Abstract
Although orthostatic hypotension (OH) has long been recognized as a manifestation of autonomic dysfunction, a growing body of literature has identified OH as a common comorbidity of hypertension. This connection is complex, related to pathophysiology in blood pressure regulation and the manner by which OH is derived as the difference between 2 blood pressure measurements. While traditional therapeutic approaches to OH among patients with neurodegenerative disorders focus on increasing upright blood pressure to prevent cerebral hypoperfusion, the management of OH among patients with hypertension is more nuanced; resting hypertension is itself associated with adverse outcomes among these patients. Although there is substantial evidence that intensive blood pressure treatment does not cause OH in the majority of patients with essential hypertension, some classes of antihypertensive agents may unmask OH in patients with an underlying autonomic impairment. Practical steps to manage OH among adults with hypertension start with (1) a thorough characterization of its patterns, triggers, and cause; (2) review and removal of aggravating factors (often pharmacological agents not related to hypertension treatment); (3) optimization of an antihypertensive regimen; and (4) adoption of a tailored treatment strategy that avoids exacerbating hypertension. These strategies include countermaneuvers and short-acting vasoactive agents (midodrine, droxidopa). Ultimately, further research is needed on the epidemiology of OH, the impact of hypertension treatment on OH, approaches to the screening and diagnosis of OH, and OH treatment among adults with hypertension to improve the care of these patients and their complex blood pressure pathophysiology.
Keywords: AHA Scientific Statements; antihypertensive agents; blood pressure; comorbidity; hypertension; hypotension, orthostatic
Hypertension affects nearly half of US adults1 and >1 billion adults worldwide.2 Although there is indisputable benefit to more intensive control of hypertension in the general population to prevent cardiovascular disease,3 hypertension treatment is often complicated by the presence of orthostatic hypotension (OH), defined as a sustained reduction in systolic blood pressure (SBP) of at least 20 mmHg or diastolic blood pressure (BP) of at least 10 mmHg within 3 minutes of standing.4 Common among adults with uncontrolled hypertension,5 OH is frequently cited as a consequence of hypertension treatment.6 Moreover, antihypertensive treatment guidelines recommend screening for OH (1) before the initiation or escalation of therapy and (2) in the setting of treatment to prevent hypotensive adverse events.7 However, recent evidence has prompted a reconsideration of OH as a limiting factor for hypertension management. The present scientific statement focuses on the latest science with respect to the epidemiology and complex pathophysiology between OH and hypertension, approaches to OH screening and monitoring, the impact of hypertension treatment on OH, treatment strategies among adults with OH and hypertension, and recommendations for further research.
EPIDEMIOLOGY
The prevalence of OH is highly variable on the basis of study inclusion criterion and screening procedures. Nevertheless, OH prevalence increases with age and with disease burden. OH is estimated to affect ≈10% of adults ≥60 years of age and increases to 16% to 30% among adults >65 years of age.8,9 Moreover, it affects 50% to 65% of institutionalized older adults10 and as many as 10% of adults with hypertension.11–13 OH also disproportionately affects adults with diabetes, heart failure, and chronic kidney disease and is strongly associated with sarcopenia and frailty.10,14–17 Population-based studies have examined the prevalence of OH by race or ethnicity and by sex in patients with hypertension with mixed findings. In the ARIC (Atherosclerosis Risk in Communities Study) cohort, OH was more common among Black adults than White adults (6.4% versus 4.4%),18 whereas OH was more prevalent among women and among White participants compared with Black participants in ACCORD (Action to Control Cardiovascular Risk in Diabetes) BP trial19 and SPRINT (Systolic Blood Pressure Intervention Trial).20
OH is an independent risk factor of mortality and cardiovascular comorbidities linked to increased hospital admissions.8,21,22 A meta-analysis of 15 cohort studies found that individuals with OH had a higher risk of developing heart failure, atrial fibrillation, coronary heart disease, and myocardial infarction.22 In a number of population-based longitudinal and prospective studies, OH has been a consistent predictor of a higher risk of coronary events, ischemic stroke, cardiovascular disease, and asymptomatic atrial fibrillation.8 These study findings suggest that OH may be a robust yet underrecognized risk factor of cardiovascular disease–related morbidity and mortality, especially among older adults.
OH is also associated with a number of noncardiovascular adverse outcomes, including falls,23–27 fractures,27–29 syncope,28,31,32 cognitive decline or dementia,27,33–35 depression,36 frailty,23 and early death.27,28 Whether OH is a causal factor in the development of these adverse outcomes is a focus of ongoing debate. It is thought that hypoperfusion of skeletal muscle, heart, and brain may cause progressive organ injury, contributing to subclinical damage and progressive declines in function. However, emerging evidence also suggests a role for comorbid hypertension in the supine or seated positions as the primary driver of injury and adverse outcomes.37 It is equally possible that the combination of high and low BP, that is, BP variability, may drive clinical events.
Last, many studies on OH identify adults with asymptomatic OH as a result of a standardized protocol. This is an important distinction from clinic cohorts in which OH is identified in response to a symptom or clinical event. Both orthostatic symptoms and asymptomatic measured OH have been associated with adverse events.34,38 Moreover, orthostatic symptoms have been associated with missing data in cohort studies (ie, being too symptomatic to stand for BP measurement),34 and there are many cases of OH not being perceived among patients.39 Together, this non-standard symptom capture and selection has led to conflicting conclusions across the OH literature.40
PATHOPHYSIOLOGY
OH is driven by gravitational redistribution of ≈300 to 800 cm3 of fluid to the lower extremities and splanchnic vessels upon standing.41,42 In the healthy adult, this drop in pressure is sensed by baroreceptors in the carotid arteries and right atrium, resulting in an autonomic reflex that promotes a number of physiological responses, most prominently sympathetic vasoconstriction (α-adrenergic response), increased heart rate (parasympathetic withdrawal and β-adrenergic response), and increased venous return through splanchnic venous bed compression and skeletal muscle contraction.42,43 Impairments in both autonomic or individual response pathways can contribute to OH. Some forms of autonomic failure are sufficient to explain OH, but in most patients, OH results from a combination of impaired autonomic reflexes and volume depletion or adverse effects of medications.44 OH should be distinguished from postural tachycardia syndrome, a chronic orthostatic intolerance condition associated with significantly increased heart rate upon standing without a drop in BP.45
OH Characterization
To individualize OH treatment, a critical first step is to consider its underlying cause and to characterize relevant BP patterns (Table). With respect to causes, the occurrence of OH implies that autonomic mechanisms are unable to compensate for the challenges of upright posture. The degree of autonomic impairment is a continuum. On one extreme is neurogenic OH, seen in neurodegenerative disorders and peripheral autonomic neuropathies and caused by neurodegeneration of central autonomic pathways or denervation of peripheral sympathetic nerves. OH is the consequence of impaired sympathetic vasoconstriction due to reduced norepinephrine release from postganglionic neurons and is independently associated with increased mortality.46 Clinically, these conditions are less common in the general population, and the pattern of OH tends to be more sustained, highly reproducible, and of larger magnitude and entails higher risk of adverse events related to cerebral hypoperfusion such as syncope or falls.
Table.
Conditions, Mechanisms, and Impact of Hypertension Treatment on Various Clinical Presentations of OH
| Diagnosis | Mechanism | Predominant pattern | Effect of hypertension treatment | Related conditions |
|---|---|---|---|---|
| Classic OH | Autonomic dysfunction | Low standing BP with or without supine hypertension Often symptomatic | Worse upright hypotension; improved supine hypertension | Parkinson disease, pure autonomic failure |
| Hypertensive OH | Reduced diastolic filling due to left ventricular hypertrophy; arterial stiffness | High supine/seated BP, normal standing BP Often asymptomatic | Reduced incidence with improved BP regulation | Hypertension, left ventricular hypertrophy, atherosclerotic disease |
| Pseudo-OH, threshold effect* (see Supplemental Figure) | Similar percent change at higher BP exceeds the BP threshold used to define OH | High supine/seated BP Asymptomatic | Reduced incidence with lower resting BP | Hypertension |
| Pseudo-OH, measurement error† (see Figure 2) | Greater measurement error with higher resting BP can cause the appearance of a large change in BP if the first error goes opposite to the second error. | Transient/nonreproducible Asymptomatic | Lower BP variability with lower resting BP | Hypertension, BP lability |
BP indicates blood pressure; and OH, orthostatic hypotension.
It has been recommended to use a systolic change threshold of 30 mm Hg among patients with hypertension to address this concern.
This issue also affects orthostatic hypertension.
The term nonneurogenic OH is often used to describe cases of OH that occur in patients who have some degree of autonomic impairment caused by aging, diabetes, or other neuropathies and in combination with aggravating factors, often volume depletion or the use of medications that impair compensatory mechanisms that maintain orthostatic tolerance.40 A more rigorous term arguably would be OH with a nonneurogenic component. There is growing enthusiasm for the ratio of orthostatic change in heart rate to change in SBP as a screening tool to indicate the presence of underlying autonomic impairment, causing an inappropriate heart rate response (a ratio <0.5 is strongly associated with neurogenic OH).47 Conversely, and of practical importance, a ratio >0.5 suggests the presence of aggravating and potentially reversible factors.
OH is also more likely to be observed among adults with hypertension, in part because of its derivation as the difference of 2 BP measurements. That is, the magnitude of changes in BP used to define OH is more likely to be observed among adults with elevated supine BPs (see Supplemental Figure). Nevertheless, OH is also associated with a number of hypertension patterns that may be informative for personalizing treatment strategies. The patterns with the most consistent evidence are (1) white-coat effect, (2) nocturnal hypertension or nondipping, and (3) morning hypotension. Multiple studies have demonstrated that OH is related to the white-coat phenomenon: elevated BP in clinic and lower BP outside of clinic.48 One study involving 4305 patients with hypertension demonstrated that early OH (OH within 1 minute of standing) was present in ≈7% of those with white-coat hypertension.49 Another study examining 897 adults (a subpopulation of SPRINT) undergoing BP treatment showed that OH was positively associated with white-coat effects.50
Another common pattern is nocturnal nondipping (ie, the absence of a normal drop in BP while asleep) or nocturnal hypertension (high BP while sleeping),50–53 which may be more pronounced in older adults because of aging-related changes in circadian BP regulation54 and could reflect an incipient neurodegenerative disorder (see the discussion of neurogenic OH).55 Furthermore, reverse dipping (nighttime BP greater than daytime BP) is associated with the most pronounced OH in elderly adults with hypertension with diabetes.56 This pattern is often coupled with yet a third common presentation of OH, morning hypotension,57 which is thought to reflect a transient hypovolemic state secondary to pressure natriuresis driven by high BP while supine overnight (Figure 1).58
Figure 1. Twenty-four–hour ambulatory BP monitor recording of a patient recently hospitalized for syncope and treated with fludrocortisone for suspected neurogenic OH.
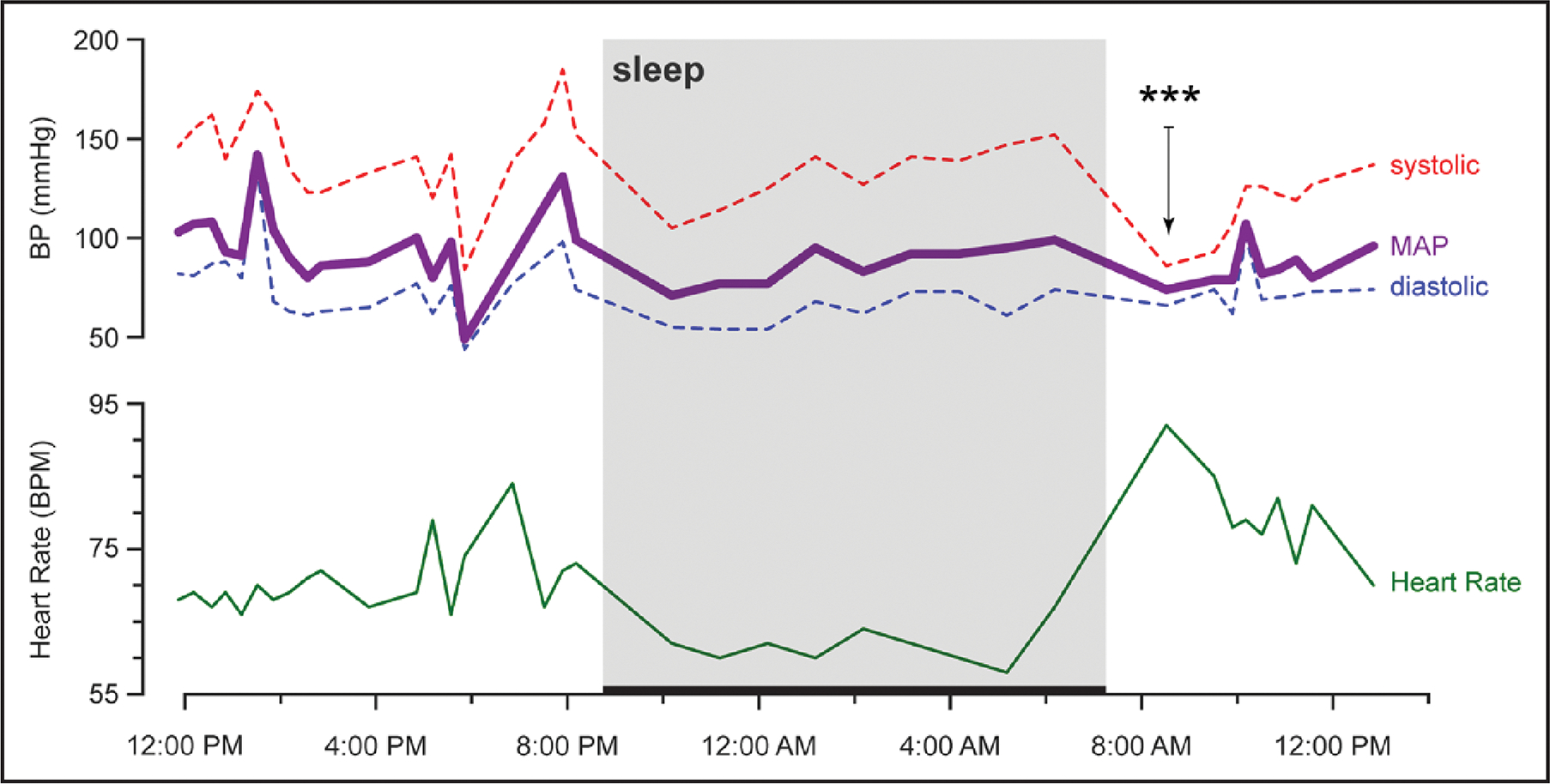
The reading is notable for blood pressure variability, nocturnal hypertension, and a morning drop in blood pressure after a short walk that correlated with severe lightheadedness (denoted by ***). Sleep period is depicted with a thick horizontal line between 8:45 pm and 7:45 am. Orthostatic vitals the day after ambulatory monitoring were consistent with orthostatic hypotension (OH) on the basis of the following systolic blood pressures/diastolic blood pressures/heart rates: 175 mm Hg/82 mm Hg/67 bpm (supine), 152 mm Hg/79 mm Hg/70 bpm (seated), 109 mm Hg/67 mm Hg/72 bpm (≈1 minute standing), and 115 mm Hg/67 mm Hg/73 bpm (≈3 minutes of standing). BP indicates blood pressure; and MAP, mean arterial pressure.
Last, it is important to consider OH in the context of BP measurement. BP varies substantially within each person, with the SD of SBPs ranging from 10 to >20 mm Hg, depending on the device and approach used. The higher the BP is, the greater its variability. Any time 2 measurements are obtained, it is possible to observe a difference that meets the criteria for OH as a result of random error (Figure 2).59 Thus, in studies that rely on single assessments of asymptomatic OH, a proportion of the OH prevalence will be secondary to measurement error and thus linked spuriously to hypertension.
Figure 2. The higher the resting SBP, the greater the variability and the potential for differences that meet the definition of OH, but are actually secondary to measurement error (±10 mm Hg).
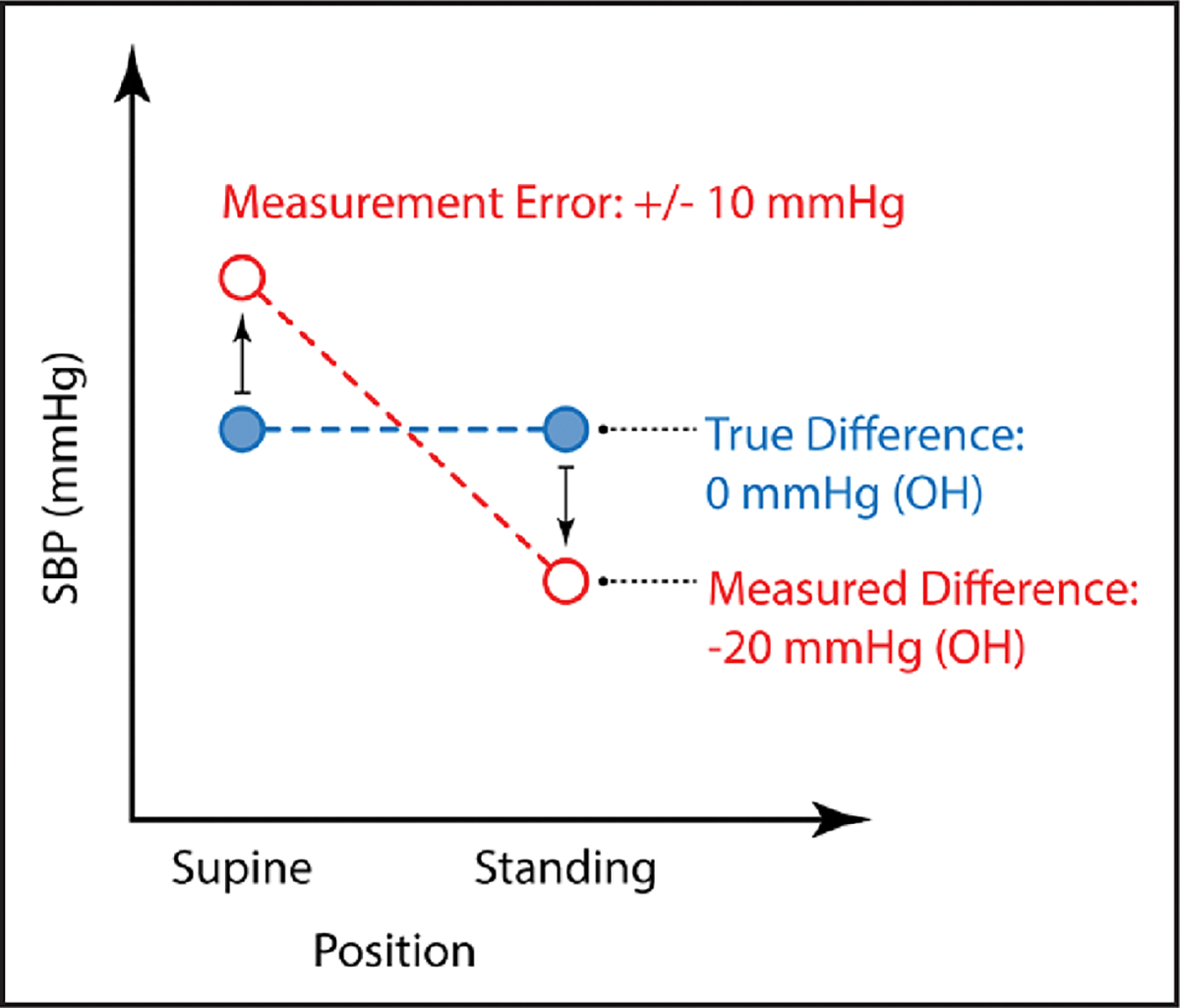
OH indicates orthostatic hypotension; and SBP, systolic blood pressure.
HYPERTENSION TREATMENT AND OH
Treatment Goal and OH
The relationship among hypertension, drug therapy, and OH is both nuanced and complex. OH is more prevalent when BP is uncontrolled than when it is controlled.60,61 Moreover, targeting a lower BP goal does not appear to cause OH in the majority of patients with essential hypertension and, in fact, may reduce its occurrence.9,19,62,63 It should be noted, however, that patients with symptomatic OH are typically excluded from hypertension trials.40 Although intensive pharmacological treatment to a goal SBP <120 mm Hg in SPRINT did not alter the association of OH (mostly asymptomatic) with hypotension-related hospitalizations/emergency department visits or bradycardia,9 intensive treatment did increase the risk for hypotensive episodes and syncope.64 Therefore, it is important to consider the severity of the patient’s autonomic impairment. Whereas the majority of patients with hypertension will tolerate and benefit from intensive hypertension treatment, the management of patients with severe forms of neurogenic OH may need to be individualized.
Questions remain as to whether clinicians should evaluate standing SBP level independently of orthostatic fall in BP during the care of patients actively treated for hypertension. One study in older community-dwelling adults found that a lower standing SBP predicted falls, whereas conventional definitions of OH did not,65 suggesting that low upright BP may be of greater concern among treated patients with hypertension than the worsening of OH, as long as upright BP is maintained above the threshold of cerebral autoregulation that triggers hypoperfusion (Supplemental Figure). Control of BP throughout the 24-hour cycle also may prove elusive given the predilection for reverse or nondipping nocturnal hypertension in patients with OH.50,52
Antihypertensive Classes and OH
Although there is substantial evidence that intensive BP lowering in randomized controlled trials reduces the risk of OH,63 few studies have addressed the effect of individual drug classes. It should also be noted that many large hypertension trials with OH measurements did not reflect the range of antihypertensive agents used in clinical practice.63 Furthermore, patients with severe forms of OH and subgroups with dementia or diabetes were generally not included in trials.40 Observational studies have shown associations between OH and number20 or class of antihypertensive agents.66 Nevertheless, this should not be conflated to imply causality because of the confounding influence of hypertension, especially uncontrolled hypertension, that accompanies hypertension treatments.
Given that the sympathetic nervous system plays a critical role in controlling compensatory responses to changes in posture, it is not surprising that drugs that impair these compensatory mechanisms are associated with OH (Figure 3). Indeed, in observational studies, peripheral α-blockers,12,66,67 β-blockers,12,66,68,69 and central sympatholytics12 are associated with OH. Among individual classes of antihypertensives, β-blocker monotherapy increased the odds of initial OH and sustained OH 2- and 3-fold, respectively.68,69 In Black adults with chronic kidney disease, metoprolol was associated with OH compared with ramipril and amlodipine.62
Figure 3. Relative ranking of hypertension classes according to risk of OH.
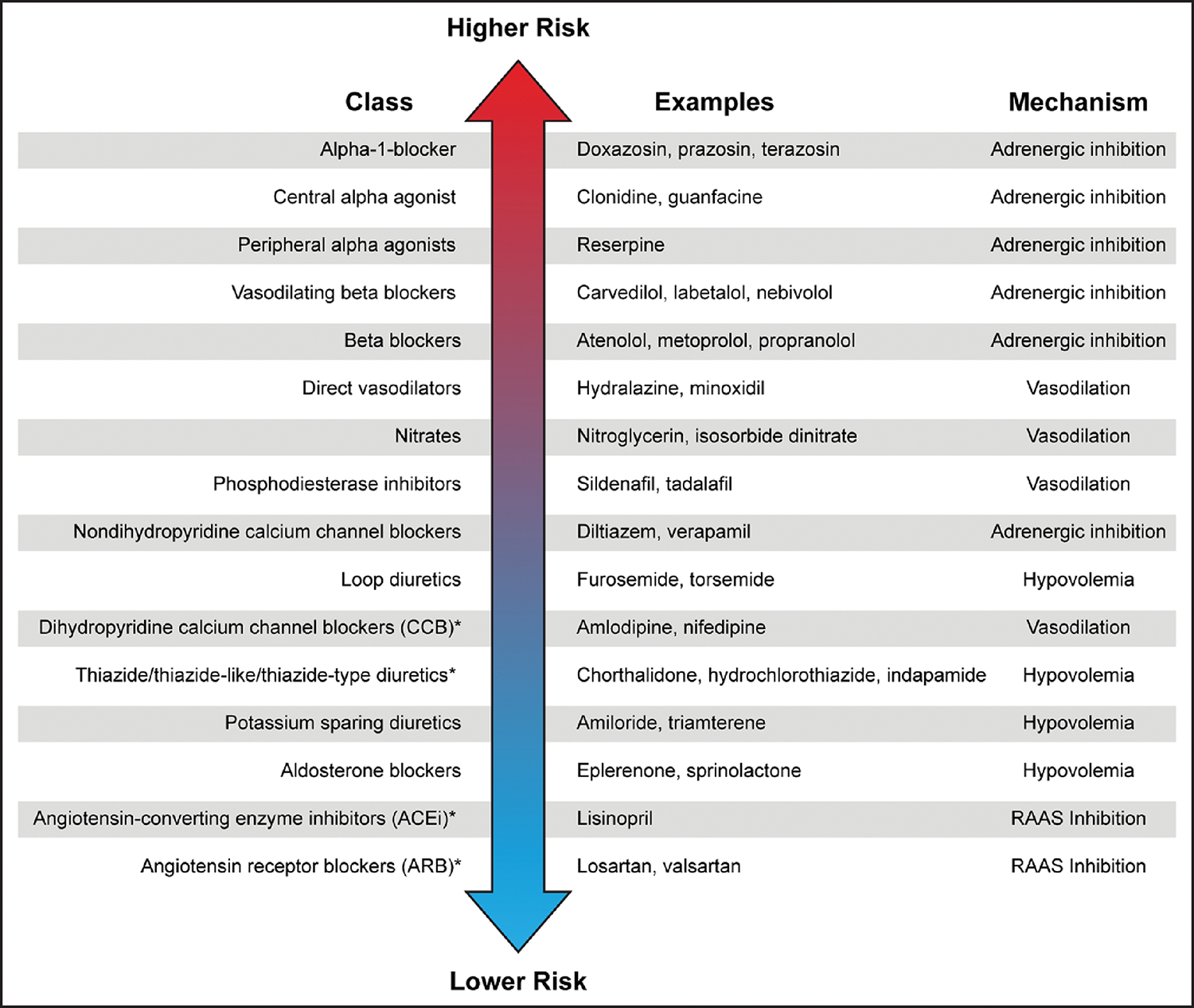
There is a scarcity of evidence on this topic, representing an important research priority. OH indicates orthostatic hypotension; and RAAS, renin-angiotensin-aldosterone system.
*A class considered first-line treatment for hypertension treatment.
Thiazide diuretics have been associated with OH in some12,66,67 but not all70 observational studies. Loop diuretics, possibly by inducing intravascular volume depletion, have also been linked to OH.66
Calcium channel blockers have been associated with greater fluctuations in OH, but these studies do not consistently differentiate between dihydropyridine and non-dihydropyridine calcium channel blockers,20 and some studies show no association.66,68 In a secondary analysis of the ALLHAT study (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack), amlodipine was not associated with a higher risk of diagnostic codes for OH compared with lisinopril or chlorthalidone, but it was associated with higher risk of falls in the short term.70
Results for renin-angiotensin system blockers (eg, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers) are less consistent, showing a reduction in OH in patients attending a hypertension clinic,12 no difference in OH in an Irish population study of adults ≥50 years of age,68 and a higher risk of OH in community-dwelling women in the United Kingdom.66 In older adults with dementia, OH was related to nitrates, combinations of angiotensin-converting enzyme inhibitors and diuretics, and combinations of angiotensin-converting enzyme inhibitors and nitrates.71
Of note, first-line drug classes in the 2017 American College of Cardiology/American Heart Association guidelines7 are all lower-risk drugs for causing OH (Figure 3). Moreover, the most effective drug combinations (renin-angiotensin system blockers and thiazides or calcium channel blockers) are lower risk for OH.
DIAGNOSTIC PROTOCOLS FOR OH AND RECOMMENDATIONS FOR SCREENING
The most sensitive and consistent orthostatic BP measurements are obtained early in the morning, when symptoms tend to be most severe. However, there is substantial variability with how OH is assessed.16 In general, it is recommended to measure BP after 5 to 10 minutes in the supine position and within 3 minutes after the standing position is assumed.4 Although this protocol may not account for all known patterns of OH, it will capture many cases of clinically significant OH. More research is needed to define the ideal timing of upright BP measurements and their association with clinical outcomes. Initial OH is a transient decrease in BP within the first 15 seconds of standing72 that can be associated with vasovagal syncope but is usually not evidence of neurogenic OH. Initial OH can be detected only by continuous beat-to-beat BP monitoring24,37,73 or by the transient nature of OH symptoms in the clinical history. Delayed-recovery OH72 is also a transient event that resolves within 2 to 3 minutes of standing and can be associated with falls. Timing of assessment directly affects the prevalence of OH detected, with earlier OH assessments identifying more OH.74 Furthermore, there are data suggesting that BP change within 1 minute of upright posture is more closely related to dizziness and adverse health outcomes.28 Last, delayed OH75 occurs after 3 minutes of standing and is associated with progression to autonomic failure.76 It should be suspected in patients with typical postural-related symptoms and a negative 3-minute postural test. This highlights the importance of both a thorough clinical history and repeated orthostatic BP measurements. Orthostatic symptom assessments are also important. In some studies, orthostatic dizziness has been a more sensitive predictor of neurological outcomes than OH measured at 3 minutes of standing,34 whereas others highlight the fact that orthostatic symptoms can be absent in patients with documented OH39 and in patients with dementia.77
Large hypertension treatment trials have used seated-to-standing rather than supine-to-standing BP protocols to detect OH.19,20,63,78 Seated-to-standing protocols are included as an alternative method in many society guidelines,16 and are more practical and easier to implement, particularly in ambulatory clinics and large clinical trials, but at the cost of decreased sensitivity79 because OH is diagnosed more frequently from supine than from seated positions.24,37,73,80–83 Moreover, 1 study found that only supine-to-standing OH was associated with falls and orthostatic symptoms in adults ≥70 years of age (62% hypertensive).83
A lower seated BP change threshold (15/7 mm Hg) has been suggested as an alternative definition for seated-to-standing protocols for detecting conventionally defined supine OH,81 although this finding, derived in a younger population of neurology patients, has not been replicated in frailer or older populations, in whom hypertension is more common.83,84 Others have recommended a more specific definition for OH in the setting of hypertension, that is, a drop in SBP of 30 mm Hg (versus 20 mm Hg). However, such a change would mitigate threshold effects at the expense of reduced sensitivity (Table and Supplemental Figure).78
The diagnosis of OH can be made with an active standing test or with a tilt table test; active stand presents a greater challenge to cardiovascular reflexes than head-up tilt85,86 and is easier to implement. Manual or preprogrammed oscillometric sphygmomanometers are the most commonly used technologies in recent antihypertensive drug trials.64,87 OH detection is also influenced by time of day (highest in the morning),88 meals,89,90 alcohol (vasodilator properties and through impaired vasoconstriction/blunted sympathetic response to standing),91–93 vigorous exercise,94 timing of hypertension medications, and duration of rest.7,95 Frail older patients may take longer to stand and thus have less marked immediate OH.96 New orthostatic symptoms or OH-related adverse clinical outcomes may herald new-onset OH in previously unaffected patients.
A practical clinical issue is who should be routinely screened for OH. The American College of Cardiology/American Heart Association guidelines7 and the European Society of Cardiology/European Society of Hypertension guidelines97 recommend obtaining orthostatic BP measurements at the initial visit in all patients with hypertension. Moreover, the American College of Cardiology/American Heart Association guidelines recommend evaluation for OH in follow-up after initiation of antihypertensive therapy and in higher-risk groups such as older adults.7 The American Autonomic Society recommends screening patients with neurodegenerative diseases such as Parkinson disease,89 and the American Diabetes Association recommends regular OH screening as part of hypertension care among adults with diabetes.98
PRACTICAL STEPS TO MANAGE OH IN ADULTS WITH HYPERTENSION
The coexistence of OH and hypertension represents a management dilemma, and the challenge is to treat one without having a significant impact on the other.
Characterize patterns, triggers, and cause. Characterization of BP pattern throughout the day and identification of OH triggers represent critical first steps for both prevention and individualized care. For example, identifying time periods or activities that trigger OH can inform plans to optimize the management of both conditions. Moreover, characterization of pattern can aid in the diagnosis of the underlying cause of OH, which can inform treatment strategy. Removing exacerbating medications, addressing aggravating factors (eg, anemia or volume depletion), and implementing nonpharmacological strategies represent first-line approaches in all cases. For symptomatic neurogenic OH, pharmacological treatment that increases upright BP may be necessary. It is important to differentiate causes of OH to avoid exacerbating comorbid hypertension.
- Optimize contributory medications
-
Nonantihypertensive medications. One of the first steps in treating OH is to remove medications with potential adverse effects. This starts with a comprehensive review of prescription and over-the-counter medications. Among nonantihypertensive medications, tricyclic antidepressants99 (notably amitriptyline), trazodone (because of its α-blocking properties),100 and dopaminergic agents are common culprits,41 in part because they are used in populations already at risk for OH (patients with peripheral neuropathies, older adults, and patients with Parkinson disease, respectively).101 Tizanidine, commonly prescribed in the United States as a central muscle relaxant, is a central sympatholytic related to clonidine that can induce a clinical picture of neurogenic OH with abnormal cardiovascular reflexes, especially among those taking strong CYP1A2 inhibitors.102,103 “Uroselective” α-1a receptor antagonists (eg, tamsulosin) that preferentially relax the smooth muscle in the bladder and urethra are commonly prescribed for benign prostatic hyperplasia and are known to increase the incidence of severe OH, requiring hospital admissions.104It is also important to consider the severity of the patient’s autonomic impairment. For example, some medications known to alter BP such as sildenafil may be generally well-tolerated in patients with hypertension but can decrease BP by ≈30 mm Hg in patients with severe autonomic failure.105
- Antihypertensive medications. Most patients with essential hypertension can be treated with recommended first-line antihypertensive agents without exacerbation of OH. Moreover, there is little evidence that a less intensive treatment goal will reduce OH.63 In addition, given the close relationship between OH and hypertension, removing antihypertensives could worsen symptoms and risk for OH. Thus, removing first-line antihypertensive agents should not be an immediate response for patients with hypertension found to have OH. Instead, regimens should be optimized, and antihypertensive medications known to be associated with OH such as α-blockers, β-blockers, and centrally acting sympatholytic agents16 should be discontinued, dose reduced, or substituted for more favorable classes (see the Antihypertensive Classes and OH section). Patients should be monitored carefully after changes in antihypertensive drug therapy are made because there may be increased risk of orthostatic symptoms and falls in the period soon after change in antihypertensive medications, particularly among older adults.106,107
-
- Nonpharmacological approaches
- Countermaneuvers. Compression garments applied to the lower body have been used to treat OH by improving venous return but are difficult for patients to apply at a constant effective pressure. An alternative option is an abdominal binder, which can be as effective in improving upright BP as the α-agonist midodrine.108 This has the advantage of selectively improving upright BP without worsening seated or supine hypertension. No clinical studies have demonstrated the tolerability and long-term efficacy of this approach, and patient compliance is often a limitation.
-
Fluid and sodium—caution. Expanding intravascular volume is thought to minimize the decline in BP while standing among patients with OH.109 Moreover, some experts recommend that patients with OH would benefit from increasing their sodium intake to 10 g sodium chloride per day.110 However, the beneficial effect of enhancing salt intake on OH has to be balanced with the risk of worsening supine hypertension and pressure natriuresis.72In patients with severe OH, drinking ≈500 mL of water has been shown to improve standing BP in small studies.111–113 Most patients in these studies had severe underlying autonomic dysfunction. For example, in a study involving 11 patients, the mean standing SBP improved from 83 mm Hg at baseline to 114 mm Hg approximately half an hour after the patient drank water. It is possible that this may relate to a pressor response resulting from water intake, rather than from a volume effect.112,113 In another small study of older adults, investigators observed an SBP increase of 12 mm Hg and a 56% response rate (SBP increase ≥10 mm Hg) in response to water intake, which was similar to abdominal compression but greater than with either physical countermaneuvers or compression stockings.114 In contrast, a distinct study of older adults with OH found no benefit in the SBP response rate when combining physical countermaneuvers with and without abdominal compression and water bolusing (480 mL room temperature tap water). However, there was a suggestion that the combination of all 3 might enhance the SBP response among those who did respond. However, these combinations did not improve symptoms.115 Additional research is needed in larger patient populations before this approach can be adapted more widely in clinical practice. Nevertheless, water bolusing may possibly be the most readily available intervention in those with OH without swallowing difficulties or gastric emptying problems.
- Pharmacological treatment of OH for specific conditions
-
Neurogenic OH. Although nonpharmacological approaches are first steps for OH in general, adults with symptomatic neurogenic OH may benefit from treatment. Thus, a stepwise approach is recommended. Medications should be used only if conservative measures fail, but medications are often needed in patients with moderate to severe neurogenic OH. Only 2 medications are approved for the treatment of OH: midodrine and droxidopa. All others are used off-label. Ideally, therapies for OH in the patient with hypertension would selectively improve upright BP without increasing supine BP. Most pressor agents used in OH, however, increase both supine and upright BPs, and some increase supine BP more than standing BP.Fludrocortisone is an example of the latter. It is a synthetic mineralocorticoid frequently added to increase the efficacy of salt supplementation to promote sodium retention, transiently expand plasma volume, and enhance the pressor effect of norepinephrine to increase peripheral vascular resistance.116 A pharmaco-epidemiological study found higher rates of all-cause hospitalizations in fludrocortisone users compared with midodrine users, especially among patients with congestive heart failure.117 Therefore, fludrocortisone is contraindicated in the presence of heart failure, a common comorbidity in patients with OH, and should be used with caution, if at all, in patients with supine hypertension. Overall evidence in support of fludrocortisone is weak.116Midodrine is an oral α-1 adrenergic receptor agonist shown in 2 randomized clinical trials to be effective in increasing upright BP.118,119 However, it also produces a greater increase in supine than standing BP, and its label includes a black-box warning about worsening supine BP.Droxidopa is a prodrug that is converted into norepinephrine by dopa decarboxylase, the enzyme that also converts levodopa into dopamine.120 It was approved for the treatment of neurogenic OH on the basis of 3 randomized clinical trials demonstrating improvement in OH symptoms.121–123 Supine hypertension is the most common side effect of droxidopa but with a low rate (≤7.9% versus ≤4.6% for placebo),124 and droxidopa is considered safer than midodrine in this regard. A recent meta-analysis found that midodrine, but not droxidopa, significantly increases risk for supine hypertension.125Arguably the safest drug to use in patients with OH and hypertension is pyridostigmine, an acetylcholinesterase inhibitor that enhances cholinergic neurotransmission at the level of the autonomic ganglia, thereby increasing both sympathetic and parasympathetic activity. The appeal of this pharmacological approach is that it is engaged only during the sympathetic activation that occurs while upright to selectively increase upright BP without worsening supine hypertension.126,127 These studies, however, involved small numbers of patients and found only small improvements in upright SBP (4 mm Hg compared with placebo). Pyridostigmine may not be effective in severely affected patients.128
-
Isolated supine hypertension in patients with severe neurogenic OH. The clinical picture of patients with neurodegenerative disorders of the autonomic nervous system is dominated by disabling OH and isolated supine hypertension. This can be due to preexisting hypertension or can occur de novo as part of their impaired autonomic regulation of BP. Most patients with normal seated BP and daytime supine hypertension can be managed by simply avoiding the supine position during the day. Nocturnal (supine) hypertension remains the main problem. Two-thirds of patients with neurogenic OH have a nondipping or reverse dipping pattern, and nocturia induces pressure natriuresis (average nighttime loss of 1.3 L of water and 70 mmol of sodium), leading to volume depletion and worsening of daytime OH.129 The ideal treatment would control nocturnal hypertension, reduce natriuresis, and improve daytime (or at least early-morning) OH. Nighttime hypertension can be effectively controlled with several short-acting antihypertensives given at bedtime, including nitroglycerin patch (removed in the morning), sildenafil, clonidine, nebivolol (but not metoprolol),130 immediate-release nifedipine, losartan, and eplerenone. Of these, only losartan reduces nighttime natriuresis, and none improve daytime OH (Figure 4).133 Notably, losartan but not captopril lowers supine BPs in patients with severe autonomic failure, suggesting extrarenal generation of angiotensin II in these patients.134,135In contrast, small proof-of-concept clinical studies have shown that sleeping in a head-up tilt position, application of local passive heat (water-recirculating heated mattress), and continuous positive airway pressure (at levels used in sleep apnea) control nocturnal hypertension (as effectively as antihypertensives), reduce nocturia, and improve early-morning OH (Figure 4).131,132 Unfortunately, to be effective, sleeping in a head-up tilt position requires levels of tilt of at least 12° (≈16-in elevation of the head of the bed), which is not practical, tolerable, or safe. Both local heat and continuous positive airway pressure reduce cardiac output by increasing venous capacitance through skin vasodilation or splanchnic venous pooling, respectively. The magnitude of the BP-lowering effect (≈30 mm Hg) is similar to that seen with antihypertensive agents133 but has been documented only in patients with severe impairment of compensatory autonomic reflexes.
- Hypertension with intermittent hypotensive events. These patients are characterized as having hypertension but have periods of often symptomatic drops in BP. Common but often underrecognized examples are postprandial hypotension and postexercise hypotension (see Supplemental Material SM1 for details). In these patients, ambulatory BP monitoring is particularly useful to characterize the mean awake-time BP, the severity of hypotensive events (including any symptoms, sequelae, or signs of end-organ damage), and the frequency of hypotensive events. These data can inform treatment strategies focused on augmenting or reducing BP to minimize the effects of BP lability. In cases of refractory hypertension, particularly when baroreflex impairment is thought to be the primary cause, guanfacine or clonidine patches have been useful.136,137
-
Figure 4. Effect of different antihypertensive medications and nonpharmacological interventions on nocturnal hypertension, diuresis, and morning orthostatic tolerance in patients with supine hypertension and OH.
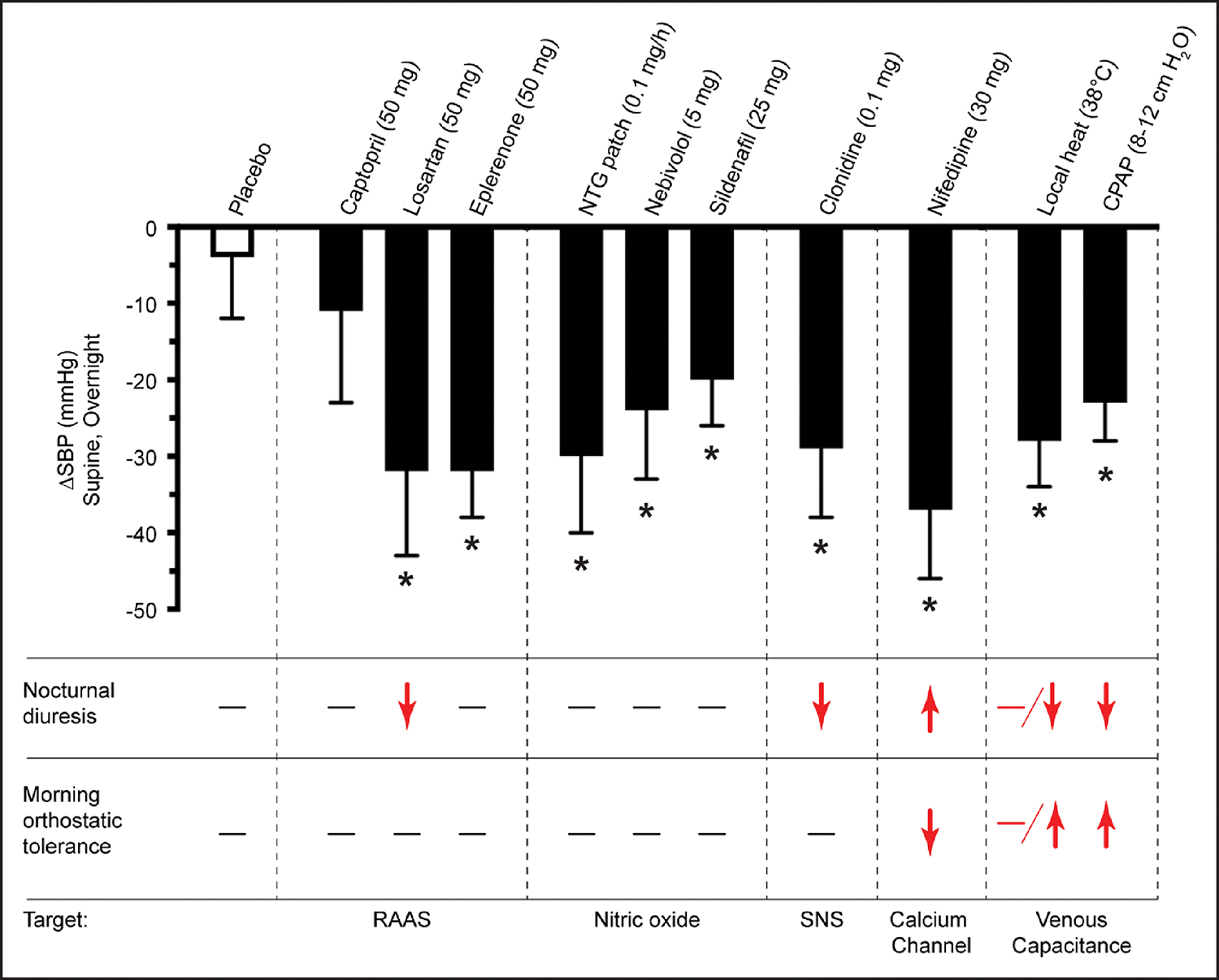
Interventions were tested on a series of single-night, placebo-controlled crossover, proof-of-concept studies.131,132 Outcomes were monitored from 8 pm to 8 am, followed by a 10-minute standing test in the morning. A single oral dose of each medication was given at 8 pm; nitroglycerin (NTG) patch was applied from 8 pm to 6 am; and local heat and continuous positive airway pressure (CPAP) were applied from 10 pm to 6 am. Vertical bar represents the maximum observed systolic blood pressure (SBP) reduction over night. Asterisk represents interventions that significantly reduced nighttime SBP. Only local heat and CPAP treatment reduce nocturnal diuresis and improve morning orthostatic tolerance. Red indicates the observed effect. Nifedipine here is the immediate-release formulation. OH indicates orthostatic hypotension; RAAS, renin-angiotensin-aldosterone system; and SNS, sympathetic nervous system. Figure courtesy of Luis E. Okamoto, Vanderbilt University Medical Center. Adapted with permission from Park et al.133
CONCLUSIONS
OH is common among adults with hypertension and increases with age as a result of impairments in autonomic reflex. Although a more aggressive hypertension treatment goal may lower the risk of OH, particularly with first-line antihypertensive agents, some second-line classes of antihypertensive agents may cause OH, and monitoring for OH may be beneficial in older patients with the initiation of new treatments. When OH is identified or in the setting of orthostatic symptoms, characterizing OH and establishing its cause are important first steps. Nonpharmacological interventions are first-line treatments regardless of cause, but modification of antihypertensive regimen and addition of medications to treat OH should take into account the cause of OH, comorbid hypertension, and cardiovascular disease risk. Ultimately, substantially more research is needed to understand and treat this form of BP dysregulation. A number of areas representing research priorities for OH are summarized in Figure 5 (Supplemental Material SM2 provides details).
Figure 5. Summary of research priorities for OH among adults with hypertension.
BP indicates blood pressure; HTN, hypertension; and OH, orthostatic hypotension.
Supplementary Material
Acknowledgments
The authors thank Luis Okamoto for contributing Figure 4 and its description. The authors also thank Jeremy Theriot, PhD, for his assistance with the graphical presentation of each figure.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on October 11, 2023, and the American Heart Association Executive Committee on December 4, 2023. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith. Edelman@wolterskluwer.com
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Stephen P Juraschek | Harvard Medical School, Beth Israel Deaconess Medical Center | NIH/NHLBI (K23HL135273 and R01 HL153191)† | None | None | None | None | None | None |
| Italo Biaggioni | Vanderbilt University Medical Center | NIH (R01 in autonomic disorders as PI and co-I, R01 HL149386 and R01 HL161095)† | None | None | None | Patent holder for the use of an automated abdominal binder in the treatment of orthostatic hypotension† | Theravance Biopharma†; Takeda*; Regeneron*; Ammeal*; Neurawell* | None |
| Melissa M. Cortez | University of Utah | NIH/NINDS (K23NS105920 and R61NS125153)† | None | None | None | None | None | None |
| John M. Flack | Southern Illinois University School of Medicine | GlaxoSmith Kline†; Indorsia†; ReCor Medical†; Quantam Genomics†; Vascular Dynamics†; Mineralys†; Cincor†; AstraZeneca† (all contracted research) | None | None | Teva† | Amphastar†; Viking Therapeutics†; Orchard Biotechnology† | Ardylex†; ReCor†; GlaxoSmithKline*; AstraZeneca†; Fibrogen†; Amgen*; Janssen* | None |
| Lama Ghazi | University of Alabama at Birmingham | None | None | None | None | None | None | None |
| Rose Anne Kenny | Trinity College Dublin (Ireland) | None | None | None | None | None | None | None |
| Mahboob Rahman | Case Western Reserve University | Bayer (PI for clinical trial, paid to institution)† | None | None | None | None | AstraZeneca* | None |
| Cyndya A. Shibao | Vanderbilt University Medical Center | Theravance (PI)†; NIH (R01 HL150350, R01 HL159203)*; AHA (967054)* | None | Symposia* | None | None | Theravance Biopharma* | None |
| Telisa Spikes | Emory University | None | None | None | None | None | None | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Kevin Heffernan | Syracuse University | None | None | None | None | None | None | None |
| Juan Kupferman | Maimonides Medical Center | None | None | None | None | None | None | None |
| Steven Shea | Columbia University | NHLBI (PI on contract NHL-BI75N9 2020D00002)* | None | None | None | None | None | None |
| Farzaneh Sorond | Northwestern University | None | None | None | None | None | None | None |
REFERENCES
- 1.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension prevalence among adults aged 18 and over: United States, 2017–2018. NCHS Data Brief. 2020:1–8. [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blood Pressure Lowering Treatment Trialists’ Collaboration. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet. 2021;398:1053–1064. doi: 10.1016/S0140-6736(21)01921-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chelimsky T, Cortelli P, Gibbons CH, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 5.Gangavati A, Hajjar I, Quach L, Jones RN, Kiely DK, Gagnon P, Lipsitz LA. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueroa JJ, Basford JR, Low PA. Preventing and treating orthostatic hypotension: as easy as A, B, C. Cleve Clin J Med. 2010;77:298–306. doi: 10.3949/ccjm.77a.09118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines [published corrections appear in Hypertension. 2018;71:e136–e139. Hypertension. 2018;72:e33]. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 8.Farrell MC, Shibao CA. Morbidity and mortality in orthostatic hypotension. Auton Neurosci. 2020;229:102717. doi: 10.1016/j.autneu.2020.102717 [DOI] [PubMed] [Google Scholar]
- 9.Juraschek SP, Taylor AA, Wright JT, Evans GW, Miller ER, Plante TB, Cushman WC, Gure TR, Haley WE, Moinuddin I, et al. Orthostatic hypotension, cardiovascular outcomes, and adverse events: results from SPRINT. Hypertension. 2020;75:660–667. doi: 10.1161/HYPERTENSIONAHA.119.14309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duggan E, Knight SP, Romero-Ortuno R. Relationship between sarcopenia and orthostatic blood pressure recovery in older falls clinic attendees. Eur Geriatr Med. 2023;14:439–446. doi: 10.1007/s41999-023-00775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fedorowski A, Burri P, Melander O. Orthostatic hypotension in genetically related hypertensive and normotensive individuals. J Hypertens. 2009;27:976–982. doi: 10.1097/hjh.0b013e3283279860 [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano C, Milazzo V, Totaro S, Sobrero G, Ravera A, Milan A, Maule S, Veglio F. Orthostatic hypotension in a cohort of hypertensive patients referring to a hypertension clinic. J Hum Hypertens. 2015;29:599–603. doi: 10.1038/jhh.2014.130 [DOI] [PubMed] [Google Scholar]
- 13.Applegate WB, Davis BR, Black HR, Smith WM, Miller ST, Burlando AJ. Prevalence of postural hypotension at baseline in the Systolic Hypertension in the Elderly Program (SHEP) cohort. J Am Geriatr Soc. 1991;39:1057–1064. doi: 10.1111/j.1532-5415.1991.tb02869.x [DOI] [PubMed] [Google Scholar]
- 14.Duggan E, Murphy CH, Knight SP, Davis JRC, O’Halloran AM, Kenny RA, Romero-Ortuno R. Differential associations between two markers of probable sarcopenia and continuous orthostatic hemodynamics in The Irish Longitudinal Study on Ageing. J Gerontol A Biol Sci Med Sci. 2022;78:glac243. doi: 10.1093/gerona/glac243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biaggioni I Orthostatic hypotension in the hypertensive patient. Am J Hypertens. 2018;31:1255–1259. doi: 10.1093/ajh/hpy089 [DOI] [PubMed] [Google Scholar]
- 16.Raber I, Belanger MJ, Farahmand R, Aggarwal R, Chiu N, Al Rifai M, Jacobsen AP, Lipsitz LA, Juraschek SP. Orthostatic hypotension in hypertensive adults: Harry Goldblatt Award for Early Career Investigators 2021. Hypertension. 2022;79:2388–2396. doi: 10.1161/hypertensionaha.122.18557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci F, De Caterina R, Fedorowski A. Orthostatic hypotension: epidemiology, prognosis, and treatment. J Am Coll Cardiol. 2015;66:848–860. doi: 10.1016/j.jacc.2015.06.1084 [DOI] [PubMed] [Google Scholar]
- 18.Rose KM, Tyroler HA, Nardo CJ, Arnett DK, Light KC, Rosamond W, Sharrett AR, Szklo M. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities study. Am J Hypertens. 2000;13(pt 1):571–578. doi: 10.1016/s0895-7061(99)00257-5 [DOI] [PubMed] [Google Scholar]
- 19.Fleg JL, Evans GW, Margolis KL, Barzilay J, Basile JN, Bigger JT, Cutler JA, Grimm R, Pedley C, Peterson K, et al. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension. 2016;68:888–895. doi: 10.1161/HYPERTENSIONAHA.116.07474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend RR, Chang TI, Cohen DL, Cushman WC, Evans GW, Glasser SP, Haley WE, Olney C, Oparil S, Del Pinto R, et al. ; SPRINT Study Research Group. Orthostatic changes in systolic blood pressure among SPRINT participants at baseline. J Am Soc Hypertens. 2016;10:847–856. doi: 10.1016/j.jash.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan J, Ricci F, Hoffmann F, Hamrefors V, Fedorowski A. Orthostatic hypertension: critical appraisal of an overlooked condition. Hypertension. 2020;75:1151–1158. doi: 10.1161/HYPERTENSIONAHA.120.14340 [DOI] [PubMed] [Google Scholar]
- 22.Min M, Shi T, Sun C, Liang M, Zhang Y, Bo G, Sun Y. Orthostatic hypotension and the risk of atrial fibrillation and other cardiovascular diseases: an updated meta-analysis of prospective cohort studies. J Clin Hypertens (Greenwich). 2019;21:1221–1227. doi: 10.1111/jch.13613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mol A, Reijnierse EM, Bui Hoang PTS, van Wezel RJA, Meskers CGM, Maier AB. Orthostatic hypotension and physical functioning in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2018;48:122–144. doi: 10.1016/j.arr.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 24.Finucane C, O’Connell MDL, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc. 2017;65:474–482. doi: 10.1111/jgs.14563 [DOI] [PubMed] [Google Scholar]
- 25.de Jong MR, Van der Elst M, Hartholt KA. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther Adv Drug Saf. 2013;4:147–154. doi: 10.1177/2042098613486829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juraschek SP, Daya N, Appel LJ, Miller ER 3rd, Windham BG, Pompeii L, Griswold ME, Kucharska-Newton A, Selvin E. Orthostatic hypotension in middle age and risk of falls. Am J Hypertens. 2017;30:188–195. doi: 10.1093/ajh/hpw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G, Boivin J-M. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32:1562–1571. doi: 10.1097/HJH.0000000000000235. discussion 1571 [DOI] [PubMed] [Google Scholar]
- 28.Juraschek SP, Daya N, Rawlings AM, Appel LJ, Miller ER, Windham BG, Griswold ME, Heiss G, Selvin E. Association of history of dizziness and long-term adverse outcomes with early vs later orthostatic hypotension assessment times in middle-aged adults. JAMA Intern Med. 2017;177:1316–1323. doi: 10.1001/jamainternmed.2017.2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle K, Lavan A, Kenny RA, Briggs R. Delayed blood pressure recovery after standing independently predicts fracture in community-dwelling older people. J Am Med Dir Assoc. 2021;22:1235–1241.e1. doi: 10.1016/j.jamda.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 30.Deleted in proof. [Google Scholar]
- 31.Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, Grubb BP, Hamdan MH, Krahn AD, Link MS. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society [published correction appears in. Circulation. 2017;136:e271–e272]. Circulation. 2017;136:e60–e122. doi: 10.1161/CIR.0000000000000499 [DOI] [PubMed] [Google Scholar]
- 32.Brignole M, Moya A, de Lange FJ, Deharo J-C, Elliott PM, Fanciulli A, Fedorowski A, Furlan R, Kenny RA, Martín A, et al. ; ESC Scientific Document Group. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39:1883–1948. doi: 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 33.Rawlings AM, Juraschek SP, Heiss G, Hughes T, Meyer ML, Selvin E, Sharrett AR, Windham BG, Gottesman RF. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. 2018;91:e759–e768. doi: 10.1212/WNL.0000000000006027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juraschek SP, Longstreth WT, Lopez OL, Gottdiener JS, Lipsitz LA, Kuller LH, Mukamal KJ. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology. 2020;95:e1941–e1950. doi: 10.1212/WNL.0000000000010456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robertson AD, Udow SJ, Espay AJ, Merola A, Camicioli R, Lang AE, Masellis M. Orthostatic hypotension and dementia incidence: links and implications. Neuropsychiatr Dis Treat. 2019;15:2181–2194. doi: 10.2147/NDT.S182123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs R, Carey D, Kennelly SP, Kenny RA. Longitudinal association between orthostatic hypotension at 30 seconds post-standing and late-life depression. Hypertension. 2018;71:946–954. doi: 10.1161/HYPERTENSIONAHA.117.10542 [DOI] [PubMed] [Google Scholar]
- 37.Donoghue OA, O’Connell MDL, Bourke R, Kenny RA. Is orthostatic hypotension and co-existing supine and seated hypertension associated with future falls in community-dwelling older adults? Results from The Irish Longitudinal Study on Ageing (TILDA). PLoS One. 2021;16:e0252212. doi: 10.1371/journal.pone.0252212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Claffey P, Pérez-Denia L, Lavan A, Kenny RA, Finucane C, Briggs R. Asymptomatic orthostatic hypotension and risk of falls in community-dwelling older people. Age Ageing. 2022;51:afac295. doi: 10.1093/ageing/afac295 [DOI] [PubMed] [Google Scholar]
- 39.Freeman R, Illigens BMW, Lapusca R, Campagnolo M, Abuzinadah AR, Bonyhay I, Sinn D-I, Miglis M, White J, Gibbons CH. Symptom recognition is impaired in patients with orthostatic hypotension. Hypertension. 2020;75:1325–1332. doi: 10.1161/HYPERTENSIONAHA.119.13619 [DOI] [PubMed] [Google Scholar]
- 40.Juraschek SP, Biaggioni I. Management of patients with hypertension and orthostatic hypotension parallel progress. Hypertension. 2022;79:2385–2387. doi: 10.1161/hypertensionaha.122.19113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivasi G, Rafanelli M, Mossello E, Brignole M, Ungar A. Drug-related orthostatic hypotension: beyond anti-hypertensive medications. Drugs Aging. 2020;37:725–738. doi: 10.1007/s40266-020-00796-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JJ, Porth CM, Erickson M. Hemodynamic response to the upright posture. J Clin Pharmacol. 1994;34:375–386. doi: 10.1002/j.1552-4604.1994.tb04977.x [DOI] [PubMed] [Google Scholar]
- 43.Rowell LB. Human Cardiovascular Control. Oxford University Press; 1993. [Google Scholar]
- 44.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Baroreflex dysfunction. N Engl J Med. 2020;382:163–178. doi: 10.1056/NEJMra1509723 [DOI] [PubMed] [Google Scholar]
- 45.Grubb BP. Postural tachycardia syndrome. Circulation. 2008;117:2814–2817. doi: 10.1161/CIRCULATIONAHA.107.761643 [DOI] [PubMed] [Google Scholar]
- 46.Gibbons CH, Freeman R. Clinical implications of delayed orthostatic hypotension: a 10-year follow-up study. Neurology. 2015;85:1362–1367. doi: 10.1212/WNL.0000000000002030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peixoto AJ. Evaluation and management of orthostatic hypotension: limited data, limitless opportunity. Cleve Clin J Med. 2022;89:36–45. doi: 10.3949/ccjm.89gr.22001 [DOI] [PubMed] [Google Scholar]
- 48.Kleipool EEF, Rozendaal ES, Mahadew SKN, Kramer MHH, van den Born BH, Serné EH, Peters MJL, Muller M. The value of ambulatory blood pressure measurement to detect masked diastolic hypotension in older patients treated for hypertension. Age Ageing. 2021;50:1229–1235. doi: 10.1093/ageing/afaa287 [DOI] [PubMed] [Google Scholar]
- 49.Yuasa S, Yamamoto H, Suzuki Y, Chin K, Ukai H, Kobayashi Y, Yano Y, Mori H. White-coat effect on orthostatic hypotension: a nationwide survey of Japanese general practitioners. Blood Press Monit. 2022;27:314–319. doi: 10.1097/MBP.0000000000000605 [DOI] [PubMed] [Google Scholar]
- 50.Ghazi L, Drawz PE, Pajewski NM, Juraschek SP. The association of orthostatic hypotension with ambulatory blood pressure phenotypes in SPRINT. Am J Hypertens. 2021;34:511–520. doi: 10.1093/ajh/hpaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kario K, Eguchi K, Hoshide S, Hoshide Y, Umeda Y, Mitsuhashi T, Shimada K. U-curve relationship between orthostatic blood pressure change and silent cerebrovascular disease in elderly hypertensives: orthostatic hypertension as a new cardiovascular risk factor. J Am Coll Cardiol. 2002;40:133–141. doi: 10.1016/s0735-1097(02)01923-x [DOI] [PubMed] [Google Scholar]
- 52.Voichanski S, Grossman C, Leibowitz A, Peleg E, Koren-Morag N, Sharabi Y, Shamiss A, Grossman E. Orthostatic hypotension is associated with nocturnal change in systolic blood pressure. Am J Hypertens. 2012;25:159–164. doi: 10.1038/ajh.2011.191 [DOI] [PubMed] [Google Scholar]
- 53.Ejaz AA, Haley WE, Wasiluk A, Meschia JF, Fitzpatrick PM. Characteristics of 100 consecutive patients presenting with orthostatic hypotension. Mayo Clin Proc. 2004;79:890–894. doi: 10.4065/79.7.890 [DOI] [PubMed] [Google Scholar]
- 54.Patetta LMA, Reffo A, Trevisan C, Curreri C, Giantin V, Franchin A, Sergi G. Orthostatic hypotension and night-time dipper patterns in geriatric outpatients. Hypertens Res. 2022;45:1468–1475. doi: 10.1038/s41440-022-00950-z [DOI] [PubMed] [Google Scholar]
- 55.Nagai M, Kato M, Dote K. Orthostatic hypotension with nondipping: phenotype of neurodegenerative disease. Hypertens Res. 2022;45:1514–1516. doi: 10.1038/s41440-022-00980-7 [DOI] [PubMed] [Google Scholar]
- 56.Costa A, Bosone D, Ramusino MC, Ghiotto N, Guaschino E, Zoppi A, D’Angelo A, Fogari R. Twenty-four-hour blood pressure profile, orthostatic hypotension, and cardiac dysautonomia in elderly type 2 diabetic hypertensive patients. Clin Auton Res. 2016;26:433–439. doi: 10.1007/s10286-016-0381-7 [DOI] [PubMed] [Google Scholar]
- 57.Fanciulli A, Jordan J, Biaggioni I, Calandra-Buonaura G, Cheshire WP, Cortelli P, Eschlboeck S, Grassi G, Hilz MJ, Kaufmann H, et al. Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS). Clin Auton Res. 2018;28:355–362. doi: 10.1007/s10286-018-0529-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uzu T, Takeji M, Yamauchi A, Kimura G. Circadian rhythm and postural change in natriuresis in non-dipper type of essential hypertension. J Hum Hypertens. 2001;15:323–327. doi: 10.1038/sj.jhh.1001185 [DOI] [PubMed] [Google Scholar]
- 59.Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. doi: 10.1136/bmj.c2289 [DOI] [PubMed] [Google Scholar]
- 60.Shen S, He T, Chu J, He J, Chen X. Uncontrolled hypertension and orthostatic hypotension in relation to standing balance in elderly hypertensive patients. Clin Interv Aging. 2015;10:897–906. doi: 10.2147/CIA.S81283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barochiner J, Alfie J, Aparicio L, Rada M, Morales M, Cuffaro P, Galarza C, Waisman G. Orthostatic hypotension in treated hypertensive patients. Rom J Intern Med. 2012;50:203–209. [PubMed] [Google Scholar]
- 62.Juraschek SP, Appel LJ, Miller ER, Mukamal KJ, Lipsitz LA. Hypertension treatment effects on orthostatic hypotension and its relationship with cardiovascular disease. Hypertension. 2018;72:986–993. doi: 10.1161/HYPERTENSIONAHA.118.11337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juraschek SP, Hu JR, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, et al. Effects of intensive blood pressure treatment on orthostatic hypotension: a systematic review and individual participant-based meta-analysis. Ann Intern Med. 2021;174:58–68. doi: 10.7326/M20-4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. ; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zia A, Kamaruzzaman SB, Myint PK, Tan MP. The association of antihypertensives with postural blood pressure and falls among seniors residing in the community: a case-control study. Eur J Clin Invest. 2015;45:1069–1076. doi: 10.1111/eci.12508 [DOI] [PubMed] [Google Scholar]
- 66.Kamaruzzaman S, Watt H, Carson C, Ebrahim S. The association between orthostatic hypotension and medication use in the British Women’s Heart and Health Study. Age Ageing. 2010;39:51–56. doi: 10.1093/ageing/afp192 [DOI] [PubMed] [Google Scholar]
- 67.Coupe MO, Clarke B, Rowland E, Barnes P, Oldershaw P, McKenna W. Adenosine and hypertrophic cardiomyopathy. Lancet. 1989;1:218–219. doi: 10.1016/s0140-6736(89)91231-2 [DOI] [PubMed] [Google Scholar]
- 68.Canney M, O’Connell MDL, Murphy CM, O’Leary N, Little MA, O’Seaghdha CM, Kenny RA. Single agent antihypertensive therapy and orthostatic blood pressure behaviour in older adults using beat-to-beat measurements: The Irish Longitudinal Study on Ageing. PLoS One. 2016;11:e0146156. doi: 10.1371/journal.pone.0146156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero-Ortuno R, O’Connell MDL, Finucane C, Soraghan C, Fan CW, Kenny RA. Insights into the clinical management of the syndrome of supine hypertension–orthostatic hypotension (SH-OH): The Irish Longitudinal Study on Ageing (TILDA). BMC Geriatr. 2013;13:73. doi: 10.1186/1471-2318-13-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juraschek SP, Simpson LM, Davis BR, Beach JL, Ishak A, Mukamal KJ. Effects of antihypertensive class on falls, syncope, and orthostatic hypotension in older adults: the ALLHAT trial. Hypertension. 2019;74:1033–1040. doi: 10.1161/HYPERTENSIONAHA.119.13445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Testa G, Ceccofiglio A, Mussi C, Bellelli G, Nicosia F, Bo M, Riccio D, Curcio F, Martone AM, Noro G, et al. Hypotensive drugs and syncope due to orthostatic hypotension in older adults with dementia (Syncope and Dementia Study). J Am Geriatr Soc. 2018;66:1532–1537. doi: 10.1111/jgs.15421 [DOI] [PubMed] [Google Scholar]
- 72.Wieling W, Kaufmann H, Claydon VE, van Wijnen VK, Harms MPM, Juraschek SP, Thijs RD. Diagnosis and treatment of orthostatic hypotension. Lancet Neurol. 2022;21:735–746. doi: 10.1016/S1474-4422(22)00169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montero-Odasso M, van der Velde N, Martin FC, Petrovic M, Tan MP, Ryg J, Aguilar-Navarro S, Alexander NB, Becker C, Blain H, et al. ; Task Force on Global Guidelines for Falls in Older Adults. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing. 2022;51:afac205. doi: 10.1093/ageing/afac205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin C, Abbott RD, Lee H, Kim J, Kimm K. Prevalence and correlates of orthostatic hypotension in middle-aged men and women in Korea: the Korean Health and Genome Study. J Hum Hypertens. 2004;18:717–723. doi: 10.1038/sj.jhh.1001732 [DOI] [PubMed] [Google Scholar]
- 75.Gibbons CH, Freeman R. Delayed orthostatic hypotension. Auton Neurosci Basic Clin. 2020;229:102724. doi: 10.1016/j.autneu.2020.102724 [DOI] [PubMed] [Google Scholar]
- 76.Lodhi HA, Peri-Okonny PA, Schesing K, Phelps K, Ngo C, Evans H, Arbique D, Price AL, Vernino S, Phillips L, et al. Usefulness of blood pressure variability indices derived from 24-hour ambulatory blood pressure monitoring in detecting autonomic failure. J Am Heart Assoc. 2019;8:e010161. doi: 10.1161/JAHA.118.010161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bengtsson-Lindberg M, Larsson V, Minthon L, Wattmo C, Londos E. Lack of orthostatic symptoms in dementia patients with orthostatic hypotension. Clin Auton Res. 2015;25:87–94. doi: 10.1007/s10286-014-0244-z [DOI] [PubMed] [Google Scholar]
- 78.Juraschek SP, Miller ER 3rd, Appel LJ. Orthostatic hypotension and symptoms in the AASK trial. Am J Hypertens. 2018;31:665–671. doi: 10.1093/ajh/hpy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grobman B, Turkson-Ocran RAN, Staessen JA, Yu Y-L, Lipsitz LA, Mukamal KJ, Juraschek SP. Body position and orthostatic hypotension in hypertensive adults: results from the Syst-Eur Trial. Hypertension. 2023;80:820–827. doi: 10.1161/hypertensionaha.122.20602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cooke J, Carew S, Costello A, Sheehy T, Lyons D. Recurrent loss of consciousness. BMJ. 2008;337:a2703. doi: 10.1136/bmj.a2703 [DOI] [PubMed] [Google Scholar]
- 81.Shaw BH, Garland EM, Black BK, Paranjape SY, Shibao CA, Okamoto LE, Gamboa A, Diedrich A, Plummer WD, Dupont WD, et al. Optimal diagnostic thresholds for diagnosis of orthostatic hypotension with a “sit-to-stand test.” J Hypertens. 2017;35:1019–1025. doi: 10.1097/HJH.0000000000001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finucane C, O’Connell MDL, Fan CW, Savva GM, Soraghan CJ, Nolan H, Cronin H, Kenny RA. Age-related normative changes in phasic orthostatic blood pressure in a large population study: findings from The Irish Longitudinal Study on Ageing (TILDA). Circulation. 2014;130:1780–1789. doi: 10.1161/CIRCULATIONAHA.114.009831 [DOI] [PubMed] [Google Scholar]
- 83.Juraschek SP, Appel LJ, Mitchell CM, Mukamal KJ, Lipsitz LA, Blackford AL, Cai Y, Guralnik JM, Kalyani RR, Michos ED, et al. Comparison of supine and seated orthostatic hypotension assessments and their association with falls and orthostatic symptoms. J Am Geriatr Soc. 2022;70:2310–2319. doi: 10.1111/jgs.17804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabriele S, Georgiopoulos I, Labat C, Kotsani M, Gautier S, Fantin F, Benetos A. Can sitting and lying blood pressure measurements be considered interchangeable in older frail adults? Eur Geriatr Med. 2022;13:1401–1415. doi: 10.1007/s41999-022-00669-7 [DOI] [PubMed] [Google Scholar]
- 85.Olufsen MS, Alston AV, Tran HT, Ottesen JT, Novak V. Modeling heart rate regulation, part I: sit-to-stand versus head-up tilt. Cardiovasc Eng. 2008;8:73–87. doi: 10.1007/s10558-007-9050-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finucane C, van Wijnen VK, Fan CW, Soraghan C, Byrne L, Westerhof BE, Freeman R, Fedorowski A, Harms MPM, Wieling W, et al. A practical guide to active stand testing and analysis using continuous beat-to-beat non-invasive blood pressure monitoring. Clin Auton Res. 2019;29:427–441. doi: 10.1007/s10286-019-00606-y [DOI] [PubMed] [Google Scholar]
- 87.Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, et al. ; ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawano Y Diurnal blood pressure variation and related behavioral factors. Hypertens Res. 2011;34:281–285. doi: 10.1038/hr.2010.241 [DOI] [PubMed] [Google Scholar]
- 89.Gibbons CH, Schmidt P, Biaggioni I, Frazier-Mills C, Freeman R, Isaacson S, Karabin B, Kuritzky L, Lew M, Low P, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264:1567–1582. doi: 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jansen RW, Lipsitz LA. Postprandial hypotension: epidemiology, pathophysiology, and clinical management. Ann Intern Med. 1995;122:286–295. doi: 10.7326/0003-4819-122-4-199502150-00009 [DOI] [PubMed] [Google Scholar]
- 91.Narkiewicz K, Cooley RL, Somers VK. Alcohol potentiates orthostatic hypotension. Circulation. 2000;101:398–402. doi: 10.1161/01.cir.101.4.398 [DOI] [PubMed] [Google Scholar]
- 92.Carter JR, Stream SF, Durocher JJ, Larson RA. Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am J Physiol Endocrinol Metab. 2011;300:E771–E778. doi: 10.1152/ajpendo.00674.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillespie JA. Vasodilator properties of alcohol. Br Med J. 1967;2:274–277. doi: 10.1136/bmj.2.5547.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Halliwill JR. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc Sport Sci Rev. 2001;29:65–70. doi: 10.1097/00003677-200104000-00005 [DOI] [PubMed] [Google Scholar]
- 95.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. J Neurol Sci. 1996;144:218–219. [PubMed] [Google Scholar]
- 96.O’Connor JD, O’Connell MDL, Nolan H, Newman L, Knight SP, Kenny RA. Impact of standing speed on the peripheral and central hemodynamic response to orthostasis: evidence from The Irish Longitudinal Study on Ageing. Hypertension. 2020;75:524–531. doi: 10.1161/HYPERTENSIONAHA.119.14040 [DOI] [PubMed] [Google Scholar]
- 97.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. ; ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 98.de Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:1273–1284. doi: 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
- 99.Press Y, Punchik B, Freud T. Orthostatic hypotension and drug therapy in patients at an outpatient comprehensive geriatric assessment unit. J Hypertens. 2016;34:351–358 doi: 10.1097/HJH.0000000000000781 [DOI] [PubMed] [Google Scholar]
- 100.Calvi A, Fischetti I, Verzicco I, Belvederi Murri M, Zanetidou S, Volpi R, Coghi P, Tedeschi S, Amore M, Cabassi A. Antidepressant drugs effects on blood pressure. Front Cardiovasc Med. 2021;8:704281. doi: 10.3389/fcvm.2021.704281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhanu C, Nimmons D, Petersen I, Orlu M, Davis D, Hussain H, Magammanage S, Walters K. Drug-induced orthostatic hypotension: a systematic review and meta-analysis of randomised controlled trials. PLoS Med. 2021;18:e1003821. doi: 10.1371/journal.pmed.1003821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farrell MC, Biaggioni I, Shibao CA. Neurogenic orthostatic hypotension induced by tizanidine. Clin Auton Res. 2020;30:173–175. doi: 10.1007/s10286-019-00637-5 [DOI] [PubMed] [Google Scholar]
- 103.Chaugai S, Dickson AL, Shuey MM, Feng QP, Barker KA, Wei W-Q, Luther JM, Stein CM, Chung CP. Co-prescription of strong CYP1A2 inhibitors and the risk of tizanidine-associated hypotension: a retrospective cohort study. Clin Pharmacol Ther. 2019;105:703–709. doi: 10.1002/cpt.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bird ST, Delaney JAC, Brophy JM, Etminan M, Skeldon SC, Hartzema AG. Tamsulosin treatment for benign prostatic hyperplasia and risk of severe hypotension in men aged 40–85 years in the United States: risk window analyses using between and within patient methodology. BMJ. 2013;347:f6320. doi: 10.1136/bmj.f6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gamboa A, Shibao C, Diedrich A, Paranjape SY, Farley G, Christman B, Raj SR, Robertson D, Biaggioni I. Excessive nitric oxide function and blood pressure regulation in patients with autonomic failure. Hypertension. 2008;51:1531–1536. doi: 10.1161/HYPERTENSIONAHA.107.105171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimbo D, Bowling CB, Levitan EB, Deng L, Sim JJ, Huang L, Reynolds K, Muntner P. Short-term risk of serious fall injuries in older adults initiating and intensifying treatment with antihypertensive medication. Circ Cardiovasc Qual Outcomes. 2016;9:222–229. doi: 10.1161/CIRCOUTCOMES.115.002524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Juraschek SP, Cluett JL, Belanger MJ, Anderson TS, Ishak A, Sahni S, Millar C, Appel LJ, Miller ER, Lipsitz LA, et al. Effects of antihypertensive deprescribing strategies on blood pressure, adverse events, and orthostatic symptoms in older adults: results from TONE. Am J Hypertens. 2022;35:337–346. doi: 10.1093/ajh/hpab171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Okamoto LE, Diedrich A, Baudenbacher FJ, Harder R, Whitfield JS, Iqbal F, Gamboa A, Shibao CA, Black BK, Raj SR, et al. Efficacy of servo- controlled splanchnic venous compression in the treatment of orthostatic hypotension: a randomized comparison with midodrine. Hypertension. 2016;68:418–426. doi: 10.1161/HYPERTENSIONAHA.116.07199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stock JM, Chelimsky G, Edwards DG, Farquhar WB. Dietary sodium and health: how much is too much for those with orthostatic disorders? Auton Neurosci. 2022;238:102947. doi: 10.1016/j.autneu.2022.102947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic hypotension: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:1294–1309. doi: 10.1016/j.jacc.2018.05.079 [DOI] [PubMed] [Google Scholar]
- 111.Shannon JR, Diedrich A, Biaggioni I, Tank J, Robertson RM, Robertson D, Jordan J. Water drinking as a treatment for orthostatic syndromes. Am J Med. 2002;112:355–360. doi: 10.1016/s0002-9343(02)01025-2 [DOI] [PubMed] [Google Scholar]
- 112.Schroeder C, Bush VE, Norcliffe LJ, Luft FC, Tank J, Jordan J, Hainsworth R. Water drinking acutely improves orthostatic tolerance in healthy subjects. Circulation. 2002;106:2806–2811. doi: 10.1161/01.cir.0000038921.64575.d0 [DOI] [PubMed] [Google Scholar]
- 113.Young TM, Mathias CJ. The effects of water ingestion on orthostatic hypotension in two groups of chronic autonomic failure: multiple system atrophy and pure autonomic failure. J Neurol Neurosurg Psychiatry. 2004;75:1737–1741. doi: 10.1136/jnnp.2004.038471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Newton JL, Frith J. The efficacy of nonpharmacologic intervention for orthostatic hypotension associated with aging. Neurology. 2018;91:e652–e656. doi: 10.1212/WNL.0000000000005994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frith J, Newton JL. Combination non-pharmacologic intervention for orthostatic hypotension in older people: a phase 2 study. Age Ageing. 2020;49:253–257. doi: 10.1093/ageing/afz173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Veazie S, Peterson K, Ansari Y, Chung KA, Gibbons CH, Raj SR, Helfand M. Fludrocortisone for orthostatic hypotension. Cochrane Database Syst Rev. 2021;5:CD012868. doi: 10.1002/14651858.CD012868.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grijalva CG, Biaggioni I, Griffin MR, Shibao CA. Fludrocortisone is associated with a higher risk of all-cause hospitalizations compared with midodrine in patients with orthostatic hypotension. J Am Heart Assoc. 2017;6:e006848. doi: 10.1161/JAHA.117.006848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Low PA, Gilden JL, Freeman R, Sheng KN, McElligott MA. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension: a randomized, double-blind multicenter study: Midodrine Study Group. JAMA. 1997;277:1046–1051. [PubMed] [Google Scholar]
- 119.Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120–124. doi: 10.1212/wnl.51.1.120 [DOI] [PubMed] [Google Scholar]
- 120.Kaufmann H, Norcliffe-Kaufmann L, Palma JA. Droxidopa in neurogenic orthostatic hypotension. Expert Rev Cardiovasc Ther. 2015;13:875–891. doi: 10.1586/14779072.2015.1057504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biaggioni I, Freeman R, Mathias CJ, Low P, Hewitt LA, Kaufmann H; Droxidopa 302 Investigators. Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension. 2015;65:101–107. doi: 10.1161/HYPERTENSIONAHA.114.04035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson’s disease (nOH306B). Mov Disord. 2015;30:646–654. doi: 10.1002/mds.26086 [DOI] [PubMed] [Google Scholar]
- 123.Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, Mauney J, Feirtag M, Mathias CJ; NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83:328–335. doi: 10.1212/WNL.0000000000000615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biaggioni I, Hewitt LA, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol. 2017;17:90. doi: 10.1186/s12883-017-0867-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen JJ, Han Y, Tang J, Portillo I, Hauser RA, Dashtipour K. Standing and supine blood pressure outcomes associated with droxidopa and midodrine in patients with neurogenic orthostatic hypotension: a bayesian meta-analysis and mixed treatment comparison of randomized trials. Ann Pharmacother. 2018;52:1182–1194. doi: 10.1177/1060028018786954 [DOI] [PubMed] [Google Scholar]
- 126.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003;74:1294–1298. doi: 10.1136/jnnp.74.9.1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O’Brien PC, Slezak J, Low PA. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–518. doi: 10.1001/archneur.63.4.noc50340 [DOI] [PubMed] [Google Scholar]
- 128.Shibao C, Okamoto LE, Gamboa A, Yu C, Diedrich A, Raj SR, Robertson D, Biaggioni I. Comparative efficacy of yohimbine against pyridostigmine for the treatment of orthostatic hypotension in autonomic failure. Hypertension. 2010;56:847–851. doi: 10.1161/HYPERTENSIONAHA.110.154898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okamoto LE, Gamboa A, Shibao C, Black BK, Diedrich A, Raj SR, Robertson D, Biaggioni I. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension. 2009;53:363–369. doi: 10.1161/HYPERTENSIONAHA.108.124552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Okamoto LE, Gamboa A, Shibao CA, Arnold AC, Choi L, Black BK, Raj SR, Robertson D, Biaggioni I. Nebivolol, but not metoprolol, lowers blood pressure in nitric oxide-sensitive human hypertension. Hypertension. 2014;64:1241–1247. doi: 10.1161/HYPERTENSIONAHA.114.04116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okamoto LE, Celedonio JE, Smith EC, Gamboa A, Shibao CA, Diedrich A, Paranjape SY, Black BK, Muldowney JAS, Peltier AC, et al. Local passive heat for the treatment of hypertension in autonomic failure. J Am Heart Assoc. 2021;10:e018979. doi: 10.1161/JAHA.120.018979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okamoto LE, Celedonio JE, Smith EC, Paranjape SY, Black BK, Wahba A, Park J-W, Shibao CA, Diedrich A, Biaggioni I. Continuous positive airway pressure for the treatment of supine hypertension and orthostatic hypotension in autonomic failure. Hypertension. 2023;80:650–658. doi: 10.1161/HYPERTENSIONAHA.122.20081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park JW, Okamoto LE, Biaggioni I. Advances in the pathophysiology and management of supine hypertension in patients with neurogenic orthostatic hypotension. Curr Hypertens Rep. 2022;24:45–54. doi: 10.1007/s11906-022-01168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biaggioni I, Garcia F, Inagami T, Haile V. Hyporeninemic normoaldosteronism in severe autonomic failure. J Clin Endocrinol Metab. 1993;76:580–586. doi: 10.1210/jcem.76.3.7680352 [DOI] [PubMed] [Google Scholar]
- 135.Arnold AC, Okamoto LE, Gamboa A, Shibao C, Raj SR, Robertson D, Biaggioni I. Angiotensin II, independent of plasma renin activity, contributes to the hypertension of autonomic failure. Hypertension. 2013;61:701–706. doi: 10.1161/HYPERTENSIONAHA.111.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biaggioni I, Shibao CA, Jordan J. Evaluation and diagnosis of afferent baroreflex failure. Hypertension. 2022;79:57–59. doi: 10.1161/HYPERTENSIONAHA.121.18372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Biaggioni I, Shibao CA, Diedrich A, Muldowney JAS, Laffer CL, Jordan J. Blood pressure management in afferent baroreflex failure: JACC review topic of the week. J Am Coll Cardiol. 2019;74:2939–2947. doi: 10.1016/j.jacc.2019.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


