Abstract
Ascorbate (vitamin C) is one of the most abundant primary metabolites in plants. Its complex chemistry enables it to function as an antioxidant, as a free radical scavenger, and as a reductant for iron and copper. Ascorbate biosynthesis occurs via the mannose/l-galactose pathway in green plants, and the evidence for this pathway being the major route is reviewed. Ascorbate accumulation is leaves is responsive to light, reflecting various roles in photoprotection. GDP-l-galactose phosphorylase (GGP) is the first dedicated step in the pathway and is important in controlling ascorbate synthesis. Its expression is determined by a combination of transcription and translation. Translation is controlled by an upstream open reading frame (uORF) which blocks translation of the main GGP-coding sequence, possibly in an ascorbate-dependent manner. GGP associates with a PAS-LOV protein, inhibiting its activity, and dissociation is induced by blue light. While low ascorbate mutants are susceptible to oxidative stress, they grow nearly normally. In contrast, mutants lacking ascorbate do not grow unless rescued by supplementation. Further research should investigate possible basal functions of ascorbate in severely deficient plants involving prevention of iron overoxidation in 2-oxoglutarate-dependent dioxygenases and iron mobilization during seed development and germination.
Keywords: GDP-mannose, iron, light response, oxidative stress, 2-oxoglutarate-dependent dioxygenases, upstream open reading frame, vitamin C, vtc mutants
Ascorbate (vitamin C) is essential for plant growth. Recent developments in understanding its functions and the control of its biosynthetic pathway via d -Man/l -Gal are reviewed.
Introduction
Ascorbate (l-ascorbic acid, vitamin C) is well known, but its functions are poorly understood (Fig. 1). As far as is known, it is restricted to eukaryotes, and it is essential for plants and mammals. Humans, other primates, bony fish, and several other groups of animals have lost the ability to synthesize ascorbate and require it in their diets. Intriguingly, this deficiency is always caused by loss of l-gulonolactone oxidase (l-GulLO), the final enzyme in the biosynthetic pathway (Duque et al., 2022). Fungi synthesize d-erythroascorbate, a 5C analogue of ascorbate which has the same chemistry as ascorbate (Loewus, 1999; Baroja-Mazo et al., 2005). Ascorbate is present in millimolar concentrations in plant and mammalian cells, although the latter are often deficient when grown in cell culture (Chepda et al., 2001; Zhitkovich, 2020). In plants, the ascorbate concentration can be exceptionally high in fruit of some species (Fenech et al., 2019), but it is generally highest in leaves and lower in roots. Typically, ascorbate concentration in lab-grown Arabidopsis thaliana leaves is 2–5 µmol g FW–1, similar in concentration to the most abundant primary metabolites sucrose, glucose, serine, and glutamate (Szecowka et al., 2013). Therefore, eukaryotic cells are bathed in a relatively high ascorbate concentration which, considering that Arabidopsis mutants with ~20% of this value and cultured mammalian cells with a few percent of normal ascorbate (Zhitkovich, 2020) are still functional, suggests that ‘excess’ is maintained, possibly as an antioxidant buffer against fluctuating or unexpected conditions.
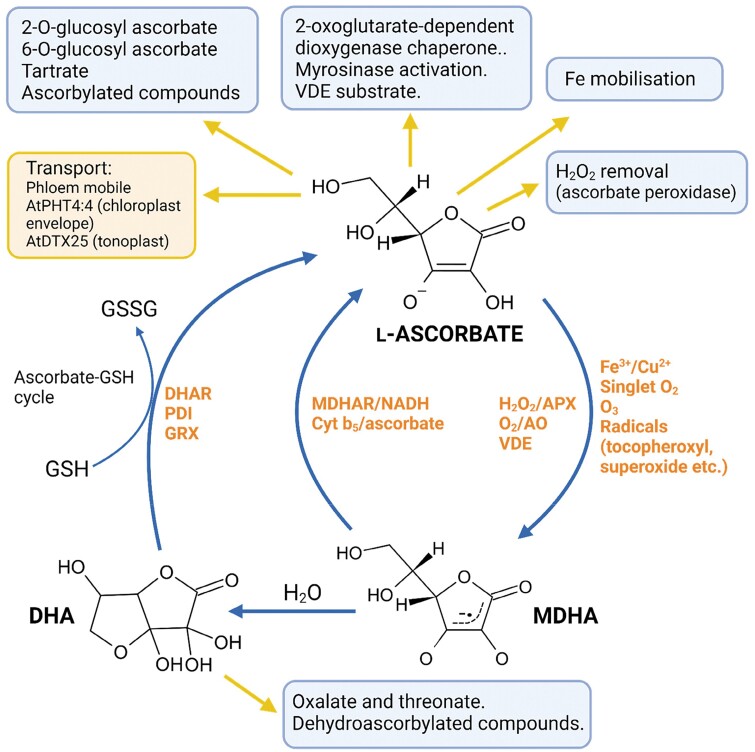
Fig. 1.
An overview of the chemistry and functions of ascorbate in plants. Ascorbic acid is predominantly present as the ascorbate anion (pKa1= 4.25). It acts as a reductant/antioxidant by reducing radicals and other reactive oxygen species by one electron transfer. H2O2 removal requires catalysis by plant-specific ascorbate peroxidases. It is unreactive with oxygen unless catalysed by ascorbate oxidase. Fe3+ and Cu2+ are readily reduced to Fe2+ and Cu+. MDHA, a resonance-stabilized radical, is the product of ascorbate oxidation. Ascorbate is an effective antioxidant because MDHA disproportionates to form DHA (most probably present as a bicyclic hemiketal form rather than the tricarbonyl structure commonly depicted) plus ascorbate. Otherwise it is reduced by MDHAR and by transmembrane reduction via cytochrome b5 which uses ascorbate as electron donor. DHA is reduced by thiols such as glutathione in the ascorbate–glutathione (Foyer–Halliwell–Asada) cycle. Ascorbate is phloem mobile and may be taken up via an unidentified plasma membrane DHA transporter, otherwise only chloroplast envelope and tonoplast ascorbate transporters have been identified. It is further metabolized to glucosides and breakdown products (in a species-dependent manner) while ascorbate and DHA can (dehydro)ascorbylate small molecules and proteins. As an antioxidant in plants, H2O2 removal using APX is the best studied function, while the physiological significance of its reactions with other radicals is less well characterized. Related to Fe, it is a protectant of the large 2-oxoglutarate-dependent dioxygen family and there is emerging evidence for a role in Fe nutrition. It is a substrate for VDE and, specifically for glucosinolate-producing species (such as Arabidopsis), it is involved in the catalytic site of myrosinases (Shikita et al., 1999) which release isothiocyanates from glucosinolates following herbivore damage. Created with BioRender.com. Abbreviations: AO, ascorbate oxidase; APX, ascorbate peroxidase; DHA, dehydroascorbate; DHAR, dehydroascorbate reductase; GRX, glutaredoxin; GSH, glutathione; GSSG, oxidized glutathione; MDHA, monodehydroascorbate radical; MDHAR, monodehydroascorbate reductase; PDI, protein disulfide isomerase; VDE, violaxanthin de-epoxidase.
Ascorbate chemistry and biochemical functions
The reactivity of ascorbate as a single electron (H) donor, with a relatively unreactive resonance-stabilized radical product, monodehydroascorbate (MDHA), is central to its biological function as a donor/chain-breaking antioxidant (Buettner, 1993; Buettner and Schafer, 2004; Smirnoff, 2018; Njus et al., 2020). MDHA disproportionates to form dehydroascorbate (DHA; rate constant 5 × 105 M–1 s–1) or is reduced to ascorbate by pyridine nucleotide-dependent MDHA reductases (MDHARs) first noted in plants by Marrè and Arrigoni (1958) (Tanaka et al., 2021). DHA most probably exists as a bicyclic hemiketal structure (Fig. 1) (Njus et al., 2020) which is readily reduced by thiols such as glutathione (GSH), catalysed by DHA reductases (DHARs) in the ascorbate–glutathione cycle (Foyer and Noctor, 2011). DHA comprises ~10% of the total ascorbate pool in healthy leaves, but its oxidation state varies with subcellular location and tissue type. MDHA radicals can be detected by EPR in plant tissue, particularly under oxidative stress conditions (Buettner and Jurkiewicz, 1993; Hideg et al., 1997). MDHA reacts with radicals such as superoxide and tocopheroxyl radical (rate constant ~108 M–1 s–1) (Njus et al., 2020). Ascorbate itself reacts with and neutralizes the following biologically relevant radicals (Buettner, 1993; Buettner and Schafer, 2004; Njus, 2020): hydroxyl radical (1 × 1010 M–1 s–1); alkoxyl radical (1.6 × 109 M–1 s–1); peroxyl radical (1 × 106 M–1 s–1); thiyl radical (6 × 108 M–1 s–1), superoxide (1 x 105 M-1 s-1) and tocopheroxyl radical (2 × 105 M–1 s–1). The biological importance of these reactions will of course depend on co-location and concentrations. It is very reactive with nitrogen dioxide radical (2 × 107 M–1 s–1), poorly reactive with peroxynitrite, and unreactive with nitric oxide (Buettner and Schafer, 2004). In terms of non-radical oxidants, it has high reactivity with singlet oxygen (3 × 108 M–1 s–1), forming hydrogen peroxide (H2O2) (Kramarenko et al., 2006), and with ozone (4.8 × 107 M–1 s–1), forming singlet oxygen (Kanofsky and Sima, 1995). Reaction with the non-radical oxidant H2O2 is very slow (2–6 M–1 s–1) (Buettner and Schafer, 2004) unless catalysed by a specialized family of ascorbate peroxidases (APXs) generally limited to photosynthetic organisms. APX acts in H2O2 removal additionally to the more widely distributed peroxiredoxins and glutathione peroxidases (Dietz, 2016; Maruta et al., 2016). Ascorbate is not directly oxidized by oxygen, but in plants apoplastic Cu-containing ascorbate oxidases (AOs) catalyse oxidation to water and MDHA. The function of AO is enigmatic, but roles in cell expansion and symbiotic interactions with nitrogen-fixing bacteria and mycorrhizal fungi have been proposed (Balsetrini et al., 2012; Garchery et al., 2013; Chatzopoulo et al., 2020).
The other key property of ascorbate is its ability to form complexes with, and reduce, higher oxidation states of transition metal ions such as Fe3+ and Cu2+. Cu2+ reduction is 80 times faster than that of Fe3+ (Buettner and Schafer, 2004). Trace concentrations of Fe and Cu oxidize ascorbate catalytically in the presence of O2 with production of H2O2 rather than via a redox reaction (Shen et al., 2021). Ascorbate maintains 2-oxoglutarate-dependent dioxygenase (2-ODD) activity by directly reducing active site Fe(IV) and Fe(III) to Fe(II), thereby avoiding irreversible inactivation (Islam et al., 2018). Famously, this is the basis of the ascorbate deficiency disease scurvy, in which loss of activity of prolyl 4-hydroxylase, an 2-ODD in the endoplasmic reticulum (ER), decreased collagen production, leading to impaired joint function and death (Arrigoni and De Tullio, 2002). 2-ODDs have many functions (Kawai et al., 2014) and this aspect is discussed later. Fe3+ reduction by ascorbate also has potential roles in iron mobilization. The ability of ascorbate to form Fe2+ is also the basis of the much-discussed Fenton reaction which generates highly reactive hydroxyl radicals from Fe2+ and H2O2, and is proposed to be the basis of the pro-oxidant effect of ascorbate under some circumstances (Castro et al., 2018). This may or may not be relevant to deleterious effects of high ascorbate on pollen function, and is discussed later.
2-O-Glucosyl ascorbate has been detected in leaves and fruit of diverse species (Toyoda-Ono et al., 2004; Richardson et al., 2020, 2021). Its concentration is generally low (<0.1 µmol g FW–1), but is substantial (4–11 µmol g FW–1) in rose hips and Lycium barbatum (goji berry) fruit. 2-O-Glycosylation (or indeed 2-O phosphate, sulfate, and palmitate esters as used in fish diets) stabilizes ascorbate (and erythroascorbate) against oxidation (Baroja-Mazo et al., 2005; Richardson et al., 2020). Identification of glucosyl transferases involved in 2-O-glucosyl ascorbate synthesis and determining its extent of hydrolysis could be useful in producing biofortified plants with a stable high ascorbate concentration. 6-O-Glucosyl ascorbate occurs in phloem of Cucurbitaceae where it is proposed to aid ascorbate translocation via their symplastic phloem loading mechanism (Hancock et al., 2008). DHA gives rise to oxalate and threonate, with 4-O-oxalyl-l-threonate as an intermediate, and tartrate is a major product from ascorbate in some species (Fig. 1) (DeBolt et al., 2006; Green and Fry, 2005; Truffault et al., 2017).
Ascorbate biosynthesis by plants: the backstory
Initial investigations into ascorbate biosynthesis date from the late 1950s when l-galactonolactone (l-GalL) was identified as the immediate precursor of ascorbate (Mapson and Isherwood, 1956; Isherwood and Mapson, 1962). Frank Loewus used [14C]glucose labelled on C1 or C6 to show that strawberry fruit produces ascorbate labelled on the same carbon atom as the glucose precursor (Loewus et al., 1956; Loewus, 1999). This labelling pattern contrasts with the ‘inversion’ of the carbon skeleton in rat, suggesting that the plant and mammalian pathways differ (Smirnoff et al., 2001; Smirnoff, 2011). The mammalian pathway uses UDP-d-glucuronate and l-gulonolactone as intermediates. The plant enzyme converting l-GalL to ascorbate was identified as mitochondrial l-GalL dehydrogenase (l-GalLDH) (Mapson and Breslow, 1958), again different from the mammalian equivalent l-GulLO. l-GalLDH was not characterized in more detail until nearly 40 years later (Oba et al., 1995; Ostergaard et al., 1997). Lycorine, an alkaloid from species in the Amaryllidaceae, was identified as an inhibitor of l-GalLDH and ascorbate synthesis (De Gara et al., 1994), although its specificity is uncertain. Following establishment of the role of l-GalLDH and the labelling pattern in the very early work, no progress was made in identifying the pathway until Loewus and Saito proposed that d-glucosone and l-sorbosone could be precursors (Loewus et al., 1990; Saito et al., 1990). However, this pathway turned out to be physiologically unimportant (Pallanca and Smirnoff, 1999) in light of the proposed d-Man/l-Gal pathway, which was subsequently named the Smirnoff–Wheeler pathway by Frank Loewus (Fig. 2) (Wheeler et al., 1998). The proposed pathway was based on radiolabelling and enzyme measurements. Meanwhile, Patricia Conklin and Rob Last were isolating ozone-sensitive Arabidopsis ethyl methanesulfonate (EMS) mutants. The first of these, originally named soz1, had decreased ascorbate content (Conklin et al., 1996). The mutant was therefore renamed vtc1, and map-based cloning identified VTC1 as GDP-mannose pyrophosphorylase (GMP), providing the first genetic evidence for the proposed d-Man/l-Gal pathway (Conklin et al., 1997, 1999). More vtc mutants were isolated with a high-throughput leaf squash assay detecting reduction of nitroblue tetrazolium by ascorbate (Conklin et al., 2000). Six mutants from 6300 EMS-mutagenized seedlings were identified (vtc1-2, vtc2-1, 2-2, 2-3, vtc3-1, and vtc4-1). VTC2 and VTC4 were eventually identified as d-Man/l-Gal pathway enzymes (Fig. 2) (Jander et al., 2002; Conklin et al., 2006). Map-based sequencing identified VTC3 as having a predicted N-terminal protein kinase domain and a C-terminal protein phosphatase C domain, a protein unique to green plants and red algae (Conklin et al., 2013). Two EMS mutants (vtc3-1 and vtc3-2) and two insertion mutants have ~35% of wild-type ascorbate and are impaired in high-light- and high-temperature-induced ascorbate accumulation. The protein phosphatase domain is predicted to be truncated in vtc3-2 but, since all the mutants have a similar decrease in ascorbate, they are all likely to be knockouts (Conklin et al., 2013). Knockout of Physcomitrium patens VTC3 decreased ascorbate by 50%, confirming its wider role in influencing ascorbate concentration (Sodeyama et al., 2021). Elucidation of the function of VTC3 in ascorbate synthesis is awaited. Because of the potential for a fully microbial-based ascorbate-manufacturing process to replace the largely chemical Reichstein process, the company Bio-Technical Resources (Wisconsin, USA) had been investigating ascorbate production in the green alga Chlorella and then in a heterotrophic relative Prototheca moriformis, filing a patent application in 1999 proposing a very similar pathway to the d-Man/l-Gal pathway (Berry et al., 1999). Prototheca moriformis secretes ascorbate which is stable in a low pH growth medium. Mutagenesis and selection produced high ascorbate strains which had higher activity of GDP-mannose-3',5'-epimerase (GME), a d-Man/l-Gal pathway enzyme (Running et al., 2002, 2003, 2004).
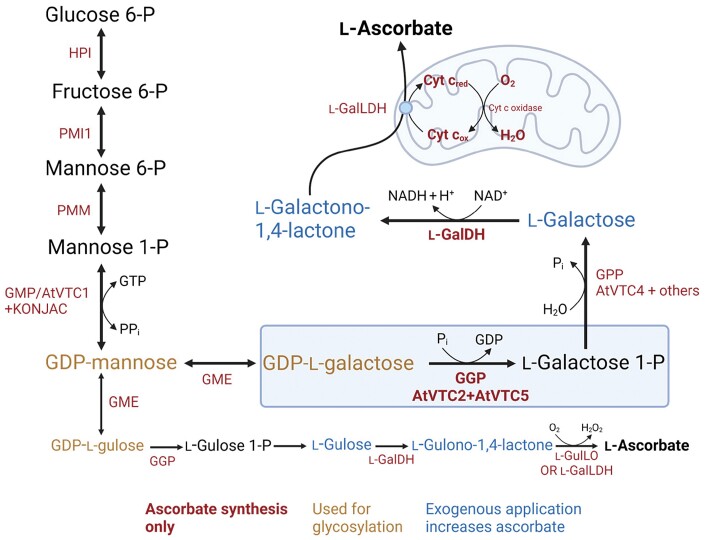
Fig. 2.
Ascorbate biosynthesis in green plants through the d-Man/l-Gal pathway. Colour coding indicates which enzymes are dedicated to ascorbate synthesis, intermediates used for glycosylation (see Fig. 3), and intermediates which are readily taken up and increase ascorbate concentration. GDP-l-galactose phosphorylase (GGP, in blue box) is the likely rate-controlling step in the pathway. Enzymes identified in ascorbate-deficient (vtc) Arabidopsis mutants are indicated. Created with BioRender.com. Abbreviations: l-GalDH, l-galactose dehydrogenase; l-GalLDH, l-galactonolactone dehydrogenase; GME, GDP-mannose-3',5'-epimerase; GGP, GDP-l-galactose phosphorylase; GMP, GDP-mannose pyrophosphorylase; GPP, l-galactose 1-P phosphatase; l-GulLO, l-gulonolactone oxidase; HPI, hexose phosphate isomerase; KONJAC, GDP-mannose pyrophosphorylase-like proteins activating GMP; PMI, phosphomannose isomerase; PMM, phosphomannose mutase.
Ascorbate biosynthesis by the d-mannose/l-galactose pathway
The d-Man/l-Gal pathway (Wheeler et al., 1998) is summarized in Fig. 2, and is reviewed below with emphasis on newer information and gaps in knowledge. The pathway can be divided into two parts. Firstly GDP-Man and GDP-l-Gal are synthesized in a series of reactions that produce GDP-sugars for protein glycosylation and polysaccharide synthesis. Second are steps dedicated to ascorbate synthesis in which GDP-l-Gal provides l-Gal and l-GalL as the unique precursors for ascorbate. It should be noted that other pathways have been proposed, for example using d-galacturonic acid or myo-inositol as precursors (Broad et al., 2020b). Indeed, overexpressing strawberry d-galacturonate reductase significantly increases ascorbate in Arabidopsis (Agius et al., 2003) suggesting that, at least in transgenic plants, an animal-like pathway can operate.
Enzymes involved in GDP-mannose synthesis: phosphomannose isomerase, phosphomannose mutase, and GDP-mannose pyrophosphorylase
A nexus of enzymes synthesizes a range of GDP-sugars [GDP-Man, GDP-l-Fuc, GDP-l-Gal, and GDP-l-gulose (l-Gul)] involved in protein glycosylation and glycan and sphingolipid synthesis in the ER and Golgi apparatus (Baldwin et al., 2001; Sharples and Fry, 2007; Sechet et al., 2018; Figueroa et al., 2021; Jing et al., 2021) (Fig. 3). GDP-l-Gal and GDP-l-Gul are also used for ascorbate synthesis, so control over the partitioning of GDP-Man between these functions is required, particularly in actively expanding cells. It is interesting to note that ascorbate itself is also required for maintaining the activity of ER-localized peptidyl prolyl/lysyl hydroxylases, 2-ODDs which are involved in hydroxyproline/lysine synthesis in hydroxyproline-rich glycoproteins (see section on ascorbate functions). There are suggestions of a role in oxidative cross-linking/folding of these proteins as well. Therefore, speculatively, the otherwise puzzling routing of ascorbate biosynthesis through GDP-sugars in plants could enable co-ordination of ascorbate-dependent protein hydroxylation, mannosylation, and folding in the ER.
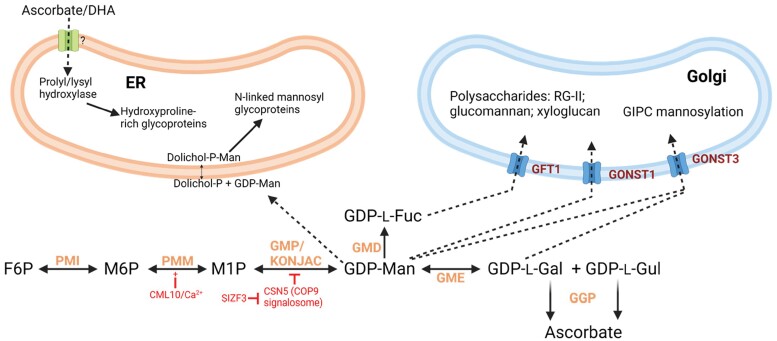
Fig. 3.
The dual role of GDP-sugars in glycosylation and ascorbate synthesis. The processing of glycoproteins destined for secretion (including structural proteins such as extensin and peptide hormones) occurs in the ER. Mannose is delivered to the ER via dolichol-P and used for protein N-glycoslyation. Additionally, the ER is the site of proline and lysine hydroxylation of glycoproteins by ascorbate-dependent 2-oxoglutarate-dependent dioxygenases (see Fig. 6). Glycan and glycosylinositol phosphoceramide synthesis in the Golgi uses GDP-sugars imported by various transporters. Considering the need to maintain a balance between the use of GDP-sugars for glycoproteins and polysaccharides required for cell wall production during growth and ascorbate synthesis, there is evidence that PMM is activated by interaction with a calmodulin-like protein (CML10) and GMP is subject to proteolytic breakdown by interaction with CSN5, which is antagonized by interaction of SIZF1 with CSN5. GMP is activated by two KONJAC proteins, which have a GMP-like sequence but no enzyme activity themselves. GDP-sugar availability will also be controlled by how much is used for ascorbate synthesis and, accordingly, GGP activity can act as a valve between ascorbate synthesis and glycoprotein/glycan synthesis. Created with BioRender.com. Abbreviations: DHA, dehydroascorbate; ER, endoplasmic reticulum; F6P, fructose 6-P; GDP-l-Fuc, GDP-l-fucose; GDP-l-Gal, GDP-l-galactose; GDP-l-Gul, GDP-l-gulose; GDP-Man, GDP-mannose; GIPC, glycosylinositol phosphoceramide; GMD, GDP-mannose-3,6-dehydratase=MUR1; GME, GDP-mannose-3',5'-epimerase; GMP, GDP-mannose pyrophosphorylase; GPP, l-galactose 1-P phosphatase; PMI, phosphomannose isomerase; M6P, mannose 6-P; M1P, mannose 1-P; PMM, phosphomannose mutase; RG-II, rhamnogalacturonan II.
Older observations suggested that GDP-Man is not synthesized via mannose 1/6-P because of the assumption that phosphomannose isomerase (PMI) is missing in plants. An extended VTC2 cycle involving GDP-Glc was proposed as a workaround for lack of PMI (Wolucka and Van Montagu, 2007). However, radiolabelling shows that GDP-Man is formed via PMI, rather than epimerization of GDP-Glc (Sharples and Fry, 2007), and two genes encoding PMIs were identified in Arabidopsis (Maruta et al., 2008). A knockdown mutant of one (PMI1) decreases ascorbate (Maruta et al., 2008), confirming its role in GDP-Man and ascorbate synthesis. Recently it was suggested that PMI1 is a moonlighting protein, interacting with the inwardly rectifying K+ channel KAT1, increasing stomatal aperture (Gonzalez-Garcia et al., 2023).
Mannose 6-phosphate (Man 6-P) produced by PMI is converted to Man 1-P by phosphomannose mutase (PMM) (Qian et al., 2007; Hoeberichts et al., 2008; Badejo et al., 2009). PMM is activated by Ca2+-dependent interaction with a calmodulin-like protein (CML10). One amiR-cml10 line has decreased ascorbate but two other amiR-cml10 lines had a greater decrease in ascorbate after H2O2 treatment (Cho et al., 2016), indicating a bottleneck if ascorbate demand is increased. It would be interesting to know if Ca-dependent PMM activation has a role in controlling Man 1-P supply and avoiding a bottleneck in GDP-Man production if demand increases. However, a kinetic model of the pathway suggests that PMI and PMM are not strong control points (Fenech et al., 2021).
GDP-mannose pyrophosphorylase (GMP) catalyses the reversible formation of GDP-Man from Man 1-P (Fig. 3). Its role in ascorbate synthesis was first demonstrated by identification of VTC1 (=CYT1, SOZ1) as a GMP in Arabidopsis (Conklin et al., 1999) and by antisense suppression in potato (Keller et al., 1999). vtc1-1 and vtc1-2 have a Pro22Ser substitution, ~30% ascorbate, and ~50% residual enzyme activity (Conklin et al., 1999). An Arabidopsis mutant with a truncated VTC1 protein (cyt1) is embryo lethal (Nickle and Meinke, 1998; Lukowitz et al., 2001). There are two other genes in Arabidopsis (At3g55590 and At4g30570) with very high sequence similarity to VTC1. Inspection of transcriptome data shows that they have very low expression compared with VTC1, explaining the embryo lethality of VTC1 knockout (Lukowitz et al., 2001). Nothing is known about the function of these homologues. Rice has three GMPs which may contribute differentially to root and leaf activity (Qin et al., 2016), and OsVTC1-1 RNAi lines have altered cell wall mannose composition as well as lower ascorbate (Lamanchai et al., 2022). Additionally, there are two proteins in Arabidopsis, KONJAC1 and 2 (KJC1/KJC2), with ~31% sequence similarity to VTC1. They have two extra amino acids in the conserved pyrophosphorylase domain, and the recombinant His-tagged proteins lack NDP-sugar pyrophosphorylase activity. kjc1 and kjc2 mutants have decreased ascorbate, and kjc1 has decreased content of GDP-Man as well as cell wall mannose. The double mutant has severe growth defects and does not flower (Sawake et al., 2015). Interestingly KJCs interact with VTC1 in pull-down assays and increase the GMP activity of VTC1 by 100% (KJC1) and 50% (KJC2). A crystal structure of VTC1 shows that it dimerizes and dodecamerizes, and will provide a useful basis for future understanding of how KJC binds and activates VTC1 (Zhang et al., 2022).
Because GDP-Man is needed for protein N-glycosylation and synthesis of Man- and l-Gal-containing polysaccharides and also Man, Fuc, and cellulose in cell walls (Lukowitz et al., 2001), the vtc1 mutants are affected in numerous functions additionally to ascorbate synthesis. Several GDP-sugar transporters are located in the Golgi membrane (Baldwin et al., 2001; Rautengarten et al., 2016; Sechet et al., 2018; Jing et al., 2021). Perturbation of glycosphingolipid synthesis in the Golgi apparatus in a mutant of the GDP-sugar transporter GONST1 increases salicylic acid (SA) and activates a constitutive hypersensitive cell death response reminiscent of other lesion mimic mutants (Mortimer et al., 2013). Therefore GDP-Man shortage in vtc1 mutants clearly impacts processes other than ascorbate biosynthesis. vtc1 is hypersensitive to ammonia (Qin et al., 2008; Barth et al., 2010; Zhang et al., 2021). This is not the case for other vtc mutants, suggesting that it is specific to GDP-Man, and the defect is suggested to be related to altered protein mannosylation or NO (Qin et al., 2008; Barth et al., 2010). Further investigation suggests that vtc1 has more NO and ammonium-induced S-nitrosoglutathione reductase (GSNOR). Since GSNOR is required for ammonium tolerance, overexpression in vtc1 improved its ammonium tolerance (Zhang et al., 2021). Therefore, the use of vtc1 alone to infer functions of ascorbate is unreliable. Given that VTC1 is at a crossroads where hexoses are shared between ascorbate and cell wall/glycosylation, it is interesting that several controls over its activity have been uncovered. Arabidopsis VTC1 interacts with CSN5B, which is part of the COP9 signalosome complex. This enables proteolysis via the 26S proteasome in the dark. Seedlings of a csn5b mutant had somewhat higher ascorbate when grown under a day–night cycle and a markedly decreased loss of ascorbate in extended dark (48 h). These results indicate that VTC1 activity is controlled via proteolysis (Wang et al., 2013; Ma et al., 2022). Further investigation showed that a D27E mutation in CSN5B stops interaction with VTC1 and increases ascorbate when expressed in Arabidopsis (Li et al., 2016). A C2H2 zinc finger protein (SIZF3) from tomato increases ascorbate when overexpressed in tomato and Arabidopsis, and appears to work by binding CSN5B, preventing it from interacting with VTC1 (based on yeast two-hybrid and transient expression studies) (Y. Li et al., 2018). Overall, although VTC1 is not dedicated to ascorbate synthesis, the control of its expression, protein turnover, and activity revealed by these studies suggest the importance of balancing GDP-Man production for use in polysaccharides required for growth, protein mannosylation, and ascorbate synthesis.
GDP-mannose 3',5'-epimerase
GDP-mannose 3',5'-epimerase (GME) is present in all green plants/algae and beyond (Beerens et al., 2021), and is encoded by one gene in Arabidopsis and either one or two genes in many other species (Watanabe et al., 2006; Mounet-Gilbert et al., 2016; Qi et al., 2017; Tao et al., 2018). GME is a cytosolic enzyme (Qi et al., 2017; Fenech et al., 2021) and reversibly converts GDP-Man into a mixture of GDP-l-Gal and a smaller amount of GDP-l-Gul (Wolucka et al., 2001; Wolucka and Van Montagu, 2003, 2007; Major et al., 2005; Watanabe et al., 2006). Its crystal structure and mechanism have been determined (Major et al., 2005). The products of the enzyme are multifunctional: GDP-l-Gal is transported into the Golgi via the GDP-sugar transporter GONST3/GGLT1 (Sechet et al., 2018) and used for synthesis of the cell wall polysaccharide rhamnogalacturonan II (RG-II) (Gilbert et al., 2009; Voxeur et al., 2011; Mounet-Gilbert et al., 2016). In tomato, which has two GME isoforms, RNAi knockdown showed that both contribute to ascorbate synthesis. In contrast, RNAi suppression of one of these (SlGME1) impacted pollen development and pollination, resulting in small fruit with few seeds (Mounet-Gilbert et al., 2016). Borate supplementation of the tomato GME RNAi lines restores growth. Similarly, in Arabidopsis, two T-DNA insertion mutants in its single GME gene have 20–50% of wild-type ascorbate. They show reduced fertility due to impaired pollen development and germination, and this phenotype is not reversed by ascorbate or borate supplementation. Vegetative growth is greatly decreased in the mutants and is rescued by borate but not ascorbate supplementation (Qi et al., 2017). GME mutants in tomato have fewer l-Gal residues in RG-II, resulting in less cross-linking in the wall and therefore impaired growth. Borate mediates cross-linking, explaining its ability to rescue growth (O’Neill et al., 2004; Voxeur et al., 2011). Squash (Cucurbita pepo) root tips have a very large decrease in growth rate and ascorbate concentration when transferred to boron-free medium (Lukaszewski and Blevins, 1996), suggesting an additional direct link between boron and ascorbate; however, this result has no simple explanation.
GDP-l-galactose phosphorylase
Production of l-Gal-1 from GDP-[14C]Man was detected in pea seedling extracts, and this activity was stimulated by phosphate (Wheeler, 2000). Subsequently GDP-l-Gal phosphorylase (GGP) was purified. Based on this work and the identification of VTC2 by map-based cloning (Jander et al., 2002), Dowdle et al. (2007) showed that GGP is encoded by two paralogous genes in Arabidopsis (VTC2/AtGGP1 and VTC5/AtGGP2). The enzyme was also characterized by Linster et al. (2007, 2008) and Laing et al. (2007). Green plants generally have two (sometimes more) paralogues, although chlorophytes have a single copy (Tao et al., 2020). In Arabidopsis, VTC2/AtGGP1 has an ~10 times higher transcript level than VTC5/AtGGP2. The corresponding knockout mutants have 20% and 80% of normal leaf ascorbate, respectively (Dowdle et al., 2007), indicating that the paralogues somehow produce a fixed proportion of ascorbate and cannot compensate each other. Double vtc2 vtc5 mutants can only grow beyond seed germination if supplemented by ascorbate, l-Gal, or l-GalL (Dowdle et al., 2007; Lim et al., 2016), indicating that GGP is specific for ascorbate synthesis in Arabidopsis and that other pathways are not quantitatively significant in germinating seedlings. There is strong evidence that GGP is often the rate-controlling enzyme in ascorbate biosynthesis (Fig. 4), and this is discussed in more detail in the section on pathway control.
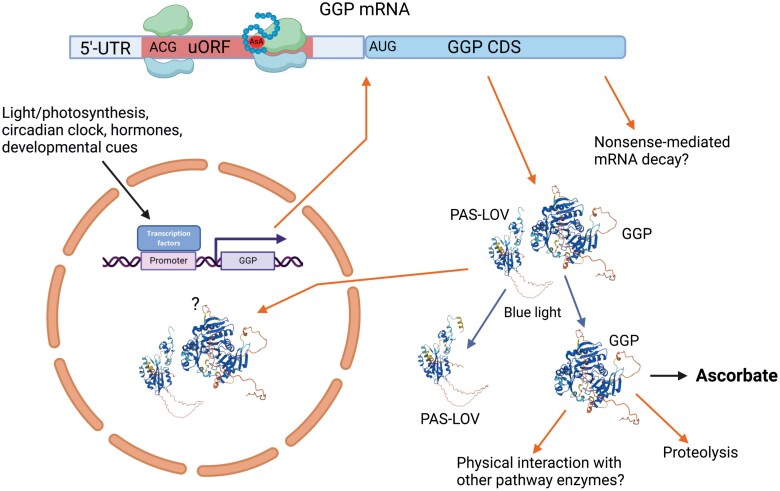
Fig. 4.
GDP-l-galactose phosphorylase (GGP) is generally the most rate-controlling step in ascorbate synthesis by the d-Man/l-Gal pathway and its activity is controlled at transcriptional, translational, and post-translational levels. Expression of GGP genes is responsive to many environmental factors (particularly light), and an increasing number of transcription factors are being identified. The 5'-UTR of the transcript has an upstream ORF (uORF) with a non-canonical ACG initiation codon. Ribosomes binding to the uORF stall and decrease translation of the GGP protein. It is proposed that the uORF produces a peptide which, in the presence of ascorbate, stalls the ribosomes, providing a mechanism for feedback control of ascorbate synthesis. The role of the peptide and whether it interacts with ascorbate are currently unknown. Transcripts harbouring uORFs are susceptible to degradation by nonsense-mediated decay, so GGP transcript levels may be influenced by this process as well as the rate of transcription. Once translated, GGP interacts with a PAS-LOV protein (PLP), with similarity to blue light-sensing phototropins. The complex is enzymatically inactive and is dissociated by blue light. This mechanism provides an additional light control over GGP. GGP and PLP are also located in the nucleus, but the significance of this is unknown. GGP and other d-Man/l-Gal pathway enzymes physically interact, again with unknown consequences. GGP also has predicted phosphorylation sites whose function has not been assessed. Created with BioRender.com. The GGP (AtVTC2) and PAS-LOV (AtPLP) protein structures were predicted by AlphaFold (https://alphafold.ebi.ac.uk/).
GGP has a dual cytosolic and nuclear localization (Muller-Moulé, 2008; Fenech et al., 2021). It is a member of the histidine triad (HIT) family of nucleoside monophosphate hydrolases/transferases but lacks one of the conserved histidines, thus favouring reaction with phosphate rather than hydrolysis (Linster et al., 2007). GGP is equally active with GDP-l-Gal and GDP-Glc, with Km values of 4–10 µM (Linster et al., 2008). It also shows guanylyltransferase activity, transferring GMP from GDP-l-Gal to hexose 1-P to form GDP-hexose and l-galactose 1-phosphate (l-Gal 1-P) (Laing et al., 2007). However, this activity is very small (<10%) compared with phosphorolysis (Linster et al., 2008). GGP is reversible, but GDP-l-Gal formation is likely to be negligible in vivo: the Km for l-Gal 1-P is 45 mM and the kcat/Km is 104 times smaller than for the forward direction (Linster et al., 2008). The Km for phosphate is 1–2 mM (Linster et al., 2008), while cytosolic concentrations are in the range 1–10 mM and do not drop until severe starvation (Versaw and Garcia, 2017). It is therefore conceivable that phosphate limits GGP activity in some cases. Arabidopsis mutants of an abscisic acid (ABA)-inducible PTP-like nucleotidase, which can hydrolyse GDP, (d)GMP, and (d)IMP, have ~30% of wild-type ascorbate in seedlings, apparently rescued by phosphate supplementation (Zhang et al., 2020). It was proposed that the nucleotidase activity increases phosphate availability. While this conclusion is somewhat plausible, VTC1, VTC2, and VTC5 transcripts are lower in the mutant, perhaps suggesting that the nucleotidase mutation has a more pervasive effect on gene expression. There is one other enzyme with similar catalytic properties to GGP (At5g18200 in Arabidopsis) with a two histidine HIT domain but otherwise low sequence similarity. It catalyses phosphorolysis of ADP-Glc and, although its crystal structure has been determined, its function is unknown (McCoy et al., 2006).
GGP is generally green plant specific (but not obviously present in rhodophytes), although similar proteins can be found in metazoans and scattered protists (Wheeler et al., 2015). The Caenorhabditis elegans enzyme is active against GDP-Glc and has a much lower kcat with GDP-Man and GDP-l-Gal. Knockout in C. elegans causes GDP-Glc accumulation and it is proposed to function in recycling GDP-Glc produced as a side reaction of GDP-mannose pyrophosphorylase (Adler et al., 2011). Synthesis or function of GDP-Glc in plants is not mentioned in a recent review on nucleotide sugars (Figueroa et al., 2021), and radiolabelling suggests that it is not a major nucleotide sugar (Sharples and Fry, 2007). However, it is possible that GGP will remove GDP-Glc (formed accidentally or otherwise), while preserving GDP, as in C. elegans. Measurement of GDP-Glc in GGP mutants would be informative and one can speculate that an enzyme more widely used for nucleotide-sugar salvage has been co-opted for ascorbate biosynthesis in plants.
l-Galactose 1-P phosphatase
l-Gal 1-P is hydrolysed to l-Gal by l-Gal 1-P phosphatase (GPP). An enzyme with this activity was purified and characterized from kiwifruit and Arabidopsis (Laing et al., 2004a). LC-MS analysis of a tryptic digest identified a myo-inositol 1-P (IMP)-type protein. The recombinant enzymes strongly prefers l-Gal 1-P, myo-inositol 1-P, and myo-inositol 3-P over other phosphates, with a Km of ~0.02–0.10 mM (Laing et al., 2004a; Torabinejad et al., 2009; Saxena et al., 2013; Nourbakhsh et al., 2014). The Arabidopsis enzyme encoded by VTC4 was shown to be the same as the enzyme purified by Laing et al. (2004a) (Conklin et al., 2006,). Identification of further vtc4 T-DNA knockout lines showed that plants retained ~30% of normal ascorbate, which suggests that other phosphatases can hydrolyse l-Gal 1-P (Conklin et al., 2006; Torabinejad et al., 2009; Saxena et al., 2013). In Arabidopsis, there are two other IMP-like (IMPL) enzymes also sensitive to Ca2+ and Li+ inhibition. One of these (IMPL1) has a preference for IMP and d-Gal 1-P, while l-Gal 1-P supports 7% of activity (Nourbakhsh et al., 2014). The ascorbate concentration of an impl1 mutant is not known but, given its predicted chloroplast location and low activity with l-Gal 1-P, it is unlikely to contribute significantly to ascorbate synthesis.
Knockout of VTC4 (vtc4-2, 4-3, and 4-4) decreases ascorbate to ~30% of wild-type levels but also decreases myo-inositol to ~70% of wild-type concentrations. Seed germination is delayed, and germination and seedling root growth are more sensitive to cold (Torabinejad et al., 2009). Sensitivity is reversed by complementation with a chickpea VTC4 (Saxena et al., 2013). Because myo-inositol and related compounds are involved in signalling/stress responses (Chaouch and Noctor, 2010), interpretation of the function of ascorbate using vtc4 mutants is not advised.
l-Galactose dehydrogenase
The key that enabled the ascorbate biosynthesis pathway to be unlocked was the discovery that l-Gal fed to plant tissues causes a rapid and large increase in ascorbate (Wheeler et al., 1998) to the same extent as observed with l-GalL some decades earlier (Isherwood et al., 1954). Therefore, an enzyme able to oxidize l-Gal to l-GalL was postulated and detected as NAD-dependent l-Gal dehydrogenase (l-GalDH) activity (Wheeler et al., 1998). Purification from pea and N-terminal sequencing identified a potential Arabidopsis gene. The recombinant enzyme had l-GalLDH activity with a relatively high affinity for l-Gal (0.1–0.4 mM) and lower affinity for l-Gul (4 mM) and l-Fuc (56 mM) (Gatzek et al., 2002). Similar properties were found for spinach and kiwifruit enzymes, with no activity supported by other sugars (Laing et al., 2004b; Mieda et al., 2004). Reversible competitive inhibition by ascorbate (Ki 0.1 mM) (Mieda et al., 2004) or slow inactivation of kiwifruit enzyme, protected by pre-addition of NAD (Laing et al., 2004b), have been reported, but no significant inhibition occurs if the pH is controlled (Vargas et al., 2022). l-GalDH is predicted to be cytosolic, confirmed by transient expression of a green fluorescent protein (GFP) fusion (Fenech et al., 2021). The crystal structure has been determined and is typical of the aldehyde-keto reductase (AKR) family (Vargas et al., 2022). The lack of a normally conserved active site arginine explains the preference for NAD over NADP. Many of the AKR family catalyse reduction (favoured by the reduced state of NADPH), while l-GalDH should operate in the oxidative direction for ascorbate synthesis, taking advantage of the predominance of NAD+ over NADH in the cytosol. Overexpression of l-GalDH in tobacco has no effect on ascorbate, while antisense suppression in Arabidopsis (to 30–50% of wild-type activity) only causes a small decrease in ascorbate, evident only in high-light conditions, which causes increased ascorbate in wild-type plants (Gatzek et al., 2002). These results indicate that l-GalDH exerts little control over ascorbate synthesis (Fenech et al., 2021). Complete knockout of l-GalDH in a T-DNA mutant results in growth arrest after germination of homozygous seedlings, and growth is fully restored by ascorbate supplementation (Fenech et al., 2021). This shows that only one enzyme in Arabidopsis can catalyse the reaction and that it is likely that its only function is ascorbate biosynthesis.
l-Galactono-1,4-lactone dehydrogenase: ascorbate synthesis and mitochondrial Complex 1 assembly
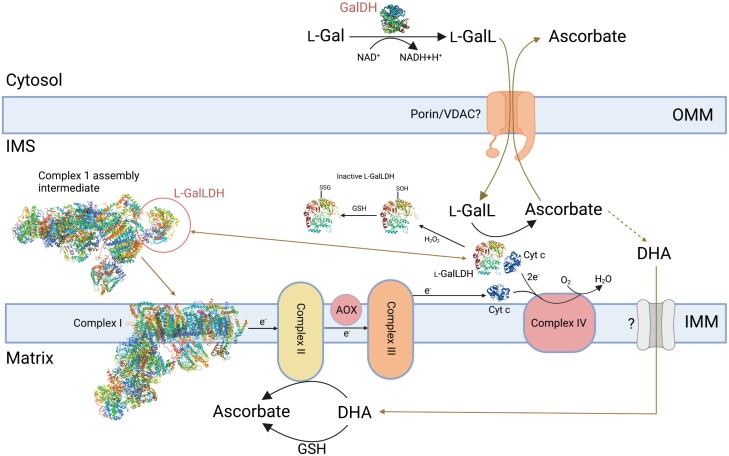
l-Galactono-1,4-lactone dehydrogenase (l-GalLDH) from mitochondria was the first plant ascorbate biosynthesis enzyme to be identified (Mapson and Breslow, 1958). Further work confirmed its location in the inner mitochondrial membrane associated with respiratory Complex 1 (Fig. 5) (Siendones et al., 1999; Bartoli et al., 2000, 2003; Millar et al., 2003). Isolated mitochondria convert l-GalL to ascorbate, although excess interferes with electron transport and phosphorylation along with increasing reactive oxygen species (ROS) production (Mazorra Morales et al., 2022). A knockout mutant reveals that l-GalDH is also required for correct assembly of Complex 1 (Pineau et al., 2008; Schertl et al., 2012; Schimmeyer et al., 2016), and a recent cryo-EM structure shows its location in a Complex 1 assembly intermediate (Soufari et al., 2020). l-GalLDH mutants are therefore compromised in Complex 1 function in addition to ascorbate deficiency.
Fig. 5.
The final step of ascorbate synthesis is localized in mitochondria. l-GalL produced in the cytosol by l-GalDH enters the intermembrane space, presumably via carriers on the outer mitochondrial membrane (e.g. porins/VDACs). l-GalL is oxidized to produce ascorbate by l-GalLDH which transfers electrons to loosely associated Cyt c via an FAD cofactor. The reduced Cyt c transfers electrons to oxygen with production of water in Complex IV. Ascorbate leaves the mitochondrion, possibly thorough porin. Ascorbate enters the mitochondrial matrix as DHA. Mitochondria take up DHA in preference to ascorbate, but the IMM transporter is not identified. DHA is reduced in the matrix by GSH or by Complex II. GalLDH is oxidized by H2O2in vitro on a specific cysteine, resulting in an inactive sulfenic acid form which can be glutathionylated. GalLDH activity increases in the light, but it is not known if oxidation has a role in vivo. Remarkably, l-GalDH is also an essential component of Complex 1 assembly and is not present in the mature complex. The mitochondrial electron transport chain is not shown in detail. The Complex 1 cryo-EM and l-GalDH crystal structure are from the Protein Data Bank (https://www.rcsb.org) (accession nos 7A24, 7A23, and 7SMI). The l-GalLDH structure was predicted by AlphaFold (https://alphafold.ebi.ac.uk/). Created with BioRender.com. Abbreviations: AOX, alternative oxidase; DHA, dehydroascorbate; GSH, glutathione; IMM, inner mitochondrial membrane; IMS, intermembrane space; l-Gal(DH), l-galactose (dehydrogenase); l-GalL(DH), l-galactonolactone (dehydrogenase); OMM, outer mitochondrial membrane; VDAC, voltage-dependent anion channel.
l-GalLDH is a member of the vanillyl-alcohol oxidase (VAO) family, as are the animal (l-GulLO) and fungal (d-arabinonolactone oxidase) enzymes. However, the key differences of the plant enzyme are that it is a dehydrogenase rather than a H2O2-producing oxidase, the FAD cofactor is not covalently linked to the enzyme (a conserved His missing), and it is mitochondrial (Leferink et al., 2008). l-GalLDH has a strong preference for l-GalL (Km=0.17 mM, kcat=134 s–1) over l-GulL (Km=13.1 mM, kcat=4.0 s–1) and other aldonolactones (Mapson and Breslow, 1958; Leferink et al., 2008). Transfer of two electrons from l-GalL reduces the associated FAD to a hydroquinone which then transfers electrons in two steps (with a semiquinone radical intermediate) to cytochrome c (Cyt c) (Leferink et al., 2008). A transient low affinity complex between Cyt c and l-GalLDH enables electron transfer from reduced FAD (Hervas et al., 2013). Leferink et al. (2008) have provided important information on Arabidopsis l-GalLDH function by using site-directed mutants. They show that mutation of a conserved valine near the flavin-binding site increases its reactivity with oxygen, explaining why the plant enzymes are dehydrogenases in contrast to other VAO enzymes (Leferink et al., 2009a). A conserved Glu386 is required for substrate binding and preference for l-GalL over l-GulL, while a neighbouring Arg388 stabilizes the negative charge of the reduced flavin (Leferink et al., 2009b).
The location of l-GalLDH in mitochondria and the ability of Cyt c to act as the electron acceptor invite the possibility that ascorbate synthesis and respiration interact with each other. Considering a typical Arabidopsis leaf respiration rate of 12 µmol O2 g–1 h–1 (O’Leary et al., 2017) and measured ascorbate turnover rate (0.1 µmol g–1 h–1 in a leaf with 5 µmol g–1 at steady state; see section on control), ascorbate synthesis is unlikely to exceed a few percent (~1%) of the respiration rate, suggesting that it is not quantitatively significant and unlikely to impact respiratory flux in vivo. However, addition of l-GalL to intact tissue or isolated mitochondria increases l-GalLDH activity greatly and, even in this case, the leaf respiration rate did not differ between the wild type and vtc2 (10–20% of wild-type ascorbate) with or without l-GalL feeding (Senn et al., 2016). Experiments with isolated mitochondria show an interaction with the mitochondrial electron transport chain (mETC) (Siendones et al., 1999; Bartoli et al., 2000; Millar et al., 2003; Matos et al., 2022; Mazorra Morales et al., 2022). Once ascorbate is synthesized in the intermembrane space, it must move to the cytoplasm and the mitochondrial matrix (Fig. 5). Presumably, l-GalL and ascorbate could move via porins/voltage-dependent anion channels (VDACs) on the outer mitochondrial membrane. Involvement of a specific transporter on the inner mitochondrial membrane has not been demonstrated but, similarly to mammals, mitochondria isolated from BY-2 cells take up DHA and not ascorbate into the matrix (Szarka et al., 2004). While enzymes of the ascorbate–GSH cycle occur in mitochondria (Jimenez et al., 1997), there is also evidence that DHA is reduced via electron transport from Complex II (Szarka et al., 2007).
It is striking that the increase in ascorbate caused by l-Gal(L) feeding is light stimulated and inhibited by photosynthetic electron transport inhibitors (Smirnoff, 2000; Bartoli et al., 2006; Yabuta et al., 2008). Also, Arabidopsis acclimated to increased light over 2 weeks had greater l-GalLDH and Cyt c oxidase activity (Bartoli et al., 2006). Furthermore, l-GalLDH activity could be influenced by the redox state of Cyt c and mETC activity. However, in intact mitochondria, provision of Complex 1 substrates stimulates ascorbate synthesis from l-GalL and is reversed (inhibited) by rotenone (Millar et al., 2003), suggesting that it is required for maximum ascorbate synthesis rather than being competitive. However, antimycin A slightly stimulates ascorbate production (Bartoli et al., 2000). These observations point to a complex picture of the control of l-GalLDH activity. Since the light response is quick, a possible explanation could be a post-translational modification. l-GalLDH is inactivated by H2O2in vitro, and removal of a critical cysteine near the active site by site-directed mutagenesis prevents inactivation. This Cys is sequentially oxidized to sulfenic, sulfinic, and sulfonic forms. The sulfenic form can be S-glutathionylated, preventing further oxidation but also switching off enzyme activity (Leferink et al., 2009c). This work was carried out in vitro, so the physiological significance is not established. However, Leferink et al. (2009c) point out that this could explain light-dependent l-GalL oxidation. We need to establish if the enzyme is more reduced in high light and oxidized/glutathionylated in the dark. The extent to which this step exerts control on ascorbate synthesis is also unclear. A kinetic model, not specifically including light control at this step, suggests that most control resides with GGP (Fenech et al., 2021), but both the mechanism and role in control should be investigated further.
Some GDP-l-Gul is formed by GME and could be a source of ascorbate (Fig. 2). GGP, l-GalDH, and GalLDH can use l-Gul-containing substrates, although less effectively than l-Gal (Gatzek et al., 2002; Linster et al., 2007; Leferink et al., 2008). Another possibility is oxidation of l-GulL by plant l-GulLO-like enzymes. Arabidopsis has seven of these, and some could be functional (Maruta et al., 2010; Aboobucker et al., 2017; Murgia et al., 2023). Overexpression of Arabidopsis l-GulLO2, 3, and 5 (as well as rat l-GulLO) in tobacco BY-2 cells increases the rate of l-GulL conversion to ascorbate (Maruta et al., 2010), although transient expression of GulLO5 in Nicotiana benthamiana had a very small effect on l-GulL conversion to ascorbate (Aboobucker et al., 2017).
Diversity and evolution of ascorbate biosynthesis
The d-Man/l-Gal pathway for ascorbate biosynthesis found in plants is entirely distinct from the biosynthetic pathway found in animals, with no shared enzymes. The two pathways result in a different orientation of the carbon chain in the ascorbate molecule. In animals, the carbon chain from hexose sugars is inverted (carbon 1 of glucose becomes carbon 6 of ascorbate), whereas in plants the carbon chain is not inverted (carbon 1 of glucose is retained as carbon 1 of ascorbate). A survey of eukaryote genomes suggests that the core d-Man/l-Gal pathway via GGP and l-GalLDH is found only in land plants and green algae (Viridiplantae) (Wheeler et al., 2015). Outside of the vascular plants, experimental evidence to support this pathway has been demonstrated in bryophytes (Marchantia and Physcomitrium) (Sodeyama et al., 2021; Ishida et al., 2023), chlorophytes (Chlamydomonas) (Urzica et al., 2012a; Vidal-Meireles et al., 2017), and trebouxiophytes (Chlorella and Prototheca) (Renstrom et al., 1983; Running et al., 2003).
Functional studies of ascorbate biosynthesis in these lineages have demonstrated that GGP plays a conserved role as the key controlling step in the d-Man/l-Gal pathway. However, these studies have highlighted important differences in the control mechanisms, particularly in response to light. The moss Physcomitrium patens contains three paralogues of GGP (VTC2-1, VTC2-2, and VTC2-3), two of which are strongly transcriptionally up-regulated by light (Sodeyama et al., 2021). The light-dependent induction of both genes is strongly suppressed by the addition of the photosynthetic electron transport inhibitor DCMU [3-(3,4-dichlorophenyl)-1,1-dimethylurea]. Knockout of either gene resulted in a substantial reduction of cellular ascorbate (46% or 17% of the wild type for vtc2-1 and vtc2-2, respectively), indicating that GGP makes a major contribution to ascorbate biosynthesis in moss. In contrast, the liverwort Marchantia polymorpha contains a single VTC2 gene whose expression is not increased by light or oxidative stress. However, MpVTC2 was essential for growth, as knockout Marchantia plants could only be maintained through supplementation with l-Gal (Ishida et al., 2023).
Control of ascorbate biosynthesis via GGP also differs substantially in the green alga C. reinhardtii. Although high light increases cellular ascorbate, transcript levels of CrVTC2 are not elevated in cells acclimated to high light (Vidal-Meireles et al., 2017). CrVTC2 transcripts are strongly increased by oxidative stress, suggesting that redox status rather than light may be the major factor controlling ascorbate biosynthesis in Chlamydomonas (Urzica et al., 2012a; Vidal-Meireles et al., 2017). Whilst inhibition of photosynthetic electron transport with DCMU decreases VTC2 expression in vascular plants and Physcomitrium, it results in an increase in CrVTC2 transcripts in Chlamydomonas. The increased expression may be triggered by the production of singlet oxygen by DCMU and demonstrates that photosynthetic electron transport is not required for elevated CrVTC2 expression. The d-Man/l-Gal pathway is the major contributor to ascorbate biosynthesis in Chlamydomonas, with CrVTC2 artificial miRNA (amiRNA) knockdown lines exhibiting just 10% of the ascorbate content of wild-type cells (Vidal-Meireles et al., 2017). The observed regulatory differences are likely to be due to differences in the cellular concentrations and roles of ascorbate between these lineages. Bryophytes and green algae have a much lower ascorbate content than vascular plants (Gest et al., 2013). Moreover, Chlamydomonas does not exhibit a strong requirement for ascorbate in the xanthophyll cycle, as severe ascorbate deficiency in Chlamydomonas CrVTC2 knockout lines does not impair energy-dependent quenching (qE) and violaxanthin de-epoxidation (Vidal-Meireles et al., 2019).
Red algae possess most of the biosynthetic enzymes of the d-Man/l-Gal pathway, including GME, l-GalDH, and l-GalLDH, but crucially lack GGP. Labelling studies demonstrated that the carbon chain of glucose is not inverted during ascorbate biosynthesis in Galdieria sulphuraria, indicating that red algae probably operate a modified d-Man/l-Gal pathway in which the conversion of GDP-l-Gal to l-Gal is catalysed by alternative enzymes that currently remain unidentified (Wheeler et al., 2015). Red algae have the capacity to produce GDP-l-Gal as an ascorbate precursor because it is a precursor for l-Gal and 3,6-anhydro-l-Gal residues in agar polysaccharides (Su and Hassid, 1962; Falshaw et al., 2023). Given that GGP plays a critical role in controlling ascorbate biosynthesis in land plants and green algae, the future identification of these enzymes in red algae will provide important insight into the evolution of these processes. A possible candidate is the ADP-glucose phosphorylase identified in Arabidopsis (McCoy et al., 2006), since red algae have similar proteins.
Outside the Archaeplastida (red and green algae), there is little evidence to support the presence of the d-Man/l-Gal pathway in any other photosynthetic eukaryote. The Archaeplastida obtained their chloroplasts from a primary endosymbiotic event with a cyanobacterium, whereas plastids in other photosynthetic eukaryotes derive from a secondary endosymbiosis with a red or green alga. Remarkably, almost all photosynthetic eukaryotes with secondary plastids possess l-GalLDH, whereas non-photosynthetic eukaryotes that are capable of ascorbate biosynthesis possess l-GulLO. Experimental evidence from Euglena, diatoms, and chrysophytes indicates that the production of l-GalL in these photosynthetic protists requires inversion of the carbon chain (C1 of glucose becomes C6 of ascorbate) (Shigeoka et al., 1979; Helsper et al., 1982; Grun and Loewus, 1984). Photosynthetic eukaryotes with secondary plastids therefore appear to possess a hybrid of the animal and plant pathways, combining inversion of the carbon chain with l-GalLDH as the terminal enzyme, using d-galacturonate and l-GalL as intermediates. The distribution of these pathways supports an evolutionary scheme in which the animal pathway represents the ancestral pathway of ascorbate biosynthesis. Red and green algae subsequently evolved an entirely novel pathway in which l-GalLDH replaced l-GulLO as the terminal enzyme, and l-Gal was utilized as the precursor for l-GalL (Wheeler et al., 2015). When other eukaryotes subsequently acquired photosynthesis via endosymbiosis with a green or red alga, it appears that only l-GalLDH was recruited by the host organism, leading to the formation of the hybrid pathway. In support of this hypothesis, two basally derived lineages in the red and green algae (the extremophile red alga Galdieria sulphuraria and the streptophyte alga, Chlorokybus atmophyticus) possess l-GulLO rather than l-GalLDH, but otherwise possess all other aspects of the d-Man/l-Gal pathway (Wheeler et al., 2015). This suggests that the d-Man/l-Gal pathway operates with l-GulLO as the terminal oxidase in these lineages, so that the recruitment of l-Gal as an intermediate in the pathway may have pre-dated the replacement of l-GulLO with l-GalLDH. Interestingly, both G. sulphuraria and C. atmophyticus usually occupy low-light environments, suggesting that selective pressure to replace l-GulLO with l-GalLDH in the red/green algal lineages may be linked to a role in photoprotection (Wheeler et al., 2015).
The strong selective pressure to replace l-GulLO with l-GalLDH in photosynthetic eukaryotes is also demonstrated by nearly all lineages that acquired their plastids via secondary endosymbiosis. Replacement of l-GulLO with l-GalLDH in these lineages would have uncoupled ascorbate production from H2O2 production, and may therefore have allowed photosynthetic eukaryotes to accumulate much larger quantities of ascorbate, enabling roles as an antioxidant and in photoprotection. Further elucidation of the nature of ascorbate biosynthesis and its cellular roles in diverse photosynthetic protists is required to test these evolutionary hypotheses.
Ascorbate transport
Subcellular fractionation and immunocytochemical detection using ascorbate-specific antibodies indicate that ascorbate occurs in chloroplasts, mitochondria, peroxisomes, and vacuoles in millimolar concentrations (Foyer and Noctor, 2011; Koffler et al., 2014). DHA predominates in the apoplast because of ascorbate oxidase (AO) activity (Pignocchi et al., 2003) coupled with the limited capacity for reduction via the thiol system as shown by full oxidation of the roGFP–Orp1 H2O2 biosensor targeted to the apoplast (Arnaud et al., 2023). The plasma membrane has a carrier-mediated ascorbate–DHA exchanger: DHA is taken up from the apoplast in exchange for ascorbate (Horemans et al., 2000), and in Betula pendula the Km for DHA uptake is 12.8 mM (Kollist et al., 2001). Critically, there has been no progress in molecular identification of any plasma membrane DHA/ascorbate transporters since the review by Horemans et al. (2000). Interestingly, H2O2 specifically induces ascorbate efflux from cultured cells, possibly via this exchanger (Parsons and Fry, 2010). Chloroplasts take up ascorbate in a carrier-dependent manner (Foyer and Lelandais, 1996) using a Δψ-dependent transporter (AtPHT4;4) from the PHOSPHATE TRANSPORTER 4 (PHT4) family. The pht4:4 knockout mutant has decreased leaf ascorbate content in high light. Chloroplast ascorbate was not measured in the mutant, but a decreased capacity for non-photochemical quenching (NPQ), which is dependent on the thylakoid lumen enzyme violaxanthin de-epoxidase (VDE) and which uses ascorbate as a substrate, suggests that chloroplast ascorbate is affected (Miyaji et al., 2015). AtPHT4:1 could be a thylakoid membrane ascorbate transporter (Miyaji et al., 2015). Mitochondrial ascorbate transport is reviewed in the discussion of l-GalLDH (Fig. 5), but specific transport proteins have not been identified. Very recently, a tonoplast-localized ascorbate transporter AtDTX25 in the multidrug and toxic compound extrusion (MATE) family was identified (Hoang et al., 2021a). It is active in ascorbate transport when expressed in yeast and Xenopus oocytes, and its role in iron mobilization is described later. In summary, there is still much to be learnt about plant ascorbate and DHA transporters, which are clearly different from those in mammals (Smirnoff, 2018).
Unlike animals, where ascorbate biosynthesis capacity is confined to liver or kidney, it is likely that ascorbate biosynthesis is cell autonomous but with differences in concentration between tissues. As a broad generalization, ascorbate concentration is higher in photosynthetic tissue than in roots. Reproductive tissues and meristems may have relatively high concentration, while fruits vary from low to exceptionally high (camu-camu, Kakadu plum, and kiwi fruit reaching 60–200 µmol g–1 FW). Ascorbate seems to move from source to sink tissues via the phloem. [14C]Ascorbate applied to source leaves of tomato, Medicago sativa, and Arabidopsis followed by autoradiography of whole plants shows label in sink tissues, including reproductive parts and root tips (Franceschi and Tarlyn, 2002; Badejo et al., 2011). Ascorbate occurs in potato and Arabidopsis phloem sap collected from aphids (Franceschi and Tarlyn, 2002; Tedone et al., 2004) and may even be synthesized in situ (Hancock et al., 2004). Furthermore, increasing source leaf ascorbate by feeding l-Gal(L) also increases ascorbate in sink tissues and supports its direct translocation (Franceschi and Tarlyn, 2002; Tedone et al., 2004). Nevertheless, it seems that translocation generally does not provide a significant amount of fruit ascorbate (Hancock et al., 2007; Li et al., 2010; Badejo et al., 2011).
Control of ascorbate concentration
The difference in ascorbate concentration between tissues, and its response to environmental (light, temperature, and mineral nutrient supply) or hormonal cues, implies that ascorbate status is sensed and then adjusted to the appropriate concentration. The final concentration is obviously dependent on the balance between synthesis and breakdown.
Leaf ascorbate concentration remains relatively constant over day/night cycles, but decreases substantially in extended dark (Smirnoff and Pallanca, 1996; Dowdle et al., 2007; Conklin et al., 2013; Truffault et al., 2017) which, in barley leaves, can be partially reversed by adding Glc or Suc (Smirnoff and Pallanca, 1996), suggesting that degradation is associated with the carbon starvation response (Pal et al., 2013). Turnover rate in leaves is ~2% of the pool size per hour as measured by breakdown of [14C]ascorbate in Arabidopsis leaves in the light (Conklin et al., 1997), potato leaves in light and dark (Imai et al., 1999), and tomato leaves over 24 h in the dark (Truffault et al., 2017). This means that an equivalent of about half the steady-state ascorbate concentration is replaced every 24 h in leaves. In contrast, turnover in embryos from germinating pea seeds is faster at 13% of pool size per hour (Pallanca and Smirnoff, 2000). Possibly this higher rate reflects the use of ascorbate in hydroxyproline-rich glycoprotein synthesis during rapid growth (see section on ascorbate functions). Breakdown is concomitant with oxalate and threonate accumulation in tomato leaves (Truffault et al., 2017). Oxalate and threonate are well-established products of DHA breakdown via 4-O-oxalyl-l-threonate (Green and Fry, 2005), but to date enzymes that catalyse these reactions are unknown, so DHA availability may be the main factor. DHA concentration (typically ~10% of the total ascorbate pool) is influenced by the rate of ascorbate oxidation and the capacity to reduce it via the ascorbate–GSH cycle. A comprehensive review of many overexpression experiments shows that increasing the recycling capacity by overexpressing DHAR tends to increase ascorbate by up to 2-fold, while MDHAR overexpression has less effect (Broad et al., 2020b).
Feedback inhibition is a common mechanism to prevent excessive accumulation of end products, and there is evidence that ascorbate synthesis is subject to feedback inhibition. The rate of [14C]ascorbate synthesis from [14C]Glc decreases as the ascorbate pool size increases (Pallanca and Smirnoff, 2000; Wolucka and Van Montagu, 2003). Although inhibition of some of the d-Man/l-Gal pathway enzymes in vitro has been reported, the effects might be artefacts due to pH or pro-oxidant effects of ascorbate, as discussed in the Introduction (Fenech et al., 2021). On the contrary, the emerging evidence suggests that flux through the d-Man/l-Gal pathway is largely controlled by GGP in a complex manner (Fig. 4). There have been many attempts to increase ascorbate by overexpressing the d-Man/l-Gal pathway enzymes. As expected, results vary between species and tissues, but a comprehensive review of these experiments indicates that GGP overexpression usually increases ascorbate while the other enzymes have a small or variable effect (Bulley et al., 2009; Yoshimura et al., 2014; Broad et al., 2020b; Fenech et al., 2021). Possibly, when overexpression of enzymes involved in GDP-Man metabolism increases ascorbate, it could be because competition with mannosylation reactions is large, for example in actively growing tissue. Added to this evidence, quantitative trait locus (QTL) analysis shows that allelic variation of MdGGP1 and MdGGP3 is associated with apple fruit ascorbate concentration (Mellidou et al., 2012). The metabolic engineering and genetic evidence is replicated by a kinetic model of the d-Man/l-Gal pathway which predicts that GGP is the only significant controlling step in the pathway (Fenech et al., 2021). For this prediction to hold, it is necessary to include feedback inhibition of GGP by ascorbate, while reported feedback of the other enzymes has no effect. Inspection of Arabidopsis transcriptome data shows that GGP1 and GGP2 (VTC2/VTC5) transcript levels are more responsive to environmental factors affecting ascorbate, such as light/darkness, than the other d-Man/l-Gal pathway enzymes, and the GGPs follow a circadian rhythm under continuous light. The increased GGP transcript level in high light is reflected by increased GGP enzyme activity in Arabidopsis (Dowdle et al., 2007). The discovery that GGP mRNA has a conserved upstream ORF (uORF) in its 5'-untranslated region (UTR) has been pivotal in understanding the control of ascorbate synthesis by opening up the possibility that the uORF controls translation in a ascorbate-dependent manner (Fig. 4) (Laing et al., 2015). uORFs control translation of the main ORF of a significant proportion of genes by causing ribosome stalling or by activating nonsense-mediated decay (NMD) of the mRNA. In some cases, the uORF encodes a peptide which aids stalling. Examples of uORF-mediated control of metabolism include feedback repression of translation of transcription factors or biosynthetic enzymes, for example in polyamine and sucrose synthesis (Kurihara et al., 2009, 2018; van der Horst et al., 2020). The key points are that the GGP uORF has a non-canonical initiation codon (ACG) and is predicted to encode a peptide. Use of transiently expressed constructs in N. benthamiana containing the 5'-UTR/uORF fused to luciferase (LUC) reporters showed that translation is increased if the uORF is deleted or mutated, and is greatly decreased if the ACG is converted to the normal AUG initiation codon. Furthermore, increasing the ascorbate content of N. benthamiana by co-infiltrating with 35S::GGP lacking the uORF represses translation of the LUC reporter. Laing et al. (2015) proposed that translation of GGP mRNA is repressed because the interaction of the uORF-encoded peptide with ascorbate causes ribosomes to stall on the uORF and thereby blocks their progression to the AUG start of the GGP-coding sequence. Currently, details of this mechanism need clarification. The peptide has not been detected but could remain bound to the ribosomes to cause stalling. There is currently no direct evidence that ascorbate itself, or a proxy of ascorbate status, is involved in enhancing stalling. Further work confirms the importance of the GGP uORF in controlling ascorbate synthesis. A high ascorbate tomato from an EMS mutagenesis screen was mapped to the predicted uORF of SlGGP1. CRISPR/Cas9 [clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated protein 9] gene editing to disrupt the SlGGP1 uORF produced tomato fruit with greatly increased ascorbate by up to 5-fold (Deslous et al., 2021). Using gene editing to increase ascorbate in this manner is clearly an effective metabolic engineering strategy, and its general applicability is confirmed by increased ascorbate in lettuce, Arabidopsis, and tomato following uORF mutation (T. Li et al., 2018; Zhang et al., 2018). However, SlGGP1 uORF edited lines of tomato with very high ascorbate have developmental defects, particularly parthenocarpy possibly caused by impaired anther development and poor pollen germination (Deslous et al., 2021). The lesson is that control of ascorbate concentration at the ‘correct’ level is important for plant function, and the uORF is a key player. Further metabolic engineering strategies using the uORF will need be tuned appropriately. Another consideration in relation to the uORF is that ribosome stalling could result in targeting of the GGP mRNA by NMD. Interestingly, the AtGGP1/VTC2 transcript level is increased in RNA helicase, UP frameshift mutants (upf1-1 and upf3-1I) in the NMD process (Kurihara et al., 2009). If this is the case, then the measured GGP transcript levels could be determined by a combination of transcription and destruction by NMD.
Considering the importance of ascorbate in photosynthesis and photoprotection (Toth, 2023), it is not surprising that ascorbate concentration in leaves is increased by high light in many species. For example, in Arabidopsis, adjustment to light intensity takes 5 d, with the final concentration saturating at a photosynthetic photon flux density (PPFD) of ~500 µmol m–2 s–1 (Page et al., 2012). Expression of VTC2 and VTC5 promoter/5'-UTR::luciferase in Arabidopsis revealed that luminescence increased after transfer to high light and showed a circadian rhythm. Overall, the VTC5 construct had lower luciferase activity than VTC2, reflecting their transcript levels (Gao et al., 2011). Since this construct contains the uORF, it is reporting the transcriptional and translational control of VTC2/5, so it will be necessary to disentangle these in future work given that transcript levels are higher in high light. Ascorbate and GGP expression are also light responsive in Physcomitrium (Sodeyama et al., 2021) but not in M. polymorpha (Ishida et al., 2023). Critically, the signals involved in high-light-induced GGP transcription/translation need to be identified. Inhibition of photosynthetic electron transport by DCMU decreases light-induced ascorbate accumulation and expression of GGP isoforms in Arabidopsis and Physcomitrium (Sodeyama et al., 2021; Yabuta et al., 2007). This observation suggests involvement of a photosynthesis-sourced signal. H2O2 could be ruled out because its production is blocked by DCMU (Exposito-Rodriguez et al., 2017). The VTC2 promoter has predicted light response elements (Gao et al., 2011). The green alga Chlamydomonas responds differently: ascorbate accumulation and GGP expression are increased by DCMU, rose Bengal (a singlet oxygen generator), and H2O2 (Vidal-Meireles et al., 2017, 2019). Chlamydomonas also accumulates ascorbate in high light, but it is suggested that this response is associated with increased H2O2 generated in the light (Vidal-Meireles et al., 2017). A recent notable development in relation to the control of GGP and ascorbate synthesis by light is the role of a physical interaction between GGP and a PAS-LOV (PLP) protein. The PAS-LOV protein contains an FMN chromophore and is a putative blue light receptor which interacts with Arabidopsis GGP1 and 2 in a yeast two-hybrid assay. The interaction is weakened by blue light (Ogura et al., 2008). This interaction was found to have functional significance following discovery of increased ascorbate (~2.5–3.5 µmol g–1 FW) in PLP mutants of tomato and Arabidopsis (Aarabi et al., 2023; Bournonville et al., 2023). Similarly, knockout or overexpression of PLP in soybean modestly increased or decreased ascorbate, respectively (Zhang et al., 2023). Critically, binding of PLP to GGP occurs in vivo and is disrupted by blue light (Aarabi et al., 2023), and this binding inhibits enzyme activity of a recombinant GGP (Bournonville et al., 2023). Physical association between sequential enzymes of the d-Man/l-Gal pathway enzymes from GMP through to l-GalDH is suggested from co-immunoprecipitation and gel filtration experiments (Fenech et al., 2021). Association of enzymes into ‘metabolons’ can sometimes improve or direct flux (Sweetlove and Fernie, 2018), but more work is needed to assess the functional significance in the d-Man/l-Gal pathway. Many other factors will influence ascorbate synthesis, and transcription factors controlling ascorbate accumulation via expression of GGP and other d-Man/l-Gal pathway enzymes are being identified (Broad et al., 2020b; Liu et al., 2022, 2023; Xu et al., 2023; Zhang et al., 2023). Jasmonic acid and methyl jasmonate increase ascorbate concentration and GGP/GME expression in Arabidopsis liquid-cultured seedlings and cell suspension cultures (Sasaki-Sekimoto et al., 2005; Wolucka et al., 2005) for up to 48 h after application, along with AtGGP1/VTC2, ATGGP2/VTC5, and GME expression. In Arabidopsis, publicly available transcriptome data indicate that GGP2 expression is specifically responsive to ozone and the PAMP flg22 additionally to light.
Metabolic engineering and biotechnology
As well as improving nutritional value, increased ascorbate might improve stress resistance. Discovery of the d-Man/l-Gal pathway resulted in a flurry of patent applications related to the use of GME, l-GalDH, and l-GalLDH in engineering plants for increased ascorbate and the possibility that l-GalDH, as a plant-specific enzyme, could be a herbicide target (Bauw et al., 1998; Berry et al., 1999; Smirnoff and Wheeler, 1999). The increasingly detailed understanding of the d-Man/l-Gal pathway and particularly the complex control of GGP activity (Fig. 4) will inform metabolic engineering strategies to increase ascorbate in specific tissues in a controlled manner. The identification of translational control by the GGP uORF, as noted in the previous section, has already provided a simple route to increasing ascorbate via gene editing. However, this approach has also shown that producing too much ascorbate can be damaging to development and fertility in tomato (Deslous et al., 2021). This effect seems to extend to Arabidopsis, where overexpression of GGP1 with a pollen-specific promoter decreases pollen production and growth (Weigand et al., 2023). However, in this case, pollen ascorbate was not increased, suggesting that increased production of degradation products or diversion of GDP-sugars from growth-critical glycosylation reactions could be the reason (Fig. 3). GME mutants in tomato and Arabidopsis have impaired pollen growth and fertility which is not rescued by ascorbate, indicating a critical role for GPP-sugars in pollen function (Mounet-Gilbert et al., 2016; Qi et al., 2017). Furthermore, the tdf1 Arabidopsis mutant has decreased expression of an ascorbate oxidase-like protein, has double wild-type ascorbate in its inflorescences, and does not develop pollen normally (Wu et al., 2023) (more details are provided in the next section). Clearly, the reason for the deleterious effect of very high ascorbate requires further investigation (Castro et al., 2018). Transgenic approaches to increasing ascorbate by overexpression of d-Man/l-Gal pathway enzymes have been well reviewed and are not covered in detail here (Ishikawa et al., 2006; Macknight et al., 2017; Broad et al., 2020b; Terzaghi and De Tullio, 2022; Castro et al., 2023). As noted in the previous section, overexpression of GGP tends to have the greatest effect. Another approach to metabolic engineering is to introduce or boost routes to l-GalL or l-GulL production via d-galacuronate or d-glucuronate, respectively, as analogues of the protist and animal pathways (Smirnoff, 2003; Wheeler et al., 2015). Overexpression of strawberry d-galacturonate reductase in Arabidopsis increases leaf ascorbate 2- to 3-fold (Agius et al., 2003). More controversially, increasing d-glucuronate production by overexpressing myo-inositol oxygenase has been reported to increase (Lorence et al., 2004) or not affect (Endres and Tenhaken, 2009) ascorbate in Arabidopsis.
Very large amounts of ascorbate are manufactured for vitamin supplements for human and fish diets, and for food/beverage manufacturing as an antioxidant preservative, so there is great interest in engineering microorganisms for ascorbate synthesis (Hancock and Viola, 2002; Running et al., 2004; Wang et al., 2018). The dominant Reichstein process, which has multiple chemical steps and one microbial conversion, is highly optimized to convert glucose to ascorbate. This is important because the price differential between precursor and product is small. A one-step fermentation to manufacture ascorbate in bacteria or yeast could be superior and cleaner. Many of the introduced pathways are synthetic, often aimed at producing the 2-keto-l-gulonate as the precursor. The existing yeast d-erythroascorbate pathway can utilize the plant intermediates l-Gal and l-GalL, which provides a useful starting point (Hancock et al., 2000; Sauer et al., 2004; Branduardi et al., 2007). Encouragingly, the entire plant d-Man/l-Gal pathway from Glc has been successfully reconstituted in Escherichia coli (Tian et al., 2022) and Saccharomyces cerevisiae (Zhou et al., 2021), although current yields are likely to be too low for commercial use.
The functions of ascorbate
Using Arabidopsis vtc mutants to understand the functions of ascorbate
The A. thaliana vtc mutants have been invaluable in elucidating ascorbate biosynthesis and, except for VTC3, their roles are now well established. Understandably, many researchers have been drawn to use these mutants to investigate the functions of ascorbate, particularly in relation to photosynthesis, photoprotection, pathogen response, and abiotic stresses (summarized in Supplementary Table S1). Complete knockout of ascorbate-specific biosynthesis genes (GGP onwards) is lethal. The exception is VTC4, whose phosphatase activity is not specific to l-Gal 1-P and because other enzymes with similar catalytic activity are present and enable ascorbate synthesis, albeit resulting in lower concentration (Conklin et al., 2006). l-GalLDH mutants are also affected in mitochondrial Complex 1 formation as discussed in the biosynthesis section. The vtc1 mutants are not just affected in ascorbate biosynthesis because GDP-Man is also needed for cell wall polysaccharide synthesis and protein glycosylation as previously discussed. The function of VTC3 is unknown, so vtc3 mutants could be pleiotropic. This leaves GGP mutants (vtc2/5), the first step dedicated to ascorbate production, and l-GalDH as the most appropriate to use for investigating ascorbate function. The original EMS mutants of GGP (vtc2-1, 2-2, and 2-3) (Conklin et al., 2000; Jander et al., 2002) were important in pathway identification. Of these, vtc2-1 and vtc2-2 have smaller rosettes than the wild type, leading to speculation that reduction of ascorbate to ~20% of the wild-type concentration affects growth and flowering (Pavet et al., 2005; Barth et al., 2006; Olmos et al., 2006; Kotchoni et al., 2009; Kerchev et al., 2011). However, the identification of insertion mutants (vtc2-4 and vtc2-5) in VTC2 which have similarly low ascorbate but are only slightly smaller, along with finding that backcrossing vtc2-1 to the wild type segregated small size from ascorbate deficiency (Lim et al., 2016), confirms that severely decreased growth in this mutant is not linked to ascorbate deficiency. A comparison of vtc2-1 and vtc2-4 showed smaller rosette biomass in both mutants in one study (Plumb et al., 2018) but no difference in another (Lim et al., 2016). The take-home message is that the vtc mutants should be used very carefully for assessing the functions of ascorbate, and of course it is very likely that observed phenotypes are highly dependent on the environment. The critical growth maintenance functions of ascorbate in plants will need mutants containing less than ~20% of wild-type ascorbate concentration since these plants can grow, while plants with no ascorbate are unable to grow (Dowdle et al., 2007; Lim et al., 2016; Fenech et al., 2021). Meanwhile, either vtc2-4 or vtc2-5 should be used, or the same phenotype should be observed in mutants from different steps in the pathway before it is attributed to ascorbate. Decreased NPQ and increased basal pathogen resistance fall into this well-supported category (Supplementary Table S1).
Antioxidant
Probably the greatest focus on ascorbate in plants has been on its antioxidant role. As noted in the Introduction, it is an effective remover of H2O2 (catalysed by the plant-specific enzyme APX) and radicals. Consequently a range of vtc and apx mutants contain more H2O2 (Mukherjee et al., 2010), potentially influencing H2O2 signalling (Smirnoff and Arnaud, 2019; Mittler et al., 2022) and stress responses. With the caveat that in some cases only one mutant has been investigated, Arabidopsis vtc mutants are generally more susceptible to a range of abiotic stresses that are presumed to increase ROS or radical production such as ozone, sulfur dioxide, UV-B and C radiation, high salinity, and temperature extremes (Conklin et al., 1996; Smirnoff, 2000; Conklin and Barth, 2004; Huang et al., 2005; Larkindale et al., 2005; Gao and Zhang, 2008; Wang et al., 2012; Yao et al., 2015; Hoang et al., 2021b). It is therefore assumed that more ascorbate will improve abiotic stress resistance, and numerous metabolic engineering attempts have this goal. Improved stress tolerance is often claimed, although the experimental conditions may not be relevant to field conditions (Smirnoff, 2018). Results are summarized by Broad et al. (2020a, b). A wide range of vtc mutants have increased basal resistance to biotrophic pathogens such as Hyaloperonospora parasitica and Pseudomonas syringae, along with increased expression of various pathogen response genes, and SA and camalexin accumulation (Pastori et al., 2003; Barth et al., 2004; Colville and Smirnoff, 2008; Mukherjee et al., 2010). It is proposed that increased H2O2 induces SA-dependent defence (Mukherjee et al., 2010). GGP2 expression and a small increase in ascorbate is induced 60–90 min after elicitation of Arabidopsis cell cultures with harpin (Czobor et al., 2017). The significance in terms of ascorbate concentration and interaction with pathogens is unknown. In contrast, vtc1 and vtc2-1 are more sensitive to the necrotrophic fungus Alternaria brassicicola (Botanga et al., 2012). It is therefore possible that ascorbate concentration is a balance between the need for antioxidant defence, photosynthesis, and pathogen resistance.
Photosynthesis and photoprotection
The involvement of ascorbate in photosynthesis was recognized some time ago (Marrè and Arrigoni, 1958; Marrè et al., 1959; Mapson, 1964). Its functions in chloroplasts are evident considering its responsiveness to light via GGP activity. These functions are: (i) the removal of H2O2 produced by oxygen photoreduction at PSI using thylakoid and stromal APXs and (ii) energy dissipation via the xanthophyll cycle (NPQ) in which thylakoid lumen VDE requires ascorbate as a substrate. Other proposed roles include as an emergency electron donor to PSII and inactivation of the oxygen-evolving complex under some conditions. Ascorbate may regenerate tocopherol from tocopheroxyl radicals resulting from singlet oxygen production in PSII. These roles in photosynthesis and photoprotection have been well reviewed and so are not considered in detail here (Foyer and Shigeoka, 2011; Foyer, 2018; Toth, 2023).
Growth and development
The role of ascorbate in growth and development requires deeper analysis. It seems likely that its key functions are not readily evident from the vtc mutants. Ascorbate disappears from seeds during maturation and desiccation, and growth following imbibition is coincident with ascorbate accumulation (Arrigoni et al., 1992, 1997; Tommasi et al., 1999; Pallanca and Smirnoff, 2000; De Tullio and Arrigoni, 2003). Fully ascorbate-deficient mutants germinate, but growth is then arrested (Lim et al., 2016; Fenech et al., 2021). Further investigation of which processes are impeded in these seedlings is needed, possibilities being the antioxidant role (i.e. ROS removal to maintain a suitable redox state for cell division) (Potters et al., 2004; Schnaubelt et al., 2015), iron mobilization, or a requirement of 2-ODDs for growth or epigenetic regulation. In roots, the quiescent centre cells have a low rate of cell division. Cell division is influenced by highly oxidized ascorbate and GSH pools which are proposed to arrest transition from the G1 to S phase of cell division. A model in which indole-3-acetic acid (IAA) induces AO activity in the QC to locally oxidize ascorbate is proposed and, intriguingly, AO could also decarboxylate IAA (Kerk and Feldman, 1995; Kerk et al., 2000; Jiang et al., 2003). These results reflect the wider picture of dependence of cell division on the redox state of ascorbate and GSH (Potters et al., 2004; Schnaubelt et al., 2015). Recent work indicates that ascorbate status could also influence the differentiation of tapetal cells for pollen production. Normal tapetal development and pollen formation in Arabidopsis requires the transcription factor DEFECTIVE IN TAPETAL DEVELOPMENT AND FUNCTION 1 (TDF1) to control the transition from cell division to differentiation. TDF1 increases expression of a copper oxidase SKS18 (in the same family as AO) and, at the same time, represses GMP (VTC1) expression (Wu et al., 2023). The authors present a model in which low ascorbate, resulting from its destruction by AO activity of SKS118 and decreased synthesis due to lower GMP activity, encourages a switch from cell division to differentiation, possibly via increased ROS production. While the evidence is compelling, two points require clarification. Firstly, although it is feasible that SKS18 has AO activity, the recombinant protein was inactive until Cu2+ was added to the assay. Unfortunately, this evidence is weak because Cu2+ alone catalytically oxidizes ascorbate (Shen et al., 2021) and, since SKS18 is likely to be a secreted glycoprotein, it will not in any case be processed correctly in E. coli. Therefore, although SKS18 could well have AO activity, the results are equivocal. Secondly, disruption of GDP-Man synthesis could itself affect differentiation.
Ascorbate and iron
Ascorbate can both chelate Fe3+ and readily reduce it to the more soluble Fe2+ (also Cu2+ to Cu+) and there is evidence that it has a pervasive role in Fe uptake, transport, and storage in animals, as well as being a cofactor/chaperone to prevent Fe over-oxidation in 2-ODDs (Lane and Richardson, 2014; Badu-Boateng and Naftalin, 2019; De Tullio, 2020). While the evolution of ascorbate in eukaryotes could have been driven by protection against ROS arising in the great oxygenation event (GOE) started by cyanobacterial oxygenic photosynthesis (Gest et al., 2013), the availability of soluble Fe2+ also plummeted following the GOE. It is perhaps not a coincidence that ascorbate is effective in Fe3+ reduction and is also an antioxidant dealing with ROS and minimizing hydroxyl radicals formed, for example, by interaction of Fe2+ and H2O2 (the Fenton reaction). Thus, ascorbate could have played a role in both maintaining Fe supply and protecting against its pro-oxidant effects. Indeed, it has been proposed that the appearance of ascorbate was an important factor in the development of multicellular organisms because of its antioxidant role but also in the function of 2-ODDs in extracellular matrix protein synthesis and epigenetic control of stem cell activity (Edgar, 2019). It is striking that ascorbate accumulation is massively increased by Fe deficiency and H2O2 in Chlamydomonas (Urzica et al., 2012a, b). This response is much greater than in vascular plants (Zaharieva et al., 1999; Zaharieva and Abadia, 2003) where the basal ascorbate concentration is much higher. Feeding ascorbate (or GSH) to Arabidopsis seedlings alleviates Fe-deficiency chlorosis (Ramirez et al., 2013). Embryos of ascorbate-deficient Arabidopsis mutants (vtc2-4 and vtc5) have low Fe (Grillet et al., 2014). Recently, a tonoplast-located ascorbate transporter in the MATE family (AtDTX25) has been identified. A knockout mutant has less ascorbate and more Fe in isolated vacuoles and is more sensitive to Fe deficiency than the wild type (Hoang et al., 2021a). These observations provide a model for mobilization of Fe in germinating seedlings which is stored as vacuolar Fe3+-citrate, malate, or phytate chelates (Fig. 6). Ascorbate is transported into the vacuole where it reduces Fe3+, and the resulting Fe2+ is transported to the cytosol via tonoplast natural resistance-associated macrophage protein (NRAMP) transporters (Bastow et al., 2018; Hoang et al., 2021a). Plasma membrane-localized Cyt b can reduce MDHA using ascorbate as electron donor (Horemans et al., 1994), although evidence for its role in vivo has been lacking. Recently a tonoplast Cyt b561 able to reduce vacuolar MDHA using cytosolic ascorbate has been identified (Gradogna et al., 2023). This activity could contribute to regeneration of vacuolar ascorbate from MDHA (Fig. 6). In summary, ascorbate is involved in Fe transport into seeds and its mobilization during germination. Possibly the arrested germination of fully ascorbate-deficient Arabidopsis mutants (Dowdle et al., 2007; Lim et al., 2016; Fenech et al., 2021) could be caused in part by impaired Fe mobilization. Further evidence for a connection between ascorbate and Fe is related to a connection with response to phosphorus deficiency. Fe deficiency decreases ascorbate in Arabidopsis seedlings and causes chlorosis. Chlorosis is reversed by ascorbate supplementation in the wild type but not in a mutant of the PHT4:4 chloroplast ascorbate transporter. Additionally VTC1, VTC2, and VTC4 expression is induced by combined Fe and P deficiency, and ascorbate concentration is maintained and chlorosis does not develop, except in pht4:4 and vtc4 mutants (Nam et al., 2021). These intriguing results indicate links between ascorbate, Fe, and P nutrition and photosynthesis which require further investigation.
Fig. 6.
The role of ascorbate in iron-related processes. Ascorbate is required to prevent irreversible over-oxidation of Fe in 2-oxoglutarate-dependent dioxygenase (2-ODD) enzymes. 2-ODDs hydroxylating prolyl and lysyl residues of glycoproteins (e.g. prolyl 4-hydroxylase) are located in the ER. Inhibition or knockout of ER-localised prolyl 4-hydroxylases increases ascorbate concentration, suggesting that 2-ODD protection in the ER is a significant sink for ascorbate. Nothing is known about uptake of ascorbate or DHA, or the processes involved in reduction of MDHA or DHA in this compartment, but possible routes are shown. DHA could be reduced by GSH using PDI or glutaredoxin or MDHA could be reduced via cytc b5 mediated transmembrane electron transport. PDI is also involved in protein folding by disulfide bond formation, along with the H2O2-generating endoplasmic reticulum oxidoreductin (ERO). DHA could also facilitate disulfide bond formation. The recent identification of a tonoplast ascorbate transporter (DTX25) and transmembrane electron transporter (CYTB561A) provides a mechanism for moving ascorbate into the vacuole and regenerating MDHA. This system facilitates Fe mobilisation from vacuoles during seed germination and aids vacuolar H2O2 scavenging by type III peroxidases. Created with BioRender.com. Abbreviations: AGP, arabinogalactan protein; AsA, ascorbate; DHA, dehydroascorbate; ERO, endoplasmic reticulum oxidoreductin; P4H, prolyl 4-hydroxylase; MDHA, monodehydroascorbate; NRAMP, Natural resistance-associated macrophage protein; PDI, protein disulfide isomerase; PRP, proline-rich protein.
The foundational biomedical interest in ascorbate came from scurvy, the severe ascorbate deficiency disease. This is largely characterized by symptoms related to defective connective tissue because of collagen deficiency. Collagen contains hydroxyproline and hydroxylysine residues required for its function and formed post-translationally by prolyl 4-hydroxylases (P4Hs) and lysyl hydroxylases in the ER (Fig. 6). These are 2-ODDs and require ascorbate to prevent overoxidation of their active site Fe. Prolyl hydroxylase is inactivated after only 15–30 reaction cycles in the absence of ascorbate and other reductants (including thiols) are much less effective at maintaining activity (Myllylä et al., 1978, 1984; Majamaa et al., 1986). Plants contain many 2-ODDs (~100 in Arabidopsis) which are involved in diverse processes (Kawai et al., 2014). ER-localized 2-ODDs hydroxylate prolyl and lysyl residues in extracellular structural proteins of the hydroxyproline-rich glycoprotein family such as extensins, proline-rich proteins, and arabinogalactan proteins (AGPs). Also, various peptide hormones are hydroxylated by unknown P4Hs (Stuhrwohldt and Schaller, 2019; Stuhrwohldt et al., 2020), while others, mostly cytosolic, are involved in synthesis and metabolism of hormones (ethylene, gibberellin, and IAA) and specialized/secondary metabolites (Smirnoff, 2018; De Tullio, 2020). Ascorbate function in mammals has experienced greatly increased interest following evidence that its concentration might influence the activity of demethylases in the 2-ODD family involved in DNA demethylation [ten eleven translocation (TET) proteins] and histone demethylation (Jumonji C-domain-containing demethylases), thereby influencing epigenetic events and processes such as stem cell differentiation (Young et al., 2015; Agathocleous et al., 2017; Cimmino et al., 2017). Ascorbate is a substrate for a Chlamydomonas TET homologue (CMD1) in the place of 2-oxoglutarate. It donates a glyceryl group to 5mC with formation of glyoxylate and carbon dioxide (Xue et al., 2019; Li et al., 2021). A CRISPR-generated knockout of CDM1 caused high light sensitivity and altered expression of various photosynthesis- and photoprotection-related genes. It will be interesting to know if this type of TET protein is more widespread, although proteins with substantial sequence similarity seem limited to a few green algae.
Ascorbate deficiency in humans and guinea pigs impairs activity of 2-ODDs, including P4H and TET. Therefore, are ascorbate-deficient plants affected in 2-ODD activity? This question was assessed by measuring the hydroxyproline content of vtc1 and vtc2-1. They do not have decreased hydroxyproline in cell wall proteins (Sultana et al., 2015) which suggests that the 20–30% of wild-type ascorbate in these mutants can maintain prolyl hydroxylase activity. Nevertheless, there are two lines of evidence suggesting that ascorbate has a major role in 2-ODDs. Firstly, inhibition of prolyl hydroxylase with 3,4-dl-dehydroproline increases ascorbate concentration in several plant species (De Gara et al., 1991; De Tullio et al., 1999). Secondly, a number of Arabidopsis 2-ODD mutants, including prolyl hydroxylases, have increased ascorbate (Mahmood and Dunwell, 2020). These observations suggest the ER-localized 2-ODDs are a major sink for ascorbate, and regeneration in the ER is not sufficient to prevent loss following oxidation. Little is known about ascorbate in the ER lumen of plants (Fig. 6). Immunocytochemical measurement of ascorbate shows that it is present in most cell compartments, including the vacuole, but staining is not seen in the ER or Golgi lumen (Zechmann et al., 2011), possibly because it is predominantly present as DHA. The ER is a relatively ‘oxidizing’ compartment to favour formation of protein disulfides common in extracellular proteins and, using a roGFP GSH/GSSG sensor functional in the ER (Grx1–roGFP2iL–HDEL), the GSH/GSSG ratio is 100 times smaller in the ER than in the cytosol (Ugalde et al., 2022). This observation supports the view that any ER localized MDHA or DHA reducing systems have limited capacity. MDHAR and DHAR are not predicted to be present in the ER, but protein disulfide isomerase (PDI) and glutaredoxin (GRX) are both present (Ugalde et al., 2022) and have DHAR activity (Wells et al., 1990). In Arabidopsis, a Cyt b561 (At5g38630) is predicted to be in the ER membrane. Since a tonoplast Cyt b561 can reduce MDHA using ascorbate (Gradogna et al., 2023), ER lumen MDHA could also be reduced to ascorbate via this electron transporter. It is not known how ascorbate (or DHA) is transported across the ER membrane.
Considering the evidence for consumption (and loss) of ascorbate in the ER due to 2-ODD activity, and the large demand for hydroxylated extracellular proteins such as extensin and AGPs during cell expansion, it is likely that growth arrest in ascorbate null mutants could be caused by limited 2-ODD activity while the 80–90% decrease in ascorbate in the vtc mutants is still sufficient to support 2-ODD activity. Overall, the role of ascorbate in Fe uptake and 2-ODD function deserves further investigation. In addition to Fe-related roles, it has been proposed in mammals that DHA could participate in protein folding via S–S formation along with endoplasmic reticulum oxidoreductase (ERO). Indeed, ascorbate deficiency in mammalian systems is suggested to contribute to ER stress (Szarka and Lorincz, 2014).
Conclusions
The biosynthetic pathway of ascorbate through GDP-Man/l-Gal (Wheeler et al., 1998) has been supported by subsequent evidence from ascorbate-deficient Arabidopsis mutants and by knockdowns in other species. The pathway is specific to the green plant lineage (Wheeler et al., 2015). The first dedicated enzyme of the pathway, GGP, funnels GDP-sugars away from their functions in protein mannosylation and glycan synthesis. Recent work has shown it is the strongest controlling step in the pathway, with its expression being the result of transcriptional and translational controls along with light-dependent interaction with a PAS-LOV protein. The translational control through a uORF could enable feedback inhibition of ascorbate synthesis. This mechanism is providing a prototypical example of the application of gene editing to biofortification, but has also revealed potential negative effects on development and fertility, particularly pollen function. The reason for this requires further investigation by unravelling direct effects of ascorbate from perturbed GDP-sugar metabolism. The mechanism of ribosome stalling on the uORF and the effect of ascorbate on this process is under investigation. GGP is usually encoded by two paralogous genes in vascular plants. Expression of both is increased by high light in Arabidopsis, but one is also responsive to jasmonate and pathogen-associated molecular patterns (PAMPs), suggesting a division of labour. Signalling mechanisms involved in light induction need to be clarified along with the specific function of the GGP isoforms. The last step of the pathway using l-GalLDH occurs in the intermembrane space of mitochondria. Indeed, a unique feature of plant mitochondria is the dual use of l-GalLDH in Complex 1 assembly and in catalysing the oxidation of l-GalL to ascorbate via an interaction with Cyt c.
Ascorbate-deficient mutants have been used to reveal roles of ascorbate in photoprotection and antioxidant defence, although care in interpretation is needed because of pleiotropic effects of many of the mutations in d-Man/l-Gal pathway genes. Despite increased stress sensitivity (and activation of basal pathogen defence), Arabidopsis mutants with ~20% normal ascorbate have few major growth defects, while full deficiency is lethal. This is puzzling given the apparently tight control of the ascorbate concentration by GGP, so possibly ‘excess’ ascorbate is needed to deal with fluctuating environmental conditions. Understanding the basal functions of ascorbate needs plants with lower ascorbate. Based on the emerging evidence, we predict that very low ascorbate will more obviously impact two iron-related processes: activity of 2-ODDs, particularly in the ER; and iron trafficking. Ascorbate is an effective reducer of higher oxidation states of Cu and Fe, thereby improving availability, and it is an antioxidant. It can be proposed that this dual capacity was the driver for the evolution of ascorbate biosynthesis capacity though diverse pathways in eukaryotes as a response to increased oxygen and decreased Fe2+/Cu+ availability following the appearance of oxygenic photosynthesis in cyanobacteria.
Supplementary data
The following supplementary data are available at JXB online.
Table S1. Growth, development, and stress responses of Arabidopsis thaliana ascorbate-deficient (vtc) mutants.
Acknowledgements
We acknowledge the input of past and present lab members (Dominique Arnaud, Louise Colville, David Cuitun-Coronado, John Dowdle, Stephan Gatzek, Mark Jones, Choon Kiat Lim, Mike Page, Jane Pallanca, Susanne Rolinski, Sorrel Hughes, and Nighat Sultana) and our collaborators (Vitor-Amorim-Silva, Miguel Botella, Patricia Conklin, Mario Fenech, Takahiro Ishikawa, Rob Last, Cathie Martin, Jeff Running, Reinhardt Rosson, and Vera Thole) to our research on ascorbate in plants. Technical assistance was provided by Quinton Cumbes, Phil Goodson, Margaret Grapes, James Kingdon, and Marjorie Raymond. We apologize to colleagues whose work was not cited due to lack of space.
Contributor Information
Nicholas Smirnoff, Biosciences, Faculty of Health and Life Sciences, Exeter EX4 4QD, UK.
Glen L Wheeler, Marine Biological Association, Plymouth PL1 2PB, UK.
Donald Ort, University of Illinois, USA.
Author contributions
NS wrote the initial draft with additional contributions and editing by GLW.
Conflict of interest
No conflict of interest declared.
Funding
Research on ascorbate in NS’s laboratory is funded by the Biotechnology and Biological Sciences Research Council (BBSRC) grant no, BB/W006553/1.
Data availability
No new data are included in this review.
References
- Aarabi F, Ghigi A, Wijesingha Ahchige M, Bulut M, Geigenberger P, Neuhaus HE, Sampathkumar A, Alseekh S, Fernie AR.. 2023. Genome-wide association study unveils ascorbate regulation by PAS/LOV PROTEIN during high light acclimation. Plant Physiology 193, 2037–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboobucker SI, Suza WP, Lorence A.. 2017. Characterization of two Arabidopsis l-gulono-1,4-lactone oxidases, AtGulLO3 and AtGulLO5, involved in ascorbate biosynthesis. Reactive Oxygen Species 4, 389–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler LN, Gomez TA, Clarke SG, Linster CL.. 2011. A novel GDP-d-glucose phosphorylase involved in quality control of the nucleoside diphosphate sugar pool in Caenorhabditis elegans and mammals. Journal of Biological Chemistry 286, 21511–21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agathocleous M, Meacham CE, Burgess RJ, et al. 2017. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius F, Gonzalez-Lamothe R, Caballero JL, Munoz-Blanco J, Botella MA, Valpuesta V.. 2003. Engineering increased vitamin C levels in plants by overexpression of a d-galacturonic acid reductase. Nature Biotechnology 21, 177–181. [DOI] [PubMed] [Google Scholar]
- Arnaud D, Deeks MJ, Smirnoff N.. 2023. Organelle-targeted biosensors reveal distinct oxidative events during pattern-triggered immune responses. Plant Physiology 191, 2551–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni O, Calabrese G, De Gara L, Bitonti MB, Liso R.. 1997. Correlation between changes in cell ascorbate and growth of Lupinus albus seedlings. Journal of Plant Physiology 150, 302–308. [Google Scholar]
- Arrigoni O, De Gara L, Tommasi F, Liso R.. 1992. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiology 99, 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC.. 2002. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta 1569, 1–9. [DOI] [PubMed] [Google Scholar]
- Badejo AA, Eltelib HA, Fukunaga K, Fujikawa Y, Esaka M.. 2009. Increase in ascorbate content of transgenic tobacco plants overexpressing the acerola (Malpighia glabra) phosphomannomutase gene. Plant and Cell Physiology 50, 423–428. [DOI] [PubMed] [Google Scholar]
- Badejo AA, Wada K, Gao Y, Maruta T, Sawa Y, Shigeoka S, Ishikawa T.. 2011. Translocation and the alternative d-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the d-mannose/l-galactose pathway. Journal of Experimental Botany 63, 229–239. [Google Scholar]
- Badu-Boateng C, Naftalin RJ.. 2019. Ascorbate and ferritin interactions: consequences for iron release in vitro and in vivo and implications for inflammation. Free Radical Biology and Medicine 133, 75–87. [DOI] [PubMed] [Google Scholar]
- Baldwin TC, Handford MG, Yuseff MI, Orellana A, Dupree P.. 2001. Identification and characterization of GONST1, a Golgi-localized GDP-mannose transporter in Arabidopsis. The Plant Cell 13, 2283–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini R, Ott T, Guther M, Bonfante P, Udvardi MK, De Tullio MC.. 2012. Ascorbate oxidase: the unexpected involvement of a ‘wasteful enzyme’ in the symbioses with nitrogen-fixing bacteria and arbuscular mycorrhizal fungi. Plant Physiology and Biochemistry 59, 71–79. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A, del Valle P, Rua J, de Cima S, Busto F, de Arriaga D, Smirnoff N.. 2005. Characterisation and biosynthesis of d-erythroascorbic acid in Phycomyces blakesleeanus. Fungal Genetics and Biology 42, 390–402. [DOI] [PubMed] [Google Scholar]
- Barth C, De Tullio M, Conklin PL.. 2006. The role of ascorbic acid in the control of flowering time and the onset of senescence. Journal of Experimental Botany 57, 1657–1665. [DOI] [PubMed] [Google Scholar]
- Barth C, Gouzd ZA, Steele HP, Imperio RM.. 2010. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. Journal of Experimental Botany 61, 379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL.. 2004. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin C-1. Plant Physiology 134, 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Millar AH, Mittova V, Kiddle G, Heazlewood JL, Theodoulou FL, Foyer CH.. 2003. Control of ascorbate synthesis by respiration and its implications for stress responses. Free Radical Research 37, 34–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Pastori GM, Foyer CH.. 2000. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiology 123, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Yu JP, Gomez F, Fernandez L, McIntosh L, Foyer CH.. 2006. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. Journal of Experimental Botany 57, 1621–1631. [DOI] [PubMed] [Google Scholar]
- Bastow EL, Garcia de la Torre VS, Maclean AE, Green RT, Merlot S, Thomine S, Balk J.. 2018. Vacuolar iron stores gated by NRAMP3 and NRAMP4 are the primary source of iron in germinating seeds. Plant Physiology 177, 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauw GJ, Davey MW, Ostergaard J, Van Montagu MC.. 1998. International Patent. Production of ascorbic acid in plants. WO 98/50558. [Google Scholar]
- Beerens K, Gevaert O, Desmet T.. 2021. GDP-mannose 3,5-epimerase: a view on structure, mechanism, and industrial potential. Frontiers in Molecular Biosciences 8, 784142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A, Running JA, Severson DK, Burlingame RP.. 1999. International Patent. Vitamin C production in microorganisms and plants. WO 99/64618. [Google Scholar]
- Botanga CJ, Bethke G, Chen Z, Gallie DR, Fiehn O, Glazebrook J.. 2012. Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Molecular Plant-Microbe Interactions 25, 1628–1638. [DOI] [PubMed] [Google Scholar]
- Bournonville C, Mori K, Deslous P, et al. 2023. Blue light promotes ascorbate synthesis by deactivating the PAS/LOV photoreceptor that inhibits GDP-l-galactose phosphorylase. The Plant Cell 35, 2615–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branduardi P, Fossati T, Sauer M, Pagani R, Mattanovich D, Porro D.. 2007. Biosynthesis of vitamin C by yeast leads to increased stress resistance. PLoS One 2, e1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad RC, Bonneau JP, Beasley JT, et al. 2020a. Effect of rice GDP-l-galactose phosphorylase constitutive overexpression on ascorbate concentration, stress tolerance, and iron bioavailability in rice. Frontiers in Plant Science 11, 595439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad RC, Bonneau JP, Hellens RP, Johnson AAT.. 2020b. Manipulation of ascorbate biosynthetic, recycling, and regulatory pathways for improved abiotic stress tolerance in plants. International Journal of Molecular Sciences 21, 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner GR. 1993. The pecking order of free-radicals and antioxidants—lipid-peroxidation, α-tocopherol, and ascorbate. Archives of Biochemistry and Biophysics 300, 535–543. [DOI] [PubMed] [Google Scholar]
- Buettner GR, Jurkiewicz BA.. 1993. Ascorbate free-radical as a marker of oxidative stress—an EPR study. Free Radical Biology and Medicine 14, 49–55. [DOI] [PubMed] [Google Scholar]
- Buettner GR, Schafer FQ.. 2004. Ascorbate as an antioxidant. In: Asard H, May JM, Smirnoff N, eds. Vitamin C: function and biochemistry in animals and plants. Oxford: BIOS Scientific Publishers, 173–188. [Google Scholar]
- Bulley SM, Rassam M, Hoser D, Otto W, Schunemann N, Wright M, MacRae E, Gleave A, Laing W.. 2009. Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-l-galactose guanyltransferase is a major control point of vitamin C biosynthesis. Journal of Experimental Botany 60, 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro JC, Castro CG, Cobos M.. 2023. Genetic and biochemical strategies for regulation of l-ascorbic acid biosynthesis in plants through the l-galactose pathway. Frontiers in Plant Science 14, 1099829. [Google Scholar]
- Castro JLS, Lima-Melo Y, Carvalho FEL, Feitosa AGS, Neto MCL, Caverzan A, Margis-Pinheiro M, Silveira JAG.. 2018. Ascorbic acid toxicity is related to oxidative stress and enhanced by high light and knockdown of chloroplast ascorbate peroxidases in rice plants. Theoretical and Experimental Plant Physiology 30, 41–55. [Google Scholar]
- Chatzopoulou F, Sanmartin M, Mellidou I, et al. 2020. Silencing of ascorbate oxidase results in reduced growth, altered ascorbic acid levels and ripening pattern in melon fruit. Plant Physiology and Biochemistry 156, 291–303. [DOI] [PubMed] [Google Scholar]
- Chaouch S, Noctor G.. 2010. myo-Inositol abolishes salicylic acid-dependent cell death and pathogen defence responses triggered by peroxisomal hydrogen peroxide. New Phytologist 188, 711–718. [DOI] [PubMed] [Google Scholar]
- Chepda T, Cadau M, Girin P, Frey J, Chamson A.. 2001. Monitoring of ascorbate at a constant rate in cell culture: effect on cell growth. In Vitro Cellular & Developmental Biology. Animal 37, 26–30. [DOI] [PubMed] [Google Scholar]
- Cho KM, Nguyen HTK, Kim SY, Shin JS, Cho DH, Hong SB, Shin JS, Ok SH.. 2016. CML10, a variant of calmodulin, modulates ascorbic acid synthesis. New Phytologist 209, 664–678. [DOI] [PubMed] [Google Scholar]
- Cimmino L, Dolgalev I, Wang Y, et al. 2017. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170, 1079–1095.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville L, Smirnoff N.. 2008. Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. Journal of Experimental Botany 59, 3857–3868. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Barth C.. 2004. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell & Environment 27, 959–970. [Google Scholar]
- Conklin PL, DePaolo D, Wintle B, Schatz C, Buckenmeyer G.. 2013. Identification of Arabidopsis VTC3 as a putative and unique dual function protein kinase::protein phosphatase involved in the regulation of the ascorbic acid pool in plants. Journal of Experimental Botany 64, 2793–2804. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Gatzek S, Wheeler GL, Dowdle J, Raymond MJ, Rolinski S, Isupov M, Littlechild JA, Smirnoff N.. 2006. Arabidopsis thaliana VTC4 encodes l-galactose-1-P phosphatase, a plant ascorbic acid biosynthetic enzyme. Journal of Biological Chemistry 281, 15662–15670. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Norris SR, Wheeler GL, Williams EH, Smirnoff N, Last RL.. 1999. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin C) biosynthesis. Proceedings of the National Academy of Sciences, USA 96, 4198–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Pallanca JE, Last RL, Smirnoff N.. 1997. l-Ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiology 115, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL.. 2000. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 154, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin PL, Williams EH, Last RL.. 1996. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proceedings of the National Academy of Sciences, USA 93, 9970–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czobor A, Hajdinak P, Szarka A.. 2017. Rapid ascorbate response to bacterial elicitor treatment in Arabidopsis thaliana cells. Acta Physiologiae Plantarum 39, 62. [Google Scholar]
- DeBolt S, Cook DR, Ford CM.. 2006. L-Tartaric acid synthesis from vitamin C in higher plants. Proceedings of the National Academy of Sciences, USA 103, 5608–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gara L, Paciolla C, Tommasi F, Liso R, Arrigoni O.. 1994. In vivo inhibition of galactono-ɣ-lactone conversion to ascorbate by lycorine. Journal of Plant Physiology 144, 649–653. [Google Scholar]
- De Gara L, Tommasi F, Liso R, Arrigoni O.. 1991. Ascorbic acid utilization by prolyl hydroxylase in vivo. Phytochemistry 30, 1397–1399. [Google Scholar]
- Deslous P, Bournonville C, Decros G, et al. 2021. Overproduction of ascorbic acid impairs pollen fertility in tomato. Journal of Experimental Botany 72, 3091–3107. [DOI] [PubMed] [Google Scholar]
- De Tullio MC. 2020. Is ascorbic acid a key signaling molecule integrating the activities of 2-oxoglutarate-dependent dioxygenases? Shifting the paradigm. Environmental and Experimental Botany 178, 104173. [Google Scholar]
- De Tullio MC, Arrigoni O.. 2003. The ascorbic acid system in seeds: to protect and to serve. Seed Science Research 13, 249–260. [Google Scholar]
- De Tullio MC, Paciolla C, la Veechia F, Rascio N, D’Emerico S, De Gara L, Liso R, Arrigoni O.. 1999. Changes in onion root development induced by the inhibition of peptidyl-prolyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209, 424–434. [DOI] [PubMed] [Google Scholar]
- Dietz KJ. 2016. Thiol-based peroxidases and ascorbate peroxidases: why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Molecules and Cells 39, 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N.. 2007. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal 52, 673–689. [DOI] [PubMed] [Google Scholar]
- Duque P, Vieira CP, Bastos B, Vieira J.. 2022. The evolution of vitamin C biosynthesis and transport in animals. BMC Ecology and Evolution 22, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar JA. 2019. l-Ascorbic acid and the evolution of multicellular eukaryotes. Journal of Theoretical Biology 476, 62–73. [DOI] [PubMed] [Google Scholar]
- Endres S, Tenhaken R.. 2009. myo-inositol oxygenase controls the level of myo-inositol in Arabidopsis, but does not increase ascorbic acid. Plant Physiology 149, 1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM.. 2017. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Communications 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falshaw R, Furneaux RH, Sims IM, Hinkley SFR, Kidgell JT, Bell TJ.. 2023. Novel 4-O-beta-d-xylopyranosyl-3,6-anhydro-l-galactopyranosyl disaccharide units in a polysaccharide from the red alga Pyrophyllon subtumens. Carbohydrate Polymers 318, 121066. [DOI] [PubMed] [Google Scholar]
- Fenech M, Amaya I, Valpuesta V, Botella MA.. 2019. Vitamin C content in fruits: biosynthesis and regulation. Frontiers in Plant Science 9, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M, Amorim-Silva V, Esteban del Valle A, Arnaud D, Ruiz-Lopez N, Castillo AG, Smirnoff N, Botella MA.. 2021. The role of GDP-l-galactose phosphorylase in the control of ascorbate biosynthesis. Plant Physiology 185, 1574–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE, Iglesias AA.. 2021. Nucleotide-sugar metabolism in plants: the legacy of Luis F Leloir. Journal of Experimental Botany 72, 4053–4067. [DOI] [PubMed] [Google Scholar]
- Foyer CH. 2018. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany 154, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M.. 1996. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. Journal of Plant Physiology 148, 391–398. [Google Scholar]
- Foyer CH, Noctor G.. 2011. Ascorbate and glutathione: the heart of the redox hub. Plant Physiology 155, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Shigeoka S.. 2011. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiology 155, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM.. 2002. l-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology 130, 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhang LX.. 2008. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. Journal of Plant Physiology 165, 138–148. [DOI] [PubMed] [Google Scholar]
- Gao Y, Badejo AA, Shibata H, Sawa Y, Maruta T, Shigeoka S, Page M, Smirnoff N, Ishikawa T.. 2011. Expression analysis of the VTC2 and VTC5 genes encoding GDP-l-galactose phosphorylase, an enzyme involved in ascorbate biosynthesis, in Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry 75, 1783–1788. [DOI] [PubMed] [Google Scholar]
- Garchery C, Gest N, Do PT, et al. 2013. A diminution in ascorbate oxidase activity affects carbon allocation and improves yield in tomato under water deficit. Plant, Cell & Environment 36, 159–175. [DOI] [PubMed] [Google Scholar]
- Gatzek S, Wheeler GL, Smirnoff N.. 2002. Antisense suppression of l-galactose dehydrogenase in Arabidopsis thaliana provides evidence for its role in ascorbate synthesis and reveals light modulated l-galactose synthesis. The Plant Journal 30, 541–553. [Google Scholar]
- Gest N, Gautier H, Stevens R.. 2013. Ascorbate as seen through plant evolution: the rise of a successful molecule? Journal of Experimental Botany 64, 33–53. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Alhagdow M, Nunes-Nesi A, et al. 2009. GDP-d-mannose 3,5-epimerase (GME) plays a key role at the intersection of ascorbate and non-cellulosic cell-wall biosynthesis in tomato. The Plant Journal 60, 499–508. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia A, Kanli M, Wisowski N, et al. 2023. Maternal Embryo Effect Arrest 31 (MEE31) is a moonlighting protein involved in GDP-l-mannose biosynthesis and KAT1 potassium channel regulation. Plant Science 338, 111897. [DOI] [PubMed] [Google Scholar]
- Gradogna A, Lagostena L, Beltrami S, Tosato E, Picco C, Scholz-Starke J, Sparla F, Trost P, Carpaneto A.. 2023. Tonoplast cytochrome b561 is a transmembrane ascorbate-dependent monodehydroascorbate reductase: functional characterisation of electron currents in plant vacuoles. New Phytologist 238, 1957–1971. [DOI] [PubMed] [Google Scholar]
- Green MA, Fry SC.. 2005. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433, 83–87. [DOI] [PubMed] [Google Scholar]
- Grillet L, Ouerdane L, Flis P, Hoang MTT, Isaure MP, Lobinski R, Curie C, Mari S.. 2014. Ascorbate efflux as a new strategy for iron reduction and transport in plants. Journal of Biological Chemistry 289, 2515–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun M, Loewus FA.. 1984. l-Ascorbic acid biosynthesis in the euryhaline diatom Cyclotella cryptica. Planta 160, 6–11. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Chudek JA, Walker PG, Pont SDA, Viola R.. 2008. Ascorbic acid conjugates isolated from the phloem of Cucurbitaceae. Phytochemistry 69, 1850–1858. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Galpin JR, Viola R.. 2000. Biosynthesis of l-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiology Letters 186, 245–250. [DOI] [PubMed] [Google Scholar]
- Hancock RD, McRae D, Haupt S, Viola R.. 2004. Synthesis of l-ascorbic acid in the phloem. BMC Plant Biology 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RD, Viola R.. 2002. Biotechnological approaches for l-ascorbic acid production. Trends in Biotechnology 20, 299–305. [DOI] [PubMed] [Google Scholar]
- Hancock RD, Walker PG, Pont SDA, Marquis N, Vivera S, Gordon SL, Brennan RM, Viola R.. 2007. l-Ascorbic acid accumulation in fruit of Ribes nigrum occurs by in situ biosynthesis via the l-galactose pathway. Functional Plant Biology 34, 1080–1091. [DOI] [PubMed] [Google Scholar]
- Helsper JP, Kagan L, Hilby CL, Maynard TM, Loewus FA.. 1982. l-Ascorbic acid biosynthesis in Ochromonas danica. Plant Physiology 69, 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas M, Bashir Q, Leferink NG, et al. 2013. Communication between l-galactono-1,4-lactone dehydrogenase and cytochrome c. FEBS Journal 280, 1830–1840. [DOI] [PubMed] [Google Scholar]
- Hideg E, Mano J, Ohno C, Asada K.. 1997. Increased levels of monodehydroascorbate radical in UV-B-irradiated broad bean leaves. Plant and Cell Physiology 38, 684–690. [Google Scholar]
- Hoang MTT, Almeida D, Chay S, Alcon C, Corratge-Faillie C, Curie C, Mari S.. 2021a. AtDTX25, a member of the multidrug and toxic compound extrusion family, is a vacuolar ascorbate transporter that controls intracellular iron cycling in Arabidopsis. New Phytologist 231, 1956–1967. [DOI] [PubMed] [Google Scholar]
- Hoang MTT, Doan MTA, Nguyen T, Tra DP, Chu TN, Dang TPT, Quach PND.. 2021b. Phenotypic characterization of Arabidopsis ascorbate and glutathione deficient mutants under abiotic stresses. Agronomy 11, 764. [Google Scholar]
- Hoeberichts FA, Vaeck E, Kiddle G, et al. 2008. A temperature-sensitive mutation in the Arabidopsis thaliana phosphomannomutase gene disrupts protein glycosylation and triggers cell death. Journal of Biological Chemistry 283, 5708–5718. [DOI] [PubMed] [Google Scholar]
- Horemans N, Asard H, Caubergs RJ.. 1994. The role of ascorbate free radical as an electron acceptor to cytochrome b-mediated trans-plasma membrane electron transport in higher plants. Plant Physiology 104, 1455–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Asard H.. 2000. Transport and action of ascorbate at the plant plasma membrane. Trends in Plant Science 5, 263–267. [DOI] [PubMed] [Google Scholar]
- Huang CH, He WL, Guo JK, Chang XX, Su PX, Zhang LX.. 2005. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. Journal of Experimental Botany 56, 3041–3049. [DOI] [PubMed] [Google Scholar]
- Imai T, Kingston-Smith AH, Foyer CH.. 1999. Ascorbate metabolism in potato leaves supplied with exogenous ascorbate. Free Radical Research 31, S171–S179. [DOI] [PubMed] [Google Scholar]
- Isherwood FA, Chen YT, Mapson LW.. 1954. Synthesis of l-ascorbic acid in plants and animals. The Biochemical Journal 56, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isherwood FA, Mapson LW.. 1962. Ascorbic acid metabolism in plants: part II. Biosynthesis. Annual Review of Plant Physiology 13, 329. [Google Scholar]
- Ishida T, Tanaka Y, Maruta T, Ishikawa T.. 2023. The d-mannose/l-galactose pathway plays a predominant role in ascorbate biosynthesis in the liverwort Marchantia polymorpha but is not regulated by light and oxidative stress. The Plant Journal doi: 10.1111/tpj.16530 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Dowdle J, Smirnoff N.. 2006. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiologia Plantarum 126, 343–355. [Google Scholar]
- Islam MS, Leissing TM, Chowdhury R, Hopkinson RJ, Schofield CJ.. 2018. 2-Oxoglutarate-dependent oxygenases. Annual Review of Biochemistry 87, 585–620. [DOI] [PubMed] [Google Scholar]
- Jander G, Norris SR, Rounsley SD, Bush DF, Levin IM, Last RL.. 2002. Arabidopsis map-based cloning in the post-genome era. Plant Physiology 129, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Meng YL, Feldman LJ.. 2003. Quiescent center formation in maize roots is associated with an auxin-regulated oxidizing environment. Development 130, 1429–1438. [DOI] [PubMed] [Google Scholar]
- Jimenez A, Hernandez JA, delRio LA, Sevilla F.. 1997. Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiology 114, 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing B, Ishikawa T, Soltis N, et al. 2021. The Arabidopsis thaliana nucleotide sugar transporter GONST2 is a functional homolog of GONST1. Plant Direct 5, e00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanofsky JR, Sima PD.. 1995. Singlet oxygen generation from the reaction of ozone with plant-leaves. Journal of Biological Chemistry 270, 7850–7852. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Ono E, Mizutani M.. 2014. Evolution and diversity of the 2-oxoglutarate-dependent dioxygenase superfamily in plants. The Plant Journal 78, 328–343. [DOI] [PubMed] [Google Scholar]
- Keller R, Springer F, Renz A, Kossmann J.. 1999. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. The Plant Journal 19, 131–141. [DOI] [PubMed] [Google Scholar]
- Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH.. 2011. The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. The Plant Cell 23, 3319–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk NM, Feldman LJ.. 1995. A biochemical model for the initiation and maintenance of the quiescent center—implications for organization of root meristems. Development 121, 2825–2833. [Google Scholar]
- Kerk NM, Jiang KN, Feldman LJ.. 2000. Auxin metabolism in the root apical meristem. Plant Physiology 122, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler BE, Luschin-Ebengreuth N, Stabentheiner E, Muller M, Zechmann B.. 2014. Compartment specific response of antioxidants to drought stress in Arabidopsis. Plant Science 227, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Moldau H, Oksanen E, Vapaavuori E.. 2001. Ascorbate transport from the apoplast to the symplast in intact leaves. Physiologia Plantarum 113, 377–383. [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C.. 2009. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiology 149, 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramarenko GG, Hummel SG, Martin SM, Buettner GR.. 2006. Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochemistry and Photobiology 82, 1634–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Makita Y, Kawashima M, Fujita T, Iwasaki S, Matsui M.. 2018. Transcripts from downstream alternative transcription start sites evade uORF-mediated inhibition of gene expression in Arabidopsis. Proceedings of the National Academy of Sciences, USA 115, 7831–7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Matsui A, Hanada K, et al. 2009. Genome-wide suppression of aberrant mRNA-like noncoding RNAs by NMD in Arabidopsis. Proceedings of the National Academy of Sciences, USA 106, 2453–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Bulley S, Wright M, Cooney J, Jensen D, Barraclough D, MacRae E.. 2004a. A highly specific l-galactose-1-phosphate phosphatase on the path to ascorbate biosynthesis. Proceedings of the National Academy of Sciences, USA 101, 16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Frearson N, Bulley S, MacRae E.. 2004b. Kiwifruit l-galactose dehydrogenase: molecular, biochemical and physiological aspects of the enzyme. Functional Plant Biology 31, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Laing WA, Martinez-Sanchez M, Wright MA, et al. 2015. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. The Plant Cell 27, 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing WA, Wright MA, Cooney J, Bulley SM.. 2007. The missing step of the l-galactose pathway of ascorbate biosynthesis in plants, an l-galactose guanyltransferase, increases leaf ascorbate content. Proceedings of the National Academy of Sciences, USA 104, 9534–9539. [Google Scholar]
- Lamanchai K, Salmon DL, Smirnoff N, Sutthinon P, Roytrakul S, Leetanasaksakul K, Kittisenachai S, Jantasuriyarat C.. 2022. OsVTC1-1 RNAi mutant with reduction of ascorbic acid synthesis alters cell wall sugar composition and cell wall-associated proteins. Agronomy 12, 1272. [Google Scholar]
- Lane DJ, Richardson DR.. 2014. The active role of vitamin C in mammalian iron metabolism: much more than just enhanced iron absorption! Free Radical Biology and Medicine 75, 69–83. [DOI] [PubMed] [Google Scholar]
- Larkindale J, Hall JD, Knight MR, Vierling E.. 2005. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiology 138, 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leferink NGH, Fraaije MW, Joosten HJ, Schaap PJ, Mattevi A, van Berkel WJH.. 2009a. Identification of a gatekeeper residue that prevents dehydrogenases from acting as oxidases. Journal of Biological Chemistry 284, 4392–4397. [DOI] [PubMed] [Google Scholar]
- Leferink NGH, Jose MDF, van den Berg WAM, van Berkel WJH.. 2009b. Functional assignment of Glu386 and Arg388 in the active site of l-galactono-γ-lactone dehydrogenase. FEBS Letters 583, 3199–3203. [DOI] [PubMed] [Google Scholar]
- Leferink NGH, van den Berg WAM, van Berkel WJH.. 2008. l-Galactono- γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. The FEBS Journal 275, 713–726. [DOI] [PubMed] [Google Scholar]
- Leferink NGH, van Duijn E, Barendregt A, Heck AJR, van Berkel WJH.. 2009c. Galactonolactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin C. Plant Physiology 150, 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ma F, Liang D, Li J, Wang Y.. 2010. Ascorbate biosynthesis during early fruit development is the main reason for its accumulation in kiwi. PLoS One 5, e14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Wang J, Yu YW, Wang FR, Dong JG, Huang RF.. 2016. D27E mutation of VTC1 impairs the interaction with CSN5B and enhances ascorbic acid biosynthesis and seedling growth in Arabidopsis. Plant Molecular Biology 92, 473–482. [DOI] [PubMed] [Google Scholar]
- Li T, Yang X, Yu Y, Si X, Zhai X, Zhang H, Dong W, Gao C, Xu C.. 2018. Domestication of wild tomato is accelerated by genome editing. Nature Biotechnology 36, 1160–1163. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang T, Sun M, Shi Y, Zhang XJ, Xu GL, Ding J.. 2021. Molecular mechanism for vitamin C-derived C5-glyceryl-methylcytosine DNA modification catalyzed by algal TET homologue CMD1. Nature Communications 12, 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chu ZN, Luo JY, Zhou YH, Cai YJ, Lu YG, Xia JH, Kuang HH, Ye ZB, Ouyang B.. 2018. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnology Journal 16, 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B, Smirnoff N, Cobbett CS, Golz JF.. 2016. Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Frontiers in Plant Science 7, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Adler LN, Webb K, Christensen KC, Brenner C, Clarke SG.. 2008. A second GDP-l-galactose phosphorylase in Arabidopsis en route to vitamin C. Covalent intermediate and substrate requirements for the conserved reaction. Journal of Biological Chemistry 283, 18483–18492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Gomez TA, Christensen KC, Adler LN, Young BD, Brenner C, Clarke SG.. 2007. Arabidopsis VTC2 encodes a GDP-l-galactose phosphorylase, the last unknown enzyme in the Smirnoff–Wheeler pathway to ascorbic acid in plants. Journal of Biological Chemistry 282, 18879–18885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bulley SM, Varkonyi-Gasic E, Zhong C, Li D.. 2023. Kiwifruit bZIP transcription factor AcePosF21 elicits ascorbic acid biosynthesis during cold stress. Plant Physiology 192, 982–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu R, Bulley SM, Zhong C, Li D.. 2022. Kiwifruit MYBS1-like and GBF3 transcription factors influence l-ascorbic acid biosynthesis by activating transcription of GDP-l-galactose phosphorylase. New Phytologist 234, 1782–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewus FA. 1999. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 52, 193–210. [Google Scholar]
- Loewus FA, Jang R, Seegmiller CG.. 1956. Conversion of C-14 labeled sugars to l-ascorbic acid in ripening strawberries. Journal of Biological Chemistry 222, 649–664. [PubMed] [Google Scholar]
- Loewus MW, Bedgar DL, Saito K, Loewus FA.. 1990. Conversion of l-sorbosone to l-ascorbic acid by a NADP-dependent dehydrogenase in bean and spinach leaf. Plant Physiology 94, 1492–1495. [Google Scholar]
- Lorence A, Chevone BI, Mendes P, Nessler CL.. 2004. myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiology 134, 1200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewski KM, Blevins DG.. 1996. Root growth inhibition in boron-deficient or aluminum-stressed squash may be a result of impaired ascorbate metabolism. Plant Physiology 112, 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last RL, Conklin PL, Somerville CR.. 2001. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proceedings of the National Academy of Sciences, USA 98, 2262–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Li H, Wang L, Li B, Wang Z, Ma B, Ma F, Li M.. 2022. F-box protein MdAMR1L1 regulates ascorbate biosynthesis in apple by modulating GDP-mannose pyrophosphorylase. Plant Physiology 188, 653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknight RC, Laing WA, Bulley SM, Broad RC, Johnson AA, Hellens RP.. 2017. Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Current Opinion in Biotechnology 44, 153–160. [DOI] [PubMed] [Google Scholar]
- Mahmood AM, Dunwell JM.. 2020. 2-Oxoglutarate-dependent dioxygenases: a renaissance in attention for ascorbic acid in plants. PLoS One 15, e0242833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majamaa K, Günzler V, Hanauske-Abel HM, Myllylä R, Kivirikko KI.. 1986. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. Journal of Biological Chemistry 261, 7819–7823. [PubMed] [Google Scholar]
- Major LL, Wolucka BA, Naismith JH.. 2005. Structure and function of GDP-mannose-3'',5''-epimerase: an enzyme which performs three chemical reactions at the same active site. Journal of the American Chemical Society 127, 18309–18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapson LW. 1964. The ascorbic acid system in leaves—further observations on photooxidation and photoreduction. Phytochemistry 3, 429–445. [Google Scholar]
- Mapson LW, Breslow E.. 1958. Biological synthesis of ascorbic acid: l-galactono-1,4-lactone dehydrogenase. The Biochemical Journal 68, 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapson LW, Isherwood FA.. 1956. Biological synthesis of l-ascorbic acid: the conversion of derivatives of d-galacturonic acid to l-ascorbate in plant extracts. The Biochemical Journal 64, 13–22. [Google Scholar]
- Marrè E, Arrigoni O.. 1958. Ascorbic acid and photosynthesis I. ‘Monodehydroascorbic acid’ reductase of chloroplasts. Biochimica et Biophysica Acta 30, 453–457. [DOI] [PubMed] [Google Scholar]
- Marrè E, Arrigoni O, Rossi G.. 1959. Ascorbic acid and photosynthesis II. Anaerobic photo-oxidation of ascorbic acid by chloroplast fragments and by whole chloroplasts. Biochimica et Biophysica Acta 36, 56–64. [DOI] [PubMed] [Google Scholar]
- Maruta T, Ichikawa Y, Mieda T, Takeda T, Tamoi M, Yabuta Y, Ishikawa T, Shigeoka S.. 2010. The contribution of Arabidopsis homologs of l-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Bioscience, Biotechnology, and Biochemistry 74, 1494–1497. [DOI] [PubMed] [Google Scholar]
- Maruta T, Sawa Y, Shigeoka S, Ishikawa T.. 2016. Diversity and evolution of ascorbate peroxidase functions in chloroplasts: more than just a classical antioxidant enzyme? Plant and Cell Physiology 57, 1377–1386. [DOI] [PubMed] [Google Scholar]
- Maruta T, Yonemitsu M, Yabuta Y, Tamoi M, Ishikawa T, Shigeoka S.. 2008. Arabidopsis phosphomannose isomerase 1, but not phosphomannose isomerase 2, is essential for ascorbic acid biosynthesis. Journal of Biological Chemistry 283, 28842–28851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos IF, Morales LMM, Santana DB, Silva GMC, Gomes MMD, Ayub RA, Costa JH, de Oliveira JG.. 2022. Ascorbate synthesis as an alternative electron source for mitochondrial respiration: possible implications for the plant performance. Frontiers in Plant Science 13, 987077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazorra Morales LM, Cosme Silva GM, Santana DB, et al. 2022. Mitochondrial dysfunction associated with ascorbate synthesis in plants. Plant Physiology and Biochemistry 185, 55–68. [DOI] [PubMed] [Google Scholar]
- McCoy JG, Arabshahi A, Bitto E, Bingham CA, Ruzicka FJ, Frey PA, Phillips GN.. 2006. Structure and mechanism of an ADP-glucose phosphorylase from Arabidopsis thaliana. Biochemistry 45, 3154–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellidou I, Chagne D, Laing WA, Keulemans J, Davey MW.. 2012. Allelic variation in paralogs of GDP-l-galactose phosphorylase is a major determinant of vitamin C concentrations in apple fruit. Plant Physiology 160, 1613–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda T, Yabuta Y, Rapolu M, Motoki T, Takeda T, Yoshimura K, Ishikawa T, Shigeoka S.. 2004. Feedback inhibition of spinach l-galactose dehydrogenase by l- ascorbate. Plant and Cell Physiology 45, 1271–1279. [Google Scholar]
- Millar AH, Mittova V, Kiddle G, Heazlewood JL, Bartoli CG, Theodoulou FL, Foyer CH.. 2003. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiology 133, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F.. 2022. Reactive oxygen species signalling in plant stress responses. Nature Reviews. Molecular Cell Biology 23, 663–679. [DOI] [PubMed] [Google Scholar]
- Miyaji T, Kuromori T, Takeuchi Y, et al. 2015. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nature Communications 6, 5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Yu X, Albrecht S, et al. 2013. Abnormal glycosphingolipid mannosylation triggers salicylic acid-mediated responses in Arabidopsis. The Plant Cell 25, 1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounet-Gilbert L, Dumont M, Ferrand C, et al. 2016. Two tomato GDP-d-mannose epimerase isoforms involved in ascorbate biosynthesis play specific roles in cell wall biosynthesis and development. Journal of Experimental Botany 67, 4767–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw MB, Barth C.. 2010. Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Molecular Plant-Microbe Interactions 23, 340–351. [DOI] [PubMed] [Google Scholar]
- Muller-Moulé P. 2008. An expression analysis of the ascorbate biosynthesis enzyme VTC2. Plant Molecular Biology 68, 31–41. [DOI] [PubMed] [Google Scholar]
- Murgia I, Midali A, Cimini S, De Gara L, Manasherova E, Cohen H, Paucelle A, Morandini P.. 2023. The Arabidopsis thaliana gulono-1,4 gamma-lactone oxidase 2 (GULLO2) facilitates iron transport from endosperm into developing embryos and affects seed coat suberization. Plant Physiology and Biochemistry 196, 712–723. [DOI] [PubMed] [Google Scholar]
- Myllylä R, Kuutti-Savolainen E-R, Kivirikko KI.. 1978. The role of ascorbate in the prolyl hydroxylase reaction. Biochemical and Biophysical Research Communications 83, 441–448. [DOI] [PubMed] [Google Scholar]
- Myllylä R, Majamaa K, Günzler V, Hanauske-Abel HM, Kivirikko KI.. 1984. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. Journal of Biological Chemistry 259, 5403–5405. [PubMed] [Google Scholar]
- Nam H-I, Shahzad Z, Dorone Y, Clowez S, Zhao K, Bouain N, Lay-Pruitt KS, Rhee SY, Rouached H.. 2021. Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling. Nature Communications 12, 7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickle TC, Meinke DW.. 1998. A cytokinesis-defective mutant of Arabidopsis (cyt1) characterized by embryonic lethality, incomplete cell walls, and excessive callose accumulation. The Plant Journal 15, 321–332. [DOI] [PubMed] [Google Scholar]
- Njus D, Kelley PM, Tu Y-J, Schlegel HB.. 2020. Ascorbic acid: the chemistry underlying its antioxidant properties. Free Radical Biology and Medicine 159, 37–43. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh A, Collakova E, Gillaspy GE.. 2014. Characterization of the inositol monophosphatase gene family in Arabidopsis. Frontiers in Plant Science 5, 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba K, Ishikawa S, Nishikawa M, Mizuno H, Yamamoto T.. 1995. Purification and properties of l-galactono-γ-lactone dehydrogenase, a key enzyme for ascorbic-acid biosynthesis, from sweet-potato roots. Journal of Biochemistry 117, 120–124. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Komatsu A, Zikihara K, Nanjo T, Tokutomi S, Wada M, Kiyosue T.. 2008. Blue light diminishes interaction of PAS/LOV proteins, putative blue light receptors in Arabidopsis thaliana, with their interacting partners. Journal of Plant Research 121, 97–105. [DOI] [PubMed] [Google Scholar]
- O’Leary BM, Lee CP, Atkin OK, Cheng R, Brown TB, Millar AH.. 2017. Variation in leaf respiration rates at night correlates with carbohydrate and amino acid supply. Plant Physiology 174, 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos E, Kiddle G, Pellny TK, Kumar S, Foyer CH.. 2006. Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. Journal of Experimental Botany 57, 1645–1655. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Ishii T, Albersheim P, Darvill AG.. 2004. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annual Review of Plant Biology 55, 109–139. [DOI] [PubMed] [Google Scholar]
- Ostergaard J, Persiau G, Davey MW, Bauw G, Van Montagu M.. 1997. Isolation of a cDNA coding for l-galactono-γ-lactone dehydrogenase, an enzyme involved in the biosynthesis of ascorbic acid in plants. Purification, characterization, cDNA cloning, and expression in yeast. Journal of Biological Chemistry 272, 30009–30016. [DOI] [PubMed] [Google Scholar]
- Page M, Sultana N, Paszkiewicz K, Florance H, Smirnoff N.. 2012. The influence of ascorbate on anthocyanin accumulation during high light acclimation in Arabidopsis thaliana: further evidence for redox control of anthocyanin synthesis. Plant, Cell & Environment 35, 388–404. [DOI] [PubMed] [Google Scholar]
- Pal SK, Liput M, Piques M, et al. 2013. Diurnal changes of polysome loading track sucrose content in the rosette of wild-type arabidopsis and the starchless pgm mutant. Plant Physiology 162, 1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanca JE, Smirnoff N.. 1999. Ascorbic acid metabolism in pea seedlings. A comparison of d-glucosone, l-sorbosone, and l-galactono-1,4-lactone as ascorbate precursors. Plant Physiology 120, 453–462. [Google Scholar]
- Pallanca JE, Smirnoff N.. 2000. The control of ascorbic acid synthesis and turnover in pea seedlings. Journal of Experimental Botany 51, 669–674. [PubMed] [Google Scholar]
- Parsons HT, Fry SC.. 2010. Reactive oxygen species-induced release of intracellular ascorbate in plant cell-suspension cultures and evidence for pulsing of net release rate. New Phytologist 187, 332–342. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH.. 2003. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. The Plant Cell 15, 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH.. 2005. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiology 139, 1291–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignocchi C, Fletcher JM, Wilkinson JE, Barnes JD, Foyer CH.. 2003. The function of ascorbate oxidase in tobacco. Plant Physiology 132, 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineau B, Layoune O, Danon A, de Paepe R.. 2008. l-Galactono-1,4-lactone dehydrogenase is required for the accumulation of plant respiratory complex I. Journal of Biological Chemistry 283, 32500–32505. [DOI] [PubMed] [Google Scholar]
- Plumb W, Townsend AJ, Rasool B, Alomrani S, Razak N, Karpinska B, Ruban AV, Foyer CH.. 2018. Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana. Journal of Experimental Botany 69, 2823–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H.. 2004. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiology 134, 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Liu Z, Fan M, Chen Y, Tian H, Wu D, Gao H, Ren C, Song S, Xie D.. 2017. GDP-d-mannose epimerase regulates male gametophyte development, plant growth and leaf senescence in Arabidopsis. Scientific Reports 7, 10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian WQ, Yu CM, Qin HJ, Liu X, Zhang AM, Johansen IE, Wang DW.. 2007. Molecular and functional analysis of phosphomannomutase (PMM) from higher plants and genetic evidence for the involvement of PMM in ascorbic acid biosynthesis in Arabidopsis and Nicotiana benthamiana. The Plant Journal 49, 399–413. [DOI] [PubMed] [Google Scholar]
- Qin C, Qian WQ, Wang WF, Wu Y, Yu CM, Jiang XH, Wang DW, Wu P.. 2008. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 105, 18308–18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Deng ZA, Zhang CY, Wang YY, Wang J, Liu H, Zhang ZL, Huang RF, Zhang ZJ.. 2016. Rice GDP-mannose pyrophosphorylase OsVTC1-1 and OsVTC1-3 play different roles in ascorbic acid synthesis. Plant Molecular Biology 90, 533–533. [DOI] [PubMed] [Google Scholar]
- Ramirez L, Bartoli CG, Lamattina L.. 2013. Glutathione and ascorbic acid protect Arabidopsis plants against detrimental effects of iron deficiency. Journal of Experimental Botany 64, 3169–3178. [DOI] [PubMed] [Google Scholar]
- Rautengarten C, Ebert B, Liu L, Stonebloom S, Smith-Moritz AM, Pauly M, Orellana A, Scheller HV, Heazlewood JL.. 2016. The Arabidopsis Golgi-localized GDP-l-fucose transporter is required for plant development. Nature Communications 7, 12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom B, Grun M, Loewus FA.. 1983. Biosynthesis of l-ascorbic acid in Chlorella pyrenoidosa. Plant Science Letters 28, 299–305. [Google Scholar]
- Richardson AT, Cho J, McGhie TK, Larsen DS, Schaffer RJ, Espley RV, Perry NB.. 2020. Discovery of a stable vitamin C glycoside in crab apples (Malus sylvestris). Phytochemistry 173, 112297. [DOI] [PubMed] [Google Scholar]
- Richardson AT, McGhie TK, Cordiner SB, Stephens TTH, Larsen DS, Laing WA, Perry NB.. 2021. 2-O-β-d-Glucopyranosyl l-ascorbic acid, a stable form of vitamin C, is widespread in crop plants. Journal of Agricultural and Food Chemistry 69, 966–973. [DOI] [PubMed] [Google Scholar]
- Running JA, Burlingame RP, Berry A.. 2003. The pathway of l-ascorbic acid biosynthesis in the colourless microalga Prototheca moriformis. Journal of Experimental Botany 54, 1841–1849. [DOI] [PubMed] [Google Scholar]
- Running JA, Peng S, Rosson RA.. 2004. The biotechnology of ascorbate manufacture. In: Asard H, May JM, Smirnoff N, eds. Vitamin C: functions and metabolism in animals and plants. Oxford: BIOS Scientific Publishers, 49–64. [Google Scholar]
- Running JA, Severson DK, Schneider KJ.. 2002. Extracellular production of l-ascorbic acid by Chlorella protothecoides, Prototheca species, and mutants of P. moriformis during aerobic culturing at low pH. Journal of Industrial Microbiology & Biotechnology 29, 93–98. [DOI] [PubMed] [Google Scholar]
- Saito K, Nick JA, Loewus FA.. 1990. d-Glucosone and l-sorbosone, putative intermediates of l-ascorbic-acid biosynthesis in detached bean and spinach leaves. Plant Physiology 94, 1496–1500. [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, et al. 2005. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. The Plant Journal 44, 653–668. [DOI] [PubMed] [Google Scholar]
- Sauer M, Branduardi P, Valli M, Porro D.. 2004. Production of l-ascorbic acid by metabolically engineered Saccharomyces cerevisiae and Zygosaccharomyces bailii. Applied and Environmental Microbiology 70, 6086–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawake S, Tajima N, Mortimer JC, et al. 2015. KONJAC1 and 2 are key factors for GDP-mannose generation and affect l-ascorbic acid and glucomannan biosynthesis in Arabidopsis. The Plant Cell 27, 3397–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena SC, Salvi P, Kaur H, Verma P, Petla BP, Rao V, Kamble N, Majee M.. 2013. Differentially expressed myo-inositol monophosphatase gene (CaIMP) in chickpea (Cicer arietinum L) encodes a lithium-sensitive phosphatase enzyme with broad substrate specificity and improves seed germination and seedling growth under abiotic stresses. Journal of Experimental Botany 64, 5623–5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertl P, Sunderhaus S, Klodmann J, Grozeff GEG, Bartoli CG, Braun H-P.. 2012. l-Galactono-1,4-lactone dehydrogenase (GLDH) forms part of three subcomplexes of mitochondrial complex I in Arabidopsis thaliana. Journal of Biological Chemistry 287, 14412–14419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmeyer J, Bock R, Meyer EH.. 2016. l-Galactono-1,4-lactone dehydrogenase is an assembly factor of the membrane arm of mitochondrial complex I in Arabidopsis. Plant Molecular Biology 90, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaubelt D, Queval G, Dong Y, Diaz-Vivancos P, Makgopa ME, Howell G, De Simone A, Bai J, Hannah MA, Foyer CH.. 2015. Low glutathione regulates gene expression and the redox potentials of the nucleus and cytosol in Arabidopsis thaliana. Plant, Cell & Environment 38, 266–279. [DOI] [PubMed] [Google Scholar]
- Sechet J, Htwe S, Urbanowicz B, et al. 2018. Suppression of Arabidopsis GGLT1 affects growth by reducing the l-galactose content and borate cross-linking of rhamnogalacturonan-II. The Plant Journal 96, 1036–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn ME, Gergoff Grozeff GE, Alegre ML, Barrile F, De Tullio MC, Bartoli CG.. 2016. Effect of mitochondrial ascorbic acid synthesis on photosynthesis. Plant Physiology and Biochemistry 104, 29–35. [DOI] [PubMed] [Google Scholar]
- Sharples SC, Fry SC.. 2007. Radioisotope ratios discriminate between competing pathways of cell wall polysaccharide and RNA biosynthesis in living plant cells. The Plant Journal 52, 252–262. [DOI] [PubMed] [Google Scholar]
- Shen J, Griffiths PT, Campbell SJ, Utinger B, Kalberer M, Paulson SE.. 2021. Ascorbate oxidation by iron, copper and reactive oxygen species: review, model development, and derivation of key rate constants. Scientific Reports 11, 7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka S, Nakano Y, Kitaoka S.. 1979. Biosynthetic pathway of l-ascorbic acid in Euglena gracilis Z. Journal of Nutritional Science and Vitaminology 25, 299–307. [DOI] [PubMed] [Google Scholar]
- Shikita M, Fahey JW, Golden TR, Holtzclaw WD, Talalay P.. 1999. An unusual case of ‘uncompetitive activation’ by ascorbic acid: purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. The Biochemical Journal 341, 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siendones E, Gonzalez-Reyes JA, Santos-Ocana C, Navas P, Cordoba F.. 1999. Biosynthesis of ascorbic acid in kidney bean l-galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiology 120, 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. 2000. Ascorbate biosynthesis and function in photoprotection. Philosophical Transactions of the Royal Society B: Biological Sciences 355, 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. 2003. Vitamin C booster. Nature Biotechnology 21, 134–136. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. 2011. Vitamin C: the metabolism and functions of ascorbic acid in plants. Advances in Botanical Research 59, 107–177. [Google Scholar]
- Smirnoff N. 2018. Ascorbic acid metabolism and functions: a comparison of plants and mammals. Free Radical Biology and Medicine 122, 116–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D.. 2019. Hydrogen peroxide metabolism and functions in plants. New Phytologist 221, 1197–1214. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA.. 2001. Biosynthesis of ascorbic acid in plants: a renaissance. Annual Review of Plant Physiology and Plant Molecular Biology 52, 437–467. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Pallanca JE.. 1996. Ascorbate metabolism in relation to oxidative stress. Biochemical Society Transactions 24, 472–478. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL.. 1999. International Patent. Plant galactose dehydrogenase. WO 99/33995. [Google Scholar]
- Sodeyama T, Nishikawa H, Harai K, Takeshima D, Sawa Y, Maruta T, Ishikawa T.. 2021. The d-mannose/l-galactose pathway is the dominant ascorbate biosynthetic route in the moss Physcomitrium patens. The Plant Journal 107, 1724–1738. [DOI] [PubMed] [Google Scholar]
- Soufari H, Parrot C, Kuhn L, Waltz F, Hashem Y.. 2020. Specific features and assembly of the plant mitochondrial complex I revealed by cryo-EM. Nature Communications 11, 5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrwohldt N, Ehinger A, Thellmann K, Schaller A.. 2020. Processing and formation of bioactive CLE40 peptide are controlled by posttranslational proline hydroxylation. Plant Physiology 184, 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrwohldt N, Schaller A.. 2019. Regulation of plant peptide hormones and growth factors by post-translational modification. Plant Biology 21, 49–63. [DOI] [PubMed] [Google Scholar]
- Su J-C, Hassid WZ.. 1962. Carbohydrates and nucleotides in the red alga Porphyra perforata II. Separation and identification of nucleotides. Biochemistry 1, 474–480. [DOI] [PubMed] [Google Scholar]
- Sultana N, Florance HV, Johns A, Smirnoff N.. 2015. Ascorbate deficiency influences the leaf cell wall glycoproteome in Arabidopsis thaliana. Plant, Cell & Environment 38, 375–384. [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Fernie AR.. 2018. The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation. Nature Communications 9, 2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka A, Horemans N, Banhegyi G, Asard H.. 2004. Facilitated glucose and dehydroascorbate transport in plant mitochondria. Archives of Biochemistry and Biophysics 428, 73–80. [DOI] [PubMed] [Google Scholar]
- Szarka A, Horemans N, Kovacs Z, Grof P, Mayer M, Banhegyi G.. 2007. Dehydroascorbate reduction in plant mitochondria is coupled to the respiratory electron transfer chain. Physiologia Plantarum 129, 225–232. [Google Scholar]
- Szarka A, Lorincz T.. 2014. The role of ascorbate in protein folding. Protoplasma 251, 489–497. [DOI] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, et al. 2013. Metabolic fluxes in an illuminated Arabidopsis rosette. The Plant Cell 25, 694–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takahashi R, Hamada A, Terai Y, Ogawa T, Sawa Y, Ishikawa T, Maruta T.. 2021. Distribution and functions of monodehydroascorbate reductases in plants: comprehensive reverse genetic analysis of Arabidopsis thaliana enzymes. Antioxidants 10, 1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Hao Z, Huang C.. 2020. Molecular evolution of GDP-l-galactose phosphorylase, a key regulatory gene in plant ascorbate biosynthesis. AoB Plants 12, plaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JJ, Wu H, Li ZY, Huang CH, Xu XB.. 2018. Molecular evolution of GDP-d-mannose epimerase (GME), a key gene in plant ascorbic acid biosynthesis. Frontiers in Plant Science 9, 1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedone L, Hancock RD, Alberino S, Haupt S, Viola R.. 2004. Long-distance transport of l-ascorbic acid in potato. BMC Plant Biology 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi M, De Tullio MC.. 2022. The perils of planning strategies to increase vitamin C content in plants: beyond the hype. Frontiers in Plant Science 13, 1096549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YS, Deng YD, Zhang WH, et al. 2022. Metabolic engineering of Escherichia coli for direct production of vitamin C from d-glucose. Biotechnology for Biofuels and Bioproducts 15, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi F, Paciolla C, Arrigoni O.. 1999. The ascorbate system in recalcitrant and orthodox seeds. Physiologia Plantarum 105, 193–198. [Google Scholar]
- Torabinejad J, Donahue JL, Gunesekera BN, Allen-Daniels MJ, Gillaspy GE.. 2009. VTC4 is a bifunctional enzyme that affects myo-inositol and ascorbate biosynthesis in plants. Plant Physiology 150, 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth SZ. 2023. The functions of chloroplastic ascorbate in vascular plants and algae. International Journal of Molecular Sciences 24, 2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda-Ono Y, Maeda M, Nakao M, Yoshimura M, Sugiura-Tomimori N, Fukami H.. 2004. 2-O-(β-d-Glucopyranosyl)ascorbic acid, a novel ascorbic acid analogue isolated from Lycium fruit. Journal of Agricultural and Food Chemistry 52, 2092–2096. [DOI] [PubMed] [Google Scholar]
- Truffault V, Fry SC, Stevens RG, Gautier H.. 2017. Ascorbate degradation in tomato leads to accumulation of oxalate, threonate and oxalyl threonate. The Plant Journal 89, 996–1008. [DOI] [PubMed] [Google Scholar]
- Ugalde JM, Aller I, Kudrjasova L, et al. 2022. Endoplasmic reticulum oxidoreductin provides resilience against reductive stress and hypoxic conditions by mediating luminal redox dynamics. The Plant Cell 34, 4007–4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzica EI, Adler LN, Page MD, Linster CL, Arbing MA, Casero D, Pellegrini M, Merchant SS, Clarke SG.. 2012a. Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-l-galactose phosphorylase. Journal of Biological Chemistry 287, 14234–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzica EI, Casero D, Yamasaki H, et al. 2012b. Systems and trans-system level analysis identifies conserved iron deficiency responses in the plant lineage. The Plant Cell 24, 3921–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst S, Filipovska T, Hanson J, Smeekens S.. 2020. Metabolite control of translation by conserved peptide uORFs: the ribosome as a metabolite multisensor. Plant Physiology 182, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JA, Leonardo DA, Pereira HD, Lopes AR, Rodriguez HN, Cobos M, Marapara JL, Castro JC, Garratt RC.. 2022. Structural characterization of l-galactose dehydrogenase: an essential enzyme for vitamin C biosynthesis. Plant and Cell Physiology 63, 1140–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versaw WK, Garcia LR.. 2017. Intracellular transport and compartmentation of phosphate in plants. Current Opinion in Plant Biology 39, 25–30. [DOI] [PubMed] [Google Scholar]
- Vidal-Meireles A, Neupert J, Zsigmond L, Rosado-Souza L, Kovacs L, Nagy V, Galambos A, Fernie AR, Bock R, Toth SZ.. 2017. Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the VTC2 gene encoding GDP-l-galactose phosphorylase. New Phytologist 214, 668–681. [DOI] [PubMed] [Google Scholar]
- Vidal-Meireles A, Toth D, Kovacs L, Neupert J, Toth SZ.. 2019. Ascorbate deficiency does not limit non-photochemical quenching in Chlamydomonas reinhardtii. Plant Physiology 182, 597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, Gilbert L, Rihouey C, Driouich A, Rothan C, Baldet P, Lerouge P.. 2011. Silencing of the GDP-d-mannose 3,5-epimerase affects the structure and cross-linking of the pectic polysaccharide rhamnogalacturonan II and plant growth in tomato. Journal of Biological Chemistry 286, 8014–8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yu YW, Zhang ZJ, Quan RD, Zhang HW, Ma LG, Deng XW, Huang RF.. 2013. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. The Plant Cell 25, 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Zhang QY, Wang F, Meng X, Meng QW.. 2012. Ascorbate plays a key role in alleviating low temperature-induced oxidative stress in Arabidopsis. Photosynthetica 50, 602–612. [Google Scholar]
- Wang P, Zeng W, Xu S, Du G, Zhou J, Chen J.. 2018. Current challenges facing one-step production of l-ascorbic acid. Biotechnology Advances 36, 1882–1899. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Suzuki K, Kitamura S.. 2006. Characterization of a GDP-d-mannose 3'',5''-epimerase from rice. Phytochemistry 67, 338–346. [DOI] [PubMed] [Google Scholar]
- Weigand C, Brady D, Davis JA, Speicher T, Bacalso J, Jones D, Miller G, Choi WG, Harper JF.. 2023. Overexpressing Vitamin C Defective 2 reduces fertility and alters Ca2+ signals in Arabidopsis pollen. Plant Physiology 191, 2276–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WW, Xu DP, Yang YF, Rocque PA.. 1990. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. Journal of Biological Chemistry 265, 15361–15364. [PubMed] [Google Scholar]
- Wheeler GL. 2000. The biosynthesis of ascorbic acid in plants. PhD Thesis, University of Exeter. [Google Scholar]
- Wheeler G, Ishikawa T, Pornsaksit V, Smirnoff N.. 2015. Evolution of alternative biosynthetic pathways for vitamin C following plastid acquisition in photosynthetic eukaryotes. eLife 4, e06369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N.. 1998. The biosynthetic pathway of vitamin C in higher plants. Nature 393, 365–369. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Goossens A, Inzé D.. 2005. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. Journal of Experimental Botany 56, 2527–2538. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Persiau G, Van Doorsselaere J, Davey MW, Demol H, Vandekerckhove J, Van Montagu M, Zabeau M, Boerjan W.. 2001. Partial purification and identification of GDP-mannose 3'', 5''-epimerase of Arabidopsis thaliana, a key enzyme of the plant vitamin C pathway. Proceedings of the National Academy of Sciences, USA 98, 14843–14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu M.. 2003. GDP-mannose 3'',5''-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. Journal of Biological Chemistry 278, 47483–47490. [DOI] [PubMed] [Google Scholar]
- Wolucka BA, Van Montagu M.. 2007. The VTC2 cycle and the de novo biosynthesis pathways for vitamin C in plants: an opinion. Phytochemistry 68, 2602–2613. [DOI] [PubMed] [Google Scholar]
- Wu SY, Hou LL, Zhu J, Wang YC, Zheng YL, Hou JQ, Yang ZN, Lou Y.. 2023. Ascorbic acid-mediated reactive oxygen species homeostasis modulates the switch from tapetal cell division to cell differentiation in Arabidopsis. The Plant Cell 35, 1474–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Huang B, Fang X, et al. 2023. SlMYB99-mediated auxin and abscisic acid antagonistically regulate ascorbic acid biosynthesis in tomato. New Phytologist 239, 949–963. [DOI] [PubMed] [Google Scholar]
- Xue JH, Chen GD, Hao F, et al. 2019. A vitamin-C-derived DNA modification catalysed by an algal TET homologue. Nature 569, 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuta Y, Maruta T, Nakamura A, Mieda T, Yoshimura K, Ishikawa T, Shigeoka S.. 2008. Conversion of l-galactono-1,4-lactone to l-ascorbate is regulated by the photosynthetic electron transport chain in Arabidopsis. Bioscience, Biotechnology, and Biochemistry 72, 2598–2607. [Google Scholar]
- Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S.. 2007. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. Journal of Experimental Botany 58, 2661–2671. [DOI] [PubMed] [Google Scholar]
- Yao YN, You JJ, Ou YB, Ma JB, Wu XL, Xu G.. 2015. Ultraviolet-B protection of ascorbate and tocopherol in plants related with their function on the stability on carotenoid and phenylpropanoid compounds. Plant Physiology and Biochemistry 90, 23–31. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Nakane T, Kume S, Shiomi Y, Maruta T, Ishikawa T, Shigeoka S.. 2014. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Bioscience, Biotechnology, and Biochemistry 78, 60–66. [DOI] [PubMed] [Google Scholar]
- Young JI, Zuchner S, Wang G.. 2015. Regulation of the epigenome by vitamin C. Annual Review of Nutrition 35, 545–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharieva T, Yamashita K, Matsumoto H.. 1999. Iron deficiency induced changes in ascorbate content and enzyme activities related to ascorbate metabolism in cucumber roots. Plant and Cell Physiology 40, 273–280. [Google Scholar]
- Zaharieva TB, Abadia J.. 2003. Iron deficiency enhances the levels of ascorbate, glutathione, and related enzymes in sugar beet roots. Protoplasma 221, 269–275. [DOI] [PubMed] [Google Scholar]
- Zechmann B, Stumpe M, Mauch F.. 2011. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 233, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhao S, Li YS, He C, Wang X, Liu L.. 2022. Crystal structures of Arabidopsis thaliana GDP-d-mannose pyrophosphorylase VITAMIN C DEFECTIVE 1. Frontiers in Plant Science 13, 899738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Si X, Ji X, Fan R, Liu J, Chen K, Wang D, Gao C.. 2018. Genome editing of upstream open reading frames enables translational control in plants. Nature Biotechnology 36, 894–898. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xiang Y, He N, et al. 2020. Enhanced vitamin C production mediated by an ABA-induced PTP-like nucleotidase improves drought tolerance of Arabidopsis and maize. Molecular Plant 13, 760–776. [DOI] [PubMed] [Google Scholar]
- Zhang L, Song HY, Li BH, Wang M, Di DW, Lin XY, Kronzucker HJ, Shi WM, Li GJ.. 2021. Induction of S-nitrosoglutathione reductase protects root growth from ammonium toxicity by regulating potassium homeostasis in Arabidopsis and rice. Journal of Experimental Botany 72, 4548–4564. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zheng J, Zhan Y, et al. 2023. GmPLP1 negatively regulates soybean resistance to high light stress by modulating photosynthetic capacity and reactive oxygen species accumulation in a blue light-dependent manner. Plant Biotechnology Journal 21, 2625–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitkovich A. 2020. Nuclear and cytoplasmic functions of vitamin C. Chemical Research in Toxicology 33, 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Bi Y, Ding M, Yuan Y.. 2021. One-step biosynthesis of vitamin C in Saccharomyces cerevisiae. Frontiers in Microbiology 12, 643472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data are included in this review.