Abstract
Long-range glutamatergic inputs originating from the cortex and thalamus are indispensable for striatal development, providing the foundation for motor and cognitive functions. Despite their significance, transcriptional regulation governing these inputs remains largely unknown. We investigated the role of a transcription factor encoded by a high-risk autism-associated gene, FOXP1, in sculpting glutamatergic inputs onto spiny projection neurons (SPNs) within the striatum. We find a neuron subtype-specific role of FOXP1 in strengthening and maturing glutamatergic inputs onto dopamine receptor 2–expressing SPNs (D2 SPNs). We also find that FOXP1 promotes synaptically driven excitability in these neurons. Using single-nuclei RNA sequencing, we identify candidate genes that mediate these cell-autonomous processes through postnatal FOXP1 function at the post-synapse. Last, we demonstrate that postnatal FOXP1 reinstatement rescues electrophysiological deficits, cell type–specific gene expression changes, and behavioral phenotypes. Together, this study enhances our understanding of striatal circuit development and provides proof of concept for a therapeutic approach for FOXP1 syndrome and other neurodevelopmental disorders.
Striatal loss of Foxp1 affects cortical striatal circuits but can be functionally rescued by gene restoration.
INTRODUCTION
Long-range excitatory glutamatergic afferents provide motor, sensory, and cognitive information into the striatum, where this information is integrated for learning and action planning (1). These inputs originate primarily from the cortex and thalamus, and the mechanisms of their development and function have been studied (2–4) [and reviewed in (5)]. Transcription factors involved in striatal development have been widely studied in the context of early developmental processes, such as cell proliferation, differentiation, migration, and fate determination of striatal neurons (6–9). Unexpectedly, the transcription factors and the genetic programming that orchestrate the construction of synaptic connectivity in striatal circuitry during development remain largely unexplored. Exceptions are autism-linked transcription factors, FOXP2 and MEF2C, which function together to regulate the formation of corticostriatal synapses (10). However, the functional relevance of transcription factors in evoked synaptic transmission has not been investigated so far. Furthermore, the striatum is composed of functionally distinct GABAergic spiny projection neurons (SPNs), dopamine receptor 1 (D1) and D2 SPNs, and our understanding of the regulation of cortical projections received by these SPN subtypes is limited.
Forkhead box P1 (FOXP1) is a key transcription factor that is highly enriched in the developing and mature striatum (11). FOXP1 haploinsufficiency causes FOXP1 syndrome with symptoms that include intellectual disability and autism (12, 13). Loss-of-function, heterozygous mutations are among the most significant recurrent de novo mutations associated with autism (14–16). FOXP1 exhibits a robust expression in SPNs and has been implicated in establishing gross striatal structure, behavior, fate determination, D1/D2 SPN physiology, and the regulation of essential genes in SPNs (8, 17–19). Unlike other transcription factors involved in early embryonic striatal development, FOXP1 expression begins later at embryonic day 14.5 (E14.5) and is most strongly expressed in postmitotic neurons (11, 20), which is maintained through adulthood. The timing of FOXP1 expression suggests that it may function in sculpting striatal circuitry during later developmental stages. While FOXP1 is expressed in both D1 and D2 SPNs, it plays a more consequential role in D2 SPNs (8, 19).
Previous investigations have reported Foxp1-dependent regulation of neuronal excitability and synaptic connections not only in the mouse cortex and hippocampus but also in the songbird brain (18, 21, 22). Our previous work demonstrated that FOXP1 is critical for maintaining normal intrinsic excitability of D2 SPNs by regulating the expression of leak and inwardly rectifying potassium channels (19). This was likely mediated by a later postnatal FOXP1 function because postnatal Foxp1 deletion could recapitulate the hyperexcitability phenotype induced by embryonic deletion.
We wanted to determine whether a similar D2 SPN locus and postnatal function of FOXP1 applies to synaptic regulation as well. It is unclear how the development of striatal circuits maintains a stable balance between the activity of D1 and D2 SPNs (3, 23). Considering the preferential role of FOXP1 in the development of D2 SPNs, we hypothesize that FOXP1 plays a key role in regulating the synaptic balance of long-range glutamatergic inputs onto striatal neurons through cell-autonomous regulation of D2 SPNs. Last, there are no reports describing the prenatal versus postnatal function of a transcription factor in sculpting striatal circuitry, which is crucial for designing successful therapeutics. Here, we hypothesize that FOXP1 functions postnatally to regulate glutamatergic synaptic inputs.
The extent and transcriptional mechanisms by which postnatal reinstatement of a transcription factor (after its absence during earlier developmental stages) can restore proper neuronal physiology are largely unexplored. A recent study has reported a rescue of electroencephalogram phenotype, behavior characteristics, and a few genes with postnatal reinstatement of Tcf4 (24). However, to our knowledge, no study has used a large-scale, unbiased approach in vivo to determine the extent of expression reversal and assess this at a cell type–specific level more rigorously. Moreover, it remains uncertain whether the rescue of neurophysiological phenotypes with the reinstatement of a transcription factor is mediated by the reversal of transcript levels induced by deletion (“genetic” rescue) or is mediated by an entirely different set of genes.
To address these questions, we conducted cell-specific embryonic and postnatal Foxp1 deletion, uncovering a vital role for FOXP1 in the development of functional glutamatergic synaptic transmission onto SPNs. Our findings reveal a robust weakening of glutamatergic inputs specifically onto D2 SPNs, highlighting the necessity of FOXP1 in strengthening these inputs. In contrast, we observed only minor changes with Foxp1 deletion in D1 SPNs. We also detected a decrease in evoked synaptic responses with postnatal Foxp1 deletion in D2 SPNs, consistent with postnatal FOXP1-mediated regulation of glutamatergic inputs. We achieved partial rescue of the synaptic transmission and complete rescue of intrinsic excitability defects in D2 SPNs through postnatal Foxp1 reinstatement. Last, single-nuclei RNA sequencing (snRNA-seq) uncovered candidate genes for FOXP1-dependent regulation of glutamatergic inputs onto D2 SPNs. The majority of differentially expressed genes (DEGs) induced by postnatal Foxp1 reinstatement exhibited rescued expression levels compared to their levels with embryonic Foxp1 deletion. These findings provide a basis for a potential therapeutic strategy for individuals with FOXP1 syndrome and other similar neurodevelopmental disorders.
RESULTS
Foxp1 deletion in D2 SPNs weakens corticostriatal inputs onto D2 SPNs
We measured the functional strength of corticostriatal inputs by using mCherry-tagged Channelrhodpsin-2 (ChR2) in callosal projecting corticostriatal axons and examined light-evoked excitatory postsynaptic currents (EPSCs) in SPNs (Fig. 1A). To do this, we expressed ChR2 through injecting Adeno-associated virus 9 (AAV9) into one cortical hemisphere on postnatal day (P) 1 and conducted recordings in the striatum of the contralateral hemisphere at P14 to P18. By studying this contralateral projection, we isolate the inputs provided by a single presynaptic, cortical neuron type—the layer 5 intratelencephalic pyramidal neurons (25). We compared EPSCs in D2 SPNs between mice with and without D2 SPN-specific Foxp1 deletion at E14 to E15 (referred to as D2 Foxp1cKO and D2 Foxp1CTL mice, respectively).
Fig. 1. Excitatory cortical inputs onto D2 SPNs are decreased with D2-specific Foxp1 deletion.
(A) pAAV-ChR2-mCherry virus was injected into the cortex of one hemisphere at P1, acute slices were prepared at P14–18, and SPNs were recorded in the contralateral striatum (created with BioRender.com). (B) Average (± SEM) EPSC responses triggered by blue light stimulation from D1 and D2 SPNs in D2 Foxp1CTL (blue and black, left) and in D2 Foxp1cKO mice (green and red, right). (C) EPSC amplitudes in D1/D2 SPN pairs in D2 Foxp1CTL and D2 Foxp1cKO mice. (D) D2/D1 ratio of EPSC amplitude for each D2/D1 pair in (C). (E) Latency to EPSC onset in D2 SPNs. (F) Time taken by the EPSC response to reach the peak amplitude in D2 SPNs. (G) Average (± SEM) EPSCs during a 5-pulse train of blue light in D2 SPNs. (H) Ratio of second to fifth response amplitude with respect to (wrt) first response amplitude during a stimulus train in D2 SPNs. (I) Example traces showing mEPSCs in D2 SPNs. (J) mEPSC frequency (left) and amplitude (right) of D2 SPNs in both D2 Foxp1CTL and D2 Foxp1cKO mice. Ns in all figures are (D2 Foxp1CTL; D2 Foxp1cKO). For (B) to (D), 20 D1/D2 pairs in five mice and 19 D1/D2 pairs in four mice. For (E) and (F), 20 D2 SPNs in five mice and 19 D2 SPNs in four mice. For (G) and (H), 18 D2 SPNs in five mice and 14 D2 SPNs in four mice. For (J), 10 D2 SPNs in three mice and 15 D2 SPNs in three mice. Statistics: (C) Two-way analysis of variance (ANOVA) with Fisher’s LSD test; (D) Mann-Whitney (MW) test; (E, F, and J) t test; and (H) Mixed effects model with Holm-Sidak corrections for multiple comparisons. **P < 0.01, ***P < 0.001, and ****P < 0.001.
For every D2 SPN examined, we recorded from a neighboring, control D1 SPN to control for variability inherent in the stimulation method (see Materials and Methods for details). EPSC amplitude of paired D1 and D2 SPNs were nearly identical in D2 Foxp1CTL mice (Fig. 1, B and C) as previously reported (26). However, in D2 Foxp1cKO mice, EPSC amplitude in D2 SPNs was roughly half of that observed in neighboring D1 SPNs (Fig. 1, B and C), leading to a diminished EPSC amplitude ratio (D2/D1) (Fig. 1D). Although we observed a trend of increased amplitude in D1 SPNs in D2 Foxp1cKO mice as compared to D2 Foxp1CTL mice (P = 0.0532), but as stated above, we focused on pairwise D1/D2 responses as we believe “absolute” comparisons between Foxp1cKO and Foxp1CTL in these experiments are unreliable (see Materials and Methods for details). We also measured EPSC rising slope in an earlier response time window to decrease contamination from disynaptic inhibition and observed a similar decline in D2/D1 ratio (fig. S1A). No changes were observed in EPSC onset latency and rise time. (Fig. 1, E and F). Furthermore, response decay time was unaltered, implying that there may not be any change in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) subunit composition (fig. S1E) (27). As reported previously, D2 SPNs displayed increased input resistance and intrinsic excitability in D2 Foxp1cKO mice (fig. S1, B and C). None of these changes were observed in the D1 SPNs (fig. S1, C, D, and F to H). In summary, FOXP1 promotes the strength of corticostriatal glutamatergic inputs onto D2 SPNs relative to D1 SPNs.
To determine whether weak synaptic function in D2 Foxp1cKO SPNs correlated with changes in presynaptic release probability, we recorded D2 SPNs while applying a train of light stimuli and measured the EPSC amplitude change during the train (Fig. 1G). The EPSC amplitudes (normalized to the first EPSC) evoked during these trains were not different in D2 SPNs between D2 Foxp1cKO and D2 Foxp1CTL mice, suggesting no changes in the presynaptic release probability (Fig. 1H). We also observed a non–cell-autonomous effect in D1 SPNs where normalized EPSC amplitude ratio of second to fifth response was higher in D2 Foxp1cKO mice, suggesting reduced release probability (fig. S1I).
To measure absolute changes in total glutamatergic inputs onto D2 SPNs with Foxp1 deletion, we blocked action potential firing using tetrodotoxin (TTX) (1 μM) and measured miniature EPSCs (mEPSCs). Although both cortical and thalamic inputs likely contribute to mEPSC measurements (1), we considered the corticostriatal evoked response alterations robust enough to be reflected in mEPSC changes (see Discussion). We found a decrease in mEPSC frequency and amplitude in D2 SPNs in D2 Foxp1cKO mice (Fig. 1, I and J). Similar results were observed for spontaneous EPSCs (sEPSCs) measurements in the absence of TTX (fig. S1J). Because we did not observe changes in release probability, the decreased mEPSC frequency is consistent with fewer excitatory synapses, while reduced amplitude suggests weaker individual synapses in D2 SPNs with the loss of Foxp1. Therefore, in addition to a shift in the balance of corticostriatal inputs onto D2 and D1 SPNs, Foxp1 deletion reduces the absolute level of glutamatergic inputs onto D2 SPNs. D1 SPNs in D2 Foxp1cKO mice did not show any mEPSC changes (fig. S1K). In summary, our findings indicate that FOXP1 is essential for the strengthening and maintenance of corticostriatal inputs onto D2 SPNs during development.
Foxp1 deletion weakens both AMPAR- and NMDAR-mediated inputs but increases the relative NMDAR synaptic composition
FOXP1 facilitates a social experience-dependent increase in the AMPAR/N-methyl-D-aspartate receptor (NMDAR) composition ratio at synapses in songbirds (22), and these changes would likely affect synaptic plasticity, strengthening, and information transfer (28). We investigated if a similar regulatory mechanism operates at corticostriatal synapses. We used a protocol that effectively eliminates disynaptic inhibitory postsynaptic current contamination, allowing us to isolate NMDAR-mediated currents. By blocking GABAergic transmission with picrotoxin (0.1 mM), action potentials with TTX (1 μM), and potassium channels by 4-Aminopyridine (4-AP), light-induced activation of ChR2 in corticostriatal presynaptic terminals resulted in isolated, action potential–independent, monosynaptic EPSCs (29). As observed above, the balance of corticostriatal excitation relative to D1 SPNs was decreased in D2 SPNs for AMPAR-mediated transmission in D2 Foxp1cKO mice (Fig. 2, A and B). NMDAR-mediated EPSCs displayed a similar pattern with decreased amplitude in D2 SPNs in D2 Foxp1cKO mice (Fig. 2C). Consequently, both AMPAR- and NMDAR-mediated EPSCs exhibited a reduced D2/D1 amplitude ratio (Fig. 2, B and C). Moreover, D2 SPNs showed a decreased ratio of AMPAR/NMDAR-mediated EPSC amplitude in D2 Foxp1cKO mice, indicating an increased relative contribution of NMDARs to corticostriatal inputs onto D2 SPNs (Fig. 2D). Higher NMDAR contribution is a signature of more immature excitatory synapses in SPNs (30). These changes were specific to loss of Foxp1 in D2 SPNs, as AMPAR/NMDAR ratio did not change for unmanipulated D1 SPNs in these mice (Fig. 2E).
Fig. 2. NMDAR-mediated component of evoked excitatory cortical inputs onto D2 SPNs is altered with D2-specific Foxp1 deletion.
(A) Monosynaptic EPSCs were evoked by depolarization of presynaptic terminals during blockade of voltage-dependent Na+ channels. Average EPSC responses (±SEM) from D1 and D2 SPNs in slices from D2 Foxp1CTL mice (blue and black, respectively, left) and from D2 Foxp1cKO mice (green and red, respectively, right). AMPAR-mediated EPSC amplitude is measured as a response peak at −70-mV holding potential, whereas for NMDAR-mediated EPSC amplitude, it is the average response in a 90- to 100-ms window while holding at +50 mV (highlighted with a brown box). (B) Amplitude of AMPAR-mediated EPSCs for each pair of D1/D2 SPNs in D2 Foxp1CTL and D2 Foxp1cKO mice (left). D2/D1 ratio of the EPSC amplitude for each pair (right). (C) Amplitude of NMDAR-mediated EPSCs and D2/D1 EPSC amplitude ratio. (D and E) Ratio of AMPAR- to NMDAR-mediated EPSC amplitudes for D2 and D1 SPNs in D2 Foxp1CTL and D2 Foxp1cKO mice. (F) Ratio of second-fifth response amplitude with respect to first response during a stimulus train in D2 SPNs. Ns in all figures are (D2 Foxp1CTL; D2 Foxp1cKO). For (A) to (C), 16 D1/D2 pairs in five mice and 16 D1/D2 pairs in three mice. For (D), 21 D2 SPNs in five mice and 18 D2 SPNs in three mice. For (E), 17 D1 SPNs in five mice and 18 D1 SPNs in three mice. For (F), 18 D2 SPNs in five mice and 14 D2 SPNs in three mice. Statistics: [B (left) and C (left)], two-way ANOVA with Fisher’s LSD test; [B (right) and C (right)] MW test; (D and E) t test; and (F) mixed effects model with Holm-Sidak correction for multiple comparisons. **P < 0.01, ***P < 0.001, and ****P < 0.001.
There was no change in the short-term dynamics of EPSC amplitude with Foxp1 deletion in D2 SPNs under these conditions, again suggesting an absence of changes in presynaptic release probability (Fig. 2F). Furthermore, mEPSC frequency and amplitude were again decreased in D2 SPNs (fig. S2, A and B). In summary, FOXP1 function is necessary for the strengthening and maturation of corticostriatal synapses, specifically in facilitating the inclusion of AMPARs in synapses onto D2 SPNs.
Germline heterozygous Foxp1 mice do not demonstrate detectable changes in synaptic properties
FOXP1 syndrome arises from heterozygous mutations leading to FOXP1 haploinsufficiency (12, 31). The Foxp1Het mouse serves as a patient-relevant model, displaying increased D2 SPN excitability and some behavioral deficits (17). To determine whether the weakening of corticostriatal inputs to D2 SPNs also occurs in Foxp1Het mouse, we again used blue light to stimulate ChR2-containing corticostriatal afferents and examined action potential-dependent synaptic responses in SPNs. The EPSC amplitude was comparable between D2 and D1 SPNs in both Foxp1WT and Foxp1Het mice, resulting in no significant difference in the EPSC amplitude ratio (D2/D1) (fig. S3, A and B). There were no changes in short-term dynamics in response to train stimuli in both D2 and D1 SPNs (fig. S3, C and D).
Note that the D1 SPNs used for normalization in Foxp1Het mice also exhibit heterozygous Foxp1 deletion and therefore may not be ideal controls for the paired recordings. Consequently, we measured the AMPAR/NMDAR ratio in D2 SPNs, which is independent of D1 SPNs. However, we did not observe any differences (fig. S3E). Responses to train stimuli and mEPSCs remained unaffected in these conditions (fig. S3, F and G). In summary, these results indicate that Foxp1 expression levels in Foxp1Het mice are adequate for the normal development of corticostriatal inputs.
Corticostriatal inputs onto D1 SPNs are much less affected by Foxp1 deletion compared to D2 SPNs
Foxp1 deletion in D1 SPNs induces changes in the cellular and electrophysiological properties of D1 SPNs and causes behavioral defects (8, 19). We examined action potential-dependent corticostriatal inputs onto D1 SPNs in D1 Foxp1cKO mice. Recordings were conducted in D1/D2 pairs. In Foxp1CTL mice, there was no significant difference in the amplitude of AMPAR-mediated EPSCs between D1 and D2 SPNs (Fig. 3, A and B). In contrast, D1 Foxp1cKO mice exhibited a significant decrease in the EPSC amplitude of D1 SPNs relative to their neighboring D2 SPNs (Fig. 3, A and B). This effect, however, was not as pronounced as the changes observed in D2 SPNs in D2 Foxp1cKO mice, considering both the smaller magnitude difference and the lack of changes in the D1/D2 EPSC amplitude ratio in D1 Foxp1cKO mice (Fig. 3C). Moreover, Foxp1 deletion did not affect the frequency and amplitude of mEPSCs in D1 SPNs (Fig. 3, D and E). These data suggest that FOXP1 does not play a substantial role in regulating the development of corticostriatal inputs onto D1 SPNs. Non–cell-autonomous effects were observed on D2 SPNs in D1 Foxp1cKO mice with D2 SPNs displaying increased sEPSC frequency, reduced intrinsic excitability, and decreased input resistance (fig. S4, A to C).
Fig. 3. Excitatory cortical inputs are less affected in D1 SPNs with D1-specific Foxp1 deletion.
(A) Average (±SEM) EPSC responses from D1 and D2 SPNs in D1 Foxp1CTL mice (blue and black, respectively, left) and from D1 Foxp1cKO mice (green and red, respectively, right). (B) Amplitude of AMPAR-mediated EPSCs for D1/D2 SPN pairs in D1 Foxp1CTL and D1 Foxp1cKO mice (interaction term, genotype × cell type; P < 0.01). (C) D1/D2 ratio of EPSC amplitude for each D1/D2 pair. (D) Example traces of sEPSC in D1 SPNs. (E) Frequency (left) and amplitude (right) of sEPSCs in D1 SPNs in D1 Foxp1CTL mice and D1 Foxp1cKO mice. Ns in all figures are (D1 Foxp1CTL; D1 Foxp1cKO). For (A) to (C), 21 D1/D2 pairs in three mice and 21 D1/D2 pairs in four mice). For (E), 17 D1 SPNs in three mice and 21 D1 SPNs in four mice. Statistics: (B) Two-way ANOVA with Fisher’s LSD test, (C) MW test, and (E) t test.
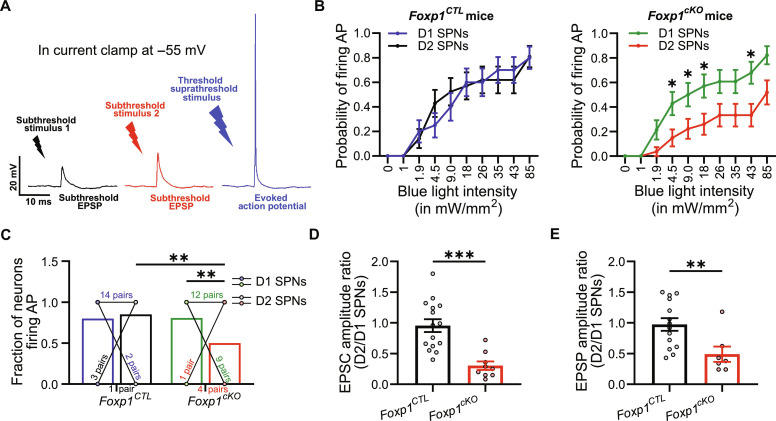
Synaptically driven excitability is reduced in D2 SPNs with Foxp1 deletion
We have previously reported increased intrinsic excitability in D2 SPNs resulting from Foxp1 deletion (19) and reproduce this finding here (fig. S1, B and C). While this might suggest heightened activity of these neurons within the intact circuit (19), the observed decrease in corticostriatal inputs suggests reduced activity. To determine how these changes are integrated to regulate synaptically driven excitability of D2 SPNs, we used blue light and ChR2 to stimulate corticostriatal afferents and measured action potential firing in SPNs. Light of variable intensity was applied that evoked subthreshold EPSPs at lower intensities and action potentials at higher intensities (Fig. 4A and Materials and Methods). For each neuron, we obtained plots of firing probability as a function of light intensity, where the firing probability refers to the fraction of trials where at least one action potential was evoked.
Fig. 4. Synaptically induced excitability from the cortex is decreased in D2 SPNs with Foxp1 deletion.
(A) Schematic of approach: blue light of different intensities was applied where subthreshold stimuli generated EPSPs, while threshold or suprathreshold stimuli generated action potentials. (B) Excitability curves from D1/D2 SPN pairs in D2 Foxp1CTL (left) and D2 Foxp1cKO mice (right). (C) Fraction of SPNs with at least one action potential evoked at the maximum possible light intensity stimulus. A total of 15% of D2 SPNs in D2 Foxp1CTL mice and 50% in D2 Foxp1cKO mice did not fire action potentials. (D) D2/D1 EPSC amplitude ratio for each D1/D2 pair at −70 mV. (E) D2/D1 ratio of EPSP amplitude for each D1/D2 pair. Ns in all figures are (D2 Foxp1CTL; D2 Foxp1cKO). For (B) and (C), 20 D1/D2 pairs in six mice and 26 D1/D2 pairs in seven mice. For (D), 15 D1/D2 pairs in three mice and 9 D1/D2 pairs in two mice. For (E), 14 D1/D2 pairs in three mice and 7 D1/D2 pairs in two mice. Statistics: (B) Mixed effects model with Sidak correction for multiple comparisons, (C) two-way ANOVA with Fisher’s LSD test, and (D and E) t test. **P < 0.01 and ***P < 0.001.
The average firing probability of D1 and D2 SPNs was equivalent in D2 Foxp1CTL mice, whereas it was significantly reduced in D2 SPNs when compared with D1 SPNs in D2 Foxp1cKO mice (Fig. 4B). Moreover, the fraction of neurons capable of firing at least one action potential with the maximum light intensity was diminished in D2 SPNs in D2 Foxp1cKO mice (Fig. 4C). In addition, the threshold intensity required to evoke an action potential was higher for D2 SPNs compared to D1 SPNs in D2 Foxp1cKO mice, whereas no difference was observed in D2 Foxp1CTL mice (fig. S5A and see Materials and Methods for calculation).
As expected, the relative EPSC amplitude was reduced (Fig. 4D and fig. S5B) and input resistance increased (19) for D2 SPNs in D2 Foxp1cKO mice (fig. S5C). The EPSP amplitude is arguably more relevant in determining synaptically driven excitability, and we would expect this to be weaker with Foxp1 deletion. This was roughly halved in D2 SPNs relative to neighboring D1 SPNs in D2 Foxp1cKO mice (Fig. 4E). Together, our results demonstrate that FOXP1 facilitates synaptically driven excitability mediated by corticostriatal inputs, which is largely caused by a decrease in EPSP amplitude.
Postnatal Foxp1 deletion weakens glutamatergic inputs onto D2 SPNs
The alterations in the synaptic physiology of D1/D2 SPNs were observed with embryonic Foxp1 deletion where Cre-expression begins at E14 to E15 (see Materials and Methods) (8). Hence, it remained unclear to what extent embryonic versus postnatal FOXP1 function underlies changes in glutamatergic inputs. Given that FOXP1 plays a postnatal role in regulating intrinsic excitability of D2 SPNs (19), we hypothesized a similar postnatal FOXP1 function in regulating synapses. To test this hypothesis, we injected an AAV–Cre–enhanced green fluorescent protein (eGFP) virus into the striatum of P1 Foxp1flox/flox mice, resulting in Foxp1 deletion by P6 to P7 (32, 33). We used extracellular electrical stimulation instead of ChR2 to stimulate long-range glutamatergic inputs as the ChR2-tagged mCherry fluorophore interfered with cell identification (see Materials and Methods and Fig. 5A). We recorded AMPAR-mediated evoked responses in pairs of GFP-positive (Foxp1D2_vcKO) and -negative (Foxp1D2_Flox) D2 SPNs.
Fig. 5. Excitatory cortical inputs onto D2 SPNs are decreased with postnatal deletion of Foxp1.
(A) Example image: A metal stimulating electrode was placed in underlying cortical white matter to stimulate the corticostriatal projections and responses were recorded in pairs of Foxp1-deleted (Foxp1D2_vcKO, eGFP positive) and control (Foxp1D2_Flox, eGFP negative) D2 SPNs. (B) Average EPSC responses (±SEM) at −70 mV from Foxp1-deleted (magenta) and control (gray) D2 SPNs. (C) EPSC amplitude for the pairs of Foxp1D2_vcKO and Foxp1D2_Flox D2 SPNs. (D) Example traces of mEPSCs in control and Foxp1-deleted D2 SPNs. (E) mEPSC frequency (left) and amplitude (right) for control and Foxp1-deleted D2 SPNs. (F) Total charge (frequency × amplitude) transfer in D2 SPNs of both groups. For (B) and (C), 7 pairs of D2 SPNs in four mice. For (E) and (F), 12 Foxp1D2_Flox and 14 Foxp1D2_vcKO D2 SPNs from three mice. Statistics: (C) Paired t test, [E (left) and F] MW test, and [E (right) t test]. *P < 0.05 and **P < 0.01.
Similar to embryonic deletion, EPSC amplitude was significantly smaller with postnatal Foxp1 deletion compared to neighboring control D2 SPNs (Fig. 5, B and C). We also observed a reduction in the frequency of mEPSCs with postnatal Foxp1 deletion; however, unlike embryonic deletion, there was an increase in amplitude (Fig. 5, D and E). This is consistent with fewer synapses but increased synaptic strength at each synapse. To assess the overall charge transfer through the mEPSCs, we multiplied the frequency and amplitude for each recording to obtain a single, “charge” parameter. Consistent with the reduced EPSC frequency, the total charge of mEPSCs was smaller with postnatal Foxp1 deletion (Fig. 5F). Similar observations were made with sEPSC measurements (fig. S6, A and B). As expected, we observed an increase in the intrinsic excitability and input resistance of these D2 SPNs (fig. S6, C and D).
Because postnatal deletion mimics the EPSC weakening observed with embryonic deletion, FOXP1-dependent positive regulation of excitatory inputs onto D2 SPNs is most likely due to postnatal FOXP1 function. Notably, while D2 SPN numbers decrease with embryonic deletion (8), this is not the case with postnatal deletion, suggesting that the observed FOXP1 regulation of glutamatergic inputs is not an indirect effect of decreased number of D2 SPNs.
Postnatal, postsynaptic reinstatement of Foxp1 is sufficient to rescue the intrinsic hyperexcitability of D2 SPNs
We next tested whether postnatal Foxp1 reinstatement is sufficient to rescue altered intrinsic excitability (19) and glutamatergic synaptic input in D2 SPNs. We cloned Foxp1 cDNA and an mCherry reporter into an AAV construct driven by the human synapsin 1 (hSyn) promoter (pAAV-hSyn-Foxp1-T2A-mCherry or Foxp1AAV; fig. S7A). Plasmids and viruses were tested in both human embryonic kidney (HEK) cells and the mouse striatum, respectively, to ensure the expression of FOXP1 protein (fig. S7, B and C, and see Materials and Methods for details). The control virus contained only mCherry reporter (pAAV-hSyn-mCherry or ControlAAV). At P1, we injected D2 Foxp1cKO and D2 Foxp1CTL mice expressing Drdr2-eGFPtg/− with either Foxp1AAV or ControlAAV into their striatum (Fig. 6A). The expression of reinstated Foxp1 was first detected at P7 through mCherry fluorescence (Fig. 6B), where we observed colocalization of eGFP and mCherry fluorescence in several neurons (highlighted with arrows in Fig. 6B). Our recordings were limited to neurons positive for both eGFP (marker of D2 SPNs) and mCherry (marker for infected neuron) (Fig. 6B and fig. S7D). We compared data among four groups: (i) D2 Foxp1CTL mice + ControlAAV, (ii) D2 Foxp1CTL mice + Foxp1AAV, (iii) D2 Foxp1cKO mice + ControlAAV, and (iv) D2 Foxp1cKO mice + Foxp1AAV.
Fig. 6. Postnatal Foxp1 reinstatement in D2 SPNs rescues electrophysiological changes due to embryonic Foxp1 deletion.
(A) Schematic describing ControlAAV and Foxp1AAV virus injection in the striatum and recording from D2 SPNs (created with BioRender.com). (B) eGFP-positive D2 SPNs and mCherry positive Foxp1AAV-infected neurons in D2 Foxp1cKO mice. Foxp1AAV-infected D2 SPNs (merge, yellow) are highlighted with arrows. (C) Example traces of action potential firing in D2 SPNs from D2 Foxp1CTL + ControlAAV; D2 Foxp1CTL + Foxp1AAV; D2 Foxp1cKO + ControlAAV; D2 Foxp1cKO + Foxp1AAV mouse groups. (D) Action potential (AP) number versus current step amplitude curves (F-I curves) for these D2 SPNs. (E) Input resistance of D2 SPNs at −55, −65, and −85 mV for all four mouse groups. (F) Current density versus voltage curves at different voltage steps (−20 to −140 mV). (G) Example traces of mEPSCs in D2 SPNs from all four mouse groups. (H) mEPSC frequency (left) and mEPSC amplitude (right) of D2 SPNs from all four mouse groups. Ns in all figures are (D2 Foxp1CTL + ControlAAV; D2 Foxp1CTL + Foxp1AAV; D2 Foxp1cKO + ControlAAV; D2 Foxp1cKO + Foxp1AAV). For (D) and (E), 36 D2 SPNs in three mice and 34 D2 SPNs in four mice and 33 D2 SPNs in five mice and 32 D2 SPNs in eight mice. For (F), 37 D2 SPNs in three mice and 34 D2 SPNs in three mice and 44 D2 SPNs in four mice and 31 D2 SPNs in three mice. For (H), 36 D2 SPNs in three mice and 31 D2 SPNs in three mice and 40 D2 SPNs in four mice and 28 D2 SPNs in three mice. Statistics: (D to F) Mixed effect analysis with Holm-Sidak correction for multiple comparisons. */**/*** show the statistics between Foxp1AAV + D2 Foxp1cKO and ControlAAV + D2 Foxp1cKO D2 SPNs. (H) One-way ANOVA with Holm-Sidak correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
We measured the intrinsic excitability of the D2 SPNs with current injections of incremental size (Fig. 6C) and obtained curves of action potential number as a function of step size for each neuron. As expected, (19), we observed intrinsic hyperexcitability with accompanying increased input resistance in D2 SPNs from the D2 Foxp1cKO + ControlAAV group (Fig. 6, C to E). With Foxp1 reinstatement, both excitability and input resistance were significantly reduced and not detectably different from D2 Foxp1CTL mice control groups (Fig. 6, C to E).
The increased intrinsic excitability in D2 SPNs resulting from Foxp1 deletion is primarily attributed to the down-regulation of two potassium currents—KIR, inward rectifying; KLeak, leak—(19). To investigate the effect of Foxp1 reinstatement on KIR and KLeak, we measured the induced currents in response to voltage steps (Fig. 6F). As anticipated, we replicated the decreased current density in D2 SPNs in the D2 Foxp1cKO + ControlAAV group at all voltage steps, and this reduction was partially rescued with Foxp1 reinstatement (Fig. 6F). We previously demonstrated that differences in the upper right quadrant largely stem from reduced KLeak, whereas differences in the lower left quadrant result from both KLeak and KIR (Fig. 6F and fig. S7, E and F) (19). While KIR is voltage dependent, KLeak is largely voltage independent (34–37). The current-voltage relation (I-V) curves in the Foxp1cKO + Foxp1AAV group exhibited similar proportionate rescue in current density at nearly all voltage steps, indicating that the rescued current was voltage independent. This suggests that postnatal Foxp1 reinstatement primarily rescues KLeak (fig. S7G).
Postnatal reinstatement of Foxp1 partially rescues the excitatory synaptic input onto D2 SPNs
We further investigated whether postnatal reinstatement of Foxp1 could rescue the weakening of glutamatergic inputs onto D2 SPNs by measuring mEPSCs and sEPSCs. As anticipated for ControlAAV groups, frequency, amplitude, and total charge transfer by mEPSCs were decreased in D2 SPNs in D2 Foxp1cKO mice (Fig. 6, G and H, and fig. S7H). Postnatal Foxp1 reinstatement partially restored the frequency and total charge of mEPSCs in D2 SPNs of the D2 Foxp1cKO + Foxp1AAV group (Fig. 6H, left, and fig. S7H). The frequency of mEPSCs was further enhanced with reinstatement of Foxp1 in D2 SPNs of Foxp1CTL mice, suggesting a direct Foxp1-mediated regulation. However, Foxp1 reinstatement did not affect mEPSC amplitude when compared to controls (Fig. 6H, right). Similar results were obtained for sEPSCs (fig. S7I). This lack of change in mEPSC/sEPSC amplitude is consistent with the idea that FOXP1 determines EPSC amplitude embryonically and not postnatally (see Discussion). To summarize, postnatal Foxp1 reinstatement in D2 SPNs partially rescues the weakened excitatory inputs induced by embryonic Foxp1 deletion.
snRNA-seq of P18 SPNs show reduced number of D2 SPNs with embryonic Foxp1 deletion
We investigated the cell-specific gene expression changes resulting from Foxp1 reinstatement using the previously described AAV strategy and performed snRNA-seq on P18 striatal tissue—the same age as for electrophysiological experiments. We used three mice from each of the following groups: (i) D2 Foxp1CTL + ControlAAV, (ii) D2 Foxp1cKO + ControlAAV, and (iii) D2 Foxp1cKO + Foxp1AAV (Fig. 7A). We obtained reads from a total of 89,745 nuclei across all three groups (fig. S8, A and B, and see Materials and Methods for details) and identified all major striatal cell types (fig. S8, C and D). Non-neuronal cells were distributed equally across all samples in the three genotypes (fig. S8E). The number of D2 SPNs was reduced with Foxp1 deletion, as previously reported (8), and this was accompanied by an increase in the number of eSPNs, progenitors, and neurogenic progenitors (fig. S8E).
Fig. 7. snRNA-seq of the striatum with postnatal reinstatement of Foxp1.
(A) Schematic showing the experimental strategy for differential gene expression (DGE) analysis of (i) “all” D2 SPNs from D2 Foxp1CTL mice and D2 Foxp1cKO mice and (ii) “selected” D2 SPNs from D2 Foxp1CTL mice infected with ControlAAV and D2 SPNs from D2 Foxp1cKO mice infected with ControlAAV and Foxp1AAV (created with BioRender.com). (B) UMAP for SPNs from the three groups of mice shows distinct clusters of the three SPN types. Different shades of red, green, and blue are indicative of subclusters in D1 SPNs, D2 SPNs, and eSPNs, respectively. (C) Volcano plot from DGE analysis of D2 SPNs from D2 Foxp1CTL and D2 Foxp1cKO demonstrate 1,538 genes down-regulated and 1222 genes up-regulated with Foxp1 deletion in D2 SPNs. [false discovery rate (FDR) ≤ 0.05, and log2 fold change ≥ 0.378]. (D) Gene Ontology plot for synaptic genes using SynGO for down-regulated DEGs. Gray color represents the subcategories of synaptic genes that do not overlap with DEGs, while blue color indicates a significant overlap (−log10 FDR > 1.3), where darker blue color implies smaller FDR values. The number of overlapping DEGs is stated below each category. (E) Gene Ontology plot for synaptic genes using SynGO for up-regulated DEGs. Gray color corresponds to no overlap, while red color demonstrates significant overlap, where darker red color depicts smaller FDR values. (F) Volcano plot from DGE analysis for D2 SPNs from D2 Foxp1cKO mice infected with ControlAAV and Foxp1AAV show 19 down-regulated and 59 up-regulated genes with Foxp1 reinstatement (FDR ≤ 0.05 and log2 fold change ≥ 0.378). (G) Expression heat map of 78 genes in each sample from three groups (Foxp1CTL + ControlAAV, Foxp1cKO + ControlAAV, and Foxp1cKO + Foxp1AAV). Gene symbols in red and blue shades are up-regulated and down-regulated DEGs, respectively, in D2 SPNs with Foxp1 reinstatement. FC, fold change.
Embryonic Foxp1 deletion in D2 SPN results in the down-regulation of postsynaptic genes
Next, we focused on examining the D2 SPNs in isolation by subclustering only the SPNs and retained 37,362 SPNs (Fig. 7B and fig. S8, F and G). To identify DEGs in D2 SPNs resulting from embryonic Foxp1 deletion, we compared D2 Foxp1CTL + ControlAAV mice with D2 Foxp1cKO + ControlAAV mice (fig. S8H). Our analysis revealed the down-regulation of 1538 genes and the up-regulation of 1222 genes in D2 SPNs of D2 Foxp1cKO + ControlAAV mice (Fig. 7C and table S1). Gene Ontology analysis of both up-regulated and down-regulated DEGs show their enrichment in pathways related to (i) postsynaptic and presynaptic transmission, especially glutamatergic synapses; (ii) ion channels, particularly potassium channels; (iii) G protein–coupled receptor signaling; and (iv) the formation of plasma membrane complexes (fig. S8I and table S2). Using SynGO for a detailed synaptic ontology, we found that most of the down-regulated genes were associated with postsynaptic functions (Fig. 7D and table S3), while up-regulated genes were enriched in both pre- and postsynaptic categories (Fig. 7E and table S3). This indicates a differential role for Foxp1-dependent regulation of synaptic genes, where positive regulation of synaptic genes has a strong postsynaptic bias.
Consistent with the SynGO findings, an analysis of down-regulated genes highlights ionotropic and metabotropic glutamate receptors as possible candidates for the reduced excitation onto D2 SPNs in D2 Foxp1cKO mice. We found a significant down-regulation of Grin1a and Grin2a transcripts, which encode the NMDAR subunits GluN1 and GluN2, respectively, as well as group I metabotropic glutamate receptors, Grm1 and Grm5. Alternatively, reduced excitatory transmission may be due to down-regulation of postsynaptic scaffold genes that are involved in glutamatergic transmission, such as Dlg4 (encoding PSD95) and Dlgap3 (also known as Sapap3 or Gkap3) (38–43). The expression of AMPAR subunit encoding genes did not change with the loss of Foxp1.
Postnatal reinstatement of Foxp1 reverses the expression changes of several genes that are dysregulated with Foxp1 deletion in D2 SPNs
Next, we examined the gene expression changes in D2 SPNs after postnatal Foxp1 reinstatement. Because of the inability to target all D2 SPNs with AAV injection, we used a strategy to isolate infected D2 SPNs (see Materials and Methods) and performed differential gene expression analyses for two comparisons: (i) “embryonic deletion” using D2 Foxp1cKO + ControlAAV and D2 Foxp1CTL + ControlAAV mice (fig. S9, A to C) and (ii) “postnatal reinstatement” using D2 Foxp1cKO + Foxp1AAV and D2 Foxp1cKO + ControlAAV mice (Fig. 7, F and G). For the embryonic deletion analysis, we identified 1196 down-regulated and 942 up-regulated genes in D2 Foxp1cKO + ControlAAV mice (fig. S9C and table S1). These DEGs greatly overlapped with the “all D2 SPNs” analysis described above (Fig. 7C). In the postnatal reinstatement analysis, we found 59 genes up-regulated and 19 genes down-regulated in Foxp1cKO + Foxp1AAV mice (Fig. 7, F and G). Sixty-eight of 78 of these postnatal reinstatement DEGS were also DEGs in the embryonic deletion analysis, but the transcript level changes for all 68 were reversed compared to deletion and hence were considered rescued (Fig. 7G). Of these, 27 DEGs were not significantly different from Foxp1CTL + ControlAAV mice indicating a full rescue (Fig. 7G). The remaining 10 of 78 DEGs were not identified as DEGs in the embryonic deletion analysis and were exclusively regulated postnatally by FOXP1.
Gene Ontology analysis of the up-regulated rescued genes revealed enrichment in pathways related to synapse regulation, AMPK signaling, cell migration, and negative regulation of apoptosis (fig. S9D and table S2). SynGO analysis suggested a preferential rescue of down-regulated postsynaptic genes and up-regulated presynaptic genes (fig. S9E and table S3)—again consistent with FOXP1-dependent positive regulation of synaptic genes having a postsynaptic bias. Notably, there was an increased expression of genes involved in regulating plasticity and other aspects of striatal glutamatergic synaptic function, such as Ppp1r1b (DARPP32), Reln, Sema3e, and Lrrk2 (44–51), suggesting potential candidates for the rescued synaptic function phenotype observed with Foxp1 reinstatement. Relevant to our observation of FOXP1-dependent regulation of excitability, Kcnk2 (Fig. 6F) (19), the gene that encodes leak potassium channel, is down-regulated with embryonic Foxp1 deletion and rescued with postnatal Foxp1 reinstatement (Fig. 7G). Last, the rescued expression of genes involved in the negative regulation of apoptosis such as Igf1r, Spry2, and Sema3e indicates a role for FOXP1 in survival of D2 SPNs as its loss leads to a reduced number of D2 SPNs (52–56).
Reinstatement of Foxp1 rescues behavioral phenotypes induced by Foxp1 deletion in both D1 and D2 SPNs
We next determined whether postnatal Foxp1 reinstatement can rescue the previously reported motor behavior alterations in D2 Foxp1cKO mice (8). We injected ControlAAV and Foxp1AAV bilaterally into the striatum of both D2 Foxp1CTL and D2 Foxp1cKO mice at P1 and tested their behavior at 8 to 10 weeks of age. D2 Foxp1cKO mice recapitulated the motor learning deficit on the rotarod; however, this phenotype was not rescued with Foxp1 reinstatement (Fig. 8A).
Fig. 8. Postnatal Foxp1 reinstatement rescues the behavior of mice with the loss of Foxp1 in D1 and D2 SPNs.
(A) Latency to fall from the rotarod in four consecutive trials for each of the 3 days (total 12 trials) in Foxp1CTL + ControlAAV, Foxp1CTL + Foxp1AAV, D2 Foxp1cKO + ControlAAV, and D2 Foxp1cKO + Foxp1AAV mice. (B and C) Velocity and time spent in the periphery of the open-field box for Foxp1CTL + ControlAAV, Foxp1CTL + Foxp1AAV, D1D2 Foxp1cKO + ControlAAV, and D1D2 Foxp1cKO + Foxp1AAV mice. (D) Nesting score of the mice from all four groups as stated in (B) and (C). We used the same control mice (Foxp1CTL) for the comparison with D2 Foxp1cKO and D1D2 Foxp1cKO mice groups. Number of mice were 27 Foxp1CTL + ControlAAV, 21 Foxp1CTL + Foxp1AAV, 20 D2 Foxp1cKO + ControlAAV, 15 D2 Foxp1cKO + Foxp1AAV, 10 D1D2 Foxp1cKO + ControlAAV, and 10 D1D2 Foxp1cKO + Foxp1AAV mice. Statistics: (A) Two-way ANOVA with Holm-Sidak correction for multiple comparisons and (B to D) one-way ANOVA with Holm-Sidak correction for multiple comparisons. *P < 0.05, ***P < 0.001, and ****P < 0.0001. ns, not significant.
To further test the potential of postnatal Foxp1 reinstatement to rescue motor deficits with loss of Foxp1, we used mice with simultaneous Foxp1 deletion in both D1 and D2 SPNs (D1D2 Foxp1cKO). These mice exhibit more robust behavioral changes, making them suitable for detecting phenotypic rescue. In line with previous work (8), D1D2 Foxp1cKO + ControlAAV mice displayed hyperactivity and spent more time in the peripheral zone of the open-field paradigm compared to Foxp1CTL + ControlAAV mice (Fig. 8, B and C). With Foxp1 reinstatement, the velocity and time spent in the periphery zone were no longer detectably different from control mice, suggesting a complete rescue of the phenotype (Fig. 8, B and C). In addition, the previously reported nesting impairment observed in D1D2 Foxp1cKO mice was partially rescued with Foxp1 reinstatement (Fig. 8D). Nevertheless, rotarod deficits were not rescued in these mice (fig. S10). In summary, postnatal reinstatement of Foxp1 was able to rescue some of the behavioral phenotypes driven by simultaneous embryonic Foxp1 deletion in both striatal SPN subtypes.
DISCUSSION
Summary
Using cell-specific embryonic deletion mouse models, we find that the transcription factor, FOXP1, promotes the maturation and strengthening of long-range glutamatergic inputs selectively onto D2 SPNs in the striatum. This process is mediated by mechanisms that are cell-autonomous, postsynaptic, and postnatal. These effects are driven by a FOXP1-dependent transcription program that involves synaptic genes. This regulation differs between pre- and postsynaptic genes where positively regulated genes showed a strong postsynaptic bias (Fig. 7, D and E). Postnatal Foxp1 reinstatement successfully rescued both physiological and behavioral phenotypes. D2 SPNs with reinstated Foxp1 exhibited a reversal in the expression of several genes that had been altered by embryonic deletion, indicating a rescued profile.
FOXP1 balances the excitatory inputs onto SPNs
Synaptogenesis and strengthening of glutamatergic inputs onto SPNs are most prominent between P8 and P20 (3, 4, 57). This process is governed by intracellular signaling pathways as well as presynaptic and postsynaptic activity (2–5). However, the role of transcription factors in directing these inputs is relatively underexplored (10). Our results were obtained during this critical developmental time window and connect FOXP1 function with strengthening of these inputs. Moreover, during this time, maturation of glutamatergic inputs onto SPNs involves a decreased contribution of NMDAR-mediated transmission (30). Our findings, illustrated by an increased proportion of NMDAR-mediated responses following Foxp1 deletion (Fig. 2D), suggest that FOXP1 plays a facilitative role in the maturation of excitatory synapses by reducing the relative proportion of synaptic NMDARs.
In addition to the direct action of FOXP1, it is possible that the glutamatergic synaptic input changes, resulting from either Foxp1 deletion or reinstatement are mediated by the change in intrinsic excitability through a FOXP1-independent process. Conversely, the inverse could also hold true, where alterations in synaptic input induce changes in intrinsic excitability. Previous studies have already established a close relationship between intrinsic excitability and synaptic transmission (58–61). Therefore, the observed reduction in synaptic excitation induced by Foxp1 deletion might be a FOXP1-independent homeostatic response to compensate the increased intrinsic excitability (62). Similarly, the combination of reduced intrinsic excitability and increased synaptic excitation in hippocampal neurons following Foxp1 deletion may also represent a similar homeostatic response (18).
In our experimental paradigm, contralateral inputs originate from a single population of intratelencephalic layer 5 pyramidal neurons (26) devoid of any thalamic contribution. Consequently, the light-evoked responses observed in contralateral SPNs were induced from well-isolated corticostriatal inputs (Figs. 1 to 5). In contrast, our measurements of mEPSC/sEPSC and responses evoked by extracellular electrical stimulation included long-range glutamatergic inputs originating from both cortical and thalamic neurons (Figs. 1 to 6). However, the fact that we observed a similar effect on both evoked (light and electrical) and spontaneous recordings suggest a common regulation of both input pathways or a strong regulation of corticostriatal inputs that can be observed even in the presence of thalamic inputs. Either way, changes observed with these measurements likely reflect changes in corticostriatal inputs.
FOXP1 is required for both embryonic and postnatal development of corticostriatal circuitry
FOXP1 is crucial for normal brain development during embryonic and postnatal stages. During early embryonic stages, it is involved in the self-renewal and differentiation of neural stem cells (63, 64), formation of midbrain dopaminergic neurons (65), and migration of several neuronal types within the developing cortex (64, 66–68). In addition, in the striatum, embryonic FOXP1 is essential for the development of D2 SPNs (8). During postnatal stages, FOXP1 continues to promote the survival of SPNs while inhibiting apoptosis and oxidative stress (20, 69, 70). Our findings are consistent with a postnatal function for FOXP1 in regulating the intrinsic excitability of D2 SPNs (19) and glutamatergic synaptic input onto these neurons (Figs. 1, 2, and 5). We show that postnatal function is both necessary and sufficient for regulation of these physiological properties by implementing postnatal deletion and reinstatement, respectively (Figs. 5 and 6). These data also indicate that the decrease in D2 SPN numbers induced by embryonic deletion is a distinct phenomenon and is independent of the accompanying electrophysiological changes.
An exception to the role of postnatal FOXP1 regulation of excitatory input is related to mEPSC/sEPSC amplitude. The frequency and amplitude of mEPSCs/sEPSCs attain maturity at different time points in SPNs during postnatal development (30). We also observe a differential FOXP1-mediated development of these properties. In contrast to embryonic deletion, postnatal Foxp1 deletion did not reduce mEPSC/sEPSC amplitude (Fig. 5 and fig. S6 versus Fig. 1 and figs. S1 and S2). Moreover, the amplitude remained unchanged with postnatal reinstatement of FOXP1 (Fig. 6 and fig. S7). On the contrary, mEPSC/sEPSC frequency decreased with both embryonic and postnatal deletion and was subsequently rescued with postnatal reinstatement (Figs. 1, 2, 5, and 6, and figs. S1, S2, and S7). Assuming the frequency of mEPSCs/sEPSCs represents the number of synapses and amplitude corresponds to the strength of individual synapses, our data suggests a dual FOXP1 function to promote both the number of synapses and synaptic strength.
snRNA-seq analysis indicates postsynaptic candidate genes for FOXP1-mediated synaptic regulation
Our data strongly suggests that FOXP1 positively regulates glutamatergic inputs onto D2 SPNs, likely by modulating the expression of key synaptic genes. A significant number of down-regulated synaptic genes in D2 SPNs following embryonic Foxp1 deletion are primarily postsynaptic and are relevant with the observed synaptic phenotypes. We found decreased expression of Grin1a, Grin2a, Grm1, and Grm5, which is consistent with the reduction in NMDAR-mediated currents. Grin1a/2a encode NMDAR subunits, while Grm1/5 encode for mGluR1/5. Both mGluR1/5 are abundantly expressed postsynaptically in striatal SPNs and are involved in excitatory synaptic transmission by activating the phospholipase C pathway (71, 72). Moreover, mGluR1/5 potentiate the activation of NMDARs in SPNs by enhancing their expression on the membrane surface (71–74). Although we observed higher expression of Grin3a (GluN3) and Grm8 (mGluR5), it is not expected to enhance glutamatergic transmission. GluN3 subunits are primarily activated by glycine, not glutamate (75), and mGluR8 is expressed presynaptically, where it decreases the NMDA receptor activity. Despite a substantial decrease in AMPAR-mediated transmission with embryonic deletion, we did not observe changes in the transcripts encoding AMPAR subunits. However, we did observe down-regulation of Dlg4 and Dlgap3, which regulate postsynaptic receptor trafficking and AMPAR-mediated transmission and therefore could account for the reduced AMPAR currents (38–43). In summary, we observe transcriptional changes in postsynaptic genes that may directly and indirectly participate in FOXP1-dependent regulation of synaptic function in D2 SPNs.
Postnatal FOXP1 reinstatement rescues gene expression and cellular electrophysiology
Foxp1 deletion in D2 Foxp1cKO mice occurs during an early phase of striatal development (E14 to E15), leading to a substantial reduction in the population of D2 SPNs. Postnatal reinstatement of Foxp1 expression occurred at P7, a stage when most neurons have largely acquired their identity. This postnatal reinstatement of FOXP1 effectively rescues most of the physiological properties of D2 SPNs while also altering the expression of certain genes. Most of these altered genes (68 of 78 postnatal reinstatement DEGs, 87%) were in fact a subset of “embryonic deletion DEGs.” Reversal in their expression levels with postnatal reinstatement thus indicates a genetic rescue. While reinstatement DEGs represent a minority among the large number of embryonic deletion DEGs, their impact is substantial enough to rescue the affected neuronal physiology of D2 SPNs. For instance, specific candidate genes observed with embryonic deletion—such as Grin1a/2a or Grm1/5—did not exhibit differential gene expression during postnatal reinstatement. This suggests that the transcriptional mechanisms underlying embryonic deletion and postnatal reinstatement may overlap but, at the same time, differ, perhaps due to differences in electrophysiological and synaptic processes that occur during embryonic versus postnatal periods.
Alternatively, up-regulation of other postsynaptic genes that were rescued with reinstatement may be more directly involved in FOXP1-dependent regulation of glutamatergic synaptic function. Some of these genes may have been more relevant to embryonic deletion as compared to the obvious receptor and scaffolding candidates discussed above. For example, Leucine-rich repeat kinase 2 (Lrkk2) is highly expressed in the SPNs (49, 76–78) and plays a critical role in regulating synaptogenesis and corticostriatal glutamatergic synaptic function (48, 79, 80). Another gene of interest, Protein phosphatase 1 regulatory inhibitor subunit 1B (Ppp1r1b, also known as DARPP32) is critical for the corticostriatal synaptic plasticity and controls both long-term potentiation and depression in the striatum (45–47). Last, Reln is another gene that has been implicated in regulating the synaptic plasticity in the striatum and hippocampus (44, 81). Together, our data suggest that these genes likely contribute to the intricate network of molecular factors that regulate synaptic function and plasticity through regulating AMPAR/NMDAR-mediated currents in D2 SPNs.
The rescued expression of Kcnk2 is particularly noteworthy, as it appears to be tightly regulated by FOXP1 and is consistently associated with its function (KLeak) in our studies (8, 17, 19). In our previous study, we showed that Foxp1 deletion in D2 SPNs leads to intrinsic hyperexcitability, primarily due to reduced KLeak and decreased expression of Kcnk2 (19). In contrast, Foxp1 reinstatement enhances Kcnk2 levels, which likely rescues the intrinsic excitability phenotype by increasing KLeak. This suggests a transcriptional mechanism through which FOXP1 directly regulates KLeak and thereby intrinsic excitability. To our knowledge, this is one of the very few clear and reproducible demonstrations of a transcriptional mechanism regulating electrophysiological function in neurons (24).
We did not observe a rescue in KIR currents in the current study. We attribute this lack of rescue to the early onset of differences in KIR currents between D2 Foxp1CTL and D2 Foxp1cKO, manifesting as early as P4 to P6 (19). The rescue construct re-expresses Foxp1 at least by P7 and possibly misses the critical developmental window for effective restoration of KIR channels. This temporal misalignment between the emergence of differences and the expression of the rescue construct could explain the observed lack of improvement in KIR currents.
Foxp1 deletion in D1 and D2 SPNs induces non–cell-autonomous effects in D2 and D1 SPNs, respectively
We observed non–cell-autonomous effects in both D1 and D2 SPNs with Foxp1 deletion in D2 and D1 Foxp1cKO mice, respectively. In D1 Foxp1cKO mice, D2 SPNs exhibited intrinsic hypoexcitability (fig. S4B) and reduced input resistance (fig. S4C). Further, these SPNs showed increased sEPSC frequency (fig. S4A). Similarly, in D2 Foxp1cKO mice, the short-term dynamics of EPSC amplitude during a 5-Hz train were altered in D1 SPNs (fig. S1I). These findings are consistent with our previous work where we found deficits in the projections of D1 SPNs to the globus pallidus in D2 Foxp1cKO mice (8). In addition, several non–cell-autonomous DEGs were identified in both D1 and D2 SPNs following Foxp1 deletion in D2 and D1 SPNs, respectively (8). The mechanisms underlying these changes are mostly unknown and may involve compensatory or homeostatic processes within the corticostriatal circuitry; however, it would require further investigation.
Limitations
Our usage of Drd2-Cre mouse line is consistent with our previous studies (8, 19). However, there may be a confound in using these mice as Drd2 receptors are also expressed in striatal interneurons and dopaminergic substantia nigral (SN) neurons (82–86), which innervate SPNs. Therefore, while we attribute changes in glutamatergic inputs to postsynaptic FOXP1 function in D2 SPNs, it is possible that there are alternative loci of FOXP1 function. For example, SN inputs are known to modulate corticostriatal synaptic plasticity, and Foxp1 deletion in these SN neurons could be the mechanism mediating our observed changes. However, the alternative loci for FOXP1 function are unlikely as Foxp1 is either lowly expressed or not expressed in these neurons (87, 88). Moreover, we recapitulated most of the embryonic phenotypes and successfully rescued them with postnatal interventions using Cre virus restricted to the striatum. The Adora2a-Cre mouse line could serve as an alternate mouse model to avoid these possible confounds (89).
Our study is primarily focused on investigating the role of FOXP1 in the early development of glutamatergic synaptic inputs onto striatal neurons. Consequently, we did not examine striatal GABAergic inhibition or the role of FOXP1 in adult striatal neurons. Moreover, as our primary aim was to investigate cellular mechanisms, we did not acquire data related to systems-level physiology. Note that while we did not observe deficits in synaptic transmission of D2 SPNs in Foxp1Het mice, we anticipate the possibility of these effects in adult mice because these mice would experience a prolonged period of reduced FOXP1 presence during development. However, because of the large scope of the study and to maintain consistency across different genetic models, we used mice aged 2 to 3 weeks. Furthermore, while we used evoked transmission for most of our assays, it was not used for all. Because of the technical constraints limiting the number of fluorophores used, we did not record evoked synaptic responses from D2 SPNs in mice for FOXP1 reinstatement experiments. Instead, we measured sEPSCs and mEPSCs, and we have provided arguments to justify this approach. Last, the links that we established between gene expression and synaptic physiology in the context of FOXP1 regulation are correlational and intended to provide candidate targets for underlying mechanisms. In summary, our study serves as a foundational framework and provides context for addressing the issues above in subsequent follow-up investigations.
MATERIALS AND METHODS
Experimental mice
We used C57BL/6J background mice for our experiments consistent with our previous study (19). We used Foxp1flox/flox mice (21, 90) and BAC-transgenic lines for fluorescence reporter [Drd2-eGFPtg/− and Drd1α-tdTomato (tdTom)tg/− (91)] and for Cre [Drd2-Cretg/− and Drd1α-Cretg/− (92)]. Drd2-specific Foxp1 conditional knockout mice were Drd2-Cretg/−:Foxp1flox/flox:Drd2-eGFPtg/− (D2 Foxp1cKO), whereas control mice were Foxp1flox/flox:Drd2-eGFPtg/− (D2 Foxp1CTL). Similarly, Drd1α-specific Foxp1cKO were also triple transgenic: Drd1α-Cretg/−:Foxp1flox/flox:Drd1α-tdTomtg/− (D1 Foxp1cKO) mice and control mice are Foxp1flox/flox:Drd1α-tdTomtg/− (D1 Foxp1CTL) (19). Both Cre lines turn on between E14 and E15, which coincides with the start of Foxp1 expression in striatal cells (8). We used both male and female mice that were maintained on 12-hour light on/12-hour off schedule with access to food and water ad libitum. All experiments were performed according to the procedures approved by the UT Southwestern Institutional Animal Care and Use Committee.
Electrophysiology methods
Brain slices and recordings
We used mice aged P15 to P18 for the electrophysiological measurements. Mice were anesthetized with a combination of ketamine (125 mg/kg) and xylazine (25 mg/kg), and brains were quickly dissected and transferred to partially frozen dissection buffer (19). Brain slices (300 μm thick) containing the dorsal striatum were prepared using a Vibratome (Leica VTS 1200S) and immediately placed in nominal artificial cerebrospinal fluid (nACSF) (bubbled with a mixture of 5% CO2 and 95% O2), first at 35°C for 30 min and then at room temperature for at least 30 min before commencement of recordings (19, 93, 94). We used glass pipettes of 5- to 8-megohm resistance to perform whole-cell patching on D1 and D2 SPNs in D1 and D2 (Foxp1cKO or Foxp1CTL) mice, which were identified based on the expression of tdTom and eGFP reporter, respectively. In certain instances, we adopted a “negative” identification approach where cells negative for eGFP and tdTom fluorescence were considered as D1 and D2 SPNs, respectively. This approach proved reliable as 95% of striatal neurons are SPNs and can be distinguished based on their smaller size (91). We included neurons with a maximum acceptable resting membrane potential of −50 mV, capacitance greater than 15 pF, stable baseline current within 15 pA, and a series resistance of less than 20 megohm in our analysis. The average series resistance did not exhibit significant differences between Foxp1cKO and Foxp1CTL neurons. The junction potential was ~10 mV and was not corrected.
All recordings were performed at a temperature of 30°C. To maintain consistency with our previous study, we adopted the same nomenclature for most of the experiments. Wild-type (WT) control neurons and Foxp1-deleted neurons were referred to as Foxp1CTL and Foxp1cKO D1/D2 SPNs, respectively. For the D2 SPNs, where Foxp1 was deleted postnatally using AAV-mediated Cre expression, uninfected neurons were named Foxp1D2_Flox D2 SPNs, while AAV-infected neurons were named Foxp1D2_vcKO SPNs.
Unless stated otherwise, we recorded and analyzed all electrophysiological parameters using custom software [Labview, National Instruments, Austin, TX; https://github.com/ColdP1228/Custom-Data-Acquisition-Program- (32); copy archived at https://github.com/elifesciences-publications/Custom-Data-Acquisition-Program-].
Current steps, input resistance, and multivoltage step protocols
For these measurements, we followed our previously published protocol (19). Briefly, we applied incremental current steps (50 pA, 500-ms duration) at the resting potential in current clamp mode to assess intrinsic excitability. The resulting number of action potentials elicited at each step was plotted to generate “firing versus current injection” curve.
To determine input resistance, we applied a single −10-mV voltage step (500-ms duration) in voltage clamp mode and measured input resistance based on the average current values within a 100-ms window before and 200 ms after the step onset. Input resistance was measured either at −85-, −65-, and − 55-mV holding potentials or solely at −65-mV holding potential depending on the specific experimental requirements.
For the IV plots at various voltages, we applied a multi-step voltage protocol (10-mV steps, 500-ms duration) while maintaining a holding potential of −70 mV (ranging from −20- to −140-mV voltages). We measured the average current within a 200-ms window at the end of the step and divided it by the capacitance to obtain the current density. Capacitance was measured as described earlier (19). For these measurements, nACSF contained TTX (1 μM).
Calculating rescued current density
For fig. S7G, we first calculated the loss of current due to Foxp1 deletion by subtracting the current densities of D2 SPNs in D2 Foxp1cKO + ControlAAV from those in D2 Foxp1CTL + ControlAAV mice at each voltage. Rescued current density was calculated as the difference between D2 Foxp1cKO + Foxp1AAV and D2 Foxp1cKO + ControlAAV measurements. Last, the ratio of rescued and lost current was the % rescued current density at respective voltages. The current density at −20 to −60 mV is exclusively KLeak, whereas it is a combination of KLeak and KIR at −90- to −140-mV voltages.
ChR2-mediated optogenetic stimulation of corticostriatal evoked postsynaptic currents EPSCs
We used commercially prepared pAAV9.CAG.hChR2(H134R)-mCherry.WPRE.SV40 (Addgene, catalog no. 100054) viral particles expressing mCherry-tagged human channel rhodopsin (hChr2) to stimulate corticostriatal inputs. Briefly, we diluted the viral particles to a titer of ~1012 viral particles (vp)/ml in sterile 1X phosphate-buffered saline (PBS) and added 1 to 2 μl of diluted Fast Green FCF dye (Sigma-Aldrich) for visualization and performed injections as described previously (19, 32, 33). P1 pups were anesthetized on ice, and we injected 350 nl of diluted viral particles into the superficial cortical layers (0.5 mm underneath the skull) on the left hemisphere using a beveled glass injection pipette and a Nanoject II injector (Drummond Scientific Inc.). The pups were allowed to recover on a heating pad until they regained consciousness and were then transferred to their home cage. Slices were prepared from P14 to P18 juvenile mice, and contralateral projections from ChR2-AAV–infected cortical neurons were stimulated with blue light to trigger synaptic responses in the striatal neurons in the right hemisphere. To avoid possible contamination by ChR2 expression in ipsilateral striatal neurons, we recorded from slices prepared from the contralateral right hemisphere.
The strength of the evoked response in SPNs using this protocol could vary due to multiple factors, including variability in slice preparation, efficiency of viral infection, density of corticostriatal inputs, and ChR2 expression in the contralateral striatum (Fig. 1A). This variability could obscure the changes in the amplitude of synaptic responses induced by Foxp1 deletion. Therefore, to mitigate this concern, we measured EPSCs in neighboring neuron pairs (<50-μm distance) consisting of a GFP-positive D2 SPN and a GFP-negative D1 SPN in D2 Foxp1cKO mice. In this design, D1 SPNs consistently expressed Foxp1 and served as a control comparison. Similarly, in D1 Foxp1cKO mice, we recorded from pairs consisting of a tdTom-positive D1 SPN and tdTom-negative D2 SPN. For each recording pair, both the D1 and D2 SPN were stimulated with blue light of identical intensity and were patched in alternating sequence (Fig. 1A). In Fig. 1 (B and C), we observed a trend for increased absolute amplitude in D1 SPNs in D2 Foxp1cKO mice compared to controls. This trend might have stemmed from the increased stimulation intensity required to elicit a measurable EPSC in D2 SPNs in D2 Foxp1cKO mice, possibly due to weaker cortical inputs. However, this speculation is not conclusive. Regardless, we refrain from making definitive conclusions regarding absolute EPSC amplitude.
AMPAR- and NMDAR-mediated EPSCs
We recorded action potential-dependent AMPAR-mediated EPSCs from D2/D1 SPNs at a holding potential of −70 mV in striatal slices incubated in nACSF containing 2 mM CaCl2 and 2 mM MgCl2. Corticostriatal axons were stimulated by a 12-ms blue light (470 nm) pulse from a fluorescent lamp (X-Cite, Lumen Dynamics) through a 40× water immersed objective (Olympus). Similar intensity light, with power ranging from 1 to 85 mW/mm2 (resulting in a final beam diameter of 340 μm and power of 0.1 to 7.6 mW), was used to record responses from D1 and D2 SPNs in each pair.
We measured the amplitude, onset latency, response rise time, and slope of the EPSC responses. Amplitude is the maximum current response in pA to the individual light pulse. Onset latency is used as a measure to filter out nonsynaptic responses. SPNs infected due to the leak of AAV-ChR2 from the ipsilateral cortex have onset latencies of 1 to 2 ms; however, all responses obtained from the SPNs in contralateral striatum had onset latency of more than 5 ms, indicative of pure synaptic currents. Response rise time is the time (in ms) to reach the peak response. Last, we used a 5- to 15-ms time window, relative to the stimulus onset, and measured the slope of the AMPAR-mediated EPSC responses between 20 and 80% of the rise time.
To study the effect of Foxp1 deletion on release probability, we stimulated afferent axons with a train of five blue light pulses of 200-ms interval and 12-ms duration and recorded the responses from both D1 and D2 SPNs (Fig. 1G). The ratio of the peak amplitude of second to the fifth response relative to the first amplitude response indicated release probability. If the relative decrease in input to the neurons is due to a decrease in release probability, then we would expect an increase in EPSC amplitudes during the train.
We also measured AMPAR- and NMDAR-mediated responses in the absence of action potential firing. This protocol isolates monosynaptic EPSCs while avoiding contamination from disynaptic inputs and was based on a previous study (29). The protocol involved recording in the presence of TTX (1 μM), picrotoxin (100 μM), and 4-AP (100 μM) to block voltage-dependent Na+ channels, γ-aminobutyric acid (GABA) type A receptors, and K+ currents, respectively. We used an NMDAR agonist, glycine (20 μM), and reduced MgCl2 (0.5 mM) to activate and record NMDAR-mediated currents while maintaining 3 mM CaCl2 concentration to balance cation levels. We also used cesium-based internal pipette solution to enhance the resolution of NMDAR-mediated currents.
We first recorded amplitude, slope, latency, and release probability of AMPAR-mediated evoked EPSCs as explained above at −70-mV holding potential. Subsequently, we depolarized the neurons to +50 mV to record NMDAR-mediated evoked EPSCs. Corticostriatal axons were stimulated with the same intensity of blue light while recording both D1 and D2 SPNs in a pair. NMDAR-mediated responses are slower in rise and decay than the AMPAR-EPSCs and initial 20- to 40-ms fast EPSC response could have contamination from AMPAR-mediated responses. Therefore, we measured the peak NMDAR-mediated amplitude in a 90- to 100-ms window after the onset of EPSCs.
Spontaneous and miniature EPSCs
The synaptic inputs onto SPNs in our measurements likely involve a combination of corticostriatal and thalamostriatal inputs. However, assuming that corticostriatal inputs contribute to the AMPAR/NMDAR differences, we can relate changes in sEPSC/mEPSC to our corticostriatal results. For sEPSC recordings, neurons were voltage clamped at −70 mV and spontaneously occurring synaptic events were recorded in 10 traces, each lasting 10 s. Similarly, mEPSCs were recorded for the same duration with nACSF supplemented with TTX (1 μM), picrotoxin (100 μM), and 4-AP (100 μM). Data analysis was performed using an automatic detection program (MiniAnalysis, Synaptosoft Inc, Decatur, GA) with a detection threshold at a value exceedingly at least five SDs (10 pA) of the baseline noise. In addition, we visually confirmed the detected events. The detection threshold remained constant throughout each recording.
Synaptically driven excitability
Synaptically driven excitability curves were generated by applying blue light (with a diameter of 300 μm, centered at the recorded cell) to depolarize corticostriatal axons expressing ChR2. D1/D2 SPNs were patched and maintained in current clamp at −55 mV, and blue light flashes (12 ms) with power ranging 1 to 85 mW/mm2 were applied efficiently to determine the threshold stimulus intensity. We started with half of the maximum power (40 mW/mm2) and adjusted the power either up or down depending on whether an action potential was evoked or not, respectively. When we approached the threshold intensity, we made smaller changes in the light intensity to precisely determine the threshold stimulus. To facilitate the comparison of the threshold intensity between two groups (fig. S5A), we calculated the log10 value of the threshold stimulus for each neuron. For neurons that did not elicit an action potential even at the maximum possible light stimulus (85 mW/mm2), we considered maximum light intensity as the threshold intensity. In addition to estimating the threshold light intensity, we also calculated the probability of firing an action potential at various stimulus intensities for these neurons.
Electrophysiology solutions
We prepared brain slices in a semi-frozen dissection buffer (bubbled with 95% O2 and 5% CO2) containing the following components: 110 mM choline-Cl, 25 mM dextrose, 25 mM NaHCO3, 3 mM ascorbic acid, 3.1 mM sodium pyruvate, 2.5 mM KCl, 1.25 mM NaH2PO4, 7 mM MgCl2, 0.5 mM CaCl2∙2H2O, and 0.2 mM kyneuric acid, with a pH of 7.3 to 7.4 and an osmolality of 280 to 285 mOsm. The slices were transferred to nACSF that contained 25 mM NaCl, 25 mM NaHCO3, 10 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM MgCl2, and 2 mM CaCl2∙2H2O with a pH of 7.3 to 7.4 and an osmolality of 300 to 305 mOsm, saturated with 95% O2/5% CO2. The pipette (internal) solution consisted of 130 mM K-Gluconate, 10 mM Hepes, 6 mM KCl, 3 mM NaCl, 0.2 mM EGTA, 14 mM phosphocreatine-tris, 4 mM adenosine 5′-triphosphate (ATP)–Mg, and 0.4 mM guanosine 5′-triphosphate (GTP)–tris with a pH of 7.25 and an osmolality of 300 mOsm.
For NMDAR-mediated currents, nACSF was modified and consisted of 125 mM NaCl, 25 mM NaHCO3, 10 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 0.5 mM MgCl2, 3 mM CaCl2∙2H2O, 0.1 mM picrotoxin, 0.1 mM 4-AP, and 0.02 mM glycine. Also, the internal pipette solution was revised and contained 125 mM Cs-gluconate, 10 mM Hepes, 5 mM TEA-Cl, 3 mM NaCl, 2.5 mM BAPTA, 0.6 mM EGTA, 14 mM phosphocreatine-tris, 4 mM ATP-Mg and, 0.4 mM GTP-tris with a pH of 7.4 and an osmolality of 290 mOsm.
Viral-mediated postnatal deletion of Foxp1
We used AAV-mediated Cre expression to perform postnatal Foxp1 deletion. AAV9 particles expressing eGFP-Cre fusion protein (pAAV.CMV.HI.eGFP-Cre.WPRE.SV40) were obtained commercially (Addgene, catalog no. 105545). Briefly, viral particles were diluted to a titer of ~1012 vp/ml in sterile 1X PBS with the addition of Fast Green FCF dye (Sigma-Aldrich) for visualization. P1 Foxp1flox/flox:Drd1α-tdTomtg/− C57BL/6J pups were anesthetized on ice, and 350 nl of diluted viral particles were injected into the striatum at a depth of 1.5 mm as explained above. For AAV-Cre mediated postnatal Foxp1 deletion, we used Foxp1flox/flox:Drd1α-tdTomtg/− mice and specifically targeted smaller, tdTom-negative neurons, assuming these to be D2 SPNs (91). The eGFP fluorescence in the nuclei served as a marker for AAV-mediated Cre expression. In each case, an infected (GFP-positive) D2 or D1 SPN was paired with an adjacent (<50-μm distance) uninfected (GFP-negative) D2 or D1 SPN, which served as a control.
We did not use ChR2-mediated stimulation of corticostriatal projections for the EPSCs measurement in D2 SPNs with postnatal Foxp1 deletion. This was because strong mCherry fluorescence in the ChR2-expressing afferents made the reliable detection of tdTom-positive D1 SPNs challenging. Instead, we induced EPSCs using an extracellular stimulating bipolar 2-conductor cluster electrode positioned in the cortical projections within approximately 50- to 100-μm distance from the recorded D2 SPN. We used monophasic current pulses (10 to 300 μA, 0.2 to 1 ms) for stimulation. The same current intensity was applied sequentially from the stimulating electrode to both infected and uninfected D2 SPN pair.
Viral-mediated postnatal reinstatement of Foxp1
Cloning of Foxp1 into the AAV-hSyn vector
We cloned Foxp1 cDNA, tagged with a fluorescence reporter mCherry (connected with T2A linker) into an AAV construct driven by the human synapsin (hSyn) promoter. Briefly, we initially amplified the vector backbone including the promoter sequence using the following primers: Vector_Fwd (5′-GTACAAGTAGACCCAGCTTTCTTGTACAAAGTG-3′) and Vector_Rev (5′-CTTGCATCATGGTGGCAGCCTGCTTTTTTG-3′). The Foxp1 and mCherry sequences were amplified, with the T2A linker sequence added in between Foxp1 and mCherry. Foxp1 was amplified from a mouse cDNA library using Foxp1_Fwd_1 (5′-ATGATGCAAGAATCTGGGTCTG-3′) and Foxp1_Rev_1 (5′-CTCCATGTCCTCATTTACTGGTTC-3′). Thereafter, part of the T2A sequence was added to the -3′end of the Foxp1 cDNA in two consecutive rounds of reactions using two reverse primers: Foxp1_Rev_2 (5′-GATGTTAGAAGACTTCCCCTGCCCTCTCCGCTTCCCTCCATGTCCTCATTTACTG-3′) and Foxp1_Rev_3 (5′-CACGTCCCCGCATGTTAGAAGACTTCCC-3′), along with the forward primer Foxp1_Fwd_2 (5′-GGCTGCCACCATGATGCAAGAATCTGGG-3′). Similarly, mCherry was amplified using another mCherry-containing plasmid as a template. The remaining part of the T2A sequence was added to the 5′-end of the mCherry sequence by two consecutive polymerase chain reaction (PCR) reactions using mCherry_Fwd_1 (5′-CGGGGACGTGGAGGAAAATCCCGGCCCCATGGTGAGCAAGGGCGAGGAGGATAA-3′) and mCherry_Fwd_2 (5′-TTCTAACATGCGGGGACGTGGAGGAAAATC-3′), along with the reverse primer mCherry_Rev (5′-AAAGCTGGGTCTACTTGTACAGCTCGTCCATG-3′), respectively. Subsequently, the three fragments were ligated using the NEB Gibson assembly cloning kit (NEB, catalog no. E5510), and the ligated product was transformed into NEB5-alpha competent Escherichia coli cells (NEB, catalog no. C2987H). Positive clones were verified through restriction digestion and PCR. The sequences of the positive clones were also confirmed with Sanger sequencing (fig. S7A).
To confirm the expression of FOXP1 protein expression in these clones, we performed transfection in HEK293 cells followed by Western blotting (fig. S7B). Briefly, HEK cells were cultured at 37°C with 5% CO2 in 1X Dulbecco’s modified Eagle’s medium (Gibco, catalog no. 12491015) supplemented with 10% fetal bovine serum and 1X penicillin-streptomycin mix (Gibco, catalog no. 15070063). Cells in a six-well plate were transfected at 70% confluency with 2 μg of the plasmid mixed with Lipofectamine 3000 transfection reagent (Invitrogen, catalog no. L3000001) as per the manufacturer’s protocol. After 48 hours of transfection, cells were washed with 1X PBS and lysed in 300 μl of 2x Laemmli sample nuffer (Bio-Rad, catalog no. 1610737), followed by incubation at 95°C for 5 min. Equal volumes of each lysate were loaded onto a 10% SDS–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane. After blocking with 5% milk in TBST (1× TBS and 0.05% Tween 20) for 1 hour at room temperature, the membranes were incubated with primary antibodies in 2% bovine serum albumin (BSA) in TBST at 4°C overnight. Following washing with TBST, the blots were incubated with horseradish peroxidase–conjugated secondary antibodies, mixed in 5% milk in TBST for 1 hour at room temperature. The membranes were washed again and then developed using the SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, catalog no. 34580). Signal acquisition was performed using a ChemiDoc MP (Bio-Rad) and analyzed using ImageLab (Bio-Rad) or Fiji.
Once the clones were confirmed, bacterial colonies for the selected plasmid were grown in 200 ml of LB broth for 14 to 15 hours at 37°C with agitation at 220 rpm in a shaking incubator to generate enough plasmid for AAV packaging. Bacterial cells were then pelleted, and plasmid isolation was carried out using NucleoBond Xtra Midi EF (Machery Nagel, catalog no. 740420.50) following the manufacturer’s protocol. A total of 140 μg of purified plasmid was packaged into AAV particles by the Neuroconnectivity Core at Baylor College of Medicine, resulting in a titer of 9.42 × 1012 genome copies (GC)/ml. The control virus for this experiment, pAAV9-hSyn-mCherry, was obtained (Addgene, catalog no. 114472) with a titer of 2.7 × 1013 GC/ml. Viral particles were diluted to a final titer of ~3 × 1012 GC/ml in sterile 1X PBS, along with Fast Green FCF dye, and were subsequently injected into the striatum of P1 pups as described above. In our experiments, we used D2 Foxp1cKO and D2 Foxp1CTL mice where eGFP-positive cells represented D2 SPNs, while mCherry marked the cells expressing either control pAAV9-hSyn-mCherry (ControlAAV) or pAAV9-hSyn-Foxp1-T2A-mCherry (Foxp1AAV) virus.
Immunoblotting from brain tissue
The striatum was dissected from mice and snap frozen in liquid nitrogen followed by storage at −80°C. For immunoblotting, tissue samples were homogenized using a Fisherbrand Pellet Pestle Cordless Motor (Thermo Fisher Scientific, catalog no. 12-141-361) in a lysis buffer composed of 20 mM tris (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.1% SDS, 0.1% sodium deoxycholate, 5% glycerol, EDTA-free protease inhibitor cocktail (Sigma-Aldrich catalog no. 11873580001), and phosphatase inhibitor mixture 2 (Sigma-Aldrich, catalog no. P5726) and phosphatase inhibitor mixture 3 (Sigma-Aldrich, catalog no. P0044). Tissue lysates were further homogenized using brief 20-s pulses of sonication until clear lysates were obtained. Lysates were then centrifuged at 10,000g for 10 min at 4°C to remove the insoluble fraction. The resulting supernatant was used for protein quantification using a BCA protein assay kit (Pierce, catalog no. 23227). Equal amounts of protein were mixed with the 4X loading dye [40% glycerol, 240 mM tris (pH 6.8), 8% SDS, 0.045 bromophenol blue, and 5% β-mercaptoethanol] and then incubated for 5 min at 95°C before being loaded on to a 10% SDS-PAGE. The subsequent steps of the immunoblotting procedure followed the same protocol as detailed above for HEK cells. To enable immunoblotting for multiple proteins on the same membrane, membranes were carefully cut at appropriate molecular weights. The primary antibodies were FOXP1 (in-house), mCherry (Proteintech, catalog no. 26765-1-AP), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Millipore, catalog no. MAB374), actin (Millipore, catalog no. MAB1501). Proteins of interest were quantified after normalization with GAPDH or actin, which served as housekeeping proteins.
Immunohistochemistry
Mice aged 1 week or older were anesthetized with ketamine/xylazine and subsequently transcardially perfused with PBS, followed by 4% paraformaldehyde (PFA) in PBS. Brains were then post-fixed overnight in 4% PFA. For mice younger than 1 week, perfusion was not conducted, and their brains were solely subjected to post-fixation. After post-fixation, brains were washed with PBS and then immersed in a 30% sucrose in PBS for 48 hours followed by embedding in tissue plus OCT compound (Fisher Health Care, catalog no. 4585) and storing at −80°C. The brains were sectioned into 30 μm thick coronal sections using a cryostat (Leica, catalog no. CM1950) at −20°C and collected in PBS. The sections containing the striatum were transferred onto Superfrost plus microscope slides (Thermo Fisher Scientific, catalog no. 12550-15) and allowed to dry. Subsequently, the sections were washed and permeabilized in 1X tris-buffered saline (TBS) followed by 1-hour incubation in a blocking solution (0.3 M glycine, 3% BSA, and 10% normal donkey serum in 1X TBS) at room temperature. Sections were again washed with TBS and incubated with primary antibodies (Rabbit anti-mCherry, Proteintech, catalog no. 26765-1-AP) in blocking solution (without glycine) overnight at 4°C. Sections were then washed in TBS and incubated with secondary antibodies [Donkey anti-Rabbit IgG (H+L), Alexa Fluor 555, Invitrogen, catalog no. A-31572] prepared in 3% BSA in 1X TBS for 2 hours at room temperature. The eGFP signal from Drd2-eGFP transgene did not require additional staining for eGFP, and it was naturally detectable. Lastly, following three more washes with 1X TBS, the sections were dried and mounted using Prolong Diamond Antifade with DAPI mountant (Invitrogen, catalog no. P36971). Fluorescence images were acquired using a confocal laser scanning microscope (Zeiss, LSM 880) with a 20× objective.
Single-nuclei RNA sequencing
Tissue collection and nuclei isolation
P18 mice were anaesthetized and sacrificed by rapid decapitation and the brain was quickly dissected and transferred to ice-cold nACSF that contained 125 mM NaCl, 25 mM NaHCO3, 10 mM dextrose, 3 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2∙2H2O, and 2 mM MgCl2 with a pH of 7.3 to 7.4 and an osmolality of 300 to 305 mOsm. We placed the brain onto an ice-cold 1.0-mm stainless steel brain matrix (Stoelting, catalog no. 51386) and obtained three 1.0-mm-thick striatal sections. These sections were then transferred into a petri dish containing cold nACSF. Under a dissection microscope, we dissected the striatum from these slices using forceps. The isolated striatal tissue was flash-frozen in liquid nitrogen and stored at −80°C. The striatal tissue was collected from mice of required genotypes at different time points.
Protocol for isolating nuclei was adapted and modified from our previously published work (8). Briefly, tissue was homogenized 20 times with pestle A, followed by 20 times with pestle B, in a glass Dounce tissue grinder (Sigma-Aldrich, catalog no. D8938) using 2 ml of ice-cold EZ Nuclei Lysis Buffer (Sigma-Aldrich, catalog no.NUC-101). The homogenized samples were then transferred into a 15-ml tube with an additional 2 ml of ice-cold EZ Nuclei lysis buffer, incubated on ice for 3 min, and centrifuged at 500g for 5 min at 4°C. The supernatant was discarded, and the nuclei pellet was resuspended in 3 ml of ice-cold nuclei suspension buffer [NSB; 1X PBS, 0.01% ultrapure BSA, and 0.1% ribonuclease inhibitor] and centrifuged again at 500g for 5 min at 4°C. The supernatant was then discarded, and nuclei pellet was resuspended in 300 to 400 μl of NSB and filtered through 40-μm size Flowmi cell strainer (Bel-Art Products, catalog no. H13680-0040). An aliquot of the filtered nuclei suspension was mixed with Trypan blue and transferred into a disposable cell counting chamber nuclei to visualize and count the healthy nuclei under a light microscope ensuring a desired nuclei concentration of 700 to 1200 nuclei/μl.
Preparation of cDNA libraries and sequencing
We prepared libraries from a total of nine mice (three mice per genotype) using the Chromium Next GEM Single Cell 3′ Reagent Kit v3.1 (10X Genomics, catalog no. 1000121) with a targeting goal of 10,000 nuclei per sample as per the manufacturer’s instructions. All mice received injections of either ControlAAV or Foxp1AAV in their striatum at P1, where only a fraction of neurons was infected that were identified on the basis of the presence of AAV transcripts. However, the relative proportion of AAV transcripts within the pool of total transcripts captured by snRNA-seq experiment was small. To address this, we used a modified approach (95) in which we specifically amplified the viral transcript sequences and prepared separate libraries. Briefly, we used 5% of the total cleaned up cDNA (amplified from the GEMs that were generated from each sample) and amplified viral transcripts using reverse primer: 10X_Enrich_R (5′-CTACACGACGCTCTTCCG-3′) and forward primers 10X_WPRE_F1 (5′-ACGAGTCGGATCTCCCTTT-3′, for ControlAAV infected mice) and 10X_mCherry_F1 (5′-GCATGGACGAGCTGTACA-3′, for Foxp1AAV infected mice). We used different forward primers for ControlAAV and Foxp1AAV infected mice because ControlAAV vector has a WPRE sequence, while Foxp1AAV has mCherry sequence at the -3′end (we removed WPRE sequence from the Foxp1AAV vector to accommodate Foxp1 and mCherry into the vector). The first round of PCR amplification was carried out for 25 cycles using Phusion High-Fidelity PCR Master Mix with HF Buffer (NEB, catalog no. M0531S). The amplified PCR product was then run on an agarose gel, where a ~250–base pair (bp) product was excised from the gel and eluted using the QIAquick Gel Extraction Kit (QIAGEN, catalog no. 28706) in 20 μl of elution buffer (NEB). A 5% of this first round amplified product was used to perform a second round of amplification using the reverse primer 10X_WPRE_F1 and forward primers 10X_WPRE_F2 (5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTACGAGTCGGATCTCCCTTT-3′ControlAAV) and 10X_mCherry_F2 (5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTGTACAAGTAGACCCAGCTT-3′ Foxp1AAV) for 20 cycles using the same Phusion polymerase. Once again, this amplified PCR product was loaded on an agarose gel where similar size product (~250 bp) was excised and eluted in 30 μl of elution buffer. The eluted PCR products were cleaned up using SPRIselect reagent and subjected to sample-index PCR amplification according to the manufacturer’s instructions (10X Genomics) to generate cDNA libraries. Last, both the total and AAV_transcript libraries were subjected to quality control assessments using the 4200 TapeStation System (Agilent, catalog no. G2991BA) and quantified using a Qubit 2.0 Fluorometer (Invitrogen). We pooled 5 nM total libraries with 0.5 nM AAV_transcript libraries (10% of the total library) for each sample and sequenced on a NovaSeq 6000 (Illumina) platform using an S4 flow cell.
Preprocessing of sequencing data
We obtained raw sequencing data for both total and AAV_transcript libraries from the McDermott Sequencing Core at UT Southwestern in the form of binary base call (BCL) files. These BCL files were subsequently demultiplexed using 10X Genomics i7 index (used during library preparation) and Illumina’s bcl2fastq v2.19.1 and mkfastq command from 10X Genomics CellRanger v3.0.2 suite. Resulting FASTQ files were assessed for quality using FASTQC (v0.11.5) (96). To facilitate alignment and quantification, a reference mouse genome annotation index was created using mouse genome (GRCm38p6) and Gencode annotation (vM17) with mkref command from 10X Genomics CellRanger v3.0.2 suite. Extracted and quality-passed FASTQ reads for primary samples were then aligned to reference mouse genome annotation index, and raw count tables were generated using count command from 10X Genomics CellRanger v3.0.2 suite. CellBender’s (97) remove-background command was then run on the raw unfiltered count tables to discard potential ambient RNA. Also, potential doublets were identified with DoubletFinder (98) using filtered count tables generated by CellBender. This comprehensive pipeline produced an expression matrix with nuclei as columns and genes as rows, encompassing all primary samples and serving as the basis for subsequent analysis. A custom reference mouse genome annotation index was separately constructed to include the amplified viral sequences along with mouse genome (GRCm38p6) and Gencode annotation (vM17) with mkref command from 10X Genomics CellRanger v3.0.2 suite. Extracted and quality-passed FASTQ reads for these samples with amplified viral transcript specific sequences were then aligned to the custom reference mouse genome annotation index and raw count tables for amplified viral transcript sequences were generated using count command from 10X Genomics CellRanger v3.0.2 suite.
Clustering analysis
Cleaned count tables for primary samples were used to run Seurat pipeline following the vignette (https://satijalab.org/seurat/articles/pbmc3k_tutorial.html). Samples across genotypes were processed through Harmony (99) using command RunHarmony to remove the effects of covariates such as batch and sex. Harmonized Seurat objects were then integrated following vignette (https://portals.broadinstitute.org/harmony/SeuratV3.html). Integrated data was clustered to identify groups of nuclei by cell types using the top 59 principal components identified using JackStraw and ScoreJackStraw approach within Seurat. Resolution of 1.2 was selected using Clustree (https://github.com/lazappi/clustree) approach. FindAllMarkers was then run to identify gene markers corresponding to each identified cluster. Cell-type annotation was executed using known marker genes and by performing a Fisher’s exact test against cell-type marker genes previously identified in a reference dataset (100). Furthermore, nuclei corresponding to SPN classes were subclustered to resolve specific SPN subtypes (D1, D2, and eccentric SPNs) with higher granularity. Cell barcodes that belong to SPN classes were also marked for presence of viral amplified sequences with at least six unique molecular identifiers (UMIs) per cell barcode from samples with viral amplified sequences. Specifically, we designated any D2 SPN that exhibited at least six or more WPRE/mCherry UMIs as ControlAAV/Foxp1AAV-positive D2 SPN, respectively (fig. S9, A and B).
Differential gene expression analysis
For the analysis of DEGs, we focused on nuclei corresponding to the D2 SPN class and grouped by genotype. To identify genes with significant differential expression within D2 SPNs in D2 Foxp1cKO mice compared to D2 Foxp1CTL mice, we used edgeR (101)–based pseudobulk approach (102). The cutoff for significance was established as a false discovery rate (FDR) < 0.05 and log2 fold change ≥ 0.378. Similarly, we also identified DEGs for AAV-infected D2 SPNs that contain a minimum of six UMIs per cell barcode for viral amplified sequences, within the same genotype groups. The number of AAV-infected D2 SPNs exhibited variability across different samples. Thus, to impart a stringent cutoff to exclude false positives, we established a threshold of six UMIs to exclude at least 50% of D2 SPNs in each sample and included the remaining D2 SPNs, with the greatest number of viral UMI’s, in our analysis. We compared AAV-infected D2 SPNs in D2 Foxp1cKO + Foxp1AAV with those in D2 Foxp1cKO + ControlAAV and identified DEGs. Gene transcripts originating from mitochondrial (chr M) and sex chromosomes (chr X and chr Y) were excluded from the differential gene expression output, given that the samples encompassed mixed genders.
Gene Ontology analysis
For the analysis of Gene Ontology associated with DEGs, we used ClusterProfiler (103). Redundant ontology terms were subsequently removed using Revigo (104). In addition, we conducted synaptic Gene Ontology analysis using SynGO (www.syngoportal.org) (105) to annotate significant DEGs across different comparisons. All expressed genes within their respective groups were used as background for the ontological analyses.
Behavior
Nest building
Mice were separated and single housed for 24 hours in new cages with intact nestlet material. Nests were scored in adult mice for their quality based on previously established criteria (8) on a scale from one to five as previously described (106).
Open-field test
This test was performed by the behavior core at UT Southwestern. Mice were individually placed in a Plexiglass box (16 inch by 16 inches) and allowed to explore the arena for 10 min. Total distance moved and the amount of time spent in the periphery was measured using EthoVision XT software package (Noldus). Average velocity was determined by dividing the total distance by the amount of time spent in the box (600 s).
Rotarod test
Rotarod test was performed as described earlier (8, 17). Mice were marked on their tails and habituated for 30 min before being placed individually on a textured rod with lanes of a Series 8 IITC Life Science Rotarod. The rod was programmed to accelerate from 4 to 40 rpm over a 5-min period. Mice were positioned facing forward on the rod, and sensors beneath the rod were activated when a mouse fell off. If a mouse managed to make one full rotation while holding onto the rod, then the sensor was manually triggered, and the mouse was removed from the rod. Mice were tested for three consecutive days, with four trials each day and at least a 10-min interval between trials and latency to fall was recorded for each trial.
Statistics
For all datasets in electrophysiology measurements, the sample size is represented by the number of neurons and number of mice. For behavior experiments, the sample size is represented by the number of mice. All data are presented as means ± SEM. Unless otherwise specified, we conducted a repeated measure two-way analysis of variance (ANOVA) (or mixed model) followed by the Holm-Sidak test for multiple comparison correction. In cases where multiple comparison correction was not applicable, we used Fisher’s LSD test. For the remaining datasets, we used a t test (if the data followed a normal distribution) or a Mann-Whitney test (for non-Gaussian distribution). For snRNA-seq dataset, statistics are mentioned in Materials and Methods.
Acknowledgments
We thank K. Huber and T. Roberts for providing critical feedback on the manuscript, S. Yamazaki and the Neuroscience Microscopy Facility, supported by the UT Southwestern Neuroscience Department and the UTSW Peter O’Donnell Jr. Brain Institute, and the Behavior Core at UT Southwestern Medical Center.
Funding: This work was supported by the National Institute of Mental Health grants MH126481 and MH102603 (to G.K.), National Institute of Neurological Disorders and Stroke grants NS126143 and NS115821 (to G.K.), James S. McDonnell Foundation 21st Century Science Initiative in Understanding Human Cognition—Scholar Award 220020467 (to G.K.), and Simons Foundation grant 573689 (to G.K. and J.G.).
Author contributions: Conceptualization: N.K., J.G., and G.K. Investigation: N.K. snRNA-seq data analysis: A.K., N.K. Mice behavior experiments: N.K. and N.I.A. Mice colony maintenance: M.H. and N.K. Supervision: J.G. and G.K. Writing—original draft: N.K. and J.G. Writing—review and editing: N.K., J.G., A.K., and G.K.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Further information and requests should be directed to and will be fulfilled by the J.G. (Jay.Gibson@utsouthwestern.edu) and G.K. (Genevieve.Konopka@utsouthwestern.edu). Animals and materials generated from this study can be provided by the J.G. and G.K.’s pending scientific review and a completed materials transfer agreement. Requests for these materials should be submitted to J.G. (Jay.Gibson@utsouthwestern.edu) or G.K. (Genevieve.Konopka@utsouthwestern.edu). All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The sequencing data reported in this paper can be accessed at NCBI GEO with accession number GSE243662. Code that was used to perform data preprocessing, clustering, and differential gene expression analysis is available at Zenodo (https://doi.org/10.5281/zenodo.10685148) and GitHub (https://github.com/konopkalab/foxp1-spn-development).
Supplementary Materials
This PDF file includes:
Figs. S1 to S10
Legends for tables S1 to S3
Other Supplementary Material for this manuscript includes the following:
Tables S1 to S3
REFERENCES AND NOTES
- 1.Haber S. N., Corticostriatal circuitry. Dialogues Clin. Neurosci. 18, 7–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lieberman O. J., McGuirt A. F., Mosharov E. V., Pigulevskiy I., Hobson B. D., Choi S., Frier M. D., Santini E., Borgkvist A., Sulzer D., Dopamine triggers the maturation of striatal spiny projection neuron excitability during a critical period. Neuron 99, 540–554.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozorovitskiy Y., Saunders A., Johnson C. A., Lowell B. B., Sabatini B. L., Recurrent network activity drives striatal synaptogenesis. Nature 485, 646–650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozorovitskiy Y., Peixoto R., Wang W., Saunders A., Sabatini B. L., Neuromodulation of excitatory synaptogenesis in striatal development. eLife 4, e10111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Pozzo-Miller L., Dysfunction of the corticostriatal pathway in autism spectrum disorders. J. Neurosci. Res. 98, 2130–2147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L., Su Z., Wang Z., Li Z., Shang Z., Du H., Liu G., Qi D., Yang Z., Xu Z., Zhang Z., Transcriptional profiling reveals the transcription factor networks regulating the survival of striatal neurons. Cell Death Dis. 12, 262 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arlotta P., Molyneaux B. J., Jabaudon D., Yoshida Y., Macklis J. D., Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J. Neurosci. 28, 622–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson A. G., Kulkarni A., Harper M., Konopka G., Single-cell analysis of Foxp1-driven mechanisms essential for striatal development. Cell Rep. 30, 3051–3066.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q., Zhang Y., Wang C., Xu Z., Liang Q., An L., Li J., Liu Z., You Y., He M., Mao Y., Chen B., Xiong Z. Q., Rubenstein J. L., Yang Z., The zinc finger transcription factor Sp9 is required for the development of striatopallidal projection neurons. Cell Rep. 16, 1431–1444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y. C., Kuo H. Y., Bornschein U., Takahashi H., Chen S. Y., Lu K. M., Yang H. Y., Chen G. M., Lin J. R., Lee Y. H., Chou Y. C., Cheng S. J., Chien C. T., Enard W., Hevers W., Paabo S., Graybiel A. M., Liu F. C., Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c. Nat. Neurosci. 19, 1513–1522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferland R. J., Cherry T. J., Preware P. O., Morrisey E. E., Walsh C. A., Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J. Comp. Neurol. 460, 266–279 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Siper P. M., De Rubeis S., Trelles M. D. P., Durkin A., Di Marino D., Muratet F., Frank Y., Lozano R., Eichler E. E., Kelly M., Beighley J., Gerdts J., Wallace A. S., Mefford H. C., Bernier R. A., Kolevzon A., Buxbaum J. D., Prospective investigation of FOXP1 syndrome. Mol. Autism. 8, 57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horn D., Kapeller J., Rivera-Brugues N., Moog U., Lorenz-Depiereux B., Eck S., Hempel M., Wagenstaller J., Gawthrope A., Monaco A. P., Bonin M., Riess O., Wohlleber E., Illig T., Bezzina C. R., Franke A., Spranger S., Villavicencio-Lorini P., Seifert W., Rosenfeld J., Klopocki E., Rappold G. A., Strom T. M., Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum. Mutat. 31, E1851–E1860 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iossifov I., O'Roak B. J., Sanders S. J., Ronemus M., Krumm N., Levy D., Stessman H. A., Witherspoon K. T., Vives L., Patterson K. E., Smith J. D., Paeper B., Nickerson D. A., Dea J., Dong S., Gonzalez L. E., Mandell J. D., Mane S. M., Murtha M. T., Sullivan C. A., Walker M. F., Waqar Z., Wei L., Willsey A. J., Yamrom B., Lee Y. H., Grabowska E., Dalkic E., Wang Z., Marks S., Andrews P., Leotta A., Kendall J., Hakker I., Rosenbaum J., Ma B., Rodgers L., Troge J., Narzisi G., Yoon S., Schatz M. C., Ye K., McCombie W. R., Shendure J., Eichler E. E., State M. W., Wigler M., The contribution of de novo coding mutations to autism spectrum disorder. Nature 515, 216–221 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders S. J., He X., Willsey A. J., Ercan-Sencicek A. G., Samocha K. E., Cicek A. E., Murtha M. T., Bal V. H., Bishop S. L., Dong S., Goldberg A. P., Jinlu C., Keaney J. F. III, Klei L., Mandell J. D., Moreno-De-Luca D., Poultney C. S., Robinson E. B., Smith L., Solli-Nowlan T., Su M. Y., Teran N. A., Walker M. F., Werling D. M., Beaudet A. L., Cantor R. M., Fombonne E., Geschwind D. H., Grice D. E., Lord C., Lowe J. K., Mane S. M., Martin D. M., Morrow E. M., Talkowski M. E., Sutcliffe J. S., Walsh C. A., Yu T. W., Autism Sequencing C., Ledbetter D. H., Martin C. L., Cook E. H., Buxbaum J. D., Daly M. J., Devlin B., Roeder K., State M. W., Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87, 1215–1233 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stessman H. A., Xiong B., Coe B. P., Wang T., Hoekzema K., Fenckova M., Kvarnung M., Gerdts J., Trinh S., Cosemans N., Vives L., Lin J., Turner T. N., Santen G., Ruivenkamp C., Kriek M., van Haeringen A., Aten E., Friend K., Liebelt J., Barnett C., Haan E., Shaw M., Gecz J., Anderlid B. M., Nordgren A., Lindstrand A., Schwartz C., Kooy R. F., Vandeweyer G., Helsmoortel C., Romano C., Alberti A., Vinci M., Avola E., Giusto S., Courchesne E., Pramparo T., Pierce K., Nalabolu S., Amaral D. G., Scheffer I. E., Delatycki M. B., Lockhart P. J., Hormozdiari F., Harich B., Castells-Nobau A., Xia K., Peeters H., Nordenskjold M., Schenck A., Bernier R. A., Eichler E. E., Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 49, 515–526 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araujo D. J., Anderson A. G., Berto S., Runnels W., Harper M., Ammanuel S., Rieger M. A., Huang H. C., Rajkovich K., Loerwald K. W., Dekker J. D., Tucker H. O., Dougherty J. D., Gibson J. R., Konopka G., FoxP1 orchestration of ASD-relevant signaling pathways in the striatum. Genes Dev. 29, 2081–2096 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacon C., Schneider M., Le Magueresse C., Froehlich H., Sticht C., Gluch C., Monyer H., Rappold G. A., Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol. Psychiatry 20, 632–639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandelwal N., Cavalier S., Rybalchenko V., Kulkarni A., Anderson A. G., Konopka G., Gibson J. R., FOXP1 negatively regulates intrinsic excitability in D2 striatal projection neurons by promoting inwardly rectifying and leak potassium currents. Mol. Psychiatry 26, 1761–1774 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowles R., Dehorter N., Ellender T., From progenitors to progeny: Shaping striatal circuit development and function. J. Neurosci. 41, 9483–9502 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araujo D. J., Toriumi K., Escamilla C. O., Kulkarni A., Anderson A. G., Harper M., Usui N., Ellegood J., Lerch J. P., Birnbaum S. G., Tucker H. O., Powell C. M., Konopka G., Foxp1 in forebrain pyramidal neurons controls gene expression required for spatial learning and synaptic plasticity. J. Neurosci. 37, 10917–10931 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Oscos F., Koch T. M. I., Pancholi H., Trusel M., Daliparthi V., Co M., Park S. E., Ayhan F., Alam D. H., Holdway J. E., Konopka G., Roberts T. F., Autism-linked gene FoxP1 selectively regulates the cultural transmission of learned vocalizations. Sci. Adv. 7, eabd2827 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhai S., Tanimura A., Graves S. M., Shen W., Surmeier D. J., Striatal synapses, circuits, and Parkinson’s disease. Curr. Opin. Neurobiol. 48, 9–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H., Gao E. B., Draper A., Berens N. C., Vihma H., Zhang X., Higashi-Howard A., Ritola K. D., Simon J. M., Kennedy A. J., Philpot B. D., Rescue of behavioral and electrophysiological phenotypes in a Pitt-Hopkins syndrome mouse model by genetic restoration of Tcf4 expression. eLife 11, e72290 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd G. M., Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci. 14, 278–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kress G. J., Yamawaki N., Wokosin D. L., Wickersham I. R., Shepherd G. M., Surmeier D. J., Convergent cortical innervation of striatal projection neurons. Nat. Neurosci. 16, 665–667 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H., Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15, 193–204 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Huang Y. H., Schluter O. M., Dong Y., Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav. Brain Res. 216, 9–18 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petreanu L., Mao T., Sternson S. M., Svoboda K., The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krajeski R. N., Macey-Dare A., van Heusden F., Ebrahimjee F., Ellender T. J., Dynamic postnatal development of the cellular and circuit properties of striatal D1 and D2 spiny projection neurons. J. Physiol. 597, 5265–5293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacon C., Rappold G. A., The distinct and overlapping phenotypic spectra of FOXP1 and FOXP2 in cognitive disorders. Hum. Genet. 131, 1687–1698 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajkovich K. E., Loerwald K. W., Hale C. F., Hess C. T., Gibson J. R., Huber K. M., Experience-dependent and differential regulation of local and long-range excitatory neocortical circuits by postsynaptic Mef2c. Neuron 93, 48–56 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adesnik H., Li G., During M. J., Pleasure S. J., Nicoll R. A., NMDA receptors inhibit synapse unsilencing during brain development. Proc. Natl. Acad. Sci. U.S.A. 105, 5597–5602 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feliciangeli S., Chatelain F. C., Bichet D., Lesage F., The family of K2P channels: Salient structural and functional properties. J. Physiol. 593, 2587–2603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mermelstein P. G., Song W. J., Tkatch T., Yan Z., Surmeier D. J., Inwardly rectifying potassium (IRK) currents are correlated with IRK subunit expression in rat nucleus accumbens medium spiny neurons. J. Neurosci. 18, 6650–6661 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nisenbaum E. S., Xu Z. C., Wilson C. J., Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J. Neurophysiol. 71, 1174–1189 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Wilson C. J., Kawaguchi Y., The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J. Neurosci. 16, 2397–2410 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prybylowski K., Wenthold R. J., N-Methyl-D-aspartate receptors: Subunit assembly and trafficking to the synapse. J. Biol. Chem. 279, 9673–9676 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Malinow R., Malenka R. C., AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Nicoll R. A., Tomita S., Bredt D. S., Auxiliary subunits assist AMPA-type glutamate receptors. Science 311, 1253–1256 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Wan Y., Ade K. K., Caffall Z., Ilcim Ozlu M., Eroglu C., Feng G., Calakos N., Circuit-selective striatal synaptic dysfunction in the Sapap3 knockout mouse model of obsessive-compulsive disorder. Biol. Psychiatry 75, 623–630 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan Y., Feng G., Calakos N., Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. J. Neurosci. 31, 16685–16691 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch J. M., Lu J., Rodriguiz R. M., Trotta N. C., Peca J., Ding J. D., Feliciano C., Chen M., Adams J. P., Luo J., Dudek S. M., Weinberg R. J., Calakos N., Wetsel W. C., Feng G., Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature 448, 894–900 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weeber E. J., Beffert U., Jones C., Christian J. M., Forster E., Sweatt J. D., Herz J., Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277, 39944–39952 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Bateup H. S., Santini E., Shen W., Birnbaum S., Valjent E., Surmeier D. J., Fisone G., Nestler E. J., Greengard P., Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl. Acad. Sci. U.S.A. 107, 14845–14850 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calabresi P., Gubellini P., Centonze D., Picconi B., Bernardi G., Chergui K., Svenningsson P., Fienberg A. A., Greengard P., Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J. Neurosci. 20, 8443–8451 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blank T., Nijholt I., Teichert U., Kugler H., Behrsing H., Fienberg A., Greengard P., Spiess J., The phosphoprotein DARPP-32 mediates cAMP-dependent potentiation of striatal N-methyl-D-aspartate responses. Proc. Natl. Acad. Sci. U.S.A. 94, 14859–14864 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen C., Soto G., Dumrongprechachan V., Bannon N., Kang S., Kozorovitskiy Y., Parisiadou L., Pathway-specific dysregulation of striatal excitatory synapses by LRRK2 mutations. eLife 9, e58997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giesert F., Hofmann A., Burger A., Zerle J., Kloos K., Hafen U., Ernst L., Zhang J., Vogt-Weisenhorn D. M., Wurst W., Expression analysis of Lrrk1, Lrrk2 and Lrrk2 splice variants in mice. PLOS ONE 8, e63778 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matikainen-Ankney B. A., Kezunovic N., Mesias R. E., Tian Y., Williams F. M., Huntley G. W., Benson D. L., Altered development of synapse structure and function in striatum caused by parkinson's disease-linked LRRK2-G2019S mutation. J. Neurosci. 36, 7128–7141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding J. B., Oh W. J., Sabatini B. L., Gu C., Semaphorin 3E-plexin-D1 signaling controls pathway-specific synapse formation in the striatum. Nat. Neurosci. 15, 215–223 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M., Liu J., Li M., Zhang S., Lu Y., Liang Y., Zhao K., Li Y., Insulin-like growth factor 1/insulin-like growth factor 1 receptor signaling protects against cell apoptosis through the PI3K/AKT pathway in glioblastoma cells. Exp. Ther. Med. 16, 1477–1482 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villalba M., Bockaert J., Journot L., Concomitant induction of apoptosis and necrosis in cerebellar granule cells following serum and potassium withdrawal. Neuroreport 8, 981–985 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Gross I., Armant O., Benosman S., de Aguilar J. L., Freund J. N., Kedinger M., Licht J. D., Gaiddon C., Loeffler J. P., Sprouty2 inhibits BDNF-induced signaling and modulates neuronal differentiation and survival. Cell Death Differ. 14, 1802–1812 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Alamri A., Soussi Gounni A., Kung S. K. P., View point: Semaphorin-3E: An emerging modulator of natural killer cell functions? Int. J. Mol. Sci. 18, 2337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luchino J., Hocine M., Amoureux M. C., Gibert B., Bernet A., Royet A., Treilleux I., Lecine P., Borg J. P., Mehlen P., Chauvet S., Mann F., Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell 24, 673–685 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Tepper J. M., Sharpe N. A., Koos T. Z., Trent F., Postnatal development of the rat neostriatum: Electrophysiological, light- and electron-microscopic studies. Dev. Neurosci. 20, 125–145 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Pratt K. G., Aizenman C. D., Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J. Neurosci. 27, 8268–8277 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sim S., Antolin S., Lin C. W., Lin Y., Lois C., Increased cell-intrinsic excitability induces synaptic changes in new neurons in the adult dentate gyrus that require Npas4. J. Neurosci. 33, 7928–7940 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corbett C. M., Miller E. N. D., Wannen E. E., Rood B. D., Chandler D. J., Loweth J. A., Cocaine exposure increases excitatory synaptic transmission and intrinsic excitability in the basolateral amygdala in male and female rats and across the estrous cycle. Neuroendocrinology 113, 1127–1139 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandez-Perez E. J., Gallegos S., Armijo-Weingart L., Araya A., Riffo-Lepe N. O., Cayuman F., Aguayo L. G., Changes in neuronal excitability and synaptic transmission in nucleus accumbens in a transgenic Alzheimer’s disease mouse model. Sci. Rep. 10, 19606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turrigiano G. G., Nelson S. B., Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Braccioli L., Vervoort S. J., Adolfs Y., Heijnen C. J., Basak O., Pasterkamp R. J., Nijboer C. H., Coffer P. J., FOXP1 promotes embryonic neural stem cell differentiation by repressing jagged1 expression. Stem Cell Rep. 9, 1530–1545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pearson C. A., Moore D. M., Tucker H. O., Dekker J. D., Hu H., Miquelajauregui A., Novitch B. G., Foxp1 regulates neural stem cell self-renewal and bias toward deep layer cortical fates. Cell Rep. 30, 1964–1981.e3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Konstantoulas C. J., Parmar M., Li M., FoxP1 promotes midbrain identity in embryonic stem cell-derived dopamine neurons by regulating Pitx3. J. Neurochem. 113, 836–847 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Li X., Han X., Tu X., Zhu D., Feng Y., Jiang T., Yang Y., Qu J., Chen J. G., An autism-related, nonsense Foxp1 mutant induces autophagy and delays radial migration of the cortical neurons. Cereb. Cortex 29, 3193–3208 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Li X., Xiao J., Frohlich H., Tu X., Li L., Xu Y., Cao H., Qu J., Rappold G. A., Chen J. G., Foxp1 regulates cortical radial migration and neuronal morphogenesis in developing cerebral cortex. PLOS ONE 10, e0127671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palmesino E., Rousso D. L., Kao T. J., Klar A., Laufer E., Uemura O., Okamoto H., Novitch B. G., Kania A., Foxp1 and lhx1 coordinate motor neuron migration with axon trajectory choice by gating Reelin signalling. PLoS Biol. 8, e1000446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J., Frohlich H., Torres F. B., Silva R. L., Poschet G., Agarwal A., Rappold G. A., Mitochondrial dysfunction and oxidative stress contribute to cognitive and motor impairment in FOXP1 syndrome. Proc. Natl. Acad. Sci. U.S.A. 119, e2112852119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J., Rappold G. A., Frohlich H., Disrupted mitochondrial network drives deficits of learning and memory in a mouse model of FOXP1 haploinsufficiency. Genes 13, 127 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pisani A., Calabresi P., Centonze D., Bernardi G., Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br. J. Pharmacol. 120, 1007–1014 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pisani A., Gubellini P., Bonsi P., Conquet F., Picconi B., Centonze D., Bernardi G., Calabresi P., Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience 106, 579–587 (2001). [DOI] [PubMed] [Google Scholar]
- 73.Jin D. Z., Xue B., Mao L. M., Wang J. Q., Metabotropic glutamate receptor 5 upregulates surface NMDA receptor expression in striatal neurons via CaMKII. Brain Res. 1624, 414–423 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen H. H., Liao P. F., Chan M. H., mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J. Biomed. Sci. 18, 19 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chatterton J. E., Awobuluyi M., Premkumar L. S., Takahashi H., Talantova M., Shin Y., Cui J., Tu S., Sevarino K. A., Nakanishi N., Tong G., Lipton S. A., Zhang D., Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature 415, 793–798 (2002). [DOI] [PubMed] [Google Scholar]
- 76.West A. B., Cowell R. M., Daher J. P., Moehle M. S., Hinkle K. M., Melrose H. L., Standaert D. G., Volpicelli-Daley L. A., Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J. Comp. Neurol. 522, 2465–2480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mandemakers W., Snellinx A., O'Neill M. J., de Strooper B., LRRK2 expression is enriched in the striosomal compartment of mouse striatum. Neurobiol. Dis. 48, 582–593 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Skelton P. D., Tokars V., Parisiadou L., LRRK2 at striatal synapses: Cell-type specificity and mechanistic insights. Cells 11, 169 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parisiadou L., Yu J., Sgobio C., Xie C., Liu G., Sun L., Gu X. L., Lin X., Crowley N. A., Lovinger D. M., Cai H., LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat. Neurosci. 17, 367–376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Volta M., Beccano-Kelly D. A., Paschall S. A., Cataldi S., MacIsaac S. E., Kuhlmann N., Kadgien C. A., Tatarnikov I., Fox J., Khinda J., Mitchell E., Bergeron S., Melrose H., Farrer M. J., Milnerwood A. J., Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. eLife 6, e28377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Durakoglugil M. S., Wasser C. R., Wong C. H., Pohlkamp T., Xian X., Lane-Donovan C., Fritschle K., Naestle L., Herz J., Reelin regulates neuronal excitability through striatal-enriched protein tyrosine phosphatase (STEP(61)) and calcium permeable AMPARs in an NMDAR-dependent manner. J. Neurosci. 41, 7340–7349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meador-Woodruff J. H., Mansour A., Bunzow J. R., Van Tol H. H., Watson S. J. Jr., Civelli O., Distribution of D2 dopamine receptor mRNA in rat brain. Proc. Natl. Acad. Sci. U.S.A. 86, 7625–7628 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mengod G., Martinez-Mir M. I., Vilaro M. T., Palacios J. M., Localization of the mRNA for the dopamine D2 receptor in the rat brain by in situ hybridization histochemistry. Proc. Natl. Acad. Sci. U.S.A. 86, 8560–8564 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiner D. M., Brann M. R., The distribution of a dopamine D2 receptor mRNA in rat brain. FEBS Lett. 253, 207–213 (1989). [DOI] [PubMed] [Google Scholar]
- 85.Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G., Dopamine receptors: From structure to function. Physiol. Rev. 78, 189–225 (1998). [DOI] [PubMed] [Google Scholar]
- 86.Alcantara A. A., Chen V., Herring B. E., Mendenhall J. M., Berlanga M. L., Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 986, 22–29 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Lein E. S., Hawrylycz M. J., Ao N., Ayres M., Bensinger A., Bernard A., Boe A. F., Boguski M. S., Brockway K. S., Byrnes E. J., Chen L., Chen L., Chen T. M., Chin M. C., Chong J., Crook B. E., Czaplinska A., Dang C. N., Datta S., Dee N. R., Desaki A. L., Desta T., Diep E., Dolbeare T. A., Donelan M. J., Dong H. W., Dougherty J. G., Duncan B. J., Ebbert A. J., Eichele G., Estin L. K., Faber C., Facer B. A., Fields R., Fischer S. R., Fliss T. P., Frensley C., Gates S. N., Glattfelder K. J., Halverson K. R., Hart M. R., Hohmann J. G., Howell M. P., Jeung D. P., Johnson R. A., Karr P. T., Kawal R., Kidney J. M., Knapik R. H., Kuan C. L., Lake J. H., Laramee A. R., Larsen K. D., Lau C., Lemon T. A., Liang A. J., Liu Y., Luong L. T., Michaels J., Morgan J. J., Morgan R. J., Mortrud M. T., Mosqueda N. F., Ng L. L., Ng R., Orta G. J., Overly C. C., Pak T. H., Parry S. E., Pathak S. D., Pearson O. C., Puchalski R. B., Riley Z. L., Rockett H. R., Rowland S. A., Royall J. J., Ruiz M. J., Sarno N. R., Schaffnit K., Shapovalova N. V., Sivisay T., Slaughterbeck C. R., Smith S. C., Smith K. A., Smith B. I., Sodt A. J., Stewart N. N., Stumpf K. R., Sunkin S. M., Sutram M., Tam A., Teemer C. D., Thaller C., Thompson C. L., Varnam L. R., Visel A., Whitlock R. M., Wohnoutka P. E., Wolkey C. K., Wong V. Y., Wood M., Yaylaoglu M. B., Young R. C., Youngstrom B. L., Yuan X. F., Zhang B., Zwingman T. A., Jones A. R., Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Zeisel A., Hochgerner H., Lonnerberg P., Johnsson A., Memic F., van der Zwan J., Haring M., Braun E., Borm L. E., La Manno G., Codeluppi S., Furlan A., Lee K., Skene N., Harris K. D., Hjerling-Leffler J., Arenas E., Ernfors P., Marklund U., Linnarsson S., Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Durieux P. F., Bearzatto B., Guiducci S., Buch T., Waisman A., Zoli M., Schiffmann S. N., de Kerchove d'Exaerde A., D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat. Neurosci. 12, 393–395 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Feng X., Ippolito G. C., Tian L., Wiehagen K., Oh S., Sambandam A., Willen J., Bunte R. M., Maika S. D., Harriss J. V., Caton A. J., Bhandoola A., Tucker P. W., Hu H., Foxp1 is an essential transcriptional regulator for the generation of quiescent naive T cells during thymocyte development. Blood 115, 510–518 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ade K. K., Wan Y., Chen M., Gloss B., Calakos N., An improved BAC transgenic fluorescent reporter line for sensitive and specific identification of striatonigral medium spiny neurons. Front Syst. Neurosci. 5, 32 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gong S., Doughty M., Harbaugh C. R., Cummins A., Hatten M. E., Heintz N., Gerfen C. R., Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agmon A., Connors B. W., Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience 41, 365–379 (1991). [DOI] [PubMed] [Google Scholar]
- 94.Hays S. A., Huber K. M., Gibson J. R., Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J. Neurosci. 31, 14223–14234 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stroud H., Yang M. G., Tsitohay Y. N., Davis C. P., Sherman M. A., Hrvatin S., Ling E., Greenberg M. E., An activity-mediated transition in transcription in early postnatal neurons. Neuron 107, 874–890.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.S. Andrews. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- 97.Fleming S. J., Chaffin M. D., Arduini A., Akkad A. D., Banks E., Marioni J. C., Philippakis A. A., Ellinor P. T., Babadi M., Unsupervised removal of systematic background noise from droplet-based single-cell experiments using CellBender. Nat. Methods 20, 1323–1335 (2023). [DOI] [PubMed] [Google Scholar]
- 98.McGinnis C. S., Murrow L. M., Gartner Z. J., DoubletFinder: Doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P. R., Raychaudhuri S., Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saunders A., Macosko E. Z., Wysoker A., Goldman M., Krienen F. M., de Rivera H., Bien E., Baum M., Bortolin L., Wang S., Goeva A., Nemesh J., Kamitaki N., Brumbaugh S., Kulp D., McCarroll S. A., Molecular diversity and specializations among the cells of the adult mouse brain. Cell 174, 1015–1030.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robinson M. D., McCarthy D. J., Smyth G. K., edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Squair J. W., Gautier M., Kathe C., Anderson M. A., James N. D., Hutson T. H., Hudelle R., Qaiser T., Matson K. J. E., Barraud Q., Levine A. J., La Manno G., Skinnider M. A., Courtine G., Confronting false discoveries in single-cell differential expression. Nat. Commun. 12, 5692 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu G., Wang L. G., Han Y., He Q. Y., clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Supek F., Bosnjak M., Skunca N., Smuc T., REVIGO summarizes and visualizes long lists of gene ontology terms. PLOS ONE 6, e21800 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koopmans F., van Nierop P., Andres-Alonso M., Byrnes A., Cijsouw T., Coba M. P., Cornelisse L. N., Farrell R. J., Goldschmidt H. L., Howrigan D. P., Hussain N. K., Imig C., de Jong A. P. H., Jung H., Kohansalnodehi M., Kramarz B., Lipstein N., Lovering R. C., MacGillavry H., Mariano V., Mi H., Ninov M., Osumi-Sutherland D., Pielot R., Smalla K. H., Tang H., Tashman K., Toonen R. F. G., Verpelli C., Reig-Viader R., Watanabe K., van Weering J., Achsel T., Ashrafi G., Asi N., Brown T. C., De Camilli P., Feuermann M., Foulger R. E., Gaudet P., Joglekar A., Kanellopoulos A., Malenka R., Nicoll R. A., Pulido C., de Juan-Sanz J., Sheng M., Sudhof T. C., Tilgner H. U., Bagni C., Bayes A., Biederer T., Brose N., Chua J. J. E., Dieterich D. C., Gundelfinger E. D., Hoogenraad C., Huganir R. L., Jahn R., Kaeser P. S., Kim E., Kreutz M. R., McPherson P. S., Neale B. M., O'Connor V., Posthuma D., Ryan T. A., Sala C., Feng G., Hyman S. E., Thomas P. D., Smit A. B., Verhage M., SynGO: An evidence-based, expert-curated knowledge base for the synapse. Neuron 103, 217–234.e214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deacon R. M., Assessing nest building in mice. Nat. Protoc. 1, 1117–1119 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S10
Legends for tables S1 to S3
Tables S1 to S3