Abstract
INTRODUCTION
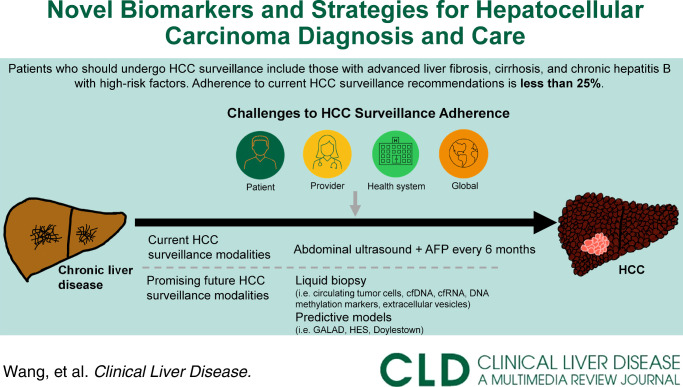
Liver cancer is the third most common cause of cancer death worldwide, the majority of which (70%–95%) is HCC that most commonly develops in the setting of chronic liver disease. While traditional risk factors for HCC (ie, viral hepatitis, alcohol use) remain important, rates of HCC in patients with metabolic dysfunction–associated liver disease are rising. Despite increasing HCC incidence, HCC surveillance rates remain low, with adherence in fewer than 25% of those at risk for HCC.1 Since HCC mortality significantly increases between early-stage and late-stage diagnoses, it is imperative to improve rates of early HCC diagnosis to decrease HCC-related mortality.1,2
DIAGNOSTIC CHALLENGES AND TREATMENT RESPONSE ASSESSMENT IN HCC
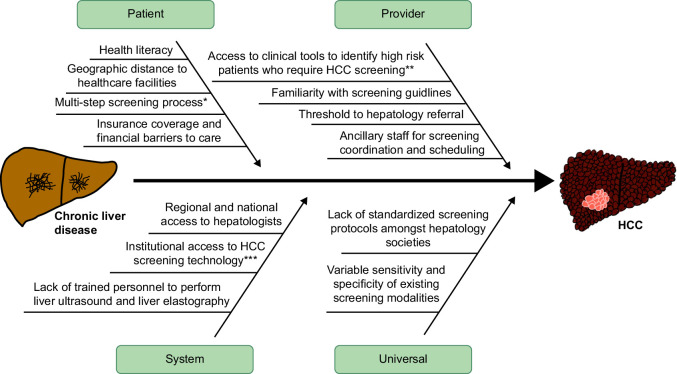
There are significant challenges to HCC surveillance at patient, provider, and system levels (Figure 1). Patient-level barriers include multi-step processes to undergo testing, adherence to biannual HCC screening, medical literacy surrounding the importance of adhering to HCC surveillance, insurance status, and access to care.1,2 On a provider level, time limitations during office visits, staying up to date with evolving hepatology society guidelines (Figure 2), and reliability of surveillance tests present barriers to both ensuring adherence to recommended surveillance in high-risk patients and early HCC diagnosis.1–3 System-level barriers to HCC surveillance include referral gaps from internists to hepatology specialists, the limited number of hepatologists geographically, insurance coverage of HCC screening tests, and institutional access to abdominal imaging and liver elastography machines.1
FIGURE 1.
Challenges in effective surveillance. *For example: obtaining an order for the exam, scheduling an imaging exam, and going to an imaging appointment **such as liver elastography, MRI, or biopsy for diagnosis of advanced fibrosis. ***CT/MRI machines, liver elastography machines, and laboratory assays.
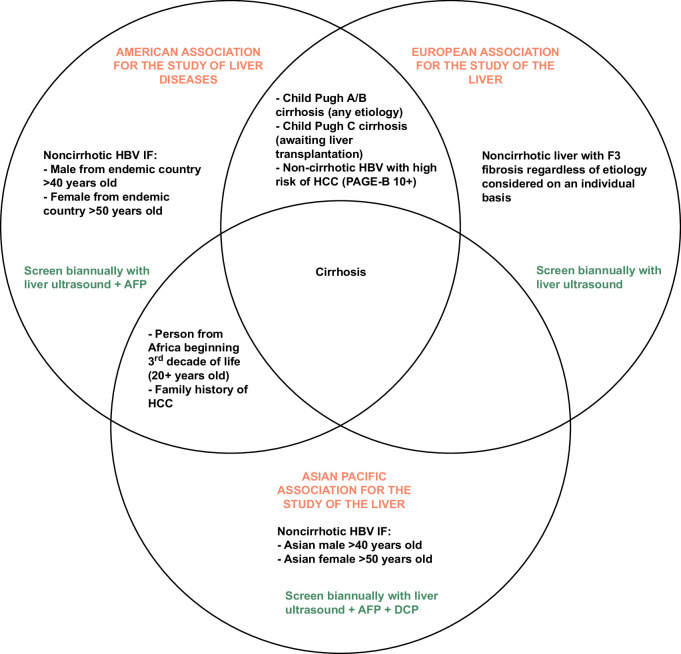
FIGURE 2.
Summary of surveillance guidelines by society. Abbreviation: AFP, alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin.
International practice guidelines for HCC surveillance remain largely unchanged in the last 2 decades, relying on some combination of abdominal ultrasound (US) and alpha-fetoprotein (AFP) (Figure 2).4–6 US is widely recommended as the initial screening test due to low cost and accessibility despite low sensitivity for early-stage HCC detection (47% for US alone, 63% for US with AFP).1 HCC treatment response is currently evaluated using serial cross-sectional imaging and serum AFP after treatment.4
CURRENT BIOMARKERS AND THEIR ROLES IN HCC CARE
The International Liver Cancer Association has published guidelines for the development and clinical application of biomarkers; many remain in the early stages of study (Table 1).19
TABLE 1.
Summary of biomarkers in use and in development
| Mechanism | Use | Sensitivity, % | Specificity, % | Phase of development | Reference | |
|---|---|---|---|---|---|---|
| Serum Biomarkers | ||||||
| AFP (cutoff 20 ng/mL) | Glycoprotein secreted by the adult liver in inflammatory states (ie, chronic HBV or HCV) Linked to the growth of HCC and evasion of intrinsic anti-tumor mechanisms Up to 20% of HCC are non-AFP producing |
Early detection, response to treatment AFP > 400 ng/mL is a poor prognostic indicator- ramicurumab effective in this population |
39–64 | 76–97 | Phase V | 7,8 |
| AFP-L3% | Subfraction of AFP | Early detection | 62 | 90 | Phase III | 9,10 |
| DCP | Prothrombin variant produced by HCC; levels vary with vitamin K levels | Early detection | 40 | 81 | Phase III | 9,10 |
| Liquid biopsy techniques | ||||||
| CTC | HCC tumor cells that enter the peripheral circulation | Early detection, Response to treatment | 95 | 73 | Phase III | 11 |
| cfDNA | Peripherally circulating nucleic acids released with cell turnover/apoptosis in malignant and inflammatory states | Early detection, Response to treatment | 91–96.8 | 43–98.8 | Phase II, III | 12–15 |
| cfRNA | Coding and noncoding RNA segments circulating in peripheral blood associated with tumor progression | Response to treatment | Variable depending on RNA segment | Variable depending on RNA segment | Phase III | 16 |
| DNA methylation markers | Changes in methylation patterns of HCC oncogenes and tumor suppressor genes | Response to treatment | Variable depending on gene | Variable depending on gene | Phase II | 15 |
| Extracellular vesicles | Membrane vesicles released by cells in both normal physiologic and pathologic states; contain bioactive molecules that can act at nearby or distant sites and are often involved in tumor growth | Early detection | 70–89 | 47–82 | Phase III | 17 |
| Clinical algorithms | ||||||
| GALAD | Gender, age, AFP-L3%, AFP, DCP | Early detection | 54–72 | 90 | Phase III published; Phase IV/V in progress | 3 |
| Doylestown Plus | AFP, age, gender, alkaline phosphatase, alanine aminotransferase | Early detection | 90 | 95 | Phase III | 18 |
| HCC early detection screening algorithm | AFP, rate of AFP change, age, alanine aminotransferase, platelets | Early detection | 53 | 48 | Phase II-III | 10 |
Abbreviations: AFP, alpha-fetoprotein; CfDNA, cell-free DNA;CfRNA, Cell-free RNA; CTC, circulating tumor cells; DCP, Des-γ-carboxy prothrombin; GALAD, Gender, Age, AFP-L3, AFP, and DCP.
AFP: The sensitivity of AFP alone to detect early-stage HCC is low (Table 1), in part because over 20% of HCC tumors do not produce AFP. In those that do, higher AFP levels are associated with more aggressive tumors, worse prognosis, and poorer treatment response. AFP trends are used for risk stratification in clinical trials and transplant eligibility. However, AFP alone is inadequate for early detection or treatment response in HCC.7
AFP-L3/DCP: AFP is fucosylated to become AFP-L3 during HCC growth.9 AFP-L3/AFP ratio (AFP-L3%) may be particularly useful in diagnosis and prognosis in patients with low-AFP–producing tumors.3 DCP has been incorporated into some HCC screening algorithms in conjunction with AFP and US because it is not elevated in chronic liver disease or cirrhosis.6
Because no biomarker has been validated as a standalone screening test, many studies have developed predictive models that utilize a combination of demographics, common laboratory values, and biomarkers, such as the Doylestown algorithm, the HCC Early Detection Screening algorithm, and the GALAD (Gender, Age, AFP-L3, AFP, and DCP) score (Table 1).3,10,18
Of these, the GALAD score is the most advanced in development. It has been validated in international populations from multiple liver disease states.3 In Phase 3 biomarker studies, GALAD has shown high sensitivity for diagnosis of early- and any-stage HCC,3 and an ongoing phase IV/V randomized control trial using GALAD for early HCC detection holds promise for incorporation into society guidelines.20
FUTURE STEPS ON BIOMARKERS IN HCC
Liquid biopsy techniques isolate and analyze tumor components in peripheral blood (eg, DNA/RNA fragments, tumor cells, and extracellular vesicles).21 Liquid biopsy is used for early detection and treatment response monitoring in acute myeloid leukemia and solid tumors such as prostate, breast, lung, colorectal, and ovarian cancer.21 Liquid biopsy in HCC is under active study (Table 1). Several whole genome sequencing and next-generation sequencing techniques of cell-free DNA have been validated with machine learning models in international patient cohorts to distinguish patients with HCC from those with cirrhosis and chronic liver disease with areas under the curve greater than 0.95.12–14 Though these are in earlier investigative phases than GALAD, their high sensitivity and specificity and relatively low-cost show promise for future use. Cell-free DNA and other liquid biopsy targets have also been studied to evaluate response to treatment—future applications may include individualized adjustments to systemic therapy or better selection of patients for liver transplantation for HCC cure.
It is worth noting that although many biomarkers and noninvasive algorithms are in development, none have adequate prospective data for adoption into guidelines. Currently, the American Association of Liver Disease recommends US with AFP every 6 months in high-risk patients.4 While we await accessible, affordable, and reliable biomarkers that might increase adherence to HCC surveillance and early detection, it is also important for practitioners to understand and identify high-risk patients for surveillance. For example, the American Association of Liver Disease recommends noninvasive fibrosis evaluations in patients with metabolic dysfunction–associated liver disease and certain comorbid conditions4 (Fig. 1).
For patients who require HCC surveillance, practitioners and health systems should work toward feasible solutions to support adherence to current guidelines—emphasizing the rationale and importance of surveillance to patients and arranging for its practical achievement (eg, scheduling imaging, lab collection, and clinic appointments on the same day; leveraging automated reminders to patients or in electronic medical records; or designing systems for local surveillance with reliable communication of findings to specialist providers).
Barriers to practical use and the rising global HCC burden necessitate better methods to risk stratify patients for HCC, detect it early, and individualize patient therapies. The last several years have brought rapid advances in the discovery and early validation of noninvasive biomarkers, whose future prospective study is likely to bring more accurate and accessible tools for HCC care.
Acknowledgments
FUNDING INFORMATION
Augusto Villanueva is funded by the NIH U01 U01CA283931.
CONFLICTS OF INTEREST
Augusto Villanueva has received consulting fees from FirstWorld, Pioneering Medicine and Genentech; advisory board fees from BMS, Roche, Astra Zeneca, Eisai, and NGM Pharmaceuticals; and research support from Eisai. He has stock options from Espervita and Atzeyo. He is listed as an inventor on a patent related to early detection of HCC (PCT/US20/61441), and funded by the NIH (1U01CA283931-01). The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AFP, alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin; GALAD, gender, Age, AFP-L3, AFP, and DCP; US, ultrasound.
Allison E. Wang and Emily A. Leven contributed equally as co-first authors.
Lauren T. Grinspan and Augusto Villanueva contributed equally as co-senior authors.
Contributor Information
Allison E. Wang, Email: allison.wang@mountsinai.org.
Emily A. Leven, Email: emily.leven@mountsinai.org.
Lauren T. Grinspan, Email: lauren.grinspan@mountsinai.org.
Augusto Villanueva, Email: augusto.villanueva@mssm.edu.
REFERENCES
- 1.Huang DQ, Singal AG, Kanwal F, Lampertico P, Buti M, Sirlin CB, et al. Hepatocellular carcinoma surveillance – utilization, barriers and the impact of changing aetiology. Nat Rev Gastroenterol Hepatol. 2023;20:797–809. [DOI] [PubMed] [Google Scholar]
- 2.Parikh ND, Tayob N, Singal AG. Blood-based biomarkers for hepatocellular carcinoma screening: Approaching the end of the ultrasound era? J Hepatol. 2023;78:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal AG, Tayob N, Mehta A, Marrero JA, El‐Serag H, Jin Q, et al. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology. 2022;75:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul JL, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 6.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11:317–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangkijvanich P, Anukulkarnkusol N, Suwangool P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: Analysis based on serum alpha-fetoprotein levels. Journal of Clinical Gastroenterology. 2000;31:302–308. [DOI] [PubMed] [Google Scholar]
- 8.Zhu AX, Finn RS, Kang Y-K, Yen CJ, Galle PR, Llovet JM, et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. British Journal of Cancer. 2021;124:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Kim GA, Han S, Lee W, Chun S, Lim YS. Longitudinal assessment of three serum biomarkers to detect very early-stage hepatocellular carcinoma. Hepatology. 2019;69:1983–1994. [DOI] [PubMed] [Google Scholar]
- 10.Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, et al. Validation of the updated hepatocellular carcinoma early detection screening algorithm in a community-based cohort of patients with cirrhosis of multiple etiologies. Clin Gastroenterol Hepatol. 2021;19:1443–1450.e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun YF, Guo W, Xu Y, Shi YH, Gong ZJ, Ji Y, et al. Circulating tumor cells from different vascular sites exhibit spatial heterogeneity in epithelial and mesenchymal composition and distinct clinical significance in hepatocellular carcinoma. Clin Cancer Res. 2018;24:547–559. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Wang Z, Tang W, Wang X, Liu R, Bao H, et al. Ultrasensitive and affordable assay for early detection of primary liver cancer using plasma cell-free DNA fragmentomics. Hepatology. 2022;76:317–329. [DOI] [PubMed] [Google Scholar]
- 13.Foda ZH, Annapragada AV, Boyapati K, Bruhm DC, Vulpescu NA, Medina JE, et al. Detecting liver cancer using cell-free DNA fragmentomes. Cancer Discov. 2023;13:616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Abou-Alfa GK, Zheng B, Liu JF, Bai J, Du LT, et al. Genome-scale profiling of circulating cell-free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res. 2021;31:589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2022;20:173–182.e177. [DOI] [PubMed] [Google Scholar]
- 16.Ning C, Cai P, Liu X, Li G, Bao P, Yan L, et al. A comprehensive evaluation of full-spectrum cell-free RNAs highlights cell-free RNA fragments for early-stage hepatocellular carcinoma detection. eBioMedicine. 2023;93:104645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun N, Zhang C, Lee YT, Tran BV, Wang J, Kim H, et al. HCC EV ECG score: An extracellular vesicle-based protein assay for detection of early-stage hepatocellular carcinoma. Hepatology. 2023;77:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Devarajan K, Singal AG, Marrero JA, Dai J, Feng Z, et al. The Doylestown Algorithm: A test to improve the performance of AFP in the detection of hepatocellular carcinoma. Cancer Prev Res (Phila). 2016;9:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singal AG, Hoshida Y, Pinato DJ, Marrero J, Nault JC, Paradis V, et al. International Liver Cancer Association (ILCA) white paper on biomarker development for hepatocellular carcinoma. Gastroenterology. 2021;160:2572–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singal AK. National Liver Cancer Biomarker Screening Trial (TRACER). Accessed December 12, 2023. Clinicaltrials.gov/study/NCT06084234.
- 21.Pelizzaro F, Cardin R, Penzo B, Pinto E, Vitale A, Cillo U, et al. Liquid biopsy in hepatocellular carcinoma: Where are we now? Cancers (Basel). 2021;13:2274. [DOI] [PMC free article] [PubMed] [Google Scholar]