Abstract
Background
Spontaneous coronary artery dissection (SCAD) is an underdiagnosed cause of acute coronary syndrome, particularly in younger women. Due to limited information about SCAD, case reports and case series can provide valuable insights into its features and management. This study aimed to comprehensively evaluate the features of SCAD patients who experienced psychophysical stress before the SCAD event.
Methods
We conducted an electronic search of PubMed, Scopus, and Web of Science from inception until January 7, 2023. We included case reports or series that described patients with SCAD who had experienced psychophysical stress before SCAD. Patients with pregnancy-associated SCAD were excluded from our analysis.
Results
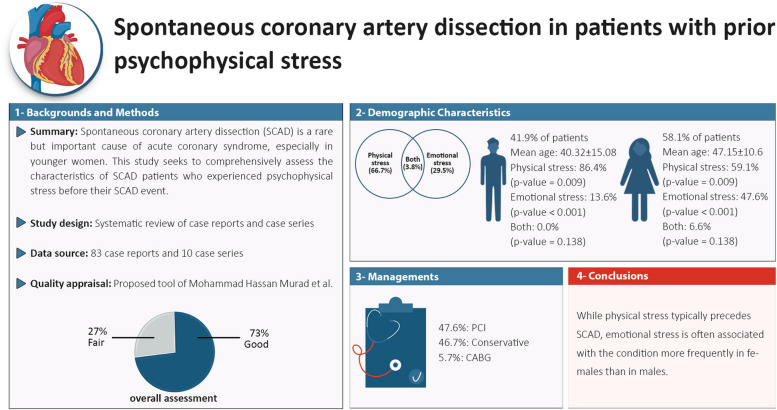
In total, we included 93 case reports or series describing 105 patients with SCAD. The average patient age was 44.29 ± 13.05 years and a total of 44 (41.9%) of patients were male. Among the included SCAD patients the most prevalent comorbidities were fibromuscular dysplasia (FMD) and hypertension with the prevalence of 36.4 and 21.9%, respectively. Preceding physical stress was more frequently reported in men than in women; 38 out of 44 (86.4%) men reported physical stress, while 36 out of 61 (59.1%) females reported physical stress (p value = 0.009). On the other hand, the opposite was true for emotional stress (men: 6 (13.6%)), women: 29 (47.6%), p value < 0.001). Coronary angiography was the main diagnostic tool. The most frequently involved artery was the left anterior descending (LAD) (62.9%). In our study, recurrence of SCAD due to either the progression of a previous lesion or new SCAD in another coronary location occurred more frequently in those treated conservatively, however the observed difference was not statistically significant (p value = 0.138).
Conclusion
While physical stress seems to precede SCAD in most cases, emotional stress is implicated in females more than males.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-03902-2.
Keywords: Spontaneous coronary artery dissection, SCAD, Acute coronary syndrome, Stress
Introduction
Spontaneous coronary artery dissection (SCAD) is becoming an increasingly recognized etiology of acute coronary syndrome (ACS), cardiac arrest, and sudden cardiac death [1]. SCAD is characterized by a non-atherosclerotic, non-iatrogenic, and non-traumatic spontaneous tear in coronary arteries, which leads to a collapsed arterial lumen (true lumen) due to the formation of an intramural hematoma (IMH) (false lumen), resulting in compromised coronary blood flow and myocardial infarction (MI) [2].
SCAD is responsible for 1–4% of ACS cases in the general population but is estimated to affect as many as 35% of women under 50 [3]. It is one of the leading causes of pregnancy-associated myocardial infarction (PAMI) [4]. Nevertheless, owing to its underdiagnosis and misdiagnosis with atherosclerotic ACS, its actual prevalence is higher than previously reported [5]. Despite this, the etiology of SCAD remains uncertain. There is increasing interest in discovering the main risk factors and triggers behind its pathogenesis. The current knowledge of SCAD has mainly remained limited to single-center case reports and case series data, with only a handful of large-scale cohorts and no randomized clinical trials investigating its pathogenesis, management, and prognosis [6–9]. Thus, exploring case reports and case series regarding SCAD could provide helpful information regarding disease characteristics and management. In addition, case reports and case series can provide insight into uncommon conditions that may be challenging for practitioners to diagnose and treat, offering guidance on how to manage these conditions.
Previous studies have suggested a minor role of traditional cardiovascular risk factors, except hypertension, in SCAD occurrence [2]. Some factors that are believed to contribute to SCAD pathogenesis potentially include underlying arteriopathies (such as fibromuscular dysplasia (FMD)), female sex, pregnancy/other hormonal changes, systemic inflammatory conditions, and connective tissue disorders [10]. One important predisposing factor includes psychophysical stress, and its role in SCAD has been introduced as a research priority and a key question by the American Heart Association [2]. More than half of the patients afflicted with SCAD had experienced emotional (including the demise of a family member or marriage and workplace-related issues) or physical stress (including extreme aerobic or anaerobic physical activities, lifting heavy objects, intense Valsalva or coughing) preceding the presentation. The stress experienced by women was predominantly emotional, while that experienced by men was primarily physical [2, 11].
In light of this information, despite the large existing cohorts of SCAD patients, we included only case series and case reports of SCAD patients because more detailed information is available in these types of studies. This systematic review aimed to exclusively evaluate the characteristics and management of patients experiencing SCAD following psychophysical stress.
Methods
Study design and search strategy
A systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We conducted a systematic search in the PubMed, Web of Science, and Scopus databases from inception to January 7, 2023. The following keywords were used: “spontaneous coronary artery dissection” and “SCAD”. No restrictions on publication date or publication status were instituted. It is important to note that the search strategy did not include any terms related to stress because the majority of the articles that met the inclusion criteria described the stressful event rather than using the term ‘stress’.
Eligibility criteria
Two independent authors (P.R. and P.F.) evaluated studies for eligibility by screening titles and abstracts. Subsequently, the full texts of potentially eligible articles were evaluated according to the inclusion criteria. Disagreements were addressed by joint discussion and consensus of the two authors. Finally, all references of the included articles were comprehensively searched to find articles that might have been missed during the initial screening.
Case reports and case series were included if they described at least one patient who had experienced psychophysical stress preceding the onset of SCAD. In our study, psychophysical stress is defined broadly to encompass both rigorous physical activity and psychological events reported by the patient preceding the occurrence of SCAD. Specifically, any intense physical exertion or notable psychological event self-reported by the patient before the SCAD incident is considered indicative of psychophysical stress. In the case series, we only selected patients with psychophysical stress in the data analysis and excluded other reported patients. Patients presenting with pregnancy-associated SCAD (due to the unique and multifaceted stressors associated with pregnancy, which encompass both psychosocial and physiological aspects) or SCAD in the context of illicit drug use, as well as studies in languages other than English, without sufficient data, or with low quality (based on quality assessment method), were excluded.
Data extraction
Data were independently extracted by two authors (P.R. and P.F.). A third author verified the accuracy of data extraction and addressed any contradictions. Using Microsoft Excel 2019 version (Microsoft Corporation, Redmond, WA, USA), the following data categories were recorded: a) study-related characteristics (first author’s name, publication year, region of the study (according to the World Health Origination regions)), b) patient characteristics (age, sex, past medical history, habitual history), c) psychophysical stress-related characteristics (type, description, time interval with the SCAD occurrence), and d) SCAD-related characteristics (signs and symptoms, clinical diagnosis, electrocardiogram (ECG) findings, dissected artery characteristics, diagnostic and therapeutic approaches, and follow-up events).
Quality appraisal
Two independent authors (P.R. and P.F.) assessed the quality of the case report/series. Disagreements were addressed by joint discussion and consensus by the two authors. The method proposed by Mohammad Hassan Murad et al. was used to assess the quality of case reports/series [12]. The methodological quality of the case reports and case series was evaluated using six of the eight questions recommended in the referenced article (see Additional file 1). Scores of 5–6, 4, and 0–3 were considered “good,” “fair,” and “poor” studies in terms of quality, respectively. Articles with a score of less than four were excluded from our study.
Statistical analysis
Data are reported as mean ± SD and/or median with interquartile range. All statistical analyses were conducted using IBM SPSS Statistics version 27 (IBM Corp., Armonk, NY, USA). Statistical analyses were conducted to investigate the relationships and differences among variables in the dataset. Chi-square and Fisher’s Exact tests were employed to assess associations among categorical variables. Comparisons between groups were performed using appropriate statistical tests such as the Independent Samples t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. The significance level for all performed tests was p value< 0.05.
Results
Study selection
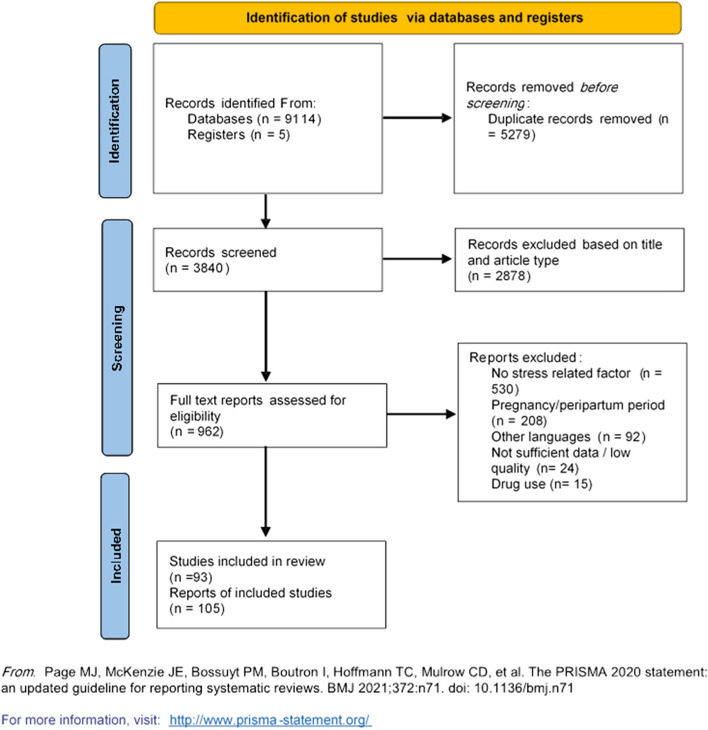
The PRISMA flow diagram is illustrated in Fig. 1. A total of 9119 articles were retrieved from PubMed, Scopus, and Web of Science. After removing 5279 duplicates using Endnote software version 20.0 (Clarivate PLC, London, United Kingdom), 3840 records were screened based on title and article type, with 2878 being excluded. Of the remaining 962 articles, full-text assessment led to the exclusion of 530 for not reporting stress-related factors prior to SCAD onset, 208 for involving patients in the pregnancy or peripartum period, 92 for being in non-English languages, 24 for low quality, and 15 for reporting illicit drug use. Five studies (eight patients) were added after reviewing the references.
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews that included searches of databases and registers only
Study characteristics
A total of 83 included case reports and 10 case series resulted in 105 patients who suffered SCAD following psychophysical stress (83 from case reports and 22 from case series) [13–105]. The largest geographical distribution of these cases was in the Region of the Americas (n = 47) and European Region (n = 37), followed by the Western Pacific Region (n = 16), South–East Asia Region (n = 3), Eastern Mediterranean Region (n = 2), and African Region (n = 1). Most reports were from the United States (n = 32), the United Kingdom (n = 13), and Canada (n = 9).
Patient characteristics
Patient characteristics are presented in Table 1. The average patient age was 44.29 ± 13.05 years. A total of 44 (41.9%) of patients were male. Female patients were older than their male counterparts (47.15 ± 10.6 vs. 40.32 ± 15.08 years, respectively, p value = 0.012).
Table 1.
Patient characteristics (N = 105)
| Total (N = 105) |
Men (N = 44) |
Women (N = 61) |
P value | |
|---|---|---|---|---|
| Age (mean ± SD) | 44.29 ± 13.05 | 40.32 ± 15.08 | 47.15 ± 10.60 | 0.012 |
| Psychophysical stress types | ||||
| Physical | 70 (66.7%) | 38 (86.4%) | 32 (52.5%) | 0.009 |
| Emotional | 31 (29.5%) | 6 (13.6%) | 25 (41.0%) | < 0.001 |
| Both | 4 (3.8%) | 0 (0.0%) | 4 (6.6%) | 0.138 |
| Past medical history and risk factors | ||||
| Hypertension | 23 (21.9%) | 7 (15.9%) | 16 (26.2%) | 0.332 |
| Diabetes | 4 (3.8%) | 2 (4.5%) | 2 (3.3%) | 1.000 |
| Dyslipidemia | 9 (8.6%) | 2 (4.5%) | 7 (11.5%) | 0.300 |
| Obesity | 4 (3.8%) | 1 (2.3%) | 3 (4.9%) | 0.640 |
| CHD | 3 (2.9%) | 1 (2.3%) | 2 (1.6%) | 1.000 |
| Heart failure | 0 (0.00%) | 0 (0.0%) | 0 (0.0%) | – |
| Mental health disorders | 12 (11.4%) | 2 (4.5%) | 10 (16.4%) | 0.061 |
| Depression | 5 (4.8%) | 0 (0.0%) | 5 (6.6%) | 0.071 |
| Anxiety | 5 (4.8%) | 0 (0.0%) | 5 (6.6%) | 0.071 |
| FMD (among 22 evaluated women and 11 evaluated men) | 12 (36.4%) | 0 (0.0%) | 12 (54.5%) | 0.001 |
| Habitual history | ||||
| Smoking | 21 (20.00%) | 10 (19.2%) | 11 (18.0%) | 0.62 |
| Alcohol consumption | 0 (0.00%) | 0 (0.0%) | 0 (0.00%) | – |
Data are presented as frequency (percentages) or mean ± SD
CHD congenital heart defects, FMD fibromuscular dysplasia
Hypertension was the most common conventional cardiovascular risk factor (21.9%), followed by dyslipidemia (8.6%), obesity (3.8%), and diabetes mellitus (3.8%). A total of 11.4% of patients had mental health disorders, including depression, anxiety disorder, attention deficit and hyperactivity disorder (ADHD), and posttraumatic stress disorder (PTSD). In terms of congenital anomalies, one patient had an anomalous origin of the coronary artery [58], and two patients had myocardial bridging [60, 95]. Heart failure and alcohol consumption were not reported in any patients. Three patients reported excessive caffeine use before experiencing SCAD [30, 45, 53]. FMD was documented in 12 of 33 patients (36.4%) who were evaluated for this condition [14, 20, 26, 38, 56, 63, 73, 99], none of whom had a known diagnosis of FMD before presenting with SCAD. All of the diagnosed FMD cases were in women. Other documented comorbidities are available in Additional file 2.
Psychophysical stress
Sole physical and emotional stress were reported among 70 (66.7%) and 31 (29.5%) patients, respectively. Four patients (3.8%) described experiencing both emotional and physical stress. Some of these stressors include aerobic or isometric physical activities, lifting heavy objects, intense Valsalva or coughing, funeral grief, work-related issues, etc. Data for each patient individually are presented in Additional file 2. Physical stress was more frequently seen among men than women; 38 out of 44 (86.4%) men reported physical stress, while 36 out of 61 (59.1%) females reported physical stress (p value = 0.009). The converse was true with regard to emotional stress (men: 6 (13.6%), women: 29 (47.6%), p value < 0.001). Psychophysical stress was experienced either immediately or for a period before the SCAD event.
Clinical manifestations
Signs and symptoms
The SCAD-related characteristics are presented in Table 2. All patients reported chest pain as their chief complaint except for three [45, 51, 97]. Furthermore, 12 (11.4%), 11 (10.5%), and 9 (8.6%) patients experienced nausea, dyspnea, and diaphoresis, respectively. Less frequent (< 5%) signs and symptoms included vomiting, fatigue, dizziness, and syncope.
Table 2.
SCAD-related characteristics
| N (%) | |
|---|---|
| ECG (N = 96)* | |
| ST-elevation | 55 (57.3%) |
| ST-depression or T-changes | 34 (35.4%) |
| Normal | 7 (7.3%) |
| Clinical Presentations | |
| Chest pain | 102 (97.1%) |
| Nausea | 12 (11.4%) |
| Dyspnea | 11 (10.5%) |
| Diaphoresis | 9 (8.6%) |
| Cardiogenic shock | 5 (4.8%) |
| Sudden cardiac death | 5 (4.8%) |
| Arrhythmia | 7 (6.7%) |
| Diagnostic imaging modalities | |
| First-line coronary angiography | 66 (62.9%) |
| CCTA-only | 3 (2.9%) |
| CCTA+ complementary coronary angiography | 4 (3.8%) |
| First-line coronary angiography+ IVUS | 18 (17.1%) |
| First-line coronary angiography+ OCT | 12 (11.4%) |
| First-line coronary angiography+ OCT + IVUS | 2 (1.9%) |
| First-line coronary angiography+ CCTA + IVUS | 1 (1.0%) |
| Repeated coronary angiography | |
| Number of arteries having SCAD (N = 93) † | |
| Single-vessel SCAD | 86 (92.5%) |
| Two-vessel SCAD | 5 (5.4%) |
| Three-vessel SCAD | 2 (2.2%) |
| Types of coronary arteries having SCAD (N = 93) † | |
| LMCA | 6 (6.5%) |
| LAD | 63 (67.7%) |
| LCX | 7 (7.5%) |
| RCA | 10 (10.8%) |
| LAD+ RCA | 4 (4.3%) |
| LAD+ LCx | 1 (1.1%) |
| LAD+ LCx + RCA | 2 (2.2%) |
| Grafted IMA artery | 3 (3.2%) |
| SCAD types (N = 34) ‡ | |
| Type 1 | 10 (29.4%) |
| Type 2 | 19 (55.9%) |
| Type 3 | 2 (5.9%) |
| Type 1 and 2 | 1 (2.9%) |
| Type 2 and 3 | 1 (2.9%) |
| Type 1, 2 and 3 | 1 (2.9%) |
| Management | |
| PCI | 50 (47.6%) |
| Complicated | 6 (12.0%) |
| Successful | 44 (88.0%) |
| Conservative | 49 (46.7%) |
| DAPT | 33 (67.3%) |
| SAPT | 12 (24.5%) |
| Not specified | 4 (8.1%) |
| CABG | 6 (5.7%) |
| Outcome | |
| In hospital mortality | 1 (1.0%) |
| Number of recurrences | |
| One | 15 (14.2%) |
| Two | 1 (1.0%) |
| Three | 1 (1.0%) |
| Four | 1 (1.0%) |
| Time of recurrence | |
| 0–10 days | 15 (60.5%) |
| 10 days-6 months | 2 (8.3%) |
| ≥6 months | 7 (29.2%) |
Data are presented as frequency (percentages)
CCTA cardiac computed tomography angiography, IVUS intravascular ultrasound, SCAD spontaneous coronary artery dissection, LMCA left main coronary artery, LAD left anterior descending artery, LCx left circumflex coronary artery, RCA right coronary artery, IMA internal mammary, PCI percutaneous coronary intervention, CABG coronary artery bypass graft, DAPT dual antiplatelet therapy, SAPT single antiplatelet therapy
*ECG evaluation was reported in 96 cases. Data for the remainder (N = 9) is unavailable
†Arteries having SCAD were reported in 93 cases. Data for the remainder (N = 12) is unavailable
‡SCAD types based on saw classification was reported in 34 cases. Three cases of multivessel SCAD presented with different types of SCAD in each artery
ECGs and other clinical diagnoses
Among 96 reported electrocardiograms (ECGs), 7 (7.3%), 34 (35.4%), and 55 (57.3%) patients presented with normal ECGs, ST depression or T segment changes, and ST elevation, respectively. Additionally, 7 (7.3%) patients showed different types of arrhythmias including atrial fibrillation (AF), ventricular fibrillation (VF), or ventricular tachycardia (VT), and a few patients presented with or were diagnosed with cardiogenic shock (n = 5) [37, 90, 92, 96, 104], concurrent cardiac tamponade (n = 1) [67], and cardiac arrest (n = 5) [14, 44, 46, 90, 96].
Diagnostic imaging modalities
Coronary angiography was used as the first diagnostic modality except for eight patients. Among the rest, cardiac computed tomography angiography (CCTA) was either used alone (n = 3) [55, 77, 106] or followed by confirmatory angiography (n = 5) [32, 43, 51, 54, 70].
In general, 13 patients were initially misdiagnosed by coronary angiography [15, 26, 49, 56, 63, 65, 72, 74, 76, 84, 85, 90, 96] and subsequently secured a diagnosis of SCAD by either intravascular ultrasound (IVUS) (n = 2), optical coherence tomography (OCT) (n = 3) or repeated angiography (n = 8). Among patients with a correct diagnosis by angiography, SCAD was confirmed by IVUS and OCT for 16 and 11 cases, respectively. Additionally, four patients were identified as having simultaneous Takotsubo syndrome [60, 66, 74, 82], and one patient was initially misdiagnosed with Takotsubo syndrome during history taking and physical examination [49].
Dissected artery characteristics
While the majority of patients had SCAD of a single artery, seven patients had multivessel SCAD [59, 63, 66, 86, 95, 99, 103], with the involvement of four (n = 1), three (n = 3), and two (n = 4) arteries. The most frequently dissected artery was the left anterior descending artery (LAD) (n = 70), followed by the right coronary artery (RCA) (n = 16), LCx (n = 10), and left main coronary artery (LMCA) (n = 6). Regarding smaller coronary branches, dissections were found in obtuse marginals (n = 6), diagonals (n = 3), posterior descending artery (n = 3), posterior left ventricular artery (n = 2), ramus intermedius (n = 1), and atrioventricular circumflex (n = 1) branches. Furthermore, three dissections occurred in internal mammary artery grafts among patients with previous coronary artery bypass graft surgery (CABG) [17, 25, 39].
Among 34 patients in whom the SCAD type was reported according to the Saw classification [107], 12 (35.3%) patients were diagnosed with type 1, 22 (64.7%) with type 2, and four (11.8%) with type 3 SCAD. One patient had three arteries having SCAD with three different classification types [86], and two patients had two dissections in two arteries with different types [20, 66]. Initial angiography confirmed type 3 dissection in one patient, which was later found to be type 1 SCAD on repeated angiography [76].
Management
A total of 49 (46.7%) patients were managed with medical therapy only, 50 (47.6%) with primary percutaneous coronary intervention (PCI), and 6 (5.7%) with CABG. Medical management included the use of either dual (n = 33) or single antiplatelet therapy (n = 12) in all patients. In addition, other medications, such as statins, beta-blockers, heparin, and warfarin, were frequently used.
Six patients had complicated PCI [14, 19, 52, 85, 90, 103], for whom stenting of the mid-LAD lesion in one patient resulted in a distal expansion of the dissection followed by a retrograde extension to the ostial LAD. A multiple stenting technique was employed to manage distal extensions, and emergent CABG was subsequently performed due to the high risk of LMCA/LCx flow being jeopardized as a result of the proximal extension [85]. PCI was terminated in three patients when the guidewire passage through the false lumen suggested a SCAD diagnosis and medical management was pursued [14, 19, 90]. Similarly, the placement of the guidewire into the false lumen suggested the diagnosis of SCAD in another patient, which was further confirmed using IVUS. Subsequently, the true lumen was found with IVUS guidance, and the stent was deployed at the dissection entry point [52]. Following the stenting of a LAD dissection in another patient, the attempt to stent the RCA lesion was terminated due to difficulties in finding the true lumen and clinical stability following the original PCI [103]. In one of the patients, IVUS imaging showed poor apposition of the stent, and therefore balloon angioplasty was redone [79]. Among the patients managed with CABG, three had LMCA dissection, and one had three arteries with SCAD.
Bioabsorbable vascular stents were utilized in four patients [40, 48, 89, 91]. Intra-aortic balloon pumps were used as additional mechanical support for three patients [90, 102, 104]. Furthermore, three patients participated in cardiac rehabilitation programs, including psychological support [16, 37, 106], and seven patients were advised to abstain from sports [51, 55, 59, 88, 97, 103]..
Outcome and follow-up events
Among the included cases only 60 cases had a follow up had a follow-up beyond the index hospitalization or initial presentation of SCAD. In-hospital mortality occurred in one of the patients. The patient underwent CABG, and a few hours after the second angiography on the fifth postoperative day, the patient experienced cardiac arrest caused by electromechanical dissociation and died [96]. Two patients experienced VF during their hospital stay and were successfully were defibrillated [15, 92]. During a median follow-up of 2 months, 16 patients experienced SCAD recurrence due to either the progression of a previous lesion or new SCAD in another coronary location, with multiple recurrences in three patients [20, 80, 102]. Just over one-half of patients had recurrence (n = 13 (59.1%)) in the first 10 days, with another two (9.1%) experiencing this between 10 days and 6 months, and the rest (n = 7 (31.8%)) after 6 months. Eighteen out of 22 recurrences were in individuals who received medical therapy. Univariable analysis of our selected cases revealed that SCAD recurrence was not significantly associated with sex (p value = 0.495), hypertension (p value = 0.594), FMD (p value = 0.261), type of stress (p value; physical stress: 0.522, emotional stress: 0.615), or type of management (p value; conservative management: 0.138, PCI: 0.141) (Table 3). Interestingly, six patients experienced a recurrent episode of chest pain after discharge but were found to be clinically stable in further evaluations [51, 62, 66, 67, 86, 99]. Dual antiplatelet therapy was stopped in one patient due to gastrointestinal bleeding and had recurrent SCAD 8 days following the index event [20].
Table 3.
Univariable analysis on predictors of SCAD recurrences
| Variables | No recurrence N (%) |
Recurrent N (%) |
P value | |
|---|---|---|---|---|
| Gender | Female | 50 (57.5%) | 11 (61.1%) | 0.495 |
| Male | 37 (42.5%) | 7 (38.9%) | ||
| HTN | No | 68 (78.2%) | 14 (77.8%) | 0.594 |
| Yes | 19 (21.8%) | 4 (22.2%) | ||
| FMD | No | 12 (57.1%) | 9 (75.0%) | 0.261 |
| Yes | 9 (42.9%) | 3 (25.0%) | ||
| Physical stress | No | 26 (29.9%) | 5 (27.8%) | 0.552 |
| Yes | 61 (70.1%) | 13 (72.2%) | ||
| Emotional stress | No | 58 (66.7%) | 12 (66.7%) | 0.615 |
| Yes | 29 (33.3%) | 6 (33.3%) | ||
| Conservative management | No | 49 (56.3%) | 7 (38.9%) | 0.138 |
| Yes | 38 (43.7%) | 11 (61.1%) | ||
| PCI | No | 43 (49.4%) | 12 (66.7%) | 0.141 |
| Yes | 44 (50.6%) | 6 (33.3%) | ||
| Arrhythmia | No | 82 (94.3%) | 16 (88.9%) | 0.344 |
| Yes | 5 (5.7%) | 2 (11.1%) | ||
FMD fibromuscular dysplasia, HTN hypertension, PCI percutaneous coronary intervention
Discussion
In this systematic review, we studied SCAD patients in whom emotional or physical stress was known as a possible trigger of SCAD. Here, we aimed to compare our findings with the overall data about SCAD from previous studies.
It is hypothesized that a combination of predisposing factors leads to higher susceptibility to having a SCAD event following a trigger. Female sex, pregnancy, physical or emotional stress, and FMD are among the known risk factors proposed by a large number of studies and are more likely to have underlying roles in its pathophysiology. The suggested hypothesis proposes that during physical or emotional stress a sudden catecholamine surge can cause an increase in arterial shear stress and lead to a stress tear of the vasa vasorum [11]. However, this mechanism has not been fully investigated. A similar mechanism has been proposed in other stress-related cardiovascular conditions such as Takotsubo syndrome [11, 108]. Endothelial dysfunction, the initial stage of atherosclerosis, is independently linked to cardiovascular events. Even individuals with few traditional risk factors but with peripheral endothelial dysfunction are at higher risk. Studies have shown mental stress impacts endothelial function, leading to oxidative stress and inflammation, which increase cardiovascular risk [109]. This suggests that the endothelium plays a critical role in translating the physiological effects of mental stress into measurable cardiovascular risk. Additionally, study of Martin et al. [110] showed that individuals with a history of apical ballooning syndrome exhibit abnormal microvascular function when stressed, leading to excessive blood vessel constriction and impaired dilation afterward. Further study revealed impaired responses to acetylcholine in the coronary arteries during mental stress, while responses to nitroglycerin remain intact, indicating endothelial dysfunction [111].
SCAD tends to predominantly affect young or middle-aged women, most often in the peripartum period [2]; surprisingly, in our study, 41.9% of reported stress-related SCAD occurred in men. This discrepancy might be potentially due to the exclusion of pregnancy-associated SCAD in our methods. In our study, the mean age of female patients was significantly higher than that of males likely due in part to excluding women in the peripartum period in the study methodology. However, a study has also reported this age difference [112]. Consistent with the results of other studies [112, 113], we found that SCAD patients whose attacks were precipitated by emotional stress were predominantly women, while physical stressors were reported more frequently in men. The study of Jaskanwal et al. [114] examined a large group of patients with chest pain and nonobstructive CAD. It found that individuals with anxiety disorders, especially women, were more likely to have coronary endothelial dysfunction. This association persisted even after adjusting for traditional cardiovascular risk factors and medication use. The findings suggest that anxiety disorders may contribute to the development of coronary endothelial dysfunction, particularly in women [114]. We witnessed that the presence of coronary artery disease risk factors was consistent with other studies [11, 112, 113, 115–118], wherein hypertension was the most common cardiovascular risk factor reported, and other classic risk factors for MI were not common.
Presently, SCAD patients are routinely screened for FMD due to a strong association, with FMD predicting major adverse cardiovascular events (MACEs) [2, 119]. In the study of Fahmy et al., approximately one-half of the male patients with SCAD had concomitant FMD [112], whereas in our study, all of the FMD cases were women. Takotsubo syndrome and SCAD share key characteristics, prompting questions about a common pathophysiology [120]. Moreover, some articles have proposed a chicken or egg causality between SCAD and Takotsubo syndrome [120]. Recent research indicates that individuals diagnosed with Takotsubo syndrome tend to be older and have a higher prevalence of specific cardiovascular risk factors when compared to those with SCAD. Additionally, Takotsubo syndrome patients, despite their older age and greater cardiovascular risk factors, exhibit lower occurrences of depressive disorder or emotional triggers than SCAD patients [121, 122]. Remarkably individuals with Takotsubo syndrome have a poorer prognosis in terms of in-hospital, mid-term, and long-term outcomes, with higher noncardiac mortality rates compared to SCAD patients [121, 122]. However, it is worth noting that in propensity score-matched cohorts of middle-aged women, SCAD diagnosis resulted in worse long-term outcomes compared to Takotsubo syndrome, primarily due to an elevated risk of cardiac-related rehospitalization [123]. Accordingly, in our study, four patients with stress-related SCAD had concomitant Takotsubo syndrome, and one was initially misdiagnosed with Takotsubo syndrome.
Regarding the clinical manifestations, some studies have reported non-ST elevation myocardial infarction (NSTEMI) as the most common presentation of SCAD [124, 125], but ST elevation myocardial infarction (STEMI) has been more prevalent in SCAD patients in other studies [112, 126]. Similarly, in our selected cases, ST elevation was the most common ECG finding. Additionally, some patients had arrhythmias, cardiogenic shock, cardiac tamponade, and cardiac arrest. These presentations were also reported in previous studies [127].
Coronary angiography has remains the diagnostic gold standard for SCAD [128], yet instances of misdiagnosis (12.4% in this study) emphasize its limitations. Intracoronary imaging (IVUS or OCT) in SCAD poses potential risks, complicating the decision on their application in diagnosis or treatment alongside PCI [129, 130]. Intracoronary imaging can be helpful for diagnostic confirmation of doubtful cases, especially type 3 SCAD [130]. In our study, seven patients had multivessel SCAD. Although one study stated that clinical outcomes and long-term follow-up were similar between single-vessel and multivessel SCAD, the stroke rate was significantly higher in patients with multivessel SCAD [131]. Overall, LAD is reported as the most frequently involved artery in SCAD [8, 125], which was also true in our study. Saw et al. reported type 2 SCAD as the most prevalent angiographic appearance of SCAD [125]. Similarly, the most common type of SCAD in stress-related cases was type 2, accounting for 64.7% of the cases in whom the SCAD type was reported.
Medical management versus revascularization in SCAD patients depends on hemodynamic stability and their thrombolysis in myocardial infarction (TIMI) flow grade found on angiography [132]. Prior meta-analyses indicated that conservative management of SCAD had similar outcomes comparing to the invasive management [133, 134], but in our study, less than one-half of the patients received medical management. It is worth noting that PCI in SCAD patients is technically challenging, given that it can lead to a propagation of dissection and cutoff of coronary flow, putting patients at a higher risk for ischemia and myocardial damage. Despite large cohorts in which SCAD patients are more managed conservatively [119], we witnessed conservative and revascularization strategies used in approximately equal numbers of patients. Therefore, this raises the question of whether this is either a bias of smaller case reports/series or whether patients with a predisposing psychosocial trigger have a slightly more severe phenotype at presentation. Studies have revealed that performing PCI in SCAD patients is challenging, and its success rate is lower than that of PCI in atherosclerotic disease [9, 127].
The incidence of early post discharge readmission following MI and SCAD is considerable. Most readmissions are because of cardiac causes [135]. Recurrence in SCAD occurs more frequently than recurrence in atherosclerotic MI [8, 136]. In our study, 16 patients experienced recurrent SCAD during a median follow-up of 2 months. This observation is consistent with prior literature [137], highlighting the importance of rigorous follow-up in the early post-SCAD period. In a large cohort of SCAD patients with low vascularization rates and high medical management, the recurrence rate was low [119]. However, in our selected SCAD cases, 18 out of 22 recurrences were in individuals who received medical therapy. This discrepancy might be due to the small size of the study population, different demographics, or probably better aggressive treatment results. Investigating the reasons behind this and finding susceptible patients is of utmost importance to reduce the associated morbidity and mortality and prevent unnecessary interventions and hospitalizations. In a meta-analysis by Gerald et al. [138], hypertension and FMD were major stressors for recurrence. In our series, stress-related SCAD recurrences were associated with neither arterial hypertension nor FMD. Additionally, Ehlers–Danlos syndrome, ADPKD, and Loeys–Dietz syndrome were present in patients with SCAD recurrence.
Limitations
There were some limitations in our study. To evaluate detailed information of each individual, we included only case reports and case series. We excluded other observational studies due to insufficient data on each individual, although they had larger populations. This is considered as the major limitation of the present study. Additionally, it should be noted that having heterogeneous follow-up durations could impact data consistency and analysis in our study and we were unable to provide a complete and comprehensive evaluation of follow-up events.
Conclusion
In the present systematic review of case reports and case series, we described the stress type, clinical features, and angiographic findings of SCAD in patients with prior psychophysical stress (see Fig. 2). While physical stress seems to precede SCAD in most cases, emotional stress is implicated in females more than males. Further studies are needed to confirm these findings.
Fig. 2.
Graphical abstract
Supplementary Information
Authors’ contributions
Conceptualization, K.H., H.S. and A.A.; methodology, K.H. and H.S.; analysis, P.F. and P.R.; validation, M.J., A.A., T.K., N.N., P.N.K.; data curation P.F. and P.R.; project administration, K.H., H.S., P.R. and P.F.; supervision, K.H., H.S., T.K., N.N. and P.N.K.; visualization, P.F., P.R., M.J., and N.H.; writing – original draft N.A.J, A.M., and P.R.; writing – reviewing and editing, K.H., H.S, M.J., Y.J., T.K., N.N. and P.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
None.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article and its additional files.
Declarations
Ethics approval and consent to participate
Since our study did not involve the collection of primary data, and we used previously published data, the need for informed consent and approval for the study was waived by Ethics Committee of Tehran University of Medical Sciences. We relied on the consent obtained from included studies involving their patients.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franke KB, Nerlekar N, Marshall H, Psaltis PJ. Systematic review and meta-analysis of the clinical characteristics and outcomes of spontanous coronary artery dissection. Int J Cardiol. 2021;322:34–39. doi: 10.1016/j.ijcard.2020.08.076. [DOI] [PubMed] [Google Scholar]
- 2.Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the american heart association. Circulation. 2018;137(19):e523–ee57. doi: 10.1161/CIR.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almasi A, Mansouri P, Jameie M, Yadangi S, Parapary SH, Mohsenizadeh SA, et al. Clinical features and prognoses of middle-aged women with ST-elevation myocardial infarction with a focus on spontaneous coronary artery dissection. Crit Pathw Cardiol. 2022;21(1):18–23. doi: 10.1097/HPC.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 4.Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129(16):1695–1702. doi: 10.1161/CIRCULATIONAHA.113.002054. [DOI] [PubMed] [Google Scholar]
- 5.Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5(3):263–270. doi: 10.1177/2048872613504310. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv. 2018;11(1):80–90. doi: 10.1016/j.jcin.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, et al. Spontaneous coronary artery dissection: angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter Cardiovasc Interv. 2017;89(1):59–68. doi: 10.1002/ccd.26383. [DOI] [PubMed] [Google Scholar]
- 8.Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol. 2017;70(9):1148–1158. doi: 10.1016/j.jacc.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Saw J, Starovoytov A, Humphries K, Sheth T, So D, Minhas K, et al. Canadian spontaneous coronary artery dissection cohort study: in-hospital and 30-day outcomes. Eur Heart J. 2019;40(15):1188–1197. doi: 10.1093/eurheartj/ehz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cereda AF, Canova PA, Soriano FS. Spontaneous coronary artery dissection after pregnancy as first manifestation of a vascular ehlers-danlos syndrome. J Invasive Cardiol. 2017;29(6):E67–Ee8. [PubMed] [Google Scholar]
- 11.Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 12.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yirerong JA, Hurlburt H. Spontaneous coronary artery dissection in a man with intense coughing spasms. BMJ Case Rep. 2018;2018:bcr-2018-225932. doi: 10.1136/bcr-2018-225932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandamme M, De Backer J, Backer T, Drieghe B, Devos D, Gevaert S. The spectrum of spontaneous coronary artery dissection: illustrated review of the literature. Acta Cardiol. 2017;72:1–11. doi: 10.1080/00015385.2017.1309095. [DOI] [PubMed] [Google Scholar]
- 15.Vandeloo B, Azzano A, Schoors D, Verstraeten A, Van Laer L, Loeys B, et al. Spontaneous coronary artery dissection in a man with a novel missense mutation in SMAD2 treated by optical coherence tomography–guided percutaneous coronary intervention. JACC: Cardiovasc Interv. 2019;12(6):e45–ee7. doi: 10.1016/j.jcin.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Verlaeckt E, Van de Bruaene L, Coeman M, Gevaert S. Spontaneous coronary artery dissection in a patient with hereditary polycystic kidney disease and a recent liver transplant: a case report. Eur Heart J Case Rep. 2019;3(4):1–5. doi: 10.1093/ehjcr/ytz216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong P, Rubenstein M, Inglessis I, Pomerantsev E, Ferrell M, Leinbach R. Spontaneous spiral dissection of a LIMA–LAD bypass graft. J Interv Cardiol. 2004;17(4):211–213. doi: 10.1111/j.1540-8183.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 18.Xin-He Y, Cheng-Jian Y, Yan J, Xin X, Jia-Ning C, Zhen-Jie Y, et al. A successful emergency management of spontaneous coronary artery dissection and review of the literature. The. Am J Emerg Med. 2013;31(7):1156.e1–1156.e3. doi: 10.1016/j.ajem.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Yang XQ, Zhu HY, Wang X, Zhao HB, Zhang W, Xiao M, et al. Spontaneous coronary artery dissection in a middle-aged woman with acute anterior myocardial infarction: A case report. Medicine (Baltimore). 2018;97(31):e11504. doi: 10.1097/MD.0000000000011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeung DF, Saw J. Multiple recurrences of spontaneous coronary artery dissection in a woman with fibromuscular dysplasia. Catheter Cardiovasc Interv. 2019;94(5):702–705. doi: 10.1002/ccd.28301. [DOI] [PubMed] [Google Scholar]
- 21.Yiangou K, Papadopoulos K, Azina C. Heavy lifting causing spontaneous coronary artery dissection with anterior myocardial infarction in a 54-year-old woman. Tex Heart Inst J. 2016;43(2):189–191. doi: 10.14503/THIJ-15-5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh GD, Nishimura M, Rogers JH, Amsterdam EA. Pain at the game: spontaneous coronary artery dissection. Am J Med. 2014;127(12):1160–1163. doi: 10.1016/j.amjmed.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Sivam S, Yozghatlian V, Dentice R, McGrady M, Moriarty C, Di Michiel J, et al. Spontaneous coronary artery dissection associated with coughing. J Cyst Fibros. 2014;13(2):235–237. doi: 10.1016/j.jcf.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Smith AAH, Wananu M, Carlson MD. Spontaneous coronary artery dissection in a healthy woman after initiating a high-intensity interval training workout program. Am J Cardiol. 2018;122(9):1588–1589. doi: 10.1016/j.amjcard.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Suresh V, Evans S. Successful stenting of stenotic lesion and spontaneous dissection of left internal mammary artery graft. Heart. 2007;93(1):44. doi: 10.1136/hrt.2006.087551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutil-Vega M, Romeu Vilar D, Barros-Membrilla AJ, Millán X, Hidalgo JA, Pons-Lladó G. Case 270: spontaneous coronary artery dissection associated with fibromuscular dysplasia. Radiology. 2019;293(1):235–240. doi: 10.1148/radiol.2019170822. [DOI] [PubMed] [Google Scholar]
- 27.Tagliari AP, Kochi AN, Rohde LEP, Wender OCB. Spontaneous left anterior descending coronary artery dissection requiring coronary artery bypass surgery. Braz J Cardiovasc Surg. 2017;32(6):536–538. doi: 10.21470/1678-9741-2017-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taha M, Latt H, Al-Khafaji J, Ali M, Seher R. A case of spontaneous coronary artery dissection presenting with acute anterior wall myocardial infarction in a young adult male - an increasingly recognized rare disease. J Community Hosp Intern Med Perspect. 2018;8(2):60–63. doi: 10.1080/20009666.2018.1440855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanabe J, Kagawa Y, Endo A, Tanabe K. Spontaneous coronary artery dissection associated with psychological stress. BMJ Case Rep. 2021;14(8):e245414. doi: 10.1136/bcr-2021-245414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unnikrishnan D, Annam R, Jacob A, Thyagarajan B, Farrugia P. STEMI in a young male after use of synephrine-containing dietary supplement. Case Rep Cardiol. 2018;2018:7074104. doi: 10.1155/2018/7074104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vale PR, Baron DW. Coronary artery stenting for spontaneous coronary artery dissection: A case report and review of the literature. Catheter Cardiovasc Diagn. 1998;45(3):280–286. doi: 10.1002/(SICI)1097-0304(199811)45:3<280::AID-CCD14>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 32.Onoda N, Izumi K. A case of spontaneous coronary artery dissection complicated with pseudoaneurysm healed by medical treatment: Follow-up by multidetector computed tomography. J Cardiol Cases. 2018;18(5):156–159. doi: 10.1016/j.jccase.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papanikolaou J, Alharthy A, Platogiannis N, Balhamar A, Alqahtani SA, Memish ZA, et al. Spontaneous coronary artery dissection in a patient with COVID-19. Coron Artery Dis. 2021;32(4):354–355. doi: 10.1097/MCA.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry R, MacConnell T, Wilde P. Case report: spontaneous coronary artery dissection. Clin Radiol. 1994;49(2):142–143. doi: 10.1016/S0009-9260(05)83460-9. [DOI] [PubMed] [Google Scholar]
- 35.Phogat V, Nepal S, Kozman H. A non-atherosclerotic heart tears apart: a case of spontaneous coronary artery dissection in a healthy postmenopausal woman. Cureus. 2022;14(5):e25459. doi: 10.7759/cureus.25459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman T, Moghadam R, Rinder M. Spontaneous coronary artery dissection: an unusual cause of st-elevation myocardial infarction in young males. Cureus. 2021;13(1):e12827. doi: 10.7759/cureus.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riaz S, Vasigh M, Mogadam E, Ganesan D, Chaudhuri D. Acute myocardial infarction due to spontaneous coronary artery dissection and plaque rupture. Cureus. 2020;12(5):e8063. doi: 10.7759/cureus.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roche AM, Klingel K, Toth K, Pepper K, Francis SA. Spontaneous coronary artery dissection in the setting of covid-19 pandemic-related stressors: a case report. Cureus. 2022;14(3):e23069. doi: 10.7759/cureus.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawami K, Natsuaki M, Hongo H, Kajiwara M, Kaneko T, Inoue Y, et al. Spontaneous internal mammary artery graft dissection triggered by emotional stress. JACC Case Rep. 2019;1(5):732–736. doi: 10.1016/j.jaccas.2019.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sengottovelu G, Rajendran R, Dattagupta A. Optical coherence tomographic image of dynamic left main coronary artery compression caused by intramural haematoma due to spontaneous coronary artery dissection – degloved artery managed with bioresorbable vascular scaffold. EuroIntervention. 2015;11(6):659. doi: 10.4244/EIJY14M12_05. [DOI] [PubMed] [Google Scholar]
- 41.Sharma H, Vetrugno V, Khan SQ. Successful treatment of a spontaneous right coronary artery dissection with a 4-mm diameter cutting balloon: a case report. Eur Heart J Case Rep. 2019;3(4):1–6. doi: 10.1093/ehjcr/ytz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma S, Raut N, Potdar A. Spontaneous coronary artery dissection: Case series and review of literature. Indian Heart J. 2016;68(4):480–485. doi: 10.1016/j.ihj.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenoy P, Tayeb T, Covas P, Temesgen N, Tracy C. Not your common athletic heart problem: using coronary cta to visualize spontaneous coronary artery dissection. Case Rep Cardiol. 2020;2020:8882561. doi: 10.1155/2020/8882561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherrid MV, Mieres J, Mogtader A, Menezes N, Steinberg G. Onset during exercise of spontaneous coronary artery dissection and sudden death: occurrence in a trained athlete: case report and review of prior cases. Chest. 1995;108(1):284–287. doi: 10.1378/chest.108.1.284. [DOI] [PubMed] [Google Scholar]
- 45.Jafri FN, Solarz D, Hjemdahl-Monsen C. Cycling induced spontaneous coronary artery dissection in a healthy male. Case Rep Emerg Med. 2018;2018:2740513. doi: 10.1155/2018/2740513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maeder M, Ammann P, Angehrn W, Rickli H. Idiopathic spontaneous coronary artery dissection: incidence, diagnosis and treatment. Int J Cardiol. 2005;101(3):363–369. doi: 10.1016/j.ijcard.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 47.Mahendiran T, Desgraz B, Antiochos P, Rubimbura V. Case report: a first case of spontaneous coronary artery dissection potentially associated with scuba diving. Front Cardiovasc Med. 2022;9. [DOI] [PMC free article] [PubMed]
- 48.Mahmood MM, Austin D. IVUS and OCT guided primary percutaneous coronary intervention for spontaneous coronary artery dissection with bioresorbable vascular scaffolds. Cardiovasc Revasc Med. 2017;18(1):53–57. doi: 10.1016/j.carrev.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Maiga H, Poropat Flerin T, Berlot B, Lipar L, Cvijic M. Challenging diagnosis of spontaneous coronary artery dissection: how to look beyond the obvious. Eur Heart J Case Rep. 2022;6(11):ytac422. doi: 10.1093/ehjcr/ytac422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayr A, Klug G, Jaschke W, Pachinger O, Metzler B. Persistent spontaneous dissection of the left anterior descending coronary artery after emotional pressure. Wien Klin Wochenschr. 2010;122(15):515–517. doi: 10.1007/s00508-010-1422-1. [DOI] [PubMed] [Google Scholar]
- 51.Mehrani M, Nematollahi A, Hatami M, Hosseini K. Coronary artery dissection in a 33-year-old man with fatigue and episodic retrosternal burning: a case report. Eur Heart J - Case Rep. 2018;2(3):yty068. doi: 10.1093/ehjcr/yty068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miki K, Fujii K, Nakata T, Shibuya M, Fukunaga M, Kawai K, et al. The utility of intravascular ultrasound for the diagnosis and management of spontaneous coronary artery dissection in a middle-aged woman with acute inferior myocardial infarction. J Cardiol Cases. 2012;6(3):e78–e80. doi: 10.1016/j.jccase.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohammadian M, Shah D, Santana M, Elkattawy S, Jesani S. Levine's sign points to spontaneous coronary artery dissection in a healthy young male. Cureus. 2022;14(5):e24893. doi: 10.7759/cureus.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oluwole AS, Virk HUH, Witzke C. Intense exercise may not be so benign: chest pain in a young athletic woman may be spontaneous coronary artery dissection (SCAD). BMJ Case Rep. 2020;13(4). [DOI] [PMC free article] [PubMed]
- 55.Kalaga RV, Malik A, Thompson PD. Exercise-related spontaneous coronary artery dissection: case report and literature review. Med Sci Sports Exerc. 2007;39(8):1218–1220. doi: 10.1249/mss.0b013e318060114f. [DOI] [PubMed] [Google Scholar]
- 56.Kalinskaya A, Skrypnik D, Kostin A, Vasilieva E, Shpektor A. Case report of an acute myocardial infarction as a result of spontaneous coronary artery dissection in a patient with fibromuscular dysplasia. Case Rep Cardiol. 2019;2019:3051616. doi: 10.1155/2019/3051616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanaroglou S, Nair V, Fernandes J. Sudden cardiac death due to coronary artery dissection as a complication of cardiac sarcoidosis. Cardiovasc Pathol: Off J Soc Cardiovasc Pathol. 2015;24. [DOI] [PubMed]
- 58.Kang G, Sarraju A, Nishi T, Rogers I, Tremmel JA, Kim JB. Spontaneous coronary artery dissection and st-segment elevation myocardial infarction in an anomalous LAD artery. JACC Case Rep. 2020;2(1):45–50. doi: 10.1016/j.jaccas.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karabag T, Dogan SM. A case of spontaneous multivessel coronary artery dissection presenting with acute myocardial infarction and ventricular tachycardia. Catheter Cardiovasc Intervent. 2012;79(1):113–116. doi: 10.1002/ccd.23255. [DOI] [PubMed] [Google Scholar]
- 60.Kegai S, Sato K, Goto K, Ozawa T, Kimura T, Kobayashi K, et al. Coexistence of spontaneous coronary artery dissection, takotsubo cardiomyopathy, and myocardial bridge. JACC Case Rep. 2021;3(2):250–254. doi: 10.1016/j.jaccas.2020.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan H, Yousaf A, Ahmad M, Munir A, Moza A. Complete recovery of an occluded coronary artery secondary to spontaneous coronary artery dissection with medical management in a young patient presenting with acute coronary syndrome. Cureus. 2022;14(10):e29980. doi: 10.7759/cureus.29980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kireev K, Genkel V, Kuznetsova A, Sadykov R. Multivessel spontaneous coronary artery dissection in a patient after mild COVID-19: A case report. SAGE Open Med Case Rep. 2020;8:2050313x20975989. doi: 10.1177/2050313X20975989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lempereur M, Gin K, Saw J. Multivessel spontaneous coronary artery dissection mimicking atherosclerosis. JACC: Cardiovasc Intervent. 2014;7(7):e87–ee8. doi: 10.1016/j.jcin.2013.12.207. [DOI] [PubMed] [Google Scholar]
- 64.Poon K, Bell B, Raffel OC, Walters DL, Jang I-K. Spontaneous coronary artery dissection. Circulation: Cardiovasc Intervent. 2011;4(2):e5–e7. doi: 10.1161/CIRCINTERVENTIONS.110.959593. [DOI] [PubMed] [Google Scholar]
- 65.Fitzpatrick J, Noman A, Ryan N, Dawson D. Recurrent spontaneous coronary artery dissection in a middle-aged male athlete patient: A case report. Eur Heart J - Case Rep. 2020;4. [DOI] [PMC free article] [PubMed]
- 66.Ghafoor HU, Bose A, El-Meligy A, Hannan J. A case report of recurrent spontaneous coronary artery dissection and Takotsubo cardiomyopathy: a treatment dilemma. Eur Heart J Case Rep. 2020;4(1):1–6. doi: 10.1093/ehjcr/ytaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goh AC, Lundstrom RJ. Spontaneous coronary artery dissection with cardiac tamponade. Tex Heart Inst J. 2015;42(5):479–482. doi: 10.14503/THIJ-14-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guess JM, Madigan CG, Hudspath CB, Hurley JT, Martinho S. Spontaneous coronary artery dissection in a 26-year-old-male soldier. Mil Med. 2019;184(5–6):e462–e4e6. doi: 10.1093/milmed/usy230. [DOI] [PubMed] [Google Scholar]
- 69.Iglesias D, Salinas P, Jiménez-Valero S. Spontaneous coronary artery dissection evaluated by optical coherence tomography. J Cardiovasc Med. 2011;12(10). [DOI] [PubMed]
- 70.Jatti K, Maurovich-Horvat P, Hasleton J, Uddin M, Ruzsics B. Cardiac computed tomography to identify and guide therapy of intramural hemorrhage in high-risk coronary anatomy. JACC Case Rep. 2021;3(1):120–124. doi: 10.1016/j.jaccas.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joy G, Eissa H. Spontaneous coronary artery dissection in a patient with a family history of fatal ascending aortic dissection: case report and discussion of diseases causing both presentations. Case Rep Cardiol. 2019;2019:7218480. doi: 10.1155/2019/7218480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin AH, Shutt BJ, Dendall RT, Bennett W. Multivessel spontaneous coronary artery dissection treated with staged percutanous coronary intervention in a non-postpartum female. BMJ Case Rep. 2012;2012. [DOI] [PMC free article] [PubMed]
- 73.Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia. Circulation: Cardiovasc Intervent. 2012;5(1):134–137. doi: 10.1161/CIRCINTERVENTIONS.111.966630. [DOI] [PubMed] [Google Scholar]
- 74.Y-Hassan S, Themudo R, Maret E. Spontaneous coronary artery dissection and takotsubo syndrome: The chicken or the egg causality dilemma. Catheter Cardiovasc Intervent. 2017;89(7):1215–1218. doi: 10.1002/ccd.26956. [DOI] [PubMed] [Google Scholar]
- 75.DeBoer R, Nasir U, Fraga JD. Spontaneous coronary artery dissection: chest pain in a young woman. J Community Hosp Intern Med Perspect. 2022;12(4):70–74. doi: 10.55729/2000-9666.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Marco M, Clemente D, Forlani D, D'Alleva A, Duronio G, Paloscia L. Spontaneous coronary artery dissection in a young woman resolved with conservative strategy. A case report. J Cardiol Cases. 2016;14(2):59–61. doi: 10.1016/j.jccase.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dwyer N, Galligan L, Harle R. Spontaneous coronary artery dissection and associated ct coronary angiographic findings: a case report and review. Heart Lung Circ. 2007;16(2):127–130. doi: 10.1016/j.hlc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 78.Ellis CJ, Haywood GA, Monro JL. Spontaneous coronary artery dissection in a young woman resulting from an intense gymnasium “work-out”. Int J Cardiol. 1994;47(2):193–194. doi: 10.1016/0167-5273(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 79.Emren SV, Şenöz O, Duygu H, Nazlı C, Ergene O. Primary spontaneous coronary dissectİon in a young male and the role of intravascular ultrasonography for diagnosis and treatment. Int J Cardiovasc Acad. 2015;1(2):66–68. doi: 10.1016/j.ijcac.2015.10.005. [DOI] [Google Scholar]
- 80.Eugène M, Siam-Tsieu V, Pillière R, Deblaise J, Dubourg O, Mansencal N. Recurrent spontaneous coronary artery dissection: Unexpected evolution and major role of emotional stress. Int J Cardiol. 2015;201:316–318. doi: 10.1016/j.ijcard.2015.08.064. [DOI] [PubMed] [Google Scholar]
- 81.Farouji I, Al-Radideh O, Abed H, DaCosta TR, Battah A, Ahmad AS, et al. Unusual presentation of post-coital spontaneous coronary artery dissection. Cureus. 2021;13(8):e17460. doi: 10.7759/cureus.17460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fitouchi S, Di Marco P, Motreff P, Lhoest N. Concomitant presentation of spontaneous coronary artery dissection with Takotsubo syndrome: a case report. Eur Heart J - Case Rep. 2022;6(5):ytac172. doi: 10.1093/ehjcr/ytac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ho Y-D, Koizumi T, Lee DP. Spontaneous coronary artery dissection in a woman with depression without coronary atherosclerotic risk factors. J Invasive Cardiol. 2007;19(6):E166–E168. [PubMed] [Google Scholar]
- 84.Souza P, Herdy AH. Spontaneous exercise-related coronary artery dissection among young patients without risk factors or atherosclerotic disease. Arq Bras Cardiol. 2019;113(5):988–998. doi: 10.36660/abc.20180446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bath AS, Aggarwal S, Gupta V, Kalavakunta JK. Slit in the coronaries: a case of spontaneous coronary artery dissection. Cureus. 2019;11(6):e4841. doi: 10.7759/cureus.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ben Ahmed H, Allouche E, Rekik A, Ouechtati W, Bezdah L. Multivessel spontaneous coronary artery dissection with simultaneously three different angiographic patterns. Ann Cardiol Angéiol. 2022;71(2):118–121. doi: 10.1016/j.ancard.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 87.Capuano C, Sesana M, Predolini S, Leonzi O, Cuccia C. Case report: a very large dissection in the left anterior descending coronary artery of a 56-year-old man. Cardiovasc Revasc Med. 2006;7(4):240–242. doi: 10.1016/j.carrev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Cascais F, Pereira A, Almeida A, Rocha L. A rare cause of chest pain in paediatric age: a teenager with acute myocardial infarction due to spontaneous coronary artery dissection. BMJ Case Rep. 2022;15:e246393. doi: 10.1136/bcr-2021-246393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cerrato E, Tomassini F, Rolfo C, Gagnor A, Varbella F. Spontaneous coronary artery dissection treated with biovascular scaffolds guided by intravascular ultrasounds imaging. Cardiovasc Interv Ther. 2017;32(2):186–189. doi: 10.1007/s12928-016-0391-3. [DOI] [PubMed] [Google Scholar]
- 90.Chang F-L, Chang W-C, Cheng Y-T, Liu T-J, Lee W-L, Lai C-H. Spontaneous coronary artery dissection causing acute myocardial infarction and cardiac arrest in a 25-year-old male. Perfusion. 2017;33(2):160–163. doi: 10.1177/0267659117727824. [DOI] [PubMed] [Google Scholar]
- 91.Cockburn J, Yan W, Bhindi R, Hansen P. Spontaneous coronary artery dissection treated with bioresorbable vascular scaffolds guided by optical coherence tomography. Can J Cardiol. 2014;30(11):1461.e1–1461.e3. doi: 10.1016/j.cjca.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 92.Cropp EM, Turner JS, Kreutz RP. Spontaneous coronary artery dissection in a 14-year-old. Am J Emerg Med. 2013;31(2):461.e5–461.e7. doi: 10.1016/j.ajem.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 93.Nourhan C, Shilpa K. Spontaneous Coronary Artery Dissection With Systemic Lupus Erythematosus. Ochsner J. 2022;22. [DOI] [PMC free article] [PubMed]
- 94.Thomas BN, Aslam S, Cullen J, Anantharaman R. Spontaneous coronary artery dissection in men presenting with acute coronary syndrome, successfully managed by intravascular ultrasound-guided percutaneous coronary intervention. BMJ Case Rep. 2014;2014:bcr2013009169. doi: 10.1136/bcr-2013-009169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aksakal A, Arslan U, Yaman M, Urumdaş M, Ateş AH. Spontaneous coronary artery dissection as a cause of myocardial infarction. World J Cardiol. 2014;6(12):1290–1292. doi: 10.4330/wjc.v6.i12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Almahmeed WA, Haykowski M, Boone J, Ling H, Allard M, Webb J, et al. Spontaneous coronary artery dissection in young women. Catheter Cardiovasc Diagn. 1996;37(2):201–205. doi: 10.1002/(SICI)1097-0304(199602)37:2<201::AID-CCD21>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 97.Altshuler E, Matthia E, Naik D, Keeley EC. Extremely heavy lifting associated with spontaneous coronary artery dissection. Cureus. 2021;13(11):e19451. doi: 10.7759/cureus.19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Álvarez-Lario B, Álvarez-Roy L, Mayordomo-Gómez S, García-García JM. Spontaneous coronary artery dissection in systemic lupus erythematosus: case-based review. Rheumatol Int. 2019;39(10):1821–1827. doi: 10.1007/s00296-019-04351-3. [DOI] [PubMed] [Google Scholar]
- 99.Anderson RD, Jayadeva PS, Wilson WM, Iyer R. Spontaneous coronary artery dissection: case series from a tertiary centre. Heart Lung Circ. 2016;25(3):e41–ee5. doi: 10.1016/j.hlc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 100.Anuwatworn A, Sethi P, Steffen K, Jonsson O, Petrasko M. Spontaneous Coronary Artery Dissection: A Rare Manifestation of Alport Syndrome. Case Rep Cardiol. 2017;2017:1705927. doi: 10.1155/2017/1705927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aqel RA, Zoghbi GJ, Iskandrian AE. Spontaneous coronary artery dissection with pseudoaneurysm formation diagnosed by intravascular ultrasound: a case report. Echocardiography. 2004;21(2):153–157. doi: 10.1111/j.0742-2822.2004.02160.x. [DOI] [PubMed] [Google Scholar]
- 102.Arrivi A, Milici C, Bock C, Placanica A, Boschetti E, Dominici M. Idiopathic, serial coronary vessels dissection in a young woman with psychological stress: a case report and review of the literature. Case Rep Vasc Med. 2012;2012:498465. doi: 10.1155/2012/498465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haq MA, Mutha V, Gaal WJv. Multivessel spontaneous coronary artery dissection of left and right coronary systems. BMJ Case Rep. 2013;2013:bcr2013201142. doi: 10.1136/bcr-2013-201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan NUA, Miller MJ, Babb JD, Ahmed S, Saha PK, Shammas RL, et al. Spontaneous coronary artery dissection. Acute Cardiac Care. 2006;8(3):162–171. doi: 10.1080/17482940600789190. [DOI] [PubMed] [Google Scholar]
- 105.Afzal AM, Sarmast SA, Weber NA, Schussler JM. Spontaneous coronary artery dissection in a 22-year-old man on lisdexamfetamine. Proc (Bayl Univ Med Cent). 2015;28(3):367–368. doi: 10.1080/08998280.2015.11929277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weber N, Weber A, Carbone P, Lawrence A, Bilbrey T, Schussler JM, et al. High-intensity, sport-specific cardiac rehabilitation training of a 22-year-old competitive cyclist after spontaneous coronary artery dissection. Bayl Univ Med Center Proc. 2018;31(2):207–209. doi: 10.1080/08998280.2017.1415509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–1122. doi: 10.1002/ccd.25293. [DOI] [PubMed] [Google Scholar]
- 108.Daoulah A, Al-Faifi SM, Hersi AS, Dinas PC, Youssef AA, Alshehri M, et al. Spontaneous coronary artery dissection in relation to physical and emotional stress: a retrospective study in 4 arab gulf countries. Curr Probl Cardiol. 2021;46(3):100484. doi: 10.1016/j.cpcardiol.2019.100484. [DOI] [PubMed] [Google Scholar]
- 109.Sara JDS, Toya T, Ahmad A, Clark MM, Gilliam WP, Lerman LO, et al. Mental stress and its effects on vascular health. Mayo Clin Proc. 2022;97(5):951–990. doi: 10.1016/j.mayocp.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56(22):1840–1846. doi: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) Eur Heart J Acute Cardiovasc Care. 2013;2(2):147–152. doi: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fahmy P, Prakash R, Starovoytov A, Boone R, Saw J. Pre-disposing and precipitating factors in men with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9(8):866–868. doi: 10.1016/j.jcin.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 113.Sharma S, Kaadan MI, Duran JM, Ponzini F, Mishra S, Tsiaras SV, et al. Risk factors, imaging findings, and sex differences in spontaneous coronary artery dissection. Am J Cardiol. 2019;123(11):1783–1787. doi: 10.1016/j.amjcard.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 114.Sara JDS, Ahmad A, Toya T, Suarez Pardo L, Lerman LO, Lerman A. Anxiety disorders are associated with coronary endothelial dysfunction in women with chest pain and nonobstructive coronary artery disease. J AmHeart Assoc. 2021;10(17):e021722. doi: 10.1161/JAHA.121.021722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alfonso F, Paulo M, Lennie V, Dutary J, Bernardo E, Jiménez-Quevedo P, et al. Spontaneous coronary artery dissection: long-term follow-up of a large series of patients prospectively managed with a "conservative" therapeutic strategy. JACC Cardiovasc Interv. 2012;5(10):1062–1070. doi: 10.1016/j.jcin.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 116.Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74(5):710–717. doi: 10.1002/ccd.22115. [DOI] [PubMed] [Google Scholar]
- 117.Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, et al. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome--A single-centre Australian experience. Int J Cardiol. 2016;202:336–338. doi: 10.1016/j.ijcard.2015.09.072. [DOI] [PubMed] [Google Scholar]
- 118.Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7(6):777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 119.Saw J, Starovoytov A, Aymong E, Inohara T, Alfadhel M, McAlister C, et al. Canadian spontaneous coronary artery dissection cohort study: 3-year outcomes. J Am Coll Cardiol. 2022;80(17):1585–1597. doi: 10.1016/j.jacc.2022.08.759. [DOI] [PubMed] [Google Scholar]
- 120.YH S. Spontaneous coronary artery dissection and takotsubo syndrome: An often overlooked association; review. Cardiovasc Revasc Med. 2018;19(6):717–723. doi: 10.1016/j.carrev.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 121.Salamanca J, García-Guimaraes M, Camacho-Freire SJ, Ojeda S, Veiga G, Jiménez-Valero S, et al. Spontaneous coronary artery dissection and Takotsubo syndrome: comparison of baseline clinical and angiographic characteristics and in-hospital outcomes. Coron Artery Dis. 2021;32(6):509–516. doi: 10.1097/MCA.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 122.Salamanca J, García-Guimaraes M, Sabaté M, Sanz-Ruiz R, Macaya F, Roura G, et al. Non-atherosclerotic acute cardiac syndromes: spontaneous coronary artery dissection and Takotsubo syndrome. Comparison of long-term clinical outcomes. Coron Artery Dis. 2024;35(1):50–58. doi: 10.1097/MCA.0000000000001304. [DOI] [PubMed] [Google Scholar]
- 123.Macaya F, Vedia Ó, Salazar CH, Mejía-Rentería H, Ruiz-Pizarro V, Salinas P, et al. Clinical outcomes of patients presenting with spontaneous coronary artery dissection versus takotsubo syndrome: a propensity score analysis. Eur Heart J Acute Cardiovasc Care. 2020;9(7):694–702. doi: 10.1177/2048872619886311. [DOI] [PubMed] [Google Scholar]
- 124.Alvarado T, García-Guimaraes M, Nogales JM, Jimenez-Kockar M, Macaya F, Alfonso F. Spontaneous coronary artery dissection: are there differences between men and women? Arq Bras Cardiol. 2023;120(1):e20210550. doi: 10.36660/abc.20210550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection. Circulation: Cardiovasc Intervent. 2014;7(5):645–655. doi: 10.1161/CIRCINTERVENTIONS.114.001760. [DOI] [PubMed] [Google Scholar]
- 126.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126(5):579–588. doi: 10.1161/CIRCULATIONAHA.112.105718. [DOI] [PubMed] [Google Scholar]
- 127.Teruzzi G, Santagostino Baldi G, Gili S, Guarnieri G, Montorsi P, Trabattoni D. Spontaneous coronary artery dissections: a systematic review. J Clin Med. 2021;10(24). [DOI] [PMC free article] [PubMed]
- 128.Adlam D, Tweet MS, Gulati R, Kotecha D, Rao P, Moss AJ, et al. Spontaneous coronary artery dissection: pitfalls of angiographic diagnosis and an approach to ambiguous cases. JACC Cardiovasc Interv. 2021;14(16):1743–1756. doi: 10.1016/j.jcin.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87(2):E54–E61. doi: 10.1002/ccd.26022. [DOI] [PubMed] [Google Scholar]
- 130.Tweet MS, Gulati R, Williamson EE, Vrtiska TJ, Hayes SN. Multimodality imaging for spontaneous coronary artery dissection in women. JACC Cardiovasc Imaging. 2016;9(4):436–450. doi: 10.1016/j.jcmg.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Salamanca J, García-Guimarães M, Sabaté M, Sanz-Ruiz R, Macaya F, Roura G, et al. Multivessel spontaneous coronary artery dissection: Clinical features, angiographic findings, management, and outcomes. Int J Cardiol. 2023;370:65–71. doi: 10.1016/j.ijcard.2022.11.011. [DOI] [PubMed] [Google Scholar]
- 132.Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection. Circulation: Cardiovasc Intervent. 2014;7(6):777–786. doi: 10.1161/CIRCINTERVENTIONS.114.001659. [DOI] [PubMed] [Google Scholar]
- 133.Bocchino PP, Angelini F, Franchin L, D’Ascenzo F, Fortuni F, De Filippo O, et al. Invasive versus conservative management in spontaneous coronary artery dissection: A meta-analysis and meta-regression study. Hell J Cardiol. 2021;62(4):297–303. doi: 10.1016/j.hjc.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 134.Jamil A, Tajrishi FZ, Kahe F, Najafi H, Montazerin SM, Shojaei F, et al. Spontaneous coronary artery dissection managed with a conservative or revascularization approach: a meta-analysis. J Cardiovasc Med (Hagerstown). 2020;21(1):42–50. doi: 10.2459/JCM.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 135.Gad MM, Mahmoud AN, Saad AM, Bazarbashi N, Ahuja KR, Karrthik AK, et al. Incidence, clinical presentation, and causes of 30-day readmission following hospitalization with spontaneous coronary artery dissection. JACC: Cardiovasc Intervent. 2020;13(8):921–932. doi: 10.1016/j.jcin.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 136.Lobo AS, Cantu SM, Sharkey SW, Grey EZ, Storey K, Witt D, et al. Revascularization in patients with spontaneous coronary artery dissection and st-segment elevation myocardial infarction. J Am Coll Cardiol. 2019;74(10):1290–1300. doi: 10.1016/j.jacc.2019.06.065. [DOI] [PubMed] [Google Scholar]
- 137.Bocchino PP, Franchin L, Angelini F, D'Ascenzo F, De Ferrari GM, Alfonso F. Outcomes during the first year following spontaneous coronary artery dissection: A systematic timeframe pooled analysis. Catheter Cardiovasc Interv. 2022;99(2):472–479. doi: 10.1002/ccd.30016. [DOI] [PubMed] [Google Scholar]
- 138.Chi G, Najafi H, Montazerin SM, Lee JJ. Factors associated with recurrent spontaneous coronary artery dissection: a systematic review and meta-analysis. Coron Artery Dis. 2022;33(7):566–573. doi: 10.1097/MCA.0000000000001168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article and its additional files.