Journal. 2019;33(9):10551–10562. doi: 10.1096/fj.201900453R. [DOI] [PubMed] [Google Scholar]
- Archundia-Herrera et al. (2017).Archundia-Herrera C, Macias-Cervantes M, Ruiz-Muñoz B, Vargas-Ortiz K, Kornhauser C, Perez-Vazquez V. Muscle irisin response to aerobic vs HIIT in overweight female adolescents. Diabetology & Metabolic Syndrome. 2017;9:101. doi: 10.1186/s13098-017-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman et al. (2007).Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5(6):426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Balducci et al. (2010).Balducci S, Zanuso S, Nicolucci A, Fernando F, Cavallo S, Cardelli P, Fallucca S, Alessi E, Letizia C, Jimenez A, Fallucca F, Pugliese G. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutrition, Metabolism and Cardiovascular Diseases. 2010;20(8):608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Banitalebi et al. (2019).Banitalebi E, Kazemi A, Faramarzi M, Nasiri S, Haghighi MM. Effects of sprint interval or combined aerobic and resistance training on myokines in overweight women with type 2 diabetes: a randomized controlled trial. Life Sciences. 2019;217(6):101–109. doi: 10.1016/j.lfs.2018.11.062. [DOI] [PubMed] [Google Scholar]
- Bertrand, Valet & Castan-Laurell (2015).Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Frontiers in Physiology. 2015;6:115. doi: 10.3389/fphys.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse-Patin et al. (2014).Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. International Journal of Obesity. 2014;38(5):707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- Bilski et al. (2022).Bilski J, Pierzchalski P, Szczepanik M, Bonior J, Zoladz JA. Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. CELLS. 2022;11(1):160. doi: 10.3390/cells11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström et al. (2012).Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher et al. (2005).Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpéné C, Audigier Y, Saulnier-Blache JS, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146(4):1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- Brandt & Pedersen (2010).Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. Journal of Biomedicine and Biotechnology. 2010;2010:520258. doi: 10.1155/2010/520258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugera et al. (2018).Bugera EM, Duhamel TA, Peeler JD, Cornish SM. The systemic myokine response of decorin, interleukin-6 (IL-6) and interleukin-15 (IL-15) to an acute bout of blood flow restricted exercise. European Journal of Applied Physiology. 2018;118(12):2679–2686. doi: 10.1007/s00421-018-3995-8. [DOI] [PubMed] [Google Scholar]
- Cariati et al. (2021).Cariati I, Bonanni R, Onorato F, Mastrogregori A, Rossi D, Iundusi R, Gasbarra E, Tancredi V, Tarantino U. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. Journal of Functional Morphology and Kinesiology. 2021;6(2):55. doi: 10.3390/jfmk6020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenphandhu et al. (2017).Charoenphandhu N, Suntornsaratoon P, Krishnamra N, Sa-Nguanmoo P, Tanajak P, Wang X, Liang G, Li X, Jiang C, Chattipakorn N, Chattipakorn S. Fibroblast growth factor-21 restores insulin sensitivity but induces aberrant bone microstructure in obese insulin-resistant rats. Journal of Bone and Mineral Metabolism. 2017;35(2):142–149. doi: 10.1007/s00774-016-0745-z. [DOI] [PubMed] [Google Scholar]
- Chau et al. (2010).Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2015).Chen D, Lee J, Gu X, Wei L, Yu SP. Intranasal delivery of apelin-13 is neuroprotective and promotes angiogenesis after ischemic stroke in mice. ASN Neuro. 2015;7(5):1759091415605114. doi: 10.1177/1759091415605114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng et al. (2012).Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nature Communications. 2012;3(1):1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlenski & Cohn (2010).Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Seminars in Cell & Developmental Biology. 2010;21(1):55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Chow et al. (2022).Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Goo Y, Haus JM, Lanza IR, Lavie CJ, Lee C-H, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nature Reviews Endocrinology. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs & Marliss (2014).Combs TP, Marliss EB. Adiponectin signaling in the liver. Reviews in Endocrine and Metabolic Disorders. 2014;15(2):137–147. doi: 10.1007/s11154-013-9280-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen et al. (2016).Cullen T, Thomas AW, Webb R, Hughes MG. Interleukin-6 and associated cytokine responses to an acute bout of high-intensity interval exercise: the effect of exercise intensity and volume. Applied Physiology, Nutrition, and Metabolism. 2016;41(8):803–808. doi: 10.1139/apnm-2015-0640. [DOI] [PubMed] [Google Scholar]
- D’Souza et al. (2017).D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Molecular Metabolism. 2017;6(9):1052–1065. doi: 10.1016/j.molmet.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa et al. (2021).de Sousa CAZ, Sierra APR, Martínez Galán BS, Maciel JFS, Manoel R, Barbeiro HV, de Souza HP, Cury-Boaventura MF. Time course and role of exercise-induced cytokines in muscle damage and repair after a marathon race. Frontiers in Physiology. 2021;12:752144. doi: 10.3389/fphys.2021.752144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty et al. (2022).Docherty S, Harley R, McAuley JJ, Crowe LAN, Pedret C, Kirwan PD, Siebert S, Millar NL. The effect of exercise on cytokines: implications for musculoskeletal health: a narrative review. BMC Sports Science, Medicine and Rehabilitation. 2022;14:5. doi: 10.1186/s13102-022-00397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2016).Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. International Journal of Obesity (lond) 2016;40(3):434–442. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray et al. (2008).Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buléon M, Cani PD, Attané C, Guigné C, Carpéné C, Burcelin R, Castan-Laurell I, Valet P. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metabolism. 2008;8(5):437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Egan & Zierath (2013).Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metabolism. 2013;17(2):162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Formigari et al. (2022).Formigari GP, Dátilo MN, Vareda B, Bonfante ILP, Cavaglieri CR, Lopes de Faria JM, Lopes de Faria JB. Renal protection induced by physical exercise may be mediated by the irisin/AMPK axis in diabetic nephropathy. Scientific Reports. 2022;12(1):9062. doi: 10.1038/s41598-022-13054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas et al. (2018).Freitas DA, Rocha-Vieira E, Soares BA, Nonato LF, Fonseca SR, Martins JB, Mendonça VA, Lacerda AC, Massensini AR, Poortamns JR, Meeusen R, Leite HR. High intensity interval training modulates hippocampal oxidative stress, BDNF and inflammatory mediators in rats. Physiology & Behavior. 2018;184:6–11. doi: 10.1016/j.physbeh.2017.10.027. [DOI] [PubMed] [Google Scholar]
- Friedrichsen et al. (2013).Friedrichsen M, Mortensen B, Pehmøller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Molecular and Cellular Endocrinology. 2013;366(2):204–214. doi: 10.1016/j.mce.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Frühbeck et al. (2014).Frühbeck G, Méndez-Giménez L, Fernández-Formoso JA, Fernández S, Rodríguez A. Regulation of adipocyte lipolysis. Nutrition Research Reviews. 2014;27(1):63–93. doi: 10.1017/S095442241400002X. [DOI] [PubMed] [Google Scholar]
- Gamas, Matafome & Seiça (2015).Gamas L, Matafome P, Seiça R. Irisin and myonectin regulation in the insulin resistant muscle: implications to adipose tissue: muscle crosstalk. Journal of Diabetes Research. 2015;2015(9):359159. doi: 10.1155/2015/359159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanemi, Yoshioka & St-Amand (2021).Ghanemi A, Yoshioka M, St-Amand J. Secreted protein acidic and rich in cysteine as a regeneration factor: beyond the tissue repair. Life. 2021;11(1):38. doi: 10.3390/life11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanemi, Yoshioka & St-Amand (2023).Ghanemi A, Yoshioka M, St-Amand J. Secreted protein acidic and rich in cysteine (SPARC)-mediated exercise effects: illustrative molecular pathways against various diseases. Diseases. 2023;11(1):33. doi: 10.3390/diseases11010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice & Taylor (2017).Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Current Opinion in Pharmacology. 2017;34:49–55. doi: 10.1016/j.coph.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson et al. (2011).Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nature Reviews Immunology. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gil & Elizondo-Montemayor (2020).Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020;12(6):1899. doi: 10.3390/nu12061899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Li & Xiao (2020).Guo A, Li K, Xiao Q. Sarcopenic obesity: myokines as potential diagnostic biomarkers and therapeutic targets? Experimental Gerontology. 2020;139(41):111022. doi: 10.1016/j.exger.2020.111022. [DOI] [PubMed] [Google Scholar]
- Hamrick & McGee-Lawrence (2018).Hamrick MW, McGee-Lawrence ME. Blocking bone loss with l-BAIBA. Trends in Endocrinology & Metabolism. 2018;29(5):284–286. doi: 10.1016/j.tem.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Handschin & Spiegelman (2008).Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves & Spriet (2018).Hargreaves M, Spriet LL. Exercise metabolism: fuels for the fire. Cold Spring Harbor Perspectives in Medicine. 2018;8(8):a029744. doi: 10.1101/cshperspect.a029744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2018).He Z, Tian Y, Valenzuela PL, Huang C, Zhao J, Hong P, He Z, Yin S, Lucia A. Myokine response to high-intensity interval vs. resistance exercise: an individual approach. Frontiers in Physiology. 2018;9:1735. doi: 10.3389/fphys.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoene & Weigert (2008).Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obesity Reviews. 2008;9(1):20–29. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- Hotamisligil (2017).Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2016).Hu H, He L, Li L, Chen L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Molecular Genetics and Metabolism. 2016;119(1–2):20–27. doi: 10.1016/j.ymgme.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Huang, Xu & Cheung (2017).Huang Z, Xu A, Cheung BMY. The potential role of fibroblast growth factor 21 in lipid metabolism and hypertension. Current Hypertension Reports. 2017;19(4):28. doi: 10.1007/s11906-017-0730-5. [DOI] [PubMed] [Google Scholar]
- Ikeda et al. (2016).Ikeda SI, Tamura Y, Kakehi S, Sanada H, Kawamori R, Watada H. Exercise-induced increase in IL-6 level enhances GLUT4 expression and insulin sensitivity in mouse skeletal muscle. Biochemical and Biophysical Research Communications. 2016;473(4):947–952. doi: 10.1016/j.bbrc.2016.03.159. [DOI] [PubMed] [Google Scholar]
- Ingerslev et al. (2017).Ingerslev B, Hansen JS, Hoffmann C, Clemmesen JO, Secher NH, Scheler M, Hrabĕ de Angelis M, Häring HU, Pedersen BK, Weigert C, Plomgaard P. Angiopoietin-like protein 4 is an exercise-induced hepatokine in humans, regulated by glucagon and cAMP. Molecular Metabolism. 2017;6(10):1286–1295. doi: 10.1016/j.molmet.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue et al. (2020).Inoue DS, Monteiro PA, Gerosa-Neto J, Santana PR, Peres FP, Edwards KM, Lira FS. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Scientific Reports. 2020;10(1):13493. doi: 10.1038/s41598-020-70326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii & Mann (2018).Ishii T, Mann GE. When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons? Neural Regeneration Research. 2018;13(5):803–804. doi: 10.4103/1673-5374.232468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez et al. (2018).Jimenez V, Jambrina C, Casana E, Sacristan V, Muñoz S, Darriba S, Rodó J, Mallol C, Garcia M, León X, Marcó S, Ribera A, Elias I, Casellas A, Grass I, Elias G, Ferré T, Motas S, Franckhauser S, Mulero F, Navarro M, Haurigot V, Ruberte J, Bosch F. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Molecular Medicine. 2018;10(8):21. doi: 10.15252/emmm.201708791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joisten et al. (2019).Joisten N, Rademacher A, Bloch W, Schenk A, Oberste M, Dalgas U, Langdon D, Caminada D, Purde MT, Gonzenbach R, Kool J, Zimmer P, Bansi J. Influence of different rehabilitative aerobic exercise programs on (anti-) inflammatory immune signalling, cognitive and functional capacity in persons with MS—study protocol of a randomized controlled trial. BMC Neurology. 2019;19:37. doi: 10.1186/s12883-019-1267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, Richter & Wojtaszewski (2006).Jørgensen SB, Richter EA, Wojtaszewski JF. Role of AMPK in skeletal muscle metabolic regulation and adaptation in relation to exercise. The Journal of Physiology. 2006;574(1):17–31. doi: 10.1113/jphysiol.2006.109942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi & Zahediasl (2018).Kazemi F, Zahediasl S. Effects of exercise training on adipose tissue apelin expression in streptozotocin-nicotinamide induced diabetic rats. Gene. 2018;662:97–102. doi: 10.1016/j.gene.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Kelly et al. (2009).Kelly M, Gauthier MS, Saha AK, Ruderman NB. Activation of AMP-activated protein kinase by interleukin-6 in rat skeletal muscle: association with changes in cAMP, energy state, and endogenous fuel mobilization. Diabetes. 2009;58(9):1953–1960. doi: 10.2337/db08-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafi et al. (2021).Khalafi M, Alamdari KA, Symonds ME, Nobari H, Carlos-Vivas J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: systematic review and meta-analysis. Hormones. 2021;20(1):23–33. doi: 10.1007/s42000-020-00245-3. [DOI] [PubMed] [Google Scholar]
- Khalafi et al. (2023).Khalafi M, Aria B, Symonds ME, Rosenkranz SK. The effects of resistance training on myostatin and follistatin in adults: a systematic review and meta-analysis. Physiology & Behavior. 2023;269(3):114272. doi: 10.1016/j.physbeh.2023.114272. [DOI] [PubMed] [Google Scholar]
- Khoramipour et al. (2021).Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, Elguindy NM, Bragazzi NL. Adiponectin: structure, physiological functions, role in diseases, and effects of nutrition. Nutrients. 2021;13(4):1180. doi: 10.3390/nu13041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2000).Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circulation Research. 2000;86(1):24–29. doi: 10.1161/01.RES.86.1.24. [DOI] [PubMed] [Google Scholar]
- Kjaer, Secher & Galbo (1987).Kjaer M, Secher NH, Galbo H. Physical stress and catecholamine release. Baillières Clinical Endocrinology and Metabolism. 1987;1(2):279–298. doi: 10.1016/S0950-351X(87)80064-2. [DOI] [PubMed] [Google Scholar]
- Korta, Pocheć & Mazur-Biały (2019).Korta P, Pocheć E, Mazur-Biały A. Irisin as a multifunctional protein: implications for health and certain diseases. Medicina. 2019;55(8):485. doi: 10.3390/medicina55080485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowiański et al. (2018).Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology. 2018;38(3):579–593. doi: 10.1007/s10571-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolopp, Thornton & Abbott (2016).Krolopp JE, Thornton SM, Abbott MJ. IL-15 activates the Jak3/STAT3 signaling pathway to mediate glucose uptake in skeletal muscle cells. Frontiers in Physiology. 2016;7:626. doi: 10.3389/fphys.2016.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Cava & Matarese (2004).La Cava A, Matarese G. The weight of leptin in immunity. Nature Reviews Immunology. 2004;4(5):371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lafontan et al. (2000).Lafontan M, Sengenes C, Galitzky J, Berlan M, De Glisezinski I, Crampes F, Stich V, Langin D, Barbe P, Rivière D. Recent developments on lipolysis regulation in humans and discovery of a new lipolytic pathway. International Journal of Obesity and Related Metabolic Disorders. 2000;24(Suppl 4):S47–52. doi: 10.1038/sj.ijo.0801505. [DOI] [PubMed] [Google Scholar]
- Landman et al. (2003).Landman RE, Puder JJ, Xiao E, Freda PU, Ferin M, Wardlaw SL. Endotoxin stimulates leptin in the human and nonhuman primate. The Journal of Clinical Endocrinology & Metabolism. 2003;88(3):1285–1291. doi: 10.1210/jc.2002-021393. [DOI] [PubMed] [Google Scholar]
- Laurens, Bergouignan & Moro (2020).Laurens C, Bergouignan A, Moro C. Exercise-released myokines in the control of energy metabolism. Frontiers in Physiology. 2020;11:91. doi: 10.3389/fphys.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin et al. (2020).Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. Journal of Applied Physiology (1985) 2020;128(1):87–99. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, Lopes & Batista (2018).Leal LG, Lopes MA, Batista ML., Jr Physical exercise-induced myokines and muscle-adipose tissue crosstalk: a review of current knowledge and the implications for health and metabolic diseases. Frontiers in Physiology. 2018;9:1307. doi: 10.3389/fphys.2018.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2015).Lee HJ, Lee JO, Kim N, Kim JK, Kim HI, Lee YW, Kim SJ, Choi JI, Oh Y, Kim JH, Suyeon H, Park SH, Kim HS. Irisin, a novel myokine, regulates glucose uptake in skeletal muscle cells via AMPK. Molecular Endocrinology. 2015;29(6):873–881. doi: 10.1210/me.2014-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al. (2014).Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metabolism. 2014;19(2):302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2022a).Li C, Cheng H, Adhikari BK, Wang S, Yang N, Liu W, Sun J, Wang Y. The role of apelin-APJ system in diabetes and obesity. Frontiers in Endocrinology. 2022a;13:820002. doi: 10.3389/fendo.2022.820002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2022b).Li XH, Liu LZ, Chen L, Pan QN, Ouyang ZY, Fan DJ, Pan X, Lu SY, Luo QH, Tao PY, Huang HQ. Aerobic exercise regulates FGF21 and NLRP3 inflammasome-mediated pyroptosis and inhibits atherosclerosis in mice. PLOS ONE. 2022b;17(8):e0273527. doi: 10.1371/journal.pone.0273527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2021).Li Q, Tan Y, Chen S, Xiao X, Zhang M, Wu Q, Dong M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-κB signaling. Journal of Receptors and Signal Transduction. 2021;41(3):294–303. doi: 10.1080/10799893.2020.1808675. [DOI] [PubMed] [Google Scholar]
- Li et al. (2006).Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, Li K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Experimental and Clinical Endocrinology & Diabetes. 2006;114(10):544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- Liang & Ward (2006).Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Advances in Physiology Education. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Loell & Lundberg (2011).Loell I, Lundberg IE. Can muscle regeneration fail in chronic inflammation: a weakness in inflammatory myopathies? Journal of Internal Medicine. 2011;269(3):243–257. doi: 10.1111/j.1365-2796.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2021).Luo J, Liu W, Feng F, Chen L. Apelin/APJ system: a novel therapeutic target for locomotor system diseases. European Journal of Pharmacology. 2021;906(Suppl. 2):174286. doi: 10.1016/j.ejphar.2021.174286. [DOI] [PubMed] [Google Scholar]
- Maeda et al. (2002).Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature Medicine. 2002;8(7):731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Magliulo et al. (2022).Magliulo L, Bondi D, Pini N, Marramiero L, Di Filippo ES. The wonder exerkines-novel insights: a critical state-of-the-art review. Molecular and Cellular Biochemistry. 2022;477(1):105–113. doi: 10.1007/s11010-021-04264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus et al. (2019).Marinus N, Hansen D, Feys P, Meesen R, Timmermans A, Spildooren J. The impact of different types of exercise training on peripheral blood brain-derived neurotrophic factor concentrations in older adults: a meta-analysis. Sports Medicine. 2019;49(10):1529–1546. doi: 10.1007/s40279-019-01148-z. [DOI] [PubMed] [Google Scholar]
- Martin et al. (2005).Martin LJ, Woo JG, Daniels SR, Goodman E, Dolan LM. The relationships of adiponectin with insulin and lipids are strengthened with increasing adiposity. The Journal of Clinical Endocrinology & Metabolism. 2005;90(7):4255–4259. doi: 10.1210/jc.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Huenchullan et al. (2020).Martinez-Huenchullan SF, Tam CS, Ban LA, Ehrenfeld-Slater P, McLennan SV, Twigg SM. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism. 2020;102:154008. doi: 10.1016/j.metabol.2019.154008. [DOI] [PubMed] [Google Scholar]
- Matsumoto et al. (2021).Matsumoto J, Takada S, Furihata T, Nambu H, Kakutani N, Maekawa S, Mizushima W, Nakano I, Fukushima A, Yokota T, Tanaka S, Handa H, Sabe H, Kinugawa S. Brain-derived neurotrophic factor improves impaired fatty acid oxidation via the activation of adenosine monophosphate-activated protein kinase-α - proliferator-activated receptor-r coactivator-1α signaling in skeletal muscle of mice with heart failure. Circulation: Heart Failure. 2021;14(1):e005890. doi: 10.1161/CIRCHEARTFAILURE.119.005890. [DOI] [PubMed] [Google Scholar]
- McPherron, Lawler & Lee (1997).McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Minokoshi et al. (2002).Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415(6869):339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mukherjee et al. (2020).Mukherjee S, Choi MJ, Kim SW, Yun JW. Secreted protein acidic and rich in cysteine (SPARC) regulates thermogenesis in white and brown adipocytes. Molecular and Cellular Endocrinology. 2020;506:110757. doi: 10.1016/j.mce.2020.110757. [DOI] [PubMed] [Google Scholar]
- Nadeau & Aguer (2019).Nadeau L, Aguer C. Interleukin-15 as a myokine: mechanistic insight into its effect on skeletal muscle metabolism. Applied Physiology, Nutrition, and Metabolism. 2019;44(3):229–238. doi: 10.1139/apnm-2018-0022. [DOI] [PubMed] [Google Scholar]
- Nadeau et al. (2019).Nadeau L, Patten DA, Caron A, Garneau L, Pinault-Masson E, Foretz M, Haddad P, Anderson BG, Quinn LS, Jardine K, McBurney MW, Pistilli EE, Harper ME, Aguer C. IL-15 improves skeletal muscle oxidative metabolism and glucose uptake in association with increased respiratory chain supercomplex formation and AMPK pathway activation. Biochimica et Biophysica Acta (BBA)—General Subjects. 2019;1863(2):395–407. doi: 10.1016/j.bbagen.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian & Takeda (2016).Nalbandian M, Takeda M. Lactate as a signaling molecule that regulates exercise-induced adaptations. Biology. 2016;5(4):38. doi: 10.3390/biology5040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash et al. (2023).Nash D, Hughes MG, Butcher L, Aicheler R, Smith P, Cullen T, Webb R. IL-6 signaling in acute exercise and chronic training: potential consequences for health and athletic performance. Scandinavian Journal of Medicine & Science in Sports. 2023;33(1):4–19. doi: 10.1111/sms.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelke et al. (2019).Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. eBioMedicine. 2019;49(12):381–388. doi: 10.1016/j.ebiom.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng et al. (2013).Ng YL, Klopcic B, Lloyd F, Forrest C, Greene W, Lawrance IC. Secreted protein acidic and rich in cysteine (SPARC) exacerbates colonic inflammatory symptoms in dextran sodium sulphate-induced murine colitis. PLOS ONE. 2013;8(10):e77575. doi: 10.1371/journal.pone.0077575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen et al. (2007).Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. The Journal of Physiology. 2007;584(1):305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori-Zadeh et al. (2019).Noori-Zadeh A, Bakhtiyari S, Khanjari S, Haghani K, Darabi S. Elevated blood apelin levels in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2019;148(11):43–53. doi: 10.1016/j.diabres.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Oliveira et al. (2013).Oliveira AG, Araujo TG, Carvalho BM, Guadagnini D, Rocha GZ, Bagarolli RA, Carvalheira JB, Saad MJ. Acute exercise induces a phenotypic switch in adipose tissue macrophage polarization in diet-induced obese rats. Obesity. 2013;21(12):2545–2556. doi: 10.1002/oby.20402. [DOI] [PubMed] [Google Scholar]
- Oost et al. (2019).Oost LJ, Kustermann M, Armani A, Blaauw B, Romanello V. Fibroblast growth factor 21 controls mitophagy and muscle mass. Journal of Cachexia, Sarcopenia and Muscle. 2019;10(3):630–642. doi: 10.1002/jcsm.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski, Schjerling & Pedersen (2000).Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans--effect of intensity of exercise. European Journal of Applied Physiology. 2000;83(6):512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- Palmer, Irwin & O’Harte (2022).Palmer ES, Irwin N, O’Harte FP. Potential therapeutic role for apelin and related peptides in diabetes: an update. Clinical Medicine Insights: Endocrinology and Diabetes. 2022;15:11795514221074679. doi: 10.1177/11795514221074679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. (2019).Pan Y, Wang B, Zheng J, Xiong R, Fan Z, Ye Y, Zhang S, Li Q, Gong F, Wu C, Lin Z, Li X, Pan X. Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner. Journal of Cellular and Molecular Medicine. 2019;23(2):1059–1071. doi: 10.1111/jcmm.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianni et al. (2023).Papagianni G, Panayiotou C, Vardas M, Balaskas N, Antonopoulos C, Tachmatzidis D, Didangelos T, Lambadiari V, Kadoglou NPE. The anti-inflammatory effects of aerobic exercise training in patients with type 2 diabetes: a systematic review and meta-analysis. Cytokine. 2023;164(3):156157. doi: 10.1016/j.cyto.2023.156157. [DOI] [PubMed] [Google Scholar]
- Pedersen (2007).Pedersen BK. IL-6 signalling in exercise and disease. Biochemical Society Transactions. 2007;35(5):1295–1297. doi: 10.1042/BST0351295. [DOI] [PubMed] [Google Scholar]
- Pedersen (2013).Pedersen BK. Muscle as a secretory organ. Comprehensive Physiology. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- Pedersen (2017).Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. European Journal of Clinical Investigation. 2017;47(8):600–611. doi: 10.1111/eci.12781. [DOI] [PubMed] [Google Scholar]
- Pedersen et al. (2007).Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. Journal of Applied Physiology (1985) 2007;103(3):1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen & Febbraio (2008).Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiological Reviews. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen & Fischer (2007).Pedersen BK, Fischer CP. Physiological roles of muscle-derived interleukin-6 in response to exercise. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(3):265–271. doi: 10.1097/MCO.0b013e3280ebb5b3. [DOI] [PubMed] [Google Scholar]
- Peng et al. (2018).Peng YJ, Shen TL, Chen YS, Mersmann HJ, Liu BH, Ding ST. Adiponectin and adiponectin receptor 1 overexpression enhance inflammatory bowel disease. Journal of Biomedical Science. 2018;25(1):24. doi: 10.1186/s12929-018-0419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perakakis et al. (2017).Perakakis N, Triantafyllou GA, Fernández-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nature Reviews Endocrinology. 2017;13(6):324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry & Hawley (2018).Perry CGR, Hawley JA. Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: historical advances, current knowledge, and future challenges. Cold Spring Harbor Perspectives in Medicine. 2018;8(9):a029686. doi: 10.1101/cshperspect.a029686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen & Pedersen (2005).Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. Journal of Applied Physiology. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Potthoff et al. (2009).Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn (2008).Quinn LS. Interleukin-15: a muscle-derived cytokine regulating fat-to-lean body composition. Journal of Animal Science. 2008;86(suppl_14):E75–83. doi: 10.2527/jas.2007-0458. [DOI] [PubMed] [Google Scholar]
- Quinn et al. (2013).Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. IL-15 overexpression promotes endurance, oxidative energy metabolism, and muscle PPARδ, SIRT1, PGC-1α, and PGC-1β expression in male mice. Endocrinology. 2013;154(1):232–245. doi: 10.1210/en.2012-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisi et al. (2016).Reisi J, Ghaedi K, Rajabi H, Marandi SM. Can resistance exercise alter irisin levels and expression profiles of FNDC5 and UCP1 in rats? Asian Journal of Sports Medicine. 2016;7(4):e35205. doi: 10.5812/asjsm.35205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahy (2024).Riahy S. The effects of 12 weeks of high-intensity interval training and moderate-intensity continuous training on FGF21, irisin, and myostatin in men with type 2 diabetes mellitus. Growth Factors. 2024;42(1):24–35. doi: 10.1080/08977194.2023.2279163. [DOI] [PubMed] [Google Scholar]
- Riley & Bradshaw (2020).Riley HJ, Bradshaw AD. The influence of the extracellular matrix in inflammation: findings from the SPARC-null mouse. The Anatomical Record. 2020;303(6):1624–1629. doi: 10.1002/ar.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnov et al. (2014).Rinnov A, Yfanti C, Nielsen S, Akerström TC, Peijs L, Zankari A, Fischer CP, Pedersen BK. Endurance training enhances skeletal muscle interleukin-15 in human male subjects. Endocrine. 2014;45(2):271–278. doi: 10.1007/s12020-013-9969-z. [DOI] [PubMed] [Google Scholar]
- Robbins & Gerszten (2023).Robbins JM, Gerszten RE. Exercise, exerkines, and cardiometabolic health: from individual players to a team sport. Journal of Clinical Investigation. 2023;133(11):e705. doi: 10.1172/jci168121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez et al. (2014).Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, Seiliez I, Picard B, Bonnieu A. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cellular and Molecular Life Sciences. 2014;71(22):4361–4371. doi: 10.1007/s00018-014-1689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar, Saleem & Tarnopolsky (2016).Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nature Reviews Endocrinology. 2016;12(9):504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- Salminen, Kauppinen & Kaarniranta (2017).Salminen A, Kauppinen A, Kaarniranta K. FGF21 activates AMPK signaling: impact on metabolic regulation and the aging process. Journal of Molecular Medicine (Berl) 2017;95(2):123–131. doi: 10.1007/s00109-016-1477-1. [DOI] [PubMed] [Google Scholar]
- Sargeant et al. (2018).Sargeant JA, Aithal GP, Takamura T, Misu H, Takayama H, Douglas JA, Turner MC, Stensel DJ, Nimmo MA, Webb DR, Yates T, King JA. The influence of adiposity and acute exercise on circulating hepatokines in normal-weight and overweight/obese men. Applied Physiology, Nutrition, and Metabolism. 2018;43(5):482–490. doi: 10.1139/apnm-2017-0639. [DOI] [PubMed] [Google Scholar]
- Sasaki et al. (2021).Sasaki S, Takeda K, Ouhara K, Shirawachi S, Kajiya M, Matsuda S, Kono S, Shiba H, Kurihara H, Mizuno N. Involvement of Rac1 in macrophage activation by brain-derived neurotrophic factor. Molecular Biology Reports. 2021;48(6):5249–5257. doi: 10.1007/s11033-021-06531-6. [DOI] [PubMed] [Google Scholar]
- Schmidt-Arras & Rose-John (2016).Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: from physiopathology to therapy. Journal of Hepatology. 2016;64(6):1403–1415. doi: 10.1016/j.jhep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Soehnlein & Lindbom (2010).Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nature Reviews Immunology. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- Solomon et al. (2013).Solomon TP, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic β-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. The Journal of Clinical Endocrinology & Metabolism. 2013;98(10):4176–4186. doi: 10.1210/jc.2013-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2010).Song H, Guan Y, Zhang L, Li K, Dong C. SPARC interacts with AMPK and regulates GLUT4 expression. Biochemical and Biophysical Research Communications. 2010;396(4):961–966. doi: 10.1016/j.bbrc.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Steensberg et al. (2000).Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. The Journal of Physiology. 2000;529(Pt 1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki (2019).Suzuki K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules. 2019;9(6):223. doi: 10.3390/biom9060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki et al. (2008).Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Molecular Endocrinology. 2008;22(4):1006–1014. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto et al. (2001).Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regulatory Peptides. 2001;99:87–92. doi: 10.1016/S0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- Thyfault & Bergouignan (2020).Thyfault JP, Bergouignan A. Exercise and metabolic health: beyond skeletal muscle. Diabetologia. 2020;63(8):1464–1474. doi: 10.1007/s00125-020-05177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg & Moschen (2010).Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- Tsuchiya et al. (2013).Tsuchiya M, Manabe Y, Yamada K, Furuichi Y, Hosaka M, Fujii NL. Chronic exercise enhances insulin secretion ability of pancreatic islets without change in insulin content in non-diabetic rats. Biochemical and Biophysical Research Communications. 2013;430(2):676–682. doi: 10.1016/j.bbrc.2012.11.092. [DOI] [PubMed] [Google Scholar]
- Vilela et al. (2017).Vilela TC, Muller AP, Damiani AP, Macan TP, da Silva S, Canteiro PB, de Sena Casagrande A, Pedroso GDS, Nesi RT, de Andrade VM, de Pinho RA. Strength and aerobic exercises improve spatial memory in aging rats through stimulating distinct neuroplasticity mechanisms. Molecular Neurobiology. 2017;54(10):7928–7937. doi: 10.1007/s12035-016-0272-x. [DOI] [PubMed] [Google Scholar]
- Vinel et al. (2018).Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradère JP, Le Gonidec S, Dortignac A, Geoffre N, Pereira O, Karaz S, Lee U, Camus M, Chaoui K, Mouisel E, Bigot A, Mouly V, Vigneau M, Pagano AF, Chopard A, Pillard F, Guyonnet S, Cesari M, Burlet-Schiltz O, Pahor M, Feige JN, Vellas B, Valet P, Dray C. The exerkine apelin reverses age-associated sarcopenia. Nature Medicine. 2018;24(9):1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- Wärnberg et al. (2010).Wärnberg J, Cunningham K, Romeo J, Marcos A. Physical activity, exercise and low-grade systemic inflammation. Proceedings of the Nutrition Society. 2010;69(3):400–406. doi: 10.1017/S0029665110001928. [DOI] [PubMed] [Google Scholar]
- Waldmann (2013).Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. Journal of Investigative Dermatology Symposium Proceedings. 2013;16(1):S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang N, Li JY, Li S, Guo XC, Wu T, Wang WF, Li DS. Fibroblast growth factor 21 regulates foam cells formation and inflammatory response in Ox-LDL-induced THP-1 macrophages. Biomedicine & Pharmacotherapy. 2018;108(Suppl B):1825–1834. doi: 10.1016/j.biopha.2018.09.143. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2022).Wang YH, Zhou HH, Luo Q, Cui S. The effect of physical exercise on circulating brain-derived neurotrophic factor in healthy subjects: a meta-analysis of randomized controlled trials. Brain and Behavior. 2022;12(4):e2544. doi: 10.1002/brb3.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White & LeBrasseur (2014).White TA, LeBrasseur NK. Myostatin and sarcopenia: opportunities and challenges—a mini-review. Gerontology. 2014;60(4):289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- Willis et al. (2019).Willis SA, Sargeant JA, Thackray AE, Yates T, Stensel DJ, Aithal GP, King JA. Effect of exercise intensity on circulating hepatokine concentrations in healthy men. Applied Physiology, Nutrition, and Metabolism. 2019;44(10):1065–1072. doi: 10.1139/apnm-2018-0818. [DOI] [PubMed] [Google Scholar]
- Wood et al. (2018).Wood J, Tse MCL, Yang X, Brobst D, Liu Z, Pang BPS, Chan WS, Zaw AM, Chow BKC, Ye K, Lee CW, Chan CB. BDNF mimetic alleviates body weight gain in obese mice by enhancing mitochondrial biogenesis in skeletal muscle. Metabolism. 2018;87:113–122. doi: 10.1016/j.metabol.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Wysocka, Pietraszek-Gremplewicz & Nowak (2018).Wysocka MB, Pietraszek-Gremplewicz K, Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Frontiers in Physiology. 2018;9:557. doi: 10.3389/fphys.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai & Yoshida (2019).Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. International Journal of Molecular Sciences. 2019;20(5):1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2019).Yang X, Brobst D, Chan WS, Tse MCL, Herlea-Pana O, Ahuja P, Bi X, Zaw AM, Kwong ZSW, Jia WH, Zhang ZG, Zhang N, Chow SKH, Cheung WH, Louie JCY, Griffin TM, Nong W, Hui JHL, Du GH, Noh HL, Saengnipanthkul S, Chow BKC, Kim JK, Lee CW, Chan CB. Muscle-generated BDNF is a sexually dimorphic myokine that controls metabolic flexibility. Science Signaling. 2019;12(594):237. doi: 10.1126/scisignal.aau1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye (2015).Ye J. Beneficial metabolic activities of inflammatory cytokine interleukin 15 in obesity and type 2 diabetes. Frontiers of Medicine. 2015;9(2):139–145. doi: 10.1007/s11684-015-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2022).Yu HC, Huang HB, Huang Tseng HY, Lu MC. Brain-derived neurotrophic factor suppressed proinflammatory cytokines secretion and enhanced MicroRNA(miR)-3168 expression in macrophages. International Journal of Molecular Sciences. 2022;23(1):570. doi: 10.3390/ijms23010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu et al. (2017).Yu N, Ruan Y, Gao X, Sun J. Systematic review and meta-analysis of randomized, controlled trials on the effect of exercise on serum leptin and adiponectin in overweight and obese individuals. Hormone and Metabolic Research. 2017;49(03):164–173. doi: 10.1055/s-0042-121605. [DOI] [PubMed] [Google Scholar]
- Zaccaria et al. (2002).Zaccaria M, Ermolao A, Roi GS, Englaro P, Tegon G, Varnier M. Leptin reduction after endurance races differing in duration and energy expenditure. European Journal of Applied Physiology. 2002;87(2):108–111. doi: 10.1007/s00421-002-0606-4. [DOI] [PubMed] [Google Scholar]
- Zaidi et al. (2021).Zaidi H, Byrkjeland R, Njerve IU, Åkra S, Solheim S, Arnesen H, Seljeflot I, Opstad TB. Adiponectin in relation to exercise and physical performance in patients with type 2 diabetes and coronary artery disease. Adipocyte. 2021;10(1):612–620. doi: 10.1080/21623945.2021.1996699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2016a).Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y, Tan Y, Feng W, Fu Y, Mellen N, Cheng R, Ma J, Zhang C, Li Z, Cai L. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of type 1 diabetic mice. Clinical Science (Lond) 2016a;130(8):625–641. doi: 10.1042/CS20150623. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2011).Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. The FASEB Journal. 2011;25(5):1653–1663. doi: 10.1096/fj.10-176917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2009b).Zhang Y, Shen C, Li X, Ren G, Fan X, Ren F, Zhang N, Sun J, Yang J. Low plasma apelin in newly diagnosed type 2 diabetes in Chinese people. Diabetes Care. 2009b;32(12):e150. doi: 10.2337/dc09-1146. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016b).Zhang L, Takara K, Yamakawa D, Kidoya H, Takakura N. Apelin as a marker for monitoring the tumor vessel normalization window during antiangiogenic therapy. Cancer Science. 2016b;107(1):36–44. doi: 10.1111/cas.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2009a).Zhang P, Wang Y, Fan Y, Tang Z, Wang N. Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009a;29(1):67–74. doi: 10.1161/ATVBAHA.108.178061. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2022).Zhang H, Wu X, Liang J, Kirberger M, Chen N. Irisin, an exercise-induced bioactive peptide beneficial for health promotion during aging process. Ageing Research Reviews. 2022;80(10):101680. doi: 10.1016/j.arr.2022.101680. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2021).Zhang S, Zhao J, Bai X, Handley M, Shan F. Biological effects of IL-15 on immune cells and its potential for the treatment of cancer. International Immunopharmacology. 2021;91(8):107318. doi: 10.1016/j.intimp.2020.107318. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review.