Abstract
Cardiogenic shock continues to portend poor outcomes, conferring short-term mortality rates of 30% to 50% despite recent scientific advances. Age is a nonmodifiable risk factor for mortality in patients with cardiogenic shock and is often considered in the decision-making process for eligibility for various therapies. Older adults have been largely excluded from analyses of therapeutic options in patients with cardiogenic shock. As a result, despite the association of advanced age with worse outcomes, focused strategies in the assessment and management of cardiogenic shock in this high-risk and growing population are lacking. Individual programs oftentimes develop upper age limits for various interventional strategies for their patients, including heart transplantation and durable left ventricular assist devices. However, age as a lone parameter should not be used to guide individual patient management decisions in cardiogenic shock. In the assessment of risk in older adults with cardiogenic shock, a comprehensive, interdisciplinary approach is central to developing best practices. In this American Heart Association scientific statement, we aim to summarize our contemporary understanding of the epidemiology, risk assessment, and in-hospital approach to management of cardiogenic shock, with a unique focus on older adults.
Keywords: AHA Scientific Statements; aging; decision making; frailty; heart transplantation; heart-assist devices; risk assessment; shock, cardiogenic
Cardiogenic shock (CS) is a complex multifactorial syndrome associated with high morbidity and confers a short-term mortality rate of 30% to 50%.1,2 Survival in patients with CS depends on multiple factors, including patient-specific baseline features, shock severity, number and degree of organ dysfunction, response to therapy, and other risk modifiers such as cardiac arrest.1,3 Age is a nonmodifiable risk factor for mortality in patients with CS and is associated with higher in-hospital mortality across all stages of shock severity.4 Commonly used in the risk assessment of CS, age has become a highly relevant factor in the decision-making process for these patients, especially when it comes to the use of invasive therapies or determining which patients will derive benefit from escalation of care. Although most transplantation centers apply an upper age limit for heart transplantation (HT) candidacy, the establishment of age cutoffs for older adults seeking candidacy for durable left ventricular assist device (LVAD) has considerable variability among different medical centers and institutions.5
Older adults are now experiencing therapeutic outcomes similar to those of their younger counterparts across various cardiovascular disease states. For example, older adults have demonstrated improved mortality with coronary revascularization after acute myocardial infarction (AMI), including AMI-CS.6,7 Recent years have shown a surge in the incidence of CS-related hospitalizations nationwide, including substantial growth in the number of older patients presenting with CS.4 There has also been a concomitant increase in the use of temporary mechanical circulatory support (t-MCS) in CS across all age groups. However, robust data to support guideline level of evidence for CS management are lacking, especially among older adults, creating a significant knowledge gap in the care of this high-risk population. The aim of this American Heart Association scientific statement is to summarize our contemporary understanding of the epidemiology, risk assessment, and in-hospital approach to management of CS in the older adult, supplementing knowledge gaps in the evaluation and management of CS with expert opinions and suggestions for clinical practice.
DEFINITIONS AND EPIDEMIOLOGY
Definitions
Older Adults
Clinical practice guidelines currently lack consistent definitions for older adults and offer limited guidance on applying evidence-based recommendations to this population. Currently, there is no standardized age threshold beyond which a patient can be classified as an older adult, nor has an unequivocal age limit been established to define futility of invasive therapies after CS.8–10 This knowledge gap is particularly pronounced in patients >75 years of age and becomes even more acute in those >80 years of age8; in these age groups, virtually no high-quality evidence is available to guide clinical decision-making. Furthermore, clinical decision-making frequently places significant emphasis on chronological age, resulting in a disregard for the critical link between in-hospital outcomes and individual patient characteristics, including the presence of geriatric conditions such as multimorbidity, polypharmacy, cognitive decline, delirium, and frailty.11 This overreliance on chronological age as the sole determining factor overlooks the complexity and variability of older patients’ health profiles and fails to account for the effect of these geriatric conditions on their outcomes.
Cardiogenic Shock
The current trial and guideline criteria for defining CS have limited standardization, a narrow focus on hypotension, and inadequate inclusion of older adults.12,13 CS is typically defined as a clinical syndrome characterized by hypotension (systolic blood pressure <90 mm Hg or the need for vasopressors to maintain systolic blood pressure ≥90 mm Hg) accompanied by signs of organ hypoperfusion such as altered mental status, liver dysfunction, renal dysfunction (urine output <30 mL/h), and elevated serum lactate levels (>2.0 mmol/L) in the presence of cardiac dysfunction.1
More recent evidence suggests that CS should be viewed as a continuum ranging from preshock to refractory shock states, involving cycles of ischemia, vascular instability, and inflammation and the potential for multiorgan dysfunction and death.14 Furthermore, emerging data strongly suggest the critical importance of incorporating additional criteria that go beyond hypotension alone to accurately reflect the severity of illness.2 In a single-center, retrospective study of >10 000 patients, inpatient mortality was higher in patients with CS with laboratory evidence of isolated hypoperfusion (eg, rising lactate and creatinine; mortality rate, 17.2%) or combined hypotension/hypoperfusion (mortality rate, 34%) compared with those defined by hypotension criterion alone (mortality rate, 9.3%).15 In addition, CS definitions based on distinct phenotypes, including noncongested, cardiorenal, or cardiometabolic subtypes, have also been described. Among these phenotypic clusters, older adults tend to be more prevalent in the cardiorenal phenotype, exhibiting greater congestion, cardiorenal dysfunction, and higher comorbidity burdens.16 In 2022, the Society for Cardiovascular Angiography & Intervention (SCAI) revised the shock classification system to incorporate these key phenotypic elements and to capture the dynamic progression of CS.10 In addition, studies have shown that patients >70 years of age have a higher representation in SCAI shock stages C/D compared with stages B/E, indicating that age acts as a modifier of mortality risk beyond the SCAI stage alone.17
Suggestions for Clinical Practice
Inconsistent definitions of older adults and CS, along with limited recommendations within clinical practice guidelines, especially for individuals ≥75 years of age, create a knowledge gap for evidence-based recommendations in older adults.
Clinical decision-making frequently places significant emphasis on chronological age, resulting in a disregard for the critical link between in-hospital outcomes and individual patient characteristics, including the presence of geriatric conditions such as multimorbidity, polypharmacy, cognitive decline, delirium, and frailty.
Epidemiology
The epidemiology of CS causes has undergone significant changes in the past decade. Although the incidence of CS associated with AMI is decreasing, there is a concurrent rise in the prevalence of CS attributed to heart failure (HF) and CS resulting from structural heart disease.18–20 In a recent report from the Critical Care Cardiology Trials Network, 46% of CS cases were attributed to HF-CS, 30% had AMI-CS, and 17% had an identified cardiac cause that was not related primarily to myocardial dysfunction (eg, incessant ventricular tachycardia or severe valve disease).21 Similar findings with a predominant incidence of HF-CS have been noted by the Cardiogenic Shock Working Group.2
Older patients with cardiac dysfunction, regardless of the underlying cause, have a higher incidence of CS. In the setting of AMI, CS has been observed in >10% of patients >75 years of age.4,22 Recent data from a European cohort revealed a significantly higher prevalence of AMI-CS among patients ≥75 years of age compared with younger patients in both ST-segment–elevation myocardial infarction (10.8% versus 3.9%; P<0.0001) and non–ST-segment–elevation myocardial infarction (4.6% versus 1.8%; P<0.0001).23 Moreover, within the subgroup of patients with cardiomyopathy, there is a growing proportion of individuals >80 years of age, representing 12% of the overall HF population, and those >75 years of age exhibit the highest rates of acute HF (30 per 1000 person-years).24 Consequently, the prevalence of HF-CS is expected to rise among older adults in the coming years.
Regardless of CS cause, the associated short-term mortality rate remains high (30%–50%),1,2 and this increases incrementally with advancing age across all SCAI stages.4 A recent analysis performed selective analyses to assess the prognostic effect of age stratified by CS cause (ie, AMI-CS versus non–AMI-CS). Although robust data remain limited, this analysis demonstrated a significantly increased mortality risk among patients with AMI-CS in the group >80 years of age, which was still evident after multivariable adjustment. Conversely, no difference in prognosis was observed when the 2 age groups were compared in patients without AMI-CS.25
Suggestions for Clinical Practice
HF-CS has emerged as the primary cause of CS. Older adults are more likely to develop AMI-CS compared with younger individuals. With the increasing prevalence of HF among the older adult population, a rise in HF-CS cases may be anticipated.
Regardless of shock cause, CS mortality remains high and increases incrementally with advancing age.
RISK ASSESSMENT AND CONSIDERATIONS FOR DECISION-MAKING
Risk Assessment
Accurately assessing the mortality risk in older patients with CS is crucial for informing care interventions and facilitating shared decision-making with patients and families. Several factors are known to be independently associated with an increased risk of adverse events in the general CS population and have been included in available contemporary risk scores.14,26–28 Although the magnitude of conferred risk varies across scores, older age remains a consistent patient-related risk factor associated with increased mortality.4,12,17,29 However, the heightened mortality risk observed in older adults is multifactorial, and a comprehensive assessment within an interdisciplinary team is essential.
When risk is being evaluated and decisions are being made for older adults, it is crucial to consider individual patient factors, the clinical trajectory, and the capabilities of the health care center (Figure 1). Older adults frequently present with more severe forms of CS and higher incidence of cardiac arrest, which serves as a CS risk modifier.3,30 This is further compounded by concurrent geriatric syndromes, including frailty, polypharmacy, multimorbidity, cognitive impairment, and socioeconomic disparities.11 According to their individual baseline risk profiles, older adults with CS can be particularly susceptible to intensive care unit (ICU)–related complications, including ventilator-associated pneumonia, delirium, critical illness myopathy, central line–associated blood-stream infection, and multiorgan dysfunction.11,31 Furthermore, the absence of pre-established care goals often adds complexity to these risks, potentially altering or delaying resuscitation efforts or the initiation of disease-modifying treatments. Last, it is imperative to recognize that in all patients with CS, including older adults, survival should not be the sole determinant guiding the care and decision-making processes. Evaluating health-related quality of life (QOL) and anticipating the potential impact of CS-related complications (eg, stroke and renal failure) on physical function, cognitive outcomes, and discharge burdens become paramount when determining the appropriate level of care aggressiveness and the use of invasive approaches. Consequently, the inclusion of an interdisciplinary approach, including a heart team when available, becomes critical in the optimal management of older adults with CS. It serves as a vital means to facilitate the implementation of evidence-based practices and has been associated with improved patient outcomes.32 In sum, the cumulative effect of all these factors, rather than age alone, contributes to an increased risk of morbidity, mortality, and prolonged hospitalization stays in older adults.
Figure 1. Decision-making in CS.
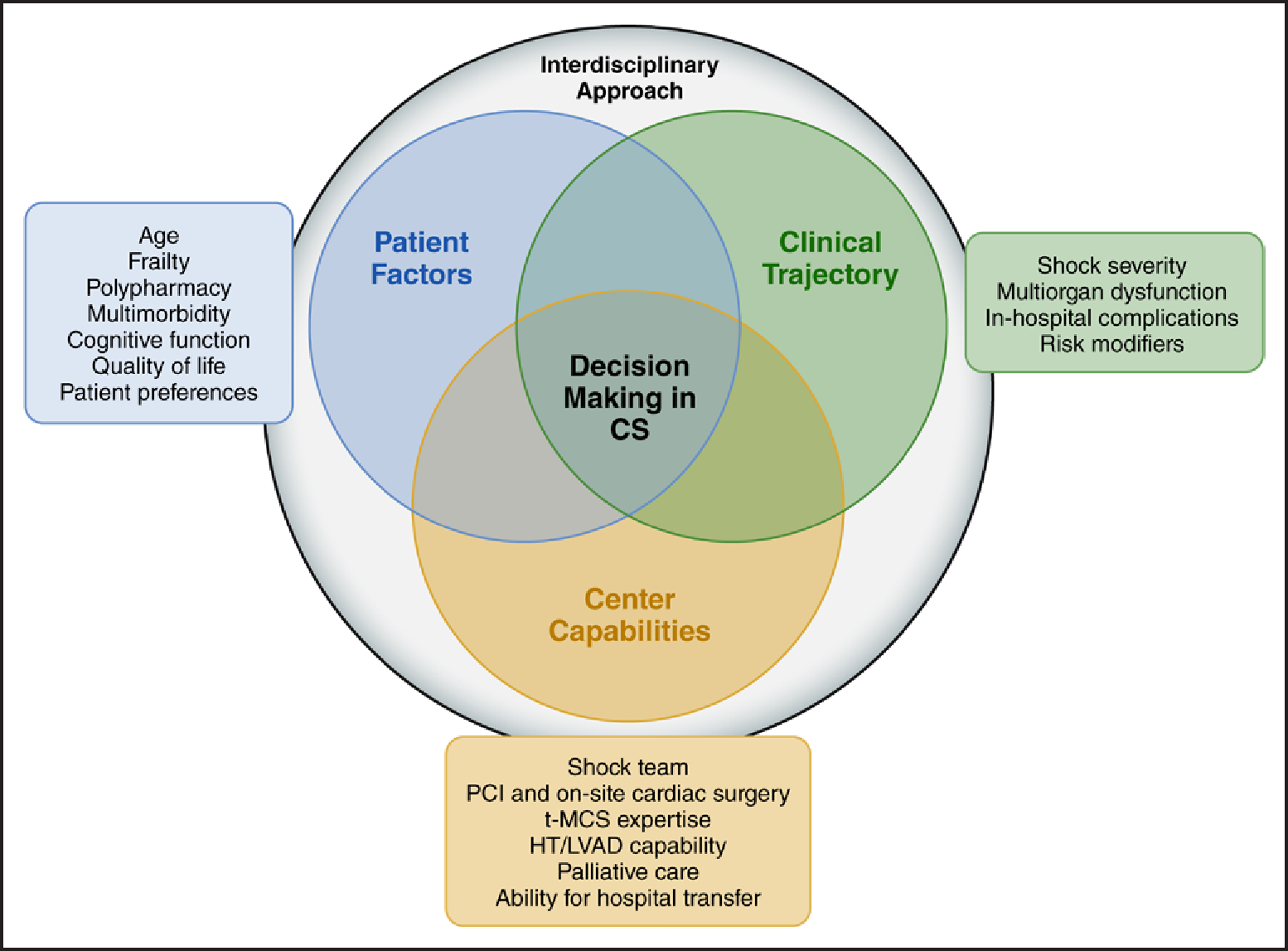
Risk assessment in cardiogenic shock (CS), especially among older adults, necessitates an interdisciplinary approach. The decision-making process involves considering individual patient factors, relevant aspects of the clinical trajectory, and the capabilities of the health care center. This comprehensive approach empowers health care professionals to customize care on the basis of each patient’s unique needs, to monitor the progression of the condition closely, and to use available resources effectively. By addressing the multifactorial challenges associated with the heightened mortality risk in older adults through an individualized and comprehensive interdisciplinary approach, health care professionals can optimize outcomes and enhance the overall management of CS in this subgroup. HT indicates heart transplantation; LVAD, left ventricular assist device; PCI, percutaneous coronary intervention; and t-MCS, temporary mechanical circulatory support.
Suggestions for Clinical Practice
Age is recognized as a contributing factor to increased mortality risk in older adults with CS; however, it should not be regarded as the sole determinant. Individualized assessments considering a range of contributing factors are necessary.
A comprehensive assessment by an interdisciplinary team is crucial in the evaluation of the multifactorial heightened mortality risk in older adults, taking into account baseline patient factors, clinical trajectory, and health care center capabilities.
Shared Decision-Making and Advance Care Planning
In older patients with CS, treatment options often involve significant tradeoffs, emphasizing the importance of shared decision-making to align therapeutic approaches with patient values and preferences. Shared decision-making has been shown to enhance outcomes, including patient satisfaction, adherence to medical therapy, and health-related QOL.33 However, decision-making in older adults is complex because of their diverse functional and comorbidity profiles, ranging from high functionality to significant frailty, cognitive impairment, and caregiver dependence. Age-related challenges such as cognitive and sensory impairments and involvement of multiple family member caregivers can complicate information sharing and decision-making.34 Nonetheless, these challenges should not hinder the implementation of care discussions.
Decision support tools have been developed specifically to enhance the shared decision-making process, proving their efficacy in facilitating informed decisions among older adults. A recently described shared decision-making intervention demonstrated a modest improvement in patient decision quality, as evidenced by increased patient knowledge and alignment between stated values and treatment choices among patients undergoing destination therapy LVAD.33 However, it is important to recognize that the potential for advancing shared decision-making extends beyond decision aids. The greatest opportunities for improvement lie in enhancing clinician communication skills and fostering interdisciplinary collaboration in the care of older adults with CS, promoting a comprehensive and patient-centered approach.34 It is important to note that the involvement of palliative care for complex shared decision-making constitutes an integral component of the delivery of shared decision-making among older adults (see the Palliative and End-of-Life Care section).
Advance care planning, including the identification of care preferences and surrogate decision-makers, should be integrated into patient-centered care, particularly in critical illnesses like CS. Clinicians can translate preferences into the approach to care, including either life-prolonging efforts or do-not-resuscitate orders, to respect patient and family wishes.35 It is equally important to recognize situations in which invasive therapies such as LVAD placement or HT may be considered futile and to align decisions with the principles of beneficence and nonmaleficence.36 These discussions become even more critical when there is no evident exit strategy and the initiation of t-MCS poses challenges in subsequent care withdrawal, the latter potentially adversely affecting both family members and health care professionals.
Suggestions for Clinical Practice
Shared decision-making is vital in older patients with CS because it facilitates aligning treatment choices with patient values and preferences, improving outcomes and health-related QOL.
Advance care planning, including the identification of care preferences and surrogate decision-makers, is essential in patient-centered care for older adults with CS, ensuring that decisions are in accordance with their wishes and the principles of beneficence and nonmaleficence.
APPROACH TO MANAGEMENT
Initial Stabilization and Resuscitation Strategies
Regardless of age, the initial treatment approach for patients with CS focuses on early recognition and stabilization. This involves a comprehensive approach to identify the underlying cause, to optimize congestion management, to improve volume status, and to address hypoperfusion to mitigate or prevent multiorgan dysfunction. Older adults may demonstrate atypical or delayed presentations, necessitating a heightened level of suspicion for timely identification of CS, stabilization, and optimal initial care pathways (Figure 2). Serial laboratory studies should be conducted to assess biological markers of end-organ function (eg, renal and hepatic biomarkers), cardiac myonecrosis (eg, cardiac troponin), and perfusion (eg, serum lactate); to monitor the patient’s response to therapies; and to detect any progression to worsening stages of shock.
Figure 2. Approach to management in CS.
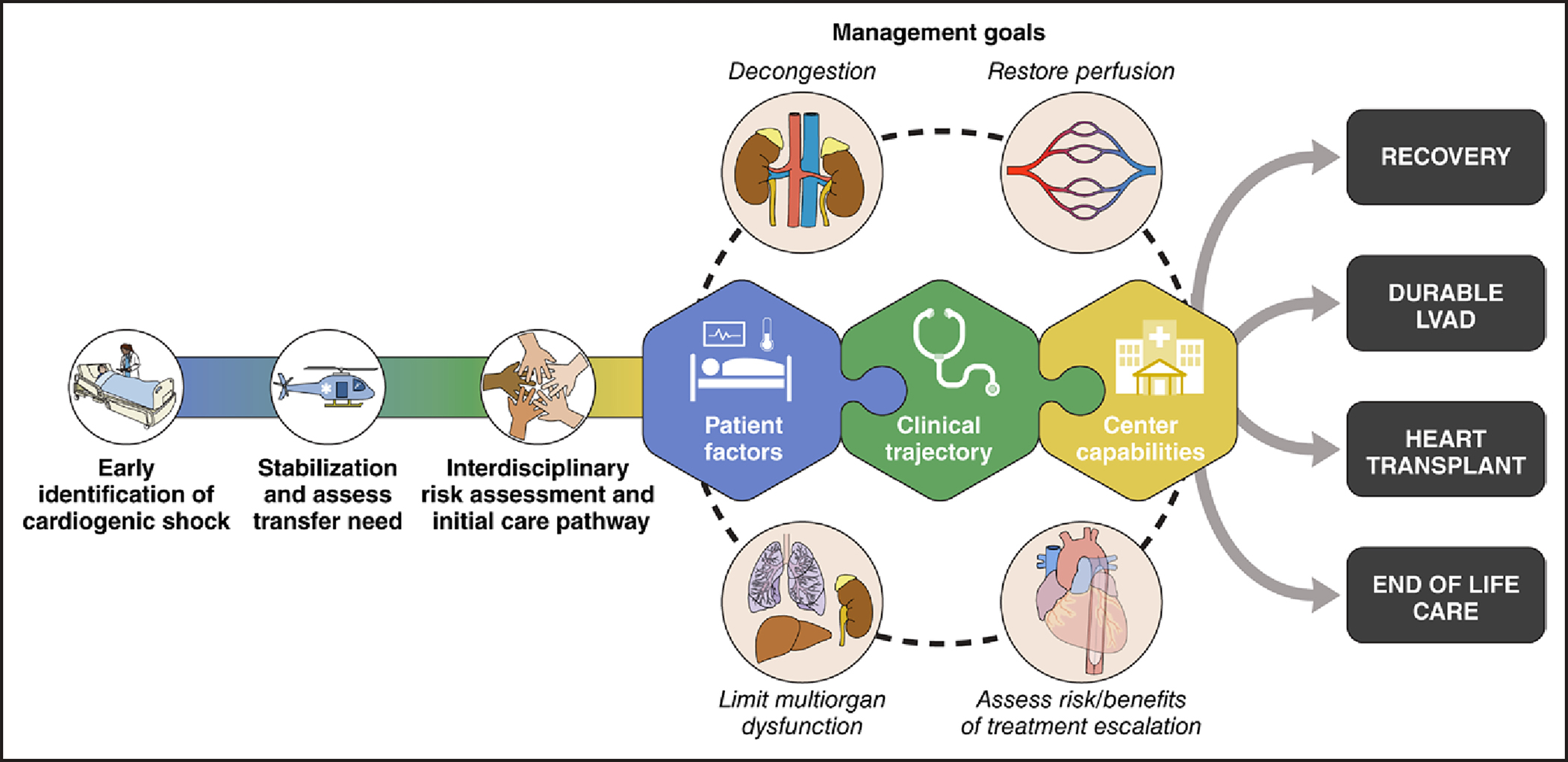
Regardless of age, the initial treatment approach for patients with CS should prioritize early recognition, initial stabilization, and timely identification of those requiring transfer to higher-level care. An interdisciplinary risk assessment and implementation of appropriate care pathways should be tailored to individual phenotypes. The management goals encompass decongestion, restoration of perfusion, limitation of multiorgan dysfunction, and evaluation of the risks and benefits of treatment escalation. Taking into account patient factors, clinical trajectory, and center capabilities, potential exit strategies may include recovery, the use of durable LVAD, heart transplantation, or transition to comfort care. CS indicates cardiogenic shock; and LVAD, left ventricular assist devices.
Although a detailed diagnostic assessment, including noninvasive and invasive monitoring, is beyond the scope of this review, it is important to highlight that patients should undergo regular assessments to gauge their response to therapies and to monitor for signs of deteriorating shock. Observational data suggest that admission to a cardiovascular-specific ICU and the use of a pulmonary artery catheter for guided therapies are associated with improved outcomes and optimal use of resources.37–39 Therefore, transfer to a facility with specialized cardiovascular care capabilities such as a cardiovascular-specific ICU should be an early consideration in the management of CS to optimize patient outcomes and to ensure appropriate monitoring and treatment. In this regard, as part of the early assessment, it is crucial to concomitantly consider the potential need for hospital transfer. Although establishing protocols for the early recognition of CS that facilitate prompt transfer of patients is recommended, the association between these protocols and improved outcomes remains unclear. Further research is needed to identify the optimal strategies for promoting standardized care and achieving better outcomes across regional CS networks.14
Parenteral vasoactive medications, including vasopressor and inotropic agents, are often the first-line therapy used to manage patients with CS. The choice of initial therapy should be guided by a patient’s pathophysiology and by a thorough understanding of the anticipated pharmacological actions of each vasoactive medication (Supplemental Table 1). It is also important to remember and expect that the presence of concomitant renal or hepatic dysfunction may potentiate the effects and prolong the action of many of these drugs, especially among older adults. Careful attention should be paid to the influence of presenting rhythm disturbances on patient hemodynamics and clinical stability. Restoration of atrioventricular synchrony may play a significant role in enhancing cardiac output and optimizing systemic perfusion. Therefore, when possible, pharmacological or electrical cardioversion of arrhythmias (or treatment of inappropriate bradyar-rhythmias) should be considered promptly in the hemodynamically unstable patient with CS.
Suggestions for Clinical Practice
Older adults presenting with CS can have atypical or delayed presentations, warranting a high index of suspicion for timely recognition, prompt evaluation, and optimal management.
Regardless of age, initial stabilization of a patient in CS consists of resuscitation ideally in an ICU, including volume expansion (if appropriate), vasopressors, inotropes, and additional therapy for decongestion; restoration of perfusion; and the prevention or treatment of multiorgan dysfunction.
Mechanical Ventilation
A common complication of CS is the development of pulmonary dysfunction; this may be the result of cardiogenic pulmonary edema, inadequate pulmonary perfusion, aspiration injury, or other pathological events. Adequate gas exchange and enhanced ventilation are critical for handling the systemic acidosis that may result from the shock state. As a result, many patients with CS will ultimately require mechanical ventilation (MV), including invasive and noninvasive MV.40 For those who need invasive MV, the decision to intubate a patient should be balanced with the potentially undesirable hemodynamic effect of the intubation process. The process of transitioning from spontaneous, negative-pressure ventilation to intubation and invasive, positive-pressure ventilation can result in atelectasis and alveolar derecruitment, hypotension due to loss of sympathetic tone with anesthetic induction, and even deleterious vagal stimulation. Special attention should be directed toward ensuring adequate ventilatory settings, need for emergency resuscitation, patient-ventilator synchrony, optimal gas exchange, and patient comfort. Age has been strongly associated with mortality among mechanically ventilated patients; however, data have shown that survival depends not only on the factors present at the start of MV but also on the development of complications and patient management in the ICU.41,42 Patient wishes in terms of their advance care planning, including cardiopulmonary resuscitation and prolonged MV preferences, should be acknowledged before initiation of invasive MV, especially in older patients.
Suggestions for Clinical Practice
Invasive MV is often needed to optimize the respiratory status in older patients with CS.
Patient wishes regarding MV are taken into consideration before the initiation of invasive MV and are periodically reviewed if an extended duration is expected.
Renal Replacement Therapy
Patients presenting with CS, especially older patients with underlying renal diseases, often require renal replacement therapy (RRT).43,44 As a result of hemodynamic instability and the potential impact of large shifts in intravascular volume, continuous RRT (CRRT) is favored over intermittent forms of dialysis for the management of acute renal failure in patients with CS. The basic goals of CRRT include decongestion, management of electrolyte disturbances, and treatment of acid-base disorders. Not surprisingly, older adults with CS who require CRRT are at higher risk for in-hospital death.43,44 A recent comparison of outcomes between older and younger patients who required an escalation of care to CRRT in the ICU demonstrated a significantly higher mortality among older patients during their entire hospital admission; however, there was not a significant difference in long-term dialysis dependence between the groups.45 For older adults who have an episode of acute kidney injury during a CS hospitalization, the decision to initiate permanent dialysis can be challenging and depends on multiple factors, including underlying renal function. Potential advantages and disadvantages of dialysis therapy, including associated morbidity and QOL, should be considered in conjunction with each patient’s unique goals and priorities.46
Suggestions for Clinical Practice
Older adults in CS requiring RRT in particular are at high risk of in-hospital mortality.
In older patients with CS requiring RRT, CRRT is favored over intermittent forms of dialysis for management of acute renal failure.
Percutaneous Revascularization
Percutaneous coronary intervention (PCI) remains the most widely used method to establish early revascularization in patients with AMI-CS. This approach to management is supported mainly by evidence from the seminal SHOCK trial (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock), which showed an improvement in survival at 6 months for patients who received early revascularization.12 However, the efficacy of PCI for adults ≥75 years of age remains an area of active debate, with conflicting evidence suggesting a derived benefit from early intervention in this patient demographic4,17,47–56 (Table). A subanalysis of the SHOCK trial showed no benefit of early revascularization compared with initial medical stabilization among the 56 older adults ≥75 years of age who were included in the analysis, with the 30-day mortality rate approaching 75%. Of note, among the 152 patients who were randomized to early revascularization, 24 patients (16%) were ≥75 years of age, and 20% of those did not undergo PCI but were nonetheless included in the intention-to-treat analysis.57 From 2005 to 2013, several studies reported high mortality rates associated with PCI during AMI-CS that approached 50%, but the rate of PCI use in practice has increased steadily over time with substantial reductions in unadjusted mortality rates.55
Table.
Studies That Examined Health Outcomes for the Older Adult Populations With AMI Complicated by CS
| Authors (year) | Study design | Older adults, n (%) | PCI rate, % | Mortality rate, % | Other outcomes |
|---|---|---|---|---|---|
| Antoniucci et al47 (2003) | Prospective, nonrandomized | 71 (35) | 100 | 6-mo: 51 | Age was an independent predictor of mortality. |
| Dauerman et al48 (2003) | Retrospective, nonrandomized | 74 (24) | 100 | Hospital mortality rate: 37 | PCI showed benefit in select patients. Older age increased risk, but presence of collateral vessel decreased the risk of death. |
| Prasad et al49 (2004) | Retrospective, nonrandomized | 61 (100) | 100 | Hospital mortality rate: 44 30 d: 47 1-y survival among those who were discharged alive: 75 |
Early invasive strategy was associated with improved survival compared with initial medical therapy. |
| Dzavik et al50 (2005) | Prospective, randomized (subanalysis of SHOCK trial) | 56 (19) | 43 | 30-d mortality rate (PCI vs no PCI): 75 vs 53.1 1-y mortality rate: 79.2 vs 65.6 |
No benefit of early revascularization vs initial medical stability. |
| White et al51 (2005) | Prospective, randomized (subanalysis of SHOCK trial with left ventricular failure and emergency PCI) | 15 (12.3) | 100 | 96 h: 65.4 30 d: 55.6 1 y: 51.9 |
Those with successful PCI had higher survival rates than those with unsuccessful PCI. There were no differences in outcomes between stents and no stents and between complete and incomplete revascularization. |
| Migliorini et al52 (2006) | Retrospective cohort | ≥75 y: 104 (37) | 92 | 6 mo: 56 | Older age and PCI failure were independent predictors of mortality. |
| Lim et al53 (2009) | Retrospective cohort (Melbourne Interventional Group) | 45 (31) | 100 | Hospital: 42.2 30 d: 43.2 1 y: 52.6 |
Older adults had higher degree of comorbidity burden but same survival rates compared with younger patients. |
| Thiele et al54 (2012) | Prospective, randomized (SHOCK II) | ≥75 y: 194 (32) | 96 | 30 d: ≈52 (IABP vs control: 53.7 vs 50.0) |
No difference between use of IABP and control by prespecified age groups. |
| Damluji et al55 (2019) | Retrospective cohort | ≥75 y: 111 901 (35) | 42 | Hospital (PCI vs no PCI): 43 vs 62 (P<0.001) | PCI rates were 65% in 2013. |
| Jentzer et al17 (2021) | Retrospective cohort | 448 (26) | 63 | 30 d for 70–79 y of age: 61.1 30 d for ≥80 y of age: 67.9 |
There was a strong and graded relationship between older age and lower 30-d survival in addition to shock severity. |
| Kanwar et al4 (2021) | Retrospective cohort | ≥75 y: 223* (82) | ... | ... | Of older adults, 34 (15%) received medical therapy, 113 (51%) received IABP alone, 3 (1%) received VA-ECMO, 6 (3%) received VA-ECMO and IABP, and 49 (22%) received Impella. |
| Ratcovich et al56 (2022) | Retrospective cohort | ≥75 y: 496 (29) | 75 | Hospital: 54.8 30 d: 27 1 y: 19 |
Lactate >4.0 mmol/L and HR >90.5 bpm were predictive of 30-d mortality. |
Data on percutaneous revascularization were not collected.
HR indicates heart rate; IABP, intra-aortic balloon pump; PCI, percutaneous coronary intervention; SHOCK, Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock; and VA-ECMO, venoarterial extracorporeal membrane oxygenation.
Recent data have demonstrated benefit of early revascularization with PCI in select older patients presenting with AMI-CS compared with all-comers and attributed their results to improved patient selection compared with the restrictive inclusion criteria used in prior randomized studies.47–49 Damluji et al55 reported that older patients who did not receive PCI had higher comorbidity burden, higher crude mortality rates, and worse CS phenotypes compared with older patients who were percutaneously revascularized. When propensity matching was used to account for these differences in treatment selection, PCI was found to be associated with significant improvements in hospital mortality among older adults.
In all, these findings highlight the influence of underlying age-associated risks in patient selection for early revascularization with PCI.58 Older patients remain at the greatest risk for complications, including in-hospital mortality, bleeding, vascular injury, prolonged hospital length of stay, multisystem dysregulation, and baseline geriatric risks.11,59 In recent years, several scientific statements on the management of CS have endorsed early revascularization.1,11 According to the 2021 American College of Cardiology/American Heart Association/SCAI coronary artery revascularization guidelines,60 the optimal treatment strategy in older adults should be patient centered and should include consultation with an interdisciplinary heart team that incorporates a geriatric specialist who can help to facilitate discussions with patients about treatment preferences in the context of age-associated impairments.
Suggestions for Clinical Practice
An optimal treatment strategy to determine benefit from PCI should be patient centered and include discussions within an interdisciplinary heart team.
Early percutaneous revascularization can be considered in select older adults with AMI-CS.
Surgical Revascularization
The benefits of surgical revascularization in AMI-CS are to achieve complete revascularization and to repair concomitant valvular or mechanical complications of AMI.6 Data derived from the Society of Thoracic Surgeons National Cardiac Database showed that of 708 593 patients undergoing coronary artery bypass graft (CABG), only 2.1% (n=14 956) had preoperative shock.61 Of those, only 26% (n=3874) were ≥75 years of age, which accounted for only ≈0.5% of the total CABGs performed in the Society of Thoracic Surgeons database.61 CS before CABG increased the risk of mortality by 7-fold, accounting for 1 in 7 deaths in patients with CABG; moreover, the mortality rate among those ≥75 years of age was 1.7-fold higher than in those <75 years of age (31% for those ≥75 years of age versus 18% for those <75 years of age).61 Although 40% of patients randomized to early revascularization in the SHOCK trial received CABG surgery, the overall rates of CABG in AMI-CS remained low, and 80% of older adults who were revascularization eligible did not receive CABG.62 Among those who receive CABG for CS, the mortality rate can approach 50%, and the morbidity among the survivors is not insignificant.
In addition to increasing rates of in-hospital mortality, older adults undergoing CABG are disproportionately affected by bleeding, hemodynamic compromise, wound healing, prolonged hospitalization, and readmission.63,64 For this reason, the decision to proceed with CABG surgery should take into account the preoperative burden of geriatric syndromes.31,58 When older patients have a low burden of age-associated risks and PCI is not feasible, surgical revascularization can be considered in select patients with CS, multivessel coronary disease, and mechanical complications.6
Suggestions for Clinical Practice
Surgical revascularization can be considered in select older adults when PCI is not feasible or when CABG is highly indicated.
The decision to proceed with CABG surgery takes into account the preoperative burden of geriatric syndromes and potential postoperative risks.
Valvular Interventions
Acute valvular heart disease can result in hemodynamic compromise and CS.6 For older adults with severe aortic stenosis and pulmonary edema or CS, percutaneous balloon valvuloplasty has been used previously as a bridge to transcatheter aortic valve replacement. Recent advancements in transcatheter heart valve technologies in high-risk patients have made immediate transcatheter aortic valve replacement possible in most circumstances.65 Transcatheter aortic valve replacement should be considered in patients with aortic stenosis CS if the patient’s burden of geriatric syndromes is not prohibitive and the patient otherwise has a good life expectancy (>1 year).
For older patients with acute aortic insufficiency secondary to aortic dissection or infective endocarditis, temporary hemodynamic stability should be obtained immediately; however, it is important to be mindful that intra-aortic balloon pump therapy is contraindicated in acute aortic sufficiency. Urgent cardiac surgery should be discussed with patients and their families as the definitive gold standard therapy.11,66
For older adults with severe acute mitral regurgitation and hemodynamic compromise, particularly those with papillary muscle rupture, t-MCS can reduce the incidence of preoperative or postanesthetic induction of hemodynamic and respiratory deterioration. Chordal-sparing mitral valve replacement is generally used because of the predictability of procedural success and durability of valve during follow-up.6 According to a recent American Heart Association scientific statement on mechanical complication of AMI, although emergency mitral valve replacement is the treatment of choice for acute mitral regurgitation, transcatheter edge-to-edge repair for patients who are not surgical candidates with prohibitive risk can be considered by the heart team.6 In all, the goals of care for acute valvular interventions in older adults should follow patient preferences and values in addition to patient- and procedure-specific risk when surgical or transcatheter approaches are considered because of their inherent higher risk for morbidity and mortality.
Suggestion for Clinical Practice and Care
Valve replacement in older adults considers patient preferences and values, as well as patient- and procedure-specific risk assessments when surgical or transcatheter approaches are considered, acknowledging the inherent higher risk for morbidity and mortality.
Temporary Mechanical Circulatory Support
Although there is lack of high-quality randomized evidence to support their use in CS, t-MCS devices are increasingly available, and patients previously considered too high risk are now being supported with t-MCS. The selection of therapies, particularly t-MCS in patients with CS, is a complex process that necessitates individualized decision-making, considering baseline characteristics, pathogenesis, clinical presentation, in-hospital trajectory, and patient preferences.67 Because high-quality data to drive clinical practice are lacking, the age cutoffs used to limit the use of t-MCS are highly variable in clinical practice. Although t-MCS is applicable across the age spectrum, the decision to proceed with t-MCS in older adults should involve a heart team discussion, accounting for any contraindications to advanced HF therapies (HT or durable LVAD) and the patient’s expressed preferences for or against aggressive care. Considering the high mortality rates associated with HT or durable LVAD placement in older patients with multiple comorbidities, careful consideration is necessary to avoid futile t-MCS interventions when there is no appropriate exit strategy in the setting of critical illness and recovery appears unlikely. Moreover, older patients with explicit do-not-resuscitate preferences should not be candidates for t-MCS; instead, pharmacological support or comfort care should be provided as appropriate. Recommendations should be conveyed during shared decision-making encounters with the patient or medical decision-makers. It is imperative to present realistic estimates of potential complications and mortality to the patient and decision-makers, allowing their input in care trajectory and frequent reassessments of care planning throughout the treatment course.
In older patients without clear contraindications to advanced HF therapies, early engagement with an HF specialist and a palliative care specialist is crucial to guide evaluation, to provide necessary education, and to facilitate a smooth transition to durable LVAD or HT. For select patients who demonstrate marked frailty of uncertain prognosis, a trial of t-MCS (or inotrope support) to achieve near normalization of cardiac output and improvement in congestion may provide an opportunity for the patient to demonstrate improvement in factors contributing to frailty, including reversal of delirium or cognitive decline, improvement in ambulation and strength, and improvement in nutritional parameters. The ability to undertake these interventions must be balanced against the associated risks (eg, infection, bleeding, stroke) of ongoing critical care management, and robust data to support this approach are lacking. Device selection for t-MCS should be based on factors similar to those guiding selection in younger patients, including the degree of required cardiac support, availability of vascular access, comorbidities, and oxygenation status.67 After device placement, careful attention should be given to complications that are more prevalent in older patients such as bleeding, critical care myopathy, progression of pre-existing organ dysfunction, infection, and worsening of cognitive impairment during nonpulsatile mechanical support.68 In addition, regular reassessment of patient progress and encountered complications is essential, with effective communication of this knowledge to the patient’s family if the anticipated clinical trajectory does not align with achieving reasonable survival, morbidity, and health-related QOL. Although an extensive analysis of individualized t-MCS devices is beyond the scope of this scientific statement, it is worth noting that compared with other modes of t-MCS, venoarterial extracorporeal membrane oxygenation support carries the highest risk of morbidity and mortality, with age being one of the leading risk factors for adverse outcomes.69
Suggestions for Clinical Practice
The decision to proceed with t-MCS in older adults is made after a heart team discussion, which factors in immediately known contraindications to advanced therapies and the patient’s known wishes for or against aggressive care.
Initiation of t-MCS in older patients is typically undertaken when there is a clear exit strategy, and daily interdisciplinary assessments are performed to monitor the escalation, de-escalation, and minimization of t-MCS–related complications.
Durable Mechanical Circulatory Support
Patients with refractory CS and an inability to be weaned from t-MCS or parenteral pharmacological circulatory support should be evaluated early for consideration of durable LVAD or HT. Criteria for patient selection for durable LVAD are beyond the scope of this scientific statement and have been discussed elsewhere in detail.5,70 In brief, patients with multimorbidity, severe end-organ dysfunction, poor physical or cognitive functional status, or malnutrition or cachexia are at increased risk for poor outcomes. Among the elderly, other considerations, including dementia, frailty, malnutrition, and the suitability of caregiver support, should be addressed. It is crucial to engage in shared decision-making discussions to ensure that patients and their caregivers fully comprehend the risks associated with surgery, the rigorous medical regimen, the need for close medical follow-up, and the potential emotional burden for all involved. It is important for patients to be provided with ample information to enable them to anticipate and understand their expected health-related QOL after the implantation of a durable LVAD.
The evolution of durable device technology over the past 2 decades has led to remarkable improvements in survival that are now in direct competition in the shorter term with HT. Survival at 2 years nationally averages 80% (equivalent to HT at 2 years), and the most recent MOMENTUM 3 (Multicenter Study of Maglev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial of the latest-generation LVAD demonstrated survival of nearly 60% at 5 years after implantation.68 Given the average mortality rate of 30% to 50% at 30 days with medical management of CS and the age limits set at most centers offering HT, durable LVAD therapy is a reasonable consideration for carefully selected older adults. Patients of any age presenting with CS requiring t-MCS have a higher mortality after durable LVAD. In an analysis of >13 000 patients with CS requiring either inotropes or t-MCS (INTERMACS [Interagency Registry for Mechanically Assisted Circulatory Support] profiles 1–3), survival at 1 year was 86% for those requiring t-MCS for stabilization compared with 91% for those supported pharmacologically. When survival was assessed by type of t-MCS, patients on venoarterial extracorporeal membrane oxygenation had the worst survival (88% at 1 year) after durable LVAD, and this held true after propensity matching (which included patient age).71 In contrast, patients stabilized with intra-aortic balloon counterpulsation or other t-MCS had a 1-year survival rate equivalent to that of individuals with severe HF stabilized with inotropes (91%).71
Suggestions for Clinical Practice
Evaluation of older adults being considered for durable LVAD should especially focus on the comorbidities of advanced HF, including frailty, end-organ dysfunction, malnutrition, and caregiver support.
Shared decision-making includes a review of the benefits, risks, and burdens associated with durable LVAD and options for palliative care with patients and caregivers.
Heart Transplantation
Advanced age has historically been viewed as a contraindication for HT; however, recent studies have shown similar survival outcomes in carefully selected older adults compared with younger patients undergoing HT.72 Based on these reassuring findings, the International Society for Heart Lung Transplantation guidelines state that patients in need can be considered for HT if they are ≤70 years of age (Class I, Level of Evidence C) and “carefully selected patients ≥70 years of age may be considered for HT” (Class IIb, Level of Evidence C).5 There is little to no guidance for older adults of extreme age (≥75 or 80 years of age). However, it is important to consider that for older patients who are of an extreme age for organ transplantation, their actuarial life expectancy has typically already been surpassed or will soon be reached, well before the expected graft survival of 15 years.
In the United States, urgent HT from the shock state is an increasingly common strategy among older adults. In 1990, only 3.4% of all HT recipients were ≥65 years old, increasing to 9.8% in 2000 and 18% in 2020. Relative to other age groups, contemporary rates of HT among adults ≥65 years of age have increased exponentially to >150 transplantations per 100 wait-list years, whereas rates of HT in other age ranges have remained mostly stable at ≤100 transplantations per 100 wait-list years.73 Pretransplantation wait-list demographics show the same upward trend in older adults; in 2020, 15.9% of all candidates were ≥65 years of age.73 Of great concern is the potential number of older patients who died while on the wait list who were eligible but did not accept LVAD therapy for definitive stabilization and recovery from acute shock. Although overall wait-list deaths are decreasing, wait-listed patients ≥65 years of age are demonstrating increased rates of wait-list deaths and an increase in death within 6 months of being removed from the wait list (regardless of the reason for removal).73
According to an analysis from the United Network for Organ Sharing/Organ Procurement and Transplantation Network database, highly selected older HT recipients had acceptable outcomes before the 2018 New Heart Allocation.74 Nevertheless, from 2011 to 2017, 86% of patients ≥65 years of age (7009 patients) were not hospitalized at the time of HT, representing an overall healthier cohort for which contemporary comparisons with urgent transplantation from a shock state might yield little guidance. Critical review of early to midterm outcomes of older patients transplanted at high urgency status after the 2018 allocation has been confounded by morbidity and mortality associated with COVID-19 infection, and published data on the incidence of patients ≥65 years of age transplanted as status 1 through 3 are lacking.
Evolving trends have indicated that fewer patients are undergoing durable LVAD as a bridge to transplantation, and the decision to offer durable LVAD to an older patient in shock effectively renders the patient destination therapy given deprioritization of stable patients with LVAD in the new allocation scheme. However, the time sensitivity inherent to the shock state can make decision-making difficult, with many turning to the use of semidurable support options (ie, t-MCS), which may allow time, reflection (team and patient/family), and the opportunity for ambulation and optimization of nutrition status before a more durable solution is considered. The confluence of transplantation trends, variable advanced therapy pathways, and the marked increase in older patients presenting in CS represents a new population of extremely ill patients undergoing a highly selective therapy, and guidelines and tailored management strategies (including immunosuppression) may require reconsideration. The incidence of older adults emergently placed on t-MCS being urgently evaluated for durable mechanical circulatory support or HT, many of whom need consideration for dual organ (eg, heart-kidney), warrants careful study.
Suggestions for Clinical Practice
Extending HT candidacy to older, often frail patients with multicomorbidities and under the conditions of intensive care and t-MCS support is challenging and controversial.
We concur with the International Society for Heart Lung Transplantation guidelines that patients can be considered for HT if they are ≤70 years of age, and carefully selected patients ≥70 years of age may be considered for HT when perceived benefits outweigh the potential risks.
Palliative and End-of-Life Care
Older adults should be considered for early integration of a palliative approach within their care, regardless of projected trajectory and eligibility for advanced therapies. Palliative services should be provided by an interdisciplinary team. The inclusion of consultative palliative care can play a crucial role in managing symptoms and facilitating shared decision-making and advance care planning, thereby incorporating patient and family preferences into specific goals and plans for end-of-life care. In the context of older adults in CS, regardless of the projected outcome, prompt offering of palliative care support should be standard practice within hospitals. This holds particular importance for individuals who are unlikely to benefit from advanced therapies, including those who are ineligible for device implantation or HT or those who choose not to undergo invasive interventions.75 Simultaneous provision of palliative care along with any advanced therapy is also essential because it not only aids in decision-making but also provides crucial support in the event of adverse outcomes.
Furthermore, when appropriate, referrals to inpatient or outpatient hospice care should be considered. Inpatient hospice can provide a dedicated and specialized environment for end-of-life care, ensuring that the patient’s needs are met comprehensively. Outpatient hospice care, on the other hand, allows patients to receive comfort-focused services while remaining in their own home or nonskilled care setting but requires that family or paid caregivers deliver daily care. The decision to refer a patient to either inpatient or outpatient hospice should be based on an individualized assessment of the patient’s clinical condition, preferences, and available resources. By integrating palliative care and considering appropriate hospice referral, health care professionals can optimize QOL for older adults facing critical illness or end-of-life situations.
Suggestion for Clinical Practice
Palliative or supportive care services are typically consulted early for older adults with CS, regardless of their projected trajectory or eligibility for advanced therapies.
FUTURE DIRECTIONS
Older adults constitute a large, heterogeneous demographic within the population of patients presenting with CS. A clear imbalance exists between CS prevalence in older adults and their representation within CS clinical trials and registries, limiting the ability to derive high-quality recommendations to guide CS care within this subgroup. Although specific recommendations pertaining to older adults are lacking, it is important to acknowledge that clinical practice guidelines do not explicitly prohibit older adults from receiving more intensive treatment. Hence, currently established age cutoffs used in clinical practice to determine eligibility for escalation of care among older adults in CS are mostly not supported by evidence.
Multiple opportunities exist to improve the care of older adults with CS. (1) Tools to better assess patients who may benefit from more aggressive treatment strategies despite older age are a major unmet need. It is fundamental to devise risk assessment tools specific to older adults that take into account patient-specific characteristics and facilitate clinical decision-making based on individualized risk and not rely on age as an isolated risk factor. (2) Representation of older adults in CS clinical trials and registries needs to be expanded to better guide clinical care recommendations specific to this patient demographic. (3) Meaningful end points specific to older adults should be considered. Although mortality is a relevant outcome to all patients with CS, other outcomes such as health-related QOL might be of particular importance among older adults when invasive therapies are considered in the setting of critical illness.
In the interim, timely recognition of shock, recognition of age-specific risk factors that might behave as risk modifiers in the older adult, and an interdisciplinary team approach to facilitate decision-making and to guide management are strongly suggested.
CONCLUSION
Data to support the development of clinical practice guidelines and recommendations for the diagnosis and management of the older adult in CS are lacking. The approach to management of CS in the older adult is nuanced and should take into account the heterogeneity of this population, including age-associated risks and individual goals of care. Throughout this scientific statement, we have provided suggestions for clinical practice, highlighting the crucial role of individualized risk assessment, an interdisciplinary approach, and patient-centered decision-making when determining escalation, de-escalation, and end-of-life care in the management of CS among older adults.
Supplementary Material
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on December 1, 2023, and the American Heart Association Executive Committee on January 26, 2024. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 215-356-2721 or email Meredith.Edelman@wolterskluwer.com
The American Heart Association requests that this document be cited as follows: Blumer V, Kanwar MK, Barnett CF, Cowger JA, Damluji AA, Farr M, Goodlin SJ, Katz JN, McIlvennan CK, Sinha SS, Wang TY; on behalf of the American Heart Association Cardiovascular Disease in Older Populations Committee of the Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Council on Cardiovascular Surgery and Anesthesia. Cardiogenic shock in older adults: a focus on age-associated risks and approach to management: a scientific statement from the American Heart Association. Circulation. 2024;149:e1051–e1065. doi: 10.1161/CIR.0000000000001214
The expert peer review of AHA-commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
Permissions: Multiple copies, modification, alteration, enhancement, and distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at https://www.heart.org/permissions. A link to the “Copyright Permissions Request Form” appears in the second paragraph (https://www.heart.org/en/about-us/statements-and-policies/copyright-request-form).
Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Vanessa Blumer | Inova Schar Heart and Vascular | None | None | None | None | None | None | None |
| Manreet Kanwar | Cardiovascular Institute at Allegheny Health Network | Cardiogenic Shock Working Group (Steering Committee member)* | None | Abiomed† | None | None | Abiomed†; CorWave (unpaid)*; Abbott* | None |
| Christopher Barnett | University of California San Francisco | None | None | None | None | None | None | None |
| Jennifer A. Cowger | Henry Ford Hospital | None | None | Zoll*; Bioventrix*; Abbott* | None | Procyrion* | Abbott†; Nuwellis (unpaid)*; Endotronix (unpaid)*; Bivacor (unpaid)*; Medtronic* | None |
| Abdulla A. Damluji | Johns Hopkins University Inova Center of Outcomes Research | None | None | None | None | None | None | None |
| Maryjane Farr | The University of Texas Southwestern Medical Center | None | None | None | None | None | None | None |
| Sarah J. Goodlin | Oregon Health and Sciences University, Patient-Centered Education and Research | None | None | None | None | None | None | None |
| Jason N. Katz | NYU Grossman School of Medicine & Bellevue Hospital Center | Abbott Corp* | None | None | None | None | None | None |
| Colleen K. McIlvennan | University of Colorado Cardiology | None | None | None | None | None | None | University of Colorado (associate professor)† |
| Shashank S. Sinha | Inova Schar Heart and Vascular, Inova Fairfax Medical Campus | None | None | None | None | None | None | None |
| Tracy Y. Wang | Duke Clinical Research Institute | None | None | None | None | None | None | None |
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Ann Gage | Cleveland Clinic Foundation | None | None | Abiomed†; Zoll Fellows Course* | None | None | None | None |
| Thomas C. Hanff | University of Utah | ISHLT/Abiomed (ISHLT Cardiogenic Shock Grant PI)* | None | None | None | None | None | None |
| Jaime A. Hernandez-Montfort | BSWH Health Advanced Heart Disease Program | None | None | Abiomed*; Abbott* | None | None | Abbott*; Abiomed* | None |
| Angela Lowenstern | Vanderbilt University | None | None | Edwards Lifesciences* | None | None | None | None |
| Van-Khue Ton | Massachusetts General Hospital and Harvard Medical School | None | None | None | None | None | None | None |
Contributor Information
Vanessa Blumer, Inova Schar Heart and Vascular.
Manreet K. Kanwar, Cardiovascular Institute at Allegheny Health Network.
Christopher F. Barnett, University of California San Francisco.
Jennifer A. Cowger, Henry Ford Hospital.
Abdulla A. Damluji, Johns Hopkins University Inova Center of Outcomes Research.
Maryjane Farr, The University of Texas Southwestern Medical Center.
Sarah J. Goodlin, Oregon Health and Sciences University, Patient-Centered Education and Research.
Jason N. Katz, NYU Grossman School of Medicine & Bellevue Hospital Center.
Colleen K. McIlvennan, University of Colorado Cardiology.
Shashank S. Sinha, Inova Schar Heart and Vascular, Inova Fairfax Medical Campus.
Tracy Y. Wang, Duke Clinical Research Institute.
REFERENCES
- 1.van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. ; on behalf of the American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. doi: 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 2.Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez-Montfort J, Zhang Y, Li B, Baca P, Dieng F, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol. 2022;80:185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 3.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 4.Kanwar M, Thayer KL, Garan AR, Hernandez-Montfort J, Whitehead E, Mahr C, Sinha SS, Vorovich E, Harwani NM, Zweck E, et al. Impact of age on outcomes in patients with cardiogenic shock. Front Cardiovasc Med. 2021;8:688098. doi: 10.3389/fcvm.2021.688098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, et al. ; International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/j.healun.2015.10.023 [DOI] [PubMed] [Google Scholar]
- 6.Damluji AA, van Diepen S, Katz JN, Menon V, Tamis-Holland JE, Bakitas M, Cohen MG, Balsam LB, Chikwe J; on behalf of the American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Surgery and Anesthesia; and Council on Cardiovascular and Stroke Nursing. Mechanical complications of acute myocardial infarction: a scientific statement from the American Heart Association. Circulation. 2021;144:e16–e35. doi: 10.1161/CIR.0000000000000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori M, Gupta A, Wang Y, Vahl T, Nazif T, Kirtane AJ, George I, Yong CM, Onuma O, Kodali S, et al. Trends in transcatheter and surgical aortic valve replacement among older adults in the United States. J Am Coll Cardiol. 2021;78:2161–2172. doi: 10.1016/j.jacc.2021.09.855 [DOI] [PubMed] [Google Scholar]
- 8.Rich MW, Chyun DA, Skolnick AH, Alexander KP, Forman DE, Kitzman DW, Maurer MS, McClurken JB, Resnick BM, Shen WK, et al. ; on behalf of the American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133:2103–2122. doi: 10.1161/CIR.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 9.Kalra S, Ranard LS, Memon S, Rao P, Garan AR, Masoumi A, O’Neill W, Kapur NK, Karmpaliotis D, Fried JA, et al. Risk prediction in cardiogenic shock: current state of knowledge, challenges and opportunities. J Card Fail. 2021;27:1099–1110. doi: 10.1016/j.cardfail.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 10.Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, Grines CL, Diercks DB, Hall S, Kapur NK, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol. 2022;79:933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 11.Damluji AA, Forman DE, van Diepen S, Alexander KP, Page RL 2nd, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, et al. ; on behalf of the American Heart Association Council on Clinical Cardiology and Council on Cardiovascular and Stroke Nursing. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation. 2020;141:e6–e32. doi: 10.1161/CIR.0000000000000741 [DOI] [PubMed] [Google Scholar]
- 12.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/nejm199908263410901 [DOI] [PubMed] [Google Scholar]
- 13.Basir MB, Pinto DS, Ziaeian B, Khandelwal A, Cowger J, Suh W, Althouse A. Mechanical circulatory support in acute myocardial infarction and cardiogenic shock: challenges and importance of randomized control trials. Catheter Cardiovasc Interv. 2021;98:1264–1274. doi: 10.1002/ccd.29593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tehrani BN, Truesdell AG, Psotka MA, Rosner C, Singh R, Sinha SS, Damluji AA, Batchelor WB. A standardized and comprehensive approach to the management of cardiogenic shock. JACC Heart Fail. 2020;8:879–891. doi: 10.1016/j.jchf.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jentzer JC, Burstein B, Van Diepen S, Murphy J, Holmes DR Jr, Bell MR, Barsness GW, Henry TD, Menon V, Rihal CS, et al. Defining shock and preshock for mortality risk stratification in cardiac intensive care unit patients. Circ Heart Fail. 2021;14:e007678. doi: 10.1161/CIRCHEARTFAILURE.120.007678 [DOI] [PubMed] [Google Scholar]
- 16.Zweck E, Thayer KL, Helgestad OKL, Kanwar M, Ayouty M, Garan AR, Hernandez-Montfort J, Mahr C, Wencker D, Sinha SS, et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10:e020085. doi: 10.1161/JAHA.120.020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jentzer JC, Schrage B, Holmes DR Jr, Dabboura S, Anavekar NS, Kirchhof P, Barsness GW, Blankenberg S, Bell MR, Westermann D. Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2021;10:604–612. doi: 10.1093/ehjacc/zuaa035 [DOI] [PubMed] [Google Scholar]
- 18.Berg DD, Bohula EA, Morrow DA. Epidemiology and causes of cardiogenic shock. Curr Opin Crit Care. 2021;27:401–408. doi: 10.1097/MCC.0000000000000845 [DOI] [PubMed] [Google Scholar]
- 19.Palacios Ordonez C, Garan AR. The landscape of cardiogenic shock: epidemiology and current definitions. Curr Opin Cardiol. 2022;37:236–240. doi: 10.1097/HCO.0000000000000957 [DOI] [PubMed] [Google Scholar]
- 20.Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, Harjola VP, Antohi EL, Arrigo M, Ben Gal T, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315–1341. doi: 10.1002/ejhf.1922 [DOI] [PubMed] [Google Scholar]
- 21.Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird-Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masoumi A, Rosenblum HR, Garan AR. Cardiogenic shock in older adults. Curr Cardiovasc Risk Rep. 2016;10:38. doi: 10.1007/s12170-016-0522-5 [DOI] [Google Scholar]
- 23.De Luca L, Olivari Z, Farina A, Gonzini L, Lucci D, Di Chiara A, Casella G, Chiarella F, Boccanelli A, Di Pasquale G, et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur J Heart Fail. 2015;17:1124–1132. doi: 10.1002/ejhf.339 [DOI] [PubMed] [Google Scholar]
- 24.Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Beaton AZ, Boehme AK, Buxton AE, et al. ; on behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2023 update: a report from the American Heart Association [published corrections appear in Circulation. 2023;147:e622 and Circulation. 2023;148:e4]. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123 [DOI] [PubMed] [Google Scholar]
- 25.Schmitt A, Weidner K, Rusnak J, Ruka M, Egner-Walter S, Mashayekhi K, Tajti P, Ayoub M, Akin I, Behnes M, et al. Age-related outcomes in patients with cardiogenic shock stratified by etiology. J Geriatr Cardiol. 2023;20:555–566. doi: 10.26599/1671-5411.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva-Cardoso J, Carubelli V, et al. ; CardShock Study Investigators. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17:501–509. doi: 10.1002/ejhf.260 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, et al. Predicting survival after ECMO for refractory cardiogenic shock: the Survival After VenoArterial-ECMO (SAVE)-score. Eur Heart J. 2015;36:2246–2256. doi: 10.1093/eurheartj/ehv194 [DOI] [PubMed] [Google Scholar]
- 28.Muller G, Flecher E, Lebreton G, Luyt CE, Trouillet JL, Brechot N, Schmidt M, Mastroianni C, Chastre J, Leprince P, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42:370–378. doi: 10.1007/s00134-016-4223-9 [DOI] [PubMed] [Google Scholar]
- 29.Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. ; CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 30.Andersen LW, Bivens MJ, Giberson T, Giberson B, Mottley JL, Gautam S, Salciccioli JD, Cocchi MN, McNally B, Donnino MW. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation. 2015;94:49–54. doi: 10.1016/j.resuscitation.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 31.Ijaz N, Buta B, Xue QL, Mohess DT, Bushan A, Tran H, Batchelor W, deFilippi CR, Walston JD, Bandeen-Roche K, et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tehrani BN, Truesdell AG, Sherwood MW, Desai S, Tran HA, Epps KC, Singh R, Psotka M, Shah P, Cooper LB, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol. 2019;73:1659–1669. doi: 10.1016/j.jacc.2018.12.084 [DOI] [PubMed] [Google Scholar]
- 33.Allen LA, McIlvennan CK, Thompson JS, Dunlay SM, LaRue SJ, Lewis EF, Patel CB, Blue L, Fairclough DL, Leister EC, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial. JAMA Intern Med. 2018;178:520–529. doi: 10.1001/jamainternmed.2017.8713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backman WD, Levine SA, Wenger NK, Harold JG. Shared decision-making for older adults with cardiovascular disease. Clin Cardiol. 2020;43:196–204. doi: 10.1002/clc.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lum HD, Sudore RL, Bekelman DB. Advance care planning in the elderly. Med Clin North Am. 2015;99:391–403. doi: 10.1016/j.mcna.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 36.Aghabarary M, Dehghan Nayeri N. Medical futility and its challenges: a review study. J Med Ethics Hist Med. 2016;9:11. [PMC free article] [PubMed] [Google Scholar]
- 37.de la Paz A, Orgel R, Hartsell SE, Pauley E, Katz JN. Getting cardiogenic shock patients to the right place: how initial intensive care unit triage decisions impact processes of care and outcomes. Am Heart J. 2020;230:66–70. doi: 10.1016/j.ahj.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 38.Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail. 2019;25:364–371. doi: 10.1016/j.cardfail.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 39.Kanwar MK, Blumer V, Zhang Y, Sinha SS, Garan AR, Hernandez-Montfort J, Khalif A, Hickey GW, Abraham J, Mahr C, et al. Pulmonary artery catheter use and risk of in-hospital death in heart failure cardiogenic shock. J Card Fail. 2023;29:1234–1244. doi: 10.1016/j.cardfail.2023.05.001 [DOI] [PubMed] [Google Scholar]
- 40.Alviar CL, Miller PE, McAreavey D, Katz JN, Lee B, Moriyama B, Soble J, van Diepen S, Solomon MA, Morrow DA; ACC Critical Care Cardiology Working Group. Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72:1532–1553. doi: 10.1016/j.jacc.2018.06.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, et al. ; Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345 [DOI] [PubMed] [Google Scholar]
- 42.Feng Y, Amoateng-Adjepong Y, Kaufman D, Gheorghe C, Manthous CA. Age, duration of mechanical ventilation, and outcomes of patients who are critically ill. Chest. 2009;136:759–764. doi: 10.1378/chest.09-0515 [DOI] [PubMed] [Google Scholar]
- 43.van Diepen S, Tymchak W, Bohula EA, Park JG, Daniels LB, Phreaner N, Barnett CF, Kenigsberg BB, DeFilippis A, Singam NS, et al. ; Critical Care Cardiology Trials Network Investigators. Incidence, underlying conditions, and outcomes of patients receiving acute renal replacement therapies in tertiary cardiac intensive care units: an analysis from the Critical Care Cardiology Trials Network Registry. Am Heart J. 2020;222:8–14. doi: 10.1016/j.ahj.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 44.Soni SS, Nagarik AP, Adikey GK, Raman A. Using continuous renal replacement therapy to manage patients of shock and acute renal failure. J Emerg Trauma Shock. 2009;2:19–22. doi: 10.4103/0974-2700.44678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conroy M, O’Flynn J, Marsh B. Mortality and long-term dialysis requirement among elderly continuous renal replacement therapy patients in a tertiary referral intensive care unit. J Intensive Care Soc. 2019;20:138–143. doi: 10.1177/1751143718784868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosansky SJ, Schell J, Shega J, Scherer J, Jacobs L, Couchoud C, Crews D, McNabney M. Treatment decisions for older adults with advanced chronic kidney disease. BMC Nephrol. 2017;18:200. doi: 10.1186/s12882-017-0617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoniucci D, Valenti R, Migliorini A, Moschi G, Parodi G, Dovellini EV, Bolognese L, Santoro GM. Comparison of impact of emergency percutaneous revascularization on outcome of patients > or =75 to those < 75 years of age with acute myocardial infarction complicated by cardiogenic shock. Am J Cardiol. 2003;91:1458–1461, A6. doi: 10.1016/s0002-9149(03)00397-7 [DOI] [PubMed] [Google Scholar]
- 48.Dauerman HL, Ryan TJ Jr, Piper WD, Kellett MA, Shubrooks SJ, Robb JF, Hearne MJ, Watkins MW, Hettleman BD, Silver MT, et al. Outcomes of percutaneous coronary intervention among elderly patients in cardiogenic shock: a multicenter, decade-long experience. J Invasive Cardiol. 2003;15:380–384. [PubMed] [Google Scholar]
- 49.Prasad A, Lennon RJ, Rihal CS, Berger PB, Holmes DR Jr. Outcomes of elderly patients with cardiogenic shock treated with early percutaneous revascularization. Am Heart J. 2004;147:1066–1070. doi: 10.1016/j.ahj.2003.07.030 [DOI] [PubMed] [Google Scholar]
- 50.Dzavik V, Sleeper LA, Picard MH, Sanborn TA, Lowe AM, Gin K, Saucedo J, Webb JG, Menon V, Slater JN, et al. ; SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK Investigators. Outcome of patients aged ≥75 years in the SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK (SHOCK) trial: do elderly patients with acute myocardial infarction complicated by cardiogenic shock respond differently to emergent revascularization? Am Heart J. 2005;149:1128–1134. doi: 10.1016/j.ahj.2005.03.045 [DOI] [PubMed] [Google Scholar]
- 51.White HD, Assmann SF, Sanborn TA, Jacobs AK, Webb JG, Sleeper LA, Wong C-K, Stewart JT, Aylward PEG, Wong S-C, et al. Comparison of percutaneous coronary intervention and coronary artery bypass grafting after acute myocardial infarction complicated by cardiogenic shock. Circulation. 2005;112:1992–2001. doi: 10.1161/CIRCULATIONAHA.105.540948 [DOI] [PubMed] [Google Scholar]
- 52.Migliorini A, Moschi G, Valenti R, Parodi G, Dovellini EV, Carrabba N, Buonamici P, Antoniucci D. Routine percutaneous coronary intervention in elderly patients with cardiogenic shock complicating acute myocardial infarction. Am Heart J. 2006;152:903–908. doi: 10.1016/j.ahj.2005.12.030 [DOI] [PubMed] [Google Scholar]
- 53.Lim HS, Farouque O, Andrianopoulos N, Yan BP, Lim CCS, Brennan AL, Reid CM, Freeman M, Charter K, Black A, et al. ; Melbourne Interventional Group. Survival of elderly patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock. JACC Cardiovasc Interv. 2009;2:146–152. doi: 10.1016/j.jcin.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 54.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. ; IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 55.Damluji AA, Bandeen-Roche K, Berkower C, Boyd CM, Al-Damluji MS, Cohen MG, Forman DE, Chaudhary R, Gerstenblith G, Walston JD, et al. Percutaneous coronary intervention in older patients with ST-segment elevation myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2019;73:1890–1900. doi: 10.1016/j.jacc.2019.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ratcovich HL, Josiassen J, Helgestad OKL, Linde L, Jensen LO, Ravn HB, Joshi FR, Engstrøm T, Schmidt H, Hassager C, et al. Outcome in elderly patients with cardiogenic shock complicating acute myocardial infarction. Shock. 2022;57:327–335. doi: 10.1097/SHK.0000000000001837 [DOI] [PubMed] [Google Scholar]
- 57.Kumar S, McDaniel M, Samady H, Forouzandeh F. Contemporary revascularization dilemmas in older adults. J Am Heart Assoc. 2020;9:e014477. doi: 10.1161/JAHA.119.014477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goyal P, Kwak MJ, Al Malouf C, Kumar M, Rohant N, Damluji AA, Denfeld QE, Bircher KK, Krishnaswami A, Alexander KP, et al. Geriatric cardiology: coming of age. JACC Adv. 2022;1:10070. doi: 10.1016/j.jacadv.2022.100070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Damluji AA, Tehrani B, Sinha SS, Samsky MD, Henry TD, Thiele H, West NEJ, Senatore FF, Truesdell AG, Dangas GD, et al. Position statement on vascular access safety for percutaneous devices in AMI complicated by cardiogenic shock. JACC Cardiovasc Interv. 2022;15:2003–2019. doi: 10.1016/j.jcin.2022.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e18–e114. doi: 10.1161/CIR.0000000000001038 [DOI] [PubMed] [Google Scholar]
- 61.Mehta RH, Grab JD, O’Brien SM, Glower DD, Haan CK, Gammie JS, Peterson ED; Society of Thoracic Surgeons National Cardiac Database Investigators. Clinical characteristics and in-hospital outcomes of patients with cardiogenic shock undergoing coronary artery bypass surgery. Circulation. 2008;117:876–885. doi: 10.1161/CIRCULATIONAHA.107.728147 [DOI] [PubMed] [Google Scholar]
- 62.Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS; NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294:448–454. doi: 10.1001/jama.294.4.448 [DOI] [PubMed] [Google Scholar]
- 63.Acharya D, Gulack BC, Loyaga-Rendon RY, Davies JE, He X, Brennan JM, Thourani VH, Williams ML. Clinical characteristics and outcomes of patients with myocardial infarction and cardiogenic shock undergoing coronary artery bypass surgery: data from the Society of Thoracic Surgeons national database. Ann Thorac Surg. 2016;101:558–566. doi: 10.1016/j.athoracsur.2015.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santarpino G, Ruggieri VG, Mariscalco G, Bounader K, Beghi C, Fischlein T, Onorati F, Faggian G, Gatti G, Pappalardo A, et al. Outcome in patients having salvage coronary artery bypass grafting. Am J Cardiol. 2015;116:1193–1198. doi: 10.1016/j.amjcard.2015.07.034 [DOI] [PubMed] [Google Scholar]
- 65.Kolte D, Khera S, Vemulapalli S, Dai D, Heo S, Goldsweig AM, Aronow HD, Elmariah S, Inglessis I, Palacios IF, et al. Outcomes following urgent/emergent transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2018;11:1175–1185. doi: 10.1016/j.jcin.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 66.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published corrections appear in Circulation. 2021;143:e229, Circulation. 202e;148:e8, and Circulation. 2023;148:e185]. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 67.Saeed D, Feldman D, Banayosy AE, Birks E, Blume E, Cowger J, Hayward C, Jorde U, Kremer J, MacGowan G, et al. The 2023 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: a 10-year update. J Heart Lung Transplant. 2023;42:e1–e222. doi: 10.1016/j.healun.2022.12.004 [DOI] [PubMed] [Google Scholar]
- 68.Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, Naka Y, Horstmanshof D, Chuang J, Wang A, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. JAMA. 2022;328:1233–1242. doi: 10.1001/jama.2022.16197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salna M, Takeda K, Kurlansky P, Ikegami H, Fan L, Han J, Stein S, Topkara V, Yuzefpolskaya M, Colombo PC, et al. The influence of advanced age on venous-arterial extracorporeal membrane oxygenation outcomes. Eur J Cardiothorac Surg. 2018;53:1151–1157. doi: 10.1093/ejcts/ezx510 [DOI] [PubMed] [Google Scholar]
- 70.Cowger JA, Grafton G. Candidate selection for durable mechanical circulatory support. Cardiol Clin. 2018;36:487–494. doi: 10.1016/j.ccl.2018.06.003 [DOI] [PubMed] [Google Scholar]
- 71.Ton VK, Xie R, Hernandez-Montfort JA, Meyns B, Nakatani T, Yanase M, Shaw S, Pettit S, Netuka I, Kirklin J, et al. Short- and long-term adverse events in patients on temporary circulatory support before durable ventricular assist device: an IMACS registry analysis. J Heart Lung Transplant. 2020;39:342–352. doi: 10.1016/j.healun.2019.12.011 [DOI] [PubMed] [Google Scholar]
- 72.Daneshvar D, Czer LS, Phan A, Schwarz ER, De Robertis M, Mirocha J, Rafiei M, Pixton JR, Kass RM, Trento A. Heart transplantation in patients aged 70 years and older: a two-decade experience. Transplant Proc. 2011;43:3851–3856. doi: 10.1016/j.transproceed.2011.08.086 [DOI] [PubMed] [Google Scholar]
- 73.Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Bradbrook K, Gauntt K, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2020 annual data report: heart. Am J Transplant. 2022;22:350–437. doi: 10.1111/ajt.16977 [DOI] [PubMed] [Google Scholar]
- 74.Cooper LB, Lu D, Mentz RJ, Rogers JG, Milano CA, Felker GM, Hernandez AF, Patel CB. Cardiac transplantation for older patients: characteristics and outcomes in the septuagenarian population. J Heart Lung Transplant. 2016;35:362–369. doi: 10.1016/j.healun.2015.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill L, Prager Geller T, Baruah R, Beattie JM, Boyne J, de Stoutz N, Di Stolfo G, Lambrinou E, Skibelund AK, Uchmanowicz I, et al. Integration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper. Eur J Heart Fail. 2020;22:2327–2339. doi: 10.1002/ejhf.1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


