Abstract
Background
The prognosis of malignant primary high-grade brain tumors, predominantly glioblastomas, is poor despite intensive multimodality treatment options. In more than 50% of patients with glioblastomas, potentially targetable mutations are present, including rearrangements, altered splicing, and/or focal amplifications of epidermal growth factor receptor (EGFR) by signaling through the RAF/RAS pathway. We studied whether treatment with the clinically available anti-EGFR monoclonal antibody panitumumab provides clinical benefit for patients with RAF/RAS-wild-type (wt) glioblastomas in the Drug Rediscovery Protocol (DRUP).
Methods
Patients with progression of treatment refractory RAF/RASwt glioblastoma were included for treatment with panitumumab in DRUP when measurable according to RANO criteria. The primary endpoints of this study are clinical benefit (CB: defined as confirmed objective response [OR] or stable disease [SD] ≥ 16 weeks) and safety. Patients were enrolled using a Simon-like 2-stage model, with 8 patients in stage 1 and up to 24 patients in stage 2 if at least 1 in 8 patients had CB in stage 1.
Results
Between 03-2018 and 02-2022, 24 evaluable patients were treated. CB was observed in 5 patients (21%), including 2 patients with partial response (8.3%) and 3 patients with SD ≥ 16 weeks (12.5%). After median follow-up of 15 months, median progression-free survival and overall survival were 1.7 months (95% CI 1.6-2.1 months) and 4.5 months (95% CI 2.9-8.6 months), respectively. No unexpected toxicities were observed.
Conclusions
Panitumumab treatment provides limited CB in patients with recurrent RAF/RASwt glioblastoma precluding further development of this therapeutic strategy.
Keywords: glioblastoma, RAF/RAS-wildtype, panitumumab, precision medicine, DRUP trial
New treatment options are needed for patients with recurrent glioblastomas. This study focused on whether treatment with the clinically available anti-EGFR monoclonal antibody panitumumab provides clinical benefit for patients with RAF/RAS -wild-type glioblastomas in the Drug Rediscovery Protocol.
Implications for Practice.
There is an unmet need for new effective treatment options for patients with recurrent glioblastomas. In 57% of patients with glioblastomas, targetable alterations in EGFR were found known to signal through the RAF/RAS pathway. However, targeting EGFR failed in patients with glioblastomas previously, potentially due to clinical trial design without target selection of RAF/RAS-wild-type tumors. In this study, we show that despite selection of patients with RAF/RAS-wild-type glioblastomas, EGFR-targeted therapy (panitumumab) is not effective. Future research should focus on the delivery of drugs through the blood-brain barrier and unravelling resistance mechanisms by broader genomic evaluation.
Introduction
Glioblastomas are the most common primary malignant brain tumors in adults, with an annual incidence of 700 patients per year in the Netherlands.1 Although aggressive first-line treatment consisting of maximal surgical resection followed by radiation with concomitant and adjuvant temozolomide, the prognosis of patients with glioblastomas remains poor.2 Five years survival is <5% and almost all glioblastomas locally recur after first-line treatment.3 Treatment options for patients with recurrent glioblastomas include re-surgery, re-irradiation, systemic therapy, and best supportive care. The role of repeated surgery or radiotherapy is controversial.4 The recommended second-line systemic therapy for patients with recurrent glioblastomas in the United States is bevacizumab since its approval in 2009 by the Food and Drug Administration (FDA) based on the results of two uncontrolled phase II trials with median progression-free survival (PFS) and median overall survival (OS) of 4.2 and 9.2 months, respectively.5,6 However, in Europe lomustine is the recommended second-line chemotherapy based on a randomized trial which showed no survival advantage of treatment with lomustine plus bevacizumab over treatment with lomustine alone in patients with progressive glioblastomas.7 This trial showed a median PFS of lomustine treatment alone in patients with glioblastomas of 1.5 months and a median OS of 8.6 months. Therefore, adequate treatment options for patients with recurrent glioblastomas is lacking, and development of new treatment strategies is warranted.8,9
Thus far, various factors may have contributed to the failure of new treatment strategies, including a high degree of tumor heterogeneity, the blood-brain barrier (BBB), and a severely immunosuppressive microenvironment.10 In 2016, a new World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) was implemented.11 In this guideline molecular parameters were incorporated into the classification of CNS tumors instead of diagnosis based on histology solely. This implementation in combination with the recent advances in genomic technology with large-scale molecular profiling of glioblastomas have led to a better understanding of their molecular landscape.12 When studying the genomic landscape of glioblastomas, 57% showed evidence of potential targetable mutations, rearrangements, altered splicing, and/or focal amplifications of epidermal growth factor receptor (EGFR).13EGFR, a receptor tyrosine kinase, was discovered as a proto-oncogene approximately 4 decades ago.14 Expression of EGFR variant III (ie, deletion of exons 2-7 of the EGFR gene), the most common EGFR mutation in patients with glioblastomas, has only been observed in tumors and not in normal tissue, suggesting that it could be a promising candidate for targeted therapy.15
EGFR-targeting antibodies have been clinically approved for the treatment of a wide variety of cancers.16 So far, efficacy data of clinical trials with anti-EGFR antibodies in patients with glioblastomas do not seem to be superior to results of the bevacizumab and lomustine trials.17-19 A phase II clinical trial in which patients with recurrent glioblastomas were treated with cetuximab in combination with bevacizumab and irinotecan showed a response rate of 26%.17 The same response rates were found for combination of bevacizumab and irinotecan. A phase II study, in which patients with recurrent glioblastomas were treated with cetuximab alone, reported a clinical benefit rate (CBR) of 35%; 3 out of 55 patients had a partial response (5.5%) and 16 patients had stable disease (29.6%). The median time to progression was 1.9 months and the PFS was shorter than 6 months in the majority of patients (50/55, 91%).18 Nimotuzumab was evaluated in a randomized phase III trial in which it was added to standard therapy for newly diagnosed glioblastomas.19 This trial failed to show additional benefit of nimotuzumab combined with standard therapy in this patient group. In all these trials, no correlation between EGFR overexpression and response or survival was found. This is in line with earlier research in patients with colorectal cancer revealing that EGFR expression is not predictive for response to anti-EGFR therapy.20 However, in other tumor types, especially colorectal cancer, it is well known that mutations in KRAS, NRAS, and BRAF genes are associated with poor outcome following anti-EGFR therapy and therefore these mutations could be used to exclude patients from treatment with anti-EGFR therapy.
The antibody-drug conjugate Depatux-M, directed against activated EGFR, as monotherapy also failed to show benefit for patients with recurrent EGFR amplified glioblastomas in a randomized controlled phase II trial. In the INTELLANCE 1 phase III study this antibody-drug conjugate was added to standard chemo-irradiation with temozolomide in patients with newly diagnosed EGFR amplified glioblastomas. Due to futility this trial was discontinued after an interim analyses.21
Panitumumab is an EGFR-targeting antibody approved by the FDA and European Medicines Agency (EMA) for treatment in wild-type (wt) RAS (no mutations in either KRAS or NRAS) metastatic colorectal cancer as first-line therapy in combination with folinic acid, fluorouracil, and oxaliplatin (FOLFOX) or in combination with folinic acid, fluorouracil, and irinotecan (FOLFIRI), in second-line in combination with FOLFIRI for patients who have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan), or as monotherapy following disease progression after prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy.22
In the ongoing Drug Rediscovery Protocol (DRUP, NCT02925234), patients with advanced cancer who have exhausted all standard of care options are being treated based on their tumor molecular profile with registered targeted treatments outside their labeled indications, systematically recording efficacy and safety data.23 In the present article, we describe treatment outcomes of a completed DRUP cohort, in which patients with treatment refractory RAF/RASwt recurrent glioblastomas were treated with panitumumab. In this cohort, patients were included based on the WHO Classification of Tumors of the CNS 2016.11 In this classification, IDH-mutant diffuse astrocytic tumors could be identified as glioblastomas based on histological parameters and therefore included in this cohort. However, in the current WHO Classification of Tumors of the CNS these tumors are considered as astrocytoma’s and graded as grade 2, 3, or 4 based on histological and genetic parameters.24 We do realize that these tumors have a different prognosis and therefore an additional exploratory analysis was performed.
Methods
Study Design
DRUP is an ongoing prospective, multicenter, non-randomized basket and umbrella trial in which patients with advanced or metastatic solid tumors, multiple myeloma, or non-Hodgkin lymphoma, who have exhausted all standard of care options, are being treated based on their tumor molecular profile, with targeted- or immunotherapy outside their registered indications. Patients are enrolled in multiple parallel cohorts, based either on tumor type combined with molecular alteration and study drug (umbrella design) or solely on molecular alteration and study drug in a tumor-agnostic cohort (basket design). Patients enrolled in the cohort “Panitumumab for RAF/RASwt glioblastomas” received 6 mg/kg panitumumab intravenously (iv) every 2 weeks until occurrence of disease progression or intolerable side effects. Dose reductions were allowed up to a minimum of 3.6 mg/kg every 2 weeks. Patients were enrolled in 9 out of 35 DRUP-participating hospitals in the Netherlands, between March 2018 and January 2022. To date, accrual in other cohorts of DRUP is still ongoing.
DRUP was approved by the Medical Ethical Committee of the Netherlands Cancer Institute in Amsterdam and is conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki’s ethical principles for medical research. The study included only adults aged ≥18 years and written informed consent was obtained from all the subjects participating in the study.
This study is registered with ClinicalTrials.gov, number NCT02925234.
Patients
Eligible patients had at least clinical and radiological evidence for refractory RAF/RASwt recurrent glioblastoma with molecular testing demonstrating no mutations in either BRAF, KRAS, or NRAS and stable or decreasing dosage of steroids for at least 7 days prior to the baseline magnetic resonance imaging (MRI). Molecular tests were performed before inclusion within DRUP and therefore not included in this trial. All molecular tests for BRAF, KRAS, or NRAS mutations were accepted, performed on new biopsies, or archived tumor material obtained from primary resection material.
Patients had progressive measurable disease according to Response Assessment in Neuro-Oncology (RANO25) and an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2. Furthermore, patients had normal organ and bone marrow function measured within 4 weeks prior to administration of study treatment and agreed to use adequate contraception for the duration of the study treatment, and for 4 months thereafter. Patients who required anti-convulsant therapy had to take non-enzyme inducing antiepileptic drugs (non-EIAED); EIAED were prohibited. Patients previously on EIAED had to be switched to non-EIAED at least 2 weeks prior to start of treatment. Patients were excluded for treatment with panitumumab if they had radiotherapy within 3 months prior to the diagnosis of progression or with a dose over 65 Gy. Additional exclusion criteria included: known hypersensitivity to panitumumab, history of interstitial pneumonitis, pulmonary fibrosis, clinically significant preexisting cardiac conditions or stroke, or acute myocardial infarction within 2 months before the first dose of study treatment; ongoing toxicity of grade 2 or higher (other than alopecia) according to “Common Terminology Criteria for Adverse Events (CTCAE, version 4.03)”, caused by previous treatments; concomitant treatment with any other anti-cancer therapy; presence of any other clinically significant medical condition which made it undesirable to participate in the study. Patients were considered evaluable for the primary endpoint if at least two treatment administrations of intravenous medication were completed. Non-evaluable patients were replaced and were excluded from efficacy analysis.
Study Endpoints
The primary endpoints of this study are clinical benefit (CB), defined as confirmed complete or partial response (CR/PR) or stable disease (SD) for 16 weeks or more, according to RANO (measured at least twice, at least 28 days apart), and treatment-related grade ≥ 3 adverse events (AEs) and serious adverse events (SAEs). Tumor response was reported by the local investigator in the electronic case record form (eCRF). MRI for tumor response assessment was performed at baseline and after every fourth treatment cycle (ie, every 8 weeks). If study treatment was continued after 3 response evaluations (ie, 24 weeks), response evaluations were performed at the end of every sixth treatment cycle (ie, every 12 weeks). Secondary endpoints included: objective response rate (ORR, defined as PR or CR), duration of response (DoR), PFS, and OS. Safety was measured by the frequency of treatment-related grade ≥ 3 AEs and SAEs occurring up to 30 days after the last dose of study drug. All AEs were graded according to the CTCAE v4.03. Safety within the trial is monitored by an Independent Data Monitoring Committee (IDMC) that is blinded for response rates per cohort during accrual.
Statistical Analysis
Cohorts in DRUP are monitored using a Simon-like 2-stage “admissible” monitoring plan to identify cohorts with evidence of activity.26 If there were no patients with CB in the first 8 participants in the cohort, the cohort would be closed. Otherwise, an additional 16 patients would be included in the cohort. If 5 or more patients met the definition of CB, further investigation would be warranted. The null hypothesis and alternative hypothesis to be tested were defined as CBR of 10% versus ≥30%. This monitoring rule had 85% power to reject the null hypothesis of a CBR 10% when the true CBR is 30%, with a one-sided alpha error rate of 7.8%. Exact 95% CIs were calculated using the Clopper-Pearson method. All statistical analyses were performed using R version 4.0.3 (https://www.R-project.org). Patient characteristics, AEs, and tumor responses were summarized using descriptive statistics. A waterfall plot was used to illustrate maximum tumor shrinkage compared to baseline. Kaplan-Meier methods were used to estimate PFS (from start treatment to progression or death from any cause, whichever came first, and censoring patients alive without progression) and OS (calculated from the first day of treatment administration to the date of death from any cause, censoring patients who were alive at follow up).
Role of Funding Source
This investigator-initiated study receives funding from the Dutch Cancer Society (KWF), Stelvio for Life Foundation and receives equal funding from multiple pharmaceutical companies, including Amgen. Study medication was made available, free of charge, by the manufacturer. Amgen had no role in the design or execution of the study and no influence on the study report.
Results
Patients
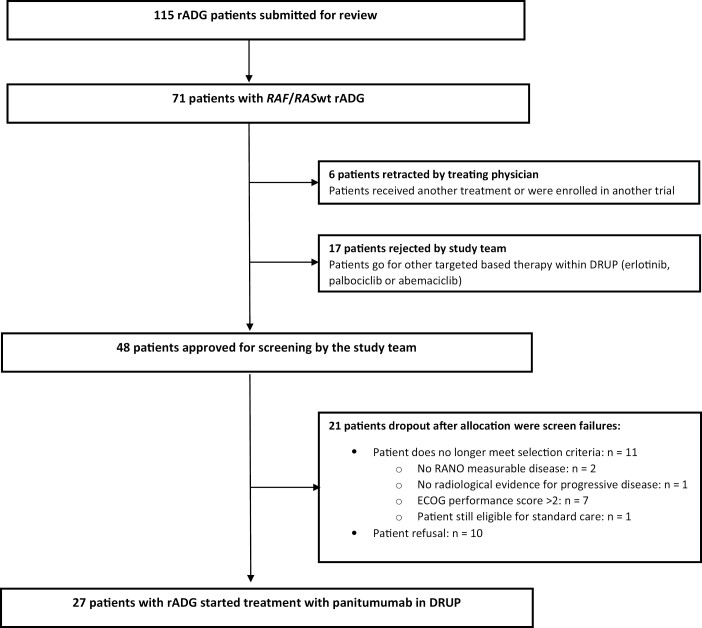
Between March 2018 and January 2022, 115 patients with recurrent adult-type diffuse gliomas who had exhausted standard treatment options were submitted to the study team for evaluation of potential study participation. Seventy-one patients (65%) had a RAF/RASwt recurrent adult-type diffuse glioma, of which 48 patients (67%) were approved by the study team to be screened for treatment with panitumumab. After screening, 27 patients (56%) were found eligible and started study treatment (Fig. 1). All 27 patients that started study treatment were included for baseline characteristics and safety analysis. Among these 27 patients, 3 patients were not evaluable for the primary endpoint and therefore excluded for the efficacy analysis. Two patients received only one complete treatment cycle due to rapid clinical deterioration and one patient had a baseline scan older than the maximum of 28 days. Baseline characteristics of the patients that were included are presented in Table 1.
Figure 1.
Flowchart of patients submitted to the study team and reasons for non-accrual. Abbreviations: rADG, recurrent adult-type diffuse gliomas; RAF/RASwt, RAF/RAS wild-type.
Table 1.
Baseline characteristics of patients enrolled in the cohort “panitumumab for patients with RAF/RASwt recurrent glioblastoma.”
| Characteristics | No. of patients (%) |
|---|---|
| Median age, years (range) | 54.5 (35-73) |
| Gender | |
| Male | 19 (70%) |
| Female | 8 (30%) |
| WHO performance status | |
| WHO 0 | 6 (22%) |
| WHO 1 | 18 (67%) |
| WHO 2 | 1 (4%) |
| Unknown | 2 (7%) |
| Prior lines of systemic therapy after SoC (“temozolomide/radiotherapy”) 2 | |
| 0 | 16 (59%) |
| 1 | 8 (30%) |
| 2 | 3 (11%) |
| WHO classification 23 | |
| IDHwt glioblastoma | 23 (85%) |
| IDH mutant, grade 3 astrocytoma | 2 (7.5%) |
| IDH mutant, grade 4 astrocytoma | 2 (7.5%) |
| Median time from first diagnosis to start study treatment, months (range) | 14 (5-197) |
| Prognostic factors | |
| 1.MGMT promoter methylation | |
| Yes | 3 (11%) |
| No | 14 (52%) |
| Unknown | 10 (37%) |
| 2. IDH1/2 mutation | |
| Yes | 4 (15%) |
| No | 23 (85%) |
Abbreviations: NO, number; WHO, World Health Organization; SoC, Standard of Care
Most of the evaluable patients (18/24, 75%) were included based on their primary diagnosis and had clinical and radiological progression; the other 6 (25%) patients had histologically proven recurrent glioblastoma. Pathological revision was performed to classify the tumors of the patients according to the fifth WHO Classification of Tumors of the CNS, the current leading guideline, while most patients were included based on older histologic diagnosis.24 After revision of the pathology diagnosis, most of the included patients had a glioblastoma, IDH wt (n = 19, 79%). There were 2 patients with astrocytoma, IDH mutant grade 3 and 2 patients with astrocytoma, IDH mutant grade 2. These 4 patients were included based on their primary diagnosis and showed clinical and radiological evidence for dedifferentiation towards grade 4 tumors and were therefore included in this cohort. For one patient revision of pathology was not possible due to missing material.
Median time from diagnosis to start study treatment was 14 months (range 5-197 months). Patients had a median age of 54.5 years (range 35-73) and 70% (N = 19) of the patients were men. Most patients (n = 25) received the standard treatment schedule, consisting of radiotherapy with concomitant and adjuvant temozolomide,2 two patients did not receive radiation therapy due to very extensive disease and were treated with only temozolomide. Eleven patients had prior second-line palliative systemic treatment of which 8 patients received bevacizumab, lomustine monotherapy, or in combination with procarbazine or hydroxyurea and 3 patients participated in a clinical trial with experimental therapy. There were no patients who received any other EGFR inhibitor drug prior to inclusion. There were no significant differences in baseline characteristics between patients with CB and without CB, as depicted in Supplementary Table S1.
Clinical Benefit and Survival
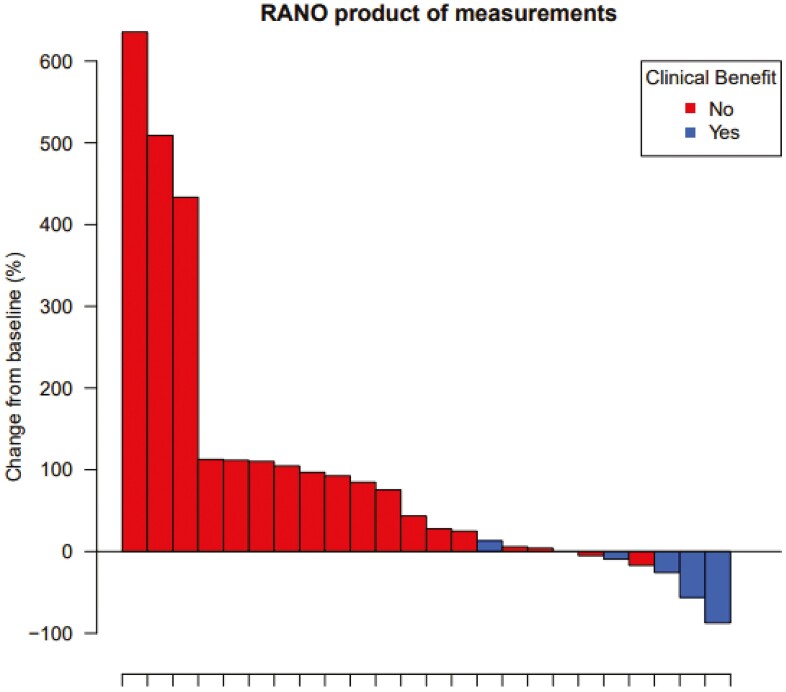
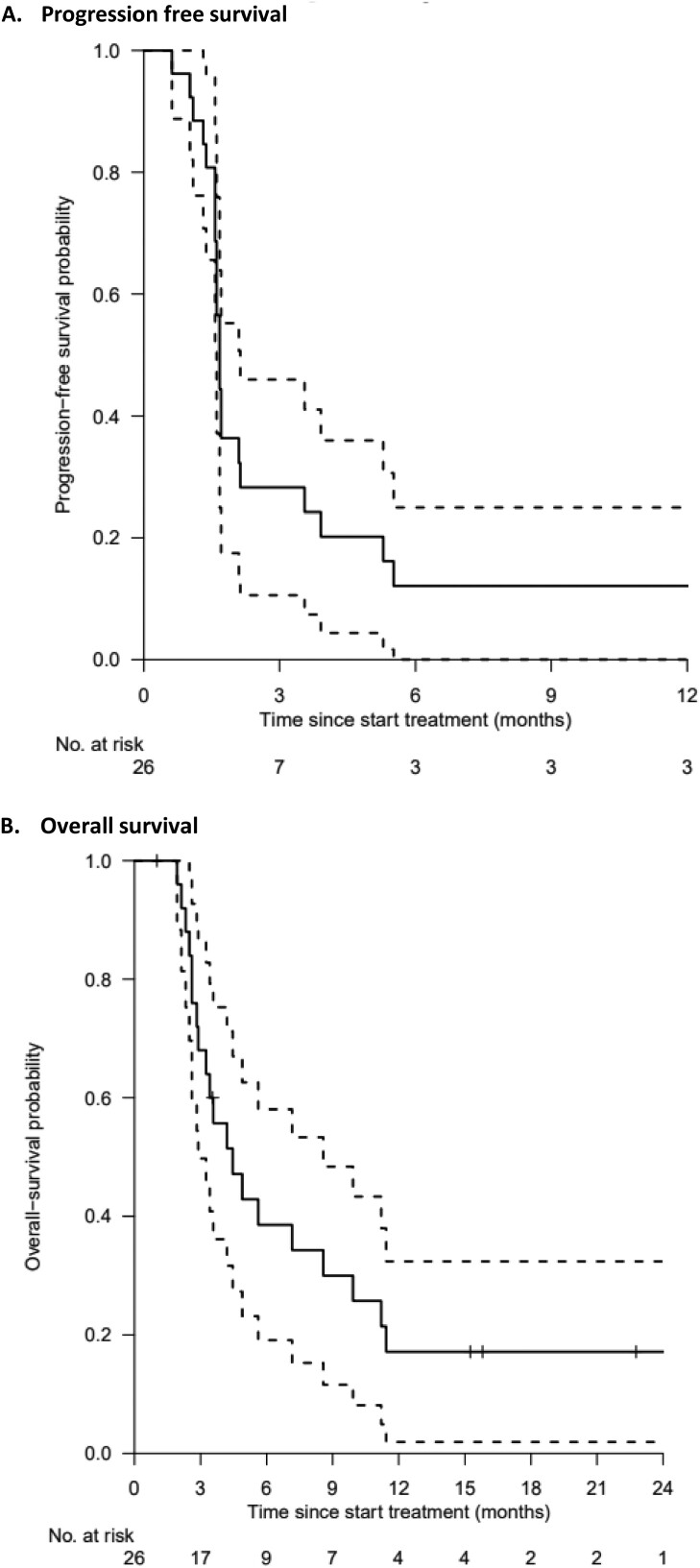
At data cutoff in August 2022, the median follow-up was 15.8 months (95% CI 15.2-NA months). The main reason for treatment discontinuation was progressive disease (n = 22, 81%). Three patients discontinued treatment due to symptomatic deterioration (12%) and the other 2 patients were still on study treatment. Of the 24 evaluable patients, 21% had CB (n = 5) upon treatment with panitumumab. Two patients achieved a PR and 3 patients had SD at 16 weeks. Figure 2 depicts the greatest changes in the sum of target lesions for each patient. The median PFS and OS were 1.7 months (95% CI 1.6-2.1 months) and 4.5 months (95% CI 2.9-8.6 months), respectively (Fig. 3A and 3B). The median time on treatment for the total group of patients was 1.4 months, while it was 12.7 months for the patient group with CB (95% CI 5.1-NA months).
Figure 2.
Waterfall plot with colors indicating the best response.
Figure 3.
Progression-free survival and overall survival curves. Kaplan-Meier curve for estimated progression-free survival (A) and overall survival (B), with 95% confidence interval (dashed lines).
An additional exploratory analysis in which we excluded the 4 patients with IDH mutated tumors showed no significant different results. All patients with IDH mutated tumors were patients with PD after 16 weeks of treatment. The median PFS and OS were 1.7 months (95% CI 1.6-3.5 months) and 4.5 months (95% CI 3.3-8.6 months), respectively (Supplementary Fig. S1A and S1B).
Results of Molecular Testing
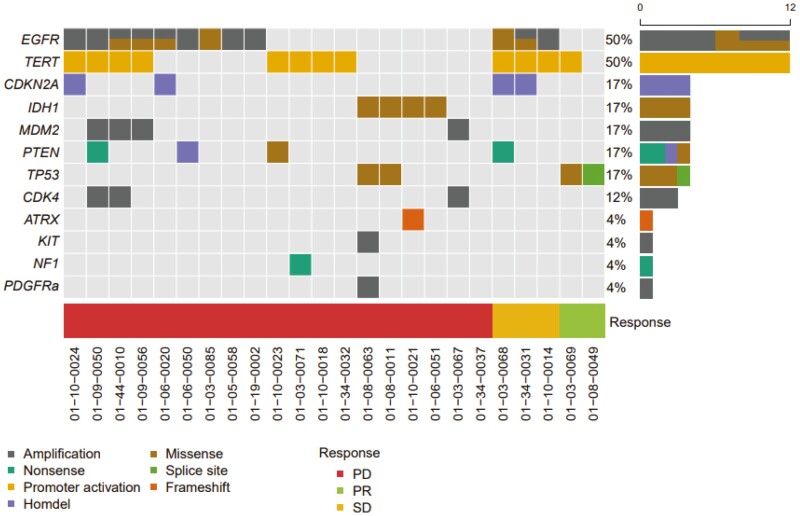
As already mentioned, all patients had an RAF/RASwt recurrent glioblastoma tumor based on molecular testing that was performed before entering DRUP. Most patients were included based on results of locally performed Next Generation Sequencing (NGS) panels (23 out of 24 evaluable patients), and one patient has had whole-genome sequencing (WGS). Figure 4 describes all detected (likely) pathogenic cancer associated other molecular alterations. In the two patients with a PR, only TP53 or TERT promoter mutations were found and no other potential oncogenic driver mutations. Of the patients with SD or PD, in 13 out of 22 patients other potential oncogenic driver mutations were detected. In half of the patients (n = 12), EGFR alterations were found. Of these 12 patients, 6 patients had only an EGFR amplification, 2 patients had only an EGFR mutation, and 4 patients had an EGFR mutation and amplification. Only one patient had the common EGFRv3 mutation, and the other patients had the following EGFR mutations: p.Ala289Thr (n = 1), p.Arg324Leu (n = 1), p.Arg108Lys (n = 1), p.Arg108Gly (n = 1), and p.Ala289Val (n = 1). There was no correlation between the presence of any EGFR alteration and clinical benefit.
Figure 4.
Oncoplot. All detected (likely) pathogenic molecular alterations by molecular testing before participating within DRUP. Abbreviations: PD, progressive disease; PR, partial response; SD, stable disease.
Safety
Overall, panitumumab was well tolerated. No AEs > grade 3 were observed in this cohort and none of the patients discontinued study treatment due to an AE. All reported AEs are shown in Table 2. For the AEs in bold, the relation to the treatment was scored as either possible, probable, or definite.
Table 2.
Adverse events
| Adverse events | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|
| Aphasia | 1 | ||
| Cerebral edema | 1 | ||
| Focal seizures | 1 | 2 | |
| Hemi-anopsia | 2 | ||
| Lymphopenia | 1 | ||
| Rash | 1 | 4 | |
| Somnolence | 1 | ||
| Thromboembolic event | 1 |
For the adverse events in bold, the relation to the treatment was scored as either “possible”, “probable”, or “definite”.
Discussion
EGFR alterations are present in 57% of patients with glioblastomas, but treatment strategies targeting EGFR have thus far failed in clinical trials. The results of this cohort also demonstrate that panitumumab, an EGFR targeting antibody, had very limited efficacy in patients with a recurrent RAF/RASwt glioblastoma. We observed SD in 3 out of 24 patients (12.5%) and only 2 out of 24 patients had a PR (8%). However, the median PFS of 1.7 months for the whole group is truly disappointing. Still, in the subgroup with CB, PFS was 13 months, which is favorable. All patients in the described cohort were selected based on the absence of alterations in the KRAS, NRAS, or BRAF gene in their tumor, as presence of these alterations lead to a well-known resistance mechanism for anti-EGFR targeting therapies in colorectal cancer.27 Despite this selection of patients with RAF/RASwt recurrent glioblastomas, our results are in line with previous reports on the activity of EGFR targeting therapies in patients with recurrent glioblastomas.17-19,28,29 In a stratified phase II trial of cetuximab, an ORR of 5.5% and disease control rate of 29.6% were reported in 55 patients with recurrent glioblastomas. No correlation between response, survival, and EGFR amplification was found in this study.18 Additionally, Westphal et al conducted a randomized, open-label phase III trial to evaluate efficacy of nimotuzumab added to standard therapy for newly diagnosed glioblastomas. Their results showed no survival benefit from adding nimotuzumab to the standard therapy, and also in this trial no correlation between EGFR amplification and clinical efficacy of nimotuzumab was found.19 Aside from the EGFR targeting antibodies cetuximab and nimotuzumab, several first generation small-molecule tyrosine kinase inhibitors of EGFR were tested in clinical trials. For example, Rich et al tested gefitinib in patients with recurrent glioblastomas in a phase II trial. No objective tumor responses were seen among 53 assessable patients and only 7 patients (13%) had a 6-month event-free survival. Again, no correlation between EGFR expression and OS was detected.28 In a randomized, controlled, phase II trial conducted by van den Bent et al, 110 patients with progressive recurrent glioblastomas after prior radiotherapy were randomly assigned to either erlotinib or a control arm receiving treatment with either temozolomide or carmustine. The 6 months PFS rate in the erlotinib arm was 11.4% vs 24% in the control arm.29 Also in our trial, no correlation between EGFR alterations and clinical benefit was found. This is in line with previous results and known from anti-EGFR therapy in patients with colorectal cancer.20
Together, these data demonstrate that even though alterations in EGFR are common in patients with glioblastomas, targeting EGFR provides limited clinical benefit. EGFR is a tyrosine kinase at the upstream end of signal transduction pathway. Mutations or deregulation of downstream molecules and upregulation of redundant receptor tyrosine kinases may bypass EGFR inhibition.30 For example, the presence of additional activating mutations in downstream effectors such as PTEN,31PIK3CA,32 or KRAS33 or co-occurrence of other amplified or mutated redundant receptor tyrosine kinases, including MET and PDGFRA/B can be responsible for treatment resistance here.34-36
A limitation of the current study is that we do not take into account other possible important molecular alterations related to resistance than RAF/RAS. Eligible patients for this trial had a treatment refractory recurrent glioblastoma with molecular testing demonstrating no mutations in either BRAF, KRAS, or NRAS. From all patients, an NGS panel or WGS was available before inclusion in the DRUP trial. Most of these molecular tests were performed on archived tumor material obtained by primary diagnosis, and therefore we do not have detailed information on molecular characteristics of the recurrent tumors in our cohort when study treatment was initiated. Importantly, in the 2 patients who had PR, no mutations in downstream effectors of the EGFR pathway were present based on the performed NGS-analyses. In 13 out of 22 patients (59%) with SD or PD, potential other oncogenic alterations were already found at primary diagnoses including mutations in downstream effectors (PTEN mutation: n = 4 and PDGFRA amplification: n = 1) and other potential driver alterations as CDK4 amplification: n = 2, NF1 mutation: n = 1, CDKN2A deletion: n = 3, ATRX mutation, n = 1 and MDM2 amplification: n = 1. Although an overall high degree of stability in the mutational status of driver glioma genes and pathways has been shown,37 in ~20% a mutational change at tumor recurrence is detected.38,39 These findings could indicate that, based on additional downstream alterations, it might be possible to further select a small group of patients with recurrent glioblastomas that might benefit from anti-EGFR therapy. Repeated biopsies could be considered before entering in targeted therapy trials to detect new driver mutations in recurrent tumors. Besides molecular alterations, the BBB may also play a role in treatment resistance in glioblastomas. Whether the BBB plays a role as a potential resistance mechanism for panitumumab treatment is unknown. There is some preclinical and clinical evidence suggesting that the BBB prohibits achievement of therapeutic drug concentrations of tyrosine kinase inhibitors (TKIs) in brain tumors.40 This kind of evidence is currently not available for monoclonal antibodies in recurrent glioblastomas from which it is expected that the BBB is disturbed; however, it is known that only 0.1%-0.2% of administered therapeutic antibodies cross the BBB when intact.41 This seems logical as monoclonal antibodies are larger molecules compared to TKIs. Previously, it has been suggested that future treatment benefits can be achieved through intra-arterial delivery of medicines into the brain across an osmotically opened BBB, instead of using the intravenous route.42 This has been demonstrated for bevacizumab, also a monoclonal antibody. Therefore, it might be that intra-arterial delivery of panitumumab could be more effective than i.v. panitumumab treatment. However, VEGF as the target of bevacizumab is mainly present in the vascularization in contrast to the target of panitumumab. Other previous research showed that intra-tumoral concentration and efficacy of antibody-drug conjugates in patients with glioblastoma inversely correlated with tumor size.43 Aside the fact that we selected patients only based on RAF/RASwt and did not take other molecular alterations, nor the glioblastomas specific intratumor heterogeneity into account, other important limitations of this study were the absence of both randomization and a control group.
Revision of pathology diagnoses revealed 4 patients with IDH mutant astrocytoma. These tumors are known to behave different compared to IDHwt glioblastomas. However, a sub-analysis in which we excluded these patients did not significantly change our results. Although we expected that patients with IDH mutated tumors might have had a more favorable outcome based on their tumor characteristics, they all had progressive disease after 8 weeks of treatment.
Conclusion
In patients with a recurrent glioblastoma, therapy selection based on RAF/RASwt genotyping for panitumumab provided insufficient CB to be further explored as a treatment strategy despite the fact that it was well tolerated. We believe that future research should focus on the delivery of drugs through the BBB and unravelling resistance mechanisms by broader genomic evaluation.
Supplementary Material
Supplementary material is available at The Oncologist online.
Acknowledgment
Names of institutions at which the work was done: Radboud University Medical Center, The Netherlands Cancer Institute, University Medical Cancer Center Utrecht, ETZ Hospital, University Medical Center Groningen, Isala Oncology Center, Maastricht University Center+, Leiden University Medical Center, Erasmus MC Cancer Institute, Hartwig Medical Foundation.
Contributor Information
Ilse A C Spiekman, Department of Medical Oncology, Erasmus MC Cancer Institute, Erasmus MC, Rotterdam, The Netherlands.
Birgit S Geurts, Oncode Institute, Utrecht, The Netherlands; Department of Molecular Oncology and Immunology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Laurien J Zeverijn, Oncode Institute, Utrecht, The Netherlands; Department of Molecular Oncology and Immunology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Gijs F de Wit, Oncode Institute, Utrecht, The Netherlands; Department of Molecular Oncology and Immunology, The Netherlands Cancer Institute, Amsterdam, The Netherlands.
Vincent van der Noort, Department of Biometrics, Netherlands Cancer Institute, Amsterdam, The Netherlands.
Paul Roepman, Hartwig Medical Foundation, Amsterdam, The Netherlands.
Wendy W J de Leng, Department of Pathology, University Medical Cancer Center Utrecht, Utrecht, The Netherlands.
Anne M L Jansen, Department of Pathology, University Medical Cancer Center Utrecht, Utrecht, The Netherlands.
Benno Kusters, Department of Pathology, Radboud University Medical Center, Nijmegen, The Netherlands.
Laurens V Beerepoot, Department of Internal Medicine, ETZ Hospital (Elisabeth-TweeSteden Ziekenhuis), Tilburg, The Netherlands.
Filip Y F L de Vos, Department of Medical Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Derk-Jan A de Groot, Department of Medical Oncology, University Medical Center Groningen, Groningen, The Netherlands.
Jan Willem B de Groot, Isala Oncology Center, Isala, Zwolle, The Netherlands.
Ann Hoeben, Division of Medical Oncology, Department of Internal Medicine, GROW School of Oncology and Development Biology, Maastricht University Center+, Maastricht, The Netherlands.
Jan Buter, Department of Medical Oncology, Amsterdam University Medical Center, Location VuMC, Amsterdam, The Netherlands.
Hans A J Gelderblom, Department of Medical Oncology, Leiden University Medical Center, Leiden, The Netherlands.
Emile E Voest, Oncode Institute, Utrecht, The Netherlands; Department of Molecular Oncology and Immunology, The Netherlands Cancer Institute, Amsterdam, The Netherlands; Center for Personalized Cancer Treatment, Rotterdam,The Netherlands.
Henk M W Verheul, Department of Medical Oncology, Erasmus MC Cancer Institute, Erasmus MC, Rotterdam, The Netherlands.
Funding
The DRUP trial was supported by the Stelvio for Life Foundation, the Dutch Cancer Society (grant number 10014), the Hartwig Medical Foundation (HMF), and all participating pharmaceutical companies: Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Eisai, Janssen, Lilly, MSD, Novartis, Pfizer, Roche.
Conflict of Interest
The authors declare the following financial interests / personal relationships which may be considered as potential competeting interests: Derk-Jan A. de Groot received funding for an investigator-driven phase II study from Hofmann la Roche and a PUSH grant for a PhD student from Siemens. All outside the submitted work and all money had been received by the UMCG. Emile E. Voest reported research funding for DRUP from Amgen. The other authors declare no conflicts of interests.
Author Contributions
Conception/design: I.A.C.S., B.S.G., L.J.Z., G.F.d.W., V.v.d.N., H.A.J.G., E.E.V., H.M.W.V. Provision of study material or patients: I.A.C.S., B.S.G., L.J.Z., G.F.d.W., P.R., W.W.J.d.L., A.M.L.J., L.V.B., F.d.V., J.A.d.G., J.W.B.d.G., A.H., J.B., H.A.J.G., E.E.V., H.M.W.V. Collection and/or assembly of data: I.A.C.S., B.S.G., L.J.Z., G.F.d.W., B.K. Data analysis and interpretation: I.A.C.S., V.v.d.N., B.K., H.M.W.V. Manuscript writing: I.A.C.S., H.M.W.V. Final approval of manuscript: All authors.
Data Availability
All data described in this study are freely available for academic use and can be obtained through a request to the corresponding author by email.
References
- 1. Registry DBT. Glioomzorg in Nederland 2019. [Google Scholar]
- 2. Roger S, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England J Med 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 3. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl. 2):ii1-i56. 10.1093/neuonc/not151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seystahl K, Wick W, Weller M.. Therapeutic options in recurrent glioblastoma--an update. Crit Rev Oncol Hematol. 2016;99:389-408. 10.1016/j.critrevonc.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 5. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733-4740. 10.1200/JCO.2008.19.8721 [DOI] [PubMed] [Google Scholar]
- 6. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740-745. 10.1200/JCO.2008.16.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954-1963. 10.1056/NEJMoa1707358 [DOI] [PubMed] [Google Scholar]
- 8. van Linde ME, Brahm CG, de Witt Hamer PC, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135(1):183-192. 10.1007/s11060-017-2564-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaff LR, Mellinghoff IK.. Glioblastoma and other primary brain malignancies in adults: a review. JAMA. 2023;329(7):574-587. 10.1001/jama.2023.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu W, Klockow JL, Zhang M, et al. Glioblastoma multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021;171:105780. 10.1016/j.phrs.2021.105780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803-820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 12. McLendon R, Friedman A, Bigner D, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477. 10.1016/j.cell.2013.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19(18 Suppl):32S-40S. [PubMed] [Google Scholar]
- 15. Gan HK, Kaye AH, Luwor RB.. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16(6):748-754. 10.1016/j.jocn.2008.12.005 [DOI] [PubMed] [Google Scholar]
- 16. Tebbutt N, Pedersen MW, Johns TG.. Targeting the ERBB family in cancer: couples therapy. Nat Rev Cancer. 2013;13(9):663-673. 10.1038/nrc3559 [DOI] [PubMed] [Google Scholar]
- 17. Hasselbalch B, Lassen U, Hansen S, et al. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol. 2010;12(5):508-516. 10.1093/neuonc/nop063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596-1603. 10.1093/annonc/mdp032 [DOI] [PubMed] [Google Scholar]
- 19. Westphal M, Heese O, Steinbach JP, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522-532. 10.1016/j.ejca.2014.12.019 [DOI] [PubMed] [Google Scholar]
- 20. Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23(9):1803-1810. 10.1200/JCO.2005.08.037 [DOI] [PubMed] [Google Scholar]
- 21. Van Den Bent M, Eoli M, Sepulveda JM, et al. INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol. 2020;22(5):684-693. 10.1093/neuonc/noz222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vectibix (panitumumab). Full Prescribing Information. Amgen Inc.; 2017. [Google Scholar]
- 23. van der Velden DL, Hoes LR, van der Wijngaart H, et al. The Drug Rediscovery protocol facilitates the expanded use of existing anticancer drugs. Nature. 2019;574(7776):127-131. 10.1038/s41586-019-1600-x [DOI] [PubMed] [Google Scholar]
- 24. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wen PY, Chang SM, Van den Bent MJ, et al. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017;35(21):2439-2449. 10.1200/JCO.2017.72.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jung SH, Lee T, Kim K, George SL.. Admissible two-stage designs for phase II cancer clinical trials. Stat Med. 2004;23(4):561-569. 10.1002/sim.1600 [DOI] [PubMed] [Google Scholar]
- 27. Allegra CJ, Rumble RB, Hamilton SR, et al. Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J Clin Oncol. 2016;34(2):179-185. 10.1200/JCO.2015.63.9674 [DOI] [PubMed] [Google Scholar]
- 28. Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22(1):133-142. 10.1200/JCO.2004.08.110 [DOI] [PubMed] [Google Scholar]
- 29. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC Brain Tumor Group Study 26034. J Clin Oncol. 2009;27(8):1268-1274. 10.1200/JCO.2008.17.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. An Z, Aksoy O, Zheng T, Fan QW, Weiss WA.. Epidermal growth factor receptor and EGFRvIII in glioblastoma: signaling pathways and targeted therapies. Oncogene. 2018;37(12):1561-1575. 10.1038/s41388-017-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012-2024. 10.1056/NEJMoa051918 [DOI] [PubMed] [Google Scholar]
- 32. Lai L, Meng W, Wei J, et al. Transformation of NSCLC to SCLC after 1st- and 3rd-generation EGFR-TKI resistance and response to EP regimen and erlotinib: 2 CARE-compliant case reports. Medicine (Baltim). 2021;100(10):e25046. 10.1097/MD.0000000000025046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diaz LA Jr, Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537-540. 10.1038/nature11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013;3(5):534-547. 10.1158/2159-8290.CD-12-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287-290. 10.1126/science.1142946 [DOI] [PubMed] [Google Scholar]
- 36. Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039-1043. 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- 37. Draaisma K, Chatzipli A, Taphoorn M, et al. Molecular evolution of IDH wild-type glioblastomas treated with standard of care affects survival and design of precision medicine trials: a report from the EORTC 1542 study. J Clin Oncol. 2020;38(1):81-99. 10.1200/JCO.19.00367 [DOI] [PubMed] [Google Scholar]
- 38. Kim J, Lee IH, Cho HJ, et al. Spatiotemporal evolution of the primary glioblastoma genome. Cancer Cell. 2015;28(3):318-328. 10.1016/j.ccell.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Cazzato E, Ladewig E, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768-776. 10.1038/ng.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Linde ME, Labots M, Brahm CG, et al. Tumor drug concentration and phosphoproteomic profiles after two weeks of treatment with sunitinib in patients with newly diagnosed glioblastoma. Clin Cancer Res. 2022;28(8):1595-1602. 10.1158/1078-0432.CCR-21-1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poduslo JF, Curran GL, Berg CT.. Macromolecular permeability across the blood-nerve and blood-brain barriers. Proc Natl Acad Sci USA. 1994;91(12):5705-5709. 10.1073/pnas.91.12.5705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lesniak WG, Chu C, Jablonska A, et al. A distinct advantage to intraarterial delivery of (89)Zr-bevacizumab in PET imaging of mice with and without osmotic opening of the blood-brain barrier. J Nucl Med. 2019;60(5):617-622. 10.2967/jnumed.118.218792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gan HK, Parakh S, Lassman AB, et al. Tumor volumes as a predictor of response to the anti-EGFR antibody drug conjugate depatuxizumab mafadotin. Neurooncol Adv. 2021;3(1):vdab102. 10.1093/noajnl/vdab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data described in this study are freely available for academic use and can be obtained through a request to the corresponding author by email.