Abstract
Striatal dopamine governs a wide range of behavioral functions, yet local dopamine concentrations can be dissociated from somatic activity. Here, we discuss how dopamine’s diverse roles in behavior may be driven by local circuit mechanisms shaping dopamine release. We first look at historical and recent work demonstrating that striatal circuits interact with dopaminergic terminals to either initiate release of dopamine or modulate release of dopamine initiated by spiking in midbrain dopamine neurons, with particular attention to GABAergic and cholinergic local circuit mechanisms. Then we discuss some of the first in vivo studies of acetylcholine-dopamine interactions in striatum and broadly discuss necessary future work in understanding the roles of midbrain versus striatal dopamine regulation.
Introduction
Our knowledge of the behavioral functions of dopamine has evolved since its discovery in 1957. The earliest investigations of dopaminergic function involved pharmacological manipulations wherein local injections of 6-Hydroxydopamine (6-OHDA, which depletes dopamine) into dopaminergic nuclei generated a motor deficit exhibited by rotational behavior. Combined with foundational work that uncovered loss of dopamine in postmortem brain tissue of patients with Parkinson’s Disease [1], this pointed to a key role in motor behavior. Hypotheses of dopamine’s function were subsequently broadened as intracranial self-stimulation studies suggested that dopamine subserved reward-related functions, with early microdialysis studies demonstrating increases in dopamine in response to both rewarding and aversive stimuli [2]. This was followed by ground-breaking electrophysiological recordings from Schultz and colleagues [3] showing midbrain dopamine neuron (DAN) firing rates encoded deviations from expected outcomes known as reward prediction errors. In recent years the number of functions attributed to dopamine signaling has continued to increase, including a range of disparate behaviors including regulation of motor vigor [4], scratching-induced reward [5], and auditory perception [6], to name just a few. This expansion of dopamine functions beyond movement production raises the question of whether this single neuromodulator utilizes diverse spatial and temporal regulatory mechanisms in mediating distinct behavioral processes.
One level of striatal dopamine regulation occurs via regulation of somatic DAN spiking in the midbrain [7,8]. However, results showing a disconnect between recorded somatic DAN activity and striatal dopamine release suggest further layers of regulation. This divergence has consistently been observed, first with microdialysis and more recently with fast scan cyclic voltammetry (FSCV) and fluorescent dopamine sensors, both of which provide higher temporal precision. Recent experiments from Berke and colleagues [9] highlight this conundrum - when simultaneously recording DAN spiking in the ventral tegmental area (VTA) and dopamine sensor activity in the nucleus accumbens (NAc), they revealed value-related increases in striatal dopamine levels that did not correlate with increased midbrain DAN spiking. Here we examine an emerging body of work that might provide mechanistic explanations for this dissociation.
To understand how local striatal regulation of dopamine may occur, we must consider the unique characteristics of dopaminergic innervation and release. Although sparse, DANs provide extensive unmyelinated innervation to target structures, particularly the striatum. A single DAN can make more than 350,000 synapses in striatum alone, and the axons are so highly branched that one axon can occupy more than 5% of the striatal volume [10]. While only ~1/3rd of DAN varicosities contain active zone machinery like that found in glutamatergic synapses, they nevertheless release dopamine with high release probability in a relatively focal manner [8]. The local mechanisms modulating dopamine release from these sites can be homosynaptic, via responses to dopamine itself, or heterosynaptic, via responses to other signaling molecules. While a thorough catalogue of these mechanisms can be seen elsewhere [11], we focus here on how striatal circuits - including both GABAergic and cholinergic interneuron subtypes as well as their cortical and thalamic drivers - interact with DAN terminals to either initiate release of dopamine or modulate release of dopamine initiated by spiking in midbrain DANs (Figure 1). Then we look at some of the first in vivo studies of acetylcholine-dopamine interactions in striatum and broadly discuss necessary future work in understanding the roles of midbrain versus striatal dopamine regulation.
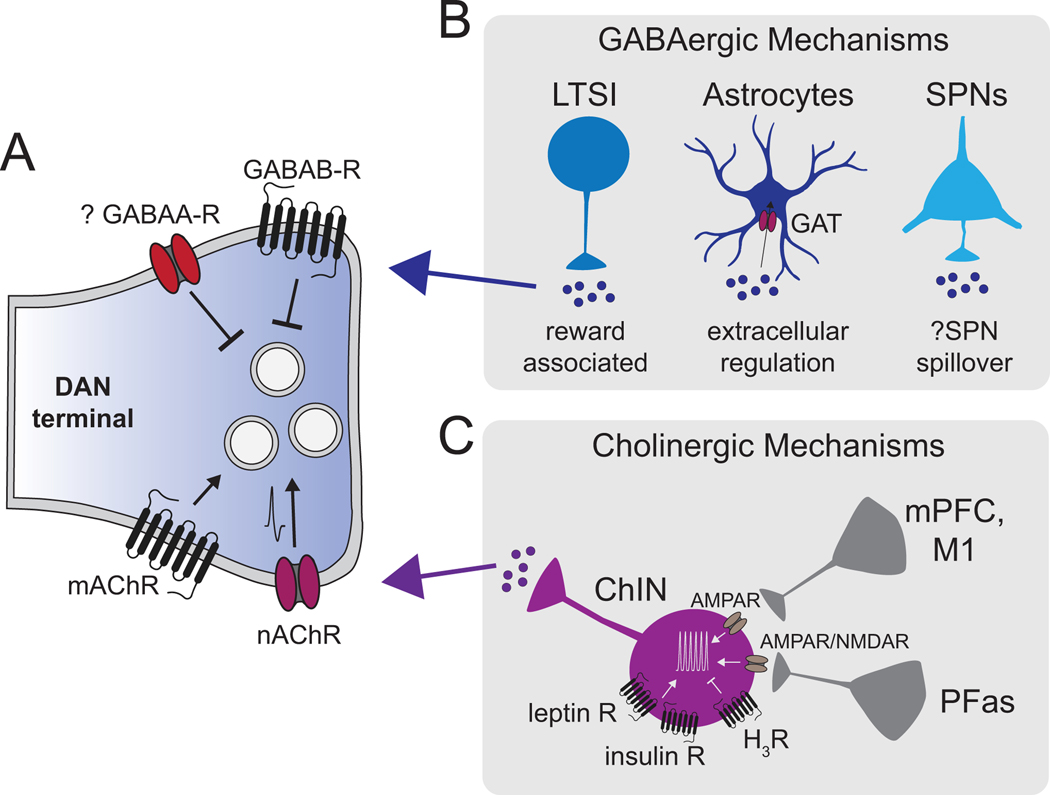
Figure 1. Cellular mechanisms for striatal control of DA release.
(A) Key neurotransmitter receptors on striatal DAN terminals that can modulate dopamine release. Arrowheads indicate facilitation of synaptic vesicle release while straight lines indicate suppression of synaptic release. (B) Potential sources of GABAergic modulation include low-threshold spiking interneurons (LTSIs), regulation of extracellular GABA levels via astrocyte GABA uptake transporters (GAT), and GABA spill-over from spiny projection neurons which comprise ~95% of striatal neurons and extensively collateralize locally. (C) Regulation of cholinergic-mediated DA release is impacted by both regulators of cholinergic interneuron excitability (including leptin, insulin and H3-histamine receptors, among others) and glutamatergic drive from both cortical and thalamic projection neurons.
Regulation by local circuit GABA
GABA has long been reported to modulate striatal dopamine [12]. As the striatum is primarily comprised of GABAergic cells, the vast majority of which participate in long loop feedback circuits to the midbrain, it is challenging to align specific GABAergic sources to local dopamine modulation in vivo. The sources of striatal GABA may include GABAergic afferents, extrasynaptic action potential-independent spillover from spiny projection neurons (SPNs), low threshold spiking interneurons (LTSIs), GABA co-released by dopamine neurons and ambient GABA tone set by uptake transporters or release of GABA from striatal astrocytes [12–16]. Within striatum, GABA may act on ionotropic GABAA and/or metabotropic GABAB receptors to attenuate dopamine release. There is ultrastructural evidence for GABAB receptors on dopamine-like axons in non-human primates [18] and rat [19]. Furthermore, striatal GABAB agonism attenuates dopamine release both in vivo and in acute slice [13,15,20], while GABAB antagonism potentiates dopamine [13,15,17]. These effects occur independently from nicotinic acetylcholine receptor (nAChRs) activity described in ‘Regulators of cholinergic-mediated dopamine regulation’ below. While this work strongly implies GABA can directly suppress dopamine release via presynaptic GABAB receptors, the evidence for modulation by GABAA has been less consistent. In vivo, effects of GABAA modulation on extracellular dopamine are ablated by local kainic acid lesions, which lesion striatal cell bodies while leaving afferents intact, suggesting the effects of GABAA are mediated by striatonigral feedback or striatal interneurons. Work from the España lab [15] later supported these findings, demonstrating GABAA-mediated reductions in evoked dopamine release are blocked by GABAB antagonism. However, recent work from the Khaliq lab [16] using whole-cell and perforated patch recordings on DAN axons reveals further complexity, with the degree of GABAA mediated modulation potentially being a function of striatal activity level.
One unresolved issue is whether there are roles for phasic GABA in the regulation of dopamine release from DAN terminals or whether all modulatory effects are a function of the ambient or tonic levels of striatal GABA. Functionally speaking, the former could permit discrete, temporally specific modulation of striatal dopamine while the latter would be less precise, influencing dopamine levels over the time course of seconds to minutes. Recently, we demonstrated dorsal striatal LTSIs, which have phasic activity to rewarded choice outcomes, can regulate dopaminergic axons, raising the possibility of more temporally discrete dopamine control. Nevertheless, two points from our study argue against precise temporal control – (1) using a slice preparation with simultaneous excitatory optogenetic control of DAN terminals and inhibitory optogenetic control of LTSIs, we found that inhibition of LTSIs increased evoked striatal dopamine even if LTSI manipulation occurred before opticallyevoked dopamine release and (2) these effects were mediated exclusively by slower GABAB receptor signaling [17]. Recent work highlighting a role of GABA uptake transporters, including those on astrocytes, which regulate tonic GABAergic control of evoked dopamine in dorsal but not ventral striatum, lends further strength to the idea of less temporally precise mechanisms for GABAergic modulation of striatal dopamine [12,14]. Resolving the respective roles of these fast and slow GABARs on dopamine release, in addition to a greater knowledge of how different striatal interneuron subtypes are modulated during behavior, will help to define the constraints of local GABAergic control of striatal dopamine.
Regulators of cholinergic-mediated dopamine regulation
The effects of cholinergic transmission on striatal dopamine release have received considerable attention (see [21,22] for review). The two primary sources of striatal acetylcholine are local cholinergic interneurons (ChINs) and long-range projections from pedunculopontine and laterodorsal tegmental nuclei [23,24]. Although these cholinergic midbrain afferents shape action strategies [25], only ChINs directly modulate dopamine release in the striatum [26]. Several landmark papers uncovered direct, local mechanisms whereby nicotinic transmission enhanced striatal dopamine release [27–29]. Dopamine evoked by single or low-frequency electrical stimulation in acute striatal slices can be partially blocked by nAChR antagonism [27–29]. In baseline conditions, repeated electrical stimulation induces a short-term depression that limits subsequent dopamine release. However, with nAChR antagonism this suppression is lifted, leading to the hypothesis that acetylcholine action on nAChRs serves as a low-pass filter at DAN terminals [28,29]. Our understanding of how nAChRs locally control dopamine has evolved substantially in the last 15 years with the power of optogenetics. Preparations using slice FSCV with selective optogenetic activation of ChINs were sufficient to drive dopamine release independent of direct stimulation of DAN axons [21,30]. Recently, elegant direct recordings of dopamine axons in the striatum have shown that ChIN activation of dopamine axons via nAChRs generates local action potentials that broaden the area of dopamine release [31,32]. Muscarinic acetylcholine receptors also have complex contributions to cholinergic control of accumbens dopamine - M5 receptors on DAN terminals enhance dopamine release while M2 and M4 auto-receptors on ChINs restrain cholinergic-mediated dopamine release [33].
Multiple neuromodulators, including leptin, insulin, and histamine, have recently been shown to exert local heterosynaptic control over dopamine release indirectly via ChIN modulation. Leptin is a metabolic hormone important for signaling satiety and regulating energy expenditure known to interact with the mesolimbic dopamine system. The Rice lab [34] has identified leptin receptors on ChIN cell bodies and processes and found that leptin increased evoked dopamine concentrations via increases in ChIN excitability. Insulin is another metabolic hormone classically considered a satiety signal which has also been recently shown to locally modulate striatal dopamine [35,36]. The striatum expresses abundant insulin receptors, and insulin can locally influence dopamine release either via increasing ChIN excitability or via astrocyte-released ATP directly mediating dopamine release from terminals [35,37,38]. Histamine has numerous regulatory functions, most notably for immune responses and sleep-wake cycle control [39,40]. Recently Trudeau and colleagues [41] demonstrated that histamine H3 receptors are expressed on ChINs, and activation of these H3Rs reduces the firing rates of ChINs in ventral striatum, concomitant with a decrease in electrically evoked dopamine release.
A wealth of evidence shows that striatal dopamine is also under glutamatergic regulation via cortical and thalamic excitatory drive of ChINs [42–44], shown via optogenetic recruitment of these structures combined with FSCV and microdialysis measures. These studies have revealed multiple levels of circuit-specific regulation that could have unique impacts on behavior – mPFC inputs drive feed-forward striatal dopamine release to specific striatal regions [45], endocannabinoid signaling within these cortical terminals constrains feed-forward dopamine release [44], and parafascicular driven ChIN-evoked dopamine release requires both NMDAR and AMPAR activation in contrast to exclusive AMPAR requirements from cortical inputs [43]. Taken together, the data present a compelling mechanistic picture for local ChIN-mediated modulation of striatal dopamine release. In these scenarios, anything that modulates ChIN output, including changes in excitability of ChINs or their drive by excitatory projections, could potentially have strong effects on uncoupling striatal dopamine from DAN somatic spiking. However, whether and under what conditions these phenomena occur in more dynamic in vivo systems and what specific computational advantages they provide remains unclear (see ‘New insights into local dopamine control from in vivo studies’ below for further discussion).
New insights into local dopamine control from in vivo studies
In vitro preps have given us insight into multiple levels of local striatal control over dopamine, which are now being expanded upon in dynamic in vivo systems. The use of sensors for acetylcholine and dopamine measured via fiber photometry has provided our first insights into striatal dopamine modulation in awake behaving animals. Mohebi and colleagues [46] showed that reward approach behaviors were marked by dopamine release ramps that coincided with ramps of ChIN calcium activity, but not changing single unit spiking activity of DANs in the lateral VTA. While this work supports the idea of local cholinergic modulation of striatal dopamine, two other studies in dorsal and ventrolateral striatum paint a more complex picture. The Sabatini and Tritsch labs [47,48] performed simultaneous imaging of dopamine and acetylcholine sensors and found these signals to be continuous anti-correlated oscillations, with the peak of the dopamine signal preceding that of acetylcholine by ~100msec. Amazingly, this relationship reproducibly appeared at rest, during locomotion, spontaneous reward delivery and in an operant task modulated by decision history and outcome. Despite the robustness of these state-independent patterns, neither team found evidence that they were supported by local cholinergic modulation of dopamine release. Targeted disruption of acetylcholine release via tetanus toxin or genetic ablation of β2-nicotinic receptors from DANs had no impact on the robust oscillatory patterns of striatal dopamine and acetylcholine. These results however contrast with the blunting of sensory-evoked dorsal striatal dopamine transients in response to pharmacological blockade of β2 nicotinic receptors via DHβE [31]. It is presently unclear what accounts for the discrepancies between in vivo experiments and their relationship to the extensive in vitro literature which would have predicted cholinergic boosting of dopamine within striatum. As with all in vivo experiments, a major caveat is that only a small subset of behavioral processes is investigated, and it remains possible that dopamine-acetylcholine interactions are relevant at discrete times or for only a subset of behavioral processes. Furthermore, these data do not invalidate the importance of local striatal dopamine-acetylcholine interactions – dopamine can inhibit striatal acetylcholine via inhibitory D2-dopamine receptor mechanisms, and perturbing these pathways disrupts choice patterns, supporting the importance of both neuromodulatory systems in regulating downstream behavior [47]. Together, these first in vivo insights remind us of the large task ahead in merging understanding from mechanistic acute slice studies with the function of these systems in the behaving brain.
Looking Forward: Untangling how local modulation of dopamine impacts behavior.
The expansive behavioral functions of dopamine have fueled searches for regulatory mechanisms that differentially impact behavior via distinct mechanisms of dopamine modulation at its striatal target. For example (Figure 2), in goal directed choice, individuals typically control which options to select and how to perform those actions (sometimes defined as motor vigor). It is possible that either tonic levels (defined as any non-phasic signal) of dopamine or ramps that precede choice would modulate action vigor while the phasic signals at outcome would mediate reward learning (noting that some have argued pre-outcome ramps reflect a form of prediction signaling) [49,50]. In this scenario, vigor-related modulation could arise largely from local striatal dopamine regulation while outcome learning could reflect prediction error signals computed by midbrain DANs. While this dissociation of dopamine regulation may help shape both ‘willingness to work’ and choice selection by dopamine in the NAc [9,51], the accuracy of this model and the potential regulatory mechanisms remain elusive.
Figure 2.
Integration of DAN somatic spiking and local DAN terminal modulation to generate behaviorally relevant DA signals in striatum. (A, bottom) Schematic of midbrain DAN somatic activity and (top) the potential local regulators of DAN terminals in striatum. Schematized striatal DA signal generated by DAN somatic spiking in response to unexpected reward (orange) and effects of local striatal modulation (blue). Three potential scenarios whereby local modulation further shapes this signal to alter baseline (tonic) dopamine levels (top), dopamine ramping prior to reward (middle) or outcome-associated dopamine.
In conclusion, the list of receptors on DAN terminals known to alter local dopamine release is large, including kappa-opioid [52], metabotropic glutamate [33], GABA [13,15] and acetylcholine [32,33] receptors. The regulated release of each cognate ligand in striatum is a potential opportunity to further sculpt the dopamine signals arising from spiking in the midbrain. As is clear from our first in vivo glimpse at acetylcholine-dopamine interactions, the combined use of viral-genetic tools to interrogate these systems while imaging dopamine within striatum during behavior will be key next steps. Furthermore, it will be necessary to delineate the potential time courses of these regulators – can local striatal modulation of dopamine only work on slow time scales, or can more phasic regulation occur as well? Addressing these two issues will provide a huge step forward in understanding how a single neuromodulator can have massively pleiotropic behavioral impacts.
Highlights.
Local mechanisms are necessary to control dopamine release across the striatum
Local GABA acts on GABAB and possibly GABAA receptors to modulate dopamine release
Cholinergic interneurons exert multiple mechanisms of control over dopamine release
Neuromodulators such as leptin, insulin, or histamine indirectly modulate dopamine
Emerging research investigates how these mechanisms modulate behavior in vivo
Acknowledgements
This work was supported by National Institute of Health (NIH) grants K01MH 127306 to ENH, R01MH 118369 to MVF, and R01NS 117061 to MVF.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Toulorge D, Schapira AHV, Hajj R: Molecular changes in the postmortem parkinsonian brain. J Neurochem 2016, 139:27–58. [DOI] [PubMed] [Google Scholar]
- 2.Holly EN, Miczek KA: Ventral tegmental area dopamine revisited: Effects of acute and repeated stress. Psychopharmacology (Berl) 2016, 233:163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz W, Dayan P, Read Montague P: A Neural Substrate of Prediction and Reward. 1990. [DOI] [PubMed] [Google Scholar]
- 4.Da Silva JA, Tecuapetla F, Paixão V, Costa RM: Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 2018, 554:244–248. [DOI] [PubMed] [Google Scholar]
- 5.Su XY, Chen M, Yuan Y, Li Y, Guo SS, Luo HQ, Huang C, Sun W, Li Y, Zhu MX, et al. : Central Processing of Itch in the Midbrain Reward Center. Neuron 2019, 102:858–872.e5. [DOI] [PubMed] [Google Scholar]
- 6.Chen APF, Malgady JM, Chen L, Shi KW, Cheng E, Plotkin JL, Ge S, Xiong Q: Nigrostriatal dopamine pathway regulates auditory discrimination behavior. Nat Commun 2022, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morikawa H, Paladini CA: Dynamic regulation of midbrain dopamine neuron activity: Intrinsic, synaptic, and plasticity mechanisms. Neuroscience 2011, 198:95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C, Kaeser PS: Mechanisms and regulation of dopamine release. Curr Opin Neurobiol 2019, 57:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohebi A, Pettibone JR, Hamid AA, Wong JMT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD: Dissociable dopamine dynamics for learning and motivation. Nature 2019, 570:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trudeau LE, Hnasko TS, Wallén-Mackenzie Å, Morales M, Rayport S, Sulzer D: The multilingual nature of dopamine neurons. In Progress in Brain Research. . Elsevier B.V.; 2014:141–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nolan SO, Zachry JE, Johnson AR, Brady LJ, Siciliano CA, Calipari ES: Direct dopamine terminal regulation by local striatal microcircuitry. J Neurochem 2020, 155:475–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts BM, Lopes EF, Cragg SJ: Axonal modulation of striatal dopamine release by local γ-aminobutyric acid (GABA) signalling. Cells 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopes EF, Roberts BM, Siddorn RE, Clements MA, Cragg SJ: Inhibition of nigrostriatal dopamine release by striatal GABA A and GABA B receptors. Journal of Neuroscience 2019, 39:1058–1065. *This study used Fast-scan Cyclic Voltammetry in acute slice with both electrical stimulation of striatum and optical stimulation of dopamine neuron terminals in striatum to assess local GABAergic modulation of dopamine release. It is shown that GABA and GABAA or GABAB receptor agonists can reduce the electrically stimulated release of dopamine independent of nicotine acetylcholine receptor activity. The optically stimulated release of dopamine is also shown to be reduced with application of GABA, and selective GABA receptor antagonism revealed receptor subtype specific modulation of dopamine release with GABAB receptor antagonism being sufficient to enhance release.
- 14.Roberts BM, Doig NM, Brimblecombe KR, Lopes EF, Siddorn RE, Threlfell S, Connor-Robson N, Bengoa-Vergniory N, Pasternack N, Wade-Martins R, et al. : GABA uptake transporters support dopamine release in dorsal striatum with maladaptive downregulation in a parkinsonism model. Nat Commun 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodnik ZD, Batra A, Oleson EB, Espana RA: Local GABAA Receptor-Mediated Suppression of Dopamine Release within the Nucleus Accumbens. ACS Chem Neurosci 2019, 10:1978–1985. [DOI] [PubMed] [Google Scholar]
- 16. Kramer PF, Twedell EL, Shin JH, Zhang R, Khaliq ZM: Axonal mechanisms mediating g-aminobutyric acid receptor type a (GABA-A) inhibition of striatal dopamine release. Elife 2020, 9:1–24. *This study used a combination of electrophysiological and photometric techniques in acute slice to identify mechanisms of dopamine axon modulation by GABAA receptors. The authors demonstrate axonal depolarization with GABAA agonism accompanied by reductions in axonal spike amplitude, propagation, and dopamine release. The mechanism is shown to involve sodium channel inactivation and shunting.
- 17. Holly EN, Davatolhagh MF, España RA, Fuccillo MV.: Striatal low-threshold spiking interneurons locally gate dopamine. Current Biology 2021, 31:4139–4147.e6. *This study used a combination of in vitro Fast-scan Cyclic Voltammetry and in vivo photometry to assess a role for low-threshold spiking interneurons (LTSIs) in the local modulation of dopamine release in striatum. In vitro, it is shown that optogenetic inhibition of LTSIs enhances optically evoked dopamine release while chemogenetic excitation of LTSIs impairs this dopamine release. Further, it is shown that this LTSI-dependent impairment of dopamine release is abolished by GABAB receptor antagonism and not GABAA. In vivo, it is shown that LTSI inhibition enhances dopamine signaling and the rate of learning.
- 18.Charara A, Heilman C, Levey AI, Smith Y: Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience 2000, 95:127–140. [DOI] [PubMed] [Google Scholar]
- 19.Yung KKL, Ng TKY, Wong CKC: Subpopulations of neurons in the rat neostriatum display GABA R1 receptor B immunoreactivity. Brain Res 1999, 830:345–352. [DOI] [PubMed] [Google Scholar]
- 20.Pitman KA, Puil E, Borgland SL: GABAB modulation of dopamine release in the nucleus accumbens core. European Journal of Neuroscience 2014, 40:3472–3480. [DOI] [PubMed] [Google Scholar]
- 21.Threlfell S, Cragg SJ: Dopamine signaling in dorsal versus ventral striatum: The dynamic role of cholinergic interneurons. Front Syst Neurosci 2011, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulzer D, Cragg SJ, Rice ME: Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016, 6:123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dautan D, Huerta-Ocampo I, Witten IB, Deisseroth K, Paul Bolam J, Gerdjikov T, Mena-Segovia J: A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the Brainstem. Journal of Neuroscience 2014, 34:4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dautan D, Bay HH, Bolam JP, Gerdjikov TV., Mena-Segovia J: Extrinsic sources of cholinergic innervation of the striatal complex: A whole-brain mapping analysis. Front Neuroanat 2016, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dautan D, Huerta-Ocampo I, Gut NK, Valencia M, Kondabolu K, Kim Y, Gerdjikov TV., Mena-Segovia J: Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Commun 2020, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brimblecombe KR, Threlfell S, Dautan D, Kosillo P, Mena-Segovia J, Cragg SJ: Targeted activation of cholinergic interneurons accounts for the modulation of dopamine by striatal nicotinic receptors. eNeuro 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou FM, Liang Y, Dani JA: Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci 2001, 4:1224–1229. [DOI] [PubMed] [Google Scholar]
- 28.Zhang H, Sulzer D: Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci 2004, 7:581–582. [DOI] [PubMed] [Google Scholar]
- 29.Rice ME, Cragg SJ: Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 2004, 7:583–584. [DOI] [PubMed] [Google Scholar]
- 30.Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF: Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: Setting the tone for reward processing. Cell Rep 2012, 2:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu C, Cai X, Ritzau-Jost A, Kramer PF, Li Y, Khaliq ZM, Hallermann S, Kaeser PS: An action potential initiation mechanism in distal axons for the control of dopamine release. Science 2022, 375:1378–1385. *This study included not only a breadth of in vitro techniques in acute slice preparations including functional imaging, amperometry, and electrophysiology, but it also included a photometric evaluation of dopamine in freely moving mice in vivo. The in vitro experiments revealed that nicotinic acetycholine receptor activity generates action potentials in dopamine axons that propagate and cause dopamine release in and beyond the area of acetylcholine binding. The in vivo experiment demonstrated impaired dopamine dynamics with the inhibition of nicotinic acetylcholine receptors.
- 32.Kramer PF, Brill-Weil SG, Cummins AC, Zhang R, Camacho-Hernandez GA, Newman AH, Eldridge MAG, Averbeck BB, Khaliq ZM: Synaptic-like axo-axonal transmission from striatal cholinergic interneurons onto dopaminergic fibers. Neuron 2022, 110:2949–2960.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin JH, Adrover MF, Wess J, Alvarez VA: Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc Natl Acad Sci U S A 2015, 112:8124–8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancini M, Patel JC, Affinati AH, Witkovsky P, Rice ME: Leptin Promotes Striatal Dopamine Release via Cholinergic Interneurons and Regionally Distinct Signaling Pathways. Journal of Neuroscience 2022, 42:6668–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel JC, Carr KD, Rice ME: Actions and Consequences of Insulin in the Striatum. Biomolecules 2023, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu S, Borgland SL: Insulin actions in the mesolimbic dopamine system. Exp Neurol 2019, 320. [DOI] [PubMed] [Google Scholar]
- 37.Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, De Vaca SC, Reith MEA, et al. : Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel JC, Stouffer MA, Mancini M, Nicholson C, Carr KD, Rice ME: Interactions between insulin and diet on striatal dopamine uptake kinetics in rodent brain slices. European Journal of Neuroscience 2019, 49:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branco ACCC, Yoshikawa FSY, Pietrobon AJ, Sato MN: Role of Histamine in Modulating the Immune Response and Inflammation. Mediators Inflamm 2018, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu X, Ma Y, Harding EC, Yustos R, Vyssotski AL, Franks NP, Wisden W: Genetic lesioning of histamine neurons increases sleep-wake fragmentation and reveals their contribution to modafinil-induced wakefulness. Sleep 2019, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varaschin RK, Osterstock G, Ducrot C, Leino S, Bourque MJ, Prado MAM, Prado VF, Salminen O, Rannanpää (née Nuutinen) S, Trudeau LE: Histamine H3 Receptors Decrease Dopamine Release in the Ventral Striatum by Reducing the Activity of Striatal Cholinergic Interneurons. Neuroscience 2018, 376:188–203. [DOI] [PubMed] [Google Scholar]
- 42.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ: Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 2012, 75:58–64. [DOI] [PubMed] [Google Scholar]
- 43.Kosillo P, Zhang YF, Threlfell S, Cragg SJ: Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cerebral Cortex 2016, 26:4160–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, Gildish I, Cachope R, Bellocchio L, Guzmán M, et al. : Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 2017, 96:1112–1126.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adrover MF, Shin JH, Quiroz C, Ferré S, Lemos JC, Alvarez VA: Prefrontal cortex-driven dopamine signals in the striatum show unique spatial and pharmacological properties. Journal of Neuroscience 2020, 40:7510–7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohebi A, Collins VL, Berke JD: Accumbens cholinergic interneurons dynamically promote dopamine release and enable motivation. Elife 2023, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chantranupong L, Beron CC, Zimmer JA, Wen MJ, Wang W, Sabatini BL: Dopamine and glutamate regulate striatal acetylcholine in decision-making. Nature 2023, 621:577–585. ** This study used in vivo photometry to assess dopamine and acetylcholine dynamics during a reward-based decision-making task. It is shown that dopamine and acetylcholine levels are multi-phasic and anticorrelated. Further, it is shown that the dopamine activity does not require acetycholine, but D2 dopamine receptor antagonism attenuates reductions in acetylcholine and impairs decision making. The authors also demonstrate a need for glutamatergic activity from cortical and thalamic afferents for acetylcholine release.
- 48. Krok AC, Maltese M, Mistry P, Miao X, Li Y, Tritsch NX: Intrinsic dopamine and acetylcholine dynamics in the striatum of mice. Nature 2023, 621:543–549. ** This study used in vivo photometry to assess dopamine and acetylcholine dynamics during a head-fixed treadmill task for water reward. The authors revealed anticorrelated dopamine and acetylcholine levels. Further, it is shown that the fluctuations in dopamine and acetylcholine levels are not interdependent. Glutamatergic afferents modulate acetylcholine and midbrain activity regulates striatal dopamine levels.
- 49.Kim HGR, Malik AN, Mikhael JG, Bech P, Tsutsui-Kimura I, Sun F, Zhang Y, Li Y, Watabe-Uchida M, Gershman SJ, et al. : A Unified Framework for Dopamine Signals across Timescales. Cell 2020, 183:1600–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikhael JG, Kim HGR, Uchida N, Gershman SJ: The role of state uncertainty in the dynamics of dopamine. Current Biology 2022, 32:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD: Mesolimbic dopamine signals the value of work. Nat Neurosci 2015, 19:117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirino BE, Spodnick MB, Gargiulo AT, Curtis GR, Barson JR, Karkhanis AN: Kappa-opioid receptor-dependent changes in dopamine and anxiety-like or approach-avoidance behavior occur differentially across the nucleus accumbens shell rostro-caudal axis. Neuropharmacology 2020, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]