Abstract
Background:
Evidence is insufficient to establish a longitudinal association between combined trajectories of body mass index (BMI) and waist circumference (WC) and dyslipidemia. Our study aimed to explore the association between multi-trajectories of BMI and WC and incident dyslipidemia and identify microbiota and metabolite signatures of these trajectories.
Methods:
Stratified by sex, we used a group-based trajectory modeling approach to identify distinct multi-trajectories of BMI and WC among 10,678 participants from the China Health and Nutrition Survey over a 24-year period. For each sex, we examined the associations between these multi-trajectories (1991–2015) and the onset dyslipidemia (2018) using multivariable logistic regression adjusting for sociodemographic and lifestyles factors. We characterized the gut microbial composition and performed LASSO and logistic regression to identify gut microbial signatures associated with these multi-trajectories in males and females, respectively.
Results:
We identified four multi-trajectories of BMI and WC among both males and females: Normal (Group 1), BMI&WC normal increasing (Group 2), BMI&WC overweight increasing (Group 3), and BMI&WC obesity increasing (Group 4). Among males, Group 2 (OR: 2.10, 95% CI: 1.28–3.46), Group 3 (OR: 2.69, 95% CI: 1.56–4.63) and Group 4 (OR: 3.56, 95% CI: 1.85–6.83) had higher odds of developing dyslipidemia. However, among females, only those in Group 2 (OR: 1.54, 95% CI: 1.03–2.30) were more likely to develop dyslipidemia. In males, compared with Group 1, we observed lower alpha-diversity within Groups 2,3, and 4, and significant beta-diversity differences within Groups 3 and 4 (p 0.001). We also identified 3, 8, and 4 characteristic bacterial genera in male Groups 2, 3 and 4, and 2 genera in female Group 2. A total of 23, 25 and 10 differential metabolites were significantly associated with the above genera, except for Group 2 in males.
Conclusions:
The ascending combined trajectories of BMI and WC are associated with a higher risk of dyslipidemia, even with normal baseline levels, especially in males. Shared and unique gut microbial and metabolic signatures among these high-risk trajectories could enhance our understanding of the mechanisms connecting obesity to dyslipidemia.
Keywords: Multi-trajectories, body mass index, waist circumference, dyslipidemia, gut microbiota, serum metabolites
Background
Dyslipidemia is a major risk factor for cardiovascular disease and a leading cause of death globally, accounting for 46.7% and 44.3% of total deaths in rural and urban areas in China in 2019[1]. Given the increasing global burden and prevalence of dyslipidemia, it is imperative to uncover novel risk factors to prevent the occurrence and development of dyslipidemia.
Obesity is a major driver of dyslipidemia. Numerous studies have explored the associations between individual obesity indicators and dyslipidemia. Individuals with higher body mass index (BMI) or waist circumference (WC) are more likely to develop dyslipidemia[2–5]. BMI, measured by combining weight and height, is widely used to assess obesity, but it cannot capture the distribution of abdominal adipose tissue as WC does. Furthermore, BMI and WC can change throughout the lifespan, and several longitudinal studies have assessed the possible effects of long-term changes in anthropometric indices on the risk of dyslipidemia[6–8]. To our knowledge, there are no longitudinal studies reporting the relationship between multi-trajectories of BMI and WC from early adulthood and dyslipidemia among the Chinese population.
Emerging evidence suggests a close connection between the gut microbiome and both human obesity[9–11] and dyslipidemia[12, 13], suggesting that the gut microbiome may play an important role in the obesitydyslipidemia relationship. However, most studies on the association between BMI/WC and gut microbiome still remain at the cross-sectional level. The longitudinal effects of the BMI/WC on the gut microbiome and related metabolites are unclear. Specific gut microbial signatures that demonstrate patterns of BMI/WC changes over time could help explain mechanistic links between obesity and dyslipidemia.
Therefore, in the present prospective cohort study followed from 1991 to 2018, we established multi-trajectories based on 24 years of BMI and WC measurement data and revealed the gut bacterial genera and serum metabolites associated with those multi-trajectories.
Methods
Study population
This study is based on the China Health and Nutrition Survey (CHNS), an ongoing population-based longitudinal study. The CHNS collects demographic information, lifestyle details, physical activity levels, dietary habits, anthropometric measurements, and biological samples [14]. Across 11 survey rounds, approximately15,000 participants were recruited in each round, representing 16 provinces and megacities across China. The most recent data available is from the 2018 survey.
According to the analysis process, our study included five sub-datasets (Fig. 1A). (1) After excluding participants < 18 years of age, pregnant or breastfeeding, or patients with cancer, stroke or disabled patients at the time of the survey, 10,678 individuals (5,222 males and 5,456 females) with at least three weight, height and WC measurements from 1991 to 2015 were included in the multi-trajectories analysis. (2) Among these participants, we further excluded those who had developed dyslipidemia in 2015 or did not continue to participate in the cohort study in 2018 (no lipid data in 2018), leaving 1,992 individuals (841 males and 1151 females) analyzed the association between the multi-trajectories and onset dyslipidemia in 2018. (3) When analyzing the relationship between multi-trajectories and gut microbiome, we included participants with stool samples collected in the trajectory population in 2015, and excluded those who had taken antibiotics within 3 months, used probiotics within the last 4 weeks, or had gastrointestinal disorders, diarrhea, and intestinal resection. 3,039 individuals (1434 males and 1605 females) were included in the discovery cohort, and 1,400 individuals (650 males and 750 females) were included in the validation cohort. (4) A total of 772 participants (334 males and 438 females) with both multi-trajectories and metabolic data in 2015 were used to analyze the differences in metabolites between multi-trajectories. (5) We selected 946 individuals (393 males and 553 females) as a subset of the validation cohort who had gut microbiome and metabolic data in 2015 to map the connection between metabolites and gut bacteria.
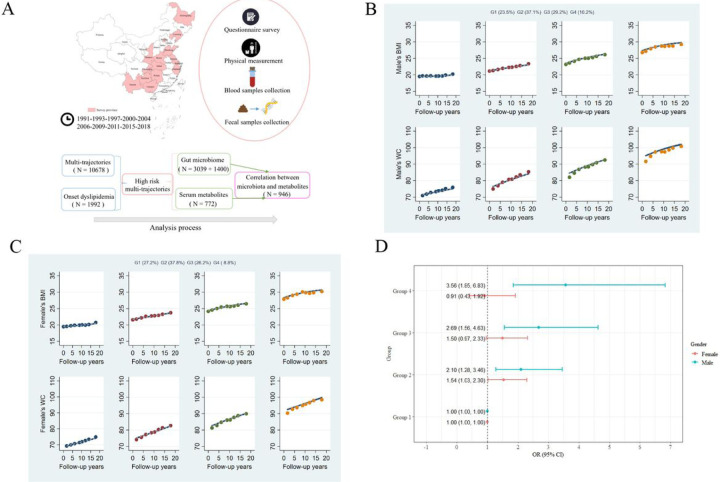
Figure 1. Multi-trajectories of BMI and WC and their associations with dyslipidemia.
(A) Summary of the study population.(B&C) Multi-trajectories of BMI and WC in CHNS cohort (1991–2015) among males (B) and females (C). The solid lines represent average estimated BMI and WC over time. The dots represent the actual data, where we weighted each individual’s responses based on posterior probabilities of group membership. (D) Associations between multi-trajectories and dyslipidemia based on binomial logistic regression model. Both models were adjusted for age, location (urban/rural), geographical area (province), education level, smoking, drinking, household income, physical activity, and dietary energy intake.
Data collection
Questionnaire survey
Sociodemographic characteristics, including location (urban or rural), geographical area (province), age, sex, education and household income, lifestyle factors (smoking status and alcohol consumption), dietary intake, physical activity, physiological and disease status (pregnancy, lactation, disability, stroke, cancer, gastrointestinal disorders, diarrhea, and intestinal resection), and medication data (the use of antibiotics and probiotics) were collected by face-to-face questionnaire interviews. Dietary energy intake was calculated by combining food intake data with the China food composition table, and the amount of physical activity was calculated by multiplying the amount of exercise time by activity intensity of various intensities.
Physical measurement
Anthropometric data, including height, weight and waist circumference, were measured on-site by trained staff. Adhering to consistent measurement standards and utilizing specialized instruments, in each survey, our trained physicians and nurses measured height and weight without shoes to the nearest 0.1 cm and 0.1 kg. We then calculated BMI as weight in kg divided by height in meters (m) squared (kg/m2). We measured waist circumference using an inelastic soft ruler with a division value of 0.1 cm.
Biological sample collection
Fasting blood samples were collected, and stored in dry ice, and sent to the laboratory for storage at −80°C within 3 hours. The plasma was centrifuged within 48 hours and stored at −80°for later use. Fecal samples were collected following standard procedures[15] and temporarily stored in a −20°C freezer within 20 minutes and then stored in a laboratory − 80°C freezer.
Assessment of dyslipidemia
Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using an automatic biochemical analyzer. The definition of dyslipidemia can be found in the Guidelines for the Prevention and Treatment of Dyslipidemia in Chinese Adults (2016 revised edition), including the flowing thresholds: TC ≥ 6.2 mmol/L, TG ≥ 2.3 mmol/L, LDL-C ≥ 4.1 mmol/L, or non-HDL-C ≥ 4.9 mmol/L.
Bioinformatics analysis of gut microbiome
Methods of DNA extraction, amplification, and sequencing have been described previously[16]. Taxonomic and functional profiles were generated using the Quantitative Insights Into Microbial Ecology 2 platform (QIIME2)[17]. Pair-end reads were assembled using the QIIME tools import command. Low-quality regions of the sequences, marker gene Illumina sequences, and chimeric sequences (“consensus”) were filtered using the DADA2 pipeline[18]. Reads were then summarized to amplicon sequence variants (ASV) in a feature table and annotated based on the naive Bayes classifier using the classify-sklearn package against the Silva-132–99 reference sequences[19].
Serum metabolome analysis
For metabolic analysis, 50 μL of the sample and 300 μL of the extraction solution (ACN: Methanol = 1:4, V/V) containing internal standards were added to a 2 mL microcentrifuge tube. The sample was vortexed for 3 minutes and then centrifuged at 12,000 rpm for 10 minutes at 4°C. Subsequently, 200 μL of the supernatant was collected and placed at −20°C for 30 minutes, followed by another centrifugation at 12,000 rpm for 3 minutes at 4°C. An aliquot of 180 μL of the supernatant was transferred for LC-ESI-MS/MS analysis. The sample extracts were analyzed using an LC-ESI-MS/MS system (UPLC, ExionLC AD, https://sciex.com.cn/; MS, QTRAP® System, https://sciex.com/) following standard protocols. The triple quadrupole-linear ion trap mass spectrometer (QTRAP) was used to perform LIT and triple quadrupole (QQQ) scans, operated and controlled by Analyst 1.6.3 software (Sciex) with standard parameters. The source temperature was 500°C; the ion spray voltage (IS) was 5500 V (positive) and 4500 V (negative); the ion source gas I (GSI), gas II (GSII), and curtain gas (CUR) were set at 55, 60, and 25.0 psi, respectively; the collision gas (CAD) was set to high. Instrument tuning and mass calibration were performed with 10 and 100 μmol/L polypropylene glycol solutions in QQQ and LIT modes, respectively. A specific set of MRM transitions was monitored for each period according to the metabolites eluted within this period.
Statistical analysis
Multi-trajectories of BMI and WC
Group-based multi-trajectory modeling (GBTM)[20] was used to determine the multi-trajectories of BMI and WC. As the WC assessment criteria are different for different sex, we performed multi-trajectory modeling with a STATA plug-in using continuous norming (cNORM) distribution for different sex[21]. We tested linear, quadratic, and cubic specifications for trajectory shape for participants in two, three, four, five, and six trajectory groups until we established the best-fitting model. We used statistically rigorous criteria to determine the best fit. (1) With the lowest Bayesian information criterion (BIC), we used the difference size (percentage change) of the BIC to choose between a more complex (with one additional specified trajectory group) and a simpler model; (2) we included at least 2% of the sample population in each trajectory class; and (3) we ascertained the average posterior probability value of membership within each group, where values greater than 0.7 indicate adequate internal reliability[22].
Association between multi-trajectories and dyslipidemia
We performed logistic regression models to explore the relationship between the sex-specific multi-trajectories (1991–2015) and onset dyslipidemia (2018). We built models for males and females, and adjusted for baseline age, location (urban/rural) and geographical area (province), smoking, alcohol use, education, household income, dietary energy intake and physical activity. Then, we used the“forestplot” and “ggplot2” functions in R to plot the forest plot and display the results of the model. We considered a two-sided p value < 0.05 to be statistically significant.
Gut microbiome analysis
We performed all gut microbiome analyses separately for different sex. Four alpha-diversity indices were calculated at a sampling depth of 6000: Shannon’s diversity index, Observed features, Pielou’s species evenness measure, and Faith’s phylogenetic diversity. In order to display the results of the four indicators on the same axis, we used the scale function in R to standardize them, then used the Wilcoxon test to compare these indicators between the dyslipidemia risk trajectory group and the normal group, and finally displayed the results in the form of box graphs. At the genus level, using the “vegdist” function from the R package “vegan”, we calculated Bray-Curtis distances between samples using genera abundance and visualized using PCoA. We then performed a permutational multivariate analysis of variance (PERMANOVA) based on the Bray-Curtis distance to determine whether there were differences between groups.
Before identifying the characteristic genera, we first preprocessed the raw genera abundance data. Genus with a presence lower than 10% were excluded, and a centered log-ratio (CLR) transformation was applied. Two cohorts were used, including the discovery cohort (3039 samples, 1434 males and 1605 females) and the validation cohort (1400 samples, 650 males and 750 females). The selection of characteristic genera for those multi-trajectories with a higher risk of dyslipidemia was based on a discovery cohort derived from Least Absolute Selection and Shrinkage Operator (LASSO) regression. LASSO regression can simplify the model by adding a penalty function and continuously compressing the coefficient to avoid collinearity and overfitting. Important variables can be efficiently screened with a smaller sample size[23]. Validations of these characteristic genera were based on both the discovery cohort and the validation cohort by logistic regression. To correct for multiple testing issue, p value was adjusted by the false discovery rate (FDR) method.
Serum metabolites analysis
Sex-specific metabolomics analyses were also performed. Wilcoxon test was used to identify differential metabolites between different multi-trajectory groups. The ratio of the median of the comparison group to the control group was used as the Fold Change (FC) value. The selection of differential metabolites was based on the following criteria: p value < 0.05 and |log2FC| > 0.5. Raw target metabolite data were log-transformed and standardized before analysis. Relationships between these differential metabolites and the characteristic microbiota of multi-trajectories were analyzed using Spearman correlation. All differential metabolites and correlation results were visualized as volcano maps and heat maps by the ggplot2 package in the R language, respectively. When the FDR adjusted p value was < 0.05, a statistical difference was considered.
Results
Sample characteristics
Table 1 presents baseline characteristics of the 10,678 enrolled participants (51.1% females). The mean (±SD) age of males and females was 39.6±14.1 years and 41.0±13.4 years, respectively. The mean follow-up time was 15.9±6.1 years for males and 15.8±6.3 years for females.
Table 1.
Baseline characteristics of the population in the multi-trajectories analysis
| Male 5222(48.9%) |
Female 5456(51.1%) |
|
|---|---|---|
| Follow-up time(years)a | 15.9±6.1 | 15.8±6.3 |
| Age(years)a | 39.6±14.1 | 41.0±13.4 |
| Urban(%) | 29.7 | 31.8 |
| Education(%) | ||
| Primary and below | 39.7 | 56.3 |
| Junior high | 35.7 | 26.6 |
| Senior high and above | 24.6 | 17.1 |
| Household income(RMB)b | 1356.5(629.7,3220.6) | 1378.1(636.7,3393.6) |
| Smoker(%) | 64.4 | 4.7 |
| Alcohol drinker (%) | 63.9 | 11.5 |
| Energy intake(kcal/d)a | 2594.1±749.2 | 2259.4±670.3 |
| Physical activity(METs/week)b | 292.0(126.0,528.0) | 336.2(144.1,589.9) |
| Body mass index(kg/m2)a | 22.1±2.9 | 22.4±3.2 |
| Waist circumference(cm)a | 78.7±9.4 | 76.5±9.2 |
Mean (SD) was reported,
Median(Q1,Q3) was reported.
Multi-trajectories of BMI and WC
Four multi-trajectories of BMI and WC among males and females were identified by GBTM (Figs. 1B and C). We named trajectories based on baseline levels (compared to adult weight criteria WS/T 428–2013) and trends. At baseline and during follow-up, 23.5% of males and 27.2% of females had BMI and WC within the normal range (BMI 18.5–23.9 kg/m2, WC <85 cm in males and <80 cm in females), were grouped into the normal trajectory (Group 1). 37.1% of males and 37.8% of females had normal BMI and WC at baseline and an upward trend during follow-up, were grouped into BMI&WC normal increasing trajectory (Group 2). 29.2% of males and 26.2% of females had a BMI in the overweight range (24–27.9 kg/m2) and WC in the precentral obesity range (males 85–89.9 cm, females 80–84.9 cm) at baseline and an upward trend during follow-up, were grouped into BMI&WC overweight increasing trajectory (Group 3). 10.2% of males and 8.8% of females had a BMI that was obese (≥ 28 kg/m2) and a WC that was central obese (male ≥ 90 cm, female ≥ 85 cm) at baseline and an upward trend during follow-up, were grouped into obesity increasing trajectory (Group 4).
Multi-trajectories and dyslipidemia
Figure 1D shows the results of logistic regression analyses exploring associations between multi-trajectories and risk of dyslipidemia. Among male participants, compared with Group 1, Group 2 (OR:2.10, 95% CI:1.28–3.46), Group 3 (OR:2.69, 95% CI:1.56–4.63) and Group 4 (OR:3.56, 95% CI:1.85–6.83) were significantly associated with a higher risk of dyslipidemia after adjusting for potential confounders. However, among females, only Group 2 (OR:1.54, 95% CI:1.03–2.30) was associated with a higher risk of dyslipidemia.
High-risk multi-trajectories and gut microbiota
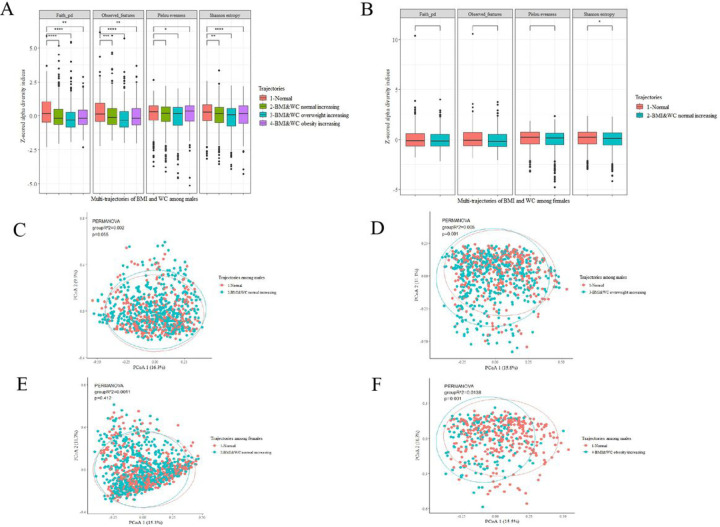
First, we evaluated overall indicators of gut microbiota composition. Among males, we found that the values of these four alpha-diversity indexes in Group 3 were lower than those in Group 1 (Fig. 2A). Among females, only the shannon’s index value was lower in Group 2 than in Group 1 (Fig. 2B). We also identified links between multi-trajectories and overall microbial structure (beta diversity). Among male participants, permutation multivariate ANOVA based on Bray-Curtis distance showed significant differences between Groups 3, 4 and 1, explaining 0.5% and 1.4% of the dissimilarities in the gut microbiota structure (P=0.001) (Figs. 2D & 2F).
Figure 2. Comparison of the diversity of gut microbiota in multi-trajectories of different sex.
(A&B) alpha -diversity analysis between males (A) and females (B). Four alpha-diversity indices were scaled: Shannon’s diversity index, observed-features, Pielou’s measure of species evenness and faith’s phylogenetic diversity. Comparison between each risk trajectory group and the normal group using Kruskal Wallis test. (C-F) β-diversity analysis between males (C, D, F) and females (E). Pairwise comparisons were determined by PERMANOVA analyses based on Bray-Curtis distance. R2 and p value were determined from 999 permutations.
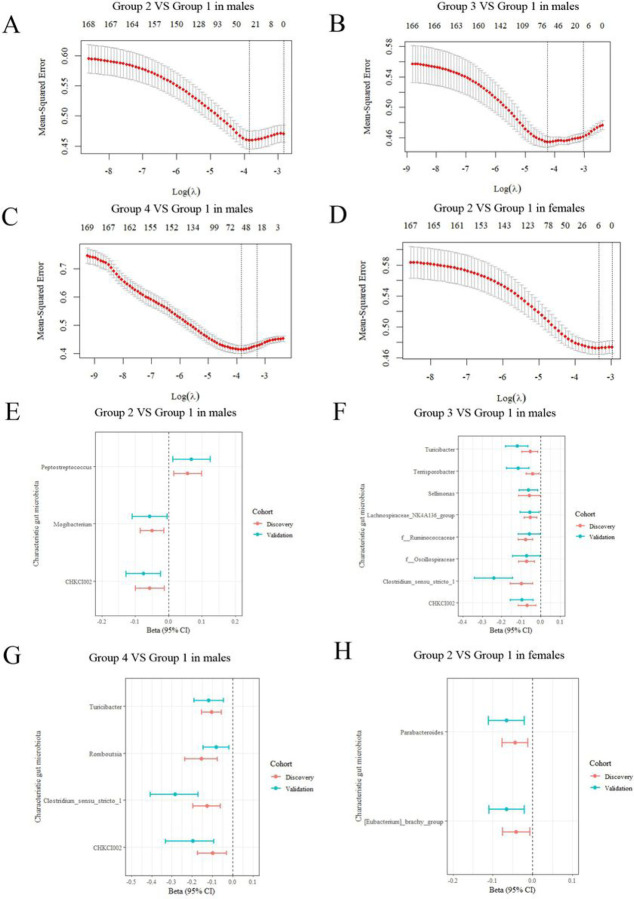
We then explored those multi-trajectories characteristic genera with a higher risk of dyslipidemia. Among males, 30, 66 and 54 microbiol genera were obtained for groups 2, 3 and 4 by LASSO regression using minimum cross-validation error as parameter in the validation dataset (Figs. 3A, 3B, and 3C). Among females, the model retained only 6 bacterial genera as characteristic genera for Group 2 (Fig. 3D). These selected bacterial genera were further validated by logistic regression. After dual verification, 3, 8, 4 and 2 characteristic bacteria genera were retained for different sex (Fig. 3E–G). Among males, both Group 3 (r = −0.10 and −0.24 in discovery and validation cohort) and Group 4 (r=−0.13 and −0.28 in discovery and validation cohort) were negatively associated with genus Clostridium_sensu_stricto_1, validation cohort with a higher absolute coefficient value. The same is true for Genus Turicibacter. Meanwhile, the genus CHKCI002 should also be mentioned, as all Groups 2, 3, and 4 were negatively correlated with it, and correlations were growing (Additional file 1: Table S1 −3). Among females, Group 2 was negatively associated with genus Parabacteroides and [Eubacterium]_brachy_group (Additional file 1: Table S4). The correlation was considered statistically significant when the FDR p value was less than 0.05. Detailed results are shown in Additional file 1: table S1–4.
Figure 3. Characteristic genera for those multi-trajectories with higher risk of dyslipidemia.
(A-D) Cross-validation curves of 169 bacteria genera for screening of characteristic genera by LASSO regression (discovery cohort). Characteristic genera selection for BMI&WC normal increasing trajectory (group 2) in male (A), BMI&WC overweight increasing trajectory (group 3) in male (B), BMI&WC obesity increasing trajectory (group 4) in male (C) and BMI&WC normal increasing trajectory (group 2) in female (D). (E-H) Characteristic genera with statistical significance after validation in both discovery cohort and validation cohort by logistic regression. Association of selected characteristic genera with BMI&WC normal increasing trajectory (group 2) in male (E), BMI&WC overweight increasing trajectory (group 3) in male (F), BMI&WC obesity increasing trajectory (group 4) in male (G), and BMI&WC normal increasing trajectory (group 2) in female (H).
Key serum metabolites associated with high-risk multi-trajectories and related gut microbiota
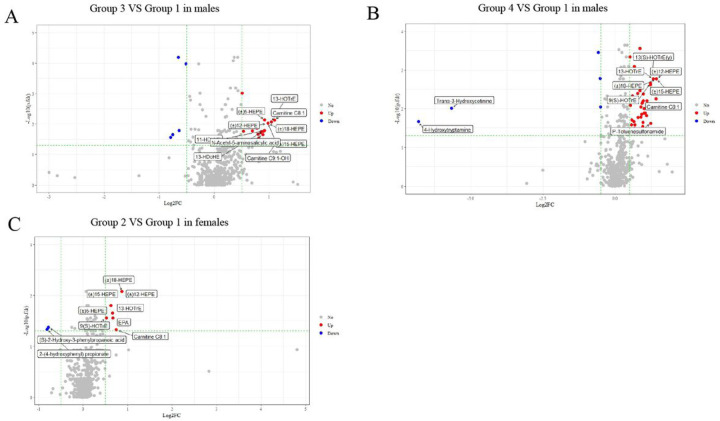
We first identified some differential metabolites of multi-trajectories with higher risk compared with the normal group. In males, no difference was found in Group 2 metabolites (Additional file 1: Table S5). Thirty-six differential metabolites were found in Group 3, of which 30 were upregulated metabolites. For Group 4, 52 out of 57 differential metabolites were upregulated (Fig. 4A&B, Additional file 1: Table S6–7). In females, 10 differential metabolites were identified in Group 2 (Fig. 4C, Additional file 1: Table S8).
Figure 4. Volcanic map of differential metabolites (P<0.05, |log2(FC)>0.5 considered to be differential).
Differential metabolites were found between the normal group (group 1) and BMI&WC overweight increasing trajectory (group 3) in male (A), BMI&WC obesity increasing trajectory (group 4) in male (B) and BMI&WC normal increasing trajectory (group 2) in female (C). Blue was the down-regulated differential metabolite, red was the up-regulated differential metabolite, and metabolites with no difference were marked as grey. The P value was further adjusted for multiple testing of pairwise comparison using the Benjamini-Hochberg method. Figure A and Figure B showed only the top 10 metabolites with significant differences respectively, and the top 10 metabolites were sorted according to the absolute value of log2FC.
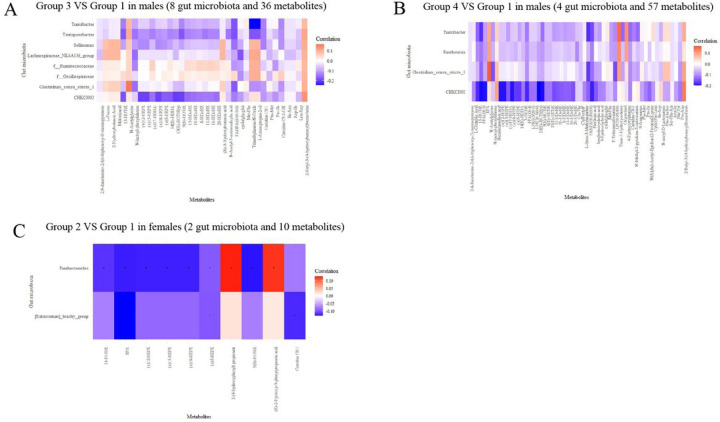
We then examined the associations between characteristic microbiota (n = 8 in Group 3, = 4 in Group 4 in males, and = 2 in Group 2 in females) and differential metabolites (n = 36 in Group 3, = 57 in Group 4 in males, and = 10 in Group 2 in females) (Fig. 5). The results showed that, in males, 23 metabolites were significantly associated with the characteristic genera of Group 3. Among them, FAHFA (8:0/10:0) was negatively associated with the genera Clostridium_sensu_stricto_1 (r=−0.17) and Turicibacter (r −0.18). N-lactoyl-phenylalanine was negatively correlated not only with the genera Clostridium_sensu_stricto_1(r −0.15), but also with two other characteristic bacteria (Lachnospiraceae_NK4A136_group and Terrisporobacter). Negative associations were also found among Trimethylamine-N-Oxide, 1-Aminopropan-2-ol and genera Turicibacter, Terrisporobacter (Fig. 5A, Additional file 1: Table S9). Twenty-five metabolites were significantly associated with characteristic genera of male Group 4. Among them, the result of FAHFA (8:0/10:0) were similar to those of Group 3. In addition, negative associations were also found among FFA (18:3), pinolenic acid and genera Turicibacter, CHKCI002 (Fig. 5B, Additional file 1: Table S10). In females, all 10 differential metabolites were significantly associated with characteristic genera in Group 2. Among them, negative associations were found among EPA, (±)5-HEPE and genera Parabacteroides, [Eubacterium]_brachy_group (Fig. 5C, Additional file 1: Table S11).
Figure 5. Spearman’s rank correlation between validated characteristic genera and differential metabolites.
(A) Association of validated 8 characteristic genera for BMI&WC overweight increasing trajectory (group 3) with 36 differential metabolites (between group 3 and group 1) in male. (B) Association of validated 4 characteristic genera for BMI&WC obesity increasing trajectory (group 4) with 57 differential metabolites (between group 4 and group 1) in male. (C) Association of validated 2 characteristic genera for BMI&WC normal increasing trajectory (group 2) with 10 differential metabolites (between group 2 and group 1) in female.
Discussion
The main results of our cohort study, based on anthropometric measurements, blood and fecal samples and 27 years of data, are as follows. First, four multi-trajectories of BMI and WC among males and females were identified, with the same increasing trend from 1991 to 2015. Most of the population was in the BMI&WC normal growth group (Group 2). In addition, male participants with increasing trends in BMI and WC had a higher risk of dyslipidemia in 2018, with OR value increasing as baseline BMI and WC shifted from normal to obese. However, in female participants, we only found an increased risk of dyslipidemia in Group 2. And second, we found several important characteristic genera negatively associated with groups that remained overweight/obesity or developed overweight/obesity, including Clostridium_sensu_stricto_1, Turicibacter, and CHKCI002 among males and Parabacteroides, [Eubacterium]_brachy_group among females. These important characteristic genera are also related to some differential metabolites, such as FAHFA(8:0/10:0), N-lactoyl-phenylalanine, Trimethylamine-N-Oxide, 1-Aminopropan-2-ol, FFA (18:3), Pinolenic acid, EPA and (±)5-HEPE; most of them belong to free fatty acids (FFAs) and oxidized lipids.
Using 24-years of BMI and WC measurements and the GBTM method, we successfully established four distinct multi-trajectories of BMI and WC in the study sample. This grouping can not only reflect the status of obesity and central obesity, but also show the baseline obesity level and long-term change trends simultaneously. To the best of our knowledge, only one study[24] reported multi-trajectory of BMI and WC, and its results were similar to our study. In addition, the BMI trajectories[25, 26] and WC trajectories[27] derived from large samples of adults only also showed four increasing trend groups. This reminds us that the BMI and WC of Chinese adults seem to be on the rise simultaneously and need to be controlled urgently.
In our study, male groups 2, 3, and 4 were associated with a higher risk of dyslipidemia. This is different with females. A cross-sectional study among Chinese primary school children also found sex differences in anthropometric indicators predicting dyslipidemia, which may be more true for boys than for girls[28]. Another study of Chinese adults showed that obese males also had a higher risk of dyslipidemia than females[29]. This sex disparity may be due to differences in lifestyle between males and females, which are risk factors of dyslipidemia[30]. A possible explanation may also be related to sexual dimorphism in fat distribution and hormone levels. A study by Nedungadi and Clegg[31] showed that females have more subcutaneous fat and males have more visceral fat, suggesting a greater risk of dyslipidemia. Additionally, studies have showed that females have higher leptin and adiponectin levels (considered cardiovascular-protective factors[32, 33]) than males[34, 35]. Therefore, the association between BMI and WC and the risk of dyslipidemia is weaker in females than in males. To date, we are not aware of any longitudinal study evaluating the relationship between multi-trajectories of BMI and WC and dyslipidemia. Limited evidence from cohort studies suggests that the odds of developing dyslipidemia are associated with increased BMI[4] and WC[6], respectively. More effective attention and interventions should be taken to manage BMI and WC to reduce the prevalence of dyslipidemia, especially in males.
Little is known about the contribution of Clostridium sensu stricto 1 to human gut health. Two mouse experiments in 2021 and 2022 suggest it may be a novel biomarker of obesity/obesity resistance [36, 37]. Our longitudinal study in adult males also confirmed this new finding, suggesting that Clostridium sensu stricto 1 might help prevent obesity. Clostridium are known producers of butyrate[38], which contributes to the integrity of the intestinal barrier, attenuates chronic inflammation by promoting regulatory T cells, and prevent pathogen proliferation [39]. Therefore, loss of butyrate-producing bacteria, such as Clostridium, induces chronic low-grade inflammation. However, there are also conflicting results for Clostridium sensu stricto 1 existed. Evidence from the animal feeding experiment[40] and intervention trails in obese patients [41] both indicate that Clostridium sensu stricto 1 is reduced after weight loss interventions. Similar to Clostridium sensu stricto 1, CHKCI002 also showed a positive correlation with butyrate in ducks[42]. Farkas V first reported the negative correlation between CHKCI002 genus and chicken body weight in 2022[43]. In this study, CHKCI002 was also negatively associated with all risk groups in males, suggesting a beneficial effect of CHKCI002 on obesity indicators. Many studies have linked Turicibacter to host lipid metabolism profile, but the results are inconsistent[44–46]. This may be a result of phenotypic diversity among Turicibacter, where hosts may experience different lipid outcomes depending on their own specific Turicibacter strains. A recent study by Lynch JB[47] further identified genes capable of altering host bile acids and lipid metabolism in Turicibacter strains, and positioned Turicibacter bacteria as modulators of host lipid biology. Our data showing that Turicibacter a negatively associated with BMI&WC in the overweight or obesity increasing trajectory groups (two groups with higher risk of dyslipidemia) support these previous studies. This also highlights the advantage of long-term trajectory characteristic microbiota of obesity indicators in predicting dyslipidemia.
In addition to the above-mentioned beneficial bacteria found in males, we also found two dominant beneficial bacteria in females: genus Parabacteroides and Eubacterium brachy group. Members of the genus Parabacteroides are saccharolytic bacteria that produce major end products of fermentation, such as acetic acid and succinic acid[48]. According to numerous studies, the relative abundance of Parabacteroides is negatively associated with BMI [49–51], which is consistent with our study. There are limited studies on the association of the Eubacterium brachy group with obesity or blood lipids. We found only one study on adult mice showing that a high-fat diet reduced the abundance of the Eubacterium brachy group at 18 weeks [52]. Members of the genus Eubacterium can undergo bile acid and cholesterol transformations in the gut, thereby contributing to its homeostasis[53]. Gut microbiologists agree that specific butyrate-producing microbial strains belonging to the genera Eubacterium may ultimately be considered as beneficial to human health as Lactobacillus and Bifidobacterium strains[54].
N-lactoyl-phenylalanine (Lac-Phe) was proposed as an “exercise hormone” that suppresses appetite and adiposity in diet-induced obese mice, however, Li et al. demonstrated complete inactivity of Lac-Phe when administered orally[55]. In this study, the amount of Lac-Phe in the overweight/obesity increasing group was higher than that in the normal group, which is inconsistent with previous animal studies. Future work will help elucidate whether Lac-Phe can help outrun obesity. As a bioactive metabolite of the gut microbiota, trimethylamine-N-oxide (TMAO) plays a critical role in the progression of many diseases, including diabetes[56], obesity[57], atherosclerosis and cardiovascular risks[58]. In these disease states, elevated circulating TMAO concentrations are commonly observed. Meta-analyses also revealed a positive dose-dependent association between circulating TMAO concentrations and obesity[59]. Mechanisms that may contribute to obesity include the role of FMO 3 (TMAO producing enzyme) in obesity regulation and adipose tissue formation[57], as well as increased hepatic insulin resistance and consequent obesity through increased TMAO concentrations[60].
γ-linolenic acid (GLA, 18:3n-6), Pinolenic acid and EPA(the first two belong to omega-6 polyunsaturated fatty acids (PUFA), and the last one belongs to omega-3 PUFA) were significantly higher in groups 3 and 4, and were negatively correlated with the beneficial bacteria Turicibacter, CHKCI002, Parabacteroides, [Eubacterium]_brachy_group in this study. A review study showed that n-6 PUFA derived eicosanoids have pro-inflammatory effects, whereas n-3 PUFA derived eicosanoids have anti-inflammatory activities[61]. Research by Sunhye Shin[62] further found that the high n-6:n-3 ratio of linoleic acid-rich oil increased lipogenesis and reduced lipid oxidation and thermogenesis. More importantly, adequate intake of n-3 PUFA can significantly influence the effects of n-6 PUFA on lipoprotein profiles[63]. Therefore, the ratio of n-6 PUFA to n-3 PUFA is more important than the amount of a single n-6 PUFA or n-3 PUFA. This study found that overweight and obese groups have higher concentrations of n-3 and n-6 fatty acids and a higher risk of dyslipidemia, which may be related to the ratio of the two.
This study has several strengths. First, we used 24-year multi-trajectories of BMI and WC to predict the risk of dyslipidemia, and long-term follow-up is a unique strength of this study. In addition, for the first time, the characteristic gut microbiota and serum metabolites of multi-trajectories of BMI and WC with higher dyslipidemia risk were analyzed. This study also has limitations. First, the characteristic bacteria or differential metabolites in the multi-trajectories were derived from observational data and the causality cannot be established at this stage. However, we conducted validation across different datasets and compared differential metabolites by p-value and screened based on FC value. Second, although we adjusted for numerous covariates, we cannot completely rule out the possibility of residual confounding. Third, the associations of obesity with functional profiles of the gut microbiome is unclear due to the use of 16S rRNA data.
Conclusions
In conclusion, our results suggest that obesity indicators influence dyslipidemia in males more than in females. We also identified some potentially gut bacteria and differential metabolites associated with long-term BMI and WC trajectories, some of which were closely related to lipid levels, revealing the role of gut bacteria and related metabolites in obesity and lipid metabolism.
Acknowledgements
We acknowledge all participants in this study and the staff responsible for the China Health and Nutrition Survey at the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, and the Carolina Population Center at the University of North Carolina at Chapel Hill. We thank Westlake University High-Performance Computing Center for computational resource and related assistance.
Funding
This study was funded by the National Key R&D Program of China (2021YFE0114200) the “Pioneer” and “Leading goose” R&D Program of Zhejiang (2024SSYS0032). This research used data from the China Health and Nutrition Survey (CHNS). We are grateful for the research grant funding from the National Institutes of Health (NIH) (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700) from 1989 to 2019. This study was also funded by The Ministry of Finance of the Republic of China from 2015 to 2017 (13103110700015005).
Funding Statement
This study was funded by the National Key R&D Program of China (2021YFE0114200) the “Pioneer” and “Leading goose” R&D Program of Zhejiang (2024SSYS0032). This research used data from the China Health and Nutrition Survey (CHNS). We are grateful for the research grant funding from the National Institutes of Health (NIH) (R01-HD30880, DK056350, R24 HD050924, and R01-HD38700) from 1989 to 2019. This study was also funded by The Ministry of Finance of the Republic of China from 2015 to 2017 (13103110700015005).
Footnotes
Ethics approval and consent to participants
The study was approved by the National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention (No. 2018–012 and No. 201524) and the Institutional Review committees of the University of North Carolina at Chapel Hill (NO. 07–1963). Participants provided their written informed consent.
Competing interests
The authors declare that they have no competing interests.
Supplementary Files
Contributor Information
Xiaofan Zhang, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Fangxu Guan, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Wanglong Gou, Zhejiang Key Laboratory of Multi-Omics in Infection and Immunity, Center for Infectious Disease Research, School of Medicine, Westlake University.
Qi Wang, Chaoyang District of Beijing Centre for Disease Control and Prevention.
Shufa Du, Department of Nutrition and Carolina Population Center, University of North Carolina at Chapel Hill.
Chang Su, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Jiguo Zhang, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Ju-Sheng Zheng, Zhejiang Key Laboratory of Multi-Omics in Infection and Immunity, Center for Infectious Disease Research, School of Medicine, Westlake University.
Huijun Wang, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Bing Zhang, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available, please contact the corresponding authors for details.
References
- 1.In China T, Hu SS. Report on cardiovascular health and diseases in China 2021: an updated summary. J Geriatr Cardiol 2023;20(6):399–430.doi: 10.26599/1671-5411.2023.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obsa MS, Ataro G, Awoke N, et al. Determinants of Dyslipidemia in Africa: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2021;8:778891.doi: 10.3389/fcvm.2021.778891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo K, Huang YQ, Shen G, et al. Effects of waist to height ratio, waist circumference, body mass index on the risk of chronic diseases, all-cause, cardiovascular and cancer mortality. Postgrad Med J 2021;97(1147):306–11.doi: 10.1136/postgradmedj-2020-137542 [DOI] [PubMed] [Google Scholar]
- 4.Cao L, Zhou J, Chen Y, et al. Effects of Body Mass Index, Waist Circumference, Waist-to-Height Ratio and Their Changes on Risks of Dyslipidemia among Chinese Adults: The Guizhou Population Health Cohort Study. Int J Environ Res Public Health 2021;19(1).doi: 10.3390/ijerph19010341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saqlain M, Akhtar Z, Karamat R, et al. Body Mass Index versus Other Adiposity Traits: Best Predictor of Cardiometabolic Risk. Iran J Public Health 2019;48(12):2224–31 [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Ren Y, Wang C, et al. Association of change in waist circumference and dyslipidaemia risk: The rural Chinese cohort study. Diabetes Metab Res Rev 2018;34(1).doi: 10.1002/dmrr.2949 [DOI] [PubMed] [Google Scholar]
- 7.Van Hemelrijck M, Ulmer H, Nagel G, et al. Longitudinal study of body mass index, dyslipidemia, hyperglycemia, and hypertension in 60,000 men and women in Sweden and Austria. PLoS One 2018;13(6):e0197830.doi: 10.1371/journal.pone.0197830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y, Bazzano LA, Juonala M, et al. Long-Term Burden of Increased Body Mass Index from Childhood on Adult Dyslipidemia: The i3C Consortium Study. J Clin Med 2019;8(10).doi: 10.3390/jcm8101725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017;23(7):859–68.doi: 10.1038/nm.4358 [DOI] [PubMed] [Google Scholar]
- 10.Tilves C, Tanaka T, Differding MK, et al. The gut microbiome and regional fat distribution: Findings from the Baltimore Longitudinal Study of Aging. Obesity (Silver Spring) 2023;31(5):1425–35.doi: 10.1002/oby.23717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song B, Zhao K, Zhou S, et al. Association of the gut microbiome with fecal short-chain fatty acids, lipopolysaccharides, and obesity in young Chinese college students. Front Nutr 2023;10:1057759.doi: 10.3389/fnut.2023.1057759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasahara K, Rey FE. The emerging role of gut microbial metabolism on cardiovascular disease. Curr Opin Microbiol 2019;50:64–70.doi: 10.1016/j.mib.2019.09.007 [DOI] [PubMed] [Google Scholar]
- 13.Matey-Hernandez ML, Williams FMK, Potter T, Valdes AM, Spector TD, Menni C. Genetic and microbiome influence on lipid metabolism and dyslipidemia. Physiol Genomics 2018;50(2):117–26.doi: 10.1152/physiolgenomics.00053.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 2010;39(6):1435–40.doi: 10.1093/ije/dyp322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogtmann E, Chen J, Amir A, et al. Comparison of Collection Methods for Fecal Samples in Microbiome Studies. Am J Epidemiol 2017;185(2):115–23.doi: 10.1093/aje/kww177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun S, Wang H, Tsilimigras MC, et al. Does geographical variation confound the relationship between host factors and the human gut microbiota: a population-based study in China. BMJ Open 2020;10(11):e038163.doi: 10.1136/bmjopen-2020-038163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019;37(8):852–7.doi: 10.1038/s41587-019-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13(7):581–3.doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yilmaz P, Parfrey LW, Yarza P, et al. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 2014;42(Database issue):D643–8.doi: 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res 2018;27(7):2015–23.doi: 10.1177/0962280216673085 [DOI] [PubMed] [Google Scholar]
- 21.Jones BL, Nagin DS. A Note on a Stata Plugin for Estimating Group-based Trajectory Models. Sociological Methods & Research 2013;42(4):608–13.doi: 10.1177/0049124113503141 [DOI] [Google Scholar]
- 22.Andruff H, Carraro N, Thompson A, Gaudreau P, Louvet B. Latent Class Growth Modelling: A Tutorial. TQMP 2009;5(1):11–24.doi: 10.20982/tqmp.05.1.p011 [DOI] [Google Scholar]
- 23.Tibshirani R. Regression Shrinkage and Selection Via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological) 1996;58(1):267–88.doi: 10.1111/j.2517-6161.1996.tb02080.x [DOI] [Google Scholar]
- 24.Wang Q, Song X, Du S, et al. Multiple Trajectories of Body Mass Index and Waist Circumference and Their Associations with Hypertension and Blood Pressure in Chinese Adults from 1991 to 2018: A Prospective Study. Nutrients 2023;15(3).doi: 10.3390/nu15030751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You D, Wang D, Wu Y, et al. Associations of genetic risk, BMI trajectories, and the risk of non-small cell lung cancer: a population-based cohort study. BMC Med 2022;20(1):203.doi: 10.1186/s12916-022-02400-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu PW, Yu T, Kukreti S, Strong C. BMI trajectory in adulthood in relation to all-cause and cause-specific mortality: A retrospective cohort study in Taiwan. PLoS One 2023;18(12):e0295919.doi: 10.1371/journal.pone.0295919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng C, Li Y, Ma W, Jia C. Trajectories of waist circumference during young adulthood and incident hypertension: the China Health and Nutrition Survey. J Hum Hypertens 2022;36(8):767–74.doi: 10.1038/s41371-021-00563-y [DOI] [PubMed] [Google Scholar]
- 28.Zheng W, Zhao A, Xue Y, et al. Gender and urban-rural difference in anthropometric indices predicting dyslipidemia in Chinese primary school children: a cross-sectional study. Lipids Health Dis 2016;15:87.doi: 10.1186/s12944-016-0255-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Zhang Y, Wu Y, et al. Obesity and Dyslipidemia in Chinese Adults: A Cross-Sectional Study in Shanghai, China. Nutrients 2022;14(11).doi: 10.3390/nu14112321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu L, Wang W, Sa R, Liu F. Prevalence and Risk Factors of Hypertension, Diabetes, and Dyslipidemia among Adults in Northwest China. Int J Hypertens 2021;2021:5528007.doi: 10.1155/2021/5528007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res 2009;2(3):321–7.doi: 10.1007/s12265-009-9101-1 [DOI] [PubMed] [Google Scholar]
- 32.Parker-Duffen JL, Walsh K. Cardiometabolic effects of adiponectin. Best Pract Res Clin Endocrinol Metab 2014;28(1):81–91.doi: 10.1016/j.beem.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta 2014;1842(3):414–23.doi: 10.1016/j.bbadis.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellström L, Wahrenberg H, Hruska K, Reynisdottir S, Arner P. Mechanisms behind gender differences in circulating leptin levels. J Intern Med 2000;247(4):457–62.doi: 10.1046/j.1365-2796.2000.00678.x [DOI] [PubMed] [Google Scholar]
- 35.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 2003;46(4):459–69.doi: 10.1007/s00125-003-1074-z [DOI] [PubMed] [Google Scholar]
- 36.Pang Y, Zheng Y, Yang N, Zan M, Zhang L, Ding W. Potential novel biomarkers in small intestine for obesity/obesity resistance revealed by multi-omics analysis. Lipids Health Dis 2022;21(1):98.doi: 10.1186/s12944-022-01711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obanda DN, Keenan MJ, Page R, et al. Gut Microbiota Composition and Predicted Microbial Metabolic Pathways of Obesity Prone and Obesity Resistant Outbred Sprague-Dawley CD Rats May Account for Differences in Their Phenotype. Front Nutr 2021;8:746515.doi: 10.3389/fnut.2021.746515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vital M, Karch A, Pieper DH. Colonic Butyrate-Producing Communities in Humans: an Overview Using Omics Data. mSystems 2017;2(6).doi: 10.1128/mSystems.00130-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer-Englar T, Barlow G, Mathur R. Obesity, diabetes, and the gut microbiome: an updated review. Expert Rev Gastroenterol Hepatol 2019;13(1):3–15.doi: 10.1080/17474124.2019.1543023 [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q, Fu Y, Zhang F, et al. Heat-Treated Adzuki Bean Protein Hydrolysates Reduce Obesity in Mice Fed a High-Fat Diet via Remodeling Gut Microbiota and Improving Metabolic Function. Mol Nutr Food Res 2022;66(8):e2100907.doi: 10.1002/mnfr.202100907 [DOI] [PubMed] [Google Scholar]
- 41.Burakova I, Smirnova Y, Gryaznova M, et al. The Effect of Short-Term Consumption of Lactic Acid Bacteria on the Gut Microbiota in Obese People. Nutrients 2022;14(16).doi: 10.3390/nu14163384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao Y, Ji Z, Shen Z, et al. Increase Dietary Fiber Intake Ameliorates Cecal Morphology and Drives Cecal Species-Specific of Short-Chain Fatty Acids in White Pekin Ducks. Front Microbiol 2022;13:853797.doi: 10.3389/fmicb.2022.853797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farkas V, Csitári G, Menyhárt L, et al. Microbiota Composition of Mucosa and Interactions between the Microbes of the Different Gut Segments Could Be a Factor to Modulate the Growth Rate of Broiler Chickens. Animals (Basel) 2022;12(10).doi: 10.3390/ani12101296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhakal S, McCormack L, Dey M. Association of the Gut Microbiota with Weight-Loss Response within a Retail Weight-Management Program. Microorganisms 2020;8(8).doi: 10.3390/microorganisms8081246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velázquez KT, Enos RT, Bader JE, et al. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J Hepatol 2019;11(8):619–37.doi: 10.4254/wjh.v11.i8.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li TT, Tong AJ, Liu YY, et al. Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem Toxicol 2019;131:110558.doi: 10.1016/j.fct.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 47.Lynch JB, Gonzalez EL, Choy K, et al. Gut microbiota Turicibacter strains differentially modify bile acids and host lipids. Nat Commun 2023;14(1):3669.doi: 10.1038/s41467-023-39403-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan HQ, Li TT, Zhu C, Zhang XQ, Wu M, Zhu XF. Parabacteroides chartae sp. nov., an obligately anaerobic species from wastewater of a paper mill. Int J Syst Evol Microbiol 2012;62(Pt 11):2613–7.doi: 10.1099/ijs.0.038000-0 [DOI] [PubMed] [Google Scholar]
- 49.Zhang S, Wu P, Tian Y, et al. Gut Microbiota Serves a Predictable Outcome of Short-Term Low-Carbohydrate Diet (LCD) Intervention for Patients with Obesity. Microbiol Spectr 2021;9(2):e0022321.doi: 10.1128/Spectrum.00223-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palmas V, Pisanu S, Madau V, et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep 2021;11(1):5532.doi: 10.1038/s41598-021-84928-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Q, Li D, He Y, et al. Discrepant gut microbiota markers for the classification of obesity-related metabolic abnormalities. Sci Rep 2019;9(1):13424.doi: 10.1038/s41598-019-49462-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pessoa J, Belew GD, Barroso C, Egas C, Jones JG. The Gut Microbiome Responds Progressively to Fat and/or Sugar-Rich Diets and Is Differentially Modified by Dietary Fat and Sugar. Nutrients 2023;15(9).doi: 10.3390/nu15092097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020;12(1):1802866.doi: 10.1080/19490976.2020.1802866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology 2017;14(8):491–502 [DOI] [PubMed] [Google Scholar]
- 55.Li VL, He Y, Contrepois K, et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022;606(7915):785–90.doi: 10.1038/s41586-022-04828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winther SA, Øllgaard JC, Tofte N, et al. Utility of Plasma Concentration of Trimethylamine N-Oxide in Predicting Cardiovascular and Renal Complications in Individuals With Type 1 Diabetes. Diabetes Care 2019;42(8):1512–20.doi: 10.2337/dc19-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schugar RC, Shih DM, Warrier M, et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep 2017;19(12):2451–61.doi: 10.1016/j.celrep.2017.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol 2018;16(3):171–81.doi: 10.1038/nrmicro.2017.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehghan P, Farhangi MA, Nikniaz L, Nikniaz Z, Asghari-Jafarabadi M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes Rev 2020;21(5):e12993.doi: 10.1111/obr.12993 [DOI] [PubMed] [Google Scholar]
- 60.DiNicolantonio JJ, McCarty M, J OK. Association of moderately elevated trimethylamine N-oxide with cardiovascular risk: is TMAO serving as a marker for hepatic insulin resistance. Open Heart 2019;6(1):e000890.doi: 10.1136/openhrt-2018-000890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saini RK, Keum YS. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - A review. Life Sci 2018;203:255–67.doi: 10.1016/j.lfs.2018.04.049 [DOI] [PubMed] [Google Scholar]
- 62.Shin S, Ajuwon KM. Effects of Diets Differing in Composition of 18-C Fatty Acids on Adipose Tissue Thermogenic Gene Expression in Mice Fed High-Fat Diets. Nutrients 2018;10(2).doi: 10.3390/nu10020256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias CB, Amigó N, Wood LG, Mallol R, Correig X, Garg ML. Improvement of the omega 3 index of healthy subjects does not alter the effects of dietary saturated fats or n-6PUFA on LDL profiles. Metabolism 2017;68:11–9.doi: 10.1016/j.metabol.2016.11.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available, please contact the corresponding authors for details.